Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 287-300 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bakizadeh F, Mokhtari S, Saeed F, Mokhtari A, Akbari Koli P, Shalbafan M. Cognitive Rehabilitation for Adult Patients With Obsessive-compulsive Disorder: A Systematic Review of Randomized Controlled Trials. BCN 2024; 15 (3) :287-300
URL: http://bcn.iums.ac.ir/article-1-2565-en.html
URL: http://bcn.iums.ac.ir/article-1-2565-en.html
Farah Bakizadeh1 

 , Saba Mokhtari2
, Saba Mokhtari2 

 , Fahime Saeed3
, Fahime Saeed3 

 , Asieh Mokhtari4
, Asieh Mokhtari4 

 , Pouria Akbari Koli5
, Pouria Akbari Koli5 

 , Mohammadreza Shalbafan *6
, Mohammadreza Shalbafan *6 




 , Saba Mokhtari2
, Saba Mokhtari2 

 , Fahime Saeed3
, Fahime Saeed3 

 , Asieh Mokhtari4
, Asieh Mokhtari4 

 , Pouria Akbari Koli5
, Pouria Akbari Koli5 

 , Mohammadreza Shalbafan *6
, Mohammadreza Shalbafan *6 


1- Department of Psychology, School of Psychology and Education, University of Tehran, Tehran, Iran.
2- Department of Psychiatry, School of Behavioral Sciences and Mental Health, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Psychiatry, Psychosis Research Center, School of Behavioral Sciences and Mental Health, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Department of Laboratory Sciences, School of Allied Medical Sciences, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Cognitive Rehabilitation, Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
6- Mental Health Research Center, Psychosocial Health Research Institute (PHRI), Iran University of Medical Sciences, Tehran, Iran.
2- Department of Psychiatry, School of Behavioral Sciences and Mental Health, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Psychiatry, Psychosis Research Center, School of Behavioral Sciences and Mental Health, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Department of Laboratory Sciences, School of Allied Medical Sciences, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Cognitive Rehabilitation, Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
6- Mental Health Research Center, Psychosocial Health Research Institute (PHRI), Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1418 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Obsessive-compulsive disorder (OCD) is a chronic and disabling mental disorder (Hollander et al., 1997). It is characterized by unwanted, repetitive, and intrusive thoughts or mental images that generate anxiety and discomfort, along with repetitive motor or mental acts aimed at preventing or reducing the associated anxiety (APA, 2013). OCD affects 1-3% of the global population (Karno et al., 1988; Sasson et al., 1997) and has a significant negative impact on public health (Shalbafan et al., 2019; Hollander et al., 1997; Murray et al., 1996). It is associated with a severe decrease in the quality of life (Arabzadeh et al., 2017; Macy et al., 2013), an impairment in all aspects of function (Hadi et al., 2021; Albert et al., 2010; DuPont et al., 1995), and an increase in suicide (Angelakis et al., 2015) and mortality rate (Meier et al., 2016). Besides these effects on personal life and public health, OCD causes a considerable economic burden (Olesen et al., 2012).
It has been shown that OCD patients have cognitive deficits in various aspects of their cognition (Suhas & Rao, 2019). Cognitive shifting ability and cognitive inflexibility during task-switching are impaired among patients with OCD (Chamberlain et al., 2021; Gruner & Pittenger, 2017; Gu et al., 2008). Compared to the general population, individuals with OCD exhibit poorer performance in planning (Van den Heuvel et al., 2005) and response inhibition (Chamberlain et al., 2006; Ghisi et al., 2013; Penades et al., 2007). Executive dysfunction is one of the most important cognitive impairments among OCD patients (Askari et al., 2022; Kashyap et al., 2013; Snyder et al., 2015; Tarafder et al., 2006), which is independent of other comorbidities, such as depression and psychomotor retardation (Snyder et al., 2015). Research suggests impairments in attention (Burdick et al., 2018; Van den Heuvel et al., 2005) and slowness in psychomotor and information processing (Harris & Dinn, 2003; Tükel et al., 2012). Additionally, verbal and non-verbal memory has been shown to be impaired in patients with OCD (Benzina et al., 2016; Muller & Roberts, 2005; Savage et al., 1999; Savage et al., 2000; Segalas et al., 2008).
Multiple cognitive impairments have been evaluated and suggested as endophenotype markers of OCD (Cavedini et al., 2010; Chamberlain et al., 2005; Rao et al., 2008; Viswanath et al., 2009). Different aspects of the importance of cognitive deficits in OCD have been assessed in recent years. The connection between symptom severity and cognitive deficits has been demonstrated in some studies (Abramovitch et al., 2011; Lacerda et al., 2003; Rao et al., 2010; Segalas et al., 2008). Cognitive impairments play a predictive role in the insight of patients with OCD (Erzegovesi et al., 2001; Kashyap et al., 2012; Ravi Kishore et al., 2004). In addition to the significant impact of insight on the prognosis of OCD patients, cognitive disturbances exhibit a specific correlation with the prognosis of OCD (Chamberlain et al., 2005).
The comorbidity of OCD with other psychiatric disorders negatively affects the severity of OCD symptoms, prognosis, and response to pharmacotherapy (Shavitt et al., 2006). Several studies have shown the association between cognitive deficits and other disorders as comorbidities in patients with OCD (Basso et al. 2001; Purcell et al., 1998; Rao et al., 2008). Regardless of comorbidities, the correlation between cognitive impairments and treatment response has been observed in patients with OCD (Cavedini et al., 2004; Cavedini et al., 2002).
Cognitive impairments can seriously repress the ability to earn, relearn, and maintain the skills that are essential for suitable performances in complicated real-life situations. The relationship between real-life functioning and neuropsychological performance has been demonstrated in patients with OCD (Perna et al., 2016).
Cognitive interventions that are expressed in articles as cognitive rehabilitation, training, and remediation are used in the management of some psychiatric and neurological disorders. Cognitive training has been effective in mitigating neurocognitive impairments associated with traumatic brain injury, schizophrenia, various types of memory impairment, Alzheimer’s disease, attention-deficit hyperactivity disorder, and mood and anxiety disorders (Keshavan et al., 2014). Therefore, this therapeutic approach is applicable for disorders that are mostly comorbid conditions with OCD. Some studies have investigated neurocognitive intervention as a new approach to the treatment of OCD. However, these studies do not provide consistent results and the benefit of the cognitive rehabilitation interventions cannot be concluded from them. For instance, two studies in 2006 assessed the effect of cognitive training in patients with OCD and even focused on the same approach (organizational strategies). Although one study has shown that cognitive training improves memory and symptom severity of OCD patients (Park et al., 2006), another one has shown no significant difference between the training and control group (Buhlmann et al., 2006).
The application of neurocognitive rehabilitation for psychiatric disorders has increased. Consequently, some review articles have been done in this context (Keshavan et al., 2014). Considering the importance of cognitive deficits in OCD patients and the inconsistency and inconsistency of the results of previous studies, a systematic review of interventions focused on cognitive deficits in OCD patients is valuable and necessary. We aimed to conduct a systematic review of studies that used cognitive rehabilitation for patients with OCD. This study will determine the quality of evidence, the effectiveness of cognitive rehabilitation on various obsessive, compulsive, and cognitive symptoms, as well as its severity.
2. Materials and Methods
Search strategy and selection criteria
This systematic review was conducted in accordance with the PRISMA guidelines (Page et al., 2021). The databases searched in this study included PubMed, Scopus, ScienceDirect, Cochrane Library, and Google Scholar. The initial search was performed on December 7, 2021, by the first author.
The search strategy, detailed in Table 1, focused on controlled clinical trials assessing the effectiveness of cognitive rehabilitation in adults with OCD, using MeSH terms and keywords, including ‘cognitive rehabilitation’ and ‘obsessive-compulsive disorder’. Included studies were required to provide measures of OCD symptom severity (using OCD scales) or conduct neuropsychological testing both at baseline and post-intervention. We included studies with adult participants, excluding those focusing on pediatric or geriatric populations, and studies involving participants with an established OCD diagnosis at baseline (excluding preclinical studies). There was no limitation for the clinical stage and studies with mild, moderate, severe, and refractory participants were included. In cases where studies included a mixed diagnostic sample (e.g. patients with both OCD and major depressive disorder), they were included only if data specific to participants diagnosed solely with OCD could be extracted from the study reports. Only studies focusing on cognitive rehabilitation, cognitive remediation, or cognitive training were included; studies involving pharmacological or non-pharmacological treatments such as cognitive-behavioral therapy or group therapies were excluded. Details of the research question and keywords are presented in Table 1.

The first author and another researcher initially screened the titles and abstracts for duplications and irrelevant studies. A third researcher then reviewed the outcomes. The full texts of the remaining studies were acquired and assessed by the first author.
The final studies exhibited significant differences in design and methodology, including different interventions, metrics or outcomes, and study designs, with some studies conducting multiple measurements per participant. These basic differences made the data unsuitable for meta-analysis.
We also evaluated the risk of bias in the studies using the Cochrane ‘risk of bias’ tool, covering six main biases.
3. Results
The database searches initially identified 200 records of interest, from which 105 duplicates were removed. From the remaining 95 studies, six articles were found to be eligible and met the inclusion criteria (Figure 1) These six articles describe individual randomized controlled trials (RCTs) that demonstrate a broad range of study designs.
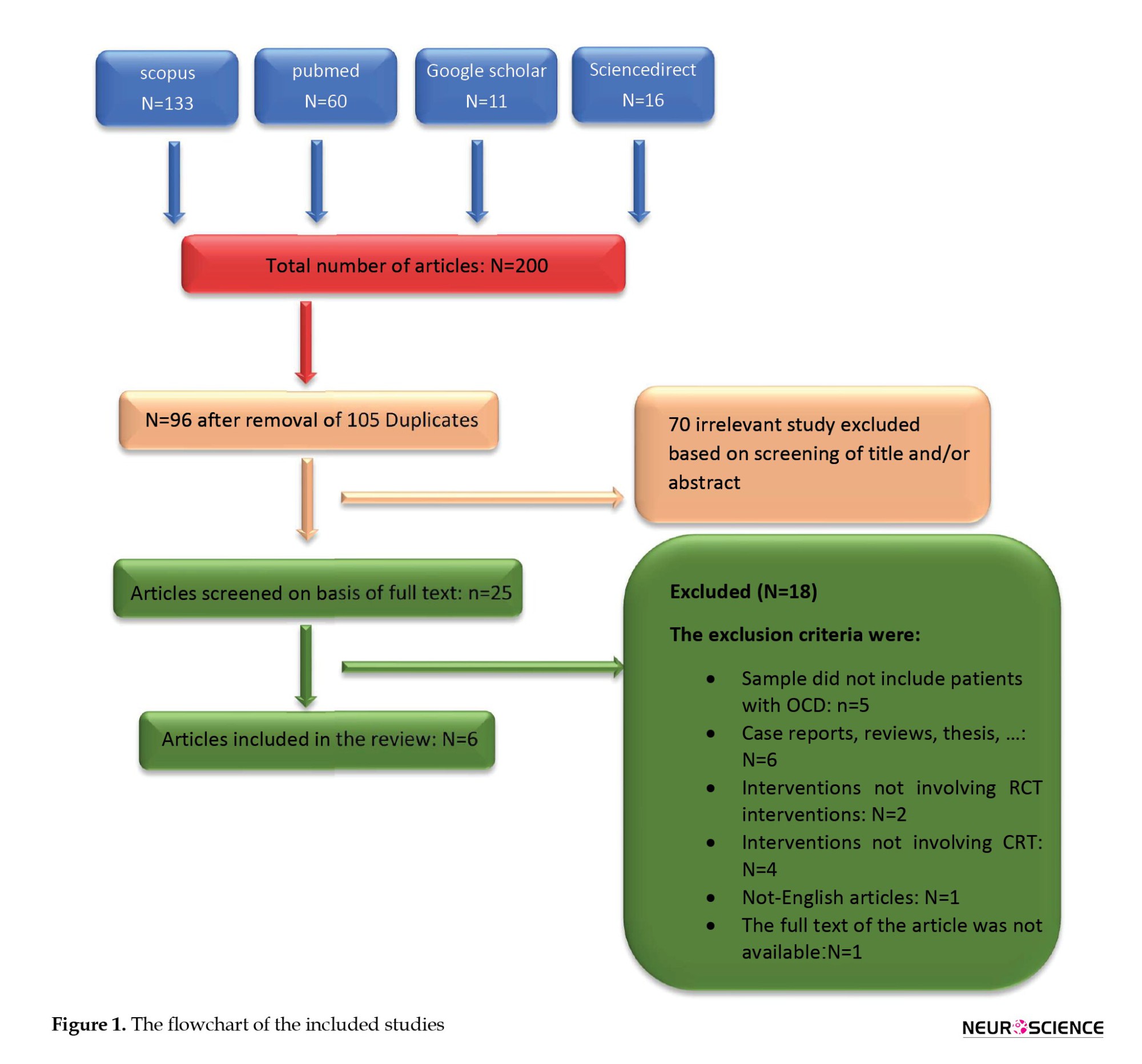
Four of these studies are randomized control trials with a passive control group, including one pilot study, while two studies are randomized control trials with an active control group. Half of the studies examined cognitive remediation, and the other half assessed cognitive training tasks.
Five of the studies evaluated the mean difference in Yale-Brown obsessive-compulsive scale (Y-BOCS) scores (Goodman et al., 1989) before and after intervention in experimental and control groups. One of these studies reported that the difference was not significant, and another only reported results from a two-way ANOVA test of group, time, and group×time interaction, without providing mean, standard deviation, standard error, or other statistics necessary for a meta-analysis. Four studies assessed the neuropsychological and cognitive symptoms of participants using various tests.
As mentioned earlier, the final studies exhibited significant differences in design, methodology, interventions, metrics or outcomes, and study designs. In some cases, there were multiple measurements per participant. These fundamental differences rendered the data unsuitable for meta-analysis.
Details of the studies are described in Table 2.
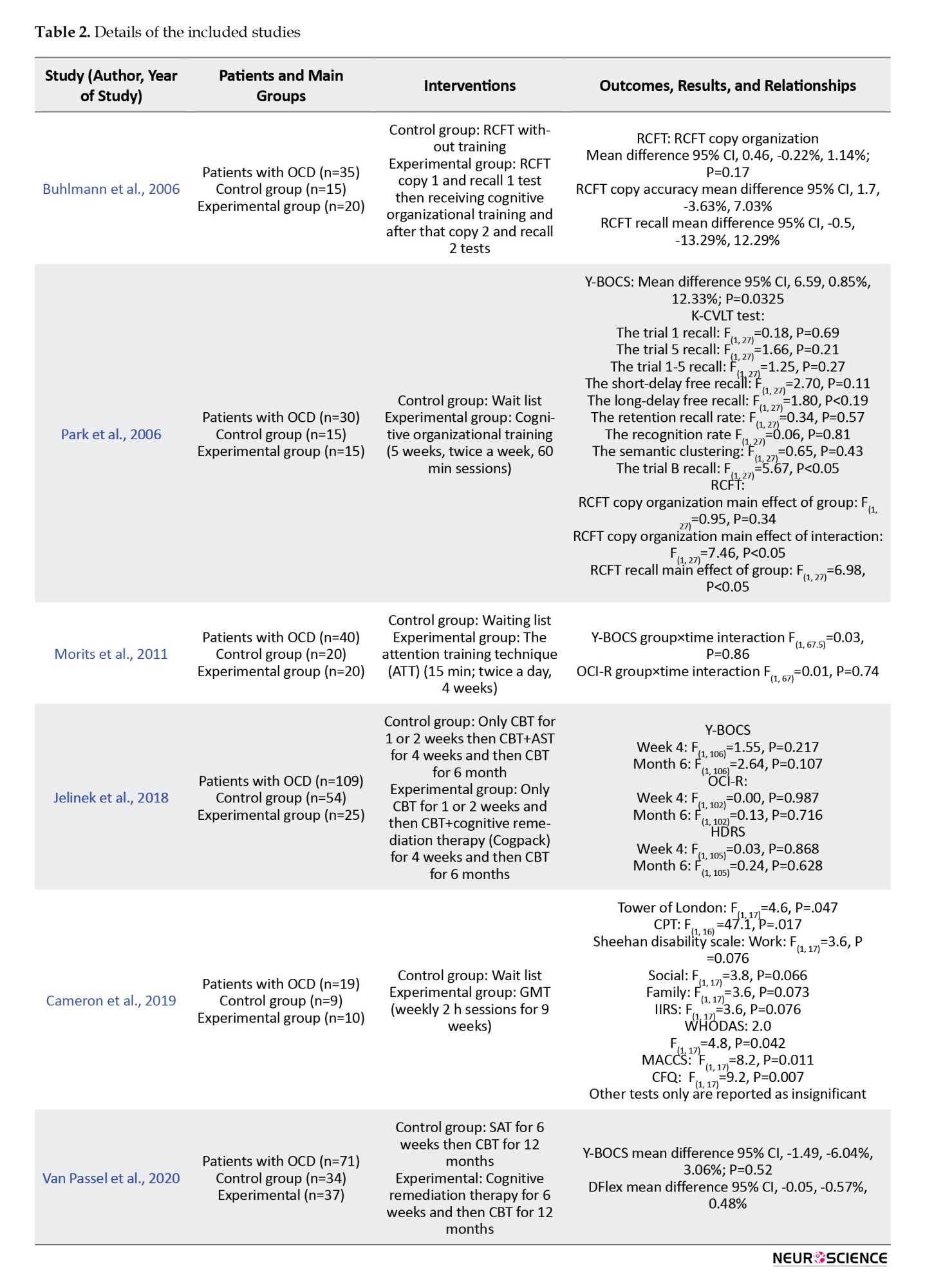
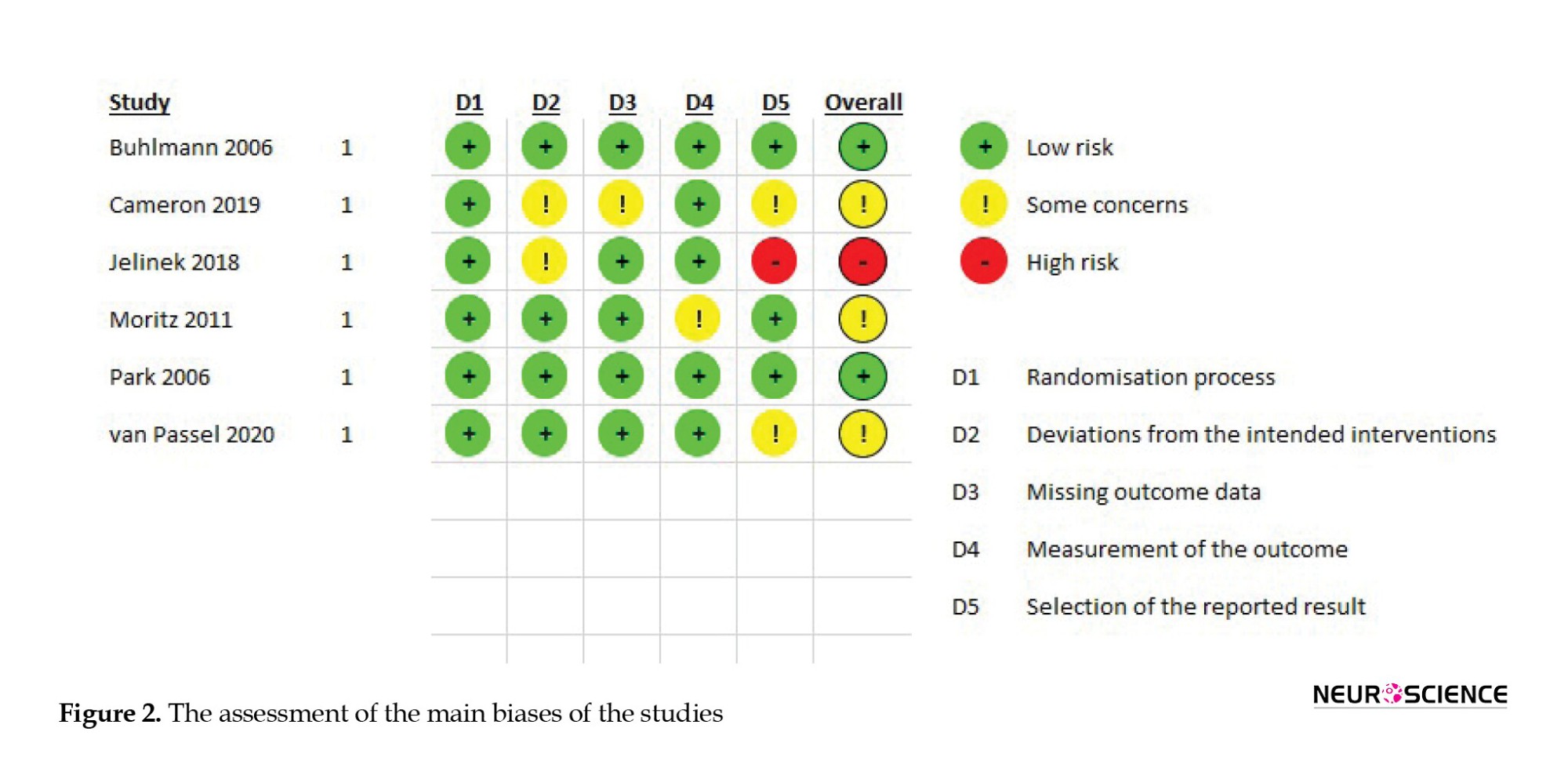
Figure 2 presents the results of assessing the main biases of the studies.
Cognitive training for organizational impairment
Two of the six studies investigated the role of organization training in OCD patients. The Rey–Rey-Osterrieth complex figure test (Shin et al., 2006) was administered before and after cognitive training. However, the training strategies and study durations varied.
In the 2006 study by Buhlmann et al., (2006), 35 OCD patients were randomized into two groups: Training and non-training.
All patients first copied the RCFT (copy I). Immediately, the patients were asked to redraw the RCFT from memory (recall I). Twenty patients received various training instructions using the Taylor complex figure (Lezak et al., 2004), which involved breaking down complex figures into simpler or meaningful parts, drawing basic units first, then adding details The remaining 15 patients (control group) received no training. Subsequently, all patients were asked to copy the RCFT (copy 2) and perform an immediate recall of the RCFT (recall 2) and a 30-minute delayed recall.
This study’s findings indicated that there were no significant differences in organizational abilities (P=0.17) or memory (P=0.93) between the two groups of subjects with OCD.
In the study by Park et al. (2006), 30 adults with OCD were divided into two groups. Fifteen participants underwent a cognitive training program consisting of nine 60-minute individual sessions held twice a week for five weeks, while the remaining fifteen were placed on a wait list (Park et al., 2006).
The cognitive training included exercises in “training for visual organizational strategies” and “training on organizational strategies in everyday life,” which incorporated problem-solving strategies.
The block design subtest of the Korean–Wechsler adult intelligence scale (Yum et al., 1992) was revised and employed as a training tool to enhance visual organizational strategies. A total of 90 patterns (10 patterns in each session, starting with simpler patterns) were used for training. During training, a total of 90 patterns (10 patterns per session, starting with simpler patterns) were used, which taught participants to integrate pattern components into a coherent and meaningful form and to understand the overall configuration of these patterns.
Training for organizational strategies applicable to everyday life was designed for this study. During each session, patients applied the information they learned to formulate structured plans and address their daily challenges.
The RCFT, Korean–California verbal learning test (Kim & Kang, 1999; Savage et al., 2000), and Y-BOCS were administered at the beginning and end of the study to assess the effects of the training.
In this study, the copy and the copy organization score of the RCFT had a significantly greater improvement in the treatment group (P<0.05). However, the immediate and delayed recall were the same. The results of K-CVLT were not significantly different. It can indicate that visual organizational training can improve visual memory and visuospatial skills in OCD patients but it does not have an effect on their verbal memory.
Regarding Y-BOCS, the obsessive-compulsive symptoms in total, obsession and compulsion subscales were significantly different between the groups and the treatment group showed a greater improvement in their symptoms (P<0.05).
The attention training technique
One study of 6 included studies investigated the role of the attention training technique in OCD patients.
The attention training technique (ATT) is a cognitive training method focused on improving intrusive thoughts (Wells, 2007). The ATT trains patients to shift their attention from internal events to external ones and can cause more attentional flexibility (Fergus & Bardeen, 2016). ATT sessions are at least 15 minutes twice a day and treatment lasts for 4 weeks (Papageorgiou & Wells, 2004). Each ATT session has four steps: 1) Step one: Several sharp noises should be deleted inside and outside a room. 2) Step two: Attention should be switched between each noise in one minute while ignoring other sounds. 3) Step 3: The attention switch between inside and outside noises should happen only when a noise has captured full attention. 4) Step 4: Attention should be on all noises with counting them (Fergus & Bardeen, 2016).
In the study by Moritz et al. (2011), 80 OCD patients were divided into two groups receiving ATT for four weeks and a control (waitlist) group. The participants were assessed via the Internet and completed questionnaires about their demographic information, medical history (they should have a previous OCD diagnosis by a healthcare professional), obsessive-compulsive inventory-revised (OCI-R) (Foa et al., 2002) and Y-BOCS. Participants were assessed by these two scales at the end of the study as well.
The treatment manual containing an introduction to ATT and a description of the treatment was sent to half of the participants via E-mail and the other half were informed that they were on a waitlist. Participants were encouraged to perform the techniques twice a day for at least 15 min.
The mixed two-way ANOVA results showed no significance of the main effects of group (P=0.72), time (P=0.07), and the group×time interaction (P=0.86) regarding the total Y-BOCS scores and its subscales. For OCI-R scores, only the effect of time was significant (P=0.04). However, none of the total or subscale scores of OCI-R achieved significance regarding the main effect of group (total score P=0.41) or group×time interaction (total score P=0.74).
One of the participants acknowledged that he did not respond honestly in post-assessment and 11 participants (10 in the ATT group) did not complete the study. In another analysis in this study, we removed the non-completer subjects, which caused no difference in results.
Cognitive remediation therapy
Two of the included studies investigated the effect of cognitive remediation therapy on OCD patients. Both studies have an active control group: One study developed a therapy with a similar structure of CRT and without its cognitive treatments, as a control group. Another study was designed to investigate the effect of association splitting therapy (AST) (a metacognition therapy) (Moritz et al., 2007) and used the CRT treatment as a control group. Due to this major and significant difference in the design of these two studies, we did not analyze the data of these studies in conjunction.
In the study by Jelinek et al. (2018), 109 OCD patients receiving CBT were randomized to two groups of cognitive remediation or association splitting. Patients were evaluated by the Y-BOCS, OCI-R, and Hamilton depression rating scale at week 0 (t0), week 4 (t1), and after 6 months (t2). Both groups of patients received CBT for one or two weeks when the CRT or the AST was added to their treatment. They received six sessions (50 minutes) of AST or CRT for three weeks (two sessions per week).
The CRT group received six sessions of the Cogpack training. The Cogpack software was designed by the marker software. It consists of 64 exercises categorized into domain-specific and non-domain-specific tasks. Domain-specific tasks work on verbal memory, verbal fluency, sustained and selective attention, motor coordination, working memory, and executive function. Non-domain-specific tasks do not focus on one cognitive function but cause a need to use different aspects, such as logical and mathematical skills or language skills, simultaneously (Caponnetto et al., 2018).
The results of this study showed no significant difference between the two groups in none of the tests or their subscale scores (t1 P: 0.21 and t2P=0.1). The t1 assessment was after an estimated two weeks of treatment and the t2 assessment was after an estimated 4.5 months from the end of CRT or AST and continuing to receive the CBT.
In the study by Van Passel in 2020 (van Passel et al., 2020), 71 adults with OCD were divided into two groups receiving CRT or SAT for five weeks (45 min, twice weekly), followed by CBT. They used Y-BOCS and the detail and flexibility questionnaire (DFlex) to evaluate the participants at week 0(t0), week 6 (t1), and after 6 (t2) and 12 months (t3) (Roberts et al., 2011).
The cognitive remediation therapy used in this study was based on the CRT model for patients with anorexia nervosa (Tchanturia et al., 2010). This intervention uses different tasks to modify cognitive flexibility and information processing.
The control group received the SAT, specifically designed for this study. The SAT shares the overall structure of the CRT, including duration and homework assignments, but excludes the cognitive training components present in the CRT.
After 5 weeks of receiving either the CRT or the SAT, all patients undergo CBT sessions (once or twice a week, lasting 45 to 90 minutes each) for the following year.
The results of this study revealed no significant main effect of group or group×time interaction at t1, t2, or t3 in Y-BOCS total or subscale scores (t1 total Y-BOCS P=0.52) or in Dflex score (t1 Dflex P=0.84).
Goal management training (GMT)
One of the included studies examined the effectiveness of goal management training (GMT) in adults with OCD.
In a pilot study by Cameron et al. (2020), 19 OCD patients were randomly assigned to two groups: GMT and waitlist control. Ten OCD patients underwent nine weeks of GMT (2-hour sessions, once weekly), with the treatment group completing an average of 7.2 sessions.
GMT is a brief, structured, present-focused cognitive remediation therapy that targets the attention system, goal-directed behaviors, and executive function. It employs a top-down processing approach that emphasizes the regulation of planning, set-shifting, goal-setting, and monitoring processes (Levine et al., 2000; Stamenova & Levine, 2018).
The assessment of participants was conducted by multiple tests and scales (Y-BOCS, depression, anxiety and stress scales (DASS-21) (Lovibond & Lovibond, 1995), cognitive failures questionnaire (CFQ) (Broadbent et al., 1982), dysexecutive questionnaire (DEX) (Simblett & Bateman, 2011), memory and cognitive confidence scale (MACCS) (Nedeljkovic & Kyrios, 2007), WHO disability assessment scale (WHODAS) 2.0 (Üstün et al., 2010), illness intrusiveness rating scale (IIRS) (Devins, 2010), Sheehan disability scale (SDS) (Sheehan, 2000), Conners’ continuous performance task (CPT) (Conners et al., 2000), Stroop color and word test (Golden, 1976), Tower of London (Culbertson & Zillmer, 2001), California verbal learning test—second edition (CVLT-II) (Delis et al., 2000), and Wechsler test of adult reading (Wechsler, 2001), however, the study provided only the significant data.
The findings of this study suggest a notable difference between groups in TOL, CPT, all functional outcome tests, and subjective cognition tests after nine weeks. However, there was no significant distinction in symptom severity tests. During the three-month follow-up, no significance was observed in any of the scales, although only six participants were accessible for this evaluation.
4. Discussion
Many studies have shown that OCD patients have cognitive deficits in various aspects of their cognition (Suhas & Rao, 2019). These neuropsychological and cognitive deficits have been identified as exacerbating factors in the severity of symptoms (Abramovitch et al., 2011; Lacerda et al., 2003; Rao et al., 2010; Segalas et al., 2008), potential endophenotypes (Rao et al., 2008; Viswanath et al., 2009), predictors of insight (Erzegovesi et al., 2001; Kashyap et al., 2012; Ravi Kishore et al., 2004) and treatment response (Cavedini et al., 2004; D’Alcante et al., 2012), potential factors in certain comorbidities (Basso et al. 2001; Purcell et al., 1998; Rao et al., 2008), and prognostic markers of OCD (Chamberlain et al., 2005; Suhas & Rao, 2019) of OCD as well.
Based on the findings of this review, current cognitive rehabilitation therapies are not successful in reducing the symptoms of OCD. The publications do not consistently report the same results, and due to substantial variations in design and methodology, they are not suitable for meta-analysis. Without a meta-analysis, there is insufficient statistical evidence to draw conclusions regarding the impact of cognitive rehabilitation on cognitive deficits and daily functioning in adults with OCD.
As it is shown in Figure 2, the overall bias of most studies is concerning and some potential biases of some studies are worth mentioning:
In the study by van Passel et al., (2020), the cognitive remediation model, the control therapy model, and the measurement scale they utilized were primarily designed for patients with anorexia nervosa. Before relying on the conclusion of this study, it seems necessary to test the validity and reliability of these models and assessment tools for OCD patients.
In the study of Moritz et al. (2011), the entire assessment and intervention were self-administered, with all procedures conducted over the Internet. This approach may raise concerns regarding the reliability and validity of the data reported by participants. The participants’ diagnoses and histories were not verified by a professional, and there is no evidence that all precipitants completed the treatment or executed the techniques in the right way.
Cameron et al., 2020 study is a pilot study with a notably small sample size. Moreover, the trial was not completed by all the treatment groups. As indicated in the manuscript, the average number of sessions completed by the treatment group was 7.2, which could limit the ability to draw definitive conclusions in a pilot study. (Cameron et al., 2020). Also, multiple scales and tests were used in the study which may lead to confusion in forming an overall assessment of this model.
The study by Jelineck et al. (2018) has some biases in design and methodology. The study requires further justification and logical reasoning for selecting cognitive remediation and metacognitive therapy for comparison. It seems that neither of these techniques can serve as a control group for the other. There are many differences in the baseline treatment of participants and all subjects were receiving different kinds of CBT, which can be a very important confounding variable. The initiation time of interventions varies among participants (as indicated in the manuscript, it is estimated to be around two weeks), resulting in differing assessment times. The first evaluation after the baseline occurs approximately two weeks after the start of treatment, which may not provide sufficient time to gauge the treatment’s effectiveness. Finally, this study incorporated six sessions of Cogpack for cognitive remediation, a method that has not been studied on OCD patients and may not be adequately effective within just six sessions.
5. Conclusion
In summary, based on the findings of this review, current cognitive rehabilitation therapies are not successful in reducing the symptoms of OCD. The results obtained from this review indicate a limited number of studies on this subject. However, due to significant variations in design and methodology among these studies, they are deemed unsuitable for meta-analysis. The absence of a meta-analysis results in insufficient statistical evidence to draw conclusions regarding the impact of cognitive rehabilitation on cognitive deficits and the daily functioning of adults with OCD. Another issue regarding the studies of this review is multiple biases and methodological errors that were addressed. Due to the importance of cognitive deficits in OCD patients, it is necessary to design and conduct standard trials to investigate the role of cognitive rehabilitation in these impairments.
Limitations and suggestions for further studies
As mentioned before, the most important limitation of this study is the limited number of clinical trials on cognitive rehabilitation for OCD patients and the methodological differences and errors of available studies. To enhance future studies, it is recommended to conduct double-blinded trials by offering comparable tasks without training components (as opposed to using a waiting list or different therapies) and ensuring the separation of operational and analysis teams. Another beneficial approach to promote study homogeneity could involve stratifying participants based on the severity of symptoms and conducting separate analyses for individuals with mild, moderate, and severe OCD. Including follow-up assessments can provide valuable insights for subsequent studies. Furthermore, given that various studies have identified cognitive deficits in OCD patients, future research endeavors should concentrate on tasks that specifically target these impaired cognitive domains.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was funded by the Cognitive Sciences & Technologies Council (Grant No.: 15745).
Authors' contributions
Conceptualization and study design: Farah Bakizadeh, Fahime Saeed, Pouria Akbari Koli and Mohammadreza Shalbafan; Data collection: Farah Bakizadeh, Saba Mokhtari, Pouria Akbari Koli, Asieh Mokhtari and Mohammadreza Shalbafan; Initial draft preparation: Farah Bakizadeh, Saba Mokhtari, Fahime Saeed and Mohammadreza Shalbafan; Editing & review: All authors. Farah Bakizadeh and Saba Mokhtari are equal contributors to this work and designated as co-first authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Sina Tavakoli for his cooperation in performing this study.
References
Obsessive-compulsive disorder (OCD) is a chronic and disabling mental disorder (Hollander et al., 1997). It is characterized by unwanted, repetitive, and intrusive thoughts or mental images that generate anxiety and discomfort, along with repetitive motor or mental acts aimed at preventing or reducing the associated anxiety (APA, 2013). OCD affects 1-3% of the global population (Karno et al., 1988; Sasson et al., 1997) and has a significant negative impact on public health (Shalbafan et al., 2019; Hollander et al., 1997; Murray et al., 1996). It is associated with a severe decrease in the quality of life (Arabzadeh et al., 2017; Macy et al., 2013), an impairment in all aspects of function (Hadi et al., 2021; Albert et al., 2010; DuPont et al., 1995), and an increase in suicide (Angelakis et al., 2015) and mortality rate (Meier et al., 2016). Besides these effects on personal life and public health, OCD causes a considerable economic burden (Olesen et al., 2012).
It has been shown that OCD patients have cognitive deficits in various aspects of their cognition (Suhas & Rao, 2019). Cognitive shifting ability and cognitive inflexibility during task-switching are impaired among patients with OCD (Chamberlain et al., 2021; Gruner & Pittenger, 2017; Gu et al., 2008). Compared to the general population, individuals with OCD exhibit poorer performance in planning (Van den Heuvel et al., 2005) and response inhibition (Chamberlain et al., 2006; Ghisi et al., 2013; Penades et al., 2007). Executive dysfunction is one of the most important cognitive impairments among OCD patients (Askari et al., 2022; Kashyap et al., 2013; Snyder et al., 2015; Tarafder et al., 2006), which is independent of other comorbidities, such as depression and psychomotor retardation (Snyder et al., 2015). Research suggests impairments in attention (Burdick et al., 2018; Van den Heuvel et al., 2005) and slowness in psychomotor and information processing (Harris & Dinn, 2003; Tükel et al., 2012). Additionally, verbal and non-verbal memory has been shown to be impaired in patients with OCD (Benzina et al., 2016; Muller & Roberts, 2005; Savage et al., 1999; Savage et al., 2000; Segalas et al., 2008).
Multiple cognitive impairments have been evaluated and suggested as endophenotype markers of OCD (Cavedini et al., 2010; Chamberlain et al., 2005; Rao et al., 2008; Viswanath et al., 2009). Different aspects of the importance of cognitive deficits in OCD have been assessed in recent years. The connection between symptom severity and cognitive deficits has been demonstrated in some studies (Abramovitch et al., 2011; Lacerda et al., 2003; Rao et al., 2010; Segalas et al., 2008). Cognitive impairments play a predictive role in the insight of patients with OCD (Erzegovesi et al., 2001; Kashyap et al., 2012; Ravi Kishore et al., 2004). In addition to the significant impact of insight on the prognosis of OCD patients, cognitive disturbances exhibit a specific correlation with the prognosis of OCD (Chamberlain et al., 2005).
The comorbidity of OCD with other psychiatric disorders negatively affects the severity of OCD symptoms, prognosis, and response to pharmacotherapy (Shavitt et al., 2006). Several studies have shown the association between cognitive deficits and other disorders as comorbidities in patients with OCD (Basso et al. 2001; Purcell et al., 1998; Rao et al., 2008). Regardless of comorbidities, the correlation between cognitive impairments and treatment response has been observed in patients with OCD (Cavedini et al., 2004; Cavedini et al., 2002).
Cognitive impairments can seriously repress the ability to earn, relearn, and maintain the skills that are essential for suitable performances in complicated real-life situations. The relationship between real-life functioning and neuropsychological performance has been demonstrated in patients with OCD (Perna et al., 2016).
Cognitive interventions that are expressed in articles as cognitive rehabilitation, training, and remediation are used in the management of some psychiatric and neurological disorders. Cognitive training has been effective in mitigating neurocognitive impairments associated with traumatic brain injury, schizophrenia, various types of memory impairment, Alzheimer’s disease, attention-deficit hyperactivity disorder, and mood and anxiety disorders (Keshavan et al., 2014). Therefore, this therapeutic approach is applicable for disorders that are mostly comorbid conditions with OCD. Some studies have investigated neurocognitive intervention as a new approach to the treatment of OCD. However, these studies do not provide consistent results and the benefit of the cognitive rehabilitation interventions cannot be concluded from them. For instance, two studies in 2006 assessed the effect of cognitive training in patients with OCD and even focused on the same approach (organizational strategies). Although one study has shown that cognitive training improves memory and symptom severity of OCD patients (Park et al., 2006), another one has shown no significant difference between the training and control group (Buhlmann et al., 2006).
The application of neurocognitive rehabilitation for psychiatric disorders has increased. Consequently, some review articles have been done in this context (Keshavan et al., 2014). Considering the importance of cognitive deficits in OCD patients and the inconsistency and inconsistency of the results of previous studies, a systematic review of interventions focused on cognitive deficits in OCD patients is valuable and necessary. We aimed to conduct a systematic review of studies that used cognitive rehabilitation for patients with OCD. This study will determine the quality of evidence, the effectiveness of cognitive rehabilitation on various obsessive, compulsive, and cognitive symptoms, as well as its severity.
2. Materials and Methods
Search strategy and selection criteria
This systematic review was conducted in accordance with the PRISMA guidelines (Page et al., 2021). The databases searched in this study included PubMed, Scopus, ScienceDirect, Cochrane Library, and Google Scholar. The initial search was performed on December 7, 2021, by the first author.
The search strategy, detailed in Table 1, focused on controlled clinical trials assessing the effectiveness of cognitive rehabilitation in adults with OCD, using MeSH terms and keywords, including ‘cognitive rehabilitation’ and ‘obsessive-compulsive disorder’. Included studies were required to provide measures of OCD symptom severity (using OCD scales) or conduct neuropsychological testing both at baseline and post-intervention. We included studies with adult participants, excluding those focusing on pediatric or geriatric populations, and studies involving participants with an established OCD diagnosis at baseline (excluding preclinical studies). There was no limitation for the clinical stage and studies with mild, moderate, severe, and refractory participants were included. In cases where studies included a mixed diagnostic sample (e.g. patients with both OCD and major depressive disorder), they were included only if data specific to participants diagnosed solely with OCD could be extracted from the study reports. Only studies focusing on cognitive rehabilitation, cognitive remediation, or cognitive training were included; studies involving pharmacological or non-pharmacological treatments such as cognitive-behavioral therapy or group therapies were excluded. Details of the research question and keywords are presented in Table 1.

The first author and another researcher initially screened the titles and abstracts for duplications and irrelevant studies. A third researcher then reviewed the outcomes. The full texts of the remaining studies were acquired and assessed by the first author.
The final studies exhibited significant differences in design and methodology, including different interventions, metrics or outcomes, and study designs, with some studies conducting multiple measurements per participant. These basic differences made the data unsuitable for meta-analysis.
We also evaluated the risk of bias in the studies using the Cochrane ‘risk of bias’ tool, covering six main biases.
3. Results
The database searches initially identified 200 records of interest, from which 105 duplicates were removed. From the remaining 95 studies, six articles were found to be eligible and met the inclusion criteria (Figure 1) These six articles describe individual randomized controlled trials (RCTs) that demonstrate a broad range of study designs.

Four of these studies are randomized control trials with a passive control group, including one pilot study, while two studies are randomized control trials with an active control group. Half of the studies examined cognitive remediation, and the other half assessed cognitive training tasks.
Five of the studies evaluated the mean difference in Yale-Brown obsessive-compulsive scale (Y-BOCS) scores (Goodman et al., 1989) before and after intervention in experimental and control groups. One of these studies reported that the difference was not significant, and another only reported results from a two-way ANOVA test of group, time, and group×time interaction, without providing mean, standard deviation, standard error, or other statistics necessary for a meta-analysis. Four studies assessed the neuropsychological and cognitive symptoms of participants using various tests.
As mentioned earlier, the final studies exhibited significant differences in design, methodology, interventions, metrics or outcomes, and study designs. In some cases, there were multiple measurements per participant. These fundamental differences rendered the data unsuitable for meta-analysis.
Details of the studies are described in Table 2.

Figure 2 presents the results of assessing the main biases of the studies.

Cognitive training for organizational impairment
Two of the six studies investigated the role of organization training in OCD patients. The Rey–Rey-Osterrieth complex figure test (Shin et al., 2006) was administered before and after cognitive training. However, the training strategies and study durations varied.
In the 2006 study by Buhlmann et al., (2006), 35 OCD patients were randomized into two groups: Training and non-training.
All patients first copied the RCFT (copy I). Immediately, the patients were asked to redraw the RCFT from memory (recall I). Twenty patients received various training instructions using the Taylor complex figure (Lezak et al., 2004), which involved breaking down complex figures into simpler or meaningful parts, drawing basic units first, then adding details The remaining 15 patients (control group) received no training. Subsequently, all patients were asked to copy the RCFT (copy 2) and perform an immediate recall of the RCFT (recall 2) and a 30-minute delayed recall.
This study’s findings indicated that there were no significant differences in organizational abilities (P=0.17) or memory (P=0.93) between the two groups of subjects with OCD.
In the study by Park et al. (2006), 30 adults with OCD were divided into two groups. Fifteen participants underwent a cognitive training program consisting of nine 60-minute individual sessions held twice a week for five weeks, while the remaining fifteen were placed on a wait list (Park et al., 2006).
The cognitive training included exercises in “training for visual organizational strategies” and “training on organizational strategies in everyday life,” which incorporated problem-solving strategies.
The block design subtest of the Korean–Wechsler adult intelligence scale (Yum et al., 1992) was revised and employed as a training tool to enhance visual organizational strategies. A total of 90 patterns (10 patterns in each session, starting with simpler patterns) were used for training. During training, a total of 90 patterns (10 patterns per session, starting with simpler patterns) were used, which taught participants to integrate pattern components into a coherent and meaningful form and to understand the overall configuration of these patterns.
Training for organizational strategies applicable to everyday life was designed for this study. During each session, patients applied the information they learned to formulate structured plans and address their daily challenges.
The RCFT, Korean–California verbal learning test (Kim & Kang, 1999; Savage et al., 2000), and Y-BOCS were administered at the beginning and end of the study to assess the effects of the training.
In this study, the copy and the copy organization score of the RCFT had a significantly greater improvement in the treatment group (P<0.05). However, the immediate and delayed recall were the same. The results of K-CVLT were not significantly different. It can indicate that visual organizational training can improve visual memory and visuospatial skills in OCD patients but it does not have an effect on their verbal memory.
Regarding Y-BOCS, the obsessive-compulsive symptoms in total, obsession and compulsion subscales were significantly different between the groups and the treatment group showed a greater improvement in their symptoms (P<0.05).
The attention training technique
One study of 6 included studies investigated the role of the attention training technique in OCD patients.
The attention training technique (ATT) is a cognitive training method focused on improving intrusive thoughts (Wells, 2007). The ATT trains patients to shift their attention from internal events to external ones and can cause more attentional flexibility (Fergus & Bardeen, 2016). ATT sessions are at least 15 minutes twice a day and treatment lasts for 4 weeks (Papageorgiou & Wells, 2004). Each ATT session has four steps: 1) Step one: Several sharp noises should be deleted inside and outside a room. 2) Step two: Attention should be switched between each noise in one minute while ignoring other sounds. 3) Step 3: The attention switch between inside and outside noises should happen only when a noise has captured full attention. 4) Step 4: Attention should be on all noises with counting them (Fergus & Bardeen, 2016).
In the study by Moritz et al. (2011), 80 OCD patients were divided into two groups receiving ATT for four weeks and a control (waitlist) group. The participants were assessed via the Internet and completed questionnaires about their demographic information, medical history (they should have a previous OCD diagnosis by a healthcare professional), obsessive-compulsive inventory-revised (OCI-R) (Foa et al., 2002) and Y-BOCS. Participants were assessed by these two scales at the end of the study as well.
The treatment manual containing an introduction to ATT and a description of the treatment was sent to half of the participants via E-mail and the other half were informed that they were on a waitlist. Participants were encouraged to perform the techniques twice a day for at least 15 min.
The mixed two-way ANOVA results showed no significance of the main effects of group (P=0.72), time (P=0.07), and the group×time interaction (P=0.86) regarding the total Y-BOCS scores and its subscales. For OCI-R scores, only the effect of time was significant (P=0.04). However, none of the total or subscale scores of OCI-R achieved significance regarding the main effect of group (total score P=0.41) or group×time interaction (total score P=0.74).
One of the participants acknowledged that he did not respond honestly in post-assessment and 11 participants (10 in the ATT group) did not complete the study. In another analysis in this study, we removed the non-completer subjects, which caused no difference in results.
Cognitive remediation therapy
Two of the included studies investigated the effect of cognitive remediation therapy on OCD patients. Both studies have an active control group: One study developed a therapy with a similar structure of CRT and without its cognitive treatments, as a control group. Another study was designed to investigate the effect of association splitting therapy (AST) (a metacognition therapy) (Moritz et al., 2007) and used the CRT treatment as a control group. Due to this major and significant difference in the design of these two studies, we did not analyze the data of these studies in conjunction.
In the study by Jelinek et al. (2018), 109 OCD patients receiving CBT were randomized to two groups of cognitive remediation or association splitting. Patients were evaluated by the Y-BOCS, OCI-R, and Hamilton depression rating scale at week 0 (t0), week 4 (t1), and after 6 months (t2). Both groups of patients received CBT for one or two weeks when the CRT or the AST was added to their treatment. They received six sessions (50 minutes) of AST or CRT for three weeks (two sessions per week).
The CRT group received six sessions of the Cogpack training. The Cogpack software was designed by the marker software. It consists of 64 exercises categorized into domain-specific and non-domain-specific tasks. Domain-specific tasks work on verbal memory, verbal fluency, sustained and selective attention, motor coordination, working memory, and executive function. Non-domain-specific tasks do not focus on one cognitive function but cause a need to use different aspects, such as logical and mathematical skills or language skills, simultaneously (Caponnetto et al., 2018).
The results of this study showed no significant difference between the two groups in none of the tests or their subscale scores (t1 P: 0.21 and t2P=0.1). The t1 assessment was after an estimated two weeks of treatment and the t2 assessment was after an estimated 4.5 months from the end of CRT or AST and continuing to receive the CBT.
In the study by Van Passel in 2020 (van Passel et al., 2020), 71 adults with OCD were divided into two groups receiving CRT or SAT for five weeks (45 min, twice weekly), followed by CBT. They used Y-BOCS and the detail and flexibility questionnaire (DFlex) to evaluate the participants at week 0(t0), week 6 (t1), and after 6 (t2) and 12 months (t3) (Roberts et al., 2011).
The cognitive remediation therapy used in this study was based on the CRT model for patients with anorexia nervosa (Tchanturia et al., 2010). This intervention uses different tasks to modify cognitive flexibility and information processing.
The control group received the SAT, specifically designed for this study. The SAT shares the overall structure of the CRT, including duration and homework assignments, but excludes the cognitive training components present in the CRT.
After 5 weeks of receiving either the CRT or the SAT, all patients undergo CBT sessions (once or twice a week, lasting 45 to 90 minutes each) for the following year.
The results of this study revealed no significant main effect of group or group×time interaction at t1, t2, or t3 in Y-BOCS total or subscale scores (t1 total Y-BOCS P=0.52) or in Dflex score (t1 Dflex P=0.84).
Goal management training (GMT)
One of the included studies examined the effectiveness of goal management training (GMT) in adults with OCD.
In a pilot study by Cameron et al. (2020), 19 OCD patients were randomly assigned to two groups: GMT and waitlist control. Ten OCD patients underwent nine weeks of GMT (2-hour sessions, once weekly), with the treatment group completing an average of 7.2 sessions.
GMT is a brief, structured, present-focused cognitive remediation therapy that targets the attention system, goal-directed behaviors, and executive function. It employs a top-down processing approach that emphasizes the regulation of planning, set-shifting, goal-setting, and monitoring processes (Levine et al., 2000; Stamenova & Levine, 2018).
The assessment of participants was conducted by multiple tests and scales (Y-BOCS, depression, anxiety and stress scales (DASS-21) (Lovibond & Lovibond, 1995), cognitive failures questionnaire (CFQ) (Broadbent et al., 1982), dysexecutive questionnaire (DEX) (Simblett & Bateman, 2011), memory and cognitive confidence scale (MACCS) (Nedeljkovic & Kyrios, 2007), WHO disability assessment scale (WHODAS) 2.0 (Üstün et al., 2010), illness intrusiveness rating scale (IIRS) (Devins, 2010), Sheehan disability scale (SDS) (Sheehan, 2000), Conners’ continuous performance task (CPT) (Conners et al., 2000), Stroop color and word test (Golden, 1976), Tower of London (Culbertson & Zillmer, 2001), California verbal learning test—second edition (CVLT-II) (Delis et al., 2000), and Wechsler test of adult reading (Wechsler, 2001), however, the study provided only the significant data.
The findings of this study suggest a notable difference between groups in TOL, CPT, all functional outcome tests, and subjective cognition tests after nine weeks. However, there was no significant distinction in symptom severity tests. During the three-month follow-up, no significance was observed in any of the scales, although only six participants were accessible for this evaluation.
4. Discussion
Many studies have shown that OCD patients have cognitive deficits in various aspects of their cognition (Suhas & Rao, 2019). These neuropsychological and cognitive deficits have been identified as exacerbating factors in the severity of symptoms (Abramovitch et al., 2011; Lacerda et al., 2003; Rao et al., 2010; Segalas et al., 2008), potential endophenotypes (Rao et al., 2008; Viswanath et al., 2009), predictors of insight (Erzegovesi et al., 2001; Kashyap et al., 2012; Ravi Kishore et al., 2004) and treatment response (Cavedini et al., 2004; D’Alcante et al., 2012), potential factors in certain comorbidities (Basso et al. 2001; Purcell et al., 1998; Rao et al., 2008), and prognostic markers of OCD (Chamberlain et al., 2005; Suhas & Rao, 2019) of OCD as well.
Based on the findings of this review, current cognitive rehabilitation therapies are not successful in reducing the symptoms of OCD. The publications do not consistently report the same results, and due to substantial variations in design and methodology, they are not suitable for meta-analysis. Without a meta-analysis, there is insufficient statistical evidence to draw conclusions regarding the impact of cognitive rehabilitation on cognitive deficits and daily functioning in adults with OCD.
As it is shown in Figure 2, the overall bias of most studies is concerning and some potential biases of some studies are worth mentioning:
In the study by van Passel et al., (2020), the cognitive remediation model, the control therapy model, and the measurement scale they utilized were primarily designed for patients with anorexia nervosa. Before relying on the conclusion of this study, it seems necessary to test the validity and reliability of these models and assessment tools for OCD patients.
In the study of Moritz et al. (2011), the entire assessment and intervention were self-administered, with all procedures conducted over the Internet. This approach may raise concerns regarding the reliability and validity of the data reported by participants. The participants’ diagnoses and histories were not verified by a professional, and there is no evidence that all precipitants completed the treatment or executed the techniques in the right way.
Cameron et al., 2020 study is a pilot study with a notably small sample size. Moreover, the trial was not completed by all the treatment groups. As indicated in the manuscript, the average number of sessions completed by the treatment group was 7.2, which could limit the ability to draw definitive conclusions in a pilot study. (Cameron et al., 2020). Also, multiple scales and tests were used in the study which may lead to confusion in forming an overall assessment of this model.
The study by Jelineck et al. (2018) has some biases in design and methodology. The study requires further justification and logical reasoning for selecting cognitive remediation and metacognitive therapy for comparison. It seems that neither of these techniques can serve as a control group for the other. There are many differences in the baseline treatment of participants and all subjects were receiving different kinds of CBT, which can be a very important confounding variable. The initiation time of interventions varies among participants (as indicated in the manuscript, it is estimated to be around two weeks), resulting in differing assessment times. The first evaluation after the baseline occurs approximately two weeks after the start of treatment, which may not provide sufficient time to gauge the treatment’s effectiveness. Finally, this study incorporated six sessions of Cogpack for cognitive remediation, a method that has not been studied on OCD patients and may not be adequately effective within just six sessions.
5. Conclusion
In summary, based on the findings of this review, current cognitive rehabilitation therapies are not successful in reducing the symptoms of OCD. The results obtained from this review indicate a limited number of studies on this subject. However, due to significant variations in design and methodology among these studies, they are deemed unsuitable for meta-analysis. The absence of a meta-analysis results in insufficient statistical evidence to draw conclusions regarding the impact of cognitive rehabilitation on cognitive deficits and the daily functioning of adults with OCD. Another issue regarding the studies of this review is multiple biases and methodological errors that were addressed. Due to the importance of cognitive deficits in OCD patients, it is necessary to design and conduct standard trials to investigate the role of cognitive rehabilitation in these impairments.
Limitations and suggestions for further studies
As mentioned before, the most important limitation of this study is the limited number of clinical trials on cognitive rehabilitation for OCD patients and the methodological differences and errors of available studies. To enhance future studies, it is recommended to conduct double-blinded trials by offering comparable tasks without training components (as opposed to using a waiting list or different therapies) and ensuring the separation of operational and analysis teams. Another beneficial approach to promote study homogeneity could involve stratifying participants based on the severity of symptoms and conducting separate analyses for individuals with mild, moderate, and severe OCD. Including follow-up assessments can provide valuable insights for subsequent studies. Furthermore, given that various studies have identified cognitive deficits in OCD patients, future research endeavors should concentrate on tasks that specifically target these impaired cognitive domains.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was funded by the Cognitive Sciences & Technologies Council (Grant No.: 15745).
Authors' contributions
Conceptualization and study design: Farah Bakizadeh, Fahime Saeed, Pouria Akbari Koli and Mohammadreza Shalbafan; Data collection: Farah Bakizadeh, Saba Mokhtari, Pouria Akbari Koli, Asieh Mokhtari and Mohammadreza Shalbafan; Initial draft preparation: Farah Bakizadeh, Saba Mokhtari, Fahime Saeed and Mohammadreza Shalbafan; Editing & review: All authors. Farah Bakizadeh and Saba Mokhtari are equal contributors to this work and designated as co-first authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Sina Tavakoli for his cooperation in performing this study.
References
Abramovitch, A., Dar, R., Schweiger, A., & Hermesh, H. (2011). Neuropsychological impairments and their association with obsessive-compulsive symptom severity in obsessive-compulsive disorder. Archives of Clinical Neuropsychology, 26(4), 364–376. [DOI:10.1093/arclin/acr022] [PMID]
Albert, U., Maina, G., Bogetto, F., Chiarle, A., & Mataix-Cols, D. (2010). Clinical predictors of health-related quality of life in obsessive-compulsive disorder. Comprehensive Psychiatry, 51(2), 193–200. [DOI:10.1016/j.comppsych.2009.03.004] [PMID]
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington: American Psychiatric Association. [DOI:10.1176/appi.books.9780890425596]
Angelakis, I., Gooding, P., Tarrier, N., & Panagioti, M. (2015). Suicidality in obsessive compulsive disorder (OCD): A systematic review and meta-analysis. Clinical Psychology Review, 39, 1–15. [DOI:10.1016/j.cpr.2015.03.002] [PMID]
Arabzadeh, S., Shahhossenie, M., Mesgarpour, B., Rezaei, F., Shalbafan, M. R., & Ghiasi, Z., et al. (2017). L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: A randomized double-blind study. Human Psychopharmacology, 32(4), 10.1002/hup.2584. [DOI:10.1002/hup.2584] [PMID]
Askari, S., Mokhtari, S., Shariat, S. V., Shariati, B., Yarahmadi, M., & Shalbafan, M. (2022). Memantine augmentation of sertraline in the treatment of symptoms and executive function among patients with obsessive-compulsive disorder: A double-blind placebo-controlled, randomized clinical trial. BMC Psychiatry, 22(1), 34. [DOI:10.1186/s12888-021-03642-z] [PMID]
Basso, M. R., Bornstein, R. A., Carona, F., & Morton, R. (2001). Depression accounts for executive function deficits in obsessive-compulsive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 14(4), 241–245. [PMID]
Benzina, N., Mallet, L., Burguière, E., N'Diaye, K., & Pelissolo, A. (2016). Cognitive dysfunction in obsessive-compulsive disorder. Current Psychiatry Reports, 18(9), 80. [DOI:10.1007/s11920-016-0720-3] [PMID]
Broadbent, D. E., Cooper, P. F., FitzGerald, P., & Parkes, K. R. (1982). The cognitive failures questionnaire (CFQ) and its correlates. The British Journal of Clinical Psychology, 21(1), 1–16. [DOI:10.1111/j.2044-8260.1982.tb01421.x] [PMID]
Buhlmann, U., Deckersbach, T., Engelhard, I., Cook, L. M., Rauch, S. L., & Kathmann, N., et al. (2006). Cognitive retraining for organizational impairment in obsessive-compulsive disorder. Psychiatry Research, 144(2-3), 109–116. [DOI:10.1016/j.psychres.2005.10.012] [PMID]
Burdick, K. E., Robinson, D. G., Malhotra, A. K., & Szeszko, P. R. (2008). Neurocognitive profile analysis in obsessive-compulsive disorder. Journal of the International Neuropsychological Societ, 14(4), 640–645. [DOI:10.1017/S1355617708080727] [PMID]
Cameron, D. H., McCabe, R. E., Rowa, K., O'Connor, C., & McKinnon, M. C. (2020). A pilot study examining the use of Goal Management Training in individuals with obsessive-compulsive disorder. Pilot and Feasibility Studies, 6, 151. [DOI:10.1186/s40814-020-00684-0] [PMID] [PMCID]
Caponnetto, P., Maglia, M., Auditore, R., Bocchieri, M., Caruso, A., & DiPiazza, J., et al. (2018). Improving neurocognitive functioning in schizophrenia by addition of cognitive remediation therapy to a standard treatment of metacognitive training. Mental Illness. 10(2), 7812. [DOI:10.4081/mi.2018.7812]
Cavedini, P., Bassi, T., Zorzi, C., & Bellodi, L. (2004). The advantages of choosing antiobsessive therapy according to decision-making functioning. Journal of Clinical Psychopharmacology, 24(6), 628–631. [DOI:10.1097/01.jcp.0000144889.51072.03] [PMID]
Cavedini, P., Riboldi, G., D'Annucci, A., Belotti, P., Cisima, M., & Bellodi, L. (2002). Decision-making heterogeneity in obsessive-compulsive disorder: Ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia, 40(2), 205–211. [DOI:10.1016/S0028-3932(01)00077-X] [PMID]
Cavedini, P., Zorzi, C., Piccinni, M., Cavallini, M. C., & Bellodi, L. (2010). Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: Searching for a new intermediate phenotype. Biological Psychiatry, 67(12), 1178–1184. [DOI:10.1016/j.biopsych.2010.02.012] [PMID]
Chamberlain, S. R., Blackwell, A. D., Fineberg, N. A., Robbins, T. W., & Sahakian, B. J. (2005). The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews, 29(3), 399–419. [DOI:10.1016/j.neubiorev.2004.11.006] [PMID]
Chamberlain, S. R., Fineberg, N. A., Blackwell, A. D., Robbins, T. W., & Sahakian, B. J. (2006). Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. The American Journal of Psychiatry, 163(7), 1282–1284. [DOI:10.1176/ajp.2006.163.7.1282] [PMID]
Chamberlain, S. R., Solly, J. E., Hook, R. W., Vaghi, M. M., & Robbins, T. W. (2021). Cognitive inflexibility in OCD and related disorders. Current Topics in Behavioral Neurosciences, 49, 125–145. [DOI:10.1007/7854_2020_198] [PMID]
Conners, C. K., Staff, M. H. S., Connelly, V., Campbell, S., MacLean, M., & Barnes, J. (2000). Conners’ continuous performance test II (CPT II v. 5). Multi-Health Systems, 29(1), 175-196. [Link]
Culbertson, WC, & Zillmer, E. A. (2001). The tower of London DX (TOLDX) manual. North Tonawanda, NY: Multi-Health Systems. [Link]
D'Alcante, C. C., Diniz, J. B., Fossaluza, V., Batistuzzo, M. C., Lopes, A. C., & Shavitt, R. G., et al. (2012). Neuropsychological predictors of response to randomized treatment in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 39(2), 310–317. [DOI:10.1016/j.pnpbp.2012.07.002] [PMID]
Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (1987-2000). California verbal learning test--second edition (CVLT –II). Washington: APA PsycTests. [DOI:10.1037/t15072-000]
Devins G. M. (2010). Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. Journal of Psychosomatic Research, 68(6), 591–602. [DOI:10.1016/j.jpsychores.2009.05.006] [PMID]
DuPont, R. L., Rice, D. P., Shiraki, S., & Rowland, C. R. (1995). Economic costs of obsessive-compulsive disorder. Medical Interface, 8(4), 102-109. [PMID]
Erzegovesi, S., Cavallini, M. C., Cavedini, P., Diaferia, G., Locatelli, M., & Bellodi, L. (2001). Clinical predictors of drug response in obsessive-compulsive disorder. Journal of Clinical Psychopharmacology, 21(5), 488–492. [DOI:10.1097/00004714-200110000-00006] [PMID]
Fergus, T. A., & Bardeen, J. R. (2016). The attention training technique: a review of a neurobehavioral therapy for emotional disorders. Cognitive and Behavioral Practice, 23(4), 502-516. [DOI:10.1016/j.cbpra.2015.11.001]
Foa, E. B., Huppert, J. D., Leiberg, S., Langner, R., Kichic, R., & Hajcak, G., et al. (2002). The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychological Assessment, 14(4), 485–496. [DOI:10.1037/1040-3590.14.4.485] [PMID]
Ghisi, M., Bottesi, G., Sica, C., Sanavio, E., & Freeston, M. H. (2013). Is performance on the Go/Nogo task related to not just right experiences in patients with obsessive compulsive disorder? Cognitive Therapy and Research, 37(6), 1121-1131. [DOI:10.1007/s10608-013-9560-1]
Golden C. J. (1976). Identification of brain disorders by the Stroop Color and Word Test. Journal of Clinical Psychology, 32(3), 654–658. [DOI:10.1002/1097-4679(197607)32:33.0.CO;2-Z] [PMID]
Goodman, W. K., Price, L. H., Rasmussen, S. A., Mazure, C., Fleischmann, R. L., & Hill, C. L., et al. (1989). The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry, 46(11), 1006–1011. [DOI:10.1001/archpsyc.1989.01810110048007] [PMID]
Gruner, P., & Pittenger, C. (2017). Cognitive inflexibility in obsessive-compulsive disorder. Neuroscience, 345, 243–255. [DOI:10.1016/j.neuroscience.2016.07.030] [PMID] [PMCID]
Gu, B. M., Park, J. Y., Kang, D. H., Lee, S. J., Yoo, S. Y., & Jo, H. J., et al. (2008). Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain, 131(Pt 1), 155–164. [DOI:10.1093/brain/awm277] [PMID]
Hadi, F., Kashefinejad, S., Kamalzadeh, L., Hoobehfekr, S., & Shalbafan, M. (2021). Glutamatergic medications as adjunctive therapy for moderate to severe obsessive-compulsive disorder in adults: A systematic review and meta-analysis. BMC Pharmacology & Toxicology, 22(1), 69. [DOI:10.1186/s40360-021-00534-6] [PMID]
Harris, C. L., & Dinn, W. M. (2003). Subtyping obsessive-compulsive disorder: Neuropsychological correlates. Behavioural Neurology, 14(3-4), 75–87. [DOI:10.1155/2003/782718] [PMID] [PMCID]
Hollander, E, Stein, DJ, Broatch, J, Himelein, C, & Rowland, C. (1997). A pharmacoeconomic and quality of life study of obsessive-compulsive disorder. CNS Spectr, 2, 16-25. [Link]
Hollander, E., Stein, D. J., Kwon, J. H., Rowland, C., Wong, C. M., & Broatch, J., et al. (1997). Psychosocial function and economic costs of obsessive-compulsive disorder. CNS Spectrums, 2(10), 16-25. [DOI:10.1017/S1092852900011068]
Jelinek, L., Hauschildt, M., Hottenrott, B., Kellner, M., & Moritz, S. (2018). "Association splitting" versus cognitive remediation in obsessive-compulsive disorder: A randomized controlled trial. Journal of Anxiety Disorders, 56, 17–25. [DOI:10.1016/j.janxdis.2018.03.012] [PMID]
Karno, M., Golding, J. M., Sorenson, S. B., & Burnam, M. A. (1988). The epidemiology of obsessive-compulsive disorder in five US communities. Archives of General Psychiatry, 45(12), 1094–1099. [DOI:10.1001/archpsyc.1988.01800360042006] [PMID]
Kashyap, H., Kumar, J. K., Kandavel, T., & Reddy, Y. C. (2013). Neuropsychological functioning in obsessive-compulsive disorder: Are executive functions the key deficit?. Comprehensive Psychiatry, 54(5), 533–540. [DOI:10.1016/j.comppsych.2012.12.003] [PMID]
Kashyap, H., Kumar, J. K., Kandavel, T., & Reddy, Y. C. (2012). Neuropsychological correlates of insight in obsessive-compulsive disorder. Acta Psychiatrica Scandinavica, 126(2), 106–114. [DOI:10.1111/j.1600-0447.2012.01845.x] [PMID]
Keshavan, M. S., Vinogradov, S., Rumsey, J., Sherrill, J., & Wagner, A. (2014). Cognitive training in mental disorders: Update and future directions. The American Journal of Psychiatry, 171(5), 510–522. [DOI:10.1176/appi.ajp.2013.13081075] [PMID] [PMCID]
Kim, J. K., & Kang, Y. (1999). Normative study of the Korean-California Verbal Learning Test (K-CVLT). The Clinical Neuropsychologist, 13(3), 365–369. [DOI:10.1076/clin.13.3.365.1740] [PMID]
Lacerda, A. L., Dalgalarrondo, P., Caetano, D., Haas, G. L., Camargo, E. E., & Keshavan, M. S. (2003). Neuropsychological performance and regional cerebral blood flow in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27(4), 657–665. [DOI:10.1016/S0278-5846(03)00076-9] [PMID]
Levine, B., Robertson, I. H., Clare, L., Carter, G., Hong, J., & Wilson, B. A., et al. (2000). Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. Journal of the International Neuropsychological Society, 6(3), 299–312. [DOI:10.1017/S1355617700633052] [PMID]
Lovibond, P. F., & Lovibond, S. H. (1995). The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33(3), 335–343. [DOI:10.1016/0005-7967(94)00075-U] [PMID]
Macy, A. S., Theo, J. N., Kaufmann, S. C., Ghazzaoui, R. B., Pawlowski, P. A., & Fakhry, H. I., et al. (2013). Quality of life in obsessive compulsive disorder. CNS Spectrums, 18(1), 21–33. [DOI:10.1017/S1092852912000697] [PMID]
Meier, S. M., Mattheisen, M., Mors, O., Schendel, D. E., Mortensen, P. B., & Plessen, K. J. (2016). Mortality among persons with obsessive-compulsive disorder in denmark. JAMA Psychiatry, 73(3), 268–274. [DOI:10.1001/jamapsychiatry.2015.3105] [PMID] [PMCID]
Moritz, S., Jelinek, L., Klinge, R., & Naber, D. (2007). Fight fire with fireflies! Association splitting: A novel cognitive technique to reduce obsessive thoughts. Behavioural and Cognitive Psychotherapy, 35(5), 631-635. [DOI:10.1017/S1352465807003931]
Moritz, S., Wess, N., Treszl, A., & Jelinek, L. (2011). The attention training technique as an attempt to decrease intrusive thoughts in obsessive-compulsive disorder (OCD): From cognitive theory to practice and back. Journal of Contemporary Psychotherapy, 41(3), 135-143. [DOI:10.1007/s10879-010-9169-6]
Muller, J., & Roberts, J. E. (2005). Memory and attention in Obsessive-Compulsive Disorder: A review. Journal of Anxiety Disorders, 19(1), 1–28. [DOI:10.1016/j.janxdis.2003.12.001] [PMID]
Murray, C. J., Lopez, A. D., & World Health Organization (WHO). (1996). The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Geneva: World Health Organization. [Link]
Nedeljkovic, M., & Kyrios, M. (2007). Confidence in memory and other cognitive processes in obsessive-compulsive disorder. Behaviour Research and Therapy, 45(12), 2899–2914. [DOI:10.1016/j.brat.2007.08.001] [PMID]
Olesen, J., Gustavsson, A., Svensson, M., Wittchen, H. U., Jönsson, B., CDBE2010 study group, & European Brain Council (2012). The economic cost of brain disorders in Europe. European Journal of Neurology, 19(1), 155–162. [DOI:10.1111/j.1468-1331.2011.03590.x] [PMID]
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., & Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160. [DOI:10.1136/bmj.n71] [PMID] [PMCID]
Papageorgiou, ., & Wells, A. (2004). Depressive rumination: Nature, theory and treatment. Hoboken: John Wiley & Sons. [DOI:10.1002/9780470713853]
Park, H. S., Shin, Y. W., Ha, T. H., Shin, M. S., Kim, Y. Y., & Lee, Y. H., et al. (2006). Effect of cognitive training focusing on organizational strategies in patients with obsessive-compulsive disorder. Psychiatry and Clinical Neurosciences, 60(6), 718–726. [DOI:10.1111/j.1440-1819.2006.01587.x] [PMID]
Penadés, R., Catalán, R., Rubia, K., Andrés, S., Salamero, M., & Gastó, C. (2007). Impaired response inhibition in obsessive compulsive disorder. European Psychiatry, 22(6), 404–410.[DOI:10.1016/j.eurpsy.2006.05.001] [PMID]
Perna, G., Cavedini, P., Harvey, P. D., Di Chiaro, N. V., Daccò, S., & Caldirola, D. (2016). Does neuropsychological performance impact on real-life functional achievements in obsessive-compulsive disorder? A preliminary study. International Journal of Psychiatry in Clinical Practice, 20(4), 224–231. [DOI:10.1080/13651501.2016.1223856] [PMID]
Purcell, R., Maruff, P., Kyrios, M., & Pantelis, C. (1998). Neuropsychological deficits in obsessive-compulsive disorder: A comparison with unipolar depression, panic disorder, and normal controls. Archives of General Psychiatry, 55(5), 415–423. [DOI:10.1001/archpsyc.55.5.415] [PMID]
Ravi Kishore, V., Samar, R., Janardhan Reddy, Y. C., Chandrasekhar, C. R., & Thennarasu, K. (2004). Clinical characteristics and treatment response in poor and good insight obsessive-compulsive disorder. European Psychiatry, 19(4), 202–208. [DOI:10.1016/j.eurpsy.2003.12.005] [PMID]
Rao, N. P., Reddy, Y. C., Kumar, K. J., Kandavel, T., & Chandrashekar, C. R. (2008). Are neuropsychological deficits trait markers in OCD?. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 32(6), 1574–1579. [DOI:10.1016/j.pnpbp.2008.05.026] [PMID]
Rao, N. P., Arasappa, R., Reddy, N. N., Venkatasubramanian, G., & Reddy, Y. C. (2010). Emotional interference in obsessive-compulsive disorder: a neuropsychological study using optimized emotional Stroop test. Psychiatry Research, 180(2-3), 99–104. [DOI:10.1016/j.psychres.2009.10.017] [PMID]
Roberts, M. E., Barthel, F. M., Lopez, C., Tchanturia, K., & Treasure, J. L. (2011). Development and validation of the detail and flexibility questionnaire (DFlex) in eating disorders. Eating Behaviors, 12(3), 168–174. [DOI:10.1016/j.eatbeh.2011.04.001] [PMID]
Sasson, Y., Zohar, J., Chopra, M., Lustig, M., Iancu, I., & Hendler, T. (1997). Epidemiology of obsessive-compulsive disorder: A world view. The Journal of Clinical Psychiatry, 58 Suppl 12, 7–10. [PMID]
Savage, C. R., Baer, L., Keuthen, N. J., Brown, H. D., Rauch, S. L., & Jenike, M. A. (1999). Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biological Psychiatry, 45(7), 905–916. [DOI:10.1016/S0006-3223(98)00278-9] [PMID]
Savage, C. R., Deckersbach, T., Wilhelm, S., Rauch, S. L., Baer, L., & Reid, T., et al. (2000). Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology, 14(1), 141–151. [DOI:10.1037/0894-4105.14.1.141] [PMID]
Segalàs, C., Alonso, P., Labad, J., Jaurrieta, N., Real, E., & Jiménez, S., et al. (2008). Verbal and nonverbal memory processing in patients with obsessive-compulsive disorder: Its relationship to clinical variables. Neuropsychology, 22(2), 262–272. [DOI:10.1037/0894-4105.22.2.262] [PMID]
Shalbafan, M., Malekpour, F., Tadayon Najafabadi, B., Ghamari, K., Dastgheib, S. A., & Mowla, A., et al. (2019). Fluvoxamine combination therapy with tropisetron for obsessive-compulsive disorder patients: A placebo-controlled, randomized clinical trial. Journal of Psychopharmacology, 33(11), 1407–1414. [DOI:10.1177/0269881119878177] [PMID]
Shavitt, R. G., Belotto, C., Curi, M., Hounie, A. G., Rosário-Campos, M. C., & Diniz, J. B., et al. (2006). Clinical features associated with treatment response in obsessive-compulsive disorder. Comprehensive Psychiatry, 47(4), 276–281. [DOI:10.1016/j.comppsych.2005.09.001] [PMID]
Sheehan, D. V. (2000). Sheehan disability scale. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association, 113-115. [Link]
Shin, M. S., Park, S. Y., Park, S. R., Seol, S. H., & Kwon, J. S. (2006). Clinical and empirical applications of the rey-osterrieth complex figure test. Nature Protocols, 1(2), 892–899. [DOI:10.1038/nprot.2006.115] [PMID]
Simblett, S. K., & Bateman, A. (2011). Dimensions of the dysexecutive questionnaire (DEX) examined using Rasch analysis. Neuropsychological Rehabilitation, 21(1), 1–25. [DOI:10.1080/09602011.2010.531216] [PMID]
Snyder, H. R., Kaiser, R. H., Warren, S. L., & Heller, W. (2015). Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clinical Psychological Science, 3(2), 301–330. [DOI:10.1177/2167702614534210] [PMID] [PMCID]
Stamenova, V., & Levine, B. (2019). Effectiveness of goal management training® in improving executive functions: A meta-analysis. Neuropsychological Rehabilitation, 29(10), 1569–1599. [DOI:10.1080/09602011.2018.1438294] [PMID]
Suhas, S., & Rao, N. P. (2019). Neurocognitive deficits in obsessive-compulsive disorder: A selective review. Indian Journal of Psychiatry, 61(Suppl 1), S30–S36. [DOI:10.4103/psychiatry.IndianJPsychiatry_517_18] [PMID] [PMCID]
Tarafder, S., Bhattacharya, P., Paul, D., Bandyopadhyay, G., & Mukhopadhyay, P. (2006). Neuropsychological disposition and its impact on the executive functions and cognitive style in patients with obsessive-compulsive disorder. Indian Journal of Psychiatry, 48(2), 102–106. [DOI:10.4103/0019-5545.31598] [PMID] [PMCID]
Tchanturia, K., Davies, H., Reeder, C., & Wykes, T. (2010). Cognitive remediation programme for anorexia nervosa: A manual for practitioners. London: Institute of Psychiatry. [Link]
Tükel, R., Gürvit, H., Ertekin, B. A., Oflaz, S., Ertekin, E., & Baran, B., et al. (2012). Neuropsychological function in obsessive-compulsive disorder. Comprehensive Psychiatry, 53(2), 167–175. [DOI:10.1016/j.comppsych.2011.03.007] [PMID]
Ustün, T. B., Chatterji, S., Kostanjsek, N., Rehm, J., Kennedy, C., & Epping-Jordan, J., et al. (2010). Developing the World Health Organization disability assessment schedule 2.0. Bulletin of the World Health Organization, 88(11), 815–823. [DOI:10.2471/BLT.09.067231] [PMID] [PMCID]
van den Heuvel, O. A., Veltman, D. J., Groenewegen, H. J., Witter, M. P., Merkelbach, J., & Cath, D. C., et al. (2005). Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry, 62(8), 922–933. [DOI:10.1001/archpsyc.62.8.922] [PMID]
van Passel, B., Danner, U. N., Dingemans, A. E., Aarts, E., Sternheim, L. C., & Becker, E. S., et al. (2020). Cognitive remediation therapy does not enhance treatment effect in obsessive-compulsive disorder and anorexia nervosa: A randomized controlled trial. Psychotherapy and Psychosomatics, 89(4), 228–241. [DOI:10.1159/000505733] [PMID]
Viswanath, B., Janardhan Reddy, Y. C., Kumar, K. J., Kandavel, T., & Chandrashekar, C. R. (2009). Cognitive endophenotypes in OCD: A study of unaffected siblings of probands with familial OCD. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(4), 610–615. [DOI:10.1016/j.pnpbp.2009.02.018] [PMID]
Type of Study: Review |
Subject:
Cellular and molecular Neuroscience
Received: 2022/10/16 | Accepted: 2022/11/12 | Published: 2024/05/1
Received: 2022/10/16 | Accepted: 2022/11/12 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





