Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 443-454 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Toloui A, Ramawad H A, Aboutaleb N, Yousefifard M. Effectiveness of Platelet-rich Plasma in Treating Spinal Cord Injuries: A Systematic Review & Meta-analysis. BCN 2024; 15 (4) :443-454
URL: http://bcn.iums.ac.ir/article-1-2638-en.html
URL: http://bcn.iums.ac.ir/article-1-2638-en.html
1- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Emergency Medicine, NYC Health & Hospitals, Coney Island, New York, United States.
2- Department of Emergency Medicine, NYC Health & Hospitals, Coney Island, New York, United States.
Full-Text [PDF 1220 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Spinal cord injuries (SCIs) primarily affect young adults and thus have devastating physical, psychological, and social impacts. This condition substantially burdens healthcare systems (Badhiwala et al, 2019; James et al., 2019). SCI leads to sensory and or motor deficits, which often present as gait disturbances, loss of coordination, severe neuropathic pain, and incontinence. Despite advances in healthcare, patients with spinal cord injuries often have a decreased quality of life (QoL) and suffer more from morbidities due to subsequent chronic symptoms (Sezer et al., 2015).
The current management strategies for SCI involve surgery, symptomatic treatment, and physical rehabilitation (Walters et al., 2013). In the acute phase of the disease, methylprednisolone is recommended during the first 8 hours. However, there is insufficient evidence to support the use of high-dose steroids 8 hours after an acute SCI (Bracken, 2012). Although physical rehabilitation and other treatment strategies can relatively improve the complications caused by spinal cord injuries, patients often face lifelong severe disabilities and chronic morbidities. Research in this field is still in progress and various therapeutic strategies from the molecular, gene, or cellular therapy or even the use of high-tech equipment such as virtual reality have been recommended to ameliorate symptoms (Janzadeh et al., 2017; Mammana et al., 2019; Miguel-Rubio et al., 2020; Nakhjavan-Shahraki et al., 2018; Sarveazad et al., 2017; Sarveazad et al., 2019; Silvestro et al., 2020). However, proposing these treatment options for Food and Drug Administration (FDA) approvals needs sufficient preclinical and clinical studies.
Platelet-rich plasma (PRP) has recently received much attention as a potential candidate for treating SCI. PRP contains several growth factors responsible for tissue regeneration and repair. The presence of growth factors and protective cytokines such as platelet-derived growth factor, transforming growth factor beta, fibroblast growth factor, insulin-like growth factor-1, insulin-like growth factor-2, vascular endothelial growth factor, epidermal growth factor, interleukin 8, keratinocyte growth factor, and connective tissue growth factor makes PRP a suitable agent for the treatment of neurodegenerative and inflammatory diseases (Marx, 2004). Regarding the pathophysiology of SCI, we observe the simultaneous occurrence of inflammation and neurodegeneration. In the acute phase of the injury, severe inflammation causes a cascade of pathophysiological events, ultimately initiating neurodegeneration and permanent lesions in the spinal cord (Alizadeh et al., 2019).
Recent research shows that PRP administration in the acute, subacute, and chronic phases of SCI improves locomotor function and reduces long-term side effects such as neuropathic pain (Salarinia et al., 2020; Salarinia et al., 2017). The administration of PRP improves angiogenesis and promotes axonal regeneration but does not significantly affect the immune system’s reaction (Chen et al., 2018). Therefore, if PRP administration can prevent the occurrence of permanent damage in spinal cord injuries, it could be used as an easy and accessible treatment in the future. Moreover, the isolation and preparation of autologous PRP are simple and fast, and these beneficial effects have created a promising window for treating spinal cord injuries. Developments in the administration of PRP for spinal cord injuries are still in the preclinical phase, and there is no conclusion on this matter. It is still not yet clear which treatment protocol of PRP has the best effectiveness, and the best time and method of administration are yet unknown. Therefore, to start clinical trials, it is necessary to provide valid preclinical evidence of PRP being a potential candidate for the treatment of SCI. The present systematic review and meta-analysis intend to collect preclinical evidence on the efficacy of different PRP administration protocols in spinal cord injuries, emphasizing functional recovery and cavity size. As a supplementary analysis, we assessed the effect of different PRP protocol treatments and the severity of spinal cord injury on the effectiveness of PRP treatment following spinal cord injury.
2. Materials and Methods
Study design
The present systematic review and meta-analysis collected preclinical evidence on the effectiveness of PRP administration in spinal cord injuries, emphasizing functional recovery and cavity size. For this purpose, an extensive literature search was conducted using the electronic databases of Medline, Embase, Scopus, and Web of Science until October 23, 2022. The search strategy was based on keywords related to PRP and SCI—the search strategy in the Medline database (Appendix 1).
Inclusion criteria
The PICO (population, intervention, comparison, outcome) components are as follows. The population includes animals (rats or mice) with SCI caused by compression, contusion, transection, or hemi-section. Intervention is the administration of PRP. A comparison was performed with a similar group that did not receive PRP, and the outcome was functional recovery and cavity size. Based on these criteria, studies conducted on animals with SCI for which PRP was administered have been included. The exclusion criteria were studies without a control group, review articles, and retracted studies.
Data gathering
Two independent researchers collected the data. After the search, articles were obtained from the mentioned databases and gray literature (Google and Google Scholar and the thesis section of the ProQuest database) in the eighth version of Endnote. These two researchers independently performed the initial screening process. The title and abstract of each article were reviewed, and if the article was relevant or likely to be relevant, the full text of the study was collected and studied. Then, the data of these studies were summarized in a checklist designed based on PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines. The extracted data included information about the study design, the characteristics of the sample and control groups (age, gender, etc.), the sample size, the type of SCI, the time interval from the occurrence of injury, and the administration of PRP, the dose of PRP, the location of SCI, the method of PRP administration, and the follow-up period.
Quality control
The quality assessment of the articles was done by two researchers independently with the SYRCLE risk of bias tool (Hooijmans et al., 2014). In case of disagreement between the researchers, the disagreement was resolved through discussion with each other or a third researcher.
Statistical analyses
The analyses were performed using the statistical program STATA 17.0. Data are recorded as Mean±SD. The presence of heterogeneity has been investigated using the I2 test. The relationships between the location of SCI, the severity of the injury, PRP dosage, PRP administration method, follow-up period, functional recovery, and cavity size were assessed using the “meta” command. Egger’s test and the funnel plot were also used to investigate publication bias.
3. Results
The search resulted in 168 articles. After removing duplicates, 105 articles were advanced for screening. After reviewing the full text of 26 articles, the data from 9 original articles were included in the present meta-analysis: Behroozi et al. (2021); Behroozi et al. (2022); Chen et al., (2018); EL-Seddawy et al., 2020; Hu et al. (2022); Lam et al. (2016); Salarinia et al., (2020); Salarinia et al. (2017); and Zhao et al., (2013) (Figure 1).
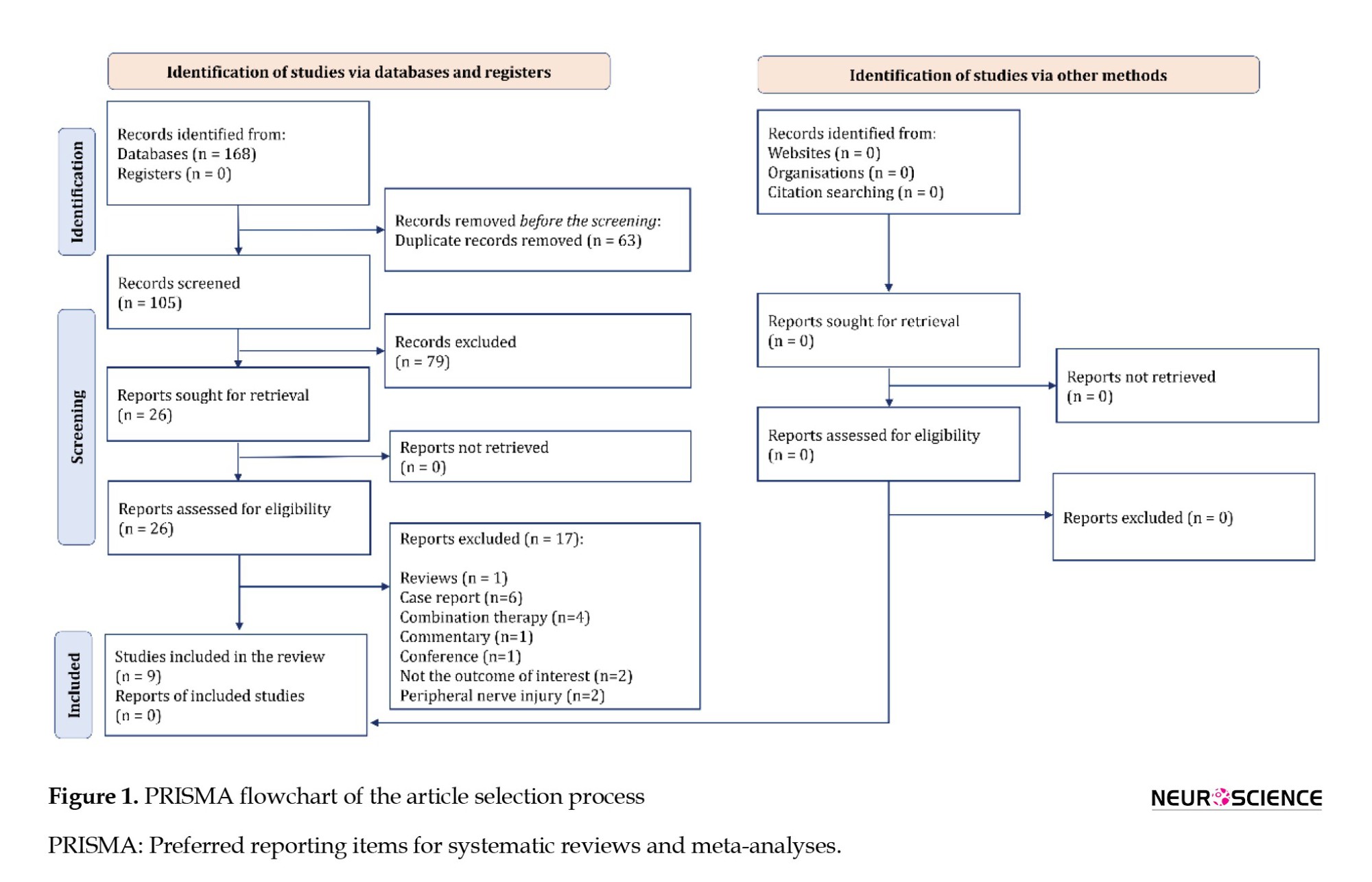
Of these 9 articles, 8 were performed on rats and 1 on mice. All studies used the SCI model in the thoracic region of the spinal cord. The injury model was compression in 3 studies, contusion in 4 studies, transection in 1 study, and hemi-section in 1 study. The severity of the injury was moderate in 3 studies and severe in 6 studies.
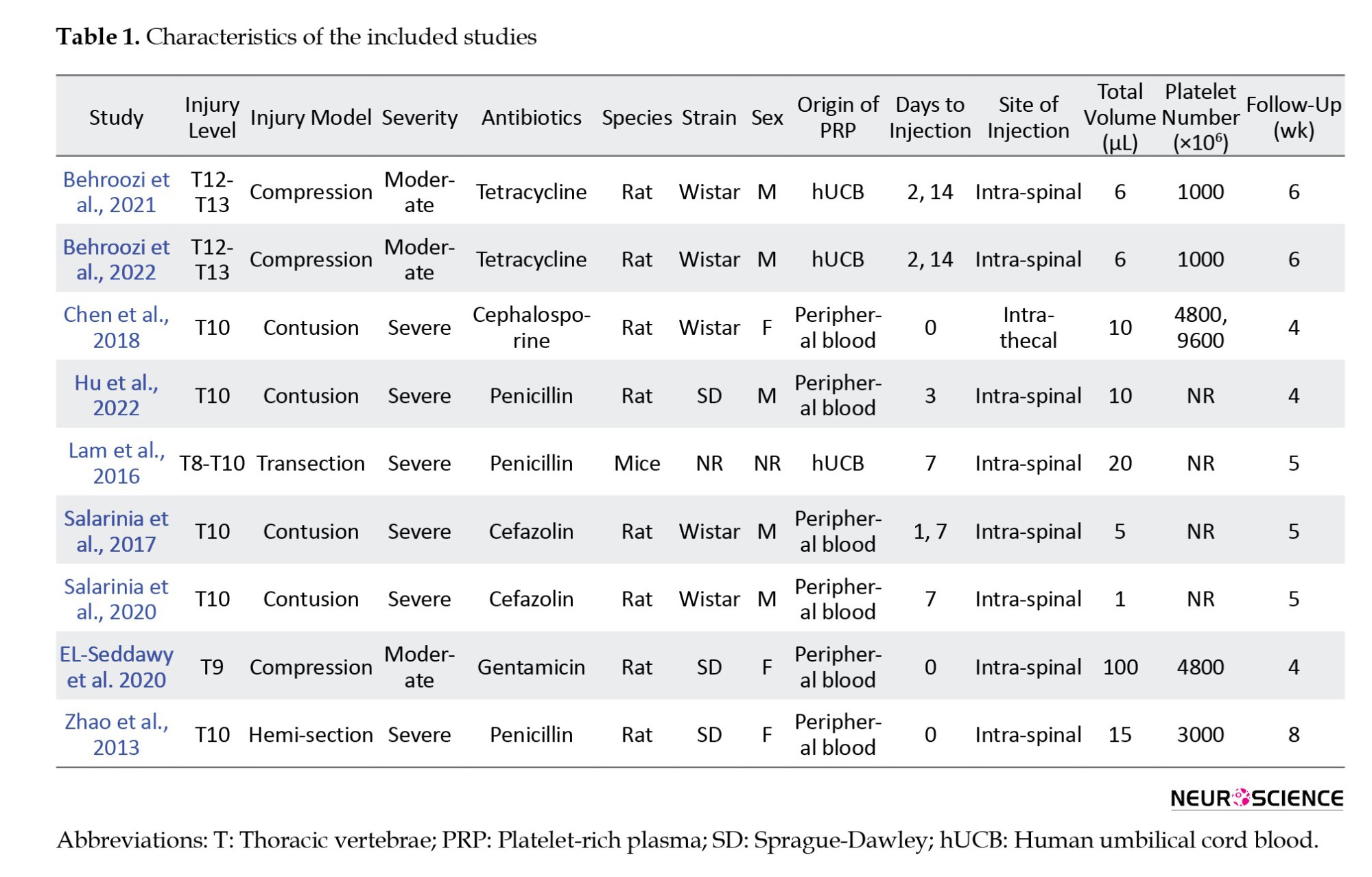
PRP was taken from human umbilical cord blood (xenograft) in 3 studies and peripheral blood (allograft/autograft) in 6 studies. In 5 studies, PRP administration was performed 24 hours after the injury, and in 3 studies, during the first 24 hours. In one study, the intervention was done in two separate groups, less than 24 hours and after 24 hours from the time of injury. The follow-up period was more than 4 weeks in 6 studies and 4 weeks or less in 3 studies. The outcome was the functional recovery in 7 studies and cavity size in one study. One article investigated the PRP administration effect on functional recovery and cavity size. All included studies examining functional recovery reported this outcome using the Basso Beattie and Bresnahan (BBB) scale. Table 1 presents the characteristics of the included articles.
The effect of PRP administration on function recovery
A total of 8 articles examined functional recovery. Using the Galbraith plot to find the outlier data, it was found that the study of EL-Seddawy et al., (2020) is an outlier. Therefore, it was excluded from the analysis. Eventually, 7 studies with 10 separate analyses were included in the current meta-analysis. The results of the pooled-data analysis showed that PRP administration has significantly improved the motor function of animals with SCI (standardized mean differences [SMD]=1.5; 95% CI, 0.9%, 2.1%; P<0.0001). Due to the moderate heterogeneity (I2=67.01%), the subgroup analyses were performed (Figure 2).
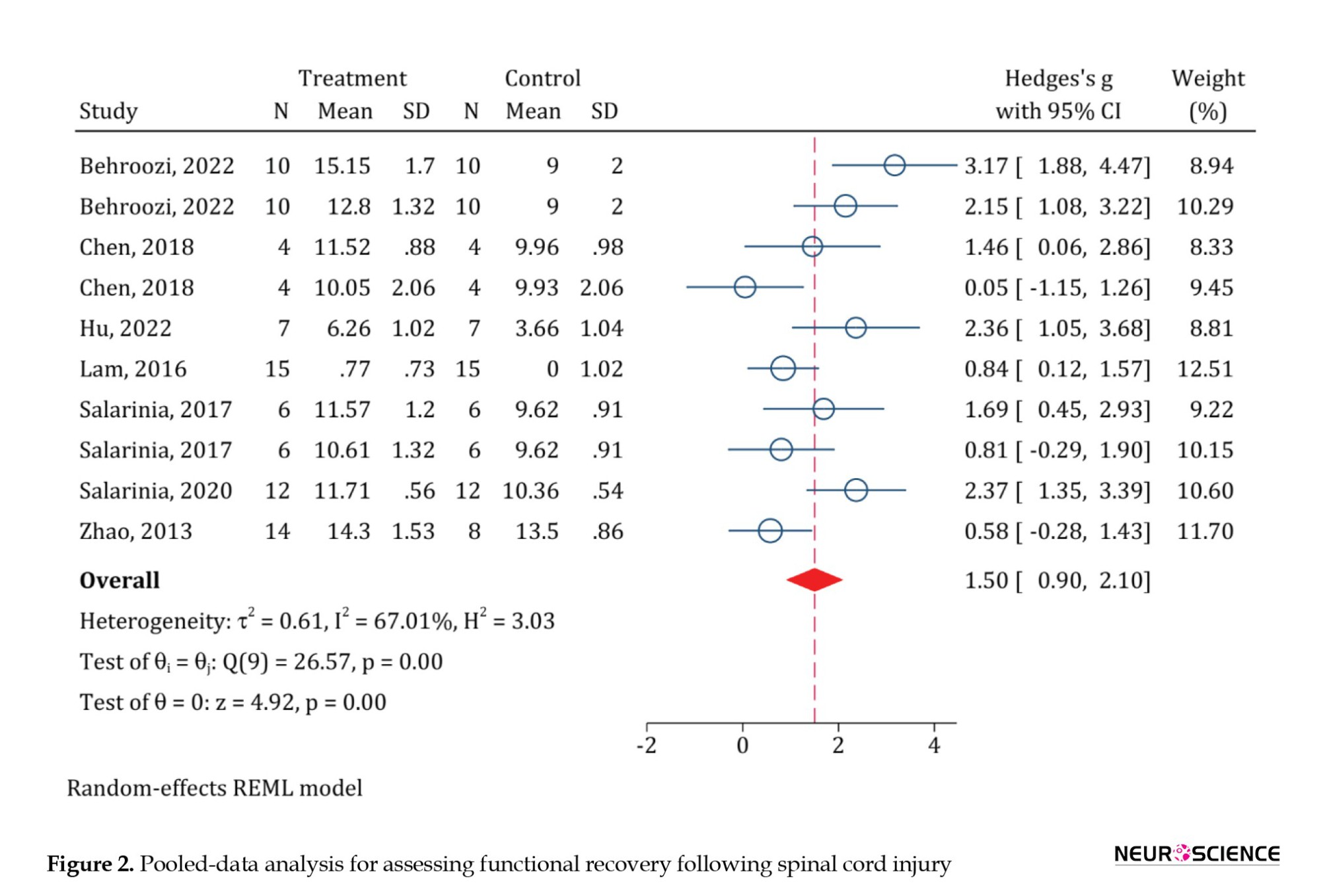
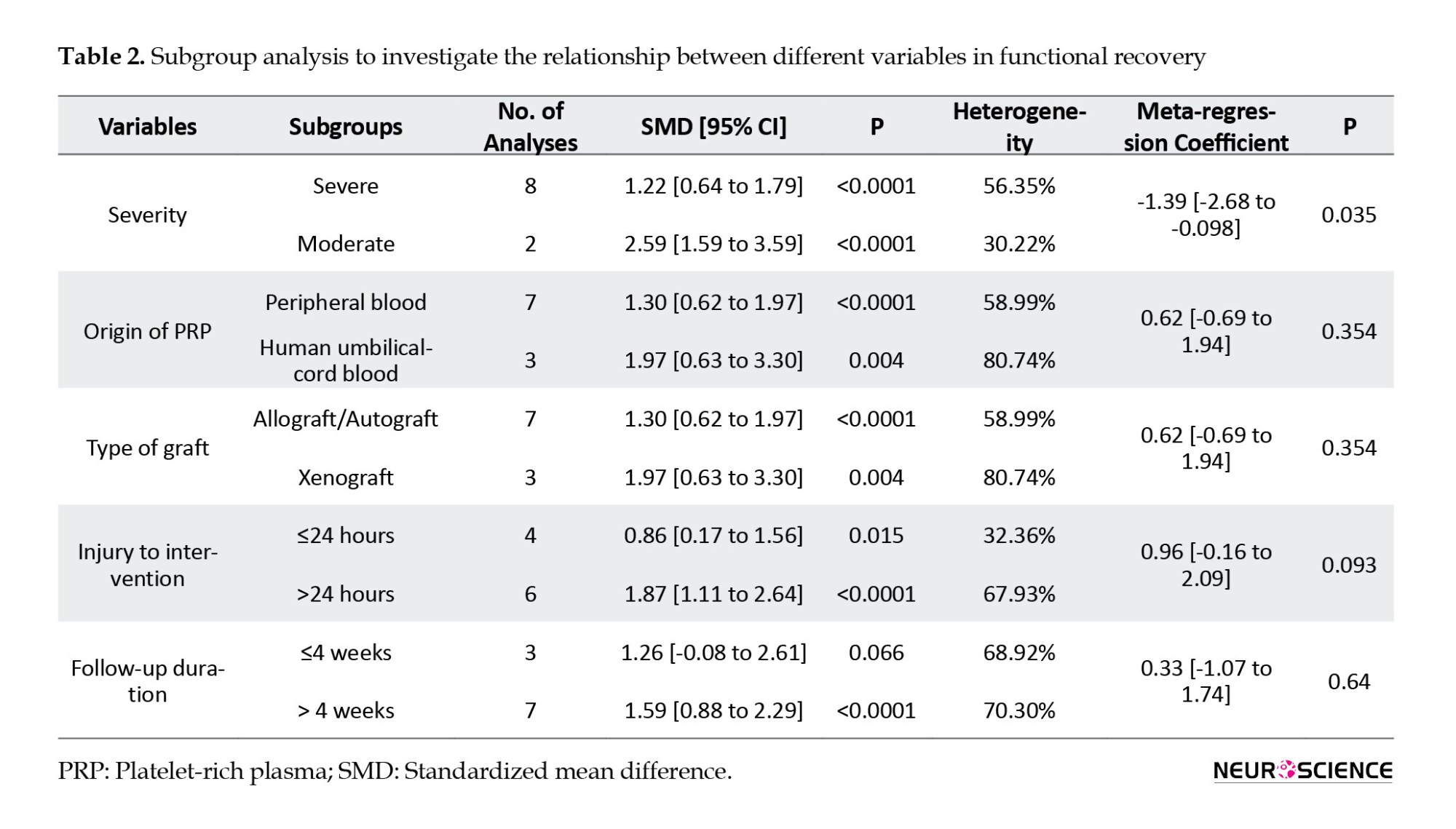
In the subgroup analysis based on the severity of SCI, treatment with PRP in both moderate injuries (SMD=2.59; 95% CI, 1.59%, 3.59%; P<0.0001; I2=30.22%) and severe ones (SMD=1.22; 95% CI, 0.64%, 1.79%; P<0.0001; I2=56.35%) has been associated with a significant improvement in the motor function of animals. However, the extent of this recovery in moderate injuries was significantly greater than in the severe injury group (meta-regression coefficient=-1.36; 95% CI, -2.68%, -0.09%; P<0.035). PRP from peripheral blood (SMD=1.3; 95% CI, 0.62, 1.97%; P<0.0001; I2=58.99%) and human umbilical cord blood (SMD=1.97; 95% CI, 0.63%, 3.3%; P<0.0001; I2=80.74%) were both effective in improving the motor function of animals. However, no significant difference was observed between the two groups (meta-regression coefficient=0.62; 95% CI, -0.69%, -1.94%; P=0.35). PRP administration in the first 24 hours after SCI (SMD=0.86; 95% CI, 0.17%, 1.56%; P=0.015; I2=32.36%) and after 24 hours (SMD=1.87; 95% CI, 1.11%, 2.64%; P<0.0001; I2=67.93%) both were effective in improving the motor function of animals. Meta-regression showed no significant difference between the administration of PRP in the first 24 hours and after 24 hours from injury (meta-regression coefficient=0.96; 95% CI, -0.16%, 2.09%; P=0.09). Finally, there was no significant difference regarding the follow-up period (meta-regression coefficient=0.33; 95% CI, -1.07%, 1.74%; P=0.64) (Table 2).
The effect of PRP administration on cavity size
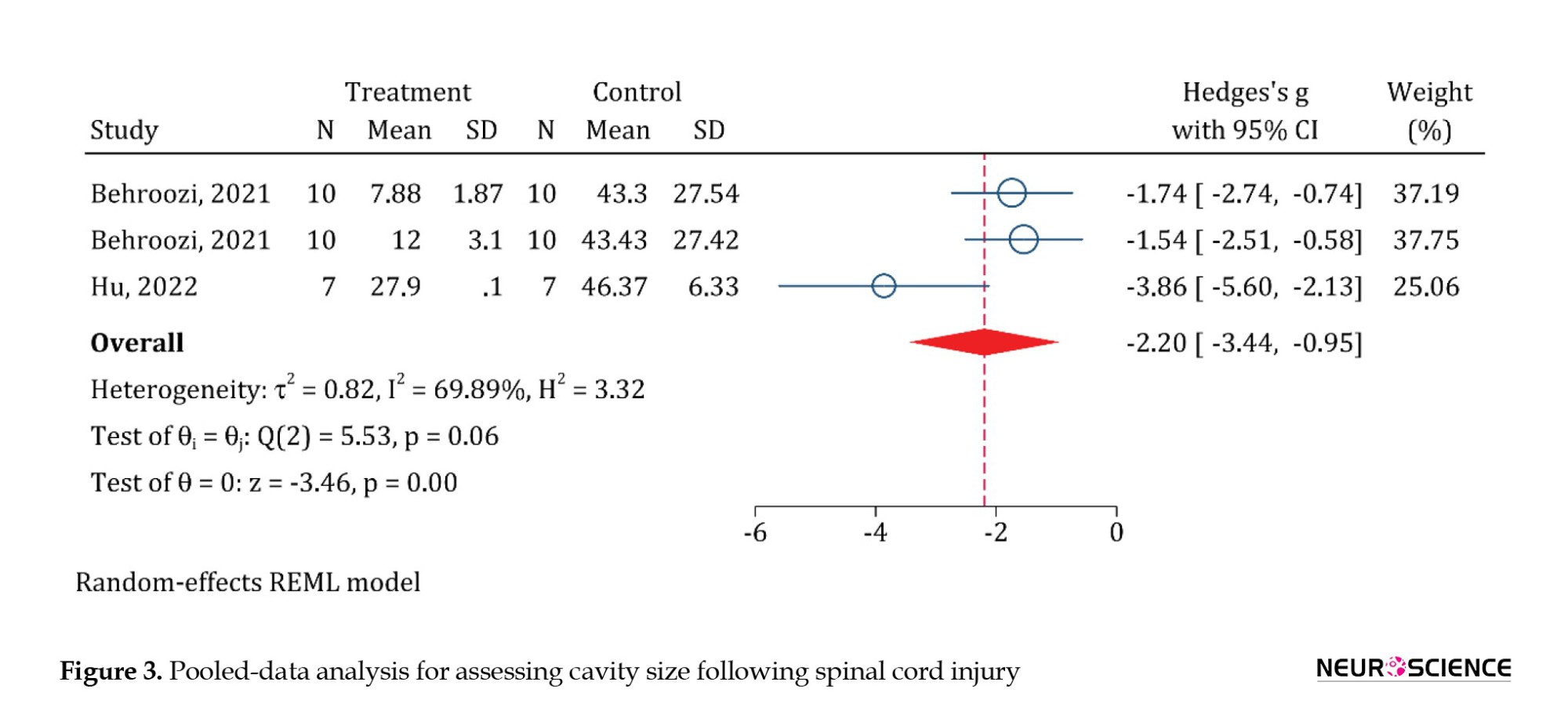
In this section, 2 articles and 3 analyses were included. Pooled-data analysis showed that PRP administration significantly reduces the cavity size in animals with SCI (SMD=-2.2; 95% CI, -3.44%,-0.95%; P<0.0001) (Figure 3).
Quality control
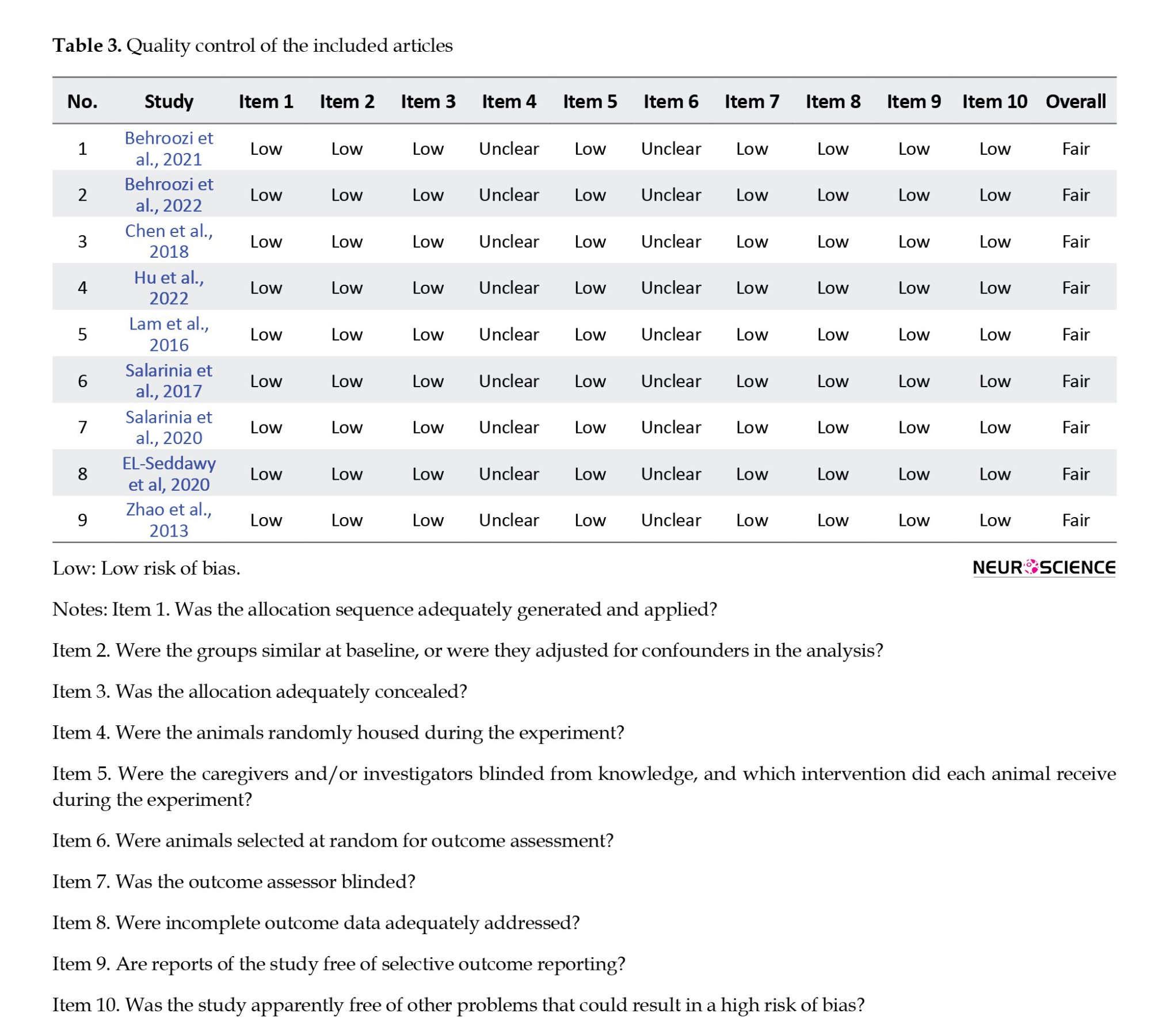
None of the articles in the quality control assessment section reported housing randomization or random outcome assessment. Therefore, the risk of bias was considered unclear in these items. The risk of bias was low in other items in the included articles. Generally, the quality of data was considered fair (Table 3).
Publication bias
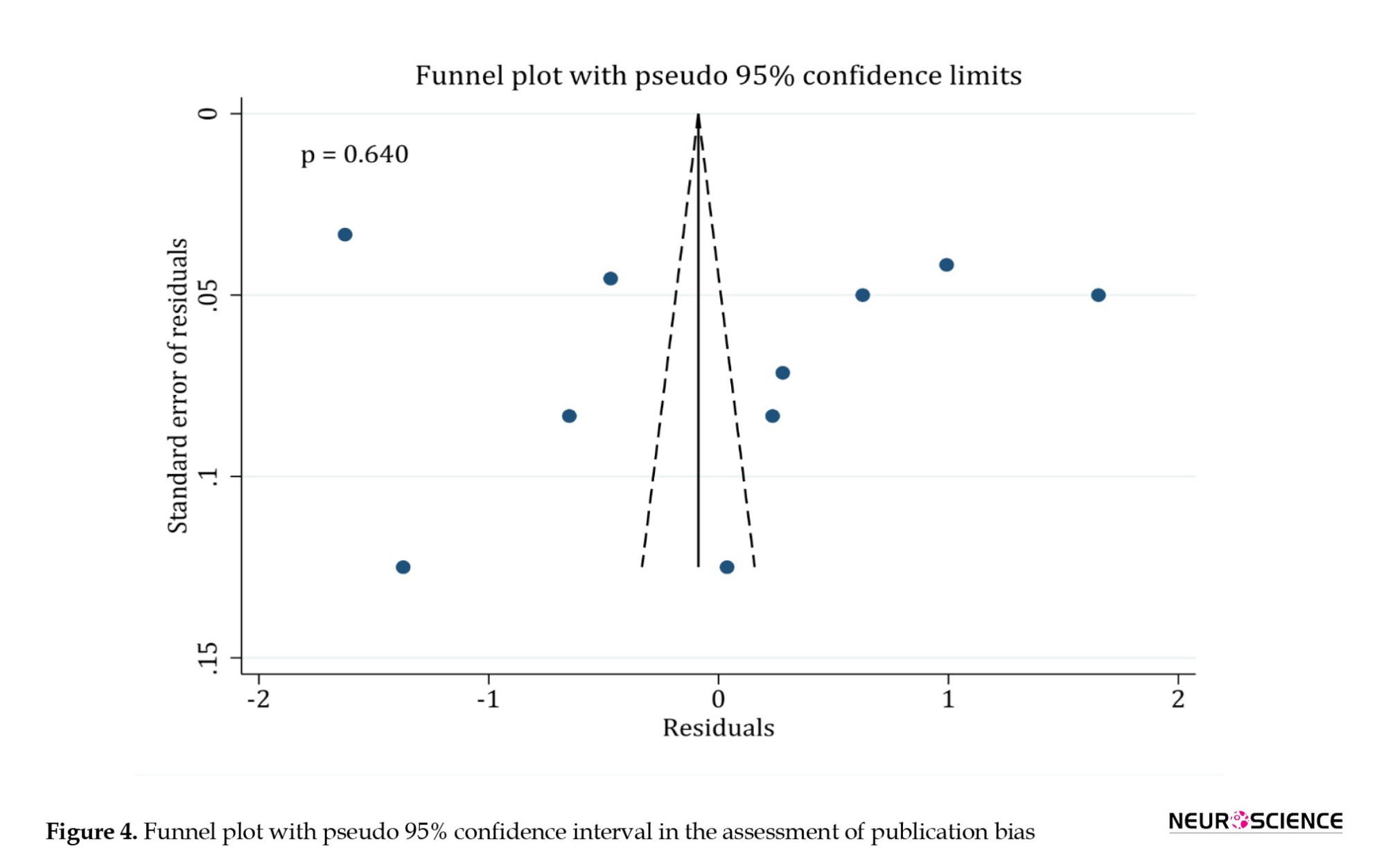
Egger’s test showed no publication bias in the reports of functional recovery (P=0.64) (Figure 4). Since the assessment of cavity size was reported in 3 analyses, publication bias assessment was not feasible in terms of methodology.
4. Discussion
The purpose of this meta-analysis was to investigate the effectiveness of PRP administration in the treatment of spinal cord injuries. By analyzing the data of current original studies, we showed that PRP administration can significantly improve motor functions and cavity size after SCI. However, there was considerable heterogeneity in the findings of the articles. To find the cause of this heterogeneity, a subgroup analysis was performed on the severity of the SCI, the origin of PRP, the time elapsed from the injury to the administration of PRP, and the length of follow-up. According to these analyses, the severity of injury was the reason for heterogeneity in the articles.
The studies conducted on moderate spinal injuries had significantly less heterogeneity than those conducted on severe injuries. Although motor function improvements were significant in both moderate and severe injuries, the improvement of motor function in animals with moderate SCI was significantly higher than in animals with severe SCI. In addition to the fact that even in the absence of therapeutic intervention, moderate SCI has a better outcome than severe injury, it should be noted that the low number of analyses performed on moderate spinal cord injuries can be one of the reasons that we observed a significant difference.
Our review shows that the studies that used the peripheral blood of animals to prepare PRP had a lower heterogeneity than those that used human umbilical cord blood. Although the difference between the two groups was not significant, the extent of improvement in the motor function of animals using human umbilical cord blood was higher than the other group. The results of the grouping of articles based on the allograft/autograft and xenograft transplantation were completely the same as the grouping based on the origin of PRP.
Cell damage and the activation of inflammatory cascades in the spinal cord are responsible for the formation of scar tissue and preventing an effective regeneration of nervous tissue by inactivating growth factors, and stem cells in the injury site, reducing the activity of glial cells and increasing the activity of macrophages (Pang et al., 2021). Current treatment strategies, both in medical treatments and surgical interventions, emphasize the greater effect of treatment in faster interventions. For example, current findings emphasize the high effectiveness of spinal decompression surgeries in the first 24 hours after SCI (Li et al., 2014; Yousefifard et al., 2017). Moreover, even more recent review studies consider surgical intervention in the first 12 hours after SCI more effective (Yousefifard et al., 2022). Nonetheless, the evidence of greater effectiveness of PRP administration after 24 hours of SCI is notable in the present study.
To illustrate this more, it can be pointed out that the SCI environment is unsuitable for the survival of growth factors in the acute phase of injury due to severe inflammation (Garcia et al., 2016). Since the effect of PRP administration on the immune response is a matter of debate (Chen et al., 2018), the possibility of intensification of the immune response due to inflammation could reduce the survival and effectiveness of PRP growth factors in the acute phase. As a result, it seems that the administration of PRP after 24 hours of injury is a potentially suitable treatment for improving motor function following SCI.
In review studies, the researcher does not control the intervention and control groups, so it is impossible to match the confounding variables between the studied groups. In the included studies, antibiotic treatment type and duration have been variable. Also, one of the included studies was conducted on mice, and the other studies were conducted on rats. The site of PRP administration in all studies was intra-spinal, while it was intra-thecal in one study. The number of platelets in the administered PRP was not mentioned in 4 studies. In all the cases mentioned, grouping the findings was impossible due to the low number of analyses.
5. Conclusion
The findings of the present meta-analysis show that PRP administration significantly improves the motor function of animals and the cavity size following SCI. Also, the present study shows the necessity of designing and implementing more comprehensive prospective studies to investigate the effectiveness of PRP treatment in spinal cord injuries. In addition, it is necessary to investigate the effectiveness of this treatment on other outcomes, such as pain and inflammation following SCI.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.FMD.REC.1400.526).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data analysis: Mahmoud Yousefifard; Study design, writing and final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
References
Spinal cord injuries (SCIs) primarily affect young adults and thus have devastating physical, psychological, and social impacts. This condition substantially burdens healthcare systems (Badhiwala et al, 2019; James et al., 2019). SCI leads to sensory and or motor deficits, which often present as gait disturbances, loss of coordination, severe neuropathic pain, and incontinence. Despite advances in healthcare, patients with spinal cord injuries often have a decreased quality of life (QoL) and suffer more from morbidities due to subsequent chronic symptoms (Sezer et al., 2015).
The current management strategies for SCI involve surgery, symptomatic treatment, and physical rehabilitation (Walters et al., 2013). In the acute phase of the disease, methylprednisolone is recommended during the first 8 hours. However, there is insufficient evidence to support the use of high-dose steroids 8 hours after an acute SCI (Bracken, 2012). Although physical rehabilitation and other treatment strategies can relatively improve the complications caused by spinal cord injuries, patients often face lifelong severe disabilities and chronic morbidities. Research in this field is still in progress and various therapeutic strategies from the molecular, gene, or cellular therapy or even the use of high-tech equipment such as virtual reality have been recommended to ameliorate symptoms (Janzadeh et al., 2017; Mammana et al., 2019; Miguel-Rubio et al., 2020; Nakhjavan-Shahraki et al., 2018; Sarveazad et al., 2017; Sarveazad et al., 2019; Silvestro et al., 2020). However, proposing these treatment options for Food and Drug Administration (FDA) approvals needs sufficient preclinical and clinical studies.
Platelet-rich plasma (PRP) has recently received much attention as a potential candidate for treating SCI. PRP contains several growth factors responsible for tissue regeneration and repair. The presence of growth factors and protective cytokines such as platelet-derived growth factor, transforming growth factor beta, fibroblast growth factor, insulin-like growth factor-1, insulin-like growth factor-2, vascular endothelial growth factor, epidermal growth factor, interleukin 8, keratinocyte growth factor, and connective tissue growth factor makes PRP a suitable agent for the treatment of neurodegenerative and inflammatory diseases (Marx, 2004). Regarding the pathophysiology of SCI, we observe the simultaneous occurrence of inflammation and neurodegeneration. In the acute phase of the injury, severe inflammation causes a cascade of pathophysiological events, ultimately initiating neurodegeneration and permanent lesions in the spinal cord (Alizadeh et al., 2019).
Recent research shows that PRP administration in the acute, subacute, and chronic phases of SCI improves locomotor function and reduces long-term side effects such as neuropathic pain (Salarinia et al., 2020; Salarinia et al., 2017). The administration of PRP improves angiogenesis and promotes axonal regeneration but does not significantly affect the immune system’s reaction (Chen et al., 2018). Therefore, if PRP administration can prevent the occurrence of permanent damage in spinal cord injuries, it could be used as an easy and accessible treatment in the future. Moreover, the isolation and preparation of autologous PRP are simple and fast, and these beneficial effects have created a promising window for treating spinal cord injuries. Developments in the administration of PRP for spinal cord injuries are still in the preclinical phase, and there is no conclusion on this matter. It is still not yet clear which treatment protocol of PRP has the best effectiveness, and the best time and method of administration are yet unknown. Therefore, to start clinical trials, it is necessary to provide valid preclinical evidence of PRP being a potential candidate for the treatment of SCI. The present systematic review and meta-analysis intend to collect preclinical evidence on the efficacy of different PRP administration protocols in spinal cord injuries, emphasizing functional recovery and cavity size. As a supplementary analysis, we assessed the effect of different PRP protocol treatments and the severity of spinal cord injury on the effectiveness of PRP treatment following spinal cord injury.
2. Materials and Methods
Study design
The present systematic review and meta-analysis collected preclinical evidence on the effectiveness of PRP administration in spinal cord injuries, emphasizing functional recovery and cavity size. For this purpose, an extensive literature search was conducted using the electronic databases of Medline, Embase, Scopus, and Web of Science until October 23, 2022. The search strategy was based on keywords related to PRP and SCI—the search strategy in the Medline database (Appendix 1).
Inclusion criteria
The PICO (population, intervention, comparison, outcome) components are as follows. The population includes animals (rats or mice) with SCI caused by compression, contusion, transection, or hemi-section. Intervention is the administration of PRP. A comparison was performed with a similar group that did not receive PRP, and the outcome was functional recovery and cavity size. Based on these criteria, studies conducted on animals with SCI for which PRP was administered have been included. The exclusion criteria were studies without a control group, review articles, and retracted studies.
Data gathering
Two independent researchers collected the data. After the search, articles were obtained from the mentioned databases and gray literature (Google and Google Scholar and the thesis section of the ProQuest database) in the eighth version of Endnote. These two researchers independently performed the initial screening process. The title and abstract of each article were reviewed, and if the article was relevant or likely to be relevant, the full text of the study was collected and studied. Then, the data of these studies were summarized in a checklist designed based on PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines. The extracted data included information about the study design, the characteristics of the sample and control groups (age, gender, etc.), the sample size, the type of SCI, the time interval from the occurrence of injury, and the administration of PRP, the dose of PRP, the location of SCI, the method of PRP administration, and the follow-up period.
Quality control
The quality assessment of the articles was done by two researchers independently with the SYRCLE risk of bias tool (Hooijmans et al., 2014). In case of disagreement between the researchers, the disagreement was resolved through discussion with each other or a third researcher.
Statistical analyses
The analyses were performed using the statistical program STATA 17.0. Data are recorded as Mean±SD. The presence of heterogeneity has been investigated using the I2 test. The relationships between the location of SCI, the severity of the injury, PRP dosage, PRP administration method, follow-up period, functional recovery, and cavity size were assessed using the “meta” command. Egger’s test and the funnel plot were also used to investigate publication bias.
3. Results
The search resulted in 168 articles. After removing duplicates, 105 articles were advanced for screening. After reviewing the full text of 26 articles, the data from 9 original articles were included in the present meta-analysis: Behroozi et al. (2021); Behroozi et al. (2022); Chen et al., (2018); EL-Seddawy et al., 2020; Hu et al. (2022); Lam et al. (2016); Salarinia et al., (2020); Salarinia et al. (2017); and Zhao et al., (2013) (Figure 1).

Of these 9 articles, 8 were performed on rats and 1 on mice. All studies used the SCI model in the thoracic region of the spinal cord. The injury model was compression in 3 studies, contusion in 4 studies, transection in 1 study, and hemi-section in 1 study. The severity of the injury was moderate in 3 studies and severe in 6 studies.
PRP was taken from human umbilical cord blood (xenograft) in 3 studies and peripheral blood (allograft/autograft) in 6 studies. In 5 studies, PRP administration was performed 24 hours after the injury, and in 3 studies, during the first 24 hours. In one study, the intervention was done in two separate groups, less than 24 hours and after 24 hours from the time of injury. The follow-up period was more than 4 weeks in 6 studies and 4 weeks or less in 3 studies. The outcome was the functional recovery in 7 studies and cavity size in one study. One article investigated the PRP administration effect on functional recovery and cavity size. All included studies examining functional recovery reported this outcome using the Basso Beattie and Bresnahan (BBB) scale. Table 1 presents the characteristics of the included articles.

The effect of PRP administration on function recovery
A total of 8 articles examined functional recovery. Using the Galbraith plot to find the outlier data, it was found that the study of EL-Seddawy et al., (2020) is an outlier. Therefore, it was excluded from the analysis. Eventually, 7 studies with 10 separate analyses were included in the current meta-analysis. The results of the pooled-data analysis showed that PRP administration has significantly improved the motor function of animals with SCI (standardized mean differences [SMD]=1.5; 95% CI, 0.9%, 2.1%; P<0.0001). Due to the moderate heterogeneity (I2=67.01%), the subgroup analyses were performed (Figure 2).

In the subgroup analysis based on the severity of SCI, treatment with PRP in both moderate injuries (SMD=2.59; 95% CI, 1.59%, 3.59%; P<0.0001; I2=30.22%) and severe ones (SMD=1.22; 95% CI, 0.64%, 1.79%; P<0.0001; I2=56.35%) has been associated with a significant improvement in the motor function of animals. However, the extent of this recovery in moderate injuries was significantly greater than in the severe injury group (meta-regression coefficient=-1.36; 95% CI, -2.68%, -0.09%; P<0.035). PRP from peripheral blood (SMD=1.3; 95% CI, 0.62, 1.97%; P<0.0001; I2=58.99%) and human umbilical cord blood (SMD=1.97; 95% CI, 0.63%, 3.3%; P<0.0001; I2=80.74%) were both effective in improving the motor function of animals. However, no significant difference was observed between the two groups (meta-regression coefficient=0.62; 95% CI, -0.69%, -1.94%; P=0.35). PRP administration in the first 24 hours after SCI (SMD=0.86; 95% CI, 0.17%, 1.56%; P=0.015; I2=32.36%) and after 24 hours (SMD=1.87; 95% CI, 1.11%, 2.64%; P<0.0001; I2=67.93%) both were effective in improving the motor function of animals. Meta-regression showed no significant difference between the administration of PRP in the first 24 hours and after 24 hours from injury (meta-regression coefficient=0.96; 95% CI, -0.16%, 2.09%; P=0.09). Finally, there was no significant difference regarding the follow-up period (meta-regression coefficient=0.33; 95% CI, -1.07%, 1.74%; P=0.64) (Table 2).

The effect of PRP administration on cavity size
In this section, 2 articles and 3 analyses were included. Pooled-data analysis showed that PRP administration significantly reduces the cavity size in animals with SCI (SMD=-2.2; 95% CI, -3.44%,-0.95%; P<0.0001) (Figure 3).

Quality control
None of the articles in the quality control assessment section reported housing randomization or random outcome assessment. Therefore, the risk of bias was considered unclear in these items. The risk of bias was low in other items in the included articles. Generally, the quality of data was considered fair (Table 3).

Publication bias
Egger’s test showed no publication bias in the reports of functional recovery (P=0.64) (Figure 4). Since the assessment of cavity size was reported in 3 analyses, publication bias assessment was not feasible in terms of methodology.

4. Discussion
The purpose of this meta-analysis was to investigate the effectiveness of PRP administration in the treatment of spinal cord injuries. By analyzing the data of current original studies, we showed that PRP administration can significantly improve motor functions and cavity size after SCI. However, there was considerable heterogeneity in the findings of the articles. To find the cause of this heterogeneity, a subgroup analysis was performed on the severity of the SCI, the origin of PRP, the time elapsed from the injury to the administration of PRP, and the length of follow-up. According to these analyses, the severity of injury was the reason for heterogeneity in the articles.
The studies conducted on moderate spinal injuries had significantly less heterogeneity than those conducted on severe injuries. Although motor function improvements were significant in both moderate and severe injuries, the improvement of motor function in animals with moderate SCI was significantly higher than in animals with severe SCI. In addition to the fact that even in the absence of therapeutic intervention, moderate SCI has a better outcome than severe injury, it should be noted that the low number of analyses performed on moderate spinal cord injuries can be one of the reasons that we observed a significant difference.
Our review shows that the studies that used the peripheral blood of animals to prepare PRP had a lower heterogeneity than those that used human umbilical cord blood. Although the difference between the two groups was not significant, the extent of improvement in the motor function of animals using human umbilical cord blood was higher than the other group. The results of the grouping of articles based on the allograft/autograft and xenograft transplantation were completely the same as the grouping based on the origin of PRP.
Cell damage and the activation of inflammatory cascades in the spinal cord are responsible for the formation of scar tissue and preventing an effective regeneration of nervous tissue by inactivating growth factors, and stem cells in the injury site, reducing the activity of glial cells and increasing the activity of macrophages (Pang et al., 2021). Current treatment strategies, both in medical treatments and surgical interventions, emphasize the greater effect of treatment in faster interventions. For example, current findings emphasize the high effectiveness of spinal decompression surgeries in the first 24 hours after SCI (Li et al., 2014; Yousefifard et al., 2017). Moreover, even more recent review studies consider surgical intervention in the first 12 hours after SCI more effective (Yousefifard et al., 2022). Nonetheless, the evidence of greater effectiveness of PRP administration after 24 hours of SCI is notable in the present study.
To illustrate this more, it can be pointed out that the SCI environment is unsuitable for the survival of growth factors in the acute phase of injury due to severe inflammation (Garcia et al., 2016). Since the effect of PRP administration on the immune response is a matter of debate (Chen et al., 2018), the possibility of intensification of the immune response due to inflammation could reduce the survival and effectiveness of PRP growth factors in the acute phase. As a result, it seems that the administration of PRP after 24 hours of injury is a potentially suitable treatment for improving motor function following SCI.
In review studies, the researcher does not control the intervention and control groups, so it is impossible to match the confounding variables between the studied groups. In the included studies, antibiotic treatment type and duration have been variable. Also, one of the included studies was conducted on mice, and the other studies were conducted on rats. The site of PRP administration in all studies was intra-spinal, while it was intra-thecal in one study. The number of platelets in the administered PRP was not mentioned in 4 studies. In all the cases mentioned, grouping the findings was impossible due to the low number of analyses.
5. Conclusion
The findings of the present meta-analysis show that PRP administration significantly improves the motor function of animals and the cavity size following SCI. Also, the present study shows the necessity of designing and implementing more comprehensive prospective studies to investigate the effectiveness of PRP treatment in spinal cord injuries. In addition, it is necessary to investigate the effectiveness of this treatment on other outcomes, such as pain and inflammation following SCI.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.FMD.REC.1400.526).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data analysis: Mahmoud Yousefifard; Study design, writing and final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
References
Alizadeh, A., Dyck, S. M., & Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Frontiers in Neurology, 10, 282. [DOI:10.3389/fneur.2019.00282] [PMID]
Badhiwala, J. H., Wilson, J. R., & Fehlings, M. G. (2019). Global burden of traumatic brain and spinal cord injury. The Lancet Neurology, 18(1), 24-25. [DOI:10.1016/S1474-4422(18)30444-7] [PMID]
Behroozi, Z., Ramezani, F., Janzadeh, A., Rahimi, B., & Nasirinezhad, F. (2021). Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiology & Behavior, 228, 113186. [DOI:10.1016/j.physbeh.2020.113186] [PMID]
Behroozi, Z., Ramezani, F., & Nasirinezhad, F. (2022). Human umbilical cord blood-derived platelet-rich plasma: A new window for motor function recovery and axonal regeneration after spinal cord injury. Physiology & Behavior, 252, 113840. [DOI:10.1016/j.physbeh.2022.113840] [PMID]
Bracken, M. B. (2012). Steroids for acute spinal cord injury. The Cochrane Database of Systematic Reviews, 1(1), CD001046.[DOI:10.1002/14651858.CD001046.pub2] [PMID]
Chen, N. F., Sung, C. S., Wen, Z. H., Chen, C. H., Feng, C. W., & Hung, H. C., et al. (2018). Therapeutic Effect Of Platelet-Rich Plasma In Rat Spinal Cord Injuries. Frontiers in Neuroscience, 12, 252. [DOI:10.3389/fnins.2018.00252] [PMID]
De Miguel-Rubio, A., Rubio, M. D., Salazar, A., Camacho, R., & Lucena-Anton, D. (2020). Effectiveness of virtual reality on functional performance after spinal cord injury: A systematic review and meta-analysis of randomized controlled trials. Journal of Clinical Medicine, 9(7), 2065. [DOI:10.3390/jcm9072065] [PMID]
EL-Seddawy, F. D., Samy, M. T. M., Mekkawy, N. H. M., Behery, A. E., & Youssef, W. O. M. (2020). Experimental trials of spinal cord injury treatment in rats. Journal of Animal Health and Production, 9(s1), 27-33. [DOI:10.17582/journal.jahp/2020/9.s1.27.33]
Garcia, E., Aguilar-Cevallos, J., Silva-Garcia, R., & Ibarra, A. (2016). Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators of Inflammation, 2016, 9476020. [DOI:10.1155/2016/9476020] [PMID]
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators (2019). Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology, 18(1), 56–87. [DOI:10.1016/S1474-4422(18)30415-0] [PMID]
Hooijmans, C. R., Rovers, M. M., De Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., & Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Medical Research Methodology, 14, 43. [DOI:10.1186/1471-2288-14-43] [PMID]
Hu, Z. B., Chen, H. C., Wei, B., Zhang, Z. M., Wu, S. K., & Sun, J. C., et al. (2022). Platelet rich plasma enhanced neuro-regeneration of human dental pulp stem cells in vitro and in rat spinal cord. Annals of Translational Medicine, 10(10), 584.[DOI:10.21037/atm-22-1745] [PMID]
Janzadeh, A., Sarveazad, A., Yousefifard, M., Dameni, S., Samani, F. S., & Mokhtarian, K., et al. (2017). Combine effect of Chondroitinase ABC and low level laser (660 nm) on spinal cord injury model in adult male rats. Neuropeptides, 65, 90-99. [DOI:10.1016/j.npep.2017.06.002] [PMID]
Lam, H. T.-M., Tran, M. N.-T., Bui, K. A., Le, T. T.-T., Bui, K. H.-T., Phan, N. K., & Van Pham, P. (2016). Adipose tissue derived stromal vascular fraction transplantation can recover spinal cord injury in mice. Progress in Stem Cell, 3(04), 144-158. [Link]
Li, Y., Walker, C. L., Zhang, Y. P., Shields, C. B., & Xu, X. M. (2014). Surgical decompression in acute spinal cord injury: a review of clinical evidence, animal model studies, and potential future directions of investigation. Frontiers in Biology, 9(2), 127-136. [DOI:10.1007/s11515-014-1297-z] [PMID]
Mammana, S., Gugliandolo, A., Cavalli, E., Diomede, F., Iori, R., & Zappacosta, R., et al. (2019). Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. Journal of Tissue Engineering and Regenerative Medicine, 13(7), 1109-1121. [DOI:10.1002/term.2857] [PMID]
Marx, R. E. (2004). Platelet-rich plasma: evidence to support its use. Journal of Oral and Maxillofacial Surgery, 62(4), 489-496. [DOI:10.1016/j.joms.2003.12.003] [PMID]
Nakhjavan-Shahraki, B., Yousefifard, M., Rahimi-Movaghar, V., Baikpour, M., Nasirinezhad, F., & Safari, S., et al. (2018). Transplantation of olfactory ensheathing cells on functional recovery and neuropathic pain after spinal cord injury; systematic review and meta-analysis. Scientific Reports, 8(1), 325. [DOI:10.1038/s41598-017-18754-4] [PMID]
Pang, Q. M., Chen, S. Y., Xu, Q. J., Fu, S. P., Yang, Y. C., & Zou, W. H., et al. (2021). Neuroinflammation and scarring after spinal cord injury: Therapeutic roles of MSCs on inflammation and glial scar. Frontiers in Immunology, 12, 751021. [DOI:10.3389/fimmu.2021.751021] [PMID]
Salarinia, R., Hosseini, M., Mohamadi, Y., Ghorbani, A., Alamdari, D. H., & Mafinezhad, A., et al. (2020). Combined use of platelet-rich plasma and adipose tissue-derived mesenchymal stem cells shows a synergistic effect in experimental spinal cord injury. Journal of Chemical Neuroanatomy, 110, 101870. [DOI:10.1016/j.jchemneu.2020.101870] [PMID]
Salarinia, R., Sadeghnia, H. R., Alamdari, D. H., Hoseini, S. J., Mafinezhad, A., & Hosseini, M. (2017). Platelet rich plasma: Effective treatment for repairing of spinal cord injury in rat. Acta Orthopaedica et Traumatologica Turcica, 51(3), 254-257. [DOI:10.1016/j.aott.2017.02.009] [PMID]
Sarveazad, A., Babahajian, A., Bakhtiari, M., Soleimani, M., Behnam, B., & Yari, A., et al. (2017). The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides, 61, 39–47. [DOI:10.1016/j.npep.2016.07.004] [PMID]
Sarveazad, A., Janzadeh, A., Taheripak, G., Dameni, S., Yousefifard, M., & Nasirinezhad, F. (2019). Co-administration of human adipose-derived stem cells and low-level laser to alleviate neuropathic pain after experimental spinal cord injury. Stem Cell Research & Therapy, 10(1), 183. [DOI:10.1186/s13287-019-1269-y] [PMID]
Sezer, N., Akkuş, S., & Uğurlu, F. G. (2015). Chronic complications of spinal cord injury. World Journal of Orthopedics, 6(1), 24-33. [DOI:10.5312/wjo.v6.i1.24] [PMID]
Silvestro, S., Bramanti, P., Trubiani, O., & Mazzon, E. (2020). Stem cells therapy for spinal cord injury: An overview of clinical trials. International Journal of Molecular Sciences, 21(2), 659. [DOI:10.3390/ijms21020659] [PMID]
Walters, B. C., Hadley, M. N., Hurlbert, R. J., Aarabi, B., Dhall, S. S., & Gelb, D. E., et al. (2013). Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery, 60(CN_suppl_1), 82-91. [DOI:10.1227/01.neu.0000430319.32247.7f] [PMID]
Yousefifard, M., Hashemi, B., Forouzanfar, M. M., Khatamian Oskooi, R., Madani Neishaboori, A., & Jalili Khoshnoud, R. (2022). Ultra-early spinal decompression surgery can improve neurological outcome of complete cervical spinal cord injury; a systematic review and meta-analysis. Archives of Academic Emergency Medicine, 10(1), e11. [PMID]
Yousefifard, M., Rahimi-Movaghar, V., Baikpour, M., Ghelichkhani, P., Hosseini, M., & Jafari, A., et al. (2017). Early versus late spinal decompression surgery in treatment of traumatic spinal cord injuries; a systematic review and meta-analysis. Emergency, 5(1), e37. [PMID]
Zhao, T., Yan, W., Xu, K., Qi, Y., Dai, X., & Shi, Z. (2013). Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy, 15(7), 792-804. [DOI:10.1016/j.jcyt.2013.04.004] [PMID]
Type of Study: Review |
Subject:
Cellular and molecular Neuroscience
Received: 2022/12/29 | Accepted: 2023/07/2 | Published: 2024/07/20
Received: 2022/12/29 | Accepted: 2023/07/2 | Published: 2024/07/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








