Volume 12, Issue 2 (March & April 2021)
BCN 2021, 12(2): 205-212 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Valian N, Heravi M, Ahmadiani A, Dargahi L. Comparison of Rat Primary Midbrain Neurons Cultured in DMEM/F12 and Neurobasal Mediums. BCN 2021; 12 (2) :205-212
URL: http://bcn.iums.ac.ir/article-1-1379-en.html
URL: http://bcn.iums.ac.ir/article-1-1379-en.html
1- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Dopaminergic neurons, Rat mesencephalon cell culture, B27-supplemented neurobasal, DMEM/F12 medium
Full-Text [PDF 1990 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
In vitro studies play an essential role in understanding the biological processes in a more isolated context (Giordano & Costa, 2011). It offers a controlled environment to test specific cellular and molecular hypothesis with a less experimental variation of in vivo models (Polikov et al., 2008). Neuronal cell lines derived from rodents and primary neuronal cultures are widely used to study the physiological properties of neurons and the potential neurotoxicity of chemicals (Giordano & Costa, 2011).
Midbrain dopaminergic neurons (mesostriatal, mesolimbic, and mesocortical pathways) are involved in various brain functions, including motor behavior, reinforcement, motivation, learning, and cognition (Iversen & Iversen, 2007). Therefore, understanding the extra- and intra-cellular signaling events that increase the development or survival of these neurons may improve the potential of therapies for dopamine-related disorders like Parkinson Disease (PD) (Orme, Bhangal, & Fricker, 2013).
Several cell lines of dopaminergic neurons, including human neuroblastoma (SH-SY5Y) (Deloncle et al., 2017; Wongprayoon & Govitrapong, 2017; Yeo et al., 2018), immortalized rat mesencephalon neuron (N27) (Kanthasamy et al., 2011; Lin et al., 2012; Selvakumar et al., 2018), mouse dopaminergic hybrid cells (Chen, Huang, & Li, 2018; Li et al., 2017), immortalized human dopaminergic precursor (Lund Human Mesencephalon; LUHMES) (Höllerhage et al., 2017; Zhang, Yin, & Zhang, 2014), and PC12 (Li, Wang, Lan, Yue, & Liu, 2011) cell lines are used in cell culture studies.
However, these cell lines may have genetic instability due to high passage numbers. Besides, neurites may not represent true axons or dendrites, and then, cell-cell interactions will be missed (Harry et al., 1998). So, primary neurons prepared directly from fresh brain tissues can be a reliable model in which neurons acquire a neuronal phenotype and differentiation and ultimately dIe (Giordano & Costa, 2011). Primary dopaminergic neurons derived from the rodent embryonic central nervous system are among the most relevant models to challenge dopaminergic neurons against various stresses and neurotoxins for evaluating the neuroprotective compounds to prevent neuronal degeneration (Gaven, Marin, & Claeysen, 2014).
Different culture mediums such as DMEM/F12 and neurobasal are used for culturing neuronal cell lines and primary cultures (Ciron, Lengacher, Dusonchet, Aebischer, & Schneider, 2012; Muneer, Alikunju, Szlachetka, & Haorah, 2011). Neurobasal medium is used extensively for primary hippocampal (Beaudoin et al., 2012; Henderson, Peng, Trojanowski, & Lee, 2018), cortical (Cui, Deng, Zhang, Yin, & Liu, 2018; Tan et al., 2017), cerebellar (Gustafsson, Katsioudi, Issazadeh-Navikas, & Kornum, 2016) and striatal (Nguyen, Rymar, & Sadikot, 2016) neurons culturing. In the case of primary dopaminergic neurons, in vitro studies have used both neurobasal (Bayer Andersen, Leander Johansen, Hentzer, Smith, & Dietz 2016; Collo et al., 2013; Orme et al., 2013) and DMEM/F12 (Choi, Kim, & Xia, 2013; Choi, Kruse, Palmiter, & Xia, 2008; Collins et al., 2016) medium. Since there is no study documenting the difference of these mediums in culturing primary dopaminergic neurons, this study was designed to compare primary midbrain cells in DMEM/F12 and neurobasal cultures.
2. Methods
2.1. Animals
Adult female and male Wistar rats, weighing 220-250 g, from our breeding colony, were used in this study. The animals were maintained and handled in compliance with the institutional guidelines approved by the Ethics Committee for animal research of the Shahid Beheshti University of Medical Sciences. Male and female rats were housed in the cage for 12 hours, and then the males were removed. Fourteen days later, the female rats were shortly anesthetized using CO2, and the pregnant rats were characterized by touching the belly. Then, they were used for the preparation of embryonic mesencephalon cells from the E14.5 embryo.
2.2. Preparation of primary mesencephalon cells
Primary mesencephalon cells were prepared as reported previously (Choi et al., 2013). The pregnant females were dammed by CO2 inhalation, and after euthanizing by cervical dislocation, the embryos were taken out from the uterus and amniotic sac. The extracted embryos were washed in sterile Phosphate Buffered Saline (PBS), and ventral midbrains were isolated. After washing in Ca2+ and Mg2+ free Hank’s Balanced Salt solution (HBSS; Sigma Aldrich, USA), the tissues were incubated with 0.05% trypsin solution (Gibco, USA) for 20 minutes at 37oC. By transferring pieces of tissue to HBSS, including 10% fetal bovine serum (FBS; Gibco, USA), trypsin was deactivated. The tissues were gently triturated in DMEM/F12 medium (Gibco, USA) with a fire-polished glass pipette 7 times to dissociate into single cells. Viable cells were counted using the hemocytometer method after diluting with trypan blue solution (1:10). The dissociated cells were plated in 0.01% poly-l-lysine (Sigma Aldrich, USA) -coated plates at density of 4×105 cells/well in 6-well plates in DMEM/F12 medium (containing 10% FBS, 1% glutamine [Gibco, USA] and 1% penicillin/streptomycin [Gibco, USA]) (Choi et al., 2013) .
or neurobasal (containing 1% B27 [Gibco, USA], 1% glutamine and 1% penicillin/streptomycin) (Bayer Andersen et al., 2016). For cells cultured in DMEM/F12 medium, half of the medium was replaced with fresh medium on Day in Vitro (DIV) 3. Then, the cultures were replenished by replacing half of the medium with serum-free DMEM/F12 containing 1% B27 (Gibco, USA) on DIV5. In the case of cells cultured in neurobasal, on DIV3, half of the medium was also replaced by a fresh medium containing Arabinose C (AraC) (3 µM) to prevent glial cell proliferation. On DIV5, half of the medium was renewed by a fresh neurobasal medium. Seven days after culturing, i.e. the time required for dopaminergic neurons stabilization and maturation, immunocytochemistry assay was performed. On DIV1, DIV3, and DIV5 of culturing, the cells were evaluated morphologically under light microscopy.
2.3. Immunocytochemistry
Seven days after culturing, immunocytochemistry was performed to visualize neuronal and glial cells (Collins et al., 2016). Cells were washed twice in PBS and then fixed in 4% paraformaldehyde for 12 min at room temperature. After three washes with TPBS (0.05% Tween 20 in PBS), cell permeabilization was performed using 0.02% Triton x-100 in PBS for 15 min. The cells were incubated with 1% Bovine Serum Albumin (BSA; Merck, Germany) in TPBS for 1 h at room temperature to block nonspecific antibody binding-sites. Primary antibody incubation was done with anti-β3-tubulin antibody (1:1000; ab18207, Abcam, USA), anti-Tyrosine Hydroxylase (TH) antibody (1:500; ab112, Abcam, USA), and anti-Glial Fibrillary Acidic Protein (GFAP) antibody (1:1000; ab7260, Abcam, USA) overnight at 4°C. Secondary antibody (1:150; anti-rabbit IgG FITC conjugated, cell signaling, USA) was added for 1 h after three washing, followed by nuclear staining with DAPI (0.4 µg/mL in PBS) just before visualization. Immunoreactive cells were observed at ×10 and ×20 magnifications under an Olympus microscope. The immunostaining assay was repeated 3 times for DMEM/F12 and neurobasal mediums.
3. Results
3.1. Morphology of primary midbrain cells in DMEM/F12 and neurobasal
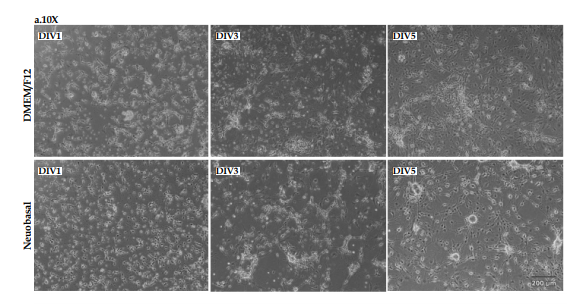
Morphology of primary midbrain cells 1, 3, and 5 days after culturing in DMEM/F12 and neurobasal mediums were evaluated using light microscopy at ×10 (Figure 1A) and ×20 (Figure 1B) magnifications.
.PNG)
As shown in Figure 1, on day 1, after culturing, the cells were almost spherical with very short processes in both mediums. On DIV3 and DIV5, the formation and branching of axons and dendrites were clearly visible, and synaptic communications were formed completely in both cultures. Our morphological findings showed that although midbrain cells grew well in both cultures and reached the final morphology, in the neurobasal medium, the cells were clustered and formed sun-like structures. It seems that mesencephalon cells have better morphology in the DMEM/F12 medium.
3.2. Beta3-tubulin-positive cells in DMEM/F12 and neurobasal mediums
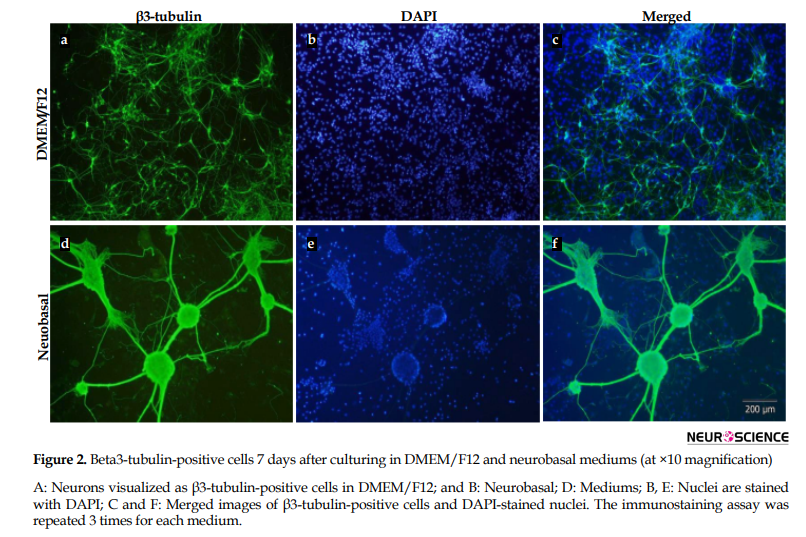
Seven days after culturing, β3-tubulin-positive cells were characterized as neurons in DMEM/F12 (Figure 2A-C) and neurobasal (Figure2D-F) using immunocytochemistry. Beta3-tubulin-positive cells (Figure 2A, D) and the nucleus of glial and neuronal cells (Figure 2B, E) were visualized using β3-tubulin antibody and DAPI, respectively. Beta3-tubulin-positive cells and DAPI-stained nucleus were merged to represent the cells better (Figure 2C, F). There was no difference between the number of immunostained neurons in DMEM/F12 and neurobasal cultures. However, the cells in the neurobasal medium were highly clustered.
3.3. GFAP-Positive neurons in DMEM/F12 and neurobasal mediums
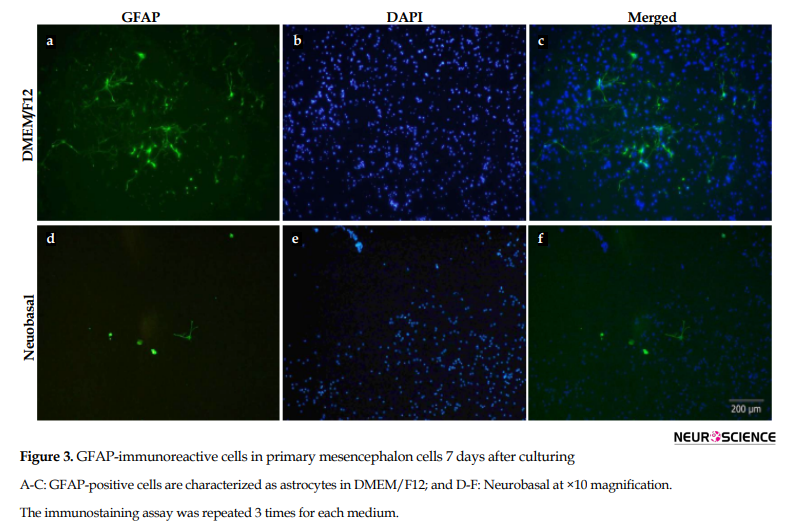
Using immunocytochemistry, astrocytes were visualized using GFAP (astroglial cell marker) antibody in DMEM/F12 (Figure 3A-C) and neurobasal (Figure 3D-F) mediums. GFAP-positive cells, DAPI-stained cell’s nucleus, and merged the nucleus with GFAP-immunoreactive cells have been demonstrated in Figure 3. Since neurobasal is the appropriate medium for culturing neurons, not astroglial cells, as well as because of adding B-27 to neurobasal medium, the number of astrocytes in neurobasal was very low compared to DMEM/F12.
3.4. TH-positive cells 7 days after culturing in DMEM/F12 and neurobasal
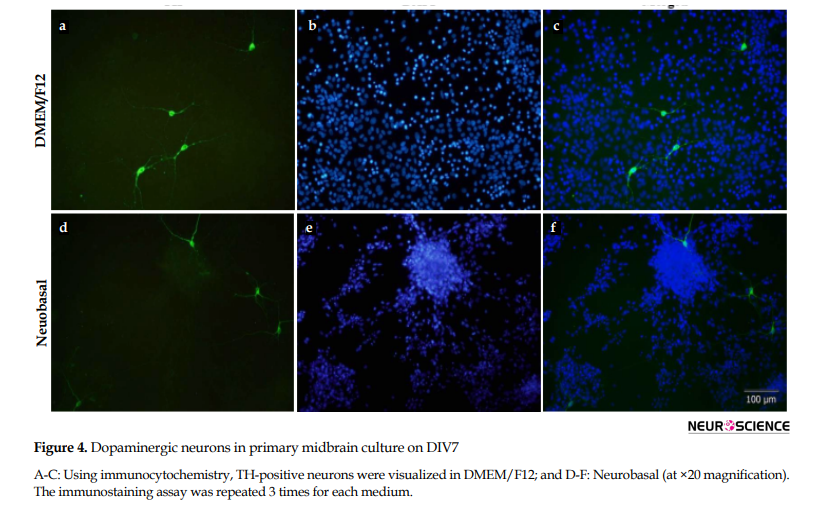
Dopaminergic neurons were characterized by immunostaining against TH antibody, 7 days following culturing in DMEM/F12 (Figure 4A-C) and neurobasal (Figure 4D-F). Similar to β3-tubulin-positive cells, despite clustering appearance in neurobasal, the number of TH+ neurons was not different in the two cultures.
4. Discussion
This study’s findings indicated that cells’ morphology was better in DMEM/F12 than that in neurobasal, but there was no difference in dopaminergic neuron number. It is suggested that primary dopaminergic neurons can grow and survive in both cultures. Consistent with our results, several in vitro studies reported survival of primary mesencephalon neurons in both cultures (Bayer Andersen et al., 2016; Choi et al., 2008; Collins et al., 2016; Collo et al., 2013). Neurobasal media is a neuron-specific culture and suppresses glial cells proliferation to less than 0.5%
of the nearly pure neuronal population, as demonstrated by immunocytochemistry for GFAP. It has been shown that glial cell growth is suppressed in a neurobasal medium. Moreover, the neurobasal medium enhances the neuronal gene expression and neuronal survival due to lower osmolarity, presence of glutamine and cysteine, and lower toxic ferrous sulfate in comparison to DMEM/F12. (Brewer, 1995; Brewer, Torricelli, Evege, & Price, 1993). Besides, B27 (an essential component of neurobasal medium) contains free radical scavenging enzymes such as catalase, superoxide dismutase, and glutathione (Nguyen et al., 2016), which provide a suitable environment for neuronal cell survival. Furthermore, using AraC in neurobasal culturing inhibits astroglial proliferation as well. So as expected, the astrocytes number was higher in DMEM/F12 compared to the neurobasal medium.
Neurotrophic factors released by astrocytes, especially Glial-Derived Neurotrophic Factor (GDNF), are critical for dopaminergic neuron survival (Kramer & Liss, 2015). In serum-supplemented DMEM/F12 media, the glial cell proliferation continued, and therefore we observed a lot of astrocytes in DMEM/F12 medium, which provided necessary neurotrophic factors. In our study, besides FBS exposure, the cells cultured in DMEM/F12 have been exposed to B27 on DIV5, which enriched the medium to survive neurons. We observed the dopaminergic neurons in DMEM/F12 similar to neurobasal and parallel with glial cells.
In general, the finding of this study indicated that primary midbrain cells can be cultured and survived in DMEM/F12 and neurobasal, with no significant differences in dopaminergic neuron number. However, DMEM/F12 was better regarding the morphology of the cells and lower cost compared to neurobasal medium as well.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This article was supported by the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences (Grant No. A-A-452-1-1392).
Authors' contributions
Methodology, bench work, writing - original draft: Neda Valian; Bench work: Mansooreh Heravi; Supervision, funding acquisition: Abolhassan Ahmadiani; Conceptualization, supervision, writing- review & editing: Leila Dargah.
Conflict of interest
The authors declared no conflict of interest.
References
Bayer Andersen, K., Leander Johansen, J., Hentzer, M., Smith, G. P., & Dietz, G. P. (2016). Protection of primary dopaminergic midbrain neurons by GPR139 agonists supports different mechanisms of MPP+ and rotenone toxicity. Frontiers in Cellular Neuroscience, 10, 164. [DOI:10.3389/fncel.2016.00164] [PMID] [PMCID]
Beaudoin, G. M., Lee, S. H., Singh, D., Yuan, Y., Ng, Y. G., & Reichardt, L. F., et al. (2012). Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nature Protocols, 7(9), 1741-54. [DOI:10.1038/nprot.2012.099] [PMID]
Brewer, G. J. (1995). Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. Journal of Neuroscience Research, 42(5), 674-83. [DOI:10.1002/jnr.490420510] [PMID]
Brewer, G. J., Torricelli, J. R., Evege, E. K., & Price, P. J. (1993). Optimized survival of hippocampal neurons in B27-supplemented neurobasal™: A new serum-free medium combination. Journal of Neuroscience Research, 35(5), 567-76. [DOI:10.1002/jnr.490350513] [PMID]
Chen, Q., Huang, X., & Li, R. (2018). lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2. American Journal of Translational Research, 10(2), 563. [PMCID] [PMID]
Choi, W. S., Kim, H. W., & Xia, Z. (2013). Preparation of primary cultured dopaminergic neurons from mouse brain. In Neural Development (pp. 61-69). Totowa, NJ: Humana Press.[DOI:10.1007/978-1-62703-444-9_6] [PMID]
Choi, W. S., Kruse, S. E., Palmiter, R. D., & Xia, Z. (2008). Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proceedings of the National Academy of Sciences, 105(39), 15136-41. [DOI:10.1073/pnas.0807581105] [PMID] [PMCID]
Ciron, C., Lengacher, S., Dusonchet, J., Aebischer, P., & Schneider, B. L. (2012). Sustained expression of PGC-1α in the rat nigrostriatal system selectively impairs dopaminergic function. Human Molecular Genetics, 21(8), 1861-76. [DOI:10.1093/hmg/ddr618] [PMID] [PMCID]
Collins, L. M., Dal Bo, G., Calcagno, M., Monzón-Sandoval, J., Sullivan, A. M., & Gutierrez, H., et al. (2016). Nociceptin/orphanin FQ inhibits the survival and axon growth of midbrain dopaminergic neurons through a p38-MAPK dependent mechanism. Molecular Neurobiology, 53(10), 7284-97.[DOI:10.1007/s12035-015-9611-6] [PMID]
Collo, G., Bono, F., Cavalleri, L., Plebani, L., Mitola, S., & Pich, E. M., et al. (2013). Nicotine-induced structural plasticity in mesencephalic dopaminergic neurons is mediated by dopamine D3 receptors and Akt-mTORC1 signaling. Molecular Pharmacology, 83(6), 1176-89. [DOI:10.1124/mol.113.084863] [PMID]
Cui, H., Deng, M., Zhang, Y., Yin, F., & Liu, J. (2018). Geniposide increases unfolded protein response-mediating HRD1 expression to accelerate APP degradation in primary cortical neurons. Neurochemical Research, 43(3), 669-80. [DOI:10.1007/s11064-018-2469-z] [PMID]
Deloncle, R., Fauconneau, B., Guillard, O., Delaval, J., Lesage, G., & Pineau, A. (2017). Copper brain protein protection against free radical-induced neuronal death: Survival ratio in SH-SY5Y neuroblastoma cell cultures. Journal of Trace Elements in Medicine and Biology, 39, 50-3. [DOI:10.1016/j.jtemb.2016.07.006] [PMID]
Gaven, F., Marin, P., & Claeysen, S. (2014). Primary culture of mouse dopaminergic neurons. Journal of Visualized Experiments: JoVE, (91), e51751. [DOI:10.3791/51751] [PMID] [PMCID]
Giordano, G., & Costa, L. G. (2011). Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods in Molecular Biology (Clifton, N.J.), 758, 13–27. [DOI:10.1007/978-1-61779-170-3_2] [PMID]
Gustafsson, J. R., Katsioudi, G., Issazadeh-Navikas, S., & Kornum, B. R. (2016). Neurobasal media facilitates increased specificity of siRNA-mediated knockdown in primary cerebellar cultures. Journal of Neuroscience Methods, 274, 116-24. [DOI:10.1016/j.jneumeth.2016.10.001] [PMID]
Harry, G. J., Billingsley, M., Bruinink, A., Campbell, I. L., Classen, W., & Dorman, D. C., et al. (1998). In vitro techniques for the assessment of neurotoxicity. Environmental Health Perspectives, 106(suppl 1), 131-58. [DOI:10.2307/3433917] [PMID] [PMCID]
Henderson, M. X., Peng, C., Trojanowski, J. Q., & Lee, V. M. (2018). LRRK2 activity does not dramatically alter α-synuclein pathology in primary neurons. Acta Neuropathologica Communications, 6(1), 1-11. [DOI:10.1186/s40478-018-0550-0] [PMID] [PMCID]
Höllerhage, M., Moebius, C., Melms, J., Chiu, W. H., Goebel, J. N., & Chakroun, T., et al. (2017). Protective efficacy of phosphodiesterase-1 inhibition against alpha-synuclein toxicity revealed by compound screening in LUHMES cells. Scientific Reports, 7(1), 1-15. [DOI:10.1038/s41598-017-11664-5] [PMID] [PMCID]
Iversen, S. D., & Iversen, L. L. (2007). Dopamine: 50 years in perspective. Trends in Neurosciences, 30(5), 188-93. [DOI:10.1016/j.tins.2007.03.002] [PMID]
Kanthasamy, K., Gordon, R., Jin, H., Anantharam, V., Ali, S., & Kanthasamy, A., et al. (2011). Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Current Neuropharmacology, 9(1), 49-53. [DOI:10.2174/157015911795017353] [PMID] [PMCID]
Kramer, E. R., & Liss, B. (2015). GDNF–Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Letters, 589(24), 3760-72. [DOI:10.1016/j.febslet.2015.11.006] [PMID]
Li, L., Liu, H., Song, H., Qin, Y., Wang, Y., & Xu, M., et al. (2017). Let-7d microRNA attenuates 6-OHDA-induced injury by targeting caspase-3 in MN9D cells. Journal of Molecular Neuroscience, 63(3), 403-11. [DOI:10.1007/s12031-017-0994-x] [PMID]
Li, L. Z., Wang, H. J., Lan, J. W., Yue, X., & Liu, C. (2011). Primary investigation of methamphetamine-induced toxicity in PC12 cells. Nan fang Yi Ke Da Xue Xue Bao, 31(1), 39-43. [PMID]
Lin, M., Chandramani-Shivalingappa, P., Jin, H., Ghosh, A., Anantharam, V., & Ali, S., et al. (2012). Methamphetamine-induced neurotoxicity linked to ubiquitin-proteasome system dysfunction and autophagy-related changes that can be modulated by protein kinase C delta in dopaminergic neuronal cells. Neuroscience, 210, 308-32. [DOI:10.1016/j.neuroscience.2012.03.004] [PMID] [PMCID]
Muneer, P. A., Alikunju, S., Szlachetka, A. M., & Haorah, J. (2011). Methamphetamine inhibits the glucose uptake by human neurons and astrocytes: Stabilization by acetyl-L-carnitine. PloS One, 6(4), e19258. [DOI:10.1371/journal.pone.0019258] [PMID] [PMCID]
Nguyen, K. Q., Rymar, V. V., & Sadikot, A. F. (2016). Impaired TrkB signaling underlies reduced BDNF-mediated trophic support of striatal neurons in the R6/2 mouse model of Huntington’s disease. Frontiers in Cellular Neuroscience, 10, 37. [DOI:10.3389/fncel.2016.00037] [PMID] [PMCID]
Orme, R. P., Bhangal, M. S., & Fricker, R. A. (2013). Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 8(4):e62040. [DOI:10.1371/journal.pone.0062040]
Polikov, V., Block, M., Zhang, C., Reichert, W. M., & Hong, J. S. (2011). In vitro models for neuroelectrodes: A paradigm for studying tissue–materials interactions in the brain. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK3934/ [PMID]
Selvakumar, G. P., Iyer, S. S., Kempuraj, D., Raju, M., Thangavel, R., & Saeed, D., et al. (2018). Glia maturation factor dependent inhibition of mitochondrial PGC-1α triggers oxidative stress-mediated apoptosis in N27 rat dopaminergic neuronal cells. Molecular Neurobiology, 55(9), 7132-52. [DOI:10.1007/s12035-018-0882-6] [PMID] [PMCID]
Tan, M. C., Widagdo, J., Chau, Y. Q., Zhu, T., Wong, J. J. L., Cheung, A., & Anggono, V. (2017). The activity-induced long non-coding RNA Meg3 modulates AMPA receptor surface expression in primary cortical neurons. Frontiers in Cellular Neuroscience, 11, 124. [DOI:10.3389/fncel.2017.00124] [PMID] [PMCID]
Wongprayoon, P., & Govitrapong, P. (2017). Melatonin protects SH-SY5Y neuronal cells against methamphetamine-induced endoplasmic reticulum stress and apoptotic cell death. Neurotoxicity Research, 31(1), 1-10. [DOI:10.1007/s12640-016-9647-z] [PMID]
Yeo, S., Sung, B., Hong, Y. M., van den Noort, M., Bosch, P., & Lee, S. H., et al. (2018). Decreased expression of serum-and glucocorticoid-inducible kinase 1 (SGK1) promotes alpha-synuclein increase related with down-regulation of dopaminergic cell in the Substantia Nigra of chronic MPTP-induced Parkinsonism mice and in SH-SY5Y cells. Gene, 661, 189-95. [DOI:10.1016/j.gene.2018.03.086] [PMID]
Zhang, X. M., Yin, M., & Zhang, M. H. (2014). Cell-based assays for Parkinson's disease using differentiated human LUHMES cells. Acta Pharmacologica Sinica, 35(7), 945-56. [DOI:10.1038/aps.2014.36] [PMID] [PMCID].
In vitro studies play an essential role in understanding the biological processes in a more isolated context (Giordano & Costa, 2011). It offers a controlled environment to test specific cellular and molecular hypothesis with a less experimental variation of in vivo models (Polikov et al., 2008). Neuronal cell lines derived from rodents and primary neuronal cultures are widely used to study the physiological properties of neurons and the potential neurotoxicity of chemicals (Giordano & Costa, 2011).
Midbrain dopaminergic neurons (mesostriatal, mesolimbic, and mesocortical pathways) are involved in various brain functions, including motor behavior, reinforcement, motivation, learning, and cognition (Iversen & Iversen, 2007). Therefore, understanding the extra- and intra-cellular signaling events that increase the development or survival of these neurons may improve the potential of therapies for dopamine-related disorders like Parkinson Disease (PD) (Orme, Bhangal, & Fricker, 2013).
Several cell lines of dopaminergic neurons, including human neuroblastoma (SH-SY5Y) (Deloncle et al., 2017; Wongprayoon & Govitrapong, 2017; Yeo et al., 2018), immortalized rat mesencephalon neuron (N27) (Kanthasamy et al., 2011; Lin et al., 2012; Selvakumar et al., 2018), mouse dopaminergic hybrid cells (Chen, Huang, & Li, 2018; Li et al., 2017), immortalized human dopaminergic precursor (Lund Human Mesencephalon; LUHMES) (Höllerhage et al., 2017; Zhang, Yin, & Zhang, 2014), and PC12 (Li, Wang, Lan, Yue, & Liu, 2011) cell lines are used in cell culture studies.
However, these cell lines may have genetic instability due to high passage numbers. Besides, neurites may not represent true axons or dendrites, and then, cell-cell interactions will be missed (Harry et al., 1998). So, primary neurons prepared directly from fresh brain tissues can be a reliable model in which neurons acquire a neuronal phenotype and differentiation and ultimately dIe (Giordano & Costa, 2011). Primary dopaminergic neurons derived from the rodent embryonic central nervous system are among the most relevant models to challenge dopaminergic neurons against various stresses and neurotoxins for evaluating the neuroprotective compounds to prevent neuronal degeneration (Gaven, Marin, & Claeysen, 2014).
Different culture mediums such as DMEM/F12 and neurobasal are used for culturing neuronal cell lines and primary cultures (Ciron, Lengacher, Dusonchet, Aebischer, & Schneider, 2012; Muneer, Alikunju, Szlachetka, & Haorah, 2011). Neurobasal medium is used extensively for primary hippocampal (Beaudoin et al., 2012; Henderson, Peng, Trojanowski, & Lee, 2018), cortical (Cui, Deng, Zhang, Yin, & Liu, 2018; Tan et al., 2017), cerebellar (Gustafsson, Katsioudi, Issazadeh-Navikas, & Kornum, 2016) and striatal (Nguyen, Rymar, & Sadikot, 2016) neurons culturing. In the case of primary dopaminergic neurons, in vitro studies have used both neurobasal (Bayer Andersen, Leander Johansen, Hentzer, Smith, & Dietz 2016; Collo et al., 2013; Orme et al., 2013) and DMEM/F12 (Choi, Kim, & Xia, 2013; Choi, Kruse, Palmiter, & Xia, 2008; Collins et al., 2016) medium. Since there is no study documenting the difference of these mediums in culturing primary dopaminergic neurons, this study was designed to compare primary midbrain cells in DMEM/F12 and neurobasal cultures.
2. Methods
2.1. Animals
Adult female and male Wistar rats, weighing 220-250 g, from our breeding colony, were used in this study. The animals were maintained and handled in compliance with the institutional guidelines approved by the Ethics Committee for animal research of the Shahid Beheshti University of Medical Sciences. Male and female rats were housed in the cage for 12 hours, and then the males were removed. Fourteen days later, the female rats were shortly anesthetized using CO2, and the pregnant rats were characterized by touching the belly. Then, they were used for the preparation of embryonic mesencephalon cells from the E14.5 embryo.
2.2. Preparation of primary mesencephalon cells
Primary mesencephalon cells were prepared as reported previously (Choi et al., 2013). The pregnant females were dammed by CO2 inhalation, and after euthanizing by cervical dislocation, the embryos were taken out from the uterus and amniotic sac. The extracted embryos were washed in sterile Phosphate Buffered Saline (PBS), and ventral midbrains were isolated. After washing in Ca2+ and Mg2+ free Hank’s Balanced Salt solution (HBSS; Sigma Aldrich, USA), the tissues were incubated with 0.05% trypsin solution (Gibco, USA) for 20 minutes at 37oC. By transferring pieces of tissue to HBSS, including 10% fetal bovine serum (FBS; Gibco, USA), trypsin was deactivated. The tissues were gently triturated in DMEM/F12 medium (Gibco, USA) with a fire-polished glass pipette 7 times to dissociate into single cells. Viable cells were counted using the hemocytometer method after diluting with trypan blue solution (1:10). The dissociated cells were plated in 0.01% poly-l-lysine (Sigma Aldrich, USA) -coated plates at density of 4×105 cells/well in 6-well plates in DMEM/F12 medium (containing 10% FBS, 1% glutamine [Gibco, USA] and 1% penicillin/streptomycin [Gibco, USA]) (Choi et al., 2013) .
or neurobasal (containing 1% B27 [Gibco, USA], 1% glutamine and 1% penicillin/streptomycin) (Bayer Andersen et al., 2016). For cells cultured in DMEM/F12 medium, half of the medium was replaced with fresh medium on Day in Vitro (DIV) 3. Then, the cultures were replenished by replacing half of the medium with serum-free DMEM/F12 containing 1% B27 (Gibco, USA) on DIV5. In the case of cells cultured in neurobasal, on DIV3, half of the medium was also replaced by a fresh medium containing Arabinose C (AraC) (3 µM) to prevent glial cell proliferation. On DIV5, half of the medium was renewed by a fresh neurobasal medium. Seven days after culturing, i.e. the time required for dopaminergic neurons stabilization and maturation, immunocytochemistry assay was performed. On DIV1, DIV3, and DIV5 of culturing, the cells were evaluated morphologically under light microscopy.
2.3. Immunocytochemistry
Seven days after culturing, immunocytochemistry was performed to visualize neuronal and glial cells (Collins et al., 2016). Cells were washed twice in PBS and then fixed in 4% paraformaldehyde for 12 min at room temperature. After three washes with TPBS (0.05% Tween 20 in PBS), cell permeabilization was performed using 0.02% Triton x-100 in PBS for 15 min. The cells were incubated with 1% Bovine Serum Albumin (BSA; Merck, Germany) in TPBS for 1 h at room temperature to block nonspecific antibody binding-sites. Primary antibody incubation was done with anti-β3-tubulin antibody (1:1000; ab18207, Abcam, USA), anti-Tyrosine Hydroxylase (TH) antibody (1:500; ab112, Abcam, USA), and anti-Glial Fibrillary Acidic Protein (GFAP) antibody (1:1000; ab7260, Abcam, USA) overnight at 4°C. Secondary antibody (1:150; anti-rabbit IgG FITC conjugated, cell signaling, USA) was added for 1 h after three washing, followed by nuclear staining with DAPI (0.4 µg/mL in PBS) just before visualization. Immunoreactive cells were observed at ×10 and ×20 magnifications under an Olympus microscope. The immunostaining assay was repeated 3 times for DMEM/F12 and neurobasal mediums.
3. Results
3.1. Morphology of primary midbrain cells in DMEM/F12 and neurobasal
Morphology of primary midbrain cells 1, 3, and 5 days after culturing in DMEM/F12 and neurobasal mediums were evaluated using light microscopy at ×10 (Figure 1A) and ×20 (Figure 1B) magnifications.

.PNG)
As shown in Figure 1, on day 1, after culturing, the cells were almost spherical with very short processes in both mediums. On DIV3 and DIV5, the formation and branching of axons and dendrites were clearly visible, and synaptic communications were formed completely in both cultures. Our morphological findings showed that although midbrain cells grew well in both cultures and reached the final morphology, in the neurobasal medium, the cells were clustered and formed sun-like structures. It seems that mesencephalon cells have better morphology in the DMEM/F12 medium.
3.2. Beta3-tubulin-positive cells in DMEM/F12 and neurobasal mediums
Seven days after culturing, β3-tubulin-positive cells were characterized as neurons in DMEM/F12 (Figure 2A-C) and neurobasal (Figure2D-F) using immunocytochemistry. Beta3-tubulin-positive cells (Figure 2A, D) and the nucleus of glial and neuronal cells (Figure 2B, E) were visualized using β3-tubulin antibody and DAPI, respectively. Beta3-tubulin-positive cells and DAPI-stained nucleus were merged to represent the cells better (Figure 2C, F). There was no difference between the number of immunostained neurons in DMEM/F12 and neurobasal cultures. However, the cells in the neurobasal medium were highly clustered.

3.3. GFAP-Positive neurons in DMEM/F12 and neurobasal mediums
Using immunocytochemistry, astrocytes were visualized using GFAP (astroglial cell marker) antibody in DMEM/F12 (Figure 3A-C) and neurobasal (Figure 3D-F) mediums. GFAP-positive cells, DAPI-stained cell’s nucleus, and merged the nucleus with GFAP-immunoreactive cells have been demonstrated in Figure 3. Since neurobasal is the appropriate medium for culturing neurons, not astroglial cells, as well as because of adding B-27 to neurobasal medium, the number of astrocytes in neurobasal was very low compared to DMEM/F12.

3.4. TH-positive cells 7 days after culturing in DMEM/F12 and neurobasal
Dopaminergic neurons were characterized by immunostaining against TH antibody, 7 days following culturing in DMEM/F12 (Figure 4A-C) and neurobasal (Figure 4D-F). Similar to β3-tubulin-positive cells, despite clustering appearance in neurobasal, the number of TH+ neurons was not different in the two cultures.

4. Discussion
This study’s findings indicated that cells’ morphology was better in DMEM/F12 than that in neurobasal, but there was no difference in dopaminergic neuron number. It is suggested that primary dopaminergic neurons can grow and survive in both cultures. Consistent with our results, several in vitro studies reported survival of primary mesencephalon neurons in both cultures (Bayer Andersen et al., 2016; Choi et al., 2008; Collins et al., 2016; Collo et al., 2013). Neurobasal media is a neuron-specific culture and suppresses glial cells proliferation to less than 0.5%
of the nearly pure neuronal population, as demonstrated by immunocytochemistry for GFAP. It has been shown that glial cell growth is suppressed in a neurobasal medium. Moreover, the neurobasal medium enhances the neuronal gene expression and neuronal survival due to lower osmolarity, presence of glutamine and cysteine, and lower toxic ferrous sulfate in comparison to DMEM/F12. (Brewer, 1995; Brewer, Torricelli, Evege, & Price, 1993). Besides, B27 (an essential component of neurobasal medium) contains free radical scavenging enzymes such as catalase, superoxide dismutase, and glutathione (Nguyen et al., 2016), which provide a suitable environment for neuronal cell survival. Furthermore, using AraC in neurobasal culturing inhibits astroglial proliferation as well. So as expected, the astrocytes number was higher in DMEM/F12 compared to the neurobasal medium.
Neurotrophic factors released by astrocytes, especially Glial-Derived Neurotrophic Factor (GDNF), are critical for dopaminergic neuron survival (Kramer & Liss, 2015). In serum-supplemented DMEM/F12 media, the glial cell proliferation continued, and therefore we observed a lot of astrocytes in DMEM/F12 medium, which provided necessary neurotrophic factors. In our study, besides FBS exposure, the cells cultured in DMEM/F12 have been exposed to B27 on DIV5, which enriched the medium to survive neurons. We observed the dopaminergic neurons in DMEM/F12 similar to neurobasal and parallel with glial cells.
In general, the finding of this study indicated that primary midbrain cells can be cultured and survived in DMEM/F12 and neurobasal, with no significant differences in dopaminergic neuron number. However, DMEM/F12 was better regarding the morphology of the cells and lower cost compared to neurobasal medium as well.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This article was supported by the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences (Grant No. A-A-452-1-1392).
Authors' contributions
Methodology, bench work, writing - original draft: Neda Valian; Bench work: Mansooreh Heravi; Supervision, funding acquisition: Abolhassan Ahmadiani; Conceptualization, supervision, writing- review & editing: Leila Dargah.
Conflict of interest
The authors declared no conflict of interest.
References
Bayer Andersen, K., Leander Johansen, J., Hentzer, M., Smith, G. P., & Dietz, G. P. (2016). Protection of primary dopaminergic midbrain neurons by GPR139 agonists supports different mechanisms of MPP+ and rotenone toxicity. Frontiers in Cellular Neuroscience, 10, 164. [DOI:10.3389/fncel.2016.00164] [PMID] [PMCID]
Beaudoin, G. M., Lee, S. H., Singh, D., Yuan, Y., Ng, Y. G., & Reichardt, L. F., et al. (2012). Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nature Protocols, 7(9), 1741-54. [DOI:10.1038/nprot.2012.099] [PMID]
Brewer, G. J. (1995). Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. Journal of Neuroscience Research, 42(5), 674-83. [DOI:10.1002/jnr.490420510] [PMID]
Brewer, G. J., Torricelli, J. R., Evege, E. K., & Price, P. J. (1993). Optimized survival of hippocampal neurons in B27-supplemented neurobasal™: A new serum-free medium combination. Journal of Neuroscience Research, 35(5), 567-76. [DOI:10.1002/jnr.490350513] [PMID]
Chen, Q., Huang, X., & Li, R. (2018). lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2. American Journal of Translational Research, 10(2), 563. [PMCID] [PMID]
Choi, W. S., Kim, H. W., & Xia, Z. (2013). Preparation of primary cultured dopaminergic neurons from mouse brain. In Neural Development (pp. 61-69). Totowa, NJ: Humana Press.[DOI:10.1007/978-1-62703-444-9_6] [PMID]
Choi, W. S., Kruse, S. E., Palmiter, R. D., & Xia, Z. (2008). Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proceedings of the National Academy of Sciences, 105(39), 15136-41. [DOI:10.1073/pnas.0807581105] [PMID] [PMCID]
Ciron, C., Lengacher, S., Dusonchet, J., Aebischer, P., & Schneider, B. L. (2012). Sustained expression of PGC-1α in the rat nigrostriatal system selectively impairs dopaminergic function. Human Molecular Genetics, 21(8), 1861-76. [DOI:10.1093/hmg/ddr618] [PMID] [PMCID]
Collins, L. M., Dal Bo, G., Calcagno, M., Monzón-Sandoval, J., Sullivan, A. M., & Gutierrez, H., et al. (2016). Nociceptin/orphanin FQ inhibits the survival and axon growth of midbrain dopaminergic neurons through a p38-MAPK dependent mechanism. Molecular Neurobiology, 53(10), 7284-97.[DOI:10.1007/s12035-015-9611-6] [PMID]
Collo, G., Bono, F., Cavalleri, L., Plebani, L., Mitola, S., & Pich, E. M., et al. (2013). Nicotine-induced structural plasticity in mesencephalic dopaminergic neurons is mediated by dopamine D3 receptors and Akt-mTORC1 signaling. Molecular Pharmacology, 83(6), 1176-89. [DOI:10.1124/mol.113.084863] [PMID]
Cui, H., Deng, M., Zhang, Y., Yin, F., & Liu, J. (2018). Geniposide increases unfolded protein response-mediating HRD1 expression to accelerate APP degradation in primary cortical neurons. Neurochemical Research, 43(3), 669-80. [DOI:10.1007/s11064-018-2469-z] [PMID]
Deloncle, R., Fauconneau, B., Guillard, O., Delaval, J., Lesage, G., & Pineau, A. (2017). Copper brain protein protection against free radical-induced neuronal death: Survival ratio in SH-SY5Y neuroblastoma cell cultures. Journal of Trace Elements in Medicine and Biology, 39, 50-3. [DOI:10.1016/j.jtemb.2016.07.006] [PMID]
Gaven, F., Marin, P., & Claeysen, S. (2014). Primary culture of mouse dopaminergic neurons. Journal of Visualized Experiments: JoVE, (91), e51751. [DOI:10.3791/51751] [PMID] [PMCID]
Giordano, G., & Costa, L. G. (2011). Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods in Molecular Biology (Clifton, N.J.), 758, 13–27. [DOI:10.1007/978-1-61779-170-3_2] [PMID]
Gustafsson, J. R., Katsioudi, G., Issazadeh-Navikas, S., & Kornum, B. R. (2016). Neurobasal media facilitates increased specificity of siRNA-mediated knockdown in primary cerebellar cultures. Journal of Neuroscience Methods, 274, 116-24. [DOI:10.1016/j.jneumeth.2016.10.001] [PMID]
Harry, G. J., Billingsley, M., Bruinink, A., Campbell, I. L., Classen, W., & Dorman, D. C., et al. (1998). In vitro techniques for the assessment of neurotoxicity. Environmental Health Perspectives, 106(suppl 1), 131-58. [DOI:10.2307/3433917] [PMID] [PMCID]
Henderson, M. X., Peng, C., Trojanowski, J. Q., & Lee, V. M. (2018). LRRK2 activity does not dramatically alter α-synuclein pathology in primary neurons. Acta Neuropathologica Communications, 6(1), 1-11. [DOI:10.1186/s40478-018-0550-0] [PMID] [PMCID]
Höllerhage, M., Moebius, C., Melms, J., Chiu, W. H., Goebel, J. N., & Chakroun, T., et al. (2017). Protective efficacy of phosphodiesterase-1 inhibition against alpha-synuclein toxicity revealed by compound screening in LUHMES cells. Scientific Reports, 7(1), 1-15. [DOI:10.1038/s41598-017-11664-5] [PMID] [PMCID]
Iversen, S. D., & Iversen, L. L. (2007). Dopamine: 50 years in perspective. Trends in Neurosciences, 30(5), 188-93. [DOI:10.1016/j.tins.2007.03.002] [PMID]
Kanthasamy, K., Gordon, R., Jin, H., Anantharam, V., Ali, S., & Kanthasamy, A., et al. (2011). Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Current Neuropharmacology, 9(1), 49-53. [DOI:10.2174/157015911795017353] [PMID] [PMCID]
Kramer, E. R., & Liss, B. (2015). GDNF–Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Letters, 589(24), 3760-72. [DOI:10.1016/j.febslet.2015.11.006] [PMID]
Li, L., Liu, H., Song, H., Qin, Y., Wang, Y., & Xu, M., et al. (2017). Let-7d microRNA attenuates 6-OHDA-induced injury by targeting caspase-3 in MN9D cells. Journal of Molecular Neuroscience, 63(3), 403-11. [DOI:10.1007/s12031-017-0994-x] [PMID]
Li, L. Z., Wang, H. J., Lan, J. W., Yue, X., & Liu, C. (2011). Primary investigation of methamphetamine-induced toxicity in PC12 cells. Nan fang Yi Ke Da Xue Xue Bao, 31(1), 39-43. [PMID]
Lin, M., Chandramani-Shivalingappa, P., Jin, H., Ghosh, A., Anantharam, V., & Ali, S., et al. (2012). Methamphetamine-induced neurotoxicity linked to ubiquitin-proteasome system dysfunction and autophagy-related changes that can be modulated by protein kinase C delta in dopaminergic neuronal cells. Neuroscience, 210, 308-32. [DOI:10.1016/j.neuroscience.2012.03.004] [PMID] [PMCID]
Muneer, P. A., Alikunju, S., Szlachetka, A. M., & Haorah, J. (2011). Methamphetamine inhibits the glucose uptake by human neurons and astrocytes: Stabilization by acetyl-L-carnitine. PloS One, 6(4), e19258. [DOI:10.1371/journal.pone.0019258] [PMID] [PMCID]
Nguyen, K. Q., Rymar, V. V., & Sadikot, A. F. (2016). Impaired TrkB signaling underlies reduced BDNF-mediated trophic support of striatal neurons in the R6/2 mouse model of Huntington’s disease. Frontiers in Cellular Neuroscience, 10, 37. [DOI:10.3389/fncel.2016.00037] [PMID] [PMCID]
Orme, R. P., Bhangal, M. S., & Fricker, R. A. (2013). Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 8(4):e62040. [DOI:10.1371/journal.pone.0062040]
Polikov, V., Block, M., Zhang, C., Reichert, W. M., & Hong, J. S. (2011). In vitro models for neuroelectrodes: A paradigm for studying tissue–materials interactions in the brain. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK3934/ [PMID]
Selvakumar, G. P., Iyer, S. S., Kempuraj, D., Raju, M., Thangavel, R., & Saeed, D., et al. (2018). Glia maturation factor dependent inhibition of mitochondrial PGC-1α triggers oxidative stress-mediated apoptosis in N27 rat dopaminergic neuronal cells. Molecular Neurobiology, 55(9), 7132-52. [DOI:10.1007/s12035-018-0882-6] [PMID] [PMCID]
Tan, M. C., Widagdo, J., Chau, Y. Q., Zhu, T., Wong, J. J. L., Cheung, A., & Anggono, V. (2017). The activity-induced long non-coding RNA Meg3 modulates AMPA receptor surface expression in primary cortical neurons. Frontiers in Cellular Neuroscience, 11, 124. [DOI:10.3389/fncel.2017.00124] [PMID] [PMCID]
Wongprayoon, P., & Govitrapong, P. (2017). Melatonin protects SH-SY5Y neuronal cells against methamphetamine-induced endoplasmic reticulum stress and apoptotic cell death. Neurotoxicity Research, 31(1), 1-10. [DOI:10.1007/s12640-016-9647-z] [PMID]
Yeo, S., Sung, B., Hong, Y. M., van den Noort, M., Bosch, P., & Lee, S. H., et al. (2018). Decreased expression of serum-and glucocorticoid-inducible kinase 1 (SGK1) promotes alpha-synuclein increase related with down-regulation of dopaminergic cell in the Substantia Nigra of chronic MPTP-induced Parkinsonism mice and in SH-SY5Y cells. Gene, 661, 189-95. [DOI:10.1016/j.gene.2018.03.086] [PMID]
Zhang, X. M., Yin, M., & Zhang, M. H. (2014). Cell-based assays for Parkinson's disease using differentiated human LUHMES cells. Acta Pharmacologica Sinica, 35(7), 945-56. [DOI:10.1038/aps.2014.36] [PMID] [PMCID].
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2019/12/10 | Accepted: 2020/05/13 | Published: 2021/03/1
Received: 2019/12/10 | Accepted: 2020/05/13 | Published: 2021/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








