Volume 15, Issue 2 (March & April 2024)
BCN 2024, 15(2): 147-156 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nejati V, Ghayerin E. Abnormal Structure and Function of Parietal Lobe in Individuals With Attention Deficit Hyperactivity Disorder (ADHD): A Systematic Review Study. BCN 2024; 15 (2) :147-156
URL: http://bcn.iums.ac.ir/article-1-1909-en.html
URL: http://bcn.iums.ac.ir/article-1-1909-en.html
1- Department of Psychology, School of Education and Psychology, Shahid Beheshti University, Tehran, Iran.
2- Department of Psychology, Faculty of Education and Psychology, Tabriz University, Tabriz, Iran.
2- Department of Psychology, Faculty of Education and Psychology, Tabriz University, Tabriz, Iran.
Keywords: Attention deficit hyperactivity disorder (ADHD), Parietal lobe, Executive functions, Systematic review
Full-Text [PDF 643 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Attention deficit hyperactivity disorder (ADHD) as a neurodevelopmental disorder is characterized by two main symptoms: Hyperactivity-impulsivity and attention deficit (American Psychiatric Association (APA), 2013) One well-documented theory to explain the behavioral symptoms in children with ADHD is the dysexecutive function theory (Barkley, 2020). Individuals with ADHD experience a variety of dysexecutive functions, such as the inability to start, focus, persist, and shift attention, keep constant effort and alertness, cope and regulate emotions, remember to-be-learned material, and finally regulate their actions (Brown, 2009; Rodríguez et al., 2021; Staff et al., 2021).
Executive functions (EFs), as an umbrella term, cover several domains such as working memory, cognitive flexibility, and inhibitory control (Burgess & Simons, 2005; Espy, 2004; Lehto et al., 2003; Miller & Cohen, 2001). Impaired EFs is a non-specific syndrome which has been identified in numerous psychiatric disorders, including autism spectrum disorder (Shiri et al., 2015), addiction (Baler & Volkow, 2006), conduct disorder (Fairchild et al., 2009), depression (Ajilchi & Nejati, 2017; Tavares et al., 2007), obsessive-compulsive disorder (Penades et al., 2007), and schizophrenia (Barch, 2005). Furthermore, some non-executive functions are impaired in individuals with ADHD, such as emotional processing (Borhani & Nejati, 2018), reward processing (Nejati et al., 2020), and spatial processing (Jung et al. 2014; Soluki et al., 2020). Furthermore, in the study of the neural correlates of ADHD, the majority of neuroimaging studies stressed the prefrontal cortex as the area of interest (Hesslinger et al., 2002; Pironti et al., 2014; Samea et al., 2019; Schulz et al., 2017; Seidman et al., 2006; Wolf et al., 2009; Wu et al., 2020)
Besides the prefrontal cortex and EFs, the parietal cortex and the respective perceptual functions play a crucial role in the psychopathology of ADHD (Dunn & Kronenberger, 2013; Schulz et al., 2017; Silk et al., 2008; Vance et al., 2007). Individuals with ADHD experience some impairments in spatial perception, spatial working memory, visual recognition, and spatial reaction time (Banaschewski et al., 2006; Rhodes et al., 2004; Coghill & Matthews, 2005). Given Posner’s attentional network, individuals with ADHD are impaired in all attentional networks, including alerting (Oberlin et al., 2005), executive (Oberlin et al., 2005), and orienting (Collings & Kwasman, 2006). Sensory processing and integration problems, as another function of the parietal cortex, are impaired in individuals with ADHD (Ghanizadeh, 2011; Mulligan, 1996). Abnormal perceptual function leads to hypersensitivity or hyposensitivity of some modalities in individuals with ADHD with respect to the deficient perceptual functions in individuals with ADHD, which fundamentally involves the parietal lobes (Kamath et al., 2020). Thus, we aimed to review abnormal structure and function of the parietal lobe in individuals with ADHD.
2. Materials and Methods
The present study was performed according to the guidelines of systematic review studies of PRISMA (the preferred reporting items for systematic reviews and meta-analyses) (Moher et al., 2009).
Search strategy
The required information was provided by reviewing scientific databases, including Web of Science, Science Direct, Springer, PubMed, and Google Scholar. Studies published in English between January 2010 and May 2021 were reviewed. We searched for resources once in July 2020 and again in June 2021.
Keywords
We searched using the following keywords: Attention deficit disorder (ADD), hyperactivity, attention deficit-hyperactivity disorder (ADHD), parietal (lobe), posterior parietal cortex (PPC), inferior parietal lobe (IPL), superior parietal lobe (SPL), angular gyrus (AG), and supramarginal gyrus (SMG).
Inclusion and exclusion criteria
Articles available in full text in English at the desired period were entered in the present review.
Data extraction
To check the relevance of the articles searched for in the present study, first the titles, then the abstracts, and finally, the authors checked the whole text. The authors agreed on which article would remain or be removed. A total of 79 articles were initially selected, and finally, 20 remained (Figure 1).

After extracting the required information, the results were summarized in Tables 1 and 2 and then analyzed.
3. Results
Twenty studies were included in the review, which examined 1207 participants, including 746 participants with ADHD and 461 healthy controls. The mean participant age range was 4.92 to 40.26 years old. Notably, the broad age range of participants could be explained by the diagnosis of ADHD from childhood to adulthood. Sample sizes ranged from 17 to 188. In what follows, we summarized the results of the included studies based on methods.

Functional magnetic resonance imaging (fMRI) studies
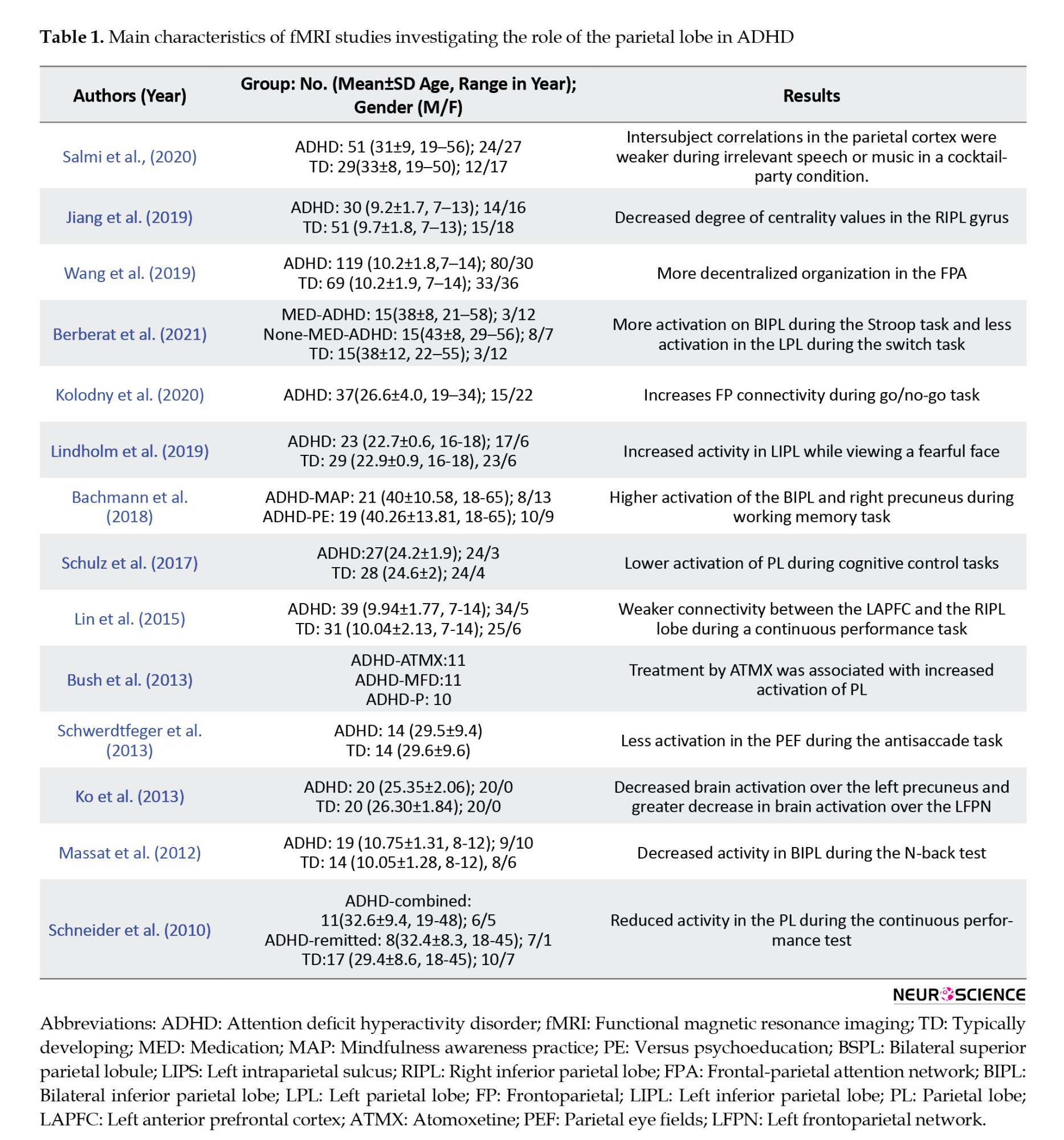
In individuals with ADHD, desynchronization of the PPC, the precuneus, and the SPL has been reported during irrelevant speech or music in the background of a film (Salmi et al., 2020). Resting-state fMRI indicates a lower degree of centrality in the default mode network (DMN) in children with ADHD (Jiang et al., 2019). This study suggests a decentralized organization (line-like topology) in the frontoparietal attention network in children with ADHD in contrast to the more centralized organization (star-like topology) in typically developing (TD) children. Another fMRI study found that drug-naïve individuals with ADHD show more activation of the bilateral inferior parietal lobe during inhibition, measured by stop-signal task, and less activation in the left parietal cortex during shifting attention, measured by switch task (Berberat et al., 2021). Kolodney et al. (2020) found that during an inhibitory control task (go/no-go), in individuals with ADHD with lower symptoms, the cooperation of the intraparietal sulcus (IPS) and the right inferior frontal gyrus (IFG) is increased. Whereas in individuals with ADHD with severe symptoms, no alteration in the activity of the parietal cortex is observed.
Also, when adults with previous ADHD express facial emotion (including happy and fearful expressions), the activity of the left IPL increases during fearful facial expressions. Besides, during visual exposure to happy faces, higher functional connectivity is detectable among the posterior cingulate cortex, right ventral frontal cortex, right dorsal parietal cortex (DPC), and left temporoparietal junction (TPJ) in individuals with former ADHD (Lindholm et al., 2019).
Bachman et al. (2018) found increased brain activation on the bilateral IPL, the right posterior insula, and the right precuneus in individuals with ADHD during performing N-back tasks, which was associated with lower scores reported in inattention and memory problems. Schulz et al. (2017) reported lower activation of the right orbitofrontal cortex, the inferior frontal cortex, and the parietal lobe in individuals with ADHD during stimulus and response conflict task. Another resting-state fMRI study found children with ADHD, compared with TD peers, showed weaker connectivity between the right anterior prefrontal cortex (PFC) and the right ventrolateral PFC and between the left anterior PFC and the right IPL during continuous performance and spatial span tests. This finding was associated with symptoms of impulsivity, opposition defiance, impaired response inhibition, and attentional control (Lin et al., 2015). Treatment with atomoxetine (ATMX) was associated with increased fMRI activation of the parietal cortex, the dorsolateral prefrontal cortex (DLPFC), and the cerebellum when completing a multi-source interference task (MSIT) in individuals with ADHD (Bush et al., 2013). Also, fMRI with a rapid event-related design in adult ADHD showed less activation in the frontal, supplementary, and parietal eye fields during the antisaccade task when preparing to execute it (Schwerdtfeger et al., 2013). Furthermore, using fMRI during phonological and visual-spatial n-back tasks has shown activation of the frontoparietal network for working memory tasks in adults with and without ADHD. It also exhibits that the intensity of the activation is more remarkable in individuals with ADHD. The control group has exhibited increased brain activation over the frontoparietal network in response to increased phonological working memory load. However, individuals with ADHD have shown a greater decrease in brain activation over the left frontoparietal network (Ko et al., 2013). Also, drug-naïve ADHD children show decreased activation of the IPL and bilateral occipital, caudate nucleus, cerebellum, and functionally connected brainstem nuclei (Massat et al., 2012). During CPT, reduced activity has also been observed in the caudate nuclei, the anterior cingulate cortex, the parietal cortical structures, the right IPL, and the left SPL in individuals with ADHD. Less activation of the left SPL is associated with impulsivity and hyperactivity, and both right IPL and left SPL have a relationship with inattention symptoms in individuals with ADHD (Schneider et al., 2010). In general, fMRI studies described an alteration in the structure and function of the parietal lobe in individuals with ADHD, which indicates lower performance of the parietal lobe in this group.
Structural magnetic resonance imaging (sMRI) study
An sMRI study reported lower global and local grey matter volumes within clusters in the bilateral frontal, right parietal, and right temporal regions in individuals with ADHD compared to TD (Vilgis et al., 2016).
Magnetization prepared rapid gradient recalled echo (MPRAGE) image study
An MPRAGE study described a reduction of cortical volume in the frontal, parietal, and temporal cortices visible in young children with ADHD (Jacobson et al., 2018).
Electroencephalogram (EEG) study
Individuals with ADHD have manifested significantly less parietal theta rhythm and event-related (de) synchronization (ERS) during inhibition and response trials during visual continuous performance test. As well as they had an increase in parietal α rhythm and ERS during inhibition and action. Furthermore, lower frontoparietal connectivity has been described in individuals with ADHD (Cowley et al., 2020). Another ERP study described less connectivity among temporal, frontal, and parietal cortices during an oddball task in individuals with ADHD (Chen et al., 2021).
Transcranial direct current stimulation (tDCS) study
Anodal tDCS over the right PPC improves attentional functioning in attention networks test (ANT), specifically in an orienting domain. Furthermore, activation of the right PPC has a destructive effect on the top-down attentional control required for selective attention measured by the Stroop test. Also, activation of the right PPC does not affect shifting attention, measured by the shifting attention test, and response inhibition, measured by the go/no-go test, which means that activation of the right PPC can improve bottom-up attentional control (Salehinejad et al., 2020).
Deep transcranial magnetic stimulation (dTMS) study
Individuals with ADHD under treatment by dTMS have improved the n-back task by increasing activation on the right parietal cortex and other areas, measured by fMRI (Bleich-Cohen et al., 2021).
4. Discussion
The present study aimed to review the role of the parietal cortex in the psychopathology of ADHD. The results of the reviewed studies with different methods, 14 fMRI studies, 1 sMRI study, 1 MPRAGE study, 2 EEG studies, 1 tDCS study, and 1 dTMS study, identified abnormal structure and function in the parietal lobe in individuals with ADHD. In the following section, the abnormal structure and function of the parietal lobes are discussed in detail.
The right parietal lobe
In the included studies, a resting-state fMRI study reported decreased centrality in the right IPL (Jiang et al., 2019). The reduced activity of the right IPL has been found during continuous performance tests (Lin et al., 2015). The right IPL, as a part of the default mode network (DMN), is impaired in individuals with ADHD (Jiang et al., 2019; Lin et al., 2015). The deficient DMN is associated with a wandering mind, which drowns individuals with ADHD in daily dreams. Furthermore, mind wandering could be considered a cognitive underpinning for ADHD symptoms (Lanier et al., 2019). Furthermore, the right IPL is a part of posterior attention networks which subserves impaired alerting and orienting attention in individuals with ADHD (Bush, 2010, 2011; Corbetta et al., 2008; Schneider et al., 2010). Moreover, memory and inattention problems are associated with hypo-activation in the right precuneus and parietal lobe (Bachmann et al., 2018; Bleich-Cohen et al., 2021). Although correlational evidence from imaging studies has found an association between hyperactivation of the right parietal cortex and executive control (Schulz et al., 2017), the causal evidence from stimulation studies does not confirm this association (Salehinejad et al., 2020).
The left parietal lobe
The activity of left IPL decreases during cocktail-party conditions (Salmi et al., 2020) and switching tasks (Berberat et al., 2021) but increases during fearful face mimicry (Lindholm et al., 2019). The left parietal region is not only involved in the obvious symptoms of ADHD, such as inattention, impulsivity, and hyperactivity but also working memory and emotional perception, such as fear and joy. Less activation of the left SPL has a relationship with impulsivity, hyperactivity, and inattention in individuals with ADHD, and previous studies have reported the role of this part in shifting attention (Berberat et al., 2021; Schneider et al., 2010). Given the impaired social cognition in individuals with ADHD (Bora & Pantelis, 2016), increasing the activity of the left IPL associated with the left TPJ improves impaired social cognition (Lindholm et al., 2019). Also, impaired working memory in children with ADHD can be attributed to the hypoactivity of the left parietal lobe (Ko et al., 2013). Thus, this lobe seems to play a crucial role in emotional processing, while the right parietal lobe has a more prominent role in processing basic emotional states. The function of this lobe seems to be more general than that of the right parietal lobe, indicating the higher specialization of the right parietal lobe versus the left parietal lobe.
