Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 649-658 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorbani M, Keykhosravi E, Hasanpour M, Abbasian Ardakani A, Mohammad Hosseini E. The Optimal Time for Postoperative Magnetic Resonance Imaging of the Sella in Patients With Pituitary Adenoma. BCN 2024; 15 (5) :649-658
URL: http://bcn.iums.ac.ir/article-1-2608-en.html
URL: http://bcn.iums.ac.ir/article-1-2608-en.html
Mohammad Ghorbani1 

 , Ehsan Keykhosravi2
, Ehsan Keykhosravi2 

 , Mohammad Hasanpour *1
, Mohammad Hasanpour *1 

 , Ali Abbasian Ardakani3
, Ali Abbasian Ardakani3 

 , Ehsan Mohammad Hosseini4
, Ehsan Mohammad Hosseini4 




 , Ehsan Keykhosravi2
, Ehsan Keykhosravi2 

 , Mohammad Hasanpour *1
, Mohammad Hasanpour *1 

 , Ali Abbasian Ardakani3
, Ali Abbasian Ardakani3 

 , Ehsan Mohammad Hosseini4
, Ehsan Mohammad Hosseini4 


1- Department of Neurosurgery, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Neurosurgery, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Radiology Technology, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Department of Neurosurgery, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Neurosurgery, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Radiology Technology, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Department of Neurosurgery, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 952 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Pituitary adenomas account for about 15% of primary intracranial tumors (Barbieri et al., 2007; Goya et al., 2004; Isobe et al., 2000; Pennacchietti et al., 2016). Except for prolactinoma, the treatment of choice in pituitary adenoma is surgical excision (Mortini et al., 2005). Postoperative imaging is important in detecting possible surgical complications, tumor residue, and treatment planning (Doerfler & Richter, 2008; Moldovan et al., 2016). Magnetic resonance imaging (MRI) is the preferred neuroradiologic method of evaluating the sellar region (Huang & Mukundan, 2016; Zaidi et al., 2016). The reason for the widespread acceptance of the MRI is the appropriate contrast among the different elements of the soft tissue (Hess & Dillon, 2012). Generally, the optimal time for early postoperative MRI in central nervous system lesions is 48 hours after surgery (Albert et al., 1994; Oser et al., 1997; Saunders et al., 2005). Nevertheless, no guidelines determine the best time for postoperative imaging in patients with pituitary adenomas (Kılıç et al., 2001b).
We analyzed the pituitary gland, adenoma, and packing material signals three months after surgery at three time points and finally proposed the optimal time for postoperative imaging after pituitary adenoma surgery.
2. Materials and Methods
Study patients
A total of 28 patients with pituitary adenoma referred to the Department of Neurosurgery at Firoozgar University Hospital in Tehran City, Iran, from July 2019 to March 2021 were examined.
The inclusion criteria were as follows: A diagnosis of pituitary adenoma, being a candidate for endoscopic transsphenoidal surgery, and no history of previous surgery or other therapies such as irradiation and dopamine agonist therapy.
All patients had preoperative contrast-enhanced MRI of the sellar region to determine the extent of the tumor and involvement of adjacent structures. Preoperative, endocrinologic, and ophthalmologic evaluations were carried out for all patients.
Surgical technique
All patients underwent an endoscopic endonasal approach under general anesthesia. The endoscopic equipment used included a 0° and 30°, 4 mm rigid endoscope (Karl Storz; Germany), a full high definition (HD) camera (Image 1, H3-Z), and a wide view HD screen. Generally, the approach was through the right nostril, but the left side was used if there was a significant septal deviation to the right. The sellar floor was reconstructed using a fat pad covered by Surgicel and gelfoam. Histopathologic analysis was performed to establish the diagnosis.
Postoperative MRI
The MRI was performed with a 1.5 T system (Siemens Medical Solutions). The imaging protocol included non-enhanced T1- and T2-weighted and post-contrast T1 images with a 5-mm section thickness of the axial, coronal, and sagittal planes. All patients had a sellar MRI before surgery and three sellar MRIs at 48 hours, two weeks, and three months after surgery.
Imaging analysis
All MR images were interpreted concerning the contents of the sella: The pituitary gland, pituitary adenoma, and adipose tissue used to pack the sella. The signal for each compartment was recorded in the T1-weighted, T2-weighted, and T1-weighted with contrast sequences at different times.
Statistical analysis
Statistical analysis, including descriptive data analysis, was performed using SPSS software, version 23; (IBM; USA). The sellar contents signal at the different time points were considered non-parametric and were evaluated using the Friedman and sign tests. Two-sided P<0.05 were considered statistically significant.
3. Results
Demographic and clinical aspects
The mean age of the participants was 45.8 years. About 60.7% were female, and 39.3% were male. The most common complaint at presentation was the enlargement of the extremities, which affected 8 patients (28.6%). A visual disturbance occurred in 7 patients (25%), while 6 patients (21.4%) had a headache. Ten patients (35.7%) had nonfunctional pituitary adenoma, 10(35.7%) were diagnosed with acromegaly, 6(21.4%) had prolactinoma, and 2(7.1%) had an ACTH-producing tumor.
Of the 28 patients, 25 had macroadenomas, and 3 had microadenomas. After surgery, a residual tumor (incomplete removal) was confirmed in 4 patients (14.2%). Postoperative cerebrospinal fluid rhinorrhea occurred in only 8 cases that improved after conservative treatment. Seven patients (25%) developed transient postoperative diabetes insipidus during hospitalization. On ophthalmological examination, 16 patients (57.1%) showed visual field deficits. The demographic and clinical characteristics are shown in Table 1.
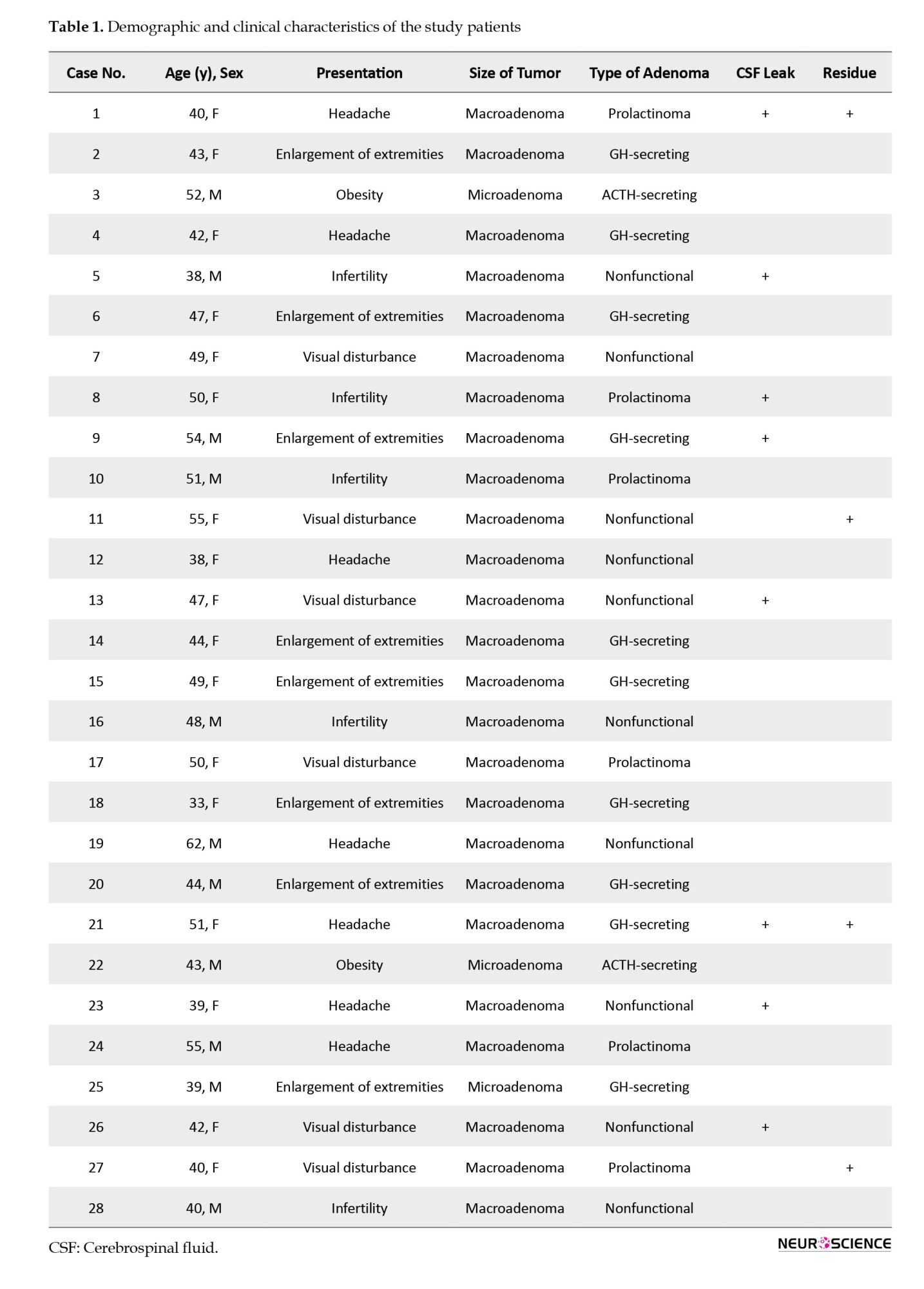
MRI of the pituitary gland
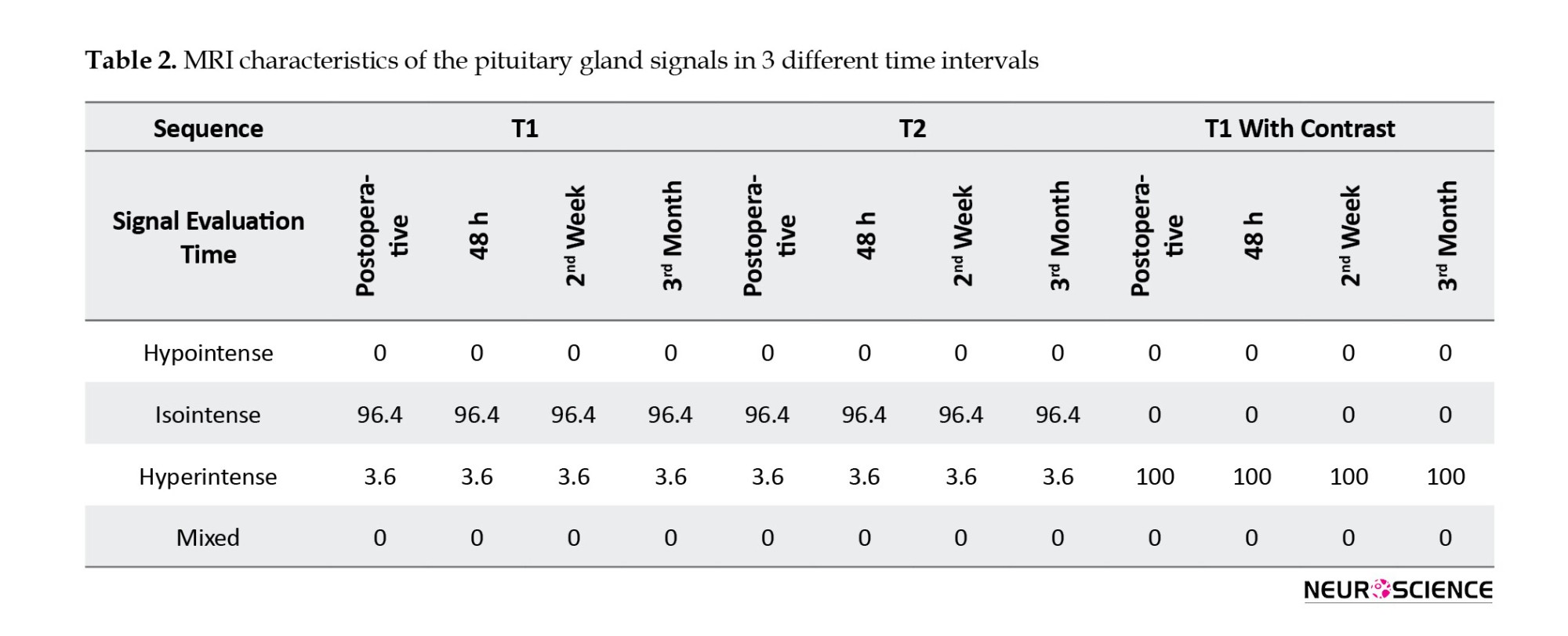
In 27 patients (96.4%), the pituitary gland in the T1-weighted sequence was isointense, and one (3.6%) was hyperintense. In the T2-weighted sequence, 27 patients (96.4%) were isointense, and one (3.6%) was hyperintense. In the T1-weighted with contrast sequence, the pituitary glands of all patients were hyperintense. The postoperative MR images had similar results at 48 hours, 2 weeks, and 3 months after surgery. An overview of the pituitary gland signal is shown in Table 2 (Figures 1, 2 and 3).
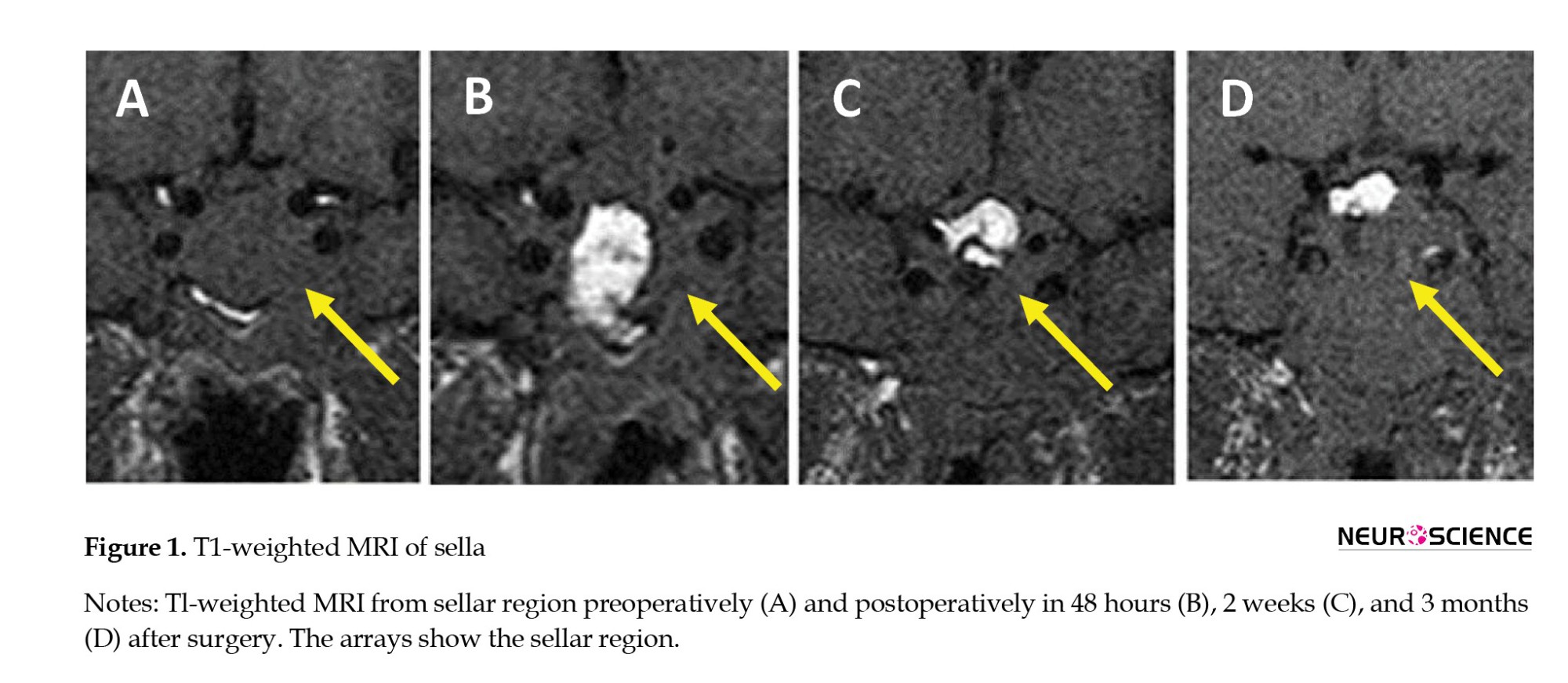
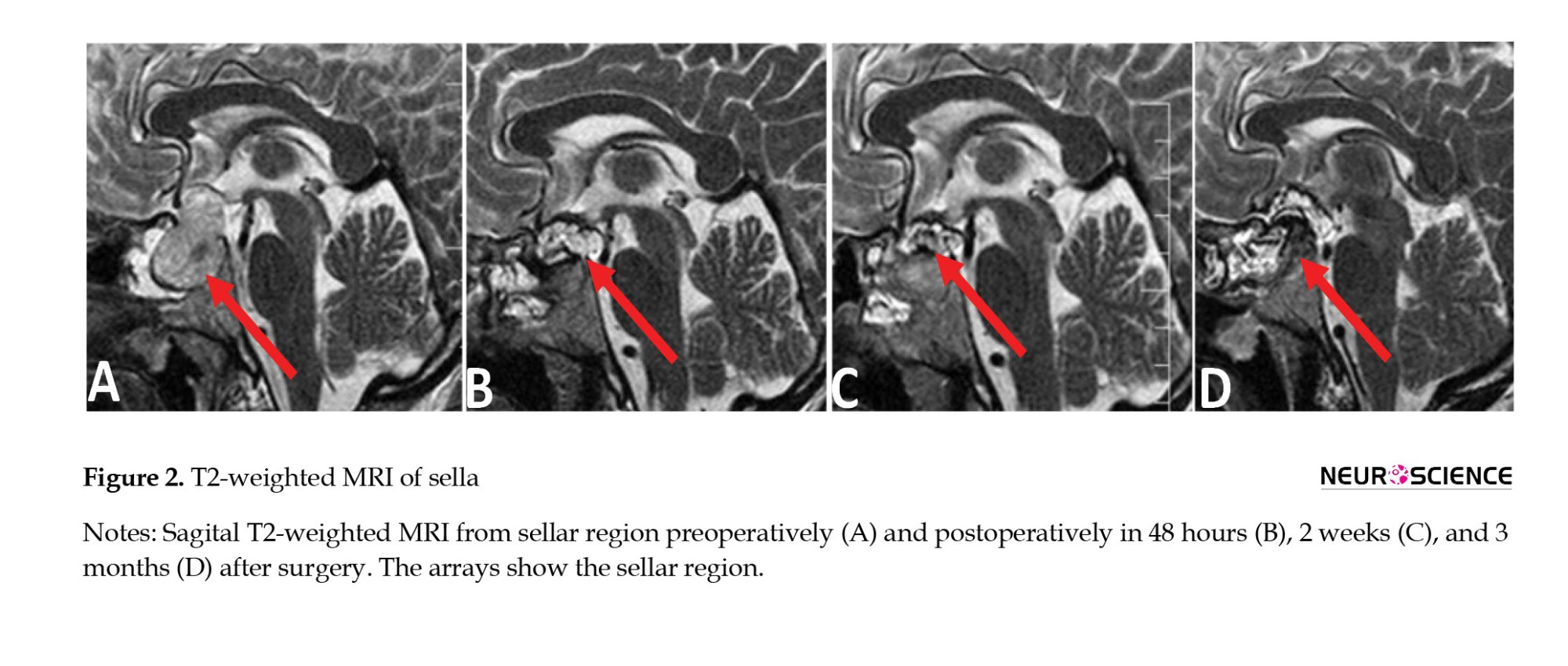
MRI of pituitary adenoma
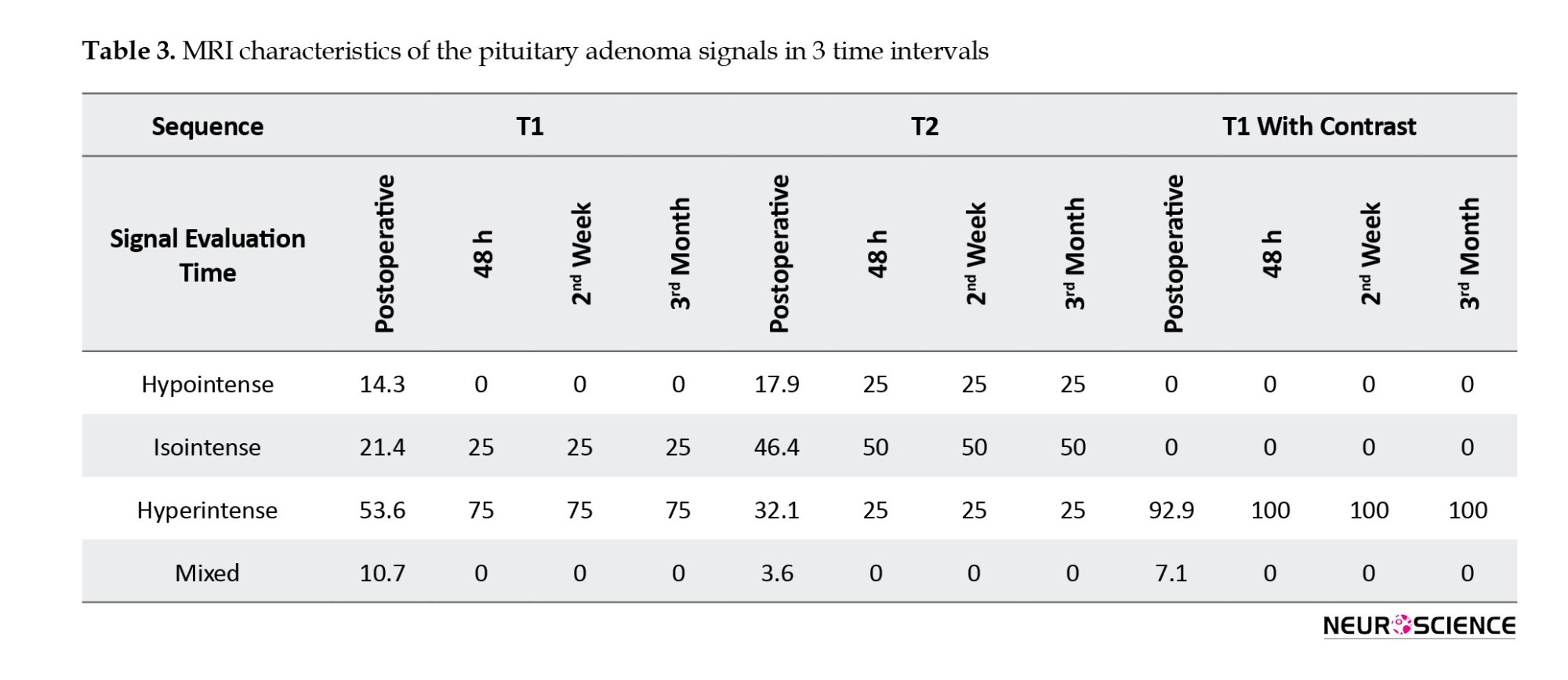
T1-weighted pituitary adenoma signals were hypointense in 14.3% of patients, isointense in 21.4%, hyperintense in 53.6%, and mixed in 10.7%. The T2-weighted sequence was hypointense in 17.9%, isointense in 46.4%, hyperintense in 32.1%, and mixed in 3.6%. The T1-weighted sequence with contrast was hyperintense in 92.9% of patients and mixed in 7.1%. In postoperative imaging, only 4 patients showed tumor residue; the signal intensity showed no difference at various times (Table 2). An overview of the MRI characteristics of the pituitary adenomas is given in Table 3 (Figures 1, 2 and 3).
MRI of packing material
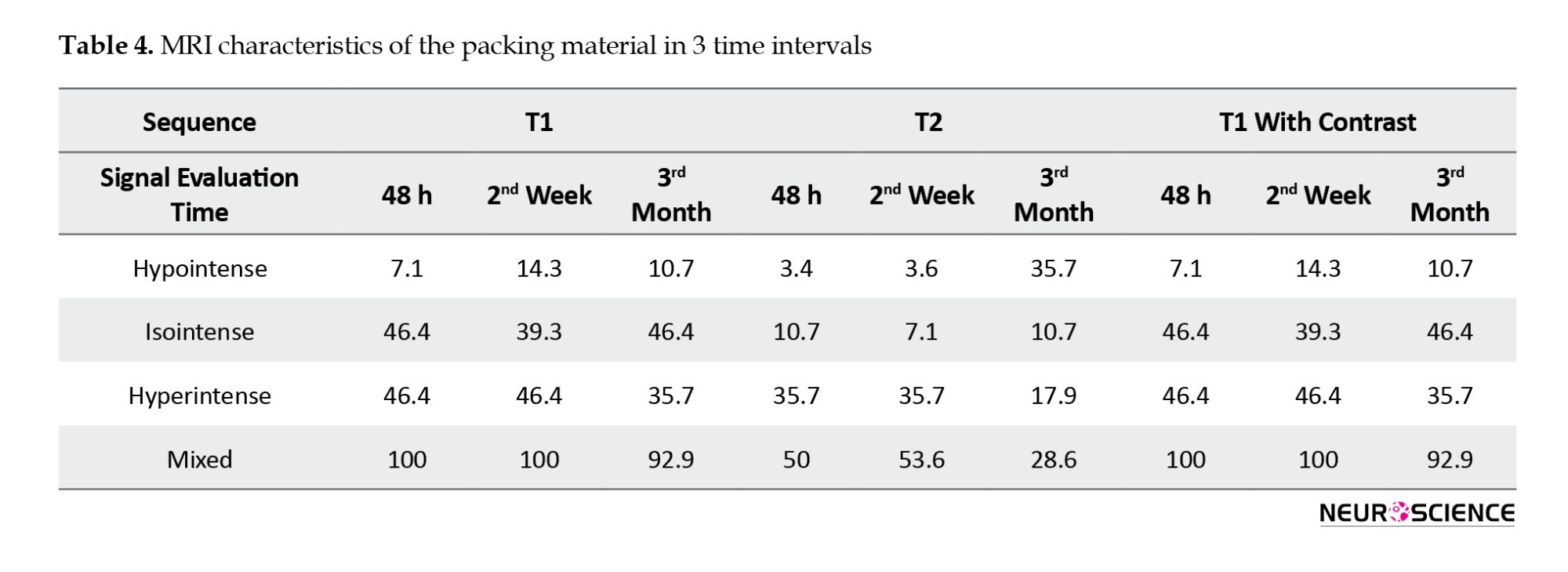
The sellar floor was reconstructed with an autografted adipose tissue covered by Surgicel and gelfoam. In the first postoperative MRI of the T1-weighted sequence, the signal of these materials in 7.1% of patients was hypointense, 46.4% was hyperintense, and 46.4% was mixed. The second MRI showed hypointense in 14.3%, hyperintense in 39.3%, and mixed intensity in 46.4%. The third MRI showed hypointense in 10.7%, hyperintense in 46.4%, and mixed intensity in 35.7%. The signal intensities of T1- and T2-weighted images and post-contrast images are indexed in Table 4.
4. Discussion
MRI is currently the preferred neuroradiologic tool for evaluating the sellar region. Postoperative MRI is routinely performed to determine residual or recurrent tumors and possible complications (Doerfler & Richter, 2008; Moldovan et al., 2016). Although the optimal time for postoperative MRI for a central nervous system neoplasm is 48 hours after surgery, there is controversy about the timing for the pituitary adenoma (Albert et al., 1994; Kılıç et al., 2001a; Oser et al., 1997; Saunders et al., 2005). Several studies recommend that the follow-up MRI should not be done immediately after transsphenoidal surgery because postoperative changes such as swelling, blood products, and packing materials inserted into the sella can lead to misinterpretation of the MRI. These studies recommend waiting for their regression (Rajaraman & Schulder, 1999; Rodriguez et al., 1996).
Steiner et al. (1992) evaluated preoperative and postoperative MR images of 25 patients diagnosed with pituitary adenomas. They recommended that a follow-up MRI be done 4-6 months after surgery. On the other hand, some studies have reported that the best time for postoperative MRI was a few days after surgery. Kilic et al. (2001a) assessed 80 patients with pituitary adenomas and found that the best follow-up imaging was 24 hours after transsphenoidal surgery. During this period, inflammation is in the early phase, and the packing material has not degraded and can easily be identified on the MR image. Blood degradation products, such as methemoglobin, have not yet formed and can easily be differentiated from a residual tumor. The current study found no time preference for the performance of postoperative MRI.
We found that the pituitary gland signals in most patients were isointense in the T1- and T2-weighted and hyperintense in the T1-weighted with contrast. The pituitary gland showed constant signals three months after the surgery and was well-demarcated from the surrounding tissue. Most previous articles reported only good delineation of the pituitary gland after surgery and did not define the signal intensity in each sequence.
Kilic et al. (2001a) concluded that the early postoperative MRI and delayed MRI delineated the pituitary gland. Yoon et al. (2001) did not explain the delineation and signal intensity of the pituitary gland and only reported changes in its configuration after surgery. Kramer et al. (2002) found that the normal gland was not visible in most patients in early postoperative MRI. Steiner et al. (1992) reported that, in 22 of 25 patients, the pituitary gland was well-delineated after surgery but did not compare this result with later follow-up imaging. Dina et al. (1993) reported that in the early postoperative period, the pituitary gland was remarkably similar to its preoperative appearance). Rodriguez et al., (1996) evaluated the size and shape of the pituitary gland after surgery and compared imaging according to these criteria.
Although only 6 patients were found to have a residual tumor, the tumor and packing material were well delineated in all of them. In all patients, the signal for the adenoma in all sequences was constant over time. Despite using the same materials for packing, their signals were different, probably because of the presence of blood that had stained the gelfoam and Surgicel. Packing material MRI signals were compared, and it was concluded that there were meaningful differences in their intensity only in the T2-weighted sequence (Table 5). We observed that the T2 signal intensity of the packing material changed from hyperintense to hypointense. This shift was probably caused by a decrease in the water content and concentration of macromolecules, which have short T2 relaxation times (Mangrum et al., 2018).

Kilic et al. (2001a) could not differentiate a residual tumor in one-third of patients in the early postoperative MRI because of blood in the tumor bed. Stiner et al. (1992) reported that the adenoma’s signal and remnant were constant in all patients except one. They performed postoperative MRI once, compared it with preoperative MRI, and could not differentiate residual tumor from implanted material. In contrast to other studies that assessed the shape of the sellar element, the current study compared post-surgical MRI statistically based on the signal intensity of the sellar contents. The change in signal intensity of the sellar elements is a more objective criterion than their shape.
We concluded that contrary to other intracranial neoplasms, there were no apparent changes in MRI signal intensity in patients with pituitary adenoma over 3 months after the operation. So, there is no advantage to the time of follow-up imaging in patients with pituitary adenoma. Consequently, unlike previous documentation, the Imaging time of the patient with pituitary adenoma was unimportant, and an MRI could be performed any time after surgery.
5. Conclusion
There are no significant differences in MRI findings between 48-hour, two-week, or three-month post-surgery periods in patients with pituitary adenoma. This indicates that the timing of post-operative imaging in pituitary adenomas is not critical for diagnostic accuracy. These findings provide flexibility in the timing of follow-up MRIs, allowing clinicians to prioritize patient stability and recovery before scheduling imaging. By reducing the urgency for immediate MRI, unnecessary stress and logistical challenges for patients and healthcare providers can be minimized. This study is the first of its kind globally, paving the way for updated post-operative care protocols specific to pituitary adenomas.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted with the approval of the Ethics Committee of Iran University of Medical Sciences. The study followed the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express our heartfelt gratitude to the MRI unit and the dedicated staff of the radiology department for their invaluable assistance during this study. We also extend our sincere thanks to the operative room team for their support and cooperation throughout the surgical procedures. Their commitment and expertise played a crucial role in the successful completion of this research.
References
Pituitary adenomas account for about 15% of primary intracranial tumors (Barbieri et al., 2007; Goya et al., 2004; Isobe et al., 2000; Pennacchietti et al., 2016). Except for prolactinoma, the treatment of choice in pituitary adenoma is surgical excision (Mortini et al., 2005). Postoperative imaging is important in detecting possible surgical complications, tumor residue, and treatment planning (Doerfler & Richter, 2008; Moldovan et al., 2016). Magnetic resonance imaging (MRI) is the preferred neuroradiologic method of evaluating the sellar region (Huang & Mukundan, 2016; Zaidi et al., 2016). The reason for the widespread acceptance of the MRI is the appropriate contrast among the different elements of the soft tissue (Hess & Dillon, 2012). Generally, the optimal time for early postoperative MRI in central nervous system lesions is 48 hours after surgery (Albert et al., 1994; Oser et al., 1997; Saunders et al., 2005). Nevertheless, no guidelines determine the best time for postoperative imaging in patients with pituitary adenomas (Kılıç et al., 2001b).
We analyzed the pituitary gland, adenoma, and packing material signals three months after surgery at three time points and finally proposed the optimal time for postoperative imaging after pituitary adenoma surgery.
2. Materials and Methods
Study patients
A total of 28 patients with pituitary adenoma referred to the Department of Neurosurgery at Firoozgar University Hospital in Tehran City, Iran, from July 2019 to March 2021 were examined.
The inclusion criteria were as follows: A diagnosis of pituitary adenoma, being a candidate for endoscopic transsphenoidal surgery, and no history of previous surgery or other therapies such as irradiation and dopamine agonist therapy.
All patients had preoperative contrast-enhanced MRI of the sellar region to determine the extent of the tumor and involvement of adjacent structures. Preoperative, endocrinologic, and ophthalmologic evaluations were carried out for all patients.
Surgical technique
All patients underwent an endoscopic endonasal approach under general anesthesia. The endoscopic equipment used included a 0° and 30°, 4 mm rigid endoscope (Karl Storz; Germany), a full high definition (HD) camera (Image 1, H3-Z), and a wide view HD screen. Generally, the approach was through the right nostril, but the left side was used if there was a significant septal deviation to the right. The sellar floor was reconstructed using a fat pad covered by Surgicel and gelfoam. Histopathologic analysis was performed to establish the diagnosis.
Postoperative MRI
The MRI was performed with a 1.5 T system (Siemens Medical Solutions). The imaging protocol included non-enhanced T1- and T2-weighted and post-contrast T1 images with a 5-mm section thickness of the axial, coronal, and sagittal planes. All patients had a sellar MRI before surgery and three sellar MRIs at 48 hours, two weeks, and three months after surgery.
Imaging analysis
All MR images were interpreted concerning the contents of the sella: The pituitary gland, pituitary adenoma, and adipose tissue used to pack the sella. The signal for each compartment was recorded in the T1-weighted, T2-weighted, and T1-weighted with contrast sequences at different times.
Statistical analysis
Statistical analysis, including descriptive data analysis, was performed using SPSS software, version 23; (IBM; USA). The sellar contents signal at the different time points were considered non-parametric and were evaluated using the Friedman and sign tests. Two-sided P<0.05 were considered statistically significant.
3. Results
Demographic and clinical aspects
The mean age of the participants was 45.8 years. About 60.7% were female, and 39.3% were male. The most common complaint at presentation was the enlargement of the extremities, which affected 8 patients (28.6%). A visual disturbance occurred in 7 patients (25%), while 6 patients (21.4%) had a headache. Ten patients (35.7%) had nonfunctional pituitary adenoma, 10(35.7%) were diagnosed with acromegaly, 6(21.4%) had prolactinoma, and 2(7.1%) had an ACTH-producing tumor.
Of the 28 patients, 25 had macroadenomas, and 3 had microadenomas. After surgery, a residual tumor (incomplete removal) was confirmed in 4 patients (14.2%). Postoperative cerebrospinal fluid rhinorrhea occurred in only 8 cases that improved after conservative treatment. Seven patients (25%) developed transient postoperative diabetes insipidus during hospitalization. On ophthalmological examination, 16 patients (57.1%) showed visual field deficits. The demographic and clinical characteristics are shown in Table 1.

MRI of the pituitary gland
In 27 patients (96.4%), the pituitary gland in the T1-weighted sequence was isointense, and one (3.6%) was hyperintense. In the T2-weighted sequence, 27 patients (96.4%) were isointense, and one (3.6%) was hyperintense. In the T1-weighted with contrast sequence, the pituitary glands of all patients were hyperintense. The postoperative MR images had similar results at 48 hours, 2 weeks, and 3 months after surgery. An overview of the pituitary gland signal is shown in Table 2 (Figures 1, 2 and 3).




MRI of pituitary adenoma
T1-weighted pituitary adenoma signals were hypointense in 14.3% of patients, isointense in 21.4%, hyperintense in 53.6%, and mixed in 10.7%. The T2-weighted sequence was hypointense in 17.9%, isointense in 46.4%, hyperintense in 32.1%, and mixed in 3.6%. The T1-weighted sequence with contrast was hyperintense in 92.9% of patients and mixed in 7.1%. In postoperative imaging, only 4 patients showed tumor residue; the signal intensity showed no difference at various times (Table 2). An overview of the MRI characteristics of the pituitary adenomas is given in Table 3 (Figures 1, 2 and 3).

MRI of packing material
The sellar floor was reconstructed with an autografted adipose tissue covered by Surgicel and gelfoam. In the first postoperative MRI of the T1-weighted sequence, the signal of these materials in 7.1% of patients was hypointense, 46.4% was hyperintense, and 46.4% was mixed. The second MRI showed hypointense in 14.3%, hyperintense in 39.3%, and mixed intensity in 46.4%. The third MRI showed hypointense in 10.7%, hyperintense in 46.4%, and mixed intensity in 35.7%. The signal intensities of T1- and T2-weighted images and post-contrast images are indexed in Table 4.

4. Discussion
MRI is currently the preferred neuroradiologic tool for evaluating the sellar region. Postoperative MRI is routinely performed to determine residual or recurrent tumors and possible complications (Doerfler & Richter, 2008; Moldovan et al., 2016). Although the optimal time for postoperative MRI for a central nervous system neoplasm is 48 hours after surgery, there is controversy about the timing for the pituitary adenoma (Albert et al., 1994; Kılıç et al., 2001a; Oser et al., 1997; Saunders et al., 2005). Several studies recommend that the follow-up MRI should not be done immediately after transsphenoidal surgery because postoperative changes such as swelling, blood products, and packing materials inserted into the sella can lead to misinterpretation of the MRI. These studies recommend waiting for their regression (Rajaraman & Schulder, 1999; Rodriguez et al., 1996).
Steiner et al. (1992) evaluated preoperative and postoperative MR images of 25 patients diagnosed with pituitary adenomas. They recommended that a follow-up MRI be done 4-6 months after surgery. On the other hand, some studies have reported that the best time for postoperative MRI was a few days after surgery. Kilic et al. (2001a) assessed 80 patients with pituitary adenomas and found that the best follow-up imaging was 24 hours after transsphenoidal surgery. During this period, inflammation is in the early phase, and the packing material has not degraded and can easily be identified on the MR image. Blood degradation products, such as methemoglobin, have not yet formed and can easily be differentiated from a residual tumor. The current study found no time preference for the performance of postoperative MRI.
We found that the pituitary gland signals in most patients were isointense in the T1- and T2-weighted and hyperintense in the T1-weighted with contrast. The pituitary gland showed constant signals three months after the surgery and was well-demarcated from the surrounding tissue. Most previous articles reported only good delineation of the pituitary gland after surgery and did not define the signal intensity in each sequence.
Kilic et al. (2001a) concluded that the early postoperative MRI and delayed MRI delineated the pituitary gland. Yoon et al. (2001) did not explain the delineation and signal intensity of the pituitary gland and only reported changes in its configuration after surgery. Kramer et al. (2002) found that the normal gland was not visible in most patients in early postoperative MRI. Steiner et al. (1992) reported that, in 22 of 25 patients, the pituitary gland was well-delineated after surgery but did not compare this result with later follow-up imaging. Dina et al. (1993) reported that in the early postoperative period, the pituitary gland was remarkably similar to its preoperative appearance). Rodriguez et al., (1996) evaluated the size and shape of the pituitary gland after surgery and compared imaging according to these criteria.
Although only 6 patients were found to have a residual tumor, the tumor and packing material were well delineated in all of them. In all patients, the signal for the adenoma in all sequences was constant over time. Despite using the same materials for packing, their signals were different, probably because of the presence of blood that had stained the gelfoam and Surgicel. Packing material MRI signals were compared, and it was concluded that there were meaningful differences in their intensity only in the T2-weighted sequence (Table 5). We observed that the T2 signal intensity of the packing material changed from hyperintense to hypointense. This shift was probably caused by a decrease in the water content and concentration of macromolecules, which have short T2 relaxation times (Mangrum et al., 2018).

Kilic et al. (2001a) could not differentiate a residual tumor in one-third of patients in the early postoperative MRI because of blood in the tumor bed. Stiner et al. (1992) reported that the adenoma’s signal and remnant were constant in all patients except one. They performed postoperative MRI once, compared it with preoperative MRI, and could not differentiate residual tumor from implanted material. In contrast to other studies that assessed the shape of the sellar element, the current study compared post-surgical MRI statistically based on the signal intensity of the sellar contents. The change in signal intensity of the sellar elements is a more objective criterion than their shape.
We concluded that contrary to other intracranial neoplasms, there were no apparent changes in MRI signal intensity in patients with pituitary adenoma over 3 months after the operation. So, there is no advantage to the time of follow-up imaging in patients with pituitary adenoma. Consequently, unlike previous documentation, the Imaging time of the patient with pituitary adenoma was unimportant, and an MRI could be performed any time after surgery.
5. Conclusion
There are no significant differences in MRI findings between 48-hour, two-week, or three-month post-surgery periods in patients with pituitary adenoma. This indicates that the timing of post-operative imaging in pituitary adenomas is not critical for diagnostic accuracy. These findings provide flexibility in the timing of follow-up MRIs, allowing clinicians to prioritize patient stability and recovery before scheduling imaging. By reducing the urgency for immediate MRI, unnecessary stress and logistical challenges for patients and healthcare providers can be minimized. This study is the first of its kind globally, paving the way for updated post-operative care protocols specific to pituitary adenomas.
Ethical Considerations
Compliance with ethical guidelines
The study was conducted with the approval of the Ethics Committee of Iran University of Medical Sciences. The study followed the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express our heartfelt gratitude to the MRI unit and the dedicated staff of the radiology department for their invaluable assistance during this study. We also extend our sincere thanks to the operative room team for their support and cooperation throughout the surgical procedures. Their commitment and expertise played a crucial role in the successful completion of this research.
References
Albert, F. K., Forsting, M., Sartor, K., Adams, H. P., & Kunze, S. (1994). Early postoperative magnetic resonance imaging after resection of malignant glioma: Objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery, 34(1), 45–61. [DOI:10.1227/00006123-199401000-00008] [PMID]
Barbieri, F., Bajetto, A., Porcile, C., Pattarozzi, A., Schettini, G., & Florio, T. (2007). Role of stromal cell-derived factor 1 (SDF1/CXCL12) in regulating anterior pituitary function. Journal of Molecular Endocrinology, 38(3), 383–389. [DOI:10.1677/JME-06-0014] [PMID]
Dina, T. S., Feaster, S. H., Laws, E. R., Jr, & Davis, D. O. (1993). MR of the pituitary gland postsurgery: Serial MR studies following transsphenoidal resection. AJNR. American Journal of Neuroradiology, 14(3), 763–769. [PMID]
Doerfler, A., & Richter, G. (2008). Lesions within and around the Pituitary. Clinical Neuroradiology, 18(1), 5-18. [DOI:10.1007/s00062-008-8001-0]
Goya, R. G., Sarkar, D. K., Brown, O. A., & Hereñú, C. B. (2004). Potential of gene therapy for the treatment of pituitary tumors. Current Gene Therapy, 4(1), 79–87. [DOI:10.2174/1566523044578086] [PMID] [PMCID]
Hess, C. P., & Dillon, W. P. (2012). Imaging the pituitary and parasellar region. Neurosurgery Clinics of North America, 23(4), 529–542.[DOI:10.1016/j.nec.2012.06.002] [PMID]
Huang, R. Y., & Mukundan, S. (2016). Imaging of the sellar region. In G. Zada, M. Lopes, S. Mukundan, & E. Laws Jr. (Eds), Atlas of sellar and parasellar lesions. Cham: Springer.[DOI:10.1007/978-3-319-22855-6_2]
Isobe, K., Ohta, M., Yasuda', S., Uno, T., Hara, R., & Machida, N., et al. (2000). Postoperative radiation therapy for pituitary adenoma. Journal of Neuro-Oncology, 48(2), 135–140. [DOI:10.1023/A:1006477905230] [PMID]
Kılıç, T., Ekinci, G., Şeker, A., Elmacı, I., Erzen, C., & Pamir, M. (2001). Determining optimal MRI follow-up after transsphenoidal surgery for pituitary adenoma: scan at 24 hours postsurgery provides reliable information. Acta Neurochirurgica, 143(11), 1103-1126. [DOI:10.1007/s007010100002] [PMID]
Kılıç, T., Ekinci, G., Şeker, A., Elmacı, I., Erzen, C., & Pamir, M. J. A. n. (2001). Determining optimal MRI follow-up after transsphenoidal surgery for pituitary adenoma: Scan at 24 hours postsurgery provides reliable information. Acta Neurochirurgica, 143(11), 1103–1126. [DOI:10.1007/s007010100002] [PMID]
Kremer, P., Forsting, M., Ranaei, G., Wüster, C., Hamer, J., & Sartor, K., et al. (2002). Magnetic resonance imaging after transsphenoidal surgery of clinically nonfunctional pituitary macroadenomas and its impact on detecting residual adenoma. Acta Neurochirurgica, 144(5), 433–443. [DOI:10.1007/s007010200064] [PMID]
Mangrum, W., Hoang, Q. B. P. B., Amrhein, T. J., Duncan, S. M., Maxfield, C. M., & Merkle, E., et al. (2018). Duke review of MRI Physics: Case review series. Amsterdam: Elsevier Science Publishers BV. [Link]
Moldovan, I. M., Melincovici, C., Mihu, C. M., Susman, S., Constantin, A. M., & Florian, S. I. J. R. N. (2016). Diagnostic criteria in invasive pituitary adenomas. Romanian Neurosurgery, 30(3), 342–355. [Link]
Mortini, P., Losa, M., Barzaghi, R., Boari, N., & Giovanelli, M. (2005). Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery, 56(6), 1222–1233. [DOI:10.1227/01.NEU.0000159647.64275.9D] [PMID]
Oser, A. B., Moran, C., Kaufman, B. A., & Park, T. J. R. (1997). Intracranial tumor in children: MR imaging findings within 24 hours of craniotomy. Radiology, 205(3), 807–812. [DOI:10.1148/radiology.205.3.9393539] [PMID]
Pennacchietti, V., Garzaro, M., Grottoli, S., Pacca, P., Garbossa, D., & Ducati, A., et al. (2016). Three-dimensional endoscopic endonasal approach and outcomes in sellar lesions: A single-center experience of 104 cases. World Neurosurgery, 89, 121–125. [DOI:10.1016/j.wneu.2016.01.049] [PMID]
Rajaraman, V., & Schulder, M. J. S. n. (1999). Postoperative MRI appearance after transsphenoidal pituitary tumor resection. Surgical Neurology, 52(6), 592–599. [DOI:10.1016/S0090-3019(99)00157-3] [PMID]
Rodríguez, O., Mateos, B., de la Pedraja, R., Villoria, R., Hernando, J. I., & Pastor, A., et al. (1996). Postoperative follow-up of pituitary adenomas after trans-sphenoidal resection: MRI and clinical correlation. Neuroradiology, 38(8), 747–754. [DOI:10.1007/s002340050341] [PMID]
Saunders, D. E., Phipps, K. P., Wade, A. M., & Hayward, R. D. (2005). Surveillance imaging strategies following surgery and/or radiotherapy for childhood cerebellar low-grade astrocytoma. Journal of Neurosurgery, 102(2 Suppl), 172–178. [DOI:10.3171/jns.2005.102.2.0172] [PMID]
Steiner, E., Knosp, E., Herold, C. J., Kramer, J., Stiglbauer, R., & Staniszewski, K., et al. (1992). Pituitary adenomas: Findings of postoperative MR imaging. Radiology, 185(2), 521–527. [DOI:10.1148/radiology.185.2.1410366] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2022/11/23 | Accepted: 2023/04/4 | Published: 2024/09/1
Received: 2022/11/23 | Accepted: 2023/04/4 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





