BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://bcn.iums.ac.ir/article-1-1693-en.html
1. Introduction
The hypothalamus widely secretes the Corticotropin-Releasing Hormone (CRH) throughout the brain under stressful conditions (Kyrou & Tsigos, 2009; Tsigos & Chrousos, 2002). Furthermore, CRH neural pathways are related to such other circuits as regulating food intake (Richard, Huang, & Timofeeva, 2000). For example, condensed CRH neurons are expressed in the hypothalamic Paraventricular Nucleus (PVN) as the main region where CRH is secreted (Kiss, Palkovits, & Aguilera, 1996; Makino et al., 2005). At the same time, the PVN has been recognized as the main region involved in food intake. CRH neurons are also found in other brain regions such as the limbic system, bed nucleus of the stria terminalis, locus coeruleus, cerebral cortex, and especially the central nucleus of the amygdala (CeA) as the extra-hypothalamic CRH region (Aguilera & Liu, 2012; Smagin, Heinrichs, & Dunn, 2001).
It is interesting to note that PVN and CeA regions are both rich in CRH receptors (Kang et al., 2011; Refojo & Holsboer, 2009). Hence, similar to PVN, the CeA has been considered an accessory nucleus capable of releasing CRH (Aguilera & Liu, 2012; Kovacs, 2013; Owens & Nemeroff, 1991).
On the other hand, the CRH neurons and receptors in both PVN and CeA are involved in regulating food intake, stress (Cai, Haubensak, Anthony, & Anderson, 2014; De Oliveira, Camboim, Diehl, Consiglio, & Quillfeldt, 2007), body weight, and metabolic control (Rabasa & Dickson, 2016). Previous studies have shown that central injection of CRH suppresses food intake and that daily intracerebroventricular administration of CRH reduces body weight (Rabasa & Dickson, 2016; Richardson, Omachi, Kermani, & Woods, 2002). Besides, various hormonal and food intake indicators (e.g. ghrelin, leptin, and glucose) regulate feeding behavior in both normal and stressful situations (Spencer, 2013). Peripheral signals from the gastrointestinal tract and adipose tissue, such as ghrelin and leptin, are also involved in food intake via the synthesis and secretion of hypothalamic neuropeptides (Carlini et al., 2004). However, controversial results have been reported on the effect of increased blood glucose levels on an enhanced sense of satiety as the result of activated glucoreceptors in the hypothalamus (Flint et al., 2007). Shiiya et al. (2002)showed that elevated blood glucose levels decrease serum ghrelin levels. Therefore, it seems that changes in food intake indicators and their interrelations might be involved in food intake regulation as their peripheral responses (Farr, Li, & Mantzoros, 2016; Fischer & O’Connell, 2017; Sobrino Crespo et al., 2014). Drawing upon previous research studies, we investigated the relationship between CRH microinjections into PVN and CeA under two types of acute psychological (social and isolation) stresses during three consecutive hours and cumulative food intake. We also checked the internal regulatory factors of food intake (e.g. serum ghrelin and leptin as well as blood glucose levels).
2. Materials and Methods
For this study, 66 male Wistar rats were obtained from the Pasteur Institute (Tehran, Iran) with an initial body weight of 200-250 g and were housed in a standard laboratory under controlled temperature (22±2°C) and humidity (50±5%) over a 12:12 h light/dark cycle (lights on at 07:00 AM–7:00 PM). Food and water were available ad libitum. The rats were allowed to adapt to the laboratory conditions (n=6 in each cage) for at least one week before surgery. The Research and Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1396.1.131) in compliance with the National Institute of Health Guide approved all the experimental conditions and procedures for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 2011 Revision). At the end of the experiments, the food intake measurement and hormonal assessment were performed in all groups (Figure 1).
.jpg)
The rats were randomly assigned to the following 11 groups (n=6 in each group):
‒ Control group (Co): rats transferred to the laboratory and received no special treatment throughout the study period.
‒ Sham-operated PVN group (Sh-PVN): rats underwent stereotaxic surgery and cannulation into the PVN for vehicle (saline) injection.
‒ Sham-operated CeA group (Sh-CeA): rats underwent stereotaxic surgery and cannulation into the CeA for vehicle (saline) injection.
‒ Group treated with CRH into their PVN (CRH-PVN): Rats received one-day microinjections of CRH into the PVN.
‒ Group treated with CRH into their CeA (CRH-CeA): rats received one-day microinjections of CRH into the CeA.
‒ Social Stress group (SS): rats were under acute social stress for one day.
‒ Isolation Stress group (IS): rats were under acute isolation stress for one day.
‒ Group subjected to social stress and receiving CRH injection into their PVN (SS-CRH-PVN): rats were under acute social stress for one day and received microinjection of CRH into their PVN.
‒ Group subjected to social stress and receiving CRH injection into their CeA (SS-CRH-CeA): rats were under acute social stress for one day and received microinjection of CRH into their CeA.
‒ Group subjected to isolation stress and receiving CRH injection into their PVN (IS-CRH-PVN): rats were under acute isolation stress for one day and received CRH microinjection into their PVN.
‒ Group subjected to isolation stress and receiving CRH injection into their CeA (IS-CRH-CeA): rats were under acute isolation stress for one day and received CRH microinjection into their CeA (Figure 1).
2.2. Experimental procedures
2.2.1. Stress paradigm
In the current study, two types (isolation and social) of stresses are induced in animals. To induce acute social stress, each rat was transferred to a new cage with 5 new neighbors, where they stayed for one day. Acute isolation stress-induced by keeping each rat in an individual cage without any neighbor (Grippo et al., 2007; Kalshetti, Alluri, Mohan, & Thakurdesai, 2015; Patki, Solanki, Atrooz, Allam, & Salim, 2013; Radahmadi, Hosseini Dastgerdi, Fallah, & Alaei, 2017; Ranjbar, Radahmadi, Alaei, & Reisi, 2014).
2.2.2. Stereotaxic surgery and cannulation
The animals were anesthetized with intraperitoneal (IP) injection of chloral hydrate (400 mg/kg) (Hosseini, Nasehi, Radahmadi, & Zarrindast, 2013) before being placed in a Stoelting stereotaxic apparatus (incisor bar±3.3 mm with symmetrically positioned ear bars). The skull was exposed to drill small holes above the PVN (AP= ‒1.92, ML= ±0.4, and DV= 8) and the central nucleus of the amygdala (AP= ‒1.8, ML= ±3.3, and DV= ‒8.8) according to the stereotaxic atlas of Paxinos and Watson (Paxinos & Watson, 2005). Then, a stainless-steel guide cannula (23 gauge) was unilaterally implanted and fixed 1 mm above the PVN or CeA before a stainless steel stylet (30 gauge) has been introduced to prevent any likely obstruction. Following the surgery, all animals received gentamycin injections (5 mg/kg; IP) to prevent infection (Bhardwaj, Deshmukh, Kaundal, & Reddy, 2016; Singh & Kumar, 2017) and were allowed for about 5 days to recover from surgery and the remnant effects of the anesthetic agent.
2.2.3. Drug microinjection into the PVN and CeA
Depending on their experimental group, the rats would receive intra-nuclear (PVN or CeA) injections of saline-dissolved corticotropin-releasing hormone (2 µg/kg in 0.5 µL saline) (CRH; Sigma-Aldrich Co., USA). After stereotaxic surgery and PVN and CeA nuclei cannulation, equal volumes of only the vehicle (0.5 µL saline) were injected into either the PVN or the CeA nuclei of both sham groups (i.e. Sh-PVN and Sh-CeA) between 7:00-8:00 AM for one day. Moreover, the animals restrained in hand for the styles to be removed from the guide cannula and replaced with dental injection needles (27 gauge) 1 mm longer than the guide cannula that would be subsequently connected to 10-µL Hamilton micro-syringes via polyethylene tubing (PE-20). CRH and or saline (for the experimental and sham groups, respectively) were injected into either PVN or CeA nuclei using an automated microinjection pump. The forward movement of a small air bubble inside the polyethylene tubing interposed between the upper end of the needle and the microinjection pump was taking as drug flow. The solutions (drug and saline) were injected in a total volume of 0.5 µL for 60 s. Following the injections, the needles were left in place for an extended 60 s to facilitate drug diffusion.
2.2.4. Food intake paradigm
The simplest paradigm used for food intake investigations is to record the mass of food eaten during a fixed time (Kristenssson et al., 2006). The food intake trials normally were conducted between 9:00‒12:00 AM for rats deprived of food for 16‒18 h after one day of stress treatment. On day 2, the rats were transferred to the laboratory at least 1 hour before the beginning of the food intake trial. Food pellets were weighed on an hourly basis over three consecutive hours (Izadi, Radahmadi, Ghasemi, & Rayatpour, 2017; Rayatpour, Ghasemi, Radahmadi, & Izadi, 2017). Subsequently, the rats were placed individually in transparent Plexiglas cages lined at the bottom with thick white paper. Then, the rats were allowed access to a pre-measured amount of regular lab chow. At the end of each hour of the 3-hour feeding periods, the leftover in the first test cage, including crumbs, were measured and reported as the amount consumed.
2.2.5. Assessment of serum leptin, ghrelin, and blood glucose levels
As planned for this study, after the food deprivation period (on day 2), blood samples (500 µL) were collected from the tail vein at 8:00-9:00 AM to assess blood glucose levels using a glucometer (On Call Plus Co., USA). Additional blood samples were collected in plastic vials and centrifuged at 6000 rpm for 20 min to separate serum samples that would be subsequently stored at -80°C until hormone (ghrelin and leptin) analysis. Serum levels of ghrelin and leptin, using the commercial Enzyme-Linked Immunosorbent Assay (ELISA) kit (Zellbio Co., Germany) with a detection limit of 0.1–20 ng/mL for rat leptin and a sensitivity of 0.05 ng/mL (intra-assay precision CV<10% and inter-assay precision CV<12%). The detection limit for rat ghrelin was set at 0.4–12.8 ng/mL and sensitivity at 0.025 ng/mL (intra-assay precision CV<10% and inter-assay precision CV<12%).
2.3. Histology
At the end of the experiments, the animals were decapitated at 13:00‒14:00. Then, their brains were removed and stored in 10% formalin for at least 3 days. Besides, frozen serial transverse sections (60 μm) were cut from the brain, and a light microscope was used to identify the PVN and CeA injection sites according to a rat brain atlas (Figure 2).
.jpg)
2.4. Statistical analysis
The feeding trials and other variables (e.g. ghrelin, leptin, and glucose levels) were analyzed using ANOVA followed by the LSD post-hoc test for multiple groups. The results were reported as Means±Standard Error of The Mean (SEM). P values less than were 0.05 considered statistically significant. Ultimately, calculations were performed using SPSS V. 24 (SPSS Inc., Chicago, IL, USA).
3. Results
.jpg)
In the current study, the different variables are presented in 5 aspects. A first aspect of the present research was investigating the food intake changes and their indicators due to either isolation or social stresses (IS and SS groups vs. control group) as naturally activated CRH because of activated hypothalamus-pituitary-adrenal (HPA) axis in stress conditions.
The second aspect of the present study investigated the effects of exogenously CRH injection into different (PVN and CeA) nuclei compared with the control, and both stressed (as naturally activated CRH) groups (the CRH-PVN and CRH-CeA groups vs. the control, IS, and SS groups).
In the third aspect of the current study, all variables in the stressed sub-groups receiving CRH injections into both PVN and CeA were compared with those in control, and both stressed groups, groups receiving CRH without stress and even each other in the same nucleus (the IS-CRH-PVN, SS-CRH-PVN, IS-CRH-CeA, SS-CRH-CeA groups vs. the control, IS, SS, CRH-PVN and CRH-CeA groups).
The fourth aspect of this study was the comparison of groups receiving CRH injections into PVN and
CeA without stress condition on all variables (the CRH-PVN group vs. the CRH-CeA group).
Finally, as a fifth aspect of the current study, all stressed sub-groups receiving CRH injections into the PVN were compared with similar groups receiving CRH injections into the CeA in terms of their anorectic effects and their impacts on food intake indicators (the IS-CRH-PVN vs. IS-CRH-CeA group; SS-CRH-PVN vs. the SS-CRH-CeA group).
3.1. Measurement of food intake
3.1.1. Effects of acute social and isolation stresses on food intake after 1, 2, and 3 hours, as well as cumulative food intake
The 1-way ANOVA followed by the LSD post-hoc test revealed no significant differences in the social and isolation stress (SS and IS) groups compared with the Co group and each other 1 and 3 hours after food intake (Figures 3A and 5A). Whereas the food intake after 2 hours was significantly lower in the IS group than in the control and SS groups (P<0.05 and P<0.01, respectively) (Figure 4A).
.jpg)
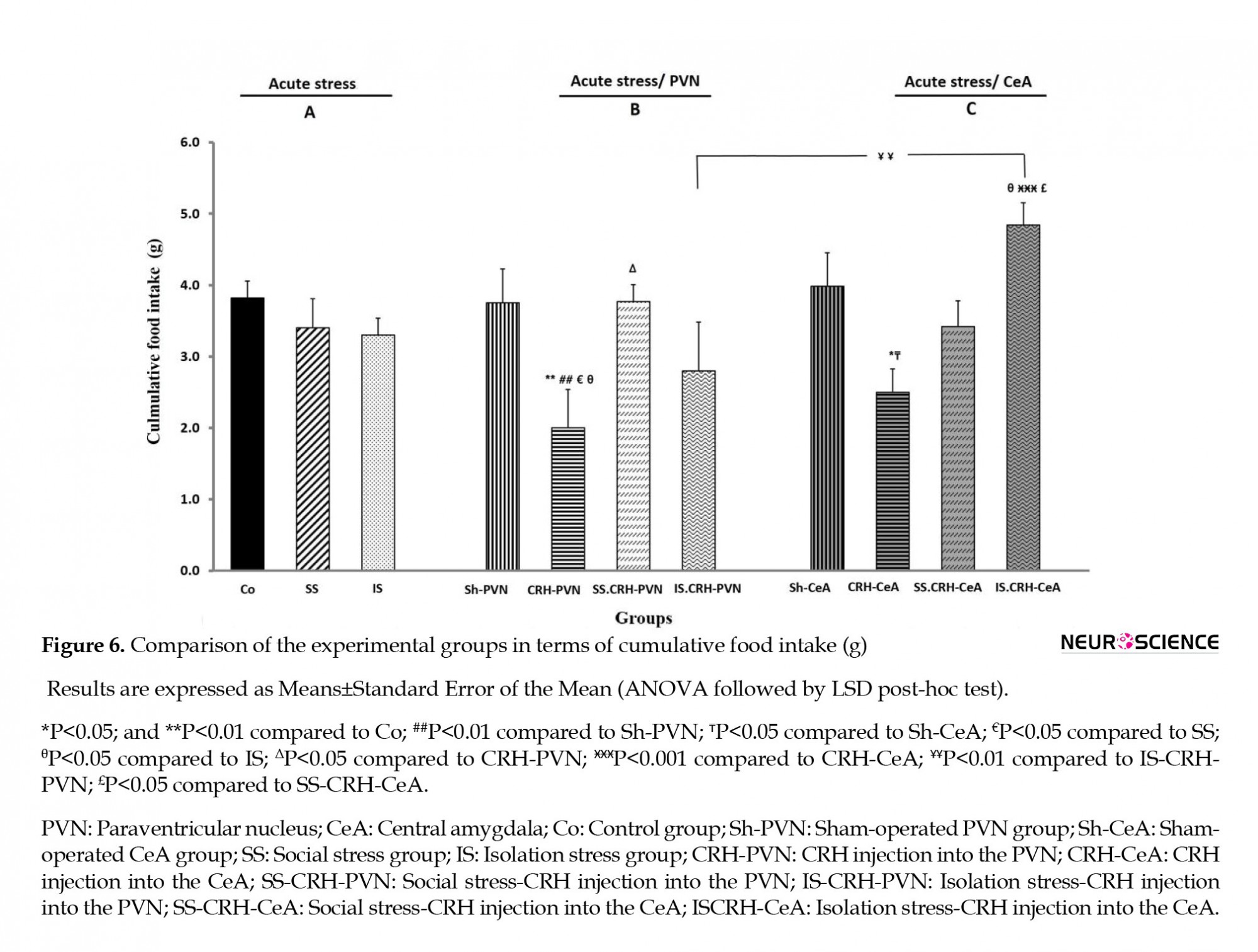
There were no significant differences in the SS and IS groups compared with the Co group and each other in cumulative food intake (Figure 6A).
3.1.2. Effects of CRH injections into PVN and CeA in the normal groups on food intake after 1, 2, and 3 hours, as well as cumulative food intake
3.1.2.1. Effects of CRH injections into PVN
There were no significant differences in food intake after 1 hour of CRH injection into the PVN (CRH-PVN) group than the Co, SS, and IS groups (Figure 3B). Whereas, food intake after 2 hours significantly decreased in the CRH-PVN group when compared with Co and SS group (P<0.001 in both) (Figure 4B). Also, compared to the Co, SS, and IS groups, the CRH-PVN group exhibited significant (P<0.01, P<0.05, and P<0.001, respectively) decreases in their food intake in the third hour (Figure 5B).
.jpg)
Significant declines were observed in cumulative food intake by the CRH-PVN group relative to the values measured in Co, SS, and IS groups (P<0.01, P<0.05, and P<0.05, respectively) (Figure 6B).
3.1.2.2. Effects of CRH injections into CeA
There were no significant differences in food intake 1 and 2 hours after CRH injection into the
CeA (CRH-CeA) group compared to the Co, SS, and IS groups (Figures 3C and 4C). The food intake after 3 hours had significant decreases in the CRH-CeA group relative to the values measured for Co, SS, and IS (P<0.001, P<0.01, and P<0.001, respectively) (Figure 5C). Significant decline (P<0.01) was observed in cumulative food intake by the CRH-CeA group compared to the Co group (Figure 6C).
3.1.3. Effects of CRH injections into PVN and CeA in stressful condition on food intake after 1, 2, and 3 hours, as well as cumulative food intake
3.1.3.1. Effects of CRH injections into PVN
The social stress group receiving CRH injections into the PVN (SS-CRH-PVN) showed significant increases in their food intake during the first hour when compared with the Co, SS, and CRH-PVN groups (P<0.05, P<0.01, and P<0.5, respectively) (Figure 3B).
Food intake in the second hour also significantly (P<0.05) decreased in the SS-CRH-PVN group compared to that in the SS group, but significantly (P<0.01) increased compared to that in the CRH-PVN group (Figure 4B). Also, significant decreases (P<0.01 and P<0.05, respectively) in food intake at the third hour were recorded for the SS-CRH-PVN group as compared with the Co and SS groups (Figure 5B).
As shown in Figure 3B, the isolation stress group receiving CRH injections into the PVN (IS-CRHPVN) did not show significant differences in food intake after 1 hour compared to Co, IS, and CRH-PVN groups. The IS-CRH-PVN group showed significant (P<0.05 and P<0.001, respectively) enhancements in their food intake after 2 hours when compared with the IS and CRH-PVN groups (Figure 4B). At the same time, the IS-CRH-PVN group exhibited significant (P<0.01 in both) decreases in food intake after 3 hours compared to the values recorded for the Co and IS groups (Figure 5B). Besides, ISCRH-PVN exhibited a significant (P<0.01) decrease compared to SS-CRH-PVN only in their food intake after 1 hour (Figure 3B).
As shown in Figure 6B, the cumulative food intake significantly (P<0.05) increased in the SS-CRH-PVN group compared to that recorded for the CRH-PVN group.
3.1.3.2 Effects of CRH injections into CeA
No significant differences were observed in food intake after 1 and 2 hours in the social stress group receiving CRH injections into the CeA (SS-CRH-CeA) compared with the Co, SS, and CRH-CeA groups (Figures 3C and 4C). At the same time, food intake after 3 hours had significantly (P<0.05) enhancement in comparison to the CRH-CeA group (Figure 5C).
As shown in Figure 3C, compared to the Co and IS groups (P<0.01 in both), the isolation stress group receiving CRH injections into the CeA (IS-CRH-CeA) showed significant enhancements in their first-hour food intake. In contrast, the IS-CRH-CeA group recorded a significant increase(P<0.001) in this parameter compared to the values measured in the CRH-CeA groups (Figure 3C). Also, the IS-CRH-CeA group showed significant (P<0.01) enhancements in food intake after 3 hours compared to the values recorded for the CRH-CeA group (Figure 5C). The IS-CRH-CeA group recorded significant increases and decreases (P<0.001 and P<0.05, respectively) in food intake after 1 and 2 hours compared with SS-CRH-CeA groups (Figures 3C and 4C). Finally, the cumulative food intake increased significantly in the IS-CRH-CeA group compared to the values measured for IS, CRH-CeA, and SS-CRH-CeA groups (P<0.05, P<0.001, and P<0.05, respectively) (Figure 6C).
3.1.4. Comparison of groups receiving CRH injections into PVN and CeA without stress on food intake after 1, 2, and 3 hours as well as cumulative food intake
As seen in Figure 4, only the food intake in the second hour had significant (P<0.01) enhancements in the CRH-CeA group when compared with the CRH-PVN group (both groups receiving similar injections). There were no significant differences in cumulative food intake of the CRH-CeA group compared to the CRH-PVN group (Figure 6).
3.1.5. Comparison of acute stress sub-groups receiving CRH injections into the PVN and CeA on food intake after 1, 2, and 3 hours as well as cumulative food intake
As shown in Figure 3B, C, food intake after 1 hour increased significantly (P<0.001) in the IS-CRH-CeA group versus the values measured in the IS-CRH-PVN group (similar in every way but receiving injections in different nuclei). Whereas, food intake after 2 hours showed significant (P<0.05) enhancement in the SS-CRH-CeA group compared with the value recorded for the SS-CRH-CeA group (Figure 4B, C). However, there were no significant differences in food intake after 3 hours between these groups (Figure 5B, C).
A Significant (P<0.01) enhancement was observed in the IS-CRH-CeA in comparison with the IS-CRH-PVN groups in cumulative food intake (Figure 5B, C).
3.3. Assessment of serum leptin and ghrelin levels as well as blood glucose level
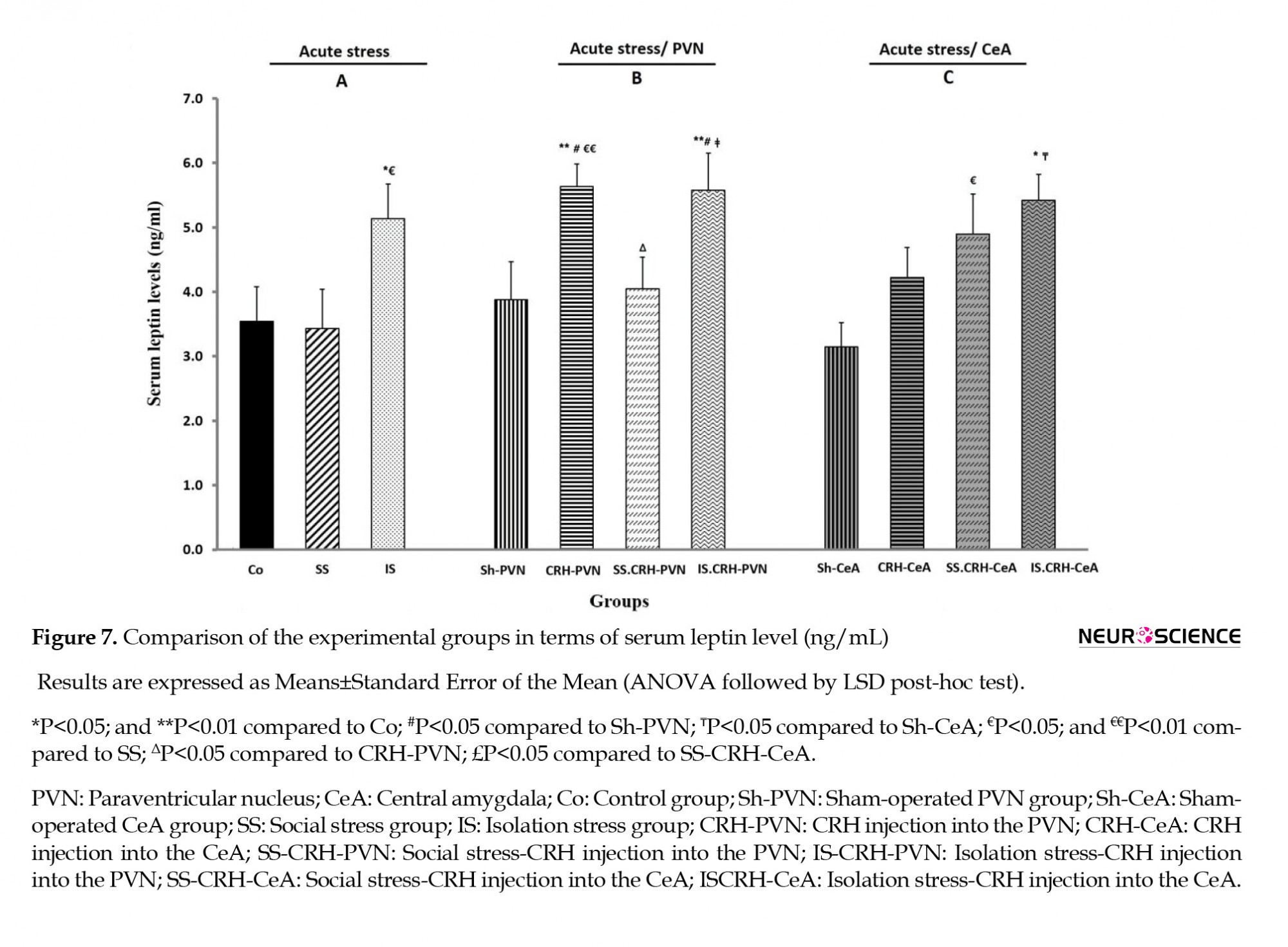
The IS, CRH-PVN, IS-CRH-PVN, and IS-CRH-CeA groups exhibited a significant increase in their serum leptin levels (P<0.05 in IS and IS-CRH-CeA, P<0.01 in both CRH-PVN and IS-CRH-PVN) compared to the Co group (Figure 7). Besides, significant enhancements were observed in this same parameter for the IS, CRH-PVN, and SS-CRH-CeA groups (P<0.05 in IS and SS-CRH-CeA, P<0.01 in CRH-PVN) compared to that measured in the SS group (Figure 7). The serum leptin level was significantly (P<0.05) lower in the SS-CRH-PVN group than in the CRH-PVN group (Figure 7B). Besides, the IS-CRH-PVN group showed a statistically significant (P<0.05) enhancement in its serum leptin level compared to that in the SS-CRH-PVN group (Figure 7B).
This is while serum ghrelin levels exhibited no significant differences among the sub-groups of PVN and CeA nuclei, although the CRH-PVN groups showed slightly elevated levels (Figure 8).

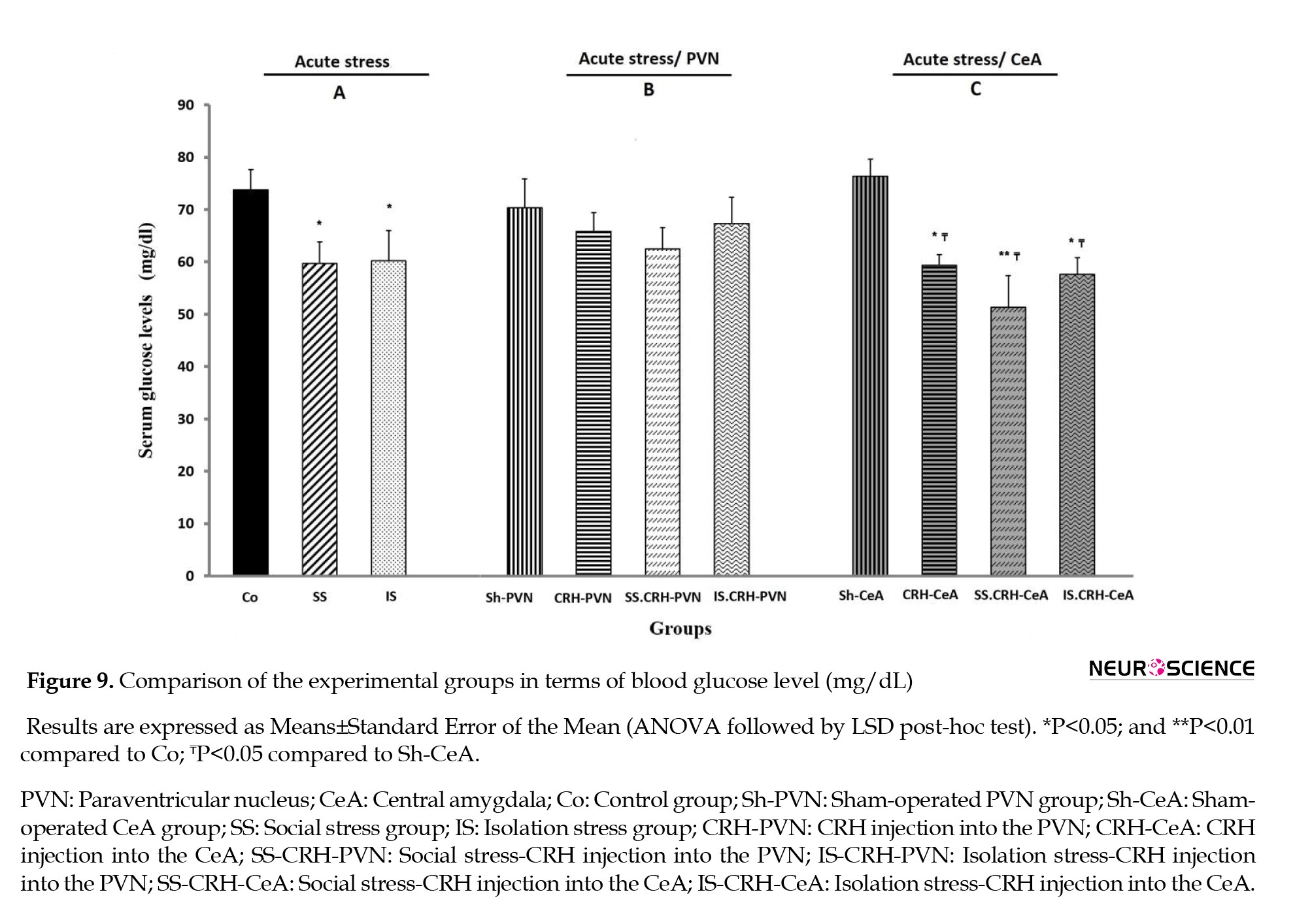
Finally, compared to the Co group, the IS, SS, and all groups receiving CRH into the CeA (CRH-CeA, SS-CRH-CeA, and IS-CRH-CeA) groups showed significant (P<0.05 in all) decreases in their blood glucose levels. An almost similar (P<0.01) decrease in blood glucose level was observed in the SS-CRH- CeA group relative to that in the Co group (Figure 9).
4. Discussion
4.1. Effects of two types of acute social and isolation stresses on all variables
The data obtained from the experiments indicated that acute isolation and social stresses led to insignificant decreases in cumulative food intake, probably due to the restricted duration of the acute stress. This finding confirmed those reported by Marquez, Belda, and Armario (2002).
On the other hand, in the isolation stress group, food intake decreased in the second hour before its increase in the third hour. This finding is much similar to the food intake behavior observed under normal conditions. More specifically, the isolated (single-housed) rats seemed to spend more time eating during the third hour, as reduction of food intake at the second hour almost completely reversed in the third hour. A previous report that demonstrated the hyper-responsiveness of individually housed rats to stressors (Perello, Chacon, Cardinali, Esquifino, & Spinedi, 2006). Food consumption inversely insignificantly and significantly changed after two and three hours in the social and isolation stressed groups, respectively. It indicates the role of the physiological system in balancing food intake in consecutive hours in the different stressed groups. A previous report demonstrated that energy balance is achieved when the input is equal to output food (dietary energy intake versus energy expenditure) (Maclean et al., 2003).
Compared to the control group, the leptin, glucose, and ghrelin levels, respectively, increased, decreased, and did not change the isolation stressed group. In contrast, the social stressed group recorded no significant changes in their serum leptin and ghrelin levels but exhibited a reduction only in their glucose levels similar to those of the isolation stress group. Furthermore, it may be claimed that identical mechanisms are involved in regulating glucose and ghrelin levels, but not leptin in both social and isolation stress groups. It seems that isolation stress had a more adverse effect on increasing serum leptin level and a probable reduction of appetite and food intake after two hours than social stress. Some studies reported that leptin inhibited appetite and food intake while also regulated energy expenditure (Kalra & Kalra, 2004; Sobrino Crespo et al., 2014). Consistent with these findings, it was reported that changes in sympathetic activation during acute stress increase the white adipose tissue and serum leptin level (Potretzke, Nakajima, Cragin, & al’Absi, 2014). Bernier, Gorissen and Flik (2012) identified leptin as a potent anorexic signal-regulating food intake under hypoxia stress. Contrary to the present findings, Ortolani, Oyama, Ferrari, Melo, and Spadari-Bratfisch (2011) showed that the anorexic effect of footshock stress was irrelevant to elevated serum leptin.
As mentioned before, blood glucose levels unexpectedly decreased in both acute isolation and social stressed groups. However, perhaps hypoglycemia is related to varied mechanisms such as involvement of different adrenergic receptors, elevated insulin release, and high glucose clearance (Armario, Castellanos, & Balasch, 1985; Nowotny et al., 2010). Current data are confirmed by decreased serum glucose levels in response to acute noise stress (Armario et al., 1985). In contrast, a study demonstrated increases in blood glucose (i.e. hyperglycemia) due to the release of glucocorticoids from the adrenal and epinephrine from the sympathetic nervous system during stress confrontation (Floyd et al., 2017). Overall, changes in food intake and its indicators in response to stress depends on stressor characteristics such as type, duration, and intensity as well as the individual’s characteristics, metabolic state, dietary restraint (Adam & Epel, 2007; Radahmadi, Alaei, Sharifi, & Hosseini, 2015; Torres & Nowson, 2007), or even time of food intake evaluation.
4.2. Effects of CRH injections into the PVN and CeA in normal individual on all variables
The cumulative food intake decreased in acute CRH injection into both PVN and CeA nuclei, particularly in that of PVN when compared to the control group. Besides, acute CRH injection into the PVN decreases food intake in the second and third hours, whereas CRH injection into the CeA decrease food intake only in the third hour. Some evidence supports that food intake reduces because of exogenously CRH injection into such parts of the brain as the third cerebral ventricle and PVN (Benoit et al., 2000; Krahn, Gosnell, Levine, & Morley, 1988; Sobrino Crespo et al., 2014) while the dosage seems not to affect. Hence, the effect of CRH on food intake might be both context- and state-dependent (Hillebrand, de Wied, & Adan, 2002). Since the food intake response to CRH injection into the PVN was observed earlier than did the CeA, the role of PVN is more important on food intake. Therefore, it seems that the anorectic effects of CRH injection into the brain nuclei must be governed by different mechanisms such as activation of neuronal circuits, receptor subtypes activated by the hormone (Benoit et al., 2000), enhanced CRH mRNA and receptor expression (Makino et al., 2005), inhibition of parasympathetic nervous system and gastric emptying (Stengel & Tache, 2009), inhibited neuropeptide Y (NPY), and stimulated proopiomelanocortin (POMC) expressions in the hypothalamus (Calvez et al., 2011). While, a previous study has indicated the important role of CeA in food intake behavior via the involvement of the hypothalamus-amygdala circuit and the transmission of inhibitory signals to the arcuate nucleus and lateral hypothalamus (Baxter & Murray, 2002; Cai et al., 2014; Lee, Gallagher, & Holland, 2010; Petrovich & Gallagher, 2003; Petrovich, Canteras, & Swanson, 2001).
The other findings showed that leptin levels rose in response to CRH injection into the PVN (CRH-PVN) while it only partially increased with CRH injection into the CeA (CRH-CeA). On the other hand, compared to the control group, blood glucose level declined only in the CRH-CeA group rather than the CRH-PVN group. Since the regulation of CRH receptor numbers might be an essential mechanism involved in modulating food intake response, the different responses to it by PVN and CeA are explained through their likely differences in CRH receptor densities under CRH injection. This is while most studies investigating the regulation of CRH receptors have been focused on the PVN at the neglect of the CeA (Lu et al., 2005). It has also been suggested that response to feeding might even depend on the nutritional state of the animal when CRH is injected in both nuclei (Hillebrand et al., 2002). Thus, when examining the effect of neuropeptides on food intake behavior, both the context and the state of the organism are recommended to be taken into account (Hillebrand et al., 2002).
4.3. Comparison of CRH injections into the PVN and CeA compared with those in both stress groups (as naturally activated CRH) on all variables
Cumulative food intake was significantly lower in the CRH-PVN group, but not in the CRH-CeA group, than in social and isolation stress groups. Besides, elevated leptin levels in the CRH-PVN group also may confirm the reduction of cumulative food intake. This is explained by the fact that the CRH receptor density was probably higher in the PVN than in the CeA, suggesting that exogenous injection of CRH into the PVN had perhaps a more anorectic effect than did that CeA. Some reports are expressed different levels of urocortin (as a CRH neuropeptide family) mRNA in the paraventricular nucleus and amygdala that are involved in stress-coping responses related to CRH (Reul & Holsboer, 2002).
4.4. Comparison of the PVN and or CeA stressed sub-groups receiving CRH injections on all variables
Based on the current findings, no major differences were detected in cumulative food intake between the social and isolation stress groups receiving CRH injections into their PVN or CeA (i.e. SS-CRH-PVN, SS-CRH-CeA, IS-CRH-PVN, and IS-CRH-CeA) when compared with the control. Although food intake over three consecutive hours showed various responses that it seems compensatory effects in consecutive hours in those groups. Furthermore, leptin level increased only in the IS-CRH-PVN and IS-CRH-CeA groups when compared with the control. Changes in serum leptin level confirmed deceased cumulative food intake only in the IS-CRH-PVN group, but not in the IS-CRH-CeA group that confirmed the role of different circuits nuclei in food intake. The observed food intake reduction might be related to the direct projection in the PVN, whereas the CeA received projection indirectly from the basolateral amygdala nucleus (Welberg & Seckl, 2001).
A significantly increased cumulative food intake was observed in the SS-CRH-PVN group compared to that measured in the CRH-PVN group. Also, the IS-CRH-CeA group exhibited a similar increase when compared with the CRH-CeA. Differences indicate that various stress types and brain nuclei were involved in the food intake responses observed (Berthoud, 2004; Harrold, Dovey, Blundell, & Halford, 2012; Izadi, Radahmadi, Ghasemi, & Rayatpour, 2018). Furthermore, reduced serum leptin levels were observed only in the SS-CRH-PVN group rather than in the CRH-PVN group, indicating that decreased leptin level was more effective than those of ghrelin and glucose in elevated food intake in the groups receiving CRH injections into their PVNs under acute social stress (SS-CRH-PVN) than in similar animals subjected to acute isolation stress (IS-CRH-PVN).
In the IS-CRH-CeA, cumulative food intake increased when compared with the values measured in the CRH-CeA group, those subjected to isolation stress, or the SS-CRH-CeA group. Our results also indicated that, compared to the control group, the IS-CRH-CeA group exhibited elevated serum leptin but decreased blood glucose levels. Changes in food intake in the groups receiving CRH into their CeAs must have occurred via mechanisms other than the leptin, glucose, and ghrelin levels. Some studies indicated some different mechanism for food intake behavior regulation by the amygdala, such as the activity of an enzyme (protein kinase c) changes, insulin signaling, dopamine, oxytocin, express of NPY and NPY receptors in this brain area (Areias & Prada, 2015; Chaves, Tilelli, Brito, & Brito, 2013; Primeaux, Wilson, Cusick, York, & Wilson, 2005).
4.5. Comparison of the sub-groups receiving CRH injections into the PVN with CeA on all variables
Moreover, the IS-CRH-CeA group, compared to the IS-CRH-PVN group, showed a significant increase in their cumulative food intake, indicating the greater role of PVN’s CRH receptors regarding the anorectic effect than that of the CeA ones. However, leptin, ghrelin, and glucose levels did not show any significant differences among these groups, suggesting different food intake mechanisms in the relevant groups.
Finally, significant decreases in blood glucose levels were detected among all the experimental groups receiving CRH injections into their CeAs. It seems that the CeA was sensitive to changes in glucose levels while PVN was sensitive to those in leptin. Stimulation of sympathetic (Beta) innervation has been reported to release insulin and diminish blood glucose levels (Hillebrand et al., 2002). Hence, the CeA seems to receive more beta-adrenergic innervation than the alpha-adrenergic one. However, such other factors as type, duration, and intensity of psychological stresses that are difficult to manipulate in humans also seem to have a bearing on the changes observed in feeding patterns as a result of metabolic responses to stress and CRH, calling for further research to shed more light on the possible mechanism(s) involved.
5. Conclusion
Ethical Considerations
Compliance with ethical guidelines
Funding
Authors' contributions
Conflict of interest
References
Adam, T. C., & Epel, E. S. (2007). Stress, eating and the reward system. Physiology & Behavior, 91(4), 449-58. [DOI:10.1016/j.physbeh.2007.04.011] [PMID]
Aguilera, G., & Liu, Y. (2012). The molecular physiology of CRH neurons. Frontiers in Neuroendocrinology, 33(1), 67-84. [DOI:10.1016/j.yfrne.2011.08.002] [PMID] [PMCID]
Areias, M. F., & Prada, P. O. (2015). Mechanisms of insulin resistance in the amygdala: Influences on food intake. Behavioural Brain Research, 282, 209-17. [DOI:10.1016/j.bbr.2015.01.003] [PMID]
Armario, A., Castellanos, J. M., & Balasch, J. (1985). Chronic noise stress and insulin secretion in male rats. Physiology & Behavior, 34(3), 359-61. [DOI:10.1016/0031-9384(85)90196-9]
Baxter, Mark G, & Murray, Elisabeth A. (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563-73. [DOI:10.1038/nrn875] [PMID]
Benoit, S. C., Thiele, T. E., Heinrichs, S. C., Rushing, P. A., Blake, K. A., & Steeley, R. J. (2000). Comparison of central administration of corticotropin-releasing hormone and urocortin on food intake, conditioned taste aversion, and c-Fos expression. Peptides, 21(3), 345-51. [DOI:10.1016/S0196-9781(00)00153-4]
Bernier, N. J., Gorissen, M., & Flik, G. (2012). Differential effects of chronic hypoxia and feed restriction on the expression of leptin and its receptor, food intake regulation and the endocrine stress response in common carp. The Journal of experimental biology, 215(Pt 13), 2273-82. [DOI:10.1242/jeb.066183] [PMID]
Berthoud, H. R. (2004). Mind versus metabolism in the control of food intake and energy balance. Physiology & Behavior, 81(5), 781-93. [DOI:10.1016/j.physbeh.2004.04.034] [PMID]
Bhardwaj, M., Deshmukh, R., Kaundal, M., & Reddy, B. K. (2016). Pharmacological induction of hemeoxygenase-1 activity attenuates intracerebroventricular streptozotocin induced neurocognitive deficit and oxidative stress in rats. European Journal of Pharmacology, 772, 43-50. [DOI:10.1016/j.ejphar.2015.12.037] [PMID]
Cai, H., Haubensak, W., Anthony, T. E., & Anderson, D. J. (2014). Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nature Neuroscience, 17(9), 1240-8. [DOI:10.1038/nn.3767] [PMID] [PMCID]
Calvez, J., Fromentin, G., Nadkarni, N., Darcel, N., Even, P., & Tome, D., et al. (2011). Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiology & behavior, 104(5), 675-83. [DOI:10.1016/j.physbeh.2011.07.012] [PMID]
Carlini, V. P., Varas, M. M., Cragnolini, A. B., Schioth, H. B., Scimonelli, T. N., & de Barioglio, S. R. (2004). Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochemical and Biophysical Research Communications, 313(3), 635-41. [DOI:10.1016/j.bbrc.2003.11.150] [PMID]
Chaves, V. E., Tilelli, C. Q., Brito, N. A., & Brito, M. N. (2013). Role of oxytocin in energy metabolism. Peptides, 45, 9-14. [DOI:10.1016/j.peptides.2013.04.010] [PMID]
De Oliveira, L. F., Camboim, C., Diehl, F., Consiglio, A. R., & Quillfeldt, J. A.. (2007). Glucocorticoid-mediated effects of systemic oxytocin upon memory retrieval. Neurobiology of Learning and Memory, 87(1), 67-71. [DOI:10.1016/j.nlm.2006.05.006] [PMID]
Ergang, P., Vodicka, M., Sotak, M., Klusonova, P., Behuliak, M., & Rehakova, L., et al. (2015). Differential impact of stress on hypothalamic-pituitary-adrenal axis: gene expression changes in Lewis and Fisher rats. Psychoneuroendocrinology, 53, 49-59. [DOI:10.1016/j.psyneuen.2014.12.013] [PMID]
Farr, O. M., Li, C. S., & Mantzoros, C. S. (2016). Central nervous system regulation of eating: Insights from human brain imaging. Metabolism, 65(5), 699-713. [DOI:10.1016/j.metabol.2016.02.002] [PMID] [PMCID]
Fischer, E. K., & O’Connell, L. A. (2017). Modification of feeding circuits in the evolution of social behavior. The Journal of Experimental Biology, 220(Pt 1), 92-102. [DOI:10.1242/jeb.143859] [PMID]
Flint, A., Gregersen, N. T., Gluud, L. L., Moller, B. K., Raben, A., & Tetens, I., et al. (2007). Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. The British Journal of Nutrition, 98(1), 17-25. [DOI:10.1017/S000711450768297X] [PMID]
Floyd, K., Veksler, A. E., McEwan, B., Hesse, C., Boren, J. P., & Dinsmore, D. R., et al. (2017). Social inclusion predicts lower blood glucose and low-density lipoproteins in healthy adults. Health Commun, 32(8), 1039-42. [DOI:10.1080/10410236.2016.1196423] [PMID]
Grippo, A. J., Gerena, D., Huang, J., Kumar, N., Shah, M., & Ughreja, R., et al. (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology, 32(8-10), 966-80. [DOI:10.1016/j.psyneuen.2007.07.004] [PMID] [PMCID]
Hillebrand, J. J., de Wied, D., & Adan, R. A. (2002). Neuropeptides, food intake and body weight regulation: A hypothalamic focus. Peptides, 23(12), 2283-306. [DOI:10.1016/S0196-9781(02)00269-3]
Hosseini, N., Nasehi, M., Radahmadi, M., & Zarrindast, M. R. (2013). Effects of CA1 glutamatergic systems upon memory impairments in cholestatic rats. Behavioural Brain Research, 256, 636-45. [DOI:10.1016/j.bbr.2013.08.018] [PMID]
Izadi, M. S., Radahmadi, M., Ghasemi, M., & Rayatpour, A. (2018). Effects of isolation and social subchronic stresses on food intake and levels of leptin, ghrelin, and glucose in male rats. Advanced Biomedical Research, 7, 118. [DOI:10.4103/abr.abr_28_18] [PMID] [PMCID]
Izadi, M. S., Radahmadi, M., Ghasemi, M., & Rayatpour, A. (2017). Effect of repeated administration of Corticotropin-Releasing Hormone (CRH) in central amygdala nucleus on feeding behavior in adult male rats. Journal of Isfahan Medical School, 35(434), 707-12. https://www.researchgate.net/publication/319246028_
Kalra, S. P., & Kalra, P. S. (2004). NPY and cohorts in regulating appetite, obesity and metabolic syndrome: Beneficial effects of gene therapy. Neuropeptides, 38(4), 201-11. [DOI:10.1016/j.npep.2004.06.003] [PMID]
Kalshetti, P. B., Alluri, R., Mohan, V., & Thakurdesai, P. A. (2015). Effects of 4-hydroxyisoleucine from fenugreek seeds on depression-like behavior in socially isolated olfactory bulbectomized rats. Pharmacognosy Magazine, 11(Suppl 3), S388-96. [DOI:10.4103/0973-1296.168980] [PMID] [PMCID]
Kang, Y. M., Zhang, A. Q., Zhao, X. F., Cardinale, J. P., Elks, C., & Cao, X. M., et al. (2011). Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Research in Cardiology, 106(3), 473-83. [DOI:10.1007/s00395-011-0155-2] [PMID] [PMCID]
Kiss, A., Palkovits, M., & Aguilera, G. (1996). Neural regulation of Corticotropin Releasing Hormone (CRH) and CRH receptor mRNA in the hypothalamic paraventricular nucleus in the rat. Journal of Neuroendocrinology, 8(2), 103-12. [DOI:10.1111/j.1365-2826.1996.tb00830.x] [PMID]
Kovacs, K. J. (2013). CRH: the link between hormonal-, metabolic- and behavioral responses to stress. Journal of Chemical Neuroanatomy, 54, 25-33. [DOI:10.1016/j.jchemneu.2013.05.003] [PMID]
Krahn, D. D., Gosnell, B. A., Levine, A. S., & Morley, J. E. (1988). Behavioral effects of corticotropinreleasing factor: localization and characterization of central effects. Brain Research, 443(1-2), 639. [DOI:10.1016/0006-8993(88)91598-3]
Kristenssson, E., Sundqvist, M., Astin, M., Kjerling, M., Mattsson, H., & Dornonville de la Cour, C., et al. (2006). Acute psychological stress raises plasma ghrelin in the rat. Regulatory Peptides, 134(2-3), 114-17. [DOI:10.1016/j.regpep.2006.02.003] [PMID]
Kyrou, I., & Tsigos, C. (2009). Stress hormones: physiological stress and regulation of metabolism. Current Opinion in Pharmacology, 9(6), 787-93. [DOI:10.1016/j.coph.2009.08.007] [PMID]
Lee, H. J., Gallagher, M., & Holland, P. C. (2010). The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learning & Memory, 17(10), 531-8. [DOI:10.1101/lm.1889510] [PMID] [PMCID]
Lu, A., Steiner, M., Engelholm, M., Wotjak, C. T., Holsboer, F., & Wurst, W., et al. (2005). Site-specific overexpression of Corticotropin-Releasing Hormone (CRH) in the mouse brain- modelling central CRH system hyperactivity. Pharmacopsychiatry, 38(05), A148. [DOI:10.1055/s-2005-918770]
Maclean, W., Harnly, J., Chen, J., Chevassus-Agnes, S., Gilani, G., & Livesey, G., et al. (2003, February). Food energy–Methods of analysis and conversion factors. Retrieved from https://www.ars.usda.gov/research/publications/publication/?seqNo115=172524
Makino, S., Tanaka, Y., Nazarloo, H. P., Noguchi, T., Nishimura, K., & Hashimoto, K. (2005). Expression of type 1 corticotropin-Releasing Hormone (CRH) receptor mRNA in the hypothalamic paraventricular nucleus following restraint stress in CRH-deficient mice. Brain Research, 1048(12), 131-7. [DOI:10.1016/j.brainres.2005.04.065] [PMID]
Marquez, C., Belda, X., & Armario, A. (2002). Post-stress recovery of pituitary-adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Research, 926(1-2), 181-5. [DOI:10.1016/S0006-8993(01)03112-2]
Nowotny, Á., Cavka, M., Herder, C., Löffler, H., Poschen, U., & Joksimovic, L., et al. (2010). Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Hormone and Metabolic Research, 42(10), 746-53. [DOI:10.1055/s-0030-1261924] [PMID]
Ortolani, D., Oyama, L. M., Ferrari, E. M., Melo, L. L., & Spadari-Bratfisch, R. C. (2011). Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiology & Behavior, 103(5), 487-92. [DOI:10.1016/j.physbeh.2011.03.028] [PMID]
Owens, M. J., & Nemeroff, C. B. (1991). Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Reviews, 43(4), 425-73. https://d1wqtxts1xzle7.cloudfront.net/53102391
Patki, G., Solanki, N., Atrooz, F., Allam, F., & Salim, S. (2013). Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Research, 1539, 73-86. [DOI:10.1016/j.brainres.2013.09.033] [PMID] [PMCID]
Perello, M., Chacon, F., Cardinali, D. P., Esquifino, A. I., & Spinedi, E. (2006). Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sciences, 78(16), 1857-62. [DOI:10.1016/j.lfs.2005.08.029] [PMID]
Petrovich, G. D., & Gallagher, M. (2003). Amygdala subsystems and control of feeding behavior by learned cues. Annals of the New York Academy of Sciences, 985, 251-62. [DOI:10.1111/j.1749-6632.2003.tb07086.x] [PMID]
Petrovich, G. D., Canteras, N. S., & Swanson, L. W. (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews, 38(1), 247-89. [DOI:10.1016/S0165-0173(01)00080-7]
Potretzke, S., Nakajima, M., Cragin, T., & al’Absi, M. (2014). Changes in circulating leptin levels during acute stress and associations with craving in abstinent smokers: A preliminary investigation. Psychoneuroendocrinology, 47, 232-40. [DOI:10.1016/j.psyneuen.2014.05.008] [PMID] [PMCID]
Primeaux, S. D., Wilson, S. P., Cusick, M. C., York, D. A., & Wilson, M. A. (2005). Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology, 30(9), 1589-97. [DOI:10.1038/sj.npp.1300705] [PMID]
Rabasa, C., & Dickson, S. L. (2016). Impact of stress on metabolism and energy balance. Current Opinion in Behavioral Sciences, 9, 71-7. [DOI:10.1016/j.cobeha.2016.01.011]
Radahmadi, M., Alaei, H., Sharifi, M. R., & Hosseini, N. (2015). Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiology & Behavior, 139, 459-67. [DOI:10.1016/j.physbeh.2014.12.004] [PMID]
Radahmadi, M., Hosseini Dastgerdi, A., Fallah, N., & Alaei, H. (2017). The effects of acute, sub-chronic and chronic psychical stress on the brain electrical activity in male rats. Physiology and Pharmacology, 21(3), 185-92. http://ppj.phypha.ir/article-1-1280-en.pdf
Ranjbar, H., Radahmadi, M., Alaei, H., & Reisi, P. (2015). Effect of different durations of stress on spatial and cognitive memory in male rats. Journal of Isfahan Medical School, 32(309), 1933-43. https://www.researchgate.net/publication/281766235_
Rayatpour, A., Ghasemi, M., Radahmadi, M., & Izadi, M. S. (2017). Effect of intraparaventricular administration of corticotropin releasing hormone on food intake in food-deprived rats. Journal of Isfahan Medical School, 35(436), 770-75. https://www.researchgate.net/publication/319620665_
Refojo, D., & Holsboer, F. (2009). CRH signaling: molecular specificity for drug targeting in the CNS. Annals of the New York Academy of Sciences, 1179(1), 106-19. [DOI:10.1111/j.1749-6632.2009.04983.x] [PMID]
Reul, J. M., & Holsboer, F. (2002). On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues in Clinical Neuroscience, 4(1), 31-46. [DOI:10.31887/DCNS.2002.4.1/jreul] [PMID] [PMCID]
Richard, D., Huang, Q., & Timofeeva, E. (2000). The corticotropin-releasing hormone system in the regulation of energy balance in obesity. International Journal of Obesity, 24(2), S36-S39. [DOI:10.1038/sj.ijo.0801275] [PMID]
Richardson, R. D., Omachi, K., Kermani, R., & Woods, S. C. (2002). Intraventricular insulin potentiates the anorexic effect of corticotropin releasing hormone in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 283(6), R1321-R1326. [DOI:10.1152/ajpregu.00521.2001]
Shiiya, T., Nakazato, M., Mizuta, M., Date, Y., Mondal, M. S., & Tanaka, M., et al. (2002). Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. The Journal of Clinical Endocrinology & Metabolism, 87(1), 240-4. [DOI:10.1210/jcem.87.1.8129] [PMID]
Singh, S., & Kumar, P. (2017). Neuroprotective potential of curcumin in combination with piperine against 6-hydroxy dopamine induced motor deficit and neurochemical alterations in rats. Inflammopharmacology, 25(1), 69-79. [DOI:10.1007/s10787-016-0297-9] [PMID]
Smagin, G. N., Heinrichs, S. C., & Dunn, A. J. (2001). The role of CRH in behavioral responses to stress. Peptides, 22(5), 713-24. [DOI:10.1016/S0196-9781(01)00384-9]
Sobrino Crespo, C., Perianes Cachero, A., Puebla Jiménez, L., Barrios, V., & Arilla Ferreiro, E. (2014). Peptides and food intake. Frontiers in Endocrinology, 5, 58. [DOI:10.3389/fendo.2014.00058] [PMID] [PMCID]
Spencer, S. J. (2013). Perinatal programming of neuroendocrine mechanisms connecting feeding behavior and stress. Frontiers in Neuroscience, 7, 109. [DOI:10.3389/fnins.2013.00109]
Spiegelman, B. M., & Flier, J. S. (2001). Obesity and the regulation of energy balance. Cell, 104(4), 531-43. [DOI:10.1016/S0092-8674(01)00240-9]
Stengel, A., & Tache, Y. (2009). Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annual Review of Physiology, 71, 219-39. [DOI:10.1146/annurev.physiol.010908.163221] [PMID] [PMCID]
Torres, S. J., & Nowson, C. A. (2007). Relationship between stress, eating behavior, and obesity. Nutrition, 23(11-12), 887-94. [DOI:10.1016/j.nut.2007.08.008] [PMID]
Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865-71. [DOI:10.1016/S0022-3999(02)00429-4]
Welberg, L. A., & Seckl, J. R. (2001). Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology, 13(2), 113-28. [DOI:10.1111/j.1365-2826.2001.00601.x] [PMID]
Received: 2020/01/18 | Accepted: 2020/06/28 | Published: 2021/01/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








