Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 633-640 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hojjati F, Rayegani S M, Nazari Nodoushan A, Bayat M, Khavari Ardestani D, Razzaghi Z. Sural/Radial Amplitude Ratio in Healthy Adults: Influence of Anatomical and Demographic Variables. BCN 2025; 16 (3) :633-640
URL: http://bcn.iums.ac.ir/article-1-2938-en.html
URL: http://bcn.iums.ac.ir/article-1-2938-en.html
Fateme Hojjati1 

 , Seyed Mansour Rayegani1
, Seyed Mansour Rayegani1 

 , Ali Nazari Nodoushan *1
, Ali Nazari Nodoushan *1 
 , Masume Bayat1
, Masume Bayat1 
 , Davood Khavari Ardestani1
, Davood Khavari Ardestani1 

 , Zahra Razzaghi2
, Zahra Razzaghi2 



 , Seyed Mansour Rayegani1
, Seyed Mansour Rayegani1 

 , Ali Nazari Nodoushan *1
, Ali Nazari Nodoushan *1 
 , Masume Bayat1
, Masume Bayat1 
 , Davood Khavari Ardestani1
, Davood Khavari Ardestani1 

 , Zahra Razzaghi2
, Zahra Razzaghi2 

1- Physical Medicine and Rehabilitation Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Biostatistics, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Biostatistics, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Radial nerve, Sural nerve, Sural-to-radial sensory nerve action potential amplitude ratio (SRAR), Healthy volunteers
Full-Text [PDF 525 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Polyneuropathy is a peripheral neuropathy that causes sensory symptoms, such as numbness, tingling, paresthesia, pain, and muscle weakness. It typically affects the distal portion of the lower limbs and can significantly reduce the patient’s quality of life. The prevalence of polyneuropathy in the population is usually between 1% and 3%, but it can increase to 7% in older adults (Teunissen et al., 2000).
Due to the high prevalence of polyneuropathy in society, timely diagnosis and treatment are crucial for improving the patient’s quality of life. Currently, the diagnosis of polyneuropathy is based on history, physical examination, and electrodiagnostic tests. Electrodiagnostic tests can be used to objectively diagnose axonal polyneuropathy, especially in the early stages when patients are asymptomatic (Hanewinckel et al., 2016).
Several electrodiagnostic criteria have been used to diagnose mild and subclinical polyneuropathy. However, the sural nerve action potential (SNAP) amplitude criterion has some disadvantages, such as the lack of early diagnosis and accuracy in mild axonal polyneuropathy cases. Additionally, the wide range of normal values in healthy individuals reduces the sensitivity and specificity of this criterion (Sreenivasan et al., 2016).
The SNAP to sural-to-radial sensory nerve action potential amplitude ratio (SRAR) is another electrodiagnostic criterion used in some studies to diagnose axonal polyneuropathy (Rutkove et al., 1997; Pastore et al., 1999; Esper et al., 2005; Overbeek et al., 2005). Based on the length-dependent nature of nerve damage in axonal polyneuropathy and the early drop in the amplitude of sural SNAP compared to radial SNAP, axonal polyneuropathy can be diagnosed in its early stages.
SRAR exhibits high sensitivity in detecting neuropathy. For instance, an SRAR cutoff of <0.4 yielded a sensitivity of 100%, while a cutoff of <0.2 provided a sensitivity of 92.86%. These metrics underscore SRAR’s reliability in distinguishing between normal and abnormal nerve function (Ramanathan et al., 2021).
Compared to other screening methods, the SRAR has a sensitivity of 100%, which is higher than that of other methods, such as the Michigan neuropathy screening instrument (MNSI) (sensitivity of 64.3%), Semmes Weinstein monofilament (SWMF) (sensitivity of 14.3%), and biogeometry (sensitivity of 78.6%). These comparisons highlight the effectiveness of the SRAR, particularly in diagnosing length-dependent neuropathy, which is common in patients with diabetes (Ramanathan et al., 2021).
Despite its potential, studies on SRAR have yielded varying and sometimes contradictory results. Some research indicates that demographic factors, such as age, influence SRAR, while other studies suggest it remains unaffected by these variables. In other words, some studies have found that the minimum normal value of SRAR is 0.4 (Rutkove et al., 1997), whereas others proposed a lower threshold of 0.21 (Esper et al., 2005; Overbeek et al., 2005).
Globally, research on SRAR is limited, and most existing studies have not adequately considered demographic variables, such as age, sex, and anatomical factors. Based on the authors’ search methods, no studies specifically addressing SRAR were found in Iran. Therefore, this study aimed to fill this gap by investigating the relationship between SRAR and demographic variables and determining the minimum normal SRAR value in healthy individuals at Shohada Tajrish and Shahid Modares Medical Centers. By enhancing the early diagnosis of axonal polyneuropathy, this study aims to provide valuable insights for improving the management of this debilitating condition.
2. Materials and Methods
Between July 2022 and December 2023, a cross-sectional study was conducted on individuals who visited the electrodiagnosis clinics of Shohada Tajrish and Shahid Modares clinical centers to investigate SRAR in healthy adults.
The participants included 108 healthy volunteers aged 20-70 years, comprising physical medicine and rehabilitation residents, volunteer partners of patients undergoing electrodiagnostic examination, and patients with unrelated conditions. The exclusion criteria included any electrodiagnostic abnormalities (e.g. radiculopathy, polyneuropathy, plexopathy, peripheral nerve damage), systemic diseases predisposing to neuropathy (e.g. diabetes, hypothyroidism, chronic liver and kidney diseases, autoimmune diseases, infectious diseases, malignancy), lower limb edema, history of alcohol consumption, trauma, leg or forearm surgery, drug use, and toxin exposure.
All participants underwent a neurological examination to assess manual muscle strength, pain, light touch, vibration, position sense, and deep tendon reflexes in both arms and legs.
The same examiner consecutively examined all participants using an electrophysiological protocol. The protocol included bilateral motor nerve conduction studies (NCS) of the tibial and peroneal nerves, as well as bilateral sensory NCS of the sural and radial nerves, using surface electrodes. These initial studies were performed to rule out polyneuropathy.
An observational method was used to collect anatomical, demographic, and electrodiagnostic data. The recorded demographic information included age, sex, height, and weight. The body mass index (BMI) was calculated by dividing the weight (kg) by the height squared (m²). Forearm length was measured from the midpoint of the antebrachial skin fold to the radius styloid, and wrist circumference was measured distal to the radius styloid. Leg length was measured from the midportion of the popliteal fossa to the top of the medial malleolus, and ankle circumference was measured proximal to the medial and lateral malleolus.
SNAPs of the radial and sural nerves were recorded using a Medelec Synergy electromyogram (Oxford Medical, UK) with standard settings (stimulation duration: 0.2 ms, nerve stimulation frequency: 1 Hz, frequency bandwidth: 3 Hz to 3 kHz, sensitivity: 20 mv/div, and sweep speed: 20 ms/div). Before the potentials were recorded, the temperature of both the upper and lower limbs was measured with a thermometer. If the temperature was below 32 °C, a warmer was used to increase using a temperature.
For radial nerve SNAP recording, participants were seated, and the active electrode was placed on the snuff box area. The reference electrode was positioned 4 cm away from the posterior surface of the first finger, and the ground electrode was placed between the stimulator and active electrode. Supramaximal electrical stimulation was applied proximal to the active electrode at a distance of 10 cm. A physiatrist performed this procedure on both hands, and the maximum radial nerve amplitude was recorded.
For sural nerve SNAP recordings, the participants were placed in a prone position. The active electrode was placed behind the lateral malleolus, and the reference electrode was positioned 4 cm distally. The stimulator was placed 14 cm proximal to the active electrodes. The procedure was performed on both legs, and the maximum amplitude of the sural nerve was recorded.
The SRAR was calculated for all participants by dividing the sural nerve amplitude by the radial nerve amplitude. Additional variables recorded included sex, age, weight, height, forearm length, leg length, and wrist and ankle circumferences. The data were systematically entered into a datasheet for subsequent analysis.
Statistical analysis:
Data were analyzed using SPSS software, version 26. Qualitative variables were described in terms of frequency (percentage), while the quantitative variables were described as Mean±SD, minimum, and maximum values. The normality of the data was assessed via the Shapiro-Wilks test. Spearman’s correlation was used to evaluate associations, and a P<0.05 was considered significant.
3. Results
One hundred eight individuals with normal electrodiagnostic test results and no exclusion criteria were included in this study. The sample consisted of 69 females (64%) and 39 males (36%) with an average age of 42.46±11.49 years. The mean BMI was 25.87±4.03 kg/m2, and the mean forearm length, wrist circumference, leg length, and ankle circumference were 25.26±2.75 cm, 16.35±7.78 cm, 37.20±2.93 cm, and 22.5±1.99 cm, respectively.
Table 1 presents the descriptive statistics of the radial and sural SNAP amplitudes, and their ratios.

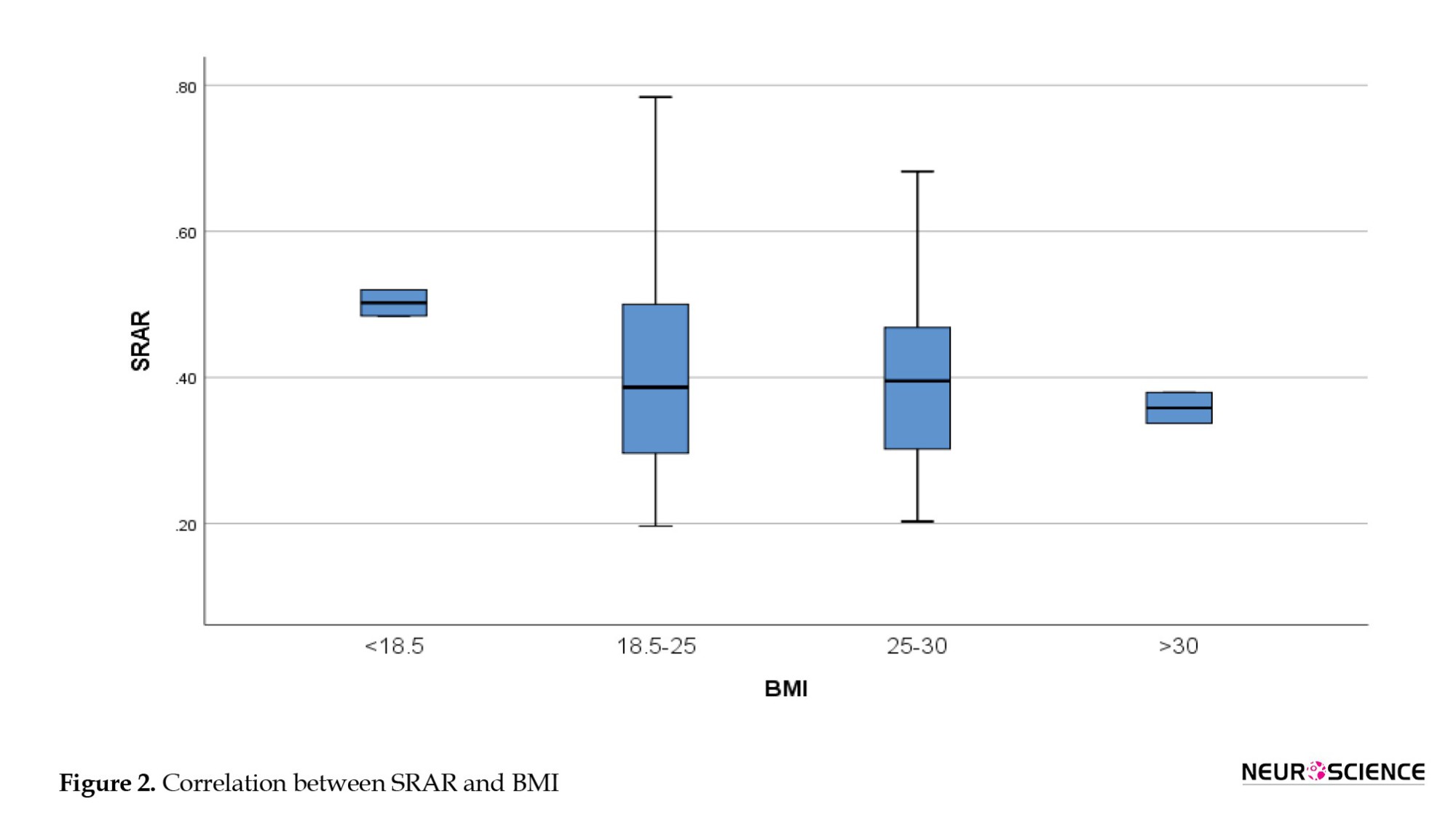
Figures 1 and 2 present the SRAR values based on age and BMI, respectively. The average SRAR for individuals aged 20-40 years was 0.45; for those aged 40-50 years, it was, and for individuals aged 50 years and older, it was 0.46. Analysis of variance (ANOVA) showed no significant difference in the SRAR between the three age groups (P>0.05). Similarly, BMI analysis indicated no significant differences between the groups (P=0.368).
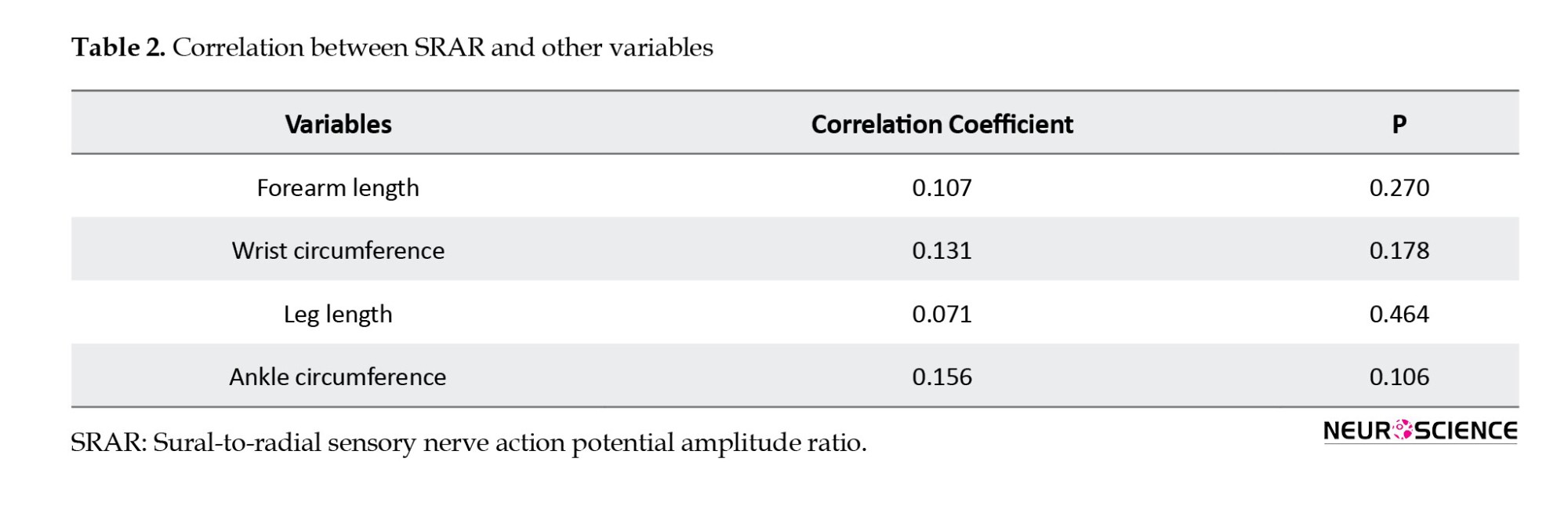
This study also investigated the relationship between SRAR and other variables, such as forearm length, wrist circumference, leg length, and ankle circumference.

Table 2 presents the correlation between SRAR and other variables. Spearman’s correlation test indicated that none of the variables (forearm length, wrist circumference, leg length, and ankle circumference) had a significant relationship with SRAR.
To determine a practical cut-off value for the SRAR, we calculated the 95th percentile of SRAR values, which was 0.21. This indicates that 95% of the SRAR measurements in our study were greater than 0.21.
4. Discussion
Polyneuropathy is a prevalent disorder with significant impacts on patients’ clinical status, socioeconomic situation, and public health. Our study identified the lowest SRAR value in normal individuals as 0.20, unaffected by demographic factors (age, sex, weight, height, BMI) or anatomical factors (forearm length, wrist circumference, leg length, ankle circumference). Based on the authors’ search methods, this is the first study to explore the relationship between SRAR and anatomical variables.
Rutkove et al. (1997) found SRAR that is a sensitive method for diagnosing mild axonal polyneuropathy, reporting the lowest normal SRAR value of 0.4, with no correlation with age. The discrepancy between their findings and ours (0.20) may stem from the smaller sample size (30 subjects) in Rutkove’s study.
Overbeek et al. (2005) argued that an SRAR value of 0.4 is too high as a minimum normal threshold, suggesting a value of 0.21, which they found to be a sensitive diagnostic tool for axonal neuropathy. They also reported that SRAR is independent of age and BMI, aligning with our findings and those of Rajabally et al. (2009) and Zis et al (2019).
Kahraman Koytak et al. (2017) found the medial plantar compound nerve action potential (CNAP) to radial SNAP amplitude ratio (MPRAR) and medial plantar CNAP were more sensitive than SRAR for diagnosing mild axonal neuropathy without clinical signs of large fiber involvement. They recommended the lowest normal SRAR value of 0.24 for all ages. However, MPRAR is impractical for individuals over 60 due to the challenging and time-consuming technique of recording the medial plantar CNAP, as well as the inability to record it in 40% of people over 60 years of age who are susceptible to neuropathy (Hemni et al., 2007; Lّseth et al., 2007).
Mansukhani et al. (2020) found the minimum normal SRAR value to be age-dependent, reporting values of 0.23, 0.20, and 0.17 for age groups 31-40, 41-50, and 51-70 years, respectively. These results are similar to ours, though they included individuals over 70 years of age, which we excluded to avoid confounding by the high prevalence of neuropathy in this age group.
Studies on the relationship between age and SRAR have yielded mixed results. Some studies have reported an inverse correlation (Overbeek et al., 2005; Esper et al., 2005; Vrancken et al., 2008; Herrmann et al., 2004; Sreenivasan et al., 2016), while others have found a direct correlation (Rutkove et al., 1997). Our study found no significant correlation between age and SRAR (rho=0.019, P=0.842), which may be attributed to differences in age range and sample distribution.
Our study found no correlation between SRAR and demographic variables, such as weight, height, and BMI, consistent with previous research (Rutkove et al., 1997; Pastore et al., 1999; Esper et al., 2005; Overbeek et al., 2005; Mansukhani et al., 2020). Additionally, we observed no correlation between SRAR and anatomical factors (wrist circumference, arm length, leg length, ankle circumference), a novel finding as no prior studies have explored this relationship.
Our results suggest that SRAR is independent of demographic (age, sex, weight) and anatomical variables (height, forearm length, wrist circumference, leg length, and ankle circumference). This reinforces SRAR’s robustness as a diagnostic tool.
Vrancken et al. (2008) evaluated the realistic yield of lower leg SNAP amplitudes and SRAR in the routine evaluation of chronic axonal polyneuropathies. They found that while SRAR can be useful in confirming distal axonal polyneuropathy, its additional diagnostic value may be limited compared to sural and superficial peroneal SNAP measurements. The results of this study align with our observation that SRAR is not influenced by demographic variables, reinforcing its potential utility as a supplementary diagnostic tool rather than a primary one.
5. Conclusion
In conclusion, our study found that the lowest SRAR value in normal individuals is 0.20, unaffected by demographic factors (age, sex, weight, height, and BMI) and anatomical factors (forearm length, wrist circumference, leg length, and ankle circumference).
We recommend further research with larger sample sizes, including healthy individuals and patients with mild axonal polyneuropathy. Future studies should investigate the cause of neuropathy and the time interval between onset and electrodiagnostic testing to better evaluate SRAR’s sensitivity and specificity in diagnosing early polyneuropathy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.1400.185). Informed consent was obtained from all participants
Funding
This study was extracted from the PhD dissertation of Ali Nazari Nodoushan, approved by the Department of Physical Medicine and Rehabilitation, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Supervision: Fateme Hojjati and Seyed Mansour Rayegani; Investigations: Ali Nazari Nodoushan and Masume Bayat; Statistical analysis: Zahra RazzaghiData collection and writing: Ali Nazari Nodoushan and Davood Khavari Ardestani.
Conflict of interest
The authors declared no conflict of interest.
References:
Polyneuropathy is a peripheral neuropathy that causes sensory symptoms, such as numbness, tingling, paresthesia, pain, and muscle weakness. It typically affects the distal portion of the lower limbs and can significantly reduce the patient’s quality of life. The prevalence of polyneuropathy in the population is usually between 1% and 3%, but it can increase to 7% in older adults (Teunissen et al., 2000).
Due to the high prevalence of polyneuropathy in society, timely diagnosis and treatment are crucial for improving the patient’s quality of life. Currently, the diagnosis of polyneuropathy is based on history, physical examination, and electrodiagnostic tests. Electrodiagnostic tests can be used to objectively diagnose axonal polyneuropathy, especially in the early stages when patients are asymptomatic (Hanewinckel et al., 2016).
Several electrodiagnostic criteria have been used to diagnose mild and subclinical polyneuropathy. However, the sural nerve action potential (SNAP) amplitude criterion has some disadvantages, such as the lack of early diagnosis and accuracy in mild axonal polyneuropathy cases. Additionally, the wide range of normal values in healthy individuals reduces the sensitivity and specificity of this criterion (Sreenivasan et al., 2016).
The SNAP to sural-to-radial sensory nerve action potential amplitude ratio (SRAR) is another electrodiagnostic criterion used in some studies to diagnose axonal polyneuropathy (Rutkove et al., 1997; Pastore et al., 1999; Esper et al., 2005; Overbeek et al., 2005). Based on the length-dependent nature of nerve damage in axonal polyneuropathy and the early drop in the amplitude of sural SNAP compared to radial SNAP, axonal polyneuropathy can be diagnosed in its early stages.
SRAR exhibits high sensitivity in detecting neuropathy. For instance, an SRAR cutoff of <0.4 yielded a sensitivity of 100%, while a cutoff of <0.2 provided a sensitivity of 92.86%. These metrics underscore SRAR’s reliability in distinguishing between normal and abnormal nerve function (Ramanathan et al., 2021).
Compared to other screening methods, the SRAR has a sensitivity of 100%, which is higher than that of other methods, such as the Michigan neuropathy screening instrument (MNSI) (sensitivity of 64.3%), Semmes Weinstein monofilament (SWMF) (sensitivity of 14.3%), and biogeometry (sensitivity of 78.6%). These comparisons highlight the effectiveness of the SRAR, particularly in diagnosing length-dependent neuropathy, which is common in patients with diabetes (Ramanathan et al., 2021).
Despite its potential, studies on SRAR have yielded varying and sometimes contradictory results. Some research indicates that demographic factors, such as age, influence SRAR, while other studies suggest it remains unaffected by these variables. In other words, some studies have found that the minimum normal value of SRAR is 0.4 (Rutkove et al., 1997), whereas others proposed a lower threshold of 0.21 (Esper et al., 2005; Overbeek et al., 2005).
Globally, research on SRAR is limited, and most existing studies have not adequately considered demographic variables, such as age, sex, and anatomical factors. Based on the authors’ search methods, no studies specifically addressing SRAR were found in Iran. Therefore, this study aimed to fill this gap by investigating the relationship between SRAR and demographic variables and determining the minimum normal SRAR value in healthy individuals at Shohada Tajrish and Shahid Modares Medical Centers. By enhancing the early diagnosis of axonal polyneuropathy, this study aims to provide valuable insights for improving the management of this debilitating condition.
2. Materials and Methods
Between July 2022 and December 2023, a cross-sectional study was conducted on individuals who visited the electrodiagnosis clinics of Shohada Tajrish and Shahid Modares clinical centers to investigate SRAR in healthy adults.
