Volume 9, Issue 1 (January & February 2018 2018)
BCN 2018, 9(1): 43-50 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hayashi K, Ikemoto T, Ueno T, Arai Y P, Shimo K, Nishihara M, et al . Discordant Relationship Between Evaluation of Facial Expression and Subjective Pain Rating Due to the Low Pain Magnitude. BCN 2018; 9 (1) :43-50
URL: http://bcn.iums.ac.ir/article-1-882-en.html
URL: http://bcn.iums.ac.ir/article-1-882-en.html
Kazuhiro Hayashi *1 
 , Tatsunori Ikemoto1
, Tatsunori Ikemoto1 
 , Takefumi Ueno2
, Takefumi Ueno2 
 , Young-Chang Park Arai1
, Young-Chang Park Arai1 
 , Kazuhiro Shimo1
, Kazuhiro Shimo1 
 , Makoto Nishihara1
, Makoto Nishihara1 
 , Shigeyuki Suzuki3
, Shigeyuki Suzuki3 
 , Takahiro Ushida1
, Takahiro Ushida1 


 , Tatsunori Ikemoto1
, Tatsunori Ikemoto1 
 , Takefumi Ueno2
, Takefumi Ueno2 
 , Young-Chang Park Arai1
, Young-Chang Park Arai1 
 , Kazuhiro Shimo1
, Kazuhiro Shimo1 
 , Makoto Nishihara1
, Makoto Nishihara1 
 , Shigeyuki Suzuki3
, Shigeyuki Suzuki3 
 , Takahiro Ushida1
, Takahiro Ushida1 

1- Multidisciplinary Pain Center, Aichi Medical University, Nagakute, Japan.
2- National Hospital Organization, Hizen Psychiatric Center, Kyushu, Japan.
3- Program in Physical and Occupational Therapy, Graduate School of Medicine, Nagoya University, Japan.
2- National Hospital Organization, Hizen Psychiatric Center, Kyushu, Japan.
3- Program in Physical and Occupational Therapy, Graduate School of Medicine, Nagoya University, Japan.
Full-Text [PDF 665 kb]
| Abstract (HTML)
Overall, the evaluation of facial expression to pain correlated with self-report pain ratings. However, the correlation was only significant when the subjects were rated as high pain (VAS≥30). In other words, when the subjects were rated as low pain (VAS<30), most of them were rated no pain by observers, despite the fact that they felt pain. The results suggest that it is difficult for observer to find whether the subjects really feel pain or not, when the pain-rating is in low range, and also there is discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating depending on pain magnitude.
It has been reported that self-report pain ratings result in poorer repeatability in mild range compared to high range over different sessions (Hayashi et al., 2015; Quiton & Greenspan, 2008). Kemp et al. has reported that pain-VAS is unreliable in experimental pain when low range or pain threshold (Kemp, Despres, & Dufour, 2012). Regarding the neuronal activities, Hayashi et al. has reported that Blood Oxygenation Level Dependent (BOLD) signal by using functional Magnetic Resonance Imaging (fMRI) was inconsistent in a session with mild pain ratings (Hayashi et al., 2016). The facial expression is related to increase activities in motor-related areas as well as in areas of the thalamocortical pain processing pathways (Kunz, Chen, Lautenbacher, Vachon-Presseau, & Rainville, 2011). These backgrounds might cause discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating in low pain range.
It has been reported that facial expression to painful stimuli is more prominent in strong stimuli compared to weak stimuli (Kunz, Mylius, Schepelmann, & Lautenbacher, 2004; Lucey et al., 2012). Kunz et al. showed only moderate correlation (r=0.4) between self-report pain and facial expression. They have discussed the possibility that stronger stimulus intensity might lead to stronger correlation between them, because low intensities elicited low frequent facial responses (Kunz et al., 2004). They have also reported that facial expression started to appear and increase with stimulus intensities from 4 kg on to the thigh, which corresponded to around 30 mm on VAS (Kunz et al., 2004). While, the present study collected various ratings of pain-VAS from 0 to 84/100 using three different mechanical stimulating forces, the frequency of evaluation as a face of mild pain or more was only 16% in 100 g stimuli, but 44% in 300 g stimuli and 66% in 600 g. It is assumed that these results lead to different correlations between facial expressions and low or high pain ratings, consistent with previous reports (Kunz et al., 2004).
Although the evaluation of facial expression varied slightly among evaluators, there was strong consistency to evaluate categorical scale of pain in the subjects both for low pain ratings and high pain ratings. Someone’s behavioral indicators of pain are usually grimacing, frowning, wincing, vocalization, and restlessness in clinical practice (Puntillo et al., 1997), and each behavior has a potential to be realized by observers as existing pain. However, our experimental results suggest that such behavioral responses may be rarely found when subjects feel mild pain. Hence it is difficult for the observer to find whether the subjects really feel pain or not, when subjective pain-ratings are limited within low range.
Firstly, the facial expression reflects not only pain behavior but also other emotional responses. The facial expression could also be managed, especially by the adult subjects. Although videos were taken of the subjects facing the front with their prior approval during the experiment, subjects were only told that “videos were taken holistically for the purposes of the experiment.” The facial expression responding to pain is influenced by social context (Vlaeyen et al., 2009), sex (Kunz, Gruber, & Lautenbacher, 2006), catastrophizing (Kunz, Chatelle, Lautenbacher, & Rainville, 2008), but not influenced by age (Kunz et al., 2008). Thus, the facial expression respond ing to pain should be assessed along with such related variables. In addition, the evaluation of facial expression to pain is affected by empathy and sympathy. The evolution of recent cognitive aspects of empathy and sympathy are closely related to processes involved in theory of mind, self-regulation, and language (Decety, 2009).
Secondly, the present study results were not compared with quantitative evaluation of facial expression such as FACS. Whether the findings of the present study are applicable to people of different ethnicities is something which should also be taken into consideration. Finally, we only investigated 12 subjects. A larger study sample might shed more light on these differences.
There is a discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating depending on pain magnitude. The evaluation of facial expression to pain was difficult for the observer when the subjects feel mild pain, even though facial expression is a fundamental way of pain communication. Characteristics of pain evaluation is important for clinical practice.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to express their gratitude to Matthew McLaughlin for his assistance in editing the manuscript.
Conflict of Interest
The authors certify that no affiliation or financial involvement exists between them and any organization with a direct interest in the subject matter or materials discussed in the article.
References
Abbey, J., Piller, N., Bellis, A. D., Esterman, A., Parker, D., Giles, L., et al. (2004). The abbey pain scale: A 1-minute numerical indicator for people with end-stage dementia. International Journal of Palliative Nursing, 10(1), 6–13. doi: 10.12968/ijpn.2004.10.1.12013
Apkarian, A. V., Bushnell, M. C., Treede, R.-D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463. doi: 10.1016/j.ejpain.2004.11.001
Apkarian, V. A., Hashmi, J. A., & Baliki, M. N. (2011). Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain, 152(Supplement), S49–S64. doi: 10.1016/j.pain.2010.11.010
Büchel, C., Bornhövd, K., Quante, M., Glauche, V., Bromm, B., & Weiller, C. (2002). Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. Journal of Neuroscience, 22(3), 970-6. PMID: 11826125
Coghill, R. C., McHaffie, J. G., & Yen, Y. F. (2003). Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences, 100(14), 8538–42. doi: 10.1073/pnas.1430684100
Collins, S. L., Moore, A. R., & McQuay, H. J. (1997). The visual analogue pain intensity scale: What is moderate pain in millimetres. Pain, 72(1), 95–7. doi: 10.1016/s0304-3959(97)00005-5
Decety, J. (2009). Empathy, sympathy and the perception of pain. Pain, 145(3), 365–6. doi: 10.1016/j.pain.2009.08.006
Derbyshire, S. W., Jones, A. K. ., Gyulai, F., Clark, S., Townsend, D., & Firestone, L. L. (1997). Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain, 73(3), 431–45. doi: 10.1016/s0304-3959(97)00138-3
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(1), 279–306. doi: 10.1093/brain/118.1.279
Deyo, K. S., Prkachin, K. M., & Mercer, S. R. (2004). Development of sensitivity to facial expression of pain. Pain, 107(1), 16–21. doi: 10.1016/s0304-3959(03)00263-x
Fishbain, D. A., Goldberg, M., Rosomoff, R. S., & Rosomoff, H. L. (1991). More Munchausen with chronic pain. The Clinical Journal of Pain, 7(3), 237–44. doi: 10.1097/00002508-199109000-00012
Gerbershagen, H. J., Rothaug, J., Kalkman, C. J., & Meissner, W. (2011). Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. British Journal of Anaesthesia, 107(4), 619–26. doi: 10.1093/bja/aer195
Goebel, R. (2012). BrainVoyager: Past, present, future. Neuro Image, 62(2), 748–56. doi: 10.1016/j.neuroimage.2012.01.083
Graven Nielsen, T., Mense, S., & Arendt Nielsen, L. (2004). Painful and non-painful pressure sensations from human skeletal muscle. Experimental Brain Research, 159(3), 273–83. doi: 10.1007/s00221-004-1937-7
Hadjistavropoulos, T., Chapelle, D. L., Hadjistavropoulos, H. D., Green, S., & Asmundson, G. J. G. (2002). Using facial expressions to assess musculoskeletal pain in older persons. European Journal of Pain, 6(3), 179–87. doi: 10.1053/eujp.2001.0327
Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2015). Regional differences of repeatability on visual analogue scale with experimental mechanical pain stimuli. Neuroscience Letters, 585, 67–71. doi: 10.1016/j.neulet.2014.11.032
Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2016). Higher pain rating results in lower variability of somatosensory cortex activation by painful mechanical stimuli: An fMRI study. Clinical Neurophysiology, 127(4), 1923–8. doi: 10.1016/j.clinph.2016.01.008
Herr, K., Coyne, P. J., Key, T., Manworren, R., McCaffery, M., Merkel, S., et al. (2006). Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Management Nursing, 7(2), 44–52. doi: 10.1016/j.pmn.2006.02.003
Hofbauer, R. K., Rainville, P., Duncan, G. H., & Bushnell, M. C. (2001). Cortical representation of the sensory dimension of pain. Journal of Neurophysiology, 86(1), 402–11. doi: 10.1152/jn.2001.86.1.402
Jackson, P. L., Meltzoff, A. N., & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–9. doi: 10.1016/j.neuroimage.2004.09.006
Kemp, J., Despres, O., & Dufour, A. (2012). Unreliability of the visual analog scale in experimental pain assessment: A sensitivity and evoked potentials study. Pain Physician, 15(5), E693-9. PMID: 22996863
Kundel, H. L., & Polansky, M. (2003). Measurement of observer agreement. Radiology, 228(2), 303–8. doi: 10.1148/radiol.2282011860
Kunz, M., Chatelle, C., Lautenbacher, S., & Rainville, P. (2008). The relation between catastrophizing and facial responsiveness to pain. Pain, 140(1), 127–34. doi: 10.1016/j.pain.2008.07.019
Kunz, M., Chen, J. I., Lautenbacher, S., Vachon-Presseau, E., & Rainville, P. (2011). Cerebral regulation of facial expressions of pain. Journal of Neuroscience, 31(24), 8730–8. doi: 10.1523/jneurosci.0217-11.2011
Kunz, M., Gruber, A., & Lautenbacher, S. (2006). Sex differences in facial encoding of pain. The Journal of Pain, 7(12), 915–28. doi: 10.1016/j.jpain.2006.04.012
Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2004). On the relationship between self-report and facial expression of pain. The Journal of Pain, 5(7), 368–76. doi: 10.1016/j.jpain.2004.06.002
Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2008). Impact of age on the facial expression of pain. Journal of Psychosomatic Research, 64(3), 311–8. doi: 10.1016/j.jpsychores.2007.09.010
Kwan, C. L., Crawley, A. P., Mikulis, D. J., & Davis, K. D. (2000). An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain, 85(3), 359-74. PMID: 10781909
LeResche, L., & Dworkin, S. F. (1984). Facial expression accompanying pain. Social Science & Medicine, 19(12), 1325–30. doi: 10.1016/0277-9536(84)90020-0
Littman, G. S., Walker, B. R., & Schneider, B. E. (1985). Reassessment of verbal and visual analog ratings in analgesic studies. Clinical Pharmacology and Therapeutics, 38(1), 16–23. doi: 10.1038/clpt.1985.127
Lord, B. (2009). Paramedic assessment of pain in the cognitively impaired adult patient. BMC Emergency Medicine, 9(1). doi: 10.1186/1471-227x-9-20
Lucey, P., Cohn, J. F., Prkachin, K. M., Solomon, P. E., Chew, S., & Matthews, I. (2012). Painful monitoring: Automatic pain monitoring using the UNBC-McMaster shoulder pain expression archive database. Image and Vision Computing, 30(3), 197–205. doi: 10.1016/j.imavis.2011.12.003
Merskey, H. (1979). Pain terms: A list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain, 6, 249-52. PMID: 460932
Mogil, J. S. (2015). Social modulation of and by pain in humans and rodents. Pain, 156, S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77
Moisset, X., & Bouhassira, D. (2007). Brain imaging of neuropathic pain. NeuroImage, 37, S80–8. doi: 10.1016/j.neuroimage.2007.03.054
Moore, R. A., Straube, S., & Aldington, D. (2013). Pain measures and cut-offs - “no worse than mild pain” as a simple, universal outcome. Anaesthesia, 68(4), 400–12. doi: 10.1111/anae.12148
Nasu, T., Taguchi, T., & Mizumura, K. (2010). Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. European Journal of Pain, 14(3), 236–44. doi: 10.1016/j.ejpain.2009.05.009
Prkachin, K. M. (1992). The consistency of facial expressions of pain: a comparison across modalities. Pain, 51(3), 297–306. doi: 10.1016/0304-3959(92)90213-u
Puntillo, K. A., Miaskowski, C., Kehrle, K., Stannard, D., Gleeson, S., & Nye, P. (1997). Relationship between behavioral and physiological indicators of pain, critical care patients' self-reports of pain, and opioid administration. Critical Care Medicine, 25(7), 1159-66. doi: 10.1097/00003246-199707000-00017
Quiton, R. L., & Greenspan, J. D. (2008). Across- and within-session variability of ratings of painful contact heat stimuli. Pain, 137(2), 245–56. doi: 10.1016/j.pain.2007.08.034
Robinson, M. E., Staud, R., & Price, D. D. (2013). Pain measurement and brain activity: Will neuroimages replace pain ratings. The Journal of Pain, 14(4), 323–7. doi: 10.1016/j.jpain.2012.05.007
Rohling, M. L., Binder, L. M., & Langhinrichsen-Rohling, J. (1995). Money matters: A meta-analytic review of the association between financial compensation and the experience and treatment of chronic pain. Health Psychology, 14(6), 537–47. doi: 10.1037/0278-6133.14.6.537
Shrout, P. E. (1998). Measurement reliability and agreement in psychiatry. Statistical Methods in Medical Research, 7(3), 301–17. doi: 10.1177/096228029800700306
Singer, T. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. doi: 10.1126/science.1093535
Takahashi, K., Taguchi, T., Itoh, K., Okada, K., Kawakita, K., & Mizumura, K. (2005). Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosensory & Motor Research, 22(4), 299–305. doi: 10.1080/08990220500420475
Takai, Y., Yamamoto Mitani, N., Chiba, Y., Nishikawa, Y., Hayashi, K., & Sugai, Y. (2010). Abbey pain scale: Development and validation of the Japanese version. Geriatrics & gerontology international, 10(2), 145-53. doi: 10.1111/j.1447-0594.2009.00568.x.
Treede, R. D., Kenshalo, D. R., Gracely, R. H., & Jones, A. K. . (1999). The cortical representation of pain. Pain, 79(2), 105–11. doi: 10.1016/s0304-3959(98)00184-5
Vlaeyen, J. W. S., Hanssen, M., Goubert, L., Vervoort, T., Peters, M., van Breukelen, G., et al. (2009). Threat of pain influences social context effects on verbal pain report and facial expression. Behaviour Research and Therapy, 47(9), 774–82. doi: 10.1016/j.brat.2009.05.008
Wallenstein, S., Heidrich d, G., Kaiko, R., & Houde, R. (1980). Clinical evaluation of mild analgesics: the measurement of clinical pain. British Journal of Clinical Pharmacology, 10(S2), 319S–27. doi: 10.1111/j.1365-2125.1980.tb01816.x
Williams, A. C. de C. (2002). Facial expression of pain: An evolutionary account. Behavioral and Brain Sciences, 25(4):439-55. doi: 10.1017/s0140525x02000080
Full-Text:
1. Introduction
Subjective pain experience is hard to understand by other people, however, objective pain evaluation is useful to understand pain in someone, especially in patients with communication difficulties (Abbey et al., 2004; Takai et al., 2010; Lord, 2009; Puntillo et al., 1997; Herr et al., 2006). Facial expression responding to pain has emerged as an important objective pain indicator in experimental research as well as in clinical practice (Prkachin, 1992). Moreover, facial expression is considered to be the most prominent way of involuntary communicating affect (Prkachin, 1992; Williams, 2002). It is also a fundamental way of pain communication by displaying and recognizing painful stimuli even in animals (Mogil, 2015). In previous studies, the facial expression is measured using Facial Action Coding System (FACS) (LeResche & Dworkin, 1984), which correlates with subjective pain ratings (Kunz, Mylius, Schepelmann, & Lautenbacher, 2004). However, using FACS requires training and it takes times to become certified as a FACS coder. It includes micro-analytic coding procedures that may be unsuitable or too cumbersome for clinical use. Hence, a simple 4-point categorical scale is commonly used on clinical practice (Abbey et al., 2004; Takai et al., 2010), and such a scale has been believed feasible for evaluating pain in others. Also, a significant correlation is reported between subjective pain, Visual Analogue Scale (VAS), and categorical scale within an individual (Wallenstein, Heidrich, Kaiko, & Houde, 1980; Littman, Walker, & Schneider, 1985).
On the other hand, it has been implied that facial expression was poor in low grade pain (Kunz et al., 2004; Lucey et al., 2012). Kunz et al. have reported that facial expression responding to pain only started when stimulus intensity became strong. Lucey et al. have showed that 62% of subjects were rated as free of pain by observers in spite of feeling pain. These findings have suggested that mild pain is hard to be evaluated by observers. Hence, we speculated that there is a discordant relationship between evaluation of facial expression and subjective pain rating when the subject perceives mild pain. A previous study has reported that when the subjects rated pain score in excess of 30 mm using 100 mm VAS, most of them had recorded at least moderate pain on a 4-point categorical scale (no pain, mild pain, moderate pain, and severe pain) (Collins, Moore, & McQuay, 1997). Therefore, facial expression responding to pain stimulus was expected different after cut-off point of 30 mm on pain-VAS.
The present study investigates the relationships between the evaluation of facial expression responding to pain and self-report ratings in cases where pain rating was categorized as mild pain rating (VAS <30 mm) and moderate to strong pain rating (VAS ≥30 mm). It was hypothesized the relationship between them is weaker in mild pain ratings compared to moderate to strong pain ratings.
2. Methods
2.1. Subjects
Twelve healthy college student volunteers (7 men and 5 women) participated in this study. Subjects were 21 to 26 years old. All subjects were right-handed, native Japanese speakers, and healthy, without any history of pain or neurological disorders. Ethical approval was obtained from the Research Ethics Committee of the Nagoya University School of Health Sciences. After being informed of the purpose and protocol of the study, all subjects provided written informed consent before undergoing the experiment.
2.2. Measurements of pain- Visual Analogue Scale (VAS)
We prepared the self-made Von Frey Monofilament (VFM) for the mechanical stimulating device. The diameter of all VFMs was 1.5 mm and the length of each monofilament (GCK-60® Mitsubishi Reyon Co. Ltd., Japan) was adjusted to produce a different force (100 g, 300 g, and 600 g) because the tissue depth affected by mechanical strain varied depending on the diameter of skin contact (Graven-Nielsen, Mense, & Arendt-Nielsen, 2004; Nasu, Taguchi, & Mizumura, 2010; Takahashi et al., 2005). The examiner practiced many times to ensure that each VFM was successfully applied perpendicular to the target surface until a VFM bending of approximately 3 to 5 mm was produced (Hayashi et al., 2015). All subjects underwent mechanical pain stimuli with von Frey hair filaments (VFHs, diameter: 1.5 mm, strain forces: 100 g, 300 g, and 600 g), on three different sessions once per week. The subjects sat in a fixed chair and placed their right hands open on the desk. Each painful stimulus was given to 3 points of inter-digital sites (second-third, third-fourth, and fourth-fifth finger) of the right hand for 5 s.
Measurements were performed with three different VFMs in 60-s intervals for each stimulus. A curtain in front of the subjects was used to prevent them from viewing the stimulating filaments so as not to predict which kinds of VFM were given during the experiment. The only information subjects were given was the site of stimulus. The pain-VAS scale consisting of 100 mm lines labeled at the anchor points with “no pain” and “worst possible pain” were measured every time at the end of each stimulus. Measurements were conducted in a quiet room with the temperature kept between 25℃-27℃, and 40%-50% humidity. Videos were taken of subjects facing the front during the experiment. Subjects were only told “videos are taken holistically for our experiment.”
2.3. Evaluation of facial expression of pain
We employed 3 evaluators (48-year-old male, 42-year-old-female, and 40-year-old-female) which were healthy and did not have pain. The evaluators had not met subjects before the study and first watched the subjects on video. Facial expression responding to pain was rated by these evaluators using a 4-point categorical scale: 0: No pain; 1: Mild pain; 2: Moderate pain; and 3: Severe pain, referring to the Abbey Scale (Abbey et al., 2004; Takai et al., 2010). The evaluators scored all facial expressions for each subject, and 9 times per subject (100 g: 3 times, 300 g: 3 times, and 600 g: 3 times). Each evaluator independently scored his or her observations. The evaluation was adopted when two of three evaluators gave the same evaluation results. If the evaluations of each evaluator were completely different, the median scale was used.
In addition, weighted kappa statistics were used for the analysis of inter-rater agreement of evaluation for facial expression of pain (Kundel & Polansky, 2003). Weighted kappa statistics were calculated between each pair of evaluators.
2.4. Statistical analyses
Normality of the data was assessed by a Shapiro-Wilk test. This test showed that the data of pain-VAS and evaluation of facial expression were not normally disturbed. Therefore, data were expressed as the median and range values, and applied to non-parametric tests. The values of weighted kappa statistics were as follows: virtually no-reliability (0.00-0.10), slight-reliability (0.11-0.40), fair-reliability (0.41-0.60), moderate-reliability (0.61-0.80), and substantial-reliability (0.81-1.00) (Shrout, 1998).
The correlation between the evaluation of facial expression and the self-report pain ratings using pain-VAS were analyzed by using Spearman’s rank correlation coefficient (ρ). Samples with VAS scores less than 30 mm were defined as a low pain group. Those with scores equal to 30 mm or greater were considered to belong to high pain group (Collins et al., 1997; Gerbershagen, Rothaug, Kalkman, & Meissner, 2011; Moore, Straube, & Aldington, 2013).
The correlation between the evaluation of facial expression and VAS was analyzed for each group, respectively. The analyses were performed using SPSS (V. 24.0J; SPSS Inc., Chicago, IL, USA), and the significance was set at P<0.05. Finally, we ran a post hoc power analysis for each analysis using G* Power software (V. 3.0.10; Franz Faul, Kiel University, Kiel, Germany).
3. Results
Subjective pain experience is hard to understand by other people, however, objective pain evaluation is useful to understand pain in someone, especially in patients with communication difficulties (Abbey et al., 2004; Takai et al., 2010; Lord, 2009; Puntillo et al., 1997; Herr et al., 2006). Facial expression responding to pain has emerged as an important objective pain indicator in experimental research as well as in clinical practice (Prkachin, 1992). Moreover, facial expression is considered to be the most prominent way of involuntary communicating affect (Prkachin, 1992; Williams, 2002). It is also a fundamental way of pain communication by displaying and recognizing painful stimuli even in animals (Mogil, 2015). In previous studies, the facial expression is measured using Facial Action Coding System (FACS) (LeResche & Dworkin, 1984), which correlates with subjective pain ratings (Kunz, Mylius, Schepelmann, & Lautenbacher, 2004). However, using FACS requires training and it takes times to become certified as a FACS coder. It includes micro-analytic coding procedures that may be unsuitable or too cumbersome for clinical use. Hence, a simple 4-point categorical scale is commonly used on clinical practice (Abbey et al., 2004; Takai et al., 2010), and such a scale has been believed feasible for evaluating pain in others. Also, a significant correlation is reported between subjective pain, Visual Analogue Scale (VAS), and categorical scale within an individual (Wallenstein, Heidrich, Kaiko, & Houde, 1980; Littman, Walker, & Schneider, 1985).
On the other hand, it has been implied that facial expression was poor in low grade pain (Kunz et al., 2004; Lucey et al., 2012). Kunz et al. have reported that facial expression responding to pain only started when stimulus intensity became strong. Lucey et al. have showed that 62% of subjects were rated as free of pain by observers in spite of feeling pain. These findings have suggested that mild pain is hard to be evaluated by observers. Hence, we speculated that there is a discordant relationship between evaluation of facial expression and subjective pain rating when the subject perceives mild pain. A previous study has reported that when the subjects rated pain score in excess of 30 mm using 100 mm VAS, most of them had recorded at least moderate pain on a 4-point categorical scale (no pain, mild pain, moderate pain, and severe pain) (Collins, Moore, & McQuay, 1997). Therefore, facial expression responding to pain stimulus was expected different after cut-off point of 30 mm on pain-VAS.
The present study investigates the relationships between the evaluation of facial expression responding to pain and self-report ratings in cases where pain rating was categorized as mild pain rating (VAS <30 mm) and moderate to strong pain rating (VAS ≥30 mm). It was hypothesized the relationship between them is weaker in mild pain ratings compared to moderate to strong pain ratings.
2. Methods
2.1. Subjects
Twelve healthy college student volunteers (7 men and 5 women) participated in this study. Subjects were 21 to 26 years old. All subjects were right-handed, native Japanese speakers, and healthy, without any history of pain or neurological disorders. Ethical approval was obtained from the Research Ethics Committee of the Nagoya University School of Health Sciences. After being informed of the purpose and protocol of the study, all subjects provided written informed consent before undergoing the experiment.
2.2. Measurements of pain- Visual Analogue Scale (VAS)
We prepared the self-made Von Frey Monofilament (VFM) for the mechanical stimulating device. The diameter of all VFMs was 1.5 mm and the length of each monofilament (GCK-60® Mitsubishi Reyon Co. Ltd., Japan) was adjusted to produce a different force (100 g, 300 g, and 600 g) because the tissue depth affected by mechanical strain varied depending on the diameter of skin contact (Graven-Nielsen, Mense, & Arendt-Nielsen, 2004; Nasu, Taguchi, & Mizumura, 2010; Takahashi et al., 2005). The examiner practiced many times to ensure that each VFM was successfully applied perpendicular to the target surface until a VFM bending of approximately 3 to 5 mm was produced (Hayashi et al., 2015). All subjects underwent mechanical pain stimuli with von Frey hair filaments (VFHs, diameter: 1.5 mm, strain forces: 100 g, 300 g, and 600 g), on three different sessions once per week. The subjects sat in a fixed chair and placed their right hands open on the desk. Each painful stimulus was given to 3 points of inter-digital sites (second-third, third-fourth, and fourth-fifth finger) of the right hand for 5 s.
Measurements were performed with three different VFMs in 60-s intervals for each stimulus. A curtain in front of the subjects was used to prevent them from viewing the stimulating filaments so as not to predict which kinds of VFM were given during the experiment. The only information subjects were given was the site of stimulus. The pain-VAS scale consisting of 100 mm lines labeled at the anchor points with “no pain” and “worst possible pain” were measured every time at the end of each stimulus. Measurements were conducted in a quiet room with the temperature kept between 25℃-27℃, and 40%-50% humidity. Videos were taken of subjects facing the front during the experiment. Subjects were only told “videos are taken holistically for our experiment.”
2.3. Evaluation of facial expression of pain
We employed 3 evaluators (48-year-old male, 42-year-old-female, and 40-year-old-female) which were healthy and did not have pain. The evaluators had not met subjects before the study and first watched the subjects on video. Facial expression responding to pain was rated by these evaluators using a 4-point categorical scale: 0: No pain; 1: Mild pain; 2: Moderate pain; and 3: Severe pain, referring to the Abbey Scale (Abbey et al., 2004; Takai et al., 2010). The evaluators scored all facial expressions for each subject, and 9 times per subject (100 g: 3 times, 300 g: 3 times, and 600 g: 3 times). Each evaluator independently scored his or her observations. The evaluation was adopted when two of three evaluators gave the same evaluation results. If the evaluations of each evaluator were completely different, the median scale was used.
In addition, weighted kappa statistics were used for the analysis of inter-rater agreement of evaluation for facial expression of pain (Kundel & Polansky, 2003). Weighted kappa statistics were calculated between each pair of evaluators.
2.4. Statistical analyses
Normality of the data was assessed by a Shapiro-Wilk test. This test showed that the data of pain-VAS and evaluation of facial expression were not normally disturbed. Therefore, data were expressed as the median and range values, and applied to non-parametric tests. The values of weighted kappa statistics were as follows: virtually no-reliability (0.00-0.10), slight-reliability (0.11-0.40), fair-reliability (0.41-0.60), moderate-reliability (0.61-0.80), and substantial-reliability (0.81-1.00) (Shrout, 1998).
The correlation between the evaluation of facial expression and the self-report pain ratings using pain-VAS were analyzed by using Spearman’s rank correlation coefficient (ρ). Samples with VAS scores less than 30 mm were defined as a low pain group. Those with scores equal to 30 mm or greater were considered to belong to high pain group (Collins et al., 1997; Gerbershagen, Rothaug, Kalkman, & Meissner, 2011; Moore, Straube, & Aldington, 2013).
The correlation between the evaluation of facial expression and VAS was analyzed for each group, respectively. The analyses were performed using SPSS (V. 24.0J; SPSS Inc., Chicago, IL, USA), and the significance was set at P<0.05. Finally, we ran a post hoc power analysis for each analysis using G* Power software (V. 3.0.10; Franz Faul, Kiel University, Kiel, Germany).
3. Results
3.1. Inter-rater agreement of evaluation of facial expression of pain
One sample (1%) of facial expression were completely different among three evaluators; no pain, mild, and moderate, respectively. It was rated as mild pain. The four level classifications of the evaluation of facial expression of pain showed a high inter-rater agreement both in mild pain ratings (VAS<30) and in strong pain ratings (VAS≥30) (kappa value=0.94-0.95) (Table 1).
3.2. The relationships between self-report pain ratings and the evaluation of facial expression
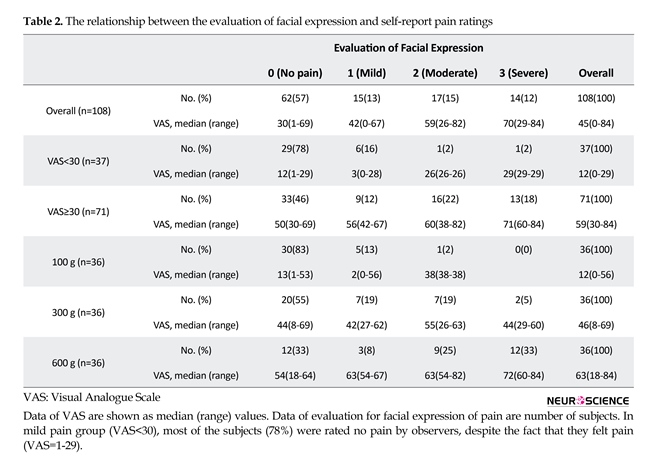
The median (range) values of VAS in overall, in mild pain ratings (VAS<30), and in strong pain ratings (VAS≥30) were 45 (0-84), 12 (0-29), and 59 (30-84), respectively (Table 2). The number of evaluations of facial expression responding to each of pain sensitivities (no pain, mild, moderate, and severe) were 62, 15, 17, 14 as overall; 29, 6, 1, 1 as low pain ratings (VAS<30); and 33, 9, 16, 13 as high pain ratings (VAS≥30); respectively (Figure 1A, Table 2). In low pain ratings (VAS<30), most subjects (78%) were rated no pain by the observers, despite the fact that they reported pain (VAS; 1-29). The number of facial expression rated mild pain or more was 6(16%) in 100 g, 16(44%) in 300 g, and 24(66%) in 600 g (Figure 1B, Table 2).
As shown in Table 3, pain ratings generally showed a moderate correlation (ρ=0.561) with the evaluation of facial expression. Next, further analysis in dichotomous group revealed that while pain ratings significantly correlated (ρ=0.611) with the evaluation of facial expression in high pain ratings; however, there was no significant correlation between them in low pain ratings. Post hoc analysis revealed that they had sufficient statistical power (>80%), respectively (Table 3).
4. DiscussionOne sample (1%) of facial expression were completely different among three evaluators; no pain, mild, and moderate, respectively. It was rated as mild pain. The four level classifications of the evaluation of facial expression of pain showed a high inter-rater agreement both in mild pain ratings (VAS<30) and in strong pain ratings (VAS≥30) (kappa value=0.94-0.95) (Table 1).
3.2. The relationships between self-report pain ratings and the evaluation of facial expression
The median (range) values of VAS in overall, in mild pain ratings (VAS<30), and in strong pain ratings (VAS≥30) were 45 (0-84), 12 (0-29), and 59 (30-84), respectively (Table 2). The number of evaluations of facial expression responding to each of pain sensitivities (no pain, mild, moderate, and severe) were 62, 15, 17, 14 as overall; 29, 6, 1, 1 as low pain ratings (VAS<30); and 33, 9, 16, 13 as high pain ratings (VAS≥30); respectively (Figure 1A, Table 2). In low pain ratings (VAS<30), most subjects (78%) were rated no pain by the observers, despite the fact that they reported pain (VAS; 1-29). The number of facial expression rated mild pain or more was 6(16%) in 100 g, 16(44%) in 300 g, and 24(66%) in 600 g (Figure 1B, Table 2).
As shown in Table 3, pain ratings generally showed a moderate correlation (ρ=0.561) with the evaluation of facial expression. Next, further analysis in dichotomous group revealed that while pain ratings significantly correlated (ρ=0.611) with the evaluation of facial expression in high pain ratings; however, there was no significant correlation between them in low pain ratings. Post hoc analysis revealed that they had sufficient statistical power (>80%), respectively (Table 3).
Overall, the evaluation of facial expression to pain correlated with self-report pain ratings. However, the correlation was only significant when the subjects were rated as high pain (VAS≥30). In other words, when the subjects were rated as low pain (VAS<30), most of them were rated no pain by observers, despite the fact that they felt pain. The results suggest that it is difficult for observer to find whether the subjects really feel pain or not, when the pain-rating is in low range, and also there is discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating depending on pain magnitude.
It has been reported that self-report pain ratings result in poorer repeatability in mild range compared to high range over different sessions (Hayashi et al., 2015; Quiton & Greenspan, 2008). Kemp et al. has reported that pain-VAS is unreliable in experimental pain when low range or pain threshold (Kemp, Despres, & Dufour, 2012). Regarding the neuronal activities, Hayashi et al. has reported that Blood Oxygenation Level Dependent (BOLD) signal by using functional Magnetic Resonance Imaging (fMRI) was inconsistent in a session with mild pain ratings (Hayashi et al., 2016). The facial expression is related to increase activities in motor-related areas as well as in areas of the thalamocortical pain processing pathways (Kunz, Chen, Lautenbacher, Vachon-Presseau, & Rainville, 2011). These backgrounds might cause discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating in low pain range.
It has been reported that facial expression to painful stimuli is more prominent in strong stimuli compared to weak stimuli (Kunz, Mylius, Schepelmann, & Lautenbacher, 2004; Lucey et al., 2012). Kunz et al. showed only moderate correlation (r=0.4) between self-report pain and facial expression. They have discussed the possibility that stronger stimulus intensity might lead to stronger correlation between them, because low intensities elicited low frequent facial responses (Kunz et al., 2004). They have also reported that facial expression started to appear and increase with stimulus intensities from 4 kg on to the thigh, which corresponded to around 30 mm on VAS (Kunz et al., 2004). While, the present study collected various ratings of pain-VAS from 0 to 84/100 using three different mechanical stimulating forces, the frequency of evaluation as a face of mild pain or more was only 16% in 100 g stimuli, but 44% in 300 g stimuli and 66% in 600 g. It is assumed that these results lead to different correlations between facial expressions and low or high pain ratings, consistent with previous reports (Kunz et al., 2004).
Although the evaluation of facial expression varied slightly among evaluators, there was strong consistency to evaluate categorical scale of pain in the subjects both for low pain ratings and high pain ratings. Someone’s behavioral indicators of pain are usually grimacing, frowning, wincing, vocalization, and restlessness in clinical practice (Puntillo et al., 1997), and each behavior has a potential to be realized by observers as existing pain. However, our experimental results suggest that such behavioral responses may be rarely found when subjects feel mild pain. Hence it is difficult for the observer to find whether the subjects really feel pain or not, when subjective pain-ratings are limited within low range.
Firstly, the facial expression reflects not only pain behavior but also other emotional responses. The facial expression could also be managed, especially by the adult subjects. Although videos were taken of the subjects facing the front with their prior approval during the experiment, subjects were only told that “videos were taken holistically for the purposes of the experiment.” The facial expression responding to pain is influenced by social context (Vlaeyen et al., 2009), sex (Kunz, Gruber, & Lautenbacher, 2006), catastrophizing (Kunz, Chatelle, Lautenbacher, & Rainville, 2008), but not influenced by age (Kunz et al., 2008). Thus, the facial expression respond ing to pain should be assessed along with such related variables. In addition, the evaluation of facial expression to pain is affected by empathy and sympathy. The evolution of recent cognitive aspects of empathy and sympathy are closely related to processes involved in theory of mind, self-regulation, and language (Decety, 2009).
Secondly, the present study results were not compared with quantitative evaluation of facial expression such as FACS. Whether the findings of the present study are applicable to people of different ethnicities is something which should also be taken into consideration. Finally, we only investigated 12 subjects. A larger study sample might shed more light on these differences.
There is a discordant relationship between the evaluation of facial expression responding to pain and self-report pain rating depending on pain magnitude. The evaluation of facial expression to pain was difficult for the observer when the subjects feel mild pain, even though facial expression is a fundamental way of pain communication. Characteristics of pain evaluation is important for clinical practice.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to express their gratitude to Matthew McLaughlin for his assistance in editing the manuscript.
Conflict of Interest
The authors certify that no affiliation or financial involvement exists between them and any organization with a direct interest in the subject matter or materials discussed in the article.
References
Abbey, J., Piller, N., Bellis, A. D., Esterman, A., Parker, D., Giles, L., et al. (2004). The abbey pain scale: A 1-minute numerical indicator for people with end-stage dementia. International Journal of Palliative Nursing, 10(1), 6–13. doi: 10.12968/ijpn.2004.10.1.12013
Apkarian, A. V., Bushnell, M. C., Treede, R.-D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463. doi: 10.1016/j.ejpain.2004.11.001
Apkarian, V. A., Hashmi, J. A., & Baliki, M. N. (2011). Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain, 152(Supplement), S49–S64. doi: 10.1016/j.pain.2010.11.010
Büchel, C., Bornhövd, K., Quante, M., Glauche, V., Bromm, B., & Weiller, C. (2002). Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. Journal of Neuroscience, 22(3), 970-6. PMID: 11826125
Coghill, R. C., McHaffie, J. G., & Yen, Y. F. (2003). Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences, 100(14), 8538–42. doi: 10.1073/pnas.1430684100
Collins, S. L., Moore, A. R., & McQuay, H. J. (1997). The visual analogue pain intensity scale: What is moderate pain in millimetres. Pain, 72(1), 95–7. doi: 10.1016/s0304-3959(97)00005-5
Decety, J. (2009). Empathy, sympathy and the perception of pain. Pain, 145(3), 365–6. doi: 10.1016/j.pain.2009.08.006
Derbyshire, S. W., Jones, A. K. ., Gyulai, F., Clark, S., Townsend, D., & Firestone, L. L. (1997). Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain, 73(3), 431–45. doi: 10.1016/s0304-3959(97)00138-3
Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(1), 279–306. doi: 10.1093/brain/118.1.279
Deyo, K. S., Prkachin, K. M., & Mercer, S. R. (2004). Development of sensitivity to facial expression of pain. Pain, 107(1), 16–21. doi: 10.1016/s0304-3959(03)00263-x
Fishbain, D. A., Goldberg, M., Rosomoff, R. S., & Rosomoff, H. L. (1991). More Munchausen with chronic pain. The Clinical Journal of Pain, 7(3), 237–44. doi: 10.1097/00002508-199109000-00012
Gerbershagen, H. J., Rothaug, J., Kalkman, C. J., & Meissner, W. (2011). Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. British Journal of Anaesthesia, 107(4), 619–26. doi: 10.1093/bja/aer195
Goebel, R. (2012). BrainVoyager: Past, present, future. Neuro Image, 62(2), 748–56. doi: 10.1016/j.neuroimage.2012.01.083
Graven Nielsen, T., Mense, S., & Arendt Nielsen, L. (2004). Painful and non-painful pressure sensations from human skeletal muscle. Experimental Brain Research, 159(3), 273–83. doi: 10.1007/s00221-004-1937-7
Hadjistavropoulos, T., Chapelle, D. L., Hadjistavropoulos, H. D., Green, S., & Asmundson, G. J. G. (2002). Using facial expressions to assess musculoskeletal pain in older persons. European Journal of Pain, 6(3), 179–87. doi: 10.1053/eujp.2001.0327
Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2015). Regional differences of repeatability on visual analogue scale with experimental mechanical pain stimuli. Neuroscience Letters, 585, 67–71. doi: 10.1016/j.neulet.2014.11.032
Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2016). Higher pain rating results in lower variability of somatosensory cortex activation by painful mechanical stimuli: An fMRI study. Clinical Neurophysiology, 127(4), 1923–8. doi: 10.1016/j.clinph.2016.01.008
Herr, K., Coyne, P. J., Key, T., Manworren, R., McCaffery, M., Merkel, S., et al. (2006). Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Management Nursing, 7(2), 44–52. doi: 10.1016/j.pmn.2006.02.003
Hofbauer, R. K., Rainville, P., Duncan, G. H., & Bushnell, M. C. (2001). Cortical representation of the sensory dimension of pain. Journal of Neurophysiology, 86(1), 402–11. doi: 10.1152/jn.2001.86.1.402
Jackson, P. L., Meltzoff, A. N., & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–9. doi: 10.1016/j.neuroimage.2004.09.006
Kemp, J., Despres, O., & Dufour, A. (2012). Unreliability of the visual analog scale in experimental pain assessment: A sensitivity and evoked potentials study. Pain Physician, 15(5), E693-9. PMID: 22996863
Kundel, H. L., & Polansky, M. (2003). Measurement of observer agreement. Radiology, 228(2), 303–8. doi: 10.1148/radiol.2282011860
Kunz, M., Chatelle, C., Lautenbacher, S., & Rainville, P. (2008). The relation between catastrophizing and facial responsiveness to pain. Pain, 140(1), 127–34. doi: 10.1016/j.pain.2008.07.019
Kunz, M., Chen, J. I., Lautenbacher, S., Vachon-Presseau, E., & Rainville, P. (2011). Cerebral regulation of facial expressions of pain. Journal of Neuroscience, 31(24), 8730–8. doi: 10.1523/jneurosci.0217-11.2011
Kunz, M., Gruber, A., & Lautenbacher, S. (2006). Sex differences in facial encoding of pain. The Journal of Pain, 7(12), 915–28. doi: 10.1016/j.jpain.2006.04.012
Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2004). On the relationship between self-report and facial expression of pain. The Journal of Pain, 5(7), 368–76. doi: 10.1016/j.jpain.2004.06.002
Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2008). Impact of age on the facial expression of pain. Journal of Psychosomatic Research, 64(3), 311–8. doi: 10.1016/j.jpsychores.2007.09.010
Kwan, C. L., Crawley, A. P., Mikulis, D. J., & Davis, K. D. (2000). An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain, 85(3), 359-74. PMID: 10781909
LeResche, L., & Dworkin, S. F. (1984). Facial expression accompanying pain. Social Science & Medicine, 19(12), 1325–30. doi: 10.1016/0277-9536(84)90020-0
Littman, G. S., Walker, B. R., & Schneider, B. E. (1985). Reassessment of verbal and visual analog ratings in analgesic studies. Clinical Pharmacology and Therapeutics, 38(1), 16–23. doi: 10.1038/clpt.1985.127
Lord, B. (2009). Paramedic assessment of pain in the cognitively impaired adult patient. BMC Emergency Medicine, 9(1). doi: 10.1186/1471-227x-9-20
Lucey, P., Cohn, J. F., Prkachin, K. M., Solomon, P. E., Chew, S., & Matthews, I. (2012). Painful monitoring: Automatic pain monitoring using the UNBC-McMaster shoulder pain expression archive database. Image and Vision Computing, 30(3), 197–205. doi: 10.1016/j.imavis.2011.12.003
Merskey, H. (1979). Pain terms: A list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain, 6, 249-52. PMID: 460932
Mogil, J. S. (2015). Social modulation of and by pain in humans and rodents. Pain, 156, S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77
Moisset, X., & Bouhassira, D. (2007). Brain imaging of neuropathic pain. NeuroImage, 37, S80–8. doi: 10.1016/j.neuroimage.2007.03.054
Moore, R. A., Straube, S., & Aldington, D. (2013). Pain measures and cut-offs - “no worse than mild pain” as a simple, universal outcome. Anaesthesia, 68(4), 400–12. doi: 10.1111/anae.12148
Nasu, T., Taguchi, T., & Mizumura, K. (2010). Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. European Journal of Pain, 14(3), 236–44. doi: 10.1016/j.ejpain.2009.05.009
Prkachin, K. M. (1992). The consistency of facial expressions of pain: a comparison across modalities. Pain, 51(3), 297–306. doi: 10.1016/0304-3959(92)90213-u
Puntillo, K. A., Miaskowski, C., Kehrle, K., Stannard, D., Gleeson, S., & Nye, P. (1997). Relationship between behavioral and physiological indicators of pain, critical care patients' self-reports of pain, and opioid administration. Critical Care Medicine, 25(7), 1159-66. doi: 10.1097/00003246-199707000-00017
Quiton, R. L., & Greenspan, J. D. (2008). Across- and within-session variability of ratings of painful contact heat stimuli. Pain, 137(2), 245–56. doi: 10.1016/j.pain.2007.08.034
Robinson, M. E., Staud, R., & Price, D. D. (2013). Pain measurement and brain activity: Will neuroimages replace pain ratings. The Journal of Pain, 14(4), 323–7. doi: 10.1016/j.jpain.2012.05.007
Rohling, M. L., Binder, L. M., & Langhinrichsen-Rohling, J. (1995). Money matters: A meta-analytic review of the association between financial compensation and the experience and treatment of chronic pain. Health Psychology, 14(6), 537–47. doi: 10.1037/0278-6133.14.6.537
Shrout, P. E. (1998). Measurement reliability and agreement in psychiatry. Statistical Methods in Medical Research, 7(3), 301–17. doi: 10.1177/096228029800700306
Singer, T. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. doi: 10.1126/science.1093535
Takahashi, K., Taguchi, T., Itoh, K., Okada, K., Kawakita, K., & Mizumura, K. (2005). Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosensory & Motor Research, 22(4), 299–305. doi: 10.1080/08990220500420475
Takai, Y., Yamamoto Mitani, N., Chiba, Y., Nishikawa, Y., Hayashi, K., & Sugai, Y. (2010). Abbey pain scale: Development and validation of the Japanese version. Geriatrics & gerontology international, 10(2), 145-53. doi: 10.1111/j.1447-0594.2009.00568.x.
Treede, R. D., Kenshalo, D. R., Gracely, R. H., & Jones, A. K. . (1999). The cortical representation of pain. Pain, 79(2), 105–11. doi: 10.1016/s0304-3959(98)00184-5
Vlaeyen, J. W. S., Hanssen, M., Goubert, L., Vervoort, T., Peters, M., van Breukelen, G., et al. (2009). Threat of pain influences social context effects on verbal pain report and facial expression. Behaviour Research and Therapy, 47(9), 774–82. doi: 10.1016/j.brat.2009.05.008
Wallenstein, S., Heidrich d, G., Kaiko, R., & Houde, R. (1980). Clinical evaluation of mild analgesics: the measurement of clinical pain. British Journal of Clinical Pharmacology, 10(S2), 319S–27. doi: 10.1111/j.1365-2125.1980.tb01816.x
Williams, A. C. de C. (2002). Facial expression of pain: An evolutionary account. Behavioral and Brain Sciences, 25(4):439-55. doi: 10.1017/s0140525x02000080
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2016/12/13 | Accepted: 2017/04/10 | Published: 2018/01/1
Received: 2016/12/13 | Accepted: 2017/04/10 | Published: 2018/01/1
References
1. Abbey, J., Piller, N., Bellis, A. D., Esterman, A., Parker, D., Giles, L., et al. (2004). The abbey pain scale: A 1-minute numerical indicator for people with end-stage dementia. International Journal of Palliative Nursing, 10(1), 6–13. doi: 10.12968/ijpn.2004.10.1.12013 [DOI:10.12968/ijpn.2004.10.1.12013]
2. Apkarian, A. V., Bushnell, M. C., Treede, R.-D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463. doi: 10.1016/j.ejpain.2004.11.001 [DOI:10.1016/j.ejpain.2004.11.001]
3. Apkarian, V. A., Hashmi, J. A., & Baliki, M. N. (2011). Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain, 152(Supplement), S49–S64. doi: 10.1016/j.pain.2010.11.010 [DOI:10.1016/j.pain.2010.11.010]
4. Büchel, C., Bornhövd, K., Quante, M., Glauche, V., Bromm, B., & Weiller, C. (2002). Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. Journal of Neuroscience, 22(3), 970-6. PMID: 11826125 [PMID]
5. Coghill, R. C., McHaffie, J. G., & Yen, Y. F. (2003). Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences, 100(14), 8538–42. doi: 10.1073/pnas.1430684100 [DOI:10.1073/pnas.1430684100]
6. Collins, S. L., Moore, A. R., & McQuay, H. J. (1997). The visual analogue pain intensity scale: What is moderate pain in millimetres. Pain, 72(1), 95–7. doi: 10.1016/s0304-3959(97)00005-5 [DOI:10.1016/S0304-3959(97)00005-5]
7. Decety, J. (2009). Empathy, sympathy and the perception of pain. Pain, 145(3), 365–6. doi: 10.1016/j.pain.2009.08.006 [DOI:10.1016/j.pain.2009.08.006]
8. Derbyshire, S. W., Jones, A. K. ., Gyulai, F., Clark, S., Townsend, D., & Firestone, L. L. (1997). Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain, 73(3), 431–45. doi: 10.1016/s0304-3959(97)00138-3 [DOI:10.1016/S0304-3959(97)00138-3]
9. Devinsky, O., Morrell, M. J., & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(1), 279–306. doi: 10.1093/brain/118.1.279 [DOI:10.1093/brain/118.1.279]
10. Deyo, K. S., Prkachin, K. M., & Mercer, S. R. (2004). Development of sensitivity to facial expression of pain. Pain, 107(1), 16–21. doi: 10.1016/s0304-3959(03)00263-x [DOI:10.1016/S0304-3959(03)00263-X]
11. Fishbain, D. A., Goldberg, M., Rosomoff, R. S., & Rosomoff, H. L. (1991). More Munchausen with chronic pain. The Clinical Journal of Pain, 7(3), 237–44. doi: 10.1097/00002508-199109000-00012 [DOI:10.1097/00002508-199109000-00012]
12. Gerbershagen, H. J., Rothaug, J., Kalkman, C. J., & Meissner, W. (2011). Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. British Journal of Anaesthesia, 107(4), 619–26. doi: 10.1093/bja/aer195 [DOI:10.1093/bja/aer195]
13. Goebel, R. (2012). BrainVoyager: Past, present, future. Neuro Image, 62(2), 748–56. doi: 10.1016/j.neuroimage.2012.01.083 [DOI:10.1016/j.neuroimage.2012.01.083]
14. Graven Nielsen, T., Mense, S., & Arendt Nielsen, L. (2004). Painful and non-painful pressure sensations from human skeletal muscle. Experimental Brain Research, 159(3), 273–83. doi: 10.1007/s00221-004-1937-7 [DOI:10.1007/s00221-004-1937-7]
15. Hadjistavropoulos, T., Chapelle, D. L., Hadjistavropoulos, H. D., Green, S., & Asmundson, G. J. G. (2002). Using facial expressions to assess musculoskeletal pain in older persons. European Journal of Pain, 6(3), 179–87. doi: 10.1053/eujp.2001.0327 [DOI:10.1053/eujp.2001.0327]
16. Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2015). Regional differences of repeatability on visual analogue scale with experimental mechanical pain stimuli. Neuroscience Letters, 585, 67–71. doi: 10.1016/j.neulet.2014.11.032 [DOI:10.1016/j.neulet.2014.11.032]
17. Hayashi, K., Ikemoto, T., Ueno, T., Arai, Y. C. P., Shimo, K., Nishihara, M., et al. (2016). Higher pain rating results in lower variability of somatosensory cortex activation by painful mechanical stimuli: An fMRI study. Clinical Neurophysiology, 127(4), 1923–8. doi: 10.1016/j.clinph.2016.01.008 [DOI:10.1016/j.clinph.2016.01.008]
18. Herr, K., Coyne, P. J., Key, T., Manworren, R., McCaffery, M., Merkel, S., et al. (2006). Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Management Nursing, 7(2), 44–52. doi: 10.1016/j.pmn.2006.02.003 [DOI:10.1016/j.pmn.2006.02.003]
19. Hofbauer, R. K., Rainville, P., Duncan, G. H., & Bushnell, M. C. (2001). Cortical representation of the sensory dimension of pain. Journal of Neurophysiology, 86(1), 402–11. doi: 10.1152/jn.2001.86.1.402 [DOI:10.1152/jn.2001.86.1.402]
20. Jackson, P. L., Meltzoff, A. N., & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–9. doi: 10.1016/j.neuroimage.2004.09.006 [DOI:10.1016/j.neuroimage.2004.09.006]
21. Kemp, J., Despres, O., & Dufour, A. (2012). Unreliability of the visual analog scale in experimental pain assessment: A sensitivity and evoked potentials study. Pain Physician, 15(5), E693-9. PMID: 22996863 [PMID]
22. Kundel, H. L., & Polansky, M. (2003). Measurement of observer agreement. Radiology, 228(2), 303–8. doi: 10.1148/radiol.2282011860 [DOI:10.1148/radiol.2282011860]
23. Kunz, M., Chatelle, C., Lautenbacher, S., & Rainville, P. (2008). The relation between catastrophizing and facial responsiveness to pain. Pain, 140(1), 127–34. doi: 10.1016/j.pain.2008.07.019 [DOI:10.1016/j.pain.2008.07.019]
24. Kunz, M., Chen, J. I., Lautenbacher, S., Vachon-Presseau, E., & Rainville, P. (2011). Cerebral regulation of facial expressions of pain. Journal of Neuroscience, 31(24), 8730–8. doi: 10.1523/jneurosci.0217-11.2011 [DOI:10.1523/JNEUROSCI.0217-11.2011]
25. Kunz, M., Gruber, A., & Lautenbacher, S. (2006). Sex differences in facial encoding of pain. The Journal of Pain, 7(12), 915–28. doi: 10.1016/j.jpain.2006.04.012 [DOI:10.1016/j.jpain.2006.04.012]
26. Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2004). On the relationship between self-report and facial expression of pain. The Journal of Pain, 5(7), 368–76. doi: 10.1016/j.jpain.2004.06.002 [DOI:10.1016/j.jpain.2004.06.002]
27. Kunz, M., Mylius, V., Schepelmann, K., & Lautenbacher, S. (2008). Impact of age on the facial expression of pain. Journal of Psychosomatic Research, 64(3), 311–8. doi: 10.1016/j.jpsychores.2007.09.010 [DOI:10.1016/j.jpsychores.2007.09.010]
28. Kwan, C. L., Crawley, A. P., Mikulis, D. J., & Davis, K. D. (2000). An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain, 85(3), 359-74. PMID: 10781909 [DOI:10.1016/S0304-3959(99)00287-0]
29. LeResche, L., & Dworkin, S. F. (1984). Facial expression accompanying pain. Social Science & Medicine, 19(12), 1325–30. doi: 10.1016/0277-9536(84)90020-0 [DOI:10.1016/0277-9536(84)90020-0]
30. Littman, G. S., Walker, B. R., & Schneider, B. E. (1985). Reassessment of verbal and visual analog ratings in analgesic studies. Clinical Pharmacology and Therapeutics, 38(1), 16–23. doi: 10.1038/clpt.1985.127 [DOI:10.1038/clpt.1985.127]
31. Lord, B. (2009). Paramedic assessment of pain in the cognitively impaired adult patient. BMC Emergency Medicine, 9(1). doi: 10.1186/1471-227x-9-20 [DOI:10.1186/1471-227X-9-20]
32. Lucey, P., Cohn, J. F., Prkachin, K. M., Solomon, P. E., Chew, S., & Matthews, I. (2012). Painful monitoring: Automatic pain monitoring using the UNBC-McMaster shoulder pain expression archive database. Image and Vision Computing, 30(3), 197–205. doi: 10.1016/j.imavis.2011.12.003 [DOI:10.1016/j.imavis.2011.12.003]
33. Merskey, H. (1979). Pain terms: A list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain, 6, 249-52. PMID: 460932
34. Mogil, J. S. (2015). Social modulation of and by pain in humans and rodents. Pain, 156, S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77 [DOI:10.1097/01.j.pain.0000460341.62094.77]
35. Moisset, X., & Bouhassira, D. (2007). Brain imaging of neuropathic pain. NeuroImage, 37, S80–8. doi: 10.1016/j.neuroimage.2007.03.054 [DOI:10.1016/j.neuroimage.2007.03.054]
36. Moore, R. A., Straube, S., & Aldington, D. (2013). Pain measures and cut-offs - "no worse than mild pain" as a simple, universal outcome. Anaesthesia, 68(4), 400–12. doi: 10.1111/anae.12148 [DOI:10.1111/anae.12148]
37. Nasu, T., Taguchi, T., & Mizumura, K. (2010). Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. European Journal of Pain, 14(3), 236–44. doi: 10.1016/j.ejpain.2009.05.009 [DOI:10.1016/j.ejpain.2009.05.009]
38. Prkachin, K. M. (1992). The consistency of facial expressions of pain: a comparison across modalities. Pain, 51(3), 297–306. doi: 10.1016/0304-3959(92)90213-u [DOI:10.1016/0304-3959(92)90213-U]
39. Puntillo, K. A., Miaskowski, C., Kehrle, K., Stannard, D., Gleeson, S., & Nye, P. (1997). Relationship between behavioral and physiological indicators of pain, critical care patients' self-reports of pain, and opioid administration. Critical Care Medicine, 25(7), 1159-66. doi: 10.1097/00003246-199707000-00017 [DOI:10.1097/00003246-199707000-00017]
40. Quiton, R. L., & Greenspan, J. D. (2008). Across- and within-session variability of ratings of painful contact heat stimuli. Pain, 137(2), 245–56. doi: 10.1016/j.pain.2007.08.034 [DOI:10.1016/j.pain.2007.08.034]
41. Robinson, M. E., Staud, R., & Price, D. D. (2013). Pain measurement and brain activity: Will neuroimages replace pain ratings. The Journal of Pain, 14(4), 323–7. doi: 10.1016/j.jpain.2012.05.007 [DOI:10.1016/j.jpain.2012.05.007]
42. Rohling, M. L., Binder, L. M., & Langhinrichsen-Rohling, J. (1995). Money matters: A meta-analytic review of the association between financial compensation and the experience and treatment of chronic pain. Health Psychology, 14(6), 537–47. doi: 10.1037/0278-6133.14.6.537 [DOI:10.1037/0278-6133.14.6.537]
43. Shrout, P. E. (1998). Measurement reliability and agreement in psychiatry. Statistical Methods in Medical Research, 7(3), 301–17. doi: 10.1177/096228029800700306 [DOI:10.1177/096228029800700306]
44. Singer, T. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. doi: 10.1126/science.1093535 [DOI:10.1126/science.1093535]
45. Takahashi, K., Taguchi, T., Itoh, K., Okada, K., Kawakita, K., & Mizumura, K. (2005). Influence of surface anesthesia on the pressure pain threshold measured with different-sized probes. Somatosensory & Motor Research, 22(4), 299–305. doi: 10.1080/08990220500420475 [DOI:10.1080/08990220500420475]
46. Takai, Y., Yamamoto Mitani, N., Chiba, Y., Nishikawa, Y., Hayashi, K., & Sugai, Y. (2010). Abbey pain scale: Development and validation of the Japanese version. Geriatrics & gerontology international, 10(2), 145-53. doi: 10.1111/j.1447-0594.2009.00568.x. [DOI:10.1111/j.1447-0594.2009.00568.x]
47. Treede, R. D., Kenshalo, D. R., Gracely, R. H., & Jones, A. K. . (1999). The cortical representation of pain. Pain, 79(2), 105–11. doi: 10.1016/s0304-3959(98)00184-5 [DOI:10.1016/S0304-3959(98)00184-5]
48. Vlaeyen, J. W. S., Hanssen, M., Goubert, L., Vervoort, T., Peters, M., van Breukelen, G., et al. (2009). Threat of pain influences social context effects on verbal pain report and facial expression. Behaviour Research and Therapy, 47(9), 774–82. doi: 10.1016/j.brat.2009.05.008 [DOI:10.1016/j.brat.2009.05.008]
49. Wallenstein, S., Heidrich d, G., Kaiko, R., & Houde, R. (1980). Clinical evaluation of mild analgesics: the measurement of clinical pain. British Journal of Clinical Pharmacology, 10(S2), 319S–27. doi: 10.1111/j.1365-2125.1980.tb01816.x [DOI:10.1111/j.1365-2125.1980.tb01816.x]
50. Williams, A. C. de C. (2002). Facial expression of pain: An evolutionary account. Behavioral and Brain Sciences, 25(4):439-55. doi: 10.1017/s0140525x02000080 [DOI:10.1017/S0140525X02000080]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |