Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 561-568 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sugumar S, Shah S, Hazeena P, Avadhani D, Murugan P, Arumugam M. A Preliminary Meta-analysis of Regulatory T-cell Reduction in Patients With Migraine. BCN 2024; 15 (5) :561-568
URL: http://bcn.iums.ac.ir/article-1-2727-en.html
URL: http://bcn.iums.ac.ir/article-1-2727-en.html
Subalakshmi Sugumar1 

 , Saman Shah2
, Saman Shah2 

 , Philo Hazeena3
, Philo Hazeena3 

 , Deepa Avadhani3
, Deepa Avadhani3 

 , Pavithra Murugan1
, Pavithra Murugan1 

 , Murugesan Arumugam *1
, Murugesan Arumugam *1 




 , Saman Shah2
, Saman Shah2 

 , Philo Hazeena3
, Philo Hazeena3 

 , Deepa Avadhani3
, Deepa Avadhani3 

 , Pavithra Murugan1
, Pavithra Murugan1 

 , Murugesan Arumugam *1
, Murugesan Arumugam *1 


1- Department of Pharmacology, Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
2- Department of Pathology, Hind Institute of Medical Sciences, Barabanki, India.
3- Department of Neurology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
2- Department of Pathology, Hind Institute of Medical Sciences, Barabanki, India.
3- Department of Neurology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
Keywords: Migraine, CD4+ T-cells, Regulatory T-cells, CD4+CD25+ regulatory T-cells, FOXP3, Autoimmunity
Full-Text [PDF 847 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Migraine is believed to be a chronic neurological disorder characterized by enervating, recurrent occurrences of unilateral throbbing headaches, afflicting about 10%-15% of the general population, with a preponderance for females (Andreou & Edvinsson, 2019). According to the global burden of disease study (2015), migraine is the most progressive neurological illness and the third leading cause of disability worldwide (Steiner et al., 2016). Migraine attacks involve a variety of neurological symptoms such as nausea, photophobia, phonophobia, osmophobia, exhaustion, and disruptions of autonomic, mental, sensory, and motor functioning, in addition to pain (Burstein, 2001). the international classification of headache disorders, third edition beta version (ICHD-III), categorizes migraine into two subtypes: Migraine without aura (MO) and migraine with aura (MA) (IHS, 2018).
The two basic ideas have dominated migraine research over the past three centuries: The vascular theory and the central neuronal theory (Isler, 1986 ; Tfelt-Hansen & Koehler, 2010). The efficacy of vasoconstrictors like ergotamine and triptans in treating acute migraine supported the vascular theory. Still, novel research shows that vasodilation is neither essential nor sufficient to trigger a migraine episode (Mason & Russo, 2018). Some studies have also indicated that migraine episodes can begin in areas of nociceptive neuromodulatory dysfunction in the brain stem (Welch, 2003; Brennan & Pietrobon, 2018). In addition, Levy and colleagues suggested that degranulation of meningeal mast cells, an inflammatory cell found in the intracranial region, may cause migraine (Levy et al., 2007). While numerous theories have been proposed for the etiology of migraine, none of these theories adequately explain the exact cause of migraine (Tfelt-Hansen & Koehler, 2010). Furthermore, there is no specific biomarker or diagnostic test for migraine, and it has often been misdiagnosed with other types of headaches.
On the other hand, there is emerging evidence that migraine may be caused by immune dysfunction (Arumugam & Narayan, 2019; Biscetti et al., 2021; Biscetti et al., 2022). In an earlier clinical study, our research group showed that CD4+CD25+ regulatory T-cells (Treg) were lower in migraine patients in comparison to healthy volunteers (Arumugam & Parthasarathy, 2016), which was the first study to identify levels of Treg variability in migraine patients. In support of this research, other clinical studies have also demonstrated a significant decrease in Treg cell levels in migraine patients (Faraji, et al., 2021; Nurkhametova et al., 2018; Li et al., 2022). However, a systematic analysis of the available data does not exist yet. Therefore, the current study attempted to analyze existing clinical data on decreasing levels of lymphocyte subsets, particularly the Treg population in migraine patients.
2. Materials and Methods
Literature search
A detailed literature search was conducted on PubMed, Scopus, Embase, ProQuest, Cochrane Review, Clinical trials, Academic thesis, American Academy of Neurology (AAN) resources, and Google Scholar databases from 2010 to 2022. The search details include “migraine in “regulatory T-cell,” “CD4+CD25+,” “inflammatory activity,” “autoimmune disorder,” “cytokines,” and “neuromodulators.” Each term’s singular and plural variations, as well as regional spelling variations, were recognized. The analysis w::::::as char::::::acterized based on the age, number of migraine patients and healthy volunteers, MO and MA, new interventions, and consequent parameters. Studies that met these criteria were used in the analysis. Meta-analysis was performed using appropriate software (Revman software, version 5.4).
Eligibility criteria
Only studies that met the following criteria were included in the analysis. Clinical studies were conducted with MO and MA patients, quantitative analyses of the T lymphocyte subset population, especially Treg cells, and studies conducted with patients without recent immune suppression therapies.
Data extraction
Data were extracted from eligible studies, and the average values and standard deviation for Treg cell levels were collected from the selected articles. The number of cases or controls, average age, gender ratio, intervention performed, and outcome parameters were taken for the analysis. Other clinically significant findings were also collected.
3. Results
Description of the included studies
Following the initial screening and eligibility criteria, 17 records were selected for qualitative analysis and 4 observational study records (Arumugam & Parthasarathy, 2016; Faraji, et al., 2021; Nurkhametova et al., 2018; Li et al., 2022) were taken for the final analysis (Figure 1). The inclusion criteria of the study were - parameters such as migraine type, age, comorbidity, and immunosuppressive treatments.
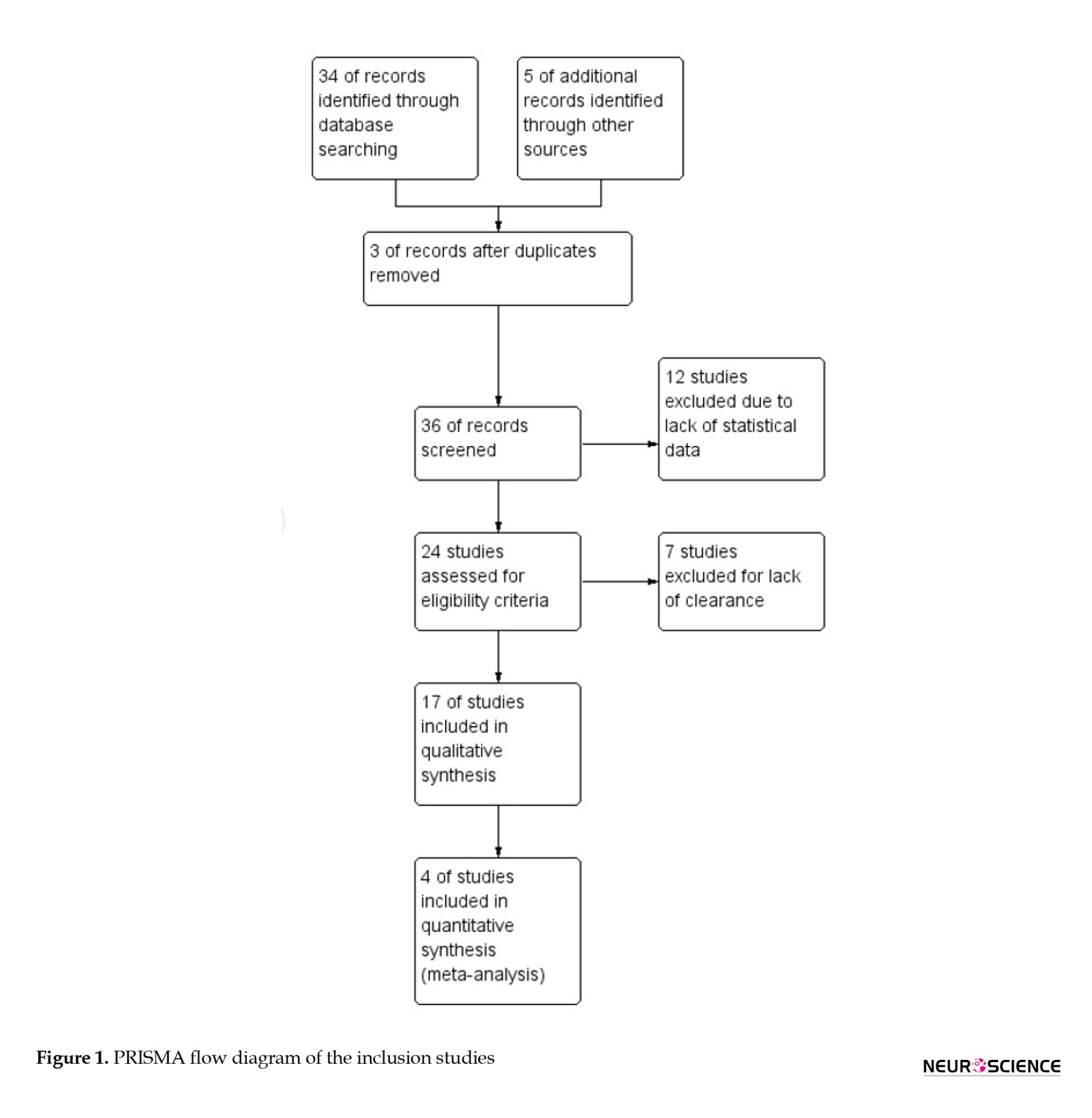
Analysis of the clinical studies in the pathophysiology of migraine
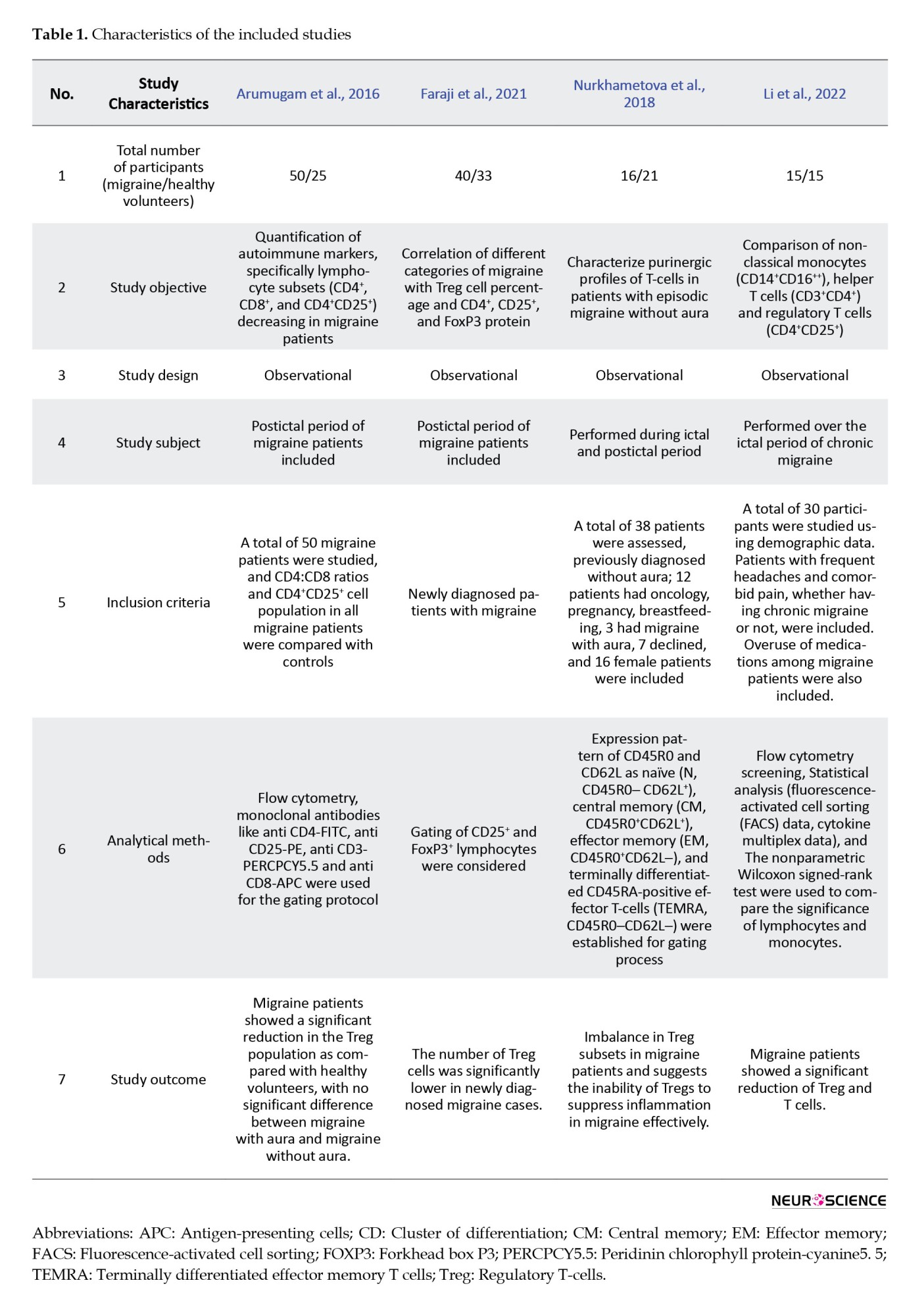
Throughout this study, 121 migraine patients (including MO and MA) and 94 healthy volunteers between the ages of 18 and 55 were included in the analysis (Table 1).
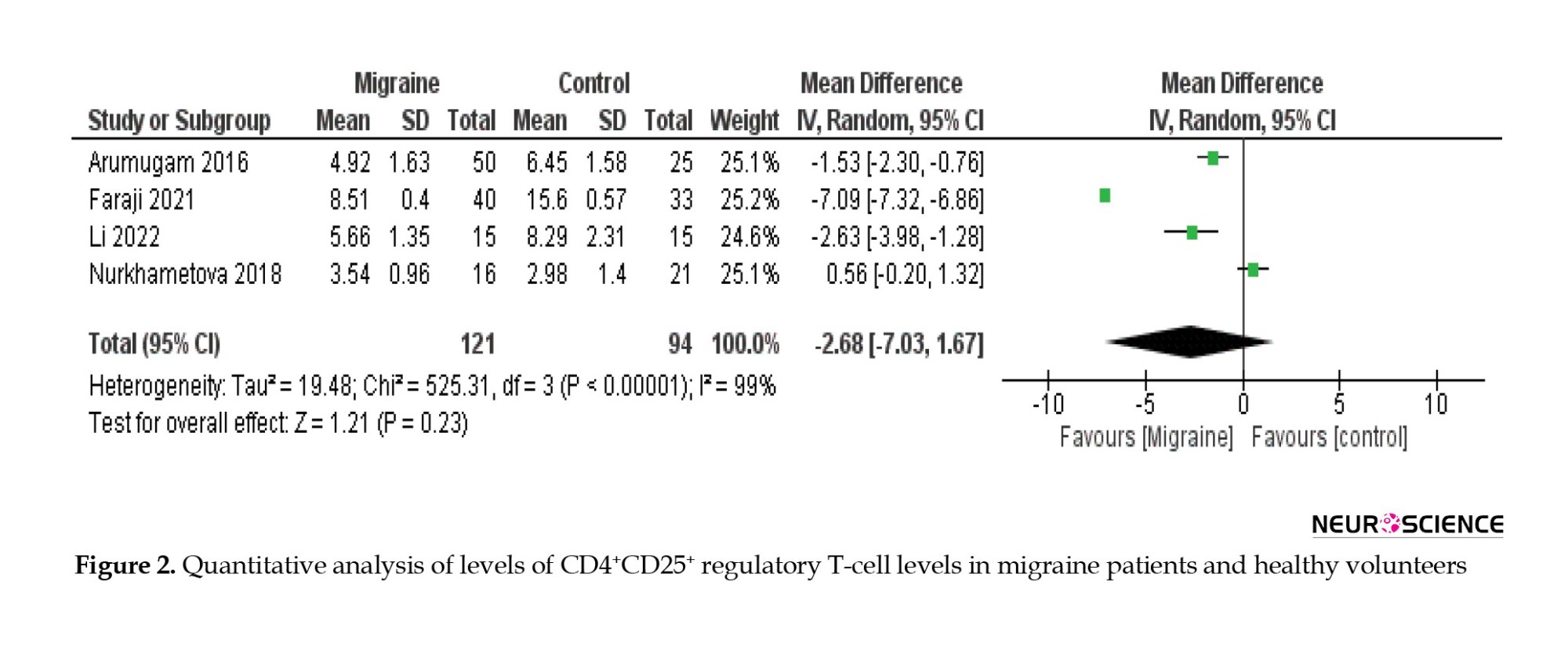
Changes in Treg cell levels were compared between migraine patients and healthy volunteers. The results show a significant reduction of Treg cell levels in migraine patients compared to healthy individuals, and the overall effect (Z=1.21; P=0.23) (Figure 2).
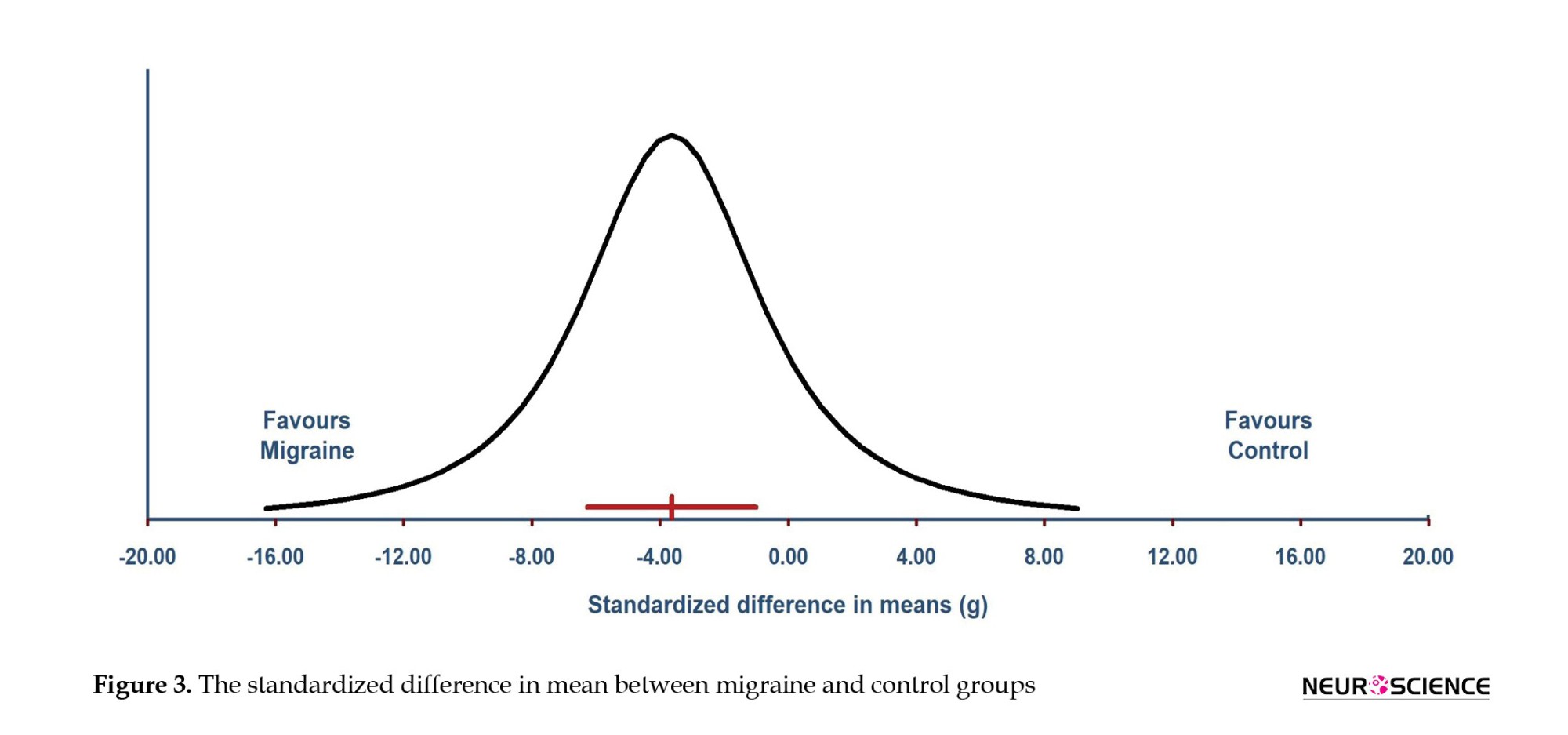
The standardized mean difference (SMD) shows -3.63 (P=0.007) with a 95% confidence interval of -6.28 to -0.99 (Figure 3). The true effect size of overall SMD in 95% CI of all comparable populations fall between the interval of -16.30 to 9.04 (Figure 3).
4. Discussion
The current meta-analysis data from 4 clinical studies show a substantial reduction of Treg cell levels in migraine patients compared to healthy volunteers. None of the studies found significant differences between MO and MA patients (Table 1). The Tregs are a specific subgroup of T cells, defined by the expression of CD4, CD25 (the IL-2 receptor a-chain), and the transcription factor forkhead box P3 (FOXP3) (Valencia & Lipsky, 2007). Treg cells are produced during thymocyte development and regulate the immunological response, preserving homeostasis and self-tolerance (Chang et al., 2005; Zhang e al., 2020; Genre et al., 2009). It can also inhibit the activation, proliferation, and effector functions of T-cells, natural killer cells, B-cells, and antigen-presenting cells (Sakaguchi et al., 2009). Thus, the reduced or abnormal regulation of Treg cells or mutation of FOXP3 enhances the probability of immunological imbalance in migraine patients. In addition, an increase in CD4+ and a decrease in CD8+ levels in migraine patients have been reported (Deng et al., 2019; Raphael et al., 2020; Arumugam & Parthasarathy, 2016). Variations in purinergic cells, such as CD4+, CD8+, CD39+, CD73+, and FOXP3 subsets, were also observed in migraine patients compared to healthy volunteers, and these outcomes are consistent with past reports (Mosnaim et al., 1998; Empl et al., 1999; Pavelek et al., 2020), indicating immune cells possible role in the physiopathology of migraine (Cseh et al., 2013; Leone et al., 1994).
However, numerous clinical studies show alteration in pro-inflammatory cytokines like IL-1, IL-6, and TNF, as well as anti-inflammatory cytokines like IL-1RA, IL-2, and IL-10, during migraine attacks (Boćkowski et al., 2010; Bruno et al., 2007; Yilmaz at al., 2010). In addition, a recent case report suggests that interferon-beta can induce or exacerbate migraine attacks during immunomodulation therapy in multiple sclerosis patients (Patti et al., 2012; Elmazny et al., 2020). These findings support the belief that immune cells, such as interferon beta, could also play a role in migraine attacks. Accordingly, contemporary approaches are opening the doors to recognizing the role of immune cells in migraine and raising the possibility of a link between immunological imbalance and migraine. Therefore, the current meta-analysis and existing evidence strongly suggest that immune cells can play a pivotal role in migraine pathogenesis.