The participants included 108 healthy volunteers aged 20-70 years, comprising physical medicine and rehabilitation residents, volunteer partners of patients undergoing electrodiagnostic examination, and patients with unrelated conditions. The exclusion criteria included any electrodiagnostic abnormalities (e.g. radiculopathy, polyneuropathy, plexopathy, peripheral nerve damage), systemic diseases predisposing to neuropathy (e.g. diabetes, hypothyroidism, chronic liver and kidney diseases, autoimmune diseases, infectious diseases, malignancy), lower limb edema, history of alcohol consumption, trauma, leg or forearm surgery, drug use, and toxin exposure.
All participants underwent a neurological examination to assess manual muscle strength, pain, light touch, vibration, position sense, and deep tendon reflexes in both arms and legs.
The same examiner consecutively examined all participants using an electrophysiological protocol. The protocol included bilateral motor nerve conduction studies (NCS) of the tibial and peroneal nerves, as well as bilateral sensory NCS of the sural and radial nerves, using surface electrodes. These initial studies were performed to rule out polyneuropathy.
An observational method was used to collect anatomical, demographic, and electrodiagnostic data. The recorded demographic information included age, sex, height, and weight. The body mass index (BMI) was calculated by dividing the weight (kg) by the height squared (m²). Forearm length was measured from the midpoint of the antebrachial skin fold to the radius styloid, and wrist circumference was measured distal to the radius styloid. Leg length was measured from the midportion of the popliteal fossa to the top of the medial malleolus, and ankle circumference was measured proximal to the medial and lateral malleolus.
SNAPs of the radial and sural nerves were recorded using a Medelec Synergy electromyogram (Oxford Medical, UK) with standard settings (stimulation duration: 0.2 ms, nerve stimulation frequency: 1 Hz, frequency bandwidth: 3 Hz to 3 kHz, sensitivity: 20 mv/div, and sweep speed: 20 ms/div). Before the potentials were recorded, the temperature of both the upper and lower limbs was measured with a thermometer. If the temperature was below 32 °C, a warmer was used to increase using a temperature.
For radial nerve SNAP recording, participants were seated, and the active electrode was placed on the snuff box area. The reference electrode was positioned 4 cm away from the posterior surface of the first finger, and the ground electrode was placed between the stimulator and active electrode. Supramaximal electrical stimulation was applied proximal to the active electrode at a distance of 10 cm. A physiatrist performed this procedure on both hands, and the maximum radial nerve amplitude was recorded.
For sural nerve SNAP recordings, the participants were placed in a prone position. The active electrode was placed behind the lateral malleolus, and the reference electrode was positioned 4 cm distally. The stimulator was placed 14 cm proximal to the active electrodes. The procedure was performed on both legs, and the maximum amplitude of the sural nerve was recorded.
The SRAR was calculated for all participants by dividing the sural nerve amplitude by the radial nerve amplitude. Additional variables recorded included sex, age, weight, height, forearm length, leg length, and wrist and ankle circumferences. The data were systematically entered into a datasheet for subsequent analysis.
Statistical analysis:
Data were analyzed using SPSS software, version 26. Qualitative variables were described in terms of frequency (percentage), while the quantitative variables were described as Mean±SD, minimum, and maximum values. The normality of the data was assessed via the Shapiro-Wilks test. Spearman’s correlation was used to evaluate associations, and a P<0.05 was considered significant.
3. Results
One hundred eight individuals with normal electrodiagnostic test results and no exclusion criteria were included in this study. The sample consisted of 69 females (64%) and 39 males (36%) with an average age of 42.46±11.49 years. The mean BMI was 25.87±4.03 kg/m2, and the mean forearm length, wrist circumference, leg length, and ankle circumference were 25.26±2.75 cm, 16.35±7.78 cm, 37.20±2.93 cm, and 22.5±1.99 cm, respectively.
Table 1 presents the descriptive statistics of the radial and sural SNAP amplitudes, and their ratios.

Figures 1 and 2 present the SRAR values based on age and BMI, respectively. The average SRAR for individuals aged 20-40 years was 0.45; for those aged 40-50 years, it was, and for individuals aged 50 years and older, it was 0.46. Analysis of variance (ANOVA) showed no significant difference in the SRAR between the three age groups (P>0.05). Similarly, BMI analysis indicated no significant differences between the groups (P=0.368).
This study also investigated the relationship between SRAR and other variables, such as forearm length, wrist circumference, leg length, and ankle circumference.

Table 2 presents the correlation between SRAR and other variables. Spearman’s correlation test indicated that none of the variables (forearm length, wrist circumference, leg length, and ankle circumference) had a significant relationship with SRAR.

To determine a practical cut-off value for the SRAR, we calculated the 95th percentile of SRAR values, which was 0.21. This indicates that 95% of the SRAR measurements in our study were greater than 0.21.

4. Discussion
Polyneuropathy is a prevalent disorder with significant impacts on patients’ clinical status, socioeconomic situation, and public health. Our study identified the lowest SRAR value in normal individuals as 0.20, unaffected by demographic factors (age, sex, weight, height, BMI) or anatomical factors (forearm length, wrist circumference, leg length, ankle circumference). Based on the authors’ search methods, this is the first study to explore the relationship between SRAR and anatomical variables.
Rutkove et al. (1997) found SRAR that is a sensitive method for diagnosing mild axonal polyneuropathy, reporting the lowest normal SRAR value of 0.4, with no correlation with age. The discrepancy between their findings and ours (0.20) may stem from the smaller sample size (30 subjects) in Rutkove’s study.
Overbeek et al. (2005) argued that an SRAR value of 0.4 is too high as a minimum normal threshold, suggesting a value of 0.21, which they found to be a sensitive diagnostic tool for axonal neuropathy. They also reported that SRAR is independent of age and BMI, aligning with our findings and those of Rajabally et al. (2009) and Zis et al (2019).
Kahraman Koytak et al. (2017) found the medial plantar compound nerve action potential (CNAP) to radial SNAP amplitude ratio (MPRAR) and medial plantar CNAP were more sensitive than SRAR for diagnosing mild axonal neuropathy without clinical signs of large fiber involvement. They recommended the lowest normal SRAR value of 0.24 for all ages. However, MPRAR is impractical for individuals over 60 due to the challenging and time-consuming technique of recording the medial plantar CNAP, as well as the inability to record it in 40% of people over 60 years of age who are susceptible to neuropathy (Hemni et al., 2007; Lّseth et al., 2007).
Mansukhani et al. (2020) found the minimum normal SRAR value to be age-dependent, reporting values of 0.23, 0.20, and 0.17 for age groups 31-40, 41-50, and 51-70 years, respectively. These results are similar to ours, though they included individuals over 70 years of age, which we excluded to avoid confounding by the high prevalence of neuropathy in this age group.
Studies on the relationship between age and SRAR have yielded mixed results. Some studies have reported an inverse correlation (Overbeek et al., 2005; Esper et al., 2005; Vrancken et al., 2008; Herrmann et al., 2004; Sreenivasan et al., 2016), while others have found a direct correlation (Rutkove et al., 1997). Our study found no significant correlation between age and SRAR (rho=0.019, P=0.842), which may be attributed to differences in age range and sample distribution.
Our study found no correlation between SRAR and demographic variables, such as weight, height, and BMI, consistent with previous research (Rutkove et al., 1997; Pastore et al., 1999; Esper et al., 2005; Overbeek et al., 2005; Mansukhani et al., 2020). Additionally, we observed no correlation between SRAR and anatomical factors (wrist circumference, arm length, leg length, ankle circumference), a novel finding as no prior studies have explored this relationship.
Our results suggest that SRAR is independent of demographic (age, sex, weight) and anatomical variables (height, forearm length, wrist circumference, leg length, and ankle circumference). This reinforces SRAR’s robustness as a diagnostic tool.
Vrancken et al. (2008) evaluated the realistic yield of lower leg SNAP amplitudes and SRAR in the routine evaluation of chronic axonal polyneuropathies. They found that while SRAR can be useful in confirming distal axonal polyneuropathy, its additional diagnostic value may be limited compared to sural and superficial peroneal SNAP measurements. The results of this study align with our observation that SRAR is not influenced by demographic variables, reinforcing its potential utility as a supplementary diagnostic tool rather than a primary one.
5. Conclusion
In conclusion, our study found that the lowest SRAR value in normal individuals is 0.20, unaffected by demographic factors (age, sex, weight, height, and BMI) and anatomical factors (forearm length, wrist circumference, leg length, and ankle circumference).
We recommend further research with larger sample sizes, including healthy individuals and patients with mild axonal polyneuropathy. Future studies should investigate the cause of neuropathy and the time interval between onset and electrodiagnostic testing to better evaluate SRAR’s sensitivity and specificity in diagnosing early polyneuropathy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.1400.185). Informed consent was obtained from all participants
Funding
This study was extracted from the PhD dissertation of Ali Nazari Nodoushan, approved by the Department of Physical Medicine and Rehabilitation, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Supervision: Fateme Hojjati and Seyed Mansour Rayegani; Investigations: Ali Nazari Nodoushan and Masume Bayat; Statistical analysis: Zahra RazzaghiData collection and writing: Ali Nazari Nodoushan and Davood Khavari Ardestani.
Conflict of interest
The authors declared no conflict of interest.
References:
Esper, G. J., Nardin, R. A., Benatar, M., Sax, T. W., Acosta, J. A., & Raynor, E. M. (2005). Sural and radial sensory responses in healthy adults: Diagnostic implications for polyneuropathy. Muscle & Nerve, 31(5), 628–632. [DOI:10.1002/mus.20313] [PMID]
Hanewinckel, R., van Oijen, M., Ikram, M. A., & van Doorn, P. A. (2016). The epidemiology and risk factors of chronic polyneuropathy. European Journal of Epidemiology, 31(1), 5–20. [DOI:10.1007/s10654-015-0094-6] [PMID]
Hemmi, S., Inoue, K., Murakami, T., & Sunada, Y. (2007). Simple and novel method to measure distal sensory nerve conduction of the medial plantar nerve. Muscle & Nerve, 36(3), 307–312. [DOI:10.1002/mus.20814] [PMID]
Herrmann, D. N., Ferguson, M. L., Pannoni, V., Barbano, R. L., Stanton, M., & Logigian, E. L. (2004). Plantar nerve AP and skin biopsy in sensory neuropathies with normal routine conduction studies. Neurology, 63(5), 879–885. [DOI:10.1212/01.wnl.0000137036.26601.84] [PMID]
Kahraman Koytak, P., Alibas, H., Omercikoglu Ozden, H., Tanridag, T., & Uluc, K. (2017). Medial plantar-to-radial amplitude ratio: Does it have electrodiagnostic utility in distal sensory polyneuropathy?. The International Journal of Neuroscience, 127(4), 356–360. [DOI:10.3109/00207454.2016.1174119] [PMID]
Løseth, S., Nebuchennykh, M., Stålberg, E., & Mellgren, S. I. (2007). Medial plantar nerve conduction studies in healthy controls and diabetics. Clinical Neurophysiology, 118(5), 1155–1161. [DOI:10.1016/j.clinph.2007.01.008] [PMID]
Mansukhani, K., Dhonde, M., Sreenivasan, A., Sharma, A., Balakrishnan, L., & Chavan, P. (2020). Sural radial amplitude ratio: A study in healthy indian subjects. Annals of Indian Academy of Neurology, 23(3), 255–260. [DOI:10.4103/aian.AIAN_321_20] [PMID]
Overbeek, B. U., van Alfen, N., Bor, J. A., & Zwarts, M. J. (2005). Sural/radial nerve amplitude ratio: Reference values in healthy subjects. Muscle & Nerve, 32(5), 613–618. [DOI:10.1002/mus.20421] [PMID]
Pastore, C., Izura, V., Geijo-Barrientos, E., & Dominguez, J. R. (1999). A comparison of electrophysiological tests for the early diagnosis of diabetic neuropathy. Muscle & Nerve, 22(12), 1667-1673. [DOI:10.1002/(sici)1097-4598(199912)22:123.0.co;2-w]
Rajabally, Y. A., Beri, S., & Bankart, J. (2009). Electrophysiological markers of large fibre sensory neuropathy: A study of sensory and motor conduction parameters. European Journal of Neurology, 16(9), 1053–1059. [DOI:10.1111/j.1468-1331.2009.02651.x] [PMID]
Ramanathan, S., Thomas, R., Chanu, A. R., Naik, D., Jebasingh, F., & Sivadasan, A., et al (2021). Standard clinical screening tests, sural radial amplitude ratio and F wave latency compared to conventional nerve conduction studies in the assessment of sensorimotor polyneuropathy in patients with type 2 diabetes mellitus. Indian Journal of Endocrinology and Metabolism, 25(6), 509–515. [DOI:10.4103/ijem.ijem_426_21] [PMID]
Rutkove, S. B., Kothari, M. J., Raynor, E. M., Levy, M. L., Fadic, R., & Nardin, R. A. (1997). Sural/radial amplitude ratio in the diagnosis of mild axonal polyneuropathy. Muscle & Nerve, 20(10), 1236-1241. [DOI:10.1002/(sici)1097-4598(199710)20:103.0.co;2-d]
Sreenivasan, A., Mansukhani, K. A., Sharma, A., & Balakrishnan, L. (2016). Sural sensory nerve action potential: A study in healthy Indian subjects. Annals of Indian Academy of Neurology, 19(3), 312–317. [DOI:10.4103/0972-2327.186786] [PMID]
Teunissen, L. L., Eurelings, M., Notermans, N. C., Hop, J. W., & van Gijn, J. (2000). Quality of life in patients with axonal polyneuropathy. Journal of Neurology, 247(3), 195–199. [DOI:10.1007/s004150050562] [PMID]
Vrancken, A. F., Notermans, N. C., Wokke, J. H., & Franssen, H. (2008). The realistic yield of lower leg SNAP amplitudes and SRAR in the routine evaluation of chronic axonal polyneuropathies. Journal of Neurology, 255(8), 1127–1135. [DOI:10.1007/s00415-008-0817-7] [PMID]
Zis, P., Hadjivassiliou, M., Rao, D. G., & Sarrigiannis, P. G. (2019). Electrophysiological determinants of the clinical severity of axonal peripheral neuropathy. Muscle & Nerve, 59(4), 491–493. [DOI:10.1002/mus.26425] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2024/05/24 | Accepted: 2025/01/22 | Published: 2025/05/1
Received: 2024/05/24 | Accepted: 2025/01/22 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





