Volume 16, Issue 5 (September & October 2025)
BCN 2025, 16(5): 949-964 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amini B, Hosini S A, Pishyareh E, Bakhshi E. The Effect of a Structured and Progressive Rhythmiccognitive Dual-task Exercise Program on Impulsivity in Children With ADHD. BCN 2025; 16 (5) :949-964
URL: http://bcn.iums.ac.ir/article-1-3202-en.html
URL: http://bcn.iums.ac.ir/article-1-3202-en.html
1- Department of Occupational Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Determinants of Health Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Determinants of Health Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Full-Text [PDF 685 kb]
| Abstract (HTML)
Full-Text:
Introduction
Impulsivity, as a multidimensional personality trait, encompasses a wide range of immature and inappropriate behaviors that often result in undesirable consequences (Bezdjian et al., 2011; Meda et al., 2009). It holds particular significance during childhood and adolescence (Chamorro et al., 2012), a developmental period during which impulsivity manifests in various clinical diagnoses, including ADHD, autism spectrum disorder, anxiety disorders, and obsessive-compulsive disorder (Amini et al., 2016; Bezdjian et al., 2011; Goya-Maldonado et al., 2010; Hamilton et al., 2015; Sebastian et al., 2013; Sebastian et al., 2014; Stahl, 2020).
According to the diagnostic and statistical manual of mental disorders (DSM-5), impulsivity is one of the core diagnostic features of attention-deficit/hyperactivity disorder (ADHD), particularly evident in preschool-aged children and those with the hyperactive-impulsive (ADHD-HI) subtype. Impulsivity often leads to behavioral difficulties in social contexts, such as an inability to wait one’s turn, frequent interruptions during conversations, or answering before a question is completed. In addition to impulsive decision-making, children and adolescents with ADHD often exhibit impaired motor coordination and increased postural sway (Newton-Howes, 2004). Research shows that children and adolescents with ADHD tend to make more impulsive decisions than their neurotypical peers (Patros et al., 2016), and disturbances in motor coordination and postural control have been linked to deficits in self-regulation and inhibitory control (Amini et al., 2018; Jahani et al., 2016).
According to Barkley’s theory, the hyperactive-impulsive subtype of ADHD is more prevalent in early childhood and is closely associated with attentional deficits. In his model, the primary underlying deficit in ADHD is a failure of response inhibition, which in turn disrupts 4 core executive functions: (a) working memory, (b) self-regulation of emotion, motivation, and arousal, (c) internalization of speech, and (d) reconstitution (i.e. behavioral analysis and synthesis). These impairments compromise the integration of the motor and perceptual systems necessary for goal-directed behavior (Barkley, 1997; Barkley et al., 1990). In this framework, executive dysfunction is posited as the primary cause of maladaptive behaviors, and response inhibition deficits are responsible for both distractibility and impulsivity in children with ADHD. Thus, inattention is considered a secondary consequence, whereas impulsivity represents a primary symptom (Barkley, 1997; Barkley et al., 1990).
Given the high prevalence of motor control impairments (approximately 50%) and postural instability (ranging from 47% to 69%) among children and adolescents with ADHD (Fliers et al., 2008; Piek et al., 2004; Williams et al., 2006), along with significant deficits in behavioral inhibition, working memory, and emotional regulation (Barkley, 1997; Barkley et al., 1990) motor-control-based physical interventions have been increasingly used as a therapeutic strategy for this population (Archer & Kostrzewa, 2011; Donnelly et al., 2016).
One fundamental cause of motor control and coordination difficulties in children with ADHD is attributed to deficits in rhythm and timing. These children often struggle to process environmental rhythms, particularly auditory ones, which are essential for achieving motor coordination in goal-directed activities (Shin et al., 2023). Notably, research suggests that humans synchronize more effectively to auditory rhythms than to visual or multimodal cues. Rhythm-based physical training has demonstrated promising effects on motor development and emotional regulation, which, in turn, enhance executive functioning and social engagement in children (Sakai et al., 2004). Accordingly, a growing body of literature has investigated the role of physical activity in this context.
Since 2014, systematic reviews have shown that most interventions rely on aerobic exercises such as treadmill running, cycling, yoga, and mixed physical activity programs. While these interventions generally enhance executive function in the short term, they typically involve 30-minute sessions focused solely on physical exertion, which elevates heart rate, respiratory output (VO₂max), and neurotransmitter levels in children with ADHD. The observed effect sizes often increase with session duration, ranging from 10 to 30 minutes (Grassmann et al., 2017). However, these studies rarely incorporate dual-task designs that combine physical and cognitive training to address impulsivity. Furthermore, such interventions frequently lack individualization, variety, or opportunities for home-based continuation. Rhythmic movement based on auditory cues has also been underexplored, and overall, the integration of auditory-motor synchronization has not been prioritized (Donnelly et al., 2016; Grassmann et al., 2017).
Impulsivity is closely associated with deficits in self-regulation (Barkley, 1997) and executive dysfunction (Bjørkly, 2013), which, from a neuroanatomical perspective, are linked to dysfunction in frontostriatal circuits involving the basal ganglia, anterior cingulate cortex, and orbitofrontal cortex regions responsible for regulating goal-directed behaviors.
Successful dual-tasking requires divided attention and the simultaneous processing of multiple internal and external stimuli (Garon et al., 2008). Neuroimaging studies highlight the role of the prefrontal cortex (PFC) in such multitasking, and underscore the basal ganglia’s importance in aligning motor behavior with auditory rhythms (Arvaniti, 2009; Thaut & Abiru, 2010).
Building on these findings, the present study employs a dual-task paradigm that involves the simultaneous performance of two independent tasks with distinct objectives (Lee et al., 2013; Pashler, 1994; Watanabe & Funahashi, 2015). This approach combines bodily movement with synchronized auditory and visual rhythms.
Recognizing the importance of fostering executive function development in children with ADHD—particularly within educational settings to promote adaptive learning behaviors (Cepeda et al., 2000) and considering the normative developmental pattern of subcortical brain circuit maturation—the dual-task format was selected as an intervention strategy. Additionally, the program was designed based on evidence showing that auditory rhythms can improve inhibitory control (Slater & Tate, 2018) and that sequential and timed motor activities enhance working memory and attentional capacity (Petrides, 1994; Smith & Jonides, 1999). Gross motor exercises have also been shown to benefit spatial awareness and response inhibition (van Der Fels et al., 2019). Accordingly, the training package developed for this study integrates these evidence-based components.
To ensure engagement and efficacy, the program incorporates principles such as task grading from simple to complex (Tomporowski & Pesce, 2019), diversity in exercise formats, and elements that promote enjoyment, empowerment, and intrinsic motivation in children (Brand & Ekkekakis, 2018; Cox et al., 2008; Diamond, 2015; Nasuti & Rhodes, 2013; Ryan & Deci, 2000). A preliminary feasibility study assessing the program’s acceptability among children has been conducted, and its findings have been published (Amini et al., 2024).
This study aimed to investigate the effect of a structured training package consisting of dual-task rhythmic physical and cognitive exercises on impulsivity, caution, alertness, and reaction time in children with ADHD. Additionally, the study examined the persistence of the effects one month after the intervention.
Materials and Methods
Study design
This study was a single-blind, randomized clinical trial. It represented the final intervention phase of a doctoral dissertation conducted in the Department of Occupational Therapy at the University of Social Welfare and Rehabilitation Sciences, Tehran City, Iran. The study was approved by the university’s Ethics Committee and was registered in the Iranian Registry of Clinical Trials (IRCT).
Study participants
The sample size was calculated to achieve sufficient statistical power to detect differences between the groups. Assuming a 5% significance level (P<0.05) and a power of 0.8, a total of 60 participants (30 per group) were required. Sixty children with ADHD, aged between 6 and 12 years, were therefore recruited and randomly assigned to either the intervention or control group.
Participants were recruited from public and private clinical centers affiliated with Tehran universities and the Tehran Medical System Organization. The inclusion criteria were as follows: A confirmed diagnosis of ADHD (combined type or hyperactive-impulsive type) by a psychiatrist and documented in the child’s medical records; chronological age between 6 and 12 years; absence of obvious physical disabilities based on therapist evaluation; elevated impulsivity scores on the short-form Connors parent questionnaire; no prior participation in rhythmic motor training programs; normal cognitive functioning as evidenced by enrollment in mainstream education; responsiveness and cooperation from the child; parental literacy; and absence of neurological, rheumatic, or orthopedic conditions that would interfere with participation in the intervention.
Covariate adaptive randomization was used to assign participants to groups, ensuring balance across key variables. Before enrollment, informed written consent was obtained from all parents or legal guardians. After the intervention period, the results were shared with families. For ethical considerations, the intervention program was offered to families in the control group free of charge upon completion of the study. The exclusion criteria included a lack of family cooperation during the intervention, withdrawal from the study, or any change in the child’s medication dosage during the trial period.
Study equipment
The intervention consisted of a graded dual-task training package combining rhythmic physical activities with age-appropriate cognitive tasks. The program was developed based on expert feedback and underwent multiple review stages before being approved.
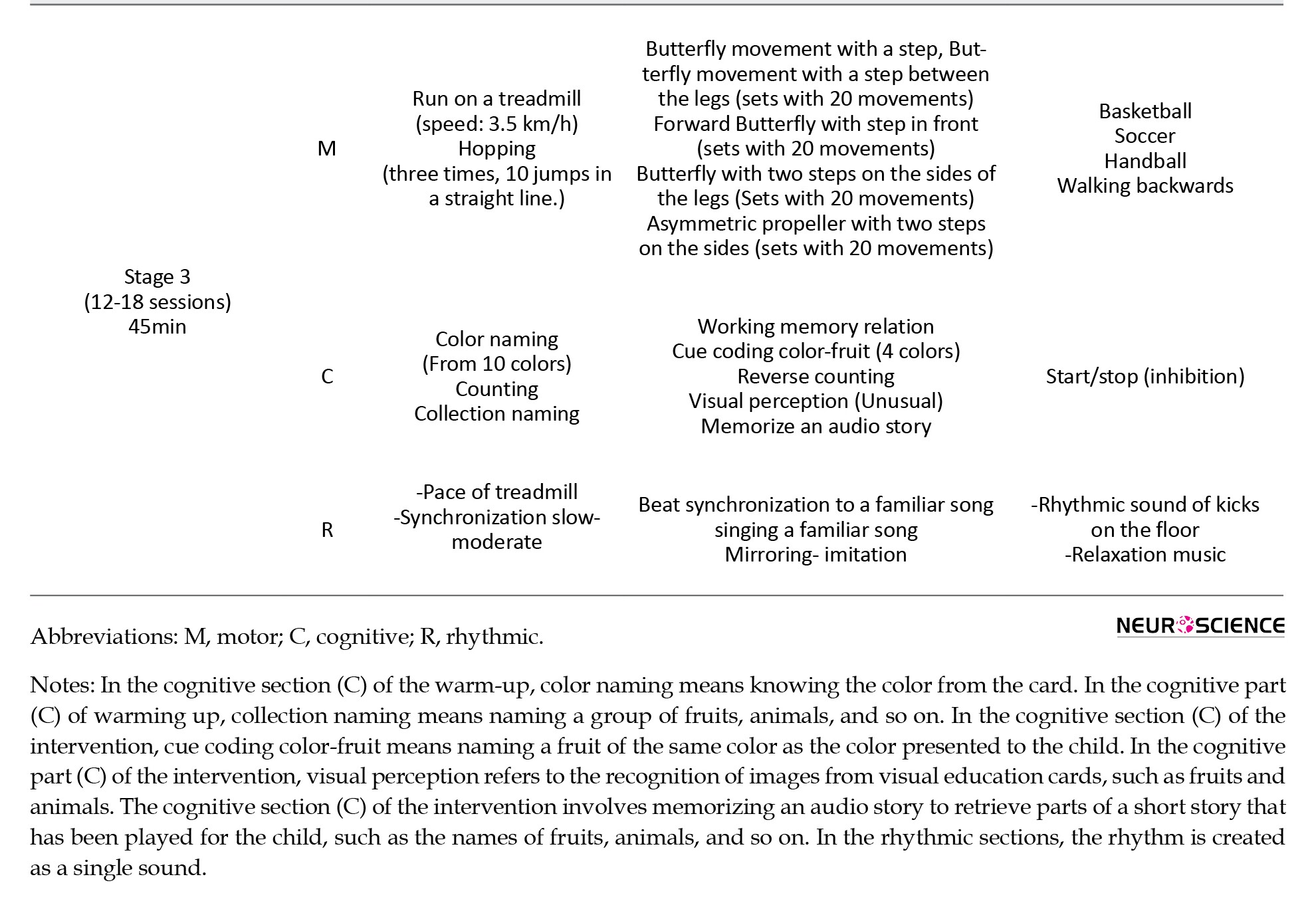
This dual-task program integrates rhythmic bodily movements with cognitive challenges, utilizing a three-level difficulty scale (from easy to hard) based on a structured dual-task paradigm (Appendix 1; the original Persian protocol has been translated into English for the appendix). The feasibility and high engagement level of the program for children were previously evaluated and confirmed in a pilot study (Amini et al., 2024).
Each phase of the program consisted of six 45-minute sessions, divided into three components: Warm-up, intervention, and cool-down. The cool-down phase included motivational elements to encourage children’s participation. Exercises were tailored to the child’s performance and developmental level, offering a set of motor-cognitive-rhythmic tasks from which the child could choose.
Assessment tools
The following assessment tools were used:
• Integrated visual and auditory continuous performance test (IVA-2): This continuous performance test (CPT) assesses five types of attention—focused, sustained, selective, divided, and shifting—across both visual and auditory modalities. IVA-2 is a specialized tool for diagnosing ADHD and measuring attention-related indices, and is currently the only CPT validated through Functional magnetic resonance imaging (fMRI) and QaEEG studies.
• Cambridge neuropsychological testing automated battery (CANTAB): A highly reliable and widely used neuropsychological assessment tool, independent of language and culture. The CANTAB battery comprises 25 tests across domains including visual memory, executive functioning, attention, verbal/semantic memory, decision-making, response inhibition, and social cognition. For this study, the reaction time (RTI) test from the CANTAB battery was used.
• Connors’ parent rating scale – short form: This questionnaire was used to evaluate ADHD symptoms and impulsivity from the perspective of the caregiver.
• Demographic information questionnaire: This form collected general information about participants.
Study procedure
Participants were selected based on the inclusion criteria. After receiving appropriate coordination with parents (completion of the consent form and demographic questionnaire), they were randomly assigned to one of two groups using covariate adaptive randomization: An intervention group (which received a graded exercise package consisting of dual physical, rhythmic, and cognitive tasks) and a control group (which received standard occupational therapy exercises). Each group consisted of 30 participants.
Four trained evaluators administered the IVA-2 and CANTAB assessments. These evaluators were senior psychometric experts and experienced occupational therapists. In the intervention phase, the occupational therapists had received training on the specific implementation of each exercise set, with the procedures practiced and refined across three preliminary sessions.
Both groups participated in 45-minute weekly sessions over 6 weeks. In the intervention group, each 45-minute session was divided into three phases: A warm-up, the intervention, and a cool-down. Each phase included a combination of physical, rhythmic, and cognitive exercises that progressively increased in difficulty. During the intervention, participants received verbal guidance, encouragement, and corrective feedback to optimize their performance. In the cool-down phase, motivational strategies, rewards, and incentives were implemented to enhance engagement and participation.
Participants in the control group continued their regular occupational therapy program, which followed the standard treatment protocol. Therapists and family members observed and recorded each child’s progress during sessions, and detailed records were maintained in individual files, each labeled with a specific code to ensure confidentiality.
All children attended the 18 planned sessions. In cases where a participant missed a session, a makeup session was scheduled. Two days after completing the final training session, the post-intervention tests (IVA-2 and CANTAB) were re-administered by the evaluators. Additionally, to assess the persistence of any potential effects, the tests were re-administered one month after the intervention concluded.
Following the final evaluations, the data were analyzed to examine the impact of the intervention on impulsivity and related cognitive outcomes.
Intervention fidelity
To ensure intervention fidelity, all sessions were implemented by trained occupational therapists who had received specific instruction on the intervention protocol. Before the intervention phase, therapists participated in a structured training program, during which the goals, procedures, and content of each exercise set were reviewed. The therapists practiced all intervention components through hands-on implementation to ensure procedural accuracy and consistency. A standardized checklist outlining the key steps of each training set was used throughout the sessions to guide implementation and maintain adherence to the protocol. Supervisory oversight and regular review of session records further supported fidelity assurance. All participants in the intervention group completed the full 18-session protocol (45 minutes per session), and missed sessions were rescheduled to ensure completeness of exposure.
Statistical analysis
Data were analyzed using both descriptive and inferential statistics with SPSS software, version 26. Descriptive statistics (Mean±SD) were used to summarize demographic characteristics and baseline scores. The normality of the data distribution was assessed using the Shapiro-Wilk test. Given the research design, which included one within-subject factor (time: Pre-test and post-test) and one between-subject factor (group: Intervention vs control), appropriate statistical tests were applied to evaluate intervention effects. Analysis of covariance (ANCOVA) was used to compare post-test scores between groups while controlling for baseline scores. Within-group changes were analyzed using a paired t-test for normally distributed data or a Wilcoxon signed-rank test for non-normally distributed data. One-way analysis of variance (ANOVA) was used to assess the retention effects within the intervention group 1 month after the intervention. Eta-squared (η²) was calculated to estimate the effect size of the intervention on each outcome variable. A significance level of P<0.05 was considered statistically significant.
Results
Participants in this study were categorized into two age groups (6–9 years and 9–12 years) and assigned to either the intervention or control group. The assessments employed included IVA and CANTAB, targeting impulsivity-related variables such as response control, audio response control, visual response control, and reaction time.
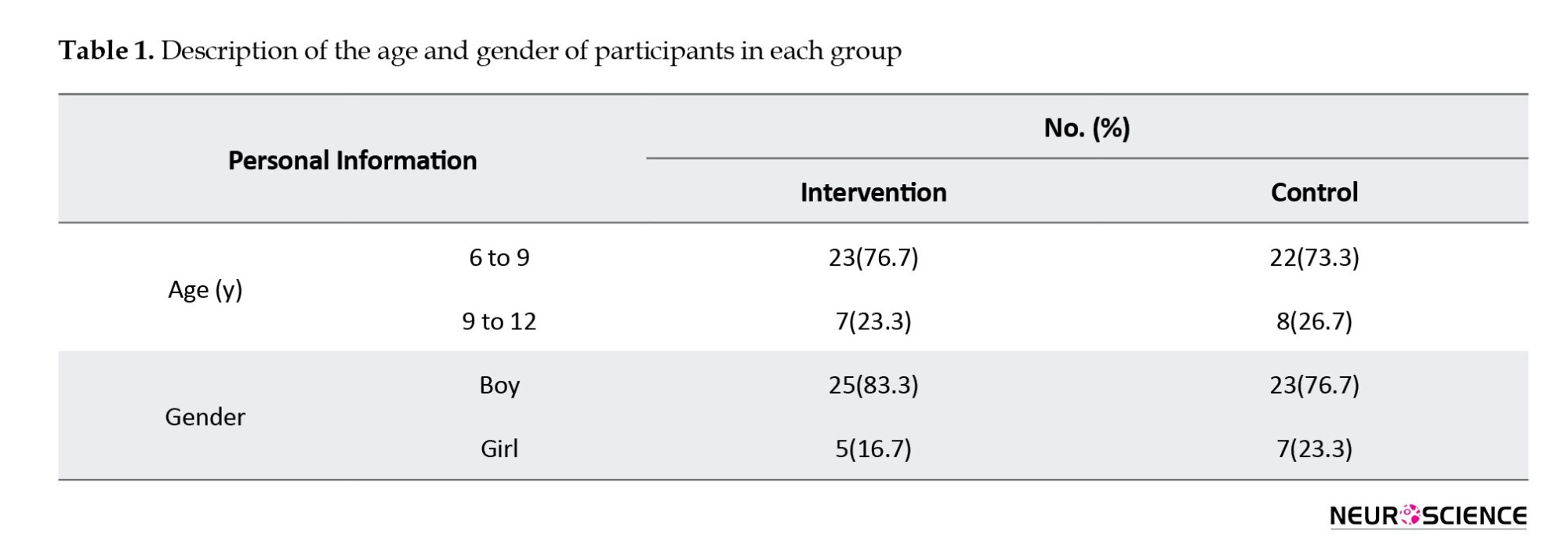
The majority of participants in both groups were aged 6 to 9 years: 76.7% (n=23) in the intervention group and 73.3% (n=22) in the control group. Conversely, the 9 to 12 age group comprised a smaller proportion: 23.3% (n=7) in the intervention group and 26.7% (n=8) in the control group. In both groups, the majority of participants were boys. Specifically, 83.3% (n=25) of the participants in the intervention group and 76.7% (n=23) in the control group were male, while the intervention and control groups included 5 and 7 girls, respectively (Table 1).
The results for the response control variable revealed a statistically significant difference before and after the intervention in both the intervention and control groups (P<0.001). Furthermore, the between-group difference was also significant (P<0.001). The effect size for this variable was η²=0.638.
Similarly, a significant pre-post intervention difference was observed in the audio response control variable for both groups (P<0.001), and the between-group comparison also yielded a statistically significant result (P<0.001). The corresponding effect size was η²=0.671.
For the visual response control variable, there was a statistically significant change from pre- to post-intervention (P<0.001) in both groups. The between-group difference was also significant (P<0.001), with an effect size of η²=0.62 (Table 2).