For the first time in the literature, Treg cell levels were taken for meta-analysis about migraine research. The limitation of the study is that only four studies were analyzed. Nevertheless, exhaustive research is required to support the notion of a link between autoimmunity and migraine.
5. Conclusion
The meta-analysis of four clinical studies shows significant reduction of Treg cells in migraine patients compared to healthy volunteers. Decreased Treg levels support the theory that migraine could be due to immune dysfunction. More specific studies on the role of immune cells in the pathophysiology of migraine can contribute to a better understanding of migraine progression.
Ethical Considerations
Compliance with ethical guidelines
This study adhered to ethical standards in research and publication. Since this was a systematic review and meta-analysis of previously published data, no new human or animal subjects were included in this study. All data sources used in this study were obtained from publicly available, peer-reviewed articles. The original studies included in our analysis followed ethical guidelines, obtaining informed consent from participants and approval from relevant institutional review boards.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Subalakshmi Sugumar and Murugesan Arumugam; Study design: Philo Hazeena and Deepa Avadhani; Writing the original draft: Subalakshmi Sugumar; Review and editing: Subalakshmi Sugumar, Saman Shah, Pavithra Murugan and Murugesan Arumugam; Methodology, data curation, visualization and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are immensely thankful to the National Knowledge Network, India for providing valuable access to scientific journals and other relevant electronic data.
References
Migraine is believed to be a chronic neurological disorder characterized by enervating, recurrent occurrences of unilateral throbbing headaches, afflicting about 10%-15% of the general population, with a preponderance for females (Andreou & Edvinsson, 2019). According to the global burden of disease study (2015), migraine is the most progressive neurological illness and the third leading cause of disability worldwide (Steiner et al., 2016). Migraine attacks involve a variety of neurological symptoms such as nausea, photophobia, phonophobia, osmophobia, exhaustion, and disruptions of autonomic, mental, sensory, and motor functioning, in addition to pain (Burstein, 2001). the international classification of headache disorders, third edition beta version (ICHD-III), categorizes migraine into two subtypes: Migraine without aura (MO) and migraine with aura (MA) (IHS, 2018).
The two basic ideas have dominated migraine research over the past three centuries: The vascular theory and the central neuronal theory (Isler, 1986 ; Tfelt-Hansen & Koehler, 2010). The efficacy of vasoconstrictors like ergotamine and triptans in treating acute migraine supported the vascular theory. Still, novel research shows that vasodilation is neither essential nor sufficient to trigger a migraine episode (Mason & Russo, 2018). Some studies have also indicated that migraine episodes can begin in areas of nociceptive neuromodulatory dysfunction in the brain stem (Welch, 2003; Brennan & Pietrobon, 2018). In addition, Levy and colleagues suggested that degranulation of meningeal mast cells, an inflammatory cell found in the intracranial region, may cause migraine (Levy et al., 2007). While numerous theories have been proposed for the etiology of migraine, none of these theories adequately explain the exact cause of migraine (Tfelt-Hansen & Koehler, 2010). Furthermore, there is no specific biomarker or diagnostic test for migraine, and it has often been misdiagnosed with other types of headaches.
On the other hand, there is emerging evidence that migraine may be caused by immune dysfunction (Arumugam & Narayan, 2019; Biscetti et al., 2021; Biscetti et al., 2022). In an earlier clinical study, our research group showed that CD4+CD25+ regulatory T-cells (Treg) were lower in migraine patients in comparison to healthy volunteers (Arumugam & Parthasarathy, 2016), which was the first study to identify levels of Treg variability in migraine patients. In support of this research, other clinical studies have also demonstrated a significant decrease in Treg cell levels in migraine patients (Faraji, et al., 2021; Nurkhametova et al., 2018; Li et al., 2022). However, a systematic analysis of the available data does not exist yet. Therefore, the current study attempted to analyze existing clinical data on decreasing levels of lymphocyte subsets, particularly the Treg population in migraine patients.
2. Materials and Methods
Literature search
A detailed literature search was conducted on PubMed, Scopus, Embase, ProQuest, Cochrane Review, Clinical trials, Academic thesis, American Academy of Neurology (AAN) resources, and Google Scholar databases from 2010 to 2022. The search details include “migraine in “regulatory T-cell,” “CD4+CD25+,” “inflammatory activity,” “autoimmune disorder,” “cytokines,” and “neuromodulators.” Each term’s singular and plural variations, as well as regional spelling variations, were recognized. The analysis w::::::as char::::::acterized based on the age, number of migraine patients and healthy volunteers, MO and MA, new interventions, and consequent parameters. Studies that met these criteria were used in the analysis. Meta-analysis was performed using appropriate software (Revman software, version 5.4).
Eligibility criteria
Only studies that met the following criteria were included in the analysis. Clinical studies were conducted with MO and MA patients, quantitative analyses of the T lymphocyte subset population, especially Treg cells, and studies conducted with patients without recent immune suppression therapies.
Data extraction
Data were extracted from eligible studies, and the average values and standard deviation for Treg cell levels were collected from the selected articles. The number of cases or controls, average age, gender ratio, intervention performed, and outcome parameters were taken for the analysis. Other clinically significant findings were also collected.
3. Results
Description of the included studies
Following the initial screening and eligibility criteria, 17 records were selected for qualitative analysis and 4 observational study records (Arumugam & Parthasarathy, 2016; Faraji, et al., 2021; Nurkhametova et al., 2018; Li et al., 2022) were taken for the final analysis (Figure 1). The inclusion criteria of the study were - parameters such as migraine type, age, comorbidity, and immunosuppressive treatments.

Analysis of the clinical studies in the pathophysiology of migraine
Throughout this study, 121 migraine patients (including MO and MA) and 94 healthy volunteers between the ages of 18 and 55 were included in the analysis (Table 1).

Changes in Treg cell levels were compared between migraine patients and healthy volunteers. The results show a significant reduction of Treg cell levels in migraine patients compared to healthy individuals, and the overall effect (Z=1.21; P=0.23) (Figure 2).

The standardized mean difference (SMD) shows -3.63 (P=0.007) with a 95% confidence interval of -6.28 to -0.99 (Figure 3). The true effect size of overall SMD in 95% CI of all comparable populations fall between the interval of -16.30 to 9.04 (Figure 3).

4. Discussion
The current meta-analysis data from 4 clinical studies show a substantial reduction of Treg cell levels in migraine patients compared to healthy volunteers. None of the studies found significant differences between MO and MA patients (Table 1). The Tregs are a specific subgroup of T cells, defined by the expression of CD4, CD25 (the IL-2 receptor a-chain), and the transcription factor forkhead box P3 (FOXP3) (Valencia & Lipsky, 2007). Treg cells are produced during thymocyte development and regulate the immunological response, preserving homeostasis and self-tolerance (Chang et al., 2005; Zhang e al., 2020; Genre et al., 2009). It can also inhibit the activation, proliferation, and effector functions of T-cells, natural killer cells, B-cells, and antigen-presenting cells (Sakaguchi et al., 2009). Thus, the reduced or abnormal regulation of Treg cells or mutation of FOXP3 enhances the probability of immunological imbalance in migraine patients. In addition, an increase in CD4+ and a decrease in CD8+ levels in migraine patients have been reported (Deng et al., 2019; Raphael et al., 2020; Arumugam & Parthasarathy, 2016). Variations in purinergic cells, such as CD4+, CD8+, CD39+, CD73+, and FOXP3 subsets, were also observed in migraine patients compared to healthy volunteers, and these outcomes are consistent with past reports (Mosnaim et al., 1998; Empl et al., 1999; Pavelek et al., 2020), indicating immune cells possible role in the physiopathology of migraine (Cseh et al., 2013; Leone et al., 1994).
However, numerous clinical studies show alteration in pro-inflammatory cytokines like IL-1, IL-6, and TNF, as well as anti-inflammatory cytokines like IL-1RA, IL-2, and IL-10, during migraine attacks (Boćkowski et al., 2010; Bruno et al., 2007; Yilmaz at al., 2010). In addition, a recent case report suggests that interferon-beta can induce or exacerbate migraine attacks during immunomodulation therapy in multiple sclerosis patients (Patti et al., 2012; Elmazny et al., 2020). These findings support the belief that immune cells, such as interferon beta, could also play a role in migraine attacks. Accordingly, contemporary approaches are opening the doors to recognizing the role of immune cells in migraine and raising the possibility of a link between immunological imbalance and migraine. Therefore, the current meta-analysis and existing evidence strongly suggest that immune cells can play a pivotal role in migraine pathogenesis.
For the first time in the literature, Treg cell levels were taken for meta-analysis about migraine research. The limitation of the study is that only four studies were analyzed. Nevertheless, exhaustive research is required to support the notion of a link between autoimmunity and migraine.
5. Conclusion
The meta-analysis of four clinical studies shows significant reduction of Treg cells in migraine patients compared to healthy volunteers. Decreased Treg levels support the theory that migraine could be due to immune dysfunction. More specific studies on the role of immune cells in the pathophysiology of migraine can contribute to a better understanding of migraine progression.
Ethical Considerations
Compliance with ethical guidelines
This study adhered to ethical standards in research and publication. Since this was a systematic review and meta-analysis of previously published data, no new human or animal subjects were included in this study. All data sources used in this study were obtained from publicly available, peer-reviewed articles. The original studies included in our analysis followed ethical guidelines, obtaining informed consent from participants and approval from relevant institutional review boards.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Subalakshmi Sugumar and Murugesan Arumugam; Study design: Philo Hazeena and Deepa Avadhani; Writing the original draft: Subalakshmi Sugumar; Review and editing: Subalakshmi Sugumar, Saman Shah, Pavithra Murugan and Murugesan Arumugam; Methodology, data curation, visualization and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are immensely thankful to the National Knowledge Network, India for providing valuable access to scientific journals and other relevant electronic data.
References
Andreou, A. P., & Edvinsson, L. (2019). Mechanisms of migraine as a chronic evolutive condition. The Journal of Headache and Pain, 20(1), 117. [DOI:10.1186/s10194-019-1066-0] [PMID] [PMCID]
Arumugam, M., & Narayan, S. K. (2019). Rethinking of the concepts: Migraine is an autoimmune disease? Neurology, Psychiatry and Brain Research, 31, 20-26. [DOI:10.1016/j.npbr.2018.11.003]
Arumugam, M., & Parthasarathy, V. (2016). Reduction of CD4(+)CD25(+) regulatory T-cells in migraine: Is migraine an autoimmune disorder?. Journal of Neuroimmunology, 290, 54–59. [DOI:10.1016/j.jneuroim.2015.11.015] [PMID]
Biscetti, L., De Vanna, G., Cresta, E., Bellotti, A., Corbelli, I., & Letizia Cupini, M., et al. (2020). Immunological findings in patients with migraine and other primary headaches: A narrative review. Clinical and Experimental Immunology, 207(1), 11–26. [DOI:10.1093/cei/uxab025] [PMID] [PMCID]
Biscetti, L., De Vanna, G., Cresta, E., Corbelli, I., Gaetani, L., & Cupini, L., et al. (2021). Headache and immunological/autoimmune disorders: A comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. Journal of Neuroinflammation, 18(1), 259. [PMID] [PMCID]
Boćkowski, L., Smigielska-Kuzia, J., Sobaniec, W., Zelazowska-Rutkowska, B., Kułak, W., & Sendrowski, K. (2010). Anti-inflammatory plasma cytokines in children and adolescents with migraine headaches. Pharmacological Reports: PR, 62(2), 287–291. [DOI:10.1016/s1734-1140(10)70268-1] [PMID]
Brennan, K. C., & Pietrobon, D. (2018). A systems neuroscience approach to migraine. Neuron, 97(5), 1004–1021. [DOI:10.1016%2Fj.neuron.2018.01.029] [PMID] [PMCID]
Bruno, P. P., Carpino, F., Carpino, G., & Zicari, A. (2007). An overview on immune system and migraine. European Review for Medical and Pharmacological Sciences, 11(4), 245–248. [PMID]
Burstein, R. (2001). Deconstructing migraine headache into peripheral and central sensitization. Pain, 89(2-3), 107–110.[DOI:10.1016/s0304-3959(00)00478-4] [PMID]
Chang, X., Zheng, P., & Liu, Y. (2006). FoxP3: A genetic link between immunodeficiency and autoimmune diseases. Autoimmunity Reviews, 5(6), 399–402. [DOI:10.1016/j.autrev.2005.10.008] [PMID]
Cseh, A., Farkas, K. M., Derzbach, L., Muller, K., Vasarhelyi, B., & Szalay, B., et al. (2013). Lymphocyte subsets in pediatric migraine. Neurological Sciences: Official Journal of The Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 34(7), 1151–1155. [DOI:10.1007/s10072-012-1218-3] [PMID]
Deng, Q., Luo, Y., Chang, C., Wu, H., Ding, Y., & Xiao, R. (2019). The emerging epigenetic role of CD8+T Cells in Autoimmune Diseases: A systematic review. Frontiers in Immunology, 10, 856. [DOI:10.3389/fimmu.2019.00856] [PMID] [PMCID]
Elmazny, A., Hamdy, S. M., Abdel-Naseer, M., Shalaby, N. M., Shehata, H. S., & Kishk, N. A., et al. (2020). Interferon-Beta-induced headache in patients with multiple sclerosis: Frequency and Characterization. Journal of Pain Research, 13, 537–545. [DOI:10.2147/JPR.S230680] [PMID] [PMCID]
Empl, M., Sostak, P., Breckner, M., Riedel, M., Müller, N., & Gruber, R., et al. (1999). T-cell subsets and expression of integrins in peripheral blood of patients with migraine. Cephalalgia: An International Journal of Headache, 19(8), 713–697. [DOI:10.1046/j.1468-2982.1999.019008713.x] [PMID]
Faraji, F., Shojapour, M., Farahani, I., Ganji, A., & Mosayebi, G. (2021). Reduced regulatory T lymphocytes in migraine patients. Neurological Research, 43(8), 677–682. [DOI:10.1080/01616412.2021.1915077] [PMID]
Genre, J., Errante, P. R., Kokron, C. M., Toledo-Barros, M., Câmara, N. O., & Rizzo, L. V. (2009). Reduced frequency of CD4(+)CD25(HIGH)FOXP3(+) cells and diminished FOXP3 expression in patients with Common Variable Immunodeficiency: A link to autoimmunity? Clinical Immunology (Orlando, Fla.), 132(2), 215–221. [DOI:10.1016/j.clim.2009.03.519] [PMID]
Isler H. (1986). Thomas Willis' two chapters on headache of 1672: a first attempt to apply the "new science" to this topic. Headache, 26(2), 95–98. [DOI:10.1111/j.1526-4610.1986.hed2602095.x] [PMID]
IHS. (2018). Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia, 38(1), 1-211. [DOI:10.1177%2F0333102417738202]
Leone, M., Biffi, M., Leoni, F., & Bussone, G. (1994). Leukocyte subsets and cortisol serum levels in patients with migraine without aura and chronic tension-type headache. Cephalalgia: An International Journal of Headache, 14(2), 139–142. [DOI:10.1046/j.1468-2982.1994.1402139.x] [PMID]
Levy, D., Burstein, R., Kainz, V., Jakubowski, M., & Strassman, A. M. (2007). Mast cell degranulation activates a pain pathway underlying migraine headache. Pain, 130(1-2), 166–176. [DOI:10.1016/j.pain.2007.03.012] [PMID] [PMCID]
Li, H., Fu, Q., Philips, K., Sun, Y., Faurot, K. R., & Gaylord, S. A., et al. (2022). Leukocyte inflammatory phenotype and function in migraine patients compared with matched non-migraine volunteers: A pilot study. BMC Neurology, 22(1), 278.[DOI:10.1186/s12883-022-02781-4] [PMID] [PMCID]
Mason, B. N., & Russo, A. F. (2018). Vascular contributions to migraine: Time to revisit? Frontiers in Cellular Neuroscience, 12, 233.[DOI:10.3389/fncel.2018.00233] [PMID] [PMCID]
Mosnaim, A. D., Kulaga, H., Adams, A. J., Wolf, M. E., Puente, J., & Freitag, F., et al. (1998). Flow cytometric analysis of lymphocyte subsets in migraine patients during and outside of an acute headache attack. Cephalalgia: An International Journal of Headache, 18(4), 197–201. [DOI:10.1046/j.1468-2982.1998.1804197.x] [PMID]
Nurkhametova, D., Kudryavtsev, I., Khayrutdinova, O., Serebryakova, M., Altunbaev, R., & Malm, T., et al. (2018). Purinergic profiling of regulatory T-cells in patients with episodic migraine. Frontiers in Cellular Neuroscience, 12, 326. [DOI:10.3389%2Ffncel.2018.00326] [PMID] [PMCID]
Patti, F., Nicoletti, A., Pappalardo, A., Castiglione, A., Lo Fermo, S., & Messina, S., et al. (2012). Frequency and severity of headache is worsened by Interferon-β therapy in patients with multiple sclerosis. Acta Neurologica Scandinavica, 125(2), 91–95. [DOI:10.1111/j.1600-0404.2011.01532.x] [PMID]
Pavelek, Z., Souček, O., Krejsek, J., Sobíšek, L., Klímová, B., & Masopust, J., et al. (2020). The role of the immune system and the biomarker CD3 + CD4 + CD45RA-CD62L- in the pathophysiology of migraine. Scientific Reports, 10(1), 12277. [DOI:10.1038%2Fs41598-020-69285-4] [PMID] [PMCID]
Raphael, I., Joern, R. R., & Forsthuber, T. G. (2020). Memory CD4+ T cells in immunity and autoimmune diseases. Cells, 9(3), 531. [DOI:10.3390/cells9030531] [PMID] [PMCID]
Sakaguchi, S., Wing, K., Onishi, Y., Prieto-Martin, P., & Yamaguchi, T. (2009). Regulatory T cells: How do they suppress immune responses? International Immunology, 21(10), 1105–1111.[DOI:10.1093/intimm/dxp095] [PMID]
Steiner, T. J., Stovner, L. J., & Vos, T. (2016). GBD 2015: Migraine is the third cause of disability in under 50s. The Journal of Headache and Pain, 17(1), 104. [DOI:10.1186%2Fs10194-016-0699-5] [PMID] [PMCID]
Tfelt-Hansen, P. C., & Koehler, P. J. (2011). One hundred years of migraine research: Major clinical and scientific observations from 1910 to 2010. Headache, 51(5), 752–778. [DOI:10.1111/j.1526-4610.2011.01892.x] [PMID]
Valencia, X., & Lipsky, P. E. (2007). CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nature Clinical Practice. Rheumatology, 3(11), 619–626. [DOI:10.1038/ncprheum0624] [PMID]
Welch, K. M. (2003). Contemporary concepts of migraine pathogenesis. Neurology, 61(8 Suppl 4), S2-S8. [DOI:10.1212/wnl.61.8_suppl_4.s2] [PMID]
Type of Study: Review |
Subject:
Cellular and molecular Neuroscience
Received: 2023/06/11 | Accepted: 2024/09/14 | Published: 2024/09/1
Received: 2023/06/11 | Accepted: 2024/09/14 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





