Volume 16, Issue 4 (July & August 2025)
BCN 2025, 16(4): 777-786 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sinaei E, Nami M, Saadat Z. Cortical Activity During Postural Recovery Under Dual-task Conditions: A Quantitative EEG Study. BCN 2025; 16 (4) :777-786
URL: http://bcn.iums.ac.ir/article-1-3070-en.html
URL: http://bcn.iums.ac.ir/article-1-3070-en.html
1- Department of Rehabilitation Sciences, MGH Institute of Health Professions, Boston, United States. & Shiraz Geriatric Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Cognitive Neuroscience Unit, Department of Social Sciences, Faculty of Communication, Arts and Science, Canadian University Dubai, Dubai, United Arab Emirates.
3- Cardiovascular Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran. & Department of Physical Therapy, School of Rehabilitation Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Cognitive Neuroscience Unit, Department of Social Sciences, Faculty of Communication, Arts and Science, Canadian University Dubai, Dubai, United Arab Emirates.
3- Cardiovascular Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran. & Department of Physical Therapy, School of Rehabilitation Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 798 kb]
| Abstract (HTML)
4. Discussion
This observational study investigated the cortical activity of young and older adults during postural adjustments following external perturbations under both ST and DT conditions. Our results revealed that older adults exhibited increased frontoparietal alpha power during the late phase of recovery from external perturbations, particularly during DT execution. These findings align with previous research, which suggests that aging is accompanied by compensatory neural mechanisms to maintain postural control despite age-related declines in sensory and motor function (Chang et al., 2016; Papegaaij et al., 2014).
We selected the alpha and beta frequency bands due to their well-established relevance in postural control and cognitive-motor interactions. Alpha activity reflects attentional engagement and cortical involvement, while beta activity is associated with motor planning and sensorimotor integration, both critical for balance recovery. The chosen channels also target cortical regions implicated in postural control, such as the sensorimotor, primary motor, and supplementary motor areas. This focused approach allowed us to assess the neural dynamics most directly involved in maintaining stability under ST and DT conditions (Ghosn et al., 2020; Protzak & Gramann, 2021).
The increased alpha power in the sensorimotor and supplementary motor areas observed in older adults during DT conditions is consistent with evidence that postural control becomes more cognitively demanding with age (Jacobs & Horak, 2007). Motor control in older individuals relies more heavily on cortical regions, including the prefrontal cortex, for compensatory strategies when regaining postural stability following perturbations (Papegaaij et al., 2014). This shift from subcortical to cortical control mechanisms may be attributed to age-related degeneration in somatosensory receptors and reduced conduction velocity, both of which compromise the automaticity of postural control (Papegaaij et al., 2014; Sturnieks et al., 2008).
This study builds upon our previous work, which investigated cortical responses to predictable and unpredictable perturbations using the same dataset (Saadat et al., 2021). While the earlier study highlighted the role of anticipation versus reaction in postural control, the current analysis shifts focus to the cognitive-motor interplay under DT conditions. By examining the influence of an additional cognitive task on cortical activity during postural recovery, this study extends our understanding of DT interference and its implications for fall risk in older adults. The present findings offer actionable insights for fall prevention strategies that integrate both motor and cognitive training, a dimension not explored in the initial analysis.
The cortical overactivation observed as increased power z-scores during DT conditions likely represents an effort to engage additional neural resources to manage the increased attentional demands (Reuter-Lorenz & Cappell, 2008). While this compensatory mechanism supports balance control, it may reduce the availability of cognitive resources for other tasks, explaining the greater DT interference observed in older adults (Palmer et al., 2021).
In line with the dedifferentiation theory, the reduced neural specificity seen in older adults may also contribute to this cortical overactivation (Morcom & Henson, 2018). With age, the brain becomes less efficient in segregating neural processes, leading to broader recruitment of cortical areas even for tasks that, in younger adults, are more localized to subcortical regions (Alizadehsaravi et al., 2020). This reduced efficiency in neural processing is particularly evident in DT scenarios, where the cognitive load further strains the brain’s limited attentional resources (Lacour et al., 2008).
Our study also found elevated beta power in the prefrontal and sensorimotor regions during DT conditions, particularly in older adults. Beta oscillations are closely linked to the cognitive aspects of motor control and are indicative of heightened cortical engagement during more demanding tasks (Teasdale & Simoneau, 2001). The increased beta power observed in older adults suggests that they require more cognitive effort to maintain balance under DT conditions, reflecting the additional neural processing required to integrate motor and cognitive tasks simultaneously.
This enhanced cortical activity in older adults may serve as a biomarker for reduced automaticity in balance control (Ghosn et al., 2020). As the task becomes more challenging, the central nervous system recruits higher-level cortical resources to compensate for the loss of subcortical control (Pizzamiglio et al., 2017). This reliance on cortical control could explain the difficulty older adults face in maintaining postural stability while concurrently performing cognitive tasks (Ghosn et al., 2020; Palmer et al., 2021).
Our results support the idea that brain oscillations, particularly in the alpha and beta frequency bands, play a critical role in modulating connectivity between different brain regions during postural tasks (Malcolm et al., 2021). Young and older adults seem to employ different neural strategies when managing DT conditions, with older adults showing less flexibility in reallocating neural resources (Malcolm et al., 2021). The greater engagement of cortical areas in older adults under DT conditions reflects an age-related shift in sensorimotor processing that compensates for the decline in subcortical mechanisms that once governed automatic postural control (Ghosn et al., 2020).
The reliance on these cortical networks may also indicate a lower threshold for eliciting stepping reactions, especially in cognitively demanding situations (Palmer et al., 2021). This condition has important implications for understanding fall risk in older adults, as greater reliance on cortical resources for postural control may lead to slower or less efficient balance recovery (Clark, 2015; Ghosn et al., 2020).
Taken together, our findings suggest the role of task complexity in potentially shaping neural responses during postural adjustments. Accordingly, depending on the cognitive demands, older adults demonstrated heightened challenges in maintaining balance. Such an observation underscores the implication of considering DT scenarios in balance assessments and interventions. Furthermore, the distinction in neural activation patterns between young and older adults proposes that age-related neural adaptations are not merely compensatory and refer to fundamental shifts in how balance is processed within the brain. In other words, since older adults engage more cortical resources in DT perturbations, an increased reliance can lead to a fragile balance system, particularly under complex conditions.
The implications of our findings extend to practical applications in fall prevention strategies. Interventions designed to enhance postural control in older adults could benefit from incorporating cognitive tasks that simulate real-world challenges. Such an approach could help mitigate the neurocognitive burden associated with DT, potentially leading to improved stability and reduced fall risk. Additionally, understanding the specific neural mechanisms underlying balance control in older adults can guide the development of targeted training programs to improve or empower both motor and cognitive functions, leading to a collective approach to maintaining functional independence in aging populations. Future research could expand on these findings by exploring additional cortical regions and frequency bands, including gamma oscillations, to provide a more comprehensive understanding of the neural dynamics underlying balance and DT performance.
5. Conclusion
The findings of this study highlight the significant role of cortical activity in postural adjustments in older adults, particularly during DT conditions. The increased alpha and beta power in the sensorimotor and prefrontal regions points to compensatory mechanisms that enable older adults to maintain balance despite age-related declines in sensorimotor function. These findings contribute to the growing body of evidence that cortical overactivation is a hallmark of aging and suggest that targeted interventions aimed at improving automaticity in balance control could mitigate DT interference and reduce fall risk in older adults.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.REC.1396.26).
Funding
This study was granted by Shiraz University of Medical Sciences, Shiraz, Iran (Grant No.: 95-01-06-13248).
Authors' contributions
Conceptualization, methodology, investigation, review, and editing: All authors; Writing the original draft: Ehsan Sinaei and Zahra Saadat; Funding acquisition and resources: Zahra Saadat and Mohammad Nami; Supervision: Mohammad Nami.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Roya Razavi, the manager of the Jahandidegan Day-Care Center, Shiraz, Iran, and all individuals who volunteered for this trial.
References
Alizadehsaravi, L., Bruijn, S. M., Maas, H., & van Dieën, J. H. (2020). Modulation of soleus muscle H-reflexes and ankle muscle co-contraction with surface compliance during unipedal balancing in young and older adults. Experimental Brain Research, 238(6), 1371–1383. [DOI:10.1007/s00221-020-05784-0] [PMID]
Brown, L. A., Shumway-Cook, A., & Woollacott, M. H. (1999). Attentional demands and postural recovery: The effects of aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 54(4), M165–M171. [DOI:10.1093/gerona/54.4.M165] [PMID]
Chang, C. J., Yang, T. F., Yang, S. W., & Chern, J. S. (2016). Cortical modulation of motor control biofeedback among the elderly with high fall risk during a posture perturbation task with augmented reality. Frontiers in Aging Neuroscience, 8, 80. [DOI:10.3389/fnagi.2016.00080] [PMID]
Clark, D. J. (2015). Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Frontiers in Human Neuroscience, 9, 246. [DOI:10.3389/fnhum.2015.00246] [PMID]
Fraizer, E. V., & Mitra, S. (2008). Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait & Posture, 27(2), 271-279. [DOI:10.1016/j.gaitpost.2007.04.002] [PMID]
Ghosn, N. J., Palmer, J. A., Borich, M. R., Ting, L. H., & Payne, A. M. (2020). Cortical beta oscillatory activity evoked during reactive balance recovery scales with perturbation difficulty and individual balance ability. Brain Sciences, 10(11), 860. [DOI:10.3390/brainsci10110860] [PMID]
Heuninckx, S., Wenderoth, N., Debaere, F., Peeters, R., & Swinnen, S. P. (2005). Neural basis of aging: The penetration of cognition into action control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(29), 6787–6796. [DOI:10.1523/JNEUROSCI.1263-05.2005] [PMID]
Heuninckx, S., Wenderoth, N., & Swinnen, S. P. (2008). Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(1), 91–99. [DOI:10.1523/JNEUROSCI.3300-07.2008] [PMID]
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339-1348. [DOI:10.1007/s00702-007-0657-0] [PMID]
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339-1348. [DOI:10.1007/s00702-007-0657-0] [PMID]
Kanekar, N., & Aruin, A. S. (2014). The effect of aging on anticipatory postural control. Experimental Brain Research, 232(4), 1127-1136. [DOI:10.1007/s00221-014-3822-3] [PMID]
Lacour, M., Bernard-Demanze, L., & Dumitrescu, M. (2008). Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiologie Clinique/Clinical Neurophysiology, 38(6), 411-421. [DOI:10.1016/j.neucli.2008.09.005] [PMID]
Laessoe, U., & Voigt, M. (2008). Anticipatory postural control strategies related to predictive perturbations. Gait & Posture, 28(1), 62-68. [DOI:10.1016/j.gaitpost.2007.10.001] [PMID]
Malcolm, B. R., Foxe, J. J., Joshi, S., Verghese, J., Mahoney, J. R., & Molholm, S., et al. (2021). Aging‐related changes in cortical mechanisms supporting postural control during base of support and optic flow manipulations. The European Journal of Neuroscience, 54(12), 8139–8157. [DOI:10.1111/ejn.15004] [PMID]
Melzer, I., Benjuya, N., & Kaplanski, J. (2001). Age-related changes of postural control: Effect of cognitive tasks. Gerontology, 47(4), 189-194. [DOI:10.1159/000052797] [PMID]
Morcom, A. M., & Henson, R. N. (2018). Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. The Journal of Neuroscience, 38(33), 7303-7313. [DOI:10.1523/JNEUROSCI.1701-17.2018] [PMID]
Ohsugi, H., Ohgi, S., Shigemori, K., & Schneider, E. B. (2013). Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neuroscience, 14, 10. [DOI:10.1186/1471-2202-14-10] [PMID]
Pai, Y. C., Wening, J. D., Runtz, E. F., Iqbal, K., & Pavol, M. J. (2003). Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. Journal of Neurophysiology, 90(2), 755-762. [DOI:10.1152/jn.01118.2002] [PMID]
Palmer, J. A., Payne, A. M., Ting, L. H., & Borich, M. R. (2021). Cortical engagement metrics during reactive balance are associated with distinct aspects of balance behavior in older adults. Frontiers in Aging Neuroscience, 13, 684743. [DOI:10.3389/fnagi.2021.684743] [PMID]
Papegaaij, S., Taube, W., Baudry, S., Otten, E., & Hortobágyi, T. (2014). Aging causes a reorganization of cortical and spinal control of posture. Frontiers in Aging Neuroscience, 6, 28. [DOI:10.3389/fnagi.2014.00028] [PMID]
Pizzamiglio, S., Naeem, U., Abdalla, H., & Turner, D. L. (2017). Neural correlates of single-and dual-task walking in the real world. Frontiers in Human Neuroscience, 11, 460. [DOI:10.3389/fnhum.2017.00460] [PMID]
Protzak, J., & Gramann, K. (2021). EEG beta-modulations reflect age-specific motor resource allocation during dual-task walking. Scientific Reports, 11(1), 16110. [DOI:10.1038/s41598-021-94874-2] [PMID]
Reid, K. F., & Fielding, R. A. (2012). Skeletal muscle power: A critical determinant of physical functioning in older adults. Exercise and Sport Sciences Reviews, 40(1), 4-12. [DOI:10.1097/JES.0b013e31823b5f13] [PMID]
Reuter-Lorenz, P. A., & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177-182. [DOI:10.1111/j.1467-8721.2008.00570.x]
Saadat, Z., Sinaei, E., Pirouzi, S., Ghofrani, M., & Nami, M. (2021). Cortical activity during postural recovery in response to predictable and unpredictable perturbations in healthy young and older adults: A quantitative eeg assessment. Basic and Clinical Neuroscience, 12(2), 291-300. [DOI:10.32598/bcn.12.2.453.1] [PMID]
Slobounov, S., Hallett, M., Stanhope, S., & Shibasaki, H. (2005). Role of cerebral cortex in human postural control: An EEG study. Clinical Neurophysiology, 116(2), 315-323. [DOI:10.1016/j.clinph.2004.09.007] [PMID]
Smith, B. A., Jacobs, J. V., & Horak, F. B. (2012). Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Experimental Brain Research, 222(4), 455-470. [DOI:10.1007/s00221-012-3232-3] [PMID]
Sturnieks, D. L., St George, R., & Lord, S. R. (2008). Balance disorders in the elderly. Neurophysiologie Clinique/Clinical Neurophysiology, 38(6), 467-478. [DOI:10.1016/j.neucli.2008.09.001] [PMID]
Teasdale, N., & Simoneau, M. (2001). Attentional demands for postural control: The effects of aging and sensory reintegration. Gait & Posture, 14(3), 203-210. [DOI:10.1016/S0966-6362(01)00134-5] [PMID]
Venkatraman, V. K., Aizenstein, H., Guralnik, J., Newman, A. B., Glynn, N. W., & Taylor, C., et al. (2010). Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage, 49(4), 3436-3442. [DOI:10.1016/j.neuroimage.2009.11.019] [PMID]
Woollacott, M. H., Shumway-Cook, A., & Nashner, L. M. (1986). Aging and posture control: Changes in sensory organization and muscular coordination. International Journal of Aging and Human Development, 23(2), 97-114. [DOI:10.2190/VXN3-N3RT-54JB-X16X] [PMID]
Full-Text:
1. Introduction
Postural instability and falls are among the most common problems in older adults. The changes in sensory, motor, and cognitive components of postural control can reduce older individuals’ postural adjustment in response to external distresses, such as slips and trips (Kanekar & Aruin, 2014; Pai et al., 2003), thus increasing the risk of falling. These changes include decreased visual, vestibular, and somatosensory inputs, reduced muscle mass, power, and torque, altered spinal reflexes, diminished attentional capacity, and functional and structural changes in the brain (Melzer et al., 2001; Reid & Fielding, 2012; Woollacott et al., 1986). Age-associated impairments in brain structures such as the supplementary motor area and the foot area of the sensorimotor cortex may also be responsible for postural instability in old age (Slobounov et al., 2005), when the postural control is less automated (Laessoe & Voigt, 2008). Hence, older adults are more reliant on cognitive input and cortical information processing during motor tasks, as shown by additional activations of sensorimotor cortical areas (Heuninckx et al., 2005; Heuninckx et al., 2008; Venkatraman et al., 2010). Postural control in older adults is attentionally demanding, and the cerebral cortex, particularly the primary motor area and premotor and prefrontal cortex, shows high activity, playing a key role in maintaining balance following perturbations (Jacobs & Horak, 2007; Papegaaij et al., 2014; Smith et al., 2012).
Neurological studies have shown that retaining postural balance against external perturbations activates cognitive processes that are also involved in complicated mental activities such as attention, concentration, perception, and learning. These cognitive tasks, therefore, share a common cognitive source with postural control tasks, which reside mostly in the frontal and parietal lobes and might undergo atrophy during aging (Fraizer & Mitra, 2008; Lacour et al., 2008; Ohsugi et al., 2013). This common capacity even assumes greater importance when one tries to control balance while concomitantly performing a cognitive task. In this condition, known as dual-task (DT), the total attentional demand is escalated. However, the attentional capacity of the brain is limited, and the increased cognitive load would reduce attentional resources for postural control (Lacour et al., 2008). Evidence suggests that older people are more susceptible than younger people during postural recovery under DT conditions (Brown et al., 1999). Incurring external perturbations while performing a cognitive task is expected to challenge the postural stability of older adults. Meanwhile, earlier research has not evaluated the cortical neurodynamic features of such an enhanced susceptibility. Therefore, this study was designed to compare the cortical activity of healthy older adults versus young individuals during postural adjustment in response to external perturbations under single-task (ST) and DT conditions.
2. Materials and Methods
Study design and setting
Volunteers who met the eligibility criteria signed a written consent form after being debriefed about the trial. Having met the ethical code standard measures as per the Declaration of Helsinki, the experiment was approved by the Institutional Review Board of Shiraz University of Medical Sciences, Shiraz, Iran. The present research also followed the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.
Asymptomatic young and older individuals were recruited through a convenience sampling method. Participants stood barefoot with their feet 24 cm apart and a load massing about 3% of their body weight attached to a belt worn at their sternum level. To induce perturbations, the examiner could release the load at random time intervals of 5 s to 15 s between trials while the participants tried to maintain their postural balance. The experiment consisted of 15 ST and 15 DT trials. For the DT assessment, the individuals were asked to perform the same test while counting backward by 3s beginning from a random two-digit number. To prevent the risk of falling, an examiner stood by the participant during all experiments.
Study participants
Twenty older adults, aged over 65 years (mean age: 65.55±4.67 years), were recruited from Jahandidegan Day-Care Center for senior citizens (Shiraz, Iran), and 19 young students between 20 and 35 years old (mean age: 24.25±3.15 years) volunteered from Shiraz University of Medical Sciences, through flyers. Older adults could stand independently without using assistive devices. Further inclusion criteria for older adults included scores of ≥24 out of 30 in the mini-mental state examination (MMSE), <7 out of 15 in the geriatric depression scale (GDS), and ≥25 out of 40 in the Fullerton advanced balance (FAB) scale. Participants were excluded if they had any history of serious neuromuscular or musculoskeletal disorders (e.g. lower extremities surgeries or fractures, neuropathy, and arthritis), uncorrected vision impairments, vestibular deficits, auditory dysfunctions, severe pain or deformity in the trunk or lower extremities (e.g. scoliosis or kyphosis), or if they had taken any medication potentially affecting their balance within the past 24 hours. Pregnant young adults and individuals with a BMI >30 kg/m2 were also excluded. This dataset was previously utilized in a separate study that investigated cortical responses to predictable and unpredictable perturbations (Saadat et al., 2021).
Quantitative electroencephalography (QEEG) measures
Thirty-two silver-chloride surface electrodes (Medico Electrodes International Ltd., Uttar Pradesh, India) mounted on an electrocap were used for EEG signal recording based on the international 10-20 system. In this study, a 32-channel NrSign 3840 EEG amplifier (NrSign Inc., Vancouver, Canada) was used to collect the EEG data at a sampling rate of 500 Hz with a 2–120 Hz bandpass filter. The impedance of skin under the electrodes was kept below 5 kΩ using conductive gel, and the FPz electrode was regarded as the reference point in a monopolar montage.
Data processing
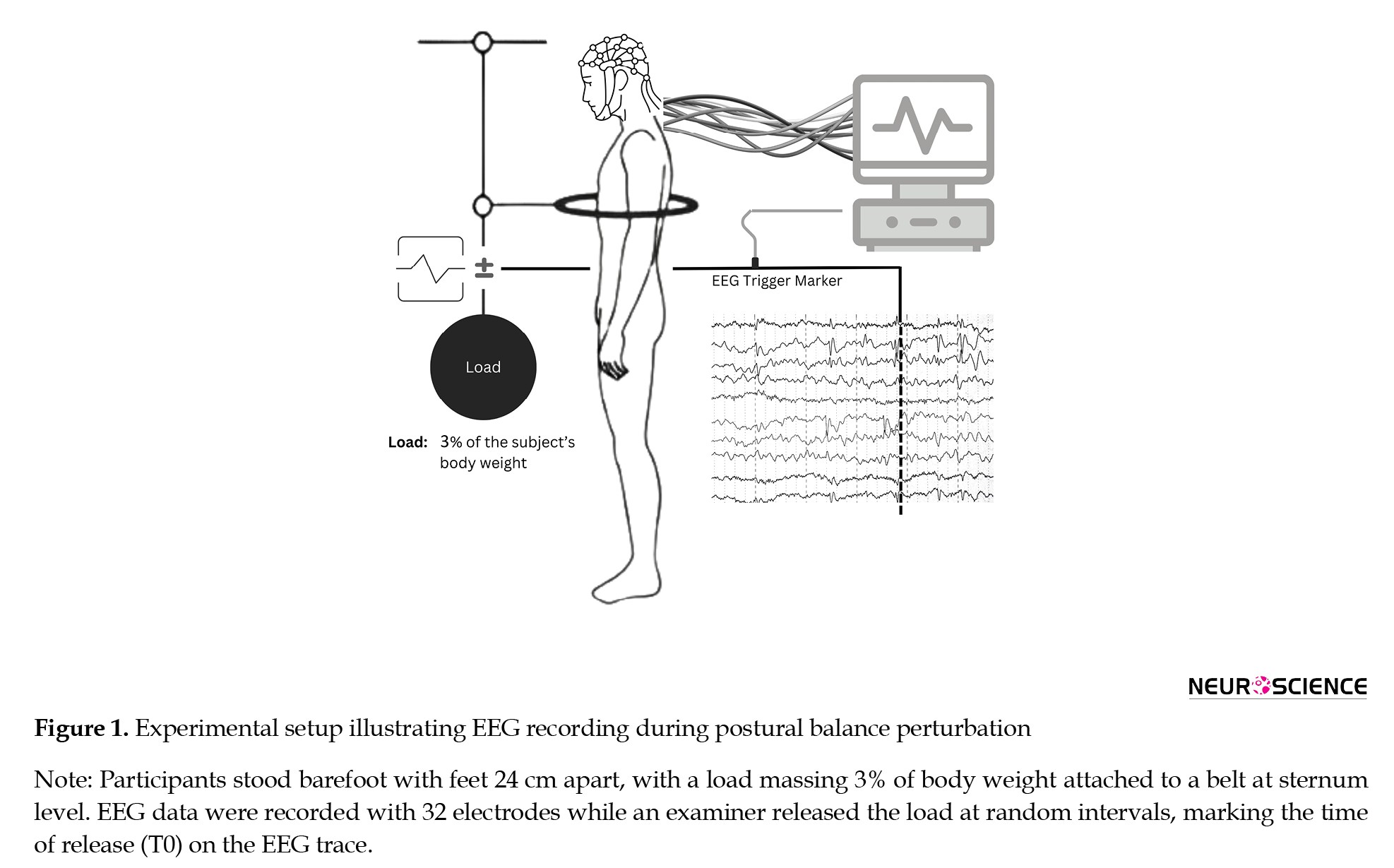
The EEG recordings were initially preprocessed for denoising using the EEGLab plugin in MATLAB software, then transformed into ASCII format to be further analyzed using the NeuroGuide Software (version 2.5.5, Applied Neuroscience, St. Petersburg, FL, USA). The synchronization of EEG data with the load release onset was achieved using a mechanical pedal system operated by the by-standing examiner. At the exact moment the load was released, the pedal generated a sharp electrical signal recorded as an event marker on a dedicated EEG channel, serving as the temporal reference (T0) for precise alignment of EEG data and perturbation onset. This marker was visually inspected during preprocessing to ensure accuracy. The analysis focused on two distinct periods: T1 (-1000 to -500 ms before T0) and T2 (-500 ms to the moment of T0) (Figure 1).
Postural instability and falls are among the most common problems in older adults. The changes in sensory, motor, and cognitive components of postural control can reduce older individuals’ postural adjustment in response to external distresses, such as slips and trips (Kanekar & Aruin, 2014; Pai et al., 2003), thus increasing the risk of falling. These changes include decreased visual, vestibular, and somatosensory inputs, reduced muscle mass, power, and torque, altered spinal reflexes, diminished attentional capacity, and functional and structural changes in the brain (Melzer et al., 2001; Reid & Fielding, 2012; Woollacott et al., 1986). Age-associated impairments in brain structures such as the supplementary motor area and the foot area of the sensorimotor cortex may also be responsible for postural instability in old age (Slobounov et al., 2005), when the postural control is less automated (Laessoe & Voigt, 2008). Hence, older adults are more reliant on cognitive input and cortical information processing during motor tasks, as shown by additional activations of sensorimotor cortical areas (Heuninckx et al., 2005; Heuninckx et al., 2008; Venkatraman et al., 2010). Postural control in older adults is attentionally demanding, and the cerebral cortex, particularly the primary motor area and premotor and prefrontal cortex, shows high activity, playing a key role in maintaining balance following perturbations (Jacobs & Horak, 2007; Papegaaij et al., 2014; Smith et al., 2012).
Neurological studies have shown that retaining postural balance against external perturbations activates cognitive processes that are also involved in complicated mental activities such as attention, concentration, perception, and learning. These cognitive tasks, therefore, share a common cognitive source with postural control tasks, which reside mostly in the frontal and parietal lobes and might undergo atrophy during aging (Fraizer & Mitra, 2008; Lacour et al., 2008; Ohsugi et al., 2013). This common capacity even assumes greater importance when one tries to control balance while concomitantly performing a cognitive task. In this condition, known as dual-task (DT), the total attentional demand is escalated. However, the attentional capacity of the brain is limited, and the increased cognitive load would reduce attentional resources for postural control (Lacour et al., 2008). Evidence suggests that older people are more susceptible than younger people during postural recovery under DT conditions (Brown et al., 1999). Incurring external perturbations while performing a cognitive task is expected to challenge the postural stability of older adults. Meanwhile, earlier research has not evaluated the cortical neurodynamic features of such an enhanced susceptibility. Therefore, this study was designed to compare the cortical activity of healthy older adults versus young individuals during postural adjustment in response to external perturbations under single-task (ST) and DT conditions.
2. Materials and Methods
Study design and setting
Volunteers who met the eligibility criteria signed a written consent form after being debriefed about the trial. Having met the ethical code standard measures as per the Declaration of Helsinki, the experiment was approved by the Institutional Review Board of Shiraz University of Medical Sciences, Shiraz, Iran. The present research also followed the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.
Asymptomatic young and older individuals were recruited through a convenience sampling method. Participants stood barefoot with their feet 24 cm apart and a load massing about 3% of their body weight attached to a belt worn at their sternum level. To induce perturbations, the examiner could release the load at random time intervals of 5 s to 15 s between trials while the participants tried to maintain their postural balance. The experiment consisted of 15 ST and 15 DT trials. For the DT assessment, the individuals were asked to perform the same test while counting backward by 3s beginning from a random two-digit number. To prevent the risk of falling, an examiner stood by the participant during all experiments.
Study participants
Twenty older adults, aged over 65 years (mean age: 65.55±4.67 years), were recruited from Jahandidegan Day-Care Center for senior citizens (Shiraz, Iran), and 19 young students between 20 and 35 years old (mean age: 24.25±3.15 years) volunteered from Shiraz University of Medical Sciences, through flyers. Older adults could stand independently without using assistive devices. Further inclusion criteria for older adults included scores of ≥24 out of 30 in the mini-mental state examination (MMSE), <7 out of 15 in the geriatric depression scale (GDS), and ≥25 out of 40 in the Fullerton advanced balance (FAB) scale. Participants were excluded if they had any history of serious neuromuscular or musculoskeletal disorders (e.g. lower extremities surgeries or fractures, neuropathy, and arthritis), uncorrected vision impairments, vestibular deficits, auditory dysfunctions, severe pain or deformity in the trunk or lower extremities (e.g. scoliosis or kyphosis), or if they had taken any medication potentially affecting their balance within the past 24 hours. Pregnant young adults and individuals with a BMI >30 kg/m2 were also excluded. This dataset was previously utilized in a separate study that investigated cortical responses to predictable and unpredictable perturbations (Saadat et al., 2021).
Quantitative electroencephalography (QEEG) measures
Thirty-two silver-chloride surface electrodes (Medico Electrodes International Ltd., Uttar Pradesh, India) mounted on an electrocap were used for EEG signal recording based on the international 10-20 system. In this study, a 32-channel NrSign 3840 EEG amplifier (NrSign Inc., Vancouver, Canada) was used to collect the EEG data at a sampling rate of 500 Hz with a 2–120 Hz bandpass filter. The impedance of skin under the electrodes was kept below 5 kΩ using conductive gel, and the FPz electrode was regarded as the reference point in a monopolar montage.
Data processing
The EEG recordings were initially preprocessed for denoising using the EEGLab plugin in MATLAB software, then transformed into ASCII format to be further analyzed using the NeuroGuide Software (version 2.5.5, Applied Neuroscience, St. Petersburg, FL, USA). The synchronization of EEG data with the load release onset was achieved using a mechanical pedal system operated by the by-standing examiner. At the exact moment the load was released, the pedal generated a sharp electrical signal recorded as an event marker on a dedicated EEG channel, serving as the temporal reference (T0) for precise alignment of EEG data and perturbation onset. This marker was visually inspected during preprocessing to ensure accuracy. The analysis focused on two distinct periods: T1 (-1000 to -500 ms before T0) and T2 (-500 ms to the moment of T0) (Figure 1).
For each group of 15 experimental trials, the alpha (8–12 Hz) and beta (12.5–25 Hz) powers were first computed for each trial individually. Then, they averaged across the 15 trials to determine the mean absolute power, expressed in square microvolts (µV²).
The EEG data recorded over bihemispheric sensorimotor and primary motor areas (C3 and C4) were scrutinized. Additionally, a power spectral analysis was conducted on the frontal (Fz, F3, F4), central (Cz), and parietal (Pz) regions to elucidate the EEG signals’ characteristics further.
Statistical analyses
The normality of data distribution was confirmed with the Shapiro–Wilk test (P>0.05), and descriptive statistics were used to present the demographic characteristics of the two groups. The QEEG data were analyzed with SPSS software, version 22 through a 3-way mixed design analysis of variance to assess the absolute power z-scores in alpha and beta frequency bands, with one ‘between-subject factor’ (group: Older vs young adults), and two ‘within-subject factors’ including condition (ST vs DT) and time (T1 and T2). A P<0.05 was established a priori to determine statistical significance.
3. Results
Demographic characteristics and baseline values of 39 participants are reported in Table 1.

As outlined in Table 2, the group×time interaction was significant for alpha power across the derivations.

Also, the main effect of the condition was significant for alpha power in the C4 and PZ regions. In addition, the post hoc analysis of conditions revealed that the power was significantly greater under the DT condition in C4 and PZ (Table 2).
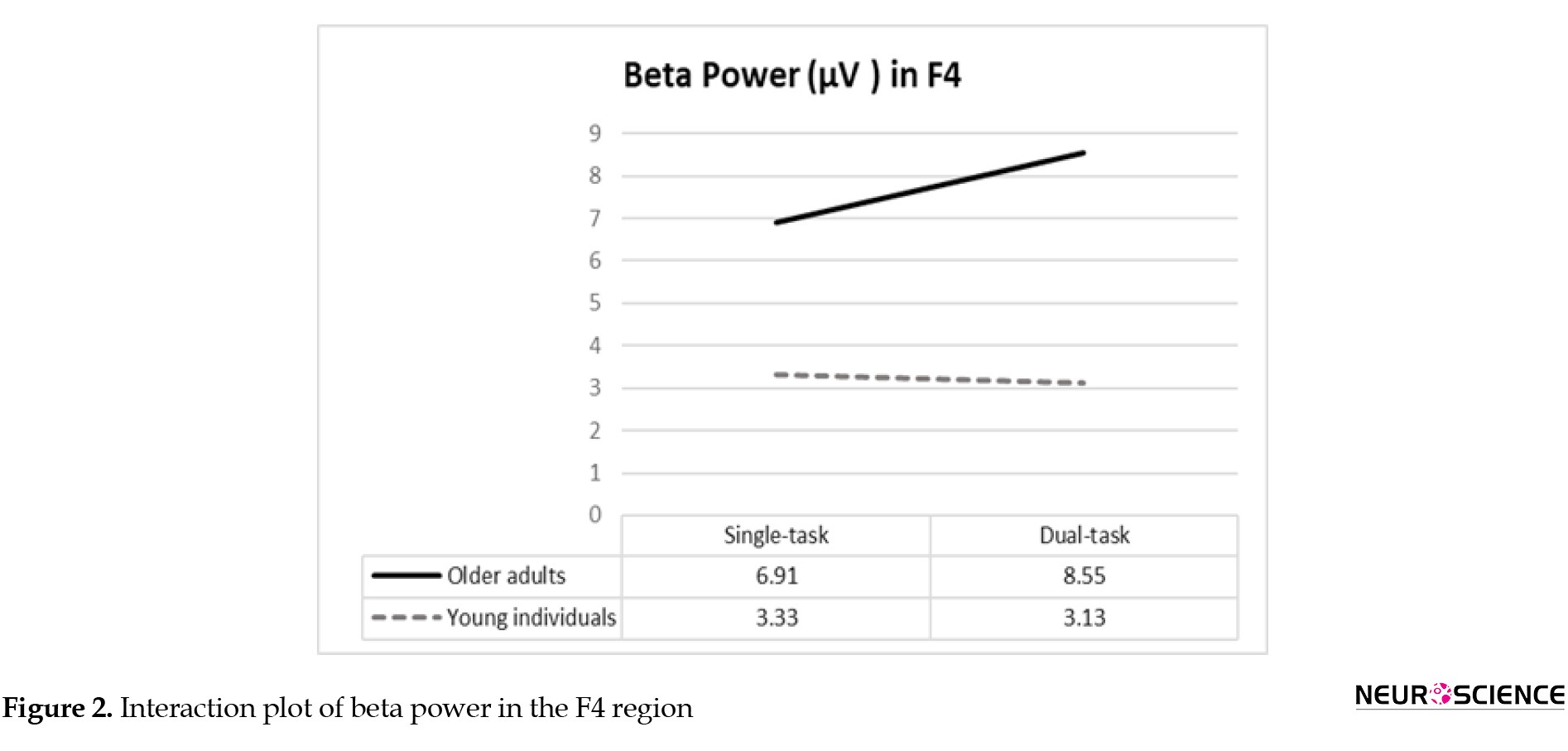
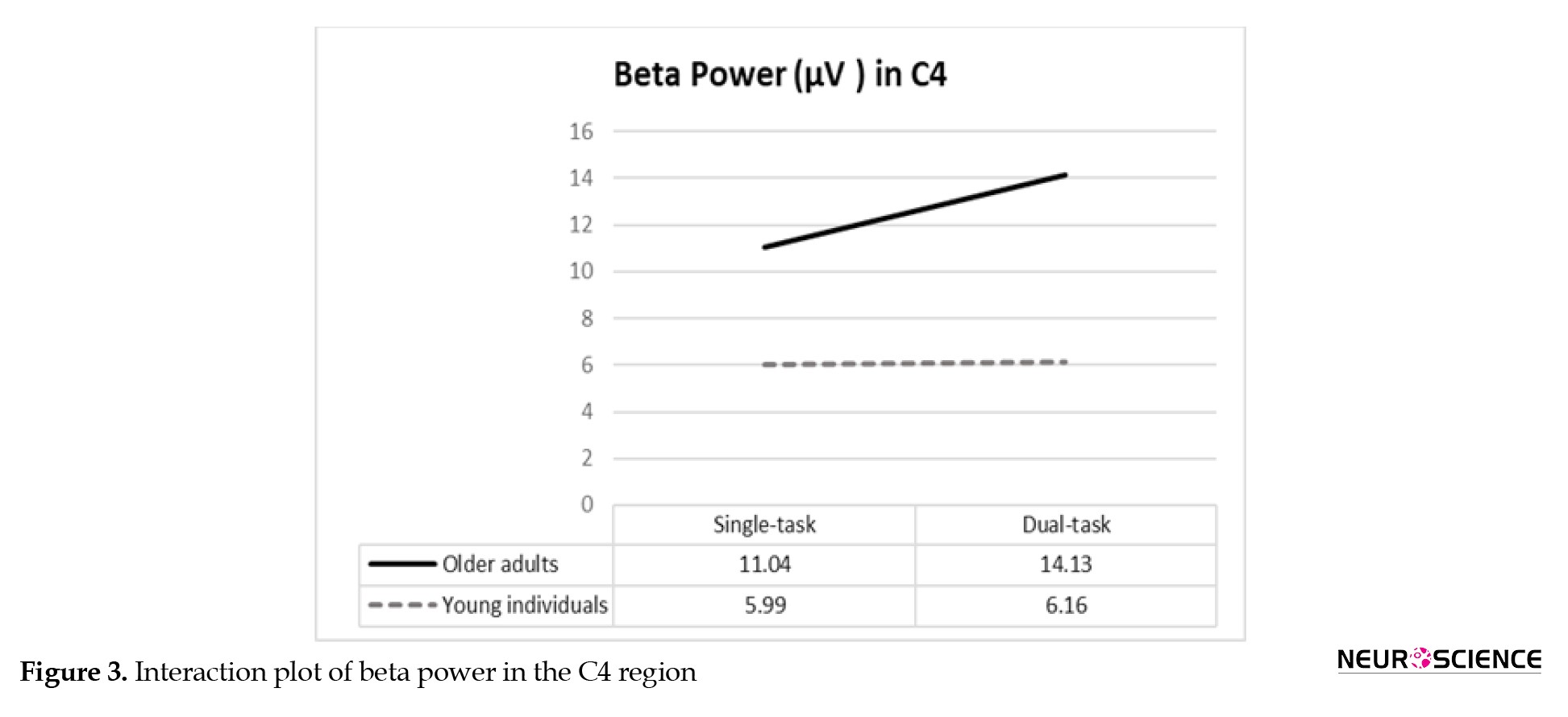
For the beta power, the group×condition interaction was significant in F4 and C4 regions, and post hoc analyses demonstrated significantly greater beta power values in older adults during dual tasks compared to the younger group (Table 3).

Findings on the group×condition for beta absolute power in F4 and C4 cortical regions are summarized in Figures 2 and 3.
The EEG data recorded over bihemispheric sensorimotor and primary motor areas (C3 and C4) were scrutinized. Additionally, a power spectral analysis was conducted on the frontal (Fz, F3, F4), central (Cz), and parietal (Pz) regions to elucidate the EEG signals’ characteristics further.
Statistical analyses
The normality of data distribution was confirmed with the Shapiro–Wilk test (P>0.05), and descriptive statistics were used to present the demographic characteristics of the two groups. The QEEG data were analyzed with SPSS software, version 22 through a 3-way mixed design analysis of variance to assess the absolute power z-scores in alpha and beta frequency bands, with one ‘between-subject factor’ (group: Older vs young adults), and two ‘within-subject factors’ including condition (ST vs DT) and time (T1 and T2). A P<0.05 was established a priori to determine statistical significance.
3. Results
Demographic characteristics and baseline values of 39 participants are reported in Table 1.

As outlined in Table 2, the group×time interaction was significant for alpha power across the derivations.

Also, the main effect of the condition was significant for alpha power in the C4 and PZ regions. In addition, the post hoc analysis of conditions revealed that the power was significantly greater under the DT condition in C4 and PZ (Table 2).
For the beta power, the group×condition interaction was significant in F4 and C4 regions, and post hoc analyses demonstrated significantly greater beta power values in older adults during dual tasks compared to the younger group (Table 3).

Findings on the group×condition for beta absolute power in F4 and C4 cortical regions are summarized in Figures 2 and 3.
4. Discussion
This observational study investigated the cortical activity of young and older adults during postural adjustments following external perturbations under both ST and DT conditions. Our results revealed that older adults exhibited increased frontoparietal alpha power during the late phase of recovery from external perturbations, particularly during DT execution. These findings align with previous research, which suggests that aging is accompanied by compensatory neural mechanisms to maintain postural control despite age-related declines in sensory and motor function (Chang et al., 2016; Papegaaij et al., 2014).
We selected the alpha and beta frequency bands due to their well-established relevance in postural control and cognitive-motor interactions. Alpha activity reflects attentional engagement and cortical involvement, while beta activity is associated with motor planning and sensorimotor integration, both critical for balance recovery. The chosen channels also target cortical regions implicated in postural control, such as the sensorimotor, primary motor, and supplementary motor areas. This focused approach allowed us to assess the neural dynamics most directly involved in maintaining stability under ST and DT conditions (Ghosn et al., 2020; Protzak & Gramann, 2021).
The increased alpha power in the sensorimotor and supplementary motor areas observed in older adults during DT conditions is consistent with evidence that postural control becomes more cognitively demanding with age (Jacobs & Horak, 2007). Motor control in older individuals relies more heavily on cortical regions, including the prefrontal cortex, for compensatory strategies when regaining postural stability following perturbations (Papegaaij et al., 2014). This shift from subcortical to cortical control mechanisms may be attributed to age-related degeneration in somatosensory receptors and reduced conduction velocity, both of which compromise the automaticity of postural control (Papegaaij et al., 2014; Sturnieks et al., 2008).
This study builds upon our previous work, which investigated cortical responses to predictable and unpredictable perturbations using the same dataset (Saadat et al., 2021). While the earlier study highlighted the role of anticipation versus reaction in postural control, the current analysis shifts focus to the cognitive-motor interplay under DT conditions. By examining the influence of an additional cognitive task on cortical activity during postural recovery, this study extends our understanding of DT interference and its implications for fall risk in older adults. The present findings offer actionable insights for fall prevention strategies that integrate both motor and cognitive training, a dimension not explored in the initial analysis.
The cortical overactivation observed as increased power z-scores during DT conditions likely represents an effort to engage additional neural resources to manage the increased attentional demands (Reuter-Lorenz & Cappell, 2008). While this compensatory mechanism supports balance control, it may reduce the availability of cognitive resources for other tasks, explaining the greater DT interference observed in older adults (Palmer et al., 2021).
In line with the dedifferentiation theory, the reduced neural specificity seen in older adults may also contribute to this cortical overactivation (Morcom & Henson, 2018). With age, the brain becomes less efficient in segregating neural processes, leading to broader recruitment of cortical areas even for tasks that, in younger adults, are more localized to subcortical regions (Alizadehsaravi et al., 2020). This reduced efficiency in neural processing is particularly evident in DT scenarios, where the cognitive load further strains the brain’s limited attentional resources (Lacour et al., 2008).
Our study also found elevated beta power in the prefrontal and sensorimotor regions during DT conditions, particularly in older adults. Beta oscillations are closely linked to the cognitive aspects of motor control and are indicative of heightened cortical engagement during more demanding tasks (Teasdale & Simoneau, 2001). The increased beta power observed in older adults suggests that they require more cognitive effort to maintain balance under DT conditions, reflecting the additional neural processing required to integrate motor and cognitive tasks simultaneously.
This enhanced cortical activity in older adults may serve as a biomarker for reduced automaticity in balance control (Ghosn et al., 2020). As the task becomes more challenging, the central nervous system recruits higher-level cortical resources to compensate for the loss of subcortical control (Pizzamiglio et al., 2017). This reliance on cortical control could explain the difficulty older adults face in maintaining postural stability while concurrently performing cognitive tasks (Ghosn et al., 2020; Palmer et al., 2021).
Our results support the idea that brain oscillations, particularly in the alpha and beta frequency bands, play a critical role in modulating connectivity between different brain regions during postural tasks (Malcolm et al., 2021). Young and older adults seem to employ different neural strategies when managing DT conditions, with older adults showing less flexibility in reallocating neural resources (Malcolm et al., 2021). The greater engagement of cortical areas in older adults under DT conditions reflects an age-related shift in sensorimotor processing that compensates for the decline in subcortical mechanisms that once governed automatic postural control (Ghosn et al., 2020).
The reliance on these cortical networks may also indicate a lower threshold for eliciting stepping reactions, especially in cognitively demanding situations (Palmer et al., 2021). This condition has important implications for understanding fall risk in older adults, as greater reliance on cortical resources for postural control may lead to slower or less efficient balance recovery (Clark, 2015; Ghosn et al., 2020).
Taken together, our findings suggest the role of task complexity in potentially shaping neural responses during postural adjustments. Accordingly, depending on the cognitive demands, older adults demonstrated heightened challenges in maintaining balance. Such an observation underscores the implication of considering DT scenarios in balance assessments and interventions. Furthermore, the distinction in neural activation patterns between young and older adults proposes that age-related neural adaptations are not merely compensatory and refer to fundamental shifts in how balance is processed within the brain. In other words, since older adults engage more cortical resources in DT perturbations, an increased reliance can lead to a fragile balance system, particularly under complex conditions.
The implications of our findings extend to practical applications in fall prevention strategies. Interventions designed to enhance postural control in older adults could benefit from incorporating cognitive tasks that simulate real-world challenges. Such an approach could help mitigate the neurocognitive burden associated with DT, potentially leading to improved stability and reduced fall risk. Additionally, understanding the specific neural mechanisms underlying balance control in older adults can guide the development of targeted training programs to improve or empower both motor and cognitive functions, leading to a collective approach to maintaining functional independence in aging populations. Future research could expand on these findings by exploring additional cortical regions and frequency bands, including gamma oscillations, to provide a more comprehensive understanding of the neural dynamics underlying balance and DT performance.
5. Conclusion
The findings of this study highlight the significant role of cortical activity in postural adjustments in older adults, particularly during DT conditions. The increased alpha and beta power in the sensorimotor and prefrontal regions points to compensatory mechanisms that enable older adults to maintain balance despite age-related declines in sensorimotor function. These findings contribute to the growing body of evidence that cortical overactivation is a hallmark of aging and suggest that targeted interventions aimed at improving automaticity in balance control could mitigate DT interference and reduce fall risk in older adults.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.REC.1396.26).
Funding
This study was granted by Shiraz University of Medical Sciences, Shiraz, Iran (Grant No.: 95-01-06-13248).
Authors' contributions
Conceptualization, methodology, investigation, review, and editing: All authors; Writing the original draft: Ehsan Sinaei and Zahra Saadat; Funding acquisition and resources: Zahra Saadat and Mohammad Nami; Supervision: Mohammad Nami.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Roya Razavi, the manager of the Jahandidegan Day-Care Center, Shiraz, Iran, and all individuals who volunteered for this trial.
References
Alizadehsaravi, L., Bruijn, S. M., Maas, H., & van Dieën, J. H. (2020). Modulation of soleus muscle H-reflexes and ankle muscle co-contraction with surface compliance during unipedal balancing in young and older adults. Experimental Brain Research, 238(6), 1371–1383. [DOI:10.1007/s00221-020-05784-0] [PMID]
Brown, L. A., Shumway-Cook, A., & Woollacott, M. H. (1999). Attentional demands and postural recovery: The effects of aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 54(4), M165–M171. [DOI:10.1093/gerona/54.4.M165] [PMID]
Chang, C. J., Yang, T. F., Yang, S. W., & Chern, J. S. (2016). Cortical modulation of motor control biofeedback among the elderly with high fall risk during a posture perturbation task with augmented reality. Frontiers in Aging Neuroscience, 8, 80. [DOI:10.3389/fnagi.2016.00080] [PMID]
Clark, D. J. (2015). Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Frontiers in Human Neuroscience, 9, 246. [DOI:10.3389/fnhum.2015.00246] [PMID]
Fraizer, E. V., & Mitra, S. (2008). Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait & Posture, 27(2), 271-279. [DOI:10.1016/j.gaitpost.2007.04.002] [PMID]
Ghosn, N. J., Palmer, J. A., Borich, M. R., Ting, L. H., & Payne, A. M. (2020). Cortical beta oscillatory activity evoked during reactive balance recovery scales with perturbation difficulty and individual balance ability. Brain Sciences, 10(11), 860. [DOI:10.3390/brainsci10110860] [PMID]
Heuninckx, S., Wenderoth, N., Debaere, F., Peeters, R., & Swinnen, S. P. (2005). Neural basis of aging: The penetration of cognition into action control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(29), 6787–6796. [DOI:10.1523/JNEUROSCI.1263-05.2005] [PMID]
Heuninckx, S., Wenderoth, N., & Swinnen, S. P. (2008). Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(1), 91–99. [DOI:10.1523/JNEUROSCI.3300-07.2008] [PMID]
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339-1348. [DOI:10.1007/s00702-007-0657-0] [PMID]
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of Neural Transmission, 114(10), 1339-1348. [DOI:10.1007/s00702-007-0657-0] [PMID]
Kanekar, N., & Aruin, A. S. (2014). The effect of aging on anticipatory postural control. Experimental Brain Research, 232(4), 1127-1136. [DOI:10.1007/s00221-014-3822-3] [PMID]
Lacour, M., Bernard-Demanze, L., & Dumitrescu, M. (2008). Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiologie Clinique/Clinical Neurophysiology, 38(6), 411-421. [DOI:10.1016/j.neucli.2008.09.005] [PMID]
Laessoe, U., & Voigt, M. (2008). Anticipatory postural control strategies related to predictive perturbations. Gait & Posture, 28(1), 62-68. [DOI:10.1016/j.gaitpost.2007.10.001] [PMID]
Malcolm, B. R., Foxe, J. J., Joshi, S., Verghese, J., Mahoney, J. R., & Molholm, S., et al. (2021). Aging‐related changes in cortical mechanisms supporting postural control during base of support and optic flow manipulations. The European Journal of Neuroscience, 54(12), 8139–8157. [DOI:10.1111/ejn.15004] [PMID]
Melzer, I., Benjuya, N., & Kaplanski, J. (2001). Age-related changes of postural control: Effect of cognitive tasks. Gerontology, 47(4), 189-194. [DOI:10.1159/000052797] [PMID]
Morcom, A. M., & Henson, R. N. (2018). Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. The Journal of Neuroscience, 38(33), 7303-7313. [DOI:10.1523/JNEUROSCI.1701-17.2018] [PMID]
Ohsugi, H., Ohgi, S., Shigemori, K., & Schneider, E. B. (2013). Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neuroscience, 14, 10. [DOI:10.1186/1471-2202-14-10] [PMID]
Pai, Y. C., Wening, J. D., Runtz, E. F., Iqbal, K., & Pavol, M. J. (2003). Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. Journal of Neurophysiology, 90(2), 755-762. [DOI:10.1152/jn.01118.2002] [PMID]
Palmer, J. A., Payne, A. M., Ting, L. H., & Borich, M. R. (2021). Cortical engagement metrics during reactive balance are associated with distinct aspects of balance behavior in older adults. Frontiers in Aging Neuroscience, 13, 684743. [DOI:10.3389/fnagi.2021.684743] [PMID]
Papegaaij, S., Taube, W., Baudry, S., Otten, E., & Hortobágyi, T. (2014). Aging causes a reorganization of cortical and spinal control of posture. Frontiers in Aging Neuroscience, 6, 28. [DOI:10.3389/fnagi.2014.00028] [PMID]
Pizzamiglio, S., Naeem, U., Abdalla, H., & Turner, D. L. (2017). Neural correlates of single-and dual-task walking in the real world. Frontiers in Human Neuroscience, 11, 460. [DOI:10.3389/fnhum.2017.00460] [PMID]
Protzak, J., & Gramann, K. (2021). EEG beta-modulations reflect age-specific motor resource allocation during dual-task walking. Scientific Reports, 11(1), 16110. [DOI:10.1038/s41598-021-94874-2] [PMID]
Reid, K. F., & Fielding, R. A. (2012). Skeletal muscle power: A critical determinant of physical functioning in older adults. Exercise and Sport Sciences Reviews, 40(1), 4-12. [DOI:10.1097/JES.0b013e31823b5f13] [PMID]
Reuter-Lorenz, P. A., & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177-182. [DOI:10.1111/j.1467-8721.2008.00570.x]
Saadat, Z., Sinaei, E., Pirouzi, S., Ghofrani, M., & Nami, M. (2021). Cortical activity during postural recovery in response to predictable and unpredictable perturbations in healthy young and older adults: A quantitative eeg assessment. Basic and Clinical Neuroscience, 12(2), 291-300. [DOI:10.32598/bcn.12.2.453.1] [PMID]
Slobounov, S., Hallett, M., Stanhope, S., & Shibasaki, H. (2005). Role of cerebral cortex in human postural control: An EEG study. Clinical Neurophysiology, 116(2), 315-323. [DOI:10.1016/j.clinph.2004.09.007] [PMID]
Smith, B. A., Jacobs, J. V., & Horak, F. B. (2012). Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Experimental Brain Research, 222(4), 455-470. [DOI:10.1007/s00221-012-3232-3] [PMID]
Sturnieks, D. L., St George, R., & Lord, S. R. (2008). Balance disorders in the elderly. Neurophysiologie Clinique/Clinical Neurophysiology, 38(6), 467-478. [DOI:10.1016/j.neucli.2008.09.001] [PMID]
Teasdale, N., & Simoneau, M. (2001). Attentional demands for postural control: The effects of aging and sensory reintegration. Gait & Posture, 14(3), 203-210. [DOI:10.1016/S0966-6362(01)00134-5] [PMID]
Venkatraman, V. K., Aizenstein, H., Guralnik, J., Newman, A. B., Glynn, N. W., & Taylor, C., et al. (2010). Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage, 49(4), 3436-3442. [DOI:10.1016/j.neuroimage.2009.11.019] [PMID]
Woollacott, M. H., Shumway-Cook, A., & Nashner, L. M. (1986). Aging and posture control: Changes in sensory organization and muscular coordination. International Journal of Aging and Human Development, 23(2), 97-114. [DOI:10.2190/VXN3-N3RT-54JB-X16X] [PMID]
Type of Study: Original |
Subject:
Computational Neuroscience
Received: 2024/10/28 | Accepted: 2025/01/18 | Published: 2025/07/1
Received: 2024/10/28 | Accepted: 2025/01/18 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |