Volume 15, Issue 4 (July & August 2024)
BCN 2024, 15(4): 455-462 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezayat E, Shayanfar F, HajiNasrollah M, Shakerian F, A. Dehaqani M. Custom-made Implants for Chronic In Vivo Electrophysiological Recording From Primate’s Brain Based on the Reconstructed Skull Model. BCN 2024; 15 (4) :455-462
URL: http://bcn.iums.ac.ir/article-1-1690-en.html
URL: http://bcn.iums.ac.ir/article-1-1690-en.html
Ehsan Rezayat1 

 , Farzad Shayanfar1
, Farzad Shayanfar1 
 , Mostafa HajiNasrollah2
, Mostafa HajiNasrollah2 
 , Farideh Shakerian3
, Farideh Shakerian3 
 , Mohammad-Reza A. Dehaqani *1
, Mohammad-Reza A. Dehaqani *1 




 , Farzad Shayanfar1
, Farzad Shayanfar1 
 , Mostafa HajiNasrollah2
, Mostafa HajiNasrollah2 
 , Farideh Shakerian3
, Farideh Shakerian3 
 , Mohammad-Reza A. Dehaqani *1
, Mohammad-Reza A. Dehaqani *1 


1- School of Cognitive Sciences, Institute for Research in Fundamental Sciences (IPM), Tehran, Iran.
2- Department of Brain and Cognitive Sciences, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran.
3- Cognitive Systems Laboratory, Department of Electrical and Computer Engineering, Control and Intelligent Processing Center of Excellence (CIPCE), School of Engineering, University of Tehran, Tehran, Iran.
2- Department of Brain and Cognitive Sciences, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran.
3- Cognitive Systems Laboratory, Department of Electrical and Computer Engineering, Control and Intelligent Processing Center of Excellence (CIPCE), School of Engineering, University of Tehran, Tehran, Iran.
Full-Text [PDF 4198 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
lthough long in use, extracellular single-unit recording in behaving animals is still the technique, and many studies have been drawn upon to investigate the temporal and spatial dimensions of cognitive activities. Several chambers and a holding headpost on the skull are required as a preparation step. The chamber provides the interface for recording electrodes and the drive system. A headpost or equivalent holding mechanism stabilizes and protects head motions. However, with this requirement comes a plethora of risks and considerations inherent in the procedure's invasiveness. Post-operative acute and chronic infections have the possibility of chronic exposure of soft tissue due to incomplete closure of the skin after surgery, leading to an almost permanent infection risk. Furthermore, chronic foreign body inflammations are the main complications and probable causes of implant failure.
While commercially available or custom-crafted versions of the implants are used, these pieces must be bent to fit into their required position after inspecting the curvature. The headposts and chambers are either screwed on the bone or, more commonly, are rooted into an acrylic base cover founded on a bolt and wire grid (Pfingst et al., 1989). The hand-bended headposts often leave gaps between the implant and bone surface, increasing the risk of infection and extending the surgery period. Acrylic resins are used extensively as denture bases and bone cement. These products, especially when leaking as monomers form the polymer complex, are known bio-incompatible and toxic materials to various tissues. Therefore, acrylic resins inhibit the successful regeneration of tissues after surgery. In addition, the heat produced while applying the exothermic variants may injure contacting cells, and resulting debris predisposes infecting bacterial growth (Jorge et al., 2003). In recent years, there has been a tendency to replace their usage with screw-only implants to reduce the risks associated with acrylic covers (Adams et al., 2011; Chen et al., 2017; McAndrew et al., 2012; Mulliken et al., 2015).
Following magnetic resonance (MR) and computerized tomography (CT) imaging, here we utilized a co-registered MR-CT reconstruction of the head region to make computer-aided design (CAD-designed) implants for two monkeys. Our suggested procedure applied the available skull areas and structures underneath the brain to be within access of the recording chamber. This procedure raises the availability of the target areas while reducing infection risks associated with an incomplete fit and longer surgical operations by providing an individualized design and a tight fit contact.
Here, we propose a protocol for custom crafting the implants based on the co-registered MR and CT series map. We hope this procedure can easily be adopted in neuroscience-based surgeries and improve some of the current methods. We also hope to increase the safety of the animals studied in neuroscience research.
2. Materials and Methods
Study animals
Two adult male rhesus monkeys (Macaca mulatta) were used. All the procedures, such as surgeries, post-operative care, behavioral studies, and management conditions, were strictly in accord with NIH guidelines for the care and use of laboratory animals and the internal regulations on animal care issued by the IPM-SCS Ethics Committee. The entire process, including the recovery phase and the time passed before fixing the headpost for the first time, took place in a 60-day interval.
CT and MR imaging
For precisely co-registering different imaging modalities on the same coordination and having the same reference frame for surgery, it is necessary to use a CT- and MRI-compatible stereotaxic frame (Tajhiz Gostar Iranian co, Iran) during imaging. In this adapter, the ear bars, the bite bar, and the eyepieces had to constrain the head precisely like a stereotaxic surgery frame. Proper frame fastening is essential, and any misplacement produces errors in computations. Figure 1 depicts the reconstruction of the head and the constraining imaging adapter. We put several marker-filled capillary tubes filled with a contrasting lipophilic material (vitamin E) in the frame, which makes it possible to set sliding steps for the geometry of the MRI image series. The marker-filled bars are in rows left and right and on the top side. When positioning the head into the MRI (CT) bed, care should be given. Using this frame, we performed the MRI (3.0 Tesla Siemens Prisma MRI Scanner) and CT (Somatom Spirit; Siemens). It is possible to perform only a T2-weighted MRI without CT imaging. In this case, the contrast of the skull surface will decrease, and more adjustments are needed to determine the threshold for reconstruction.
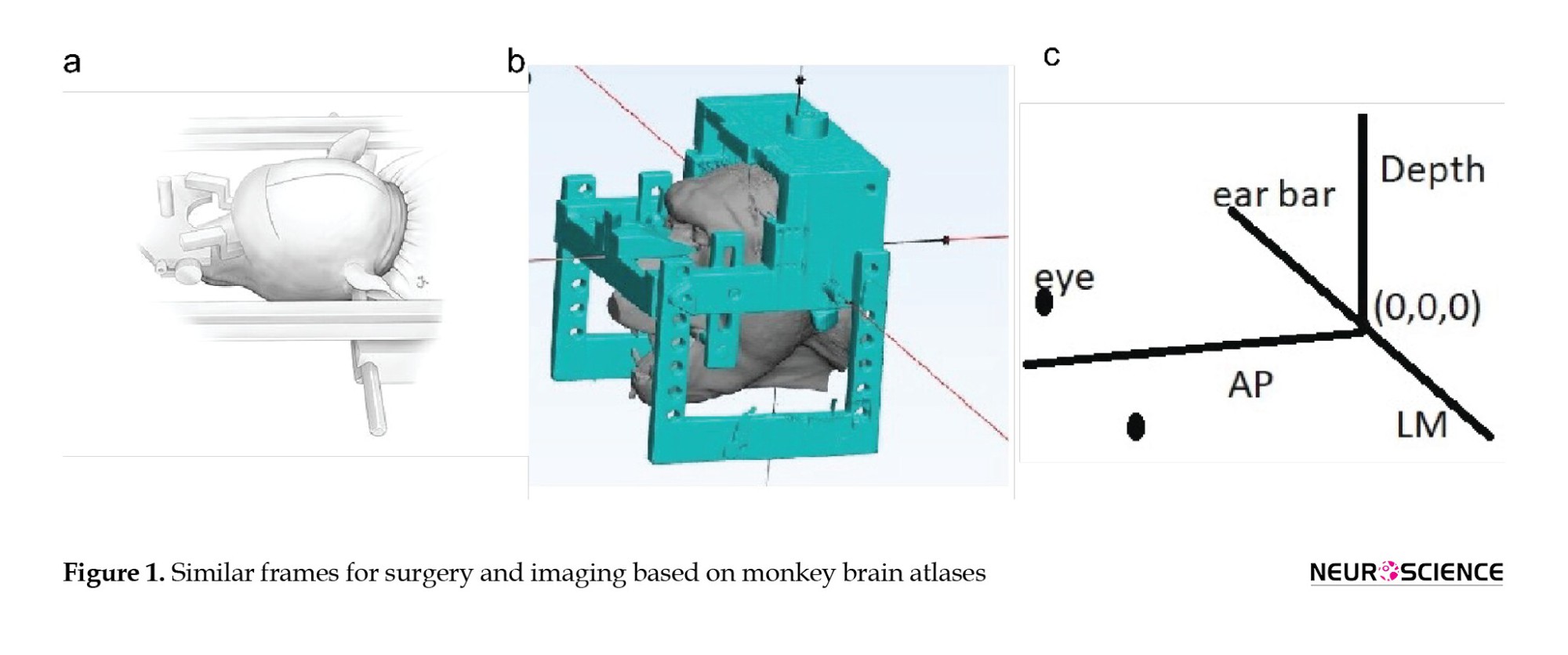
a) An animated surgery stereotaxic monkey head is fixed by ear, eye, and mouth bars; b) A monkey head fastened in the imaging adaptor similar to surgery stereotaxic frame; c) An animated 3D graph shoes coordination in both stereotaxic frames (the plane with depth=0 is the plane in which ear bar and eyepieces exist).
Preparing mesh model
We used the Materialise Innovation Suite research use version (Materialise Co, Belgium) to produce the initial 3D model of the skull and brain tissue. First, imaging series were segmented based on the alpha intensity band to isolate tissues of interest (bone/brain tissue).
a) The MR image series was reconstructed using software (Figure 2). Markers of imaging adaptors are shown as white lines beside the monkey’s head; b) Different views of segmented brain tissue and extracted 3D model of the brain in the bottom; c) Similar to b for skull tissue.

Using different masks, we extracted skull and brain tissue separately. It might be necessary to edit the masks manually if the imaging contrast is not perfect in some regions of the images. After preparing the 3D model, we smoothed the skull surface to remove unwanted distortions (Figure 3). It was needed to mesh the skull surface for CAD design. Mesh islands formed after threshold segmentation in the previous step can be removed through the edge connection network. Connection angle mesh extraction was used to remove the inner table of the skull. Later, it was necessary to reduce mesh size and to smooth out distortions. Mesh size reduction makes working with mesh easier for lower-end processing units, and smoothing distortions allow for better surface fitting when designing the implants. Quadratic reduction and Laplacian smoothing were used for mesh reduction and smoothing, respectively (Chen et al., 2003). If necessary, we could apply manual mesh triangle removal and welding in some areas to prevent the smoothing algorithm from rerunning over the already appropriate neighboring areas. It is reasonable to keep the mesh size and surface as close to the original reconstruction as possible since, in either case, excessive change reduces precision. In this stage, the boundaries of the areas of interest were extruded right to the skull surface to guide the design process. In this study, we targeted the frontal eye field (FEF) and V4 in one monkey and the prefrontal cortex (PFC) and IT in another monkey. Then, the mesh was exported in the ‘.stp ‘format, allowing for superior cross-platform compatibility between CAD software (Lanz et al., 2013).

The implants are CAD-designed, considering their position relative to the skull curvature. Monkey JK was used for FEF and V4 recording, and monkey Z for PFC and IT recording. a) FEF is located anterior to the arcuate sulcus, and V4 is the anterior bank of the lunate sulcus; b) For PFC and IT, one chamber was used from the lunate sulcus to the anterior of the principal sulcus.
Implant design and manufacturing
There are several good software options for implant CAD, and the choice depends on the expertise, finance, or access to the software. While computer graphics experts may find OpenGL their solution, most researchers do not consider this an accessible option. An ideal solution must provide users with an integrated environment for the design and assembly of the parts and with tools to allow the simulation of real geometry. Fortunately, Autodesk provides a three-year free license for students, and during this study, we had access to the top-of-the-range Autodesk Inventor Pro 2018 (Autodesk Co, USA). We imported the prepared mesh model into Autodesk Inventor, and a surface was fitted over the relevant areas of the skull. However, it is possible to fit a single surface on the area of interest, sometimes especially when you plan to exhaust a large area, or there is some problem with smoothing. It will be better to fit a separate surface for each implant. This procedure takes some time but ensures a surface closely following the model’s curvature and avoids extending the surface beyond the skull area and overlying brain areas of interest. It works best, especially when you are aiming for lower tolerance levels. Using this method, the fitting function works smoothly and does not try to achieve the coverage at the cost of reducing the precision (depending on the function definition and in case no exception is raised if you go beyond a specific limit). An axis through an arbitrary point on a vertex, preferably on the center of the skull mesh confined within the extruded brain area, defines a plane perpendicular to the axis. The implants are sketched on such planes and are extruded to the fitted surfaces. After extruding all implants, it becomes necessary to check if they are completely disjoint (if your design does not report or prevent geometry interference) and modify them if needed. The next step is defining the features of the implants. Sometimes, achieving the exact dimensions requires local mesh manipulations, which may result in an overall design crash depending on your design platform. After such local manipulations, check the entire implant mesh for any deviation from the design in every step. This is the most time-consuming part of the procedure and depends on its complexity. The model of implants for one monkey exists in supplementary materials.
The implants were machined using a 3-axis computer numerical control (CNC) machine from medical grade titanium (Ti-6Al-4V ELI - grade 23) in a single piece for each part (De Rezende & Johansson, 1993). To reduce the cost of fabrication, screw holes were created manually. This decision reduces the complexity of each part and the precision for the location of holes, which is in order of 1 mm. To cut the costs, you can also 3D-print the skull model and craft aluminum implants to test the design before crafting the main titanium ones (Figure 4) (Chen et al., 2017).

Surgery
The subject was anesthetized using diazepam and a ketamine cocktail (7 mg/kg) for induction. For maintenance of anesthesia, the animal was intubated and ventilated with 0.8%–1.5% isoflurane (20% O2 and 80% air). The head region was placed and fixed in a stereotaxic frame (Toos Bioresearch Co., Iran). Tetracycline ointment was applied to the eye and ear. The heart rate, SpO2, and the animal’s temperature were checked during surgery (Chen et al., 2017). The skull’s skin was shaved and rinsed with 5% povidone-iodine scrub solution, and a coronal section across the midsagittal plane exposed the underlying bone. Temporalis muscles on both sides were scraped off to make room for implant foot pieces. After cleaning the field with cotton swabs, the stereotaxic coordinates of each piece were identified and outlined with a surgical pen. The implants slipped to their places without any trouble or modification. A power drill with spotting drill bits (1 and 1.3 mm diameter) was used to mark entries. The self-drilling, self-tapping cortical titanium screws (2-mm diameter with different lengths 5, 7, and 9 mm; Synthes) were hand screwed in place muscle and skin flaps were restored to their original positions and stitched. The chamber’s inner spaces were rinsed several times, and a thin layer of cold-cure dental acrylic was applied to each chamber piece to prevent infection and save the underlying skull from necrosis before craniotomy. The craniotomy will be done in the subsequent surgery. One day before and two weeks after surgery, topical antibiotics were applied, and the skin-implant margins were treated with topical tetracycline ointment. After two weeks, no maintenance was performed, and 50 days after the surgery, the head post was fixed for the first time in the frame.
3. Results
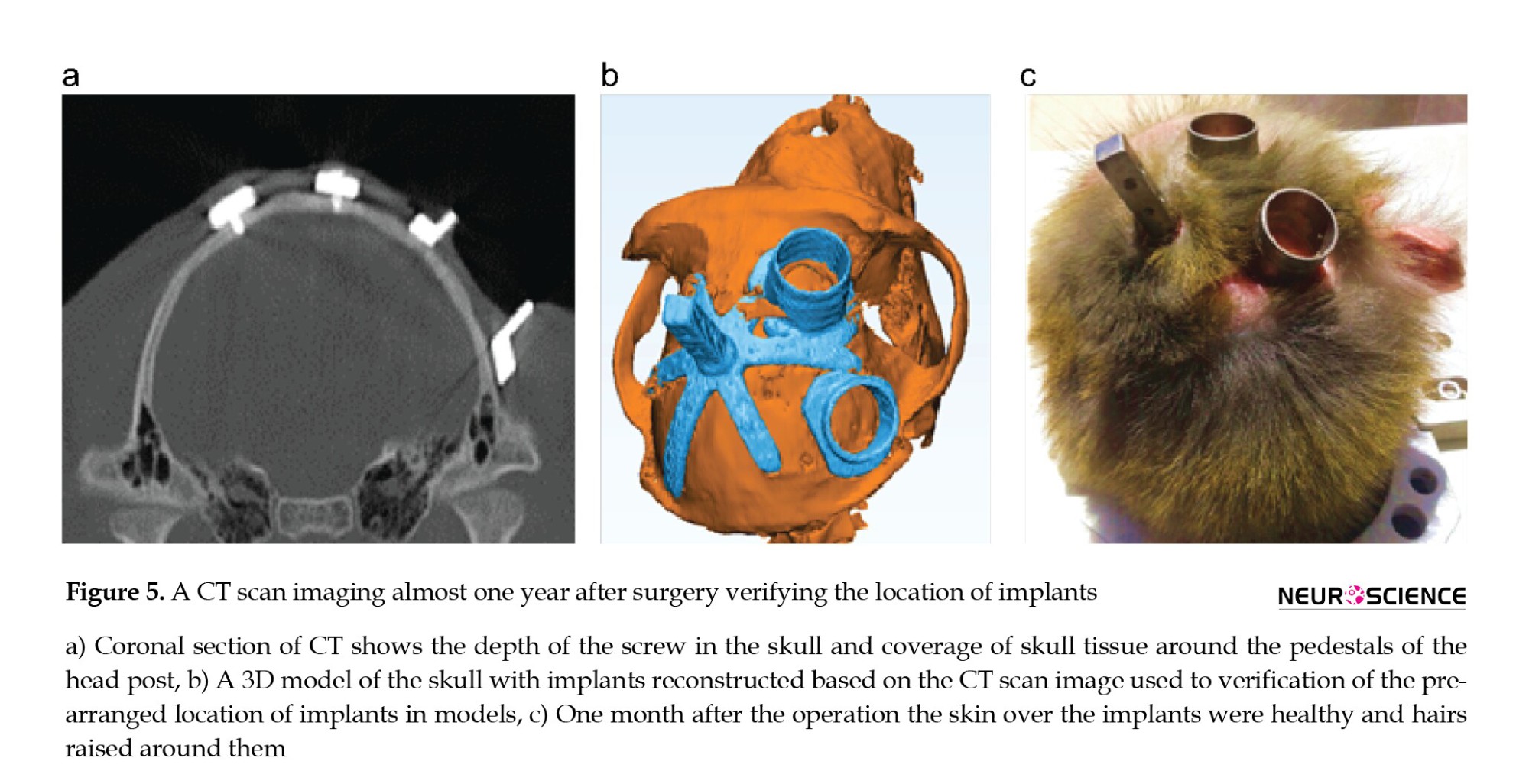
One day after surgery, the monkeys regained their normal activity. The surgery inflammation disappeared in 5 days. The marginal gaps started to be filled by fibrosis, and all the areas were covered by fibrotic tissue after a month. There was no visible manifestation of infection at the site of surgery. There has been no sign of implants coming loose or any implication of infection or failure. We did not need to clean around the chamber and the head post because we used biocompatible materials. There are advantages to the proposed method compared to the technique that uses acrylic material to fix the head post and chamber. One year after surgery, we performed a CT imaging. Figure 5 shows that the fibrotic tissue cores around the implants. Another observation was a significant reduction in surgery time (4-6 hours).
4. Discussion
Regarding the considerable time and cost spent preparing monkey subjects for behavioral neuroscience experiments, it is always a good idea to safeguard against probable sources of failure. Depending on the cognitive task, there is a long period before running the recording experiment that monkeys are trained enough to participate in the actual task. As explained earlier, implants used during recording studies may become sources of failure. Until recent decades, the most applied protocol required the application of acrylic cement as a base for chambers and as a gap filler for both chambers and headposts (Betelak et al., 2001). Many attempts, therefore, have been made to optimize each step, including the choice of material used, the shape of the implants, and the method to fix them in place (Adams et al., 2011; McAndrew et al., 2012; Mulliken et al., 2015).
On the other hand, commercially available versions are generic and do not completely conform to the specific research requirements regarding the researcher's obsession with head motion or the ideal collocation of the implants for more efficient utilization of the skull area. Here, we showed that designing and installing implants limited by the skull area is possible. For instance, using our described method and to achieve better stability, we extended the legs of the headpost both laterally to the temporal region and medially tangentially in between the edges of the chambers. At the same time, we designed the chambers large enough to allow full access to the desired brain areas. The implemented parts held high fidelity to the design and slipped onto their predicted places. This is practically impossible, not to mention its complications when you try to shape a nonspecific post or chamber during the surgery.
In two recent papers, custom-design approaches are proposed. In the first one (Mulliken et al., 2015), it is suggested to use specific PEEK (polyetheretherketone) materials for fabricating implants which our experience shows that these materials are not suitable for chronic usage, especially for the headposts which receive large tension. The second study recommended titanium 3D printing, which is an expensive method (Chen et al., 2017). Although we presented the procedure for implants used for typical single-unit recording studies, there is no limitation in extending its application to other similar or less similar studies or procedures where an implant is planned to be installed on the skull for animal studies or even for human surgical procedures.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Institute for Research in Fundamental Sciences (IPM).
Funding
This work was supported by Cognitive Sciences & Technologies Council (Grant No.: 2698). Monkeys were supplied by the Institute for Research in Fundamental Sciences (IPM), Primate Research Center, supported by the IPM research grant.
Authors' contributions
Conceptualization and supervision: Ehsan Rezayat, Frideh Shakerian and Mohammad-Reza A. Dehaqani; Methodology: Ehsan Rezayat and Farzad Shanfar; Investigation and writing: All authors; Funding acquisition and resources: Mohammad-Reza A. Dehaqani and Mostafa HajiNasrollah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Behrad Noudoost, Reza Rajimehr and Arash Afraz, who commented on the design and surgery plan. The authors thank colleagues in other laboratories, especially Jafar Dehghani and Ali Esmailpour, who generously shared their experience and expertise in developing titanium headposts and chambers. The authors thank Mohsen Moradi, who lent the macaque skulls for one monkey, and Mohammad Rabiee, who did that for another monkey. Thanks to the veterinarians, technicians, and husbandry staff at the IPM Primate Research Center for their superb care of the animals, and thanks to the veterinarians, technicians, and husbandry staff at the Royan Primate Research Center of Royan Institute for their assistance during and after the surgery.
References
lthough long in use, extracellular single-unit recording in behaving animals is still the technique, and many studies have been drawn upon to investigate the temporal and spatial dimensions of cognitive activities. Several chambers and a holding headpost on the skull are required as a preparation step. The chamber provides the interface for recording electrodes and the drive system. A headpost or equivalent holding mechanism stabilizes and protects head motions. However, with this requirement comes a plethora of risks and considerations inherent in the procedure's invasiveness. Post-operative acute and chronic infections have the possibility of chronic exposure of soft tissue due to incomplete closure of the skin after surgery, leading to an almost permanent infection risk. Furthermore, chronic foreign body inflammations are the main complications and probable causes of implant failure.
While commercially available or custom-crafted versions of the implants are used, these pieces must be bent to fit into their required position after inspecting the curvature. The headposts and chambers are either screwed on the bone or, more commonly, are rooted into an acrylic base cover founded on a bolt and wire grid (Pfingst et al., 1989). The hand-bended headposts often leave gaps between the implant and bone surface, increasing the risk of infection and extending the surgery period. Acrylic resins are used extensively as denture bases and bone cement. These products, especially when leaking as monomers form the polymer complex, are known bio-incompatible and toxic materials to various tissues. Therefore, acrylic resins inhibit the successful regeneration of tissues after surgery. In addition, the heat produced while applying the exothermic variants may injure contacting cells, and resulting debris predisposes infecting bacterial growth (Jorge et al., 2003). In recent years, there has been a tendency to replace their usage with screw-only implants to reduce the risks associated with acrylic covers (Adams et al., 2011; Chen et al., 2017; McAndrew et al., 2012; Mulliken et al., 2015).
Following magnetic resonance (MR) and computerized tomography (CT) imaging, here we utilized a co-registered MR-CT reconstruction of the head region to make computer-aided design (CAD-designed) implants for two monkeys. Our suggested procedure applied the available skull areas and structures underneath the brain to be within access of the recording chamber. This procedure raises the availability of the target areas while reducing infection risks associated with an incomplete fit and longer surgical operations by providing an individualized design and a tight fit contact.
Here, we propose a protocol for custom crafting the implants based on the co-registered MR and CT series map. We hope this procedure can easily be adopted in neuroscience-based surgeries and improve some of the current methods. We also hope to increase the safety of the animals studied in neuroscience research.
2. Materials and Methods
Study animals
Two adult male rhesus monkeys (Macaca mulatta) were used. All the procedures, such as surgeries, post-operative care, behavioral studies, and management conditions, were strictly in accord with NIH guidelines for the care and use of laboratory animals and the internal regulations on animal care issued by the IPM-SCS Ethics Committee. The entire process, including the recovery phase and the time passed before fixing the headpost for the first time, took place in a 60-day interval.
CT and MR imaging
For precisely co-registering different imaging modalities on the same coordination and having the same reference frame for surgery, it is necessary to use a CT- and MRI-compatible stereotaxic frame (Tajhiz Gostar Iranian co, Iran) during imaging. In this adapter, the ear bars, the bite bar, and the eyepieces had to constrain the head precisely like a stereotaxic surgery frame. Proper frame fastening is essential, and any misplacement produces errors in computations. Figure 1 depicts the reconstruction of the head and the constraining imaging adapter. We put several marker-filled capillary tubes filled with a contrasting lipophilic material (vitamin E) in the frame, which makes it possible to set sliding steps for the geometry of the MRI image series. The marker-filled bars are in rows left and right and on the top side. When positioning the head into the MRI (CT) bed, care should be given. Using this frame, we performed the MRI (3.0 Tesla Siemens Prisma MRI Scanner) and CT (Somatom Spirit; Siemens). It is possible to perform only a T2-weighted MRI without CT imaging. In this case, the contrast of the skull surface will decrease, and more adjustments are needed to determine the threshold for reconstruction.

a) An animated surgery stereotaxic monkey head is fixed by ear, eye, and mouth bars; b) A monkey head fastened in the imaging adaptor similar to surgery stereotaxic frame; c) An animated 3D graph shoes coordination in both stereotaxic frames (the plane with depth=0 is the plane in which ear bar and eyepieces exist).
Preparing mesh model
We used the Materialise Innovation Suite research use version (Materialise Co, Belgium) to produce the initial 3D model of the skull and brain tissue. First, imaging series were segmented based on the alpha intensity band to isolate tissues of interest (bone/brain tissue).
a) The MR image series was reconstructed using software (Figure 2). Markers of imaging adaptors are shown as white lines beside the monkey’s head; b) Different views of segmented brain tissue and extracted 3D model of the brain in the bottom; c) Similar to b for skull tissue.

Using different masks, we extracted skull and brain tissue separately. It might be necessary to edit the masks manually if the imaging contrast is not perfect in some regions of the images. After preparing the 3D model, we smoothed the skull surface to remove unwanted distortions (Figure 3). It was needed to mesh the skull surface for CAD design. Mesh islands formed after threshold segmentation in the previous step can be removed through the edge connection network. Connection angle mesh extraction was used to remove the inner table of the skull. Later, it was necessary to reduce mesh size and to smooth out distortions. Mesh size reduction makes working with mesh easier for lower-end processing units, and smoothing distortions allow for better surface fitting when designing the implants. Quadratic reduction and Laplacian smoothing were used for mesh reduction and smoothing, respectively (Chen et al., 2003). If necessary, we could apply manual mesh triangle removal and welding in some areas to prevent the smoothing algorithm from rerunning over the already appropriate neighboring areas. It is reasonable to keep the mesh size and surface as close to the original reconstruction as possible since, in either case, excessive change reduces precision. In this stage, the boundaries of the areas of interest were extruded right to the skull surface to guide the design process. In this study, we targeted the frontal eye field (FEF) and V4 in one monkey and the prefrontal cortex (PFC) and IT in another monkey. Then, the mesh was exported in the ‘.stp ‘format, allowing for superior cross-platform compatibility between CAD software (Lanz et al., 2013).

The implants are CAD-designed, considering their position relative to the skull curvature. Monkey JK was used for FEF and V4 recording, and monkey Z for PFC and IT recording. a) FEF is located anterior to the arcuate sulcus, and V4 is the anterior bank of the lunate sulcus; b) For PFC and IT, one chamber was used from the lunate sulcus to the anterior of the principal sulcus.
Implant design and manufacturing
There are several good software options for implant CAD, and the choice depends on the expertise, finance, or access to the software. While computer graphics experts may find OpenGL their solution, most researchers do not consider this an accessible option. An ideal solution must provide users with an integrated environment for the design and assembly of the parts and with tools to allow the simulation of real geometry. Fortunately, Autodesk provides a three-year free license for students, and during this study, we had access to the top-of-the-range Autodesk Inventor Pro 2018 (Autodesk Co, USA). We imported the prepared mesh model into Autodesk Inventor, and a surface was fitted over the relevant areas of the skull. However, it is possible to fit a single surface on the area of interest, sometimes especially when you plan to exhaust a large area, or there is some problem with smoothing. It will be better to fit a separate surface for each implant. This procedure takes some time but ensures a surface closely following the model’s curvature and avoids extending the surface beyond the skull area and overlying brain areas of interest. It works best, especially when you are aiming for lower tolerance levels. Using this method, the fitting function works smoothly and does not try to achieve the coverage at the cost of reducing the precision (depending on the function definition and in case no exception is raised if you go beyond a specific limit). An axis through an arbitrary point on a vertex, preferably on the center of the skull mesh confined within the extruded brain area, defines a plane perpendicular to the axis. The implants are sketched on such planes and are extruded to the fitted surfaces. After extruding all implants, it becomes necessary to check if they are completely disjoint (if your design does not report or prevent geometry interference) and modify them if needed. The next step is defining the features of the implants. Sometimes, achieving the exact dimensions requires local mesh manipulations, which may result in an overall design crash depending on your design platform. After such local manipulations, check the entire implant mesh for any deviation from the design in every step. This is the most time-consuming part of the procedure and depends on its complexity. The model of implants for one monkey exists in supplementary materials.
The implants were machined using a 3-axis computer numerical control (CNC) machine from medical grade titanium (Ti-6Al-4V ELI - grade 23) in a single piece for each part (De Rezende & Johansson, 1993). To reduce the cost of fabrication, screw holes were created manually. This decision reduces the complexity of each part and the precision for the location of holes, which is in order of 1 mm. To cut the costs, you can also 3D-print the skull model and craft aluminum implants to test the design before crafting the main titanium ones (Figure 4) (Chen et al., 2017).

Surgery
The subject was anesthetized using diazepam and a ketamine cocktail (7 mg/kg) for induction. For maintenance of anesthesia, the animal was intubated and ventilated with 0.8%–1.5% isoflurane (20% O2 and 80% air). The head region was placed and fixed in a stereotaxic frame (Toos Bioresearch Co., Iran). Tetracycline ointment was applied to the eye and ear. The heart rate, SpO2, and the animal’s temperature were checked during surgery (Chen et al., 2017). The skull’s skin was shaved and rinsed with 5% povidone-iodine scrub solution, and a coronal section across the midsagittal plane exposed the underlying bone. Temporalis muscles on both sides were scraped off to make room for implant foot pieces. After cleaning the field with cotton swabs, the stereotaxic coordinates of each piece were identified and outlined with a surgical pen. The implants slipped to their places without any trouble or modification. A power drill with spotting drill bits (1 and 1.3 mm diameter) was used to mark entries. The self-drilling, self-tapping cortical titanium screws (2-mm diameter with different lengths 5, 7, and 9 mm; Synthes) were hand screwed in place muscle and skin flaps were restored to their original positions and stitched. The chamber’s inner spaces were rinsed several times, and a thin layer of cold-cure dental acrylic was applied to each chamber piece to prevent infection and save the underlying skull from necrosis before craniotomy. The craniotomy will be done in the subsequent surgery. One day before and two weeks after surgery, topical antibiotics were applied, and the skin-implant margins were treated with topical tetracycline ointment. After two weeks, no maintenance was performed, and 50 days after the surgery, the head post was fixed for the first time in the frame.
3. Results
One day after surgery, the monkeys regained their normal activity. The surgery inflammation disappeared in 5 days. The marginal gaps started to be filled by fibrosis, and all the areas were covered by fibrotic tissue after a month. There was no visible manifestation of infection at the site of surgery. There has been no sign of implants coming loose or any implication of infection or failure. We did not need to clean around the chamber and the head post because we used biocompatible materials. There are advantages to the proposed method compared to the technique that uses acrylic material to fix the head post and chamber. One year after surgery, we performed a CT imaging. Figure 5 shows that the fibrotic tissue cores around the implants. Another observation was a significant reduction in surgery time (4-6 hours).

4. Discussion
Regarding the considerable time and cost spent preparing monkey subjects for behavioral neuroscience experiments, it is always a good idea to safeguard against probable sources of failure. Depending on the cognitive task, there is a long period before running the recording experiment that monkeys are trained enough to participate in the actual task. As explained earlier, implants used during recording studies may become sources of failure. Until recent decades, the most applied protocol required the application of acrylic cement as a base for chambers and as a gap filler for both chambers and headposts (Betelak et al., 2001). Many attempts, therefore, have been made to optimize each step, including the choice of material used, the shape of the implants, and the method to fix them in place (Adams et al., 2011; McAndrew et al., 2012; Mulliken et al., 2015).
On the other hand, commercially available versions are generic and do not completely conform to the specific research requirements regarding the researcher's obsession with head motion or the ideal collocation of the implants for more efficient utilization of the skull area. Here, we showed that designing and installing implants limited by the skull area is possible. For instance, using our described method and to achieve better stability, we extended the legs of the headpost both laterally to the temporal region and medially tangentially in between the edges of the chambers. At the same time, we designed the chambers large enough to allow full access to the desired brain areas. The implemented parts held high fidelity to the design and slipped onto their predicted places. This is practically impossible, not to mention its complications when you try to shape a nonspecific post or chamber during the surgery.
In two recent papers, custom-design approaches are proposed. In the first one (Mulliken et al., 2015), it is suggested to use specific PEEK (polyetheretherketone) materials for fabricating implants which our experience shows that these materials are not suitable for chronic usage, especially for the headposts which receive large tension. The second study recommended titanium 3D printing, which is an expensive method (Chen et al., 2017). Although we presented the procedure for implants used for typical single-unit recording studies, there is no limitation in extending its application to other similar or less similar studies or procedures where an implant is planned to be installed on the skull for animal studies or even for human surgical procedures.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Institute for Research in Fundamental Sciences (IPM).
Funding
This work was supported by Cognitive Sciences & Technologies Council (Grant No.: 2698). Monkeys were supplied by the Institute for Research in Fundamental Sciences (IPM), Primate Research Center, supported by the IPM research grant.
Authors' contributions
Conceptualization and supervision: Ehsan Rezayat, Frideh Shakerian and Mohammad-Reza A. Dehaqani; Methodology: Ehsan Rezayat and Farzad Shanfar; Investigation and writing: All authors; Funding acquisition and resources: Mohammad-Reza A. Dehaqani and Mostafa HajiNasrollah.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Behrad Noudoost, Reza Rajimehr and Arash Afraz, who commented on the design and surgery plan. The authors thank colleagues in other laboratories, especially Jafar Dehghani and Ali Esmailpour, who generously shared their experience and expertise in developing titanium headposts and chambers. The authors thank Mohsen Moradi, who lent the macaque skulls for one monkey, and Mohammad Rabiee, who did that for another monkey. Thanks to the veterinarians, technicians, and husbandry staff at the IPM Primate Research Center for their superb care of the animals, and thanks to the veterinarians, technicians, and husbandry staff at the Royan Primate Research Center of Royan Institute for their assistance during and after the surgery.
References
Adams, D. L., Economides, J. R., Jocson, C. M., Parker, J. M., & Horton, J. C. (2011). A watertight acrylic-free titanium recording chamber for electrophysiology in behaving monkeys. Journal of Neurophysiology, 106(3), 1581-1590. [DOI:10.1152/jn.00405.2011] [PMID]
Betelak, K. F., Margiotti, E. A., Wohlford, M. E., & Suzuki, D. A. (2001). The use of titanium implants and prosthodontic techniques in the preparation of non-human primates for long-term neuronal recording studies. Journal of Neuroscience Methods, 112(1), 9-20. [DOI:10.1016/S0165-0270(01)00442-3] [PMID]
Chen, X., Possel, J. K., Wacongne, C., van Ham, A. F., Klink, P. C., & Roelfsema, P. R. (2017). 3D printing and modelling of customized implants and surgical guides for non-human primates. Journal of Neuroscience Methods, 286, 38-55. [DOI:10.1016/j.jneumeth.2017.05.013] [PMID]
Chen, Z., Tristano, J. R., & Kwok, W. (2003). Combined Laplacian and Optimization-based Smoothing for Quadratic Mixed Surface Meshes. IMR.
De Rezende, M. L. R., & Johansson, C. B. (1993). Quantitative bone tissue response to commercially pure titanium implants. Journal of Materials Science: Materials in Medicine, 4(3), 233-239. [DOI:10.1007/BF00122274]
Jorge, J. H., Giampaolo, E. T., Machado, A. L., & Vergani, C. E. (2003). Cytotoxicity of denture base acrylic resins: A literature review. The Journal of Prosthetic Dentistry, 90(2), 190-193. [DOI:10.1016/S0022-3913(03)00349-4] [PMID]
Lanz, F., Lanz, X., Scherly, A., Moret, V., Gaillard, A., & Gruner, P., et al.(2013). Refined methodology for implantation of a head fixation device and chronic recording chambers in non-human primates. Journal of Neuroscience Methods, 219(2), 262-270. [DOI:10.1016/j.jneumeth.2013.07.015] [PMID]
McAndrew, R. M., Lingo VanGilder, J. L., Naufel, S. N., & Helms Tillery, S. I. (2012). Individualized recording chambers for non-human primate neurophysiology. Journal of Neuroscience Methods, 207(1), 86-90. [DOI:10.1016/j.jneumeth.2012.03.014] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2020/01/15 | Accepted: 2020/05/9 | Published: 2018/03/15
Received: 2020/01/15 | Accepted: 2020/05/9 | Published: 2018/03/15
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