Bilateral and central parietal lobe
In the included studies, fMRI results reported a reduction in the bilateral parietal lobe during the cocktail-party condition (Salmi et al., 2020), cognitive control task (Schulz et al., 2017), and continuous performance test (Schneider et al., 2010). This finding was confirmed by MPRAGE, which supported a reduction in the volume of the parietal lobes (Jacobson et al., 2018). Furthermore, EEG showed decreased parietal lobes activity (Cowley et al., 2020). Finally, interventional studies confirm these findings. In children with ADHD, atomoxetine increases the activity of the parietal lobe (Bush et al., 2013), anodal tDCS improves working memory performance (Salehinejad et al., 2020), and dTMS increases the activity of the right parietal lobe and improves working memory performance (Bleich-Cohen et al., 2021). Lower connectivity of the IPL and the PPC is associated with ADHD symptoms, which are associated with weakness in the control of irrelevant auditory and visual stimuli and inattention symptoms (Chen et al., 2021; Kolodny et al., 2020; Salmi et al., 2020; Schneider et al., 2010; Schwerdtfeger et al., 2013).
Based on EEG results, less theta rhythm and more α rhythm are shown during the control of irrelevant visual stimuli (Bush et al., 2013; Cowley et al., 2020). However, boosting the right PPC through tDCS did not alter cognitive control (Salehinejad et al., 2020), so it is likely that increased right parietal cortex activity depends on increased left parietal cortex activity during irrelevant stimulus inhibition, indicating the role of bilateral parietal lobes in cognitive control (Berberat et al., 2021). Also, memory problems are associated with less activation of the bilateral IPL (Bachmann et al., 2018).
Along with reduced interaction and activity, a decline in the cortical volume of the parietal lobe is obvious in individuals with ADHD (Jacobson et al., 2018; Massat et al., 2012; Wang et al., 2019). With respect to these results, increasing the activity of the parietal lobes and training of respective functions should be taken into account in the rehabilitation of ADHD.
5. Conclusion
According to the reviewed studies, there is an association between abnormal parietal structure and function and cognitive dysfunction in individuals with ADHD. The impaired structure and function of the parietal lobe could be followed in this cortex’s activity, size, connections, and processes. A variety of cognitive domains, including attention, working memory, inhibitory control, social cognition, and inner thinking, are controlled by the parietal cortex, which is impaired in individuals with ADHD. Functional and structural alteration of the parietal cortex has been described in ADHD, which has a causal relationship with cognitive impairments. All included studies reported abnormal parietal lobe structural, functional, or connectivity or improvement of cognitive functions with parietal lobe stimulation. Some limitations should be taken into account in the present study. The heterogeneity of the tasks used in the included studies and the variety of methods did not allow us to perform a meta-analysis.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Vahid Nejati, Investigation, Writing the original draft: Elnaz Ghayerin, Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Ajilchi, B., & Nejati, V. (2017). Executive Functions in Students With Depression, Anxiety, and Stress Symptoms. Basic and Clinical Neuroscience, 8(3), 223–232. [DOI:10.18869/nirp.bcn.8.3.223] [PMID] [PMCID]
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5TM). Washington: American Psychiatric Association. [Link]
Bachmann, K., Lam, A. P., Sörös, P., Kanat, M., Hoxhaj, E., & Matthies, S., et al. (2018). Effects of mindfulness and psychoeducation on working memory in adult ADHD: A randomised, controlled fMRI study. Behaviour Research and Therapy, 106, 47–56. [DOI:10.1016/j.brat.2018.05.002] [PMID]
Baler, R. D., & Volkow, N. D. (2006). Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine, 12(12), 559–566. [DOI:10.1016/j.molmed.2006.10.005] [PMID]
Banaschewski, T., Ruppert, S., Tannock, R., Albrecht, B., Becker, A., & Uebel, H., et al. (2006). Colour perception in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 568–572. [DOI:10.1111/j.1469-7610.2005.01540.x] [PMID]
Barch, D. M. (2005). The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology, 1, 321–353. [DOI:10.1146/annurev.clinpsy.1.102803.143959] [PMID]
Barkley, R. A. (2020). Taking charge of ADHD: The complete, authoritative guide for parents. New York: Guilford Publications. [Link]
Berberat, J., Huggenberger, R., Montali, M., Gruber, P., Pircher, A., & Lövblad, K. O., et al. (2021). Brain activation patterns in medicated versus medication-naïve adults with attention-deficit hyperactivity disorder during fMRI tasks of motor inhibition and cognitive switching. BMC Medical Imaging, 21(1), 53. [DOI:10.1186/s12880-021-00579-3] [PMID] [PMCID]
Bleich-Cohen, M., Gurevitch, G., Carmi, N., Medvedovsky, M., Bregman, N., & Nevler, N., et al. (2021). A functional magnetic resonance imaging investigation of prefrontal cortex deep transcranial magnetic stimulation efficacy in adults with attention deficit/hyperactive disorder: A double blind, randomized clinical trial. NeuroImage. Clinical, 30, 102670. [DOI:10.1016/j.nicl.2021.102670] [PMID] [PMCID]
Bora, E., & Pantelis, C. (2016). Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): Comparison with healthy controls and autistic spectrum disorder. Psychological Medicine, 46(4), 699–716. [DOI:10.1017/S0033291715002573] [PMID]
Borhani, K., & Nejati, V. (2018). Emotional face recognition in individuals withattention-deficit/hyperactivity disorder: A review article. Developmental Neuropsychology, 43(3), 256–277. [DOI:10.1080/87565641.2018.1440295] [PMID]
Brown, T. E. (2009). ADD/ADHD and impaired executive function in clinical practice. Current Attention Disorders Reports, 1, 37-41. [DOI:10.1007/s12618-009-0006-3]
Burgess, P. W., & Simons, J. S. (2005). Theories of frontal lobe executive function: Clinical applications. In PW. Halligan, & DT. Wade (eds.) The effectiveness of rehabilitation for cognitive deficits (pp.211–232). Oxford: Oxford University. [DOI:10.1093/acprof:oso/9780198526544.003.0018]
Bush, G. (2010). Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology, 35(1), 278–300. [DOI:10.1038/npp.2009.120] [PMID] [PMCID]
Bush G. (2011). Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biological Psychiatry, 69(12), 1160–1167. [DOI:10.1016/j.biopsych.2011.01.022] [PMID] [PMCID]
Bush, G., Holmes, J., Shin, L. M., Surman, C., Makris, N., & Mick, E., et al. (2013). Atomoxetine increases fronto-parietal functional MRI activation in attention-deficit/hyperactivity disorder: A pilot study. Psychiatry Research, 211(1), 88–91. [DOI:10.1016/j.pscychresns.2012.09.004] [PMID] [PMCID]
Chen, C., Yang, H., Du, Y., Zhai, G., Xiong, H., & Yao, D., et al. (2021). Altered functional connectivity in children with ADHD revealed by scalp EEG: An ERP study. Neural Plasticity, 2021, 6615384. [DOI:10.1155/2021/6615384] [PMID] [PMCID]
Collings, R. D., & Kwasman, A. (2006). Visual orienting deficits among boys with ADHD-inattentive type. Individual Differences Research, 4(2), 111-122. [Link]
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI:10.1016/j.neuron.2008.04.017] [PMID] [PMCID]
Cowley, B. U., Juurmaa, K., & Palomaki, J. (2020). Strength of attention-sampling parietal EEG theta rhythm is linked to impaired inhibition in adult ADHD. Eneuro, 9(1), 1-20. [DOI:10.1523/ENEURO.0028-21.2021]
Dunn, D. W., & Kronenberger, W. G. (2013). Attention deficit. Handbook of Clinical Neurology, 111, 257–261. [DOI:10.1016/B978-0-444-52891-9.00028-2] [PMID]
Espy K. A. (2004). Using developmental, cognitive, and neuroscience approaches to understand executive control in young children. Developmental Neuropsychology, 26(1), 379–384. [DOI:10.1207/s15326942dn2601_1] [PMID]
Fairchild, G., van Goozen, S. H., Stollery, S. J., Aitken, M. R., Savage, J., & Moore, S. C., et al. (2009). Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological Psychiatry, 66(2), 162–168. [DOI:10.1016/j.biopsych.2009.02.024] [PMID] [PMCID]
Ghanizadeh A. (2011). Sensory processing problems in children with ADHD, A systematic review. Psychiatry Investigation, 8(2), 89–94. [DOI:10.4306/pi.2011.8.2.89] [PMID] [PMCID]
Hesslinger, B., Tebartz van Elst, L., Thiel, T., Haegele, K., Hennig, J., & Ebert, D. (2002). Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters, 328(3), 319–321. [DOI:10.1016/S0304-3940(02)00554-2] [PMID]
Jacobson, L. A., Crocetti, D., Dirlikov, B., Slifer, K., Denckla, M. B., & Mostofsky, S. H., et al. (2018). Anomalous brain development is evident in preschoolers with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 24(6), 531–539. [DOI:10.1017/S1355617718000103] [PMID] [PMCID]
Jiang, K., Yi, Y., Li, L., Li, H., Shen, H., & Zhao, F., et al. (2019). Functional network connectivity changes in children with attention-deficit hyperactivity disorder: A resting-state fMRI study. International Journal of Developmental Neuroscience, 78, 1–6. [DOI:10.1016/j.ijdevneu.2019.07.003] [PMID]
Jung, H., Woo, Y. J., Kang, J. W., Choi, Y. W., & Kim, K. M. (2014). Visual perception of ADHD children with sensory processing disorder. Psychiatry Investigation, 11(2), 119–123. [DOI:10.4306/pi.2014.11.2.119] [PMID] [PMCID]
Kamath, M. S., Dahm, C. R., Tucker, J. R., Huang-Pollock, C. L., Etter, N. M., & Neely, K. A. (2020). Sensory profiles in adults with and without ADHD. Research in Developmental Disabilities, 104, 103696. [DOI:10.1016/j.ridd.2020.103696] [PMID] [PMCID]
Ko, C. H., Yen, J. Y., Yen, C. F., Chen, C. S., Lin, W. C., & Wang, P. W., et al. (2013). Brain activation deficit in increased-load working memory tasks among adults with ADHD using fMRI. European Archives of Psychiatry and Clinical Neuroscience, 263(7), 561–573. [DOI:10.1007/s00406-013-0407-2] [PMID]
Kolodny, T., Mevorach, C., Stern, P., Biderman, N., Ankaoua, M., & Tsafrir, S., et al. (2020). Fronto-parietal engagement in response inhibition is inversely scaled with attention-deficit/hyperactivity disorder symptom severity. NeuroImage. Clinical, 25, 102119. [DOI:10.1016/j.nicl.2019.102119] [PMID] [PMCID]
Lanier, J., Noyes, E., & Biederman, J. (2021). Mind wandering (internal distractibility) in ADHD: A literature review. Journal of Attention Disorders, 25(6), 885–890. [DOI:10.1177/1087054719865781] [PMID]
Lehto, J. E., Juujärvi, P., Kooistra, L., & Pulkkinen, L. (2003). Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology, 21, 59-80. [DOI:10.1348/026151003321164627]
Lin, H. Y., Tseng, W. Y., Lai, M. C., Matsuo, K., & Gau, S. S. (2015). Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 21(4), 271–284. [DOI:10.1017/S135561771500020X] [PMID]
Lindholm, P., Lieslehto, J., Nikkinen, J., Moilanen, I., Hurtig, T., & Veijola, J., et al. (2019). Brain response to facial expressions in adults with adolescent ADHD. Psychiatry Research. Neuroimaging, 292, 54–61. [DOI:10.1016/j.pscychresns.2019.09.003] [PMID]
Massat, I., Slama, H., Kavec, M., Linotte, S., Mary, A., & Baleriaux, D., et al. (2012). Working memory-related functional brain patterns in never medicated children with ADHD. Plos One, 7(11), e49392. [DOI:10.1371/journal.pone.0049392] [PMID] [PMCID]
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI:10.1146/annurev.neuro.24.1.167] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Reprint--preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Physical Therapy, 89(9), 873–880. [DOI:10.1093/ptj/89.9.873] [PMID]
Mulligan S. (1996). An analysis of score patterns of children with attention disorders on the sensory integration and praxis tests. The American Journal of Occupational Therapy, 50(8), 647–654. [DOI:10.5014/ajot.50.8.647] [PMID]
Nejati, V., Sarraj Khorrami, A., & Nitsche, M. A. (2021). Transcranial direct current stimulation improves reward processing in children with ADHD. Journal of Attention Disorders, 25(11), 1623–1631. [DOI:10.1177/1087054720923094] [PMID]
Oberlin, B. G., Alford, J. L., & Marrocco, R. T. (2005). Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research, 165(1), 1–11. [DOI:10.1016/j.bbr.2005.06.041] [PMID]
Penadés, R., Catalán, R., Rubia, K., Andrés, S., Salamero, M., & Gastó, C. (2007). Impaired response inhibition in obsessive compulsive disorder. European Psychiatry, 22(6), 404–410.[DOI:10.1016/j.eurpsy.2006.05.001] [PMID]
Pironti, V. A., Lai, M. C., Müller, U., Dodds, C. M., Suckling, J., & Bullmore, E. T., et al. (2014). Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biological Psychiatry, 76(8), 639–647. [DOI:10.1016/j.biopsych.2013.09.025] [PMID] [PMCID]
Rhodes, S. M., Coghill, D. R., & Matthews, K. (2004). Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology, 175(3), 319–330. [DOI:10.1007/s00213-004-1833-7] [PMID]
Rhodes, S. M., Coghill, D. R., & Matthews, K. (2005). Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychological Medicine, 35(8), 1109–1120. [DOI:10.1017/S0033291705004599] [PMID]
Rodríguez, C., García, T., Areces, D., Rodríguez, J., Arteaga-Henriquez, G., & Ramos-Quiroga, A. (2021). Retrospective symptoms and learning difficulties predicting ADHD in adults: Differences between prison inmates and the clinical population. Scandinavian Journal of Psychology, 62(3), 301–311. [DOI:10.1111/sjop.12716] [PMID]
Salehinejad, M. A., Ghayerin, E., Nejati, V., Yavari, F., & Nitsche, M. A. (2020). Domain-specific involvement of the right posterior parietal cortex in attention network and attentional control of ADHD: A randomized, cross-over, sham-controlled tDCS study. Neuroscience, 444, 149–159. [DOI:10.1016/j.neuroscience.2020.07.037] [PMID]
Salmi, J., Metwaly, M., Tohka, J., Alho, K., Leppämäki, S., & Tani, P., et al. (2020). ADHD desynchronizes brain activity during watching a distracted multi-talker conversation. NeuroImage, 216, 116352. [DOI:10.1016/j.neuroimage.2019.116352] [PMID]
Samea, F., Soluki, S., Nejati, V., Zarei, M., Cortese, S., & Eickhoff, S. B., et al. (2019). Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neuroscience and Biobehavioral Reviews, 100, 1–8. [DOI:10.1016/j.neubiorev.2019.02.011] [PMID] [PMCID]
Schneider, M. F., Krick, C. M., Retz, W., Hengesch, G., Retz-Junginger, P., & Reith, W., et al. (2010). Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults-A functional magnetic resonance imaging (fMRI) study. Psychiatry Research, 183(1), 75–84.[DOI:10.1016/j.pscychresns.2010.04.005] [PMID]
Schulz, K. P., Li, X., Clerkin, S. M., Fan, J., Berwid, O. G., & Newcorn, J. H., et al. (2017). Prefrontal and parietal correlates of cognitive control related to the adult outcome of attention-deficit/hyperactivity disorder diagnosed in childhood. Cortex, 90, 1–11. [DOI:10.1016/j.cortex.2017.01.019] [PMID] [PMCID]
Hakvoort Schwerdtfeger, R. M., Alahyane, N., Brien, D. C., Coe, B. C., Stroman, P. W., & Munoz, D. P. (2012). Preparatory neural networks are impaired in adults with attention-deficit/hyperactivity disorder during the antisaccade task. NeuroImage. Clinical, 2, 63–78. [DOI:10.1016/j.nicl.2012.10.006] [PMID] [PMCID]
Seidman, L. J., Valera, E. M., Makris, N., Monuteaux, M. C., Boriel, D. L., & Kelkar, K., et al. (2006). Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry, 60(10), 1071–1080. [DOI:10.1016/j.biopsych.2006.04.031] [PMID]
Shiri, V., Hosseini, S. A., Pishyareh, E., Nejati, V., & Biglarian, A. (2015). [Studying the relationship of executive functions with behavioral symptoms in children with high-functioning Autism (Persian)]. Journal of Rehabilitaion, 16(3), 208-217. [Link]
Silk, T. J., Vance, A., Rinehart, N., Bradshaw, J. L., & Cunnington, R. (2008). Dysfunction in the fronto-parietal network in attention deficit hyperactivity disorder (ADHD): An fMRI study. Brain Imaging and Behavior, 2, 123-131. [DOI:10.1007/s11682-008-9021-8]
Soluki, M., Mahmoudi, F., Abdolmaleki, A., Asadi, A., & Sabahi Namini, A. (2024). Cerium oxide nanoparticles as a new neuroprotective agent to promote functional recovery in a rat model of sciatic nerve crush injury. British Journal of Neurosurgery, 38(2), 301–306. [DOI:10.21203/rs.3.rs-41923/v1] [PMID]
Staff, A. I., Luman, M., van der Oord, S., Bergwerff, C. E., van den Hoofdakker, B. J., & Oosterlaan, J. (2022). Facial emotion recognition impairment predicts social and emotional problems in children with (subthreshold) ADHD. European Child & Adolescent Psychiatry, 31(5), 715–727. [DOI:10.1007/s00787-020-01709-y] [PMID] [PMCID]
Taylor Tavares, J. V., Clark, L., Cannon, D. M., Erickson, K., Drevets, W. C., & Sahakian, B. J. (2007). Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biological Psychiatry, 62(8), 917–924. [DOI:10.1016/j.biopsych.2007.05.034] [PMID]
Vance, A., Silk, T. J., Casey, M., Rinehart, N. J., Bradshaw, J. L., & Bellgrove, M. A., et al. (2007). Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Molecular Psychiatry, 12(9), 826–793. [DOI:10.1038/sj.mp.4001999] [PMID]
Vilgis, V., Sun, L., Chen, J., Silk, T. J., & Vance, A. (2016). Global and local grey matter reductions in boys with ADHD combined type and ADHD inattentive type. Psychiatry Research. Neuroimaging, 254, 119–126. [DOI:10.1016/j.pscychresns.2016.06.008] [PMID]
Wang, Y., Tao, F., Zuo, C., Kanji, M., Hu, M., & Wang, D. (2019). Disrupted resting frontal-parietal attention network topology is associated with a clinical measure in children with attention-deficit/hyperactivity disorder. Frontiers in Psychiatry, 10, 300. [DOI:10.3389/fpsyt.2019.00300] [PMID] [PMCID]
Wolf, R. C., Plichta, M. M., Sambataro, F., Fallgatter, A. J., Jacob, C., & Lesch, K. P., et al. (2009). Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping, 30(7), 2252–2266. [DOI:10.1002/hbm.20665] [PMID] [PMCID]
Wu, Z., Luo, Y., Gao, Y., Han, Y., Wu, K., & Li, X. (2020). The role of frontal and occipital cortices in processing sustained visual attention in young adults with attention-deficit/hyperactivity disorder: A functional near-infrared spectroscopy study. Neuroscience Bulletin, 36(6), 659–663. [DOI:10.1007/s12264-020-00492-9] [PMID] [PMCID]
Attention deficit hyperactivity disorder (ADHD) as a neurodevelopmental disorder is characterized by two main symptoms: Hyperactivity-impulsivity and attention deficit (American Psychiatric Association (APA), 2013) One well-documented theory to explain the behavioral symptoms in children with ADHD is the dysexecutive function theory (Barkley, 2020). Individuals with ADHD experience a variety of dysexecutive functions, such as the inability to start, focus, persist, and shift attention, keep constant effort and alertness, cope and regulate emotions, remember to-be-learned material, and finally regulate their actions (Brown, 2009; Rodríguez et al., 2021; Staff et al., 2021).
Executive functions (EFs), as an umbrella term, cover several domains such as working memory, cognitive flexibility, and inhibitory control (Burgess & Simons, 2005; Espy, 2004; Lehto et al., 2003; Miller & Cohen, 2001). Impaired EFs is a non-specific syndrome which has been identified in numerous psychiatric disorders, including autism spectrum disorder (Shiri et al., 2015), addiction (Baler & Volkow, 2006), conduct disorder (Fairchild et al., 2009), depression (Ajilchi & Nejati, 2017; Tavares et al., 2007), obsessive-compulsive disorder (Penades et al., 2007), and schizophrenia (Barch, 2005). Furthermore, some non-executive functions are impaired in individuals with ADHD, such as emotional processing (Borhani & Nejati, 2018), reward processing (Nejati et al., 2020), and spatial processing (Jung et al. 2014; Soluki et al., 2020). Furthermore, in the study of the neural correlates of ADHD, the majority of neuroimaging studies stressed the prefrontal cortex as the area of interest (Hesslinger et al., 2002; Pironti et al., 2014; Samea et al., 2019; Schulz et al., 2017; Seidman et al., 2006; Wolf et al., 2009; Wu et al., 2020)
Besides the prefrontal cortex and EFs, the parietal cortex and the respective perceptual functions play a crucial role in the psychopathology of ADHD (Dunn & Kronenberger, 2013; Schulz et al., 2017; Silk et al., 2008; Vance et al., 2007). Individuals with ADHD experience some impairments in spatial perception, spatial working memory, visual recognition, and spatial reaction time (Banaschewski et al., 2006; Rhodes et al., 2004; Coghill & Matthews, 2005). Given Posner’s attentional network, individuals with ADHD are impaired in all attentional networks, including alerting (Oberlin et al., 2005), executive (Oberlin et al., 2005), and orienting (Collings & Kwasman, 2006). Sensory processing and integration problems, as another function of the parietal cortex, are impaired in individuals with ADHD (Ghanizadeh, 2011; Mulligan, 1996). Abnormal perceptual function leads to hypersensitivity or hyposensitivity of some modalities in individuals with ADHD with respect to the deficient perceptual functions in individuals with ADHD, which fundamentally involves the parietal lobes (Kamath et al., 2020). Thus, we aimed to review abnormal structure and function of the parietal lobe in individuals with ADHD.
2. Materials and Methods
The present study was performed according to the guidelines of systematic review studies of PRISMA (the preferred reporting items for systematic reviews and meta-analyses) (Moher et al., 2009).
Search strategy
The required information was provided by reviewing scientific databases, including Web of Science, Science Direct, Springer, PubMed, and Google Scholar. Studies published in English between January 2010 and May 2021 were reviewed. We searched for resources once in July 2020 and again in June 2021.
Keywords
We searched using the following keywords: Attention deficit disorder (ADD), hyperactivity, attention deficit-hyperactivity disorder (ADHD), parietal (lobe), posterior parietal cortex (PPC), inferior parietal lobe (IPL), superior parietal lobe (SPL), angular gyrus (AG), and supramarginal gyrus (SMG).
Inclusion and exclusion criteria
Articles available in full text in English at the desired period were entered in the present review.
Data extraction
To check the relevance of the articles searched for in the present study, first the titles, then the abstracts, and finally, the authors checked the whole text. The authors agreed on which article would remain or be removed. A total of 79 articles were initially selected, and finally, 20 remained (Figure 1).

After extracting the required information, the results were summarized in Tables 1 and 2 and then analyzed.

3. Results
Twenty studies were included in the review, which examined 1207 participants, including 746 participants with ADHD and 461 healthy controls. The mean participant age range was 4.92 to 40.26 years old. Notably, the broad age range of participants could be explained by the diagnosis of ADHD from childhood to adulthood. Sample sizes ranged from 17 to 188. In what follows, we summarized the results of the included studies based on methods.

Functional magnetic resonance imaging (fMRI) studies
In individuals with ADHD, desynchronization of the PPC, the precuneus, and the SPL has been reported during irrelevant speech or music in the background of a film (Salmi et al., 2020). Resting-state fMRI indicates a lower degree of centrality in the default mode network (DMN) in children with ADHD (Jiang et al., 2019). This study suggests a decentralized organization (line-like topology) in the frontoparietal attention network in children with ADHD in contrast to the more centralized organization (star-like topology) in typically developing (TD) children. Another fMRI study found that drug-naïve individuals with ADHD show more activation of the bilateral inferior parietal lobe during inhibition, measured by stop-signal task, and less activation in the left parietal cortex during shifting attention, measured by switch task (Berberat et al., 2021). Kolodney et al. (2020) found that during an inhibitory control task (go/no-go), in individuals with ADHD with lower symptoms, the cooperation of the intraparietal sulcus (IPS) and the right inferior frontal gyrus (IFG) is increased. Whereas in individuals with ADHD with severe symptoms, no alteration in the activity of the parietal cortex is observed.
Also, when adults with previous ADHD express facial emotion (including happy and fearful expressions), the activity of the left IPL increases during fearful facial expressions. Besides, during visual exposure to happy faces, higher functional connectivity is detectable among the posterior cingulate cortex, right ventral frontal cortex, right dorsal parietal cortex (DPC), and left temporoparietal junction (TPJ) in individuals with former ADHD (Lindholm et al., 2019).
Bachman et al. (2018) found increased brain activation on the bilateral IPL, the right posterior insula, and the right precuneus in individuals with ADHD during performing N-back tasks, which was associated with lower scores reported in inattention and memory problems. Schulz et al. (2017) reported lower activation of the right orbitofrontal cortex, the inferior frontal cortex, and the parietal lobe in individuals with ADHD during stimulus and response conflict task. Another resting-state fMRI study found children with ADHD, compared with TD peers, showed weaker connectivity between the right anterior prefrontal cortex (PFC) and the right ventrolateral PFC and between the left anterior PFC and the right IPL during continuous performance and spatial span tests. This finding was associated with symptoms of impulsivity, opposition defiance, impaired response inhibition, and attentional control (Lin et al., 2015). Treatment with atomoxetine (ATMX) was associated with increased fMRI activation of the parietal cortex, the dorsolateral prefrontal cortex (DLPFC), and the cerebellum when completing a multi-source interference task (MSIT) in individuals with ADHD (Bush et al., 2013). Also, fMRI with a rapid event-related design in adult ADHD showed less activation in the frontal, supplementary, and parietal eye fields during the antisaccade task when preparing to execute it (Schwerdtfeger et al., 2013). Furthermore, using fMRI during phonological and visual-spatial n-back tasks has shown activation of the frontoparietal network for working memory tasks in adults with and without ADHD. It also exhibits that the intensity of the activation is more remarkable in individuals with ADHD. The control group has exhibited increased brain activation over the frontoparietal network in response to increased phonological working memory load. However, individuals with ADHD have shown a greater decrease in brain activation over the left frontoparietal network (Ko et al., 2013). Also, drug-naïve ADHD children show decreased activation of the IPL and bilateral occipital, caudate nucleus, cerebellum, and functionally connected brainstem nuclei (Massat et al., 2012). During CPT, reduced activity has also been observed in the caudate nuclei, the anterior cingulate cortex, the parietal cortical structures, the right IPL, and the left SPL in individuals with ADHD. Less activation of the left SPL is associated with impulsivity and hyperactivity, and both right IPL and left SPL have a relationship with inattention symptoms in individuals with ADHD (Schneider et al., 2010). In general, fMRI studies described an alteration in the structure and function of the parietal lobe in individuals with ADHD, which indicates lower performance of the parietal lobe in this group.
Structural magnetic resonance imaging (sMRI) study
An sMRI study reported lower global and local grey matter volumes within clusters in the bilateral frontal, right parietal, and right temporal regions in individuals with ADHD compared to TD (Vilgis et al., 2016).
Magnetization prepared rapid gradient recalled echo (MPRAGE) image study
An MPRAGE study described a reduction of cortical volume in the frontal, parietal, and temporal cortices visible in young children with ADHD (Jacobson et al., 2018).
Electroencephalogram (EEG) study
Individuals with ADHD have manifested significantly less parietal theta rhythm and event-related (de) synchronization (ERS) during inhibition and response trials during visual continuous performance test. As well as they had an increase in parietal α rhythm and ERS during inhibition and action. Furthermore, lower frontoparietal connectivity has been described in individuals with ADHD (Cowley et al., 2020). Another ERP study described less connectivity among temporal, frontal, and parietal cortices during an oddball task in individuals with ADHD (Chen et al., 2021).
Transcranial direct current stimulation (tDCS) study
Anodal tDCS over the right PPC improves attentional functioning in attention networks test (ANT), specifically in an orienting domain. Furthermore, activation of the right PPC has a destructive effect on the top-down attentional control required for selective attention measured by the Stroop test. Also, activation of the right PPC does not affect shifting attention, measured by the shifting attention test, and response inhibition, measured by the go/no-go test, which means that activation of the right PPC can improve bottom-up attentional control (Salehinejad et al., 2020).
Deep transcranial magnetic stimulation (dTMS) study
Individuals with ADHD under treatment by dTMS have improved the n-back task by increasing activation on the right parietal cortex and other areas, measured by fMRI (Bleich-Cohen et al., 2021).
4. Discussion
The present study aimed to review the role of the parietal cortex in the psychopathology of ADHD. The results of the reviewed studies with different methods, 14 fMRI studies, 1 sMRI study, 1 MPRAGE study, 2 EEG studies, 1 tDCS study, and 1 dTMS study, identified abnormal structure and function in the parietal lobe in individuals with ADHD. In the following section, the abnormal structure and function of the parietal lobes are discussed in detail.
The right parietal lobe
In the included studies, a resting-state fMRI study reported decreased centrality in the right IPL (Jiang et al., 2019). The reduced activity of the right IPL has been found during continuous performance tests (Lin et al., 2015). The right IPL, as a part of the default mode network (DMN), is impaired in individuals with ADHD (Jiang et al., 2019; Lin et al., 2015). The deficient DMN is associated with a wandering mind, which drowns individuals with ADHD in daily dreams. Furthermore, mind wandering could be considered a cognitive underpinning for ADHD symptoms (Lanier et al., 2019). Furthermore, the right IPL is a part of posterior attention networks which subserves impaired alerting and orienting attention in individuals with ADHD (Bush, 2010, 2011; Corbetta et al., 2008; Schneider et al., 2010). Moreover, memory and inattention problems are associated with hypo-activation in the right precuneus and parietal lobe (Bachmann et al., 2018; Bleich-Cohen et al., 2021). Although correlational evidence from imaging studies has found an association between hyperactivation of the right parietal cortex and executive control (Schulz et al., 2017), the causal evidence from stimulation studies does not confirm this association (Salehinejad et al., 2020).
The left parietal lobe
The activity of left IPL decreases during cocktail-party conditions (Salmi et al., 2020) and switching tasks (Berberat et al., 2021) but increases during fearful face mimicry (Lindholm et al., 2019). The left parietal region is not only involved in the obvious symptoms of ADHD, such as inattention, impulsivity, and hyperactivity but also working memory and emotional perception, such as fear and joy. Less activation of the left SPL has a relationship with impulsivity, hyperactivity, and inattention in individuals with ADHD, and previous studies have reported the role of this part in shifting attention (Berberat et al., 2021; Schneider et al., 2010). Given the impaired social cognition in individuals with ADHD (Bora & Pantelis, 2016), increasing the activity of the left IPL associated with the left TPJ improves impaired social cognition (Lindholm et al., 2019). Also, impaired working memory in children with ADHD can be attributed to the hypoactivity of the left parietal lobe (Ko et al., 2013). Thus, this lobe seems to play a crucial role in emotional processing, while the right parietal lobe has a more prominent role in processing basic emotional states. The function of this lobe seems to be more general than that of the right parietal lobe, indicating the higher specialization of the right parietal lobe versus the left parietal lobe.
Bilateral and central parietal lobe
In the included studies, fMRI results reported a reduction in the bilateral parietal lobe during the cocktail-party condition (Salmi et al., 2020), cognitive control task (Schulz et al., 2017), and continuous performance test (Schneider et al., 2010). This finding was confirmed by MPRAGE, which supported a reduction in the volume of the parietal lobes (Jacobson et al., 2018). Furthermore, EEG showed decreased parietal lobes activity (Cowley et al., 2020). Finally, interventional studies confirm these findings. In children with ADHD, atomoxetine increases the activity of the parietal lobe (Bush et al., 2013), anodal tDCS improves working memory performance (Salehinejad et al., 2020), and dTMS increases the activity of the right parietal lobe and improves working memory performance (Bleich-Cohen et al., 2021). Lower connectivity of the IPL and the PPC is associated with ADHD symptoms, which are associated with weakness in the control of irrelevant auditory and visual stimuli and inattention symptoms (Chen et al., 2021; Kolodny et al., 2020; Salmi et al., 2020; Schneider et al., 2010; Schwerdtfeger et al., 2013).
Based on EEG results, less theta rhythm and more α rhythm are shown during the control of irrelevant visual stimuli (Bush et al., 2013; Cowley et al., 2020). However, boosting the right PPC through tDCS did not alter cognitive control (Salehinejad et al., 2020), so it is likely that increased right parietal cortex activity depends on increased left parietal cortex activity during irrelevant stimulus inhibition, indicating the role of bilateral parietal lobes in cognitive control (Berberat et al., 2021). Also, memory problems are associated with less activation of the bilateral IPL (Bachmann et al., 2018).
Along with reduced interaction and activity, a decline in the cortical volume of the parietal lobe is obvious in individuals with ADHD (Jacobson et al., 2018; Massat et al., 2012; Wang et al., 2019). With respect to these results, increasing the activity of the parietal lobes and training of respective functions should be taken into account in the rehabilitation of ADHD.
5. Conclusion
According to the reviewed studies, there is an association between abnormal parietal structure and function and cognitive dysfunction in individuals with ADHD. The impaired structure and function of the parietal lobe could be followed in this cortex’s activity, size, connections, and processes. A variety of cognitive domains, including attention, working memory, inhibitory control, social cognition, and inner thinking, are controlled by the parietal cortex, which is impaired in individuals with ADHD. Functional and structural alteration of the parietal cortex has been described in ADHD, which has a causal relationship with cognitive impairments. All included studies reported abnormal parietal lobe structural, functional, or connectivity or improvement of cognitive functions with parietal lobe stimulation. Some limitations should be taken into account in the present study. The heterogeneity of the tasks used in the included studies and the variety of methods did not allow us to perform a meta-analysis.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Vahid Nejati, Investigation, Writing the original draft: Elnaz Ghayerin, Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Ajilchi, B., & Nejati, V. (2017). Executive Functions in Students With Depression, Anxiety, and Stress Symptoms. Basic and Clinical Neuroscience, 8(3), 223–232. [DOI:10.18869/nirp.bcn.8.3.223] [PMID] [PMCID]
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5TM). Washington: American Psychiatric Association. [Link]
Bachmann, K., Lam, A. P., Sörös, P., Kanat, M., Hoxhaj, E., & Matthies, S., et al. (2018). Effects of mindfulness and psychoeducation on working memory in adult ADHD: A randomised, controlled fMRI study. Behaviour Research and Therapy, 106, 47–56. [DOI:10.1016/j.brat.2018.05.002] [PMID]
Baler, R. D., & Volkow, N. D. (2006). Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine, 12(12), 559–566. [DOI:10.1016/j.molmed.2006.10.005] [PMID]
Banaschewski, T., Ruppert, S., Tannock, R., Albrecht, B., Becker, A., & Uebel, H., et al. (2006). Colour perception in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 568–572. [DOI:10.1111/j.1469-7610.2005.01540.x] [PMID]
Barch, D. M. (2005). The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology, 1, 321–353. [DOI:10.1146/annurev.clinpsy.1.102803.143959] [PMID]
Barkley, R. A. (2020). Taking charge of ADHD: The complete, authoritative guide for parents. New York: Guilford Publications. [Link]
Berberat, J., Huggenberger, R., Montali, M., Gruber, P., Pircher, A., & Lövblad, K. O., et al. (2021). Brain activation patterns in medicated versus medication-naïve adults with attention-deficit hyperactivity disorder during fMRI tasks of motor inhibition and cognitive switching. BMC Medical Imaging, 21(1), 53. [DOI:10.1186/s12880-021-00579-3] [PMID] [PMCID]
Bleich-Cohen, M., Gurevitch, G., Carmi, N., Medvedovsky, M., Bregman, N., & Nevler, N., et al. (2021). A functional magnetic resonance imaging investigation of prefrontal cortex deep transcranial magnetic stimulation efficacy in adults with attention deficit/hyperactive disorder: A double blind, randomized clinical trial. NeuroImage. Clinical, 30, 102670. [DOI:10.1016/j.nicl.2021.102670] [PMID] [PMCID]
Bora, E., & Pantelis, C. (2016). Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): Comparison with healthy controls and autistic spectrum disorder. Psychological Medicine, 46(4), 699–716. [DOI:10.1017/S0033291715002573] [PMID]
Borhani, K., & Nejati, V. (2018). Emotional face recognition in individuals withattention-deficit/hyperactivity disorder: A review article. Developmental Neuropsychology, 43(3), 256–277. [DOI:10.1080/87565641.2018.1440295] [PMID]
Brown, T. E. (2009). ADD/ADHD and impaired executive function in clinical practice. Current Attention Disorders Reports, 1, 37-41. [DOI:10.1007/s12618-009-0006-3]
Burgess, P. W., & Simons, J. S. (2005). Theories of frontal lobe executive function: Clinical applications. In PW. Halligan, & DT. Wade (eds.) The effectiveness of rehabilitation for cognitive deficits (pp.211–232). Oxford: Oxford University. [DOI:10.1093/acprof:oso/9780198526544.003.0018]
Bush, G. (2010). Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology, 35(1), 278–300. [DOI:10.1038/npp.2009.120] [PMID] [PMCID]
Bush G. (2011). Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biological Psychiatry, 69(12), 1160–1167. [DOI:10.1016/j.biopsych.2011.01.022] [PMID] [PMCID]
Bush, G., Holmes, J., Shin, L. M., Surman, C., Makris, N., & Mick, E., et al. (2013). Atomoxetine increases fronto-parietal functional MRI activation in attention-deficit/hyperactivity disorder: A pilot study. Psychiatry Research, 211(1), 88–91. [DOI:10.1016/j.pscychresns.2012.09.004] [PMID] [PMCID]
Chen, C., Yang, H., Du, Y., Zhai, G., Xiong, H., & Yao, D., et al. (2021). Altered functional connectivity in children with ADHD revealed by scalp EEG: An ERP study. Neural Plasticity, 2021, 6615384. [DOI:10.1155/2021/6615384] [PMID] [PMCID]
Collings, R. D., & Kwasman, A. (2006). Visual orienting deficits among boys with ADHD-inattentive type. Individual Differences Research, 4(2), 111-122. [Link]
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI:10.1016/j.neuron.2008.04.017] [PMID] [PMCID]
Cowley, B. U., Juurmaa, K., & Palomaki, J. (2020). Strength of attention-sampling parietal EEG theta rhythm is linked to impaired inhibition in adult ADHD. Eneuro, 9(1), 1-20. [DOI:10.1523/ENEURO.0028-21.2021]
Dunn, D. W., & Kronenberger, W. G. (2013). Attention deficit. Handbook of Clinical Neurology, 111, 257–261. [DOI:10.1016/B978-0-444-52891-9.00028-2] [PMID]
Espy K. A. (2004). Using developmental, cognitive, and neuroscience approaches to understand executive control in young children. Developmental Neuropsychology, 26(1), 379–384. [DOI:10.1207/s15326942dn2601_1] [PMID]
Fairchild, G., van Goozen, S. H., Stollery, S. J., Aitken, M. R., Savage, J., & Moore, S. C., et al. (2009). Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological Psychiatry, 66(2), 162–168. [DOI:10.1016/j.biopsych.2009.02.024] [PMID] [PMCID]
Ghanizadeh A. (2011). Sensory processing problems in children with ADHD, A systematic review. Psychiatry Investigation, 8(2), 89–94. [DOI:10.4306/pi.2011.8.2.89] [PMID] [PMCID]
Hesslinger, B., Tebartz van Elst, L., Thiel, T., Haegele, K., Hennig, J., & Ebert, D. (2002). Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neuroscience Letters, 328(3), 319–321. [DOI:10.1016/S0304-3940(02)00554-2] [PMID]
Jacobson, L. A., Crocetti, D., Dirlikov, B., Slifer, K., Denckla, M. B., & Mostofsky, S. H., et al. (2018). Anomalous brain development is evident in preschoolers with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 24(6), 531–539. [DOI:10.1017/S1355617718000103] [PMID] [PMCID]
Jiang, K., Yi, Y., Li, L., Li, H., Shen, H., & Zhao, F., et al. (2019). Functional network connectivity changes in children with attention-deficit hyperactivity disorder: A resting-state fMRI study. International Journal of Developmental Neuroscience, 78, 1–6. [DOI:10.1016/j.ijdevneu.2019.07.003] [PMID]
Jung, H., Woo, Y. J., Kang, J. W., Choi, Y. W., & Kim, K. M. (2014). Visual perception of ADHD children with sensory processing disorder. Psychiatry Investigation, 11(2), 119–123. [DOI:10.4306/pi.2014.11.2.119] [PMID] [PMCID]
Kamath, M. S., Dahm, C. R., Tucker, J. R., Huang-Pollock, C. L., Etter, N. M., & Neely, K. A. (2020). Sensory profiles in adults with and without ADHD. Research in Developmental Disabilities, 104, 103696. [DOI:10.1016/j.ridd.2020.103696] [PMID] [PMCID]
Ko, C. H., Yen, J. Y., Yen, C. F., Chen, C. S., Lin, W. C., & Wang, P. W., et al. (2013). Brain activation deficit in increased-load working memory tasks among adults with ADHD using fMRI. European Archives of Psychiatry and Clinical Neuroscience, 263(7), 561–573. [DOI:10.1007/s00406-013-0407-2] [PMID]
Kolodny, T., Mevorach, C., Stern, P., Biderman, N., Ankaoua, M., & Tsafrir, S., et al. (2020). Fronto-parietal engagement in response inhibition is inversely scaled with attention-deficit/hyperactivity disorder symptom severity. NeuroImage. Clinical, 25, 102119. [DOI:10.1016/j.nicl.2019.102119] [PMID] [PMCID]
Lanier, J., Noyes, E., & Biederman, J. (2021). Mind wandering (internal distractibility) in ADHD: A literature review. Journal of Attention Disorders, 25(6), 885–890. [DOI:10.1177/1087054719865781] [PMID]
Lehto, J. E., Juujärvi, P., Kooistra, L., & Pulkkinen, L. (2003). Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology, 21, 59-80. [DOI:10.1348/026151003321164627]
Lin, H. Y., Tseng, W. Y., Lai, M. C., Matsuo, K., & Gau, S. S. (2015). Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 21(4), 271–284. [DOI:10.1017/S135561771500020X] [PMID]
Lindholm, P., Lieslehto, J., Nikkinen, J., Moilanen, I., Hurtig, T., & Veijola, J., et al. (2019). Brain response to facial expressions in adults with adolescent ADHD. Psychiatry Research. Neuroimaging, 292, 54–61. [DOI:10.1016/j.pscychresns.2019.09.003] [PMID]
Massat, I., Slama, H., Kavec, M., Linotte, S., Mary, A., & Baleriaux, D., et al. (2012). Working memory-related functional brain patterns in never medicated children with ADHD. Plos One, 7(11), e49392. [DOI:10.1371/journal.pone.0049392] [PMID] [PMCID]
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI:10.1146/annurev.neuro.24.1.167] [PMID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Reprint--preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Physical Therapy, 89(9), 873–880. [DOI:10.1093/ptj/89.9.873] [PMID]
Mulligan S. (1996). An analysis of score patterns of children with attention disorders on the sensory integration and praxis tests. The American Journal of Occupational Therapy, 50(8), 647–654. [DOI:10.5014/ajot.50.8.647] [PMID]
Nejati, V., Sarraj Khorrami, A., & Nitsche, M. A. (2021). Transcranial direct current stimulation improves reward processing in children with ADHD. Journal of Attention Disorders, 25(11), 1623–1631. [DOI:10.1177/1087054720923094] [PMID]
Oberlin, B. G., Alford, J. L., & Marrocco, R. T. (2005). Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research, 165(1), 1–11. [DOI:10.1016/j.bbr.2005.06.041] [PMID]
Penadés, R., Catalán, R., Rubia, K., Andrés, S., Salamero, M., & Gastó, C. (2007). Impaired response inhibition in obsessive compulsive disorder. European Psychiatry, 22(6), 404–410.[DOI:10.1016/j.eurpsy.2006.05.001] [PMID]
Pironti, V. A., Lai, M. C., Müller, U., Dodds, C. M., Suckling, J., & Bullmore, E. T., et al. (2014). Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biological Psychiatry, 76(8), 639–647. [DOI:10.1016/j.biopsych.2013.09.025] [PMID] [PMCID]
Rhodes, S. M., Coghill, D. R., & Matthews, K. (2004). Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology, 175(3), 319–330. [DOI:10.1007/s00213-004-1833-7] [PMID]
Rhodes, S. M., Coghill, D. R., & Matthews, K. (2005). Neuropsychological functioning in stimulant-naive boys with hyperkinetic disorder. Psychological Medicine, 35(8), 1109–1120. [DOI:10.1017/S0033291705004599] [PMID]
Rodríguez, C., García, T., Areces, D., Rodríguez, J., Arteaga-Henriquez, G., & Ramos-Quiroga, A. (2021). Retrospective symptoms and learning difficulties predicting ADHD in adults: Differences between prison inmates and the clinical population. Scandinavian Journal of Psychology, 62(3), 301–311. [DOI:10.1111/sjop.12716] [PMID]
Salehinejad, M. A., Ghayerin, E., Nejati, V., Yavari, F., & Nitsche, M. A. (2020). Domain-specific involvement of the right posterior parietal cortex in attention network and attentional control of ADHD: A randomized, cross-over, sham-controlled tDCS study. Neuroscience, 444, 149–159. [DOI:10.1016/j.neuroscience.2020.07.037] [PMID]
Salmi, J., Metwaly, M., Tohka, J., Alho, K., Leppämäki, S., & Tani, P., et al. (2020). ADHD desynchronizes brain activity during watching a distracted multi-talker conversation. NeuroImage, 216, 116352. [DOI:10.1016/j.neuroimage.2019.116352] [PMID]
Samea, F., Soluki, S., Nejati, V., Zarei, M., Cortese, S., & Eickhoff, S. B., et al. (2019). Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neuroscience and Biobehavioral Reviews, 100, 1–8. [DOI:10.1016/j.neubiorev.2019.02.011] [PMID] [PMCID]
Schneider, M. F., Krick, C. M., Retz, W., Hengesch, G., Retz-Junginger, P., & Reith, W., et al. (2010). Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults-A functional magnetic resonance imaging (fMRI) study. Psychiatry Research, 183(1), 75–84.[DOI:10.1016/j.pscychresns.2010.04.005] [PMID]
Schulz, K. P., Li, X., Clerkin, S. M., Fan, J., Berwid, O. G., & Newcorn, J. H., et al. (2017). Prefrontal and parietal correlates of cognitive control related to the adult outcome of attention-deficit/hyperactivity disorder diagnosed in childhood. Cortex, 90, 1–11. [DOI:10.1016/j.cortex.2017.01.019] [PMID] [PMCID]
Hakvoort Schwerdtfeger, R. M., Alahyane, N., Brien, D. C., Coe, B. C., Stroman, P. W., & Munoz, D. P. (2012). Preparatory neural networks are impaired in adults with attention-deficit/hyperactivity disorder during the antisaccade task. NeuroImage. Clinical, 2, 63–78. [DOI:10.1016/j.nicl.2012.10.006] [PMID] [PMCID]
Seidman, L. J., Valera, E. M., Makris, N., Monuteaux, M. C., Boriel, D. L., & Kelkar, K., et al. (2006). Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry, 60(10), 1071–1080. [DOI:10.1016/j.biopsych.2006.04.031] [PMID]
Shiri, V., Hosseini, S. A., Pishyareh, E., Nejati, V., & Biglarian, A. (2015). [Studying the relationship of executive functions with behavioral symptoms in children with high-functioning Autism (Persian)]. Journal of Rehabilitaion, 16(3), 208-217. [Link]
Silk, T. J., Vance, A., Rinehart, N., Bradshaw, J. L., & Cunnington, R. (2008). Dysfunction in the fronto-parietal network in attention deficit hyperactivity disorder (ADHD): An fMRI study. Brain Imaging and Behavior, 2, 123-131. [DOI:10.1007/s11682-008-9021-8]
Soluki, M., Mahmoudi, F., Abdolmaleki, A., Asadi, A., & Sabahi Namini, A. (2024). Cerium oxide nanoparticles as a new neuroprotective agent to promote functional recovery in a rat model of sciatic nerve crush injury. British Journal of Neurosurgery, 38(2), 301–306. [DOI:10.21203/rs.3.rs-41923/v1] [PMID]
Staff, A. I., Luman, M., van der Oord, S., Bergwerff, C. E., van den Hoofdakker, B. J., & Oosterlaan, J. (2022). Facial emotion recognition impairment predicts social and emotional problems in children with (subthreshold) ADHD. European Child & Adolescent Psychiatry, 31(5), 715–727. [DOI:10.1007/s00787-020-01709-y] [PMID] [PMCID]
Taylor Tavares, J. V., Clark, L., Cannon, D. M., Erickson, K., Drevets, W. C., & Sahakian, B. J. (2007). Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biological Psychiatry, 62(8), 917–924. [DOI:10.1016/j.biopsych.2007.05.034] [PMID]
Vance, A., Silk, T. J., Casey, M., Rinehart, N. J., Bradshaw, J. L., & Bellgrove, M. A., et al. (2007). Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Molecular Psychiatry, 12(9), 826–793. [DOI:10.1038/sj.mp.4001999] [PMID]
Vilgis, V., Sun, L., Chen, J., Silk, T. J., & Vance, A. (2016). Global and local grey matter reductions in boys with ADHD combined type and ADHD inattentive type. Psychiatry Research. Neuroimaging, 254, 119–126. [DOI:10.1016/j.pscychresns.2016.06.008] [PMID]
Wang, Y., Tao, F., Zuo, C., Kanji, M., Hu, M., & Wang, D. (2019). Disrupted resting frontal-parietal attention network topology is associated with a clinical measure in children with attention-deficit/hyperactivity disorder. Frontiers in Psychiatry, 10, 300. [DOI:10.3389/fpsyt.2019.00300] [PMID] [PMCID]
Wolf, R. C., Plichta, M. M., Sambataro, F., Fallgatter, A. J., Jacob, C., & Lesch, K. P., et al. (2009). Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping, 30(7), 2252–2266. [DOI:10.1002/hbm.20665] [PMID] [PMCID]
Wu, Z., Luo, Y., Gao, Y., Han, Y., Wu, K., & Li, X. (2020). The role of frontal and occipital cortices in processing sustained visual attention in young adults with attention-deficit/hyperactivity disorder: A functional near-infrared spectroscopy study. Neuroscience Bulletin, 36(6), 659–663. [DOI:10.1007/s12264-020-00492-9] [PMID] [PMCID]
Type of Study: Review |
Subject:
Cognitive Neuroscience
Received: 2020/08/19 | Accepted: 2022/07/13 | Published: 2024/03/1
Received: 2020/08/19 | Accepted: 2022/07/13 | Published: 2024/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








