Volume 13, Issue 6 (November & December 2022)
BCN 2022, 13(6): 893-900 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirfazeli F S, Mohebi F, Jahanbakhshi A, Aryani O, Almasi-Dooghaee M. Repetitive Suicidal Behaviors in a Case With a New Mutation of Wolfram Syndrome: A Jump From the Gene to the Behavior. BCN 2022; 13 (6) :893-900
URL: http://bcn.iums.ac.ir/article-1-2233-en.html
URL: http://bcn.iums.ac.ir/article-1-2233-en.html
Fatemeh Sadat Mirfazeli1 

 , Fatemeh Mohebi2
, Fatemeh Mohebi2 
 , Amin Jahanbakhshi3
, Amin Jahanbakhshi3 
 , Omid Aryani4
, Omid Aryani4 

 , Mostafa Almasi-Dooghaee *5
, Mostafa Almasi-Dooghaee *5 




 , Fatemeh Mohebi2
, Fatemeh Mohebi2 
 , Amin Jahanbakhshi3
, Amin Jahanbakhshi3 
 , Omid Aryani4
, Omid Aryani4 

 , Mostafa Almasi-Dooghaee *5
, Mostafa Almasi-Dooghaee *5 


1- Mental Health Research Center, Psychosocial Health Research Institute, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Psychiatry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Skull Base Research Center, Department of Neurosurgery, Rasool Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Neurology, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Psychiatry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Skull Base Research Center, Department of Neurosurgery, Rasool Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Neurology, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1465 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Wolfram syndrome (WS), a rare autosomal recessive neurodegenerative disease, was first described by Wolfram and Wagener in 1938 (Rigoli, et al., 2018; Wolfram & Wagener, 1938). This syndrome is characterized by diabetes insipidus (DI), diabetes mellitus (DM), optic atrophy, deafness (DIDMOAD), ataxia, urinary tract complications, and psychiatric manifestations that present in 60% of cases (Bitoun, 1994; Dreyer et al., 1982; Swift, et al, 1990). WS shows a variety of psychiatric symptoms, such as mood disorders and impulsivity (Bischoff et al., 2015; Crawford, et al., 2002; Sequeira et al., 2003; Swift & Swift, 2005). Among these, some of them reported suicidal behavior as a clinical feature of WS (Aluclu, et al., 2006; Chatterjee, et al., 2017; Lodha, et al., 2018; Nickl-Jockschat, et al., 2009; Swift et al., 1990).
WS is caused by mutations in the WFS1 gene. The WFS1 gene is located on chromosome 4p16 (Inoue et al., 1998) and encodes Wolframin, one of the membrane glycoproteins of the endoplasmic reticulum (ER) that prevents ER stress-related apoptosis and regulates calcium channel activity. Wolframin is expressed in the liver and also brain regions, such as the hippocampus (Osman et al., 2003; Riggs et al., 2005; Takeda et al., 2001; Yamada et al., 2006), which may contribute to some of its psychiatric disturbances. To date, more than 357 mutations of the WFS1 gene have been documented in the Human Gene Mutation Database (HGMD) (Stenson et al., 2014)
Some authors have proposed a genotype-phenotype correlation despite the similarity between different cases (Matsunaga et al., 2014). However, it is not yet confirmed whether there is any correlation between these mutations and psychiatric manifestations, including suicidal attempts (Aluclu et al., 2006).
In this article, we described a 26-year-old man with a new mutation of WS with repetitive suicidal behaviors and psychiatric hospitalizations added to a brief review of the connection between suicidal behaviors and WS.
2. Case Presentation
A twenty-six-year-old man was taken to the emergency room with an altered level of consciousness because of a suicidal attempt by injecting 150IU NovoRapid® insulin in December 2019. After initial management, he was admitted to the psychiatry ward of Rasoul-Akram hospital, affiliated with the Iran University of Medical Sciences, for his depressed mood and suicidal ideation. Medical records revealed that he is a known case of WS. In his past psychiatric history, he had several suicidal attempts both impulsive and planned, and several episodes of depression, poor drug compliance after remission despite thorough psychoeducation, and frequent medication response failure despite temporary symptom relief. There was a negative substance history except for opium, which he gave up years ago. His family history was negative for any mental illnesses and suicide in first-degree relatives except for opium addiction in both his parents.
He was accidentally diagnosed to have DM at age three, then, he progressively lost nearly all of his vision at age 11 when he gave up his education. He had recurrent hospitalizations for his uncontrolled DM. During one of them at age 16, psychotherapy was recommended for him because of his aggressive behaviors. Two years later at 18 years old, he committed his first impulsive suicidal attempt, after a fight with his brother.
His first psychiatric admission was at age 20 due to a depressive episode with DM as its precipitating factor. He was treated with sertraline and fluoxetine (concurrently). Despite getting better, after one year, he interrupted medication for an unknown reason, which was the trigger for the relapse of the second depressive episode. However, he did not use any medication until age 24. At age 24, he developed headaches and committed a second suicidal attempt. Another selective serotonergic reuptake inhibitor medication was initiated. Looking for the cause of the headache, the neurologic examination revealed bilateral optic atrophy, cerebellar ataxia, and bilateral sensorineural hearing loss. Brain magnetic resonance imaging (MRI) was also requested that showed diffuse atrophy in the cerebrum, cerebellum, and brain stem, in addition to an incidental finding of a 2-cm cavernous malformation in the left frontal lobe (Figure 1).
.jpg)
In addition, diabetes insipidus (DI) was also diagnosed according to lab data. Based on cumulative findings of DM, DI, optic atrophy, sensorineural hearing loss, and neuropsychiatric manifestations, he was finally diagnosed with WS. The diagnosis was confirmed with genetic testing.
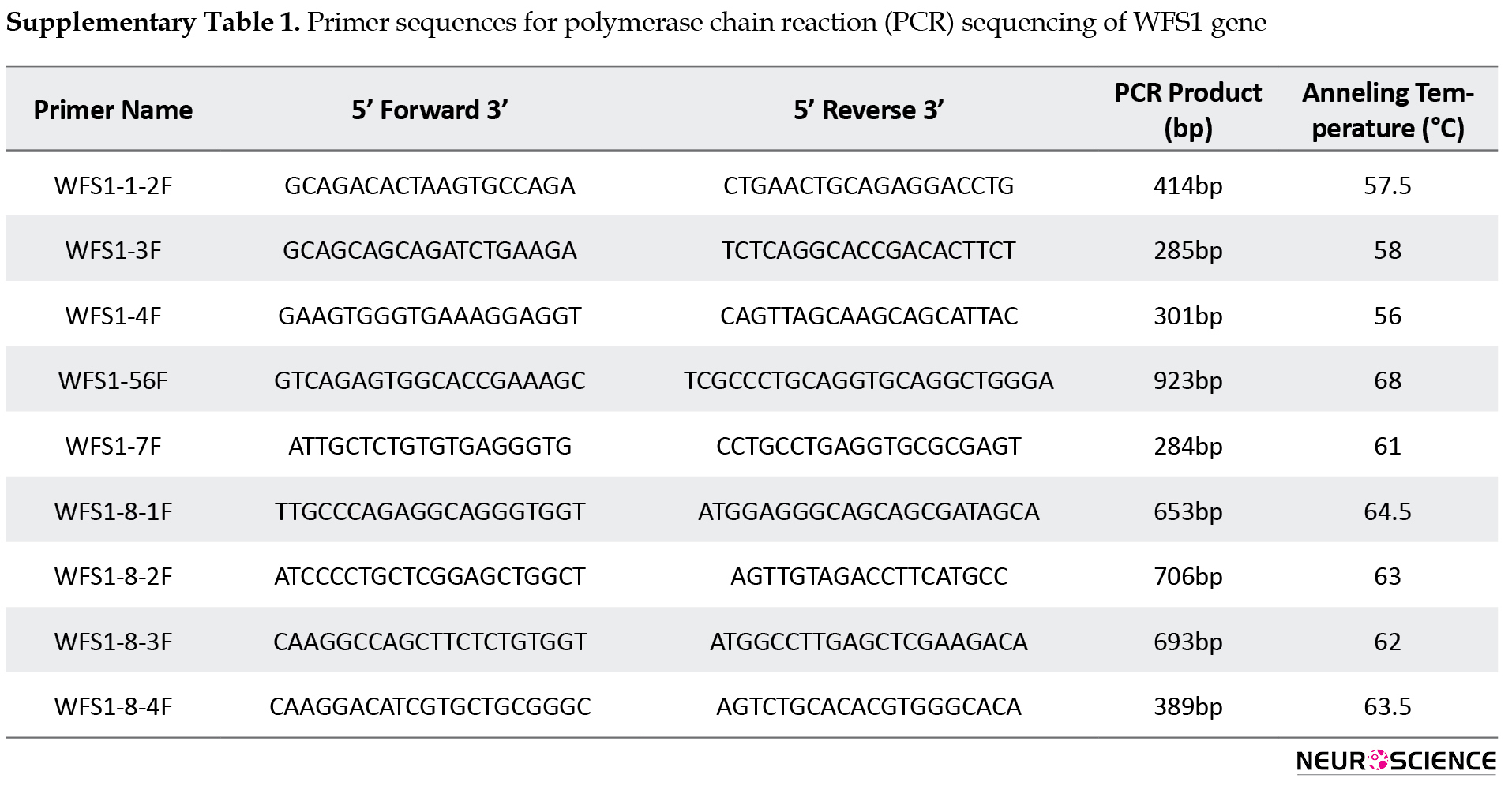
The genomic DNA of the patient was extracted from peripheral blood leukocytes and genetic studies were performed by polymerase chain reaction (PCR) sequencing of the coding regions and exon–intron boundaries of the WFS1 gene (Primer sequences are given in Suppl Table 1).
Molecular analysis of the WFS1 gene revealed a homozygous c.493C>T mutation, which alters a glutamine amino acid to a premature stop codon at codon 165(p.Q165X) leading to truncation of Wolframin protein (Figure 2).
.jpg)
The p.Q165X mutation had not been reported previously and both parents were heterozygous carriers of this mutation.
After about six months, he committed a serious suicidal attempt (the third one) by insulin injection that caused hospitalization. He received sertraline for depression and desmopressin for DI. But again, despite symptom recovery, he interrupted his medication for an unknown reason. Around six months later, he was admitted for depression and suicidal ideation and treated with escitalopram and buspirone. Two months later, he had a severe major depressive episode while taking escitalopram. thus, he was admitted and received electroshock therapy. Thereafter, the depressive episode was recovered but he developed severe headaches that did not respond to analgesics.
After discharge, he committed three suicidal attempts and regressed to a depressed mood, mentioning despair of pain relief as the main reason for suicide. He had at least another eight planned suicidal attempts mostly by insulin injection during the last year. They were all treated at home and did not lead to hospitalization as his family became more experienced in managing his suicide attempts. In his current hospitalization, he was treated with fluoxetine, gabapentin, and sodium valproate for depression and migraine headaches. He was discharged with partial remission of headaches and depression. The follow-up visit within one year of the last hospitalization demonstrated some transient suicidal ideas but no additional suicidal attempt (Figure 2).
3. Discussion
We described a novel stop-gain mutation in the WFS1 gene in a young man with repetitive suicidal behavior. He had at least 16 reported suicide attempts between the age of 18 to 26. In addition, the genetic study revealed a novel homozygous stop-gain mutation as c.493C>T (p.Q165X) in the WFS1 gene. This variation has not been reported in control databases, such as the 1,000 Genomes Project, Exome Variant Server, Exome Aggregation Consortium, dbSNP Database, or literature.
In this case, the classic symptoms of WS, including endocrine, ophthalmologic, and neurologic manifestations were conducted before the emergence of most of the suicidal attempts. One may assume that these later manifestations are reactive to the diagnosis of a non-treatable disease, at least in this case. However, the psychiatric symptoms, including mood disorder and impulsive and aggressive behaviors were begun simultaneously with other symptoms. In one study, psychiatric hospitalizations, suicide, and subjective complaints of mental illness were compared between family members of WS and Ataxia-Telangiectasia, which revealed more frequency in the blood relatives of the former syndrome, significantly (Swift, et al., 1991). As both disorders are non-treatable diseases with some common manifestations, this was against the assumption of the reactive nature of the psychiatric manifestation of WS. In addition, there are several other case reports, which suggest the probable relationship between the WFS1 gene and suicide. To our knowledge, before this case, there are at least sixteen patients with confirmed WS in the literature who had suicidal behavior (Aluclu et al., 2006; Nickl-Jockschat et al., 2009; Swift et al., 1990).
There are some genetic studies on the contribution of the WFS1 gene in suicides. In a controlled study, Crawford et al. found no increased incidence of WFS1 carrier in 100 patients with completed suicides (Crawford et al., 2002). In contrast, in another study, the frequency of the 611R/611R genotype, one of the three most common variants of exon 8 mutation in WFS1, in suicide victims was much higher than controls and the score of impulsivity and novelty seeking were also significantly higher in this group (Sequeira et al., 2003). The contrary results were reported by Zalsman et al. They found an association between H611R polymorphism and mood disorders but aggression/impulsivity and suicidal behavior did not find a such association (Zalsman et al., 2009). However, based on some indirect data analysis, Swift et al. declared that the carriers of the WFS1 gene have a greater than the eight-fold risk for psychiatric hospitalization or suicide (Swift et al., 1991). Several years later, Swift et al. reported that heterozygote individuals for the WFS1 gene had even more chances (26 folds) to require psychiatric hospitalization (Swift, et al., 1998).
Alongside gene mutation, epigenetic factors that change the contribution of genes may play a role in suicide. For instance, McGowan et al. showed significant hypermethylation in the brain of suicide subjects led to rRNA expression reduction observed in the hippocampus, but not the cerebellum (McGowan et al., 2008).
The genetic study, in this case, revealed a new unreported stop-codon mutation on the WFS1 gene. Although the previous documents affirm the role of the WFS1 gene contribution in suicide, the high amount of suicidal attempts in our case is unusual. He had at least 16 suicidal attempts. This high tendency for suicide may be due to the newly detected mutation, which was found in our patient. The variability in a tendency for suicide and genotype variations of WS was suggested by Sequeira et al. They found that there was a significantly higher frequency of suicide in carriers of H611R, R456H, and I333V, which are three major locus variations of WFS1 at the locus of H611R (Sequeira et al., 2003). Furthermore, the patient had an incidental finding of a small cavernoma in neuroimaging. It is not clear if this structural lesion located in the left frontal cortex may have a role in the high tendency for suicidal behavior or impulsivity observed in the patient.
Furthermore, twin and family studies revealed that suicidal behavior could be heritable (Baldessarini & Hennen, 2004; Voracek & Loibl, 2007). As of now, besides genetic and epigenetic factors discussed above, some studies demonstrated some other candidate genes to play a role in the etiology of suicidal behaviors (Baldessarini & Hennen, 2004). For example, the effect of various serotonergic and dopaminergic genes, dysregulation of fibroblast growth factor genes, glial cells, and oxytocin–related genes have all been proposed for the possible connection of the role of genes in suicidal behavior (Baldessarini & Hennen, 2004; Roy, et al., 1997).
4. Conclusion
We reported a novel gene mutation in a young man who suffered from WS with repetitive suicidal attempts. We suppose that this new mutation might be responsible for the high tendency to suicide in our patient. Also, it is recommended that psychological support should be a routine practice in patients with WS.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article including being anonymous of the patient in all stages of the research and taking consent from his mother as the main caregiver.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Fatemeh Sadat Mirfazeli, Amin Jahanbakhshi, Mostafa Almasi-Dooghaee; Writing–original draft: Fatemeh Mohebi, Amin Jahanbakhshi, Mostafa Almasi-Dooghaee, Omid Aryani; Recourses: Fatemeh Sadat Mirfazeli, Mostafa Almasi-Dooghaee, Fatemeh Mohebi, Omid Aryani.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the Endocrinology and Metabolism Research Institute affiliated with the Tehran University of Medical Sciences, for providing genetic study. In addition, we acknowledged respectfully our patient and his kind mother for sharing their data to complete this research.
References
Aluclu, M. U., Bahceci, M., Tuzcu, A., Arikan, S., & Gokalp, D. (2006). A new mutation in WFS1 gene (C. 1522-1523delTA, Y508fsX421) may be responsible for early appearance of clinical features of Wolfram syndrome and suicidal behaviour. Neuro Endocrinology Letters, 27(6), 691–694. [PMID]
Baldessarini, R. J., & Hennen, J. (2004). Genetics of suicide: An overview. Harvard Review of Psychiatry, 12(1), 1–13. [DOI:10.1080/10673220490425915] [PMID]
Bischoff, A. N., Reiersen, A. M., Buttlaire, A., Al-Lozi, A., Doty, T., & Marshall, B. A., et al. (2015). Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet Journal of Rare Diseases, 10, 66. [DOI:10.1186/s13023-015-0282-1] [PMID] [PMCID]
Bitoun, P. (1994). Wolfram syndrome: A report of four cases and review of the literature. Ophthalmic Genetics, 15(2), 77-85. [DOI:10.3109/13816819409098867]
Chatterjee, S. S., Mitra, S., & Pal, S. K. (2017). Mania in wolfram’s disease: From bedside to bench. Clinical Psychopharmacology and Neuroscience, 15(1), 70-72. [DOI:10.9758/cpn.2017.15.1.70] [PMCID]
Crawford, J., Zielinski, M. A., Fisher, L. J., Sutherland, G. R., & Goldney, R. D. (2002). Is there a relationship between Wolfram syndrome carrier status and suicide? American Journal of Medical Genetics, 114(3), 343-346. [DOI:10.1002/ajmg.10256]
Dreyer, M., Rüdiger, H. W., Bujara, K., Herberhold, C., Kühnau, J., & Maack, P., et al. (1982). The syndrome of diabetes insipidus, diabetes mellitus, optic atrophy, deafness, and other abnormalities (DIDMOAD-syndrome). Two affected sibs and a short review of the literature (98 cases). Klinische Wochenschrift, 60(9), 471-475. [DOI:10.1007/bf01720362] [PMID]
Inoue, H., Tanizawa, Y., Wasson, J., Behn, P., Kalidas, K., & Bernal-Mizrachi, E., et al. (1998). A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nature Genetics, 20(2), 143–148. [DOI:10.1038/2441] [PMID]
Lodha, S., Das, L., Ramchandani, G. D., & Bhansali, A. (2018). A case of young diabetes and parasuicide. BMJ Case Reports, 2018, bcr2018225839. [DOI:10.1136/bcr-2018-225839] [PMID] [PMCID]
Matsunaga, K., Tanabe, K., Inoue, H., Okuya, S., Ohta, Y., & Akiyama, M., et al. (2014). Wolfram syndrome in the Japanese population; Molecular analysis of WFS1 gene and characterization of clinical features. Plos One, 9(9), e106906. [DOI:10.1371/journal.pone.0106906] [PMID] [PMCID]
McGowan, P. O., Sasaki, A., Huang, T. C., Unterberger, A., Suderman, M., & Ernst, C., et al. (2008). Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. Plos One, 3(5), e2085. [DOI:10.1371/journal.pone.0002085] [PMID] [PMCID]
Nickl-Jockschat, T., Kunert, H. J., Herpertz-Dahlmann, B., & Grözinger, M. (2008). Psychiatric symptoms in a patient with Wolfram syndrome caused by a combination of thalamic deficit and endocrinological pathologies. Neurocase, 15(1), 47-52. [PMID]
Osman, A. A., Saito, M., Makepeace, C., Permutt, M. A., Schlesinger, P., & Mueckler, M. (2003). Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. The Journal of biological chemistry, 278(52), 52755-52762. [DOI:10.1074/jbc.m310331200] [PMID]
Riggs, A. C., Bernal-Mizrachi, E., Ohsugi, M., Wasson, J., Fatrai, S., & Welling, C., et al. (2005). Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia, 48(11), 2313-2321. [DOI:10.1007/s00125-005-1947-4] [PMID]
Rigoli, L., Bramanti, P., Di Bella, C., & De Luca, F. (2018). Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatric Research, 83(5), 921–929.[DOI:10.1038/pr.2018.17] [PMID]
Roy, A., Rylander, G., & Sarchiapone, M. (1997). Genetics of suicide: Family studies and molecular genetics. Annals of the New York Academy of Sciences, 836, 135–157. [DOI:10.1111/j.1749-6632.1997.tb52358.x] [PMID]
Sequeira, A., Kim, C., Seguin, M., Lesage, A., Chawky, N., & Desautels, A., et al. (2003). Wolfram syndrome and suicide: Evidence for a role of WFS1 in suicidal and impulsive behavior. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of The International Society of Psychiatric Genetics, 119B(1), 108–113. [DOI:10.1002/ajmg.b.20011] [PMID]
