Volume 10, Issue 4 (July & August 2019)
BCN 2019, 10(4): 355-362 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehdizadeh M, Martinez-Martin P, Habibi S A, Nikbakht N, Alvandi F, Bazipoor P, et al . The Association of Balance, Fear of Falling, and Daily Activities With Drug Phases and Severity of Disease in Patients With Parkinson. BCN 2019; 10 (4) :355-362
URL: http://bcn.iums.ac.ir/article-1-1177-en.html
URL: http://bcn.iums.ac.ir/article-1-1177-en.html
Maryam Mehdizadeh1 
 , Pablo Martinez-Martin2
, Pablo Martinez-Martin2 
 , Seyed Amirhasan Habibi3
, Seyed Amirhasan Habibi3 
 , Negar Nikbakht4
, Negar Nikbakht4 
 , Faeze Alvandi5
, Faeze Alvandi5 
 , Parvane Bazipoor6
, Parvane Bazipoor6 
 , Ailin Panahi7
, Ailin Panahi7 
 , Ghorban Taghizadeh *8
, Ghorban Taghizadeh *8 


 , Pablo Martinez-Martin2
, Pablo Martinez-Martin2 
 , Seyed Amirhasan Habibi3
, Seyed Amirhasan Habibi3 
 , Negar Nikbakht4
, Negar Nikbakht4 
 , Faeze Alvandi5
, Faeze Alvandi5 
 , Parvane Bazipoor6
, Parvane Bazipoor6 
 , Ailin Panahi7
, Ailin Panahi7 
 , Ghorban Taghizadeh *8
, Ghorban Taghizadeh *8 

1- Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran.; Department of Neuroscience, School of Advance Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- National Center of Epidemiology and CIBERNED, Carlos III Institute of Health, Madrid, Spain.
3- Department of Neurology, School of Medicine, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Occupational Therapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
5- Department of Occupational Therapy, School of Paramedical and Health, Zanjan University of Medical Sciences, Zanjan, Iran.
6- Department of Pathology and Sport Biomechanics, Faculty of Literature and Humanities, Bu-Ali Sina University, Hamadan, Iran.
7- Department of Occupational Therapy, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
8- Department of Occupational Therapy, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.; Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
2- National Center of Epidemiology and CIBERNED, Carlos III Institute of Health, Madrid, Spain.
3- Department of Neurology, School of Medicine, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Occupational Therapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran.
5- Department of Occupational Therapy, School of Paramedical and Health, Zanjan University of Medical Sciences, Zanjan, Iran.
6- Department of Pathology and Sport Biomechanics, Faculty of Literature and Humanities, Bu-Ali Sina University, Hamadan, Iran.
7- Department of Occupational Therapy, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
8- Department of Occupational Therapy, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.; Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 650 kb]
| Abstract (HTML)
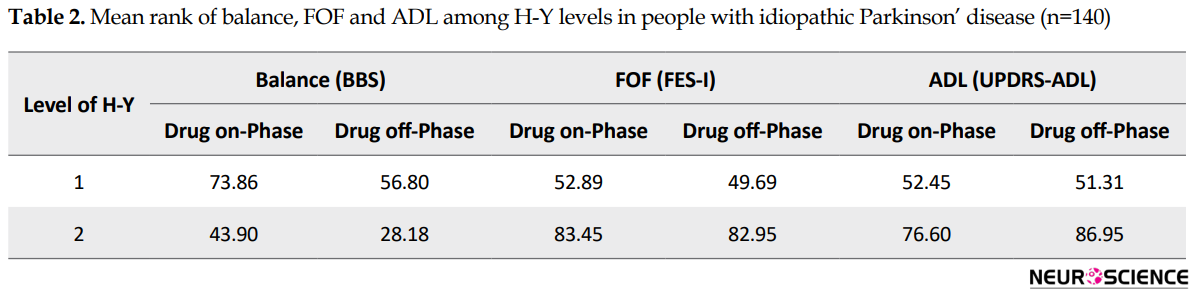
The results of Man Whitney indicated that the distribution of BBS, FES-I, and UPDRS-ADL scores in drug on-and off-phase were significantly different among HY levels of PD severity. Table 2 presents the mean rank scores of BBS, FES-I, and UPDRS-ADL in the drug on-phase and drug off-phase at varying levels of PD severity. In all tests, P values were less than 0.001.
4. Discussion
The purpose of this study was to investigate the association of functional balance, FOF, and independence in ADL in people with idiopathic PD with drug on-phase and drug off-phase. The results of this study showed a significant difference between various levels of functional balance and severity of the disease, as well as FOF and independence in ADL in both drug on- and drug offphases.
According to studies of Franchignoni et al. (2005), Bryant, Rintala, Hou, & Prota (2015), and Landers et al. (2017), people with greater damage in balance show more FOF during their functions (Bryant et al., 2015; Franchignoni et al., 2005; Landers et al., 2017). Also according to results of Landers et al. (2017) study, FOF is significantly different at varying stages of disease severity (Landers et al., 2017), which is consistent with the results of our research. Also, as suggested by Foreman et al. (2011) who used a correlation analysis, evaluation of FOF and independence in ADL at different levels of functional balance in drug off-phase gives more accurate information to therapists in these cases (Foreman et al., 2011). On the other hand, results at different levels of functional balance showed a significant change in independence in ADL in drug on-phase and drug off-phase. This outcome in our study may be due to a decrease in physical activity, as a result of a reduction in the effect of dopamine medications in the brain, which needs further investigation.
One of the important factors for independence in ADL, especially in those needing a standing position, is to maintain the balance and to control the posture. Landers et al. (2017) found that patients with PD avoid doing such activities because of the lack of functional balance to prevent falling (Landers et al., 2017). The results of this study also showed that at better levels of functional balance, independence in ADL was higher. These results were more pronounced in drug off-phase, which alters the functional state of the patients with PD.
Finally, the findings of this study indicate that taking a plan (rehabilitation and medication) to improve the balance of people with idiopathic PD can help reduce FOF. It is noteworthy that when individuals are less worried about falling during their ADL, their level of independence will become relatively higher.
Additionally, increasing dependence on others due to the reduction of independence in ADL can increase the feeling of tiredness and burden among the caregivers. Since PD is a chronic and progressive disorder, interventions designed to improve the level of independence in ADL can indirectly help the caregivers (Bhatia et al., 2003).
Maintaining balance and posture in a standing position is essential for most of the daily activities (Dunsky, Zeev, & Netz, 2017). According to the results of this study, in people with more balance problem, the fear of falling and dependence on others were higher. This case was shown in this study with the progression of the disease as well as in the off-phase drug.
We believe that consideration of balance training in the rehabilitation program of these individuals can help maintain independence in everyday activities, even at advanced levels of disease and in the drug off-phase (in which symptoms of disease reappear). Of course, more comprehensive studies are needed to confirm this conclusion.
This study has some limitations. First, psychological factors were not considered in this study. Moreover, individuals were selected with a convenience sampling method, and, thus, most of the samples belonged to stage 1 of PD severity. These limitations may reduce the degree of generalizability of the results. Hence, it is suggested that future studies consider these issues.
In conclusion, considering the association of functional balance, FOF, and independence in ADL in patients with PD with drug on-off phase, it is critical to design an effective treatment plan. Identification and management of difficulties in balance and ADL, as well as FOF, may increase the quality of life and the level of participation of individuals in social activities (regardless of the severity of disease and drug phases).
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Student Research Center of Iran University of Medical Sciences (Code Number: IR.IUMS.REC.1394.26664).
Funding
This work was supported by the Student Research Committee in Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Maryam Mehdizadeh, Ghorban Taghizadeh; Methodology: Maryam Mehdizadeh, Ghorban Taghizadeh and Seyed Amir Hassan Habibi; Investigation, Maryam Mehdizadeh, Faeze Alvandi, Parvane Bazipoor and Negar Nikbakht; Writing-original draft: All author; Writing –review & editing: Maryam Mehdizadeh, Pablo Martinez-Martin; Funding acquisition: Maryam Mehdizadeh, Ghorban Taghizadeh; Supervision: Ghorban Taghizadeh and Pablo Martinez-Martin.
Conflict of interest
The authors declared no conflict of interest.
References
Full-Text:
1. Introduction
Parkinson Disease (PD) is a neurodegenerative disorder. One of its main symptoms is the inability to maintain posture and balance (Herman, Weiss, Brozgol, Giladi, & Hausdorff, 2014), a disturbance potentially associated with falling and Fear of Falling (FOF) (Adkin, Frank, & Jog, 2003). FOF is defined as a concern of falling during performing everyday life activities, which may prevent an individual from carrying out his or her activities (Lindholm, Hagell, Hansson, & Nilsson, 2014). According to previous studies in patients with PD, the decreased daily activities due to falling fear is considerable (about 44.1%) (Bloem et al., 2001). Avoiding daily activities because of FOF can increase dependence on others and social isolation while reducing the physical activity and quality of life (Deshpande et al., 2008; Kader, Iwarsson, Odin, & Nilsson, 2016; Rosqvist et al., 2017).
Levodopa is the primary treatment of PD and can be beneficial for almost all patients with PD. As the disease progresses, continuous use of the drug can lead to a series of motor complications (i.e. motor fluctuations). These people experience motor fluctuations between two drug phases called “on” (after taking the first dose of medication while symptoms of the disease are being controlled) and “off” (when symptoms re-emerge, and the patient shows problems in motor function) (Ahlskog, & Muenter, 2001; Rodríguez-Molinero et al., 2017). Previous studies have examined the effects of motor fluctuations on various performances (such as balance, falling, etc.) in both drug phases. The best treatment response, medical interventions, and rehabilitation programs must vary according to the patient’s condition patient’s motor performance and drug phase. Hence, understanding the relationship between factors such as FOF, functional balance abilities, and independence in Activities of Daily Living (ADL) could be useful for planning the most appropriate therapeutic interventions for these patients (Morris, Iansek, & Churchyard, 1998; Morris, Morris, & Iansek, 2001).
However, the relationship between these factors (i.e. functional balance, FOF, and independence in ADL) has not been investigated concerning the severity of the PD. Also, most studies in this area have been conducted in the drug on-phase period. However, functional balance, FOF, and independence in ADL may be significantly different in drug off-phase compared with drug on-phase (Foreman, Addison, Kim, & Dibble, 2011; Franchignoni, Martignoni, Ferriero, & Pasetti, 2005). This study aims to investigate the association of functional balance, FOF, and independence in ADL in people with idiopathic PD with drug on- and drug off-phase.
2.Methods
2.1. Study participants
The study included 140 subjects with idiopathic PD (age: Mean±SD; 60.51±12.32 y; 66.42% male). The inclusion criteria were having idiopathic PD diagnosed by a neurologist based on UK Parkinson Disease Society Brain Bank clinical diagnostic criteria for PD (Hughes, Daniel, Kilford, & Lees, 1992), lacking any cognitive impairment by obtaining score of >21 on mini-mental status examination (Godefroy et al., 2011), and understanding test instructions in Persian language. All patients with a history of receiving rehabilitation services were excluded from the study.
This study was approved by the Ethics Committee of the Student Research Center at Iran University of Medical Sciences. The participants signed informed consent before participating in this study.
2.2. Study procedure
At the beginning of the study, the patients were asked not to use levodopa before coming to the clinic. Assessments were first performed in the drug off-phase (i.e. 12 hours after the last dose of levodopa and before the morning dose) and later in the drug on-phase (i.e. 1 hour after taking the first dose of levodopa in the morning) (Morris et al., 2001). Demographic information (age, sex, duration of disease, the severity of disease based on the Hoehn and Yahr scale, type of drugs, etc.) was obtained using a demographic questionnaire. Functional balance, FOF, and independence in ADL were evaluated using the Berg Balance Scale (BBS), Fall Efficacy Scale-International (FES-I), and Unified Parkinson Disease Rating Scale-ADL (UPDRS-ADL), respectively. The duration of evaluations varied between 30 and 60 minutes (depending on duration of the drug effect).
2.3. Study tools
2.3.1. Hoehn and Yahr (HY) Scale
This scale was used to determine the level of PD progression. Based on this scale, the severity of the disease is divided into 5 levels, where level 1 indicates the patient’s normal condition and level 5 indicates patient’s use of a wheelchair (Goetz et al., 2004).
2.3.2. Berg Balance Scale (BBS)
This scale is a basic test for evaluating functional balance and consists of 14 items; each of these items is scored from 0 to 4. The total BBS score ranges from 0 to 56, and the higher score indicates a better balance. BBS evaluates two key dimensions of balance in everyday activities; dynamic and static. The instruments used include chronometer, ruler, chair, and a step. The Persian version of this scale has high inter-rater reliability (ICC=0.99) and internal consistency (the Cronbach α=0.92) in patients with PD (Babaei-Ghazani et al., 2017).
2.3.3. Fall Efficacy Scale-International (FES-I)
This scale has 16 items that measure the FOF. The total score of FES-I ranges between 0 and 64, with a higher score indicating more FOF. The Persian version of this scale has good test-retest reliability (Pearson correlation coefficient=0.70) in the elderly population (Khajavi, 2013).
2.3.4. Unified Parkinson Disease Rating Scale–Activity of Daily Living (UPDRS–ADL)
The second subset of the UPDRS scale (i.e. UPDRS-ADL) was used to measure individuals’ ability and independence in ADL, which contains 13 items (total score range: 0-52). A higher score indicates more dependency on others to carry out daily activities (Shulman et al., 2016).
2.4. Statistical analyses
Normality of data was explored by the Kolmogorov-Smirnov test, which indicates the non-normal distribution of data. The Spearman correlation coefficient was used to examine the relationship between functional balance (i.e. BBS score), FOF (i.e. FES-I score), and independence in ADL (i.e. UPDRS-ADL score). The Spearman rank correlation coefficient values of more than 0.60, 0.30-0.59, and less than 0.30 indicate strong, moderate, and weak correlations, respectively (Xie et al., 2006).
Kruskal-Wallis H was investigated between the patients’ FOF and UPDRS-ADL score considering functional balance, which was divided into three levels using BBS score as follows: scores from 56 to 41, level 1; 40 to 21, level 2; less than 20, level 3 (Schneider et al., 2012).
Mann-Whitney test was used to examine the differences in functional balance, FOF, and independence in ADL with regard to PD severity. Different levels of PD severity were determined according to HY stages (stages 1 and 2 or mild were considered as level 1; and stages 3 and 4, or moderate/severe were considered as level 2). Statistical analyses were performed separately for both drug phases (on and off).
3. Results
Based on HY scale, 94(67.14%) participants were in stage 1, 46(32.85%) in stage 2. The Mean±SD scores of BBS in drug on-phase were 53.14±5.91, 44.25±13.90 at stages 1 and 2 based on HY scale, respectively. Also, the Mean±SD scores of BBS in drug off-phase were 51.67±7.76, 37.84±16.27 at stages 1 and 2 based on HY scale, respectively.
A moderate correlation was found between functional balance and FOF in the drug on-phase (r=-0.61, P<0.001) and the drug off-phase (r=-0.70, P<0.001). Also, the correlation between functional balance and independence in ADL was moderate in drug on-phase (r=-0.52, P<0.001) and drug off-phase (r=-0.62, P<0.001).
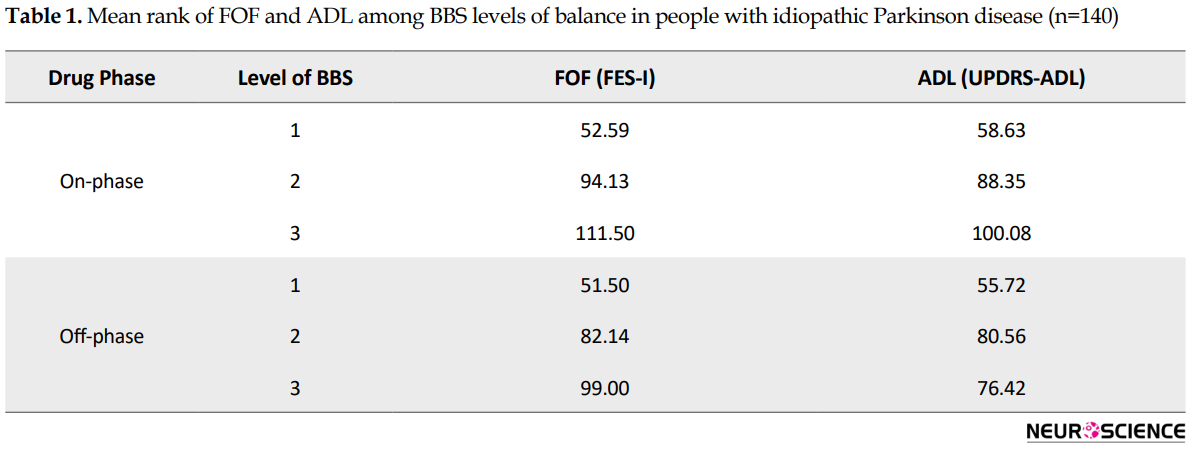
Based on the results of Kruskal-Wallis H test, the distribution of the FES-I and UPDRS-ADL scores in drug on-phase (H=27.44 and H=12.80, respectively) and drug off-phase (H=32.69 and H=21.09, respectively) have significant differences in different levels of functional balance. Mean rank FES-I and UPDRS-ADL scores are presented in Table 1 for BBS levels of functional balance in the drug on-phase and dug off-phase. In all Kruskal-Wallis H tests, P values were less than 0.001.
Parkinson Disease (PD) is a neurodegenerative disorder. One of its main symptoms is the inability to maintain posture and balance (Herman, Weiss, Brozgol, Giladi, & Hausdorff, 2014), a disturbance potentially associated with falling and Fear of Falling (FOF) (Adkin, Frank, & Jog, 2003). FOF is defined as a concern of falling during performing everyday life activities, which may prevent an individual from carrying out his or her activities (Lindholm, Hagell, Hansson, & Nilsson, 2014). According to previous studies in patients with PD, the decreased daily activities due to falling fear is considerable (about 44.1%) (Bloem et al., 2001). Avoiding daily activities because of FOF can increase dependence on others and social isolation while reducing the physical activity and quality of life (Deshpande et al., 2008; Kader, Iwarsson, Odin, & Nilsson, 2016; Rosqvist et al., 2017).
Levodopa is the primary treatment of PD and can be beneficial for almost all patients with PD. As the disease progresses, continuous use of the drug can lead to a series of motor complications (i.e. motor fluctuations). These people experience motor fluctuations between two drug phases called “on” (after taking the first dose of medication while symptoms of the disease are being controlled) and “off” (when symptoms re-emerge, and the patient shows problems in motor function) (Ahlskog, & Muenter, 2001; Rodríguez-Molinero et al., 2017). Previous studies have examined the effects of motor fluctuations on various performances (such as balance, falling, etc.) in both drug phases. The best treatment response, medical interventions, and rehabilitation programs must vary according to the patient’s condition patient’s motor performance and drug phase. Hence, understanding the relationship between factors such as FOF, functional balance abilities, and independence in Activities of Daily Living (ADL) could be useful for planning the most appropriate therapeutic interventions for these patients (Morris, Iansek, & Churchyard, 1998; Morris, Morris, & Iansek, 2001).
However, the relationship between these factors (i.e. functional balance, FOF, and independence in ADL) has not been investigated concerning the severity of the PD. Also, most studies in this area have been conducted in the drug on-phase period. However, functional balance, FOF, and independence in ADL may be significantly different in drug off-phase compared with drug on-phase (Foreman, Addison, Kim, & Dibble, 2011; Franchignoni, Martignoni, Ferriero, & Pasetti, 2005). This study aims to investigate the association of functional balance, FOF, and independence in ADL in people with idiopathic PD with drug on- and drug off-phase.
2.Methods
2.1. Study participants
The study included 140 subjects with idiopathic PD (age: Mean±SD; 60.51±12.32 y; 66.42% male). The inclusion criteria were having idiopathic PD diagnosed by a neurologist based on UK Parkinson Disease Society Brain Bank clinical diagnostic criteria for PD (Hughes, Daniel, Kilford, & Lees, 1992), lacking any cognitive impairment by obtaining score of >21 on mini-mental status examination (Godefroy et al., 2011), and understanding test instructions in Persian language. All patients with a history of receiving rehabilitation services were excluded from the study.
This study was approved by the Ethics Committee of the Student Research Center at Iran University of Medical Sciences. The participants signed informed consent before participating in this study.
2.2. Study procedure
At the beginning of the study, the patients were asked not to use levodopa before coming to the clinic. Assessments were first performed in the drug off-phase (i.e. 12 hours after the last dose of levodopa and before the morning dose) and later in the drug on-phase (i.e. 1 hour after taking the first dose of levodopa in the morning) (Morris et al., 2001). Demographic information (age, sex, duration of disease, the severity of disease based on the Hoehn and Yahr scale, type of drugs, etc.) was obtained using a demographic questionnaire. Functional balance, FOF, and independence in ADL were evaluated using the Berg Balance Scale (BBS), Fall Efficacy Scale-International (FES-I), and Unified Parkinson Disease Rating Scale-ADL (UPDRS-ADL), respectively. The duration of evaluations varied between 30 and 60 minutes (depending on duration of the drug effect).
2.3. Study tools
2.3.1. Hoehn and Yahr (HY) Scale
This scale was used to determine the level of PD progression. Based on this scale, the severity of the disease is divided into 5 levels, where level 1 indicates the patient’s normal condition and level 5 indicates patient’s use of a wheelchair (Goetz et al., 2004).
2.3.2. Berg Balance Scale (BBS)
This scale is a basic test for evaluating functional balance and consists of 14 items; each of these items is scored from 0 to 4. The total BBS score ranges from 0 to 56, and the higher score indicates a better balance. BBS evaluates two key dimensions of balance in everyday activities; dynamic and static. The instruments used include chronometer, ruler, chair, and a step. The Persian version of this scale has high inter-rater reliability (ICC=0.99) and internal consistency (the Cronbach α=0.92) in patients with PD (Babaei-Ghazani et al., 2017).
2.3.3. Fall Efficacy Scale-International (FES-I)
This scale has 16 items that measure the FOF. The total score of FES-I ranges between 0 and 64, with a higher score indicating more FOF. The Persian version of this scale has good test-retest reliability (Pearson correlation coefficient=0.70) in the elderly population (Khajavi, 2013).
2.3.4. Unified Parkinson Disease Rating Scale–Activity of Daily Living (UPDRS–ADL)
The second subset of the UPDRS scale (i.e. UPDRS-ADL) was used to measure individuals’ ability and independence in ADL, which contains 13 items (total score range: 0-52). A higher score indicates more dependency on others to carry out daily activities (Shulman et al., 2016).
2.4. Statistical analyses
Normality of data was explored by the Kolmogorov-Smirnov test, which indicates the non-normal distribution of data. The Spearman correlation coefficient was used to examine the relationship between functional balance (i.e. BBS score), FOF (i.e. FES-I score), and independence in ADL (i.e. UPDRS-ADL score). The Spearman rank correlation coefficient values of more than 0.60, 0.30-0.59, and less than 0.30 indicate strong, moderate, and weak correlations, respectively (Xie et al., 2006).
Kruskal-Wallis H was investigated between the patients’ FOF and UPDRS-ADL score considering functional balance, which was divided into three levels using BBS score as follows: scores from 56 to 41, level 1; 40 to 21, level 2; less than 20, level 3 (Schneider et al., 2012).
Mann-Whitney test was used to examine the differences in functional balance, FOF, and independence in ADL with regard to PD severity. Different levels of PD severity were determined according to HY stages (stages 1 and 2 or mild were considered as level 1; and stages 3 and 4, or moderate/severe were considered as level 2). Statistical analyses were performed separately for both drug phases (on and off).
3. Results
Based on HY scale, 94(67.14%) participants were in stage 1, 46(32.85%) in stage 2. The Mean±SD scores of BBS in drug on-phase were 53.14±5.91, 44.25±13.90 at stages 1 and 2 based on HY scale, respectively. Also, the Mean±SD scores of BBS in drug off-phase were 51.67±7.76, 37.84±16.27 at stages 1 and 2 based on HY scale, respectively.
A moderate correlation was found between functional balance and FOF in the drug on-phase (r=-0.61, P<0.001) and the drug off-phase (r=-0.70, P<0.001). Also, the correlation between functional balance and independence in ADL was moderate in drug on-phase (r=-0.52, P<0.001) and drug off-phase (r=-0.62, P<0.001).
Based on the results of Kruskal-Wallis H test, the distribution of the FES-I and UPDRS-ADL scores in drug on-phase (H=27.44 and H=12.80, respectively) and drug off-phase (H=32.69 and H=21.09, respectively) have significant differences in different levels of functional balance. Mean rank FES-I and UPDRS-ADL scores are presented in Table 1 for BBS levels of functional balance in the drug on-phase and dug off-phase. In all Kruskal-Wallis H tests, P values were less than 0.001.
The results of Man Whitney indicated that the distribution of BBS, FES-I, and UPDRS-ADL scores in drug on-and off-phase were significantly different among HY levels of PD severity. Table 2 presents the mean rank scores of BBS, FES-I, and UPDRS-ADL in the drug on-phase and drug off-phase at varying levels of PD severity. In all tests, P values were less than 0.001.
4. Discussion
The purpose of this study was to investigate the association of functional balance, FOF, and independence in ADL in people with idiopathic PD with drug on-phase and drug off-phase. The results of this study showed a significant difference between various levels of functional balance and severity of the disease, as well as FOF and independence in ADL in both drug on- and drug offphases.
According to studies of Franchignoni et al. (2005), Bryant, Rintala, Hou, & Prota (2015), and Landers et al. (2017), people with greater damage in balance show more FOF during their functions (Bryant et al., 2015; Franchignoni et al., 2005; Landers et al., 2017). Also according to results of Landers et al. (2017) study, FOF is significantly different at varying stages of disease severity (Landers et al., 2017), which is consistent with the results of our research. Also, as suggested by Foreman et al. (2011) who used a correlation analysis, evaluation of FOF and independence in ADL at different levels of functional balance in drug off-phase gives more accurate information to therapists in these cases (Foreman et al., 2011). On the other hand, results at different levels of functional balance showed a significant change in independence in ADL in drug on-phase and drug off-phase. This outcome in our study may be due to a decrease in physical activity, as a result of a reduction in the effect of dopamine medications in the brain, which needs further investigation.
One of the important factors for independence in ADL, especially in those needing a standing position, is to maintain the balance and to control the posture. Landers et al. (2017) found that patients with PD avoid doing such activities because of the lack of functional balance to prevent falling (Landers et al., 2017). The results of this study also showed that at better levels of functional balance, independence in ADL was higher. These results were more pronounced in drug off-phase, which alters the functional state of the patients with PD.
Finally, the findings of this study indicate that taking a plan (rehabilitation and medication) to improve the balance of people with idiopathic PD can help reduce FOF. It is noteworthy that when individuals are less worried about falling during their ADL, their level of independence will become relatively higher.
Additionally, increasing dependence on others due to the reduction of independence in ADL can increase the feeling of tiredness and burden among the caregivers. Since PD is a chronic and progressive disorder, interventions designed to improve the level of independence in ADL can indirectly help the caregivers (Bhatia et al., 2003).
Maintaining balance and posture in a standing position is essential for most of the daily activities (Dunsky, Zeev, & Netz, 2017). According to the results of this study, in people with more balance problem, the fear of falling and dependence on others were higher. This case was shown in this study with the progression of the disease as well as in the off-phase drug.
We believe that consideration of balance training in the rehabilitation program of these individuals can help maintain independence in everyday activities, even at advanced levels of disease and in the drug off-phase (in which symptoms of disease reappear). Of course, more comprehensive studies are needed to confirm this conclusion.
This study has some limitations. First, psychological factors were not considered in this study. Moreover, individuals were selected with a convenience sampling method, and, thus, most of the samples belonged to stage 1 of PD severity. These limitations may reduce the degree of generalizability of the results. Hence, it is suggested that future studies consider these issues.
In conclusion, considering the association of functional balance, FOF, and independence in ADL in patients with PD with drug on-off phase, it is critical to design an effective treatment plan. Identification and management of difficulties in balance and ADL, as well as FOF, may increase the quality of life and the level of participation of individuals in social activities (regardless of the severity of disease and drug phases).
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Student Research Center of Iran University of Medical Sciences (Code Number: IR.IUMS.REC.1394.26664).
Funding
This work was supported by the Student Research Committee in Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Maryam Mehdizadeh, Ghorban Taghizadeh; Methodology: Maryam Mehdizadeh, Ghorban Taghizadeh and Seyed Amir Hassan Habibi; Investigation, Maryam Mehdizadeh, Faeze Alvandi, Parvane Bazipoor and Negar Nikbakht; Writing-original draft: All author; Writing –review & editing: Maryam Mehdizadeh, Pablo Martinez-Martin; Funding acquisition: Maryam Mehdizadeh, Ghorban Taghizadeh; Supervision: Ghorban Taghizadeh and Pablo Martinez-Martin.
Conflict of interest
The authors declared no conflict of interest.
References
- Adkin, A. L., Frank, J. S., & Jog, M. S. (2003). Fear of falling and postural control in Parkinson’s disease. Movement Disorders, 18(5), 496-502. [DOI:10.1002/mds.10396] [PMID]
- Ahlskog, J. E., & Muenter, M. D. (2001). Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Movement Disorders, 16(3), 448-58. [DOI:10.1002/mds.1090] [PMID]
- Babaei-Ghazani, A., Mohammadi, H., Shahidi, G. A., Habibi, S. A. H., Forogh, B., Ahadi, T., et al. (2017). Reliability and validity of the Persian translation of Berg Balance scale in parkinson disease. Aging clinical and Experimental Research, 29(5), 857-62. [DOI:10.1007/s40520-016-0682-7] [PMID]
- Bhatia, S., & Gupta, A. (2003). Impairments in activities of daily living in Parkinson’s disease: Implications for management. NeuroRehabilitation, 18(3), 209-14. [PMID]
- Bloem, B. R., Grimbergen, Y. A., Cramer, M., Willemsen, M., & Zwinderman, A. H. (2001). Prospective assessment of falls in Parkinson’s disease. Journal of Neurology, 248(11), 950-8. [DOI:10.1007/s004150170047] [PMID]
- Bryant, M. S., Rintala, D. H., Hou, J. G., & Protas, E. J. (2015). Relationship of falls and fear of falling to activity limitations and physical inactivity in Parkinson’s disease. Journal of Aging and Physical Activity, 23(2), 187-93. [DOI:10.1123/japa.2013-0244] [PMID]
- Deshpande, N., Metter, E. J., Lauretani, F., Bandinelli, S., Guralnik, J., & Ferrucci, L. (2008). Activity restriction induced by fear of falling and objective and subjective measures of physical function: A prospective cohort study. Journal of the American Geriatrics Society, 56(4), 615-20. [DOI:10.1111/j.1532-5415.2007.01639.x] [PMID] [PMCID]
- Dunsky, A., Zeev, A., & Netz, Y. (2017). Balance performance is task specific in older adults. BioMed research international, 2017(6987017), 1-7. [DOI:10.1155/2017/6987017] [PMID] [PMCID]
- Foreman, K. B., Addison, O., Kim, H. S., & Dibble, L. E. (2011). Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism & Related Disorders, 17(3), 166-71. [DOI:10.1016/j.parkreldis.2010.12.007] [PMID] [PMCID]
- Franchignoni, F., Martignoni, E., Ferriero, G., & Pasetti, C. (2005). Balance and fear of falling in parkinson’s disease. Parkinsonism & Related Disorders, 11(7), 427-33. [DOI:10.1016/j.parkreldis.2005.05.005] [PMID]
- Godefroy, O., Fickl, A., Roussel, M., Auribault, C., Bugnicourt, J. M., Lamy, C., et al. (2011). Is the montreal cognitive assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? Stroke, 42(6), 1712-6. [DOI:10.1161/STROKEAHA.110.606277] [PMID]
- Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., et al. (2004). Movement disorder society task force report on the hoehn and yahr staging scale: Status and recommendations the movement disorder society task force on rating scales for Parkinson’s disease. Movement Disorders, 19(9), 1020-8. [DOI:10.1002/mds.20213] [PMID]
- Herman, T., Weiss, A., Brozgol, M., Giladi, N., & Hausdorff, J. M. (2014). Gait and balance in Parkinson’s disease subtypes: Objective measures and classification considerations. Journal of Neurology, 261(12), 2401-10. [DOI:10.1007/s00415-014-7513-6] [PMID]
- Hughes, A. J., Daniel, S. E., Kilford, L., & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry, 55(3), 181-4. [DOI:10.1136/jnnp.55.3.181] [PMID] [PMCID]
- Kader, M., Iwarsson, S., Odin, P., & Nilsson, M. H. (2016). Fall-related activity avoidance in relation to a history of falls or near falls, fear of falling and disease severity in people with Parkinson’s disease. BMC Neurology, 16, 84. [DOI:10.1186/s12883-016-0612-5] [PMID] [PMCID]
- Khajavi, D. (2013). Validation and reliability of Persian version of fall Efficacy Scale-International (FES-I) in community-dwelling older adults. Iranian Journal of Ageing, 8(2), 39-47.
- Landers, M. R., Lopker, M., Newman, M., Gourlie, R., Sorensen, S., & Vong, R. (2017). A cross-sectional analysis of the characteristics of individuals with Parkinson disease who avoid activities and participation due to fear of falling. Journal of Neurologic Physical Therapy, 41(1), 31-42. [DOI:10.1097/NPT.0000000000000162] [PMID]
- Lindholm, B., Hagell, P., Hansson, O., & Nilsson, M. H. (2014). Factors associated with fear of falling in people with Parkinson’s disease. BMC neurology, 14, 19. [DOI:10.1186/1471-2377-14-19] [PMID] [PMCID]
- Mak, M. K., & Pang, M. Y. (2009). Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: A 1-year prospective study. Journal of Neurology, 256(10), 1689-95. [DOI:10.1007/s00415-009-5184-5] [PMID]
- Morris, M., Iansek, R., & Churchyard, A. (1998). The role of the physiotherapist in quantifying movement fluctuations in Parkinson’s disease. Australian Journal of Physiotherapy, 44(2), 105109-14. [DOI:10.1016/S0004-9514(14)60370-6]
- Morris, S., Morris, M. E., & Iansek, R. (2001). Reliability of measurements obtained with the timed “Up & Go” test in people with parkinson disease. Physical Therapy, 81(2), 810-8. [DOI:10.1093/ptj/81.2.810] [PMID]
- Rodríguez-Molinero, A., Samà, A., Pérez-López, C., Rodríguez-Martín, D., Alcaine, S., Mestre, B., et al. (2017). Analysis of correlation between an accelerometer-based algorithm for detecting parkinsonian gait and UPDRS subscales. Frontiers in Nurology, 8, 431. [DOI:10.3389/fneur.2017.00431] [PMID] [PMCID]
- Rosqvist, K., Hagell, P., Odin, P., Ekström, H., Iwarsson, S., & Nilsson, M. (2017). Factors associated with life satisfaction in parkinson’s disease. Acta Neurologica Scandinavica, 136(1), 64-71. [DOI:10.1111/ane.12695] [PMID]
- Schneider, J. C., Qu, H. D., Lowry, J., Walker, J., Vitale, E., & Zona, M. (2012). Efficacy of inpatient burn rehabilitation: A prospective pilot study examining range of motion, hand function and balance. Burns, 38(2), 164-71. [DOI:10.1016/j.burns.2011.11.002] [PMID]
- Shulman, L. M., Armstrong, M., Ellis, T., Gruber‐Baldini, A., Horak, F., Nieuwboer, A., et al. (2016). Disability rating scales in Parkinson’s disease: Critique and recommendations. Movement Disorders, 31(10), 1455-65. [DOI:10.1002/mds.26649] [PMID]
- Xie, F., Li, S. C., Roos, E. M., Fong, K. Y., Lo, N. N., Yeo, S. J., et al. (2006). Cross-cultural adaptation and validation of Singapore English and Chinese versions of the Knee injury and Osteoarthritis Outcome Score (KOOS) in Asians with knee osteoarthritis in Singapore. Osteoarthritis and Cartilage, 14(11), 1098-103. [DOI:10.1016/j.joca.2006.05.005] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2018/04/30 | Accepted: 2018/11/5 | Published: 2019/07/1
Received: 2018/04/30 | Accepted: 2018/11/5 | Published: 2019/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |