Volume 16, Issue 2 (March & April 2025)
BCN 2025, 16(2): 393-402 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zarei P, Raoufy M R, Shojaei A, Fathollahi Y, Mirnajafi-Zadeh J. Effect of High-frequency Electrical Stimulation of Olfactory Bulb on Spontaneous Excitatory Postsynaptic Currents in Hippocampal CA1 Pyramidal Cells of Kindling Rats. BCN 2025; 16 (2) :393-402
URL: http://bcn.iums.ac.ir/article-1-2930-en.html
URL: http://bcn.iums.ac.ir/article-1-2930-en.html
Parisa Zarei1 

 , Mohammad Reza Raoufy1
, Mohammad Reza Raoufy1 

 , Amir Shojaei1
, Amir Shojaei1 

 , Yaghoub Fathollahi1
, Yaghoub Fathollahi1 

 , Javad Mirnajafi-Zadeh *2
, Javad Mirnajafi-Zadeh *2 




 , Mohammad Reza Raoufy1
, Mohammad Reza Raoufy1 

 , Amir Shojaei1
, Amir Shojaei1 

 , Yaghoub Fathollahi1
, Yaghoub Fathollahi1 

 , Javad Mirnajafi-Zadeh *2
, Javad Mirnajafi-Zadeh *2 


1- Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
2- Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. & Faculty of Medical Sciences, Institute for Brain and Cognition, Tarbiat Modares University, Tehran, Iran.
2- Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. & Faculty of Medical Sciences, Institute for Brain and Cognition, Tarbiat Modares University, Tehran, Iran.
Full-Text [PDF 834 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Approximately 50 million people worldwide have epilepsy, making it the third most prevalent chronic neurological condition (Ghosh et al., 2021). Despite the widespread occurrence of epilepsy, there remains a lack of definitive treatment for epilepsy patients. While anticonvulsant medication serves as the predominant therapeutic approach, its effectiveness lies in suppressing seizures rather than addressing the fundamental brain irregularities responsible for epilepsy (Macdonald & Kelly, 1995). Consequently, there is a need to explore novel methods aimed at mitigating the adverse effects of seizures on the brain.
The kindling model represents a progressive evolution of focal seizures followed by focal to bilateral tonic seizures triggered by repetitive electrical or chemical stimulation (Cheng et al., 2020). The mechanism underlying epileptogenesis is thought to involve excessive activation of excitatory pathways in the brain, which includes heightened glutamate transmission and an imbalance between neuronal excitation and inhibition. It has been widely recognized that the increase in glutamate receptor activity plays a significant role in initiating and propagating epileptic discharges throughout the kindling process (McNamara et al., 1988b).
Deep brain stimulation (DBS) has undergone extensive clinical investigation as a treatment modality for various brain disorders, including Parkinson disease, epilepsy, pain management, addiction, stroke recovery, obsessive-compulsive disorder, and depression (Kuhn et al., 2011; Sandoval-Pistorius et al., 2023). One probable mechanism of DBS is its ability to induce depressive effects, which may prevent neurons from over-firing, particularly in certain applications.
DBS is usually applied at high frequencies (i.e. high-frequency stimulation: HFS), and 130 Hz is one of its most common frequencies in seizure control in both clinical and experimental studies (Covolan et al., 2014; Lee et al., 2006; Wyckhuys et al., 2010). Three probable mechanisms of HFS on neuronal activity have been proposed: Inhibition, excitation, and modulation. Previous research has indicated that HFS may reduce neuronal firing by activating inhibitory synapses within neural circuits (Alhourani et al., 2015) or depleting neurotransmitters at excitatory synapses (Iremonger et al., 2006). Nonetheless, in some investigations incorporating animal experiments and mathematical simulations, the researchers have demonstrated that HFS may elevate the firing rate of neuronal action potentials (Deniau et al., 2010a). These controversies in the effects of HFS on neuronal activity may be explained by the third possible mechanism, i.e., modulation. HFS may alter neurons’ firing patterns or rhythm rather than solely modifying neuronal firing rates (Florence et al., 2016; Herrington et al., 2016; McConnell et al., 2012). Enhancing the presynaptic inhibition is also one of the proposed DBS anticonvulsant mechanisms. This enhancement may occur through stimulation, hyperpolarizing local neuronal somas and dendrites, inducing depolarization blockage, and neurotransmitter depletion (Deniau et al., 2010b; Montgomery Jr & Gale, 2008).
Knowing little about the precise anticonvulsant mechanisms of DBS made it difficult to widely use epilepsy treatment (Chiken & Nambu, 2016). Therefore, these mechanisms need to be investigated. In the present study, we attempted to examine the effect of high-frequency-DBS (HFS) on kindling-induced changes in excitatory postsynaptic currents (EPSCs) in CA1 hippocampal pyramidal cells.
2. Materials and Methods
Study animals
In this experimental investigation, 17 male Wistar rats were individually housed in animal cages (each rat was kept separately in one cage) maintained at an ambient temperature ranging from 22 ˚C to 25 ˚C and subjected to a 12-hour light/12-hour dark cycle with lights on from 6:00 AM to 6:00 PM. The animals were 2-4 months old (200-280 g) at surgery and had access to water and food ad libitum.
Experimental design
We assessed how HFS influenced synaptic currents in fully kindling animals. Subjects were divided into four groups: a) The control group (6 rats), in which animals underwent the surgical procedure but were not subjected to either HFS or kindling stimulations; b) The control+HFS group (3 rats), in which animals underwent similar experimental procedures to the control group but received HFS; c) Kindling group (5 rats) that received kindling stimulation and after achieving complete kindled state, their hippocampal slices were prepared 48 h after the last kindling stimulation for patch clamp recording (these animals did not receive DBS), and d) kindled+HFS group (3 rats) that received HFS after achieving complete kindled state and their hippocampal slices were prepared similar to the kindled group. In the control and control+HFS groups, hippocampal slices were prepared at a time duration similar to the kindled and kindled+HFS groups.
In this study, the number of recorded cells in each experimental group was 13 cells/12 slices in the control group, 9 cells/5 slices in the control+HFS group, 10 cells/9 slices in the kindled group, and 8 cells/5 slices in the kindled+HFS group.
Surgery
The rats underwent deep anesthesia by a mixture of ketamine and xylazine (Sigma, England, 100/10 mg/kg, intraperitoneal injection). They were securely positioned in a horizontally flat skull configuration in a stereotaxic apparatus. The animal skull was exposed following a cut in the scalp. A bipolar stimulating electrode and a monopolar recording electrode were carefully implanted into the hippocampal CA1 region of the right hemisphere at coordinates of 5.3 mm posterior, 5.2 mm lateral to the bregma, and 6.5 mm below the dura. These electrodes served for both kindling stimulations and after-discharge recording. Additional bipolar stimulating electrodes were inserted into the right and left olfactory bulbs (OBs) (coordinated as 7.5 mm anterior to bregma, 1.0 mm lateral to right or left, and 1.6 mm below the dura) for HFS. Electrodes were Teflon-coated stainless-steel electrodes (A-M Systems, Inc., WA, USA, 127 μm in diameter). The electrodes were checked to be completely insulated except at their tips. Additionally, a monopolar electrode connected to a stainless-steel screw was positioned in the skull above the occipital cortex to serve as a reference and or ground electrode.
To induce kindled seizures in animals, after discharge (AD) threshold was measured by applying a monophasic square wave with a pulse duration of 1 ms and frequency of 50 Hz for 2 s (Ghafouri et al., 2016a). In the first step, the stimulus was administered at 30 µA. ADs were identified as spikes with amplitudes higher than twice the baseline activity and frequencies of at least 1 Hz. If ADs were recorded for at least 8 seconds, the applied stimulating current was considered AD threshold. If AD were not recorded, the stimulus intensity was increased in the steps of 10 µA at 10 min intervals until the ADs were recorded for 8 s or more (Khodadadi et al., 2022). In the kindling model of seizure, the presence of ADs at the AD threshold is crucial for seizure progression. Therefore, AD recording is necessary to confirm the proper development of the kindling procedure.
Kindling induction
As explained previously (Khodadadi et al., 2022), the AD threshold was determined following a 7-day recovery period post-surgery. Rats were electrically stimulated at the AD threshold 6 times per day at inter-stimulus intervals of 20 min. Epileptiform ADs were recorded from the hippocampal CA1 region, followed by kindling stimulations using a PC-based data acquisition system (BIODAC ES1721, TRITA Health Technology Co., Tehran, Iran). Seizure severity was assessed using Racine’s scale (Racine et al., 1977): Stage 0 (no convulsions), stage 1 (facial automatism), stage 2 (head nodding), stage 3 (unilateral forelimb clonus), stage 4 (bilateral forelimb clonus), and stage 5 (rearing, falling, and generalized convulsions). Animals were considered fully kindled when they displayed at least one stage 5 seizure activity in three consecutive days.
Applying HFS in OB
To investigate the impact of applying HFS in the OB on hippocampal kindled seizures, fully kindled rats underwent four sets of bilateral HFS in the OB using stimulating electrodes. The first set of HFS was administered in fully kindled animals immediately after the last kindling stimulation once the ADs were finished. The second set of HFS was delivered 6 hours later. The third set of HFS was applied on the subsequent day (24 h after the first HFS), and the fourth set of HFS was administered 6 h after the third HFS. Each set of HFS contained 4 trains of monophasic square waves with a pulse width of 0.1 ms. The duration of each train was 200 s, with an inter-train interval of 100 s. These trains were applied as high-frequency, at 130 Hz; each train contained 26,000 pulses. HFS parameters were determined based on our previous experiments (Ghafouri et al., 2016b; Sadeghian et al., 2020).
Whole-cell patch clamp recording
Whole-cell patch clamp recordings were run in dorsal and ventral hippocampal slices. The procedure of brain slice preparation was similar to our previous experiment (Ghafouri et al., 2016a). Briefly, rats were killed by decapitation while anesthetized with CO2. Then, the right hemisphere was rapidly removed and submerged in an ice-cold cutting solution that contained 2.5 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 1 mM NaH2PO4, 26.2 mM NaHCO3, 238 mM sucrose, and 11 mM D-glucose and bubbled with 95% O2- 5% CO2. The osmolarity was adjusted to 290-300 mOsm. We used a vibratome (1000 Plus Sectioning System, Vibratome, MO, USA) to prepare the transverse slices (300 μm). Subsequently, the right hippocampi were dissected out and transferred to standard artificial cerebrospinal fluid (ACSF) (continuously bubbled with 95% O2- 5% CO2) that consisted of 125 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 10 mM D-glucose, 2 mM CaCl2, and 1.3 mM MgCl2. The osmolality was in the 290-300 mOsm range, and pH was adjusted to 7.2-7.35 by 1 M NaOH. Slices were then incubated for one hour at 35 ˚C and stored at room temperature (23-25 ˚C) until individually transferred to a submerged recording chamber. All chemicals were purchased from Sigma, England.
Slices were moved to a plexiglas recording chamber in a fixed-stage upright microscope (Axioskop 2 FS MOT, Carl Zeiss, Germany). The chamber was continuously perfused with ACSF at a 1.5-2.5 mL/min rate. The solution was bubbled by carbogen (95% O2 and 5% CO2), and its pH was adjusted to 7.2-7.4. All recordings were conducted at room temperature (23-25 ˚C). To visualize the pyramidal cells in the hippocampal CA1 area, an IR-CCD camera (IR-1000, MTI, USA) with a ×40 water immersion objective lens was employed. Recorded cells were chosen based on their characteristic pyramidal shape and smooth, low-contrast appearance. Whole-cell patch clamp recordings were conducted in voltage-clamp mode using recording microelectrodes (1.5 mm outer diameter, borosilicate glass, GC150-11, Harvard Apparatus, UK) pulled with a horizontal puller (P-97, Sutter Instruments, USA) and filled with intracellular solutions tailored for spontaneous EPSCs (sEPSCs) recordings. The internal solution filled the microelectrode contained 135 mM K-gluconate, 20 mM KCl, 10 mM HEPES, 0.2 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetra acetic acid (EGTA), 7 mM disodium-phosphocreatine, 2 mM MgATP, 0.3 mM NaGTP and 1 mM QX-314 for the sEPSC recording.
Electrode tip resistance in the bath ranged from 5 to 8 MΩ, and series resistance varied from 18 to 30 MΩ. Cells were discarded if the series resistance changed by more than 20% during the experiment. Capacitance compensation was performed during recordings. Data were low-pass filtered at 3 kHz and sampled at 10 kHz using a MultiClamp 700B amplifier equipped with a Digidata 1440A/D converter (Molecular Devices, CA, USA). The recorded signal was stored on a PC for offline analysis using MiniAnalysis and pCLAMP10 software. After establishing a gigaseal (more than 2 GΩ), brief suction achieved whole-cell configuration.
We investigated the impact of HFS application in kindling animals on sEPSC of CA1 pyramidal neurons by recording sEPSC of CA1 pyramidal cells for 5 min in voltage-clamp mode. For the sEPSC recording, the voltage was clamped on -52 mV as GABAA reverse potential to omit the GABAergic inhibitory currents. The GABAA reverse potential was obtained by adding CNQX (20 μM, Tocris Bioscience, England) as AMPA (the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) receptor antagonist and AP-5 (50 μM, Tocris Bioscience, England) as NMDA receptor antagonist to ACSF. In this situation, the I-V curve of GABAA receptors was obtained, and the GABAA reverse potential was calculated (Sadeghian et al., 2020). sEPSCs were recorded at least 10 min after achieving the whole-cell configuration. The measured parameters of sEPSC included amplitude and inter-event interval.
Statistical analysis
Obtained data were averaged and presented as Mean±SEM. Statistical analysis was conducted using GraphPad Prism software, version 6.01 for Windows (GraphPad Software, Ca, USA). To assess the impact of HFS application on various parameters of sEPSC, we employed one-way ANOVA followed by post-hoc Tukey’s test to compare different parameters of sEPSC among different groups. The significant difference was considered when the measured P<0.05.
3. Results
No significant difference was observed in the number of stimulating days required to achieve full kindled seizures among the kindled (7-12 days) and kindled+HFS (7-12 days) groups. The AD threshold was also in a similar range (60-80 μA) in the two groups. Additionally, there was no significant difference in AD duration following the first stimulation at the AD threshold between kindled (93.19±6.86 s) and kindled+HFS groups (86.15±4.86 s). These findings indicated no significant differences in seizure susceptibility among the two experimental groups.
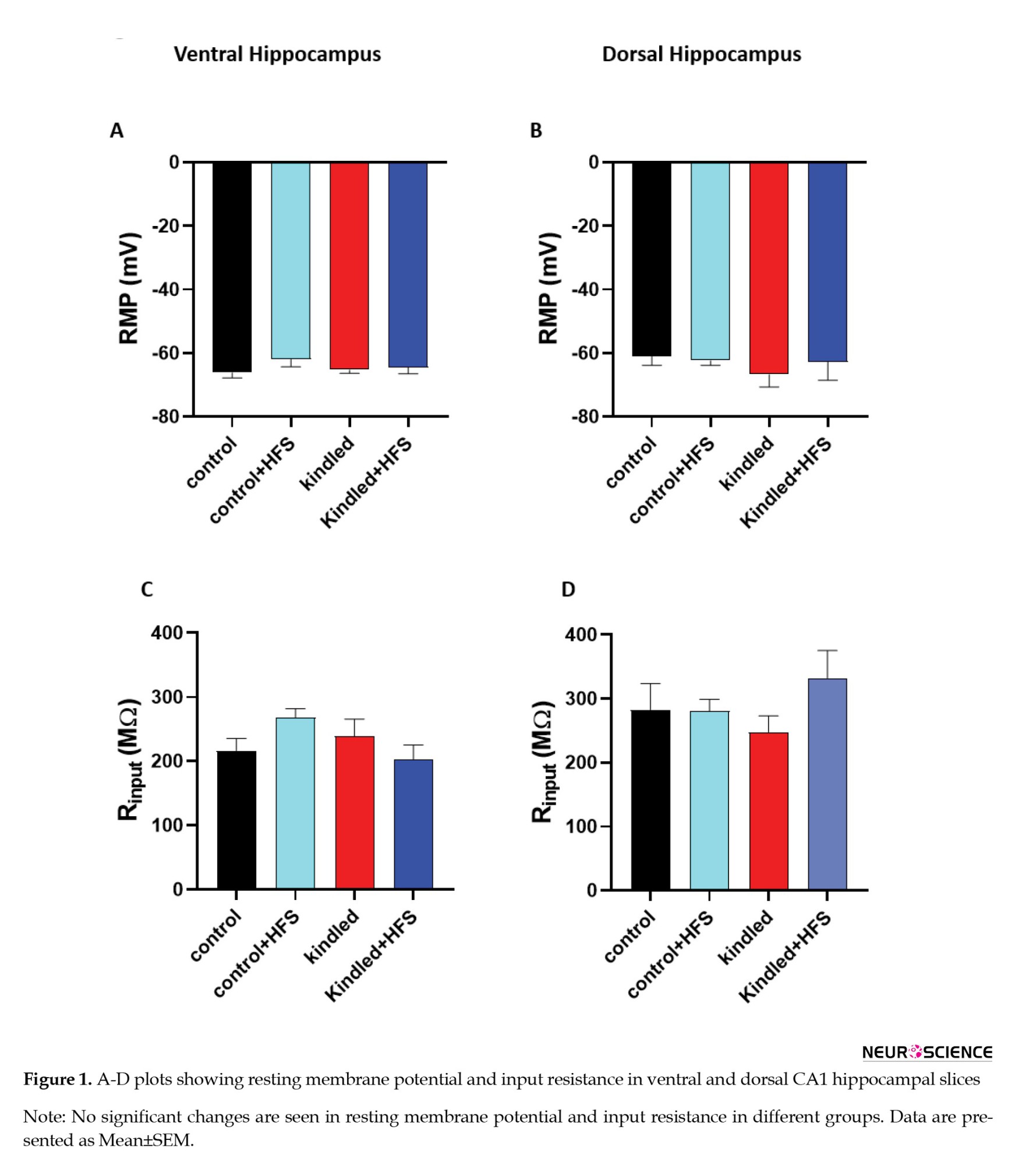
In the first experiment, we examined the effect of HFS application on sEPSC in hippocampal pyramidal cells of kindling animals. One-way ANOVA showed no significant difference in input resistance and resting membrane potential of ventral and dorsal hippocampal CA1 pyramidal cells among the different experimental groups (Figures 1A, 1B, 1C, and 1D).
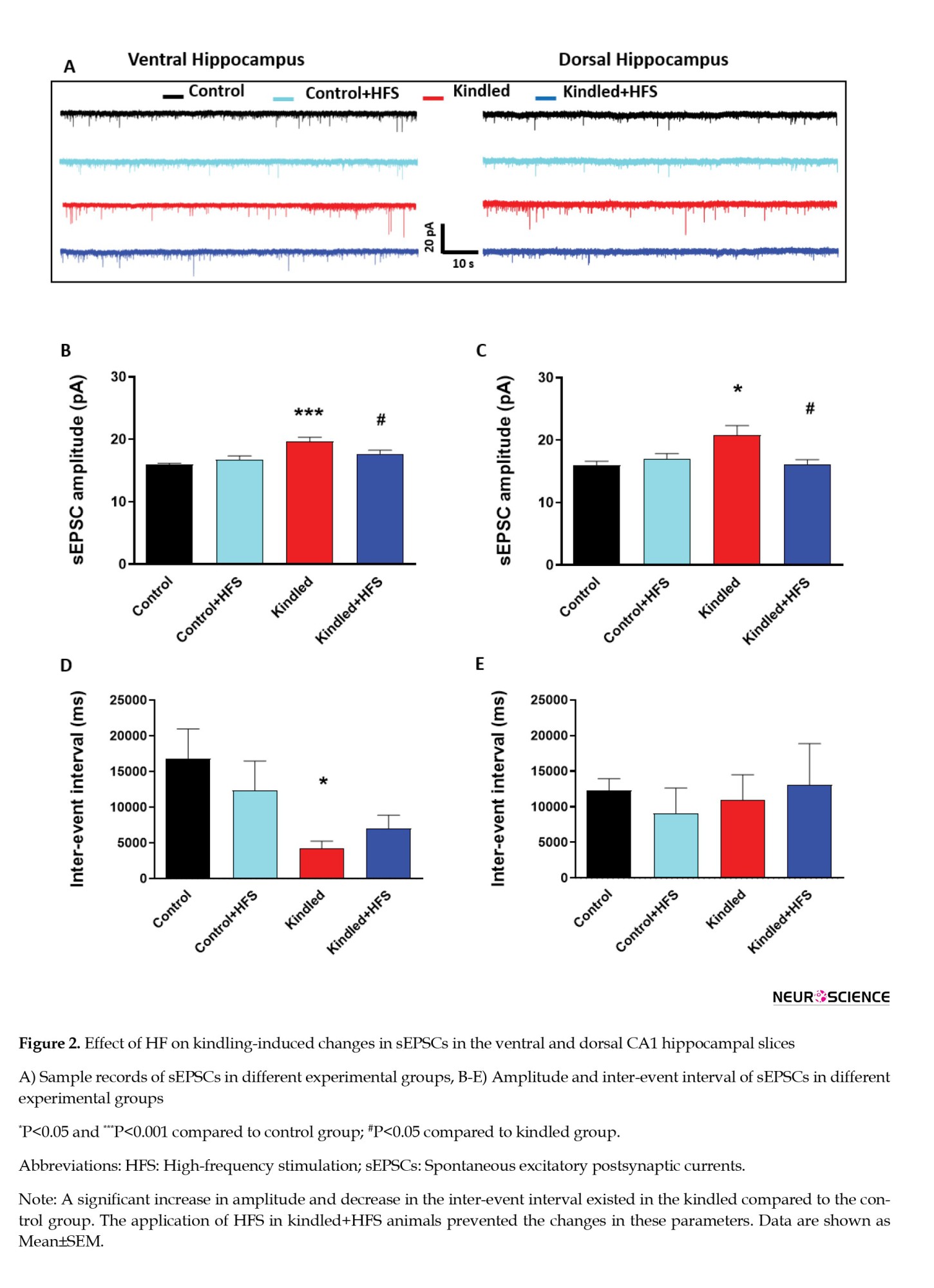
However, there was a significant increase in the amplitude of sEPSCs in ventral hippocampal CA1 pyramidal cells in kindled rats compared to the control group (P<0.001). Also, there was a significant decrease in the inter-event interval of sEPSCs in pyramidal cells in kindled rats compared to the control group (P<0.05). Applying HFS in the kindled+HFS group restored the changes in sEPSC amplitude and inter-event intervals; there was no significant difference between the kindled+HFS and the control group. Application of HFS alone had no significant effect on these parameters compared to the control group (Figures 2B, and 2D).
The parameters mentioned above were also evaluated in the dorsal hippocampal slices. There was a significant increase in the amplitude of sEPSCs in dorsal hippocampal CA1 pyramidal cells in kindled rats compared to the control group (P<0.001). There was also a significant decrease in the inter-event interval of sEPSCs in pyramidal cells in kindled rats compared to the control group (P<0.01). These parameters were restored to control values following applying HFS in the kindled+HFS group. There was no significant difference between the kindled+HFS and the control group. The application of HFS alone had no significant effect on these parameters compared to the control group (Figures 2C, and 2E).
4. Discussion
Our results suggested that applying HFS in the OB of kindled animals restored the seizure-induced changes in sEPSC parameters in pyramidal cells of the dorsal and ventral hippocampal CA1 area. sEPSCs indicate neuronal excitability and spontaneous firing (Meyer et al., 2008). Evaluating the parameters of sEPSCs provides a suitable approach to investigating the excitability of the nervous system. Increasing evidence indicates the participation of the N-methyl-d-aspartate (NMDA) subtype of excitatory amino acid receptor in the progression of experimental epilepsy. The special voltage-dependent activation of NMDA receptors gives synaptic transmission regenerative qualities, causing increased depolarization and burst firing, much like what is seen in epileptiform discharges (Herron et al., 1986; McNamara et al., 1988a; Mei et al., 2020; Nowak et al., 1984). Accordingly, it may suggest that applying HFS in the OB reduced the kindling-induced increment in neuronal excitability in both the dorsal and ventral hippocampus.
The OB has been shown to have a strong connection with the ventral hippocampus through the entorhinal cortex (Vanderwolf, 1992). This means that high-frequency electrical stimulation of OB may indirectly modulate the neuronal activation of hippocampal neurons. Our previous study shows that applying DBS in OB has an anticonvulsant effect on hippocampal kindled seizures (Khodadadi et al., 2022). Therefore, restoring the sEPSCs parameters in hippocampal CA1 neurons may be considered a possible mechanism of DBS’s anticonvulsant action when applied in the OB.
The cellular mechanism of this effectiveness and the effect of DBS on excitatory and inhibitory synaptic transmissions is unknown. It may be postulated that following the application of HFS, the release of GABA and glutamate from presynaptic terminals increases. Glutamate causes depolarization of the postsynaptic neuron terminal through AMPA receptors, and this depolarization is reduced by the inhibition caused by the stimulation of GABAA receptors. With the continuation of stimulation, the inhibitory mechanisms are lost. Depolarization continues, and secondarily, it causes the activation of NMDA receptors and the entry of calcium through voltage-dependent calcium channels, and as a result, the subsequent discharge waves begin (Morimoto et al., 2004). Therefore, a limitation of the present study was that we could not find the mechanism of HFS action. Accordingly, it can be suggested that a calcium voltage-dependent channel antagonist or a GABA receptor antagonist be used to determine their roles in the effects of HFS on seizure. Moreover, the effect of HFS may also be related to the activation of microglia and their anti-inflammatory effects, which have been implicated in epilepsy (Chen et al., 2020; Peng et al., 2019).
Also, HFS increases the release of GABA by activating GABAA receptors on the cell body, dendrites, and axonal ends of GABAergic neurons (Mantovani et al., 2009). HFS leads to reduced excitability, inhibition of epileptic activities, and inhibition of the nerve network of the target tissue (Bikson et al., 2001). However, further studies are required to reveal the exact mechanism(s) involved in the effect of HFS on sEPSCs.
The findings of this study revealed a notable increase in the amplitude and reduction in the inter-event interval of sEPSCs in pyramidal cells of the CA1 hippocampus in kindled animals. These changes in the parameters indicated a rise in the occurrence of sEPSCs and glutamatergic transmission in the CA1 hippocampus of kindled animals. These results supported previous reports that showed that NMDA responses of dentate gyrus granule cells and CA3 pyramidal neurons (Kraus et al., 1994) are profoundly altered following chronic epilepsy (kindling) (Hellier et al., 2009). The documented rise in glutamatergic transmission in our study and in previously mentioned reports could be associated with alterations in AMPA and or NMDA receptor functions. These alterations may arise from changes in the open duration of NMDA receptors and their inhibition by Mg2+, upregulation, and increased affinity of both NMDA and AMPA receptors (Ekonomou et al., 2001; Yeh et al., 1989) in epileptic neurons (Ghasemi & Schachter, 2011). Therefore, according to the observed changes in the amplitude of excitatory currents, postsynaptic mechanisms are probably involved. However, since our results showed the change of inter-event interval in the ventral part, presynaptic mechanisms can also be involved.
Interestingly, the changes in sEPSCs were observed in both dorsal and ventral hippocampus. The OB has many connections with the ventral hippocampus (Vanderwolf, 1992), and the role of the hippocampus in odor emotion and odor learning is exerted through the ventral hippocampus (Fanselow & Dong, 2010). Therefore, it may be expected that the anticonvulsant action of DBS in OB is exerted via the ventral hippocampus. However, significant changes in synaptic currents in the dorsal hippocampus revealed that applying DBS in OB has a widespread action in all hippocampal regions. Considering the important role of the dorsal hippocampus in seizure propagation (Fujita et al., 2014), the restoring effect of DBS on dorsal hippocampal neurons had an essential role in DBS anticonvulsant action.
Our findings indicate that applying HFS to the OB at a frequency of 130 Hz effectively reduced excitability in the hippocampal slices of kindling animals. As HFS is generally inhibitory, one hypothesis is that the inhibition of pro-seizure glutamatergic neurons may mediate the antiseizure effect of HFS (Wang et al., 2020). Further, patch clamp recording results showed that HFS in the OB significantly decreased the excitatory currents, primarily by reducing the amplitude and increasing the inter-event intervals of EPSCs. These effects show that both presynaptic and postsynaptic mechanisms may be involved in the inhibitory effects of HFS on kindling animals.
5. Conclusion
The present study showed that applying HFS in fully kindled animals could prevent increased spontaneous glutamatergic transmission in hippocampal CA1 pyramidal cells. This preventive effect of HFS could be considered a mechanism for HFS anticonvulsive action in kindled animals. Also, given both the connectivity between the olfactory system and the hippocampus and the fact that OB stimulation can be done through the olfactory epithelium, the olfactory system may be a noninvasive site for DBS administration in medically refractory epilepsy patients.
Ethical Considerations
Compliance with ethical guidelines
All experimental work and animal care procedures were conducted per international guidelines governing the use of laboratory animals. They were approved by the Tarbiat Modares University Ethics Committee for Animal Research, Tehran, Iran (Code: IR.MODARES.REC.1399.088). Measures were taken to minimize the number of animals utilized and any distress they experienced.
Funding
This study was supported by grants from the Vice Chancellor for Research at Tarbiat Modares University, Tehran, Iran (Grant No.: IG-39709), and the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant No.: 4030727).
Authors' contributions
Conceptualization: Javad Mirnajafi-Zadeh, Mohammad Reza Raoufy, Amir Shojaei, and Yaghoub Fathollahi; Writing the original draft: Parisa Zarei and Javad Mirnajafi-Zadeh; Methodology, investigation, review, and editing: All authors; Funding acquisition and supervision: Javad Mirnajafi-Zadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciated the Vice Chancellor for Research at Tarbiat Modares University, Tehran, Iran for supporting the project.
References
Approximately 50 million people worldwide have epilepsy, making it the third most prevalent chronic neurological condition (Ghosh et al., 2021). Despite the widespread occurrence of epilepsy, there remains a lack of definitive treatment for epilepsy patients. While anticonvulsant medication serves as the predominant therapeutic approach, its effectiveness lies in suppressing seizures rather than addressing the fundamental brain irregularities responsible for epilepsy (Macdonald & Kelly, 1995). Consequently, there is a need to explore novel methods aimed at mitigating the adverse effects of seizures on the brain.
The kindling model represents a progressive evolution of focal seizures followed by focal to bilateral tonic seizures triggered by repetitive electrical or chemical stimulation (Cheng et al., 2020). The mechanism underlying epileptogenesis is thought to involve excessive activation of excitatory pathways in the brain, which includes heightened glutamate transmission and an imbalance between neuronal excitation and inhibition. It has been widely recognized that the increase in glutamate receptor activity plays a significant role in initiating and propagating epileptic discharges throughout the kindling process (McNamara et al., 1988b).
Deep brain stimulation (DBS) has undergone extensive clinical investigation as a treatment modality for various brain disorders, including Parkinson disease, epilepsy, pain management, addiction, stroke recovery, obsessive-compulsive disorder, and depression (Kuhn et al., 2011; Sandoval-Pistorius et al., 2023). One probable mechanism of DBS is its ability to induce depressive effects, which may prevent neurons from over-firing, particularly in certain applications.
DBS is usually applied at high frequencies (i.e. high-frequency stimulation: HFS), and 130 Hz is one of its most common frequencies in seizure control in both clinical and experimental studies (Covolan et al., 2014; Lee et al., 2006; Wyckhuys et al., 2010). Three probable mechanisms of HFS on neuronal activity have been proposed: Inhibition, excitation, and modulation. Previous research has indicated that HFS may reduce neuronal firing by activating inhibitory synapses within neural circuits (Alhourani et al., 2015) or depleting neurotransmitters at excitatory synapses (Iremonger et al., 2006). Nonetheless, in some investigations incorporating animal experiments and mathematical simulations, the researchers have demonstrated that HFS may elevate the firing rate of neuronal action potentials (Deniau et al., 2010a). These controversies in the effects of HFS on neuronal activity may be explained by the third possible mechanism, i.e., modulation. HFS may alter neurons’ firing patterns or rhythm rather than solely modifying neuronal firing rates (Florence et al., 2016; Herrington et al., 2016; McConnell et al., 2012). Enhancing the presynaptic inhibition is also one of the proposed DBS anticonvulsant mechanisms. This enhancement may occur through stimulation, hyperpolarizing local neuronal somas and dendrites, inducing depolarization blockage, and neurotransmitter depletion (Deniau et al., 2010b; Montgomery Jr & Gale, 2008).
Knowing little about the precise anticonvulsant mechanisms of DBS made it difficult to widely use epilepsy treatment (Chiken & Nambu, 2016). Therefore, these mechanisms need to be investigated. In the present study, we attempted to examine the effect of high-frequency-DBS (HFS) on kindling-induced changes in excitatory postsynaptic currents (EPSCs) in CA1 hippocampal pyramidal cells.
2. Materials and Methods
Study animals
In this experimental investigation, 17 male Wistar rats were individually housed in animal cages (each rat was kept separately in one cage) maintained at an ambient temperature ranging from 22 ˚C to 25 ˚C and subjected to a 12-hour light/12-hour dark cycle with lights on from 6:00 AM to 6:00 PM. The animals were 2-4 months old (200-280 g) at surgery and had access to water and food ad libitum.
Experimental design
We assessed how HFS influenced synaptic currents in fully kindling animals. Subjects were divided into four groups: a) The control group (6 rats), in which animals underwent the surgical procedure but were not subjected to either HFS or kindling stimulations; b) The control+HFS group (3 rats), in which animals underwent similar experimental procedures to the control group but received HFS; c) Kindling group (5 rats) that received kindling stimulation and after achieving complete kindled state, their hippocampal slices were prepared 48 h after the last kindling stimulation for patch clamp recording (these animals did not receive DBS), and d) kindled+HFS group (3 rats) that received HFS after achieving complete kindled state and their hippocampal slices were prepared similar to the kindled group. In the control and control+HFS groups, hippocampal slices were prepared at a time duration similar to the kindled and kindled+HFS groups.
In this study, the number of recorded cells in each experimental group was 13 cells/12 slices in the control group, 9 cells/5 slices in the control+HFS group, 10 cells/9 slices in the kindled group, and 8 cells/5 slices in the kindled+HFS group.
Surgery
The rats underwent deep anesthesia by a mixture of ketamine and xylazine (Sigma, England, 100/10 mg/kg, intraperitoneal injection). They were securely positioned in a horizontally flat skull configuration in a stereotaxic apparatus. The animal skull was exposed following a cut in the scalp. A bipolar stimulating electrode and a monopolar recording electrode were carefully implanted into the hippocampal CA1 region of the right hemisphere at coordinates of 5.3 mm posterior, 5.2 mm lateral to the bregma, and 6.5 mm below the dura. These electrodes served for both kindling stimulations and after-discharge recording. Additional bipolar stimulating electrodes were inserted into the right and left olfactory bulbs (OBs) (coordinated as 7.5 mm anterior to bregma, 1.0 mm lateral to right or left, and 1.6 mm below the dura) for HFS. Electrodes were Teflon-coated stainless-steel electrodes (A-M Systems, Inc., WA, USA, 127 μm in diameter). The electrodes were checked to be completely insulated except at their tips. Additionally, a monopolar electrode connected to a stainless-steel screw was positioned in the skull above the occipital cortex to serve as a reference and or ground electrode.
To induce kindled seizures in animals, after discharge (AD) threshold was measured by applying a monophasic square wave with a pulse duration of 1 ms and frequency of 50 Hz for 2 s (Ghafouri et al., 2016a). In the first step, the stimulus was administered at 30 µA. ADs were identified as spikes with amplitudes higher than twice the baseline activity and frequencies of at least 1 Hz. If ADs were recorded for at least 8 seconds, the applied stimulating current was considered AD threshold. If AD were not recorded, the stimulus intensity was increased in the steps of 10 µA at 10 min intervals until the ADs were recorded for 8 s or more (Khodadadi et al., 2022). In the kindling model of seizure, the presence of ADs at the AD threshold is crucial for seizure progression. Therefore, AD recording is necessary to confirm the proper development of the kindling procedure.
Kindling induction
As explained previously (Khodadadi et al., 2022), the AD threshold was determined following a 7-day recovery period post-surgery. Rats were electrically stimulated at the AD threshold 6 times per day at inter-stimulus intervals of 20 min. Epileptiform ADs were recorded from the hippocampal CA1 region, followed by kindling stimulations using a PC-based data acquisition system (BIODAC ES1721, TRITA Health Technology Co., Tehran, Iran). Seizure severity was assessed using Racine’s scale (Racine et al., 1977): Stage 0 (no convulsions), stage 1 (facial automatism), stage 2 (head nodding), stage 3 (unilateral forelimb clonus), stage 4 (bilateral forelimb clonus), and stage 5 (rearing, falling, and generalized convulsions). Animals were considered fully kindled when they displayed at least one stage 5 seizure activity in three consecutive days.
Applying HFS in OB
To investigate the impact of applying HFS in the OB on hippocampal kindled seizures, fully kindled rats underwent four sets of bilateral HFS in the OB using stimulating electrodes. The first set of HFS was administered in fully kindled animals immediately after the last kindling stimulation once the ADs were finished. The second set of HFS was delivered 6 hours later. The third set of HFS was applied on the subsequent day (24 h after the first HFS), and the fourth set of HFS was administered 6 h after the third HFS. Each set of HFS contained 4 trains of monophasic square waves with a pulse width of 0.1 ms. The duration of each train was 200 s, with an inter-train interval of 100 s. These trains were applied as high-frequency, at 130 Hz; each train contained 26,000 pulses. HFS parameters were determined based on our previous experiments (Ghafouri et al., 2016b; Sadeghian et al., 2020).
Whole-cell patch clamp recording
Whole-cell patch clamp recordings were run in dorsal and ventral hippocampal slices. The procedure of brain slice preparation was similar to our previous experiment (Ghafouri et al., 2016a). Briefly, rats were killed by decapitation while anesthetized with CO2. Then, the right hemisphere was rapidly removed and submerged in an ice-cold cutting solution that contained 2.5 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 1 mM NaH2PO4, 26.2 mM NaHCO3, 238 mM sucrose, and 11 mM D-glucose and bubbled with 95% O2- 5% CO2. The osmolarity was adjusted to 290-300 mOsm. We used a vibratome (1000 Plus Sectioning System, Vibratome, MO, USA) to prepare the transverse slices (300 μm). Subsequently, the right hippocampi were dissected out and transferred to standard artificial cerebrospinal fluid (ACSF) (continuously bubbled with 95% O2- 5% CO2) that consisted of 125 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 10 mM D-glucose, 2 mM CaCl2, and 1.3 mM MgCl2. The osmolality was in the 290-300 mOsm range, and pH was adjusted to 7.2-7.35 by 1 M NaOH. Slices were then incubated for one hour at 35 ˚C and stored at room temperature (23-25 ˚C) until individually transferred to a submerged recording chamber. All chemicals were purchased from Sigma, England.
Slices were moved to a plexiglas recording chamber in a fixed-stage upright microscope (Axioskop 2 FS MOT, Carl Zeiss, Germany). The chamber was continuously perfused with ACSF at a 1.5-2.5 mL/min rate. The solution was bubbled by carbogen (95% O2 and 5% CO2), and its pH was adjusted to 7.2-7.4. All recordings were conducted at room temperature (23-25 ˚C). To visualize the pyramidal cells in the hippocampal CA1 area, an IR-CCD camera (IR-1000, MTI, USA) with a ×40 water immersion objective lens was employed. Recorded cells were chosen based on their characteristic pyramidal shape and smooth, low-contrast appearance. Whole-cell patch clamp recordings were conducted in voltage-clamp mode using recording microelectrodes (1.5 mm outer diameter, borosilicate glass, GC150-11, Harvard Apparatus, UK) pulled with a horizontal puller (P-97, Sutter Instruments, USA) and filled with intracellular solutions tailored for spontaneous EPSCs (sEPSCs) recordings. The internal solution filled the microelectrode contained 135 mM K-gluconate, 20 mM KCl, 10 mM HEPES, 0.2 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetra acetic acid (EGTA), 7 mM disodium-phosphocreatine, 2 mM MgATP, 0.3 mM NaGTP and 1 mM QX-314 for the sEPSC recording.
Electrode tip resistance in the bath ranged from 5 to 8 MΩ, and series resistance varied from 18 to 30 MΩ. Cells were discarded if the series resistance changed by more than 20% during the experiment. Capacitance compensation was performed during recordings. Data were low-pass filtered at 3 kHz and sampled at 10 kHz using a MultiClamp 700B amplifier equipped with a Digidata 1440A/D converter (Molecular Devices, CA, USA). The recorded signal was stored on a PC for offline analysis using MiniAnalysis and pCLAMP10 software. After establishing a gigaseal (more than 2 GΩ), brief suction achieved whole-cell configuration.
We investigated the impact of HFS application in kindling animals on sEPSC of CA1 pyramidal neurons by recording sEPSC of CA1 pyramidal cells for 5 min in voltage-clamp mode. For the sEPSC recording, the voltage was clamped on -52 mV as GABAA reverse potential to omit the GABAergic inhibitory currents. The GABAA reverse potential was obtained by adding CNQX (20 μM, Tocris Bioscience, England) as AMPA (the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) receptor antagonist and AP-5 (50 μM, Tocris Bioscience, England) as NMDA receptor antagonist to ACSF. In this situation, the I-V curve of GABAA receptors was obtained, and the GABAA reverse potential was calculated (Sadeghian et al., 2020). sEPSCs were recorded at least 10 min after achieving the whole-cell configuration. The measured parameters of sEPSC included amplitude and inter-event interval.
Statistical analysis
Obtained data were averaged and presented as Mean±SEM. Statistical analysis was conducted using GraphPad Prism software, version 6.01 for Windows (GraphPad Software, Ca, USA). To assess the impact of HFS application on various parameters of sEPSC, we employed one-way ANOVA followed by post-hoc Tukey’s test to compare different parameters of sEPSC among different groups. The significant difference was considered when the measured P<0.05.
3. Results
No significant difference was observed in the number of stimulating days required to achieve full kindled seizures among the kindled (7-12 days) and kindled+HFS (7-12 days) groups. The AD threshold was also in a similar range (60-80 μA) in the two groups. Additionally, there was no significant difference in AD duration following the first stimulation at the AD threshold between kindled (93.19±6.86 s) and kindled+HFS groups (86.15±4.86 s). These findings indicated no significant differences in seizure susceptibility among the two experimental groups.
In the first experiment, we examined the effect of HFS application on sEPSC in hippocampal pyramidal cells of kindling animals. One-way ANOVA showed no significant difference in input resistance and resting membrane potential of ventral and dorsal hippocampal CA1 pyramidal cells among the different experimental groups (Figures 1A, 1B, 1C, and 1D).

However, there was a significant increase in the amplitude of sEPSCs in ventral hippocampal CA1 pyramidal cells in kindled rats compared to the control group (P<0.001). Also, there was a significant decrease in the inter-event interval of sEPSCs in pyramidal cells in kindled rats compared to the control group (P<0.05). Applying HFS in the kindled+HFS group restored the changes in sEPSC amplitude and inter-event intervals; there was no significant difference between the kindled+HFS and the control group. Application of HFS alone had no significant effect on these parameters compared to the control group (Figures 2B, and 2D).
The parameters mentioned above were also evaluated in the dorsal hippocampal slices. There was a significant increase in the amplitude of sEPSCs in dorsal hippocampal CA1 pyramidal cells in kindled rats compared to the control group (P<0.001). There was also a significant decrease in the inter-event interval of sEPSCs in pyramidal cells in kindled rats compared to the control group (P<0.01). These parameters were restored to control values following applying HFS in the kindled+HFS group. There was no significant difference between the kindled+HFS and the control group. The application of HFS alone had no significant effect on these parameters compared to the control group (Figures 2C, and 2E).

4. Discussion
Our results suggested that applying HFS in the OB of kindled animals restored the seizure-induced changes in sEPSC parameters in pyramidal cells of the dorsal and ventral hippocampal CA1 area. sEPSCs indicate neuronal excitability and spontaneous firing (Meyer et al., 2008). Evaluating the parameters of sEPSCs provides a suitable approach to investigating the excitability of the nervous system. Increasing evidence indicates the participation of the N-methyl-d-aspartate (NMDA) subtype of excitatory amino acid receptor in the progression of experimental epilepsy. The special voltage-dependent activation of NMDA receptors gives synaptic transmission regenerative qualities, causing increased depolarization and burst firing, much like what is seen in epileptiform discharges (Herron et al., 1986; McNamara et al., 1988a; Mei et al., 2020; Nowak et al., 1984). Accordingly, it may suggest that applying HFS in the OB reduced the kindling-induced increment in neuronal excitability in both the dorsal and ventral hippocampus.
The OB has been shown to have a strong connection with the ventral hippocampus through the entorhinal cortex (Vanderwolf, 1992). This means that high-frequency electrical stimulation of OB may indirectly modulate the neuronal activation of hippocampal neurons. Our previous study shows that applying DBS in OB has an anticonvulsant effect on hippocampal kindled seizures (Khodadadi et al., 2022). Therefore, restoring the sEPSCs parameters in hippocampal CA1 neurons may be considered a possible mechanism of DBS’s anticonvulsant action when applied in the OB.
The cellular mechanism of this effectiveness and the effect of DBS on excitatory and inhibitory synaptic transmissions is unknown. It may be postulated that following the application of HFS, the release of GABA and glutamate from presynaptic terminals increases. Glutamate causes depolarization of the postsynaptic neuron terminal through AMPA receptors, and this depolarization is reduced by the inhibition caused by the stimulation of GABAA receptors. With the continuation of stimulation, the inhibitory mechanisms are lost. Depolarization continues, and secondarily, it causes the activation of NMDA receptors and the entry of calcium through voltage-dependent calcium channels, and as a result, the subsequent discharge waves begin (Morimoto et al., 2004). Therefore, a limitation of the present study was that we could not find the mechanism of HFS action. Accordingly, it can be suggested that a calcium voltage-dependent channel antagonist or a GABA receptor antagonist be used to determine their roles in the effects of HFS on seizure. Moreover, the effect of HFS may also be related to the activation of microglia and their anti-inflammatory effects, which have been implicated in epilepsy (Chen et al., 2020; Peng et al., 2019).
Also, HFS increases the release of GABA by activating GABAA receptors on the cell body, dendrites, and axonal ends of GABAergic neurons (Mantovani et al., 2009). HFS leads to reduced excitability, inhibition of epileptic activities, and inhibition of the nerve network of the target tissue (Bikson et al., 2001). However, further studies are required to reveal the exact mechanism(s) involved in the effect of HFS on sEPSCs.
The findings of this study revealed a notable increase in the amplitude and reduction in the inter-event interval of sEPSCs in pyramidal cells of the CA1 hippocampus in kindled animals. These changes in the parameters indicated a rise in the occurrence of sEPSCs and glutamatergic transmission in the CA1 hippocampus of kindled animals. These results supported previous reports that showed that NMDA responses of dentate gyrus granule cells and CA3 pyramidal neurons (Kraus et al., 1994) are profoundly altered following chronic epilepsy (kindling) (Hellier et al., 2009). The documented rise in glutamatergic transmission in our study and in previously mentioned reports could be associated with alterations in AMPA and or NMDA receptor functions. These alterations may arise from changes in the open duration of NMDA receptors and their inhibition by Mg2+, upregulation, and increased affinity of both NMDA and AMPA receptors (Ekonomou et al., 2001; Yeh et al., 1989) in epileptic neurons (Ghasemi & Schachter, 2011). Therefore, according to the observed changes in the amplitude of excitatory currents, postsynaptic mechanisms are probably involved. However, since our results showed the change of inter-event interval in the ventral part, presynaptic mechanisms can also be involved.
Interestingly, the changes in sEPSCs were observed in both dorsal and ventral hippocampus. The OB has many connections with the ventral hippocampus (Vanderwolf, 1992), and the role of the hippocampus in odor emotion and odor learning is exerted through the ventral hippocampus (Fanselow & Dong, 2010). Therefore, it may be expected that the anticonvulsant action of DBS in OB is exerted via the ventral hippocampus. However, significant changes in synaptic currents in the dorsal hippocampus revealed that applying DBS in OB has a widespread action in all hippocampal regions. Considering the important role of the dorsal hippocampus in seizure propagation (Fujita et al., 2014), the restoring effect of DBS on dorsal hippocampal neurons had an essential role in DBS anticonvulsant action.
Our findings indicate that applying HFS to the OB at a frequency of 130 Hz effectively reduced excitability in the hippocampal slices of kindling animals. As HFS is generally inhibitory, one hypothesis is that the inhibition of pro-seizure glutamatergic neurons may mediate the antiseizure effect of HFS (Wang et al., 2020). Further, patch clamp recording results showed that HFS in the OB significantly decreased the excitatory currents, primarily by reducing the amplitude and increasing the inter-event intervals of EPSCs. These effects show that both presynaptic and postsynaptic mechanisms may be involved in the inhibitory effects of HFS on kindling animals.
5. Conclusion
The present study showed that applying HFS in fully kindled animals could prevent increased spontaneous glutamatergic transmission in hippocampal CA1 pyramidal cells. This preventive effect of HFS could be considered a mechanism for HFS anticonvulsive action in kindled animals. Also, given both the connectivity between the olfactory system and the hippocampus and the fact that OB stimulation can be done through the olfactory epithelium, the olfactory system may be a noninvasive site for DBS administration in medically refractory epilepsy patients.
Ethical Considerations
Compliance with ethical guidelines
All experimental work and animal care procedures were conducted per international guidelines governing the use of laboratory animals. They were approved by the Tarbiat Modares University Ethics Committee for Animal Research, Tehran, Iran (Code: IR.MODARES.REC.1399.088). Measures were taken to minimize the number of animals utilized and any distress they experienced.
Funding
This study was supported by grants from the Vice Chancellor for Research at Tarbiat Modares University, Tehran, Iran (Grant No.: IG-39709), and the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant No.: 4030727).
Authors' contributions
Conceptualization: Javad Mirnajafi-Zadeh, Mohammad Reza Raoufy, Amir Shojaei, and Yaghoub Fathollahi; Writing the original draft: Parisa Zarei and Javad Mirnajafi-Zadeh; Methodology, investigation, review, and editing: All authors; Funding acquisition and supervision: Javad Mirnajafi-Zadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciated the Vice Chancellor for Research at Tarbiat Modares University, Tehran, Iran for supporting the project.
References
Alhourani, A., McDowell, M. M., Randazzo, M. J., Wozny, T. A., Kondylis, E. D., & Lipski, W. J., et al. (2015). Network effects of deep brain stimulation. Journal of Neurophysiology, 114(4), 2105–2117. [DOI:10.1152/jn.00275.2015] [PMID]
Bikson, M., Lian, J., Hahn, P. J., Stacey, W. C., Sciortino, C., & Durand, D. M. (2001). Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. The Journal of Physiology, 531(Pt 1), 181-191. [DOI:10.1111/j.1469-7793.2001.0181j.x] [PMID]
Chen, Q. L., Xia, L., Zhong, S. P., Wang, Q., Ding, J., & Wang, X. (2020). Bioinformatic analysis identifies key transcriptome signatures in temporal lobe epilepsy. CNS Neuroscience & Therapeutics, 26(12), 1266–1277. [DOI:10.1111/cns.13470] [PMID]
Chen, Q. L., Xia, L., Zhong, S. P., Wang, Q., Ding, J., & Wang, X. (2020). Bioinformatic analysis identifies key transcriptome signatures in temporal lobe epilepsy. CNS Neuroscience & Therapeutics, 26(12), 1266–1277. [DOI:10.1111/cns.13470] [PMID]
Chiken, S., & Nambu, A. (2016). Mechanism of deep brain stimulation: Inhibition, excitation, or disruption? The Neuroscientist, 22(3), 313-322. [DOI:10.1177/1073858415581986] [PMID]
Covolan, L., de Almeida, A. C., Amorim, B., Cavarsan, C., Miranda, M. F., & Aarão, M. C., et al. (2014). Effects of anterior thalamic nucleus deep brain stimulation in chronic epileptic rats. Plos One, 9(6), e97618. [DOI:10.1371/journal.pone.0097618] [PMID]
Deniau, J. M., Degos, B., Bosch, C., & Maurice, N. (2010). Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. The European Journal of Neuroscience, 32(7), 1080–1091. [DOI:10.1111/j.1460-9568.2010.07413.x] [PMID]
Deniau, J. M., Degos, B., Bosch, C., & Maurice, N. (2010). Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. The European Journal of Neuroscience, 32(7), 1080–1091. [DOI:10.1111/j.1460-9568.2010.07413.x] [PMID]
Ekonomou, A., Smith, A. L., & Angelatou, F. (2001). Changes in AMPA receptor binding and subunit messenger RNA expression in hippocampus and cortex in the pentylenetetrazole-induced ‘kindling’model of epilepsy. Molecular Brain Research, 95(1-2), 27-35. [DOI:10.1016/S0169-328X(01)00230-3]
Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7-19. [DOI:10.1016/j.neuron.2009.11.031] [PMID]
Florence, G., Sameshima, K., Fonoff, E. T., & Hamani, C. (2016). Deep brain stimulation: More complex than the inhibition of cells and excitation of fibers. The Neuroscientist, 22(4), 332-345. [DOI:10.1177/1073858415591964] [PMID]
Fujita, S., Toyoda, I., Thamattoor, A. K., & Buckmaster, P. S. (2014). Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. The Journal of Neuroscience, 34(50), 16671-16687. [DOI:10.1523/JNEUROSCI.0584-14.2014] [PMID]
Ghafouri, S., Fathollahi, Y., Javan, M., Shojaei, A., Asgari, A., & Mirnajafi-Zadeh, J. (2016). Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Research, 126, 37-44. [DOI:10.1016/j.eplepsyres.2016.06.010] [PMID]
Ghafouri, S., Fathollahi, Y., Javan, M., Shojaei, A., Asgari, A., & Mirnajafi-Zadeh, J. (2016). Effect of low frequency stimulation on impaired spontaneous alternation behavior of kindled rats in Y-maze test. Epilepsy Research, 126, 37-44. [DOI:10.1016/j.eplepsyres.2016.06.010] [PMID]
Ghasemi, M., & Schachter, S. C. (2011). The NMDA receptor complex as a therapeutic target in epilepsy: A review. Epilepsy & Behavior, 22(4), 617-640. [DOI:10.1016/j.yebeh.2011.07.024] [PMID]
Ghosh, S., Sinha, J. K., Khan, T., Devaraju, K. S., Singh, P., & Vaibhav, K., et al. (2021). Pharmacological and therapeutic approaches in the treatment of epilepsy. Biomedicines, 9(5), 470. [DOI:10.3390/biomedicines9050470] [PMID]
Hellier, J. L., White, A., Williams, P. A., Dudek, F. E., & Staley, K. J. (2009). NMDA receptor-mediated long-term alterations in epileptiform activity in experimental chronic epilepsy. Neuropharmacology, 56(2), 414-421. [DOI:10.1016/j.neuropharm.2008.09.009] [PMID]
Herrington, T. M., Cheng, J. J., & Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. Journal of Neurophysiology, 115(1), 19-38. [DOI:10.1152/jn.00281.2015] [PMID]
Herron, C. E., Lester, R. A. J., Coan, E. J., & Collingridge, G. L. (1986). Frequency-dependent involvement of NMDA receptors in the hippocampus: A novel synaptic mechanism. Nature, 322(6076), 265-268. [DOI:10.1038/322265a0] [PMID]
Iremonger, K. J., Anderson, T. R., Hu, B., & Kiss, Z. H. (2006). Cellular mechanisms preventing sustained activation of cortex during subcortical high-frequency stimulation. Journal of Neurophysiology, 96(2), 613-621. [DOI:10.1152/jn.00105.2006] [PMID]
Khodadadi, M., Zare, M., Rezaei, M., Bakhtiarzadeh, F., Barkley, V., & Shojaei, A., et al. (2022). Effect of low frequency stimulation of olfactory bulb on seizure severity, learning, and memory in kindled rats. Epilepsy Research, 188, 107055. [DOI:10.1016/j.eplepsyres.2022.107055] [PMID]
Kraus, J. E., Yeh, G. C., Bonhaus, D. W., Nadler, J. V., & McNamara, J. O. (1994). Kindling induces the long-lasting expression of a novel population of NMDA receptors in hippocampal region CA3. The Journal of Neuroscience, 14(7), 4196-4205. [DOI:10.1523/JNEUROSCI.14-07-04196.1994] [PMID]
Kuhn, J., Möller, M., Mueller, U., Bogerts, B., Mann, K., & Gründler, T. O. J. (2011). Deep brain stimulation for the treatment of addiction. Addiction, 106(8), 1536-1538. [DOI:10.1111/j.1360-0443.2011.03452.x] [PMID]
Lee, K. J., Jang, K. S., & Shon, Y. M. (2006). Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochirurgica. Supplement, 99, 87–91. [DOI:10.1007/978-3-211-35205-2_17] [PMID]
Macdonald, R. L., & Kelly, K. M. (1995). Antiepileptic drug mechanisms of action. Epilepsia, 36(Suppl 2), S2–S12.[DOI:10.1111/j.1528-1157.1995.tb05996.x] [PMID]
Mantovani, M., Moser, A., Haas, C. A., Zentner, J., & Feuerstein, T. J. (2009). GABA(A) autoreceptors enhance GABA release from human neocortex: towards a mechanism for high-frequency stimulation (HFS) in brain?. Naunyn-Schmiedeberg's archives of Pharmacology, 380(1), 45–58. [DOI:10.1007/s00210-009-0410-3] [PMID]
McConnell, G. C., So, R. Q., Hilliard, J. D., Lopomo, P., & Grill, W. M. (2012). Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. The Journal of Neuroscience, 32(45), 15657-15668. [DOI:10.1523/JNEUROSCI.2824-12.2012] [PMID]
McNamara, J. O., Russell, R. D., Rigsbee, L. C., & Bonhaus, D. W. (1988). Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology, 27(6), 563-568. [DOI:10.1016/0028-3908(88)90176-1] [PMID]
McNamara, J. O., Russell, R. D., Rigsbee, L., & Bonhaus, D. W. (1988). Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology, 27(6), 563-568. [DOI:10.1016/0028-3908(88)90176-1] [PMID]
Mei, Y. Y., Lee, M. H., Cheng, T. C., Hsiao, I. H., Wu, D. C., & Zhou, N. (2020). NMDA receptors sustain but do not initiate neuronal depolarization in spreading depolarization. Neurobiology of Disease, 145, 105071. [DOI:10.1016/j.nbd.2020.105071] [PMID]
Meyer, D. A., Carter, J. M., Johnstone, A. F. M., & Shafer, T. J. (2008). Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. Neurotoxicology, 29(2), 213-225. [DOI:10.1016/j.neuro.2007.11.005] [PMID]
Montgomery, E. B., Jr, & Gale, J. T. (2008). Mechanisms of action of deep brain stimulation(DBS). Neuroscience and Biobehavioral Reviews, 32(3), 388–407. [DOI:10.1016/j.neubiorev.2007.06.003] [PMID]
Morimoto, K., Fahnestock, M., & Racine, R. J. (2004). Kindling and status epilepticus models of epilepsy: Rewiring the brain. Progress in Neurobiology, 73(1), 1-60. [DOI:10.1016/j.pneurobio.2004.03.009] [PMID]
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A., & Prochiantz, A. (1984). Magnesium gates glutamate-activated channels in mouse central neurones. Nature, 307(5950), 462-465. [DOI:10.1038/307462a0] [PMID]
Peng, J., Wang, K., Xiang, W., Li, Y., Hao, Y., & Guan, Y. (2019). Rosiglitazone polarizes microglia and protects against pilocarpine‐induced status epilepticus. CNS Neuroscience & Therapeutics, 25(12), 1363-1372. [DOI:10.1111/cns.13265] [PMID]
Racine, R., Rose, P. A., & Burnham, W. M. (1977). Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques, 4(4), 273–278. [DOI:10.1017/S0317167100025117] [PMID]
Sadeghian, A., Salari, Z., Azizi, H., Raoufy, M. R., Shojaei, A., & Kosarmadar, N., et al. (2020). The role of dopamine D2-like receptors in a “depotentiation-like effect” of deep brain stimulation in kindled rats. Brain Research, 1738, 146820. [DOI:10.1016/j.brainres.2020.146820] [PMID]
Sandoval-Pistorius, S. S., Hacker, M. L., Waters, A. C., Wang, J., Provenza, N. R., & Hemptinne, C. et al. (2023). Advances in deep brain stimulation: From mechanisms to applications. The Journal of Neuroscience, 43(45), 7575-7586. [DOI:10.1523/JNEUROSCI.1427-23.2023] [PMID]
Vanderwolf, C. H. (1992). Hippocampal activity, olfaction, and sniffing: an olfactory input to the dentate gyrus. Brain Research, 593(2), 197-208. [DOI:10.1016/0006-8993(92)91308-2] [PMID]
Wang, Y., Wang, Y., Xu, C., Wang, S., Tan, N., & Chen, C., et al. (2020). Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biological Psychiatry, 87(9), 843-856. [DOI:10.1016/j.biopsych.2019.11.014] [PMID]
Wyckhuys, T., Raedt, R., Vonck, K., Wadman, W., & Boon, P. (2010). Comparison of hippocampal deep brain stimulation with high (130 Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Research, 88(2-3), 239-246. [DOI:10.1016/j.eplepsyres.2009.11.014] [PMID]
Yeh, G. C., Bonhaus, D. W., Nadler, J. V., & McNamara, J. O. (1989). N-methyl-D-aspartate receptor plasticity in kindling: quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proceedings of the National Academy of Sciences of the United States of America, 86(20), 8157–8160. [DOI:10.1073/pnas.86.20.8157] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2024/05/7 | Accepted: 2024/07/28 | Published: 2025/03/1
Received: 2024/05/7 | Accepted: 2024/07/28 | Published: 2025/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





