Volume 12, Issue 3 (May & June 2021)
BCN 2021, 12(3): 339-348 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahrami A, Rezaeitalab F, Farahmand S K, Mazloum Khorasani Z, Arabi S M, Bahrami-Taghanaki H, et al . High-dose Vitamin D Supplementation and Improvement in Cognitive Abilities, Insomnia, and Daytime Sleepiness in Adolescent Girls. BCN 2021; 12 (3) :339-348
URL: http://bcn.iums.ac.ir/article-1-1538-en.html
URL: http://bcn.iums.ac.ir/article-1-1538-en.html
Afsane Bahrami1 

 , Fariborz Rezaeitalab2
, Fariborz Rezaeitalab2 

 , Seyed Kazem Farahmand3
, Seyed Kazem Farahmand3 

 , Zahra Mazloum Khorasani4
, Zahra Mazloum Khorasani4 

 , Seyed Mostafa Arabi5
, Seyed Mostafa Arabi5 

 , Hamidreza Bahrami-Taghanaki6
, Hamidreza Bahrami-Taghanaki6 

 , Gordon A. Ferns7
, Gordon A. Ferns7 

 , Majid Ghayour-Mobarhan *8
, Majid Ghayour-Mobarhan *8 




 , Fariborz Rezaeitalab2
, Fariborz Rezaeitalab2 

 , Seyed Kazem Farahmand3
, Seyed Kazem Farahmand3 

 , Zahra Mazloum Khorasani4
, Zahra Mazloum Khorasani4 

 , Seyed Mostafa Arabi5
, Seyed Mostafa Arabi5 

 , Hamidreza Bahrami-Taghanaki6
, Hamidreza Bahrami-Taghanaki6 

 , Gordon A. Ferns7
, Gordon A. Ferns7 

 , Majid Ghayour-Mobarhan *8
, Majid Ghayour-Mobarhan *8 


1- Cellular and Molecular Research Center, School of Medicine, Birjand University of Medical Sciences, Bijand, Iran.
2- Department of Neurology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Traditional Medicine, School of Persian and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Endocrine Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Biochemistry of Nutrition Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Chinese and Complementary Medicine Research Center, School of Traditional and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
7- Division of Medical Education, School of Brighton & Sussex Medical, University of Brighton and the University of Sussex, Falmer, Brighton, Sussex, UK.
8- Metabolic Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Neurology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Traditional Medicine, School of Persian and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Endocrine Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Biochemistry of Nutrition Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Chinese and Complementary Medicine Research Center, School of Traditional and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
7- Division of Medical Education, School of Brighton & Sussex Medical, University of Brighton and the University of Sussex, Falmer, Brighton, Sussex, UK.
8- Metabolic Research Center, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 759 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Nuclear steroid hormone Vitamin D (VitD) contributes to various physiological processes in the body. The role of VitD in calcium homeostasis has already known. Also, VitD affects neuronal functioning (Eyles et al., 2009; McCann & Ames, 2008; McGrath, Feron, Eyles & Mackay-Sim, 2001). VitD exhibits neurosteroid properties, essential to protect the Central Nervous System (CNS), and helps in neurotransmission, neuroimmunomodulation, and brain processes (Briones & Darwish, 2012).
Vitamin D Receptors (VDR) are widely distributed in many cell types of the human brain, such as neurons and glial cells. High levels of VDR is found in the cortex and hippocampus (Eyles, Smith, Kinobe, Hewison & McGrath, 2005; Langub, Herman, Malluche & Koszewski, 2001; Prüfer, Veenstra, Jirikowski & Kumar, 1999), which are essential areas for regulation of cognitive functions, such as learning, memory, and behavior (Becker, Eyles, McGrath & Grecksch, 2005). Moreover, enzyme (1-α-hydroxylase) related to the synthesis of the active form of VitD is prevalent in the brain, and its gene expression has been found in neural tissues (Zehnder et al., 2001). VDR also
contributes to neuroprotective pathways, decreasing oxidative damages and amyloid-β formation and accumulation (Morley, 2014). Animal studies have shown that VitD is crucial for brain development (Eyles et al., 2009). These studies have reported that VitD may support the structure and integrity of neurons within neurotrophin synthesis and detoxification pathways, which is mandatory for neuronal survival (Kang & Schuman, 2000).
Inadequate VitD levels in prenatal rats dysregulate 36 brain proteins implicated in multiple biological activities, such as oxidative phosphorylation, redox balance, cytoskeleton reservation, post-translational modifications, synaptic plasticity, calcium homeostasis, and neurotransmission in adult (Almeras et al., 2007). Furthermore, evidence shows that VitD deficiency may cause mental disorders such as major and minor depression, schizophrenia, anxiety, and sleep disorders (Garcion, Wion-Barbot, Montero-Menei, Berger & Wion, 2002; Gominak & Stumpf, 2012; Hoogendijk et al., 2008; Jorde, Waterloo, Saleh, Haug & Svartberg, 2006). Risen dietary consumption of VitD has been related to fewer incidences of self-reported psychotic-like events (Hedelin et al., 2010).
Recent studies have suggested a negative association between VitD status and adverse sleep outcomes (Bellia et al., 2013; Khoo et al., 2011). The negative impacts of sleep disorders include sleepiness, neurocognitive deficits, decrease mental performance, daytime fatigue, and altered mood in adolescents. In recent years, sleep disorders have become very common worldwide and contribute to the etiology of heart disease, diabetes, depression, and other complications (Bagai, 2010; Skaer & Sclar, 2010).
The effects of VitD on cognitive function and sleep patterns have been reported in several studies, but the results of these studies are inconsistent (Rossom et al., 2012; Stein, Scherer, Ladd & Harrison, 2011). Epidemiological studies show an association between low concentration of VitD and impairments in cognitive performances such as memory and orientation (Llewellyn, Langa & Lang, 2009; Przybelski & Binkley, 2007; Wilkins, Sheline, Roe, Birge & Morris, 2006), psychomotor speed, and executive function (Buell et al., 2009; Lee et al., 2009), also diagnosis of vascular dementia and Alzheimer disease (Buell et al., 2010). However, other studies found no relationship between VitD levels and performance on cognitive testing (Slinin et al., 2010), or they showed worse cognitive function by the elderly individuals in the high 25-hydroxy vitamin D [25(OH)D] quintile (McGrath et al., 2007). Also, it has been reported that individuals with sleep disorders have lower serum 25(OH)D levels (McCarty, Reddy, Keigley, Kim & Marino, 2012; Mete et al., 2013).
We have previously shown that VitD supplementation positively affects systemic inflammation, cardio-metabolic profile, and mood or emotional performance in adolescents (Bahrami et al., 2018; Khayyatzadeh et al., 2018; Tabatabaeizadeh et al., 2017). The present study aimed to further assess supplementation with high dose VitD on cognitive abilities, insomnia, and daytime sleepiness in adolescents.
2. Methods
2.1. Study design
This study was performed in the cities of Mashhad and Sabzevar, Iran, between January and April 2015, as described previously (Khayyatzadeh et al., 2018; Tabatabaeizadeh et al., 2017). In a large VitD project, 1026 adolescents aged between 12 and 18 years were called. Of whom, 988 met the inclusion criteria. All participants received 9 50000 IU of VitD capsules for 9 weeks (1 per week). Finally, 940 adolescents completed the trial, with a dropout rate of 4.8%. The Ethics Committee approved the study and informed written consent was provided by all participants and their parents (IR.MUMS.FM.REC.1395.12).
2.2. Study instruments
Assessment of cognitive abilities and sleep disorders was performed by standard self-report questionnaires mentioned as follows. An expert assistant aids participants to fulfill all of the questions.
2.3. The Insomnia Severity Index (ISI)
The Insomnia Severity Index (ISI) questionnaire is a reliable instrument that yields a quantitative index of perceived insomnia severity. The instrument involves 7 items focusing on sleep disorder severity, sleep-related satisfaction, and anxiety related to the sleeping problem. Each item is rated on a 5-point Likert-type scale (0-4) and provides a total score ranging from 0 to 28. Higher scores indicate more severe insomnia. Scores may be categorized as 0-7 (no clinically significant insomnia), 8–14 (sub-threshold insomnia or mild insomnia), 15-21 (moderate insomnia), and 22-28 (severe insomnia). ISI-Persian was previously validated among Iranian subjects and was found to have a high internal consistency (The Cronbach alpha= 0.8 and test-retest reliability = 0.7) (Yazdi, Sadeghniiat-Haghighi, Zohal & Elmizadeh, 2012).
2.4. Epworth Sleepiness Scale (ESS)
The Epworth Sleepiness Scale (ESS) is a questionnaire-derived scale of sleepiness that can assess the degree of daytime sleepiness. It is an 8-item questionnaire scored on a 4-point Likert-type scale that measures the habitual likelihood to fall asleep in common situations of daily living. The total score ranges from 0 to 24. Normal is less than 10. Mild to moderate obstructive sleep apnea get a score between 10 and 16. Also, severe obstructive sleep apnea or narcolepsy scores over 16. ESS has been validated and found to have a high internal consistency (the Cronbach alpha= 0.88) and acceptable test-retest reliability (r=0.81; 95% confidence interval 0.74–0.86, P<0.001) among Iranian population (Sadeghniiat Haghighi et al., 2013).
2.5. Cognitive abilities task
Cognitive performances were evaluated using the Cognitive Abilities Questionnaire (CAQ), which comprises 30 items. Each item is rated on a 5-point scale (1-5) and summed up to provide a total score ranging from 30 to 150. The validity of CAQ with high internal consistency (the Cronbach α=0.83) and good test-retest reliability (r=0.86) have been established in Iranian population (Nejati, 2013). Higher scores represent better cognition abilities. The CAQ assesses memory, inhibitory control, selective attention, decision making, planning, sustained attention, social cognition, and cognitive flexibility.
2.6. Depression and aggression
To assess the score of depression, the subjects were asked to complete the Beck depression inventory II (BDI II) questionnaire (Beck, Steer & Brown, 1996). Higher scores represent a high degree of depression. The validity of the BDI Persian version with high internal consistency (the Cronbach α=0.87) and good test-retest reliability (r=0.74) has been established in Iranian students (Mohammadi, 2007).
2.7. The Buss-Perry Aggression Questionnaire (BPAQ)
The Buss-Perry Aggression Questionnaire (BPAQ) is a 29-item questionnaire, which evaluates four aggression scores of physical aggression, verbal aggression, anger, and hostility. They are scored with a 5-point rating system. It was used to determine the aggression level of the subjects (Elliott, Baughan & Sexton, 2007). A high BPAQ score indicates higher aggression. The test-retest reliability and validity of this questionnaire have been verified among the Iranian population (Motevalian, Asadi-Lari, Rahimi & Eftekhar, 2011).
2.8. Vitamin D measurements
Fasting blood samples were collected in the early morning at baseline and post nine weeks’ trial, after a 12 hour overnight fasting. Blood samples were centrifuged (Hettich model D-78532) to separate serum. An Electrochemical-Luminescence (ECL; Roche) method was used to measure serum 25(OH)D.
2.9. Assessment of other variables
An expert interviewer collected demographic data, and anthropometric parameters were determined using the standard techniques at baseline and after 9 weeks of intervention. The question related to smoking was, “How many hours are you expose to cigarettes?” If reported 30 minutes and above, the subject was considered exposed to smoking. Physical activity was measured by a validated questionnaire and represented as Metabolic Equivalents (METs) in hours/day (Delshad et al., 2015).
2.10. Statistical method
The Kolmogorov-Smirnov test was used to determine whether the variables had a normal distribution. Results were expressed as frequency, percentage, and Mean±Standard Deviation. The Chi-square or ANOVA tests and post hoc Turkey’s test were applied to compare quantitative variables in different subject groups (insomnia, sleepiness, both insomnia, and sleepiness, as well as normal), followed by Tukey HSD. Cochran’s Q tests or paired-sample t test was used to compare variables at baseline and after the intervention. P < 0.05 was set at statistical significance in all tests. SPSS for Windows (v. 16, SPSS Inc., Chicago, IL) was used for statistical analysis.
3. Results
A total of 940 subjects were entered the study. At baseline, 222 participants presented some degree of sleepiness (ESS score ≥10), and 216 subjects presented some degree of insomnia (ISI score ≥8). Altogether, 15.0%, 15.6%, and 8.0% of subjects had some degree of insomnia, sleepiness, both insomnia, and sleepiness, respectively. In contrast, 61.4% had no insomnia and or sleepiness. Baseline characteristics of responders who completed the intervention are presented in Table 1.
.png)
Analysis of Variance (ANOVA) test results showed the four groups of participants (insomnia, sleepiness, both insomnia, and sleepiness, or control) were well-matched regarding age, body mass index, and physical activity. The subjects with some degree of insomnia and daytime sleepiness are more exposed to smoke than normal subjects (P=0.005). Furthermore, the subjects with both insomnia and sleepiness have higher depression and aggression scores than the normal population (P<0.05; Table 1).
During and after the supplementation period, no subject reported any significant adverse effects. The Mean±SD serum 25(OH)D level exhibited a significant increase from 9.46±8.83 ng/mL at baseline to 35.91±15.56 ng/mL after supplementation.
After VitD supplementation, significant improvements were seen in most cognitive abilities, including scores at the memory, inhibitory control and selective attention, decision making, planning, sustained attention, and cognitive flexibility after supplementation (P<0.001). But the positive effect on social cognition was not significant (Table 2).
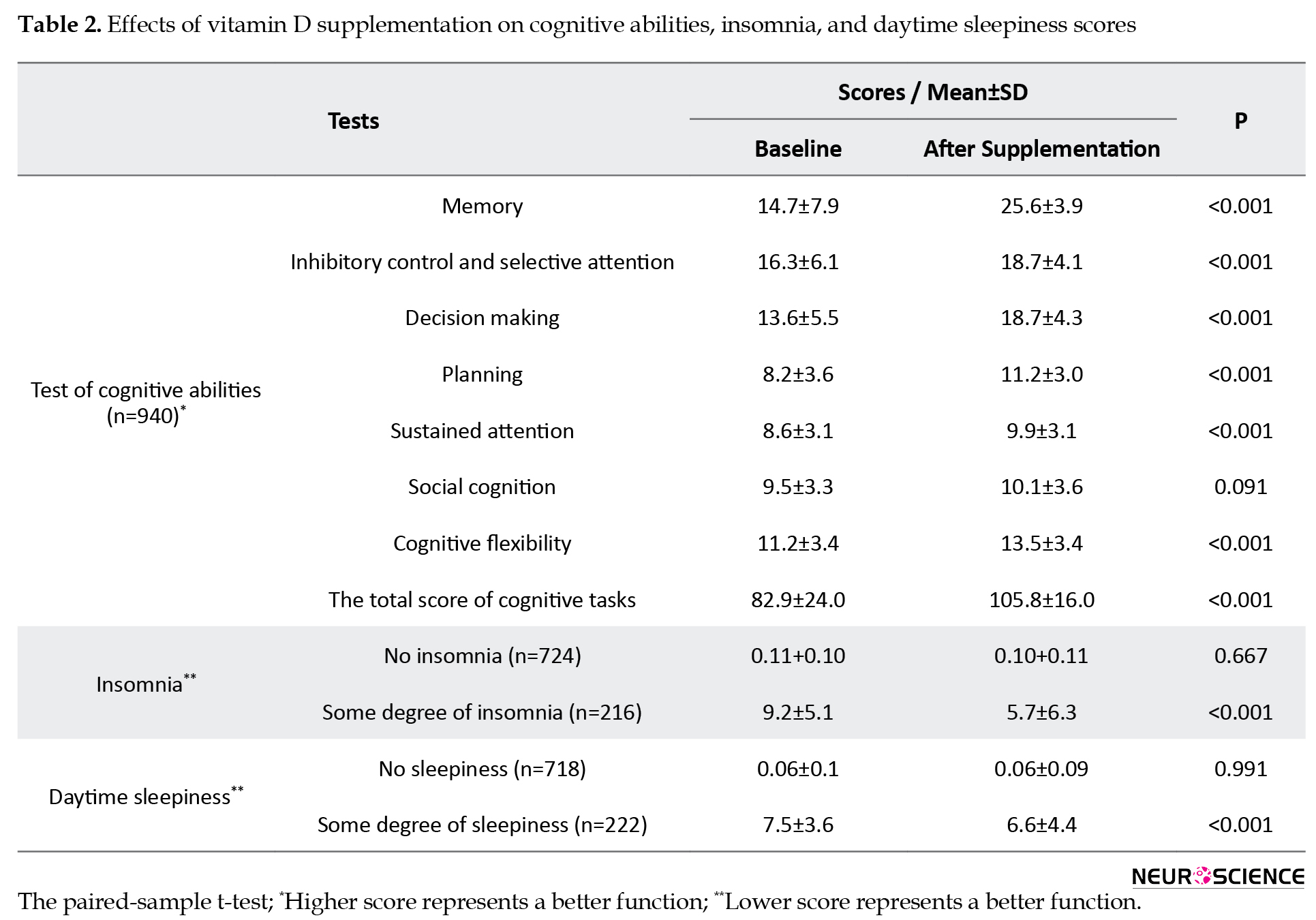
VitD supplementation caused to significant reduction in total score of insomnia in cases with insomnia (9.2±5.1 to 5.7±6.3; P<0.001). Day time sleepiness score was also decreased after supplementation in adolescents with some degree of sleepiness (7.5±3.6 to 6.6±4.4; P<0.001).
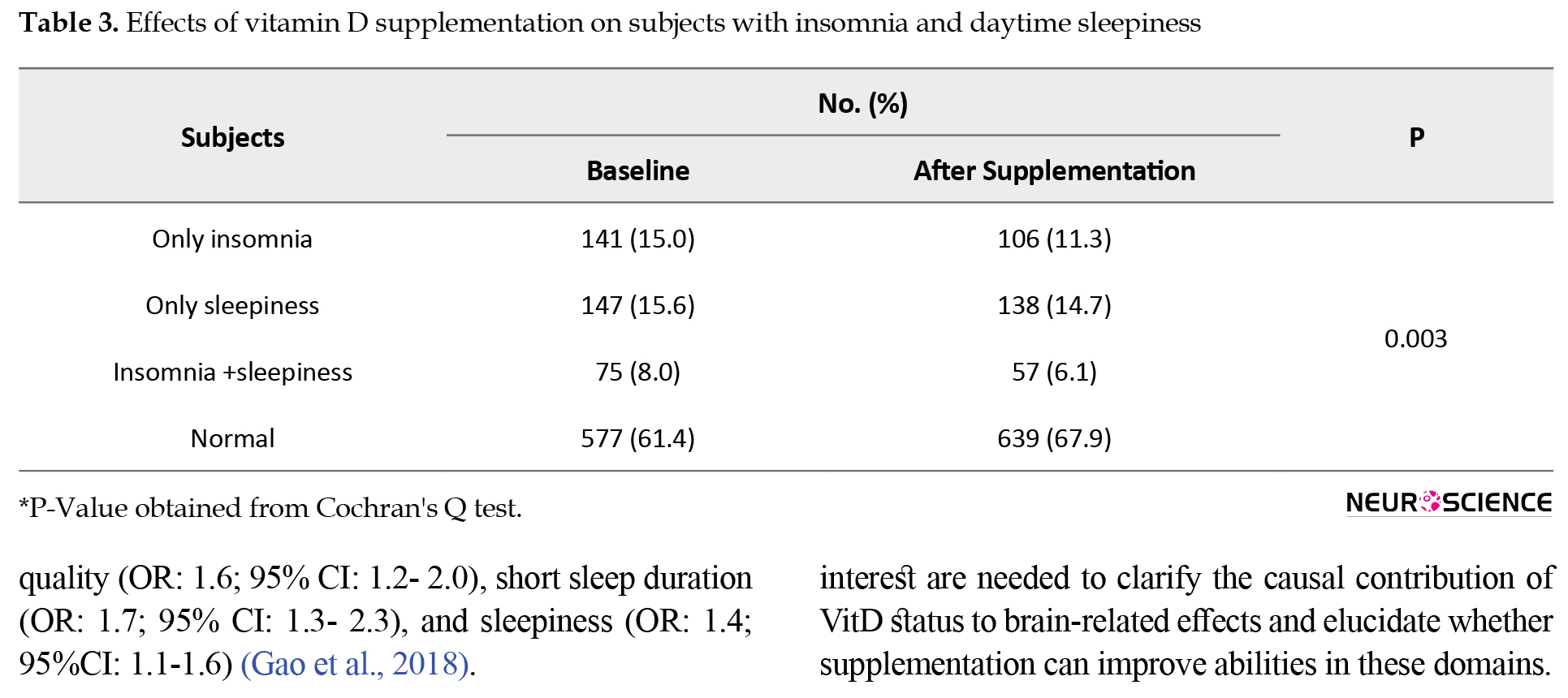
At baseline, the prevalence of subjects with insomnia alone was 15.0%, while this value after supplementation was decreased to 11.3%. Similar results were also found for the prevalence of subjects with only sleepiness (15.6% reduced to 14.7%), and the cases with both insomnia and sleepiness (8.0% reduced to 6.1%), while in normal cases, the sleep disorders increased from 61.4% to 67.9% after supplementation (P=0.003; Table 3).
4. Discussion
This interventional study is the first to investigate the effects of high-dose VitD supplementation on cognitive abilities and sleep disorders. The results showed that VitD supplementation significantly improved subjective measures of insomnia, daytime sleepiness, and cognitive abilities such as memory, inhibitory control, and selective attention, decision making, planning, sustained attention, and cognitive flexibility in adolescent girls.
The present study was conducted to validate or refute the effects of VitD supplementation on the cognitive function observed in previous studies (van der Schaft et al., 2013). This study used a large sample size with a rigorous method to assess cognitive abilities. The results are consistent with the amount of data indicating that low VitD status is associated with impairments in cognitive function (Lanham-New et al., 2011; Vieth et al., 2007). The neurocognitive effect of VitD is well-supported. The VDR and catalytic enzymes are present in the brain areas involved in forming new memories, processing, and complex planning (Buell & Dawson-Hughes, 2008). VitD is neuroprotective because it modulates the expression of enzymes (tyrosine hydroxylase) related to dopamine’s synthesis and norepinephrine/epinephrine. Also, it enhances neuron survival through the prevention of oxidative pathways responsible for free radical production in the brain via the elevation of antioxidant (γ-glutamyl transpeptidase) generation and the suppression of the synthesis of inducible Nitric Oxide Synthase (iNOS), a toxic enzyme for the brain (Vaidya & Williams, 2012). The Institute of Medicine (2011) claimed a biologically confirmed association between VitD level and brain-related outcomes.
