Volume 16, Issue 1 (January & February 2025)
BCN 2025, 16(1): 81-94 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmad Siddiqui S, Singh S, Ahmad M K, Arshad M. Altered Serotonin 5HT-1B Receptor Expression Regulate Sex-dependent Difference for Stress and Anxiety. BCN 2025; 16 (1) :81-94
URL: http://bcn.iums.ac.ir/article-1-2476-en.html
URL: http://bcn.iums.ac.ir/article-1-2476-en.html
1- Department of Biochemistry, Faculty of Medicine, King George’s Medical University, Lucknow, India.
2- Department of Biotechnology, School of Sciences, Babasaheb Bhimrao Ambedkar University, Lucknow, India.
3- Department of Zoology, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, India.
2- Department of Biotechnology, School of Sciences, Babasaheb Bhimrao Ambedkar University, Lucknow, India.
3- Department of Zoology, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, India.
Full-Text [PDF 3177 kb]
| Abstract (HTML)
EPM test
The EPM was used to analyze the effect of stress or anxiety on body physiology in rats. The apparatus of EPM consists of four arms, of which two are open, and two closed (each 40×10 cm dimension), all joined at the center, thus creating a plus-shaped structure, elevated 50 cm above the ground and located in a room with low-intensity light. During the experiments, the rats were positioned at the center of the maze, and the face of the animal was positioned towards a closed arm. The animal’s activity was observed for up to 5 minutes and analyzed. The entries in open arms were analyzed as a percentage of open arm entries concerning the time spent in each arm during 5 minutes of exposure in all groups. The analysis for the result obtained by EPM was performed using any-maze analysis software (ANY-maze, Stoelting Co., US).
OFT
The OFT is used to analyze physiological conditions during animal stress and anxiety (Seibenhener et al., 2015). The OFT apparatus was done in a square box (120×120×60 cm) made of non-transparent material in a soundproof room. The arena was divided into inner central and outer areas (Seibenhener et al., 2015). All animals were positioned arbitrarily at one of the corners. These animals explored the arena for 10-15 min, and their behaviors were analyzed with the help of a monochromic camera situated on the top. The overall time used and the number of entries at the center arena were analyzed manually for complete session time.
Body weight
For identification and analysis of stress in these animals, they were weighted after each day of the stress session. Previous studies on stress suggest a continuously decreased body weight in experimental animals (Harris, 2015; Yau & Potenza, 2013).
Plasma corticosterone
Blood samples (about 1 mL) from the stress and control groups were collected through aortic puncture and transferred into ice-cooled centrifugal tubes. Blood was kept at room temperature for 1 hour for clotting in a collection tube, followed by centrifugation at 1700 ×g, for 10 min, at 4 °C to separate serum from blood. The serum was then stored at -80 °C freezer until further experiment.
Brain subregions
The whole amygdala (including BLA and CeA, bregma -2.6 to -3.2), hippocampus (including CA1, CA2, CA3, and DG, bregma -2.6 to -3.2), and PFC (including PL and IL, bregma 2.76 to 3.24) brain regions were used for the analysis of serotonin receptor expression through transcription analysis using Q-PCR and translation analysis using IHC. The brain area analyzed in this study is the key partner for emotional and cognitive responses (Stults-Kolehmainen & Sinha, 2014; Albert et al., 2014; McEwen et al., 2016).
Tissue preparation for IHC and transcription analysis
After stress experiments (2 h post-experiment), the rats were anesthetized by pentobarbital (60 mg/kg, IP) and were transcardially perfused with a normal saline solution. It was followed by the introduction of ice-cold 4% paraformaldehyde solution (in 0.1 M phosphate buffer solution, pH 7.4), brains removed after decapitation. Isolated brains were further post-fixed in a 4% paraformaldehyde solution for 24 h, followed by keeping them in sucrose solution (10%, 20%, and 30% solutions serially in 0.1 M phosphate buffer, pH 7.4). Then, the brains were frozen at -30 °C to -35 °C in isopentane solution for 30 min and stored in a deep freezer at -80 °C for IHC. For the transcription study, the rats were transcardially perfused with chilled normal saline solution only, and the brains were collected and stored in a deep freezer at -80 °C for Q-PCR.
Isolation of mRNA and cDNA preparation
The hippocampus, PFC, and amygdala regions were dissected from all groups for transcription analysis. The serotonin receptor mRNA expression was analyzed between male and female chronic restrained groups and their respective control groups. The sample size from each group was 5-6, which was used for the mRNA expression study.
The tissue from each animal (5 mg) was homogenized in 100 µL of cell lysis buffer and β-mercaptoethanol (0.7 µL).
From these tissue samples, total cellular RNA was isolated using a GeneJET RNA isolation kit (Thermo, Catalog No. K0731), followed by genomic DNA removal using DNase I treatment. The purity of RNA was analyzed using a spectrophotometer at 260/280 nm, around OD=1.75. cDNA was prepared from these isolated mRNA and oligo-dT primers using the RevertAid First Strand cDNA synthesis kit (Thermo, K1622). These generated cDNAs were further used as a template in real-time PCR amplification. The reaction mixture for PCR analysis was prepared in a final volume of 15 µL as follows: 5x reaction buffer (3 µL), dNTP mixture (1.5 µL), oligo dT primer (1 µL), reverse transcriptase (0.75 µL), RNase inhibitor (0.75 µL) and template RNA (3 µL). Reaction conditions for cDNA preparation were as follows: 60 min at 42 °C, 5 min at 70 °C and holding at 4 °C. The cDNA generated in this reaction was stored at -80 deep freezer.
Q-PCR
The level of serotonin receptor mRNA was analyzed using Q-PCR. mRNA expression analysis was performed in Stratagene Max-Pro Real-Time PCR detection System using maxima SYBR Green/ROX Master Mix (Thermo). The quantity of expression was represented as fold change through the Ct method. The primers sequences are as follows: c-fos, 5’-CCGACTCCTTCTCCAGCAT-3’ (forward), 5’ –TCACCGTGGGGATAAAGTTG-3’ (reverse); 5HT-1Br, 5`-GGAAAGTCCTGCTGGTTGCT-3` (forward), 5`- CGATCAGGTAGTTAGCCGGG-3` (reverse) and control GAPDH, 5`-AGTGCCAGCCTCGTCTCATA-3` (forward), 5`-TCCCGTTGATGACCAGCTTC-3` (reverse). Each study group had 5-6 samples analyzed in triplicate. The reaction mixture consists of 10 µL SYBR Green Master Mix (2x), forward and reverse primers (1µl each), cDNA (3 µL), and a final volume of 20 µL with ddH2O. The reaction condition used was as follows: Initial denaturation (95 °C, 10 min), denaturation (95 °C, 30 s), annealing temperature (57 °C, 30 s), and extension (72 °C, 30 s), total 45 cycles.
Immunohistochemistry (IHC)
The brain was sliced into 20-μm thick brain sections containing different brain regions such as PFC and hippocampus, and the serial sections were collected with the help of a cryostat (Microm HM 525, Germany). The collected sections were washed in PBS (phosphate-buffered saline) solution (0.01 M) and blocked in PBST (phosphate-buffered saline containing 1% Tween 20) containing 1% normal horse serum solution (VECTASTAIN kit). After blockage, the sections were incubated in anti-5HT-1Br (1:250 dilution, ASR-022, Thermo), anti-c-fos (1:500, cat. PA5-143600, Invitro) primary antibody, overnight at room temperature. Afterward, the sections were incubated for 2 h at room temperature in a biotinylated linked secondary antibody (1:500 dilution, Vector Lab.). Afterward, the sections were washed and incubated with avidin-biotinylated-peroxidase complex (VECTASTAIN Elite ABC Kit) and stained by the DAB staining solution (Vector lab.). After staining, the sections were mounted on glass slides and covered with a coverslip using DPX Mountant. The images from each section were acquired using a compound light microscope (Nikon). The level of serotonin receptor expression was analyzed as several positive neurons in different brain subregions using the NIS-Basic Research image analysis system (Nikon).
Statistical analysis
For statistical analysis, the data were presented as Mean±SD of the means and analyzed by analysis of variance (ANOVA; one-way, two-way) or student t-test using GraphPad Prism statistical software, version 7.
3. Results
Behavioral study
EPM test
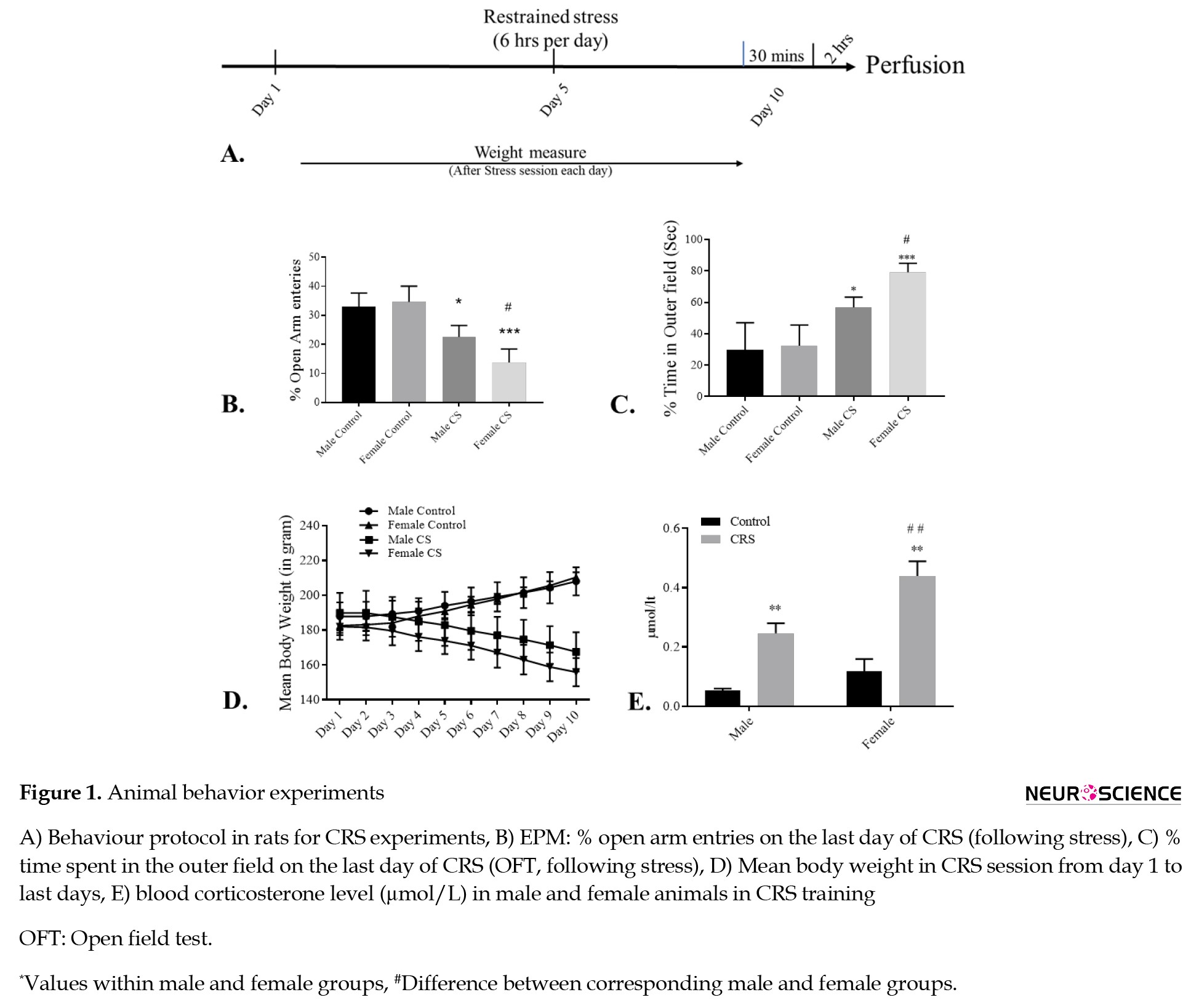
Both male and female animals underwent 6 h of chronic stress training for 10 days (Figure 1A). All animals explored the environment, but anxiogenic factors caused a decreased exploration of the environment in open arms. Entries in the open arm were measured as the percentage of entries in the open arm for both groups for an overall duration of time. The Tukey post hoc analysis exhibited decreased entries in the open arm by the male and female chronic stress groups on day 10. However, control groups exhibited an enhanced open arm entry at day 10 compared to day 1 (F(3, 16)=21.15, P<0.001; Figure 1C). The statistical analysis exhibited a significant effect of chronic stress on body physiology in both the male and female CRS rats when compared with their respective control groups (Figure 1B).
OFT for anxiety measurement
When compared, the OFT showed significant differences between CRS and respective control groups. The time spent in the central area was longer for the control groups than for the CRS groups. The Tukey post hoc analysis suggested that the female CRS group exhibited comparatively fewer entries in the central field than the male CRS group (P<0.05). Here, the result suggested a significant change in the time spent in the central arena (F(3, 16)=19.42, P<0.001). Also, the number of entries in the central area is significantly lower in male and female CRS groups than in the control groups (Figure 1C).
Body weight measurement
Following the stress session, the body weight was measured in the CRS and control groups (every day). There was an alteration in mean body weight in all groups when comparing day 1 with day 10 (P<0.05). In the CRS groups (males and females), the body weight declined significantly and continuously from day 1 to day 10 (P<0.0001). However, the control groups (males and females) exhibited significantly enhanced body weight from day 1 to day 10 (P<0.0001; Tukey post hoc analysis). The test compared the first and last day trials of all groups. The result suggested a significant effect of stress (F(3, 15)=15.95, P<0.0001) and the interaction of stress with the number of trials (F(3, 15)=431.4, P<0.0001) with the body weight in these rats (Figure 1D).
Plasma corticosterone level
Theesponse caused by stress on the animal is measured by analyzing plasma corticosterone levels from their blood. The corticosterone in the male and female animals was higher under CRS than in the control animals; however, it was much higher in female rats than in the male animals under similar stress conditions. The result was confirmed by the statistical analysis using Tukey post hoc examination. The result revealed a significant outcome of gender (F(1, 4)=80.09, P=0.0009), stress (F(1, 4)=445.6, P<0.0001), and interaction effect of gender with the chronic stress (F(1, 4)=9.9, P=0.05) when corticosterone level was analyzed in the male and female rats under chronic stress (Figure 1E).
Transcriptional analysis
Amygdala
c-Fos mRNA expression
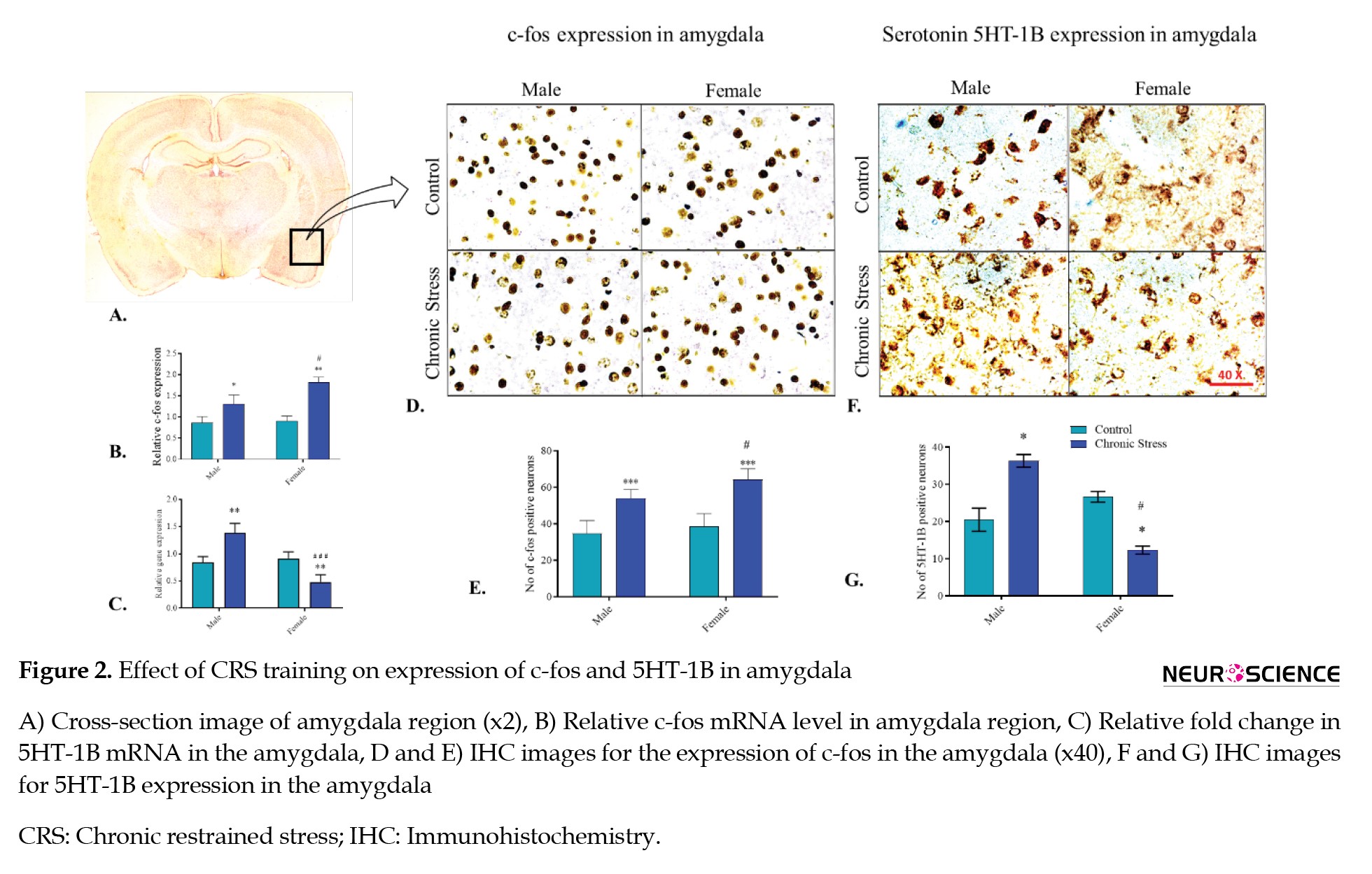
In the amygdala of the male and female rats, the c-fos expression was amplified significantly in chronic stress groups compared with their respective controls (P<0.05 and P<0.01, respectively) (Tukey post hoc analysis). Interestingly, the change in c-fos mRNA was significantly more in the female CRS group than in the male CRS group (P<0.05). The result exhibited significant outcomes of gender (F(1, 4)=26.25, P<0.01), stress (F(1, 4)=58.79, P<0.01), and the interaction of sex with the chronic stress (F(1, 4)=16.78, P<0.05) on c-fos mRNA expression in the amygdala compared to control groups (Figure 2B).
5HT-1B mRNa expression
The 5HT-1B expression showed a significant fold-change in 5HT-1B mRNA expression in male and female rats compared to their respective control groups. In male SD rats, the 5HT-1B mRNA was amplified (P<0.01) significantly, while in female rats, transcription diminished (P<0.01) in the CRS groups compared with their respective control groups, confirmed by the Tukey post hoc analysis. The result presented the significant effect of gender (F(1, 4)=21.31, P<0.01) and the interaction of sex with stress (F(1, 4)=156.3, P<0.001) for serotonin 5HT-1B mRNA expression in the amygdala region, compared to the control animals (Figure 2C).
mRNA expression in the prefrontal cortex
c-Fos mRNA expression
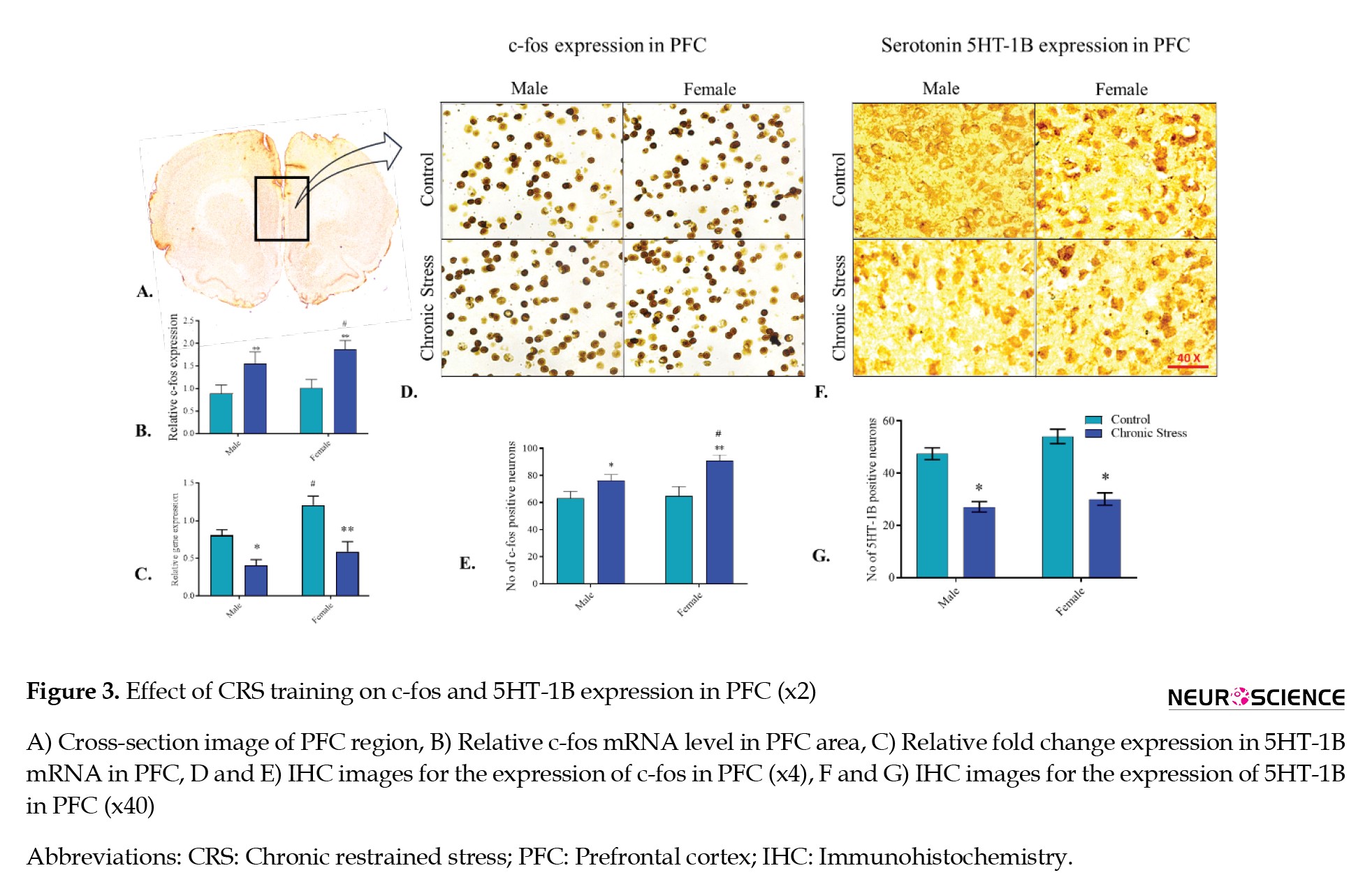
In the PFC of the male and female rats, the c-fos expression was significantly amplified in CRS groups compared to their respective control groups, confirmed by the Tukey post hoc analysis (P<0.01 and P<0.01, respectively). The c-fos expression was higher in the female stress group than in the male stress group (P<0.05). The outcome exhibited a significant consequence of stress (F(1, 4)=85.84, P<0.001) on c-fos mRNA expression in PFC (Figure 3B).
5HT-1B mRNA expression
In male and female SD rats, the 5HT-1B mRNA declined (P<0.01 and P<0.01, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. The result displayed the significant effect of chronic stress (F(1, 4)=97.04, P<0.001) and gender (F(1, 4)=326, P<0.0001) for the expression of 5HT-1B mRNA in the PFC. However, basal serotonin 5HT-1B receptor mRNA level was higher for the female group when compared with the male stress group (Figure 3C).
mRNA expression in the hippocampus
c-Fos mRNA expression
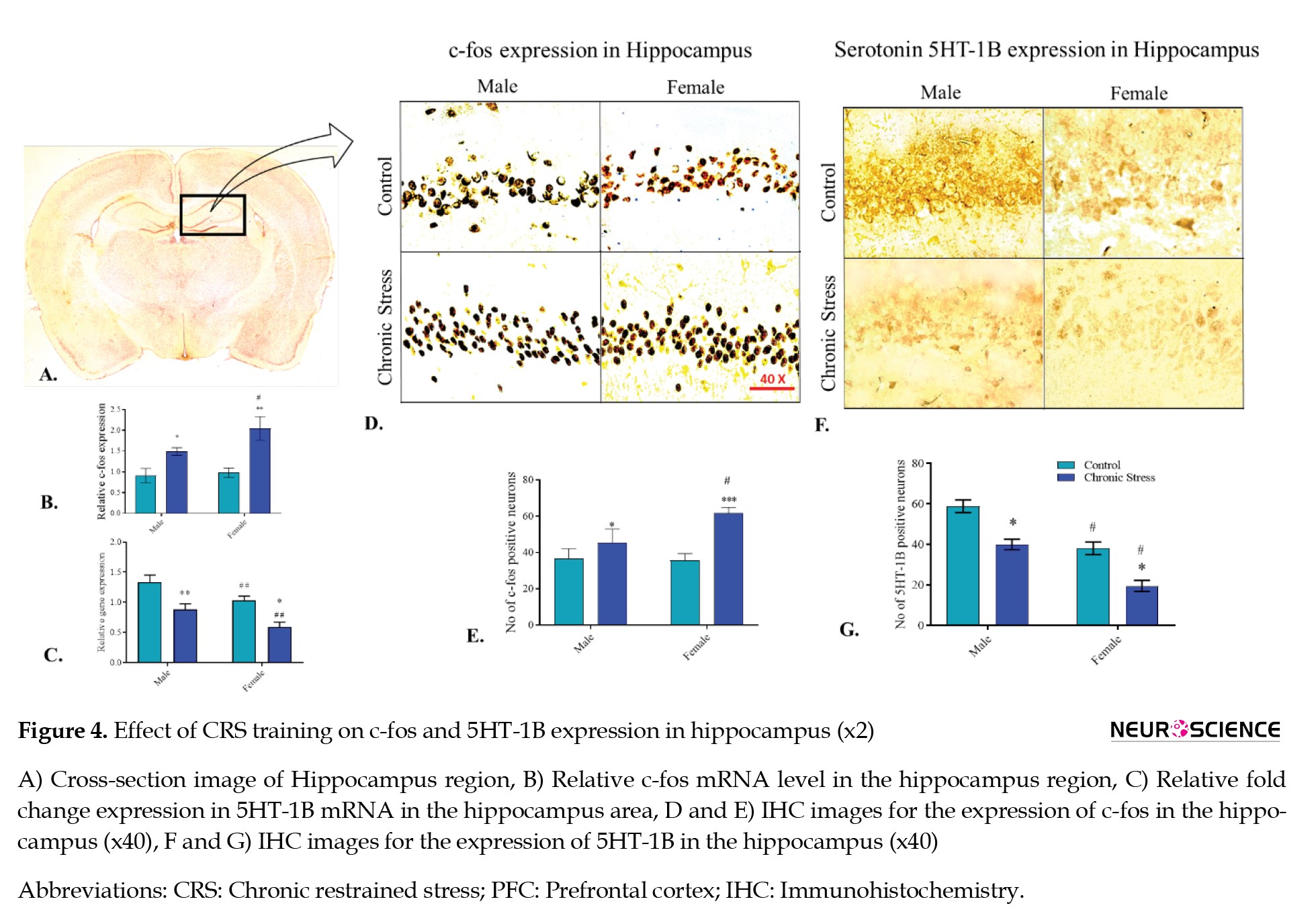
In the hippocampus of the male and female rats, the c-fos expression was significantly amplified in chronic stress groups compared to their respective control groups, confirmed by the Tukey post hoc analysis (P<0.05 and P<0.01, respectively).
However, the change in expression was significantly greater in female chronic stress groups than in male chronic stress group rats (P<0.05). The ANOVA revealed the significant effect of chronic stress (F(1, 4)=13.61, P<0.05), gender of animals (F(1, 4)=56.15, P<0.01), and the interaction of gender and the chronic stress condition (F(1, 4)=9.62, P<0.05) for the expression of c-fos mRNA in hippocampus region compared with the control (Figure 4B).
5HT-1B mRNA expression
In male and female SD rats, the 5HT-1B mRNA declined (P<0.01 and P<0.05, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. The ANOVA revealed the significant effect of chronic stress (F(1, 4)=32.61, P<0.01) and gender (F(1, 4)=133.1, P<0.001) for the expression of 5HT-1B mRNA in the hippocampus region when compared with the control. The 5HT-1B level was significantly lower in the female chronic stress group than in the male chronic stress group, suggesting a sex-dependent association of this receptor subtype in the hippocampus under stress conditions (Figure 4C).
Immunohistochemistry
c-Fos expression in amygdala
c-Fos protein expression was analyzed in the amygdala for both male and female groups to understand amygdala activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the c-fos level increased (P<0.001 and P<0.001, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals significantly differed in c-fos levels when exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=24.71, P<0.01) and gender (F(1, 5)=54.05, P<0.001) on the expression of c-fos in the amygdala region when compared with the control. The c-fos level suggested a sex-dependent association of this receptor subtype in the amygdala under stress conditions (Figures 2D, and 2E).
Serotonin 5HT-1B receptor expression in the amygdala
The serotonin receptor 5HT-1B expression was analyzed in the amygdala between male and female animals. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male SD rats, the 5HT-1B level increased (P<0.05) in the CRS groups compared to their respective control animal groups. However, it declined in female rats (P<0.05) when exposed to stress, confirmed by the Tukey post hoc analysis. ANOVA revealed the significant effect of gender (F(1, 5)=40.98, P<0.01) and the interaction of chronic stress and gender (F(1, 5)=35.14, P<0.01) for the expression of 5HT-1B in the amygdala region when compared with the control. The 5HT-1B level suggested a sex-based association of this receptor subtype in the amygdala under stress conditions (Figures 2F, and 2G).
c-Fos expression in PFC
The c-fos expression in PFC between male and female animals was analyzed to understand PFC activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In the male and female SD rats, the c-fos level increased (P<0.05 and P<0.01, respectively) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals had a significant difference in c-fos levels when animals were exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=21.44, P<0.01), gender (F(1, 5)=39.89, P<0.01), and the interaction of chronic stress with gender (F(1, 5)=11.26, P<0.05) for the expression of c-fos in PFC region compared with the control. The c-fos level suggested a sex-dependent association of c-fos activity in PFC under stress conditions (Figures 3D, and 3E).
Serotonin 5HT-1B receptor expression in the PFC
The 5HT-1B expression was analyzed in the PFC between male and female animals to understand its activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In the male and female SD rats, the 5HT-1B level decreased (P<0.05 and P<0.05, respectively) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. ANOVA revealed the significant effect of chronic stress (F(1, 5)=70.25, P<0.001) and gender (F(1, 5)=7.6, P<0.05) for the expression of 5HT-1B in the PFC region compared with the control. The 5HT-1B level suggested a sex-dependent association of 5HT-1B activity in PFC under stress conditions (Figures 3F, and 3G).
c-Fos expression in the hippocampus
The c-fos expression was analyzed between male and female animals in the hippocampus to understand its activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the c-fos level increased (P<0.05 and P<0.01, respectively) significantly in CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals had a significant difference in c-fos levels when animals were exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=14.78, P<0.05), gender (F(1, 5)=25.85, P<0.01), and the interaction of chronic stress with gender (F(1, 5)=43.03, P<0.01) for the expression of c-fos in hippocampus region when compared with the control. The c-fos level suggested a sex-based association of c-fos activity in the hippocampus under stress conditions (Figures 4D, and 4E).
Serotonin 5HT-1B receptor expression in hippocampus
The 5HT-1B expression was analyzed between male and female animals in the hippocampus to understand its activity under stress conditions. The chronic restrained group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the 5HT-1B level declined (P<0.05; P<0.05) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, a significant difference was observed among male and female animals for 5HT-1B level when animals were exposed to stress (P<0.05). Although both the stress groups exhibit a reduction in serotonin 5HT-1B receptor expression, the basal level for serotonin 5HT-1B receptor was lower in female stress groups (P<0.05). The ANOVA result revealed the significant effect of chronic stress (F(1, 5)=29.19, P<0.05) and gender (F(1, 5)=153.7, P<0.0001) for the expression of 5HT-1B in the hippocampus region when compared with the control. The 5HT-1B level suggested a sex-based association of 5HT-1B activity in the hippocampus under stress conditions (Figures 4F, and 4G).
4. Discussion
The current study investigated the role of the serotonin 5HT-1B receptor subtype on sex-dependent stress and anxiety variation in SD rats. Our rodent model-based study found a sex-based spatial function played by the serotonin 5HT-1B receptor subtype in CRS conditions among male and female SD rats. To better understand the difference between stress circuitry and involvement of the 5HT-1B receptor, we first investigated the activity of the amygdala, hippocampus, and PFC regions of the brain under CRS condition using c-fos expression (an IEG [immediate early gene] used as a neuronal activity marker gene) (Gallo et al., 2018). We observed a sex-dependent difference in activation patterns in these brain areas under CRS training.
The c-fos expression analysis confirmed activation of the hippocampus, PFC, and amygdala brain regions during chronic stress in male and female rats. However, the expression was comparatively higher in female CRS animals than in males, which correlated with a comparatively higher stress response in females. The result suggests that enhanced activity of trio brain areas (i.e. PFC, amygdala, and hippocampus) causes hyperactivation of stress circuitry in the female stress group compared to the male stress group. Although other regions, such as the hypothalamic-pituitary-adrenal axis, are associated with the stress circuitry, our primary focus was on trio partners due to their central role in emotional responses (McEwen et al., 2016). The stress circuitry involves the DRN, which innervates the amygdala, PFC, and hippocampus through direct and indirect neuronal connections (McEwen et al., 2016; Huang et al., 2019). Through its serotonergic innervation, DRN regulates the activity of the amygdala, PFC, and hippocampus regions under stressful conditions (Bocchio et al., 2016; Huang et al., 2019).
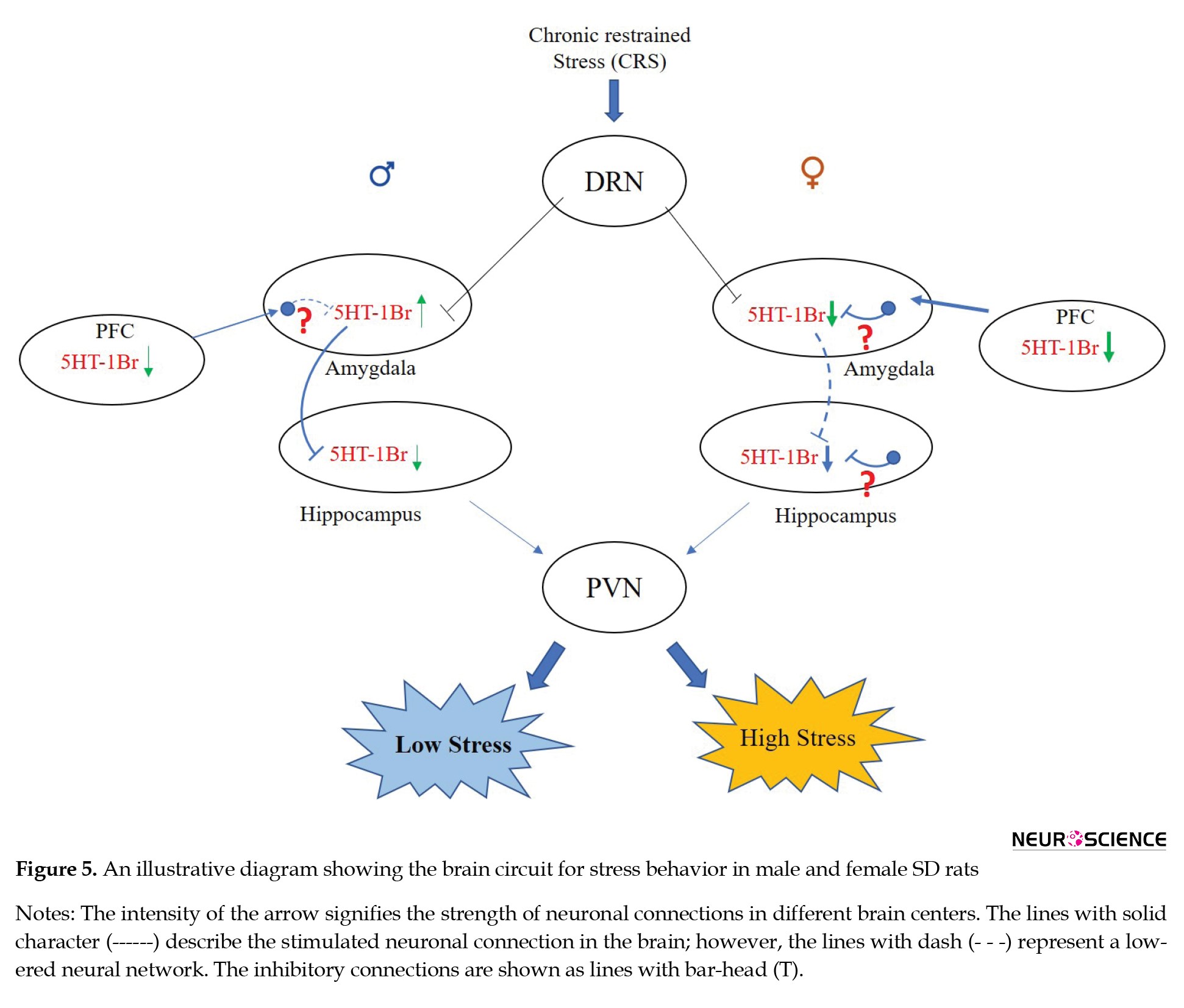
The current study hypothesized that the difference in serotonergic 5HT-1B receptor between both sexes might cause differential activation of these brain regions, resulting in differences in stress response (Figure 5).
Full-Text:
1. Introduction
Our daily lifestyle exposes us to several environmental factors that may be challenging and problematic. Negative situations we face in life, such as heavy workloads, our socioeconomic conditions, accidents, traumas, wars, sexual abuse, and terrorist attacks, sometimes have deleterious effects (Stults-Kolehmainen & Sinha, 2014). Stress creates multidimensional adverse effects that cause physiological as well as behavioral and psychological dysfunctions (Schneiderman et al., 2005; Segerstrom & Miller, 2004). If not managed properly, there is a considerable possibility for the development of various types of psychological disorders, such as depression and other related stress and anxiety disorders (Yang et al., 2015).
The consequences of stress vary among people; some people are less affected by it, while others face difficulty. Likewise, the effect of stress and anxiety is also not the same between the sexes, as females are more susceptible to stress and anxiety conditions (Verma et al., 2011; Bahrami & Yousefi, 2011). The severity of stress and anxiety is also higher in females than in males, and women are comparatively more likely to experience higher stress and anxiety (Verma et al., 2011; McLean et al., 2011). The number of women facing work-related stress is nearly 50% higher compared with same-age men (Gino et al., 2015).
The serotonin receptor system is an intricate arrangement of neuronal networks involved in the regulation of various important brain functions like learning, memory, emotional responses, sleep-wake cycle, anxiety, and mood control (Jenkins et al., 2016; Bacqué-Cazenave et al., 2020; Charnay & Léger, 2010). This system is formed by the neurotransmitter (5-hydroxytryptamine) serotonin, synthesized by neurons in the nervous system, and 14 different serotonin receptors (e.g. 5HT-1 to 5HT-7 receptor) located all over the brain (Yohn et al., 2017; Pithadia & Jain, 2006). The system regulates the release of different neurotransmitters (like acetylcholine, gamma-aminobutyric acid [GABA], epinephrine/norepinephrine, and glutamate) and some hormones, thereby controlling different brain functions such as sleep-wake cycle, mood, learning, anxiety, memory, and sleep (Ciranna, 2006). Contrary to humans, the rodent brain contains 13 serotonin receptors (except 5HT-1E) (Berumen et al., 2012; Osredkar & Krzan, 2009). Different animal studies have suggested the involvement of the serotonin receptor system in anxiety and stress conditions (Overstreet et al., 2003; Akimova et al., 2009; Bacqué-Cazenave et al., 2020; Karayol et al., 2021). Studies involving 5HT-2C receptor overexpression in rodent forebrain regions have shown increased anxiety in these animals (Kimura et al., 2009), while other studies have suggested the role of 5HT-1A, 5HT-2A, and 5HT4 receptors in stress and anxiety conditions (Akimova et al., 2009; Xiang et al., 2019, Karayol et al., 2021). More interestingly, Bhatnagar et al. (2004) found a difference in the role of 5HT-3r between male and female mice. 5HT-1B receptor, which is an inhibitory receptor, is highly expressed in the substantia nigra and globus pallidus area of the brain, while in moderate to low levels in the amygdala, prefrontal cortex (PFC), and hippocampus (Tiger et al., 2008; Švob Štrac et al., 2016). These studies suggest a specialized function by serotonin receptors regulating stress differently between the sexes.
The stress circuitry starts from the dorsal raphe nucleus (DRN), which innervates rodents’ hippocampus, amygdala, PFC, and other related brain regions (Shin et al., 2010). The hippocampus, amygdala, and PFC form an intricate network known as the limbic system, the area mainly associated with the regulation of emotional response in stress conditions (Rajmohan et al., 2007; Tiger et al., 2008; Šimić et al., 2021). The circuitry system formed by these components is called “stress circuitry.” The hippocampus region expresses a comparatively high level of glucocorticoid and mineralocorticoid hormone receptors (Koning et al., 2019). Furthermore, the hippocampus, combined with other brain parts such as the amygdala, PFC, and brain nuclear stria terminalis, regulates stress circuitry in the brain (Rajmohan et al., 2007).
Although there are known physiological differences between male and female individuals, the difference in the serotonin receptor (5HT receptor) is crucial. Despite this difference, little information is available that specifies the function of serotonin receptors with stress and anxiety-dependent differences in male and female animals. For a better understanding of such differences, it is necessary to study serotonin receptors in detail, with special emphasis on sex-dependent anxiety differences. Unless we understand the biological differences between the brains of male and female individuals, developing better therapeutic
applications is challenging
Materials and Methods
Animals and groups
The research experiments were executed with healthy adult Sprague–Dawley (SD) rats aged 2–3 months. All the animals were kept under standard 12-h light/dark laboratory conditions, a temperature of 23b °C, and food and water ad libitum. The study included four animal groups (male control, female control, male CRS, and female CRS group). Regarding the behavior experiments, there were 10-12 animals in each group (half underwent an elevated plus maze [EPM] test while half took an open field test [OFT]), and these animals were not used for molecular study. Another group was used for molecular study using immunohistochemistry (IHC) and quantitative polymerase chain reaction (Q-PCR) (n=10 to 12 animals in each group, half used for IHC and the rest for PCR), and no OFT or EPM test was performed.
Chronic restrained stress (CRS) in rats
The CRS was performed on males and females with the use of 10 days of chronic restrained immobilization stress sessions, 6 h/d (8:00 AM to 2:00 PM) (Buynitsky et al., 2009). The immobilization process in CRS was done using a cone made up of polythene (8-10 inches). After a stress session, the OFT and EPM test was performed in animals to analyze the effect of stress on body physiology. After the final behavior experiment, the animals were perfused transcardially, and their brains and blood samples were collected during the perfusion of animals. The samples were stored at -80 °C for further molecular analysis (Figure 1A).
Our daily lifestyle exposes us to several environmental factors that may be challenging and problematic. Negative situations we face in life, such as heavy workloads, our socioeconomic conditions, accidents, traumas, wars, sexual abuse, and terrorist attacks, sometimes have deleterious effects (Stults-Kolehmainen & Sinha, 2014). Stress creates multidimensional adverse effects that cause physiological as well as behavioral and psychological dysfunctions (Schneiderman et al., 2005; Segerstrom & Miller, 2004). If not managed properly, there is a considerable possibility for the development of various types of psychological disorders, such as depression and other related stress and anxiety disorders (Yang et al., 2015).
The consequences of stress vary among people; some people are less affected by it, while others face difficulty. Likewise, the effect of stress and anxiety is also not the same between the sexes, as females are more susceptible to stress and anxiety conditions (Verma et al., 2011; Bahrami & Yousefi, 2011). The severity of stress and anxiety is also higher in females than in males, and women are comparatively more likely to experience higher stress and anxiety (Verma et al., 2011; McLean et al., 2011). The number of women facing work-related stress is nearly 50% higher compared with same-age men (Gino et al., 2015).
The serotonin receptor system is an intricate arrangement of neuronal networks involved in the regulation of various important brain functions like learning, memory, emotional responses, sleep-wake cycle, anxiety, and mood control (Jenkins et al., 2016; Bacqué-Cazenave et al., 2020; Charnay & Léger, 2010). This system is formed by the neurotransmitter (5-hydroxytryptamine) serotonin, synthesized by neurons in the nervous system, and 14 different serotonin receptors (e.g. 5HT-1 to 5HT-7 receptor) located all over the brain (Yohn et al., 2017; Pithadia & Jain, 2006). The system regulates the release of different neurotransmitters (like acetylcholine, gamma-aminobutyric acid [GABA], epinephrine/norepinephrine, and glutamate) and some hormones, thereby controlling different brain functions such as sleep-wake cycle, mood, learning, anxiety, memory, and sleep (Ciranna, 2006). Contrary to humans, the rodent brain contains 13 serotonin receptors (except 5HT-1E) (Berumen et al., 2012; Osredkar & Krzan, 2009). Different animal studies have suggested the involvement of the serotonin receptor system in anxiety and stress conditions (Overstreet et al., 2003; Akimova et al., 2009; Bacqué-Cazenave et al., 2020; Karayol et al., 2021). Studies involving 5HT-2C receptor overexpression in rodent forebrain regions have shown increased anxiety in these animals (Kimura et al., 2009), while other studies have suggested the role of 5HT-1A, 5HT-2A, and 5HT4 receptors in stress and anxiety conditions (Akimova et al., 2009; Xiang et al., 2019, Karayol et al., 2021). More interestingly, Bhatnagar et al. (2004) found a difference in the role of 5HT-3r between male and female mice. 5HT-1B receptor, which is an inhibitory receptor, is highly expressed in the substantia nigra and globus pallidus area of the brain, while in moderate to low levels in the amygdala, prefrontal cortex (PFC), and hippocampus (Tiger et al., 2008; Švob Štrac et al., 2016). These studies suggest a specialized function by serotonin receptors regulating stress differently between the sexes.
The stress circuitry starts from the dorsal raphe nucleus (DRN), which innervates rodents’ hippocampus, amygdala, PFC, and other related brain regions (Shin et al., 2010). The hippocampus, amygdala, and PFC form an intricate network known as the limbic system, the area mainly associated with the regulation of emotional response in stress conditions (Rajmohan et al., 2007; Tiger et al., 2008; Šimić et al., 2021). The circuitry system formed by these components is called “stress circuitry.” The hippocampus region expresses a comparatively high level of glucocorticoid and mineralocorticoid hormone receptors (Koning et al., 2019). Furthermore, the hippocampus, combined with other brain parts such as the amygdala, PFC, and brain nuclear stria terminalis, regulates stress circuitry in the brain (Rajmohan et al., 2007).
Although there are known physiological differences between male and female individuals, the difference in the serotonin receptor (5HT receptor) is crucial. Despite this difference, little information is available that specifies the function of serotonin receptors with stress and anxiety-dependent differences in male and female animals. For a better understanding of such differences, it is necessary to study serotonin receptors in detail, with special emphasis on sex-dependent anxiety differences. Unless we understand the biological differences between the brains of male and female individuals, developing better therapeutic
applications is challenging
Materials and Methods
Animals and groups
The research experiments were executed with healthy adult Sprague–Dawley (SD) rats aged 2–3 months. All the animals were kept under standard 12-h light/dark laboratory conditions, a temperature of 23b °C, and food and water ad libitum. The study included four animal groups (male control, female control, male CRS, and female CRS group). Regarding the behavior experiments, there were 10-12 animals in each group (half underwent an elevated plus maze [EPM] test while half took an open field test [OFT]), and these animals were not used for molecular study. Another group was used for molecular study using immunohistochemistry (IHC) and quantitative polymerase chain reaction (Q-PCR) (n=10 to 12 animals in each group, half used for IHC and the rest for PCR), and no OFT or EPM test was performed.
Chronic restrained stress (CRS) in rats
The CRS was performed on males and females with the use of 10 days of chronic restrained immobilization stress sessions, 6 h/d (8:00 AM to 2:00 PM) (Buynitsky et al., 2009). The immobilization process in CRS was done using a cone made up of polythene (8-10 inches). After a stress session, the OFT and EPM test was performed in animals to analyze the effect of stress on body physiology. After the final behavior experiment, the animals were perfused transcardially, and their brains and blood samples were collected during the perfusion of animals. The samples were stored at -80 °C for further molecular analysis (Figure 1A).
EPM test
The EPM was used to analyze the effect of stress or anxiety on body physiology in rats. The apparatus of EPM consists of four arms, of which two are open, and two closed (each 40×10 cm dimension), all joined at the center, thus creating a plus-shaped structure, elevated 50 cm above the ground and located in a room with low-intensity light. During the experiments, the rats were positioned at the center of the maze, and the face of the animal was positioned towards a closed arm. The animal’s activity was observed for up to 5 minutes and analyzed. The entries in open arms were analyzed as a percentage of open arm entries concerning the time spent in each arm during 5 minutes of exposure in all groups. The analysis for the result obtained by EPM was performed using any-maze analysis software (ANY-maze, Stoelting Co., US).
OFT
The OFT is used to analyze physiological conditions during animal stress and anxiety (Seibenhener et al., 2015). The OFT apparatus was done in a square box (120×120×60 cm) made of non-transparent material in a soundproof room. The arena was divided into inner central and outer areas (Seibenhener et al., 2015). All animals were positioned arbitrarily at one of the corners. These animals explored the arena for 10-15 min, and their behaviors were analyzed with the help of a monochromic camera situated on the top. The overall time used and the number of entries at the center arena were analyzed manually for complete session time.
Body weight
For identification and analysis of stress in these animals, they were weighted after each day of the stress session. Previous studies on stress suggest a continuously decreased body weight in experimental animals (Harris, 2015; Yau & Potenza, 2013).
Plasma corticosterone
Blood samples (about 1 mL) from the stress and control groups were collected through aortic puncture and transferred into ice-cooled centrifugal tubes. Blood was kept at room temperature for 1 hour for clotting in a collection tube, followed by centrifugation at 1700 ×g, for 10 min, at 4 °C to separate serum from blood. The serum was then stored at -80 °C freezer until further experiment.
Brain subregions
The whole amygdala (including BLA and CeA, bregma -2.6 to -3.2), hippocampus (including CA1, CA2, CA3, and DG, bregma -2.6 to -3.2), and PFC (including PL and IL, bregma 2.76 to 3.24) brain regions were used for the analysis of serotonin receptor expression through transcription analysis using Q-PCR and translation analysis using IHC. The brain area analyzed in this study is the key partner for emotional and cognitive responses (Stults-Kolehmainen & Sinha, 2014; Albert et al., 2014; McEwen et al., 2016).
Tissue preparation for IHC and transcription analysis
After stress experiments (2 h post-experiment), the rats were anesthetized by pentobarbital (60 mg/kg, IP) and were transcardially perfused with a normal saline solution. It was followed by the introduction of ice-cold 4% paraformaldehyde solution (in 0.1 M phosphate buffer solution, pH 7.4), brains removed after decapitation. Isolated brains were further post-fixed in a 4% paraformaldehyde solution for 24 h, followed by keeping them in sucrose solution (10%, 20%, and 30% solutions serially in 0.1 M phosphate buffer, pH 7.4). Then, the brains were frozen at -30 °C to -35 °C in isopentane solution for 30 min and stored in a deep freezer at -80 °C for IHC. For the transcription study, the rats were transcardially perfused with chilled normal saline solution only, and the brains were collected and stored in a deep freezer at -80 °C for Q-PCR.
Isolation of mRNA and cDNA preparation
The hippocampus, PFC, and amygdala regions were dissected from all groups for transcription analysis. The serotonin receptor mRNA expression was analyzed between male and female chronic restrained groups and their respective control groups. The sample size from each group was 5-6, which was used for the mRNA expression study.
The tissue from each animal (5 mg) was homogenized in 100 µL of cell lysis buffer and β-mercaptoethanol (0.7 µL).
From these tissue samples, total cellular RNA was isolated using a GeneJET RNA isolation kit (Thermo, Catalog No. K0731), followed by genomic DNA removal using DNase I treatment. The purity of RNA was analyzed using a spectrophotometer at 260/280 nm, around OD=1.75. cDNA was prepared from these isolated mRNA and oligo-dT primers using the RevertAid First Strand cDNA synthesis kit (Thermo, K1622). These generated cDNAs were further used as a template in real-time PCR amplification. The reaction mixture for PCR analysis was prepared in a final volume of 15 µL as follows: 5x reaction buffer (3 µL), dNTP mixture (1.5 µL), oligo dT primer (1 µL), reverse transcriptase (0.75 µL), RNase inhibitor (0.75 µL) and template RNA (3 µL). Reaction conditions for cDNA preparation were as follows: 60 min at 42 °C, 5 min at 70 °C and holding at 4 °C. The cDNA generated in this reaction was stored at -80 deep freezer.
Q-PCR
The level of serotonin receptor mRNA was analyzed using Q-PCR. mRNA expression analysis was performed in Stratagene Max-Pro Real-Time PCR detection System using maxima SYBR Green/ROX Master Mix (Thermo). The quantity of expression was represented as fold change through the Ct method. The primers sequences are as follows: c-fos, 5’-CCGACTCCTTCTCCAGCAT-3’ (forward), 5’ –TCACCGTGGGGATAAAGTTG-3’ (reverse); 5HT-1Br, 5`-GGAAAGTCCTGCTGGTTGCT-3` (forward), 5`- CGATCAGGTAGTTAGCCGGG-3` (reverse) and control GAPDH, 5`-AGTGCCAGCCTCGTCTCATA-3` (forward), 5`-TCCCGTTGATGACCAGCTTC-3` (reverse). Each study group had 5-6 samples analyzed in triplicate. The reaction mixture consists of 10 µL SYBR Green Master Mix (2x), forward and reverse primers (1µl each), cDNA (3 µL), and a final volume of 20 µL with ddH2O. The reaction condition used was as follows: Initial denaturation (95 °C, 10 min), denaturation (95 °C, 30 s), annealing temperature (57 °C, 30 s), and extension (72 °C, 30 s), total 45 cycles.
Immunohistochemistry (IHC)
The brain was sliced into 20-μm thick brain sections containing different brain regions such as PFC and hippocampus, and the serial sections were collected with the help of a cryostat (Microm HM 525, Germany). The collected sections were washed in PBS (phosphate-buffered saline) solution (0.01 M) and blocked in PBST (phosphate-buffered saline containing 1% Tween 20) containing 1% normal horse serum solution (VECTASTAIN kit). After blockage, the sections were incubated in anti-5HT-1Br (1:250 dilution, ASR-022, Thermo), anti-c-fos (1:500, cat. PA5-143600, Invitro) primary antibody, overnight at room temperature. Afterward, the sections were incubated for 2 h at room temperature in a biotinylated linked secondary antibody (1:500 dilution, Vector Lab.). Afterward, the sections were washed and incubated with avidin-biotinylated-peroxidase complex (VECTASTAIN Elite ABC Kit) and stained by the DAB staining solution (Vector lab.). After staining, the sections were mounted on glass slides and covered with a coverslip using DPX Mountant. The images from each section were acquired using a compound light microscope (Nikon). The level of serotonin receptor expression was analyzed as several positive neurons in different brain subregions using the NIS-Basic Research image analysis system (Nikon).
Statistical analysis
For statistical analysis, the data were presented as Mean±SD of the means and analyzed by analysis of variance (ANOVA; one-way, two-way) or student t-test using GraphPad Prism statistical software, version 7.
3. Results
Behavioral study
EPM test
Both male and female animals underwent 6 h of chronic stress training for 10 days (Figure 1A). All animals explored the environment, but anxiogenic factors caused a decreased exploration of the environment in open arms. Entries in the open arm were measured as the percentage of entries in the open arm for both groups for an overall duration of time. The Tukey post hoc analysis exhibited decreased entries in the open arm by the male and female chronic stress groups on day 10. However, control groups exhibited an enhanced open arm entry at day 10 compared to day 1 (F(3, 16)=21.15, P<0.001; Figure 1C). The statistical analysis exhibited a significant effect of chronic stress on body physiology in both the male and female CRS rats when compared with their respective control groups (Figure 1B).
OFT for anxiety measurement
When compared, the OFT showed significant differences between CRS and respective control groups. The time spent in the central area was longer for the control groups than for the CRS groups. The Tukey post hoc analysis suggested that the female CRS group exhibited comparatively fewer entries in the central field than the male CRS group (P<0.05). Here, the result suggested a significant change in the time spent in the central arena (F(3, 16)=19.42, P<0.001). Also, the number of entries in the central area is significantly lower in male and female CRS groups than in the control groups (Figure 1C).
Body weight measurement
Following the stress session, the body weight was measured in the CRS and control groups (every day). There was an alteration in mean body weight in all groups when comparing day 1 with day 10 (P<0.05). In the CRS groups (males and females), the body weight declined significantly and continuously from day 1 to day 10 (P<0.0001). However, the control groups (males and females) exhibited significantly enhanced body weight from day 1 to day 10 (P<0.0001; Tukey post hoc analysis). The test compared the first and last day trials of all groups. The result suggested a significant effect of stress (F(3, 15)=15.95, P<0.0001) and the interaction of stress with the number of trials (F(3, 15)=431.4, P<0.0001) with the body weight in these rats (Figure 1D).
Plasma corticosterone level
Theesponse caused by stress on the animal is measured by analyzing plasma corticosterone levels from their blood. The corticosterone in the male and female animals was higher under CRS than in the control animals; however, it was much higher in female rats than in the male animals under similar stress conditions. The result was confirmed by the statistical analysis using Tukey post hoc examination. The result revealed a significant outcome of gender (F(1, 4)=80.09, P=0.0009), stress (F(1, 4)=445.6, P<0.0001), and interaction effect of gender with the chronic stress (F(1, 4)=9.9, P=0.05) when corticosterone level was analyzed in the male and female rats under chronic stress (Figure 1E).
Transcriptional analysis
Amygdala
c-Fos mRNA expression
In the amygdala of the male and female rats, the c-fos expression was amplified significantly in chronic stress groups compared with their respective controls (P<0.05 and P<0.01, respectively) (Tukey post hoc analysis). Interestingly, the change in c-fos mRNA was significantly more in the female CRS group than in the male CRS group (P<0.05). The result exhibited significant outcomes of gender (F(1, 4)=26.25, P<0.01), stress (F(1, 4)=58.79, P<0.01), and the interaction of sex with the chronic stress (F(1, 4)=16.78, P<0.05) on c-fos mRNA expression in the amygdala compared to control groups (Figure 2B).
5HT-1B mRNa expression
The 5HT-1B expression showed a significant fold-change in 5HT-1B mRNA expression in male and female rats compared to their respective control groups. In male SD rats, the 5HT-1B mRNA was amplified (P<0.01) significantly, while in female rats, transcription diminished (P<0.01) in the CRS groups compared with their respective control groups, confirmed by the Tukey post hoc analysis. The result presented the significant effect of gender (F(1, 4)=21.31, P<0.01) and the interaction of sex with stress (F(1, 4)=156.3, P<0.001) for serotonin 5HT-1B mRNA expression in the amygdala region, compared to the control animals (Figure 2C).
mRNA expression in the prefrontal cortex
c-Fos mRNA expression
In the PFC of the male and female rats, the c-fos expression was significantly amplified in CRS groups compared to their respective control groups, confirmed by the Tukey post hoc analysis (P<0.01 and P<0.01, respectively). The c-fos expression was higher in the female stress group than in the male stress group (P<0.05). The outcome exhibited a significant consequence of stress (F(1, 4)=85.84, P<0.001) on c-fos mRNA expression in PFC (Figure 3B).
5HT-1B mRNA expression
In male and female SD rats, the 5HT-1B mRNA declined (P<0.01 and P<0.01, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. The result displayed the significant effect of chronic stress (F(1, 4)=97.04, P<0.001) and gender (F(1, 4)=326, P<0.0001) for the expression of 5HT-1B mRNA in the PFC. However, basal serotonin 5HT-1B receptor mRNA level was higher for the female group when compared with the male stress group (Figure 3C).
mRNA expression in the hippocampus
c-Fos mRNA expression
In the hippocampus of the male and female rats, the c-fos expression was significantly amplified in chronic stress groups compared to their respective control groups, confirmed by the Tukey post hoc analysis (P<0.05 and P<0.01, respectively).
However, the change in expression was significantly greater in female chronic stress groups than in male chronic stress group rats (P<0.05). The ANOVA revealed the significant effect of chronic stress (F(1, 4)=13.61, P<0.05), gender of animals (F(1, 4)=56.15, P<0.01), and the interaction of gender and the chronic stress condition (F(1, 4)=9.62, P<0.05) for the expression of c-fos mRNA in hippocampus region compared with the control (Figure 4B).
5HT-1B mRNA expression
In male and female SD rats, the 5HT-1B mRNA declined (P<0.01 and P<0.05, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. The ANOVA revealed the significant effect of chronic stress (F(1, 4)=32.61, P<0.01) and gender (F(1, 4)=133.1, P<0.001) for the expression of 5HT-1B mRNA in the hippocampus region when compared with the control. The 5HT-1B level was significantly lower in the female chronic stress group than in the male chronic stress group, suggesting a sex-dependent association of this receptor subtype in the hippocampus under stress conditions (Figure 4C).
Immunohistochemistry
c-Fos expression in amygdala
c-Fos protein expression was analyzed in the amygdala for both male and female groups to understand amygdala activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the c-fos level increased (P<0.001 and P<0.001, respectively) in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals significantly differed in c-fos levels when exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=24.71, P<0.01) and gender (F(1, 5)=54.05, P<0.001) on the expression of c-fos in the amygdala region when compared with the control. The c-fos level suggested a sex-dependent association of this receptor subtype in the amygdala under stress conditions (Figures 2D, and 2E).
Serotonin 5HT-1B receptor expression in the amygdala
The serotonin receptor 5HT-1B expression was analyzed in the amygdala between male and female animals. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male SD rats, the 5HT-1B level increased (P<0.05) in the CRS groups compared to their respective control animal groups. However, it declined in female rats (P<0.05) when exposed to stress, confirmed by the Tukey post hoc analysis. ANOVA revealed the significant effect of gender (F(1, 5)=40.98, P<0.01) and the interaction of chronic stress and gender (F(1, 5)=35.14, P<0.01) for the expression of 5HT-1B in the amygdala region when compared with the control. The 5HT-1B level suggested a sex-based association of this receptor subtype in the amygdala under stress conditions (Figures 2F, and 2G).
c-Fos expression in PFC
The c-fos expression in PFC between male and female animals was analyzed to understand PFC activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In the male and female SD rats, the c-fos level increased (P<0.05 and P<0.01, respectively) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals had a significant difference in c-fos levels when animals were exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=21.44, P<0.01), gender (F(1, 5)=39.89, P<0.01), and the interaction of chronic stress with gender (F(1, 5)=11.26, P<0.05) for the expression of c-fos in PFC region compared with the control. The c-fos level suggested a sex-dependent association of c-fos activity in PFC under stress conditions (Figures 3D, and 3E).
Serotonin 5HT-1B receptor expression in the PFC
The 5HT-1B expression was analyzed in the PFC between male and female animals to understand its activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In the male and female SD rats, the 5HT-1B level decreased (P<0.05 and P<0.05, respectively) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. ANOVA revealed the significant effect of chronic stress (F(1, 5)=70.25, P<0.001) and gender (F(1, 5)=7.6, P<0.05) for the expression of 5HT-1B in the PFC region compared with the control. The 5HT-1B level suggested a sex-dependent association of 5HT-1B activity in PFC under stress conditions (Figures 3F, and 3G).
c-Fos expression in the hippocampus
The c-fos expression was analyzed between male and female animals in the hippocampus to understand its activity under stress conditions. The CRS group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the c-fos level increased (P<0.05 and P<0.01, respectively) significantly in CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, male and female animals had a significant difference in c-fos levels when animals were exposed to stress (P<0.05). ANOVA revealed the significant effect of chronic stress (F(1, 5)=14.78, P<0.05), gender (F(1, 5)=25.85, P<0.01), and the interaction of chronic stress with gender (F(1, 5)=43.03, P<0.01) for the expression of c-fos in hippocampus region when compared with the control. The c-fos level suggested a sex-based association of c-fos activity in the hippocampus under stress conditions (Figures 4D, and 4E).
Serotonin 5HT-1B receptor expression in hippocampus
The 5HT-1B expression was analyzed between male and female animals in the hippocampus to understand its activity under stress conditions. The chronic restrained group of male and female rats revealed a significant variance compared to their control group animals. In male and female SD rats, the 5HT-1B level declined (P<0.05; P<0.05) significantly in the CRS groups compared with their respective control animal groups, confirmed by the Tukey post hoc analysis. However, a significant difference was observed among male and female animals for 5HT-1B level when animals were exposed to stress (P<0.05). Although both the stress groups exhibit a reduction in serotonin 5HT-1B receptor expression, the basal level for serotonin 5HT-1B receptor was lower in female stress groups (P<0.05). The ANOVA result revealed the significant effect of chronic stress (F(1, 5)=29.19, P<0.05) and gender (F(1, 5)=153.7, P<0.0001) for the expression of 5HT-1B in the hippocampus region when compared with the control. The 5HT-1B level suggested a sex-based association of 5HT-1B activity in the hippocampus under stress conditions (Figures 4F, and 4G).
4. Discussion
The current study investigated the role of the serotonin 5HT-1B receptor subtype on sex-dependent stress and anxiety variation in SD rats. Our rodent model-based study found a sex-based spatial function played by the serotonin 5HT-1B receptor subtype in CRS conditions among male and female SD rats. To better understand the difference between stress circuitry and involvement of the 5HT-1B receptor, we first investigated the activity of the amygdala, hippocampus, and PFC regions of the brain under CRS condition using c-fos expression (an IEG [immediate early gene] used as a neuronal activity marker gene) (Gallo et al., 2018). We observed a sex-dependent difference in activation patterns in these brain areas under CRS training.
The c-fos expression analysis confirmed activation of the hippocampus, PFC, and amygdala brain regions during chronic stress in male and female rats. However, the expression was comparatively higher in female CRS animals than in males, which correlated with a comparatively higher stress response in females. The result suggests that enhanced activity of trio brain areas (i.e. PFC, amygdala, and hippocampus) causes hyperactivation of stress circuitry in the female stress group compared to the male stress group. Although other regions, such as the hypothalamic-pituitary-adrenal axis, are associated with the stress circuitry, our primary focus was on trio partners due to their central role in emotional responses (McEwen et al., 2016). The stress circuitry involves the DRN, which innervates the amygdala, PFC, and hippocampus through direct and indirect neuronal connections (McEwen et al., 2016; Huang et al., 2019). Through its serotonergic innervation, DRN regulates the activity of the amygdala, PFC, and hippocampus regions under stressful conditions (Bocchio et al., 2016; Huang et al., 2019).
The current study hypothesized that the difference in serotonergic 5HT-1B receptor between both sexes might cause differential activation of these brain regions, resulting in differences in stress response (Figure 5).
We started our investigation by finding the potential role of serotonin receptors in CRS condition which affects the internal cellular and molecular difference between both the sexes. Our examination of different serotonin receptors suggested an interesting role of the 5HT-1B receptor subtype in both sexes, which correlated with the difference in stress responses.
It is observed through the current study that the expression of serotonin 5HT-1B receptor declined in the PFC and hippocampus in both male and female stress groups, but in the amygdala, it increased in the male CRS group and decreased in the female CRS group. Although basal 5HT-1B level in the amygdala was similar between both sexes, the hippocampus exhibited comparatively lower 5HT-1B expression in the female control group than in the male control group. Compared to males, the female CRS group exhibited comparatively lower 5HT-1B receptor expression in the hippocampus, which might be one of the reasons why females experience higher stress than males. Some of the recent animal studies have suggested an anxiolytic function for 5HT-1B receptor activation (Yohn et al., 2017; Tiger et al., 2018), which is also in line with our study results where decreased serotonin 5HT-1B receptor correlated with higher stress response. Likewise, the hippocampus, a key brain region regulating stress response (Levone et al., 2014; McEwen et al., 2016), exhibited a comparatively reduced 5HT-1B level in the female chronic stress group than the male stress group. The anti-anxiety activity of the serotonin 5HT-1B receptor might be the main reason why the hippocampus exhibited such a reduced 5HT-1B level in females than in male rats.
In the amygdala, the expression of the 5HT-1B receptor exhibited a different pattern among male and female rats. In the male CRS group, 5HT-1B expression increased; in females, the expression decreased significantly in the amygdala region. This dropping of the 5HT-1B receptor in the female amygdala might be the reason for higher amygdala activity in females under stressful conditions, as confirmed by c-fos expression. However, this differential expression for the 5HT-1B receptor did not correlate with stress response, as both sexes exhibited an enhanced stress response under the CRS condition. The result suggests a difference in intra-amygdala stress circuitry for similar stress conditions. This intra-amygdala difference of the 5HT-1B receptor might be crucial in determining the severity of stress between the sexes. Furthermore, a decreased 5HT-1B expression in the amygdala could not maintain the 5HT-1B level in the hippocampus, resulting in decreased 5HT-1B expression, causing a higher stress response in female rats. Contrary to this, an increased 5HT-1B expression in the male amygdala instigated moderate hippocampus inhibition, resulting in a comparatively lower stress response.
Overall, the male CRS group exhibited direct neuronal connectivity between the amygdala and hippocampus, where an enhanced 5HT-1B expression in the amygdala inhibited 5HT-1B expression in the hippocampus as this serotonergic receptor is inhibitory and functions by decreasing the release of other neurotransmitters through postsynaptic neurons once activated (Ciranna, 2006; Tiger et al., 2018). Moreover, in female rats, the system exhibited an indirect serotonergic receptor-based stress circuitry where a decreased 5HT-1B expression in the amygdala might activate those neurons that inhibit 5HT-1B receptor expression on postsynaptic neurons in the hippocampus.
The result further suggests that PFC modulated the hippocampus activity by regulating amygdala activity. Under the CRS condition, PFC was active in both sexes, but the female group exhibited relatively higher activation. Although this PFC activation correlated with decreased 5HT-1B levels in the PFC of both animals, the female chronic stress group revealed significantly lower expression compared to its control group. This difference caused comparatively lower 5HT-1B levels and increased amygdala activity in female animals. This enhanced amygdala activity in females caused a decreased 5HT-1B level in the hippocampus and increased hippocampal activity, causing a higher stress response in the female CRS groups.
Contrary to this, comparatively lower amygdala activity in male animals was correlated with an increased 5HT-1B expression, resulting in enhanced hippocampal activity. The hippocampal activity exhibited a sex-dependent variation for stress response and the 5HT-1B expression. The current study proposed a sex-dependent variation in stress circuitry, where, more interestingly, an inhibitory network in the female hippocampus might be involved in regulating hippocampus activity under stress. As a result of decreased 5HT-1B receptor expression, serotonergic neurons might not be able to disinhibit 5HT-1B receptor expression in the hippocampus, causing higher activation of the hippocampus and, finally, a higher stress in females.
This sex-dependent variation in anxiety and stress character might be an evolutionary response acquired differently by the females and males based on differences in environmental contingencies. As we know, by the start of human civilization, the condition of women in our society was not so good due to the dominance of males over females (Zhu & Chang, 2019; Martin et al., 2009). However, the condition of women has improved in our society nowadays, but not as much as men’s. The external environmental factors and internal molecular biology of the brain affect our life’s functionality and behavioral aspects equally.
In conclusion, a sex-dependent difference in the stress circuitry among male and female rats incorporating the serotonin 5HT-1B receptor system may play an important role in regulating this circuitry. The anatomical and neural circuitry-based differences between both sexes are important aspects for the characterization of stress and anxiety.
Some important outcomes from the study include the following. First, male and female individuals have an internal neuronal difference in stress circuitry. Second, the serotonin 5HT-1B receptor is accompanied by anti-anxiety and anti-stress functions irrespective of the sex of the individual. Third, the stress circuitry in females is more complex than in males. Fourth, the variation in stress response among male and female animals is associated with the difference in serotonin 5HT-1B receptor-based stress circuitry. Overall, the difference in the serotonin receptor system between males and females is of great importance, which promises the development of a better therapeutic strategy for stress disorders, keeping in mind the importance of sex differences. This research is the first study where we studied a spatial association of serotonin receptors in stressful situations among male and female animals. Future research incorporating other serotonin receptors linked with sex-dependent stress and anxiety will help characterize this condition better and develop potent therapeutic approaches, including pharmacological intervention.
Ethical Considerations
Compliance with ethical guidelines
The study experiments were approved by the Institutional Animal Ethics Committee, King George’s Medical University, Lucknow, India (Code: 169/IAEC/2022).
Funding
The research was supported by the Indian Council of Medical Research, New Delhi, India (Grant No.: 2021-1133 under the ICMR-DHR scheme).
Authors' contributions
Conceptualization, investigation, writing the original draft and funding acquisition: Sarfraj Ahmad Siddiqui; Methodology, software and validation: Sarfraj Ahmad Siddiqui and Sanjay Singh; Formal Analysis: Sanjay Singh and Md Arshad; Data curation and resources: Sarfraj Ahmad Siddiqui and Md Arshad; Visualization, supervision, project administration, review and editing: Sanjay Singh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank DHR-ICMR Young Scientist under the HRD Scheme in New Delhi, India (Proposal No. 2021-1133) for the current work.
References
Akimova, E., Lanzenberger, R., & Kasper, S. (2009). The serotonin-1A receptor in anxiety disorders. Biological Psychiatry, 66(7), 627–635. [DOI:10.1016/j.biopsych.2009.03.012] [PMID]
Albert, P. R., Vahid-Ansari, F., & Luckhart, C. (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Frontiers in Behavioral Neuroscience, 8, 199. [DOI:10.3389/fnbeh.2014.00199] [PMID]
Bacqué-Cazenave, J., Bharatiya, R., Barrière, G., Delbecque, J. P., Bouguiyoud, N., & Di Giovanni, G., et al. (2020). Serotonin in animal cognition and behavior. International Journal of Molecular Sciences, 21(5), 1649. [DOI:10.3390/ijms21051649] [PMID]
Bahrami, F., & Yousefi, N. (2011). Females are more anxious than males: A metacognitive perspective. Iranian Journal of Psychiatry and Behavioral Sciences, 5(2), 83–90. [PMID]
Berumen, L. C., Rodríguez, A., Miledi, R., & García-Alcocer, G. (2012). Serotonin receptors in hippocampus. TheScientificWorldJournal, 2012, 823493. [DOI:10.1100/2012/823493] [PMID]
Bhatnagar, S., Nowak, N., Babich, L., & Bok, L. (2004). Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behavioural Brain Research, 153(2), 527–535. [DOI:10.1016/j.bbr.2004.01.018] [PMID]
Bocchio, M., McHugh, S. B., Bannerman, D. M., Sharp, T., & Capogna, M. (2016). Serotonin, Amygdala and Fear: Assembling the Puzzle. Frontiers in Neural Circuits, 10, 24. [DOI:10.3389/fncir.2016.00024] [PMID]
Buynitsky, T., & Mostofsky, D. I. (2009). Restraint stress in biobehavioral research: Recent developments. Neuroscience and Biobehavioral Reviews, 33(7), 1089–1098. [DOI:10.1016/j.neubiorev.2009.05.004] [PMID]
Charnay, Y., & Léger, L. (2010). Brain serotonergic circuitries. Dialogues in Clinical Neuroscience, 12(4), 471–487. [DOI:10.31887/DCNS.2010.12.4/ycharnay] [PMID]
Ciranna L. (2006). Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: Implications in physiological functions and in pathology. Current Neuropharmacology, 4(2), 101–114. [DOI:10.2174/157015906776359540] [PMID]
Gallo, F. T., Katche, C., Morici, J. F., Medina, J. H., & Weisstaub, N. V. (2018). Immediate early genes, memory and psychiatric disorders: Focus on c-Fos, Egr1 and Arc. Frontiers in Behavioral Neuroscience, 12, 79. [DOI:10.3389/fnbeh.2018.00079] [PMID]
Gino, F., Wilmuth, C. A., & Brooks, A. W. (2015). Compared to men, women view professional advancement as equally attainable, but less desirable. Proceedings of the National Academy of Sciences of the United States of America, 112(40), 12354–12359. [DOI:10.1073/pnas.1502567112] [PMID]
Harris R. B. (2015). Chronic and acute effects of stress on energy balance: Are there appropriate animal models? American Journal of Physiology, 308(4), R250–R265. [DOI:10.1152/ajpregu.00361.2014] [PMID]
Huang, K. W., Ochandarena, N. E., Philson, A. C., Hyun, M., Birnbaum, J. E., & Cicconet, M., et al. (2019). Molecular and anatomical organization of the dorsal raphe nucleus. eLife, 8, e46464. [DOI:10.7554/eLife.46464] [PMID]
Jenkins, T. A., Nguyen, J. C., Polglaze, K. E., & Bertrand, P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients, 8(1), 56. [DOI:10.3390/nu8010056] [PMID]
Karayol, R., Medrihan, L., Warner-Schmidt, J. L., Fait, B. W., Rao, M. N., & Holzner, E. B., et al. (2021). Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Molecular Psychiatry, 26(6), 2334–2349. [DOI:10.1038/s41380-020-00994-y] [PMID]
Kimura, A., Stevenson, P. L., Carter, R. N., Maccoll, G., French, K. L., & Simons, J. P., et al. (2009). Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. The European Journal of Neuroscience, 30(2), 299–306. [DOI:10.1111/j.1460-9568.2009.06831.x] [PMID]
Koning, A. C. A. M., Buurstede, J. C., van Weert, L. T. C. M., & Meijer, O. C. (2019). Glucocorticoid and mineralocorticoid receptors in the brain: A transcriptional perspective. Journal of the Endocrine Society, 3(10), 1917–1930. [DOI:10.1210/js.2019-00158] [PMID]
Levone, B. R., Cryan, J. F., & O'Leary, O. F. (2014). Role of adult hippocampal neurogenesis in stress resilience. Neurobiology of Stress, 1, 147–155. [DOI:10.1016/j.ynstr.2014.11.003] [PMID]
Martin, E. I., Ressler, K. J., Binder, E., & Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: Brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America, 32(3), 549–575. [DOI:10.1016/j.psc.2009.05.004] [PMID]
McEwen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23. [DOI:10.1038/npp.2015.171] [PMID]
Osredkar, D., Krzan, M. (2009). Expression of serotonin receptor subtypes in rat brain and astrocyte cell cultures: an age and tissue-dependent process. Periodicum Biologorum, 111(1), 129-135. [Link]
Overstreet, D. H., Commissaris, R. C., De La Garza, R., 2nd, File, S. E., Knapp, D. J., & Seiden, L. S. (2003). Involvement of 5-HT1A receptors in animal tests of anxiety and depression: Evidence from genetic models. Stress, 6(2), 101–110. [DOI:10.1080/1025389031000111311] [PMID]
Pithadia, A. B., & Jain, S. M. (2009). 5-hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. Journal of Clinical Medicine Research, 1(2), 72–80. [DOI:10.4021/jocmr2009.05.1237] [PMID]
Rajmohan, V., & Mohandas, E. (2007). The limbic system. Indian Journal of Psychiatry, 49(2), 132–139. [DOI:10.4103/0019-5545.33264] [PMID]
Schneiderman, N., Ironson, G., & Siegel, S. D. (2005). Stress and health: psychological, behavioral, and biological determinants. Annual Review of clinical psychology, 1, 607–628. [DOI:10.1146/annurev.clinpsy.1.102803.144141] [PMID]
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. [DOI:10.1037/0033-2909.130.4.601] [PMID]
Seibenhener, M. L., & Wooten, M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, (96), e52434. [DOI:10.3791/52434] [PMID]
Shin, L. M., & Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. [DOI:10.1038/npp.2009.83] [PMID]
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., & Šagud, M., et al. (2021). Understanding Emotions: Origins and roles of the amygdala. Biomolecules, 11(6), 823. [DOI:10.3390/biom11060823] [PMID]
Stults-Kolehmainen, M. A., & Sinha, R. (2014). The effects of stress on physical activity and exercise. Sports Medicine, 44(1), 81–121. [DOI:10.1007/s40279-013-0090-5] [PMID]
Švob Štrac, D., Pivac, N., & Mück-Šeler, D. (2016). The serotonergic system and cognitive function. Translational Neuroscience, 7(1), 35–49. [DOI:10.1515/tnsci-2016-0007] [PMID]
Tiger, M., Varnäs, K., Okubo, Y., & Lundberg, J. (2018). The 5-HT1B receptor-A potential target for antidepressant treatment. Psychopharmacology, 235(5), 1317–1334. [DOI:10.1007/s00213-018-4872-1] [PMID]
Verma, R., Balhara, Y. P., & Gupta, C. S. (2011). Gender differences in stress response: Role of developmental and biological determinants. Industrial Psychiatry Journal, 20(1), 4–10. [DOI:10.4103/0972-6748.98407] [PMID]
Xiang, M., Jiang, Y., Hu, Z., Yang, Y., Du, X., Botchway, B. O., et al. (2019). Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. American Journal of Translational Research, 11(4), 2288–2303. [PMID]
Yang, L., Zhao, Y., Wang, Y., Liu, L., Zhang, X., & Li, B., et al. (2015). The effects of psychological stress on depression. Current Neuropharmacology, 13(4), 494–504. [DOI:10.2174/1570159X1304150831150507] [PMID]
Yau, Y. H., & Potenza, M. N. (2013). Stress and eating behaviors. Minerva Endocrinologica, 38(3), 255–267.
Yohn, C. N., Gergues, M. M., & Samuels, B. A. (2017). The role of 5-HT receptors in depression. Molecular Brain, 10(1), 28. [DOI:10.1186/s13041-017-0306-y] [PMID]
Zhu, N., & Chang, L. (2019). Evolved but not fixed: A life history account of gender roles and gender inequality. Frontiers in Psychology, 10, 1709. [DOI:10.3389/fpsyg.2019.01709] [PMID]
It is observed through the current study that the expression of serotonin 5HT-1B receptor declined in the PFC and hippocampus in both male and female stress groups, but in the amygdala, it increased in the male CRS group and decreased in the female CRS group. Although basal 5HT-1B level in the amygdala was similar between both sexes, the hippocampus exhibited comparatively lower 5HT-1B expression in the female control group than in the male control group. Compared to males, the female CRS group exhibited comparatively lower 5HT-1B receptor expression in the hippocampus, which might be one of the reasons why females experience higher stress than males. Some of the recent animal studies have suggested an anxiolytic function for 5HT-1B receptor activation (Yohn et al., 2017; Tiger et al., 2018), which is also in line with our study results where decreased serotonin 5HT-1B receptor correlated with higher stress response. Likewise, the hippocampus, a key brain region regulating stress response (Levone et al., 2014; McEwen et al., 2016), exhibited a comparatively reduced 5HT-1B level in the female chronic stress group than the male stress group. The anti-anxiety activity of the serotonin 5HT-1B receptor might be the main reason why the hippocampus exhibited such a reduced 5HT-1B level in females than in male rats.
In the amygdala, the expression of the 5HT-1B receptor exhibited a different pattern among male and female rats. In the male CRS group, 5HT-1B expression increased; in females, the expression decreased significantly in the amygdala region. This dropping of the 5HT-1B receptor in the female amygdala might be the reason for higher amygdala activity in females under stressful conditions, as confirmed by c-fos expression. However, this differential expression for the 5HT-1B receptor did not correlate with stress response, as both sexes exhibited an enhanced stress response under the CRS condition. The result suggests a difference in intra-amygdala stress circuitry for similar stress conditions. This intra-amygdala difference of the 5HT-1B receptor might be crucial in determining the severity of stress between the sexes. Furthermore, a decreased 5HT-1B expression in the amygdala could not maintain the 5HT-1B level in the hippocampus, resulting in decreased 5HT-1B expression, causing a higher stress response in female rats. Contrary to this, an increased 5HT-1B expression in the male amygdala instigated moderate hippocampus inhibition, resulting in a comparatively lower stress response.
Overall, the male CRS group exhibited direct neuronal connectivity between the amygdala and hippocampus, where an enhanced 5HT-1B expression in the amygdala inhibited 5HT-1B expression in the hippocampus as this serotonergic receptor is inhibitory and functions by decreasing the release of other neurotransmitters through postsynaptic neurons once activated (Ciranna, 2006; Tiger et al., 2018). Moreover, in female rats, the system exhibited an indirect serotonergic receptor-based stress circuitry where a decreased 5HT-1B expression in the amygdala might activate those neurons that inhibit 5HT-1B receptor expression on postsynaptic neurons in the hippocampus.
The result further suggests that PFC modulated the hippocampus activity by regulating amygdala activity. Under the CRS condition, PFC was active in both sexes, but the female group exhibited relatively higher activation. Although this PFC activation correlated with decreased 5HT-1B levels in the PFC of both animals, the female chronic stress group revealed significantly lower expression compared to its control group. This difference caused comparatively lower 5HT-1B levels and increased amygdala activity in female animals. This enhanced amygdala activity in females caused a decreased 5HT-1B level in the hippocampus and increased hippocampal activity, causing a higher stress response in the female CRS groups.
Contrary to this, comparatively lower amygdala activity in male animals was correlated with an increased 5HT-1B expression, resulting in enhanced hippocampal activity. The hippocampal activity exhibited a sex-dependent variation for stress response and the 5HT-1B expression. The current study proposed a sex-dependent variation in stress circuitry, where, more interestingly, an inhibitory network in the female hippocampus might be involved in regulating hippocampus activity under stress. As a result of decreased 5HT-1B receptor expression, serotonergic neurons might not be able to disinhibit 5HT-1B receptor expression in the hippocampus, causing higher activation of the hippocampus and, finally, a higher stress in females.
This sex-dependent variation in anxiety and stress character might be an evolutionary response acquired differently by the females and males based on differences in environmental contingencies. As we know, by the start of human civilization, the condition of women in our society was not so good due to the dominance of males over females (Zhu & Chang, 2019; Martin et al., 2009). However, the condition of women has improved in our society nowadays, but not as much as men’s. The external environmental factors and internal molecular biology of the brain affect our life’s functionality and behavioral aspects equally.
In conclusion, a sex-dependent difference in the stress circuitry among male and female rats incorporating the serotonin 5HT-1B receptor system may play an important role in regulating this circuitry. The anatomical and neural circuitry-based differences between both sexes are important aspects for the characterization of stress and anxiety.
Some important outcomes from the study include the following. First, male and female individuals have an internal neuronal difference in stress circuitry. Second, the serotonin 5HT-1B receptor is accompanied by anti-anxiety and anti-stress functions irrespective of the sex of the individual. Third, the stress circuitry in females is more complex than in males. Fourth, the variation in stress response among male and female animals is associated with the difference in serotonin 5HT-1B receptor-based stress circuitry. Overall, the difference in the serotonin receptor system between males and females is of great importance, which promises the development of a better therapeutic strategy for stress disorders, keeping in mind the importance of sex differences. This research is the first study where we studied a spatial association of serotonin receptors in stressful situations among male and female animals. Future research incorporating other serotonin receptors linked with sex-dependent stress and anxiety will help characterize this condition better and develop potent therapeutic approaches, including pharmacological intervention.
Ethical Considerations
Compliance with ethical guidelines
The study experiments were approved by the Institutional Animal Ethics Committee, King George’s Medical University, Lucknow, India (Code: 169/IAEC/2022).
Funding
The research was supported by the Indian Council of Medical Research, New Delhi, India (Grant No.: 2021-1133 under the ICMR-DHR scheme).
Authors' contributions
Conceptualization, investigation, writing the original draft and funding acquisition: Sarfraj Ahmad Siddiqui; Methodology, software and validation: Sarfraj Ahmad Siddiqui and Sanjay Singh; Formal Analysis: Sanjay Singh and Md Arshad; Data curation and resources: Sarfraj Ahmad Siddiqui and Md Arshad; Visualization, supervision, project administration, review and editing: Sanjay Singh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank DHR-ICMR Young Scientist under the HRD Scheme in New Delhi, India (Proposal No. 2021-1133) for the current work.
References
Akimova, E., Lanzenberger, R., & Kasper, S. (2009). The serotonin-1A receptor in anxiety disorders. Biological Psychiatry, 66(7), 627–635. [DOI:10.1016/j.biopsych.2009.03.012] [PMID]
Albert, P. R., Vahid-Ansari, F., & Luckhart, C. (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Frontiers in Behavioral Neuroscience, 8, 199. [DOI:10.3389/fnbeh.2014.00199] [PMID]
Bacqué-Cazenave, J., Bharatiya, R., Barrière, G., Delbecque, J. P., Bouguiyoud, N., & Di Giovanni, G., et al. (2020). Serotonin in animal cognition and behavior. International Journal of Molecular Sciences, 21(5), 1649. [DOI:10.3390/ijms21051649] [PMID]
Bahrami, F., & Yousefi, N. (2011). Females are more anxious than males: A metacognitive perspective. Iranian Journal of Psychiatry and Behavioral Sciences, 5(2), 83–90. [PMID]
Berumen, L. C., Rodríguez, A., Miledi, R., & García-Alcocer, G. (2012). Serotonin receptors in hippocampus. TheScientificWorldJournal, 2012, 823493. [DOI:10.1100/2012/823493] [PMID]
Bhatnagar, S., Nowak, N., Babich, L., & Bok, L. (2004). Deletion of the 5-HT3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behavioural Brain Research, 153(2), 527–535. [DOI:10.1016/j.bbr.2004.01.018] [PMID]
Bocchio, M., McHugh, S. B., Bannerman, D. M., Sharp, T., & Capogna, M. (2016). Serotonin, Amygdala and Fear: Assembling the Puzzle. Frontiers in Neural Circuits, 10, 24. [DOI:10.3389/fncir.2016.00024] [PMID]
Buynitsky, T., & Mostofsky, D. I. (2009). Restraint stress in biobehavioral research: Recent developments. Neuroscience and Biobehavioral Reviews, 33(7), 1089–1098. [DOI:10.1016/j.neubiorev.2009.05.004] [PMID]
Charnay, Y., & Léger, L. (2010). Brain serotonergic circuitries. Dialogues in Clinical Neuroscience, 12(4), 471–487. [DOI:10.31887/DCNS.2010.12.4/ycharnay] [PMID]
Ciranna L. (2006). Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: Implications in physiological functions and in pathology. Current Neuropharmacology, 4(2), 101–114. [DOI:10.2174/157015906776359540] [PMID]
Gallo, F. T., Katche, C., Morici, J. F., Medina, J. H., & Weisstaub, N. V. (2018). Immediate early genes, memory and psychiatric disorders: Focus on c-Fos, Egr1 and Arc. Frontiers in Behavioral Neuroscience, 12, 79. [DOI:10.3389/fnbeh.2018.00079] [PMID]
Gino, F., Wilmuth, C. A., & Brooks, A. W. (2015). Compared to men, women view professional advancement as equally attainable, but less desirable. Proceedings of the National Academy of Sciences of the United States of America, 112(40), 12354–12359. [DOI:10.1073/pnas.1502567112] [PMID]
Harris R. B. (2015). Chronic and acute effects of stress on energy balance: Are there appropriate animal models? American Journal of Physiology, 308(4), R250–R265. [DOI:10.1152/ajpregu.00361.2014] [PMID]
Huang, K. W., Ochandarena, N. E., Philson, A. C., Hyun, M., Birnbaum, J. E., & Cicconet, M., et al. (2019). Molecular and anatomical organization of the dorsal raphe nucleus. eLife, 8, e46464. [DOI:10.7554/eLife.46464] [PMID]
Jenkins, T. A., Nguyen, J. C., Polglaze, K. E., & Bertrand, P. P. (2016). Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients, 8(1), 56. [DOI:10.3390/nu8010056] [PMID]
Karayol, R., Medrihan, L., Warner-Schmidt, J. L., Fait, B. W., Rao, M. N., & Holzner, E. B., et al. (2021). Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Molecular Psychiatry, 26(6), 2334–2349. [DOI:10.1038/s41380-020-00994-y] [PMID]
Kimura, A., Stevenson, P. L., Carter, R. N., Maccoll, G., French, K. L., & Simons, J. P., et al. (2009). Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. The European Journal of Neuroscience, 30(2), 299–306. [DOI:10.1111/j.1460-9568.2009.06831.x] [PMID]
Koning, A. C. A. M., Buurstede, J. C., van Weert, L. T. C. M., & Meijer, O. C. (2019). Glucocorticoid and mineralocorticoid receptors in the brain: A transcriptional perspective. Journal of the Endocrine Society, 3(10), 1917–1930. [DOI:10.1210/js.2019-00158] [PMID]
Levone, B. R., Cryan, J. F., & O'Leary, O. F. (2014). Role of adult hippocampal neurogenesis in stress resilience. Neurobiology of Stress, 1, 147–155. [DOI:10.1016/j.ynstr.2014.11.003] [PMID]
Martin, E. I., Ressler, K. J., Binder, E., & Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: Brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America, 32(3), 549–575. [DOI:10.1016/j.psc.2009.05.004] [PMID]
McEwen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23. [DOI:10.1038/npp.2015.171] [PMID]
Osredkar, D., Krzan, M. (2009). Expression of serotonin receptor subtypes in rat brain and astrocyte cell cultures: an age and tissue-dependent process. Periodicum Biologorum, 111(1), 129-135. [Link]
Overstreet, D. H., Commissaris, R. C., De La Garza, R., 2nd, File, S. E., Knapp, D. J., & Seiden, L. S. (2003). Involvement of 5-HT1A receptors in animal tests of anxiety and depression: Evidence from genetic models. Stress, 6(2), 101–110. [DOI:10.1080/1025389031000111311] [PMID]
Pithadia, A. B., & Jain, S. M. (2009). 5-hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. Journal of Clinical Medicine Research, 1(2), 72–80. [DOI:10.4021/jocmr2009.05.1237] [PMID]
Rajmohan, V., & Mohandas, E. (2007). The limbic system. Indian Journal of Psychiatry, 49(2), 132–139. [DOI:10.4103/0019-5545.33264] [PMID]
Schneiderman, N., Ironson, G., & Siegel, S. D. (2005). Stress and health: psychological, behavioral, and biological determinants. Annual Review of clinical psychology, 1, 607–628. [DOI:10.1146/annurev.clinpsy.1.102803.144141] [PMID]
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. [DOI:10.1037/0033-2909.130.4.601] [PMID]
Seibenhener, M. L., & Wooten, M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, (96), e52434. [DOI:10.3791/52434] [PMID]
Shin, L. M., & Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. [DOI:10.1038/npp.2009.83] [PMID]
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., & Šagud, M., et al. (2021). Understanding Emotions: Origins and roles of the amygdala. Biomolecules, 11(6), 823. [DOI:10.3390/biom11060823] [PMID]
Stults-Kolehmainen, M. A., & Sinha, R. (2014). The effects of stress on physical activity and exercise. Sports Medicine, 44(1), 81–121. [DOI:10.1007/s40279-013-0090-5] [PMID]
Švob Štrac, D., Pivac, N., & Mück-Šeler, D. (2016). The serotonergic system and cognitive function. Translational Neuroscience, 7(1), 35–49. [DOI:10.1515/tnsci-2016-0007] [PMID]
Tiger, M., Varnäs, K., Okubo, Y., & Lundberg, J. (2018). The 5-HT1B receptor-A potential target for antidepressant treatment. Psychopharmacology, 235(5), 1317–1334. [DOI:10.1007/s00213-018-4872-1] [PMID]
Verma, R., Balhara, Y. P., & Gupta, C. S. (2011). Gender differences in stress response: Role of developmental and biological determinants. Industrial Psychiatry Journal, 20(1), 4–10. [DOI:10.4103/0972-6748.98407] [PMID]
Xiang, M., Jiang, Y., Hu, Z., Yang, Y., Du, X., Botchway, B. O., et al. (2019). Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. American Journal of Translational Research, 11(4), 2288–2303. [PMID]
Yang, L., Zhao, Y., Wang, Y., Liu, L., Zhang, X., & Li, B., et al. (2015). The effects of psychological stress on depression. Current Neuropharmacology, 13(4), 494–504. [DOI:10.2174/1570159X1304150831150507] [PMID]
Yau, Y. H., & Potenza, M. N. (2013). Stress and eating behaviors. Minerva Endocrinologica, 38(3), 255–267.
Yohn, C. N., Gergues, M. M., & Samuels, B. A. (2017). The role of 5-HT receptors in depression. Molecular Brain, 10(1), 28. [DOI:10.1186/s13041-017-0306-y] [PMID]
Zhu, N., & Chang, L. (2019). Evolved but not fixed: A life history account of gender roles and gender inequality. Frontiers in Psychology, 10, 1709. [DOI:10.3389/fpsyg.2019.01709] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2022/05/15 | Accepted: 2024/04/13 | Published: 2025/01/1
Received: 2022/05/15 | Accepted: 2024/04/13 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |