Volume 8, Issue 5 (September & October 2017)
BCN 2017, 8(5): 419-427 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sarraf Razavi M, Tehranidoost M, Ghassemi F, Purabassi P, Taymourtash A. Emotional Face Recognition in Children With Attention Deficit/Hyperactivity Disorder: Evidence From Event Related Gamma Oscillation. BCN 2017; 8 (5) :419-427
URL: http://bcn.iums.ac.ir/article-1-938-en.html
URL: http://bcn.iums.ac.ir/article-1-938-en.html
Mahdiyeh Sarraf Razavi1 
 , Mehdi Tehranidoost1
, Mehdi Tehranidoost1 
 , Farnaz Ghassemi *2
, Farnaz Ghassemi *2 
 , Parivash Purabassi2
, Parivash Purabassi2 
 , Athena Taymourtash2
, Athena Taymourtash2 


 , Mehdi Tehranidoost1
, Mehdi Tehranidoost1 
 , Farnaz Ghassemi *2
, Farnaz Ghassemi *2 
 , Parivash Purabassi2
, Parivash Purabassi2 
 , Athena Taymourtash2
, Athena Taymourtash2 

1- Department of Neurosciences and Addiction Studies, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
2- Department of Biomedical Engineering, Amirkabir University of Technology, Tehran, Iran.
Keywords: Emotional face recognition, Event-Related Oscillation (ERO), Gamma band activity, Attention Deficit Hyperactivity Disorder (ADHD)
Full-Text [PDF 688 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
ADHD is a common neurodevelopmental disorder characterized by inattentiveness and hyperactivity/impulsivity (American Psychiatric Association, 2013). Individuals with ADHD also show problems in social and emotional functions, including the effective assessment of the emotional state of others. It is important to set the adaptive behavior of human facial expressions in social interactions (Cadesky, Mota, & Schachar, 2000; Corbett & Glidden, 2000). Based on the evidence, frontotem-poral-posterior and fronto striatal cerebellar systems are involved in emotional functions. These regions may contribute to impairments of emotional recognition in ADHD (Corbett & Glidden, 2000; Dickstein, Bannon, Xavier Castellanos, & Milham, 2006; Durston, Van Belle, & De Zeeuw, 2011).
However, neural studies to investigate these impairments are rare. Results of recent studies revealed that gamma band activity (GBA) in electroencephalography (EEG) as well as Event-Related Potentials (ERP) play an important role in evaluation of higher cognitive processes such as attention, memory, language, and emotion (Fell et al., 2001; Keil et al., 2001; Sebastiani, Simoni, Gemignani, Ghelarducci, & Santarcange-lo, 2005; Matsumoto, Ichikawa, Kanayama, Ohira, & Iidaka, 2006; Martini et al., 2012). Several studies report that the gamma band activity at 40 Hz oscillation is a correlate of selective attention (Lutzenberger, Pulvermüller, Elbert, & Birbaumer, 1995; Keil et al., 2001; Keil, Stolarova, Moratti, & Ray, 2007; Martini et al., 2012).
Also results of studies indicate that an increase in gamma band activity at 40 Hz frequency is associated with the tuning of novelty processing by negative emotions (Singer, 1995; Garcia-Garcia, Yordanova, Kolev, Domínguez-Borràs, & Escera, 2010). Interestingly, results of Janik, Rezlescu, & Banissy (2015) study indicates that modulating occipital gamma with 40 Hz using transcranial Alternating Current Stimulation (tACS) enhances facial anger perception. Balconi and Pozoli (2009) showed an increased gamma band activity in response to emotions (happiness, sadness, fear, and anger) compared with neutral stimuli. Keil et al., (2007) found an increase in synchrony of very early cortical oscillations at 20–35 Hz aversive visual stimuli.
Müller, Gruber, & Keil (2000) showed an increased gamma band activity (30±50 Hz) for negative valence over the left temporal region as compared to the right one and a lateral shift towards the right hemisphere for positive valence. Martini et al., (2012) found that unpleasant images compared to neutral visual stimuli, elicited an increase of gamma power (30–45 Hz) at 200 ms and 850 ms after stimulus presentation.
Time course and specific topography of affective gamma band activity modulations during cognitive stimuli demonstrate discrimination between early and late processing stages. Basar (2012) reported possibility of 3-4 phase/time-locked gamma responses in 28–45 Hz frequency window during presentation of stimuli. According to their explanation, the early response, starts at 100 ms, in the primary occipital cortex which is probably the direct response over the short pathway via lateral geniculate nucleus and reflects the early visual processing.
According to Keil et al., (2007), the early gamma response could be sensory in origin and sensitive to simple features associated with emotions. Balconi and Pozilli (2009) reported an increasing early gamma band response for emotional stimuli between 150 ms and 250 ms. On the other hand, according to Basar (2012), late processing stage, starting around 300 ms, can be associated with the conscious perception and discrimination of the emotional stimuli. According to Polich (2007) study, time window 300-500 ms onset stimuli is modulated by allocation of attention, initial memory storage, and processing of cognitive tasks.
Martini et al., (2012) found two peaks of weak activation at high gamma frequencies (65–80 Hz) between 200 and 400 ms after stimulus onset; it is interpreted to be less sensitive to image features and more dependent on the conceptual processing of the stimulus identity. Balconi and Lucchiari (2008) reported higher GBA for emotional and neutral stimuli and this increase was more pronounced between 250 and 350 ms. And finally, time window around 400 to 800 ms reflect more strategic high-level processes that require the conscious awareness such as decision making, response criterion, and deep and elaborate processing of emotion (Schupp et al., 2004; Williams et al., 2007). Accordingly, these findings show an important role of gamma oscillations in evaluating early and late stages of cognitive stimuli.
On the other hand, abnormalities in gamma band response and phase synchronization have been shown to be related to various neurological and psychiatric disorders such as schizophre-nia, Alzheimer, and ADHD (Matsumoto et al., 2006; Basar & Guntekin, 2013). Gross et al., (2012) reported reduced gamma oscillations in emotional face perception in Autism Spectrum Disorder. Studies show that ADHD patients have lower absolute and/or relative gamma power in comparison with age-matched healthy controls (Cited in Basar & Guntekin, 2013). Few studies indicate impaired gamma band responses related to cognitive stimuli in ADHD patients compared to healthy controls (Yordanova, Banaschewski, Kolev, Woerner, & Rothenberger, 2001; Lenz et al., 2008; Lenz et al., 2010). Lenz et al., (2010) reported ADHD group (11-17 years old) showed no differentiation between known and unknown stimuli during application of forced-choice–reaction task. However, normal group revealed increased evoked gamma response following familiar stimuli compared to new images.
Based on the mentioned studies, gamma band activity could reflect the characteristics of emotional integration or emotional utilization processes in individuals with ADHD. To our knowledge and according to review articles by Basar and Guntekin (2013) and Güntekin and Başar (2014), our study is the first one that evaluates gamma-band responses with stages of neural activity of emotional face recognition in children with ADHD. We expected that gamma band activity would be diminished for emotional faces in these patients compared to typically developing children during stages of facial emotional recognition. The present study aimed to gain better understanding of the neurobiological basis of children with ADHD in emotional face processing.
2. Methods
2.1. Participants
Nineteen boys, aged between 7 and 11 years (Mean±SD age: 9.21(1.13) years) diagnosed with ADHD were compared with 19 typically developing ones (9.73±1.04 years) matched with age, sex, and years of education. The control group was recruited from elementary schools in Tehran. Children with ADHD were recruited from patients referred to a child and adolescent psychiatric clinic. The individuals with ADHD were diagnosed according to DSM-IV (Statistical Manual of Mental Disorders, Fourth edition) (American Psychiatric Association, 2013). The patients were diagnosed as combined type and were drug naive.
