Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 533-550 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Balali M R, Hosseini M, Khanboloki M, Mohammadi M, Khorshidvand A M, Taghi Joghataei M. Pathways Mediating Blindsight After V1 Injury: A Systematic Review of Human and Monkey Studies. BCN 2025; 16 (3) :533-550
URL: http://bcn.iums.ac.ir/article-1-3057-en.html
URL: http://bcn.iums.ac.ir/article-1-3057-en.html
Mohammad Reza Balali1 

 , Mohaddeseh Hosseini1
, Mohaddeseh Hosseini1 

 , Maryam Khanboloki1
, Maryam Khanboloki1 

 , Mohammad Mohammadi1
, Mohammad Mohammadi1 

 , Amir Mohammad Khorshidvand *1
, Amir Mohammad Khorshidvand *1 

 , Mohammad Taghi Joghataei2
, Mohammad Taghi Joghataei2 




 , Mohaddeseh Hosseini1
, Mohaddeseh Hosseini1 

 , Maryam Khanboloki1
, Maryam Khanboloki1 

 , Mohammad Mohammadi1
, Mohammad Mohammadi1 

 , Amir Mohammad Khorshidvand *1
, Amir Mohammad Khorshidvand *1 

 , Mohammad Taghi Joghataei2
, Mohammad Taghi Joghataei2 


1- Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Anatomy, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Anatomy, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 927 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
After specific receptors in the eye trigger the sensation, visual data travel through a pathway to the lateral geniculate nucleus (LGN) in the thalamus, then to the visual area (V1) and higher cortical regions. This main pathway is known as the reticulogeniculostriate pathway. Damage to area V1 interrupts this pathway, resulting in the loss of “conscious vision” (Celesia, 2010). However, extensive research has shown that some patients retain the ability to respond to stimuli within their scotoma-localized area of diminished vision, even if not consciously, a phenomenon known as blindsight (Weiskrantz et al., 1974). For example, studies have shown a wide range of residual functions, including shape discrimination, object recognition (Trevethan et al., 2007; Van den Stock et al., 2015; Van den Stock et al., 2014; Weiskrantz, 1987), color perception (Kentridge et al., 2007; Morland et al., 1999), recognition of emotions (Bertini et al., 2013; Gerbella et al., 2019; Pegna et al., 2005; Van den Stock et al., 2011), manual localization, actions towards or spontaneous anti-pointing of unseen targets (de Gelder et al., 2008; Smits et al., 2019), processing gaze direction (Burra et al., 2013), and movement detection (Grasso et al., 2020; Hervais-Adelman et al., 2015) when the examiner applies pressure. In this context, the ability to perceive emotions unconsciously is referred to as affective blindsight, while other types are termed non-affective. The question here is which path or pathways in the brain can be attributed to blindsight. Various hypotheses and ideas have been proposed to explain the blindsight phenomenon.
A group of studies believes that area V1 is not obliterated after damage and that the small remaining islands continue to function. These islands are not large enough to elicit conscious vision, but they are sufficient to allow a person to respond to a stimulus unconsciously (Kalat, 2015; Radoeva et al., 2008). For example, through functional magnetic resonance imaging (MRI) (fMRI) studies of a patient, it was found that the patient could unconsciously perceive movements through the tiny islands left in his V1 area (Morland et al., 2004). Some researchers have also claimed a role for the remaining V1 islands in blindsight (Papanikolaou et al., 2019). Contrary to this hypothesis, some individuals still remain unconsciously aware of their vision despite the complete loss of the V1 area (Morland et al., 2004; Papanikolaou et al., 2019; Radoeva et al., 2008; Tran et al., 2019), questioning the sufficiency of V1 islands in mediating blindsight.
The second group claims that the LGN is central to this phenomenon. Two pathways extend from LGN to higher brain areas: One transmits information to V1, or striate cortex (the striate cortical area responsible for processing visual information), and the other bypasses V1, sending information directly to the extrastriate cortex. These articles highlight the role of LGN-extrastriate pathways in the emergence of blindsight (Schmid et al., 2010). A study on a person with bilateral V1 damage showed that pathways from the LGN to the middle temporal (MT) region facilitate motion detection (Ajina & Bridge, 2019).
The last group of researchers considered the role of the superior colliculus (SC) in the emergence of blindsight. They believe that pathways passing through this area, which transfer information directly from the eye to regions above V1, play a prominent role. After surgical removal of the V1 area in two monkeys, Kato et al. (2011) demonstrated the role of retinotectal pathways through SC in blindsight. However, another study that used artificial induction of blindsight through transcranial magnetic stimulation (TMS), rejected this role (Allen et al., 2014).
The phenomenon of blindsight has been extensively examined by neuroscientists for decades, resulting in a substantial body of research. Each article analyzes the topic from diverse perspectives, utilizing distinct methodologies. Considering methodological, chronological, and technical disparities, essential components must be identified and conclusions formulated accordingly. Thus, conducting a systematic review in this domain is necessary to direct future research toward these pathways, thereby enhancing our understanding and developing rehabilitation treatments for individuals experiencing V1 injury.
2. Materials and Methods
A systematic review of published studies up to August 10, 2024, was conducted. No language limit was considered. The inclusion criteria required studies to be original research articles that demonstrated precise methodology and robust evidence of pathway activation in blindsight. Due to the anatomical and functional similarities in the visual systems, only studies on humans and monkeys were included to provide a comprehensive understanding of the blindsight pathways. Research on monkeys enables more controlled experimental conditions, allowing for the observation of V1 damage effects in ways that are not ethically or practically feasible in human studies. This strengthens our understanding of how these pathways operate across species, ultimately supporting the translational potential of these findings in clinical settings.
The exclusion criteria involved studies in which the damage to the V1 region was ambiguous, and the proposed pathways of blindsight were not mentioned or, if mentioned, did not provide significant evidence. We excluded cases of hemispherectomy due to the inability to examine the interaction between both hemispheres and accurately trace compensatory pathways in this phenomenon. These criteria aimed to ensure repeatability and clarity in data selection, enhancing the rigor of this systematic review.
Search in database, screening, and data extraction
We searched online databases, such as PubMed, Scopus, Web of Science, and Embase, to extract all relevant articles. The research syntax is provided in the Supplementary Material.
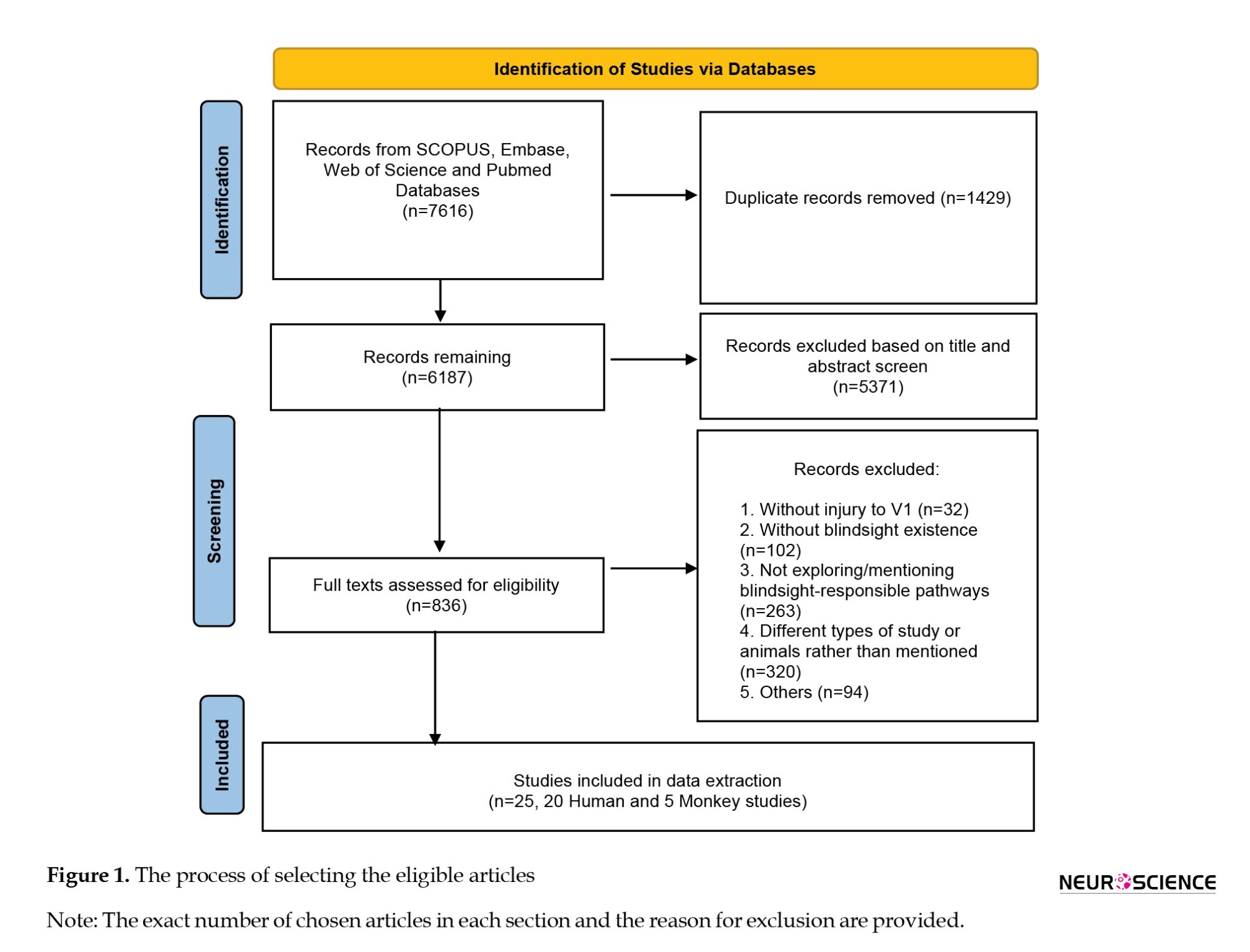
In the first step, articles were assessed based on their titles, and those with irrelevant titles were excluded. In the second step, the abstracts of the chosen articles were reviewed. Then, full texts of approved articles were reviewed, selecting studies on humans or monkeys that mentioned pathways facilitating blindsight. After reading the full texts, we used the JBI critical appraisal checklist for human studies (Moola et al., 2019; Munn et al., 2020) and the SYRCLE tool (Hooijmans et al., 2014) for animal studies to assess the risk. Two authors independently evaluated studies, resolving disagreements through discussion and consensus. The primary search retrieved 7616 articles from the databases, 1429 of which were identified as duplicates. After reviewing the titles and abstracts, 836 articles were selected for full-text analysis. Based on the inclusion criteria, 25 articles were selected for data extraction. Figure 1 shows the process of selecting eligible articles.
The reported data included publication year and country, first author’s name, study type, age, sex, and number of cases/controls, V1 injury mechanism, injury onset age, time elapsed since injury, injury location, type of task, and stimuli used to assess blindsight existence and its responsible pathways, imaging tool, and proposed pathways mediating blindsight. Two reviewers independently extracted data using pre-structured data sheets. Tables 1-6 present the data.
Quality assessment
The JBI critical appraisal checklist was used to assess the quality of human studies, and the SYRCLE risk assessment tool was used for animal studies. Two authors independently evaluated all the studies. All differences of opinion were settled by discussion and mutual agreement. The case-control studies (n=9) were assessed based on 10 criteria for study design, participant selection, exposure and outcome measurement, and statistical analyses. Studies with a high risk of bias in two or more domains were considered to have an overall high risk of bias. Overall, the quality assessment revealed that most case-control studies had a low or unclear risk of bias, indicating good study design and conduct. Although the quality of reporting in case-control studies was good, some studies did not adequately consider confounding factors or employ appropriate grouping techniques. Ten case reports and the only case series were evaluated on eight criteria for clear, detailed patient descriptions and presentations. Most studies were assessed as low-risk and did not have significant issues. Animal studies (n=5) were evaluated using the SYRCLE risk assessment tool, which assessed 10 criteria related to study design, grouping, exposure, and outcome measurements. Overall, the quality assessment of experimental studies revealed that most had a moderate risk of bias.
3. Results
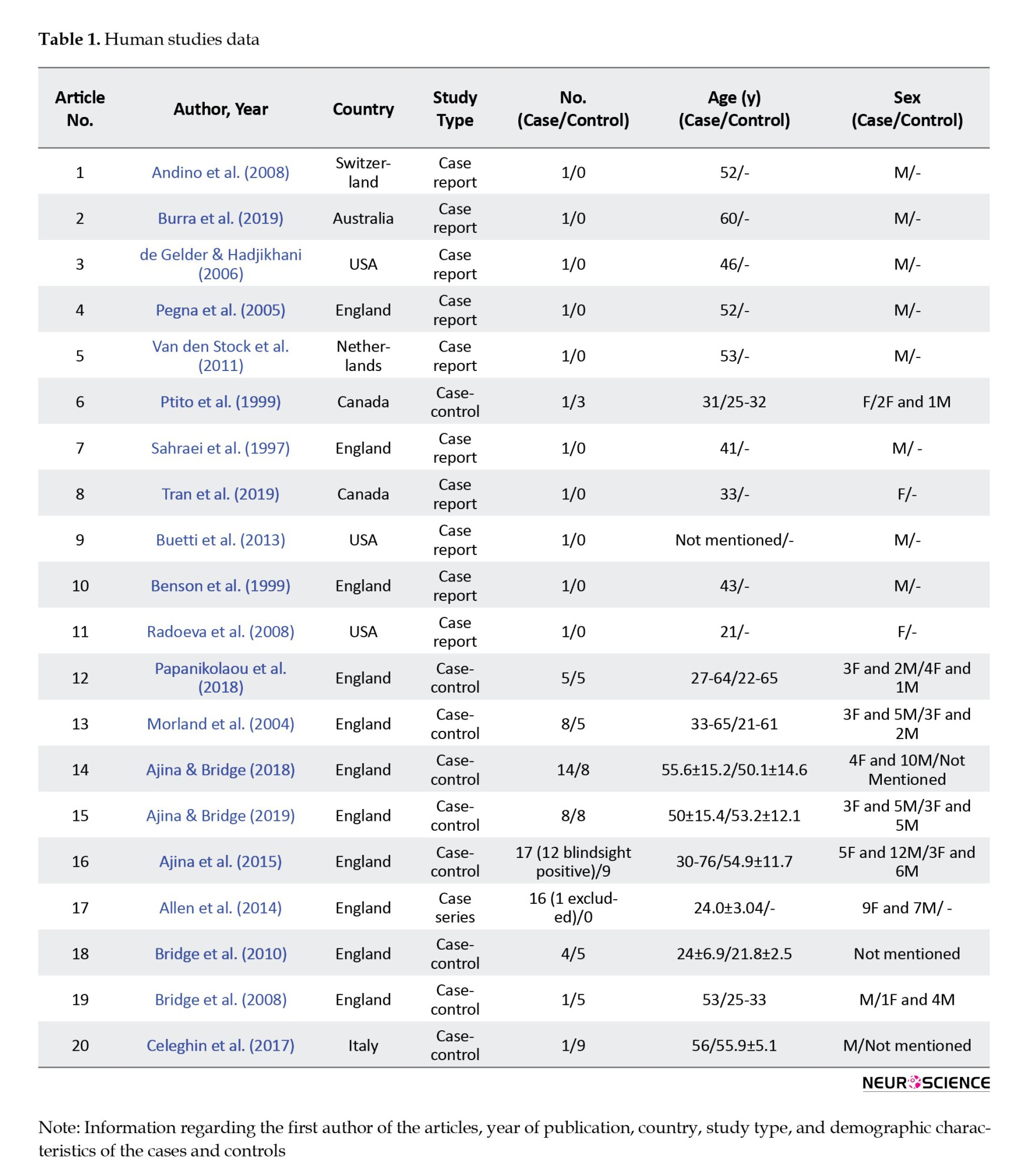
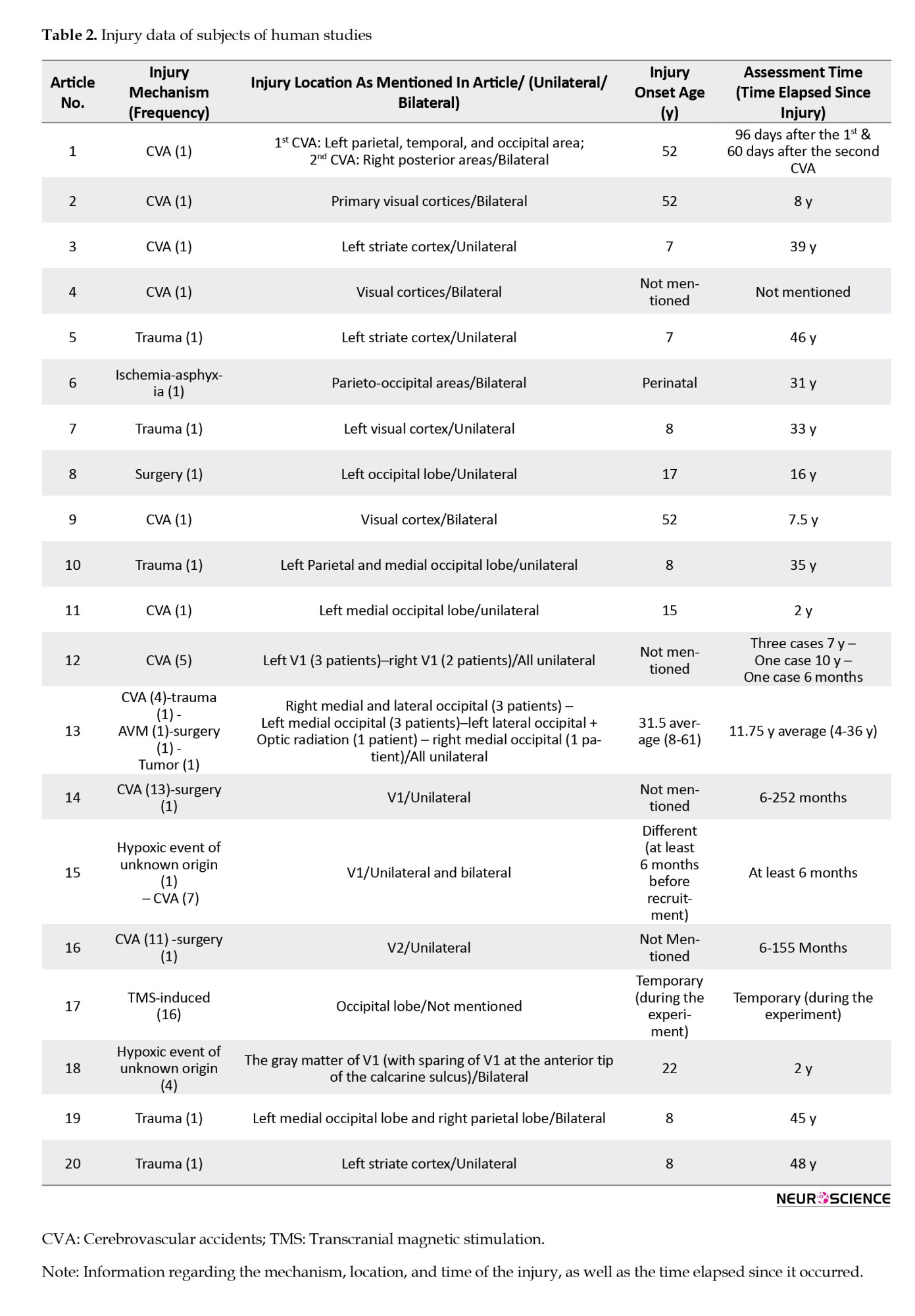
We assessed 25 articles, including 20 human and five monkey studies. Five of the 20 human studies focused on affective blindsight and 15 on non-affective blindsight. Among all human studies, 10 were case reports, nine were case-control studies, and one was a case series (Table 1). The mechanisms by which area V1 was injured or inactivated are as follows: 46 patients had V1 injury due to cerebrovascular accidents (CVA), six had trauma, four had undergone surgery, one had an arteriovenous malformation, one experienced ischemia due to asphyxia, one had a tumor, and five had lesions due to hypoxia of unknown origin. Additionally, there were 16 cases of TMS-induced V1 inactivation (Table 2).
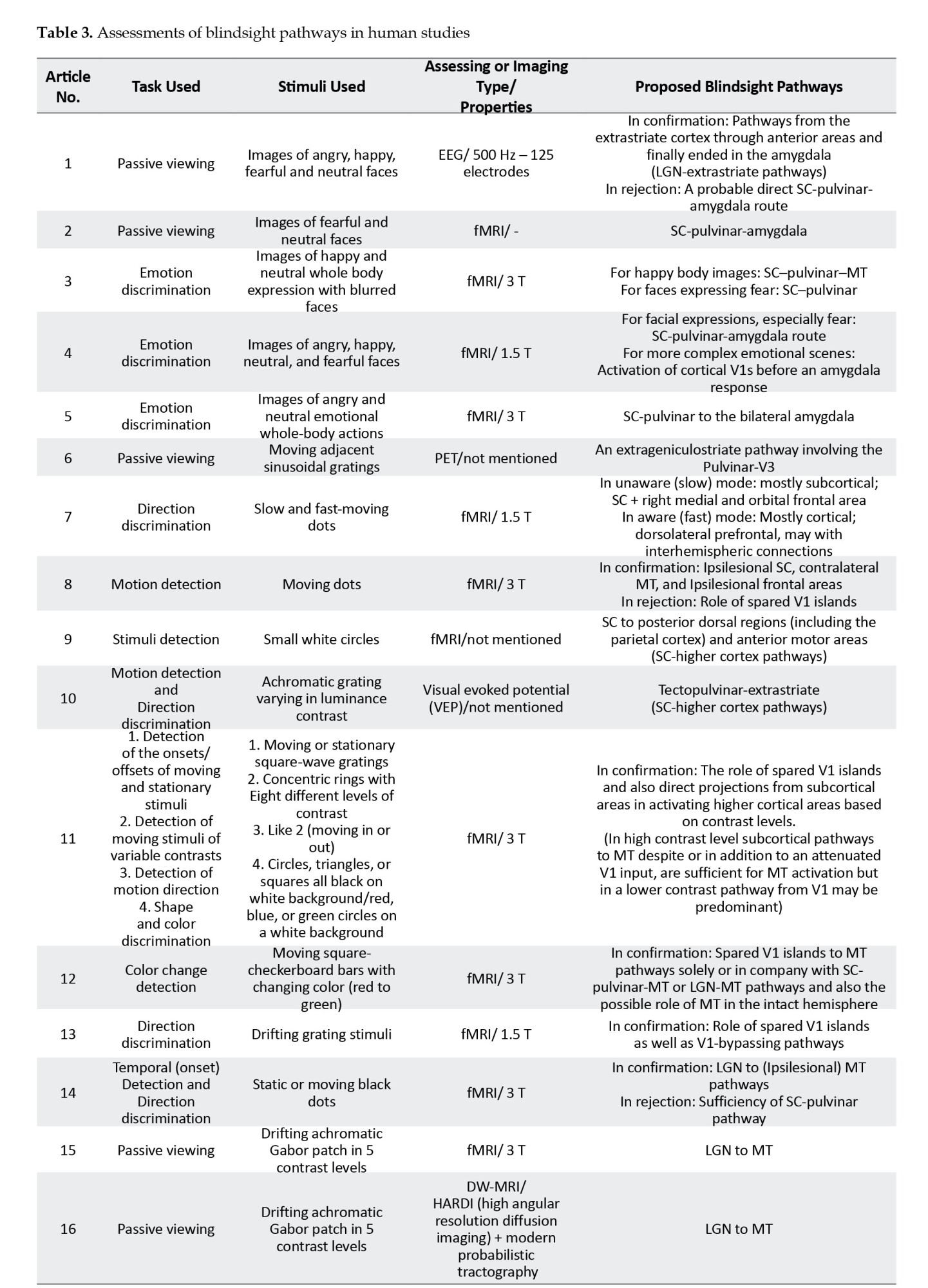
To categorize the tasks used to detect the stimuli in the cases, the articles were divided into the following categories: Seven articles used passive viewing, three used emotion discrimination, five used direction discrimination, five used stimulus (onset) detection, and two used color discrimination to examine the blindsight-mediating pathways. Regarding the imaging tools used, 14 studies chose fMRI to identify pathways; two used electroencephalography (EEG) and visual evoked potential, one used PET, and two used diffusion-weighted MRI (DW-MRI) (Table 3).
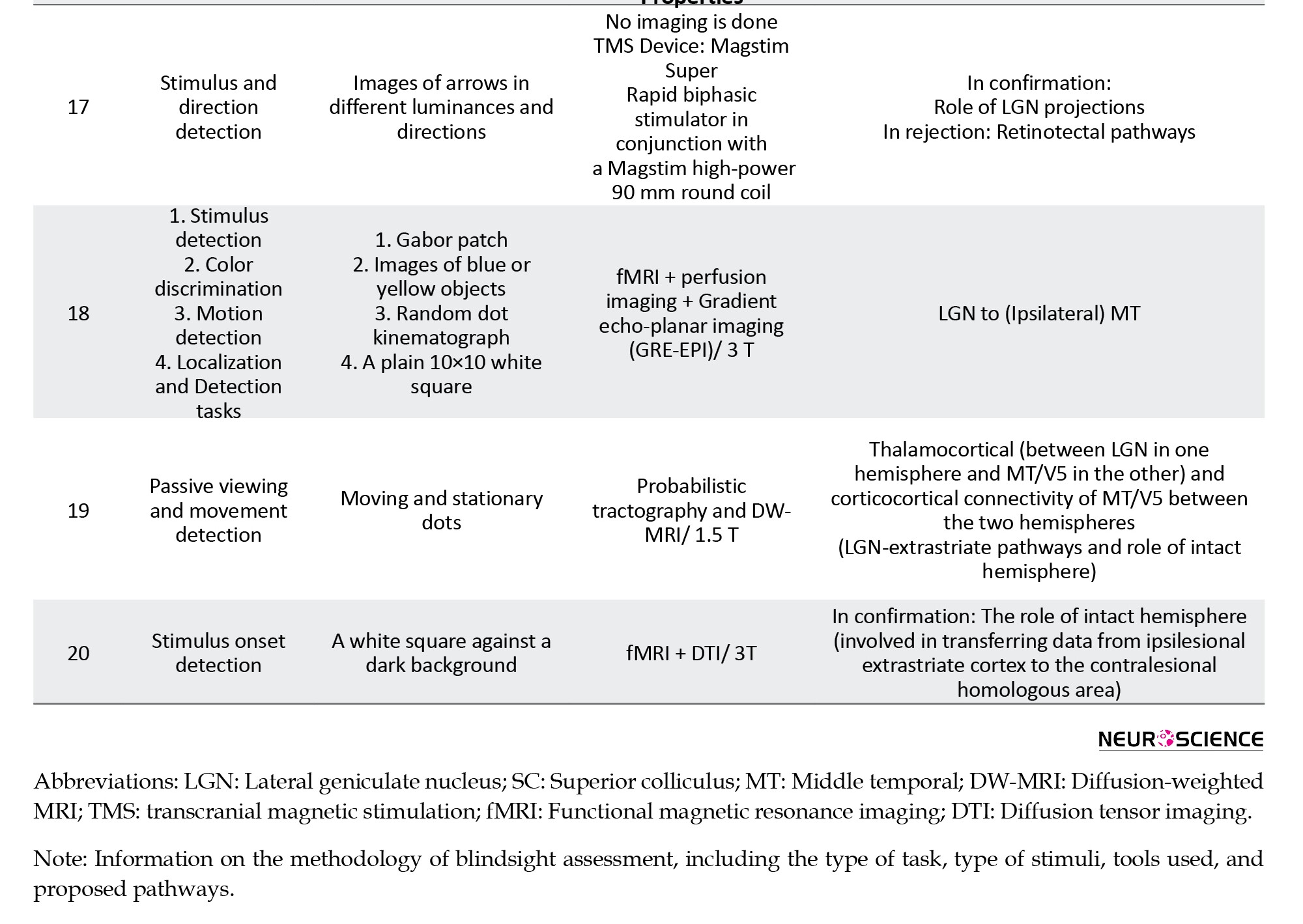
There were also five monkey studies, with eight monkeys participating (Table 4).
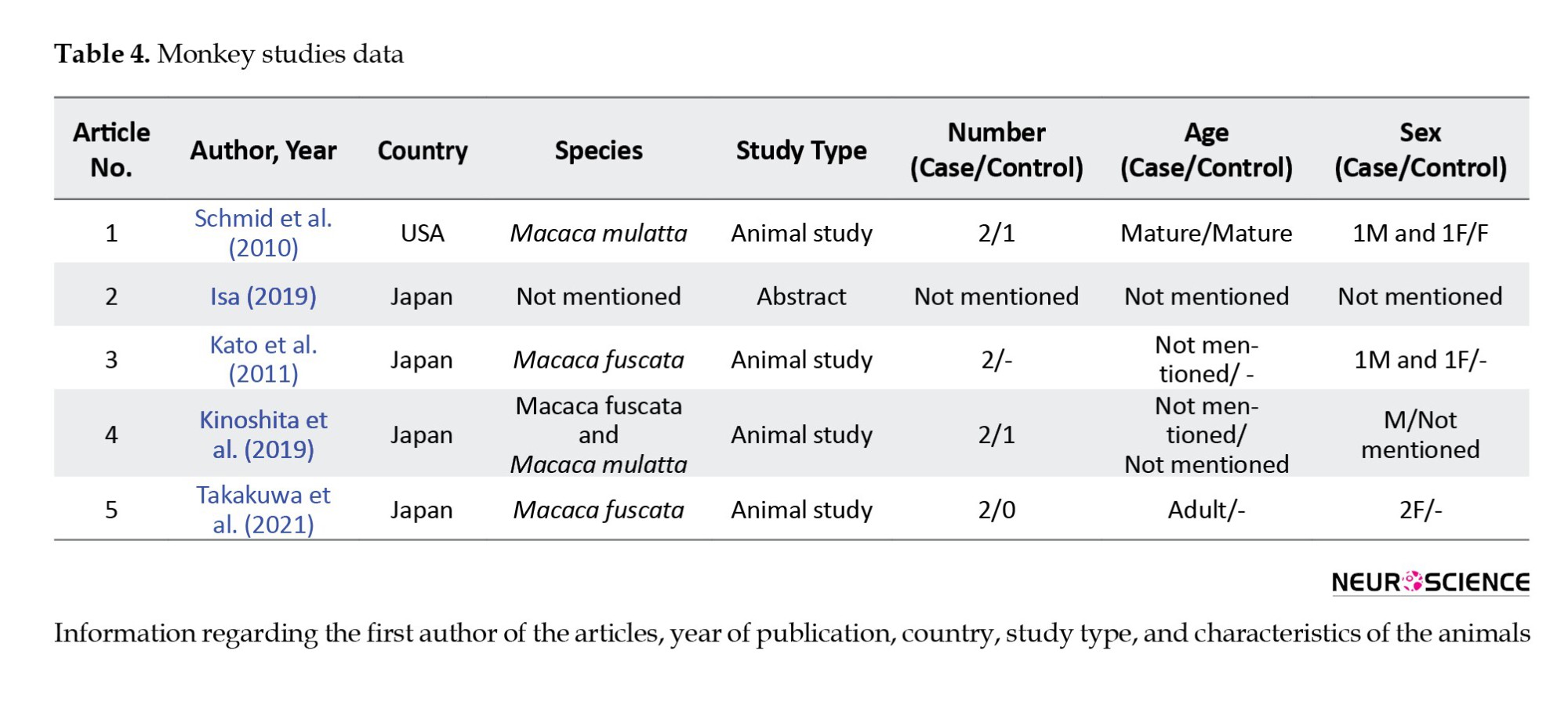
The injury mechanism in four of these studies was surgical removal of the V1 area by aspiration. All injuries were unilateral. One article did not mention the number of cases and injury mechanisms (Table 5).
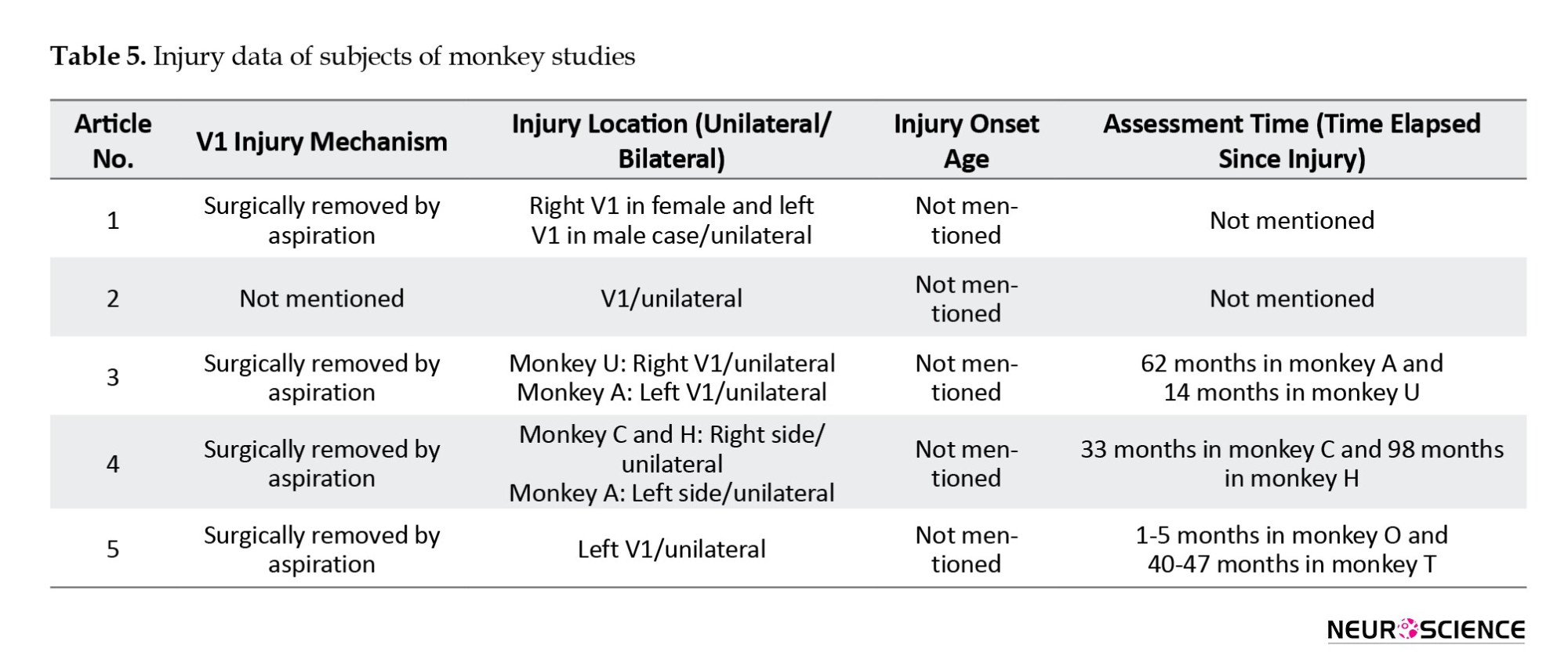
The task involved stimulation detection through visually guided saccades. Each study used the inactivation of different brain parts to assess the effects on behavior; one study also employed fMRI for pathway assessment (Table 6).
Main findings
Human studies
Four articles supported that the SC-pulvinar-amygdala pathway facilitates emotion detection in affective blindsight. Among those, three studies used emotion discrimination, and one used a passive viewing task. Pegna et al. (2005) showed that facial expressions, especially fear, are processed via this route, as evidenced by increased activation in the right amygdala. The role of higher cortical areas was also emphasized in more complex emotional processing before it reaches the amygdala. However, another study involving a single patient who performed a passive viewing task and was evaluated using EEG presented different results. The results of this study question the existence of a direct SC-pulvinar-amygdala route. Similar to previous research on more complex emotions, this study suggests an indirect pathway that transits from the extrastriate cortex through anterior areas and ultimately terminates in the amygdala (Andino et al., 2009).
In cases of non-affective blindsight, three studies supported the idea that blindsight occurs due to the function of spared islands of V1. However, all these studies also found V1 bypassing pathways that sometimes reached the MT. The tasks performed by the patients were as follows: One-directional discrimination, one-color change detection, one-stimulus and direction detection along with shape and color discrimination, and detection of moving stimuli with variable contrast levels. All these studies used fMRI to determine the pathways.
Six articles supported the pathway from the LGN to the MT (in both the injured and non-injured hemispheres), with 55 cases. The tasks were as follows: Two studies used stimulus and direction detection, one study used both passive viewing and movement detection, one study combined stimulus and motion detection with color discrimination and localization tasks, and two studies involved passive viewing. The tools used were as follows: Three studies used fMRI, two used DW-MRI, and one used TMS.
A study demonstrated that in addition to the ipsilesional pathway from LGN to MT, which was similarly observed in control cases, two other projections also exist in G.Y.: a contralateral pathway from right LGN to left MT/V5 and a projection from the MT of the intact hemisphere to the MT of the injured hemisphere, created through interhemispheric connections (Bridge et al., 2008). The study by Tran et al. (2019) also pointed to the pathway that passes through hemispheres from ipsilesional SC to contralateral MT.
Five articles suggested that higher cortical areas play a significant role in mediating blindsight. For example, Tran et al. (2019) showed that in motion detection, the pathway passes from the ipsilesional SC to the contralateral MT and ipsilesional frontal area, suggesting a robust alternative route for visual processing. By incorporating the variable of awareness into the investigation of pathways leading to blindsight, Sahraie et al. (1997) demonstrated that higher cortical regions can effectively create the phenomenon of blindsight. Buetti et al. (2013) showed that in the stimulus detection task, the V1-bypassing pathway extends from the SC to the posterior dorsal areas. In the study by Ptito et al. (1999), patients performed a passive viewing task. Positron emission tomography (PET) showed that the data passed from the pulvinar to the extrastriate cortex. Benson et al. (1999) performed two tasks: Motion detection and direction discrimination. These results suggest that the pathway from tectopulvinar to extrastriate areas serves as a mediating pathway in blindsight.
Monkey studies
One article emphasized the role of the LGN in mediating blindsight through extrastriate pathways. In a study by Schmid et al. (2010), monkeys’ brains were analyzed using fMRI during a detection task involving rotating checkerboard stimuli. The results showed that in monkeys with V1 damage, visual data passed through the LGN to the extrastriate pathway.
Four studies highlighted the importance of the SC-pulvinar pathway. In a survey by Kato et al. (2011), V1 was surgically removed by aspiration, and a visual detection task was conducted while fMRI images were obtained from the monkeys’ brains. The researchers identified two key pathways responsible for blindsight ability: The SC-to-pulvinar and the SC-to-LGN pathways. The study rejects the notion that the LGN plays a sole role in blindsight. In another study Isa (2019) claimed that the main pathway for blindsight is the SC-pulvinar-extrastriate pathway. In a study by Kinoshita et al. (2019), the monkeys’ V1 areas were aspirated, and several months later, their brains were assessed histologically. The researchers recommended that the SC-to-pulvinar pathway is responsible for blindsight. Takakuwa et al. (2021) claimed that the SC and pulvinar areas play a significant role in mediating blindsight, as the pathway from the SC to the cortical areas through the pulvinar is the primary pathway of blindsight. This study also claimed that the LGN is an essential part of the blindsight-mediating pathways.
4. Discussion
Blindsight continues to challenge traditional views on how vision is processed in the brain, particularly following damage to the primary visual cortex. This discussion integrates recent findings, emphasizes discrepancies among studies, proposes hypotheses to explain these discrepancies, and critically evaluates the studies’ methodologies to enhance our understanding of the pathways underlying blindsight.
Review of evidence regarding different blindsight pathways
The spared visual (V1) islands
The hypothesis that small, spared islands within the damaged V1 area contribute to blindsight is widely debated. Three studies support the role of spared V1 islands in blindsight (Morland et al., 2004; Papanikolaou et al., 2019; Radoeva et al., 2008). Two studies suggests that these islands enable unconscious visual processing, using high-resolution fMRI to identify active V1 tissue (Morland et al., 2004; Papanikolaou et al., 2019). A potential weakness is the assumption that fMRI signals equate to functional processing, which may not always be accurate. Conversely, de Gelder et al. (2008). and Tran et al. (2019) provided evidence that blindsight can occur with complete V1 damage, questioning the necessity of V1 islands for residual visual function. The former used behavioral tests and anatomical imaging to demonstrate complete V1 damage, though minimal functional V1 tissue may still exist (de Gelder et al., 2008). Different imaging modalities could introduce inconsistencies in detecting small functional areas.
A hypothesis that might reconcile these findings is that while spared V1 islands can enhance certain types of blindsight, their presence is not strictly necessary, and other neural mechanisms can effectively compensate for their absence. For example, in one study, the pathways responsible for blindsight were categorized based on the contrast of stimuli, showing that subcortical pathways ending in MT are the primary pathways for recognizing a high-contrast stimulus in the impaired visual field. In contrast, V1 is more efficient in recognizing low-contrast stimuli (Radoeva et al., 2008).
The LGN-extrastriate pathway
The LGN to extrastriate cortex (also known as the geniculoextrastriate) pathway is increasingly recognized as crucial for blindsight. Studies have shown that direct pathways from the LGN to the MT are vital for the unconscious detection of movement in the damaged visual field (Ajina & Bridge, 2018, Ajina & Bridge, 2019; Ajina et al., 2015; Allen et al., 2014; Bridge et al., 2010, Bridge et al., 2008). One study demonstrated this pathway’s role using advanced neuroimaging, showing that direct LGN-MT connections are critical for motion detection in the absence of V1 (Ajina et al., 2015). Another study used diffusion weighted imaging (DWI) and probabilistic tractography to map the LGN-MT pathways, providing clear anatomical evidence (Ajina & Bridge, 2019). Schmid et al. (2010) validated these findings with precise lesion techniques and behavioral testing in monkeys, offering clear causal evidence. However, studies on non-human primates may not be directly applicable to humans.
Although the experiment conducted by Allen et al. (2014) using TMS-induced blindsight primarily favored the dominance of LGN-based pathways, it also indicated that the existence of other pathways, including tectopulvinar pathways, in creating blindsight is entirely plausible, suggesting that additional structures may be crucial. This argument is strengthened by the fact that TMS’s transient effects might not fully replicate chronic V1 damage. Functional redundancy in the visual system ensures robust visual processing even when primary routes are compromised. Therefore, this discrepancy suggests that the LGN-extrastriate pathway is vital but works alongside other pathways, such as those containing the pulvinar and SC, to support blindsight.
The SC and pulvinar contributions
According to this hypothesis, pathways passing through the SC to the pulvinar, transferring information directly from the eye to regions above V1, play a significant role in the emergence of blindsight (Benson et al., 1999; Buetti et al., 2013; Ptito et al., 1999; Tran et al., 2019). Research by Kato et al. (2011) and Isa (2019) underscores the significance of SC-based pathways in rerouting visual information after V1 damage. Kato et al. (2011) conducted lesion studies in monkeys, using behavioral assessments and fMRI to provide strong evidence for these pathways. However, generalizability to humans is uncertain due to species differences, and behavioral tasks in monkeys may not fully capture the complexities of human visual processing. Isa (2019) used reversible inactivation of the SC-pulvinar pathway in monkeys to show its role in blindsight, offering robust causal evidence. However, reversibility may not perfectly simulate permanent human lesions. Conversely, Ajina and Bridge (2018) argued that the SC-pulvinar pathway alone is insufficient, emphasizing the need for LGN-extrastriate cortex connectivity. They combined human neuroimaging and case studies to provide a comprehensive overview of the pathway, though reliance on correlational data limits the inference of causality. A methodological question is whether these imaging techniques detect all relevant subcortical activities. A hypothesis to reconcile these findings is that SC and pulvinar pathways act synergistically with the LGN-extrastriate route, collectively supporting various aspects of blindsight. Individual differences in brain architecture and the nature of visual tasks performed could determine the relative contributions of these pathways.
The intact hemisphere
The potential involvement of the intact hemisphere in compensating for damage to V1 has been the subject of considerable debate. Ptito et al. (1999) found that activation of extrageniculostriate pathways after damage to area V1 suggests the involvement of interhemispheric pathways. Some researchers concluded that the connection between the two hemispheres is a fundamental component in compensating for damage to V1 (Bridge et al., 2008; Celeghin et al., 2017; Papanikolaou et al., 2019; Tran et al., 2019). Two studies suggested that increased connectivity between the intact and damaged hemispheres contributes to blindsight (Bridge et al., 2008; Celeghin et al., 2017). The former used DWI and functional connectivity analyses to show increased interhemispheric connections, providing strong anatomical and functional evidence (Bridge et al., 2008). A potential question is whether the observed connectivity changes directly result from V1 damage or pre-existing conditions. The latter employed detailed case studies with advanced neuroimaging, offering a nuanced view of the intact hemisphere’s role. However, the small sample size of the case studies limits their generalizability. On the other hand, some articles believe that the healthy hemisphere may not play a role in creating blindsight (Ajina et al., 2015; Bridge et al., 2010; Buetti et al., 2013). One study downplayed the intact hemisphere's role and by emphasizing direct LGN-MT pathways, suggested that interhemispheric connectivity may not be as critical (Ajina et al.,2015). This study also suggests that blindsight relies on functional connections between the MT and LGN, rather than interhemispheric connections Ajina et al. (2015). A methodological question is whether these imaging techniques can detect subtle changes in interhemispheric connectivity. This discrepancy might be justified by proposing that the intact hemisphere’s contribution varies depending on the specific visual tasks and the extent of interhemispheric communication established through neuroplasticity. Pre-existing individual differences in brain lateralization and connectivity may also influence the degree of compensation in the intact hemisphere.
Role of higher-order cortical areas
A study used neuroimaging to show that blindsight depends on an operational link between the MT and the LGN, rather than the pulvinar, suggesting the involvement of higher-order cortical areas. This study provides robust anatomical evidence, though the correlational data limit causality (Ajina & Bridge, 2015). Another study identified dentified extrastriate cortex activation without V1 activation, highlighting the role of higher cortical areas in visual processing. This study demonstrated the involvement of higher-order regions using fMRI (Bridge et al., 2010). Contrary to these articles, Sahraie et al. (1997) emphasized the role of subcortical pathways, suggesting that higher-order areas may not be necessary for all aspects of blindsight.
Cooperative pathways
As mentioned earlier, G.Y. is probably the most well-known case of blindsight, which has been extensively tested. Numerous studies on this individual provide a unique picture of the simultaneous activities of multiple pathways in creating blindsight. Two studies identified the SC-pulvinar pathway as the main route for affective blindsight in G.Y. (de Gelder & Hadjikhani, 2006; Van den Stock et al., 2011). Another two studies (Bridge et al., 2008; Celeghin et al., 2017) highlighted the role of the intact hemisphere in blindsight, with the former noting increased thalamocortical (e.g. LGN to MT) and corticocortical (e.g. MT/V5 between hemispheres) connections in G.Y. The presence of articles proposing different pathways in G.Y. suggests that multiple blindsight pathways can be simultaneously activated in one person. Papanikolaou et al. (2019) concluded that residual blindsight abilities may result from fine coordination between residual V1 areas and MT, as well as connections from SC and LGN areas to MT, confirming the presence of multiple pathways in damaged individuals. An explanation is that some of these pathways may potentially develop after brain injury, adding to previously existing pathways that did not emerge due to the dominance of the primary visual system, becoming active only after damage to V1. The presence of older pathways alongside those formed after an injury can result in more than one pathway being simultaneously active in an individual. In some cases, these pathways may cooperate and overlap with each other.
Human and animal study parallels
The articles that studied blindsight in monkeys also yielded results similar to those in humans. Among animal studies, the first group emphasizes SC’s significant role in creating blindsight, particularly the pathway from SC to pulvinar (Kato et al., 2011; Kinoshita et al., 2019; Takakuwa et al., 2021). Schmid et al. (2010) support the involvement of the LGN-extrastriate pathway in monkeys. Human studies often employ non-invasive imaging and correlational methodologies, offering valuable insights, although they lack the experimental rigor typically found in animal research. Discrepancies, such as a heightened focus on SC-pulvinar pathways in animals compared to a more equitable consideration of LGN and SC contributions in humans, may be attributed to species-specific variations in visual processing or methodological disparities. A suggestion to explain this mismatch is that. In contrast, core pathways are preserved across species, the dependence on individual channels may vary due to evolutionary adaptations and differences in cortical complexity between humans and animals.
Causes of differences in blindsight pathway activation
The activation of blindsight pathways can differ significantly among individuals due to several factors discussed below.
Individual variability in neuroanatomy
Variations in brain structure and connectivity can lead to individual differences in the activation of specific pathways. Certain studies suggest structural variations in the areas associated with blindsight pathways among among (Bridge et al., 2008), potentially influencing the efficacy of each pathway in compensating for V1 loss. Structural variations may result in discrepancies in the activation of several cortical and subcortical circuits.
The extent of visual (V1) damage
The severity and exact location of the lesion in the V1 region can influence the routes employed for blindsight. The residual functionality in V1 may lead to different degrees of pathway activation, affecting the extent of blindsight. Ajina and Bridge showed that individuals with residual V1 function effectively utilize direct pathways from the LGN to higher s, such as the MT region. Functional V1 remnants may influence the balance between cortical and subcortical pathways.
Age at the time of injury and time elapsed since injury
The significance of time is well-established; however, examining its impact presents difficulties. While acknowledging the importance of time in improving our comprehension of the brain’s adaptive functions post-V1 loss, the variety in individual recovery rates, disparities in the severity of V1 damage, and methodological inconsistencies among studies can mislead interpretations.
The active pathways are strongly influenced by the age at which the V1 injury occurs, suggesting that different pathways are preferred in younger brains compared to older ones and that these preferences change with time. Increased neuroplasticity in developing brains makes it easier for young children to rewire their visual circuits. Celeghin et al. (2017) highlighted that younger patients with stronger neuroplasticity could create more robust compensatory networks, allowing them to maintain some visuomotor functions even after severe damage to critical s. This group exhibited enhanced visual information transmission due to enhanced transcallosal connections between the unaffected and damaged hemispheres. While still capable of some neuroplastic adaptation, older brains may exhibit different patterns of compensatory mechanisms due to reduced plasticity.
The time elapsed since a V1 lesion is another critical factor in the emergence of blindsight abilities. Longitudinal studies, detailed case analyses, and animal research have all highlighted that neuroplastic changes occur gradually and require significant time to manifest fully. Therefore, patients assessed soon after injury may show different activation patterns than those evaluated after a longer period, reflecting ongoing neuroplastic changes. Studies conducted immediately after T.N. injury indicated limited blindsight capabilities, whereas follow-up studies years later revealed much more pronounced visual functions. Andino et al. (2009) conducted their study shortly after T.N.’s bilateral V1 damage, suggesting that the LGN-to-extrastriate areas-to-amygdala pathway is the main route for affective blindsight, thereby questioning the direct SC-pulvinar-amygdala route. However, another study (Burra et al., 2019) on T.N. eight years post-injury suggested the SC-pulvinar-amygdala pathway is the main route for affective blindsight. This discrepancy may be due to neuroplasticity, providing T.N. with a new pathway that was absent at the time of injury. This further substantiates the concept that fundamental blindsight pathways may be replaced over time and that the brain’s compensatory mechanisms necessitate significant time for complete development. Animal studies further support the significance of temporal factors in the development of blindsight. Kinoshita et al. (2019) researched monkeys with V1 lesions and found that alternative visual pathways, including the SC-pulvinar-extrastriate route, progressively gained prominence over the months following the injury. The gradual enhancement in route functionality aligns with documented neuroplastic alterations in human research, suggesting that temporal factors are essential in humans and non-human primates. These findings underscore the dynamic characteristics of neuroplastic adaptation and the significance of individualized strategies, dependent on the patient’s age and duration since injury, in the management and rehabilitation of blindsight.
Cognitive and attentional states during the test and the stimulus type
The potential impact of experimental conditions on the traced pathways should not be overlooked. Individuals’ cognitive and attentional states can influence the activation of blindsight pathways. Ptito et al. (1999) discovered that attentional states can affect motion processing. Buetti et al. (2013) demonstrated a distinction between goal-directed and discrete response localization in a patient, suggesting that the activation of specific pathways is significantly influenced by cognitive burden and attentional focus. However, the solitary case study restricts generalizability. Sahraie et al. (1997) discovered that neural activity patterns associated with the conscious and unconscious processing of visual stimuli fluctuate with cognitive and attentional states, influencing the preferred utilization of specific pathways.
The type of visual stimuli, especially those loaded with emotional content, may activate blindsight circuits. Pegna et al. (2005) demonstrated that affective blindsight, represented by the non-conscious processing of frightening facial expressions, primarily involves the SC-pulvinar-amygdala pathway, as indicated by heightened activation of the right amygdala. This suggests that emotionally charged inputs may selectively activate specific subcortical pathways associated with affective processing.
The implications of neuroplasticity for rehabilitation
After considering all these variables, it is possible to infer that neuroplasticity plays a substantial role in blindsight following an injury to the V1 region. Pre-existing pathways may account for initial forms of blindsight, but substantial neuroplastic alterations over time may markedly enhance and fortify these capabilities (Figure 2). The potential for neuroplasticity and reorganization of neural circuits is likely affected by genetic variations, the age at which injury occurs, and the extent of the damage.
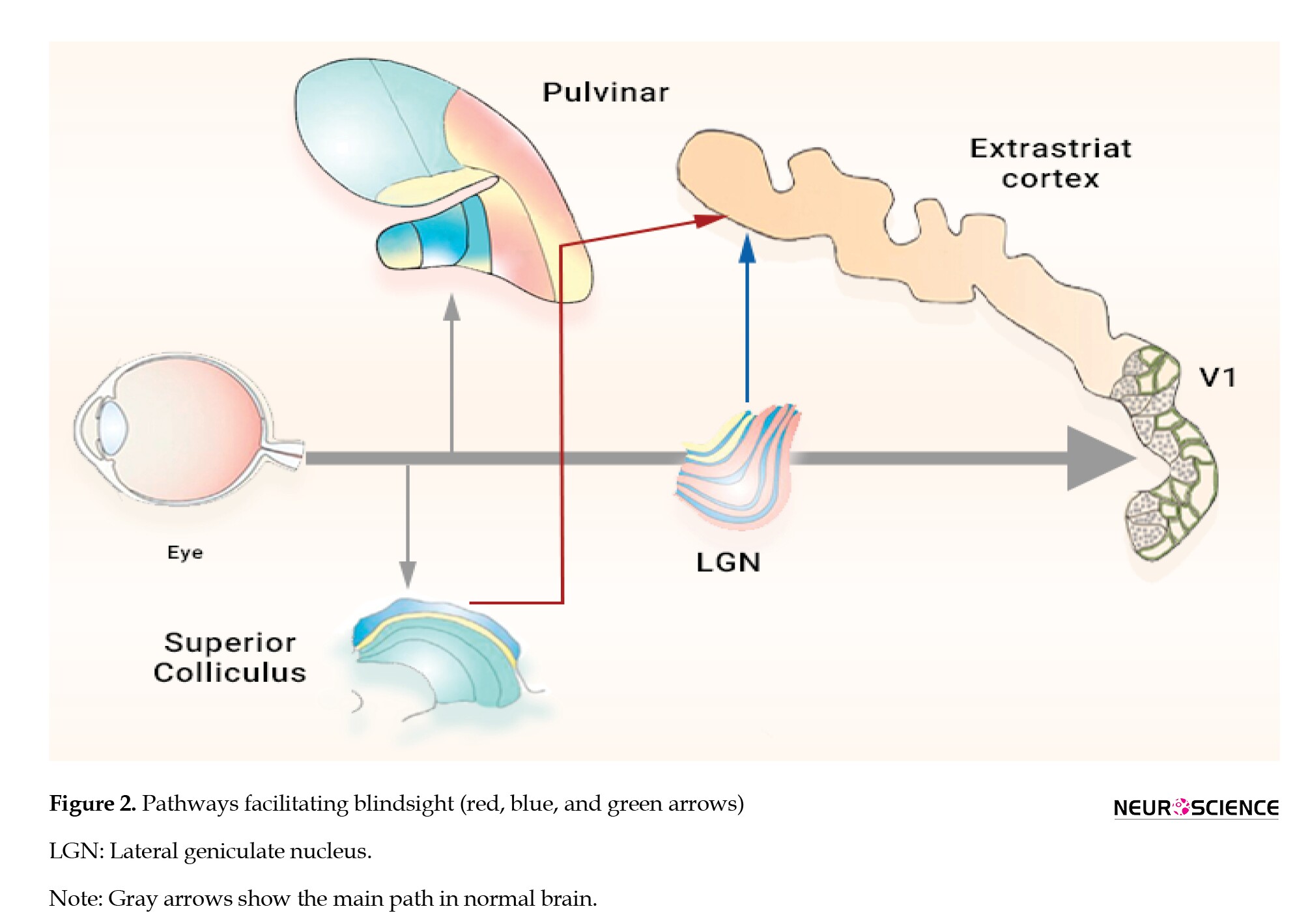
Highlighting the roles of the LGN-extrastriate and SC-pulvinar pathways, these findings significantly impact clinical interventions aimed at enhancing visual function in patients with V1 damage. From a therapeutic perspective, blindsight-mediated pathways can serve as targets for rehabilitation strategies. For example, targeted therapies that activate the SC-pulvinar route could help enhance emotional and spatial awareness, particularly in patients with affective blindsight Targeted visual training programs focusing on motion detection tasks may stimulate the LGN to MT pathway, thereby improving patients’ ability to detect motion in the blind field. Having comprehended the importance of repetitive practice and targeted interventions in fostering neuroplasticity, it is evident that both short- and long-term strategies are crucial for optimizing rehabilitation efficacy. The best practical therapy approach may be one that integrates multiple perspectives. Cross-modal sensory stimulation, such as combining auditory or tactile cues with visual tasks, can also leverage cross-modal plasticity to aid visual processing. By stimulating multiple sensory pathways, rehabilitation can enhance the brain’s adaptive mechanisms, reinforcing the neural circuits involved in blindsight. These clinical interventions offer a promising avenue for restoring vision or improving visual tasks, enhancing patients’ independence and well-being.
Recent data underscore the diversity and plasticity of the visual system, indicating that numerous mechanisms may facilitate blindsight. Although spared V1 islands may contribute to this phenomenon, the LGN-extrastriate, and SC-pulvinar pathways are increasingly recognized as critical pathways for residual visual function. While also introducing diversity in the manifestation of blindsight, the brain’s adaptability and the potential involvement of the intact hemisphere enhance our comprehension, introducing variety in the manifestation of blindsight. The age at which a V1 injury occurs and the time since the injury is critical for identifying the neural pathways associated with blindsight. These findings underscore the dynamic characteristics of neuroplastic adaptation and the significance of individualized strategies in managing and rehabilitating blindsight. To enhance the recovery of individuals with cortical blindness, future research should explore these pathways in rehabilitation protocols, potentially integrating neuromodulation, visual retraining, and cross-modal sensory stimulation to activate residual pathways and enhance adaptive neuroplastic responses. By advancing our understanding of these fields, we can create more effective rehabilitation strategies that will ultimately leverage the brain’s inherent plasticity to restore vision.
Due to the low prevalence of blindsight, most articles are case reports involving a few patients, such as G.Y. and T.N., whose data is often repeated, potentially biasing our understanding of alternative pathways. While providing detailed insights, small sample sizes and case reports may not be generalizable to larger populations and are limited by their anecdotal nature and lack of control groups. Particular research lacks specificity regarding the characteristics of V1 lesions, impeding repeatability and systematic comparisons. Furthermore, our data were inappropriate for meta-analyses, and all human investigations were retrospective, hindering our ability to monitor individual variations in most instances. The investigations utilized a variety of methodologies, such as imaging modalities (fMRI, EEG, PET, and DW-MRI) and behavioral activities, which hindered the ability to make definitive conclusions about blindsight pathways. Future research should prioritize longitudinal studies that track pathway activation over time. Such studies enable a deeper understanding of compensatory mechanisms as they evolve, thereby enhancing our knowledge of long-term visual recovery. Standardizing methodologies, including consistent imaging techniques and outcome measures, will also improve comparability across studies. Expanding the sample size could further strengthen the statistical reliability and enhance the generalizability of the findings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1404.117)
Funding
This research was supported by the research project funded by the Research Deputy of the Iran University of Medical Sciences, Tehran, Iran (Project No.: 20989).
Authors' contributions
Conceptualization: All authors; Funding acquisition, resources, and validation: Mohammad Taghi Joghataei; Investigation, Methodology, Writing the original draft: Mohammad Reza Balali, Mohaddeseh Hosseini, Maryam Khanboloki, Mohammad Mohammadi, and Amir Mohammad Khorshidvand; Project administration: Mohammad Reza Balali and Amir Mohammad Khorshidvand; Review and editing: Mohammad Reza Balali, Amir Mohammad Khorshidvand, and Mohammad Mohammadi; Supervision: Mohammad Taghi Joghataei and Amir Mohammad Khorshidvand; Visualization: Amir Mohammad Khorshidvand and Mohammad Mohammadi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their gratitude to Mahmoud Yousefi-Fard, Arash Ketabforoush, and Kiarash Tanha for their precious technical help and also to Zahra Rekabi and Maryam Ashjaazadeh for their indispensable contributions to the composition of this publication. We thank the Research Deputy of Iran University of Medical Sciences for funding this research.
References
After specific receptors in the eye trigger the sensation, visual data travel through a pathway to the lateral geniculate nucleus (LGN) in the thalamus, then to the visual area (V1) and higher cortical regions. This main pathway is known as the reticulogeniculostriate pathway. Damage to area V1 interrupts this pathway, resulting in the loss of “conscious vision” (Celesia, 2010). However, extensive research has shown that some patients retain the ability to respond to stimuli within their scotoma-localized area of diminished vision, even if not consciously, a phenomenon known as blindsight (Weiskrantz et al., 1974). For example, studies have shown a wide range of residual functions, including shape discrimination, object recognition (Trevethan et al., 2007; Van den Stock et al., 2015; Van den Stock et al., 2014; Weiskrantz, 1987), color perception (Kentridge et al., 2007; Morland et al., 1999), recognition of emotions (Bertini et al., 2013; Gerbella et al., 2019; Pegna et al., 2005; Van den Stock et al., 2011), manual localization, actions towards or spontaneous anti-pointing of unseen targets (de Gelder et al., 2008; Smits et al., 2019), processing gaze direction (Burra et al., 2013), and movement detection (Grasso et al., 2020; Hervais-Adelman et al., 2015) when the examiner applies pressure. In this context, the ability to perceive emotions unconsciously is referred to as affective blindsight, while other types are termed non-affective. The question here is which path or pathways in the brain can be attributed to blindsight. Various hypotheses and ideas have been proposed to explain the blindsight phenomenon.
A group of studies believes that area V1 is not obliterated after damage and that the small remaining islands continue to function. These islands are not large enough to elicit conscious vision, but they are sufficient to allow a person to respond to a stimulus unconsciously (Kalat, 2015; Radoeva et al., 2008). For example, through functional magnetic resonance imaging (MRI) (fMRI) studies of a patient, it was found that the patient could unconsciously perceive movements through the tiny islands left in his V1 area (Morland et al., 2004). Some researchers have also claimed a role for the remaining V1 islands in blindsight (Papanikolaou et al., 2019). Contrary to this hypothesis, some individuals still remain unconsciously aware of their vision despite the complete loss of the V1 area (Morland et al., 2004; Papanikolaou et al., 2019; Radoeva et al., 2008; Tran et al., 2019), questioning the sufficiency of V1 islands in mediating blindsight.
The second group claims that the LGN is central to this phenomenon. Two pathways extend from LGN to higher brain areas: One transmits information to V1, or striate cortex (the striate cortical area responsible for processing visual information), and the other bypasses V1, sending information directly to the extrastriate cortex. These articles highlight the role of LGN-extrastriate pathways in the emergence of blindsight (Schmid et al., 2010). A study on a person with bilateral V1 damage showed that pathways from the LGN to the middle temporal (MT) region facilitate motion detection (Ajina & Bridge, 2019).
The last group of researchers considered the role of the superior colliculus (SC) in the emergence of blindsight. They believe that pathways passing through this area, which transfer information directly from the eye to regions above V1, play a prominent role. After surgical removal of the V1 area in two monkeys, Kato et al. (2011) demonstrated the role of retinotectal pathways through SC in blindsight. However, another study that used artificial induction of blindsight through transcranial magnetic stimulation (TMS), rejected this role (Allen et al., 2014).
The phenomenon of blindsight has been extensively examined by neuroscientists for decades, resulting in a substantial body of research. Each article analyzes the topic from diverse perspectives, utilizing distinct methodologies. Considering methodological, chronological, and technical disparities, essential components must be identified and conclusions formulated accordingly. Thus, conducting a systematic review in this domain is necessary to direct future research toward these pathways, thereby enhancing our understanding and developing rehabilitation treatments for individuals experiencing V1 injury.
2. Materials and Methods
A systematic review of published studies up to August 10, 2024, was conducted. No language limit was considered. The inclusion criteria required studies to be original research articles that demonstrated precise methodology and robust evidence of pathway activation in blindsight. Due to the anatomical and functional similarities in the visual systems, only studies on humans and monkeys were included to provide a comprehensive understanding of the blindsight pathways. Research on monkeys enables more controlled experimental conditions, allowing for the observation of V1 damage effects in ways that are not ethically or practically feasible in human studies. This strengthens our understanding of how these pathways operate across species, ultimately supporting the translational potential of these findings in clinical settings.
The exclusion criteria involved studies in which the damage to the V1 region was ambiguous, and the proposed pathways of blindsight were not mentioned or, if mentioned, did not provide significant evidence. We excluded cases of hemispherectomy due to the inability to examine the interaction between both hemispheres and accurately trace compensatory pathways in this phenomenon. These criteria aimed to ensure repeatability and clarity in data selection, enhancing the rigor of this systematic review.
Search in database, screening, and data extraction
We searched online databases, such as PubMed, Scopus, Web of Science, and Embase, to extract all relevant articles. The research syntax is provided in the Supplementary Material.
In the first step, articles were assessed based on their titles, and those with irrelevant titles were excluded. In the second step, the abstracts of the chosen articles were reviewed. Then, full texts of approved articles were reviewed, selecting studies on humans or monkeys that mentioned pathways facilitating blindsight. After reading the full texts, we used the JBI critical appraisal checklist for human studies (Moola et al., 2019; Munn et al., 2020) and the SYRCLE tool (Hooijmans et al., 2014) for animal studies to assess the risk. Two authors independently evaluated studies, resolving disagreements through discussion and consensus. The primary search retrieved 7616 articles from the databases, 1429 of which were identified as duplicates. After reviewing the titles and abstracts, 836 articles were selected for full-text analysis. Based on the inclusion criteria, 25 articles were selected for data extraction. Figure 1 shows the process of selecting eligible articles.

The reported data included publication year and country, first author’s name, study type, age, sex, and number of cases/controls, V1 injury mechanism, injury onset age, time elapsed since injury, injury location, type of task, and stimuli used to assess blindsight existence and its responsible pathways, imaging tool, and proposed pathways mediating blindsight. Two reviewers independently extracted data using pre-structured data sheets. Tables 1-6 present the data.

Quality assessment
The JBI critical appraisal checklist was used to assess the quality of human studies, and the SYRCLE risk assessment tool was used for animal studies. Two authors independently evaluated all the studies. All differences of opinion were settled by discussion and mutual agreement. The case-control studies (n=9) were assessed based on 10 criteria for study design, participant selection, exposure and outcome measurement, and statistical analyses. Studies with a high risk of bias in two or more domains were considered to have an overall high risk of bias. Overall, the quality assessment revealed that most case-control studies had a low or unclear risk of bias, indicating good study design and conduct. Although the quality of reporting in case-control studies was good, some studies did not adequately consider confounding factors or employ appropriate grouping techniques. Ten case reports and the only case series were evaluated on eight criteria for clear, detailed patient descriptions and presentations. Most studies were assessed as low-risk and did not have significant issues. Animal studies (n=5) were evaluated using the SYRCLE risk assessment tool, which assessed 10 criteria related to study design, grouping, exposure, and outcome measurements. Overall, the quality assessment of experimental studies revealed that most had a moderate risk of bias.
3. Results
We assessed 25 articles, including 20 human and five monkey studies. Five of the 20 human studies focused on affective blindsight and 15 on non-affective blindsight. Among all human studies, 10 were case reports, nine were case-control studies, and one was a case series (Table 1). The mechanisms by which area V1 was injured or inactivated are as follows: 46 patients had V1 injury due to cerebrovascular accidents (CVA), six had trauma, four had undergone surgery, one had an arteriovenous malformation, one experienced ischemia due to asphyxia, one had a tumor, and five had lesions due to hypoxia of unknown origin. Additionally, there were 16 cases of TMS-induced V1 inactivation (Table 2).

To categorize the tasks used to detect the stimuli in the cases, the articles were divided into the following categories: Seven articles used passive viewing, three used emotion discrimination, five used direction discrimination, five used stimulus (onset) detection, and two used color discrimination to examine the blindsight-mediating pathways. Regarding the imaging tools used, 14 studies chose fMRI to identify pathways; two used electroencephalography (EEG) and visual evoked potential, one used PET, and two used diffusion-weighted MRI (DW-MRI) (Table 3).


There were also five monkey studies, with eight monkeys participating (Table 4).

The injury mechanism in four of these studies was surgical removal of the V1 area by aspiration. All injuries were unilateral. One article did not mention the number of cases and injury mechanisms (Table 5).

The task involved stimulation detection through visually guided saccades. Each study used the inactivation of different brain parts to assess the effects on behavior; one study also employed fMRI for pathway assessment (Table 6).

Main findings
Human studies
Four articles supported that the SC-pulvinar-amygdala pathway facilitates emotion detection in affective blindsight. Among those, three studies used emotion discrimination, and one used a passive viewing task. Pegna et al. (2005) showed that facial expressions, especially fear, are processed via this route, as evidenced by increased activation in the right amygdala. The role of higher cortical areas was also emphasized in more complex emotional processing before it reaches the amygdala. However, another study involving a single patient who performed a passive viewing task and was evaluated using EEG presented different results. The results of this study question the existence of a direct SC-pulvinar-amygdala route. Similar to previous research on more complex emotions, this study suggests an indirect pathway that transits from the extrastriate cortex through anterior areas and ultimately terminates in the amygdala (Andino et al., 2009).
In cases of non-affective blindsight, three studies supported the idea that blindsight occurs due to the function of spared islands of V1. However, all these studies also found V1 bypassing pathways that sometimes reached the MT. The tasks performed by the patients were as follows: One-directional discrimination, one-color change detection, one-stimulus and direction detection along with shape and color discrimination, and detection of moving stimuli with variable contrast levels. All these studies used fMRI to determine the pathways.
Six articles supported the pathway from the LGN to the MT (in both the injured and non-injured hemispheres), with 55 cases. The tasks were as follows: Two studies used stimulus and direction detection, one study used both passive viewing and movement detection, one study combined stimulus and motion detection with color discrimination and localization tasks, and two studies involved passive viewing. The tools used were as follows: Three studies used fMRI, two used DW-MRI, and one used TMS.
A study demonstrated that in addition to the ipsilesional pathway from LGN to MT, which was similarly observed in control cases, two other projections also exist in G.Y.: a contralateral pathway from right LGN to left MT/V5 and a projection from the MT of the intact hemisphere to the MT of the injured hemisphere, created through interhemispheric connections (Bridge et al., 2008). The study by Tran et al. (2019) also pointed to the pathway that passes through hemispheres from ipsilesional SC to contralateral MT.
Five articles suggested that higher cortical areas play a significant role in mediating blindsight. For example, Tran et al. (2019) showed that in motion detection, the pathway passes from the ipsilesional SC to the contralateral MT and ipsilesional frontal area, suggesting a robust alternative route for visual processing. By incorporating the variable of awareness into the investigation of pathways leading to blindsight, Sahraie et al. (1997) demonstrated that higher cortical regions can effectively create the phenomenon of blindsight. Buetti et al. (2013) showed that in the stimulus detection task, the V1-bypassing pathway extends from the SC to the posterior dorsal areas. In the study by Ptito et al. (1999), patients performed a passive viewing task. Positron emission tomography (PET) showed that the data passed from the pulvinar to the extrastriate cortex. Benson et al. (1999) performed two tasks: Motion detection and direction discrimination. These results suggest that the pathway from tectopulvinar to extrastriate areas serves as a mediating pathway in blindsight.
Monkey studies
One article emphasized the role of the LGN in mediating blindsight through extrastriate pathways. In a study by Schmid et al. (2010), monkeys’ brains were analyzed using fMRI during a detection task involving rotating checkerboard stimuli. The results showed that in monkeys with V1 damage, visual data passed through the LGN to the extrastriate pathway.
Four studies highlighted the importance of the SC-pulvinar pathway. In a survey by Kato et al. (2011), V1 was surgically removed by aspiration, and a visual detection task was conducted while fMRI images were obtained from the monkeys’ brains. The researchers identified two key pathways responsible for blindsight ability: The SC-to-pulvinar and the SC-to-LGN pathways. The study rejects the notion that the LGN plays a sole role in blindsight. In another study Isa (2019) claimed that the main pathway for blindsight is the SC-pulvinar-extrastriate pathway. In a study by Kinoshita et al. (2019), the monkeys’ V1 areas were aspirated, and several months later, their brains were assessed histologically. The researchers recommended that the SC-to-pulvinar pathway is responsible for blindsight. Takakuwa et al. (2021) claimed that the SC and pulvinar areas play a significant role in mediating blindsight, as the pathway from the SC to the cortical areas through the pulvinar is the primary pathway of blindsight. This study also claimed that the LGN is an essential part of the blindsight-mediating pathways.
4. Discussion
Blindsight continues to challenge traditional views on how vision is processed in the brain, particularly following damage to the primary visual cortex. This discussion integrates recent findings, emphasizes discrepancies among studies, proposes hypotheses to explain these discrepancies, and critically evaluates the studies’ methodologies to enhance our understanding of the pathways underlying blindsight.
Review of evidence regarding different blindsight pathways
The spared visual (V1) islands
The hypothesis that small, spared islands within the damaged V1 area contribute to blindsight is widely debated. Three studies support the role of spared V1 islands in blindsight (Morland et al., 2004; Papanikolaou et al., 2019; Radoeva et al., 2008). Two studies suggests that these islands enable unconscious visual processing, using high-resolution fMRI to identify active V1 tissue (Morland et al., 2004; Papanikolaou et al., 2019). A potential weakness is the assumption that fMRI signals equate to functional processing, which may not always be accurate. Conversely, de Gelder et al. (2008). and Tran et al. (2019) provided evidence that blindsight can occur with complete V1 damage, questioning the necessity of V1 islands for residual visual function. The former used behavioral tests and anatomical imaging to demonstrate complete V1 damage, though minimal functional V1 tissue may still exist (de Gelder et al., 2008). Different imaging modalities could introduce inconsistencies in detecting small functional areas.
A hypothesis that might reconcile these findings is that while spared V1 islands can enhance certain types of blindsight, their presence is not strictly necessary, and other neural mechanisms can effectively compensate for their absence. For example, in one study, the pathways responsible for blindsight were categorized based on the contrast of stimuli, showing that subcortical pathways ending in MT are the primary pathways for recognizing a high-contrast stimulus in the impaired visual field. In contrast, V1 is more efficient in recognizing low-contrast stimuli (Radoeva et al., 2008).
The LGN-extrastriate pathway
The LGN to extrastriate cortex (also known as the geniculoextrastriate) pathway is increasingly recognized as crucial for blindsight. Studies have shown that direct pathways from the LGN to the MT are vital for the unconscious detection of movement in the damaged visual field (Ajina & Bridge, 2018, Ajina & Bridge, 2019; Ajina et al., 2015; Allen et al., 2014; Bridge et al., 2010, Bridge et al., 2008). One study demonstrated this pathway’s role using advanced neuroimaging, showing that direct LGN-MT connections are critical for motion detection in the absence of V1 (Ajina et al., 2015). Another study used diffusion weighted imaging (DWI) and probabilistic tractography to map the LGN-MT pathways, providing clear anatomical evidence (Ajina & Bridge, 2019). Schmid et al. (2010) validated these findings with precise lesion techniques and behavioral testing in monkeys, offering clear causal evidence. However, studies on non-human primates may not be directly applicable to humans.
Although the experiment conducted by Allen et al. (2014) using TMS-induced blindsight primarily favored the dominance of LGN-based pathways, it also indicated that the existence of other pathways, including tectopulvinar pathways, in creating blindsight is entirely plausible, suggesting that additional structures may be crucial. This argument is strengthened by the fact that TMS’s transient effects might not fully replicate chronic V1 damage. Functional redundancy in the visual system ensures robust visual processing even when primary routes are compromised. Therefore, this discrepancy suggests that the LGN-extrastriate pathway is vital but works alongside other pathways, such as those containing the pulvinar and SC, to support blindsight.
The SC and pulvinar contributions
According to this hypothesis, pathways passing through the SC to the pulvinar, transferring information directly from the eye to regions above V1, play a significant role in the emergence of blindsight (Benson et al., 1999; Buetti et al., 2013; Ptito et al., 1999; Tran et al., 2019). Research by Kato et al. (2011) and Isa (2019) underscores the significance of SC-based pathways in rerouting visual information after V1 damage. Kato et al. (2011) conducted lesion studies in monkeys, using behavioral assessments and fMRI to provide strong evidence for these pathways. However, generalizability to humans is uncertain due to species differences, and behavioral tasks in monkeys may not fully capture the complexities of human visual processing. Isa (2019) used reversible inactivation of the SC-pulvinar pathway in monkeys to show its role in blindsight, offering robust causal evidence. However, reversibility may not perfectly simulate permanent human lesions. Conversely, Ajina and Bridge (2018) argued that the SC-pulvinar pathway alone is insufficient, emphasizing the need for LGN-extrastriate cortex connectivity. They combined human neuroimaging and case studies to provide a comprehensive overview of the pathway, though reliance on correlational data limits the inference of causality. A methodological question is whether these imaging techniques detect all relevant subcortical activities. A hypothesis to reconcile these findings is that SC and pulvinar pathways act synergistically with the LGN-extrastriate route, collectively supporting various aspects of blindsight. Individual differences in brain architecture and the nature of visual tasks performed could determine the relative contributions of these pathways.
The intact hemisphere
The potential involvement of the intact hemisphere in compensating for damage to V1 has been the subject of considerable debate. Ptito et al. (1999) found that activation of extrageniculostriate pathways after damage to area V1 suggests the involvement of interhemispheric pathways. Some researchers concluded that the connection between the two hemispheres is a fundamental component in compensating for damage to V1 (Bridge et al., 2008; Celeghin et al., 2017; Papanikolaou et al., 2019; Tran et al., 2019). Two studies suggested that increased connectivity between the intact and damaged hemispheres contributes to blindsight (Bridge et al., 2008; Celeghin et al., 2017). The former used DWI and functional connectivity analyses to show increased interhemispheric connections, providing strong anatomical and functional evidence (Bridge et al., 2008). A potential question is whether the observed connectivity changes directly result from V1 damage or pre-existing conditions. The latter employed detailed case studies with advanced neuroimaging, offering a nuanced view of the intact hemisphere’s role. However, the small sample size of the case studies limits their generalizability. On the other hand, some articles believe that the healthy hemisphere may not play a role in creating blindsight (Ajina et al., 2015; Bridge et al., 2010; Buetti et al., 2013). One study downplayed the intact hemisphere's role and by emphasizing direct LGN-MT pathways, suggested that interhemispheric connectivity may not be as critical (Ajina et al.,2015). This study also suggests that blindsight relies on functional connections between the MT and LGN, rather than interhemispheric connections Ajina et al. (2015). A methodological question is whether these imaging techniques can detect subtle changes in interhemispheric connectivity. This discrepancy might be justified by proposing that the intact hemisphere’s contribution varies depending on the specific visual tasks and the extent of interhemispheric communication established through neuroplasticity. Pre-existing individual differences in brain lateralization and connectivity may also influence the degree of compensation in the intact hemisphere.
Role of higher-order cortical areas
A study used neuroimaging to show that blindsight depends on an operational link between the MT and the LGN, rather than the pulvinar, suggesting the involvement of higher-order cortical areas. This study provides robust anatomical evidence, though the correlational data limit causality (Ajina & Bridge, 2015). Another study identified dentified extrastriate cortex activation without V1 activation, highlighting the role of higher cortical areas in visual processing. This study demonstrated the involvement of higher-order regions using fMRI (Bridge et al., 2010). Contrary to these articles, Sahraie et al. (1997) emphasized the role of subcortical pathways, suggesting that higher-order areas may not be necessary for all aspects of blindsight.
Cooperative pathways
As mentioned earlier, G.Y. is probably the most well-known case of blindsight, which has been extensively tested. Numerous studies on this individual provide a unique picture of the simultaneous activities of multiple pathways in creating blindsight. Two studies identified the SC-pulvinar pathway as the main route for affective blindsight in G.Y. (de Gelder & Hadjikhani, 2006; Van den Stock et al., 2011). Another two studies (Bridge et al., 2008; Celeghin et al., 2017) highlighted the role of the intact hemisphere in blindsight, with the former noting increased thalamocortical (e.g. LGN to MT) and corticocortical (e.g. MT/V5 between hemispheres) connections in G.Y. The presence of articles proposing different pathways in G.Y. suggests that multiple blindsight pathways can be simultaneously activated in one person. Papanikolaou et al. (2019) concluded that residual blindsight abilities may result from fine coordination between residual V1 areas and MT, as well as connections from SC and LGN areas to MT, confirming the presence of multiple pathways in damaged individuals. An explanation is that some of these pathways may potentially develop after brain injury, adding to previously existing pathways that did not emerge due to the dominance of the primary visual system, becoming active only after damage to V1. The presence of older pathways alongside those formed after an injury can result in more than one pathway being simultaneously active in an individual. In some cases, these pathways may cooperate and overlap with each other.
Human and animal study parallels
The articles that studied blindsight in monkeys also yielded results similar to those in humans. Among animal studies, the first group emphasizes SC’s significant role in creating blindsight, particularly the pathway from SC to pulvinar (Kato et al., 2011; Kinoshita et al., 2019; Takakuwa et al., 2021). Schmid et al. (2010) support the involvement of the LGN-extrastriate pathway in monkeys. Human studies often employ non-invasive imaging and correlational methodologies, offering valuable insights, although they lack the experimental rigor typically found in animal research. Discrepancies, such as a heightened focus on SC-pulvinar pathways in animals compared to a more equitable consideration of LGN and SC contributions in humans, may be attributed to species-specific variations in visual processing or methodological disparities. A suggestion to explain this mismatch is that. In contrast, core pathways are preserved across species, the dependence on individual channels may vary due to evolutionary adaptations and differences in cortical complexity between humans and animals.
Causes of differences in blindsight pathway activation
The activation of blindsight pathways can differ significantly among individuals due to several factors discussed below.
Individual variability in neuroanatomy
Variations in brain structure and connectivity can lead to individual differences in the activation of specific pathways. Certain studies suggest structural variations in the areas associated with blindsight pathways among among (Bridge et al., 2008), potentially influencing the efficacy of each pathway in compensating for V1 loss. Structural variations may result in discrepancies in the activation of several cortical and subcortical circuits.
The extent of visual (V1) damage
The severity and exact location of the lesion in the V1 region can influence the routes employed for blindsight. The residual functionality in V1 may lead to different degrees of pathway activation, affecting the extent of blindsight. Ajina and Bridge showed that individuals with residual V1 function effectively utilize direct pathways from the LGN to higher s, such as the MT region. Functional V1 remnants may influence the balance between cortical and subcortical pathways.
Age at the time of injury and time elapsed since injury
The significance of time is well-established; however, examining its impact presents difficulties. While acknowledging the importance of time in improving our comprehension of the brain’s adaptive functions post-V1 loss, the variety in individual recovery rates, disparities in the severity of V1 damage, and methodological inconsistencies among studies can mislead interpretations.
The active pathways are strongly influenced by the age at which the V1 injury occurs, suggesting that different pathways are preferred in younger brains compared to older ones and that these preferences change with time. Increased neuroplasticity in developing brains makes it easier for young children to rewire their visual circuits. Celeghin et al. (2017) highlighted that younger patients with stronger neuroplasticity could create more robust compensatory networks, allowing them to maintain some visuomotor functions even after severe damage to critical s. This group exhibited enhanced visual information transmission due to enhanced transcallosal connections between the unaffected and damaged hemispheres. While still capable of some neuroplastic adaptation, older brains may exhibit different patterns of compensatory mechanisms due to reduced plasticity.
The time elapsed since a V1 lesion is another critical factor in the emergence of blindsight abilities. Longitudinal studies, detailed case analyses, and animal research have all highlighted that neuroplastic changes occur gradually and require significant time to manifest fully. Therefore, patients assessed soon after injury may show different activation patterns than those evaluated after a longer period, reflecting ongoing neuroplastic changes. Studies conducted immediately after T.N. injury indicated limited blindsight capabilities, whereas follow-up studies years later revealed much more pronounced visual functions. Andino et al. (2009) conducted their study shortly after T.N.’s bilateral V1 damage, suggesting that the LGN-to-extrastriate areas-to-amygdala pathway is the main route for affective blindsight, thereby questioning the direct SC-pulvinar-amygdala route. However, another study (Burra et al., 2019) on T.N. eight years post-injury suggested the SC-pulvinar-amygdala pathway is the main route for affective blindsight. This discrepancy may be due to neuroplasticity, providing T.N. with a new pathway that was absent at the time of injury. This further substantiates the concept that fundamental blindsight pathways may be replaced over time and that the brain’s compensatory mechanisms necessitate significant time for complete development. Animal studies further support the significance of temporal factors in the development of blindsight. Kinoshita et al. (2019) researched monkeys with V1 lesions and found that alternative visual pathways, including the SC-pulvinar-extrastriate route, progressively gained prominence over the months following the injury. The gradual enhancement in route functionality aligns with documented neuroplastic alterations in human research, suggesting that temporal factors are essential in humans and non-human primates. These findings underscore the dynamic characteristics of neuroplastic adaptation and the significance of individualized strategies, dependent on the patient’s age and duration since injury, in the management and rehabilitation of blindsight.
Cognitive and attentional states during the test and the stimulus type
The potential impact of experimental conditions on the traced pathways should not be overlooked. Individuals’ cognitive and attentional states can influence the activation of blindsight pathways. Ptito et al. (1999) discovered that attentional states can affect motion processing. Buetti et al. (2013) demonstrated a distinction between goal-directed and discrete response localization in a patient, suggesting that the activation of specific pathways is significantly influenced by cognitive burden and attentional focus. However, the solitary case study restricts generalizability. Sahraie et al. (1997) discovered that neural activity patterns associated with the conscious and unconscious processing of visual stimuli fluctuate with cognitive and attentional states, influencing the preferred utilization of specific pathways.
The type of visual stimuli, especially those loaded with emotional content, may activate blindsight circuits. Pegna et al. (2005) demonstrated that affective blindsight, represented by the non-conscious processing of frightening facial expressions, primarily involves the SC-pulvinar-amygdala pathway, as indicated by heightened activation of the right amygdala. This suggests that emotionally charged inputs may selectively activate specific subcortical pathways associated with affective processing.
The implications of neuroplasticity for rehabilitation
After considering all these variables, it is possible to infer that neuroplasticity plays a substantial role in blindsight following an injury to the V1 region. Pre-existing pathways may account for initial forms of blindsight, but substantial neuroplastic alterations over time may markedly enhance and fortify these capabilities (Figure 2). The potential for neuroplasticity and reorganization of neural circuits is likely affected by genetic variations, the age at which injury occurs, and the extent of the damage.

Highlighting the roles of the LGN-extrastriate and SC-pulvinar pathways, these findings significantly impact clinical interventions aimed at enhancing visual function in patients with V1 damage. From a therapeutic perspective, blindsight-mediated pathways can serve as targets for rehabilitation strategies. For example, targeted therapies that activate the SC-pulvinar route could help enhance emotional and spatial awareness, particularly in patients with affective blindsight Targeted visual training programs focusing on motion detection tasks may stimulate the LGN to MT pathway, thereby improving patients’ ability to detect motion in the blind field. Having comprehended the importance of repetitive practice and targeted interventions in fostering neuroplasticity, it is evident that both short- and long-term strategies are crucial for optimizing rehabilitation efficacy. The best practical therapy approach may be one that integrates multiple perspectives. Cross-modal sensory stimulation, such as combining auditory or tactile cues with visual tasks, can also leverage cross-modal plasticity to aid visual processing. By stimulating multiple sensory pathways, rehabilitation can enhance the brain’s adaptive mechanisms, reinforcing the neural circuits involved in blindsight. These clinical interventions offer a promising avenue for restoring vision or improving visual tasks, enhancing patients’ independence and well-being.
Recent data underscore the diversity and plasticity of the visual system, indicating that numerous mechanisms may facilitate blindsight. Although spared V1 islands may contribute to this phenomenon, the LGN-extrastriate, and SC-pulvinar pathways are increasingly recognized as critical pathways for residual visual function. While also introducing diversity in the manifestation of blindsight, the brain’s adaptability and the potential involvement of the intact hemisphere enhance our comprehension, introducing variety in the manifestation of blindsight. The age at which a V1 injury occurs and the time since the injury is critical for identifying the neural pathways associated with blindsight. These findings underscore the dynamic characteristics of neuroplastic adaptation and the significance of individualized strategies in managing and rehabilitating blindsight. To enhance the recovery of individuals with cortical blindness, future research should explore these pathways in rehabilitation protocols, potentially integrating neuromodulation, visual retraining, and cross-modal sensory stimulation to activate residual pathways and enhance adaptive neuroplastic responses. By advancing our understanding of these fields, we can create more effective rehabilitation strategies that will ultimately leverage the brain’s inherent plasticity to restore vision.
Due to the low prevalence of blindsight, most articles are case reports involving a few patients, such as G.Y. and T.N., whose data is often repeated, potentially biasing our understanding of alternative pathways. While providing detailed insights, small sample sizes and case reports may not be generalizable to larger populations and are limited by their anecdotal nature and lack of control groups. Particular research lacks specificity regarding the characteristics of V1 lesions, impeding repeatability and systematic comparisons. Furthermore, our data were inappropriate for meta-analyses, and all human investigations were retrospective, hindering our ability to monitor individual variations in most instances. The investigations utilized a variety of methodologies, such as imaging modalities (fMRI, EEG, PET, and DW-MRI) and behavioral activities, which hindered the ability to make definitive conclusions about blindsight pathways. Future research should prioritize longitudinal studies that track pathway activation over time. Such studies enable a deeper understanding of compensatory mechanisms as they evolve, thereby enhancing our knowledge of long-term visual recovery. Standardizing methodologies, including consistent imaging techniques and outcome measures, will also improve comparability across studies. Expanding the sample size could further strengthen the statistical reliability and enhance the generalizability of the findings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1404.117)
Funding
This research was supported by the research project funded by the Research Deputy of the Iran University of Medical Sciences, Tehran, Iran (Project No.: 20989).
Authors' contributions
Conceptualization: All authors; Funding acquisition, resources, and validation: Mohammad Taghi Joghataei; Investigation, Methodology, Writing the original draft: Mohammad Reza Balali, Mohaddeseh Hosseini, Maryam Khanboloki, Mohammad Mohammadi, and Amir Mohammad Khorshidvand; Project administration: Mohammad Reza Balali and Amir Mohammad Khorshidvand; Review and editing: Mohammad Reza Balali, Amir Mohammad Khorshidvand, and Mohammad Mohammadi; Supervision: Mohammad Taghi Joghataei and Amir Mohammad Khorshidvand; Visualization: Amir Mohammad Khorshidvand and Mohammad Mohammadi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their gratitude to Mahmoud Yousefi-Fard, Arash Ketabforoush, and Kiarash Tanha for their precious technical help and also to Zahra Rekabi and Maryam Ashjaazadeh for their indispensable contributions to the composition of this publication. We thank the Research Deputy of Iran University of Medical Sciences for funding this research.
References
Ajina, S., & Bridge, H. (2018). Blindsight relies on a functional connection between hMT+ and the lateral geniculate nucleus, not the pulvinar. PLoS biology, 16(7), e2005769. [DOI:10.1371/journal.pbio.2005769] [PMID]
Ajina, S., & Bridge, H. (2019). Subcortical pathways to extrastriate visual cortex underlie residual vision following bilateral damage to V1. Neuropsychologia, 128, 140–149. [DOI:10.1016/j.neuropsychologia.2018.01.007] [PMID]
Ajina, S., Pestilli, F., Rokem, A., Kennard, C., & Bridge, H. (2015). Human blindsight is mediated by an intact geniculo-extrastriate pathway. eLife, 4, e08935. [DOI:10.7554/eLife.08935] [PMID]
Allen, C. P., Sumner, P., & Chambers, C. D. (2014). The timing and neuroanatomy of conscious vision as revealed by TMS-induced blindsight. Journal of Cognitive Neuroscience, 26(7), 1507–1518. [DOI:10.1162/jocn_a_00557] [PMID]
Andino, S. L., Menendez, R. G., Khateb, A., Landis, T., & Pegna, A. J. (2009). Electrophysiological correlates of affective blindsight. NeuroImage, 44(2), 581–589. [DOI:10.1016/j.neuroimage.2008.09.002] [PMID]
Benson, P. J., Guo, K., & Hardiman, M. J. (1999). Cortical evoked potentials due to motion contrast in the blind hemifield. Neuroreport, 10(17), 3595–3600. [DOI:10.1097/00001756-199911260-00024] [PMID]
Bertini, C., Cecere, R., & Làdavas, E. (2013). I am blind, but I "see" fear. Cortex, 49(4), 985–993. [DOI:https://doi.org/10.1016/j.cortex.2012.02.006] [PMID]
Bridge, H., Hicks, S. L., Xie, J., Okell, T. W., Mannan, S., & Alexander, I., et al. (2010). Visual activation of extra-striate cortex in the absence of V1 activation. Neuropsychologia, 48(14), 4148–4154. [DOI:10.1016/j.neuropsychologia.2010.10.022] [PMID]
Bridge, H., Thomas, O., Jbabdi, S., & Cowey, A. (2008). Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain, 131(Pt 6), 1433–1444. [DOI:10.1093/brain/awn063] [PMID]
Buetti, S., Tamietto, M., Hervais-Adelman, A., Kerzel, D., de Gelder, B., & Pegna, A. J. (2013). Dissociation between goal-directed and discrete response localization in a patient with bilateral cortical blindness. Journal of Cognitive Neuroscience, 25(10), 1769–1775. [DOI:10.1162/jocn_a_00404] [PMID]
Burra, N., Hervais-Adelman, A., Celeghin, A., de Gelder, B., & Pegna, A. J. (2019). Affective blindsight relies on low spatial frequencies. Neuropsychologia, 128, 44–49. [DOI:10.1016/j.neuropsychologia.2017.10.009] [PMID]
Burra, N., Hervais-Adelman, A., Kerzel, D., Tamietto, M., de Gelder, B., & Pegna, A. J. (2013). Amygdala activation for eye contact despite complete cortical blindness. The Journal of Neuroscience, 33(25), 10483–10489. [DOI:10.1523/jneurosci.3994-12.2013] [PMID]
Celeghin, A., Diano, M., de Gelder, B., Weiskrantz, L., Marzi, C. A., & Tamietto, M. (2017). Intact hemisphere and corpus callosum compensate for visuomotor functions after early visual cortex damage. Proceedings of the National Academy of Sciences of the United States of America, 114(48), E10475–E10483. [DOI:10.1073/pnas.1714801114] [PMID]
Celesia, G. G. (2010). Visual perception and awareness: A modular system. Journal of Psychophysiology, 24(2), 62-67. [DOI:10.1027/0269-8803/a000014]
de Gelder, B., & Hadjikhani, N. (2006). Non-conscious recognition of emotional body language. Neuroreport, 17(6), 583–586. [DOI:10.1097/00001756-200604240-00006] [PMID]
de Gelder, B., Tamietto, M., van Boxtel, G., Goebel, R., Sahraie, A., & van den Stock, J., et al. (2008). Intact navigation skills after bilateral loss of striate cortex. Current Biology, 18(24), R1128–R1129. [DOI:10.1016/j.cub.2008.11.002] [PMID]
Gerbella, M., Caruana, F., & Rizzolatti, G. (2019). Pathways for smiling, disgust and fear recognition in blindsight patients. Neuropsychologia, 128, 6–13. [DOI:10.1016/j.neuropsychologia.2017.08.028] [PMID]
Grasso, P. A., Pietrelli, M., Zanon, M., Làdavas, E., & Bertini, C. (2020). Alpha oscillations reveal implicit visual processing of motion in hemianopia. Cortex, 122, 81–96. [DOI:10.1016/j.cortex.2018.08.009] [PMID]
Hervais-Adelman, A., Legrand, L. B., Zhan, M., Tamietto, M., de Gelder, B., & Pegna, A. J. (2015). Looming sensitive cortical regions without V1 input: Evidence from a patient with bilateral cortical blindness. Frontiers in Integrative Neuroscience, 9, 51. [DOI:10.3389/fnint.2015.00051] [PMID]
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., & Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Medical Research Methodology, 14, 43. [DOI: 10.1186/1471-2288-14-43] [PMID]
Isa, T. (2019). Neural mechanisms and functions of blindsight. IBRO Reports, 6, S44-S45. [DOI: 10.1016/j.ibror.2019.07.135]
Kalat, J. W. (2015). Biological psychology. Boston: Cengage Learning. [Link]
Kato, R., Takaura, K., Ikeda, T., Yoshida, M., & Isa, T. (2011). Contribution of the retino-tectal pathway to visually guided saccades after lesion of the primary visual cortex in monkeys. The European Journal of Neuroscience, 33(11), 1952–1960. [DOI:10.1111/j.1460-9568.2011.07729.x] [PMID]
Kentridge, R. W., Heywood, C. A., & Weiskrantz, L. (2007). Color contrast processing in human striate cortex. Proceedings of the National Academy of Sciences of the United States of America, 104(38), 15129–15131. [DOI:10.1073/pnas.0706603104] [PMID]
Kinoshita, M., Kato, R., Isa, K., Kobayashi, K., Kobayashi, K., & Onoe, H., et al. (2019). Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nature Communications, 10(1), 135. [DOI:10.1038/s41467-018-08058-0] [PMID]
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., & Sfetic, R., et al. (2019). Chapter 7: Systematic reviews of etiology and risk. In: E. Aromataris and Z. Munn (Ed.). Joanna Briggs Institute Reviewer's Manual. North Adelaide: The Joanna Briggs Institute. [DOI:10.46658/jbirm-17-06]
Morland, A. B., Jones, S. R., Finlay, A. L., Deyzac, E., Lê, S., & Kemp, S. (1999). Visual perception of motion, luminance and colour in a human hemianope. Brain, 122(Pt 6), 1183–1198. [DOI:10.1093/brain/122.6.1183] [PMID]
Morland, A. B., Lê, S., Carroll, E., Hoffmann, M. B., & Pambakian, A. (2004). The role of spared calcarine cortex and lateral occipital cortex in the responses of human hemianopes to visual motion. Journal of Cognitive Neuroscience, 16(2), 204–218. [DOI:10.1162/089892904322984517] [PMID]
Munn, Z., Barker, T. H., Moola, S., Tufanaru, C., Stern, C., McArthur, A., Stephenson, M., & Aromataris, E. (2020). Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evidence Synthesis, 18(10):p 2127-2133. [DOI:10.11124/jbisrir-d-19-00099]
Papanikolaou, A., Keliris, G. A., Papageorgiou, T. D., Schiefer, U., Logothetis, N. K., & Smirnakis, S. M. (2019). Organization of area hV5/MT+ in subjects with homonymous visual field defects. NeuroImage, 190, 254–268. [DOI:10.1016/j.neuroimage.2018.03.062] [PMID]
Pegna, A. J., Khateb, A., Lazeyras, F., & Seghier, M. L. (2005). Discriminating emotional faces without primary visual cortices involves the right amygdala. Nature Neuroscience, 8(1), 24–25. [DOI:10.1038/nn1364] [PMID]
Ptito, M., Johannsen, P., Faubert, J., & Gjedde, A. (1999). Activation of human extrageniculostriate pathways after damage to area V1. Neuroimage, 9(1), 97-107. [DOI:10.1006/nimg.1998.0390] [PMID]
Radoeva, P. D., Prasad, S., Brainard, D. H., & Aguirre, G. K. (2008). Neural activity within area V1 reflects unconscious visual performance in a case of blindsight. Journal of Cognitive Neuroscience, 20(11), 1927–1939. [DOI:10.1162/jocn.2008.20139] [PMID]
Sahraie, A., Weiskrantz, L., Barbur, J. L., Simmons, A., Williams, S. C., & Brammer, M. J. (1997). Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proceedings of the National Academy of Sciences of the United States of America, 94(17), 9406–9411. [DOI:10.1073/pnas.94.17.9406] [PMID]
Schmid, M. C., Mrowka, S. W., Turchi, J., Saunders, R. C., Wilke, M., & Peters, A. J., et al. (2010). Blindsight depends on the lateral geniculate nucleus. Nature, 466(7304), 373–377. [DOI:10.1038/nature09179] [PMID]
Smits, A. R., Seijdel, N., Scholte, H. S., Heywood, C. A., Kentridge, R. W., & de Haan, E. H. F. (2019). Action blindsight and antipointing in a hemianopic patient. Neuropsychologia, 128, 270–275. [DOI:10.1016/j.neuropsychologia.2018.03.029] [PMID]
Takakuwa, N., Isa, K., Onoe, H., Takahashi, J., & Isa, T. (2021). Contribution of the pulvinar and lateral geniculate nucleus to the control of visually guided saccades in blindsight monkeys. The Journal of Neuroscience, 41(8), 1755–1768. [DOI:10.1523/jneurosci.2293-20.2020] [PMID]
Tran, A., MacLean, M. W., Hadid, V., Lazzouni, L., Nguyen, D. K., & Tremblay, J., et al. (2019). Neuronal mechanisms of motion detection underlying blindsight assessed by functional magnetic resonance imaging (fMRI). Neuropsychologia, 128, 187–197. [DOI:10.1016/j.neuropsychologia.2019.02.012] [PMID]
Trevethan, C. T., Sahraie, A., & Weiskrantz, L. (2007). Form discrimination in a case of blindsight. Neuropsychologia, 45(9), 2092–2103. [DOI:10.1016/j.neuropsychologia.2007.01.022] [PMID]
Van den Stock, J., Tamietto, M., Hervais-Adelman, A., Pegna, A. J., & de Gelder, B. (2015). Body recognition in a patient with bilateral primary visual cortex lesions. Biological Psychiatry, 77(7), e31–e33. [DOI:10.1016/j.biopsych.2013.06.023] [PMID]
Van den Stock, J., Tamietto, M., Sorger, B., Pichon, S., Grézes, J., & de Gelder, B. (2011). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proceedings of the National Academy of Sciences of the United States of America, 108(39), 16188–16193. [DOI:10.1073/pnas.1107214108] [PMID]
Van den Stock, J., Tamietto, M., Zhan, M., Heinecke, A., Hervais-Adelman, A., & Legrand, L. B., et al. (2014). Neural correlates of body and face perception following bilateral destruction of the primary visual cortices. Frontiers in Behavioral Neuroscience, 8, 30. [DOI:10.3389/fnbeh.2014.00030] [PMID]
Weiskrantz L. (1987). Residual vision in a scotoma. A follow-up study of 'form' discrimination. Brain, 110(Pt 1), 77–92. [DOI:10.1093/brain/110.1.77] [PMID]
Weiskrantz, L., Warrington, E. K., Sanders, M. D., & Marshall, J. (1974). Visual capacity in the hemianopic field following a restricted occipital ablation. Brain, 97(4), 709–728. [DOI:10.1093/brain/97.1.709] [PMID]
Type of Study: Review |
Subject:
Behavioral Neuroscience
Received: 2024/10/16 | Accepted: 2024/11/19 | Published: 2025/05/1
Received: 2024/10/16 | Accepted: 2024/11/19 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





