Volume 16, Issue 5 (September & October 2025)
BCN 2025, 16(5): 987-1002 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Heydari M, Jalali Kondori B, Raei M, Eftekhari Moghadam A R. Protective Effects of Adipose-derived Stem Cell Exosomes vs Dexamethasone on Neuromotor Deficits in Focal Cerebral Ischemia. BCN 2025; 16 (5) :987-1002
URL: http://bcn.iums.ac.ir/article-1-3226-en.html
URL: http://bcn.iums.ac.ir/article-1-3226-en.html
1- Department of Anatomical Science, School of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran.
2- Department of Anatomical Science, School of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran. & Baqiyatallah Research Center for Gastroenterology and Liver Diseases (BRCGL), Baqiyatallah University of Medical Sciences, Tehran, Iran.
3- Health Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran.
2- Department of Anatomical Science, School of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran. & Baqiyatallah Research Center for Gastroenterology and Liver Diseases (BRCGL), Baqiyatallah University of Medical Sciences, Tehran, Iran.
3- Health Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Keywords: Cerebral ischemia, Exosomes, Mesenchymal stem cells (MSCs), Dexamethasone, Neuroprotection, Inflammation, Stroke therapy
Full-Text [PDF 7511 kb]
| Abstract (HTML)
Full-Text:
Introduction
Cerebral ischemia is a pathological condition characterized by insufficient oxygen supply to the brain tissue, which can manifest transiently as transient ischemic attacks due to cerebral vasoconstriction, or permanently as cerebral infarction or stroke, typically resulting from thrombosis or embolism in major cerebral arteries. Stroke is recognized globally as a leading cause of mortality and long-term disability, imposing substantial clinical and socioeconomic burdens. Thus, there remains an urgent necessity for novel therapeutic interventions that can effectively mitigate ischemic damage and improve patient outcomes (Amantea et al., 2009).
Ischemic injury induces severe tissue edema and increased intracranial pressure, subsequently leading to irreversible neuronal cell death. A comprehensive understanding of these pathophysiological processes is crucial for developing effective preventive strategies and therapeutic approaches for patients at risk (Amantea et al., 2009). Current therapeutic interventions, primarily antiplatelet and thrombolytic treatments, offer limited efficacy by merely targeting restoration or preservation of cerebral blood flow, rather than directly addressing the underlying cellular and molecular mechanisms of ischemic neuronal death (Amantea et al., 2009; Gladstone et al., 2002).
Shortly after ischemic onset, substantial damage occurs within the core ischemic region, characterized by a significant reduction in cerebral blood flow (greater than 80%), energy depletion, oxygen deprivation, and loss of ionic gradients resulting from neuronal depolarization. These conditions primarily induce cell death through excitotoxicity, mitochondrial dysfunction, excessive production of reactive oxygen species, and activation of programmed cell death pathways (Lo et al., 2003; Jayaraj et al., 2019).
Recently, exosomes have emerged as promising therapeutic vehicles due to their capability to cross the blood-brain barrier efficiently and deliver bioactive cargo into neural tissue. Exosomes significantly contribute to reducing inflammation in the central nervous system and enhancing recovery of neuromotor function post-injury (Console et al., 2019). Notably, research has demonstrated that brain injury induces upregulation of miR-124-3p, a microRNA known to alleviate neuronal inflammation and improve neurological function. Given their inherent capacity to transport anti-inflammatory modulators such as microRNAs and proteins, exosomes represent a compelling strategy for targeted therapeutic delivery to the CNS, addressing inflammation-related pathological conditions (Gao et al., 2020).
Stem cell-derived exosomes, acting as key paracrine mediators, have shown promising therapeutic effects in ischemic stroke through their roles in promoting angiogenesis and neurogenesis, alongside exhibiting significant neuroprotective and neuroregenerative properties (Huang et al., 2020). Recent findings further indicate that exosomes secreted by various stem cells, including adipose-derived stem cells (ADSCs), can carry bioactive molecules such as lipids, proteins, and genetic materials into the extracellular environment, effectively modulating pathological conditions in neurodegenerative diseases. Specifically, ADSC-derived exosomes have been demonstrated to attenuate inflammation and oxidative stress by modulating the Nrf2/HO-1 signaling pathway, thereby highlighting their therapeutic potential for ischemic and degenerative brain injuries (Shen et al., 2021).
The innovation of this study lies in utilizing stem cell-derived exosomes, particularly those derived from ADSCs, as novel therapeutic agents targeting the underlying molecular and cellular mechanisms of cerebral ischemia. The primary goal of this research is to investigate the therapeutic potential and efficacy of ADSC-derived exosomes in reducing inflammation, oxidative stress, and neuronal damage associated with ischemic stroke, to establish their role as a viable and advanced therapeutic intervention for ischemic cerebral injuries.
Materials and Methods
Study design
This experimental study evaluated the neuroprotective effects of adipose tissue–mesenchymal stem cell (AT-MSC)-derived exosomes in a rat model of transient focal cerebral ischemia. To assess the therapeutic impact, behavioral, biochemical, and histopathological analyses were performed.
Animal grouping
Twenty adult male Wistar rats (250–280 g) were randomly divided into four groups (n=5 per group):
1. Sham group: Underwent surgical procedures without ischemia induction and received normal saline orally for 7 days.
2. MCAO control group: Underwent middle cerebral artery occlusion (MCAO) without receiving any treatment.
3. Exosome-treated group: Administered 0.2 mL phosphate-buffered saline (PBS)-diluted exosomes intravenously via the tail vein 1 hour before ischemia, followed by daily injections for 7 days.
Dexamethasone group: Treated with daily intraperitoneal injections of dexamethasone (1 mg/kg) starting 24 hours after ischemia.
Induction of transient focal cerebral ischemia
Transient focal cerebral ischemia was induced using the MCAO method. Rats were anesthetized with 5% isoflurane (induction) and maintained with 2% isoflurane in 30% oxygen and 70% nitrogen. A midline neck incision exposed the common, external, and internal carotid arteries. A silicone-coated 3-0 nylon filament was inserted through the ICA to occlude the MCA for 90 minutes. Afterward, the filament was removed to allow reperfusion. Body temperature was kept at 37±0.5 °C throughout the procedure.
Exosome isolation and characterization
Exosomes were isolated from AT-MSCs harvested from a young Wistar rat via ultracentrifugation. NanoSight analysis was used to assess particle size and concentration. Western blotting confirmed the presence of specific surface markers indicative of exosomes.
Behavioral assessments
Bederson neurological score: Neurological deficits were scored at 24 hours and 7 days post-ischemia based on forelimb flexion, lateral push resistance, and circling. Scores ranged from 0 (normal) to 5 (severe deficit) (Figure 1).

Garcia neurological score: A comprehensive assessment was conducted at 24 hours, 3 days, and 7 days post-ischemia using the Garcia scale, which includes six categories:
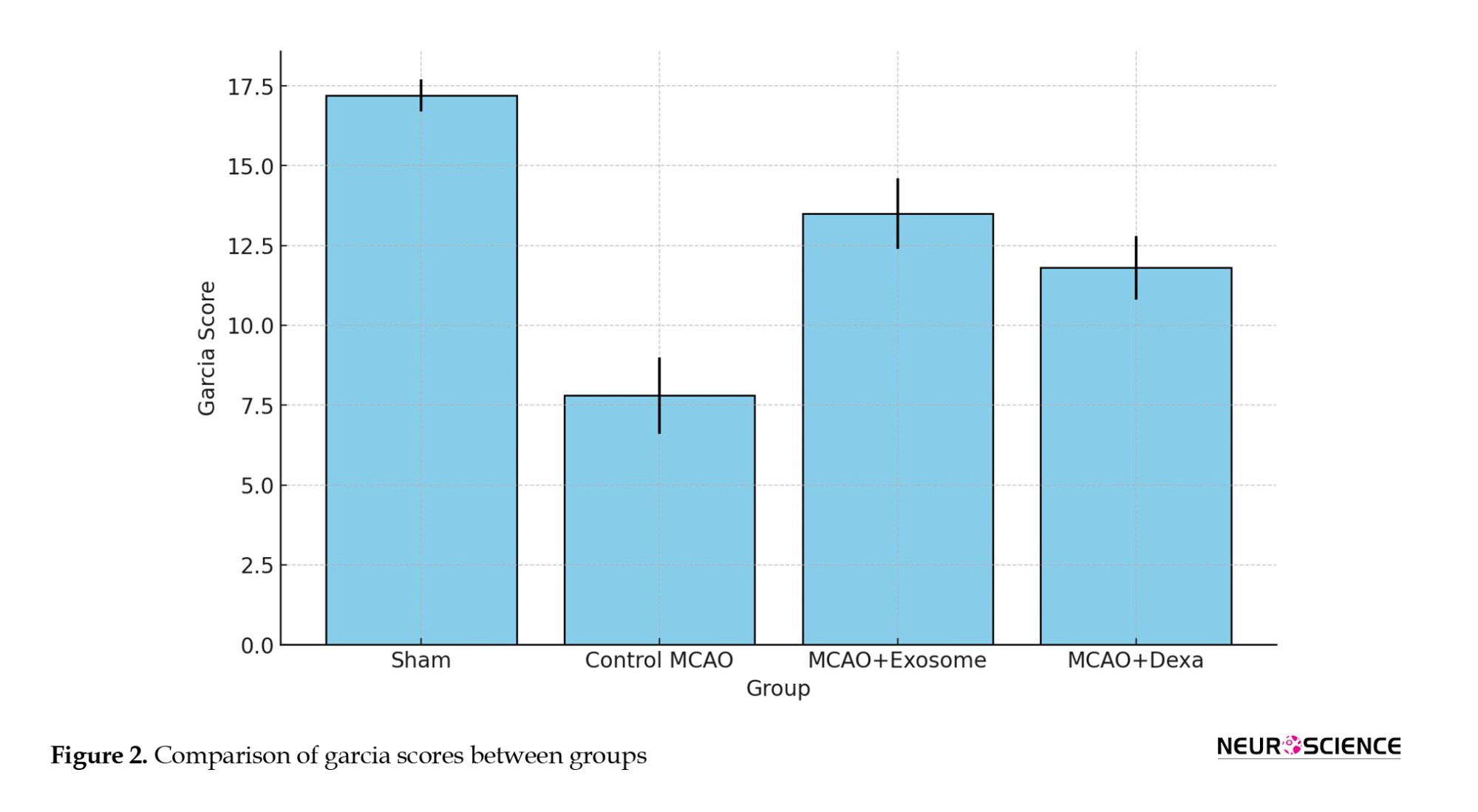
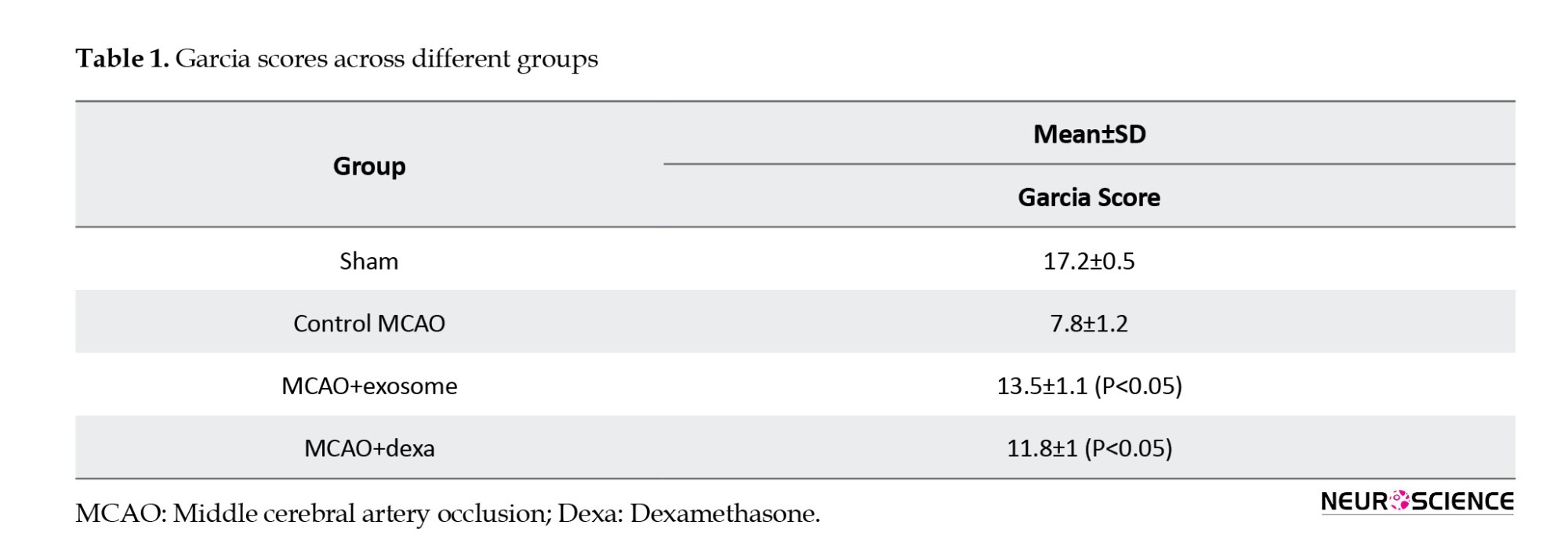
Spontaneous activity, symmetry of limb movement, climbing ability, response to touch/pain, and body proprioception (Figure 2, Table 1).
Vibrissae response
Total scores ranged from 3 (severe deficit) to 18 (normal).
Biochemical analysis
Serum levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were measured using ELISA kits (Jiamei Biotech Co. Ltd., Beijing, China). Absorbance was read at 450 nm, and concentrations were calculated using standard curves.
Infarct volume assessment
At 24 hours post-MCAO, rats were euthanized (pentobarbital 100 mg/kg, IP). Brains were removed, rinsed in cold saline, and cut into 2-mm coronal sections. Slices were incubated in 2% 2,3,5-Triphenyltetrazolium chloride (TTC) at 37 °C for 20 minutes, then fixed in 10% formalin. Digital images were analyzed with ImageJ software, version 1.54p using:
Infarct volume=Σ (Infarct area×Slice thickness).
Histopathological analysis
On day 7 post-ischemia, brain samples were fixed in 10% buffered formalin, processed through ethanol and xylene, and embedded in paraffin. Five-micron sections were stained with H&E to assess necrosis and inflammation. Nissl staining (1% toluidine blue at 45 °C for 30 minutes) was used to assess neuronal survival. Sections were analyzed using a light microscope (DMI4000B, Leica, Germany).
Statistical analysis
Data were analyzed using SPSS, version 28 (IBM Corp. NY, US). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for comparisons. Results are presented as Mean±SD. A P<0.05 was considered statistically significant.
Ethical considerations
All procedures were approved by the Animal Ethics Committee of Baqiyatallah University of Medical Sciences in accordance with national guidelines. Animals were euthanized humanely at the end of the study using a high-dose anesthetic (IP).
Results
Neurological performance evaluation
The Bederson test is a standard tool for evaluating the severity of neurological deficits following focal cerebral ischemia. Its score ranges from 0 (no neurological deficit) to 5 (severe motor and neurological impairment), reflecting the level of motor and neurological deficits in animals.
The sham group showed the least mean neurological deficits (0.5±0.2), which confirmed normal brain function.
● The control MCAO group exhibited the highest mean neurological deficit (4.2±0.5), indicating severe damage caused by MCAO.
● The MCAO+exosome and MCAO+dexa groups had significantly lower scores than the MCAO control group, but still showed a significant difference compared to the sham group.
● Exosome treatment showed a greater reduction in motor deficits than dexamethasone, although the difference between these two treatments was not statistically significant.
The Garcia test evaluates neurological function within a range of 3 (severe damage) to 18 (normal function), with higher scores indicating improved neurological function and reduced motor deficits.
● The sham group received the highest score, reflecting normal function.
● The MCAO (untreated) group received the lowest score, confirming severe motor and neurological deficits.
● The exosome-treated group had higher scores than the dexa-treated group, suggesting better neurological recovery.
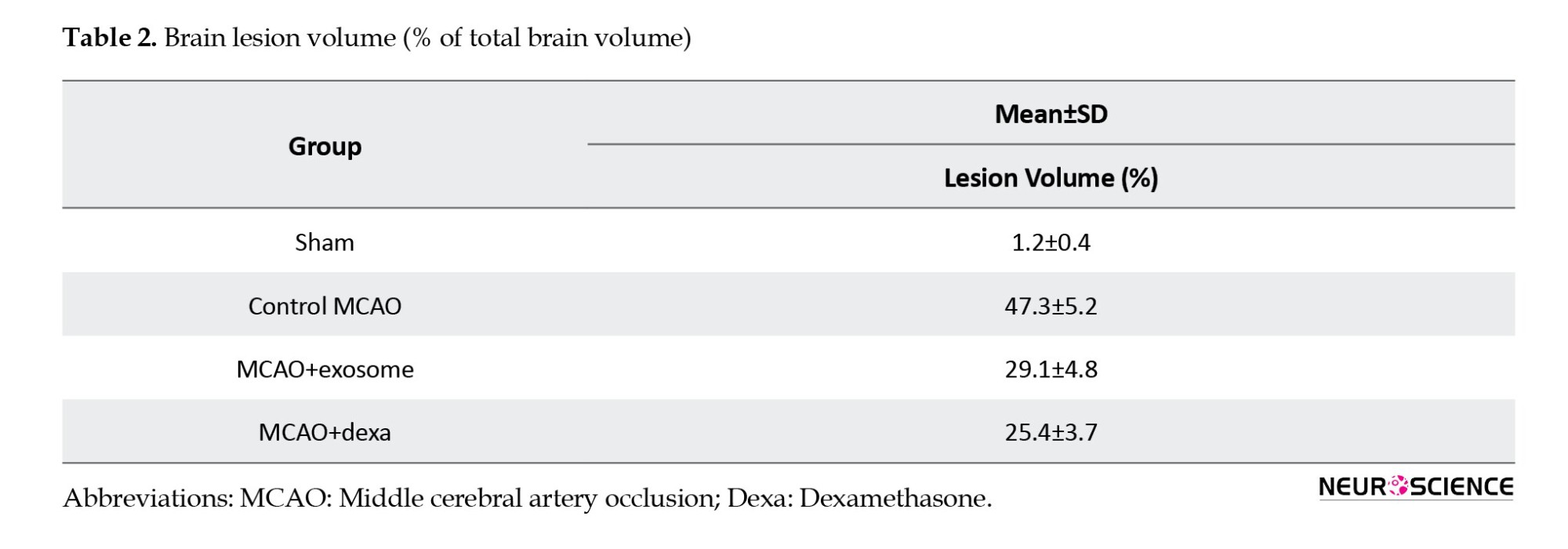
Brain lesion volume (staining analysis)
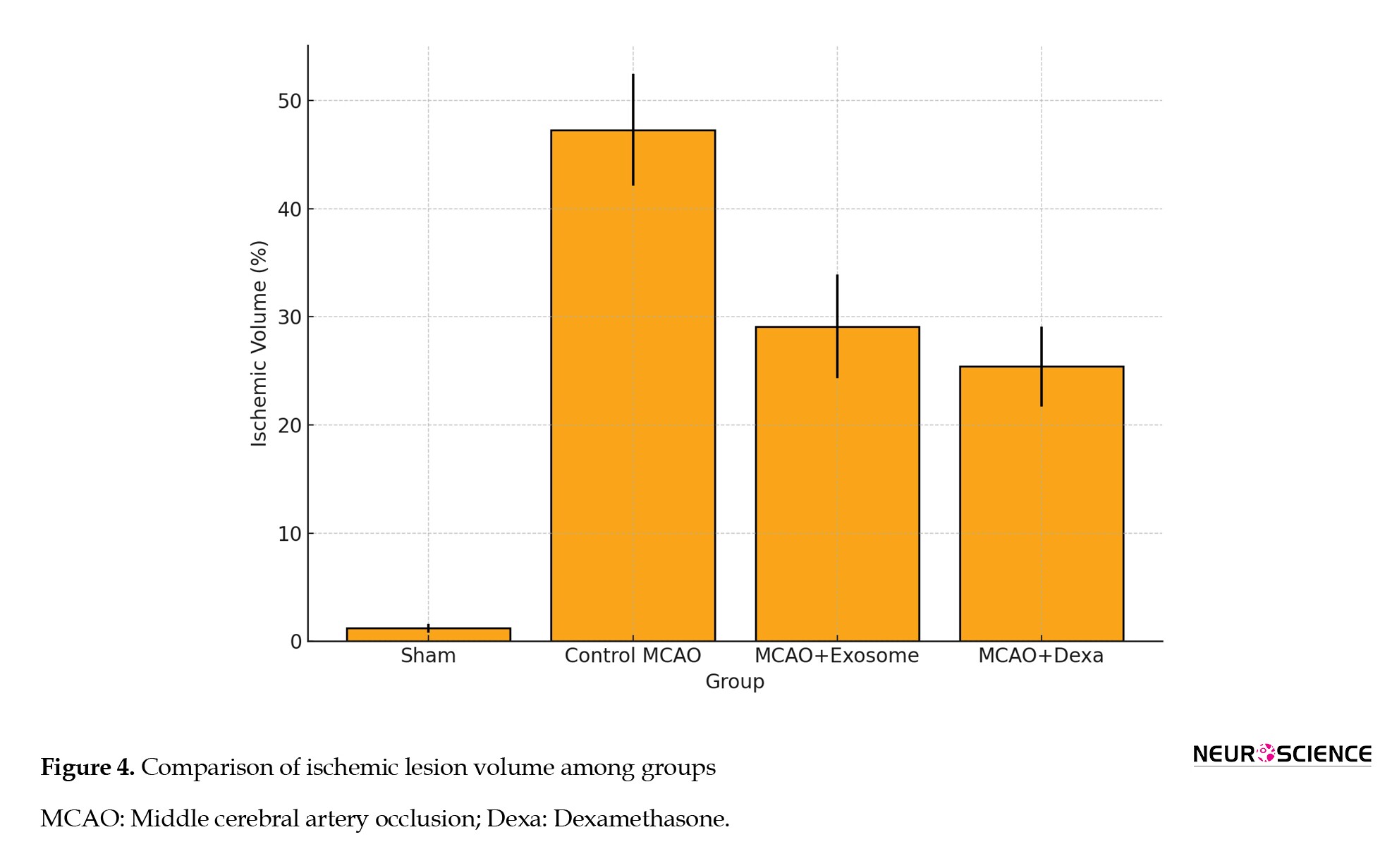
TTC staining was used to assess the extent of brain tissue necrosis following ischemia. In this method, necrotic areas appeared white, while healthy tissue appeared dark red (Figure 3, Table 2).
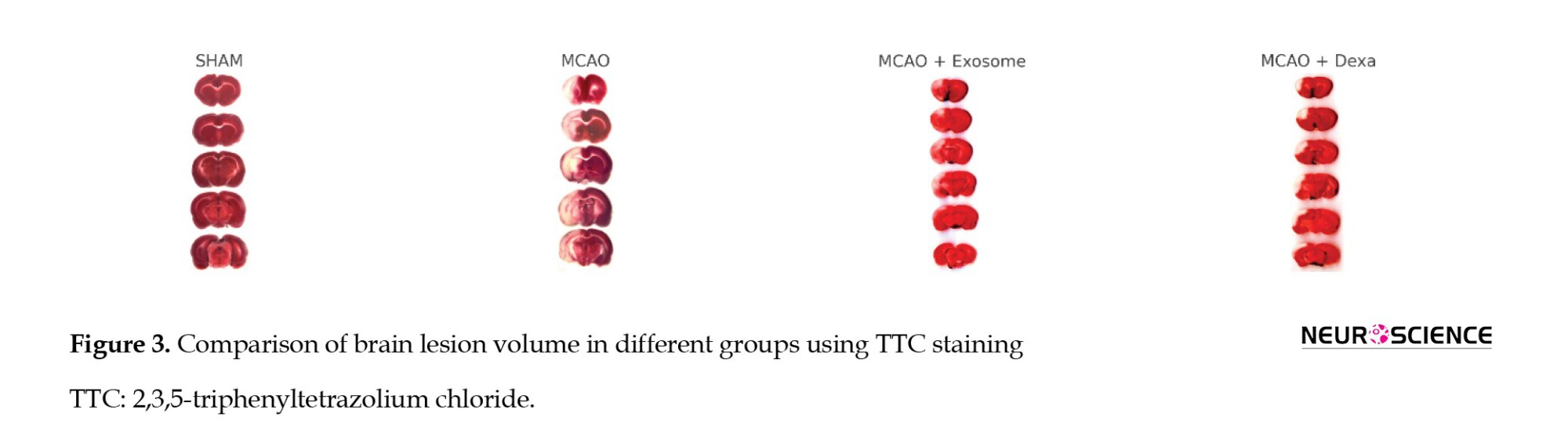
● In the sham group, no white areas (ischemic damage) were observed, indicating healthy brain tissue.
● In the sham group, no white areas (ischemic damage) were observed, indicating healthy brain tissue.
● In the MCAO group, a large white area was observed in the brain, indicating infarction and severe neuronal damage.
● In the treatment groups (e.g. exosome and dexamethasone groups), infarct areas were significantly reduced compared to the MCAO group (Figure 4).
● Treatment groups showed fewer ischemic lesions, while the MCAO group exhibited the largest infarct area.
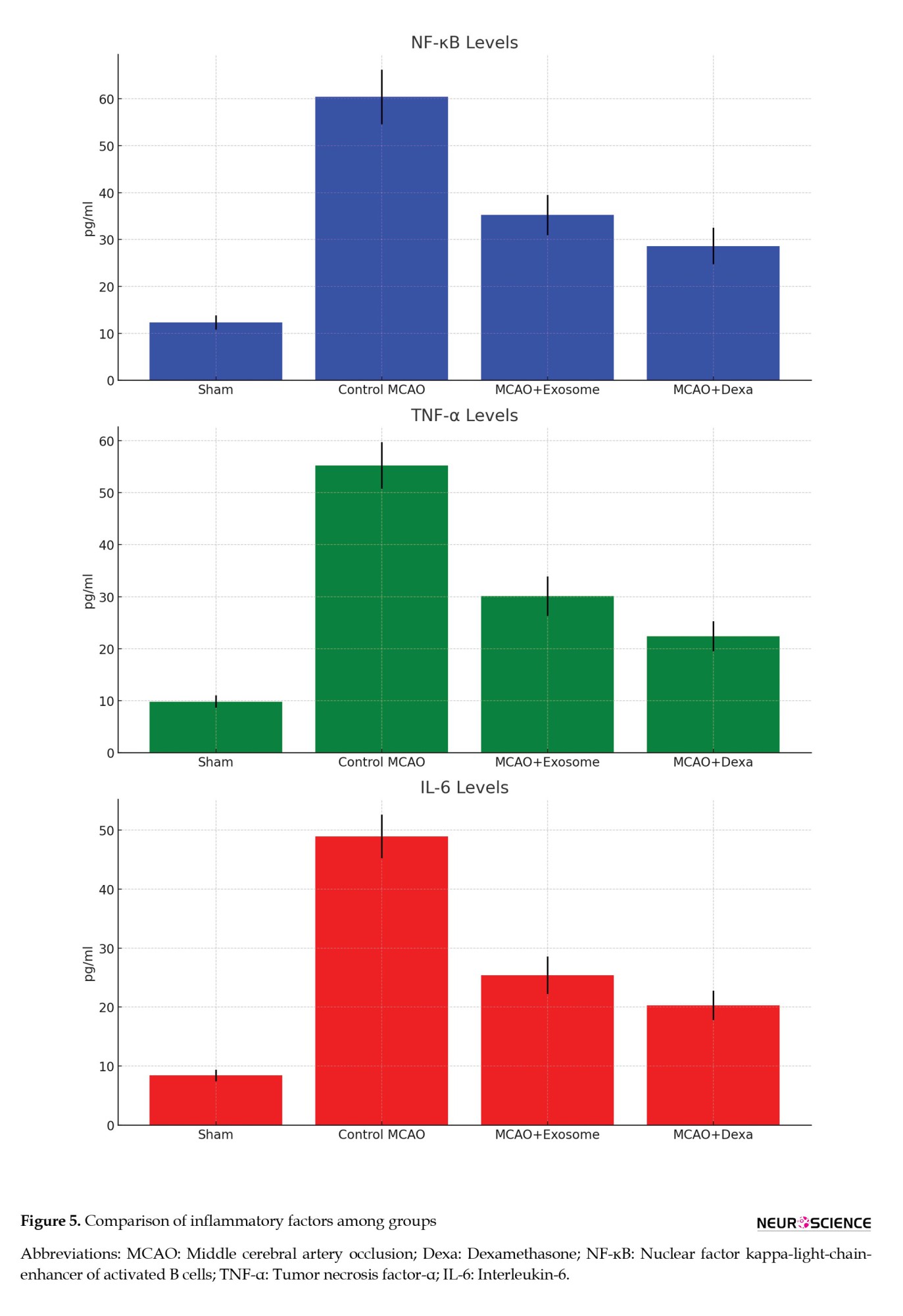
Inflammatory factor levels in serum
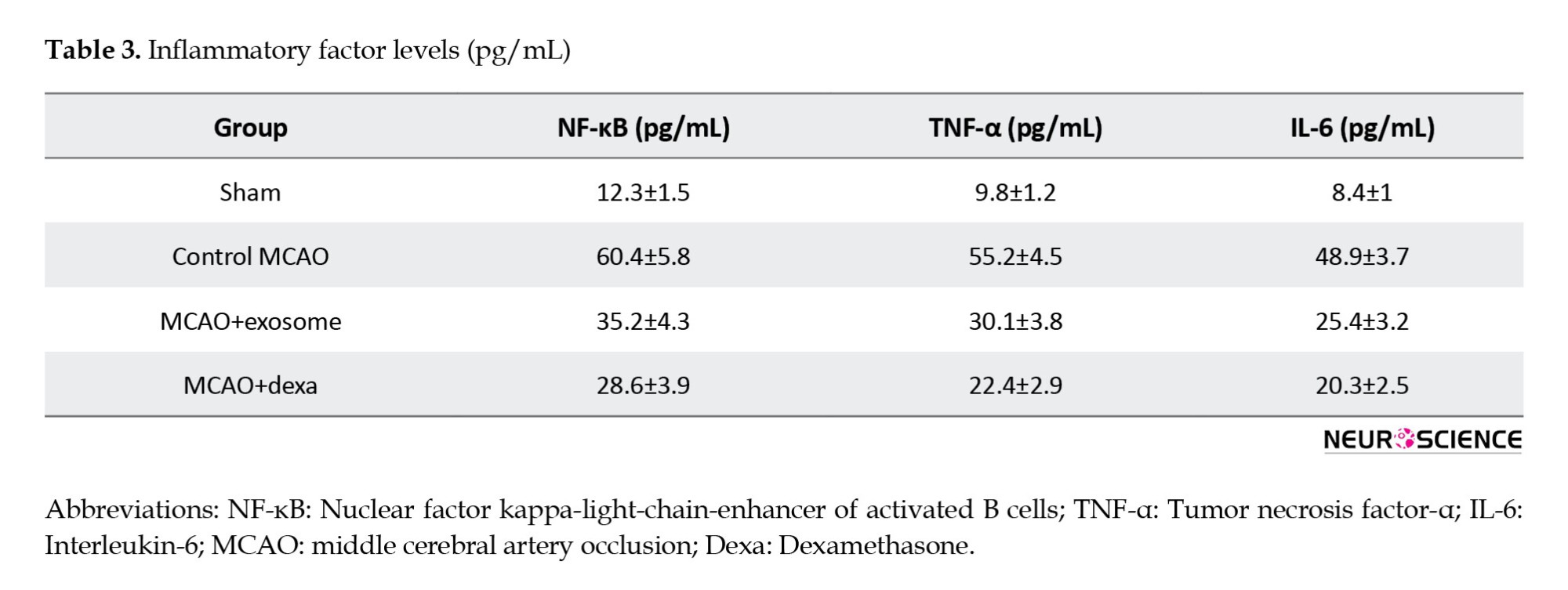
The inflammatory response plays a critical role in the pathophysiology of ischemic brain injury. This section examines the levels of key inflammatory markers (NF-κB, TNF-α, IL-6) in the serum of animals from different treatment groups. These factors are often elevated in response to ischemic injury and are associated with neuronal damage and neuroinflammation. The analysis of these factors helps in understanding the anti-inflammatory effects of the exosome and dexamethasone treatments (Figure 5, Table 3).
Histopathological findings
To evaluate tissue changes resulting from cerebral ischemia and the therapeutic effects of exosomes and dexamethasone, Hematoxylin and Eosin (H&E) and Nissl staining techniques were employed (Figure 6). Microscopic examinations were performed on coronal brain sections of the animals, and the extent of necrosis, tissue edema, the number of damaged neurons, and neuronal structure preservation were compared across different groups.

● In the sham group, the brain tissue structure appeared normal, with no signs of necrosis, tissue edema, or neuronal degeneration. Neurons exhibited normal morphology with healthy nuclei and uniform distribution. Additionally, no evidence of inflammatory responses was observed in the tissue.
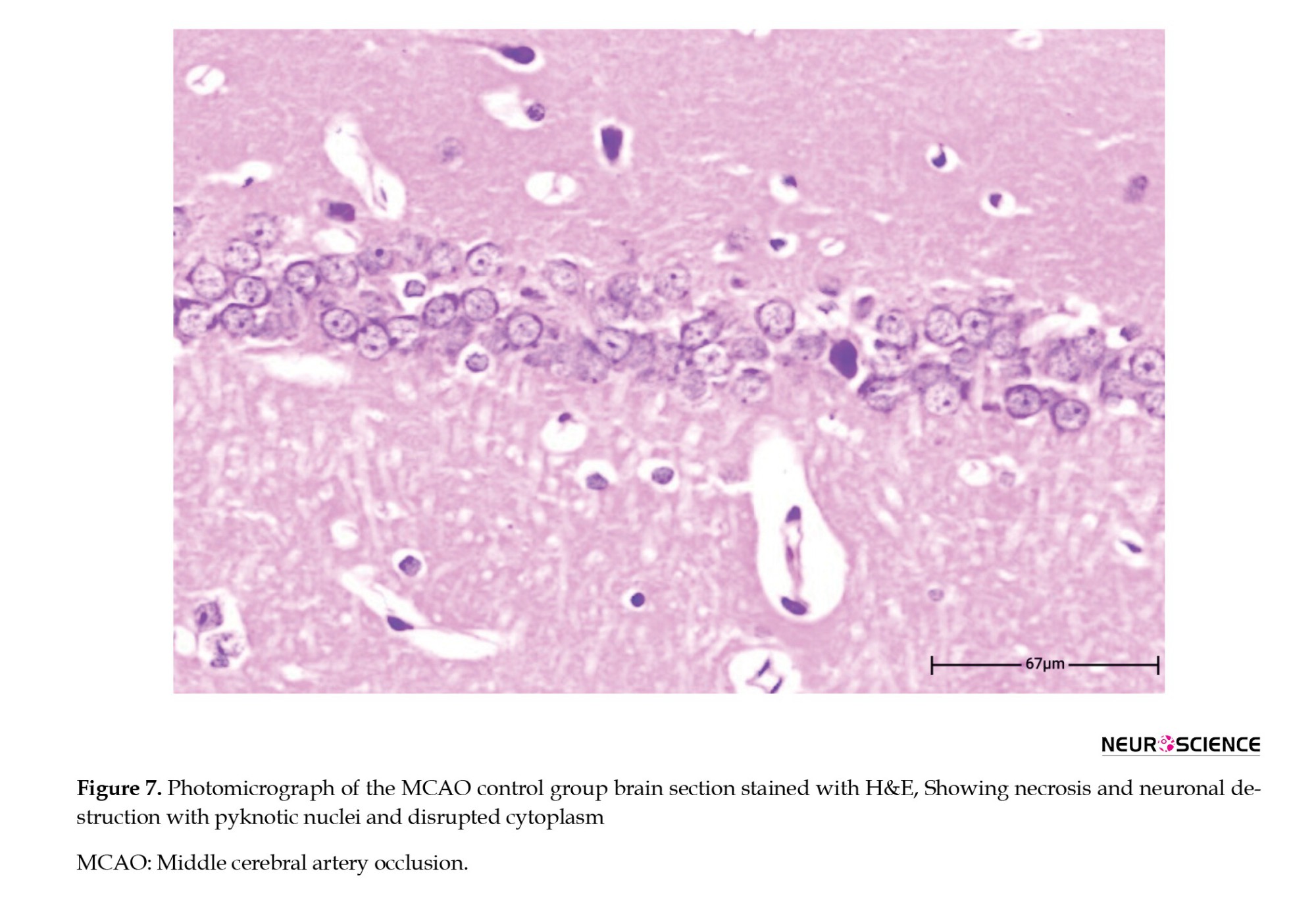
● In the control MCAO group, extensive necrosis, loss of tissue integrity, severe edema, and neuronal death were evident. Necrotic neurons exhibited shrunken nuclei and dense cytoplasm. Increased numbers of inflammatory cells, cellular swelling, and severe tissue changes were observed in the ischemic regions. These changes indicated significant damage caused by cerebral ischemia (Figure 7).
● In the exosome treatment group, necrosis and tissue edema were significantly reduced. A greater number of neurons exhibited healthy nuclei, and tissue integrity was improved compared to the MCAO group. In this group, inflammatory responses and immune cell accumulation were reduced, resulting in less cell death (Table 4).
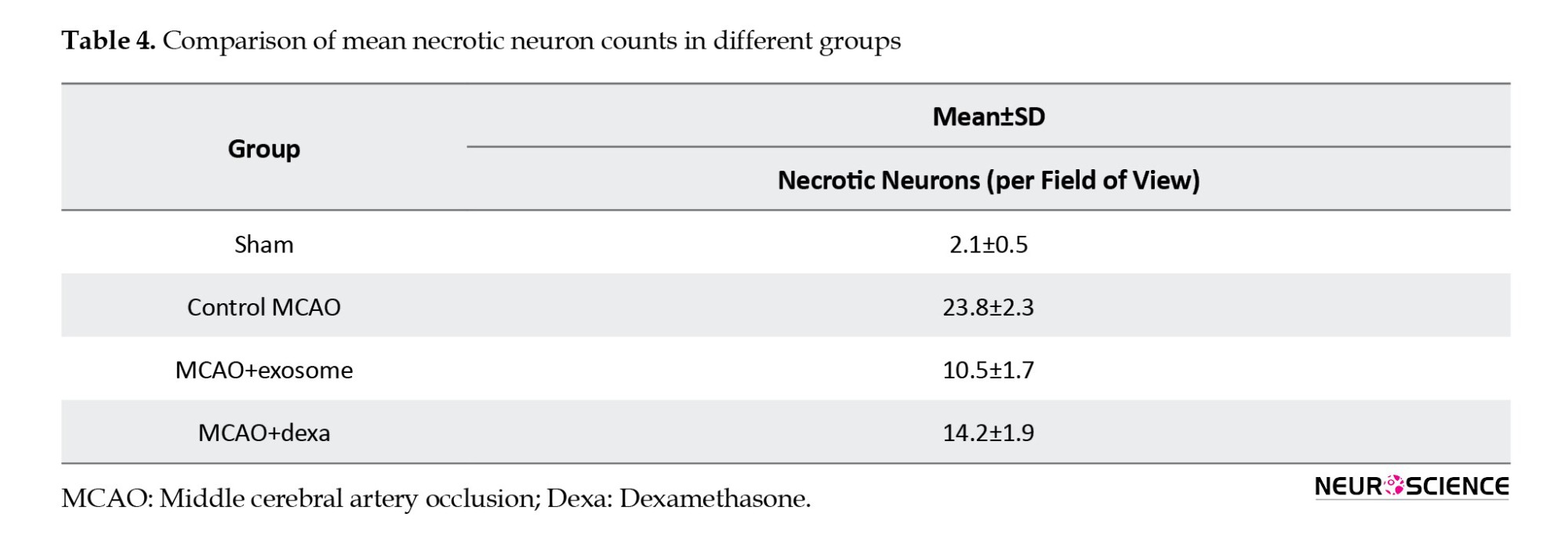
These results suggest that exosomes have protective effects in reducing neuronal damage and inflammation resulting from cerebral ischemia (Figure 8).

● In the dexamethasone treatment group, there was a reduction in necrosis and tissue edema compared to the MCAO group. However, the degree of reduction was less than in the exosome-treated group. Although fewer necrotic cells were observed in this group, the inflammatory responses remained significant. Compared to the exosome group, dexamethasone had a lesser effect on reducing inflammation and promoting neuronal reconstruction (Figure 9).

Histopathological findings – Nissl staining
To evaluate the preservation and degeneration of neurons after cerebral ischemia, Nissl staining was used (Figure 10). This staining technique was used to assess the number of healthy neurons and the extent of neuronal degeneration in damaged regions. In all groups, Nissl bodies (rough endoplasmic reticulum) were observed in the cytoplasm, appearing purple-blue, and in the nucleus, they seemed light blue.
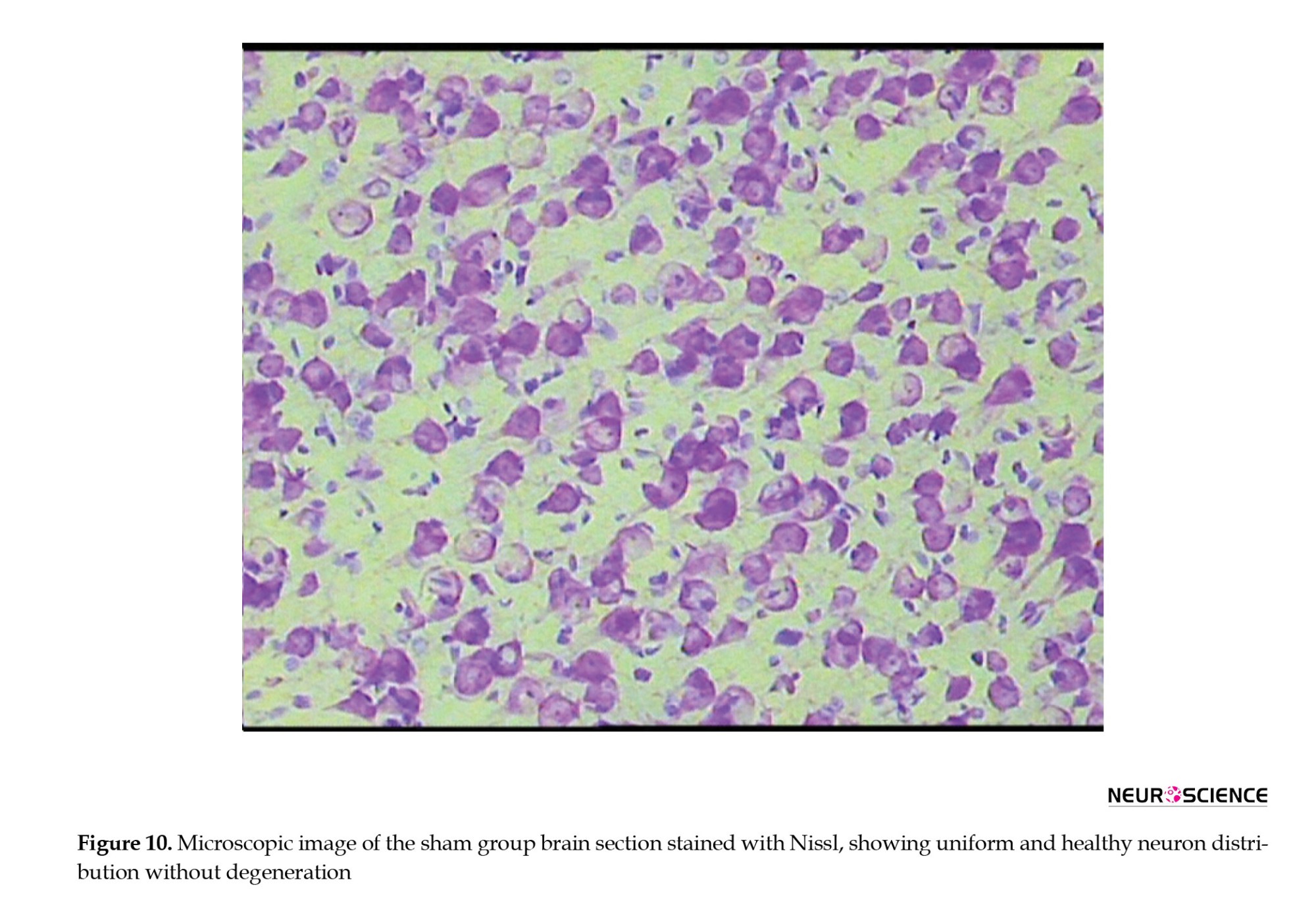
● In the sham group, neurons exhibited healthy nuclei and well-defined cytoplasm. No neuronal degeneration or loss was observed. The distribution of neurons was uniform, with no signs of damage.
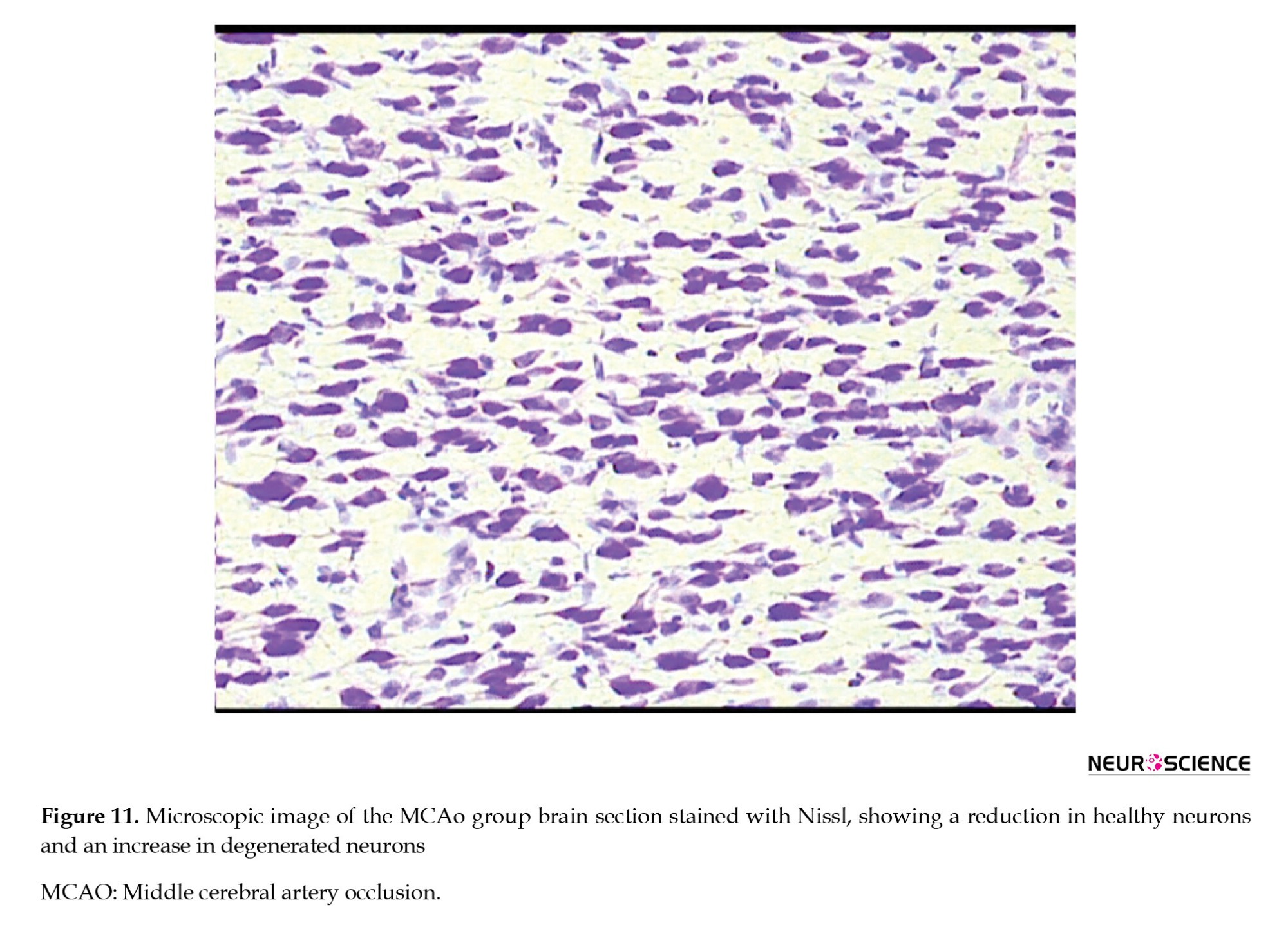
● In the MCAO control group, a significant reduction in the number of healthy neurons was observed. Many neurons had shrunken and pyknotic nuclei, and basophilic material accumulated in the cytoplasm of degenerated neurons. These degenerative changes indicated severe damage caused by ischemia (Figure 11).
● In the exosome treatment group, a significant increase in the number of healthy neurons was observed. The cellular structure of neurons in the control group was more organized than that of the MCAO group. The number of pyknotic neurons decreased, and less neuronal degeneration was observed. These results suggest that exosomes have protective effects against ischemic damage (Figure 12).
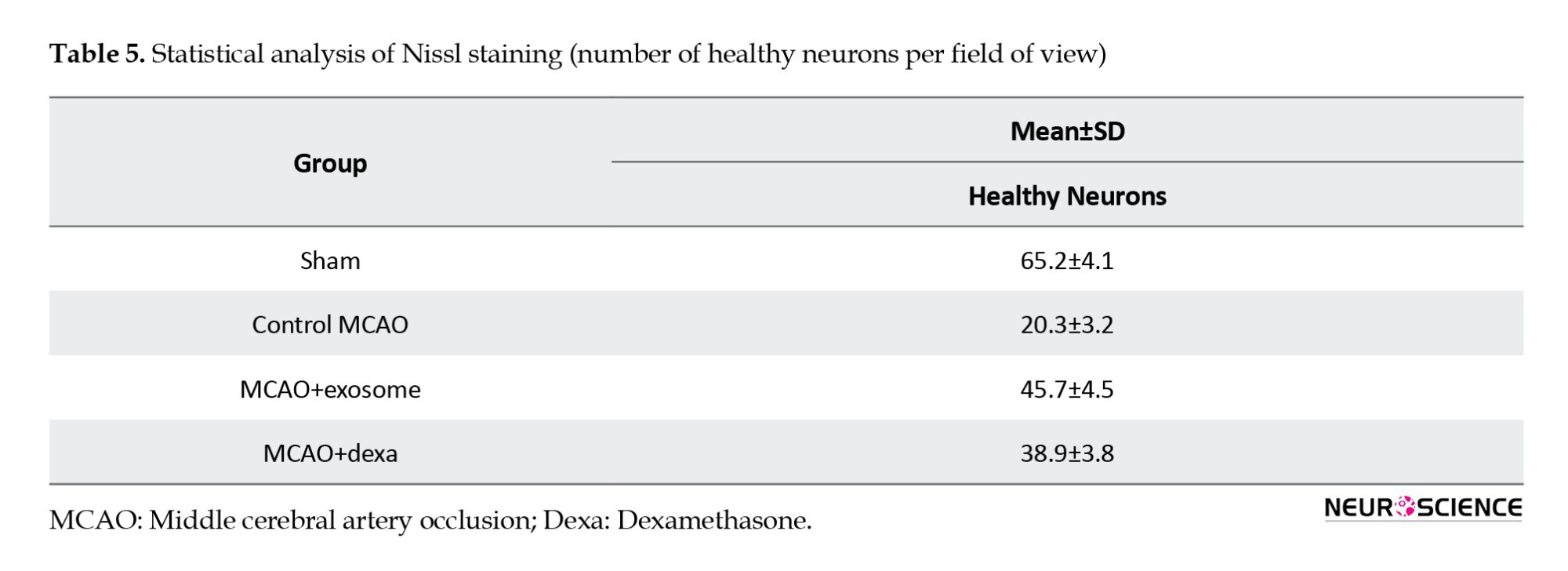
● In the dexamethasone treatment group, an increase in the number of healthy neurons was observed compared to the MCAO group; however, the improvement was less pronounced than that in the exosome group (Table 5). Some neurons still exhibited signs of degeneration and reduced Nissl substance, but the severity of these changes was less than in the MCAO group (Figure 13).
Discussion
Comparison of results with previous studies
Neurological function and motor recovery
Neurological deficits are a common consequence of stroke, primarily due to neuronal death, inflammation, and damage to neural circuits. In the present study, a significant reduction in Bederson scores was observed in both the exosome- and dexamethasone-treated groups compared to the MCAO control group, indicating improved neurological outcomes.
Xin et al. (2013) demonstrated that MSC-derived exosomes could enhance neurological function and reduce motor impairment in a stroke animal model (Xin et al., 2013). Similarly, Doeppner et al. reported that exosomes reduce neuronal necrosis and promote motor recovery by transferring neuroprotective microRNAs (Doeppner et al., 2015).
Reduction of infarct volume and pathological findings
TTC staining revealed that infarct size and necrotic damage were significantly reduced in the exosome-treated groups. Jiao et al. found that stem cell-derived exosomes can mitigate necrosis and promote neuronal survival and regeneration in ischemic brains by delivering microRNAs and growth factors (Jiao et al., 2022). Xian et al. also reported that exosomes suppress the expression of inflammatory mediators, such as NF-κB, TNF-α, and interleukin (IL)-1β, leading to reduced tissue necrosis and smaller infarct volumes (Xian et al., 2019).
Our results support the notion that exosomes exert superior neuroprotective effects compared to conventional treatments, such as dexamethasone, largely through the modulation of inflammatory signaling and stimulation of cellular repair mechanisms.
Exosomes have also been shown to inhibit apoptosis and increase the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which are crucial for neuronal regeneration and reducing secondary damage after ischemia (Zhu et al., 2023).
Furthermore, recent evidence suggests that exosome therapy improves mitochondrial function and reduces oxidative stress by decreasing reactive oxygen species (ROS), thereby enhancing neuronal survival and recovery (Wang et al., 2020).
Another mechanism includes the modulation of microglial activity. Exosomes can attenuate chronic inflammation by suppressing pro-inflammatory responses in microglia, creating a more favorable environment for brain tissue regeneration (Zhang et al., 2025).
In contrast to dexamethasone, which mainly acts through anti-inflammatory pathways, exosome treatment not only reduces inflammation but also promotes neuroregeneration. While further research is needed to elucidate precise mechanisms, current evidence highlights the high therapeutic potential of exosomes in ischemic brain injury.
Mechanisms of therapeutic effects
Potential mechanisms of exosome action
Stem cell-derived exosomes exert their therapeutic effects through a variety of mechanisms due to their cargo of neuroprotective microRNAs, growth factors, and anti-inflammatory proteins, including:
● Delivery of anti-inflammatory microRNAs such as miR-124 and miR-21, which suppress inflammatory pathways and enhance neuronal viability.
● Inhibition of the NF-κB and IL-6 signaling pathways reduces inflammation and prevents necrosis.
● Upregulation of neurotrophic factors, such as BDNF and NGF, facilitates neuronal repair.
● Promotion of angiogenesis and increased blood supply in the ischemic region via VEGF expression.
Inhibition of apoptosis, leading to improved cell survival (Wei et al., 2025).
Mechanisms of dexamethasone
Dexamethasone is a potent corticosteroid with well-known anti-inflammatory properties. Its actions include: Inhibition of the NF-κB and MAPK (mitogen-activated protein kinase) pathways leads to reduced production of pro-inflammatory cytokines. Reduced infiltration of immune cells into the ischemic region and suppression of cerebral edema. Decreased chronic inflammation and necrosis (Hossmann, 2006).
Conclusion
The findings of this study revealed that both treatments—stem cell-derived exosomes and dexamethasone—were effective in reducing ischemic damage, inflammation, and neurological deficits. However, exosomes demonstrated superior efficacy due to their multifaceted mechanisms, including neuroprotection, angiogenesis, and anti-inflammatory effects.
Exosomes can promote neural tissue repair by delivering bioactive factors, including BDNF, NGF, and VEGF. They mitigate neuroinflammation and oxidative stress by downregulating the expression of inflammatory genes (e.g. IL-1β, TNF-α) and inhibiting NF-κB activation, resulting in decreased cell death and enhanced brain repair.
Compared to dexamethasone, exosomes act through more complex signaling pathways, exerting broader effects on neurons, endothelial cells, and immune cells. While dexamethasone mainly suppresses inflammation, exosomes stimulate neurogenesis, synaptic plasticity, and vascular remodeling. This condition gives them a therapeutic edge in treating stroke.
Animal studies have confirmed their efficacy in reducing brain lesions and improving cognitive and motor functions. For example, recent studies demonstrated that exosome injection leads to decreased infarct volume, enhanced behavioral performance, and upregulation of protective factors in the brain.
Future research should focus on optimizing exosome production, purification, and delivery methods to enhance their therapeutic potential. Key challenges remain in determining the optimal dosage and administration route, as well as assessing long-term safety in stroke patients.
Overall, this study highlights the therapeutic promise of exosome-based strategies in stroke treatment. By delivering biological agents that reduce inflammation and promote neuroregeneration, exosomes represent a novel and potentially transformative approach for managing neurological disorders.
Study limitations
1. Lack of long-term assessment of neuronal regeneration and cognitive function.
2. Absence of detailed investigation into molecular signaling pathways involved in inflammation and neurorepair.
3. Use of a single animal model (rat) may limit the generalizability of the results.
Recommendations for future research
1. Evaluate the effects of these therapies in other animal models and over extended time periods.
2. Utilize advanced techniques, such as RNA sequencing, to analyze molecular mechanisms and identify key signaling pathways.
3. Compare exosome therapy with other regenerative approaches, such as direct MSC transplantation.
4. Investigate combinatory strategies involving exosomes and other neuroprotective agents to enhance therapeutic efficacy.
Ethical Considerations
Compliance with ethical guidelines
All animal procedures were approved by the Institutional Animal Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran (Code: (IR.BMSU.AEC.1403.001), and performed in accordance with the national guidelines for animal research. All efforts were made to minimize animal suffering and the number of animals used.
Funding
This research was supported by the Research and Technology of Baqiyatallah University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Ali Reza Eftekhari Moghadam and Mahdi Heydari; Methodology: Mahdi Heydari, Bahman Jalali Kondori, Mehdi Raei, and Ali Reza Eftekhari Moghadam; Nvestigation: Mahdi Heydari, Bahman Jalali Kondori, and Mehdi Raei; Data curation: Mahdi Heydari and Bahman Jalali Kondori; Writing the original draft: Mahdi Heydari and Mehdi Raei; Review and editing: Ali Reza Eftekhari Moghadam and Bahman Jalali Kondori; Visualization: Mahdi Heydari; Funding acquisition, project administration, and supervision: Ali Reza Eftekhari Moghadam.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors like to express their heartfelt gratitude to the esteemed faculty and staff of the Department of Anatomical Science at Baqiyatallah University of Medical Sciences, Tehran, Iran. Their unwavering support and guidance have been instrumental in the successful completion of this research. Special thanks to the Research Deputy of Baqiyatallah University of Medical Sciences, for their encouragement and invaluable insights, which have significantly enriched their understanding and approach to the subject matter.
References
Cerebral ischemia is a pathological condition characterized by insufficient oxygen supply to the brain tissue, which can manifest transiently as transient ischemic attacks due to cerebral vasoconstriction, or permanently as cerebral infarction or stroke, typically resulting from thrombosis or embolism in major cerebral arteries. Stroke is recognized globally as a leading cause of mortality and long-term disability, imposing substantial clinical and socioeconomic burdens. Thus, there remains an urgent necessity for novel therapeutic interventions that can effectively mitigate ischemic damage and improve patient outcomes (Amantea et al., 2009).
Ischemic injury induces severe tissue edema and increased intracranial pressure, subsequently leading to irreversible neuronal cell death. A comprehensive understanding of these pathophysiological processes is crucial for developing effective preventive strategies and therapeutic approaches for patients at risk (Amantea et al., 2009). Current therapeutic interventions, primarily antiplatelet and thrombolytic treatments, offer limited efficacy by merely targeting restoration or preservation of cerebral blood flow, rather than directly addressing the underlying cellular and molecular mechanisms of ischemic neuronal death (Amantea et al., 2009; Gladstone et al., 2002).
Shortly after ischemic onset, substantial damage occurs within the core ischemic region, characterized by a significant reduction in cerebral blood flow (greater than 80%), energy depletion, oxygen deprivation, and loss of ionic gradients resulting from neuronal depolarization. These conditions primarily induce cell death through excitotoxicity, mitochondrial dysfunction, excessive production of reactive oxygen species, and activation of programmed cell death pathways (Lo et al., 2003; Jayaraj et al., 2019).
Recently, exosomes have emerged as promising therapeutic vehicles due to their capability to cross the blood-brain barrier efficiently and deliver bioactive cargo into neural tissue. Exosomes significantly contribute to reducing inflammation in the central nervous system and enhancing recovery of neuromotor function post-injury (Console et al., 2019). Notably, research has demonstrated that brain injury induces upregulation of miR-124-3p, a microRNA known to alleviate neuronal inflammation and improve neurological function. Given their inherent capacity to transport anti-inflammatory modulators such as microRNAs and proteins, exosomes represent a compelling strategy for targeted therapeutic delivery to the CNS, addressing inflammation-related pathological conditions (Gao et al., 2020).
Stem cell-derived exosomes, acting as key paracrine mediators, have shown promising therapeutic effects in ischemic stroke through their roles in promoting angiogenesis and neurogenesis, alongside exhibiting significant neuroprotective and neuroregenerative properties (Huang et al., 2020). Recent findings further indicate that exosomes secreted by various stem cells, including adipose-derived stem cells (ADSCs), can carry bioactive molecules such as lipids, proteins, and genetic materials into the extracellular environment, effectively modulating pathological conditions in neurodegenerative diseases. Specifically, ADSC-derived exosomes have been demonstrated to attenuate inflammation and oxidative stress by modulating the Nrf2/HO-1 signaling pathway, thereby highlighting their therapeutic potential for ischemic and degenerative brain injuries (Shen et al., 2021).
The innovation of this study lies in utilizing stem cell-derived exosomes, particularly those derived from ADSCs, as novel therapeutic agents targeting the underlying molecular and cellular mechanisms of cerebral ischemia. The primary goal of this research is to investigate the therapeutic potential and efficacy of ADSC-derived exosomes in reducing inflammation, oxidative stress, and neuronal damage associated with ischemic stroke, to establish their role as a viable and advanced therapeutic intervention for ischemic cerebral injuries.
Materials and Methods
Study design
This experimental study evaluated the neuroprotective effects of adipose tissue–mesenchymal stem cell (AT-MSC)-derived exosomes in a rat model of transient focal cerebral ischemia. To assess the therapeutic impact, behavioral, biochemical, and histopathological analyses were performed.
Animal grouping
Twenty adult male Wistar rats (250–280 g) were randomly divided into four groups (n=5 per group):
1. Sham group: Underwent surgical procedures without ischemia induction and received normal saline orally for 7 days.
2. MCAO control group: Underwent middle cerebral artery occlusion (MCAO) without receiving any treatment.
3. Exosome-treated group: Administered 0.2 mL phosphate-buffered saline (PBS)-diluted exosomes intravenously via the tail vein 1 hour before ischemia, followed by daily injections for 7 days.
Dexamethasone group: Treated with daily intraperitoneal injections of dexamethasone (1 mg/kg) starting 24 hours after ischemia.
Induction of transient focal cerebral ischemia
Transient focal cerebral ischemia was induced using the MCAO method. Rats were anesthetized with 5% isoflurane (induction) and maintained with 2% isoflurane in 30% oxygen and 70% nitrogen. A midline neck incision exposed the common, external, and internal carotid arteries. A silicone-coated 3-0 nylon filament was inserted through the ICA to occlude the MCA for 90 minutes. Afterward, the filament was removed to allow reperfusion. Body temperature was kept at 37±0.5 °C throughout the procedure.
Exosome isolation and characterization
Exosomes were isolated from AT-MSCs harvested from a young Wistar rat via ultracentrifugation. NanoSight analysis was used to assess particle size and concentration. Western blotting confirmed the presence of specific surface markers indicative of exosomes.
Behavioral assessments
Bederson neurological score: Neurological deficits were scored at 24 hours and 7 days post-ischemia based on forelimb flexion, lateral push resistance, and circling. Scores ranged from 0 (normal) to 5 (severe deficit) (Figure 1).

Garcia neurological score: A comprehensive assessment was conducted at 24 hours, 3 days, and 7 days post-ischemia using the Garcia scale, which includes six categories:
Spontaneous activity, symmetry of limb movement, climbing ability, response to touch/pain, and body proprioception (Figure 2, Table 1).


Vibrissae response
Total scores ranged from 3 (severe deficit) to 18 (normal).
Biochemical analysis
Serum levels of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were measured using ELISA kits (Jiamei Biotech Co. Ltd., Beijing, China). Absorbance was read at 450 nm, and concentrations were calculated using standard curves.
Infarct volume assessment
At 24 hours post-MCAO, rats were euthanized (pentobarbital 100 mg/kg, IP). Brains were removed, rinsed in cold saline, and cut into 2-mm coronal sections. Slices were incubated in 2% 2,3,5-Triphenyltetrazolium chloride (TTC) at 37 °C for 20 minutes, then fixed in 10% formalin. Digital images were analyzed with ImageJ software, version 1.54p using:
Infarct volume=Σ (Infarct area×Slice thickness).
Histopathological analysis
On day 7 post-ischemia, brain samples were fixed in 10% buffered formalin, processed through ethanol and xylene, and embedded in paraffin. Five-micron sections were stained with H&E to assess necrosis and inflammation. Nissl staining (1% toluidine blue at 45 °C for 30 minutes) was used to assess neuronal survival. Sections were analyzed using a light microscope (DMI4000B, Leica, Germany).
Statistical analysis
Data were analyzed using SPSS, version 28 (IBM Corp. NY, US). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for comparisons. Results are presented as Mean±SD. A P<0.05 was considered statistically significant.
Ethical considerations
All procedures were approved by the Animal Ethics Committee of Baqiyatallah University of Medical Sciences in accordance with national guidelines. Animals were euthanized humanely at the end of the study using a high-dose anesthetic (IP).
Results
Neurological performance evaluation
The Bederson test is a standard tool for evaluating the severity of neurological deficits following focal cerebral ischemia. Its score ranges from 0 (no neurological deficit) to 5 (severe motor and neurological impairment), reflecting the level of motor and neurological deficits in animals.
The sham group showed the least mean neurological deficits (0.5±0.2), which confirmed normal brain function.
● The control MCAO group exhibited the highest mean neurological deficit (4.2±0.5), indicating severe damage caused by MCAO.
● The MCAO+exosome and MCAO+dexa groups had significantly lower scores than the MCAO control group, but still showed a significant difference compared to the sham group.
● Exosome treatment showed a greater reduction in motor deficits than dexamethasone, although the difference between these two treatments was not statistically significant.
The Garcia test evaluates neurological function within a range of 3 (severe damage) to 18 (normal function), with higher scores indicating improved neurological function and reduced motor deficits.
● The sham group received the highest score, reflecting normal function.
● The MCAO (untreated) group received the lowest score, confirming severe motor and neurological deficits.
● The exosome-treated group had higher scores than the dexa-treated group, suggesting better neurological recovery.
Brain lesion volume (staining analysis)
TTC staining was used to assess the extent of brain tissue necrosis following ischemia. In this method, necrotic areas appeared white, while healthy tissue appeared dark red (Figure 3, Table 2).

● In the sham group, no white areas (ischemic damage) were observed, indicating healthy brain tissue.
● In the sham group, no white areas (ischemic damage) were observed, indicating healthy brain tissue.
● In the MCAO group, a large white area was observed in the brain, indicating infarction and severe neuronal damage.
● In the treatment groups (e.g. exosome and dexamethasone groups), infarct areas were significantly reduced compared to the MCAO group (Figure 4).
● Treatment groups showed fewer ischemic lesions, while the MCAO group exhibited the largest infarct area.


Inflammatory factor levels in serum
The inflammatory response plays a critical role in the pathophysiology of ischemic brain injury. This section examines the levels of key inflammatory markers (NF-κB, TNF-α, IL-6) in the serum of animals from different treatment groups. These factors are often elevated in response to ischemic injury and are associated with neuronal damage and neuroinflammation. The analysis of these factors helps in understanding the anti-inflammatory effects of the exosome and dexamethasone treatments (Figure 5, Table 3).


Histopathological findings
To evaluate tissue changes resulting from cerebral ischemia and the therapeutic effects of exosomes and dexamethasone, Hematoxylin and Eosin (H&E) and Nissl staining techniques were employed (Figure 6). Microscopic examinations were performed on coronal brain sections of the animals, and the extent of necrosis, tissue edema, the number of damaged neurons, and neuronal structure preservation were compared across different groups.

● In the sham group, the brain tissue structure appeared normal, with no signs of necrosis, tissue edema, or neuronal degeneration. Neurons exhibited normal morphology with healthy nuclei and uniform distribution. Additionally, no evidence of inflammatory responses was observed in the tissue.
● In the control MCAO group, extensive necrosis, loss of tissue integrity, severe edema, and neuronal death were evident. Necrotic neurons exhibited shrunken nuclei and dense cytoplasm. Increased numbers of inflammatory cells, cellular swelling, and severe tissue changes were observed in the ischemic regions. These changes indicated significant damage caused by cerebral ischemia (Figure 7).

● In the exosome treatment group, necrosis and tissue edema were significantly reduced. A greater number of neurons exhibited healthy nuclei, and tissue integrity was improved compared to the MCAO group. In this group, inflammatory responses and immune cell accumulation were reduced, resulting in less cell death (Table 4).

These results suggest that exosomes have protective effects in reducing neuronal damage and inflammation resulting from cerebral ischemia (Figure 8).

● In the dexamethasone treatment group, there was a reduction in necrosis and tissue edema compared to the MCAO group. However, the degree of reduction was less than in the exosome-treated group. Although fewer necrotic cells were observed in this group, the inflammatory responses remained significant. Compared to the exosome group, dexamethasone had a lesser effect on reducing inflammation and promoting neuronal reconstruction (Figure 9).

Histopathological findings – Nissl staining
To evaluate the preservation and degeneration of neurons after cerebral ischemia, Nissl staining was used (Figure 10). This staining technique was used to assess the number of healthy neurons and the extent of neuronal degeneration in damaged regions. In all groups, Nissl bodies (rough endoplasmic reticulum) were observed in the cytoplasm, appearing purple-blue, and in the nucleus, they seemed light blue.

● In the sham group, neurons exhibited healthy nuclei and well-defined cytoplasm. No neuronal degeneration or loss was observed. The distribution of neurons was uniform, with no signs of damage.
● In the MCAO control group, a significant reduction in the number of healthy neurons was observed. Many neurons had shrunken and pyknotic nuclei, and basophilic material accumulated in the cytoplasm of degenerated neurons. These degenerative changes indicated severe damage caused by ischemia (Figure 11).

● In the exosome treatment group, a significant increase in the number of healthy neurons was observed. The cellular structure of neurons in the control group was more organized than that of the MCAO group. The number of pyknotic neurons decreased, and less neuronal degeneration was observed. These results suggest that exosomes have protective effects against ischemic damage (Figure 12).

● In the dexamethasone treatment group, an increase in the number of healthy neurons was observed compared to the MCAO group; however, the improvement was less pronounced than that in the exosome group (Table 5). Some neurons still exhibited signs of degeneration and reduced Nissl substance, but the severity of these changes was less than in the MCAO group (Figure 13).


Discussion
Comparison of results with previous studies
Neurological function and motor recovery
Neurological deficits are a common consequence of stroke, primarily due to neuronal death, inflammation, and damage to neural circuits. In the present study, a significant reduction in Bederson scores was observed in both the exosome- and dexamethasone-treated groups compared to the MCAO control group, indicating improved neurological outcomes.
Xin et al. (2013) demonstrated that MSC-derived exosomes could enhance neurological function and reduce motor impairment in a stroke animal model (Xin et al., 2013). Similarly, Doeppner et al. reported that exosomes reduce neuronal necrosis and promote motor recovery by transferring neuroprotective microRNAs (Doeppner et al., 2015).
Reduction of infarct volume and pathological findings
TTC staining revealed that infarct size and necrotic damage were significantly reduced in the exosome-treated groups. Jiao et al. found that stem cell-derived exosomes can mitigate necrosis and promote neuronal survival and regeneration in ischemic brains by delivering microRNAs and growth factors (Jiao et al., 2022). Xian et al. also reported that exosomes suppress the expression of inflammatory mediators, such as NF-κB, TNF-α, and interleukin (IL)-1β, leading to reduced tissue necrosis and smaller infarct volumes (Xian et al., 2019).
Our results support the notion that exosomes exert superior neuroprotective effects compared to conventional treatments, such as dexamethasone, largely through the modulation of inflammatory signaling and stimulation of cellular repair mechanisms.
Exosomes have also been shown to inhibit apoptosis and increase the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which are crucial for neuronal regeneration and reducing secondary damage after ischemia (Zhu et al., 2023).
Furthermore, recent evidence suggests that exosome therapy improves mitochondrial function and reduces oxidative stress by decreasing reactive oxygen species (ROS), thereby enhancing neuronal survival and recovery (Wang et al., 2020).
Another mechanism includes the modulation of microglial activity. Exosomes can attenuate chronic inflammation by suppressing pro-inflammatory responses in microglia, creating a more favorable environment for brain tissue regeneration (Zhang et al., 2025).
In contrast to dexamethasone, which mainly acts through anti-inflammatory pathways, exosome treatment not only reduces inflammation but also promotes neuroregeneration. While further research is needed to elucidate precise mechanisms, current evidence highlights the high therapeutic potential of exosomes in ischemic brain injury.
Mechanisms of therapeutic effects
Potential mechanisms of exosome action
Stem cell-derived exosomes exert their therapeutic effects through a variety of mechanisms due to their cargo of neuroprotective microRNAs, growth factors, and anti-inflammatory proteins, including:
● Delivery of anti-inflammatory microRNAs such as miR-124 and miR-21, which suppress inflammatory pathways and enhance neuronal viability.
● Inhibition of the NF-κB and IL-6 signaling pathways reduces inflammation and prevents necrosis.
● Upregulation of neurotrophic factors, such as BDNF and NGF, facilitates neuronal repair.
● Promotion of angiogenesis and increased blood supply in the ischemic region via VEGF expression.
Inhibition of apoptosis, leading to improved cell survival (Wei et al., 2025).
Mechanisms of dexamethasone
Dexamethasone is a potent corticosteroid with well-known anti-inflammatory properties. Its actions include: Inhibition of the NF-κB and MAPK (mitogen-activated protein kinase) pathways leads to reduced production of pro-inflammatory cytokines. Reduced infiltration of immune cells into the ischemic region and suppression of cerebral edema. Decreased chronic inflammation and necrosis (Hossmann, 2006).
Conclusion
The findings of this study revealed that both treatments—stem cell-derived exosomes and dexamethasone—were effective in reducing ischemic damage, inflammation, and neurological deficits. However, exosomes demonstrated superior efficacy due to their multifaceted mechanisms, including neuroprotection, angiogenesis, and anti-inflammatory effects.
Exosomes can promote neural tissue repair by delivering bioactive factors, including BDNF, NGF, and VEGF. They mitigate neuroinflammation and oxidative stress by downregulating the expression of inflammatory genes (e.g. IL-1β, TNF-α) and inhibiting NF-κB activation, resulting in decreased cell death and enhanced brain repair.
Compared to dexamethasone, exosomes act through more complex signaling pathways, exerting broader effects on neurons, endothelial cells, and immune cells. While dexamethasone mainly suppresses inflammation, exosomes stimulate neurogenesis, synaptic plasticity, and vascular remodeling. This condition gives them a therapeutic edge in treating stroke.
Animal studies have confirmed their efficacy in reducing brain lesions and improving cognitive and motor functions. For example, recent studies demonstrated that exosome injection leads to decreased infarct volume, enhanced behavioral performance, and upregulation of protective factors in the brain.
Future research should focus on optimizing exosome production, purification, and delivery methods to enhance their therapeutic potential. Key challenges remain in determining the optimal dosage and administration route, as well as assessing long-term safety in stroke patients.
Overall, this study highlights the therapeutic promise of exosome-based strategies in stroke treatment. By delivering biological agents that reduce inflammation and promote neuroregeneration, exosomes represent a novel and potentially transformative approach for managing neurological disorders.
Study limitations
1. Lack of long-term assessment of neuronal regeneration and cognitive function.
2. Absence of detailed investigation into molecular signaling pathways involved in inflammation and neurorepair.
3. Use of a single animal model (rat) may limit the generalizability of the results.
Recommendations for future research
1. Evaluate the effects of these therapies in other animal models and over extended time periods.
2. Utilize advanced techniques, such as RNA sequencing, to analyze molecular mechanisms and identify key signaling pathways.
3. Compare exosome therapy with other regenerative approaches, such as direct MSC transplantation.
4. Investigate combinatory strategies involving exosomes and other neuroprotective agents to enhance therapeutic efficacy.
Ethical Considerations
Compliance with ethical guidelines
All animal procedures were approved by the Institutional Animal Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran (Code: (IR.BMSU.AEC.1403.001), and performed in accordance with the national guidelines for animal research. All efforts were made to minimize animal suffering and the number of animals used.
Funding
This research was supported by the Research and Technology of Baqiyatallah University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Ali Reza Eftekhari Moghadam and Mahdi Heydari; Methodology: Mahdi Heydari, Bahman Jalali Kondori, Mehdi Raei, and Ali Reza Eftekhari Moghadam; Nvestigation: Mahdi Heydari, Bahman Jalali Kondori, and Mehdi Raei; Data curation: Mahdi Heydari and Bahman Jalali Kondori; Writing the original draft: Mahdi Heydari and Mehdi Raei; Review and editing: Ali Reza Eftekhari Moghadam and Bahman Jalali Kondori; Visualization: Mahdi Heydari; Funding acquisition, project administration, and supervision: Ali Reza Eftekhari Moghadam.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors like to express their heartfelt gratitude to the esteemed faculty and staff of the Department of Anatomical Science at Baqiyatallah University of Medical Sciences, Tehran, Iran. Their unwavering support and guidance have been instrumental in the successful completion of this research. Special thanks to the Research Deputy of Baqiyatallah University of Medical Sciences, for their encouragement and invaluable insights, which have significantly enriched their understanding and approach to the subject matter.
References
Amantea, D., Nappi, G., Bernardi, G., Bagetta, G., & Corasaniti, M. T. (2009). Post‐ischemic brain damage: Pathophysiology and role of inflammatory mediators. The FEBS Journal, 276(1), 13–26. [DOI:10.1111/j.1742-4658.2008.06766.x] [PMID]
Console, L., Scalise, M., & Indiveri, C. (2019). Exosomes in inflammation and role as biomarkers. Clinica Chimica Acta; International Journal of Clinical Chemistry, 488, 165–171. [DOI:10.1016/j.cca.2018.11.009] [PMID]
Doeppner, T. R., Herz, J., Görgens, A., Schlechter, J., Ludwig, A. K., & Radtke, S., et al. (2015). Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translational Medicine, 4(10), 1131–1143. [DOI:10.5966/sctm.2015-0078] [PMID]
Gao, B., Zhou, S., Sun, C., Cheng, D., Zhang, Y., & Li, X., et al. (2020). Brain endothelial cell-derived exosomes induce neuroplasticity in rats with ischemia/reperfusion injury. ACS Chemical Neuroscience, 11(15), 2201-2213. [DOI:10.1021/acschemneuro.0c00089] [PMID]
Gladstone, D. J., Black, S. E., Hakim, A. M., & Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery (2002). Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke, 33(8), 2123–2136. [DOI:10.1161/01.STR.0000025518.34157.51] [PMID]
Hossmann, K. A. (2006). Pathophysiology and therapy of experimental stroke. Cellular and Molecular Neurobiology, 26(7-8), 1057–1083. [DOI:10.1007/s10571-006-9008-1] [PMID]
Huang, M., Hong, Z., Xiao, C., Li, L., Chen, L., & Cheng, S., et al. (2020). Effects of exosomes on neurological function recovery for ischemic stroke in pre-clinical studies: A meta-analysis. Frontiers in Cellular Neuroscience, 14, 593130. [DOI: 10.3389/fncel.2020.59313] [PMID]
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., & Rosenberg, G. A. (2019). Neuroinflammation: Friend and foe for ischemic stroke. Journal of Neuroinflammation, 16(1), 1-24. [DOI:10.1186/s12974-019-1516-2] [PMID]
Jiao, Y., Sun, Y. T., Chen, N. F., Zhou, L. N., Guan, X., & Wang, J. Y., et al. (2022). Human umbilical cord-derived mesenchymal stem cells promote repair of neonatal brain injury caused by hypoxia/ischemia in rats. Neural regeneration research, 17(11), 2518–2525. [DOI: 10.4103/1673-5374.339002] [PMID]
Lo, E. H., Dalkara, T., & Moskowitz, M. A. (2003). Mechanisms, challenges and opportunities in stroke. Nature Reviews Neuroscience, 4(5), 399-414. [DOI:10.1038/nrn1106] [PMID]
Shen, K., Jia, Y., Wang, X., Zhang, J., Liu, K., & Wang, J., et al. (2021). Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radical Biology and Medicine, 165, 54-66. [DOI:10.1016/j.freeradbiomed.2021.01.023] [PMID]
Wang, X., Zhou, Y., Gao, Q., Ping, D., Wang, Y., & Wu, W., et al. (2020). The role of exosomal micrornas and oxidative stress in neurodegenerative diseases. Oxidative Medicine and cellular Longevity, 2020, 3232869. [DOI:10.1155/2020/3232869] [PMID]
Wei, D., Li, F., Guo, C., Chen, J., & You, Y. (2025). Exosomes and non-coding RNAs in the regulation of neuroinflammation after ischemic stroke: mechanisms and therapeutic perspectives. Frontiers in Immunology, 16, 1601843. [DOI: 10.3389/fimmu.2025.1601843] [PMID]
Xian, P., Hei, Y., Wang, R., Wang, T., Yang, J., Li, J., & Di, Z., et al. (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics, 9(20), 5956–5975. [DOI:10.7150/thno.33872] [PMID]
Xin, H., Li, Y., Cui, Y., Yang, J. J., Zhang, Z. G., & Chopp, M. (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 33(11), 1711–1715.[DOI:10.1038/jcbfm.2013.152] [PMID]
Zhang, Z., Ji, R., Liu, Z., Jiang, Z., Chu, M., & Wang, Y., et al. (2025). hUMSC-exosomes suppress TREM1-p38 MAPK signaling via HMGB1-dependent mechanisms to reprogram microglial function and promote neuroprotection in ischemic stroke. Journal of Nanobiotechnology, 23(1), 572. [DOI:10.1186/s12951-025-03652-z] [PMID]
Zhu, Z. H., Jia, F., Ahmed, W., Zhang, G. L., Wang, H., & Lin, C. Q., et al. (2023). Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regeneration Research, 18(2), 404–409. [DOI: 10.4103/1673-5374.346466] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2025/05/21 | Accepted: 2025/07/11 | Published: 2025/09/1
Received: 2025/05/21 | Accepted: 2025/07/11 | Published: 2025/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








