Volume 13, Issue 5 (September & October 2022)
BCN 2022, 13(5): 695-708 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Igbo E J, Okoliko U, Aminu I, Kopada A, Olorunnado S, Akinola O B. Structural Changes in the Medial Prefrontal Cortex and Anterior Cingulate Cortex of Dehydroepiandrosterone-Induced Wistar Rat Model of Polycystic Ovarian Syndrome. BCN 2022; 13 (5) :695-708
URL: http://bcn.iums.ac.ir/article-1-1975-en.html
URL: http://bcn.iums.ac.ir/article-1-1975-en.html
Enya Joseph Igbo *1 

 , Ukwenya Okoliko2
, Ukwenya Okoliko2 
 , Imam Aminu1
, Imam Aminu1 

 , Aisha Kopada1
, Aisha Kopada1 

 , Samson Olorunnado3
, Samson Olorunnado3 

 , Oluwole B. Akinola1
, Oluwole B. Akinola1 




 , Ukwenya Okoliko2
, Ukwenya Okoliko2 
 , Imam Aminu1
, Imam Aminu1 

 , Aisha Kopada1
, Aisha Kopada1 

 , Samson Olorunnado3
, Samson Olorunnado3 

 , Oluwole B. Akinola1
, Oluwole B. Akinola1 


1- Department of Anatomy, University of Ilorin, Ilorin, Kwara State, Nigeria.
2- Department of Human Anatomy, Federal University of Technology, Akure, Ondo, Nigeria.
3- Department of Human Anatomy, School of Medicine and Pharmacy, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda.
2- Department of Human Anatomy, Federal University of Technology, Akure, Ondo, Nigeria.
3- Department of Human Anatomy, School of Medicine and Pharmacy, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda.
Keywords: Polycystic ovary syndrome, Medial Prefrontal Cortex (mPFC), Anterior Cingulate Cortex, Dehydroepiandrosterone
Full-Text [PDF 2017 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
olycystic Ovary Syndrome (PCOS) is considered an ovarian disease. It is a prevalent multifactorial endocrine and metabolic disorder associated with many psychiatric disorders (Khan et al., 2019). PCOS is estimated to affect approximately 5% to 10% of women in their reproductive ages, leading to anovulatory infertility, characterized by hyperandrogenism, insulin resistance, menstrual dysfunction, and polycystic ovaries (Moore & Campbell, 2017). The etiology of PCOS is not clearly understood, however, lipid imbalance, oxidative stress, insulin resistance, genetics, and the brain are some of the contributing factors (ÇINar & EryIlMaz, 2016; Moore & Campbell, 2017).
Studies have shown that women with PCOS have a dysregulated Hypothalamic Pituitary Adrenal (HPA) axis, resulting in the high secretion of the Luteinizing Hormone (LH) and the gonadotropin-releasing hormone (GnRH) (Silva, et al., 2018). This elevates the estradiol and progesterone concentration in others to suppress and modulates the upsurge of LH and GnRH (Reddy et al., 2016).
Such hormonal imbalance results in higher activities in the brain’s parietal and temporal lobe of women with PCOS compared to women without PCOS; however, it does not relate to the performance during a working memory task (Chaudhari et al., 2018). Compromised working memory broadly affects cognitive functioning, the quality of life, and psychological well-being; however, it is unclear whether changes in the androgen levels affect the working memory processes as well (Blay et al., 2016). Women with PCOS are also highly susceptible to social phobia and suicidal ideation and have intense dysphoric feelings, which affect their quality of life (Blay, et al., 2016).
Accordingly, this study uses dehydroepiandrosterone (DHEA) to induce PCOS in rat models and focus on understanding the structural changes that occur in the ovary of PCOS female when compared to normal female and also evaluate the neurobehavioral (anxiety and depression) and neurochemical (oxidative stress and inflammatory markers) changes, and the associated changes in the medial prefrontal cortex (mPFC) and the anterior cingulate cortex (ACC) of DHEA-induced PCOS model rats.
2. Materials and Methods
Study animals
A total of 12 female juvenile Wistar rats (30 to 50 g) about 22 to 44 days old were used in this study. Firstly, 5 male and 10 adult female Wistar rats were acquired from the animal house, Department of Biochemistry, Faculty of Life Sciences, University of Ilorin, and bred in the College of Health Sciences Animal Holdings, University of Ilorin. Female pops were separated from their mothers after 21 days (3 weeks), caged in standard polypropylene cages, allowed to acclimatize to their new environment for 2 weeks (14 days), maintained in 12 h light-dark cycles at room temperature, and were fed with standard diet and water provided ad libitum. The rats were fed daily with commercially available rat pellets feed which was bought from Ogooluwa feed and Flour Mill Limited, Sango, Ilorin, Kwara State, Nigeria.
Procurement of dehydroepiandrosterone and sesame oil
DHEA was obtained from Sigma-Aldrich, USA, and sesame oil used was procured from Aromokeye pharmacy, Ilorin Kwara State, Nigeria.
Experimental design
The experimental animals were randomly divided into 2 groups, namely the vehicle group and the PCOS group, each containing 6 rats (Table 1).
.jpg)
Drug administration
All the experimental animals in the vehicle group subcutaneously (SC) received 0.2 mL of sesame oil, while rats in the PCOS group SC received DHEA (6 mg/100 g body weight, dissolved in 0.2 mL of sesame oil) (Ikeda et al., 2014). All substance administration was done daily for 21 days. Vaginal smears were collected daily after a week (week 1) of DHEA administration and evaluated microscopically to confirm the induction of PCOS.
Confirmation of polycystic ovary syndrome using vaginal smear
Vaginal smears were used to determine the regularity or irregularity of the animal’s estrus cycle. First, 0.3 mL of normal saline was injected slowly into the vagina of the animal using a pipette and then withdrawn. One to two drops of the withdrawn fluid, containing the vaginal fluid, were removed to prepare the smear. Samples were examined using a light binocular microscope. Rats that were in the estrus phase of the reproductive cycle were selected for the next stages of the study. From consistent observation, control animals showed a constant change in the phase of their estrous cycle while treated animals were characterized by the presence of persistent vaginal cornification (Figure 1); their vaginal smear had more cornified (horn) cells in the estrus stage (Ajayi et al, 2020).
.jpg)
Neurobehavioral analysis
Following 24 h after the last administration, a neurobehavioral investigation was done to evaluate the anxiety and depressive behavior using various behavioral paradigms.
Open-field test
The open-field test (OFT) requires the use of a large cubic box, usually measuring 1m×1m×1m, and the top of the cube is typically left uncovered. Each rat is separately placed in the middle of the box and its explorative movements are recorded. At the end of the experiment, the video capturing each rat movement is analyzed (Seibenhener & Wooten, 2015; Yu, Hao et al., 2016).
Y-Maze test
The Y-maze test requires a cardboard apparatus that consists of three enclosed arms, which converge on an equilateral triangular center platform. At the beginning of each experimental session, each rat is placed in the center platform of the maze, facing all three arms immediately before the session (Akanmu, et al., 2011). The total number of entries and the number of continual triple entries into each of the three arms without repetition, known as “the number of spontaneous alteration performance (SAP)” is recorded and evaluated. This test lasts for 6 min (Akanmu, et al., 2011).
Forced swim test
The forced swim test (FST) has 2 sessions, 24 h apart from each other. The first session is the pretest stage which lasts 15 min and the second session is the test stage which lasts 5 min. Firstly, transparent cylinders are filled with tap water at 23°C±1°C and the water depth is measured above the rat’s length so that the rat cannot touch the bottom of the container with its hind legs. Hence, each rat is placed in a water-filled cylinder. After the stipulated duration, the rats are removed and cleaned up with dry cloths. The duration of immobility during the 5-min test is reordered (Akanmu, et al., 2011; Yankelevitch-Yahav et al., 2015).
Black and white box (light-dark box)
The black and white box test (light-dark box) is an experimental procedure, developed for testing anxiety and depression in laboratory rodents. The apparatus has two chambers. One of the chambers is made of clear or white plastic walls and is highly illuminated while the other chamber is painted black. The light and dark chambers are connected by a small passage through which the animals can move freely (Sestakova, et al., 2013).
A testing session starts by placing the animal in the center of the illuminated compartment, facing the opening to the dark compartment for a period lasting usually 5 min. The floor of both compartments is divided into squares to determine the animal’s locomotion (Sestakova, et al., 2013; Seibenhener, et al., 2015).
Excision of rat brain and collection of blood samples
Immediately after all neurobehavioral analyses, two rats from each group were anesthetized with an intraperitoneal (IP) injection of ketamine (3 mg/kg) and perfused intracardially using phosphate-buffered saline (PBS) followed by 40% of neutral buffered formalin. The brain and ovarian tissue were excised following craniotomy and laparoscopy, respectively. Thereafter, both tissues were fixed in 10% neutral buffered formalin for histological evaluation using hematoxylin and eosin (H&E) staining. The coronal section of the brain was obtained at the level of the central sulcus, separating the prefrontal cortex from the other region of the brain. Also, the remaining four rats in each group were sacrificed by cervical dislocation, and the blood sample was taken by cardiac puncture and transferred immediately into a tube containing ethylenediaminetetraacetic acid. Thus, the plasma was separated by cold-centrifugation for biochemical analysis.
Biochemical assays
The levels of reproductive hormones (Follicle Stimulating Hormone [FSH] and LH), brain’s modulatory biomarkers (Norepinephrine [NE]), and brain-derived neurotrophic factor (BDNF), a Marker of Lipid Peroxidation (MDA), ROS, and pro-inflammatory cytokine (interleukin 6 [IL-6]) were quantified using the competitive enzyme immunoassay technique via a polyclonal anti-LH, anti-FSH, anti-NE, anti-BDNF, anti-MDA, anti-ROS with anti-IL-6 antibody and an LH, FSH, NE, BDNF, MDA, ROS with IL-6-horseradish peroxidase (HRP) conjugate, respectively.
Study procedure
The analysis was done according to the manufacturer’s instructions in the ELISA kits. During each probe, the plasma and buffer were incubated together with LH, FSH, NE, BDNF, MDA, ROS, and IL-6-HRP conjugate in a pre-coated plate for 1 h. Subsequently, the wells were decanted and washed 5 times. The wells were further incubated with a substrate for the HRP enzyme. The enzyme-substrate reaction end product formed a blue-colored complex. Lastly, a stop solution was added to stop the reaction, and it turned the solution color yellow. The intensity of the color was measured spectrophotometrically at 450 nm in a microplate reader. The solution color intensity was inversely proportional to the LH, FSH, NE, BDNF, MDA, and ROS with IL-6 concentration since LH, FSH, NE, BDNF, MDA, and ROS with IL-6 from plasma samples and LH, FSH, NE, BDNF, MDA, ROS with IL-6-HRP conjugate compete for the anti-LH, anti-FSH, anti-NE, anti-BDNF, anti-MDA, anti-ROS with anti-IL-6 antibody binding site.
Statistical analysis
The statistical analysis was done via 1-way analysis of variance (ANOVA) using the GraphPad Prism® software (v. 5). A value of <0.05 was considered to indicate the significant difference between the groups.
Ethical considerations
All protocol and treatment procedures were done according to the Institutional Animal Care and Use Committee (IACUC) Guideline and approved by the Ethics Committee of the University of Ilorin, Kwara State, Nigeria (approval number: UERC/ASN/2019/1743), which is in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2010) (National Research Council, 2011).
3. Results
Exploratory activities, depressive and anxiety behaviors of polycystic ovary syndrome model rats
The results of various behavioral tests that were conducted are evaluated below.
Open-field test
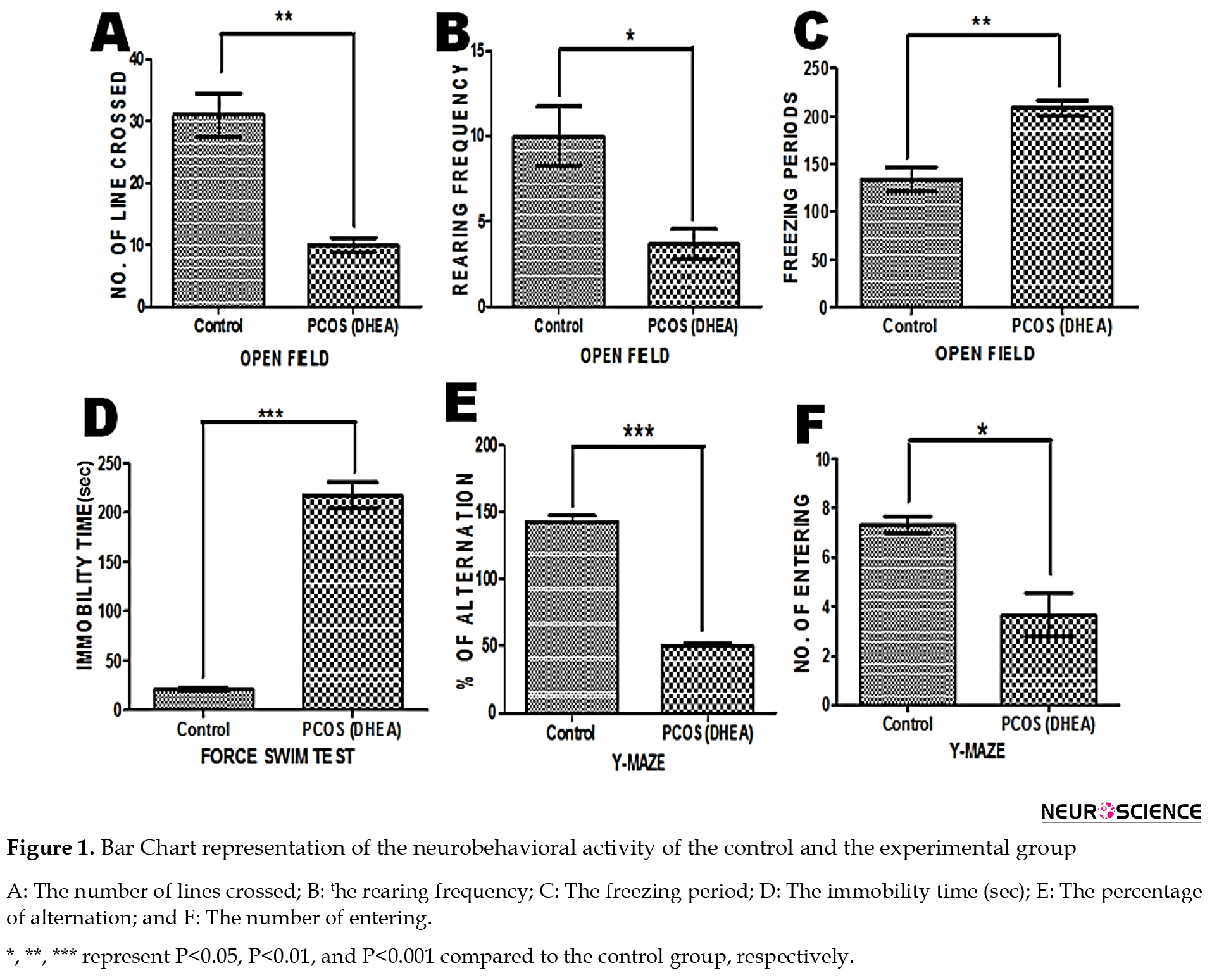
Number of line crossing: The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (10.00±1.155) was significantly lower than the control group (31.00±3.512) (P<0.05) (Figure 1A).
Number of rearing: The Mean±SD latency for PCOS rats (3.667±0.882) was significantly depleted when compared to control rats (10.00±1.732) (P<0.05) (Figure 1B).
Freezing period: The Mean±SD latency for control rats (134.0±12.50) was significantly lower than for PCOS rats (208.7±7.839) (P<0.05) (Figure 1C).
Black and White Box Test
Number of line crossing: The Mean±SD latency for PCOS rats (2.333±0.333) was significantly lower than the control group (20.33±2.028) (P<0.05) (Figure 2A).
.jpg)
Number of rearing: The Mean±SD latency for PCOS rats (0.333±0.333) was significantly depleted when compared to control rats (5.000±0.577) (P<0.05) (Figure 2B).
Number of peeping: The Mean±SD latency for PCOS rats (2.667±0.333) was significantly lower than for control rats (8.333±0.667) (P<0.05) (Figure 2C).
Percentage of time in white box: The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (10.67±0.882) was significantly lower compared to the control rats (125.0±4.000) (P<0.05) (Figure 2D).
Percentage of time in black box: The Mean±SD latency for the control rats (175.0±17.35) was significantly lower than for the PCOS rats (289.3±3.180) (P<0.05) (Figure 2E).
Y-Maze Test
Number of entrances. The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (3.667±0.8819) was significantly reduced compared to the control rats (7.333±0.3333) (P<0.05) (Figure 1F).
Percentage of Alternation. The Mean±SD latency for PCOS rats (50.00±2.646) was significantly lower than the control rats (143.0±4.359) (P<0.05) (Figure 1E).
Forced swim test
Immobility time (Sec). The unpaired t test analysis revealed that the Mean±SD latency for the control rats (20.67±1.453) was significantly reduced compared to the PCOS rats (217.3±13.35), indicating depressive manifestations (P<0.05) (Figure 1D).
Black and white box (close)
Number of Rearing. The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (13.33±1.202) was significantly lower compared to the control rats (5.333±0.667) (P<0.05) (Figure 3B).
.jpg)
Freezing Period. The unpaired t test analysis revealed that the Mean±SD latency for the control rats (113.7±3.283) was significantly lower compared to the PCOS rats (191.7±2.028) (P<0.05) (Figure 3A).
PReproductive hormones in dehydroepiandrosterone-induced polycystic ovary syndrome rat model
According to Figure 4, the Mean±SD latency for (A) the control rats was 111.4±3.400 and the Mean±SD latency for the PCOS rats was 138.1±4.135 in FSH, showing that the Mean±SD was significantly higher than the control; (B) the control was 108.0±6.961 and the Mean±SD for PCOS rats were 167.7±5.217 in LH which shows that it was significantly higher compared to the control (P˂0.05). The data on the reproductive hormone concentrations of the experimental rats are expressed in Figure 4.
.jpg)
Expression of oxidative function/lipid peroxidation and inflammatory responses in dehydroepiandrosterone-induced polycystic ovary syndrome rat model
According to Figure 5, there was no significant difference in various biochemical parameters that were measured, of which there was a noticeable reduction in the Mean±SD latency for the control group across oxidative stress and inflammatory markers (MDA [0.632±0.069], ROS [3.506±0.270], and IL-6 [69.46±7.922]) when compared to the PCOS group (MDA [0.790±0.087], ROS [3.773±0.081], and IL-6 [86.58±2.155]). Hence, the data from the 1-way ANOVA showed a significant increase in the inflammatory marker (IL-6) when compared to oxidative stress markers (MDA and ROS).
.jpg)
Brains modulatory neurotransmitter marker
According to Figure 6, the Mean±SD latency for (A) NE level in the control group (7.159±0.265) was significantly higher than the PCOS group (5.374±0.673); whereas, (B) the BDNF level in the control group (496.5±16.68) was not significantly higher than the PCOS group (422.2±7.411).
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
General histology
Plate 7 shows the general histoarchitectural in the ovarian sections of the control group (A) and the PCOS group (B). The section of the ovary from a control rat showed follicles at various stages (pre-antral follicle, antral follicle, corpus luteum, and atretic follicle), while the section of the ovary from a PCOS rat showed increased cystic, atretic, and antral follicles as well as decreased corpus luteum.
In Plate 8 and Plate 9, the mPFC showed hypertrophy and hyperplasia of cells, especially in the cortical plate of the PCOS group (B). The pyramidal cells were also reduced, which revealed vacuolated neurons and degenerating neurons, as well as the loss of cell membranes leaving a hollow around them (Plate 10).
4. Discussion
PCOS is the most commonly diagnosed human endocrine and metabolic disorder among other endocrine disorders in women during their reproductive ages. As a result, understanding the connection between PCOS as a biological disorder and its psychological components is crucial (Kumarendran, et al., 2018; Peecher, 2018). The findings from various literature have documented an increased risk of depressive disorders in women with PCOS (Rassi, et al., 2010; Yu, et al., 2016; Tan, Grigg, Kulkarni, 2018). The deleterious effects of DHEA leading to PCOS are established, however, there is a need to examine the state of PFC and the ACC, its associated neurobehavioral changes, and the correlating role of oxidative stress and inflammatory markers in establishing PCOS. Thus, this might help to unravel some unknown manifestations of DHEA-induced PCOS.
As noted in this study, the neurobehavioral assessment conducted following PCOS revealed a decrease in the general locomotory movement activities which suggests depressive disorders as well as a cognitive decline, as there was a statistical significant depletion in the number of lines crossed and the rearing frequency in the OFT performance, similar to the number of lines crossed, the rearing frequency, and the percentage of time in the white box, using the black and white box test; in addition to the number of entering and the percentage of alternation in the Y-maze test performance, with a statistically significant increase in the immobility time using the FST neurobehavioral paradigm. Also, the increased anxiety behavior was observed, as there was a statistically significant reduction in the number of peeping using the black and white box test paradigm and the rearing frequency in black and white box (close) test performance, with a significant increase in the freezing period using OFT and the black and white box (close) tests; also, the percentage of time in the black box was increased in the black and white box test performance. The changes observed in the significant decline and increases across the behavioral apparatus suggest the role of DHEA-induced PCOS in initiating depressive and anxiety behaviors. The findings from this study are also in line with Ressler et al. (2015), Yu et al. (2016), and Peecher (2018).
Hypersecretion of LH and FSH is a well‐established biomarker associated with PCOS women, and the measurement of serum LH concentration and LH/FSH ratio is commonly used to assess these women. In clinical studies, Milsom and colleagues have reported the elevation of LH or LH/FSH ratio to correlate with the increased risk of infertility and menstrual cycle disturbance Milsom et al. (2003). The hormonal level of LH and FSH in PCOS rats was significantly increased when compared to control rats in this study. Meanwhile, comparing the LH/FSH ratio, LH significantly increased over FSH, confirming what is overwhelmingly reported in the literature (Milsom et al., 2003; Zhang et al., 2013; Fu et al., 2013; Zadehmodarres et al., 2015).
Based on the fact that oxidative damage is mediated by free radicals (ROS, reactive nitrogen species, and other reactive species), this research involved evaluating MDA, and ROS levels, and correlating the possible pro-inflammatory effect (measuring IL-6), leading to decreased general movement activities/anxiety expression and depressive manifestation in PCOS rats. There was a noticeable yet statistically not significant increase in the level of ROS following PCOS when compared to the control group. Also, lipid peroxidation products are one of the main outcomes associated with oxidative stress, as there was an obvious yet not statistically significant increase in the MDA level, following PCOS when compared to the control group. Various studies have reported similar findings in oxidative stress biomarkers, following PCOS (Ghowsi et al., 2019). Furthermore, recent evidence suggests that PCOS may be associated with chronic inflammation, where the inflammatory cytokines show a positive correlation with anovulation and other PCOS symptoms (Wang, et al., 2017). Thus, our study reported an increase in the level of a pro-inflammatory and immunoregulatory cytokine biomarker (IL-6) in PCOS rats which was not statistically significant when compared to the control group. The study results are in line with the results of Ressler et al. (2015).
This study also revealed the expression of brain modulatory neurotransmitters (NE and BDNF) in PCOS model rats, of which NE exhibited a statistically significant reduction in PCOS rats when compared to the control group. Yu et al. (2016) found that the levels of NE, dopamine (DA), serotonin (5-HT), and their metabolites were significantly decreased following DHEA treatment, reflecting depressive behavior. This indicates that potential neurochemical mechanisms could underlie the observed behavioral changes. Chaudhari and Nampoothiri also found the expression levels DA, NE, 5HT, and epinephrine to be significantly low in testosterone propionate PCOS model rats. Therefore, the decrease in neurotransmitters, mainly NE, 5-HT, and DA attributes to mood disorders, such as depression and anxiety in PCOS (Chaudhari et al., 2018). Accordingly, the activity of BDNF was slightly depleted in PCOS rats, however, it was not statistically significant when compared to control rats. Although BDNF plays an important role in neural plasticity, enhances long-term potentiation, and promotes learning and memory, mutation or decreased level of BDNF in rodents result in learning deficits, long-term potentiation impairment, and declined learning and memory (Sarkaki et al., 2014).
Furthermore, histological photomicrographs results of the mPFC /ACC that are much involved in mediating emotional responses showed normal cytoarchitecture, normal neuronal population, and regular neuronal morphology in the control rats; however, rats treated with DHEA for 21 days to induce PCOS showed extensive neuronal degeneration evidenced by reduced pyramidal cells, which revealed vacuolated neurons, degenerating neurons, and the loss of cell membranes leaving a hollow around them.
5. Conclusion
In this study, PCOS rats exhibited alteration across several neurobehavioral tests, hormonal parameters (LH and FSH), and specific oxidative stress and inflammatory biomarkers. This could attribute to the impairment in emotional and executive function in the mPFC and ACC as observed by the neurobehavioral assessments.
Ethical Considerations
Compliance with ethical guidelines
The Ethical Committee of the University of Ilorin, College of Health Sciences approved the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
Akanmu, M. A., Olowookere, T. A., Atunwa, S. A., Ibrahim, B. O., Lamidi, O. F., & Adams, P. A., et al. (2011). Neuropharmacological effects of Nigerian honey in mice. African Journal of Traditional, Complementary and Alternative Medicines, 8(3), 230–249. [PMID] [PMCID]
Atef, M. M., Abd-Ellatif, R. N., Emam, M. N., Abo El Gheit, R. E., Amer, A. I., & Hafez, Y. M. (2011). Therapeutic potential of sodium selenite in letrozole induced polycystic ovary syndrome rat model: Targeting mitochondrial approach (selenium in PCOS). Archives of Biochemistry and Biophysics, 671, 245-254. [PMID]
Azizi, M., Elyasi, F. (2017). Psychosomatic aspects of polycystic ovarian syndrome: A review. Iranian Journal of Psychiatry and Behavioral Sciences, 11(2), e6595. [DOI:10.5812/ijpbs.6595]
Blay, S. L., Aguiar, J. V., & Passos, I. C. (2016). Polycystic ovary syndrome and mental disorders: A systematic review and exploratory meta-analysis. Neuropsychiatric Disease and Treatment, 12, 2895–2903. [PMID] [PMCID]
Chaudhari, N., Dawalbhakta, M., & Nampoothiri, L. (2018).GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reproductive Biology and Endocrinology, 16(1), 37. [PMID] [PMCID]
ÇINar, M., & EryIlMaz, Ö. G. (2016). Experimental models of polycystic ovary syndrome. Medeniyet Medical Journal, 31(1), 53-57. [DOI:10.5222/MMJ.2016.053]
Cowan, N. (2014). Working memory underpins cognitive development, learning, and education. Educational Psychology Review, 26(2), 197-223. [PMID]
Fu, L., Zhang, Z., Zhang, A., Xu, J., Huang, X., & Zheng, Q., et al. (2013). Association study between FSHR Ala307Thr and Ser680Asn variants and polycystic ovary syndrome (PCOS) in Northern Chinese Han women. Journal of Assisted Reproduction and Genetics, 30(5), 717-721. [PMID]
Ghowsi, M., Khazali, H., & Sisakhtnezhad, S.(2018). The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. International Journal of Reproductive BioMedicine, 16(3), 149–158. [PMID]
Ikeda, K., Baba, T., Morishita, M., Honnma, H., Endo, T., Kiya, T., et al. (2014). Long-term treatment with dehydroepiandrosterone may lead to follicular atresia through interaction with anti-Mullerian hormone. Journal of Ovarian Research, 7, 46. [PMID]
Khan, M. J., Ullah, A., & Basit, S. (2019). Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. The Application of Clinical Genetics, 12, 249-260. [PMID] [PMCID]
Kumarendran, B., O'Reilly, M. W., Manolopoulos, K. N., Toulis, K. A., Gokhale, K. M., & Sitch, A. J., et al. (2018). Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Medicine, 15(3), e1002542. [PMID] [PMCID]
Milsom, S. R., Sowter, M. C., Carter, M. A., Knox, B. S., & Gunn, A. J. (2003) LH levels in women with polycystic ovarian syndrome: have modern assays made them irrelevant? BJOG: An International Journal of Obstetrics and Gynaecology, 110(8), 760-760-764. [DOI:10.1111/j.1471-0528.2003.02528.x]
Moore, A. M., & Campbell, R. E. (2017). Polycystic ovary syndrome: Understanding the role of the brain. Frontiers in Neuroendocrinology, 46, 1-14. [PMID]
National Research Council. (2011). Guide for the care and use of laboratory animals. Washington, DC: The National Academies Press. [Link]
Peecher, D. (2018). Modeling the psychiatric aspects of polycystic ovary syndrome and induced stress [MA thesis]. Washington: Central Washington University. [Link]
Rassi, A., Veras, A. B., dos Reis, M., Pastore, D. L., Bruno, L. M., & Bruno, R. V., et al. (2010). Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Comprehensive Psychiatry, 51(6), 599-602. [PMID]
Reddy, P. S., Begum, N., Mutha, S., & Bakshi, V. (2016). Beneficial effect of curcumin in letrozole-induced polycystic ovary syndrome. Asian Pacific Journal of Reproduction, 5(2), 116-122. [DOI:10.1016/j.apjr.2016.01.006]
Ressler, I. B., Grayson, B. E., Ulrich-Lai, Y. M., & Seeley, R. J. (2015). Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. American Journal of Physiology-Endocrinology and Metabolism, 308(12), E1076–E1084. [PMID]
Sarkaki, A., Fathimoghaddam, H., Mansouri, S. M., Korrani, M. S., Saki, G., & Farbood, Y. (2014). Gallic acid improves cognitive, hippocampal long-term potentiation deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Pakistan Journal of Biological Sciences, 17(8), 978-990. [PMID]
Seibenhener, M. L., & Wooten, M. C. (2015). Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments : JoVE, (96), e52434.[PMID]
Sestakova, N., Puzserova, A., Kluknavsky, M., & Bernatova, I. (2013). Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdisciplinary Toxicology, 6(3), 126-135. [PMID] [PMCID]
Silva, M. S., Prescott, M., & Campbell, R. E. (2018). Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight, 3(7), e99405. [PMID] [PMCID]
Tan, R. Y., Grigg, J., & Kulkarni, J. (2018). Borderline personality disorder and polycystic ovary syndrome: A review of the literature. The Australian and New Zealand journal of psychiatry, 52(2), 117-128. [PMID]
Wang, L., Qi, H., Baker, P. N., Zhen, Q., Zeng, Q., & Shi, R., et al. (2017). Altered circulating inflammatory cytokines are associated with anovulatory polycystic ovary syndrome (PCOS) women resistant to clomiphene citrate treatment. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 23, 1083-1089. [PMID] [PMCID]
Yankelevitch-Yahav, R., Franko, M., Huly, A., & Doron, R. (2015). The forced swim test as a model of depressive-like behavior. Journal of Visualized Experiments: JoVE, (97), 52587. [PMID]
Yu, Q., Hao, S., Wang, H., Song, X., Shen, Q., & Kang, J. (2016).Depression-like behavior in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Biology of Reproduction, 95(4), 79. [PMID]
Zadehmodarres, S., Heidar, Z., Razzaghi, Z., Ebrahimi, L., Soltanzadeh, K., & Abed, F. (2015). Anti-mullerian hormon level and polycystic ovarian syndrome diagnosis. Iranian Journal of Reproductive Medicine, 13(4), 227-230. [PMID]
Zhang, H. Y., Guo, C. X., Zhu, F. F., Qu, P. P., Lin, W. J., & Xiong, J. (2013). Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Archives of Gynecology and Obstetrics, 287(3), 525-531. [PMID]
olycystic Ovary Syndrome (PCOS) is considered an ovarian disease. It is a prevalent multifactorial endocrine and metabolic disorder associated with many psychiatric disorders (Khan et al., 2019). PCOS is estimated to affect approximately 5% to 10% of women in their reproductive ages, leading to anovulatory infertility, characterized by hyperandrogenism, insulin resistance, menstrual dysfunction, and polycystic ovaries (Moore & Campbell, 2017). The etiology of PCOS is not clearly understood, however, lipid imbalance, oxidative stress, insulin resistance, genetics, and the brain are some of the contributing factors (ÇINar & EryIlMaz, 2016; Moore & Campbell, 2017).
Studies have shown that women with PCOS have a dysregulated Hypothalamic Pituitary Adrenal (HPA) axis, resulting in the high secretion of the Luteinizing Hormone (LH) and the gonadotropin-releasing hormone (GnRH) (Silva, et al., 2018). This elevates the estradiol and progesterone concentration in others to suppress and modulates the upsurge of LH and GnRH (Reddy et al., 2016).
Such hormonal imbalance results in higher activities in the brain’s parietal and temporal lobe of women with PCOS compared to women without PCOS; however, it does not relate to the performance during a working memory task (Chaudhari et al., 2018). Compromised working memory broadly affects cognitive functioning, the quality of life, and psychological well-being; however, it is unclear whether changes in the androgen levels affect the working memory processes as well (Blay et al., 2016). Women with PCOS are also highly susceptible to social phobia and suicidal ideation and have intense dysphoric feelings, which affect their quality of life (Blay, et al., 2016).
Accordingly, this study uses dehydroepiandrosterone (DHEA) to induce PCOS in rat models and focus on understanding the structural changes that occur in the ovary of PCOS female when compared to normal female and also evaluate the neurobehavioral (anxiety and depression) and neurochemical (oxidative stress and inflammatory markers) changes, and the associated changes in the medial prefrontal cortex (mPFC) and the anterior cingulate cortex (ACC) of DHEA-induced PCOS model rats.
2. Materials and Methods
Study animals
A total of 12 female juvenile Wistar rats (30 to 50 g) about 22 to 44 days old were used in this study. Firstly, 5 male and 10 adult female Wistar rats were acquired from the animal house, Department of Biochemistry, Faculty of Life Sciences, University of Ilorin, and bred in the College of Health Sciences Animal Holdings, University of Ilorin. Female pops were separated from their mothers after 21 days (3 weeks), caged in standard polypropylene cages, allowed to acclimatize to their new environment for 2 weeks (14 days), maintained in 12 h light-dark cycles at room temperature, and were fed with standard diet and water provided ad libitum. The rats were fed daily with commercially available rat pellets feed which was bought from Ogooluwa feed and Flour Mill Limited, Sango, Ilorin, Kwara State, Nigeria.
Procurement of dehydroepiandrosterone and sesame oil
DHEA was obtained from Sigma-Aldrich, USA, and sesame oil used was procured from Aromokeye pharmacy, Ilorin Kwara State, Nigeria.
Experimental design
The experimental animals were randomly divided into 2 groups, namely the vehicle group and the PCOS group, each containing 6 rats (Table 1).
.jpg)
Drug administration
All the experimental animals in the vehicle group subcutaneously (SC) received 0.2 mL of sesame oil, while rats in the PCOS group SC received DHEA (6 mg/100 g body weight, dissolved in 0.2 mL of sesame oil) (Ikeda et al., 2014). All substance administration was done daily for 21 days. Vaginal smears were collected daily after a week (week 1) of DHEA administration and evaluated microscopically to confirm the induction of PCOS.
Confirmation of polycystic ovary syndrome using vaginal smear
Vaginal smears were used to determine the regularity or irregularity of the animal’s estrus cycle. First, 0.3 mL of normal saline was injected slowly into the vagina of the animal using a pipette and then withdrawn. One to two drops of the withdrawn fluid, containing the vaginal fluid, were removed to prepare the smear. Samples were examined using a light binocular microscope. Rats that were in the estrus phase of the reproductive cycle were selected for the next stages of the study. From consistent observation, control animals showed a constant change in the phase of their estrous cycle while treated animals were characterized by the presence of persistent vaginal cornification (Figure 1); their vaginal smear had more cornified (horn) cells in the estrus stage (Ajayi et al, 2020).
.jpg)

Neurobehavioral analysis
Following 24 h after the last administration, a neurobehavioral investigation was done to evaluate the anxiety and depressive behavior using various behavioral paradigms.
Open-field test
The open-field test (OFT) requires the use of a large cubic box, usually measuring 1m×1m×1m, and the top of the cube is typically left uncovered. Each rat is separately placed in the middle of the box and its explorative movements are recorded. At the end of the experiment, the video capturing each rat movement is analyzed (Seibenhener & Wooten, 2015; Yu, Hao et al., 2016).
Y-Maze test
The Y-maze test requires a cardboard apparatus that consists of three enclosed arms, which converge on an equilateral triangular center platform. At the beginning of each experimental session, each rat is placed in the center platform of the maze, facing all three arms immediately before the session (Akanmu, et al., 2011). The total number of entries and the number of continual triple entries into each of the three arms without repetition, known as “the number of spontaneous alteration performance (SAP)” is recorded and evaluated. This test lasts for 6 min (Akanmu, et al., 2011).
Forced swim test
The forced swim test (FST) has 2 sessions, 24 h apart from each other. The first session is the pretest stage which lasts 15 min and the second session is the test stage which lasts 5 min. Firstly, transparent cylinders are filled with tap water at 23°C±1°C and the water depth is measured above the rat’s length so that the rat cannot touch the bottom of the container with its hind legs. Hence, each rat is placed in a water-filled cylinder. After the stipulated duration, the rats are removed and cleaned up with dry cloths. The duration of immobility during the 5-min test is reordered (Akanmu, et al., 2011; Yankelevitch-Yahav et al., 2015).
Black and white box (light-dark box)
The black and white box test (light-dark box) is an experimental procedure, developed for testing anxiety and depression in laboratory rodents. The apparatus has two chambers. One of the chambers is made of clear or white plastic walls and is highly illuminated while the other chamber is painted black. The light and dark chambers are connected by a small passage through which the animals can move freely (Sestakova, et al., 2013).
A testing session starts by placing the animal in the center of the illuminated compartment, facing the opening to the dark compartment for a period lasting usually 5 min. The floor of both compartments is divided into squares to determine the animal’s locomotion (Sestakova, et al., 2013; Seibenhener, et al., 2015).
Excision of rat brain and collection of blood samples
Immediately after all neurobehavioral analyses, two rats from each group were anesthetized with an intraperitoneal (IP) injection of ketamine (3 mg/kg) and perfused intracardially using phosphate-buffered saline (PBS) followed by 40% of neutral buffered formalin. The brain and ovarian tissue were excised following craniotomy and laparoscopy, respectively. Thereafter, both tissues were fixed in 10% neutral buffered formalin for histological evaluation using hematoxylin and eosin (H&E) staining. The coronal section of the brain was obtained at the level of the central sulcus, separating the prefrontal cortex from the other region of the brain. Also, the remaining four rats in each group were sacrificed by cervical dislocation, and the blood sample was taken by cardiac puncture and transferred immediately into a tube containing ethylenediaminetetraacetic acid. Thus, the plasma was separated by cold-centrifugation for biochemical analysis.
Biochemical assays
The levels of reproductive hormones (Follicle Stimulating Hormone [FSH] and LH), brain’s modulatory biomarkers (Norepinephrine [NE]), and brain-derived neurotrophic factor (BDNF), a Marker of Lipid Peroxidation (MDA), ROS, and pro-inflammatory cytokine (interleukin 6 [IL-6]) were quantified using the competitive enzyme immunoassay technique via a polyclonal anti-LH, anti-FSH, anti-NE, anti-BDNF, anti-MDA, anti-ROS with anti-IL-6 antibody and an LH, FSH, NE, BDNF, MDA, ROS with IL-6-horseradish peroxidase (HRP) conjugate, respectively.
Study procedure
The analysis was done according to the manufacturer’s instructions in the ELISA kits. During each probe, the plasma and buffer were incubated together with LH, FSH, NE, BDNF, MDA, ROS, and IL-6-HRP conjugate in a pre-coated plate for 1 h. Subsequently, the wells were decanted and washed 5 times. The wells were further incubated with a substrate for the HRP enzyme. The enzyme-substrate reaction end product formed a blue-colored complex. Lastly, a stop solution was added to stop the reaction, and it turned the solution color yellow. The intensity of the color was measured spectrophotometrically at 450 nm in a microplate reader. The solution color intensity was inversely proportional to the LH, FSH, NE, BDNF, MDA, and ROS with IL-6 concentration since LH, FSH, NE, BDNF, MDA, and ROS with IL-6 from plasma samples and LH, FSH, NE, BDNF, MDA, ROS with IL-6-HRP conjugate compete for the anti-LH, anti-FSH, anti-NE, anti-BDNF, anti-MDA, anti-ROS with anti-IL-6 antibody binding site.
Statistical analysis
The statistical analysis was done via 1-way analysis of variance (ANOVA) using the GraphPad Prism® software (v. 5). A value of <0.05 was considered to indicate the significant difference between the groups.
Ethical considerations
All protocol and treatment procedures were done according to the Institutional Animal Care and Use Committee (IACUC) Guideline and approved by the Ethics Committee of the University of Ilorin, Kwara State, Nigeria (approval number: UERC/ASN/2019/1743), which is in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2010) (National Research Council, 2011).
3. Results
Exploratory activities, depressive and anxiety behaviors of polycystic ovary syndrome model rats
The results of various behavioral tests that were conducted are evaluated below.
Open-field test
Number of line crossing: The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (10.00±1.155) was significantly lower than the control group (31.00±3.512) (P<0.05) (Figure 1A).
Number of rearing: The Mean±SD latency for PCOS rats (3.667±0.882) was significantly depleted when compared to control rats (10.00±1.732) (P<0.05) (Figure 1B).
Freezing period: The Mean±SD latency for control rats (134.0±12.50) was significantly lower than for PCOS rats (208.7±7.839) (P<0.05) (Figure 1C).
Black and White Box Test
Number of line crossing: The Mean±SD latency for PCOS rats (2.333±0.333) was significantly lower than the control group (20.33±2.028) (P<0.05) (Figure 2A).
.jpg)
Number of rearing: The Mean±SD latency for PCOS rats (0.333±0.333) was significantly depleted when compared to control rats (5.000±0.577) (P<0.05) (Figure 2B).
Number of peeping: The Mean±SD latency for PCOS rats (2.667±0.333) was significantly lower than for control rats (8.333±0.667) (P<0.05) (Figure 2C).
Percentage of time in white box: The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (10.67±0.882) was significantly lower compared to the control rats (125.0±4.000) (P<0.05) (Figure 2D).
Percentage of time in black box: The Mean±SD latency for the control rats (175.0±17.35) was significantly lower than for the PCOS rats (289.3±3.180) (P<0.05) (Figure 2E).
Y-Maze Test
Number of entrances. The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (3.667±0.8819) was significantly reduced compared to the control rats (7.333±0.3333) (P<0.05) (Figure 1F).
Percentage of Alternation. The Mean±SD latency for PCOS rats (50.00±2.646) was significantly lower than the control rats (143.0±4.359) (P<0.05) (Figure 1E).
Forced swim test
Immobility time (Sec). The unpaired t test analysis revealed that the Mean±SD latency for the control rats (20.67±1.453) was significantly reduced compared to the PCOS rats (217.3±13.35), indicating depressive manifestations (P<0.05) (Figure 1D).
Black and white box (close)
Number of Rearing. The unpaired t test analysis revealed that the Mean±SD latency for PCOS rats (13.33±1.202) was significantly lower compared to the control rats (5.333±0.667) (P<0.05) (Figure 3B).
.jpg)
Freezing Period. The unpaired t test analysis revealed that the Mean±SD latency for the control rats (113.7±3.283) was significantly lower compared to the PCOS rats (191.7±2.028) (P<0.05) (Figure 3A).
PReproductive hormones in dehydroepiandrosterone-induced polycystic ovary syndrome rat model
According to Figure 4, the Mean±SD latency for (A) the control rats was 111.4±3.400 and the Mean±SD latency for the PCOS rats was 138.1±4.135 in FSH, showing that the Mean±SD was significantly higher than the control; (B) the control was 108.0±6.961 and the Mean±SD for PCOS rats were 167.7±5.217 in LH which shows that it was significantly higher compared to the control (P˂0.05). The data on the reproductive hormone concentrations of the experimental rats are expressed in Figure 4.
.jpg)
Expression of oxidative function/lipid peroxidation and inflammatory responses in dehydroepiandrosterone-induced polycystic ovary syndrome rat model
According to Figure 5, there was no significant difference in various biochemical parameters that were measured, of which there was a noticeable reduction in the Mean±SD latency for the control group across oxidative stress and inflammatory markers (MDA [0.632±0.069], ROS [3.506±0.270], and IL-6 [69.46±7.922]) when compared to the PCOS group (MDA [0.790±0.087], ROS [3.773±0.081], and IL-6 [86.58±2.155]). Hence, the data from the 1-way ANOVA showed a significant increase in the inflammatory marker (IL-6) when compared to oxidative stress markers (MDA and ROS).
.jpg)
Brains modulatory neurotransmitter marker
According to Figure 6, the Mean±SD latency for (A) NE level in the control group (7.159±0.265) was significantly higher than the PCOS group (5.374±0.673); whereas, (B) the BDNF level in the control group (496.5±16.68) was not significantly higher than the PCOS group (422.2±7.411).
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
General histology
Plate 7 shows the general histoarchitectural in the ovarian sections of the control group (A) and the PCOS group (B). The section of the ovary from a control rat showed follicles at various stages (pre-antral follicle, antral follicle, corpus luteum, and atretic follicle), while the section of the ovary from a PCOS rat showed increased cystic, atretic, and antral follicles as well as decreased corpus luteum.
In Plate 8 and Plate 9, the mPFC showed hypertrophy and hyperplasia of cells, especially in the cortical plate of the PCOS group (B). The pyramidal cells were also reduced, which revealed vacuolated neurons and degenerating neurons, as well as the loss of cell membranes leaving a hollow around them (Plate 10).
4. Discussion
PCOS is the most commonly diagnosed human endocrine and metabolic disorder among other endocrine disorders in women during their reproductive ages. As a result, understanding the connection between PCOS as a biological disorder and its psychological components is crucial (Kumarendran, et al., 2018; Peecher, 2018). The findings from various literature have documented an increased risk of depressive disorders in women with PCOS (Rassi, et al., 2010; Yu, et al., 2016; Tan, Grigg, Kulkarni, 2018). The deleterious effects of DHEA leading to PCOS are established, however, there is a need to examine the state of PFC and the ACC, its associated neurobehavioral changes, and the correlating role of oxidative stress and inflammatory markers in establishing PCOS. Thus, this might help to unravel some unknown manifestations of DHEA-induced PCOS.
As noted in this study, the neurobehavioral assessment conducted following PCOS revealed a decrease in the general locomotory movement activities which suggests depressive disorders as well as a cognitive decline, as there was a statistical significant depletion in the number of lines crossed and the rearing frequency in the OFT performance, similar to the number of lines crossed, the rearing frequency, and the percentage of time in the white box, using the black and white box test; in addition to the number of entering and the percentage of alternation in the Y-maze test performance, with a statistically significant increase in the immobility time using the FST neurobehavioral paradigm. Also, the increased anxiety behavior was observed, as there was a statistically significant reduction in the number of peeping using the black and white box test paradigm and the rearing frequency in black and white box (close) test performance, with a significant increase in the freezing period using OFT and the black and white box (close) tests; also, the percentage of time in the black box was increased in the black and white box test performance. The changes observed in the significant decline and increases across the behavioral apparatus suggest the role of DHEA-induced PCOS in initiating depressive and anxiety behaviors. The findings from this study are also in line with Ressler et al. (2015), Yu et al. (2016), and Peecher (2018).
Hypersecretion of LH and FSH is a well‐established biomarker associated with PCOS women, and the measurement of serum LH concentration and LH/FSH ratio is commonly used to assess these women. In clinical studies, Milsom and colleagues have reported the elevation of LH or LH/FSH ratio to correlate with the increased risk of infertility and menstrual cycle disturbance Milsom et al. (2003). The hormonal level of LH and FSH in PCOS rats was significantly increased when compared to control rats in this study. Meanwhile, comparing the LH/FSH ratio, LH significantly increased over FSH, confirming what is overwhelmingly reported in the literature (Milsom et al., 2003; Zhang et al., 2013; Fu et al., 2013; Zadehmodarres et al., 2015).
Based on the fact that oxidative damage is mediated by free radicals (ROS, reactive nitrogen species, and other reactive species), this research involved evaluating MDA, and ROS levels, and correlating the possible pro-inflammatory effect (measuring IL-6), leading to decreased general movement activities/anxiety expression and depressive manifestation in PCOS rats. There was a noticeable yet statistically not significant increase in the level of ROS following PCOS when compared to the control group. Also, lipid peroxidation products are one of the main outcomes associated with oxidative stress, as there was an obvious yet not statistically significant increase in the MDA level, following PCOS when compared to the control group. Various studies have reported similar findings in oxidative stress biomarkers, following PCOS (Ghowsi et al., 2019). Furthermore, recent evidence suggests that PCOS may be associated with chronic inflammation, where the inflammatory cytokines show a positive correlation with anovulation and other PCOS symptoms (Wang, et al., 2017). Thus, our study reported an increase in the level of a pro-inflammatory and immunoregulatory cytokine biomarker (IL-6) in PCOS rats which was not statistically significant when compared to the control group. The study results are in line with the results of Ressler et al. (2015).
This study also revealed the expression of brain modulatory neurotransmitters (NE and BDNF) in PCOS model rats, of which NE exhibited a statistically significant reduction in PCOS rats when compared to the control group. Yu et al. (2016) found that the levels of NE, dopamine (DA), serotonin (5-HT), and their metabolites were significantly decreased following DHEA treatment, reflecting depressive behavior. This indicates that potential neurochemical mechanisms could underlie the observed behavioral changes. Chaudhari and Nampoothiri also found the expression levels DA, NE, 5HT, and epinephrine to be significantly low in testosterone propionate PCOS model rats. Therefore, the decrease in neurotransmitters, mainly NE, 5-HT, and DA attributes to mood disorders, such as depression and anxiety in PCOS (Chaudhari et al., 2018). Accordingly, the activity of BDNF was slightly depleted in PCOS rats, however, it was not statistically significant when compared to control rats. Although BDNF plays an important role in neural plasticity, enhances long-term potentiation, and promotes learning and memory, mutation or decreased level of BDNF in rodents result in learning deficits, long-term potentiation impairment, and declined learning and memory (Sarkaki et al., 2014).
Furthermore, histological photomicrographs results of the mPFC /ACC that are much involved in mediating emotional responses showed normal cytoarchitecture, normal neuronal population, and regular neuronal morphology in the control rats; however, rats treated with DHEA for 21 days to induce PCOS showed extensive neuronal degeneration evidenced by reduced pyramidal cells, which revealed vacuolated neurons, degenerating neurons, and the loss of cell membranes leaving a hollow around them.
5. Conclusion
In this study, PCOS rats exhibited alteration across several neurobehavioral tests, hormonal parameters (LH and FSH), and specific oxidative stress and inflammatory biomarkers. This could attribute to the impairment in emotional and executive function in the mPFC and ACC as observed by the neurobehavioral assessments.
Ethical Considerations
Compliance with ethical guidelines
The Ethical Committee of the University of Ilorin, College of Health Sciences approved the study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no conflict of interest.
References
Akanmu, M. A., Olowookere, T. A., Atunwa, S. A., Ibrahim, B. O., Lamidi, O. F., & Adams, P. A., et al. (2011). Neuropharmacological effects of Nigerian honey in mice. African Journal of Traditional, Complementary and Alternative Medicines, 8(3), 230–249. [PMID] [PMCID]
Atef, M. M., Abd-Ellatif, R. N., Emam, M. N., Abo El Gheit, R. E., Amer, A. I., & Hafez, Y. M. (2011). Therapeutic potential of sodium selenite in letrozole induced polycystic ovary syndrome rat model: Targeting mitochondrial approach (selenium in PCOS). Archives of Biochemistry and Biophysics, 671, 245-254. [PMID]
Azizi, M., Elyasi, F. (2017). Psychosomatic aspects of polycystic ovarian syndrome: A review. Iranian Journal of Psychiatry and Behavioral Sciences, 11(2), e6595. [DOI:10.5812/ijpbs.6595]
Blay, S. L., Aguiar, J. V., & Passos, I. C. (2016). Polycystic ovary syndrome and mental disorders: A systematic review and exploratory meta-analysis. Neuropsychiatric Disease and Treatment, 12, 2895–2903. [PMID] [PMCID]
Chaudhari, N., Dawalbhakta, M., & Nampoothiri, L. (2018).GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reproductive Biology and Endocrinology, 16(1), 37. [PMID] [PMCID]
ÇINar, M., & EryIlMaz, Ö. G. (2016). Experimental models of polycystic ovary syndrome. Medeniyet Medical Journal, 31(1), 53-57. [DOI:10.5222/MMJ.2016.053]
Cowan, N. (2014). Working memory underpins cognitive development, learning, and education. Educational Psychology Review, 26(2), 197-223. [PMID]
Fu, L., Zhang, Z., Zhang, A., Xu, J., Huang, X., & Zheng, Q., et al. (2013). Association study between FSHR Ala307Thr and Ser680Asn variants and polycystic ovary syndrome (PCOS) in Northern Chinese Han women. Journal of Assisted Reproduction and Genetics, 30(5), 717-721. [PMID]
Ghowsi, M., Khazali, H., & Sisakhtnezhad, S.(2018). The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. International Journal of Reproductive BioMedicine, 16(3), 149–158. [PMID]
Ikeda, K., Baba, T., Morishita, M., Honnma, H., Endo, T., Kiya, T., et al. (2014). Long-term treatment with dehydroepiandrosterone may lead to follicular atresia through interaction with anti-Mullerian hormone. Journal of Ovarian Research, 7, 46. [PMID]
Khan, M. J., Ullah, A., & Basit, S. (2019). Genetic basis of polycystic ovary syndrome (PCOS): Current perspectives. The Application of Clinical Genetics, 12, 249-260. [PMID] [PMCID]
Kumarendran, B., O'Reilly, M. W., Manolopoulos, K. N., Toulis, K. A., Gokhale, K. M., & Sitch, A. J., et al. (2018). Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Medicine, 15(3), e1002542. [PMID] [PMCID]
Milsom, S. R., Sowter, M. C., Carter, M. A., Knox, B. S., & Gunn, A. J. (2003) LH levels in women with polycystic ovarian syndrome: have modern assays made them irrelevant? BJOG: An International Journal of Obstetrics and Gynaecology, 110(8), 760-760-764. [DOI:10.1111/j.1471-0528.2003.02528.x]
Moore, A. M., & Campbell, R. E. (2017). Polycystic ovary syndrome: Understanding the role of the brain. Frontiers in Neuroendocrinology, 46, 1-14. [PMID]
National Research Council. (2011). Guide for the care and use of laboratory animals. Washington, DC: The National Academies Press. [Link]
Peecher, D. (2018). Modeling the psychiatric aspects of polycystic ovary syndrome and induced stress [MA thesis]. Washington: Central Washington University. [Link]
Rassi, A., Veras, A. B., dos Reis, M., Pastore, D. L., Bruno, L. M., & Bruno, R. V., et al. (2010). Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Comprehensive Psychiatry, 51(6), 599-602. [PMID]
Reddy, P. S., Begum, N., Mutha, S., & Bakshi, V. (2016). Beneficial effect of curcumin in letrozole-induced polycystic ovary syndrome. Asian Pacific Journal of Reproduction, 5(2), 116-122. [DOI:10.1016/j.apjr.2016.01.006]
Ressler, I. B., Grayson, B. E., Ulrich-Lai, Y. M., & Seeley, R. J. (2015). Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. American Journal of Physiology-Endocrinology and Metabolism, 308(12), E1076–E1084. [PMID]
Sarkaki, A., Fathimoghaddam, H., Mansouri, S. M., Korrani, M. S., Saki, G., & Farbood, Y. (2014). Gallic acid improves cognitive, hippocampal long-term potentiation deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Pakistan Journal of Biological Sciences, 17(8), 978-990. [PMID]
Seibenhener, M. L., & Wooten, M. C. (2015). Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments : JoVE, (96), e52434.[PMID]
Sestakova, N., Puzserova, A., Kluknavsky, M., & Bernatova, I. (2013). Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdisciplinary Toxicology, 6(3), 126-135. [PMID] [PMCID]
Silva, M. S., Prescott, M., & Campbell, R. E. (2018). Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight, 3(7), e99405. [PMID] [PMCID]
Tan, R. Y., Grigg, J., & Kulkarni, J. (2018). Borderline personality disorder and polycystic ovary syndrome: A review of the literature. The Australian and New Zealand journal of psychiatry, 52(2), 117-128. [PMID]
Wang, L., Qi, H., Baker, P. N., Zhen, Q., Zeng, Q., & Shi, R., et al. (2017). Altered circulating inflammatory cytokines are associated with anovulatory polycystic ovary syndrome (PCOS) women resistant to clomiphene citrate treatment. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 23, 1083-1089. [PMID] [PMCID]
Yankelevitch-Yahav, R., Franko, M., Huly, A., & Doron, R. (2015). The forced swim test as a model of depressive-like behavior. Journal of Visualized Experiments: JoVE, (97), 52587. [PMID]
Yu, Q., Hao, S., Wang, H., Song, X., Shen, Q., & Kang, J. (2016).Depression-like behavior in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Biology of Reproduction, 95(4), 79. [PMID]
Zadehmodarres, S., Heidar, Z., Razzaghi, Z., Ebrahimi, L., Soltanzadeh, K., & Abed, F. (2015). Anti-mullerian hormon level and polycystic ovarian syndrome diagnosis. Iranian Journal of Reproductive Medicine, 13(4), 227-230. [PMID]
Zhang, H. Y., Guo, C. X., Zhu, F. F., Qu, P. P., Lin, W. J., & Xiong, J. (2013). Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case-control study. Archives of Gynecology and Obstetrics, 287(3), 525-531. [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2020/10/22 | Accepted: 2020/12/26 | Published: 2022/09/11
Received: 2020/10/22 | Accepted: 2020/12/26 | Published: 2022/09/11
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