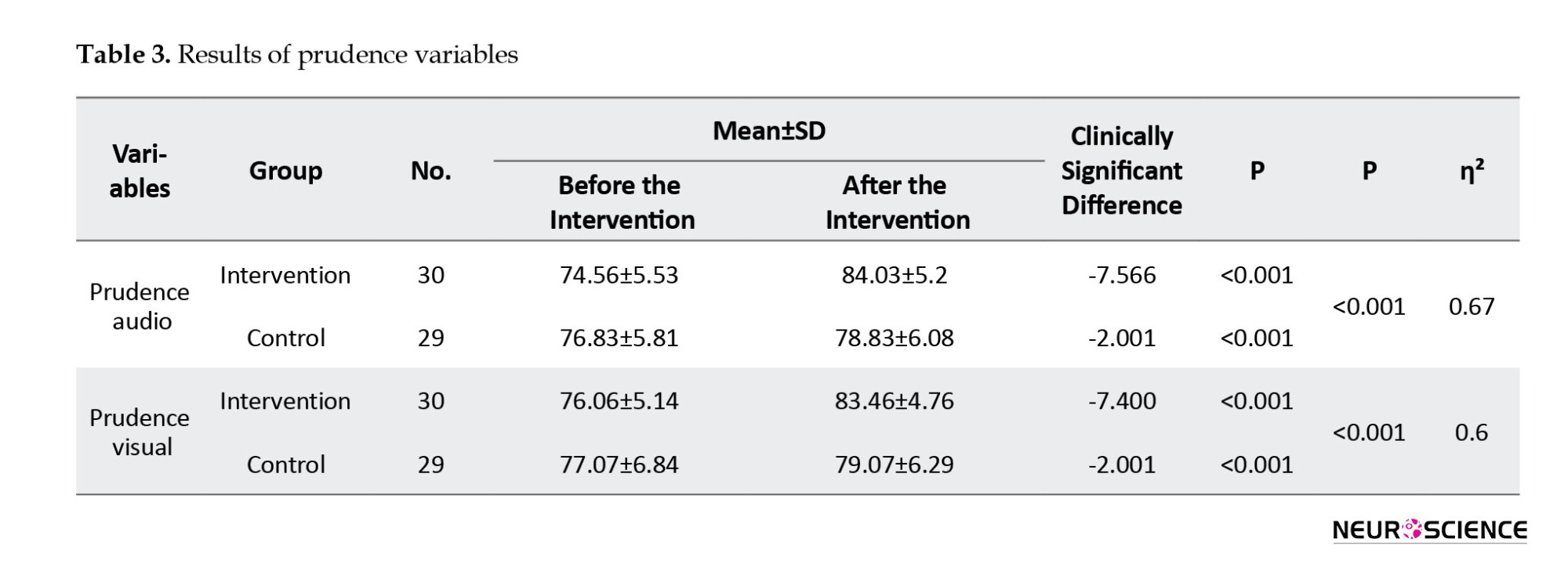
The response control audio prudence variable showed a statistically significant pre-post intervention difference in both the intervention and control groups (P<0.001). The between-group difference was also significant (P<0.001), with an effect size of η²=0.673.
Similarly, the response control visual prudence variable demonstrated a significant change from pre- to post-intervention across both groups (P<0.001). The difference between the two groups was also statistically significant (P<0.001), with an effect size of η²=0.605 (Table 3).
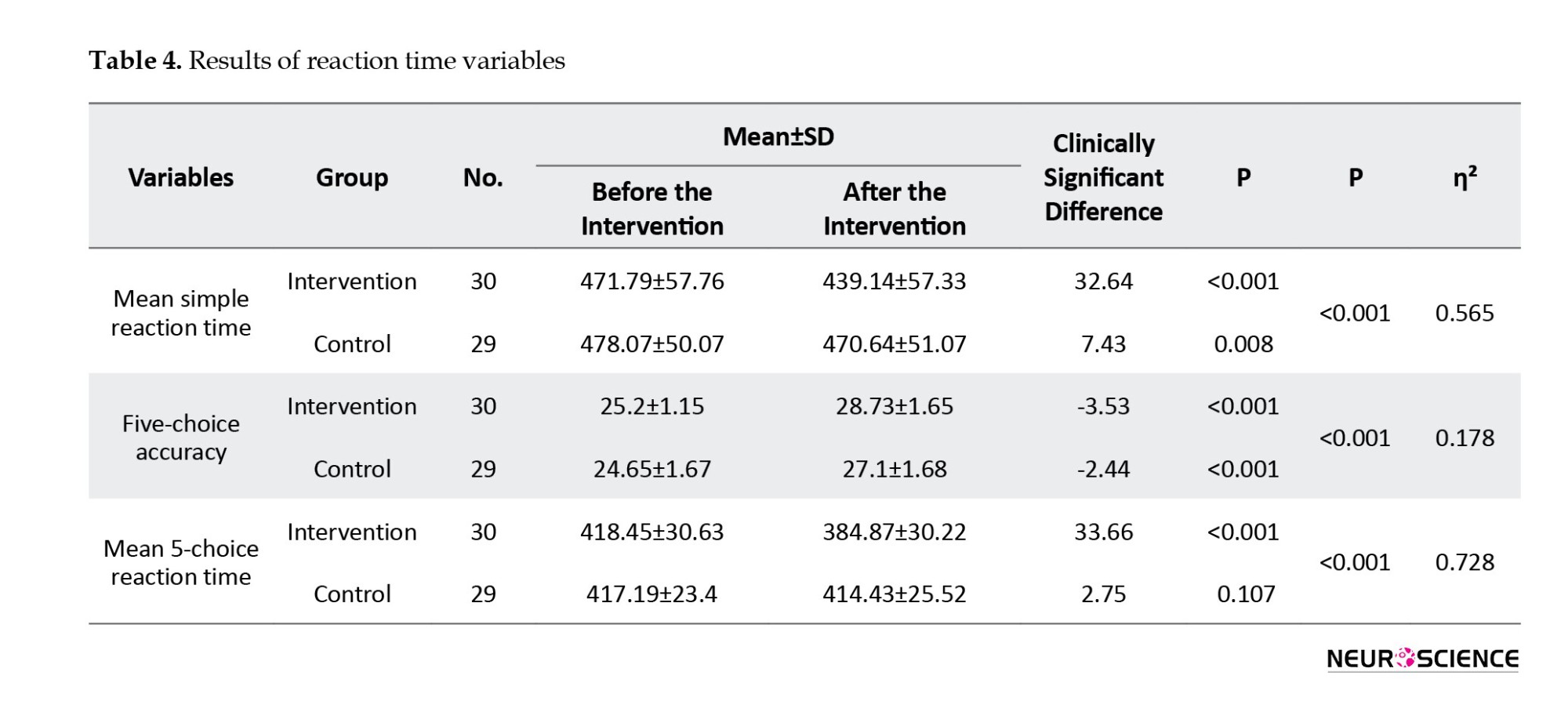
The mean simple reaction time variable revealed significant changes before and after the intervention in both the intervention (P<0.001) and control groups (P=0.008). A significant between-group difference was also observed (P<0.001).
Regarding the mean 5-choice reaction time variable, the intervention group exhibited a significant improvement post-intervention (P<0.001), whereas the control group did not show a significant change (P=0.107). The between-group difference remained significant (P<0.001).
For the 5-choice accuracy variable, a statistically significant difference was observed between the pre- and post-intervention periods in both groups (P<0.001). The difference between the intervention and control groups was also significant (P<0.001) (Table 4).
No statistically significant differences were found one month after the intervention in the indicators of response control (P=0.379) and audio response control (P=0.4983), indicating the persistence of improvements in these variables (Table 5).

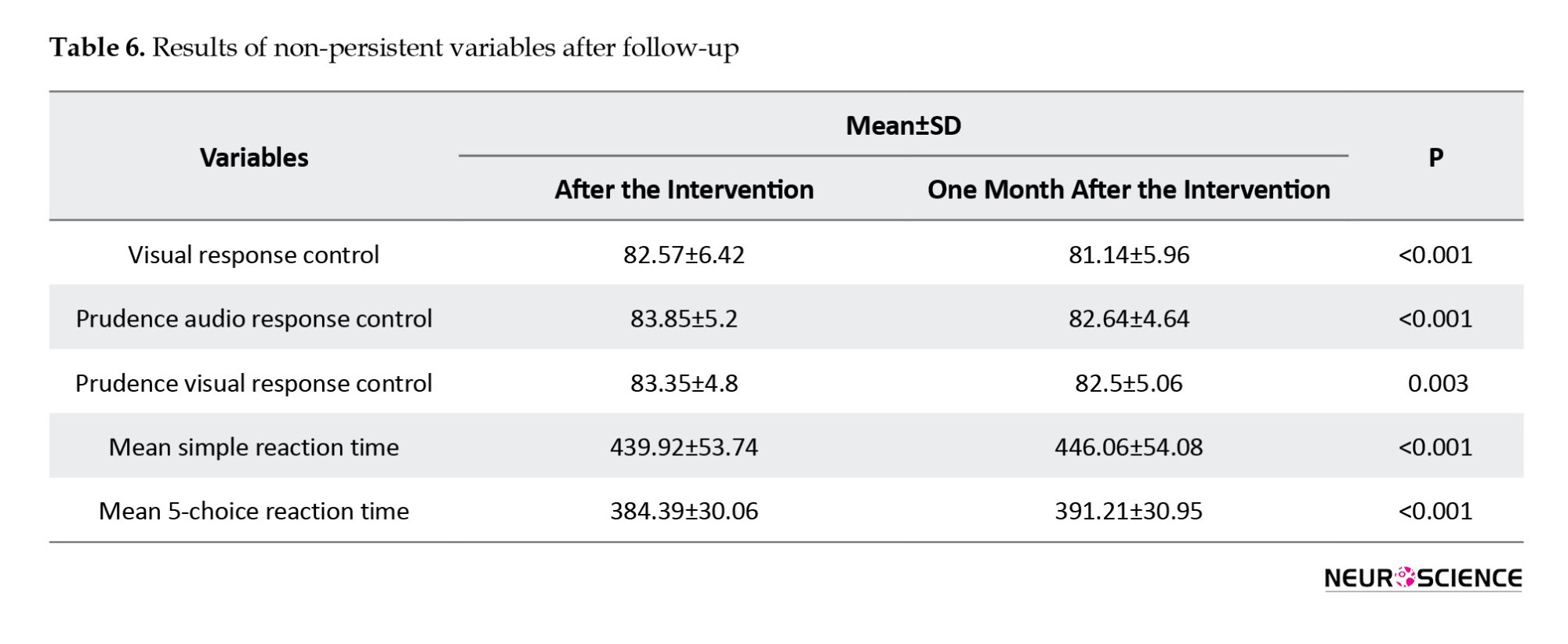
Conversely, a significant decline in performance was observed one month after the intervention in the following variables: Visual response control (P<0.001), prudence audio response control (P<0.001), prudence visual response control (P=0.003), mean simple reaction time (P<0.001), and mean five-choice reaction time (P<0.001) (Table 6).
Discussion
Although the occurrence of visible and noticeable differences in these variables between the intervention and control groups was consistent with the results of previous studies, identifying the possible reasons for these behaviors can be effective in clarifying the underlying causes and applying them in clinical settings.
ADHD is characterized by weakness in the functions of the frontal lobe of the brain, particularly in executive functions, as well as a defect in the connection between the specialized circuit of this part and subcortical structures, such as the basal ganglia.
The basal ganglia are known as one of the main processing centers of the brain, which is effective in producing and presenting the final output of motor behavior and causes the convergence and integration of received environmental information.
The importance of paying attention to these two components in modern perspectives has become so strong that Gabriel (1997) refers to the basal ganglia as “cognitive pattern generators” to emphasize the role of this structure in the scaffolding of cognitive performance.
It seems that basal ganglia play a key role in explaining the results of this study because, considering the five main circuits of basal ganglia (Pesce et al., 2019) and their role in both verbal functions and auditory processing in terms of sound sequences and rhythms (Ben-Soussan et al., 2015; Koziol et al., 2014) and the production and continuity of rhythm production (Thaut et al., 2009), it also plays a role in nonverbal functions such as memory and attention (Bhide et al., 2013) or the perception of timing (Repp & Su, 2013; Thaut & Abiru, 2010), especially in the perception of motor rhythms and the regulation of motor sequences (Vogt, 2019) and the fragmentation of skillful motor functions and their segmentation (Dahan & Reiner, 2017) and the construction of meaningful and purposeful behavioral sequences (Ferguson et al., 2024).
Of course, in the meantime, it is necessary to consider the function over arching (Pesce et al., 2019) also considered the integration of all circuits and networks related to the basal ganglia and providing an integrated and unified function, on the other hand, it overlooked the importance of the fifth basal ganglia circuit (the hyperdirect pathway) in the rapid functions of inhibiting and controlling impulsive behaviors (Pesce et al., 2019).
