Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 605-614 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghazvini H, Tirgar F, Khodamoradi M, Seyedhosseini Tamijani S M, Niknamfar S, Akbari E, et al . Investigating Facilitatory Effects of Lithium on Methamphetamine-induced Spatial Memory Impairments in Rat. BCN 2023; 14 (5) :605-614
URL: http://bcn.iums.ac.ir/article-1-2348-en.html
URL: http://bcn.iums.ac.ir/article-1-2348-en.html
Hamed Ghazvini *1 

 , Fatemeh Tirgar2
, Fatemeh Tirgar2 

 , Mehdi Khodamoradi3
, Mehdi Khodamoradi3 

 , Seyedeh Masoumeh Seyedhosseini Tamijani1
, Seyedeh Masoumeh Seyedhosseini Tamijani1 

 , Saba Niknamfar4
, Saba Niknamfar4 

 , Esmaeil Akbari5
, Esmaeil Akbari5 

 , Mohammad Nekahi4
, Mohammad Nekahi4 

 , Nabiollah Tarjani1
, Nabiollah Tarjani1 

 , Hossein Ghalehnoei1
, Hossein Ghalehnoei1 

 , Motahareh Rouhi Ardeshiri6
, Motahareh Rouhi Ardeshiri6 




 , Fatemeh Tirgar2
, Fatemeh Tirgar2 

 , Mehdi Khodamoradi3
, Mehdi Khodamoradi3 

 , Seyedeh Masoumeh Seyedhosseini Tamijani1
, Seyedeh Masoumeh Seyedhosseini Tamijani1 

 , Saba Niknamfar4
, Saba Niknamfar4 

 , Esmaeil Akbari5
, Esmaeil Akbari5 

 , Mohammad Nekahi4
, Mohammad Nekahi4 

 , Nabiollah Tarjani1
, Nabiollah Tarjani1 

 , Hossein Ghalehnoei1
, Hossein Ghalehnoei1 

 , Motahareh Rouhi Ardeshiri6
, Motahareh Rouhi Ardeshiri6 


1- Psychiatry and Behavioral Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Substance Abuse Prevention Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Department of Neuroscience, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
5- Department of Physiology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
6- Immunogenetics Research Center, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Substance Abuse Prevention Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Department of Neuroscience, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
5- Department of Physiology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
6- Immunogenetics Research Center, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 1513 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Methamphetamine (METH) is one of the synthetic psychostimulants with deleterious effects on brain and behavior (Camarasa et al., 2010; Khalifeh et al., 2019). A growing body of literature indicates that exposure to METH results in long-lasting damage to dopaminergic and serotonergic nerve terminals (Thomas et al., 2010; Thomas et al., 2004). One of the significant effects of METH abuse is the neurocognitive deficit that persists long after drug withdrawal. Recently, studies demonstrated that METH-exposed animals showed significant impairments in performance on hippocampus-dependent spatial tasks, such as the Morris water maze (MWM) (Ghalehnoei et al., 2020; Ghazvini et al., 2016).
In recent years, numerous studies have been conducted on the role of lithium in neurological dysfunction in various brain diseases (Chuang, 2004). Lithium has neuroprotective effects, such as hippocampal neurogenesis, that ameliorate neurological deficits and carry long-term potentiation in the hippocampus of rat dentate gyrus (Contestabile et al., 2012; Kim et al., 2004; Yan et al., 2007). In addition, it has been suggested that lithium is recognized as an appropriate agent for hippocampal-dependent cognitive enhancement. For example, Yu et al. (2012) have shown that treatment with lithium in animals exposed to traumatic brain injury reduces deficits in spatial learning in the MWM.
On the other hand, lithium has neuroprotective properties by inhibiting apoptosis and promoting neuronal survival (Fan et al., 2015; Zhong et al., 2006). In this regard, animals treated with lithium exhibit a reduced apoptosis of neural progenitor cells in the hippocampus and improved memory deficits (Huo et al., 2012). Meanwhile, Fan et al. (2015) indicated that lithium chloride administration prevented spatial learning and memory deficits in mice with cerebral ischemia by inhibiting apoptosis and increasing BDNF expression in the hippocampus.
There are a few relevant documents on the neurocognitive effects of lithium on METH-induced behavioral toxicities; however, recent work has demonstrated that lithium modulates METH toxicity through its impact on monoaminergic release. For example, Ago et al. have shown that treatment with lithium attenuates METH-induced hyper-locomotion and behavioral sensitization by modulating the prefrontal monoamine release in mice (Ago et al., 2012). Nevertheless, the effect of lithium on METH-induced memory impairment is still largely unknown.
Accordingly, given the importance of hippocampal apoptosis in cognitive deficits, the present study aims to understand the possible effects of lithium on preventing deficits in spatial learning and memory induced by repeated administration of METH in male Wistar rats.
2. Materials and Methods
Study animals
This study was performed on male Wistar rats weighing between 200 g and 250 g at the beginning of the test. Sufficient effort was taken to reduce the number and suffering of the study animals. During the experimental procedure, the rats had access to free food and water and were housed in groups of 4 in a cage. Its temperature was maintained at 23±1°C with a 12 h light/dark cycle (light beginning at 7:00 AM).
Experimental protocols
The animals were randomly assigned into four groups (n=8 in each group). The experimental groups were as follows: 1) Saline group: The rats that received normal saline subcutaneously (SC); 2) METH-exposed group: The rats that received METH (6 mg/kg, SC); 3) Lithium group: The rats that received a lithium injection; 4) METH+lithium group: Rats that received lithium (20 mg/kg, intraperitoneal [IP]) and METH (6 mg/kg, SC). The animals were examined for spatial learning and memory using the MWM one day after the treatments. Finally, the rats were sacrificed for histological examination and measurement of brain water content. For histological analysis, hippocampal tissue was prepared for the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay.
Morris water maze
MWM behavioral task was used to assess spatial learning memory. As previously described (Hajali et al., 2015; Hajali et al., 2012), the aspects of the apparatus were a stainless steel water tank 160 cm in diameter and 80 cm in height, filled with water to a depth of 40 cm. The water temperature was maintained at 22±1°C. The maze was geographically separated into four equal quadrants. The hidden platform (10 cm in diameter) was submerged 1.5 cm below the water’s surface in the center of quadrant 4. A camera was located above the water tank and attached to a computer. The performance of the animals was recorded by a smart video tracking system (Noldus Ethovision® software, version 5. The rats were trained to find the hidden platform according to the visual cues (squares, circles, or triangles) placed around the room.
Spatial learning and memory
The acquisition phase consisted of three training blocks parted by a 30 min rest interval. Every block included four 60 s consecutive trials and three 60 s inter-trial intervals. Each trial was started by placing the animal into the water with its face toward the wall of the water pool. The starting point varied randomly in different quadrants from trial to trial, with an equal number of trials starting from each quadrant. The animal was released into the maze and allowed to swim for a maximum of 60 s in each trial to find the hidden escape platform. After the animal found the platform, it was allowed to remain there for 20 to 30 s and was then removed and put into an animal cage to wait for 30 to 35 s before the subsequent trial. Each animal that failed to find the platform within 60 s was guided to the platform. The time and distance to find the hidden platform were collected, analyzed, and interpreted later.
Subsequently, a probe test was carried out 2 h after the training to test. In the probe test, the hidden platform was removed, and the animal was placed in quadrant 2, across from quadrant 4 (target quadrant). The animal was permitted to swim for 60 s in the water maze. The animal’s time in the target quadrant was analyzed to measure spatial memory retention. At the end of the probe trial, rats did a visible platform test to determine any possibility of intervention with sensory and motor coordination or motivation. In this test, an apparent platform covered with aluminum foil was raised 2 cm above the water surface, and the animal’s ability to escape to a visible platform was calculated (Ghazvini et al., 2016).
Determination of brain water content
To determine the water content of the brain, rats were deeply anesthetized and decapitated 24 h after drug administration, and then the brain was removed. The wet tissue’s weight was calculated and placed in an oven for 72 h to obtain the dry weight. The water content was measured as the difference between the wet and dry weights of the brain according to the Equation 1:
1. Water content=([Wet mass-Dry mass]/[Wet mass]) ×100 (Ahmad et al., 2008).
Tissue preparation
Following the behavioral tests, animals were profoundly anesthetized, and transcranial perfusion was carried out with 0.9% saline followed by fixative solution (glutaraldehyde 1.25% and paraformaldehyde %1 in 0.01 M. phosphate-buffered saline [PBS] at pH=7.4) for 1 h. The brain was carefully extracted after the perfusion, washed in normal saline, fixed in the same fixative overnight, and eventually embedded in paraffin. Coronal sections (5 µm thick) were taken using a microtome from 3.3 mm to 4.2 mm posterior to bregma according to the Paxinos atlas.
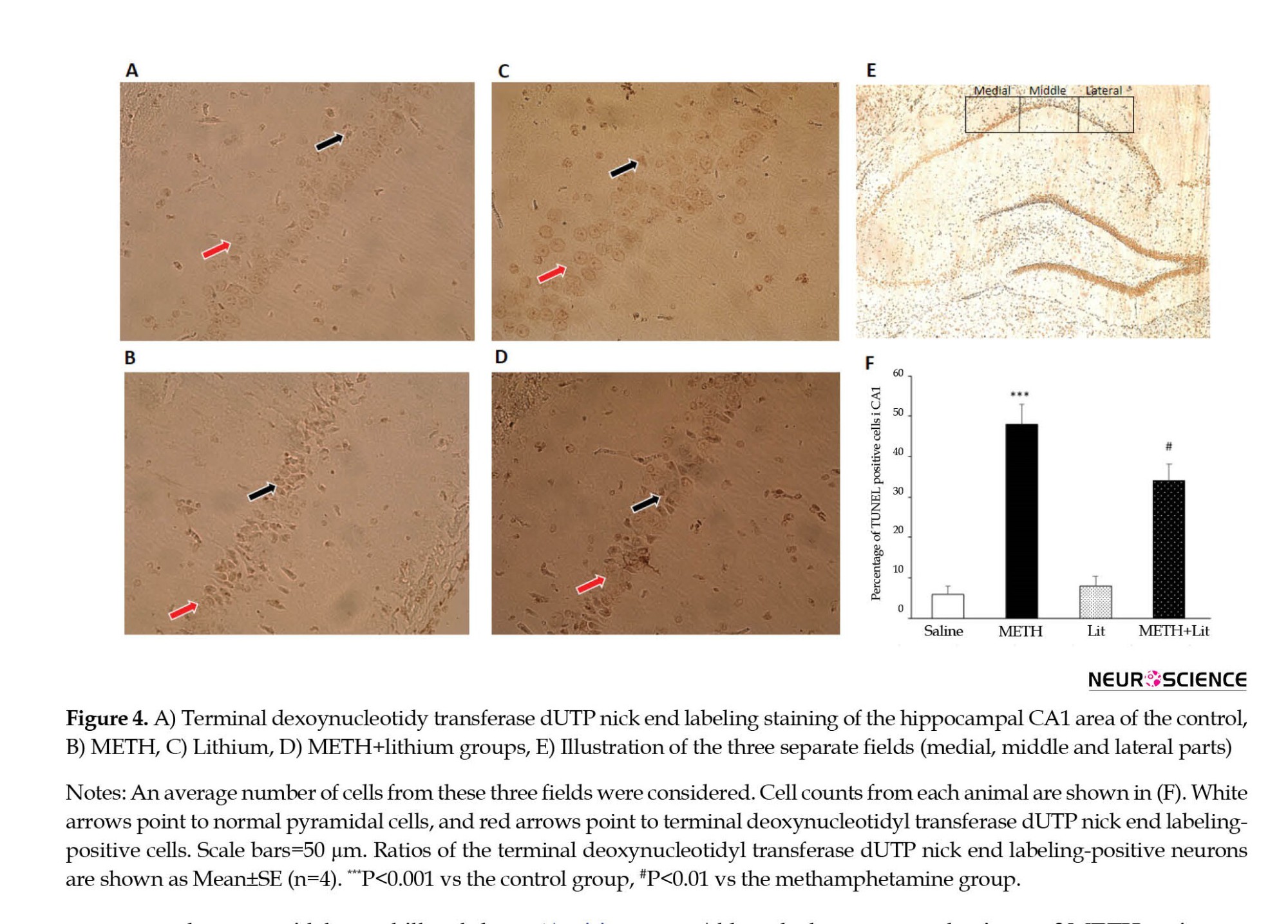
The TUNEL assay was performed to evaluate apoptosis in the pyramidal neurons of the hippocampal CA1 area, as described previously. To do so, a commercial kit (In Situ Cell Death Detection Kit; Roche, Mannheim, Germany) was used based on the instructions provided by the manufacturer. In brief, the paraffin-embedded sections were incubated in xylene for deparaffinization, dehydrated in descending ethanol concentrations, and rinsed in PBS. Permeabilization was done by 10 mM proteinase K for 30 min at room temperature. Subsequently, the sections were washed and incubated with 3% H2O2 in methanol for 10 min in a dark place to block endogenous peroxidase activity, leading to false-positive results. After adding the TUNEL reaction mixture, the sections were incubated at 37°C for 60 min in a chamber with appropriate humidity, washed with PBS, and visualized using a converter‒POD at 37°C for 30 min in a dark place with moderate humidity. Afterward, the sections were rinsed in PBS and incubated with 50–100 μL 0.05% 3, 3′-diaminobenzidine as a chromogen for 10 min. After washing with PBS, the sections were mounted on slides. Using a light microscope, the number of TUNEL-positive neurons was counted (400 μm long, 0.160 mm2) in the CA1 area of the right hippocampus. The number of pyramidal neurons in the hippocampal CA1 area was calculated in three fields (medial, middle, and lateral parts). An average number of cells from these three fields was considered as cell counts from each animal (Ghazvini et al., 2021).
Data analysis
The data from the acquisition phase, distance, and time spent to find the hidden platform were analyzed by a two-way analysis of variance (ANOVA) along with repeated measures to determine differences in the learning rates of the groups (group and block being the factors). All comparisons between the groups for the data collected in the MWM probe trials, swimming speed, and histological data were analyzed via ANOVA. The analyses of variances were followed by the Tukey test for multiple comparisons when required. The data were expressed as Mean±SE, and the level of statistical significance was considered P<0.05.
3. Results
Spatial learning
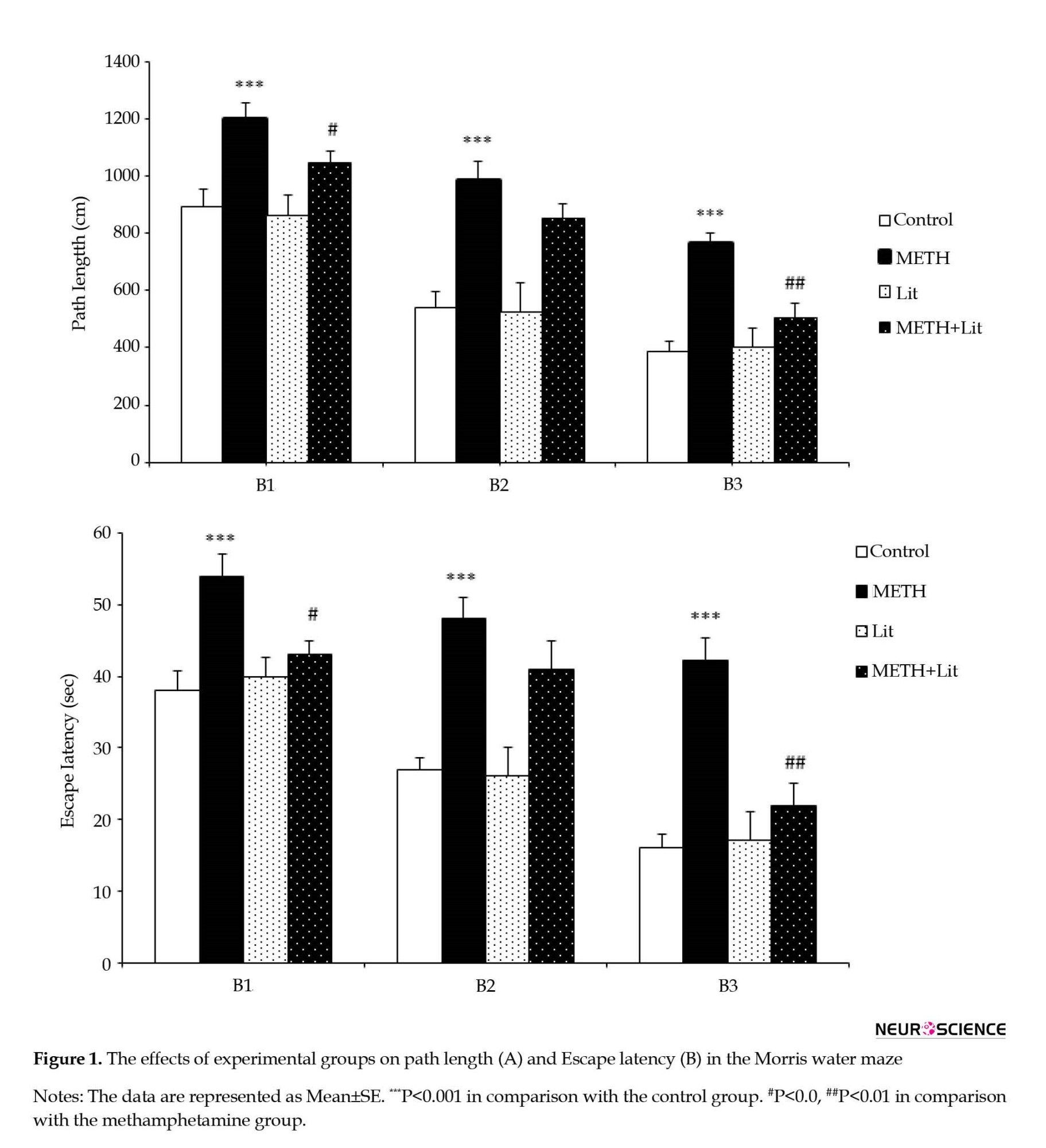
As shown in Figure 1A and Figure 1B, repeated measures analysis of ANOVA revealed that the path length and escape latency of the animals in the METH group significantly increased in block 1 (P<0.001), block 2 (P<0.001), and block 3 (P<0.001) compared to the control group, indicating an impairment in spatial learning after exposure to METH. The current results show that lithium reduces the path length and escape latency to find the hidden platform compared to the METH group. The mean distance traveled by the METH+lithium group in block 1 (P<0.05) and block 3 (P<0.01) decreased in comparison with the METH group (Figure 1A). The analysis also showed that the mean escape latency of the METH+lithium group in block 1 (P<0.05) and block 3 (P<0.01) decreased compared to the METH group (Figure 1B). Therefore, the cognitive ability of animals in the METH group to find hidden platforms was partially improved by lithium therapy.
Spatial short-term memory
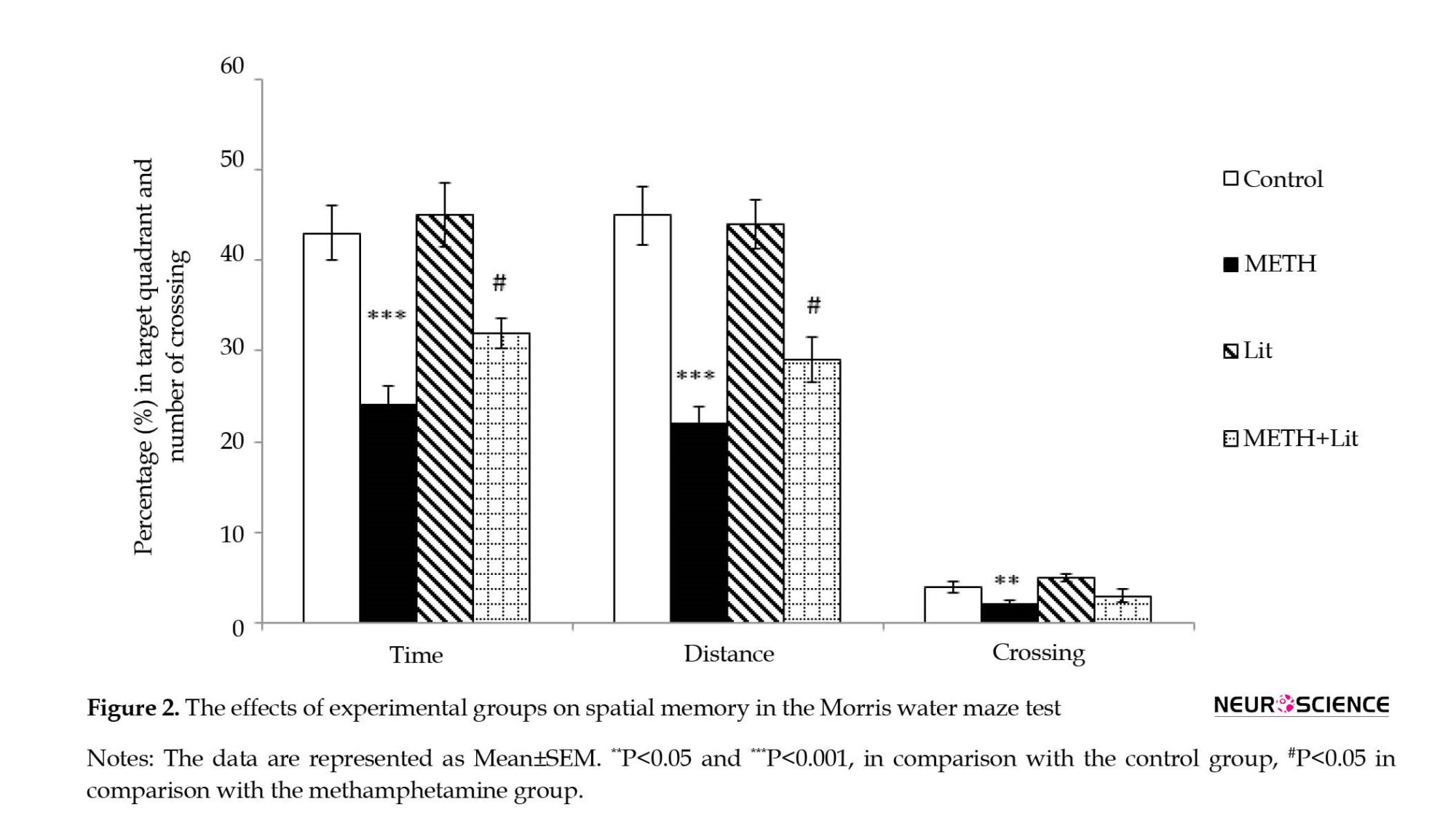
In a probe test, 2 h after the acquisition phase, the percentage of the time, distance, and the number of crossings in the target quadrant (quadrant 4) were calculated for short-term spatial memory retrieval. As illustrated in Figure 2, the one-way ANOVA showed that animals that received METH (6 mg/kg) did not recall the location of the platform well since the time spent, distance, and the number of crossing in the target quadrant was higher than that of the control group (P<0.001). The analysis also revealed that the METH+lithium group exhibits a decrease in spatial short-term memory impairment and spatial learning. The rats of the METH+lithium group spent more time (P<0.05) and distance (P<0.05) in quadrant four compared to the METH group. Further analysis also showed that the number of crossings in the target quadrant in the METH group significantly decreased compared to the control group (P<0.01). However, the number of crossing in the target quadrant in the METH+lithium group was not significantly different compared to the METH group.
Latency to the visible platform and swimming speed
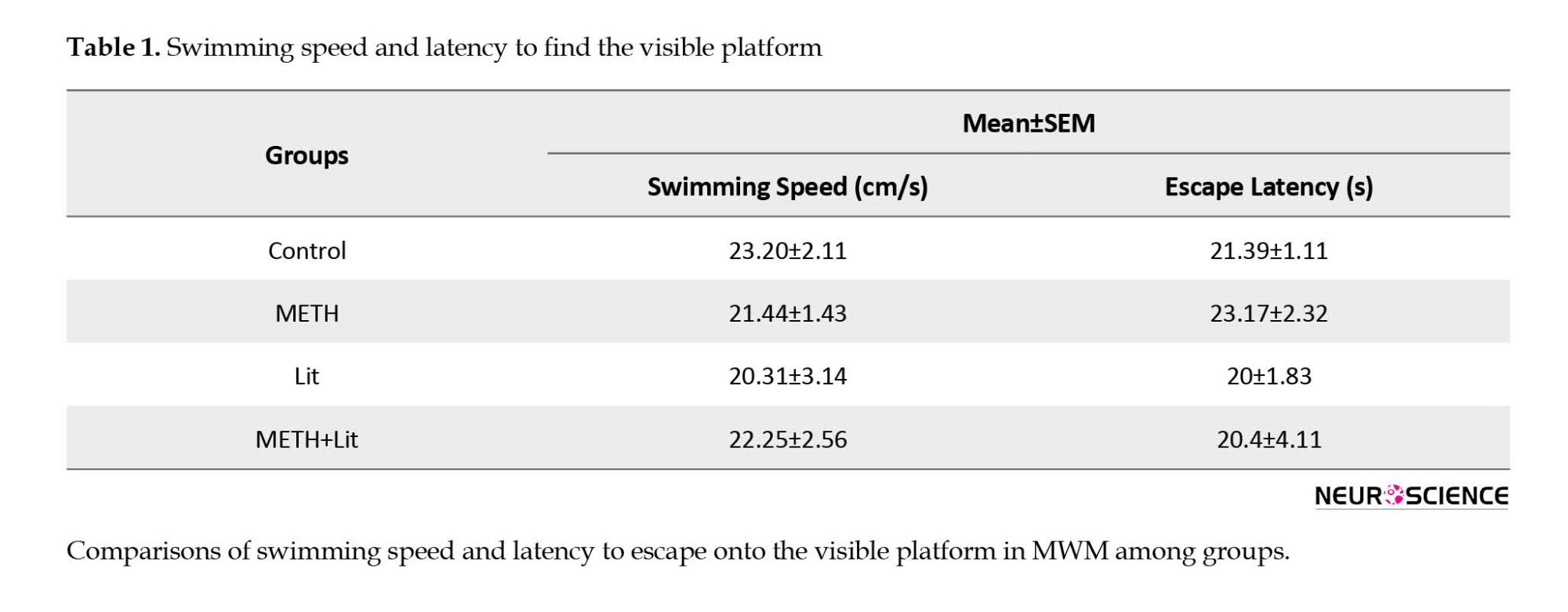
The analysis of escape latency to find the visible platform and swimming speed showed no difference between groups (P>0.05, Table 1). METH and lithium administration did not affect the visible test and swimming speed. Thus, visual and motor functions did not differ between groups.
Effects of lithium and methamphetamine regimen on brain water content
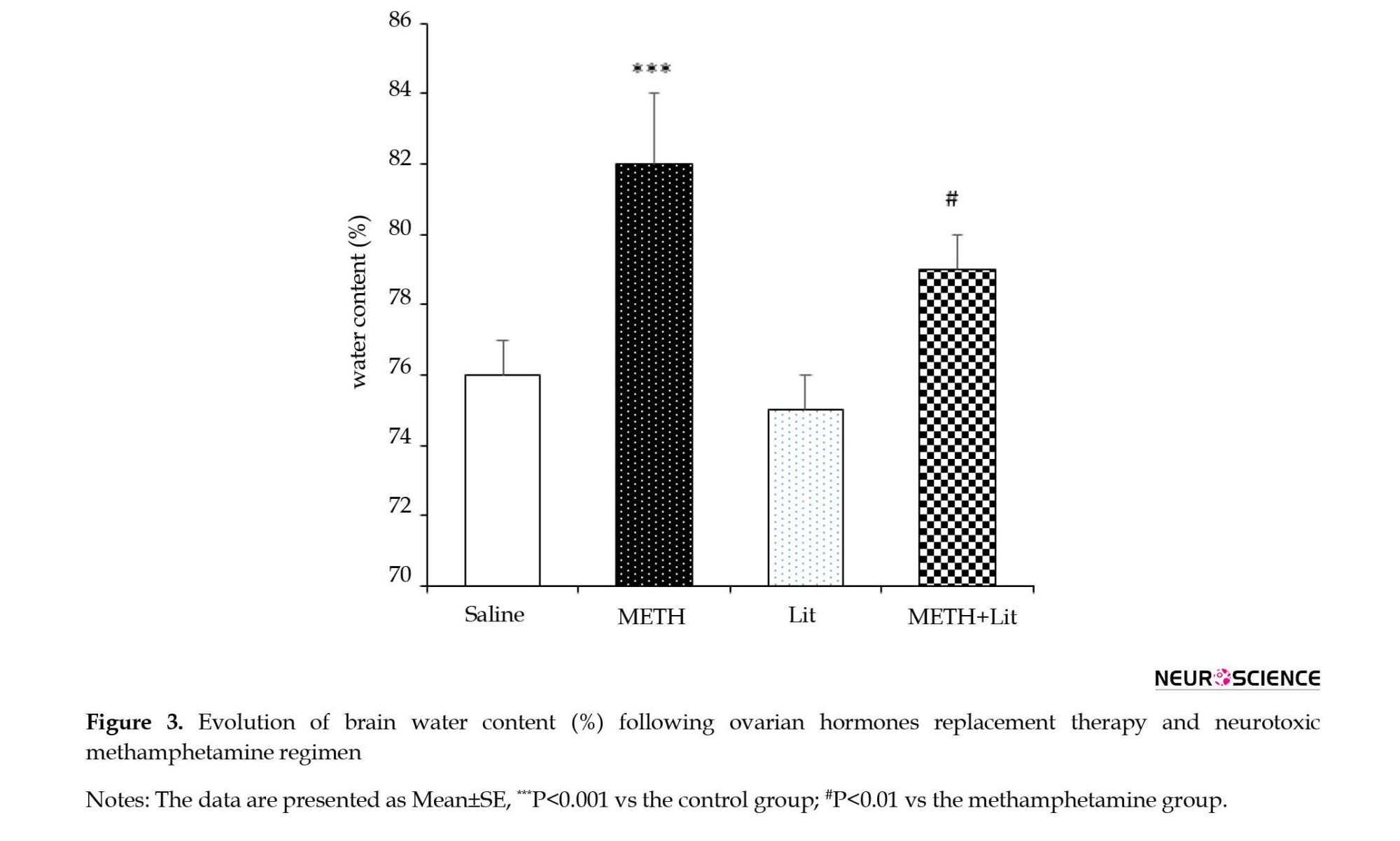
The rats were examined for brain edema based on the calculated brain water content. As shown in Figure 3, the brain water content was significantly increased in the METH group compared to the control group (P<0.001). In addition, the brain water content in the METH+lithium group was significantly lower than that in the METH group (P<0.05).
Effects of lithium and methamphetamine regimen on neuronal apoptosis in the hippocampal CA1 area
As illustrated in Figure 4, the number of TUNEL-positive cells in the CA1 region of the hippocampus in the METH group showed a significant increase compared to the control group (P<0.001; Figure 4B). In addition, the METH+lithium group revealed a substantial decrease in TUNEL-positive cells compared to the METH group (P<0.05; Figure 4D). However, the results showed that lithium administration alone did not affect the number of TUNEL-positive cells in the CA1 region.
4. Discussion
The current study aimed to investigate the possible effects of lithium on METH-induced neuronal damage and memory impairment. The results showed that the administration of METH had a disruptive impact on spatial learning and memory in male Wistar rats. In addition, treatment with lithium significantly improved spatial learning and memory impairment in METH-exposed rats. Moreover, the data showed that the neurotoxic regimen of METH (6 mg/kg, four injections with two h intervals) resulted in long-lasting cognitive deficits and was accompanied by neuronal cell death in the CA1 area of the hippocampus and brain edema, which was partially reversed by lithium.
A growing body of literature suggests that amphetamines lead to long-lasting cognitive impairment and are among the most widely used illegal drugs (Amiri et al., 2016; Robinson & Becker, 1986; Tirgar et al., 2014). The data presented here are in line with similar studies that have found an association between long-term cognitive impairment and neurotoxic regimens of METH (Friedman et al., 1998; Khodamoradi et al., 2022; Nagai et al., 2007). For example, Ghazvini et al. (2021) have shown that exposure to METH in rats leads to various cognitive problems, such as learning and memory impairment, anxiety-like behavior, and deficits in synaptic plasticity.
Although the exact mechanisms of METH action remain unclear and controversial, previous studies point to some possible mechanisms for behavioral neurotoxicity: a reduction in dopamine and serotonin transporter activity following METH administration (Nagai et al., 2007; Khodamoradi et al., 2022). In this point of view, studies suggest that exposure to a neurotoxic METH treatment leads to glial activation and increased neuronal apoptosis, which may contribute to METH cognitive impairment. A growing literature emphasizes that hippocampal activity is related to various neurocognitive actions (Khalifeh et al., 2017; Khodamoradi et al., 2018; Azimi Sanavi et al., 2022; Shahveisi et al., 2022). Moreover, our results and other authors’ results have demonstrated that the animals receiving METH exhibit neuronal cell death in the hippocampus, a key link between neuronal cell death and memory performance. In this regard, the present data showed, in line with previous results, that the administration of METH leads to a significant increase in TUNEL-positive cells in the CA1 region.
Recently, there has been increasing evidence that lithium has a neuroprotective effect on cognitive performance in some neurological disorders. For example, when injected IP daily for 2 weeks (1 mmol/kg) in male ischemic rats, lithium significantly improved spatial learning and memory in the MWM task (Yan et al., 2007). Moreover, daily treatment with lithium improved spatial memory functions in a mouse model of Alzheimer disease (Toledo & Inestrosa, 2010). These results confirm the existing evidence on the ability of lithium to prevent spatial learning and memory in METH-exposed rats.
Another concern about METH-induced neurotoxicity is related to brain edema. Recently, we reported that METH induces brain hyperthermia and brain edema, possibly due to blood-brain barrier breakdown. Based on our results, it is reasonable to speculate that the behavioral deficits observed in animals receiving METH are partly due to brain edema. In line with this hypothesis, previous studies have shown a close association between brain edema and memory impairment (Tominaga & Ohnishi, 1989). In agreement with our results, Kiyatkin et al. (2007) showed that METH 9 mg/kg leads to brain hyperthermia and brain edema in male Long-Evans rats. In the present study, one of the probable mechanisms involved in improving memory performance is somehow mediated by the effect of lithium on the reduction of brain edema; however, the exact mechanism is unclear.
Our results also demonstrated the beneficial effects of lithium on neuronal apoptosis cell death in METH-exposed animals. In agreement with our findings, Deng et al. (2001) found that a single injection of 40 mg/kg METH caused neuronal cell death in several brain areas, including striatum, cortex, and hippocampus. In addition, administration of METH to male Wistar rats caused dose-dependent neuronal apoptosis in the hippocampus (Shahidi et al., 2019). Thus, these data confirm the presence of METH-induced deleterious effects in non-dopaminergic neurons, which may contribute to the learning and memory processes. A large body of evidence suggests that neuronal apoptosis in the brain area is involved in cognitive performance. The cortex and hippocampus disrupt learning and memory function, and excessive neuronal apoptosis in the hippocampus contributes to memory dysfunction (Kim et al., 2013). From this point of view, recent evidence suggests that a neuroprotective agent may reduce neuronal apoptosis in the hippocampus and, in some way, improve memory function (Yang et al., 2013; Zarifkar et al., 2010). Thus, another likely mechanism for the cognitive enhancing effects of lithium observed in the current study may lie in the reduction of neuronal apoptosis in the hippocampus.
In addition, previous studies have reported that lithium can significantly improve learning and memory through its effects on hippocampal synaptic plasticity and neurogenesis. However, as revealed by previous studies, the exact mechanisms of how lithium exerts its effects may involve the modulation of signaling cascades in the brain, one of which is the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway (Yan et al., 2007). Nevertheless, the effects of lithium on METH neurotoxicity have been controversial in previous reports and require further investigation to be elucidated.
5. Conclusions
The present results suggest that treatment with lithium can partially alleviate the METH-induced spatial memory impairment in male rats. In addition, treatment with lithium significantly reduced brain edema and apoptosis of neuronal damage in the CA1 region in METH-exposed rats. The results also indicate that these neurocognitive effects of lithium may be associated with its anti-apoptotic effects in the hippocampus and reduction of brain edema, which may be influenced by numerous factors, such as the applied dose, duration and timing of treatment, age, and so on. However, further studies are needed to determine which mechanisms are involved in the protective effect of lithium against methamphetamine-induced cognitive impairment, such as decision-making, anxiety, etc.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Mazandaran University of Medical Sciences approved the study (Code: IR.MAZUMS.REC.1398.5168).
Funding
This research was supported by the research project (No.: 5168), Funded by the Mazandaran University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Shafagh Shariati for the critical proofreading of the manuscript.
References
Ago, Y., Tanaka, T., Kita, Y., Tokumoto, H., Takuma, K., & Matsuda, T. (2012). Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacology, 62(4), 1634–1639. [DOI:10.1016/j.neuropharm.2011.10.004] [PMID]
Ahmad, M. L., Khaksari, M., Sepehri, G. R., Dabiri, S., Asadi, K. G. R., & Mahmoudi, M., et al. (2008). [Comparison of the effects of progesterone, allopregnanolone and gender on supressing edema formation after traumatic brain injury in rats (Persian)]. Journal of Kerman University of Medical Sciences, 15(1), 47-59. [Link]
Amiri, S., Alijanpour, S., Tirgar, F., Haj-Mirzaian, A., Amini-Khoei, H., & Rahimi-Balaei, M., et al. (2016). NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience, 329, 122–133. [DOI:10.1016/j.neuroscience.2016.05.003] [PMID]
Azimi Sanavi, M., Ghazvini, H., Zargari, M., Ghalehnoei, H., & Hosseini-Khah, Z. (2022). Effects of clozapine and risperidone antipsychotic drugs on the expression of CACNA1C and behavioral changes in rat ‘Ketamine model of schizophrenia. Neuroscience Letters, 770, 136354. [DOI:10.1016/j.neulet.2021.136354] [PMID]
Camarasa, J., Rodrigo, T., Pubill, D., & Escubedo, E. (2010). Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacological Research, 62(5), 450–456. [DOI:10.1016/j.phrs.2010.05.004] [PMID]
Chuang D. M. (2004). Neuroprotective and neurotrophic actions of the mood stabilizer lithium: Can it be used to treat neurodegenerative diseases? Critical Reviews in Neurobiology, 16(1-2), 83–90. [DOI:10.1615/CritRevNeurobiol.v16.i12.90] [PMID]
Contestabile, A., Greco, B., Ghezzi, D., Tucci, V., Benfenati, F., & Gasparini, L. (2013). Lithium rescues synaptic plasticity and memory in Down syndrome mice. The Journal of Clinical Investigation, 123(1), 348–361. [DOI:10.1172/JCI64650] [PMID]
Deng, X., Wang, Y., Chou, J., & Cadet, J. L. (2001). Methamphetamine causes widespread apoptosis in the mouse brain: Evidence from using an improved TUNEL histochemical method. Brain Research. Molecular Brain Research, 93(1), 64–69. [DOI:10.1016/S0169-328X(01)00184-X] [PMID]
Fan, M., Jin, W., Zhao, H., Xiao, Y., Jia, Y., & Yin, Y., et al. (2015).Lithium chloride administration prevents spatial learning and memory impairment in repeated cerebral ischemia-reperfusion mice by depressing apoptosis and increasing BDNF expression in hippocampus. Behavioural Brain Research, 291, 399–406. [DOI:10.1016/j.bbr.2015.05.047] [PMID]
Fan, M., Song, C., Wang, T., Li, L., Dong, Y., & Jin, W., et al. (2015). Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurological Sciences, 36(2), 315–321. [DOI:10.1007/s10072-014-1943-x] [PMID]
Friedman, S. D., Castañeda, E., & Hodge, G. K. (1998). Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacology, Biochemistry, and Behavior, 61(1), 35–44. [DOI:10.1016/S0091-3057(98)00066-5] [PMID]
Ghalehnoei, H., Ghazvini, H., Mellati, A., Seyedhosseini Tamijani, S. M., Rafaiee, R., & Elyasi, L., et al. (2020). [Effects of estrogen and progesterone on behavioral impairment and neuronal death in ovariectomized rats induced by methamphetamine (Persian)]. Journal of Mazandaran University of Medical Sciences, 30(186), 1-12. [Link]
Ghazvini, H., Khaksari, M., Esmaeilpour, K., Shabani, M., Asadi-Shekaari, M., & Khodamoradi, M., et al. (2016). Effects of treatment with estrogen and progesterone on the methamphetamine-induced cognitive impairment in ovariectomized rats. Neuroscience Letters, 619, 60–67. [DOI:10.1016/j.neulet.2016.02.057] [PMID]
Ghazvini, H., Tirgar, F., Khodamoradi, M., Akbarnejad, Z., Rafaiee, R., & Seyedhosseini Tamijani, S. M., et al. (2021). Ovarian hormones prevent methamphetamine-induced anxiety-related behaviors and neuronal damage in ovariectomized rats. Neuroscience Letters, 746, 135652. [DOI:10.1016/j.neulet.2021.135652] [PMID]
Hajali, V., Sheibani, V., Esmaeili-Mahani, S., & Shabani, M. (2012). Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behavioural Brain Research, 228(2), 311–318.[DOI:10.1016/j.bbr.2011.12.008] [PMID]
Hajali, V., Sheibani, V., Ghazvini, H., Ghadiri, T., Valizadeh, T., & Saadati, H., et al. (2015). Effect of castration on the susceptibility of male rats to the sleep deprivation-induced impairment of behavioral and synaptic plasticity. Neurobiology of Learning and Memory, 123, 140–148. [DOI:10.1016/j.nlm.2015.05.008] [PMID]
Huo, K., Sun, Y., Li, H., Du, X., Wang, X., & Karlsson, N., et al. (2012). Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Molecular and Cellular Neurosciences, 51(1-2), 32–42. [DOI:10.1016/j.mcn.2012.07.002] [PMID]
Khalifeh, S., Khodagholi, F., Shaerzadeh, F., Ghazvini, H., Zarrindast, M. R., & Azizi, V. (2017). Brain region specificity of mitochondrial biogenesis and bioenergetics response to Nrf2 knockdown: A comparison among hippocampus, prefrontal cortex and amygdala of male rat brain. Brazilian Archives of Biology and Technology, 60. [DOI:10.1590/1678-4324-2017160744]
Khalifeh, S., Khodamoradi, M., Hajali, V., Ghazvini, H., Eliasy, L., & Kheradmand, A., et al. (2019). Naloxone ameliorates spatial memory deficits and hyperthermia induced by a neurotoxic methamphetamine regimen in male rats. Galen Medical Journal, 8, e1182. [DOI:10.31661/gmj.v8i0.1182] [PMID]
Khodamoradi, M., Ghazvini, H., Esmaeili-Mahani, S., Shahveisi, K., Farnia, V., & Zhaleh, H., et al. (2018). Genistein attenuates seizure-induced hippocampal brain-derived neurotrophic factor overexpression in ovariectomized rats. Journal of Chemical Neuroanatomy, 89, 43–50. [DOI:10.1016/j.jchemneu.2018.03.002] [PMID]
Khodamoradi, M., Tirgar, F., Ghazvini, H., Rafaiee, R., Tamijani, S. M. S., & Karimi, N., et al. (2022). Role of the cannabinoid CB1 receptor in methamphetamine-induced social and recognition memory impairment. Neuroscience Letters, 779, 136634.[DOI:10.1016/j.neulet.2022.136634] [PMID]
Kim, B. K., Ko, I. G., Kim, S. E., Kim, C. J., Yoon, J. S., & Baik, H. H., et al. (2013). Impact of several types of stresses on short-term memory and apoptosis in the hippocampus of rats. International Neurourology Journal, 17(3), 114–120. [DOI:10.5213/inj.2013.17.3.114] [PMID]
Kim, J. S., Chang, M. Y., Yu, I. T., Kim, J. H., Lee, S. H., & Lee, Y. S., et al. (2004). Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. Journal of Neurochemistry, 89(2), 324–336.[DOI:10.1046/j.1471-4159.2004.02329.x]
Kiyatkin, E. A., Brown, P. L., & Sharma, H. S. (2007). Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: Critical role of brain hyperthermia. European Journal of Neuroscience, 26(5), 1242–1253.[DOI:10.1111/j.1460-9568.2007.05741.x] [PMID]
Nagai, T., Takuma, K., Dohniwa, M., Ibi, D., Mizoguchi, H., & Kamei, H., et al. (2007). Repeated methamphetamine treatment impairs spatial working memory in rats: Reversal by clozapine but not haloperidol. Psychopharmacology, 194(1), 21–32. [DOI:10.1007/s00213-007-0820-1] [PMID]
Robinson, T. E., & Becker, J. B. (1986). Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Research, 396(2), 157–198. [PMID]
Shahidi, S., Komaki, A., Sadeghian, R., & Asl, S. S. (2019). Different doses of methamphetamine alter long-term potentiation, level of BDNF and neuronal apoptosis in the hippocampus of reinstated rats. The Journal of Physiological Sciences, 69(2), 409–419. [DOI:10.1007/s12576-019-00660-1] [PMID]
Shahveisi, K., Abdoli, N., Farnia, V., Khazaie, H., Hosseini, M., & Ghazvini, H., et al. (2022). REM sleep deprivation before extinction or reinstatement alters methamphetamine reward memory via D1-like dopamine receptors. Pharmacology, Biochemistry, and Behavior, 213, 173319. [DOI:10.1016/j.pbb.2021.173319] [PMID]
Thomas, D. M., Angoa Pérez, M., Francescutti-Verbeem, D. M., Shah, M. M., & Kuhn, D. M. (2010). The role of endogenous serotonin in methamphetamine‐induced neurotoxicity to dopamine nerve endings of the striatum. Journal of Neurochemistry, 115(3), 595–605. [DOI:10.1111/j.1471-4159.2010.06950.x] [PMID]
Thomas, D. M., Walker, P. D., Benjamins, J. A., Geddes, T. J., & Kuhn, D. M. (2004). Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. The Journal of Pharmacology and Experimental Therapeutics, 311(1), 1–7. [PMID]
Tirgar, F., Rezayof, A., & Zarrindast, M. R. (2014). Central amygdala nicotinic and 5-HT1A receptors mediate the reversal effect of nicotine and MDMA on morphine-induced amnesia. Neuroscience, 277, 392–402. [DOI:10.1016/j.neuroscience.2014.07.014] [PMID]
Toledo, E. M., & Inestrosa, N. C. (2010). Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Molecular Psychiatry, 15(3), 228- 272. [PMID]
Tominaga, T., & Ohnishi, S. T. (1989). Interrelationship of brain edema, motor deficits, and memory impairment in rats exposed to focal ischemia. Stroke, 20(4), 513–518. [DOI:10.1161/01.STR.20.4.513] [PMID]
Yan, X. B., Hou, H. L., Wu, L. M., Liu, J., & Zhou, J. N. (2007). Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology, 53(4), 487–495. [DOI:10.1016/j.neuropharm.2007.06.020] [PMID]
Yang, S., Zhou, G., Liu, H., Zhang, B., Li, J., & Cui, R., et al. (2013). Protective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. BioMed Research International, 2013, 215798. [DOI:10.1155/2013/215798] [PMID]
Yu, F., Zhang, Y., & Chuang, D. M. (2012). Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. Journal of Neurotrauma, 29(13), 2342–2351. [DOI:10.1089/neu.2012.2449]
Zarifkar, A., Choopani, S., Ghasemi, R., Naghdi, N., Maghsoudi, A. H., & Maghsoudi, N., et al. (2010). Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. European Journal of Pharmacology, 634(1-3), 84–88. [DOI:10.1016/j.ejphar.2010.02.029] [PMID]
Zhong, J., Yang, X., Yao, W., & Lee, W. (2006). Lithium protects ethanol-induced neuronal apoptosis. Biochemical and Biophysical Research Communications, 350(4), 905–910.[DOI:10.1016/j.bbrc.2006.09.138] [PMID]
Methamphetamine (METH) is one of the synthetic psychostimulants with deleterious effects on brain and behavior (Camarasa et al., 2010; Khalifeh et al., 2019). A growing body of literature indicates that exposure to METH results in long-lasting damage to dopaminergic and serotonergic nerve terminals (Thomas et al., 2010; Thomas et al., 2004). One of the significant effects of METH abuse is the neurocognitive deficit that persists long after drug withdrawal. Recently, studies demonstrated that METH-exposed animals showed significant impairments in performance on hippocampus-dependent spatial tasks, such as the Morris water maze (MWM) (Ghalehnoei et al., 2020; Ghazvini et al., 2016).
In recent years, numerous studies have been conducted on the role of lithium in neurological dysfunction in various brain diseases (Chuang, 2004). Lithium has neuroprotective effects, such as hippocampal neurogenesis, that ameliorate neurological deficits and carry long-term potentiation in the hippocampus of rat dentate gyrus (Contestabile et al., 2012; Kim et al., 2004; Yan et al., 2007). In addition, it has been suggested that lithium is recognized as an appropriate agent for hippocampal-dependent cognitive enhancement. For example, Yu et al. (2012) have shown that treatment with lithium in animals exposed to traumatic brain injury reduces deficits in spatial learning in the MWM.
On the other hand, lithium has neuroprotective properties by inhibiting apoptosis and promoting neuronal survival (Fan et al., 2015; Zhong et al., 2006). In this regard, animals treated with lithium exhibit a reduced apoptosis of neural progenitor cells in the hippocampus and improved memory deficits (Huo et al., 2012). Meanwhile, Fan et al. (2015) indicated that lithium chloride administration prevented spatial learning and memory deficits in mice with cerebral ischemia by inhibiting apoptosis and increasing BDNF expression in the hippocampus.
There are a few relevant documents on the neurocognitive effects of lithium on METH-induced behavioral toxicities; however, recent work has demonstrated that lithium modulates METH toxicity through its impact on monoaminergic release. For example, Ago et al. have shown that treatment with lithium attenuates METH-induced hyper-locomotion and behavioral sensitization by modulating the prefrontal monoamine release in mice (Ago et al., 2012). Nevertheless, the effect of lithium on METH-induced memory impairment is still largely unknown.
Accordingly, given the importance of hippocampal apoptosis in cognitive deficits, the present study aims to understand the possible effects of lithium on preventing deficits in spatial learning and memory induced by repeated administration of METH in male Wistar rats.
2. Materials and Methods
Study animals
This study was performed on male Wistar rats weighing between 200 g and 250 g at the beginning of the test. Sufficient effort was taken to reduce the number and suffering of the study animals. During the experimental procedure, the rats had access to free food and water and were housed in groups of 4 in a cage. Its temperature was maintained at 23±1°C with a 12 h light/dark cycle (light beginning at 7:00 AM).
Experimental protocols
The animals were randomly assigned into four groups (n=8 in each group). The experimental groups were as follows: 1) Saline group: The rats that received normal saline subcutaneously (SC); 2) METH-exposed group: The rats that received METH (6 mg/kg, SC); 3) Lithium group: The rats that received a lithium injection; 4) METH+lithium group: Rats that received lithium (20 mg/kg, intraperitoneal [IP]) and METH (6 mg/kg, SC). The animals were examined for spatial learning and memory using the MWM one day after the treatments. Finally, the rats were sacrificed for histological examination and measurement of brain water content. For histological analysis, hippocampal tissue was prepared for the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay.
Morris water maze
MWM behavioral task was used to assess spatial learning memory. As previously described (Hajali et al., 2015; Hajali et al., 2012), the aspects of the apparatus were a stainless steel water tank 160 cm in diameter and 80 cm in height, filled with water to a depth of 40 cm. The water temperature was maintained at 22±1°C. The maze was geographically separated into four equal quadrants. The hidden platform (10 cm in diameter) was submerged 1.5 cm below the water’s surface in the center of quadrant 4. A camera was located above the water tank and attached to a computer. The performance of the animals was recorded by a smart video tracking system (Noldus Ethovision® software, version 5. The rats were trained to find the hidden platform according to the visual cues (squares, circles, or triangles) placed around the room.
Spatial learning and memory
The acquisition phase consisted of three training blocks parted by a 30 min rest interval. Every block included four 60 s consecutive trials and three 60 s inter-trial intervals. Each trial was started by placing the animal into the water with its face toward the wall of the water pool. The starting point varied randomly in different quadrants from trial to trial, with an equal number of trials starting from each quadrant. The animal was released into the maze and allowed to swim for a maximum of 60 s in each trial to find the hidden escape platform. After the animal found the platform, it was allowed to remain there for 20 to 30 s and was then removed and put into an animal cage to wait for 30 to 35 s before the subsequent trial. Each animal that failed to find the platform within 60 s was guided to the platform. The time and distance to find the hidden platform were collected, analyzed, and interpreted later.
Subsequently, a probe test was carried out 2 h after the training to test. In the probe test, the hidden platform was removed, and the animal was placed in quadrant 2, across from quadrant 4 (target quadrant). The animal was permitted to swim for 60 s in the water maze. The animal’s time in the target quadrant was analyzed to measure spatial memory retention. At the end of the probe trial, rats did a visible platform test to determine any possibility of intervention with sensory and motor coordination or motivation. In this test, an apparent platform covered with aluminum foil was raised 2 cm above the water surface, and the animal’s ability to escape to a visible platform was calculated (Ghazvini et al., 2016).
Determination of brain water content
To determine the water content of the brain, rats were deeply anesthetized and decapitated 24 h after drug administration, and then the brain was removed. The wet tissue’s weight was calculated and placed in an oven for 72 h to obtain the dry weight. The water content was measured as the difference between the wet and dry weights of the brain according to the Equation 1:
1. Water content=([Wet mass-Dry mass]/[Wet mass]) ×100 (Ahmad et al., 2008).
Tissue preparation
Following the behavioral tests, animals were profoundly anesthetized, and transcranial perfusion was carried out with 0.9% saline followed by fixative solution (glutaraldehyde 1.25% and paraformaldehyde %1 in 0.01 M. phosphate-buffered saline [PBS] at pH=7.4) for 1 h. The brain was carefully extracted after the perfusion, washed in normal saline, fixed in the same fixative overnight, and eventually embedded in paraffin. Coronal sections (5 µm thick) were taken using a microtome from 3.3 mm to 4.2 mm posterior to bregma according to the Paxinos atlas.
The TUNEL assay was performed to evaluate apoptosis in the pyramidal neurons of the hippocampal CA1 area, as described previously. To do so, a commercial kit (In Situ Cell Death Detection Kit; Roche, Mannheim, Germany) was used based on the instructions provided by the manufacturer. In brief, the paraffin-embedded sections were incubated in xylene for deparaffinization, dehydrated in descending ethanol concentrations, and rinsed in PBS. Permeabilization was done by 10 mM proteinase K for 30 min at room temperature. Subsequently, the sections were washed and incubated with 3% H2O2 in methanol for 10 min in a dark place to block endogenous peroxidase activity, leading to false-positive results. After adding the TUNEL reaction mixture, the sections were incubated at 37°C for 60 min in a chamber with appropriate humidity, washed with PBS, and visualized using a converter‒POD at 37°C for 30 min in a dark place with moderate humidity. Afterward, the sections were rinsed in PBS and incubated with 50–100 μL 0.05% 3, 3′-diaminobenzidine as a chromogen for 10 min. After washing with PBS, the sections were mounted on slides. Using a light microscope, the number of TUNEL-positive neurons was counted (400 μm long, 0.160 mm2) in the CA1 area of the right hippocampus. The number of pyramidal neurons in the hippocampal CA1 area was calculated in three fields (medial, middle, and lateral parts). An average number of cells from these three fields was considered as cell counts from each animal (Ghazvini et al., 2021).
Data analysis
The data from the acquisition phase, distance, and time spent to find the hidden platform were analyzed by a two-way analysis of variance (ANOVA) along with repeated measures to determine differences in the learning rates of the groups (group and block being the factors). All comparisons between the groups for the data collected in the MWM probe trials, swimming speed, and histological data were analyzed via ANOVA. The analyses of variances were followed by the Tukey test for multiple comparisons when required. The data were expressed as Mean±SE, and the level of statistical significance was considered P<0.05.
3. Results
Spatial learning
As shown in Figure 1A and Figure 1B, repeated measures analysis of ANOVA revealed that the path length and escape latency of the animals in the METH group significantly increased in block 1 (P<0.001), block 2 (P<0.001), and block 3 (P<0.001) compared to the control group, indicating an impairment in spatial learning after exposure to METH. The current results show that lithium reduces the path length and escape latency to find the hidden platform compared to the METH group. The mean distance traveled by the METH+lithium group in block 1 (P<0.05) and block 3 (P<0.01) decreased in comparison with the METH group (Figure 1A). The analysis also showed that the mean escape latency of the METH+lithium group in block 1 (P<0.05) and block 3 (P<0.01) decreased compared to the METH group (Figure 1B). Therefore, the cognitive ability of animals in the METH group to find hidden platforms was partially improved by lithium therapy.

Spatial short-term memory
In a probe test, 2 h after the acquisition phase, the percentage of the time, distance, and the number of crossings in the target quadrant (quadrant 4) were calculated for short-term spatial memory retrieval. As illustrated in Figure 2, the one-way ANOVA showed that animals that received METH (6 mg/kg) did not recall the location of the platform well since the time spent, distance, and the number of crossing in the target quadrant was higher than that of the control group (P<0.001). The analysis also revealed that the METH+lithium group exhibits a decrease in spatial short-term memory impairment and spatial learning. The rats of the METH+lithium group spent more time (P<0.05) and distance (P<0.05) in quadrant four compared to the METH group. Further analysis also showed that the number of crossings in the target quadrant in the METH group significantly decreased compared to the control group (P<0.01). However, the number of crossing in the target quadrant in the METH+lithium group was not significantly different compared to the METH group.

Latency to the visible platform and swimming speed
The analysis of escape latency to find the visible platform and swimming speed showed no difference between groups (P>0.05, Table 1). METH and lithium administration did not affect the visible test and swimming speed. Thus, visual and motor functions did not differ between groups.

Effects of lithium and methamphetamine regimen on brain water content
The rats were examined for brain edema based on the calculated brain water content. As shown in Figure 3, the brain water content was significantly increased in the METH group compared to the control group (P<0.001). In addition, the brain water content in the METH+lithium group was significantly lower than that in the METH group (P<0.05).

Effects of lithium and methamphetamine regimen on neuronal apoptosis in the hippocampal CA1 area
As illustrated in Figure 4, the number of TUNEL-positive cells in the CA1 region of the hippocampus in the METH group showed a significant increase compared to the control group (P<0.001; Figure 4B). In addition, the METH+lithium group revealed a substantial decrease in TUNEL-positive cells compared to the METH group (P<0.05; Figure 4D). However, the results showed that lithium administration alone did not affect the number of TUNEL-positive cells in the CA1 region.

4. Discussion
The current study aimed to investigate the possible effects of lithium on METH-induced neuronal damage and memory impairment. The results showed that the administration of METH had a disruptive impact on spatial learning and memory in male Wistar rats. In addition, treatment with lithium significantly improved spatial learning and memory impairment in METH-exposed rats. Moreover, the data showed that the neurotoxic regimen of METH (6 mg/kg, four injections with two h intervals) resulted in long-lasting cognitive deficits and was accompanied by neuronal cell death in the CA1 area of the hippocampus and brain edema, which was partially reversed by lithium.
A growing body of literature suggests that amphetamines lead to long-lasting cognitive impairment and are among the most widely used illegal drugs (Amiri et al., 2016; Robinson & Becker, 1986; Tirgar et al., 2014). The data presented here are in line with similar studies that have found an association between long-term cognitive impairment and neurotoxic regimens of METH (Friedman et al., 1998; Khodamoradi et al., 2022; Nagai et al., 2007). For example, Ghazvini et al. (2021) have shown that exposure to METH in rats leads to various cognitive problems, such as learning and memory impairment, anxiety-like behavior, and deficits in synaptic plasticity.
Although the exact mechanisms of METH action remain unclear and controversial, previous studies point to some possible mechanisms for behavioral neurotoxicity: a reduction in dopamine and serotonin transporter activity following METH administration (Nagai et al., 2007; Khodamoradi et al., 2022). In this point of view, studies suggest that exposure to a neurotoxic METH treatment leads to glial activation and increased neuronal apoptosis, which may contribute to METH cognitive impairment. A growing literature emphasizes that hippocampal activity is related to various neurocognitive actions (Khalifeh et al., 2017; Khodamoradi et al., 2018; Azimi Sanavi et al., 2022; Shahveisi et al., 2022). Moreover, our results and other authors’ results have demonstrated that the animals receiving METH exhibit neuronal cell death in the hippocampus, a key link between neuronal cell death and memory performance. In this regard, the present data showed, in line with previous results, that the administration of METH leads to a significant increase in TUNEL-positive cells in the CA1 region.
Recently, there has been increasing evidence that lithium has a neuroprotective effect on cognitive performance in some neurological disorders. For example, when injected IP daily for 2 weeks (1 mmol/kg) in male ischemic rats, lithium significantly improved spatial learning and memory in the MWM task (Yan et al., 2007). Moreover, daily treatment with lithium improved spatial memory functions in a mouse model of Alzheimer disease (Toledo & Inestrosa, 2010). These results confirm the existing evidence on the ability of lithium to prevent spatial learning and memory in METH-exposed rats.
Another concern about METH-induced neurotoxicity is related to brain edema. Recently, we reported that METH induces brain hyperthermia and brain edema, possibly due to blood-brain barrier breakdown. Based on our results, it is reasonable to speculate that the behavioral deficits observed in animals receiving METH are partly due to brain edema. In line with this hypothesis, previous studies have shown a close association between brain edema and memory impairment (Tominaga & Ohnishi, 1989). In agreement with our results, Kiyatkin et al. (2007) showed that METH 9 mg/kg leads to brain hyperthermia and brain edema in male Long-Evans rats. In the present study, one of the probable mechanisms involved in improving memory performance is somehow mediated by the effect of lithium on the reduction of brain edema; however, the exact mechanism is unclear.
Our results also demonstrated the beneficial effects of lithium on neuronal apoptosis cell death in METH-exposed animals. In agreement with our findings, Deng et al. (2001) found that a single injection of 40 mg/kg METH caused neuronal cell death in several brain areas, including striatum, cortex, and hippocampus. In addition, administration of METH to male Wistar rats caused dose-dependent neuronal apoptosis in the hippocampus (Shahidi et al., 2019). Thus, these data confirm the presence of METH-induced deleterious effects in non-dopaminergic neurons, which may contribute to the learning and memory processes. A large body of evidence suggests that neuronal apoptosis in the brain area is involved in cognitive performance. The cortex and hippocampus disrupt learning and memory function, and excessive neuronal apoptosis in the hippocampus contributes to memory dysfunction (Kim et al., 2013). From this point of view, recent evidence suggests that a neuroprotective agent may reduce neuronal apoptosis in the hippocampus and, in some way, improve memory function (Yang et al., 2013; Zarifkar et al., 2010). Thus, another likely mechanism for the cognitive enhancing effects of lithium observed in the current study may lie in the reduction of neuronal apoptosis in the hippocampus.
In addition, previous studies have reported that lithium can significantly improve learning and memory through its effects on hippocampal synaptic plasticity and neurogenesis. However, as revealed by previous studies, the exact mechanisms of how lithium exerts its effects may involve the modulation of signaling cascades in the brain, one of which is the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway (Yan et al., 2007). Nevertheless, the effects of lithium on METH neurotoxicity have been controversial in previous reports and require further investigation to be elucidated.
5. Conclusions
The present results suggest that treatment with lithium can partially alleviate the METH-induced spatial memory impairment in male rats. In addition, treatment with lithium significantly reduced brain edema and apoptosis of neuronal damage in the CA1 region in METH-exposed rats. The results also indicate that these neurocognitive effects of lithium may be associated with its anti-apoptotic effects in the hippocampus and reduction of brain edema, which may be influenced by numerous factors, such as the applied dose, duration and timing of treatment, age, and so on. However, further studies are needed to determine which mechanisms are involved in the protective effect of lithium against methamphetamine-induced cognitive impairment, such as decision-making, anxiety, etc.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Mazandaran University of Medical Sciences approved the study (Code: IR.MAZUMS.REC.1398.5168).
Funding
This research was supported by the research project (No.: 5168), Funded by the Mazandaran University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Shafagh Shariati for the critical proofreading of the manuscript.
References
Ago, Y., Tanaka, T., Kita, Y., Tokumoto, H., Takuma, K., & Matsuda, T. (2012). Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacology, 62(4), 1634–1639. [DOI:10.1016/j.neuropharm.2011.10.004] [PMID]
Ahmad, M. L., Khaksari, M., Sepehri, G. R., Dabiri, S., Asadi, K. G. R., & Mahmoudi, M., et al. (2008). [Comparison of the effects of progesterone, allopregnanolone and gender on supressing edema formation after traumatic brain injury in rats (Persian)]. Journal of Kerman University of Medical Sciences, 15(1), 47-59. [Link]
Amiri, S., Alijanpour, S., Tirgar, F., Haj-Mirzaian, A., Amini-Khoei, H., & Rahimi-Balaei, M., et al. (2016). NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience, 329, 122–133. [DOI:10.1016/j.neuroscience.2016.05.003] [PMID]
Azimi Sanavi, M., Ghazvini, H., Zargari, M., Ghalehnoei, H., & Hosseini-Khah, Z. (2022). Effects of clozapine and risperidone antipsychotic drugs on the expression of CACNA1C and behavioral changes in rat ‘Ketamine model of schizophrenia. Neuroscience Letters, 770, 136354. [DOI:10.1016/j.neulet.2021.136354] [PMID]
Camarasa, J., Rodrigo, T., Pubill, D., & Escubedo, E. (2010). Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacological Research, 62(5), 450–456. [DOI:10.1016/j.phrs.2010.05.004] [PMID]
Chuang D. M. (2004). Neuroprotective and neurotrophic actions of the mood stabilizer lithium: Can it be used to treat neurodegenerative diseases? Critical Reviews in Neurobiology, 16(1-2), 83–90. [DOI:10.1615/CritRevNeurobiol.v16.i12.90] [PMID]
Contestabile, A., Greco, B., Ghezzi, D., Tucci, V., Benfenati, F., & Gasparini, L. (2013). Lithium rescues synaptic plasticity and memory in Down syndrome mice. The Journal of Clinical Investigation, 123(1), 348–361. [DOI:10.1172/JCI64650] [PMID]
Deng, X., Wang, Y., Chou, J., & Cadet, J. L. (2001). Methamphetamine causes widespread apoptosis in the mouse brain: Evidence from using an improved TUNEL histochemical method. Brain Research. Molecular Brain Research, 93(1), 64–69. [DOI:10.1016/S0169-328X(01)00184-X] [PMID]
Fan, M., Jin, W., Zhao, H., Xiao, Y., Jia, Y., & Yin, Y., et al. (2015).Lithium chloride administration prevents spatial learning and memory impairment in repeated cerebral ischemia-reperfusion mice by depressing apoptosis and increasing BDNF expression in hippocampus. Behavioural Brain Research, 291, 399–406. [DOI:10.1016/j.bbr.2015.05.047] [PMID]
Fan, M., Song, C., Wang, T., Li, L., Dong, Y., & Jin, W., et al. (2015). Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurological Sciences, 36(2), 315–321. [DOI:10.1007/s10072-014-1943-x] [PMID]
Friedman, S. D., Castañeda, E., & Hodge, G. K. (1998). Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacology, Biochemistry, and Behavior, 61(1), 35–44. [DOI:10.1016/S0091-3057(98)00066-5] [PMID]
Ghalehnoei, H., Ghazvini, H., Mellati, A., Seyedhosseini Tamijani, S. M., Rafaiee, R., & Elyasi, L., et al. (2020). [Effects of estrogen and progesterone on behavioral impairment and neuronal death in ovariectomized rats induced by methamphetamine (Persian)]. Journal of Mazandaran University of Medical Sciences, 30(186), 1-12. [Link]
Ghazvini, H., Khaksari, M., Esmaeilpour, K., Shabani, M., Asadi-Shekaari, M., & Khodamoradi, M., et al. (2016). Effects of treatment with estrogen and progesterone on the methamphetamine-induced cognitive impairment in ovariectomized rats. Neuroscience Letters, 619, 60–67. [DOI:10.1016/j.neulet.2016.02.057] [PMID]
Ghazvini, H., Tirgar, F., Khodamoradi, M., Akbarnejad, Z., Rafaiee, R., & Seyedhosseini Tamijani, S. M., et al. (2021). Ovarian hormones prevent methamphetamine-induced anxiety-related behaviors and neuronal damage in ovariectomized rats. Neuroscience Letters, 746, 135652. [DOI:10.1016/j.neulet.2021.135652] [PMID]
Hajali, V., Sheibani, V., Esmaeili-Mahani, S., & Shabani, M. (2012). Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behavioural Brain Research, 228(2), 311–318.[DOI:10.1016/j.bbr.2011.12.008] [PMID]
Hajali, V., Sheibani, V., Ghazvini, H., Ghadiri, T., Valizadeh, T., & Saadati, H., et al. (2015). Effect of castration on the susceptibility of male rats to the sleep deprivation-induced impairment of behavioral and synaptic plasticity. Neurobiology of Learning and Memory, 123, 140–148. [DOI:10.1016/j.nlm.2015.05.008] [PMID]
Huo, K., Sun, Y., Li, H., Du, X., Wang, X., & Karlsson, N., et al. (2012). Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Molecular and Cellular Neurosciences, 51(1-2), 32–42. [DOI:10.1016/j.mcn.2012.07.002] [PMID]
Khalifeh, S., Khodagholi, F., Shaerzadeh, F., Ghazvini, H., Zarrindast, M. R., & Azizi, V. (2017). Brain region specificity of mitochondrial biogenesis and bioenergetics response to Nrf2 knockdown: A comparison among hippocampus, prefrontal cortex and amygdala of male rat brain. Brazilian Archives of Biology and Technology, 60. [DOI:10.1590/1678-4324-2017160744]
Khalifeh, S., Khodamoradi, M., Hajali, V., Ghazvini, H., Eliasy, L., & Kheradmand, A., et al. (2019). Naloxone ameliorates spatial memory deficits and hyperthermia induced by a neurotoxic methamphetamine regimen in male rats. Galen Medical Journal, 8, e1182. [DOI:10.31661/gmj.v8i0.1182] [PMID]
Khodamoradi, M., Ghazvini, H., Esmaeili-Mahani, S., Shahveisi, K., Farnia, V., & Zhaleh, H., et al. (2018). Genistein attenuates seizure-induced hippocampal brain-derived neurotrophic factor overexpression in ovariectomized rats. Journal of Chemical Neuroanatomy, 89, 43–50. [DOI:10.1016/j.jchemneu.2018.03.002] [PMID]
Khodamoradi, M., Tirgar, F., Ghazvini, H., Rafaiee, R., Tamijani, S. M. S., & Karimi, N., et al. (2022). Role of the cannabinoid CB1 receptor in methamphetamine-induced social and recognition memory impairment. Neuroscience Letters, 779, 136634.[DOI:10.1016/j.neulet.2022.136634] [PMID]
Kim, B. K., Ko, I. G., Kim, S. E., Kim, C. J., Yoon, J. S., & Baik, H. H., et al. (2013). Impact of several types of stresses on short-term memory and apoptosis in the hippocampus of rats. International Neurourology Journal, 17(3), 114–120. [DOI:10.5213/inj.2013.17.3.114] [PMID]
Kim, J. S., Chang, M. Y., Yu, I. T., Kim, J. H., Lee, S. H., & Lee, Y. S., et al. (2004). Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. Journal of Neurochemistry, 89(2), 324–336.[DOI:10.1046/j.1471-4159.2004.02329.x]
Kiyatkin, E. A., Brown, P. L., & Sharma, H. S. (2007). Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: Critical role of brain hyperthermia. European Journal of Neuroscience, 26(5), 1242–1253.[DOI:10.1111/j.1460-9568.2007.05741.x] [PMID]
Nagai, T., Takuma, K., Dohniwa, M., Ibi, D., Mizoguchi, H., & Kamei, H., et al. (2007). Repeated methamphetamine treatment impairs spatial working memory in rats: Reversal by clozapine but not haloperidol. Psychopharmacology, 194(1), 21–32. [DOI:10.1007/s00213-007-0820-1] [PMID]
Robinson, T. E., & Becker, J. B. (1986). Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Research, 396(2), 157–198. [PMID]
Shahidi, S., Komaki, A., Sadeghian, R., & Asl, S. S. (2019). Different doses of methamphetamine alter long-term potentiation, level of BDNF and neuronal apoptosis in the hippocampus of reinstated rats. The Journal of Physiological Sciences, 69(2), 409–419. [DOI:10.1007/s12576-019-00660-1] [PMID]
Shahveisi, K., Abdoli, N., Farnia, V., Khazaie, H., Hosseini, M., & Ghazvini, H., et al. (2022). REM sleep deprivation before extinction or reinstatement alters methamphetamine reward memory via D1-like dopamine receptors. Pharmacology, Biochemistry, and Behavior, 213, 173319. [DOI:10.1016/j.pbb.2021.173319] [PMID]
Thomas, D. M., Angoa Pérez, M., Francescutti-Verbeem, D. M., Shah, M. M., & Kuhn, D. M. (2010). The role of endogenous serotonin in methamphetamine‐induced neurotoxicity to dopamine nerve endings of the striatum. Journal of Neurochemistry, 115(3), 595–605. [DOI:10.1111/j.1471-4159.2010.06950.x] [PMID]
Thomas, D. M., Walker, P. D., Benjamins, J. A., Geddes, T. J., & Kuhn, D. M. (2004). Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. The Journal of Pharmacology and Experimental Therapeutics, 311(1), 1–7. [PMID]
Tirgar, F., Rezayof, A., & Zarrindast, M. R. (2014). Central amygdala nicotinic and 5-HT1A receptors mediate the reversal effect of nicotine and MDMA on morphine-induced amnesia. Neuroscience, 277, 392–402. [DOI:10.1016/j.neuroscience.2014.07.014] [PMID]
Toledo, E. M., & Inestrosa, N. C. (2010). Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Molecular Psychiatry, 15(3), 228- 272. [PMID]
Tominaga, T., & Ohnishi, S. T. (1989). Interrelationship of brain edema, motor deficits, and memory impairment in rats exposed to focal ischemia. Stroke, 20(4), 513–518. [DOI:10.1161/01.STR.20.4.513] [PMID]
Yan, X. B., Hou, H. L., Wu, L. M., Liu, J., & Zhou, J. N. (2007). Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology, 53(4), 487–495. [DOI:10.1016/j.neuropharm.2007.06.020] [PMID]
Yang, S., Zhou, G., Liu, H., Zhang, B., Li, J., & Cui, R., et al. (2013). Protective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. BioMed Research International, 2013, 215798. [DOI:10.1155/2013/215798] [PMID]
Yu, F., Zhang, Y., & Chuang, D. M. (2012). Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. Journal of Neurotrauma, 29(13), 2342–2351. [DOI:10.1089/neu.2012.2449]
Zarifkar, A., Choopani, S., Ghasemi, R., Naghdi, N., Maghsoudi, A. H., & Maghsoudi, N., et al. (2010). Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. European Journal of Pharmacology, 634(1-3), 84–88. [DOI:10.1016/j.ejphar.2010.02.029] [PMID]
Zhong, J., Yang, X., Yao, W., & Lee, W. (2006). Lithium protects ethanol-induced neuronal apoptosis. Biochemical and Biophysical Research Communications, 350(4), 905–910.[DOI:10.1016/j.bbrc.2006.09.138] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2021/11/13 | Accepted: 2023/07/27 | Published: 2023/09/1
Received: 2021/11/13 | Accepted: 2023/07/27 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





