Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 667-676 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moarrefzadeh A, Sarandili S, Motamed-Gorji N, Majdolashrafi F, Bahardoust M, Mousavi S, et al . Predictors of Quality of Life in Patients With Parkinson’s Disease: A Multicenter Case-control Study. BCN 2025; 16 (3) :667-676
URL: http://bcn.iums.ac.ir/article-1-2987-en.html
URL: http://bcn.iums.ac.ir/article-1-2987-en.html
Predictors of Quality of Life in Patients With Parkinson’s Disease: A Multicenter Case-control Study
Aida Moarrefzadeh1 

 , Sadra Sarandili2
, Sadra Sarandili2 

 , Nogol Motamed-Gorji3
, Nogol Motamed-Gorji3 

 , Fatemeh Majdolashrafi4
, Fatemeh Majdolashrafi4 

 , Mansour Bahardoust4
, Mansour Bahardoust4 

 , Safa Mousavi5
, Safa Mousavi5 

 , Neda Hashemi6
, Neda Hashemi6 

 , Arash Sarveazad7
, Arash Sarveazad7 

 , Mohammadhossein Vazirizadeh-Mahabadi8
, Mohammadhossein Vazirizadeh-Mahabadi8 

 , Seyed Amirhassan Habibi *9
, Seyed Amirhassan Habibi *9 




 , Sadra Sarandili2
, Sadra Sarandili2 

 , Nogol Motamed-Gorji3
, Nogol Motamed-Gorji3 

 , Fatemeh Majdolashrafi4
, Fatemeh Majdolashrafi4 

 , Mansour Bahardoust4
, Mansour Bahardoust4 

 , Safa Mousavi5
, Safa Mousavi5 

 , Neda Hashemi6
, Neda Hashemi6 

 , Arash Sarveazad7
, Arash Sarveazad7 

 , Mohammadhossein Vazirizadeh-Mahabadi8
, Mohammadhossein Vazirizadeh-Mahabadi8 

 , Seyed Amirhassan Habibi *9
, Seyed Amirhassan Habibi *9 


1- Department of Psychology, Faculty of Humanities, Ahar Branch, Islamic Azad University, Ahar, Iran.
2- Department of Health Sciences, Curtin Medical School, Curtin University, Perth, Australia.
3- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Epidemiology, School of Public Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Department of Public Health, College of Health and Human Services, California State University, Fresno, United States.
6- Department of Perinatology, Endometriosis Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
7- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran. & Nursing Care Research Center, Iran University of Medical Sciences, Tehran, Iran.
8- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
9- Department of Movement Disorders, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Health Sciences, Curtin Medical School, Curtin University, Perth, Australia.
3- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Epidemiology, School of Public Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Department of Public Health, College of Health and Human Services, California State University, Fresno, United States.
6- Department of Perinatology, Endometriosis Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
7- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran. & Nursing Care Research Center, Iran University of Medical Sciences, Tehran, Iran.
8- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
9- Department of Movement Disorders, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Keywords: Parkinson, Quality of life (QoL), Parkinson’s disease questionnaire (PDQ)-39, Prognostic factors
Full-Text [PDF 623 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, with rapidly increasing prevalence among other neurological diseases (de Lau & Breteler, 2006; Feigin et al., 2017). More than 4 million people suffer from this incurable disease, and the number is predicted to double by 2030 (Dorsey et al., 2007). The disease is characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra region of the brain, leading to typical motor symptoms, such as tremors, bradykinesia (slowness of movement), rigidity, and postural instability. However, PD is not limited to motor symptoms; it also has substantial non-motor manifestations that significantly impact the quality of life (QoL) of affected individuals (Garcia-Ruiz et al., 2014; Wirdefeldt et al., 2011). By identifying and addressing the prognostic factors associated with QoL, healthcare professionals can improve the patient’s overall well-being and ultimately help them live a more fulfilling life (Morris et al., 2009). Low Qol in patients with idiopathic PD seems to be associated with a fear of falls in patients with PD, which is demonstrative of mobility and activities of daily living (Mehdizadeh et al., 2016). However, there has been growing concern regarding non-motor symptoms, including dementia, sexual dysfunction, mood disturbance, and insomnia, because they are thought to have an even more detrimental effect on QoL than classic motor deficits (Chaudhuri et al., 2006; Forsaa et al., 2008; Weintraub et al., 2004).
The World Health Organization (WHO) defines QoL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and about their goals, expectations, standards, and concerns” (Group, 1995). Assessing QoL in PD requires reliable and validated measurement tools. One widely used instrument is the PD questionnaire (PDQ-39). The PDQ-39 is a self-administered questionnaire to assess health-related QoL in individuals with PD. It consists of eight domains or sub-scales: Mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort (Jenkinson et al., 1997; Peto et al., 1995). By evaluating these domains, the PDQ-39 comprehensively assesses the disease’s impact on different aspects of QoL. A poorer QoL in patients with PD has been linked to several demographic parameters, including age (Hendred & Foster, 2016), sex (Valeikienė et al., 2008), education (Cubo et al., 2002), and several clinical characteristics of the disease, such as its duration and stage (Dogan et al., 2015). In this case-control study, we aimed to assess the QoL in patients with PD using the PDQ-39 questionnaire and identify the prognostic factors associated with their QoL.
2. Materials and Methods
The present observational (case-control) study was conducted in a multicenter manner after approval by the Ethics Committee of Iran University of Medical Sciences. The cases included 211 patients with PD who had been referred to the Neurology Clinic of Taleghani and Rasoul Akram hospitals affiliated with Shahid Beheshti University of Medical Sciences and Iran University of Medical Sciences (2019-2022). The sampling method for patients in two centers was conducted as available among patients who met the study’s inclusion criteria. To control for confounding variables between the two groups, cases and controls were matched in terms of demographic characteristics, including age, sex, physical profile, and comorbidities, using the frequency matching method.
Eligibility criteria
The inclusion criteria included a definitive diagnosis of PD based on clinical findings and examination by a neurologist, mainly based on the UK PD Society brain bank diagnostic criteria (Clarke et al., 2016); being alive at the time of follow-up; undergoing intervention with deep brain stimulation (DBS); at least six months had passed since the surgery; completeness of the file; and informed cooperation of the patients to participate in the study and complete the QoL questionnaire.
The exclusion criteria included cancer, drug and alcohol addiction, untreated severe depression or other neuropsychiatric diseases, including multiple sclerosis (MS) and Alzheimer’s disease, chronic viral infection, such as viral hepatitis or HIV, and dead Patients.
Data gathering
The study was conducted in two formats: Retrospective (collecting demographic, clinical, and radiographic information from patients’ files) and prospective (completion of a QoL questionnaire). Data were collected using a two-part checklist after visiting the archive department and accessing the patients’ files. The first part included the patient’s demographic information form (age, sex, body mass index (IBM), education, number of morbidities, and smoking history). The second part included clinical information (age at onset, duration, disease severity, and DBS surgery). The severity of PD was classified into four stages based on the Hoehn and Yahr index (Bhidayasiri & Tarsy, 2012). A higher stage indicates a more severe disease.
The PD questionnaire (PDQ-39) was used to evaluate QoL. This questionnaire has eight separate dimensions: movement (10 questions), daily life activities (6 questions), feeling good (6 questions), stigma (4 questions), social support (3 questions), recognition (4 questions), communication (3 questions), and physical discomfort (3 questions). Each questionnaire question had five options on the Likert scale; only one option was marked. The first option is the sign of the best situation (score 0), and the fifth option is the sign of the worst (score 4). The range of scores for each dimension is reported from 0 to 100, where zero means no problem and 100 indicates the worst health condition. The score of each dimension is calculated as follows: The sum of the raw scores of each dimension divided by the sum of the maximum possible raw score of that dimension, multiplied by 100. The average scores of these dimensions were combined to create a single index called the PD summary index (PDSI). The range of the PDSI is also reported to be 0-100. The validity and reliability of the Persian version of this questionnaire for Iranian patients have been confirmed by Dehghan et al. (2016). The same questionnaire was used to evaluate QoL in the control group. After obtaining consent from the patients to participate in the study, the patients or the researcher completed the QoL questionnaire in person (in cases where the patients were unable to complete the questionnaire).
Statistical analysis
Data were analyzed using SPSS software, version 22. Descriptive statistics (frequency and %) were used to report qualitative variables. Quantitative variables were reported as Mean±SD. The normality of the distribution of quantitative variables was evaluated using the Shapiro-Wilk test. The chi-square test was used to compare qualitative variables in two groups. To compare the quantitative variables between two groups, a t-test was used when the quantitative variables had a normal distribution, and a Mann-Whitney test was used if the assumption of normality was not met. The analysis of single variables in more than two groups was performed using a one-way variance test. To control for confounding variables, all variables with a P<0.05 in the univariate analysis were included in the multivariate logistic regression analysis using the backward model. The effect size index was reported along with the adjusted odds ratio and its 95% confidence interval (CI). Multivariate logistic regression analysis was used to estimate the predictor variables of QoL in patients with PD. A P<0.05 was considered statistically significant.
3. Results
Demographic data
Four hundred twenty-two participants (211 cases and 211 controls) were included in the study. The mean age of the patients was 59.8±13.7 years. The median age was 60 years. One hundred twenty-eight patients (60.8%) were male. Regarding disease severity based on the H and Y stage index, most patients were in stages 2 and 3. The average duration of the disease since its onset was 4.28±3.85 years. 86(40.8%) patients underwent DBS intervention. No statistically significant difference was observed for the demographic variables in the two groups (Table 1).
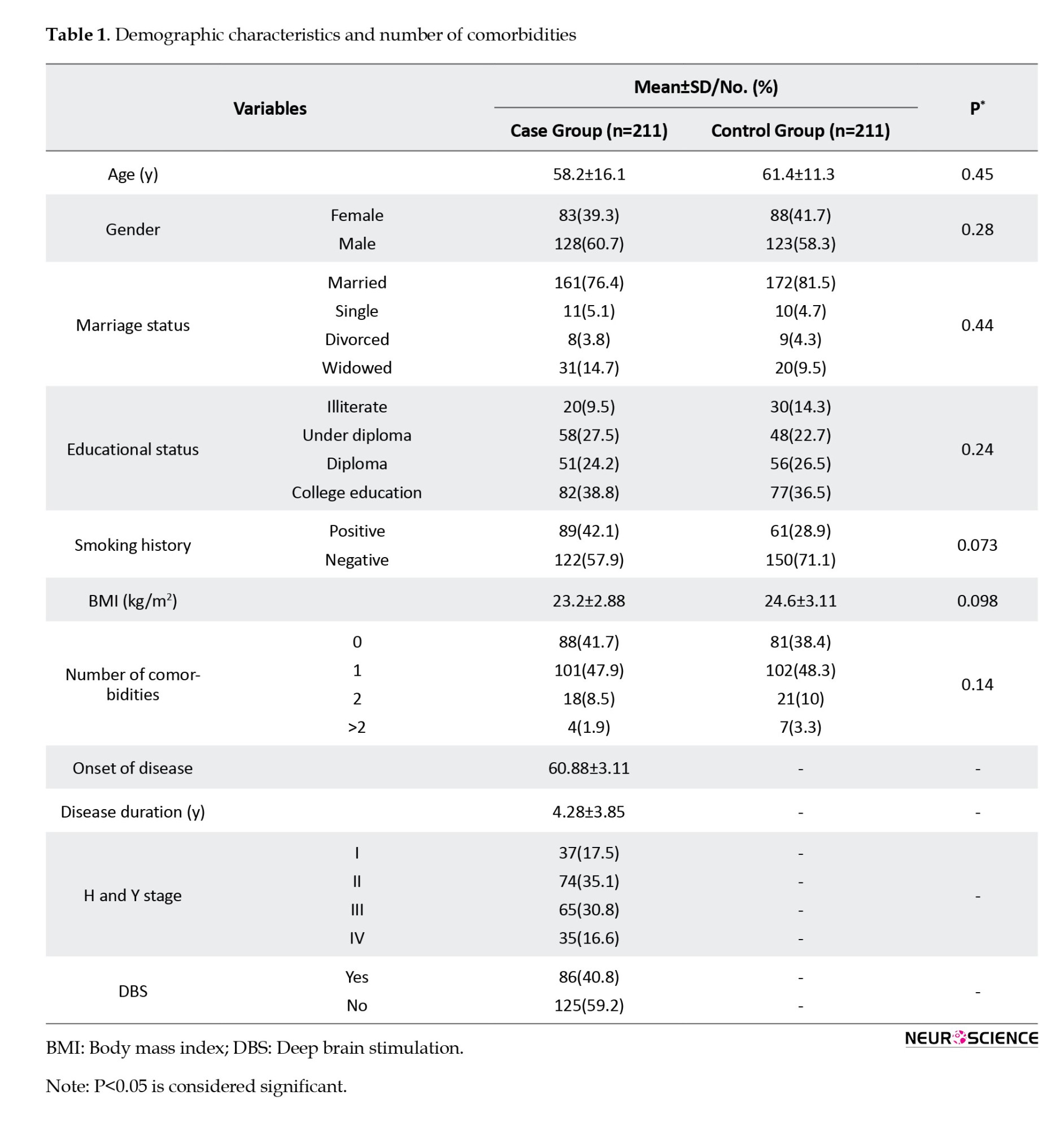
Comparing QoL of patients with PD compared to the control group
The mean PDQ-39 score in the case group was significantly higher than that in the control group (P=0.001). The mean PDQ-39 score in all subscales, except for social support, was significantly lower in the control group than in the case group (P<0.001). The highest and lowest mean scores in patients with PD were 60.33±12.1 and 31.26±20.5 for mobility and social support subscales, respectively (Table 2).
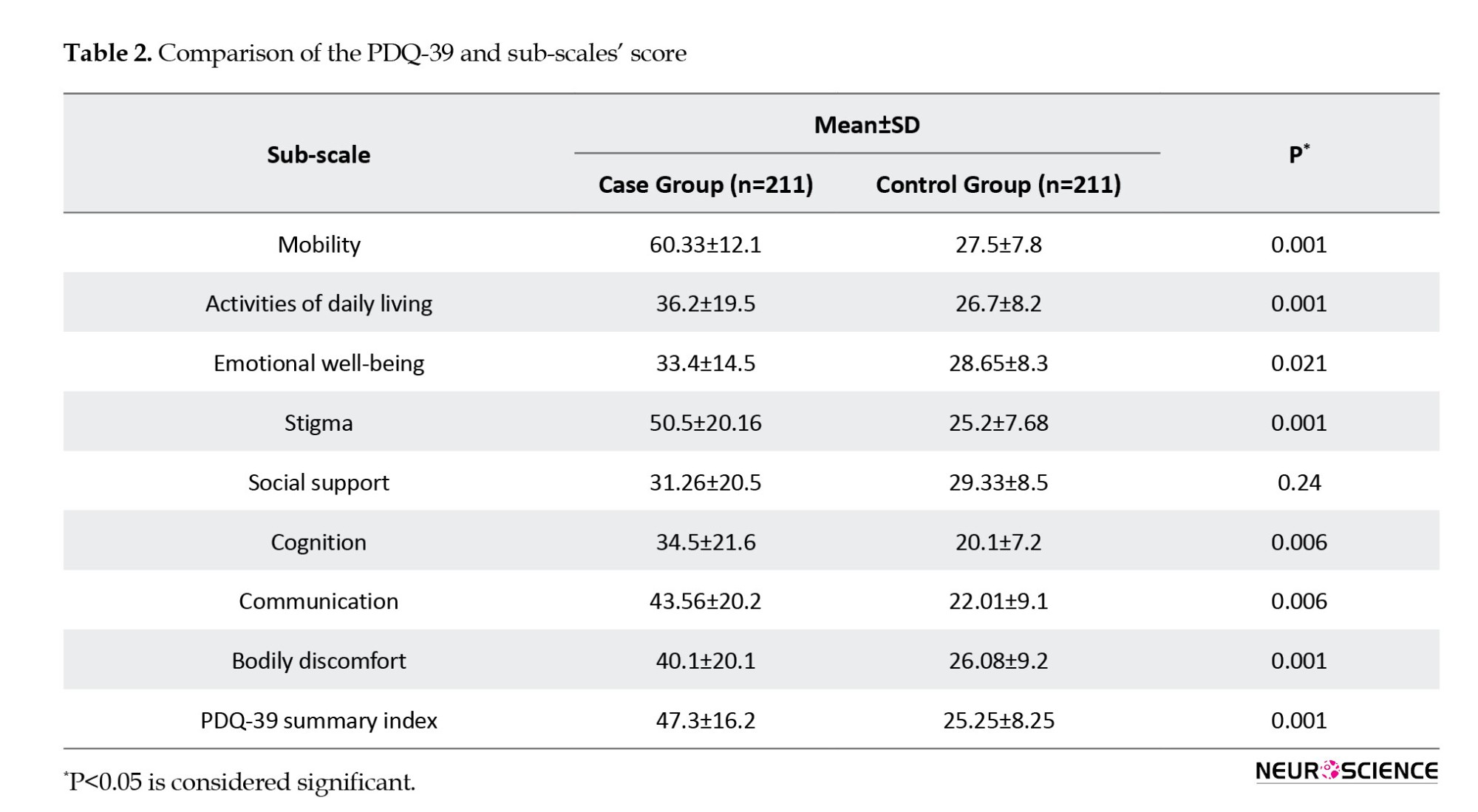
Comparing QoL of patients with PD based on DBS or non-DBS
The mean PDQ-39 score in the group of patients with DBS and those without DBS was 42.22±18.1 and 53.9±21.1, respectively, and this difference was statistically significant (P<0.001). The mean QoL score in the sub-scales of mobility, activities of daily living, cognition, and communication in the intervention group were significantly better than those in the non-intervention group (P<0.05). Although the mean QoL score for the emotional well-being, stigma, social support, and bodily discomfort subscales were better in the intervention group, this difference was not statistically significant (P>0.05) (Table 3).
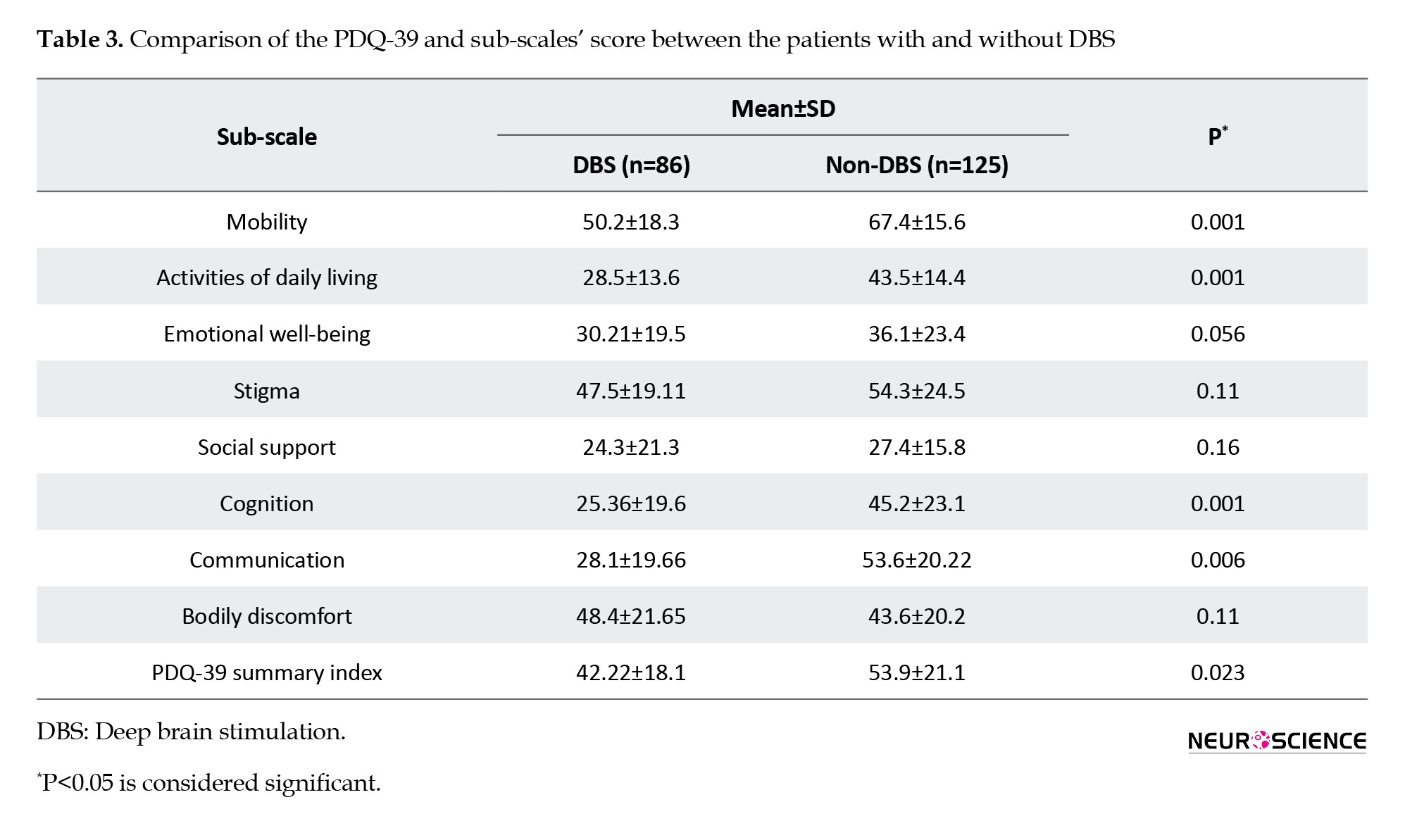
Comparing QoL of patients with PD based on the stage of the disease
The comparison of the mean QoL score and sub-scales showed that QoL was significantly different in stages of the disease (P<0.05) (Table 4).
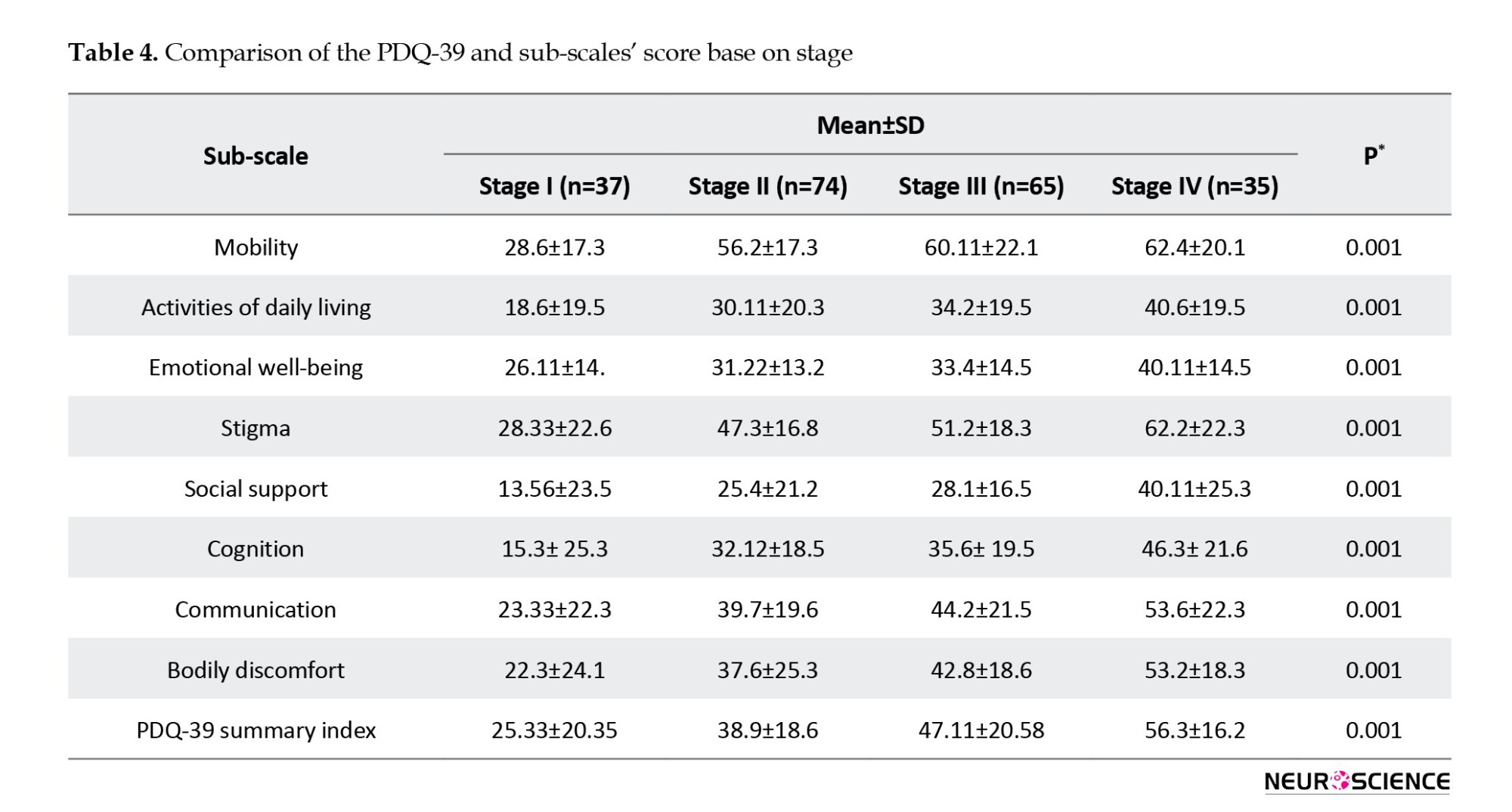
Prognostic factors associated with their QoL in patients with PD
The results of multivariate analysis showed that sex, patient age, smoking, education level, duration of disease, patient stage, and intervention with DBS were significantly related to patients’ QoL (P<0.05) (Table 5).
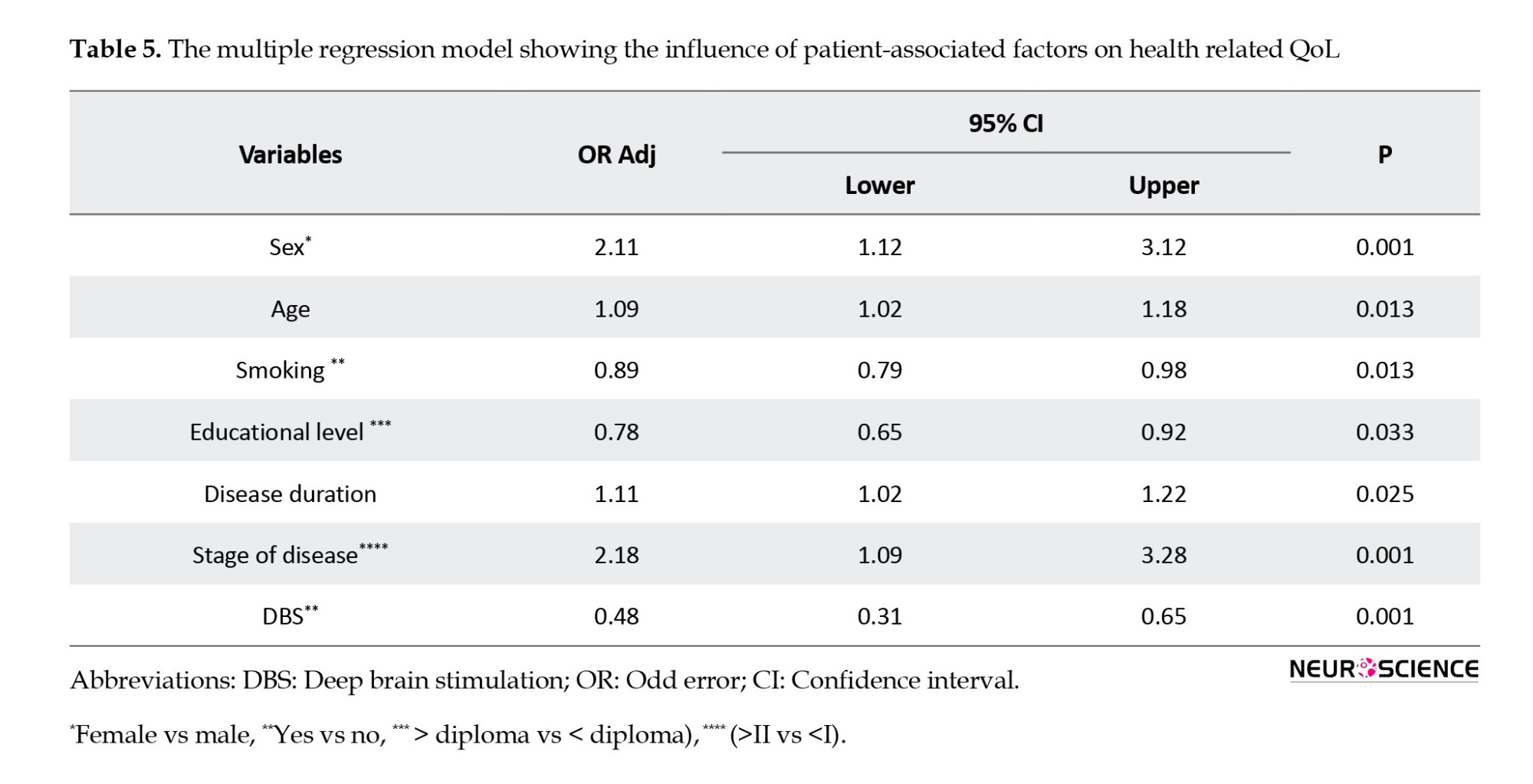
4. Discussion
This study aimed to assess the QoL of patients with PD using the PDQ-39 and identify the prognostic factors associated with their QoL. Aside from various motor dysfunctions, at the neuroscience level, PD is associated with neuropsychological dysfunctions, such as sleep disturbance, depression, fatigue, and cognitive disorders, all of which can adversely affect QoL (Zhao et al., 2021). In this study, the assessment of QoL using the PDQ-39 questionnaire showed that the overall and sub-scale scores, except for social support, were significantly higher in the case group than in the control group. The factors most strongly associated with lower QoL in patients with PD, ranked in order of importance, were mobility, stigma, communication, bodily discomfort, activities of daily living, cognition, and emotional well-being. These results are consistent with most previous research highlighting PD’s negative impact on overall QoL. (Hariz & Forsgren, 2011; Paolucci et al., 2018; Park et al., 2020; Schrag et al., 2000a; Vossius et al., 2009). Similar to our study, Chu and Tan identified mobility as one of the dimensions with the highest impact on QOL in patients with PD, and social support did not differ significantly between case and control groups (Chu & Tan, 2018). In contrast to our study, Park et al. stated that PD patients had significantly lower QOL in all dimensions of the PDQ-39 compared to healthy controls, except for bodily discomfort (Park et al., 2014).
This difference could be due to the lower mean age of patients with PD in our study compared to Park. With advancing age, even healthy individuals experience general physical decline, increasing the likelihood of lower QoL due to bodily discomfort (Leplège & Hunt, 1997). Comparing QoL outcomes between PD patients with and without DBS intervention, we observed a significant difference in the PDQ-39 summary index scores. Patients who underwent DBS intervention exhibited a significantly better QoL, as indicated by a lower PDQ-39 summary index score, than those without DBS. This finding suggests that DBS positively impacts the overall QoL of PD patients. Consistent with numerous previous studies (Bohlega et al., 2016; Bratsos et al., 2018; Nijhuis et al., 2021; Perestelo-Pérez et al., 2014), our findings showed that DBS-recipient PD patients presented better QoL (lower PDQ-39 score). In our study, we examined all aspects of QoL after DBS. Since DBS improves the motor circuits in speech and language in patients with PD, it is unsurprising to see enhancements in indicators such as mobility, daily activities, and communication (Bratsos et al., 2018; Follett et al., 2010; Krack et al., 2003; Weaver et al., 2012; Xie et al., 2016). Our study results support this improvement, consistent with previous studies (Baudouin et al., 2023; Follett et al., 2010; Perestelo-Pérez et al., 2014; Weaver et al., 2012; Zahodne et al., 2009). In our study, social support scores, emotional well-being, and stigma did not significantly differ between the two groups. According to these criteria, the results can vary across studies due to differences in cultural contexts. For example, receiving DBS may be perceived as a form of electric shock therapy in some countries, which could increase stigma levels. In contrast to our study, some results have demonstrated that DBS can alleviate bodily discomfort by reducing pain (Follett et al., 2010; Weaver et al., 2012; Xie et al., 2016; Zahodne et al., 2009). The discrepancy may be attributed to several factors, including variations in study populations (differences in disease severity, duration of PD, and comorbidities) and variations in surgical technique, target location, and stimulation parameters used in DBS procedures. Our results align with previous studies (Koplas et al., 1999; Park et al., 2014; Schrag et al., 2000b), indicating a clear association between PD stage and QoL. As PD progressed from stage I to stage IV, we observed a gradual decline in QoL. The QoL was lowest in mobility, followed by stigma sub-scales in all stages of the disease. However, in some studies, a deteriorating trend has not yet been observed in all aspects of QoL (Fitzpatrick et al., 1997; Schrag et al., 2000b).
The potential explanation for this discrepancy may lie in the differences in the healthcare system, such as variations in geriatric medicine and palliative care practices. According to the multiple regression results, female gender, older age, non-smoking status, lower educational level, longer disease duration, and advanced disease stage are the main prognostic factors associated with lower QoL. Among the factors considered, sex had the second strongest relationship with QoL. Similar to previous studies (Balzer-Geldsetzer et al., 2018; Dluzen & McDermott, 2000; Kuopio et al., 2000; Meng et al., 2022), our results indicated that females experience a lower QoL. The reason behind this difference could be the older age of onset of PD among women due to the neuroprotective effect of estrogen before menopause (Haaxma et al., 2007). Our study, in contrast to the findings of Hendred and Foster, (2016) in the USA population, showed a negative relationship between age and QoL. Our results can be explained by the differences in retirement support and healthcare coverage in developing countries (Hendred & Foster, 2016; Huang et al., 2020; Netuveli & Blane, 2008). The positive relationship between educational level and QoL can be explained by the crucial role of this prognostic factor in providing individuals with access to economic resources, employment opportunities, and stable, supportive social relationships (Cubo et al., 2002; Hendred & Foster, 2016; Ross & Van Willigen, 1997).
5. Conclusion
In conclusion, this study highlights the significant impact of DBS on PD patients’ QoL, especially in sub-scales of mobility, daily activities, emotional well-being, and cognition. Moreover, identifying the main prognostic factors of QoL (sex, age, smoking status, educational level, disease duration, and stage) can lead to avenues for improving the QoL for these patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1402.141). The research team adhered to the ethical principles outlined in the Declaration of Helsinki regarding clinical studies at all stages of the present study. Consent was obtained from all participants and/or their legal guardian(s) in the case of minors (below 16 years of age). Since no interventions were performed on patients, the condition for maintaining the confidentiality of patient information was not a moral restriction, as per the Ethics Committee.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Seyed Amirhassan Habibi, Mansour Bahardoust, Aida Moarrefzadeh, Safa Mousavi, and Arash Sarveazad; Data collection: Sadra Sarandili, Mansour Bahardoust, Nogol Motamed-Gorji, Neda Hashemi, Mohammadhossein Vazirizadeh-Mahabadi, and Arash Sarveazad; Data analysis and interpretation: Mansour Bahardoust and Safa Mousavi; Writing the original draft: Aida Moarrefzadeh, Arash Sarveazad, and Mohammadhossein Vazirizadeh-Mahabadi; Review and editing: Seyed Amirhassan Habibi, Neda Hashemi, Mansour Bahardoust, Arash Sarveazad, Safa Mousavi, Mohammadhossein Vazirizadeh-Mahabadi, and Nogol Motamed-Gorji; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors sincerely appreciate all participants who contributed to this research and completed the questionnaires.
References
Parkinson’s disease (PD) is the second most common neurodegenerative disease, with rapidly increasing prevalence among other neurological diseases (de Lau & Breteler, 2006; Feigin et al., 2017). More than 4 million people suffer from this incurable disease, and the number is predicted to double by 2030 (Dorsey et al., 2007). The disease is characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra region of the brain, leading to typical motor symptoms, such as tremors, bradykinesia (slowness of movement), rigidity, and postural instability. However, PD is not limited to motor symptoms; it also has substantial non-motor manifestations that significantly impact the quality of life (QoL) of affected individuals (Garcia-Ruiz et al., 2014; Wirdefeldt et al., 2011). By identifying and addressing the prognostic factors associated with QoL, healthcare professionals can improve the patient’s overall well-being and ultimately help them live a more fulfilling life (Morris et al., 2009). Low Qol in patients with idiopathic PD seems to be associated with a fear of falls in patients with PD, which is demonstrative of mobility and activities of daily living (Mehdizadeh et al., 2016). However, there has been growing concern regarding non-motor symptoms, including dementia, sexual dysfunction, mood disturbance, and insomnia, because they are thought to have an even more detrimental effect on QoL than classic motor deficits (Chaudhuri et al., 2006; Forsaa et al., 2008; Weintraub et al., 2004).
The World Health Organization (WHO) defines QoL as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and about their goals, expectations, standards, and concerns” (Group, 1995). Assessing QoL in PD requires reliable and validated measurement tools. One widely used instrument is the PD questionnaire (PDQ-39). The PDQ-39 is a self-administered questionnaire to assess health-related QoL in individuals with PD. It consists of eight domains or sub-scales: Mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort (Jenkinson et al., 1997; Peto et al., 1995). By evaluating these domains, the PDQ-39 comprehensively assesses the disease’s impact on different aspects of QoL. A poorer QoL in patients with PD has been linked to several demographic parameters, including age (Hendred & Foster, 2016), sex (Valeikienė et al., 2008), education (Cubo et al., 2002), and several clinical characteristics of the disease, such as its duration and stage (Dogan et al., 2015). In this case-control study, we aimed to assess the QoL in patients with PD using the PDQ-39 questionnaire and identify the prognostic factors associated with their QoL.
2. Materials and Methods
The present observational (case-control) study was conducted in a multicenter manner after approval by the Ethics Committee of Iran University of Medical Sciences. The cases included 211 patients with PD who had been referred to the Neurology Clinic of Taleghani and Rasoul Akram hospitals affiliated with Shahid Beheshti University of Medical Sciences and Iran University of Medical Sciences (2019-2022). The sampling method for patients in two centers was conducted as available among patients who met the study’s inclusion criteria. To control for confounding variables between the two groups, cases and controls were matched in terms of demographic characteristics, including age, sex, physical profile, and comorbidities, using the frequency matching method.
Eligibility criteria
The inclusion criteria included a definitive diagnosis of PD based on clinical findings and examination by a neurologist, mainly based on the UK PD Society brain bank diagnostic criteria (Clarke et al., 2016); being alive at the time of follow-up; undergoing intervention with deep brain stimulation (DBS); at least six months had passed since the surgery; completeness of the file; and informed cooperation of the patients to participate in the study and complete the QoL questionnaire.
The exclusion criteria included cancer, drug and alcohol addiction, untreated severe depression or other neuropsychiatric diseases, including multiple sclerosis (MS) and Alzheimer’s disease, chronic viral infection, such as viral hepatitis or HIV, and dead Patients.
Data gathering
The study was conducted in two formats: Retrospective (collecting demographic, clinical, and radiographic information from patients’ files) and prospective (completion of a QoL questionnaire). Data were collected using a two-part checklist after visiting the archive department and accessing the patients’ files. The first part included the patient’s demographic information form (age, sex, body mass index (IBM), education, number of morbidities, and smoking history). The second part included clinical information (age at onset, duration, disease severity, and DBS surgery). The severity of PD was classified into four stages based on the Hoehn and Yahr index (Bhidayasiri & Tarsy, 2012). A higher stage indicates a more severe disease.
The PD questionnaire (PDQ-39) was used to evaluate QoL. This questionnaire has eight separate dimensions: movement (10 questions), daily life activities (6 questions), feeling good (6 questions), stigma (4 questions), social support (3 questions), recognition (4 questions), communication (3 questions), and physical discomfort (3 questions). Each questionnaire question had five options on the Likert scale; only one option was marked. The first option is the sign of the best situation (score 0), and the fifth option is the sign of the worst (score 4). The range of scores for each dimension is reported from 0 to 100, where zero means no problem and 100 indicates the worst health condition. The score of each dimension is calculated as follows: The sum of the raw scores of each dimension divided by the sum of the maximum possible raw score of that dimension, multiplied by 100. The average scores of these dimensions were combined to create a single index called the PD summary index (PDSI). The range of the PDSI is also reported to be 0-100. The validity and reliability of the Persian version of this questionnaire for Iranian patients have been confirmed by Dehghan et al. (2016). The same questionnaire was used to evaluate QoL in the control group. After obtaining consent from the patients to participate in the study, the patients or the researcher completed the QoL questionnaire in person (in cases where the patients were unable to complete the questionnaire).
Statistical analysis
Data were analyzed using SPSS software, version 22. Descriptive statistics (frequency and %) were used to report qualitative variables. Quantitative variables were reported as Mean±SD. The normality of the distribution of quantitative variables was evaluated using the Shapiro-Wilk test. The chi-square test was used to compare qualitative variables in two groups. To compare the quantitative variables between two groups, a t-test was used when the quantitative variables had a normal distribution, and a Mann-Whitney test was used if the assumption of normality was not met. The analysis of single variables in more than two groups was performed using a one-way variance test. To control for confounding variables, all variables with a P<0.05 in the univariate analysis were included in the multivariate logistic regression analysis using the backward model. The effect size index was reported along with the adjusted odds ratio and its 95% confidence interval (CI). Multivariate logistic regression analysis was used to estimate the predictor variables of QoL in patients with PD. A P<0.05 was considered statistically significant.
3. Results
Demographic data
Four hundred twenty-two participants (211 cases and 211 controls) were included in the study. The mean age of the patients was 59.8±13.7 years. The median age was 60 years. One hundred twenty-eight patients (60.8%) were male. Regarding disease severity based on the H and Y stage index, most patients were in stages 2 and 3. The average duration of the disease since its onset was 4.28±3.85 years. 86(40.8%) patients underwent DBS intervention. No statistically significant difference was observed for the demographic variables in the two groups (Table 1).

Comparing QoL of patients with PD compared to the control group
The mean PDQ-39 score in the case group was significantly higher than that in the control group (P=0.001). The mean PDQ-39 score in all subscales, except for social support, was significantly lower in the control group than in the case group (P<0.001). The highest and lowest mean scores in patients with PD were 60.33±12.1 and 31.26±20.5 for mobility and social support subscales, respectively (Table 2).

Comparing QoL of patients with PD based on DBS or non-DBS
The mean PDQ-39 score in the group of patients with DBS and those without DBS was 42.22±18.1 and 53.9±21.1, respectively, and this difference was statistically significant (P<0.001). The mean QoL score in the sub-scales of mobility, activities of daily living, cognition, and communication in the intervention group were significantly better than those in the non-intervention group (P<0.05). Although the mean QoL score for the emotional well-being, stigma, social support, and bodily discomfort subscales were better in the intervention group, this difference was not statistically significant (P>0.05) (Table 3).

Comparing QoL of patients with PD based on the stage of the disease
The comparison of the mean QoL score and sub-scales showed that QoL was significantly different in stages of the disease (P<0.05) (Table 4).

Prognostic factors associated with their QoL in patients with PD
The results of multivariate analysis showed that sex, patient age, smoking, education level, duration of disease, patient stage, and intervention with DBS were significantly related to patients’ QoL (P<0.05) (Table 5).

4. Discussion
This study aimed to assess the QoL of patients with PD using the PDQ-39 and identify the prognostic factors associated with their QoL. Aside from various motor dysfunctions, at the neuroscience level, PD is associated with neuropsychological dysfunctions, such as sleep disturbance, depression, fatigue, and cognitive disorders, all of which can adversely affect QoL (Zhao et al., 2021). In this study, the assessment of QoL using the PDQ-39 questionnaire showed that the overall and sub-scale scores, except for social support, were significantly higher in the case group than in the control group. The factors most strongly associated with lower QoL in patients with PD, ranked in order of importance, were mobility, stigma, communication, bodily discomfort, activities of daily living, cognition, and emotional well-being. These results are consistent with most previous research highlighting PD’s negative impact on overall QoL. (Hariz & Forsgren, 2011; Paolucci et al., 2018; Park et al., 2020; Schrag et al., 2000a; Vossius et al., 2009). Similar to our study, Chu and Tan identified mobility as one of the dimensions with the highest impact on QOL in patients with PD, and social support did not differ significantly between case and control groups (Chu & Tan, 2018). In contrast to our study, Park et al. stated that PD patients had significantly lower QOL in all dimensions of the PDQ-39 compared to healthy controls, except for bodily discomfort (Park et al., 2014).
This difference could be due to the lower mean age of patients with PD in our study compared to Park. With advancing age, even healthy individuals experience general physical decline, increasing the likelihood of lower QoL due to bodily discomfort (Leplège & Hunt, 1997). Comparing QoL outcomes between PD patients with and without DBS intervention, we observed a significant difference in the PDQ-39 summary index scores. Patients who underwent DBS intervention exhibited a significantly better QoL, as indicated by a lower PDQ-39 summary index score, than those without DBS. This finding suggests that DBS positively impacts the overall QoL of PD patients. Consistent with numerous previous studies (Bohlega et al., 2016; Bratsos et al., 2018; Nijhuis et al., 2021; Perestelo-Pérez et al., 2014), our findings showed that DBS-recipient PD patients presented better QoL (lower PDQ-39 score). In our study, we examined all aspects of QoL after DBS. Since DBS improves the motor circuits in speech and language in patients with PD, it is unsurprising to see enhancements in indicators such as mobility, daily activities, and communication (Bratsos et al., 2018; Follett et al., 2010; Krack et al., 2003; Weaver et al., 2012; Xie et al., 2016). Our study results support this improvement, consistent with previous studies (Baudouin et al., 2023; Follett et al., 2010; Perestelo-Pérez et al., 2014; Weaver et al., 2012; Zahodne et al., 2009). In our study, social support scores, emotional well-being, and stigma did not significantly differ between the two groups. According to these criteria, the results can vary across studies due to differences in cultural contexts. For example, receiving DBS may be perceived as a form of electric shock therapy in some countries, which could increase stigma levels. In contrast to our study, some results have demonstrated that DBS can alleviate bodily discomfort by reducing pain (Follett et al., 2010; Weaver et al., 2012; Xie et al., 2016; Zahodne et al., 2009). The discrepancy may be attributed to several factors, including variations in study populations (differences in disease severity, duration of PD, and comorbidities) and variations in surgical technique, target location, and stimulation parameters used in DBS procedures. Our results align with previous studies (Koplas et al., 1999; Park et al., 2014; Schrag et al., 2000b), indicating a clear association between PD stage and QoL. As PD progressed from stage I to stage IV, we observed a gradual decline in QoL. The QoL was lowest in mobility, followed by stigma sub-scales in all stages of the disease. However, in some studies, a deteriorating trend has not yet been observed in all aspects of QoL (Fitzpatrick et al., 1997; Schrag et al., 2000b).
The potential explanation for this discrepancy may lie in the differences in the healthcare system, such as variations in geriatric medicine and palliative care practices. According to the multiple regression results, female gender, older age, non-smoking status, lower educational level, longer disease duration, and advanced disease stage are the main prognostic factors associated with lower QoL. Among the factors considered, sex had the second strongest relationship with QoL. Similar to previous studies (Balzer-Geldsetzer et al., 2018; Dluzen & McDermott, 2000; Kuopio et al., 2000; Meng et al., 2022), our results indicated that females experience a lower QoL. The reason behind this difference could be the older age of onset of PD among women due to the neuroprotective effect of estrogen before menopause (Haaxma et al., 2007). Our study, in contrast to the findings of Hendred and Foster, (2016) in the USA population, showed a negative relationship between age and QoL. Our results can be explained by the differences in retirement support and healthcare coverage in developing countries (Hendred & Foster, 2016; Huang et al., 2020; Netuveli & Blane, 2008). The positive relationship between educational level and QoL can be explained by the crucial role of this prognostic factor in providing individuals with access to economic resources, employment opportunities, and stable, supportive social relationships (Cubo et al., 2002; Hendred & Foster, 2016; Ross & Van Willigen, 1997).
5. Conclusion
In conclusion, this study highlights the significant impact of DBS on PD patients’ QoL, especially in sub-scales of mobility, daily activities, emotional well-being, and cognition. Moreover, identifying the main prognostic factors of QoL (sex, age, smoking status, educational level, disease duration, and stage) can lead to avenues for improving the QoL for these patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1402.141). The research team adhered to the ethical principles outlined in the Declaration of Helsinki regarding clinical studies at all stages of the present study. Consent was obtained from all participants and/or their legal guardian(s) in the case of minors (below 16 years of age). Since no interventions were performed on patients, the condition for maintaining the confidentiality of patient information was not a moral restriction, as per the Ethics Committee.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and study design: Seyed Amirhassan Habibi, Mansour Bahardoust, Aida Moarrefzadeh, Safa Mousavi, and Arash Sarveazad; Data collection: Sadra Sarandili, Mansour Bahardoust, Nogol Motamed-Gorji, Neda Hashemi, Mohammadhossein Vazirizadeh-Mahabadi, and Arash Sarveazad; Data analysis and interpretation: Mansour Bahardoust and Safa Mousavi; Writing the original draft: Aida Moarrefzadeh, Arash Sarveazad, and Mohammadhossein Vazirizadeh-Mahabadi; Review and editing: Seyed Amirhassan Habibi, Neda Hashemi, Mansour Bahardoust, Arash Sarveazad, Safa Mousavi, Mohammadhossein Vazirizadeh-Mahabadi, and Nogol Motamed-Gorji; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors sincerely appreciate all participants who contributed to this research and completed the questionnaires.
References
Balzer-Geldsetzer, M., Klotsche, J., LANDSCAPE Consortium, Dodel, R., & Riedel, O. (2018). Quality of life in a German cohort of Parkinson's patients assessed with three different measures. Journal of Neurology, 265(11), 2713–2722. [DOI:10.1007/s00415-018-9047-9] [PMID]
Baudouin, R., Lechien, J. R., Carpentier, L., Gurruchaga, J. M., Lisan, Q., & Hans, S. (2023). Deep brain stimulation impact on voice and speech quality in parkinson's disease: A systematic review. Otolaryngology--Head And Neck Surgery, 168(3), 307–318. [DOI:10.1177/01945998221120189] [PMID]
Bhidayasiri, R., & Tarsy, D. (2012). Movement disorders: A video atlas. Berlin: Springer Science & Business Media. [DOI:10.1007/978-1-60327-426-5]
Bohlega, S., Abou Al-Shaar, H., Alkhairallah, T., Al-Ajlan, F., Hasan, N., & Alkahtani, K. (2015). Levodopa-carbidopa intestinal gel infusion therapy in advanced parkinson's disease: Single middle eastern center experience. European Neurology, 74(5-6), 227–236. [DOI:10.1159/000442151] [PMID]
Bratsos, S., Karponis, D., & Saleh, S. N. (2018). Efficacy and safety of deep brain stimulation in the treatment of parkinson's disease: A systematic review and meta-analysis of randomized controlled trials. Cureus, 10(10), e3474. [DOI:10.7759/cureus.3474] [PMID]
Chaudhuri, K. R., Healy, D. G., Schapira, A. H., & National Institute for Clinical Excellence (2006). Non-motor symptoms of Parkinson's disease: Diagnosis and management. The Lancet. Neurology, 5(3), 235–245. [DOI:10.1016/S1474-4422(06)70373-8] [PMID]
Chu, S. Y., & Tan, C. L. (2018). Subjective self-rated speech intelligibility and quality of life in patients with parkinson’s disease in a Malaysian sample. The Open Public Health Journal, 11(1), 485. [DOI:10.2174/1874944501811010485]
Clarke, C. E., Patel, S., & Ives, N.; On behalf of the PD REHAB Collaborative Group. (2016). Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson’s disease: A large pragmatic randomised controlled trial (PD REHAB). Southampton (UK): NIHR Journals Librar (Health Technology Assessment, No. 20.63.) Appendix 1, UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria. [Link]
Cubo, E., Rojo, A., Ramos, S., Quintana, S., Gonzalez, M., & Kompoliti, K., et al. (2002). The importance of educational and psychological factors in Parkinson's disease quality of life. European Journal of Neurology, 9(6), 589–593. [DOI:10.1046/j.1468-1331.2002.00484.x] [PMID]
de Lau, L. M., & Breteler, M. M. (2006). Epidemiology of parkinson's disease. The Lancet. Neurology, 5(6), 525–535. [DOI:10.1016/s1474-4422(06)70471-9] [PMID]
Dehghan, A., Ghaem, H., Borhani-Haghighi, A., Safari-Faraman, R., Moosazadeh, M., & Gholami, A. (2016). Evaluation of reliability and validity of PDQ-39: Questionnaire in iranian patients with Parkinson’s disease. Zahedan Journal of Research in Medical Sciences, 18(3), e6245. [DOI:10.17795/zjrms-6245]
Dluzen, D. E., & McDermott, J. L. (2000). Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: implications for Parkinson's disease. The Journal of Gender-Specific Medicine, 3(6), 36-42. [PMID]
Dogan, V. B., Koksal, A., Dirican, A., Baybas, S., Dirican, A., & Dogan, G. B. (2015). Independent effect of fatigue on health-related quality of life in patients with idiopathic Parkinson's disease. Neurological Sciences, 36(12), 2221–2226. [DOI:10.1007/s10072-015-2340-9] [PMID]
Dorsey, E. R., Constantinescu, R., Thompson, J. P., Biglan, K. M., Holloway, R. G., & Kieburtz, K., et al. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 68(5), 384–386. [DOI:10.1212/01.wnl.0000247740.47667.03] [PMID]
GBD 2015 Neurological Disorders Collaborator Group (2017). Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the global burden of disease study 2015. The Lancet. Neurology, 16(11), 877–897. [DOI:10.1016/S1474-4422(17)30299-5] [PMID]
Fitzpatrick, R., Peto, V., Jenkinson, C., Greenhall, R., & Hyman, N. (1997). Health-related quality of life in Parkinson's disease: A study of outpatient clinic attenders. Movement Disorders, 12(6), 916–922. [DOI:10.1002/mds.870120613] [PMID]
Follett, K. A., Weaver, F. M., Stern, M., Hur, K., Harris, C. L., & Luo, P., et al. (2010). Pallidal versus subthalamic deep-brain stimulation for parkinson's disease. The New England Journal of Medicine, 362(22), 2077–2091. [DOI:10.1056/NEJMoa0907083] [PMID]
Forsaa, E. B., Larsen, J. P., Wentzel-Larsen, T., Herlofson, K., & Alves, G. (2008). Predictors and course of health-related quality of life in Parkinson's disease. Movement Disorders, 23(10), 1420–1427. [DOI:10.1002/mds.22121] [PMID]
Garcia-Ruiz, P. J., Chaudhuri, K. R., & Martinez-Martin, P. (2014). Non-motor symptoms of Parkinson's disease A review…from the past. Journal of the Neurological Sciences, 338(1-2), 30–33. [DOI:10.1016/j.jns.2014.01.002] [PMID]
No Aithor. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. (1995). Social Science & Medicine, 41(10), 1403–1409. [DOI:10.1016/0277-9536(95)00112-K] [PMID]
Haaxma, C. A., Bloem, B. R., Borm, G. F., Oyen, W. J., Leenders, K. L., & Eshuis, S., et al. (2007). Gender differences in parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 78(8), 819–824. [DOI:10.1136/jnnp.2006.103788] [PMID]
Hariz, G. M., & Forsgren, L. (2011). Activities of daily living and quality of life in persons with newly diagnosed parkinson's disease according to subtype of disease, and in comparison to healthy controls. Acta Neurologica Scandinavica, 123(1), 20–27. [DOI:10.1111/j.1600-0404.2010.01344.x] [PMID]
Hendred, S. K., & Foster, E. R. (2016). Use of the World Health Organization quality of life assessment short version in mild to moderate parkinson disease. Archives of Physical Medicine and Rehabilitation, 97(12), 2123–2129.e1. [DOI:10.1016/j.apmr.2016.05.020] [PMID]
Huang, R., Ghose, B., & Tang, S. (2020). Effect of financial stress on self-rereported health and quality of life among older adults in five developing countries: A cross sectional analysis of WHO-SAGE survey. BMC Geriatrics, 20(1), 288. [DOI:10.1186/s12877-020-01687-5] [PMID]
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R., & Hyman, N. (1997). The parkinson's disease questionnaire (PDQ-39): Development and validation of a Parkinson's disease summary index score. Age and Ageing, 26(5), 353–357. [DOI:10.1093/ageing/26.5.353] [PMID]
Koplas, P. A., Gans, H. B., Wisely, M. P., Kuchibhatla, M., Cutson, T. M., & Gold, D. T., et al. (1999). Quality of life and Parkinson's disease. The journals of gerontology. Series A, Biological Sciences and Medical Sciences, 54(4), M197–M202. [DOI:10.1093/gerona/54.4.M197] [PMID]
Krack, P., Batir, A., Van Blercom, N., Chabardes, S., Fraix, V., & Ardouin, C., et al. (2003). Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. The New England Journal of Medicine, 349(20), 1925–1934. [DOI:10.1056/NEJMoa035275] [PMID]
Kuopio, A. M., Marttila, R. J., Helenius, H., Toivonen, M., & Rinne, U. K. (2000). The quality of life in Parkinson's disease. Movement Disorders, 15(2), 216–223. [DOI:10.1002/1531-8257(200003)15:23.0.CO;2-#] [PMID]
Leplège, A., & Hunt, S. (1997). The problem of quality of life in medicine. JAMA, 278(1), 47–50. [DOI:10.1001/jama.1997.03550010061041] [PMID]
Mehdizadeh, M., Lajevardi, L., Habibi, S. A. H., ArabBaniasad, M., Baghoori, D., & Daneshjoo, F., et al. (2016). The association between fear of falling and quality of life for balance impairments based on hip and ankle strategies in the drug On- and Off-phase of patients with idiopathic Parkinson' disease. Medical Journal of the Islamic Republic of Iran, 30, 453. [PMID]
Meng, D., Jin, Z., Gao, L., Wang, Y., Wang, R., & Fang, J., et al. (2022). The quality of life in patients with Parkinson's disease: Focus on gender difference. Brain and Behavior, 12(3), e2517. [DOI:10.1002/brb3.2517] [PMID]
Morris, M. E., Watts, J. J., Iansek, R., Jolley, D., Campbell, D., & Murphy, A. T., et al. (2009). Quantifying the profile and progression of impairments, activity, participation, and quality of life in people with Parkinson disease: Protocol for a prospective cohort study. BMC Geriatrics, 9, 2. [DOI:10.1186/1471-2318-9-2] [PMID]
Netuveli, G., & Blane, D. (2008). Quality of life in older ages. British Medical Bulletin, 85, 113–126. [DOI:10.1093/bmb/ldn003] [PMID]
Nijhuis, F. A. P., Esselink, R., de Bie, R. M. A., Groenewoud, H., Bloem, B. R., & Post, B., et al. (2021). Translating evidence to advanced parkinson's disease patients: A systematic review and meta-analysis. Movement Disorders, 36(6), 1293–1307. [DOI:10.1002/mds.28599] [PMID]
Paolucci, T., Iosa, M., Morone, G., Fratte, M. D., Paolucci, S., & Saraceni, V. M., et al (2018). Romberg ratio coefficient in quiet stance and postural control in Parkinson's disease. Neurological Sciences, 39(8), 1355–1360. [DOI:10.1007/s10072-018-3423-1] [PMID]
Park, H. J., Sohng, K. Y., & Kim, S. (2014). Validation of the korean version of the 39-item parkinson's disease questionnaire (PDQ-39). Asian Nursing Research, 8(1), 67–74. [DOI:10.1016/j.anr.2014.02.004] [PMID]
Park, S., Kim, R., Shin, J. H., Kim, H. J., Paek, S. H., & Jeon, B. (2020). The probable REM sleep behavior disorder negatively affects health-related quality of life in Parkinson's disease with bilateral subthalamic nucleus stimulation. Parkinsonism & Related Disorders, 81, 136–139. [DOI:10.1016/j.parkreldis.2020.06.031] [PMID]
Perestelo-Pérez, L., Rivero-Santana, A., Pérez-Ramos, J., Serrano-Pérez, P., Panetta, J., & Hilarion, P. (2014). Deep brain stimulation in Parkinson's disease: Meta-analysis of randomized controlled trials. Journal of Neurology, 261(11), 2051–2060. [DOI:10.1007/s00415-014-7254-6] [PMID]
Peto, V., Jenkinson, C., Fitzpatrick, R., & Greenhall, R. (1995). The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Quality of Life Research, 4(3), 241–248. [DOI:10.1007/bf02260863] [PMID]
Ross, C. E., & Van Willigen, M. (1997). Education and the subjective quality of life. Journal of Health and Social Behavior, 38(3), 275–297. [DOI:10.2307/2955371] [PMID]
Schrag, A., Jahanshahi, M., & Quinn, N. (2000). How does parkinson's disease affect quality of life? A comparison with quality of life in the general population. Movement Disorders, 15(6), 1112–1118. [DOI:10.1002/1531-8257(200011)15:63.0.CO;2-A] [PMID]
Schrag, A., Jahanshahi, M., & Quinn, N. (2000). What contributes to quality of life in patients with Parkinson's disease?. Journal of Neurology, Neurosurgery, and Psychiatry, 69(3), 308–312. [DOI:10.1136/jnnp.69.3.308] [PMID]
Valeikienė, V., Čeremnych-Aleksejenko, J., Alekna, V., & Juozulynas, J. A. (2008). Differences in WHOQOL-100 domain scores in Parkinson’s disease and osteoarthritis. Medical Science Monitor, 14(4), 221-227. [Link]
Vossius, C., Nilsen, O. B., & Larsen, J. P. (2009). Health state values during the first year of drug treatment in early-stage Parkinson's disease: A prospective, population-based, cohort study. Drugs & Aging, 26(11), 973–980. [DOI:10.2165/11318750-000000000-00000] [PMID]
Weaver, F. M., Follett, K. A., Stern, M., Luo, P., Harris, C. L., & Hur, K., et al. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology, 79(1), 55–65. [DOI:10.1212/WNL.0b013e31825dcdc1] [PMID]
Weintraub, D., Moberg, P. J., Duda, J. E., Katz, I. R., & Stern, M. B. (2004). Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's disease. Journal of the American Geriatrics Society, 52(5), 784–788. [DOI:10.1111/j.1532-5415.2004.52219.x] [PMID]
Wirdefeldt, K., Adami, H. O., Cole, P., Trichopoulos, D., & Mandel, J. (2011). Epidemiology and etiology of parkinson's disease: A review of the evidence. European Journal of Epidemiology, 26(Suppl 1), S1–S58. [DOI:10.1007/s10654-011-9581-6] [PMID]
Xie, C. L., Shao, B., Chen, J., Zhou, Y., Lin, S. Y., & Wang, W. W. (2016). Effects of neurostimulation for advanced Parkinson's disease patients on motor symptoms: A multiple-treatments meta-analysas of randomized controlled trials. Scientific Reports, 6, 25285. [DOI:10.1038/srep25285] [PMID]
Zahodne, L. B., Okun, M. S., Foote, K. D., Fernandez, H. H., Rodriguez, R. L., & Wu, S. S., et al. (2009). Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. Journal of Neurology, 256(8), 1321–1329. [DOI:10.1007/s00415-009-5121-7] [PMID]
Zhao, N., Yang, Y., Zhang, L., Zhang, Q., Balbuena, L., & Ungvari, G. S., et al. (2021). Quality of life in parkinson's disease: A systematic review and meta-analysis of comparative studies. CNs Neuroscience & Therapeutics, 27(3), 270–279. [DOI:10.1111/cns.13549] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2024/07/28 | Accepted: 2024/08/18 | Published: 2025/05/1
Received: 2024/07/28 | Accepted: 2024/08/18 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





