Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 463-476 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soleimani E, Ahmadiani A, Bazrgar M, Khodagholi F, Eliassi A. The 40-Hz White Light Emitting Diode to Alleviate Psychiatric Symptoms Induced by Streptozotocin In Vivo. BCN 2024; 15 (4) :463-476
URL: http://bcn.iums.ac.ir/article-1-2855-en.html
URL: http://bcn.iums.ac.ir/article-1-2855-en.html
Elham Soleimani1 

 , Abolhassan Ahmadiani *1
, Abolhassan Ahmadiani *1 

 , Maryam Bazrgar1
, Maryam Bazrgar1 

 , Fariba Khodagholi1
, Fariba Khodagholi1 

 , Afsaneh Eliassi2
, Afsaneh Eliassi2 




 , Abolhassan Ahmadiani *1
, Abolhassan Ahmadiani *1 

 , Maryam Bazrgar1
, Maryam Bazrgar1 

 , Fariba Khodagholi1
, Fariba Khodagholi1 

 , Afsaneh Eliassi2
, Afsaneh Eliassi2 


1- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Neurophysiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Neurophysiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Alzheimer disease (AD), 40-Hz white light emitting diode (WLED), Psychiatric symptoms (PS), mtDNA, Monoamine oxidase (MAO), Catalase (CAT)
Full-Text [PDF 1451 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Alzheimer disease (AD), a neurodegenerative disease that progresses with age, accounts for the largest proportion of dementia globally (Chakraborty et al., 2019) present in almost 90% of patients with AD. Most patients with AD suffer from various behavioral and mood disorders that accelerate the progression of the disease. Studies have recently shown that psychiatric symptoms (PS), such as depression, anxiety, and apathy, occur before the onset of cognitive symptoms in AD patients (Vuic et al., 2022; Zhang et al., 2018) which frequently develop during the course and different stages of dementia. The diagnosis of BPSD is complex due to symptom variety, and relies on detailed clinical evaluation and medical history. Accurate assessment of BPSD is crucial in order to tailor therapeutic intervention (non-pharmacological and pharmacological. Currently, there are no FDA-approved interventions for PS, and the available pharmacological treatments, such as monoamine oxidase inhibitors prescribed for depression and anxiety, have toxic side effects, including hepatotoxicity, orthostatic hypotension, and hypertensive crisis (Chakraborty et al., 2019; Dhapola et al., 2022; Nandi et al., 2023) present in almost 90% of patients with AD.
Scientists are investigating nonpharmacological, noninvasive alternatives, such as photobiomodulation (PBM) using red light (RL) or near-infrared light (NIR), for treating brain diseases because current treatments have limited effectiveness and more side effects (Li et al., 2023). A 40-Hz white light scintillator through the eyes has been shown to attenuate the pathological characteristics of AD (Tian et al., 2023). The PBM has multiple benefits, such as reducing inflammatory factors, improving memory in AD model mice (Yu et al., 2022), colocalizing microglia with Aβ and eliminating senile plaques (Iaccarino et al., 2016; Tao et al., 2021) and reducing superoxide and Aβ-induced inflammation in astrocytes (Lu et al., 2017).
The impact of a 40-Hz white light emitting diode (WLED) on PS in AD has not been proven, and the underlying mechanisms are not fully understood despite the hypothesis that it may affect certain structures and biomolecules in mitochondria. As demonstrated by researchers, the use of 40-Hz WLED in the Aβ1-42 toxicity model increases mitochondrial respiratory chain complex I and IV activity, decreases reactive oxygen species (ROS) production and mitochondrial membrane potential, and improves the structure-function of potassium channels (Nazari et al., 2022).
Moreover, the mitochondrial dysfunction is acknowledged as an early event in the development of AD (Wang et al., 2020). Excessive production of ROS through intracerebroventricular (ICV) -streptozotocin (STZ) in the sporadic AD (sAD) model targets mitochondria, which are the main source of ROS production. It results in a reduction in mtDNA frequency and contributes to AD pathology and cognitive and non-cognitive behavioral disorders (Ansari Dezfouli et al., 2019; Dhapola et al., 2022; Harerimana et al., 2022; Wan Chik et al., 2023).
Previous studies have acknowledged the significance of Aβ in generating H2O2 as the primary free radical produced in mitochondria. They suggest that H2O2 is rapidly catalyzed by catalase (CAT) and preserves the mitochondrial function in AD (Reddy, 2006).
In addition, monoamine oxidases, found in the mitochondria’s outer membrane, are associated with AD PS pathophysiology because of their impact on monoamine neurotransmitter levels, as well as their role in oxidative stress through hydrogen peroxide production (Behl et al., 2021; Emilsson et al., 2002). Therefore, it has been proposed that inhibiting monoamine oxidases could enhance PS by decreasing oxidative stress and the breakdown of amine neurotransmitters.
Therefore, the objective of the current research is to provide answers to the following questions: Does 40-Hz flashing light improve depression, anxiety, and social interaction at an early stage in STZ-induced AD-like rats? Was the effect of 40-Hz flashing light on behavioral symptoms due to the regulation of monoaminoxidase A (MAOA), monoaminoxidase B (MAOB), and CAT enzyme activity as well as the abundance of mitochondrial DNA in the hippocampus (HIP), amygdala (AMY), and prefrontal cortex (PFC).
2. Materials and Methods
Animal model
Male Wistar rats (mean weight: 250±10g) were kept in the laboratory under controlled conditions (12:12-h light: Dark cycle, steady temperature of 23±2 °C, and free access to water and food), and all procedures followed ethical guidelines approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Induction of STZ toxicity
STZ (Sigma-Aldrich) was used to create an Alzheimer-like animal model. According to the Paxinos Watson atlas, a stereotactic surgery was performed to inject STZ (3 mg/kg) or normal saline (3 μL by gentle infusion over 5 min) into bilateral intraventricular (AP=0.8, ML=1.5, DV=3) after inducing anesthesia in rats with ketamine/xylazine (Alluri et al., 2020; Paxinos & Watson, 2006).
Experimental design
The intervention was started one week after the surgery, given once a day for 15 min, and continued for 7 days. Following a 24-h interval since the last session, a group of rats were subjected to behavioral testing, while the other rats had their brain tissue collected for molecular and enzyme studies (Figure 1).
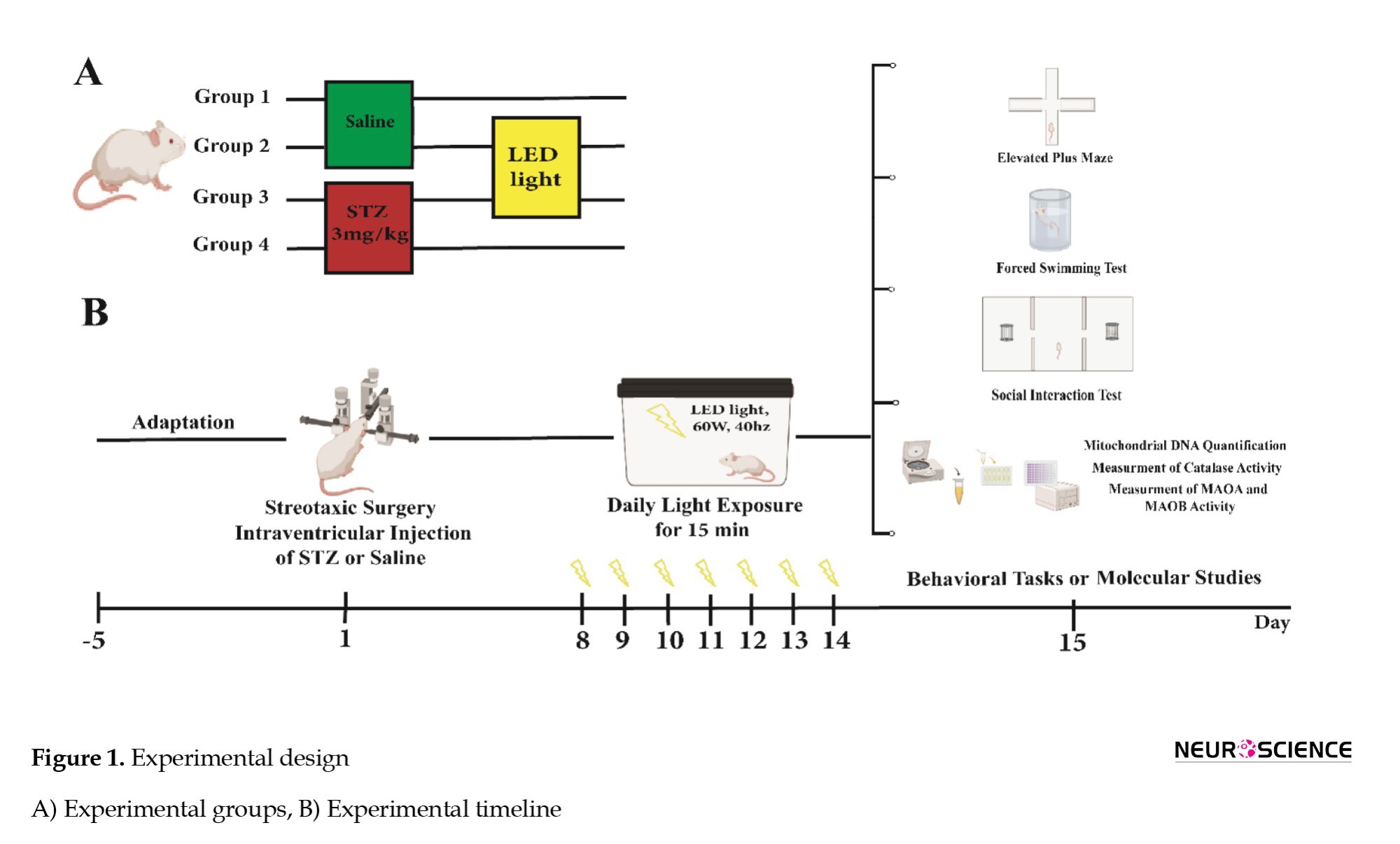
The experimental groups were randomly divided as follows.
1. Saline (control): Bilateral ICV injection of normal saline,
2. Saline + light (40-Hz WLED),
3. STZ (Alzheimer-like group): Bilateral ICV injection of STZ (3 mg/kg) and
4. STZ + light (40-Hz WLED).
The rats were exposed to white light with a visible wavelength of 425-550 nm, intensity of 60 W, frequency of 40 Hz, and average irradiance of 12 mW/cm2 in a dark chamber containing LED strip lamps (Iaccarino et al., 2016; Jones et al., 2019; Nazari et al., 2022).
Elevated plus maze test (EPM)
The EPM behavioral model measures rodent anxiety based on their searching and avoidance instincts. It features four arms in the shape of a positive sign, each measuring 50×10 cm, with a high wall surrounding the closed arm and 1 cm high edges around the open arms. The central area, with dimensions of 10×10 cm, intersects the arms, and the maze is illuminated by a dim glow (Rodgers & Johnson 1995; Walf & Frye 2007) we have used a large database comprising the behavioral profiles of 90 undrugged mice to examine the relationship between the standard spatiotemporal measures and a range of specific behaviors related to the defensive repertoire of the mouse. A factor analysis applied to the standard measures revealed two factors related to anxiety and locomotor activity. The simple addition of center time (an infrequently recorded measure. Animals were individually positioned in the maze’s center, facing the open arms and freely moving among them for 5 min. A decrease in the percentage of entries and or time spent in open arms indicates an anxiolytic index (Beirami et al., 2017).
Forced swim test
In this test, it is thought that inactivity reflects the state of depression. In this study, the animal was placed individually in a plexiglass cylinder containing tap water to a depth of 30 cm, which was kept for 15 min (usually at 25 °C). Twenty-four hours later, the subjects were placed in the cylinder, and their immobility time was counted in a 5-min test period. A subject is considered motionless when it is passively floating in water in a little bent but vertical status with its head just above the water surface (Tucci et al., 2022) GRA displayed antidepressant activity in preclinical models. We have previously demonstrated that a single ICV administration of soluble amyloid-beta 1-42 (sAβ 1-42).
Social interaction test (three chamber paradigm)
In this trial, animals unfamiliar with one another were chosen, with two control rats required in each experiment, one for the first stage and the other for the second stage of the trial. A social interaction test was performed for 25 min in an unfamiliar rectangular space divided into three parts by walls. During habituation, the rats could move freely between the rooms for 5 min. In the first stage (10 min), one of the control rats was placed in one of the chambers to test the animalʼs social tendencies. The duration of direct contact with stranger rat 1 was recorded. In the second stage (10 min), the level of the animalʼs desire to create new social relationships was tested, with the parameters stated in the previous step measured, and the ratʼs behavioral differences in interaction with stranger 1 and stranger 2 were observed. This test investigates sociability, the desire for new connections, and social isolation (Davoudi et al., 2023) memory impairment, and anxiety-like behavior are characterized in many people identified with autism spectrum disorder (ASD).
Quantification of mitochondrial DNA
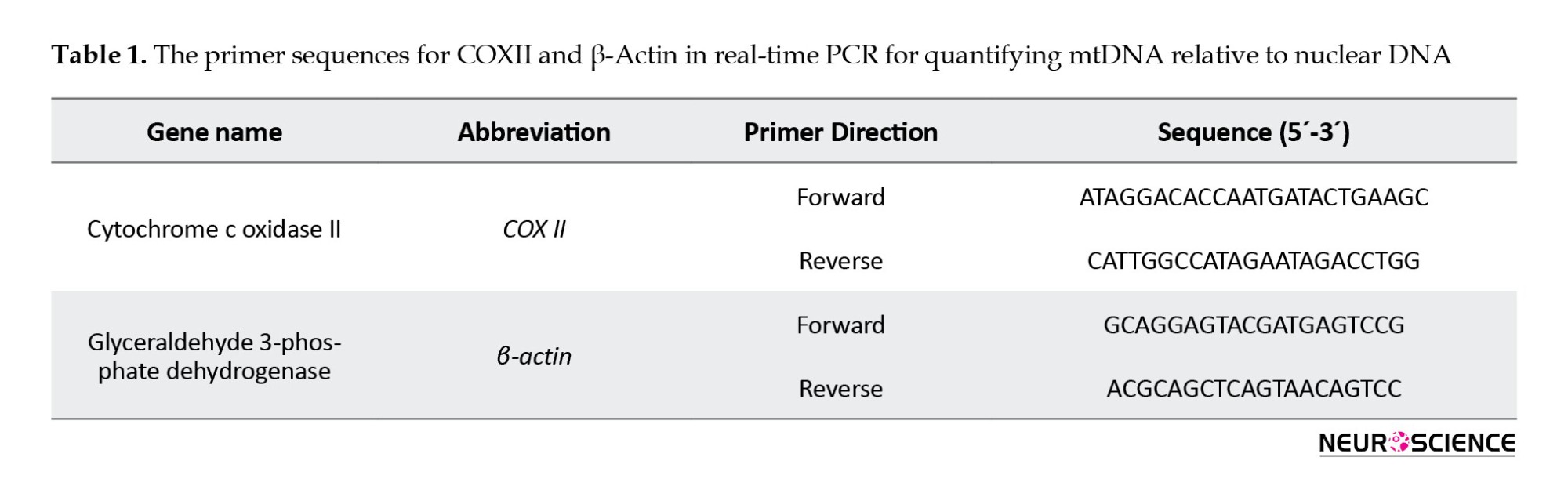
DNA was extracted from the HIP, AMY, and PFC using the tissue genomic DNA extraction mini kit (Favorgen, Taiwan) following the provided instructions. The quantification of mtDNA relative amount was performed by implementing real-time PCR methodology with SYBR Green detection. The quantification of mtDNA was performed using PCR Master Mix reagents (Ampliqon, Denmark), following specific cycling conditions, including activation at 95 °C for 10 min, denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, extension at 72 °C for 30 s, and a total of 40 cycles in the ABi step-one System (USA) (Ansari Dezfouli et al., 2019; Sheng et al., 2012). The primers used for the mitochondrial gene cytochrome c oxidase II and the nuclear gene β-actin are listed in Table 1.
Measurement of the CAT activity
We used TPR’s CAT activity assay kit (Teb Pazhouhan Razi (TPR), Iran) to measure CA activity in tissues.
Measurement of monoamine oxidase A and monoamine oxidase B activity
To evaluate the activity of monoamine oxidase A (MAOA) and monoamine oxidase B (MAOB) enzymes, mitochondria were isolated using the Clark and Nicklas method and then measured by spectrophotometry (Clark & Nicklas 1970). Sodium phosphate buffer (100 mM) and 5-hydroxytryptamine (4 mM) were combined in each well to estimate MAOA activity. Subsequently, the enzymatic reaction was initiated by adding solutions of the mitochondrial fraction, and the resulting change in absorbance was monitored at 280 nm using a spectrophotometer (Thermo Scientific/USA) against a blank consisting of sodium phosphate buffer (100 mM) and 5-hydroxytryptamine (4 mM). To estimate MAOB activity, a mixture of sodium phosphate buffer (100 mM) and 0.1 M benzylamine was prepared in each well at a wavelength of 249.5 nm, and a blank containing sodium phosphate buffer and benzylamine was used as a reference. The next step was adding mitochondrial fraction solutions to start the enzymatic reaction and recording the resulting change in absorbance (Bahaeddin et al., 2022).
Statistical analysis
The processing and visualization of the data were conducted using GraphPad Prism software, version 9 and the results were presented as the Mean±SEM. The significant difference was set as a P<0.05. The study groups were compared using a one-way analysis of variance (ANOVA) and a Tukey post hoc test.
3. Results
Effect of 40-Hz WLED on STZ-induced anxiety-like responses
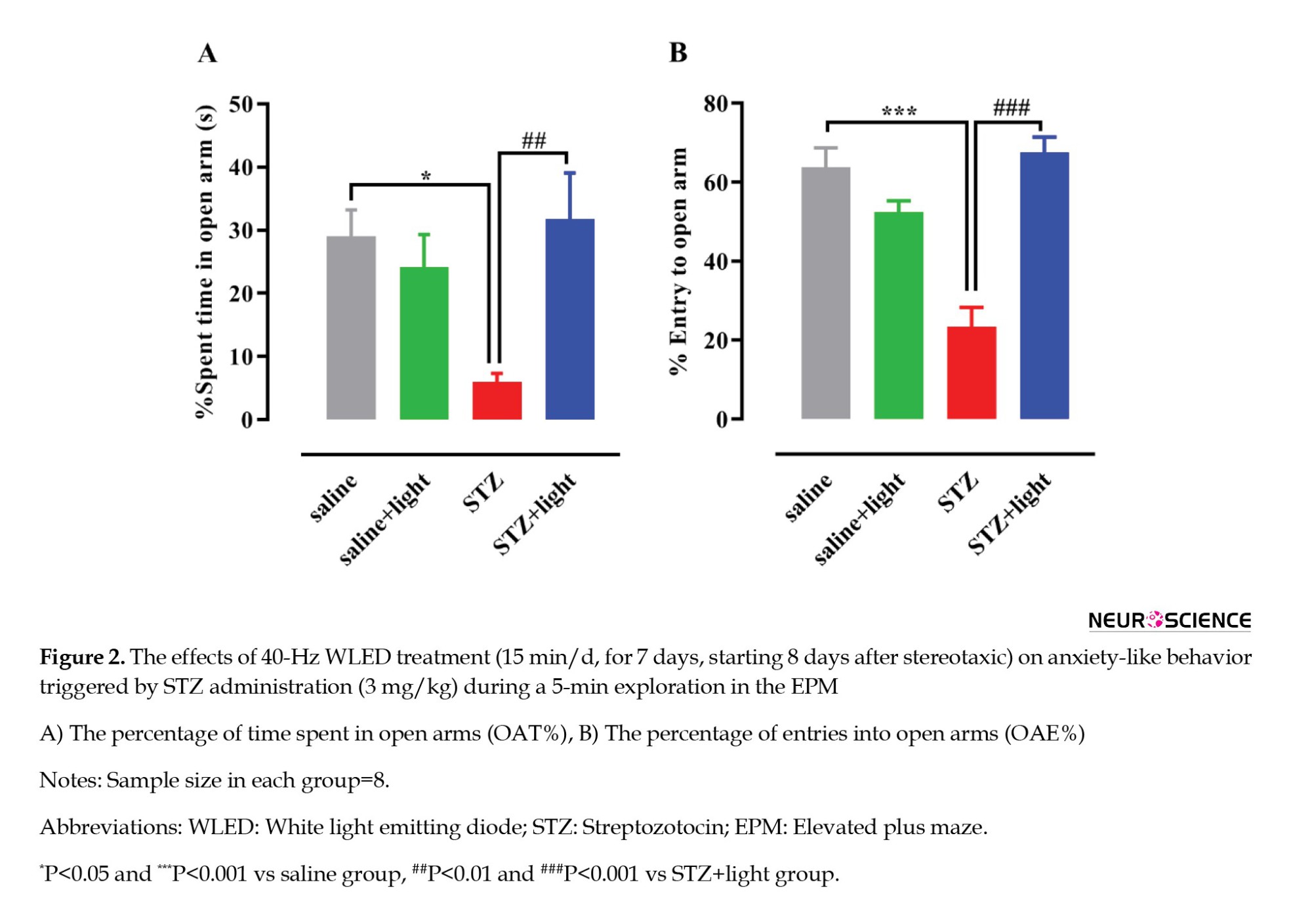
One-way ANOVA revealed significant differences in the percentage of time spent on the open arms (OAT%) (F(3, 28)=5.403, P=0.0046) (Figure 2A) and the percentage of entries into the open arms (OAE %) (F(3, 28)=22.33, P<0.001) (Figure 2B) during the EPM test. Post-hoc analysis revealed that the levels of OAT% (P<0.05) and OAE% (P<0.0001) in the STZ-injected rats were significantly reduced compared with those in the saline group, suggesting an anxiogenic reaction. In the 40-Hz WLED treatment group (STZ+light), there was a notable rise in OAT% (P<0.01) and OAE% (P<0.0001) compared with the STZ-injected rats, indicating that the 40-Hz WLED reversed the anxiogenic response induced by STZ administration.
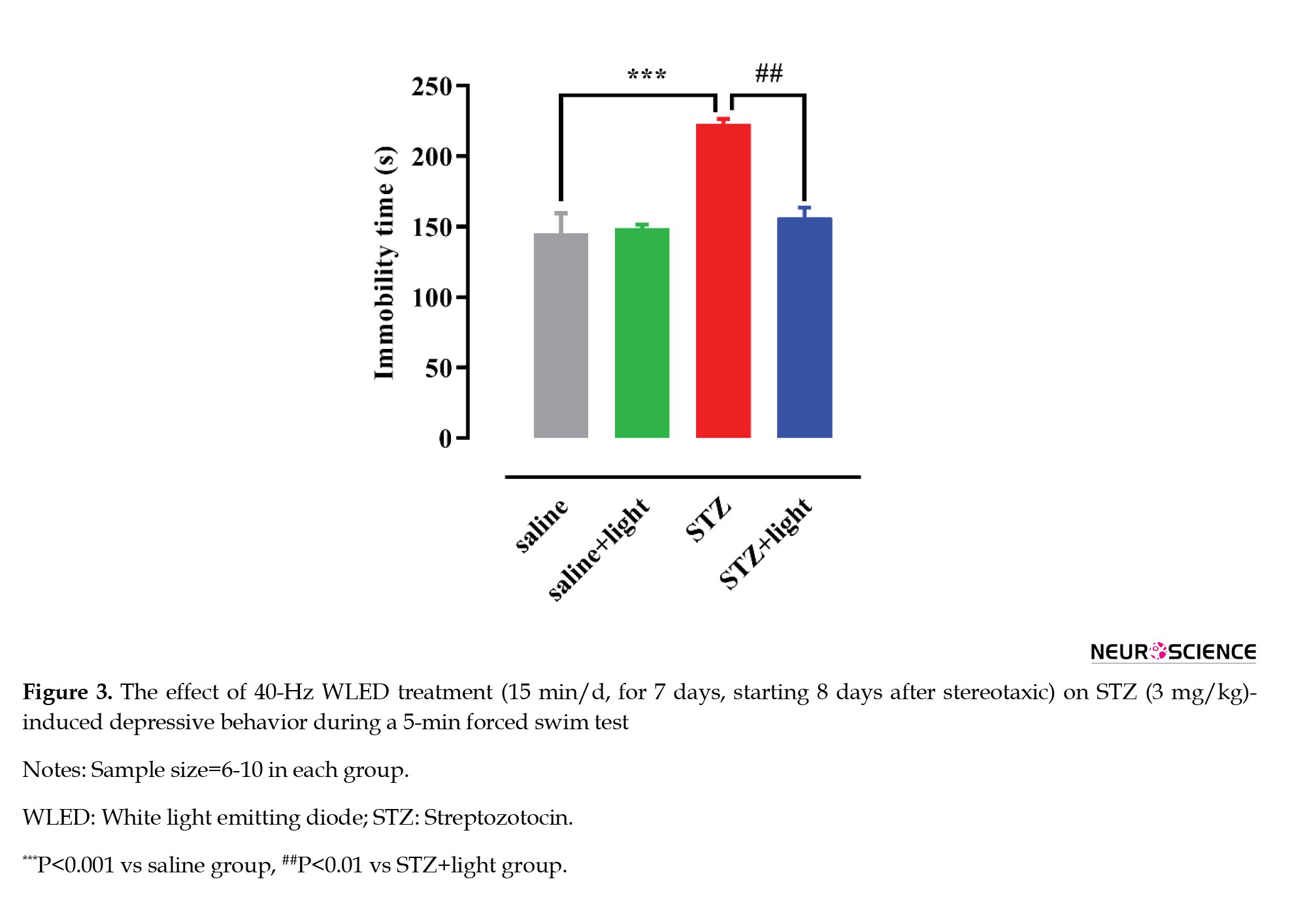
Effect of 40-Hz WLED on STZ-induced depressive-like responses
Figure 3 shows the behavior of rats subjected to a forced swim test (FST). One-way ANOVA showed significant differences in immobility time between the groups (F(3, 24)=9.967, P=0.0002) (Figure 3). Post-hoc analysis (Tukey test) indicated that STZ induced a depression-like response as evidenced by a significant increase in immobility time (P<0.001) compared with the saline group. Applying 40-Hz WLED led to a significant decrease in immobility time (P<0.01) and removed the STZ-induced depression in rats.
Effect of 40-Hz WLED on STZ-induced impairment of social interactions
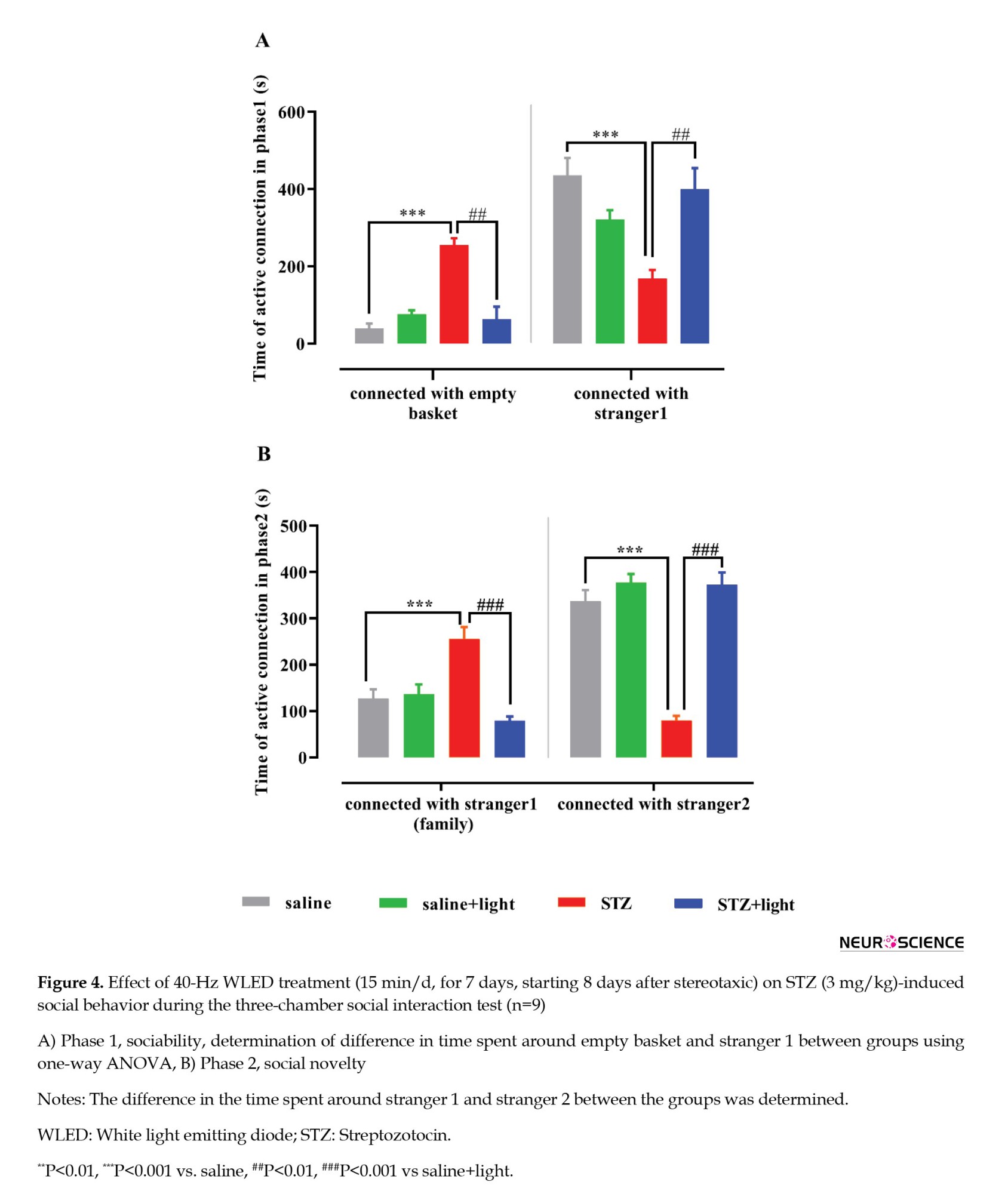
One-way ANOVA analysis in phase 1 of the experiment showed a significant group difference in the time spent with the empty basket and stranger 1 (F(3, 32)=25.04, P<0.0001; and F(3, 32)=9.261, P=0.0001; respectively). In the control group, the increase in time spent around stranger 1 and the decrease in time spent around the empty basket indicate normal sociability. In contrast, the opposite behavior in the STZ group suggests a decrease in the desire to make social connections (Figure 4A). On the other hand, we showed that light significantly improved social relationships in the STZ+light group. One-way ANOVA analysis in phase 2 shows a significant group difference in the time spent with stranger 1 (F(3, 32)=26.68, P<0.0001) and stranger 2 (F(3, 32)=28.52, P<0.0001). The STZ group showed less desire to create new relationships and preferred social isolation by spending less time with stranger 2 than the control group. While light therapy could have a significant positive effect on social novelty in STZ+light by increasing the time spent around the stranger 2. It is possible that the results were influenced by the decrease in anxiety that occurred following exposure to light (Figure 4B).
Effect of 40-Hz WLED on CAT activity in the HIP, AMY, and PFC
A significant difference in CAT activity between different groups was observed in the AMY (F(3, 14)=60.37, P<0.001) (Figure 5C) and PFC (F(3, 10)=4.588, P=0.0288) (Figure 5B). The mean CAT activity of the STZ group in the AMY and PFC was significantly lower than that of the saline group, (P<0.0001 and P<0.05, respectively). Also, 40-Hz WLED treatment in the AMY prevented the decrease of CAT activation in the STZ+light group compared with the STZ group (P<0.01). However, in the other two brain regions, HIP, PFC, and CAT activity did not show any significant difference in the light-treated rats with respect to the STZ group.

Effect of 40-Hz WLED on the activity of MAOA and MAOB
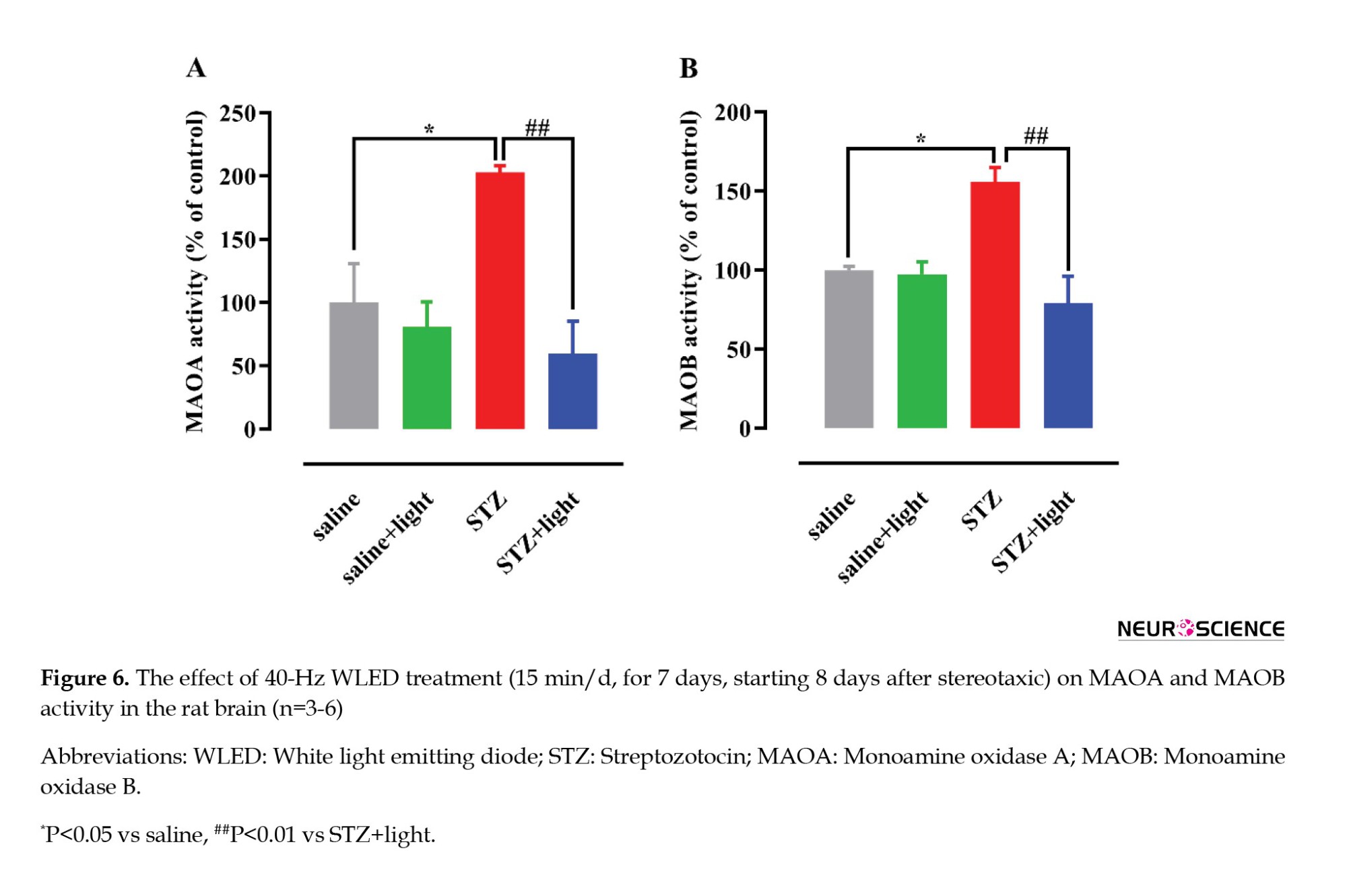
One-way ANOVA showed significant differences in MAOA and MAOB enzymatic activity between the study groups (F(3, 20)=8.035, P=0.001 and F(3, 8)=9.857, P=0.0046; respectively) (Figure 6). STZ administration resulted in a significant increase in MAOA and MAOB enzymatic activity (P<0.05) compared with the saline group. Also, 40-Hz WLED significantly reduced MAOA and MAOB (P<0.01) enzymatic activity in the STZ-light group compared to STZ-treated rats. The decrease in anxiety and depression in animals through WLED therapy may be due to the decline in MAOA and MAOB activity, leading to the reduction of monoamine oxidation involved in anxiety and depression.
Effect of 40-Hz WLED on mtDNA relative amount in the HIP, AMY, and PFC
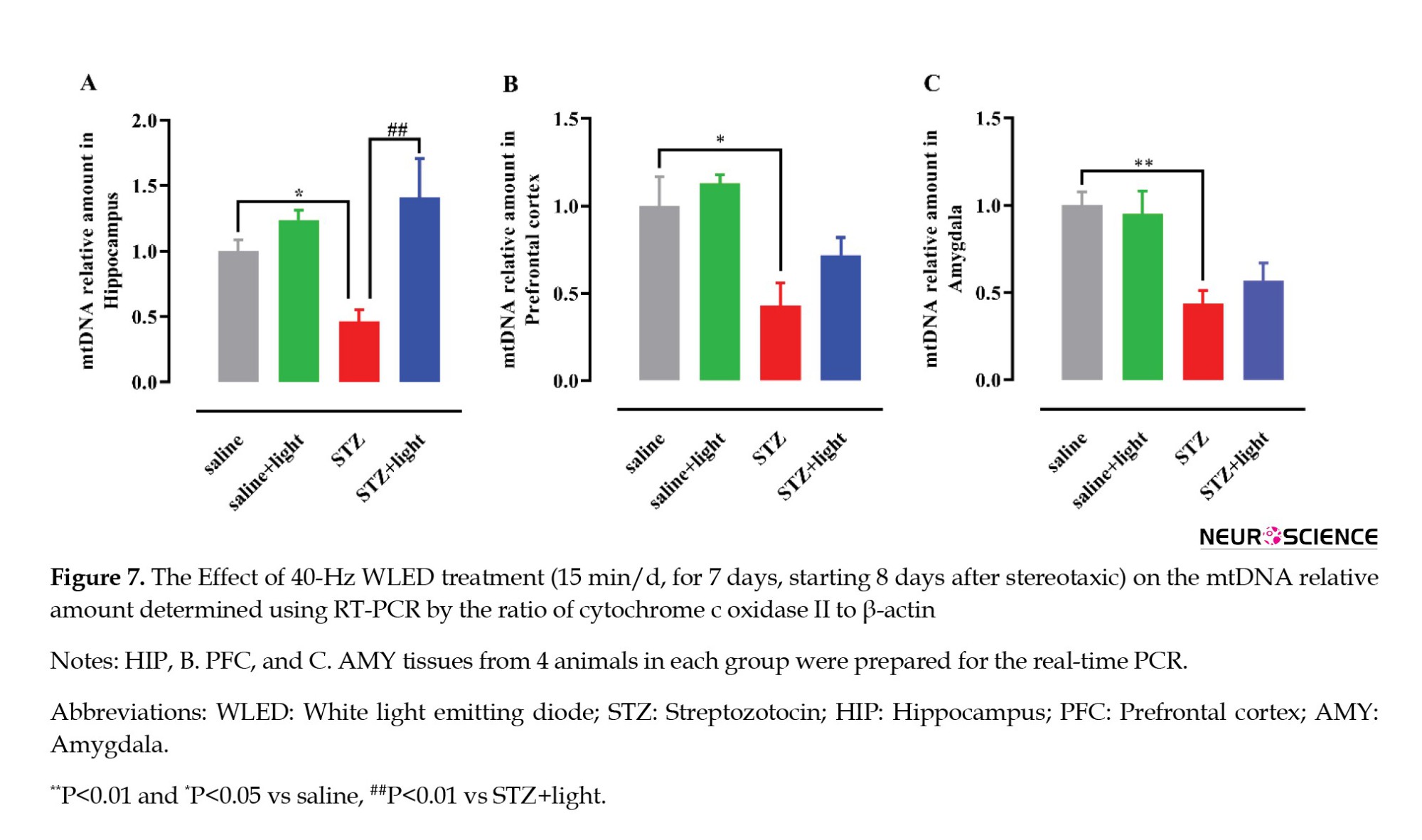
The mtDNA to total DNA ratio was measured using real-time PCR to evaluate the mtDNA relative amount in brain tissue. A significant difference between the groups was observed in the HIP (F(3, 12)=6.414, P=0.0077) (Figure 7A), PFC (F(3, 12)=6.645, P=0.0068), (Figure 7B) and AMY (F(3, 12)=8.135, P=0.0032) (Figure 7C). STZ injection decreased mtDNA relative amount in all three brain regions compared to saline (control) groups (P<0.01 and P<0.05) (Figure 7A). Also, 40-Hz WLED treatment in HIP prevented the decrease of mtDNA relative amount in STZ+light group compared with STZ group (P<0.01). However, the observed increase in mtDNA relative amount in the PFC and AMY following 40 Hz WLED treatment did not show statistical significance.
4. Discussion
The increasing prevalence of AD and the adverse effects of PS, including anxiety and depression on patients, highlight searching for more effective and also low-cost treatments (Degawa et al., 2021; Kenwood et al., 2022). Recent research suggests that investigating the involvement of the AMY, HIP, and PFC in anxiety reactions and the potential role of mitochondrial dysfunction in the pathogenesis of AD may improve treatment options for psychiatric disorders in Alzheimer patients (Ashleigh et al., 2023; Chen et al., 2021; Duval et al., 2015).
Our results indicate these results: 1) Anxiety and depressive-like behaviors and social isolation occur in STZ-induced AD model rats (on day 14), 2) In the STZ toxicity model (on day 14), a decrease in the frequency of mtDNA in the HIP, PFC, and AMY, a reduction in the activity of CAT in the PFC and AMY, and an increase in the activity of MAOA and MAOB enzymes in the whole brain were observed compared to control animals, 3) 40-Hz WLED improves depression and anxiety-like behaviors and social interactions, and 4) 40-Hz WLED increases the mtDNA relative amount in the HIP, improves the activity of CAT enzyme in the AMY and also decreases the activity of MAOA and MAOB.
STZ mimics several pathological and behavioral changes observed in humans with AD, including cognitive dysfunction, depression, anxiety, oxidative stress, and mitochondrial dysfunction. According to independent research studies, the cognitive performance and memory of rats decreased on days 21 (Kumar et al., 2016), 35 (Correia et al., 2012), and 60 (Roy et al., 2022) after receiving a single dose of ICV-STZ (3 mg/kg). Also, Chen et al. (2013) reported an increase in anxiety-like behaviors three weeks after STZ treatment. In this study, behavioral, enzymatic, and molecular tests were performed in the early stage of AD, 14 days after STZ injection, which distinguishes that from previous research. PS was observed and documented in a 14-day AD model, indicating a novel finding in the current study. In our research, STZ-injected rats showed a ~23% and ~40% decrease in the anxiety indices OAT% and OAE% (in EPM), respectively. In addition, the STZ-injected rats demonstrated a notable rise of ~53% in immobility time, indicating hopelessness and depression (in FST). Moreover, in this study, animals showed reduced sociability in the three-chamber test 14 days after exposure to STZ, which was a novel finding. This study yielded significant results regarding the early onset of PS 14 days after STZ administration. Hence, manifestations of anxiety and depression and social withdrawal are evident not only in the later phases but also in the initial stages of sAD. Thus, early diagnosis of AD through these symptoms before the onset of proteinopathy can prevent the progression of the disease and minimize its speed.
There is significant evidence of oxidant-antioxidant enzyme imbalance in a sAD model induced by ICV-STZ, which leads to increased impairment in cognitive and non-cognitive behaviors and potentially plays a role in the pathophysiology of depression and anxiety (Akhtar et al., 2021; Ghaderi et al., 2022; Postu et al., 2022). Neurons are more vulnerable to oxidative damage caused by hydroxyl free radicals because of the low level of CAT (Lee et al., 2020). In contrast, mitochondria are more sensitive because of the abundant production of H2O2 and lack of CAT production (Palma et al., 2020). Hence, in the current study, an assessment was conducted on the CAT activity in the AMY, HIP, and PFC, which are regions that contribute to the development of anxiety and depression (Duval et al., 2015). For example, Hajizadeh et al. (2020) demonstrated that a reduction in cerebral cortex antioxidant enzymes, including superoxide dismutase (SOD), CAT, and glutathione peroxidase and a subsequent increase in anxiety behaviors occurred 21 days after ICV-STZ (3 mg/kg). In addition, a study conducted by Abu-Taweel et al. (2021) revealed that depression and anxiety behaviors were observed along with reduced CAT activity in the HIP after 35 days in a sporadic model caused by AlCl3 in mice. This study investigated the timing of oxidative disorders to expedite the diagnosis of sAD by examining CAT activity 14 days after model induction. It revealed a significant reduction in the AMY and PFC of STZ-injected rats (~83% and ~40%, respectively). Based on our findings, further investigation is needed to explore the possible influence of CAT-mediated oxidative stress modulation on the initial phase of AD.
Furthermore, it has been suggested that changes in mitochondrial structure and function contribute to the progression of AD and related behavioral changes. For example, MAOA and MAOB potentially play a significant role in the pathophysiology of AD through the oxidation of mood-related neurotransmitters (Santin et al., 2021). In the present study, 14 days after STZ administration, an elevation of over two times in MAOA activity and a ~56% rise in MAOB were noticed in the brains of STZ-injected rats. Hafez et al. and Abdelghany et al. documented that AD rats had reduced serotonin and increased MAO activity in the HIP and cerebral cortex three months after AlCl3 administration compared with control rats (Abdelghany et al., 2023; Hafez et al., 2021). The study findings reveal that MAOA and MAOB activity changes report earlier than what was observed before, aligning with the emergence of anxiety and depression-like behaviors in the initial phase.
In addition, mtDNA abnormalities and copy number reduction are other mitochondrial factors that may be associated with AD-related cognitive and psychological changes. For example, Ansari Dezfouli et al., (2019)demonstrated that the injection of 1-42 Aβ into the CA1 region of the HIP results in anxiety-like behavior, impaired working memory, and a decline in mtDNA copy number. The present study showed that mtDNA relative amounts in the HIP, PFC, and AMY decreased by ~53%, ~57%, and ~56%, respectively, in STZ-injected rats (on day 14). According to the reviewed literature, mtDNA is more susceptible to oxidative stress due to its proximity to high concentrations of ROS, inefficient DNA repair mechanisms, and lack of protective histones (Phillips et al., 2014). Damage to mtDNA and loss of mitochondria disturb oxidative phosphorylation (OXPHOS), alterations in brain energy metabolism, increased ROS levels and decreased mtDNA abundance have been directly linked to poor cognitive performance in AD (Ansari Dezfouli et al., 2019; Klein et al., 2021; Shang et al., 2022). Nazari et al. also showed that Aβ1-42 amyloid toxicity reduces the activity of mitochondrial complexes I and IV (OXPHOS members), increases ROS production, and leads to memory deficits (Nazari et al., 2022). In this study, we propose that decreased mtDNA relative amount, probably through the alterations mentioned above, can result in depressive and anxiety-related behavior, as well as social seclusion in STZ-injected rats after 14 days. Thus, the utilization of mtDNA relative amount as an early biomarker for AD, along with interventions that maintain balanced mtDNA levels, may effectively manage AD progression and associated mood disorders.
In recent years, researchers have introduced PBM as a nonpharmacological and noninvasive approach with less cost and side effects and safer to treat AD. PBM uses different wavelengths of light as a noninvasive neuroprotective strategy (Heinig et al., 2020; Tian et al., 2023). PBM is employed as a novel technique for enhancing brain function by stimulating neural activity through increased activity of complexes in the mitochondrial electron transport chain (Pope & Denton 2020; Salehpour et al., 2018). In recent research, light emission diodes (LEDs) have been recommended over lasers because lasers have a narrow beam width and are not well-suited for treating large areas, in addition to generating excessive heat on the tissue (Hamblin, 2016; Nazari et al., 2022; Rojas & Gonzalez-Lima, 2013). In 2018, Singer et al. (2018) introduced 40-Hz flickering white light exposure as a protocol to non-invasively reduce Aβ levels by changing the morphology of microglia to a phagocytic state, leading to improved memory in transgenic Alzheimer rats. In a study by Park et al. (2020) conducted a study on the 40-Hz flickering light treatment group and found a reduction in tau and Aβ phosphorylation in the HIP and improved spatial learning, memory, long-term memory, mitochondrial function, and neural plasticity.
In this study, it was shown for the first time that exposure of the whole body to 40-Hz WLED for 15 min a day for 7 days improves anxiety-like and depression-like behaviors and social interactions in STZ-induced AD. Therefore, it can be claimed that treatment with 40-Hz WLED improves not only the cognitive performance of the animals but also PS in the Alzheimer rat model. Nevertheless, the mechanism of 40-Hz WLED effects on mitochondrial activity in PS is still unclear. Recent studies have shown that 630-nm RL can activate CAT by photoelectric coupling to the tyrosine residue, leading to H2O2 degradation (Park et al., 2020). However, more research is needed to justify the increase in CAT activity after applying 40-Hz WLED. In addition, no convincing evidence has been discovered to explain the mechanism by which light reduces the activity of monoamine oxidase A and B enzymes.
In another study, the COX II gene, one of the subunits of complex IV, was evaluated as an indicator for measuring mtDNA relative amount by RT-PCR (Ansari Dezfouli et al., 2019). Our findings indicate that the decrease in mtDNA relative amount caused by STZ was more than two-fold by 40-Hz WLED irradiation in the HIP. However, no significant increases were observed in other experimental regions. The PBM effects are related to the capacity of brain tissue chromophores to absorb photon energy, depending on the wavelength and the impact on cellular metabolism and brain physiology (Cardoso et al., 2021). Nazari et al., (2022) showed that exposure of Aβ-injected rats to 40-Hz WLED with a wavelength of ~425-550 nm significantly increased complex I and complex IV activity and reduced ΔΨm and ROS production. Also, other studies have shown that mitochondrial cytochrome c oxidase (COX; complex IV) as a chromophore absorbs light at about 420-450 nm (Covian et al., 2023), suggesting that the beneficial effects of the 40 Hz WLEDs in this study may be attributed to these factors. However, many questions about the impact of 40-Hz WLED on mitochondrial activity have remained unanswered, and more experimental research is needed to make a conclusive statement about the underlying mechanism behind the beneficial effect of 40-Hz WLED in an AD model.
5. Conclusion
This study provides the first documentation of the effect of light therapy (40-Hz WLED) on PS related to AD and the possible effect of 40-Hz WLED on some mitochondrial factors involved in it. Light improves anxiety, depression, and social interaction in the early stages of sAD by increasing CAT activity, decreasing MAOA and MAOB activity, and increasing the mtDNA relative amount. In the future, 40-Hz WLED may serve as a noninvasive therapeutic option for mitigating early-stage Alzheimer PS.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.2018.973).
Funding
This study was supported by Neuroscience Research Center of Shahid Beheshti University of Medical Sciences (No.: 23397) as well as by a research grant (Grant No.: 22241) from Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Methodology: Fariba Khodagholi and Afsaneh Eliassi; Visualization: Maryam Bazrgar; Investigation: Elham Soleimani and Maryam Bazrgar; Validation: Maryam Bazrgar: Fariba Khodagholi and Afsaneh Eliassi; Formal analysis and writing the original draft: Elham Soleimani; Review and editing: Fariba Khodagholi, Afsaneh Eliassi and Abolhassan Ahmadiani; Funding acquisition, supervisor, and project administration: Abolhassan Ahmadiani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This work was a part of the PhD dissertation of Elham Soleimani, approved by the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
Alzheimer disease (AD), a neurodegenerative disease that progresses with age, accounts for the largest proportion of dementia globally (Chakraborty et al., 2019) present in almost 90% of patients with AD. Most patients with AD suffer from various behavioral and mood disorders that accelerate the progression of the disease. Studies have recently shown that psychiatric symptoms (PS), such as depression, anxiety, and apathy, occur before the onset of cognitive symptoms in AD patients (Vuic et al., 2022; Zhang et al., 2018) which frequently develop during the course and different stages of dementia. The diagnosis of BPSD is complex due to symptom variety, and relies on detailed clinical evaluation and medical history. Accurate assessment of BPSD is crucial in order to tailor therapeutic intervention (non-pharmacological and pharmacological. Currently, there are no FDA-approved interventions for PS, and the available pharmacological treatments, such as monoamine oxidase inhibitors prescribed for depression and anxiety, have toxic side effects, including hepatotoxicity, orthostatic hypotension, and hypertensive crisis (Chakraborty et al., 2019; Dhapola et al., 2022; Nandi et al., 2023) present in almost 90% of patients with AD.
Scientists are investigating nonpharmacological, noninvasive alternatives, such as photobiomodulation (PBM) using red light (RL) or near-infrared light (NIR), for treating brain diseases because current treatments have limited effectiveness and more side effects (Li et al., 2023). A 40-Hz white light scintillator through the eyes has been shown to attenuate the pathological characteristics of AD (Tian et al., 2023). The PBM has multiple benefits, such as reducing inflammatory factors, improving memory in AD model mice (Yu et al., 2022), colocalizing microglia with Aβ and eliminating senile plaques (Iaccarino et al., 2016; Tao et al., 2021) and reducing superoxide and Aβ-induced inflammation in astrocytes (Lu et al., 2017).
The impact of a 40-Hz white light emitting diode (WLED) on PS in AD has not been proven, and the underlying mechanisms are not fully understood despite the hypothesis that it may affect certain structures and biomolecules in mitochondria. As demonstrated by researchers, the use of 40-Hz WLED in the Aβ1-42 toxicity model increases mitochondrial respiratory chain complex I and IV activity, decreases reactive oxygen species (ROS) production and mitochondrial membrane potential, and improves the structure-function of potassium channels (Nazari et al., 2022).
Moreover, the mitochondrial dysfunction is acknowledged as an early event in the development of AD (Wang et al., 2020). Excessive production of ROS through intracerebroventricular (ICV) -streptozotocin (STZ) in the sporadic AD (sAD) model targets mitochondria, which are the main source of ROS production. It results in a reduction in mtDNA frequency and contributes to AD pathology and cognitive and non-cognitive behavioral disorders (Ansari Dezfouli et al., 2019; Dhapola et al., 2022; Harerimana et al., 2022; Wan Chik et al., 2023).
Previous studies have acknowledged the significance of Aβ in generating H2O2 as the primary free radical produced in mitochondria. They suggest that H2O2 is rapidly catalyzed by catalase (CAT) and preserves the mitochondrial function in AD (Reddy, 2006).
In addition, monoamine oxidases, found in the mitochondria’s outer membrane, are associated with AD PS pathophysiology because of their impact on monoamine neurotransmitter levels, as well as their role in oxidative stress through hydrogen peroxide production (Behl et al., 2021; Emilsson et al., 2002). Therefore, it has been proposed that inhibiting monoamine oxidases could enhance PS by decreasing oxidative stress and the breakdown of amine neurotransmitters.
Therefore, the objective of the current research is to provide answers to the following questions: Does 40-Hz flashing light improve depression, anxiety, and social interaction at an early stage in STZ-induced AD-like rats? Was the effect of 40-Hz flashing light on behavioral symptoms due to the regulation of monoaminoxidase A (MAOA), monoaminoxidase B (MAOB), and CAT enzyme activity as well as the abundance of mitochondrial DNA in the hippocampus (HIP), amygdala (AMY), and prefrontal cortex (PFC).
2. Materials and Methods
Animal model
Male Wistar rats (mean weight: 250±10g) were kept in the laboratory under controlled conditions (12:12-h light: Dark cycle, steady temperature of 23±2 °C, and free access to water and food), and all procedures followed ethical guidelines approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Induction of STZ toxicity
STZ (Sigma-Aldrich) was used to create an Alzheimer-like animal model. According to the Paxinos Watson atlas, a stereotactic surgery was performed to inject STZ (3 mg/kg) or normal saline (3 μL by gentle infusion over 5 min) into bilateral intraventricular (AP=0.8, ML=1.5, DV=3) after inducing anesthesia in rats with ketamine/xylazine (Alluri et al., 2020; Paxinos & Watson, 2006).
Experimental design
The intervention was started one week after the surgery, given once a day for 15 min, and continued for 7 days. Following a 24-h interval since the last session, a group of rats were subjected to behavioral testing, while the other rats had their brain tissue collected for molecular and enzyme studies (Figure 1).

The experimental groups were randomly divided as follows.
1. Saline (control): Bilateral ICV injection of normal saline,
2. Saline + light (40-Hz WLED),
3. STZ (Alzheimer-like group): Bilateral ICV injection of STZ (3 mg/kg) and
4. STZ + light (40-Hz WLED).
The rats were exposed to white light with a visible wavelength of 425-550 nm, intensity of 60 W, frequency of 40 Hz, and average irradiance of 12 mW/cm2 in a dark chamber containing LED strip lamps (Iaccarino et al., 2016; Jones et al., 2019; Nazari et al., 2022).
Elevated plus maze test (EPM)
The EPM behavioral model measures rodent anxiety based on their searching and avoidance instincts. It features four arms in the shape of a positive sign, each measuring 50×10 cm, with a high wall surrounding the closed arm and 1 cm high edges around the open arms. The central area, with dimensions of 10×10 cm, intersects the arms, and the maze is illuminated by a dim glow (Rodgers & Johnson 1995; Walf & Frye 2007) we have used a large database comprising the behavioral profiles of 90 undrugged mice to examine the relationship between the standard spatiotemporal measures and a range of specific behaviors related to the defensive repertoire of the mouse. A factor analysis applied to the standard measures revealed two factors related to anxiety and locomotor activity. The simple addition of center time (an infrequently recorded measure. Animals were individually positioned in the maze’s center, facing the open arms and freely moving among them for 5 min. A decrease in the percentage of entries and or time spent in open arms indicates an anxiolytic index (Beirami et al., 2017).
Forced swim test
In this test, it is thought that inactivity reflects the state of depression. In this study, the animal was placed individually in a plexiglass cylinder containing tap water to a depth of 30 cm, which was kept for 15 min (usually at 25 °C). Twenty-four hours later, the subjects were placed in the cylinder, and their immobility time was counted in a 5-min test period. A subject is considered motionless when it is passively floating in water in a little bent but vertical status with its head just above the water surface (Tucci et al., 2022) GRA displayed antidepressant activity in preclinical models. We have previously demonstrated that a single ICV administration of soluble amyloid-beta 1-42 (sAβ 1-42).
Social interaction test (three chamber paradigm)
In this trial, animals unfamiliar with one another were chosen, with two control rats required in each experiment, one for the first stage and the other for the second stage of the trial. A social interaction test was performed for 25 min in an unfamiliar rectangular space divided into three parts by walls. During habituation, the rats could move freely between the rooms for 5 min. In the first stage (10 min), one of the control rats was placed in one of the chambers to test the animalʼs social tendencies. The duration of direct contact with stranger rat 1 was recorded. In the second stage (10 min), the level of the animalʼs desire to create new social relationships was tested, with the parameters stated in the previous step measured, and the ratʼs behavioral differences in interaction with stranger 1 and stranger 2 were observed. This test investigates sociability, the desire for new connections, and social isolation (Davoudi et al., 2023) memory impairment, and anxiety-like behavior are characterized in many people identified with autism spectrum disorder (ASD).
Quantification of mitochondrial DNA
DNA was extracted from the HIP, AMY, and PFC using the tissue genomic DNA extraction mini kit (Favorgen, Taiwan) following the provided instructions. The quantification of mtDNA relative amount was performed by implementing real-time PCR methodology with SYBR Green detection. The quantification of mtDNA was performed using PCR Master Mix reagents (Ampliqon, Denmark), following specific cycling conditions, including activation at 95 °C for 10 min, denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, extension at 72 °C for 30 s, and a total of 40 cycles in the ABi step-one System (USA) (Ansari Dezfouli et al., 2019; Sheng et al., 2012). The primers used for the mitochondrial gene cytochrome c oxidase II and the nuclear gene β-actin are listed in Table 1.

Measurement of the CAT activity
We used TPR’s CAT activity assay kit (Teb Pazhouhan Razi (TPR), Iran) to measure CA activity in tissues.
Measurement of monoamine oxidase A and monoamine oxidase B activity
To evaluate the activity of monoamine oxidase A (MAOA) and monoamine oxidase B (MAOB) enzymes, mitochondria were isolated using the Clark and Nicklas method and then measured by spectrophotometry (Clark & Nicklas 1970). Sodium phosphate buffer (100 mM) and 5-hydroxytryptamine (4 mM) were combined in each well to estimate MAOA activity. Subsequently, the enzymatic reaction was initiated by adding solutions of the mitochondrial fraction, and the resulting change in absorbance was monitored at 280 nm using a spectrophotometer (Thermo Scientific/USA) against a blank consisting of sodium phosphate buffer (100 mM) and 5-hydroxytryptamine (4 mM). To estimate MAOB activity, a mixture of sodium phosphate buffer (100 mM) and 0.1 M benzylamine was prepared in each well at a wavelength of 249.5 nm, and a blank containing sodium phosphate buffer and benzylamine was used as a reference. The next step was adding mitochondrial fraction solutions to start the enzymatic reaction and recording the resulting change in absorbance (Bahaeddin et al., 2022).
Statistical analysis
The processing and visualization of the data were conducted using GraphPad Prism software, version 9 and the results were presented as the Mean±SEM. The significant difference was set as a P<0.05. The study groups were compared using a one-way analysis of variance (ANOVA) and a Tukey post hoc test.
3. Results
Effect of 40-Hz WLED on STZ-induced anxiety-like responses
One-way ANOVA revealed significant differences in the percentage of time spent on the open arms (OAT%) (F(3, 28)=5.403, P=0.0046) (Figure 2A) and the percentage of entries into the open arms (OAE %) (F(3, 28)=22.33, P<0.001) (Figure 2B) during the EPM test. Post-hoc analysis revealed that the levels of OAT% (P<0.05) and OAE% (P<0.0001) in the STZ-injected rats were significantly reduced compared with those in the saline group, suggesting an anxiogenic reaction. In the 40-Hz WLED treatment group (STZ+light), there was a notable rise in OAT% (P<0.01) and OAE% (P<0.0001) compared with the STZ-injected rats, indicating that the 40-Hz WLED reversed the anxiogenic response induced by STZ administration.

Effect of 40-Hz WLED on STZ-induced depressive-like responses
Figure 3 shows the behavior of rats subjected to a forced swim test (FST). One-way ANOVA showed significant differences in immobility time between the groups (F(3, 24)=9.967, P=0.0002) (Figure 3). Post-hoc analysis (Tukey test) indicated that STZ induced a depression-like response as evidenced by a significant increase in immobility time (P<0.001) compared with the saline group. Applying 40-Hz WLED led to a significant decrease in immobility time (P<0.01) and removed the STZ-induced depression in rats.

Effect of 40-Hz WLED on STZ-induced impairment of social interactions
One-way ANOVA analysis in phase 1 of the experiment showed a significant group difference in the time spent with the empty basket and stranger 1 (F(3, 32)=25.04, P<0.0001; and F(3, 32)=9.261, P=0.0001; respectively). In the control group, the increase in time spent around stranger 1 and the decrease in time spent around the empty basket indicate normal sociability. In contrast, the opposite behavior in the STZ group suggests a decrease in the desire to make social connections (Figure 4A). On the other hand, we showed that light significantly improved social relationships in the STZ+light group. One-way ANOVA analysis in phase 2 shows a significant group difference in the time spent with stranger 1 (F(3, 32)=26.68, P<0.0001) and stranger 2 (F(3, 32)=28.52, P<0.0001). The STZ group showed less desire to create new relationships and preferred social isolation by spending less time with stranger 2 than the control group. While light therapy could have a significant positive effect on social novelty in STZ+light by increasing the time spent around the stranger 2. It is possible that the results were influenced by the decrease in anxiety that occurred following exposure to light (Figure 4B).

Effect of 40-Hz WLED on CAT activity in the HIP, AMY, and PFC
A significant difference in CAT activity between different groups was observed in the AMY (F(3, 14)=60.37, P<0.001) (Figure 5C) and PFC (F(3, 10)=4.588, P=0.0288) (Figure 5B). The mean CAT activity of the STZ group in the AMY and PFC was significantly lower than that of the saline group, (P<0.0001 and P<0.05, respectively). Also, 40-Hz WLED treatment in the AMY prevented the decrease of CAT activation in the STZ+light group compared with the STZ group (P<0.01). However, in the other two brain regions, HIP, PFC, and CAT activity did not show any significant difference in the light-treated rats with respect to the STZ group.

Effect of 40-Hz WLED on the activity of MAOA and MAOB
One-way ANOVA showed significant differences in MAOA and MAOB enzymatic activity between the study groups (F(3, 20)=8.035, P=0.001 and F(3, 8)=9.857, P=0.0046; respectively) (Figure 6). STZ administration resulted in a significant increase in MAOA and MAOB enzymatic activity (P<0.05) compared with the saline group. Also, 40-Hz WLED significantly reduced MAOA and MAOB (P<0.01) enzymatic activity in the STZ-light group compared to STZ-treated rats. The decrease in anxiety and depression in animals through WLED therapy may be due to the decline in MAOA and MAOB activity, leading to the reduction of monoamine oxidation involved in anxiety and depression.

Effect of 40-Hz WLED on mtDNA relative amount in the HIP, AMY, and PFC
The mtDNA to total DNA ratio was measured using real-time PCR to evaluate the mtDNA relative amount in brain tissue. A significant difference between the groups was observed in the HIP (F(3, 12)=6.414, P=0.0077) (Figure 7A), PFC (F(3, 12)=6.645, P=0.0068), (Figure 7B) and AMY (F(3, 12)=8.135, P=0.0032) (Figure 7C). STZ injection decreased mtDNA relative amount in all three brain regions compared to saline (control) groups (P<0.01 and P<0.05) (Figure 7A). Also, 40-Hz WLED treatment in HIP prevented the decrease of mtDNA relative amount in STZ+light group compared with STZ group (P<0.01). However, the observed increase in mtDNA relative amount in the PFC and AMY following 40 Hz WLED treatment did not show statistical significance.

4. Discussion
The increasing prevalence of AD and the adverse effects of PS, including anxiety and depression on patients, highlight searching for more effective and also low-cost treatments (Degawa et al., 2021; Kenwood et al., 2022). Recent research suggests that investigating the involvement of the AMY, HIP, and PFC in anxiety reactions and the potential role of mitochondrial dysfunction in the pathogenesis of AD may improve treatment options for psychiatric disorders in Alzheimer patients (Ashleigh et al., 2023; Chen et al., 2021; Duval et al., 2015).
Our results indicate these results: 1) Anxiety and depressive-like behaviors and social isolation occur in STZ-induced AD model rats (on day 14), 2) In the STZ toxicity model (on day 14), a decrease in the frequency of mtDNA in the HIP, PFC, and AMY, a reduction in the activity of CAT in the PFC and AMY, and an increase in the activity of MAOA and MAOB enzymes in the whole brain were observed compared to control animals, 3) 40-Hz WLED improves depression and anxiety-like behaviors and social interactions, and 4) 40-Hz WLED increases the mtDNA relative amount in the HIP, improves the activity of CAT enzyme in the AMY and also decreases the activity of MAOA and MAOB.
STZ mimics several pathological and behavioral changes observed in humans with AD, including cognitive dysfunction, depression, anxiety, oxidative stress, and mitochondrial dysfunction. According to independent research studies, the cognitive performance and memory of rats decreased on days 21 (Kumar et al., 2016), 35 (Correia et al., 2012), and 60 (Roy et al., 2022) after receiving a single dose of ICV-STZ (3 mg/kg). Also, Chen et al. (2013) reported an increase in anxiety-like behaviors three weeks after STZ treatment. In this study, behavioral, enzymatic, and molecular tests were performed in the early stage of AD, 14 days after STZ injection, which distinguishes that from previous research. PS was observed and documented in a 14-day AD model, indicating a novel finding in the current study. In our research, STZ-injected rats showed a ~23% and ~40% decrease in the anxiety indices OAT% and OAE% (in EPM), respectively. In addition, the STZ-injected rats demonstrated a notable rise of ~53% in immobility time, indicating hopelessness and depression (in FST). Moreover, in this study, animals showed reduced sociability in the three-chamber test 14 days after exposure to STZ, which was a novel finding. This study yielded significant results regarding the early onset of PS 14 days after STZ administration. Hence, manifestations of anxiety and depression and social withdrawal are evident not only in the later phases but also in the initial stages of sAD. Thus, early diagnosis of AD through these symptoms before the onset of proteinopathy can prevent the progression of the disease and minimize its speed.
There is significant evidence of oxidant-antioxidant enzyme imbalance in a sAD model induced by ICV-STZ, which leads to increased impairment in cognitive and non-cognitive behaviors and potentially plays a role in the pathophysiology of depression and anxiety (Akhtar et al., 2021; Ghaderi et al., 2022; Postu et al., 2022). Neurons are more vulnerable to oxidative damage caused by hydroxyl free radicals because of the low level of CAT (Lee et al., 2020). In contrast, mitochondria are more sensitive because of the abundant production of H2O2 and lack of CAT production (Palma et al., 2020). Hence, in the current study, an assessment was conducted on the CAT activity in the AMY, HIP, and PFC, which are regions that contribute to the development of anxiety and depression (Duval et al., 2015). For example, Hajizadeh et al. (2020) demonstrated that a reduction in cerebral cortex antioxidant enzymes, including superoxide dismutase (SOD), CAT, and glutathione peroxidase and a subsequent increase in anxiety behaviors occurred 21 days after ICV-STZ (3 mg/kg). In addition, a study conducted by Abu-Taweel et al. (2021) revealed that depression and anxiety behaviors were observed along with reduced CAT activity in the HIP after 35 days in a sporadic model caused by AlCl3 in mice. This study investigated the timing of oxidative disorders to expedite the diagnosis of sAD by examining CAT activity 14 days after model induction. It revealed a significant reduction in the AMY and PFC of STZ-injected rats (~83% and ~40%, respectively). Based on our findings, further investigation is needed to explore the possible influence of CAT-mediated oxidative stress modulation on the initial phase of AD.
Furthermore, it has been suggested that changes in mitochondrial structure and function contribute to the progression of AD and related behavioral changes. For example, MAOA and MAOB potentially play a significant role in the pathophysiology of AD through the oxidation of mood-related neurotransmitters (Santin et al., 2021). In the present study, 14 days after STZ administration, an elevation of over two times in MAOA activity and a ~56% rise in MAOB were noticed in the brains of STZ-injected rats. Hafez et al. and Abdelghany et al. documented that AD rats had reduced serotonin and increased MAO activity in the HIP and cerebral cortex three months after AlCl3 administration compared with control rats (Abdelghany et al., 2023; Hafez et al., 2021). The study findings reveal that MAOA and MAOB activity changes report earlier than what was observed before, aligning with the emergence of anxiety and depression-like behaviors in the initial phase.
In addition, mtDNA abnormalities and copy number reduction are other mitochondrial factors that may be associated with AD-related cognitive and psychological changes. For example, Ansari Dezfouli et al., (2019)demonstrated that the injection of 1-42 Aβ into the CA1 region of the HIP results in anxiety-like behavior, impaired working memory, and a decline in mtDNA copy number. The present study showed that mtDNA relative amounts in the HIP, PFC, and AMY decreased by ~53%, ~57%, and ~56%, respectively, in STZ-injected rats (on day 14). According to the reviewed literature, mtDNA is more susceptible to oxidative stress due to its proximity to high concentrations of ROS, inefficient DNA repair mechanisms, and lack of protective histones (Phillips et al., 2014). Damage to mtDNA and loss of mitochondria disturb oxidative phosphorylation (OXPHOS), alterations in brain energy metabolism, increased ROS levels and decreased mtDNA abundance have been directly linked to poor cognitive performance in AD (Ansari Dezfouli et al., 2019; Klein et al., 2021; Shang et al., 2022). Nazari et al. also showed that Aβ1-42 amyloid toxicity reduces the activity of mitochondrial complexes I and IV (OXPHOS members), increases ROS production, and leads to memory deficits (Nazari et al., 2022). In this study, we propose that decreased mtDNA relative amount, probably through the alterations mentioned above, can result in depressive and anxiety-related behavior, as well as social seclusion in STZ-injected rats after 14 days. Thus, the utilization of mtDNA relative amount as an early biomarker for AD, along with interventions that maintain balanced mtDNA levels, may effectively manage AD progression and associated mood disorders.
In recent years, researchers have introduced PBM as a nonpharmacological and noninvasive approach with less cost and side effects and safer to treat AD. PBM uses different wavelengths of light as a noninvasive neuroprotective strategy (Heinig et al., 2020; Tian et al., 2023). PBM is employed as a novel technique for enhancing brain function by stimulating neural activity through increased activity of complexes in the mitochondrial electron transport chain (Pope & Denton 2020; Salehpour et al., 2018). In recent research, light emission diodes (LEDs) have been recommended over lasers because lasers have a narrow beam width and are not well-suited for treating large areas, in addition to generating excessive heat on the tissue (Hamblin, 2016; Nazari et al., 2022; Rojas & Gonzalez-Lima, 2013). In 2018, Singer et al. (2018) introduced 40-Hz flickering white light exposure as a protocol to non-invasively reduce Aβ levels by changing the morphology of microglia to a phagocytic state, leading to improved memory in transgenic Alzheimer rats. In a study by Park et al. (2020) conducted a study on the 40-Hz flickering light treatment group and found a reduction in tau and Aβ phosphorylation in the HIP and improved spatial learning, memory, long-term memory, mitochondrial function, and neural plasticity.
In this study, it was shown for the first time that exposure of the whole body to 40-Hz WLED for 15 min a day for 7 days improves anxiety-like and depression-like behaviors and social interactions in STZ-induced AD. Therefore, it can be claimed that treatment with 40-Hz WLED improves not only the cognitive performance of the animals but also PS in the Alzheimer rat model. Nevertheless, the mechanism of 40-Hz WLED effects on mitochondrial activity in PS is still unclear. Recent studies have shown that 630-nm RL can activate CAT by photoelectric coupling to the tyrosine residue, leading to H2O2 degradation (Park et al., 2020). However, more research is needed to justify the increase in CAT activity after applying 40-Hz WLED. In addition, no convincing evidence has been discovered to explain the mechanism by which light reduces the activity of monoamine oxidase A and B enzymes.
In another study, the COX II gene, one of the subunits of complex IV, was evaluated as an indicator for measuring mtDNA relative amount by RT-PCR (Ansari Dezfouli et al., 2019). Our findings indicate that the decrease in mtDNA relative amount caused by STZ was more than two-fold by 40-Hz WLED irradiation in the HIP. However, no significant increases were observed in other experimental regions. The PBM effects are related to the capacity of brain tissue chromophores to absorb photon energy, depending on the wavelength and the impact on cellular metabolism and brain physiology (Cardoso et al., 2021). Nazari et al., (2022) showed that exposure of Aβ-injected rats to 40-Hz WLED with a wavelength of ~425-550 nm significantly increased complex I and complex IV activity and reduced ΔΨm and ROS production. Also, other studies have shown that mitochondrial cytochrome c oxidase (COX; complex IV) as a chromophore absorbs light at about 420-450 nm (Covian et al., 2023), suggesting that the beneficial effects of the 40 Hz WLEDs in this study may be attributed to these factors. However, many questions about the impact of 40-Hz WLED on mitochondrial activity have remained unanswered, and more experimental research is needed to make a conclusive statement about the underlying mechanism behind the beneficial effect of 40-Hz WLED in an AD model.
5. Conclusion
This study provides the first documentation of the effect of light therapy (40-Hz WLED) on PS related to AD and the possible effect of 40-Hz WLED on some mitochondrial factors involved in it. Light improves anxiety, depression, and social interaction in the early stages of sAD by increasing CAT activity, decreasing MAOA and MAOB activity, and increasing the mtDNA relative amount. In the future, 40-Hz WLED may serve as a noninvasive therapeutic option for mitigating early-stage Alzheimer PS.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.MSP.REC.2018.973).
Funding
This study was supported by Neuroscience Research Center of Shahid Beheshti University of Medical Sciences (No.: 23397) as well as by a research grant (Grant No.: 22241) from Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Methodology: Fariba Khodagholi and Afsaneh Eliassi; Visualization: Maryam Bazrgar; Investigation: Elham Soleimani and Maryam Bazrgar; Validation: Maryam Bazrgar: Fariba Khodagholi and Afsaneh Eliassi; Formal analysis and writing the original draft: Elham Soleimani; Review and editing: Fariba Khodagholi, Afsaneh Eliassi and Abolhassan Ahmadiani; Funding acquisition, supervisor, and project administration: Abolhassan Ahmadiani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This work was a part of the PhD dissertation of Elham Soleimani, approved by the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
Abdelghany, A. K., Gamal, A., Abdel-Wahab, A., Abdel-Razik, A. H., El-Samannoudy, S. I., & Ibrahim, M. A., et al. (2023). RETRACTED ARTICLE: Evaluating the neuroprotective effect of Spirulina platensis-loaded niosomes against Alzheimer's disease induced in rats. Drug Delivery and Translational Research, 13(10), 2690. [DOI:10.1007/s13346-023-01301-2] [PMID]
Abu-Taweel, G. M., & Al-Mutary, M. G. (2021). “Pomegranate juice moderates anxiety- and depression-like behaviors in AlCl3-treated male mice.” Journal of Trace Elements in Medicine and Biology: Organ of the Society for Minerals and Trace Elements (GMS), 68, 126842. [DOI:10.1016/j.jtemb.2021.126842] [PMID]
Akhtar, A., Dhaliwal, J., & Sah, S. P. (2021).“7,8-Dihydroxyflavone improves cognitive functions in ICV-STZ rat model of sporadic Alzheimer's disease by reversing oxidative stress, mitochondrial dysfunction, and insulin resistance. Psychopharmacology, 238(7), 1991–2009. [DOI:10.1007/s00213-021-05826-7] [PMID]
Alluri, R., Ambati, S. R., Routhu, K., Kopalli, S. R., & Koppula, S. (2020).“Phosphoinositide 3-Kinase Inhibitor AS605240 Ameliorates Streptozotocin-Induced Alzheimer’s disease like sporadic dementia in experimental rats.” EXCLI Journal, 19, 71–85. [PMID]
Ansari Dezfouli, M., Zahmatkesh, M., Farahmandfar, M., & Khodagholi, F. (2019). “Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal sirtuin-1 signaling pathway.” Physiology & Behavior, 204, 65–75. [DOI:10.1016/j.physbeh.2019.02.016] [PMID]
Ashleigh, T., Swerdlow, R. H., & Beal, M. F. (2023). “The role of mitochondrial dysfunction in alzheimer’s disease pathogenesis.” Alzheimer’s and Dementia, 19(1), 333-342. [DOI:10.1002/alz.12683] [PMID]
Bahaeddin, Z., Khodagholi, F., Foolad, F., Emadi, F., Alijaniha, F., & Zareh Shahamati, S., et al. (2023). “Almond intake during pregnancy in rats improved the cognitive performance of adult male offspring.” Nutritional Neuroscience, 26(9), 888–900. [DOI:10.1080/1028415X.2022.2108255] [PMID]
Behl, T., Kaur, D., Sehgal, A., Singh, S., Sharma, N., & Zengin, G., et al. (2021). “Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An insight into the therapeutic potential of inhibitors.” Molecules (Basel, Switzerland), 26(12), 3724. [DOI:10.3390/molecules26123724] [PMID]
Beirami, E., Oryan, S., Seyedhosseini Tamijani, S. M., Ahmadiani, A., & Dargahi, L. (2017). “Intranasal insulin treatment alleviates methamphetamine induced anxiety-like behavior and neuroinflammation.” Neuroscience Letters, 660, 122–129.[DOI:10.1016/j.neulet.2017.09.026] [PMID]
Caardoso, F. D. S., Gonzalez-Lima, F., & Gomes da Silva, S. (2021). Photobiomodulation for the aging brain. Ageing Research Reviews, 70, 101415.“ [DOI:10.1016/j.arr.2021.101415] [PMID]
Chakraborty, S., Lennon, J. C., Malkaram, S. A., Zeng, Y., Fisher, D. W., & Dong, H. (2019). “Serotonergic system, cognition, and BPSD in Alzheimer’s Disease.” Neuroscience Letters, 704, 36-44. [DOI:10.1016/j.neulet.2019.03.050] [PMID]
Chen, Y., Liang, Z., Blanchard, J., Dai, C. L., Sun, S., & Lee, M. H., et al. (2013). “A non-transgenic mouse model (Icv-STZ Mouse) of Alzheimer’s Disease: Similarities to and differences from the transgenic model (3xTg-AD Mouse).” Molecular Neurobiology, 47(2), 711-725. [DOI:10.1007/s12035-012-8375-5] [PMID]
Chen, Y., Dang, M., & Zhang, Z. (2021). “Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s Disease: A systematic review of symptom-general and -specific lesion patterns.” Molecular Neurodegeneration, 16(1), 38. [DOI:10.1186/s13024-021-00456-1] [PMID]
Clark, J. B., & Nicklas, W. J. (1970). The metabolism of rat brain mitochondria. Preparation and characterization. The Journal of Biological Chemistry, 245(18), 4724–4731. [DOI:10.1016/S0021-9258(18)62854-6] [PMID]
Correia, S. C., Santos, R. X., Santos, M. S., Casadesus, G., Lamanna, J. C., & Perry, G., et al. (2013). “Mitochondrial abnormalities in a streptozotocin-induced rat model of sporadic Alzheimer's disease. Current Alzheimer Research, 10(4), 406–419. [PMID]
Covian, R., Edwards, L. O., Lucotte, B. M., & Balaban, R. S. (2023). “Spectroscopic identification of the catalytic intermediates of cytochrome c oxidase in respiring heart mitochondria.” Biochimica et Biophysica acta. Bioenergetics, 1864(2), 148934. [DOI:10.1016/j.bbabio.2022.148934] [PMID]
Davoudi, S., Rahdar, M., Hosseinmardi, N., Behzadi, G., & Janahmadi, M. (2023). “Chronic inhibition of astrocytic aquaporin-4 induces autistic-like behavior in control rat offspring similar to maternal exposure to valproic acid.” Physiology & Behavior, 269, 114286. [DOI:10.1016/j.physbeh.2023.114286] [PMID]
Degawa, T., Kawahata, I., Izumi, H., Shinoda, Y., & Fukunaga, K. (2021).“T-Type Ca2+ channel enhancer SAK3 Administration Improves the BPSD-like behaviors in AppNL−G-F/NL−G-F Knock-in Mice.” Journal of Pharmacological Sciences, 146(1), 1-9. [DOI:10.1016/j.jphs.2021.02.006] [PMID]
Dhapola, R., Sarma, P., Medhi, B., Prakash, A., & Reddy, D. H. (2022). Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer's Disease. Molecular Neurobiology, 59(1), 535–555. [DOI:10.1007/s12035-021-02612-6] [PMID]
Duval, E. R., Javanbakht, A., & Liberzon, I. (2015).“Neural circuits in anxiety and stress disorders: A Focused review.” Therapeutics and Clinical Risk Management, 11, 115–126.[DOI:10.2147/TCRM.S48528] [PMID]
Emilsson, L., Saetre, P., Balciuniene, J., Castensson, A., Cairns, N., & Jazin, E. E. (2002). “Increased monoamine oxidase messenger RNA expression levels in frontal cortex of Alzheimer’s Disease Patients.” Neuroscience Letters, 326(1), 56-60. [DOI:10.1016/S0304-3940(02)00307-5] [PMID]
Ghaderi, S., Gholipour, P., Komaki, A., Salehi, I., Rashidi, K., & Esmaeil Khoshnam, S., et al. (2022). “P-Coumaric acid ameliorates cognitive and non-cognitive disturbances in a rat model of Alzheimer’s Disease: The role of oxidative stress and inflammation.” International Immunopharmacology, 112, 109295. [DOI:10.1016/j.intimp.2022.109295] [PMID]
Hafez, H. A., Kamel, M. A., Osman, M. Y., Osman, H. M., Elblehi, S. S., & Mahmoud, S. A. (2021). “Ameliorative effects of astaxanthin on brain tissues of alzheimer’s disease-like model: Cross talk between neuronal-specific MicroRNA-124 and related pathways.” Molecular and Cellular Biochemistry, 476(5), 2233–2249. [DOI:10.1007/s11010-021-04079-4] [PMID]
Hajizadeh Moghaddam, A., Ahmadnia, H., Jelodar, S. K., & Ranjbar, M. (2020). “Hesperetin nanoparticles attenuate anxiogenic-like behavior and cerebral oxidative stress through the upregulation of antioxidant enzyme expression in experimental dementia of Alzheimer’s Type.” Neurological Research, 42(6), 477-486. [PMID]
Hamblin M. R. (2016). “Shining light on the head: Photobiomodulation for brain disorders.” BBA Clinical, 6, 113–124.[DOI:10.1016/j.bbacli.2016.09.002] [PMID]
Harerimana, N. V., Paliwali, D., Romero-Molina, C., Bennett, D. A., Pa, J., & Goate, A., et al. (2022). The role of mitochondrial genome abundance in Alzheimer’s Disease. Alzheimer's & Dementia: The Journal of The Alzheimer's Association, 19(5), 2069–2083. [DOI:10.1002/alz.12812] [PMID]
Heinig, N., Schumann, U., Calzia, D., Panfoli, I., Ader, M., & Schmidt, M. H. H., et al. (2020). Photobiomodulation mediates neuroprotection against blue light induced retinal photoreceptor degeneration.” International Journal of Molecular Sciences, 21(7), 2370. [DOI:10.3390/ijms21072370] [PMID]
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., & Gillingham, T. Z., et al. (2016.). “Gamma frequency entrainment attenuates amyloid load and modifies microglia.” Nature, 540(7632), 230–235. [DOI:10.1038/nature20587] [PMID]
Jones, M., McDermott, B., Oliveira, B. L., O'Brien, A., Coogan, D., & Lang, M., et al. (2019). “Gamma band light stimulation in human case studies: Groundwork for Potential Alzheimer’s Disease Treatment.” Journal of Alzheimer’s Disease, 70(1), 171-185. [DOI:10.3233/JAD-190299] [PMID]
Kenwood, M. M., Kalin, N. H., & Barbas, H. (2022).“The prefrontal cortex, pathological anxiety, and anxiety disorders.” Neuropsychopharmacology, 47(1), 260-275. [DOI:10.1038/s41386-021-01109-z] [PMID]
Klein, H. U., Trumpff, C., Yang, H. S., Lee, A. J., Picard, M., & Bennett, D. A., et al. (2021). “Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain.” Molecular Neurodegeneration, 16(1), 75. [DOI:10.1186/s13024-021-00495-8] [PMID]
Kumar, A., Ekavali, Mishra, J., Chopra, K., & Dhull, D. K. (2016). “Possible role of P-Glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction.” Psychopharmacology, 233(1), 137-152. [DOI:10.1007/s00213-015-4095-7] [PMID]
Lee, K. H., Cha, M., & Lee, B. H. (2020). Neuroprotective effect of antioxidants in the brain. International Journal of Molecular Sciences, 21(19), 7152. [DOI:10.3390/ijms21197152] [PMID]
Li, X., Ji, M., Zhang, H., Liu, Z., Chai, Y., & Cheng, Q., et al. (2023). “Non-drug therapies for Alzheimer’s Disease: A review.” Neurology and Therapy, 12(1), 39-72. [DOI:10.1007/s40120-022-00416-x] [PMID]
Lu, Y., Wang, R., Dong, Y., Tucker, D., Zhao, N., & Ahmed, M. E., et al. (2017). “Low-level laser therapy for beta amyloid toxicity in rat hippocampus.” Neurobiology of Aging, 49, 165-182. [DOI:10.1016/j.neurobiolaging.2016.10.003] [PMID]
Nandi, N. K., Bhatia, R., Saini, S., Rawat, R., Sharma, S., & Raj, K., et al. (2023). “Design, synthesis, pharmacological and in silico screening of disubstituted-piperazine derivatives as selective and reversible MAO-A inhibitors for treatment of depression.” Journal of Molecular Structure, 1276, 134671. [DOI:10.1016/j.molstruc.2022.134671]
Nazari, M., Vajed-Samiei, T., Torabi, N., Fahanik-Babaei, J., Saghiri, R., & Khodagholi, F., et al. (2022). “The 40-Hz White Light-Emitting Diode (LED) improves the structure-function of the brain mitochondrial KATP channel and respiratory chain activities in amyloid beta toxicity.” Molecular Neurobiology 59(4), 2424-40. [DOI:10.1007/s12035-021-02681-7] [PMID]
Palma, F. R., He, C., Danes, J. M., Paviani, V., Coelho, D. R., & Gantner, B. N., et al. (2020). “Mitochondrial superoxide dismutase: What the established, the intriguing, and the novel reveal about a key cellular redox switch.” Antioxidants and Redox Signaling, 32(10), 701-714. [DOI:10.1089/ars.2019.7962] [PMID]
Park, S. S., Park, H. S., Kim, C. J., Kang, H. S., Kim, D. H., & Baek, S. S., et al. (2020). “Physical exercise during exposure to 40-hz light flicker improves cognitive functions in the 3xtg mouse model of Alzheimer’s Disease.” Alzheimer’s Research and Therapy, 12(1), 62. [DOI:10.1186/s13195-020-00631-4] [PMID]
Paxinos, G., & Watson, C. (2006). The rat brain- in stereotaxic coordinates. Elsevier Science. [Link]
Phillips, N. R., Simpkins, J. W., & Roby, R. K. (2014).“Mitochondrial DNA deletions in Alzheimer’s brains: A review.” Alzheimer’s and Dementia, 10(3), 393-400. [DOI:10.1016/j.jalz.2013.04.508] [PMID]
Pope, N. J., & Denton, M. L. (2020). Low irradiance light exposure alters the activity of key enzymes in the mitochondrial electron transport chain. Proceedings Volume 11221, Mechanisms of Photobiomodulation Therapy XV; 112210E. [DOI:10.1117/12.2546624]
Postu, P. A., Mihasan, M., Gorgan, D. L., Sadiki, F. Z., El Idrissi, M., & Hritcu, L. (2022). “Pinus halepensis essential oil ameliorates Aβ1-42-Induced brain injury by diminishing anxiety, oxidative stress, and neuroinflammation in rats. Biomedicines, 10(9), 2300. [DOI:10.3390/biomedicines10092300] [PMID]
Reddy, P. H. (2006).“Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s Disease.” Journal of Neurochemistry, 96(1), 1-13. [DOI:10.1111/j.1471-4159.2005.03530.x] [PMID]
Rodgers, R. J., & Johnson, N. J. (1995). “Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety.” Pharmacology, Biochemistry and Behavior, 52(2), 297-303. [DOI:10.1016/0091-3057(95)00138-M] [PMID]
Rojas, J. C., & Gonzalez-Lima, F. (2013).“Neurological and psychological applications of transcranial lasers and LEDs.” Biochemical Pharmacology, 86(4), 447-457. [DOI:10.1016/j.bcp.2013.06.012] [PMID]
Roy, A., Sharma, S., Nag, T. C., Katyal, J., Gupta, Y. K., & Jain, S. (2022). “Cognitive dysfunction and anxiety resulting from synaptic downscaling, hippocampal atrophy, and ventricular enlargement with intracerebroventricular streptozotocin injection in male wistar rats.” Neurotoxicity Research, 40(6), 2179-2202. [DOI:10.1007/s12640-022-00563-x] [PMID]
Salehpour, F., Mahmoudi, J., Kamari, F., Sadigh-Eteghad, S., Rasta, S. H., & Hamblin, M. R. (2018).“Brain photobiomodulation therapy: A narrative review.” Molecular Neurobiology, 55(8), 6601-6636. [DOI:10.1007/s12035-017-0852-4] [PMID]
Santin, Y., Resta, J., Parini, A., & Mialet-Perez, J. (2021).“Monoamine oxidases in age-associated diseases: New perspectives for old enzymes.” Ageing Research Reviews, 66, 101256. [DOI:10.1016/j.arr.2021.101256] [PMID]
Shang, D., Huang, M., Wang, B., Yan, X., Wu, Z., & Zhang, X. (2023).“MtDNA maintenance and alterations in the pathogenesis of neurodegenerative diseases.” Current Neuropharmacology, 21(3), 578-598. [DOI:10.2174/1570159X20666220810114644] [PMID]
Sheng, B., Wang, X., Su, B., Lee, H. G., Casadesus, G., & Perry, G., et al. (2012). “Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s Disease.” Journal of Neurochemistry, 120(3), 419-429. [DOI:10.1111/j.1471-4159.2011.07581.x] [PMID]
Singer, A. C., Martorell, A. J., Douglas, J. M., Abdurrob, F., Attokaren, M. K., & Tipton, J., et al. (2018). “Noninvasive 40-hz light flicker to recruit microglia and reduce amyloid Beta Load.” Nature Protocols, 13(8), 1850-1868. [DOI:10.1038/s41596-018-0021-x] [PMID]
Tao, L., Liu, Q., Zhang, F., Fu, Y., Zhu, X., & Weng, X., et al. (2021). “Microglia modulation with 1070-Nm light attenuates Aβ burden and cognitive impairment in Alzheimer’s disease mouse model.” Light: Science and Applications, 10(1), 179.[DOI:10.1038/s41377-021-00617-3] [PMID]
Tian, Z., Wang, P., Huang, K., Yu, J., Zhang, M., & Liu, Y., et al. (2023). “Photobiomodulation for Alzheimer’s Disease: Photoelectric coupling effect on attenuating Aβ neurotoxicity.” Lasers in Medical Science, 38(1), 39. [DOI:10.1007/s10103-022-03692-z] [PMID]
Tucci, P., Bove, M., Sikora, V., Dimonte, S., Morgese, M. G., Schiavone, S., et al. (2022). “Glucoraphanin triggers rapid antidepressant responses in a rat model of Beta Amyloid-induced depressive-like behaviour.” Pharmaceuticals, 15(9), 1054. [DOI:10.3390/ph15091054] [PMID]
Vuic, B., Konjevod, M., Tudor, L., Milos, T., Nikolac Perkovic, M., & Nedic Erjavec, G., et al. (2022). “Tailoring the therapeutic interventions for behavioral and psychological symptoms of dementia.” Expert Review of Neurotherapeutics, 22(8), 707–720. [DOI:10.1080/14737175.2022.2112668] [PMID]
Walf, A. A., & Frye, C. A. (2007). “The use of the elevated plus maze as an assay of anxiety-related behavior in rodents.” Nature Protocols, 2(2), 322-328. [DOI:10.1038/nprot.2007.44] [PMID]
Wan Chik, Mazzura, Nur Adiilah Ramli, Nurul Aqmar Mohamad Nor Hazalin, and Gurmeet Kaur Surindar Singh. 2023. Streptozotocin mechanisms and its role in rodent models for alzheimer’s disease. Toxin Reviews 42(1): 491-502. [DOI: 10.1080/15569543.2022.2150646]
Wang, W., Zhao, F., Ma, X., Perry, G., & Zhu, X. (2020). “Mitochondria dysfunction in the pathogenesis of Alzheimer’s Disease: Recent advances.” Molecular Neurodegeneration, 15(1), 30. [DOI:10.1186/s13024-020-00376-6] [PMID]
Yu, Y., Jiang, X., Fang, X., Wang, Y., Liu, P., & Ling, J., et al. (2023). “Transauricular vagal nerve stimulation at 40 Hz Inhibits Hippocampal P2X7R/NLRP3/Caspase-1 signaling and improves spatial learning and memory in 6-month-old APP/PS1 Mice.” Neuromodulation: Journal of the International Neuromodulation Society, 26(3), 589–600. [DOI:10.1016/j.neurom.2022.03.011] [PMID]
Zhang, Q., Yang, C., Liu, T., Liu, L., Li, F., & Cai, Y., et al. (2018).“Citalopram restores short-term memory deficit and non-cognitive behaviors in APP/PS1 mice while halting the advance of Alzheimer’s Disease-like Pathology.” Neuropharmacology, 131, 475–486. [DOI:10.1016/j.neuropharm.2017.12.021] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2024/01/8 | Accepted: 2024/01/16 | Published: 2024/07/20
Received: 2024/01/8 | Accepted: 2024/01/16 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





