Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 641-656 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zhang P, Xiao H. Vitis Vinifera L. Flavones Preserve Mitophagy in the Amyloid-beta 1-42-induced Model of Alzheimer’s Disease Neurodegeneration. BCN 2025; 16 (3) :641-656
URL: http://bcn.iums.ac.ir/article-1-2934-en.html
URL: http://bcn.iums.ac.ir/article-1-2934-en.html
1- College of Public Health, Xinjiang Medical University, Urumqi, China.
Keywords: Alzheimer’s disease, Vitis vinifera L. flavones (VTF), Chloroquine (CQ), Amyloid-beta (Aβ)1-42-Aβ1-42-induced neurodegeneration, Mitophagy, Neuroprotective efficacy
Full-Text [PDF 3013 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease, affecting 18% of the Chinese population (Chan et al., 2013; Jia et al., 2020). The National Aged Population Center of China predicts a significant increase in the impact of AD among individuals aged >60 years, reaching approximately 60 million over the next decade. This highlights AD as a major concern in China’s public healthcare system (Kolachala et al., 2021; Li et al., 2018; Wang et al., 2008).
AD is characterized by a complex network of pathophysiological pathways influenced by genetic, environmental, and lifestyle factors. Inflammation, heightened oxidative stress, and compromised synaptic function play significant roles in disease progression, complicating therapeutic interventions (De-Paula et al., 2012; Li et al., 2023). A nuanced understanding of the molecular intricacies of AD is imperative to develop targeted strategies for this multifaceted neurodegenerative disorder (Skaper, 2012).
Autophagy, driven by the formation of autophagosomes, plays a central role in self-protection during AD progression, eliminating surplus intracellular peptides and damaged organelles (Chung et al., 2019; Klionsky et al., 2021; Krishnan et al., 2020). A significant focus revolves around the initiation of amyloid-beta (Aβ) clusters and entangled Tau proteins, considered pivotal biomarkers in AD therapy (Bloom, 2014; Fonseca et al., 2013). Aβ, derived from amyloid precursor protein (APP), undergoes abnormal aggregation to form plaques (Salminen et al., 2013). These Aβ clusters, characterized by misfolded proteins, act as focal points in the observed neurodegenerative cascade in AD. Aβ peptides in AD include Aβ1-42 and Aβ1, the former being a pathogenic variant with 42 amino acids (Aβ1-42), exhibiting an elevated propensity for aggregation and the formation of toxic oligomers, which significantly contribute to neurodegeneration (Sepulcre et al., 2017). In contrast, Aβ1, a shorter counterpart, is generated during APP cleavage, displaying a comparatively lower inclination for aggregation. The interplay between these forms underscores their distinct roles in the complex Aβ cascade, influencing the pathophysiology of AD (Jackson et al., 2016). However, in advanced stages of AD, impaired autophagy may lead to Aβ1-42 and Aβ1 accumulation, exacerbating neurodegenerative processes. Balancing the interplay between Aβ clusters and autophagy dynamics holds potential for targeted therapeutic intervention. Modulating autophagy to enhance Aβ clearance or inhibit Aβ cluster formation presents promising avenues for further exploration in AD research (Dunyset al., 2018).
Numerous in vitro and in vivo studies have investigated polyphenolic compounds from Vitis vinifera L. flavones (VTF), a herb rich in cholinergic neurotransmitters, widely used in traditional Chinese medicine (Rodriguez-Mateos et al., 2014). These flavonoids have been shown to prevent damage to hippocampal neurons by inhibiting autophagy and promoting anti-neurodegenerative effects (Benavente-García & Castillo, 2008). Flavonoids from VTF act as oxygen-free radical scavengers and antioxidants, stimulating synaptic plasticity and improving cognitive impairment in AD-model mice (Ma et al., 2018). Our latest report demonstrated that VTF can influence the pathological changes of AD by regulating hippocampal neurons via autophagy in APP/presenilin 1 (PS1) transgenic sedentary Alzheimer model mice. However, it remains unclear whether VTF can prevent hippocampal neuron damage by inhibiting autophagy and Aβ clustering (Joseph et al., 2023; Lopresti et al., 2023).
This study aimed to investigate the anti-neurodegenerative role of VTF in greater detail, exploring its relationship with autophagy and Aβ aggregation. This study aimed to elucidate the in vitro mechanism by which VTF protects neurons from Aβ1-42-induced neurodegeneration. Analyzing changes in Aβ1-42-induced autophagy-related protein expression in brain tissues will shed light on VTF’s potential to suppress excessive autophagy in Aβ1-42-induced SH-SY5Y cells. These findings may offer promising avenues for AD research, indicating that VTF’s neuroprotective effects could be attributed to the inhibition of excessive autophagy in Aβ1-42-induced SH-SY5Y cells.
2. Materials and Methods
Preparation of flavones from V. vinifera L.
V. vinifera L. grapes from the Vitaceae family were sourced from a reputable Uyghur medicine market in Turpan, Xinjiang Province, China, one week before initiating the experiment. The seeds were meticulously collected in August 2023 from the Gaochang District, Turpan City, located in the northwest region of the city, by PZ (GPS coordinates: 42.9225° N, 89.1913° E). The botanical identification of the plant was confirmed as V. vinifera L., commonly known as “European grapes” in Chinese (Ōuzhōu pútáo), at the National Herbarium of China. After collection, the seeds were carefully air-dried to eliminate excess moisture, ensuring optimal extraction efficiency. Subsequently, the dried seeds were finely ground into a powder with a particle size of approximately 20 mg, ensuring uniformity and consistency in the extraction process. The powder underwent meticulous extraction using 95% ethanol as the solvent for a precisely controlled duration of 2 h, following standard extraction protocols (Li et al., 2020). Following extraction, the resulting mixture underwent rotary evaporation under controlled conditions to remove the ethanol solvent, yielding a crude extract with enhanced purity and concentration. The yield of the crude extract was determined to be 170 g, designated as DCB. The supernatant obtained after rotary evaporation was carefully preserved for subsequent analysis to prevent the loss of valuable components. To further enrich and purify the extracted flavonoids, a series of sophisticated purification steps were meticulously executed. This purification process involved suspending the crude extract in water, followed by purification with AB-8 resin, a highly efficient adsorbent material known for its excellent selectivity and flavonoid adsorption capacity. The purification process was carried out using a precise gradient of 5% water and 95% ethanol to achieve optimal separation and purification of the target compounds. Subsequently, a 50% ethanol elution fraction containing highly enriched flavonoids was collected for processing. Finally, the purified VTF was obtained as a high-quality brown-yellow powder through meticulous vacuum drying at a controlled temperature of 60 °C after freeze-drying of the purified fraction. This final purification step, with a yield of extracts exceeding 92%, ensured the removal of any residual moisture and solvent traces, resulting in the production of highly pure and concentrated flavones ready for further analysis and biological evaluation. As detailed in previous results (Abdul Manap et al., 2020), chemical analysis of VTF extracts revealed a rich presence of flavonoids and stilbenes, with resveratrol (3,5,4’-trihydroxy-trans-stilbene) standing out prominently. Additionally, compounds like Quercetin (2-[3,4-dihydroxyphenyl]-3,5,7-trihydroxychromen-4-one), Kaempferol (3,5,7-trihydroxy-2-[4-hydroxyphenyl]chromen-4-one), and Myricetin (3,5,7-trihydroxy-2-[3,4,5-trihydroxyphenyl]chromen-4-one) were consistently identified as key components within VTF. This comprehensive examination significantly advances our understanding of the potential health benefits and therapeutic applications of these compounds.
Preparation of Aβ1-42 oligomers
Aβ1–42 oligomers were prepared according to the protocol described by Stine et al. (2003). The flavonoid treatment approach was based on the neuroprotective findings of Rezai-Zadeh et al., 2009. Initially, 5 mg of lyophilized Aβ1-42 (Sigma-Aldrich, St. Louis, MO, USA) was equilibrated at room temperature for 30 minutes to prevent condensation upon unsealing. The peptide was then suspended in ice-cold 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to achieve a one mM solution. After brief vortexing, the Aβ1-42/HFIP solution was incubated in polypropylene vials using a glass GasTight Hamilton syringe with a Teflon plug for 2 h to monomerize Aβ. The subsequent concentration of the Aβ1-42/HFIP solution under vacuum in a SpeedVac centrifuge (800 g, room temperature) produced a clear peptide film. Stringent temperature control (maintained below 25 °C) prevented peptide degradation during this process. The Aβ1-42 film was resuspended in dimethyl sulfoxide (DMSO) containing 10% fetal bovine serum, yielding a concentration of 400 μmol/L.
Preparation of chloroquine (CQ)
In this study, CQ, a lysosomal inhibitor, was used to modulate autophagy. A 50 mM stock solution of CQ diphosphate salt (Sigma-Aldrich, St. Louis, MO, USA) was prepared, dissolved in DMSO, sterilized by filtration (0.2 micrometers), and stored in aliquots at -20 ºC until use. Work solutions were diluted in 84% (v/v) mouse embryonic fibroblasts (MEFs; Thermo Fisher Scientific, Waltham, MA, USA), with 15.0% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (P/S; Sigma-Aldrich, St Louis, MO, USA). The cells were treated with CQ in a dose-dependent manner, ranging from 0 to 100 μM.
Cell culture and treatment
The SH-SY5Y human-derived neuroblastoma cell line (American Type Culture Collection [ATCC] CRL-2266) was sourced from the American Type Culture Collection (ATCC) (Manassas, VA, United States) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (P/S; Sigma-Aldrich, St Louis, MO, USA). The culture conditions were maintained in a constant temperature incubator at 37 °C with 5% carbon dioxide (CO2) to optimize cell growth. Regular monitoring for mycoplasma contamination was implemented to preserve the integrity of the cell lines, ensuring that SH-SY5Y cells remained free of potential contamination during experimental procedures. Stock solutions were diluted in DMEM with 1.0% (v/v) FBS to prepare working solutions. The study included the following treatment groups: Control (60 μM phosphate-buffered saline [PBS]), vehicle control (20 μM Aβ1-42 oligomer), VTF (80 mg/L VTF + 20 μM Aβ1-42 oligomer), CQ (40 μM CQ+20 μM Aβ1-42 oligomer), and VTF+CQ (80 mg/L VTF+40 μM CQ+20 μM Aβ1-42 oligomer).
Cell proliferation assays
The induction of cell proliferation by Aβ1-42 was assessed using a cell counting kit (CCK-8 assay, A311-02, Vazyme, Nanjing, China). Initially, cells (5×104 cells/well) were seeded in 96-well plates at a predetermined density. After treatments with VTF and CQ, CCK-8 solution was added to each well, followed by incubation. Absorbance was measured at 450 nm using a multimode microplate reader (Thermo Fisher Scientific Inc., MA, United States). Cell viability was quantified as a percentage relative to the absorbance of control cells.
Immunofluorescent (IFC) assay
An IFC assay was conducted following established protocols to assess the impact of VTF on Aβ1-42-induced Aβ deposition in SH-SY5Y cells (Abdul Manap et al., 2020). SH-SY5Y cells were seeded at a density of 5×104 cells/well in IbiTreat chamber slides (Ibidi GmbH, Martinsried, Germany) and incubated at 37 °C in a humidified 5% carbon dioxide (CO2) incubator. Once the cells reached 80% confluency, they were treated with 25 mM Aβ fibrils for 24 h. Following treatment, the cells were washed thrice with PBS, fixed with 4% paraformaldehyde (Aldrich, Steinheim, Germany) for 15 min, and then washed thrice with PBS. The fixed cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, US), diluted in PBS for 15 minutes on ice, and washed three times with PBS. To minimize non-specific binding, cells were blocked with 10% bovine serum albumin (BSA; normal goat serum, Abcam, Cambridge, UK) diluted in PBS + 0.1% Tween 20 for 60 min at room temperature. Next, cells were incubated with the primary anti-Aβ1-42 antibody (5 µL, 1:100; Abcam, Cambridge, UK) diluted in 1% blocking buffer (PBS + 0.1% Tween 20 + 1% bovine serum albumin [BSA]) overnight at 4 °C. Subsequently, cells were washed three times with PBS containing 0.1% Tween 20 and then incubated with the secondary Cy3-labeled goat anti-rabbit IgG antibody (3 µL; 1:100, Promega, Madison, WI) diluted in 1% blocking buffer for 60 minutes at room temperature. Following three washes with PBS + 0.1% Tween 20 in the dark, cells were incubated with Fluoroshield mounting medium containing 6-diamidino-2-phenylindole (DAPI) (AB104139, AbCam, Cambridge, UK) for 5 minutes at room temperature in the dark. Finally, cells were observed using a Nikon NIS-Elements fluorescence microscope (Nikon, Tokyo, Japan). Immunofluorescent signals were captured and analyzed using a fluorescence microscope connected to a computerized imaging system (Image-Pro plus V6.0; Silver Spring, MD).
Transmission electron microscopy (TEM) assay
For an in-depth analysis of cellular ultrastructure and autophagosomes across different groups, TEM observations were performed using a CM12 TEM (Philips, Amsterdam, Netherlands). SH-SY5Y cells (3×104 cells/well) were washed twice with 0.2 M PBS (pH 7.4), gently scraped, and fixed with 2.5% glutaraldehyde (v/v, in 0.1 M cacodylate buffer, pH 7.4) for 60 minutes on ice. After thorough washing, cells were post-fixed with 1% osmium tetroxide (OsO4) (w/v, in 0.1 M cacodylate buffer, pH 7.4) for 60 minutes at 4 °C. Subsequent steps involved dehydration, embedding in Spurr’s resin (TAAB Laboratories Equipment Ltd, Aldermaston, England), and examination under TEM at 80 kV.
Western blot (WB) analysis
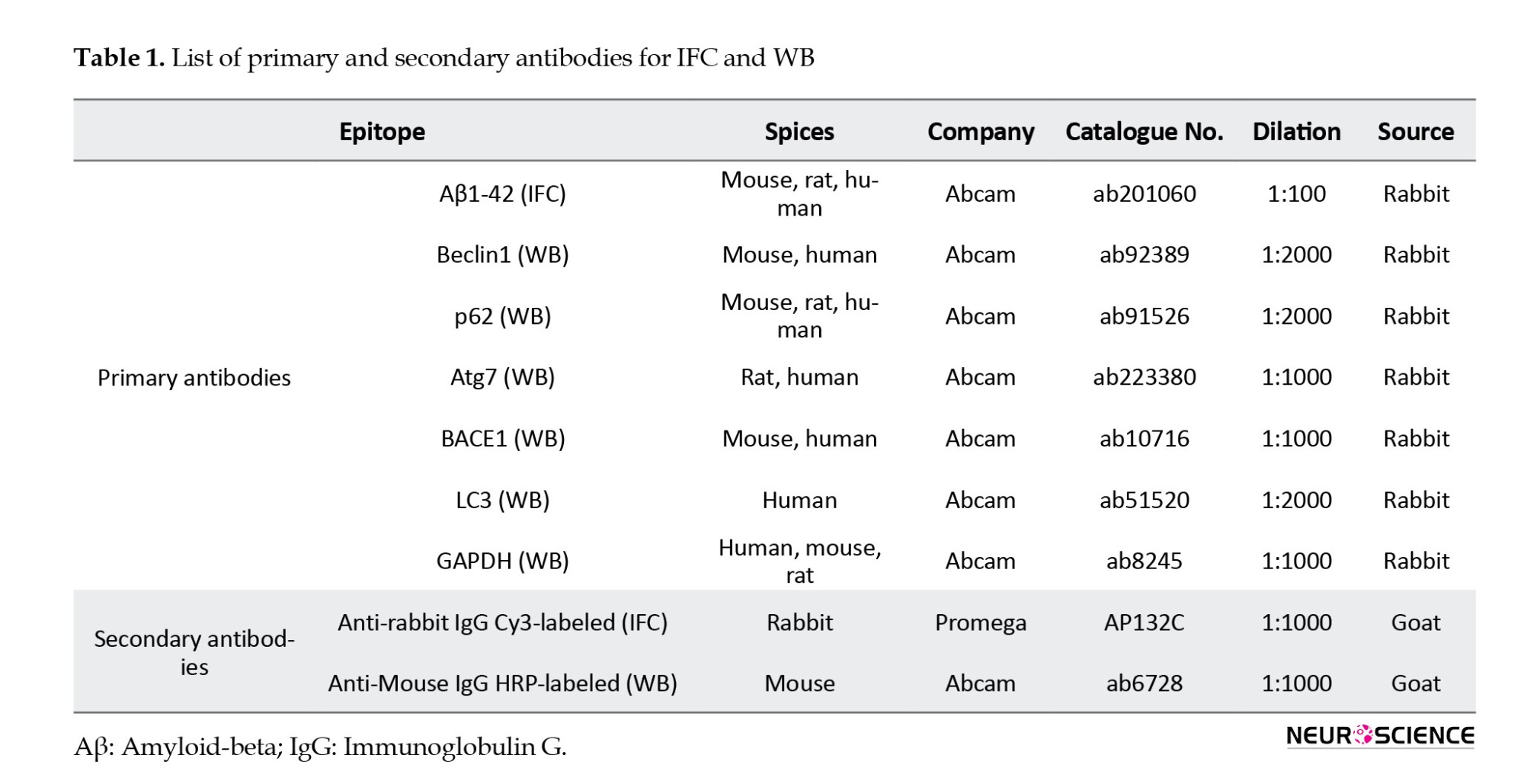
To dissect the cleaved Aβ1-42-induced pathways, we performed Western blot analysis to assess the expression of BACE1, Atg7, p62, LC3-Ⅰ, LC3-Ⅱ, and Beclin-1 proteins in various treated SH-SY5Y cell groups. Lysates from 2×106 SH-SY5Y cells/well in each group were extracted using RIPA buffer (Roche Diagnostics, Mannheim, Germany). Protein samples were boiled with a loading buffer and separated using standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis methods. Subsequently, proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, followed by blocking with 5% bovine serum albumin for 30 min at room temperature. The PVDF membrane was probed with primary antibodies against Beclin1 (1:2000), p62 (1:2000), Atg7 (1:1000), BACE1 (1:1000), LC3 (1:2000), and GAPDH (1:1000), all purchased from Abcam, Cambridge, UK. Specific antibody binding was detected by incubating the membrane with a goat anti-mouse immunoglobulin G (IgG) conjugated to biotin (1:2000 dilution) at 4 °C for 2 hours. Following extensive washing with TBS buffer, 3-39DiAminoBenzidine solution was applied for 20 minutes in the dark, and the membrane was incubated with ExtrAvidin peroxidase (diluted 1:1500) at 4 °C for 60 minutes. WB for human GAPDH (36 kDa) was used as a control. To visualize the protein bands, an enhanced chemiluminescence detection system was utilized, and quantitative analysis was performed using ImageJ software. Table 1 provides comprehensive information on the primary and secondary antibodies employed in immunohistochemistry (IHC) staining, facilitating the accurate detection and visualization of target proteins.
Statistical analysis
Statistical analyses were conducted utilizing SPSS software, version 26 (Chicago, Illinois, USA). Measurement data were expressed as Mean±SD. Graphs were generated using GraphPad Prism software, version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Statistical comparisons between experimental groups employed either one-way analysis of variance or least significant difference (LSD) analysis of variance, depending on the study design. Post-hoc analysis was used to determine statistical significance (P) among groups, with significance levels set at P<0.05 and P<0.001. All charts were created using Prism.
3. Results
VTF enhances proliferation in Aβ1-42-induced SH-SY5Y cells
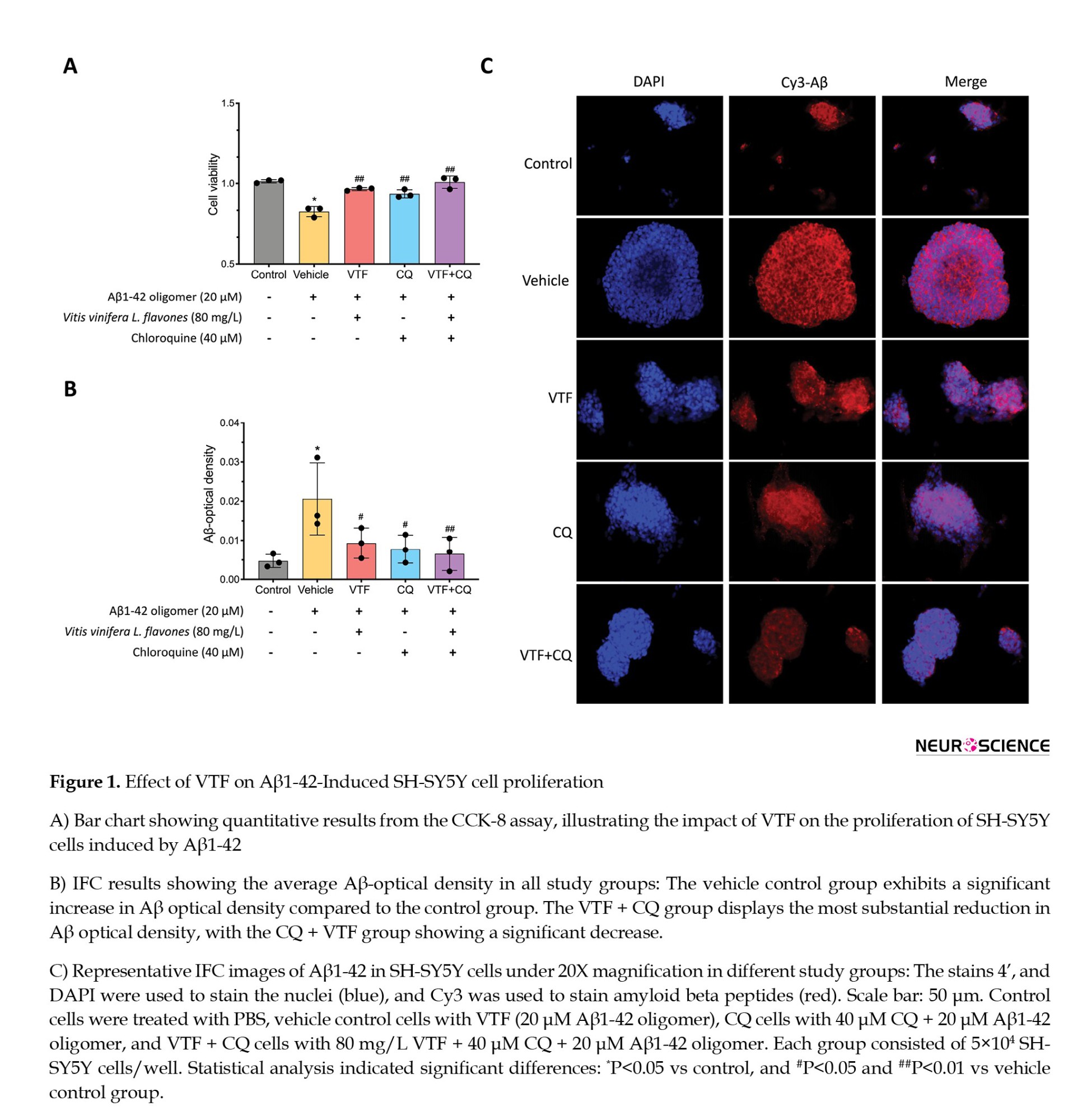
We investigated the protective impact of VTF against Aβ1-42-induced cellular stress in SH-SY5Y cells. The CCK-8 assay highlighted a substantial reduction in cell proliferation upon exposure to 20 μM Aβ1-42 oligomers compared to the control group (1.10±0.02 vs 0.83±0.03; P<0.01). This underscores the neurotoxicity of Aβ1-42 oligomers and their ability to inhibit cell proliferation. Conversely, VTF, CQ, and CQ + VTF demonstrated a marked increase in viable cell count, emphasizing the reparative capabilities of VTF and CQ against Aβ-induced toxicity (Figure 1A; P<0.01). To unravel the impact of VTF on Aβ1-42-induced Aβ deposition, IFC measured Aβ optical density values (Figures 1B and 1C). The vehicle control group exhibited a significant increase in IFC Aβ optical density values compared to the control group (Figure 1B, P<0.01). Both the VTF and CQ groups showed decreased optical density values compared to the vehicle control group (P<0.05). Notably, the VTF + CQ group displayed the most substantial reduction in Aβ optical density, with the CQ + VTF group showing a significant decrease (0.007±0.0004 vs 0.021±0.009; P<0.01). As shown in Figure 1C, Aβ protein expression was prominent outside and within the nucleus of the cells. Aβ protein deposition was the lowest in the control group and highest in the vehicle control group (P<0.01). Compared to the vehicle control group, the VTF, CQ, and VTF + CQ groups displayed a gradual increase in red-fluorescently labeled Aβ protein deposition (P<0.01). The results indicated a significant increase in the red-fluorescent labeling area and IFC Aβ optical density values in the vehicle control group compared to the control group (P<0.01), signifying substantial Aβ protein deposition. Furthermore, compared to the vehicle control group, the VTF and CQ groups exhibited a reduction in the area of red fluorescent labeling and a decrease in Aβ optical density values (P<0.05). The CQ + VTF group demonstrated a significant reduction in Aβ optical density values (P<0.01). These findings suggest that both VTF and CQ can mitigate intracellular Aβ protein deposition, enhancing cell vitality and protecting against Aβ-induced organelle damage. Overall, these results underscore the robust reparative potential of both VTF and CQ in mitigating the aging process and enhancing cell vitality.
VTF preserves Aβ1-42-induced mitophagy alterations
To structurally analyze the impact of VTF on hippocampal autophagy, we utilized TEM to examine Aβ1-42-induced alterations in mitochondria and autophagosomes across the study groups (Figure 2). As depicted in Figure 2A, the control group displayed well-defined mitochondria with orderly arranged cristae and minimal autophagosomes. In contrast, Aβ1-42 induction led to disrupted mitochondrial morphology, increased autophagosome levels, and cellular stress (Figure 2B). Both VTF and CQ treatments significantly improved mitochondrial characteristics, including increased numbers, regular morphology, reduced swelling, enhanced cristae, and fewer autophagosomes, compared to the vehicle control group (Figures 2C and 2D, respectively). Notably, the combined treatment with VTF and CQ demonstrated a robust protective effect, preserving mitochondrial integrity and cellular homeostasis (Figure 2E). The TEM results underscore the detrimental impact of Aβ1-42 oligomers, which intensify cell senescence, induce mitochondrial dysfunction, and promote aberrant autophagy. Collectively, these findings highlight the multifaceted protective attributes of VTF and CQ, contributing to the maintenance of normal mitochondrial structure, functional homeostasis, autophagy homeostasis, and overall cellular well-being under Aβ1-42-induced cellular stress.

VTF modulates mitophagy via the autophagy–lysosomal pathway
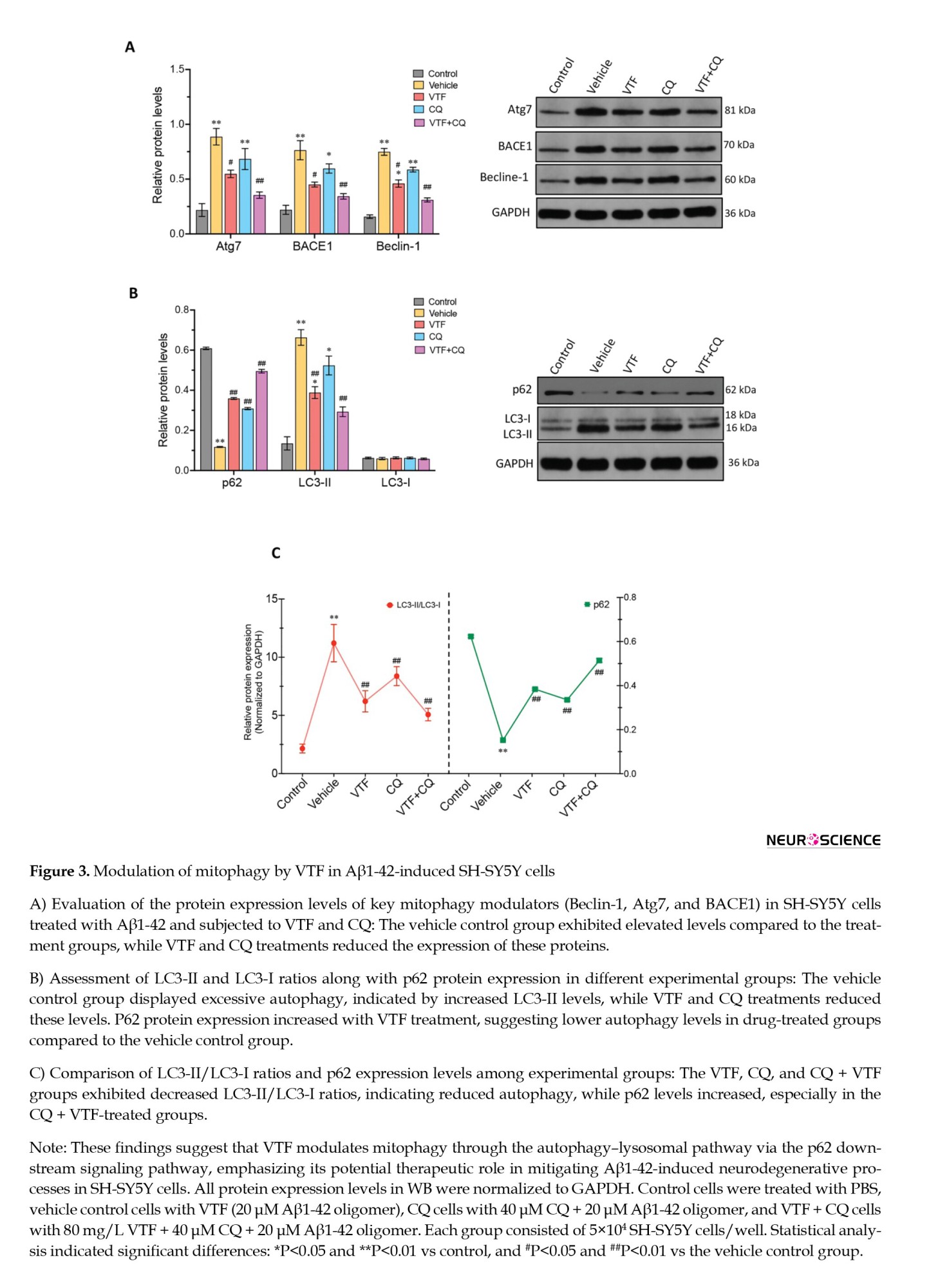
To investigate the influence of VTF on mitophagy induced by Aβ1-42 in SH-SY5Y cells, we examined crucial proteins associated with the autophagy–lysosomal pathway, including Beclin-1, p62, Atg7, and BACE1 (Figure 3). Western blot analysis revealed a substantial increase in the protein expression of autophagy–lysosomal pathway components, Beclin-1, Atg7, and BACE1, in the vehicle control group compared to the treatment groups (Figure 3A; P<0.05). Upon VTF and CQ treatment, a significant reduction in Beclin-1, Atg7, and BACE1 protein levels was observed in the VTF, CQ, and CQ+VTF groups compared to the vehicle control group. This suggests that VTF may protect neuronal cells by inhibiting the autophagy-lysosomal pathway. To delve deeper into the VTF + CQ mechanism in the p62 pathway, our analysis of LC3-II/LC3-I levels uncovered excessive autophagy and altered p62 expression across different groups, corroborating our earlier observations. Compared to the control group, the vehicle control group exhibited significantly elevated LC3-II levels, accompanied by a notable decrease in p62 protein expression (Figure 3B; P<0.01), indicating heightened autophagy. The VTF and CQ groups showed a significant increase in p62 protein expression (P<0.01), indicating lower autophagy levels in these drug-treated groups than in the vehicle control group. Figure 3C illustrates the comparison of LC3-II/LC3-I ratios and p62 expression. The VTF, CQ, and CQ + VTF groups displayed significantly reduced LC3-II/LC3-I levels compared to the vehicle control groups. In contrast, p62 levels were higher in the CQ + VTF-treated groups than in the vehicle control group (Figure 3C; P<0.01). This supports our hypothesis of excessive autophagy in the vehicle control group cells, emphasizing that VTF protects against the inhibition of abnormally activated autophagy, thereby preserving nerve cell function. This suggests that both VTF alone and the combination of VTF and CQ upregulate p62 protein expression, modulate mitophagy, and suppress the autophagy–lysosomal pathway, involving Beclin-1, Atg7, BACE1, LC3-II, and the ratio of LC3-II to LC3-I protein levels. While both VTF alone and the combined treatment of VTF and CQ upregulated p62 protein expression, modulated mitophagy, and suppressed the autophagy–lysosomal pathway involving Beclin-1, Atg7, BACE1, LC3-II, and LC3-II/LC3-I protein levels, as previously stated, subtle differences emerged in the expression levels of these proteins between the treatment groups. Further analysis revealed nuanced variations in the regulatory mechanisms underlying the response to VTF alone compared to the combination treatment with CQ, suggesting the potential synergistic effects of the combination treatment on autophagy regulation.
4. Discussion
This study investigated the in vitro mechanism by which VTF safeguards neurons against Aβ1-42-induced neurodegeneration. Our results, for the first time, collectively demonstrate that VTF and CQ contribute to maintaining normal mitochondrial structure, functional homeostasis, autophagy homeostasis, and overall cellular well-being under Aβ1-42-induced cellular stress. Our data provide additional support for the neuroprotective role of VTF through the regulation of autophagy, specifically by modulating mitophagy via the autophagy–lysosomal pathway. Notably, our investigation highlights the significant contribution of VTF in exerting anti-neurodegenerative effects by inhibiting excessive autophagy and preserving neuronal function.
The selection of VTF for investigation in our study was based on their well-documented antioxidant and neuroprotective properties. Numerous studies have suggested that polyphenolic compounds derived from flavonoids act as effective oxygen-free radical scavengers and antioxidants in AD (Joseph et al., 2023; Lopresti et al., 2023). Moreover, VTF has been reported to exhibit neuroprotective effects by preserving mitochondrial function and regulating autophagy, thereby promoting cell survival and maintaining cellular homeostasis. These properties make VTF an attractive candidate for investigating its potential therapeutic benefits in neurodegenerative diseases, such as AD. Recent studies have revealed the multifaceted properties of flavonoids, indicating their neuroprotective functions and anti-inflammatory effects. These compounds, known for their ability to cross the blood-brain barrier, have demonstrated promising outcomes in enhancing learning and memory in mice with cognitive impairment (Kim et al., 2009; Prakash & Sudhandiran, 2015; Spencer et al., 2012). Onozuka et al. (2008) reported that Nobiletin, a citrus flavonoid can ameliorate memory impairment and reduce Aβ pathology in a transgenic mouse model of AD. Notably, this effect is attributed to its role in decreasing amyloid beta production through the mediation of presenilin-1 phosphorylation (Abdul Manap et al., 2020; Rezai-Zadeh et al., 2009). Furthermore, our choice of VTF is supported by a growing body of literature highlighting its efficacy in attenuating neurodegeneration and cognitive decline in preclinical models of AD. Therefore, by focusing on VTF in this study, we aimed to contribute to the expanding knowledge of its neuroprotective mechanisms and therapeutic potential in AD and related disorders. Although existing studies demonstrated VTF’s ability to promote synaptic plasticity and indirectly influence the expression of cholinergic neurotransmitters, the precise mechanisms, particularly from the perspective of autophagy, remain elusive (Kim et al., 2009; Prakash & Sudhandiran, 2015; Spencer et al., 2012). Our in vitro findings aim to underscore the profound anti-neurodegenerative effects of VTF. In contrast to CQ, a prototypical lysosome inhibitor, VTF plays a distinct role in modulating mitophagy. In the context of AD pathogenesis, the aggregation of Aβ resulting from abnormal APP hydrolysis by β- and γ-secretases is considered a valuable and prognostic biomarker (Xiao et al., 2017).
The selection of CQ as a lysosome inhibitor in our study was based on its well-documented pharmacological properties and established role in modulating autophagy. CQ disrupts lysosomal acidification and impairs autophagic flux, leading to the accumulation of autophagosomes and inhibition of protein degradation within lysosomes (Fedele & Proud, 2020; Ke, 2024; Redmann et al., 2017). In AD, impaired autophagy and dysfunctional lysosomal degradation contribute to the accumulation of protein aggregates, including Aβ and tau, which are hallmark features of the disease pathology. By inhibiting lysosomal function, CQ exacerbates autophagy dysfunction and accelerates disease progression (Varma et al., 2023). Furthermore, CQ has been widely used as a pharmacological tool in preclinical studies to investigate the role of autophagy in neurodegenerative diseases and explore potential therapeutic interventions (Halcrow et al., 2021; Rainsford et al., 2015). Its well-characterized mechanism of action and established safety profile make it a valuable tool for dissecting autophagy-related pathways and identifying novel therapeutic targets (Pedrioli et al., 2020; Rainsford et al., 2015). The inclusion of CQ in our study provides a unique opportunity to investigate its synergistic effects with VTF in modulating autophagy and neuroprotection in AD models (Caporaso et al, 1992). By combining CQ’s lysosome-inhibiting properties with the antioxidant and neuroprotective effects of VTF, we aim to elucidate the mechanisms underlying their therapeutic potential and advance our understanding of AD pathogenesis and treatment (Furst, 1996; Rainsford et al., 2015).
Our study shows the potential therapeutic effects of VTF against neurodegeneration, focusing specifically on AD. We found that VTF exhibited promising anti-neurodegenerative properties by modulating mitophagy and suppressing the autophagy–lysosomal pathway. One key finding of our study was the modulation. These results highlight the potential of VTF as a therapeutic intervention for AD. However, further research is required to understand the precise mechanisms involved and assess the safety and efficacy of VTF in clinical settings. Nonetheless, our study provides valuable insights into the neuroprotective effects of VTF, offering hope for the development of novel treatments for neurodegenerative diseases.f mitophagy by VTF, which plays a crucial role in maintaining mitochondrial homeostasis and cellular health (Moldovan et al., 2020). Mitophagy, the selective degradation of damaged mitochondria, is impaired in AD and other neurodegenerative disorders, leading to mitochondrial dysfunction and oxidative stress. By promoting mitophagy, VTF may facilitate the removal of dysfunctional mitochondria, thereby mitigating oxidative stress and apoptotic signaling pathways implicated in neurodegeneration (Balea Ş et al., 2020). Additionally, VTF suppressed the autophagy–lysosomal pathway, which is dysregulated in AD pathology. By downregulating key autophagy-related proteins, such as Beclin-1, Atg7, BACE1, LC3-II, and LC3-II/LC3-I, VTF may reduce the accumulation of neurotoxic protein aggregates associated with AD (Chifenti et al., 2013; He et al, 2015). However, the molecular mechanisms underlying this modulation, in vitro and in vivo, remain elusive. It remains unclear whether VTF can prevent hippocampal neuron damage by inhibiting autophagy and Aβ clustering. We observed that VTF preserves Aβ1-42-induced alterations in mitochondrial and autophagosomal structures. Our investigation further delves into the intricate molecular pathways, highlighting VTF’s role in modulating mitophagy through the autophagy–lysosomal pathway, involving key players, such as Beclin-1, p62, Atg7, and BACE1. Specifically, we emphasize the impact on BACE1, a crucial orchestrator of mitochondrial and autophagosomal processes, demonstrating its elevated levels in the vehicle control group and subsequent attenuation upon VTF treatment (Lee et al., 2021; Liu et al., 2023). This dynamic modulation suggests that VTF has the potential to mitigate Aβ production and, alleviate neurodegeneration. Within the autophagy cascade, Atg7 and Beclin-1 emerge as a pivotal regulator, representing a focal point for understanding VTF’s influence on autophagy levels (Caballero & Coto-Montes, 2012; Fîlfan et al., 2017). Our results reveal a significant reduction in Atg7 expression in Aβ1-42-induced SH-SY5Y cells, indicating increased autophagy. Notably, the subsequent decrease in Atg7 levels following VTF treatment signifies a potential mechanistic facet through which VTF maintains autophagy homeostasis, strengthening its neuroprotective role (Li et al., 2012). Our study revealed a notable increase in p62 expression following VTF treatment. This elevation aligns with our hypothesis that VTF inhibits excessive autophagy, thereby preserving nerve cell function (Caccamo et al., 2017). The observed inverse correlation between autophagy and p62 underscores the delicately orchestrated balance potentially sustained by VTF. These results provide valuable insights into the molecular underpinnings of VTF’s neuroprotective effects in the context of AD, highlighting its potential therapeutic significance.
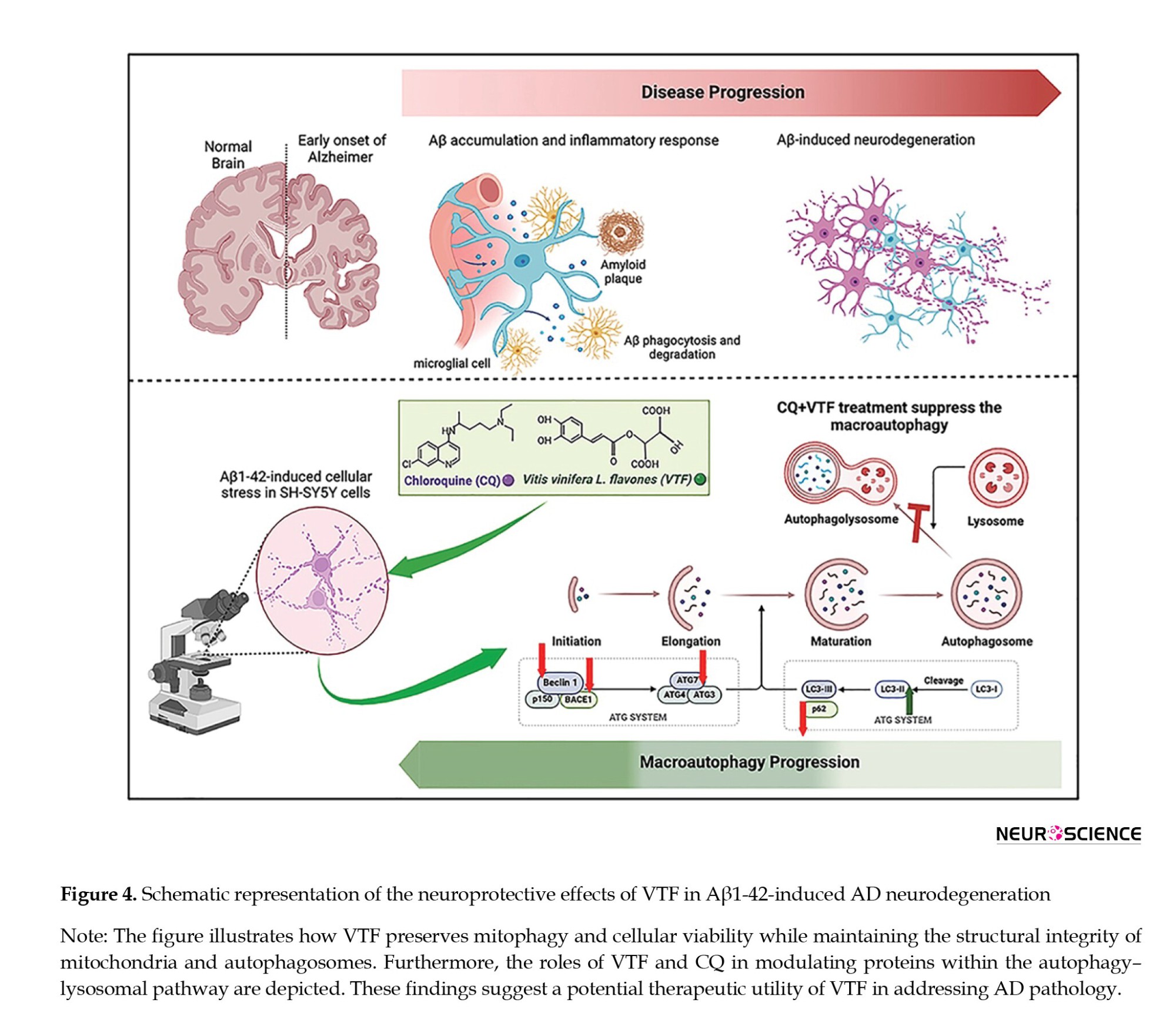
Excessive autophagy is a notable concern in Aβ1-42-induced SH-SY5Y cells and AD, emphasizing the crucial need for effective therapeutic interventions. LC3, specifically the LC3-II/LC3-I ratios has emerged as a reliable indicator of autophagy levels (Chai et al., 2022; Heckmann et al., 2019; Pradeepkiran & Reddy, 2020). Our study underscores the significance of this crucial marker, revealing a discernible decrease in LC3-II/LC3-I ratios following VTF treatment. This substantial attenuation of excessive autophagy further enhances the neuroprotective potential of VTF in the intricate landscape of AD. The nuanced regulation of autophagy levels by VTF not only illuminates the molecular intricacies involved but also unveils promising avenues for therapeutic strategies targeting cellular homeostasis in neurodegenerative diseases. The upregulation of p62 seamlessly aligns with our hypothesis that VTF inhibits excessive autophagy, providing additional evidence of its potential to preserve nerve cell function. This delicate balance between autophagy markers, LC3, and p62 highlights the multifaceted impact of VTF on cellular homeostasis. This not only enriches our understanding of the molecular intricacies involved in neurodegenerative conditions but also lays the groundwork for targeted therapeutic strategies aimed at restoring autophagic equilibrium (Liu & Li, 2019; Ułamek-Kozioł et al., 2013). In Figure 4, we illustrate how VTF preserves mitophagy in Aβ1-42-induced AD neurodegeneration. Our findings highlight the significant neuroprotective effects of VTF and CQ against Aβ-induced damage. VTF treatment maintained cellular viability and preserved the integrity of mitochondria and autophagosomes integrity. Additionally, our study revealed the role of VTF and CQ in modulating key proteins in the autophagy–lysosomal pathway. These findings warrant further investigation of VTF’s therapeutic potential in AD.
These results highlight the potential of VTF as a therapeutic intervention for AD. However, further research is needed to understand the precise mechanisms involved and assess the safety and efficacy of VTF in clinical settings. Nonetheless, our study provides valuable insights into the neuroprotective effects of VTF, offering hope for the development of novel treatments for neurodegenerative diseases (Balea Ş et al., 2020; Chifenti et al., 2013; He et al., 2015).
Our study highlights the neuroprotective potential of VTF but acknowledges the limitations of our primarily in vitro approach. To better understand VTF’s mechanisms and therapeutic implications, we recognize the need for more extensive cellular and molecular in vitro investigations. These studies should focus on specific aspects, such as how flavonoids modulate autophagy and interact with key molecular pathways involved in neurodegeneration. Furthermore, structural elucidation of VTF’s effects at the cellular level, combined with comprehensive molecular analyses, will offer valuable insights into is therapeutic efficacy. Additionally, while alternative models, such as Aβ-induced mitochondrial dysfunction, could provide complementary evidence, resource constraints and the scope of our study prevented us from exploring these models in this study. Also, our study did not specifically assess alterations in autophagy flow, and we did not include a control experiment to evaluate whether the introduction of autophagy inhibitors could reserve the observed positive effects of VTF. Future research endeavors should aim to address these limitations by incorporating more comprehensive in vivo studies, including animal models, and conducting controlled experiments to unravel the intricate dynamics of autophagy modulation by VTF. Furthermore, the complex interplay between various autophagic markers, the specific mechanisms of flavonoids, and the multifaceted nature of AD pathology warrant further detailed exploration in subsequent investigations to enhance our understanding and pave the way for potential therapeutic applications (Funderburk et al., 2010; Nixon, 2007).
Exploring additional markers and signaling pathways associated with autophagy modulation by VTF could provide a more comprehensive understanding of its therapeutic potential. Moreover, extending these investigations to in vivo models and clinical studies would bridge the translational gap, offering a clearer picture of VTF’s efficacy in a more complex physiological context. The delineation of VTF’s influence on synaptic plasticity and cholinergic neurotransmitters, coupled with its role in autophagy regulation, opens avenues for targeted interventions that address multiple facets of AD pathology. This study lays the groundwork for further research aimed at harnessing the full potential of VTF as a promising candidate for neuroprotective strategies in the context of neurodegenerative disorders. By addressing these limitations and discussing avenues for further investigation, we aim to provide a transparent interpretation of our results and contribute to advancing our understanding of neuroprotective compounds for the treatment of neurodegenerative diseases.
5. Conclusion
In conclusion, our study reveals VTF’s novel role, alongside CQ, in preserving cellular health under Aβ1-42-induced stress, emphasizing its anti-neurodegenerative effects through modulation of mitophagy. While our results open avenues for further in vivo exploration, the study underscores VTF’s potential in targeting multiple facets of AD pathology, presenting a promising candidate for future neuroprotective interventions.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design, investigations, experiments, and final approval: All authors; Data analysis: Peng Zhang; Writing: Hui Xiao.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank College of Public Health, Xinjiang Medical University, Urumqi, China.
References
Alzheimer’s disease (AD) is the most common neurodegenerative disease, affecting 18% of the Chinese population (Chan et al., 2013; Jia et al., 2020). The National Aged Population Center of China predicts a significant increase in the impact of AD among individuals aged >60 years, reaching approximately 60 million over the next decade. This highlights AD as a major concern in China’s public healthcare system (Kolachala et al., 2021; Li et al., 2018; Wang et al., 2008).
AD is characterized by a complex network of pathophysiological pathways influenced by genetic, environmental, and lifestyle factors. Inflammation, heightened oxidative stress, and compromised synaptic function play significant roles in disease progression, complicating therapeutic interventions (De-Paula et al., 2012; Li et al., 2023). A nuanced understanding of the molecular intricacies of AD is imperative to develop targeted strategies for this multifaceted neurodegenerative disorder (Skaper, 2012).
Autophagy, driven by the formation of autophagosomes, plays a central role in self-protection during AD progression, eliminating surplus intracellular peptides and damaged organelles (Chung et al., 2019; Klionsky et al., 2021; Krishnan et al., 2020). A significant focus revolves around the initiation of amyloid-beta (Aβ) clusters and entangled Tau proteins, considered pivotal biomarkers in AD therapy (Bloom, 2014; Fonseca et al., 2013). Aβ, derived from amyloid precursor protein (APP), undergoes abnormal aggregation to form plaques (Salminen et al., 2013). These Aβ clusters, characterized by misfolded proteins, act as focal points in the observed neurodegenerative cascade in AD. Aβ peptides in AD include Aβ1-42 and Aβ1, the former being a pathogenic variant with 42 amino acids (Aβ1-42), exhibiting an elevated propensity for aggregation and the formation of toxic oligomers, which significantly contribute to neurodegeneration (Sepulcre et al., 2017). In contrast, Aβ1, a shorter counterpart, is generated during APP cleavage, displaying a comparatively lower inclination for aggregation. The interplay between these forms underscores their distinct roles in the complex Aβ cascade, influencing the pathophysiology of AD (Jackson et al., 2016). However, in advanced stages of AD, impaired autophagy may lead to Aβ1-42 and Aβ1 accumulation, exacerbating neurodegenerative processes. Balancing the interplay between Aβ clusters and autophagy dynamics holds potential for targeted therapeutic intervention. Modulating autophagy to enhance Aβ clearance or inhibit Aβ cluster formation presents promising avenues for further exploration in AD research (Dunyset al., 2018).
Numerous in vitro and in vivo studies have investigated polyphenolic compounds from Vitis vinifera L. flavones (VTF), a herb rich in cholinergic neurotransmitters, widely used in traditional Chinese medicine (Rodriguez-Mateos et al., 2014). These flavonoids have been shown to prevent damage to hippocampal neurons by inhibiting autophagy and promoting anti-neurodegenerative effects (Benavente-García & Castillo, 2008). Flavonoids from VTF act as oxygen-free radical scavengers and antioxidants, stimulating synaptic plasticity and improving cognitive impairment in AD-model mice (Ma et al., 2018). Our latest report demonstrated that VTF can influence the pathological changes of AD by regulating hippocampal neurons via autophagy in APP/presenilin 1 (PS1) transgenic sedentary Alzheimer model mice. However, it remains unclear whether VTF can prevent hippocampal neuron damage by inhibiting autophagy and Aβ clustering (Joseph et al., 2023; Lopresti et al., 2023).
This study aimed to investigate the anti-neurodegenerative role of VTF in greater detail, exploring its relationship with autophagy and Aβ aggregation. This study aimed to elucidate the in vitro mechanism by which VTF protects neurons from Aβ1-42-induced neurodegeneration. Analyzing changes in Aβ1-42-induced autophagy-related protein expression in brain tissues will shed light on VTF’s potential to suppress excessive autophagy in Aβ1-42-induced SH-SY5Y cells. These findings may offer promising avenues for AD research, indicating that VTF’s neuroprotective effects could be attributed to the inhibition of excessive autophagy in Aβ1-42-induced SH-SY5Y cells.
2. Materials and Methods
Preparation of flavones from V. vinifera L.
V. vinifera L. grapes from the Vitaceae family were sourced from a reputable Uyghur medicine market in Turpan, Xinjiang Province, China, one week before initiating the experiment. The seeds were meticulously collected in August 2023 from the Gaochang District, Turpan City, located in the northwest region of the city, by PZ (GPS coordinates: 42.9225° N, 89.1913° E). The botanical identification of the plant was confirmed as V. vinifera L., commonly known as “European grapes” in Chinese (Ōuzhōu pútáo), at the National Herbarium of China. After collection, the seeds were carefully air-dried to eliminate excess moisture, ensuring optimal extraction efficiency. Subsequently, the dried seeds were finely ground into a powder with a particle size of approximately 20 mg, ensuring uniformity and consistency in the extraction process. The powder underwent meticulous extraction using 95% ethanol as the solvent for a precisely controlled duration of 2 h, following standard extraction protocols (Li et al., 2020). Following extraction, the resulting mixture underwent rotary evaporation under controlled conditions to remove the ethanol solvent, yielding a crude extract with enhanced purity and concentration. The yield of the crude extract was determined to be 170 g, designated as DCB. The supernatant obtained after rotary evaporation was carefully preserved for subsequent analysis to prevent the loss of valuable components. To further enrich and purify the extracted flavonoids, a series of sophisticated purification steps were meticulously executed. This purification process involved suspending the crude extract in water, followed by purification with AB-8 resin, a highly efficient adsorbent material known for its excellent selectivity and flavonoid adsorption capacity. The purification process was carried out using a precise gradient of 5% water and 95% ethanol to achieve optimal separation and purification of the target compounds. Subsequently, a 50% ethanol elution fraction containing highly enriched flavonoids was collected for processing. Finally, the purified VTF was obtained as a high-quality brown-yellow powder through meticulous vacuum drying at a controlled temperature of 60 °C after freeze-drying of the purified fraction. This final purification step, with a yield of extracts exceeding 92%, ensured the removal of any residual moisture and solvent traces, resulting in the production of highly pure and concentrated flavones ready for further analysis and biological evaluation. As detailed in previous results (Abdul Manap et al., 2020), chemical analysis of VTF extracts revealed a rich presence of flavonoids and stilbenes, with resveratrol (3,5,4’-trihydroxy-trans-stilbene) standing out prominently. Additionally, compounds like Quercetin (2-[3,4-dihydroxyphenyl]-3,5,7-trihydroxychromen-4-one), Kaempferol (3,5,7-trihydroxy-2-[4-hydroxyphenyl]chromen-4-one), and Myricetin (3,5,7-trihydroxy-2-[3,4,5-trihydroxyphenyl]chromen-4-one) were consistently identified as key components within VTF. This comprehensive examination significantly advances our understanding of the potential health benefits and therapeutic applications of these compounds.
Preparation of Aβ1-42 oligomers
Aβ1–42 oligomers were prepared according to the protocol described by Stine et al. (2003). The flavonoid treatment approach was based on the neuroprotective findings of Rezai-Zadeh et al., 2009. Initially, 5 mg of lyophilized Aβ1-42 (Sigma-Aldrich, St. Louis, MO, USA) was equilibrated at room temperature for 30 minutes to prevent condensation upon unsealing. The peptide was then suspended in ice-cold 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to achieve a one mM solution. After brief vortexing, the Aβ1-42/HFIP solution was incubated in polypropylene vials using a glass GasTight Hamilton syringe with a Teflon plug for 2 h to monomerize Aβ. The subsequent concentration of the Aβ1-42/HFIP solution under vacuum in a SpeedVac centrifuge (800 g, room temperature) produced a clear peptide film. Stringent temperature control (maintained below 25 °C) prevented peptide degradation during this process. The Aβ1-42 film was resuspended in dimethyl sulfoxide (DMSO) containing 10% fetal bovine serum, yielding a concentration of 400 μmol/L.
Preparation of chloroquine (CQ)
In this study, CQ, a lysosomal inhibitor, was used to modulate autophagy. A 50 mM stock solution of CQ diphosphate salt (Sigma-Aldrich, St. Louis, MO, USA) was prepared, dissolved in DMSO, sterilized by filtration (0.2 micrometers), and stored in aliquots at -20 ºC until use. Work solutions were diluted in 84% (v/v) mouse embryonic fibroblasts (MEFs; Thermo Fisher Scientific, Waltham, MA, USA), with 15.0% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (P/S; Sigma-Aldrich, St Louis, MO, USA). The cells were treated with CQ in a dose-dependent manner, ranging from 0 to 100 μM.
Cell culture and treatment
The SH-SY5Y human-derived neuroblastoma cell line (American Type Culture Collection [ATCC] CRL-2266) was sourced from the American Type Culture Collection (ATCC) (Manassas, VA, United States) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (P/S; Sigma-Aldrich, St Louis, MO, USA). The culture conditions were maintained in a constant temperature incubator at 37 °C with 5% carbon dioxide (CO2) to optimize cell growth. Regular monitoring for mycoplasma contamination was implemented to preserve the integrity of the cell lines, ensuring that SH-SY5Y cells remained free of potential contamination during experimental procedures. Stock solutions were diluted in DMEM with 1.0% (v/v) FBS to prepare working solutions. The study included the following treatment groups: Control (60 μM phosphate-buffered saline [PBS]), vehicle control (20 μM Aβ1-42 oligomer), VTF (80 mg/L VTF + 20 μM Aβ1-42 oligomer), CQ (40 μM CQ+20 μM Aβ1-42 oligomer), and VTF+CQ (80 mg/L VTF+40 μM CQ+20 μM Aβ1-42 oligomer).
Cell proliferation assays
The induction of cell proliferation by Aβ1-42 was assessed using a cell counting kit (CCK-8 assay, A311-02, Vazyme, Nanjing, China). Initially, cells (5×104 cells/well) were seeded in 96-well plates at a predetermined density. After treatments with VTF and CQ, CCK-8 solution was added to each well, followed by incubation. Absorbance was measured at 450 nm using a multimode microplate reader (Thermo Fisher Scientific Inc., MA, United States). Cell viability was quantified as a percentage relative to the absorbance of control cells.
Immunofluorescent (IFC) assay
An IFC assay was conducted following established protocols to assess the impact of VTF on Aβ1-42-induced Aβ deposition in SH-SY5Y cells (Abdul Manap et al., 2020). SH-SY5Y cells were seeded at a density of 5×104 cells/well in IbiTreat chamber slides (Ibidi GmbH, Martinsried, Germany) and incubated at 37 °C in a humidified 5% carbon dioxide (CO2) incubator. Once the cells reached 80% confluency, they were treated with 25 mM Aβ fibrils for 24 h. Following treatment, the cells were washed thrice with PBS, fixed with 4% paraformaldehyde (Aldrich, Steinheim, Germany) for 15 min, and then washed thrice with PBS. The fixed cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, US), diluted in PBS for 15 minutes on ice, and washed three times with PBS. To minimize non-specific binding, cells were blocked with 10% bovine serum albumin (BSA; normal goat serum, Abcam, Cambridge, UK) diluted in PBS + 0.1% Tween 20 for 60 min at room temperature. Next, cells were incubated with the primary anti-Aβ1-42 antibody (5 µL, 1:100; Abcam, Cambridge, UK) diluted in 1% blocking buffer (PBS + 0.1% Tween 20 + 1% bovine serum albumin [BSA]) overnight at 4 °C. Subsequently, cells were washed three times with PBS containing 0.1% Tween 20 and then incubated with the secondary Cy3-labeled goat anti-rabbit IgG antibody (3 µL; 1:100, Promega, Madison, WI) diluted in 1% blocking buffer for 60 minutes at room temperature. Following three washes with PBS + 0.1% Tween 20 in the dark, cells were incubated with Fluoroshield mounting medium containing 6-diamidino-2-phenylindole (DAPI) (AB104139, AbCam, Cambridge, UK) for 5 minutes at room temperature in the dark. Finally, cells were observed using a Nikon NIS-Elements fluorescence microscope (Nikon, Tokyo, Japan). Immunofluorescent signals were captured and analyzed using a fluorescence microscope connected to a computerized imaging system (Image-Pro plus V6.0; Silver Spring, MD).
Transmission electron microscopy (TEM) assay
For an in-depth analysis of cellular ultrastructure and autophagosomes across different groups, TEM observations were performed using a CM12 TEM (Philips, Amsterdam, Netherlands). SH-SY5Y cells (3×104 cells/well) were washed twice with 0.2 M PBS (pH 7.4), gently scraped, and fixed with 2.5% glutaraldehyde (v/v, in 0.1 M cacodylate buffer, pH 7.4) for 60 minutes on ice. After thorough washing, cells were post-fixed with 1% osmium tetroxide (OsO4) (w/v, in 0.1 M cacodylate buffer, pH 7.4) for 60 minutes at 4 °C. Subsequent steps involved dehydration, embedding in Spurr’s resin (TAAB Laboratories Equipment Ltd, Aldermaston, England), and examination under TEM at 80 kV.
Western blot (WB) analysis
To dissect the cleaved Aβ1-42-induced pathways, we performed Western blot analysis to assess the expression of BACE1, Atg7, p62, LC3-Ⅰ, LC3-Ⅱ, and Beclin-1 proteins in various treated SH-SY5Y cell groups. Lysates from 2×106 SH-SY5Y cells/well in each group were extracted using RIPA buffer (Roche Diagnostics, Mannheim, Germany). Protein samples were boiled with a loading buffer and separated using standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis methods. Subsequently, proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane, followed by blocking with 5% bovine serum albumin for 30 min at room temperature. The PVDF membrane was probed with primary antibodies against Beclin1 (1:2000), p62 (1:2000), Atg7 (1:1000), BACE1 (1:1000), LC3 (1:2000), and GAPDH (1:1000), all purchased from Abcam, Cambridge, UK. Specific antibody binding was detected by incubating the membrane with a goat anti-mouse immunoglobulin G (IgG) conjugated to biotin (1:2000 dilution) at 4 °C for 2 hours. Following extensive washing with TBS buffer, 3-39DiAminoBenzidine solution was applied for 20 minutes in the dark, and the membrane was incubated with ExtrAvidin peroxidase (diluted 1:1500) at 4 °C for 60 minutes. WB for human GAPDH (36 kDa) was used as a control. To visualize the protein bands, an enhanced chemiluminescence detection system was utilized, and quantitative analysis was performed using ImageJ software. Table 1 provides comprehensive information on the primary and secondary antibodies employed in immunohistochemistry (IHC) staining, facilitating the accurate detection and visualization of target proteins.

Statistical analysis
Statistical analyses were conducted utilizing SPSS software, version 26 (Chicago, Illinois, USA). Measurement data were expressed as Mean±SD. Graphs were generated using GraphPad Prism software, version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Statistical comparisons between experimental groups employed either one-way analysis of variance or least significant difference (LSD) analysis of variance, depending on the study design. Post-hoc analysis was used to determine statistical significance (P) among groups, with significance levels set at P<0.05 and P<0.001. All charts were created using Prism.
3. Results
VTF enhances proliferation in Aβ1-42-induced SH-SY5Y cells
We investigated the protective impact of VTF against Aβ1-42-induced cellular stress in SH-SY5Y cells. The CCK-8 assay highlighted a substantial reduction in cell proliferation upon exposure to 20 μM Aβ1-42 oligomers compared to the control group (1.10±0.02 vs 0.83±0.03; P<0.01). This underscores the neurotoxicity of Aβ1-42 oligomers and their ability to inhibit cell proliferation. Conversely, VTF, CQ, and CQ + VTF demonstrated a marked increase in viable cell count, emphasizing the reparative capabilities of VTF and CQ against Aβ-induced toxicity (Figure 1A; P<0.01). To unravel the impact of VTF on Aβ1-42-induced Aβ deposition, IFC measured Aβ optical density values (Figures 1B and 1C). The vehicle control group exhibited a significant increase in IFC Aβ optical density values compared to the control group (Figure 1B, P<0.01). Both the VTF and CQ groups showed decreased optical density values compared to the vehicle control group (P<0.05). Notably, the VTF + CQ group displayed the most substantial reduction in Aβ optical density, with the CQ + VTF group showing a significant decrease (0.007±0.0004 vs 0.021±0.009; P<0.01). As shown in Figure 1C, Aβ protein expression was prominent outside and within the nucleus of the cells. Aβ protein deposition was the lowest in the control group and highest in the vehicle control group (P<0.01). Compared to the vehicle control group, the VTF, CQ, and VTF + CQ groups displayed a gradual increase in red-fluorescently labeled Aβ protein deposition (P<0.01). The results indicated a significant increase in the red-fluorescent labeling area and IFC Aβ optical density values in the vehicle control group compared to the control group (P<0.01), signifying substantial Aβ protein deposition. Furthermore, compared to the vehicle control group, the VTF and CQ groups exhibited a reduction in the area of red fluorescent labeling and a decrease in Aβ optical density values (P<0.05). The CQ + VTF group demonstrated a significant reduction in Aβ optical density values (P<0.01). These findings suggest that both VTF and CQ can mitigate intracellular Aβ protein deposition, enhancing cell vitality and protecting against Aβ-induced organelle damage. Overall, these results underscore the robust reparative potential of both VTF and CQ in mitigating the aging process and enhancing cell vitality.

VTF preserves Aβ1-42-induced mitophagy alterations
To structurally analyze the impact of VTF on hippocampal autophagy, we utilized TEM to examine Aβ1-42-induced alterations in mitochondria and autophagosomes across the study groups (Figure 2). As depicted in Figure 2A, the control group displayed well-defined mitochondria with orderly arranged cristae and minimal autophagosomes. In contrast, Aβ1-42 induction led to disrupted mitochondrial morphology, increased autophagosome levels, and cellular stress (Figure 2B). Both VTF and CQ treatments significantly improved mitochondrial characteristics, including increased numbers, regular morphology, reduced swelling, enhanced cristae, and fewer autophagosomes, compared to the vehicle control group (Figures 2C and 2D, respectively). Notably, the combined treatment with VTF and CQ demonstrated a robust protective effect, preserving mitochondrial integrity and cellular homeostasis (Figure 2E). The TEM results underscore the detrimental impact of Aβ1-42 oligomers, which intensify cell senescence, induce mitochondrial dysfunction, and promote aberrant autophagy. Collectively, these findings highlight the multifaceted protective attributes of VTF and CQ, contributing to the maintenance of normal mitochondrial structure, functional homeostasis, autophagy homeostasis, and overall cellular well-being under Aβ1-42-induced cellular stress.

VTF modulates mitophagy via the autophagy–lysosomal pathway
To investigate the influence of VTF on mitophagy induced by Aβ1-42 in SH-SY5Y cells, we examined crucial proteins associated with the autophagy–lysosomal pathway, including Beclin-1, p62, Atg7, and BACE1 (Figure 3). Western blot analysis revealed a substantial increase in the protein expression of autophagy–lysosomal pathway components, Beclin-1, Atg7, and BACE1, in the vehicle control group compared to the treatment groups (Figure 3A; P<0.05). Upon VTF and CQ treatment, a significant reduction in Beclin-1, Atg7, and BACE1 protein levels was observed in the VTF, CQ, and CQ+VTF groups compared to the vehicle control group. This suggests that VTF may protect neuronal cells by inhibiting the autophagy-lysosomal pathway. To delve deeper into the VTF + CQ mechanism in the p62 pathway, our analysis of LC3-II/LC3-I levels uncovered excessive autophagy and altered p62 expression across different groups, corroborating our earlier observations. Compared to the control group, the vehicle control group exhibited significantly elevated LC3-II levels, accompanied by a notable decrease in p62 protein expression (Figure 3B; P<0.01), indicating heightened autophagy. The VTF and CQ groups showed a significant increase in p62 protein expression (P<0.01), indicating lower autophagy levels in these drug-treated groups than in the vehicle control group. Figure 3C illustrates the comparison of LC3-II/LC3-I ratios and p62 expression. The VTF, CQ, and CQ + VTF groups displayed significantly reduced LC3-II/LC3-I levels compared to the vehicle control groups. In contrast, p62 levels were higher in the CQ + VTF-treated groups than in the vehicle control group (Figure 3C; P<0.01). This supports our hypothesis of excessive autophagy in the vehicle control group cells, emphasizing that VTF protects against the inhibition of abnormally activated autophagy, thereby preserving nerve cell function. This suggests that both VTF alone and the combination of VTF and CQ upregulate p62 protein expression, modulate mitophagy, and suppress the autophagy–lysosomal pathway, involving Beclin-1, Atg7, BACE1, LC3-II, and the ratio of LC3-II to LC3-I protein levels. While both VTF alone and the combined treatment of VTF and CQ upregulated p62 protein expression, modulated mitophagy, and suppressed the autophagy–lysosomal pathway involving Beclin-1, Atg7, BACE1, LC3-II, and LC3-II/LC3-I protein levels, as previously stated, subtle differences emerged in the expression levels of these proteins between the treatment groups. Further analysis revealed nuanced variations in the regulatory mechanisms underlying the response to VTF alone compared to the combination treatment with CQ, suggesting the potential synergistic effects of the combination treatment on autophagy regulation.

4. Discussion
This study investigated the in vitro mechanism by which VTF safeguards neurons against Aβ1-42-induced neurodegeneration. Our results, for the first time, collectively demonstrate that VTF and CQ contribute to maintaining normal mitochondrial structure, functional homeostasis, autophagy homeostasis, and overall cellular well-being under Aβ1-42-induced cellular stress. Our data provide additional support for the neuroprotective role of VTF through the regulation of autophagy, specifically by modulating mitophagy via the autophagy–lysosomal pathway. Notably, our investigation highlights the significant contribution of VTF in exerting anti-neurodegenerative effects by inhibiting excessive autophagy and preserving neuronal function.
The selection of VTF for investigation in our study was based on their well-documented antioxidant and neuroprotective properties. Numerous studies have suggested that polyphenolic compounds derived from flavonoids act as effective oxygen-free radical scavengers and antioxidants in AD (Joseph et al., 2023; Lopresti et al., 2023). Moreover, VTF has been reported to exhibit neuroprotective effects by preserving mitochondrial function and regulating autophagy, thereby promoting cell survival and maintaining cellular homeostasis. These properties make VTF an attractive candidate for investigating its potential therapeutic benefits in neurodegenerative diseases, such as AD. Recent studies have revealed the multifaceted properties of flavonoids, indicating their neuroprotective functions and anti-inflammatory effects. These compounds, known for their ability to cross the blood-brain barrier, have demonstrated promising outcomes in enhancing learning and memory in mice with cognitive impairment (Kim et al., 2009; Prakash & Sudhandiran, 2015; Spencer et al., 2012). Onozuka et al. (2008) reported that Nobiletin, a citrus flavonoid can ameliorate memory impairment and reduce Aβ pathology in a transgenic mouse model of AD. Notably, this effect is attributed to its role in decreasing amyloid beta production through the mediation of presenilin-1 phosphorylation (Abdul Manap et al., 2020; Rezai-Zadeh et al., 2009). Furthermore, our choice of VTF is supported by a growing body of literature highlighting its efficacy in attenuating neurodegeneration and cognitive decline in preclinical models of AD. Therefore, by focusing on VTF in this study, we aimed to contribute to the expanding knowledge of its neuroprotective mechanisms and therapeutic potential in AD and related disorders. Although existing studies demonstrated VTF’s ability to promote synaptic plasticity and indirectly influence the expression of cholinergic neurotransmitters, the precise mechanisms, particularly from the perspective of autophagy, remain elusive (Kim et al., 2009; Prakash & Sudhandiran, 2015; Spencer et al., 2012). Our in vitro findings aim to underscore the profound anti-neurodegenerative effects of VTF. In contrast to CQ, a prototypical lysosome inhibitor, VTF plays a distinct role in modulating mitophagy. In the context of AD pathogenesis, the aggregation of Aβ resulting from abnormal APP hydrolysis by β- and γ-secretases is considered a valuable and prognostic biomarker (Xiao et al., 2017).
The selection of CQ as a lysosome inhibitor in our study was based on its well-documented pharmacological properties and established role in modulating autophagy. CQ disrupts lysosomal acidification and impairs autophagic flux, leading to the accumulation of autophagosomes and inhibition of protein degradation within lysosomes (Fedele & Proud, 2020; Ke, 2024; Redmann et al., 2017). In AD, impaired autophagy and dysfunctional lysosomal degradation contribute to the accumulation of protein aggregates, including Aβ and tau, which are hallmark features of the disease pathology. By inhibiting lysosomal function, CQ exacerbates autophagy dysfunction and accelerates disease progression (Varma et al., 2023). Furthermore, CQ has been widely used as a pharmacological tool in preclinical studies to investigate the role of autophagy in neurodegenerative diseases and explore potential therapeutic interventions (Halcrow et al., 2021; Rainsford et al., 2015). Its well-characterized mechanism of action and established safety profile make it a valuable tool for dissecting autophagy-related pathways and identifying novel therapeutic targets (Pedrioli et al., 2020; Rainsford et al., 2015). The inclusion of CQ in our study provides a unique opportunity to investigate its synergistic effects with VTF in modulating autophagy and neuroprotection in AD models (Caporaso et al, 1992). By combining CQ’s lysosome-inhibiting properties with the antioxidant and neuroprotective effects of VTF, we aim to elucidate the mechanisms underlying their therapeutic potential and advance our understanding of AD pathogenesis and treatment (Furst, 1996; Rainsford et al., 2015).
Our study shows the potential therapeutic effects of VTF against neurodegeneration, focusing specifically on AD. We found that VTF exhibited promising anti-neurodegenerative properties by modulating mitophagy and suppressing the autophagy–lysosomal pathway. One key finding of our study was the modulation. These results highlight the potential of VTF as a therapeutic intervention for AD. However, further research is required to understand the precise mechanisms involved and assess the safety and efficacy of VTF in clinical settings. Nonetheless, our study provides valuable insights into the neuroprotective effects of VTF, offering hope for the development of novel treatments for neurodegenerative diseases.f mitophagy by VTF, which plays a crucial role in maintaining mitochondrial homeostasis and cellular health (Moldovan et al., 2020). Mitophagy, the selective degradation of damaged mitochondria, is impaired in AD and other neurodegenerative disorders, leading to mitochondrial dysfunction and oxidative stress. By promoting mitophagy, VTF may facilitate the removal of dysfunctional mitochondria, thereby mitigating oxidative stress and apoptotic signaling pathways implicated in neurodegeneration (Balea Ş et al., 2020). Additionally, VTF suppressed the autophagy–lysosomal pathway, which is dysregulated in AD pathology. By downregulating key autophagy-related proteins, such as Beclin-1, Atg7, BACE1, LC3-II, and LC3-II/LC3-I, VTF may reduce the accumulation of neurotoxic protein aggregates associated with AD (Chifenti et al., 2013; He et al, 2015). However, the molecular mechanisms underlying this modulation, in vitro and in vivo, remain elusive. It remains unclear whether VTF can prevent hippocampal neuron damage by inhibiting autophagy and Aβ clustering. We observed that VTF preserves Aβ1-42-induced alterations in mitochondrial and autophagosomal structures. Our investigation further delves into the intricate molecular pathways, highlighting VTF’s role in modulating mitophagy through the autophagy–lysosomal pathway, involving key players, such as Beclin-1, p62, Atg7, and BACE1. Specifically, we emphasize the impact on BACE1, a crucial orchestrator of mitochondrial and autophagosomal processes, demonstrating its elevated levels in the vehicle control group and subsequent attenuation upon VTF treatment (Lee et al., 2021; Liu et al., 2023). This dynamic modulation suggests that VTF has the potential to mitigate Aβ production and, alleviate neurodegeneration. Within the autophagy cascade, Atg7 and Beclin-1 emerge as a pivotal regulator, representing a focal point for understanding VTF’s influence on autophagy levels (Caballero & Coto-Montes, 2012; Fîlfan et al., 2017). Our results reveal a significant reduction in Atg7 expression in Aβ1-42-induced SH-SY5Y cells, indicating increased autophagy. Notably, the subsequent decrease in Atg7 levels following VTF treatment signifies a potential mechanistic facet through which VTF maintains autophagy homeostasis, strengthening its neuroprotective role (Li et al., 2012). Our study revealed a notable increase in p62 expression following VTF treatment. This elevation aligns with our hypothesis that VTF inhibits excessive autophagy, thereby preserving nerve cell function (Caccamo et al., 2017). The observed inverse correlation between autophagy and p62 underscores the delicately orchestrated balance potentially sustained by VTF. These results provide valuable insights into the molecular underpinnings of VTF’s neuroprotective effects in the context of AD, highlighting its potential therapeutic significance.
Excessive autophagy is a notable concern in Aβ1-42-induced SH-SY5Y cells and AD, emphasizing the crucial need for effective therapeutic interventions. LC3, specifically the LC3-II/LC3-I ratios has emerged as a reliable indicator of autophagy levels (Chai et al., 2022; Heckmann et al., 2019; Pradeepkiran & Reddy, 2020). Our study underscores the significance of this crucial marker, revealing a discernible decrease in LC3-II/LC3-I ratios following VTF treatment. This substantial attenuation of excessive autophagy further enhances the neuroprotective potential of VTF in the intricate landscape of AD. The nuanced regulation of autophagy levels by VTF not only illuminates the molecular intricacies involved but also unveils promising avenues for therapeutic strategies targeting cellular homeostasis in neurodegenerative diseases. The upregulation of p62 seamlessly aligns with our hypothesis that VTF inhibits excessive autophagy, providing additional evidence of its potential to preserve nerve cell function. This delicate balance between autophagy markers, LC3, and p62 highlights the multifaceted impact of VTF on cellular homeostasis. This not only enriches our understanding of the molecular intricacies involved in neurodegenerative conditions but also lays the groundwork for targeted therapeutic strategies aimed at restoring autophagic equilibrium (Liu & Li, 2019; Ułamek-Kozioł et al., 2013). In Figure 4, we illustrate how VTF preserves mitophagy in Aβ1-42-induced AD neurodegeneration. Our findings highlight the significant neuroprotective effects of VTF and CQ against Aβ-induced damage. VTF treatment maintained cellular viability and preserved the integrity of mitochondria and autophagosomes integrity. Additionally, our study revealed the role of VTF and CQ in modulating key proteins in the autophagy–lysosomal pathway. These findings warrant further investigation of VTF’s therapeutic potential in AD.
These results highlight the potential of VTF as a therapeutic intervention for AD. However, further research is needed to understand the precise mechanisms involved and assess the safety and efficacy of VTF in clinical settings. Nonetheless, our study provides valuable insights into the neuroprotective effects of VTF, offering hope for the development of novel treatments for neurodegenerative diseases (Balea Ş et al., 2020; Chifenti et al., 2013; He et al., 2015).

Our study highlights the neuroprotective potential of VTF but acknowledges the limitations of our primarily in vitro approach. To better understand VTF’s mechanisms and therapeutic implications, we recognize the need for more extensive cellular and molecular in vitro investigations. These studies should focus on specific aspects, such as how flavonoids modulate autophagy and interact with key molecular pathways involved in neurodegeneration. Furthermore, structural elucidation of VTF’s effects at the cellular level, combined with comprehensive molecular analyses, will offer valuable insights into is therapeutic efficacy. Additionally, while alternative models, such as Aβ-induced mitochondrial dysfunction, could provide complementary evidence, resource constraints and the scope of our study prevented us from exploring these models in this study. Also, our study did not specifically assess alterations in autophagy flow, and we did not include a control experiment to evaluate whether the introduction of autophagy inhibitors could reserve the observed positive effects of VTF. Future research endeavors should aim to address these limitations by incorporating more comprehensive in vivo studies, including animal models, and conducting controlled experiments to unravel the intricate dynamics of autophagy modulation by VTF. Furthermore, the complex interplay between various autophagic markers, the specific mechanisms of flavonoids, and the multifaceted nature of AD pathology warrant further detailed exploration in subsequent investigations to enhance our understanding and pave the way for potential therapeutic applications (Funderburk et al., 2010; Nixon, 2007).
Exploring additional markers and signaling pathways associated with autophagy modulation by VTF could provide a more comprehensive understanding of its therapeutic potential. Moreover, extending these investigations to in vivo models and clinical studies would bridge the translational gap, offering a clearer picture of VTF’s efficacy in a more complex physiological context. The delineation of VTF’s influence on synaptic plasticity and cholinergic neurotransmitters, coupled with its role in autophagy regulation, opens avenues for targeted interventions that address multiple facets of AD pathology. This study lays the groundwork for further research aimed at harnessing the full potential of VTF as a promising candidate for neuroprotective strategies in the context of neurodegenerative disorders. By addressing these limitations and discussing avenues for further investigation, we aim to provide a transparent interpretation of our results and contribute to advancing our understanding of neuroprotective compounds for the treatment of neurodegenerative diseases.
5. Conclusion
In conclusion, our study reveals VTF’s novel role, alongside CQ, in preserving cellular health under Aβ1-42-induced stress, emphasizing its anti-neurodegenerative effects through modulation of mitophagy. While our results open avenues for further in vivo exploration, the study underscores VTF’s potential in targeting multiple facets of AD pathology, presenting a promising candidate for future neuroprotective interventions.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design, investigations, experiments, and final approval: All authors; Data analysis: Peng Zhang; Writing: Hui Xiao.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank College of Public Health, Xinjiang Medical University, Urumqi, China.
References
Abdul Manap, A. S., Madhavan, P., Vijayabalan, S., Chia, A., & Fukui, K. (2020). Explicating anti-amyloidogenic role of curcumin and piperine via amyloid beta (Aβ) explicit pathway: Recovery and reversal paradigm effects. PeerJ, 8, e10003. [DOI:10.7717/peerj.10003] [PMID]
Balea, Ş. S., Pârvu, A. E., Pârvu, M., Vlase, L., Dehelean, C. A., & Pop, T. I. (2020). Antioxidant, anti-inflammatory and antiproliferative effects of the vitis vinifera l. var. fetească neagră and pinot noir pomace extracts. Frontiers in Pharmacology, 11, 990. [DOI:10.3389/fphar.2020.00990] [PMID]
Benavente-García, O., & Castillo, J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of Agricultural and Food Chemistry, 56(15), 6185–6205. [DOI:10.1021/jf8006568] [PMID]
Bloom G. S. (2014). Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurology, 71(4), 505–508. [DOI:10.1001/jamaneurol.2013.5847] [PMID]
Caballero García, B., & Coto Montes, A. M. (2012). An insight into the role of autophagy in cell responses in the aging and neurodegenerative braenn. Histology and Histopathology, 27(3), 263-275. [Link]
Caccamo, A., Ferreira, E., Branca, C., & Oddo, S. (2017). p62 improves AD-like pathology by increasing autophagy. Molecular Psychiatry, 22(6), 865–873. [DOI:10.1038/mp.2016.139] [PMID]
Caporaso, G. L., Gandy, S. E., Buxbaum, J. D., & Greengard, P. (1992). Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America, 89(6), 2252–2256. [DOI:10.1073/pnas.89.6.2252] [PMID]
Chai, G. S., Wu, J. J., Gong, J., Zhou, J. L., Jiang, Z. Q., Yi, H. Y., . . . Nie, Y. J. (2022). Activation of β2-adrenergic Receptor Ameliorates Amyloid-β-induced Mitophagy Defects and Tau Pathology in Mice. Neuroscience, 505, 34-50. [DOI:10.1016/j.neuroscience.2022.09.020] [PMID]
Chan, K. Y., Wang, W., Wu, J. J., Liu, L., Theodoratou, E., & Car, J., et al. (2013). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: A systematic review and analysis. Lancet, 381(9882), 2016–2023. [DOI:10.1016/S0140-6736(13)60221-4] [PMID]
Chifenti, B., Locci, M. T., Lazzeri, G., Guagnozzi, M., Dinucci, D., & Chiellini, F., et al. (2013). Autophagy-related protein LC3 and Beclin-1 in the first trimester of pregnancy. Clinical and Experimental Reproductive Medicine, 40(1), 33–37. [DOI:10.5653/cerm.2013.40.1.33] [PMID]
Chung, K. M., Hernández, N., Sproul, A. A., & Yu, W. H. (2019). Alzheimer's disease and the autophagic-lysosomal system. Neuroscience Letters, 697, 49–58. [DOI:10.1016/j.neulet.2018.05.017] [PMID]
De-Paula, V. J., Radanovic, M., Diniz, B. S., & Forlenza, O. V. (2012). Alzheimer's disease. Sub-Cellular Biochemistry, 65, 329–352. [DOI:10.1007/978-94-007-5416-4_14] [PMID]
Dunys, J., Valverde, A., & Checler, F. (2018). Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?. The Journal of Biological Chemistry, 293(40), 15419–15428. [DOI:10.1074/jbc.R118.003999] [PMID]
Fedele, A. O., & Proud, C. G. (2020). Chloroquine and bafilomycin A mimic lysosomal storage disorders and impair mTORC1 signalling. Bioscience Reports, 40(4), BSR20200905. [DOI:10.1042/BSR20200905] [PMID]
Fîlfan, M., Sandu, R. E., Zăvăleanu, A. D., Greşiţă, A., Glăvan, D. G., & Olaru, D. G., et al. (2017). Autophagy in aging and disease. Romanian Journal of Morphology and Embryology, 58(1), 27-31. [Link]
Fonseca, M. B., Solá, S., Xavier, J. M., Dionísio, P. A., & Rodrigues, C. M. (2013). Amyloid β peptides promote autophagy-dependent differentiation of mouse neural stem cells: Aβ-mediated neural differentiation. Molecular Neurobiology, 48(3), 829–840. [DOI:10.1007/s12035-013-8471-1] [PMID]
Funderburk, S. F., Marcellino, B. K., & Yue, Z. (2010). Cell "self-eating" (autophagy) mechanism in Alzheimer's disease. The Mount Sinai Journal of Medicine, New York, 77(1), 59–68. [DOI:10.1002/msj.20161] [PMID]
Furst, D. E. (1996). Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus, 5(Suppl 1), S11-15. [DOI:10.1177/0961203396005001041]
Halcrow, P. W., Geiger, J. D., & Chen, X. (2021). Overcoming chemoresistance: Altering pH of cellular compartments by chloroquine and hydroxychloroquine. Frontiers in cell and Developmental Biology, 9, 627639. [DOI:10.3389/fcell.2021.627639] [PMID]
He, R., Peng, J., Yuan, P., Xu, F., & Wei, W. (2015). Divergent roles of BECN1 in LC3 lipidation and autophagosomal function. Autophagy, 11(5), 740–747. [DOI:10.1080/15548627.2015.1034404] [PMID]
Heckmann, B. L., Teubner, B. J. W., Tummers, B., Boada-Romero, E., Harris, L., & Yang, M., et al. (2019). LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine alzheimer's disease. Cell, 178(3), 536–51.e14. [DOI:10.1016/j.cell.2019.05.056] [PMID]
Jackson, R. J., Rudinskiy, N., Herrmann, A. G., Croft, S., Kim, J. M., & Petrova, V., et al. (2016). Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease. The European Journal of Neuroscience, 44(12), 3056–3066. [DOI:10.1111/ejn.13442] [PMID]
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., & Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. The Lancet. Public Health, 5(12), e661–e671. [DOI:10.1016/S2468-2667(20)30185-7] [PMID]
Joseph, D. K., Mat Ludin, A. F., Ibrahim, F. W., Ahmadazam, A., Che Roos, N. A., & Shahar, S., et al. (2023). Effects of aerobic exercise and dietary flavonoids on cognition: A systematic review and meta-analysis. Frontiers in Physiology, 14, 1216948. [DOI:10.3389/fphys.2023.1216948] [PMID]
Ke P. Y. (2024). Molecular mechanism of autophagosome-lysosome fusion in mammalian cells. Cells, 13(6), 500. [DOI:10.3390/cells13060500] [PMID]
Kim, D. H., Kim, S., Jeon, S. J., Son, K. H., Lee, S., & Yoon, B. H., et al. (2009). Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathway. British Journal of Pharmacology, 158(4), 1131–1142. [DOI:10.1111/j.1476-5381.2009.00378.x] [PMID]
Klionsky, D. J., Petroni, G., Amaravadi, R. K., Baehrecke, E. H., Ballabio, A., & Boya, P., et al. (2021). Autophagy in major human diseases. The EMBO Journal, 40(19), e108863. [DOI:10.15252/embj.2021108863] [PMID]
Kolachala, V. L., Lopez, C., Shen, M., Shayakhmetov, D., & Gupta, N. A. (2021). Ischemia reperfusion injury induces pyroptosis and mediates injury in steatotic liver thorough Caspase 1 activation. Apoptosis, 26(5-6), 361–370. [DOI:10.1007/s10495-021-01673-1] [PMID]
Krishnan, S., Shrestha, Y., Jayatunga, D. P. W., Rea, S., Martins, R., & Bharadwaj, P. (2020). Activate or inhibit? Implications of autophagy modulation as a therapeutic strategy for alzheimer's disease. International Journal of Molecular Sciences, 21(18), 6739. [DOI:10.3390/ijms21186739] [PMID]
Lee, J. H., Ahn, N. H., Choi, S. B., Kwon, Y., & Yang, S. H. (2021). Natural products targeting amyloid beta in alzheimer's disease. International Journal of Molecular Sciences, 22(5), 2341. [DOI:10.3390/ijms22052341] [PMID]
Li, H., Fu, X., Deng, G., David, A., & Huang, L. (2020). Extraction of oil from grape seeds (Vitis vinifera L.) using recyclable CO2-expanded ethanol. Chemical Engineering and Processing - Process Intensification, 157, 108147. [DOI:10.1016/j.cep.2020.108147]
Li, K., Wei, S., Liu, Z., Hu, L., Lin, J., & Tan, S., et al. (2018). The prevalence of alzheimer's disease in China: A systematic review and meta-analysis. Iranian Journal of Public Health, 47(11), 1615–1626. [PMID]
Li, Q., Chen, M., Liu, H., Yang, L., & Yang, G. (2012). Expression of APP, BACE1, AChE and ChAT in an AD model in rats and the effect of donepezil hydrochloride treatment. Molecular Medicine Reports, 6(6), 1450–1454. [DOI:10.3892/mmr.2012.1102] [PMID]
Li, X., Quan, M., Wei, Y., Wang, W., Xu, L., & Wang, Q., et al. (2023). Critical thinking of Alzheimer's transgenic mouse model: Current research and future perspective. Science China. Life Sciences, 66(12), 2711–2754. [DOI:10.1007/s11427-022-2357-x] [PMID]
Liu, J., & Li, L. (2019). Targeting autophagy for the treatment of alzheimer's disease: Challenges and opportunities. Frontiers in Molecular Neuroscience, 12, 203. [DOI:10.3389/fnmol.2019.00203] [PMID]
Liu, J., Li, T., Zhong, G., Pan, Y., Gao, M., & Su, S., et al. (2023). Exploring the therapeutic potential of natural compounds for Alzheimer's disease: Mechanisms of action and pharmacological properties. Biomedicine & Pharmacotherapy, 166, 115406. [DOI:10.1016/j.biopha.2023.115406] [PMID]
Lopresti, A. L., Smith, S. J., Pouchieu, C., Pourtau, L., Gaudout, D., & Pallet, V., et al. (2023). Effects of a polyphenol-rich grape and blueberry extract (Memophenol™) on cognitive function in older adults with mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Frontiers in Psychology, 14, 1144231. [DOI:10.3389/fpsyg.2023.1144231] [PMID]
Ma, L., Xiao, H., Wen, J., Liu, Z., He, Y., & Yuan, F. (2018). Possible mechanism of Vitis vinifera L. flavones on neurotransmitters, synaptic transmission and related learning and memory in Alzheimer model rats. Lipids in Health and Disease, 17(1), 152. [DOI:10.1186/s12944-018-0708-6] [PMID]
Moldovan, M. L., Carpa, R., Fize-an, I., Vlase, L., Bogdan, C., & Iurian, S. M., et al. (2020). Phytochemical profile and biological activities of tendrils and leaves extracts from a variety of vitis vinifera L. Antioxidants, 9(5), 373. [DOI:10.3390/antiox9050373] [PMID]
Nixon R. A. (2007). Autophagy, amyloidogenesis and Alzheimer disease. Journal of Cell Science, 120(Pt 23), 4081–4091. [DOI:10.1242/jcs.019265] [PMID]
Onozuka, H., Nakajima, A., Matsuzaki, K., Shin, R. W., Ogino, K., Saigusa, D., ... & Ohizumi, Y. (2008). Nobiletin, a citrus flavonoid, improves memory impairment and Aβ pathology in a transgenic mouse model of Alzheimer’s disease. The Journal of Pharmacology And Experimental Therapeutics, 326(3), 739-744. [DOI:10.1124/jpet.108.140293] [PMID]
Pedrioli, G., Patani, R., & Paganetti, P. (2020). Chloroquine, the coronavirus crisis, and neurodegeneration: A perspective. Frontiers in Neurology, 11, 596528. [DOI:10.3389/fneur.2020.596528] [PMID]
Pradeepkiran, J. A., & Reddy, P. H. (2020). Defective mitophagy in Alzheimer's disease. Ageing Research Reviews, 64, 101191.[DOI:10.1016/j.arr.2020.101191] [PMID]
Prakash, D., & Sudhandiran, G. (2015). Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. The Journal of Nutritional Biochemistry, 26(12), 1527–1539. [DOI:10.1016/j.jnutbio.2015.07.017] [PMID]
Rainsford, K. D., Parke, A. L., Clifford-Rashotte, M., & Kean, W. F. (2015). Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology, 23(5), 231–269. [DOI:10.1007/s10787-015-0239-y] [PMID]
Redmann, M., Benavides, G. A., Berryhill, T. F., Wani, W. Y., Ouyang, X., & Johnson, M. S., et al. (2017). Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biology, 11, 73–81. [DOI:10.1016/j.redox.2016.11.004] [PMID]
Rezai-Zadeh, K., Douglas Shytle, R., Bai, Y., Tian, J., Hou, H., & Mori, T., et al. (2009). Flavonoid-mediated presenilin-1 phosphorylation reduces alzheimer's disease beta-amyloid production. Journal of Cellular and Molecular Medicine, 13(3), 574–588. [DOI:10.1111/j.1582-4934.2008.00344.x] [PMID]
Rodriguez-Mateos, A., Vauzour, D., Krueger, C. G., Shanmuganayagam, D., Reed, J., & Calani, L., et al. (2014). Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Archives of Toxicology, 88(10), 1803–1853. [DOI:10.1007/s00204-014-1330-7] [PMID]
Salminen, A., Kaarniranta, K., Kauppinen, A., Ojala, J., Haapasalo, A., & Soininen, H., et al. (2013). Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome. Progress in Neurobiology, 106-107. [DOI:10.1016/j.pneurobio.2013.06.002] [PMID]
Sepulcre, J., Grothe, M. J., Sabuncu, M., Chhatwal, J., Schultz, A. P., & Hanseeuw, B., et al. (2017). Hierarchical organization of tau and amyloid deposits in the cerebral cortex. JAMA Neurology, 74(7), 813–820. [DOI:10.1001/jamaneurol.2017.0263] [PMID]
Skaper S. D. (2012). Alzheimer's disease and amyloid: Culprit or coincidence?. International Review of Neurobiology, 102, 277–316. [DOI:10.1016/B978-0-12-386986-9.00011-9] [PMID]
Spencer, J. P., Vafeiadou, K., Williams, R. J., & Vauzour, D. (2012). Neuroinflammation: Modulation by flavonoids and mechanisms of action. Molecular Aspects of Medicine, 33(1), 83–97. [DOI:10.1016/j.mam.2011.10.016] [PMID]
Stine, W. B., Jr, Dahlgren, K. N., Krafft, G. A., & LaDu, M. J. (2003). In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. The Journal of Biological Chemistry, 278(13), 11612–11622. [DOI: 10.1074/jbc.M210207200] [PMID]
Ułamek-Kozioł, M., Furmaga-Jabłońska, W., Januszewski, S., Brzozowska, J., Sciślewska, M., & Jabłoński, M., et al. (2013). Neuronal autophagy: Self-eating or self-cannibalism in Alzheimer's disease. Neurochemical Research, 38(9), 1769–1773. [DOI:10.1007/s11064-013-1082-4] [PMID]
Varma, V. R., Desai, R. J., Navakkode, S., Wong, L. W., Anerillas, C., & Loeffler, T., et al. (2023). Hydroxychloroquine lowers Alzheimer's disease and related dementias risk and rescues molecular phenotypes related to Alzheimer's disease. Molecular Psychiatry, 28(3), 1312–1326. [DOI:10.1038/s41380-022-01912-0] [PMID]
Wang, W. Z., Fang, X. H., Stephenson, L. L., Khiabani, K. T., & Zamboni, W. A. (2008). Ischemia/reperfusion-induced necrosis and apoptosis in the cells isolated from rat skeletal muscle. Journal of Orthopaedic Research, 26(3), 351–356. [DOI:10.1002/jor.20493] [PMID]
Xiao, H., Ma, L., Li, Y., Wu, X., & Yuan, F. (2017). Flavones from vitis vinifera L inhibits Aβ. International Journal of Clinical and Experimental Medicine, 10(6), 8866-8874. [Link]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2024/05/19 | Accepted: 2024/12/4 | Published: 2025/05/1
Received: 2024/05/19 | Accepted: 2024/12/4 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