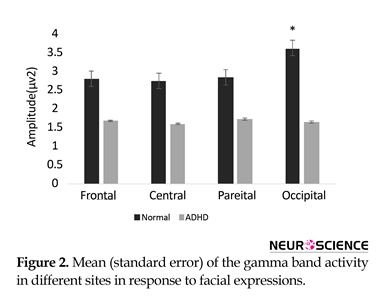
Conners’ Parent Rating Scale-Revised (CPRS-R, short version) was administered to the participants to confirm the diagnosis of ADHD and determine severity of their symptoms. If the T-scores of CPRS-R subscales were above 65, the participant was excluded from the normal group. The two groups were right-handed, had corrected to normal visual acuity. Intelligence Quotient (IQ) of all participants were evaluated according to the WISC-R IQ test (ADHD group: 106±4.36, Control group: 122±10.71) (Table 1).
2.2. Task and stimuli
A compilation of 6 Caucasian faces (3 females and 3 males) in JPG format expressing happy, angry, sad, and neutral expressions were collected from Cohn Kanade AU-coded Facial Expressions Database (Kanade, Cohn, & Tian, 2000). Luminance and contrast of all images were equivalent across stimuli using Photoshop (version 7). The photos were in black and white and positioned within a rectangular frame (261×365 pixel array). The pictures were presented for 2000 ms at the center of the monitor screen and instantly replaced by a white fixation point in the light gray background (1024×768 pixels). The inter stimulus interval (ISI) was 1400±100 ms. The task was designed using Eevoke software (version 3.1).
The main task included 1 practice and 5 experimental blocks. Each experimental block comprised with 48 trials; 4 emotions (anger, happiness, sadness, and neutral) of each faces (3 female and 3 males) that repeated two times. Thus, there were 60 repeats of each expression in a random way. We defined four buttons for each facial expressions procedure (anger, happiness, sadness, and neutral) on a joy stick.
All participants were invited to the laboratory of EEG recording. The parents completed a consent form before starting the examination. During the EEG session, participants were seated in a comfortable chair in a dimly lit room 60 cm from a 17-inch LG computer screen. The participants were asked to look at the center of the screen during the recordings, if possible without making eye movements, and to blink only during the intervals. To ensure that they attend to stimuli, they were monitored by camera in another control room during task performance. All children were instructed to press on the button for each expression when they recognized the target stimuli during each trial (Stimuli presentation until the end of fixation).
2.3. Electrophysiological recording and analysis
Continuous EEG signals were recorded by 64 Ag/AgCl electrodes mounted in an electrode cap (Waveguard, ANT, Netherlands) according to the international 10-20 standard and additional intermediate positions in neuropsychology laboratory of Payam-e Noor University. ASA 4.7.1 software was used for data acquisition. Electrode impedances were maintained below 10 kΩ. The sampling rate was 512 Hz. A 50-Hz notch filter of the recording system, eliminated the line noise during signal acquisition. Event-related oscillations data were analyzed offline using MATLAB R2013a software.
Raw data were filtered with a band-pass filter of 0.1 to 80 Hz and referenced to the mastoids average. The eye movement artifacts were canceled using the independent component analysis. In addition, the remaining artifacts with deflection amplitudes of ±100 μV from the baseline were eliminated (primarily through automatic artifact reduction). Artifact-free EEG recordings were then segmented into epochs ranging from 200 ms prestimulus to 800 ms poststimulus. Each channel baseline epoch was corrected by prestimulus average voltage subtraction. Several studies reported that the lower frequencies of Gamma band oscillation (about 40 Hz), modulated with emotional stimuli indicated attentional processing and discrimination of the emotional stimuli (Keil et al., 2001; Keil et al., 2007; Martini et al., 2012; Herrmann, Munk, & Engel, 2004).
To investigate facial emotion processing based on gamma oscillations, we constructed a new time series
ADHD is a common neurodevelopmental disorder characterized by inattentiveness and hyperactivity/impulsivity (American Psychiatric Association, 2013). Individuals with ADHD also show problems in social and emotional functions, including the effective assessment of the emotional state of others. It is important to set the adaptive behavior of human facial expressions in social interactions (Cadesky, Mota, & Schachar, 2000; Corbett & Glidden, 2000). Based on the evidence, frontotem-poral-posterior and fronto striatal cerebellar systems are involved in emotional functions. These regions may contribute to impairments of emotional recognition in ADHD (Corbett & Glidden, 2000; Dickstein, Bannon, Xavier Castellanos, & Milham, 2006; Durston, Van Belle, & De Zeeuw, 2011).
However, neural studies to investigate these impairments are rare. Results of recent studies revealed that gamma band activity (GBA) in electroencephalography (EEG) as well as Event-Related Potentials (ERP) play an important role in evaluation of higher cognitive processes such as attention, memory, language, and emotion (Fell et al., 2001; Keil et al., 2001; Sebastiani, Simoni, Gemignani, Ghelarducci, & Santarcange-lo, 2005; Matsumoto, Ichikawa, Kanayama, Ohira, & Iidaka, 2006; Martini et al., 2012). Several studies report that the gamma band activity at 40 Hz oscillation is a correlate of selective attention (Lutzenberger, Pulvermüller, Elbert, & Birbaumer, 1995; Keil et al., 2001; Keil, Stolarova, Moratti, & Ray, 2007; Martini et al., 2012).
Also results of studies indicate that an increase in gamma band activity at 40 Hz frequency is associated with the tuning of novelty processing by negative emotions (Singer, 1995; Garcia-Garcia, Yordanova, Kolev, Domínguez-Borràs, & Escera, 2010). Interestingly, results of Janik, Rezlescu, & Banissy (2015) study indicates that modulating occipital gamma with 40 Hz using transcranial Alternating Current Stimulation (tACS) enhances facial anger perception. Balconi and Pozoli (2009) showed an increased gamma band activity in response to emotions (happiness, sadness, fear, and anger) compared with neutral stimuli. Keil et al., (2007) found an increase in synchrony of very early cortical oscillations at 20–35 Hz aversive visual stimuli.
Müller, Gruber, & Keil (2000) showed an increased gamma band activity (30±50 Hz) for negative valence over the left temporal region as compared to the right one and a lateral shift towards the right hemisphere for positive valence. Martini et al., (2012) found that unpleasant images compared to neutral visual stimuli, elicited an increase of gamma power (30–45 Hz) at 200 ms and 850 ms after stimulus presentation.
Time course and specific topography of affective gamma band activity modulations during cognitive stimuli demonstrate discrimination between early and late processing stages. Basar (2012) reported possibility of 3-4 phase/time-locked gamma responses in 28–45 Hz frequency window during presentation of stimuli. According to their explanation, the early response, starts at 100 ms, in the primary occipital cortex which is probably the direct response over the short pathway via lateral geniculate nucleus and reflects the early visual processing.
According to Keil et al., (2007), the early gamma response could be sensory in origin and sensitive to simple features associated with emotions. Balconi and Pozilli (2009) reported an increasing early gamma band response for emotional stimuli between 150 ms and 250 ms. On the other hand, according to Basar (2012), late processing stage, starting around 300 ms, can be associated with the conscious perception and discrimination of the emotional stimuli. According to Polich (2007) study, time window 300-500 ms onset stimuli is modulated by allocation of attention, initial memory storage, and processing of cognitive tasks.
Martini et al., (2012) found two peaks of weak activation at high gamma frequencies (65–80 Hz) between 200 and 400 ms after stimulus onset; it is interpreted to be less sensitive to image features and more dependent on the conceptual processing of the stimulus identity. Balconi and Lucchiari (2008) reported higher GBA for emotional and neutral stimuli and this increase was more pronounced between 250 and 350 ms. And finally, time window around 400 to 800 ms reflect more strategic high-level processes that require the conscious awareness such as decision making, response criterion, and deep and elaborate processing of emotion (Schupp et al., 2004; Williams et al., 2007). Accordingly, these findings show an important role of gamma oscillations in evaluating early and late stages of cognitive stimuli.
On the other hand, abnormalities in gamma band response and phase synchronization have been shown to be related to various neurological and psychiatric disorders such as schizophre-nia, Alzheimer, and ADHD (Matsumoto et al., 2006; Basar & Guntekin, 2013). Gross et al., (2012) reported reduced gamma oscillations in emotional face perception in Autism Spectrum Disorder. Studies show that ADHD patients have lower absolute and/or relative gamma power in comparison with age-matched healthy controls (Cited in Basar & Guntekin, 2013). Few studies indicate impaired gamma band responses related to cognitive stimuli in ADHD patients compared to healthy controls (Yordanova, Banaschewski, Kolev, Woerner, & Rothenberger, 2001; Lenz et al., 2008; Lenz et al., 2010). Lenz et al., (2010) reported ADHD group (11-17 years old) showed no differentiation between known and unknown stimuli during application of forced-choice–reaction task. However, normal group revealed increased evoked gamma response following familiar stimuli compared to new images.
Based on the mentioned studies, gamma band activity could reflect the characteristics of emotional integration or emotional utilization processes in individuals with ADHD. To our knowledge and according to review articles by Basar and Guntekin (2013) and Güntekin and Başar (2014), our study is the first one that evaluates gamma-band responses with stages of neural activity of emotional face recognition in children with ADHD. We expected that gamma band activity would be diminished for emotional faces in these patients compared to typically developing children during stages of facial emotional recognition. The present study aimed to gain better understanding of the neurobiological basis of children with ADHD in emotional face processing.
2. Methods
2.1. Participants
Nineteen boys, aged between 7 and 11 years (Mean±SD age: 9.21(1.13) years) diagnosed with ADHD were compared with 19 typically developing ones (9.73±1.04 years) matched with age, sex, and years of education. The control group was recruited from elementary schools in Tehran. Children with ADHD were recruited from patients referred to a child and adolescent psychiatric clinic. The individuals with ADHD were diagnosed according to DSM-IV (Statistical Manual of Mental Disorders, Fourth edition) (American Psychiatric Association, 2013). The patients were diagnosed as combined type and were drug naive.
Conners’ Parent Rating Scale-Revised (CPRS-R, short version) was administered to the participants to confirm the diagnosis of ADHD and determine severity of their symptoms. If the T-scores of CPRS-R subscales were above 65, the participant was excluded from the normal group. The two groups were right-handed, had corrected to normal visual acuity. Intelligence Quotient (IQ) of all participants were evaluated according to the WISC-R IQ test (ADHD group: 106±4.36, Control group: 122±10.71) (Table 1).
2.2. Task and stimuli
A compilation of 6 Caucasian faces (3 females and 3 males) in JPG format expressing happy, angry, sad, and neutral expressions were collected from Cohn Kanade AU-coded Facial Expressions Database (Kanade, Cohn, & Tian, 2000). Luminance and contrast of all images were equivalent across stimuli using Photoshop (version 7). The photos were in black and white and positioned within a rectangular frame (261×365 pixel array). The pictures were presented for 2000 ms at the center of the monitor screen and instantly replaced by a white fixation point in the light gray background (1024×768 pixels). The inter stimulus interval (ISI) was 1400±100 ms. The task was designed using Eevoke software (version 3.1).
The main task included 1 practice and 5 experimental blocks. Each experimental block comprised with 48 trials; 4 emotions (anger, happiness, sadness, and neutral) of each faces (3 female and 3 males) that repeated two times. Thus, there were 60 repeats of each expression in a random way. We defined four buttons for each facial expressions procedure (anger, happiness, sadness, and neutral) on a joy stick.
All participants were invited to the laboratory of EEG recording. The parents completed a consent form before starting the examination. During the EEG session, participants were seated in a comfortable chair in a dimly lit room 60 cm from a 17-inch LG computer screen. The participants were asked to look at the center of the screen during the recordings, if possible without making eye movements, and to blink only during the intervals. To ensure that they attend to stimuli, they were monitored by camera in another control room during task performance. All children were instructed to press on the button for each expression when they recognized the target stimuli during each trial (Stimuli presentation until the end of fixation).
2.3. Electrophysiological recording and analysis
Continuous EEG signals were recorded by 64 Ag/AgCl electrodes mounted in an electrode cap (Waveguard, ANT, Netherlands) according to the international 10-20 standard and additional intermediate positions in neuropsychology laboratory of Payam-e Noor University. ASA 4.7.1 software was used for data acquisition. Electrode impedances were maintained below 10 kΩ. The sampling rate was 512 Hz. A 50-Hz notch filter of the recording system, eliminated the line noise during signal acquisition. Event-related oscillations data were analyzed offline using MATLAB R2013a software.
Raw data were filtered with a band-pass filter of 0.1 to 80 Hz and referenced to the mastoids average. The eye movement artifacts were canceled using the independent component analysis. In addition, the remaining artifacts with deflection amplitudes of ±100 μV from the baseline were eliminated (primarily through automatic artifact reduction). Artifact-free EEG recordings were then segmented into epochs ranging from 200 ms prestimulus to 800 ms poststimulus. Each channel baseline epoch was corrected by prestimulus average voltage subtraction. Several studies reported that the lower frequencies of Gamma band oscillation (about 40 Hz), modulated with emotional stimuli indicated attentional processing and discrimination of the emotional stimuli (Keil et al., 2001; Keil et al., 2007; Martini et al., 2012; Herrmann, Munk, & Engel, 2004).
To investigate facial emotion processing based on gamma oscillations, we constructed a new time series
by concatenating a specific time window from all clean epochs. Then we estimated the signal power spec-trum in frequency band of 35–45 HZ for three different time windows based on literature (Martini et al., 2012) and following current data analysis: 0–250 ms as early processing (can be sensitive to simple features associated with emotions (Keil et al., 2007)), 250–500 ms as late processing (can indicate allocation of attention, initial memory storage the processing of cognitive tasks (Polich, 2007)), and 500–750 ms as later ones (can reflect more strategic high-level processes that require the conscious awareness and deeper and elaborate processing of emotion (Schupp et al., 2004; Williams et al., 2007)). Based on the previous studies, F3, F4, C3, C4, P3, P4, O1, O2 electrodes were used for the statistical analysis (Ramos-Loyo, González-Garrido, Sánchez-Loyo, Medina, & Basar-Eroglu, 2009).
2.4. Statistical analysis
Frequency-band measures were statistically analyzed using repeated-measure analysis of variance (ANOVA) with the following core factors: facial expression (happiness, anger, sadness, and neutral), site (area) (anterior [F3, F4], central [C3, C4], occipital [O1, O2], and parietal [P3, P4]), side (lateralization) (left [F3, C3, P3, O1], and right [F4, C4, P4, O2]) as the within subjects factors, and groups (patients and controls) as the between subject factor. Greenhouse Geisser correction was used for the degrees of freedom. In the next step, paired t-test were used to break down between main effects such as site and independent sample t test were used to break down between-subject and interaction effects such as facial expression×group. Throughout the experiment, P<0.05 were considered significant.
