Volume 9, Issue 4 (July & August 2018 2018)
BCN 2018, 9(4): 269-274 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shareghi Brojeni M, Salimi M, Mirmohammadsadeghi Z, Haghparast A, Eliassi A. Comparison of Effects of Light Anesthetics, Diethyl Ether and Carbon Dioxide, on Hypothalamic Paraventricular Nucleus D1 and D2 Dopamine Receptors- and Glucosensitive Neurons-Induced Food Intake in Fasted Conscious Rats. BCN 2018; 9 (4) :269-274
URL: http://bcn.iums.ac.ir/article-1-942-en.html
URL: http://bcn.iums.ac.ir/article-1-942-en.html
Masoud Shareghi Brojeni1 

 , Morteza Salimi2
, Morteza Salimi2 
 , Zahra Mirmohammadsadeghi2
, Zahra Mirmohammadsadeghi2 
 , Abbas Haghparast3
, Abbas Haghparast3 
 , Afsaneh Eliassi *2
, Afsaneh Eliassi *2 



 , Morteza Salimi2
, Morteza Salimi2 
 , Zahra Mirmohammadsadeghi2
, Zahra Mirmohammadsadeghi2 
 , Abbas Haghparast3
, Abbas Haghparast3 
 , Afsaneh Eliassi *2
, Afsaneh Eliassi *2 

1- Neurophysiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Physiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Physiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Carbon dioxide, Diethyl ether, Paraventricular Nucleus (PVN), Food intake, Dopamine receptors, Glucosensing neurons
Full-Text [PDF 673 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
In experimental research, inhalation of Carbon Dioxide (CO2) or diethyl ether is used as light anesthetic agents. For example, in our previous studies that we considered the role of the Ventromedial Hypothalamus (VMH) (Eliassi, Nazari, & Naghdi, 2009) and paraventricular (Chaleek, Kermani, Eliassi, & Haghparast, 2012; Kermani & Eliassi, 2012) hypothalamic orexin-1 receptors in the regulation of gastric acid secretion, the ventromedial hypothalamus VMH or PVN drug injections were performed under brief diethyl ether anesthesia.
In addition, Zaringhalam, Tekieh, Manaheji, and Akhtari (2013) considered the cellular events during arthritis-induced hyperalgesia under brief CO2 anesthesia. However, little is known about the effect of light anesthetic agents on experimental results. Van Herck et al. (1991) demonstrated that rat plasma corticosterone and glucose increased after two minutes exposure to diethyl ether anesthesia. Furthermore, Zardooz et al. (2010) showed that a brief exposure to either diethyl ether or CO2 affected the plasma corticosterone, glucose, and insulin levels in fed or fasted rats. These data and others (Tanaka, Nabatame, & Tanifuji, 2005) support that light anesthetic agents affect the experimental data.
Recently, the effects of CO2 on insects and plants behavior have been shown. For example, Majeed, Hill and Ignell (2013) demonstrated that the take-off and source contact behavior of Aedes aegypti (female yellow fever mosquitoes) is impeded at elevated background levels of CO2 as a result of masking of the stimulus signal. Furthermore, saprophagous insects often use CO2 as a cue for finding food (Kojima, 2015) and elevated atmospheric CO2 increases fiber fractions of a mammalian herbivore, Microtus ochrogaster (Habeck & Lindroth, 2013).
To control the homeostatic feeding motivation, a number of neurons project to hypothalamic Paraventricular Nucleus (PVN) (Morton, Cummings, Baskin, Barsh, & Schwartz, 2006; Saper et al. 2002; Schwartz et al., 2000). Dopamine is also considered to be the main catecholamine in the brain and serves an important regulatory role in the control of feeding behavior (Szczypka, Rainey, & Palmiter, 2000; Steele et al., 2010). Dopamine signaling is mediated by five receptors, termed D1-D5 receptors. Administration of D2 receptor agonist decreases plasma leptin levels in an obese woman and increases food intake (Kim, Shin, Kim, Lee, & Baik, 2005).
Furthermore, according to Yu and Kim (2012) study, D4 receptors in PVN may be a pharmacological target for obesity. Recently, we also reported that D1 and D2 Dopamine Receptors (DR) and also Glucosensitive Neurons (GSNs) in the hypothalamic Paraventricular Nucleus (PVN) increased food intake in 18 hours food-deprived rats (data is preparing to be submitted). In our experiments, dopamine agonists, antagonists and glucose were injected into the hypothalamic Paraventricular Nucleus under light diethyl ether anesthesia. However, little is known about the effect of brief diethyl ether or CO2 anesthesia on experimental food intake results. Therefore, in this study, we evaluated whether inhalation of diethyl ether and CO2 as light anesthetic agents is able to affect food intake in conscious rats.
Furthermore, we considered and compared the effect of these two anesthetic agents on PVN D1 and D2 dopamine receptors-induced and glucose-induced food intake in 18 hours food-deprived rats.
2. Methods
2.1. Animals
Male Wistar rats, weighing 220-250 g (Neuroscience Research Center, Tehran, Iran) were housed in 12:12 h light:dark cycle at 22ºC-24ºC. They were deprived of food, but not water, for 18-20 h prior to experiments.
2.2. Drugs
Ketamine (Rotex, Levallois-Perret, France) and xylazine (Alfasan, Woerden, The Netherlands) were used to anaesthetize rats. Quinpirole, SKF38393 and glucose were purchased from Sigma (St Louis, MO, USA).
2.3. Injection of compounds
Drugs or vehicle were injected in a volume of 0.3 µL into the PVN. The drug injections were performed under brief diethyl ether or CO2 anesthesia using a 0.5-µL Hamilton syringe. Animals were exposed to CO2 or diethyl ether inhalation for 30 s and obtained full consciousness after 1 minute.
2.4. Operation
After anesthetizing by ketamine and xylazine, animals were fitted with a 23-gauge stainless steel cannula. Cannula was inserted into right PVN according to the stereotaxic atlas of Paxinos and Watson (2007) as follows: lateral, 0.4 mm from midline; dorsoventral, 7 mm from skull surface; and anteroposterior, 1.8 mm from the bregma. The injector was extended 1 mm beyond the end of the guide cannula. Experimental trials were performed after 7-day recovery period. For histological examination, the brains were fixed in formalin and 100- µM thick sections were taken and examined with light microscopy.
2.5. Measurements of food intake
The weight of food pellets used were measured by a Sartorius scale, TE3135 (Gottingen, Germany), with d=0.001 mg accuracy. Feeding trials normally conducted from Saturday to Wednesday between 9:00 AM and 12:00 PM. On the test day, the fasted rats were transported to the laboratory at least 1 h before the beginning of the feeding trial. After injecting the test compound, the rats were placed in a clear plastic cage and allowed access to a premeasured amount of their regular lab feeding chow. The amount of food and crumbs left in the test cages was measured. Rats received no more than two feeding trials per week. All experiments were approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.33).
2.6. Statistical analysis
Results are presented as the mean±SEM. The differences between two and more than two groups were evaluated by the Student t test and 1-way ANOVA followed by Tukey HSD test, respectively. P<0.05 was considered significant.
3. Results
3.1. Influence of light CO2 and diethyl ether anesthesia on food intake
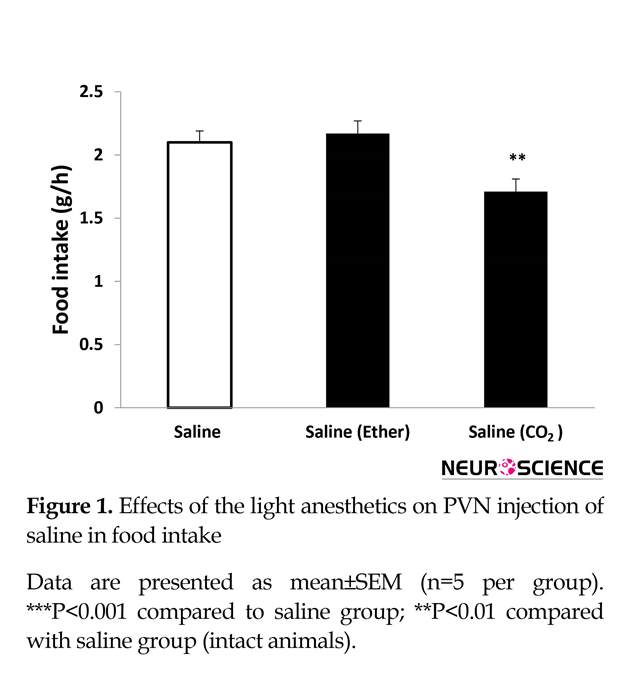
Inhalation of CO2 but not diethyl ether (for 30 s) had a significant effect on food intake. After light CO2 anesthesia, food intake decreased significantly (Figure 1). Our results indicate a reduction of approximately 20% with a value of 1.71±0.1 g/h from light CO2 test group compared to 2.1±0.1 g/h for the control group (n=5) (P<0.01). Furthermore, Figure 1 demonstrates that light diethyl ether anesthesia has no effect on food intake.
3.2. Effects of light CO2 and diethyl ether anesthesia on glucose-induced food intake
Our study showed that the PVN injection of glucose induced dose-dependent increase of gastric acid secretion and glucose 0.8 µg had maximum stimulatory effect (Chaleek, et al, 2012). Acid secretion is a part of feeding behavior. As shown in Figure 2, in light CO2 test groups, glucose (0.8
In experimental research, inhalation of Carbon Dioxide (CO2) or diethyl ether is used as light anesthetic agents. For example, in our previous studies that we considered the role of the Ventromedial Hypothalamus (VMH) (Eliassi, Nazari, & Naghdi, 2009) and paraventricular (Chaleek, Kermani, Eliassi, & Haghparast, 2012; Kermani & Eliassi, 2012) hypothalamic orexin-1 receptors in the regulation of gastric acid secretion, the ventromedial hypothalamus VMH or PVN drug injections were performed under brief diethyl ether anesthesia.
In addition, Zaringhalam, Tekieh, Manaheji, and Akhtari (2013) considered the cellular events during arthritis-induced hyperalgesia under brief CO2 anesthesia. However, little is known about the effect of light anesthetic agents on experimental results. Van Herck et al. (1991) demonstrated that rat plasma corticosterone and glucose increased after two minutes exposure to diethyl ether anesthesia. Furthermore, Zardooz et al. (2010) showed that a brief exposure to either diethyl ether or CO2 affected the plasma corticosterone, glucose, and insulin levels in fed or fasted rats. These data and others (Tanaka, Nabatame, & Tanifuji, 2005) support that light anesthetic agents affect the experimental data.
Recently, the effects of CO2 on insects and plants behavior have been shown. For example, Majeed, Hill and Ignell (2013) demonstrated that the take-off and source contact behavior of Aedes aegypti (female yellow fever mosquitoes) is impeded at elevated background levels of CO2 as a result of masking of the stimulus signal. Furthermore, saprophagous insects often use CO2 as a cue for finding food (Kojima, 2015) and elevated atmospheric CO2 increases fiber fractions of a mammalian herbivore, Microtus ochrogaster (Habeck & Lindroth, 2013).
To control the homeostatic feeding motivation, a number of neurons project to hypothalamic Paraventricular Nucleus (PVN) (Morton, Cummings, Baskin, Barsh, & Schwartz, 2006; Saper et al. 2002; Schwartz et al., 2000). Dopamine is also considered to be the main catecholamine in the brain and serves an important regulatory role in the control of feeding behavior (Szczypka, Rainey, & Palmiter, 2000; Steele et al., 2010). Dopamine signaling is mediated by five receptors, termed D1-D5 receptors. Administration of D2 receptor agonist decreases plasma leptin levels in an obese woman and increases food intake (Kim, Shin, Kim, Lee, & Baik, 2005).
Furthermore, according to Yu and Kim (2012) study, D4 receptors in PVN may be a pharmacological target for obesity. Recently, we also reported that D1 and D2 Dopamine Receptors (DR) and also Glucosensitive Neurons (GSNs) in the hypothalamic Paraventricular Nucleus (PVN) increased food intake in 18 hours food-deprived rats (data is preparing to be submitted). In our experiments, dopamine agonists, antagonists and glucose were injected into the hypothalamic Paraventricular Nucleus under light diethyl ether anesthesia. However, little is known about the effect of brief diethyl ether or CO2 anesthesia on experimental food intake results. Therefore, in this study, we evaluated whether inhalation of diethyl ether and CO2 as light anesthetic agents is able to affect food intake in conscious rats.
Furthermore, we considered and compared the effect of these two anesthetic agents on PVN D1 and D2 dopamine receptors-induced and glucose-induced food intake in 18 hours food-deprived rats.
2. Methods
2.1. Animals
Male Wistar rats, weighing 220-250 g (Neuroscience Research Center, Tehran, Iran) were housed in 12:12 h light:dark cycle at 22ºC-24ºC. They were deprived of food, but not water, for 18-20 h prior to experiments.
2.2. Drugs
Ketamine (Rotex, Levallois-Perret, France) and xylazine (Alfasan, Woerden, The Netherlands) were used to anaesthetize rats. Quinpirole, SKF38393 and glucose were purchased from Sigma (St Louis, MO, USA).
2.3. Injection of compounds
Drugs or vehicle were injected in a volume of 0.3 µL into the PVN. The drug injections were performed under brief diethyl ether or CO2 anesthesia using a 0.5-µL Hamilton syringe. Animals were exposed to CO2 or diethyl ether inhalation for 30 s and obtained full consciousness after 1 minute.
2.4. Operation
After anesthetizing by ketamine and xylazine, animals were fitted with a 23-gauge stainless steel cannula. Cannula was inserted into right PVN according to the stereotaxic atlas of Paxinos and Watson (2007) as follows: lateral, 0.4 mm from midline; dorsoventral, 7 mm from skull surface; and anteroposterior, 1.8 mm from the bregma. The injector was extended 1 mm beyond the end of the guide cannula. Experimental trials were performed after 7-day recovery period. For histological examination, the brains were fixed in formalin and 100- µM thick sections were taken and examined with light microscopy.
2.5. Measurements of food intake
The weight of food pellets used were measured by a Sartorius scale, TE3135 (Gottingen, Germany), with d=0.001 mg accuracy. Feeding trials normally conducted from Saturday to Wednesday between 9:00 AM and 12:00 PM. On the test day, the fasted rats were transported to the laboratory at least 1 h before the beginning of the feeding trial. After injecting the test compound, the rats were placed in a clear plastic cage and allowed access to a premeasured amount of their regular lab feeding chow. The amount of food and crumbs left in the test cages was measured. Rats received no more than two feeding trials per week. All experiments were approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.33).
2.6. Statistical analysis
Results are presented as the mean±SEM. The differences between two and more than two groups were evaluated by the Student t test and 1-way ANOVA followed by Tukey HSD test, respectively. P<0.05 was considered significant.
3. Results
3.1. Influence of light CO2 and diethyl ether anesthesia on food intake
Inhalation of CO2 but not diethyl ether (for 30 s) had a significant effect on food intake. After light CO2 anesthesia, food intake decreased significantly (Figure 1). Our results indicate a reduction of approximately 20% with a value of 1.71±0.1 g/h from light CO2 test group compared to 2.1±0.1 g/h for the control group (n=5) (P<0.01). Furthermore, Figure 1 demonstrates that light diethyl ether anesthesia has no effect on food intake.
3.2. Effects of light CO2 and diethyl ether anesthesia on glucose-induced food intake
Our study showed that the PVN injection of glucose induced dose-dependent increase of gastric acid secretion and glucose 0.8 µg had maximum stimulatory effect (Chaleek, et al, 2012). Acid secretion is a part of feeding behavior. As shown in Figure 2, in light CO2 test groups, glucose (0.8
µg) did not affect food intake compared to the control rats (animals received saline without anesthetic agents).
In diethyl ether group, food intake increased from 2.1 g/h in saline group to 2.6±0.08 g/h in glucose 0.8 µg-treated rats (n=5) (P<0.01). In the absence of anesthetic agents, however, the magnitude of glucose-induced food intake was approximately 2-fold more, compared to the control group (Figure 2).
3.3. Effects of light CO2 and diethyl ether anesthesia on SKF3833 (D1 receptor agonist)-induced and quinpirole (D2 receptor agonist)-induced food intake
In our previous study, we showed that PVN-microinjection of SKF38393 and quinpirole increased food intake in a dose-dependent manner and the maximum effects were observed at doses of 3 and 5 µg, respectively (data is preparing to be submitted). PVN injection of SKF38393 (P<0.001) or quinpirole (P<0.01) decreased food intake after light CO2 anesthesia (Figures 3 and 4). Compared to CO2 group, light diethyl ether anesthesia had reverse effect on D1 and D2 receptors-induced food intake. As shown in Figures 3 and 4, PVN microinjection of SKF38393 (5 µg) and quinpirole (0.3 µg) increased food intake compared to saline group (P<0.001 and P<0.0001, respectively).
4. Discussion
In this study, we demonstrated that brief inhalation of CO2, but not diethyl ether, as light anesthetic agents, decrease food intake compared to saline-treated rats. Furthermore, in the current study, we found that D1 and D2 dopamine receptors-induced food intake decreases under light CO2 anesthesia. However, despite the negative effect of CO2 on D1 and D2-induced food intake, we observed D1 and D2 agonist increased feeding behavior under brief diethyl ether anesthesia. Our result has also shown that glucose-stimulated food intake has remained at high level under light diethyl ether anesthesia similar to intact animals (without light anesthetic agents). However, this effect was not observed in CO2 group with the same drug condition.
The hypothalamic Paraventricular Nucleus (PVN) receives a number of central pathways to control the eating behavior (Blouet & Schwartz, 2010; Morton et al., 2006; Schwartz et al., 2000). These studies have demonstrated that the neuropeptides and neurotransmitters are involved in these phenomena. For example, anorectic agent induces its effects through the cerebral release of
In diethyl ether group, food intake increased from 2.1 g/h in saline group to 2.6±0.08 g/h in glucose 0.8 µg-treated rats (n=5) (P<0.01). In the absence of anesthetic agents, however, the magnitude of glucose-induced food intake was approximately 2-fold more, compared to the control group (Figure 2).
3.3. Effects of light CO2 and diethyl ether anesthesia on SKF3833 (D1 receptor agonist)-induced and quinpirole (D2 receptor agonist)-induced food intake
In our previous study, we showed that PVN-microinjection of SKF38393 and quinpirole increased food intake in a dose-dependent manner and the maximum effects were observed at doses of 3 and 5 µg, respectively (data is preparing to be submitted). PVN injection of SKF38393 (P<0.001) or quinpirole (P<0.01) decreased food intake after light CO2 anesthesia (Figures 3 and 4). Compared to CO2 group, light diethyl ether anesthesia had reverse effect on D1 and D2 receptors-induced food intake. As shown in Figures 3 and 4, PVN microinjection of SKF38393 (5 µg) and quinpirole (0.3 µg) increased food intake compared to saline group (P<0.001 and P<0.0001, respectively).
4. Discussion
In this study, we demonstrated that brief inhalation of CO2, but not diethyl ether, as light anesthetic agents, decrease food intake compared to saline-treated rats. Furthermore, in the current study, we found that D1 and D2 dopamine receptors-induced food intake decreases under light CO2 anesthesia. However, despite the negative effect of CO2 on D1 and D2-induced food intake, we observed D1 and D2 agonist increased feeding behavior under brief diethyl ether anesthesia. Our result has also shown that glucose-stimulated food intake has remained at high level under light diethyl ether anesthesia similar to intact animals (without light anesthetic agents). However, this effect was not observed in CO2 group with the same drug condition.
The hypothalamic Paraventricular Nucleus (PVN) receives a number of central pathways to control the eating behavior (Blouet & Schwartz, 2010; Morton et al., 2006; Schwartz et al., 2000). These studies have demonstrated that the neuropeptides and neurotransmitters are involved in these phenomena. For example, anorectic agent induces its effects through the cerebral release of
dopamine, and the consequent activation of D1-like and D2-like receptors (Leibowitz, 1975; Chen et al., 2001; Kuo, 2002; Kuo, 2003), decreasing the level of hypothalamic Neuropeptide Y (NPY) (Hsie, Yang, & Kuo, 2005; Kuo, 2005). Furthermore, we have shown that PVN-microinjected SKF38393 (a dopamine D1 agonist) and quinpirole (a dopamine D2 agonist) increased food intake at doses more than 0.07 µg. These effects were inhibited by D1 and D2 dopamine receptor antagonists, SCH23390 and sulpiride, respectively (data are preparing to be submitted).
Within the hypothalamus, glucosensitive neurons are found in the arcuate and paraventricular nuclei (Silver & Erecinska, 1998). Our results showed that the PVN-microinjected glucose increased gastric acid secretion at doses of 350-750 nM in 18-24 h fasted conscious rats (Chaleek, et al, 2012). Gastric acid secretion is a part of feeding behavior. Therefore, we suggest that the PVN-glucose sensing neurons might be involved in central regulatory mechanism of acid secretion and the control of energy homeostasis.
All our experiments were done under brief diethyl ether anesthesia. Although it is well established that PVN D1 and D2 dopamine receptors and glucosensing neurons are involved in regulatory mechanisms of feeding behavior, the specific effects of inhalation of light anesthetic agents including CO2 and diethyl ether during experimental approaches have remained unexplored. Our results show that glucose and D1 and D2 agonists increase food intake under brief diethyl ether anesthesia whereas light CO2 inhalation inhibits the effect of glucose and changes the stimulatory effects of D1 and D2 agonists to inhibitory effects in feeding behavior. At the present time, we do not know the exact CO2 and diethyl ether mechanisms on food intake.
Probably the food intake decreases under brief anesthesia as a result of masking of the stimulus signal. For example, PVN and lateral hypothalamus received NPY-containing neuron projections from arcuate nucleus. It has been shown that NPY increases food intake by activating NPY1 and NPY5 receptors within the hypothalamus (Levens & Della-Zuana, 2003; Mashiko et al., 2006). Furthermore, our previous studies indicate that the orexin-A-induced gastric acid secretion in PVN (Chaleek, et al, 2012) is blocked by Intracerebroventricular (ICV) administration of NPY1- and NPY5-receptor antagonists (Kermani & Eliassi, 2012).
Gastric acid secretion is a part of feeding behavior. Meguid et al. (2000) demonstrated that afferent information from the autonomic nervous system affects gastrointestinal mediators, and circulatory concentrations of nutrients and hormones are transmitted to the presynaptic monoaminergic system of the hypothalamus. These presynaptic afferent neurons influence postsynaptic cells by releasing dopamine. According to their model, postsynaptic neurons may express both D1 and D2 receptors which are involved in food intake by activation of stimulatory and inhibitory food intake neuropeptides, including NPY. Therefore, we suggest that masking of the first or second order hypothalamic neurons in response to the light anesthetic agents may be one mechanism by which food intake decreases.
In conclusion, the present study demonstrates that light CO2 but not diethyl ether anesthetics decreases food intake. Our results suggest that dopamine receptors and glucosensing neurons in PVN may be, at least in part, one of targets of light anesthetic agents. Whether these effects result from masking of inhibitory or stimulatory neurons which originate in the PVN or is mediated by fibers of passage is yet to be determined. The current study also suggests that feeding experimental results may be affected by any experimental approach using these anesthetics
Ethical Considerations
Compliance with ethical guidelines
All experiments were approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.33).
Funding
This work was supported by a grant from the Neurophysiology Research Center of Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors confirm that there is no conflict of interest.
References
Within the hypothalamus, glucosensitive neurons are found in the arcuate and paraventricular nuclei (Silver & Erecinska, 1998). Our results showed that the PVN-microinjected glucose increased gastric acid secretion at doses of 350-750 nM in 18-24 h fasted conscious rats (Chaleek, et al, 2012). Gastric acid secretion is a part of feeding behavior. Therefore, we suggest that the PVN-glucose sensing neurons might be involved in central regulatory mechanism of acid secretion and the control of energy homeostasis.
All our experiments were done under brief diethyl ether anesthesia. Although it is well established that PVN D1 and D2 dopamine receptors and glucosensing neurons are involved in regulatory mechanisms of feeding behavior, the specific effects of inhalation of light anesthetic agents including CO2 and diethyl ether during experimental approaches have remained unexplored. Our results show that glucose and D1 and D2 agonists increase food intake under brief diethyl ether anesthesia whereas light CO2 inhalation inhibits the effect of glucose and changes the stimulatory effects of D1 and D2 agonists to inhibitory effects in feeding behavior. At the present time, we do not know the exact CO2 and diethyl ether mechanisms on food intake.
Probably the food intake decreases under brief anesthesia as a result of masking of the stimulus signal. For example, PVN and lateral hypothalamus received NPY-containing neuron projections from arcuate nucleus. It has been shown that NPY increases food intake by activating NPY1 and NPY5 receptors within the hypothalamus (Levens & Della-Zuana, 2003; Mashiko et al., 2006). Furthermore, our previous studies indicate that the orexin-A-induced gastric acid secretion in PVN (Chaleek, et al, 2012) is blocked by Intracerebroventricular (ICV) administration of NPY1- and NPY5-receptor antagonists (Kermani & Eliassi, 2012).
Gastric acid secretion is a part of feeding behavior. Meguid et al. (2000) demonstrated that afferent information from the autonomic nervous system affects gastrointestinal mediators, and circulatory concentrations of nutrients and hormones are transmitted to the presynaptic monoaminergic system of the hypothalamus. These presynaptic afferent neurons influence postsynaptic cells by releasing dopamine. According to their model, postsynaptic neurons may express both D1 and D2 receptors which are involved in food intake by activation of stimulatory and inhibitory food intake neuropeptides, including NPY. Therefore, we suggest that masking of the first or second order hypothalamic neurons in response to the light anesthetic agents may be one mechanism by which food intake decreases.
In conclusion, the present study demonstrates that light CO2 but not diethyl ether anesthetics decreases food intake. Our results suggest that dopamine receptors and glucosensing neurons in PVN may be, at least in part, one of targets of light anesthetic agents. Whether these effects result from masking of inhibitory or stimulatory neurons which originate in the PVN or is mediated by fibers of passage is yet to be determined. The current study also suggests that feeding experimental results may be affected by any experimental approach using these anesthetics
Ethical Considerations
Compliance with ethical guidelines
All experiments were approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNS.REC.1396.33).
Funding
This work was supported by a grant from the Neurophysiology Research Center of Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors confirm that there is no conflict of interest.
References
- Blouet, C., & Schwartz, G. J. (2010). Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural Brain Research, 209(1), 1–12. [DOI:10.1016/j.bbr.2009.12.024] [PMID]
- Chaleek, N., Kermani, M., Eliassi, A., & Haghparast, A. (2012). Effects of orexin and glucose microinjected into the hypothalamic Paraventricular Nucleus on gastric acid secretion in conscious rats. Neurogastroenterology & Motility, 24(2), e94–e102. [DOI:10.1111/j.1365-2982.2011.01789.x] [PMID]
- Chen, T. Y., Duh, S. L., Huang, C. C., Lin, T. B., & Kuo, D. Y. (2001). Evidence for the involvement of dopamine D1 and D2 receptors in mediating the decrease of food intake during repeated treatment with amphetamine. Journal of Biomedical Science, 8(6), 462-6. [DOI:10.1007/BF02256608] [PMID]
- Eliassi, A., Nazari, M., & Naghdi N. (2009). Role of the ventromedial hypothalamic orexin-1 receptors in regulation of gastric acid secretion in conscious rats. Journal of Neuroendocrinology, 21(3), 177-82. [DOI:10.1111/j.1365-2826.2009.01824.x] [PMID]
- Habeck, C. W., & Lindroth, R. L. (2013). Influence of global atmospheric change on the feeding behavior and growth performance of a mammalian herbivore, microtus ochrogaster. PLOS ONE, 8(8), e72717. [PMCID] [PMID] [DOI:10.1371/journal.pone.0072717]
- Hsieh, Y. S., Yang, S. F., & Kuo, D. Y. (2005). Amphetamine, an appetite suppressant, decreases neuropeptide Y immunoreactivity in rat hypothalamic paraventriculum. Regulatory Peptides, 127(1–3), 169–76. [DOI:10.1016/j.regpep.2004.11.007] [PMID]
- Kermani, M., & Eliassi, A. (2012). Gastric acid secretion induced by Paraventricular Nucleus microinjection of orexin A is mediated through activation of neuropeptide yergic system. Neuroscience, 226, 81–8. [DOI:10.1016/j.neuroscience.2012.08.052] [PMID]
- Kim, R. Y., Shin, S. W., Kim, B. J., Lee, W., & Baik, J. H. (2005). Dynamic regulation of hypothalamic neuropeptide gene expression and food intake by melanocortin analogues and reversal with melanocortin-4 receptor antagonist. Biochemical and Biophysical Research Communications, 329(4), 1178-85. [DOI:10.1016/j.bbrc.2005.06.023] [PMID]
- Kojima, W. (2015). Attraction to carbon dioxide from feeding resources and conspecific neighbours in larvae of the rhinoceros beetle Trypoxylus dichotomus. PLOS ONE, 10(11), e0141733. [DOI:10.1371/journal.pone.0141733]
- Kuo, D. Y. (2002). Co-administration of dopamine D1 and D2 agonists additively decreases daily food intake, body weight and hypothalamic neuropeptide Y level in rats. Journal of Biomedical Science, 9(2), 126–32. [DOI:10.1159/000048208] [PMID]
- Kuo, D. Y. (2003). Further evidence for the mediation of both subtypes of dopamine D1/D2 receptors and cerebral Neuropeptide Y (NPY) in amphetamine-induced appetite suppression. Behavioural Brain Research, 147(1-2), 149–55. [DOI:10.1016/j.bbr.2003.04.001]
- Kuo, D. Y. (2005). Involvement of hypothalamic neuropeptide Y in regulating the amphetamine induced appetite suppression in streptozotocin diabetic rats. Regulatory Peptides, 127(1-3), 19–26. [DOI:10.1016/j.regpep.2004.10.008] [PMID]
- Leibowitz, S. (1975). Amphetamine: possible site and mode of action for producing anorexia in the rat. Brain Research, 84(1), 160–7. [DOI:10.1016/0006-8993(75)90811-2]
- Levens, N. R, & Della-Zuana, O. (2003). Neuropeptide Y Y5 receptor antagonists as anti-obesity drugs. Current Opinion in Investigational Drugs, 4(10), 1198–204. [PMID]
- Majeed, S., Hill, S. R., & Ignell, R. (2013). Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. Journal of Experimental Biology, 217(4), 598–604. [DOI:10.1242/jeb.092718] [PMID]
- Mashiko, S., Ishihara, A., Iwaasa, H., Sano, H., Ito, J., Gomori, A., et al. (2006). A pair-feeding study reveals that a Y5 antagonist causes weight loss in diet-induced obese mice by modulating food intake and energy expenditure. Molecular Pharmacology, 71(2), 602–8. [DOI:10.1124/mol.106.029991] [PMID]
- Meguid, M. M., Fetissov, S. O., Varma, M., Sato, T., Zhang, L., Laviano, A., et al. (2000). Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition, 16(10), 843– 57. [DOI:10.1016/S0899-9007(00)00449-4]
- Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S., & Schwartz, M. W. (2006). Central nervous system control of food intake and body weight. Nature. 443(7109), 289-95. [DOI:10.1038/nature05026] [PMID]
- Paxinos, G., & Watson, C. H. (2007). The rat brain in stereotaxic coordinates. New York: Academic Press. [PMCID]
- Saper, C. B., Chou, T. C., & Elmquist, J. K. (2002). The need to feed: Homeostatic and hedonic control of eating. Neuron, 36(2), 199-211. [DOI:10.1016/S0896-6273(02)00969-8]
- Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J., & Baskin, D. G. (2000). Central nervous system control of food intake. Nature, 404(6778), 661-71. [DOI:10.1038/35007534] [PMID]
- Silver, I. A., & Erecinska, M. (1998). Glucose-induced intracellular ion changes in sugar sensitive hypothalamic neurons. Journal of Neurophysiology, 79(4), 1733–45. [DOI:10.1152/jn.1998.79.4.1733] [PMID]
- Steele, K. E., Prokopowicz, G. P., Schweitzer, M. A., Magunsuon, T. H., Lidor, A. O., Kuwabawa, H., et al. (2010). Alterations of central dopamine receptors before and after gastric bypass surgery. Obesity Surgery, 20(3), 369-74. [DOI:10.1007/s11695-009-0015-4] [PMID]
- Tanaka, T., Nabatam, H., & Tanifuji, Y.(2005). Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. Journal of Anesthesia, 19(4), 277-28. [DOI:10.1007/s00540-005-0341-1] [PMID]
- Van Herck, H., Baumans, V., De Boer, S. F., Van Der Gugten, J., Van Woerkom, A. B., & Beynen, A. C. (1991). Endocrine stress response in rats subjected to singular orbital puncture while under diethyl-ether anaesthesia. Laboratory Animals, 25(4), 325-9. [DOI:10.1258/002367791780809931] [PMID]
- Yu, J. H., & Kim, M. S. (2012). Molecular mechanisms of appetite regulation. Diabetes & Metabolism Journal, 36(6), 391-8. [DOI:10.4093/dmj.2012.36.6.391] [PMID] [PMCID]
- Zardooz, H., Rostamkhani, F., Zaringhalam, J., & Shahrivar, F. F. (2010). Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiological Research, 59(6), 973-8. [PMID]
- Zaringhalam, J., Tekieh E., Manaheji, H., & Akhtari Z. (2013). Cellular events during arthritis-induced hyperalgesia are mediated by interlukin-6 and p38 MAPK and their effects on the expression of spinal mu-opioid receptors. Rheumatology International, 33(9), 2291-9. [DOI:10.1007/s00296-013-2715-2] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2017/04/17 | Accepted: 2017/07/30 | Published: 2018/07/1
Received: 2017/04/17 | Accepted: 2017/07/30 | Published: 2018/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





.jpg)


