Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 683-702 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Emmanuel I O, Alabi B, Sotunde O, Olowoparija S, Obaro M, Badejo A, et al . Evaluation of Anxiolytic Effects of Justicia secunda Methanol Leaf Extract and Its Chemical Constituents in Mice. BCN 2024; 15 (5) :683-702
URL: http://bcn.iums.ac.ir/article-1-2897-en.html
URL: http://bcn.iums.ac.ir/article-1-2897-en.html
Ileri Oluwa Emmanuel1 

 , Babatunde Alabi *2
, Babatunde Alabi *2 

 , Olasubomi Sotunde1
, Olasubomi Sotunde1 
 , Segun Olowoparija1
, Segun Olowoparija1 
 , Michael Obaro1
, Michael Obaro1 
 , Ayotunde Badejo1
, Ayotunde Badejo1 
 , Olufunmilayo Ologe3
, Olufunmilayo Ologe3 
 , Abayomi Ajayi1
, Abayomi Ajayi1 
 , Ifeoluwa Oguntoye4
, Ifeoluwa Oguntoye4 
 , Opeyemi Hammed5
, Opeyemi Hammed5 
 , Olugbenga Iwalewa1
, Olugbenga Iwalewa1 



 , Babatunde Alabi *2
, Babatunde Alabi *2 

 , Olasubomi Sotunde1
, Olasubomi Sotunde1 
 , Segun Olowoparija1
, Segun Olowoparija1 
 , Michael Obaro1
, Michael Obaro1 
 , Ayotunde Badejo1
, Ayotunde Badejo1 
 , Olufunmilayo Ologe3
, Olufunmilayo Ologe3 
 , Abayomi Ajayi1
, Abayomi Ajayi1 
 , Ifeoluwa Oguntoye4
, Ifeoluwa Oguntoye4 
 , Opeyemi Hammed5
, Opeyemi Hammed5 
 , Olugbenga Iwalewa1
, Olugbenga Iwalewa1 

1- Neuropharmacology Unit, Department of Pharmacology and Therapeutics, College of Medicine, University of Ibadan, Ibadan, Nigeria.
2- Department of Pharmacology & Therapeutics, Faculty of Basic Clinical Sciences, Bowen University, Iwo, Nigeria.
3- Department of Pharmacology and Therapeutics, College of Health Sciences, University of Ilorin, Ilorin, Nigeria.
4- Oranfe and Osun State Hospital, Ile Ife, Nigeria.
5- Department of Physiology, College of Health Science, Ogbomoso, Nigeria.
2- Department of Pharmacology & Therapeutics, Faculty of Basic Clinical Sciences, Bowen University, Iwo, Nigeria.
3- Department of Pharmacology and Therapeutics, College of Health Sciences, University of Ilorin, Ilorin, Nigeria.
4- Oranfe and Osun State Hospital, Ile Ife, Nigeria.
5- Department of Physiology, College of Health Science, Ogbomoso, Nigeria.
Full-Text [PDF 3026 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
With a lifetime prevalence of over 10%, anxiety and depression are major public health issues that impact a large portion of the general population and greatly increase the worldwide burden of disease. Anxiety disorders are thought to be responsible for 26.8 million disability-adjusted life years, according to the global burden of disease research, and depression is thought to affect 300 million people worldwide (Khosa, 2020). Anxiety is a physiological reaction that protects an organism against harm. In severe cases, it becomes pathological and can result in psychiatric and or cardiovascular illnesses (Filatova et al., 2021). Treatments for anxiety and depression are frequently associated with side effects like tachycardia, orthostatic hypotension, digestive problems, weight gain, sexual problems, and visual problems. Memory impairment is one of the main issues that arises after longer exposure (Garakani et al., 2020).
It is thought that dysregulation of some central nervous system neurotransmitters, including dopamine, serotonin, and gamma amino butyric acid (GABA), causes anxiety and depression (Filatova et al., 2021). Behavioral interventions, medication therapy, and psychotherapy are the current methods used to treat depression and anxiety (Beard & Delgadillo, 2019). In clinical practice, imipramine, desipramine, amoxapine, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) have proven to be the most effective medications for managing anxiety and depression (Garakani et al., 2020).
However, the two groups displayed several adverse outcomes, including addiction, increased suicidal thoughts, lower alertness, sexual dysfunction, and high costs (Garakani et al., 2020).
It is well known that benzodiazepines interact with GABA receptors’ allosteric sites. By binding to GABA receptors, benzodiazepines reduce excitability in the central nervous system. Although long-term benzodiazepine use is associated with adverse effects such as memory loss and cognitive decline, this action will reduce anxiety (Garakani et al., 2020).
Researchers are revaluing several plant species for their therapeutic chemical principles due to the many issues with conventional medications. Justicia secunda is one such plant species. In traditional medicine, leaf extracts treat various illnesses, including anemia, depression, sickle cell disease, and diabetes (Kitadi et al., 2020). When J. secunda leaves were screened for phytochemical content, alkaloids and polyphenols, including flavonoids, quinones, tannins, and anthocyanins, were found (Fagbohoun et al., 2022). According to Kamso et al. (2023), luteolin derivatives are the main secondary metabolites in the leaves. Strong anxiolytic substances are therefore expected, with fewer side effects and an earlier onset of action than those found in present medications. This study uses gas chromatography-mass spectrometry (GC-MS) to examine the bioactive compounds found in methanol leaf extract of J. secunda (MLEJS). It also looks at the anxiolytic-like and anti-depressant activities of MLEJS, the role of GABAa/benzodiazepine mechanisms, and MLEJS’s impact on proinflammatory cytokines, acetylcholinesterase activity, and biomarkers of depressive-like behavior induced by lipopolysaccharide.
2. Materials and Methods
Chemicals and reagents
Flumazenil (Neon Laboratories Ltd, India), diazepam (DZP) (Roche, Basel Switzerland), thiopental sodium (Kwality, India), lipopolysaccharide (LPS) (Escherichia coli serotype, 055:B5 Sigma-Aldrich, USA) are the chemicals used in this study. Imipramine hydrochloride (Sigma-Aldrich, USA), trichloroacetic acid (TCA), thiobarbituric acid (TBA), acetylthiocholine, Ellman reagent [5′, 5′-Dithiobis- (2-nitrobenzoate) DTNB], hydrogen peroxide, ammonium sulfate, phosphate buffered saline, distilled water and all other chemicals used were of analytical grade.
Extract preparation
The fresh, healthy leaves of J. secunda were obtained from the staff quarters of Obafemi Awolowo University Ile-Ife, Osun state; it was identified and authenticated at the Department of Forest Herbarium with the voucher specimen FHI number 112604.
After being shade-dried and ground into a powder using a high-capacity grinder, the leaves were immersed in 70% aqueous methanol at a 1:10 (w/v) ratio for three days. A solid extract yielding 23.3 g was produced by filtering it with Whitman filter paper and then drying it off in a rotary evaporator. After that, the dry extract was reconstituted in regular saline at quantities suitable for the different tests.
GC-MS phytochemical analysis
The Agilent Technologies GC-MS (GC-7820A, MS 5975C) was used for the analysis. It was applied to an HP‐5 capillary column coated with 5% phenyl methyl siloxane (30 mm length ×0.32 mm diameter ×0.25 μm film thickness). The instrument was initially set to 110 ºC and kept there for two minutes. After that, the oven temperature was raised to 280 ºC, at a rate of 4 ºC/min, and kept there for nine minutes. The injection port temperature was guaranteed 250 ºC, and the helium flow rate was 1.573 mL/min. The ionization voltage was 70 eV with an ion source temperature of 230 °C, quadrupole temperature of 150 °C, and transfer line temperature of 280 °C. The samples were injected in splitless mode, one µL at 260 °C injection temperature. A mass spectral scan range of 30-550 (m/z) was used. Compounds included in the plant sample were identified by matching the spectrum acquired from GC-MS analysis with a computer search on a NIST 14 mass spectra data repository. Mass spectrum GC-MS interpretation was done with the National Institute of Standards and Technology (NIST) database, which has over 62000 patterns. A comparison was made between the spectrums of the unknown and known components found in the NIST collection. We determined the components of the test materials’ names, molecular weights, and structures.
Study animals
This study employed male Swiss mice weighing 20–25 g. The rats were housed in clean, well-ventilated polypropylene plastic cages with bedding made of wood shavings. They were also given free access to water and fed commercial rat chow pellets. The ambient conditions were kept standard. The 3Rs (replacement, reduction, and refinement) of animal experiments were followed in the treatment of the animals, and pain and discomfort were minimized by providing enriching environments.
Experimental designs
The study design comprises an acute toxicity study (6 mice were used), an anxiolytic-like study (77 mice were used), and an antidepressant-like study (30 mice were used).
Acute toxicity test
An acute oral toxicity study was conducted using the Organization for Economic Co-operation and Development (OECD) 423 guideline. The mice (n=3) that had fasted overnight were given MLEJS orally at a maximum dosage of 2000 mg/kg. They were then monitored closely for two hours, and then for 24, 72, and finally for up to 14 days for any signs of lethality, a state of morbidity, or death concerning their behavioral, neurological, and autonomic profiles. To confirm the results and determine the dangerous class of LD50, the limit test was conducted on three additional mice in another group.
An acute oral toxicity investigation reveals that the MLEJS therapy had no toxicity or moribund state. This condition implies that the approximate LD50 was more than 2500 mg/kg and that the non-observable adverse effect dose level was larger than 2000 mg/kg.
Anxiolytic-like experiment
The animals were randomly divided into five groups, with seven animals apiece. Sixty minutes before the experiment, mice in groups 2-4 were given different doses of MLEJS (12.5, 25, and 50 mg/kg orally, respectively), while group 5 got a dose of DZP (1 mg/kg PO). Group 1 mice were given normal saline 10 mL/kg orally and served as the control. Benzodiazepines are known to have anxiolytic effects at low doses and cause drowsiness and myorelaxant effects at higher dosages (Galdino et al., 2012). To check for anxiolytic-like effects, we employed DZP (1 mg/kg) as a positive control. To clarify the potential participation of GABAa/benzodiazepine mechanisms of the extract, six further groups of mice (n=7) were pretreated with 6 mg/kg flumazenil intraperitoneally 15 minutes before the treatment with the most effective anxiolytic-like dose of MLEJS (50 mg/kg) or DZP. Group 1 mice were given orally normal saline (10 mL/kg) as the control. Group 2 mice received 6 mL/kg of flumazenil alone. Group 3 mice received 50 mL/kg of MLEJS only. Group 4 mice received 6 mL/kg of flumazenil + MLEJS (50 mg/kg). Group 5 mice received 1 mL/kg of DZP, and group 6 mice received 1 mL/kg of DZP + 6 mg/kg of flumazenil.
Antidepressant-like experiment
Mice were assigned to six different experimental groups (n=5). They were semi-randomized, so all groups’ mean body weights were comparable. After that, mice received orally MLEJS (12.5, 25, and 50 mg/kg) or imipramine (10 mg/kg) as positive control and vehicle (saline, 10 mL/kg as normal control) once daily for 7 days. On the last (seventh) day of the treatment, lipopolysaccharide (LPS) (0.83 mg/kg) was injected intraperitoneally after 30 minutes of respective treatment to all groups except the vehicle control group. After 24 hours of LPS administration, a battery of behavioral tests, including an open field test (OFT), forced swimming test (FST), and tail suspension test (TST), were performed to evaluate depressive-like behavior in mice (Barua et al., 2018).
Behavioral procedures
Myorelaxant and sedative anxiolytic tests
Test for motor coordination using rotarod
The animals were pre-selected in a training session 24 hours before the test based on their ability to stay on the bar (at 12 revolutions per minute) for 120 seconds. The test was conducted using a horizontal rotating rod (Ugo Basile). All four paws of the animals were placed onto the bar 60 minutes after the treatment with vehicle (10 mL/kg), MLEJS (12.5, 25, or 50 mg/kg), or DZP (1 or 5 mg/kg). The time it took for each mouse to fall was recorded, with a maximum of 120 seconds on the bar, and the number of falls was calculated within 60 seconds, with a maximum of three falls permitted (Kokubo et al., 2018).
Sedative test using thiopental sodium sleeping time
All groups received thiopental sodium (20 mg/kg, IP) an hour after oral dose of MLEJS (12.5, 25, and 50 mg/kg), DZP (1 and 5 mg/kg), or vehicle. Sleep latency is the amount of time after thiopental sodium delivery before the righting reflex is lost; sleeping time is the time between the loss and the reflex’s voluntary recovery. The apparatus was cleaned with 70% ethanol after each animal to avoid odor bias.
Anxiolytic activity tests
Test for anxiety using hole-board
The animals were placed in the middle of a perforated board and divided into 9 equal-sized squares 60 minutes after treatment; during 5 minutes, the number of head dips into the holes, and the number of squares crossed (with all four paws) were recorded. The apparatus was cleaned with 70% ethanol after every animal to avoid odor bias.
Test for anxiety using light and dark box
The participant is exposed to a novel environment consisting of protected (dark compartment) and unprotected (light compartment) locations during the light↔dark exploration test. It is believed that risk aversion and exploratory desire are inherently at odds, which prevents exploration from occurring. The animals were positioned in the middle of the light area facing the dark area entrance sixty minutes after treatment; the number of times they switched between the two compartments and the amount of time they spent in the light area was counted over five minutes.
Test for anxiolytic/anxiogenic properties using elevated plus maze
The experiment capitalizes on mice’s innate curiosity to investigate new surroundings. The arms of the maze, which were raised to a height of about one meter above the floor, were either open or exposed to the mouse or enclosed and protected. The animals were individually placed in the middle of a plus maze 60 minutes after treatment and watched for 5 minutes. The animal was tested by counting the number of times it entered and exited the open and enclosed arms and the amount of time it spent there. Anxiety was measured by converting the number of entries into open arms and the time spent there into percentages of all entries and time, respectively.
Involvement of GABAa/benzodiazepine mechanisms
The rats were intraperitoneally pre-treated with flumazenil (6 mg/kg; 15 min pretreatment), an antagonist of GABAa/benzodiazepine receptors, to look into potential mechanisms underpinning the anxiolytic actions of MLEJS.
Antidepressant activity tests
Test for spontaneous movement activity using the open field
This test was run to see if the MLEJS may impact the mice’s locomotor activity during the open-field test. After the mouse was put within the device, the number of times it crossed lines and reared over 5 minutes was used to calculate its locomotor activity. After testing each mouse, the equipment was washed with 70% ethanol and dried to remove any traces of the preceding animal’s scent.
Test for antidepressant activity using FST
Mice were used in the experiment, and an open cylindrical container with a diameter of 10 cm and a height of 25 cm holding 19 cm of water was used. During the test session, mice were kept in this unavoidable cylinder for 6 minutes, with the final 4 minutes of immobility being recorded. They were deemed immobile when they stopped fighting, floated still, and made only the motions required to maintain their heads above the water.
Test for antidepressant activity using TST
The mice could be individually suspended 50 cm above the floor on the table’s edge by applying sticky tape about 1 cm from the tip of the tail. The immobility periods for the different groups were recorded at intervals of 6 minutes. An animal was hanged if it did not exhibit any movement. It was considered immobile.
Assessment of oxidative stress and antioxidant status
Lipid peroxidation ((LPO)) and nitric oxide
According to Afolabi et al. (2022), the thiobarbituric reacting substance (TBARS) assay was used to determine the concentration of the LPO end product malondialdehyde (MDA). The amount of nitrite in the brain tissues was calculated using the Griess reagent method, as reported by Odebiyi et al. (2021).
Antioxidant status
As explained by Alabi et al., a reduced glutathione (GSH) concentration was found in the mice’s brain supernatant by applying an older Ellam technique (Alabi et al., 2023). Superoxide dismutase (SOD) activity was measured according to the Misra and Fridovich procedure, which Campos-Shimada et al. (2020) reported. The procedure that Hadwan and Kadhum (2018) had previously outlined was used to measure the amount of catalase.
Estimation of brain cytokines and enzyme
The manufacturer’s instructions were followed to determine the amounts of tumor necrosis factor (TNF)-α (BioLegend, USA; CAT NO 430904) and IL-6 (BioLegend, USA; CAT NO 431304) in the brain supernatant. The Ellman method was used to measure the amount of acetylcholinesterase in brain tissue, as explained by Khalil and Abass (2017).
Statistical analysis
The data were presented as Mean±SEM. All behavioral data were subjected to a normality test, a one-way analysis of variance (ANOVA) was used for all analyses (nonparametric analysis was used for the rotarod test), and a Tukey post-hoc test was used for multiple comparisons when necessary. One-way ANOVA was used to examine the biochemical data, and Tukey’s post-hoc test was then performed. Version 8 of GraphPad Prism (GraphPad Software, Inc. La Jolla, CA 92037 USA) was used to analyze the data. A P<0.05 was deemed statistically significant for every test. Since the study is exploratory overall, the P should be interpreted as descriptive rather than as a means of testing a hypothesis. The specification of all intergroup comparisons preceded data collection.
3. Results
GC-MS phytochemical analysis
A full-scan gas chromatogram of J. secunda’s methanol leaf extract is displayed in Figure 1.

It verified the existence of several bioactive substances with varying retention periods (RT). Table 1 lists the major chemicals determined by their RT and % peak area. Also, 9,12,15-octadecatrienoic acid (18.11%) was the most abundant component by peak area. It was followed by n-hexadecanoic acid (14.94%), phytol (7.92%), stigmasterol (5.39%), squalene (4.83%), neophytadiene (3.95%), campesterol (2.96%), vitamin E (2.51%), methyl stearate (1.21%), β-amyrin (1.17%), 9-octadecenamide (1.10%), 2-palmitoyl glycerol (0.91%), and 4a (2H)-naphthalenol (0.86%).

Effect of MLEJS on motor coordination in mice using rotarod apparatus
DZP (at 1 mg/kg) significantly increased the number of falls in the rotarod test, while MLEJS (12.5, 25, and 50 mg/kg) did not affect the number of falls (the Kruskal-Wallis [KW] test=14.60, P<0.01; Dunn test, P<0.001 vs vehicle group, Figure 2A). DZP (5 mg/kg) significantly prolonged the falling latency from the revolving rod (ANOVA: F(5, 24)=3.779, P<0.01, Figure 2B) (P<0.0001 vs vehicle).

Effect of MLEJS on sleeping test
DZP (1 or 5 mg/kg) reduced the sleep latency (ANOVA: F(5, 24)=13.17, P<0.001; Figure 3A) (P<0.05 and P<0.001 vs vehicle, respectively), while the MLEJS treatments did not significantly reduce the sleep latency (P>0.05 vs vehicle).

DZP (1 and 5 mg/kg) also enhanced the amount of time spent sleeping (ANOVA: F(5, 24)=128.53, P<0.001; Figure 3B) (P<0.01 and P<0.001 vs vehicle, respectively).
Anxiolytic-like effect of MLEJS in mice using light and dark boxes
In the light-dark test, the number of transitions between the light and dark compartments after oral treatment with MLEJS (25 and 50 mg/kg (P<0.05 and P<0.0001 vs vehicle, respectively) and DZP 1 mg/kg (P<0.001 vs vehicle) increased (ANOVA: F(4, 30)=11.74, P<0.0001; Figure 4). The duration of time in the light compartment was higher for all MLEJS treatments (12.5, 25, and 500 mg/kg) (P<0.05, P<0.05, and P<0.001 vs vehicle, respectively) (ANOVA: F(4, 30)=6.371, P<0.0008; Figure 4) and DZP (1 mg/kg) (P<0.001 vs vehicle).

Anxiolytic-like effect of MLEJS in mice using the hole board
Following oral treatment with MLEJS (25 and 50 mg/kg (P<0.05 and P<0.0001 vs vehicle, respectively) and DZP (1 mg/kg) (P<0.001 vs vehicle) (ANOVA: F(4, 30)=11.74, P<0.0001; Figure 4) anxiolytic effect was enhanced. All treatments with MLEJS (12.5, 25, and 500 mg/kg) (P<0.05, P<0.05 and P<0.001 vs vehicle, respectively) increased the amount of time spent in the light compartment (ANOVA: F(4, 30)=6.371, P<0.0008; Figure 4) and 1 mg/kg of DZP (P<0.001 compared to vehicle). Treatment with MLEJS (50 mg/kg) and DZP (P<0.001 vs vehicle, respectively) increased the head dipping (Figure 5A). No significant effect on square crossed in the holeboard across all treatment groups (Figure 5B).

Anxiolytic-like effect of acute and subacute administration of MLEJS in mice using elevated plus maze
Figures 6A, 6B, 6C, 6D, 6E And 6F, depict the outcomes of administering the J. secunda methanol leaf extract acutely (Figures 6A, 6B, 6C and 6D) and sub-acutely (Figures 6E and 6F) on the elevated plus maze. The percentage of entries in the open arms with acute and subacute treatments (acute: F(4, 30)=12.49, P<0.0001; chronic: F(4, 30)=17.16, P<0.0001) and the percentage of time spent in the open arms (acute: F(4, 30)=91.83, P<0.0001; subacute: F(4, 30)=85.29, P<0.0001) differed between groups. According to a one-way ANOVA, compared to the vehicle and the lower dose of MLEJS (12.5 mg/kg), DZP and MLEJS (25 and 50 mg/kg) enhanced the percentage of entrances into and time spent on the open arms in the acute treatment groups (all P<0.05). Additionally, in the subacute trials, the proportion of entry into and duration spent on the open arms compared with the vehicle increased with DZP and all doses of MLEJS (12.5, 25, and 50 mg/kg) (all P<0.05). Following acute administration (F(4, 30)=1.639, P>0.05) and subacute administration (F(4, 30)=2.486, P>0.05), there were no variations in the overall number of arm entries.

Effects of flumazenil pretreatment on the anxiolytic-like effect of acute administration of MLEJS in the elevated plus maze
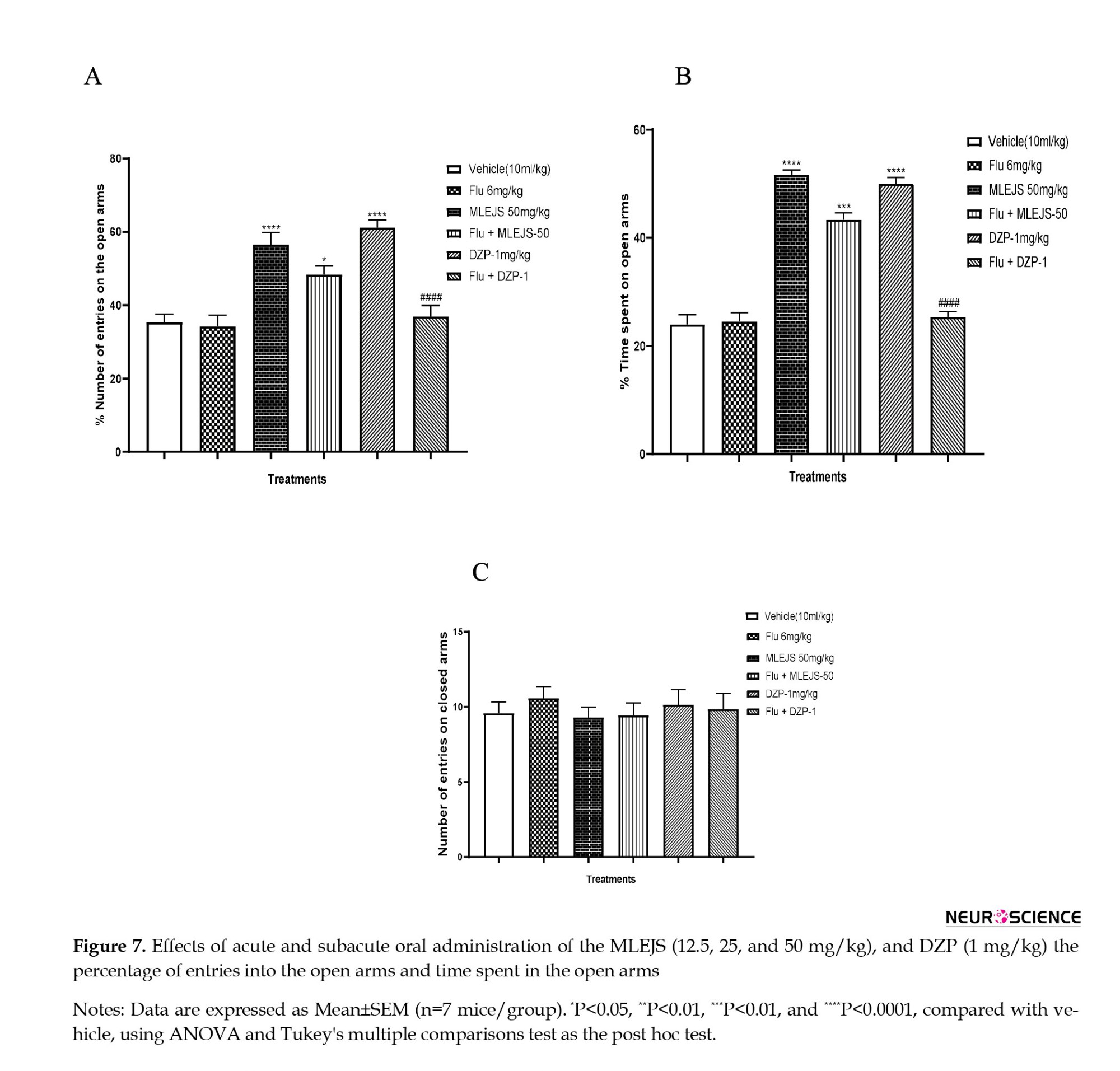
The effects of J. secunda leaf extract diluted with methanol in the raised plus maze following flumazenil pretreatment are as follows. The percentage of entries into the open arms varied between groups (ANOVA: F(4, 30)=18.17, P<0.0001; Figure 7A] with MLEJS (50 mg/kg) (P<0.001 vs vehicle group), flumazenil + MLEJS (50 mg/kg) (P<0.05 vs vehicle group), and DZP (1 mg/kg) (and P<0.0001 vs vehicle group) and flumazenil + MLEJS (50 mg/kg) (P>0.05 vs MLEJS, 50 mg/kg).
The percentage of time spent in the open arms was also examined using ANOVA (F(4, 30)=101.2, P<0.0001; Figure 7B) with the following groups: DZP (1 mg/kg) (P<0.0001 vs vehicle group), flumazenil + MLEJS (50 mg/kg) (P>0.05 vs MLEJS (50 mg/kg) group, and MLEJS (50 mg/kg) (P<0.001 vs vehicle group). There was no significant difference in the total number of entries in closed arms between the groups (ANOVA: F(4, 30)=0.3166, P>0.05; Figure 7C).
Antidepressant-like effect of MLEJS on LPS-treated mice using open field test
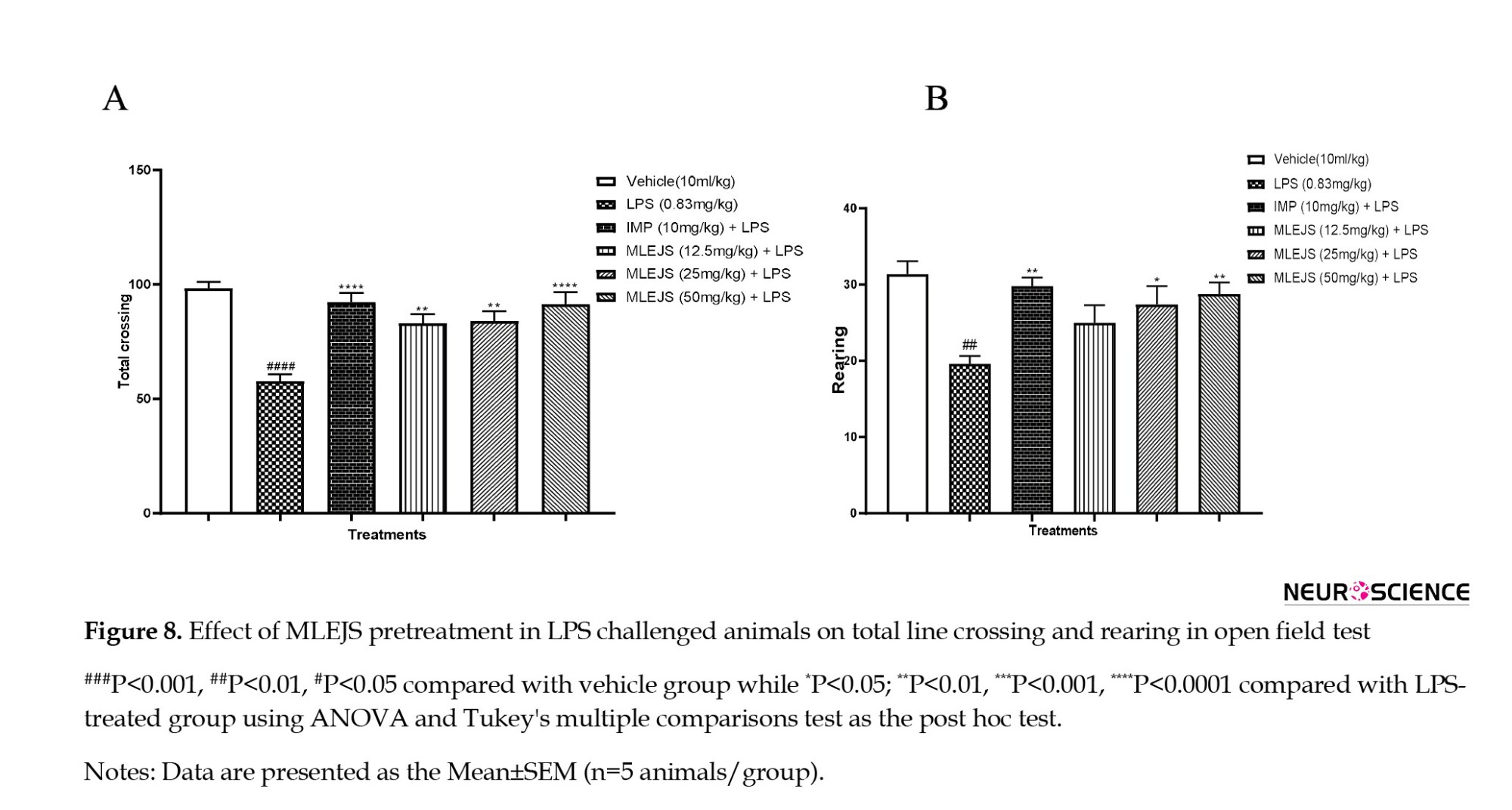
The effects of J. secunda leaf extract in methanol on rearing and total line crossing in an open-field experiment are as follows. Total line crossing (ANOVA: F(5, 24)=12.84, P<0.0001; Figure 8A) showed differences between groups when it came to imipramine (10 mg/kg) + LPS (P<0.0001 vs LPS group), MLEJS + LPS (12.5, 25, and 50 mg/kg) (P<0.01, P<0.01, and P<0.001 vs LPS group), and significantly lower LPS (0.83 mg/kg) (P<0.0001 vs vehicle group). Additionally, MLEJS + LPS (25 and 50 mg/kg) (P<0.05 and P<0.001 vs LPS group, respectively), imipramine (10 mg/kg) + LPS (P<0.001 vs LPS group), and a significant decrease in LPS (0.83 mg/kg) (P<0.001 vs vehicle group) were found in rearing (ANOVA: F(5, 24)=5.656, P<0.001; Figure 8B).
Antidepressant-like effect of MLEJS on LPS-treated mice using FST
The impact of J. secunda leaf extract in methanol on forced swimming immobility time is as follows. There were differences between the groups (ANOVA: F(5, 24)=27.12, P<0.0001; Figure 9) for imipramine (10 mg/kg) + LPS (P<0.001 vs LPS group), MLEJS+LPS (12.5, 25, and 50 mg/kg) (P<0.05, P<0.01, and P<0.01 vs LPS group), and a significant increase in LPS (0.83 mg/kg) (P<0.001 vs vehicle group).

Antidepressant-like effect of MLEJS on LPS-treated mice using TST
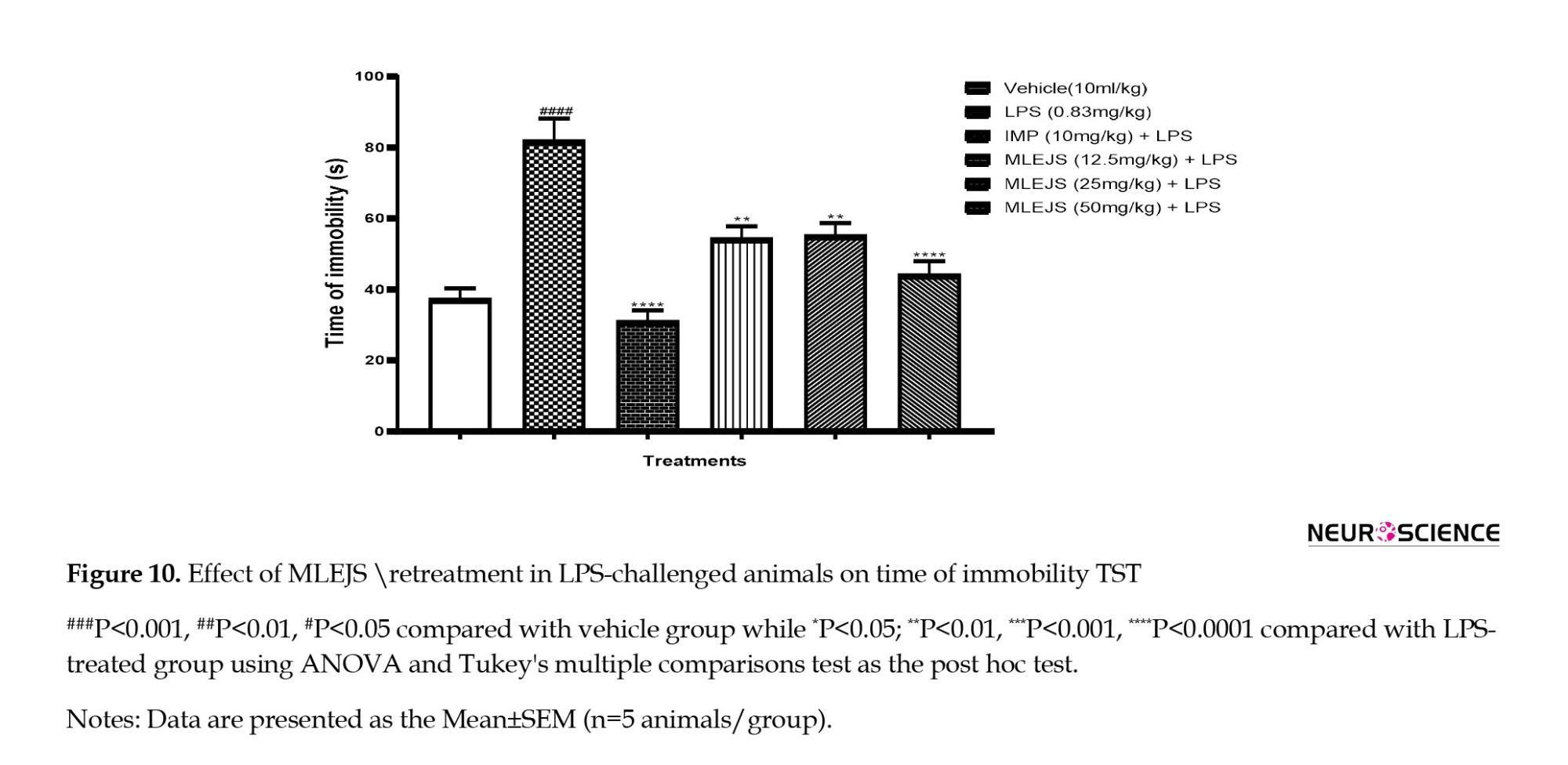
MLEJS + LPS (12.5, 25, and 50 mg/kg) (P<0.01, P<0.01, and P<0.0001 vs LPS group, respectively), imipramine (10 mg/kg) + LPS (P<0.0001 vs LPS group), and a significant increase in LPS (0.83 mg/kg) (P<0.0001 vs vehicle group) showed differences (ANOVA: F(5, 24)=19.11, P<0.0001; Figure 10).
Antidepressant-like effect of MLEJS on lps-treated mice on in vivo oxidants and antioxidant parameters
When compared to the vehicle-treated group, Figure 11A demonstrates that 24 hours later, LPS significantly (P<0.05) increased the level of TBARS (ANOVA: F(5, 24)=5.222, P<0.001). The pretreatment with imipramine (10 mg/kg) and MLEJS (50 mg/kg) significantly decreased the amount of TBARS (P<0.05). Additionally, pretreatment with MLEJS (12.5 and 25 mg/kg) had a positive but non-significant impact on the amount of TBARS in brain homogenate. When compared to the LPS-treated group, Figure 11B demonstrates that MLEJS (50 mg/kg) pretreatment considerably (P<0.05) increased GSH levels. However, MLEJS (12.5, 25 mg/kg) pretreatment was not significant (ANOVA: F(5, 24)=5.280, P<0.001). On the other hand, compared to mice treated with LPS, imipramine markedly (P<0.001) increased GSH levels.
Figure 11C shows that pretreatment with imipramine (P<0.05) and MLEJS (50 mg/kg) significantly increased SOD activity after LPS induced a considerable drop in it (ANOVA: F(5, 24)=4.988, P<0.01). However, neither of the MLEJS concentrations (12.5 or 25 mg/kg) significantly countered the decline in brain homogenate SOD caused by LPS.

Moreover, imipramine administration increased the CAT activity of LPS-challenged mice (P<0.05) (ANOVA: F(5, 24)=6.474, P<0.01; Figure 11D). However, no significant effect was observed with MLEJS pretreatment at any dose. After administering LPS to mice for 24 hours, Figure 11E demonstrated a significant increase in nitrite levels (P<0.001) in their brain homogenate compared to the vehicle-treated group. Pretreatment with imipramine (10 mg/kg) significantly (P<0.001) inhibited the increase in nitrite levels in the brain homogenate caused by LPS (ANOVA: F(5, 24)=14.88, P<0.0001). Additionally, MLEJS (12.5, 25, and 50 mg/kg) showed a protective effect against the LPS-induced decrease in nitrite levels in mice (P<0.01, P<0.01, and P<0.001 vs LPS group, respectively).
Antidepressant-like effect of MLEJS on LPS-treated mice on cytokines (IL-6 and TNF-α) level
LPS after 24 hours showed a significant (P<0.0001) increase in the brain TNF-α level, another proinflammatory cytokine in mice (ANOVA: F(5, 24)=9.344, P<0.001; Figure 12B]. Pretreatment with imipramine and MLEJS (12.5, 25, and 50 mg/kg) significantly (P<0.001, P<0.01, and P<0.001 vs LPS group, respectively) decreased TNF-α level.

Antidepressant-like effect of MLEJS on LPS-treated mice on acetylcholinesterase activity
Compared to the vehicle-treated group, the acetylcholinesterase (AchE) activity was considerably higher (P<0.01) in the mice that were challenged with LPS (ANOVA: F(5, 24)=4.260, P<0.001; Figure 13). AChE activity was considerably decreased (P<0.05, P<0.05, and P<0.01 vs LPS group, respectively) by imipramine and MLEJS (12.5 and 50 mg/kg).

Discussion
Globally, anxiety and depression are the primary causes of disability due to their complex and varied psychiatric illnesses. A motor coordination deficiency would most likely impact the results of the behavioral tests. The rotarod test was used in this investigation to examine the motor effects of MLEJS. The findings indicate that unlike DZP (5 mg/kg), MLEJS (12.5–50 mg/kg) has no discernible effect on motor coordination.
The elevated plus maze test is among the most popular for assessing anxiolytic/anxiogenic qualities. Based on the observation that rats and mice naturally dislike open spaces, this test interprets avoidance of open arms as anxiogenic behavior (Acikgoz et al., 2022). An increase in open-arms exploration (time and entry into open arms) is a sign of anxiolytic-like efficacy, but this is not the case for medications with anxiogenic-like effects (Acikgoz et al., 2022). In both the acute and subacute administrations of this trial, MLEJS increased the number of entries into open arms and the amount of time spent in them without changing the number of entries into closed arms, suggesting an anxiolytic-like effect without compromising motor function. Anxiety in the light-dark box test is measured by counting the number of transitions into and out of the light chamber; an increase in these parameters is thought to indicate anxiolytic-like qualities. Anxiety is caused by the tension between the urge to explore and to withdraw from an unfamiliar, well-lit environment. This investigation demonstrates that these two parameters rise with MLEJS treatment.
An increase in head-dipping behaviors may be an indication of an anxiolytic-like condition in the hole-board test, which is helpful for simulating anxiety in animals (Galdino et al., 2012). The data obtained supported the anxiolytic-like effect previously demonstrated in the elevated plus maze and light-dark box tests, demonstrating that MLEJS increased the frequency of head dips without changing the number of squares crossed. Consequently, these three techniques demonstrate that MLEJS exhibits anxiolytic-like activity without altering the sedative effects’ typical locomotion pattern. The crossing administration did not change the MLEJS number, according to the open-field test results (Table 1), confirming the prior observation of no change in motor coordination from the rotarod test. The reduction in rearing behavior appears to be due to a central depressive activity rather than a loss of motor coordination or neuromuscular blockade. An increase in these parameters may be a sign of anxiolytic-like effects (Galdino et al., 2012). MLEJS treatment also raised the preference for the central area, improving the crossing number and time spent in the apparatus’s central section (Table 1). The anxiolytic effect of Pimpinella anisum leaf was reported by Es-Safi et al. (2021) using open-field, elevated plus maze, and light-dark box tests. These results are comparable to their work.
The benzodiazepine system is involved in the underlying mechanism of MLEJS’s anxiolytic-like actions. Flumazenil, a well-known competitive antagonist that binds to the GABAa receptor’s benzodiazepine site and is known to counteract the anxiolytic, sedative, and hypnotic effects of benzodiazepines, was administered to the animals before their pretreatment (Engin, 2023). Although not considerably, flumazenil (6 mg/kg) partially reduced the overall anxiolytic-like action of MLEJS. Our findings demonstrate that flumazenil administration had no discernible impact on the effects of MLEJS, suggesting that the benzodiazepine site of the GABAa receptor may not be involved in MLEJS’s anxiolytic-like effects.
In the second part of the investigation, which was the anti-depressant phase, mice that had been exposed to LPS for 24 hours showed depressive-like behavior, which was examined using OFT, FST, and TST. The antioxidant biomarkers, proinflammatory cytokines, and acetylcholinesterase activity in the mice’s brains were also measured.
The primary criterion proposed to show an animal’s hopelessness and poor mood (behavioral despair), linked to depressive-like behavior, is the length of immobility or despair behavior produced in both FST and TST. Both of these behavioral despair tests are dependable and have been widely utilized in the screening process for novel antidepressants (Barua et al., 2018).
According to Barua et al., (2018), an LPS dose of 0.83 mg/kg (IP) was used in this investigation because it can cause the entire spectrum of the acute illness response and depressive-like behavior, and it has been demonstrated to cause depression 24 hours after LPS injection (O'Connor et al., 2009).
The current study showed that MLEJS shortened the time that LPS-treated mice underwent TST and FST immobility. According to Ohgi et al.’s (2013) and Ji et al.’s (2014) research, MLEJS’s capacity to shorten the period of immobility in LPS-treated mice implies that animals may have antidepressant-like action.
Increased levels of oxidative stress and neuroinflammation have been linked to the creation of major depression, even though altered brain levels of monoamines have long been recognized as the primary pathological hallmark of the condition (Vaváková et al., 2015). Cell death, decreased neurogenesis, decreased neural plasticity, and increased autoimmune responses have been linked to oxidative stress. These factors then start and spread neuroinflammation, intensifying tissue destruction (Bakunina et al., 2015).
It is well known that brain cells are particularly vulnerable to the harmful effects of free radicals and that the degree of tissue damage is correlated with elevated MDA and nitrite levels and reduced antioxidant defense systems in the cells (Vaváková et al., 2015). Patients suffering from depressive diseases have been found to have elevated levels of MDA, a significant indicator of oxidative stress (Ait Tayeb et al., 2023). Antidepressant therapies significantly decreased oxidative stress, which corresponds with clinical outcome indicators in preclinical research (Behr et al., 2012). Our findings demonstrate that oxidative and nitrosative stress caused by LPS impacted the mice’s brains. When comparing the brains of the LPS-treated mice to the vehicle-treated group, the MDA level, a hallmark of LPO, was considerably higher in the former group.
Numerous past research projects have indicated that NO plays a major role in the pathophysiology of depression. Numerous NO inhibitors have antidepressant effects by either decreasing the amount of cyclic guanosine monophosphate or inhibiting the NO synthase enzyme in the HC (Lindqvist et al., 2017).
Additionally, it has been revealed that several neuroinflammation biomarkers, such as TNF-α and interleukin (IL)-6, are up-regulated in depressive disorders, indicating that inflammation is the primary underlying cause of the condition (Murrough et al., 2018).
These cytokines cause microglial activation and neuroinflammation, which alter the pathophysiology of depression (Ghasemi, 2019). We have demonstrated in this study that treating LPS causes a rise in the levels of proinflammatory cytokines (TNF-α and IL-6). Here, our findings unequivocally demonstrate that MLEJS pretreatment inhibits proinflammatory reactions and lessens the depressive-like behavior that the administration of LPS subsequently brought on.
Stress and inflammation can cause the enzymes that break down or inactivate many neuromodulators to become sensitive. It has been demonstrated that oxidative stress and the proinflammatory cytokine IL-1 increase acetylcholinesterase expression (Joca et al., 2019). The effects of LPS-mediated neuroinflammation on cortical inhibition and elevated AChE activity have an impact on neuromodulation.
According to the chromatogram, MLEJS is a complex mixture of several metabolites, including linolenic acid, triterpenoids, fatty acids, vitamins, aromatic compounds, and steroids. The presence of multiple compounds that may act in an additive manner to enhance the potency of active constituents or multiple active ingredients that may act synergistically, acting through independent but ideally complementary pathways to confer maximal effects, may be the cause of J. secunda’s anti-anxiety and anti-depressant effect.
Also, 9,12,15-Octadecatrienoic acid, known as linolenic acid, is the main compound in MLEJS by GC-MS. It is an essential fatty acid derived from plants and a member of the omega-3 (τ-3) polyunsaturated fatty acid group, from which humans can synthesize other omega-3, including eicosapentaenoic acid and docosahexaenoic acid. According to a study by Perez et al. (2013), omega-3 PUFAs cause a strong anxiolytic-like effect in the elevated plus maze (EPM) when combined with picrotoxin pretreatment. This finding suggests that GABAa receptors are involved in these effects. Additionally, the forced swim and TSTs (Park et al., 2012)demonstrate an antidepressant-like effect. Additionally, it has been demonstrated that omega-3 PUFAs ameliorate anxiety- and depressive-like phenotypes in a variety of animal models of depression (Vines et al., 2012; Tang et al., 2015). Additionally, SSRIs, in combination with omega-3 PUFAs, seem to be more efficacious than antidepressant medications alone in lowering behaviors associated with depression. Additionally, the study demonstrates that MLEJS has similar concentrations of other unsaturated fatty acids, such as oleic acid (omega-9 τ-9) and linoleic acid (omega-6 τ-6). Anxiolytic, antidepressant, anti-inflammatory, hypocholesterolemic, hepatoprotective, cancer-preventive, antihistaminic, 5-alpha reductase inhibitor, antiandrogenic, antiarthritic, and anticoronary properties have been documented for this (Able et al., 2014). By increasing the brain’s antioxidant state, this compound’s capacity to scavenge free radicals may indirectly affect the MLEJS’s depressive and anxiolytic properties. The previous study by Onochie et al. (2020), who also showed the abundant content of flavonoids in the leaves of J. secunda, is supported by the association between the antioxidant role of MLEJS and its depressive and anxiolytic effects.
Another component found in GCMS is squalene, a naturally occurring triterpene with neuroprotective and oxygen-scavenging properties. Because squalene lowers neuroinflammation and increases brain antioxidants, it may help explain J. secunda’s reported antidepressant potential. It has anticancer qualities as well. A diterpene called phytol, a branched-chain unsaturated alcohol molecule also present in J. secunda, suggests plants’ potential antibacterial, anti-inflammatory, and anticancer effects. According to Fedotova et al. (2017), phytol interacts with and involves the GABAa/benzodiazepine receptor to provide sedative and anxiolytic-like effects in mice.
The extract contains a triterpene called alpha (α) amyrin, which may also have antidepressant and anxiolytic properties. Prior studies confirm that α-amyrin shortens the mice’s immobility period during the behavior despair test (Kun & Zuhua, 2019). Different animal models have revealed the anxiolytic effects of the mixture of α/β-amyrin, which was isolated from the stem bark resin of Protium heptaphyllum. It has been claimed that the positive allosteric regulation of BDZ-sensitive and -insensitive types of GABAa receptors mediates these effects (Frota Aragão et al., 2023). The study’s findings demonstrated that while α/β-amyrin enhanced the period of permanence and the number of entrances in the open arms, it dramatically lowered the number of crossings, grooming, rearing, and crossings in the closed arms.
5. Conclusion
The study’s findings suggest that, without affecting motor coordination, acute administration of J. secunda methanol leaf extract had anxiolytic-like effects in the elevated plus maze, light/dark, and hole-board tests. This effect is probably not mediated by GABAa/BDZ receptors because it neither showed tolerance after brief repeated dosing nor caused peripheral neuromuscular blocking or sedation. Furthermore, in the mouse model of LPS-induced depression, MLEJS shows a great ability to reverse depressive-like behavior. The antidepressant effect can be achieved by reducing proinflammatory mediators or blocking oxidative and nitrosative stress. As a result, our research raises the possibility that MLEJS could be a helpful therapeutic strategy for the management of mental illnesses linked to oxidative damage and neuroinflammation.
Ethical Considerations
Compliance with ethical guidelines
The ethical permission number of the University of Ibadan (UI-ACUREC/19/0158) was granted for using animals in this investigation.
Funding
This manuscript was extracted from the master's thesis Ileri Oluwa Emmanuel, approved by the Department of Pharmacology & Therapeutics, Faculty of Basic Medical Sciences, University of Ibadan, Ibadan, Nigeria.
Authors' contributions
Conceptualization and supervision: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Babatunde Alabi and Ayotunde Badejo; Methodology: Ileri Oluwa Emmanuel, Abayomi Ajayi, Babatunde Alabi, and Olasubomi Sotunde; Investigation: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, and Olufunmilayo Ologe; Resources: Segun Olowoparija, Michael Obaro; Data collection and analysis; Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Olugbenga Iwalewa, Babatunde Alabi, and Abayomi Ajayi; Writing the original draft: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Babatunde Alabi; Review and editing: Babatunde Alabi, Opeyemi Hammed, Abayomi Ajayi, & Ifeoluwa Oguntoye.
Conflict of interest
The authors declared no conflict of interest.
References
With a lifetime prevalence of over 10%, anxiety and depression are major public health issues that impact a large portion of the general population and greatly increase the worldwide burden of disease. Anxiety disorders are thought to be responsible for 26.8 million disability-adjusted life years, according to the global burden of disease research, and depression is thought to affect 300 million people worldwide (Khosa, 2020). Anxiety is a physiological reaction that protects an organism against harm. In severe cases, it becomes pathological and can result in psychiatric and or cardiovascular illnesses (Filatova et al., 2021). Treatments for anxiety and depression are frequently associated with side effects like tachycardia, orthostatic hypotension, digestive problems, weight gain, sexual problems, and visual problems. Memory impairment is one of the main issues that arises after longer exposure (Garakani et al., 2020).
It is thought that dysregulation of some central nervous system neurotransmitters, including dopamine, serotonin, and gamma amino butyric acid (GABA), causes anxiety and depression (Filatova et al., 2021). Behavioral interventions, medication therapy, and psychotherapy are the current methods used to treat depression and anxiety (Beard & Delgadillo, 2019). In clinical practice, imipramine, desipramine, amoxapine, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) have proven to be the most effective medications for managing anxiety and depression (Garakani et al., 2020).
However, the two groups displayed several adverse outcomes, including addiction, increased suicidal thoughts, lower alertness, sexual dysfunction, and high costs (Garakani et al., 2020).
It is well known that benzodiazepines interact with GABA receptors’ allosteric sites. By binding to GABA receptors, benzodiazepines reduce excitability in the central nervous system. Although long-term benzodiazepine use is associated with adverse effects such as memory loss and cognitive decline, this action will reduce anxiety (Garakani et al., 2020).
Researchers are revaluing several plant species for their therapeutic chemical principles due to the many issues with conventional medications. Justicia secunda is one such plant species. In traditional medicine, leaf extracts treat various illnesses, including anemia, depression, sickle cell disease, and diabetes (Kitadi et al., 2020). When J. secunda leaves were screened for phytochemical content, alkaloids and polyphenols, including flavonoids, quinones, tannins, and anthocyanins, were found (Fagbohoun et al., 2022). According to Kamso et al. (2023), luteolin derivatives are the main secondary metabolites in the leaves. Strong anxiolytic substances are therefore expected, with fewer side effects and an earlier onset of action than those found in present medications. This study uses gas chromatography-mass spectrometry (GC-MS) to examine the bioactive compounds found in methanol leaf extract of J. secunda (MLEJS). It also looks at the anxiolytic-like and anti-depressant activities of MLEJS, the role of GABAa/benzodiazepine mechanisms, and MLEJS’s impact on proinflammatory cytokines, acetylcholinesterase activity, and biomarkers of depressive-like behavior induced by lipopolysaccharide.
2. Materials and Methods
Chemicals and reagents
Flumazenil (Neon Laboratories Ltd, India), diazepam (DZP) (Roche, Basel Switzerland), thiopental sodium (Kwality, India), lipopolysaccharide (LPS) (Escherichia coli serotype, 055:B5 Sigma-Aldrich, USA) are the chemicals used in this study. Imipramine hydrochloride (Sigma-Aldrich, USA), trichloroacetic acid (TCA), thiobarbituric acid (TBA), acetylthiocholine, Ellman reagent [5′, 5′-Dithiobis- (2-nitrobenzoate) DTNB], hydrogen peroxide, ammonium sulfate, phosphate buffered saline, distilled water and all other chemicals used were of analytical grade.
Extract preparation
The fresh, healthy leaves of J. secunda were obtained from the staff quarters of Obafemi Awolowo University Ile-Ife, Osun state; it was identified and authenticated at the Department of Forest Herbarium with the voucher specimen FHI number 112604.
After being shade-dried and ground into a powder using a high-capacity grinder, the leaves were immersed in 70% aqueous methanol at a 1:10 (w/v) ratio for three days. A solid extract yielding 23.3 g was produced by filtering it with Whitman filter paper and then drying it off in a rotary evaporator. After that, the dry extract was reconstituted in regular saline at quantities suitable for the different tests.
GC-MS phytochemical analysis
The Agilent Technologies GC-MS (GC-7820A, MS 5975C) was used for the analysis. It was applied to an HP‐5 capillary column coated with 5% phenyl methyl siloxane (30 mm length ×0.32 mm diameter ×0.25 μm film thickness). The instrument was initially set to 110 ºC and kept there for two minutes. After that, the oven temperature was raised to 280 ºC, at a rate of 4 ºC/min, and kept there for nine minutes. The injection port temperature was guaranteed 250 ºC, and the helium flow rate was 1.573 mL/min. The ionization voltage was 70 eV with an ion source temperature of 230 °C, quadrupole temperature of 150 °C, and transfer line temperature of 280 °C. The samples were injected in splitless mode, one µL at 260 °C injection temperature. A mass spectral scan range of 30-550 (m/z) was used. Compounds included in the plant sample were identified by matching the spectrum acquired from GC-MS analysis with a computer search on a NIST 14 mass spectra data repository. Mass spectrum GC-MS interpretation was done with the National Institute of Standards and Technology (NIST) database, which has over 62000 patterns. A comparison was made between the spectrums of the unknown and known components found in the NIST collection. We determined the components of the test materials’ names, molecular weights, and structures.
Study animals
This study employed male Swiss mice weighing 20–25 g. The rats were housed in clean, well-ventilated polypropylene plastic cages with bedding made of wood shavings. They were also given free access to water and fed commercial rat chow pellets. The ambient conditions were kept standard. The 3Rs (replacement, reduction, and refinement) of animal experiments were followed in the treatment of the animals, and pain and discomfort were minimized by providing enriching environments.
Experimental designs
The study design comprises an acute toxicity study (6 mice were used), an anxiolytic-like study (77 mice were used), and an antidepressant-like study (30 mice were used).
Acute toxicity test
An acute oral toxicity study was conducted using the Organization for Economic Co-operation and Development (OECD) 423 guideline. The mice (n=3) that had fasted overnight were given MLEJS orally at a maximum dosage of 2000 mg/kg. They were then monitored closely for two hours, and then for 24, 72, and finally for up to 14 days for any signs of lethality, a state of morbidity, or death concerning their behavioral, neurological, and autonomic profiles. To confirm the results and determine the dangerous class of LD50, the limit test was conducted on three additional mice in another group.
An acute oral toxicity investigation reveals that the MLEJS therapy had no toxicity or moribund state. This condition implies that the approximate LD50 was more than 2500 mg/kg and that the non-observable adverse effect dose level was larger than 2000 mg/kg.
Anxiolytic-like experiment
The animals were randomly divided into five groups, with seven animals apiece. Sixty minutes before the experiment, mice in groups 2-4 were given different doses of MLEJS (12.5, 25, and 50 mg/kg orally, respectively), while group 5 got a dose of DZP (1 mg/kg PO). Group 1 mice were given normal saline 10 mL/kg orally and served as the control. Benzodiazepines are known to have anxiolytic effects at low doses and cause drowsiness and myorelaxant effects at higher dosages (Galdino et al., 2012). To check for anxiolytic-like effects, we employed DZP (1 mg/kg) as a positive control. To clarify the potential participation of GABAa/benzodiazepine mechanisms of the extract, six further groups of mice (n=7) were pretreated with 6 mg/kg flumazenil intraperitoneally 15 minutes before the treatment with the most effective anxiolytic-like dose of MLEJS (50 mg/kg) or DZP. Group 1 mice were given orally normal saline (10 mL/kg) as the control. Group 2 mice received 6 mL/kg of flumazenil alone. Group 3 mice received 50 mL/kg of MLEJS only. Group 4 mice received 6 mL/kg of flumazenil + MLEJS (50 mg/kg). Group 5 mice received 1 mL/kg of DZP, and group 6 mice received 1 mL/kg of DZP + 6 mg/kg of flumazenil.
Antidepressant-like experiment
Mice were assigned to six different experimental groups (n=5). They were semi-randomized, so all groups’ mean body weights were comparable. After that, mice received orally MLEJS (12.5, 25, and 50 mg/kg) or imipramine (10 mg/kg) as positive control and vehicle (saline, 10 mL/kg as normal control) once daily for 7 days. On the last (seventh) day of the treatment, lipopolysaccharide (LPS) (0.83 mg/kg) was injected intraperitoneally after 30 minutes of respective treatment to all groups except the vehicle control group. After 24 hours of LPS administration, a battery of behavioral tests, including an open field test (OFT), forced swimming test (FST), and tail suspension test (TST), were performed to evaluate depressive-like behavior in mice (Barua et al., 2018).
Behavioral procedures
Myorelaxant and sedative anxiolytic tests
Test for motor coordination using rotarod
The animals were pre-selected in a training session 24 hours before the test based on their ability to stay on the bar (at 12 revolutions per minute) for 120 seconds. The test was conducted using a horizontal rotating rod (Ugo Basile). All four paws of the animals were placed onto the bar 60 minutes after the treatment with vehicle (10 mL/kg), MLEJS (12.5, 25, or 50 mg/kg), or DZP (1 or 5 mg/kg). The time it took for each mouse to fall was recorded, with a maximum of 120 seconds on the bar, and the number of falls was calculated within 60 seconds, with a maximum of three falls permitted (Kokubo et al., 2018).
Sedative test using thiopental sodium sleeping time
All groups received thiopental sodium (20 mg/kg, IP) an hour after oral dose of MLEJS (12.5, 25, and 50 mg/kg), DZP (1 and 5 mg/kg), or vehicle. Sleep latency is the amount of time after thiopental sodium delivery before the righting reflex is lost; sleeping time is the time between the loss and the reflex’s voluntary recovery. The apparatus was cleaned with 70% ethanol after each animal to avoid odor bias.
Anxiolytic activity tests
Test for anxiety using hole-board
The animals were placed in the middle of a perforated board and divided into 9 equal-sized squares 60 minutes after treatment; during 5 minutes, the number of head dips into the holes, and the number of squares crossed (with all four paws) were recorded. The apparatus was cleaned with 70% ethanol after every animal to avoid odor bias.
Test for anxiety using light and dark box
The participant is exposed to a novel environment consisting of protected (dark compartment) and unprotected (light compartment) locations during the light↔dark exploration test. It is believed that risk aversion and exploratory desire are inherently at odds, which prevents exploration from occurring. The animals were positioned in the middle of the light area facing the dark area entrance sixty minutes after treatment; the number of times they switched between the two compartments and the amount of time they spent in the light area was counted over five minutes.
Test for anxiolytic/anxiogenic properties using elevated plus maze
The experiment capitalizes on mice’s innate curiosity to investigate new surroundings. The arms of the maze, which were raised to a height of about one meter above the floor, were either open or exposed to the mouse or enclosed and protected. The animals were individually placed in the middle of a plus maze 60 minutes after treatment and watched for 5 minutes. The animal was tested by counting the number of times it entered and exited the open and enclosed arms and the amount of time it spent there. Anxiety was measured by converting the number of entries into open arms and the time spent there into percentages of all entries and time, respectively.
Involvement of GABAa/benzodiazepine mechanisms
The rats were intraperitoneally pre-treated with flumazenil (6 mg/kg; 15 min pretreatment), an antagonist of GABAa/benzodiazepine receptors, to look into potential mechanisms underpinning the anxiolytic actions of MLEJS.
Antidepressant activity tests
Test for spontaneous movement activity using the open field
This test was run to see if the MLEJS may impact the mice’s locomotor activity during the open-field test. After the mouse was put within the device, the number of times it crossed lines and reared over 5 minutes was used to calculate its locomotor activity. After testing each mouse, the equipment was washed with 70% ethanol and dried to remove any traces of the preceding animal’s scent.
Test for antidepressant activity using FST
Mice were used in the experiment, and an open cylindrical container with a diameter of 10 cm and a height of 25 cm holding 19 cm of water was used. During the test session, mice were kept in this unavoidable cylinder for 6 minutes, with the final 4 minutes of immobility being recorded. They were deemed immobile when they stopped fighting, floated still, and made only the motions required to maintain their heads above the water.
Test for antidepressant activity using TST
The mice could be individually suspended 50 cm above the floor on the table’s edge by applying sticky tape about 1 cm from the tip of the tail. The immobility periods for the different groups were recorded at intervals of 6 minutes. An animal was hanged if it did not exhibit any movement. It was considered immobile.
Assessment of oxidative stress and antioxidant status
Lipid peroxidation ((LPO)) and nitric oxide
According to Afolabi et al. (2022), the thiobarbituric reacting substance (TBARS) assay was used to determine the concentration of the LPO end product malondialdehyde (MDA). The amount of nitrite in the brain tissues was calculated using the Griess reagent method, as reported by Odebiyi et al. (2021).
Antioxidant status
As explained by Alabi et al., a reduced glutathione (GSH) concentration was found in the mice’s brain supernatant by applying an older Ellam technique (Alabi et al., 2023). Superoxide dismutase (SOD) activity was measured according to the Misra and Fridovich procedure, which Campos-Shimada et al. (2020) reported. The procedure that Hadwan and Kadhum (2018) had previously outlined was used to measure the amount of catalase.
Estimation of brain cytokines and enzyme
The manufacturer’s instructions were followed to determine the amounts of tumor necrosis factor (TNF)-α (BioLegend, USA; CAT NO 430904) and IL-6 (BioLegend, USA; CAT NO 431304) in the brain supernatant. The Ellman method was used to measure the amount of acetylcholinesterase in brain tissue, as explained by Khalil and Abass (2017).
Statistical analysis
The data were presented as Mean±SEM. All behavioral data were subjected to a normality test, a one-way analysis of variance (ANOVA) was used for all analyses (nonparametric analysis was used for the rotarod test), and a Tukey post-hoc test was used for multiple comparisons when necessary. One-way ANOVA was used to examine the biochemical data, and Tukey’s post-hoc test was then performed. Version 8 of GraphPad Prism (GraphPad Software, Inc. La Jolla, CA 92037 USA) was used to analyze the data. A P<0.05 was deemed statistically significant for every test. Since the study is exploratory overall, the P should be interpreted as descriptive rather than as a means of testing a hypothesis. The specification of all intergroup comparisons preceded data collection.
3. Results
GC-MS phytochemical analysis
A full-scan gas chromatogram of J. secunda’s methanol leaf extract is displayed in Figure 1.

It verified the existence of several bioactive substances with varying retention periods (RT). Table 1 lists the major chemicals determined by their RT and % peak area. Also, 9,12,15-octadecatrienoic acid (18.11%) was the most abundant component by peak area. It was followed by n-hexadecanoic acid (14.94%), phytol (7.92%), stigmasterol (5.39%), squalene (4.83%), neophytadiene (3.95%), campesterol (2.96%), vitamin E (2.51%), methyl stearate (1.21%), β-amyrin (1.17%), 9-octadecenamide (1.10%), 2-palmitoyl glycerol (0.91%), and 4a (2H)-naphthalenol (0.86%).

Effect of MLEJS on motor coordination in mice using rotarod apparatus
DZP (at 1 mg/kg) significantly increased the number of falls in the rotarod test, while MLEJS (12.5, 25, and 50 mg/kg) did not affect the number of falls (the Kruskal-Wallis [KW] test=14.60, P<0.01; Dunn test, P<0.001 vs vehicle group, Figure 2A). DZP (5 mg/kg) significantly prolonged the falling latency from the revolving rod (ANOVA: F(5, 24)=3.779, P<0.01, Figure 2B) (P<0.0001 vs vehicle).

Effect of MLEJS on sleeping test
DZP (1 or 5 mg/kg) reduced the sleep latency (ANOVA: F(5, 24)=13.17, P<0.001; Figure 3A) (P<0.05 and P<0.001 vs vehicle, respectively), while the MLEJS treatments did not significantly reduce the sleep latency (P>0.05 vs vehicle).

DZP (1 and 5 mg/kg) also enhanced the amount of time spent sleeping (ANOVA: F(5, 24)=128.53, P<0.001; Figure 3B) (P<0.01 and P<0.001 vs vehicle, respectively).
Anxiolytic-like effect of MLEJS in mice using light and dark boxes
In the light-dark test, the number of transitions between the light and dark compartments after oral treatment with MLEJS (25 and 50 mg/kg (P<0.05 and P<0.0001 vs vehicle, respectively) and DZP 1 mg/kg (P<0.001 vs vehicle) increased (ANOVA: F(4, 30)=11.74, P<0.0001; Figure 4). The duration of time in the light compartment was higher for all MLEJS treatments (12.5, 25, and 500 mg/kg) (P<0.05, P<0.05, and P<0.001 vs vehicle, respectively) (ANOVA: F(4, 30)=6.371, P<0.0008; Figure 4) and DZP (1 mg/kg) (P<0.001 vs vehicle).

Anxiolytic-like effect of MLEJS in mice using the hole board
Following oral treatment with MLEJS (25 and 50 mg/kg (P<0.05 and P<0.0001 vs vehicle, respectively) and DZP (1 mg/kg) (P<0.001 vs vehicle) (ANOVA: F(4, 30)=11.74, P<0.0001; Figure 4) anxiolytic effect was enhanced. All treatments with MLEJS (12.5, 25, and 500 mg/kg) (P<0.05, P<0.05 and P<0.001 vs vehicle, respectively) increased the amount of time spent in the light compartment (ANOVA: F(4, 30)=6.371, P<0.0008; Figure 4) and 1 mg/kg of DZP (P<0.001 compared to vehicle). Treatment with MLEJS (50 mg/kg) and DZP (P<0.001 vs vehicle, respectively) increased the head dipping (Figure 5A). No significant effect on square crossed in the holeboard across all treatment groups (Figure 5B).

Anxiolytic-like effect of acute and subacute administration of MLEJS in mice using elevated plus maze
Figures 6A, 6B, 6C, 6D, 6E And 6F, depict the outcomes of administering the J. secunda methanol leaf extract acutely (Figures 6A, 6B, 6C and 6D) and sub-acutely (Figures 6E and 6F) on the elevated plus maze. The percentage of entries in the open arms with acute and subacute treatments (acute: F(4, 30)=12.49, P<0.0001; chronic: F(4, 30)=17.16, P<0.0001) and the percentage of time spent in the open arms (acute: F(4, 30)=91.83, P<0.0001; subacute: F(4, 30)=85.29, P<0.0001) differed between groups. According to a one-way ANOVA, compared to the vehicle and the lower dose of MLEJS (12.5 mg/kg), DZP and MLEJS (25 and 50 mg/kg) enhanced the percentage of entrances into and time spent on the open arms in the acute treatment groups (all P<0.05). Additionally, in the subacute trials, the proportion of entry into and duration spent on the open arms compared with the vehicle increased with DZP and all doses of MLEJS (12.5, 25, and 50 mg/kg) (all P<0.05). Following acute administration (F(4, 30)=1.639, P>0.05) and subacute administration (F(4, 30)=2.486, P>0.05), there were no variations in the overall number of arm entries.

Effects of flumazenil pretreatment on the anxiolytic-like effect of acute administration of MLEJS in the elevated plus maze
The effects of J. secunda leaf extract diluted with methanol in the raised plus maze following flumazenil pretreatment are as follows. The percentage of entries into the open arms varied between groups (ANOVA: F(4, 30)=18.17, P<0.0001; Figure 7A] with MLEJS (50 mg/kg) (P<0.001 vs vehicle group), flumazenil + MLEJS (50 mg/kg) (P<0.05 vs vehicle group), and DZP (1 mg/kg) (and P<0.0001 vs vehicle group) and flumazenil + MLEJS (50 mg/kg) (P>0.05 vs MLEJS, 50 mg/kg).
The percentage of time spent in the open arms was also examined using ANOVA (F(4, 30)=101.2, P<0.0001; Figure 7B) with the following groups: DZP (1 mg/kg) (P<0.0001 vs vehicle group), flumazenil + MLEJS (50 mg/kg) (P>0.05 vs MLEJS (50 mg/kg) group, and MLEJS (50 mg/kg) (P<0.001 vs vehicle group). There was no significant difference in the total number of entries in closed arms between the groups (ANOVA: F(4, 30)=0.3166, P>0.05; Figure 7C).

Antidepressant-like effect of MLEJS on LPS-treated mice using open field test
The effects of J. secunda leaf extract in methanol on rearing and total line crossing in an open-field experiment are as follows. Total line crossing (ANOVA: F(5, 24)=12.84, P<0.0001; Figure 8A) showed differences between groups when it came to imipramine (10 mg/kg) + LPS (P<0.0001 vs LPS group), MLEJS + LPS (12.5, 25, and 50 mg/kg) (P<0.01, P<0.01, and P<0.001 vs LPS group), and significantly lower LPS (0.83 mg/kg) (P<0.0001 vs vehicle group). Additionally, MLEJS + LPS (25 and 50 mg/kg) (P<0.05 and P<0.001 vs LPS group, respectively), imipramine (10 mg/kg) + LPS (P<0.001 vs LPS group), and a significant decrease in LPS (0.83 mg/kg) (P<0.001 vs vehicle group) were found in rearing (ANOVA: F(5, 24)=5.656, P<0.001; Figure 8B).

Antidepressant-like effect of MLEJS on LPS-treated mice using FST
The impact of J. secunda leaf extract in methanol on forced swimming immobility time is as follows. There were differences between the groups (ANOVA: F(5, 24)=27.12, P<0.0001; Figure 9) for imipramine (10 mg/kg) + LPS (P<0.001 vs LPS group), MLEJS+LPS (12.5, 25, and 50 mg/kg) (P<0.05, P<0.01, and P<0.01 vs LPS group), and a significant increase in LPS (0.83 mg/kg) (P<0.001 vs vehicle group).

Antidepressant-like effect of MLEJS on LPS-treated mice using TST
MLEJS + LPS (12.5, 25, and 50 mg/kg) (P<0.01, P<0.01, and P<0.0001 vs LPS group, respectively), imipramine (10 mg/kg) + LPS (P<0.0001 vs LPS group), and a significant increase in LPS (0.83 mg/kg) (P<0.0001 vs vehicle group) showed differences (ANOVA: F(5, 24)=19.11, P<0.0001; Figure 10).

Antidepressant-like effect of MLEJS on lps-treated mice on in vivo oxidants and antioxidant parameters
When compared to the vehicle-treated group, Figure 11A demonstrates that 24 hours later, LPS significantly (P<0.05) increased the level of TBARS (ANOVA: F(5, 24)=5.222, P<0.001). The pretreatment with imipramine (10 mg/kg) and MLEJS (50 mg/kg) significantly decreased the amount of TBARS (P<0.05). Additionally, pretreatment with MLEJS (12.5 and 25 mg/kg) had a positive but non-significant impact on the amount of TBARS in brain homogenate. When compared to the LPS-treated group, Figure 11B demonstrates that MLEJS (50 mg/kg) pretreatment considerably (P<0.05) increased GSH levels. However, MLEJS (12.5, 25 mg/kg) pretreatment was not significant (ANOVA: F(5, 24)=5.280, P<0.001). On the other hand, compared to mice treated with LPS, imipramine markedly (P<0.001) increased GSH levels.
Figure 11C shows that pretreatment with imipramine (P<0.05) and MLEJS (50 mg/kg) significantly increased SOD activity after LPS induced a considerable drop in it (ANOVA: F(5, 24)=4.988, P<0.01). However, neither of the MLEJS concentrations (12.5 or 25 mg/kg) significantly countered the decline in brain homogenate SOD caused by LPS.

Moreover, imipramine administration increased the CAT activity of LPS-challenged mice (P<0.05) (ANOVA: F(5, 24)=6.474, P<0.01; Figure 11D). However, no significant effect was observed with MLEJS pretreatment at any dose. After administering LPS to mice for 24 hours, Figure 11E demonstrated a significant increase in nitrite levels (P<0.001) in their brain homogenate compared to the vehicle-treated group. Pretreatment with imipramine (10 mg/kg) significantly (P<0.001) inhibited the increase in nitrite levels in the brain homogenate caused by LPS (ANOVA: F(5, 24)=14.88, P<0.0001). Additionally, MLEJS (12.5, 25, and 50 mg/kg) showed a protective effect against the LPS-induced decrease in nitrite levels in mice (P<0.01, P<0.01, and P<0.001 vs LPS group, respectively).
Antidepressant-like effect of MLEJS on LPS-treated mice on cytokines (IL-6 and TNF-α) level
LPS after 24 hours showed a significant (P<0.0001) increase in the brain TNF-α level, another proinflammatory cytokine in mice (ANOVA: F(5, 24)=9.344, P<0.001; Figure 12B]. Pretreatment with imipramine and MLEJS (12.5, 25, and 50 mg/kg) significantly (P<0.001, P<0.01, and P<0.001 vs LPS group, respectively) decreased TNF-α level.

Antidepressant-like effect of MLEJS on LPS-treated mice on acetylcholinesterase activity
Compared to the vehicle-treated group, the acetylcholinesterase (AchE) activity was considerably higher (P<0.01) in the mice that were challenged with LPS (ANOVA: F(5, 24)=4.260, P<0.001; Figure 13). AChE activity was considerably decreased (P<0.05, P<0.05, and P<0.01 vs LPS group, respectively) by imipramine and MLEJS (12.5 and 50 mg/kg).

Discussion
Globally, anxiety and depression are the primary causes of disability due to their complex and varied psychiatric illnesses. A motor coordination deficiency would most likely impact the results of the behavioral tests. The rotarod test was used in this investigation to examine the motor effects of MLEJS. The findings indicate that unlike DZP (5 mg/kg), MLEJS (12.5–50 mg/kg) has no discernible effect on motor coordination.
The elevated plus maze test is among the most popular for assessing anxiolytic/anxiogenic qualities. Based on the observation that rats and mice naturally dislike open spaces, this test interprets avoidance of open arms as anxiogenic behavior (Acikgoz et al., 2022). An increase in open-arms exploration (time and entry into open arms) is a sign of anxiolytic-like efficacy, but this is not the case for medications with anxiogenic-like effects (Acikgoz et al., 2022). In both the acute and subacute administrations of this trial, MLEJS increased the number of entries into open arms and the amount of time spent in them without changing the number of entries into closed arms, suggesting an anxiolytic-like effect without compromising motor function. Anxiety in the light-dark box test is measured by counting the number of transitions into and out of the light chamber; an increase in these parameters is thought to indicate anxiolytic-like qualities. Anxiety is caused by the tension between the urge to explore and to withdraw from an unfamiliar, well-lit environment. This investigation demonstrates that these two parameters rise with MLEJS treatment.
An increase in head-dipping behaviors may be an indication of an anxiolytic-like condition in the hole-board test, which is helpful for simulating anxiety in animals (Galdino et al., 2012). The data obtained supported the anxiolytic-like effect previously demonstrated in the elevated plus maze and light-dark box tests, demonstrating that MLEJS increased the frequency of head dips without changing the number of squares crossed. Consequently, these three techniques demonstrate that MLEJS exhibits anxiolytic-like activity without altering the sedative effects’ typical locomotion pattern. The crossing administration did not change the MLEJS number, according to the open-field test results (Table 1), confirming the prior observation of no change in motor coordination from the rotarod test. The reduction in rearing behavior appears to be due to a central depressive activity rather than a loss of motor coordination or neuromuscular blockade. An increase in these parameters may be a sign of anxiolytic-like effects (Galdino et al., 2012). MLEJS treatment also raised the preference for the central area, improving the crossing number and time spent in the apparatus’s central section (Table 1). The anxiolytic effect of Pimpinella anisum leaf was reported by Es-Safi et al. (2021) using open-field, elevated plus maze, and light-dark box tests. These results are comparable to their work.
The benzodiazepine system is involved in the underlying mechanism of MLEJS’s anxiolytic-like actions. Flumazenil, a well-known competitive antagonist that binds to the GABAa receptor’s benzodiazepine site and is known to counteract the anxiolytic, sedative, and hypnotic effects of benzodiazepines, was administered to the animals before their pretreatment (Engin, 2023). Although not considerably, flumazenil (6 mg/kg) partially reduced the overall anxiolytic-like action of MLEJS. Our findings demonstrate that flumazenil administration had no discernible impact on the effects of MLEJS, suggesting that the benzodiazepine site of the GABAa receptor may not be involved in MLEJS’s anxiolytic-like effects.
In the second part of the investigation, which was the anti-depressant phase, mice that had been exposed to LPS for 24 hours showed depressive-like behavior, which was examined using OFT, FST, and TST. The antioxidant biomarkers, proinflammatory cytokines, and acetylcholinesterase activity in the mice’s brains were also measured.
The primary criterion proposed to show an animal’s hopelessness and poor mood (behavioral despair), linked to depressive-like behavior, is the length of immobility or despair behavior produced in both FST and TST. Both of these behavioral despair tests are dependable and have been widely utilized in the screening process for novel antidepressants (Barua et al., 2018).
According to Barua et al., (2018), an LPS dose of 0.83 mg/kg (IP) was used in this investigation because it can cause the entire spectrum of the acute illness response and depressive-like behavior, and it has been demonstrated to cause depression 24 hours after LPS injection (O'Connor et al., 2009).
The current study showed that MLEJS shortened the time that LPS-treated mice underwent TST and FST immobility. According to Ohgi et al.’s (2013) and Ji et al.’s (2014) research, MLEJS’s capacity to shorten the period of immobility in LPS-treated mice implies that animals may have antidepressant-like action.
Increased levels of oxidative stress and neuroinflammation have been linked to the creation of major depression, even though altered brain levels of monoamines have long been recognized as the primary pathological hallmark of the condition (Vaváková et al., 2015). Cell death, decreased neurogenesis, decreased neural plasticity, and increased autoimmune responses have been linked to oxidative stress. These factors then start and spread neuroinflammation, intensifying tissue destruction (Bakunina et al., 2015).
It is well known that brain cells are particularly vulnerable to the harmful effects of free radicals and that the degree of tissue damage is correlated with elevated MDA and nitrite levels and reduced antioxidant defense systems in the cells (Vaváková et al., 2015). Patients suffering from depressive diseases have been found to have elevated levels of MDA, a significant indicator of oxidative stress (Ait Tayeb et al., 2023). Antidepressant therapies significantly decreased oxidative stress, which corresponds with clinical outcome indicators in preclinical research (Behr et al., 2012). Our findings demonstrate that oxidative and nitrosative stress caused by LPS impacted the mice’s brains. When comparing the brains of the LPS-treated mice to the vehicle-treated group, the MDA level, a hallmark of LPO, was considerably higher in the former group.
Numerous past research projects have indicated that NO plays a major role in the pathophysiology of depression. Numerous NO inhibitors have antidepressant effects by either decreasing the amount of cyclic guanosine monophosphate or inhibiting the NO synthase enzyme in the HC (Lindqvist et al., 2017).
Additionally, it has been revealed that several neuroinflammation biomarkers, such as TNF-α and interleukin (IL)-6, are up-regulated in depressive disorders, indicating that inflammation is the primary underlying cause of the condition (Murrough et al., 2018).
These cytokines cause microglial activation and neuroinflammation, which alter the pathophysiology of depression (Ghasemi, 2019). We have demonstrated in this study that treating LPS causes a rise in the levels of proinflammatory cytokines (TNF-α and IL-6). Here, our findings unequivocally demonstrate that MLEJS pretreatment inhibits proinflammatory reactions and lessens the depressive-like behavior that the administration of LPS subsequently brought on.
Stress and inflammation can cause the enzymes that break down or inactivate many neuromodulators to become sensitive. It has been demonstrated that oxidative stress and the proinflammatory cytokine IL-1 increase acetylcholinesterase expression (Joca et al., 2019). The effects of LPS-mediated neuroinflammation on cortical inhibition and elevated AChE activity have an impact on neuromodulation.
According to the chromatogram, MLEJS is a complex mixture of several metabolites, including linolenic acid, triterpenoids, fatty acids, vitamins, aromatic compounds, and steroids. The presence of multiple compounds that may act in an additive manner to enhance the potency of active constituents or multiple active ingredients that may act synergistically, acting through independent but ideally complementary pathways to confer maximal effects, may be the cause of J. secunda’s anti-anxiety and anti-depressant effect.
Also, 9,12,15-Octadecatrienoic acid, known as linolenic acid, is the main compound in MLEJS by GC-MS. It is an essential fatty acid derived from plants and a member of the omega-3 (τ-3) polyunsaturated fatty acid group, from which humans can synthesize other omega-3, including eicosapentaenoic acid and docosahexaenoic acid. According to a study by Perez et al. (2013), omega-3 PUFAs cause a strong anxiolytic-like effect in the elevated plus maze (EPM) when combined with picrotoxin pretreatment. This finding suggests that GABAa receptors are involved in these effects. Additionally, the forced swim and TSTs (Park et al., 2012)demonstrate an antidepressant-like effect. Additionally, it has been demonstrated that omega-3 PUFAs ameliorate anxiety- and depressive-like phenotypes in a variety of animal models of depression (Vines et al., 2012; Tang et al., 2015). Additionally, SSRIs, in combination with omega-3 PUFAs, seem to be more efficacious than antidepressant medications alone in lowering behaviors associated with depression. Additionally, the study demonstrates that MLEJS has similar concentrations of other unsaturated fatty acids, such as oleic acid (omega-9 τ-9) and linoleic acid (omega-6 τ-6). Anxiolytic, antidepressant, anti-inflammatory, hypocholesterolemic, hepatoprotective, cancer-preventive, antihistaminic, 5-alpha reductase inhibitor, antiandrogenic, antiarthritic, and anticoronary properties have been documented for this (Able et al., 2014). By increasing the brain’s antioxidant state, this compound’s capacity to scavenge free radicals may indirectly affect the MLEJS’s depressive and anxiolytic properties. The previous study by Onochie et al. (2020), who also showed the abundant content of flavonoids in the leaves of J. secunda, is supported by the association between the antioxidant role of MLEJS and its depressive and anxiolytic effects.
Another component found in GCMS is squalene, a naturally occurring triterpene with neuroprotective and oxygen-scavenging properties. Because squalene lowers neuroinflammation and increases brain antioxidants, it may help explain J. secunda’s reported antidepressant potential. It has anticancer qualities as well. A diterpene called phytol, a branched-chain unsaturated alcohol molecule also present in J. secunda, suggests plants’ potential antibacterial, anti-inflammatory, and anticancer effects. According to Fedotova et al. (2017), phytol interacts with and involves the GABAa/benzodiazepine receptor to provide sedative and anxiolytic-like effects in mice.
The extract contains a triterpene called alpha (α) amyrin, which may also have antidepressant and anxiolytic properties. Prior studies confirm that α-amyrin shortens the mice’s immobility period during the behavior despair test (Kun & Zuhua, 2019). Different animal models have revealed the anxiolytic effects of the mixture of α/β-amyrin, which was isolated from the stem bark resin of Protium heptaphyllum. It has been claimed that the positive allosteric regulation of BDZ-sensitive and -insensitive types of GABAa receptors mediates these effects (Frota Aragão et al., 2023). The study’s findings demonstrated that while α/β-amyrin enhanced the period of permanence and the number of entrances in the open arms, it dramatically lowered the number of crossings, grooming, rearing, and crossings in the closed arms.
5. Conclusion
The study’s findings suggest that, without affecting motor coordination, acute administration of J. secunda methanol leaf extract had anxiolytic-like effects in the elevated plus maze, light/dark, and hole-board tests. This effect is probably not mediated by GABAa/BDZ receptors because it neither showed tolerance after brief repeated dosing nor caused peripheral neuromuscular blocking or sedation. Furthermore, in the mouse model of LPS-induced depression, MLEJS shows a great ability to reverse depressive-like behavior. The antidepressant effect can be achieved by reducing proinflammatory mediators or blocking oxidative and nitrosative stress. As a result, our research raises the possibility that MLEJS could be a helpful therapeutic strategy for the management of mental illnesses linked to oxidative damage and neuroinflammation.
Ethical Considerations
Compliance with ethical guidelines
The ethical permission number of the University of Ibadan (UI-ACUREC/19/0158) was granted for using animals in this investigation.
Funding
This manuscript was extracted from the master's thesis Ileri Oluwa Emmanuel, approved by the Department of Pharmacology & Therapeutics, Faculty of Basic Medical Sciences, University of Ibadan, Ibadan, Nigeria.
Authors' contributions
Conceptualization and supervision: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Babatunde Alabi and Ayotunde Badejo; Methodology: Ileri Oluwa Emmanuel, Abayomi Ajayi, Babatunde Alabi, and Olasubomi Sotunde; Investigation: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, and Olufunmilayo Ologe; Resources: Segun Olowoparija, Michael Obaro; Data collection and analysis; Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Olugbenga Iwalewa, Babatunde Alabi, and Abayomi Ajayi; Writing the original draft: Ileri Oluwa Emmanuel, Olugbenga Iwalewa, Babatunde Alabi; Review and editing: Babatunde Alabi, Opeyemi Hammed, Abayomi Ajayi, & Ifeoluwa Oguntoye.
Conflict of interest
The authors declared no conflict of interest.
References
COVID-19 Mental Disorders Collaborators. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet (London, England), 398(10312), 1700–1712. [DOI:10.1016/S0140-6736(21)02143-7] [PMID]
Khosa, J. (2020). An exploration of the predictors of depression and anxiety in older people (60+ years) using secondary data collected from the Malawi longitudinal study on families and health [PhD dissertation]. Malawi: Kamuzu University of Health Sciences. [Link]
Filatova, E. V., Shadrina, M. I., & Slominsky, P. A. (2021). Major depression: One brain, one disease, one set of intertwined processes. Cells, 10(6), 1283. [DOI:10.3390/cells10061283] [PMID] [PMCID]
Beard, J. I., & Delgadillo, J. (2019). Early response to psychological therapy as a predictor of depression and anxiety treatment outcomes: A systematic review and meta-analysis. Depression and Anxiety, 36(9), 866-878. [DOI:10.1002/da.22931] [PMID]
Garakani, A., Murrough, J. W., Freire, R. C., Thom, R. P., Larkin, K., & Buono, F. D., et al. (2020). Pharmacotherapy of anxiety disorders: Current and emerging treatment options. Frontiers in Psychiatry, 11, 595584. [DOI:10.3389/fpsyt.2020.595584] [PMID] [PMCID]
Kitadi, J. M., Mazasa, P. P., Sha-Tshibey Tshibangu, D., Kasali, F. M., Tshilanda, D. D., & Ngbolua, K. T., et al. (2020). Ethnopharmacological survey and antisickling activity of plants used in the management of sickle cell disease in Kikwit city, DR Congo. Evidence-Based Complementary and Alternative Medicine: eCAM, 2020, 1346493. [DOI:10.1155/2020/1346493] [PMID] [PMCID]
Fagbohoun, L., Nonvidé, G. C., Orou, A. S., Houngbèmè, A., Sakirigui, A., & Gunin, F., et al. (2022). Anti-anaemic activity and potential toxicity of extracts of four tinctorial plants used in the treatment of anemia in Benin: Gossypium barbadense, Sorghum bicolor, Hibiscus sabdariffa and Justicia secunda. American Journal of Plant Sciences, 13(12), 1460-1477. [DOI:10.4236/ajps.2022.1312100]
Kamlo Kamso, V. F., Dongmo Melogmo, Y. K., Tchegnitegni, B. T., Tchatat Tali, M. B., Dize, D., & Ngansop, C. N., et al. (2023). New lignan glycosides from Justicia secunda Vahl (Acanthaceae) with antimicrobial and antiparasitic properties. Heliyon, 9(12), e22897. [DOI:10.1016/j.heliyon.2023.e22897] [PMID] [PMCID]
Galdino, P. M., Nascimento, M. V., Florentino, I. F., Lino, R. C., Fajemiroye, J. O., & Chaibub, B. A., et al. (2012). The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 38(2), 276-284. [DOI:10.1016/j.pnpbp.2012.04.012] [PMID]
Barua, C. C., Haloi, P., Saikia, B., Sulakhiya, K., Pathak, D. C., & Tamuli, S., et al. (2018). Zanthoxylum alatum abrogates lipopolysaccharide-induced depression-like behaviours in mice by modulating neuroinflammation and monoamine neurotransmitters in the hippocampus. Pharmaceutical Biology, 56(1), 245-252. [DOI:10.1080/13880209.2017.1391298] [PMID] [PMCID]
Kokubo, M., Toya, S., Amano, I., & Takatsuru, Y. (2018). Early-life stress induces motor coordination dysfunction in adult mice. The Journal of Physiological Sciences, 68(5), 663–669.[DOI:10.1007/s12576-017-0580-6] [PMID] [PMCID]
Afolabi, O., Alabi, B., Omobowale, T., Oluranti, O., & Iwalewa, O. (2022). Cysteamine mitigates torsion/detorsion-induced reperfusion injury via inhibition of apoptosis, oxidative stress and inflammatory responses in experimental rat model. Andrologia, 54(1), e14243. [DOI:10.1111/and.14243] [PMID]
Odebiyi, O., Badejo, J., Alabi, B., Ajayi, A., Iwalewa, O., & Fagbemi, O. (2021). Evaluation of modulatory effects of taurine in the aortic and myocardial tissue of nitroglycerin-induced tolerance Wistar rats. Nutrire, 46(2), 12. [DOI:10.1186/s41110-021-00141-9]
Alabi, B., Iwalewa, O., Omobowale, T., Adedapo, A., Hammed, O., & Ajike, R., et al. (2023). Cysteamine attenuates intestinal reperfusion injury induced by mesenteric artery occlusion by enhancing intracellular thiol activities. Drug Research, 73(03), 137-145. [DOI:10.1055/a-1974-9132] [PMID]
Campos-Shimada, L. B., Hideo Gilglioni, E., Fernandes Garcia, R., Rizato Martins-Maciel, E., Luiza Ishii-Iwamoto, E., & Luzia Salgueiro-Pagadigorria, C. (2020). Superoxide dismutase: A review and a modified protocol for activities measurements in rat livers. Archives of Physiology and Biochemistry, 126(4), 292-299. [DOI:10.1080/13813455.2018.1520891] [PMID]
Hadwan, M. H., & kadhum Ali, S. (2018). New spectrophotometric assay for assessments of catalase activity in biological samples. Analytical Biochemistry, 542, 29-33. [DOI:10.1016/j.ab.2017.11.013] [PMID]
Khalil, K. A., & Abass, K. S. (2017). The measurements of the cholinesterase activity of brain and plasma in rabbits by using modified Michel and Ellman assays. Insights in Enzyme Research, 1, 2. [DOI:10.21767/2573-4466.100009]
Acikgoz, B., Dalkiran, B., & Dayi, A. (2022). An overview of the currency and usefulness of behavioral tests used from past to present to assess anxiety, social behavior and depression in rats and mice. Behavioural Processes, 200, 104670. [DOI:10.1016/j.beproc.2022.104670] [PMID]
Engin, E. (2023). GABAA receptor subtypes and benzodiazepine use, misuse, and abuse. Frontiers in Psychiatry, 13, 1060949. [DOI:10.3389/fpsyt.2022.1060949] [PMID] [PMCID]
Barua, C. C., Saikia, B., Ren, X., Elancheran, R., Pathak, D. C., & Tamuli, S., et al. (2018). Zanthoxylum alatum attenuates lipopolysaccharide-induced depressive-like behavior in mice hippocampus. Pharmacognosy Magazine, 14(59s), s673-682s. [DOI:10.4103/pm.pm_606_17]
O’Connor, J. C., Lawson, M. A., André, C., Moreau, M., Lestage, J., & Castanon, N., et al. (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2, 3-dioxygenase activation in mice. Molecular Psychiatry, 14(5), 511-522. [DOI:10.1038/sj.mp.4002148] [PMID] [PMCID]
Ohgi, Y., Futamura, T., Kikuchi, T., & Hashimoto, K. (2013). Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacology Biochemistry and Behavior, 103(4), 853-859. [DOI:10.1016/j.pbb.2012.12.003] [PMID]
Ji, W. W., Wang, S. Y., Ma, Z. Q., Li, R. P., Li, S. S., & Xue, J. S., et al. (2014). Effects of perillaldehyde on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacology Biochemistry and Behavior, 116, 1-8. [PMID]
Vaváková, M., Ďuračková, Z., & Trebatická, J. (2015). Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Medicine and Cellular Longevity, 2015, 898393. [DOI:10.1155/2015/898393] [PMID] [PMCID]
Bakunina, N., Pariante, C. M., & Zunszain, P. A. (2015). Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology, 144(3), 365-373. [DOI:10.1111/imm.12443] [PMID] [PMCID]
Ait Tayeb, A. E. K., Poinsignon, V., Chappell, K., Bouligand, J., Becquemont, L., & Verstuyft, C. (2023). Major depressive disorder and oxidative stress: A review of peripheral and genetic biomarkers according to clinical characteristics and disease stages. Antioxidants, 12(4), 942. [DOI:10.3390/antiox12040942] [PMID] [PMCID]
Behr, G. A., Moreira, J. C., & Frey, B. N. (2012). Preclinical and clinical evidence of antioxidant effects of antidepressant agents: Implications for the pathophysiology of major depressive disorder. Oxidative Medicine and Cellular Longevity, 2012, 609421. [DOI:10.1155/2012/609421] [PMID] [PMCID]
Lindqvist, D., Dhabhar, F. S., James, S. J., Hough, C. M., Jain, F. A., & Bersani, F. S., et al. (2017). Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology, 76, 197-205. [DOI:10.1016/j.psyneuen.2016.11.031] [PMID] [PMCID]
Murrough, J. W., Huryk, K. M., Mao, X., Iacoviello, B., Collins, K., & Nierenberg, A. A., et al. (2018). A pilot study of minocycline for the treatment of bipolar depression: Effects on cortical glutathione and oxidative stress in vivo. Journal of Affective Disorders, 230, 56-64. [DOI:10.1016/j.jad.2017.12.067] [PMID]
Ghasemi, M. (2019). Nitric oxide: Antidepressant mechanisms and inflammation. Advances in Pharmacology, 86, 121-152. [DOI:10.1016/bs.apha.2019.04.004] [PMID]
Joca, S. R., Sartim, A. G., Roncalho, A. L., Diniz, C. F., & Wegener, G. (2019). Nitric oxide signalling and antidepressant action revisited. Cell and Tissue Research, 377, 45-58. [DOI:10.1007/s00441-018-02987-4] [PMID]
Pérez, M. Á., Terreros, G., & Dagnino-Subiabre, A. (2013). Long-term ω-3 fatty acid supplementation induces anti-stress effects and improves learning in rats. Behavioral and Brain Functions, 9, 25. [DOI:10.1186/1744-9081-9-25] [PMID] [PMCID]
Park, Y., Moon, H. J., & Kim, S. H. (2012). N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. The Journal of Nutritional Biochemistry, 23(8), 924-928. [DOI:10.1016/j.jnutbio.2011.04.018] [PMID]
Vines, A., Delattre, A. M., Lima, M. M., Rodrigues, L. S., Suchecki, D., & Machado, R. B., et al. (2012). The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology, 62(1), 184-191. [DOI:10.1016/j.neuropharm.2011.06.017] [PMID]
Tang, M., Jiang, P., Li, H., Cai, H., Liu, Y., & Gong, H. et al. (2015). Antidepressant-like effect of n-3 PUFAs in CUMS rats: Role of tPA/PAI-1 system. Physiology & Behavior, 139, 210-215. [DOI:10.1016/j.physbeh.2014.11.054] [PMID]
Able, J. A., Liu, Y., Jandacek, R., Rider, T., Tso, P., & McNamara, R. K. (2014). Omega-3 fatty acid deficient male rats exhibit abnormal behavioral activation in the forced swim test following chronic fluoxetine treatment: association with altered 5-HT1A and alpha2A adrenergic receptor expression. Journal of Psychiatric Research, 50, 42-50. [DOI:10.1016/j.jpsychires.2013.11.008] [PMID] [PMCID]
Fedotova, J., Kubatka, P., Büsselberg, D., Shleikin, A. G., Caprnda, M., & Dragasek, J., et al. (2017). Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomedicine & Pharmacotherapy, 95, 437-446. [DOI:10.1016/j.biopha.2017.08.107] [PMID]
Kun, X., & Zuhua, G. (2019). Amyrin exerts potent anxiolytic and antidepressant effects via mechanisms involving monoamine oxidase and γ-aminobutyric acid in mouse hippocampus. Tropical Journal of Pharmaceutical Research, 18(8), 1673-1681. [Link]
Frota Aragão, G., Oliveira Nogueira, A., Félix Xavier Júnior, F. A., Azul Monteiro Evangelista, J. S., Nogueira Bandeira, P., & Fernandes, C., et al. (2023). Acute toxicity study of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum (Aubl.) Marchand. Acta Scientiarum: Biological Sciences, 45(1), e66144. [DOI:10.4025/actascibiolsci.v45i1.66144]
Es-Safi, I., Mechchate, H., Amaghnouje, A., Elbouzidi, A., Bouhrim, M., & Bencheikh, N., et al. (2021). Assessment of antidepressant-like, anxiolytic effects and impact on memory of pimpinella anisum l. Total extract on swiss albino mice. Plants, 10(8), 1573. [DOI:10.3390/plants10081573] [PMID] [PMCID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2024/03/1 | Accepted: 2024/04/8 | Published: 2024/09/1
Received: 2024/03/1 | Accepted: 2024/04/8 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





