Volume 15, Issue 3 (May & Jun 2024)
BCN 2024, 15(3): 273-286 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Noroozian M, Shakiba A, Mohammadian F. Effect of Controlling the Cardiovascular Risk Factors on the Cognitive Decline Prevention in the Elderly: A Systematic Review. BCN 2024; 15 (3) :273-286
URL: http://bcn.iums.ac.ir/article-1-2391-en.html
URL: http://bcn.iums.ac.ir/article-1-2391-en.html
1- Department of Psychiatry, Roozbeh Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 683 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
The increased life expectancy throughout the world has led to a rise in the number of people affected by chronic diseases, which have become major health challenges (Brown, 2015). Evidence suggests that many chronic diseases in the elderly share common risk factors. Among these, dementia and cognitive impairment are the most prevalent causes of morbidity and mortality in the elderly. Cognitive decline in older adults is a major public health problem and can diminish independence and quality of life (Livingston et al., 2020). Approximately 47 million people worldwide were affected by dementia in 2015, a number that is expected to triple by 2050 (Patterson & International, 2018). In the absence of a cure for the disease or treatment, reducing the risk of dementia is doubly important (Cummings et al., 2016). Even in cases where effective treatments are available, reducing the risk of disease occurrence remains a fundamental solution. For many non-communicable diseases with existing treatments, such as diabetes, cancer, and heart disease, risk reduction plays a vital role in disease prevention (Bloom et al., 2017).
The main risk factors for the onset of Alzheimer’s disease and other dementias are age, family history, and predisposed genes, such as apolipoprotein E allele ε4 (Hsiung & Sadovnick, 2007). However, none of these risk factors can be altered or modified by medical interventions or individual behavior. There is ample evidence supporting the association between multiple variable risk factors and decreased cognitive decline risk, as discussed in this review (Baumgart et al., 2015. Vascular risk factors are increasingly recognized as significant contributors to dementia and are thus targets for future treatments. Vascular risk factors in middle age appear to be most closely associated with cognitive decline in old age (Whitmer et al., 2005). The US National Institutes of Health emphasizes that diabetes mellitus, smoking, depression, mental or physical inactivity, and poor diet are related to the risk of cognitive decline. This list has expanded to include high blood pressure, obesity, and low education (Barnes & Yaffe, 2011). While the association between high blood pressure and AD risk is complex and age-dependent, some evidence suggests that in middle-aged, but not older, populations, blood pressure is associated with a 50% increase in AD and dementia risk (Lee et al., 2022). High blood pressure can increase the risk of AD by reducing the vascular integrity of the blood-brain barrier, which leads to extravasation or leakage of protein into brain tissue, which in turn leads to cell damage, apoptosis, and increased Aβ accumulation. However, the direct causal relationship between blood pressure and subsequent cognitive decline is debatable, as there is also emerging evidence suggesting that blood pressure may represent a protective response to cerebral hypoperfusion, evident a decade before AD onset (Corrada et al., 2017). According to the obesity epidemic and growing evidence of the relationship between body mass index (BMI) and cognition, several studies have found that being overweight or obese were independent risk factors for cognitive decline (Doruk et al., 2010; Lee et al., 2010; Naderali et al., 2009; Nilsson & Nilsson, 2009). Obese individuals exhibit smaller whole brain and total gray matter volume than those with normal weight (Gunstad et al., 2008). On the other hand, there is a U-shaped relationship between weight and cognitive function: Both low and high weight are associated with a heightened risk of AD and cognitive impairment. This relationship may also exhibit an age-related component (Bae & Park, 2021). Conversely, there is data suggesting an inverse relationship in the years preceding disease onset; for instance, weight loss, which may result from cognitive deficits during the pre-dementia phase of AD (Luchsinger et al., 2007). Diabetes has been demonstrated to directly increase dementia risk by impacting Aβ accumulation in the brain. Other studies indicate that diabetes may elevate the risk of cerebrovascular disease but not AD pathology (Biessels & Despa, 2018). Despite proper diabetes control being approved and recommended for preventing most diabetes-related diseases, its effect on preventing or delaying the onset of dementia remains unclear (Ravona-Springer & Schnaider-Beeri, 2011). In addition to glycemic control, several factors associated with diabetes may interact with dementia clinical manifestations and neuropathology, as well as the rate of functional and cognitive decline (Biessels et al., 2008). A review study discovered that severe hypoglycemia did not benefit young people with type 1 diabetes in terms of cognitive function, whereas older individuals with type 2 diabetes benefited from treatment in terms of information processing speed and executive function (Moheet et al., 2015). Systematic reviews and prospective studies on the association between cholesterol levels in middle age, old age, and dementia have yielded mixed results, including no association between cholesterol levels and vascular dementia (Park et al., 2013). While some observational studies have indicated that statin use to control cholesterol levels reduces dementia risk, a review in Cochrane (McGuinness et al., 2016) and other review studies found no evidence supporting the reduction of dementia risk through statin use.
2. Materials and Methods
Search strategy
This review was conducted according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (Moher et al., 2009). A systematic search of scientific article databases, including PubMed, Scopus, Google Scholar, SCIELO, and Cochrane Central was performed using the appropriate keywords and search protocol for each database. For example, in the PubMed database, relevant search keywords and strategies were determined based on MeSH terms.
The inclusion criteria encompassed clinical studies published in English, targeting elderly populations and intervening to modify at least one cardiovascular risk factor (such as diabetes, dyslipidemia, hypertension, sedentary lifestyle, and obesity), with cognitive status evaluated as an outcome measure. The general search model employed phrases such as “intervention” OR “modification” OR “modify” OR “control” OR “change” in combination with terms like “vascular” OR “hypertension” OR “hyperlipidemia” OR “dyslipidemia” OR “diabetes” OR “obesity” OR “overweight,” along with terms like “cognitive” OR “memory” OR “dementia” OR “mind,” excluding non-human studies. Studies published after 2000 were accepted. This search strategy was then adapted to the characteristics of other databases. We described the search strategy in Figure 1 in detail. The search terms with similar meanings were combined using the OR logic, and the search terms were linked using the AND logic. The search syntax was written separately for each database.
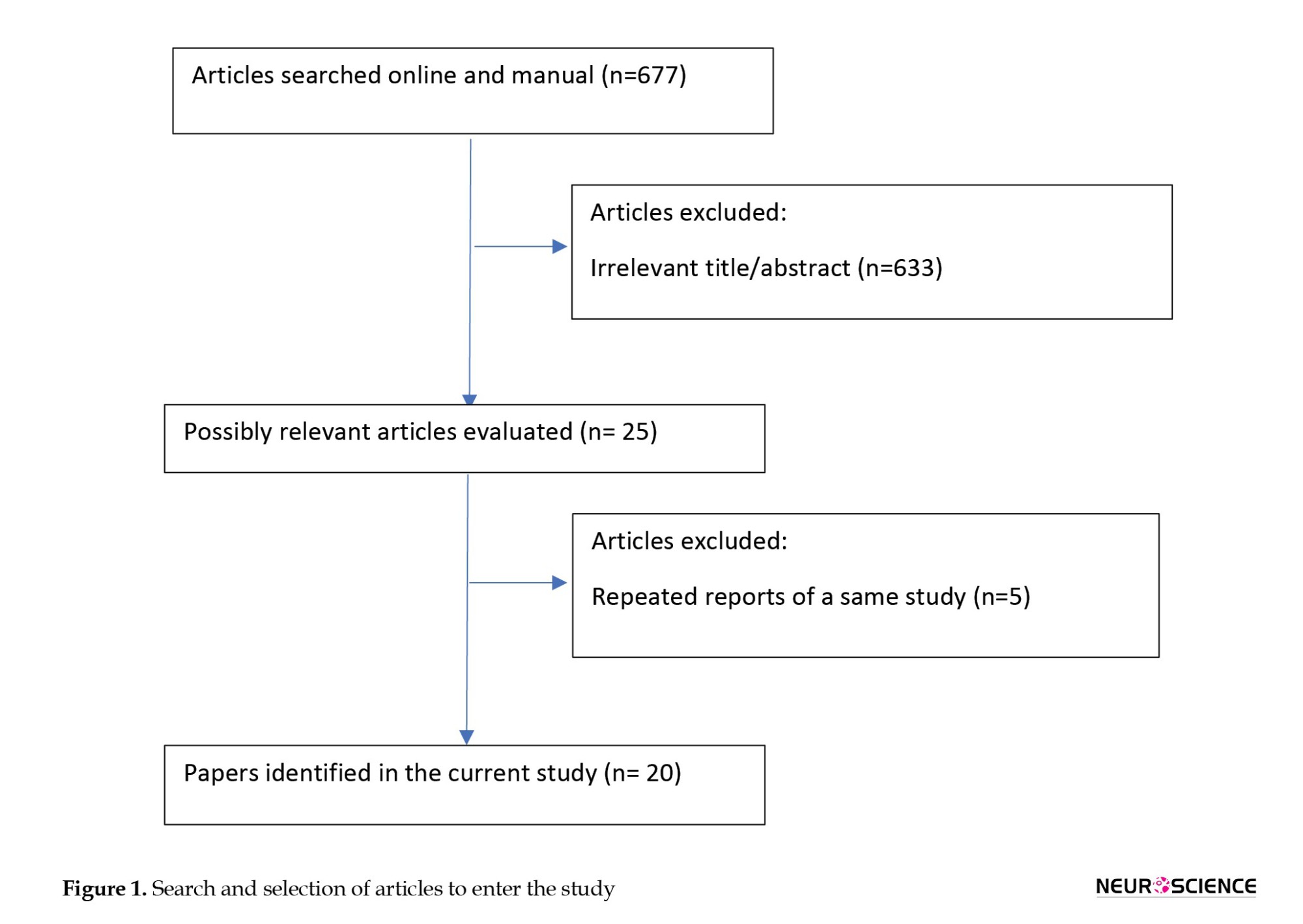
After removing duplicates, a total of 677 articles were identified across the four databases. Two researchers independently screened the searched studies and evaluated them based on inclusion criteria, titles, and abstracts. In cases of uncertainty regarding article selection, the full text was reviewed. Disagreements were resolved through consultation with another independent researcher, ultimately determining whether to include the article. Then, the required data were extracted from qualified articles according to the data collection form for RCT studies.
3. Results
The PRISMA flow chart shows the process of identifying, screening, and evaluating selected studies (Figure 1). The initial search resulted in 677 eligible articles, which were scrutinized by two independent researchers. Out of these 677 articles, 633 were excluded from further research for various reasons, including duplicates. Studies were included in the review if they met the following inclusion and exclusion criteria: Inclusion criteria encompassed randomized controlled trials published as full-text articles in scientific journals. Exclusion criteria involved studies reporting insufficient data and those published solely as abstracts for conferences and proceedings.
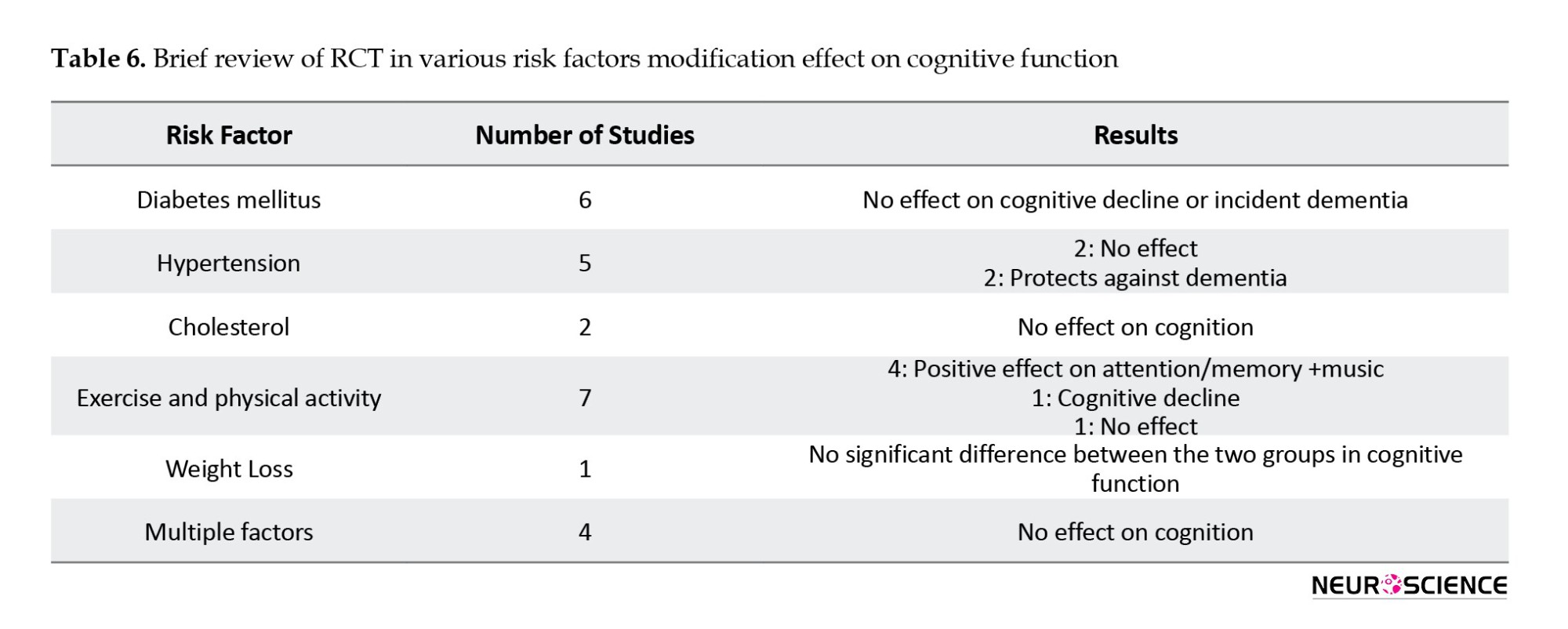
Finally, 25 articles remained for data analysis. These studies examined the effect of controlling cardiovascular risk factors and lifestyle on the risk of cognitive impairment, with risk factors including diabetes mellitus, high blood pressure, high cholesterol levels, and exercise and physical activity being studied as lifestyle factors affecting cardiovascular disease.
Diabetes
Six articles were related to the study of intensive blood-glucose control effect on cognitive function (Table 1). Of these articles, five were related to the results of one study. This study is called Look AHEAD, and its protocol was published in 2003 (Ryan et al., 2003).
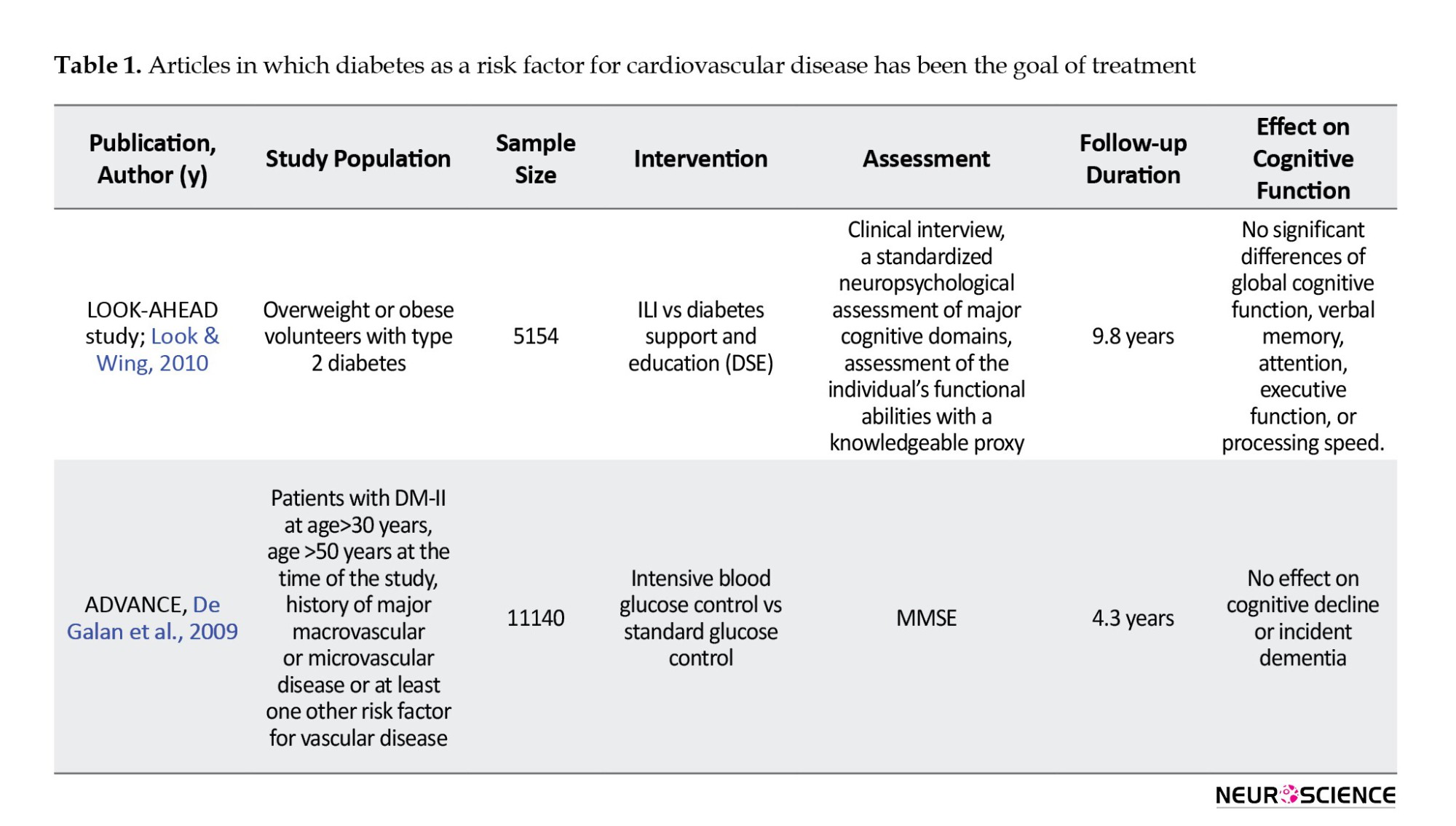
The primary purpose of this study was to evaluate the long-term effects of a severe weight loss program in patients with type 2 diabetes over 4 years. Approximately 5,000 men and women with type 2 diabetes, aged 45 to 47 years, participated in this study, undergoing two types of interventions: Intensive lifestyle intervention (ILI) and diabetic support and education (Look & Wing, 2010). In the ILI intervention, participants adhered to a diet with 1200-1800 calories per day and engaged in over 175 minutes per week of physical activity, with a target of 7% weight loss. Participants were followed for an average of 9.8 years. In this study, participants were evaluated with cognitive batteries including, modified mini-mental state examination (MMSE), Rey auditory verbal learning test, digit symbol coding, trail-making test, modified Stroop color-word test, and brain imaging assessments. In this study, no significant difference was seen between the two intervention groups in terms of cognitive function. However, in the ILI group, the rate of brain hyperintensity lesions was lower, which could mean better overall brain health. Negative effects on cognitive function were observed in the subgroup of very obese patients with body mass index above 40 kg/m2, and patients with a positive history of cardiovascular disease.
Another study was called ADVANCE, in which participants also had type 2 diabetes. A total of 5,571 patients over the age of 55 (mean age 65 years) were included in the study. In the intervention group, treatment with slow-release glycoside at a dose of 120-30 mg plus metformin, thiazolidinediones, acarbose, or insulin was performed to achieve HBA1c less than 6.5%. In this study, no cognitive changes based on MMSE were seen after five years, and a non-significant increase in dementia incidence was seen in the study group (Look & Wing, 2010).
Hypertension
There were five articles on the effects of blood pressure control on the incidence of cognitive disorders, and the results of four studies were reported (Table 2), (Forette et al., 2002; Lithell et al., 2003; Peters et al., 2008; Tzourio et al., 2003).
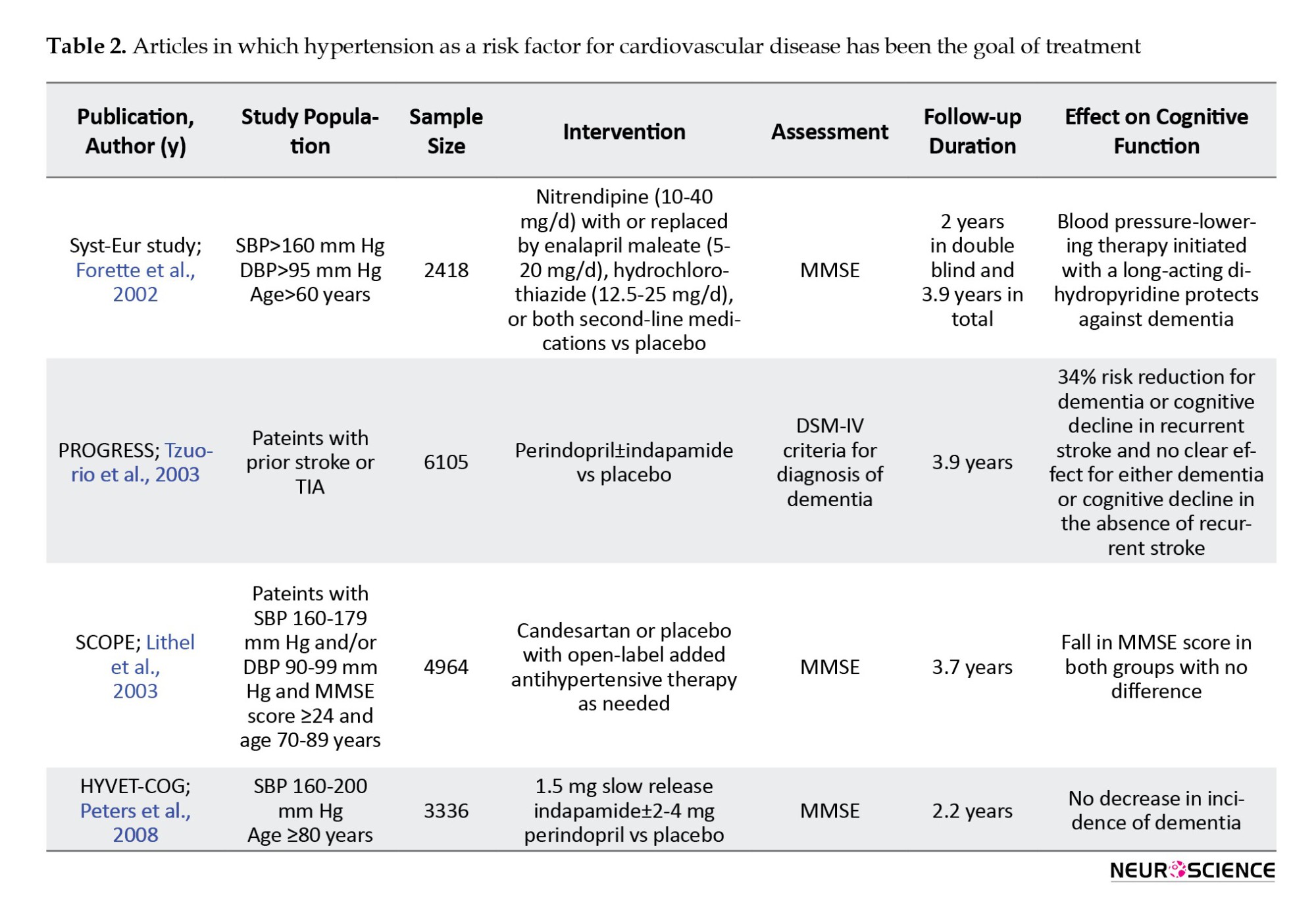
In all these studies, cognitive status assessment was one of the secondary goals of the study. The primary purpose was to investigate adverse vascular consequences, such as stroke, and cardiovascular events, such as myocardial infarction or cardiac death. The sample size was between 2418 and 6105 people. All studies assessed the elderly and one (HYVET-COG) assessed the oldest-old group (Peters et al., 2008). The MMSE tool was used to assess cognitive function. The follow-up period in these studies was between 2.2 and 3.9 years.
In these studies, a 24% to 42% reduction in stroke incidence was seen. The HYVET-COG study, which targeted the oldest-old group, also showed a significant reduction in stroke incidence. In all these studies, no significant effect was seen on cognitive function. A SCOPE study on data reanalysis found an impact on some cognitive domains, including episodic memory (Lithell et al., 2003).
Lipid-profile disorders
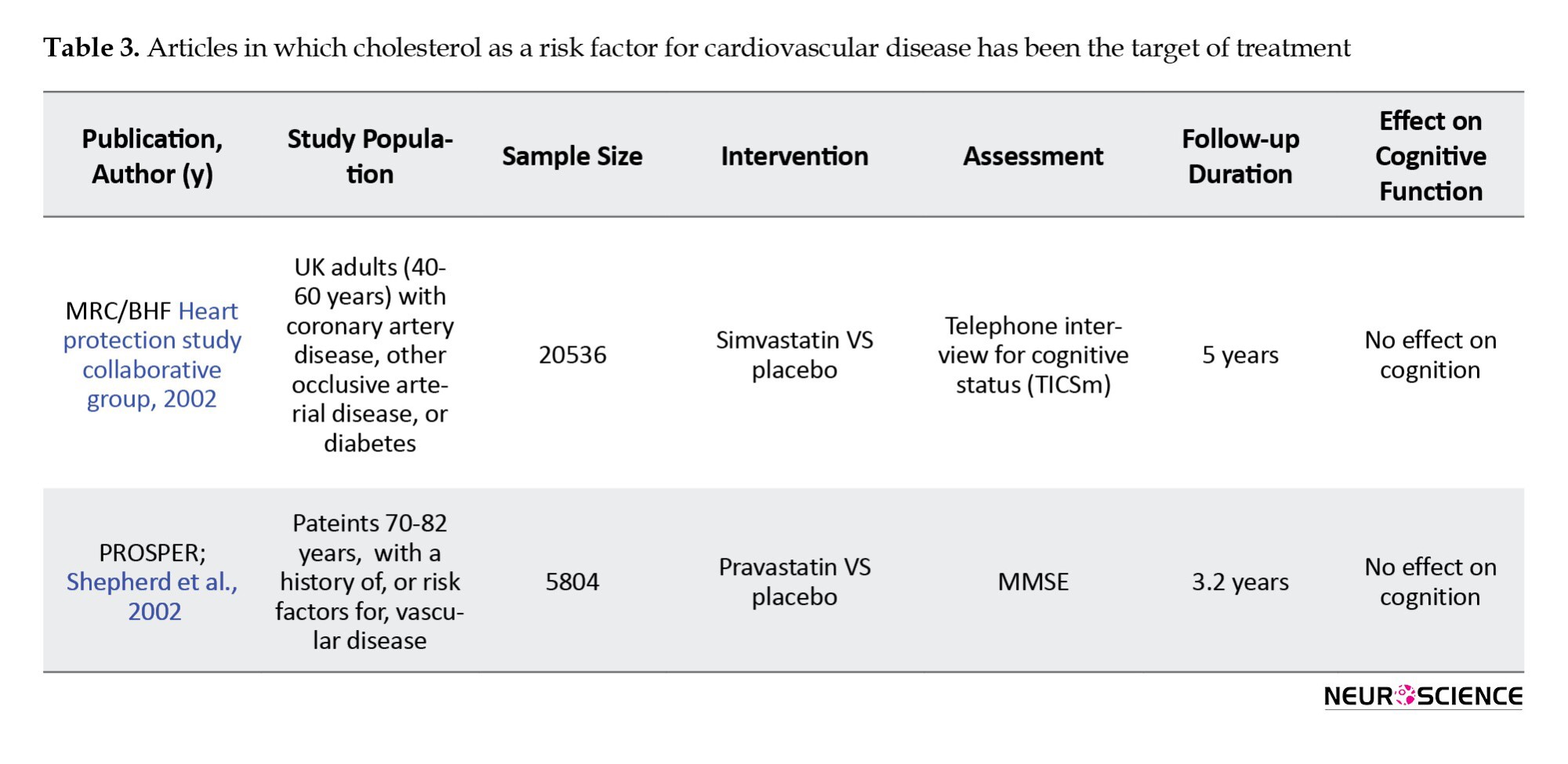
Two studies specifically examined the effect of LDL-reducing therapies on cognitive disorders (Table 3). The first study was the MRC/BHF Heart Protection Study, which compared simvastatin with a placebo. In this study, with a 5-year follow-up, cognitive function as a secondary outcome was assessed by the cognitive assessment telephone interview test. In the second PROSPER study, 5804 people in the two randomized groups received pravastatin or placebo. Cognitive status in this study was evaluated as a secondary outcome by the MMSE test. Both studies showed positive effects of treatment on primary outcomes, such as all-cause mortality, coronary death, non-vascular death, non-fatal myocardial infarction, and stroke. However, in both studies, no effects were seen on cognitive function (Shepherd et al., 2002).
Physical activity and exercise
Seven studies examined the effects of exercise and physical activity on cognitive function (Table 4) (Carles et al., 2007; Emery et al., 2003; Fiocco et al., 2013; Teixeira et al., 2013; Xu et al., 2017; Yamamoto et al., 2009). In 2013, Fiocco et al. measured the effect of exercise and lifestyle intervention programs on the cardiovascular and metabolic status of middle-aged people with type 2 diabetes. In this pilot study, 17 middle-aged patients underwent a 24-week exercise intervention and were assessed using the California verbal learning test (CVLT), DSST, and Fluency test cognitive test. This study showed that despite the improvement of cardiovascular endurance, reduction of BMI, and improvement of depression symptoms, there was no change in the level of glucose and fat, and, contrary to expectation, it decreased after performing cognitive tests. However, this decrease in CVLT was limited to patients who simultaneously had diabetes and high blood pressure (Fiocco et al., 2013).
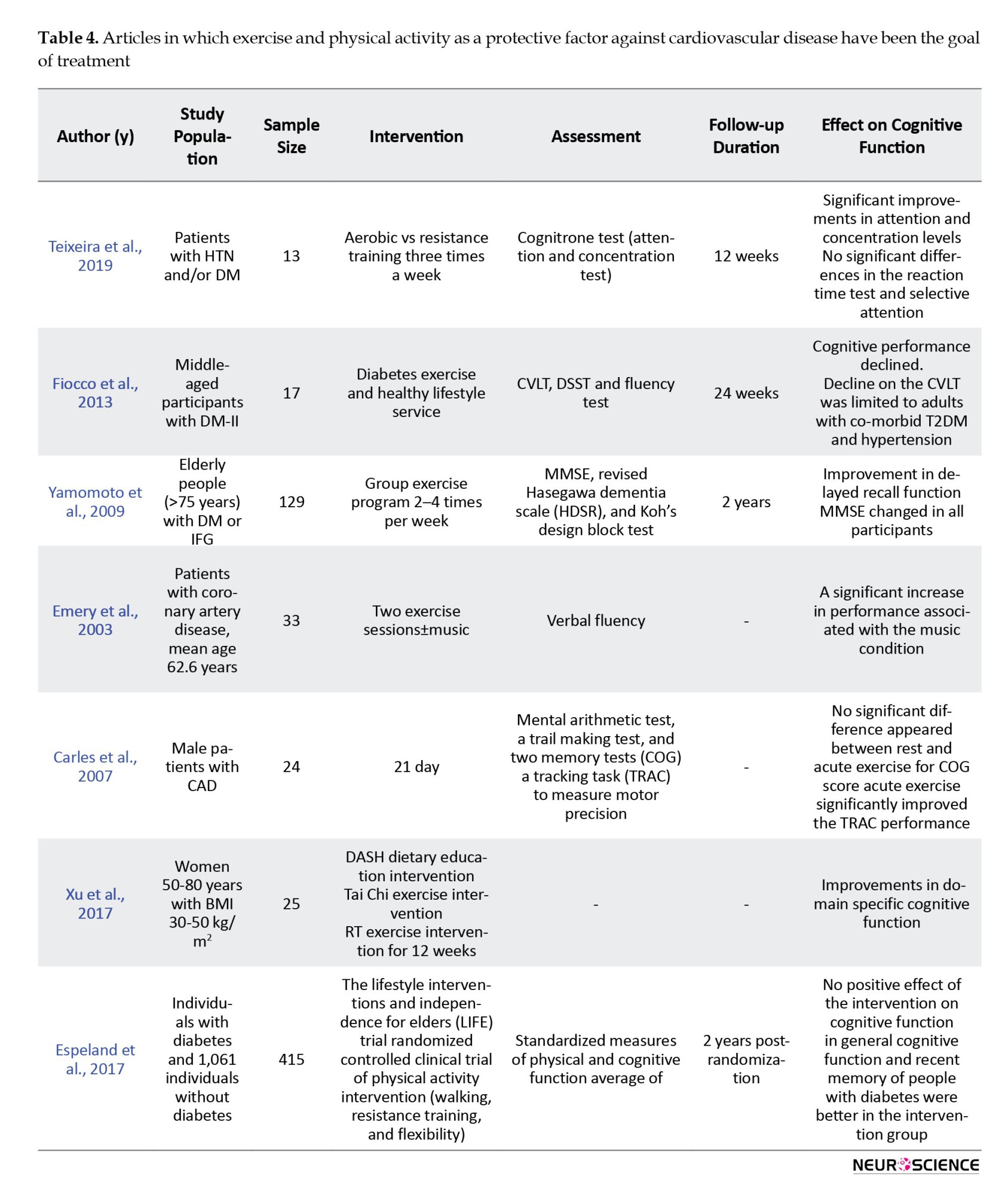
The Yamamoto study cognitively assessed 129 individuals over the age of 75 with MMSE and Hasegawa dementia scale revised (HDSR). In this study, three groups of people with diabetes, impaired glucose tolerance (IGT), and normal glucose tolerance (NGT) participated in an exercise program 2-4 times a week and received a nutrition training program. In subjects with MMSE and diabetes, baseline HDSR was lower than NGT, which returned to NGT after lifestyle intervention (Yamamoto et al., 2009).
Carles et al. study examined the short-term exercise effect on cognitive function in patients with coronary heart disease and heart failure. In this study, 24 men with a mean age of 51.6 years were cognitively assessed by an exclusively cognitive test (COG) and a tracking task after exercise. This study showed that exercise training increases the positive effect of exercise on cognition. (Carles et al., 2007). In general, aerobic exercise seems to improve cognitive function. The positive effect of exercise is characterized by increased cerebral blood flow and levels of neurotransmitters.
Espeland et al. assessed the effect of physical activity on the cognitive and physical function of sedentary people. They measured the physical activity effect intervention in 415 patients with diabetes and 1061 patients without diabetes aged 70 to 89 years, and two years later, performed the cognitive and physical evaluation. Although the intervention on cognitive function showed no improvement, in general, the overall cognitive function and recent memory of people with diabetes were better compared to the intervention group (Espeland et al., 2017).
In another study, Teixeira et al. examined the effect of aerobic/resistance training on diabetic and hypertensive patients over 12 weeks. This study showed improved attention and concentration in patients without affecting reaction time, which could be justified by increasing perfusion and oxygen delivery to the brain due to exercise. In this study, 21 patients were assessed; however, eight patients left, and the remaining 13 patients underwent exercise according to the Ramp protocol. A cognitive assessment-based MTTS (mental test and training system) test was performed. In this study, there was an improvement in attention and concentration, which will be very effective in diabetic patients to manage their various medications and improve social relationships. Nonetheless, a limited period of exercise was considered (Teixeira et al., 2019).
Compared to the studies reviewed in the previous sections, studies examining the effect of exercise often had a smaller sample size (129-13) and a shorter follow-up duration (immediately after two sessions of an exercise program for up to 24 weeks). In these studies, it was found that exercise improves some areas of cognitive activity, such as attention and concentration or accuracy in motor movements. In patients with comorbid diabetes and hypertension, an exercise program was even associated with cognitive function decrease.
Weight loss
One study investigated the effect of weight loss with cognitive rehabilitation on cognitive function. In Beck et al.’s study, participants were divided into two groups: Cognitive rehabilitation interventions and weight loss programs. At the end of the study, there was no significant difference between the two groups in cognitive function changes. At the end of the study, there was no significant difference between the two groups in cognitive function changes (Beck et al., 2013).
Multiple interventions
Four studies implemented interventions to control several risk factors for cardiovascular disease (Murra et al., 2017; Shepherd et al., 2002; Strandberg et al., 2006), (Table 5).
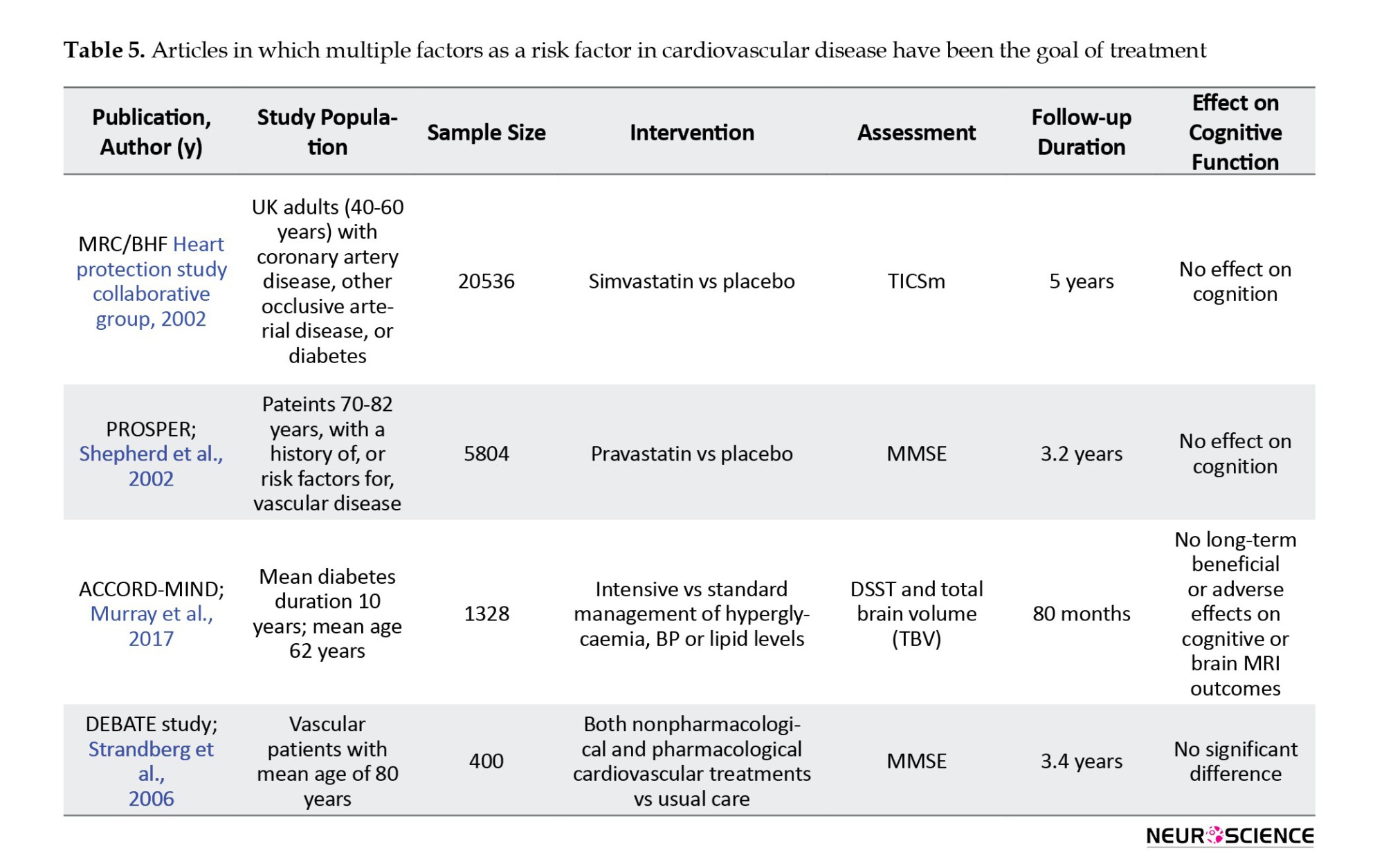
In the MIND study, which was part of the ACCORD study, 2977 patients with type 2 diabetes underwent standard or intensive glycemic, lipid, and blood pressure control. In this study, the digit symbol substitution test (DSST) and total brain volume were measured by MRI. At the 80-month follow-up, there was no significant difference in DSST test scores or brain structure between the two groups (Murray et al., 2017).
The largest sample size in these studies was related to the MRC/BHF study. However, it had the highest dropout rate, which was significantly related to the participants who had lower scores on cognitive tests at the baseline assessment. Both the MRC/BHF and ACCORD-MIND studies had long follow-up periods of 5 years or more.
None of these studies, which targeted several cardiovascular risk factors, showed a significant reduction in cognitive impairment or cognitive function improvement. In the only study that examined the effect of weight loss, the cognitive performance improvement was similar to the cognitive rehabilitation program (control group) (Table 6).
4. Discussion
The present study reviewed the medical treatment of four common cardiovascular risk factors and lifestyle interventions, including physical activity and diet. The aim was to evaluate the results of all randomized clinical trials (RCTs) of appropriate quality to assess dementia as a primary or secondary outcome.
Over the past 20 years, several RCTs have been conducted on the effect of medical treatment on cardiovascular risk factors for dementia. Most of the studies mentioned in the present review had a large sample size and considerable follow-up time.
These studies evaluated the effect of controlling cardiovascular risk factors on mortality and a range of vascular outcomes, including stroke, myocardial infarction, and peripheral arterial disease, as the core purpose. However, the evaluation of cognitive impairment, dementia, or improvement in cognitive function was considered a secondary outcome. There is only one positive study (Syst-Eur) that shows the protective consequence of hypertension treatment. Because different antihypertensive regimens have been used in these studies, the cognitive effect of a particular class of antihypertensive medications was not obvious (Forette et al., 2002).
There is no evidence of prophylactic effects on dementia for type 2 diabetes management and statin therapy. The long-term effect of subclinical hypoglycemia and functional disorders on brain autoregulation may be the cause of cognitive function exacerbation in the ILI group. A similar mechanism is seen in tight control in type 1 diabetes. Another reason may be related to the reduced neuroprotective effects of leptin in this group. Another reason may be related to the reduced neuroprotective effects of leptin in this group. The effects of physical activity on improving cognitive function were promising. However, these studies often had a smaller sample size and a much shorter setting time than other reviewed studies (Emery et al., 2003; Espeland et al., 2017; Fiocco et al., 2013; Teixeira et al., 2019; Xu et al., 2017; Yamamoto et al., 2009). However, these studies often had a smaller sample size and a much shorter setting time than other reviewed studies (Murray et al., 2017; Shepherd et al., 2002; Strandberg et al., 2006).
The most important bias that may have affected the study results is the exclusion of samples due to cognitive impairment, especially if a study is not explicitly designed to monitor cognitive function, as cognitive impairment may lead to cessation of informed consent or hospitalization. This omission from the selected study may reduce the potential treatment effect and consequently lead to a type 2 error, particularly if the intervention is effective and dropout occurs more frequently in the control group. In all studies on hypertension, further treatment with other antihypertensive medications was allowed in both the intervention and placebo groups if needed to achieve acceptable blood pressure levels. As a result, many patients in the control group received antihypertensive medications, which may reduce experimental differentiation and its effect on cardiovascular outcomes and dementia (Forette et al., 2002; Lithell et al., 2003; Peters et al., 2008; Tzourio et al., 2003). In cholesterol studies, this additional treatment with statins was performed less frequently than the study medications. In many cases, placebo-controlled trials are often not possible for ethical reasons (Shepherd et al., 2002). The age of the participants can also be another factor affecting the results. The study population is relatively young in most studies, and therefore, its effect on cognitive impairment and the incidence of dementia during follow-up was not recognizable. In the age range of 65-69, dementia is still relatively rare, with an incidence is approximately 2.4 cases per 1000 people, which increases sharply with age to 70.2 cases per 1000 people over the age of 90 (Rizzi, Rosset et al., 2014). However, it was expected that the number of participants with dementia or significant cognitive impairment would be low in most studies, limiting the study’s ability to detect such an effect.
Another point is the effect of mortality on the probability of disease. Dementia and cognitive disorders are age-related. The incidence of dementia may be increased by preventing cardiovascular disease, which reduces mortality. In many studies, interventions reduced mortality. However, none of the studies investigated the role of premature mortality in reducing the incidence of dementia.
One of the limitations of this study was the lack of a baseline cognitive assessment, which makes changes after the intervention not well interpretable. Another limitation was the cognitive assessment time, which was up to 3 months between the cognitive assessment and the end time of the program, and future studies should reduce this time to less than two weeks. Clinical dementia is the result of the interaction of brain injuries secondary to various risk factors, and brain resilience and cognitive reserve and the presence of a high cognitive reserve can correct this damage. Cognitive reserve was not initially measured in many of these studies; thus, the effectiveness of interventions in people with different cognitive reserves cannot be assured.
On the other hand, the cognitive assessment, which was performed in the AHEAD study was years after the initial randomization. Studies should take into account the potential loss of effective follow-up in a percentage of patients; given the time gap between study randomization and cognitive assessment, it is possible that some patients with cognitive impairment in the study may have passed away due to old age.
Some studies found that weight loss can be a sign of the onset of cognitive impairment. In contrast, some trials on weight loss suggested that obesity heightens the risk of dementia in midlife but may reduce the risk of dementia in later stages of life and during the aging process. These studies suggest that neurodegenerative diseases and dementia by affecting hormones, mood, and smell sense can lead to reduced oral intake and weight loss. The weight loss caused in these studies should be considered in two categories of desire (due to intervention) and unwanted (due to the onset of dementia). It is difficult to differentiate the effects of weight loss due to intervention from the weight loss impact due to the inflammatory and neurodegenerative process. Another hypothesis suggests that leptin can have positive effects on neurogenesis and reduce apoptosis, and due to the decrease in leptin in the ILI group, these positive effects are also reduced. It can be suggested that in the ILI group, with a decrease in leptin, a decrease in neurogenesis in the hippocampus, potentially affecting the molecular and structural aspects of the brain.
In general, brain structural alterations are utilized to evaluate cognitive function outcomes. Another aspect to consider is that the ILI group exhibits fewer microvascular changes and less cerebral atrophy, with a 9% reduction in ventricular volume, lower global atrophy, and a 28% decrease in white matter changes. Unexpectedly, there is no association between decreased cognitive function in the ILI group and atrophy and vascular lesions of the brain (Look & Wing, 2010). As a result, functional imaging techniques may be able to answer our question of why more cognitive decline occurs despite fewer brain changes in the ILI group.
The ACCORD-MIND study also showed that strict control of blood sugar leads to greater overall brain volume despite no difference in cognitive function. On the other hand, it should be borne in mind that ILI can lead to subclinical hypoglycemia and this hypoglycemia has long-term negative effects on brain function and cognition (Murray et al., 2017). In other words, it is essential to explore the concept of self-regulation of cerebral arteries and whether a comparable mechanism exists for organ damage resulting from tight blood sugar control in diabetic patients, potentially causing modifications or harm to cerebral arteries in the elderly. Another issue to consider is the effect of legacy, which is the lag time between the intervention time that affects the metabolic process in diabetic patients and the benefits that the patient achieves. On the other hand, most of the mentioned studies designed short-term interventions, while the process of dementia and cognitive decline is much longer. From midlife, where risk factors become apparent, to old age when the dementia process unfolds, a considerable amount of time elapses. A short study duration can pose a significant limitation in observing the impact of metabolic disorders. Moreover, in long-term studies, the cost-effectiveness of interventions should also be regarded as a crucial challenge.
In general, it seems that exercise can improve cognitive function by improving glucose metabolism in the elderly with complaints of memory impairment. Exercise may increase the oxidative load, which is high in type 2 diabetes. Studies have shown that even moderate exercise levels can increase the level of free radicals and lead to oxidative effects. In contrast, regular physical activity can protect against oxidative effects. Diet is another factor that should be considered in relation to cognitive function and physical activity in patients with type 2 diabetes. There must be a control group to determine that cognitive decline can be part of normal aging apart from the intervention.
Lastly, it is imperative to contemplate the methodology employed for cognitive assessment and the diverse nature of testing, especially when certain interventions fail to influence cognitive function. While the MMSE is commonly utilized in numerous studies for diagnosing established dementia, it may lack sensitivity in cognitively healthy individuals and younger age groups. We need to use a more sensitive test whenever we cannot find a significant change in clinical trials and prevention studies. Thus, it is recommended to use a more comprehensive battery, such as AVLT in future studies.
Similar to all review studies, publication bias could impact our search outcomes. Typically, studies do not publish negative results in the abstract section, potentially leading to the exclusion of certain articles during the initial search phase.
5. Conclusion
After reviewing the studies, it can be inferred that there is inadequate evidence to support the notion that treating cardiovascular risk factors can prevent dementia. Although exercise, and conceivably blood pressure management, exhibit a preventive impact, the effectiveness is less apparent for statin therapy and intensive treatment of type 2 diabetes. The discontinuation of studies, the inability to sustain study participation, competitive risks, or various forms of selection or treatment bias could have reduced the potential effects of interventions in the trials mentioned. Future RCTs involving diverse populations with alternative interventions and extended follow-up periods, particularly aimed at assessing their impact on cognitive function or dementia, can tackle the fundamental question of whether the associations observed in cohort studies can translate into substantial clinical therapeutic benefits for cognition.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflicts of interest.
References
The increased life expectancy throughout the world has led to a rise in the number of people affected by chronic diseases, which have become major health challenges (Brown, 2015). Evidence suggests that many chronic diseases in the elderly share common risk factors. Among these, dementia and cognitive impairment are the most prevalent causes of morbidity and mortality in the elderly. Cognitive decline in older adults is a major public health problem and can diminish independence and quality of life (Livingston et al., 2020). Approximately 47 million people worldwide were affected by dementia in 2015, a number that is expected to triple by 2050 (Patterson & International, 2018). In the absence of a cure for the disease or treatment, reducing the risk of dementia is doubly important (Cummings et al., 2016). Even in cases where effective treatments are available, reducing the risk of disease occurrence remains a fundamental solution. For many non-communicable diseases with existing treatments, such as diabetes, cancer, and heart disease, risk reduction plays a vital role in disease prevention (Bloom et al., 2017).
The main risk factors for the onset of Alzheimer’s disease and other dementias are age, family history, and predisposed genes, such as apolipoprotein E allele ε4 (Hsiung & Sadovnick, 2007). However, none of these risk factors can be altered or modified by medical interventions or individual behavior. There is ample evidence supporting the association between multiple variable risk factors and decreased cognitive decline risk, as discussed in this review (Baumgart et al., 2015. Vascular risk factors are increasingly recognized as significant contributors to dementia and are thus targets for future treatments. Vascular risk factors in middle age appear to be most closely associated with cognitive decline in old age (Whitmer et al., 2005). The US National Institutes of Health emphasizes that diabetes mellitus, smoking, depression, mental or physical inactivity, and poor diet are related to the risk of cognitive decline. This list has expanded to include high blood pressure, obesity, and low education (Barnes & Yaffe, 2011). While the association between high blood pressure and AD risk is complex and age-dependent, some evidence suggests that in middle-aged, but not older, populations, blood pressure is associated with a 50% increase in AD and dementia risk (Lee et al., 2022). High blood pressure can increase the risk of AD by reducing the vascular integrity of the blood-brain barrier, which leads to extravasation or leakage of protein into brain tissue, which in turn leads to cell damage, apoptosis, and increased Aβ accumulation. However, the direct causal relationship between blood pressure and subsequent cognitive decline is debatable, as there is also emerging evidence suggesting that blood pressure may represent a protective response to cerebral hypoperfusion, evident a decade before AD onset (Corrada et al., 2017). According to the obesity epidemic and growing evidence of the relationship between body mass index (BMI) and cognition, several studies have found that being overweight or obese were independent risk factors for cognitive decline (Doruk et al., 2010; Lee et al., 2010; Naderali et al., 2009; Nilsson & Nilsson, 2009). Obese individuals exhibit smaller whole brain and total gray matter volume than those with normal weight (Gunstad et al., 2008). On the other hand, there is a U-shaped relationship between weight and cognitive function: Both low and high weight are associated with a heightened risk of AD and cognitive impairment. This relationship may also exhibit an age-related component (Bae & Park, 2021). Conversely, there is data suggesting an inverse relationship in the years preceding disease onset; for instance, weight loss, which may result from cognitive deficits during the pre-dementia phase of AD (Luchsinger et al., 2007). Diabetes has been demonstrated to directly increase dementia risk by impacting Aβ accumulation in the brain. Other studies indicate that diabetes may elevate the risk of cerebrovascular disease but not AD pathology (Biessels & Despa, 2018). Despite proper diabetes control being approved and recommended for preventing most diabetes-related diseases, its effect on preventing or delaying the onset of dementia remains unclear (Ravona-Springer & Schnaider-Beeri, 2011). In addition to glycemic control, several factors associated with diabetes may interact with dementia clinical manifestations and neuropathology, as well as the rate of functional and cognitive decline (Biessels et al., 2008). A review study discovered that severe hypoglycemia did not benefit young people with type 1 diabetes in terms of cognitive function, whereas older individuals with type 2 diabetes benefited from treatment in terms of information processing speed and executive function (Moheet et al., 2015). Systematic reviews and prospective studies on the association between cholesterol levels in middle age, old age, and dementia have yielded mixed results, including no association between cholesterol levels and vascular dementia (Park et al., 2013). While some observational studies have indicated that statin use to control cholesterol levels reduces dementia risk, a review in Cochrane (McGuinness et al., 2016) and other review studies found no evidence supporting the reduction of dementia risk through statin use.
2. Materials and Methods
Search strategy
This review was conducted according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines (Moher et al., 2009). A systematic search of scientific article databases, including PubMed, Scopus, Google Scholar, SCIELO, and Cochrane Central was performed using the appropriate keywords and search protocol for each database. For example, in the PubMed database, relevant search keywords and strategies were determined based on MeSH terms.
The inclusion criteria encompassed clinical studies published in English, targeting elderly populations and intervening to modify at least one cardiovascular risk factor (such as diabetes, dyslipidemia, hypertension, sedentary lifestyle, and obesity), with cognitive status evaluated as an outcome measure. The general search model employed phrases such as “intervention” OR “modification” OR “modify” OR “control” OR “change” in combination with terms like “vascular” OR “hypertension” OR “hyperlipidemia” OR “dyslipidemia” OR “diabetes” OR “obesity” OR “overweight,” along with terms like “cognitive” OR “memory” OR “dementia” OR “mind,” excluding non-human studies. Studies published after 2000 were accepted. This search strategy was then adapted to the characteristics of other databases. We described the search strategy in Figure 1 in detail. The search terms with similar meanings were combined using the OR logic, and the search terms were linked using the AND logic. The search syntax was written separately for each database.

After removing duplicates, a total of 677 articles were identified across the four databases. Two researchers independently screened the searched studies and evaluated them based on inclusion criteria, titles, and abstracts. In cases of uncertainty regarding article selection, the full text was reviewed. Disagreements were resolved through consultation with another independent researcher, ultimately determining whether to include the article. Then, the required data were extracted from qualified articles according to the data collection form for RCT studies.
3. Results
The PRISMA flow chart shows the process of identifying, screening, and evaluating selected studies (Figure 1). The initial search resulted in 677 eligible articles, which were scrutinized by two independent researchers. Out of these 677 articles, 633 were excluded from further research for various reasons, including duplicates. Studies were included in the review if they met the following inclusion and exclusion criteria: Inclusion criteria encompassed randomized controlled trials published as full-text articles in scientific journals. Exclusion criteria involved studies reporting insufficient data and those published solely as abstracts for conferences and proceedings.
Finally, 25 articles remained for data analysis. These studies examined the effect of controlling cardiovascular risk factors and lifestyle on the risk of cognitive impairment, with risk factors including diabetes mellitus, high blood pressure, high cholesterol levels, and exercise and physical activity being studied as lifestyle factors affecting cardiovascular disease.
Diabetes
Six articles were related to the study of intensive blood-glucose control effect on cognitive function (Table 1). Of these articles, five were related to the results of one study. This study is called Look AHEAD, and its protocol was published in 2003 (Ryan et al., 2003).

The primary purpose of this study was to evaluate the long-term effects of a severe weight loss program in patients with type 2 diabetes over 4 years. Approximately 5,000 men and women with type 2 diabetes, aged 45 to 47 years, participated in this study, undergoing two types of interventions: Intensive lifestyle intervention (ILI) and diabetic support and education (Look & Wing, 2010). In the ILI intervention, participants adhered to a diet with 1200-1800 calories per day and engaged in over 175 minutes per week of physical activity, with a target of 7% weight loss. Participants were followed for an average of 9.8 years. In this study, participants were evaluated with cognitive batteries including, modified mini-mental state examination (MMSE), Rey auditory verbal learning test, digit symbol coding, trail-making test, modified Stroop color-word test, and brain imaging assessments. In this study, no significant difference was seen between the two intervention groups in terms of cognitive function. However, in the ILI group, the rate of brain hyperintensity lesions was lower, which could mean better overall brain health. Negative effects on cognitive function were observed in the subgroup of very obese patients with body mass index above 40 kg/m2, and patients with a positive history of cardiovascular disease.
Another study was called ADVANCE, in which participants also had type 2 diabetes. A total of 5,571 patients over the age of 55 (mean age 65 years) were included in the study. In the intervention group, treatment with slow-release glycoside at a dose of 120-30 mg plus metformin, thiazolidinediones, acarbose, or insulin was performed to achieve HBA1c less than 6.5%. In this study, no cognitive changes based on MMSE were seen after five years, and a non-significant increase in dementia incidence was seen in the study group (Look & Wing, 2010).
Hypertension
There were five articles on the effects of blood pressure control on the incidence of cognitive disorders, and the results of four studies were reported (Table 2), (Forette et al., 2002; Lithell et al., 2003; Peters et al., 2008; Tzourio et al., 2003).

In all these studies, cognitive status assessment was one of the secondary goals of the study. The primary purpose was to investigate adverse vascular consequences, such as stroke, and cardiovascular events, such as myocardial infarction or cardiac death. The sample size was between 2418 and 6105 people. All studies assessed the elderly and one (HYVET-COG) assessed the oldest-old group (Peters et al., 2008). The MMSE tool was used to assess cognitive function. The follow-up period in these studies was between 2.2 and 3.9 years.
In these studies, a 24% to 42% reduction in stroke incidence was seen. The HYVET-COG study, which targeted the oldest-old group, also showed a significant reduction in stroke incidence. In all these studies, no significant effect was seen on cognitive function. A SCOPE study on data reanalysis found an impact on some cognitive domains, including episodic memory (Lithell et al., 2003).
Lipid-profile disorders
Two studies specifically examined the effect of LDL-reducing therapies on cognitive disorders (Table 3). The first study was the MRC/BHF Heart Protection Study, which compared simvastatin with a placebo. In this study, with a 5-year follow-up, cognitive function as a secondary outcome was assessed by the cognitive assessment telephone interview test. In the second PROSPER study, 5804 people in the two randomized groups received pravastatin or placebo. Cognitive status in this study was evaluated as a secondary outcome by the MMSE test. Both studies showed positive effects of treatment on primary outcomes, such as all-cause mortality, coronary death, non-vascular death, non-fatal myocardial infarction, and stroke. However, in both studies, no effects were seen on cognitive function (Shepherd et al., 2002).

Physical activity and exercise
Seven studies examined the effects of exercise and physical activity on cognitive function (Table 4) (Carles et al., 2007; Emery et al., 2003; Fiocco et al., 2013; Teixeira et al., 2013; Xu et al., 2017; Yamamoto et al., 2009). In 2013, Fiocco et al. measured the effect of exercise and lifestyle intervention programs on the cardiovascular and metabolic status of middle-aged people with type 2 diabetes. In this pilot study, 17 middle-aged patients underwent a 24-week exercise intervention and were assessed using the California verbal learning test (CVLT), DSST, and Fluency test cognitive test. This study showed that despite the improvement of cardiovascular endurance, reduction of BMI, and improvement of depression symptoms, there was no change in the level of glucose and fat, and, contrary to expectation, it decreased after performing cognitive tests. However, this decrease in CVLT was limited to patients who simultaneously had diabetes and high blood pressure (Fiocco et al., 2013).

The Yamamoto study cognitively assessed 129 individuals over the age of 75 with MMSE and Hasegawa dementia scale revised (HDSR). In this study, three groups of people with diabetes, impaired glucose tolerance (IGT), and normal glucose tolerance (NGT) participated in an exercise program 2-4 times a week and received a nutrition training program. In subjects with MMSE and diabetes, baseline HDSR was lower than NGT, which returned to NGT after lifestyle intervention (Yamamoto et al., 2009).
Carles et al. study examined the short-term exercise effect on cognitive function in patients with coronary heart disease and heart failure. In this study, 24 men with a mean age of 51.6 years were cognitively assessed by an exclusively cognitive test (COG) and a tracking task after exercise. This study showed that exercise training increases the positive effect of exercise on cognition. (Carles et al., 2007). In general, aerobic exercise seems to improve cognitive function. The positive effect of exercise is characterized by increased cerebral blood flow and levels of neurotransmitters.
Espeland et al. assessed the effect of physical activity on the cognitive and physical function of sedentary people. They measured the physical activity effect intervention in 415 patients with diabetes and 1061 patients without diabetes aged 70 to 89 years, and two years later, performed the cognitive and physical evaluation. Although the intervention on cognitive function showed no improvement, in general, the overall cognitive function and recent memory of people with diabetes were better compared to the intervention group (Espeland et al., 2017).
In another study, Teixeira et al. examined the effect of aerobic/resistance training on diabetic and hypertensive patients over 12 weeks. This study showed improved attention and concentration in patients without affecting reaction time, which could be justified by increasing perfusion and oxygen delivery to the brain due to exercise. In this study, 21 patients were assessed; however, eight patients left, and the remaining 13 patients underwent exercise according to the Ramp protocol. A cognitive assessment-based MTTS (mental test and training system) test was performed. In this study, there was an improvement in attention and concentration, which will be very effective in diabetic patients to manage their various medications and improve social relationships. Nonetheless, a limited period of exercise was considered (Teixeira et al., 2019).
Compared to the studies reviewed in the previous sections, studies examining the effect of exercise often had a smaller sample size (129-13) and a shorter follow-up duration (immediately after two sessions of an exercise program for up to 24 weeks). In these studies, it was found that exercise improves some areas of cognitive activity, such as attention and concentration or accuracy in motor movements. In patients with comorbid diabetes and hypertension, an exercise program was even associated with cognitive function decrease.
Weight loss
One study investigated the effect of weight loss with cognitive rehabilitation on cognitive function. In Beck et al.’s study, participants were divided into two groups: Cognitive rehabilitation interventions and weight loss programs. At the end of the study, there was no significant difference between the two groups in cognitive function changes. At the end of the study, there was no significant difference between the two groups in cognitive function changes (Beck et al., 2013).
Multiple interventions
Four studies implemented interventions to control several risk factors for cardiovascular disease (Murra et al., 2017; Shepherd et al., 2002; Strandberg et al., 2006), (Table 5).

In the MIND study, which was part of the ACCORD study, 2977 patients with type 2 diabetes underwent standard or intensive glycemic, lipid, and blood pressure control. In this study, the digit symbol substitution test (DSST) and total brain volume were measured by MRI. At the 80-month follow-up, there was no significant difference in DSST test scores or brain structure between the two groups (Murray et al., 2017).
The largest sample size in these studies was related to the MRC/BHF study. However, it had the highest dropout rate, which was significantly related to the participants who had lower scores on cognitive tests at the baseline assessment. Both the MRC/BHF and ACCORD-MIND studies had long follow-up periods of 5 years or more.
None of these studies, which targeted several cardiovascular risk factors, showed a significant reduction in cognitive impairment or cognitive function improvement. In the only study that examined the effect of weight loss, the cognitive performance improvement was similar to the cognitive rehabilitation program (control group) (Table 6).

4. Discussion
The present study reviewed the medical treatment of four common cardiovascular risk factors and lifestyle interventions, including physical activity and diet. The aim was to evaluate the results of all randomized clinical trials (RCTs) of appropriate quality to assess dementia as a primary or secondary outcome.
Over the past 20 years, several RCTs have been conducted on the effect of medical treatment on cardiovascular risk factors for dementia. Most of the studies mentioned in the present review had a large sample size and considerable follow-up time.
These studies evaluated the effect of controlling cardiovascular risk factors on mortality and a range of vascular outcomes, including stroke, myocardial infarction, and peripheral arterial disease, as the core purpose. However, the evaluation of cognitive impairment, dementia, or improvement in cognitive function was considered a secondary outcome. There is only one positive study (Syst-Eur) that shows the protective consequence of hypertension treatment. Because different antihypertensive regimens have been used in these studies, the cognitive effect of a particular class of antihypertensive medications was not obvious (Forette et al., 2002).
There is no evidence of prophylactic effects on dementia for type 2 diabetes management and statin therapy. The long-term effect of subclinical hypoglycemia and functional disorders on brain autoregulation may be the cause of cognitive function exacerbation in the ILI group. A similar mechanism is seen in tight control in type 1 diabetes. Another reason may be related to the reduced neuroprotective effects of leptin in this group. Another reason may be related to the reduced neuroprotective effects of leptin in this group. The effects of physical activity on improving cognitive function were promising. However, these studies often had a smaller sample size and a much shorter setting time than other reviewed studies (Emery et al., 2003; Espeland et al., 2017; Fiocco et al., 2013; Teixeira et al., 2019; Xu et al., 2017; Yamamoto et al., 2009). However, these studies often had a smaller sample size and a much shorter setting time than other reviewed studies (Murray et al., 2017; Shepherd et al., 2002; Strandberg et al., 2006).
The most important bias that may have affected the study results is the exclusion of samples due to cognitive impairment, especially if a study is not explicitly designed to monitor cognitive function, as cognitive impairment may lead to cessation of informed consent or hospitalization. This omission from the selected study may reduce the potential treatment effect and consequently lead to a type 2 error, particularly if the intervention is effective and dropout occurs more frequently in the control group. In all studies on hypertension, further treatment with other antihypertensive medications was allowed in both the intervention and placebo groups if needed to achieve acceptable blood pressure levels. As a result, many patients in the control group received antihypertensive medications, which may reduce experimental differentiation and its effect on cardiovascular outcomes and dementia (Forette et al., 2002; Lithell et al., 2003; Peters et al., 2008; Tzourio et al., 2003). In cholesterol studies, this additional treatment with statins was performed less frequently than the study medications. In many cases, placebo-controlled trials are often not possible for ethical reasons (Shepherd et al., 2002). The age of the participants can also be another factor affecting the results. The study population is relatively young in most studies, and therefore, its effect on cognitive impairment and the incidence of dementia during follow-up was not recognizable. In the age range of 65-69, dementia is still relatively rare, with an incidence is approximately 2.4 cases per 1000 people, which increases sharply with age to 70.2 cases per 1000 people over the age of 90 (Rizzi, Rosset et al., 2014). However, it was expected that the number of participants with dementia or significant cognitive impairment would be low in most studies, limiting the study’s ability to detect such an effect.
Another point is the effect of mortality on the probability of disease. Dementia and cognitive disorders are age-related. The incidence of dementia may be increased by preventing cardiovascular disease, which reduces mortality. In many studies, interventions reduced mortality. However, none of the studies investigated the role of premature mortality in reducing the incidence of dementia.
One of the limitations of this study was the lack of a baseline cognitive assessment, which makes changes after the intervention not well interpretable. Another limitation was the cognitive assessment time, which was up to 3 months between the cognitive assessment and the end time of the program, and future studies should reduce this time to less than two weeks. Clinical dementia is the result of the interaction of brain injuries secondary to various risk factors, and brain resilience and cognitive reserve and the presence of a high cognitive reserve can correct this damage. Cognitive reserve was not initially measured in many of these studies; thus, the effectiveness of interventions in people with different cognitive reserves cannot be assured.
On the other hand, the cognitive assessment, which was performed in the AHEAD study was years after the initial randomization. Studies should take into account the potential loss of effective follow-up in a percentage of patients; given the time gap between study randomization and cognitive assessment, it is possible that some patients with cognitive impairment in the study may have passed away due to old age.
Some studies found that weight loss can be a sign of the onset of cognitive impairment. In contrast, some trials on weight loss suggested that obesity heightens the risk of dementia in midlife but may reduce the risk of dementia in later stages of life and during the aging process. These studies suggest that neurodegenerative diseases and dementia by affecting hormones, mood, and smell sense can lead to reduced oral intake and weight loss. The weight loss caused in these studies should be considered in two categories of desire (due to intervention) and unwanted (due to the onset of dementia). It is difficult to differentiate the effects of weight loss due to intervention from the weight loss impact due to the inflammatory and neurodegenerative process. Another hypothesis suggests that leptin can have positive effects on neurogenesis and reduce apoptosis, and due to the decrease in leptin in the ILI group, these positive effects are also reduced. It can be suggested that in the ILI group, with a decrease in leptin, a decrease in neurogenesis in the hippocampus, potentially affecting the molecular and structural aspects of the brain.
In general, brain structural alterations are utilized to evaluate cognitive function outcomes. Another aspect to consider is that the ILI group exhibits fewer microvascular changes and less cerebral atrophy, with a 9% reduction in ventricular volume, lower global atrophy, and a 28% decrease in white matter changes. Unexpectedly, there is no association between decreased cognitive function in the ILI group and atrophy and vascular lesions of the brain (Look & Wing, 2010). As a result, functional imaging techniques may be able to answer our question of why more cognitive decline occurs despite fewer brain changes in the ILI group.
The ACCORD-MIND study also showed that strict control of blood sugar leads to greater overall brain volume despite no difference in cognitive function. On the other hand, it should be borne in mind that ILI can lead to subclinical hypoglycemia and this hypoglycemia has long-term negative effects on brain function and cognition (Murray et al., 2017). In other words, it is essential to explore the concept of self-regulation of cerebral arteries and whether a comparable mechanism exists for organ damage resulting from tight blood sugar control in diabetic patients, potentially causing modifications or harm to cerebral arteries in the elderly. Another issue to consider is the effect of legacy, which is the lag time between the intervention time that affects the metabolic process in diabetic patients and the benefits that the patient achieves. On the other hand, most of the mentioned studies designed short-term interventions, while the process of dementia and cognitive decline is much longer. From midlife, where risk factors become apparent, to old age when the dementia process unfolds, a considerable amount of time elapses. A short study duration can pose a significant limitation in observing the impact of metabolic disorders. Moreover, in long-term studies, the cost-effectiveness of interventions should also be regarded as a crucial challenge.
In general, it seems that exercise can improve cognitive function by improving glucose metabolism in the elderly with complaints of memory impairment. Exercise may increase the oxidative load, which is high in type 2 diabetes. Studies have shown that even moderate exercise levels can increase the level of free radicals and lead to oxidative effects. In contrast, regular physical activity can protect against oxidative effects. Diet is another factor that should be considered in relation to cognitive function and physical activity in patients with type 2 diabetes. There must be a control group to determine that cognitive decline can be part of normal aging apart from the intervention.
Lastly, it is imperative to contemplate the methodology employed for cognitive assessment and the diverse nature of testing, especially when certain interventions fail to influence cognitive function. While the MMSE is commonly utilized in numerous studies for diagnosing established dementia, it may lack sensitivity in cognitively healthy individuals and younger age groups. We need to use a more sensitive test whenever we cannot find a significant change in clinical trials and prevention studies. Thus, it is recommended to use a more comprehensive battery, such as AVLT in future studies.
Similar to all review studies, publication bias could impact our search outcomes. Typically, studies do not publish negative results in the abstract section, potentially leading to the exclusion of certain articles during the initial search phase.
5. Conclusion
After reviewing the studies, it can be inferred that there is inadequate evidence to support the notion that treating cardiovascular risk factors can prevent dementia. Although exercise, and conceivably blood pressure management, exhibit a preventive impact, the effectiveness is less apparent for statin therapy and intensive treatment of type 2 diabetes. The discontinuation of studies, the inability to sustain study participation, competitive risks, or various forms of selection or treatment bias could have reduced the potential effects of interventions in the trials mentioned. Future RCTs involving diverse populations with alternative interventions and extended follow-up periods, particularly aimed at assessing their impact on cognitive function or dementia, can tackle the fundamental question of whether the associations observed in cohort studies can translate into substantial clinical therapeutic benefits for cognition.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflicts of interest.
References
Bae, E. M., & Park, S. M. (2021). Association between Variations in Body Mass Index and Cognitive Function in Older Korean Adults. Journal of Obesity & Metabolic Syndrome, 30(3), 271-278. [DOI:10.7570/jomes21044] [PMID] [PMCID]
Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet. Neurology, 10(9), 819-828. [DOI:10.1016/S1474-4422(11)70072-2] [PMID]
Baumgart, M., Snyder, H. M., Carrillo, M. C., Fazio, S., Kim, H., & Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11(6), 718-726. doi: [DOI:10.1016/j.jalz.2015.05.016] [PMID]
Beck, C., Fausett, J. K., Krukowski, R. A., Cornell, C. E., Prewitt, T. E., & Lensing, S., et al. (2013). A randomized trial of a community-based cognitive intervention for obese senior adults. Journal of Aging and Health, 25(1), 97–118. [DOI:10.1177/0898264312467374] [PMID] [PMCID]
Biessels, G. J., Deary, I. J., & Ryan, C. M. (2008). Cognition and diabetes: A lifespan perspective. The Lancet. Neurology, 7(2), 184–190.[DOI:10.1016/S1474-4422(08)70021-8] [PMID]
Biessels, G. J., & Despa, F. (2018). Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nature Reviews. Endocrinology, 14(10), 591–604. [DOI:10.1038/s41574-018-0048-7] [PMID] [PMCID]
Bloom, R., Schnaider-Beeri, M., Ravona-Springer, R., Heymann, A., & Dabush, H., Bar, L., et al. (2017). Computerized cognitive training for older diabetic adults at risk of dementia: Study protocol for a randomized controlled trial. Alzheimer’s & Dementia (New York, N. Y.), 3(4), 636-650. [DOI:10.1016/j.trci.2017.10.003] [PMID] [PMCID]
Brown G. C. (2015). Living too long: The current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Reports, 16(2), 137–141. [DOI:10.15252/embr.201439518] [PMID] [PMCID]
Carles, S., Jr, Curnier, D., Pathak, A., Roncalli, J., Bousquet, M., & Garcia, J. L., et al. (2007). Effects of short-term exercise and exercise training on cognitive function among patients with cardiac disease. Journal of Cardiopulmonary Rehabilitation and Prevention, 27(6), 395–399. [DOI:10.1097/01.HCR.0000300268.00140.e6] [PMID]
Corrada, M. M., Hayden, K. M., Paganini-Hill, A., Bullain, S. S., DeMoss, J., & Aguirre, C., et al. (2017). Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimer's & Dementia, 13(2), 103-110. [DOI:10.1016/j.jalz.2016.09.007] [PMID] [PMCID]
Cummings, J., Morstorf, T., & Lee, G. (2016). Alzheimer’s drug-development pipeline: 2016. Alzheimer’s & Dementia (New York, N. Y.), 2(4), 222-232. [DOI:10.1016/j.trci.2016.07.001] [PMID] [PMCID]
De Galan, B. E., Zoungas, S., Chalmers, J., Anderson, C., Dufouil, C., Pillai, A., ... & ADVANCE Collaborative Group. (2009). Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: The action in diabetes and vascular disease: Preterax and diamicron modified release controlled evaluation (ADVANCE) trial. Diabetologia, 52, 2328-2336. [DOI:10.1007/s00125-009-1484-7]
Doruk, H., Naharci, M. I., Bozoglu, E., Isik, A. T., & Kilic, S. (2010). The relationship between body mass index and incidental mild cognitive impairment, Alzheimer’s disease and vascular dementia in elderly. The Journal of Nutrition, Health & Aging, 14(10), 834–838. [DOI:10.1007/s12603-010-0113-y] [PMID]
Emery, C. F., Hsiao, E. T., Hill, S. M., & Frid, D. J. (2003). Short-term effects of exercise and music on cognitive performance among participants in a cardiac rehabilitation program. Heart & Lung: The Journal of Critical Care, 32(6), 368–373. [DOI:10.1016/S0147-9563(03)00120-1] [PMID]
Espeland, M. A., Lipska, K., Miller, M. E., Rushing, J., Cohen, R. A., & Verghese, J., et al. (2017). Effects of Physical Activity Intervention on Physical and Cognitive Function in Sedentary Adults With and Without Diabetes. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(6), 861–866. [DOI:10.1093/gerona/glw179] [PMID] [PMCID]
Fiocco, A. J., Scarcello, S., Marzolini, S., Chan, A., Oh, P., & Proulx, G., et al. (2013). The effects of an exercise and lifestyle intervention program on cardiovascular, metabolic factors and cognitive performance in middle-aged adults with type II diabetes: A pilot study. Canadian Journal of Diabetes, 37(4), 214–219. [DOI:10.1016/j.jcjd.2013.03.369] [PMID]
Forette, F., Seux, M. L., Staessen, J. A., Thijs, L., Babarskiene, M. R., & Babeanu, S., et al. (2002). The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Archives of Internal Medicine, 162(18), 2046-2052. [DOI:10.1001/archinte.162.18.2046] [PMID]
Gunstad, J., Paul, R. H., Cohen, R. A., Tate, D. F., Spitznagel, M. B., & Grieve, S., et al. (2008). Relationship between body mass index and brain volume in healthy adults. The International Journal of Neuroscience, 118(11), 1582–1593. [DOI:10.1080/00207450701392282] [PMID]
Heart Protection Study Collaborative Group (2002). MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet (London, England), 360(9326), 7–22. [DOI:10.1016/S0140-6736(02)09327-3] [PMID]
Hsiung, G. Y., & Sadovnick, A. D. (2007). Genetics and dementia: Risk factors, diagnosis, and management. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 3(4), 418–427. [DOI:10.1016/j.jalz.2007.07.010] [PMID]
Lee, C. J., Lee, J. Y., Han, K., Kim, D. H., Cho, H., & Kim, K. J., et al. (2022). Blood pressure levels and risks of dementia: A nationwide study of 4.5 million people. Hypertension, 79(1), 218-229. [DOI:10.1161/HYPERTENSIONAHA.121.17283] [PMID]
Lee, Y., Back, J. H., Kim, J., Kim, S. H., Na, D. L., & Cheong, H. K., et al. (2010). Systematic review of health behavioral risks and cognitive health in older adults. International Psychogeriatrics, 22(2), 174–187. [DOI:10.1017/S1041610209991189] [PMID]
Lithell, H., Hansson, L., Skoog, I., Elmfeldt, D., Hofman, A., & Olofsson, B., et al. (2003). The study on cognition and prognosis in the elderly (SCOPE): Principal results of a randomized double-blind intervention trial. Journal of Hypertension, 21(5), 875–886. [DOI:10.1097/00004872-200305000-00011] [PMID]
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., & Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413-446. [DOI:10.1016/S0140-6736(20)30367-6] [PMID]
Look, A. R. G., & Wing, R. R. (2010). Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Archives of Internal Medicine, 170(17), 1566-1575. [DOI:10.1001/archinternmed.2010.334]
Luchsinger, J. A., Patel, B., Tang, M. X., Schupf, N., & Mayeux, R. (2007). Measures of adiposity and dementia risk in elderly persons. Archives of Neurology, 64(3), 392–398. [DOI:10.1001/archneur.64.3.392] [PMID] [PMCID]
McGuinness, B., Craig, D., Bullock, R., & Passmore, P. (2016).Statins for the prevention of dementia. The Cochrane Database of Systematic Reviews, 2016(1), CD003160. [DOI:10.1002/14651858.CD003160.pub3] [PMID]
Moheet, A., Mangia, S., & Seaquist, E. R. (2015). Impact of diabetes on cognitive function and brain structure. Annals of the New York Academy of Sciences, 1353, 60–71. [DOI:10.1111/nyas.12807] [PMID] [PMCID]
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Clinical research ed.), 339, b2535. [DOI:10.1136/bmj.b2535] [PMID] [PMCID]
Murray, A. M., Hsu, F. C., Williamson, J. D., Bryan, R. N., Gerstein, H. C., & Sullivan, M. D., et al. (2017). ACCORDION MIND: Results of the observational extension of the ACCORD MIND randomised trial. Diabetologia, 60(1), 69-80. [DOI:10.1007/s00125-016-4118-x] [PMID] [PMCID]
Naderali, E. K., Ratcliffe, S. H., & Dale, M. C. (2009). Obesity and Alzheimer’s disease: A link between body weight and cognitive function in old age. American Journal of Alzheimer's Disease and Other Dementias, 24(6), 445–449. [DOI:10.1177/1533317509348208] [PMID] [PMCID]
Nilsson, L. G., & Nilsson, E. (2009). Overweight and cognition. Scandinavian Journal of Psychology, 50(6), 660–667 [DOI:10.1111/j.1467-9450.2009.00777.x] [PMID]
Park, S. H., Kim, J. H., Choi, K. H., Jang, Y. J., Bae, S. S., & Choi, B. T., et al. (2013). Hypercholesterolemia accelerates amyloid β-induced cognitive deficits. International Journal of Molecular Medicine, 31(3), 577–582. [DOI:10.3892/ijmm.2013.1233] [PMID]
Patterson, C., & Alzheimer’s Disease International (ADI). (2018). World Alzheimer report 2018. London: Alzheimer’s Disease International. [Link]
Peters, R., Beckett, N., Forette, F., Tuomilehto, J., Clarke, R., & Ritchie, C., et al. (2008). Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. The Lancet. Neurology, 7(8), 683-689. [DOI:10.1016/S1474-4422(08)70143-1] [PMID]
Ravona-Springer, R., & Schnaider-Beeri, M. (2011). The association of diabetes and dementia and possible implications for nondiabetic populations. Expert Review of Neurotherapeutics, 11(11), 1609–1617. [DOI:10.1586/ern.11.152] [PMID] [PMCID]
Rizzi, L., Rosset, I., & Roriz-Cruz, M. (2014). Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Research International, 2014, 908915. [DOI:10.1155/2014/908915] [PMID] [PMCID]
Ryan, D. H., Espeland, M. A., Foster, G. D., Haffner, S. M., Hubbard, V. S., & Johnson, K. C., et al. (2003). Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials, 24(5), 610–628. [DOI:10.1016/S0197-2456(03)00064-3] [PMID]
Shepherd, J., Blauw, G. J., Murphy, M. B., Bollen, E. L., Buckley, B. M., & Cobbe, S. M., et al. (2002). Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet (London, England), 360(9346), 1623-1630. [DOI:10.1016/S0140-6736(02)11600-X] [PMID]
Strandberg, T. E., Pitkala, K. H., Berglind, S., Nieminen, M. S., & Tilvis, R. S. (2006). Multifactorial intervention to prevent recurrent cardiovascular events in patients 75 years or older: The drugs and evidence-based medicine in the elderly (DEBATE) study: A randomized, controlled trial. American Heart Journal, 152(3), 585–592. [DOI:10.1016/j.ahj.2006.02.006] [PMID]
Teixeira, C. V., Gobbi, S., Pereira, J. R., Vital, T. M., Hernandéz, S. S., & Shigematsu, R., et al. (2013). Effects of square-stepping exercise on cognitive functions of older people. Psychogeriatrics, 13(3), 148-156. [DOI:10.1111/psyg.12017] [PMID]
Teixeira, R. B., Marins, J. C. B., Amorim, P. R. S., Teoldo, I., Cupeiro, R., & Andrade, M. O. C., et al. (2019). Evaluating the effects of exercise on cognitive function in hypertensive and diabetic patients using the mental test and training system. The World Journal of Biological Psychiatry, 20(3), 209–218. [DOI:10.1080/15622975.2017.1337222] [PMID]
Tzourio, C., Anderson, C., Chapman, N., Woodward, M., Neal, B., & MacMahon, S., et al. (2003). Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Archives of Internal Medicine, 163(9), 1069-1075. [DOI:10.1001/archinte.163.9.1069] [PMID]
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C., & Yaffe, K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277-281. [DOI:10.1212/01.WNL.0000149519.47454.F2] [PMID]
Xu, F., Delmonico, M. J., Lofgren, I. E., Uy, K. M., Maris, S. A., & Quintanilla, D., et al. (2017). Effect of a Combined Tai Chi, resistance training and dietary intervention on cognitive function in obese older women. The Journal of Frailty & Aging, 6(3), 167–171. [PMID]
Yamamoto, N., Yamanaka, G., Takasugi, E., Ishikawa, M., Yamanaka, T., & Murakami, S., et al. (2009). Lifestyle intervention reversed cognitive function in aged people with diabetes mellitus: two-year follow up. Diabetes Research and Clinical Practice, 85(3), 343–346. [DOI:10.1016/j.diabres.2009.05.014] [PMID]
Type of Study: Review |
Subject:
Clinical Neuroscience
Received: 2022/01/17 | Accepted: 2022/02/23 | Published: 2024/05/1
Received: 2022/01/17 | Accepted: 2022/02/23 | Published: 2024/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








