Volume 16, Issue 2 (March & April 2025)
BCN 2025, 16(2): 519-532 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fazeli A, Zolghadriha A, Pirzeh R, Ramezani S, Dadashi M. Effectiveness of Low-frequency rTMS and CBT in Reducing Symptoms Severity and Improving Cognitive Flexibility in Adults With OCD: A Clinical Trial. BCN 2025; 16 (2) :519-532
URL: http://bcn.iums.ac.ir/article-1-2616-en.html
URL: http://bcn.iums.ac.ir/article-1-2616-en.html
1- Department of Psychiatry, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
2- Department of Educational Sciences, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran.
3- Department of Clinical Psychology, Social Determinants of Health Research Center, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
2- Department of Educational Sciences, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran.
3- Department of Clinical Psychology, Social Determinants of Health Research Center, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
Keywords: Obsessive-compulsive disorder (OCD), Cognitive behavioral therapy (CBT), Repetitive transcranial magnetic stimulation (rTMS), Cognitive flexibility
Full-Text [PDF 772 kb]
| Abstract (HTML)
Full-Text:
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating and severe mental disorder characterized by varying degrees of obsessive thoughts and behaviors. Obsessive thoughts are intrusive, unwanted, and annoying thoughts or images that people experience spontaneously incompatible with the person’s obvious and perceived feelings. Compulsions are repetitive and time-consuming behaviors or mental acts that are used to neutralize anxiety caused by obsessive thoughts (Rapinesi et al., 2019; Robbins et al., 2019). According to the World Health Organization (WHO), it is among the 10 disabling disorders (Melchior et al., 2019). Its lifelong prevalence is 1%-3% worldwide (Kessler et al., 2005) and 5.1%-1.8% in Iran (Mohammadi et al., 2004; Vandad Sharifi et al., 2015). OCD has a gradual onset and becomes chronic if people do not receive treatment (Olatunji et al., 2013), and its symptoms change over time due to stressors in life (Stewart et al., 2004). People with OCD tend to engage in obsessive actions, and even if they know that obsessive actions are useless, they cannot stop it (Sternheim et al., 2014). Impaired executive functioning has been observed frequently in OCD individuals (Fournet et al., 2019). Executive functioning is defined as managing intervening components in goal-directed behaviors and predicting the consequences of behavior (Pajouhinia et al., 2020). Executive functions include cognitive processes, such as working memory, inhibitory control, and cognitive flexibility, essential for goal-directed behavior (Miyake et al., 2000; Nejati et al., 2020). Clinically, people with OCD have difficulty switching between mental processes to generate adaptive behavioral responses to their symptoms. Many neurological studies have shown reduced cognitive flexibility in people with OCD (Gruner & Pittenger, 2017; Vriend et al., 2013). The ability to modify cognitive sets to adapt to variable environmental stimuli is a key component in most operational definitions of cognitive flexibility. It is considered a wide range of behaviors that enable people to adapt adaptively to stressful events instead of having maladaptive behaviors (Kurginyan & Osavolyuk, 2018). Recently, neurological models of OCD have suggested cognitive inflexibility as a significant feature of OCD patients, which can also be present in their relatives (Chamberlain et al., 2007). Although OCD patients have many cognitive impairments, impaired cognitive flexibility may be an essential trait for understanding the neural basis of OCD (Tomiyama et al., 2019).
Cognitive-behavioral therapy (CBT) based on exposure and response prevention (ERP) is currently the standard treatment for OCD. In CBT, individuals are believed to respond to the cognitive representation of stressful events instead of responding to these events (Porto et al., 2009). ERP involves gradual and long-term exposure to intimidating stimuli and avoiding obsessive actions (Olatunji et al., 2013). CBT can reduce cortico-striato-thalamo-cortical circuit hyperactivity and ultimately help improve the symptoms of OCD (Moody et al., 2017). However, CBT is much less common than drug therapy. According to surveys in the United Kingdom and USA, only 5% of adults with OCD receive CBT (O’Neill & Feusner, 2015). On average, 30% of these patients refuse ERP therapy or drop out of treatment (Olatunji et al., 2010; Melchior et al., 2019). Therefore, complementary interventions have been suggested as an alternative to overcome CBT limitations in treating OCD. A potential new treatment option, repetitive transcranial magnetic stimulation (rTMS), can modulate neural activity in brain circuits (Elbeh et al., 2016; Husain et al., 2002). First introduced by Barker et al. in 1985, rTMS is a non-invasive technique that delivers electromagnetic pulses to selected areas of the cerebral cortex (Barker et al., 1985; Jaafari et al., 2012). The stimulation can be applied at either high (≥5 Hz) or low (≤1 Hz) frequencies, which have stimulatory and inhibitory effects on cortical excitability, respectively (Lefaucheur et al., 2014). Studies have shown that the dorsolateral prefrontal cortex (DLPFC) is essential in cognitive flexibility (Borwick et al., 2020; Quiñones-Camacho et al., 2019). Thus, improvement in DLPFC neuronal function may help improve the cognitive flexibility of patients with OCD. In a clinical trial, Seo et al. reported the effectiveness of low-frequency rTMS (LF-rTMS) over the right DLPFC in relieving the symptoms of OCD and depression in OCD patients (Seo et al., 2016).
Due to the high involvement of networks in the pathophysiology of OCD and the rTMS’s ability to adjust cortical and subcortical structures and its potential therapeutic effectiveness in modulating inactive or hyperactive areas of the brain by targeting cortical circuits in patients with OCD, and lack of study on comparing the efficacy of CBT and LF-rTMS in treating OCD patients, the present study aims to compare the effects of CBT with ERP and rTMS over the right DLPFC on symptoms and cognitive flexibility in people with OCD. It is hypothesized that (a) there is a difference between LF-rTMS and CBT in reducing the severity of OCD symptoms and (b) there is a difference between LF-rTMS and CBT in improving the cognitive flexibility of OCD patients.
Materials and Methods
Study design and participants
This randomized clinical trial employed a pre-test/post-test/follow-up design. The study population consists of all adults with OCD referred to the clinic of Shahid Beheshti Hospital in Zanjan City, Iran, in 2020 (during the COVID-19 pandemic) (n=41). The sample size was determined to be 13 for each group using G*Power software by considering α=0.05, an error probability of 0.95, and an effect size of 0.6 according to previous studies in the literature which reported middle-size to large-size effects of rTMS and CBT on patients with OCD (Perera et al., 2021; Hoppen et al., 2021). Due to the COVID-19 pandemic and considering the 20% dropout, the sample size was increased to 17 for each group. In this regard, 34 patients were selected using a convenience sampling method and randomly (by drawing cards) assigned into two parallel groups of CBT (n=17) and rTMS (n=17). Each group of patients was acknowledged which group s/he was assigned to. The randomization was conducted using a lottery method by the last author. All samples were diagnosed with OCD by a psychiatrist and re-evaluated by a psychologist through a structured clinical interview for diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) (SCID-5) and using the Millon clinical multiaxial inventory-III (MCMI-III). The inclusion criteria were as follows: Having OCD according to the psychiatrist and based on SCID-5, bearing at least a middle-school education, being 18-50 years old, signing a written consent, and lacking a history of psychological therapies, transcranial direct current stimulation, or neurofeedback.
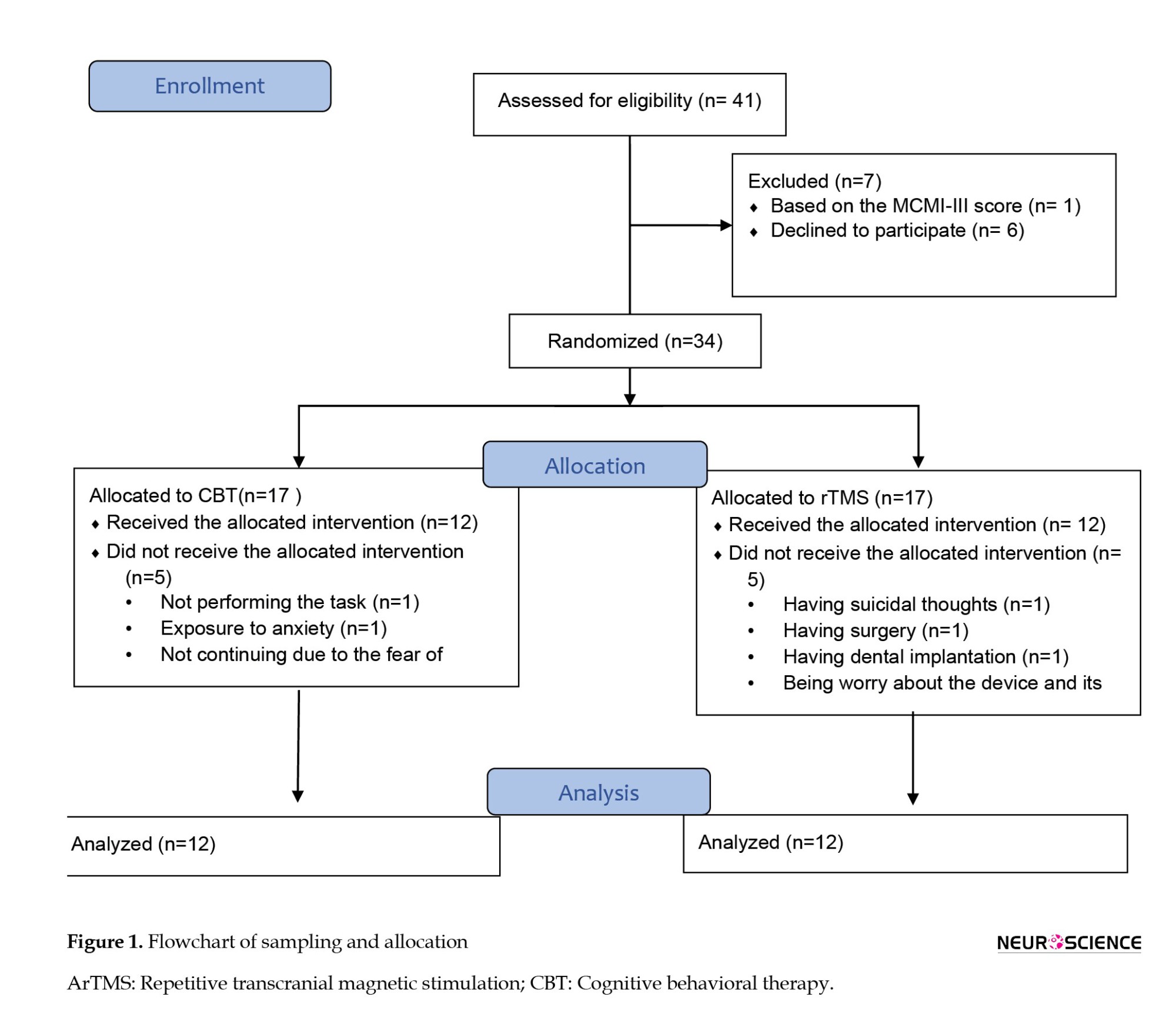
On the other hand, the exclusion criteria were the existence of suicidal thoughts, personality disorders according to the SCID-5 and MCMI-III, psychotic disorders, history of seizures and epilepsy, the existence of an electrical or metal object in the body (e.g. pacemaker), and having bipolar disorder. Before entering the study, the participants received medication whose dosage had been stabilized for four weeks. After and during the study, the psychiatrist kept the dosage the same. Ten patients were excluded from the study (5 from the rTMS group and 5 from the CBT group). Therefore, 12 patients in each group completed the study. Figure 1 shows the sampling and allocation processes.
Study measures
After obtaining written informed consent from the participants, they completed a demographic form, the Yale-Brown obsessive-compulsive scale (Y-BOCS) to assess their OCD symptoms, and the cognitive flexibility inventory (CFI) to evaluate their cognitive flexibility. The Y-BOCS is a semi-structured interview and the gold standard for assessing OCD symptoms. It has two primary scales: Symptom checklist (SC) and severity scale (SS). The SC has 16 self-report items rated on a 5-point scale. In the SS, the severity of obsessions and compulsions is measured in five areas: Distress, frequency, intervention, resistance, and symptom control. In this study, we used the Persian version of Y-BOCS validated by Rajezi Esfahani et al. (2021), who reported internal consistency of 0.97 for the SC and 0.95 for the SS, a split-half reliability of 0.93 for the SC and 0.89 for the SS, and a test re-test reliability of 0.99. In our study, patients completed the SS scale only.
Dennis and Vander Wal (2010) developed the CFI, which is a 20-item self-report tool using a 7-point Likert scale to measure three aspects of cognitive flexibility, including the ability to perceive multiple alternative explanations for life occurrences and human behavior, the ability to generate multiple alternative solutions to difficult situations, and the desire to perceive difficult situations as controllable (Control subscale). The CFI has excellent internal consistency and high test re-test reliability (Dennis & Vander Wal, 2010). They reported Cronbach α values of 0.90, 0.86, and 0.91, as well as test re-test reliability values of 0.81, 0.77, and 0.75 for the overall scale, control, and alternatives subscales, respectively. For its Persian version, Shareh et al. (2014)reported a three-factor structure: control, alternative solutions, and alternative explanations. They reported the Cronbach α and test re-test coefficients for the Persian CFI reliability as 0.90 and 0.71, respectively. The mentioned tools were completed again immediately and one month after the intervention.
Study interventions
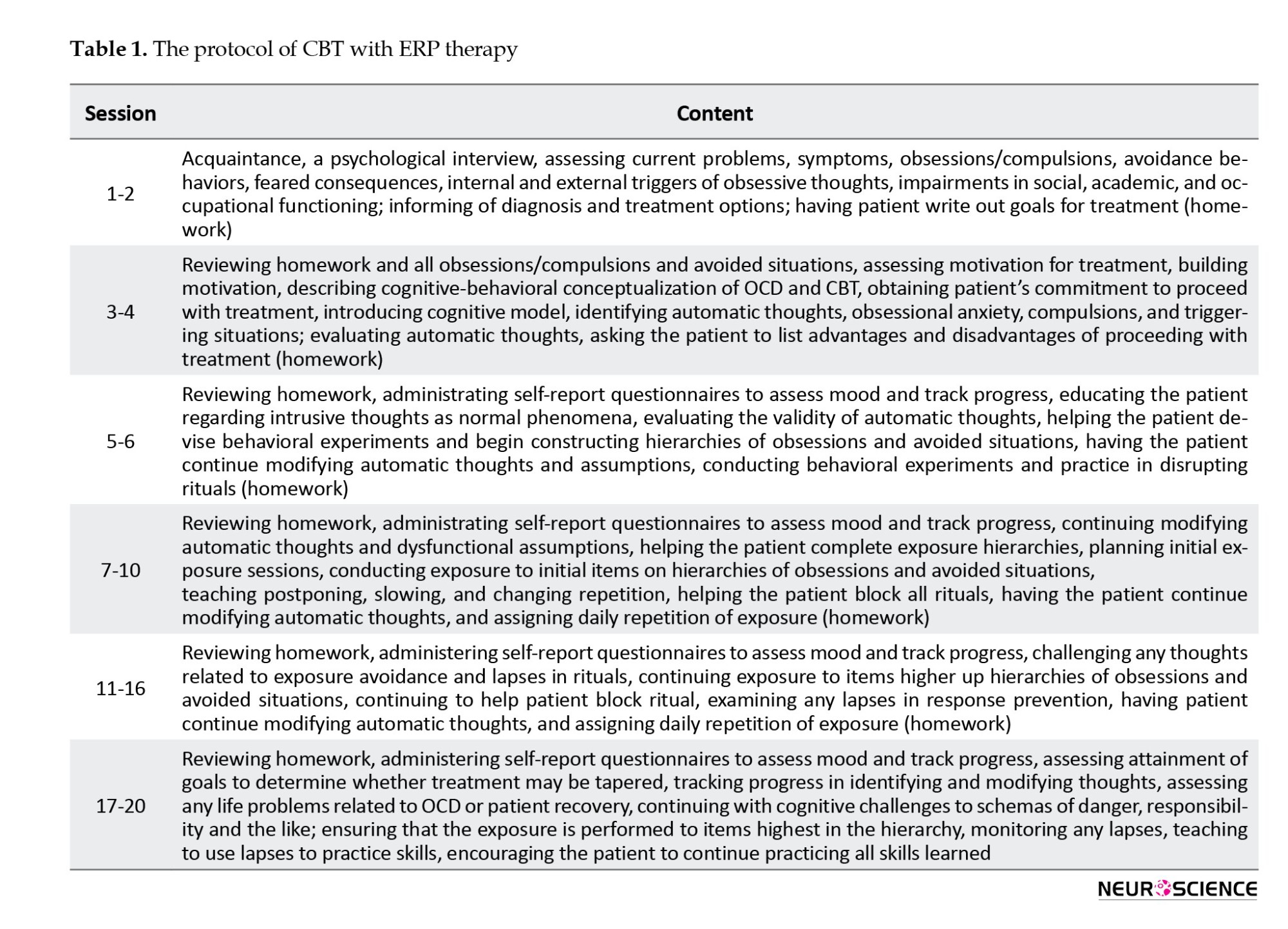
The CBT group individually received CBT with ERP at 20 sessions twice a week, each for 45-90 minutes, according to the protocol proposed by Leahy et al. (2011)(Table 1). According to Jaurrieta et al. (2008), individual CBT is more effective in reducing OCD symptoms than group CBT. Treatment was performed by the researcher (MS student in clinical psychology) under the supervision of a therapist.
The rTMS group received rTMS for 2 weeks at 10 sessions (5 consecutive days per week, each for 20 minutes) according to the protocol proposed by Gomes et al. (2012). Each person received 1-Hz rTMS at 100% of resting MT (1200 pulses per day with a 10-min rest interval between each 300 pulses) using a focal 8-shaped 70-mm coil (Neuro-MS/D Advanced Therapeutic, Neurosoft Ltd., Russia), which was positioned on the right DLPFC (F4, according to the EEG 10–20 International System) such that there was no space between the skin and the coil. The rTMS was conducted by an expert who was unaware of the results.
Data analysis
Descriptive statistics such as Mean±SD, frequency, and percentage were used to analyze the collected data. Also, inferential statistics such as the chi-square test and independent t-test (to examine the differences in demographic factors and pre-test means), multivariate analysis of covariance (MANCOVA) (to compare the groups in terms of Y-BOCS and CFI scores), repeated-measures analysis of variance (ANOVA) (to compare the means of Y-BOCS and CFI between the time points), and the Fisher least significant difference (LSD) test for pairwise comparison were used in SPSS software, version 22. According to the results of the Kolmogorov-Smirnov test, the assumption of normal distribution of data in all three stages of pre-test, post-test, and follow-up was confirmed (P>0.05). According to the results of Levene’s test, the assumption of the quality of variances in the studied groups was not observed in the post-test data of obsessions (a component of Y-BOCS) and the pre-test data of alternatives subscale of CFI (P<0.05). Therefore, the degree of corrected freedom was used to compare the two groups in the mentioned variables. In other variables, the equality of variances was confirmed (P>0.05).
Results
Characteristics of participants
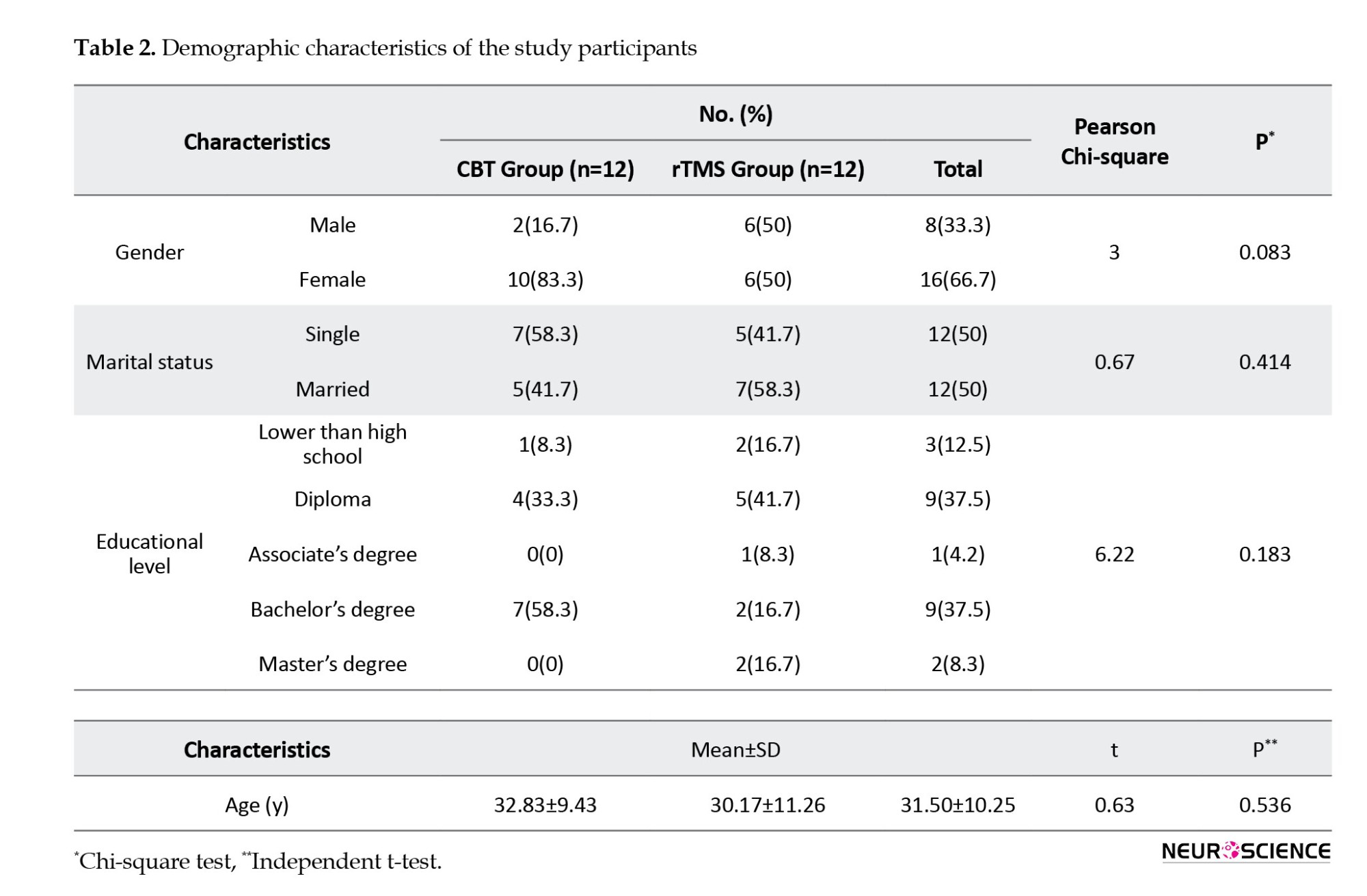
Table 2 presents the demographic characteristics of patients. In the CBT group, with a mean age of 32.83±9.43 years, there were two males and 10 females; 7 were single, 5 were married, and most had a bachelor’s degree (n=7, 58.3%). In the rTMS group with a mean age of 30.17±11.26 years, there were 6 males and 6 females; 5 were single, 7 were married, and most had a high school diploma (n=5, 41.7%). No significant difference was found between the two groups in terms of gender (P=0.083), marital status (P= 0.414), and level of education (P=0.183) according to the results of the chi-square test, and in terms of age (P=0.536) according to the results of independent t-test (Table 2). In the CBT group, 9 patients (75%) had contamination obsessions with washing/cleaning compulsion, 2(16.16%) had harm obsessions with checking compulsions, and one (8.33%) had symmetry obsessions with ordering. In the rTMS group, 9(75%) had contamination obsessions with washing/cleaning compulsions, and 3(25%) had harm obsessions with checking compulsions. However, this difference between groups was not significant according to the chi-square test results (P>0.05).
Comparing OCD symptoms in two study groups
As seen in Figure 2, the pre-test scores of Y-BOCS and its components were higher in the CBT group than in the rTMS group.
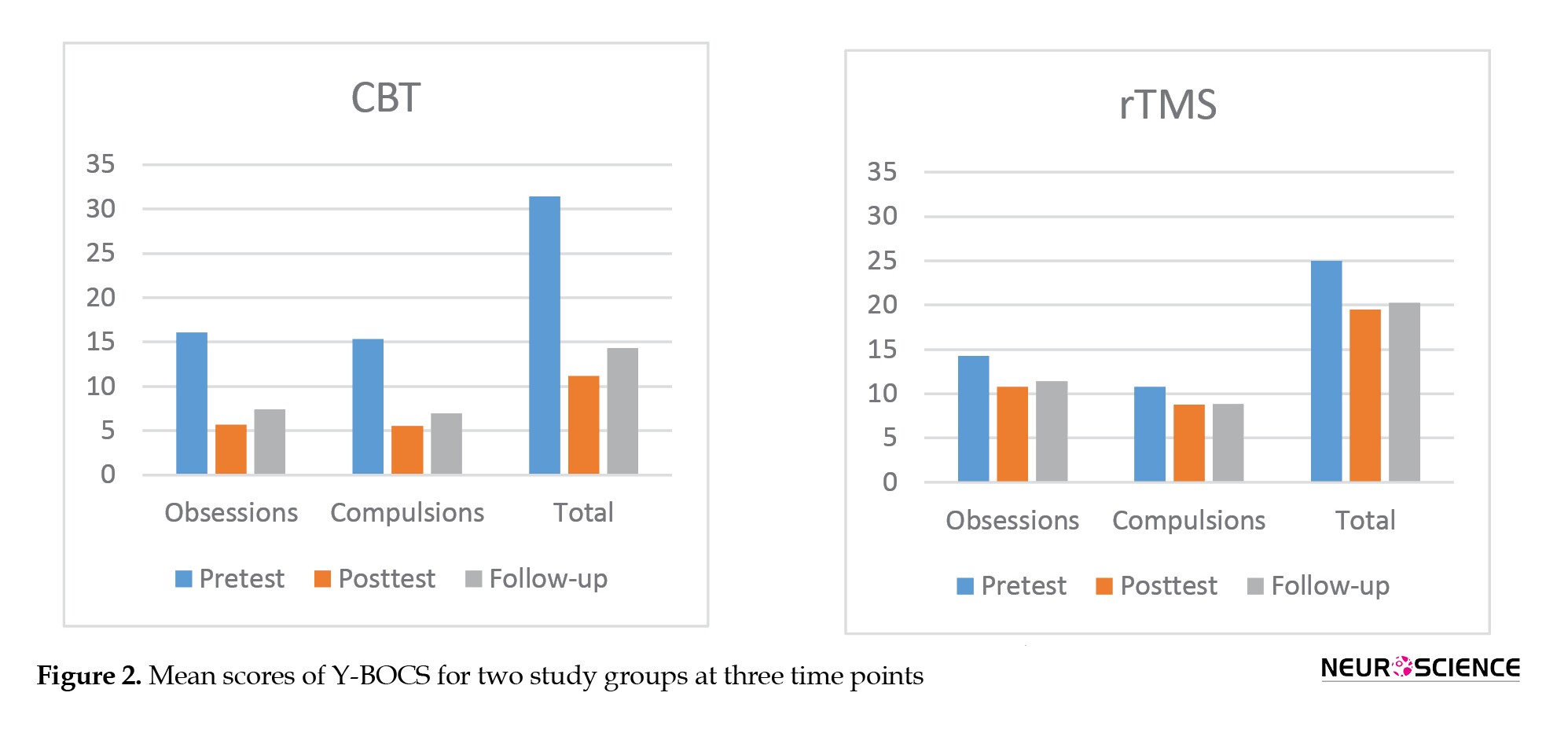
This difference was statistically significant only in the total score (P=0.011) and the compulsions domain (P=0.017). Immediately after the intervention, the scores decreased significantly in both groups, whereas the decrease was higher in the CBT group. Results of MANCOVA (Table 3) showed that, after controlling the pre-test scores, the difference between groups was statistically significant in post-test obsessions (F1, 21=23.645, P<0.001, η2=0.53); post-test compulsions (F1, 21=20.920, P<0.001, η2=0.45); and post-test total score (F1, 21=25.565, P<0.001, η2=0.55). One month after the intervention, these scores slightly increased in both groups.
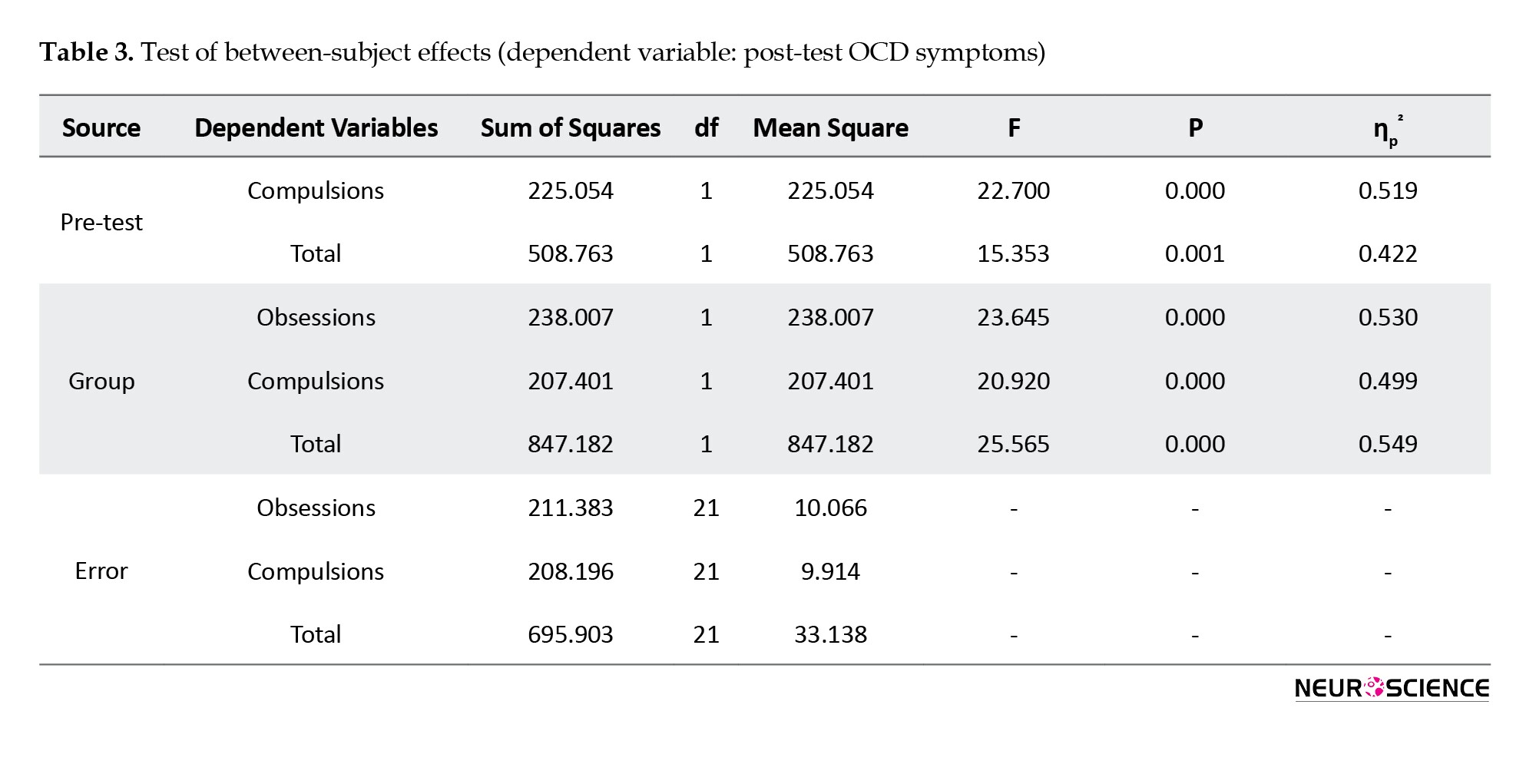
Results of repeated-measures ANOVA (Table 4) showed a significant difference in the Y-BOCS scores within three time points of pre-test, post-test, and follow-up, where the main and interaction effects were significant (P<0.001).
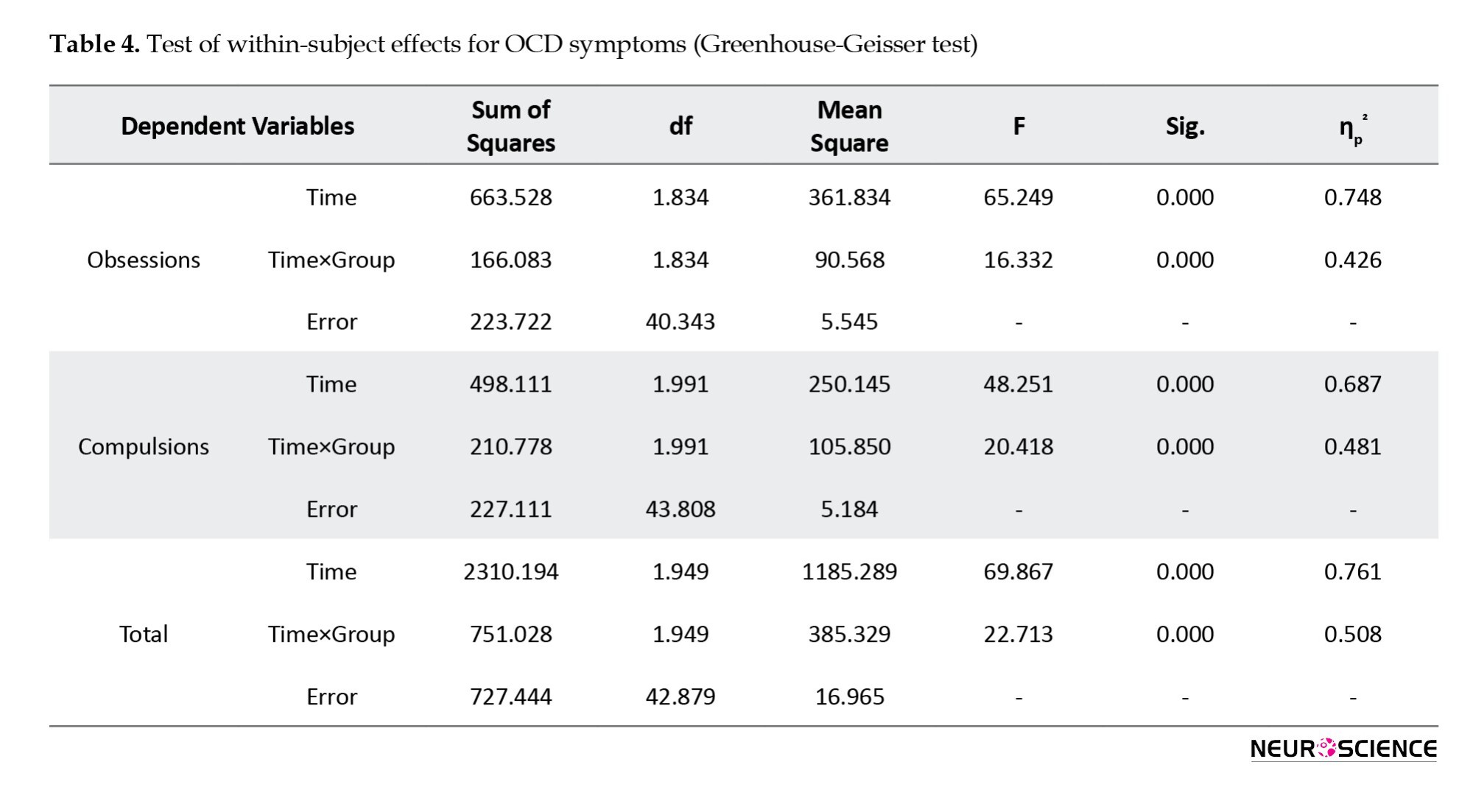
To assess between which time points this difference was observed, the LSD test was conducted. In the CBT group, the results (Table 5) showed a significant difference between pre-test and post-test scores and between pre-test and follow-up scores of obsessions, compulsions, and total scores (P<0.001). Still, there was no significant difference between post-test and follow-up scores (P>0.05). In the rTMS group, there was a significant difference between pre-test and post-test scores and between pre-test and follow-up scores of obsessions and total score (P<0.05) but not in compulsions. No significant differences between the post-test and follow-up scores of any variables were observed in this group (Table 5).
Comparing cognitive flexibility in two study groups
As seen in Figure 3, the pre-test scores of total CFI and its three subscales were higher in the CBT group than in the rTMS group, but there was no significant difference between the pre-test CFI scores of the two groups (P>0.05).
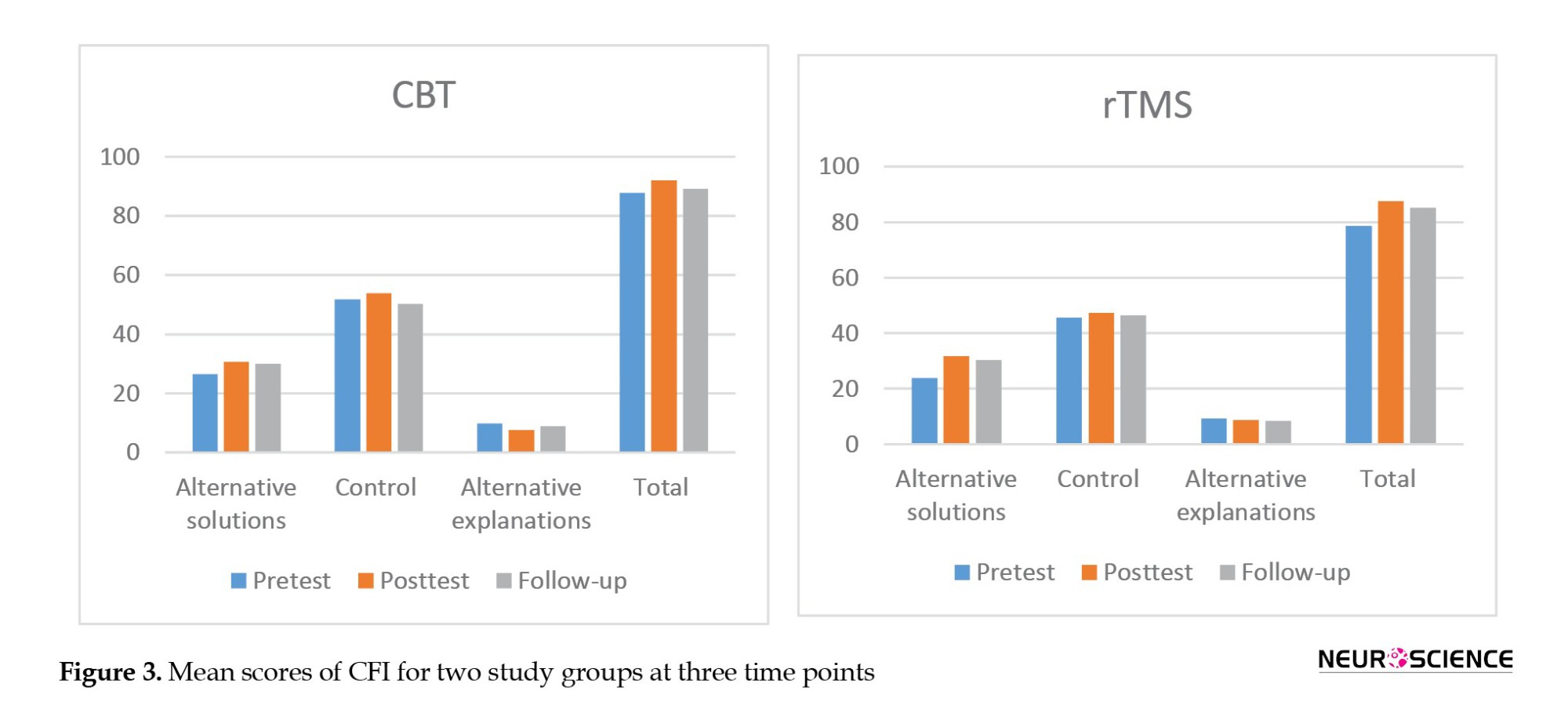
Immediately after the intervention, both groups’ total scores and scores of “alternative solutions” and “control” increased. In contrast, the “alternative explanations” subscale score decreased in both groups. Results of MANCOVA (Table 6) showed that these differences between groups were not statistically significant in any domains (P>0.05).
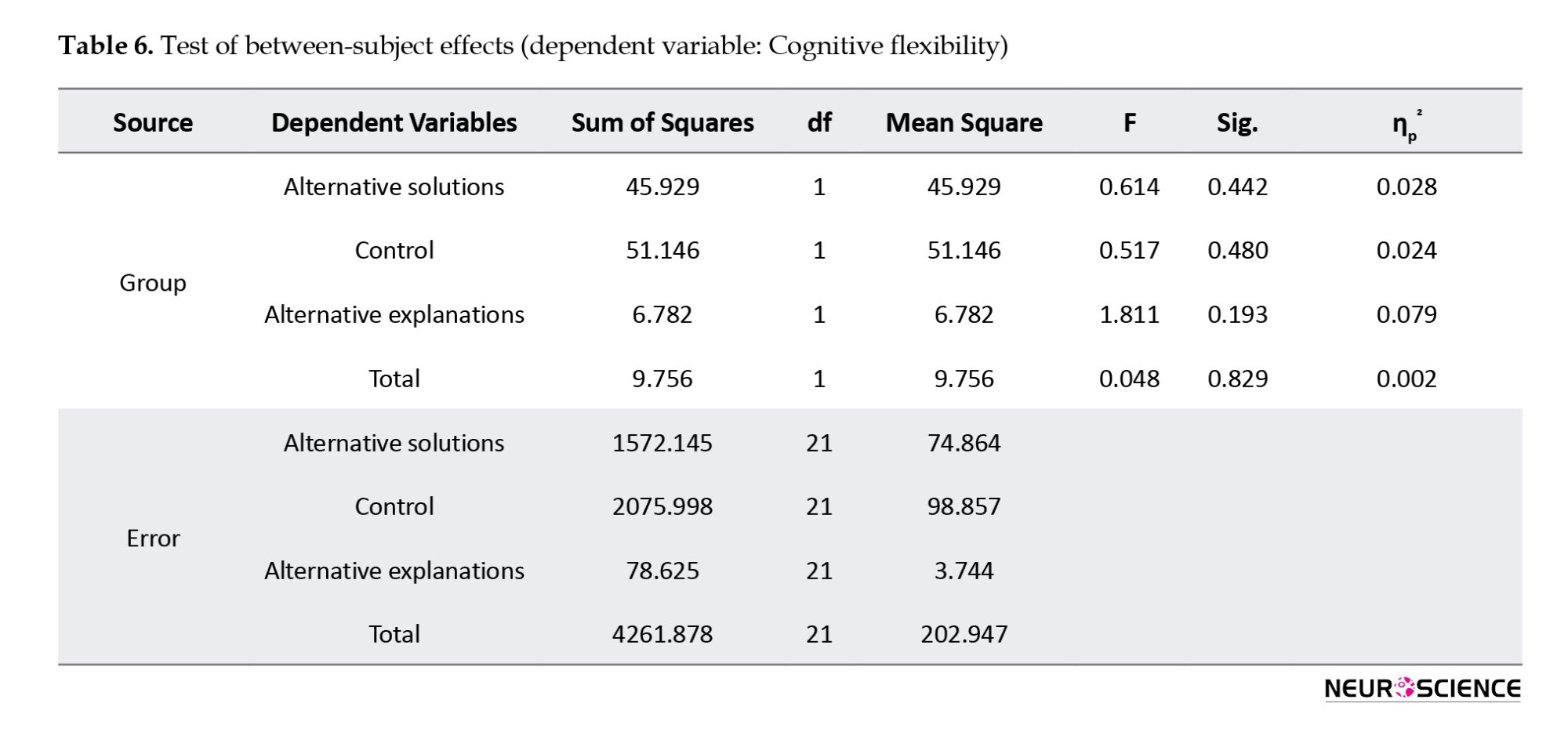
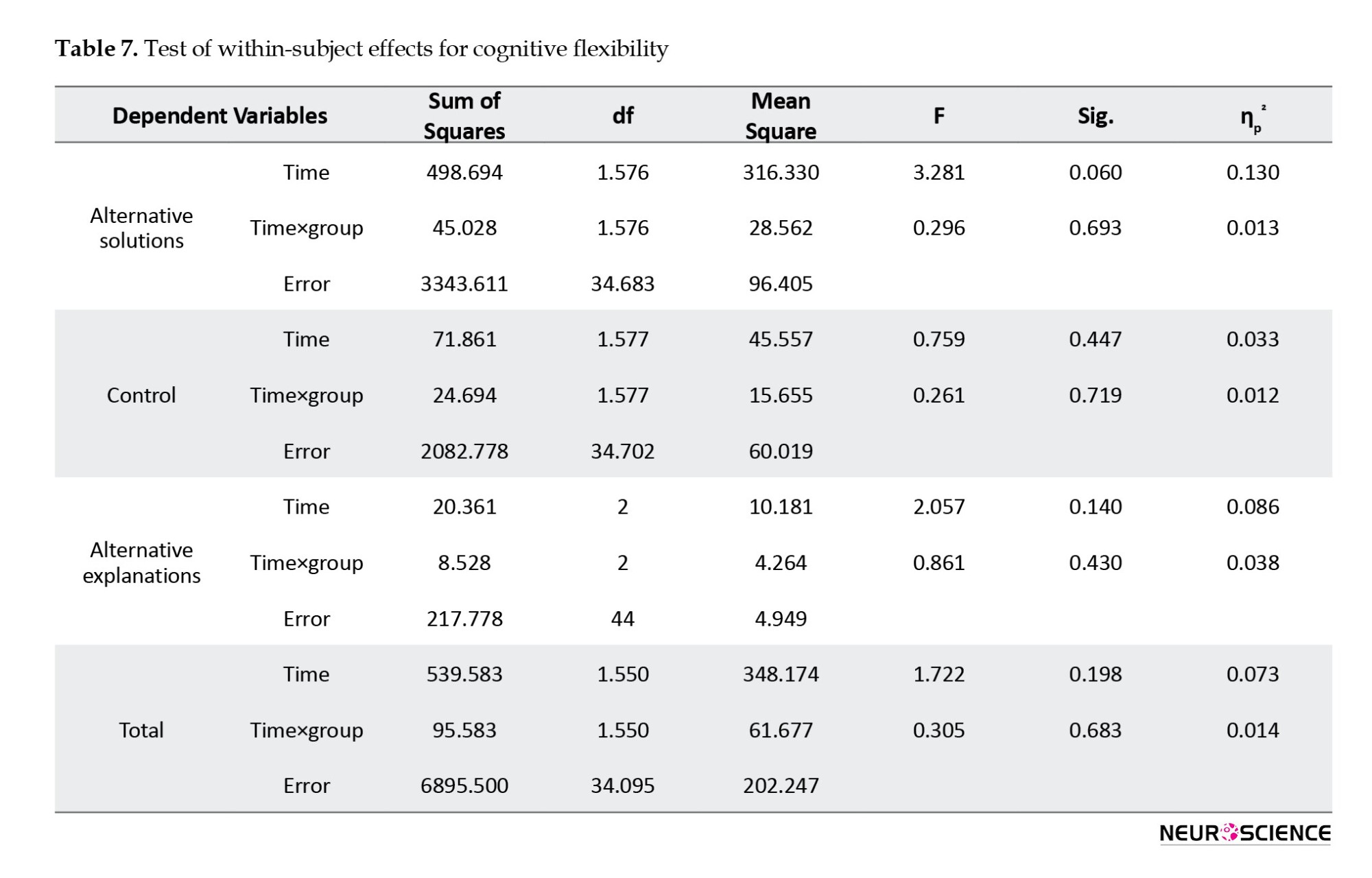
One month after intervention, a slight decrease was reported in the total score of CFI and its subscales in both groups. Results of repeated-measures ANOVA (Table 7) showed no significant overall difference between the two groups in any variables over three time points of pre-test, post-test, and follow-up (P>0.05).
