Volume 16, Issue 1 (January & February 2025)
BCN 2025, 16(1): 19-30 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jahanshahi M, Elyasi L, Nikmahzar E. L-α-aminoadipic Acid-induced Astrocytes Inhibition in the Hippocampal CA1 Region, Anxiety-like Behavior, and Memory Impairment. BCN 2025; 16 (1) :19-30
URL: http://bcn.iums.ac.ir/article-1-2792-en.html
URL: http://bcn.iums.ac.ir/article-1-2792-en.html
1- Department of Anatomy, Faculty of Medicine, Neuroscience Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
2- Neuroscience Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
2- Neuroscience Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
Keywords: Astrocyte, Glial fibrillary acidic protein (GFAP), L-α-aminoadipic acid, Anxiety, Memory, Hippocampus
Full-Text [PDF 2118 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Astrocytes, the most abundant neuroglial in the central nervous system (CNS), have vital roles in brain homeostasis, neuroprotection, synaptic plasticity, uptake and release of neurotransmitters, and innate immunity (Acosta et al., 2017; Dallérac & Rouach, 2016; Navarrete & Araque, 2014; Olsen et al., 2018; Shang et al., 2015; Soung & Klein, 2020; Zorec et al., 2015). Astrocytes modulate anti-inflammatory processes and regulate microglia’s function during brain injury through secreting cytokines (Lima et al., 2014; Tarasov et al., 2020). Moreover, they maintain the glutamate and GABA (gamma-aminobutyric acid) neurotransmitter reservoirs by expressing pyruvate carboxylase, the enzyme required to synthesize these two amino acids (Schousboe et al., 2013). Inflammatory cytokines control astrocytes-released glutamate. Hence, glia-to-neuron signaling may be affected by cytokine mediators in pathological conditions (Vesce et al., 2007). Astrocytes respond to neuronal activity by expressing several receptors and altering homeostasis, creating a neuron-astrocyte cross-talk. Abnormal conditions could affect the neuron-astrocyte integrity and disturb cognitive functions and final behavior output. In addition to the typical roles of astrocytes, recent research has been focusing on their cognitive functions (Hosseini et al., 2020; Santello et al., 2019; Suzuki et al., 2011).
Previous studies found astrocyte plasticity in rat hippocampus after spatial working memory (Jahanshahi et al., 2008; Mehrdad et al., 2007). Indeed, enhanced expression of glial fibrillary acidic protein (GFAP) and astrocyte density after learning showed astrocytes’ recruitment in cognition (Dallérac & Rouach, 2016). GFAP is an intermediate filament mainly found in mature astrocytes’ cytoskeleton structure (Bronzuoli et al., 2018; Guillamón Vivancos et al., 2015). The mutations and abnormal expression of GFAP have been observed in neurodegeneration, neuroinflammation, and psychiatric disorders (Li et al., 2020). Therefore, specific inhibition of astrocytes may help investigate the contribution of reactive astrocytes to some neurodegenerative diseases.
L-α-aminoadipic acid (L-α-AAA) is a homolog of the excitatory amino acid glutamate, generally used to exert astrocyte-specific toxicity in vitro and in vivo studies (Guidetti & Schwarcz, 2003). Microinjection of L-α-AAA inhibits the glutamine synthetase that plays an essential role in the learning and consolidation of memories (Guidetti & Schwarcz, 2003; Robinson et al., 2015). It decreased long-term potentiation magnitude, which underlies memory impairment. It also reported that altered glutamate neurotransmission occurred in anxiety-like behavior related to traumatic brain injury in the amygdala (Beitchman et al., 2020). It is not clear how the ablation of astrocytes changes the memory and behavior. However, a combination of alternations in trophic support for neurons and glutamate cycle, astrocytes typically control that, has been proposed (David et al., 2019). The effect of hippocampal astrocytes on anxiety and memory has not been extensively investigated (Leitão, 2018). In this study, we aimed to evaluate the effect of astrocyte inhibition with L-α-AAA on inhibitory passive avoidance memory, anxiety-like behavior, and the density of GFAP-ir astrocytes in rat hippocampus.
2. Materials and Methods
Study animals
A total of 21 adult male rats of the Wistar strain (180–220 g) were obtained from the animal house of Golestan University of Medical Sciences. The rats were placed in the cages at 22±2 ºC, 12 hours light/dark cycle with access to food and water ad libitum. All procedures followed the principles of the Ethical Board of Golestan University of Medical Sciences (Gorgan, Iran).
Stereotaxic procedure and L-α-AAA microinjection into the hippocampal CA1 subfield
According to our previous studies, the intrahippocampal injection was performed with minor modifications (Azami et al., 2010; Jahanshahi et al., 2018; Moghadami et al., 2016). Briefly, the rats were anesthetized for stereotaxic surgery (David Kopf Instruments, USA) using an intraperitoneal injection of ketamine (100 mg/mL) and xylazine (20 mg/mL). Stainless guide cannulas (21-gauge) were bilaterally implanted at the dorsal CA1 hippocampal area; the coordinates were anterior-posterior: −3 mm from bregma; medial-lateral: ±2 mm from midline; and dorsal-ventral: −2 mm from the skull surface (Paxinos & Watson, 2007). At the end of the procedure, each cannula was temporarily closed with a stainless-steel wire to preserve it from occlusion. Seven days after the surgery, 1 μL/rat (0.5 μL on each side) of L-α-AAA and or vehicle were injected into the CA1 hippocampal area by a Hamilton micro-syringe over one minute. The needle was kept in a cannula to prevent backflow. L-α-AAA (Sigma-Aldrich, China) was dissolved in normal saline containing 6% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, China) with pH=7. One day before and after the last injection of drugs, learning and memory were assessed by passive avoidance memory tasks. The EPM was performed 1 day before and 30 minutes after the last injection of drugs.
Experimental design
The rats were randomly assigned into three groups (n=7): Control, with no intervention; vehicle, with stereotaxic surgery, received 6% 2-hydroxypropyl-β-cyclodextrin (vehicle) once per day for three consecutive days and evaluated for behavioral tests; experimental group (L-α-AAA), with stereotaxic surgery, received L-α-AAA at dose of 25 µg/µL once daily for three consecutive days and evaluated for behavioral test.
All drugs were injected into the dorsal CA1 hippocampal area. Rats subjected to inhibitory passive avoidance memory and anxiety assessments were habituated to the condition of the testing room for 1 h before performing the behavioral tests. The two behavioral tests were not performed on the same rats, and behavioral tests were done blind to treatment.
Behavioral tests
Passive avoidance memory test
The passive avoidance memory test was performed as described in our previous studies (Mahakizadeh et al., 2015; Seifhosseini et al., 2011). The apparatus (the shuttle box) was composed of two light and dark compartments (20×20×30 cm) with identical sizes that were divided by a manually operated gate (7×9 cm). The dark compartment had a stainless steel shock grid floor. Electric shocks were given using a stimulator (50 Hz, 1.5 mA intensity, 3 s). It has three steps: Habituation, training, and prob.
Habituation
Each rat was gently placed into the light compartment, and after 5 seconds, the gate was opened, and the rat was permitted to go into the dark compartment. Rats were excluded from the experiments if they delayed for more than 120 seconds to cross the dark compartment.
Training
The training was done 30 minutes after habituation. The rat was placed in the light compartment, and after 5 seconds, the gate was opened. When the rat entered the dark compartment with all four feet, the gate was closed, and the rat received an electrical foot shock (50 Hz, 1.5 mA intensity, 3 s). After 20 seconds, the rat was returned to the cage. One hundred and twenty seconds later, the rat was re-tested using a method similar to the one used in the prior trials; successful learning was defined as the rat remaining in the light compartment for 120 seconds.
Prob
One day after the last drug injection, the rat was placed in the light compartment, and then the step-through latency time to enter the dark compartment was recorded. The cut-off time for the retention trial was 300 seconds.
Elevated plus maze test (EPM)
The EPM test was used to assess anxiety-related behavior in rats (Karimi et al., 2014; Nikmahzar et al., 2016). Briefly, this plus-shaped apparatus was located at 50 cm in height. It is made up of two open arms (50×10 cm) and two closed arms (50×10×40 cm) opposite to each other, linked by a central square zone (10×10 cm). The test was performed by placing the rat on the center of EPM facing an open arm, and the rat was permitted to explore the maze for 5 minutes. During the step, the number of entries into both arms and the time spent in each arm was recorded. Arm entries were recorded when the rat entered all four paws into the EPM arm. The maze was cleaned with 70% ethanol between each step for each rat. Finally, the percentage of open and closed arms time, the percentage of open and closed arm entries, open arm latency, and pure index of locomotor activity were measured. Total arm entries into the open and closed arms were measured as a pure index of locomotor activity.
Tissue preparation
Twenty-four hours after the behavioral test, the rats were deeply anesthetized with chloroform and transcardially perfused by injecting 0.9% saline and 4% paraformaldehyde (Scharlau, Spain). The brains were collected and kept in a fixative solution (4% paraformaldehyde) for the next 7 days. Afterward, the automated tissue processor (Did Sabz, Iran) was used to histologically process brain samples, which were finally embedded in paraffin blocks. Paraffin blocks were cut using a rotary microtome to a thickness of 6 µm coronal sections from the hippocampus at 20 μm intervals between each two successive sections (Moghadami et al., 2016). The sections were immunostained for GFAP, a specific marker for astrocytes.
Immunohistochemistry staining for GFAP-ir astrocytes
GFAP-ir astrocytes were evaluated by immunohistochemistry staining (Nikmahzar et al., 2019; Shaabani et al., 2011). In this regard, the sections were immersed in xylene to deparaffinize and rehydrate in graded ethanol. The antigen was retrieved in retrieval solution (pH= 9, Tashkhis Baft Arajen, Iran) for 20 minutes at 90-95 °C using a laboratory water bath. Next, the slides were cooled at room temperature and rinsed with washing buffer (phosphate buffered saline [PBS]/Tween 20 in 0.1% Triton X-100). In order to quench endogenous peroxidase activity, brain sections were incubated in 0.3% hydrogen peroxide and methanol for 10 minutes at room temperature. After rinsing in the washing buffer, brain sections were incubated with avidin/biotin blocking solution (Dako, Denmark) for 30 minutes at room temperature and rinsed in the washing buffer. To block nonspecific reactivity, brain sections were incubated with 1% bovine serum albumin (BSA) blocking solution for 60 minutes at 37 °C temperature. Then, brain sections were incubated in primary anti-rabbit polyclonal GFAP antibody (ab16997, 1:100, Abcam Inc., USA) for 120 minutes at 37 °C. After washing in buffer, the sections were incubated in secondary biotinylated goat anti-rabbit IgG antibody (ab64256, Abcam Inc., USA) for 60 minutes at 37 °C and then rinsed with washing buffer. Afterward, the brain sections were probed with streptavidin HRP protein (1:5000, Abcam Inc., USA) for 30 minutes at room temperature. Finally, the brain sections were covered with the diaminobenzidine solution (Dako, Denmark) and rinsed gently with distilled water. After background staining with Meyer’s hematoxylin for 3-4 seconds, the brain sections were dehydrated in graded ethanol, cleared in xylene, and mounted with entellan glue (Merck, Germany).
Imaging and counting GFAP-ir astrocytes
Pictures were captured using a digital camera (Model: DP73, Olympus, Japan) connected to a light microscope (Model: BX 53, Olympus, Japan) with 40× magnification for CA1, CA3, and DG regions of the hippocampus. The number of GFAP-ir astrocytes was counted in a 30000 μm2 area at three regions of the hippocampus by the cellSens standard 1.14 software (Olympus, Japan). Imaging and counting were performed blinded to treatment.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16 (Armonk, NY, USA). All data were expressed as Mean±SD, and the Shapiro-Wilk test assessed data normality. Behavioral assessments and the histological data were analyzed by one-way analysis of variance (ANOVA) with LSD post-hoc test. P<0.05 was regarded as a significant difference.
Results
L-α-AAA administration into the hippocampal CA1 Area and the passive avoidance memory function
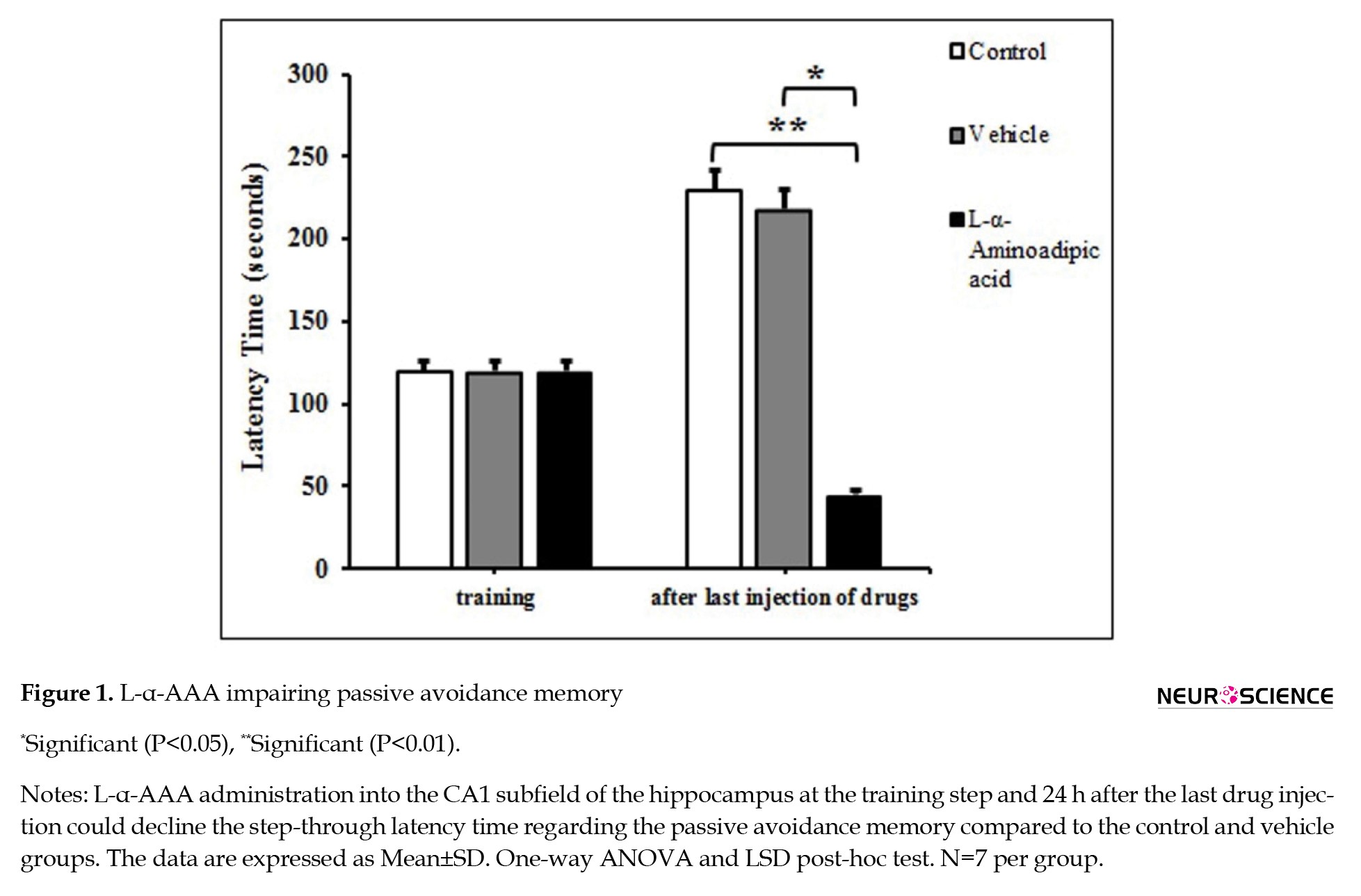
Figure 1 shows that all groups’ step-through latency time in the training step was 120 seconds.
Astrocytes, the most abundant neuroglial in the central nervous system (CNS), have vital roles in brain homeostasis, neuroprotection, synaptic plasticity, uptake and release of neurotransmitters, and innate immunity (Acosta et al., 2017; Dallérac & Rouach, 2016; Navarrete & Araque, 2014; Olsen et al., 2018; Shang et al., 2015; Soung & Klein, 2020; Zorec et al., 2015). Astrocytes modulate anti-inflammatory processes and regulate microglia’s function during brain injury through secreting cytokines (Lima et al., 2014; Tarasov et al., 2020). Moreover, they maintain the glutamate and GABA (gamma-aminobutyric acid) neurotransmitter reservoirs by expressing pyruvate carboxylase, the enzyme required to synthesize these two amino acids (Schousboe et al., 2013). Inflammatory cytokines control astrocytes-released glutamate. Hence, glia-to-neuron signaling may be affected by cytokine mediators in pathological conditions (Vesce et al., 2007). Astrocytes respond to neuronal activity by expressing several receptors and altering homeostasis, creating a neuron-astrocyte cross-talk. Abnormal conditions could affect the neuron-astrocyte integrity and disturb cognitive functions and final behavior output. In addition to the typical roles of astrocytes, recent research has been focusing on their cognitive functions (Hosseini et al., 2020; Santello et al., 2019; Suzuki et al., 2011).
Previous studies found astrocyte plasticity in rat hippocampus after spatial working memory (Jahanshahi et al., 2008; Mehrdad et al., 2007). Indeed, enhanced expression of glial fibrillary acidic protein (GFAP) and astrocyte density after learning showed astrocytes’ recruitment in cognition (Dallérac & Rouach, 2016). GFAP is an intermediate filament mainly found in mature astrocytes’ cytoskeleton structure (Bronzuoli et al., 2018; Guillamón Vivancos et al., 2015). The mutations and abnormal expression of GFAP have been observed in neurodegeneration, neuroinflammation, and psychiatric disorders (Li et al., 2020). Therefore, specific inhibition of astrocytes may help investigate the contribution of reactive astrocytes to some neurodegenerative diseases.
L-α-aminoadipic acid (L-α-AAA) is a homolog of the excitatory amino acid glutamate, generally used to exert astrocyte-specific toxicity in vitro and in vivo studies (Guidetti & Schwarcz, 2003). Microinjection of L-α-AAA inhibits the glutamine synthetase that plays an essential role in the learning and consolidation of memories (Guidetti & Schwarcz, 2003; Robinson et al., 2015). It decreased long-term potentiation magnitude, which underlies memory impairment. It also reported that altered glutamate neurotransmission occurred in anxiety-like behavior related to traumatic brain injury in the amygdala (Beitchman et al., 2020). It is not clear how the ablation of astrocytes changes the memory and behavior. However, a combination of alternations in trophic support for neurons and glutamate cycle, astrocytes typically control that, has been proposed (David et al., 2019). The effect of hippocampal astrocytes on anxiety and memory has not been extensively investigated (Leitão, 2018). In this study, we aimed to evaluate the effect of astrocyte inhibition with L-α-AAA on inhibitory passive avoidance memory, anxiety-like behavior, and the density of GFAP-ir astrocytes in rat hippocampus.
2. Materials and Methods
Study animals
A total of 21 adult male rats of the Wistar strain (180–220 g) were obtained from the animal house of Golestan University of Medical Sciences. The rats were placed in the cages at 22±2 ºC, 12 hours light/dark cycle with access to food and water ad libitum. All procedures followed the principles of the Ethical Board of Golestan University of Medical Sciences (Gorgan, Iran).
Stereotaxic procedure and L-α-AAA microinjection into the hippocampal CA1 subfield
According to our previous studies, the intrahippocampal injection was performed with minor modifications (Azami et al., 2010; Jahanshahi et al., 2018; Moghadami et al., 2016). Briefly, the rats were anesthetized for stereotaxic surgery (David Kopf Instruments, USA) using an intraperitoneal injection of ketamine (100 mg/mL) and xylazine (20 mg/mL). Stainless guide cannulas (21-gauge) were bilaterally implanted at the dorsal CA1 hippocampal area; the coordinates were anterior-posterior: −3 mm from bregma; medial-lateral: ±2 mm from midline; and dorsal-ventral: −2 mm from the skull surface (Paxinos & Watson, 2007). At the end of the procedure, each cannula was temporarily closed with a stainless-steel wire to preserve it from occlusion. Seven days after the surgery, 1 μL/rat (0.5 μL on each side) of L-α-AAA and or vehicle were injected into the CA1 hippocampal area by a Hamilton micro-syringe over one minute. The needle was kept in a cannula to prevent backflow. L-α-AAA (Sigma-Aldrich, China) was dissolved in normal saline containing 6% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, China) with pH=7. One day before and after the last injection of drugs, learning and memory were assessed by passive avoidance memory tasks. The EPM was performed 1 day before and 30 minutes after the last injection of drugs.
Experimental design
The rats were randomly assigned into three groups (n=7): Control, with no intervention; vehicle, with stereotaxic surgery, received 6% 2-hydroxypropyl-β-cyclodextrin (vehicle) once per day for three consecutive days and evaluated for behavioral tests; experimental group (L-α-AAA), with stereotaxic surgery, received L-α-AAA at dose of 25 µg/µL once daily for three consecutive days and evaluated for behavioral test.
All drugs were injected into the dorsal CA1 hippocampal area. Rats subjected to inhibitory passive avoidance memory and anxiety assessments were habituated to the condition of the testing room for 1 h before performing the behavioral tests. The two behavioral tests were not performed on the same rats, and behavioral tests were done blind to treatment.
Behavioral tests
Passive avoidance memory test
The passive avoidance memory test was performed as described in our previous studies (Mahakizadeh et al., 2015; Seifhosseini et al., 2011). The apparatus (the shuttle box) was composed of two light and dark compartments (20×20×30 cm) with identical sizes that were divided by a manually operated gate (7×9 cm). The dark compartment had a stainless steel shock grid floor. Electric shocks were given using a stimulator (50 Hz, 1.5 mA intensity, 3 s). It has three steps: Habituation, training, and prob.
Habituation
Each rat was gently placed into the light compartment, and after 5 seconds, the gate was opened, and the rat was permitted to go into the dark compartment. Rats were excluded from the experiments if they delayed for more than 120 seconds to cross the dark compartment.
Training
The training was done 30 minutes after habituation. The rat was placed in the light compartment, and after 5 seconds, the gate was opened. When the rat entered the dark compartment with all four feet, the gate was closed, and the rat received an electrical foot shock (50 Hz, 1.5 mA intensity, 3 s). After 20 seconds, the rat was returned to the cage. One hundred and twenty seconds later, the rat was re-tested using a method similar to the one used in the prior trials; successful learning was defined as the rat remaining in the light compartment for 120 seconds.
Prob
One day after the last drug injection, the rat was placed in the light compartment, and then the step-through latency time to enter the dark compartment was recorded. The cut-off time for the retention trial was 300 seconds.
Elevated plus maze test (EPM)
The EPM test was used to assess anxiety-related behavior in rats (Karimi et al., 2014; Nikmahzar et al., 2016). Briefly, this plus-shaped apparatus was located at 50 cm in height. It is made up of two open arms (50×10 cm) and two closed arms (50×10×40 cm) opposite to each other, linked by a central square zone (10×10 cm). The test was performed by placing the rat on the center of EPM facing an open arm, and the rat was permitted to explore the maze for 5 minutes. During the step, the number of entries into both arms and the time spent in each arm was recorded. Arm entries were recorded when the rat entered all four paws into the EPM arm. The maze was cleaned with 70% ethanol between each step for each rat. Finally, the percentage of open and closed arms time, the percentage of open and closed arm entries, open arm latency, and pure index of locomotor activity were measured. Total arm entries into the open and closed arms were measured as a pure index of locomotor activity.
Tissue preparation
Twenty-four hours after the behavioral test, the rats were deeply anesthetized with chloroform and transcardially perfused by injecting 0.9% saline and 4% paraformaldehyde (Scharlau, Spain). The brains were collected and kept in a fixative solution (4% paraformaldehyde) for the next 7 days. Afterward, the automated tissue processor (Did Sabz, Iran) was used to histologically process brain samples, which were finally embedded in paraffin blocks. Paraffin blocks were cut using a rotary microtome to a thickness of 6 µm coronal sections from the hippocampus at 20 μm intervals between each two successive sections (Moghadami et al., 2016). The sections were immunostained for GFAP, a specific marker for astrocytes.
Immunohistochemistry staining for GFAP-ir astrocytes
GFAP-ir astrocytes were evaluated by immunohistochemistry staining (Nikmahzar et al., 2019; Shaabani et al., 2011). In this regard, the sections were immersed in xylene to deparaffinize and rehydrate in graded ethanol. The antigen was retrieved in retrieval solution (pH= 9, Tashkhis Baft Arajen, Iran) for 20 minutes at 90-95 °C using a laboratory water bath. Next, the slides were cooled at room temperature and rinsed with washing buffer (phosphate buffered saline [PBS]/Tween 20 in 0.1% Triton X-100). In order to quench endogenous peroxidase activity, brain sections were incubated in 0.3% hydrogen peroxide and methanol for 10 minutes at room temperature. After rinsing in the washing buffer, brain sections were incubated with avidin/biotin blocking solution (Dako, Denmark) for 30 minutes at room temperature and rinsed in the washing buffer. To block nonspecific reactivity, brain sections were incubated with 1% bovine serum albumin (BSA) blocking solution for 60 minutes at 37 °C temperature. Then, brain sections were incubated in primary anti-rabbit polyclonal GFAP antibody (ab16997, 1:100, Abcam Inc., USA) for 120 minutes at 37 °C. After washing in buffer, the sections were incubated in secondary biotinylated goat anti-rabbit IgG antibody (ab64256, Abcam Inc., USA) for 60 minutes at 37 °C and then rinsed with washing buffer. Afterward, the brain sections were probed with streptavidin HRP protein (1:5000, Abcam Inc., USA) for 30 minutes at room temperature. Finally, the brain sections were covered with the diaminobenzidine solution (Dako, Denmark) and rinsed gently with distilled water. After background staining with Meyer’s hematoxylin for 3-4 seconds, the brain sections were dehydrated in graded ethanol, cleared in xylene, and mounted with entellan glue (Merck, Germany).
Imaging and counting GFAP-ir astrocytes
Pictures were captured using a digital camera (Model: DP73, Olympus, Japan) connected to a light microscope (Model: BX 53, Olympus, Japan) with 40× magnification for CA1, CA3, and DG regions of the hippocampus. The number of GFAP-ir astrocytes was counted in a 30000 μm2 area at three regions of the hippocampus by the cellSens standard 1.14 software (Olympus, Japan). Imaging and counting were performed blinded to treatment.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16 (Armonk, NY, USA). All data were expressed as Mean±SD, and the Shapiro-Wilk test assessed data normality. Behavioral assessments and the histological data were analyzed by one-way analysis of variance (ANOVA) with LSD post-hoc test. P<0.05 was regarded as a significant difference.
Results
L-α-AAA administration into the hippocampal CA1 Area and the passive avoidance memory function
Figure 1 shows that all groups’ step-through latency time in the training step was 120 seconds.
Injection of L-α-AAA into the hippocampal CA1 area for three consecutive days significantly decreased the step-through latency time compared to the vehicle group (F(2, 15)=6.213, P=0.011). However, there was no significant difference in step-through latency time between the control and vehicle groups (Figure 1).
L-α-AAA administration into the hippocampal CA1 and increased anxiety-like behavior
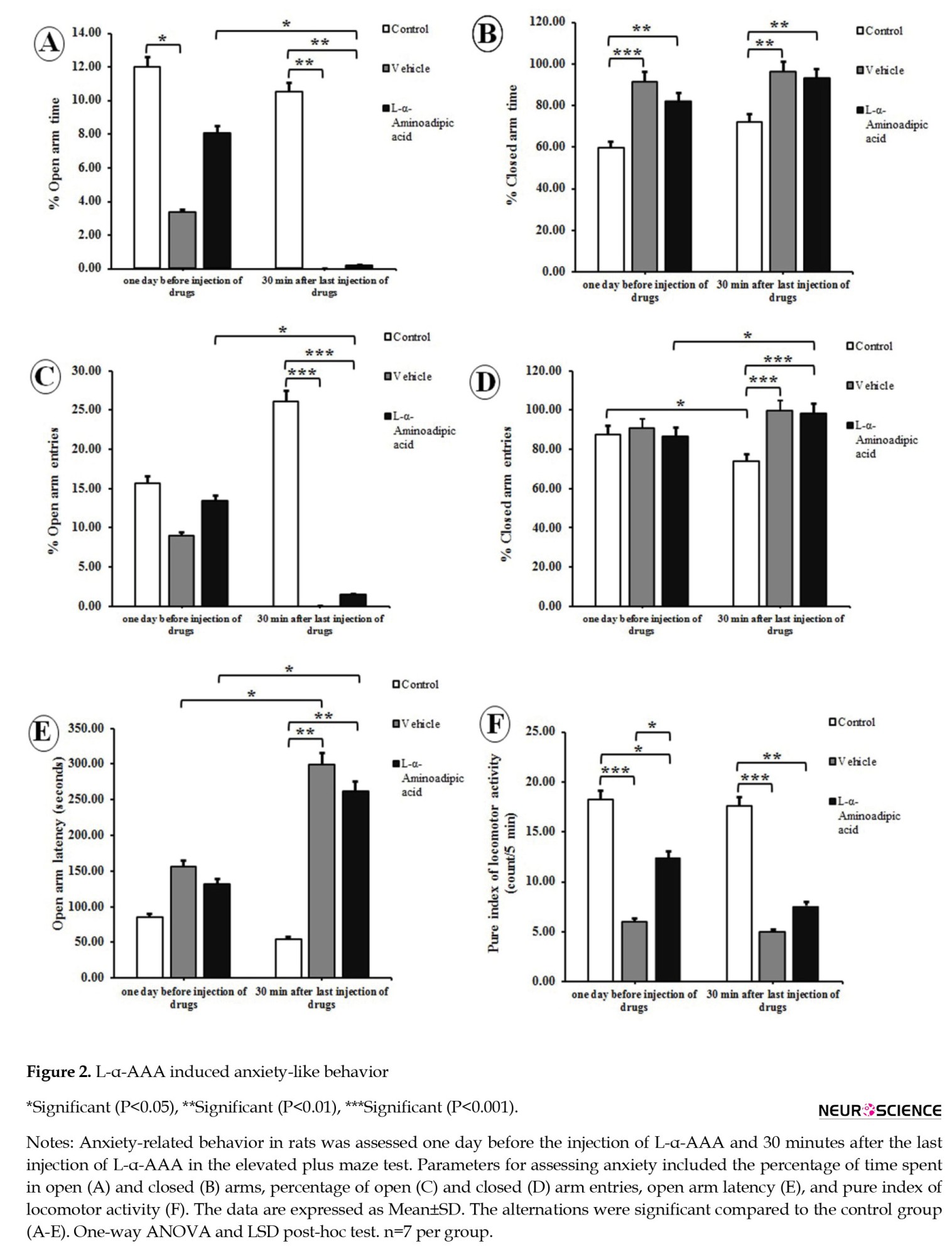
The data of the elevated plus maze revealed that L-α-AAA significantly decreased the percentage of time spent in the open arm (F(2, 12)=7.940; P=0.006) and significantly increased the percentage of time spent in the closed arm (F(2, 12)=7.280; P=0.009) compared to control rats (Figures 2 A and 2B).
L-α-AAA administration into the hippocampal CA1 and increased anxiety-like behavior
The data of the elevated plus maze revealed that L-α-AAA significantly decreased the percentage of time spent in the open arm (F(2, 12)=7.940; P=0.006) and significantly increased the percentage of time spent in the closed arm (F(2, 12)=7.280; P=0.009) compared to control rats (Figures 2 A and 2B).
L-α-AAA significantly reduced the percentage of time spent in the open arm 30 minutes after last injection compared to one day before injection of L-α-AAA (P<0.05, Figure 2A). Three consecutive daily doses of L-α-AAA significantly decreased the percentage of open arm entries (F(2, 12)=34.932; P=0.000) and increased the percentage of closed arm entries (F(2, 12)=34.932; P=0.000) compared to control rats (Figures 2C and 2D). It also significantly decreased the percentage of open-arm entries and increased the percentage of closed-arm entries 30 minutes after the last injection compared to one day before injection of L-α-AAA (P<0.05, Figures 2C and 2D). A significant increase in the open arm latency (F(2, 12)=22.107; P=0.000) was observed following the administration of L-α-AAA compared to control rats (Figure 2E). The data showed a significant reduction in the pure index of locomotor activity in L-α-AAA group (F(2, 12)=14.647; P=0.001) compared to control rats (Figue 2F). Moreover, there was significant difference in anxiety parameters in the elevated plus maze between control and vehicle-treated group rats (Figure 2).
L-α-AAA administration and the number of GFAP-ir astrocytes in the hippocampus
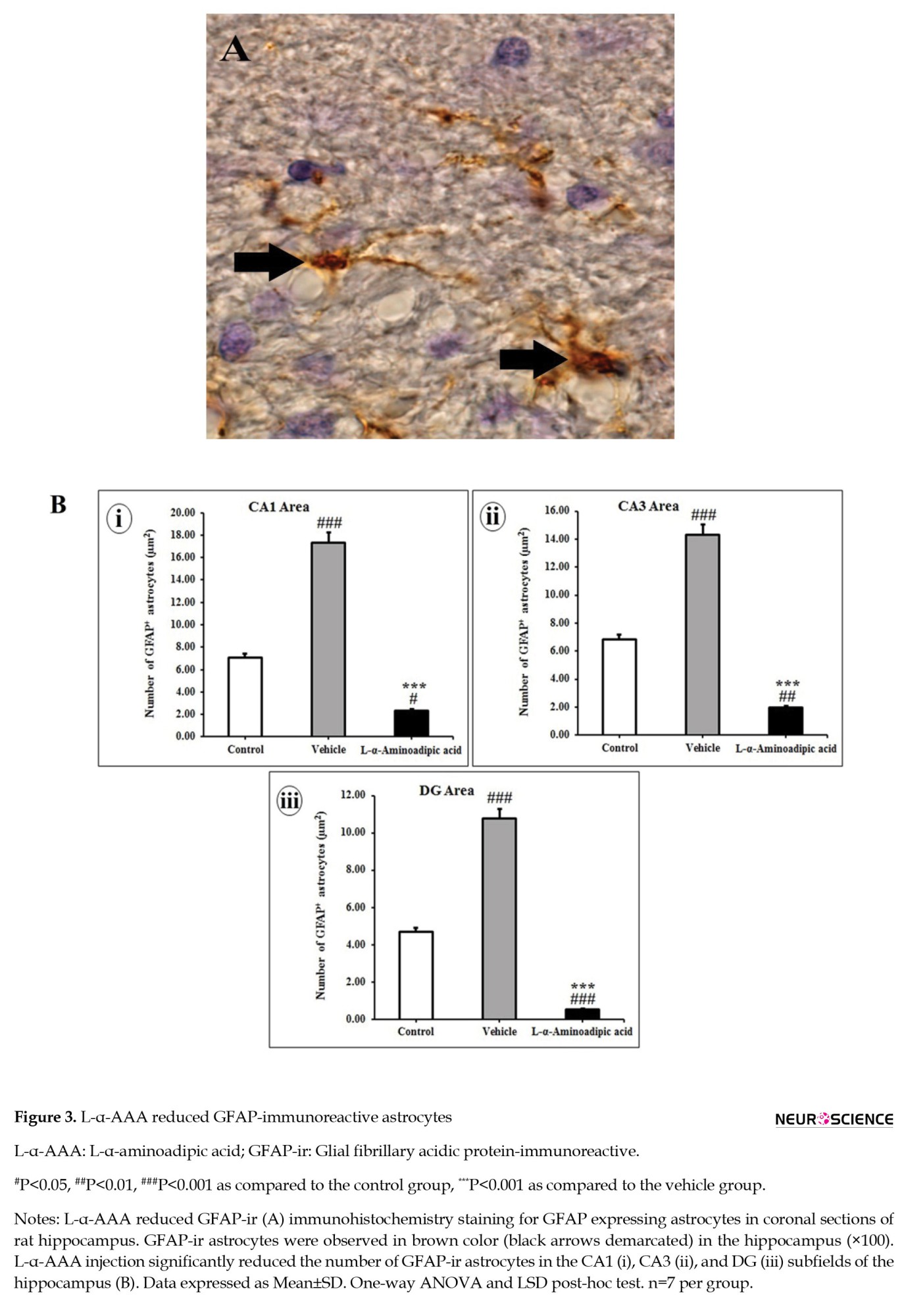
Three consecutive daily doses of vehicle administration into the hippocampal CA1 area significantly increased the number of GFAP-ir astrocytes in the CA1, CA3, and DG subfields of the hippocampus compared with control rats (P<0.001, Figures 3B, i, ii, and iii).
L-α-AAA administration and the number of GFAP-ir astrocytes in the hippocampus
Three consecutive daily doses of vehicle administration into the hippocampal CA1 area significantly increased the number of GFAP-ir astrocytes in the CA1, CA3, and DG subfields of the hippocampus compared with control rats (P<0.001, Figures 3B, i, ii, and iii).
L-α-AAA injected rats exhibited a significant decline in the number of GFAP-ir astrocytes in the CA1, CA3, and DG subfields of the hippocampus three days after administration compared to vehicle rats (P<0.001; Figures 3Bi, 3Bii, and 3Biii). The number of GFAP-ir astrocytes significantly decreased after administration of L-α-AAA compared to control rats in the CA1 (F(2, 55)=29.742; P=0.000), CA3 (F(2, 55)=39.497; P=0.000) and DG (F(2, 55)=61.143; P=0.000) subfields of hippocampus (Figures 3Bi, 3Bii, and 3Biii).
4. Discussion
The present study revealed that L-α-AAA microinjection into the CA1 subfield of the hippocampus could impair the passive avoidance memory and increase the anxiety-like behavior in male rats. The number of GFAP-ir astrocytes in the hippocampus decreased after astrocyte ablation.
Substances such as fluoroacetate, fluorocitrate, methionine sulfoximine, and ethacrynic acid are metabolic inhibitors in astrocytes and in association with memory impairment (Dallérac & Rouach, 2016; Fonnum et al., 1997). It has been proven that the gliotoxin, L-α-AAA, impaired medial prefrontal cortex-depended cognitive functions, working memory, and reversal learning (Lima et al., 2014). Pereira et al. (2021) reported that L-α-AAA significantly decreased recognition memory, a hippocampal memory, in mice. In agreement with these findings, our data showed that inhibition of astrocytes by injection of L-α-AAA diminished passive avoidance memory, a hippocampus-related memory.
Inside the brain, glutamine synthetase, a regulator enzyme of the glutamate/glutamine cycle in neurons, is produced by astrocytes (Son et al., 2019). The enzyme produces glutamine, a glutamate neurotransmitter involved in learning and memory. Studies revealed that inhibiting the enzyme caused memory impairment (Kant et al., 2014; Kulijewicz Nawrot et al., 2013; Lima et al., 2014; Robinson et al., 2015). L-α-AAA, as a glutamate analog, inhibits glutamine synthetase (David et al., 2018; Guidetti & Schwarcz, 2003; Pereira et al., 2021). Hence, the cause of memory impairment that we observed in our study may be the inhibition of glutamine synthetase by L-α-AAA.
The glutamatergic system plays a significant role in anxiety disorders (Kaur & Singh, 2017). Banasr and Duman reported that L-α-AAA infusion (100 µg/µL) in the rat prefrontal cortex induced anxiety in the novelty-suppressed feeding test (Banasr & Duman, 2008). According to this report, we observed that L-α-AAA microinjection into the CA1 subfield of the hippocampus could increase the anxiety-like behavior in the elevated plus maze.
Previous studies have displayed that the number of hippocampal astrocytes increased after different vehicle microinjections (such as dimethyl sulfoxide and normal saline) in rat hippocampus (Emamian et al., 2010; Jahanshahi et al., 2012). Also, it is reported that 2-hydroxypropyl-β-cyclodextrin injection enhanced astrocytic activity (GFAP intensity) in the cerebellum (Jeong et al., 2019). The current study found that intra-CA1 injection of vehicle (6% 2-Hydroxypropyl-β-cyclodextrin) increased the density of GFAP-ir astrocytes in the hippocampus compared to control rats. While L-α-AAA reduced the density of GFAP-ir astrocytes in the hippocampus after three consecutive daily doses. It is claimed that L-α-AAA downregulates the mRNA expression of GFAP in astrocytes moreover, induces cell death in cultured astrocytes (David et al., 2018; Nishimura et al., 2000). After L-α-AAA injection into the substantia nigra, locus coeruleus, and amygdala, the amount of GFAP-ir astrocytes reduces (Chang et al., 1993; Khurgel et al., 1996). Microinjection of 6.4 nmol L-α-AAA within 3 days caused a transient loss of GFAP-ir astrocytes in the rat hippocampus (Rodríguez et al., 2004). Also, the density of GFAP-ir astrocytes decreased after microinjection of L-α-AAA (50 and 100 µg/µL) to the rodent pre-limbic and medial prefrontal cortex (Banasr & Duman, 2008; David et al., 2018; Domin et al., 2014; Lee et al., 2013). In another study, L-α-AAA injection (20 µg/µL) led to the ablation of GFAP-ir astrocytes in the rat medial prefrontal cortex (Lima et al., 2014) and one day after injection of L-α-AAA at a dose of 25 nmol, GFAP mRNA expression declined in the rat anterior cingulate cortex (Chen et al., 2012). Injection of L-α-AAA (50 µg/µL, 2 injections) into the corpus callosum reduced the density of GFAP-ir astrocytes and expression of GFAP in demyelination model mice (Madadi et al., 2019).
Alterations of hippocampal astrocytes may be linked to cognitive deficits (David et al., 2019). Moreover, it is reported that the density of GFAP-ir astrocytes decreased in the pre-limbic cortex and CA3 area of the hippocampus for up to 72 h after administration of L-α-AAA in rodents (David et al., 2019). A more recent study found that ICV-injected L-α-aminoadipate reduced the immunoreactivity of GFAP but also declined the S100β, the other marker of astrocytes in CA1 and CA3 regions. Taken together, these findings support the gliotoxicity of L-α-AAA on hippocampal astrocytes, leading to memory deficiency and anxiety-like behavior. Further, noticing the important role of astrocytes in anxiety-like behaviors will emerge new ideas about anxiety and the brain.
5. Conclusion
This study focused on the role of astrocytes in memory function and anxiety-like behaviors. It concluded that L-α-AAA-related inhibited astrocytes impaired passive avoidance memory and increased anxiety-like behavior, which can be considered in future therapeutic strategies.
Ethical Considerations
Compliance with ethical guidelines
All experiments followed the Ethical Committee of Golestan University of Medical Sciences, Gorgan, Iran (Code: 1394.206).
Funding
This work was supported by the Deputy of Research and Technology, Golestan University of Medical Sciences, Gorgan, Iran (Grant No.: 940819197).
Authors' contributions
Conceptualisation, study design, review and editing: Mehrdad Jahanshahi; Data collection: Mehrdad Jahanshahi, Leila Elyasi, and Emsehgol Nikmahzar; Data analysis and writing the original draft: Leila Elyasi and Emsehgol Nikmahzar; Data interpretation: Emsehgol Nikmahzar; final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Neuroscience Research Center for the Histological and Behavioral Experiments and the Research and Technology Deputy of Golestan University of Medical Sciences, Gorgan, Iran for financial support.
References
Acosta, C., Anderson, H. D., & Anderson, C. M. (2017). Astrocyte dysfunction in Alzheimer disease. Journal of Neuroscience Research, 95(12), 2430–2447. [DOI:10.1002/jnr.24075] [PMID]
Azami, N. S., Piri, M., Oryan, S., Jahanshahi, M., Babapour, V., & Zarrindast, M. R. (2010). Involvement of dorsal hippocampal alpha-adrenergic receptors in the effect of scopolamine on memory retrieval in inhibitory avoidance task. Neurobiology of Learning and Memory, 93(4), 455–462. [DOI:10.1016/j.nlm.2010.01.003] [PMID]
Banasr, M., & Duman, R. S. (2008). Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry, 64(10), 863–870. [DOI:10.1016/j.biopsych.2008.06.008] [PMID]
Beitchman, J. A., Griffiths, D. R., Hur, Y., Ogle, S. B., Bromberg, C. E., & Morrison, H. W., et al. (2020). Experimental traumatic brain injury induces chronic glutamatergic dysfunction in amygdala circuitry known to regulate anxiety-like behavior. Frontiers in Neuroscience, 13, 1434. [DOI:10.3389/fnins.2020.00212] [PMID]
Bronzuoli, M. R., Facchinetti, R., Steardo, L., Jr, Romano, A., Stecca, C., & Passarella, S., et al. (2018). Palmitoylethanolamide dampens reactive astrogliosis and improves neuronal trophic support in a triple transgenic Model of Alzheimer's Disease: In vitro and in vivo evidence. Oxidative Medicine and Cellular Longevity, 2018, 4720532. [DOI:10.1155/2018/4720532] [PMID]
Chang, F. W., Wang, S. D., Lu, K. T., & Lee, E. H. (1993). Differential interactive effects of gliotoxin and MPTP in the substantia nigra and the locus coeruleus in BALB/c mice. Brain Research Bulletin, 31(3-4), 253–266. [DOI:10.1016/0361-9230(93)90215-w] [PMID]
Chen, F. L., Dong, Y. L., Zhang, Z. J., Cao, D. L., Xu, J., & Hui, J., et al. (2012). Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Research Bulletin, 87(1), 60–66. [DOI:10.1016/j.brainresbull.2011.09.022] [PMID]
Dallérac, G., & Rouach, N. (2016). Astrocytes as new targets to improve cognitive functions. Progress in Neurobiology, 144, 48–67. [DOI:10.1016/j.pneurobio.2016.01.003] [PMID]
David, J., Gormley, S., McIntosh, A. L., Kebede, V., Thuery, G., & Varidaki, A., et al. (2019). L-alpha-amino adipic acid provokes depression-like behaviour and a stress related increase in dendritic spine density in the pre-limbic cortex and hippocampus in rodents. Behavioural Brain Research, 362, 90–102. [DOI:10.1016/j.bbr.2019.01.015] [PMID]
David, J., O'Toole, E., O'Reilly, K., Thuery, G., Assmann, N., & Finlay, D., et al. (2018). Inhibitors of the NMDA-nitric oxide signaling pathway protect against neuronal atrophy and synapse loss provoked by l-alpha aminoadipic acid-treated astrocytes. Neuroscience, 392, 38–56. [DOI:10.1016/j.neuroscience.2018.09.023] [PMID]
Domin, H., Szewczyk, B., Woźniak, M., Wawrzak-Wleciał, A., & Śmiałowska, M. (2014). Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behavioural Brain Research, 273, 23–33. [DOI:10.1016/j.bbr.2014.07.019] [PMID]
Emamian, S., Naghdi, N., Sepehri, H., Jahanshahi, M., Sadeghi, Y., & Choopani, S. (2010). Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behavioural Brain Research, 208(1), 30–37. [DOI:10.1016/j.bbr.2009.11.004] [PMID]
Fonnum, F., Johnsen, A., & Hassel, B. (1997). Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia, 21(1), 106-113. [DOI:10.1002/(SICI)1098-1136(199709)21:13.0.CO;2-W]
Guidetti, P., & Schwarcz, R. (2003). Determination of alpha-aminoadipic acid in brain, peripheral tissues, and body fluids using GC/MS with negative chemical ionization. Brain Research. Molecular Brain Research, 118(1-2), 132–139. [DOI:10.1016/j.molbrainres.2003.08.004] [PMID]
Guillamón-Vivancos, T., Gómez-Pinedo, U., & Matías-Guiu, J. (2015). Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia, 30(2), 119–129. [DOI:10.1016/j.nrl.2012.12.007] [PMID]
Hosseini, S., Michaelsen-Preusse, K., Grigoryan, G., Chhatbar, C., Kalinke, U., & Korte, M. (2020). Type I interferon receptor signaling in astrocytes regulates hippocampal synaptic plasticity and cognitive function of the healthy CNS. Cell Reports, 31(7), 107666. [DOI:10.1016/j.celrep.2020.107666] [PMID]
Jahanshahi, M., Azami, N. S., & Nickmahzar, E. (2012). Effect of scopolamine-based amnesia on the number of astrocytes in the rat’s hippocampus. International Journal of Morphology, 30(2), 388-393. [DOI:10.4067/S0717-95022012000200004]
Jahanshahi, M., Nikmahzar, E., Elyasi, L., Babakordi, F., & Hooshmand, E. (2018). α2-Adrenoceptor-ir neurons' density changes after single dose of clonidine and yohimbine administration in the hippocampus of male rat. The International Journal of Neuroscience, 128(5), 404–411. [DOI:10.1080/00207454.2017.1389926] [PMID]
Jahanshahi, M., Sadeghi, Y., Hosseini, A., Naghdi, N., & Marjani, A. (2008). The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singapore Medical Journal, 49(5), 388-391. [Link]
Mehrdad, J., Sadeghi, Y., Hosseini, A., & Naghdi, N. (2007). The astrocytes number in different subfield of rat's hippocampus in reference memory learning method. Pakistan Journal of Biological Sciences, 10(21), 3964–3966. [DOI:10.3923/pjbs.2007.3964.3966] [PMID]
Jeong, M. S., Bae, J. S., & Jin, H. K. (2019). Vascular endothelial growth factor improves the therapeutic effects of cyclodextrin in Niemann-Pick type C mice. Animal Cells and Systems, 23(5), 346–354. [DOI:10.1080/19768354.2019.1651768] [PMID]
Kant, D., Tripathi, S., 1st, Qureshi, M. F., Tripathi, S., 2nd, Pandey, S., Singh, G., et al. (2014). The effect of glial glutamine synthetase inhibition on recognition and temporal memories in the rat. Neuroscience Letters, 560, 98–102. [DOI:10.1016/j.neulet.2013.12.033] [PMID]
Karimi, S., Jahanshahi, M., & Golalipour, M. J. (2014). The effect of MDMA-induced anxiety on neuronal apoptosis in adult male rats' hippocampus. Folia Biologica, 60(4), 187–191. [DOI:10.14712/fb2014060040187] [PMID]
Kaur, S., & Singh, R. (2017). Role of different neurotransmitters in anxiety: A systemic review. International Journal of Pharmaceutical Sciences and Research, 8(2), 411. [DOI:10.25258/ijcprr.v8i01.9088]
Khurgel, M., Koo, A. C., & Ivy, G. O. (1996). Selective ablation of astrocytes by intracerebral injections of α‐aminoadipate. Glia, 16(4), 351-358. [DOI:10.1002/(SICI)1098-1136(199604)16:43.0.CO;2-2]
Kulijewicz-Nawrot, M., Syková, E., Chvátal, A., Verkhratsky, A., & Rodríguez, J. J. (2013). Astrocytes and glutamate homoeostasis in Alzheimer's disease: A decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro, 5(4), 273–282. [DOI:10.1042/AN20130017] [PMID]
Lee, Y., Son, H., Kim, G., Kim, S., Lee, D. H., & Roh, G. S., et al. (2013). Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. Journal of Psychiatry & Neuroscience, 38(3), 183–191. [DOI:10.1503/jpn.120024] [PMID]
Leitão, C. I. M. (2018). [Impact of an astrocytic pathology on hippocampal memory [MA thesis] (Portuguese)]. Coimbra: University of Coimbra. [Link]
Li, D., Liu, X., Liu, T., Liu, H., Tong, L., & Jia, S., et al. (2020). Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia, 68(5), 878-897. [DOI:10.1002/glia.23734] [PMID]
Lima, A., Sardinha, V. M., Oliveira, A. F., Reis, M., Mota, C., & Silva, M. A., et al. (2014). Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Molecular Psychiatry, 19(7), 834–841. [DOI:10.1038/mp.2013.182] [PMID]
Madadi, S., Pasbakhsh, P., Tahmasebi, F., Mortezaee, K., Khanehzad, M., & Boroujeni, F. B., et al. (2019). Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metabolic Brain Disease, 34(2), 593–603. [DOI:10.1007/s11011-019-0385-9] [PMID]
Mahakizadeh, S., Jahanshahi, M., Haidari, K., & Shahbazi, M. (2015). Dopamine receptors gene expression in male rat hippocampus after administration of MDMA (Ecstasy). International Journal of Morphology, 33(1), 301-308. [DOI:10.4067/S0717-95022015000100048]
Moghadami, S., Jahanshahi, M., Sepehri, H., & Amini, H. (2016). Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat's hippocampus: Testosterone replacement compensates it. Behavioral and Brain Functions, 12(1), 5. [DOI:10.1186/s12993-016-0089-9] [PMID]
Navarrete, M., & Araque, A. (2014). The cajal school and the physiological role of astrocytes: a way of thinking. Frontiers in Neuroanatomy, 8, 33. [DOI:10.3389/fnana.2014.00033] [PMID]
Nikmahzar, E., Jahanshahi, M., Elyasi, L., Saeidi, M., Babakordi, F., & Bahlakeh, G. (2019). Human chorionic gonadotropin attenuates amyloid-β plaques induced by streptozotocin in the rat brain by affecting cytochrome c-ir neuron density. Iranian Journal of Basic Medical Sciences, 22(2), 166–172. [DOI:10.22038/ijbms.2018.31412.7569] [PMID]
Nikmahzar, E., Jahanshahi, M., Ghaemi, A., Naseri, G. R., Moharreri, A. R., & Lotfinia, A. A. (2016). Hippocampal serotonin-2A receptor-immunoreactive neurons density increases after testosterone therapy in the gonadectomized male mice. Anatomy & Cell Biology, 49(4), 259–272. [DOI:10.5115/acb.2016.49.4.259] [PMID]
Nishimura, R. N., Santos, D., Fu, S. T., & Dwyer, B. E. (2000). Induction of cell death by L-alpha-aminoadipic acid exposure in cultured rat astrocytes: Relationship to protein synthesis. Neurotoxicology, 21(3), 313–320. [PMID]
Olsen, M., Aguilar, X., Sehlin, D., Fang, X. T., Antoni, G., & Erlandsson, A., et al. (2018). Astroglial responses to amyloid-beta progression in a mouse model of alzheimer's disease. Molecular Imaging and Biology, 20(4), 605–614. [DOI:10.1007/s11307-017-1153-z] [PMID]
Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic coordinates. Edinburgh: Elsevier. [Link]
Pereira, M. F., Amaral, I. M., Lopes, C., Leitão, C., Madeira, D., & Lopes, J. P., et al. (2021). l-α-aminoadipate causes astrocyte pathology with negative impact on mouse hippocampal synaptic plasticity and memory. FASEB Journal, 35(8), e21726. [DOI:10.1096/fj.202100336R] [PMID]
Robinson, S. R., Lee, A., Bishop, G. M., Czerwinska, H., & Dringen, R. (2015). Inhibition of astrocytic glutamine synthetase by lead is associated with a slowed clearance of hydrogen peroxide by the glutathione system. Frontiers in Integrative Neuroscience, 9, 61. [DOI:10.3389/fnint.2015.00061] [PMID]
Rodríguez, M. J., Martínez-Sánchez, M., Bernal, F., & Mahy, N. (2004). Heterogeneity between hippocampal and septal astroglia as a contributing factor to differential in vivo AMPA excitotoxicity. Journal of Neuroscience Research, 77(3), 344–353. [DOI:10.1002/jnr.20177] [PMID]
Santello, M., Toni, N., & Volterra, A. (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nature Neuroscience, 22(2), 154–166. [DOI:10.1038/s41593-018-0325-8] [PMID]
Schousboe, A., Bak, L. K., & Waagepetersen, H. S. (2013). Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Frontiers in Endocrinology, 4, 102. [DOI:10.3389/fendo.2013.00102] [PMID]
Seifhosseini, S., Jahanshahi, M., Moghimi, A., & Aazami, N. S. (2011). The effect of scopolamine on avoidance memory and hippocampal neurons in male Wistar rats. Basic and Clinical Neuroscience, 3(1), 9-15. [Link]
Shaabani, R., Jahanshahi, M., Nowrouzian, M., Sadeghi, Y., & Azami, N. (2011). Effect of morphine based CPP on the hippocampal astrocytes of male Wistar rats. Asian Journal of Cell Biology, 6(3), 89-96. [DOI:10.3923/ajcb.2011.89.96]
Shang, X. L., Wang, Q. B., Liu, X. P., Yao, X. Q., Cao, F. Y., & Wang, Q., et al. (2015). Fluorocitrate induced the alterations of memory-related proteins and tau hyperphosphorylation in SD rats. Neuroscience Letters, 584, 230–235. [DOI:10.1016/j.neulet.2014.10.036] [PMID]
Son, H., Kim, S., Jung, D. H., Baek, J. H., Lee, D. H., & Roh, G. S., et al. (2019). Insufficient glutamine synthetase activity during synaptogenesis causes spatial memory impairment in adult mice. Scientific Reports, 9(1), 252. [DOI:10.1038/s41598-018-36619-2] [PMID]
Soung, A., & Klein, R. S. (2020). Astrocytes: Initiators of and responders to inflammation. In T. Spohr (Ed.), Glia in health and disease (pp. 1-22). London: IntechOpen. [DOI:10.5772/intechopen.89760]
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., & Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell, 144(5), 810–823. [DOI:10.1016/j.cell.2011.02.018] [PMID]
Tarasov, V. V., Svistunov, A. A., Chubarev, V. N., Sologova, S. S., Mukhortova, P., & Levushkin, D., et al. (2020). Alterations of astrocytes in the context of schizophrenic dementia. Frontiers in Pharmacology, 10, 1612. [DOI:10.3389/fphar.2019.01612] [PMID]
Vesce, S., Rossi, D., Brambilla, L., & Volterra, A. (2007). Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. International Review of Neurobiology, 82, 57–71. [DOI:10.1016/S0074-7742(07)82003-4] [PMID]
Zorec, R., Horvat, A., Vardjan, N., & Verkhratsky, A. (2015). Memory Formation Shaped by Astroglia. Frontiers in Integrative Neuroscience, 9, 56. [DOI:10.3389/fnint.2015.00056] [PMID]
4. Discussion
The present study revealed that L-α-AAA microinjection into the CA1 subfield of the hippocampus could impair the passive avoidance memory and increase the anxiety-like behavior in male rats. The number of GFAP-ir astrocytes in the hippocampus decreased after astrocyte ablation.
Substances such as fluoroacetate, fluorocitrate, methionine sulfoximine, and ethacrynic acid are metabolic inhibitors in astrocytes and in association with memory impairment (Dallérac & Rouach, 2016; Fonnum et al., 1997). It has been proven that the gliotoxin, L-α-AAA, impaired medial prefrontal cortex-depended cognitive functions, working memory, and reversal learning (Lima et al., 2014). Pereira et al. (2021) reported that L-α-AAA significantly decreased recognition memory, a hippocampal memory, in mice. In agreement with these findings, our data showed that inhibition of astrocytes by injection of L-α-AAA diminished passive avoidance memory, a hippocampus-related memory.
Inside the brain, glutamine synthetase, a regulator enzyme of the glutamate/glutamine cycle in neurons, is produced by astrocytes (Son et al., 2019). The enzyme produces glutamine, a glutamate neurotransmitter involved in learning and memory. Studies revealed that inhibiting the enzyme caused memory impairment (Kant et al., 2014; Kulijewicz Nawrot et al., 2013; Lima et al., 2014; Robinson et al., 2015). L-α-AAA, as a glutamate analog, inhibits glutamine synthetase (David et al., 2018; Guidetti & Schwarcz, 2003; Pereira et al., 2021). Hence, the cause of memory impairment that we observed in our study may be the inhibition of glutamine synthetase by L-α-AAA.
The glutamatergic system plays a significant role in anxiety disorders (Kaur & Singh, 2017). Banasr and Duman reported that L-α-AAA infusion (100 µg/µL) in the rat prefrontal cortex induced anxiety in the novelty-suppressed feeding test (Banasr & Duman, 2008). According to this report, we observed that L-α-AAA microinjection into the CA1 subfield of the hippocampus could increase the anxiety-like behavior in the elevated plus maze.
Previous studies have displayed that the number of hippocampal astrocytes increased after different vehicle microinjections (such as dimethyl sulfoxide and normal saline) in rat hippocampus (Emamian et al., 2010; Jahanshahi et al., 2012). Also, it is reported that 2-hydroxypropyl-β-cyclodextrin injection enhanced astrocytic activity (GFAP intensity) in the cerebellum (Jeong et al., 2019). The current study found that intra-CA1 injection of vehicle (6% 2-Hydroxypropyl-β-cyclodextrin) increased the density of GFAP-ir astrocytes in the hippocampus compared to control rats. While L-α-AAA reduced the density of GFAP-ir astrocytes in the hippocampus after three consecutive daily doses. It is claimed that L-α-AAA downregulates the mRNA expression of GFAP in astrocytes moreover, induces cell death in cultured astrocytes (David et al., 2018; Nishimura et al., 2000). After L-α-AAA injection into the substantia nigra, locus coeruleus, and amygdala, the amount of GFAP-ir astrocytes reduces (Chang et al., 1993; Khurgel et al., 1996). Microinjection of 6.4 nmol L-α-AAA within 3 days caused a transient loss of GFAP-ir astrocytes in the rat hippocampus (Rodríguez et al., 2004). Also, the density of GFAP-ir astrocytes decreased after microinjection of L-α-AAA (50 and 100 µg/µL) to the rodent pre-limbic and medial prefrontal cortex (Banasr & Duman, 2008; David et al., 2018; Domin et al., 2014; Lee et al., 2013). In another study, L-α-AAA injection (20 µg/µL) led to the ablation of GFAP-ir astrocytes in the rat medial prefrontal cortex (Lima et al., 2014) and one day after injection of L-α-AAA at a dose of 25 nmol, GFAP mRNA expression declined in the rat anterior cingulate cortex (Chen et al., 2012). Injection of L-α-AAA (50 µg/µL, 2 injections) into the corpus callosum reduced the density of GFAP-ir astrocytes and expression of GFAP in demyelination model mice (Madadi et al., 2019).
Alterations of hippocampal astrocytes may be linked to cognitive deficits (David et al., 2019). Moreover, it is reported that the density of GFAP-ir astrocytes decreased in the pre-limbic cortex and CA3 area of the hippocampus for up to 72 h after administration of L-α-AAA in rodents (David et al., 2019). A more recent study found that ICV-injected L-α-aminoadipate reduced the immunoreactivity of GFAP but also declined the S100β, the other marker of astrocytes in CA1 and CA3 regions. Taken together, these findings support the gliotoxicity of L-α-AAA on hippocampal astrocytes, leading to memory deficiency and anxiety-like behavior. Further, noticing the important role of astrocytes in anxiety-like behaviors will emerge new ideas about anxiety and the brain.
5. Conclusion
This study focused on the role of astrocytes in memory function and anxiety-like behaviors. It concluded that L-α-AAA-related inhibited astrocytes impaired passive avoidance memory and increased anxiety-like behavior, which can be considered in future therapeutic strategies.
Ethical Considerations
Compliance with ethical guidelines
All experiments followed the Ethical Committee of Golestan University of Medical Sciences, Gorgan, Iran (Code: 1394.206).
Funding
This work was supported by the Deputy of Research and Technology, Golestan University of Medical Sciences, Gorgan, Iran (Grant No.: 940819197).
Authors' contributions
Conceptualisation, study design, review and editing: Mehrdad Jahanshahi; Data collection: Mehrdad Jahanshahi, Leila Elyasi, and Emsehgol Nikmahzar; Data analysis and writing the original draft: Leila Elyasi and Emsehgol Nikmahzar; Data interpretation: Emsehgol Nikmahzar; final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Neuroscience Research Center for the Histological and Behavioral Experiments and the Research and Technology Deputy of Golestan University of Medical Sciences, Gorgan, Iran for financial support.
References
Acosta, C., Anderson, H. D., & Anderson, C. M. (2017). Astrocyte dysfunction in Alzheimer disease. Journal of Neuroscience Research, 95(12), 2430–2447. [DOI:10.1002/jnr.24075] [PMID]
Azami, N. S., Piri, M., Oryan, S., Jahanshahi, M., Babapour, V., & Zarrindast, M. R. (2010). Involvement of dorsal hippocampal alpha-adrenergic receptors in the effect of scopolamine on memory retrieval in inhibitory avoidance task. Neurobiology of Learning and Memory, 93(4), 455–462. [DOI:10.1016/j.nlm.2010.01.003] [PMID]
Banasr, M., & Duman, R. S. (2008). Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry, 64(10), 863–870. [DOI:10.1016/j.biopsych.2008.06.008] [PMID]
Beitchman, J. A., Griffiths, D. R., Hur, Y., Ogle, S. B., Bromberg, C. E., & Morrison, H. W., et al. (2020). Experimental traumatic brain injury induces chronic glutamatergic dysfunction in amygdala circuitry known to regulate anxiety-like behavior. Frontiers in Neuroscience, 13, 1434. [DOI:10.3389/fnins.2020.00212] [PMID]
Bronzuoli, M. R., Facchinetti, R., Steardo, L., Jr, Romano, A., Stecca, C., & Passarella, S., et al. (2018). Palmitoylethanolamide dampens reactive astrogliosis and improves neuronal trophic support in a triple transgenic Model of Alzheimer's Disease: In vitro and in vivo evidence. Oxidative Medicine and Cellular Longevity, 2018, 4720532. [DOI:10.1155/2018/4720532] [PMID]
Chang, F. W., Wang, S. D., Lu, K. T., & Lee, E. H. (1993). Differential interactive effects of gliotoxin and MPTP in the substantia nigra and the locus coeruleus in BALB/c mice. Brain Research Bulletin, 31(3-4), 253–266. [DOI:10.1016/0361-9230(93)90215-w] [PMID]
Chen, F. L., Dong, Y. L., Zhang, Z. J., Cao, D. L., Xu, J., & Hui, J., et al. (2012). Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Research Bulletin, 87(1), 60–66. [DOI:10.1016/j.brainresbull.2011.09.022] [PMID]
Dallérac, G., & Rouach, N. (2016). Astrocytes as new targets to improve cognitive functions. Progress in Neurobiology, 144, 48–67. [DOI:10.1016/j.pneurobio.2016.01.003] [PMID]
David, J., Gormley, S., McIntosh, A. L., Kebede, V., Thuery, G., & Varidaki, A., et al. (2019). L-alpha-amino adipic acid provokes depression-like behaviour and a stress related increase in dendritic spine density in the pre-limbic cortex and hippocampus in rodents. Behavioural Brain Research, 362, 90–102. [DOI:10.1016/j.bbr.2019.01.015] [PMID]
David, J., O'Toole, E., O'Reilly, K., Thuery, G., Assmann, N., & Finlay, D., et al. (2018). Inhibitors of the NMDA-nitric oxide signaling pathway protect against neuronal atrophy and synapse loss provoked by l-alpha aminoadipic acid-treated astrocytes. Neuroscience, 392, 38–56. [DOI:10.1016/j.neuroscience.2018.09.023] [PMID]
Domin, H., Szewczyk, B., Woźniak, M., Wawrzak-Wleciał, A., & Śmiałowska, M. (2014). Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behavioural Brain Research, 273, 23–33. [DOI:10.1016/j.bbr.2014.07.019] [PMID]
Emamian, S., Naghdi, N., Sepehri, H., Jahanshahi, M., Sadeghi, Y., & Choopani, S. (2010). Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behavioural Brain Research, 208(1), 30–37. [DOI:10.1016/j.bbr.2009.11.004] [PMID]
Fonnum, F., Johnsen, A., & Hassel, B. (1997). Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia, 21(1), 106-113. [DOI:10.1002/(SICI)1098-1136(199709)21:13.0.CO;2-W]
Guidetti, P., & Schwarcz, R. (2003). Determination of alpha-aminoadipic acid in brain, peripheral tissues, and body fluids using GC/MS with negative chemical ionization. Brain Research. Molecular Brain Research, 118(1-2), 132–139. [DOI:10.1016/j.molbrainres.2003.08.004] [PMID]
Guillamón-Vivancos, T., Gómez-Pinedo, U., & Matías-Guiu, J. (2015). Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia, 30(2), 119–129. [DOI:10.1016/j.nrl.2012.12.007] [PMID]
Hosseini, S., Michaelsen-Preusse, K., Grigoryan, G., Chhatbar, C., Kalinke, U., & Korte, M. (2020). Type I interferon receptor signaling in astrocytes regulates hippocampal synaptic plasticity and cognitive function of the healthy CNS. Cell Reports, 31(7), 107666. [DOI:10.1016/j.celrep.2020.107666] [PMID]
Jahanshahi, M., Azami, N. S., & Nickmahzar, E. (2012). Effect of scopolamine-based amnesia on the number of astrocytes in the rat’s hippocampus. International Journal of Morphology, 30(2), 388-393. [DOI:10.4067/S0717-95022012000200004]
Jahanshahi, M., Nikmahzar, E., Elyasi, L., Babakordi, F., & Hooshmand, E. (2018). α2-Adrenoceptor-ir neurons' density changes after single dose of clonidine and yohimbine administration in the hippocampus of male rat. The International Journal of Neuroscience, 128(5), 404–411. [DOI:10.1080/00207454.2017.1389926] [PMID]
Jahanshahi, M., Sadeghi, Y., Hosseini, A., Naghdi, N., & Marjani, A. (2008). The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singapore Medical Journal, 49(5), 388-391. [Link]
Mehrdad, J., Sadeghi, Y., Hosseini, A., & Naghdi, N. (2007). The astrocytes number in different subfield of rat's hippocampus in reference memory learning method. Pakistan Journal of Biological Sciences, 10(21), 3964–3966. [DOI:10.3923/pjbs.2007.3964.3966] [PMID]
Jeong, M. S., Bae, J. S., & Jin, H. K. (2019). Vascular endothelial growth factor improves the therapeutic effects of cyclodextrin in Niemann-Pick type C mice. Animal Cells and Systems, 23(5), 346–354. [DOI:10.1080/19768354.2019.1651768] [PMID]
Kant, D., Tripathi, S., 1st, Qureshi, M. F., Tripathi, S., 2nd, Pandey, S., Singh, G., et al. (2014). The effect of glial glutamine synthetase inhibition on recognition and temporal memories in the rat. Neuroscience Letters, 560, 98–102. [DOI:10.1016/j.neulet.2013.12.033] [PMID]
Karimi, S., Jahanshahi, M., & Golalipour, M. J. (2014). The effect of MDMA-induced anxiety on neuronal apoptosis in adult male rats' hippocampus. Folia Biologica, 60(4), 187–191. [DOI:10.14712/fb2014060040187] [PMID]
Kaur, S., & Singh, R. (2017). Role of different neurotransmitters in anxiety: A systemic review. International Journal of Pharmaceutical Sciences and Research, 8(2), 411. [DOI:10.25258/ijcprr.v8i01.9088]
Khurgel, M., Koo, A. C., & Ivy, G. O. (1996). Selective ablation of astrocytes by intracerebral injections of α‐aminoadipate. Glia, 16(4), 351-358. [DOI:10.1002/(SICI)1098-1136(199604)16:43.0.CO;2-2]
Kulijewicz-Nawrot, M., Syková, E., Chvátal, A., Verkhratsky, A., & Rodríguez, J. J. (2013). Astrocytes and glutamate homoeostasis in Alzheimer's disease: A decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro, 5(4), 273–282. [DOI:10.1042/AN20130017] [PMID]
Lee, Y., Son, H., Kim, G., Kim, S., Lee, D. H., & Roh, G. S., et al. (2013). Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. Journal of Psychiatry & Neuroscience, 38(3), 183–191. [DOI:10.1503/jpn.120024] [PMID]
Leitão, C. I. M. (2018). [Impact of an astrocytic pathology on hippocampal memory [MA thesis] (Portuguese)]. Coimbra: University of Coimbra. [Link]
Li, D., Liu, X., Liu, T., Liu, H., Tong, L., & Jia, S., et al. (2020). Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia, 68(5), 878-897. [DOI:10.1002/glia.23734] [PMID]
Lima, A., Sardinha, V. M., Oliveira, A. F., Reis, M., Mota, C., & Silva, M. A., et al. (2014). Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Molecular Psychiatry, 19(7), 834–841. [DOI:10.1038/mp.2013.182] [PMID]
Madadi, S., Pasbakhsh, P., Tahmasebi, F., Mortezaee, K., Khanehzad, M., & Boroujeni, F. B., et al. (2019). Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metabolic Brain Disease, 34(2), 593–603. [DOI:10.1007/s11011-019-0385-9] [PMID]
Mahakizadeh, S., Jahanshahi, M., Haidari, K., & Shahbazi, M. (2015). Dopamine receptors gene expression in male rat hippocampus after administration of MDMA (Ecstasy). International Journal of Morphology, 33(1), 301-308. [DOI:10.4067/S0717-95022015000100048]
Moghadami, S., Jahanshahi, M., Sepehri, H., & Amini, H. (2016). Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat's hippocampus: Testosterone replacement compensates it. Behavioral and Brain Functions, 12(1), 5. [DOI:10.1186/s12993-016-0089-9] [PMID]
Navarrete, M., & Araque, A. (2014). The cajal school and the physiological role of astrocytes: a way of thinking. Frontiers in Neuroanatomy, 8, 33. [DOI:10.3389/fnana.2014.00033] [PMID]
Nikmahzar, E., Jahanshahi, M., Elyasi, L., Saeidi, M., Babakordi, F., & Bahlakeh, G. (2019). Human chorionic gonadotropin attenuates amyloid-β plaques induced by streptozotocin in the rat brain by affecting cytochrome c-ir neuron density. Iranian Journal of Basic Medical Sciences, 22(2), 166–172. [DOI:10.22038/ijbms.2018.31412.7569] [PMID]
Nikmahzar, E., Jahanshahi, M., Ghaemi, A., Naseri, G. R., Moharreri, A. R., & Lotfinia, A. A. (2016). Hippocampal serotonin-2A receptor-immunoreactive neurons density increases after testosterone therapy in the gonadectomized male mice. Anatomy & Cell Biology, 49(4), 259–272. [DOI:10.5115/acb.2016.49.4.259] [PMID]
Nishimura, R. N., Santos, D., Fu, S. T., & Dwyer, B. E. (2000). Induction of cell death by L-alpha-aminoadipic acid exposure in cultured rat astrocytes: Relationship to protein synthesis. Neurotoxicology, 21(3), 313–320. [PMID]
Olsen, M., Aguilar, X., Sehlin, D., Fang, X. T., Antoni, G., & Erlandsson, A., et al. (2018). Astroglial responses to amyloid-beta progression in a mouse model of alzheimer's disease. Molecular Imaging and Biology, 20(4), 605–614. [DOI:10.1007/s11307-017-1153-z] [PMID]
Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic coordinates. Edinburgh: Elsevier. [Link]
Pereira, M. F., Amaral, I. M., Lopes, C., Leitão, C., Madeira, D., & Lopes, J. P., et al. (2021). l-α-aminoadipate causes astrocyte pathology with negative impact on mouse hippocampal synaptic plasticity and memory. FASEB Journal, 35(8), e21726. [DOI:10.1096/fj.202100336R] [PMID]
Robinson, S. R., Lee, A., Bishop, G. M., Czerwinska, H., & Dringen, R. (2015). Inhibition of astrocytic glutamine synthetase by lead is associated with a slowed clearance of hydrogen peroxide by the glutathione system. Frontiers in Integrative Neuroscience, 9, 61. [DOI:10.3389/fnint.2015.00061] [PMID]
Rodríguez, M. J., Martínez-Sánchez, M., Bernal, F., & Mahy, N. (2004). Heterogeneity between hippocampal and septal astroglia as a contributing factor to differential in vivo AMPA excitotoxicity. Journal of Neuroscience Research, 77(3), 344–353. [DOI:10.1002/jnr.20177] [PMID]
Santello, M., Toni, N., & Volterra, A. (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nature Neuroscience, 22(2), 154–166. [DOI:10.1038/s41593-018-0325-8] [PMID]
Schousboe, A., Bak, L. K., & Waagepetersen, H. S. (2013). Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Frontiers in Endocrinology, 4, 102. [DOI:10.3389/fendo.2013.00102] [PMID]
Seifhosseini, S., Jahanshahi, M., Moghimi, A., & Aazami, N. S. (2011). The effect of scopolamine on avoidance memory and hippocampal neurons in male Wistar rats. Basic and Clinical Neuroscience, 3(1), 9-15. [Link]
Shaabani, R., Jahanshahi, M., Nowrouzian, M., Sadeghi, Y., & Azami, N. (2011). Effect of morphine based CPP on the hippocampal astrocytes of male Wistar rats. Asian Journal of Cell Biology, 6(3), 89-96. [DOI:10.3923/ajcb.2011.89.96]
Shang, X. L., Wang, Q. B., Liu, X. P., Yao, X. Q., Cao, F. Y., & Wang, Q., et al. (2015). Fluorocitrate induced the alterations of memory-related proteins and tau hyperphosphorylation in SD rats. Neuroscience Letters, 584, 230–235. [DOI:10.1016/j.neulet.2014.10.036] [PMID]
Son, H., Kim, S., Jung, D. H., Baek, J. H., Lee, D. H., & Roh, G. S., et al. (2019). Insufficient glutamine synthetase activity during synaptogenesis causes spatial memory impairment in adult mice. Scientific Reports, 9(1), 252. [DOI:10.1038/s41598-018-36619-2] [PMID]
Soung, A., & Klein, R. S. (2020). Astrocytes: Initiators of and responders to inflammation. In T. Spohr (Ed.), Glia in health and disease (pp. 1-22). London: IntechOpen. [DOI:10.5772/intechopen.89760]
Suzuki, A., Stern, S. A., Bozdagi, O., Huntley, G. W., Walker, R. H., & Magistretti, P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell, 144(5), 810–823. [DOI:10.1016/j.cell.2011.02.018] [PMID]
Tarasov, V. V., Svistunov, A. A., Chubarev, V. N., Sologova, S. S., Mukhortova, P., & Levushkin, D., et al. (2020). Alterations of astrocytes in the context of schizophrenic dementia. Frontiers in Pharmacology, 10, 1612. [DOI:10.3389/fphar.2019.01612] [PMID]
Vesce, S., Rossi, D., Brambilla, L., & Volterra, A. (2007). Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. International Review of Neurobiology, 82, 57–71. [DOI:10.1016/S0074-7742(07)82003-4] [PMID]
Zorec, R., Horvat, A., Vardjan, N., & Verkhratsky, A. (2015). Memory Formation Shaped by Astroglia. Frontiers in Integrative Neuroscience, 9, 56. [DOI:10.3389/fnint.2015.00056] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2023/09/6 | Accepted: 2024/04/8 | Published: 2025/01/1
Received: 2023/09/6 | Accepted: 2024/04/8 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |