Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 617-630 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Takhshid M A, Mahmoodazdeh A, Shafiee S M, Sisakht M, khosdel Z. Adrenomedullin and Protecting Spinal Motor Neurons Against Doxorubicin-induced Toxicity. BCN 2024; 15 (5) :617-630
URL: http://bcn.iums.ac.ir/article-1-2257-en.html
URL: http://bcn.iums.ac.ir/article-1-2257-en.html
Mohammad Ali Takhshid *1 

 , Amir Mahmoodazdeh2
, Amir Mahmoodazdeh2 

 , Sayed Mohammad Shafiee2
, Sayed Mohammad Shafiee2 

 , Mohsen Sisakht2
, Mohsen Sisakht2 
 , Zahra Khosdel2
, Zahra Khosdel2 




 , Amir Mahmoodazdeh2
, Amir Mahmoodazdeh2 

 , Sayed Mohammad Shafiee2
, Sayed Mohammad Shafiee2 

 , Mohsen Sisakht2
, Mohsen Sisakht2 
 , Zahra Khosdel2
, Zahra Khosdel2 


1- Division of Medical Biotechnology, Department of Laboratory Sciences, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Keywords: Spinal motor neuron (SMN), Doxorubicin (DOX), Adrenomedullin (AM), Oxidative stress, Inflammation
Full-Text [PDF 1477 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Spinal motor neurons (SMNs) are essential to the spinal cord neural circuit and play crucial roles in spinal reflexes. SMNs also transmit signals from the brain to the muscles (Bäumer et al., 2014). Progressive loss of SMNs that occurs in amyotrophic lateral sclerosis and the use of chemotherapeutic drugs leads to muscle atrophy and disability (Drechsel et al., 2012; Starobova & Vetter, 2017; Verma et al., 2017). Doxorubicin (DOX) is among the potent chemotherapeutic drugs used to treat several cancers (Khan et al., 2014; McGowan et al., 2017). DOX exerts its therapeutic effects through the inhibition of replication and an increase in the production of inflammatory mediators and reactive oxygen species (ROS) (Capelôa et al., 2014; Zhao & Zhang, 2017). Despite its beneficial effects, irreversible toxic effects of DOX on the induction of cognitive and motor impairments such as learning disabilities and memory loss have been reported (Moruno-Manchon et al., 2016). DOX-mediated degeneration of the dorsal root ganglion (DRG) and SMNs have also been shown in the spinal cord in both animal models (Wu et al., 2015) and human studies (Saito et al., 2019).
Adrenomedullin (AM), a peptide belonging to the calcitonin family, is widely expressed in peripheral tissues and the central nervous system (CNS) in the brain and the spinal cord (Hong et al., 2009). Considering the peripheral tissues, AM has revealed protective effects against Leydig cells pyroptosis (Li et al., 2019), heart failure (Voors et al., 2019), pulmonary and renal diseases (Holmes et al., 2013), sepsis (Geven et sl., 2018), and ulcerative colitis (Martinez-Herrero et al., 2017). An in vitro study on mesenchymal stem cells showed that AM overexpression could protect the cells from serum deprivation and hypoxia-induced apoptosis (Si et al., 2018). Moreover, AM inhibited streptozotocin-induced cell death in cultured human renal tubule cells (Uetake et al., 2014). In the CNS, calcitonin-like receptor (CLR), receptor activities modifying protein-2 (RAMP-2), and RAMP-3, as AM receptor components, are expressed in both glial and nerve cells. Animal studies have suggested AM’s role in transmitting nociceptive impulses (Ma et al., 2006; Takhshid et al., 2004). The neuroprotective effects of AM against ischemic brain damage and acute brain injury were mediated through decreasing oxidative stress and pro-inflammatory cytokines production (Demir et al., 2013). A recent study also disclosed the protective effects of AM against the neurotoxic effects of DOX in DRG neurons (Mahmoodazdeh et al., 2020). The present study aims to determine AM, CLR, and RAMPs expressions in rat embryonic SMNs and assess AM’s protective and anti-oxidant effects against DOX-induced neurotoxicity.
2. Materials and Methods
Reagents and chemicals
Rat AM was purchased from Bachem. Cell culture materials were bought from Gibco BRL (Life Technologies, Renfrewshire, UK), except for poly-D-lysine, Trypsin-EDTA solution, penicillin/streptomycin solution, MTT assay kit, OptiPrep™ density gradient medium (D1556-250ML, Sigma, USA), and DOX that were the products of Sigma (USA). BCA protein assay kit and nitric oxide (NO) generation kit were purchased from Thermo Fisher Scientific, Inc. (The USA). ROS detection kit was provided by Molecular Probes (The USA), and an 8-iso prostaglandin F2α (iPF2α) ELISA kit was obtained from Cayman Chemical Co. (The USA). All primers were prepared by Metabion Co. (Germany). Apoptosis detection kits were purchased from Bio Vision (Switzerland), and Syber green polymerase chain reaction (PCR) master mix was bought from Ampliqon A/S, Odense (Denmark).
Isolation and culture of SMNs
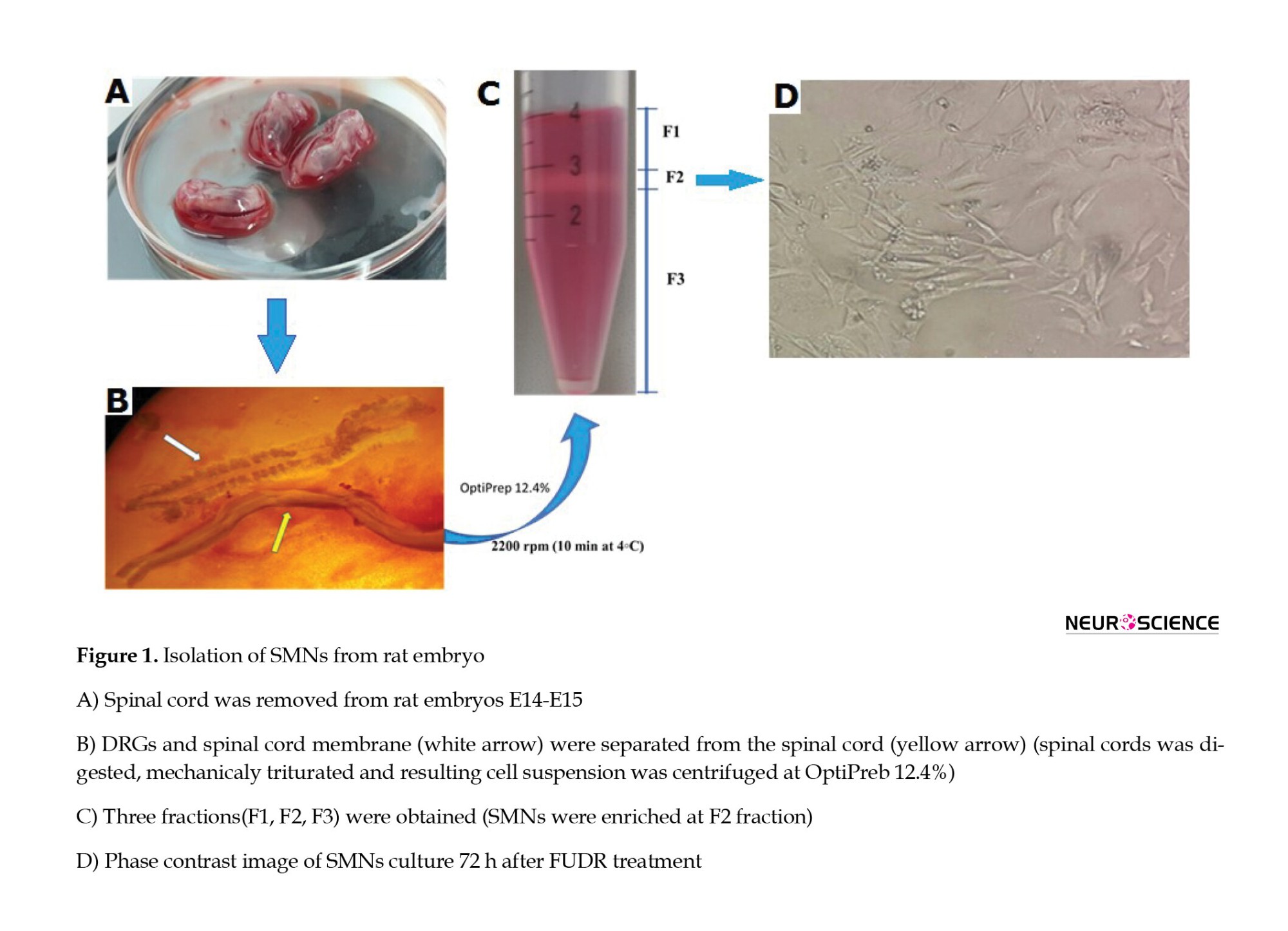
SMNs were cultured according to Wang’s method (Wang et al., 2017). As illustrated in Figure 1, the spinal cord was separated from the DRGs, rinsed in L15 medium containing penicillin G (300 U/mL) and gentamycin (75 μg/mL), and digested in 0.025% trypsin (Invitrogen) at 37 ºC for 40 min. The spinal cells were then dissociated using trituration, and the suspended cells were put on a single gradient of OptiPrep™ and centrifuged for 10 min (4 ºC) to separate SMNs from debris (F1), SMNs(F2), and non-neural cells (Figure 1). The SMNs (F2 layer) were carefully separated, re-suspended in an appropriate volume of culture medium, transferred to the poly-D-lysine coated culture plates, and cultured in Neurobasal-A medium supplemented with B27 (2%), GlutaMAX (2 mM), streptomycin (100 mg), and penicillin (100 units) at 37 °C, 5% CO2, and 80% relative humidity. After that, the cultured cells were treated with 5-fluorodeoxyuridine (20 mM; 72 h) to prevent the growth of non-neural cells (Liu et al., 2013). Finally, the purity of the SMNs in the culture was evaluated using phase contrast microscopy. Based on the results, SMNs comprised 87±5% of the cultured cells.
Cell viability assay
To evaluate DOX toxicity, the SMNs were treated with DOX (0-100 µM), and their viability was measured for 12, 24, and 48 h. To evaluate the possible toxicity of AM, the SMNs were treated with AM (3.125-100 nM) for 24 h. The doses of AM were selected according to a previous study (Mahmoodazdeh et al., 2020). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5- diphenyl-2H-tetrazolium bromide) was used to assess the effects of treatment on cell survival (Mahmoodazdeh et al., 2020). IC50 value of DOX was calculated using Prism 6 software. For further evaluations, the SMNs were cultured in 48-well plates (2×105 cells/well) and treated with AM (50 nM), DOX (10.54 µM; IC50), and AM + DOX (50 nM+10.54 µM). SMNs were pretreated with AM for two h, DOX was added to the wells, and cell viability was measured after 24 h.
Apoptosis assay
The effects of the treatments on SMNs apoptosis were determined and evaluated using annexin V-FITC/PI staining and flow cytometry. Briefly, the treated SMNs were harvested, stained, and analyzed using a flow cytometer (BD) at 488 nm.
ROS assay
Intracellular ROS were measured using 2’,7’-dichloro fluorescein diacetate (DCFH-DA, Molecular Probes, Eugene, OR, USA) probe and flowcytometry (Chen et al., 2010). Briefly, the SMNs were incubated with 10 μM of DCF-DA at 37 °C for 30 min. The cells were then washed three times with phosphate-buffered saline (PBS), and the fluorescence intensity was measured (excitation: 488 and emission:525 nm) using a Multimode Plate Reader.
Oxidative markers assay
NO levels in the cell lysate were assayed using the Griess-reduction method and nitrite standard curve (1−100 nmol/mL) (Hare et al., 2008). The Griess reagent (5% H3PO4 containing 1% sulfanilamide and 0.1% naphthyl ethylenediamine) was mixed with the cell lysates and incubated at room temperature in the dark. The absorbance of the samples was then measured at 540 nm. The thiobarbituric acid method (Tsikas, 2017) was used to measure MDA levels in SMNs. To quantify iPF2α levels, the SMNs were lysed and treated with KOH (15% at 40 °C for 60 min) to release esterified forms of iPF2α. iPF2α was then measured using an ELISA kit (Cayman, item No. 51635) and normalized based on each sample’s total protein concentration.
Mitochondrial membrane potential (MMP) assay
Staining with Rhodamine 123 (Rh123) was used to MMP (Sakamuru et al., 2016). Briefly, 24 h after the treatments, the SMNs were incubated with Rh 123 (1 μM) at 37 °C for 30 min. SMNs were then washed with PBS, and the fluorescence intensity of Rh123 was measured (excitation 488 and emission 525 nm).
Gene expression analysis by qPCR
The relative expressions of AM, CRL, RAMPs, MMP-3, MMP-13, iNOS, TNF-α, IL-1β, and SOX9 were measured using qPCR (ABI Biosystems), specific primers (Table 1), and β-actin as the housekeeping gene. Qiagen and Thermo Fisher kits (USA) were used for RNA extraction and cDNA synthesis.

Statistical analysis
SPSS software, version 16 was used to analyze the data. According to the Shapiro-Wilk test, the data were normally distributed (P<0.05). Therefore, one-way analysis of variance (ANOVA) and Student-Newman-Keuls post-hoc test was used to analyze the data. The data were presented as Mean±SEM, and P<0.05 was considered a significant difference.
3. Results
AM protected SMNs against DOX-induced cell death
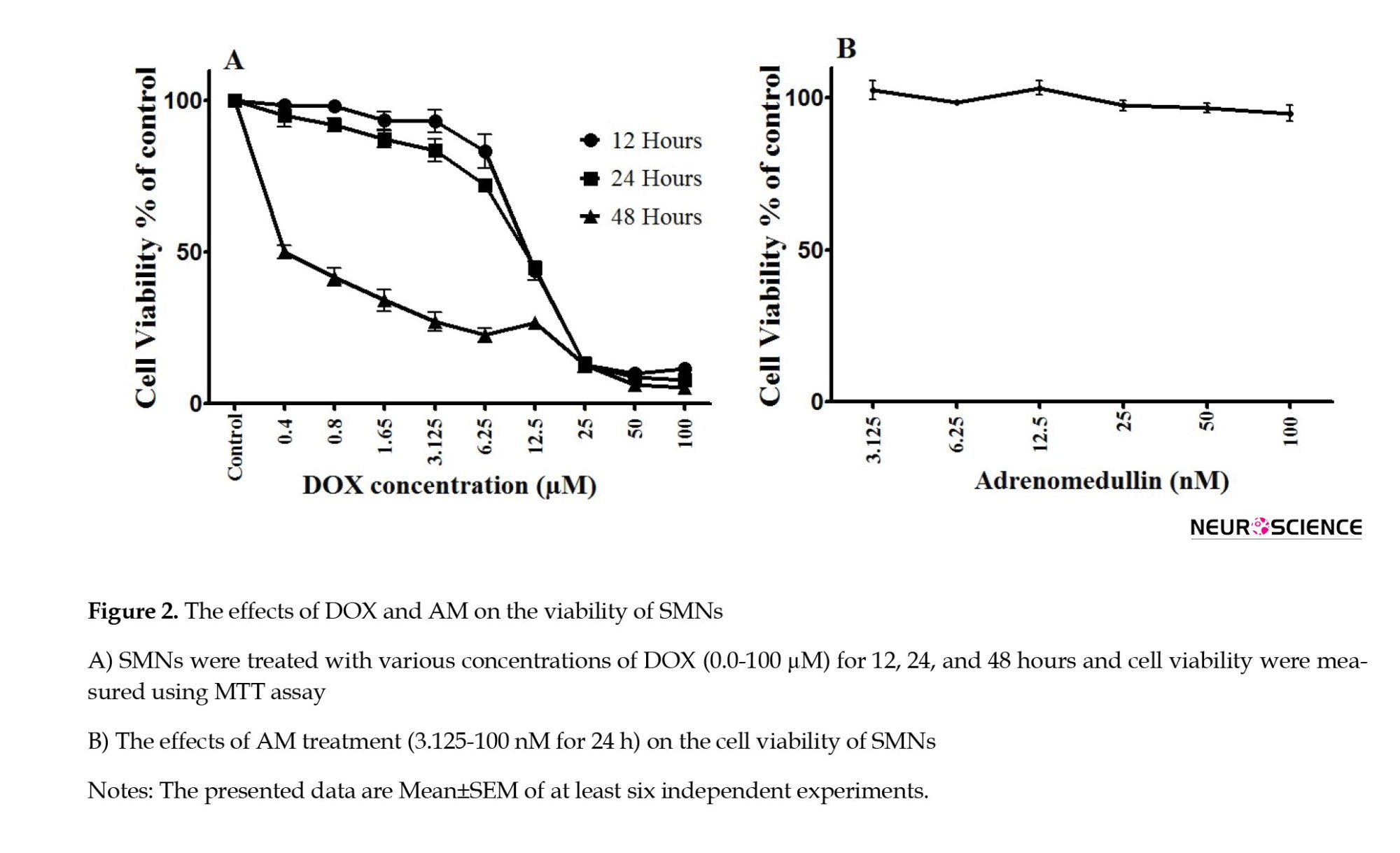
The MTT assay results (Figure 2) revealed a dose- and time-dependent increase in SMN cell death following DOX treatment (IC50 values=10.72±3.42, 10.54±2.59, and 0.95±0.14 µM for 12, 24, and 48 h of treatment, respectively). AM exerted no significant toxic effects on the viability of SMNs (Figure 2B).
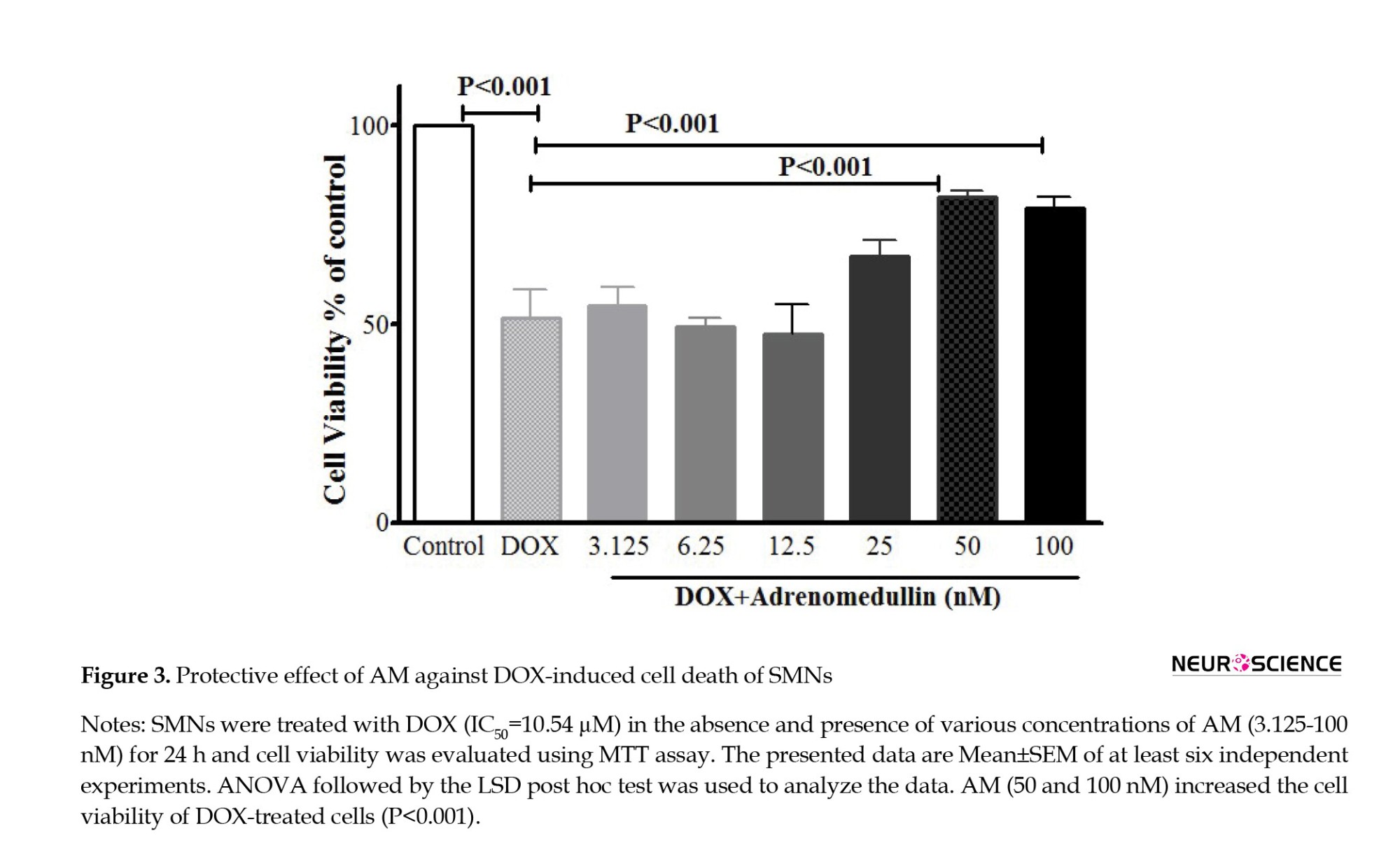
AM reversed DOX (10.54 µM for 24 h)-induced cell death in a concentration-dependent manner (P<0.0001) (Figure 3). Based on the MTT results, one concentration of DOX (10.54 µM) and AM (50 nM) AM was chosen for the subsequent experiments.
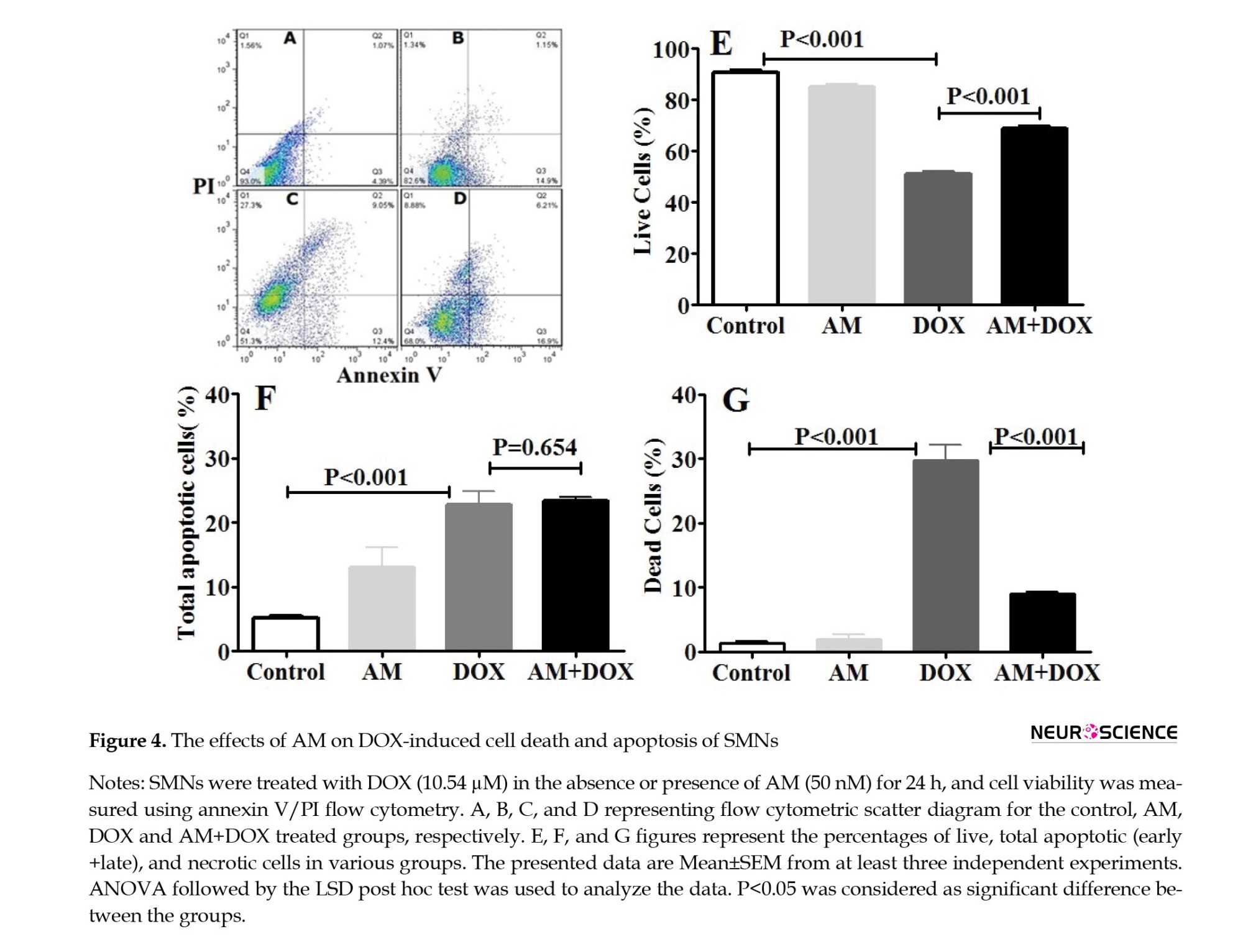
AM reversed DOX-induced SMNs cell death
Based on the apoptosis data, the SMNs viability decreased to 49.3% in the DOX-treated neurons (10.54 µM for 24 h). The decrease in the viable SMNs population in the DOX-treated cells was accompanied by a significant increase in the percentage of necrotic cells to 33.8%. However, no significant change was observed in the percentage of live cells in the AM group (50 nM).
AM also reduced the DOX-induced necrosis of SMNs (P<0.0001) (Figure 4).
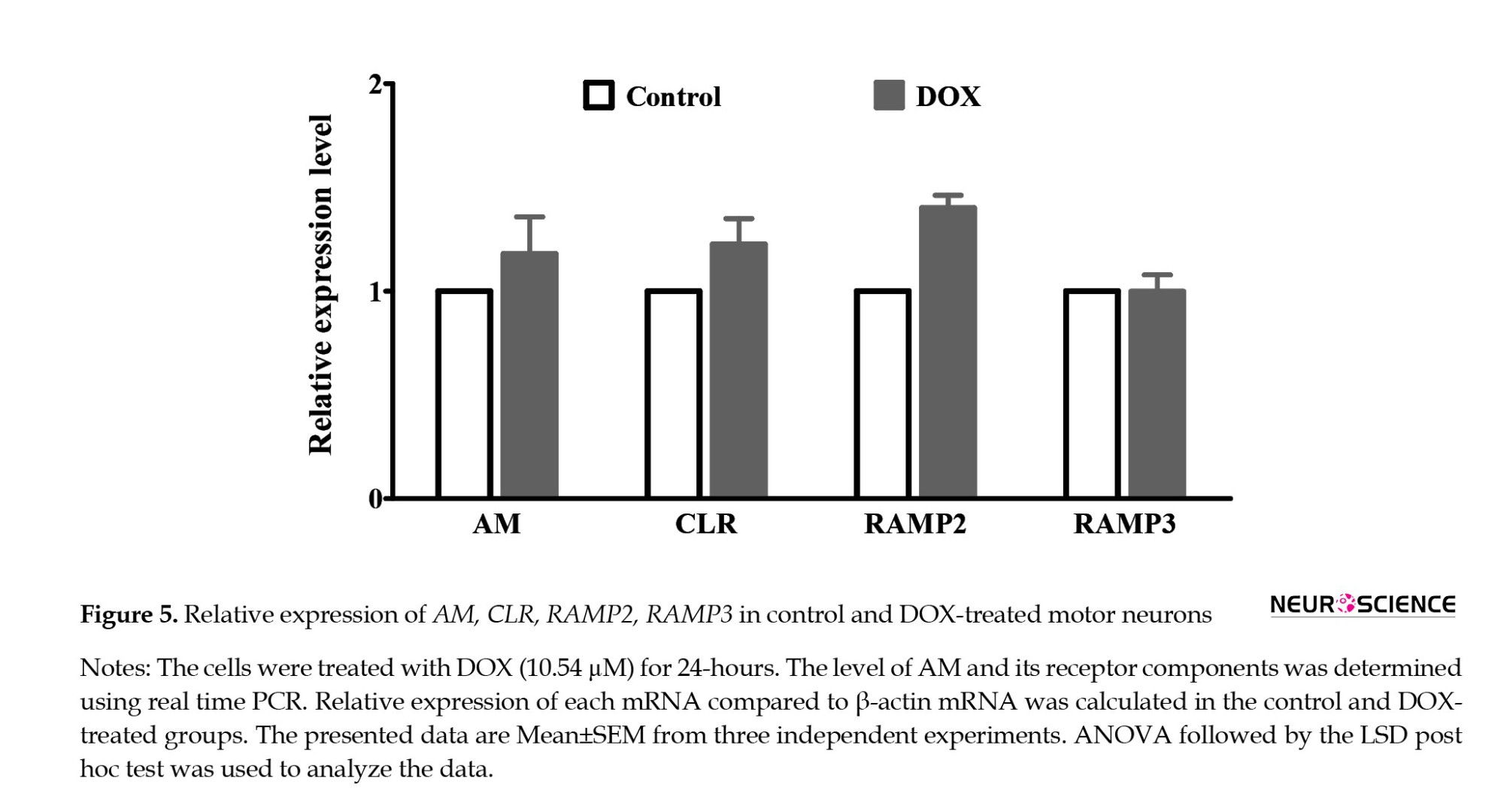
SMNs expressed AM, CLR, and RAMPs
The results of q-PCR revealed the expressions of CLR, RAMPs, and AM in the SMNs (Figure 5) DOX increased the expression of RAMP2 (P=0.001), while it had no significant effects on the expressions of AM, CLR, and RAMP3.
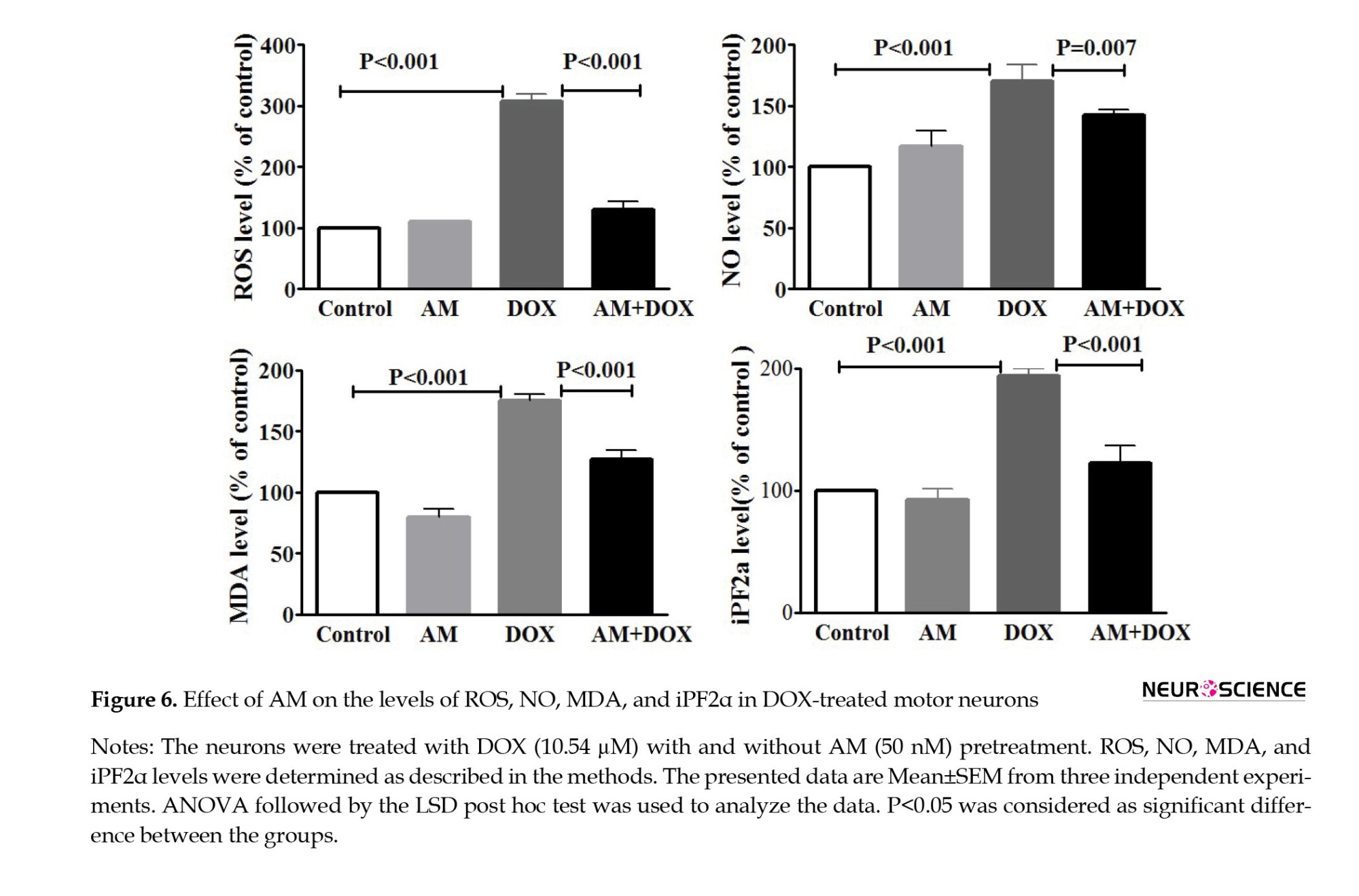
DOX-Induced oxidative stress ameliorated by AM
The levels of various markers were measured to assess the effects of DOX on oxidative stress (Figure 6). As illustrated in Figure 6A, DOX elevated intracellular ROS in the SMNs compared to the controls (P<0.001). Pretreatment with AM (50 nM) significantly reduced DOX-induced ROS generation (P<0.001). Furthermore, AM pretreatment significantly (P=0.004) decreased DOX-induced NO production in the SMNs (Figure 6B). Moreover, the levels of MDA (P<0.001) and iPF2α (P=0.003) were elevated in the DOX-treated SMNs. Similarly, AM decreased the DOX-elevated levels of iPF2α (P=0.003) and MDA (P=0.007) (Figure 6).
AM suppressed the effects of DOX on MMP
MMP dissipation can lead to mitochondrial dysfunction and cell death (Webster, 2012). The results of the MMP assay revealed a significant decline in MMP DOX-treated SMNs compared to the controls (P<0.001). AM treatment alone had no significant effects on the MMP level but significantly reduced the effect of DOX (10.54 µM for 24 h) on the MMP loss (Figure 7).

The effects of AM and DOX-induced gene expression
Following treatment with DOX (10.54 µM for 24 h), the mRNA levels of TNF-α, iNOS, and IL-1β (P<0.001) increased compared to the control SMNs (Figure 8).
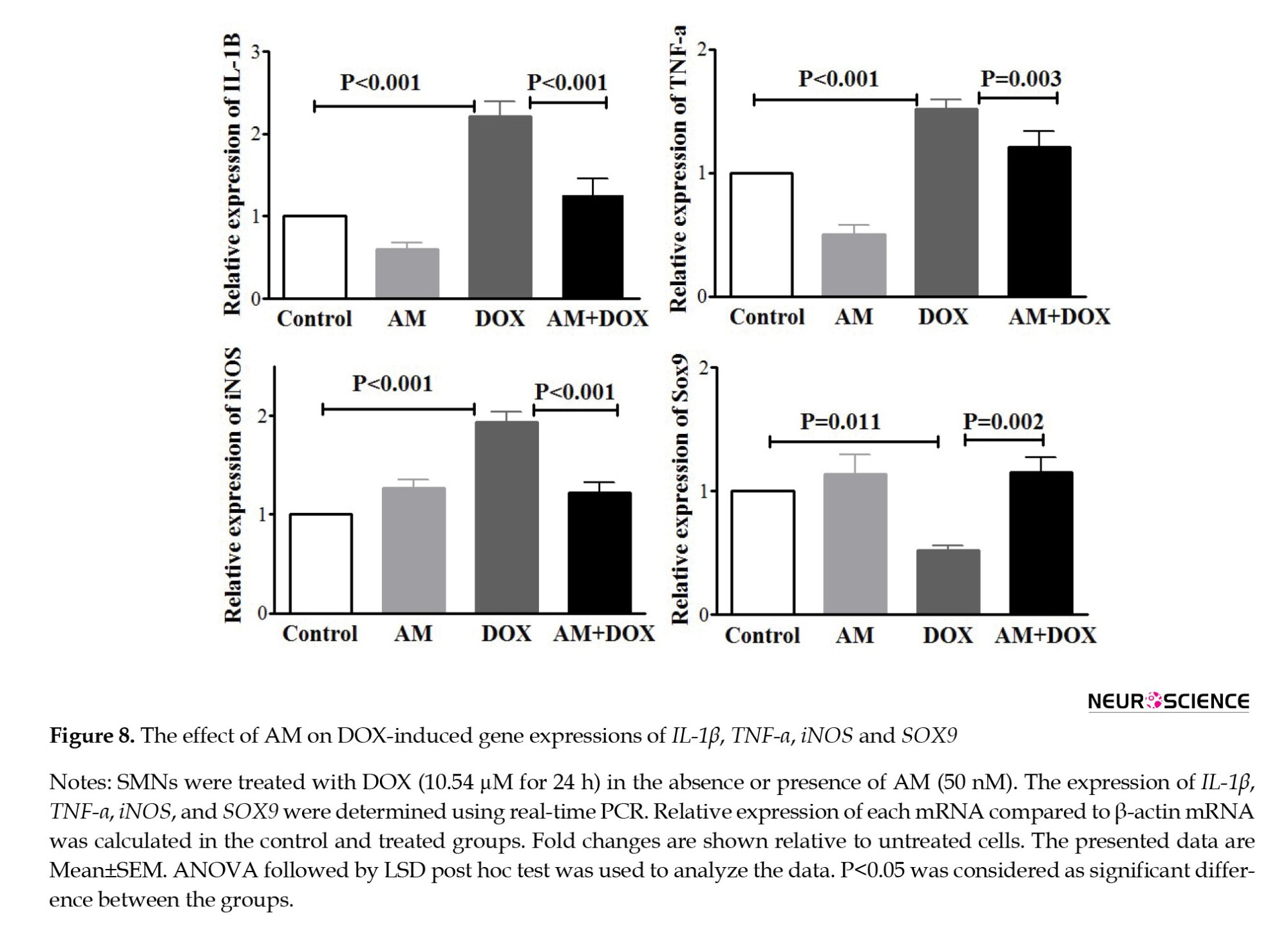
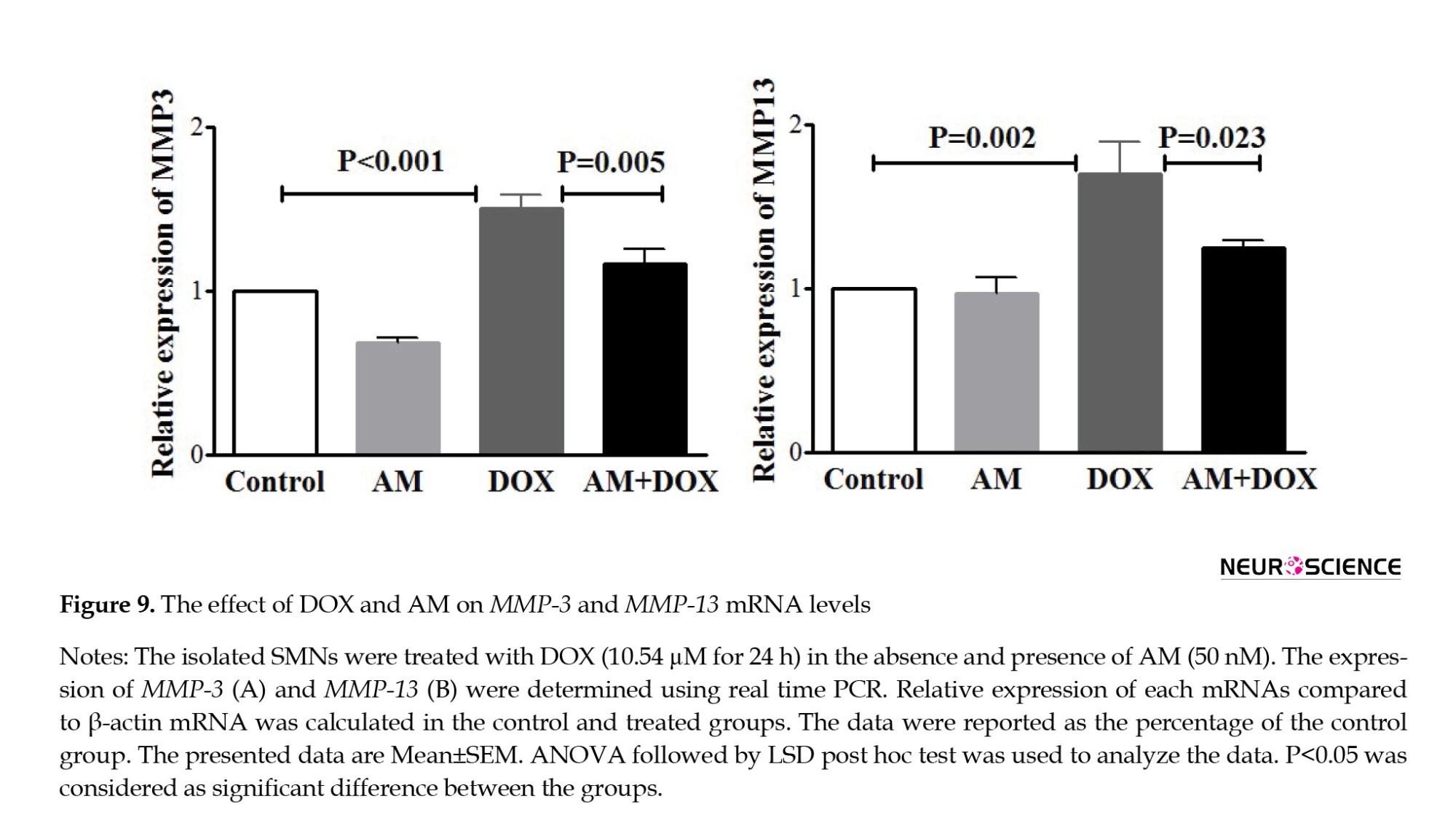
AM blocked the effects of DOX on iNOS, TNF-α, SOX9, and IL-1β expressions. As shown in Figure 9, DOX also increased the expression levels of MMP-3 (P<0.001) and MMP-13 (P=0.007). AM pretreatment revealed no significant effects on MMP-13 mRNA while decreasing the DOX-induced MMP-3 expression.
4. Discussion
The cultures of isolated SMNs provide a valuable model for studying the mechanisms involved in motor neuron survival, degeneration, and regeneration (Bucchia et al., 2018). This research utilized this model to determine AM’s possible neuroprotective role. The findings revealed the protective effects of AM against DOX-induced cell death in the SMNs. AM also ameliorated the DOX-induced oxidative stress and MMP dissipation. Finally, DOX-induced expressions of inflammatory mediators were reversed by AM. These findings suggested that AM might be considered a protective factor in the conditions associated with the degeneration of SMNs.
Although DOX poorly crosses the blood-brain barrier, there is evidence that it can reach the motor neurons through retrograde axoplasmic transport (Sabri, 2018). In the current study, treatment of the isolated SMNs with DOX led to oxidative stress, increased expression of inflammatory markers, and cell death. These findings were in line with the observed toxicity of DOX in DRG neurons (Mahmoodazdeh et al., 2020) and cardiac cells (Yoshizawa et al., 2016).
Several studies have shown the protective effects of AM in different cells mediated through antioxidant and anti-inflammatory activities (Yoshimoto et al., 2004). The current study results revealed the expressions of AM, CLR, and RAMP-2 and -3 in the embryonic SMNs, which was in accordance with the findings of the research carried out by Montuenga et al. (1997) that indicated the expressions of AM and CLR in the ventral spinal cells from day 14 of the embryonic life. In the present investigation, the results of the flow cytometric assay demonstrated that AM (50 nM) reversed the DOX-induced necrosis of the SMNs while it had no significant effects on DOX-induced apoptosis. AM also reduced the DOX-induced increase in the intracellular levels of oxidative stress markers (ROS, NO, MDA, and iPFα), suggesting the antioxidant effects of AM. Although the underlying mechanisms were not explored in our investigation, it has been revealed that AM could block the IL-1β-induced inflammation by decreasing the expressions of iNOS and cyclooxygenase-2 as well as the concentrations of NO and prostaglandin E2 (Hu et al., 2015).
In line with other studies, the findings showed that DOX significantly induced MMP dissipation. AM ameliorated DOX-induced mitochondrial damage in cardiac muscle cells (Yoshizawa et al., 2016). Therefore, prevention of mitochondria damage may play a role in the observed AM effects. Some reports regarding the anti-inflammatory properties of AM are also available. Hu et al. showed that AM could attenuate inflammation-induced apoptosis of rats’ Leydig cells (Hu et al., 2015). This finding agreed with the present study results, which demonstrated a significant reduction in the expressions of inflammatory mediators following AM pretreatment in the SMNs, suggesting that AM may exert anti-inflammatory action against toxicity induced by DOX. Several mechanisms have been proposed for the anti-inflammatory properties of AM. For example, AM may inhibit the release of cytokine-induced neutrophil chemoattractants, possibly via a cAMP-dependent pathway (Yu et al., 2009).
Matrix metalloproteinase enzymes are actively involved in the inflammatory process, mainly through their role in regulating the availability and activity of inflammatory mediators (Nissinen & Kähäri, 2014). It has been shown that MMPs, especially MMP-3 and -13, have a role in cell toxicities induced by anti-cancer agents. The present study findings revealed a significant increase in the expression levels of these enzymes following DOX treatment. The same results were reported as the side effect of another chemotherapeutic agent, i.e. paclitaxel (Cirrincione et al., 2019; Nishida et al., 2008), suggesting that MMP-3 may play a role in chemotherapy-induced cell death. ROS (Haorah et al., 2007) and inflammatory mediators (Fingleton, 2017) were also critical in activating MMPs. Thus, antioxidant and anti-inflammatory agents could act as possible inhibitors of MMPs in abnormal states. Although the current study results proved the inhibitory effect of AM on the expression levels of MMPs, more studies are recommended to clarify the exact pathways.
SOX9 has been found to play a role in various physiological pathways, such as CNS development (Pevny & Placzek, 2005) and neural stem cell survival (Scott et al., 2010). Proteasome-induced degradation of SOX9 was revealed following DNA damage by several genotoxic agents (Hong et al., 2016). However, SOX9 upregulation increased cell viability, suggesting its role in cell survival (Roche et al., 2015). In the present study, DOX decreased the SOX9 expression in the SMNs, while AM blocked the action of DOX, suggesting that SOX may play a role in AM’s protective effects. CREB binds to its regulatory elements in SOX promoter, leading to SOX upregulation (Piera-Velazquez et al., 2007), while its expression is downregulated by IL-1β and TNF-α (Piera-Velazquez et al., 2007; Yu et al., 2009). Moreover, the BMP-2 signaling pathway increases SOX9 gene expression. In DRG neurons, AM increases BMP-2 expression, suggesting the possible role of BMP-2 signaling in AM-induced SOX9 expression.
5. Conclusion
In conclusion, the present study provided substantial evidence that AM ameliorated the DOX-induced toxicity in isolated motor neurons. Based on the results, a decrease in cell viability mainly mediated through necrotic cell death, increased level of oxidative stress markers, loss of MMPs, and increased expressions of genes involved in inflammation were the toxic effects detected following the treatment of motor neurons with DOX. The findings indicated that pretreatment with a low dose of AM (50 nM) could protect the motor neurons from the DOX-induced cytotoxic effect, most probably through its anti-oxidant effects.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Shiraz University of Medical Sciences approved this study (Code: IR.SUMS.REC.1396.582).
Funding
This article was extracted from the PhD dissertation of Amir Mahmoodzadeh, approved by the Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Vice Chancellor for Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran, financially supported this research (Grant No.: 95-01-01-13898).
Authors' contributions
Conceptualization and supervision: Mohammad Ali Takhshid and Sayed Mohammad Shafiee; Methodology: Amir Mahmoodzadeh, Mohsen Sisakht, and Zahra Khoshdel; Investigation: Amir Mahmoodzadeh, Mohsen Sisakht, and Zahra Khoshdel; Data analysis: Amir Mahmoodzadeh and Zahra Khoshdel; Writing the original draft: Amir Mahmoodzadeh and Mohammad Ali Takhshid; Review and editing: Mohammad Ali Takhshid and Sayed Mohammad Shafiee; Funding acquisition and resources: Mohammad Ali Takhshid and Sayed Mohammad Shafiee.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express their gratitude to the Vice Chancellor for Research Affairs at Shiraz University of Medical Sciences for the financial endorsement provided for this research endeavor.
References
Spinal motor neurons (SMNs) are essential to the spinal cord neural circuit and play crucial roles in spinal reflexes. SMNs also transmit signals from the brain to the muscles (Bäumer et al., 2014). Progressive loss of SMNs that occurs in amyotrophic lateral sclerosis and the use of chemotherapeutic drugs leads to muscle atrophy and disability (Drechsel et al., 2012; Starobova & Vetter, 2017; Verma et al., 2017). Doxorubicin (DOX) is among the potent chemotherapeutic drugs used to treat several cancers (Khan et al., 2014; McGowan et al., 2017). DOX exerts its therapeutic effects through the inhibition of replication and an increase in the production of inflammatory mediators and reactive oxygen species (ROS) (Capelôa et al., 2014; Zhao & Zhang, 2017). Despite its beneficial effects, irreversible toxic effects of DOX on the induction of cognitive and motor impairments such as learning disabilities and memory loss have been reported (Moruno-Manchon et al., 2016). DOX-mediated degeneration of the dorsal root ganglion (DRG) and SMNs have also been shown in the spinal cord in both animal models (Wu et al., 2015) and human studies (Saito et al., 2019).
Adrenomedullin (AM), a peptide belonging to the calcitonin family, is widely expressed in peripheral tissues and the central nervous system (CNS) in the brain and the spinal cord (Hong et al., 2009). Considering the peripheral tissues, AM has revealed protective effects against Leydig cells pyroptosis (Li et al., 2019), heart failure (Voors et al., 2019), pulmonary and renal diseases (Holmes et al., 2013), sepsis (Geven et sl., 2018), and ulcerative colitis (Martinez-Herrero et al., 2017). An in vitro study on mesenchymal stem cells showed that AM overexpression could protect the cells from serum deprivation and hypoxia-induced apoptosis (Si et al., 2018). Moreover, AM inhibited streptozotocin-induced cell death in cultured human renal tubule cells (Uetake et al., 2014). In the CNS, calcitonin-like receptor (CLR), receptor activities modifying protein-2 (RAMP-2), and RAMP-3, as AM receptor components, are expressed in both glial and nerve cells. Animal studies have suggested AM’s role in transmitting nociceptive impulses (Ma et al., 2006; Takhshid et al., 2004). The neuroprotective effects of AM against ischemic brain damage and acute brain injury were mediated through decreasing oxidative stress and pro-inflammatory cytokines production (Demir et al., 2013). A recent study also disclosed the protective effects of AM against the neurotoxic effects of DOX in DRG neurons (Mahmoodazdeh et al., 2020). The present study aims to determine AM, CLR, and RAMPs expressions in rat embryonic SMNs and assess AM’s protective and anti-oxidant effects against DOX-induced neurotoxicity.
2. Materials and Methods
Reagents and chemicals
Rat AM was purchased from Bachem. Cell culture materials were bought from Gibco BRL (Life Technologies, Renfrewshire, UK), except for poly-D-lysine, Trypsin-EDTA solution, penicillin/streptomycin solution, MTT assay kit, OptiPrep™ density gradient medium (D1556-250ML, Sigma, USA), and DOX that were the products of Sigma (USA). BCA protein assay kit and nitric oxide (NO) generation kit were purchased from Thermo Fisher Scientific, Inc. (The USA). ROS detection kit was provided by Molecular Probes (The USA), and an 8-iso prostaglandin F2α (iPF2α) ELISA kit was obtained from Cayman Chemical Co. (The USA). All primers were prepared by Metabion Co. (Germany). Apoptosis detection kits were purchased from Bio Vision (Switzerland), and Syber green polymerase chain reaction (PCR) master mix was bought from Ampliqon A/S, Odense (Denmark).
Isolation and culture of SMNs
SMNs were cultured according to Wang’s method (Wang et al., 2017). As illustrated in Figure 1, the spinal cord was separated from the DRGs, rinsed in L15 medium containing penicillin G (300 U/mL) and gentamycin (75 μg/mL), and digested in 0.025% trypsin (Invitrogen) at 37 ºC for 40 min. The spinal cells were then dissociated using trituration, and the suspended cells were put on a single gradient of OptiPrep™ and centrifuged for 10 min (4 ºC) to separate SMNs from debris (F1), SMNs(F2), and non-neural cells (Figure 1). The SMNs (F2 layer) were carefully separated, re-suspended in an appropriate volume of culture medium, transferred to the poly-D-lysine coated culture plates, and cultured in Neurobasal-A medium supplemented with B27 (2%), GlutaMAX (2 mM), streptomycin (100 mg), and penicillin (100 units) at 37 °C, 5% CO2, and 80% relative humidity. After that, the cultured cells were treated with 5-fluorodeoxyuridine (20 mM; 72 h) to prevent the growth of non-neural cells (Liu et al., 2013). Finally, the purity of the SMNs in the culture was evaluated using phase contrast microscopy. Based on the results, SMNs comprised 87±5% of the cultured cells.

Cell viability assay
To evaluate DOX toxicity, the SMNs were treated with DOX (0-100 µM), and their viability was measured for 12, 24, and 48 h. To evaluate the possible toxicity of AM, the SMNs were treated with AM (3.125-100 nM) for 24 h. The doses of AM were selected according to a previous study (Mahmoodazdeh et al., 2020). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5- diphenyl-2H-tetrazolium bromide) was used to assess the effects of treatment on cell survival (Mahmoodazdeh et al., 2020). IC50 value of DOX was calculated using Prism 6 software. For further evaluations, the SMNs were cultured in 48-well plates (2×105 cells/well) and treated with AM (50 nM), DOX (10.54 µM; IC50), and AM + DOX (50 nM+10.54 µM). SMNs were pretreated with AM for two h, DOX was added to the wells, and cell viability was measured after 24 h.
Apoptosis assay
The effects of the treatments on SMNs apoptosis were determined and evaluated using annexin V-FITC/PI staining and flow cytometry. Briefly, the treated SMNs were harvested, stained, and analyzed using a flow cytometer (BD) at 488 nm.
ROS assay
Intracellular ROS were measured using 2’,7’-dichloro fluorescein diacetate (DCFH-DA, Molecular Probes, Eugene, OR, USA) probe and flowcytometry (Chen et al., 2010). Briefly, the SMNs were incubated with 10 μM of DCF-DA at 37 °C for 30 min. The cells were then washed three times with phosphate-buffered saline (PBS), and the fluorescence intensity was measured (excitation: 488 and emission:525 nm) using a Multimode Plate Reader.
Oxidative markers assay
NO levels in the cell lysate were assayed using the Griess-reduction method and nitrite standard curve (1−100 nmol/mL) (Hare et al., 2008). The Griess reagent (5% H3PO4 containing 1% sulfanilamide and 0.1% naphthyl ethylenediamine) was mixed with the cell lysates and incubated at room temperature in the dark. The absorbance of the samples was then measured at 540 nm. The thiobarbituric acid method (Tsikas, 2017) was used to measure MDA levels in SMNs. To quantify iPF2α levels, the SMNs were lysed and treated with KOH (15% at 40 °C for 60 min) to release esterified forms of iPF2α. iPF2α was then measured using an ELISA kit (Cayman, item No. 51635) and normalized based on each sample’s total protein concentration.
Mitochondrial membrane potential (MMP) assay
Staining with Rhodamine 123 (Rh123) was used to MMP (Sakamuru et al., 2016). Briefly, 24 h after the treatments, the SMNs were incubated with Rh 123 (1 μM) at 37 °C for 30 min. SMNs were then washed with PBS, and the fluorescence intensity of Rh123 was measured (excitation 488 and emission 525 nm).
Gene expression analysis by qPCR
The relative expressions of AM, CRL, RAMPs, MMP-3, MMP-13, iNOS, TNF-α, IL-1β, and SOX9 were measured using qPCR (ABI Biosystems), specific primers (Table 1), and β-actin as the housekeeping gene. Qiagen and Thermo Fisher kits (USA) were used for RNA extraction and cDNA synthesis.

Statistical analysis
SPSS software, version 16 was used to analyze the data. According to the Shapiro-Wilk test, the data were normally distributed (P<0.05). Therefore, one-way analysis of variance (ANOVA) and Student-Newman-Keuls post-hoc test was used to analyze the data. The data were presented as Mean±SEM, and P<0.05 was considered a significant difference.
3. Results
AM protected SMNs against DOX-induced cell death
The MTT assay results (Figure 2) revealed a dose- and time-dependent increase in SMN cell death following DOX treatment (IC50 values=10.72±3.42, 10.54±2.59, and 0.95±0.14 µM for 12, 24, and 48 h of treatment, respectively). AM exerted no significant toxic effects on the viability of SMNs (Figure 2B).

AM reversed DOX (10.54 µM for 24 h)-induced cell death in a concentration-dependent manner (P<0.0001) (Figure 3). Based on the MTT results, one concentration of DOX (10.54 µM) and AM (50 nM) AM was chosen for the subsequent experiments.

AM reversed DOX-induced SMNs cell death
Based on the apoptosis data, the SMNs viability decreased to 49.3% in the DOX-treated neurons (10.54 µM for 24 h). The decrease in the viable SMNs population in the DOX-treated cells was accompanied by a significant increase in the percentage of necrotic cells to 33.8%. However, no significant change was observed in the percentage of live cells in the AM group (50 nM).
AM also reduced the DOX-induced necrosis of SMNs (P<0.0001) (Figure 4).

SMNs expressed AM, CLR, and RAMPs
The results of q-PCR revealed the expressions of CLR, RAMPs, and AM in the SMNs (Figure 5) DOX increased the expression of RAMP2 (P=0.001), while it had no significant effects on the expressions of AM, CLR, and RAMP3.

DOX-Induced oxidative stress ameliorated by AM
The levels of various markers were measured to assess the effects of DOX on oxidative stress (Figure 6). As illustrated in Figure 6A, DOX elevated intracellular ROS in the SMNs compared to the controls (P<0.001). Pretreatment with AM (50 nM) significantly reduced DOX-induced ROS generation (P<0.001). Furthermore, AM pretreatment significantly (P=0.004) decreased DOX-induced NO production in the SMNs (Figure 6B). Moreover, the levels of MDA (P<0.001) and iPF2α (P=0.003) were elevated in the DOX-treated SMNs. Similarly, AM decreased the DOX-elevated levels of iPF2α (P=0.003) and MDA (P=0.007) (Figure 6).

AM suppressed the effects of DOX on MMP
MMP dissipation can lead to mitochondrial dysfunction and cell death (Webster, 2012). The results of the MMP assay revealed a significant decline in MMP DOX-treated SMNs compared to the controls (P<0.001). AM treatment alone had no significant effects on the MMP level but significantly reduced the effect of DOX (10.54 µM for 24 h) on the MMP loss (Figure 7).

The effects of AM and DOX-induced gene expression
Following treatment with DOX (10.54 µM for 24 h), the mRNA levels of TNF-α, iNOS, and IL-1β (P<0.001) increased compared to the control SMNs (Figure 8).

AM blocked the effects of DOX on iNOS, TNF-α, SOX9, and IL-1β expressions. As shown in Figure 9, DOX also increased the expression levels of MMP-3 (P<0.001) and MMP-13 (P=0.007). AM pretreatment revealed no significant effects on MMP-13 mRNA while decreasing the DOX-induced MMP-3 expression.

4. Discussion
The cultures of isolated SMNs provide a valuable model for studying the mechanisms involved in motor neuron survival, degeneration, and regeneration (Bucchia et al., 2018). This research utilized this model to determine AM’s possible neuroprotective role. The findings revealed the protective effects of AM against DOX-induced cell death in the SMNs. AM also ameliorated the DOX-induced oxidative stress and MMP dissipation. Finally, DOX-induced expressions of inflammatory mediators were reversed by AM. These findings suggested that AM might be considered a protective factor in the conditions associated with the degeneration of SMNs.
Although DOX poorly crosses the blood-brain barrier, there is evidence that it can reach the motor neurons through retrograde axoplasmic transport (Sabri, 2018). In the current study, treatment of the isolated SMNs with DOX led to oxidative stress, increased expression of inflammatory markers, and cell death. These findings were in line with the observed toxicity of DOX in DRG neurons (Mahmoodazdeh et al., 2020) and cardiac cells (Yoshizawa et al., 2016).
Several studies have shown the protective effects of AM in different cells mediated through antioxidant and anti-inflammatory activities (Yoshimoto et al., 2004). The current study results revealed the expressions of AM, CLR, and RAMP-2 and -3 in the embryonic SMNs, which was in accordance with the findings of the research carried out by Montuenga et al. (1997) that indicated the expressions of AM and CLR in the ventral spinal cells from day 14 of the embryonic life. In the present investigation, the results of the flow cytometric assay demonstrated that AM (50 nM) reversed the DOX-induced necrosis of the SMNs while it had no significant effects on DOX-induced apoptosis. AM also reduced the DOX-induced increase in the intracellular levels of oxidative stress markers (ROS, NO, MDA, and iPFα), suggesting the antioxidant effects of AM. Although the underlying mechanisms were not explored in our investigation, it has been revealed that AM could block the IL-1β-induced inflammation by decreasing the expressions of iNOS and cyclooxygenase-2 as well as the concentrations of NO and prostaglandin E2 (Hu et al., 2015).
In line with other studies, the findings showed that DOX significantly induced MMP dissipation. AM ameliorated DOX-induced mitochondrial damage in cardiac muscle cells (Yoshizawa et al., 2016). Therefore, prevention of mitochondria damage may play a role in the observed AM effects. Some reports regarding the anti-inflammatory properties of AM are also available. Hu et al. showed that AM could attenuate inflammation-induced apoptosis of rats’ Leydig cells (Hu et al., 2015). This finding agreed with the present study results, which demonstrated a significant reduction in the expressions of inflammatory mediators following AM pretreatment in the SMNs, suggesting that AM may exert anti-inflammatory action against toxicity induced by DOX. Several mechanisms have been proposed for the anti-inflammatory properties of AM. For example, AM may inhibit the release of cytokine-induced neutrophil chemoattractants, possibly via a cAMP-dependent pathway (Yu et al., 2009).
Matrix metalloproteinase enzymes are actively involved in the inflammatory process, mainly through their role in regulating the availability and activity of inflammatory mediators (Nissinen & Kähäri, 2014). It has been shown that MMPs, especially MMP-3 and -13, have a role in cell toxicities induced by anti-cancer agents. The present study findings revealed a significant increase in the expression levels of these enzymes following DOX treatment. The same results were reported as the side effect of another chemotherapeutic agent, i.e. paclitaxel (Cirrincione et al., 2019; Nishida et al., 2008), suggesting that MMP-3 may play a role in chemotherapy-induced cell death. ROS (Haorah et al., 2007) and inflammatory mediators (Fingleton, 2017) were also critical in activating MMPs. Thus, antioxidant and anti-inflammatory agents could act as possible inhibitors of MMPs in abnormal states. Although the current study results proved the inhibitory effect of AM on the expression levels of MMPs, more studies are recommended to clarify the exact pathways.
SOX9 has been found to play a role in various physiological pathways, such as CNS development (Pevny & Placzek, 2005) and neural stem cell survival (Scott et al., 2010). Proteasome-induced degradation of SOX9 was revealed following DNA damage by several genotoxic agents (Hong et al., 2016). However, SOX9 upregulation increased cell viability, suggesting its role in cell survival (Roche et al., 2015). In the present study, DOX decreased the SOX9 expression in the SMNs, while AM blocked the action of DOX, suggesting that SOX may play a role in AM’s protective effects. CREB binds to its regulatory elements in SOX promoter, leading to SOX upregulation (Piera-Velazquez et al., 2007), while its expression is downregulated by IL-1β and TNF-α (Piera-Velazquez et al., 2007; Yu et al., 2009). Moreover, the BMP-2 signaling pathway increases SOX9 gene expression. In DRG neurons, AM increases BMP-2 expression, suggesting the possible role of BMP-2 signaling in AM-induced SOX9 expression.
5. Conclusion
In conclusion, the present study provided substantial evidence that AM ameliorated the DOX-induced toxicity in isolated motor neurons. Based on the results, a decrease in cell viability mainly mediated through necrotic cell death, increased level of oxidative stress markers, loss of MMPs, and increased expressions of genes involved in inflammation were the toxic effects detected following the treatment of motor neurons with DOX. The findings indicated that pretreatment with a low dose of AM (50 nM) could protect the motor neurons from the DOX-induced cytotoxic effect, most probably through its anti-oxidant effects.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Shiraz University of Medical Sciences approved this study (Code: IR.SUMS.REC.1396.582).
Funding
This article was extracted from the PhD dissertation of Amir Mahmoodzadeh, approved by the Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. Vice Chancellor for Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran, financially supported this research (Grant No.: 95-01-01-13898).
Authors' contributions
Conceptualization and supervision: Mohammad Ali Takhshid and Sayed Mohammad Shafiee; Methodology: Amir Mahmoodzadeh, Mohsen Sisakht, and Zahra Khoshdel; Investigation: Amir Mahmoodzadeh, Mohsen Sisakht, and Zahra Khoshdel; Data analysis: Amir Mahmoodzadeh and Zahra Khoshdel; Writing the original draft: Amir Mahmoodzadeh and Mohammad Ali Takhshid; Review and editing: Mohammad Ali Takhshid and Sayed Mohammad Shafiee; Funding acquisition and resources: Mohammad Ali Takhshid and Sayed Mohammad Shafiee.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express their gratitude to the Vice Chancellor for Research Affairs at Shiraz University of Medical Sciences for the financial endorsement provided for this research endeavor.
References
Bäumer, D., Talbot, K., & Turner, M. R. (2014). Advances in motor neurone disease. Journal of the Royal Society of Medicine, 107(1), 14-21. [DOI:10.1177/0141076813511451] [PMID] [PMCID]
Bucchia, M., Merwin, S. J., Re, D. B., & Kariya, S. (2018). Limitations and challenges in modeling diseases involving spinal motor neuron degeneration in vitro. Frontiers in Cellular Neuroscience, 12, 61. [DOI:10.3389/fncel.2018.00061] [PMID] [PMCID]
Capelôa, T., Caramelo, F., Fontes-Ribeiro, C., Gomes, C., & Silva, A. P. (2014). Role of methamphetamine on glioblastoma cytotoxicity induced by doxorubicin and methotrexate. Neurotoxicity Research, 26(3), 216-227. [DOI:10.1007/s12640-014-9464-1] [PMID]
Chen, X., Zhong, Z., Xu, Z., Chen, L., & Wang, Y. (2010). 2’,7’-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radical Research, 44(6), 587–604.[DOI:10.3109/10715761003709802] [PMID]
Cirrincione, A. M., Pellegrini, A. D., Dominy, J. R., Benjamin, M. E., Utkina-Sosunova, I., & Lotti, F., et al (2019). Paclitaxel-induced peripheral neuropathy is caused by epidermal ROS and mitochondrial damage through conserved MMP-13 activation. bioRxiv. [DOI:10.1101/743419]
Demir, H., Onur, O. E., Denizbasi, A., Akoglu, H., Eroglu, S. E., & Ozpolat, C., et al. (2013). The effects of adrenomedullin in traumatic brain injury. Peptides, 43, 27-31. [DOI:10.1016/j.peptides.2013.02.018] [PMID]
Drechsel, D. A., Estévez, A. G., Barbeito, L., & Beckman, J. S. (2012). Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotoxicity Research, 22(4), 251-264. [DOI:10.1007/s12640-012-9322-y] [PMID] [PMCID]
Fingleton, B. (2017). Matrix metalloproteinases as regulators of inflammatory processes. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1864(11), 2036-2042. [DOI:10.1016/j.bbamcr.2017.05.010] [PMID]
Geven, C., Kox, M., & Pickkers, P. (2018). Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Frontiers in Immunology, 9, 292. [DOI:10.3389/fimmu.2018.00292] [PMID] [PMCID]
Haorah, J., Ramirez, S. H., Schall, K., Smith, D., Pandya, R., & Persidsky, Y. (2007). Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. Journal of Neurochemistry, 101(2), 566-576. [DOI:10.1111/j.1471-4159.2006.04393.x] [PMID]
Hare, J. M., Beigi, F., & Tziomalos, K. (2008). Nitric oxide and cardiobiology-methods for intact hearts and isolated myocytes. Methods in Enzymology, 441, 369–392. [DOI:10.1016/S0076-6879(08)01221-4] [PMID]
Holmes, D., Campbell, M., Harbinson, M., & Bell, D. (2013). Protective effects of intermedin on cardiovascular, pulmonary and renal diseases: Comparison with adrenomedullin and CGRP. Current Protein & Peptide Science, 14(4), 294–329. [DOI:10.2174/13892037113149990049] [PMID]
Hong, X., Liu, W., Song, R., Shah, J. J., Feng, X., & Tsang, C. K., et al. (2016). SOX9 is targeted for proteasomal degradation by the E3 ligase FBW7 in response to DNA damage. Nucleic Acids Research, 44(18), 8855–8869. [DOI:10.1093/nar/gkw748] [PMID] [PMCID]
Hong, Y., Liu, Y., Chabot, J. G., Fournier, A., & Quirion, R. (2009). Upregulation of adrenomedullin in the spinal cord and dorsal root ganglia in the early phase of CFA-induced inflammation in rats. Pain, 146(1-2), 105-113. [DOI:10.1016/j.pain.2009.07.015] [PMID]
Hu, W., Zhou, P. H., Rao, T., Zhang, X. B., Wang, W., & Zhang, L. J. (2015). Adrenomedullin attenuates interleukin-1β-induced inflammation and apoptosis in rat Leydig cells via inhibition of NF-κB signaling pathway. Experimental Cell Research, 339(2), 220-230. [DOI:10.1016/j.yexcr.2015.10.024] [PMID]
Khan, M. S., Singh, M., Khan, M. A., Arya, D., & Ahmad, S. (2014). Scientific validation of cardioprotective attribute by standardized extract of Bombyx mori against doxorubicin-induced cardiotoxicity in murine model. EXCLI Journal, 13, 1043–1054. [PMID]
Li, M. Y., Zhu, X. L., Zhao, B. X., Shi, L., Wang, W., & Hu, W., et al. (2019). Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS-AMPK-mTOR axis. Cell Death & Disease, 10(7), 489. [DOI:10.1038/s41419-019-1728-5] [PMID] [PMCID]
Liu, R., Lin, G., & Xu, H. (2013). An efficient method for dorsal root ganglia neurons purification with a one-time anti-mitotic reagent treatment. Plos One, 8(4), e60558. [DOI:10.1371/journal.pone.0060558] [PMID] [PMCID]
Ma, W., Chabot, J. G., & Quirion, R. (2006). A role for adrenomedullin as a pain-related peptide in the rat. Proceedings of the National Academy of Sciences of the United States of America, 103(43), 16027–16032. [DOI:10.1073/pnas.0602488103] [PMID] [PMCID]
Mahmoodazdeh, A., Shafiee, S. M., Sisakht, M., Khoshdel, Z., & Takhshid, M. A. (2020). Adrenomedullin protects rat dorsal root ganglion neurons against doxorubicin-induced toxicity by ameliorating oxidative stress. Iranian Journal of Basic Medical Sciences, 23(9), 1197–1206. [PMID]
Martinez-Herrero, S., Larrayoz, I. M., Narro-Iniguez, J., Rubio-Mediavilla, S., & Martinez, A. (2017). Lack of adrenomedullin aggravates acute TNBS-induced colitis symptoms in mice, especially in females. Frontiers in Physiology, 8, 1058. [DOI:10.3389/fphys.2017.01058] [PMID] [PMCID]
McGowan, J. V., Chung, R., Maulik, A., Piotrowska, I., Walker, J. M., & Yellon, D. M. (2017). Anthracycline chemotherapy and cardiotoxicity. Cardiovascular Drugs and Therapy, 31(1), 63-75. [DOI:10.1007/s10557-016-6711-0] [PMID] [PMCID]
Montuenga, L. M., Martínez, A., Miller, M. J., Unsworth, E. J., & Cuttitta, F. (1997). Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinology, 138(1), 440-451. [DOI:10.1210/endo.138.1.4881] [PMID]
Moruno-Manchon, J. F., Uzor, N. E., Kesler, S. R., Wefel, J. S., Townley, D. M., & Nagaraja, A. S., et al. (2016). TFEB ameliorates the impairment of the autophagy-lysosome pathway in neurons induced by doxorubicin. Aging, 8(12), 3507-3519. [DOI:10.18632/aging.101144] [PMID] [PMCID]
Nishida, K., Kuchiiwa, S., Oiso, S., Futagawa, T., Masuda, S., & Takeda, Y., et al. (2008). Up-regulation of matrix metalloproteinase-3 in the dorsal root ganglion of rats with paclitaxel-induced neuropathy. Cancer Science, 99(8), 1618–1625.[DOI:10.1111/j.1349-7006.2008.00877.x] [PMID] [PMCID]
Nissinen, L., & Kähäri, V. M. (2014). Matrix metalloproteinases in inflammation. Biochimica et Biophysica Acta, 1840(8), 2571–2580. [DOI:10.1016/j.bbagen.2014.03.007] [PMID]
Pevny, L., & Placzek, M. (2005). SOX genes and neural progenitor identity. Current Opinion in Neurobiology, 15(1), 7–13. [DOI:10.1016/j.conb.2005.01.016] [PMID]
Piera-Velazquez, S., Hawkins, D. F., Whitecavage, M. K., Colter, D. C., Stokes, D. G., & Jimenez, S. A. (2007). Regulation of the human SOX9 promoter by Sp1 and CREB. Experimental cell Research, 313(6), 1069–1079. [DOI:10.1016/j.yexcr.2007.01.001] [PMID] [PMCID]
Roche, K. C., Gracz, A. D., Liu, X. F., Newton, V., Akiyama, H., & Magness, S. T. (2015). SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology, 149(6), 1553-1563.e1510. [DOI:10.1053/j.gastro.2015.07.004] [PMID] [PMCID]
Sabri, M. I. (2018). Chemical neurotoxins and disruption of the axonal transport system. In Z. Iqbal (Ed.), Axoplasmic Transport (pp. 185-207)., Florida: CRC press. [DOI:10.1201/9781351070003-15]
Saito, T., Okamura, A., Inoue, J., Makiura, D., Doi, H., & Yakushijin, K., et al. (2019). Anemia is a novel predictive factor for the onset of severe chemotherapy-induced peripheral neuropathy in lymphoma patients receiving rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy. Oncology Research, 27(4), 469–474. [DOI:10.3727/096504018X15267574931782] [PMID] [PMCID]
Sakamuru, S., Attene-Ramos, M. S., & Xia, M. (2016). Mitochondrial membrane potential assay. Methods in Molecular Biology (Clifton, N.J.), 1473, 17–22. [DOI:10.1007/978-1-4939-6346-1_2] [PMID] [PMCID]
Scott, C. E., Wynn, S. L., Sesay, A., Cruz, C., Cheung, M., & Gomez Gaviro, M. V., et al. (2010). SOX9 induces and maintains neural stem cells. Nature Neuroscience, 13(10), 1181–1189. [DOI:10.1038/nn.2646] [PMID]
Si, H., Zhang, Y., Song, Y., & Li, L. (2018). Overexpression of adrenomedullin protects mesenchymal stem cells against hypoxia and serum deprivationinduced apoptosis via the Akt/GSK3β and Bcl2 signaling pathways. International Journal of Molecular Medicine, 41(6), 3342-3352. [DOI:10.3892/ijmm.2018.3533] [PMID] [PMCID]
Starobova, H., & Vetter, I. (2017). Pathophysiology of chemotherapy-induced peripheral neuropathy. Frontiers in Molecular Neuroscience, 10, 174. [DOI:10.3389/fnmol.2017.00174] [PMID] [PMCID]
Takhshid, M. A., Owji, A. A., Vasei, M., Panjehshahin, M. R., Tabei, S. M., & Tabatabaee, H. R., et al. (2004). Expression of spinal cord Fos protein in response to intrathecal adrenomedullin and CGRP in conscious rats. Brain Research, 1020(1-2), 30–36. [DOI:10.1016/j.brainres.2004.05.112] [PMID]
Tsikas, D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Analytical Biochemistry, 524, 13–30. [DOI:10.1016/j.ab.2016.10.021] [PMID]
Uetake, R., Sakurai, T., Kamiyoshi, A., Ichikawa-Shindo, Y., Kawate, H., & Iesato, Y., et al. (2014). Adrenomedullin-RAMP2 system suppresses ER stress-induced tubule cell death and is involved in kidney protection. Plos One, 9(2), e87667. [DOI:10.1371/journal.pone.0087667] [PMID] [PMCID]
Verma, T., Mallik, S. B., Ramalingayya, G. V., Nayak, P. G., Kishore, A., & Pai, K. S. R., et al. (2017). Sodium valproate enhances doxorubicin-induced cognitive dysfunction in Wistar rats. Biomedicine & Pharmacotherapy, 96, 736-741. [DOI:10.1016/j.biopha.2017.09.150] [PMID]
Voors, A. A., Kremer, D., Geven, C., Ter Maaten, J. M., Struck, J., & Bergmann, A., et al. (2019). Adrenomedullin in heart failure: Pathophysiology and therapeutic application. European Journal of Heart Failure, 21(2), 163–171. [DOI:10.1002/ejhf.1366] [PMID] [PMCID]
Wang, W., Qi, B., Lv, H., Wu, F., Liu, L., & Wang, W., et al. (2017). A new method of isolating spinal motor neurons from fetal mouse. Journal of Neuroscience Methods, 288, 57-61. [DOI:10.1016/j.jneumeth.2017.06.014] [PMID]
Webster, K. A. (2012). Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiology, 8(6), 863-884. [DOI:10.2217/fca.12.58] [PMID] [PMCID]
Wu, S. N., So, E. C., Liao, Y. K., & Huang, Y. M. (2015). Reversal by ranolazine of doxorubicin-induced prolongation in the inactivation of late sodium current in rat dorsal root ganglion neurons. Pain Medicine, 16(5), 1032-1034. [DOI:10.1111/pme.12681] [PMID]
Yoshimoto, T., Fukai, N., Sato, R., Sugiyama, T., Ozawa, N., & Shichiri, M., et al. (2004). Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology, 145(7), 3331-3337. [DOI:10.1210/en.2003-1583] [PMID]
Yoshizawa, T., Takizawa, S., Shimada, S., Tokudome, T., Shindo, T., & Matsumoto, K. (2016). Effects of adrenomedullin on doxorubicin-induced cardiac damage in mice. Biological & Pharmaceutical Bulletin, 39(5), 737–746. [DOI:10.1248/bpb.b15-00832] [PMID]
Yoshizawa, T., Takizawa, S., Shimada, S., Tokudome, T., Shindo, T., & Matsumoto, K. (2016). Effects of adrenomedullin on doxorubicin-induced cardiac damage in mice. Biological & Pharmaceutical Bulletin, 39(5), 737–746. [DOI:10.1248/bpb.b15-00832] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2021/08/18 | Accepted: 2022/04/12 | Published: 2024/09/1
Received: 2021/08/18 | Accepted: 2022/04/12 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





