Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 157-164 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Razmkon A, Abdollahifard S, Rezaei H, Bahadori A R, Eskandarzadeh P, Rastegar Kazerooni A. Effect of Deep Brain Stimulation on Parkinson Disease Dementia: A Systematic Review and Meta-analysis. BCN 2024; 15 (2) :157-164
URL: http://bcn.iums.ac.ir/article-1-2177-en.html
URL: http://bcn.iums.ac.ir/article-1-2177-en.html
Ali Razmkon1 

 , Saeed Abdollahifard *1
, Saeed Abdollahifard *1 

 , Hirad Rezaei1
, Hirad Rezaei1 

 , Amir Reza Bahadori1
, Amir Reza Bahadori1 

 , Parham Eskandarzadeh1
, Parham Eskandarzadeh1 

 , AmirAli Rastegar Kazerooni1
, AmirAli Rastegar Kazerooni1 




 , Saeed Abdollahifard *1
, Saeed Abdollahifard *1 

 , Hirad Rezaei1
, Hirad Rezaei1 

 , Amir Reza Bahadori1
, Amir Reza Bahadori1 

 , Parham Eskandarzadeh1
, Parham Eskandarzadeh1 

 , AmirAli Rastegar Kazerooni1
, AmirAli Rastegar Kazerooni1 


1- Research Center for Neuromodulation and Pain, Shiraz University of Medical Sciences, Shiraz, Iran.
Keywords: Subthalamic nucleus, Parkinson disease, Deep brain stimulation, Parkinson disease dementia
Full-Text [PDF 600 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Parkinson disease (PD) is one of the most common neurodegenerative conditions, which is characterized by bradykinesia, rigidity, and tremor (Groiss et al., 2009). Patients in the early stages of PD may have subtle cognitive deficits, while overt cognitive deficits are usually manifestations of late-stage PD (Hanagasi et al., 2017). Pathologically, the cardinal features of PD are dopaminergic cell degeneration in the nigrostriatal system, aggregation of Lewy bodies in the cell cytoplasm, and Lewy neurites (Weil et al., 2017).
According to a population-based cohort study, nearly 80% of patients with PD will develop cognitive dysfunction (Aarsland et al., 2003). Mild cognitive impairment as a transition state between normal aging and dementia can be converted to Parkinson disease dementia (PDD) in about 50% of cases (Broeders et al., 2013). Furthermore, according to the Sydney multicenter study, 10 years after diagnosis of PD, dementia may develop in 75% of patients and up to 83% after 20 years (Hely et al., 2008). Although the pathogenesis of PDD is still not completely known, some studies assumed that dysfunction in memory circuits may explain PDD (Lv et al., 2018). Other studies claim that PDD can be related to the presence of Lewy bodies, amyloid plaques, and neurofibrillary tangles in the neocortex and limbic system (Delgado-Alvarado et al., 2016; Weil et al., 2017). Atrophy in the front striatal area and cholinergic structures associated with frontal executive dysfunction are also considered predictors of PDD (Sunwoo et al., 2014; Weil et al., 2017). Deep brain stimulation (DBS) of either the subthalamic nucleus (STN) or globus pallidus interna (GPi) has been tried for treatment of PD patients (Radhakrishnan & Goyal, 2018). Some of the potential contraindications for DBS include some psychiatric disorders like psychosis or depression and uncompensated personality disorders, but the role of DBS in the progression and advancement of PDD is still unknown (Bronstein et al., 2011; Groiss et al., 2009; Kogan et al., 2019).
Appleby et al. reviewed PD patients and showed the controversial effect of DBS on PDD; while some patient’s conditions improved, others worsened or remained unchanged (Appleby et al., 2007). On the other hand, a long-term follow-up of PD patients illustrated no significant changes in dementia scores compared to the baseline (Sunwoo et al., 2014).
There is still a debate on the outcome of DBS on the cognitive function of PD patients. Based on the controversy mentioned above, this study aimed to investigate the effect of DBS on the cognition of PD patients after surgery compared to medical therapy. We also compared the effect of stimulation of different targets on PDD.
2. Materials and Methods
We searched the keywords ((“DBS” OR “deep brain stimulation,”), (“PD” OR “Parkinson’s disease”) AND (“memory” or “dementia”)) in PubMed, Scopus, Cochrane Library, and Web of Science database on October 22, 2020. Articles were added to an endnote database, and two separate researchers deleted duplicate articles and screened the studies; then, the conflicts were discussed with a third person.
The exclusion criteria were as follows:
- Reviews, case series or case reports, letters, Commentaries,
- Articles that were not written in English or included less than five cases,
- No randomization methods were used in the study for advocating patients into the case and control groups, and
- Articles with inadequate data for the assessment of global dementia.
Again, two separate researchers extracted the Mattis dementia rating scale (MDRS) score and descriptive characteristics of the studies, including the author’s name, publication date, the stimulated targets, and the age, and sex of the patients. If any conflict was noted, the issue was discussed with a third researcher.
We used comprehensive meta-analysis version 2 to analyze the data. The raw mean difference was used as the articles studied the same questionnaire and score. A random model was used, and as the correlation coefficient (r) was not reported in the articles, the data were analyzed three times using r=0.1, 0.5, and 0.9. The final result remained significant, so it was not affected by “r.” We calculated the heterogeneity of the data, which was assessed using I2 scores. The scores less than 25% were considered low, 26%-50% mild, 51%-75% high, and 75%-100% very high. The Egger test was used to assess the publication bias. Also, the sensitivity of the results to each article was assessed by excluding each study and analyzing the effect size again.
Two different analyses were conducted. The first analysis compared the best medical treatment (BMT) to subthalamic nucleus- deep brain stimulation (STN-DBS), and the second analysis was performed to compare STN-DBS with other procedures and targets.
3. Results
Study selection
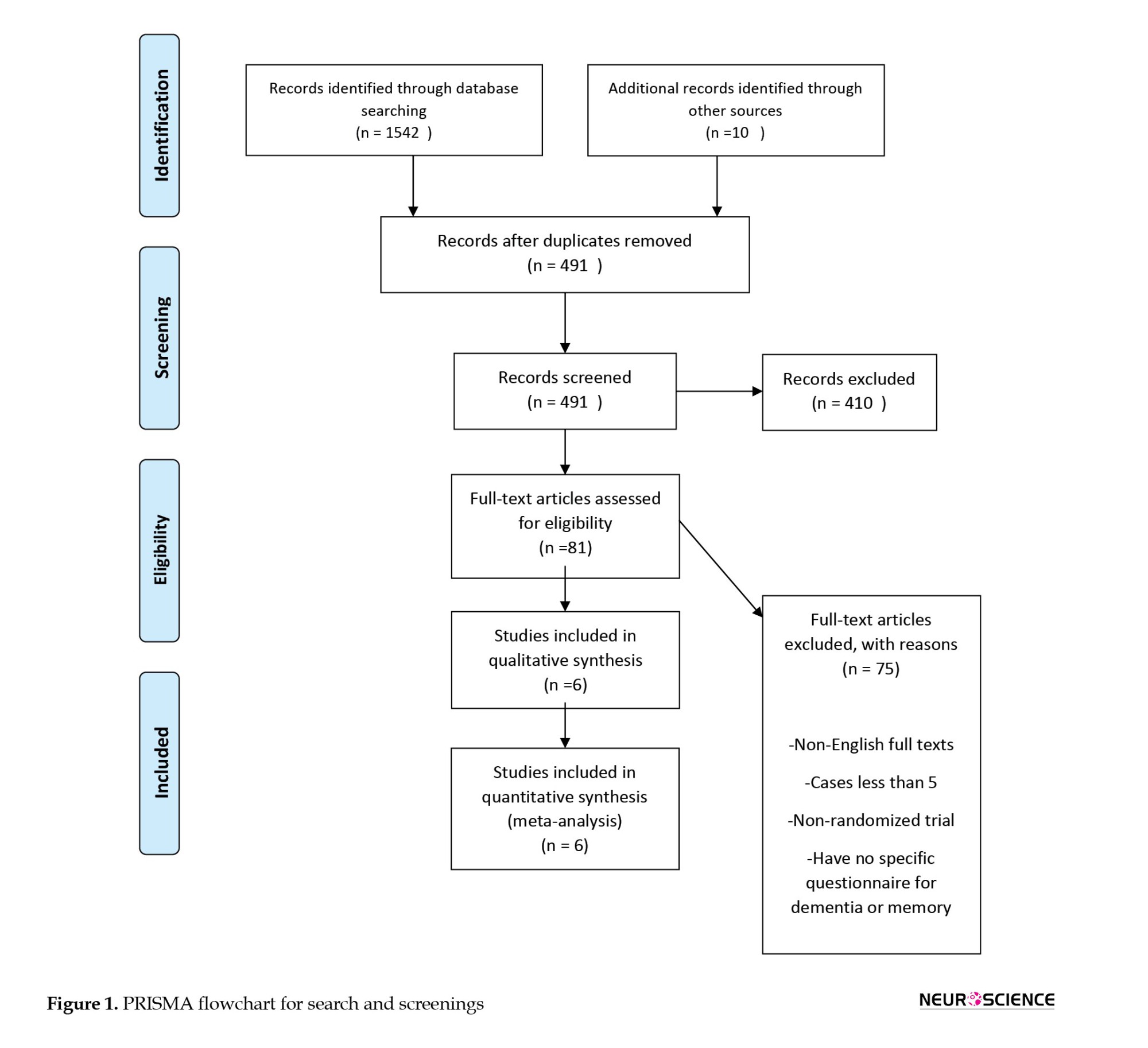
In total, 491 studies were screened after removing the duplicates. The screening results yielded 81 articles to be checked for eligibility. Finally, six studies were included in this meta-analysis for synthesis. The PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart was used for other details (Figure 1).
Study characteristics
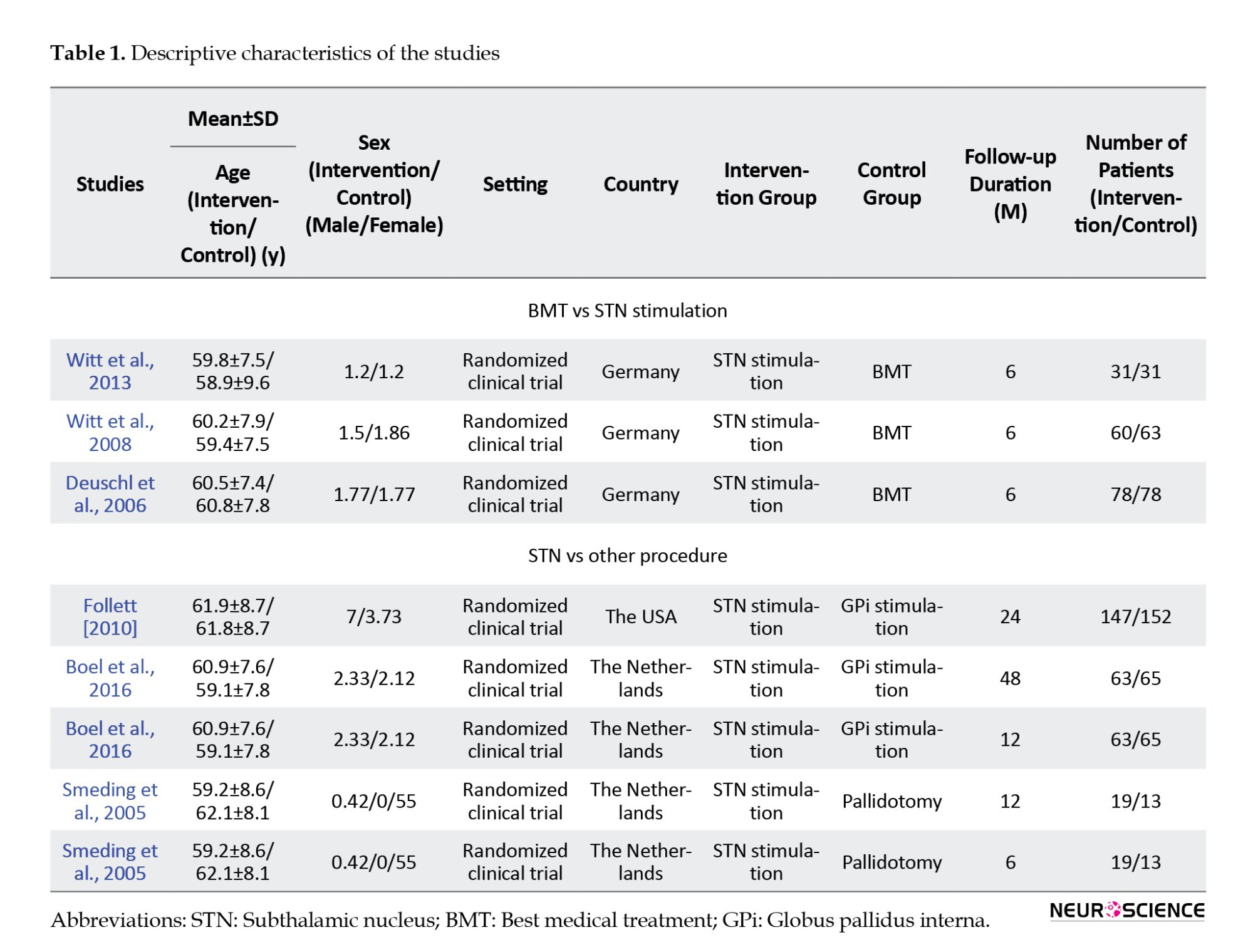
Overall, 800 patients were included in this meta-analysis (341 patients in the first analysis and 459 patients in the second one). For the assessment of global dementia, MDRS, along with descriptive data from the articles, were extracted. Patients with Parkinson disease comprised our study population. In comparing BMT and STN, all studies followed the patients for 6 months, and in the second analysis, the follow-up time varied from 6 months to 48 months. In the latter analysis, the control groups were pallidal stimulation (GPi stimulation) and pallidotomy. It is worth mentioning that two articles were used twice as they had two different follow-up times and provided adequate information for analysis. Other characteristics of these articles are shown in Table 1.
Results of analysis
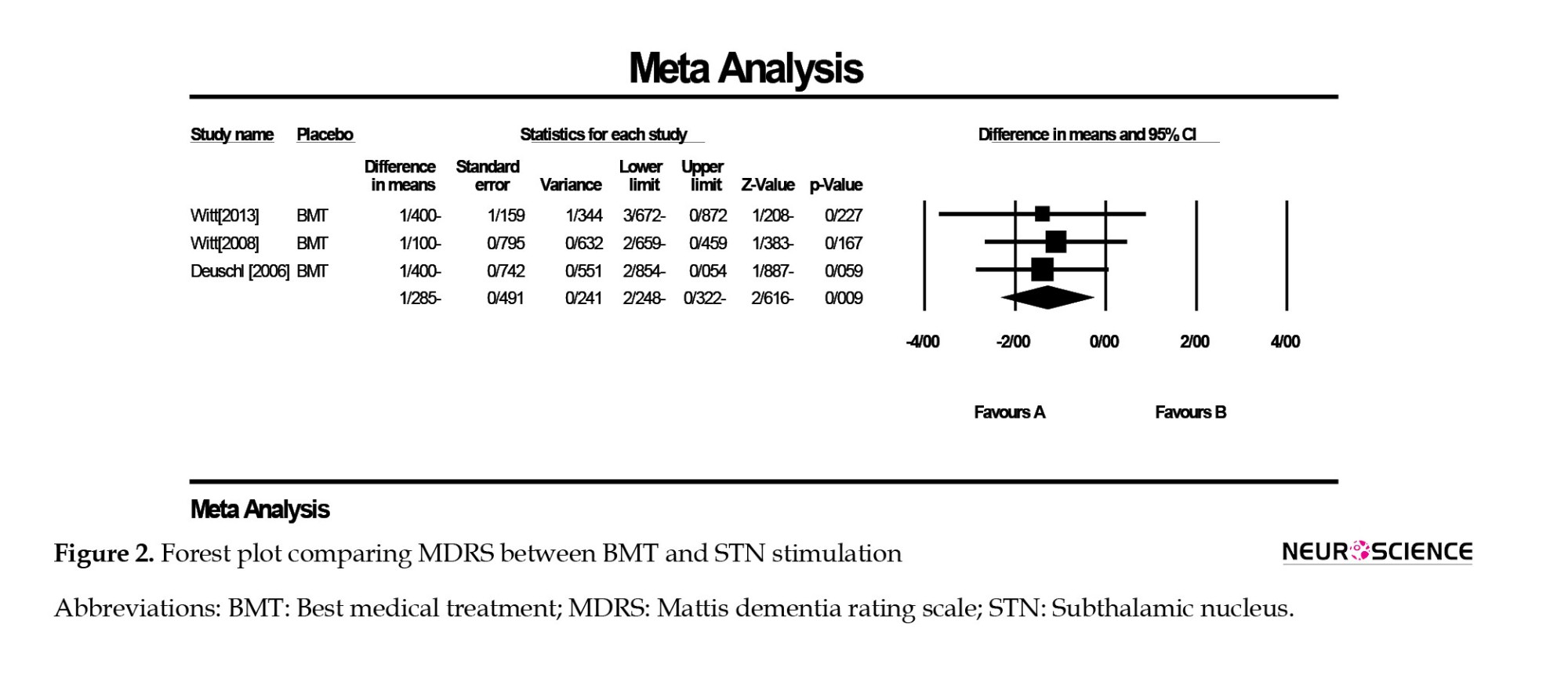
In a comparison of BMT and STN stimulation, the results revealed a significant decrease in MDRS in the STN group compared to BMT (difference in means=-1.285, 95% CI, -2.24%, -0.32%; P=0.009). The publication bias was not significant in Egger test (P=0.860), and no heterogeneity was found (I2=0.00). The forest plot of this analysis is displayed in Figure 2.
In the analysis comparing STN vs other procedures, the result was not significant, and stimulation of STN had no superior effect on dementia of patients with PD (difference in means=-1.071; 95% CI, -2.25%, 0.11%; P=0.77). Also, the Egger test was not significant (P=0.71), and no heterogeneity was observed (I2=0.00). Other details are reported in Figure 3.

4. Discussion
Overall, our results indicated the STN-DBS group showed a larger cognitive decline than the BMT. It should be noted that these articles only followed the patients for a short period after surgery, and the duration of follow-up was not longer than 48 months.
Long-term studies have been conducted to investigate the progression of dementia in DBS-implanted patients and general PD patients and confirmed that the incidence of dementia was increasing as the disease advanced in both the DBS group and general PD patients (Gruber et al., 2019; Jellinger, 2018). In two cohort studies, PD patients were followed after STN-DBS implantation surgery for 3-10 years. The incidence of new-onset dementia in these two studies was approximately equal to the patients who were medically treated (Aybek et al., 2007; Bove et al., 2020). It should be considered that the onset of PD and age of DBS-implanted patients were different in these two studies and in other studies, which may lead to incongruency in the results (Bove et al., 2020).
The explanation for the short- and long-term effects of STN-DBS on dementia in PD patients is challenging. The deficit in the cholinergic output of the nucleus basalis of Meynert to the cortex is assumed to be a major cause of dementia in PD (Bohnen et al., 2006). Gielow et al. (2017) conducted a study on cholinergic input and output of the forebrain. The study showed that STN had a cholinergic output to the motor cortex and ventral and lateral orbitofrontal cortices. On the other hand, DBS may inhibit the neighboring neurons (Chiken & Nambu, 2016). Based on these articles, this hypothesis might be developed that cholinergic output to the cortex will be decreased even more by the stimulation of the STN. The hypothesis mentioned above may explain the decrease in MDRS in the short term. Still, as the PD progresses, it may be assumed that the degenerative processes of the disease may worsen the situation.
Other researchers have proposed different theories about cognitive decline after DBS implantation surgery. Witt et al. (2013) discussed that physical insult, especially to the caudate nucleus during surgery, may negatively affect global cognition. Also, STN stimulation changes the cerebral blood flow of the cortex and plays a role in impairing response inhibition. Another study hypothesized that the role of DBS lead in interrupting connections in the white matter and resulting cognitive decline (Blume et al., 2017). Erasmi et al. (2018) argued that the controversial effect of hyperintense lesioning around magnetic resonance imaging (MRI) of the brain after surgery, classified as gliosis, may negatively affect cognition. The study investigated 30 patients who conducted MRIs for different reasons for hyperintense lesions around the DBS lead. Finally, 26 out of 30 patients had these lesions. In contrast, Liu et al. (2020) denied the correlation between white matter hyperintensities and cognitive decline.
5. Conclusion
To sum up, our study showed that STN-DBS may have a negative effect on the global dementia of patients with PD compared with patients receiving only BMT in a short follow-up period. However, our study could not demonstrate such an effect comparing STN-DBS with other procedures like pallidotomy or GPi stimulation.
Study limitations
The major limitation of our study was the small number of randomized clinical trial articles compared with each other. Also, the number of patients in the included trials could be more. In addition, more long-term studies with larger sample sizes are needed to validate current findings.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A., & Kragh-Sørensen, P. (2003). Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology, 60(3), 387–392. [DOI:10.1001/archneur.60.3.387] [PMID]
Appleby, B. S., Duggan, P. S., Regenberg, A., & Rabins, P. V. (2007). Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years' experience. Movement Disorders : Official Journal of the Movement Disorder Society, 22(12), 1722–1728. [DOI:10.1002/mds.21551] [PMID]
Aybek, S., Gronchi-Perrin, A., Berney, A., Chiuvé, S. C., Villemure, J. G., & Burkhard, P. R., et al. (2007). Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson's disease. Movement Disorders : Official Journal of the Movement Disorder Society, 22(7), 974–981. [DOI:10.1002/mds.21478] [PMID]
Blume, J., Lange, M., Rothenfusser, E., Doenitz, C., Bogdahn, U., & Brawanski, A., et al. (2017). The impact of white matter lesions on the cognitive outcome of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Clinical Neurology and Neurosurgery, 159, 87–92. [DOI:10.1016/j.clineuro.2017.05.023] [PMID]
Boel, J. A., Odekerken, V. J., Schmand, B. A., Geurtsen, G. J., Cath, D. C., Figee, M., ... & Bour, L. J. (2016). Cognitive and psychiatric outcome 3 years after globus pallidus pars interna or subthalamic nucleus deep brain stimulation for Parkinson's disease. Parkinsonism & Related Disorders, 33, 90-95. [DOI: 10.1016/j.parkreldis.2016.09.018] [PMID]
Bohnen, N. I., Kaufer, D. I., Hendrickson, R., Ivanco, L. S., Lopresti, B. J., & Constantine, G. M., et al. (2006). Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. Journal of Neurology, 253(2), 242–247. [DOI:10.1007/s00415-005-0971-0] [PMID]
Bove, F., Fraix, V., Cavallieri, F., Schmitt, E., Lhommée, E., & Bichon, A., et al. (2020). Dementia and subthalamic deep brain stimulation in Parkinson disease: A long-term overview. Neurology, 95(4), e384-e392. [DOI:10.1212/wnl.0000000000009822] [PMID]
Broeders, M., de Bie, R. M., Velseboer, D. C., Speelman, J. D., Muslimovic, D., & Schmand, B. (2013). Evolution of mild cognitive impairment in Parkinson disease. Neurology, 81(4), 346-352. [DOI:10.1212/WNL.0b013e31829c5c86] [PMID]
Bronstein, J. M., Tagliati, M., Alterman, R. L., Lozano, A. M., Volkmann, J., & Stefani, A., et al. (2011). Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Archives of Neurology, 68(2), 165. [DOI:10.1001/archneurol.2010.260] [PMID]
Chiken, S., & Nambu, A. (2016). Mechanism of deep brain stimulation: Inhibition, excitation, or disruption? The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 22(3), 313-322. [DOI:10.1177/1073858415581986] [PMID]
Delgado-Alvarado, M., Gago, B., Navalpotro-Gomez, I., Jiménez-Urbieta, H., & Rodriguez-Oroz, M. C. (2016). Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Movement Disorders, 31(6), 861-881. [PMID]
Deuschl, G., Schade-Brittinger, C., Krack, P., Volkmann, J., Schäfer, H., Bötzel, K., ... & Voges, J. (2006). A randomized trial of deep-brain stimulation for Parkinson's disease. New England Journal of Medicine, 355(9), 896-908. [DOI: 10.1056/NEJMoa060281] [PMID]
Erasmi, R., Granert, O., Zorenkov, D., Falk, D., Wodarg, F., & Deuschl, G., et al. (2018). White matter changes along the electrode lead in patients treated with deep brain stimulation. Frontiers in Neurology, 9, 983. [DOI:10.3389/fneur.2018.00983] [PMID]
Gielow, M. R., & Zaborszky, L. (2017). The input-output relationship of the cholinergic basal forebrain. Cell Reports, 18(7), 1817-1830. [DOI:10.1016/j.celrep.2017.01.060] [PMID]
Groiss, S. J., Wojtecki, L., Südmeyer, M., & Schnitzler, A. (2009). Deep brain stimulation in Parkinson's disease. Therapeutic Advances in Neurological Disorders, 2(6), 20–28. [DOI:10.1177/1756285609339382] [PMID]
Gruber, D., Calmbach, L., Kühn, A. A., Krause, P., Kopp, U. A., & Schneider, G. H., et al. (2019). Longterm outcome of cognition, affective state, and quality of life following subthalamic deep brain stimulation in Parkinson’s disease. Journal of Neural Transmission (Vienna, Austria : 1996), 126(3), 309–318. [DOI:10.1007/s00702-019-01972-7] [PMID]
Hanagasi, H. A., Tufekcioglu, Z., & Emre, M. (2017). Dementia in Parkinson's disease. Journal of The Neurological Sciences, 374, 26–31. [DOI:10.1016/j.jns.2017.01.012] [PMID]
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., & Morris, J. G. (2008). The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders, 23(6), 837-844. [DOI:10.1002/mds.21956] [PMID]
Jellinger, K. A. (2018). Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. Journal of Neural Transmission (Vienna, Austria : 1996), 125(4), 615–650. [DOI:10.1007/s00702-017-1821-9] [PMID]
Kogan, M., McGuire, M., & Riley, J. (2019). Deep brain stimulation for Parkinson Disease. Neurosurgery Clinics of North America, 30(2), 137–146. [DOI:10.1016/j.nec.2019.01.001] [PMID]
Liu, Y., Wu, L., Yang, C., Xian, W., Zheng, Y., & Zhang, C., et al. (2020). The white matter hyperintensities within the cholinergic pathways and cognitive performance in patients with Parkinson’s disease after bilateral STN DBS. Journal of The Neurological Sciences, 418, 117121. [PMID]
Lv, Q., Du, A., Wei, W., Li, Y., Liu, G., & Wang, X. P. (2018). Deep brain stimulation: A potential treatment for Dementia in Alzheimer’s Disease (AD) and Parkinson’s Disease Dementia (PDD). Frontiers in Neuroscience, 12, 360. [DOI:10.3389/fnins.2018.00360] [PMID]
Radhakrishnan, D. M., & Goyal, V. (2018). Parkinson’s disease: A review. Neurology India, 66(Supplement), S26–S35.[DOI:10.4103/0028-3886.226451] [PMID]
Smeding, H. M., Esselink, R. A., Schmand, B., Koning-Haanstra, M., Nijhuis, I., Wijnalda, E. M., & Speelman, J. D. (2005). Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: A comparison of neuropsychological effects. Journal of Neurology, 252, 176-182. [DOI: 10.1007/s00415-005-0628-z] [PMID]
Sunwoo, M. K., Jeon, S., Ham, J. H., Hong, J. Y., Lee, J. E., & Lee, J. M., et al. (2014). The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with P arkinson’s disease. European Journal of Neurology, 21(6), 922-e950. [DOI:10.1111/ene.12412] [PMID]
Weil, R. S., Lashley, T. L., Bras, J., Schrag, A. E., & Schott, J. M. (2017). Current concepts and controversies in the pathogenesis of Parkinson’s disease dementia and Dementia with Lewy Bodies. F1000Research, 6, 1604. [DOI:10.12688/f1000research.11725.1] [PMID]
Witt, K., Daniels, C., Reiff, J., Krack, P., Volkmann, J., Pinsker, M. O., ... & Deuschl, G. (2008). Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. The Lancet Neurology, 7(7), 605-614. [DOI: 10.1016/S1474-4422(08)70114-5] [PMID]
Witt, K., Granert, O., Daniels, C., Volkmann, J., Falk, D., & van Eimeren, T., et al. (2013). Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: Results from a randomized trial. Brain, 136(Pt 7), 2109-2119. [DOI:10.1093/brain/awt151] [PMID]
Parkinson disease (PD) is one of the most common neurodegenerative conditions, which is characterized by bradykinesia, rigidity, and tremor (Groiss et al., 2009). Patients in the early stages of PD may have subtle cognitive deficits, while overt cognitive deficits are usually manifestations of late-stage PD (Hanagasi et al., 2017). Pathologically, the cardinal features of PD are dopaminergic cell degeneration in the nigrostriatal system, aggregation of Lewy bodies in the cell cytoplasm, and Lewy neurites (Weil et al., 2017).
According to a population-based cohort study, nearly 80% of patients with PD will develop cognitive dysfunction (Aarsland et al., 2003). Mild cognitive impairment as a transition state between normal aging and dementia can be converted to Parkinson disease dementia (PDD) in about 50% of cases (Broeders et al., 2013). Furthermore, according to the Sydney multicenter study, 10 years after diagnosis of PD, dementia may develop in 75% of patients and up to 83% after 20 years (Hely et al., 2008). Although the pathogenesis of PDD is still not completely known, some studies assumed that dysfunction in memory circuits may explain PDD (Lv et al., 2018). Other studies claim that PDD can be related to the presence of Lewy bodies, amyloid plaques, and neurofibrillary tangles in the neocortex and limbic system (Delgado-Alvarado et al., 2016; Weil et al., 2017). Atrophy in the front striatal area and cholinergic structures associated with frontal executive dysfunction are also considered predictors of PDD (Sunwoo et al., 2014; Weil et al., 2017). Deep brain stimulation (DBS) of either the subthalamic nucleus (STN) or globus pallidus interna (GPi) has been tried for treatment of PD patients (Radhakrishnan & Goyal, 2018). Some of the potential contraindications for DBS include some psychiatric disorders like psychosis or depression and uncompensated personality disorders, but the role of DBS in the progression and advancement of PDD is still unknown (Bronstein et al., 2011; Groiss et al., 2009; Kogan et al., 2019).
Appleby et al. reviewed PD patients and showed the controversial effect of DBS on PDD; while some patient’s conditions improved, others worsened or remained unchanged (Appleby et al., 2007). On the other hand, a long-term follow-up of PD patients illustrated no significant changes in dementia scores compared to the baseline (Sunwoo et al., 2014).
There is still a debate on the outcome of DBS on the cognitive function of PD patients. Based on the controversy mentioned above, this study aimed to investigate the effect of DBS on the cognition of PD patients after surgery compared to medical therapy. We also compared the effect of stimulation of different targets on PDD.
2. Materials and Methods
We searched the keywords ((“DBS” OR “deep brain stimulation,”), (“PD” OR “Parkinson’s disease”) AND (“memory” or “dementia”)) in PubMed, Scopus, Cochrane Library, and Web of Science database on October 22, 2020. Articles were added to an endnote database, and two separate researchers deleted duplicate articles and screened the studies; then, the conflicts were discussed with a third person.
The exclusion criteria were as follows:
- Reviews, case series or case reports, letters, Commentaries,
- Articles that were not written in English or included less than five cases,
- No randomization methods were used in the study for advocating patients into the case and control groups, and
- Articles with inadequate data for the assessment of global dementia.
Again, two separate researchers extracted the Mattis dementia rating scale (MDRS) score and descriptive characteristics of the studies, including the author’s name, publication date, the stimulated targets, and the age, and sex of the patients. If any conflict was noted, the issue was discussed with a third researcher.
We used comprehensive meta-analysis version 2 to analyze the data. The raw mean difference was used as the articles studied the same questionnaire and score. A random model was used, and as the correlation coefficient (r) was not reported in the articles, the data were analyzed three times using r=0.1, 0.5, and 0.9. The final result remained significant, so it was not affected by “r.” We calculated the heterogeneity of the data, which was assessed using I2 scores. The scores less than 25% were considered low, 26%-50% mild, 51%-75% high, and 75%-100% very high. The Egger test was used to assess the publication bias. Also, the sensitivity of the results to each article was assessed by excluding each study and analyzing the effect size again.
Two different analyses were conducted. The first analysis compared the best medical treatment (BMT) to subthalamic nucleus- deep brain stimulation (STN-DBS), and the second analysis was performed to compare STN-DBS with other procedures and targets.
3. Results
Study selection
In total, 491 studies were screened after removing the duplicates. The screening results yielded 81 articles to be checked for eligibility. Finally, six studies were included in this meta-analysis for synthesis. The PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart was used for other details (Figure 1).

Study characteristics
Overall, 800 patients were included in this meta-analysis (341 patients in the first analysis and 459 patients in the second one). For the assessment of global dementia, MDRS, along with descriptive data from the articles, were extracted. Patients with Parkinson disease comprised our study population. In comparing BMT and STN, all studies followed the patients for 6 months, and in the second analysis, the follow-up time varied from 6 months to 48 months. In the latter analysis, the control groups were pallidal stimulation (GPi stimulation) and pallidotomy. It is worth mentioning that two articles were used twice as they had two different follow-up times and provided adequate information for analysis. Other characteristics of these articles are shown in Table 1.

Results of analysis
In a comparison of BMT and STN stimulation, the results revealed a significant decrease in MDRS in the STN group compared to BMT (difference in means=-1.285, 95% CI, -2.24%, -0.32%; P=0.009). The publication bias was not significant in Egger test (P=0.860), and no heterogeneity was found (I2=0.00). The forest plot of this analysis is displayed in Figure 2.

In the analysis comparing STN vs other procedures, the result was not significant, and stimulation of STN had no superior effect on dementia of patients with PD (difference in means=-1.071; 95% CI, -2.25%, 0.11%; P=0.77). Also, the Egger test was not significant (P=0.71), and no heterogeneity was observed (I2=0.00). Other details are reported in Figure 3.

4. Discussion
Overall, our results indicated the STN-DBS group showed a larger cognitive decline than the BMT. It should be noted that these articles only followed the patients for a short period after surgery, and the duration of follow-up was not longer than 48 months.
Long-term studies have been conducted to investigate the progression of dementia in DBS-implanted patients and general PD patients and confirmed that the incidence of dementia was increasing as the disease advanced in both the DBS group and general PD patients (Gruber et al., 2019; Jellinger, 2018). In two cohort studies, PD patients were followed after STN-DBS implantation surgery for 3-10 years. The incidence of new-onset dementia in these two studies was approximately equal to the patients who were medically treated (Aybek et al., 2007; Bove et al., 2020). It should be considered that the onset of PD and age of DBS-implanted patients were different in these two studies and in other studies, which may lead to incongruency in the results (Bove et al., 2020).
The explanation for the short- and long-term effects of STN-DBS on dementia in PD patients is challenging. The deficit in the cholinergic output of the nucleus basalis of Meynert to the cortex is assumed to be a major cause of dementia in PD (Bohnen et al., 2006). Gielow et al. (2017) conducted a study on cholinergic input and output of the forebrain. The study showed that STN had a cholinergic output to the motor cortex and ventral and lateral orbitofrontal cortices. On the other hand, DBS may inhibit the neighboring neurons (Chiken & Nambu, 2016). Based on these articles, this hypothesis might be developed that cholinergic output to the cortex will be decreased even more by the stimulation of the STN. The hypothesis mentioned above may explain the decrease in MDRS in the short term. Still, as the PD progresses, it may be assumed that the degenerative processes of the disease may worsen the situation.
Other researchers have proposed different theories about cognitive decline after DBS implantation surgery. Witt et al. (2013) discussed that physical insult, especially to the caudate nucleus during surgery, may negatively affect global cognition. Also, STN stimulation changes the cerebral blood flow of the cortex and plays a role in impairing response inhibition. Another study hypothesized that the role of DBS lead in interrupting connections in the white matter and resulting cognitive decline (Blume et al., 2017). Erasmi et al. (2018) argued that the controversial effect of hyperintense lesioning around magnetic resonance imaging (MRI) of the brain after surgery, classified as gliosis, may negatively affect cognition. The study investigated 30 patients who conducted MRIs for different reasons for hyperintense lesions around the DBS lead. Finally, 26 out of 30 patients had these lesions. In contrast, Liu et al. (2020) denied the correlation between white matter hyperintensities and cognitive decline.
5. Conclusion
To sum up, our study showed that STN-DBS may have a negative effect on the global dementia of patients with PD compared with patients receiving only BMT in a short follow-up period. However, our study could not demonstrate such an effect comparing STN-DBS with other procedures like pallidotomy or GPi stimulation.
Study limitations
The major limitation of our study was the small number of randomized clinical trial articles compared with each other. Also, the number of patients in the included trials could be more. In addition, more long-term studies with larger sample sizes are needed to validate current findings.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A., & Kragh-Sørensen, P. (2003). Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology, 60(3), 387–392. [DOI:10.1001/archneur.60.3.387] [PMID]
Appleby, B. S., Duggan, P. S., Regenberg, A., & Rabins, P. V. (2007). Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years' experience. Movement Disorders : Official Journal of the Movement Disorder Society, 22(12), 1722–1728. [DOI:10.1002/mds.21551] [PMID]
Aybek, S., Gronchi-Perrin, A., Berney, A., Chiuvé, S. C., Villemure, J. G., & Burkhard, P. R., et al. (2007). Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson's disease. Movement Disorders : Official Journal of the Movement Disorder Society, 22(7), 974–981. [DOI:10.1002/mds.21478] [PMID]
Blume, J., Lange, M., Rothenfusser, E., Doenitz, C., Bogdahn, U., & Brawanski, A., et al. (2017). The impact of white matter lesions on the cognitive outcome of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Clinical Neurology and Neurosurgery, 159, 87–92. [DOI:10.1016/j.clineuro.2017.05.023] [PMID]
Boel, J. A., Odekerken, V. J., Schmand, B. A., Geurtsen, G. J., Cath, D. C., Figee, M., ... & Bour, L. J. (2016). Cognitive and psychiatric outcome 3 years after globus pallidus pars interna or subthalamic nucleus deep brain stimulation for Parkinson's disease. Parkinsonism & Related Disorders, 33, 90-95. [DOI: 10.1016/j.parkreldis.2016.09.018] [PMID]
Bohnen, N. I., Kaufer, D. I., Hendrickson, R., Ivanco, L. S., Lopresti, B. J., & Constantine, G. M., et al. (2006). Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. Journal of Neurology, 253(2), 242–247. [DOI:10.1007/s00415-005-0971-0] [PMID]
Bove, F., Fraix, V., Cavallieri, F., Schmitt, E., Lhommée, E., & Bichon, A., et al. (2020). Dementia and subthalamic deep brain stimulation in Parkinson disease: A long-term overview. Neurology, 95(4), e384-e392. [DOI:10.1212/wnl.0000000000009822] [PMID]
Broeders, M., de Bie, R. M., Velseboer, D. C., Speelman, J. D., Muslimovic, D., & Schmand, B. (2013). Evolution of mild cognitive impairment in Parkinson disease. Neurology, 81(4), 346-352. [DOI:10.1212/WNL.0b013e31829c5c86] [PMID]
Bronstein, J. M., Tagliati, M., Alterman, R. L., Lozano, A. M., Volkmann, J., & Stefani, A., et al. (2011). Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Archives of Neurology, 68(2), 165. [DOI:10.1001/archneurol.2010.260] [PMID]
Chiken, S., & Nambu, A. (2016). Mechanism of deep brain stimulation: Inhibition, excitation, or disruption? The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 22(3), 313-322. [DOI:10.1177/1073858415581986] [PMID]
Delgado-Alvarado, M., Gago, B., Navalpotro-Gomez, I., Jiménez-Urbieta, H., & Rodriguez-Oroz, M. C. (2016). Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Movement Disorders, 31(6), 861-881. [PMID]
Deuschl, G., Schade-Brittinger, C., Krack, P., Volkmann, J., Schäfer, H., Bötzel, K., ... & Voges, J. (2006). A randomized trial of deep-brain stimulation for Parkinson's disease. New England Journal of Medicine, 355(9), 896-908. [DOI: 10.1056/NEJMoa060281] [PMID]
Erasmi, R., Granert, O., Zorenkov, D., Falk, D., Wodarg, F., & Deuschl, G., et al. (2018). White matter changes along the electrode lead in patients treated with deep brain stimulation. Frontiers in Neurology, 9, 983. [DOI:10.3389/fneur.2018.00983] [PMID]
Gielow, M. R., & Zaborszky, L. (2017). The input-output relationship of the cholinergic basal forebrain. Cell Reports, 18(7), 1817-1830. [DOI:10.1016/j.celrep.2017.01.060] [PMID]
Groiss, S. J., Wojtecki, L., Südmeyer, M., & Schnitzler, A. (2009). Deep brain stimulation in Parkinson's disease. Therapeutic Advances in Neurological Disorders, 2(6), 20–28. [DOI:10.1177/1756285609339382] [PMID]
Gruber, D., Calmbach, L., Kühn, A. A., Krause, P., Kopp, U. A., & Schneider, G. H., et al. (2019). Longterm outcome of cognition, affective state, and quality of life following subthalamic deep brain stimulation in Parkinson’s disease. Journal of Neural Transmission (Vienna, Austria : 1996), 126(3), 309–318. [DOI:10.1007/s00702-019-01972-7] [PMID]
Hanagasi, H. A., Tufekcioglu, Z., & Emre, M. (2017). Dementia in Parkinson's disease. Journal of The Neurological Sciences, 374, 26–31. [DOI:10.1016/j.jns.2017.01.012] [PMID]
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., & Morris, J. G. (2008). The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders, 23(6), 837-844. [DOI:10.1002/mds.21956] [PMID]
Jellinger, K. A. (2018). Dementia with Lewy bodies and Parkinson’s disease-dementia: Current concepts and controversies. Journal of Neural Transmission (Vienna, Austria : 1996), 125(4), 615–650. [DOI:10.1007/s00702-017-1821-9] [PMID]
Kogan, M., McGuire, M., & Riley, J. (2019). Deep brain stimulation for Parkinson Disease. Neurosurgery Clinics of North America, 30(2), 137–146. [DOI:10.1016/j.nec.2019.01.001] [PMID]
Liu, Y., Wu, L., Yang, C., Xian, W., Zheng, Y., & Zhang, C., et al. (2020). The white matter hyperintensities within the cholinergic pathways and cognitive performance in patients with Parkinson’s disease after bilateral STN DBS. Journal of The Neurological Sciences, 418, 117121. [PMID]
Lv, Q., Du, A., Wei, W., Li, Y., Liu, G., & Wang, X. P. (2018). Deep brain stimulation: A potential treatment for Dementia in Alzheimer’s Disease (AD) and Parkinson’s Disease Dementia (PDD). Frontiers in Neuroscience, 12, 360. [DOI:10.3389/fnins.2018.00360] [PMID]
Radhakrishnan, D. M., & Goyal, V. (2018). Parkinson’s disease: A review. Neurology India, 66(Supplement), S26–S35.[DOI:10.4103/0028-3886.226451] [PMID]
Smeding, H. M., Esselink, R. A., Schmand, B., Koning-Haanstra, M., Nijhuis, I., Wijnalda, E. M., & Speelman, J. D. (2005). Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: A comparison of neuropsychological effects. Journal of Neurology, 252, 176-182. [DOI: 10.1007/s00415-005-0628-z] [PMID]
Sunwoo, M. K., Jeon, S., Ham, J. H., Hong, J. Y., Lee, J. E., & Lee, J. M., et al. (2014). The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with P arkinson’s disease. European Journal of Neurology, 21(6), 922-e950. [DOI:10.1111/ene.12412] [PMID]
Weil, R. S., Lashley, T. L., Bras, J., Schrag, A. E., & Schott, J. M. (2017). Current concepts and controversies in the pathogenesis of Parkinson’s disease dementia and Dementia with Lewy Bodies. F1000Research, 6, 1604. [DOI:10.12688/f1000research.11725.1] [PMID]
Witt, K., Daniels, C., Reiff, J., Krack, P., Volkmann, J., Pinsker, M. O., ... & Deuschl, G. (2008). Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. The Lancet Neurology, 7(7), 605-614. [DOI: 10.1016/S1474-4422(08)70114-5] [PMID]
Witt, K., Granert, O., Daniels, C., Volkmann, J., Falk, D., & van Eimeren, T., et al. (2013). Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: Results from a randomized trial. Brain, 136(Pt 7), 2109-2119. [DOI:10.1093/brain/awt151] [PMID]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2021/05/24 | Accepted: 2022/09/27 | Published: 2024/03/1
Received: 2021/05/24 | Accepted: 2022/09/27 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





