Volume 14, Issue 5 (September & October 2023)
BCN 2023, 14(5): 675-686 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fatehi F, Khaghani P, Okhovat A A, Moradi K, Teimouri F, Mortaja M, et al . Investigating the Association Between Muscular Ultrasonographic Alterations and Clinical Symptoms in Patients With Inflammatory Myopathy. BCN 2023; 14 (5) :675-686
URL: http://bcn.iums.ac.ir/article-1-2229-en.html
URL: http://bcn.iums.ac.ir/article-1-2229-en.html
Farzad Fatehi *1 

 , Parisa Khaghani1
, Parisa Khaghani1 

 , Ali Asghar Okhovat1
, Ali Asghar Okhovat1 

 , Kamyar Moradi1
, Kamyar Moradi1 

 , Farzad Teimouri1
, Farzad Teimouri1 

 , Mahsa Mortaja1
, Mahsa Mortaja1 

 , Mahsa Layegh1
, Mahsa Layegh1 

 , Akram Panahi1
, Akram Panahi1 

 , Shahriar Nafissi1
, Shahriar Nafissi1 




 , Parisa Khaghani1
, Parisa Khaghani1 

 , Ali Asghar Okhovat1
, Ali Asghar Okhovat1 

 , Kamyar Moradi1
, Kamyar Moradi1 

 , Farzad Teimouri1
, Farzad Teimouri1 

 , Mahsa Mortaja1
, Mahsa Mortaja1 

 , Mahsa Layegh1
, Mahsa Layegh1 

 , Akram Panahi1
, Akram Panahi1 

 , Shahriar Nafissi1
, Shahriar Nafissi1 


1- Neuromuscular Research Center, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1274 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Inflammatory myopathies (IMs) are a diverse group of muscular autoimmune disorders characterized by muscle weakness as the initial symptom. According to the new classification system for IMs, these disorders include inclusion body myositis, dermatomyositis (DM), polymyositis (PM), immune-mediated necrotizing myopathy, anti-synthetase syndrome, and overlap myositis (Selva-O’Callaghan et al., 2018). Most IM patients respond well to the immunosuppressive treatment except for including body myositis. Nevertheless, it is essential to point out that many IM patients progress despite intense treatment (Schmidt, 2018). The development of many outcome measures for myositis activity has been standardized in the field, which has aided our understanding of the long-term effects of such diseases and the development of new therapies (Rider et al., 2018).
Currently, most clinicians use manual muscle testing (MMT), electrodiagnostic tests, including electromyography (EMG), and serum markers, such as creatine kinase (CK) for diagnosis and follow-up of the patients. Other outcome measures used in the referral center for follow-up include additional clinical assessment tools, such as hand-held dynamometry (Allenbach et al., 2012), muscle magnetic resonance imaging (MRI), and muscle ultrasound (MUS). The lack of definite tools to measure muscle activity may develop some diagnostic problems that may cause some diagnostic issues. The MMT test may be affected by inter-observer differences (Miller et al., 2001). Therefore, there is growing attention to the application of imaging modalities. Muscle MRI has been broadly used in IMs to assess the extent of muscle involvement and determine the best location for muscle biopsy and patients’ follow-up; however, this modality is expensive, non-feasible, and time-consuming. Thus, the application of MUS has been increased due to its widespread availability, easier techniques, non-invasiveness, and cost-effectiveness for real-time imaging of the muscles (Pillen et al., 2006).
Both qualitative and quantitative methods are applied for muscle evaluations in MUS (Heckmatt et al., 1980, 1982). MUS benefits from an acceptable sensitivity to detect muscle changes even in the early stages of the disease, reflected by increased echo intensity (EI) due to acute inflammation and edema within the muscle tissue (Habers et al., 2015). As one of the main parameters of MUS, EI is an outcome of choice in patients with IMs; the region of interest (ROI) determination for EI calculation is quantitative (Pillen & Van Alfen, 2015); therefore, the findings are less operator-dependent with higher sensitivity than subjective analysis (Pillen et al., 2006).
The muscles of patients with PM/DM have higher EI compared to healthy muscles (Mittal et al., 2003; Noto et al., 2014). However, there is limited data on MUS’s diagnostic utility for differentiating PM/DM subjects from healthy ones. Besides, the association between MUS parameters and bedside clinical characteristics remains unknown. This study compares MUS parameters in IM to healthy subjects and measures the associations between clinical scores and MUS scores in the patients.
2. Materials and Methods
Study design and participants
In this cross-sectional case-control study, we enrolled 17 adult patients (>18 years old) diagnosed with IM, confirmed with a muscle biopsy, and 17 normal subjects in a neuromuscular referral center from September 2018 to January 2020. The inclusion criteria were defined as the presence of clinical evidence of IM, including bilateral symmetric proximal muscle weakness, disease duration of fewer than five years, history of elevation in serum skeletal muscle enzymes (CK >300 IU/L), or electromyography (EMG) results, indicating myopathic pattern along with irritation on needle EMG and definite evidence of inflammatory changes in muscle biopsy. Characteristic rashes on clinical examination and perifascicular atrophy in muscle biopsy were considered for the classification of DM. We excluded the patients with possible evidence of inclusion-body myositis, muscular dystrophy, metabolic or endocrine myopathy, toxic myopathy, and granulomatous and infectious myositis. Informed consent was obtained from all participants.
Functional measures
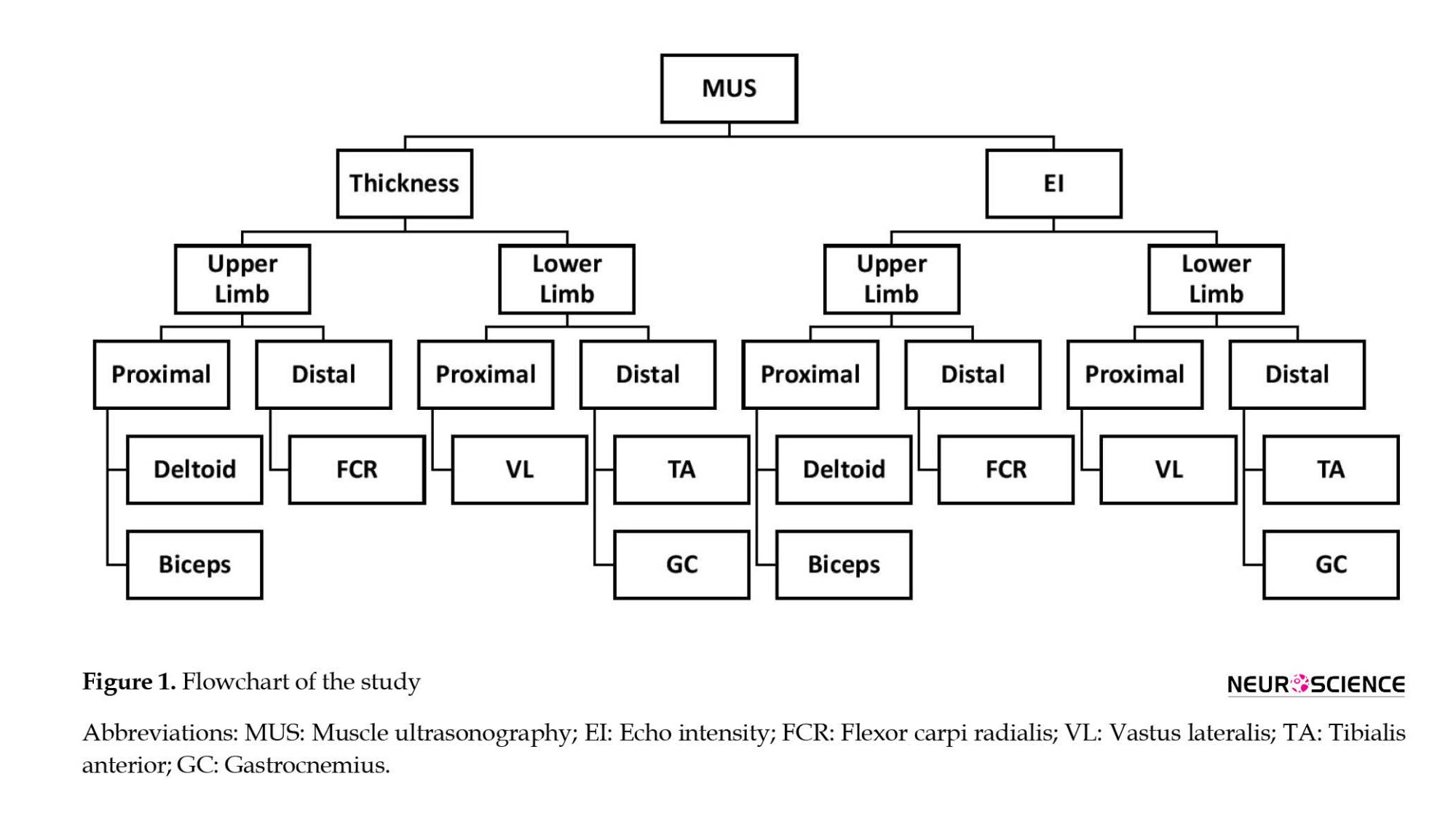
MMT was conducted by an expert neurologist using the medical research council scale, scoring muscle groups that are responsible for the following activities from 0 to 5: Arm abduction, elbow flexion/extension, wrist flexion, hip flexion, knee flexion/extension, and ankle dorsiflexion/plantar flexion. Moreover, a calibrated hand-held dynamometer (microFET®2, Hoggan Scientific, USA) quantitively measured the muscle forces. We calculated functional measures of MMT and dynamometry for each muscle separately. Also, we defined the average sum-score of muscle groups for proximal extremities muscles, distal extremities muscles, upper extremities muscles, lower extremities muscles, and average total sum-score (Figure 1). To calculate each average sum-score, we initially added the scores of all muscles or actions and then divided them by the number of muscles or actions.
Muscle ultrasound
A standard ultrasound protocol was applied for MUS, using a Sonosite M-Turbo C machine with a 15-6 MHz linear probe (Sonosite, Fujifilm) by an expert in neuromuscular ultrasound (AP). Ultrasound scans were made from the following muscles on both sides: Biceps brachii, deltoid, flexor carpi radialis, vastus lateralis, gastrocnemius, and tibialis anterior. Before the ultrasound examination, dirt and debris were cleaned from the skin. The areas were prepped with alcohol. A generous amount of contact gel was used to minimize the transducer’s required pressure on the skin.
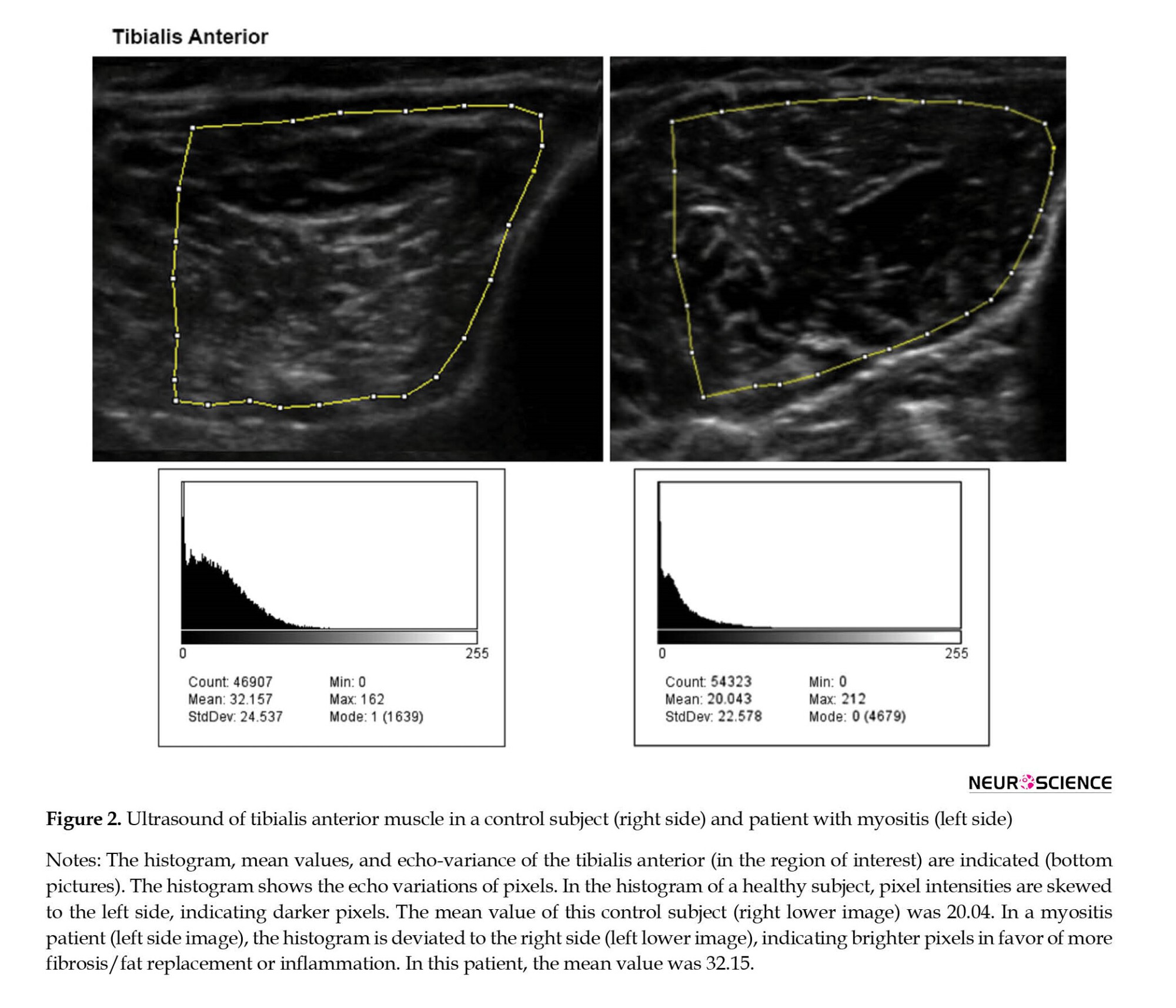
Each muscle underwent MUS three consecutive times, and the means of EI and thickness scores were calculated to minimize the variations. All scans were made in the transverse plane with a standard transducer location corresponding to the same investigator (PK) of the muscle belly, with three years of experience in neuromuscular ultrasound. We adjusted the probe’s angle to avoid oblique scanning until the best bone EI was acquired. The ultrasound machine was set on musculoskeletal mode and autogain function for ultrasonographic evaluations. Furthermore, we considered the depth of 4 cm for biceps brachii, deltoid, flexor carpi radialis, gastrocnemius, and tibialis anterior muscles and 6 cm for vastus lateralis muscle. Afterward, images were imported to the ImageJ software (Fiji version) (Schindelin et al., 2012), and the maximal thickness of individual muscles was measured at the standardized locations (Figure 2). For each muscle, the ROI was determined as the region with the highest intensity of muscle tissue devoid of bone or surrounding tissue. The mean gray-scale level for the EI was obtained using the ImageJ software histogram function (resolution: 32-bit, black=0, white=255) (Figure 2). We separately calculated EI and thickness for each muscle and defined the average sum-score of muscle groups for muscle groups.
Statistical analysis
Data analysis was performed using the R Studio (version 3.2.2). We used the Shapiro-Wilk test to test the normality of the data. Since the variables (MMT, dynamometry, EI, and thickness) did not follow a normal distribution pattern, we used the non-parametric tests for the analysis. For the comparison of scales between the patients and healthy subjects, we used the Mann-Whitney test. The data for the tools are presented as median (interquartile [IQR]: 25th–75th percentiles). The diagnostic accuracy of MUS (EI and thickness), hand-held dynamometry, and MMT methods for distinguishing IM patients and healthy controls were assessed using the receiver operating characteristic curve (ROC) analysis. The significance level of <0.05 was regarded as significant.
3. Results
Demographic characteristics of the participants
We enrolled 17 patients with IM and 17 healthy subjects. IMs included DM (12 patients), PM, and non-specific myositis (5 patients). The median disease duration in the patients was 7 (IQR: 3-11) months. The patients and controls were matched in age (patients: 45.8±16.8 years; control: 41.7±16.4 years; P=0.47) and gender (patients: 13 females, control: 13 females; P=1.00). All patients received prednisolone (median dose: 25 [IQR: 15-40] mg/day). In addition, two patients received concurrent mycophenolate mofetil, while three received concurrent methotrexate. The mean CK level at the visit was 680 (250-1890) IU/L (maximum=8550 IU/L).
Functional measures
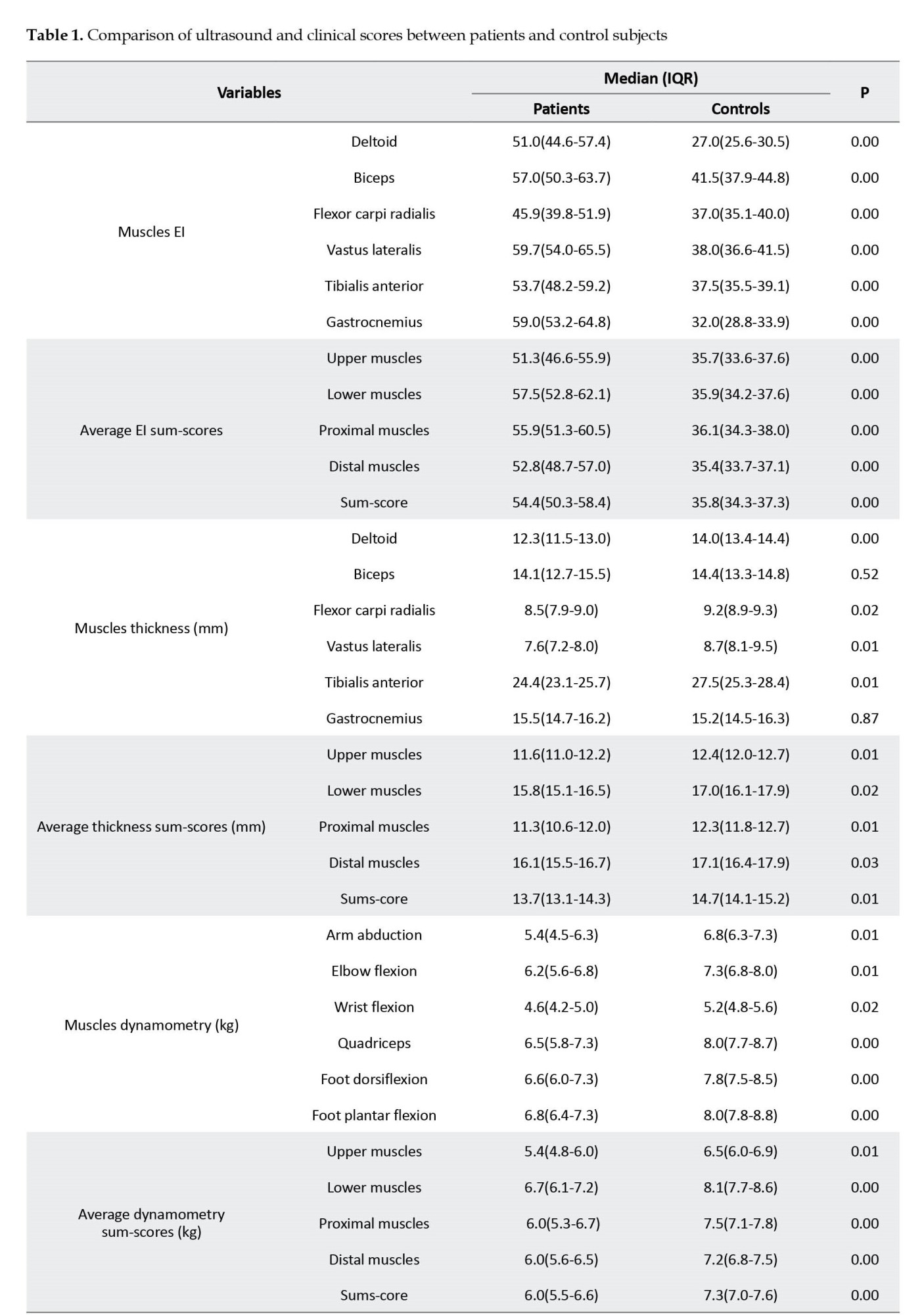
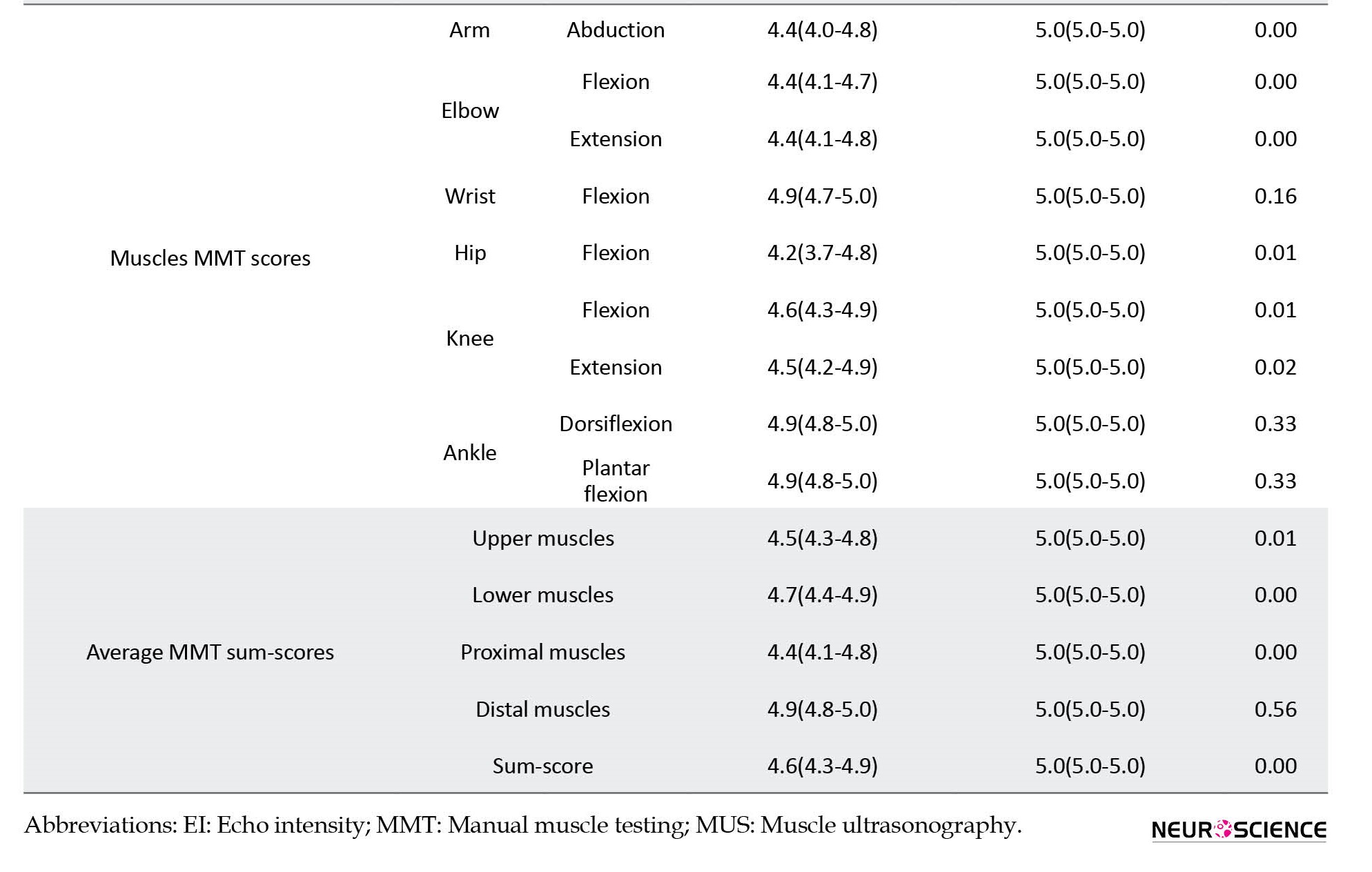
In terms of muscle strength, patients showed lower muscle strength compared to healthy subjects (Table 1). However, distal power, including wrist flexion, foot plantar flexion, and dorsiflexion, was not different between the study groups.
Muscle ultrasound parameters
All patients’ muscles showed significantly higher EI than control subjects’ corresponding muscles (Table 1). Other than biceps and gastrocnemius muscles, all muscles and compartments demonstrated a lower thickness than the controls (Table 1). The average EI sum-score for patients was 54.4 (50.3-58.4) vs 35.8 (34.3-37.3) for healthy subjects (P<0.01). Moreover, among patients, the EI sum-score of lower extremities muscles was higher than upper extremities muscles (57.5 [52.8-62.1] vs 51.3 [46.6-55.9], P=0.07) and EI of proximal muscles was higher than distal muscles (55.9 [51.3-60.5] vs 52.8 [48.7-57.0], P=0.37]; however, the differences were not significant.
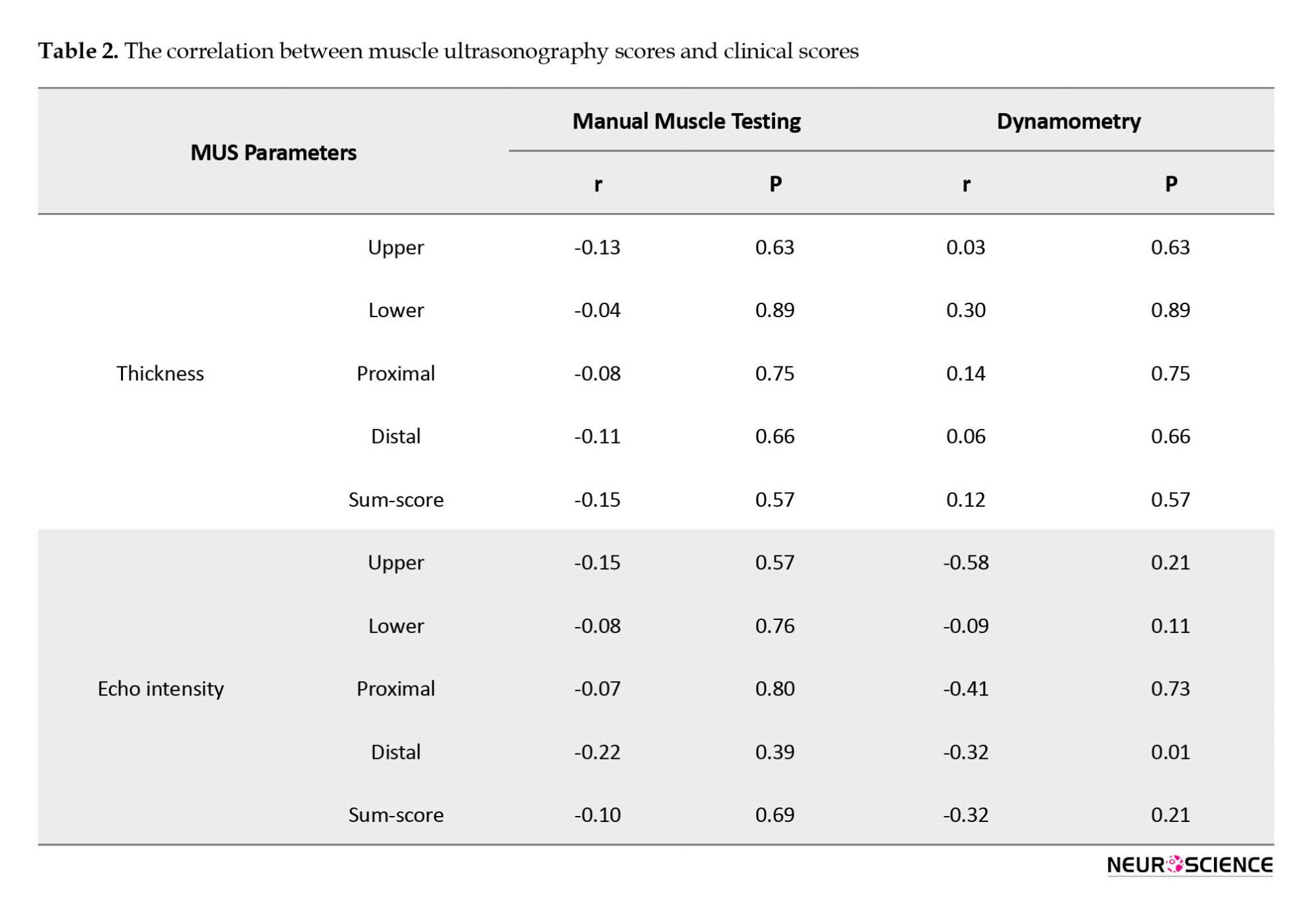
Association between muscle ultrasound and clinical parameters
The correlations between MUS parameters (EI and thickness) and clinical parameters (MMT and dynamometry) are indicated in Table 2. We found no significant correlation between MUS (EI and thickness) and clinical parameters (MMT and dynamometry), except for the correlation between distal dynamometry and EI.
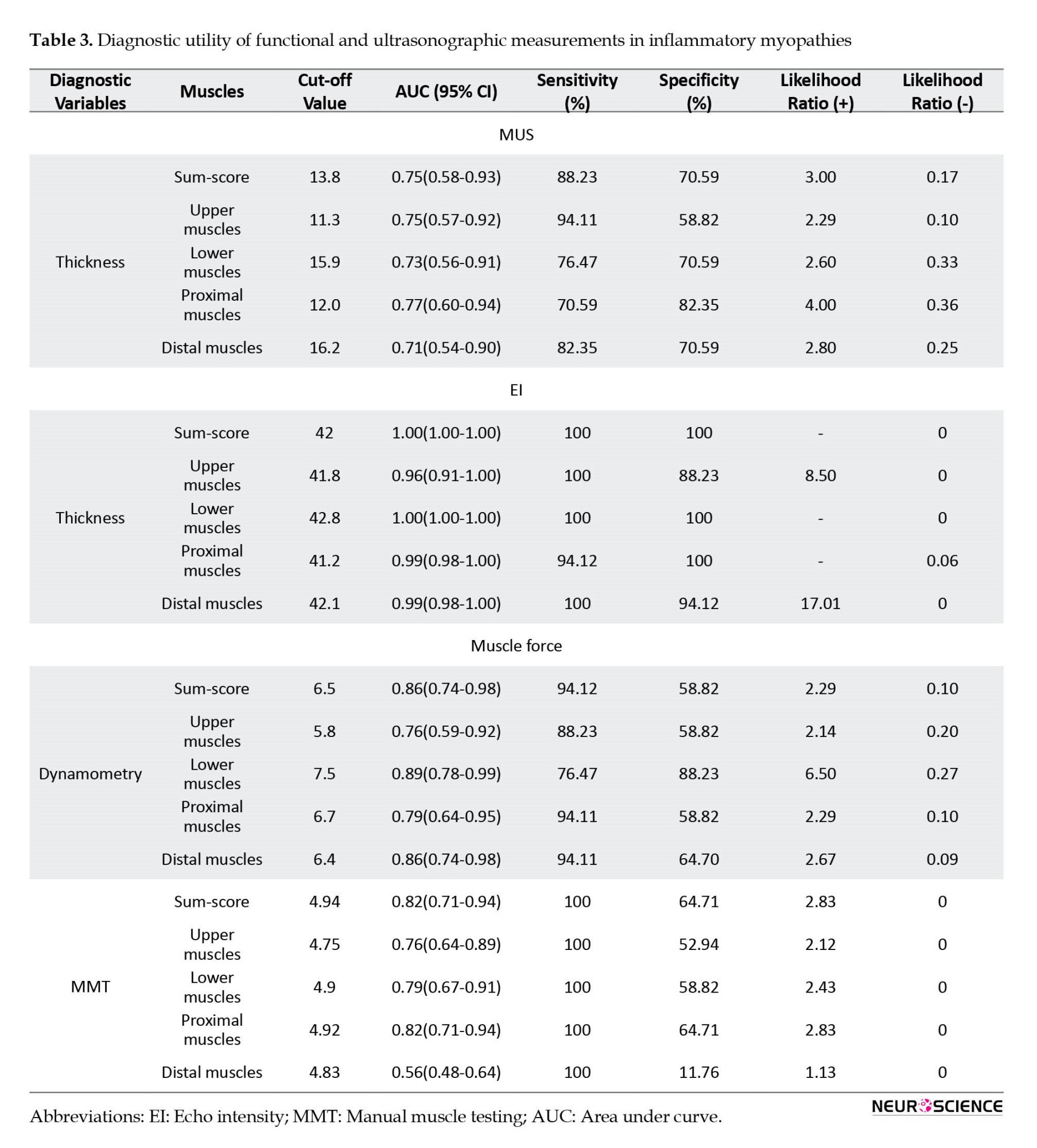
Diagnostic utility of muscle ultrasound
The ROC curve analysis assessed the diagnostic accuracy of muscle ultrasound sum-scores (average EI and thickness sum-score) to distinguish IM from healthy status (Table 3, Figure 3). In evaluating the average EI sum-score, scores of 39.7 or higher corresponded to a sensitivity of 100 and a specificity of 88.2 (likelihood ratio [LR]+: 8.5, LR-=0) to discriminate patients from control subjects, and the area under the ROC curve was 0.97 (Figure 3).
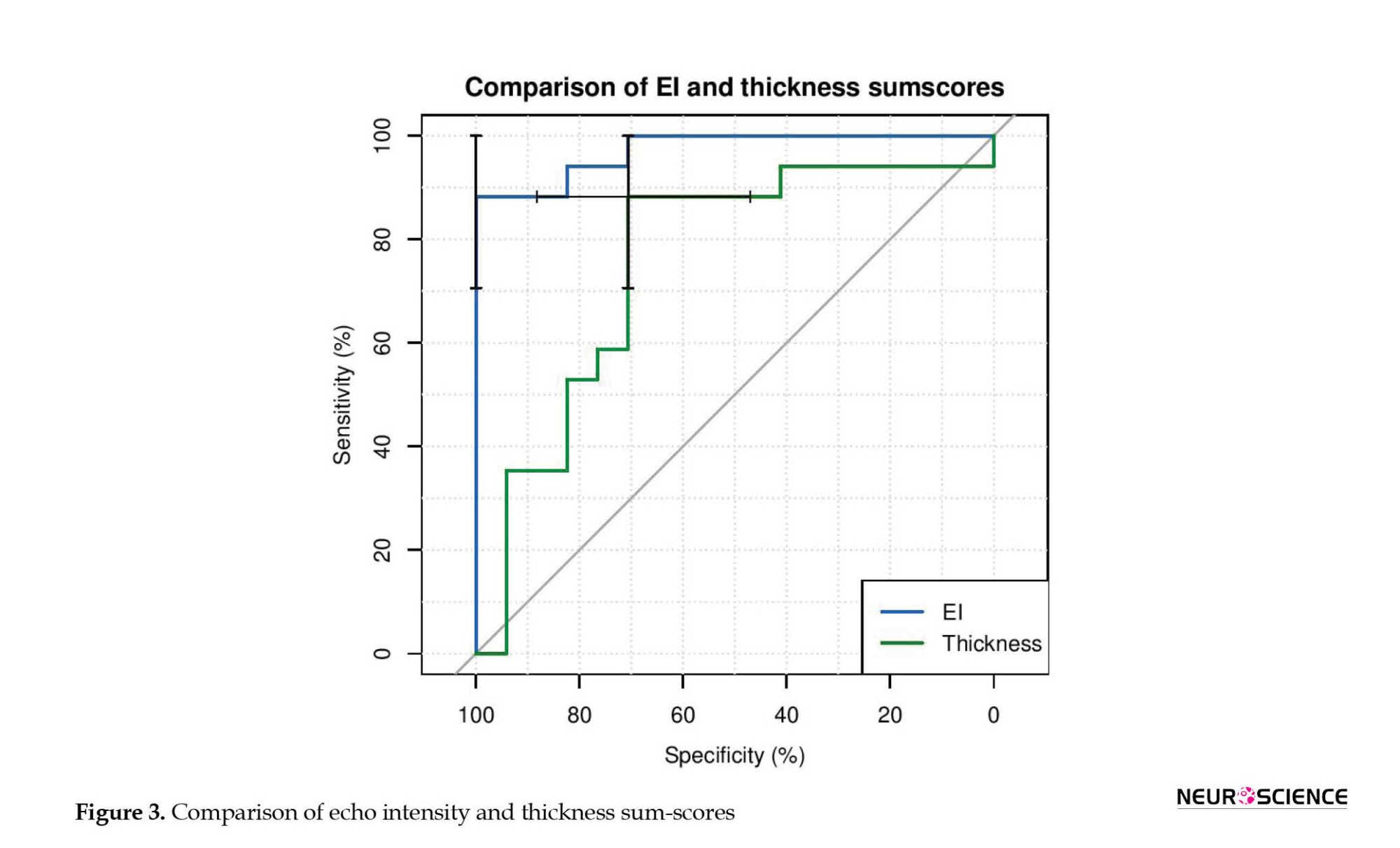
For the average thickness sum-score, scores of 13.6 mm or lesser corresponded to a sensitivity of 70.6 and a specificity of 88.2 (LR+: 6.0, LR-: 0.33) to discriminate patients from control subjects, and the area under the ROC curve was 0.75. The area under the curve significantly differed between EI and thickness (difference between areas=0.22, P=0.03).
4. Discussion
In the present study, we evaluated the association between MUS variables (muscle EI and thickness) and clinical (MMT, dynamometry) measures in patients with IM. Although clinical and MUS variables significantly differed between the patients and healthy individuals, there was nearly no relationship between their scores. Accordingly, we performed ROC analysis to understand better MUS’s ability to distinguish IM patients from healthy subjects. We observed good diagnostic utility for this ancillary method, especially for the echo intensity parameter.
Although there is no trace of imaging methods in the current classification criteria of IMs, such modalities are useful in diagnosing and monitoring such disorders. MRI imaging is the gold standard, showing acceptable sensitivity and specificity (Day et al., 2017; Walker, 2008). However, MRI has significant disadvantages as it is expensive, difficult to tolerate for some patients, and contraindicated in individuals with ferromagnetic biomedical implants (Adler & Garofalo, 2009). On the other hand, ultrasonography is a simple, non-invasive, and cost-effective alternative that allows real-time analysis of the muscle condition with high spatial resolution and has recently been used in the neuromuscular field, such as polyneuropathies and motor neuron disease as well as myopathies (Rajabkhah et al., 2020; Wijntjes & Alfen, 2021).
Healthy muscles are hypoechoic in MUS’s cross-sectional view, probably due to the high abundance of blood in the muscle tissue (Campbell et al., 2005; Whittaker & Stokes, 2011). Reimers et al. showed that fat replacement is the leading cause of increased muscle echogenicity (Reimers et al., 1993). Also, it has been demonstrated that PM/DM muscles exhibit higher echo intensity than healthy muscles (Mittal et al., 2003; Noto et al., 2014). Meanwhile, there is evidence that muscle size is altered in patients with PM/DM (Bhansing et al., 2015; Mittal et al., 2003). In acute stages, muscles might be normal in size or might even represent slight edema. Accordingly, fat tissue deposition or edema could mask the muscles’ reduced size in the early stages (Adler & Garofalo, 2009). In contrast, affected muscles experience a remarkable size reduction in chronic stages when atrophy is a prominent part. Our results support the previous literature, demonstrating higher EI and lower extremities muscle thickness in patients with PM/DM.
IM patients might benefit from an efficient therapeutic approach in case of insight into their disease activity. Expert consensus indicates that clinical and laboratory examinations are unreliable enough to estimate the disease activity. Other paraclinical methods are warranted, including muscle biopsy, EMG, and imaging modalities (Adler & Garofalo, 2009; Meng et al., 2001). Muscle biopsy is considered the gold standard for providing information regarding the disease activity; however, it is invasive. Therefore, other non-invasive modalities have been suggested as appropriate alternatives. In this respect, the diagnostic utility of MUS for IMs has been previously investigated. Reimers et al. showed that MUS has a similar sensitivity to EMG to detect the histo-pathologically proven PM/DM (Reimers et al., 1993).
Evidence concerns the significant association between some clinical assessments and imaging modalities. For instance, Meng et al. indicated that abnormal gray-scale US scores were correlated with lower CK levels and diseases of longer duration (Meng et al., 2001). Meanwhile, the values obtained from the combination of MUS and elastography showed partial concordance with laboratory markers (Botar-Jid et al., 2010). However, the presence of an association between bedside disease activity assessments and muscle imaging parameters remains controversial. For instance, in a recent study of radiologic patterns in inflammatory myopathies disorders, no significant correlation was found between MRI features and bedside measures, including MMT, myositis disease activity assessment tool (MDAAT-muscle), patient global visual analog scale, and physician global visual analog scale (Day et al., 2019). A similar result was observed in this study; accordingly, an association was merely between EI and the dynamometry score of the distal compartment. Further investigations are necessary to evaluate the association between clinical and imaging parameters.
5. Conclusion
In summary, we showed that MUS variables differ considerably between IM patients and healthy individuals, including muscle thickness and echo intensity. No one technique (EMG, CK, MRI, US, MMT, etc.) is an ideal diagnostic/treatment biomarker. Ultrasound is inexpensive, user-friendly, objective, and non-invasive, so further study is merited.
Study limitations
This study faced several limitations. First, the cross-sectional design of this investigation restricts the abiity to discover the predictive capacity of MUS in IMs. Second, our limited sample size questions the generalizability of the findings. Third, all patients were under treatment; this issue might vary IM patients’ clinical and paraclinical features compared to drug-naïve ones. Fourth, patient selection was relatively heterogeneous. Fifth, muscle blood flow and elastography were not evaluated in our study.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the local Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.MEDICINE.REC.1398.367).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization and supervision: Farzad Fatehi, Ali Asghar Okhovat and Shahriar Nafissi; Methodology: Farzad Fatehi and Shahriar Nafissi; Investigation: Parisa Khaghani, Mahsa Layegh and Akram Panahi; Writing the original draft: Kamyar Moradi, Farzad Teimouri and Mahsa Mortaja; Review and editing: Ali Asghar Okhovat and Farzad Fatehi.
Conflict of interest
The authors declared that no conflict of interest.
Acknowledgments
The authors appreciate Neurophysiology Clinic of Shariati Hospital for conducting this study.
References
Adler, R. S., & Garofalo, G. (2009). Ultrasound in the evaluation of the inflammatory myopathies. Current Rheumatology Reports, 11(4), 302-308. [DOI:10.1007/s11926-009-0042-0] [PMID]
Allenbach, Y., Benveniste, O., Decostre, V., Canal, A., Eymard, B., & Herson, S., et al. (2012). Quadriceps strength is a sensitive marker of disease progression in sporadic inclusion body myositis. Neuromuscular Disorders: NMD, 22(11), 980-986. [DOI:10.1016/j.nmd.2012.05.004] [PMID]
Bhansing, K. J., Van Rosmalen, M. H., Van Engelen, B. G., Vonk, M. C., Van Riel, P. L., & Pillen, S. (2015). Increased fascial thickness of the deltoid muscle in dermatomyositis and polymyositis: An ultrasound study. Muscle & Nerve, 52(4), 534-539. [DOI:10.1002/mus.24595] [PMID]
Botar-Jid, C., Damian, L., Dudea, S. M., Vasilescu, D., Rednic, S., & Badea, R. (2010). The contribution of ultrasonography and sonoelastography in assessment of myositis. Medical Ultrasonography, 12(2), 120-126. [PMID]
Campbell, S. E., Adler, R., & Sofka, C. M. (2005). Ultrasound of muscle abnormalities. Ultrasound Quarterly, 21(2), 87–154. [PMID]
Day, J. A., Bajic, N., Gentili, S., Patel, S., & Limaye, V. (2019). Radiographic patterns of muscle involvement in the idiopathic inflammatory myopathies. Muscle & Nerve, 60(5), 549-557. [DOI:10.1002/mus.26660] [PMID]
Day, J., Patel, S., & Limaye, V. (2017). The role of magnetic resonance imaging techniques in evaluation and management of the idiopathic inflammatory myopathies. Seminars in Arthritis and Rheumatism, 46(5), 642-649. [DOI:10.1016/j.semarthrit.2016.11.001] [PMID]
Habers, G. E., Van Brussel, M., Bhansing, K. J., Hoppenreijs, E. P., Janssen, A. J., & Van Royen-Kerkhof, A., et al. (2015). Quantitative muscle ultrasonography in the follow-up of juvenile dermatomyositis. Muscle & Nerve, 52(4), 540-546. [DOI:10.1002/mus.24564] [PMID]
Heckmatt, J. Z., Dubowitz, V., & Leeman, S. (1980). Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet (London, England), 1(8183), 1389-1390. [DOI:10.1016/s0140-6736(80)92656-2] [PMID]
Heckmatt, J. Z., Leeman, S., & Dubowitz, V. (1982). Ultrasound imaging in the diagnosis of muscle disease. The Journal of Pediatrics, 101(5), 656-660. [DOI:10.1016/s0022-3476(82)80286-2] [PMID]
Meng, C., Adler, R., Peterson, M., & Kagen, L. (2001). Combined use of power Doppler and gray-scale sonography: A new technique for the assessment of inflammatory myopathy. The Journal of Rheumatology, 28(6), 1271-1282. [PMID]
Miller, F. W., Rider, L. G., Chung, Y. L., Cooper, R., Danko, K., & Farewell, V., et al. (2001). Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford, England), 40(11), 1262-1273. [DOI:10.1093/rheumatology/40.11.1262] [PMID]
Mittal, G. A., Wadhwani, R., Shroff, M., Sukthankar, R., Pathan, E., & Joshi, V. R. (2003). Ultrasonography in the diagnosis and follow-up of idiopathic inflammatory myopathies-A preliminary study. The Journal of the Association of Physicians of India, 51, 252–256. [PMID]
Noto, Y., Shiga, K., Tsuji, Y., Kondo, M., Tokuda, T., & Mizuno, T., et al. (2014). Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: A diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle & Nerve, 49(5), 745-748. [DOI:10.1002/mus.24056] [PMID]
Pillen, S., & Van Alfen, N. (2015). Muscle ultrasound from diagnostic tool to outcome measure-Quantification is the challenge. Muscle & Nerve, 52(3), 319-320. [DOI:10.1002/mus.24613] [PMID]
Pillen, S., van Keimpema, M., Nievelstein, R. A., Verrips, A., van Kruijsbergen-Raijmann, W., & Zwarts, M. J. (2006). Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound in Medicine & Biology, 32(9), 1315-1321. [DOI:10.1016/j.ultrasmedbio.2006.05.028] [PMID]
Rajabkhah, S., Moradi, K., Okhovat, A. A., Van Alfen, N., Fathi, D., & Aghaghazvini, L., et al. (2020). Application of muscle ultrasound for the evaluation of patients with amyotrophic lateral sclerosis: An observational cross-sectional study. Muscle & Nerve, 62(4), 516-521. [DOI:10.1002/mus.27036] [PMID]
Reimers, C. D., Fleckenstein, J. L., Witt, T. N., Müller-Felber, W., & Pongratz, D. E. (1993). Muscular ultrasound in idiopathic inflammatory myopathies of adults. Journal of the Neurological Sciences, 116(1), 82-92. [DOI:10.1016/0022-510X(93)90093-E] [PMID]
Reimers, K., Reimers, C. D., Wagner, S., Paetzke, I., & Pongratz, D. E. (1993). Skeletal muscle sonography: A correlative study of echogenicity and morphology. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine, 12(2), 73-77. [DOI:10.7863/jum.1993.12.2.73] [PMID]
Rider, L. G., Aggarwal, R., Machado, P. M., Hogrel, J. Y., Reed, A. M., & Christopher-Stine, L., et al. (2018). Update on outcome assessment in myositis. Nature Reviews. Rheumatology, 14(5), 303-318. [DOI:10.1038/nrrheum.2018.33] [PMID]
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., & Pietzsch, T., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676-682. [DOI:10.1038/nmeth.2019] [PMID]
Schmidt, J. (2018). Current classification and management of inflammatory myopathies. Journal of Neuromuscular Diseases, 5(2), 109-129. [DOI:10.3233/JND-180308] [PMID]
Selva-O'Callaghan, A., Pinal-Fernandez, I., Trallero-Araguás, E., Milisenda, J. C., Grau-Junyent, J. M., & Mammen, A. L. (2018).Classification and management of adult inflammatory myopathies. The Lancet. Neurology, 17(9), 816-828. [DOI:10.1016/S1474-4422(18)30254-0] [PMID]
Walker, U. A. (2008). Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Current Opinion in Rheumatology, 20(6), 656-661. [DOI:10.1097/BOR.0b013e3283118711] [PMID]
Whittaker, J. L., & Stokes, M. (2011). Ultrasound imaging and muscle function. The Journal of Orthopaedic and Sports Physical Therapy, 41(8), 572-580. [DOI:10.2519/jospt.2011.3682] [PMID]
Wijntjes, J., & van Alfen, N. (2021). Muscle ultrasound: Present state and future opportunities. Muscle & Nerve, 63(4), 455-466. [DOI:10.1002/mus.27081] [PMID]
Inflammatory myopathies (IMs) are a diverse group of muscular autoimmune disorders characterized by muscle weakness as the initial symptom. According to the new classification system for IMs, these disorders include inclusion body myositis, dermatomyositis (DM), polymyositis (PM), immune-mediated necrotizing myopathy, anti-synthetase syndrome, and overlap myositis (Selva-O’Callaghan et al., 2018). Most IM patients respond well to the immunosuppressive treatment except for including body myositis. Nevertheless, it is essential to point out that many IM patients progress despite intense treatment (Schmidt, 2018). The development of many outcome measures for myositis activity has been standardized in the field, which has aided our understanding of the long-term effects of such diseases and the development of new therapies (Rider et al., 2018).
Currently, most clinicians use manual muscle testing (MMT), electrodiagnostic tests, including electromyography (EMG), and serum markers, such as creatine kinase (CK) for diagnosis and follow-up of the patients. Other outcome measures used in the referral center for follow-up include additional clinical assessment tools, such as hand-held dynamometry (Allenbach et al., 2012), muscle magnetic resonance imaging (MRI), and muscle ultrasound (MUS). The lack of definite tools to measure muscle activity may develop some diagnostic problems that may cause some diagnostic issues. The MMT test may be affected by inter-observer differences (Miller et al., 2001). Therefore, there is growing attention to the application of imaging modalities. Muscle MRI has been broadly used in IMs to assess the extent of muscle involvement and determine the best location for muscle biopsy and patients’ follow-up; however, this modality is expensive, non-feasible, and time-consuming. Thus, the application of MUS has been increased due to its widespread availability, easier techniques, non-invasiveness, and cost-effectiveness for real-time imaging of the muscles (Pillen et al., 2006).
Both qualitative and quantitative methods are applied for muscle evaluations in MUS (Heckmatt et al., 1980, 1982). MUS benefits from an acceptable sensitivity to detect muscle changes even in the early stages of the disease, reflected by increased echo intensity (EI) due to acute inflammation and edema within the muscle tissue (Habers et al., 2015). As one of the main parameters of MUS, EI is an outcome of choice in patients with IMs; the region of interest (ROI) determination for EI calculation is quantitative (Pillen & Van Alfen, 2015); therefore, the findings are less operator-dependent with higher sensitivity than subjective analysis (Pillen et al., 2006).
The muscles of patients with PM/DM have higher EI compared to healthy muscles (Mittal et al., 2003; Noto et al., 2014). However, there is limited data on MUS’s diagnostic utility for differentiating PM/DM subjects from healthy ones. Besides, the association between MUS parameters and bedside clinical characteristics remains unknown. This study compares MUS parameters in IM to healthy subjects and measures the associations between clinical scores and MUS scores in the patients.
2. Materials and Methods
Study design and participants
In this cross-sectional case-control study, we enrolled 17 adult patients (>18 years old) diagnosed with IM, confirmed with a muscle biopsy, and 17 normal subjects in a neuromuscular referral center from September 2018 to January 2020. The inclusion criteria were defined as the presence of clinical evidence of IM, including bilateral symmetric proximal muscle weakness, disease duration of fewer than five years, history of elevation in serum skeletal muscle enzymes (CK >300 IU/L), or electromyography (EMG) results, indicating myopathic pattern along with irritation on needle EMG and definite evidence of inflammatory changes in muscle biopsy. Characteristic rashes on clinical examination and perifascicular atrophy in muscle biopsy were considered for the classification of DM. We excluded the patients with possible evidence of inclusion-body myositis, muscular dystrophy, metabolic or endocrine myopathy, toxic myopathy, and granulomatous and infectious myositis. Informed consent was obtained from all participants.
Functional measures
MMT was conducted by an expert neurologist using the medical research council scale, scoring muscle groups that are responsible for the following activities from 0 to 5: Arm abduction, elbow flexion/extension, wrist flexion, hip flexion, knee flexion/extension, and ankle dorsiflexion/plantar flexion. Moreover, a calibrated hand-held dynamometer (microFET®2, Hoggan Scientific, USA) quantitively measured the muscle forces. We calculated functional measures of MMT and dynamometry for each muscle separately. Also, we defined the average sum-score of muscle groups for proximal extremities muscles, distal extremities muscles, upper extremities muscles, lower extremities muscles, and average total sum-score (Figure 1). To calculate each average sum-score, we initially added the scores of all muscles or actions and then divided them by the number of muscles or actions.

Muscle ultrasound
A standard ultrasound protocol was applied for MUS, using a Sonosite M-Turbo C machine with a 15-6 MHz linear probe (Sonosite, Fujifilm) by an expert in neuromuscular ultrasound (AP). Ultrasound scans were made from the following muscles on both sides: Biceps brachii, deltoid, flexor carpi radialis, vastus lateralis, gastrocnemius, and tibialis anterior. Before the ultrasound examination, dirt and debris were cleaned from the skin. The areas were prepped with alcohol. A generous amount of contact gel was used to minimize the transducer’s required pressure on the skin.
Each muscle underwent MUS three consecutive times, and the means of EI and thickness scores were calculated to minimize the variations. All scans were made in the transverse plane with a standard transducer location corresponding to the same investigator (PK) of the muscle belly, with three years of experience in neuromuscular ultrasound. We adjusted the probe’s angle to avoid oblique scanning until the best bone EI was acquired. The ultrasound machine was set on musculoskeletal mode and autogain function for ultrasonographic evaluations. Furthermore, we considered the depth of 4 cm for biceps brachii, deltoid, flexor carpi radialis, gastrocnemius, and tibialis anterior muscles and 6 cm for vastus lateralis muscle. Afterward, images were imported to the ImageJ software (Fiji version) (Schindelin et al., 2012), and the maximal thickness of individual muscles was measured at the standardized locations (Figure 2). For each muscle, the ROI was determined as the region with the highest intensity of muscle tissue devoid of bone or surrounding tissue. The mean gray-scale level for the EI was obtained using the ImageJ software histogram function (resolution: 32-bit, black=0, white=255) (Figure 2). We separately calculated EI and thickness for each muscle and defined the average sum-score of muscle groups for muscle groups.

Statistical analysis
Data analysis was performed using the R Studio (version 3.2.2). We used the Shapiro-Wilk test to test the normality of the data. Since the variables (MMT, dynamometry, EI, and thickness) did not follow a normal distribution pattern, we used the non-parametric tests for the analysis. For the comparison of scales between the patients and healthy subjects, we used the Mann-Whitney test. The data for the tools are presented as median (interquartile [IQR]: 25th–75th percentiles). The diagnostic accuracy of MUS (EI and thickness), hand-held dynamometry, and MMT methods for distinguishing IM patients and healthy controls were assessed using the receiver operating characteristic curve (ROC) analysis. The significance level of <0.05 was regarded as significant.
3. Results
Demographic characteristics of the participants
We enrolled 17 patients with IM and 17 healthy subjects. IMs included DM (12 patients), PM, and non-specific myositis (5 patients). The median disease duration in the patients was 7 (IQR: 3-11) months. The patients and controls were matched in age (patients: 45.8±16.8 years; control: 41.7±16.4 years; P=0.47) and gender (patients: 13 females, control: 13 females; P=1.00). All patients received prednisolone (median dose: 25 [IQR: 15-40] mg/day). In addition, two patients received concurrent mycophenolate mofetil, while three received concurrent methotrexate. The mean CK level at the visit was 680 (250-1890) IU/L (maximum=8550 IU/L).
Functional measures
In terms of muscle strength, patients showed lower muscle strength compared to healthy subjects (Table 1). However, distal power, including wrist flexion, foot plantar flexion, and dorsiflexion, was not different between the study groups.


Muscle ultrasound parameters
All patients’ muscles showed significantly higher EI than control subjects’ corresponding muscles (Table 1). Other than biceps and gastrocnemius muscles, all muscles and compartments demonstrated a lower thickness than the controls (Table 1). The average EI sum-score for patients was 54.4 (50.3-58.4) vs 35.8 (34.3-37.3) for healthy subjects (P<0.01). Moreover, among patients, the EI sum-score of lower extremities muscles was higher than upper extremities muscles (57.5 [52.8-62.1] vs 51.3 [46.6-55.9], P=0.07) and EI of proximal muscles was higher than distal muscles (55.9 [51.3-60.5] vs 52.8 [48.7-57.0], P=0.37]; however, the differences were not significant.
Association between muscle ultrasound and clinical parameters
The correlations between MUS parameters (EI and thickness) and clinical parameters (MMT and dynamometry) are indicated in Table 2. We found no significant correlation between MUS (EI and thickness) and clinical parameters (MMT and dynamometry), except for the correlation between distal dynamometry and EI.

Diagnostic utility of muscle ultrasound
The ROC curve analysis assessed the diagnostic accuracy of muscle ultrasound sum-scores (average EI and thickness sum-score) to distinguish IM from healthy status (Table 3, Figure 3). In evaluating the average EI sum-score, scores of 39.7 or higher corresponded to a sensitivity of 100 and a specificity of 88.2 (likelihood ratio [LR]+: 8.5, LR-=0) to discriminate patients from control subjects, and the area under the ROC curve was 0.97 (Figure 3).

For the average thickness sum-score, scores of 13.6 mm or lesser corresponded to a sensitivity of 70.6 and a specificity of 88.2 (LR+: 6.0, LR-: 0.33) to discriminate patients from control subjects, and the area under the ROC curve was 0.75. The area under the curve significantly differed between EI and thickness (difference between areas=0.22, P=0.03).

4. Discussion
In the present study, we evaluated the association between MUS variables (muscle EI and thickness) and clinical (MMT, dynamometry) measures in patients with IM. Although clinical and MUS variables significantly differed between the patients and healthy individuals, there was nearly no relationship between their scores. Accordingly, we performed ROC analysis to understand better MUS’s ability to distinguish IM patients from healthy subjects. We observed good diagnostic utility for this ancillary method, especially for the echo intensity parameter.
Although there is no trace of imaging methods in the current classification criteria of IMs, such modalities are useful in diagnosing and monitoring such disorders. MRI imaging is the gold standard, showing acceptable sensitivity and specificity (Day et al., 2017; Walker, 2008). However, MRI has significant disadvantages as it is expensive, difficult to tolerate for some patients, and contraindicated in individuals with ferromagnetic biomedical implants (Adler & Garofalo, 2009). On the other hand, ultrasonography is a simple, non-invasive, and cost-effective alternative that allows real-time analysis of the muscle condition with high spatial resolution and has recently been used in the neuromuscular field, such as polyneuropathies and motor neuron disease as well as myopathies (Rajabkhah et al., 2020; Wijntjes & Alfen, 2021).
Healthy muscles are hypoechoic in MUS’s cross-sectional view, probably due to the high abundance of blood in the muscle tissue (Campbell et al., 2005; Whittaker & Stokes, 2011). Reimers et al. showed that fat replacement is the leading cause of increased muscle echogenicity (Reimers et al., 1993). Also, it has been demonstrated that PM/DM muscles exhibit higher echo intensity than healthy muscles (Mittal et al., 2003; Noto et al., 2014). Meanwhile, there is evidence that muscle size is altered in patients with PM/DM (Bhansing et al., 2015; Mittal et al., 2003). In acute stages, muscles might be normal in size or might even represent slight edema. Accordingly, fat tissue deposition or edema could mask the muscles’ reduced size in the early stages (Adler & Garofalo, 2009). In contrast, affected muscles experience a remarkable size reduction in chronic stages when atrophy is a prominent part. Our results support the previous literature, demonstrating higher EI and lower extremities muscle thickness in patients with PM/DM.
IM patients might benefit from an efficient therapeutic approach in case of insight into their disease activity. Expert consensus indicates that clinical and laboratory examinations are unreliable enough to estimate the disease activity. Other paraclinical methods are warranted, including muscle biopsy, EMG, and imaging modalities (Adler & Garofalo, 2009; Meng et al., 2001). Muscle biopsy is considered the gold standard for providing information regarding the disease activity; however, it is invasive. Therefore, other non-invasive modalities have been suggested as appropriate alternatives. In this respect, the diagnostic utility of MUS for IMs has been previously investigated. Reimers et al. showed that MUS has a similar sensitivity to EMG to detect the histo-pathologically proven PM/DM (Reimers et al., 1993).
Evidence concerns the significant association between some clinical assessments and imaging modalities. For instance, Meng et al. indicated that abnormal gray-scale US scores were correlated with lower CK levels and diseases of longer duration (Meng et al., 2001). Meanwhile, the values obtained from the combination of MUS and elastography showed partial concordance with laboratory markers (Botar-Jid et al., 2010). However, the presence of an association between bedside disease activity assessments and muscle imaging parameters remains controversial. For instance, in a recent study of radiologic patterns in inflammatory myopathies disorders, no significant correlation was found between MRI features and bedside measures, including MMT, myositis disease activity assessment tool (MDAAT-muscle), patient global visual analog scale, and physician global visual analog scale (Day et al., 2019). A similar result was observed in this study; accordingly, an association was merely between EI and the dynamometry score of the distal compartment. Further investigations are necessary to evaluate the association between clinical and imaging parameters.
5. Conclusion
In summary, we showed that MUS variables differ considerably between IM patients and healthy individuals, including muscle thickness and echo intensity. No one technique (EMG, CK, MRI, US, MMT, etc.) is an ideal diagnostic/treatment biomarker. Ultrasound is inexpensive, user-friendly, objective, and non-invasive, so further study is merited.
Study limitations
This study faced several limitations. First, the cross-sectional design of this investigation restricts the abiity to discover the predictive capacity of MUS in IMs. Second, our limited sample size questions the generalizability of the findings. Third, all patients were under treatment; this issue might vary IM patients’ clinical and paraclinical features compared to drug-naïve ones. Fourth, patient selection was relatively heterogeneous. Fifth, muscle blood flow and elastography were not evaluated in our study.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the local Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.MEDICINE.REC.1398.367).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization and supervision: Farzad Fatehi, Ali Asghar Okhovat and Shahriar Nafissi; Methodology: Farzad Fatehi and Shahriar Nafissi; Investigation: Parisa Khaghani, Mahsa Layegh and Akram Panahi; Writing the original draft: Kamyar Moradi, Farzad Teimouri and Mahsa Mortaja; Review and editing: Ali Asghar Okhovat and Farzad Fatehi.
Conflict of interest
The authors declared that no conflict of interest.
Acknowledgments
The authors appreciate Neurophysiology Clinic of Shariati Hospital for conducting this study.
References
Adler, R. S., & Garofalo, G. (2009). Ultrasound in the evaluation of the inflammatory myopathies. Current Rheumatology Reports, 11(4), 302-308. [DOI:10.1007/s11926-009-0042-0] [PMID]
Allenbach, Y., Benveniste, O., Decostre, V., Canal, A., Eymard, B., & Herson, S., et al. (2012). Quadriceps strength is a sensitive marker of disease progression in sporadic inclusion body myositis. Neuromuscular Disorders: NMD, 22(11), 980-986. [DOI:10.1016/j.nmd.2012.05.004] [PMID]
Bhansing, K. J., Van Rosmalen, M. H., Van Engelen, B. G., Vonk, M. C., Van Riel, P. L., & Pillen, S. (2015). Increased fascial thickness of the deltoid muscle in dermatomyositis and polymyositis: An ultrasound study. Muscle & Nerve, 52(4), 534-539. [DOI:10.1002/mus.24595] [PMID]
Botar-Jid, C., Damian, L., Dudea, S. M., Vasilescu, D., Rednic, S., & Badea, R. (2010). The contribution of ultrasonography and sonoelastography in assessment of myositis. Medical Ultrasonography, 12(2), 120-126. [PMID]
Campbell, S. E., Adler, R., & Sofka, C. M. (2005). Ultrasound of muscle abnormalities. Ultrasound Quarterly, 21(2), 87–154. [PMID]
Day, J. A., Bajic, N., Gentili, S., Patel, S., & Limaye, V. (2019). Radiographic patterns of muscle involvement in the idiopathic inflammatory myopathies. Muscle & Nerve, 60(5), 549-557. [DOI:10.1002/mus.26660] [PMID]
Day, J., Patel, S., & Limaye, V. (2017). The role of magnetic resonance imaging techniques in evaluation and management of the idiopathic inflammatory myopathies. Seminars in Arthritis and Rheumatism, 46(5), 642-649. [DOI:10.1016/j.semarthrit.2016.11.001] [PMID]
Habers, G. E., Van Brussel, M., Bhansing, K. J., Hoppenreijs, E. P., Janssen, A. J., & Van Royen-Kerkhof, A., et al. (2015). Quantitative muscle ultrasonography in the follow-up of juvenile dermatomyositis. Muscle & Nerve, 52(4), 540-546. [DOI:10.1002/mus.24564] [PMID]
Heckmatt, J. Z., Dubowitz, V., & Leeman, S. (1980). Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet (London, England), 1(8183), 1389-1390. [DOI:10.1016/s0140-6736(80)92656-2] [PMID]
Heckmatt, J. Z., Leeman, S., & Dubowitz, V. (1982). Ultrasound imaging in the diagnosis of muscle disease. The Journal of Pediatrics, 101(5), 656-660. [DOI:10.1016/s0022-3476(82)80286-2] [PMID]
Meng, C., Adler, R., Peterson, M., & Kagen, L. (2001). Combined use of power Doppler and gray-scale sonography: A new technique for the assessment of inflammatory myopathy. The Journal of Rheumatology, 28(6), 1271-1282. [PMID]
Miller, F. W., Rider, L. G., Chung, Y. L., Cooper, R., Danko, K., & Farewell, V., et al. (2001). Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford, England), 40(11), 1262-1273. [DOI:10.1093/rheumatology/40.11.1262] [PMID]
Mittal, G. A., Wadhwani, R., Shroff, M., Sukthankar, R., Pathan, E., & Joshi, V. R. (2003). Ultrasonography in the diagnosis and follow-up of idiopathic inflammatory myopathies-A preliminary study. The Journal of the Association of Physicians of India, 51, 252–256. [PMID]
Noto, Y., Shiga, K., Tsuji, Y., Kondo, M., Tokuda, T., & Mizuno, T., et al. (2014). Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: A diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle & Nerve, 49(5), 745-748. [DOI:10.1002/mus.24056] [PMID]
Pillen, S., & Van Alfen, N. (2015). Muscle ultrasound from diagnostic tool to outcome measure-Quantification is the challenge. Muscle & Nerve, 52(3), 319-320. [DOI:10.1002/mus.24613] [PMID]
Pillen, S., van Keimpema, M., Nievelstein, R. A., Verrips, A., van Kruijsbergen-Raijmann, W., & Zwarts, M. J. (2006). Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound in Medicine & Biology, 32(9), 1315-1321. [DOI:10.1016/j.ultrasmedbio.2006.05.028] [PMID]
Rajabkhah, S., Moradi, K., Okhovat, A. A., Van Alfen, N., Fathi, D., & Aghaghazvini, L., et al. (2020). Application of muscle ultrasound for the evaluation of patients with amyotrophic lateral sclerosis: An observational cross-sectional study. Muscle & Nerve, 62(4), 516-521. [DOI:10.1002/mus.27036] [PMID]
Reimers, C. D., Fleckenstein, J. L., Witt, T. N., Müller-Felber, W., & Pongratz, D. E. (1993). Muscular ultrasound in idiopathic inflammatory myopathies of adults. Journal of the Neurological Sciences, 116(1), 82-92. [DOI:10.1016/0022-510X(93)90093-E] [PMID]
Reimers, K., Reimers, C. D., Wagner, S., Paetzke, I., & Pongratz, D. E. (1993). Skeletal muscle sonography: A correlative study of echogenicity and morphology. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine, 12(2), 73-77. [DOI:10.7863/jum.1993.12.2.73] [PMID]
Rider, L. G., Aggarwal, R., Machado, P. M., Hogrel, J. Y., Reed, A. M., & Christopher-Stine, L., et al. (2018). Update on outcome assessment in myositis. Nature Reviews. Rheumatology, 14(5), 303-318. [DOI:10.1038/nrrheum.2018.33] [PMID]
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., & Pietzsch, T., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676-682. [DOI:10.1038/nmeth.2019] [PMID]
Schmidt, J. (2018). Current classification and management of inflammatory myopathies. Journal of Neuromuscular Diseases, 5(2), 109-129. [DOI:10.3233/JND-180308] [PMID]
Selva-O'Callaghan, A., Pinal-Fernandez, I., Trallero-Araguás, E., Milisenda, J. C., Grau-Junyent, J. M., & Mammen, A. L. (2018).Classification and management of adult inflammatory myopathies. The Lancet. Neurology, 17(9), 816-828. [DOI:10.1016/S1474-4422(18)30254-0] [PMID]
Walker, U. A. (2008). Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Current Opinion in Rheumatology, 20(6), 656-661. [DOI:10.1097/BOR.0b013e3283118711] [PMID]
Whittaker, J. L., & Stokes, M. (2011). Ultrasound imaging and muscle function. The Journal of Orthopaedic and Sports Physical Therapy, 41(8), 572-580. [DOI:10.2519/jospt.2011.3682] [PMID]
Wijntjes, J., & van Alfen, N. (2021). Muscle ultrasound: Present state and future opportunities. Muscle & Nerve, 63(4), 455-466. [DOI:10.1002/mus.27081] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2021/07/14 | Accepted: 2023/06/20 | Published: 2023/09/1
Received: 2021/07/14 | Accepted: 2023/06/20 | Published: 2023/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





