Volume 14, Issue 6 (November & December 2023)
BCN 2023, 14(6): 805-812 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Panahi Y, Monazzah M A, Vafaei Saiah G. Menthol Dissolved in Dimethyl Sulfoxide Protects Against Epileptiform Activity Induced by Pentylenetetrazol in Male Rats. BCN 2023; 14 (6) :805-812
URL: http://bcn.iums.ac.ir/article-1-1995-en.html
URL: http://bcn.iums.ac.ir/article-1-1995-en.html
1- Department of Basic Science, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran.
Full-Text [PDF 649 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Menthol is fragrant monoterpenoid alcohol obtained from peppermint (Mentha piperita L.) oil. For centuries, it has been used as a food additive, local anesthetic, topical analgesic, antipruritic agent, and gastric sedative (Galeotti et al., 2002). Among the different optical isomers, (-)-menthol predominates the others in nature. Due to its peculiar flavor and fragrance, menthol is widely used in cosmetics (Eccles, 1994). Additionally, it is one of the ingredients of antiseptic and cooling formulations in pharmaceutical dosage forms. This optical isomer can also be found in eutectic formulations of local anesthetic agents. Menthol affects both the peripheral nervous system and the central nervous system (CNS) because its intra-cerebro-ventricular and systemic administration may produce sedation or anesthesia (Watt & Betts, 2008), anticonvulsant effects (Zhang et al., 2008), analgesic effects (Su et al., 2011), improvement in learning (McKenzie, 2018), and cognitive functions (Bhadania et al., 2012). Cellular targets of menthol in the CNS include voltage-gated sodium channels (Pan et al., 2012), voltage-gated calcium channels (San Cheang et al., 2013), serotonin receptors, nicotinic acetylcholine receptors (Ashoor et al., 2013), glycine receptors (Hall et al., 2013), and gamma-aminobutyric acid (GABA) receptors. Although behavioral studies have claimed that menthol can influence CNS (Umezu et al., 2001), no definite target has been determined for menthol in the CNS, yet. Nevertheless, menthol has been shown to inhibit hippocampal neurons by increasing the tone of the GABAA nervous system (Taniguchi et al., 1994). Dimethyl sulfoxide (DMSO) was used as the solvent of menthol in this study because most drugs tested for their antiepileptic effects on animal models are dissolved in DMSO, which is an amphipathic molecule. Due to its physicochemical properties, DMSO is regarded as an extremely efficient solvent for water-insoluble compounds (Santos et al., 2003). Several biological functions have been attributed to DMSO in different CNS models (Sardo et al., 2006). For instance, data from initial in vivo studies have shown the antiepileptic effect of DMSO on an animal model of temporal lobe epilepsy and different studies have demonstrated its dose-dependent anticonvulsant and proconvulsant effects. DMSO has also been reported to have antiepileptic effects at moderate and high doses (Carletti et al., 2013). However, a study showed that the moderate and high doses of DMSO produced proconvulsant effects (Kovács et al., 2011). Given the results of the involvement of DMSO in seizure-induced neuropathology, its widespread use as a solvent and vehicle for effective agents in mammalian systems cannot be easily justified (Noel et al., 1975). According to our previous study (Panahi et al., 2020), we observed that the effects of DMSO in seizure activities are biphasic, i.e. it had protective effects at a dose of 10% and stimulant effects at doses of 50% and above. Therefore, in this study, we used 100% of it as a solvent to make sure that it has no protective effects on seizure activity, and if an effect is observed, we can easily attribute it to menthol. Considering that, we utilized DMSO as the solvent despite its controversial effects on the CNS. Therefore, the present study was designed to investigate the possible protective effects of different concentrations of menthol on pentylenetetrazol (PTZ)-induced seizures in adult male rats.
2. Materials and Methods
Animals
In the present study, 30 male Wistar rats weighing 200-250 g were purchased from the Laboratory Animal Breeding Center in the Faculty of Pharmacy at Urmia University of Medical Sciences. The animals were acclimatized to their new surroundings for a week before the experiment. The rats were housed in special cages at a temperature of 22°C and humidity of 45%-55% under standard laboratory conditions with a 12 h dark-light cycle. They had ad libitum access to food and water.
The animals were randomly divided into five equal groups (n=6 group), one group was considered as control and the rest as treatments. The control group received normal saline (200 µL) and the first, second, and third treatments received an intraperitoneal injection of menthol dissolved in DMSO at the doses of 100, 200 and 400 mg/kg (M100, M200, and M400. 200 µL), respectively. The fourth group was intraperitoneally injected with 200 µL of DMSO. Doses of menthol were used according to the pilot research work and because doses above 600 mg/kg caused the killing of the studied animals, in the present study, lower doses were used and doses of 100, 200, and 400 mg/kg were selected.
Chemicals
The flowing substances were used, diazepam (diazepam chemidarou 10 mg/2ml amp, it was purchased from the pharmacy of Tabriz University of Medical Sciences, Tabriz City, Iran), ketamine (Alfasan Inc., Utrecht, Holland), Zylazine (Alfasan, Woerden-Holland), DMSO (liquid concentrate 99%), menthol (Porsina Pharmacy, Tehran City, Iran) and PTZ (was prepared from Sigma Aldrich and was dissolved in saline).
Electrophysiology
Then, rats were anesthetized by IP injection of a combination of ketamine (Alfasan, Netherland) (80 mg/kg) and xylazine (Alfasan, Netherland) (5 mg/kg) (Panahi et al., 2020). Next, a stereotaxic device was used to keep their heads in a fixed position and, according to the atlas of Paxinos & Watson (2006), a specific location (AP=-0.27, ML=-0.14, DV=-0.3) was selected for stereotaxic surgery to access the stratum radiatum of the hippocampal carbonic anhydrase 1 (CA1) region. According to our previous study (Panahi et al., 2017) due to the structure and neural circuit of the hippocampus and the high density of pyramidal cells in the striatum layer of CA1, the intensity of the impulse recorded (field action potentials) in this part can be at its highest and best, which is the reason for placing the recording electrode in this part. Finally, using a dental drill, a hole was drilled in the position to implant a tungsten dipole recording electrode and record extracellular field potentials.
In the control group, baseline field potentials were recorded for 10 minutes, and afterward, normal saline (at the same volume as menthol) was intraperitoneally injected. PTZ (80 mg/kg) was injected 30 minutes later. To suppress PTZ-induced seizures, diazepam at the dose of 10 mg/kg (Wu & Wang, 2018) was intraperitoneally injected after 10 minutes.
In the treatment groups, after recording the baseline field potentials, menthol was intraperitoneally injected at doses of 100, 200, and 400 mg/kg. Its action on the baseline activity was monitored for 30 minutes and then PTZ (80 mg/kg) was intraperitoneally injected to induce the experimental epileptiform activity. Considering that epileptic activities were performed on live animals, diazepam (10 mg/kg, IP) (A routine drug for the treatment of seizure symptoms) was used to suppress these activities at the end of the procedure.
Experimental design
1) Anesthetizing animals; 2) Placement in a stereotaxic device; 3)Insert the recording electrode into the hippocampus; 4) Basic record for 10 minutes; 5) Menthol injection (normal saline in the control group); 6) After 30 minutes, injection of pentylenetetrazol; 7) After 10 minutes, injection of diazepam.
Statistical analysis
The packaged SPSS software, version 22 was used for statistical analysis. To compare differences between groups, the one-way analysis of variance (ANOVA) and Tukey’s post-hoc test were performed. Using the repeated measures analysis of variance (rANOVA) and Tukey’s post-hoc test, differences in each group were explored. A P<0.05 was considered statistically significant.
3. Results
DMSO induced a proconvulsant effect on seizure activity and significantly (P<0.001) increased spike counts. M100 also induced stimulatory effects on the seizure activity and increased spike counts (P<0.001). Results related to M200 and M400 showed an inhibitory effect on seizure activity and decreased spike counts (P<0.05). As presented in Figures 1 and 2 following the PTZ injection, the number of field action potentials was significantly increased (P<0.001) in the DMSO group compared to the control, indicating its proconvulsant effects on PTZ-induced seizure activity.
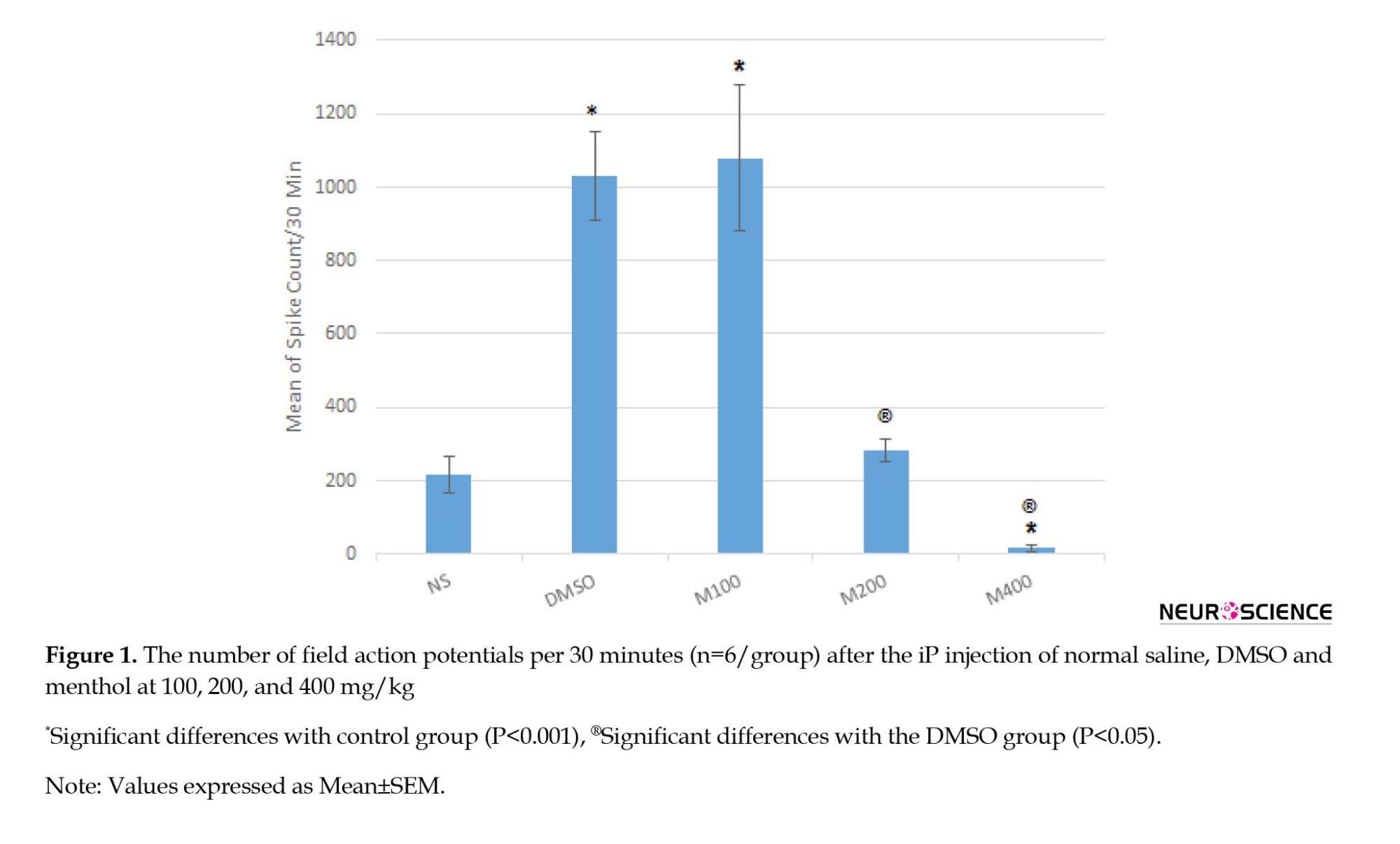
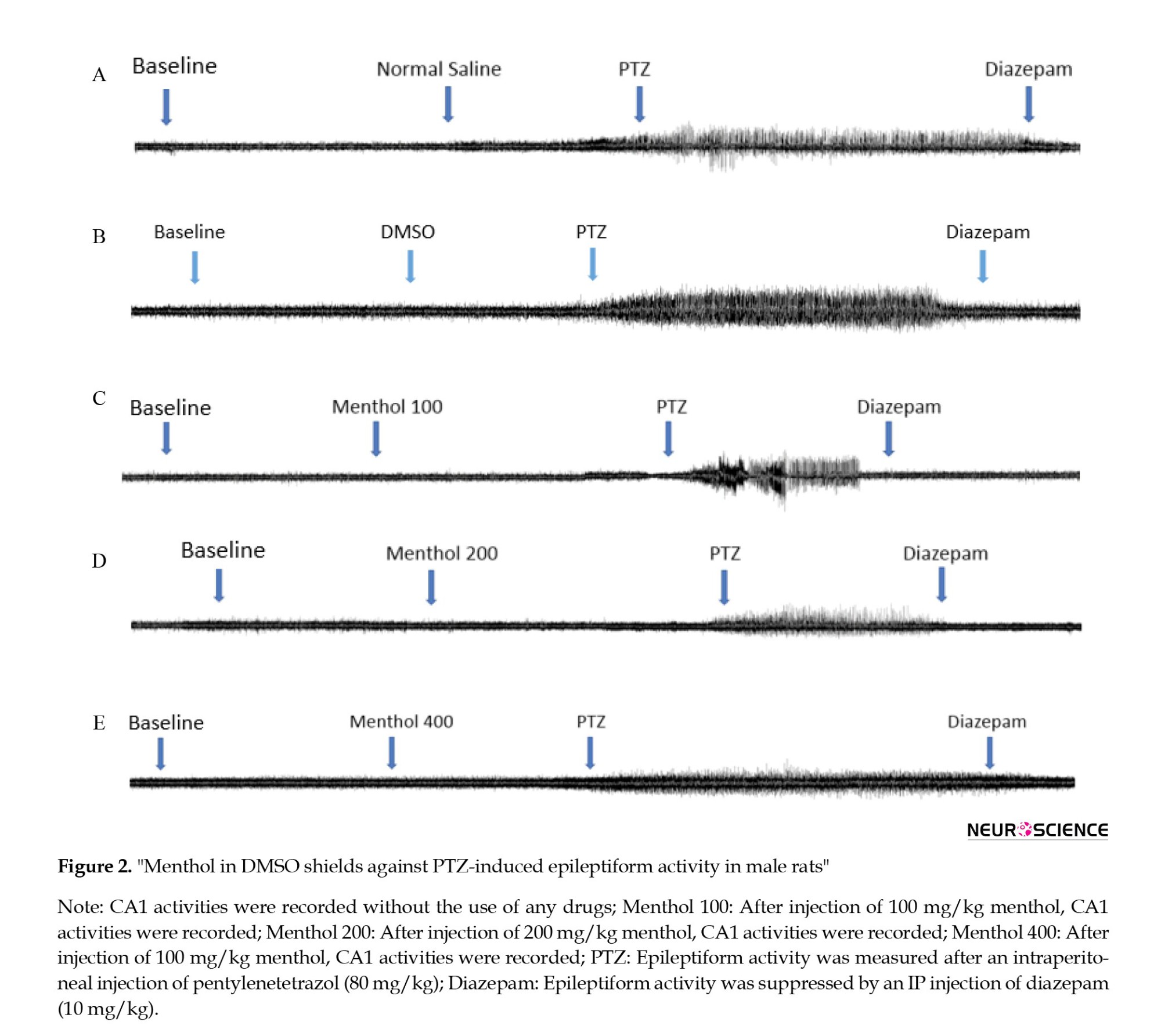
The spike count was also significantly higher in the M100 group than the control (Figures 2a, 2b, 2c and 2d) suggesting that menthol at the lower dose cannot inhibit the proconvulsant effects of the solvent on PTZ-induced seizure activity. However, the middle dose (200 mg/kg) can inhibit the proconvulsant effects of DMSO because no significant difference was observed between the M200 group and the control on spike counts (Figures 2a, 2b, 2c and 2d). On the other side, the PTZ-induced seizure activity was significantly reduced in the M400 group (Figure 2a and 2e) and the proconvulsant effects of DMSO were also suppressed. Therefore, menthol exerts protective effects in a dose-dependent manner on the PTZ-induced seizure activity, which is potentiated by DMSO.
4. Discussion
The results of the present study revealed that DMSO at the absolute concentration (100%) similar to the study conducted by Panahi et al., (2020) and Kumari et al. (2018) exacerbated the PTZ-induced seizure activity but menthol alleviated it in a dose-dependent manner. As mentioned, we aimed to explore the protective effects of menthol on the PTZ-induced seizure activity; therefore we used DMSO as a solvent for menthol regardless of its controversial effects on the CNS. It was essential to select a dose of DMSO at which menthol can be easily dissolved and not cause any problem in the biological system, therefore we did not adjust the dose for which DMSO had no antiepileptic effect. Therefore, we used DMSO at a concentration of 100% and observed that it had proconvulsant effects on adult male rats evidenced by a significant increase in the number of spikes per unit of time. Nevertheless, using different concentrations of DMSO, other researchers, such as Panahi et al., (2020) have demonstrated its dose-dependent anticonvulsant or proconvulsant effects. With this aspect, DMSO has been claimed to have antiepileptic effects at moderate and high doses. On the contrary, a study showed that moderate and high doses of DMSO produced proconvulsant effects (Kovács et al., 2011). To some extent, this is consistent with the results of the present study in which DMSO was used at a concentration of 100% and increased the PTZ-induced seizure activity in adult male rats. Meanwhile, it has been reported that DMSO can injure nerves and induce histopathological changes. For example, DMSO caused neuropathologic damage and exacerbated soman-induced seizures when it was used as a solvent and vehicle (0.5-1 mL/kg). On the other side, some studies have reported the protective effects of DMSO on the CNS. For instance, in a study conducted by Noel et al, DMSO at 9 mL/kg/day demonstrated no toxic signs (Noel et al., 1975). It has also been indicated that DMSO at doses of 1-6 mL/kg possesses antioxidant and free radical scavenging properties in rats (Wang et al., 2000). Moreover, the nontoxicity of DMSO in the nervous system has been reported in previous studies (Bodjarian et al., 1995; Maooz et al., 2020).
Therefore, despite its controversial actions in the CNS, we used DMSO as a solvent in our study, because we focused on the investigation of the antiepileptic properties of menthol and the effects of DMSO on the CNS were not crucial to us. Nevertheless, proconvulsant effects of DMSO and the suppression of the DMSO-augmented seizure activity by menthol suggest the protective effects of menthol on the PTZ-induced seizure activity.
Evidence shows that the administration of menthol produces different behavioral effects in the CNS on animal models and some of them are GABAA-receptor-mediated effects (Tani et al., 2010); although this receptor is a target for many general anesthetics and analgesics (Hemmings et al., 2005). The enhancement of the GABAA-receptor-mediated inhibition plays a central role in suppressing neuronal excitability by anesthetics (Bieda & MacIver, 2004). The enhancing effect of menthol on cultured hippocampal neurons occurs only when the GABA concentration is 3 µM, implying that menthol preferentially acts on GABAA receptors. However, these results demonstrate that menthol is a selective enhancer of the tonic GABAergic inhibition in CA1 pyramidal neurons of the hippocampus (Zhang et al., 2008). Recent studies show that extrasynaptic GABAA receptors are optimally activated by GABA in physiological conditions and an additional increase in the GABA concentration significantly suppresses neuronal excitability (Bieda & MacIver, 2004). Hence, the enhancement of the function of activated GABAA receptors may be a therapeutic approach to diseases, such as epilepsy in which neuronal excitability is increased.
Different animal models of epilepsy exist to assess the effects of anticonvulsant drugs and PTZ, a GABA receptor antagonist, is one of them (Lau et al., 2014). We used the PTZ-induced seizure model to explore the in vivo action of menthol and determine whether menthol can suppress the epileptic activity induced by inhibiting GABA. According to the previous study, the systemic administration of menthol can induce anticonvulsant effects and suppress the prolongation of the seizure latency induced by PTZ (Zhang et al., 2008); however, in the present study, no significant difference was observed between the menthol-treated groups and the control group in terms of the onset of the seizure activity. Meanwhile, the studies conducted by (Zhang et al., 2008) indicated that the IP administration of menthol had ambulation-promoting effects on house mice, implying that it could enter the brain and reach an influential concentration (Haeseler et al., 2002). Therefore, considering that menthol can penetrate the blood-brain barrier, it can be used for the treatment of epilepsy. As a suitable solvent, menthol may contribute to the development of new drugs effective in treating epilepsy; although it has been demonstrated that its administration can be potentially beneficial for lowering the neural network hyperactivity in pathological and normal conditions. Menthol has been shown to raise the after-discharge threshold, and after-discharge duration, and reduce the susceptibility of rats to hippocampal seizures. These results and the anticonvulsant action of menthol on the PTZ model highlight the role of the menthol-mediated enhancement of tonic inhibition in preventing epileptic activity (Zhang et al., 2008).
Evidence shows that extrasynaptic GABAA receptors have subunit compositions that differ from those of synaptic receptors. Menthol has been indicated to selectively enhance the tonic inhibition of hippocampal pyramidal neurons. Hence, the suppression of the epileptic activity and neuronal hyperexcitability via the selective enhancement of the tonic inhibition in pyramidal neurons emphasizes the prominent role of tonic inhibition in controlling network excitability, including physiological oscillations and the pathological propagation of the epileptic activity. Nevertheless, it is unclear which receptor (tonic or phasic) is activated by menthol (Zhang et al., 2008). In this regard, a study explored the effects of menthol on GABA receptors compared to benzodiazepines and intravenous anesthetics, such as steroids, barbiturates, etomidate, and propofol. According to the results of this study, menthol affects GABA receptors through sites distinct from steroids, benzodiazepines, and barbiturates. Considering structural affinities between propofol and menthol, these results are expected. At clinically relevant low concentrations, propofol selectively enhances GABA-activated tonic currents in hippocampal neurons (Bhadania et al., 2012), confirming that menthol and propofol may modulate GABA receptors with a similar mechanism (Zhang et al., 2008). Therefore, menthol selectively enhances the inhibition mediated by high-affinity GABA receptors in hippocampal CA1 pyramidal neurons of rats, resulting in the inhibition of in vitro and in vivo neuronal excitability (Zhang et al., 2008); however, menthol has been proven to activate GABA-medicated tonic and phasic currents in vitro. The action of menthol on tonic currents seems to be mediated by δ subunit-lacking extrasynaptic GABAA receptors (Lau et al., 2014). Therefore, further studies are needed to confirm the precise mechanism of menthol action.
5. Conclusion
Menthol, as a natural compound, has inhibitory effects on PTZ-induced seizure activity in a dose-dependent manner. Therefore, it may be used as a prophylactic agent and an adjuvant to treat epileptic patients or those disposed to epilepsy. However, further mechanistic studies should be conducted to elucidate the precise mechanism of action of menthol on the seizure activity. Such determinations should be covered in future research.
Ethical Considerations
Compliance with ethical guidelines
According to the Helsinki Declaration, researchers of the present study considered all ethical considerations regarding the safe handling, restraint of laboratory animals, and the application of suitable anesthesia to minimize animal pain.
Funding
This study was performed as a research project in the Department of Basic Science, Faculty of Veterinary Medicine, University of Tabriz.
Authors' contributions
Conceptualization, software, validation, formal analysis, resources, data curation, writing, visualization, supervision, project administration, and funding acquisition: Yousef Panahi; Investigation: Mohammad Amin Monazzah and Gholamreza Vafaei Saiah.
Conflict of interest
The authors declared no conflict of interest.
References
Ashoor, A., Nordman, J. C., Veltri, D., Yang, K. H., Shuba, Y., & Al Kury, L., et al. (2013). Menthol inhibits 5-HT3 receptor-mediated currents. The Journal of Pharmacology and Experimental Therapeutics, 347(2), 398–409. [DOI:10.1124/jpet.113.203976] [PMID]
Awan, M., Buriak, I., Fleck, R., Fuller, B., Goltsev, A., & Kerby, J., et al. (2020). Dimethyl sulfoxide: A central player since the dawn of cryobiology, is efficacy balanced by toxicity?. Regenerative Medicine, 15(3), 1463–1491.[DOI:10.2217/rme-2019-0145] [PMID]
Bhadania, M., Joshi, H., Patel, P., & Kulkarni, V. H. (2012). Protective effect of menthol on β-amyloid peptide induced cognitive deficits in mice. European Journal of Pharmacology, 681(1-3), 50–54. [DOI:10.1016/j.ejphar.2012.01.035] [PMID]
Bieda, M. C., & MacIver, M. B. (2004). Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. Journal of Neurophysiology, 92(3), 1658–1667. [DOI:10.1152/jn.00223.2004] [PMID]
Bodjarian, N., Carpentier, P., Baubichon, D., Blanchet, G., & Lallement, G. (1995). Involvement of non-muscarinic receptors in phosphoinositide signalling during soman-induced seizures. European Journal of Pharmacology, 289(2), 291–297. [DOI:10.1016/0922-4106(95)90106-X] [PMID]
Carletti, F., Ferraro, G., Rizzo, V., Cannizzaro, C., & Sardo, P. (2013). Antiepileptic effect of dimethyl sulfoxide in a rat model of temporal lobe epilepsy. Neuroscience Letters, 546, 31–35.[DOI:10.1016/j.neulet.2013.04.031] [PMID]
Eccles R. (1994). Menthol and related cooling compounds. The Journal of Pharmacy and Pharmacology, 46(8), 618–630.[DOI:10.1111/j.2042-7158.1994.tb03871.x] [PMID]
Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., Bartolini, A., & Ghelardini, C. (2002). Menthol: A natural analgesic compound. Neuroscience Letters, 322(3), 145–148. [DOI:10.1016/S0304-3940(01)02527-7] [PMID]
Haeseler, G., Maue, D., Grosskreutz, J., Bufler, J., Nentwig, B., & Piepenbrock, S., et al. (2002). Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. European Journal of Anaesthesiology, 19(8), 571–579. [DOI:10.1017/S0265021502000923] [PMID]
Hall, A. C., Turcotte, C. M., Betts, B. A., Yeung, W. Y., Agyeman, A. S., & Burk, L. A. (2004). Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. European Journal of Pharmacology, 506(1), 9–16. [DOI:10.1016/0014-2999(91)90630-9] [PMID]
Hemmings, H. C., Jr, Akabas, M. H., Goldstein, P. A., Trudell, J. R., Orser, B. A., & Harrison, N. L. (2005). Emerging molecular mechanisms of general anesthetic action. Trends in Pharmacological Sciences, 26(10), 503–510. [DOI:10.1016/j.tips.2005.08.006] [PMID]
Kovács, Z., Czurkó, A., Kékesi, K. A., & Juhász, G. (2011). The effect of intraperitoneally administered dimethyl sulfoxide on absence-like epileptic activity of freely moving WAG/Rij rats. Journal of Neuroscience Methods, 197(1), 133–136. [DOI:10.1016/j.jneumeth.2011.02.005] [PMID]
Kumari, P., Singh, N., & Saha, L. (2018). Potentiation of pentylenetetrazole-induced neuronal damage by dimethyl sulfoxide in chemical kindling model in rats. Indian Journal of Pharmacology, 50(2), 84–87. [DOI:10.4103/ijp.IJP_559_17] [PMID] [PMCID]
Lau, B. K., Karim, S., Goodchild, A. K., Vaughan, C. W., & Drew, G. M. (2014). Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. British Journal of Pharmacology, 171(11), 2803–2813. [DOI:10.1111/bph.12602] [PMID]
McKenzie S. (2018). Inhibition shapes the organization of hippocampal representations. Hippocampus, 28(9), 659–671.[DOI:10.1002/hipo.22803] [PMID] [PMCID]
Noel, P. R., Barnett, K. C., Davies, R. E., Jolly, D. W., Leahy, J. S., & Mawdesley-Thomas, L. E., et al. (1975). The toxicity of dimethyl sulphoxide (DMSO) for the dog, pig, rat and rabbit. Toxicology, 3(2), 143–169. [DOI:10.1016/0300-483X(75)90081-5] [PMID]
Pan, R., Tian, Y., Gao, R., Li, H., Zhao, X., & Barrett, J. E., et al. (2012). Central mechanisms of menthol-induced analgesia. The Journal of Pharmacology and Experimental Therapeutics, 343(3), 661–672. [DOI:10.1124/jpet.112.196717] [PMID]
Panahi, Y., Monnazah M. A., & Vafaei, G. (2020). [Study of the use of dimethyl sulfoxide (DMSO) as a solvent in the administration of antiepileptic drugs (Persian)]. Studies in Medical Sciences, 31(4), 316-324. [Link]
Panahi, Y., Saboory, E., Rassouli, A., Sadeghi-Hashjin, G., Roshan-Milani, S., & Derafshpour, L., et al. (2017). The effect of selective opioid receptor agonists and antagonists on epileptiform activity in morphine-dependent infant mice hippocampal slices. International Journal of Developmental Neuroscience, 60, 56–62. [DOI:10.1016/j.ijdevneu.2017.04.003] [PMID]
Paxinos, G., & Watson, C. (2006). The rat brain in stereotaxic coordinates: Hard cover edition. Amsterdam: Elsevier. [Link]
Cheang, W. S., Lam, M. Y., Wong, W. T., Tian, X. Y., Lau, C. W., & Zhu, Z., et al. (2013). Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. European Journal of Pharmacology, 702(1-3), 79–84. [DOI:10.1016/j.ejphar.2013.01.028] [PMID]
Santos, N. C., Figueira-Coelho, J., Martins-Silva, J., & Saldanha, C. (2003). Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochemical Pharmacology, 65(7), 1035–1041. [DOI:10.1016/S0006-2952(03)00002-9] [PMID]
Sardo, P., Carletti, F., D'Agostino, S., Rizzo, V., & Ferraro, G. (2006). Involvement of nitric oxide-soluble guanylyl cyclase pathway in the control of maximal dentate gyrus activation in the rat. Journal of Neural Transmission, 113(12), 1855–1861. [DOI:10.1007/s00702-006-0491-9] [PMID]
Su, L., Wang, C., Yu, Y. H., Ren, Y. Y., Xie, K. L., & Wang, G. L. (2011). Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neuroscience, 12, 120. [DOI:10.1186/1471-2202-12-120] [PMID] [PMCID]
Tani, M., Onimaru, H., Ikeda, K., Kawakami, K., & Homma, I. (2010). Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport, 21(17), 1095–1099. [DOI:10.1097/WNR.0b013e3283405bad] [PMID]
Taniguchi, Y., Deguchi, Y., Saita, M., & Noda, K. (1994). [Antinociceptive effects of counterirritants (Japanese)]. Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica, 104(6), 433–446. [DOI:10.1254/fpj.104.433] [PMID]
Umezu, T., Sakata, A., & Ito, H. (2001). Ambulation-promoting effect of peppermint oil and identification of its active constituents. Pharmacology Biochemistry and Behavior, 69(3-4), 383-90. [DOI:10.1016/S0091-3057(01)00543-3]
Wang, H., Ma, L., Li, Y., & Cho, C. H. (2000). Exposure to cigarette smoke increases apoptosis in the rat gastric mucosa through a reactive oxygen species-mediated and p53-independent pathway. Free Radical Biology & Medicine, 28(7), 1125–1131. [DOI:10.1016/S0891-5849(00)00207-0] [PMID]
Watt, E. E., Betts, B. A., Kotey, F. O., Humbert, D. J., Griffith, T. N., & Kelly, E. W., et al. (2008). Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. European Journal of Pharmacology, 590(1-3), 120–126. [DOI:10.1016/j.ejphar.2008.06.003] [PMID]
Wu, Q., & Wang, H. (2018). The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Research, 148, 8–16. [DOI:10.1016/j.eplepsyres.2018.10.002] [PMID]
Zhang, X. B., Jiang, P., Gong, N., Hu, X. L., Fei, D., & Xiong, Z. Q., et al. (2008). A-type GABA receptor as a central target of TRPM8 agonist menthol. Plos One, 3(10), e3386. [DOI:10.1371/journal.pone.0003386] [PMID] [PMCID]
Menthol is fragrant monoterpenoid alcohol obtained from peppermint (Mentha piperita L.) oil. For centuries, it has been used as a food additive, local anesthetic, topical analgesic, antipruritic agent, and gastric sedative (Galeotti et al., 2002). Among the different optical isomers, (-)-menthol predominates the others in nature. Due to its peculiar flavor and fragrance, menthol is widely used in cosmetics (Eccles, 1994). Additionally, it is one of the ingredients of antiseptic and cooling formulations in pharmaceutical dosage forms. This optical isomer can also be found in eutectic formulations of local anesthetic agents. Menthol affects both the peripheral nervous system and the central nervous system (CNS) because its intra-cerebro-ventricular and systemic administration may produce sedation or anesthesia (Watt & Betts, 2008), anticonvulsant effects (Zhang et al., 2008), analgesic effects (Su et al., 2011), improvement in learning (McKenzie, 2018), and cognitive functions (Bhadania et al., 2012). Cellular targets of menthol in the CNS include voltage-gated sodium channels (Pan et al., 2012), voltage-gated calcium channels (San Cheang et al., 2013), serotonin receptors, nicotinic acetylcholine receptors (Ashoor et al., 2013), glycine receptors (Hall et al., 2013), and gamma-aminobutyric acid (GABA) receptors. Although behavioral studies have claimed that menthol can influence CNS (Umezu et al., 2001), no definite target has been determined for menthol in the CNS, yet. Nevertheless, menthol has been shown to inhibit hippocampal neurons by increasing the tone of the GABAA nervous system (Taniguchi et al., 1994). Dimethyl sulfoxide (DMSO) was used as the solvent of menthol in this study because most drugs tested for their antiepileptic effects on animal models are dissolved in DMSO, which is an amphipathic molecule. Due to its physicochemical properties, DMSO is regarded as an extremely efficient solvent for water-insoluble compounds (Santos et al., 2003). Several biological functions have been attributed to DMSO in different CNS models (Sardo et al., 2006). For instance, data from initial in vivo studies have shown the antiepileptic effect of DMSO on an animal model of temporal lobe epilepsy and different studies have demonstrated its dose-dependent anticonvulsant and proconvulsant effects. DMSO has also been reported to have antiepileptic effects at moderate and high doses (Carletti et al., 2013). However, a study showed that the moderate and high doses of DMSO produced proconvulsant effects (Kovács et al., 2011). Given the results of the involvement of DMSO in seizure-induced neuropathology, its widespread use as a solvent and vehicle for effective agents in mammalian systems cannot be easily justified (Noel et al., 1975). According to our previous study (Panahi et al., 2020), we observed that the effects of DMSO in seizure activities are biphasic, i.e. it had protective effects at a dose of 10% and stimulant effects at doses of 50% and above. Therefore, in this study, we used 100% of it as a solvent to make sure that it has no protective effects on seizure activity, and if an effect is observed, we can easily attribute it to menthol. Considering that, we utilized DMSO as the solvent despite its controversial effects on the CNS. Therefore, the present study was designed to investigate the possible protective effects of different concentrations of menthol on pentylenetetrazol (PTZ)-induced seizures in adult male rats.
2. Materials and Methods
Animals
In the present study, 30 male Wistar rats weighing 200-250 g were purchased from the Laboratory Animal Breeding Center in the Faculty of Pharmacy at Urmia University of Medical Sciences. The animals were acclimatized to their new surroundings for a week before the experiment. The rats were housed in special cages at a temperature of 22°C and humidity of 45%-55% under standard laboratory conditions with a 12 h dark-light cycle. They had ad libitum access to food and water.
The animals were randomly divided into five equal groups (n=6 group), one group was considered as control and the rest as treatments. The control group received normal saline (200 µL) and the first, second, and third treatments received an intraperitoneal injection of menthol dissolved in DMSO at the doses of 100, 200 and 400 mg/kg (M100, M200, and M400. 200 µL), respectively. The fourth group was intraperitoneally injected with 200 µL of DMSO. Doses of menthol were used according to the pilot research work and because doses above 600 mg/kg caused the killing of the studied animals, in the present study, lower doses were used and doses of 100, 200, and 400 mg/kg were selected.
Chemicals
The flowing substances were used, diazepam (diazepam chemidarou 10 mg/2ml amp, it was purchased from the pharmacy of Tabriz University of Medical Sciences, Tabriz City, Iran), ketamine (Alfasan Inc., Utrecht, Holland), Zylazine (Alfasan, Woerden-Holland), DMSO (liquid concentrate 99%), menthol (Porsina Pharmacy, Tehran City, Iran) and PTZ (was prepared from Sigma Aldrich and was dissolved in saline).
Electrophysiology
Then, rats were anesthetized by IP injection of a combination of ketamine (Alfasan, Netherland) (80 mg/kg) and xylazine (Alfasan, Netherland) (5 mg/kg) (Panahi et al., 2020). Next, a stereotaxic device was used to keep their heads in a fixed position and, according to the atlas of Paxinos & Watson (2006), a specific location (AP=-0.27, ML=-0.14, DV=-0.3) was selected for stereotaxic surgery to access the stratum radiatum of the hippocampal carbonic anhydrase 1 (CA1) region. According to our previous study (Panahi et al., 2017) due to the structure and neural circuit of the hippocampus and the high density of pyramidal cells in the striatum layer of CA1, the intensity of the impulse recorded (field action potentials) in this part can be at its highest and best, which is the reason for placing the recording electrode in this part. Finally, using a dental drill, a hole was drilled in the position to implant a tungsten dipole recording electrode and record extracellular field potentials.
In the control group, baseline field potentials were recorded for 10 minutes, and afterward, normal saline (at the same volume as menthol) was intraperitoneally injected. PTZ (80 mg/kg) was injected 30 minutes later. To suppress PTZ-induced seizures, diazepam at the dose of 10 mg/kg (Wu & Wang, 2018) was intraperitoneally injected after 10 minutes.
In the treatment groups, after recording the baseline field potentials, menthol was intraperitoneally injected at doses of 100, 200, and 400 mg/kg. Its action on the baseline activity was monitored for 30 minutes and then PTZ (80 mg/kg) was intraperitoneally injected to induce the experimental epileptiform activity. Considering that epileptic activities were performed on live animals, diazepam (10 mg/kg, IP) (A routine drug for the treatment of seizure symptoms) was used to suppress these activities at the end of the procedure.
Experimental design
1) Anesthetizing animals; 2) Placement in a stereotaxic device; 3)Insert the recording electrode into the hippocampus; 4) Basic record for 10 minutes; 5) Menthol injection (normal saline in the control group); 6) After 30 minutes, injection of pentylenetetrazol; 7) After 10 minutes, injection of diazepam.
Statistical analysis
The packaged SPSS software, version 22 was used for statistical analysis. To compare differences between groups, the one-way analysis of variance (ANOVA) and Tukey’s post-hoc test were performed. Using the repeated measures analysis of variance (rANOVA) and Tukey’s post-hoc test, differences in each group were explored. A P<0.05 was considered statistically significant.
3. Results
DMSO induced a proconvulsant effect on seizure activity and significantly (P<0.001) increased spike counts. M100 also induced stimulatory effects on the seizure activity and increased spike counts (P<0.001). Results related to M200 and M400 showed an inhibitory effect on seizure activity and decreased spike counts (P<0.05). As presented in Figures 1 and 2 following the PTZ injection, the number of field action potentials was significantly increased (P<0.001) in the DMSO group compared to the control, indicating its proconvulsant effects on PTZ-induced seizure activity.

The spike count was also significantly higher in the M100 group than the control (Figures 2a, 2b, 2c and 2d) suggesting that menthol at the lower dose cannot inhibit the proconvulsant effects of the solvent on PTZ-induced seizure activity. However, the middle dose (200 mg/kg) can inhibit the proconvulsant effects of DMSO because no significant difference was observed between the M200 group and the control on spike counts (Figures 2a, 2b, 2c and 2d). On the other side, the PTZ-induced seizure activity was significantly reduced in the M400 group (Figure 2a and 2e) and the proconvulsant effects of DMSO were also suppressed. Therefore, menthol exerts protective effects in a dose-dependent manner on the PTZ-induced seizure activity, which is potentiated by DMSO.

4. Discussion
The results of the present study revealed that DMSO at the absolute concentration (100%) similar to the study conducted by Panahi et al., (2020) and Kumari et al. (2018) exacerbated the PTZ-induced seizure activity but menthol alleviated it in a dose-dependent manner. As mentioned, we aimed to explore the protective effects of menthol on the PTZ-induced seizure activity; therefore we used DMSO as a solvent for menthol regardless of its controversial effects on the CNS. It was essential to select a dose of DMSO at which menthol can be easily dissolved and not cause any problem in the biological system, therefore we did not adjust the dose for which DMSO had no antiepileptic effect. Therefore, we used DMSO at a concentration of 100% and observed that it had proconvulsant effects on adult male rats evidenced by a significant increase in the number of spikes per unit of time. Nevertheless, using different concentrations of DMSO, other researchers, such as Panahi et al., (2020) have demonstrated its dose-dependent anticonvulsant or proconvulsant effects. With this aspect, DMSO has been claimed to have antiepileptic effects at moderate and high doses. On the contrary, a study showed that moderate and high doses of DMSO produced proconvulsant effects (Kovács et al., 2011). To some extent, this is consistent with the results of the present study in which DMSO was used at a concentration of 100% and increased the PTZ-induced seizure activity in adult male rats. Meanwhile, it has been reported that DMSO can injure nerves and induce histopathological changes. For example, DMSO caused neuropathologic damage and exacerbated soman-induced seizures when it was used as a solvent and vehicle (0.5-1 mL/kg). On the other side, some studies have reported the protective effects of DMSO on the CNS. For instance, in a study conducted by Noel et al, DMSO at 9 mL/kg/day demonstrated no toxic signs (Noel et al., 1975). It has also been indicated that DMSO at doses of 1-6 mL/kg possesses antioxidant and free radical scavenging properties in rats (Wang et al., 2000). Moreover, the nontoxicity of DMSO in the nervous system has been reported in previous studies (Bodjarian et al., 1995; Maooz et al., 2020).
Therefore, despite its controversial actions in the CNS, we used DMSO as a solvent in our study, because we focused on the investigation of the antiepileptic properties of menthol and the effects of DMSO on the CNS were not crucial to us. Nevertheless, proconvulsant effects of DMSO and the suppression of the DMSO-augmented seizure activity by menthol suggest the protective effects of menthol on the PTZ-induced seizure activity.
Evidence shows that the administration of menthol produces different behavioral effects in the CNS on animal models and some of them are GABAA-receptor-mediated effects (Tani et al., 2010); although this receptor is a target for many general anesthetics and analgesics (Hemmings et al., 2005). The enhancement of the GABAA-receptor-mediated inhibition plays a central role in suppressing neuronal excitability by anesthetics (Bieda & MacIver, 2004). The enhancing effect of menthol on cultured hippocampal neurons occurs only when the GABA concentration is 3 µM, implying that menthol preferentially acts on GABAA receptors. However, these results demonstrate that menthol is a selective enhancer of the tonic GABAergic inhibition in CA1 pyramidal neurons of the hippocampus (Zhang et al., 2008). Recent studies show that extrasynaptic GABAA receptors are optimally activated by GABA in physiological conditions and an additional increase in the GABA concentration significantly suppresses neuronal excitability (Bieda & MacIver, 2004). Hence, the enhancement of the function of activated GABAA receptors may be a therapeutic approach to diseases, such as epilepsy in which neuronal excitability is increased.
Different animal models of epilepsy exist to assess the effects of anticonvulsant drugs and PTZ, a GABA receptor antagonist, is one of them (Lau et al., 2014). We used the PTZ-induced seizure model to explore the in vivo action of menthol and determine whether menthol can suppress the epileptic activity induced by inhibiting GABA. According to the previous study, the systemic administration of menthol can induce anticonvulsant effects and suppress the prolongation of the seizure latency induced by PTZ (Zhang et al., 2008); however, in the present study, no significant difference was observed between the menthol-treated groups and the control group in terms of the onset of the seizure activity. Meanwhile, the studies conducted by (Zhang et al., 2008) indicated that the IP administration of menthol had ambulation-promoting effects on house mice, implying that it could enter the brain and reach an influential concentration (Haeseler et al., 2002). Therefore, considering that menthol can penetrate the blood-brain barrier, it can be used for the treatment of epilepsy. As a suitable solvent, menthol may contribute to the development of new drugs effective in treating epilepsy; although it has been demonstrated that its administration can be potentially beneficial for lowering the neural network hyperactivity in pathological and normal conditions. Menthol has been shown to raise the after-discharge threshold, and after-discharge duration, and reduce the susceptibility of rats to hippocampal seizures. These results and the anticonvulsant action of menthol on the PTZ model highlight the role of the menthol-mediated enhancement of tonic inhibition in preventing epileptic activity (Zhang et al., 2008).
Evidence shows that extrasynaptic GABAA receptors have subunit compositions that differ from those of synaptic receptors. Menthol has been indicated to selectively enhance the tonic inhibition of hippocampal pyramidal neurons. Hence, the suppression of the epileptic activity and neuronal hyperexcitability via the selective enhancement of the tonic inhibition in pyramidal neurons emphasizes the prominent role of tonic inhibition in controlling network excitability, including physiological oscillations and the pathological propagation of the epileptic activity. Nevertheless, it is unclear which receptor (tonic or phasic) is activated by menthol (Zhang et al., 2008). In this regard, a study explored the effects of menthol on GABA receptors compared to benzodiazepines and intravenous anesthetics, such as steroids, barbiturates, etomidate, and propofol. According to the results of this study, menthol affects GABA receptors through sites distinct from steroids, benzodiazepines, and barbiturates. Considering structural affinities between propofol and menthol, these results are expected. At clinically relevant low concentrations, propofol selectively enhances GABA-activated tonic currents in hippocampal neurons (Bhadania et al., 2012), confirming that menthol and propofol may modulate GABA receptors with a similar mechanism (Zhang et al., 2008). Therefore, menthol selectively enhances the inhibition mediated by high-affinity GABA receptors in hippocampal CA1 pyramidal neurons of rats, resulting in the inhibition of in vitro and in vivo neuronal excitability (Zhang et al., 2008); however, menthol has been proven to activate GABA-medicated tonic and phasic currents in vitro. The action of menthol on tonic currents seems to be mediated by δ subunit-lacking extrasynaptic GABAA receptors (Lau et al., 2014). Therefore, further studies are needed to confirm the precise mechanism of menthol action.
5. Conclusion
Menthol, as a natural compound, has inhibitory effects on PTZ-induced seizure activity in a dose-dependent manner. Therefore, it may be used as a prophylactic agent and an adjuvant to treat epileptic patients or those disposed to epilepsy. However, further mechanistic studies should be conducted to elucidate the precise mechanism of action of menthol on the seizure activity. Such determinations should be covered in future research.
Ethical Considerations
Compliance with ethical guidelines
According to the Helsinki Declaration, researchers of the present study considered all ethical considerations regarding the safe handling, restraint of laboratory animals, and the application of suitable anesthesia to minimize animal pain.
Funding
This study was performed as a research project in the Department of Basic Science, Faculty of Veterinary Medicine, University of Tabriz.
Authors' contributions
Conceptualization, software, validation, formal analysis, resources, data curation, writing, visualization, supervision, project administration, and funding acquisition: Yousef Panahi; Investigation: Mohammad Amin Monazzah and Gholamreza Vafaei Saiah.
Conflict of interest
The authors declared no conflict of interest.
References
Ashoor, A., Nordman, J. C., Veltri, D., Yang, K. H., Shuba, Y., & Al Kury, L., et al. (2013). Menthol inhibits 5-HT3 receptor-mediated currents. The Journal of Pharmacology and Experimental Therapeutics, 347(2), 398–409. [DOI:10.1124/jpet.113.203976] [PMID]
Awan, M., Buriak, I., Fleck, R., Fuller, B., Goltsev, A., & Kerby, J., et al. (2020). Dimethyl sulfoxide: A central player since the dawn of cryobiology, is efficacy balanced by toxicity?. Regenerative Medicine, 15(3), 1463–1491.[DOI:10.2217/rme-2019-0145] [PMID]
Bhadania, M., Joshi, H., Patel, P., & Kulkarni, V. H. (2012). Protective effect of menthol on β-amyloid peptide induced cognitive deficits in mice. European Journal of Pharmacology, 681(1-3), 50–54. [DOI:10.1016/j.ejphar.2012.01.035] [PMID]
Bieda, M. C., & MacIver, M. B. (2004). Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. Journal of Neurophysiology, 92(3), 1658–1667. [DOI:10.1152/jn.00223.2004] [PMID]
Bodjarian, N., Carpentier, P., Baubichon, D., Blanchet, G., & Lallement, G. (1995). Involvement of non-muscarinic receptors in phosphoinositide signalling during soman-induced seizures. European Journal of Pharmacology, 289(2), 291–297. [DOI:10.1016/0922-4106(95)90106-X] [PMID]
Carletti, F., Ferraro, G., Rizzo, V., Cannizzaro, C., & Sardo, P. (2013). Antiepileptic effect of dimethyl sulfoxide in a rat model of temporal lobe epilepsy. Neuroscience Letters, 546, 31–35.[DOI:10.1016/j.neulet.2013.04.031] [PMID]
Eccles R. (1994). Menthol and related cooling compounds. The Journal of Pharmacy and Pharmacology, 46(8), 618–630.[DOI:10.1111/j.2042-7158.1994.tb03871.x] [PMID]
Galeotti, N., Di Cesare Mannelli, L., Mazzanti, G., Bartolini, A., & Ghelardini, C. (2002). Menthol: A natural analgesic compound. Neuroscience Letters, 322(3), 145–148. [DOI:10.1016/S0304-3940(01)02527-7] [PMID]
Haeseler, G., Maue, D., Grosskreutz, J., Bufler, J., Nentwig, B., & Piepenbrock, S., et al. (2002). Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. European Journal of Anaesthesiology, 19(8), 571–579. [DOI:10.1017/S0265021502000923] [PMID]
Hall, A. C., Turcotte, C. M., Betts, B. A., Yeung, W. Y., Agyeman, A. S., & Burk, L. A. (2004). Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. European Journal of Pharmacology, 506(1), 9–16. [DOI:10.1016/0014-2999(91)90630-9] [PMID]
Hemmings, H. C., Jr, Akabas, M. H., Goldstein, P. A., Trudell, J. R., Orser, B. A., & Harrison, N. L. (2005). Emerging molecular mechanisms of general anesthetic action. Trends in Pharmacological Sciences, 26(10), 503–510. [DOI:10.1016/j.tips.2005.08.006] [PMID]
Kovács, Z., Czurkó, A., Kékesi, K. A., & Juhász, G. (2011). The effect of intraperitoneally administered dimethyl sulfoxide on absence-like epileptic activity of freely moving WAG/Rij rats. Journal of Neuroscience Methods, 197(1), 133–136. [DOI:10.1016/j.jneumeth.2011.02.005] [PMID]
Kumari, P., Singh, N., & Saha, L. (2018). Potentiation of pentylenetetrazole-induced neuronal damage by dimethyl sulfoxide in chemical kindling model in rats. Indian Journal of Pharmacology, 50(2), 84–87. [DOI:10.4103/ijp.IJP_559_17] [PMID] [PMCID]
Lau, B. K., Karim, S., Goodchild, A. K., Vaughan, C. W., & Drew, G. M. (2014). Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. British Journal of Pharmacology, 171(11), 2803–2813. [DOI:10.1111/bph.12602] [PMID]
McKenzie S. (2018). Inhibition shapes the organization of hippocampal representations. Hippocampus, 28(9), 659–671.[DOI:10.1002/hipo.22803] [PMID] [PMCID]
Noel, P. R., Barnett, K. C., Davies, R. E., Jolly, D. W., Leahy, J. S., & Mawdesley-Thomas, L. E., et al. (1975). The toxicity of dimethyl sulphoxide (DMSO) for the dog, pig, rat and rabbit. Toxicology, 3(2), 143–169. [DOI:10.1016/0300-483X(75)90081-5] [PMID]
Pan, R., Tian, Y., Gao, R., Li, H., Zhao, X., & Barrett, J. E., et al. (2012). Central mechanisms of menthol-induced analgesia. The Journal of Pharmacology and Experimental Therapeutics, 343(3), 661–672. [DOI:10.1124/jpet.112.196717] [PMID]
Panahi, Y., Monnazah M. A., & Vafaei, G. (2020). [Study of the use of dimethyl sulfoxide (DMSO) as a solvent in the administration of antiepileptic drugs (Persian)]. Studies in Medical Sciences, 31(4), 316-324. [Link]
Panahi, Y., Saboory, E., Rassouli, A., Sadeghi-Hashjin, G., Roshan-Milani, S., & Derafshpour, L., et al. (2017). The effect of selective opioid receptor agonists and antagonists on epileptiform activity in morphine-dependent infant mice hippocampal slices. International Journal of Developmental Neuroscience, 60, 56–62. [DOI:10.1016/j.ijdevneu.2017.04.003] [PMID]
Paxinos, G., & Watson, C. (2006). The rat brain in stereotaxic coordinates: Hard cover edition. Amsterdam: Elsevier. [Link]
Cheang, W. S., Lam, M. Y., Wong, W. T., Tian, X. Y., Lau, C. W., & Zhu, Z., et al. (2013). Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. European Journal of Pharmacology, 702(1-3), 79–84. [DOI:10.1016/j.ejphar.2013.01.028] [PMID]
Santos, N. C., Figueira-Coelho, J., Martins-Silva, J., & Saldanha, C. (2003). Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochemical Pharmacology, 65(7), 1035–1041. [DOI:10.1016/S0006-2952(03)00002-9] [PMID]
Sardo, P., Carletti, F., D'Agostino, S., Rizzo, V., & Ferraro, G. (2006). Involvement of nitric oxide-soluble guanylyl cyclase pathway in the control of maximal dentate gyrus activation in the rat. Journal of Neural Transmission, 113(12), 1855–1861. [DOI:10.1007/s00702-006-0491-9] [PMID]
Su, L., Wang, C., Yu, Y. H., Ren, Y. Y., Xie, K. L., & Wang, G. L. (2011). Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neuroscience, 12, 120. [DOI:10.1186/1471-2202-12-120] [PMID] [PMCID]
Tani, M., Onimaru, H., Ikeda, K., Kawakami, K., & Homma, I. (2010). Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport, 21(17), 1095–1099. [DOI:10.1097/WNR.0b013e3283405bad] [PMID]
Taniguchi, Y., Deguchi, Y., Saita, M., & Noda, K. (1994). [Antinociceptive effects of counterirritants (Japanese)]. Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica, 104(6), 433–446. [DOI:10.1254/fpj.104.433] [PMID]
Umezu, T., Sakata, A., & Ito, H. (2001). Ambulation-promoting effect of peppermint oil and identification of its active constituents. Pharmacology Biochemistry and Behavior, 69(3-4), 383-90. [DOI:10.1016/S0091-3057(01)00543-3]
Wang, H., Ma, L., Li, Y., & Cho, C. H. (2000). Exposure to cigarette smoke increases apoptosis in the rat gastric mucosa through a reactive oxygen species-mediated and p53-independent pathway. Free Radical Biology & Medicine, 28(7), 1125–1131. [DOI:10.1016/S0891-5849(00)00207-0] [PMID]
Watt, E. E., Betts, B. A., Kotey, F. O., Humbert, D. J., Griffith, T. N., & Kelly, E. W., et al. (2008). Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. European Journal of Pharmacology, 590(1-3), 120–126. [DOI:10.1016/j.ejphar.2008.06.003] [PMID]
Wu, Q., & Wang, H. (2018). The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Research, 148, 8–16. [DOI:10.1016/j.eplepsyres.2018.10.002] [PMID]
Zhang, X. B., Jiang, P., Gong, N., Hu, X. L., Fei, D., & Xiong, Z. Q., et al. (2008). A-type GABA receptor as a central target of TRPM8 agonist menthol. Plos One, 3(10), e3386. [DOI:10.1371/journal.pone.0003386] [PMID] [PMCID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2020/11/14 | Accepted: 2022/05/30 | Published: 2023/11/1
Received: 2020/11/14 | Accepted: 2022/05/30 | Published: 2023/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