Discussion
The purpose of this study was to compare the effectiveness of CBT with ERP (presented individually) and low frequency (1-Hz) rTMS in reducing symptom severity (Y-BOCS score) and improving cognitive inflexibility (CFI score) in 24 patients with OCD. The results showed that both treatment methods highly reduced the severity of OCD symptoms immediately after intervention, where CBT had a higher effect. The difference between the results of the two methods was statistically significant. This finding confirms our first hypothesis. After one month, the severity of symptoms was slightly increased in both groups, but it was not statistically significant. Grassi et al. (2018) evaluated the potential CBT-enhancing effect of high-frequency rTMS over the left DLPFC in patients with OCD. They reported that the rTMS could be an adequate tool to enhance the impact of CBT with ERP technique in these patients. In their study, at the end of the 16 CBT sessions (once a week), patients showed a 35% and 30% symptom reduction in obsessions and compulsions, respectively. In our study, the means of obsessions and compulsions were reduced from 16.08 to 5.67 and from 15.33 to 5.50, respectively, after 20 CBT sessions. In a meta-analysis by Reid et al. (2021), the effect of CBT with ERP on reducing OCD symptoms was reported as high, which is consistent with our results. Elbeh et al. (2016), in a clinical trial, evaluated the effect of 1-Hz (low frequency) and 10-Hz (high frequency) rTMS on the right DLPFC in people with OCD. Their results showed that LF-rTMS significantly reduced the Y-BOCS score, while this effect was insignificant at 10 Hz. Hence, they concluded that 1Hz-rTMS, targeting the right DLPFC, is a promising tool for treating OCD. Shayganfard et al. (2016), Liang et al. (2021), and Khedr et al. (2022) also reported that LF rTMS over right DLPFC improved symptoms of OCD. These are consistent with our results. Seo et al. (2016) examined the effect of rTMS on the right DLPFC (1 Hz, 1200 pulses per session, 100% of resting motor threshold) for three weeks at 15 sessions in people with OCD. Their results also showed a significant decrease in the Y-BOCS score. In Alonso et al.’s study (2001), each OCD patient was given LF-rTMS (1 Hz, 110% of resting motor threshold) over the right DLPFC three times a week for 6 weeks. Their results did not show a significant decrease in the Y-BOCS score at the post-test and follow-up phases, which is against our results. The discrepancy may be due to the shape of the coil used for stimulation. They used a circular coil, while we used a figure-of-eight butterfly coil. The difference in the shape of coils can affect the inhibitory effect of 1-Hz rTMS (Lang et al., 2066). According to Ørskov et al. (2021), the figure-of-eight coil may have better applicability in patients due to the lower incidence of lack of inhibition in healthy subjects and the lower experience of pain or discomfort. Another reason for the discrepancy can be the difference in treatment sessions (10 sessions vs 18 sessions) and stimulation intensity (100% vs 110% of resting motor threshold). In our study, the LF-rTMS could significantly reduce obsessions in patients (from 14.25 to 10.75) but had no significant effect on their compulsions from the pre-test to follow-up phases; this may be due to the stimulated area (right DLPFC), which is related to the cognitive circuit that influences obsessive thoughts, or not simulating the left DLPFC which has a role in inhibitory control of OCD patients (Menzies et al., 2008). Fremont et al. (2022) showed that volume loss in the left DLPFC is associated with developing compulsive behaviors.
In comparing the effects of CBT and LF rTMS on the cognitive flexibility of adults with OCD, our results showed no significant difference between the two methods in improving the cognitive flexibility of patients. This finding rejects the second hypothesis of this study. Although the total and scores of “alternative solutions” and “control” components of the cognitive flexibility increased in both groups, the difference was not statistically significant. In one study, Shayganfard et al. (2016) found that executive functions (Wisconsin card sorting test performance) did not improve after rTMS in 10 adults with OCD, which is consistent with our results. No more related studies on OCD patients were found for the comparison of the results. The non-significant effect of LF-rTMS on the cognitive flexibility of OCD patients in our study may be because the ability of rTMS is limited to penetrate and stimulate the subcortical regions, such as the thalamus and caudate nucleus, which have been suggested as anatomical neural substrates involved in OCD (Menzies et al., 2008).
Regarding the non-significant effect of LF-rTMS on the cognitive flexibility of OCD patients, the reason may be that a self-report tool is used to assess cognitive flexibility (i.e. the CFI). Compared to neuropsychological tests, self-report tools assess a different aspect of cognitive flexibility (Johnco et al., 2014). People with lower cognitive flexibility can still benefit from CBT, even though they cannot use cognitive restructuring (Johnco et al., 2014).
The present study had limitations, such as a low sample size, no placebo or control group (since it was difficult to recruit patients during the COVID-19 pandemic), and not using objective assessment tools for assessing cognitive flexibility in patients. Most tests used in neuropsychological assessments to measure cognitive flexibility, such as the Wisconsin test, may not show the more subtle cognitive problems that occur due to mental disorders well (Eling et al., 2008). Moreover, significant practical limitations reduce the clinical application of these tests. The Wisconsin test, for example, is time-consuming in execution and scoring, has a training effect, and requires an interactive relationship between the rater and the subject. As a result of the training effect, patients’ responses are not solely due to the intervention effect. In this regard, we used a questionnaire (CFI) to measure the cognitive flexibility of OCD patients. Moreover, the existence of comorbid diseases (i.e. depression) was not assessed. The parameters of LF-rTMS (10 sessions, 1 Hz, 100% of motor threshold, and 1200 pulses/day) may be suboptimal. They may also not be enough to generate antidepressant effects in patients. Furthermore, the generalization of the results to all OCD patients in Iran should be done with caution since this study was conducted on patients attending a clinic in a city of Iran (Zanjan). Further studies are recommended by stimulating emotional and cognitive circuits in the brain and using a larger sample size, a control/placebo group, and objective measurement tools such as functional magnetic resonance imaging and electroencephalography. We applied LF-rTMS over the right DLPFC of patients. Future studies can use high-frequency rTMS or apply it over the left DLPFC to assess its efficacy compared to CBT with ERP.
Conclusion
There is a significant difference between CBT and LF-rTMS techniques in reducing the severity of OCD symptoms. Still, there is no difference between them in improving the cognitive flexibility of patients with OCD.
Ethical Considerations
Compliance with ethical guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975. Written informed consent was obtained from all patients to be included in the study. Ethical approval was obtained from the Research Ethics Committee of Zanjan University of Medical Sciences, Zanjan, Iran (Code: IR.ZUMS.REC.1399.180). This study was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20200805048316N1).
Funding
This study was extracted from the master thesis of Arash Fazeli, approved by the Department of Psychiatry, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
Authors' contributions
Conceptualization, data analysis, review and editing: Mohsen Dadashi; Supervision: Mohsen Dadashi, Ahmad Zolghadriha, and Reza Pirzeh; Methodology, data collection and investigation: Arash Fazeli; Writing the original draft: Arash Fazeli, and Shokoufe Ramezani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all the patients who participated in this study for their cooperation.
References
Obsessive-compulsive disorder (OCD) is a debilitating and severe mental disorder characterized by varying degrees of obsessive thoughts and behaviors. Obsessive thoughts are intrusive, unwanted, and annoying thoughts or images that people experience spontaneously incompatible with the person’s obvious and perceived feelings. Compulsions are repetitive and time-consuming behaviors or mental acts that are used to neutralize anxiety caused by obsessive thoughts (Rapinesi et al., 2019; Robbins et al., 2019). According to the World Health Organization (WHO), it is among the 10 disabling disorders (Melchior et al., 2019). Its lifelong prevalence is 1%-3% worldwide (Kessler et al., 2005) and 5.1%-1.8% in Iran (Mohammadi et al., 2004; Vandad Sharifi et al., 2015). OCD has a gradual onset and becomes chronic if people do not receive treatment (Olatunji et al., 2013), and its symptoms change over time due to stressors in life (Stewart et al., 2004). People with OCD tend to engage in obsessive actions, and even if they know that obsessive actions are useless, they cannot stop it (Sternheim et al., 2014). Impaired executive functioning has been observed frequently in OCD individuals (Fournet et al., 2019). Executive functioning is defined as managing intervening components in goal-directed behaviors and predicting the consequences of behavior (Pajouhinia et al., 2020). Executive functions include cognitive processes, such as working memory, inhibitory control, and cognitive flexibility, essential for goal-directed behavior (Miyake et al., 2000; Nejati et al., 2020). Clinically, people with OCD have difficulty switching between mental processes to generate adaptive behavioral responses to their symptoms. Many neurological studies have shown reduced cognitive flexibility in people with OCD (Gruner & Pittenger, 2017; Vriend et al., 2013). The ability to modify cognitive sets to adapt to variable environmental stimuli is a key component in most operational definitions of cognitive flexibility. It is considered a wide range of behaviors that enable people to adapt adaptively to stressful events instead of having maladaptive behaviors (Kurginyan & Osavolyuk, 2018). Recently, neurological models of OCD have suggested cognitive inflexibility as a significant feature of OCD patients, which can also be present in their relatives (Chamberlain et al., 2007). Although OCD patients have many cognitive impairments, impaired cognitive flexibility may be an essential trait for understanding the neural basis of OCD (Tomiyama et al., 2019).
Cognitive-behavioral therapy (CBT) based on exposure and response prevention (ERP) is currently the standard treatment for OCD. In CBT, individuals are believed to respond to the cognitive representation of stressful events instead of responding to these events (Porto et al., 2009). ERP involves gradual and long-term exposure to intimidating stimuli and avoiding obsessive actions (Olatunji et al., 2013). CBT can reduce cortico-striato-thalamo-cortical circuit hyperactivity and ultimately help improve the symptoms of OCD (Moody et al., 2017). However, CBT is much less common than drug therapy. According to surveys in the United Kingdom and USA, only 5% of adults with OCD receive CBT (O’Neill & Feusner, 2015). On average, 30% of these patients refuse ERP therapy or drop out of treatment (Olatunji et al., 2010; Melchior et al., 2019). Therefore, complementary interventions have been suggested as an alternative to overcome CBT limitations in treating OCD. A potential new treatment option, repetitive transcranial magnetic stimulation (rTMS), can modulate neural activity in brain circuits (Elbeh et al., 2016; Husain et al., 2002). First introduced by Barker et al. in 1985, rTMS is a non-invasive technique that delivers electromagnetic pulses to selected areas of the cerebral cortex (Barker et al., 1985; Jaafari et al., 2012). The stimulation can be applied at either high (≥5 Hz) or low (≤1 Hz) frequencies, which have stimulatory and inhibitory effects on cortical excitability, respectively (Lefaucheur et al., 2014). Studies have shown that the dorsolateral prefrontal cortex (DLPFC) is essential in cognitive flexibility (Borwick et al., 2020; Quiñones-Camacho et al., 2019). Thus, improvement in DLPFC neuronal function may help improve the cognitive flexibility of patients with OCD. In a clinical trial, Seo et al. reported the effectiveness of low-frequency rTMS (LF-rTMS) over the right DLPFC in relieving the symptoms of OCD and depression in OCD patients (Seo et al., 2016).
Due to the high involvement of networks in the pathophysiology of OCD and the rTMS’s ability to adjust cortical and subcortical structures and its potential therapeutic effectiveness in modulating inactive or hyperactive areas of the brain by targeting cortical circuits in patients with OCD, and lack of study on comparing the efficacy of CBT and LF-rTMS in treating OCD patients, the present study aims to compare the effects of CBT with ERP and rTMS over the right DLPFC on symptoms and cognitive flexibility in people with OCD. It is hypothesized that (a) there is a difference between LF-rTMS and CBT in reducing the severity of OCD symptoms and (b) there is a difference between LF-rTMS and CBT in improving the cognitive flexibility of OCD patients.
Materials and Methods
Study design and participants
This randomized clinical trial employed a pre-test/post-test/follow-up design. The study population consists of all adults with OCD referred to the clinic of Shahid Beheshti Hospital in Zanjan City, Iran, in 2020 (during the COVID-19 pandemic) (n=41). The sample size was determined to be 13 for each group using G*Power software by considering α=0.05, an error probability of 0.95, and an effect size of 0.6 according to previous studies in the literature which reported middle-size to large-size effects of rTMS and CBT on patients with OCD (Perera et al., 2021; Hoppen et al., 2021). Due to the COVID-19 pandemic and considering the 20% dropout, the sample size was increased to 17 for each group. In this regard, 34 patients were selected using a convenience sampling method and randomly (by drawing cards) assigned into two parallel groups of CBT (n=17) and rTMS (n=17). Each group of patients was acknowledged which group s/he was assigned to. The randomization was conducted using a lottery method by the last author. All samples were diagnosed with OCD by a psychiatrist and re-evaluated by a psychologist through a structured clinical interview for diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) (SCID-5) and using the Millon clinical multiaxial inventory-III (MCMI-III). The inclusion criteria were as follows: Having OCD according to the psychiatrist and based on SCID-5, bearing at least a middle-school education, being 18-50 years old, signing a written consent, and lacking a history of psychological therapies, transcranial direct current stimulation, or neurofeedback.
On the other hand, the exclusion criteria were the existence of suicidal thoughts, personality disorders according to the SCID-5 and MCMI-III, psychotic disorders, history of seizures and epilepsy, the existence of an electrical or metal object in the body (e.g. pacemaker), and having bipolar disorder. Before entering the study, the participants received medication whose dosage had been stabilized for four weeks. After and during the study, the psychiatrist kept the dosage the same. Ten patients were excluded from the study (5 from the rTMS group and 5 from the CBT group). Therefore, 12 patients in each group completed the study. Figure 1 shows the sampling and allocation processes.

Study measures
After obtaining written informed consent from the participants, they completed a demographic form, the Yale-Brown obsessive-compulsive scale (Y-BOCS) to assess their OCD symptoms, and the cognitive flexibility inventory (CFI) to evaluate their cognitive flexibility. The Y-BOCS is a semi-structured interview and the gold standard for assessing OCD symptoms. It has two primary scales: Symptom checklist (SC) and severity scale (SS). The SC has 16 self-report items rated on a 5-point scale. In the SS, the severity of obsessions and compulsions is measured in five areas: Distress, frequency, intervention, resistance, and symptom control. In this study, we used the Persian version of Y-BOCS validated by Rajezi Esfahani et al. (2021), who reported internal consistency of 0.97 for the SC and 0.95 for the SS, a split-half reliability of 0.93 for the SC and 0.89 for the SS, and a test re-test reliability of 0.99. In our study, patients completed the SS scale only.
Dennis and Vander Wal (2010) developed the CFI, which is a 20-item self-report tool using a 7-point Likert scale to measure three aspects of cognitive flexibility, including the ability to perceive multiple alternative explanations for life occurrences and human behavior, the ability to generate multiple alternative solutions to difficult situations, and the desire to perceive difficult situations as controllable (Control subscale). The CFI has excellent internal consistency and high test re-test reliability (Dennis & Vander Wal, 2010). They reported Cronbach α values of 0.90, 0.86, and 0.91, as well as test re-test reliability values of 0.81, 0.77, and 0.75 for the overall scale, control, and alternatives subscales, respectively. For its Persian version, Shareh et al. (2014)reported a three-factor structure: control, alternative solutions, and alternative explanations. They reported the Cronbach α and test re-test coefficients for the Persian CFI reliability as 0.90 and 0.71, respectively. The mentioned tools were completed again immediately and one month after the intervention.
Study interventions
The CBT group individually received CBT with ERP at 20 sessions twice a week, each for 45-90 minutes, according to the protocol proposed by Leahy et al. (2011)(Table 1). According to Jaurrieta et al. (2008), individual CBT is more effective in reducing OCD symptoms than group CBT. Treatment was performed by the researcher (MS student in clinical psychology) under the supervision of a therapist.

The rTMS group received rTMS for 2 weeks at 10 sessions (5 consecutive days per week, each for 20 minutes) according to the protocol proposed by Gomes et al. (2012). Each person received 1-Hz rTMS at 100% of resting MT (1200 pulses per day with a 10-min rest interval between each 300 pulses) using a focal 8-shaped 70-mm coil (Neuro-MS/D Advanced Therapeutic, Neurosoft Ltd., Russia), which was positioned on the right DLPFC (F4, according to the EEG 10–20 International System) such that there was no space between the skin and the coil. The rTMS was conducted by an expert who was unaware of the results.
Data analysis
Descriptive statistics such as Mean±SD, frequency, and percentage were used to analyze the collected data. Also, inferential statistics such as the chi-square test and independent t-test (to examine the differences in demographic factors and pre-test means), multivariate analysis of covariance (MANCOVA) (to compare the groups in terms of Y-BOCS and CFI scores), repeated-measures analysis of variance (ANOVA) (to compare the means of Y-BOCS and CFI between the time points), and the Fisher least significant difference (LSD) test for pairwise comparison were used in SPSS software, version 22. According to the results of the Kolmogorov-Smirnov test, the assumption of normal distribution of data in all three stages of pre-test, post-test, and follow-up was confirmed (P>0.05). According to the results of Levene’s test, the assumption of the quality of variances in the studied groups was not observed in the post-test data of obsessions (a component of Y-BOCS) and the pre-test data of alternatives subscale of CFI (P<0.05). Therefore, the degree of corrected freedom was used to compare the two groups in the mentioned variables. In other variables, the equality of variances was confirmed (P>0.05).
Results
Characteristics of participants
Table 2 presents the demographic characteristics of patients. In the CBT group, with a mean age of 32.83±9.43 years, there were two males and 10 females; 7 were single, 5 were married, and most had a bachelor’s degree (n=7, 58.3%). In the rTMS group with a mean age of 30.17±11.26 years, there were 6 males and 6 females; 5 were single, 7 were married, and most had a high school diploma (n=5, 41.7%). No significant difference was found between the two groups in terms of gender (P=0.083), marital status (P= 0.414), and level of education (P=0.183) according to the results of the chi-square test, and in terms of age (P=0.536) according to the results of independent t-test (Table 2). In the CBT group, 9 patients (75%) had contamination obsessions with washing/cleaning compulsion, 2(16.16%) had harm obsessions with checking compulsions, and one (8.33%) had symmetry obsessions with ordering. In the rTMS group, 9(75%) had contamination obsessions with washing/cleaning compulsions, and 3(25%) had harm obsessions with checking compulsions. However, this difference between groups was not significant according to the chi-square test results (P>0.05).

Comparing OCD symptoms in two study groups
As seen in Figure 2, the pre-test scores of Y-BOCS and its components were higher in the CBT group than in the rTMS group.

This difference was statistically significant only in the total score (P=0.011) and the compulsions domain (P=0.017). Immediately after the intervention, the scores decreased significantly in both groups, whereas the decrease was higher in the CBT group. Results of MANCOVA (Table 3) showed that, after controlling the pre-test scores, the difference between groups was statistically significant in post-test obsessions (F1, 21=23.645, P<0.001, η2=0.53); post-test compulsions (F1, 21=20.920, P<0.001, η2=0.45); and post-test total score (F1, 21=25.565, P<0.001, η2=0.55). One month after the intervention, these scores slightly increased in both groups.

Results of repeated-measures ANOVA (Table 4) showed a significant difference in the Y-BOCS scores within three time points of pre-test, post-test, and follow-up, where the main and interaction effects were significant (P<0.001).

To assess between which time points this difference was observed, the LSD test was conducted. In the CBT group, the results (Table 5) showed a significant difference between pre-test and post-test scores and between pre-test and follow-up scores of obsessions, compulsions, and total scores (P<0.001). Still, there was no significant difference between post-test and follow-up scores (P>0.05). In the rTMS group, there was a significant difference between pre-test and post-test scores and between pre-test and follow-up scores of obsessions and total score (P<0.05) but not in compulsions. No significant differences between the post-test and follow-up scores of any variables were observed in this group (Table 5).

Comparing cognitive flexibility in two study groups
As seen in Figure 3, the pre-test scores of total CFI and its three subscales were higher in the CBT group than in the rTMS group, but there was no significant difference between the pre-test CFI scores of the two groups (P>0.05).

Immediately after the intervention, both groups’ total scores and scores of “alternative solutions” and “control” increased. In contrast, the “alternative explanations” subscale score decreased in both groups. Results of MANCOVA (Table 6) showed that these differences between groups were not statistically significant in any domains (P>0.05).

One month after intervention, a slight decrease was reported in the total score of CFI and its subscales in both groups. Results of repeated-measures ANOVA (Table 7) showed no significant overall difference between the two groups in any variables over three time points of pre-test, post-test, and follow-up (P>0.05).

Discussion
The purpose of this study was to compare the effectiveness of CBT with ERP (presented individually) and low frequency (1-Hz) rTMS in reducing symptom severity (Y-BOCS score) and improving cognitive inflexibility (CFI score) in 24 patients with OCD. The results showed that both treatment methods highly reduced the severity of OCD symptoms immediately after intervention, where CBT had a higher effect. The difference between the results of the two methods was statistically significant. This finding confirms our first hypothesis. After one month, the severity of symptoms was slightly increased in both groups, but it was not statistically significant. Grassi et al. (2018) evaluated the potential CBT-enhancing effect of high-frequency rTMS over the left DLPFC in patients with OCD. They reported that the rTMS could be an adequate tool to enhance the impact of CBT with ERP technique in these patients. In their study, at the end of the 16 CBT sessions (once a week), patients showed a 35% and 30% symptom reduction in obsessions and compulsions, respectively. In our study, the means of obsessions and compulsions were reduced from 16.08 to 5.67 and from 15.33 to 5.50, respectively, after 20 CBT sessions. In a meta-analysis by Reid et al. (2021), the effect of CBT with ERP on reducing OCD symptoms was reported as high, which is consistent with our results. Elbeh et al. (2016), in a clinical trial, evaluated the effect of 1-Hz (low frequency) and 10-Hz (high frequency) rTMS on the right DLPFC in people with OCD. Their results showed that LF-rTMS significantly reduced the Y-BOCS score, while this effect was insignificant at 10 Hz. Hence, they concluded that 1Hz-rTMS, targeting the right DLPFC, is a promising tool for treating OCD. Shayganfard et al. (2016), Liang et al. (2021), and Khedr et al. (2022) also reported that LF rTMS over right DLPFC improved symptoms of OCD. These are consistent with our results. Seo et al. (2016) examined the effect of rTMS on the right DLPFC (1 Hz, 1200 pulses per session, 100% of resting motor threshold) for three weeks at 15 sessions in people with OCD. Their results also showed a significant decrease in the Y-BOCS score. In Alonso et al.’s study (2001), each OCD patient was given LF-rTMS (1 Hz, 110% of resting motor threshold) over the right DLPFC three times a week for 6 weeks. Their results did not show a significant decrease in the Y-BOCS score at the post-test and follow-up phases, which is against our results. The discrepancy may be due to the shape of the coil used for stimulation. They used a circular coil, while we used a figure-of-eight butterfly coil. The difference in the shape of coils can affect the inhibitory effect of 1-Hz rTMS (Lang et al., 2066). According to Ørskov et al. (2021), the figure-of-eight coil may have better applicability in patients due to the lower incidence of lack of inhibition in healthy subjects and the lower experience of pain or discomfort. Another reason for the discrepancy can be the difference in treatment sessions (10 sessions vs 18 sessions) and stimulation intensity (100% vs 110% of resting motor threshold). In our study, the LF-rTMS could significantly reduce obsessions in patients (from 14.25 to 10.75) but had no significant effect on their compulsions from the pre-test to follow-up phases; this may be due to the stimulated area (right DLPFC), which is related to the cognitive circuit that influences obsessive thoughts, or not simulating the left DLPFC which has a role in inhibitory control of OCD patients (Menzies et al., 2008). Fremont et al. (2022) showed that volume loss in the left DLPFC is associated with developing compulsive behaviors.
In comparing the effects of CBT and LF rTMS on the cognitive flexibility of adults with OCD, our results showed no significant difference between the two methods in improving the cognitive flexibility of patients. This finding rejects the second hypothesis of this study. Although the total and scores of “alternative solutions” and “control” components of the cognitive flexibility increased in both groups, the difference was not statistically significant. In one study, Shayganfard et al. (2016) found that executive functions (Wisconsin card sorting test performance) did not improve after rTMS in 10 adults with OCD, which is consistent with our results. No more related studies on OCD patients were found for the comparison of the results. The non-significant effect of LF-rTMS on the cognitive flexibility of OCD patients in our study may be because the ability of rTMS is limited to penetrate and stimulate the subcortical regions, such as the thalamus and caudate nucleus, which have been suggested as anatomical neural substrates involved in OCD (Menzies et al., 2008).
Regarding the non-significant effect of LF-rTMS on the cognitive flexibility of OCD patients, the reason may be that a self-report tool is used to assess cognitive flexibility (i.e. the CFI). Compared to neuropsychological tests, self-report tools assess a different aspect of cognitive flexibility (Johnco et al., 2014). People with lower cognitive flexibility can still benefit from CBT, even though they cannot use cognitive restructuring (Johnco et al., 2014).
The present study had limitations, such as a low sample size, no placebo or control group (since it was difficult to recruit patients during the COVID-19 pandemic), and not using objective assessment tools for assessing cognitive flexibility in patients. Most tests used in neuropsychological assessments to measure cognitive flexibility, such as the Wisconsin test, may not show the more subtle cognitive problems that occur due to mental disorders well (Eling et al., 2008). Moreover, significant practical limitations reduce the clinical application of these tests. The Wisconsin test, for example, is time-consuming in execution and scoring, has a training effect, and requires an interactive relationship between the rater and the subject. As a result of the training effect, patients’ responses are not solely due to the intervention effect. In this regard, we used a questionnaire (CFI) to measure the cognitive flexibility of OCD patients. Moreover, the existence of comorbid diseases (i.e. depression) was not assessed. The parameters of LF-rTMS (10 sessions, 1 Hz, 100% of motor threshold, and 1200 pulses/day) may be suboptimal. They may also not be enough to generate antidepressant effects in patients. Furthermore, the generalization of the results to all OCD patients in Iran should be done with caution since this study was conducted on patients attending a clinic in a city of Iran (Zanjan). Further studies are recommended by stimulating emotional and cognitive circuits in the brain and using a larger sample size, a control/placebo group, and objective measurement tools such as functional magnetic resonance imaging and electroencephalography. We applied LF-rTMS over the right DLPFC of patients. Future studies can use high-frequency rTMS or apply it over the left DLPFC to assess its efficacy compared to CBT with ERP.
Conclusion
There is a significant difference between CBT and LF-rTMS techniques in reducing the severity of OCD symptoms. Still, there is no difference between them in improving the cognitive flexibility of patients with OCD.
Ethical Considerations
Compliance with ethical guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975. Written informed consent was obtained from all patients to be included in the study. Ethical approval was obtained from the Research Ethics Committee of Zanjan University of Medical Sciences, Zanjan, Iran (Code: IR.ZUMS.REC.1399.180). This study was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20200805048316N1).
Funding
This study was extracted from the master thesis of Arash Fazeli, approved by the Department of Psychiatry, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
Authors' contributions
Conceptualization, data analysis, review and editing: Mohsen Dadashi; Supervision: Mohsen Dadashi, Ahmad Zolghadriha, and Reza Pirzeh; Methodology, data collection and investigation: Arash Fazeli; Writing the original draft: Arash Fazeli, and Shokoufe Ramezani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all the patients who participated in this study for their cooperation.
References
Alonso, P., Pujol, J., Cardoner, N., Benlloch, L., Deus, J., & Menchón, J. M., et al. (2001). Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A double-blind, placebo-controlled study. The American Journal of Psychiatry, 158(7), 1143–1145. [DOI:10.1176/appi.ajp.158.7.1143] [PMID]
Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet, 1(8437), 1106–1107. [DOI: 10.1016/s0140-6736(85)92413-4] [PMID]
Borwick, C., Lal, R., Lim, L. W., Stagg, C. J., & Aquili, L. (2020). Dopamine depletion effects on cognitive flexibility as modulated by tDCS of the dlPFC. Brain Stimulation, 13(1), 105–108. [DOI:10.1016/j.brs.2019.08.016] [PMID]
Chamberlain, S. R., Fineberg, N. A., Menzies, L. A., Blackwell, A. D., Bullmore, E. T., & Robbins, T. W., et al. (2007). Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. The American Journal of Psychiatry, 164(2), 335–338. [DOI:10.1176/ajp.2007.164.2.335] [PMID]
Dennis, J. P., & Vander Wal, J. S. (2010). The cognitive flexibility inventory: Instrument development and estimates of reliability and validity. Cognitive Therapy and Research, 34(3), 241-253. [DOI:10.1007/s10608-009-9276-4]
Elbeh, K. A. M., Elserogy, Y. M. B., Khalifa, H. E., Ahmed, M. A., Hafez, M. H., & Khedr, E. M. (2016). Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: Double blind randomized clinical trial. Psychiatry Research, 238, 264–269. [DOI:10.1016/j.psychres.2016.02.031] [PMID]
Esfahani, S. R., Motaghipour, Y., Kamkari, K., Zahiredin, A., & Janbozorgi, M. (2012). Reliability and Validity of the Persian version of the Yale-Brown Obsessive-Compulsive scale (Y-BOCS). Iranian Journal of Psychiatry and Clinical Psychology, 17(4), 297-303. [Link]
Eling, P., Derckx, K., & Maes, R. (2008). On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain and Cognition, 67(3), 247–253. [DOI:10.1016/j.bandc.2008.01.006] [PMID]
Fremont, R., Dworkin, J., Manoochehri, M., Krueger, F., Huey, E., & Grafman, J. (2022). Damage to the dorsolateral prefrontal cortex is associated with repetitive compulsive behaviors in patients with penetrating brain injury. BMJ Neurology Open, 4(1), e000229. [DOI:10.1136/bmjno-2021-000229] [PMID]
Fournet, N., Achachi, O., Roy, A., Besnard, J., Lancelot, C., & Le Gall, D., et al. (2019). Impaired Executive Function in Everyday Life: A Predictor of OCD Relapse? Journal of Behavioral and Brain Science, 9(3), 90-107. [DOI:10.4236/jbbs.2019.93008]
Gomes, P. V., Brasil-Neto, J. P., Allam, N., & Rodrigues de Souza, E. (2012). A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. The Journal of Neuropsychiatry and Clinical Neurosciences, 24(4), 437–443. [DOI:10.1176/appi.neuropsych.11100242] [PMID]
Grassi, G., Pacini, S., Cecchelli, C., & Pallanti, S. (2018). Enhancing cognitive-behavioral therapy for obsessive-compulsive disorder with transcranic magnetic stimulation: A proof of concept. European Neuropsychopharmacology, 28(6), 766-767. [DOI:10.1016/j.euroneuro.2017.10.008]
Gruner, P., & Pittenger, C. (2017). Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience, 345, 243–255.[DOI:10.1016/j.neuroscience.2016.07.030] [PMID]
Hoppen, L. M., Kuck, N., Bürkner, P. C., Karin, E., Wootton, B. M., & Buhlmann, U. (2021). Low intensity technology-delivered cognitive behavioral therapy for obsessive-compulsive disorder: A meta-analysis. BMC Psychiatry, 21(1), 322. [DOI:10.1186/s12888-021-03272-5] [PMID]
Husain, F. T., Nandipati, G., Braun, A. R., Cohen, L. G., Tagamets, M. A., & Horwitz, B. (2002). Simulating transcranial magnetic stimulation during PET with a large-scale neural network model of the prefrontal cortex and the visual system. NeuroImage, 15(1), 58–73. [DOI:10.1006/nimg.2001.0966] [PMID]
Jaafari, N., Rachid, F., Rotge, J. Y., Polosan, M., El-Hage, W., & Belin, D., et al. (2012). Safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorder: A review. The world Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 13(3), 164–177. [DOI:10.3109/15622975.2011.575177] [PMID]
Jaurrieta, N., Jimenez-Murcia, S., Menchón, J. M., Del Pino Alonso, M., Segalas, C., & Alvarez-Moya, E. M., et al. (2008). Individual versus group cognitive-behavioral treatment for obsessive-compulsive disorder: a controlled pilot study. Psychotherapy Research: Journal of the Society for Psychotherapy Research, 18(5), 604–614. [DOI:10.1080/10503300802192141] [PMID]
Johnco, C., Wuthrich, V. M., & Rapee, R. M. (2014). Reliability and validity of two self-report measures of cognitive flexibility. Psychological Assessment, 26(4), 1381–1387. [DOI:10.1037/a0038009] [PMID]
Johnco, C., Wuthrich, V. M., & Rapee, R. M. (2014). The influence of cognitive flexibility on treatment outcome and cognitive restructuring skill acquisition during cognitive behavioural treatment for anxiety and depression in older adults: Results of a pilot study. Behaviour Research and Therapy, 57, 55–64. [DOI:10.1016/j.brat.2014.04.005] [PMID]
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [PMID]
Khedr, E. M., Elbeh, K., Saber, M., Abdelrady, Z., & Abdelwarith, A. (2022). A double blind randomized clinical trial of the effectiveness of low frequency rTMS over right DLPFC or OFC for treatment of obsessive-compulsive disorder. Journal of Psychiatric Research, 156, 122–131. [DOI:10.1016/j.jpsychires.2022.10.025] [PMID]
Kurginyan, S. S., & Osavolyuk, E. Y. (2018). Psychometric Properties of a Russian Version of the Cognitive Flexibility Inventory (CFI-R). Frontiers in Psychology, 9, 845. [DOI:10.3389/fpsyg.2018.00845] [PMID]
Lang, N., Harms, J., Weyh, T., Lemon, R. N., Paulus, W., & Rothwell, J. C., et al. (2006). Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(10), 2292–2301. [DOI:10.1016/j.clinph.2006.05.030] [PMID]
Lefaucheur, J. P., André-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., & Benninger, D. H., et al. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 125(11), 2150–2206. [DOI:10.1016/j.clinph.2014.05.021] [PMID]
Leahy, R. L., Holland, S. J., & McGinn, L. K. (2011). Treatment plans and interventions for depression and anxiety disorders. New York: Guilford Press; 2011. [Link]
Liang, K., Li, H., Bu, X., Li, X., Cao, L., & Liu, J., et al. (2021). Efficacy and tolerability of repetitive transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder in adults: A systematic review and network meta-analysis. Translational Psychiatry, 11(1), 332. [DOI:10.1038/s41398-021-01453-0] [PMID]
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI:10.1006/cogp.1999.0734] [PMID]
Melchior, K., Franken, I., Deen, M., & van der Heiden, C. (2019). Metacognitive therapy versus exposure and response prevention for obsessive-compulsive disorder: study protocol for a randomized controlled trial. Trials, 20(1), 277. [DOI:10.1186/s13063-019-3381-9] [PMID]
Menzies, L., Chamberlain, S. R., Laird, A. R., Thelen, S. M., Sahakian, B. J., & Bullmore, E. T. (2008). Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews, 32(3), 525–549. [DOI:10.1016/j.neubiorev.2007.09.005] [PMID]
Mohammadi, M. R., Ghanizadeh, A., Rahgozar, M., Noorbala, A. A., Davidian, H., & Afzali, H. M., et al. (2004). Prevalence of obsessive-compulsive disorder in Iran. BMC Psychiatry, 4, 2. [DOI:10.1186/1471-244X-4-2] [PMID]
Moody, T. D., Morfini, F., Cheng, G., Sheen, C., Tadayonnejad, R., & Reggente, N., et al. (2017). Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity. Translational Psychiatry, 7(9), e1230. [DOI:10.1038/tp.2017.192] [PMID]
Nejati, V., Salehinejad, M. A., Nitsche, M. A., Najian, A., & Javadi, A. H. (2020). Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. Journal of Attention Disorders, 24(13), 1928–1943. [DOI:10.1177/1087054717730611] [PMID]
Olatunji, B. O., Davis, M. L., Powers, M. B., & Smits, J. A. (2013). Cognitive-behavioral therapy for obsessive-compulsive disorder: a meta-analysis of treatment outcome and moderators. Journal of Psychiatric Research, 47(1), 33–41. [DOI:10.1016/j.jpsychires.2012.08.020] [PMID]
O'Neill, J., & Feusner, J. D. (2015). Cognitive-behavioral therapy for obsessive-compulsive disorder: Access to treatment, prediction of long-term outcome with neuroimaging. Psychology Research and Behavior Management, 8, 211–223. [DOI:10.2147/PRBM.S75106] [PMID]
Ørskov, S., Bostock, H., Howells, J., Pugdahl, K., Fuglsang-Frederiksen, A., & Nielsen, C. S., et al. (2021). Comparison of figure-of-8 and circular coils for threshold tracking transcranial magnetic stimulation measurements. Neurophysiologie Clinique = Clinical Neurophysiology, 51(2), 153–160. [DOI:10.1016/j.neucli.2021.01.001] [PMID]
Olatunji, B. O., Cisler, J. M., & Deacon, B. J. (2010). Efficacy of cognitive behavioral therapy for anxiety disorders: A review of meta-analytic findings. The Psychiatric Clinics of North America, 33(3), 557–577. [DOI:10.1016/j.psc.2010.04.002] [PMID]
Pajouhinia, S., Abavisani,Y., & Rezazadeh, Z. (2020). Explaining the obsessive-compulsive symptoms based on cognitive flexibility and social cognition. Practice in Clinical Psychology, 8(3), 233-242. [DOI:10.32598/jpcp.8.3.10.717.1]
Perera, M. P. N., Mallawaarachchi, S., Miljevic, A., Bailey, N. W., Herring, S. E., & Fitzgerald, P. B. (2021). Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A meta-analysis of randomized, sham-controlled trials. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 6(10), 947–960. [DOI:10.1016/j.bpsc.2021.03.010] [PMID]
Porto, P. R., Oliveira, L., Mari, J., Volchan, E., Figueira, I., & Ventura, P. (2009). Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(2), 114–125. [DOI:10.1176/jnp.2009.21.2.114] [PMID]
Quiñones-Camacho, L. E., Fishburn, F. A., Camacho, M. C., Wakschlag, L. S., & Perlman, S. B. (2019). Cognitive flexibility-related prefrontal activation in preschoolers: A biological approach to temperamental effortful control. Developmental Cognitive Neuroscience, 38, 100651. [DOI:10.1016/j.dcn.2019.100651] [PMID]
Rapinesi, C., Kotzalidis, G. D., Ferracuti, S., Sani, G., Girardi, P., & Del Casale, A. (2019). Brain Stimulation in Obsessive-Compulsive Disorder (OCD): A systematic review. Current Neuropharmacology, 17(8), 787–807. [DOI:10.2174/1570159X17666190409142555] [PMID]
Reid, J. E., Laws, K. R., Drummond, L., Vismara, M., Grancini, B., & Mpavaenda, D., et al. (2021). Cognitive behavioural therapy with exposure and response prevention in the treatment of obsessive-compulsive disorder: A systematic review and meta-analysis of randomised controlled trials. Comprehensive Psychiatry, 106, 152223. [DOI:10.1016/j.comppsych.2021.152223] [PMID]
Robbins, T. W., Vaghi, M. M., & Banca, P. (2019). Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron, 102(1), 27–47. [DOI:10.1016/j.neuron.2019.01.046] [PMID]
Seo, H. J., Jung, Y. E., Lim, H. K., Um, Y. H., Lee, C. U., & Chae, J. H. (2016). Adjunctive Low-frequency Repetitive Transcranial Magnetic Stimulation over the Right Dorsolateral Prefrontal Cortex in Patients with Treatment-resistant Obsessive-compulsive Disorder: A randomized controlled trial. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 14(2), 153–160. [DOI:10.9758/cpn.2016.14.2.153] [PMID]
Sharifi, V., Amin-Esmaeili, M., Hajebi, A., Motevalian, A., Radgoodarzi, R., & Hefazi, M., et al. (2015). Twelve-month prevalence and correlates of psychiatric disorders in Iran: The Iranian Mental Health Survey, 2011. Archives of Iranian Medicine, 18(2), 76–84. [PMID]
Shayganfard, M., Jahangard, L., Nazaribadie, M., Haghighi, M., Ahmadpanah, M., & Sadeghi Bahmani, D., et al. (2016). Repetitive transcranial magnetic stimulation improved symptoms of obsessive-compulsive disorders but not executive functions: Results from a randomized clinical trial with crossover design and sham condition. Neuropsychobiology, 74(2), 115–124. [DOI:10.1159/000457128] [PMID]
Shareh, H., Farmani, A., & Soltani, E. (2014). Investigating the Reliability and Validity of the Cognitive Flexibility Inventory (CFI-I) among Iranian University Students. Practice in Clinical Psychology, 2(1), 43-50. [Link]
Stewart, S. E., Geller, D. A., Jenike, M., Pauls, D., Shaw, D., & Mullin, B., et al. (2004). Long-term outcome of pediatric obsessive-compulsive disorder: A meta-analysis and qualitative review of the literature. Acta Psychiatrica Scandinavica, 110(1), 4–13.. [DOI:10.1111/j.1600-0447.2004.00302.x] [PMID]
Sternheim, L., van der Burgh, M., Berkhout, L. J., Dekker, M. R., & Ruiter, C. (2014). Poor cognitive flexibility, and the experience thereof, in a subclinical sample of female students with obsessive-compulsive symptoms. Scandinavian Journal of Psychology, 55(6), 573–577. [DOI:10.1111/sjop.12163] [PMID]
Tomiyama, H., Nakao, T., Murayama, K., Nemoto, K., Ikari, K., & Yamada, S., et al. (2019). Dysfunction between dorsal caudate and salience network associated with impaired cognitive flexibility in obsessive-compulsive disorder: A resting-state fMRI study. NeuroImage. Clinical, 24, 102004. [DOI:10.1016/j.nicl.2019.102004] [PMID]
Vriend, C., de Wit, S. J., Remijnse, P. L., van Balkom, A. J., Veltman, D. J., & van den Heuvel, O. A. (2013). Switch the itch: a naturalistic follow-up study on the neural correlates of cognitive flexibility in obsessive-compulsive disorder. Psychiatry Research, 213(1), 31–38. [DOI:10.1016/j.pscychresns.2012.12.006] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/12/4 | Accepted: 2023/08/16 | Published: 2025/04/21
Received: 2022/12/4 | Accepted: 2023/08/16 | Published: 2025/04/21
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








