Volume 16, Issue 6 (November & December 2025)
BCN 2025, 16(6): 1169-1178 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sadeghi H S, Rezaei Tavirani M, Abrishami M, Hamzeloo-Moghadam M. Flaxseed (Linum usitatissimum) Modulates Oxytocin Signaling Pathway via CALM3-mediated Calcium Signaling: A Protein-protein Interaction Network and Functional Enrichment Analysis. BCN 2025; 16 (6) :1169-1178
URL: http://bcn.iums.ac.ir/article-1-3232-en.html
URL: http://bcn.iums.ac.ir/article-1-3232-en.html
1- Department of Traditional Pharmacy, Traditional Medicine and Materia Medica Research Center, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Proteomics Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Proteomics Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Linum usitatissimum, Oxytocin (OXT) signaling, Anxiety, CALM3, Protein-protein interaction (PPI) network
Full-Text [PDF 2114 kb]
| Abstract (HTML)
Full-Text:
Introduction
Linum usitatissimum (Linn.), commonly known as flax, belongs to the Linaceae family (Watson & Preedy, 2012). This species is known for its striking pale-blue flowers with five distinct petals. The seeds produced by flax plants, referred to as flaxseeds, are characterized by their flat, pointed, oval shapes (Ansari et al., 2019). The entire plant is commercially utilized, either in its raw state or after undergoing processing (Singh et al., 2011). Flaxseed has been incorporated into the human diet since the dawn of the first civilizations. In ancient Egypt and Greece, it was used for medicinal purposes (University of Wisconsin--Extension et al., 1990). Flaxseeds are known for their significant nutritional benefits and are a primary source of omega-3 fatty acids, particularly α-linolenic acid. They are also abundant in short-chain polyunsaturated fatty acids, soluble and insoluble fibers, phytoestrogenic lignans, such as secoisolariciresinol diglycoside, proteins, and a range of antioxidants (Ivanov et al., 2011; Oomah, 2001; Singh et al., 2011; Touré & Xueming, 2010). The properties of these compounds make L. usitatissimum a suitable choice for addressing various health concerns, including respiratory issues, neurological disorders, diabetes, gastrointestinal problems, constipation, abdominal pain, urinary infections, and skin inflammation (Al-Madhagy et al., 2023; Mueed et al., 2022). A notable feature of flaxseed, as demonstrated by multiple studies, is its capacity to reduce anxiety (Al-Madhagy et al., 2023; Anis et al., 2016).
Generalized anxiety disorder (GAD) is a persistent and incapacitating condition that impacts approximately 6.1% of people throughout their lives. Sensations of fear, unease, and discomfort define anxiety. It may present physically with symptoms, such as perspiration, restlessness, muscle tension, and an elevated heart rate (DeMartini et al., 2019). Available treatment options encompass psychological therapies and pharmacological interventions (Hoge et al., 2012). Global guidelines for the management of GAD recommend selective serotonin reuptake inhibitors, serotonin and noradrenaline reuptake inhibitors, and pregabalin as primary treatment options, due to their proven effectiveness and favorable safety profiles (Bandelow et al., 2023).
Oxytocin (OXT), a neuropeptide composed of nine amino acids, was the first human hormone to be identified (Carter, 2022). This molecule is particularly known for its role in stimulating uterine contractions. Furthermore, OXT has been proposed as an anti-anxiety agent in various studies (Gully et al., 2024). Although OXT is released under various physiological conditions, research has indicated that its secretion can also be triggered by external stimuli, such as flaxseed extract (Lecová et al., 2024; Sirotkin, 2023).
Natural products constitute a substantial portion of modern pharmaceutical agents, especially in disease treatment (Pal & Shukla, 2003). A unique strategy to deepen our understanding of the therapeutic mechanisms of active compounds present in medicinal plants involves predicting the gene networks they influence (Shao & Zhang, 2013). The analysis of protein-protein interactions (PPI) is beneficial for pinpointing this essential aspect (Tang et al., 2023). PPI network analysis elucidates the PPIs within a biological context (Rezaei-Tavirani et al., 2022). Although the literature has documented the anti-anxiety effects of flaxseed, its molecular mechanism remains poorly understood. This study aimed to demonstrate its molecular mechanism through the analysis of PPI networks.
Materials and Methods
This study examined the gene expression profiles triggered by flaxseed using a thorough bioinformatics methodology. Gene expression data were meticulously gathered from the Gene Expression Omnibus (GEO) dataset GSE36422 (National Center for Biotechnology Information, 2025), utilizing Gene Expression Omnibus (GEO)2R (National Center for Biotechnology Information, 2025) for the comparative analysis of expressed genes among various experimental groups (Subramanian et al., 2023). The initial data processing included a detailed Venn diagram (Figure 1) to clarify the gene expression profiles uniquely linked to flaxseed intervention. Bioinformatics techniques were employed to elucidate the molecular mechanisms underlying flaxseed-induced gene expression.

A comprehensive analytical approach was adopted, beginning with functional enrichment analysis using KEGG pathway and gene ontology (GO) methodologies. To gain a thorough understanding of molecular interactions, the genes overlapping with flaxseed differentially expressed genes (DEGs) and OXT signaling pathways were subsequently analyzed. To validate the targeted genes, a PPI network for the OXT signaling pathway was constructed using STRING (version 11.5), with interaction confidence carefully filtered to a minimum score of 0.4 (Szklarczyk et al., 2023). Subsequently, network analysis was performed. Cytoscape (version 3.10.3) was utilized (Shannon et al., 2023), employing CytoHubba’s Maximal Clique Centrality (MCC) algorithm to rank hub proteins; betweenness centrality was used to identify bottlenecks (threshold: Top 5 nodes), and functional analysis was conducted with the ClueGo plugin, utilizing a kappa score of 0.4. Furthermore, the key genes associated with OXT in the context of flaxseed were investigated within the network.
Results
The Venn diagram depicting gene expression induced by flaxseed from GSE36422 shows 98 significant genes linked to flaxseed, of which 56 were retained after data cleaning. The 56 genes were further analyzed. The data were assessed using Venn diagrams comparing Radiation–(flaxseed-radiation), control-radiation, flaxseed-control, and (flaxseed-radiation). The Venn diagram revealed that 98 DEGs were significantly different between the flaxseed and the control groups (Figure 1). Analysis using a volcano plot indicated a significant difference in gene expression profiles between samples fed a 10% flaxseed diet for three weeks (flaxseed group) and untreated samples. The box plot for the flaxseed-control comparison demonstrated that all gene expression profiles were median-centered and comparable. Likewise, the density plot exhibited a consistent pattern in the flaxseed-control analysis. After eliminating duplicate genes and uncharacterized entries, the 98 significant DEGs were reduced to 56, representing genes significantly influenced by the presence of flaxseed.
Pathway analysis identifies three significant genes: CALM3 (calmodulin3), NFATC4 (nuclear factor of activated T cells 4), and RAF1 (Raf-1 Proto-Oncogene), which are prevalent in both the flaxseed and OXT signaling pathways (Figure 2 and Figure 3).

KEGG pathway enrichment analysis revealed several pathways characterized by high counts and the most elevated logP values, including the C-type lectin receptor signaling pathway, the OXT signaling pathway, cellular senescence, and the cyclic guanosine monophosphate-activated protein kinase G (cGMP-PKG) signaling pathway. These genes are crucial across all pathways, with CALM3 up-regulated (overexpressed) and NFATC4 and RAF1 down-regulated (underexpressed).

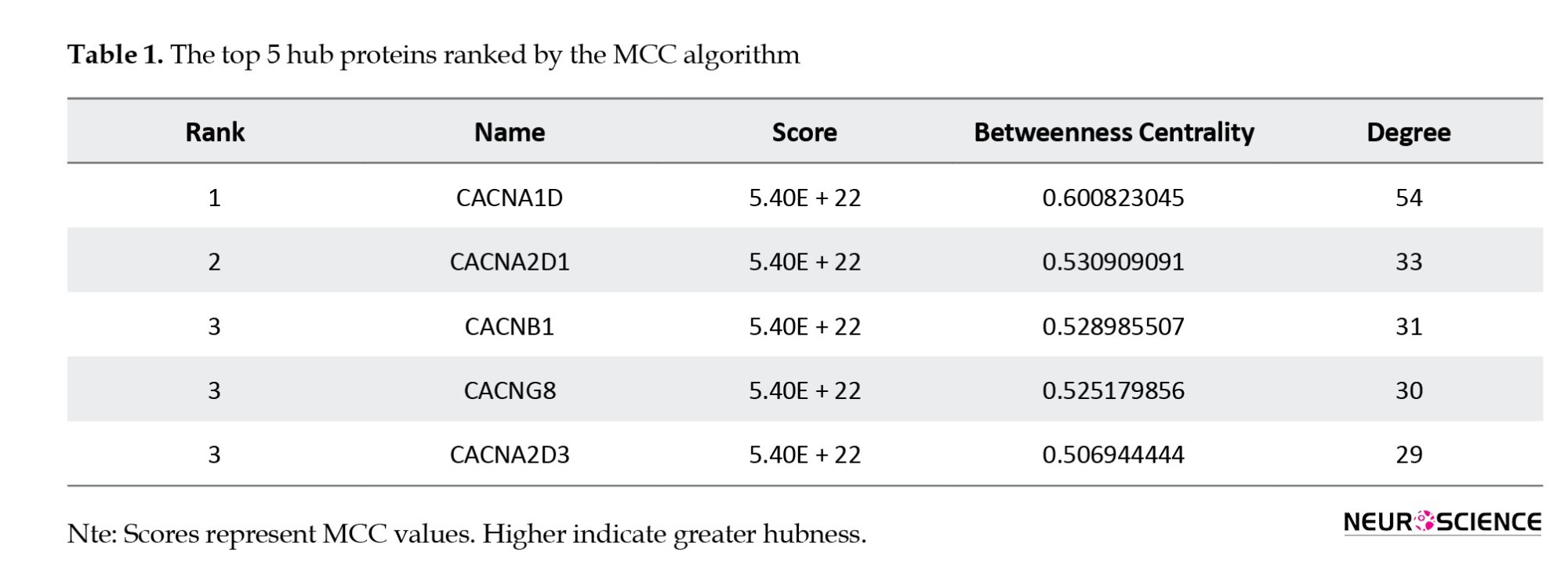
GO analysis, depicted with various colored lines, illustrates distinct biological pathways, where the size of each node reflects the relative significance or connectivity of each gene within the network. CALM3 is represented as a red node, indicating its involvement in multiple signaling pathways, such as cellular senescence, OXT signaling, C-type lectin receptor signaling, and GnRH signaling, and its associations with viral infection pathways (specifically HIV-1 and cytomegalovirus). Color coding indicates upregulation (red on the log2 fold change scale). NFATC4, depicted as a purple node within the network, is linked to pathways associated with long-term potentiation, viral infections, and GnRH signaling. This transcription factor is essential for immune response and cellular development. Color coding indicates downregulation (purple in the log2 fold change scale). RAF1, illustrated as a blue node, is significantly connected to various pathways: cGMP-PKG signaling, responses to viral infections, and long-term potentiation. The color coding also suggests it is downregulated (purple in the log2 fold change scale) (Figure 2). The genes related to the OXT signaling pathway included CALM3, NFATC4, and RAF1, with a p-adj value of 0.0017. RAF1 is involved in the MAPK signaling pathway, CALM3 regulates calcium channels, and NFATC4 negatively regulates the OXT pathway (Figure 2). The precise positioning of each gene within the OXT signaling pathway is as follows: RAF1 is integrated into the MAPK signaling pathway, activated downstream of rat sarcoma (Ras), which is stimulated by the activation of the OXT receptor. It phosphorylates and activates MEK1/2, which subsequently activates ERK1/2. The activation of ERK1/2 results in the transcriptional regulation of genes crucial for cardiomyocyte proliferation, differentiation, and survival. RAF1 is vital in enhancing the parturition cascade (the process of uterine contraction during labor). NFATC4 operates downstream of calcium signaling, activated by calcium-dependent signaling via calcineurin (CALN). Once dephosphorylated by calcineurin, NFATC4 migrates to the nucleus to influence gene expression. It plays a role in the negative regulation of OXT signaling in myometrial cells by regulating genes, such as RCAN1 and RGS2, that modulate OXT receptor signaling and calcium dynamics. CALM3 plays a pivotal role in calcium signaling by binding to calcium ions, thereby functioning as a calcium sensor. Additionally, it activates downstream targets, such as calmodulin-dependent kinase and calcineurin (CALN). Through these interactions, CALM3 regulates myosin light chain kinase, an essential regulator of muscle contraction in both myometrial and mammary gland cells. In summary, RAF1 is instrumental in mediating MAPK signaling pathways that facilitate proliferation and differentiation. NFATC4 regulates transcription in response to calcium signaling, thereby modulating OXT signaling. CALM3 serves as a calcium sensor that governs various downstream pathways, including those involved in contraction and metabolism. These genes are vital for the physiological responses triggered by OXT, which encompass uterine contraction, cardiovascular effects, and cellular proliferation (Figure 3). A collection of 147 proteins associated with the OXT signaling pathway was assembled utilizing STRING and subsequently examined in Cytoscape. Summary statistics of the network: Number of nodes: 147, number of edges: 2172, average number of neighbors: 29.551, network density: 0.202, network heterogeneity: 0.644, network centralization: 0.406. Among these proteins, 86 (highlighted in red) were the primary neighbors of CALM3, and 91 of the 147 proteins were the primary neighbors of the three specified genes. The top five hub and bottleneck proteins were identified using the Cyto-Hubba plugin (Tables 1 and 2).

Within the first neighbor nodes, there was one hub protein (denoted by a diamond shape) and five bottleneck proteins, including CALM3 (denoted by a rectangle shape) (Figure 4).

Discussion
Anxiety is recognized as one of the most prevalent mental disorders in society. In the context of Iranian traditional medicine literature, anxiety is perceived differently (Kaplan et al., 2007). The term “Khuf” refers to the unease concerning a potentially adverse situation that may occur in the future (Motovaseliyan et al., 2016). Avicenna identified flaxseed as a valuable therapeutic approach for managing anxiety (Avecinna, 2005). Flaxseed (L. usitatissimum L., Linaceae), rich in biologically active compounds, is recognized for its nutritional, physiological, and therapeutic properties (Sirotkin, 2023). Research conducted both in vivo and in vitro has provided evidence of hormonal regulation by flaxseed, including stimulation of OXT expression (Sirotkin, 2023; Tou et al., 1999). Lecová et al. (2024) found that pigs fed a diet enriched with 10% flaxseed showed increased OXT levels. Additionally, Vlčková et al. (2022) indicated that flaxseed has a positive impact on OXT ’s physiological effects by offering a protective mechanism for OXT receptors in the presence of xylene. In this study, we illustrated the regulation of various biological pathways by flaxseed-induced DEGs, including the C-type lectin receptor signaling pathway, the OXT signaling pathway, cellular senescence, and the cGMP-PKG signaling pathway, as determined by functional enrichment analysis. The anti-anxiety properties of OXT are well-documented and extensively researched in medical sciences. Tang et al. (2024) demonstrated that the intranasal administration of OXT alleviated post-surgical anxiety in mice. Furthermore, Chaipunko et al. (2025) revealed that OXT can modulate the hypothalamic-pituitary-adrenal (HPA) axis and mitigate anxiety.
GO analysis identified three genes (CALM3, RAF1, NFACT4) among 56 flaxseed-induced DEGs as the most significant and relevant common genes linking flaxseed to the OXT pathway. Among these three pivotal genes, CALM3 stands out as the most crucial, exhibiting distinct characteristics and regulatory behaviors. In contrast to its counterparts, RAF1 and NFATC4, which were downregulated, CALM3 was upregulated, and its remarkable position within the network designates it as a vital mediator in this molecular cascade. This interpretation is further reinforced by NFATC4’s established role as a negative regulator of the OXT pathway. Consequently, its downregulation is anticipated to lead to diminished inhibition and enhanced OXT signaling. The network analysis further highlighted CALM3 as a bottleneck, sustaining first-neighbor interactions with five other essential proteins (four bottleneck proteins and one hub protein). Furthermore, it exhibited remarkable connectivity with 85 first neighbors, accounting for 58% of all proteins involved in the OXT signaling pathway. This approach implies that CALM3 functions as a master regulator within the network, orchestrating various signaling cascades and ensuring proper information flow throughout the system. The significant impact of CALM3 within the network strongly supports its position as the primary molecular mediator, through which flaxseed may exert its anxiolytic effects via the OXT pathway. OXT, a neuropeptide that plays a role in social bonding, reproductive functions, and stress responses, primarily exerts its effects by activating its receptors, thereby increasing intracellular calcium levels. The rise in calcium activates CALM3, which facilitates downstream signaling processes, such as the activation of the MAPK pathway and the RhoA/Rho kinase pathway, both of which are associated with smooth muscle contraction and other physiological responses to OXT (Bakos et al., 2018; Munk et al., 2022; Uvnaes Moberg et al., 2022). Moreover, CALM3 may also affect cardiovascular functions regulated by OXT, underscoring its potential role in integrating calcium signaling with OXT ’s effects on the cardiovascular system (Ayar et al., 2014; Hirasawa et al., 2001; Rabow et al., 2023). Therefore, CALM3 acts as a vital link between calcium signaling and the OXT ’s physiological effects. Additionally, it offers potentially significant insights for therapeutic strategies in anxiety management through dietary interventions.
Conclusion
This study clarifies a new mechanism by which flaxseed influences anxiety via the OXT pathway. In silico analysis identifies three common genes associated with both anxiety and flaxseed. These genes are crucial in the OXT signaling pathway, with CALM3 identified as a primary calcium-sensing regulator. Simultaneously, the downregulation of NFATC4 and RAF1 further facilitates the disinhibition of OXT signaling and the modulation of the MAPK cascade. These results not only affirm the traditional applications of flaxseed but also underscore its potential as a dietary supplement for anxiety disorders. Future investigations should confirm these mechanisms through clinical trials and examine the synergistic effects of conventional treatments.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was derived from the PhD dissertation of Hajar Sadat Sadeghi, approved by the Department of Traditional Pharmacy, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This study was financially supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No.: 43006118).
Authors' contributions
Supervision: Mostafa Rezaei Tavirani and Maryam Hamzeloo-Moghadam; Methodology: Hajar Sadat Sadeghi and Melika Abrishami; Data collection: Hajar Sadat Sadeghi Sadeghi and Melika Abrishami; Data analysis: Hajar Sadat Sadeghi Sadeghi and Melika Abrishami; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Linum usitatissimum (Linn.), commonly known as flax, belongs to the Linaceae family (Watson & Preedy, 2012). This species is known for its striking pale-blue flowers with five distinct petals. The seeds produced by flax plants, referred to as flaxseeds, are characterized by their flat, pointed, oval shapes (Ansari et al., 2019). The entire plant is commercially utilized, either in its raw state or after undergoing processing (Singh et al., 2011). Flaxseed has been incorporated into the human diet since the dawn of the first civilizations. In ancient Egypt and Greece, it was used for medicinal purposes (University of Wisconsin--Extension et al., 1990). Flaxseeds are known for their significant nutritional benefits and are a primary source of omega-3 fatty acids, particularly α-linolenic acid. They are also abundant in short-chain polyunsaturated fatty acids, soluble and insoluble fibers, phytoestrogenic lignans, such as secoisolariciresinol diglycoside, proteins, and a range of antioxidants (Ivanov et al., 2011; Oomah, 2001; Singh et al., 2011; Touré & Xueming, 2010). The properties of these compounds make L. usitatissimum a suitable choice for addressing various health concerns, including respiratory issues, neurological disorders, diabetes, gastrointestinal problems, constipation, abdominal pain, urinary infections, and skin inflammation (Al-Madhagy et al., 2023; Mueed et al., 2022). A notable feature of flaxseed, as demonstrated by multiple studies, is its capacity to reduce anxiety (Al-Madhagy et al., 2023; Anis et al., 2016).
Generalized anxiety disorder (GAD) is a persistent and incapacitating condition that impacts approximately 6.1% of people throughout their lives. Sensations of fear, unease, and discomfort define anxiety. It may present physically with symptoms, such as perspiration, restlessness, muscle tension, and an elevated heart rate (DeMartini et al., 2019). Available treatment options encompass psychological therapies and pharmacological interventions (Hoge et al., 2012). Global guidelines for the management of GAD recommend selective serotonin reuptake inhibitors, serotonin and noradrenaline reuptake inhibitors, and pregabalin as primary treatment options, due to their proven effectiveness and favorable safety profiles (Bandelow et al., 2023).
Oxytocin (OXT), a neuropeptide composed of nine amino acids, was the first human hormone to be identified (Carter, 2022). This molecule is particularly known for its role in stimulating uterine contractions. Furthermore, OXT has been proposed as an anti-anxiety agent in various studies (Gully et al., 2024). Although OXT is released under various physiological conditions, research has indicated that its secretion can also be triggered by external stimuli, such as flaxseed extract (Lecová et al., 2024; Sirotkin, 2023).
Natural products constitute a substantial portion of modern pharmaceutical agents, especially in disease treatment (Pal & Shukla, 2003). A unique strategy to deepen our understanding of the therapeutic mechanisms of active compounds present in medicinal plants involves predicting the gene networks they influence (Shao & Zhang, 2013). The analysis of protein-protein interactions (PPI) is beneficial for pinpointing this essential aspect (Tang et al., 2023). PPI network analysis elucidates the PPIs within a biological context (Rezaei-Tavirani et al., 2022). Although the literature has documented the anti-anxiety effects of flaxseed, its molecular mechanism remains poorly understood. This study aimed to demonstrate its molecular mechanism through the analysis of PPI networks.
Materials and Methods
This study examined the gene expression profiles triggered by flaxseed using a thorough bioinformatics methodology. Gene expression data were meticulously gathered from the Gene Expression Omnibus (GEO) dataset GSE36422 (National Center for Biotechnology Information, 2025), utilizing Gene Expression Omnibus (GEO)2R (National Center for Biotechnology Information, 2025) for the comparative analysis of expressed genes among various experimental groups (Subramanian et al., 2023). The initial data processing included a detailed Venn diagram (Figure 1) to clarify the gene expression profiles uniquely linked to flaxseed intervention. Bioinformatics techniques were employed to elucidate the molecular mechanisms underlying flaxseed-induced gene expression.

A comprehensive analytical approach was adopted, beginning with functional enrichment analysis using KEGG pathway and gene ontology (GO) methodologies. To gain a thorough understanding of molecular interactions, the genes overlapping with flaxseed differentially expressed genes (DEGs) and OXT signaling pathways were subsequently analyzed. To validate the targeted genes, a PPI network for the OXT signaling pathway was constructed using STRING (version 11.5), with interaction confidence carefully filtered to a minimum score of 0.4 (Szklarczyk et al., 2023). Subsequently, network analysis was performed. Cytoscape (version 3.10.3) was utilized (Shannon et al., 2023), employing CytoHubba’s Maximal Clique Centrality (MCC) algorithm to rank hub proteins; betweenness centrality was used to identify bottlenecks (threshold: Top 5 nodes), and functional analysis was conducted with the ClueGo plugin, utilizing a kappa score of 0.4. Furthermore, the key genes associated with OXT in the context of flaxseed were investigated within the network.
Results
The Venn diagram depicting gene expression induced by flaxseed from GSE36422 shows 98 significant genes linked to flaxseed, of which 56 were retained after data cleaning. The 56 genes were further analyzed. The data were assessed using Venn diagrams comparing Radiation–(flaxseed-radiation), control-radiation, flaxseed-control, and (flaxseed-radiation). The Venn diagram revealed that 98 DEGs were significantly different between the flaxseed and the control groups (Figure 1). Analysis using a volcano plot indicated a significant difference in gene expression profiles between samples fed a 10% flaxseed diet for three weeks (flaxseed group) and untreated samples. The box plot for the flaxseed-control comparison demonstrated that all gene expression profiles were median-centered and comparable. Likewise, the density plot exhibited a consistent pattern in the flaxseed-control analysis. After eliminating duplicate genes and uncharacterized entries, the 98 significant DEGs were reduced to 56, representing genes significantly influenced by the presence of flaxseed.
Pathway analysis identifies three significant genes: CALM3 (calmodulin3), NFATC4 (nuclear factor of activated T cells 4), and RAF1 (Raf-1 Proto-Oncogene), which are prevalent in both the flaxseed and OXT signaling pathways (Figure 2 and Figure 3).

KEGG pathway enrichment analysis revealed several pathways characterized by high counts and the most elevated logP values, including the C-type lectin receptor signaling pathway, the OXT signaling pathway, cellular senescence, and the cyclic guanosine monophosphate-activated protein kinase G (cGMP-PKG) signaling pathway. These genes are crucial across all pathways, with CALM3 up-regulated (overexpressed) and NFATC4 and RAF1 down-regulated (underexpressed).

GO analysis, depicted with various colored lines, illustrates distinct biological pathways, where the size of each node reflects the relative significance or connectivity of each gene within the network. CALM3 is represented as a red node, indicating its involvement in multiple signaling pathways, such as cellular senescence, OXT signaling, C-type lectin receptor signaling, and GnRH signaling, and its associations with viral infection pathways (specifically HIV-1 and cytomegalovirus). Color coding indicates upregulation (red on the log2 fold change scale). NFATC4, depicted as a purple node within the network, is linked to pathways associated with long-term potentiation, viral infections, and GnRH signaling. This transcription factor is essential for immune response and cellular development. Color coding indicates downregulation (purple in the log2 fold change scale). RAF1, illustrated as a blue node, is significantly connected to various pathways: cGMP-PKG signaling, responses to viral infections, and long-term potentiation. The color coding also suggests it is downregulated (purple in the log2 fold change scale) (Figure 2). The genes related to the OXT signaling pathway included CALM3, NFATC4, and RAF1, with a p-adj value of 0.0017. RAF1 is involved in the MAPK signaling pathway, CALM3 regulates calcium channels, and NFATC4 negatively regulates the OXT pathway (Figure 2). The precise positioning of each gene within the OXT signaling pathway is as follows: RAF1 is integrated into the MAPK signaling pathway, activated downstream of rat sarcoma (Ras), which is stimulated by the activation of the OXT receptor. It phosphorylates and activates MEK1/2, which subsequently activates ERK1/2. The activation of ERK1/2 results in the transcriptional regulation of genes crucial for cardiomyocyte proliferation, differentiation, and survival. RAF1 is vital in enhancing the parturition cascade (the process of uterine contraction during labor). NFATC4 operates downstream of calcium signaling, activated by calcium-dependent signaling via calcineurin (CALN). Once dephosphorylated by calcineurin, NFATC4 migrates to the nucleus to influence gene expression. It plays a role in the negative regulation of OXT signaling in myometrial cells by regulating genes, such as RCAN1 and RGS2, that modulate OXT receptor signaling and calcium dynamics. CALM3 plays a pivotal role in calcium signaling by binding to calcium ions, thereby functioning as a calcium sensor. Additionally, it activates downstream targets, such as calmodulin-dependent kinase and calcineurin (CALN). Through these interactions, CALM3 regulates myosin light chain kinase, an essential regulator of muscle contraction in both myometrial and mammary gland cells. In summary, RAF1 is instrumental in mediating MAPK signaling pathways that facilitate proliferation and differentiation. NFATC4 regulates transcription in response to calcium signaling, thereby modulating OXT signaling. CALM3 serves as a calcium sensor that governs various downstream pathways, including those involved in contraction and metabolism. These genes are vital for the physiological responses triggered by OXT, which encompass uterine contraction, cardiovascular effects, and cellular proliferation (Figure 3). A collection of 147 proteins associated with the OXT signaling pathway was assembled utilizing STRING and subsequently examined in Cytoscape. Summary statistics of the network: Number of nodes: 147, number of edges: 2172, average number of neighbors: 29.551, network density: 0.202, network heterogeneity: 0.644, network centralization: 0.406. Among these proteins, 86 (highlighted in red) were the primary neighbors of CALM3, and 91 of the 147 proteins were the primary neighbors of the three specified genes. The top five hub and bottleneck proteins were identified using the Cyto-Hubba plugin (Tables 1 and 2).


Within the first neighbor nodes, there was one hub protein (denoted by a diamond shape) and five bottleneck proteins, including CALM3 (denoted by a rectangle shape) (Figure 4).

Discussion
Anxiety is recognized as one of the most prevalent mental disorders in society. In the context of Iranian traditional medicine literature, anxiety is perceived differently (Kaplan et al., 2007). The term “Khuf” refers to the unease concerning a potentially adverse situation that may occur in the future (Motovaseliyan et al., 2016). Avicenna identified flaxseed as a valuable therapeutic approach for managing anxiety (Avecinna, 2005). Flaxseed (L. usitatissimum L., Linaceae), rich in biologically active compounds, is recognized for its nutritional, physiological, and therapeutic properties (Sirotkin, 2023). Research conducted both in vivo and in vitro has provided evidence of hormonal regulation by flaxseed, including stimulation of OXT expression (Sirotkin, 2023; Tou et al., 1999). Lecová et al. (2024) found that pigs fed a diet enriched with 10% flaxseed showed increased OXT levels. Additionally, Vlčková et al. (2022) indicated that flaxseed has a positive impact on OXT ’s physiological effects by offering a protective mechanism for OXT receptors in the presence of xylene. In this study, we illustrated the regulation of various biological pathways by flaxseed-induced DEGs, including the C-type lectin receptor signaling pathway, the OXT signaling pathway, cellular senescence, and the cGMP-PKG signaling pathway, as determined by functional enrichment analysis. The anti-anxiety properties of OXT are well-documented and extensively researched in medical sciences. Tang et al. (2024) demonstrated that the intranasal administration of OXT alleviated post-surgical anxiety in mice. Furthermore, Chaipunko et al. (2025) revealed that OXT can modulate the hypothalamic-pituitary-adrenal (HPA) axis and mitigate anxiety.
GO analysis identified three genes (CALM3, RAF1, NFACT4) among 56 flaxseed-induced DEGs as the most significant and relevant common genes linking flaxseed to the OXT pathway. Among these three pivotal genes, CALM3 stands out as the most crucial, exhibiting distinct characteristics and regulatory behaviors. In contrast to its counterparts, RAF1 and NFATC4, which were downregulated, CALM3 was upregulated, and its remarkable position within the network designates it as a vital mediator in this molecular cascade. This interpretation is further reinforced by NFATC4’s established role as a negative regulator of the OXT pathway. Consequently, its downregulation is anticipated to lead to diminished inhibition and enhanced OXT signaling. The network analysis further highlighted CALM3 as a bottleneck, sustaining first-neighbor interactions with five other essential proteins (four bottleneck proteins and one hub protein). Furthermore, it exhibited remarkable connectivity with 85 first neighbors, accounting for 58% of all proteins involved in the OXT signaling pathway. This approach implies that CALM3 functions as a master regulator within the network, orchestrating various signaling cascades and ensuring proper information flow throughout the system. The significant impact of CALM3 within the network strongly supports its position as the primary molecular mediator, through which flaxseed may exert its anxiolytic effects via the OXT pathway. OXT, a neuropeptide that plays a role in social bonding, reproductive functions, and stress responses, primarily exerts its effects by activating its receptors, thereby increasing intracellular calcium levels. The rise in calcium activates CALM3, which facilitates downstream signaling processes, such as the activation of the MAPK pathway and the RhoA/Rho kinase pathway, both of which are associated with smooth muscle contraction and other physiological responses to OXT (Bakos et al., 2018; Munk et al., 2022; Uvnaes Moberg et al., 2022). Moreover, CALM3 may also affect cardiovascular functions regulated by OXT, underscoring its potential role in integrating calcium signaling with OXT ’s effects on the cardiovascular system (Ayar et al., 2014; Hirasawa et al., 2001; Rabow et al., 2023). Therefore, CALM3 acts as a vital link between calcium signaling and the OXT ’s physiological effects. Additionally, it offers potentially significant insights for therapeutic strategies in anxiety management through dietary interventions.
Conclusion
This study clarifies a new mechanism by which flaxseed influences anxiety via the OXT pathway. In silico analysis identifies three common genes associated with both anxiety and flaxseed. These genes are crucial in the OXT signaling pathway, with CALM3 identified as a primary calcium-sensing regulator. Simultaneously, the downregulation of NFATC4 and RAF1 further facilitates the disinhibition of OXT signaling and the modulation of the MAPK cascade. These results not only affirm the traditional applications of flaxseed but also underscore its potential as a dietary supplement for anxiety disorders. Future investigations should confirm these mechanisms through clinical trials and examine the synergistic effects of conventional treatments.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This study was derived from the PhD dissertation of Hajar Sadat Sadeghi, approved by the Department of Traditional Pharmacy, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. This study was financially supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No.: 43006118).
Authors' contributions
Supervision: Mostafa Rezaei Tavirani and Maryam Hamzeloo-Moghadam; Methodology: Hajar Sadat Sadeghi and Melika Abrishami; Data collection: Hajar Sadat Sadeghi Sadeghi and Melika Abrishami; Data analysis: Hajar Sadat Sadeghi Sadeghi and Melika Abrishami; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Al-Madhagy, S., Ashmawy, N. S., Mamdouh, A., Eldahshan, O. A., & Farag, M. A. (2023). A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. European Journal of Medical Research, 28(1), 240. [DOI:10.1186/s40001-023-01203-6] [PMID]
Anis, L., Haider, S., & Sajid, I. (2016). Repeated oral administration of flaxseeds induced antidepressant and anxiolytic effects in rats. FUUAST Journal of Biology, 6(1), 41-46. [Link]
Ansari, R., Zarshenas, M. M., & Dadbakhsh, A. H. (2019). A review on pharmacological and clinical aspects of linum usitatissimum L. Current Drug Discovery Technologies, 16(2), 148-158. [DOI:10.2174/1570163815666180521101136] [PMID]
Avicenna, Abu-Asab M, Amri H, Micozzi MS. (2013). Avicenna's medicine: A new translation of the 11th-century canon with practical applications for integrative health care. Rochester: Healing Arts Press.
Ayar, A., Ozcan, M., Alcin, E., Serhatlioglu, I., Ozcan, S., & Kutlu, S., et al. (2014). Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. Journal of Physiology and Biochemistry, 70(1), 43-48. [DOI:10.1007/s13105-013-0278-z] [PMID]
Bakos, J., Srancikova, A., Havranek, T., & Bacova, Z. (2018). Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plasticity, 2018, 4864107. [DOI:10.1155/2018/4864107] [PMID]
Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D. L. D. C., Denys, D., & Dilbaz, N., et al. (2023). World federation of societies of biological psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders - Version 3. Part I: Anxiety disorders. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 24(2), 79–117. [PMID]
Carter, C. S. (2022). Oxytocin and love: Myths, metaphors and mysteries. Comprehensive Psychoneuroendocrinology, 9, 100107. [DOI:10.1016/j.cpnec.2021.100107] [PMID]
Chaipunko, S., Sookkua, T., Nopparat, C., & Chutabhakdikul, N. (2024). Oxytocin Protects Against Corticosterone-Induced DA Dysfunction: An Involvement of the PKA/CREB Pathway. Neurochemical Research, 50(1), 38. [DOI:10.1007/s11064-024-04294-7] [PMID]
DeMartini, J., Patel, G., & Fancher, T. L. (2019). Generalized anxiety disorder. Annals of Internal Medicine, 170(7), ITC49-ITC64. [DOI:10.7326/AITC201904020] [PMID]
Gully, B. J., Brown, Z. E., Hornbacher, R., Brown, J. C., Back, S. E., & McCance-Katz, E. F., et al. (2024). Oxytocin reduces noradrenergic-induced opioid-like withdrawal symptoms in individuals on opioid agonist therapy. Biological Psychiatry Global Open Science, 5(1), 100395. [DOI:10.1016/j.bpsgos.2024.100395] [PMID]
Hirasawa, M., Kombian, S. B., & Pittman, Q. J. (2001). Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: Role of N-and P/Q-type calcium channels. The Journal of Physiology, 532(Pt 3), 595-607. [DOI:10.1111/j.1469-7793.2001.0595e.x] [PMID]
Hoge, E. A., Ivkovic, A., & Fricchione, G. L. (2012). Generalized anxiety disorder: Diagnosis and treatment. BMJ (Clinical research ed.), 345, e7500. [DOI:10.1136/bmj.e7500] [PMID]
Ivanov, S., Rashevskaya, T., & Makhonina, M. (2011). Flaxseed additive application in dairy products production. Procedia Food Science, 1, 275-280. [DOI:10.1016/j.profoo.2011.09.043]
Kaplan, H., Sadock, B., & Sadock, V. (2007). Synopsis of psychiatry behavioral sciences/clinical psychiatry [F. Rezaei, Persian trans]. Tehran: Arjmand publications. [Link]
Lecová, M., Babjáková, D., Sopková, D., Andrejčáková, Z., Hertelyová, Z., & Petrilla, V., et al. (2024). Different lengths of diet supplementation with 10% flaxseed alter the hormonal profile and the follicular fluid fatty acid content of fattening gilts. Life, 14(2), 240. [DOI:10.3390/life14020240] [PMID]
Motovaseliyan, M., Ghafari, F., & Kakhaki, M. T. (2016). [ equivalent to anxiety in Iranian traditional medicine texts (Persian)]. Journal of Medical-Scientific Research History, 8(27), 47-72. [Link]
Mueed, A., Shibli, S., Korma, S. A., Madjirebaye, P., Esatbeyoglu, T., & Deng, Z. (2022). Flaxseed bioactive compounds: Chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods (Basel, Switzerland), 11(20), 3307. [DOI:10.3390/foods11203307] [PMID]
Munk, M., Villalobo, E., Villalobo, A., & Berchtold, M. W. (2022). Differential expression of the three independent CaM genes coding for an identical protein: Potential relevance of distinct mRNA stability by different codon usage. Cell Calcium, 107, 102656. [DOI:10.1016/j.ceca.2022.102656] [PMID]
National Center for Biotechnology Information. (2025). Gene Expression Omnibus. Rockville: National Center for Biotechnology Information. [Link]
National Center for Biotechnology Information. (2025). GEO2R. Rockville: National Center for Biotechnology Information. [Link]
Oomah, B. D. (2001). Flaxseed as a functional food source. Journal of The Science of Food and Agriculture, 81(9), 889-894. [DOI:10.1002/jsfa.898]
Pal, S. K., & Shukla, Y. (2003). Herbal medicine: Current status and the future. Asian Pacific Journal of Cancer Prevention, 4(4), 281-288. [PMID]
Rabow, S., Jonsson, H., Bro, E., & Olofsson, P. (2023). Cardiovascular effects of oxytocin and carbetocin at cesarean section. A prospective double-blind randomized study using noninvasive pulse wave analysis. The Journal of Maternal-Fetal & Neonatal Medicine, 36(1), 2208252. [DOI:10.1080/14767058.2023.2208252] [PMID]
Rezaei-Tavirani,M., Razzaghi, Z., Arjmand, B., & Vafaee, R. (2022). Cholesterol metabolism pathway, the main target of coffee. Research Journal of Pharmacognosy, 9(4), 39-47. [DOI:10.22127/rjp.2022.350680.1937]
University of Wisconsin--Extension., Cooperative Extension Service and University of Minnesota., & Center for Alternative Crops and Products and Minnesota Extension Service. (1990). Alternative Field Crops Manual. Madison: University of Wisconsin-Extension, Cooperative Extension. [Link]
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Researvh. 2003; 13(11):2498-504. [DOI: 10.1101/gr.1239303] [PMID]
Shao, L., & Zhang, B. (2013). Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chinese Journal of Natural Medicines, 11(2), 110-120. [DOI:10.1016/S1875-5364(13)60037-0] [PMID]
Singh, K., Mridula, D., Rehal, J., & Barnwal, P. (2011). Flaxseed: A potential source of food, feed and fiber. Critical Reviews in Food Science and Nutrition, 51(3), 210-222. [DOI:10.1080/10408390903537241] [PMID]
Sirotkin, A. V. (2023). Influence of flaxseed (Linum usitatissimum) on female reproduction. Planta Medica, 89(06), 608-615. [DOI:10.1055/a-2013-2966] [PMID]
Subramanian, S., Thoms, J. A. I., Huang, Y., Cornejo-Páramo, P., Koch, F. C., Jacquelin, S., et al. (2023). Genome-wide transcription factor-binding maps reveal cell-specific changes in the regulatory architecture of human HSPCs. Blood, 142(17), 1448–1462. [DOI:10.1182/blood.2023021120] [PMID]
Szklarczyk, D., Kirsch, R., Koutrouli, M., Nastou, K., Mehryary, F., et al. (2023). The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Research, 51(D1), D638–D646. [DOI:10.1093/nar/gkac1000] [PMID]
Tang, T., Zhang, X., Liu, Y., Peng, H., Zheng, B., & Yin, Y., et al. (2023). Machine learning on protein-protein interaction prediction: models, challenges and trends. Briefings in Bioinformatics, 24(2), bbad076. [DOI:10.1093/bib/bbad076] [PMID]
Tang, Y. B., Tang, L., Chen, B., Fan, M. J., Chen, G. J., & Ou, Y. N., et al. (2025). Intranasal oxytocin alleviates postsurgical pain and comorbid anxiety in mice: Participation of BK(Ca) channels in the hippocampus. Neuropharmacology, 265, 110243. [DOI:10.1016/j.neuropharm.2024.110243] [PMID]
Tou, J. C., Chen, J., & Thompson, L. U. (1999). Dose, timing, and duration of flaxseed exposure affect reproductive indices and sex hormone levels in rats. Journal of Toxicology and Environmental Health, Part A, 56(8), 555-570. [DOI:10.1080/00984109909350177] [PMID]
Touré, A., & Xueming, X. (2010). Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio‐active components, and health benefits. Comprehensive Reviews in Food Science and Food Safety, 9(3), 261-269. [DOI:10.1111/j.1541-4337.2009.00105.x] [PMID]
Uvnaes Moberg, K., Julius, H., Handlin, L., & Petersson, M. (2022). Sensory stimulation and oxytocin: their roles in social interaction and health promotion. Frontiers in Psychology, 13, 929741. [DOI:10.3389/fpsyg.2022.929741] [PMID]
Vlčková, R., Sopková, D., Andrejčáková, Z., Lecová, M., Fabian, D., & Šefčíková, Z., et al. (2022). Dietary supplementation of flaxseed (Linum usitatissimum L.) alters ovarian functions of xylene-exposed mice. Life, 12(8), 1152. [DOI:10.3390/life12081152] [PMID]
Watson, R. R., & Preedy, V. R. (2012). Bioactive Food as Dietary Interventions for Diabetes: Bioactive Foods in Chronic Disease States. Amsterdam: Elsevier Science. [Link]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2025/05/26 | Accepted: 2025/10/19 | Published: 2025/11/28
Received: 2025/05/26 | Accepted: 2025/10/19 | Published: 2025/11/28
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








