Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 677-690 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Asri N, Ahmadi B, Taraghikhah N, Houri H, Jahani-Sherafat S, Rezaei-Tavirani M, et al . Tryptophan and Sleep Disruptions in Patients With Celiac Disease. BCN 2025; 16 (3) :677-690
URL: http://bcn.iums.ac.ir/article-1-2997-en.html
URL: http://bcn.iums.ac.ir/article-1-2997-en.html
Nastaran Asri1 

 , Behrooz Ahmadi2
, Behrooz Ahmadi2 

 , Nazanin Taraghikhah2
, Nazanin Taraghikhah2 

 , Hamidreza Houri3
, Hamidreza Houri3 

 , Somayeh Jahani-Sherafat4
, Somayeh Jahani-Sherafat4 

 , Mostafa Rezaei-Tavirani5
, Mostafa Rezaei-Tavirani5 

 , Mohadeseh Mahmoudi Ghehsareh2
, Mohadeseh Mahmoudi Ghehsareh2 

 , Ayad Bahadorimonfared6
, Ayad Bahadorimonfared6 

 , Mohammad Rostami-Nejad *2
, Mohammad Rostami-Nejad *2 




 , Behrooz Ahmadi2
, Behrooz Ahmadi2 

 , Nazanin Taraghikhah2
, Nazanin Taraghikhah2 

 , Hamidreza Houri3
, Hamidreza Houri3 

 , Somayeh Jahani-Sherafat4
, Somayeh Jahani-Sherafat4 

 , Mostafa Rezaei-Tavirani5
, Mostafa Rezaei-Tavirani5 

 , Mohadeseh Mahmoudi Ghehsareh2
, Mohadeseh Mahmoudi Ghehsareh2 

 , Ayad Bahadorimonfared6
, Ayad Bahadorimonfared6 

 , Mohammad Rostami-Nejad *2
, Mohammad Rostami-Nejad *2 


1- Student Research Committee, Celiac Disease and Gluten Related Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Laser Application in Medical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Proteomics Research Center, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Health and Community Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Foodborne and Waterborne Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Laser Application in Medical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Proteomics Research Center, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Department of Health and Community Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Celiac disease (CD), Sleep quality, Pittsburgh sleep quality index (PSQI) questionnaire, Tryptophan (Trp), Immune system
Full-Text [PDF 916 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Celiac disease (CD) is a chronic autoimmune disorder characterized by an inflammatory response to gluten, a protein found in wheat, barley, and rye (Gujral et al., 2012). The CD is a complex disorder with a wide range of symptoms, including gastrointestinal manifestations, such as abdominal pain and diarrhea, and extra-intestinal presentations, including dermatitis herpetiformis and reproductive issues (Therrien et al., 2020). Patients with CD also commonly report anxiety, depression, and other mood disorders, which may be linked to poor subjective sleep quality (Rostami-Nejad et al., 2020; Sharifnejad et al., 2023; Zingone et al., 2010). Prolonged inadequate sleep is associated with unfavorable outcomes, including the onset of chronic systemic inflammation, frequently observed in individuals with CD (Palumbo & Wyse, 2020; Sobolewska-Włodarczyk et al., 2021).
The intricate interplay between sleep and the immune system highlights how immune activation and inflammation disrupt sleep patterns. Inadequate sleep can impact immune responses and the production of pro/anti-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-2, IL-4, and IL-10 (Besedovsky et al., 2019; Gottshall et al., 2021; Hurtado-Alvarado et al., 2013; Irwin, 2023; Rockstrom et al., 2018). Additionally, sleep deprivation can elevate cortisol levels by activating the hypothalamic-pituitary-adrenal axis, leading to immune activation and the release of inflammatory cytokines, such as TNF-α and IL-2 (Garbarino et al., 2021; Irwin & Opp, 2017). The relationship between imbalanced inflammatory cytokines and sleep disorders is frequently observed in individuals with CD (Westerholm-Ormio et al., 2002; Zingone et al., 2010).
Tryptophan (Trp), an essential amino acid, is deficient in individuals with CD, as indicated by previous studies (Addolorato et al., 2004; Khalkhal et al., 2022). The primary breakdown of Trp occurs within the kynurenine pathway, mediated by the enzymes Trp 2,3-dioxygenase and indoleamine 2,3-dioxygenase. This metabolic pathway plays a crucial role in regulating both sleep and immune responses (Fallah et al., 2024; Heimberger & Lukas, 2023). Moreover, Trp serves as a precursor for the production of serotonin and melatonin, both of which are essential for modulating the sleep-wake cycle (Bhat et al., 2020). Recent research also suggests that in addition to their involvement in sleep regulation, serotonin and melatonin influence immune responses by binding to specific receptors, such as the 5-hydroxytryptamine receptor 1A, which is present on various immune cells, including macrophages and T lymphocytes (Arioz et al., 2019; Herr et al., 2017).
The relationship between CD and sleep disorders is reciprocal. CD can negatively impact sleep quality, and inadequate sleep can worsen the symptoms of CD through immune and inflammatory mechanisms (Ranjbaran et al., 2007). This study aimed to evaluate the sleep quality of individuals with CD. To achieve this, we utilized the Pittsburgh sleep quality index (PSQI) questionnaire, a widely recognized tool for assessing subjective sleep quality across various populations. Through the implementation of this questionnaire, we gathered data concerning participants’ sleep patterns, duration, problems, and overall sleep quality. Furthermore, we sought to investigate the potential connections between sleep disorder and inflammatory cytokines implicated in CD. Specifically, we focused on TNF-α, IL-4, IL-10, and IL-2, as well as the plasma levels of Trp.
2. Materials and Methods
We conducted a prospective study involving a cohort of 76 adults from the CD and Gluten-Related Disorders Research Center at Shahid Beheshti University of Medical Sciences, Tehran, Iran. The study was conducted between March 20, 2022, and December 28, 2022. Participants were selected based on specific inclusion and exclusion criteria to ensure the integrity of the research. The inclusion criteria included adults aged >18 years with a confirmed diagnosis of CD based on positive serological tests and histological biopsies of the small intestine, as per the modified Marsh criteria (Villanacci et al., 2020). Additionally, participants must not have consumed any Trp supplements in the month preceding the study. Individuals were excluded from the study if they had undergone any medical or surgical intervention for CD in the prior three months, had pre-existing sleep disorders (e.g. sleep apnea, insomnia) unrelated to CD, were currently taking medications that could affect sleep patterns, or inflammatory responses (such as corticosteroids), or were pregnant or breastfeeding. Written informed consent was obtained from all participants before their involvement, and each participant underwent the aseptic collection of 15 m/L of venous blood utilizing specialized vacuum tubes.
Sleep quality assessment
This study employed the PSQI to evaluate participants’ sleep quality. The PSQI questionnaire comprises 19 items, classified into seven subscales: Sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep problems, use of sleep medication, and daytime dysfunction. Each component is rated on a scale of 0–3, with a total score ranging from 0 to 21. A cumulative score of ≥6 indicates poor sleep quality (Buysse et al, 1989; Farrahi Moghaddam et al., 2012).
Evaluation of Trp plasma levels
To evaluate plasma Trp levels, peripheral blood samples were centrifuged at 3500 revolutions per minute (rpm) for 15 minutes. The resulting plasma fraction was then stored at -80 °C until needed for analysis by high-performance liquid chromatography (HPLC). The ACME 9000 system (Younglin, Anyang, Korea), equipped with a fluorescence detector essential for determining Trp levels, was utilized in this study. A methanol solution was used to remove proteins from the plasma samples. The deproteinization process involved vortexing the samples for 30 s and subsequent centrifugation at 5000 rpm for 7 minutes. The resulting clear supernatant was then prepared for further analysis.
Chromatographic separation of Trp was achieved by injecting 100 µL of the clear supernatant into a GL Sciences column with dimensions of 250×3.0 mm and a particle size of 3 µm. The mobile phase for this process comprised a mixture of methanol and tetrahydrofuran in a volumetric ratio of 4:1. Throughout the analysis, the fluorescence signals were monitored and recorded at optimal excitation and emission wavelengths of 340 nm and 450 nm, respectively.
Evaluation of pro-inflammatory cytokines in serum
The blood samples were allowed to clot for 30 minutes at room temperature before centrifugation at 1500×g for 10 minutes. Subsequently, serum was collected and stored at -80 °C for further biochemical analysis. The concentrations of human TNF-α and IL-10 in the serum were determined using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kit (Karmania Pars Gene, Iran), following the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader. Each determination was performed in triplicate by established laboratory principles.
Expression analysis of pro-inflammatory cytokines
Total ribonucleic acid (RNA) was isolated from whole blood samples of all participants using the YTA Total RNA Purification Mini kit for Blood/Cultured Cell/Tissue (Yekta Tajhiz Azma, Iran) according to the manufacturer’s instructions. RNA concentration and quality were assessed using a Nanodrop 1000 spectrophotometer (Fisher Thermo, Wilmington, DE, USA). After adjusting the RNA concentrations, complementary DNA (cDNA) synthesis was performed using the 2 Step 2X RTPCR Premix (Taq) kit (Biofact, South Korea), and the resulting complementary DNA (cDNA) was stored at -20 °C for quantitative real-time polymerase chain reaction (qPCR).
Specific primers for amplifying IL-2, IL-4, and beta-2-microglobulin (B2M), a housekeeping gene, were designed using Gene Runner software (version 3.05). The primer sequences were as follows: IL-2 forward: 5’-TACATGCCCAAGAAGGCCAC-3’, IL-2 reverse: 5’-AGCACTTCCTCCAGAGGTTTG-3’; IL-4 forward: 5’-CTTTGCTGCCTCCAAGAACAC-3’, IL-4 reverse: 5’-TTCCTGTCGAGCCGTTTCAG-3’; B2M forward: 5’-CCAGCGTACTCCAAAGATTC-3’, B2M reverse: 5’-ATGTCGGATGGATGAAACCC-3’.
The messenger ribonucleic acid (mRNA) expression levels of the target genes were evaluated using SYBR Premix Ex Taq (Real Q Plus 2x Master Mix Green-Amplicon, Japan) with the Rotor-Gene® Q real-time PCR system (Qiagen, Germany). All qPCR reactions were conducted in duplicate, and the mRNA expression level of each gene was calculated following the 2-ΔΔCt method (ΔCt=ΔCt target -ΔCt endogenous).
Statistical analysis
Data were analyzed using SPSS software version 25, developed by IBM (Chicago, IL, USA). Graphical representations were generated using GraphPad Prism 8.4.0, software (GraphPad Software, Inc.) (San Diego, CA, USA). Descriptive statistics were employed to summarize the characteristics of the study participants, including demographics, clinical features, and sleep quality parameters. An independent sample t-test compared two groups of continuous variables that followed a normal distribution. All results were reported with a 95% confidence interval. Statistical significance was set at P<0.05. The correlations between variables were assessed using Pearson’s correlation tests.
3. Results
Demographic and clinical characteristics of CD participants
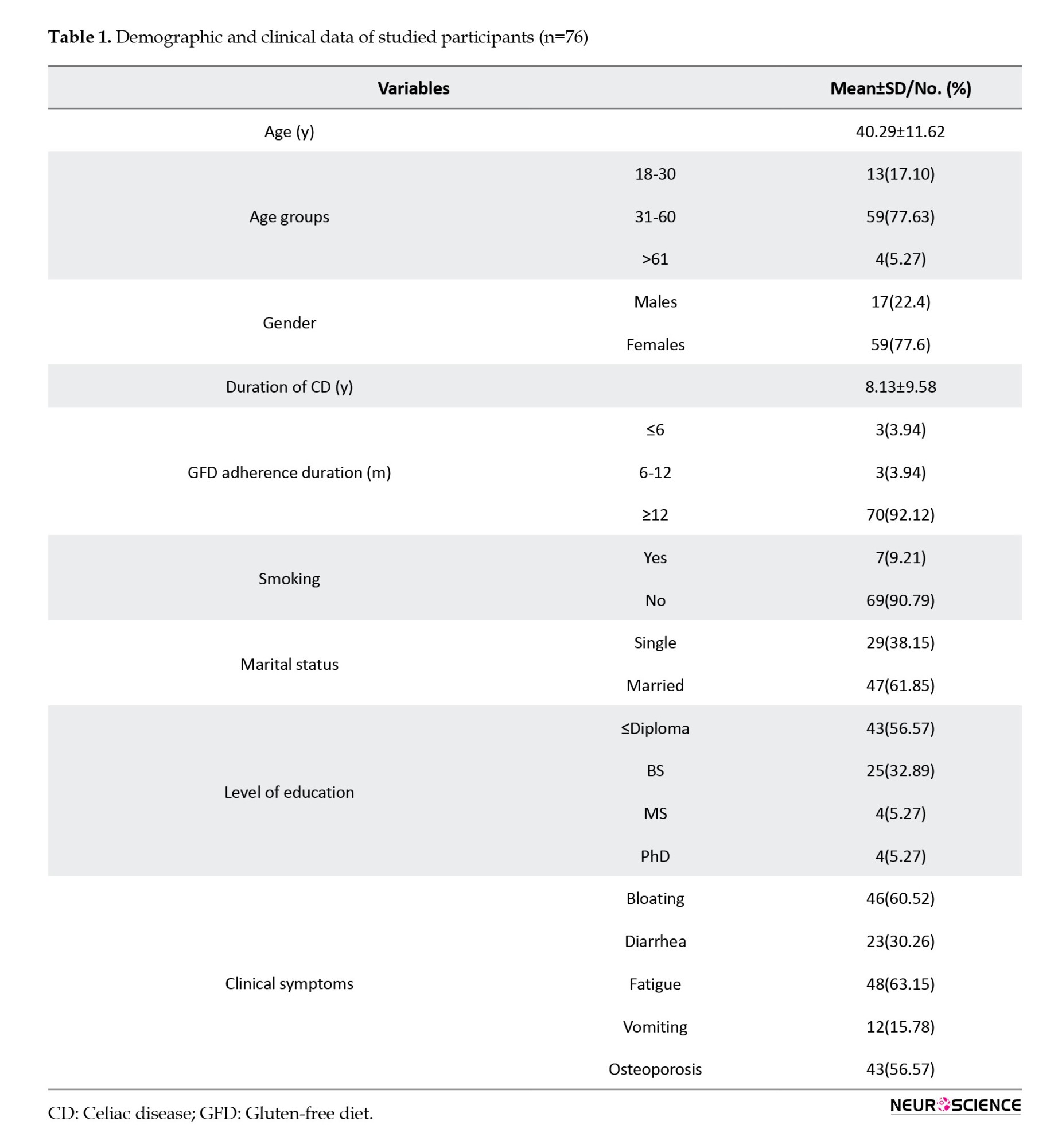
The study primarily involved adult participants, with a mean age of 40.29±11.62 years. Most of the samples were female (77.6%). Fatigue emerged as the predominant symptom among participants, affecting 63.15% of cases.
Comprehensive demographic and clinical data were collected, including information on CD duration, adherence to a gluten-free diet (GFD), smoking habits, marital status, and educational attainment. Table 1 presents a detailed breakdown of additional symptoms, such as bloating, diarrhea, vomiting, and osteoporosis.
Assessment of sleep quality in CD participants
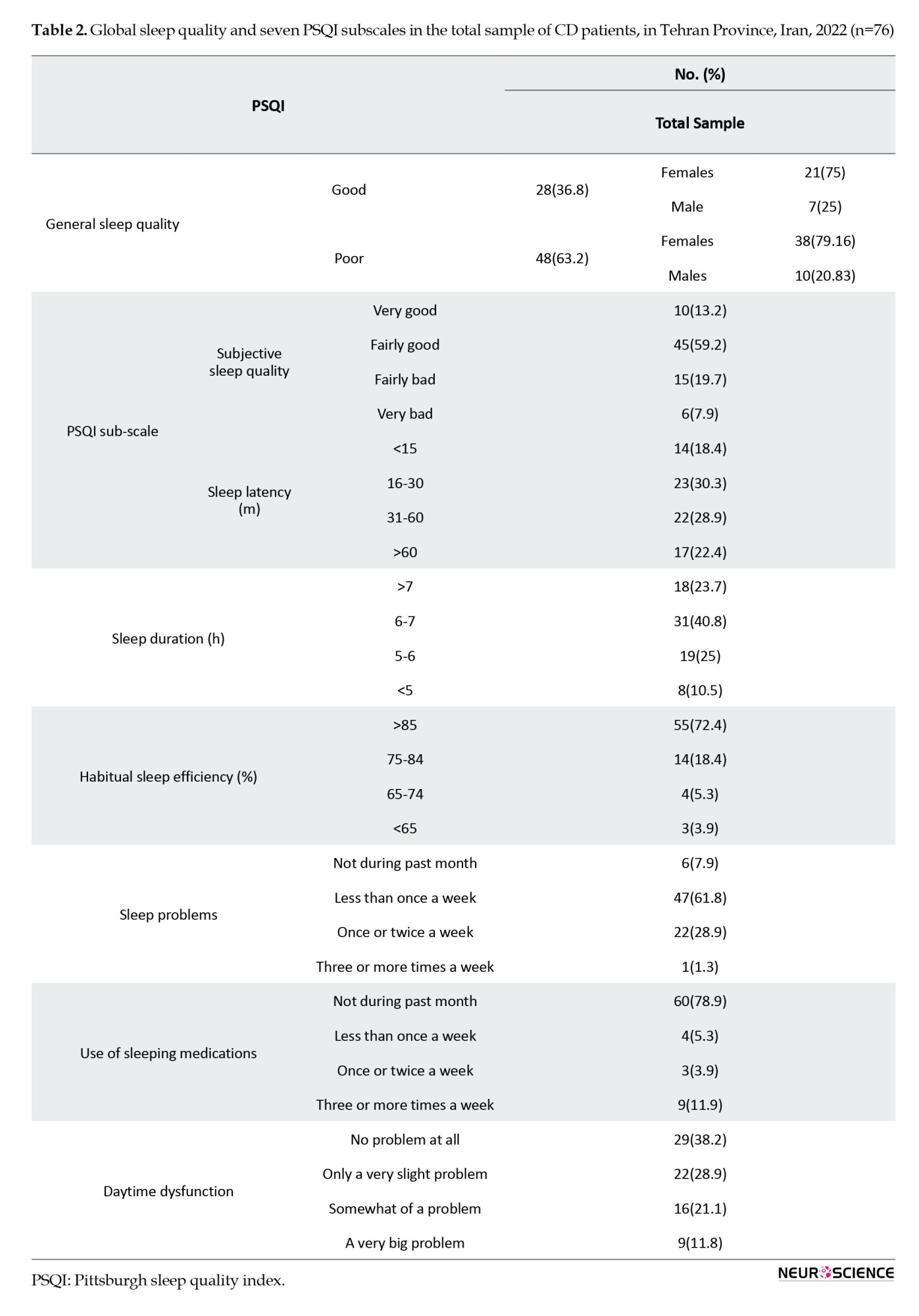
Table 2 presents a comprehensive analysis of overall sleep quality and seven specific PSQI subscales among patients with CD. Notably, 63.2% of the participants displayed signs of poor sleep quality based on the total questionnaire scores (most of whom were women [79.16%]). Concerning subjective sleep quality, 59.2% of the participants reported experiencing fairly good quality. Analysis of sleep latency distribution revealed that 30.3% experienced a latency period ranging from 16-30 minutes. Regarding sleep duration, the majority (40.8%) reported sleeping for 6-7 hours per night. Habitual sleep efficiency was notably high, with a majority (72.4%) of participants reporting an efficiency of over 85%. Sleep problems were reported by a considerable percentage (61.8%), occurring less than once a week. Furthermore, the majority (78.9%) of the participants reported not using any sleep medications. Daytime dysfunction showed variability, with the highest percentage (38.2%) of patients reporting no difficulties.
Plasma Trp concentration in poor and good sleepers
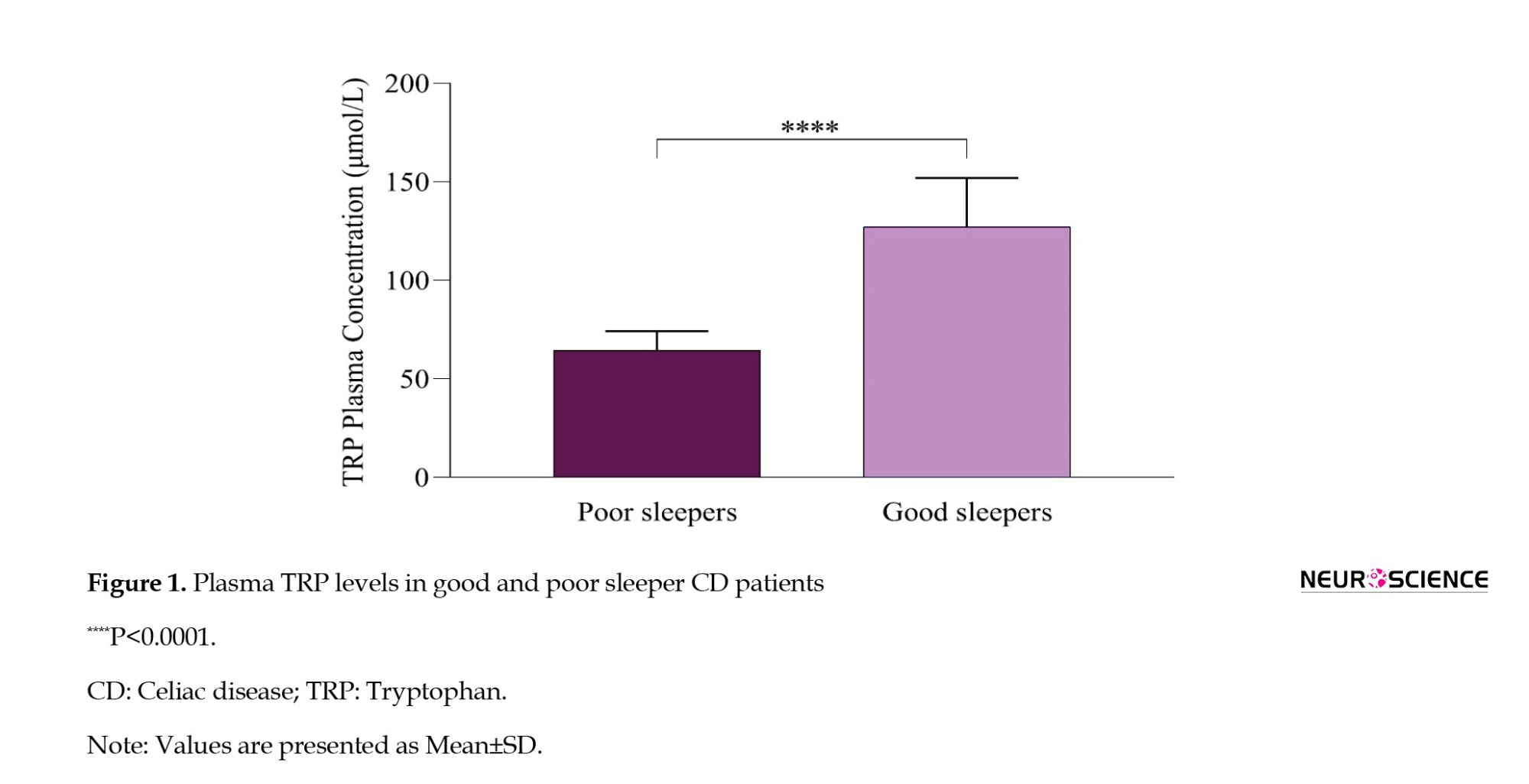
We assessed the plasma Trp concentrations in individuals diagnosed with CD, categorizing them based on their reported sleep quality as either poor or good. The results, illustrated in Figure 1, revealed a statistically significant lower plasma Trp concentration among participants who reported poor sleep compared to those with good sleep quality (P<0.0001).
Serum levels of NF-α and IL-10 in poor and good sleepers
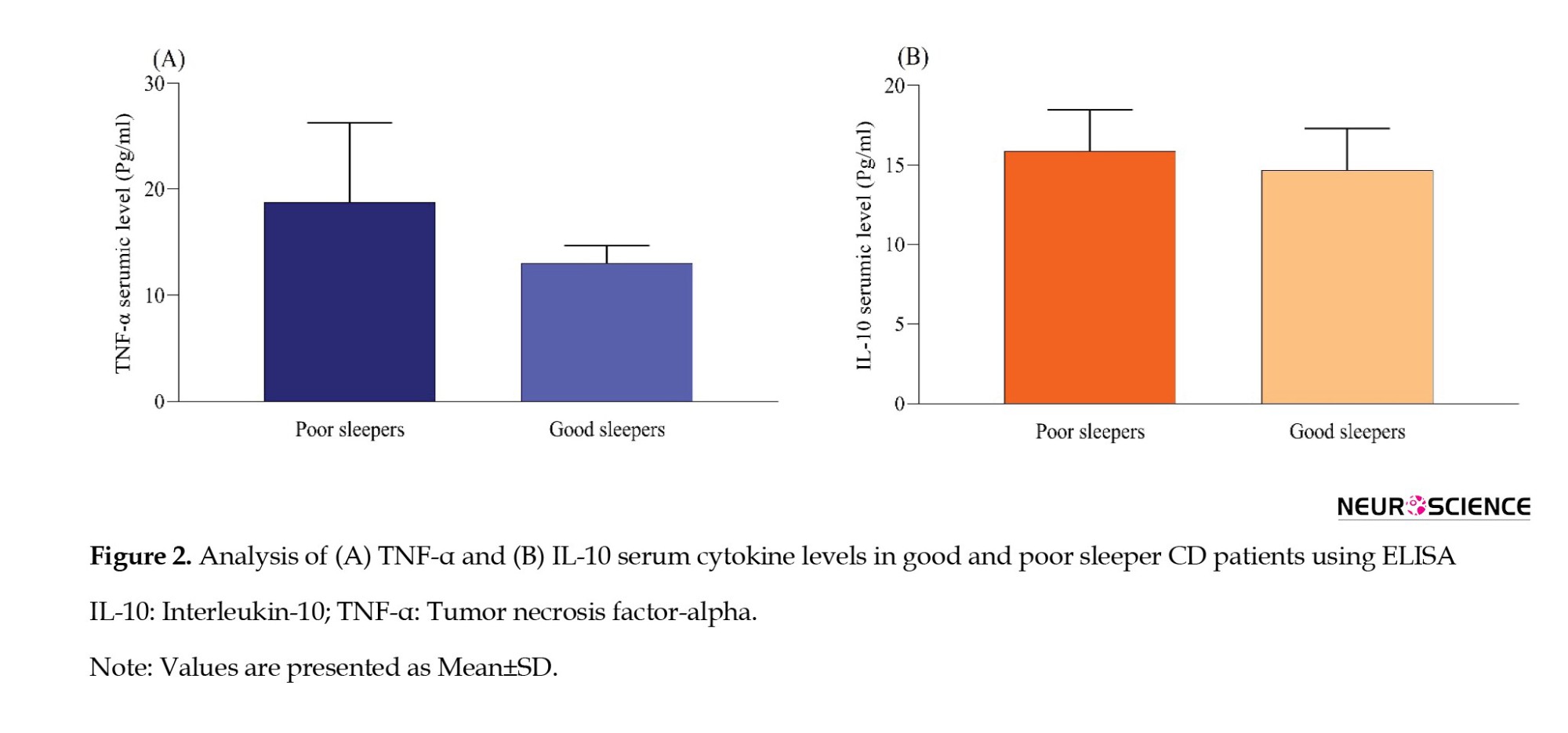
Serum TNF-α and IL-10 levels were measured in patients with CD with either poor or good sleep quality. No statistically significant difference was observed in the serum TNF-α and IL-10 levels between the group of individuals experiencing poor sleep and the group reporting good sleep quality (P>0.05) (Figure 2).
The expression levels of IL-2 and IL-4 mRNA in poor and good sleepers
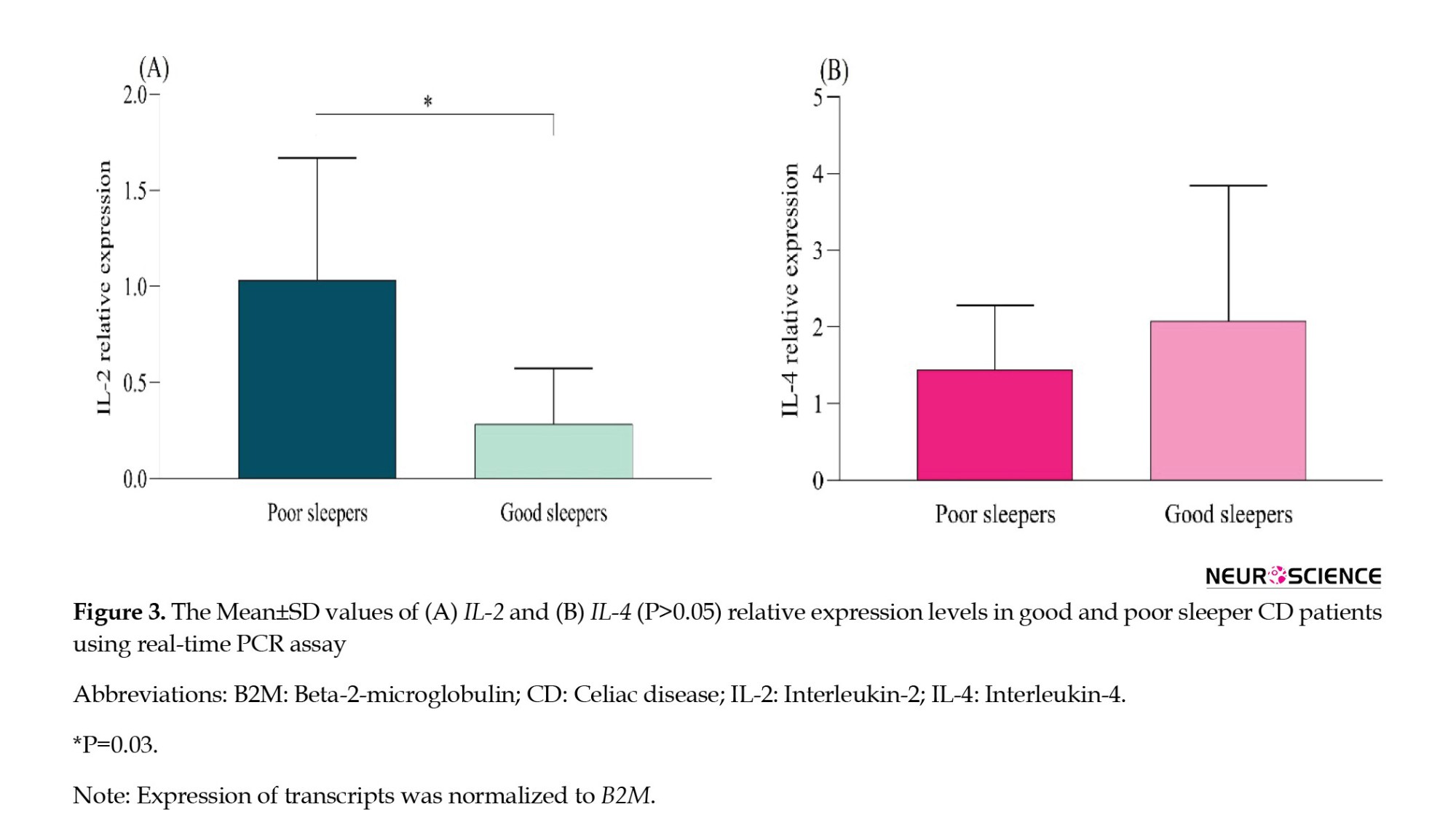
We examined the relative expression of IL-2 and IL-4 genes in individuals diagnosed with CD who reported varying levels of sleep quality. As depicted in Figure 3, our results revealed a significant increase in IL-2 expression in the group experiencing poor sleep compared to those reporting good sleep (P=0.03). Conversely, the change in the expression level of IL-4 was not statistically significant (P>0.05).
Correlation analysis
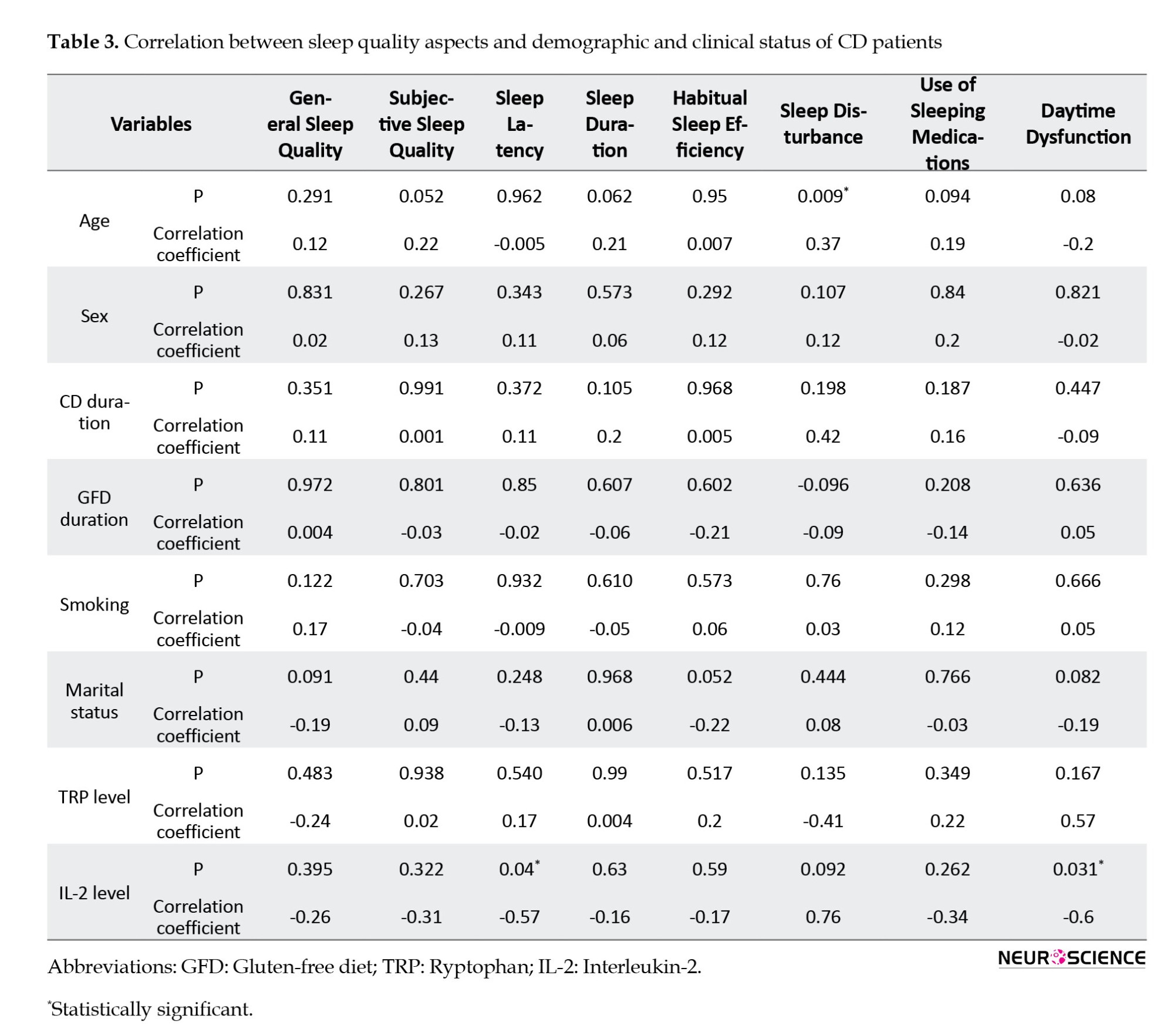
Pearson’s correlation analysis was conducted to assess the relationships between various factors, including age, sex, duration of CD, adherence to a GFD, smoking habits, marital status, IL-2 expression, plasma Trp levels, and different aspects of sleep quality. The results showed a significant positive correlation between participants’ age and sleep problems (P=0.009, r=0.37). Moreover, IL-2 mRNA levels were negatively correlated with sleep latency (P=0.04, r=-0.57) and daytime dysfunction (P=0.03, r=-0.6). No significant correlations were found between the other variables and different aspects of sleep quality (Table 3).
Primary awareness sources of CD patients
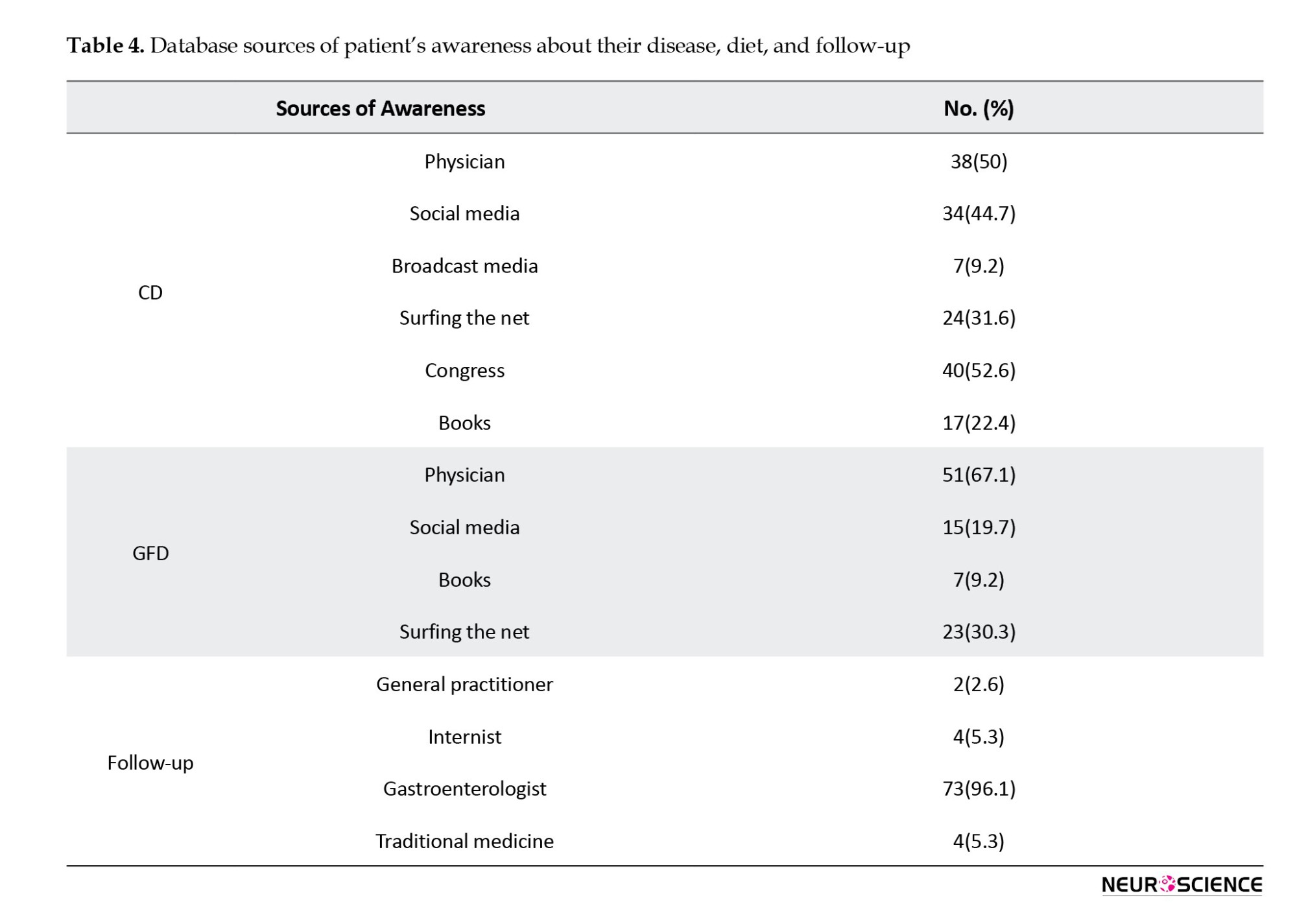
This study examined the primary sources of information regarding CD, dietary considerations, and follow-up for participants. Attendance at medical congresses was identified as a substantial source of information, representing 52.6% of the sample. This result underscores the crucial role of professional gatherings in disseminating knowledge within the medical community.
Consultations with physicians were crucial, with 67.1% of participants depending on this source. Most of the participants were observed and followed up by gastroenterologists. Table 4 presents the distribution of awareness from other sources.
4. Discussion
This study was conducted to investigate the quality of sleep in Iranian patients with CD. This study explores the relationship between CD and sleep quality, alongside the possible involvement of inflammatory cytokines and Trp in this relationship. The results revealed that a notable proportion (63.2%) of participants experienced poor sleep quality.
Consistent with these results, a previous study by Zingone et al. (2010) also noted high PSQI scores among patients with CD, indicating poor sleep quality characterized by prolonged sleep latency and short sleep duration. Ballou et al. (2018) similarly observed poor sleep quality in 61% of patients with CD, aligning closely with our results. Furthermore, our study identified a significant positive correlation between the age of patients with CD and sleep problems, underscoring the importance of integrating sleep evaluations into routine follow-up protocols as patients age. In alignment with this, a study by Mårild et al. (2015) demonstrated that patients with CD are 33% more likely to use hypnotic drugs than healthy controls. In our study, 21.1% of patients with CD reported using sleep medications. Importantly, patients with CD and poor sleep quality exhibited lower plasma Trp levels than those with good sleep quality. Since Trp plays a crucial role in sleep regulation through its involvement in the kynurenine pathway and serotonin synthesis, assessing Trp levels before prescribing hypnotic drugs may benefit to patients with CD (Bhat et al., 2020; Heimberger & Lukas, 2023). Considering Trp supplementation as a potential primary approach for improving sleep quality in these patients is noteworthy. A study conducted by Sutanto et al. (2022) demonstrated that the inclusion of Trp effectively reduced sleep latency. Specifically, participants consuming >1 g Trp exhibited a significantly shorter time to fall asleep compared to those consuming less than 1 g. However, Trp supplementation did not have significant effects on other aspects of sleep.
In our study, individuals with poor sleep quality demonstrated higher levels of IL-2, while no changes were observed in TNF-α, IL-4, and IL-10 compared to those with good sleep quality. These cytokines play a crucial role in immune signaling and have been implicated in both CD pathogenesis and sleep regulation (Imeri & Opp, 2009; Redwine et al., 2000). Prior research investigating cytokine levels in individuals with sleep problems has had varied results. For instance, Taraz et al. found elevated levels of TNF-α in the serum of patients undergoing hemodialysis with poor sleep quality, which is consistent with our results (Taraz et al., 2013). Several studies have demonstrated a correlation between sleep deprivation and elevated levels of TNF-α in the subsequent days (Kaushal et al., 2012). Elevated TNF-α concentrations following sleep deprivation or fragmentation contributes to excessive daytime sleepiness in patients with sleep apnea (Kaushal et al., 2012). Yang et al. demonstrated a positive correlation between poor sleep quality and TNF-α levels and a negative correlation with IL-2 levels (Yang et al., 2023). Kaartinen et al. associated good overall sleep quality with higher logarithmic cytokine concentrations of IL-2, IL-4, IL-6, IL-10, IL-12, and IL-13 (Kaartinen et al., 2019). In our study, elevated levels of IL-2 were observed in participants with poor sleep quality, consistent with previous research indicating that sleep deprivation reduces lymphocyte blastogenesis, natural killer cell activity, and upregulates IL-1 and IL-2 (Ibarra-Coronado et al., 2015). Furthermore, a negative correlation was observed between IL-2 mRNA levels and sleep latency and daytime dysfunction, emphasizing the importance of this gene in sleep disorders. Kaartinen et al. (2019) suggested that good overall sleep quality is associated with high logarithmic concentrations of cytokines IL-4 and IL-10. Previous studies have also demonstrated a decrease in the production of stimulated IL-10 and IL-4 during sleep in humans, indicating a decline in anti-inflammatory activity (Poluektov, 2021).
Given the notable female predominance in our cohort (77.6%), it is essential to consider how sex may influence sleep quality and immune responses in patients with CD. Research indicates that women often report poorer sleep quality and are more susceptible to sleep disorders than men, which may be attributed to hormonal fluctuations related to the menstrual cycle, pregnancy, or menopause (Nowakowski et al., 2013). Furthermore, the immune response differs between the sexes, with females exhibiting stronger immune reactions, which can influence inflammatory markers, such as cytokines (Klein & Flanagan, 2016). In our study, the observed elevation of IL-2 levels among individuals with poor sleep quality highlights a potential pathophysiological link that may be amplified in women due to their distinct immune profiles and increased susceptibility to psychosocial stressors.
Moreover, besides the observed relationships between sleep quality, Trp levels, and pro-inflammatory cytokines in patients with CD, several confounding factors may also significantly influence sleep outcomes. Psychological stressors, including anxiety and depression, are prevalent among individuals with CD and have been shown to disrupt sleep patterns (Moawad et al., 2024; Staner, 2003). Furthermore, dietary adherence to a GFD can play a critical role; variations in adherence levels may influence not only inflammatory responses but also nutritional intake and overall well-being, thereby impacting sleep quality (Cotton et al., 2023). Additionally, the presence of comorbid conditions, such as autoimmune disorders or gastrointestinal symptoms, could contribute to sleep disturbances through mechanisms involving chronic inflammation or pain (Khanijow et al., 2015; Zielinski et al., 2019). Hence, controlling for these psychological, dietary, and health-related factors in future research is crucial for elucidating the multifaceted relationship between CD, immune dysregulation, and sleep quality.
To further explore the intricate relationship between CD, sleep quality, and immune function, future research should focus on several key areas. Longitudinal studies are essential to assess how sleep quality evolves in response to dietary changes or interventions, such as Trp supplementation, and to investigate the long-term effects on inflammatory markers and overall health. Moreover, specific attention should be given to the role of other dietary components and their interactions with sleep quality in CD patients, as dietary adherence can significantly impact both gut health and sleep regulation. Additionally, examining potential differences in sleep quality and inflammatory responses across diverse populations with CD might provide insights into the influence of genetic, cultural, and environmental factors. Such studies could pave the way for tailored therapeutic strategies aimed at enhancing sleep quality and, consequently, the quality of life for individuals living with CD.
This study has several limitations. First, the study sample consisted of only 76 adult participants from a specific research center, which may have limited the generalizability of the results to a broader population. Including a larger and more diverse sample would offer a more representative understanding of the relationship between CD, sleep quality, and inflammatory markers. Second, the assessment of sleep quality relied on self-reported measures, specifically the PSQI questionnaire. Self-report measures are prone to recall bias and individual interpretation, which can potentially impact the accuracy and reliability of the results. Incorporating subjective measures of sleep, such as polysomnography, would yield more robust and precise data on sleep quality.
5. Conclusion
Our results revealed that a substantial proportion of patients with CD experienced poor sleep quality, underscoring the importance of incorporating sleep assessments into routine care for these individuals. Patients with CD may experience deleterious effects on sleep, resulting in disturbances that can profoundly impact their overall well-being. The observed correlations between age, sleep problems, and cytokine expression underscore the multi-faceted nature of sleep quality and its potential impact on immune and inflammatory responses in individuals with CD. The observed correlation between low plasma Trp levels and impaired sleep quality suggests a potential therapeutic avenue through Trp supplementation to alleviate sleep problems in patients with CD. Future research should elucidate the underlying mechanisms linking CD, sleep quality, and immune function.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval for the study was obtained from the Ethics Committee of the Research Institute for Gastroenterology and Liver Diseases (RIGLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP. REC.1397.564). Written informed consent was obtained from all participants before their involvement.
Funding
Financial support for this study was provided by the Student Research Committee affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No.: 1403/50494).
Authors' contributions
Conceptualization: Mohammad Rostami-Nejad; Writing the original draft: Nastaran Asri, Nastaran Asri, Mohadeseh Mahmoudi Ghehsareh M Nazanin Taraghikhah; Review and editing: Mohammad Rostami-Nejad, Somayeh Jahani-Sherafat, Hamidreza Houri, Mostafa Rezaei-Tavirani, and Ayad Bahadorimonfared.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This study is related to the project No.: 1403/50494 from the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We appreciate the support of the “Student Research Committee” and “Celiac Disease and Gluten-Related Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences”.
References
Celiac disease (CD) is a chronic autoimmune disorder characterized by an inflammatory response to gluten, a protein found in wheat, barley, and rye (Gujral et al., 2012). The CD is a complex disorder with a wide range of symptoms, including gastrointestinal manifestations, such as abdominal pain and diarrhea, and extra-intestinal presentations, including dermatitis herpetiformis and reproductive issues (Therrien et al., 2020). Patients with CD also commonly report anxiety, depression, and other mood disorders, which may be linked to poor subjective sleep quality (Rostami-Nejad et al., 2020; Sharifnejad et al., 2023; Zingone et al., 2010). Prolonged inadequate sleep is associated with unfavorable outcomes, including the onset of chronic systemic inflammation, frequently observed in individuals with CD (Palumbo & Wyse, 2020; Sobolewska-Włodarczyk et al., 2021).
The intricate interplay between sleep and the immune system highlights how immune activation and inflammation disrupt sleep patterns. Inadequate sleep can impact immune responses and the production of pro/anti-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-2, IL-4, and IL-10 (Besedovsky et al., 2019; Gottshall et al., 2021; Hurtado-Alvarado et al., 2013; Irwin, 2023; Rockstrom et al., 2018). Additionally, sleep deprivation can elevate cortisol levels by activating the hypothalamic-pituitary-adrenal axis, leading to immune activation and the release of inflammatory cytokines, such as TNF-α and IL-2 (Garbarino et al., 2021; Irwin & Opp, 2017). The relationship between imbalanced inflammatory cytokines and sleep disorders is frequently observed in individuals with CD (Westerholm-Ormio et al., 2002; Zingone et al., 2010).
Tryptophan (Trp), an essential amino acid, is deficient in individuals with CD, as indicated by previous studies (Addolorato et al., 2004; Khalkhal et al., 2022). The primary breakdown of Trp occurs within the kynurenine pathway, mediated by the enzymes Trp 2,3-dioxygenase and indoleamine 2,3-dioxygenase. This metabolic pathway plays a crucial role in regulating both sleep and immune responses (Fallah et al., 2024; Heimberger & Lukas, 2023). Moreover, Trp serves as a precursor for the production of serotonin and melatonin, both of which are essential for modulating the sleep-wake cycle (Bhat et al., 2020). Recent research also suggests that in addition to their involvement in sleep regulation, serotonin and melatonin influence immune responses by binding to specific receptors, such as the 5-hydroxytryptamine receptor 1A, which is present on various immune cells, including macrophages and T lymphocytes (Arioz et al., 2019; Herr et al., 2017).
The relationship between CD and sleep disorders is reciprocal. CD can negatively impact sleep quality, and inadequate sleep can worsen the symptoms of CD through immune and inflammatory mechanisms (Ranjbaran et al., 2007). This study aimed to evaluate the sleep quality of individuals with CD. To achieve this, we utilized the Pittsburgh sleep quality index (PSQI) questionnaire, a widely recognized tool for assessing subjective sleep quality across various populations. Through the implementation of this questionnaire, we gathered data concerning participants’ sleep patterns, duration, problems, and overall sleep quality. Furthermore, we sought to investigate the potential connections between sleep disorder and inflammatory cytokines implicated in CD. Specifically, we focused on TNF-α, IL-4, IL-10, and IL-2, as well as the plasma levels of Trp.
2. Materials and Methods
We conducted a prospective study involving a cohort of 76 adults from the CD and Gluten-Related Disorders Research Center at Shahid Beheshti University of Medical Sciences, Tehran, Iran. The study was conducted between March 20, 2022, and December 28, 2022. Participants were selected based on specific inclusion and exclusion criteria to ensure the integrity of the research. The inclusion criteria included adults aged >18 years with a confirmed diagnosis of CD based on positive serological tests and histological biopsies of the small intestine, as per the modified Marsh criteria (Villanacci et al., 2020). Additionally, participants must not have consumed any Trp supplements in the month preceding the study. Individuals were excluded from the study if they had undergone any medical or surgical intervention for CD in the prior three months, had pre-existing sleep disorders (e.g. sleep apnea, insomnia) unrelated to CD, were currently taking medications that could affect sleep patterns, or inflammatory responses (such as corticosteroids), or were pregnant or breastfeeding. Written informed consent was obtained from all participants before their involvement, and each participant underwent the aseptic collection of 15 m/L of venous blood utilizing specialized vacuum tubes.
Sleep quality assessment
This study employed the PSQI to evaluate participants’ sleep quality. The PSQI questionnaire comprises 19 items, classified into seven subscales: Sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep problems, use of sleep medication, and daytime dysfunction. Each component is rated on a scale of 0–3, with a total score ranging from 0 to 21. A cumulative score of ≥6 indicates poor sleep quality (Buysse et al, 1989; Farrahi Moghaddam et al., 2012).
Evaluation of Trp plasma levels
To evaluate plasma Trp levels, peripheral blood samples were centrifuged at 3500 revolutions per minute (rpm) for 15 minutes. The resulting plasma fraction was then stored at -80 °C until needed for analysis by high-performance liquid chromatography (HPLC). The ACME 9000 system (Younglin, Anyang, Korea), equipped with a fluorescence detector essential for determining Trp levels, was utilized in this study. A methanol solution was used to remove proteins from the plasma samples. The deproteinization process involved vortexing the samples for 30 s and subsequent centrifugation at 5000 rpm for 7 minutes. The resulting clear supernatant was then prepared for further analysis.
Chromatographic separation of Trp was achieved by injecting 100 µL of the clear supernatant into a GL Sciences column with dimensions of 250×3.0 mm and a particle size of 3 µm. The mobile phase for this process comprised a mixture of methanol and tetrahydrofuran in a volumetric ratio of 4:1. Throughout the analysis, the fluorescence signals were monitored and recorded at optimal excitation and emission wavelengths of 340 nm and 450 nm, respectively.
Evaluation of pro-inflammatory cytokines in serum
The blood samples were allowed to clot for 30 minutes at room temperature before centrifugation at 1500×g for 10 minutes. Subsequently, serum was collected and stored at -80 °C for further biochemical analysis. The concentrations of human TNF-α and IL-10 in the serum were determined using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kit (Karmania Pars Gene, Iran), following the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader. Each determination was performed in triplicate by established laboratory principles.
Expression analysis of pro-inflammatory cytokines
Total ribonucleic acid (RNA) was isolated from whole blood samples of all participants using the YTA Total RNA Purification Mini kit for Blood/Cultured Cell/Tissue (Yekta Tajhiz Azma, Iran) according to the manufacturer’s instructions. RNA concentration and quality were assessed using a Nanodrop 1000 spectrophotometer (Fisher Thermo, Wilmington, DE, USA). After adjusting the RNA concentrations, complementary DNA (cDNA) synthesis was performed using the 2 Step 2X RTPCR Premix (Taq) kit (Biofact, South Korea), and the resulting complementary DNA (cDNA) was stored at -20 °C for quantitative real-time polymerase chain reaction (qPCR).
Specific primers for amplifying IL-2, IL-4, and beta-2-microglobulin (B2M), a housekeeping gene, were designed using Gene Runner software (version 3.05). The primer sequences were as follows: IL-2 forward: 5’-TACATGCCCAAGAAGGCCAC-3’, IL-2 reverse: 5’-AGCACTTCCTCCAGAGGTTTG-3’; IL-4 forward: 5’-CTTTGCTGCCTCCAAGAACAC-3’, IL-4 reverse: 5’-TTCCTGTCGAGCCGTTTCAG-3’; B2M forward: 5’-CCAGCGTACTCCAAAGATTC-3’, B2M reverse: 5’-ATGTCGGATGGATGAAACCC-3’.
The messenger ribonucleic acid (mRNA) expression levels of the target genes were evaluated using SYBR Premix Ex Taq (Real Q Plus 2x Master Mix Green-Amplicon, Japan) with the Rotor-Gene® Q real-time PCR system (Qiagen, Germany). All qPCR reactions were conducted in duplicate, and the mRNA expression level of each gene was calculated following the 2-ΔΔCt method (ΔCt=ΔCt target -ΔCt endogenous).
Statistical analysis
Data were analyzed using SPSS software version 25, developed by IBM (Chicago, IL, USA). Graphical representations were generated using GraphPad Prism 8.4.0, software (GraphPad Software, Inc.) (San Diego, CA, USA). Descriptive statistics were employed to summarize the characteristics of the study participants, including demographics, clinical features, and sleep quality parameters. An independent sample t-test compared two groups of continuous variables that followed a normal distribution. All results were reported with a 95% confidence interval. Statistical significance was set at P<0.05. The correlations between variables were assessed using Pearson’s correlation tests.
3. Results
Demographic and clinical characteristics of CD participants
The study primarily involved adult participants, with a mean age of 40.29±11.62 years. Most of the samples were female (77.6%). Fatigue emerged as the predominant symptom among participants, affecting 63.15% of cases.
Comprehensive demographic and clinical data were collected, including information on CD duration, adherence to a gluten-free diet (GFD), smoking habits, marital status, and educational attainment. Table 1 presents a detailed breakdown of additional symptoms, such as bloating, diarrhea, vomiting, and osteoporosis.

Assessment of sleep quality in CD participants
Table 2 presents a comprehensive analysis of overall sleep quality and seven specific PSQI subscales among patients with CD. Notably, 63.2% of the participants displayed signs of poor sleep quality based on the total questionnaire scores (most of whom were women [79.16%]). Concerning subjective sleep quality, 59.2% of the participants reported experiencing fairly good quality. Analysis of sleep latency distribution revealed that 30.3% experienced a latency period ranging from 16-30 minutes. Regarding sleep duration, the majority (40.8%) reported sleeping for 6-7 hours per night. Habitual sleep efficiency was notably high, with a majority (72.4%) of participants reporting an efficiency of over 85%. Sleep problems were reported by a considerable percentage (61.8%), occurring less than once a week. Furthermore, the majority (78.9%) of the participants reported not using any sleep medications. Daytime dysfunction showed variability, with the highest percentage (38.2%) of patients reporting no difficulties.

Plasma Trp concentration in poor and good sleepers
We assessed the plasma Trp concentrations in individuals diagnosed with CD, categorizing them based on their reported sleep quality as either poor or good. The results, illustrated in Figure 1, revealed a statistically significant lower plasma Trp concentration among participants who reported poor sleep compared to those with good sleep quality (P<0.0001).

Serum levels of NF-α and IL-10 in poor and good sleepers
Serum TNF-α and IL-10 levels were measured in patients with CD with either poor or good sleep quality. No statistically significant difference was observed in the serum TNF-α and IL-10 levels between the group of individuals experiencing poor sleep and the group reporting good sleep quality (P>0.05) (Figure 2).

The expression levels of IL-2 and IL-4 mRNA in poor and good sleepers
We examined the relative expression of IL-2 and IL-4 genes in individuals diagnosed with CD who reported varying levels of sleep quality. As depicted in Figure 3, our results revealed a significant increase in IL-2 expression in the group experiencing poor sleep compared to those reporting good sleep (P=0.03). Conversely, the change in the expression level of IL-4 was not statistically significant (P>0.05).

Correlation analysis
Pearson’s correlation analysis was conducted to assess the relationships between various factors, including age, sex, duration of CD, adherence to a GFD, smoking habits, marital status, IL-2 expression, plasma Trp levels, and different aspects of sleep quality. The results showed a significant positive correlation between participants’ age and sleep problems (P=0.009, r=0.37). Moreover, IL-2 mRNA levels were negatively correlated with sleep latency (P=0.04, r=-0.57) and daytime dysfunction (P=0.03, r=-0.6). No significant correlations were found between the other variables and different aspects of sleep quality (Table 3).

Primary awareness sources of CD patients
This study examined the primary sources of information regarding CD, dietary considerations, and follow-up for participants. Attendance at medical congresses was identified as a substantial source of information, representing 52.6% of the sample. This result underscores the crucial role of professional gatherings in disseminating knowledge within the medical community.
Consultations with physicians were crucial, with 67.1% of participants depending on this source. Most of the participants were observed and followed up by gastroenterologists. Table 4 presents the distribution of awareness from other sources.

4. Discussion
This study was conducted to investigate the quality of sleep in Iranian patients with CD. This study explores the relationship between CD and sleep quality, alongside the possible involvement of inflammatory cytokines and Trp in this relationship. The results revealed that a notable proportion (63.2%) of participants experienced poor sleep quality.
Consistent with these results, a previous study by Zingone et al. (2010) also noted high PSQI scores among patients with CD, indicating poor sleep quality characterized by prolonged sleep latency and short sleep duration. Ballou et al. (2018) similarly observed poor sleep quality in 61% of patients with CD, aligning closely with our results. Furthermore, our study identified a significant positive correlation between the age of patients with CD and sleep problems, underscoring the importance of integrating sleep evaluations into routine follow-up protocols as patients age. In alignment with this, a study by Mårild et al. (2015) demonstrated that patients with CD are 33% more likely to use hypnotic drugs than healthy controls. In our study, 21.1% of patients with CD reported using sleep medications. Importantly, patients with CD and poor sleep quality exhibited lower plasma Trp levels than those with good sleep quality. Since Trp plays a crucial role in sleep regulation through its involvement in the kynurenine pathway and serotonin synthesis, assessing Trp levels before prescribing hypnotic drugs may benefit to patients with CD (Bhat et al., 2020; Heimberger & Lukas, 2023). Considering Trp supplementation as a potential primary approach for improving sleep quality in these patients is noteworthy. A study conducted by Sutanto et al. (2022) demonstrated that the inclusion of Trp effectively reduced sleep latency. Specifically, participants consuming >1 g Trp exhibited a significantly shorter time to fall asleep compared to those consuming less than 1 g. However, Trp supplementation did not have significant effects on other aspects of sleep.
In our study, individuals with poor sleep quality demonstrated higher levels of IL-2, while no changes were observed in TNF-α, IL-4, and IL-10 compared to those with good sleep quality. These cytokines play a crucial role in immune signaling and have been implicated in both CD pathogenesis and sleep regulation (Imeri & Opp, 2009; Redwine et al., 2000). Prior research investigating cytokine levels in individuals with sleep problems has had varied results. For instance, Taraz et al. found elevated levels of TNF-α in the serum of patients undergoing hemodialysis with poor sleep quality, which is consistent with our results (Taraz et al., 2013). Several studies have demonstrated a correlation between sleep deprivation and elevated levels of TNF-α in the subsequent days (Kaushal et al., 2012). Elevated TNF-α concentrations following sleep deprivation or fragmentation contributes to excessive daytime sleepiness in patients with sleep apnea (Kaushal et al., 2012). Yang et al. demonstrated a positive correlation between poor sleep quality and TNF-α levels and a negative correlation with IL-2 levels (Yang et al., 2023). Kaartinen et al. associated good overall sleep quality with higher logarithmic cytokine concentrations of IL-2, IL-4, IL-6, IL-10, IL-12, and IL-13 (Kaartinen et al., 2019). In our study, elevated levels of IL-2 were observed in participants with poor sleep quality, consistent with previous research indicating that sleep deprivation reduces lymphocyte blastogenesis, natural killer cell activity, and upregulates IL-1 and IL-2 (Ibarra-Coronado et al., 2015). Furthermore, a negative correlation was observed between IL-2 mRNA levels and sleep latency and daytime dysfunction, emphasizing the importance of this gene in sleep disorders. Kaartinen et al. (2019) suggested that good overall sleep quality is associated with high logarithmic concentrations of cytokines IL-4 and IL-10. Previous studies have also demonstrated a decrease in the production of stimulated IL-10 and IL-4 during sleep in humans, indicating a decline in anti-inflammatory activity (Poluektov, 2021).
Given the notable female predominance in our cohort (77.6%), it is essential to consider how sex may influence sleep quality and immune responses in patients with CD. Research indicates that women often report poorer sleep quality and are more susceptible to sleep disorders than men, which may be attributed to hormonal fluctuations related to the menstrual cycle, pregnancy, or menopause (Nowakowski et al., 2013). Furthermore, the immune response differs between the sexes, with females exhibiting stronger immune reactions, which can influence inflammatory markers, such as cytokines (Klein & Flanagan, 2016). In our study, the observed elevation of IL-2 levels among individuals with poor sleep quality highlights a potential pathophysiological link that may be amplified in women due to their distinct immune profiles and increased susceptibility to psychosocial stressors.
Moreover, besides the observed relationships between sleep quality, Trp levels, and pro-inflammatory cytokines in patients with CD, several confounding factors may also significantly influence sleep outcomes. Psychological stressors, including anxiety and depression, are prevalent among individuals with CD and have been shown to disrupt sleep patterns (Moawad et al., 2024; Staner, 2003). Furthermore, dietary adherence to a GFD can play a critical role; variations in adherence levels may influence not only inflammatory responses but also nutritional intake and overall well-being, thereby impacting sleep quality (Cotton et al., 2023). Additionally, the presence of comorbid conditions, such as autoimmune disorders or gastrointestinal symptoms, could contribute to sleep disturbances through mechanisms involving chronic inflammation or pain (Khanijow et al., 2015; Zielinski et al., 2019). Hence, controlling for these psychological, dietary, and health-related factors in future research is crucial for elucidating the multifaceted relationship between CD, immune dysregulation, and sleep quality.
To further explore the intricate relationship between CD, sleep quality, and immune function, future research should focus on several key areas. Longitudinal studies are essential to assess how sleep quality evolves in response to dietary changes or interventions, such as Trp supplementation, and to investigate the long-term effects on inflammatory markers and overall health. Moreover, specific attention should be given to the role of other dietary components and their interactions with sleep quality in CD patients, as dietary adherence can significantly impact both gut health and sleep regulation. Additionally, examining potential differences in sleep quality and inflammatory responses across diverse populations with CD might provide insights into the influence of genetic, cultural, and environmental factors. Such studies could pave the way for tailored therapeutic strategies aimed at enhancing sleep quality and, consequently, the quality of life for individuals living with CD.
This study has several limitations. First, the study sample consisted of only 76 adult participants from a specific research center, which may have limited the generalizability of the results to a broader population. Including a larger and more diverse sample would offer a more representative understanding of the relationship between CD, sleep quality, and inflammatory markers. Second, the assessment of sleep quality relied on self-reported measures, specifically the PSQI questionnaire. Self-report measures are prone to recall bias and individual interpretation, which can potentially impact the accuracy and reliability of the results. Incorporating subjective measures of sleep, such as polysomnography, would yield more robust and precise data on sleep quality.
5. Conclusion
Our results revealed that a substantial proportion of patients with CD experienced poor sleep quality, underscoring the importance of incorporating sleep assessments into routine care for these individuals. Patients with CD may experience deleterious effects on sleep, resulting in disturbances that can profoundly impact their overall well-being. The observed correlations between age, sleep problems, and cytokine expression underscore the multi-faceted nature of sleep quality and its potential impact on immune and inflammatory responses in individuals with CD. The observed correlation between low plasma Trp levels and impaired sleep quality suggests a potential therapeutic avenue through Trp supplementation to alleviate sleep problems in patients with CD. Future research should elucidate the underlying mechanisms linking CD, sleep quality, and immune function.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval for the study was obtained from the Ethics Committee of the Research Institute for Gastroenterology and Liver Diseases (RIGLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP. REC.1397.564). Written informed consent was obtained from all participants before their involvement.
Funding
Financial support for this study was provided by the Student Research Committee affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No.: 1403/50494).
Authors' contributions
Conceptualization: Mohammad Rostami-Nejad; Writing the original draft: Nastaran Asri, Nastaran Asri, Mohadeseh Mahmoudi Ghehsareh M Nazanin Taraghikhah; Review and editing: Mohammad Rostami-Nejad, Somayeh Jahani-Sherafat, Hamidreza Houri, Mostafa Rezaei-Tavirani, and Ayad Bahadorimonfared.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This study is related to the project No.: 1403/50494 from the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We appreciate the support of the “Student Research Committee” and “Celiac Disease and Gluten-Related Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences”.
References
Addolorato, G., De Lorenzi, G., Abenavoli, L., Leggio, L., Capristo, E., & Gasbarrini, G. (2004). Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Alimentary Pharmacology & Therapeutics, 20(7), 777–782. [DOI:10.1111/j.1365-2036.2004.02193.x] [PMID]
Arioz, B. I., Tastan, B., Tarakcioglu, E., Tufekci, K. U., Olcum, M., & Ersoy, N., et al. (2019). Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Frontiers in Immunology, 10, 1511. [DOI:10.3389/fimmu.2019.01511] [PMID]
Ballou, S., Alhassan, E., Hon, E., Lembo, C., Rangan, V., & Singh, P., et al. (2018). Sleep disturbances are commonly reported among patients presenting to a gastroenterology clinic. Digestive Diseases and Sciences, 63(11), 2983–2991. [DOI:10.1007/s10620-018-5237-7] [PMID]
Besedovsky, L., Lange, T., & Haack, M. (2019). The sleep-immune crosstalk in health and disease. Physiological Reviews, 99(3), 1325–1380. [DOI:10.1152/physrev.00010.2018] [PMID]
Bhat, A., Pires, A. S., Tan, V., Babu Chidambaram, S., & Guillemin, G. J. (2020). Effects of sleep deprivation on the tryptophan metabolism. International Journal of Tryptophan Research, 13, 1178646920970902. [DOI:10.1177/1178646920970902] [PMID]
Buysse, D. J., Reynolds, C. F., 3rd, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI:10.1016/0165-1781(89)90047-4] [PMID]
Cotton, C., Raju, S. A., Ahmed, H., Webster, G., Hallam, R., & Croall, I., et al. (2023). Does a gluten-free diet improve quality of life and sleep in patients with non-coeliac gluten/wheat sensitivity?. Nutrients, 15(15), 3461. [DOI:10.3390/nu15153461] [PMID]
Fallah, S., Asri, N., Nikzamir, A., Ahmadipour, S., Sadeghi, A., & Rostami, K., et al. (2024). Investigating the impact of vitamin a and amino acids on immune responses in celiac disease patients. Diseases, 12(1), 13. [DOI:10.3390/diseases12010013] [PMID]
Farrahi Moghaddam, J., Nakhaee, N., Sheibani, V., Garrusi, B., & Amirkafi, A. (2012). Reliability and validity of the Persian version of the pittsburgh sleep quality index (PSQI-P). Sleep & Breathing, 16(1), 79–82. [DOI:10.1007/s11325-010-0478-5] [PMID]
Garbarino, S., Lanteri, P., Bragazzi, N. L., Magnavita, N., & Scoditti, E. (2021). Role of sleep deprivation in immune-related disease risk and outcomes. Communications Biology, 4(1), 1304. [DOI:10.1038/s42003-021-02825-4] [PMID]
Gottshall, J. L., Guedes, V. A., Pucci, J. U., Brooks, D., Watson, N., & Sheth, P., et al. (2022). Poor sleep quality is linked to elevated extracellular vesicle-associated inflammatory cytokines in warfighters with chronic mild traumatic brain injuries. Frontiers in Pharmacology, 12, 762077. [DOI:10.3389/fphar.2021.762077] [PMID]
Gujral, N., Freeman, H. J., & Thomson, A. B. (2012). Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World Journal of Gastroenterology, 18(42), 6036–6059. [DOI:10.3748/wjg.v18.i42.6036] [PMID]
Heimberger, A. B., & Lukas, R. V. (2023). The kynurenine pathway implicated in patient delirium: Possible indications for indoleamine 2,3 dioxygenase inhibitors. The Journal of Clinical Investigation, 133(2), e164577. [DOI:10.1172/JCI164577] [PMID]
Herr, N., Bode, C., & Duerschmied, D. (2017). The effects of serotonin in immune cells. Frontiers in Cardiovascular Medicine, 4, 48. [DOI:10.3389/fcvm.2017.00048] [PMID]
Hurtado-Alvarado, G., Pavón, L., Castillo-García, S. A., Hernández, M. E., Domínguez-Salazar, E., & Velázquez-Moctezuma, J., et al. (2013). Sleep loss as a factor to induce cellular and molecular inflammatory variations. Clinical & Developmental Immunology, 2013, 801341. [DOI:10.1155/2013/801341] [PMID]
Ibarra-Coronado, E. G., Pantaleón-Martínez, A. M., Velazquéz-Moctezuma, J., Prospéro-García, O., Méndez-Díaz, M., & Pérez-Tapia, M., et al. (2015). The bidirectional relationship between sleep and immunity against infections. Journal of Immunology Research, 2015, 678164. [DOI:10.1155/2015/678164] [PMID]
Imeri, L., & Opp, M. R. (2009). How (and why) the immune system makes us sleep. Nature reviews. Neuroscience, 10(3), 199–210. [DOI:10.1038/nrn2576] [PMID]
Irwin M. R. (2022). Sleep disruption induces activation of inflammation and heightens risk for infectious disease: Role of impairments in thermoregulation and elevated ambient temperature. Temperature, 10(2), 198–234. [DOI:10.1080/23328940.2022.2109932] [PMID]
Irwin, M. R., & Opp, M. R. (2017). Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology, 42(1), 129–155. [DOI:10.1038/npp.2016.148] [PMID]
Kaartinen, M., Karlsson, L., Paavonen, E. J., Polo-Kantola, P., Pelto, J., & Nousiainen, N., et al. (2019). Maternal tiredness and cytokine concentrations in mid-pregnancy. Journal of Psychosomatic Research, 127, 109843. [DOI:10.1016/j.jpsychores.2019.109843] [PMID]
Kaushal, N., Ramesh, V., & Gozal, D. (2012). TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. Plos One, 7(9), e45610. [DOI:10.1371/journal.pone.0045610] [PMID]
Khalkhal, E., Rezaei-Tavirani, M., Asri, N., Nobakht, F., Jahani-Sherafat, S., & Haidari, M. H., et al. (2022). Introducing new potential biomarkers for celiac disease among the genes extracted from general databases. Middle East Journal of Digestive Diseases, 14(2), 192–199. [DOI:10.34172/mejdd.2022.272] [PMID]
Khanijow, V., Prakash, P., Emsellem, H. A., Borum, M. L., & Doman, D. B. (2015). Sleep dysfunction and gastrointestinal diseases. Gastroenterology & Hepatology, 11(12), 817–825. [PMID]
Klein, S. L., & Flanagan, K. L. (2016). Sex differences in immune responses. Nature Reviews. Immunology, 16(10), 626–638. [DOI:10.1038/nri.2016.90] [PMID]
Mårild, K., Morgenthaler, T. I., Somers, V. K., Kotagal, S., Murray, J. A., & Ludvigsson, J. F. (2015). Increased use of hypnotics in individuals with celiac disease: A nationwide case-control study. BMC Gastroenterology, 15, 10. [DOI:10.1186/s12876-015-0236-z] [PMID]
Moawad, M. H., Serag, I., Shalaby, M. M., Aissani, M. S., Sadeq, M. A., & Hendi, N. I., et al. (2024). Anxiety and depression among adults and children with celiac disease: A meta-analysis of different psychiatry scales. Psychiatric Research and Clinical Practice, 6(4), 124–133. [DOI:10.1176/appi.prcp.20230076] [PMID]
Nowakowski, S., Meers, J., & Heimbach, E. (2013). Sleep and women's health. Sleep Medicine Research, 4(1), 1–22. [DOI:10.17241/smr.2013.4.1.1] [PMID]
Palumbo, C. S., & Wyse, J. (2020). Markers of systemic and gut-specific inflammation in celiac disease. The Turkish Journal of Gastroenterology, 31(2), 187–189. [DOI:10.5152/tjg.2020.19081] [PMID]
Poluektov M. G. (2021). Sleep and immunity. Neuroscience and Behavioral Physiology, 51(5), 609–615. [DOI:10.1007/s11055-021-01113-2] [PMID]
Ranjbaran, Z., Keefer, L., Farhadi, A., Stepanski, E., Sedghi, S., & Keshavarzian, A. (2007). Impact of sleep disturbances in inflammatory bowel disease. Journal of Gastroenterology and Hepatology, 22(11), 1748–1753. [DOI:10.1111/j.1440-1746.2006.04820.x] [PMID]
Redwine, L., Hauger, R. L., Gillin, J. C., & Irwin, M. (2000). Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. The Journal of Clinical Endocrinology and Metabolism, 85(10), 3597–3603. [DOI:10.1210/jcem.85.10.6871] [PMID]
Rockstrom, M. D., Chen, L., Taishi, P., Nguyen, J. T., Gibbons, C. M., & Veasey, S. C., et al. (2018). Tumor necrosis factor alpha in sleep regulation. Sleep Medicine Reviews, 40, 69–78.[DOI:10.1016/j.smrv.2017.10.005] [PMID]
Rostami-Nejad, M., Taraghikhah, N., Ciacci, C., Pourhoseingholi, M. A., Barzegar, F., & Rezaei-Tavirani, M., et al. (2020). Anxiety symptoms in adult celiac patients and the effect of a gluten-free diet: An Iranian nationwide study. Inflammatory Intestinal Diseases, 5(1), 42–47. [DOI:10.1159/000505657] [PMID]
Sharifnejad, Y., Amanpour, F., Rostami, K., Tavirani, M. R., Pourhoseingholi, M. A., & Rostami-Nejad, M. (2023). Relationship between anxiety and quality of life in the presence of other factors in adult celiac patients; a nationwide study. Gastroenterology and Hepatology From Bed to Bench, 16(2), 151. [DOI:10.22037/ghfbb.v16i2.2134] [PMID]
Sobolewska-Włodarczyk, A., Włodarczyk, M., Talar, M., Wiśniewska-Jarosińska, M., Gąsiorowska, A., & Fichna, J. (2021). The association of the quality of sleep with proinflammatory cytokine profile in inflammatory bowel disease patients. Pharmacological Reports, 73(6), 1660–1669. [DOI:10.1007/s43440-021-00333-0] [PMID]
Staner L. (2003). Sleep and anxiety disorders. Dialogues in Clinical Neuroscience, 5(3), 249–258. [DOI:10.31887/DCNS.2003.5.3/lstaner] [PMID]
Sutanto, C. N., Loh, W. W., & Kim, J. E. (2022). The impact of tryptophan supplementation on sleep quality: A systematic review, meta-analysis, and meta-regression. Nutrition Reviews, 80(2), 306–316. [DOI:10.1093/nutrit/nuab027] [PMID]
Taraz, M., Khatami, M. R., Hajiseyedjavadi, M., Farrokhian, A., Amini, M., & Khalili, H., et al. (2013). Association between antiinflammatory cytokine, IL-10, and sleep quality in patients on maintenance hemodialysis. Hemodialysis international. International Symposium on Home Hemodialysis, 17(3), 382–390. [DOI:10.1111/hdi.12035] [PMID]
Therrien, A., Kelly, C. P., & Silvester, J. A. (2020). Celiac disease: Extraintestinal manifestations and associated conditions. Journal of Clinical Gastroenterology, 54(1), 8–21. [DOI:10.1097/MCG.0000000000001267] [PMID]
Villanacci, V., Vanoli, A., Leoncini, G., Arpa, G., Salviato, T., Bonetti, L. R., Baronchelli, C., Saragoni, L., & Parente, P. (2020). Celiac disease: histology-differential diagnosis-complications. A practical approach. Pathologica, 112(3), 186–196. [DOI: 10.32074/1591-951x-157] [PMID]
Westerholm-Ormio, M., Garioch, J., Ketola, I., & Savilahti, E. (2002). Inflammatory cytokines in small intestinal mucosa of patients with potential coeliac disease. Clinical and Experimental Immunology, 128(1), 94–101. [DOI:10.1046/j.1365-2249.2002.01798.x] [PMID]
Yang, Y., Gu, K., Meng, C., Li, J., Lu, Q., & Zhou, X., et al. (2023). Relationship between sleep and serum inflammatory factors in patients with major depressive disorder. Psychiatry Research, 329, 115528. [DOI:10.1016/j.psychres.2023.115528] [PMID]
Zielinski, M. R., Systrom, D. M., & Rose, N. R. (2019). Fatigue, sleep, and autoimmune and related disorders. Frontiers in Immunology, 10, 1827. [DOI:10.3389/fimmu.2019.01827] [PMID]
Zingone, F., Siniscalchi, M., Capone, P., Tortora, R., Andreozzi, P., & Capone, E., et al. (2010). The quality of sleep in patients with coeliac disease. Alimentary Pharmacology & Therapeutics, 32(8), 1031–1036. [DOI:10.1111/j.1365-2036.2010.04432.x] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2024/08/10 | Accepted: 2024/09/14 | Published: 2025/05/1
Received: 2024/08/10 | Accepted: 2024/09/14 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





