Volume 16, Issue 4 (July & August 2025)
BCN 2025, 16(4): 751-762 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Momeni J, Hosseini E, Khaleghi Ghadiri M, Samini F, Tabibkhooei A, Gorji A et al . The Impact of Small Molecule on the Astrocyte's Viability Derived From Epileptic Brain Tissues. BCN 2025; 16 (4) :751-762
URL: http://bcn.iums.ac.ir/article-1-3098-en.html
URL: http://bcn.iums.ac.ir/article-1-3098-en.html
Javad Momeni1 

 , Elham Hosseini1
, Elham Hosseini1 

 , Maryam Khaleghi Ghadiri2
, Maryam Khaleghi Ghadiri2 

 , Fariborz Samini3
, Fariborz Samini3 

 , Alireza Tabibkhooei4
, Alireza Tabibkhooei4 

 , Ali Gorji5
, Ali Gorji5 

 , Sajad Sahab Negah *6
, Sajad Sahab Negah *6 




 , Elham Hosseini1
, Elham Hosseini1 

 , Maryam Khaleghi Ghadiri2
, Maryam Khaleghi Ghadiri2 

 , Fariborz Samini3
, Fariborz Samini3 

 , Alireza Tabibkhooei4
, Alireza Tabibkhooei4 

 , Ali Gorji5
, Ali Gorji5 

 , Sajad Sahab Negah *6
, Sajad Sahab Negah *6 


1- Department of Neuroscience, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. & Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Neurosurgery, Münster University, Münster, Germany.
3- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Neurosurgery, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
5- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. & Shefa Neuroscience Research Center, Khatam Alanbia Hospital, Tehran, Iran.
6- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. & Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Neurosurgery, Münster University, Münster, Germany.
3- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Neurosurgery, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
5- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. & Shefa Neuroscience Research Center, Khatam Alanbia Hospital, Tehran, Iran.
6- Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. & Multiple Sclerosis Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 2616 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Epilepsy is a neurological condition defined by repeated seizures caused by the synchronized, abnormal firing of groups of neurons. Glial cells are deeply involved in the genesis, development, and progression of epilepsy pathophysiology, mainly through their contribution to the synchronization of neural networks (Rosciszewski et al., 2019). These cells closely interact with neurons, regulate synaptic communication, and are involved in inflammatory processes and immune reactions (Diaz Verdugo et al., 2019; Heuser et al., 2014). Each type of glial cell possesses different properties that could be targeted to preserve neuronal health (Rasband, 2016). Several studies revealed significant phenotypical and functional heterogeneity of glial cells across various regions of the mammalian central nervous system (Tan et al., 2020). Emerging evidence suggests that glial cells undergo apoptosis and exhibit neuroinflammation in the epileptic focus, potentially contributing to epileptogenesis (Sokolova et al., 2022). Specifically, oligodendrocyte apoptosis has been linked to myelin damage in epilepsy (Sokolova et al., 2022). Furthermore, glial cells are involved in epileptogenesis through their interactions with neurons (Egaña-Huguet et al., 2021). Astrocytes can sense and respond to neuronal activity, allowing them to regulate synaptic interactions (Tavassoli et al., 2022). Given the importance of astrocytes in epilepsy, modulating their function may offer therapeutic potential.
The longer duration of epilepsy is associated with decreased neuronal production and sustained astrogenesis (Ammothumkandy et al., 2022). Although immature neurons in mesial temporal lobe epilepsy (MTLE) remain inactive and are not present during local epileptiform activity, immature astroglia persist in all cases of MTLE. This astroglia, rather than new neurons, could be a potential target for regulating the hyperactivity of adult human neurons (Ammothumkandy et al., 2022). For example, the endocannabinoid system in glial cells has been studied as a target for treating epilepsy in animal models (Egaña-Huguet et al., 2021). Glial cells could be considered valuable next-generation targets for designing anti-epileptic drugs, offering the potential to enhance outcomes for medically refractory epilepsy (Shen et al., 2023).
Several studies have investigated small molecules’ (SMs) ability to influence glial cell properties in epilepsy models. Recently, there have been reports indicating that SMs capable of binding to and activating K+/Cl− co-transporter (KCC2) have shown promise in reducing neuronal Cl− accumulation and excitability. When KCC2 is selectively disabled in the hippocampus, it leads to the buildup of neuronal Cl− and depolarizes GABAAR (gamma-aminobutyric acid type A receptor) currents. These impairments in inhibition are associated with the beginning of unprovoked seizures, neuronal cell death, and the activation of reactive astrocytes. The activation of KCC2 by compound 350, a small molecule, has been found to both prevent the development of and halt ongoing benzodiazepine resistance (BDZ-RSE) status epilepticus (SE) in a mouse model of epilepsy. Furthermore, KCC2 activation decreases neuronal cell death following BDZ-RSE (Jarvis et al., 2023).
Recently, several SMs have been investigated during glial pathogenesis in epilepsy. For instance, valproate (VPA), an antiepileptic drug, modulates glial cell function. Research has indicated that VPA can influence the development and differentiation of neural crest progenitors as well as hippocampal neural stem cells. Earlier research on VPA’s effects on neural differentiation has mainly focused on how it influences transcription by inhibiting histone deacetylases (HDACs) (Almutawaa et al., 2014; Hsieh et al., 2004; Santos et al., 2020; Yu et al., 2009). Other SMs, such as Forskolin and CHIR, can influence cell signaling pathways (Han et al., 2023; Wang et al., 2022; Yan et al., 2016). Forskolin activates the enzyme adenylate cyclase (AC) directly, leading to the production of cAMP from ATP and raising the levels of intracellular cAMP (Sapio et al., 2017). Prolonged cAMP activation in astrocytes increases glutamate transporter expression, while rapid cAMP spikes can reduce glutamate uptake by triggering the internalization of these transporters. Inhibiting AC or lowering intracellular cAMP can enhance glutamate uptake by preventing the internalization of GLT-1 and GLAST (Li et al., 2015). On the other hand, the WNT/β-catenin pathway is activated to prevent astrocytes from changing into different cell types by increasing the expression of Ngn2. After a stroke, a protein called WNT2 is released from dying neurons, activating WNT/β-linked protein signaling in reactive astrocytes. This leads to the reversal of the specialized functions of astrocytes, which in turn supports the creation of new neurons in the cortex, indirectly activates the WNT pathway by inhibiting GSK-3 using CHIR99021, kenpaullone, and other small molecule compounds, leading to the expression of Ngn2 and encouraging astrocytes to transform into neurons (Cheng et al., 2015; Huang et al., 2024; Yin et al., 2019).
Mardones et al. (2024) investigate the WNT signaling pathway in epileptogenesis using the activator Chir99021. They induced mesial temporal lobe epilepsy in wild-type and POMC-eGFP transgenic mice through intrahippocampal kainate injection, followed by 21 days of daily Chir99021 treatment starting 3 h post-induction. The study revealed that intrahippocampal kainate (IHK) resulted in epilepsy after a 14-day latent period. WNT activation significantly reduced seizure frequency and duration, with lasting effects. Additionally, dendritic structures were preserved, and object location memory improved post-treatment. These findings suggest that canonical WNT activation may prevent epileptogenesis in the IHK mouse model, presenting a novel prevention strategy for epilepsy (Mardones et al., 2024). A study by Feng et al. (2024) examined the effects of VPA on hippocampal astrogliosis in a rat model of temporal lobe epilepsy. Results showed that chronic VPA administration at 200 mg/kg significantly reduced astrogliosis and neuronal loss in the hippocampus after SE and improved cognitive impairments in KA-SE rats. Long-term administration at 400 mg/kg reduced astrogliosis at the middle stage post-SE but worsened cognitive impairments later. These findings suggest that VPA, at the right dosage, could reduce hippocampal astrogliosis and offer a potential new antiepileptic mechanism for long-term use (Feng et al., 2024). To date, no in vitro studies have examined the effects of SMs on human astrocytes derived from epileptic patients. Therefore, our study sought to evaluate the impact of three SMs, valproate, forskolin, and a GSK3 inhibitor/WNT activator (document_number_1; CHIR), on the viability of astrocyte cells obtained from individuals with epilepsy. By investigating the effects of these compounds on astrocytes from epileptic patients, this study demonstrates their behavior across different brain regions in epilepsy.
2. Materials and Methods
Tissue preparation
Tissues from temporal lobe resections were collected from seven patients with drug-resistant temporal lobe epilepsy who underwent surgery at Khatam Alanbia Hospital in Tehran and Imam Reza Hospital in Mashhad City, Iran.
Primary cell culture
After the neurosurgeon resected the tissue in the operating room, the tissue was transferred to the cell culture laboratory in a conical tube containing cold phosphate-buffered saline (PBS) (Gibco, Germany) and Pen/Strep (Sigma, Germany) 10% solution. In the laboratory, the tissue was transferred to a petri dish. Then, the necrotic tissues, blood vessels, and white matter were separated using a scalpel and forceps. Next, the mechanical digestion was performed for 3 min. The tissue was overlaid with cold PBS. Then, the tissue was transferred to a falcon and centrifuged at 1500 round per min (rpm). After removing the supernatant, trypsin (Gibco, Germany) was added to the tissue (at twice the amount of the tissue) for 5 min at 37 °C. To neutralize the medium, twice the amount of culture medium containing fetal bovine serum (Gibco, Germany) was added to a falcon and centrifuged at 1200 rpm for 3 min. After removing the supernatant, we added a medium and passed it through a 70 µm cell strainer. Finally, cells were counted and transferred to the flask.
Following a 70% confluency, we isolated astrocytes using the MD-astrocyte model. This model is predicated on the death of neurons, which triggers the rapid proliferation of astrocytes and oligodendrocytes in culture. It also involves the selective detachment of the upper oligodendrocytes, which occurs when the cultures are subjected to shear forces generated by shaking (Creighton et al., 2019; Liddelow, 2017; Schildge et al., 2013).
Cell counting and plating
The medium for expansion contained Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen, Germany) with the addition of 0.5% N2 supplement (Invitrogen, Germany), 1% B27 supplement (Invitrogen, Germany), 2 μg/mL heparin (Sigma, Germany), 1% penicillin/streptomycin, 1% glutamine (Invitrogen, Germany), 20 ng/mL essential fibroblast growth factor (bFGF; Millipore, Germany), and 20 ng/mL epidermal growth factor (EGF; (Sigma, Germany). The medium was changed every three days, and cells were transferred every seven days and re-cultured in a fresh growth medium. The cell cultures were kept in an incubator (Memmert, Germany) at 37 °C with 95% air and 5% CO2. Astrocyte cells were cultured separately and expanded to the third passage under similar culture conditions.
SMs treatment
In this research, we utilized three distinct SMs, namely CHIR99021 (CHIR, Sigma, SML1046), valproic acid (VPA, Sigma, P4543), and forskolin (FOR, Sigma, F3917). Additionally, BDNF (Sigma, B3795) was employed as a growth factor in certain groups. The astrocyte cells were exposed to BDNF at 50 ng/mL, CHIR at 3 µM, VPA at 1 mM, and FORS at 100 µM. Cell viability was then measured at 48 and 72 h post-treatment. Combining these SMs, we structured 12 groups, detailed in Table 1.

In each group, we used neural basal medium containing B27: 1%, L-Glu: 1%, Pen/Strep: 1%, and N2: 0.5%.
Immunocytochemistry assay
We performed immunocytochemistry to characterize cells. Initially, we discarded the culture medium and rinsed the cells with PBS. Next, we treated the cells with 4% paraformaldehyde for 30 min to fix them. Following that, we permeabilized the cells using a Triton X-100 solution and applied normal goat serum and BSA as blocking agents. We then applied primary antibodies, such as GFAP (Sigma, Germany) at a 1:200 dilution, vimentin (Santa Cruz, Germany) at a 1:100 dilution, and Fibronectin (Merck, Germany) at a 1:100 dilution, as markers for astrocytic cells at 4 °C. After this, we applied secondary antibodies, including goat anti-mouse fluorescein isothiocyanate (Abcam, UK), for 1.5 h at room temperature. We used propidium iodide to stain cell nuclei and incubated secondary antibodies alone to identify negative controls. Finally, we examined the data using fluorescent microscopy (Olympus, Germany). The tissue derived from patient number 1 for the amygdala, number 7 for the neocortex, and number 4 for the hippocampus was used for cell characterization.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Atocel, Austria). Astrocyte cells (1×104/well) were incubated in 96-well culture dishes overnight. Subsequently, the cells were exposed to various SMs for 48 or 72 h. After the treatment, 10 μL of MTT solution in PBS (5 mg/mL) was applied to each well to achieve a final concentration of 0.05%. Following a 3-h incubation, the supernatant was discarded, and 100 μL of dimethyl sulfoxide (Sigma, Germany) was introduced to dissolve the formazan crystals. The microplates were gently agitated without light for 60 min, and the absorbance was gauged between 570 and 630 nm using a Stat FAX303 plate reader (Rasti et al., 2024).
Statistical analysis
The current study presented data as Mean±SD, utilizing GraphPad Prism software, version 5 for analysis. Statistical variations among the groups were determined through one-way analysis of variance (ANOVA). A threshold of P<0.05 was used to signify the levels of statistical significance.
3. Results
Demographic data
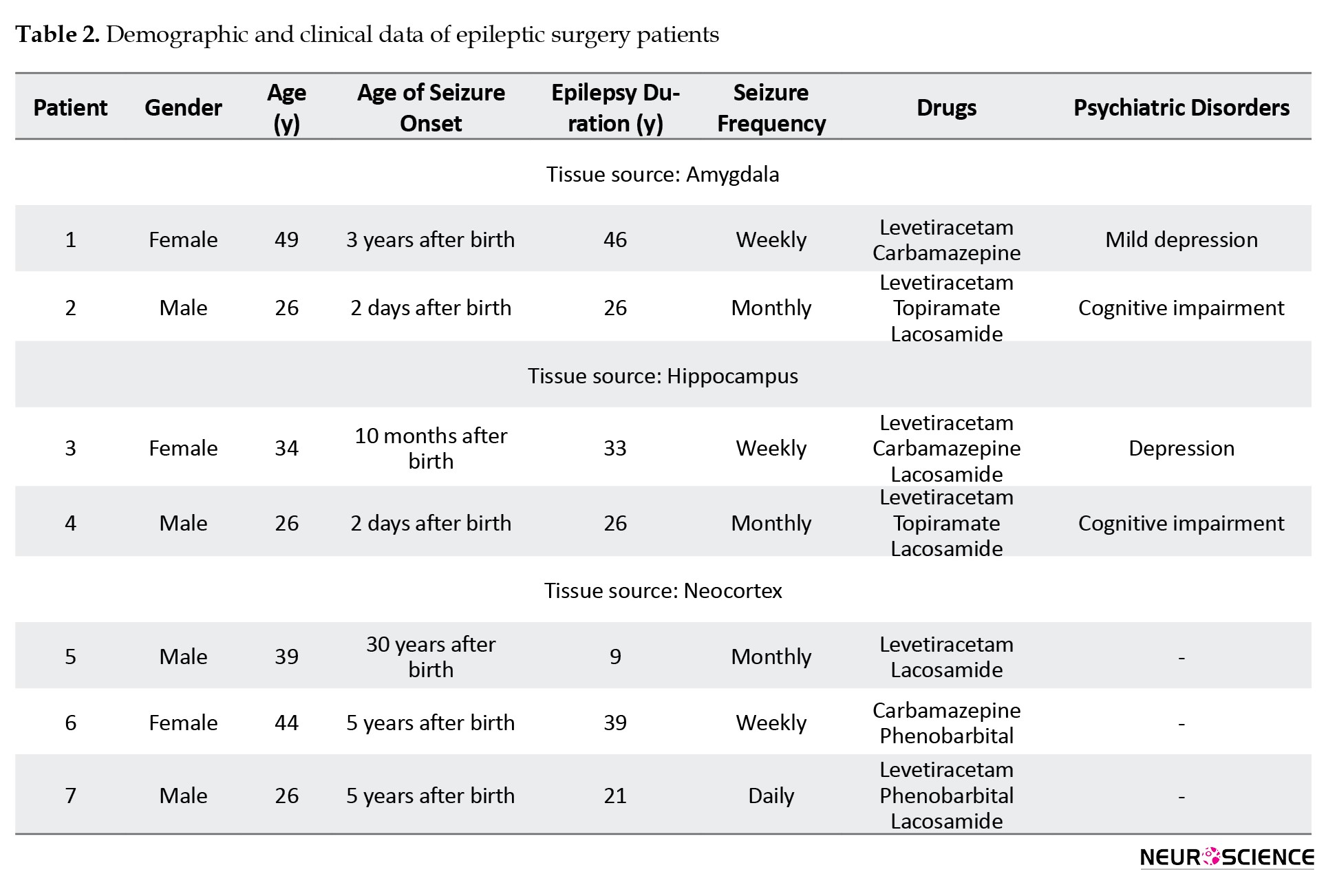
This study was conducted on brain tissue from seven individuals with medically intractable epilepsy who had undergone surgery. Four participants were male, with an mean age of 35.37±8.87 years, ranging from 26 to 44 years. The duration of their seizures varied between 9 and 46 years. All patients had been using two or three medications before surgery. Neuropsychiatric symptoms, including cognitive impairments and depression, were observed in four patients (Table 2).
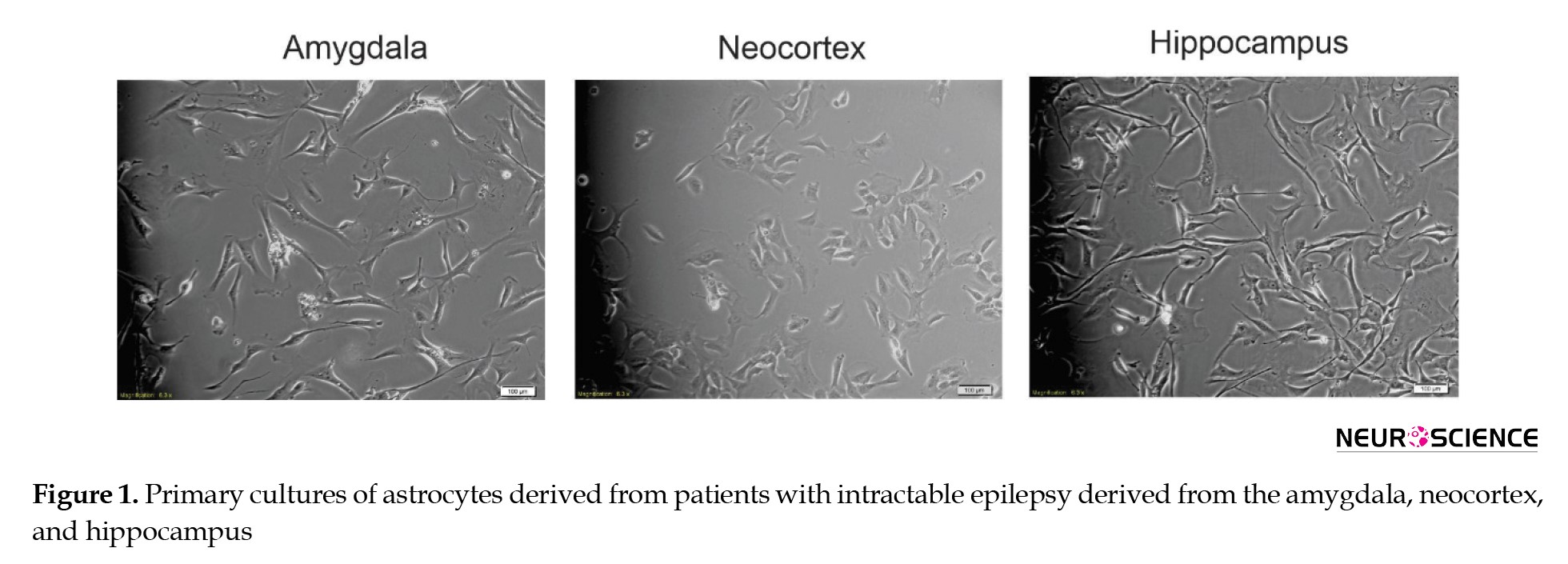
Isolation and expansion of human astrocyte cells
Human astrocyte cells were isolated and cultured from resected mesial temporal lobe tissues during epilepsy surgery. Following the primary culture, the cells achieved 80% confluence within ten days (Figure 1).
Epilepsy is a neurological condition defined by repeated seizures caused by the synchronized, abnormal firing of groups of neurons. Glial cells are deeply involved in the genesis, development, and progression of epilepsy pathophysiology, mainly through their contribution to the synchronization of neural networks (Rosciszewski et al., 2019). These cells closely interact with neurons, regulate synaptic communication, and are involved in inflammatory processes and immune reactions (Diaz Verdugo et al., 2019; Heuser et al., 2014). Each type of glial cell possesses different properties that could be targeted to preserve neuronal health (Rasband, 2016). Several studies revealed significant phenotypical and functional heterogeneity of glial cells across various regions of the mammalian central nervous system (Tan et al., 2020). Emerging evidence suggests that glial cells undergo apoptosis and exhibit neuroinflammation in the epileptic focus, potentially contributing to epileptogenesis (Sokolova et al., 2022). Specifically, oligodendrocyte apoptosis has been linked to myelin damage in epilepsy (Sokolova et al., 2022). Furthermore, glial cells are involved in epileptogenesis through their interactions with neurons (Egaña-Huguet et al., 2021). Astrocytes can sense and respond to neuronal activity, allowing them to regulate synaptic interactions (Tavassoli et al., 2022). Given the importance of astrocytes in epilepsy, modulating their function may offer therapeutic potential.
The longer duration of epilepsy is associated with decreased neuronal production and sustained astrogenesis (Ammothumkandy et al., 2022). Although immature neurons in mesial temporal lobe epilepsy (MTLE) remain inactive and are not present during local epileptiform activity, immature astroglia persist in all cases of MTLE. This astroglia, rather than new neurons, could be a potential target for regulating the hyperactivity of adult human neurons (Ammothumkandy et al., 2022). For example, the endocannabinoid system in glial cells has been studied as a target for treating epilepsy in animal models (Egaña-Huguet et al., 2021). Glial cells could be considered valuable next-generation targets for designing anti-epileptic drugs, offering the potential to enhance outcomes for medically refractory epilepsy (Shen et al., 2023).
Several studies have investigated small molecules’ (SMs) ability to influence glial cell properties in epilepsy models. Recently, there have been reports indicating that SMs capable of binding to and activating K+/Cl− co-transporter (KCC2) have shown promise in reducing neuronal Cl− accumulation and excitability. When KCC2 is selectively disabled in the hippocampus, it leads to the buildup of neuronal Cl− and depolarizes GABAAR (gamma-aminobutyric acid type A receptor) currents. These impairments in inhibition are associated with the beginning of unprovoked seizures, neuronal cell death, and the activation of reactive astrocytes. The activation of KCC2 by compound 350, a small molecule, has been found to both prevent the development of and halt ongoing benzodiazepine resistance (BDZ-RSE) status epilepticus (SE) in a mouse model of epilepsy. Furthermore, KCC2 activation decreases neuronal cell death following BDZ-RSE (Jarvis et al., 2023).
Recently, several SMs have been investigated during glial pathogenesis in epilepsy. For instance, valproate (VPA), an antiepileptic drug, modulates glial cell function. Research has indicated that VPA can influence the development and differentiation of neural crest progenitors as well as hippocampal neural stem cells. Earlier research on VPA’s effects on neural differentiation has mainly focused on how it influences transcription by inhibiting histone deacetylases (HDACs) (Almutawaa et al., 2014; Hsieh et al., 2004; Santos et al., 2020; Yu et al., 2009). Other SMs, such as Forskolin and CHIR, can influence cell signaling pathways (Han et al., 2023; Wang et al., 2022; Yan et al., 2016). Forskolin activates the enzyme adenylate cyclase (AC) directly, leading to the production of cAMP from ATP and raising the levels of intracellular cAMP (Sapio et al., 2017). Prolonged cAMP activation in astrocytes increases glutamate transporter expression, while rapid cAMP spikes can reduce glutamate uptake by triggering the internalization of these transporters. Inhibiting AC or lowering intracellular cAMP can enhance glutamate uptake by preventing the internalization of GLT-1 and GLAST (Li et al., 2015). On the other hand, the WNT/β-catenin pathway is activated to prevent astrocytes from changing into different cell types by increasing the expression of Ngn2. After a stroke, a protein called WNT2 is released from dying neurons, activating WNT/β-linked protein signaling in reactive astrocytes. This leads to the reversal of the specialized functions of astrocytes, which in turn supports the creation of new neurons in the cortex, indirectly activates the WNT pathway by inhibiting GSK-3 using CHIR99021, kenpaullone, and other small molecule compounds, leading to the expression of Ngn2 and encouraging astrocytes to transform into neurons (Cheng et al., 2015; Huang et al., 2024; Yin et al., 2019).
Mardones et al. (2024) investigate the WNT signaling pathway in epileptogenesis using the activator Chir99021. They induced mesial temporal lobe epilepsy in wild-type and POMC-eGFP transgenic mice through intrahippocampal kainate injection, followed by 21 days of daily Chir99021 treatment starting 3 h post-induction. The study revealed that intrahippocampal kainate (IHK) resulted in epilepsy after a 14-day latent period. WNT activation significantly reduced seizure frequency and duration, with lasting effects. Additionally, dendritic structures were preserved, and object location memory improved post-treatment. These findings suggest that canonical WNT activation may prevent epileptogenesis in the IHK mouse model, presenting a novel prevention strategy for epilepsy (Mardones et al., 2024). A study by Feng et al. (2024) examined the effects of VPA on hippocampal astrogliosis in a rat model of temporal lobe epilepsy. Results showed that chronic VPA administration at 200 mg/kg significantly reduced astrogliosis and neuronal loss in the hippocampus after SE and improved cognitive impairments in KA-SE rats. Long-term administration at 400 mg/kg reduced astrogliosis at the middle stage post-SE but worsened cognitive impairments later. These findings suggest that VPA, at the right dosage, could reduce hippocampal astrogliosis and offer a potential new antiepileptic mechanism for long-term use (Feng et al., 2024). To date, no in vitro studies have examined the effects of SMs on human astrocytes derived from epileptic patients. Therefore, our study sought to evaluate the impact of three SMs, valproate, forskolin, and a GSK3 inhibitor/WNT activator (document_number_1; CHIR), on the viability of astrocyte cells obtained from individuals with epilepsy. By investigating the effects of these compounds on astrocytes from epileptic patients, this study demonstrates their behavior across different brain regions in epilepsy.
2. Materials and Methods
Tissue preparation
Tissues from temporal lobe resections were collected from seven patients with drug-resistant temporal lobe epilepsy who underwent surgery at Khatam Alanbia Hospital in Tehran and Imam Reza Hospital in Mashhad City, Iran.
Primary cell culture
After the neurosurgeon resected the tissue in the operating room, the tissue was transferred to the cell culture laboratory in a conical tube containing cold phosphate-buffered saline (PBS) (Gibco, Germany) and Pen/Strep (Sigma, Germany) 10% solution. In the laboratory, the tissue was transferred to a petri dish. Then, the necrotic tissues, blood vessels, and white matter were separated using a scalpel and forceps. Next, the mechanical digestion was performed for 3 min. The tissue was overlaid with cold PBS. Then, the tissue was transferred to a falcon and centrifuged at 1500 round per min (rpm). After removing the supernatant, trypsin (Gibco, Germany) was added to the tissue (at twice the amount of the tissue) for 5 min at 37 °C. To neutralize the medium, twice the amount of culture medium containing fetal bovine serum (Gibco, Germany) was added to a falcon and centrifuged at 1200 rpm for 3 min. After removing the supernatant, we added a medium and passed it through a 70 µm cell strainer. Finally, cells were counted and transferred to the flask.
Following a 70% confluency, we isolated astrocytes using the MD-astrocyte model. This model is predicated on the death of neurons, which triggers the rapid proliferation of astrocytes and oligodendrocytes in culture. It also involves the selective detachment of the upper oligodendrocytes, which occurs when the cultures are subjected to shear forces generated by shaking (Creighton et al., 2019; Liddelow, 2017; Schildge et al., 2013).
Cell counting and plating
The medium for expansion contained Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen, Germany) with the addition of 0.5% N2 supplement (Invitrogen, Germany), 1% B27 supplement (Invitrogen, Germany), 2 μg/mL heparin (Sigma, Germany), 1% penicillin/streptomycin, 1% glutamine (Invitrogen, Germany), 20 ng/mL essential fibroblast growth factor (bFGF; Millipore, Germany), and 20 ng/mL epidermal growth factor (EGF; (Sigma, Germany). The medium was changed every three days, and cells were transferred every seven days and re-cultured in a fresh growth medium. The cell cultures were kept in an incubator (Memmert, Germany) at 37 °C with 95% air and 5% CO2. Astrocyte cells were cultured separately and expanded to the third passage under similar culture conditions.
SMs treatment
In this research, we utilized three distinct SMs, namely CHIR99021 (CHIR, Sigma, SML1046), valproic acid (VPA, Sigma, P4543), and forskolin (FOR, Sigma, F3917). Additionally, BDNF (Sigma, B3795) was employed as a growth factor in certain groups. The astrocyte cells were exposed to BDNF at 50 ng/mL, CHIR at 3 µM, VPA at 1 mM, and FORS at 100 µM. Cell viability was then measured at 48 and 72 h post-treatment. Combining these SMs, we structured 12 groups, detailed in Table 1.

In each group, we used neural basal medium containing B27: 1%, L-Glu: 1%, Pen/Strep: 1%, and N2: 0.5%.
Immunocytochemistry assay
We performed immunocytochemistry to characterize cells. Initially, we discarded the culture medium and rinsed the cells with PBS. Next, we treated the cells with 4% paraformaldehyde for 30 min to fix them. Following that, we permeabilized the cells using a Triton X-100 solution and applied normal goat serum and BSA as blocking agents. We then applied primary antibodies, such as GFAP (Sigma, Germany) at a 1:200 dilution, vimentin (Santa Cruz, Germany) at a 1:100 dilution, and Fibronectin (Merck, Germany) at a 1:100 dilution, as markers for astrocytic cells at 4 °C. After this, we applied secondary antibodies, including goat anti-mouse fluorescein isothiocyanate (Abcam, UK), for 1.5 h at room temperature. We used propidium iodide to stain cell nuclei and incubated secondary antibodies alone to identify negative controls. Finally, we examined the data using fluorescent microscopy (Olympus, Germany). The tissue derived from patient number 1 for the amygdala, number 7 for the neocortex, and number 4 for the hippocampus was used for cell characterization.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Atocel, Austria). Astrocyte cells (1×104/well) were incubated in 96-well culture dishes overnight. Subsequently, the cells were exposed to various SMs for 48 or 72 h. After the treatment, 10 μL of MTT solution in PBS (5 mg/mL) was applied to each well to achieve a final concentration of 0.05%. Following a 3-h incubation, the supernatant was discarded, and 100 μL of dimethyl sulfoxide (Sigma, Germany) was introduced to dissolve the formazan crystals. The microplates were gently agitated without light for 60 min, and the absorbance was gauged between 570 and 630 nm using a Stat FAX303 plate reader (Rasti et al., 2024).
Statistical analysis
The current study presented data as Mean±SD, utilizing GraphPad Prism software, version 5 for analysis. Statistical variations among the groups were determined through one-way analysis of variance (ANOVA). A threshold of P<0.05 was used to signify the levels of statistical significance.
3. Results
Demographic data
This study was conducted on brain tissue from seven individuals with medically intractable epilepsy who had undergone surgery. Four participants were male, with an mean age of 35.37±8.87 years, ranging from 26 to 44 years. The duration of their seizures varied between 9 and 46 years. All patients had been using two or three medications before surgery. Neuropsychiatric symptoms, including cognitive impairments and depression, were observed in four patients (Table 2).

Isolation and expansion of human astrocyte cells
Human astrocyte cells were isolated and cultured from resected mesial temporal lobe tissues during epilepsy surgery. Following the primary culture, the cells achieved 80% confluence within ten days (Figure 1).
The astrocytes exhibit diverse shapes and intricate structures. The main processes, called “branches,” originate from the soma, with their precise number being diverse. The neocortex cells have fewer branches than those in the amygdala and hippocampus.
Immunocytochemistry assay
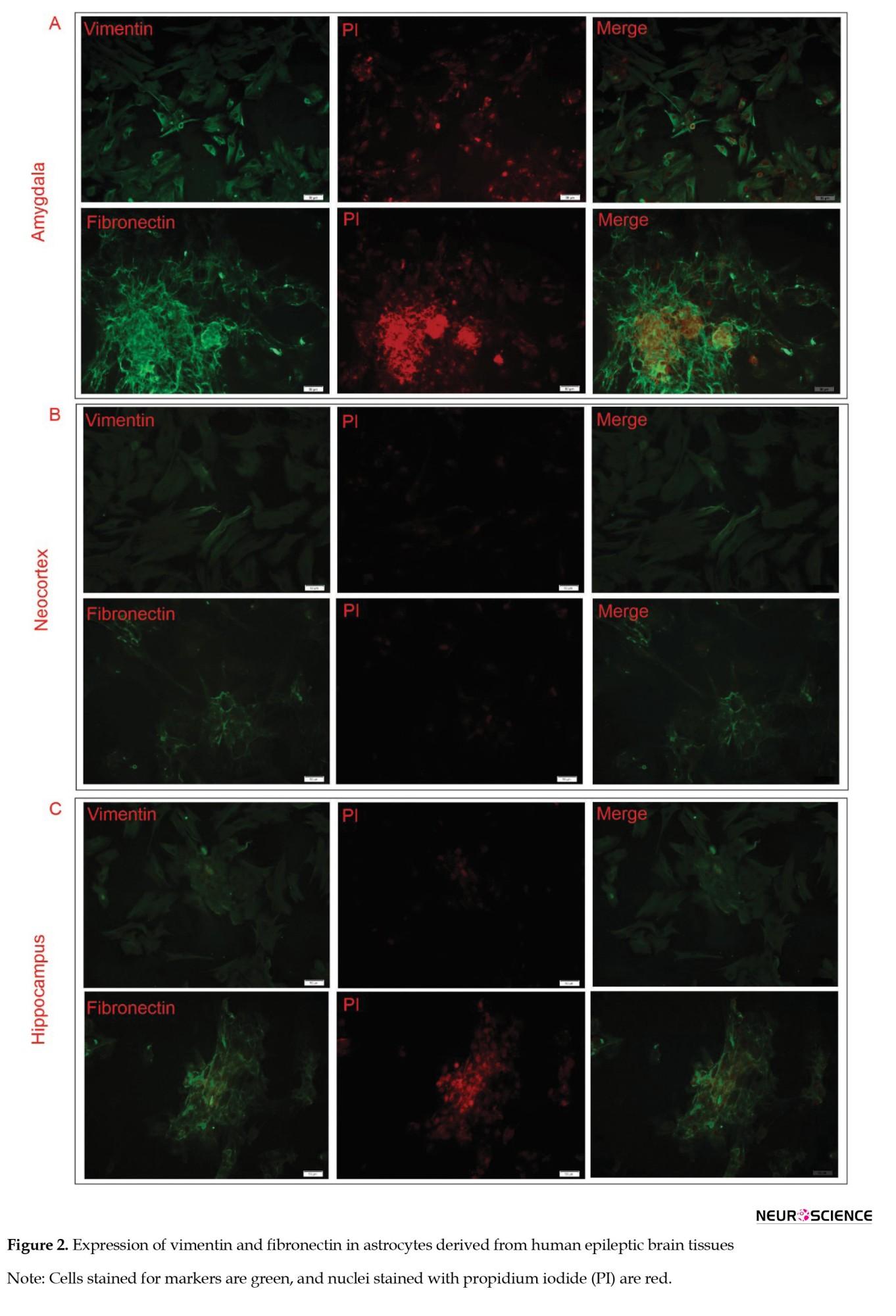
We analyzed the expression levels of GFAP, vimentin, and fibronectin markers. Our findings revealed a negative expression of the GFAP marker and a positive expression of vimentin and fibronectin (Figure 2).
Immunocytochemistry assay
We analyzed the expression levels of GFAP, vimentin, and fibronectin markers. Our findings revealed a negative expression of the GFAP marker and a positive expression of vimentin and fibronectin (Figure 2).
The findings are consistent with previous studies (Macikova et al., 2009; Perzelova et al., 2007), which shows that astrocytes derived from human brain tissues predominantly express vimentin (100%) and fibronectin (95%), with only 0.1% testing positive for GFAP. Building on these findings, we effectively isolated and purified astrocytes for further analysis.
Cell viability assay
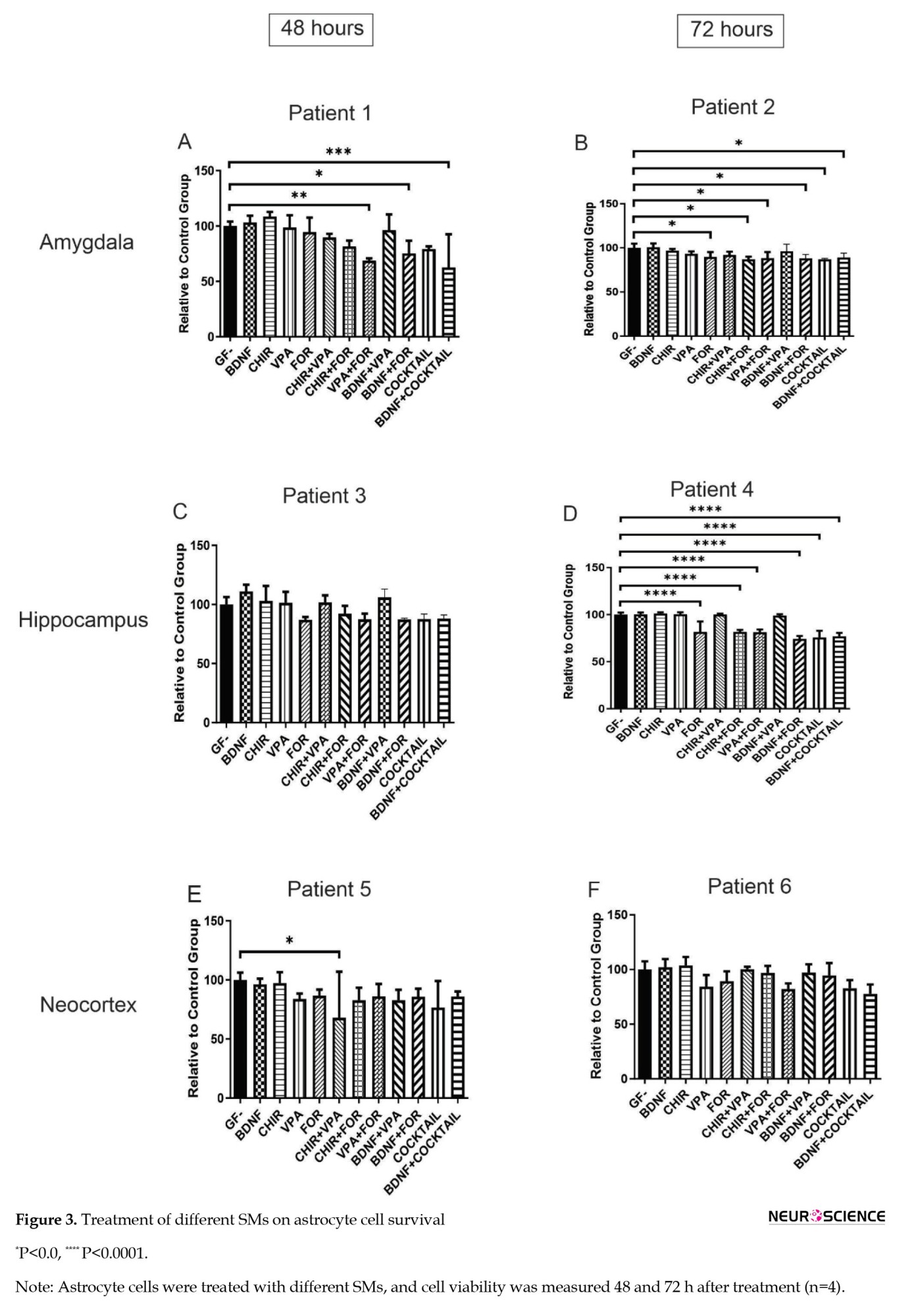
The viability of astrocytes derived from epileptic tissue was evaluated after treatment with SMs and compared to cells that were not treated. The findings indicate that specific SMs can inhibit the viability of astrocytes when used alone or in combination with others, depending on the cell source and individual patients. For instance, two patients’ astrocytes from the amygdala exhibited more robust responses to SMs (Figures 3A and 3B).
Cell viability assay
The viability of astrocytes derived from epileptic tissue was evaluated after treatment with SMs and compared to cells that were not treated. The findings indicate that specific SMs can inhibit the viability of astrocytes when used alone or in combination with others, depending on the cell source and individual patients. For instance, two patients’ astrocytes from the amygdala exhibited more robust responses to SMs (Figures 3A and 3B).
In contrast, astrocytes from the neocortex showed reduced viability in only one group (CHIR+VPA; Figures 3E and 3F). Astrocytes from the hippocampus exhibited a significant decrease in viability for one patient across all groups, while another showed no substantial changes (Figure 3C and 3D).
4. Discussion
Astroglial cells make up around 30% of all cells in the adult mammalian central nervous system, making them the most prevalent cell type (Liddelow & Barres, 2017). These cells share early progenitor cells with neurons. They are highly likely to be converted into neurons in neurological disorders, where there is neuronal loss or a high number of astrocytes due to uncontrolled proliferation. Thus, inhibiting astrocyte proliferation, mainly astrogliosis, occurs in epilepsy and creates a chaotic environment; targeting the proliferation and viability of astrocytes could be a novel approach to managing MTLE (Chandrasekaran et al., 2016; Westergard & Rothstein, 2020). The present study demonstrates that specific SMs can reduce the viability of astrocytes in some epileptic patients. To arrive at a valid conclusion, we conducted viability assays on astrocytes isolated from different tissues (amygdala, hippocampus, and cortex) obtained from intractable epileptic patients individually. Notably, tissue heterogeneity, brain localization, and the timing of epilepsy onset and seizure duration may influence the cellular response to our intervention (Wong, 2019). However, the results of two studies have indicated that the differentiation of astrocytes derived from human fetal brain tissue and cell lines was taken into neurons (exposed to TTNPB, SB431542, LDN193189, Thiazovivin, CHIR99021, DAPT, VPA, SAG, purmophamine) and oligodendrocytes (exposed to CHIR99021, Forskolin, Repsox, LDN, VPA, and Thiazovivin), respectively, when exposed to SMs (Sharifi-Kelishadi et al., 2024; Zhang et al., 2015). This means SMs can regulate astrocyte molecular mechanisms. The difference between this study and our findings was that we used several tissues, while these two studies assessed their response on one cell type or cell line. The heterogeneity of cells was evident in our study; this might affect the response to SMs. Another difference was that the outcome of our study was viability for a maximum of 72 h, while those studies evaluated differentiation during 10 days. Additionally, we just used three SMs, while they used 9 SMs for neuronal differentiation and 6 SMs for oligodendrogenesis.
Recent studies indicate that WNT/β-catenin signaling is involved in neurogenesis and neuronal reorganization during epileptogenesis (Huang et al., 2015; Moncion et al., 2024; Qu et al., 2017). Rawat et al. (2023) used a lithium-pilocarpine model of status epilepsy and found that this signaling pathway was inactive during the acute phase but activated in the chronic stage. This activation may increase neuronal count, synaptic density, astrogliosis, and apoptosis in chronic epilepsy. The findings suggest that targeting GSK-3β could be beneficial in the acute stage, while addressing the chronic stage may require targeting β-catenin and disheveled proteins (Rawat et al., 2023). Recent studies show that VPA exerts anti-epileptic effects by modulating the excitatory/inhibitory (E/I) balance through astrocytes (Takeda et al., 2021). As a histone deacetylase (HDAC) inhibitor, VPA influences gene expression in astrocytes, affecting messenger ribonucleic acid levels of cell adhesion molecules and extracellular matrix proteins in a time- and concentration-dependent manner. VPA also increases levels of excitatory (PSD-95) and inhibitory (gephyrin) proteins in mixed cultures, indicating its role in regulating E/I balance through astrocytic mechanisms, which warrant further research on astroglial contributions to synapse formation (Wang et al., 2012). We found that the combination of CHIR and VPA reduces astrocyte viability, suggesting that these SMs may inhibit astrogliosis in the context of epilepsy.
Consistent with the present results, it has been reported that SMs could inhibit neuroinflammation by inhibiting proinflammatory cytokines. In this regard, SMs could inhibit the survival of astrocytes, which is a primary source of proinflammatory production (Dey et al., 2016).
We have found that specific drug/small molecule screenings are achievable using cells obtained from epileptic tissue. This discovery is a significant step forward in personalized medicine. The findings demonstrate that patient responses vary, and intriguingly, we observed varied reactions in terms of cell localization. The complexity of epilepsy is attributed to various mechanisms, such as the imbalance between inhibitory and excitatory neurotransmission, heightened inflammation, specific brain region neuronal damage, and irregularities in neuronal and cortical development. These mechanisms result in varied responses to pharmacological interventions (do Canto et al., 2021). For example, following the analysis of 57 temporal lobe epilepsy samples along with one control through histochemistry and electron microscopy, researchers identified considerable variation among the samples. Some patients experienced pronounced segmental loss in the hippocampus, while others showed moderate neuronal loss in the hippocampus, with a few not exhibiting any pathological changes whatsoever (Kunz et al., 2000).
Another crucial mechanism in epilepsy, recently highlighted, is biophysical heterogeneity. This phenomenon significantly reduces neuronal diversity within seizure-generating regions (Rich et al., 2022). Within this context, using SMs could boost neuronal diversity by stimulating the creation of new neurons in regions prone to seizures. In particular, compounds like retinoic acid, Notch inhibitors, WNT activators, and DNMT inhibitors can prompt the transformation of neural cells from human fibroblasts by triggering autophagy and reducing reactive oxygen species production (Rujanapun et al., 2019). Thus, SMs can inhibit astrogliosis while conversely having the potential to enhance neuronal production. This presents a novel approach in epilepsy for modifying cellular architecture and addressing neuronal heterogeneity. The primary limitation of this investigation was the limited sample size (n=7). Future research should aim to involve a larger cohort of participants to enhance the robustness and generalizability of the findings.
5. Conclusion
In summary, targeting the proliferation and viability of astrocyte cells represents a promising approach to managing MTLE. Our study, which involved viability assays on cells from different tissues obtained from intractable epileptic patients, highlights the importance of considering tissue heterogeneity, brain localization, and timing of epilepsy onset. Notably, patient responses vary, and the complexity of epilepsy involves multiple mechanisms. Finally, to better understand the effects of SMs, the following recommendations are suggested: 1) Conduct large-scale studies to analyze patient responses to different SMs, considering factors such as genetic variations, comorbidities, and lifestyle; 2) Investigate the synergistic effects of combining SMs with other therapeutic approaches, such as antiepileptic drugs, immunomodulators, or stem cell therapies; 3) Explore specific SMs that can selectively modulate glial cell viability without affecting neuronal health.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.REC.1400.056). Using resected patient tissue and the subsequent procedures were conducted followed by the Declaration of Helsink.
Funding
This study was financially supported by the Council for Development of Stem Cell Sciences and Technologies, Tehran, Iran (Grant No.: 104552). This study was supported by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 991796).
Authors' contributions
Conceptualization and supervision: Sajad Sahab Negah and Ali Gorji; Methodology and funding acquisition: Sajad Sahab Negah; Data collection: Javad Momeni; Data analysis: Sajad Sahab Negah, Ali Gorji, and Javad Momeni; Funding acquisition: Sajad Sahab Negah; Resources: Sajad Sahab Negah, Ali Gorji, Maryam Khaleghi Ghadiri, Fariborz Samini, and Alireza Tabibkhooei; Investigation and writing: All authors.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
The authors thank the Imam Reza Hospital and Khatam Alanbia Hospital patients for cooperating with this manuscript.
References
Almutawaa, W., Kang, N. H., Pan, Y., & Niles, L. P. (2014). Induction of neurotrophic and differentiation factors in neural stem cells by valproic acid. Basic & Clinical Pharmacology & Toxicology, 115(2), 216–221. [DOI:10.1111/bcpt.12201] [PMID]
Ammothumkandy, A., Ravina, K., Wolseley, V., Tartt, A. N., Yu, P. N., & Corona, L., et al. (2022). Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nature Neuroscience, 25(4), 493–503. [DOI:10.1038/s41593-022-01044-2] [PMID]
Chandrasekaran, A., Avci, H. X., Leist, M., Kobolák, J., & Dinnyés, A. (2016). Astrocyte differentiation of human pluripotent stem cells: New tools for neurological disorder research. Frontiers in Cellular Neuroscience, 10, 215. [DOI:10.3389/fncel.2016.00215] [PMID]
Cheng, L., Gao, L., Guan, W., Mao, J., Hu, W., & Qiu, B., et al. (2015). Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Research, 25(11), 1269–1272. [DOI:10.1038/cr.2015.120] [PMID]
Creighton, B. A., Ruffins, T. W., & Lorenzo, D. N. (2019). Visualizing and analyzing intracellular transport of organelles and other cargos in astrocytes. Journal of Visualized Experiments: JoVE, (150), 10.3791/60230.[DOI:10.3791/60230] [PMID]
Dey, A., Kang, X., Qiu, J., Du, Y., & Jiang, J. (2016). Anti-inflammatory small molecules to treat seizures and epilepsy: From bench to bedside. Trends in Pharmacological Sciences, 37(6), 463–484. [DOI:10.1016/j.tips.2016.03.001] [PMID]
Diaz Verdugo, C., Myren-Svelstad, S., Aydin, E., Van Hoeymissen, E., Deneubourg, C., & Vanderhaeghe, S., et al. (2019). Glia-neuron interactions underlie state transitions to generalized seizures. Nature Communications, 10(1), 3830. [DOI:10.1038/s41467-019-11739-z] [PMID]
do Canto, A. M., Donatti, A., Geraldis, J. C., Godoi, A. B., da Rosa, D. C., & Lopes-Cendes, I. (2021). Neuroproteomics in epilepsy: What do we know so far? Frontiers in Molecular Neuroscience, 13, 604158. [DOI:10.3389/fnmol.2020.604158] [PMID]
Egaña-Huguet, J., Soria-Gómez, E., & Grandes, P. (2021). The Endocannabinoid System in glial cells and their profitable interactions to treat epilepsy: Evidence from animal models. International Journal of Molecular Sciences, 22(24), 13231. [DOI:10.3390/ijms222413231] [PMID]
Feng, H., Luo, J., Li, Z., Zhao, Y., Liu, Y., & Zhu, H. (2024). Valproic acid attenuates the severity of astrogliosis in the hippocampus of animal models of temporal lobe epilepsy. IBRO Neuroscience Reports, 17, 471–479. [DOI:10.1016/j.ibneur.2024.11.003] [PMID]
Han, Y., He, Y., Jin, X., Xie, J., Yu, P., & Gao, G., et al. (2023). CHIR99021 maintenance of the cell stemness by regulating cellular iron metabolism. Antioxidants (Basel), 12(2), 377. [DOI:10.3390/antiox12020377] [PMID]
Heuser, K., Szokol, K., & Taubøll, E. (2014). The role of glial cells in epilepsy. Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke, 134(1), 37–41. [DOI:10.4045/tidsskr.12.1344] [PMID]
Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E., & Gage, F. H. (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(47), 16659–16664. [DOI:10.1073/pnas.0407643101] [PMID]
Huang, C., Fu, X. H., Zhou, D., & Li, J. M. (2015). The Role of Wnt/β-Catenin Signaling pathway in disrupted hippocampal neurogenesis of temporal lobe epilepsy: A potential therapeutic target? Neurochemical Research, 40(7), 1319–1332. [DOI:10.1007/s11064-015-1614-1] [PMID]
Huang, L., Lai, X., Liang, X., Chen, J., Yang, Y., & Xu, W., et al. (2024). A promise for neuronal repair: Reprogramming astrocytes into neurons in vivo. Bioscience Reports, 44(1), BSR20231717. [DOI:10.1042/BSR20231717] [PMID]
Jarvis, R., Josephine Ng, S. F., Nathanson, A. J., Cardarelli, R. A., Abiraman, K., & Wade, F., et al. (2023). Direct activation of KCC2 arrests benzodiazepine refractory status epilepticus and limits the subsequent neuronal injury in mice. Cell Reports. Medicine, 4(3), 100957. [DOI:10.1016/j.xcrm.2023.100957] [PMID]
Kunz, W. S., Kudin, A. P., Vielhaber, S., Blümcke, I., Zuschratter, W., & Schramm, J., et al. (2000). Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Annals of Neurology, 48(5), 766–773. [PMID]
Li, D., Hérault, K., Zylbersztejn, K., Lauterbach, M. A., Guillon, M., & Oheim, M., et al. (2015). Astrocyte VAMP3 vesicles undergo Ca2+ -independent cycling and modulate glutamate transporter trafficking. The Journal of Physiology, 593(13), 2807–2832. [DOI:10.1113/JP270362] [PMID]
Liddelow, S. (2017). Purification and culture methods for astrocytes. Washington: Society of Neuroscience. [Link]
Liddelow, S. A., & Barres, B. A. (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity, 46(6), 957-967. [DOI:10.1016/j.immuni.2017.06.006] [PMID]
Macikova, I., Perzelova, A., Mraz, P., Bizik, I., & Steno, J. (2009). GFAP-positive astrocytes are rare or absent in primary adult human brain tissue cultures. Biologia, 64(4), 833-839. [DOI:10.2478/s11756-009-0136-1]
Mardones, M. D., Rostam, K. D., Nickerson, M. C., & Gupta, K. (2024). Canonical Wnt activator Chir99021 prevents epileptogenesis in the intrahippocampal kainate mouse model of temporal lobe epilepsy. Experimental Neurology, 376, 114767.[DOI:10.1016/j.expneurol.2024.114767] [PMID]
Moncion, C., West, P. J., & Metcalf, C. S. (2024). Wnt Signaling: The next step on the road to anti-epileptogenic therapies? Epilepsy Currents, 24(5), 355-357. [DOI:10.1177/15357597241280467] [PMID]
Perzelova, A., Macikova, I., Tardy, M., Mraz, P., Bizik, I., & Steno, J. (2007). Subpopulation of nestin positive glial precursor cells occur in primary adult human brain cultures. Biologia, 62(5), 633-640. [DOI:10.2478/s11756-007-0123-3]
Qu, Z., Su, F., Qi, X., Sun, J., Wang, H., & Qiao, Z., et al. (2017). Wnt/β-catenin signalling pathway mediated aberrant hippocampal neurogenesis in kainic acid-induced epilepsy. Cell Biochemistry and Function, 35(7), 472–476. [DOI:10.1002/cbf.3306] [PMID]
Rasband, M. N. (2016). Glial contributions to neural function and disease. Molecular & Cellular Proteomics: MCP, 15(2), 355–361. [DOI:10.1074/mcp.R115.053744] [PMID]
Rasti, M., Parniaei, A. H., Dehghani, L., Nasr Esfahani, S., Mirhendi, H., & Yazdani, V., et al. (2024). Enhancing the wound healing process through local injection of exosomes derived from blood serum: An in vitro and in vivo assessment. Regenerative Therapy, 26, 281–289. [DOI:10.1016/j.reth.2024.06.004] [PMID]
Rawat, K., Gautam, V., Sandhu, A., Bhatia, A., & Saha, L. (2023). Differential regulation of Wnt/β-catenin signaling in acute and chronic epilepsy in repeated low dose lithium-pilocarpine rat model of status epilepticus. Neuroscience, 535, 36-49. [DOI:10.1016/j.neuroscience.2023.10.019] [PMID]
Rich, S., Moradi Chameh, H., Lefebvre, J., & Valiante, T. A. (2022). Loss of neuronal heterogeneity in epileptogenic human tissue impairs network resilience to sudden changes in synchrony. Cell Reports, 39(8), 110863. [DOI:10.1016/j.celrep.2022.110863] [PMID]
Rosciszewski, G., Cadena, V., Auzmendi, J., Cieri, M. B., Lukin, J., & Rossi, A. R., et al. (2019). Detrimental effects of HMGB-1 require microglial-astroglial interaction: Implications for the status epilepticus -induced neuroinflammation. Frontiers in Cellular Neuroscience, 13, 380. [DOI:10.3389/fncel.2019.00380] [PMID]
Rujanapun, N., Heebkaew, N., Promjantuek, W., Sotthibundhu, A., Kunhorm, P., & Chaicharoenaudomrung, N., et al. (2019). Small molecules re-establish neural cell fate of human fibroblasts via autophagy activation. In Vitro Cellular & Developmental Biology. Animal, 55(8), 622–632. [DOI:10.1007/s11626-019-00381-0] [PMID]
Santos, J., Hubert, T., & Milthorpe, B. K. (2020). Valproic acid promotes early neural differentiation in adult mesenchymal stem cells through protein signalling pathways. Cells, 9(3), 619. [DOI:10.3390/cells9030619] [PMID]
Sapio, L., Gallo, M., Illiano, M., Chiosi, E., Naviglio, D., & Spina, A., et al. (2017). The Natural cAMP elevating compound forskolin in cancer therapy: Is it time? Journal of Cellular Physiology, 232(5), 922–927. [DOI:10.1002/jcp.25650] [PMID]
Schildge, S., Bohrer, C., Beck, K., & Schachtrup, C. (2013). Isolation and culture of mouse cortical astrocytes. Journal of Visualized Experiments: JoVE, (71), 50079. [DOI:10.3791/50079] [PMID]
Sharifi-Kelishadi, M., Zare, L., Fathollahi, Y., & Javan, M. (2024). Conversion of astrocyte cell lines to oligodendrocyte progenitor cells using small molecules and transplantation to animal model of multiple sclerosis. Journal of Molecular Neuroscience: MN, 74(2), 40. [DOI:10.1007/s12031-024-02206-6] [PMID]
Shen, W., Pristov, J. B., Nobili, P., & Nikolić, L. (2023). Can glial cells save neurons in epilepsy? Neural Regeneration Research, 18(7), 1417–1422. [DOI:10.4103/1673-5374.360281] [PMID]
Sokolova, T. V., Zabrodskaya, Y. M., Litovchenko, A. V., Paramonova, N. M., Kasumov, V. R., & Kravtsova, S. V., et al. (2022). Relationship between neuroglial apoptosis and neuroinflammation in the epileptic focus of the brain and in the blood of patients with drug-resistant epilepsy. International Journal of Molecular Sciences, 23(20), 12561. [DOI:10.3390/ijms232012561] [PMID]
Takeda, K., Watanabe, T., Oyabu, K., Tsukamoto, S., Oba, Y., & Nakano, T., et al. (2021). Valproic acid-exposed astrocytes impair inhibitory synapse formation and function. Scientific Reports, 11(1), 23. [DOI:10.1038/s41598-020-79520-7] [PMID]
Tan, Y. L., Yuan, Y., & Tian, L. (2020). Microglial regional heterogeneity and its role in the brain. Molecular Psychiatry, 25(2), 351–367. [DOI:10.1038/s41380-019-0609-8] [PMID]
Tavassoli, Z., Giahi, M., Janahmadi, M., & Hosseinmardi, N. (2022). Glial cells inhibition affects the incidence of metaplasticity in the hippocampus of Pentylentetrazole-induced kindled rats. Epilepsy & Behavior: E&B, 135, 108907. [DOI:10.1016/j.yebeh.2022.108907] [PMID]
Wang, B., Khan, S., Wang, P., Wang, X., Liu, Y., & Chen, J., et al. (2022). A Highly Selective GSK-3β Inhibitor CHIR99021 promotes osteogenesis by activating canonical and autophagy-mediated wnt signaling. Frontiers in Endocrinology, 13, 926622. [DOI:10.3389/fendo.2022.926622] [PMID]
Wang, C. C., Chen, P. S., Hsu, C. W., Wu, S. J., Lin, C. T., & Gean, P. W. (2012). Valproic acid mediates the synaptic excitatory/inhibitory balance through astrocytes--a preliminary study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 37(1), 111–120. [DOI:10.1016/j.pnpbp.2012.01.017] [PMID]
Westergard, T., & Rothstein, J. D. (2020). Astrocyte Diversity: Current insights and future directions. Neurochemical Research, 45(6), 1298–1305. [DOI:10.1007/s11064-020-02959-7] [PMID]
Wong, M. (2019). The role of glia in epilepsy, intellectual disability, and other neurodevelopmental disorders in tuberous sclerosis complex. Journal of Neurodevelopmental Disorders, 11(1), 30. [DOI:10.1186/s11689-019-9289-6] [PMID]
Yan, K., Gao, L. N., Cui, Y. L., Zhang, Y., & Zhou, X. (2016). The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (review). Molecular Medicine Reports, 13(5), 3715–3723.[DOI:10.3892/mmr.2016.5005] [PMID]
Yin, J. C., Zhang, L., Ma, N. X., Wang, Y., Lee, G., & Hou, X. Y., et al. (2019). Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Reports, 12(3), 488–501. [DOI:10.1016/j.stemcr.2019.01.003] [PMID]
Yu, I. T., Park, J. Y., Kim, S. H., Lee, J. S., Kim, Y. S., & Son, H. (2009). Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology, 56(2), 473-480. [DOI:10.1016/j.neuropharm.2008.09.019] [PMID]
Zhang, L., Yin, J. C., Yeh, H., Ma, N. X., Lee, G., & Chen, X. A., et al. (2015). Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell, 17(6), 735–747. [DOI:10.1016/j.stem.2015.09.012] [PMID]
4. Discussion
Astroglial cells make up around 30% of all cells in the adult mammalian central nervous system, making them the most prevalent cell type (Liddelow & Barres, 2017). These cells share early progenitor cells with neurons. They are highly likely to be converted into neurons in neurological disorders, where there is neuronal loss or a high number of astrocytes due to uncontrolled proliferation. Thus, inhibiting astrocyte proliferation, mainly astrogliosis, occurs in epilepsy and creates a chaotic environment; targeting the proliferation and viability of astrocytes could be a novel approach to managing MTLE (Chandrasekaran et al., 2016; Westergard & Rothstein, 2020). The present study demonstrates that specific SMs can reduce the viability of astrocytes in some epileptic patients. To arrive at a valid conclusion, we conducted viability assays on astrocytes isolated from different tissues (amygdala, hippocampus, and cortex) obtained from intractable epileptic patients individually. Notably, tissue heterogeneity, brain localization, and the timing of epilepsy onset and seizure duration may influence the cellular response to our intervention (Wong, 2019). However, the results of two studies have indicated that the differentiation of astrocytes derived from human fetal brain tissue and cell lines was taken into neurons (exposed to TTNPB, SB431542, LDN193189, Thiazovivin, CHIR99021, DAPT, VPA, SAG, purmophamine) and oligodendrocytes (exposed to CHIR99021, Forskolin, Repsox, LDN, VPA, and Thiazovivin), respectively, when exposed to SMs (Sharifi-Kelishadi et al., 2024; Zhang et al., 2015). This means SMs can regulate astrocyte molecular mechanisms. The difference between this study and our findings was that we used several tissues, while these two studies assessed their response on one cell type or cell line. The heterogeneity of cells was evident in our study; this might affect the response to SMs. Another difference was that the outcome of our study was viability for a maximum of 72 h, while those studies evaluated differentiation during 10 days. Additionally, we just used three SMs, while they used 9 SMs for neuronal differentiation and 6 SMs for oligodendrogenesis.
Recent studies indicate that WNT/β-catenin signaling is involved in neurogenesis and neuronal reorganization during epileptogenesis (Huang et al., 2015; Moncion et al., 2024; Qu et al., 2017). Rawat et al. (2023) used a lithium-pilocarpine model of status epilepsy and found that this signaling pathway was inactive during the acute phase but activated in the chronic stage. This activation may increase neuronal count, synaptic density, astrogliosis, and apoptosis in chronic epilepsy. The findings suggest that targeting GSK-3β could be beneficial in the acute stage, while addressing the chronic stage may require targeting β-catenin and disheveled proteins (Rawat et al., 2023). Recent studies show that VPA exerts anti-epileptic effects by modulating the excitatory/inhibitory (E/I) balance through astrocytes (Takeda et al., 2021). As a histone deacetylase (HDAC) inhibitor, VPA influences gene expression in astrocytes, affecting messenger ribonucleic acid levels of cell adhesion molecules and extracellular matrix proteins in a time- and concentration-dependent manner. VPA also increases levels of excitatory (PSD-95) and inhibitory (gephyrin) proteins in mixed cultures, indicating its role in regulating E/I balance through astrocytic mechanisms, which warrant further research on astroglial contributions to synapse formation (Wang et al., 2012). We found that the combination of CHIR and VPA reduces astrocyte viability, suggesting that these SMs may inhibit astrogliosis in the context of epilepsy.
Consistent with the present results, it has been reported that SMs could inhibit neuroinflammation by inhibiting proinflammatory cytokines. In this regard, SMs could inhibit the survival of astrocytes, which is a primary source of proinflammatory production (Dey et al., 2016).
We have found that specific drug/small molecule screenings are achievable using cells obtained from epileptic tissue. This discovery is a significant step forward in personalized medicine. The findings demonstrate that patient responses vary, and intriguingly, we observed varied reactions in terms of cell localization. The complexity of epilepsy is attributed to various mechanisms, such as the imbalance between inhibitory and excitatory neurotransmission, heightened inflammation, specific brain region neuronal damage, and irregularities in neuronal and cortical development. These mechanisms result in varied responses to pharmacological interventions (do Canto et al., 2021). For example, following the analysis of 57 temporal lobe epilepsy samples along with one control through histochemistry and electron microscopy, researchers identified considerable variation among the samples. Some patients experienced pronounced segmental loss in the hippocampus, while others showed moderate neuronal loss in the hippocampus, with a few not exhibiting any pathological changes whatsoever (Kunz et al., 2000).
Another crucial mechanism in epilepsy, recently highlighted, is biophysical heterogeneity. This phenomenon significantly reduces neuronal diversity within seizure-generating regions (Rich et al., 2022). Within this context, using SMs could boost neuronal diversity by stimulating the creation of new neurons in regions prone to seizures. In particular, compounds like retinoic acid, Notch inhibitors, WNT activators, and DNMT inhibitors can prompt the transformation of neural cells from human fibroblasts by triggering autophagy and reducing reactive oxygen species production (Rujanapun et al., 2019). Thus, SMs can inhibit astrogliosis while conversely having the potential to enhance neuronal production. This presents a novel approach in epilepsy for modifying cellular architecture and addressing neuronal heterogeneity. The primary limitation of this investigation was the limited sample size (n=7). Future research should aim to involve a larger cohort of participants to enhance the robustness and generalizability of the findings.
5. Conclusion
In summary, targeting the proliferation and viability of astrocyte cells represents a promising approach to managing MTLE. Our study, which involved viability assays on cells from different tissues obtained from intractable epileptic patients, highlights the importance of considering tissue heterogeneity, brain localization, and timing of epilepsy onset. Notably, patient responses vary, and the complexity of epilepsy involves multiple mechanisms. Finally, to better understand the effects of SMs, the following recommendations are suggested: 1) Conduct large-scale studies to analyze patient responses to different SMs, considering factors such as genetic variations, comorbidities, and lifestyle; 2) Investigate the synergistic effects of combining SMs with other therapeutic approaches, such as antiepileptic drugs, immunomodulators, or stem cell therapies; 3) Explore specific SMs that can selectively modulate glial cell viability without affecting neuronal health.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.REC.1400.056). Using resected patient tissue and the subsequent procedures were conducted followed by the Declaration of Helsink.
Funding
This study was financially supported by the Council for Development of Stem Cell Sciences and Technologies, Tehran, Iran (Grant No.: 104552). This study was supported by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 991796).
Authors' contributions
Conceptualization and supervision: Sajad Sahab Negah and Ali Gorji; Methodology and funding acquisition: Sajad Sahab Negah; Data collection: Javad Momeni; Data analysis: Sajad Sahab Negah, Ali Gorji, and Javad Momeni; Funding acquisition: Sajad Sahab Negah; Resources: Sajad Sahab Negah, Ali Gorji, Maryam Khaleghi Ghadiri, Fariborz Samini, and Alireza Tabibkhooei; Investigation and writing: All authors.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
The authors thank the Imam Reza Hospital and Khatam Alanbia Hospital patients for cooperating with this manuscript.
References
Almutawaa, W., Kang, N. H., Pan, Y., & Niles, L. P. (2014). Induction of neurotrophic and differentiation factors in neural stem cells by valproic acid. Basic & Clinical Pharmacology & Toxicology, 115(2), 216–221. [DOI:10.1111/bcpt.12201] [PMID]
Ammothumkandy, A., Ravina, K., Wolseley, V., Tartt, A. N., Yu, P. N., & Corona, L., et al. (2022). Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nature Neuroscience, 25(4), 493–503. [DOI:10.1038/s41593-022-01044-2] [PMID]
Chandrasekaran, A., Avci, H. X., Leist, M., Kobolák, J., & Dinnyés, A. (2016). Astrocyte differentiation of human pluripotent stem cells: New tools for neurological disorder research. Frontiers in Cellular Neuroscience, 10, 215. [DOI:10.3389/fncel.2016.00215] [PMID]
Cheng, L., Gao, L., Guan, W., Mao, J., Hu, W., & Qiu, B., et al. (2015). Direct conversion of astrocytes into neuronal cells by drug cocktail. Cell Research, 25(11), 1269–1272. [DOI:10.1038/cr.2015.120] [PMID]
Creighton, B. A., Ruffins, T. W., & Lorenzo, D. N. (2019). Visualizing and analyzing intracellular transport of organelles and other cargos in astrocytes. Journal of Visualized Experiments: JoVE, (150), 10.3791/60230.[DOI:10.3791/60230] [PMID]
Dey, A., Kang, X., Qiu, J., Du, Y., & Jiang, J. (2016). Anti-inflammatory small molecules to treat seizures and epilepsy: From bench to bedside. Trends in Pharmacological Sciences, 37(6), 463–484. [DOI:10.1016/j.tips.2016.03.001] [PMID]
Diaz Verdugo, C., Myren-Svelstad, S., Aydin, E., Van Hoeymissen, E., Deneubourg, C., & Vanderhaeghe, S., et al. (2019). Glia-neuron interactions underlie state transitions to generalized seizures. Nature Communications, 10(1), 3830. [DOI:10.1038/s41467-019-11739-z] [PMID]
do Canto, A. M., Donatti, A., Geraldis, J. C., Godoi, A. B., da Rosa, D. C., & Lopes-Cendes, I. (2021). Neuroproteomics in epilepsy: What do we know so far? Frontiers in Molecular Neuroscience, 13, 604158. [DOI:10.3389/fnmol.2020.604158] [PMID]
Egaña-Huguet, J., Soria-Gómez, E., & Grandes, P. (2021). The Endocannabinoid System in glial cells and their profitable interactions to treat epilepsy: Evidence from animal models. International Journal of Molecular Sciences, 22(24), 13231. [DOI:10.3390/ijms222413231] [PMID]
Feng, H., Luo, J., Li, Z., Zhao, Y., Liu, Y., & Zhu, H. (2024). Valproic acid attenuates the severity of astrogliosis in the hippocampus of animal models of temporal lobe epilepsy. IBRO Neuroscience Reports, 17, 471–479. [DOI:10.1016/j.ibneur.2024.11.003] [PMID]
Han, Y., He, Y., Jin, X., Xie, J., Yu, P., & Gao, G., et al. (2023). CHIR99021 maintenance of the cell stemness by regulating cellular iron metabolism. Antioxidants (Basel), 12(2), 377. [DOI:10.3390/antiox12020377] [PMID]
Heuser, K., Szokol, K., & Taubøll, E. (2014). The role of glial cells in epilepsy. Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, ny Raekke, 134(1), 37–41. [DOI:10.4045/tidsskr.12.1344] [PMID]
Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E., & Gage, F. H. (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(47), 16659–16664. [DOI:10.1073/pnas.0407643101] [PMID]
Huang, C., Fu, X. H., Zhou, D., & Li, J. M. (2015). The Role of Wnt/β-Catenin Signaling pathway in disrupted hippocampal neurogenesis of temporal lobe epilepsy: A potential therapeutic target? Neurochemical Research, 40(7), 1319–1332. [DOI:10.1007/s11064-015-1614-1] [PMID]
Huang, L., Lai, X., Liang, X., Chen, J., Yang, Y., & Xu, W., et al. (2024). A promise for neuronal repair: Reprogramming astrocytes into neurons in vivo. Bioscience Reports, 44(1), BSR20231717. [DOI:10.1042/BSR20231717] [PMID]
Jarvis, R., Josephine Ng, S. F., Nathanson, A. J., Cardarelli, R. A., Abiraman, K., & Wade, F., et al. (2023). Direct activation of KCC2 arrests benzodiazepine refractory status epilepticus and limits the subsequent neuronal injury in mice. Cell Reports. Medicine, 4(3), 100957. [DOI:10.1016/j.xcrm.2023.100957] [PMID]
Kunz, W. S., Kudin, A. P., Vielhaber, S., Blümcke, I., Zuschratter, W., & Schramm, J., et al. (2000). Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Annals of Neurology, 48(5), 766–773. [PMID]
Li, D., Hérault, K., Zylbersztejn, K., Lauterbach, M. A., Guillon, M., & Oheim, M., et al. (2015). Astrocyte VAMP3 vesicles undergo Ca2+ -independent cycling and modulate glutamate transporter trafficking. The Journal of Physiology, 593(13), 2807–2832. [DOI:10.1113/JP270362] [PMID]
Liddelow, S. (2017). Purification and culture methods for astrocytes. Washington: Society of Neuroscience. [Link]
Liddelow, S. A., & Barres, B. A. (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity, 46(6), 957-967. [DOI:10.1016/j.immuni.2017.06.006] [PMID]
Macikova, I., Perzelova, A., Mraz, P., Bizik, I., & Steno, J. (2009). GFAP-positive astrocytes are rare or absent in primary adult human brain tissue cultures. Biologia, 64(4), 833-839. [DOI:10.2478/s11756-009-0136-1]
Mardones, M. D., Rostam, K. D., Nickerson, M. C., & Gupta, K. (2024). Canonical Wnt activator Chir99021 prevents epileptogenesis in the intrahippocampal kainate mouse model of temporal lobe epilepsy. Experimental Neurology, 376, 114767.[DOI:10.1016/j.expneurol.2024.114767] [PMID]
Moncion, C., West, P. J., & Metcalf, C. S. (2024). Wnt Signaling: The next step on the road to anti-epileptogenic therapies? Epilepsy Currents, 24(5), 355-357. [DOI:10.1177/15357597241280467] [PMID]
Perzelova, A., Macikova, I., Tardy, M., Mraz, P., Bizik, I., & Steno, J. (2007). Subpopulation of nestin positive glial precursor cells occur in primary adult human brain cultures. Biologia, 62(5), 633-640. [DOI:10.2478/s11756-007-0123-3]
Qu, Z., Su, F., Qi, X., Sun, J., Wang, H., & Qiao, Z., et al. (2017). Wnt/β-catenin signalling pathway mediated aberrant hippocampal neurogenesis in kainic acid-induced epilepsy. Cell Biochemistry and Function, 35(7), 472–476. [DOI:10.1002/cbf.3306] [PMID]
Rasband, M. N. (2016). Glial contributions to neural function and disease. Molecular & Cellular Proteomics: MCP, 15(2), 355–361. [DOI:10.1074/mcp.R115.053744] [PMID]
Rasti, M., Parniaei, A. H., Dehghani, L., Nasr Esfahani, S., Mirhendi, H., & Yazdani, V., et al. (2024). Enhancing the wound healing process through local injection of exosomes derived from blood serum: An in vitro and in vivo assessment. Regenerative Therapy, 26, 281–289. [DOI:10.1016/j.reth.2024.06.004] [PMID]
Rawat, K., Gautam, V., Sandhu, A., Bhatia, A., & Saha, L. (2023). Differential regulation of Wnt/β-catenin signaling in acute and chronic epilepsy in repeated low dose lithium-pilocarpine rat model of status epilepticus. Neuroscience, 535, 36-49. [DOI:10.1016/j.neuroscience.2023.10.019] [PMID]
Rich, S., Moradi Chameh, H., Lefebvre, J., & Valiante, T. A. (2022). Loss of neuronal heterogeneity in epileptogenic human tissue impairs network resilience to sudden changes in synchrony. Cell Reports, 39(8), 110863. [DOI:10.1016/j.celrep.2022.110863] [PMID]
Rosciszewski, G., Cadena, V., Auzmendi, J., Cieri, M. B., Lukin, J., & Rossi, A. R., et al. (2019). Detrimental effects of HMGB-1 require microglial-astroglial interaction: Implications for the status epilepticus -induced neuroinflammation. Frontiers in Cellular Neuroscience, 13, 380. [DOI:10.3389/fncel.2019.00380] [PMID]
Rujanapun, N., Heebkaew, N., Promjantuek, W., Sotthibundhu, A., Kunhorm, P., & Chaicharoenaudomrung, N., et al. (2019). Small molecules re-establish neural cell fate of human fibroblasts via autophagy activation. In Vitro Cellular & Developmental Biology. Animal, 55(8), 622–632. [DOI:10.1007/s11626-019-00381-0] [PMID]
Santos, J., Hubert, T., & Milthorpe, B. K. (2020). Valproic acid promotes early neural differentiation in adult mesenchymal stem cells through protein signalling pathways. Cells, 9(3), 619. [DOI:10.3390/cells9030619] [PMID]
Sapio, L., Gallo, M., Illiano, M., Chiosi, E., Naviglio, D., & Spina, A., et al. (2017). The Natural cAMP elevating compound forskolin in cancer therapy: Is it time? Journal of Cellular Physiology, 232(5), 922–927. [DOI:10.1002/jcp.25650] [PMID]
Schildge, S., Bohrer, C., Beck, K., & Schachtrup, C. (2013). Isolation and culture of mouse cortical astrocytes. Journal of Visualized Experiments: JoVE, (71), 50079. [DOI:10.3791/50079] [PMID]
Sharifi-Kelishadi, M., Zare, L., Fathollahi, Y., & Javan, M. (2024). Conversion of astrocyte cell lines to oligodendrocyte progenitor cells using small molecules and transplantation to animal model of multiple sclerosis. Journal of Molecular Neuroscience: MN, 74(2), 40. [DOI:10.1007/s12031-024-02206-6] [PMID]
Shen, W., Pristov, J. B., Nobili, P., & Nikolić, L. (2023). Can glial cells save neurons in epilepsy? Neural Regeneration Research, 18(7), 1417–1422. [DOI:10.4103/1673-5374.360281] [PMID]
Sokolova, T. V., Zabrodskaya, Y. M., Litovchenko, A. V., Paramonova, N. M., Kasumov, V. R., & Kravtsova, S. V., et al. (2022). Relationship between neuroglial apoptosis and neuroinflammation in the epileptic focus of the brain and in the blood of patients with drug-resistant epilepsy. International Journal of Molecular Sciences, 23(20), 12561. [DOI:10.3390/ijms232012561] [PMID]
Takeda, K., Watanabe, T., Oyabu, K., Tsukamoto, S., Oba, Y., & Nakano, T., et al. (2021). Valproic acid-exposed astrocytes impair inhibitory synapse formation and function. Scientific Reports, 11(1), 23. [DOI:10.1038/s41598-020-79520-7] [PMID]
Tan, Y. L., Yuan, Y., & Tian, L. (2020). Microglial regional heterogeneity and its role in the brain. Molecular Psychiatry, 25(2), 351–367. [DOI:10.1038/s41380-019-0609-8] [PMID]
Tavassoli, Z., Giahi, M., Janahmadi, M., & Hosseinmardi, N. (2022). Glial cells inhibition affects the incidence of metaplasticity in the hippocampus of Pentylentetrazole-induced kindled rats. Epilepsy & Behavior: E&B, 135, 108907. [DOI:10.1016/j.yebeh.2022.108907] [PMID]
Wang, B., Khan, S., Wang, P., Wang, X., Liu, Y., & Chen, J., et al. (2022). A Highly Selective GSK-3β Inhibitor CHIR99021 promotes osteogenesis by activating canonical and autophagy-mediated wnt signaling. Frontiers in Endocrinology, 13, 926622. [DOI:10.3389/fendo.2022.926622] [PMID]
Wang, C. C., Chen, P. S., Hsu, C. W., Wu, S. J., Lin, C. T., & Gean, P. W. (2012). Valproic acid mediates the synaptic excitatory/inhibitory balance through astrocytes--a preliminary study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 37(1), 111–120. [DOI:10.1016/j.pnpbp.2012.01.017] [PMID]
Westergard, T., & Rothstein, J. D. (2020). Astrocyte Diversity: Current insights and future directions. Neurochemical Research, 45(6), 1298–1305. [DOI:10.1007/s11064-020-02959-7] [PMID]
Wong, M. (2019). The role of glia in epilepsy, intellectual disability, and other neurodevelopmental disorders in tuberous sclerosis complex. Journal of Neurodevelopmental Disorders, 11(1), 30. [DOI:10.1186/s11689-019-9289-6] [PMID]
Yan, K., Gao, L. N., Cui, Y. L., Zhang, Y., & Zhou, X. (2016). The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (review). Molecular Medicine Reports, 13(5), 3715–3723.[DOI:10.3892/mmr.2016.5005] [PMID]
Yin, J. C., Zhang, L., Ma, N. X., Wang, Y., Lee, G., & Hou, X. Y., et al. (2019). Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Reports, 12(3), 488–501. [DOI:10.1016/j.stemcr.2019.01.003] [PMID]
Yu, I. T., Park, J. Y., Kim, S. H., Lee, J. S., Kim, Y. S., & Son, H. (2009). Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology, 56(2), 473-480. [DOI:10.1016/j.neuropharm.2008.09.019] [PMID]
Zhang, L., Yin, J. C., Yeh, H., Ma, N. X., Lee, G., & Chen, X. A., et al. (2015). Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell, 17(6), 735–747. [DOI:10.1016/j.stem.2015.09.012] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2024/12/14 | Accepted: 2025/01/22 | Published: 2025/07/1
Received: 2024/12/14 | Accepted: 2025/01/22 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |