Volume 15, Issue 6 (November & December 2024)
BCN 2024, 15(6): 713-734 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gilanchi S, Rezaei Tavirani M, Daskareh M. The Necessity for Biomarker-based Personalized Medicine in Major Depression Disorder: A Comprehensive Literature Review. BCN 2024; 15 (6) :713-734
URL: http://bcn.iums.ac.ir/article-1-2824-en.html
URL: http://bcn.iums.ac.ir/article-1-2824-en.html
1- Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran.
2- Department of Radiology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Radiology, Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1015 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Depression is a heterogeneous disorder, covering numerous diseases with different causes and pathophysiologies (Nestler et al., 2002) and a broad spectrum from minor/subthreshold to major (Jani et al., 2015). Major depressive disorder (MDD) is a serious disorder of enormous sociological and clinical relevance. MDD patients present a wide range of symptoms like low energy, depressed mood, lack of interest or pleasure, guilt or feelings of low self-worth, abnormal sleep or appetite, and poor concentration. All of these symptoms can cause significant distress and loss of normal function. A person cannot be diagnosed as having MDD, following the diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) unless he or she shows 5 of the symptoms mentioned above, one of which must be a depressed mood or anhedonia that impairs his or her ability to function in social or occupational settings (Bains & Abdijadid, 2022; Brigitta, 2022; Marcus et al., 2012). Depression and melancholia are known to have originated in Hippocrates’ writings, the father of modern medicine, in the fifth century BC (Spielberger et al., 2003).
Depression is the most prevalent psychiatric disorder and represents one of the leading causes of disability in the world (Newby et al., 2015; Penninx et al., 2013). According to the World Health Organization (WHO), MDD will account for 13% of the global disease burden by 2030, displacing cardiovascular diseases, with a lifetime prevalence of 10% and 15% (Lopizzo et al., 2015). Given its widespread prevalence, MDD has been called the “common cold” of mental illness (Spielberger et al., 2003). Every year, approximately one million people die in Western societies due to a lack of comprehension of disease pathophysiology and laboratory tests to help with accurate diagnosis and antidepressant (AD) treatment strategies (Martins-de-Souza et al., 2012).
There are two major hypotheses based on the classical monoaminergic hypothesis of depression: The cytokine hypothesis (dysfunction in the immune-inflammatory system) and the neurotrophic hypothesis (neuronal plasticity) (Cattaneo et al., 2015). Epidemiologic studies indicate that approximately 40% to 50% of depression etiology is hereditary (Nestler et al., 2002). Generally, genetic, epigenetic, physiological, and psychological components make up the biological basis of mood disorders (Saltiel & Silvershein, 2015). Genetic research indicates that polygenes with small effects and infrequent mutations are probably the genetic causes of the condition (Lee et al., 2014).
However, non-genetic factors are important, too. These variables are incredibly diverse. A variety of these factors range from stress and emotional trauma to viral infections and even random processes during brain development (Nestler et al., 2002). Additionally, undeniable documentation indicates that people with physical disorders—and particularly those with numerous physical disorders—are more likely to develop depression (Kang et al., 2015). Moreover, socioeconomic status is a risk factor for depression (Liang et al., 2012).
Based on the DSM’s symptomatic criteria, depression has been classified as “major depression” since the 1960s. According to these criteria, the diagnosis of depression, in contrast to the majority of disorders of other organ systems, i.e. cancer and diabetes, is not based on quantitative clinical testing like serum chemistry, organ imaging, and biopsies but rather on highly variable symptoms (Lakhan et al., 2010; Nestler et al., 2002).
The clinical overlap of Alzheimer-type dementia, vascular dementia, frontotemporal dementia, and MDD presents considerable diagnostic problems (Braaten et al., 2006). Also, patients with bipolar disorder experience significant depressive symptoms (Chang, 2009; Lin et al., 2008).
If depression is not controlled, significant aftereffects, such as economic, social, physical, and psychological consequences, will be seen. Several studies demonstrate that treating depression is efficient and cost-effective (Evans-Lacko et al., 2016; Greenberg et al., 2003). Several factors contribute to the economic burden of depression, including the prevalence of the disease, the rate and degree of impairment, and the treatment success (Greenberg et al., 2003). In 1990, studies indicated an average loss of 5.6 productive hours per week for depressed workers in the United States. On the other hand, treatment has its costs. The same studies estimate that the annual economic burden for direct treatment, missed wages, indirect workplace costs, and labor costs related to short-term and long-term disability ranges from $44 to $53 billion (Stewart et al., 2003; Tierney, 2007). According to estimates, indirect costs to society are seven times the direct costs (Organization, 2005).
The additional direct cost per person decreased between 2010 and 2018 despite a rise in the entire population of MDD sufferers. In the meantime, the percentage of people with MDD who received therapy remained the same over the preceding 10 years, indicating that this population still has significant unmet treatment needs (Greenberg et al., 2021).
Up to now, attempts to define biomarkers for MDD have not yet led to robust biomarkers. This outcome is due to an incomplete knowledge of the molecular mechanisms underlying MDD and how these mechanisms react and interact in a dynamic environment. Further studies are required to understand the MDD pathophysiology fully.
Researchers are looking for new approaches to treat psychiatric problems because the available traditional treatments are not ideal (Larijani et al., 2021). Moreover, due to the overlapping clinical features of mental disorders and MDD, the diagnosis and treatment of depression and determining its subgroups are facing many problems. Even though significant findings have been made relating to the effective treatment of depression, there are still large gaps in our understanding. Recognizing the neurobiological basis of MDD has remained one of the most significant challenges for modern psychiatry. A better understanding of the molecular mechanisms of MDD can open new horizons in managing, treating, and remission of MDD. The current study highlighted the significance of identifying novel biomarkers for diagnosing and predicting treatment responses and evaluating MDD and its subtypes.
Search strategy
The search strategies for this review are divided into two sections. A different set of search techniques was used for each of the two sections of this evaluation. At first, a literature search was conducted through PubMed and Google Scholar using the search keywords (“major depressive disorder” OR “MDD”) up to June 1, 2022. The second part included articles on each part of the study topic. PubMed and Google Scholar were extensively searched utilizing combinations of the following keywords: (MDD [AND] history), (MDD [AND] mechanism), (MDD [AND] diagnosis), (MDD [AND] treatment), (MDD [AND] drug resistance), and (MDD [AND] biomarker). The title and abstract of each retrieved study were checked to determine whether it met the inclusion or exclusion criteria. However, animal experiments, pediatric MDD, and postpartum depression were excluded. Subsequently, a search was made with more specific keywords for the parts that needed more study. Figure 1 represents the workflow of the study.
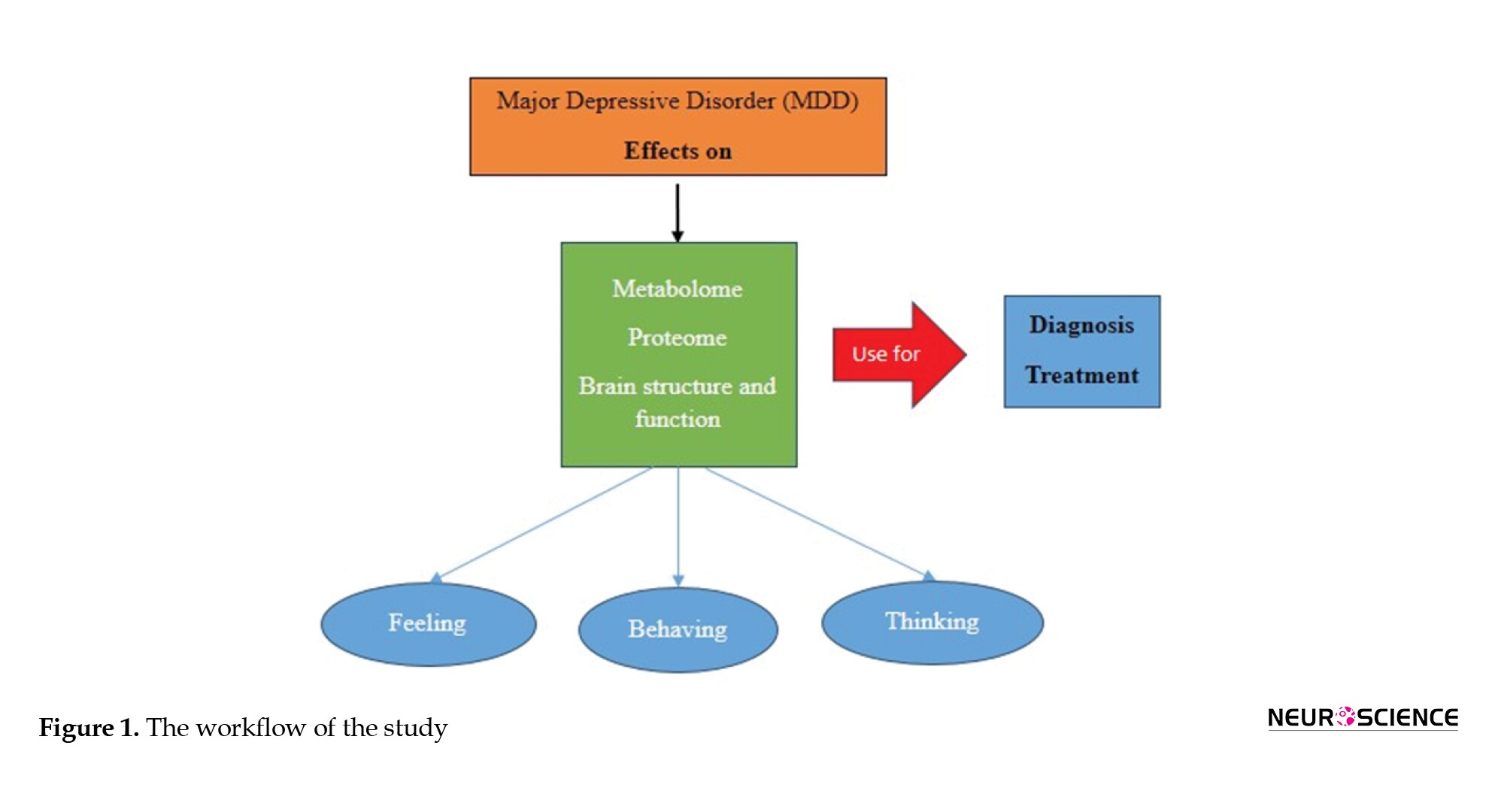
History
The concepts of depression and melancholia can be traced back to the fifth century BC in Hippocrates writings, the father of modern medicine. “Black mood” is a description of the Greek-Latin term “melancholia,” which Hippocrates attributed to excessive black bile in the brain (Marsella et al., 1987; Onions, 1965). Melancholia was regarded as a mental disorder characterized by prolonged sadness and fear, “despondency, sleeplessness, irritability, restlessness,” and an aversion to food (Hirshbein, 2009).
In the second century AD, Galen’s restatement of Hippocrates’ description of melancholia prevailed for the next 1500 years. Galen believed that contrary to earlier authorities, yellow bile, nutritional deficiencies, the suppression of menstrual or hemorrhoidal flow, and emotional elements might also contribute to melancholia’s origin (Ariza et al., 2010; Marsella et al., 1987). During the 17th and 18th centuries, more modern, special theories about melancholy began appearing by physicians and Christian Church pastors in Europe. The Latin verb deprimere, which means “to press down,” inspired the current English term for depression (Kanter et al., 2008; Roystonn et al., 2021).
Five writers—Griesinger, Sankey, Maudsley, Krafft-Ebing, and Kraepelin—addressed the root of delusional melancholy during the 1860s and 1880s. The authors all came to the same conclusion—that melancholia was a basic mood disorder—and maintained that the delusions naturally resulted from the aberrant mood. During this century, the model for explaining delusional melancholia in terms of faculty psychology reversed from one that linked intellect to mood to one that linked mood to intellect (Kendler, 2020). In the 1980s and late 1970s, many researchers looked at the effects of norepinephrine, precursors tyrosine, and phenylalanine, as well as serotonin precursors L-tryptophan and 5-hydroxytryptophan on depressive patients (Meyers, 2000). Depressive illness was thought of as a recurrent episodic disorder with complete remission between episodes for several decades in the 20th century (Ban, 2014). At the end of the 20th century, depression, or melancholy, was recognized as a true illness of the time, comparable to the hysteria Charcot had witnessed at the Salpêtrière Hospital in Paris in the 19th century (Barroso, 2003).
Many different treatment approaches have been tried in depressive states. Historically, there have been three phases in the development of the psychiatric treatment of depression.
The first phase, which lasted until the mid-1930s, w:::::::::as char:::::::::acterized by ineffective somatic treatment and psychotherapy. Although many different drugs and physical techniques were used, none of these treatments had a consistent therapeutic effect, even though they occasionally benefited some patients. The second phase is convulsive therapy and lobotomy, which lasted from the mid-1930s to the mid-1950s of the 21st century. Convulsions are brought on either chemically by pentamethylene tetrazol (metrazol) or electrically by a current sent through the brain at this phase of psychotherapy development. For all intents and purposes, this therapy is recommended for acute severe depression. Around the same time, frontal lobotomies were invented, and they were successful in treating depressive disorders, especially chronic ones that had resisted electroconvulsive therapy (ECT). The third phase is pharmacotherapy. This phase began in the middle of 1950, and the development of this phase has not yet been completed (Ban, 2014; Lehmann, 1965; López-Muñoz & Alamo, 2009). An improved comprehension of the molecular mechanisms of MDD can help discover valuable treatments for this disease.
Mechanism
Identifying the cause and pathogenesis of MDD or the prediction of the treatment response in these patients is more important, and for achieving these aims, understanding biological changes during MDD is necessary. MDD may be associated with neurotransmitters and biochemical factors, such as neurophysiologic, neuroimaging, and inflammatory markers. The main neurotransmitters of depression include the serotonergic, noradrenergic, and dopaminergic systems (Lee & Kim, 2013). Patients with depression have decreased hippocampal volume, which is strongly correlated with the frequency and duration of depressive episodes. Despite structural abnormalities in the brain, the neurobiology of MDD is thought to be influenced by changes in brain neuronal function (Saltiel & Silvershein, 2015). Positron emission tomography (PET) studies in mood disorders have discovered several abnormalities in regional cerebral blood flow (CBF) and glucose metabolism in limbic structures and the prefrontal cortex (PFC) (Mössner et al., 2007). Most neurotransmitter synthesis is regulated by the brain (Meyers, 2000). In addition, these brain regions’ structure and function are controlled by monoaminergic neurotransmission (Saltiel & Silvershein, 2015). Monoamine neurotransmitters, particularly serotonin (5-HT), dopamine (DA), and noradrenaline (NA), are hypoactive in MDD patients’ midbrains, and these agents have received more attention because nearly all of the AD drugs’ mechanisms of action act through these systems (Drevets et al., 2008; Werner & Covenas, 2010). In addition to the classical neurotransmitters, neuropeptides are changed in specific brain regions during major depression. Major depression is associated with the hyperactivity of some neuropeptides, including substance P corticotropin-releasing hormone and thyrotropin-releasing hormone, and the hypoactivity of other neuropeptides, including neuropeptide Y and galanin (Werner & Covenas, 2010). Although the monoamine transmitter is important to fully comprehend the disease’s pathophysiology, the monoamine theory is insufficient. Many studies indicate that immune mechanisms, mainly cytokines, are implicated in depression’s pathogenesis (Sahin & Aricioglu, 2013). The cytokine hypothesis of depression posits that the cytokines have a critical function in its cause. Cytokines are immune system hormones. They are composed of proteins and glycoproteins secreted by immune cells and act as signals among the immune cells (Dunn et al., 2005). Several clinical studies reveal that people with depression indicate higher plasma levels of pro-inflammatory cytokines like interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (Dunn et al., 2005). The interaction between peripheral inflammation and the central nervous system has been thoroughly studied. Several mechanisms for entering cytokines, particularly TNF-α and IL-1β, in the brain influence central neuronal function and cause behavioral alterations known as “sickness behavior.” These mechanisms include passing through circumventricular organs, projection with peripheral vagal nerve afferents, uptaking by active transport systems, crossing of cytokine induced immune cells like macrophages, monocytes, and T cells directly through the blood brain barrier and direct passage. These cytokines, after entering the brain, trigger their own production, particularly in the amygdala, dentate gyrus of the hippocampus, hypothalamus, and other parts of the brain. Also, recent studies indicate that the Toll-like receptors, which have a role in neuronal function and the production of cytokines and chemokines in response to inflammation or stressful situations, have altered protein and mRNA levels in the hypothalamus of people with depression (Cattaneo et al., 2015; Sahin & Aricioglu, 2013). The neurotoxic mechanisms and the modulation of neurotransmitter metabolism are two examples of how cytokines in the brain can impact brain function (Capuron & Dantzer, 2003; Cattaneo et al., 2015).
It has been suggested that the pro-inflammatory cytokines IL-1β and IL-6, which increase during infection, are crucial for synaptic plasticity, neurogenesis, and neuromodulation. These pro-inflammatory cytokines stimulate the paraventricular nucleus of the hypothalamus to secrete corticotropin-releasing hormone (CRH), activate the hypothalamic-pituitary-adrenal (HPA) axis, and encourage the release of adreno-corticotrophin hormone (ACTH) and glucocorticoids. Understanding how IL-1β functions in the pathophysiology of depression may help explain how it affects changes in amine metabolism, neurogenesis, and neuroinflammation (Farooq et al., 2017; Jeon & Kim, 2016; Zunszain et al., 2011).
Additionally, it has been demonstrated that cytokines can cause dysfunction of the neurotrophic system and decrease neurogenesis in several brain regions, most notably in the hippocampus. Free radicals, oxidants, and glucocorticoids are overproduced due to the excessive inflammatory response sparked by pro-inflammatory cytokines in the peripheral nervous system, which can disrupt glial cell activities and damage neurons in the brain. As a result, neural plasticity may eventually decline, which is a key component of depression-related dysfunction. The term “neuronal plasticity” describes several mechanisms essential for brain function, including the capacity to recognize, respond to, and adapt to a wide range of external and internal stimuli. It is believed that these processes can be dysfunctional in various psychiatric diseases, which may ultimately increase disease vulnerability (Ariza et al., 2010; Cattaneo et al., 2015).
Furthermore, the stress hormones, such as cortisol, ACTH, and CRH, can all be increased by cytokines. These hormones have been linked to HPA dysfunction and are elevated in depressed patients. Disrupted microglia function has been linked to neurologic and psychiatric disease and could significantly impact neuronal activity and function (Cattaneo et al., 2015; Pariante & Miller, 2001; Zunszain et al., 2011).
Brain-derived neurotrophic factor (BDNF) has received significant attention as a growth factor in MDD. BDNF controls neuronal plasticity, migration, and survival in the central and peripheral neurological systems. It is hypothesized that higher corticosteroid dosages will likely cause decreased levels of BDNF in depression because activation of the glucocorticoid receptors (GRs) negatively affects the BDNF gene. Although BDNF levels in MDD may serve as a diagnostic and prognostic marker, there are still unresolved issues, such as whether peripheral BDNF can pass the blood-brain barrier and cause behavioral effects (Hacimusalar & Eşel, 2018).
According to neuroimaging research, depression affects several structurally and functionally dysfunctional brain regions, the majority of which are related to the limbic system, default mode network, central execution network, and salience network. They contribute to several clinical depressive symptoms (Dai et al., 2019).
The lateral PFC activity is abnormally decreased in MDD patients, particularly during express voluntary control, when the emotional experience is already occurring. On the other hand, it is possible that (medicated) MDD patients can control their emotions in the early, automatic stages by drawing on additional lateral prefrontal neuronal resources. Medial may act as a mediator in this action plan (Rive et al., 2013).
The neuropharmacological mechanisms that have been proposed as the final common pathways for AD responses are as follows: Increase in the gene expression of BDNF and other neurotrophic/neuroprotective factors in the hippocampus and PFC, enhancement of postsynaptic serotonin type 1A (5-HT1A) receptor function, and attenuation of the sensitivity or transmission of NMDA-glutamatergic receptors (Drevets et al., 2008).
2. Diagnosis
Comprehensive evaluation and accurate diagnosis are essential components of managing depression. The evaluation must be based on a thorough history, physical exam, and mental state examination. All sources, especially the family, must be consulted for history. The diagnosis must be noted using the most recent diagnostic criteria (Gautam et al., 2017).
The diagnosis of MDD is made using internationally accepted diagnostic criteria. The International Classification of Diseases (Mental and Behavioral Disorders, ICD-10) and the classification of the American Psychiatric Association (DSM-4) are two of the most commonly used criteria (Farah & Gillihan, 2012). The ICD-10 classifies depression as mild, moderate, or severe (with or without psychotic symptoms) based on a list of 10 depressive symptoms (Ariza et al., 2010; Kessing, 2004). The disease criteria of the DSM-5 have come under harsh criticism from Hyman (2011), a former director of the National Institute of Mental Health (NIMH). He stated: “The problem is that the DSM has been introduced into an understudied area, which has been accepted without question”. It was formed with a focus on a paradigm shift in the system. Given DSM-IV's limitations and lack of foundation other than disease essentialism, there is a need to amend DSM-4 to be upgraded to DSM-5 with a more thorough approach to identify the heterogeneity of major depressive disorder (Kim & Park, 2021). The ICD-10 and DSM-4 diagnostic criteria for depressive episodes overlap, but there are some differences in emphasis. ICD-10 requires that the patient exhibit two of the first three symptoms (depressed mood, loss of interest in daily activities, and decreased energy) and at least two of the remaining seven symptoms. For DSM-4, at least five of the nine symptoms must be present in the patient, and at least one of the first two symptoms (depressed mood and loss of interest). For both diagnostic methods, a diagnosis may only be made when the symptoms have existed for at least two weeks (Nonetheless, it might be shortened for the ICD-10 if symptoms are very severe or present quickly). Both ICD-10 and DSM-4 require that symptoms result in functional impairment that increases with the episode’s severity (NCCMH, 2010). Table 1 shows the diagnostic criteria of ICD-10.

Some minor changes were made to the DSM-4 diagnostic criteria for some disorders, making it easier for attending physicians and psychologists to make diagnoses. The goal of the DSM-5 was to make a subtle shift towards “bridging the gap between etiology-based symptomatology and identifiable pathophysiological etiology” (Kupfer & Regier, 2011; Svenaeus, 2014). Table 2 presents the diagnostic criteria for DSM-5.

First, in the context of mood disorders, depressive disorders are considered a separate entity from bipolar disorders. Second, diagnostic thresholds for MDD were lowered in DSM-5 compared with those in DSM-4. The term “hopelessness” was changed to a more individualized way of describing depressed mood, and the term “bereavement exclusion” was removed from the diagnostic criteria. In the ICD-10 diagnostic criteria for depressive episodes, the deletion of the item ‘bereavement exclusion’ was partially supported because clinical or genetic aspects of major depressive episodes caused by bereavement were not significantly different from those caused in other contexts of major depressive episodes. Thirdly, the transdiagnostic specifiers, such as those with psychotic features, those with mixed features, and those with anxious features, were adjusted in DSM-5. This change was done to show the quantitative, not qualitative, overlapping symptoms of major depressive disorder related to anxiety disorder, schizophrenia, and bipolar disorder. According to the severity-psychosis hypothesis, the specifier was coded with psychotic features only in MDD. This hypothesis emphasizes psychotic symptoms as factors that depend on the severity of MDD. By rejecting the severity-psychosis hypothesis in several studies, the DSM-5, encoding the specifier with psychotic features, was approved for major depressive disorder as well as mild and moderate MDD and dysthymic disorder (Kim & Park, 2021; Svenaeus, 2014).
The inclusion and exclusion criteria for each diagnostic category are being reevaluated and revised in light of recent discoveries. The addition of genetic and neurobiological measures has been taken into consideration regarding this most recent adjustment (Farah & Gillihan, 2012).
The progression of depression and how effectively a patient responds to treatment are significantly influenced by a wide range of biological, psychological, and social aspects that are not well recognized by existing diagnostic frameworks. When doing a diagnostic examination, it is crucial to take into account both the patient’s past experiences with depression as well as any family history of the condition (NICE, 2009).
Some practitioners already use brain imaging to make psychiatric diagnoses (Farah & Gillihan, 2012). Although the pathoanatomic foundation of mental diseases could be defined significantly with the use of modern imaging, a key challenge in using neuroimaging for psychiatric diagnosis is that the clinical usefulness of such tests depends in part on their ability to differentiate between various conditions. As the number of diagnostic categories regarded as clinically important increases, generally, both inter-subject and intra-subject variability in interpretation rises (NICE, 2009; Savitz et al., 2013). PET imaging research has identified multiple anomalies in regional CBF and glucose metabolism in different brain regions. Recent studies on neuroimaging have concentrated on the neurobiological abnormalities related to MDD, such as malfunctioning or structural variations in cerebral regions, due to reactive microglia’s association with some molecular modifications that can be detected by different radiotracers, morphological and functional changes in reactive microglia allowed for in vivo detection of microglial activation as a biomarker of central inflammation in a variety of pathological conditions, including MDD (Gritti et al., 2021; Lee & Kim, 2013).
Single photon emission computed tomography (SPECT) is the functional imaging method used as an ancillary diagnostic tool in clinical psychiatry. In particular, it helps differentiate depression from neurodegenerative diseases when making a differential diagnosis of depression. This imaging technique measures regional CBF by tracers that emit gamma rays in the blood. This local blood flow data creates a low-resolution, three-dimensional image of brain activity (Cho et al., 2002; Farah & Gillihan, 2012; Nagafusa et al., 2012).
The following conditions can be measured with magnetic resonance imaging (MRI): Brain structure volume (structural MRI), white matter integrity and density (diffusion tensor imaging [DTI]), or functional metabolic activity patterns (fMRI), either at rest or in response to a specific task or challenge. Moreover, fMRI can investigate activity in specific brain regions or coordinated temporal activity patterns across several regions (fcMRI) (Dunlop & Mayberg, 2014; Pilmeyer et al., 2022).
Treatments
Achieving complete remission in MDD is difficult because of the chronic nature of this disease. Typical response rates in AD trials are 60% to 70%, while remission rates are much lower (between 30% and 50%) (McIntyre & O’Donovan, 2004; Trivedi & Daly, 2008).
There are three phases of depression: mild, moderate, and severe. These three phases can be divided into two general group treatment strategies for achieving remission in MDD: pharmacological and nonpharmacological (Trivedi & Daly, 2008).
Numerous distinct pharmacologic classes of medications may be utilized to treat depression, and each of them can modulate depression symptoms via different mechanisms. The mechanisms of action of these drugs are all involved with or dependent upon the alteration of several neuromediators. The neurotransmitters serotonin and norepinephrine are the main targets of most major classes of ADs (Gold et al., 2015; Tierney, 2007).
Despite higher acquisition costs, combination therapy for MDD can be cheaper than monotherapy. Any initial monotherapy has a low remission rate, and AD combinations are currently utilized in practice at the second or following steps when relapse occurs over a longer time or, in some situations, even acutely as a first step where rapidity of impact is a clinical priority. If employed as initial therapies, these combinations may be more effective than monotherapy in terms of higher rates of remission, lower attrition, or longer-term benefits (McIntyre & O’Donovan, 2004; Trivedi & Daly, 2008).
First-line AD treatment is ineffective in up to half of MDD patients, and two or more treatments are ineffective in one-third of them. Nonpharmacological treatments can be treatment options for treatment-resistant patients (Trivedi & Daly, 2008).
Nonpharmacological treatments can be divided into two groups: Somatic treatments and psychotherapy. Somatic treatments include vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation, magnetic seizure therapy, ECT, and ablative neurosurgery (Moreines et al., 2011; Trivedi & Daly, 2008).
Creating novel therapeutic alternatives based on the pathophysiology of a certain psychiatric condition and permitting a more targeted treatment approach is one of the main objectives. Invasive or noninvasive brain and cranial nerve stimulation procedures are one group of such therapy possibilities. VNS uses an implanted pulse generator in the left anterior chest wall to deliver intermittent electrical stimulation to the left cervical vagus nerve. The US Food and Drug Administration (FDA) approved VNS in 2005 for “the adjunctive long-term treatment of chronic or recurrent depression for patients 18 years of age or older who are experiencing a major depressive episode and have not had an adequate response to four or more adequate AD treatments” (Christmas et al., 2013; Cimpianu et al., 2017; Nemeroff et al., 2006).
To stimulate the cerebral cortex with rTMS, an electric coil is used to produce a magnetic field. Contrary to ECT, patients tolerate rTMS well, do not require anesthetics, and do not appear to affect cognitive function adversely. The US FDA has approved using 3000 daily stimuli to treat MDD (or 15000 stimuli per week) (Mirabzadeh & Khodaei, 2012; Pan et al., 2020).
Usual treatments for severe TRD have some known side effects that may limit their use. ECT and ablative neurosurgery are two common solutions for severe TRD. Under general anesthesia, an electrical current is serially administered through the brain in the ECT procedure to cause a generalized tonic-clonic seizure. Gray matter volume in the medial temporal lobes significantly increased after ECT, indicating that it may have a neurotrophic effect contributing to its therapeutic efficacy. Also, the longitudinal experience with ablative techniques demonstrates that precise, distinct lesions continue to play a significant role in the disruption of affective circuitry in the management of TRD (Moreines et al., 2011; Ota et al., 2015; Volpini et al., 2017).
The earliest surgical attempt to treat TRD was an ablative neurosurgery technique. There are several surgeries today, including anterior cingulotomy, anterior capsulotomy, limbic leucotomy, and subcaudal tractotomy (Moreines et al., 2011). According to the evidence, even after surgeries, residual symptoms remain frequently, which leads to psychosocial dysfunction. The chances of full recovery are reduced when patients remain more symptomatic. There are numerous ongoing initiatives to deploy cutting-edge ablative therapy for the brain. Recent advances in cerebral ablation techniques have led to new methods that enable precise targeting, accurate thermal dose delivery, and real-time visualization of induced tissue damage. Examples of these methods include magnetic resonance-guided focused ultrasound and laser interstitial thermal therapy. The accuracy of ablative operations may be further enhanced by new modalities, including MRI-guided focused ultrasound surgery, while safety is increased by avoiding open-brain surgery. This technique has gained popularity over the past 10 years, and its therapeutic uses are expanding. Nonetheless, more traditional procedures like stereotactic radiosurgery and radiofrequency thermal ablation continue to play a crucial part in the treatment of numerous neurological illnesses (Franzini et al., 2020; Franzini et al., 2019; Volpini et al., 2017).
According to the theoretical base, brief psychotherapy interventions can be divided into four primary categories: Psychodynamic therapy (grounded in psychoanalytic principles), interpersonal therapy (IPT), supportive counseling (Rogerian person-centered therapy), and cognitive behavioral therapy (CBT) (Möller & Henkel, 2005).
Two effective short-term psychotherapies for major depression are IPT and CBT (Aronson & Ayres, 2000). Specifically, in depression, CBT seems to decrease residual symptoms and lead to reduce the risk of relapse. Also, for the first-line treatment of MDD in children and adolescents, CBT is the most efficient and cost-effective approach. It has been proposed that combining AD medication with psychotherapy may be more successful than using either approach separately (Haby et al., 2004; Trivedi & Daly, 2008).
According to IPT, there is a correlation between interpersonal issues and depressive symptoms that has a significant impact on the development and maintenance of depressive disorders. Hence, the primary therapy goals of this strategy are interpersonal issues. The four areas of interpersonal difficulty include role disputes, role transitions, complicated bereavement, and interpersonal deficits (Brakemeier & Frase, 2012).
Although both CBT and IPT can be successful therapies for MDDs, it is still unclear which therapy is superior to the other (Zhou et al., 2017).
Clinical decision-making is made easier and more consistent using treatment algorithms that offer specific steps. In psychiatry, treatment algorithms (TAs) are designed to enhance patient outcomes by reducing prescribing variance, boosting the appropriateness of medications and dosages, and avoiding “pseudoresistance” (Vaccarino & Kennedy, 2022). TAs, consisting of sequential treatment strategies and standardized therapeutic-choice instructions, have been created to solve this issue. These TAs are made to prevent treatment resistance and increase the quality of care to improve results (Ricken et al., 2018). The Texas medication algorithm project compared the clinical and financial results of algorithm-guided treatment (ALGO) and treatment as usual (TAU) using predetermined medication algorithms, clinical support, and a predetermined patient and family educational package. Based on clinician-rated and patient-reported symptoms and total mental functioning during a year, the ALGO intervention package was more effective for patients with MDD than TAU (Trivedi et al., 2004). The ALGO group included patients who needed to start AD therapy or change their AD medications. Initially, the TAU group followed similar criteria. However, the TAU group patient selection was mainly based on whether they required fewer medication changes and whether their overall score on the Brief Psychiatric Rating Scale was greater than the median for each patient's routine quarterly evaluation at the clinic (Trivedi, 2007).
Drug resistance
ADs, in turn, ameliorate many neurobiological disturbances in depression and thereby alleviate depressive symptoms, but multiple therapies are ineffective for up to one-third of individuals with MDD who are receiving sufficient care (Anacker et al., 2011; De Carlo et al., 2016). MDD patients’ responses to AD drugs are different (Table 3) (El-Hage et al., 2013).
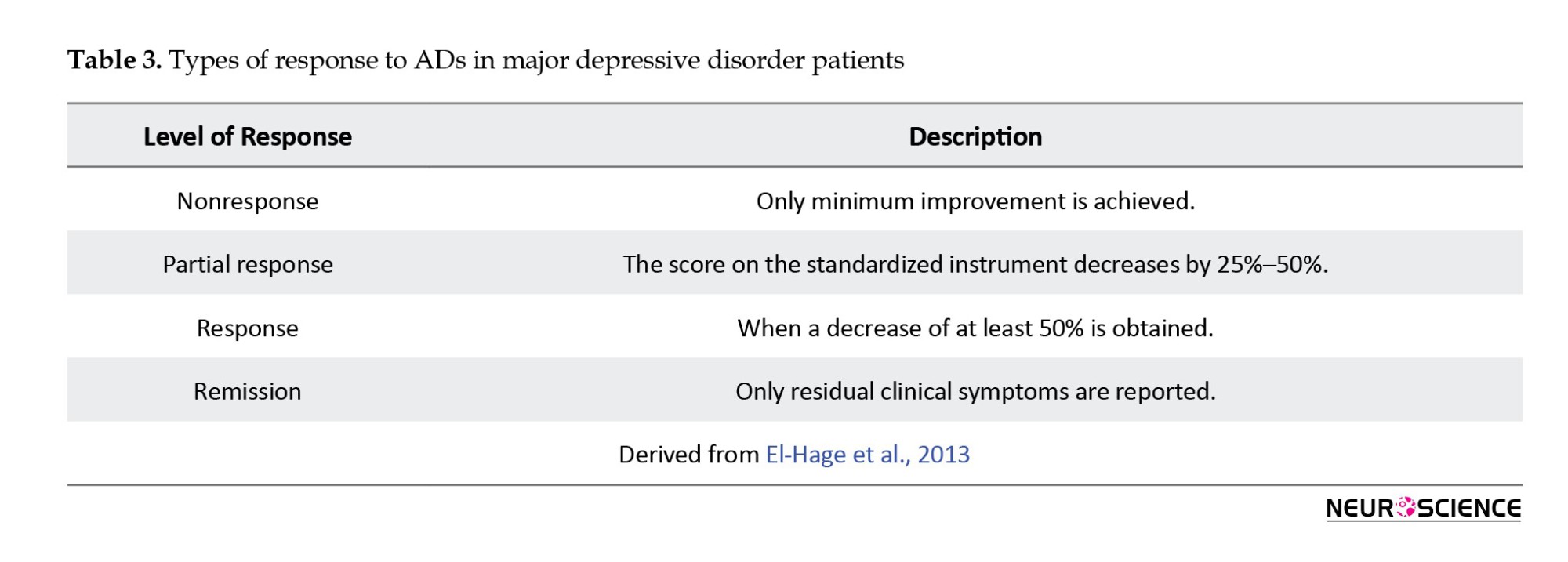
Although first-line AD therapy significantly alleviates the symptoms of depression in numerous cases, only 50% to 60% of MDD patients respond to the treatment. In addition, 30% to 40% of MDD patients never get symptom remission after taking standard AD medication (Rogóż, 2013). Although numerous definitions for TRD patients have been proposed, no validated consensus definition of TRD exists. To define depressed disorders that did not sufficiently remit following treatment, one of the most basic TRD concepts is resistance. It is most commonly defined as the inability of a current depressive episode to respond to at least two sufficient trials (Berlim & Turecki, 2007; El-Hage et al., 2013; Guilloux et al., 2012). The European Agency describes TRD for the evaluation of medicinal products as the inability to respond to two medications from different classes that have been administered for long enough at a sufficient dose without any particular mention of an appropriate dosage or duration by regulatory authorities. TRD is not defined by the US FDA (Mathew, 2008). TRD is widespread, increasing the risk of suicide, disability, and patient suffering (Moreines et al., 2011).
Numerous comprehensive studies have been conducted on the issue of AD-resistant depression (Rogóż, 2013). There are some strategies for dealing with these problems. Combination therapy is one of these strategies, which involves giving patients who partially respond to one AD a second medication. This strategy is commonly used to improve the response to initial treatment (Ables & Baughman III, 2003). Also, for patients who have partially responded, adding a non-AD medication to an AD is a helpful method. Thyroid hormone, lithium, beta-blocker pindolol (Visken), and buspirone (Buspar) are four substances that are widely used in augmentation therapy. Lithium and triiodothyronine are the best-documented options. Studies indicated that lithium decreases the activity of postsynaptic serotonin or 5-HT receptors, leading to enhanced serotonin transmission. Triiodothyronine may be used to improve the effects of tricyclic ADs, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors (Ables & Baughman III, 2003; Cadieux, 1998; Joffe et al., 2006).
A more successful treatment for individuals who have severe resistance to AD therapy or those with psychotic depression is ECT, a nonpharmacological intervention that is routinely evaluated as a measure of TRD (Ables & Baughman III, 2003; Trevino et al., 2014).
Four staging methods have been proposed for classifications of TRD. These classifications include the “Thase and Rush staging method” (Thase & Rush, 1997), which involves graduating stages of resistance according to response to one or more different therapeutic approaches (Table 4).
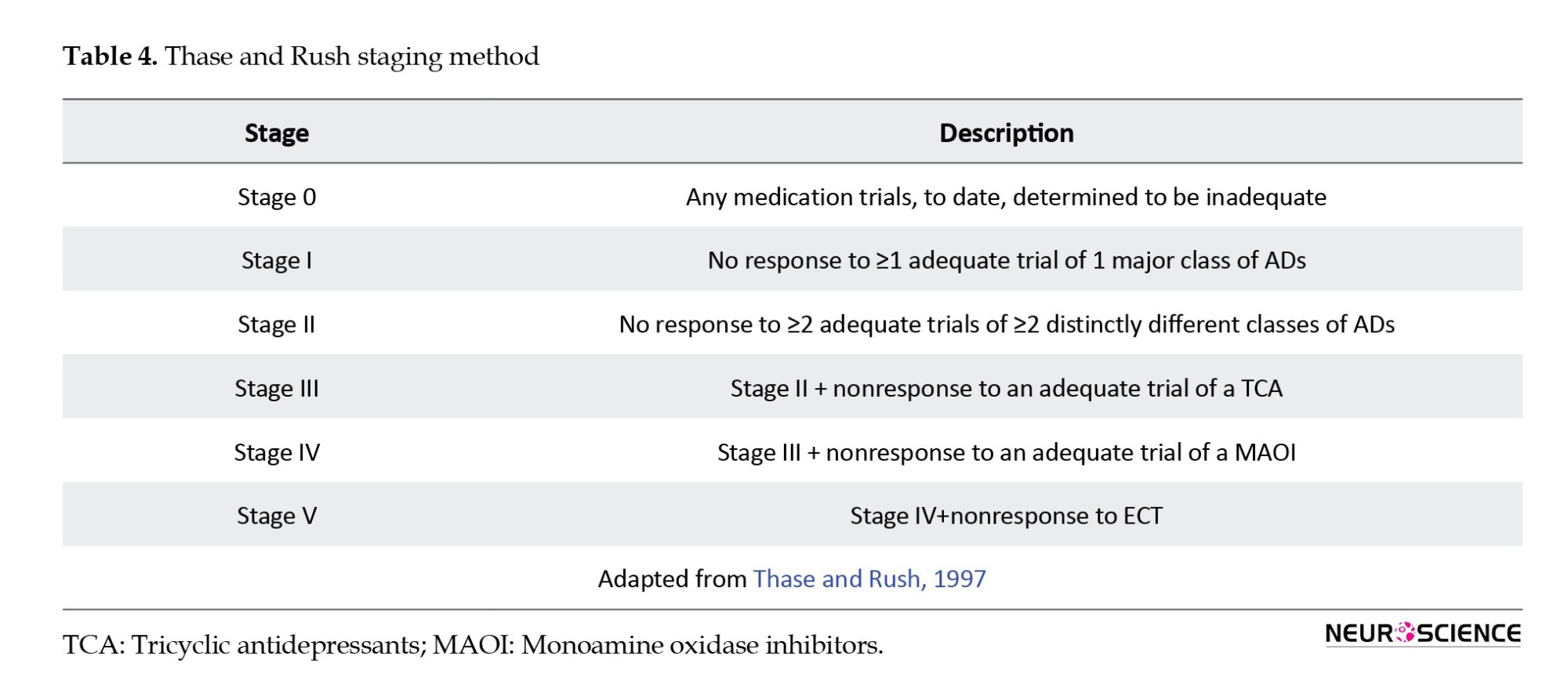
The Massachusetts general hospital staging method (MGH-S) (Fava, 2003) is based on the number of AD trials carried out and their possible alteration but disregards the variation in pharmacological classes among the trials. The MGH-S method evaluates the dose and duration of each prior therapy to consider the intensity and optimization of that particular treatment (Table 5).
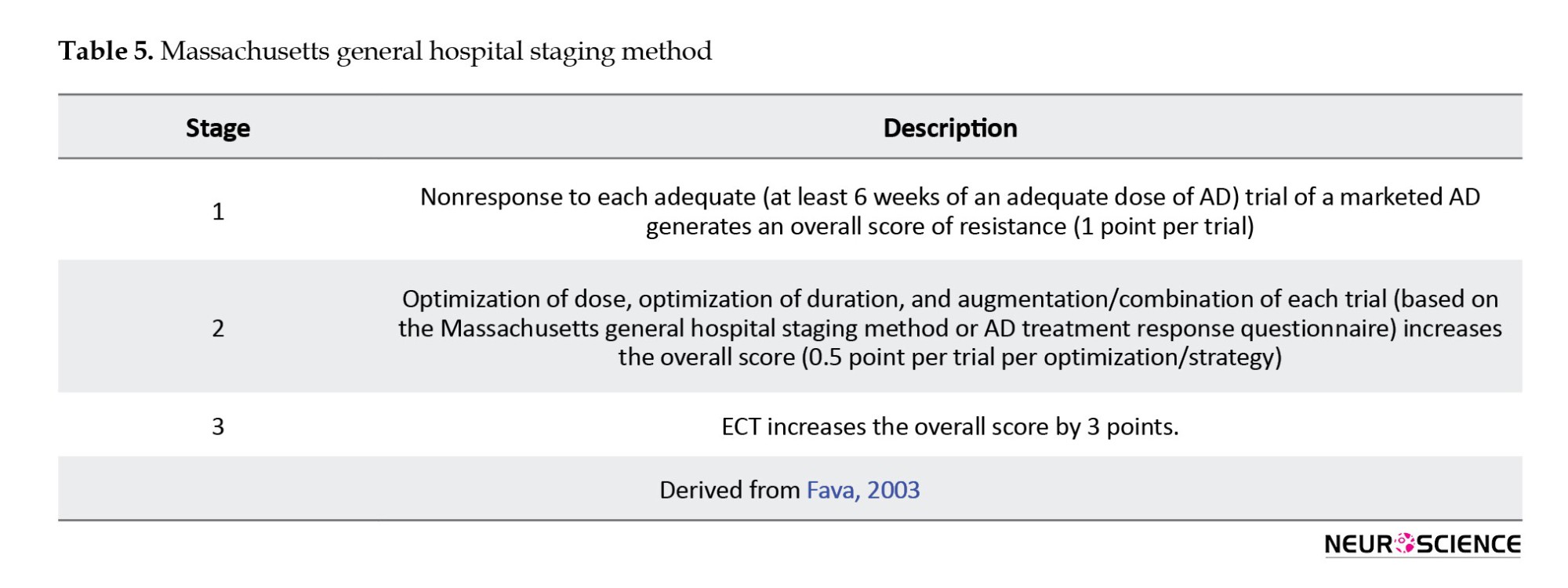
The “European staging method” (Souery et al., 1999) includes both classification and staging approaches to TRD as well as chronic aspects of resistance (Table 6).

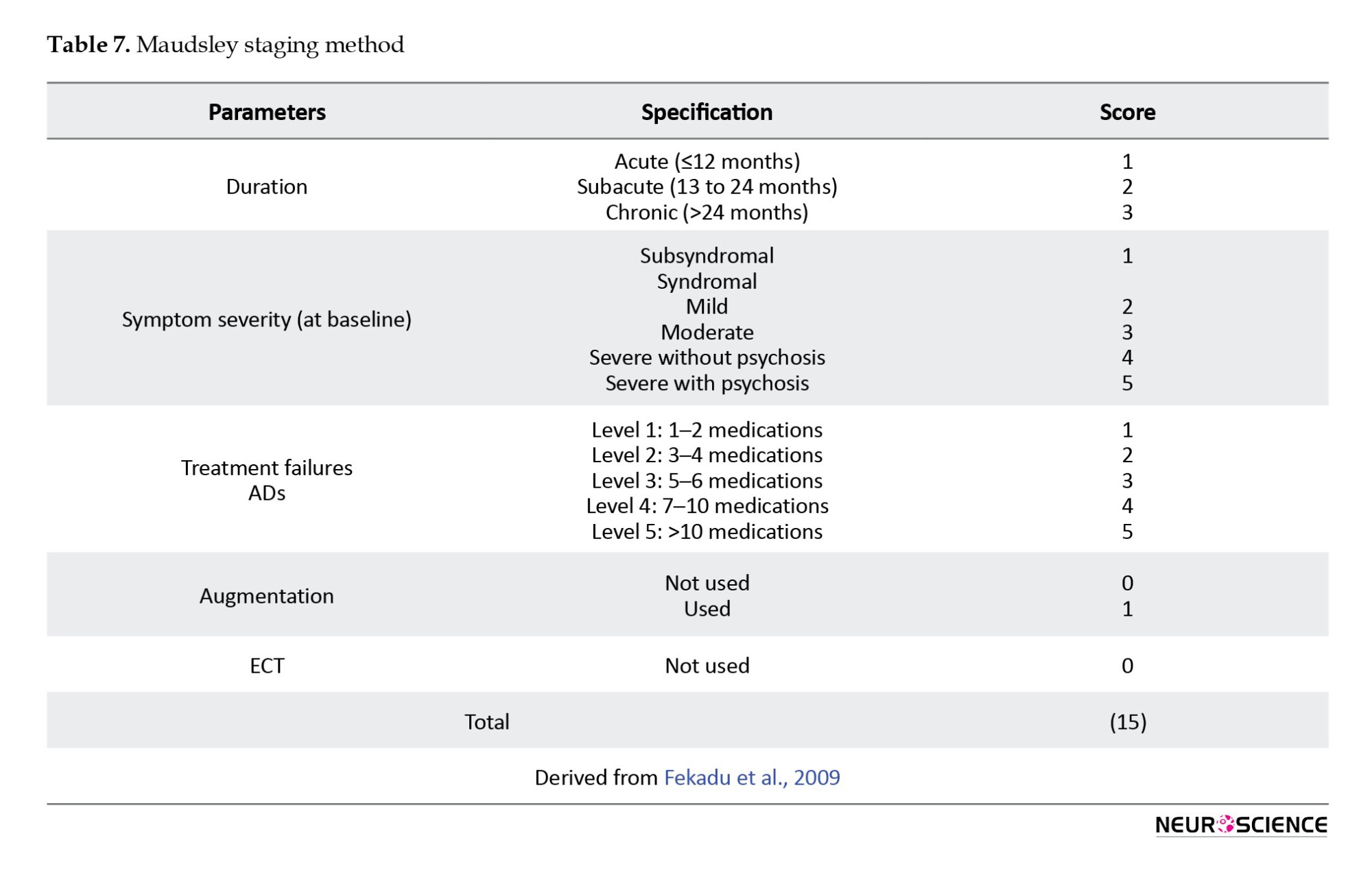
The “Maudsley staging method (MSM)”(Fekadu et al., 2009) includes the number of unsuccessful treatment trials and the chronicity of the disease. In addition, it incorporates measurements of disease severity as a significant cofactor (Table 7) (Guilloux et al., 2012; Trevino et al., 2014).
In contrast to treatment resistance, there is pseudoresistance. Pseudoresistance can result from inadequate dosage or treatment length, patient noncompliance, or uncommon pharmacokinetics, as well as a misdiagnosis of the primary disorder due to failure to recognize a secondary mood disorder or a depressive subtype (Mirabzadeh & Khodaei, 2012). Differentiating between true treatment-resistant depression and pseudoresistance is the clinician’s initial task. During the clinical assessment, three areas are concentrated on to rule out pseudoresistance: Physician-related factors, patient-related factors, and accuracy of diagnosis (Kornstein & Schneider, 2001).
3. Discussion
Clinical observations of behavioral changes are often used to characterize psychiatric disorders (Niciu et al., 2014). Clinical medicine is interested in developing objective, biologically-based tests for psychiatric disorders (First et al., 2018). Obtaining personalized medicine in psychiatry is a valuable goal because its success could significantly reduce morbidity and mortality (Ozomaro et al., 2013). MDD is a major contributor to disability worldwide and an important risk factor for noncompliance with medical treatment. It is a complicated phenotype driven by multiple biological disruptions and may result in numerous physiological changes (Lee et al., 2015; Martins-de-Souza et al., 2014).
Our study shows that pro-inflammatory cytokines enter the brain through various mechanisms, affecting central neuronal function and causing “sickness behavior.” These cytokines trigger their production in the brain’s regions, including the amygdala, dentate gyrus of the hippocampus, and hypothalamus. Also, PET studies have identified abnormalities in regional CBF and glucose metabolism in limbic structures and PFC in mood disorders. So, to gain a more comprehensive understanding of the brain circuitry underlying MDD, more research is needed to integrate studies on the different parts of the brain, like the hippocampus and amygdala, as well as hypothalamic circuits.
Our study revealed that decreased levels of BDNF in MDD may serve as a diagnostic marker, but unresolved issues remain, such as whether peripheral BDNF can pass the blood-brain barrier and cause behavioral effects. According to a study, electroconvulsive treatment (ECT) increases the levels of serum BDNF in patients with depression (Rocha et al., 2016). Also, several studies show that ADs may be crucial in activating tropomyosin receptor kinase B (TrkB) receptors and raising brain levels of BDNF (Rana et al., 2021). Depressive behaviors do not appear to be caused by genetic disruption of the signaling pathways involving BDNF and its receptor, TrkB, but it does reduce the effectiveness of AD medications. Therefore, while BDNF is not the only mediator of depression or anxiety, it may be a target for ADs (Martinowich et al., 2007).
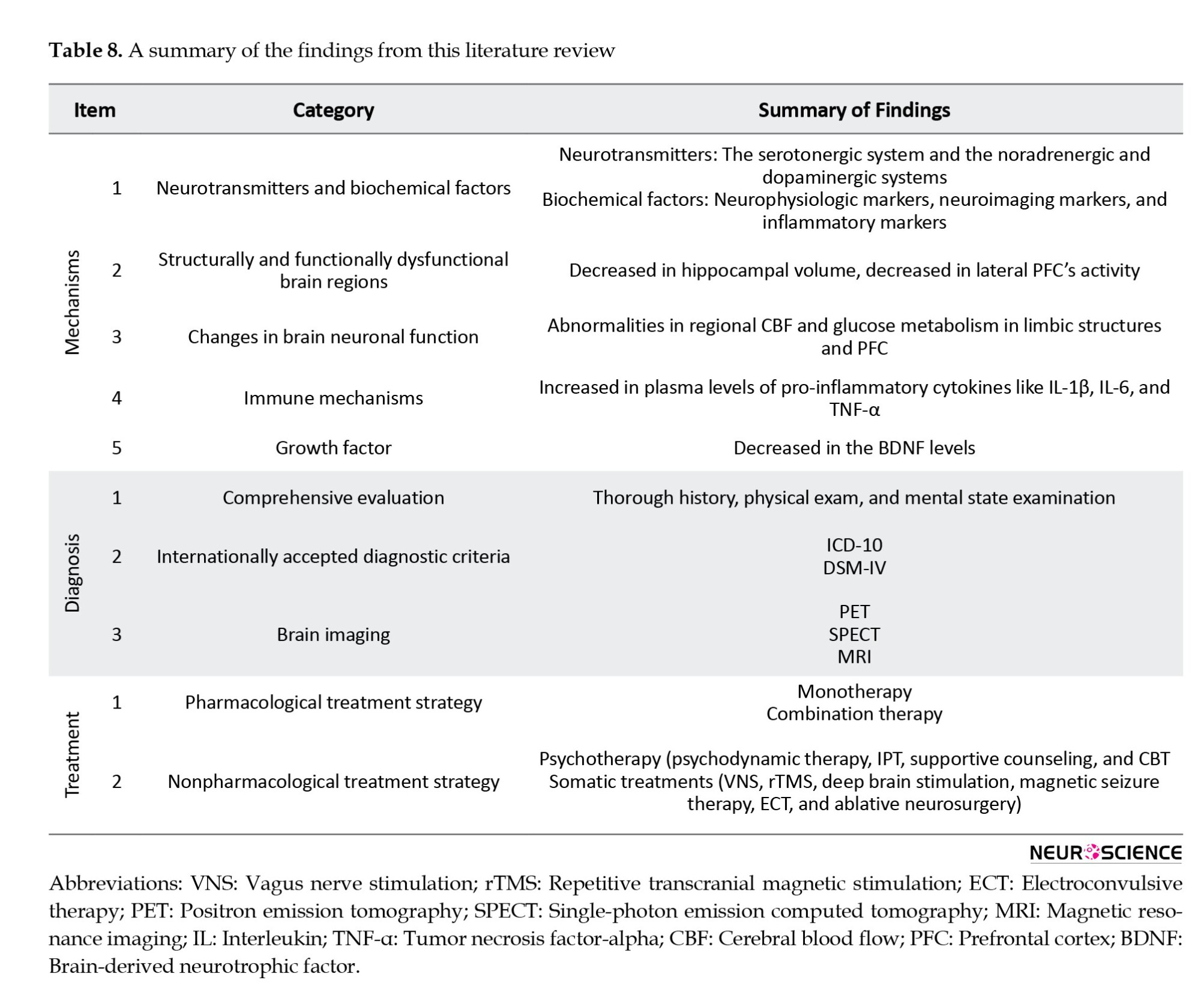
Despite all the advancements made thus far, as summarized in Table 8, many unresolved issues still need to be answered (Brigitta, 2022).
The pharmaceutical treatments that are currently available are generally designed to improve monoamine-dependent neurotransmission, but pharmacotherapy is not always effective, so new non-monoamine-based approaches, such as melatonergic mechanisms and glutamate methods, are becoming increasingly important. Other therapies, including psychotherapy, ECT, magnetic transcription, activating the vagus nerve, and deep brain stimulation, are imprecise but, to varying degrees, effective (Malik et al., 2021). Also, a staging model for TRD should ideally categorize patients based on their level of MDD treatment resistance. Psychopathological and biological indicators for staging TRD, similar to those used in oncology, may help predict better the course and prognosis of the disease (Ruhé et al., 2012).
The lack of biomarkers that can effectively diagnose MDD and predict treatment responses, as well as evaluate MDD and its subtypes, is critical in clinical practice and continues to be a bottleneck in the pharmaceutical industry (Ding et al., 2014; Huang & Lin, 2015; Mora et al., 2018).
In recent years, there has been much interest in applying omics technology to determine the underlying mechanisms of diseases and find biomarkers (Gilanchi et al., 2020). A systems biology-based method can help discover predictive biomarkers in mood disorders because it compiles and integrates several hierarchical levels or domains, making it a useful tool for future studies (Niciu et al., 2014). Diagnostic categories based on symptom patterns that have a distant relationship to biological mechanisms restrict the development of biomarkers for mental disorders. In diseases with high heritability (schizophrenia, autism, and Alzheimer disease), genomic research has produced significant genome-wide association study results. In contrast, in disorders with moderate heritability (anxiety disorders, unipolar major depression), the development of symptoms relies more on environmental risk factors. Methods to identify biologically more homogeneous subgroups are needed to advance biomarker research (Bagdy & Juhasz, 2013). To better comprehend the mechanisms driving brain phenotypes and neuropsychiatric disorders, a field known as “neuroimaging genomics” has emerged that combines genomic and imaging data (Mufford et al., 2017). Significant genetic-neuroimaging connections have been discovered for the monoaminergic, BDNF, glutamatergic, HPA axis, and other common genes, which were in line with theories about the pathophysiology of MDD (Zhang et al., 2018).
Further information regarding the function of the genome may be provided by proteomic research. Systems biology analysis can better evaluate proteome-generated data to understand the origins and effects of complex psychiatric disorders like MDD (Martins-de-Souza et al., 2010). A proteomic study on the brains of post-mortem depression patients in 2012 discovered changes in the expression of arachidonic acid and phospholipase D2, both of which are components of membrane structure and function and are key components of synaptic vesicle membranes. These changes have also been linked to depression (Martins-de-Souza et al., 2012). Proteomics is a helpful tool for identifying disease-specific biomarkers in body fluids by examining global protein profiling (Amiri-Dashatan et al., 2018). Ditzen et al. (2012) researched MDD CSF and found 11 differentially expressed proteins as biomarker candidates for MDD. A proteome study of the plasma presented by Xu et al., (2012) identified 9 proteins expressed differently in MDD patients and the control group. However, most proteomic studies have provided an understanding of the molecular aspects of MDD, and very limited studies have identified differentially expressed proteins as candidate biomarkers for MDD. It is still possible to identify proteins in the field of proteomic research that may aid in not only diagnosis but also patient classification according to various types of MDD (such as atypical depression and psychotic depression), prognosis, treatment monitoring, and response evaluation, and potential drug targets to be used (Martins-de-Souza, 2012).
Moreover, recent investigations have revealed changes in epigenetic markers in suicide victims, raising the possibility of a connection between epigenetics and depression. Advances in proteomic technology have made it possible to explore epigenetic mechanisms in a high-throughput way (Xu et al., 2012). In addition, to functionally validate changes in protein expression, targeted analysis of metabolites has been used (Martins-de-Souza et al., 2010). Also, in MDD patients, the use of neuroimaging techniques to predict anticipated therapeutic results is quickly evolving. The fronto-insular cortex’s pretreatment resting state metabolic activity can distinguish between individuals who are likely to respond to psychotherapy and those who are more likely to respond to medication, and it may serve as a biomarker for treatment selection (Dunlop & Mayberg, 2014).
Also, many medical scientists have been interested in bioinformatics, particularly in investigating disease-related protein networks. Many bioinformatics studies have been conducted on various diseases thus far to identify therapeutic and diagnostic biomarkers. There have been some studies done on diseases, including psychological disorders and depression. This method may result in new diagnostic guidelines for the early identification and assessment of these conditions (Maghvan et al., 2017). Since disease conditions change the biological pathways by which proteins are expressed, studying these changes in tissue, blood, urine, or other biological samples could reveal disease markers (Amiri-Dashatan et al., 2018). Disease-related tissue damage may also sometimes alter body fluids’ metabolic profiles (Khalkhal et al., 2021).
Since tissue alterations are known to manifest in body fluids, considering that human brain samples cannot be obtained, and because of the heterogeneity of MDD, patients’ brains undergo extensive changes. Examining all body fluids, including cerebrospinal fluid, serum, plasma, and urine, will be very helpful in identifying biomarkers. Moreover, psychiatric disorders are so diverse that a single biomarker is insufficient to determine the cellular and molecular pathways involved in a specific person. The most efficient method for diagnostic and treatment decision-making will be identifying a panel of biomarkers with high sensitivity and specificity for different disease subtypes based on their underlying biological mechanisms.
These findings opened a new window to personal medicine because these data suggest that new biomarkers may make it possible to divide MDD patients into different subtypes. Validating such robust biomarkers might result in novel personalized medicine strategies based on patient classification. However, limiting the study to non-imaging markers would be a mistake, as imaging techniques offer the most accurate evaluation of the organ that is the origin of the disease. However, identifying new biomarkers for MDD using molecular profiles may also result in drug discovery and more targeted treatment for MDD patients.
It is essential to point out that the current review contains certain limitations. For instance, some methodological considerations, such as the gender of the patients in studies, were not considered while determining the inclusion and exclusion criteria. Also, as studies do not always clarify the drug treatment of patients, this information was not considered for the inclusion or exclusion criteria of the study.
4. Conclusion
Our findings provide a comprehensive profile of the MDD. The results of this study demonstrate that, despite the high prevalence of MDD, there are still many unresolved issues with regard to the diagnosis, the course of treatment, and the identification of the primary mechanisms underlying the disease. Our study indicates a need to find novel biomarkers for this disease because, due to the lack of biomarkers based on physiological measures or diagnostic tests, objective diagnosis and prognosis in MDD remain difficult. Additionally, high-quality and prospective studies are necessary to accurately identify biomarkers. Systems biology studies can play a significant role in the disease’s future. Also, a more comprehensive and multivariable strategy that combines several approaches, including bioinformatics, proteomics, metabolomics, and neuroimaging techniques, would allow the diagnosis and treatment of depression to be personalized and play a significant role in monitoring therapy efficacy and follow-up of disease. Also, biomarkers help us better understand the neurobiology of different depression subtypes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran, supported this project.
Authors' contributions
Conceptualization, methodology and writing the original draft: Mostafa Rezaei Tavirani and Samira Gilanchi; Investigation: Samira Gilanchi and Mahyar Daskareh; Review, and editing: All authors; Supervision: Samira Gilanchi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran.
References:
Depression is a heterogeneous disorder, covering numerous diseases with different causes and pathophysiologies (Nestler et al., 2002) and a broad spectrum from minor/subthreshold to major (Jani et al., 2015). Major depressive disorder (MDD) is a serious disorder of enormous sociological and clinical relevance. MDD patients present a wide range of symptoms like low energy, depressed mood, lack of interest or pleasure, guilt or feelings of low self-worth, abnormal sleep or appetite, and poor concentration. All of these symptoms can cause significant distress and loss of normal function. A person cannot be diagnosed as having MDD, following the diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) unless he or she shows 5 of the symptoms mentioned above, one of which must be a depressed mood or anhedonia that impairs his or her ability to function in social or occupational settings (Bains & Abdijadid, 2022; Brigitta, 2022; Marcus et al., 2012). Depression and melancholia are known to have originated in Hippocrates’ writings, the father of modern medicine, in the fifth century BC (Spielberger et al., 2003).
Depression is the most prevalent psychiatric disorder and represents one of the leading causes of disability in the world (Newby et al., 2015; Penninx et al., 2013). According to the World Health Organization (WHO), MDD will account for 13% of the global disease burden by 2030, displacing cardiovascular diseases, with a lifetime prevalence of 10% and 15% (Lopizzo et al., 2015). Given its widespread prevalence, MDD has been called the “common cold” of mental illness (Spielberger et al., 2003). Every year, approximately one million people die in Western societies due to a lack of comprehension of disease pathophysiology and laboratory tests to help with accurate diagnosis and antidepressant (AD) treatment strategies (Martins-de-Souza et al., 2012).
There are two major hypotheses based on the classical monoaminergic hypothesis of depression: The cytokine hypothesis (dysfunction in the immune-inflammatory system) and the neurotrophic hypothesis (neuronal plasticity) (Cattaneo et al., 2015). Epidemiologic studies indicate that approximately 40% to 50% of depression etiology is hereditary (Nestler et al., 2002). Generally, genetic, epigenetic, physiological, and psychological components make up the biological basis of mood disorders (Saltiel & Silvershein, 2015). Genetic research indicates that polygenes with small effects and infrequent mutations are probably the genetic causes of the condition (Lee et al., 2014).
However, non-genetic factors are important, too. These variables are incredibly diverse. A variety of these factors range from stress and emotional trauma to viral infections and even random processes during brain development (Nestler et al., 2002). Additionally, undeniable documentation indicates that people with physical disorders—and particularly those with numerous physical disorders—are more likely to develop depression (Kang et al., 2015). Moreover, socioeconomic status is a risk factor for depression (Liang et al., 2012).
Based on the DSM’s symptomatic criteria, depression has been classified as “major depression” since the 1960s. According to these criteria, the diagnosis of depression, in contrast to the majority of disorders of other organ systems, i.e. cancer and diabetes, is not based on quantitative clinical testing like serum chemistry, organ imaging, and biopsies but rather on highly variable symptoms (Lakhan et al., 2010; Nestler et al., 2002).
The clinical overlap of Alzheimer-type dementia, vascular dementia, frontotemporal dementia, and MDD presents considerable diagnostic problems (Braaten et al., 2006). Also, patients with bipolar disorder experience significant depressive symptoms (Chang, 2009; Lin et al., 2008).
If depression is not controlled, significant aftereffects, such as economic, social, physical, and psychological consequences, will be seen. Several studies demonstrate that treating depression is efficient and cost-effective (Evans-Lacko et al., 2016; Greenberg et al., 2003). Several factors contribute to the economic burden of depression, including the prevalence of the disease, the rate and degree of impairment, and the treatment success (Greenberg et al., 2003). In 1990, studies indicated an average loss of 5.6 productive hours per week for depressed workers in the United States. On the other hand, treatment has its costs. The same studies estimate that the annual economic burden for direct treatment, missed wages, indirect workplace costs, and labor costs related to short-term and long-term disability ranges from $44 to $53 billion (Stewart et al., 2003; Tierney, 2007). According to estimates, indirect costs to society are seven times the direct costs (Organization, 2005).
The additional direct cost per person decreased between 2010 and 2018 despite a rise in the entire population of MDD sufferers. In the meantime, the percentage of people with MDD who received therapy remained the same over the preceding 10 years, indicating that this population still has significant unmet treatment needs (Greenberg et al., 2021).
Up to now, attempts to define biomarkers for MDD have not yet led to robust biomarkers. This outcome is due to an incomplete knowledge of the molecular mechanisms underlying MDD and how these mechanisms react and interact in a dynamic environment. Further studies are required to understand the MDD pathophysiology fully.
Researchers are looking for new approaches to treat psychiatric problems because the available traditional treatments are not ideal (Larijani et al., 2021). Moreover, due to the overlapping clinical features of mental disorders and MDD, the diagnosis and treatment of depression and determining its subgroups are facing many problems. Even though significant findings have been made relating to the effective treatment of depression, there are still large gaps in our understanding. Recognizing the neurobiological basis of MDD has remained one of the most significant challenges for modern psychiatry. A better understanding of the molecular mechanisms of MDD can open new horizons in managing, treating, and remission of MDD. The current study highlighted the significance of identifying novel biomarkers for diagnosing and predicting treatment responses and evaluating MDD and its subtypes.
Search strategy
The search strategies for this review are divided into two sections. A different set of search techniques was used for each of the two sections of this evaluation. At first, a literature search was conducted through PubMed and Google Scholar using the search keywords (“major depressive disorder” OR “MDD”) up to June 1, 2022. The second part included articles on each part of the study topic. PubMed and Google Scholar were extensively searched utilizing combinations of the following keywords: (MDD [AND] history), (MDD [AND] mechanism), (MDD [AND] diagnosis), (MDD [AND] treatment), (MDD [AND] drug resistance), and (MDD [AND] biomarker). The title and abstract of each retrieved study were checked to determine whether it met the inclusion or exclusion criteria. However, animal experiments, pediatric MDD, and postpartum depression were excluded. Subsequently, a search was made with more specific keywords for the parts that needed more study. Figure 1 represents the workflow of the study.

History
The concepts of depression and melancholia can be traced back to the fifth century BC in Hippocrates writings, the father of modern medicine. “Black mood” is a description of the Greek-Latin term “melancholia,” which Hippocrates attributed to excessive black bile in the brain (Marsella et al., 1987; Onions, 1965). Melancholia was regarded as a mental disorder characterized by prolonged sadness and fear, “despondency, sleeplessness, irritability, restlessness,” and an aversion to food (Hirshbein, 2009).
In the second century AD, Galen’s restatement of Hippocrates’ description of melancholia prevailed for the next 1500 years. Galen believed that contrary to earlier authorities, yellow bile, nutritional deficiencies, the suppression of menstrual or hemorrhoidal flow, and emotional elements might also contribute to melancholia’s origin (Ariza et al., 2010; Marsella et al., 1987). During the 17th and 18th centuries, more modern, special theories about melancholy began appearing by physicians and Christian Church pastors in Europe. The Latin verb deprimere, which means “to press down,” inspired the current English term for depression (Kanter et al., 2008; Roystonn et al., 2021).
Five writers—Griesinger, Sankey, Maudsley, Krafft-Ebing, and Kraepelin—addressed the root of delusional melancholy during the 1860s and 1880s. The authors all came to the same conclusion—that melancholia was a basic mood disorder—and maintained that the delusions naturally resulted from the aberrant mood. During this century, the model for explaining delusional melancholia in terms of faculty psychology reversed from one that linked intellect to mood to one that linked mood to intellect (Kendler, 2020). In the 1980s and late 1970s, many researchers looked at the effects of norepinephrine, precursors tyrosine, and phenylalanine, as well as serotonin precursors L-tryptophan and 5-hydroxytryptophan on depressive patients (Meyers, 2000). Depressive illness was thought of as a recurrent episodic disorder with complete remission between episodes for several decades in the 20th century (Ban, 2014). At the end of the 20th century, depression, or melancholy, was recognized as a true illness of the time, comparable to the hysteria Charcot had witnessed at the Salpêtrière Hospital in Paris in the 19th century (Barroso, 2003).
Many different treatment approaches have been tried in depressive states. Historically, there have been three phases in the development of the psychiatric treatment of depression.
The first phase, which lasted until the mid-1930s, w:::::::::as char:::::::::acterized by ineffective somatic treatment and psychotherapy. Although many different drugs and physical techniques were used, none of these treatments had a consistent therapeutic effect, even though they occasionally benefited some patients. The second phase is convulsive therapy and lobotomy, which lasted from the mid-1930s to the mid-1950s of the 21st century. Convulsions are brought on either chemically by pentamethylene tetrazol (metrazol) or electrically by a current sent through the brain at this phase of psychotherapy development. For all intents and purposes, this therapy is recommended for acute severe depression. Around the same time, frontal lobotomies were invented, and they were successful in treating depressive disorders, especially chronic ones that had resisted electroconvulsive therapy (ECT). The third phase is pharmacotherapy. This phase began in the middle of 1950, and the development of this phase has not yet been completed (Ban, 2014; Lehmann, 1965; López-Muñoz & Alamo, 2009). An improved comprehension of the molecular mechanisms of MDD can help discover valuable treatments for this disease.
Mechanism
Identifying the cause and pathogenesis of MDD or the prediction of the treatment response in these patients is more important, and for achieving these aims, understanding biological changes during MDD is necessary. MDD may be associated with neurotransmitters and biochemical factors, such as neurophysiologic, neuroimaging, and inflammatory markers. The main neurotransmitters of depression include the serotonergic, noradrenergic, and dopaminergic systems (Lee & Kim, 2013). Patients with depression have decreased hippocampal volume, which is strongly correlated with the frequency and duration of depressive episodes. Despite structural abnormalities in the brain, the neurobiology of MDD is thought to be influenced by changes in brain neuronal function (Saltiel & Silvershein, 2015). Positron emission tomography (PET) studies in mood disorders have discovered several abnormalities in regional cerebral blood flow (CBF) and glucose metabolism in limbic structures and the prefrontal cortex (PFC) (Mössner et al., 2007). Most neurotransmitter synthesis is regulated by the brain (Meyers, 2000). In addition, these brain regions’ structure and function are controlled by monoaminergic neurotransmission (Saltiel & Silvershein, 2015). Monoamine neurotransmitters, particularly serotonin (5-HT), dopamine (DA), and noradrenaline (NA), are hypoactive in MDD patients’ midbrains, and these agents have received more attention because nearly all of the AD drugs’ mechanisms of action act through these systems (Drevets et al., 2008; Werner & Covenas, 2010). In addition to the classical neurotransmitters, neuropeptides are changed in specific brain regions during major depression. Major depression is associated with the hyperactivity of some neuropeptides, including substance P corticotropin-releasing hormone and thyrotropin-releasing hormone, and the hypoactivity of other neuropeptides, including neuropeptide Y and galanin (Werner & Covenas, 2010). Although the monoamine transmitter is important to fully comprehend the disease’s pathophysiology, the monoamine theory is insufficient. Many studies indicate that immune mechanisms, mainly cytokines, are implicated in depression’s pathogenesis (Sahin & Aricioglu, 2013). The cytokine hypothesis of depression posits that the cytokines have a critical function in its cause. Cytokines are immune system hormones. They are composed of proteins and glycoproteins secreted by immune cells and act as signals among the immune cells (Dunn et al., 2005). Several clinical studies reveal that people with depression indicate higher plasma levels of pro-inflammatory cytokines like interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (Dunn et al., 2005). The interaction between peripheral inflammation and the central nervous system has been thoroughly studied. Several mechanisms for entering cytokines, particularly TNF-α and IL-1β, in the brain influence central neuronal function and cause behavioral alterations known as “sickness behavior.” These mechanisms include passing through circumventricular organs, projection with peripheral vagal nerve afferents, uptaking by active transport systems, crossing of cytokine induced immune cells like macrophages, monocytes, and T cells directly through the blood brain barrier and direct passage. These cytokines, after entering the brain, trigger their own production, particularly in the amygdala, dentate gyrus of the hippocampus, hypothalamus, and other parts of the brain. Also, recent studies indicate that the Toll-like receptors, which have a role in neuronal function and the production of cytokines and chemokines in response to inflammation or stressful situations, have altered protein and mRNA levels in the hypothalamus of people with depression (Cattaneo et al., 2015; Sahin & Aricioglu, 2013). The neurotoxic mechanisms and the modulation of neurotransmitter metabolism are two examples of how cytokines in the brain can impact brain function (Capuron & Dantzer, 2003; Cattaneo et al., 2015).
It has been suggested that the pro-inflammatory cytokines IL-1β and IL-6, which increase during infection, are crucial for synaptic plasticity, neurogenesis, and neuromodulation. These pro-inflammatory cytokines stimulate the paraventricular nucleus of the hypothalamus to secrete corticotropin-releasing hormone (CRH), activate the hypothalamic-pituitary-adrenal (HPA) axis, and encourage the release of adreno-corticotrophin hormone (ACTH) and glucocorticoids. Understanding how IL-1β functions in the pathophysiology of depression may help explain how it affects changes in amine metabolism, neurogenesis, and neuroinflammation (Farooq et al., 2017; Jeon & Kim, 2016; Zunszain et al., 2011).
Additionally, it has been demonstrated that cytokines can cause dysfunction of the neurotrophic system and decrease neurogenesis in several brain regions, most notably in the hippocampus. Free radicals, oxidants, and glucocorticoids are overproduced due to the excessive inflammatory response sparked by pro-inflammatory cytokines in the peripheral nervous system, which can disrupt glial cell activities and damage neurons in the brain. As a result, neural plasticity may eventually decline, which is a key component of depression-related dysfunction. The term “neuronal plasticity” describes several mechanisms essential for brain function, including the capacity to recognize, respond to, and adapt to a wide range of external and internal stimuli. It is believed that these processes can be dysfunctional in various psychiatric diseases, which may ultimately increase disease vulnerability (Ariza et al., 2010; Cattaneo et al., 2015).
Furthermore, the stress hormones, such as cortisol, ACTH, and CRH, can all be increased by cytokines. These hormones have been linked to HPA dysfunction and are elevated in depressed patients. Disrupted microglia function has been linked to neurologic and psychiatric disease and could significantly impact neuronal activity and function (Cattaneo et al., 2015; Pariante & Miller, 2001; Zunszain et al., 2011).
Brain-derived neurotrophic factor (BDNF) has received significant attention as a growth factor in MDD. BDNF controls neuronal plasticity, migration, and survival in the central and peripheral neurological systems. It is hypothesized that higher corticosteroid dosages will likely cause decreased levels of BDNF in depression because activation of the glucocorticoid receptors (GRs) negatively affects the BDNF gene. Although BDNF levels in MDD may serve as a diagnostic and prognostic marker, there are still unresolved issues, such as whether peripheral BDNF can pass the blood-brain barrier and cause behavioral effects (Hacimusalar & Eşel, 2018).
According to neuroimaging research, depression affects several structurally and functionally dysfunctional brain regions, the majority of which are related to the limbic system, default mode network, central execution network, and salience network. They contribute to several clinical depressive symptoms (Dai et al., 2019).
The lateral PFC activity is abnormally decreased in MDD patients, particularly during express voluntary control, when the emotional experience is already occurring. On the other hand, it is possible that (medicated) MDD patients can control their emotions in the early, automatic stages by drawing on additional lateral prefrontal neuronal resources. Medial may act as a mediator in this action plan (Rive et al., 2013).
The neuropharmacological mechanisms that have been proposed as the final common pathways for AD responses are as follows: Increase in the gene expression of BDNF and other neurotrophic/neuroprotective factors in the hippocampus and PFC, enhancement of postsynaptic serotonin type 1A (5-HT1A) receptor function, and attenuation of the sensitivity or transmission of NMDA-glutamatergic receptors (Drevets et al., 2008).
2. Diagnosis
Comprehensive evaluation and accurate diagnosis are essential components of managing depression. The evaluation must be based on a thorough history, physical exam, and mental state examination. All sources, especially the family, must be consulted for history. The diagnosis must be noted using the most recent diagnostic criteria (Gautam et al., 2017).
The diagnosis of MDD is made using internationally accepted diagnostic criteria. The International Classification of Diseases (Mental and Behavioral Disorders, ICD-10) and the classification of the American Psychiatric Association (DSM-4) are two of the most commonly used criteria (Farah & Gillihan, 2012). The ICD-10 classifies depression as mild, moderate, or severe (with or without psychotic symptoms) based on a list of 10 depressive symptoms (Ariza et al., 2010; Kessing, 2004). The disease criteria of the DSM-5 have come under harsh criticism from Hyman (2011), a former director of the National Institute of Mental Health (NIMH). He stated: “The problem is that the DSM has been introduced into an understudied area, which has been accepted without question”. It was formed with a focus on a paradigm shift in the system. Given DSM-IV's limitations and lack of foundation other than disease essentialism, there is a need to amend DSM-4 to be upgraded to DSM-5 with a more thorough approach to identify the heterogeneity of major depressive disorder (Kim & Park, 2021). The ICD-10 and DSM-4 diagnostic criteria for depressive episodes overlap, but there are some differences in emphasis. ICD-10 requires that the patient exhibit two of the first three symptoms (depressed mood, loss of interest in daily activities, and decreased energy) and at least two of the remaining seven symptoms. For DSM-4, at least five of the nine symptoms must be present in the patient, and at least one of the first two symptoms (depressed mood and loss of interest). For both diagnostic methods, a diagnosis may only be made when the symptoms have existed for at least two weeks (Nonetheless, it might be shortened for the ICD-10 if symptoms are very severe or present quickly). Both ICD-10 and DSM-4 require that symptoms result in functional impairment that increases with the episode’s severity (NCCMH, 2010). Table 1 shows the diagnostic criteria of ICD-10.

Some minor changes were made to the DSM-4 diagnostic criteria for some disorders, making it easier for attending physicians and psychologists to make diagnoses. The goal of the DSM-5 was to make a subtle shift towards “bridging the gap between etiology-based symptomatology and identifiable pathophysiological etiology” (Kupfer & Regier, 2011; Svenaeus, 2014). Table 2 presents the diagnostic criteria for DSM-5.

First, in the context of mood disorders, depressive disorders are considered a separate entity from bipolar disorders. Second, diagnostic thresholds for MDD were lowered in DSM-5 compared with those in DSM-4. The term “hopelessness” was changed to a more individualized way of describing depressed mood, and the term “bereavement exclusion” was removed from the diagnostic criteria. In the ICD-10 diagnostic criteria for depressive episodes, the deletion of the item ‘bereavement exclusion’ was partially supported because clinical or genetic aspects of major depressive episodes caused by bereavement were not significantly different from those caused in other contexts of major depressive episodes. Thirdly, the transdiagnostic specifiers, such as those with psychotic features, those with mixed features, and those with anxious features, were adjusted in DSM-5. This change was done to show the quantitative, not qualitative, overlapping symptoms of major depressive disorder related to anxiety disorder, schizophrenia, and bipolar disorder. According to the severity-psychosis hypothesis, the specifier was coded with psychotic features only in MDD. This hypothesis emphasizes psychotic symptoms as factors that depend on the severity of MDD. By rejecting the severity-psychosis hypothesis in several studies, the DSM-5, encoding the specifier with psychotic features, was approved for major depressive disorder as well as mild and moderate MDD and dysthymic disorder (Kim & Park, 2021; Svenaeus, 2014).
The inclusion and exclusion criteria for each diagnostic category are being reevaluated and revised in light of recent discoveries. The addition of genetic and neurobiological measures has been taken into consideration regarding this most recent adjustment (Farah & Gillihan, 2012).
The progression of depression and how effectively a patient responds to treatment are significantly influenced by a wide range of biological, psychological, and social aspects that are not well recognized by existing diagnostic frameworks. When doing a diagnostic examination, it is crucial to take into account both the patient’s past experiences with depression as well as any family history of the condition (NICE, 2009).
Some practitioners already use brain imaging to make psychiatric diagnoses (Farah & Gillihan, 2012). Although the pathoanatomic foundation of mental diseases could be defined significantly with the use of modern imaging, a key challenge in using neuroimaging for psychiatric diagnosis is that the clinical usefulness of such tests depends in part on their ability to differentiate between various conditions. As the number of diagnostic categories regarded as clinically important increases, generally, both inter-subject and intra-subject variability in interpretation rises (NICE, 2009; Savitz et al., 2013). PET imaging research has identified multiple anomalies in regional CBF and glucose metabolism in different brain regions. Recent studies on neuroimaging have concentrated on the neurobiological abnormalities related to MDD, such as malfunctioning or structural variations in cerebral regions, due to reactive microglia’s association with some molecular modifications that can be detected by different radiotracers, morphological and functional changes in reactive microglia allowed for in vivo detection of microglial activation as a biomarker of central inflammation in a variety of pathological conditions, including MDD (Gritti et al., 2021; Lee & Kim, 2013).
Single photon emission computed tomography (SPECT) is the functional imaging method used as an ancillary diagnostic tool in clinical psychiatry. In particular, it helps differentiate depression from neurodegenerative diseases when making a differential diagnosis of depression. This imaging technique measures regional CBF by tracers that emit gamma rays in the blood. This local blood flow data creates a low-resolution, three-dimensional image of brain activity (Cho et al., 2002; Farah & Gillihan, 2012; Nagafusa et al., 2012).
The following conditions can be measured with magnetic resonance imaging (MRI): Brain structure volume (structural MRI), white matter integrity and density (diffusion tensor imaging [DTI]), or functional metabolic activity patterns (fMRI), either at rest or in response to a specific task or challenge. Moreover, fMRI can investigate activity in specific brain regions or coordinated temporal activity patterns across several regions (fcMRI) (Dunlop & Mayberg, 2014; Pilmeyer et al., 2022).
Treatments
Achieving complete remission in MDD is difficult because of the chronic nature of this disease. Typical response rates in AD trials are 60% to 70%, while remission rates are much lower (between 30% and 50%) (McIntyre & O’Donovan, 2004; Trivedi & Daly, 2008).
There are three phases of depression: mild, moderate, and severe. These three phases can be divided into two general group treatment strategies for achieving remission in MDD: pharmacological and nonpharmacological (Trivedi & Daly, 2008).
Numerous distinct pharmacologic classes of medications may be utilized to treat depression, and each of them can modulate depression symptoms via different mechanisms. The mechanisms of action of these drugs are all involved with or dependent upon the alteration of several neuromediators. The neurotransmitters serotonin and norepinephrine are the main targets of most major classes of ADs (Gold et al., 2015; Tierney, 2007).
Despite higher acquisition costs, combination therapy for MDD can be cheaper than monotherapy. Any initial monotherapy has a low remission rate, and AD combinations are currently utilized in practice at the second or following steps when relapse occurs over a longer time or, in some situations, even acutely as a first step where rapidity of impact is a clinical priority. If employed as initial therapies, these combinations may be more effective than monotherapy in terms of higher rates of remission, lower attrition, or longer-term benefits (McIntyre & O’Donovan, 2004; Trivedi & Daly, 2008).
First-line AD treatment is ineffective in up to half of MDD patients, and two or more treatments are ineffective in one-third of them. Nonpharmacological treatments can be treatment options for treatment-resistant patients (Trivedi & Daly, 2008).
Nonpharmacological treatments can be divided into two groups: Somatic treatments and psychotherapy. Somatic treatments include vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation, magnetic seizure therapy, ECT, and ablative neurosurgery (Moreines et al., 2011; Trivedi & Daly, 2008).
Creating novel therapeutic alternatives based on the pathophysiology of a certain psychiatric condition and permitting a more targeted treatment approach is one of the main objectives. Invasive or noninvasive brain and cranial nerve stimulation procedures are one group of such therapy possibilities. VNS uses an implanted pulse generator in the left anterior chest wall to deliver intermittent electrical stimulation to the left cervical vagus nerve. The US Food and Drug Administration (FDA) approved VNS in 2005 for “the adjunctive long-term treatment of chronic or recurrent depression for patients 18 years of age or older who are experiencing a major depressive episode and have not had an adequate response to four or more adequate AD treatments” (Christmas et al., 2013; Cimpianu et al., 2017; Nemeroff et al., 2006).
To stimulate the cerebral cortex with rTMS, an electric coil is used to produce a magnetic field. Contrary to ECT, patients tolerate rTMS well, do not require anesthetics, and do not appear to affect cognitive function adversely. The US FDA has approved using 3000 daily stimuli to treat MDD (or 15000 stimuli per week) (Mirabzadeh & Khodaei, 2012; Pan et al., 2020).
Usual treatments for severe TRD have some known side effects that may limit their use. ECT and ablative neurosurgery are two common solutions for severe TRD. Under general anesthesia, an electrical current is serially administered through the brain in the ECT procedure to cause a generalized tonic-clonic seizure. Gray matter volume in the medial temporal lobes significantly increased after ECT, indicating that it may have a neurotrophic effect contributing to its therapeutic efficacy. Also, the longitudinal experience with ablative techniques demonstrates that precise, distinct lesions continue to play a significant role in the disruption of affective circuitry in the management of TRD (Moreines et al., 2011; Ota et al., 2015; Volpini et al., 2017).
The earliest surgical attempt to treat TRD was an ablative neurosurgery technique. There are several surgeries today, including anterior cingulotomy, anterior capsulotomy, limbic leucotomy, and subcaudal tractotomy (Moreines et al., 2011). According to the evidence, even after surgeries, residual symptoms remain frequently, which leads to psychosocial dysfunction. The chances of full recovery are reduced when patients remain more symptomatic. There are numerous ongoing initiatives to deploy cutting-edge ablative therapy for the brain. Recent advances in cerebral ablation techniques have led to new methods that enable precise targeting, accurate thermal dose delivery, and real-time visualization of induced tissue damage. Examples of these methods include magnetic resonance-guided focused ultrasound and laser interstitial thermal therapy. The accuracy of ablative operations may be further enhanced by new modalities, including MRI-guided focused ultrasound surgery, while safety is increased by avoiding open-brain surgery. This technique has gained popularity over the past 10 years, and its therapeutic uses are expanding. Nonetheless, more traditional procedures like stereotactic radiosurgery and radiofrequency thermal ablation continue to play a crucial part in the treatment of numerous neurological illnesses (Franzini et al., 2020; Franzini et al., 2019; Volpini et al., 2017).
According to the theoretical base, brief psychotherapy interventions can be divided into four primary categories: Psychodynamic therapy (grounded in psychoanalytic principles), interpersonal therapy (IPT), supportive counseling (Rogerian person-centered therapy), and cognitive behavioral therapy (CBT) (Möller & Henkel, 2005).
Two effective short-term psychotherapies for major depression are IPT and CBT (Aronson & Ayres, 2000). Specifically, in depression, CBT seems to decrease residual symptoms and lead to reduce the risk of relapse. Also, for the first-line treatment of MDD in children and adolescents, CBT is the most efficient and cost-effective approach. It has been proposed that combining AD medication with psychotherapy may be more successful than using either approach separately (Haby et al., 2004; Trivedi & Daly, 2008).
According to IPT, there is a correlation between interpersonal issues and depressive symptoms that has a significant impact on the development and maintenance of depressive disorders. Hence, the primary therapy goals of this strategy are interpersonal issues. The four areas of interpersonal difficulty include role disputes, role transitions, complicated bereavement, and interpersonal deficits (Brakemeier & Frase, 2012).
Although both CBT and IPT can be successful therapies for MDDs, it is still unclear which therapy is superior to the other (Zhou et al., 2017).
Clinical decision-making is made easier and more consistent using treatment algorithms that offer specific steps. In psychiatry, treatment algorithms (TAs) are designed to enhance patient outcomes by reducing prescribing variance, boosting the appropriateness of medications and dosages, and avoiding “pseudoresistance” (Vaccarino & Kennedy, 2022). TAs, consisting of sequential treatment strategies and standardized therapeutic-choice instructions, have been created to solve this issue. These TAs are made to prevent treatment resistance and increase the quality of care to improve results (Ricken et al., 2018). The Texas medication algorithm project compared the clinical and financial results of algorithm-guided treatment (ALGO) and treatment as usual (TAU) using predetermined medication algorithms, clinical support, and a predetermined patient and family educational package. Based on clinician-rated and patient-reported symptoms and total mental functioning during a year, the ALGO intervention package was more effective for patients with MDD than TAU (Trivedi et al., 2004). The ALGO group included patients who needed to start AD therapy or change their AD medications. Initially, the TAU group followed similar criteria. However, the TAU group patient selection was mainly based on whether they required fewer medication changes and whether their overall score on the Brief Psychiatric Rating Scale was greater than the median for each patient's routine quarterly evaluation at the clinic (Trivedi, 2007).
Drug resistance
ADs, in turn, ameliorate many neurobiological disturbances in depression and thereby alleviate depressive symptoms, but multiple therapies are ineffective for up to one-third of individuals with MDD who are receiving sufficient care (Anacker et al., 2011; De Carlo et al., 2016). MDD patients’ responses to AD drugs are different (Table 3) (El-Hage et al., 2013).

Although first-line AD therapy significantly alleviates the symptoms of depression in numerous cases, only 50% to 60% of MDD patients respond to the treatment. In addition, 30% to 40% of MDD patients never get symptom remission after taking standard AD medication (Rogóż, 2013). Although numerous definitions for TRD patients have been proposed, no validated consensus definition of TRD exists. To define depressed disorders that did not sufficiently remit following treatment, one of the most basic TRD concepts is resistance. It is most commonly defined as the inability of a current depressive episode to respond to at least two sufficient trials (Berlim & Turecki, 2007; El-Hage et al., 2013; Guilloux et al., 2012). The European Agency describes TRD for the evaluation of medicinal products as the inability to respond to two medications from different classes that have been administered for long enough at a sufficient dose without any particular mention of an appropriate dosage or duration by regulatory authorities. TRD is not defined by the US FDA (Mathew, 2008). TRD is widespread, increasing the risk of suicide, disability, and patient suffering (Moreines et al., 2011).
Numerous comprehensive studies have been conducted on the issue of AD-resistant depression (Rogóż, 2013). There are some strategies for dealing with these problems. Combination therapy is one of these strategies, which involves giving patients who partially respond to one AD a second medication. This strategy is commonly used to improve the response to initial treatment (Ables & Baughman III, 2003). Also, for patients who have partially responded, adding a non-AD medication to an AD is a helpful method. Thyroid hormone, lithium, beta-blocker pindolol (Visken), and buspirone (Buspar) are four substances that are widely used in augmentation therapy. Lithium and triiodothyronine are the best-documented options. Studies indicated that lithium decreases the activity of postsynaptic serotonin or 5-HT receptors, leading to enhanced serotonin transmission. Triiodothyronine may be used to improve the effects of tricyclic ADs, monoamine oxidase inhibitors, and selective serotonin reuptake inhibitors (Ables & Baughman III, 2003; Cadieux, 1998; Joffe et al., 2006).
A more successful treatment for individuals who have severe resistance to AD therapy or those with psychotic depression is ECT, a nonpharmacological intervention that is routinely evaluated as a measure of TRD (Ables & Baughman III, 2003; Trevino et al., 2014).
Four staging methods have been proposed for classifications of TRD. These classifications include the “Thase and Rush staging method” (Thase & Rush, 1997), which involves graduating stages of resistance according to response to one or more different therapeutic approaches (Table 4).

The Massachusetts general hospital staging method (MGH-S) (Fava, 2003) is based on the number of AD trials carried out and their possible alteration but disregards the variation in pharmacological classes among the trials. The MGH-S method evaluates the dose and duration of each prior therapy to consider the intensity and optimization of that particular treatment (Table 5).

The “European staging method” (Souery et al., 1999) includes both classification and staging approaches to TRD as well as chronic aspects of resistance (Table 6).

The “Maudsley staging method (MSM)”(Fekadu et al., 2009) includes the number of unsuccessful treatment trials and the chronicity of the disease. In addition, it incorporates measurements of disease severity as a significant cofactor (Table 7) (Guilloux et al., 2012; Trevino et al., 2014).

In contrast to treatment resistance, there is pseudoresistance. Pseudoresistance can result from inadequate dosage or treatment length, patient noncompliance, or uncommon pharmacokinetics, as well as a misdiagnosis of the primary disorder due to failure to recognize a secondary mood disorder or a depressive subtype (Mirabzadeh & Khodaei, 2012). Differentiating between true treatment-resistant depression and pseudoresistance is the clinician’s initial task. During the clinical assessment, three areas are concentrated on to rule out pseudoresistance: Physician-related factors, patient-related factors, and accuracy of diagnosis (Kornstein & Schneider, 2001).
3. Discussion
Clinical observations of behavioral changes are often used to characterize psychiatric disorders (Niciu et al., 2014). Clinical medicine is interested in developing objective, biologically-based tests for psychiatric disorders (First et al., 2018). Obtaining personalized medicine in psychiatry is a valuable goal because its success could significantly reduce morbidity and mortality (Ozomaro et al., 2013). MDD is a major contributor to disability worldwide and an important risk factor for noncompliance with medical treatment. It is a complicated phenotype driven by multiple biological disruptions and may result in numerous physiological changes (Lee et al., 2015; Martins-de-Souza et al., 2014).
Our study shows that pro-inflammatory cytokines enter the brain through various mechanisms, affecting central neuronal function and causing “sickness behavior.” These cytokines trigger their production in the brain’s regions, including the amygdala, dentate gyrus of the hippocampus, and hypothalamus. Also, PET studies have identified abnormalities in regional CBF and glucose metabolism in limbic structures and PFC in mood disorders. So, to gain a more comprehensive understanding of the brain circuitry underlying MDD, more research is needed to integrate studies on the different parts of the brain, like the hippocampus and amygdala, as well as hypothalamic circuits.
Our study revealed that decreased levels of BDNF in MDD may serve as a diagnostic marker, but unresolved issues remain, such as whether peripheral BDNF can pass the blood-brain barrier and cause behavioral effects. According to a study, electroconvulsive treatment (ECT) increases the levels of serum BDNF in patients with depression (Rocha et al., 2016). Also, several studies show that ADs may be crucial in activating tropomyosin receptor kinase B (TrkB) receptors and raising brain levels of BDNF (Rana et al., 2021). Depressive behaviors do not appear to be caused by genetic disruption of the signaling pathways involving BDNF and its receptor, TrkB, but it does reduce the effectiveness of AD medications. Therefore, while BDNF is not the only mediator of depression or anxiety, it may be a target for ADs (Martinowich et al., 2007).
Despite all the advancements made thus far, as summarized in Table 8, many unresolved issues still need to be answered (Brigitta, 2022).

The pharmaceutical treatments that are currently available are generally designed to improve monoamine-dependent neurotransmission, but pharmacotherapy is not always effective, so new non-monoamine-based approaches, such as melatonergic mechanisms and glutamate methods, are becoming increasingly important. Other therapies, including psychotherapy, ECT, magnetic transcription, activating the vagus nerve, and deep brain stimulation, are imprecise but, to varying degrees, effective (Malik et al., 2021). Also, a staging model for TRD should ideally categorize patients based on their level of MDD treatment resistance. Psychopathological and biological indicators for staging TRD, similar to those used in oncology, may help predict better the course and prognosis of the disease (Ruhé et al., 2012).
The lack of biomarkers that can effectively diagnose MDD and predict treatment responses, as well as evaluate MDD and its subtypes, is critical in clinical practice and continues to be a bottleneck in the pharmaceutical industry (Ding et al., 2014; Huang & Lin, 2015; Mora et al., 2018).
In recent years, there has been much interest in applying omics technology to determine the underlying mechanisms of diseases and find biomarkers (Gilanchi et al., 2020). A systems biology-based method can help discover predictive biomarkers in mood disorders because it compiles and integrates several hierarchical levels or domains, making it a useful tool for future studies (Niciu et al., 2014). Diagnostic categories based on symptom patterns that have a distant relationship to biological mechanisms restrict the development of biomarkers for mental disorders. In diseases with high heritability (schizophrenia, autism, and Alzheimer disease), genomic research has produced significant genome-wide association study results. In contrast, in disorders with moderate heritability (anxiety disorders, unipolar major depression), the development of symptoms relies more on environmental risk factors. Methods to identify biologically more homogeneous subgroups are needed to advance biomarker research (Bagdy & Juhasz, 2013). To better comprehend the mechanisms driving brain phenotypes and neuropsychiatric disorders, a field known as “neuroimaging genomics” has emerged that combines genomic and imaging data (Mufford et al., 2017). Significant genetic-neuroimaging connections have been discovered for the monoaminergic, BDNF, glutamatergic, HPA axis, and other common genes, which were in line with theories about the pathophysiology of MDD (Zhang et al., 2018).
Further information regarding the function of the genome may be provided by proteomic research. Systems biology analysis can better evaluate proteome-generated data to understand the origins and effects of complex psychiatric disorders like MDD (Martins-de-Souza et al., 2010). A proteomic study on the brains of post-mortem depression patients in 2012 discovered changes in the expression of arachidonic acid and phospholipase D2, both of which are components of membrane structure and function and are key components of synaptic vesicle membranes. These changes have also been linked to depression (Martins-de-Souza et al., 2012). Proteomics is a helpful tool for identifying disease-specific biomarkers in body fluids by examining global protein profiling (Amiri-Dashatan et al., 2018). Ditzen et al. (2012) researched MDD CSF and found 11 differentially expressed proteins as biomarker candidates for MDD. A proteome study of the plasma presented by Xu et al., (2012) identified 9 proteins expressed differently in MDD patients and the control group. However, most proteomic studies have provided an understanding of the molecular aspects of MDD, and very limited studies have identified differentially expressed proteins as candidate biomarkers for MDD. It is still possible to identify proteins in the field of proteomic research that may aid in not only diagnosis but also patient classification according to various types of MDD (such as atypical depression and psychotic depression), prognosis, treatment monitoring, and response evaluation, and potential drug targets to be used (Martins-de-Souza, 2012).
Moreover, recent investigations have revealed changes in epigenetic markers in suicide victims, raising the possibility of a connection between epigenetics and depression. Advances in proteomic technology have made it possible to explore epigenetic mechanisms in a high-throughput way (Xu et al., 2012). In addition, to functionally validate changes in protein expression, targeted analysis of metabolites has been used (Martins-de-Souza et al., 2010). Also, in MDD patients, the use of neuroimaging techniques to predict anticipated therapeutic results is quickly evolving. The fronto-insular cortex’s pretreatment resting state metabolic activity can distinguish between individuals who are likely to respond to psychotherapy and those who are more likely to respond to medication, and it may serve as a biomarker for treatment selection (Dunlop & Mayberg, 2014).
Also, many medical scientists have been interested in bioinformatics, particularly in investigating disease-related protein networks. Many bioinformatics studies have been conducted on various diseases thus far to identify therapeutic and diagnostic biomarkers. There have been some studies done on diseases, including psychological disorders and depression. This method may result in new diagnostic guidelines for the early identification and assessment of these conditions (Maghvan et al., 2017). Since disease conditions change the biological pathways by which proteins are expressed, studying these changes in tissue, blood, urine, or other biological samples could reveal disease markers (Amiri-Dashatan et al., 2018). Disease-related tissue damage may also sometimes alter body fluids’ metabolic profiles (Khalkhal et al., 2021).
Since tissue alterations are known to manifest in body fluids, considering that human brain samples cannot be obtained, and because of the heterogeneity of MDD, patients’ brains undergo extensive changes. Examining all body fluids, including cerebrospinal fluid, serum, plasma, and urine, will be very helpful in identifying biomarkers. Moreover, psychiatric disorders are so diverse that a single biomarker is insufficient to determine the cellular and molecular pathways involved in a specific person. The most efficient method for diagnostic and treatment decision-making will be identifying a panel of biomarkers with high sensitivity and specificity for different disease subtypes based on their underlying biological mechanisms.
These findings opened a new window to personal medicine because these data suggest that new biomarkers may make it possible to divide MDD patients into different subtypes. Validating such robust biomarkers might result in novel personalized medicine strategies based on patient classification. However, limiting the study to non-imaging markers would be a mistake, as imaging techniques offer the most accurate evaluation of the organ that is the origin of the disease. However, identifying new biomarkers for MDD using molecular profiles may also result in drug discovery and more targeted treatment for MDD patients.
It is essential to point out that the current review contains certain limitations. For instance, some methodological considerations, such as the gender of the patients in studies, were not considered while determining the inclusion and exclusion criteria. Also, as studies do not always clarify the drug treatment of patients, this information was not considered for the inclusion or exclusion criteria of the study.
4. Conclusion
Our findings provide a comprehensive profile of the MDD. The results of this study demonstrate that, despite the high prevalence of MDD, there are still many unresolved issues with regard to the diagnosis, the course of treatment, and the identification of the primary mechanisms underlying the disease. Our study indicates a need to find novel biomarkers for this disease because, due to the lack of biomarkers based on physiological measures or diagnostic tests, objective diagnosis and prognosis in MDD remain difficult. Additionally, high-quality and prospective studies are necessary to accurately identify biomarkers. Systems biology studies can play a significant role in the disease’s future. Also, a more comprehensive and multivariable strategy that combines several approaches, including bioinformatics, proteomics, metabolomics, and neuroimaging techniques, would allow the diagnosis and treatment of depression to be personalized and play a significant role in monitoring therapy efficacy and follow-up of disease. Also, biomarkers help us better understand the neurobiology of different depression subtypes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran, supported this project.
Authors' contributions
Conceptualization, methodology and writing the original draft: Mostafa Rezaei Tavirani and Samira Gilanchi; Investigation: Samira Gilanchi and Mahyar Daskareh; Review, and editing: All authors; Supervision: Samira Gilanchi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of Proteomics Research Center, Shahid Beheshti University of Medical Science, Tehran, Iran.
References:
Ables, A. Z., &Baughman, O. L., 3rd. (2003). Antidepressants: Update on new agents and indications. American Family Physician, 67(3), 547-554. [PMID]
Amiri-Dashatan, N., Koushki, M., Abbaszadeh, H. A., Rostami-Nejad, M., & Rezaei-Tavirani, M. (2018). Proteomics applications in health: Biomarker and drug discovery and food industry. Iranian Journal of Pharmaceutical Research: IJPR, 17(4), 1523-1536. [PMID]
Anacker, C., Zunszain, P. A., Carvalho, L. A., & Pariante, C. M. (2011). The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology, 36(3), 415-425. [DOI:10.1016/j.psyneuen.2010.03.007] [PMID]
Ariza, M., Merino, G., & Linero, E. (2010). Clinical practice guideline on major depression in childhood and adolescence. Madrid, Spain: Ministry of Health and Social Policy, Galician Health Technology Assessment Agency. [Link]
Aronson, S. C., & Ayres, V. E. (2000). Depression: A treatment algorithm for the family physician. Hospital Physician, 36(7), 21-38. [Link]
Bagdy, G., & Juhasz, G. (2013). Biomarkers for personalised treatment in psychiatric diseases. Expert Opinion on Medical Diagnostics, 7(5), 417-422. [DOI:10.1517/17530059.2013.821979] [PMID]
Bains, N., & Abdijadid, S. (2022). Major depressive disorder. In StatPearls. StatPearls Publishing. [PMID]
Ban, T. A. (2014). From melancholia to depression: A history of diagnosis and treatment. Mexico City: International Network for the History of Neuropsychopharmacology. [Link]
Barroso, M. G. (2003). Depression: Clinical definition and case histories. International Journal of Transpersonal Studies, 22(1), 89-99. [DOI:10.24972/ijts.2003.22.1.89]
Berlim, M. T., & Turecki, G. (2007). Definition, assessment, and staging of treatment-resistant refractory major depression: A review of current concepts and methods. Canadian Journal of Psychiatry, 52(1), 46-54. [DOI:10.1177/070674370705200108] [PMID]
Braaten, A. J., Parsons, T. D., McCue, R., Sellers, A., & Burns, W. J. (2006). Neurocognitive differential diagnosis of dementing diseases: Alzheimer’s dementia, vascular dementia, frontotemporal dementia, and major depressive disorder. International Journal of Neuroscience, 116(11), 1271-1293. [DOI:10.1080/00207450600920928] [PMID]
Brakemeier, E. L., & Frase, L. (2012). Interpersonal psychotherapy (IPT) in major depressive disorder. European Archives of Psychiatry and Clinical Neuroscience, 262(Suppl 2), S117–S121.[DOI:10.1007/s00406-012-0357-0] [PMID]
Brigitta, B. (2022). Pathophysiology of depression and mechanisms of treatment. Dialogues in Clinical Neuroscience, 4(1), 7–20. [PMID]
Cadieux, R. J. (1998). Practical management of treatment-resistant depression. American Family Physician, 58(9), 2059-2062. [PMID]
Capuron, L., & Dantzer, R. (2003). Cytokines and depression: The need for a new paradigm. Brain, Behavior, and Immunity, 17(1), 119-124. [DOI:10.1016/S0889-1591(02)00078-8] [PMID]
Cattaneo, A., Macchi, F., Plazzotta, G., Veronica, B., Bocchio-Chiavetto, L., & Riva, M. A., et al. (2015). Inflammation and neuronal plasticity: A link between childhood trauma and depression pathogenesis. Frontiers in Cellular Neuroscience, 9, 40. [DOI:10.3389/fncel.2015.00040] [PMID]
Chang, K. (2009). Challenges in the diagnosis and treatment of pediatric bipolar depression. Dialogues in Clinical Neuroscience, 11(1), 73-80. [DOI:10.31887/DCNS.2009.11.1/kchang] [PMID]
Cho, M. J., Lyoo, I. K., Lee, D. W., Kwon, J. S., Lee, J. S., & Lee, D. S., et al. (2002). Brain single photon emission computed tomography findings in depressive pseudodementia patients. Journal of Affective Disorders, 69(1-3), 159-166. [DOI:10.1016/S0165-0327(01)00301-9]
Christmas, D., Steele, J. D., Tolomeo, S., Eljamel, M. S., & Matthews, K. (2013). Vagus nerve stimulation for chronic major depressive disorder: 12-month outcomes in highly treatment-refractory patients. Journal of Affective Disorders, 150(3), 1221-1225. [DOI:10.1016/j.jad.2013.05.080] [PMID]
Cimpianu, C. L., Strube, W., Falkai, P., Palm, U., & Hasan, A. (2017). Vagus nerve stimulation in psychiatry: A systematic review of the available evidence. Journal of Neural Transmission, 124(1), 145-158. [DOI:10.1007/s00702-016-1642-2] [PMID]
Dai, L., Zhou, H., Xu, X., & Zuo, Z. (2019). Brain structural and functional changes in patients with major depressive disorder: A literature review. PeerJ, 7, e8170. [DOI:10.7717/peerj.8170] [PMID]
De Carlo, V., Calati, R., & Serretti, A. (2016). Socio-demographic and clinical predictors of nonresponse/non-remission in treatment resistant depressed patients: A systematic review. Psychiatry Research, 240, 421-430. [DOI:10.1016/j.psychres.2016.04.034] [PMID]
National Institute of Clinical Excellence (NICE). (2009). The treatment and management of depression in adults (partial update of NICE clinical guideline 23). London: NICE. [Link]
Ding, X., Yang, S., Li, W., Liu, Y., Li, Z., & Zhang, Y., et al. (2014). The potential biomarker panels for identification of major depressive disorder (MDD) patients with and without early life stress (ELS) by metabonomic analysis. Plos One, 9(5), e97479. [DOI:10.1371/journal.pone.0097479] [PMID]
Ditzen, C., Tang, N., Jastorff, A. M., Teplytska, L., Yassouridis, A., & Maccarrone, G., et al. (2012). Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology, 37(4), 1013-1025. [DOI:10.1038/npp.2011.285] [PMID]
Drevets, W. C., Price, J. L., & Furey, M. L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure and Function, 213(1), 93-118. [DOI:10.1007/s00429-008-0189-x] [PMID]
Dunlop, B. W., & Mayberg, H. S. (2014). Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues in Clinical Neuroscience, 16(4), 479-490. [DOI:10.31887/DCNS.2014.16.4/bdunlop] [PMID]
Dunlop, B. W., & Mayberg, H. S. (2014). Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues in Clinical Neuroscience, 16(4), 479–490. [PMID]
Dunn, A. J., Swiergiel, A. H., & de Beaurepaire, R. (2005). Cytokines as mediators of depression: What can we learn from animal studies? Neuroscience & Biobehavioral Reviews, 29(4-5), 891-909. [DOI:10.1016/j.neubiorev.2005.03.023] [PMID]
El-Hage, W., Leman, S., Camus, V., & Belzung, C. (2013). Mechanisms of antidepressant resistance. Frontiers in Pharmacology, 4, 146. [DOI:10.3389/fphar.2013.00146] [PMID]
Evans-Lacko, S., Koeser, L., Knapp, M., Longhitano, C., Zohar, J., & Kuhn, K. (2016). Evaluating the economic impact of screening and treatment for depression in the workplace. European Neuropsychopharmacology, 26(6), 1004-1013. [DOI:10.1016/j.euroneuro.2016.03.005] [PMID]
Farah, M. J., & Gillihan, S. J. (2012). The puzzle of neuroimaging and psychiatric diagnosis: Technology and nosology in an evolving discipline. AJOB Neuroscience, 3(4), 31-41. [DOI:10.1080/21507740.2012.713072] [PMID]
Farooq, R. K., Asghar, K., Kanwal, S., & Zulqernain, A. (2017). Role of inflammatory cytokines in depression: Focus on interleukin-1β. Biomedical Reports, 6(1), 15-20. [DOI:10.3892/br.2016.807] [PMID]
Fava, M. (2003). Diagnosis and definition of treatment-resistant depression. Biological Psychiatry, 53(8), 649-659. [DOI:10.1016/S0006-3223(03)00231-2] [PMID]
Fekadu, A., Wooderson, S., Donaldson, C., Markopoulou, K., Masterson, B., & Poon, L., et al. (2009). A multidimensional tool to quantify treatment resistance in depression: The Maudsley staging method. The Journal of Clinical Psychiatry, 70(2), 177-184. [DOI:10.4088/JCP.08m04309] [PMID]
First, M. B., Drevets, W. C., Carter, C., Dickstein, D. P., Kasoff, L., & Kim, K. L., et al. (2018). Clinical applications of neuroimaging in psychiatric disorders. The American Journal of Psychiatry, 175(9), 915-916. [DOI:10.1176/appi.ajp.2018.1750701] [PMID]
Franzini, A., Moosa, S., Prada, F., & Elias, W. J. (2020). Ultrasound ablation in neurosurgery: Current clinical applications and future perspectives. Neurosurgery, 87(1), 1-10. [DOI:10.1093/neuros/nyz407] [PMID]
Franzini, A., Moosa, S., Servello, D., Small, I., DiMeco, F., & Xu, Z., et al. (2019). Ablative brain surgery: An overview. International Journal of Hyperthermia, 36(2), 64-80. [DOI:10.1080/02656736.2019.1616833] [PMID]
Gautam, S., Jain, A., Gautam, M., Vahia, V. N., & Grover, S. (2017). Clinical practice guidelines for the management of depression. Indian Journal of Psychiatry, 59(Suppl 1), S34–S50.[DOI:10.4103/0019-5545.196973] [PMID]
Gilanchi, S., Zali, H., Faranoush, M., Tavirani, M. R., Shahriary, K., & Daskareh, M. (2020). Identification of candidate biomarkers for idiopathic thrombocytopenic purpura by bioinformatics analysis of microarray data. Iranian Journal of Pharmaceutical Research: IJPR, 19(4), 275-289. [PMID]
Gold, P. W., Machado-Vieira, R., & Pavlatou, M. G. (2015). Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plasticity, 2015, 581976.[DOI:10.1155/2015/581976] [PMID]
Greenberg, P. E., Fournier, A. A., Sisitsky, T., Simes, M., Berman, R., & Koenigsberg, S. H., et al. (2021). The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics, 39(6), 653-665. [DOI:10.1007/s40273-021-01019-4] [PMID]
Greenberg, P. E., Kessler, R. C., Birnbaum, H. G., Leong, S. A., Lowe, S. W., & Berglund, P. A., et al. (2003). The economic burden of depression in the United States: How did it change between 1990 and 2000? The Journal of Clinical Psychiatry, 64(12), 5373. [DOI:10.4088/JCP.v64n1211] [PMID]
Gritti, D., Delvecchio, G., Ferro, A., Bressi, C., & Brambilla, P. (2021). Neuroinflammation in major depressive disorder: A review of PET imaging studies examining the 18-kDa translocator protein. Journal of Affective Disorders, 292, 642–651.[DOI:10.1016/j.jad.2021.06.001] [PMID]
Guilloux, J., David, D., Samuels, B., David, I., Gardier, A., & Guiard, B. (2012). Non-response to initial antidepressant therapy. In G. Rossi (Ed), Psychology-selected papers. London: InTech. [DOI:10.5772/37783]
Haby, M. M., Tonge, B., Littlefield, L., Carter, R., & Vos, T. (2004). Cost-effectiveness of cognitive behavioural therapy and selective serotonin reuptake inhibitors for major depression in children and adolescents. The Australian and New Zealand Journal of Psychiatry, 38(8), 579–591. [DOI:10.1080/j.1440-1614.2004.01421.x] [PMID]
Hacimusalar, Y., & Eşel, E. (2018). Suggested biomarkers for major depressive disorder. Noro Psikiyatri Arsivi, 55(3), 280–290. [DOI:10.29399/npa.19482] [PMID]
National Collaborating Centre for Mental Health (NCCMH). (2010). The classification of depression and depression rating scales/questionnaires. In NCCMH (Eds) Depression in adults with a chronic physical health problem: Treatment and management. Leicester: British Psychological Society. [Link]
Hirshbein, L. (2009). History of psychiatry and medical psychology: With an epilogue on psychiatry and the mind-body relation. Bulletin of the History of Medicine, 83(2), 391-392. [DOI:10.1353/bhm.0.0234]
Huang, T. L., & Lin, C. C. (2015). Advances in biomarkers of major depressive disorder. Advances in Clinical Chemistry, 68, 177-204. [DOI:10.1016/bs.acc.2014.11.003] [PMID]
Hyman, S. E. (2011). Diagnosing the DSM: Diagnostic classification needs fundamental reform. Cerebrum, 2011(6). [PMID]
Jani, B. D., McLean, G., Nicholl, B. I., Barry, S. J., Sattar, N., & Mair, F. S., et al. (2015). Risk assessment and predicting outcomes in patients with depressive symptoms: A review of potential role of peripheral blood based biomarkers. Frontiers in Human Neuroscience, 9, 18. [DOI:10.3389/fnhum.2015.00018] [PMID]
Jeon, S. W., & Kim, Y. K. (2016). Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World Journal of Psychiatry, 6(3), 283-293. [DOI:10.5498/wjp.v6.i3.283] [PMID]
Joffe, R. T., Sokolov, S. T., & Levitt, A. J. (2006). Lithium and triiodothyronine augmentation of antidepressants. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 51(12), 791–793. [DOI:10.1177/070674370605101209] [PMID]
Kang, H. J., Kim, S. Y., Bae, K. Y., Kim, S. W., Shin, I. S., & Yoon, J. S., et al. (2015). Comorbidity of depression with physical disorders: Research and clinical implications. Chonnam Medical Journal, 51(1), 8-18. [DOI:10.4068/cmj.2015.51.1.8] [PMID]
Kanter, J. W., Busch, A. M., Weeks, C. E., & Landes, S. J. (2008). The nature of clinical depression: Symptoms, syndromes, and behavior analysis. The Behavior Analyst, 31(1), 1-21. [PMID]
Kendler, K. S. (2020). The origin of our modern concept of depression-The history of melancholia from 1780-1880: A review. JAMA Psychiatry, 77(8), 863-868. [DOI:10.1001/jamapsychiatry.2019.4709] [PMID]
Kessing, L. V. (2004). Severity of depressive episodes according to ICD-10: Prediction of risk of relapse and suicide. The British Journal of Psychiatry: The Journal of Mental Science, 184, 153–156. [DOI:10.1192/bjp.184.2.153] [PMID]
Khalkhal, E., Rezaei-Tavirani, M., Fathi, F., Nobakht M Gh, B. F., Taherkhani, A., & Rostami-Nejad, M., et al. (2021). Screening of altered metabolites and metabolic pathways in celiac disease using NMR spectroscopy. BioMed Research International, 2021, 1798783. [DOI:10.1155/2021/1798783] [PMID]
Kim, Y. K., & Park, S. C. (2021). An alternative approach to future diagnostic standards for major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 105, 110133. [DOI:10.1016/j.pnpbp.2020.110133] [PMID]
Kornstein, S. G., & Schneider, R. K. (2001). Clinical features of treatment-resistant depression. The Journal of Clinical Psychiatry, 62 (Suppl 16), 18–25. [PMID]
Kupfer, D. J., & Regier, D. A. (2011). Neuroscience, clinical evidence, and the future of psychiatric classification in DSM-5. The American Journal of Psychiatry, 168(7), 672–674. [DOI:10.1176/appi.ajp.2011.11020219] [PMID]
Lakhan, S. E., Vieira, K., & Hamlat, E. (2010). Biomarkers in psychiatry: Drawbacks and potential for misuse. International Archives of Medicine, 3, 1. [DOI:10.1186/1755-7682-3-1] [PMID]
Larijani, B., Parhizkar Roudsari, P., Hadavandkhani, M., Alavi-Moghadam, S., Rezaei-Tavirani, M., & Goodarzi, P., et al. (2021). Stem cell-based models and therapies: A key approach into schizophrenia treatment. Cell and Tissue Banking, 22(2), 207–223. [DOI:10.1007/s10561-020-09888-3] [PMID]
Lee, H. Y., & Kim, Y. K. (2013). Biological markers and genetic factors of major depressive disorder. In N. Kocabasoglu (eD.), Mood disorders. London: InTech [DOI:10.5772/54388]
Lee, J., Joo, E. J., Lim, H. J., Park, J. M., Lee, K. Y., & Park, A., et al. (2015). Proteomic analysis of serum from patients with major depressive disorder to compare their depressive and remission statuses. Psychiatry Investigation, 12(2), 249-259. [DOI:10.4306/pi.2015.12.2.249] [PMID]
Lee, K. Y., Jeong, S. H., Kim, S. H., Ahn, Y. M., Kim, Y. S., & Jung, H. Y., et al. (2014). Genetic role of BDNF Val66Met and 5-HTTLPR polymorphisms on depressive disorder. Psychiatry Investigation, 11(2), 192-199. [DOI:10.4306/pi.2014.11.2.192] [PMID]
Lehmann, H. (1965). The pharmacotherapy of the depressive syndrome. Canadian Medical Association Journal, 92(15), 821-828. [PMID]
Liang, Y., Gong, Y. H., Wen, X. P., Guan, C. P., Li, M. C., & Yin, P., et al. (2012). Social determinants of health and depression: A preliminary investigation from rural China. Plos One, 7(1), e30553. [DOI:10.1371/journal.pone.0030553] [PMID]
Lin, K. M., Perlis, R. H., & Wan, Y. J. (2008). Pharmacogenomic strategy for individualizing antidepressant therapy. Dialogues in Clinical Neuroscience, 10(4), 401-408. [DOI:10.31887/DCNS.2008.10.4/kmlin] [PMID]
López-Muñoz, F., & Alamo, C. (2009). Monoaminergic neurotransmission: The history of the discovery of antidepressants from 1950s until today. Current Pharmaceutical Design, 15(14), 1563-1586. [DOI:10.2174/138161209788168001] [PMID]
Lopizzo, N., Bocchio Chiavetto, L., Cattane, N., Plazzotta, G., Tarazi, F. I., & Pariante, C. M., et al. (2015). Gene-environment interaction in major depression: Focus on experience-dependent biological systems. Frontiers in Psychiatry, 6, 68. [DOI:10.3389/fpsyt.2015.00068] [PMID]
Maghvan, P., Rezaei-Tavirani, M., Zali, H., Nikzamir, A., Abdi, S., & Khodadoostan, M., et al. (2017). Network analysis of common genes related to esophageal, gastric, and colon cancers. Gastroenterology and Hepatology from Bed to Bench, 10(4), 295-302. [PMID]
Malik, S., Singh, R., Arora, G., Dangol, A., & Goyal, S. (2021). Biomarkers of major depressive disorder: Knowing is half the battle. Clinical Psychopharmacology and Neuroscience, 19(1), 12-25. [DOI:10.9758/cpn.2021.19.1.12] [PMID]
Marcus, M., Yasamy, M. T., van Ommeren, M. Chisholm, D., & Saxena, S. (2012). Depression: A global public health concern. London: World Federation for Mental Health. [Link]
Marsella, A. J., Hirschfeld, R., & Katz, M. M. (1987). The measurement of depression. New York: Guilford Press. [Link]
Martinowich, K., Manji, H., & Lu, B. (2007). New insights into BDNF function in depression and anxiety. Nature Neuroscience, 10(9), 1089-1093. [DOI:10.1038/nn1971] [PMID]
Martins-de-Souza, D. (2012). Comprehending depression through proteomics. The International Journal of Neuropsychopharmacology, 15(10), 1373–1374. [DOI:10.1017/S146114571200034X] [PMID]
Martins-de-Souza, D., Guest, P. C., Harris, L. W., Vanattou-Saifoudine, N., Webster, M. J., & Rahmoune, H., et al. (2012). Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Translational Psychiatry, 2(3), e87. [DOI:10.1038/tp.2012.13] [PMID]
Martins-de-Souza, D., Harris, L. W., Guest, P. C., Turck, C. W., & Bahn, S. (2010). The role of proteomics in depression research. European Archives of Psychiatry and Clinical Neuroscience, 260(6), 499–506. [DOI:10.1007/s00406-009-0093-2] [PMID]
Martins-de-Souza, D., Maccarrone, G., Ising, M., Kloiber, S., Lucae, S., & Holsboer, F., et al. (2014). Plasma fibrinogen: Now also an antidepressant response marker? Translational Psychiatry, 4(1), e352. [DOI:10.1038/tp.2013.129] [PMID]
Mathew, S. J. (2008). Treatment-resistant depression: Recent developments and future directions. Depression and Anxiety, 25(12), 989–992. [DOI:10.1002/da.20540] [PMID]
McIntyre, R. S., & O’Donovan, C. (2004). The human cost of not achieving full remission in depression. Canadian Journal of Psychiatry, 49(1), 10-16. [PMID]
Meyers, S. (2000). Use of neurotransmitter precursors for treatment of depression. Alternative Medicine Review, 5(1), 64-71. [PMID]
Mirabzadeh, A., & Khodaei, M. R. (2012). Treatment of depression in the elderly: A systematic review. Iranian Rehabilitation Journal, 10(3), 76-91. [Link]
Möller, H.,& Henkel, V. (2005). What are the most effective diagnostic and therapeutic strategies for the management of depression in specialist care. Copenhagen, WHO Regional Office for Europe. [Link]
Mora, C., Zonca, V., Riva, M. A., & Cattaneo, A. (2018). Blood biomarkers and treatment response in major depression. Expert Review of Molecular Diagnostics, 18(6), 513-529. [DOI:10.1080/14737159.2018.1470927] [PMID]
Moreines, J. L., McClintock, S. M., & Holtzheimer, P. E. (2011). Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: A review. Brain Stimulation, 4(1), 17-27. [DOI:10.1016/j.brs.2010.01.005] [PMID]
Mössner, R., Mikova, O., Koutsilieri, E., Saoud, M., Ehlis, A. C., & Müller, N., et al. (2007). Consensus paper of the WFSBP Task Force on Biological Markers: Biological markers in depression. The world Journal of Biological Psychiatry, 8(3), 141-174. [DOI:10.1080/15622970701263303] [PMID]
Mufford, M. S., Stein, D. J., Dalvie, S., Groenewold, N. A., Thompson, P. M., & Jahanshad, N. (2017). Neuroimaging genomics in psychiatry-a translational approach. Genome Medicine, 9(1), 102. [DOI:10.1186/s13073-017-0496-z] [PMID]
Nagafusa, Y., Okamoto, N., Sakamoto, K., Yamashita, F., Kawaguchi, A., & Higuchi, T., et al. (2012). Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. Journal of Affective Disorders, 140(3), 296-299. [DOI:10.1016/j.jad.2012.03.026] [PMID]
Nemeroff, C. B., Mayberg, H. S., Krahl, S. E., McNamara, J., Frazer, A., & Henry, T. R., et al. (2006). VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology, 31(7), 1345-1355. [DOI:10.1038/sj.npp.1301082] [PMID]
Nestler, E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J., & Monteggia, L. M. (2002). Neurobiology of depression. Neuron, 34(1), 13-25. [DOI:10.1016/S0896-6273(02)00653-0] [PMID]
Newby, J. M., McKinnon, A., Kuyken, W., Gilbody, S., & Dalgleish, T. (2015). Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clinical Psychology Review, 40, 91-110. [DOI:10.1016/j.cpr.2015.06.002] [PMID]
Niciu, M. J., Mathews, D. C., Ionescu, D. F., Richards, E. M., Furey, M. L., & Yuan, P., et al. (2014). Biomarkers in mood disorders research: Developing new and improved therapeutics. Revista de Psiquiatria Clinica, 41(5), 131–134.[DOI:10.1590/0101-60830000000027] [PMID]
Onions, C. T. (1965). Oxford dictionary of English Etymology. Canadian Journal of Linguistics, 11(1). [DOI: 10.1017/S0008413100005661]
Ota, M., Noda, T., Sato, N., Okazaki, M., Ishikawa, M., & Hattori, K., et al. (2015). Effect of electroconvulsive therapy on gray matter volume in major depressive disorder. Journal of Affective Disorders, 186, 186-191. [DOI:10.1016/j.jad.2015.06.051] [PMID]
Ozomaro, U., Wahlestedt, C., & Nemeroff, C. B. (2013). Personalized medicine in psychiatry: Problems and promises. BMC Medicine, 11, 132. [DOI:10.1186/1741-7015-11-132] [PMID]
Pan, F., Shen, Z., Jiao, J., Chen, J., Li, S., & Lu, J., et al. (2020). Neuronavigation‐guided rTMS for the treatment of depressive patients with suicidal ideation: A double‐blind, randomized, sham‐controlled trial. Clinical Pharmacology & Therapeutics, 108(4), 826-832. [DOI:10.1002/cpt.1858] [PMID]
Pariante, C. M., & Miller, A. H. (2001). Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391-404. [DOI:10.1016/S0006-3223(00)01088-X] [PMID]
Penninx, B. W., Milaneschi, Y., Lamers, F., & Vogelzangs, N. (2013). Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Medicine, 11, 129. [DOI:10.1186/1741-7015-11-129] [PMID]
Pilmeyer, J., Huijbers, W., Lamerichs, R., Jansen, J. F. A., Breeuwer, M., & Zinger, S. (2022). Functional MRI in major depressive disorder: A review of findings, limitations, and future prospects. Journal of Neuroimaging, 32(4), 582-595. [DOI:10.1111/jon.13011] [PMID]
Rana, T., Behl, T., Sehgal, A., Srivastava, P., & Bungau, S. (2021). Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. Journal of Molecular Neuroscience, 71(10), 2008–2021. [DOI:10.1007/s12031-020-01754-x] [PMID]
Ricken, R., Wiethoff, K., Reinhold, T., Stamm, T. J., Baghai, T. C., & Fisher, R., et al. (2018). A standardized stepwise drug treatment algorithm for depression reduces direct treatment costs in depressed inpatients‐Results from the German Algorithm Project (GAP3). Journal of Affective Disorders, 228, 173-177. [DOI:10.1016/j.jad.2017.11.051] [PMID]
Rive, M. M., van Rooijen, G., Veltman, D. J., Phillips, M. L., Schene, A. H., & Ruhé, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10), 2529-2553. [DOI:10.1016/j.neubiorev.2013.07.018] [PMID]
Rocha, R. B., Dondossola, E. R., Grande, A. J., Colonetti, T., Ceretta, L. B., & Passos, I. C., et al. (2016). Increased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: A meta-analysis study. Journal of Psychiatric Research, 83, 47-53. [DOI:10.1016/j.jpsychires.2016.08.004] [PMID]
Rogóż, Z. (2013). Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: Preclinical and clinical efficacy. Pharmacological Reports, 65(6), 1535-1544. [DOI:10.1016/S1734-1140(13)71515-9] [PMID]
Roystonn, K., Teh, W. L., Samari, E., Cetty, L., Devi, F., & Shahwan, S., et al. (2021). Analysis and interpretation of metaphors: Exploring young adults’ subjective experiences with depression. Qualitative Health Research, 31(8), 1437-1447. [DOI:10.1177/10497323211004104] [PMID]
Ruhé, H. G., van Rooijen, G., Spijker, J., Peeters, F. P., & Schene, A. H. (2012). Staging methods for treatment resistant depression. A systematic review. Journal of Affective Disorders, 137(1-3), 35-45. [DOI:10.1016/j.jad.2011.02.020] [PMID]
Sahin, C., & Aricioglu, F. (2013). Future directions of cytokine hypothesis in depression:’NLRP3 inflamazomu’. Psychiatry and Clinical Psychopharmacology, 23(3), 280-288. [DOI:10.5455/bcp.20130927070724]
Saltiel, P. F., & Silvershein, D. I. (2015). Major depressive disorder: Mechanism-based prescribing for personalized medicine. Neuropsychiatric Disease and Treatment, 11, 875-888. [DOI:10.2147/NDT.S73261] [PMID]
Savitz, J., Rauch, S. L., & Drevets, W. (2013). Clinical application of brain imaging for the diagnosis of mood disorders: the current state of play. Molecular Psychiatry, 18(5), 528-539. [DOI:10.1038/mp.2013.25] [PMID]
Souery, D., Amsterdam, J., De Montigny, C., Lecrubier, Y., Montgomery, S., & Lipp, O., et al. (1999). Treatment resistant depression: Methodological overview and operational criteria. European Neuropsychopharmacology, 9(1-2), 83-91. [DOI:10.1016/S0924-977X(98)00004-2]
Spielberger, C. D., Ritterband, L. M., Reheiser, E. C., & Brunner, T. M. (2003). The nature and measurement of depression. International Journal of Clinical and Health Psychology, 3(2), 209–234. [Link]
Stewart, W. F., Ricci, J. A., Chee, E., Hahn, S. R., & Morganstein, D. (2003). Cost of lost productive work time among US workers with depression. JAMA, 289(23), 3135-3144. [DOI:10.1001/jama.289.23.3135] [PMID]
Svenaeus, F. (2014). Diagnosing mental disorders and saving the normal, Medicine, Health Care and Philosophy, 17, 241-244. [DOI: 110.1007/s11019-013-9529-6]
Thase, M. E., & Rush, A. J. (1997). When at first you don’t succeed: Sequential strategies for antidepressant nonresponders. Journal of Clinical Psychiatry, 58(13), 23-29. [Link]
Tierney, J. G. (2007). Treatment-resistant depression: Managed care considerations. Journal of Managed Care Pharmacy, 13(6 Suppl A), S2–S7. [DOI:10.18553/jmcp.2007.13.s6-a.2] [PMID]
Trevino, K., McClintock, S. M., McDonald Fischer, N., Vora, A., & Husain, M. M. (2014). Defining treatment-resistant depression: A comprehensive review of the literature. Annals of clinical psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 26(3), 222–232. [PMID]
Trivedi, M. H (2007). Use of decision support tools for treatment algorithms. CNS Spectrums, 12(S13), 9-16. [DOI: 10.1017/S1092852900003746]
Trivedi, M. H., & Daly, E. J. (2008). Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues in Clinical Neuroscience, 10(4), 377-384. [DOI:10.31887/DCNS.2008.10.4/mhtrivedi] [PMID]
Trivedi, M. H., Rush, A. J., Crismon, M. L., Kashner, T. M., Toprac, M. G., & Carmody, T. J., et al. (2004). Clinical results for patients with major depressive disorder in thetexas medication algorithm project. Archives of General Psychiatry, 61(7), 669-680. [DOI:10.1001/archpsyc.61.7.669] [PMID]
Vaccarino, S. R., & Kennedy, S. H. (2022). Treatment algorithms for treatment-resistant depression. In J. Quevedo, Riva-Posse. P, Bobo. WV (Eds), Managing Treatment-Resistant Depression (pp. 81-98): Elsevier. [Link]
Volpini, M., Giacobbe, P., Cosgrove, G. R., Levitt, A., Lozano, A. M., & Lipsman, N. (2017). The history and future of ablative neurosurgery for major depressive disorder. Stereotactic and Functional Neurosurgery, 95(4), 216-228. [DOI:10.1159/000478025] [PMID]
Werner, F.-M., & Covenas, R. (2010). Classical neurotransmitters and neuropeptides involved in major depression: A review. The International Journal of Neuroscience, 120(7), 455–470. [DOI:10.3109/00207454.2010.483651] [PMID]
WHO. (2005). What are the most effective diagnostic and therapeutic strategies for the management of depression in specialist care? Geneva: WHO. [Link]
WHO. (2019). International statistical classification of diseases and related health problems (10th ed.). Genava: WHO. [Link]
Xu, H. B., Zhang, R. F., Luo, D., Zhou, Y., Wang, Y., & Fang, L., et al. (2012). Comparative proteomic analysis of plasma from major depressive patients: Identification of proteins associated with lipid metabolism and immunoregulation. The International Journal of Neuropsychopharmacology, 15(10), 1413–1425. [DOI:10.1017/S1461145712000302] [PMID]
Zhang, H. F., Mellor, D., & Peng, D. H. (2018). Neuroimaging genomic studies in major depressive disorder: A systematic review. CNS Neuroscience & Therapeutics, 24(11), 1020–1036.[DOI:10.1111/cns.12829] [PMID]
Zhou, S. G., Hou, Y. F., Liu, D., & Zhang, X. Y. (2017). Effect of cognitive behavioral therapy versus interpersonal psychotherapy in patients with major depressive disorder: A meta-analysis of randomized controlled trials. Chinese Medical Journal, 130(23), 2844-2851. [DOI:10.4103/0366-6999.219149] [PMID]
Type of Study: Review |
Subject:
Behavioral Neuroscience
Received: 2023/11/13 | Accepted: 2024/01/2 | Published: 2024/11/1
Received: 2023/11/13 | Accepted: 2024/01/2 | Published: 2024/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