Stenson, P. D., Mort, M., Ball, E. V., Shaw, K., Phillips, A., & Cooper, D. N. (2014). The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Human Genetics, 133(1), 1–9. [DOI:10.1007/s00439-013-1358-4] [PMID] [PMCID]
Swift, M., & Swift, R. (2005). Wolframin mutations and hospitalization for psychiatric illness. Molecular Psychiatry, 10(8), 799–803. [DOI:10.1038/sj.mp.4001681] [PMID]
Swift, R. G., Polymeropoulos, M. H., Torres, R., & Swift, M. (1998). Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Molecular Psychiatry, 3(1), 86–91. [DOI:10.1038/sj.mp.4000344] [PMID]
Swift, R. G., Sadler, D. B., & Swift, M. (1990). Psychiatric findings in Wolfram syndrome homozygotes. Lancet, 336(8716), 667-669. [DOI:10.1016/0140-6736(90)92157-d] [PMID]
Swift, R. G., Perkins, D. O., Chase, C. L., Sadler, D. B., & Swift, M. (1991). Psychiatric disorders in 36 families with Wolfram syndrome. The American Journal of Psychiatry, 148(6), 775–779. [DOI:10.1176/ajp.148.6.775] [PMID]
Takeda, K., Inoue, H., Tanizawa, Y., Matsuzaki, Y., Oba, J., & Watanabe, Y., et al. (2001). WFS1 (Wolfram syndrome 1) gene product: Predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Human Molecular Genetics, 10(5), 477–484. [DOI:10.1093/hmg/10.5.477] [PMID]
Voracek, M., & Loibl, L. M. (2007). Genetics of suicide: A systematic review of twin studies. Wiener Klinische Wochenschrift, 119(15-16), 463–475. [DOI:10.1007/s00508-007-0823-2] [PMID]
Wolfram, D. J., & Wagener, H. P. (1938). Diabetes mellitus and simple optic atrophy among siblings: Report of four cases. Mayo Clinic Proceedings, 13, 715-718. [Link]
Yamada, T., Ishihara, H., Tamura, A., Takahashi, R., Yamaguchi, S., & Takei, D., et al. (2006). WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Human Molecular Genetics, 15(10), 1600–1609. [DOI:10.1093/hmg/ddl081] [PMID]
Zalsman, G., Mann, M. J., Huang, Y. Y., Oquendo, M. A., Brent, D. A., & Burke, A. K., et al. (2009). Wolframin gene H611R polymorphism: No direct association with suicidal behavior but possible link to mood disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(4), 707–710. [DOI:10.1016/j.pnpbp.2009.03.017] [PMID] [PMCID]
Wolfram syndrome (WS), a rare autosomal recessive neurodegenerative disease, was first described by Wolfram and Wagener in 1938 (Rigoli, et al., 2018; Wolfram & Wagener, 1938). This syndrome is characterized by diabetes insipidus (DI), diabetes mellitus (DM), optic atrophy, deafness (DIDMOAD), ataxia, urinary tract complications, and psychiatric manifestations that present in 60% of cases (Bitoun, 1994; Dreyer et al., 1982; Swift, et al, 1990). WS shows a variety of psychiatric symptoms, such as mood disorders and impulsivity (Bischoff et al., 2015; Crawford, et al., 2002; Sequeira et al., 2003; Swift & Swift, 2005). Among these, some of them reported suicidal behavior as a clinical feature of WS (Aluclu, et al., 2006; Chatterjee, et al., 2017; Lodha, et al., 2018; Nickl-Jockschat, et al., 2009; Swift et al., 1990).
WS is caused by mutations in the WFS1 gene. The WFS1 gene is located on chromosome 4p16 (Inoue et al., 1998) and encodes Wolframin, one of the membrane glycoproteins of the endoplasmic reticulum (ER) that prevents ER stress-related apoptosis and regulates calcium channel activity. Wolframin is expressed in the liver and also brain regions, such as the hippocampus (Osman et al., 2003; Riggs et al., 2005; Takeda et al., 2001; Yamada et al., 2006), which may contribute to some of its psychiatric disturbances. To date, more than 357 mutations of the WFS1 gene have been documented in the Human Gene Mutation Database (HGMD) (Stenson et al., 2014)
Some authors have proposed a genotype-phenotype correlation despite the similarity between different cases (Matsunaga et al., 2014). However, it is not yet confirmed whether there is any correlation between these mutations and psychiatric manifestations, including suicidal attempts (Aluclu et al., 2006).
In this article, we described a 26-year-old man with a new mutation of WS with repetitive suicidal behaviors and psychiatric hospitalizations added to a brief review of the connection between suicidal behaviors and WS.
2. Case Presentation
A twenty-six-year-old man was taken to the emergency room with an altered level of consciousness because of a suicidal attempt by injecting 150IU NovoRapid® insulin in December 2019. After initial management, he was admitted to the psychiatry ward of Rasoul-Akram hospital, affiliated with the Iran University of Medical Sciences, for his depressed mood and suicidal ideation. Medical records revealed that he is a known case of WS. In his past psychiatric history, he had several suicidal attempts both impulsive and planned, and several episodes of depression, poor drug compliance after remission despite thorough psychoeducation, and frequent medication response failure despite temporary symptom relief. There was a negative substance history except for opium, which he gave up years ago. His family history was negative for any mental illnesses and suicide in first-degree relatives except for opium addiction in both his parents.
He was accidentally diagnosed to have DM at age three, then, he progressively lost nearly all of his vision at age 11 when he gave up his education. He had recurrent hospitalizations for his uncontrolled DM. During one of them at age 16, psychotherapy was recommended for him because of his aggressive behaviors. Two years later at 18 years old, he committed his first impulsive suicidal attempt, after a fight with his brother.
His first psychiatric admission was at age 20 due to a depressive episode with DM as its precipitating factor. He was treated with sertraline and fluoxetine (concurrently). Despite getting better, after one year, he interrupted medication for an unknown reason, which was the trigger for the relapse of the second depressive episode. However, he did not use any medication until age 24. At age 24, he developed headaches and committed a second suicidal attempt. Another selective serotonergic reuptake inhibitor medication was initiated. Looking for the cause of the headache, the neurologic examination revealed bilateral optic atrophy, cerebellar ataxia, and bilateral sensorineural hearing loss. Brain magnetic resonance imaging (MRI) was also requested that showed diffuse atrophy in the cerebrum, cerebellum, and brain stem, in addition to an incidental finding of a 2-cm cavernous malformation in the left frontal lobe (Figure 1).
.jpg)
In addition, diabetes insipidus (DI) was also diagnosed according to lab data. Based on cumulative findings of DM, DI, optic atrophy, sensorineural hearing loss, and neuropsychiatric manifestations, he was finally diagnosed with WS. The diagnosis was confirmed with genetic testing.
The genomic DNA of the patient was extracted from peripheral blood leukocytes and genetic studies were performed by polymerase chain reaction (PCR) sequencing of the coding regions and exon–intron boundaries of the WFS1 gene (Primer sequences are given in Suppl Table 1).

Molecular analysis of the WFS1 gene revealed a homozygous c.493C>T mutation, which alters a glutamine amino acid to a premature stop codon at codon 165(p.Q165X) leading to truncation of Wolframin protein (Figure 2).
.jpg)
The p.Q165X mutation had not been reported previously and both parents were heterozygous carriers of this mutation.
After about six months, he committed a serious suicidal attempt (the third one) by insulin injection that caused hospitalization. He received sertraline for depression and desmopressin for DI. But again, despite symptom recovery, he interrupted his medication for an unknown reason. Around six months later, he was admitted for depression and suicidal ideation and treated with escitalopram and buspirone. Two months later, he had a severe major depressive episode while taking escitalopram. thus, he was admitted and received electroshock therapy. Thereafter, the depressive episode was recovered but he developed severe headaches that did not respond to analgesics.
After discharge, he committed three suicidal attempts and regressed to a depressed mood, mentioning despair of pain relief as the main reason for suicide. He had at least another eight planned suicidal attempts mostly by insulin injection during the last year. They were all treated at home and did not lead to hospitalization as his family became more experienced in managing his suicide attempts. In his current hospitalization, he was treated with fluoxetine, gabapentin, and sodium valproate for depression and migraine headaches. He was discharged with partial remission of headaches and depression. The follow-up visit within one year of the last hospitalization demonstrated some transient suicidal ideas but no additional suicidal attempt (Figure 2).
3. Discussion
We described a novel stop-gain mutation in the WFS1 gene in a young man with repetitive suicidal behavior. He had at least 16 reported suicide attempts between the age of 18 to 26. In addition, the genetic study revealed a novel homozygous stop-gain mutation as c.493C>T (p.Q165X) in the WFS1 gene. This variation has not been reported in control databases, such as the 1,000 Genomes Project, Exome Variant Server, Exome Aggregation Consortium, dbSNP Database, or literature.
In this case, the classic symptoms of WS, including endocrine, ophthalmologic, and neurologic manifestations were conducted before the emergence of most of the suicidal attempts. One may assume that these later manifestations are reactive to the diagnosis of a non-treatable disease, at least in this case. However, the psychiatric symptoms, including mood disorder and impulsive and aggressive behaviors were begun simultaneously with other symptoms. In one study, psychiatric hospitalizations, suicide, and subjective complaints of mental illness were compared between family members of WS and Ataxia-Telangiectasia, which revealed more frequency in the blood relatives of the former syndrome, significantly (Swift, et al., 1991). As both disorders are non-treatable diseases with some common manifestations, this was against the assumption of the reactive nature of the psychiatric manifestation of WS. In addition, there are several other case reports, which suggest the probable relationship between the WFS1 gene and suicide. To our knowledge, before this case, there are at least sixteen patients with confirmed WS in the literature who had suicidal behavior (Aluclu et al., 2006; Nickl-Jockschat et al., 2009; Swift et al., 1990).
There are some genetic studies on the contribution of the WFS1 gene in suicides. In a controlled study, Crawford et al. found no increased incidence of WFS1 carrier in 100 patients with completed suicides (Crawford et al., 2002). In contrast, in another study, the frequency of the 611R/611R genotype, one of the three most common variants of exon 8 mutation in WFS1, in suicide victims was much higher than controls and the score of impulsivity and novelty seeking were also significantly higher in this group (Sequeira et al., 2003). The contrary results were reported by Zalsman et al. They found an association between H611R polymorphism and mood disorders but aggression/impulsivity and suicidal behavior did not find a such association (Zalsman et al., 2009). However, based on some indirect data analysis, Swift et al. declared that the carriers of the WFS1 gene have a greater than the eight-fold risk for psychiatric hospitalization or suicide (Swift et al., 1991). Several years later, Swift et al. reported that heterozygote individuals for the WFS1 gene had even more chances (26 folds) to require psychiatric hospitalization (Swift, et al., 1998).
Alongside gene mutation, epigenetic factors that change the contribution of genes may play a role in suicide. For instance, McGowan et al. showed significant hypermethylation in the brain of suicide subjects led to rRNA expression reduction observed in the hippocampus, but not the cerebellum (McGowan et al., 2008).
The genetic study, in this case, revealed a new unreported stop-codon mutation on the WFS1 gene. Although the previous documents affirm the role of the WFS1 gene contribution in suicide, the high amount of suicidal attempts in our case is unusual. He had at least 16 suicidal attempts. This high tendency for suicide may be due to the newly detected mutation, which was found in our patient. The variability in a tendency for suicide and genotype variations of WS was suggested by Sequeira et al. They found that there was a significantly higher frequency of suicide in carriers of H611R, R456H, and I333V, which are three major locus variations of WFS1 at the locus of H611R (Sequeira et al., 2003). Furthermore, the patient had an incidental finding of a small cavernoma in neuroimaging. It is not clear if this structural lesion located in the left frontal cortex may have a role in the high tendency for suicidal behavior or impulsivity observed in the patient.
Furthermore, twin and family studies revealed that suicidal behavior could be heritable (Baldessarini & Hennen, 2004; Voracek & Loibl, 2007). As of now, besides genetic and epigenetic factors discussed above, some studies demonstrated some other candidate genes to play a role in the etiology of suicidal behaviors (Baldessarini & Hennen, 2004). For example, the effect of various serotonergic and dopaminergic genes, dysregulation of fibroblast growth factor genes, glial cells, and oxytocin–related genes have all been proposed for the possible connection of the role of genes in suicidal behavior (Baldessarini & Hennen, 2004; Roy, et al., 1997).
4. Conclusion
We reported a novel gene mutation in a young man who suffered from WS with repetitive suicidal attempts. We suppose that this new mutation might be responsible for the high tendency to suicide in our patient. Also, it is recommended that psychological support should be a routine practice in patients with WS.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article including being anonymous of the patient in all stages of the research and taking consent from his mother as the main caregiver.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Fatemeh Sadat Mirfazeli, Amin Jahanbakhshi, Mostafa Almasi-Dooghaee; Writing–original draft: Fatemeh Mohebi, Amin Jahanbakhshi, Mostafa Almasi-Dooghaee, Omid Aryani; Recourses: Fatemeh Sadat Mirfazeli, Mostafa Almasi-Dooghaee, Fatemeh Mohebi, Omid Aryani.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the Endocrinology and Metabolism Research Institute affiliated with the Tehran University of Medical Sciences, for providing genetic study. In addition, we acknowledged respectfully our patient and his kind mother for sharing their data to complete this research.
References
Aluclu, M. U., Bahceci, M., Tuzcu, A., Arikan, S., & Gokalp, D. (2006). A new mutation in WFS1 gene (C. 1522-1523delTA, Y508fsX421) may be responsible for early appearance of clinical features of Wolfram syndrome and suicidal behaviour. Neuro Endocrinology Letters, 27(6), 691–694. [PMID]
Baldessarini, R. J., & Hennen, J. (2004). Genetics of suicide: An overview. Harvard Review of Psychiatry, 12(1), 1–13. [DOI:10.1080/10673220490425915] [PMID]
Bischoff, A. N., Reiersen, A. M., Buttlaire, A., Al-Lozi, A., Doty, T., & Marshall, B. A., et al. (2015). Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet Journal of Rare Diseases, 10, 66. [DOI:10.1186/s13023-015-0282-1] [PMID] [PMCID]
Bitoun, P. (1994). Wolfram syndrome: A report of four cases and review of the literature. Ophthalmic Genetics, 15(2), 77-85. [DOI:10.3109/13816819409098867]
Chatterjee, S. S., Mitra, S., & Pal, S. K. (2017). Mania in wolfram’s disease: From bedside to bench. Clinical Psychopharmacology and Neuroscience, 15(1), 70-72. [DOI:10.9758/cpn.2017.15.1.70] [PMCID]
Crawford, J., Zielinski, M. A., Fisher, L. J., Sutherland, G. R., & Goldney, R. D. (2002). Is there a relationship between Wolfram syndrome carrier status and suicide? American Journal of Medical Genetics, 114(3), 343-346. [DOI:10.1002/ajmg.10256]
Dreyer, M., Rüdiger, H. W., Bujara, K., Herberhold, C., Kühnau, J., & Maack, P., et al. (1982). The syndrome of diabetes insipidus, diabetes mellitus, optic atrophy, deafness, and other abnormalities (DIDMOAD-syndrome). Two affected sibs and a short review of the literature (98 cases). Klinische Wochenschrift, 60(9), 471-475. [DOI:10.1007/bf01720362] [PMID]
Inoue, H., Tanizawa, Y., Wasson, J., Behn, P., Kalidas, K., & Bernal-Mizrachi, E., et al. (1998). A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nature Genetics, 20(2), 143–148. [DOI:10.1038/2441] [PMID]
Lodha, S., Das, L., Ramchandani, G. D., & Bhansali, A. (2018). A case of young diabetes and parasuicide. BMJ Case Reports, 2018, bcr2018225839. [DOI:10.1136/bcr-2018-225839] [PMID] [PMCID]
Matsunaga, K., Tanabe, K., Inoue, H., Okuya, S., Ohta, Y., & Akiyama, M., et al. (2014). Wolfram syndrome in the Japanese population; Molecular analysis of WFS1 gene and characterization of clinical features. Plos One, 9(9), e106906. [DOI:10.1371/journal.pone.0106906] [PMID] [PMCID]
McGowan, P. O., Sasaki, A., Huang, T. C., Unterberger, A., Suderman, M., & Ernst, C., et al. (2008). Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. Plos One, 3(5), e2085. [DOI:10.1371/journal.pone.0002085] [PMID] [PMCID]
Nickl-Jockschat, T., Kunert, H. J., Herpertz-Dahlmann, B., & Grözinger, M. (2008). Psychiatric symptoms in a patient with Wolfram syndrome caused by a combination of thalamic deficit and endocrinological pathologies. Neurocase, 15(1), 47-52. [PMID]
Osman, A. A., Saito, M., Makepeace, C., Permutt, M. A., Schlesinger, P., & Mueckler, M. (2003). Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. The Journal of biological chemistry, 278(52), 52755-52762. [DOI:10.1074/jbc.m310331200] [PMID]
Riggs, A. C., Bernal-Mizrachi, E., Ohsugi, M., Wasson, J., Fatrai, S., & Welling, C., et al. (2005). Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia, 48(11), 2313-2321. [DOI:10.1007/s00125-005-1947-4] [PMID]
Rigoli, L., Bramanti, P., Di Bella, C., & De Luca, F. (2018). Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatric Research, 83(5), 921–929.[DOI:10.1038/pr.2018.17] [PMID]
Roy, A., Rylander, G., & Sarchiapone, M. (1997). Genetics of suicide: Family studies and molecular genetics. Annals of the New York Academy of Sciences, 836, 135–157. [DOI:10.1111/j.1749-6632.1997.tb52358.x] [PMID]
Sequeira, A., Kim, C., Seguin, M., Lesage, A., Chawky, N., & Desautels, A., et al. (2003). Wolfram syndrome and suicide: Evidence for a role of WFS1 in suicidal and impulsive behavior. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of The International Society of Psychiatric Genetics, 119B(1), 108–113. [DOI:10.1002/ajmg.b.20011] [PMID]
Stenson, P. D., Mort, M., Ball, E. V., Shaw, K., Phillips, A., & Cooper, D. N. (2014). The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Human Genetics, 133(1), 1–9. [DOI:10.1007/s00439-013-1358-4] [PMID] [PMCID]
Swift, M., & Swift, R. (2005). Wolframin mutations and hospitalization for psychiatric illness. Molecular Psychiatry, 10(8), 799–803. [DOI:10.1038/sj.mp.4001681] [PMID]
Swift, R. G., Polymeropoulos, M. H., Torres, R., & Swift, M. (1998). Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Molecular Psychiatry, 3(1), 86–91. [DOI:10.1038/sj.mp.4000344] [PMID]
Swift, R. G., Sadler, D. B., & Swift, M. (1990). Psychiatric findings in Wolfram syndrome homozygotes. Lancet, 336(8716), 667-669. [DOI:10.1016/0140-6736(90)92157-d] [PMID]
Swift, R. G., Perkins, D. O., Chase, C. L., Sadler, D. B., & Swift, M. (1991). Psychiatric disorders in 36 families with Wolfram syndrome. The American Journal of Psychiatry, 148(6), 775–779. [DOI:10.1176/ajp.148.6.775] [PMID]
Takeda, K., Inoue, H., Tanizawa, Y., Matsuzaki, Y., Oba, J., & Watanabe, Y., et al. (2001). WFS1 (Wolfram syndrome 1) gene product: Predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Human Molecular Genetics, 10(5), 477–484. [DOI:10.1093/hmg/10.5.477] [PMID]
Voracek, M., & Loibl, L. M. (2007). Genetics of suicide: A systematic review of twin studies. Wiener Klinische Wochenschrift, 119(15-16), 463–475. [DOI:10.1007/s00508-007-0823-2] [PMID]
Wolfram, D. J., & Wagener, H. P. (1938). Diabetes mellitus and simple optic atrophy among siblings: Report of four cases. Mayo Clinic Proceedings, 13, 715-718. [Link]
Yamada, T., Ishihara, H., Tamura, A., Takahashi, R., Yamaguchi, S., & Takei, D., et al. (2006). WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Human Molecular Genetics, 15(10), 1600–1609. [DOI:10.1093/hmg/ddl081] [PMID]
Zalsman, G., Mann, M. J., Huang, Y. Y., Oquendo, M. A., Brent, D. A., & Burke, A. K., et al. (2009). Wolframin gene H611R polymorphism: No direct association with suicidal behavior but possible link to mood disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(4), 707–710. [DOI:10.1016/j.pnpbp.2009.03.017] [PMID] [PMCID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/04/18 | Accepted: 2021/08/23 | Published: 2022/11/1
Received: 2021/04/18 | Accepted: 2021/08/23 | Published: 2022/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