The selection of variables related to response control (response control, audio response control, visual response control) and also regarding response selection time (five-choice accuracy, mean five-choice reaction time, choice movement time mean five) has been observed in the accepted definition and acceptance of impulsivity, which refers to the occurrence of a behavior (motor and automatic dimension) without thinking about the consequences (cognitive dimension and behavior selection). Today, it has been determined that each of the motor qualities and components can have the greatest impact on which aspect of the cases mentioned above (Chamorro et al., 2012; Deslandes et al., 2009), for example, gross skillful movements are related to working memory, spatial perception, and response inhibition (Kramer & Erickson, 2007). The role of rhythmic movements in facilitating social relationships has been mentioned. Additionally, sequential and timing exercises impact working memory and attention (Buchanan et al., 1997; McAuley et al., 2005), whereas rhythmic movements and movements related to rhythmic hearing have a distinct effect on inhibitory functions (Ohgi et al., 2008). Perhaps the overall outcome of these cases can be considered as one of the reasons that the audio response control variable in this study had a much larger effect size than the visual response control variable, because in this proposed set of exercises, the role of auditory stimulation and corresponding and proportionate body rhythm has been particularly emphasized.
Other studies have also shown that if an auditory or motor rhythmic sequence is maintained, the complementary premotor areas of the frontal cortex can compensate for the dysfunction of the basal ganglia (Jamey et al., 2024), and this two-way interaction can play a more significant role in rehabilitation models (Myers et al., 2019).
Considering the age range of the participants, it is also crucial to acknowledge the role of neural maturation in this process. Given that the age of the participants in this study was over six years old, it can be expected that after the second year, connections between the limbic circuit and the anterior cingulate in addition to the prefrontal region have been established (Mathias et al., 2020), and the self-regulatory circuit is maturing and developing, and these connections can cause a connection between cognition and emotion (Deforche & De Bourdeaudhuij, 2015; Laffere et al., 2021), and this age after 5 years of age (Janmohammadi et al., 2020; Koziol et al., 2014) can be very valuable in ensuring the completion of the self-regulatory circuit, which seems to have been useful in achieving improvements related to response inhibition and laying the groundwork for controlling impulsivity (Mathias et al., 2020).
Some researchers have also emphasized the importance of a step-by-step and progressively challenging level of difficulty in exercises, such as homework, to facilitate executive functions (Amrani & Golumbic, 2019). Some have also emphasized the importance of this feature in such a way that if physical exercises do not possess it, they cannot affect executive function (Deslandes et al., 2009). On the other hand, some researchers have also emphasized the diversity of exercise combinations and their ability to attract and interest the child and instill a sense of empowerment (Ahmed & Mohamed, 2011; Burnham, 2012; Chamorro et al., 2012) and pleasure (Bustamante, 2013; Chen et al., 2012), and have considered these issues ::as char::acteristics of a motor exercise package (Chamorro et al., 2012; Cornelius et al., 2017; Hoza et al., 2016; Kang et al., 2011) that can affect the child’s self-regulatory functions.
Research has also focused on the use of various rhythms in the form of rhythmic movements or auditory rhythms from the surrounding environment (Chamorro et al., 2012; Lufi & Parish-Plass, 2011; Medina et al., 2010; Piepmeier et al., 2015) and their effects through body-centered cognitive theories (Donnelly et al., 2016; Tse et al., 2021), as well as the theory of the influence of the musician (Diamond, 2015; Ohgi et al., 2008) on the child’s learning, cognitive (van Der Fels et al., 2019) and self-regulation functions, and examples of these have been presented in the form of a combination of rhythm and movement.
ADHD has been closely associated with deficits in core executive functions, such as inhibitory control, cognitive flexibility, and working memory (Barkley, 1997). Specific deficits related to inhibition include impairments in working memory, inner speech, self-regulation, emotional modulation, motivation, and arousal. In the proposed training package, cognitive demands and ultimately working memory capacity within the dual-task paradigm are progressively increased in a structured and hierarchical manner, with external rhythm-based self-regulation emphasized throughout all major training components (Myers et al., 2019).
In general, all aspects of impulse control were considered in the exercises, and the results align with this effort and goal setting. The first topic is presented from new perspectives that refer to the sensory-motor paradigm and the role of continuity in sensory functions in decision-making and problem-solving. These perspectives also highlight the interaction between subcortical areas and cortical regions (Pesce et al., 2019). The signs and traces of this neural cooperation are evident in all types of mammals, and it is considered the most specialized sensory and motor information processing system, playing an important role in cooperation with subcortical areas, such as the basal ganglia and the cerebellum, as well as the PFC. In some sources, this collaboration has been referred to as the “dual-tiered model,” emphasizing the importance of these regions collaborating with higher cortical areas to achieve complex behavioral outcomes, such as problem-solving and decision-making. This model of collaboration explains both the monitoring and regulation of automatic behaviors and higher-level cognitive behaviors that require inhibitory patterns (Heyder et al., 2004; Rae et al., 2015).
It is essential to note that more than 95% of human behavior is performed automatically, and focusing on this aspect underscores the significance of the unconscious and involuntary subcortical parts of the central nervous system. The self-regulation circuit and neural network, which plays a central role in controlling impulses, making decisions, and resolving conflicts and tensions (Sylvester et al., 2018), is also composed of two cortical parts, prefrontal and anterior cingulate regions (Deforche & De Bourdeaudhuij, 2015), and subcortical parts (basal nuclei and cerebellum).
From a pathological and pathophysiological perspective, structural and functional damage in these regions also has the greatest burden and contribution in children with attention deficit hyperactivity disorder (Jaschke et al., 2018; Vazou et al., 2019; Wilson, 2002).
It seems that considering the two aspects of motor activity (Boes et al., 2009) and interest and attractiveness (Ahmed & Mohamed, 2011; Chamorro et al., 2012; Rothbart et al., 2011) for participants, the role of the neurotransmitter “dopamine” should also be mentioned, because these are considered part of the proven functions of this neurochemical carrier. The secretion and increased presence of this substance in synaptic spaces can play a role in the construction and formation of neural circuits, their stabilization, and the strengthening of these currents (Aylward et al., 1996).
The “musician’s advantage” perspective (Ohgi et al., 2008) is also one of the modern psychological perspectives that, by emphasizing body-centered cognition (Tse et al., 2021) and the function of the cerebellum in the self-regulation and control network of motor behaviors (Diamond & Lee, 2011; Donnelly et al., 2016), refers to the clinical use of motor rhythm and the harmony of physical movement patterns with the emergence of observable behaviors and the generalization of these rhythmic and coordinated patterns to the entire motor and physical behaviors of the individual, and its effects on behavioral inhibition in different individuals and clinical groups and considering the use of balance exercises and their combination with movement rhythms in this training package, a portion of the changes and improvements achieved can be attributed to this.
Another aspect of the improvements achieved could be related to the mediating role of working memory in this regard (Buchanan et al., 1997; Krain & Castellanos, 2006; McAuley et al., 2005), because it has been shown that generalization and organization of information in the working memory section improves the attentional control system as well as emotional control and improves the individual’s self-regulation. Another aspect is the use of ball exercises in combination with the proposed exercise package, which, as noted in another study (Hoza et al., 2016), has also been highlighted for its effect on the development of restraint, self-control, and monitoring of one’s own behaviors.
Additionally, in the motivation section, an incentive was provided to the child at the final stage of each phase and upon completion of the main activity. This approach not only reinforced motivation throughout the exercises but also taught the concept of reward delay.
Conclusion
In this study, the effectiveness of a package of rhythmic physical dual-task exercises combined with cognitive activities on impulsivity and self-regulation in children with ADHD was investigated. Given the diversity of treatment approaches and the common parental concerns about the side effects of pharmacological interventions, implementing a structured, leveled program of rhythmic physical-cognitive dual tasks appears to offer a promising alternative. Such an approach, which is both comprehensive and feasible, may enhance family engagement and increase the child’s intrinsic motivation due to its inherent attractiveness. Overall, this intervention could contribute to effective symptom management, particularly in reducing impulsivity, in children with ADHD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Human Studies Ethics Committee of the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (Code: IR.USWR.REC.1400.207). This study was also registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20220212054004N1). The studies were conducted in accordance with local laws and institutional requirements. Written informed consent to participate in this study was provided by the legal guardians/close relatives of the participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and investigation: Ebrahim Pishyareh, Behzad Amini, and Seyed Ali Hosseini; Study design, and methodology: All authors; Experiments, statistical analysis, and writing the original draft: Behzad Amini and Ebrahim Pishyareh; Review, and editing: Behzad Amini, Ebrahim Pishyareh, and Seyed Ali Hosseini.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their deepest gratitude to all participants in this study and their families for their collaboration with the research team. The authors also thank the Department of Occupational Therapy at the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. Additionally, The authors would like to express their appreciation to Ofogh Occupational Therapy Clinic, Tehran, Iran, for providing the intervention space and conducting the assessment.
References
Impulsivity, as a multidimensional personality trait, encompasses a wide range of immature and inappropriate behaviors that often result in undesirable consequences (Bezdjian et al., 2011; Meda et al., 2009). It holds particular significance during childhood and adolescence (Chamorro et al., 2012), a developmental period during which impulsivity manifests in various clinical diagnoses, including ADHD, autism spectrum disorder, anxiety disorders, and obsessive-compulsive disorder (Amini et al., 2016; Bezdjian et al., 2011; Goya-Maldonado et al., 2010; Hamilton et al., 2015; Sebastian et al., 2013; Sebastian et al., 2014; Stahl, 2020).
According to the diagnostic and statistical manual of mental disorders (DSM-5), impulsivity is one of the core diagnostic features of attention-deficit/hyperactivity disorder (ADHD), particularly evident in preschool-aged children and those with the hyperactive-impulsive (ADHD-HI) subtype. Impulsivity often leads to behavioral difficulties in social contexts, such as an inability to wait one’s turn, frequent interruptions during conversations, or answering before a question is completed. In addition to impulsive decision-making, children and adolescents with ADHD often exhibit impaired motor coordination and increased postural sway (Newton-Howes, 2004). Research shows that children and adolescents with ADHD tend to make more impulsive decisions than their neurotypical peers (Patros et al., 2016), and disturbances in motor coordination and postural control have been linked to deficits in self-regulation and inhibitory control (Amini et al., 2018; Jahani et al., 2016).
According to Barkley’s theory, the hyperactive-impulsive subtype of ADHD is more prevalent in early childhood and is closely associated with attentional deficits. In his model, the primary underlying deficit in ADHD is a failure of response inhibition, which in turn disrupts 4 core executive functions: (a) working memory, (b) self-regulation of emotion, motivation, and arousal, (c) internalization of speech, and (d) reconstitution (i.e. behavioral analysis and synthesis). These impairments compromise the integration of the motor and perceptual systems necessary for goal-directed behavior (Barkley, 1997; Barkley et al., 1990). In this framework, executive dysfunction is posited as the primary cause of maladaptive behaviors, and response inhibition deficits are responsible for both distractibility and impulsivity in children with ADHD. Thus, inattention is considered a secondary consequence, whereas impulsivity represents a primary symptom (Barkley, 1997; Barkley et al., 1990).
Given the high prevalence of motor control impairments (approximately 50%) and postural instability (ranging from 47% to 69%) among children and adolescents with ADHD (Fliers et al., 2008; Piek et al., 2004; Williams et al., 2006), along with significant deficits in behavioral inhibition, working memory, and emotional regulation (Barkley, 1997; Barkley et al., 1990) motor-control-based physical interventions have been increasingly used as a therapeutic strategy for this population (Archer & Kostrzewa, 2011; Donnelly et al., 2016).
One fundamental cause of motor control and coordination difficulties in children with ADHD is attributed to deficits in rhythm and timing. These children often struggle to process environmental rhythms, particularly auditory ones, which are essential for achieving motor coordination in goal-directed activities (Shin et al., 2023). Notably, research suggests that humans synchronize more effectively to auditory rhythms than to visual or multimodal cues. Rhythm-based physical training has demonstrated promising effects on motor development and emotional regulation, which, in turn, enhance executive functioning and social engagement in children (Sakai et al., 2004). Accordingly, a growing body of literature has investigated the role of physical activity in this context.
Since 2014, systematic reviews have shown that most interventions rely on aerobic exercises such as treadmill running, cycling, yoga, and mixed physical activity programs. While these interventions generally enhance executive function in the short term, they typically involve 30-minute sessions focused solely on physical exertion, which elevates heart rate, respiratory output (VO₂max), and neurotransmitter levels in children with ADHD. The observed effect sizes often increase with session duration, ranging from 10 to 30 minutes (Grassmann et al., 2017). However, these studies rarely incorporate dual-task designs that combine physical and cognitive training to address impulsivity. Furthermore, such interventions frequently lack individualization, variety, or opportunities for home-based continuation. Rhythmic movement based on auditory cues has also been underexplored, and overall, the integration of auditory-motor synchronization has not been prioritized (Donnelly et al., 2016; Grassmann et al., 2017).
Impulsivity is closely associated with deficits in self-regulation (Barkley, 1997) and executive dysfunction (Bjørkly, 2013), which, from a neuroanatomical perspective, are linked to dysfunction in frontostriatal circuits involving the basal ganglia, anterior cingulate cortex, and orbitofrontal cortex regions responsible for regulating goal-directed behaviors.
Successful dual-tasking requires divided attention and the simultaneous processing of multiple internal and external stimuli (Garon et al., 2008). Neuroimaging studies highlight the role of the prefrontal cortex (PFC) in such multitasking, and underscore the basal ganglia’s importance in aligning motor behavior with auditory rhythms (Arvaniti, 2009; Thaut & Abiru, 2010).
Building on these findings, the present study employs a dual-task paradigm that involves the simultaneous performance of two independent tasks with distinct objectives (Lee et al., 2013; Pashler, 1994; Watanabe & Funahashi, 2015). This approach combines bodily movement with synchronized auditory and visual rhythms.
Recognizing the importance of fostering executive function development in children with ADHD—particularly within educational settings to promote adaptive learning behaviors (Cepeda et al., 2000) and considering the normative developmental pattern of subcortical brain circuit maturation—the dual-task format was selected as an intervention strategy. Additionally, the program was designed based on evidence showing that auditory rhythms can improve inhibitory control (Slater & Tate, 2018) and that sequential and timed motor activities enhance working memory and attentional capacity (Petrides, 1994; Smith & Jonides, 1999). Gross motor exercises have also been shown to benefit spatial awareness and response inhibition (van Der Fels et al., 2019). Accordingly, the training package developed for this study integrates these evidence-based components.
To ensure engagement and efficacy, the program incorporates principles such as task grading from simple to complex (Tomporowski & Pesce, 2019), diversity in exercise formats, and elements that promote enjoyment, empowerment, and intrinsic motivation in children (Brand & Ekkekakis, 2018; Cox et al., 2008; Diamond, 2015; Nasuti & Rhodes, 2013; Ryan & Deci, 2000). A preliminary feasibility study assessing the program’s acceptability among children has been conducted, and its findings have been published (Amini et al., 2024).
This study aimed to investigate the effect of a structured training package consisting of dual-task rhythmic physical and cognitive exercises on impulsivity, caution, alertness, and reaction time in children with ADHD. Additionally, the study examined the persistence of the effects one month after the intervention.
Materials and Methods
Study design
This study was a single-blind, randomized clinical trial. It represented the final intervention phase of a doctoral dissertation conducted in the Department of Occupational Therapy at the University of Social Welfare and Rehabilitation Sciences, Tehran City, Iran. The study was approved by the university’s Ethics Committee and was registered in the Iranian Registry of Clinical Trials (IRCT).
Study participants
The sample size was calculated to achieve sufficient statistical power to detect differences between the groups. Assuming a 5% significance level (P<0.05) and a power of 0.8, a total of 60 participants (30 per group) were required. Sixty children with ADHD, aged between 6 and 12 years, were therefore recruited and randomly assigned to either the intervention or control group.
Participants were recruited from public and private clinical centers affiliated with Tehran universities and the Tehran Medical System Organization. The inclusion criteria were as follows: A confirmed diagnosis of ADHD (combined type or hyperactive-impulsive type) by a psychiatrist and documented in the child’s medical records; chronological age between 6 and 12 years; absence of obvious physical disabilities based on therapist evaluation; elevated impulsivity scores on the short-form Connors parent questionnaire; no prior participation in rhythmic motor training programs; normal cognitive functioning as evidenced by enrollment in mainstream education; responsiveness and cooperation from the child; parental literacy; and absence of neurological, rheumatic, or orthopedic conditions that would interfere with participation in the intervention.
Covariate adaptive randomization was used to assign participants to groups, ensuring balance across key variables. Before enrollment, informed written consent was obtained from all parents or legal guardians. After the intervention period, the results were shared with families. For ethical considerations, the intervention program was offered to families in the control group free of charge upon completion of the study. The exclusion criteria included a lack of family cooperation during the intervention, withdrawal from the study, or any change in the child’s medication dosage during the trial period.
Study equipment
The intervention consisted of a graded dual-task training package combining rhythmic physical activities with age-appropriate cognitive tasks. The program was developed based on expert feedback and underwent multiple review stages before being approved.
This dual-task program integrates rhythmic bodily movements with cognitive challenges, utilizing a three-level difficulty scale (from easy to hard) based on a structured dual-task paradigm (Appendix 1; the original Persian protocol has been translated into English for the appendix). The feasibility and high engagement level of the program for children were previously evaluated and confirmed in a pilot study (Amini et al., 2024).
Each phase of the program consisted of six 45-minute sessions, divided into three components: Warm-up, intervention, and cool-down. The cool-down phase included motivational elements to encourage children’s participation. Exercises were tailored to the child’s performance and developmental level, offering a set of motor-cognitive-rhythmic tasks from which the child could choose.
Assessment tools
The following assessment tools were used:
• Integrated visual and auditory continuous performance test (IVA-2): This continuous performance test (CPT) assesses five types of attention—focused, sustained, selective, divided, and shifting—across both visual and auditory modalities. IVA-2 is a specialized tool for diagnosing ADHD and measuring attention-related indices, and is currently the only CPT validated through Functional magnetic resonance imaging (fMRI) and QaEEG studies.
• Cambridge neuropsychological testing automated battery (CANTAB): A highly reliable and widely used neuropsychological assessment tool, independent of language and culture. The CANTAB battery comprises 25 tests across domains including visual memory, executive functioning, attention, verbal/semantic memory, decision-making, response inhibition, and social cognition. For this study, the reaction time (RTI) test from the CANTAB battery was used.
• Connors’ parent rating scale – short form: This questionnaire was used to evaluate ADHD symptoms and impulsivity from the perspective of the caregiver.
• Demographic information questionnaire: This form collected general information about participants.
Study procedure
Participants were selected based on the inclusion criteria. After receiving appropriate coordination with parents (completion of the consent form and demographic questionnaire), they were randomly assigned to one of two groups using covariate adaptive randomization: An intervention group (which received a graded exercise package consisting of dual physical, rhythmic, and cognitive tasks) and a control group (which received standard occupational therapy exercises). Each group consisted of 30 participants.
Four trained evaluators administered the IVA-2 and CANTAB assessments. These evaluators were senior psychometric experts and experienced occupational therapists. In the intervention phase, the occupational therapists had received training on the specific implementation of each exercise set, with the procedures practiced and refined across three preliminary sessions.
Both groups participated in 45-minute weekly sessions over 6 weeks. In the intervention group, each 45-minute session was divided into three phases: A warm-up, the intervention, and a cool-down. Each phase included a combination of physical, rhythmic, and cognitive exercises that progressively increased in difficulty. During the intervention, participants received verbal guidance, encouragement, and corrective feedback to optimize their performance. In the cool-down phase, motivational strategies, rewards, and incentives were implemented to enhance engagement and participation.
Participants in the control group continued their regular occupational therapy program, which followed the standard treatment protocol. Therapists and family members observed and recorded each child’s progress during sessions, and detailed records were maintained in individual files, each labeled with a specific code to ensure confidentiality.
All children attended the 18 planned sessions. In cases where a participant missed a session, a makeup session was scheduled. Two days after completing the final training session, the post-intervention tests (IVA-2 and CANTAB) were re-administered by the evaluators. Additionally, to assess the persistence of any potential effects, the tests were re-administered one month after the intervention concluded.
Following the final evaluations, the data were analyzed to examine the impact of the intervention on impulsivity and related cognitive outcomes.
Intervention fidelity
To ensure intervention fidelity, all sessions were implemented by trained occupational therapists who had received specific instruction on the intervention protocol. Before the intervention phase, therapists participated in a structured training program, during which the goals, procedures, and content of each exercise set were reviewed. The therapists practiced all intervention components through hands-on implementation to ensure procedural accuracy and consistency. A standardized checklist outlining the key steps of each training set was used throughout the sessions to guide implementation and maintain adherence to the protocol. Supervisory oversight and regular review of session records further supported fidelity assurance. All participants in the intervention group completed the full 18-session protocol (45 minutes per session), and missed sessions were rescheduled to ensure completeness of exposure.
Statistical analysis
Data were analyzed using both descriptive and inferential statistics with SPSS software, version 26. Descriptive statistics (Mean±SD) were used to summarize demographic characteristics and baseline scores. The normality of the data distribution was assessed using the Shapiro-Wilk test. Given the research design, which included one within-subject factor (time: Pre-test and post-test) and one between-subject factor (group: Intervention vs control), appropriate statistical tests were applied to evaluate intervention effects. Analysis of covariance (ANCOVA) was used to compare post-test scores between groups while controlling for baseline scores. Within-group changes were analyzed using a paired t-test for normally distributed data or a Wilcoxon signed-rank test for non-normally distributed data. One-way analysis of variance (ANOVA) was used to assess the retention effects within the intervention group 1 month after the intervention. Eta-squared (η²) was calculated to estimate the effect size of the intervention on each outcome variable. A significance level of P<0.05 was considered statistically significant.
Results
Participants in this study were categorized into two age groups (6–9 years and 9–12 years) and assigned to either the intervention or control group. The assessments employed included IVA and CANTAB, targeting impulsivity-related variables such as response control, audio response control, visual response control, and reaction time.
The majority of participants in both groups were aged 6 to 9 years: 76.7% (n=23) in the intervention group and 73.3% (n=22) in the control group. Conversely, the 9 to 12 age group comprised a smaller proportion: 23.3% (n=7) in the intervention group and 26.7% (n=8) in the control group. In both groups, the majority of participants were boys. Specifically, 83.3% (n=25) of the participants in the intervention group and 76.7% (n=23) in the control group were male, while the intervention and control groups included 5 and 7 girls, respectively (Table 1).

The results for the response control variable revealed a statistically significant difference before and after the intervention in both the intervention and control groups (P<0.001). Furthermore, the between-group difference was also significant (P<0.001). The effect size for this variable was η²=0.638.
Similarly, a significant pre-post intervention difference was observed in the audio response control variable for both groups (P<0.001), and the between-group comparison also yielded a statistically significant result (P<0.001). The corresponding effect size was η²=0.671.
For the visual response control variable, there was a statistically significant change from pre- to post-intervention (P<0.001) in both groups. The between-group difference was also significant (P<0.001), with an effect size of η²=0.62 (Table 2).

The response control audio prudence variable showed a statistically significant pre-post intervention difference in both the intervention and control groups (P<0.001). The between-group difference was also significant (P<0.001), with an effect size of η²=0.673.
Similarly, the response control visual prudence variable demonstrated a significant change from pre- to post-intervention across both groups (P<0.001). The difference between the two groups was also statistically significant (P<0.001), with an effect size of η²=0.605 (Table 3).

The mean simple reaction time variable revealed significant changes before and after the intervention in both the intervention (P<0.001) and control groups (P=0.008). A significant between-group difference was also observed (P<0.001).
Regarding the mean 5-choice reaction time variable, the intervention group exhibited a significant improvement post-intervention (P<0.001), whereas the control group did not show a significant change (P=0.107). The between-group difference remained significant (P<0.001).
For the 5-choice accuracy variable, a statistically significant difference was observed between the pre- and post-intervention periods in both groups (P<0.001). The difference between the intervention and control groups was also significant (P<0.001) (Table 4).

No statistically significant differences were found one month after the intervention in the indicators of response control (P=0.379) and audio response control (P=0.4983), indicating the persistence of improvements in these variables (Table 5).

Conversely, a significant decline in performance was observed one month after the intervention in the following variables: Visual response control (P<0.001), prudence audio response control (P<0.001), prudence visual response control (P=0.003), mean simple reaction time (P<0.001), and mean five-choice reaction time (P<0.001) (Table 6).

Discussion
Although the occurrence of visible and noticeable differences in these variables between the intervention and control groups was consistent with the results of previous studies, identifying the possible reasons for these behaviors can be effective in clarifying the underlying causes and applying them in clinical settings.
ADHD is characterized by weakness in the functions of the frontal lobe of the brain, particularly in executive functions, as well as a defect in the connection between the specialized circuit of this part and subcortical structures, such as the basal ganglia.
The basal ganglia are known as one of the main processing centers of the brain, which is effective in producing and presenting the final output of motor behavior and causes the convergence and integration of received environmental information.
The importance of paying attention to these two components in modern perspectives has become so strong that Gabriel (1997) refers to the basal ganglia as “cognitive pattern generators” to emphasize the role of this structure in the scaffolding of cognitive performance.
It seems that basal ganglia play a key role in explaining the results of this study because, considering the five main circuits of basal ganglia (Pesce et al., 2019) and their role in both verbal functions and auditory processing in terms of sound sequences and rhythms (Ben-Soussan et al., 2015; Koziol et al., 2014) and the production and continuity of rhythm production (Thaut et al., 2009), it also plays a role in nonverbal functions such as memory and attention (Bhide et al., 2013) or the perception of timing (Repp & Su, 2013; Thaut & Abiru, 2010), especially in the perception of motor rhythms and the regulation of motor sequences (Vogt, 2019) and the fragmentation of skillful motor functions and their segmentation (Dahan & Reiner, 2017) and the construction of meaningful and purposeful behavioral sequences (Ferguson et al., 2024).
Of course, in the meantime, it is necessary to consider the function over arching (Pesce et al., 2019) also considered the integration of all circuits and networks related to the basal ganglia and providing an integrated and unified function, on the other hand, it overlooked the importance of the fifth basal ganglia circuit (the hyperdirect pathway) in the rapid functions of inhibiting and controlling impulsive behaviors (Pesce et al., 2019).
The selection of variables related to response control (response control, audio response control, visual response control) and also regarding response selection time (five-choice accuracy, mean five-choice reaction time, choice movement time mean five) has been observed in the accepted definition and acceptance of impulsivity, which refers to the occurrence of a behavior (motor and automatic dimension) without thinking about the consequences (cognitive dimension and behavior selection). Today, it has been determined that each of the motor qualities and components can have the greatest impact on which aspect of the cases mentioned above (Chamorro et al., 2012; Deslandes et al., 2009), for example, gross skillful movements are related to working memory, spatial perception, and response inhibition (Kramer & Erickson, 2007). The role of rhythmic movements in facilitating social relationships has been mentioned. Additionally, sequential and timing exercises impact working memory and attention (Buchanan et al., 1997; McAuley et al., 2005), whereas rhythmic movements and movements related to rhythmic hearing have a distinct effect on inhibitory functions (Ohgi et al., 2008). Perhaps the overall outcome of these cases can be considered as one of the reasons that the audio response control variable in this study had a much larger effect size than the visual response control variable, because in this proposed set of exercises, the role of auditory stimulation and corresponding and proportionate body rhythm has been particularly emphasized.
Other studies have also shown that if an auditory or motor rhythmic sequence is maintained, the complementary premotor areas of the frontal cortex can compensate for the dysfunction of the basal ganglia (Jamey et al., 2024), and this two-way interaction can play a more significant role in rehabilitation models (Myers et al., 2019).
Considering the age range of the participants, it is also crucial to acknowledge the role of neural maturation in this process. Given that the age of the participants in this study was over six years old, it can be expected that after the second year, connections between the limbic circuit and the anterior cingulate in addition to the prefrontal region have been established (Mathias et al., 2020), and the self-regulatory circuit is maturing and developing, and these connections can cause a connection between cognition and emotion (Deforche & De Bourdeaudhuij, 2015; Laffere et al., 2021), and this age after 5 years of age (Janmohammadi et al., 2020; Koziol et al., 2014) can be very valuable in ensuring the completion of the self-regulatory circuit, which seems to have been useful in achieving improvements related to response inhibition and laying the groundwork for controlling impulsivity (Mathias et al., 2020).
Some researchers have also emphasized the importance of a step-by-step and progressively challenging level of difficulty in exercises, such as homework, to facilitate executive functions (Amrani & Golumbic, 2019). Some have also emphasized the importance of this feature in such a way that if physical exercises do not possess it, they cannot affect executive function (Deslandes et al., 2009). On the other hand, some researchers have also emphasized the diversity of exercise combinations and their ability to attract and interest the child and instill a sense of empowerment (Ahmed & Mohamed, 2011; Burnham, 2012; Chamorro et al., 2012) and pleasure (Bustamante, 2013; Chen et al., 2012), and have considered these issues ::as char::acteristics of a motor exercise package (Chamorro et al., 2012; Cornelius et al., 2017; Hoza et al., 2016; Kang et al., 2011) that can affect the child’s self-regulatory functions.
Research has also focused on the use of various rhythms in the form of rhythmic movements or auditory rhythms from the surrounding environment (Chamorro et al., 2012; Lufi & Parish-Plass, 2011; Medina et al., 2010; Piepmeier et al., 2015) and their effects through body-centered cognitive theories (Donnelly et al., 2016; Tse et al., 2021), as well as the theory of the influence of the musician (Diamond, 2015; Ohgi et al., 2008) on the child’s learning, cognitive (van Der Fels et al., 2019) and self-regulation functions, and examples of these have been presented in the form of a combination of rhythm and movement.
ADHD has been closely associated with deficits in core executive functions, such as inhibitory control, cognitive flexibility, and working memory (Barkley, 1997). Specific deficits related to inhibition include impairments in working memory, inner speech, self-regulation, emotional modulation, motivation, and arousal. In the proposed training package, cognitive demands and ultimately working memory capacity within the dual-task paradigm are progressively increased in a structured and hierarchical manner, with external rhythm-based self-regulation emphasized throughout all major training components (Myers et al., 2019).
In general, all aspects of impulse control were considered in the exercises, and the results align with this effort and goal setting. The first topic is presented from new perspectives that refer to the sensory-motor paradigm and the role of continuity in sensory functions in decision-making and problem-solving. These perspectives also highlight the interaction between subcortical areas and cortical regions (Pesce et al., 2019). The signs and traces of this neural cooperation are evident in all types of mammals, and it is considered the most specialized sensory and motor information processing system, playing an important role in cooperation with subcortical areas, such as the basal ganglia and the cerebellum, as well as the PFC. In some sources, this collaboration has been referred to as the “dual-tiered model,” emphasizing the importance of these regions collaborating with higher cortical areas to achieve complex behavioral outcomes, such as problem-solving and decision-making. This model of collaboration explains both the monitoring and regulation of automatic behaviors and higher-level cognitive behaviors that require inhibitory patterns (Heyder et al., 2004; Rae et al., 2015).
It is essential to note that more than 95% of human behavior is performed automatically, and focusing on this aspect underscores the significance of the unconscious and involuntary subcortical parts of the central nervous system. The self-regulation circuit and neural network, which plays a central role in controlling impulses, making decisions, and resolving conflicts and tensions (Sylvester et al., 2018), is also composed of two cortical parts, prefrontal and anterior cingulate regions (Deforche & De Bourdeaudhuij, 2015), and subcortical parts (basal nuclei and cerebellum).
From a pathological and pathophysiological perspective, structural and functional damage in these regions also has the greatest burden and contribution in children with attention deficit hyperactivity disorder (Jaschke et al., 2018; Vazou et al., 2019; Wilson, 2002).
It seems that considering the two aspects of motor activity (Boes et al., 2009) and interest and attractiveness (Ahmed & Mohamed, 2011; Chamorro et al., 2012; Rothbart et al., 2011) for participants, the role of the neurotransmitter “dopamine” should also be mentioned, because these are considered part of the proven functions of this neurochemical carrier. The secretion and increased presence of this substance in synaptic spaces can play a role in the construction and formation of neural circuits, their stabilization, and the strengthening of these currents (Aylward et al., 1996).
The “musician’s advantage” perspective (Ohgi et al., 2008) is also one of the modern psychological perspectives that, by emphasizing body-centered cognition (Tse et al., 2021) and the function of the cerebellum in the self-regulation and control network of motor behaviors (Diamond & Lee, 2011; Donnelly et al., 2016), refers to the clinical use of motor rhythm and the harmony of physical movement patterns with the emergence of observable behaviors and the generalization of these rhythmic and coordinated patterns to the entire motor and physical behaviors of the individual, and its effects on behavioral inhibition in different individuals and clinical groups and considering the use of balance exercises and their combination with movement rhythms in this training package, a portion of the changes and improvements achieved can be attributed to this.
Another aspect of the improvements achieved could be related to the mediating role of working memory in this regard (Buchanan et al., 1997; Krain & Castellanos, 2006; McAuley et al., 2005), because it has been shown that generalization and organization of information in the working memory section improves the attentional control system as well as emotional control and improves the individual’s self-regulation. Another aspect is the use of ball exercises in combination with the proposed exercise package, which, as noted in another study (Hoza et al., 2016), has also been highlighted for its effect on the development of restraint, self-control, and monitoring of one’s own behaviors.
Additionally, in the motivation section, an incentive was provided to the child at the final stage of each phase and upon completion of the main activity. This approach not only reinforced motivation throughout the exercises but also taught the concept of reward delay.
Conclusion
In this study, the effectiveness of a package of rhythmic physical dual-task exercises combined with cognitive activities on impulsivity and self-regulation in children with ADHD was investigated. Given the diversity of treatment approaches and the common parental concerns about the side effects of pharmacological interventions, implementing a structured, leveled program of rhythmic physical-cognitive dual tasks appears to offer a promising alternative. Such an approach, which is both comprehensive and feasible, may enhance family engagement and increase the child’s intrinsic motivation due to its inherent attractiveness. Overall, this intervention could contribute to effective symptom management, particularly in reducing impulsivity, in children with ADHD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Human Studies Ethics Committee of the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (Code: IR.USWR.REC.1400.207). This study was also registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20220212054004N1). The studies were conducted in accordance with local laws and institutional requirements. Written informed consent to participate in this study was provided by the legal guardians/close relatives of the participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and investigation: Ebrahim Pishyareh, Behzad Amini, and Seyed Ali Hosseini; Study design, and methodology: All authors; Experiments, statistical analysis, and writing the original draft: Behzad Amini and Ebrahim Pishyareh; Review, and editing: Behzad Amini, Ebrahim Pishyareh, and Seyed Ali Hosseini.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their deepest gratitude to all participants in this study and their families for their collaboration with the research team. The authors also thank the Department of Occupational Therapy at the University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. Additionally, The authors would like to express their appreciation to Ofogh Occupational Therapy Clinic, Tehran, Iran, for providing the intervention space and conducting the assessment.
References
Ahmed, G. M., & Mohamed, S. (2011). Effect of regular aerobic exercises on behavioral, cognitive and psychological response in patients with attention deficit-hyperactivity disorder. Life Science Journal, 8, 366-371. [Link]
Amini, B., Hosseini, S. A., & Akbarfahimi, N. (2018). Balance performance disorders and sway of the center of gravity in children with ADHD. Journal of Modern Rehabilitation, 12(1), 3-12. [DOI:10.32598/jmr.12.1.3]
Amini, B., Hosseini, S. A., Biglarian, A., Amiri, N., & Pishyareh, E. (2016). Comparative study of mobility quality and walking parameters in children with ADHD and normal controls. Journal of Biomedical Science, 1(3), e8105. [Link]
Amini, B., Hosseini, S. A., Pishyareh, E., Bakhshi, E., & Haghgoo, H. A. (2024). [Designing an exercise protocol to improve impulsivity control in children with attention deficit hyperactivity disorder: A pilot study (Persian)]. Archives of Rehabilitation, 25, 702-725. [DOI:10.32598/RJ.25.specialissue.3833.1]
Amrani, A. K., & Golumbic, E. Z. (2019). The preferred period hypothesis revisited: Rhythmic preferences and motor tapping precision in ADHD adults and controls. bioRxiv, 2019.2012. 2024.887802.
Archer, T., & Kostrzewa, R. M. (2011). Physical exercise alleviates ADHD symptoms: Regional deficits and development trajectory. Neurotoxicity Research, 21(2), 195-209. [DOI:10.1007/s12640-011-9260-0] [PMID]
Arvaniti, A. (2009). Rhythm, timing and the timing of rhythm. Phonetica, 66(1-2), 46-63. [DOI:10.1159/000208930] [PMID]
Aylward, E. H., Reiss, A. L., Reader, M. J., Singer, H. S., Brown, J. E., & Denckla, M. B. (1996). Basal ganglia volumes in children with attention-deficit hyperactivity disorder. Journal of Child Neurology, 11(2), 112-115. [DOI:10.1177/088307389601100210] [PMID]
Barkley, R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65-94. [DOI:10.1037/0033-2909.121.1.65] [PMID]
Barkley, R. A., DuPaul, G. J., & McMurray, M. B. (1990). Comprehensive evaluation of attention deficit disorder with and without hyperactivity as defined by research criteria. Journal of Consulting and Clinical Psychology, 58(6), 775-789. [DOI:10.1037/0022-006X.58.6.775] [PMID]
Ben-Soussan, T. D., Berkovich-Ohana, A., Piervincenzi, C., Glicksohn, J., & Carducci, F. (2015). Embodied cognitive flexibility and neuroplasticity following Quadrato Motor Training. Frontiers in Psychology, 6, 1021. [DOI:10.3389/fpsyg.2015.01021] [PMID]
Bezdjian, S., Baker, L. A., & Tuvblad, C. (2011). Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clinical Psychology Review, 31(7), 1209-1223. [DOI:10.1016/j.cpr.2011.07.005] [PMID]
Bhide, A., Power, A., & Goswami, U. (2013). A rhythmic musical intervention for poor readers: A comparison of efficacy with a letter‐based intervention. Mind, Brain, and Education, 7(2), 113-123. [DOI:10.1111/mbe.12016]
Bjørkly, S. (2013). A systematic review of the relationship between impulsivity and violence in persons with psychosis: Evidence or spin cycle? Aggression and Violent Behavior, 18(6), 753-760. [DOI:10.1016/j.avb.2013.08.001]
Boes, A. D., Bechara, A., Tranel, D., Anderson, S. W., Richman, L., & Nopoulos, P. (2009). Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience, 4(1), 1-9. [DOI:10.1093/scan/nsn035] [PMID]
Brand, R., & Ekkekakis, P. (2018). Affective-reflective theory of physical inactivity and exercise. German Journal of Exercise and Sport Research, 48(1), 48-58. [DOI:10.1007/s12662-017-0477-9]
Buchanan, J., Kelso, J., DeGuzman, G., & Ding, M. (1997). The spontaneous recruitment and suppression of degrees of freedom in rhythmic hand movements. Human Movement Science, 16(1), 1-32. [DOI:10.1016/S0167-9457(96)00040-1]
Burnham, B. (2012). Make a move: A multi-sensory, movement coordinated furnishing support system for children with ADHD: A thesis presented in partial fulfillment of the degree of Master of Design [PhD dissertation]. Turitea: Design Massey University. [Link]
Bustamante, E. E. (2013). Physical activity intervention for ADHD and DBD [Phd dissertation]. Chicago: University of Illinois. [Link]
Cepeda, N. J., Cepeda, M. L., & Kramer, A. F. (2000). Task switching and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 28(3), 213–226.[DOI:10.1023/A:1005143419092] [PMID]
Chamorro, J., Bernardi, S., Potenza, M. N., Grant, J. E., & Marsh, R., Wang, S., et al. (2012). Impulsivity in the general population: a national study. Journal of Psychiatric Research, 46(8), 994-1001. [DOI:10.1016/j.jpsychires.2012.04.023] [PMID]
Chen, L. J., Stevinson, C., Ku, P. W., Chang, Y. K., & Chu, D. C. (2012). Relationships of leisure-time and non-leisure-time physical activity with depressive symptoms: A population-based study of Taiwanese older adults. The International Journal of Behavioral Nutrition and Physical Activity, 9, 28. [DOI:10.1186/1479-5868-9-28] [PMID]
Cornelius, C., Fedewa, A. L., & Ahn, S. (2017). The effect of physical activity on children with ADHD: A quantitative review of the literature. Journal of Applied School Psychology, 33(2), 136-170. [DOI:10.1080/15377903.2016.1265622]
Cox, E. R., Halloran, D. R., Homan, S. M., Welliver, S., & Mager, D. E. (2008). Trends in the prevalence of chronic medication use in children: 2002-2005. Pediatrics, 122(5), e1053-e1061. [DOI:10.1542/peds.2008-0214] [PMID]
Dahan, A., & Reiner, M. (2017). Evidence for deficient motor planning in ADHD. Scientific Reports, 7(1), 9631. [DOI:10.1038/s41598-017-09984-7] [PMID]
Deforche, B., & De Bourdeaudhuij, I. (2015). Attentional distraction during exercise in overweight and normal-weight boys. International Journal of Environmental Research and Public Health, 12(3), 3077-3090. [DOI:10.3390/ijerph120303077] [PMID]
Deslandes, A., Moraes, H., Ferreira, C., Veiga, H., Silveira, H., & Mouta, R., et al. (2009). Exercise and mental health: Many reasons to move. Neuropsychobiology, 59(4), 191-198. [DOI:10.1159/000223730] [PMID]
Diamond, A., & Lee, K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333(6045), 959-964. [DOI:10.1126/science.1204529] [PMID]
Diamond, L. (2015). Facing up to the democratic recession. Journal of Democracy, 26(1), 141-155. [DOI:10.1353/jod.2015.0009]
Donnelly, J. E., Hillman, C. H., Castelli, D., Etnier, J. L., Lee, S., & Tomporowski, P., et al. (2016). Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Medicine and Science in Sports and Exercise, 48(6), 1197. [DOI:10.1249/MSS.0000000000000966] [PMID]
Ferguson, C., Hobson, C., Hedge, C., Waters, C., Anning, K., & Van Goozen, S. (2024). Disentangling the relationships between motor control and cognitive control in young children with symptoms of ADHD. Child Neuropsychology, 30(2), 289-314. [DOI:10.1080/09297049.2023.2190965] [PMID]
Fliers, E., Rommelse, N., Vermeulen, S. H., Altink, M., Buschgens, C. J., & Faraone, S. V., et al. (2008). Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: Effects of age and gender. Journal of Neural Transmission, 115(2), 211-220. [DOI:10.1007/s00702-007-0827-0] [PMID]
Graybiel, A. M. (1997). The basal ganglia and cognitive pattern generators. Schizophrenia Bulletin, 23(3), 459-469. [DOI:10.1093/schbul/23.3.459]
Garon, N., Bryson, S. E., & Smith, I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134(1), 31-60. [DOI:10.1037/0033-2909.134.1.31] [PMID]
Goya-Maldonado, R., Walther, S., Simon, J., Stippich, C., Weisbrod, M., & Kaiser, S. (2010). Motor impulsivity and the ventrolateral prefrontal cortex. Psychiatry Research, 183(1), 89–91. [DOI:10.1016/j.pscychresns.2010.04.006] [PMID]
Grassmann, V., Alves, M. V., Santos-Galduróz, R. F., & Galduróz, J. C. F. (2017). Possible cognitive benefits of acute physical exercise in children with ADHD: A systematic review. Journal of Attention Disorders, 21(5), 367-371. [DOI:10.1177/1087054714526041] [PMID]
Hamilton, K. R., Mitchell, M. R., Wing, V. C., Balodis, I. M., Bickel, W. K., & Fillmore, M., et al. (2015). Choice impulsivity: Definitions, measurement issues, and clinical implications. Personality Disorders: Theory, Research, and Treatment, 6(2), 182-198. [DOI:10.1037/per0000099]
Heyder, K., Suchan, B., & Daum, I. (2004). Cortico-subcortical contributions to executive control. Acta Psychologica, 115(2-3), 271-289. [DOI:10.1016/j.actpsy.2003.12.010] [PMID]
Hoza, B., Martin, C. P., Pirog, A., & Shoulberg, E. K. (2016). Using physical activity to manage ADHD symptoms: The state of the evidence. Current Psychiatry Reports, 18(12), 113. [DOI:10.1007/s11920-016-0749-3] [PMID]
Jahani, M., Pishyareh, E., Haghgoo, H. A., Hosseini, S. A., & Ghadamgahi Sani, S. N. (2016). Neurofeedback effect on perceptual-motor skills of children with ADHD. Iranian Rehabilitation Journal, 14(1), 43-50. [DOI:10.15412/J.IRJ.08140107]
Jamey, K., Laflamme, H., Foster, N. E., Rigoulot, S., Kotz, S. A., & Bella, S. D. (2024). Can you beat the music? Validation of a gamified rhythmic training in children with ADHD. medRxiv. [DOI:10.1101/2024.03.19.24304539]
Janmohammadi, S., Haghgoo, H. A., Farahbod, M., Overton, P. G., & Pishyareh, E. (2020). Effect of a visual tracking intervention on attention and behavior of children with Attention Deficit Hyperactivity Disorder. Journal of Eye Movement Research, 12(8), 10.16910/jemr.12.8.6. [DOI:10.16910/jemr.12.8.6] [PMID]
Jaschke, A. C., Honing, H., & Scherder, E. J. A. (2018). Longitudinal analysis of music education on executive functions in primary school children. Frontiers in Neuroscience, 12, 103. [DOI:10.3389/fnins.2018.00103] [PMID]
Kang, K. D., Choi, J. W., Kang, S. G., & Han, D. H. (2011). Sports therapy for attention, cognitions and sociality. International Journal of Sports Medicine, 32(12), 953–959. [DOI:10.1055/s-0031-1283175] [PMID]
Koziol, L. F., Budding, D., Andreasen, N., D’Arrigo, S., Bulgheroni, S., & Imamizu, H., et al. (2014). Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum (London, England), 13(1), 151–177. [DOI:10.1007/s12311-013-0511-x] [PMID]
Krain, A. L., & Castellanos, F. X. (2006). Brain development and ADHD. Clinical Psychology Review, 26(4), 433-444. [DOI:10.1016/j.cpr.2006.01.005] [PMID]
Kramer, A. F., & Erickson, K. I. (2007). Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends in Cognitive Sciences, 11(8), 342-348. [DOI:10.1016/j.tics.2007.06.009] [PMID]
Laffere, A., Dick, F., Holt, L. L., & Tierney, A. (2021). Attentional modulation of neural entrainment to sound streams in children with and without ADHD. NeuroImage, 224, 117396. [DOI:10.1016/j.neuroimage.2020.117396] [PMID]
Lee, H., Sullivan, S. J., & Schneiders, A. G. (2013). The use of the dual-task paradigm in detecting gait performance deficits following a sports-related concussion: A systematic review and meta-analysis. Journal of Science and Medicine in Sport, 16(1), 2-7. [DOI:10.1016/j.jsams.2012.03.013] [PMID]
Lufi, D., & Parish-Plass, J. (2011). Sport-based group therapy program for boys with ADHD or with other behavioral disorders. Child & Family Behavior Therapy, 33(3), 217-230. [DOI:10.1080/07317107.2011.596000]
Mathias, B., Zamm, A., Gianferrara, P. G., Ross, B., & Palmer, C. (2020). Rhythm complexity modulates behavioral and neural dynamics during auditory-motor synchronization. Journal of Cognitive Neuroscience, 32(10), 1864-1880. [DOI:10.1162/jocn_a_01601] [PMID]
McAuley, E., Elavsky, S., Jerome, G. J., Konopack, J. F., & Marquez, D. X. (2005). Physical activity-related well-being in older adults: Social cognitive influences. Psychology and Aging, 20(2), 295-302. [DOI:10.1037/0882-7974.20.2.295] [PMID]
Meda, S. A., Stevens, M. C., Potenza, M. N., Pittman, B., Gueorguieva, R., & Andrews, M. M., et al. (2009). Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology, 20(5-6), 390–399. [DOI:10.1097/FBP.0b013e32833113a3] [PMID]
Medina, J. A., Netto, T. L., Muszkat, M., Medina, A. C., Botter, D., & Orbetelli, R., et al. (2010). Exercise impact on sustained attention of ADHD children, methylphenidate effects. Attention Deficit and Hyperactivity Disorders, 2(1), 49–58. [DOI:10.1007/s12402-009-0018-y] [PMID]
Myers, J., Kokkinos, P., & Nyelin, E. (2019). Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients, 11(7), 1652. [DOI:10.3390/nu11071652] [PMID]
Nasuti, G., & Rhodes, R. E. (2013). Affective judgment and physical activity in youth: Review and meta-analyses. Annals of Behavioral Medicine, 45(3), 357-376. [DOI:10.1007/s12160-012-9462-6] [PMID]
Newton-Howes, G. (2004). What happens when children with attention deficit/hyperactivity disorder grow up? Journal of the Royal Society of Medicine, 97(11), 531-535. [DOI:10.1177/014107680409701105] [PMID]
Ohgi, S., Morita, S., Loo, K. K., & Mizuike, C. (2008). Time series analysis of spontaneous upper-extremity movements of premature infants with brain injuries. Physical Therapy, 88(9), 1022-1033. [DOI:10.2522/ptj.20070171] [PMID]
Pashler, H. (1994). Dual-task interference in simple tasks: data and theory. Psychological Bulletin, 116(2), 220–244.[DOI:10.1037/0033-2909.116.2.220] [PMID]
Patros, C. H., Alderson, R. M., Kasper, L. J., Tarle, S. J., Lea, S. E., & Hudec, K. L. (2016). Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review, 43, 162–174. [DOI:10.1016/j.cpr.2015.11.001] [PMID]
Pesce, C., Croce, R., Ben-Soussan, T. D., Vazou, S., McCullick, B., & Tomporowski, P. D., et al. (2019). Variability of practice as an interface between motor and cognitive development. International Journal of Sport and Exercise Psychology, 17(2), 133-152. [DOI:10.1080/1612197X.2016.1223421]
Petrides, M. (1994). Frontal lobes and behaviour. Current Opinion in Neurobiology, 4(2), 207–211. [DOI:10.1016/0959-4388(94)90074-4] [PMID]
Piek, J. P., Dyck, M. J., Nieman, A., Anderson, M., Hay, D., & Smith, L. M., et al.(2004). The relationship between motor coordination, executive functioning and attention in school aged children. Archives of clinical Neuropsychology, 19(8), 1063-1076. [DOI:10.1016/j.acn.2003.12.007] [PMID]
Piepmeier, A. T., Shih, C. H., Whedon, M., Williams, L. M., Davis, M. E., & Henning, D. A., et al. (2015). The effect of acute exercise on cognitive performance in children with and without ADHD. Journal of Sport and Health science, 4(1), 97-104. [DOI:10.1016/j.jshs.2014.11.004]
Rae, C. L., Hughes, L. E., Anderson, M. C., & Rowe, J. B. (2015). The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. Journal of Neuroscience, 35(2), 786-794. [DOI:10.1523/JNEUROSCI.3093-13.2015] [PMID]
Repp, B. H., & Su, Y. H. (2013). Sensorimotor synchronization: A review of recent research (2006-2012). Psychonomic Bulletin & Review, 20(3), 403–452. [DOI:10.3758/s13423-012-0371-2] [PMID]
Rothbart, M. K., Sheese, B. E., Rueda, M. R., & Posner, M. I. (2011). Developing mechanisms of self-regulation in early life. Emotion Review, 3(2), 207-213. [DOI:10.1177/1754073910387943]
Ryan, R. M., & Deci, E. L. (2000). Intrinsic and extrinsic motivations: Classic definitions and new directions. Contemporary Educational Psychology, 25(1), 54-67. [DOI:10.1006/ceps.1999.1020] [PMID]
Sakai, K., Hikosaka, O., & Nakamura, K. (2004). Emergence of rhythm during motor learning. Trends in Cognitive Sciences, 8(12), 547-553. [DOI:10.1016/j.tics.2004.10.005] [PMID]
Sebastian, A., Jacob, G., Lieb, K., & Tüscher, O. (2013). Impulsivity in borderline personality disorder: A matter of disturbed impulse control or a facet of emotional dysregulation? Current Psychiatry Reports, 15(2), 339. [DOI:10.1007/s11920-012-0339-y] [PMID]
Sebastian, A., Jung, P., Krause-Utz, A., Lieb, K., Schmahl, C., & Tüscher, O. (2014). Frontal dysfunctions of impulse control-a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Frontiers in Human Neuroscience, 8, 698. [DOI:10.3389/fnhum.2014.00698] [PMID]
Shin, H. J., Lee, H. J., Kang, D., Kim, J. I., & Jeong, E. (2023). Rhythm-based assessment and training for children with attention deficit hyperactivity disorder (ADHD): A feasibility study protocol. Frontiers in Human Neuroscience, 17, 1190736. [DOI:10.3389/fnhum.2023.1190736] [PMID]
Slater, J. L., & Tate, M. C. (2018). Timing deficits in ADHD: Insights from the neuroscience of musical rhythm. Frontiers in Computational Neuroscience, 12, 51. [DOI:10.3389/fncom.2018.00051] [PMID]
Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657-1661. [DOI:10.1126/science.283.5408.1657] [PMID]
Stahl, J. L. (2020). Psychiatric diagnoses in children and adolescents with chronic kidney disease [MA thesis]. Washington: University of Washington. [Link]
Sylvester, B. D., Curran, T., Standage, M., Sabiston, C. M., & Beauchamp, M. R. (2018). Predicting exercise motivation and exercise behavior: A moderated mediation model testing the interaction between perceived exercise variety and basic psychological needs satisfaction. Psychology of Sport and Exercise, 36, 50-56. [DOI:10.1016/j.psychsport.2018.01.004]
Thaut, M. H., & Abiru, M. (2010). Rhythmic auditory stimulation in rehabilitation of movement disorders: A review of current research. Music Perception, 27(4), 263-269. [DOI:10.1525/mp.2010.27.4.263]
Thaut, M. H., Gardiner, J. C., Holmberg, D., Horwitz, J., Kent, L., & Andrews, G., et al. (2009). Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Annals of the New York Academy of Sciences, 1169, 406-416. [DOI:10.1111/j.1749-6632.2009.04585.x] [PMID]
Tomporowski, P. D., & Pesce, C. (2019). Exercise, sports, and performance arts benefit cognition via a common process. Psychological Bulletin, 145(9), 929-951. [DOI:10.1037/bul0000200] [PMID]
Tse, A. C. Y., Anderson, D. I., Liu, V. H. L., & Tsui, S. S. L. (2021). Improving executive function of children with autism spectrum disorder through cycling skill acquisition. Medicine & Science in Sports & Exercise, 53(7), 1417-1424. [DOI:10.1249/MSS.0000000000002609] [PMID]
van der Fels, I. M. J., Smith, J., de Bruijn, A. G. M., Bosker, R. J., Königs, M., & Oosterlaan, J., et al. (2019). Relations between gross motor skills and executive functions, controlling for the role of information processing and lapses of attention in 8-10 year old children. Plos One, 14(10), e0224219.[DOI:10.1371/journal.pone.0224219] [PMID]
Vazou, S., Pesce, C., Lakes, K., & Smiley-Oyen, A. (2019). More than one road leads to Rome: A narrative review and meta-analysis of physical activity intervention effects on cognition in youth. International Journal of Sport and Exercise Psychology, 17(2), 153-178. [DOI:10.1080/1612197X.2016.1223423] [PMID]
Vogt, B. A. (2019). Cingulate impairments in ADHD: Comorbidities, connections, and treatment. Handbook of Clinical Neurology, 166, 297-314. [DOI:10.1016/B978-0-444-64196-0.00016-9] [PMID]
Watanabe, K., & Funahashi, S. (2015). A dual-task paradigm for behavioral and neurobiological studies in nonhuman primates. Journal of Neuroscience Methods, 246, 1-12. [DOI:10.1016/j.jneumeth.2015.03.006] [PMID]
Williams, M., Maschette, W., & Lythgo, N. (2006). Timing error by children identified with DCD leads to inefficient jump performance. Paper presented at: 24 International Symposium on Biomechanics in Sports, 15 July 2006, Salzburg, Austria. [Link]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2025/04/27 | Accepted: 2025/08/20 | Published: 2025/09/1
Received: 2025/04/27 | Accepted: 2025/08/20 | Published: 2025/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |