Volume 12, Issue 6 (November & December 2021)
BCN 2021, 12(6): 789-804 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tahamtan M, Nazari A, Aghaei I, Shabani M. Ischemic Postconditioning Attenuates Bilateral Renal Ischemia-induced Cognitive Impairments. BCN 2021; 12 (6) :789-804
URL: http://bcn.iums.ac.ir/article-1-1559-en.html
URL: http://bcn.iums.ac.ir/article-1-1559-en.html
1- Department of Neuroscience, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Biology, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
3- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Neuroscience Research Center, Neuropharmacology Institute, Kerman University of Medical Sciences, Kerman, Iran.
2- Department of Biology, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
3- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Neuroscience Research Center, Neuropharmacology Institute, Kerman University of Medical Sciences, Kerman, Iran.
Keywords: Cognitive impairments, Acute kidney injury, Postconditioning, Brain-derived Neurotrophic Factor, Bilateral renal ischemia
Full-Text [PDF 919 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Acute Kidney Injury (AKI) is a frequent complication, i.e., associated with increased hospital stay and mortality (Hussein, Barakat, Awadalla, & Shokeir, 2012; Khatri, Himmelfarb, Adams, Becker, K., Longstreth, & Tirschwell, 2014). Renal Ischemia-Reperfusion Injury (Renal IRI) is one of the most important causes of AKI and can lead to remote organ dysfunction like lung, brain, and heart (Hassoun et al., 2007).
Patients with AKI suffer from high levels of mental and cognitive dysfunctions. Synthesizing the results of the previous studies, AKI caused cellular and soluble inflammation, edema, alterations in water transport, and increased microvascular protein leakage in the brain (Arieff & Massry, 1974; Arieff, Massry, Barrientos, & Kleeman, 1973; Liu et al., 2008). Due to the inflammatory response, the disruption of the BBB, endothelial injury, and stimulation of the coagulation cascades within the brain have been observed, leading to alterations in neuronal cell protein transcription and cellular activation. Furthermore, an increased number of pyknotic neuronal cells and microgliosis following 60 min of renal ischemia in the CA1 region of the brain hippocampus, compared to the sham group, has been indicated (Kinsey, Li, & Okusa, 2008; Liu et al., 2008). Another research also reported increased GFAP and microglia cells as distant organ consequences of AKI (Kinsey et al., 2008; Liu et al., 2008; Ratliff, Rabadi, Vasko, Yasuda, & Goligorsky, 2013; TSAO, Hsu, Wu, Liu, & Lei, 2001). These changes were implicated in causing altered brain function (Nongnuch, Panorchan, & Davenport, 2014).
Bilateral renal ischemia has been introduced as the leading cause of AKI in patients. Since ischemia/reperfusion can cause irreversible damages to tissue and mechanisms contributing to the pathogenesis of ischemia-reperfusion injury are complex, new strategies like ischemic conditioning have been explained to increase tolerance and protect against ischemia-reperfusion injury (Seifi, Kadkhodaee, Najafi, & Mahmoudi, 2014). In ischemic conditioning, brief intermittent and reversible episodes of ischemia with reperfusion can induce protective effects against ischemia/reperfusion injury. These processes were defined based on this phenomenon: ischemic preconditioning and Ischemic Postconditioning (IPo).
Ischemic preconditioning is a powerful intervention against IRI; however, it is clinically feasible because, in most cases, ischemia is not predictable (Zhao, Sapolsky, & Steinberg, 2006). Several studies introduced IPo as a new neuroprotective strategy to deal with IRI (Penna, Tullio, Moro, Folino, Merlino, & Pagliaro, 2010; Wever, Menting, Masereeuw, van der Vliet, Rongen, & Warlé, 2012; Zhao et al., 2003). It is the phenomenon that brief, repetitive cycles of ischemia during the onset of reperfusion can be protective in different systems (Penna et al., 2010; Wang et al., 2008; Wever et al., 2012) and can attenuate the potential additional injury which may be induced by reperfusion. Thus, IPo has become a clinical application to significantly decrease IRI (Deftereos et al., 2013; Liu et al., 2009; Lønborg et al., 2010). However, the detailed mechanism of postconditioning remains unknown. Discovering involved mechanisms in postconditioning may shed light on finding effective treatment against injuries.
Neurotrophins are neuroprotective factors that exert their effects through intracellular signaling pathways. Brain-Derived Neurotrophic Factor (BDNF) is a member of the neurotrophin family, which plays a prominent role in neuronal survival, plasticity, learning, and memory. Moreover, the vital role of BDNF in neurological function following brain injury has been indicated. Several studies discriminated the protective role of BDNF following neonatal hypoxic_ ischemic brain injury, transient, and focal cerebral ischemia, and stroke (Melo et al., 2013; Singh & Su, 2013; Vetrovoĭ, Rybnikova, & Samoĭlov, 2013).
There is a growing body of evidence that hypoxic and ischemic postconditioning up-regulate the expression of BDNF, particularly in hippocampal CA1 neurons in rats (Vetrovoi, Rybnikova, Glushchenko, & Samoilov, 2015; Vetrovoĭ et al., 2013; Zen’ko, Rybnikova, & Glushchenko, 2013; Zhang et al., 2015).
Several studies outlined the protective effects of postconditioning in the heart (Kin et al., 2005; Kin et al., 2004; Tsang, Hausenloy, Mocanu, & Yellon, 2004), brain (Ren et al., 2008; Zhao, 2009; Zhao, Sapolsky, & Steinberg, 2006), liver (Sun, Liu, & Sun, 2004), and kidney (Liu et al., 2007); however, there is no study to examine the effects of renal IPo on brain damage induced by renal IRI. The study aimed to investigate: 1) whether IPo will be able to prevent memory and motor deficits, 2) whether IPo causes protective effects by alteration in BDNF level.
2. Methods
Animal preparation and experimental design
Adult male Wistar rats (8-10 weeks old, 180-220 g) were used in this experiment and fed with standard rat chow and water ad libitum. The animals were housed under a controlled temperature (21-23 °C) and 12:12h light/dark cycle. All animals’ procedures were observed to minimize pain and were approved by the Kerman Medical University Ethics Committee (EC/KNRC/92-6). There were 6 different groups of rats in the current study. The rats subjected to Bilateral Renal Ischemia/reperfusion (BRI) were divided into 2 groups of n=10, in which the right and left renal arteries and veins were occluded for 1h that followed by the reperfusion periods of 24 hours (BRI-24h group) and 1 week (BRI-1w group). The sham-operated rats were divided into 2 groups of n=10 that animals underwent only anesthesia without occlusion with periods equivalent to reperfusion of 24 hours (sham-24h group) or 1 week (sham-1w group).
IPo animals were also divided into 2 groups (n=10/group) subjected to 60 min ischemia, then 3 cycles of 10s of reperfusion followed by 10s ischemia in the reperfusion periods of 24 hours (IPo-24h group) and 1 week (IPo-1w group). This model of postconditioning was based on the methods described before (Figure 1) (Guo et al., 2014).
.jpg)
Inducing bilateral renal ischemia/reperfusion and ischemic postconditioning
The study rats were anesthetized with ether, and a midline laparotomy was performed. The renal arteries and veins were occluded with non-traumatic clamps. The occlusion was verified visually by a color change of the kidney to a paler shade. The clamps were isolated at the end of the ischemic period, and blood was reperfused. The kidneys were treated identically in sham-operated control rats, except for clamping. In ischemic postconditioning-operated rats after 60 min of occlusion to induce ischemia, 3 cycles of ischemia (10 sec) followed by reperfusion (10 sec) exerted.
The abdominal incision was sutured at two layers by 2-0 silk. All surgical procedure was done under sterile condition, and the rat was allowed to recover from the anesthesia before returning to an individual cage. The Kerman University approved the surgical protocol of Medical Sciences. Behavioral assays were performed 24h or 1 week after surgery with a 2h interval among each assay in the following order: open field test, rotarod, wire grip test, and shuttle box or Morris water maze (the last two assays were performed in separate groups of study due to the nature of the procedure).
Behavioral experiments
Open-field test
The open-field test was used to screen anxiety-like behavior 24h and 1w after reperfusion. The rats were adapted in the experimental room 1 h before the test, then were placed in the center of the apparatus, i.e., made of a Plexiglas square arena [90×90×30 (H) cm], its floor was divided into 16 squares, so the field was divided into central and peripheral squares. The vertical and horizontal activities of the animals were observed directly and continuously for 5 min. They then were analyzed using Ethovision software, v. 7.1, a video tracking software to automate the behavioral paradigms (Noldus Information Technology, the Netherlands). The following behavioral components were measured: total distance moved [TDM, cm], number of rearing (as a measure of vertical activity), and velocity. At the end of each test, the examined animals were removed from the chamber, and the field was cleaned with a damp cloth (Razavinasab et al., 2013).
Rotarod
The effects of BRI and postconditioning on motor balance and coordination of the animals by performing accelerating rotarod (Hugo Sachs Electronic, Germany) 24h and 1w after reperfusion were observed.
The rotarod performance test measures motor parameters, including motor balance and coordination based on a rotating rod with forced motor activity. The rotarod started from a speed of 10 Revolutions Per Min (RPM) to the maximum speed of 60 RPM. It was divided into 4 equal sections, thus enabling 4 rats to walk on the rod simultaneously. Three trials were performed for each rat (intertrial interval=30 min), with each trial lasting for a total maximum of 300s. Intervals between the mounting of the rat on the rod and falling off of it were recorded as a measure of motor coordination and balance. The length of time a given animal stays on this rotating rod is known as its balance index (Shabani et al., 2012).
Wire grip test
To evaluate the muscle strength of the animals, the hanging-wire-grip test was performed 1 week after the operation. The rat was placed in a vertical posture while its forepaws were put in contact with the steel wire (80 cm long, with a diameter of 7 mm). The animal was released whenever it grasped the wire. The total time each rat could hang suspended from a wire grid was recorded for three trials with a 30 min inter-trial interval (Haghani, Shabani, & Moazzami, 2013).
Passive Avoidance (PA) learning
PA learning was selected as the tool for assessing fear learning in BRI rats and the possible effect of postconditioning on 24h and 1w after reperfusion. For this purpose, an apparatus with the dimensions of 100∗25∗25 consisted of two identical illuminated and dark boxes separated by a guillotine door and a stainless rod grid serving as the base. The adaptation phase was followed by a single trial in which the animals were placed in the illuminated compartment. After 10s, the door was opened, and the animal was allowed to go to the dark compartment. After that, the door was closed without electric shock, and after the 20s, the animal was placed in the cage. The rats without the tendency to enter the dark chamber were excluded. After 30 min of adaptation, for the learning trial, this procedure was repeated, and animals were given an inescapable foot-shock (0.5 mA, 1.5 s) immediately after entering the darkroom. This procedure was repeated until the animal did not enter the dark box. The number of shocks received was recorded in this phase. The retention tests were conducted 24h after the training phase (without shock) to evaluate memory. The latency time (cut off: 300 sec) to enter the dark compartment as retention time [Step-Through Latency (STL)] was recorded (Abbassian et al., 2016).
Morris Water Maze (MWM)
The rats were tested by an MWM as previously described (Aghaei, Shabani, Doustar, Nazeri, & Dehpour, 2014). The test chamber was a circular tank (140 cm diameter, 45 cm height), surrounded by extra-tank visual cues. A visible or submerged platform (15 cm wide, 35 cm height) was placed 1.5 cm above or below the water surface. Water temperature was maintained at 21-23°C. Rats’ behavior was recorded with the Ethovision system. The following parameters were computed for each animal: total distance and time spent to reach the platform in three consecutive trials, the number of crosses in the correct quadrant in the retention phase, percentage of the time, and distance traveled in the correct quadrant. Each rat underwent three blocks of trials in the training phase, each including four trials (inter-trial interval= 30s). In each trial, animals were put in one of the 4 quadrants facing the maze. The examined animals were allowed to swim the 60s to find the platform, and if they did not find it, they were put on the platform by the examiner. After 30 s, the rat was again put to trial. After 2h of the last block, the rats underwent a probe trial during which the platform was removed from the tank and the number of crosses in the correct quadrant and total time spent in the target quadrant was recorded and analyzed per rat (Shabani, et sl., 2012).
Molecular experiment
Tissue dissection and preparation for Western blot
The rats were divided into sham, BRI, and IPo groups for the molecular experiment, decapitated 24h after surgery. All of the rats were anesthetized with atmospheric CO2. After decapitation, the brains were rapidly extracted and placed on ice. Both whole hippocampi were freshly harvested and placed in a microtube and stored at −80 until homogenization for further western blot assay.
BDNF immunoblot analysis
The dissected hippocampus tissues were homogenized in ice-cold buffer containing 10 mM Tris–HCl (pH=7.4), 1 mM EDTA, 0.1% SDS, 0.1% Na-deoxycholate, 1% NP-40 with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2.5 μg/mL of leupeptin, 10 μg/mL of aprotinin) and 1 mM sodium orthovanadate (a phosphatase inhibitor). The homogenate was centrifuged at 15,000 RPM for 20 min at 4°C. The resulting supernatant was retained as the whole-cell fraction. Protein concentrations were measured using the Bradford method (Bio-Rad Laboratories, Muenchen, Germany). Equal amounts of protein were resolved electrophoretically on a 9% SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE) SDS-PAGE gel and transferred to PVDF (polyvinylidenefluoride) membranes. After blocking with 5%, non-fat dried milk in Tris-buffered saline with Tween 20 (blocking buffer, TBS-T, 150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 0.1% Tween 20) for 2h, at room temperature and then, the membranes were incubated overnight with a primary rabbit polyclonal antibody for BDNF (1:1000, sc-20981; Santa Cruz Biotechnology, Santa Cruz, USA) at 4°C. After washing in TBS-T buffer (3 times for 5 min each, at room temperature), the blots were incubated for 2 h at room temperature with an anti-rabbit IgG secondary antibody conjugated with horseradish peroxidase (1:15,000; GE Healthcare Bio-Sciences). The primary and secondary antibodies were diluted in blocking buffer. The antibody-antigen complexes were revealed using the ECL system (Amersham Biosciences). Images were captured on a Gel Doc imaging system (Bio-Rad, Hercules, CA, US) converted to a tiff file. Lab Work analyzing software (UVP, UK) was used to analyze the intensity of the expression. β-actin immunoblotting (antibody from Cell Signaling Technology, INC. Beverly, MA, USA; 1:1000) was used to control loading. The timeline applied for the experimental protocol has been indicated in Figure 2.
.jpg)
Statistical analysis was performed in SPSS. All data were expressed as Mean±SEM. Analysis of Variance (ANOVA) followed by Tukey’s post-hoc analysis was used to compare the differences between the study groups. Repeated-measures ANOVA was used to analyze the data of the MWM task in the learning phase. The band density values were expressed as BDNF/β-actin ratio for each sample. The mean differences for the study groups were compared using one-way ANOVA, followed by the Newman–Keuls test. P<0.05 was considered significant.
3. Results
Open field test
The open field has been used to investigate locomotion and anxiety-related behaviors. Rats were subjected to 60 min of renal ischemia followed by reperfusion presented no significant differences in all parameters (velocity, rearing number, & total distance moved) measured in this test 24h after operation (P>0.05, Figure 3-A, B, and C).
.jpg)
Moreover, an open field test also was performed 1 week after surgery. There was no significant alteration in mentioned parameters 1 week after surgery (Figure 4-A, B, & C).
.jpg)
The effect of BRI on balance function, motor learning, and muscle strength
Compared with the sham group, BRI reduced duration on the rod 24h after surgery (P<0.01). Postconditioning rats had no significant difference with sham and BRI groups (P>0.05) (Figure 5).
.jpg)
No significant difference in duration on the rod was observed between the groups of study 1 week after reperfusion (P>0.05) (Figure 6).
.jpg)
BRI animals showed reduced muscle strength compared to the postconditioning group 1 week after reperfusion (P<0.05, Figure 7).
.jpg)
Postconditioning could significantly reverse this effect of BRI.
The effect of BRI and IPo on PA learning 24h after reperfusion
There was no significant difference in the number of shocks received among the three groups (P>0.05, Figure 8-A). BRI rats had impairment in memory retrieval compared to the sham group (P<0.001, ANOVA); postconditioning could reverse this impairment in BRI rats (p<0.05, ANOVA) (Figure 8-B).
.jpg)
The effect of BRI and IPo on PA learning 1 week after reperfusion
No significant alteration in the parameters measured was observed amongst three groups of study 1 week after reperfusion (P>0.05, Figure 9-A & B).
.jpg)
The effect of BRI and IPo on spatial learning 1 week after reperfusion
There were no significant differences in the parameters measured across blocks of trials (Figure 10-A &C) and probe trial (Figure 10-D & F) between groups in all periods, indicating that BRI and IPo did not influence the spatial learning.
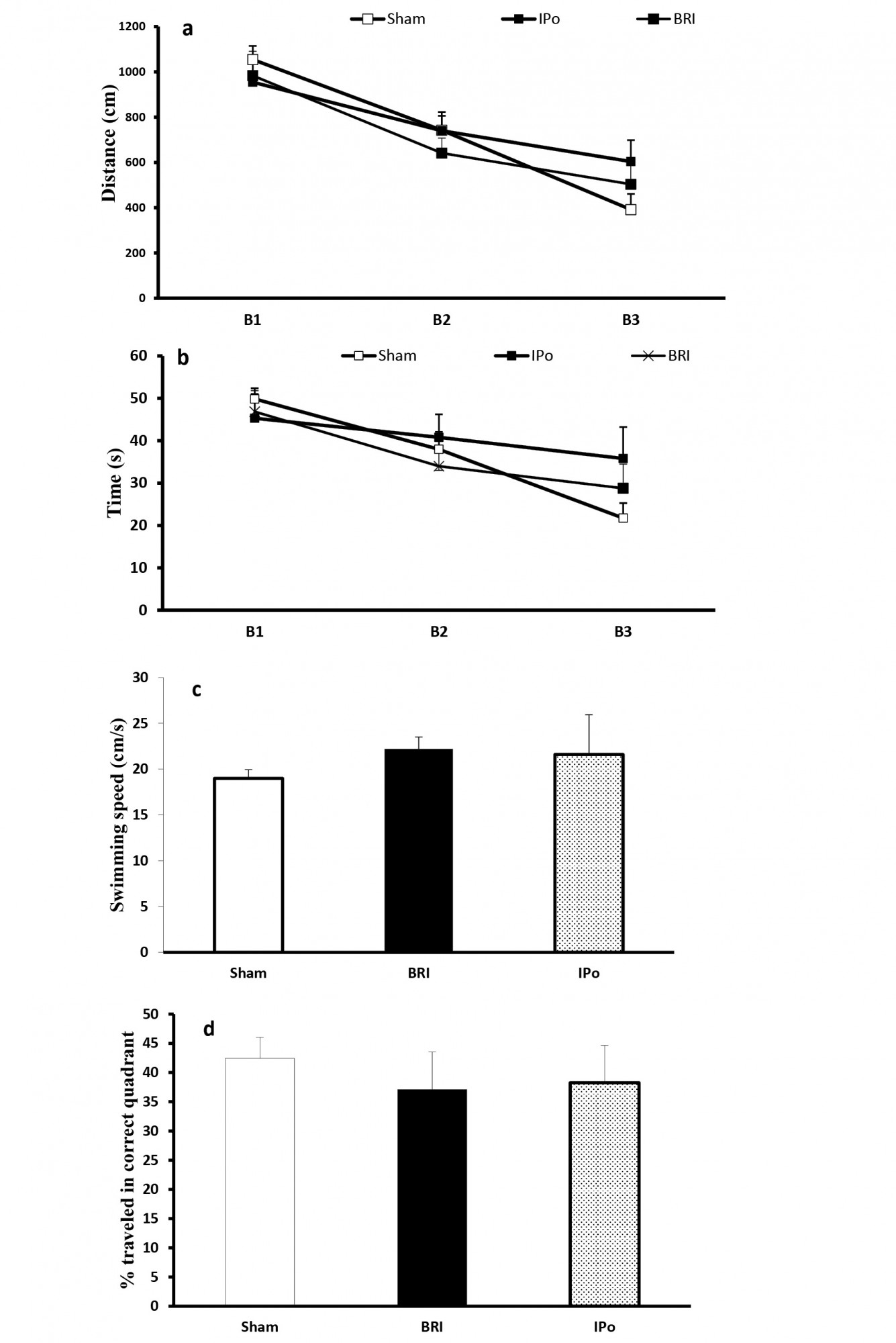
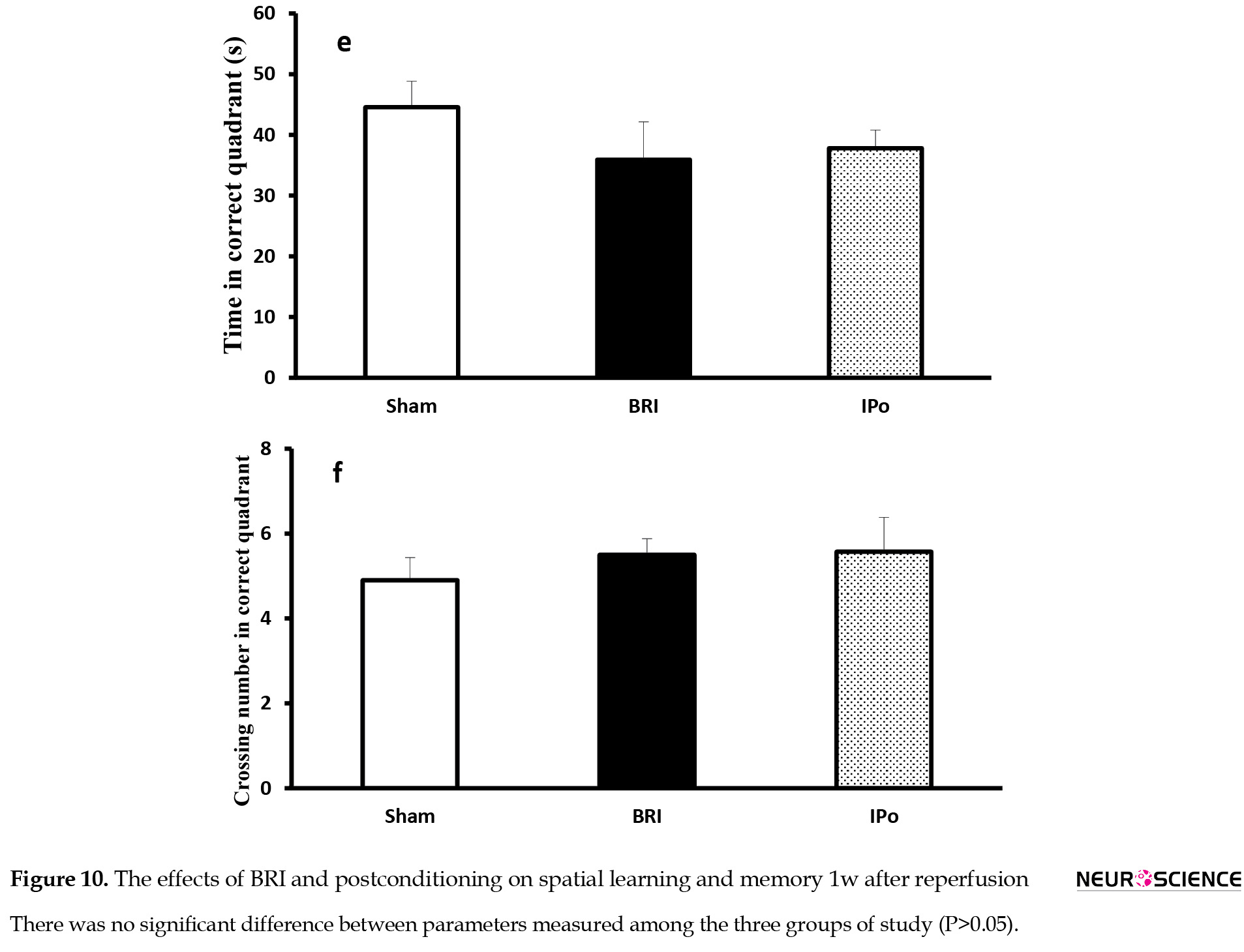
BDNF protein expression
Next, we sought to identify a potential molecular mechanism that underlies memory impairments. We evaluate the expression of BDNF 24h after reperfusion because the IR-induced memory dysfunction was observed just at this time point. As per Figure 11, IR rats indicated a decreased hippocampal BDNF protein expression level than the sham-operated group (P<0.05). Surprisingly, the postconditioning situation could significantly (P<0.05) prevent the decreasing effect of renal IRI on the hippocampal BDNF expression (Figure 11).
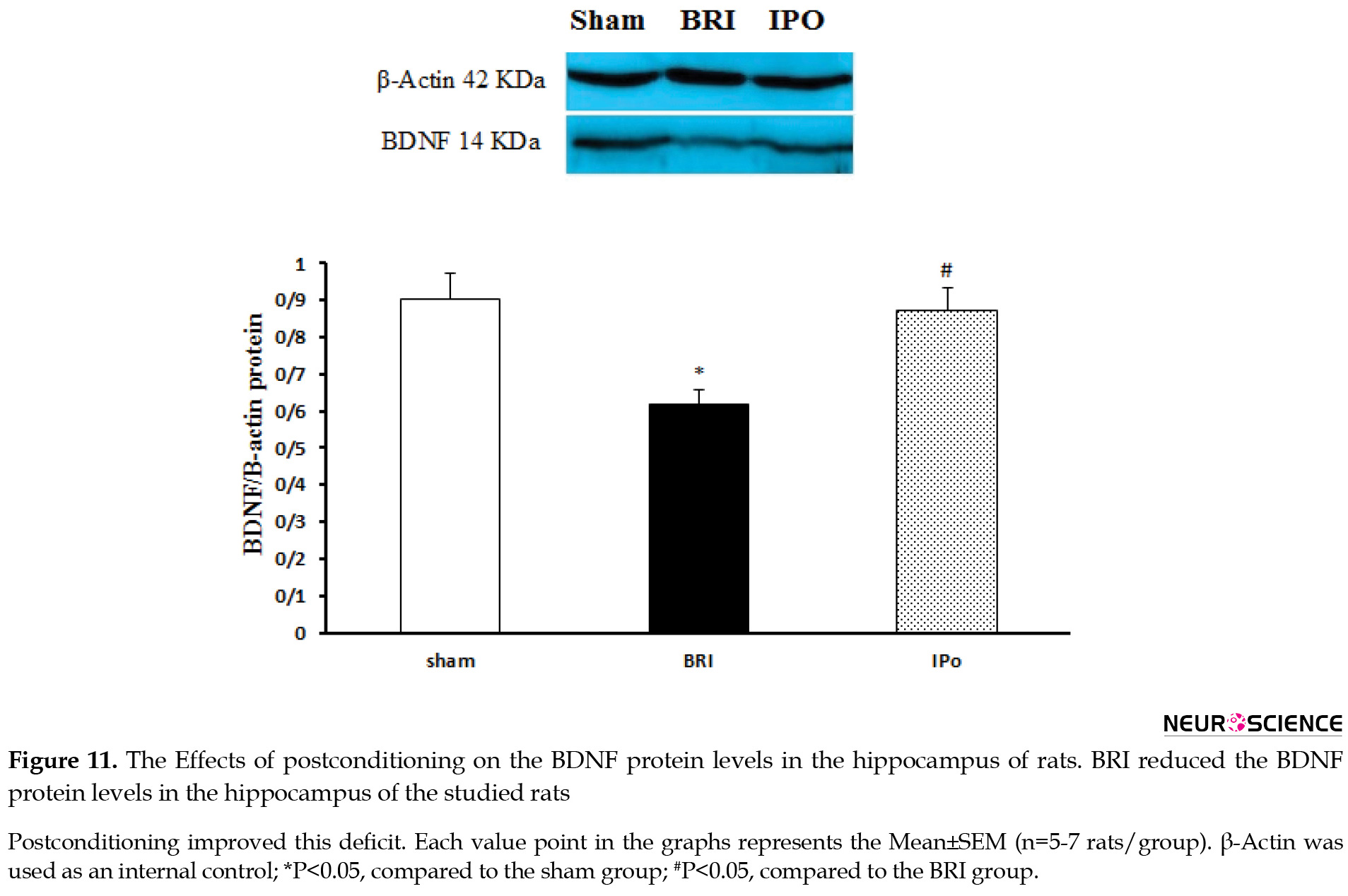
Therefore, the present results indicated that the significant down-regulation of the BDNF protein in BRI rats was prevented by inducing renal ischemic postconditioning.
4. Discussion
Ischemia causes tissue damage in various clinical situations, such as acute infarctions or hypoperfusion, and the restoration of suitable blood flow is the primary treatment. Remote ischemic conditioning is an approach of conditioning that is not the target organ, but a more accessible tissue is submitted to a conditioning stimulus.
We observed that 1h of BRI, as a model of acute kidney injury in rats, impairs memory and balance function 24h after reperfusion and muscle strength of the animals 1w after reperfusion. Postconditioning indicated a promising effect against these impairments induced by BRI. Moreover, we observed no significant memory impairment and balanced disability in the BRI group, compared to the other groups 1w after the operation.
The high morbidity and mortality in patients with AKI and the non-existence of effective therapeutic modalities necessitate strategies to alleviate the impact of kidney injury on distant organs. Ischemic renal tissue’s reperfusion (sudden restoration of blood flow) is highly harmful, especially at ischemia. The reperfusion phase initiates a wide range of cellular events responsible for transendothelial migration of inflammatory cells, tissue edema, necrotic and apoptotic cell death (Edelstein, Ling, & Schrier, 1997). Thus, interventions given only at the beginning of reperfusion can reduce post-ischemic injury (Zhao, 2010).
BRI reliably induced balance and muscle strength impairments in the behavioral tasks employed in the present study. Previous studies revealed that the alterations in locomotor activity are related to the ischemic damage of CA1 area of the hippocampus in the open-field test (Chandler, DeLeo, & Carney, 1985; Gerhardt & Boast, 1988; Liu et al., 2008; Skelly, Hennessy, Dansereau, & Cunningham, 2013; Wang & Corbett, 1990) a region which is involved in anxiety and depression (Heldt, Stanek, Chhatwal, & Ressler, 2007). Increased circulating inflammatory cytokines and the number of pyknotic neuronal cells in the hippocampus have been indicated due to AKI (Liu et al., 2008; Skelly et al., 2013). No significant difference was observed between total distances moved, velocity, and rearing numbers in both time points assessed by open field test 24h and 1w after reperfusion.
The current study confirmed that bilateral renal ischemia followed by 24h of reperfusion resulted in memory impairment, i.e., assessed by a PA learning test. Postconditioning could improve this impairment in BRI rats. BRI followed by 1w of reperfusion couldn’t impair PA learning, and no significant difference was observed between the groups. Several studies on alterations in brain function following cerebral ischemia verify impairments in motor coordination, learning, and memory abilities (Norio et al., 1990; Tahamtan et al., 2013; Yan, Hou, Wu, Liu, & Zhou, 2007).
The total distances moved, and velocity is not significant between groups in different time points; therefore, it can indicate that the memory defect shown in the PA test is not due to the impaired ability of animals to move freely.
One promising finding of the previous studies is postconditioning. The term “postconditioning” refers to intermittent interruptions of blood flow at the onset of reperfusion which can activate signaling pathways and effectors (Zhao et al., 2003). However, the detailed mechanisms underlying these actions remain to be determined. To our knowledge, for the first time, we report that postconditioning protects some aspects of brain function against reperfusion injury induced by bilateral renal ischemia. Remote ischemic conditioning activates protective pathways in distant organs, such as the brain, heart, and kidney, representing an exciting new neuroprotection pattern. The exact mechanism of signal transmission from the periphery to the brain is unknown; however, humoral factors and an intact nervous system play critical roles.
One of the critical objectives of the experiments was to examine the effect of renal IRI and renal IRI Plus postconditioning on hippocampal BDNF level. Immunoblotting data indicated that postconditioning preserved BDNF protein level in the hippocampus region of BRI rats. BDNF, a crucial trophic factor in synaptic plasticity (Cunha, Brambilla, & Thomas, 2010), is present in high concentration in the hippocampus and cerebral cortex (Cotman & Berchtold, 2002). BDNF plays a crucial role in hippocampal-dependent learning and memory. It also may indicate a promising neuromodulatory therapeutic agent, increasing the survival of neurons, regeneration, and differentiation (Bella, Lin, Cagiannos, & Lue, 2008; Cotman & Berchtold, 2002; Hariri et al., 2003). Furthermore, BRI-induced changes in PA learning and memory seem to correlate with the decrease in hippocampal BDNF level.
A growing body of literature demonstrates that BDNF has not only potent neuroprotective effects but also is a strong inducer of recovery after various types of ischemic lesions (Kiprianova, Sandkühler, Schwab, Hoyer, & Spranger, 1999; Schäbitz et al., 2004; Schäbitz, Schwab, Spranger, & Hacke, 1997; Schäbitz, Sommer, Zoder, Kiessling, Schwaninger, & Schwab, 2000; Wu & Pardridge, 1999).
The present study data agree with the previous studies. Seifi et al. reported that postconditioning protected the liver, as a remote organ, from renal IRI may be through reducing oxidative stress markers (Seifi et al., 2014). Additionally, another study indicated that postconditioning reduced the systemic damage intensity after a small intestinal ischemic reperfusion episode (Onody et al., 2012).
5. Conclusion
The current study results suggested that AKI triggers distant organ dysfunction and postconditioning protects some aspects of brain function, as a remote organ from the renal ischemia-reperfusion injury. These beneficial effects of postconditioning against IR-induced memory impairment may be associated with preventing IR-induced hippocampal BDNF down-regulation. Moreover, the present study adds to the literature supporting the concept that modifying reperfusion conditions at the early moments may be a valuable strategy to reduce injuries induced by ischemia-reperfusion.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the ethical committee of the Kerman Medical University.
Funding
The study was funded by Kerman Neuroscience Research Center.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no conflict of interest.
Refrences
Abbassian, H., Esmaeili, P., Tahamtan, M., Aghaei, I., Vaziri, Z., & Sheibani, V., et al. (2016). Cannabinoid receptor agonism suppresses tremor, cognition disturbances and anxiety-like behaviors in a rat model of essential tremor. Physiology & Behavior, 164(PartA), 314-20. [DOI:10.1016/j.physbeh.2016.06.013] [PMID]
Aghaei, I., Shabani, M., Doustar, N., Nazeri, M., & Dehpour, A. (2014). Peroxisome proliferator-activated receptor-γ activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacology Biochemistry and Behavior, 120, 133-9. [DOI:10.1016/j.pbb.2014.03.002] [PMID]
Arieff, A. I., & Massry, S. G. (1974). Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. The Journal of Clinical Investigation, 53(2), 387-92. [DOI:10.1172/JCI107571] [PMID] [PMCID]
Arieff, A. I., Massry, S. G., Barrientos, A., & Kleeman, C. R. (1973). Brain water and electrolyte metabolism in uremia: Effects of slow and rapid hemodialysis. Kidney International, 4(3), 177-87. [DOI:10.1038/ki.1973.100] [PMID]
Bella, A. J., Lin, G., Cagiannos, I., & Lue, T. F. (2008). Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian Journal of Andrology, 10(1), 54-59. [DOI:10.1111/j.1745-7262.2008.00368.x] [PMID]
Chandler, M. J., DeLeo, J., & Carney, J. M. (1985). An unanesthetized-gerbil model of cerebral ischemia-induced behavioral changes. Journal of Pharmacological Methods, 14(2), 137-46. [DOI:10.1016/0160-5402(85)90051-8] [PMID]
Cotman, C. W., & Berchtold, N. C. (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences, 25(6), 295-301. [DOI:10.1016/S0166-2236(02)02143-4][PMID]
Cunha, C., Brambilla, R., & Thomas, K. L. (2010). A simple role for BDNF in learning and memory? Frontiers in Molecular Neuroscience, 3, 1. [DOI:10.3389/neuro.02.001.2010] [PMID] [PMCID]
Deftereos, S., Giannopoulos, G., Tzalamouras, V., Raisakis, K., Kossyvakis, C., & Kaoukis, A., et al. (2013). Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. Journal of the American College of Cardiology, 61(19), 1949-55. [DOI:10.1016/j.jacc.2013.02.023] [PMID]
Edelstein, C. L., Ling, H., & Schrier, R. W. (1997). The nature of renal cell injury. Kidney International, 51(5), 1341-51. [DOI:10.1038/ki.1997.183] [PMID]
Gerhardt, S. C., & Boast, C. A. (1988). Motor activity changes following cerebral ischemia in gerbils are correlated with the degree of neuronal degeneration in hippocampus. Behavioral Neuroscience, 102(2), 301-3. [DOI:10.1037/0735-7044.102.2.301] [PMID]
Guo, Q., Du, X., Zhao, Y., Zhang, D., Yue, L., & Wang, Z. (2014). Ischemic postconditioning prevents renal ischemia reperfusion injury through the induction of heat shock proteins in rats. Molecular Medicine Reports, 10(6), 2875-81. [DOI:10.3892/mmr.2014.2641] [PMID] [PMCID]
Haghani, M., Shabani, M., & Moazzami, K. (2013). Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience, 250, 588-98. [DOI:10.1016/j.neuroscience.2013.07.049] [PMID]
Hariri, A. R., Goldberg, T. E., Mattay, V. S., Kolachana, B. S., Callicott, J. H., & Egan, M. F., et al. (2003). Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience, 23(17), 6690-4. [DOI:10.1523/JNEUROSCI.23-17-06690.2003] [PMID] [PMCID]
Hassoun, H. T., Grigoryev, D. N., Lie, M. L., Liu, M., Cheadle, C., & Tuder, R. M., et al. (2007). Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. American Journal of Physiology-Renal Physiology, 293(1), F30-40. [DOI:10.1152/ajprenal.00023.2007] [PMID]
Heldt, S. A., Stanek, L., Chhatwal, J. P., & Ressler, K. J. (2007). Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular Psychiatry, 12(7), 656-70. [DOI:10.1038/sj.mp.4001957] [PMID] [PMCID]
Hussein, A. A. M., Barakat, N., Awadalla, A., & Shokeir, A. A. (2012). Systemic and renal haemodynamic changes in renal schemia/reperfusion injury: Impact of erythropoietin. Canadian Journal of Physiology and Pharmacology, 90(11), 1535-43. [DOI:10.1139/y2012-120] [PMID]
Khatri, M., Himmelfarb, J., Adams, D., Becker, K., Longstreth, W. T., & Tirschwell, D. L. (2014). Acute kidney injury is associated with increased hospital mortality after stroke. Journal of Stroke and Cerebrovascular Diseases, 23(1), 25-30. [DOI:10.1016/j.jstrokecerebrovasdis.2012.06.005] [PMID] [PMCID]
Kin, H., Zatta, A. J., Lofye, M. T., Amerson, B. S., Halkos, M. E., & Kerendi, F., et al. (2005). Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovascular Research, 67(1), 124-33. [DOI:10.1016/j.cardiores.2005.02.015] [PMID]
Kin, H., Zhao, Z. Q., Sun, H.-Y., Wang, N.-P., Corvera, J. S., & Halkos, M. E., et al. (2004). Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovascular Research, 62(1), 74-85. [DOI:10.1016/j.cardiores.2004.01.006] [PMID]
Kinsey, G. R., Li, L., & Okusa, M. D. (2008). Inflammation in acute kidney injury. Nephron Experimental Nephrology, 109(4), e102-e107. [DOI:10.1159/000142934] [PMID] [PMCID]
Kiprianova, I., Sandkühler, J., Schwab, S., Hoyer, S., & Spranger, M. (1999). Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Experimental Neurology, 159(2), 511-9. [DOI:10.1006/exnr.1999.7109] [PMID]
Liu, K. X., Li, Y. S., Huang, W. Q., Chen, S. Q., Wang, Z. X., & Liu, J. X., et al. (2009). Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Medicine, 35(5), 933-42. [DOI:10.1007/s00134-009-1428-1] [PMID]
Liu, M., Liang, Y., Chigurupati, S., Lathia, J. D., Pletnikov, M., & Sun, Z., et al. (2008). Acute kidney injury leads to inflammation and functional changes in the brain. Journal of the American Society of Nephrology, 19(7), 1360-70. [DOI:10.1681/ASN.2007080901] [PMID] [PMCID]
Liu, X., Chen, H., Zhan, B., Xing, B., Zhou, J., & Zhu, H., et al. (2007). Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochemical and Biophysical Research Communications, 359(3), 628-34. [DOI:10.1016/j.bbrc.2007.05.129] [PMID]
Lønborg, J., Kelbæk, H., Vejlstrup, N., Jørgensen, E., Helqvist, S., & Saunamäki, K., et al. (2010). Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circulation Cardiovascular Interventions, 3(1), 34-41. [DOI:10.1161/CIRCINTERVENTIONS.109.905521] [PMID]
Melo, C. V., Okumoto, S., Gomes, J. R., Baptista, M. S., Bahr, B. A., & Frommer, W. B., et al. (2013). Spatiotemporal resolution of BDNF neuroprotection against glutamate excitotoxicity in cultured hippocampal neurons. Neuroscience, 237, 66-86. [DOI:10.1016/j.neuroscience.2013.01.054] [PMID]
Nongnuch, A., Panorchan, K., & Davenport, A. (2014). Brain-kidney crosstalk. Crit Care, 18(3), 225. [DOI:10.1186/cc13907] [PMID] [PMCID]
Norio, H., Hiroshi, W., Nobuhide, A., Mitsue, K., Jiro, I., & Yushiro, T. (1990). Cerebral ischemia model with conscious mice: Involvement of NMDA receptor activation and derangement of learning and memory ability. Journal of Pharmacological Methods, 23(4), 311-27. [DOI:10.1016/0160-5402(90)90059-T] [PMID]
Onody, P., Rosero, O., Kovacs, T., Gabbais, D., Hegedüs, V., & Lotz, G., et al. (2012). Postconditioning--effective method against distant organ dysfunction? Magyar Sebeszet, 65(4), 222-9. [DOI:10.1556/maseb.65.2012.4.9] [PMID]
Penna, C., Tullio, F., Moro, F., Folino, A., Merlino, A., & Pagliaro, P. (2010). Effects of a protocol of ischemic postconditioning and/or captopril in hearts of normotensive and hypertensive rats. Basic Research in Cardiology, 105(2), 181-92. [DOI:10.1007/s00395-009-0075-6] [PMID]
Ratliff, B. B., Rabadi, M. M., Vasko, R., Yasuda, K., & Goligorsky, M. S. (2013). Messengers without borders: Mediators of systemic inflammatory response in AKI. Journal of the American Society of Nephrology, 24(4), 529-36. [DOI:10.1681/ASN.2012060633] [PMID]
Razavinasab, M., Shamsizadeh, A., Shabani, M., Nazeri, M., Allahtavakoli, M., & Asadi‐Shekaari, M., et al. (2013). Pharmacological blockade of TRPV1 receptors modulates the effects of 6‐OHDA on motor and cognitive functions in a rat model of parkinson’s disease. Fundamental & Clinical Pharmacology, 27(6), 632-40. [DOI:10.1111/fcp.12015] [PMID]
Ren, C., Gao, X., Niu, G., Yan, Z., Chen, X., & Zhao, H. (2008). Delayed postconditioning protects against focal ischemic brain injury in rats. PloS one, 3(12), e3851. [DOI:10.1371/journal.pone.0003851] [PMID] [PMCID]
Schäbitz, W. R., Berger, C., Kollmar, R., Seitz, M., Tanay, E., & Kiessling, M., et al. (2004). Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke, 35(4), 992-7. [DOI:10.1161/01.STR.0000119754.85848.0D] [PMID]
Schäbitz, W. R., Schwab, S., Spranger, M., & Hacke, W. (1997). Intraventricular brain-derived neurotrophic factor size after focal cerebral ischemia in rats. Journal of Cerebral Blood Flow & Metabolism, 17(5), 500-6. [DOI:10.1097/00004647-199705000-00003] [PMID]
Schäbitz, W. R., Sommer, C., Zoder, W., Kiessling, M., Schwaninger, M., & Schwab, S. (2000). Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke, 31(9), 2212-7. [DOI:10.1161/01.STR.31.9.2212] [PMID]
Seifi, B., Kadkhodaee, M., Najafi, A., & Mahmoudi, A. (2014). Protection of liver as a remote organ after renal ischemia-reperfusion injury by renal ischemic postconditioning. International Journal of Nephrology, 2014, 120391. [DOI:10.1155/2014/120391] [PMID] [PMCID]
Shabani, M., Larizadeh, M. H., Parsania, S., Asadi Shekaari, M., & Shahrokhi, N. (2012). Profound destructive effects of adolescent exposure to vincristine accompanied with some sex differences in motor and memory performance. Canadian Journal of Physiology and Pharmacology, 90(4), 379-86. [DOI:10.1139/y11-132] [PMID]
Shabani, M., Nazeri, M., Parsania, S., Razavinasab, M., Zangiabadi, N., & Esmaeilpour, K., et al. (2012). Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology, 33(5), 1314-21. [DOI:10.1016/j.neuro.2012.08.004] [PMID]
Singh, M., & Su, C. (2013). Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience, 239, 84-91. [DOI:10.1016/j.neuroscience.2012.09.056] [PMID] [PMCID]
Skelly, D. T., Hennessy, E., Dansereau, M. A., & Cunningham, C. (2013). A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57BL/6 mice. PloS one, 8(7), e69123. [DOI:10.1371/journal.pone.0069123] [PMID] [PMCID]
Sun, K., Liu, Z. S., & Sun, Q. (2004). Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World Journal of Gastroenterology, 10(13), 1934-8. [DOI:10.3748/wjg.v10.i13.1934] [PMID] [PMCID]
Tahamtan, M., Allahtavakoli, M., Abbasnejad, M., Roohbakhsh, A., Taghipour, Z., &Taghavi, M., et al. (2013). Exercise preconditioning improves behavioral functions following transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in rats. Archives Of Iranian Medicine, 16(12), 697-704. [PMID]
Tsang, A., Hausenloy, D. J., Mocanu, M. M., & Yellon, D. M. (2004). Postconditioning: A form of modified reperfusion protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation Research, 95(3), 230-2. [DOI:10.1161/01.RES.0000138303.76488.fe] [PMID]
Tsao, N., Hsu, H. P., Wu, C. M., Liu, C. C., & Lei, H. Y. (2001). Tumour necrosis factor-α causes an increase in blood-brain barrier permeability during sepsis. Journal of Medical Microbiology, 50(9), 812-21. [DOI:10.1099/0022-1317-50-9-812] [PMID]
Vetrovoi, O. V., Rybnikova, E. A., Glushchenko, T. S., & Samoilov, M. O. (2015). Effects of hypoxic postconditioning on the expression of antiapoptotic protein Bcl-2 and neurotrophin BDNF in hippocampal field CA1 in rats subjected to severe hypoxia. Neuroscience and Behavioral Physiology, 45(4), 367-70. [DOI:10.1007/s11055-015-0083-y]
Vetrovoĭ, O., Rybnikova, T., & Samoĭlov, M. (2013). [Effect of hypoxic postconditioning on the expression of antiapoptotic protein Bcl-2 and neurotrophin BDNF in CA1 hippocampal field of rats surviving severe hypoxia (Russian)]. Morfologiia, 145(2), 16-20. [PMID]
Wang, D., & Corbett, D. (1990). Cerebral ischemia, locomotor activity and spatial mapping. Brain Research, 533(1), 78-82. [DOI:10.1016/0006-8993(90)91798-L] [PMID]
Wang, J. Y., Shen, J., Gao, Q., Ye, Z. g., Yang, S. Y., & Liang, H. W., et al. (2008). Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke, 39(3), 983-90. [DOI:10.1161/STROKEAHA.107.499079] [PMID]
Wever, K. E., Menting, T., Masereeuw, R., van der Vliet, J. A., Rongen, G. A., & Warlé, M. C. (2012). Local and remote ischemic postconditionings have synergistic protective effects on renal ischemia-reperfusion injury. Transplantation, 94(1), e1-e2. [DOI:10.1097/TP.0b013e318257ad76] [PMID]
Wu, D., & Pardridge, W. M. (1999). Neuroprotection with noninvasive neurotrophin delivery to the brain. Proceedings of the National Academy of Sciences, 96(1), 254-9. [DOI:10.1073/pnas.96.1.254] [PMID] [PMCID]
Yan, X. B., Hou, H. L., Wu, L. M., Liu, J., & Zhou, J. N. (2007). Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology, 53(4), 487-95. [DOI:10.1016/j.neuropharm.2007.06.020] [PMID]
Zen’ko, M., Rybnikova, Y., & Glushchenko, T. S. (2014). [Expression of BDNF neurotrophin in the hippocampus and neocortex of rats during the development of post-stress anxiety and its correction by hypoxic postconditioning (Russian)]. Morfologiia, 146(5), 14-18. [PMID]
Zhang, X., Zhang, Q., Tu, J., Zhu, Y., Yang, F., & Liu, B., et al. (2015). Prosurvival NMDA 2A receptor signaling mediates postconditioning neuroprotection in the hippocampus. Hippocampus, 25(3), 286-96. [DOI:10.1002/hipo.22372] [PMID]
Zhao, H. (2009). Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. Journal of Cerebral Blood Flow & Metabolism, 29(5), 873-85. [DOI:10.1038/jcbfm.2009.13] [PMID] [PMCID]
Zhao, H., Sapolsky, R. M., & Steinberg, G. K. (2006). Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. Journal of Cerebral Blood Flow & Metabolism, 26(9), 1114-21. [DOI:10.1038/sj.jcbfm.9600348] [PMID]
Zhao, Z. Q. (2010). Postconditioning in reperfusion injury: A status report. Cardiovascular Drugs and Therapy, 24(3), 265-79. [DOI:10.1007/s10557-010-6240-1] [PMID]
Zhao, Z. Q., Corvera, J. S., Halkos, M. E., Kerendi, F., Wang, N. P., & Guyton, R. A., et al. (2003). Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. American Journal of Physiology-Heart and Circulatory Physiology, 285(2), H579-H588. [DOI:10.1152/ajpheart.01064.2002] [PMID]
Acute Kidney Injury (AKI) is a frequent complication, i.e., associated with increased hospital stay and mortality (Hussein, Barakat, Awadalla, & Shokeir, 2012; Khatri, Himmelfarb, Adams, Becker, K., Longstreth, & Tirschwell, 2014). Renal Ischemia-Reperfusion Injury (Renal IRI) is one of the most important causes of AKI and can lead to remote organ dysfunction like lung, brain, and heart (Hassoun et al., 2007).
Patients with AKI suffer from high levels of mental and cognitive dysfunctions. Synthesizing the results of the previous studies, AKI caused cellular and soluble inflammation, edema, alterations in water transport, and increased microvascular protein leakage in the brain (Arieff & Massry, 1974; Arieff, Massry, Barrientos, & Kleeman, 1973; Liu et al., 2008). Due to the inflammatory response, the disruption of the BBB, endothelial injury, and stimulation of the coagulation cascades within the brain have been observed, leading to alterations in neuronal cell protein transcription and cellular activation. Furthermore, an increased number of pyknotic neuronal cells and microgliosis following 60 min of renal ischemia in the CA1 region of the brain hippocampus, compared to the sham group, has been indicated (Kinsey, Li, & Okusa, 2008; Liu et al., 2008). Another research also reported increased GFAP and microglia cells as distant organ consequences of AKI (Kinsey et al., 2008; Liu et al., 2008; Ratliff, Rabadi, Vasko, Yasuda, & Goligorsky, 2013; TSAO, Hsu, Wu, Liu, & Lei, 2001). These changes were implicated in causing altered brain function (Nongnuch, Panorchan, & Davenport, 2014).
Bilateral renal ischemia has been introduced as the leading cause of AKI in patients. Since ischemia/reperfusion can cause irreversible damages to tissue and mechanisms contributing to the pathogenesis of ischemia-reperfusion injury are complex, new strategies like ischemic conditioning have been explained to increase tolerance and protect against ischemia-reperfusion injury (Seifi, Kadkhodaee, Najafi, & Mahmoudi, 2014). In ischemic conditioning, brief intermittent and reversible episodes of ischemia with reperfusion can induce protective effects against ischemia/reperfusion injury. These processes were defined based on this phenomenon: ischemic preconditioning and Ischemic Postconditioning (IPo).
Ischemic preconditioning is a powerful intervention against IRI; however, it is clinically feasible because, in most cases, ischemia is not predictable (Zhao, Sapolsky, & Steinberg, 2006). Several studies introduced IPo as a new neuroprotective strategy to deal with IRI (Penna, Tullio, Moro, Folino, Merlino, & Pagliaro, 2010; Wever, Menting, Masereeuw, van der Vliet, Rongen, & Warlé, 2012; Zhao et al., 2003). It is the phenomenon that brief, repetitive cycles of ischemia during the onset of reperfusion can be protective in different systems (Penna et al., 2010; Wang et al., 2008; Wever et al., 2012) and can attenuate the potential additional injury which may be induced by reperfusion. Thus, IPo has become a clinical application to significantly decrease IRI (Deftereos et al., 2013; Liu et al., 2009; Lønborg et al., 2010). However, the detailed mechanism of postconditioning remains unknown. Discovering involved mechanisms in postconditioning may shed light on finding effective treatment against injuries.
Neurotrophins are neuroprotective factors that exert their effects through intracellular signaling pathways. Brain-Derived Neurotrophic Factor (BDNF) is a member of the neurotrophin family, which plays a prominent role in neuronal survival, plasticity, learning, and memory. Moreover, the vital role of BDNF in neurological function following brain injury has been indicated. Several studies discriminated the protective role of BDNF following neonatal hypoxic_ ischemic brain injury, transient, and focal cerebral ischemia, and stroke (Melo et al., 2013; Singh & Su, 2013; Vetrovoĭ, Rybnikova, & Samoĭlov, 2013).
There is a growing body of evidence that hypoxic and ischemic postconditioning up-regulate the expression of BDNF, particularly in hippocampal CA1 neurons in rats (Vetrovoi, Rybnikova, Glushchenko, & Samoilov, 2015; Vetrovoĭ et al., 2013; Zen’ko, Rybnikova, & Glushchenko, 2013; Zhang et al., 2015).
Several studies outlined the protective effects of postconditioning in the heart (Kin et al., 2005; Kin et al., 2004; Tsang, Hausenloy, Mocanu, & Yellon, 2004), brain (Ren et al., 2008; Zhao, 2009; Zhao, Sapolsky, & Steinberg, 2006), liver (Sun, Liu, & Sun, 2004), and kidney (Liu et al., 2007); however, there is no study to examine the effects of renal IPo on brain damage induced by renal IRI. The study aimed to investigate: 1) whether IPo will be able to prevent memory and motor deficits, 2) whether IPo causes protective effects by alteration in BDNF level.
2. Methods
Animal preparation and experimental design
Adult male Wistar rats (8-10 weeks old, 180-220 g) were used in this experiment and fed with standard rat chow and water ad libitum. The animals were housed under a controlled temperature (21-23 °C) and 12:12h light/dark cycle. All animals’ procedures were observed to minimize pain and were approved by the Kerman Medical University Ethics Committee (EC/KNRC/92-6). There were 6 different groups of rats in the current study. The rats subjected to Bilateral Renal Ischemia/reperfusion (BRI) were divided into 2 groups of n=10, in which the right and left renal arteries and veins were occluded for 1h that followed by the reperfusion periods of 24 hours (BRI-24h group) and 1 week (BRI-1w group). The sham-operated rats were divided into 2 groups of n=10 that animals underwent only anesthesia without occlusion with periods equivalent to reperfusion of 24 hours (sham-24h group) or 1 week (sham-1w group).
IPo animals were also divided into 2 groups (n=10/group) subjected to 60 min ischemia, then 3 cycles of 10s of reperfusion followed by 10s ischemia in the reperfusion periods of 24 hours (IPo-24h group) and 1 week (IPo-1w group). This model of postconditioning was based on the methods described before (Figure 1) (Guo et al., 2014).
.jpg)
Inducing bilateral renal ischemia/reperfusion and ischemic postconditioning
The study rats were anesthetized with ether, and a midline laparotomy was performed. The renal arteries and veins were occluded with non-traumatic clamps. The occlusion was verified visually by a color change of the kidney to a paler shade. The clamps were isolated at the end of the ischemic period, and blood was reperfused. The kidneys were treated identically in sham-operated control rats, except for clamping. In ischemic postconditioning-operated rats after 60 min of occlusion to induce ischemia, 3 cycles of ischemia (10 sec) followed by reperfusion (10 sec) exerted.
The abdominal incision was sutured at two layers by 2-0 silk. All surgical procedure was done under sterile condition, and the rat was allowed to recover from the anesthesia before returning to an individual cage. The Kerman University approved the surgical protocol of Medical Sciences. Behavioral assays were performed 24h or 1 week after surgery with a 2h interval among each assay in the following order: open field test, rotarod, wire grip test, and shuttle box or Morris water maze (the last two assays were performed in separate groups of study due to the nature of the procedure).
Behavioral experiments
Open-field test
The open-field test was used to screen anxiety-like behavior 24h and 1w after reperfusion. The rats were adapted in the experimental room 1 h before the test, then were placed in the center of the apparatus, i.e., made of a Plexiglas square arena [90×90×30 (H) cm], its floor was divided into 16 squares, so the field was divided into central and peripheral squares. The vertical and horizontal activities of the animals were observed directly and continuously for 5 min. They then were analyzed using Ethovision software, v. 7.1, a video tracking software to automate the behavioral paradigms (Noldus Information Technology, the Netherlands). The following behavioral components were measured: total distance moved [TDM, cm], number of rearing (as a measure of vertical activity), and velocity. At the end of each test, the examined animals were removed from the chamber, and the field was cleaned with a damp cloth (Razavinasab et al., 2013).
Rotarod
The effects of BRI and postconditioning on motor balance and coordination of the animals by performing accelerating rotarod (Hugo Sachs Electronic, Germany) 24h and 1w after reperfusion were observed.
The rotarod performance test measures motor parameters, including motor balance and coordination based on a rotating rod with forced motor activity. The rotarod started from a speed of 10 Revolutions Per Min (RPM) to the maximum speed of 60 RPM. It was divided into 4 equal sections, thus enabling 4 rats to walk on the rod simultaneously. Three trials were performed for each rat (intertrial interval=30 min), with each trial lasting for a total maximum of 300s. Intervals between the mounting of the rat on the rod and falling off of it were recorded as a measure of motor coordination and balance. The length of time a given animal stays on this rotating rod is known as its balance index (Shabani et al., 2012).
Wire grip test
To evaluate the muscle strength of the animals, the hanging-wire-grip test was performed 1 week after the operation. The rat was placed in a vertical posture while its forepaws were put in contact with the steel wire (80 cm long, with a diameter of 7 mm). The animal was released whenever it grasped the wire. The total time each rat could hang suspended from a wire grid was recorded for three trials with a 30 min inter-trial interval (Haghani, Shabani, & Moazzami, 2013).
Passive Avoidance (PA) learning
PA learning was selected as the tool for assessing fear learning in BRI rats and the possible effect of postconditioning on 24h and 1w after reperfusion. For this purpose, an apparatus with the dimensions of 100∗25∗25 consisted of two identical illuminated and dark boxes separated by a guillotine door and a stainless rod grid serving as the base. The adaptation phase was followed by a single trial in which the animals were placed in the illuminated compartment. After 10s, the door was opened, and the animal was allowed to go to the dark compartment. After that, the door was closed without electric shock, and after the 20s, the animal was placed in the cage. The rats without the tendency to enter the dark chamber were excluded. After 30 min of adaptation, for the learning trial, this procedure was repeated, and animals were given an inescapable foot-shock (0.5 mA, 1.5 s) immediately after entering the darkroom. This procedure was repeated until the animal did not enter the dark box. The number of shocks received was recorded in this phase. The retention tests were conducted 24h after the training phase (without shock) to evaluate memory. The latency time (cut off: 300 sec) to enter the dark compartment as retention time [Step-Through Latency (STL)] was recorded (Abbassian et al., 2016).
Morris Water Maze (MWM)
The rats were tested by an MWM as previously described (Aghaei, Shabani, Doustar, Nazeri, & Dehpour, 2014). The test chamber was a circular tank (140 cm diameter, 45 cm height), surrounded by extra-tank visual cues. A visible or submerged platform (15 cm wide, 35 cm height) was placed 1.5 cm above or below the water surface. Water temperature was maintained at 21-23°C. Rats’ behavior was recorded with the Ethovision system. The following parameters were computed for each animal: total distance and time spent to reach the platform in three consecutive trials, the number of crosses in the correct quadrant in the retention phase, percentage of the time, and distance traveled in the correct quadrant. Each rat underwent three blocks of trials in the training phase, each including four trials (inter-trial interval= 30s). In each trial, animals were put in one of the 4 quadrants facing the maze. The examined animals were allowed to swim the 60s to find the platform, and if they did not find it, they were put on the platform by the examiner. After 30 s, the rat was again put to trial. After 2h of the last block, the rats underwent a probe trial during which the platform was removed from the tank and the number of crosses in the correct quadrant and total time spent in the target quadrant was recorded and analyzed per rat (Shabani, et sl., 2012).
Molecular experiment
Tissue dissection and preparation for Western blot
The rats were divided into sham, BRI, and IPo groups for the molecular experiment, decapitated 24h after surgery. All of the rats were anesthetized with atmospheric CO2. After decapitation, the brains were rapidly extracted and placed on ice. Both whole hippocampi were freshly harvested and placed in a microtube and stored at −80 until homogenization for further western blot assay.
BDNF immunoblot analysis
The dissected hippocampus tissues were homogenized in ice-cold buffer containing 10 mM Tris–HCl (pH=7.4), 1 mM EDTA, 0.1% SDS, 0.1% Na-deoxycholate, 1% NP-40 with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2.5 μg/mL of leupeptin, 10 μg/mL of aprotinin) and 1 mM sodium orthovanadate (a phosphatase inhibitor). The homogenate was centrifuged at 15,000 RPM for 20 min at 4°C. The resulting supernatant was retained as the whole-cell fraction. Protein concentrations were measured using the Bradford method (Bio-Rad Laboratories, Muenchen, Germany). Equal amounts of protein were resolved electrophoretically on a 9% SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE) SDS-PAGE gel and transferred to PVDF (polyvinylidenefluoride) membranes. After blocking with 5%, non-fat dried milk in Tris-buffered saline with Tween 20 (blocking buffer, TBS-T, 150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 0.1% Tween 20) for 2h, at room temperature and then, the membranes were incubated overnight with a primary rabbit polyclonal antibody for BDNF (1:1000, sc-20981; Santa Cruz Biotechnology, Santa Cruz, USA) at 4°C. After washing in TBS-T buffer (3 times for 5 min each, at room temperature), the blots were incubated for 2 h at room temperature with an anti-rabbit IgG secondary antibody conjugated with horseradish peroxidase (1:15,000; GE Healthcare Bio-Sciences). The primary and secondary antibodies were diluted in blocking buffer. The antibody-antigen complexes were revealed using the ECL system (Amersham Biosciences). Images were captured on a Gel Doc imaging system (Bio-Rad, Hercules, CA, US) converted to a tiff file. Lab Work analyzing software (UVP, UK) was used to analyze the intensity of the expression. β-actin immunoblotting (antibody from Cell Signaling Technology, INC. Beverly, MA, USA; 1:1000) was used to control loading. The timeline applied for the experimental protocol has been indicated in Figure 2.
.jpg)
Statistical analysis was performed in SPSS. All data were expressed as Mean±SEM. Analysis of Variance (ANOVA) followed by Tukey’s post-hoc analysis was used to compare the differences between the study groups. Repeated-measures ANOVA was used to analyze the data of the MWM task in the learning phase. The band density values were expressed as BDNF/β-actin ratio for each sample. The mean differences for the study groups were compared using one-way ANOVA, followed by the Newman–Keuls test. P<0.05 was considered significant.
3. Results
Open field test
The open field has been used to investigate locomotion and anxiety-related behaviors. Rats were subjected to 60 min of renal ischemia followed by reperfusion presented no significant differences in all parameters (velocity, rearing number, & total distance moved) measured in this test 24h after operation (P>0.05, Figure 3-A, B, and C).
.jpg)
Moreover, an open field test also was performed 1 week after surgery. There was no significant alteration in mentioned parameters 1 week after surgery (Figure 4-A, B, & C).
.jpg)
The effect of BRI on balance function, motor learning, and muscle strength
Compared with the sham group, BRI reduced duration on the rod 24h after surgery (P<0.01). Postconditioning rats had no significant difference with sham and BRI groups (P>0.05) (Figure 5).
.jpg)
No significant difference in duration on the rod was observed between the groups of study 1 week after reperfusion (P>0.05) (Figure 6).
.jpg)
BRI animals showed reduced muscle strength compared to the postconditioning group 1 week after reperfusion (P<0.05, Figure 7).
.jpg)
Postconditioning could significantly reverse this effect of BRI.
The effect of BRI and IPo on PA learning 24h after reperfusion
There was no significant difference in the number of shocks received among the three groups (P>0.05, Figure 8-A). BRI rats had impairment in memory retrieval compared to the sham group (P<0.001, ANOVA); postconditioning could reverse this impairment in BRI rats (p<0.05, ANOVA) (Figure 8-B).
.jpg)
The effect of BRI and IPo on PA learning 1 week after reperfusion
No significant alteration in the parameters measured was observed amongst three groups of study 1 week after reperfusion (P>0.05, Figure 9-A & B).
.jpg)
The effect of BRI and IPo on spatial learning 1 week after reperfusion
There were no significant differences in the parameters measured across blocks of trials (Figure 10-A &C) and probe trial (Figure 10-D & F) between groups in all periods, indicating that BRI and IPo did not influence the spatial learning.


BDNF protein expression
Next, we sought to identify a potential molecular mechanism that underlies memory impairments. We evaluate the expression of BDNF 24h after reperfusion because the IR-induced memory dysfunction was observed just at this time point. As per Figure 11, IR rats indicated a decreased hippocampal BDNF protein expression level than the sham-operated group (P<0.05). Surprisingly, the postconditioning situation could significantly (P<0.05) prevent the decreasing effect of renal IRI on the hippocampal BDNF expression (Figure 11).

Therefore, the present results indicated that the significant down-regulation of the BDNF protein in BRI rats was prevented by inducing renal ischemic postconditioning.
4. Discussion
Ischemia causes tissue damage in various clinical situations, such as acute infarctions or hypoperfusion, and the restoration of suitable blood flow is the primary treatment. Remote ischemic conditioning is an approach of conditioning that is not the target organ, but a more accessible tissue is submitted to a conditioning stimulus.
We observed that 1h of BRI, as a model of acute kidney injury in rats, impairs memory and balance function 24h after reperfusion and muscle strength of the animals 1w after reperfusion. Postconditioning indicated a promising effect against these impairments induced by BRI. Moreover, we observed no significant memory impairment and balanced disability in the BRI group, compared to the other groups 1w after the operation.
The high morbidity and mortality in patients with AKI and the non-existence of effective therapeutic modalities necessitate strategies to alleviate the impact of kidney injury on distant organs. Ischemic renal tissue’s reperfusion (sudden restoration of blood flow) is highly harmful, especially at ischemia. The reperfusion phase initiates a wide range of cellular events responsible for transendothelial migration of inflammatory cells, tissue edema, necrotic and apoptotic cell death (Edelstein, Ling, & Schrier, 1997). Thus, interventions given only at the beginning of reperfusion can reduce post-ischemic injury (Zhao, 2010).
BRI reliably induced balance and muscle strength impairments in the behavioral tasks employed in the present study. Previous studies revealed that the alterations in locomotor activity are related to the ischemic damage of CA1 area of the hippocampus in the open-field test (Chandler, DeLeo, & Carney, 1985; Gerhardt & Boast, 1988; Liu et al., 2008; Skelly, Hennessy, Dansereau, & Cunningham, 2013; Wang & Corbett, 1990) a region which is involved in anxiety and depression (Heldt, Stanek, Chhatwal, & Ressler, 2007). Increased circulating inflammatory cytokines and the number of pyknotic neuronal cells in the hippocampus have been indicated due to AKI (Liu et al., 2008; Skelly et al., 2013). No significant difference was observed between total distances moved, velocity, and rearing numbers in both time points assessed by open field test 24h and 1w after reperfusion.
The current study confirmed that bilateral renal ischemia followed by 24h of reperfusion resulted in memory impairment, i.e., assessed by a PA learning test. Postconditioning could improve this impairment in BRI rats. BRI followed by 1w of reperfusion couldn’t impair PA learning, and no significant difference was observed between the groups. Several studies on alterations in brain function following cerebral ischemia verify impairments in motor coordination, learning, and memory abilities (Norio et al., 1990; Tahamtan et al., 2013; Yan, Hou, Wu, Liu, & Zhou, 2007).
The total distances moved, and velocity is not significant between groups in different time points; therefore, it can indicate that the memory defect shown in the PA test is not due to the impaired ability of animals to move freely.
One promising finding of the previous studies is postconditioning. The term “postconditioning” refers to intermittent interruptions of blood flow at the onset of reperfusion which can activate signaling pathways and effectors (Zhao et al., 2003). However, the detailed mechanisms underlying these actions remain to be determined. To our knowledge, for the first time, we report that postconditioning protects some aspects of brain function against reperfusion injury induced by bilateral renal ischemia. Remote ischemic conditioning activates protective pathways in distant organs, such as the brain, heart, and kidney, representing an exciting new neuroprotection pattern. The exact mechanism of signal transmission from the periphery to the brain is unknown; however, humoral factors and an intact nervous system play critical roles.
One of the critical objectives of the experiments was to examine the effect of renal IRI and renal IRI Plus postconditioning on hippocampal BDNF level. Immunoblotting data indicated that postconditioning preserved BDNF protein level in the hippocampus region of BRI rats. BDNF, a crucial trophic factor in synaptic plasticity (Cunha, Brambilla, & Thomas, 2010), is present in high concentration in the hippocampus and cerebral cortex (Cotman & Berchtold, 2002). BDNF plays a crucial role in hippocampal-dependent learning and memory. It also may indicate a promising neuromodulatory therapeutic agent, increasing the survival of neurons, regeneration, and differentiation (Bella, Lin, Cagiannos, & Lue, 2008; Cotman & Berchtold, 2002; Hariri et al., 2003). Furthermore, BRI-induced changes in PA learning and memory seem to correlate with the decrease in hippocampal BDNF level.
A growing body of literature demonstrates that BDNF has not only potent neuroprotective effects but also is a strong inducer of recovery after various types of ischemic lesions (Kiprianova, Sandkühler, Schwab, Hoyer, & Spranger, 1999; Schäbitz et al., 2004; Schäbitz, Schwab, Spranger, & Hacke, 1997; Schäbitz, Sommer, Zoder, Kiessling, Schwaninger, & Schwab, 2000; Wu & Pardridge, 1999).
The present study data agree with the previous studies. Seifi et al. reported that postconditioning protected the liver, as a remote organ, from renal IRI may be through reducing oxidative stress markers (Seifi et al., 2014). Additionally, another study indicated that postconditioning reduced the systemic damage intensity after a small intestinal ischemic reperfusion episode (Onody et al., 2012).
5. Conclusion
The current study results suggested that AKI triggers distant organ dysfunction and postconditioning protects some aspects of brain function, as a remote organ from the renal ischemia-reperfusion injury. These beneficial effects of postconditioning against IR-induced memory impairment may be associated with preventing IR-induced hippocampal BDNF down-regulation. Moreover, the present study adds to the literature supporting the concept that modifying reperfusion conditions at the early moments may be a valuable strategy to reduce injuries induced by ischemia-reperfusion.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the ethical committee of the Kerman Medical University.
Funding
The study was funded by Kerman Neuroscience Research Center.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declare no conflict of interest.
Refrences
Abbassian, H., Esmaeili, P., Tahamtan, M., Aghaei, I., Vaziri, Z., & Sheibani, V., et al. (2016). Cannabinoid receptor agonism suppresses tremor, cognition disturbances and anxiety-like behaviors in a rat model of essential tremor. Physiology & Behavior, 164(PartA), 314-20. [DOI:10.1016/j.physbeh.2016.06.013] [PMID]
Aghaei, I., Shabani, M., Doustar, N., Nazeri, M., & Dehpour, A. (2014). Peroxisome proliferator-activated receptor-γ activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacology Biochemistry and Behavior, 120, 133-9. [DOI:10.1016/j.pbb.2014.03.002] [PMID]
Arieff, A. I., & Massry, S. G. (1974). Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. The Journal of Clinical Investigation, 53(2), 387-92. [DOI:10.1172/JCI107571] [PMID] [PMCID]
Arieff, A. I., Massry, S. G., Barrientos, A., & Kleeman, C. R. (1973). Brain water and electrolyte metabolism in uremia: Effects of slow and rapid hemodialysis. Kidney International, 4(3), 177-87. [DOI:10.1038/ki.1973.100] [PMID]
Bella, A. J., Lin, G., Cagiannos, I., & Lue, T. F. (2008). Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian Journal of Andrology, 10(1), 54-59. [DOI:10.1111/j.1745-7262.2008.00368.x] [PMID]
Chandler, M. J., DeLeo, J., & Carney, J. M. (1985). An unanesthetized-gerbil model of cerebral ischemia-induced behavioral changes. Journal of Pharmacological Methods, 14(2), 137-46. [DOI:10.1016/0160-5402(85)90051-8] [PMID]
Cotman, C. W., & Berchtold, N. C. (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences, 25(6), 295-301. [DOI:10.1016/S0166-2236(02)02143-4][PMID]
Cunha, C., Brambilla, R., & Thomas, K. L. (2010). A simple role for BDNF in learning and memory? Frontiers in Molecular Neuroscience, 3, 1. [DOI:10.3389/neuro.02.001.2010] [PMID] [PMCID]
Deftereos, S., Giannopoulos, G., Tzalamouras, V., Raisakis, K., Kossyvakis, C., & Kaoukis, A., et al. (2013). Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. Journal of the American College of Cardiology, 61(19), 1949-55. [DOI:10.1016/j.jacc.2013.02.023] [PMID]
Edelstein, C. L., Ling, H., & Schrier, R. W. (1997). The nature of renal cell injury. Kidney International, 51(5), 1341-51. [DOI:10.1038/ki.1997.183] [PMID]
Gerhardt, S. C., & Boast, C. A. (1988). Motor activity changes following cerebral ischemia in gerbils are correlated with the degree of neuronal degeneration in hippocampus. Behavioral Neuroscience, 102(2), 301-3. [DOI:10.1037/0735-7044.102.2.301] [PMID]
Guo, Q., Du, X., Zhao, Y., Zhang, D., Yue, L., & Wang, Z. (2014). Ischemic postconditioning prevents renal ischemia reperfusion injury through the induction of heat shock proteins in rats. Molecular Medicine Reports, 10(6), 2875-81. [DOI:10.3892/mmr.2014.2641] [PMID] [PMCID]
Haghani, M., Shabani, M., & Moazzami, K. (2013). Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience, 250, 588-98. [DOI:10.1016/j.neuroscience.2013.07.049] [PMID]
Hariri, A. R., Goldberg, T. E., Mattay, V. S., Kolachana, B. S., Callicott, J. H., & Egan, M. F., et al. (2003). Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of Neuroscience, 23(17), 6690-4. [DOI:10.1523/JNEUROSCI.23-17-06690.2003] [PMID] [PMCID]
Hassoun, H. T., Grigoryev, D. N., Lie, M. L., Liu, M., Cheadle, C., & Tuder, R. M., et al. (2007). Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. American Journal of Physiology-Renal Physiology, 293(1), F30-40. [DOI:10.1152/ajprenal.00023.2007] [PMID]
Heldt, S. A., Stanek, L., Chhatwal, J. P., & Ressler, K. J. (2007). Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular Psychiatry, 12(7), 656-70. [DOI:10.1038/sj.mp.4001957] [PMID] [PMCID]
Hussein, A. A. M., Barakat, N., Awadalla, A., & Shokeir, A. A. (2012). Systemic and renal haemodynamic changes in renal schemia/reperfusion injury: Impact of erythropoietin. Canadian Journal of Physiology and Pharmacology, 90(11), 1535-43. [DOI:10.1139/y2012-120] [PMID]
Khatri, M., Himmelfarb, J., Adams, D., Becker, K., Longstreth, W. T., & Tirschwell, D. L. (2014). Acute kidney injury is associated with increased hospital mortality after stroke. Journal of Stroke and Cerebrovascular Diseases, 23(1), 25-30. [DOI:10.1016/j.jstrokecerebrovasdis.2012.06.005] [PMID] [PMCID]
Kin, H., Zatta, A. J., Lofye, M. T., Amerson, B. S., Halkos, M. E., & Kerendi, F., et al. (2005). Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovascular Research, 67(1), 124-33. [DOI:10.1016/j.cardiores.2005.02.015] [PMID]
Kin, H., Zhao, Z. Q., Sun, H.-Y., Wang, N.-P., Corvera, J. S., & Halkos, M. E., et al. (2004). Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovascular Research, 62(1), 74-85. [DOI:10.1016/j.cardiores.2004.01.006] [PMID]
Kinsey, G. R., Li, L., & Okusa, M. D. (2008). Inflammation in acute kidney injury. Nephron Experimental Nephrology, 109(4), e102-e107. [DOI:10.1159/000142934] [PMID] [PMCID]
Kiprianova, I., Sandkühler, J., Schwab, S., Hoyer, S., & Spranger, M. (1999). Brain-derived neurotrophic factor improves long-term potentiation and cognitive functions after transient forebrain ischemia in the rat. Experimental Neurology, 159(2), 511-9. [DOI:10.1006/exnr.1999.7109] [PMID]
Liu, K. X., Li, Y. S., Huang, W. Q., Chen, S. Q., Wang, Z. X., & Liu, J. X., et al. (2009). Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Medicine, 35(5), 933-42. [DOI:10.1007/s00134-009-1428-1] [PMID]
Liu, M., Liang, Y., Chigurupati, S., Lathia, J. D., Pletnikov, M., & Sun, Z., et al. (2008). Acute kidney injury leads to inflammation and functional changes in the brain. Journal of the American Society of Nephrology, 19(7), 1360-70. [DOI:10.1681/ASN.2007080901] [PMID] [PMCID]
Liu, X., Chen, H., Zhan, B., Xing, B., Zhou, J., & Zhu, H., et al. (2007). Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochemical and Biophysical Research Communications, 359(3), 628-34. [DOI:10.1016/j.bbrc.2007.05.129] [PMID]
Lønborg, J., Kelbæk, H., Vejlstrup, N., Jørgensen, E., Helqvist, S., & Saunamäki, K., et al. (2010). Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circulation Cardiovascular Interventions, 3(1), 34-41. [DOI:10.1161/CIRCINTERVENTIONS.109.905521] [PMID]
Melo, C. V., Okumoto, S., Gomes, J. R., Baptista, M. S., Bahr, B. A., & Frommer, W. B., et al. (2013). Spatiotemporal resolution of BDNF neuroprotection against glutamate excitotoxicity in cultured hippocampal neurons. Neuroscience, 237, 66-86. [DOI:10.1016/j.neuroscience.2013.01.054] [PMID]
Nongnuch, A., Panorchan, K., & Davenport, A. (2014). Brain-kidney crosstalk. Crit Care, 18(3), 225. [DOI:10.1186/cc13907] [PMID] [PMCID]
Norio, H., Hiroshi, W., Nobuhide, A., Mitsue, K., Jiro, I., & Yushiro, T. (1990). Cerebral ischemia model with conscious mice: Involvement of NMDA receptor activation and derangement of learning and memory ability. Journal of Pharmacological Methods, 23(4), 311-27. [DOI:10.1016/0160-5402(90)90059-T] [PMID]
Onody, P., Rosero, O., Kovacs, T., Gabbais, D., Hegedüs, V., & Lotz, G., et al. (2012). Postconditioning--effective method against distant organ dysfunction? Magyar Sebeszet, 65(4), 222-9. [DOI:10.1556/maseb.65.2012.4.9] [PMID]
Penna, C., Tullio, F., Moro, F., Folino, A., Merlino, A., & Pagliaro, P. (2010). Effects of a protocol of ischemic postconditioning and/or captopril in hearts of normotensive and hypertensive rats. Basic Research in Cardiology, 105(2), 181-92. [DOI:10.1007/s00395-009-0075-6] [PMID]
Ratliff, B. B., Rabadi, M. M., Vasko, R., Yasuda, K., & Goligorsky, M. S. (2013). Messengers without borders: Mediators of systemic inflammatory response in AKI. Journal of the American Society of Nephrology, 24(4), 529-36. [DOI:10.1681/ASN.2012060633] [PMID]
Razavinasab, M., Shamsizadeh, A., Shabani, M., Nazeri, M., Allahtavakoli, M., & Asadi‐Shekaari, M., et al. (2013). Pharmacological blockade of TRPV1 receptors modulates the effects of 6‐OHDA on motor and cognitive functions in a rat model of parkinson’s disease. Fundamental & Clinical Pharmacology, 27(6), 632-40. [DOI:10.1111/fcp.12015] [PMID]
Ren, C., Gao, X., Niu, G., Yan, Z., Chen, X., & Zhao, H. (2008). Delayed postconditioning protects against focal ischemic brain injury in rats. PloS one, 3(12), e3851. [DOI:10.1371/journal.pone.0003851] [PMID] [PMCID]
Schäbitz, W. R., Berger, C., Kollmar, R., Seitz, M., Tanay, E., & Kiessling, M., et al. (2004). Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke, 35(4), 992-7. [DOI:10.1161/01.STR.0000119754.85848.0D] [PMID]
Schäbitz, W. R., Schwab, S., Spranger, M., & Hacke, W. (1997). Intraventricular brain-derived neurotrophic factor size after focal cerebral ischemia in rats. Journal of Cerebral Blood Flow & Metabolism, 17(5), 500-6. [DOI:10.1097/00004647-199705000-00003] [PMID]
Schäbitz, W. R., Sommer, C., Zoder, W., Kiessling, M., Schwaninger, M., & Schwab, S. (2000). Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke, 31(9), 2212-7. [DOI:10.1161/01.STR.31.9.2212] [PMID]
Seifi, B., Kadkhodaee, M., Najafi, A., & Mahmoudi, A. (2014). Protection of liver as a remote organ after renal ischemia-reperfusion injury by renal ischemic postconditioning. International Journal of Nephrology, 2014, 120391. [DOI:10.1155/2014/120391] [PMID] [PMCID]
Shabani, M., Larizadeh, M. H., Parsania, S., Asadi Shekaari, M., & Shahrokhi, N. (2012). Profound destructive effects of adolescent exposure to vincristine accompanied with some sex differences in motor and memory performance. Canadian Journal of Physiology and Pharmacology, 90(4), 379-86. [DOI:10.1139/y11-132] [PMID]
Shabani, M., Nazeri, M., Parsania, S., Razavinasab, M., Zangiabadi, N., & Esmaeilpour, K., et al. (2012). Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology, 33(5), 1314-21. [DOI:10.1016/j.neuro.2012.08.004] [PMID]
Singh, M., & Su, C. (2013). Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience, 239, 84-91. [DOI:10.1016/j.neuroscience.2012.09.056] [PMID] [PMCID]
Skelly, D. T., Hennessy, E., Dansereau, M. A., & Cunningham, C. (2013). A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, TNF-α and IL-6 challenges in C57BL/6 mice. PloS one, 8(7), e69123. [DOI:10.1371/journal.pone.0069123] [PMID] [PMCID]
Sun, K., Liu, Z. S., & Sun, Q. (2004). Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World Journal of Gastroenterology, 10(13), 1934-8. [DOI:10.3748/wjg.v10.i13.1934] [PMID] [PMCID]
Tahamtan, M., Allahtavakoli, M., Abbasnejad, M., Roohbakhsh, A., Taghipour, Z., &Taghavi, M., et al. (2013). Exercise preconditioning improves behavioral functions following transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in rats. Archives Of Iranian Medicine, 16(12), 697-704. [PMID]
Tsang, A., Hausenloy, D. J., Mocanu, M. M., & Yellon, D. M. (2004). Postconditioning: A form of modified reperfusion protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation Research, 95(3), 230-2. [DOI:10.1161/01.RES.0000138303.76488.fe] [PMID]
Tsao, N., Hsu, H. P., Wu, C. M., Liu, C. C., & Lei, H. Y. (2001). Tumour necrosis factor-α causes an increase in blood-brain barrier permeability during sepsis. Journal of Medical Microbiology, 50(9), 812-21. [DOI:10.1099/0022-1317-50-9-812] [PMID]
Vetrovoi, O. V., Rybnikova, E. A., Glushchenko, T. S., & Samoilov, M. O. (2015). Effects of hypoxic postconditioning on the expression of antiapoptotic protein Bcl-2 and neurotrophin BDNF in hippocampal field CA1 in rats subjected to severe hypoxia. Neuroscience and Behavioral Physiology, 45(4), 367-70. [DOI:10.1007/s11055-015-0083-y]
Vetrovoĭ, O., Rybnikova, T., & Samoĭlov, M. (2013). [Effect of hypoxic postconditioning on the expression of antiapoptotic protein Bcl-2 and neurotrophin BDNF in CA1 hippocampal field of rats surviving severe hypoxia (Russian)]. Morfologiia, 145(2), 16-20. [PMID]
Wang, D., & Corbett, D. (1990). Cerebral ischemia, locomotor activity and spatial mapping. Brain Research, 533(1), 78-82. [DOI:10.1016/0006-8993(90)91798-L] [PMID]
Wang, J. Y., Shen, J., Gao, Q., Ye, Z. g., Yang, S. Y., & Liang, H. W., et al. (2008). Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke, 39(3), 983-90. [DOI:10.1161/STROKEAHA.107.499079] [PMID]
Wever, K. E., Menting, T., Masereeuw, R., van der Vliet, J. A., Rongen, G. A., & Warlé, M. C. (2012). Local and remote ischemic postconditionings have synergistic protective effects on renal ischemia-reperfusion injury. Transplantation, 94(1), e1-e2. [DOI:10.1097/TP.0b013e318257ad76] [PMID]
Wu, D., & Pardridge, W. M. (1999). Neuroprotection with noninvasive neurotrophin delivery to the brain. Proceedings of the National Academy of Sciences, 96(1), 254-9. [DOI:10.1073/pnas.96.1.254] [PMID] [PMCID]
Yan, X. B., Hou, H. L., Wu, L. M., Liu, J., & Zhou, J. N. (2007). Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology, 53(4), 487-95. [DOI:10.1016/j.neuropharm.2007.06.020] [PMID]
Zen’ko, M., Rybnikova, Y., & Glushchenko, T. S. (2014). [Expression of BDNF neurotrophin in the hippocampus and neocortex of rats during the development of post-stress anxiety and its correction by hypoxic postconditioning (Russian)]. Morfologiia, 146(5), 14-18. [PMID]
Zhang, X., Zhang, Q., Tu, J., Zhu, Y., Yang, F., & Liu, B., et al. (2015). Prosurvival NMDA 2A receptor signaling mediates postconditioning neuroprotection in the hippocampus. Hippocampus, 25(3), 286-96. [DOI:10.1002/hipo.22372] [PMID]
Zhao, H. (2009). Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. Journal of Cerebral Blood Flow & Metabolism, 29(5), 873-85. [DOI:10.1038/jcbfm.2009.13] [PMID] [PMCID]
Zhao, H., Sapolsky, R. M., & Steinberg, G. K. (2006). Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. Journal of Cerebral Blood Flow & Metabolism, 26(9), 1114-21. [DOI:10.1038/sj.jcbfm.9600348] [PMID]
Zhao, Z. Q. (2010). Postconditioning in reperfusion injury: A status report. Cardiovascular Drugs and Therapy, 24(3), 265-79. [DOI:10.1007/s10557-010-6240-1] [PMID]
Zhao, Z. Q., Corvera, J. S., Halkos, M. E., Kerendi, F., Wang, N. P., & Guyton, R. A., et al. (2003). Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. American Journal of Physiology-Heart and Circulatory Physiology, 285(2), H579-H588. [DOI:10.1152/ajpheart.01064.2002] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2019/07/20 | Accepted: 2020/06/14 | Published: 2021/11/1
Received: 2019/07/20 | Accepted: 2020/06/14 | Published: 2021/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








