Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 179-192 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fadaei A, Najafi M, Miladi-Gorji H, Tajik-Mansoury M A, Afkar M. Effect of Neurofeedback Training and Swimming Exercise on the Electroencephalographic Changes and Craving in Methamphetamine-dependent Patients. BCN 2025; 16 (S1) :179-192
URL: http://bcn.iums.ac.ir/article-1-2587-en.html
URL: http://bcn.iums.ac.ir/article-1-2587-en.html
Atefeh Fadaei1 

 , Mahmoud Najafi1
, Mahmoud Najafi1 

 , Hossein Miladi-Gorji *2
, Hossein Miladi-Gorji *2 

 , Mohammad Ali Tajik-Mansoury3
, Mohammad Ali Tajik-Mansoury3 

 , Mohammad Afkar4
, Mohammad Afkar4 




 , Mahmoud Najafi1
, Mahmoud Najafi1 

 , Hossein Miladi-Gorji *2
, Hossein Miladi-Gorji *2 

 , Mohammad Ali Tajik-Mansoury3
, Mohammad Ali Tajik-Mansoury3 

 , Mohammad Afkar4
, Mohammad Afkar4 


1- Department of Clinical Psychology, Faculty of Psychology & Educational Sciences, Semnan University, Semnan, Iran.
2- Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran.
3- Department of Medical Physics, Faculty of Medicine, Semnan University of Medical Science, Semnan, Iran.
4- Department of Emergency Medicine, Torbat Jam Faculty of Medical Science, Torbat- e Jam, Iran.
2- Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran.
3- Department of Medical Physics, Faculty of Medicine, Semnan University of Medical Science, Semnan, Iran.
4- Department of Emergency Medicine, Torbat Jam Faculty of Medical Science, Torbat- e Jam, Iran.
Keywords: Methamphetamine (METH), Neurofeedback (NFB), Swimming exercise, Electroencephalography (EEG), Craving
Full-Text [PDF 2239 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Methamphetamine (METH) abuse is a widespread issue and a significant public health problem (Burns, 2014), which affects the central nervous system (CNS) (Cherner et al., 2010). METH abuse is associated with dependence, persistent craving, and withdrawal syndrome, including depression, anxiety, and cognitive deficits in humans (Dean et al., 2013; McGregor et al., 2005) and animal models (Bigdeli et al., 2015; Hajheidari et al., 2015). Also, previous studies have shown that METH abuse makes the electroencephalographic (EEG) abnormal (Newton et al., 2004).
The neurotoxic effects of METH persisted even after prolonged abstinence (Ernst et al., 2000; Hajheidari et al., 2017).
Previous studies have shown that METH abuse increased delta and theta band powers after 4 days of abstinence (Newton et al., 2003). Also, it increased beta band power after 1–3 months of abstinence (Zhao et al., 2021). EEG abnormalities may be related to METH-induced behavioral disorders (Khajehpour et al., 2019; Shafiee-Kandjani et al., 2020). Therefore, it provides a potential insight that therapeutic interventions may occur via modulation of the EEG pattern of brain waves in patients with METH dependence.
Previous studies have shown that neurofeedback training (NFB) normalizes or optimizes brain activity (LaVaque, 2003) and decreases craving for METH (Hashemian, 2015). They also demonstrate that treating METH-dependent patients is rather complex (Brands et al., 2012).
So, it seems that NFB as a stand-alone therapy may have weak outcomes for the treatment of METH abuse and may need a complementary therapy approach to treatment. In this line, previous studies have shown that NFB training during swimming exercises improves and optimizes mental/psychomotor performances (Mikicin et al., 2020). In further notes, unlike other sports, swimming exercises simultaneously activate a great number of muscles, delay fatigue, and increase the chest movement amplitude (Eider et al., 2014) as well as the motivation and mood of the patient (Stan, 2012). Also, exercise increases alpha (Schneider et al., 2009) and beta (Moraes et al., 2007) power. Given the high risk of relapse in these patients, it needs a combined treatment to provide comprehensive treatment. Thus, this study aims to investigate the effect of NFB training and swimming exercise, both alone and combined, on the EEG changes and visual image-induced craving in METH-dependent patients.
2. Materials and Methods
Study participants
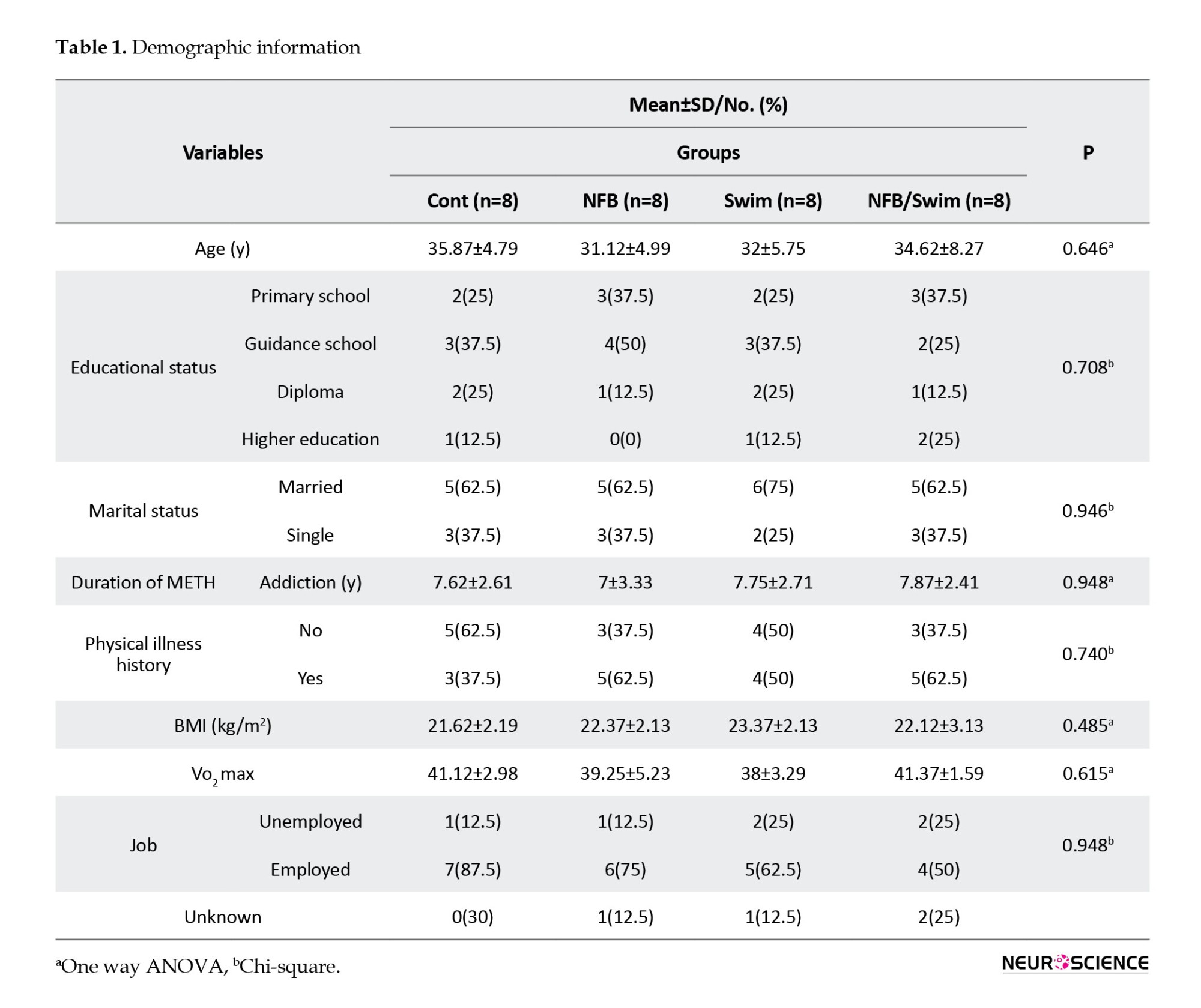
Patients with METH dependence were 32 men aged 25–45 years who were staying in an addiction treatment camp (Taybad, Iran). They were invited to participate in the study between 2020 and 2021, and then a two-week screening phase was started. During the screening, the medical history of the participants, physical examination, and criteria for METH dependence had assessed by the treatment camp’s physician. Patients had no history of drug treatment for withdrawal, professional sports, and the use of psychiatric drugs. They had no cognitive impairment, head trauma, stroke, cardiovascular, or psychiatric disorders. The treatment groups were matched concerning their age, education level, and type of drug abuse. Demographic variables, including age, educational status, marital status, duration of METH addiction, physical illness history, body mass index (BMI), VO2 max, and job, were evaluated (Table 1).
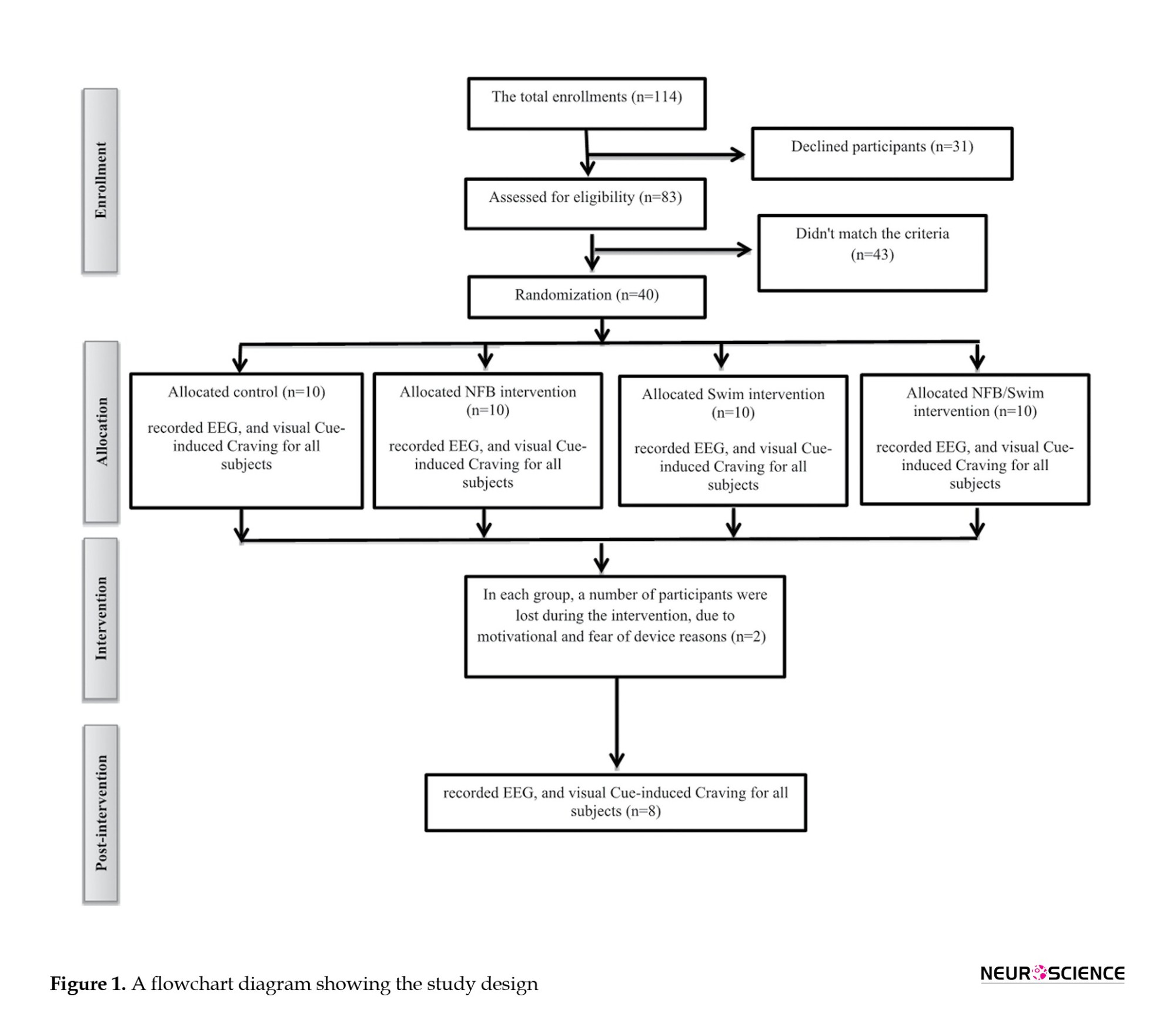
A total of 114 subjects were enrolled and screened. Thirty-one patients declined to participate, and 43 did not match the criteria. Finally, 40 patients met all inclusion criteria. We first informed the patients with a brief description of the study programs and their rights, and all participants provided written informed consent. The patients were then randomized into four groups using a pretest and post-test design: Control (no medication based on the camp’s protocol), NFB training (three days per week, alternate days for 10 weeks), regular swimming exercises (three days per week, alternate days for 10 weeks), and simultaneous NFB and swimming training (three days swim and three days NFB in alternate days one week for 10 weeks). However, 8 patients showed little motivation to continue and were excluded from the analysis. Finally, 32 patients completed the study (Figure 1). The Ethics Committee guidelines and regulations approved 32 patients. EEG and visual image-induced craving were recorded in all 32 patients (Figure 1).
Experimental protocol
NFB training method
Patients underwent the NFB protocol for 10 weeks, which was three interval sessions per week, and each session lasted 30 minutes using the Thought Technology ProComp 2 system, as previously described in the Iranian population (Dehghani-Arani et al., 2013; Kober et al., 2015). This protocol in every session was focused on sensory-motor rhythm (SMR) training in the central brain cortex (Cz region) and alpha-theta in the parietal cortex (Pz region). The protocol involves placing the active, reference, and grand electrodes in the Cz, left, and right ears, respectively. The primary purpose of our protocol design was to reinforce the SMR band (12–15 Hz) and suppress the alpha (8–12 Hz), theta (5–8 Hz), delta (2–5 Hz), and high beta (18–30 Hz). A visual threshold-dependent protocol in the form of a computer game was used as reward stimuli for the patients. Patients were advised to focus on feedback to find the most successful mental strategy and to get as many rewards as possible. When the EEG frequencies were within the defined thresholds, the patient obtained the reward, and the image was changed. The image would remain unchanged if the patient could not adjust to the thresholds. In this study, if the patient could increase the SMR wave by 80% of the time and decrease the delta, theta, and high beta by 20%, he received many rewards of visual feedback. Feedback in the alpha-theta training protocol on the Pz area was presented only in an auditory format. In this protocol, the patients close their eyes and only listen to the sound being played to them. The active, reference, and grand electrodes are placed in the Pz, left, and right ears, respectively, and two distinct tones of sounds were used to amplify alpha and theta under eyes-closed conditions. If the delta wave increases, the system could prevent the patient from falling asleep using a special sound. In this session, if the theta/beta ratio decreases, voice feedback is provided as a reward stimulus, which consists of continuing to play the music.
Swimming protocol
The swimming training protocol was done for 10 weeks with 3 sessions per week and 60 minutes per session. This training was conducted in a swimming pool (Taybad, Iran) under the supervision of an experienced exercise trainer. To start, we calculated the maximum heart rate (HRmax) with the formula (220- Age) (Bragada et al., 2009; Gellish et al., 2007). We used average HRmax to approximate the exercise intensity, which was determined at a moderate intensity (60%-70% heart rate reserve -12 beats/min). Each swimming session included three parts; the first was 10-minute warm-up, then there was a 40 minute swim, and the last part was a 10-minute cool down (Karapolat et al., 2009). In this study, the water temperature was about 32 °C.
EEG protocol
All EEG recordings were carried out after completing a neuropsychological test between 12 PM and 4 PM, under laboratory-controlled sound and light conditions, as previously described (Choi et al., 2013). The EEG recording lasted 10 minutes and included the following conditions: 5 minutes with eyes closed, 5 minutes with eyes open. EEG recordings were obtained from the scalp using SynAmps 2 with a 19-channel Quik-Cap and a NeuroScan system. The signals were recorded at a sampling frequency of 500 Hz, and electrode impedance was below 5 kΩ. The EEG was band-pass filtered at 0.1–60 Hz using the signal processing software of the NeuroScan system for spectral analysis in 32-bit file format, and 19 electrode sites of 19 channels were driven by the NeuroGuide montage set as follows: FP1, F3, F7, Fz, FP2, F4, F8, T3, C3, Cz, T4, C4, T5, P3, O1, Pz, T6, P4, and O2. Artifacts were eliminated using the artifact detection of the NeuroGuide software. Here, we analyzed the eyes closed EEG in resting conditions. Accepted epochs of EEG data for absolute (uV2) power were calculated using fast Fourier transforms and averaged by NeuroGuide’s spectral analysis system in four frequency bands: Delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz). The activity at 19 sites was divided into five regions: Frontal, parietal, temporal, central, and occipital. Relative power was calculated as a percentage of the total power for each frequency band. Also, the delta/alpha ratio was computed as the mean scalp delta to alpha power ratio.
Visual image-induced craving
Participants were asked to rate their craving intensity and their subjective desire for METH-induced by each picture on the self-report visual analog scale (VAS) from 0 to 100 (Ekhtiari et al., 2010). The time interval of each picture exposure in the task was between 15 and 20 s. In each category of pictures, there were 2 pictures as follows: METH outside or inside the package without instruments (2 pictures), METH with instruments (2 pictures), instruments (2 pictures), cues accompanied by drug use (2 pictures), an act of abuse without a seen instrument (2 pictures), and neutral pictures (2 pictures).
Statistical analysis
We used the Kolmogorov-Smirnov test to evaluate the normality of the data. As required, demographic data between groups were compared using the chi-square test and one-way analysis of variance (ANOVA). The multivariate analysis of covariance (MANCOVA) was used to determine whether pretesting participants influenced their post-test scores on the dependent measures with the post hoc Bonferroni test. Results are expressed as Mean±SEM and Mean±SD. Differences were considered statistically significant at P<0.05.
EEG data were processed with custom-made scripts using MATLAB version R2012a. First, data from the resting-state measurements were imported into EEGLAB, bandpass filtered between 1-40 Hz, and inspected for gross movement artifacts that were manually removed. Subsequently, epochs of 4 s in length were created. Epochs containing amplitudes exceeding ±100 μV at any scalp electrode and/or epochs containing abnormally distributed data (joint probability or kurtosis >5 SD from expected mean values) were rejected. Because absolute EEG power data are skewed, data were log-transformed before analysis. We then utilized several procedures to reduce the likelihood of type I error. To identify EEG bands of interest for further analysis, we calculated a grand mean of 19 electrodes for each frequency band, thus providing an overall index of power for each band. The MANCOVA test compared the grand mean power in each group. Power at each electrode site was subsequently examined using MANCOVA.
3. Results
Demographic variables
Patients revealed no significant differences in any demographic variable. All 32 participants were male, 65% of the population were married, and 35% were single, with a mean age of 33. Most participants were employed (64%) and from secondary school (37%). The mean duration of METH use was 7 years. More than half of the participants reported a physical illness history (52%). ANOVA showed no statistically significant difference between groups in the mean age (F(3, 28)=1, P=NS), duration of METH use (F(3, 28)=0.15, P=NS), BMI (F(3, 28)=0.72, P=NS), and VO2 max (F(3, 28)=1.66, P=NS) in METH addicts. The mean body mass index (BMI) and VO2 max in groups were 22.37 kg/m2 and 39.93 mL/kg/min, respectively (Table 1).
Effect of NFB training and swimming exercise on the EEG power changes in METH-dependent patients
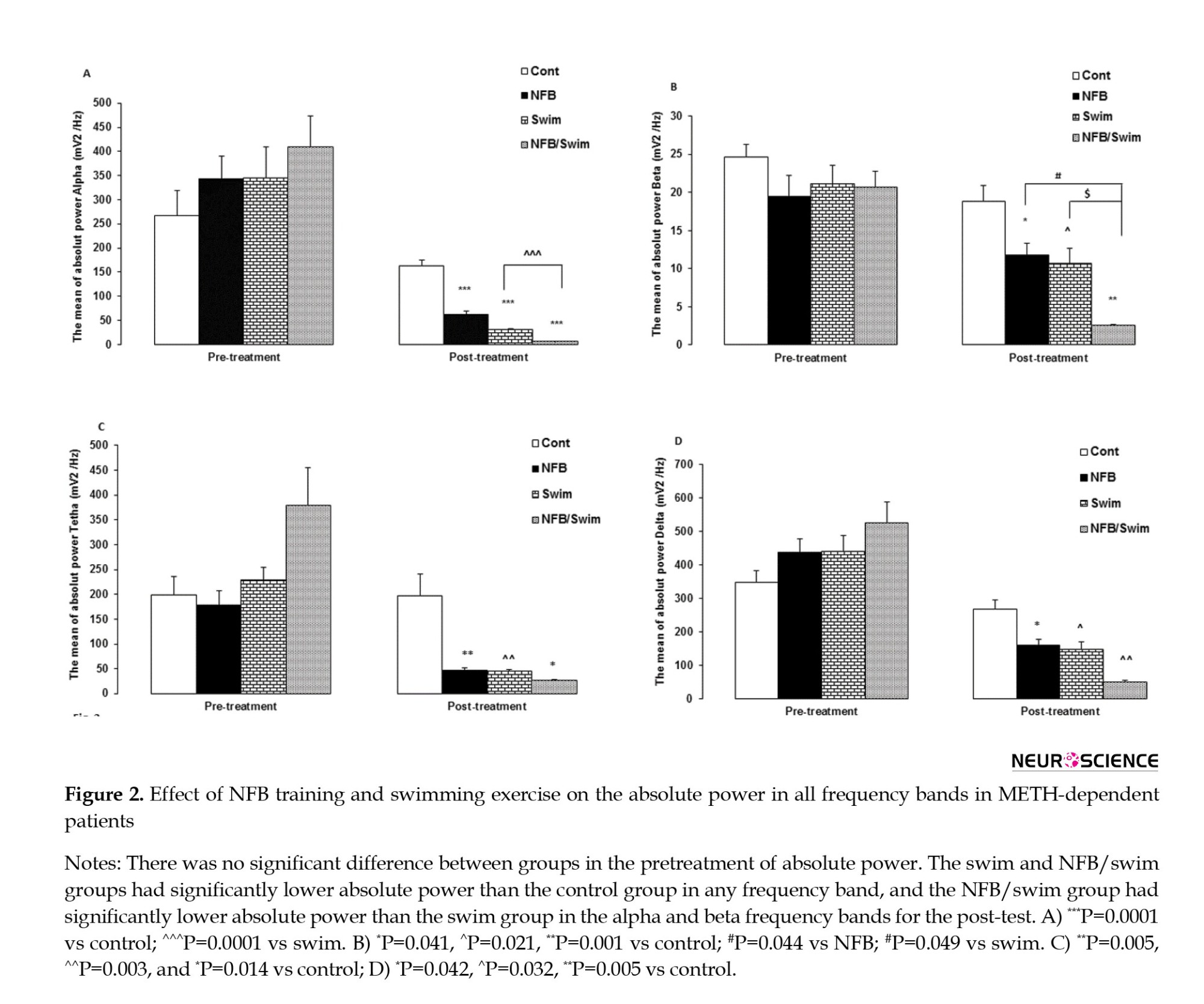
The MANCOVA revealed a significant interaction effect from pretreatment to posttreatment in absolute power (Wilks Lambda=0.039, F=11.22, P=0.0001, partial η2=0.66). There was no significant difference in pretreatment of the absolute power between groups. We observed significant differences among the three treatment conditions for the absolute power in alpha (F=68.04, P=0.0001), beta (F=7.25, P=0.001), theta (F=6.62, P=0.002), and delta (F=5.1, P=0.007) frequency bands.
Comparing the absolute power across posttreatment between groups showed that the alpha band in the NFB, swim, and NFB/swim groups had lower absolute power than the control group (P=0.0001, for all comparisons). Also, the NFB/swim group showed lower alpha power than the swim group (P=0.0001). Comparing the absolute power for posttreatment between groups showed that the beta band in the NFB (P=0.041), swim (P=0.021), and NFB/swim (P=0.001) groups had lower absolute power than the control group, also the NFB/swim group showed lower absolute power than the NFB (P=0.044) and swim (P=0.049) groups. Comparisons between groups in the theta band showed that the NFB (P=0.005), swim (P=0.003), and NFB/swim (P=0.014) groups had lower absolute power than the control group. Also, comparisons between groups in the delta band showed that the NFB (P=0.042), swim (P=0.032), and NFB/swim (P=0.006) groups had lower absolute power than the control group (Figure 2).
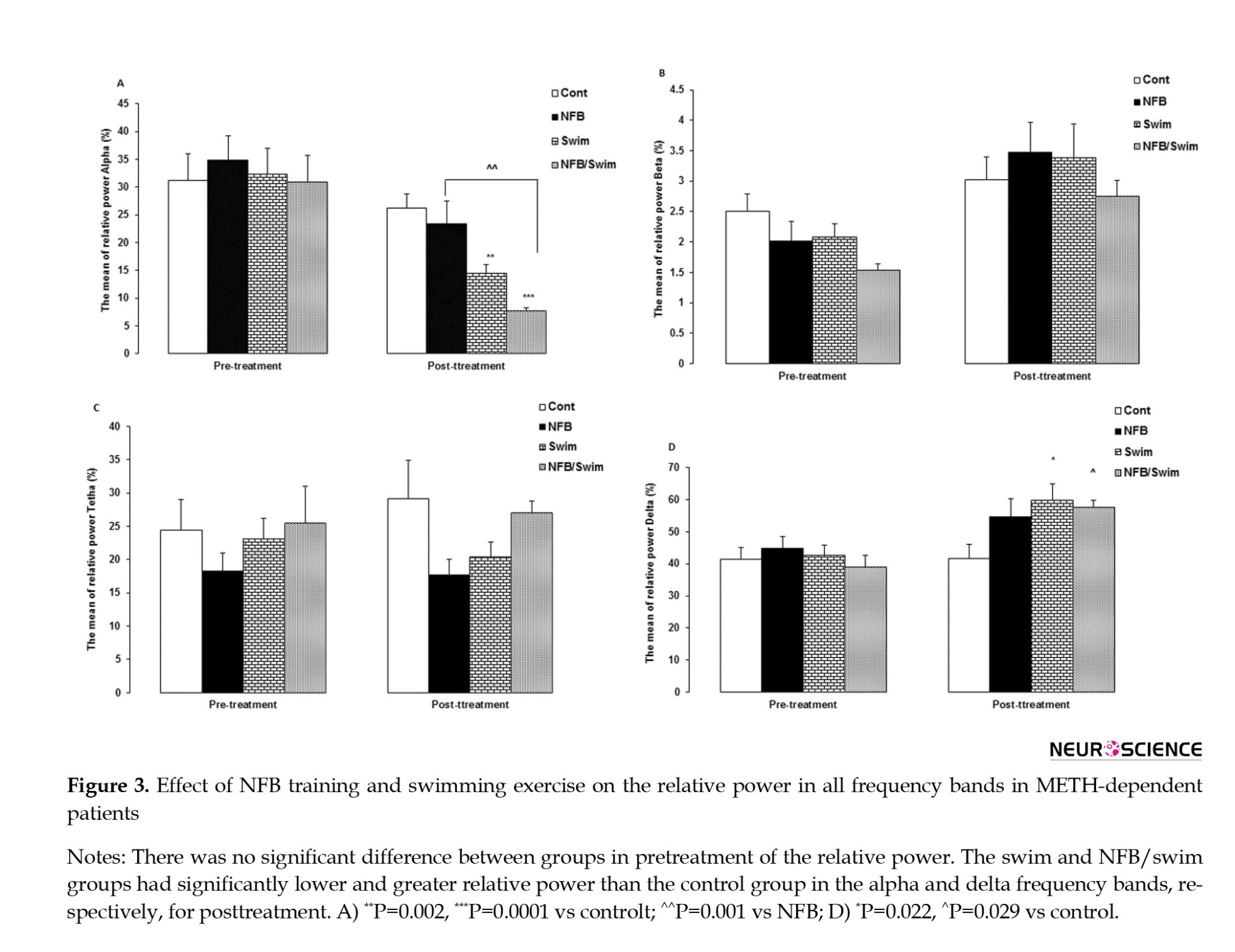
The MANCOVA revealed a significant interaction effect from pretreatment to posttreatment in relative power (Wilks Lambda=0.214, F=5.51, P=0.0001, partial η2=0.41). There was no significant difference in pretreatment of the relative power between groups. We observed significant differences among the three treatment conditions for the relative power in alpha (F=12.21, P=0.0001) and delta (F=4.1, P=0.017) frequency bands.
Comparing the relative power across for posttreatment between groups showed that the alpha band in the swim (P=0.002) and NFB/swim (P=0.0001) groups had lower power than the control group. Also, the NFB/swim group showed lower relative power than the NFB group (P=0.001). Comparing the relative power for posttreatment between groups showed that the delta band in the swim (P=0.022) and NFB/swim (P=0.029) groups had greater relative power than the control group (Figure 3).
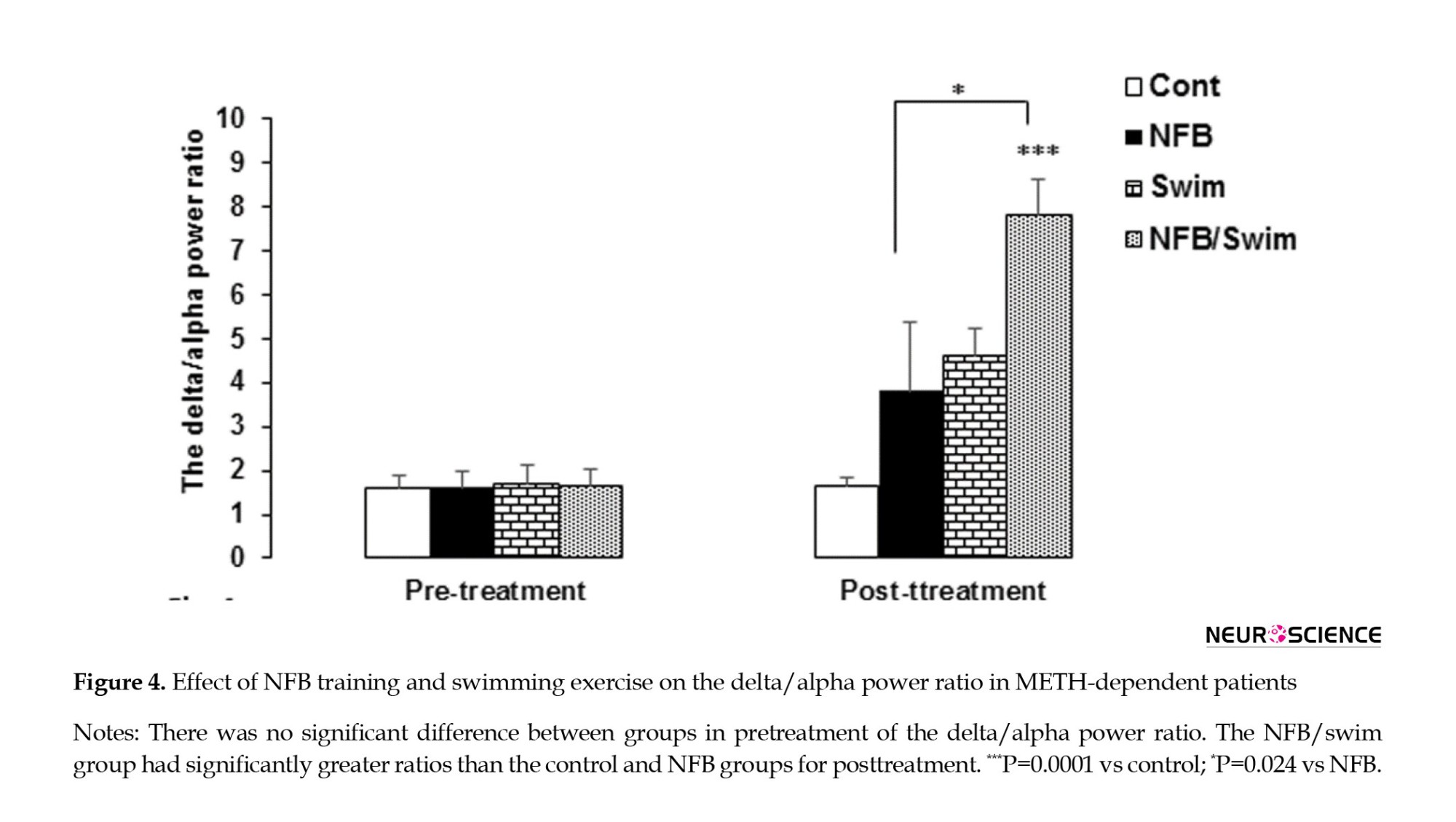
The MANCOVA revealed a statistically significant interaction effect from pretreatment to posttreatment in the delta/alpha power ratio (Wilks Lambda=0.551, F=7.35, P=0.001, Partial η2=0.449). There was no significant difference between groups in pretreatment of the delta/alpha power ratio. We observed significant differences among the three treatment conditions for the delta/alpha power ratio (F=7.61, P=0.001). Comparing the delta/alpha power ratio for posttreatment between groups showed that the NFB/swim group had greater ratios than the control (P=0.0001) and the NFB (P=0.024) groups (Figure 4).
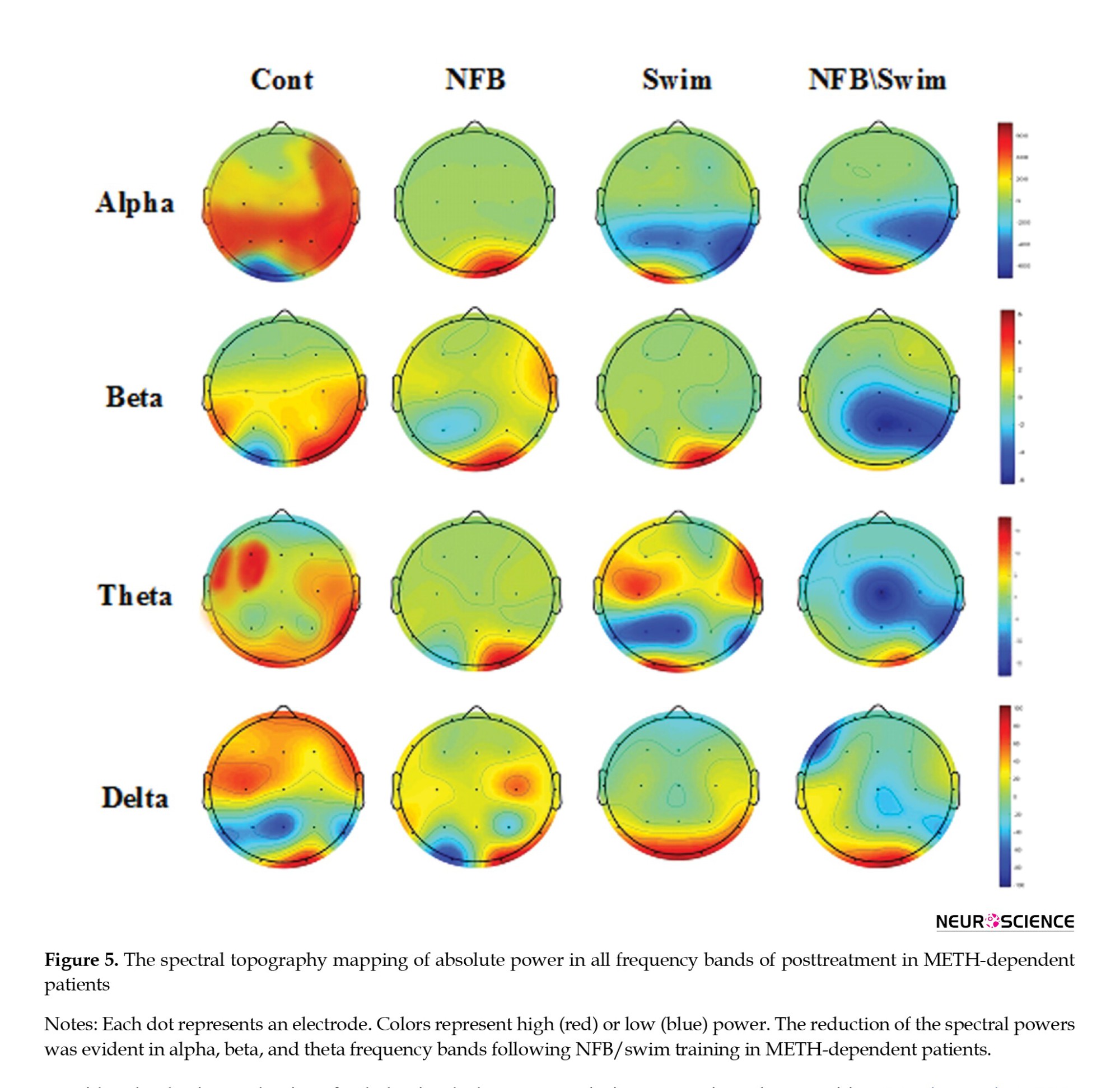
The reduction of the spectral powers was pretty evident in the frequency bands of alpha, beta, and theta following NFB/swim training in METH-dependent patients, which are shown in blue on the spectral topography mapping of absolute power (Figure 5).
Effect of NFB training and swimming exercise on visual image-induced craving in METH-dependent patients
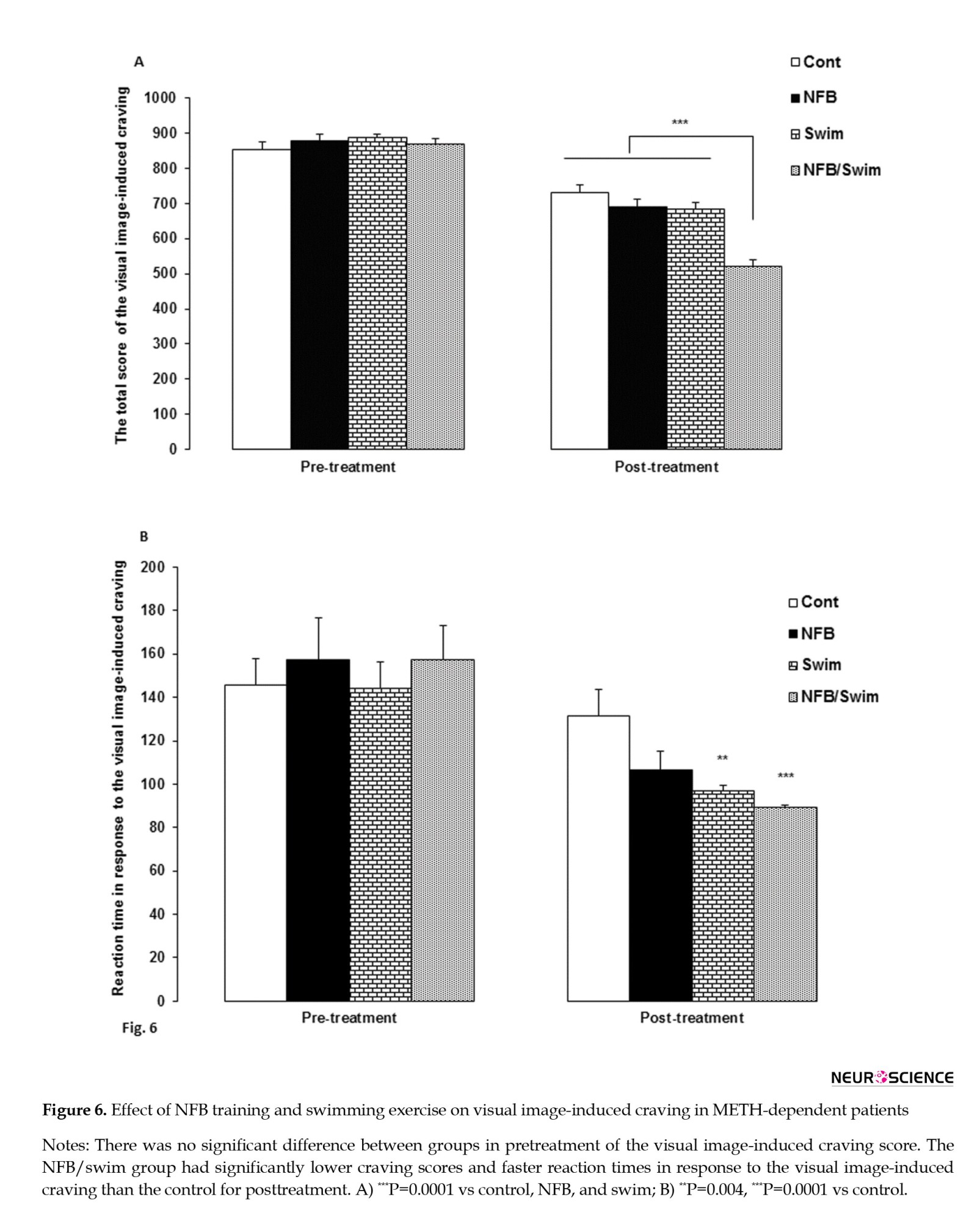
The MANCOVA revealed a significant interaction effect from pre- to posttreatment in visual image-induced craving (Wilks Lambda=0.25, F=11.07, P=0.0001, partial η2=0.50). There was no significant difference between groups in pretreatment of the visual image-induced craving score. We observed significant differences among the three treatment conditions for the visual image-induced craving score (F=20.51, P=0.0001) and reaction time in response to the visual image-induced craving (F=4.41, P=0.01).
Comparisons between groups in the visual image-induced craving showed that the NFB/swim group (P=0.0001) had a lower craving score than the control, swim, and NFB groups (P=0.0001, for all comparisons) (Figure 6A). Also, comparisons between groups showed that the swim (P=0.004) and NFB/swim (P=0.0001) groups had faster reaction times in response to the visual image-induced craving than the control group (Figure 6B).
4. Discussion
We found no significant difference in the pretreatment scores of the absolute and relative powers in frequency bands between groups in METH-dependent patients. Here, we found the greater power of 4 frequency bands for pretreatment in METH-dependent patients.
Similar to our findings, a previous study has shown an increased EEG power in the delta and theta for all areas of the brain after 4 days of METH abstinence, and EEG was abnormal in 64% of patients (Newton et al., 2003). In line with our findings, a previous study shows that whole brain metabolism in the METH abusers is 14% higher as a sensitive indicator of brain dysfunction (Volkow et al., 2001).
The increase in the power of all 4 frequency bands in the present findings suggests increased cortical activation in resting conditions, impaired inhibitory function, and loss of neural efficiency that may indicate a “noisy” brain (Prashad et al., 2018), leading to the interruption of the cognitive-affective-behavioral processes, including depression, psychosomatic disorders, psychomotor agitation, neuro-psycho-physiological abnormalities, encephalopathy, cognitive and memory impairments (Begić et al., 2011; Glasner-Edwards & Mooney, 2014; Kalechstein et al., 2009; Zorick et al., 2010). Also, it has been shown that increased delta and theta EEG activity may reflect the rewarding properties of drug use, whereas the increased alpha power may be associated with anxiety and nervousness (Reid et al., 2006), as it is observed in the patients of the current study.
Our findings have shown that the NFB training, swimming exercise, and combining NFB and swimming exercise for 10 weeks reduced EEG absolute power for 4 frequency bands. The combination of the NFB and swimming exercise (NFB/swim group) significantly reduced EEG absolute power for alpha and beta frequency bands in METH-dependent patients during withdrawal.
Following NFB training, patients learn to voluntarily modulate their electrical brain activity, which has beneficial effects on cognitive functions (Kober et al., 2013) and is already used as a therapeutic tool to treat different types of disorders. The NFB helps to adjust disruptive wave patterns, restore a normal pattern of brain activity, and correct abnormal brain function without any lasting side effects (Scott et al., 2005) in substance use disorders. In this regard, previous studies have shown that NFB training as a mood-enhancing and energizing experience (Raymond et al., 2005) can induce neural plasticity in the dorsal anterior and mid-cingulate (Lanius et al., 2012; Yamashita et al., 2017), involved in the cognitive functioning and emotional processing. Also, NFB-induced brain and neural plasticity is a phenomenon considered a basic mechanism for behavioral changes (Rostami & Dehghani-Arani, 2015).
The interesting finding of our study was that NFB/swim and swim reduced alpha’s relative power and increased delta’s relative power. Our study also showed that NFB/swim caused a significant increase in the delta/alpha power ratio for posttreatment in METH-dependent patients. Previous experience has shown that delta and alpha oscillations are reciprocally related (Knyazev, 2007).
Previous studies have shown that functionally delta oscillations may integrate and synchronize cerebral activity with homeostatic processes, including autonomic functions and motivational and cognitive processes (Harmony, 2013; Knyazev, 2007; Knazav, 2012). For example, increases in delta power were also reported during semantic tasks, cognitive (Franke et al., 2022), and motivation (Knyazev et al., 2004) processing. Delta inhibitory oscillations would modulate the activity of those networks that should be inactive to perform the task (Harmony, 2013).
Another potential explanation of our results could be the use of relative EEG power as a sensitive and stable measure to provide a useful neurophysiological biomarker to determine psychotic disorders and age-related changes in EEG (Howells et al., 2018; Jelic et al., 2000) in patients. A previous study has shown that regional cerebral blood flow decreases when delta power increases (Dang-Vu et al., 2005). Another study has shown that detoxified METH abusers had significantly lower rates of metabolism in the thalamus and striatum (Volkow et al., 2001). This finding emphasizes the importance and presence of an extra-thalamic delta rhythm (Dang-Vu et al., 2005), including the cortex (Steriade et al., 1993) in METH-dependent patients. It seems the brains of METH-dependent patients, following the NFB training and swimming exercise in the present study, are calm and relaxed. This condition produces more delta (Dunn et al., 1999).
The increased delta/alpha power ratio following the NFB training and swimming exercise may reflect decreased cortical activation, increased neural efficiency, and reduced effort in METH-dependent patients with a “noisy” brain and stress before treatment. Our finding indicates that the brains of METH-dependent patients following the NFB training along with swimming exercises are quiet and calm in a relaxed state with lower anxiety. Maybe this reduced effort in a calm brain caused a decrease in the visual image-induced craving score in METH-dependent patients following the NFB training and swimming exercise. These patients also had faster reaction times in response to the visual image-induced craving in the NFB/swim and swim groups. Similar to our findings, previous studies have shown that exercise reduces METH (Wang et al., 2016) and cannabis (Buchowski et al., 2011) cravings.
A previous study has shown that the craving in substance-dependence patients is almost all related to abnormalities in alpha and beta, particularly in the parietal and central lobes of the brain (Sokhadze et al., 2008), as observed in our study of patients before treatment. However, there is some evidence that the greater delta power increases ratings of cocaine craving (Reid et al., 2006). Thus, a more important question would be whether the increase in delta/alpha power ratio could decrease the visual image-induced craving for METH after abstinence in NFB/swim group. Our present data cannot answer this question and requires a different experimental protocol.
Therefore, the NFB training along with swimming exercise (NFB/swim group) may more effectively reduce the craving for METH after abstinence. Previous studies have documented associations between NFB training and physical activity. In this regard, it has been shown that NFB training may be useful in enabling athletes to perform optimally (Dupee et al., 2016). Also, NFB training during swimming exercises improves and optimizes mental/psychomotor performances (Mikicin et al., 2020).
At present, the neurobiological mechanisms to reduce EEG absolute power for 4 frequency bands and METH craving following the effectiveness of swimming exercise combined with NFB training are still unknown. It may be due to an ability to self-regulate dopaminergic neurons of the substantia nigra/ventral tegmental (Sulzer et al., 2013), the endogenous release of key neuromodulators (Ros et al., 2021), the regulation of serotonin (Konareva, 2006), and β-endorphin (Akbariet al., 2016) following NFB training. Also, the regulation of the above-mentioned key neuromodulators following swimming exercise might have enhanced the effects of NFB. Future studies need to examine the neurobiological mechanisms induced by NFB training and swimming exercise.
5. Conclusion
This study provides novel evidence that the NFB training and swimming exercise during METH-abstinence could decrease EEG absolute power for 4 frequency bands and the relative power of alpha while increasing the relative power of delta and the delta/alpha power ratio. Also, our findings have shown that the NFB training and swimming exercise decreased the visual image-induced craving score in METH-dependent patients. Our results could suggest the application of the NFB training along with swimming exercise as a useful therapeutic strategy for the treatment of METH use disorder with follow-up studies that utilize a larger number of samples and the most suitable type and intensity of exercise (voluntary, treadmill, and swimming).
Study limitations
This study also had some limitations. Larger sample sizes in future studies will increase power, decrease estimation error, and increase the ability to evaluate subgroup differences further. It is suggested that different types of exercise and other NFB protocols be used in the treatment of METH-dependent patients.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants followed the ethical standards of the Ethics Committee of the Semnan University of Medical Sciences, Semnan, Iran (Code: IR.SEMUMS.REC.1397.308) and with the 1964 Helsinki Declaration. Also, This study received the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20190703044092N1).
Funding
This article was taken from the PhD dissertation of Atefeh Fadaei, approved by Department of Clinical Psychology, Faculty of Psychology & Educational Sciences, Semnan University, Semnan, Iran. This work was supported by a personal research grant from Mahmoud Najafi, Faculty of Semnan University, Semnan, Iran.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Semnan University and the Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran, for its support.
References
Methamphetamine (METH) abuse is a widespread issue and a significant public health problem (Burns, 2014), which affects the central nervous system (CNS) (Cherner et al., 2010). METH abuse is associated with dependence, persistent craving, and withdrawal syndrome, including depression, anxiety, and cognitive deficits in humans (Dean et al., 2013; McGregor et al., 2005) and animal models (Bigdeli et al., 2015; Hajheidari et al., 2015). Also, previous studies have shown that METH abuse makes the electroencephalographic (EEG) abnormal (Newton et al., 2004).
The neurotoxic effects of METH persisted even after prolonged abstinence (Ernst et al., 2000; Hajheidari et al., 2017).
Previous studies have shown that METH abuse increased delta and theta band powers after 4 days of abstinence (Newton et al., 2003). Also, it increased beta band power after 1–3 months of abstinence (Zhao et al., 2021). EEG abnormalities may be related to METH-induced behavioral disorders (Khajehpour et al., 2019; Shafiee-Kandjani et al., 2020). Therefore, it provides a potential insight that therapeutic interventions may occur via modulation of the EEG pattern of brain waves in patients with METH dependence.
Previous studies have shown that neurofeedback training (NFB) normalizes or optimizes brain activity (LaVaque, 2003) and decreases craving for METH (Hashemian, 2015). They also demonstrate that treating METH-dependent patients is rather complex (Brands et al., 2012).
So, it seems that NFB as a stand-alone therapy may have weak outcomes for the treatment of METH abuse and may need a complementary therapy approach to treatment. In this line, previous studies have shown that NFB training during swimming exercises improves and optimizes mental/psychomotor performances (Mikicin et al., 2020). In further notes, unlike other sports, swimming exercises simultaneously activate a great number of muscles, delay fatigue, and increase the chest movement amplitude (Eider et al., 2014) as well as the motivation and mood of the patient (Stan, 2012). Also, exercise increases alpha (Schneider et al., 2009) and beta (Moraes et al., 2007) power. Given the high risk of relapse in these patients, it needs a combined treatment to provide comprehensive treatment. Thus, this study aims to investigate the effect of NFB training and swimming exercise, both alone and combined, on the EEG changes and visual image-induced craving in METH-dependent patients.
2. Materials and Methods
Study participants
Patients with METH dependence were 32 men aged 25–45 years who were staying in an addiction treatment camp (Taybad, Iran). They were invited to participate in the study between 2020 and 2021, and then a two-week screening phase was started. During the screening, the medical history of the participants, physical examination, and criteria for METH dependence had assessed by the treatment camp’s physician. Patients had no history of drug treatment for withdrawal, professional sports, and the use of psychiatric drugs. They had no cognitive impairment, head trauma, stroke, cardiovascular, or psychiatric disorders. The treatment groups were matched concerning their age, education level, and type of drug abuse. Demographic variables, including age, educational status, marital status, duration of METH addiction, physical illness history, body mass index (BMI), VO2 max, and job, were evaluated (Table 1).

A total of 114 subjects were enrolled and screened. Thirty-one patients declined to participate, and 43 did not match the criteria. Finally, 40 patients met all inclusion criteria. We first informed the patients with a brief description of the study programs and their rights, and all participants provided written informed consent. The patients were then randomized into four groups using a pretest and post-test design: Control (no medication based on the camp’s protocol), NFB training (three days per week, alternate days for 10 weeks), regular swimming exercises (three days per week, alternate days for 10 weeks), and simultaneous NFB and swimming training (three days swim and three days NFB in alternate days one week for 10 weeks). However, 8 patients showed little motivation to continue and were excluded from the analysis. Finally, 32 patients completed the study (Figure 1). The Ethics Committee guidelines and regulations approved 32 patients. EEG and visual image-induced craving were recorded in all 32 patients (Figure 1).

Experimental protocol
NFB training method
Patients underwent the NFB protocol for 10 weeks, which was three interval sessions per week, and each session lasted 30 minutes using the Thought Technology ProComp 2 system, as previously described in the Iranian population (Dehghani-Arani et al., 2013; Kober et al., 2015). This protocol in every session was focused on sensory-motor rhythm (SMR) training in the central brain cortex (Cz region) and alpha-theta in the parietal cortex (Pz region). The protocol involves placing the active, reference, and grand electrodes in the Cz, left, and right ears, respectively. The primary purpose of our protocol design was to reinforce the SMR band (12–15 Hz) and suppress the alpha (8–12 Hz), theta (5–8 Hz), delta (2–5 Hz), and high beta (18–30 Hz). A visual threshold-dependent protocol in the form of a computer game was used as reward stimuli for the patients. Patients were advised to focus on feedback to find the most successful mental strategy and to get as many rewards as possible. When the EEG frequencies were within the defined thresholds, the patient obtained the reward, and the image was changed. The image would remain unchanged if the patient could not adjust to the thresholds. In this study, if the patient could increase the SMR wave by 80% of the time and decrease the delta, theta, and high beta by 20%, he received many rewards of visual feedback. Feedback in the alpha-theta training protocol on the Pz area was presented only in an auditory format. In this protocol, the patients close their eyes and only listen to the sound being played to them. The active, reference, and grand electrodes are placed in the Pz, left, and right ears, respectively, and two distinct tones of sounds were used to amplify alpha and theta under eyes-closed conditions. If the delta wave increases, the system could prevent the patient from falling asleep using a special sound. In this session, if the theta/beta ratio decreases, voice feedback is provided as a reward stimulus, which consists of continuing to play the music.
Swimming protocol
The swimming training protocol was done for 10 weeks with 3 sessions per week and 60 minutes per session. This training was conducted in a swimming pool (Taybad, Iran) under the supervision of an experienced exercise trainer. To start, we calculated the maximum heart rate (HRmax) with the formula (220- Age) (Bragada et al., 2009; Gellish et al., 2007). We used average HRmax to approximate the exercise intensity, which was determined at a moderate intensity (60%-70% heart rate reserve -12 beats/min). Each swimming session included three parts; the first was 10-minute warm-up, then there was a 40 minute swim, and the last part was a 10-minute cool down (Karapolat et al., 2009). In this study, the water temperature was about 32 °C.
EEG protocol
All EEG recordings were carried out after completing a neuropsychological test between 12 PM and 4 PM, under laboratory-controlled sound and light conditions, as previously described (Choi et al., 2013). The EEG recording lasted 10 minutes and included the following conditions: 5 minutes with eyes closed, 5 minutes with eyes open. EEG recordings were obtained from the scalp using SynAmps 2 with a 19-channel Quik-Cap and a NeuroScan system. The signals were recorded at a sampling frequency of 500 Hz, and electrode impedance was below 5 kΩ. The EEG was band-pass filtered at 0.1–60 Hz using the signal processing software of the NeuroScan system for spectral analysis in 32-bit file format, and 19 electrode sites of 19 channels were driven by the NeuroGuide montage set as follows: FP1, F3, F7, Fz, FP2, F4, F8, T3, C3, Cz, T4, C4, T5, P3, O1, Pz, T6, P4, and O2. Artifacts were eliminated using the artifact detection of the NeuroGuide software. Here, we analyzed the eyes closed EEG in resting conditions. Accepted epochs of EEG data for absolute (uV2) power were calculated using fast Fourier transforms and averaged by NeuroGuide’s spectral analysis system in four frequency bands: Delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz). The activity at 19 sites was divided into five regions: Frontal, parietal, temporal, central, and occipital. Relative power was calculated as a percentage of the total power for each frequency band. Also, the delta/alpha ratio was computed as the mean scalp delta to alpha power ratio.
Visual image-induced craving
Participants were asked to rate their craving intensity and their subjective desire for METH-induced by each picture on the self-report visual analog scale (VAS) from 0 to 100 (Ekhtiari et al., 2010). The time interval of each picture exposure in the task was between 15 and 20 s. In each category of pictures, there were 2 pictures as follows: METH outside or inside the package without instruments (2 pictures), METH with instruments (2 pictures), instruments (2 pictures), cues accompanied by drug use (2 pictures), an act of abuse without a seen instrument (2 pictures), and neutral pictures (2 pictures).
Statistical analysis
We used the Kolmogorov-Smirnov test to evaluate the normality of the data. As required, demographic data between groups were compared using the chi-square test and one-way analysis of variance (ANOVA). The multivariate analysis of covariance (MANCOVA) was used to determine whether pretesting participants influenced their post-test scores on the dependent measures with the post hoc Bonferroni test. Results are expressed as Mean±SEM and Mean±SD. Differences were considered statistically significant at P<0.05.
EEG data were processed with custom-made scripts using MATLAB version R2012a. First, data from the resting-state measurements were imported into EEGLAB, bandpass filtered between 1-40 Hz, and inspected for gross movement artifacts that were manually removed. Subsequently, epochs of 4 s in length were created. Epochs containing amplitudes exceeding ±100 μV at any scalp electrode and/or epochs containing abnormally distributed data (joint probability or kurtosis >5 SD from expected mean values) were rejected. Because absolute EEG power data are skewed, data were log-transformed before analysis. We then utilized several procedures to reduce the likelihood of type I error. To identify EEG bands of interest for further analysis, we calculated a grand mean of 19 electrodes for each frequency band, thus providing an overall index of power for each band. The MANCOVA test compared the grand mean power in each group. Power at each electrode site was subsequently examined using MANCOVA.
3. Results
Demographic variables
Patients revealed no significant differences in any demographic variable. All 32 participants were male, 65% of the population were married, and 35% were single, with a mean age of 33. Most participants were employed (64%) and from secondary school (37%). The mean duration of METH use was 7 years. More than half of the participants reported a physical illness history (52%). ANOVA showed no statistically significant difference between groups in the mean age (F(3, 28)=1, P=NS), duration of METH use (F(3, 28)=0.15, P=NS), BMI (F(3, 28)=0.72, P=NS), and VO2 max (F(3, 28)=1.66, P=NS) in METH addicts. The mean body mass index (BMI) and VO2 max in groups were 22.37 kg/m2 and 39.93 mL/kg/min, respectively (Table 1).
Effect of NFB training and swimming exercise on the EEG power changes in METH-dependent patients
The MANCOVA revealed a significant interaction effect from pretreatment to posttreatment in absolute power (Wilks Lambda=0.039, F=11.22, P=0.0001, partial η2=0.66). There was no significant difference in pretreatment of the absolute power between groups. We observed significant differences among the three treatment conditions for the absolute power in alpha (F=68.04, P=0.0001), beta (F=7.25, P=0.001), theta (F=6.62, P=0.002), and delta (F=5.1, P=0.007) frequency bands.
Comparing the absolute power across posttreatment between groups showed that the alpha band in the NFB, swim, and NFB/swim groups had lower absolute power than the control group (P=0.0001, for all comparisons). Also, the NFB/swim group showed lower alpha power than the swim group (P=0.0001). Comparing the absolute power for posttreatment between groups showed that the beta band in the NFB (P=0.041), swim (P=0.021), and NFB/swim (P=0.001) groups had lower absolute power than the control group, also the NFB/swim group showed lower absolute power than the NFB (P=0.044) and swim (P=0.049) groups. Comparisons between groups in the theta band showed that the NFB (P=0.005), swim (P=0.003), and NFB/swim (P=0.014) groups had lower absolute power than the control group. Also, comparisons between groups in the delta band showed that the NFB (P=0.042), swim (P=0.032), and NFB/swim (P=0.006) groups had lower absolute power than the control group (Figure 2).

The MANCOVA revealed a significant interaction effect from pretreatment to posttreatment in relative power (Wilks Lambda=0.214, F=5.51, P=0.0001, partial η2=0.41). There was no significant difference in pretreatment of the relative power between groups. We observed significant differences among the three treatment conditions for the relative power in alpha (F=12.21, P=0.0001) and delta (F=4.1, P=0.017) frequency bands.
Comparing the relative power across for posttreatment between groups showed that the alpha band in the swim (P=0.002) and NFB/swim (P=0.0001) groups had lower power than the control group. Also, the NFB/swim group showed lower relative power than the NFB group (P=0.001). Comparing the relative power for posttreatment between groups showed that the delta band in the swim (P=0.022) and NFB/swim (P=0.029) groups had greater relative power than the control group (Figure 3).

The MANCOVA revealed a statistically significant interaction effect from pretreatment to posttreatment in the delta/alpha power ratio (Wilks Lambda=0.551, F=7.35, P=0.001, Partial η2=0.449). There was no significant difference between groups in pretreatment of the delta/alpha power ratio. We observed significant differences among the three treatment conditions for the delta/alpha power ratio (F=7.61, P=0.001). Comparing the delta/alpha power ratio for posttreatment between groups showed that the NFB/swim group had greater ratios than the control (P=0.0001) and the NFB (P=0.024) groups (Figure 4).

The reduction of the spectral powers was pretty evident in the frequency bands of alpha, beta, and theta following NFB/swim training in METH-dependent patients, which are shown in blue on the spectral topography mapping of absolute power (Figure 5).

Effect of NFB training and swimming exercise on visual image-induced craving in METH-dependent patients
The MANCOVA revealed a significant interaction effect from pre- to posttreatment in visual image-induced craving (Wilks Lambda=0.25, F=11.07, P=0.0001, partial η2=0.50). There was no significant difference between groups in pretreatment of the visual image-induced craving score. We observed significant differences among the three treatment conditions for the visual image-induced craving score (F=20.51, P=0.0001) and reaction time in response to the visual image-induced craving (F=4.41, P=0.01).
Comparisons between groups in the visual image-induced craving showed that the NFB/swim group (P=0.0001) had a lower craving score than the control, swim, and NFB groups (P=0.0001, for all comparisons) (Figure 6A). Also, comparisons between groups showed that the swim (P=0.004) and NFB/swim (P=0.0001) groups had faster reaction times in response to the visual image-induced craving than the control group (Figure 6B).

4. Discussion
We found no significant difference in the pretreatment scores of the absolute and relative powers in frequency bands between groups in METH-dependent patients. Here, we found the greater power of 4 frequency bands for pretreatment in METH-dependent patients.
Similar to our findings, a previous study has shown an increased EEG power in the delta and theta for all areas of the brain after 4 days of METH abstinence, and EEG was abnormal in 64% of patients (Newton et al., 2003). In line with our findings, a previous study shows that whole brain metabolism in the METH abusers is 14% higher as a sensitive indicator of brain dysfunction (Volkow et al., 2001).
The increase in the power of all 4 frequency bands in the present findings suggests increased cortical activation in resting conditions, impaired inhibitory function, and loss of neural efficiency that may indicate a “noisy” brain (Prashad et al., 2018), leading to the interruption of the cognitive-affective-behavioral processes, including depression, psychosomatic disorders, psychomotor agitation, neuro-psycho-physiological abnormalities, encephalopathy, cognitive and memory impairments (Begić et al., 2011; Glasner-Edwards & Mooney, 2014; Kalechstein et al., 2009; Zorick et al., 2010). Also, it has been shown that increased delta and theta EEG activity may reflect the rewarding properties of drug use, whereas the increased alpha power may be associated with anxiety and nervousness (Reid et al., 2006), as it is observed in the patients of the current study.
Our findings have shown that the NFB training, swimming exercise, and combining NFB and swimming exercise for 10 weeks reduced EEG absolute power for 4 frequency bands. The combination of the NFB and swimming exercise (NFB/swim group) significantly reduced EEG absolute power for alpha and beta frequency bands in METH-dependent patients during withdrawal.
Following NFB training, patients learn to voluntarily modulate their electrical brain activity, which has beneficial effects on cognitive functions (Kober et al., 2013) and is already used as a therapeutic tool to treat different types of disorders. The NFB helps to adjust disruptive wave patterns, restore a normal pattern of brain activity, and correct abnormal brain function without any lasting side effects (Scott et al., 2005) in substance use disorders. In this regard, previous studies have shown that NFB training as a mood-enhancing and energizing experience (Raymond et al., 2005) can induce neural plasticity in the dorsal anterior and mid-cingulate (Lanius et al., 2012; Yamashita et al., 2017), involved in the cognitive functioning and emotional processing. Also, NFB-induced brain and neural plasticity is a phenomenon considered a basic mechanism for behavioral changes (Rostami & Dehghani-Arani, 2015).
The interesting finding of our study was that NFB/swim and swim reduced alpha’s relative power and increased delta’s relative power. Our study also showed that NFB/swim caused a significant increase in the delta/alpha power ratio for posttreatment in METH-dependent patients. Previous experience has shown that delta and alpha oscillations are reciprocally related (Knyazev, 2007).
Previous studies have shown that functionally delta oscillations may integrate and synchronize cerebral activity with homeostatic processes, including autonomic functions and motivational and cognitive processes (Harmony, 2013; Knyazev, 2007; Knazav, 2012). For example, increases in delta power were also reported during semantic tasks, cognitive (Franke et al., 2022), and motivation (Knyazev et al., 2004) processing. Delta inhibitory oscillations would modulate the activity of those networks that should be inactive to perform the task (Harmony, 2013).
Another potential explanation of our results could be the use of relative EEG power as a sensitive and stable measure to provide a useful neurophysiological biomarker to determine psychotic disorders and age-related changes in EEG (Howells et al., 2018; Jelic et al., 2000) in patients. A previous study has shown that regional cerebral blood flow decreases when delta power increases (Dang-Vu et al., 2005). Another study has shown that detoxified METH abusers had significantly lower rates of metabolism in the thalamus and striatum (Volkow et al., 2001). This finding emphasizes the importance and presence of an extra-thalamic delta rhythm (Dang-Vu et al., 2005), including the cortex (Steriade et al., 1993) in METH-dependent patients. It seems the brains of METH-dependent patients, following the NFB training and swimming exercise in the present study, are calm and relaxed. This condition produces more delta (Dunn et al., 1999).
The increased delta/alpha power ratio following the NFB training and swimming exercise may reflect decreased cortical activation, increased neural efficiency, and reduced effort in METH-dependent patients with a “noisy” brain and stress before treatment. Our finding indicates that the brains of METH-dependent patients following the NFB training along with swimming exercises are quiet and calm in a relaxed state with lower anxiety. Maybe this reduced effort in a calm brain caused a decrease in the visual image-induced craving score in METH-dependent patients following the NFB training and swimming exercise. These patients also had faster reaction times in response to the visual image-induced craving in the NFB/swim and swim groups. Similar to our findings, previous studies have shown that exercise reduces METH (Wang et al., 2016) and cannabis (Buchowski et al., 2011) cravings.
A previous study has shown that the craving in substance-dependence patients is almost all related to abnormalities in alpha and beta, particularly in the parietal and central lobes of the brain (Sokhadze et al., 2008), as observed in our study of patients before treatment. However, there is some evidence that the greater delta power increases ratings of cocaine craving (Reid et al., 2006). Thus, a more important question would be whether the increase in delta/alpha power ratio could decrease the visual image-induced craving for METH after abstinence in NFB/swim group. Our present data cannot answer this question and requires a different experimental protocol.
Therefore, the NFB training along with swimming exercise (NFB/swim group) may more effectively reduce the craving for METH after abstinence. Previous studies have documented associations between NFB training and physical activity. In this regard, it has been shown that NFB training may be useful in enabling athletes to perform optimally (Dupee et al., 2016). Also, NFB training during swimming exercises improves and optimizes mental/psychomotor performances (Mikicin et al., 2020).
At present, the neurobiological mechanisms to reduce EEG absolute power for 4 frequency bands and METH craving following the effectiveness of swimming exercise combined with NFB training are still unknown. It may be due to an ability to self-regulate dopaminergic neurons of the substantia nigra/ventral tegmental (Sulzer et al., 2013), the endogenous release of key neuromodulators (Ros et al., 2021), the regulation of serotonin (Konareva, 2006), and β-endorphin (Akbariet al., 2016) following NFB training. Also, the regulation of the above-mentioned key neuromodulators following swimming exercise might have enhanced the effects of NFB. Future studies need to examine the neurobiological mechanisms induced by NFB training and swimming exercise.
5. Conclusion
This study provides novel evidence that the NFB training and swimming exercise during METH-abstinence could decrease EEG absolute power for 4 frequency bands and the relative power of alpha while increasing the relative power of delta and the delta/alpha power ratio. Also, our findings have shown that the NFB training and swimming exercise decreased the visual image-induced craving score in METH-dependent patients. Our results could suggest the application of the NFB training along with swimming exercise as a useful therapeutic strategy for the treatment of METH use disorder with follow-up studies that utilize a larger number of samples and the most suitable type and intensity of exercise (voluntary, treadmill, and swimming).
Study limitations
This study also had some limitations. Larger sample sizes in future studies will increase power, decrease estimation error, and increase the ability to evaluate subgroup differences further. It is suggested that different types of exercise and other NFB protocols be used in the treatment of METH-dependent patients.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants followed the ethical standards of the Ethics Committee of the Semnan University of Medical Sciences, Semnan, Iran (Code: IR.SEMUMS.REC.1397.308) and with the 1964 Helsinki Declaration. Also, This study received the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20190703044092N1).
Funding
This article was taken from the PhD dissertation of Atefeh Fadaei, approved by Department of Clinical Psychology, Faculty of Psychology & Educational Sciences, Semnan University, Semnan, Iran. This work was supported by a personal research grant from Mahmoud Najafi, Faculty of Semnan University, Semnan, Iran.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Semnan University and the Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran, for its support.
References
Akbari, Y. Z., Dolatshahee, B., & Rezaee, D. E. (2016). The effectiveness of neurofeedback training on reducing symptoms of war veterans with posttraumatic stress disorder. Practice in Clinical Psychology, 4(1), 17-23. [Link]
Begic, D., Popovic-Knapic, V., Grubisin, J., Kosanovic-Rajacic, B., Filipcic, I., Telarovic, I., & Jakovljevic, M. (2011). Quantitative electroencephalography in schizophrenia and depression. Psychiatr Danub, 23(4), 355-362. [PMID]
Bigdeli, I., Asia, M. N., Miladi-Gorji, H., & Fadaei, A. (2015). The spatial learning and memory performance in methamphetamine-sensitized and withdrawn rats. Iranian Journal of Basic Medical Sciences, 18(3), 234–239. [PMID]
Bragada, J. A., Pedro, P. M., Vasques, C. S., Tiago, M. B., & Vítor, P. L. (2009). Net heart rate to prescribe physical activity in middle-aged to older active adults. Journal of Sports Science & Medicine, 8(4), 616–621. [PMID]
Brands, B., Corea, L., Strike, C., Singh, V. A. S., Behrooz, R. C., & Rush, B. (2012). Demand for substance abuse treatment related to use of crystal methamphetamine in Ontario: An observational study. International Journal of Mental Health and Addiction, 10(5), 696-709. [DOI:10.1007/s11469-011-9362-1]
Buchowski, M. S., Meade, N. N., Charboneau, E., Park, S., Dietrich, M. S., & Cowan, R. L., et al. (2011). Aerobic exercise training reduces cannabis craving and use in non-treatment-seeking cannabis-dependent adults. Plos One, 6(3), e17465.[DOI:10.1371/journal.pone.0017465] [PMID]
Burns, L. (2014). World drug report 2013 by United Nations Office on Drugs and Crime, United nations. Drug and Alcohol Review, 33(2), 216-216. [DOI:10.1111/dar.12110]
Cherner, M., Suarez, P., Casey, C., Deiss, R., Letendre, S., & Marcotte, T., et al. (2010). Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug and Alcohol Dependence, 106(2-3), 154–163. [DOI:10.1016/j.drugalcdep.2009.08.010] [PMID]
Choi, J. S., Park, S. M., Lee, J., Hwang, J. Y., Jung, H. Y., & Choi, S. W., et al. (2013). Resting-state beta and gamma activity in Internet addiction. International Journal of Psychophysiology, 89(3), 328-333. [DOI:10.1016/j.ijpsycho.2013.06.007] [PMID]
Dang-Vu, T. T., Desseilles, M., Laureys, S., Degueldre, C., Perrin, F., & Phillips, C., et al. (2005). Cerebral correlates of delta waves during non-REM sleep revisited. NeuroImage, 28(1), 14-21. [DOI:10.1016/j.neuroimage.2005.05.028] [PMID]
Dean, A. C., Groman, S. M., Morales, A. M., & London, E. D. (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(2), 259–274. [DOI:10.1038/npp.2012.179] [PMID]
Dehghani-Arani, F., Rostami, R., & Nadali, H. (2013). Neurofeedback training for opiate addiction: Improvement of mental health and craving. Applied Psychophysiology and Biofeedback, 38(2), 133–141. [DOI:10.1007/s10484-013-9218-5] [PMID]
Dunn, B. R., Hartigan, J. A., & Mikulas, W. L. (1999). Concentration and mindfulness meditations: Unique forms of consciousness? Applied Psychophysiology and Biofeedback, 24(3), 147–165. [DOI:10.1023/A:1023498629385] [PMID]
Dupee, M., Forneris, T., & Werthner, P. (2016). Perceived outcomes of a biofeedback and neurofeedback training intervention for optimal performance: Learning to enhance self-awareness and self-regulation with Olympic athletes. The Sport Psychologist, 30(4), 339-349. [DOI:10.1123/tsp.2016-0028]
Łubkowska, W., Paczyńska-Jędrycka, M., & Eider, J. (2014). The significance of swimming and corrective exercises in water in treatment of postural deficits and scoliosis. Central European Journal of Sport Sciences and Medicine, 6(2), 93-101. [Link]
Ekhtiari, H., Alam-Mehrjerdi, Z., Nouri, M., George, S., & Mokri, A. (2010). Designing and evaluation of reliability and validity of visual cue-induced craving assessment task for methamphetamine smokers. Basic and Clinical Neuroscience, 1(4), 34-37.[Link]
Ernst, T., Chang, L., Leonido-Yee, M., & Speck, O. (2000). Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology, 54(6), 1344–1349. [DOI:10.1212/WNL.54.6.1344] [PMID]
Franke, L. M., Gitchel, G. T., Perera, R. A., Hadimani, R. L., Holloway, K. L., & Walker, W. C. (2022). Randomized trial of rTMS in traumatic brain injury: Improved subjective neurobehavioral symptoms and increases in EEG delta activity. Brain Injury, 36(5), 683-692. [DOI:10.1080/02699052.2022.2033845] [PMID]
Gellish, R. L., Goslin, B. R., Olson, R. E., McDonald, A., Russi, G. D., & Moudgil, V. K. (2007). Longitudinal modeling of the relationship between age and maximal heart rate. Medicine and Science in Sports and Exercise, 39(5), 822–829. [DOI:10.1097/mss.0b013e31803349c6] [PMID]
Glasner-Edwards, S., & Mooney, L. J. (2014). Methamphetamine psychosis: Epidemiology and management. CNS Drugs, 28(12), 1115–1126. [DOI:10.1007/s40263-014-0209-8] [PMID]
Hajheidari, S., Miladi-Gorji, H., & Bigdeli, I. (2015). Effect of environmental enrichment on the severity of psychological dependence and voluntary methamphetamine consumption in methamphetamine-withdrawn rats. Neuroscience Letters, 584, 151–155. [DOI:10.1016/j.neulet.2014.10.017] [PMID]
Hajheidari, S., Sameni, H. R., Bandegi, A. R., & Miladi-Gorji, H. (2017). Effects of prolonged abstinence from METH on the hippocampal BDNF levels, neuronal numbers, and apoptosis in methamphetamine-sensitized rats. Neuroscience Letters, 645, 80–85. [DOI:10.1016/j.neulet.2017.02.051] [PMID]
Harmony, T. (2013). The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience, 7, 83. [DOI:10.3389/fnint.2013.00083] [PMID]
Hashemian, P. (2015). The effectiveness of neurofeedback therapy in craving of methamphetamine use. Open Journal of Psychiatry, 5(2), 177-179. [DOI:10.4236/ojpsych.2015.52020]
Howells, F. M., Temmingh, H. S., Hsieh, J. H., van Dijen, A. V., Baldwin, D. S., & Stein, D. J. (2018). Electroencephalographic delta/alpha frequency activity differentiates psychotic disorders: A study of schizophrenia, bipolar disorder, and methamphetamine-induced psychotic disorder. Translational Psychiatry, 8(1), 75. [DOI:10.1038/s41398-018-0105-y] [PMID]
Jelic, V., Johansson, S. E., Almkvist, O., Shigeta, M., Julin, P., & Nordberg, A., et al. (2000). Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer's disease. Neurobiology of Aging, 21(4), 533–540. [DOI:10.1016/S0197-4580(00)00153-6] [PMID]
Kalechstein, A. D., De la Garza, R., 2nd, Newton, T. F., Green, M. F., Cook, I. A., & Leuchter, A. F. (2009). Quantitative EEG abnormalities are associated with memory impairment in recently abstinent methamphetamine-dependent individuals. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(3), 254–258. [DOI:10.1176/jnp.2009.21.3.254] [PMID]
Karapolat, H., Eyigor, S., Zoghi, M., Akkoc, Y., Kirazli, Y., & Keser, G. (2009). Are swimming or aerobic exercise better than conventional exercise in ankylosing spondylitis patients? A randomized controlled study. European Journal of Physical and Rehabilitation Medicine, 45(4), 449–457. [PMID]
Khajehpour, H., Makkiabadi, B., Ekhtiari, H., Bakht, S., Noroozi, A., & Mohagheghian, F. (2019). Disrupted resting-state brain functional network in methamphetamine abusers: A brain source space study by EEG. Plos One, 14(12), e0226249.[DOI:10.1371/journal.pone.0226249] [PMID]
Knyazev, G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews, 31(3), 377–395. [DOI:10.1016/j.neubiorev.2006.10.004] [PMID]
Knyazev, G. G. (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neuroscience and Biobehavioral Reviews, 36(1), 677–695. [DOI:10.1016/j.neubiorev.2011.10.002] [PMID]
Knyazev, G. G., Savostyanov, A. N., & Levin, E. A. (2004). Alpha oscillations as a correlate of trait anxiety. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 53(2), 147–160. [DOI:10.1016/j.ijpsycho.2004.03.001] [PMID]
Kober, S. E., Witte, M., Ninaus, M., Neuper, C., & Wood, G. (2013). Learning to modulate one’s own brain activity: The effect of spontaneous mental strategies. Frontiers in Human Neuroscience, 7, 695. [DOI:10.3389/fnhum.2013.00695] [PMID]
Kober, S. E., Witte, M., Stangl, M., Väljamäe, A., Neuper, C., & Wood, G. (2015). Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 126(1), 82–95. [DOI:10.1016/j.clinph.2014.03.031] [PMID]
Konareva, I. (2006). Correlations between the psychological peculiarities of an individual and the efficacy of a single neurofeedback session (by the EEG characteristics). Neurophysiology, 38(3), 201-208. [DOI:10.1007/s11062-006-0047-5]
Lanius, R., Ros, T., Theberge, J., Frewen, P., Kluetsch, R., & Densmore, M., et al. (2012). Mind over chatter: Plastic up-regulation of the fMRI alertness network by EEG neurofeedback. Nature Precedings, 1, 1. [DOI:10.1038/npre.2012.7060.1]
LaVaque, T. J. (2003). Neurofeedback, neurotherapy, and quantitative EEG. In D. Moss, A. McGrady, T. C. Davies & I. Wickramasekera (Eds.), Handbook of mind-body medicine for primary care (pp. 123-136). California: SAGE Publications, Inc.[DOI:10.4135/9781452232607.n9]
McGregor, C., Srisurapanont, M., Jittiwutikarn, J., Laobhripatr, S., Wongtan, T., & White, J. M. (2005). The nature, time course, and severity of methamphetamine withdrawal. Addiction (Abingdon, England), 100(9), 1320–1329. [DOI:10.1111/j.1360-0443.2005.01160.x] [PMID]
Mikicin, M., Mróz, A., Karczewska-Lindinger, M., Malinowska, K., Mastalerz, A., & Kowalczyk, M. (2020). Effect of the neurofeedback-EEG training during physical exercise on the range of mental work performance and individual physiological parameters in swimmers. Applied Psychophysiology and Biofeedback, 45(2), 49-55. [DOI:10.1007/s10484-020-09456-1]
Moraes, H., Ferreira, C., Deslandes, A., Cagy, M., Pompeu, F., & Ribeiro, P., et al. (2007). Beta and alpha electroencephalographic activity changes after acute exercise. Arquivos de Neuro-Psiquiatria, 65(3A), 637–641. [DOI:10.1590/S0004-282X2007000400018] [PMID]
Newton, T. F., Cook, I. A., Kalechstein, A. D., Duran, S., Monroy, F., & Ling, W., et al. (2003). Quantitative EEG abnormalities in recently abstinent methamphetamine-dependent individuals. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(3), 410–415. [DOI:10.1016/S1388-2457(02)00409-1] [PMID]
Newton, T. F., Kalechstein, A. D., Hardy, D. J., Cook, I. A., Nestor, L., & Ling, W., et al. (2004). Association between quantitative EEG and neurocognition in methamphetamine-dependent volunteers. Clinical Neurophysiology: Official Journal of The International Federation of Clinical Neurophysiology, 115(1), 194–198. [DOI:10.1016/S1388-2457(03)00314-6] [PMID]
Prashad, S., Dedrick, E. S., & Filbey, F. M. (2018). Cannabis users exhibit increased cortical activation during resting state compared to non-users. NeuroImage, 179, 176–186.[DOI:10.1016/j.neuroimage.2018.06.031] [PMID]
Raymond, J., Varney, C., Parkinson, L. A., & Gruzelier, J. H. (2005). The effects of alpha/theta neurofeedback on personality and mood. Brain Research. Cognitive Brain Research, 23(2-3), 287–292. [DOI:10.1016/j.cogbrainres.2004.10.023] [PMID]
Reid, M. S., Flammino, F., Howard, B., Nilsen, D., & Prichep, L. S. (2006). Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 31(4), 872–884. [DOI:10.1038/sj.npp.1300888] [PMID]
Ros, T., Kwiek, J., Andriot, T., Michela, A., Vuilleumier, P., Garibotto, V., & Ginovart, N. (2021). PET imaging of dopamine neurotransmission during EEG neurofeedback. Frontiers in Physiology, 11, 590503. [DOI:10.3389/fphys.2020.590503] [PMID]
Rostami, R., & Dehghani-Arani, F. (2015). Neurofeedback training as a new method in the treatment of crystal methamphetamine-dependent patients: A preliminary study. Applied Psychophysiology and Biofeedback, 40(3), 151–161. [DOI:10.1007/s10484-015-9281-1] [PMID]
Schneider, S., Askew, C. D., Diehl, J., Mierau, A., Kleinert, J., & Abel, T., et al. (2009). EEG activity and mood in health-orientated runners after different exercise intensities. Physiology & Behavior, 96(4-5), 709–716. [DOI:10.1016/j.physbeh.2009.01.007] [PMID]
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance-abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455–469. [DOI:10.1081/ADA-200056807] [PMID]
Shafiee-Kandjani, A. R., Jahan, A., Moghadam-Salimi, M., Fakhari, A., Nazari, M. A., & Sadeghpour, S. (2020). Resting-state electroencephalographic coherence in recently abstinent methamphetamine users. International Journal of High Risk Behaviors and Addiction, 9(4), e103606. [DOI:10.5812/ijhrba.103606]
Stan, A. E. (2012). The benefits of participation in aquatic activities for people with disabilities. Medicina Sportivă, 8(1), 1737-1742. [Link]
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1-28. [DOI:10.1007/s10484-007-9047-5]
Steriade, M., Nunez, A., & Amzica, F. (1993). A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13(8), 3252–3265. [DOI:10.1523/JNEUROSCI.13-08-03252.1993] [PMID]
Sulzer, J., Sitaram, R., Blefari, M. L., Kollias, S., Birbaumer, N., & Stephan, K. E., et al. (2013). Neurofeedback-mediated self-regulation of the dopaminergic midbrain. NeuroImage, 83, 817–825. [DOI:10.1016/j.neuroimage.2013.05.115] [PMID]
Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Franceschi, D., & Sedler, M. J., et al. (2001). Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. The American Journal of Psychiatry, 158(3), 383–389. [DOI:10.1176/appi.ajp.158.3.383] [PMID]
Wang, D., Zhou, C., Zhao, M., Wu, X., & Chang, Y. K. (2016).Dose-response relationships between exercise intensity, cravings, and inhibitory control in methamphetamine dependence: An ERPs study. Drug and Alcohol Dependence, 161, 331–339. [DOI:10.1016/j.drugalcdep.2016.02.023] [PMID]
Yamashita, A., Hayasaka, S., Kawato, M., & Imamizu, H. (2017).Connectivity neurofeedback training can differentially change functional connectivity and cognitive performance. Cerebral Cortex (New York, N.Y.: 1991), 27(10), 4960–4970. [DOI:10.1093/cercor/bhx177] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2022/11/6 | Accepted: 2023/08/12 | Published: 2025/03/18
Received: 2022/11/6 | Accepted: 2023/08/12 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