A wealth of evidence supports that low vitamin D may be associated with greater odds of cognitive impairment and reduced cognitive functioning in older adults (Breitling et al., 2012; Llewellyn, Lang, Langa & Melzer, 2011; Przybelski & Binkley, 2007). In HIV-positive patients, severe VitD deficiency was reported to be independently related with a higher odds of neurocognitive decline (OR=2.1; 95% CI:1.04–4.05) (Vergori et al., 2019). Low serum 25(OH)D concentration may independently predict worsening cognitive functions in diabetic patients (Rui-hua, Yong-de, Xiao-zhen, Chen & Bin, 2019). However, there are some discrepancies in the study results. Some studies have examined the association between VitD status and cognitive functions in young adults with different results (Hansen, Bakke, Dahl & Thayer, 2011; Lašaite, Gailyte, Puzinas, Preikša & Kazanavičius, 2011; Tolppanen, Williams & Lawlor, 2011). For example, the NHANES study expressed no relationship between VitD concentration and neurocognitive functioning in adolescents and adults (McGrath et al., 2007). In a longitudinal study on Puerto Rican adults from the Boston Area, the basal serum 25(OH)D level was not associated with a 2-year change in cognitive test scores, executive function, and memory domains (Palacios, Scott, Sahasrabudhe, Gao & Tucker, 2020).
A double-blind controlled trial reported that VitD supplementation does not influence cognitive function and mood in healthy young adults. In these participants, despite significant increases in VitD consumption, no significant changes were observed in working memory (F=1.09; P=0.30), cognitive flexibility (F=1.37; P=0.24) and response inhibition (F=0.82; P=0.37) (Dean et al., 2011). In another study, no treatment-induced improvement in cognition or behavior was observed in nursing home residents (Przybelski et al., 2008). In a randomized clinical trial among 422 subjects, no significant associations were found between serum 25(OH)D level and the three independent cognitive tasks (verbal recall test, coding test, and tapping test) at baseline. After four months, VitD supplementation did not promote cognition in mid-aged and older individuals (Jorde et al., 2019). The small sample size, the presence or absence of confounding variables, short intervention duration, and test reproducibility may preclude the observation of positive effects in these studies.
The improvement in sleep disorders is important because sleep disorders are associated with several health problems (Bagai, 2010; Goel, Rao, Durmer & Dinges, 2009; Skaer & Sclar, 2010). Although VitD is crucial to many physiologic processes in man, there are limited data in relation to its effects on sleep disorders. There is a wealth of evidence that VitD is involved in sleep regulation by decreasing prostaglandin D2 and cytokine (Feldman et al., 2007; Jablonski, Chonchol, Pierce, Walker & Seals, 2011; Krueger, Majde & Rector, 2011). VitD deficiency may be contributed to the development of symptoms of wakefulness most frequently associated with sleep disorders (McCarty et al., 2012). Our study showed that high-dose VitD supplementation significantly reduced the severity of insomnia among adolescents. These findings are compatible with the study of Khoo et al., who reported that serum VitD levels in healthy individuals were inversely associated with sleep-regulating factors such as tumor necrosis factor-alpha and interleukin-1 (Khoo et al., 2011).
Our results are similar to those of Bellia et al., who reported that a low VitD status might contribute to sleepiness through central nervous system signaling and inflammatory mediators (Bellia et al., 2013). McCarty et al. have also announced that serum 25(OH)D concentrations are linked with ESS in patients with sleep disorders (McCarty et al., 2012). One possible mechanism for this inverse correlation may be the reduced levels of sympathetic stimulation or deactivation of the hypothalamic-pituitary-adrenal stress response axis. A recent systematic review and meta-analysis have reported that those with low vitamin D had a significantly higher risk of sleep disorders (OR:1.5; 95%CI: 1.3-1.7), poor sleep quality (OR: 1.6; 95% CI: 1.2- 2.0), short sleep duration (OR: 1.7; 95% CI: 1.3- 2.3), and sleepiness (OR: 1.4; 95%CI: 1.1-1.6) (Gao et al., 2018).
The strong points of this study are its large number of carefully selected participants, comprehensive measures of the baseline characteristics, and its powerful assessment of cognitive abilities and detection of insomnia and sleepiness. To detect any possible beneficial effects of VitD supplementation on cognitive abilities and sleep disorders, characteristics of the study population, the dose of VitD, duration of intervention, and methods of cognitive testing are important. The given oral mega-doses effectively corrected severe VitD deficiency in peripheral blood, but it is still unclear whether normalization of impaired plasma level of VitD reflects the status of VitD in the Cerebrospinal Fluid (CSF) and cells in CNS.
Our study has several limitations. Our participants consisted of healthy adolescent girls who were free of cognitive impairment and major psychiatric illness. Therefore, our findings may not be generalized to clinical patients demonstrating emotional disorders or cognitive impairment. Because all observations received supplementation in the main study, we could not use a control group for comparison.
5. Conclusion
Our findings reveal that VitD supplementation improves cognitive abilities and sleep problems in healthy adolescents with a high prevalence of VitD insufficiency and deficiency. This approach may be a valuable means of improving cognitive abilities and sleep problems. Besides, treating VitD deficiency may benefit the general population other than the teenagers if other larger studies confirmed similar effects on different age and ethnic groups. Although detection and treatment of VitD deficiency are crucial for a range of health outcomes, future randomized controlled trials in key populations of interest are needed to clarify the causal contribution of VitD status to brain-related effects and elucidate whether supplementation can improve abilities in these domains.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed following the ethical standards of the Institutional and or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all study participants.
Funding
This study was supported by grants No. 931188 (Majid Ghayour-Mobarhan) from Mashhad University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Almeras, L., Eyles, D., Benech, Ph., Laffite, D., Villard, C., & Patatian, A., et al. (2007). Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics, 7(5), 769-80. [DOI:10.1002/pmic.200600392] [PMID]
Bagai, K. (2010). Obstructive sleep apnea, stroke, and cardiovascular diseases. The Neurologist, 16(6), 329-39. [DOI:10.1097/NRL.0b013e3181f097cb] [PMID]
Bahrami, A., Mazloum, S. R., Maghsoudi, Sh., Soleimani, D., Khayyatzadeh, S. S., & Arekhi, S., et al. (2018). High dose vitamin D supplementation is associated with a reduction in depression score among adolescent girls: A nine-week follow-up study. Journal of Diatery Supplemens, 15(2), 173-82. [DOI:10.1080/19390211.2017.1334736, In press.] [PMID]
Beck, A. T., Steer, R. A., & Brown, G. (1996). Beck depression inventory-II. Retrieved from https://doi.apa.org/doiLanding?doi=10.1037%2Ft00742-000 [DOI:10.1037/t00742-000]
Becker, A., Eyles, D. W., McGrath, J. J., & Grecksch, G. (2005). Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behavioural Brain Research, 161(2), 306-12. [DOI:10.1016/j.bbr.2005.02.015] [PMID]
Bellia, A., Garcovich, C., D’Adamo, M., Lombardo, M., Tesauro, M., & Donadel, G., et al. (2013). Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Internal and Emergency Medicine, 8(1), 33-40. [DOI:10.1007/s11739-011-0559-x] [PMID]
Breitling, L. P., Perna, L., Müller, H., Raum, E., Kliegel, M., & Brenner, H. (2012). Vitamin D and cognitive functioning in the elderly population in Germany. Experimental Gerontology, 47(1), 122-7. [DOI:10.1016/j.exger.2011.11.004] [PMID]
Briones, T. L., & Darwish, H. (2012). Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. Journal of Neuroinflammation, 9, 244. [DOI:10.1186/1742-2094-9-244] [PMID] [PMCID]
Buell, J. S., Dawson-Hughes, B., Scott, T. M., Weiner, D. E., Dallal, G. E., & Qui, W. Q., et al. (2010). 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology, 74(1), 18-26. [DOI:10.1212/WNL.0b013e3181beecb7] [PMID] [PMCID]
Buell, J. S., & Dawson-Hughes, B. (2008). Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Molecular Aspects of Medicine, 29(6), 415-22. [DOI:10.1016/j.mam.2008.05.001] [PMID] [PMCID]
Buell, J. S., Scott, T. M., Dawson-Hughes, B., Dallal, G. E., Rosenberg, I. H., & Folstein, M. F., et al. (2009). Vitamin D is associated with cognitive function in elders receiving home health services. The Journals of Gerontology Series A, 64A(8), 888-95. [DOI:10.1093/gerona/glp032] [PMID] [PMCID]
Dean, A. J., Bellgrove, M. A., Hall, T., Phan, W. M. J., Eyles, D. W., & Kvaskoff, D., et al. (2011). Effects of vitamin D supplementation on cognitive and emotional functioning in young adults-a randomised controlled trial. PLoS One, 6(11), e25966. [DOI:10.1371/journal.pone.0025966] [PMID] [PMCID]
Delshad, M., Ghanbarian, A., Rezaei Ghaleh, N., Amirshekari, G., Askari, S., & Azizi, F. (2015). Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. International Journal of Preventive Medicine, 6(1), 3. [DOI:10.4103/2008-7802.151433] [PMID] [PMCID]
Elliott, M. A., Baughan, Ch. J., & Sexton, B. F. (2007). Errors and violations in relation to motorcyclists’ crash risk. Accident Analysis & Prevention, 39(3), 491-9. [DOI:10.1016/j.aap.2006.08.012] [PMID]
Eyles, D. W., Feron, F., Cui, X., Kesby, J. P., Harms, L. H., & Ko, P., et al. (2009). Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology, 34(Suppl 1), S247-57. [DOI:10.1016/j.psyneuen.2009.04.015] [PMID]
Eyles, D. W., Smith, S., Kinobe, R., Hewison, M., & McGrath, J. J. (2005). Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. Journal of Chemical Neuroanatomy, 29(1), 21-30. [DOI:10.1016/j.jchemneu.2004.08.006] [PMID]
Feldman, D., Krishnan, A., Moreno, J., Swami, S., Peehl, D. M., & Srinivas, S. (2007). Vitamin D inhibition of the prostaglandin pathway as therapy for prostate cancer. Nutrition Reviews, 65(Suppl_2), S113-5. [DOI:10.1111/j.1753-4887.2007.tb00335.x] [PMID]
Gao, Q., Kou, T., Zhuang, B., Ren, Y., Dong, X., & Wang, Q. (2018). The association between vitamin D deficiency and sleep disorders: A systematic review and meta-analysis. Nutrients, 10(10), 1395. [DOI:10.3390/nu10101395] [PMID] [PMCID]
Garcion, E., Wion-Barbot, N., Montero-Menei, C. N., Berger, F., & Wion, D. (2002). New clues about vitamin D functions in the nervous system. Trends in Endocrinology & Metabolism, 13(3), 100-5. [DOI:10.1016/S1043-2760(01)00547-1]
Goel, N., Rao, H., Durmer, J. S., & Dinges, D. F. (2009). Neurocognitive consequences of sleep deprivation. Seminars in neurology, 29(4), 320-39. [DOI:10.1055/s-0029-1237117] [PMID] [PMCID]
Gominak, S. C., & Stumpf, W. E. (2012). The world epidemic of sleep disorders is linked to vitamin D deficiency. Medical Hypotheses, 79(2), 132-5. [DOI:10.1016/j.mehy.2012.03.031] [PMID]
Hansen, A. L., Bakke, L., Dahl, L., & Thayer, J. F. (2011). Vitamin D and executive function: A preliminary report. Perceptual and Motor Skills, 113(2), 677-85. [DOI:10.2466/02.09.13.15.16.PMS.113.5.677-685] [PMID]
Hedelin, M., Löf, M., Olsson, M., Lewander, T., Nilsson, B., & Hultman, Ch. M., et al. (2010). Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33 000 women from the general population. BMC Psychiatry, 10(1), 38. [DOI:10.1186/1471-244X-10-38] [PMID] [PMCID]
Hoogendijk, W. J. G., Lips, P., Dik, M. G., Deeg, D. J. H., Beekman, A. T. F., & Penninx, B. W. J. H. (2008). Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Archives of General Psychiatry, 65(5), 508-12. [DOI:10.1001/archpsyc.65.5.508] [PMID]
Institute of Medicine. (2011). Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press. [DOI:10.17226/13050] [PMID]
Jablonski, K. L., Chonchol, M., Pierce, G. L., Walker, A. E., & Seals, D. R. (2011). 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension, 57(1), 63-9. [DOI:10.1161/HYPERTENSIONAHA.110.160929] [PMID] [PMCID]
Jorde, R., Kubiak, J., Svartberg, J., Fuskevåg, O. M., Figenschau, Y., & Martinaityte, I., et al. (2019). Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. Journal of the Neurological Sciences, 396, 165-71. [DOI:10.1016/j.jns.2018.11.020] [PMID]
Jorde, R., Waterloo, K., Saleh, F., Haug, E., & Svartberg, J. (2006). Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. Journal of Neurology, 253(4), 464-70. [DOI:10.1007/s00415-005-0027-5] [PMID]
Kang, H., & Schuman, E. M. (2000). Intracellular Ca2+ signaling is required for neurotrophin-induced potentiation in the adult rat hippocampus. Neuroscience Letters, 282(3), 141-4. [DOI:10.1016/S0304-3940(00)00893-4]
Khayyatzadeh, S. S., Mirmousavi, S. J., Fazeli, M., Abasalti, Z., Avan, A., & Javandoost, A., et al. (2018). High-dose vitamin D supplementation is associated with an improvement in several cardio-metabolic risk factors in adolescent girls: a nine-week follow-up study. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 55(2), 227-35. [DOI:10.1177/0004563217707784] [PMID]
Khoo, A. L., Chai, L. Y. A., Koenen, H. J. P. M., Sweep, F. C. G. J., Joosten, I., & Netea, M. G., et al. (2011). Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clinical & Experimental Immunology, 164(1), 72-9. [DOI:10.1111/j.1365-2249.2010.04315.x] [PMID] [PMCID]
Krueger, J. M., Majde, J. A., & Rector, D. M. (2011). Cytokines in immune function and sleep regulation. In P. Montagna, & S. Chokroverty (Eds.), Sleep disorders part I. Handbook of clinical neurology (pp. 229-240). Vol. 98. Amsterdam: Elsevier. [DOI:10.1016/B978-0-444-52006-7.00015-0] [PMID] [PMCID]
Langub, M. C., Herman, J. P., Malluche, H. H., & Koszewski, N. J. (2001). Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience, 104(1), 49-56. [DOI:10.1016/S0306-4522(01)00049-5]
Lanham-New, S. A., Buttriss, J. L., Miles, L. M., Ashwell, M., Berry, J. L., & Boucher, B. J., et al. (2011). Proceedings of the rank forum on vitamin D. British Journal of Nutrition, 105(1), 144-56. [DOI:10.1017/S0007114510002576] [PMID] [PMCID]
Lašaite, L., Gailyte, I., Puzinas, P., Preikša, R., & Kazanavičius, G. (2011). Vitamin D deficiency is related to worse emotional state. Central European Journal of Medicine, 6(5), 558-66. [DOI:10.2478/s11536-011-0061-x]
Lee, D. M., Tajar, A., Ulubaev, A., Pendleton, N., O’Neill, T. W., & O’Connor, D. B., et al. (2009). Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. Journal of Neurology, Neurosurgery & Psychiatry, 80(7), 722-9. [DOI:10.1136/jnnp.2008.165720] [PMID]
Llewellyn, D. J., Lang, I. A., Langa, K. M., & Melzer, D. (2011). Vitamin D and cognitive impairment in the elderly U.S. population. The Journals of Gerontology: Series A, 66A(1), 59-65. [DOI:10.1093/gerona/glq185] [PMID] [PMCID]
Llewellyn, D. J., Langa, K. M., & Lang, I. A. (2009). Serum 25-hydroxyvitamin D concentration and cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 22(3), 188-95. [DOI:10.1177/0891988708327888] [PMID] [PMCID]
McCann, J. C., & Ames, B. N. (2008). Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? The FASEB Journal, 22(4), 982-1001. [DOI:10.1096/fj.07-9326rev] [PMID]
McCarty, D. E., Reddy, A., Keigley, Q., Kim, P. Y., & Marino, A. A. (2012). Vitamin D, race, and excessive daytime sleepiness. Journal of Clinical Sleep Medicine, 8(6), 693-7. [DOI:10.5664/jcsm.2266] [PMID] [PMCID]
McGrath, J., Feron, F., Eyles, D., & Mackay-Sim, A. (2001). Vitamin D: The neglected neurosteroid? Trends in Neurosciences, 24(10), 570-1. [DOI:10.1016/S0166-2236(00)01949-4]
McGrath, J., Scragg, R., Chant, D., Eyles, D., Burne, T., & Obradovic, D. (2007). No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology, 29(1-2), 49-54. [DOI:10.1159/000108918] [PMID]
Mete, T., Yalcin, Y., Berker, D., Ciftci, B., Guven, S. F., & Topaloglu, O., et al. (2013). Obstructive sleep apnea syndrome and its association with vitamin D deficiency. Journal of Endocrinological Investigation, 36(9), 681-5. [DOI:10.3275/8923] [PMID]
Mohammadi, N. (2007). [A perliminary study of the psychometric properties of Buss and Perry's aggression questionnaire (Persian)]. Journal of Social Sciences and Humanities of Shiraz University, 25(4), 135-51. https://www.sid.ir/fa/journal/ViewPaper.aspx?id=69211
Morley, J. E. (2014). Dementia: Does vitamin D modulate cognition? Nature Reviews Neurology, 10(11), 613-4. [DOI:10.1038/nrneurol.2014.193] [PMID]
Motevalian, S. A., Asadi-Lari, M., Rahimi, H., & Eftekhar, M. (2011). Validation of a persian version of motorcycle rider behavior questionnaire. Annals of Advances in Automotive Medicine, 55, 91-8. [PMID] [PMCID]
Nejati, V. (2013). [Cognitive abilities questionnaire: Development and evaluation of psychometric properties (Persian)]. Advances in Cognitive Sciences, 15(2), 11-9. http://icssjournal.ir/article-1-289-en.html
Palacios, N., Scott, T., Sahasrabudhe, N., Gao, X., & Tucker, K. L. (2020). Serum vitamin D and cognition in a cohort of Boston-area Puerto Ricans. Nutritional Neuroscience, 23(9), 1-8. [DOI:10.1080/1028415X.2018.1545291] [PMID]
Prüfer, K., Veenstra, T. D., Jirikowski, G. F., & Kumar, R. (1999). Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. Journal of Chemical Neuroanatomy, 16(2), 135-45. [DOI:10.1016/S0891-0618(99)00002-2]
Przybelski, R., Agrawal, S., Krueger, D., Engelke, J. A., Walbrun, F., & Binkley, N. (2008). Rapid correction of low vitamin D status in nursing home residents. Osteoporosis International, 19(11), 1621-8. [DOI:10.1007/s00198-008-0619-x] [PMID]
Przybelski, R. J., & Binkley, N. C. (2007). Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Archives of Biochemistry and Biophysics, 460(2), 202-5. [DOI:10.1016/j.abb.2006.12.018] [PMID]
Rossom, R. C., Espeland, M. A., Manson, J. E., Dysken, M. W., Johnson, K. C., & Lane, D. S., et al. (2012). Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. Journal of the American Geriatrics Society, 60(12), 2197-205. [DOI:10.1111/jgs.12032] [PMID] [PMCID]
Rui-hua, Ch., Yong-de, P., Xiao-zhen, J., Chen, J., & Bin, Zh. (2019). Decreased levels of serum IGF-1 and vitamin D Are associated with cognitive impairment in patients with type 2 diabetes. American Journal of Alzheimer’s Disease & Other Dementias®, 34(7-8), 450-6. [DOI:10.1177/1533317519860334] [PMID]
Sadeghniiat Haghighi, Kh., Montazeri, A., Khajeh Mehrizi, A., Aminian, O., Rahimi Golkhandan, A., & Saraei, M., et al. (2013). The epworth sleepiness scale: Translation and validation study of the Iranian version. Sleep and Breathing, 17(1), 419-26. [DOI:10.1007/s11325-012-0646-x] [PMID]
Skaer, T. L., & Sclar, D. A. (2010). Economic implications of sleep disorders. PharmacoEconomics, 28(11), 1015-23. [DOI:10.2165/11537390-000000000-00000] [PMID]
Slinin, Y., Paudel, M. L., Taylor, B. C., Fink, H. A., Ishani, A., & Canales, M. T., et al. (2010). 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology, 74(1), 33-41. [DOI:10.1212/WNL.0b013e3181c7197b] [PMID] [PMCID]
Stein, M. S., Scherer, S. C., Ladd, K. S., & Harrison, L. C. (2011). A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. Journal of Alzheimer’s Disease, 26(3), 477-84. [DOI:10.3233/JAD-2011-110149] [PMID]
Tabatabaeizadeh, S. A., Avan, A., Bahrami, A., Khodashenas, E., Esmaeili, H., & Ferns, G. A., et al. (2017). High‐dose supplementation of vitamin D affects measures of systemic inflammation: Reductions in high‐sensitivity C‐reactive protein level and Neutrophil to Lymphocyte Ratio (NLR) distribution. Journal of Cellular Biochemistry, 118(12), 4317-22. [DOI:10.1002/jcb.26084] [PMID]
Tolppanen, A. M., Williams, D. M., & Lawlor, D. A. (2011). The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology, 22(1), 113-7. [DOI:10.1097/EDE.0b013e3181f74683] [PMID] [PMCID]
Vaidya, A., & Williams, J. S. (2012). The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism, 61(4), 450-8. [DOI:10.1016/j.metabol.2011.09.007] [PMID] [PMCID]
van der Schaft, J., Koek, H. L., Dijkstra, E., Verhaar, H. J. J., van der Schouw, Y. T., & Emmelot-Vonk, M. H. (2013). The association between vitamin D and cognition: A systematic review. Ageing Research Reviews, 12(4), 1013-23. [DOI:10.1016/j.arr.2013.05.004] [PMID]
Vergori, A., Pinnetti, C., Lorenzini, P., Brita, A. C., Libertone, R., & Mastrorosa, I., et al. (2019). Vitamin D deficiency is associated with neurocognitive impairment in HIV-infected subjects. Retrieved from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3320268 [DOI:10.2139/ssrn.3320268]
Vieth, R., Bischoff-Ferrari, H., Boucher, B. J., Dawson-Hughes, B., Garland, C. F., & Heaney, R. P., et al. (2007). The urgent need to recommend an intake of vitamin D that is effective. The American Journal of Clinical Nutrition, 85(3), 649-50. [DOI:10.1093/ajcn/85.3.649] [PMID]
Wilkins, C. H., Sheline, Y. I., Roe, C. M., Birge, S. J., & Morris, J. C. (2006). Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. The American Journal of Geriatric Psychiatry, 14(12), 1032-40. [DOI:10.1097/01.JGP.0000240986.74642.7c] [PMID]
Yazdi, Z., Sadeghniiat-Haghighi, Kh., Zohal, M. A., & Elmizadeh, Kh. (2012). Validity and reliability of the Iranian version of the Insomnia Severity Index. The Malaysian Journal of Medical Sciences, 19(4), 31-6. [PMID] [PMCID]
Zehnder, D., Bland, R., Williams, M. C., McNinch, R. W., Howie, A. J., & Stewart, P. M., et al. (2001). Extrarenal expression of 25-Hydroxyvitamin D3-1α-Hydroxylase. The Journal of Clinical Endocrinology & Metabolism, 86(2), 888-94. [DOI:10.1210/jcem.86.2.7220] [PMID]
Nuclear steroid hormone Vitamin D (VitD) contributes to various physiological processes in the body. The role of VitD in calcium homeostasis has already known. Also, VitD affects neuronal functioning (Eyles et al., 2009; McCann & Ames, 2008; McGrath, Feron, Eyles & Mackay-Sim, 2001). VitD exhibits neurosteroid properties, essential to protect the Central Nervous System (CNS), and helps in neurotransmission, neuroimmunomodulation, and brain processes (Briones & Darwish, 2012).
Vitamin D Receptors (VDR) are widely distributed in many cell types of the human brain, such as neurons and glial cells. High levels of VDR is found in the cortex and hippocampus (Eyles, Smith, Kinobe, Hewison & McGrath, 2005; Langub, Herman, Malluche & Koszewski, 2001; Prüfer, Veenstra, Jirikowski & Kumar, 1999), which are essential areas for regulation of cognitive functions, such as learning, memory, and behavior (Becker, Eyles, McGrath & Grecksch, 2005). Moreover, enzyme (1-α-hydroxylase) related to the synthesis of the active form of VitD is prevalent in the brain, and its gene expression has been found in neural tissues (Zehnder et al., 2001). VDR also
contributes to neuroprotective pathways, decreasing oxidative damages and amyloid-β formation and accumulation (Morley, 2014). Animal studies have shown that VitD is crucial for brain development (Eyles et al., 2009). These studies have reported that VitD may support the structure and integrity of neurons within neurotrophin synthesis and detoxification pathways, which is mandatory for neuronal survival (Kang & Schuman, 2000).
Inadequate VitD levels in prenatal rats dysregulate 36 brain proteins implicated in multiple biological activities, such as oxidative phosphorylation, redox balance, cytoskeleton reservation, post-translational modifications, synaptic plasticity, calcium homeostasis, and neurotransmission in adult (Almeras et al., 2007). Furthermore, evidence shows that VitD deficiency may cause mental disorders such as major and minor depression, schizophrenia, anxiety, and sleep disorders (Garcion, Wion-Barbot, Montero-Menei, Berger & Wion, 2002; Gominak & Stumpf, 2012; Hoogendijk et al., 2008; Jorde, Waterloo, Saleh, Haug & Svartberg, 2006). Risen dietary consumption of VitD has been related to fewer incidences of self-reported psychotic-like events (Hedelin et al., 2010).
Recent studies have suggested a negative association between VitD status and adverse sleep outcomes (Bellia et al., 2013; Khoo et al., 2011). The negative impacts of sleep disorders include sleepiness, neurocognitive deficits, decrease mental performance, daytime fatigue, and altered mood in adolescents. In recent years, sleep disorders have become very common worldwide and contribute to the etiology of heart disease, diabetes, depression, and other complications (Bagai, 2010; Skaer & Sclar, 2010).
The effects of VitD on cognitive function and sleep patterns have been reported in several studies, but the results of these studies are inconsistent (Rossom et al., 2012; Stein, Scherer, Ladd & Harrison, 2011). Epidemiological studies show an association between low concentration of VitD and impairments in cognitive performances such as memory and orientation (Llewellyn, Langa & Lang, 2009; Przybelski & Binkley, 2007; Wilkins, Sheline, Roe, Birge & Morris, 2006), psychomotor speed, and executive function (Buell et al., 2009; Lee et al., 2009), also diagnosis of vascular dementia and Alzheimer disease (Buell et al., 2010). However, other studies found no relationship between VitD levels and performance on cognitive testing (Slinin et al., 2010), or they showed worse cognitive function by the elderly individuals in the high 25-hydroxy vitamin D [25(OH)D] quintile (McGrath et al., 2007). Also, it has been reported that individuals with sleep disorders have lower serum 25(OH)D levels (McCarty, Reddy, Keigley, Kim & Marino, 2012; Mete et al., 2013).
We have previously shown that VitD supplementation positively affects systemic inflammation, cardio-metabolic profile, and mood or emotional performance in adolescents (Bahrami et al., 2018; Khayyatzadeh et al., 2018; Tabatabaeizadeh et al., 2017). The present study aimed to further assess supplementation with high dose VitD on cognitive abilities, insomnia, and daytime sleepiness in adolescents.
2. Methods
2.1. Study design
This study was performed in the cities of Mashhad and Sabzevar, Iran, between January and April 2015, as described previously (Khayyatzadeh et al., 2018; Tabatabaeizadeh et al., 2017). In a large VitD project, 1026 adolescents aged between 12 and 18 years were called. Of whom, 988 met the inclusion criteria. All participants received 9 50000 IU of VitD capsules for 9 weeks (1 per week). Finally, 940 adolescents completed the trial, with a dropout rate of 4.8%. The Ethics Committee approved the study and informed written consent was provided by all participants and their parents (IR.MUMS.FM.REC.1395.12).
2.2. Study instruments
Assessment of cognitive abilities and sleep disorders was performed by standard self-report questionnaires mentioned as follows. An expert assistant aids participants to fulfill all of the questions.
2.3. The Insomnia Severity Index (ISI)
The Insomnia Severity Index (ISI) questionnaire is a reliable instrument that yields a quantitative index of perceived insomnia severity. The instrument involves 7 items focusing on sleep disorder severity, sleep-related satisfaction, and anxiety related to the sleeping problem. Each item is rated on a 5-point Likert-type scale (0-4) and provides a total score ranging from 0 to 28. Higher scores indicate more severe insomnia. Scores may be categorized as 0-7 (no clinically significant insomnia), 8–14 (sub-threshold insomnia or mild insomnia), 15-21 (moderate insomnia), and 22-28 (severe insomnia). ISI-Persian was previously validated among Iranian subjects and was found to have a high internal consistency (The Cronbach alpha= 0.8 and test-retest reliability = 0.7) (Yazdi, Sadeghniiat-Haghighi, Zohal & Elmizadeh, 2012).
2.4. Epworth Sleepiness Scale (ESS)
The Epworth Sleepiness Scale (ESS) is a questionnaire-derived scale of sleepiness that can assess the degree of daytime sleepiness. It is an 8-item questionnaire scored on a 4-point Likert-type scale that measures the habitual likelihood to fall asleep in common situations of daily living. The total score ranges from 0 to 24. Normal is less than 10. Mild to moderate obstructive sleep apnea get a score between 10 and 16. Also, severe obstructive sleep apnea or narcolepsy scores over 16. ESS has been validated and found to have a high internal consistency (the Cronbach alpha= 0.88) and acceptable test-retest reliability (r=0.81; 95% confidence interval 0.74–0.86, P<0.001) among Iranian population (Sadeghniiat Haghighi et al., 2013).
2.5. Cognitive abilities task
Cognitive performances were evaluated using the Cognitive Abilities Questionnaire (CAQ), which comprises 30 items. Each item is rated on a 5-point scale (1-5) and summed up to provide a total score ranging from 30 to 150. The validity of CAQ with high internal consistency (the Cronbach α=0.83) and good test-retest reliability (r=0.86) have been established in Iranian population (Nejati, 2013). Higher scores represent better cognition abilities. The CAQ assesses memory, inhibitory control, selective attention, decision making, planning, sustained attention, social cognition, and cognitive flexibility.
2.6. Depression and aggression
To assess the score of depression, the subjects were asked to complete the Beck depression inventory II (BDI II) questionnaire (Beck, Steer & Brown, 1996). Higher scores represent a high degree of depression. The validity of the BDI Persian version with high internal consistency (the Cronbach α=0.87) and good test-retest reliability (r=0.74) has been established in Iranian students (Mohammadi, 2007).
2.7. The Buss-Perry Aggression Questionnaire (BPAQ)
The Buss-Perry Aggression Questionnaire (BPAQ) is a 29-item questionnaire, which evaluates four aggression scores of physical aggression, verbal aggression, anger, and hostility. They are scored with a 5-point rating system. It was used to determine the aggression level of the subjects (Elliott, Baughan & Sexton, 2007). A high BPAQ score indicates higher aggression. The test-retest reliability and validity of this questionnaire have been verified among the Iranian population (Motevalian, Asadi-Lari, Rahimi & Eftekhar, 2011).
2.8. Vitamin D measurements
Fasting blood samples were collected in the early morning at baseline and post nine weeks’ trial, after a 12 hour overnight fasting. Blood samples were centrifuged (Hettich model D-78532) to separate serum. An Electrochemical-Luminescence (ECL; Roche) method was used to measure serum 25(OH)D.
2.9. Assessment of other variables
An expert interviewer collected demographic data, and anthropometric parameters were determined using the standard techniques at baseline and after 9 weeks of intervention. The question related to smoking was, “How many hours are you expose to cigarettes?” If reported 30 minutes and above, the subject was considered exposed to smoking. Physical activity was measured by a validated questionnaire and represented as Metabolic Equivalents (METs) in hours/day (Delshad et al., 2015).
2.10. Statistical method
The Kolmogorov-Smirnov test was used to determine whether the variables had a normal distribution. Results were expressed as frequency, percentage, and Mean±Standard Deviation. The Chi-square or ANOVA tests and post hoc Turkey’s test were applied to compare quantitative variables in different subject groups (insomnia, sleepiness, both insomnia, and sleepiness, as well as normal), followed by Tukey HSD. Cochran’s Q tests or paired-sample t test was used to compare variables at baseline and after the intervention. P < 0.05 was set at statistical significance in all tests. SPSS for Windows (v. 16, SPSS Inc., Chicago, IL) was used for statistical analysis.
3. Results
A total of 940 subjects were entered the study. At baseline, 222 participants presented some degree of sleepiness (ESS score ≥10), and 216 subjects presented some degree of insomnia (ISI score ≥8). Altogether, 15.0%, 15.6%, and 8.0% of subjects had some degree of insomnia, sleepiness, both insomnia, and sleepiness, respectively. In contrast, 61.4% had no insomnia and or sleepiness. Baseline characteristics of responders who completed the intervention are presented in Table 1.
.png)
Analysis of Variance (ANOVA) test results showed the four groups of participants (insomnia, sleepiness, both insomnia, and sleepiness, or control) were well-matched regarding age, body mass index, and physical activity. The subjects with some degree of insomnia and daytime sleepiness are more exposed to smoke than normal subjects (P=0.005). Furthermore, the subjects with both insomnia and sleepiness have higher depression and aggression scores than the normal population (P<0.05; Table 1).
During and after the supplementation period, no subject reported any significant adverse effects. The Mean±SD serum 25(OH)D level exhibited a significant increase from 9.46±8.83 ng/mL at baseline to 35.91±15.56 ng/mL after supplementation.
After VitD supplementation, significant improvements were seen in most cognitive abilities, including scores at the memory, inhibitory control and selective attention, decision making, planning, sustained attention, and cognitive flexibility after supplementation (P<0.001). But the positive effect on social cognition was not significant (Table 2).

VitD supplementation caused to significant reduction in total score of insomnia in cases with insomnia (9.2±5.1 to 5.7±6.3; P<0.001). Day time sleepiness score was also decreased after supplementation in adolescents with some degree of sleepiness (7.5±3.6 to 6.6±4.4; P<0.001).
At baseline, the prevalence of subjects with insomnia alone was 15.0%, while this value after supplementation was decreased to 11.3%. Similar results were also found for the prevalence of subjects with only sleepiness (15.6% reduced to 14.7%), and the cases with both insomnia and sleepiness (8.0% reduced to 6.1%), while in normal cases, the sleep disorders increased from 61.4% to 67.9% after supplementation (P=0.003; Table 3).

4. Discussion
This interventional study is the first to investigate the effects of high-dose VitD supplementation on cognitive abilities and sleep disorders. The results showed that VitD supplementation significantly improved subjective measures of insomnia, daytime sleepiness, and cognitive abilities such as memory, inhibitory control, and selective attention, decision making, planning, sustained attention, and cognitive flexibility in adolescent girls.
The present study was conducted to validate or refute the effects of VitD supplementation on the cognitive function observed in previous studies (van der Schaft et al., 2013). This study used a large sample size with a rigorous method to assess cognitive abilities. The results are consistent with the amount of data indicating that low VitD status is associated with impairments in cognitive function (Lanham-New et al., 2011; Vieth et al., 2007). The neurocognitive effect of VitD is well-supported. The VDR and catalytic enzymes are present in the brain areas involved in forming new memories, processing, and complex planning (Buell & Dawson-Hughes, 2008). VitD is neuroprotective because it modulates the expression of enzymes (tyrosine hydroxylase) related to dopamine’s synthesis and norepinephrine/epinephrine. Also, it enhances neuron survival through the prevention of oxidative pathways responsible for free radical production in the brain via the elevation of antioxidant (γ-glutamyl transpeptidase) generation and the suppression of the synthesis of inducible Nitric Oxide Synthase (iNOS), a toxic enzyme for the brain (Vaidya & Williams, 2012). The Institute of Medicine (2011) claimed a biologically confirmed association between VitD level and brain-related outcomes.
A wealth of evidence supports that low vitamin D may be associated with greater odds of cognitive impairment and reduced cognitive functioning in older adults (Breitling et al., 2012; Llewellyn, Lang, Langa & Melzer, 2011; Przybelski & Binkley, 2007). In HIV-positive patients, severe VitD deficiency was reported to be independently related with a higher odds of neurocognitive decline (OR=2.1; 95% CI:1.04–4.05) (Vergori et al., 2019). Low serum 25(OH)D concentration may independently predict worsening cognitive functions in diabetic patients (Rui-hua, Yong-de, Xiao-zhen, Chen & Bin, 2019). However, there are some discrepancies in the study results. Some studies have examined the association between VitD status and cognitive functions in young adults with different results (Hansen, Bakke, Dahl & Thayer, 2011; Lašaite, Gailyte, Puzinas, Preikša & Kazanavičius, 2011; Tolppanen, Williams & Lawlor, 2011). For example, the NHANES study expressed no relationship between VitD concentration and neurocognitive functioning in adolescents and adults (McGrath et al., 2007). In a longitudinal study on Puerto Rican adults from the Boston Area, the basal serum 25(OH)D level was not associated with a 2-year change in cognitive test scores, executive function, and memory domains (Palacios, Scott, Sahasrabudhe, Gao & Tucker, 2020).
A double-blind controlled trial reported that VitD supplementation does not influence cognitive function and mood in healthy young adults. In these participants, despite significant increases in VitD consumption, no significant changes were observed in working memory (F=1.09; P=0.30), cognitive flexibility (F=1.37; P=0.24) and response inhibition (F=0.82; P=0.37) (Dean et al., 2011). In another study, no treatment-induced improvement in cognition or behavior was observed in nursing home residents (Przybelski et al., 2008). In a randomized clinical trial among 422 subjects, no significant associations were found between serum 25(OH)D level and the three independent cognitive tasks (verbal recall test, coding test, and tapping test) at baseline. After four months, VitD supplementation did not promote cognition in mid-aged and older individuals (Jorde et al., 2019). The small sample size, the presence or absence of confounding variables, short intervention duration, and test reproducibility may preclude the observation of positive effects in these studies.
The improvement in sleep disorders is important because sleep disorders are associated with several health problems (Bagai, 2010; Goel, Rao, Durmer & Dinges, 2009; Skaer & Sclar, 2010). Although VitD is crucial to many physiologic processes in man, there are limited data in relation to its effects on sleep disorders. There is a wealth of evidence that VitD is involved in sleep regulation by decreasing prostaglandin D2 and cytokine (Feldman et al., 2007; Jablonski, Chonchol, Pierce, Walker & Seals, 2011; Krueger, Majde & Rector, 2011). VitD deficiency may be contributed to the development of symptoms of wakefulness most frequently associated with sleep disorders (McCarty et al., 2012). Our study showed that high-dose VitD supplementation significantly reduced the severity of insomnia among adolescents. These findings are compatible with the study of Khoo et al., who reported that serum VitD levels in healthy individuals were inversely associated with sleep-regulating factors such as tumor necrosis factor-alpha and interleukin-1 (Khoo et al., 2011).
Our results are similar to those of Bellia et al., who reported that a low VitD status might contribute to sleepiness through central nervous system signaling and inflammatory mediators (Bellia et al., 2013). McCarty et al. have also announced that serum 25(OH)D concentrations are linked with ESS in patients with sleep disorders (McCarty et al., 2012). One possible mechanism for this inverse correlation may be the reduced levels of sympathetic stimulation or deactivation of the hypothalamic-pituitary-adrenal stress response axis. A recent systematic review and meta-analysis have reported that those with low vitamin D had a significantly higher risk of sleep disorders (OR:1.5; 95%CI: 1.3-1.7), poor sleep quality (OR: 1.6; 95% CI: 1.2- 2.0), short sleep duration (OR: 1.7; 95% CI: 1.3- 2.3), and sleepiness (OR: 1.4; 95%CI: 1.1-1.6) (Gao et al., 2018).
The strong points of this study are its large number of carefully selected participants, comprehensive measures of the baseline characteristics, and its powerful assessment of cognitive abilities and detection of insomnia and sleepiness. To detect any possible beneficial effects of VitD supplementation on cognitive abilities and sleep disorders, characteristics of the study population, the dose of VitD, duration of intervention, and methods of cognitive testing are important. The given oral mega-doses effectively corrected severe VitD deficiency in peripheral blood, but it is still unclear whether normalization of impaired plasma level of VitD reflects the status of VitD in the Cerebrospinal Fluid (CSF) and cells in CNS.
Our study has several limitations. Our participants consisted of healthy adolescent girls who were free of cognitive impairment and major psychiatric illness. Therefore, our findings may not be generalized to clinical patients demonstrating emotional disorders or cognitive impairment. Because all observations received supplementation in the main study, we could not use a control group for comparison.
5. Conclusion
Our findings reveal that VitD supplementation improves cognitive abilities and sleep problems in healthy adolescents with a high prevalence of VitD insufficiency and deficiency. This approach may be a valuable means of improving cognitive abilities and sleep problems. Besides, treating VitD deficiency may benefit the general population other than the teenagers if other larger studies confirmed similar effects on different age and ethnic groups. Although detection and treatment of VitD deficiency are crucial for a range of health outcomes, future randomized controlled trials in key populations of interest are needed to clarify the causal contribution of VitD status to brain-related effects and elucidate whether supplementation can improve abilities in these domains.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed following the ethical standards of the Institutional and or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all study participants.
Funding
This study was supported by grants No. 931188 (Majid Ghayour-Mobarhan) from Mashhad University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Almeras, L., Eyles, D., Benech, Ph., Laffite, D., Villard, C., & Patatian, A., et al. (2007). Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders. Proteomics, 7(5), 769-80. [DOI:10.1002/pmic.200600392] [PMID]
Bagai, K. (2010). Obstructive sleep apnea, stroke, and cardiovascular diseases. The Neurologist, 16(6), 329-39. [DOI:10.1097/NRL.0b013e3181f097cb] [PMID]
Bahrami, A., Mazloum, S. R., Maghsoudi, Sh., Soleimani, D., Khayyatzadeh, S. S., & Arekhi, S., et al. (2018). High dose vitamin D supplementation is associated with a reduction in depression score among adolescent girls: A nine-week follow-up study. Journal of Diatery Supplemens, 15(2), 173-82. [DOI:10.1080/19390211.2017.1334736, In press.] [PMID]
Beck, A. T., Steer, R. A., & Brown, G. (1996). Beck depression inventory-II. Retrieved from https://doi.apa.org/doiLanding?doi=10.1037%2Ft00742-000 [DOI:10.1037/t00742-000]
Becker, A., Eyles, D. W., McGrath, J. J., & Grecksch, G. (2005). Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behavioural Brain Research, 161(2), 306-12. [DOI:10.1016/j.bbr.2005.02.015] [PMID]
Bellia, A., Garcovich, C., D’Adamo, M., Lombardo, M., Tesauro, M., & Donadel, G., et al. (2013). Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Internal and Emergency Medicine, 8(1), 33-40. [DOI:10.1007/s11739-011-0559-x] [PMID]
Breitling, L. P., Perna, L., Müller, H., Raum, E., Kliegel, M., & Brenner, H. (2012). Vitamin D and cognitive functioning in the elderly population in Germany. Experimental Gerontology, 47(1), 122-7. [DOI:10.1016/j.exger.2011.11.004] [PMID]
Briones, T. L., & Darwish, H. (2012). Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. Journal of Neuroinflammation, 9, 244. [DOI:10.1186/1742-2094-9-244] [PMID] [PMCID]
Buell, J. S., Dawson-Hughes, B., Scott, T. M., Weiner, D. E., Dallal, G. E., & Qui, W. Q., et al. (2010). 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology, 74(1), 18-26. [DOI:10.1212/WNL.0b013e3181beecb7] [PMID] [PMCID]
Buell, J. S., & Dawson-Hughes, B. (2008). Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Molecular Aspects of Medicine, 29(6), 415-22. [DOI:10.1016/j.mam.2008.05.001] [PMID] [PMCID]
Buell, J. S., Scott, T. M., Dawson-Hughes, B., Dallal, G. E., Rosenberg, I. H., & Folstein, M. F., et al. (2009). Vitamin D is associated with cognitive function in elders receiving home health services. The Journals of Gerontology Series A, 64A(8), 888-95. [DOI:10.1093/gerona/glp032] [PMID] [PMCID]
Dean, A. J., Bellgrove, M. A., Hall, T., Phan, W. M. J., Eyles, D. W., & Kvaskoff, D., et al. (2011). Effects of vitamin D supplementation on cognitive and emotional functioning in young adults-a randomised controlled trial. PLoS One, 6(11), e25966. [DOI:10.1371/journal.pone.0025966] [PMID] [PMCID]
Delshad, M., Ghanbarian, A., Rezaei Ghaleh, N., Amirshekari, G., Askari, S., & Azizi, F. (2015). Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. International Journal of Preventive Medicine, 6(1), 3. [DOI:10.4103/2008-7802.151433] [PMID] [PMCID]
Elliott, M. A., Baughan, Ch. J., & Sexton, B. F. (2007). Errors and violations in relation to motorcyclists’ crash risk. Accident Analysis & Prevention, 39(3), 491-9. [DOI:10.1016/j.aap.2006.08.012] [PMID]
Eyles, D. W., Feron, F., Cui, X., Kesby, J. P., Harms, L. H., & Ko, P., et al. (2009). Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology, 34(Suppl 1), S247-57. [DOI:10.1016/j.psyneuen.2009.04.015] [PMID]
Eyles, D. W., Smith, S., Kinobe, R., Hewison, M., & McGrath, J. J. (2005). Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. Journal of Chemical Neuroanatomy, 29(1), 21-30. [DOI:10.1016/j.jchemneu.2004.08.006] [PMID]
Feldman, D., Krishnan, A., Moreno, J., Swami, S., Peehl, D. M., & Srinivas, S. (2007). Vitamin D inhibition of the prostaglandin pathway as therapy for prostate cancer. Nutrition Reviews, 65(Suppl_2), S113-5. [DOI:10.1111/j.1753-4887.2007.tb00335.x] [PMID]
Gao, Q., Kou, T., Zhuang, B., Ren, Y., Dong, X., & Wang, Q. (2018). The association between vitamin D deficiency and sleep disorders: A systematic review and meta-analysis. Nutrients, 10(10), 1395. [DOI:10.3390/nu10101395] [PMID] [PMCID]
Garcion, E., Wion-Barbot, N., Montero-Menei, C. N., Berger, F., & Wion, D. (2002). New clues about vitamin D functions in the nervous system. Trends in Endocrinology & Metabolism, 13(3), 100-5. [DOI:10.1016/S1043-2760(01)00547-1]
Goel, N., Rao, H., Durmer, J. S., & Dinges, D. F. (2009). Neurocognitive consequences of sleep deprivation. Seminars in neurology, 29(4), 320-39. [DOI:10.1055/s-0029-1237117] [PMID] [PMCID]
Gominak, S. C., & Stumpf, W. E. (2012). The world epidemic of sleep disorders is linked to vitamin D deficiency. Medical Hypotheses, 79(2), 132-5. [DOI:10.1016/j.mehy.2012.03.031] [PMID]
Hansen, A. L., Bakke, L., Dahl, L., & Thayer, J. F. (2011). Vitamin D and executive function: A preliminary report. Perceptual and Motor Skills, 113(2), 677-85. [DOI:10.2466/02.09.13.15.16.PMS.113.5.677-685] [PMID]
Hedelin, M., Löf, M., Olsson, M., Lewander, T., Nilsson, B., & Hultman, Ch. M., et al. (2010). Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33 000 women from the general population. BMC Psychiatry, 10(1), 38. [DOI:10.1186/1471-244X-10-38] [PMID] [PMCID]
Hoogendijk, W. J. G., Lips, P., Dik, M. G., Deeg, D. J. H., Beekman, A. T. F., & Penninx, B. W. J. H. (2008). Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Archives of General Psychiatry, 65(5), 508-12. [DOI:10.1001/archpsyc.65.5.508] [PMID]
Institute of Medicine. (2011). Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press. [DOI:10.17226/13050] [PMID]
Jablonski, K. L., Chonchol, M., Pierce, G. L., Walker, A. E., & Seals, D. R. (2011). 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension, 57(1), 63-9. [DOI:10.1161/HYPERTENSIONAHA.110.160929] [PMID] [PMCID]
Jorde, R., Kubiak, J., Svartberg, J., Fuskevåg, O. M., Figenschau, Y., & Martinaityte, I., et al. (2019). Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. Journal of the Neurological Sciences, 396, 165-71. [DOI:10.1016/j.jns.2018.11.020] [PMID]
Jorde, R., Waterloo, K., Saleh, F., Haug, E., & Svartberg, J. (2006). Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. Journal of Neurology, 253(4), 464-70. [DOI:10.1007/s00415-005-0027-5] [PMID]
Kang, H., & Schuman, E. M. (2000). Intracellular Ca2+ signaling is required for neurotrophin-induced potentiation in the adult rat hippocampus. Neuroscience Letters, 282(3), 141-4. [DOI:10.1016/S0304-3940(00)00893-4]
Khayyatzadeh, S. S., Mirmousavi, S. J., Fazeli, M., Abasalti, Z., Avan, A., & Javandoost, A., et al. (2018). High-dose vitamin D supplementation is associated with an improvement in several cardio-metabolic risk factors in adolescent girls: a nine-week follow-up study. Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, 55(2), 227-35. [DOI:10.1177/0004563217707784] [PMID]
Khoo, A. L., Chai, L. Y. A., Koenen, H. J. P. M., Sweep, F. C. G. J., Joosten, I., & Netea, M. G., et al. (2011). Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clinical & Experimental Immunology, 164(1), 72-9. [DOI:10.1111/j.1365-2249.2010.04315.x] [PMID] [PMCID]
Krueger, J. M., Majde, J. A., & Rector, D. M. (2011). Cytokines in immune function and sleep regulation. In P. Montagna, & S. Chokroverty (Eds.), Sleep disorders part I. Handbook of clinical neurology (pp. 229-240). Vol. 98. Amsterdam: Elsevier. [DOI:10.1016/B978-0-444-52006-7.00015-0] [PMID] [PMCID]
Langub, M. C., Herman, J. P., Malluche, H. H., & Koszewski, N. J. (2001). Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience, 104(1), 49-56. [DOI:10.1016/S0306-4522(01)00049-5]
Lanham-New, S. A., Buttriss, J. L., Miles, L. M., Ashwell, M., Berry, J. L., & Boucher, B. J., et al. (2011). Proceedings of the rank forum on vitamin D. British Journal of Nutrition, 105(1), 144-56. [DOI:10.1017/S0007114510002576] [PMID] [PMCID]
Lašaite, L., Gailyte, I., Puzinas, P., Preikša, R., & Kazanavičius, G. (2011). Vitamin D deficiency is related to worse emotional state. Central European Journal of Medicine, 6(5), 558-66. [DOI:10.2478/s11536-011-0061-x]
Lee, D. M., Tajar, A., Ulubaev, A., Pendleton, N., O’Neill, T. W., & O’Connor, D. B., et al. (2009). Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. Journal of Neurology, Neurosurgery & Psychiatry, 80(7), 722-9. [DOI:10.1136/jnnp.2008.165720] [PMID]
Llewellyn, D. J., Lang, I. A., Langa, K. M., & Melzer, D. (2011). Vitamin D and cognitive impairment in the elderly U.S. population. The Journals of Gerontology: Series A, 66A(1), 59-65. [DOI:10.1093/gerona/glq185] [PMID] [PMCID]
Llewellyn, D. J., Langa, K. M., & Lang, I. A. (2009). Serum 25-hydroxyvitamin D concentration and cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 22(3), 188-95. [DOI:10.1177/0891988708327888] [PMID] [PMCID]
McCann, J. C., & Ames, B. N. (2008). Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? The FASEB Journal, 22(4), 982-1001. [DOI:10.1096/fj.07-9326rev] [PMID]
McCarty, D. E., Reddy, A., Keigley, Q., Kim, P. Y., & Marino, A. A. (2012). Vitamin D, race, and excessive daytime sleepiness. Journal of Clinical Sleep Medicine, 8(6), 693-7. [DOI:10.5664/jcsm.2266] [PMID] [PMCID]
McGrath, J., Feron, F., Eyles, D., & Mackay-Sim, A. (2001). Vitamin D: The neglected neurosteroid? Trends in Neurosciences, 24(10), 570-1. [DOI:10.1016/S0166-2236(00)01949-4]
McGrath, J., Scragg, R., Chant, D., Eyles, D., Burne, T., & Obradovic, D. (2007). No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology, 29(1-2), 49-54. [DOI:10.1159/000108918] [PMID]
Mete, T., Yalcin, Y., Berker, D., Ciftci, B., Guven, S. F., & Topaloglu, O., et al. (2013). Obstructive sleep apnea syndrome and its association with vitamin D deficiency. Journal of Endocrinological Investigation, 36(9), 681-5. [DOI:10.3275/8923] [PMID]
Mohammadi, N. (2007). [A perliminary study of the psychometric properties of Buss and Perry's aggression questionnaire (Persian)]. Journal of Social Sciences and Humanities of Shiraz University, 25(4), 135-51. https://www.sid.ir/fa/journal/ViewPaper.aspx?id=69211
Morley, J. E. (2014). Dementia: Does vitamin D modulate cognition? Nature Reviews Neurology, 10(11), 613-4. [DOI:10.1038/nrneurol.2014.193] [PMID]
Motevalian, S. A., Asadi-Lari, M., Rahimi, H., & Eftekhar, M. (2011). Validation of a persian version of motorcycle rider behavior questionnaire. Annals of Advances in Automotive Medicine, 55, 91-8. [PMID] [PMCID]
Nejati, V. (2013). [Cognitive abilities questionnaire: Development and evaluation of psychometric properties (Persian)]. Advances in Cognitive Sciences, 15(2), 11-9. http://icssjournal.ir/article-1-289-en.html
Palacios, N., Scott, T., Sahasrabudhe, N., Gao, X., & Tucker, K. L. (2020). Serum vitamin D and cognition in a cohort of Boston-area Puerto Ricans. Nutritional Neuroscience, 23(9), 1-8. [DOI:10.1080/1028415X.2018.1545291] [PMID]
Prüfer, K., Veenstra, T. D., Jirikowski, G. F., & Kumar, R. (1999). Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. Journal of Chemical Neuroanatomy, 16(2), 135-45. [DOI:10.1016/S0891-0618(99)00002-2]
Przybelski, R., Agrawal, S., Krueger, D., Engelke, J. A., Walbrun, F., & Binkley, N. (2008). Rapid correction of low vitamin D status in nursing home residents. Osteoporosis International, 19(11), 1621-8. [DOI:10.1007/s00198-008-0619-x] [PMID]
Przybelski, R. J., & Binkley, N. C. (2007). Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Archives of Biochemistry and Biophysics, 460(2), 202-5. [DOI:10.1016/j.abb.2006.12.018] [PMID]
Rossom, R. C., Espeland, M. A., Manson, J. E., Dysken, M. W., Johnson, K. C., & Lane, D. S., et al. (2012). Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. Journal of the American Geriatrics Society, 60(12), 2197-205. [DOI:10.1111/jgs.12032] [PMID] [PMCID]
Rui-hua, Ch., Yong-de, P., Xiao-zhen, J., Chen, J., & Bin, Zh. (2019). Decreased levels of serum IGF-1 and vitamin D Are associated with cognitive impairment in patients with type 2 diabetes. American Journal of Alzheimer’s Disease & Other Dementias®, 34(7-8), 450-6. [DOI:10.1177/1533317519860334] [PMID]
Sadeghniiat Haghighi, Kh., Montazeri, A., Khajeh Mehrizi, A., Aminian, O., Rahimi Golkhandan, A., & Saraei, M., et al. (2013). The epworth sleepiness scale: Translation and validation study of the Iranian version. Sleep and Breathing, 17(1), 419-26. [DOI:10.1007/s11325-012-0646-x] [PMID]
Skaer, T. L., & Sclar, D. A. (2010). Economic implications of sleep disorders. PharmacoEconomics, 28(11), 1015-23. [DOI:10.2165/11537390-000000000-00000] [PMID]
Slinin, Y., Paudel, M. L., Taylor, B. C., Fink, H. A., Ishani, A., & Canales, M. T., et al. (2010). 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology, 74(1), 33-41. [DOI:10.1212/WNL.0b013e3181c7197b] [PMID] [PMCID]
Stein, M. S., Scherer, S. C., Ladd, K. S., & Harrison, L. C. (2011). A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. Journal of Alzheimer’s Disease, 26(3), 477-84. [DOI:10.3233/JAD-2011-110149] [PMID]
Tabatabaeizadeh, S. A., Avan, A., Bahrami, A., Khodashenas, E., Esmaeili, H., & Ferns, G. A., et al. (2017). High‐dose supplementation of vitamin D affects measures of systemic inflammation: Reductions in high‐sensitivity C‐reactive protein level and Neutrophil to Lymphocyte Ratio (NLR) distribution. Journal of Cellular Biochemistry, 118(12), 4317-22. [DOI:10.1002/jcb.26084] [PMID]
Tolppanen, A. M., Williams, D. M., & Lawlor, D. A. (2011). The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology, 22(1), 113-7. [DOI:10.1097/EDE.0b013e3181f74683] [PMID] [PMCID]
Vaidya, A., & Williams, J. S. (2012). The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism, 61(4), 450-8. [DOI:10.1016/j.metabol.2011.09.007] [PMID] [PMCID]
van der Schaft, J., Koek, H. L., Dijkstra, E., Verhaar, H. J. J., van der Schouw, Y. T., & Emmelot-Vonk, M. H. (2013). The association between vitamin D and cognition: A systematic review. Ageing Research Reviews, 12(4), 1013-23. [DOI:10.1016/j.arr.2013.05.004] [PMID]
Vergori, A., Pinnetti, C., Lorenzini, P., Brita, A. C., Libertone, R., & Mastrorosa, I., et al. (2019). Vitamin D deficiency is associated with neurocognitive impairment in HIV-infected subjects. Retrieved from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3320268 [DOI:10.2139/ssrn.3320268]
Vieth, R., Bischoff-Ferrari, H., Boucher, B. J., Dawson-Hughes, B., Garland, C. F., & Heaney, R. P., et al. (2007). The urgent need to recommend an intake of vitamin D that is effective. The American Journal of Clinical Nutrition, 85(3), 649-50. [DOI:10.1093/ajcn/85.3.649] [PMID]
Wilkins, C. H., Sheline, Y. I., Roe, C. M., Birge, S. J., & Morris, J. C. (2006). Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. The American Journal of Geriatric Psychiatry, 14(12), 1032-40. [DOI:10.1097/01.JGP.0000240986.74642.7c] [PMID]
Yazdi, Z., Sadeghniiat-Haghighi, Kh., Zohal, M. A., & Elmizadeh, Kh. (2012). Validity and reliability of the Iranian version of the Insomnia Severity Index. The Malaysian Journal of Medical Sciences, 19(4), 31-6. [PMID] [PMCID]
Zehnder, D., Bland, R., Williams, M. C., McNinch, R. W., Howie, A. J., & Stewart, P. M., et al. (2001). Extrarenal expression of 25-Hydroxyvitamin D3-1α-Hydroxylase. The Journal of Clinical Endocrinology & Metabolism, 86(2), 888-94. [DOI:10.1210/jcem.86.2.7220] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2019/06/27 | Accepted: 2020/09/2 | Published: 2021/05/1
Received: 2019/06/27 | Accepted: 2020/09/2 | Published: 2021/05/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