3. Results
We carried out repeated-measure ANOVA (facial expression×site×side×group [4×4×2×2]) for three time windows separately. In the first time window (0–250 ms), this analyses revealed a significant main effect of group (F1, 36=12.36, P=0.001; partial η2=0.155), and a significant main effect of site (F2.1, 76.26=3.78, P=0.02; partial η2=0.013). Also the results showed a significant interaction effect of site×group (F2.1, 76.26=3.79, P=0.02; partial η2=0.059), and a significant interaction effect of facial expression×group (F2.1, 77.47=4.68, P=0.01; partial η2=0.069). In the second (250–500 ms) and third (500–750 ms) time windows, repeated-measure ANOVA revealed only main effect of group which showed smaller gamma band activity in ADHD group compared to healthy control one (P<0.05). We observed no significant main effect or interaction effect in terms of other factors of side, site, face expression, and group (P>0.05) in the second and third time windows. Therefore, our results focused on first time window that indicated early facial emotion processing.
2.4. Statistical analysis
Frequency-band measures were statistically analyzed using repeated-measure analysis of variance (ANOVA) with the following core factors: facial expression (happiness, anger, sadness, and neutral), site (area) (anterior [F3, F4], central [C3, C4], occipital [O1, O2], and parietal [P3, P4]), side (lateralization) (left [F3, C3, P3, O1], and right [F4, C4, P4, O2]) as the within subjects factors, and groups (patients and controls) as the between subject factor. Greenhouse Geisser correction was used for the degrees of freedom. In the next step, paired t-test were used to break down between main effects such as site and independent sample t test were used to break down between-subject and interaction effects such as facial expression×group. Throughout the experiment, P<0.05 were considered significant.
3. Results
We carried out repeated-measure ANOVA (facial expression×site×side×group [4×4×2×2]) for three time windows separately. In the first time window (0–250 ms), this analyses revealed a significant main effect of group (F1, 36=12.36, P=0.001; partial η2=0.155), and a significant main effect of site (F2.1, 76.26=3.78, P=0.02; partial η2=0.013). Also the results showed a significant interaction effect of site×group (F2.1, 76.26=3.79, P=0.02; partial η2=0.059), and a significant interaction effect of facial expression×group (F2.1, 77.47=4.68, P=0.01; partial η2=0.069). In the second (250–500 ms) and third (500–750 ms) time windows, repeated-measure ANOVA revealed only main effect of group which showed smaller gamma band activity in ADHD group compared to healthy control one (P<0.05). We observed no significant main effect or interaction effect in terms of other factors of side, site, face expression, and group (P>0.05) in the second and third time windows. Therefore, our results focused on first time window that indicated early facial emotion processing.
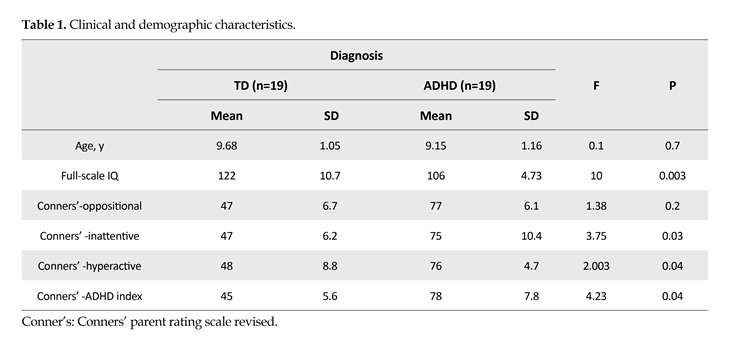
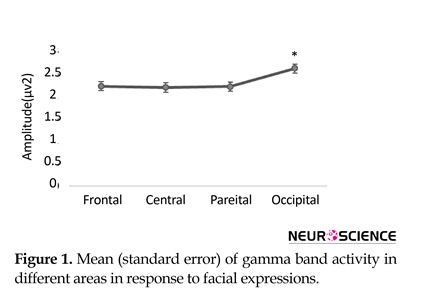
Also post hoc analysis showed that gamma oscillation measures were smaller in ADHD group (1.6±0.2) compared to normal group (2.9±0.2) in the first time window. Follow-up paired t test showed greater gamma band activity in occipital site compared to frontal (P=0.05, t(37)=1.97), central (P=0.01, t(37)=2.47), and parietal (P=0.02, t(37)=2.28) areas in ADHD group (Figure 1). Follow-up independent t test revealed a significant lower gamma band activity in ADHD group compared to normal children in occipital regions (F4.2, 36=14.8, P=0.001) (Figure 2). Also follow-up independent t test showed a significant decrease for happiness (F2.6, 36=6.63, P=0.01), (F3.2, 36=6.8, P=0.01) and anger (F4.2, 36=13.7, P=0.001), (F6, 36=8.25, P=0.007) in ADHD group compared to typically developing children in left and right occipital, respectively (Figure 3).
4. Discussion
This study aimed to compare the gamma band oscillations among patients with ADHD and healthy controls during facial emotion recognition. We expected to observe emotion recognition deficits in ADHD children. The results showed a significant (P<0.05) increased gamma band activity within occipital regions during the first time window (0–250 ms) compared to other sites. Also, we observed a significant (P<0.05) reduction in gamma band activity in ADHD children compared to normal group in response to facial expressions within occipital lobe. This study supported our hypothesis that individuals with ADHD were different from typically developing children during facial expression recognition, especially in early stage of facial emotion processing.
Consistent with our findings, several studies have shown a similar gamma-band increase in response to emotional pictures with occipital distribution (Keil et al., 2001; Keil et al., 2007; Garcia-Garcia et al., 2010). Balconi and Lucchiari (2008) as well as Aftanas, Varlamov, Pavlov, Makhnev and Reva (2002) reported a more emotional compared to non-emotional stimuli in posterior distribution of gamma oscillations. The results of the current study revealed that gamma band activity modulated by emotions compared to neutral, only in first time interval (0–250 ms) indicating early stage of facial emotion recognition but not late stage. Consistent with the current results about early gamma response (<250 ms), Martini et al., (2012) revealed the increase of gamma activity in the low gamma frequency band (30–45 Hz) for unpleasant picture compared to neutral visual stimuli at the shortest latencies (0–250 ms).
Also, Balconi and Pozoli (2009) showed an increased gamma band activity in response to emotions compared to neutral at 150–250 ms time interval with a peak at around 240 ms of latency. Keil et al., (2001) reported gamma band activity (30–45 Hz) at 80 ms
4. Discussion
This study aimed to compare the gamma band oscillations among patients with ADHD and healthy controls during facial emotion recognition. We expected to observe emotion recognition deficits in ADHD children. The results showed a significant (P<0.05) increased gamma band activity within occipital regions during the first time window (0–250 ms) compared to other sites. Also, we observed a significant (P<0.05) reduction in gamma band activity in ADHD children compared to normal group in response to facial expressions within occipital lobe. This study supported our hypothesis that individuals with ADHD were different from typically developing children during facial expression recognition, especially in early stage of facial emotion processing.
Consistent with our findings, several studies have shown a similar gamma-band increase in response to emotional pictures with occipital distribution (Keil et al., 2001; Keil et al., 2007; Garcia-Garcia et al., 2010). Balconi and Lucchiari (2008) as well as Aftanas, Varlamov, Pavlov, Makhnev and Reva (2002) reported a more emotional compared to non-emotional stimuli in posterior distribution of gamma oscillations. The results of the current study revealed that gamma band activity modulated by emotions compared to neutral, only in first time interval (0–250 ms) indicating early stage of facial emotion recognition but not late stage. Consistent with the current results about early gamma response (<250 ms), Martini et al., (2012) revealed the increase of gamma activity in the low gamma frequency band (30–45 Hz) for unpleasant picture compared to neutral visual stimuli at the shortest latencies (0–250 ms).
Also, Balconi and Pozoli (2009) showed an increased gamma band activity in response to emotions compared to neutral at 150–250 ms time interval with a peak at around 240 ms of latency. Keil et al., (2001) reported gamma band activity (30–45 Hz) at 80 ms
poststimulus enhanced in response to unpleasant stimuli compared to neutral. These findings are consistent with our results that supported the role of early gamma response in assessing early stage of facial emotion processing. However, our findings did not reveal modulation of gamma band activity by emotion compared to neutral during the late stage. Unlike the current results, Martini et al., (2012) reported an increased gamma band activity (lower gamma frequency) in response to emotions (unpleasant compared to neutral) at the time interval of 250–500 ms. Also, they found two peaks of weak activation at high gamma frequencies (65–85 Hz) at 200 ms and 850 ms after stimulus onset. Keil et al., (2001) reported an increased gamma band activity (46–65 Hz) in response to unpleasant compared to neutral pictures at 500 ms. In fact, we did not analyze the 45–65 Hz or 65–85 Hz interval, that may be the reason for inconsistent results.
Also, the current results showed a significant diminished evoked gamma band responses only to anger and happiness emotions compared to neutral ones during early stage of processing (time window 0–250 ms) within occipital regions in ADHD group compared to healthy control ones. However, the current findings did not reveal any difference between groups in response to sad faces. Behavioral studies showed that ADHD children have deficits in emotional face recognition, especially negative (fear, anger, sadness) ones compared to healthy children (Singh et al., 1998; Cadesky et al., 2000; Corbett& Glidden, 2000, Dan & Raz, 2015). However, few behavioral studies did not report facial emotion recognition impairment (anger, happiness, sadness) in ADHD group compared to normal ones (Boakes, Chapman, Houghton, & West, 2007; Schwenck, et al., 2013).
Thus, the current results should be discussed with respect to early visual processing. Unfortunately, the relevant literature is scarce. Inconsistent with current results, William et al., (2008) reported adolescents with ADHD [(mean±SD) age: 13.79(2.33) years; range 8–17)] have been shown to display reduced occipital P1 component during all of expressions (fear, anger, sadness, disgust, happiness, or neutral) compared to normal group. The P1 component (in the ERP studies)is primarily involved in visual attention and initial sensory encoding (similar to role of early gamma responses according to Basar, 2012), localized in bilateral occipital areas and fusiform gyrus (Hillyard, Mangun, Woldorff, & Luck, 1995; Eimer & Holmes, 2002).
Few studies report reduced P100 amplitude in occipital regions in ADHD group (Barry et al., 2009; Nazari et al., 2010), probably indicating dysfunction of early visual pathways, which provide sensory input to the amygdala and may cause emotion recognition deficits. In the present study, participants’ Mean(SD) age was 9.21(1.13) years (age range was 7-11 years), which may be the reason for inconsistent results especially with William et al., (2008). In this regard, further research with other age groups or other gamma band frequencies (for happy and angry faces recognition) are recommended.
These findings show the important role of occipital gamma oscillations in facial emotion perception. The present study shows that children with ADHD have abnormality in brain function for early stage of emotional face recognition compared to normal children that can be caused by deficits in selective and sustained attention and early visual processing. These findings might provide preliminary evidence for future planning of interventional approaches for children with ADHD.
Acknowledgments
This study is a part of first author PhD thesis in neuroscience entitled Evaluation of brain function in recognizing emotional faces in children with attention-deficit /hyperactivity disorder (ADHD) compared with normal children using the Event Related Potentials (ERP). It was supported by a grant from Tehran university of Medical sciences. We thank Miss. Zamani and Dr.Rahmanian, supervisor of neuropsychology laboratory of Payame Noor University, and all children and their parents for their contribution to this study.
Conflict of Interest
All authors certify that this manuscript has neither been published in whole nor in part nor being considered for publication elsewhere. The authors have no conflicts of interest to declare.
References
Aftanas, L. I., Varlamov, A. A., Pavlov, S. V., Makhnev, V. P., & Reva, N. V. (2002). Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. International Journal of Psychophysiology, 44(1), 67–82. doi: 10.1016/s0167-8760(01)00194-5
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Missouri: American Psychiatric Pub.
Balconi, M., & Lucchiari, C. (2008). Consciousness and arousal effects on emotional face processing as revealed by brain oscillations: A gamma band analysis. International Journal of Psychophysiology, 67(1), 41–6. doi: 10.1016/j.ijpsycho.2007.10.002
Balconi, M., & Pozzoli, U. (2009). Arousal effect on emotional face comprehension. Physiology & Behavior, 97(3-4), 455–62. doi: 10.1016/j.physbeh.2009.03.023
Barry, R. J., Clarke, A. R., & Johnstone, S. J. (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology, 114(2), 171–83. doi: 10.1016/s1388-2457(02)00362-0
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., & Dupuy, F. E. (2010). Resting-state EEG gamma activity in children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 121(11), 1871–7. doi: 10.1016/j.clinph.2010.04.022
Barry, R. J., Clarke, A. R., McCarthy, R., Selikowitz, M., Brown, C. R., & Heaven, P. C. L. (2009). Event-related potentials in adults with Attention-Deficit/Hyperactivity Disorder: An investigation using an inter-modal auditory/visual oddball task. International Journal of Psychophysiology, 71(2), 124–31. doi: 10.1016/j.ijpsycho.2008.09.009
Başar, E. (2012). Multiple oscillations and phase locking in human gamma responses: An essay in search of eigenvalues. NeuroQuantology, 10(4). doi: 10.14704/nq.2012.10.4.603
Başar, E., & Güntekin, B. (2013). Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. In E. Başar, C. Başar-Eroglu, A. O zerdem, P. M. Rossini, & G. G. Yener (Eds.). Supplements to Clinical Neurophysiology (pp. 303–41). Cambridge, Massachusetts: Academic Press. doi: 10.1016/b978-0-7020-5307-8.00019-3
Boakes, J., Chapman, E., Houghton, S., & West, J. (2007). Facial affect interpretation in boys with attention deficit/hyperactivity disorder. Child Neuropsychology, 14(1), 82–96. doi: 10.1080/09297040701503327
Cadesky, E. B., Mota, V. L., & Schachar, R. J. (2000). Beyond words: how do children with ADHD and/or conduct problems process nonverbal information about affect. Journal of the American Academy of Child & Adolescent Psychiatry, 39(9), 1160–7. doi: 10.1097/00004583-200009000-00016
Corbett, B., & Glidden, H. (2000). Processing affective stimuli in children with Attention-Deficit Hyperactivity Disorder. Child Neuropsychology (Neuropsychology, Development and Cognition: Section C), 6(2), 144–55. doi: 10.1076/chin.6.2.144.7056
Dan, O., & Raz, S. (2015). Response patterns to emotional faces among adolescents diagnosed with ADHD. Journal of Attention Disorders. doi: 10.1177/1087054715606215
Dickstein, S. G., Bannon, K., Xavier Castellanos, F., & Milham, M. P. (2006). The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. Journal of Child Psychology and Psychiatry, 47(10), 1051–62. doi: 10.1111/j.1469-7610.2006.01671.x
Durston, S., van Belle, J., & de Zeeuw, P. (2011). Differentiating frontostriatal and fronto-cerebellar circuits in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 69(12), 1178–84. doi: 10.1016/j.biopsych.2010.07.037
Eimer, M., & Holmes, A. (2002). An ERP study on the time course of emotional face processing. Neuroreport, 13(4), 427-31. doi: 10.1097/00001756-200203250-00013
Fell, J., Klaver, P., Lehnertz, K., Grunwald, T., Schaller, C., Elger, C. E., et al. (2001). Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nature Neuroscience, 4(12), 1259-64. doi: 10.1038/nn759
Garcia-Garcia, M., Yordanova, J., Kolev, V., Domínguez-Borràs, J., & Escera, C. (2010). Tuning the brain for novelty detection under emotional threat: The role of increasing gamma phase-synchronization. NeuroImage, 49(1), 1038–44. doi: 10.1016/j.neuroimage.2009.07.059
Gross, E., El-Baz, A. S., Sokhadze, G. E., Sears, L., Casanova, M. F., & Sokhadze, E. M. (2012). Induced EEG gamma oscillation alignment improves differentiation between autism and ADHD group responses in a facial categorization task. Journal of Neurotherapy, 16(2), 78–91. doi: 10.1080/10874208.2012.677631
Güntekin, B., & Başar, E. (2014). A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia, 58, 33–51. doi: 10.1016/j.neuropsychologia.2014.03.014
Herrmann, C. S., Munk, M. H. J., & Engel, A. K. (2004). Cognitive functions of gamma-band activity: Memory match and utilization. Trends in Cognitive Sciences, 8(8), 347–55. doi: 10.1016/j.tics.2004.06.006
Hillyard, S. A., Mangun, G. R., Woldorff, M. G., & Luck, S. J. (1995). Neural mechanisms mediating selective attention. In M. S. Gazzaniga (Ed.). The Cognitive Neurosciences. Massachussets: MIT Press.
Janik, A. B., Rezlescu, C., & Banissy, M. J. (2015). Enhancing anger perception with transcranial alternating current stimulation induced gamma oscillations. Brain Stimulation, 8(6), 1138–43. doi: 10.1016/j.brs.2015.07.032
Kanade, T., Cohn, J. F., & Yingli Tian. (2000). Comprehensive database for facial expression analysis. Paper presented at the 4th IEEE International Conference on Automatic Face and Gesture Recognition (Cat. No. PR00580). Grenoble, France, 28-30 March 2000. doi: 10.1109/afgr.2000.840611
Keil, A., Müller, M. M., Gruber, T., Wienbruch, C., Stolarova, M., & Elbert, T. (2001). Effects of emotional arousal in the cerebral hemispheres: A study of oscillatory brain activity and event-related potentials. Clinical Neurophysiology, 112(11), 2057–68. doi: 10.1016/s1388-2457(01)00654-x
Keil, A., Stolarova, M., Moratti, S., & Ray, W. J. (2007). Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. NeuroImage, 36(2), 472–9. doi: 10.1016/j.neuroimage.2007.02.048
Lenz, D., Krauel, K., Flechtner, H. H., Schadow, J., Hinrichs, H., & Herrmann, C. S. (2010). Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia, 48(7), 1985–93. doi: 10.1016/j.neuropsychologia.2010.03.019
Lenz, D., Krauel, K., Schadow, J., Baving, L., Duzel, E., & Herrmann, C. S. (2008). Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Research, 1235, 117–32. doi: 10.1016/j.brainres.2008.06.023
Luo, Q., Mitchell, D., Cheng, X., Mondillo, K., Mccaffrey, D., Holroyd, T., et al. (2008). Visual awareness, emotion, and gamma band synchronization. Cerebral Cortex, 19(8), 1896–904. doi: 10.1093/cercor/bhn216
Lutzenberger, W., Pulvermüller, F., Elbert, T., & Birbaumer, N. (1995). Visual stimulation alters local 40-Hz responses in humans: an EEG-study. Neuroscience Letters, 183(1-2), 39–42. doi: 10.1016/0304-3940(94)11109-v
Martini, N., Menicucci, D., Sebastiani, L., Bedini, R., Pingitore, A., Vanello, N., et al. (2012). The dynamics of EEG gamma responses to unpleasant visual stimuli: From local activity to functional connectivity. NeuroImage, 60(2), 922–32. doi: 10.1016/j.neuroimage.2012.01.060
Matsumoto, A., Ichikawa, Y., Kanayama, N., Ohira, H., & Iidaka, T. (2006). Gamma band activity and its synchronization reflect the dysfunctional emotional processing in alexithymic persons. Psychophysiology, 43(6), 533–40. doi: 10.1111/j.1469-8986.2006.00461.x
Müller, M. M., Gruber, T., & Keil, A. (2000). Modulation of induced gamma band activity in the human EEG by attention and visual information processing. International Journal of Psychophysiology, 38(3), 283–99. doi: 10.1016/s0167-8760(00)00171-9
Nazari, M. A., Berquin, P., Missonnier, P., Aarabi, A., Debatisse, D., De Broca, A., et al. (2010). Visual sensory processing deficit in the occipital region in children with attention-deficit/hyperactivity disorder as revealed by event-related potentials during cued continuous performance test. Neurophysiologie Clinique/Clinical Neurophysiology, 40(3), 137–49. doi: 10.1016/j.neucli.2010.03.001
Pelc, K., Kornreich, C., Foisy, M.-L., & Dan, B. (2006). Recognition of emotional facial expressions in Attention-Deficit Hyperactivity Disorder. Pediatric Neurology, 35(2), 93–7. doi: 10.1016/j.pediatrneurol.2006.01.014
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–48. doi: 10.1016/j.clinph.2007.04.019
Ramos Loyo, J., González Garrido, A. A., Sánchez Loyo, L. M., Medina, V., & Basar-Eroglu, C. (2009). Event-related potentials and event-related oscillations during identity and facial emotional processing in schizophrenia. International Journal of Psychophysiology, 71(1), 84–90. doi: 10.1016/j.ijpsycho.2008.07.008
Schupp, H. T., Öhman, A., Junghöfer, M., Weike, A. I., Stockburger, J., & Hamm, A. O. (2004). The facilitated processing of threatening faces: An ERP analysis. Emotion, 4(2), 189–200. doi: 10.1037/1528-3542.4.2.189
Schwenck, C., Schneider, T., Schreckenbach, J., Zenglein, Y., Gensthaler, A., Taurines, R., et al. (2013). Emotion recognition in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). ADHD Attention Deficit and Hyperactivity Disorders, 5(3), 295–302. doi: 10.1007/s12402-013-0104-z
Sebastiani, L., Simoni, A., Gemignani, A., Ghelarducci, B., & Santarcangelo, E. L. (2005). Relaxation as a cognitive task. Archives Italiennes De Biologie, 143(1), 1-12. doi: 10.4449/aib.v143i1.336
Singer, W. (1995). Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience, 18(1), 555–86. doi: 10.1146/annurev.neuro.18.1.555
Singh, S. D., Ellis, C. R., Winton, A. S. W., Singh, N. N., Leung, J. P., & Oswald, D. P. (1998). Recognition of facial expressions of emotion by children with Attention-Deficit Hyperactivity Disorder. Behavior Modification, 22(2), 128–42. doi: 10.1177/01454455980222002
Williams, L. M., Hermens, D. F., Palmer, D., Kohn, M., Clarke, S., Keage, H., et al. (2008). Misinterpreting emotional expressions in Attention-Deficit/Hyperactivity Disorder: Evidence for a neural marker and stimulant effects. Biological Psychiatry, 63(10), 917–26. doi: 10.1016/j.biopsych.2007.11.022
Williams, L. M., Kemp, A. H., Felmingham, K., Liddell, B. J., Palmer, D. M., & Bryant, R. A. (2007). Neural biases to covert and overt signals of fear: Dissociation by trait anxiety and depression. Journal of Cognitive Neuroscience, 19(10), 1595–1608. doi: 10.1162/jocn.2007.19.10.1595
Yordanova, J., Banaschewski, T., Kolev, V., Woerner, W., & Rothenberger, A. (2001). Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder – evidence from event-related gamma oscillations. Clinical Neurophysiology, 112(6), 1096–108. doi: 10.1016/s1388-2457(01)00524-7
Also, the current results showed a significant diminished evoked gamma band responses only to anger and happiness emotions compared to neutral ones during early stage of processing (time window 0–250 ms) within occipital regions in ADHD group compared to healthy control ones. However, the current findings did not reveal any difference between groups in response to sad faces. Behavioral studies showed that ADHD children have deficits in emotional face recognition, especially negative (fear, anger, sadness) ones compared to healthy children (Singh et al., 1998; Cadesky et al., 2000; Corbett& Glidden, 2000, Dan & Raz, 2015). However, few behavioral studies did not report facial emotion recognition impairment (anger, happiness, sadness) in ADHD group compared to normal ones (Boakes, Chapman, Houghton, & West, 2007; Schwenck, et al., 2013).
Thus, the current results should be discussed with respect to early visual processing. Unfortunately, the relevant literature is scarce. Inconsistent with current results, William et al., (2008) reported adolescents with ADHD [(mean±SD) age: 13.79(2.33) years; range 8–17)] have been shown to display reduced occipital P1 component during all of expressions (fear, anger, sadness, disgust, happiness, or neutral) compared to normal group. The P1 component (in the ERP studies)is primarily involved in visual attention and initial sensory encoding (similar to role of early gamma responses according to Basar, 2012), localized in bilateral occipital areas and fusiform gyrus (Hillyard, Mangun, Woldorff, & Luck, 1995; Eimer & Holmes, 2002).
Few studies report reduced P100 amplitude in occipital regions in ADHD group (Barry et al., 2009; Nazari et al., 2010), probably indicating dysfunction of early visual pathways, which provide sensory input to the amygdala and may cause emotion recognition deficits. In the present study, participants’ Mean(SD) age was 9.21(1.13) years (age range was 7-11 years), which may be the reason for inconsistent results especially with William et al., (2008). In this regard, further research with other age groups or other gamma band frequencies (for happy and angry faces recognition) are recommended.
These findings show the important role of occipital gamma oscillations in facial emotion perception. The present study shows that children with ADHD have abnormality in brain function for early stage of emotional face recognition compared to normal children that can be caused by deficits in selective and sustained attention and early visual processing. These findings might provide preliminary evidence for future planning of interventional approaches for children with ADHD.
Acknowledgments
This study is a part of first author PhD thesis in neuroscience entitled Evaluation of brain function in recognizing emotional faces in children with attention-deficit /hyperactivity disorder (ADHD) compared with normal children using the Event Related Potentials (ERP). It was supported by a grant from Tehran university of Medical sciences. We thank Miss. Zamani and Dr.Rahmanian, supervisor of neuropsychology laboratory of Payame Noor University, and all children and their parents for their contribution to this study.
Conflict of Interest
All authors certify that this manuscript has neither been published in whole nor in part nor being considered for publication elsewhere. The authors have no conflicts of interest to declare.
References
Aftanas, L. I., Varlamov, A. A., Pavlov, S. V., Makhnev, V. P., & Reva, N. V. (2002). Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. International Journal of Psychophysiology, 44(1), 67–82. doi: 10.1016/s0167-8760(01)00194-5
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Missouri: American Psychiatric Pub.
Balconi, M., & Lucchiari, C. (2008). Consciousness and arousal effects on emotional face processing as revealed by brain oscillations: A gamma band analysis. International Journal of Psychophysiology, 67(1), 41–6. doi: 10.1016/j.ijpsycho.2007.10.002
Balconi, M., & Pozzoli, U. (2009). Arousal effect on emotional face comprehension. Physiology & Behavior, 97(3-4), 455–62. doi: 10.1016/j.physbeh.2009.03.023
Barry, R. J., Clarke, A. R., & Johnstone, S. J. (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology, 114(2), 171–83. doi: 10.1016/s1388-2457(02)00362-0
Barry, R. J., Clarke, A. R., Hajos, M., McCarthy, R., Selikowitz, M., & Dupuy, F. E. (2010). Resting-state EEG gamma activity in children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology, 121(11), 1871–7. doi: 10.1016/j.clinph.2010.04.022
Barry, R. J., Clarke, A. R., McCarthy, R., Selikowitz, M., Brown, C. R., & Heaven, P. C. L. (2009). Event-related potentials in adults with Attention-Deficit/Hyperactivity Disorder: An investigation using an inter-modal auditory/visual oddball task. International Journal of Psychophysiology, 71(2), 124–31. doi: 10.1016/j.ijpsycho.2008.09.009
Başar, E. (2012). Multiple oscillations and phase locking in human gamma responses: An essay in search of eigenvalues. NeuroQuantology, 10(4). doi: 10.14704/nq.2012.10.4.603
Başar, E., & Güntekin, B. (2013). Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. In E. Başar, C. Başar-Eroglu, A. O zerdem, P. M. Rossini, & G. G. Yener (Eds.). Supplements to Clinical Neurophysiology (pp. 303–41). Cambridge, Massachusetts: Academic Press. doi: 10.1016/b978-0-7020-5307-8.00019-3
Boakes, J., Chapman, E., Houghton, S., & West, J. (2007). Facial affect interpretation in boys with attention deficit/hyperactivity disorder. Child Neuropsychology, 14(1), 82–96. doi: 10.1080/09297040701503327
Cadesky, E. B., Mota, V. L., & Schachar, R. J. (2000). Beyond words: how do children with ADHD and/or conduct problems process nonverbal information about affect. Journal of the American Academy of Child & Adolescent Psychiatry, 39(9), 1160–7. doi: 10.1097/00004583-200009000-00016
Corbett, B., & Glidden, H. (2000). Processing affective stimuli in children with Attention-Deficit Hyperactivity Disorder. Child Neuropsychology (Neuropsychology, Development and Cognition: Section C), 6(2), 144–55. doi: 10.1076/chin.6.2.144.7056
Dan, O., & Raz, S. (2015). Response patterns to emotional faces among adolescents diagnosed with ADHD. Journal of Attention Disorders. doi: 10.1177/1087054715606215
Dickstein, S. G., Bannon, K., Xavier Castellanos, F., & Milham, M. P. (2006). The neural correlates of attention deficit hyperactivity disorder: An ALE meta-analysis. Journal of Child Psychology and Psychiatry, 47(10), 1051–62. doi: 10.1111/j.1469-7610.2006.01671.x
Durston, S., van Belle, J., & de Zeeuw, P. (2011). Differentiating frontostriatal and fronto-cerebellar circuits in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 69(12), 1178–84. doi: 10.1016/j.biopsych.2010.07.037
Eimer, M., & Holmes, A. (2002). An ERP study on the time course of emotional face processing. Neuroreport, 13(4), 427-31. doi: 10.1097/00001756-200203250-00013
Fell, J., Klaver, P., Lehnertz, K., Grunwald, T., Schaller, C., Elger, C. E., et al. (2001). Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nature Neuroscience, 4(12), 1259-64. doi: 10.1038/nn759
Garcia-Garcia, M., Yordanova, J., Kolev, V., Domínguez-Borràs, J., & Escera, C. (2010). Tuning the brain for novelty detection under emotional threat: The role of increasing gamma phase-synchronization. NeuroImage, 49(1), 1038–44. doi: 10.1016/j.neuroimage.2009.07.059
Gross, E., El-Baz, A. S., Sokhadze, G. E., Sears, L., Casanova, M. F., & Sokhadze, E. M. (2012). Induced EEG gamma oscillation alignment improves differentiation between autism and ADHD group responses in a facial categorization task. Journal of Neurotherapy, 16(2), 78–91. doi: 10.1080/10874208.2012.677631
Güntekin, B., & Başar, E. (2014). A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia, 58, 33–51. doi: 10.1016/j.neuropsychologia.2014.03.014
Herrmann, C. S., Munk, M. H. J., & Engel, A. K. (2004). Cognitive functions of gamma-band activity: Memory match and utilization. Trends in Cognitive Sciences, 8(8), 347–55. doi: 10.1016/j.tics.2004.06.006
Hillyard, S. A., Mangun, G. R., Woldorff, M. G., & Luck, S. J. (1995). Neural mechanisms mediating selective attention. In M. S. Gazzaniga (Ed.). The Cognitive Neurosciences. Massachussets: MIT Press.
Janik, A. B., Rezlescu, C., & Banissy, M. J. (2015). Enhancing anger perception with transcranial alternating current stimulation induced gamma oscillations. Brain Stimulation, 8(6), 1138–43. doi: 10.1016/j.brs.2015.07.032
Kanade, T., Cohn, J. F., & Yingli Tian. (2000). Comprehensive database for facial expression analysis. Paper presented at the 4th IEEE International Conference on Automatic Face and Gesture Recognition (Cat. No. PR00580). Grenoble, France, 28-30 March 2000. doi: 10.1109/afgr.2000.840611
Keil, A., Müller, M. M., Gruber, T., Wienbruch, C., Stolarova, M., & Elbert, T. (2001). Effects of emotional arousal in the cerebral hemispheres: A study of oscillatory brain activity and event-related potentials. Clinical Neurophysiology, 112(11), 2057–68. doi: 10.1016/s1388-2457(01)00654-x
Keil, A., Stolarova, M., Moratti, S., & Ray, W. J. (2007). Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. NeuroImage, 36(2), 472–9. doi: 10.1016/j.neuroimage.2007.02.048
Lenz, D., Krauel, K., Flechtner, H. H., Schadow, J., Hinrichs, H., & Herrmann, C. S. (2010). Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia, 48(7), 1985–93. doi: 10.1016/j.neuropsychologia.2010.03.019
Lenz, D., Krauel, K., Schadow, J., Baving, L., Duzel, E., & Herrmann, C. S. (2008). Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Research, 1235, 117–32. doi: 10.1016/j.brainres.2008.06.023
Luo, Q., Mitchell, D., Cheng, X., Mondillo, K., Mccaffrey, D., Holroyd, T., et al. (2008). Visual awareness, emotion, and gamma band synchronization. Cerebral Cortex, 19(8), 1896–904. doi: 10.1093/cercor/bhn216
Lutzenberger, W., Pulvermüller, F., Elbert, T., & Birbaumer, N. (1995). Visual stimulation alters local 40-Hz responses in humans: an EEG-study. Neuroscience Letters, 183(1-2), 39–42. doi: 10.1016/0304-3940(94)11109-v
Martini, N., Menicucci, D., Sebastiani, L., Bedini, R., Pingitore, A., Vanello, N., et al. (2012). The dynamics of EEG gamma responses to unpleasant visual stimuli: From local activity to functional connectivity. NeuroImage, 60(2), 922–32. doi: 10.1016/j.neuroimage.2012.01.060
Matsumoto, A., Ichikawa, Y., Kanayama, N., Ohira, H., & Iidaka, T. (2006). Gamma band activity and its synchronization reflect the dysfunctional emotional processing in alexithymic persons. Psychophysiology, 43(6), 533–40. doi: 10.1111/j.1469-8986.2006.00461.x
Müller, M. M., Gruber, T., & Keil, A. (2000). Modulation of induced gamma band activity in the human EEG by attention and visual information processing. International Journal of Psychophysiology, 38(3), 283–99. doi: 10.1016/s0167-8760(00)00171-9
Nazari, M. A., Berquin, P., Missonnier, P., Aarabi, A., Debatisse, D., De Broca, A., et al. (2010). Visual sensory processing deficit in the occipital region in children with attention-deficit/hyperactivity disorder as revealed by event-related potentials during cued continuous performance test. Neurophysiologie Clinique/Clinical Neurophysiology, 40(3), 137–49. doi: 10.1016/j.neucli.2010.03.001
Pelc, K., Kornreich, C., Foisy, M.-L., & Dan, B. (2006). Recognition of emotional facial expressions in Attention-Deficit Hyperactivity Disorder. Pediatric Neurology, 35(2), 93–7. doi: 10.1016/j.pediatrneurol.2006.01.014
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–48. doi: 10.1016/j.clinph.2007.04.019
Ramos Loyo, J., González Garrido, A. A., Sánchez Loyo, L. M., Medina, V., & Basar-Eroglu, C. (2009). Event-related potentials and event-related oscillations during identity and facial emotional processing in schizophrenia. International Journal of Psychophysiology, 71(1), 84–90. doi: 10.1016/j.ijpsycho.2008.07.008
Schupp, H. T., Öhman, A., Junghöfer, M., Weike, A. I., Stockburger, J., & Hamm, A. O. (2004). The facilitated processing of threatening faces: An ERP analysis. Emotion, 4(2), 189–200. doi: 10.1037/1528-3542.4.2.189
Schwenck, C., Schneider, T., Schreckenbach, J., Zenglein, Y., Gensthaler, A., Taurines, R., et al. (2013). Emotion recognition in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). ADHD Attention Deficit and Hyperactivity Disorders, 5(3), 295–302. doi: 10.1007/s12402-013-0104-z
Sebastiani, L., Simoni, A., Gemignani, A., Ghelarducci, B., & Santarcangelo, E. L. (2005). Relaxation as a cognitive task. Archives Italiennes De Biologie, 143(1), 1-12. doi: 10.4449/aib.v143i1.336
Singer, W. (1995). Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience, 18(1), 555–86. doi: 10.1146/annurev.neuro.18.1.555
Singh, S. D., Ellis, C. R., Winton, A. S. W., Singh, N. N., Leung, J. P., & Oswald, D. P. (1998). Recognition of facial expressions of emotion by children with Attention-Deficit Hyperactivity Disorder. Behavior Modification, 22(2), 128–42. doi: 10.1177/01454455980222002
Williams, L. M., Hermens, D. F., Palmer, D., Kohn, M., Clarke, S., Keage, H., et al. (2008). Misinterpreting emotional expressions in Attention-Deficit/Hyperactivity Disorder: Evidence for a neural marker and stimulant effects. Biological Psychiatry, 63(10), 917–26. doi: 10.1016/j.biopsych.2007.11.022
Williams, L. M., Kemp, A. H., Felmingham, K., Liddell, B. J., Palmer, D. M., & Bryant, R. A. (2007). Neural biases to covert and overt signals of fear: Dissociation by trait anxiety and depression. Journal of Cognitive Neuroscience, 19(10), 1595–1608. doi: 10.1162/jocn.2007.19.10.1595
Yordanova, J., Banaschewski, T., Kolev, V., Woerner, W., & Rothenberger, A. (2001). Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder – evidence from event-related gamma oscillations. Clinical Neurophysiology, 112(6), 1096–108. doi: 10.1016/s1388-2457(01)00524-7
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2017/04/12 | Accepted: 2017/07/15 | Published: 2017/09/19
Received: 2017/04/12 | Accepted: 2017/07/15 | Published: 2017/09/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |