Volume 16, Issue 2 (March & April 2025)
BCN 2025, 16(2): 379-392 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nazari S, Gheibi M, Salehi Sangani P, Felehkari F, Gohardehi F, Niknamfar S, et al . Coenzyme Q10 Suppression of Cue-related Reinstatement Through Cognition Improvement and Hippocampal Increase in BDNF Expression in Morphine-dependent Male Rats. BCN 2025; 16 (2) :379-392
URL: http://bcn.iums.ac.ir/article-1-2912-en.html
URL: http://bcn.iums.ac.ir/article-1-2912-en.html
Somayeh Nazari1 

 , Mobina Gheibi1
, Mobina Gheibi1 

 , Pouria Salehi Sangani2
, Pouria Salehi Sangani2 

 , Farzaneh Felehkari3
, Farzaneh Felehkari3 

 , Farnam Gohardehi4
, Farnam Gohardehi4 

 , Saba Niknamfar1
, Saba Niknamfar1 

 , Hamid Jomehpour5
, Hamid Jomehpour5 

 , Hamed Ghazvini6
, Hamed Ghazvini6 

 , Seyedeh Masoumeh Seyedhosseini Tamijani7
, Seyedeh Masoumeh Seyedhosseini Tamijani7 

 , Mohammad Vahabzadeh-Kebria4
, Mohammad Vahabzadeh-Kebria4 

 , Fahimeh Mohseni8
, Fahimeh Mohseni8 

 , Raheleh Rafaiee *6
, Raheleh Rafaiee *6 




 , Mobina Gheibi1
, Mobina Gheibi1 

 , Pouria Salehi Sangani2
, Pouria Salehi Sangani2 

 , Farzaneh Felehkari3
, Farzaneh Felehkari3 

 , Farnam Gohardehi4
, Farnam Gohardehi4 

 , Saba Niknamfar1
, Saba Niknamfar1 

 , Hamid Jomehpour5
, Hamid Jomehpour5 

 , Hamed Ghazvini6
, Hamed Ghazvini6 

 , Seyedeh Masoumeh Seyedhosseini Tamijani7
, Seyedeh Masoumeh Seyedhosseini Tamijani7 

 , Mohammad Vahabzadeh-Kebria4
, Mohammad Vahabzadeh-Kebria4 

 , Fahimeh Mohseni8
, Fahimeh Mohseni8 

 , Raheleh Rafaiee *6
, Raheleh Rafaiee *6 


1- Student Research Committee, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Student Research Committee, School of Allied Medical, Mazandaran University of Medical Sciences, Sari, Iran.
4- Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
5- Psychiatry and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Department of Neuroscience, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Psychiatry and Behavioral Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
8- Center for Health Related Social and Behavioral Sciences Research, Shahroud University of Medical Sciences, Shahroud, Iran.
2- Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Student Research Committee, School of Allied Medical, Mazandaran University of Medical Sciences, Sari, Iran.
4- Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
5- Psychiatry and Behavioral Sciences Research Center, Mashhad University of Medical Sciences, Mashhad, Iran.
6- Department of Neuroscience, School of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Psychiatry and Behavioral Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
8- Center for Health Related Social and Behavioral Sciences Research, Shahroud University of Medical Sciences, Shahroud, Iran.
Keywords: Coenzyme Q10 (CoQ10), Cognitive dysfunction, Recurrence, Morphine (MOR), Opioid dependence
Full-Text [PDF 1357 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Opioids prove to be the most efficient pain relievers for treating various forms of pain (Jaremko et al., 2014). However, the use of opioids is limited by the fears of addiction and tolerance (Heshmatzad et al., 2021). One of the most potent substances that is obtained from opium poppy (Papaver somniferum plant) is morphine (MOR), which can lead to relaxation and euphoria (Raehal & Bohn, 2005). Therefore, MOR and other opioids, such as heroin, have extremely high abusive potential (Ghamati et al., 2014). In recent decades, studies have considered opioid addiction to be a “relapsing chronic disease” that severely affects public health (Leshner, 1998). Of note, the persistence of opioid use disorder is known by the resumption of drug-seeking and drug-abusing behaviors that are activated by stimuli associated with drug use, even long after the withdrawal period (Han et al., 2015). Cue-related reinstatement in MOR-addicted rats refers to the phenomenon where environmental cues associated with drug use trigger a resurgence of drug-seeking behavior after a period of abstinence. Studies show that the insular cortex (IC) and the nucleus accumbens (NAc) are critical in mediating cue-induced reinstatement of MOR-seeking behavior. Activation of glutamatergic projections from the IC to the NAc core is essential for this reinstatement (Zhang et al., 2019).
Opioid addiction severely disrupts cognitive activities such as learning, memory, and mood processes, which can be the reason for returning to drug use. For example, a past study has revealed that chronic MOR significantly impairs working memory in rats (Sala et al., 1994). Also, a previous study has shown that the inhibitory memory of rats is significantly impaired by post-training administration of MOR (2.5, 5, and 7.5 mg/kg) (Tavassoli et al., 2017). Furthermore, it has been shown that long-term MOR addiction strongly attenuates neurogenesis, disrupts memory performance, and changes emotional reactivity and anxiety levels in male rats (Famitafreshi et al., 2015). Previous research has shown that rats exposed to long-term MOR exhibit deficits in spatial memory as assessed by the Morris water maze task (Brolin et al., 2018). In addition, reports indicate that MOR can increase oxidative stress in the rodent brain (Milanesi et al., 2023). As we know, oxidative stress can lead to a wide range of cognitive impairments (Kholghi et al., 2023; Mehrabanifar et al., 2023).
Coenzyme Q10 (2, 3-dimethoxy-5-methyl-6-multiprenyl-1, 4-benzoquinone, [CoQ10]) is a fat-soluble vitamin-like substance and an essential electron carrier in the respiratory chain of the inner mitochondrial membrane for ATP metabolic processes. The nature of CoQ10 in the rapid acquisition and loss of electrons causes the potent antioxidant properties of this compound. CoQ10 prevents lipid peroxidation and protein oxidation by avoiding the generation of peroxyl radicals. Every cell is well equipped with an enzymatic antioxidant defense system to deal with oxidative stress. CoQ10 passes through the blood-brain barrier if taken orally or by intravenous injection. CoQ10 prevents tissue damage and causes exogenous preservation and survival of nerve cells. The medical literature highlights the recurring emphasis on the beneficial impact of CoQ10 on learning and memory. The positive effects of CoQ10 on the learning and memory process are emphasized in the medical literature. A recent study has shown that CoQ10 significantly improves amyloid-beta (Aβ)-induced decline in discrimination index (DI) in the novel object recognition (NOR) test, learning, and spatial memory tested in the Morris water maze, passive avoidance memory and learning, and long-term potentiation deficit in the hippocampus of aged animals (Asadbegi et al., 2023). In another study, CoQ10 showed anti-inflammatory and antioxidant properties.
The change of brain-derived neurotrophic factor (BDNF) in MOR-addicted rats is a significant area of research, revealing complex interactions between substance use and neuroplasticity. MOR dependence leads to a decrease in BDNF levels of cerebrospinal fluid during active addiction. At the same time, withdrawal triggers an increase in BDNF levels (Rezamohammadi et al., 2020). In the hippocampus, BDNF levels were found to enhance during MOR withdrawal, suggesting a compensatory mechanism following addiction (Fatahi et al., 2020). Increased BDNF expression in the ventral tegmental area (VTA) was associated with reduced behavioral sensitization to MOR, indicating a protective role against addiction (Deng et al., 2023). CoQ10 was observed to stimulate the production of BDNF, an essential protein involved in neuroplasticity associated with learning and memory, and SOX2, a transcription factor critical for maintaining self-renewal, in the hippocampus of a rat model of Alzheimer disease (Sheykhhasan et al., 2022). A study has shown that treatment with CoQ10 may lead to neuroprotection against the detrimental effects of Aβ on synaptic plasticity in the hippocampus of rats by improving antioxidant activity (Komaki et al., 2019). Furthermore, it has been shown that CoQ10 can alleviate cognitive impairments induced by Intracerebroventricular (ICV) injection of streptozotocin in experimental rodents (Ishrat et al., 2006). According to these findings, we aim to investigate the effect of CoQ10 on cognitive impairments, cue-related reinstatement, and expression of BDNF in MOR-dependent male rats.
2. Materials and Methods
Study animals
This study used 48 male Wistar rats (200-220 g). Each Plexiglas cage consisted of 4 rats housed under a 12-h light cycle (lights beginning at 7:00 AM), constant humidity, and temperature (23±2 °C). All the rats had free access to food and water, and all the experiments were conducted during the light hours (8:00 AM to 3:00 PM). Also, the rats were bred at the Neuroscience Research Center, Mazandaran University of Medical Sciences, Sari, Iran. Our experimental protocol was designed under the National Institutes of Health Guide for the Care and Use of Animals Lab (Politis et al., 2011).
Drugs
Experimental protocol
This study included 6 experimental groups (n=8) as follows:
1) Oil group: Received sesame oil for one month through oral gavage.
2) MOR+oil group: Received MOR for 3 weeks and normal saline by oral gavage during one month of withdrawal.
3) MOR+CoQ10-100 group: Received MOR for 3 weeks and CoQ10 (100 mg/kg) by oral gavage.
4) MOR+CoQ10-200 group: Received MOR for 3 weeks and CoQ10 (200 mg/kg) for a month by oral gavage.
5) MOR+CoQ10-400 group: Received MOR for 3 weeks and CoQ10 (400 mg/kg) for a month by oral gavage.
6) CoQ10-400 group: Received CoQ10 (400 mg/kg) for a month by oral gavage.
MOR sulfate powder was dissolved in distilled water (DW). Rats were administered increasing doses of MOR (25 to 100 mg/kg, SC) once a day for 21 days: Days 1-5, 5 mg (25 mg/kg, SC); days 6-10, 10 mg (50 mg/kg, SC); days 11-15, 15 mg (75 mg/kg, SC); days 16-21, 20 mg (100 mg/kg/SC). CoQ10 was dissolved in sesame oil and administered through gavage. The volume of oral gavage was 1 mL (Ebrahimi & Esmaeili-Mahani, 2020; Matthews et al., 1998; Paul & Gueven, 2021; Rauscher et al., 2001; Shibani et al., 2019).
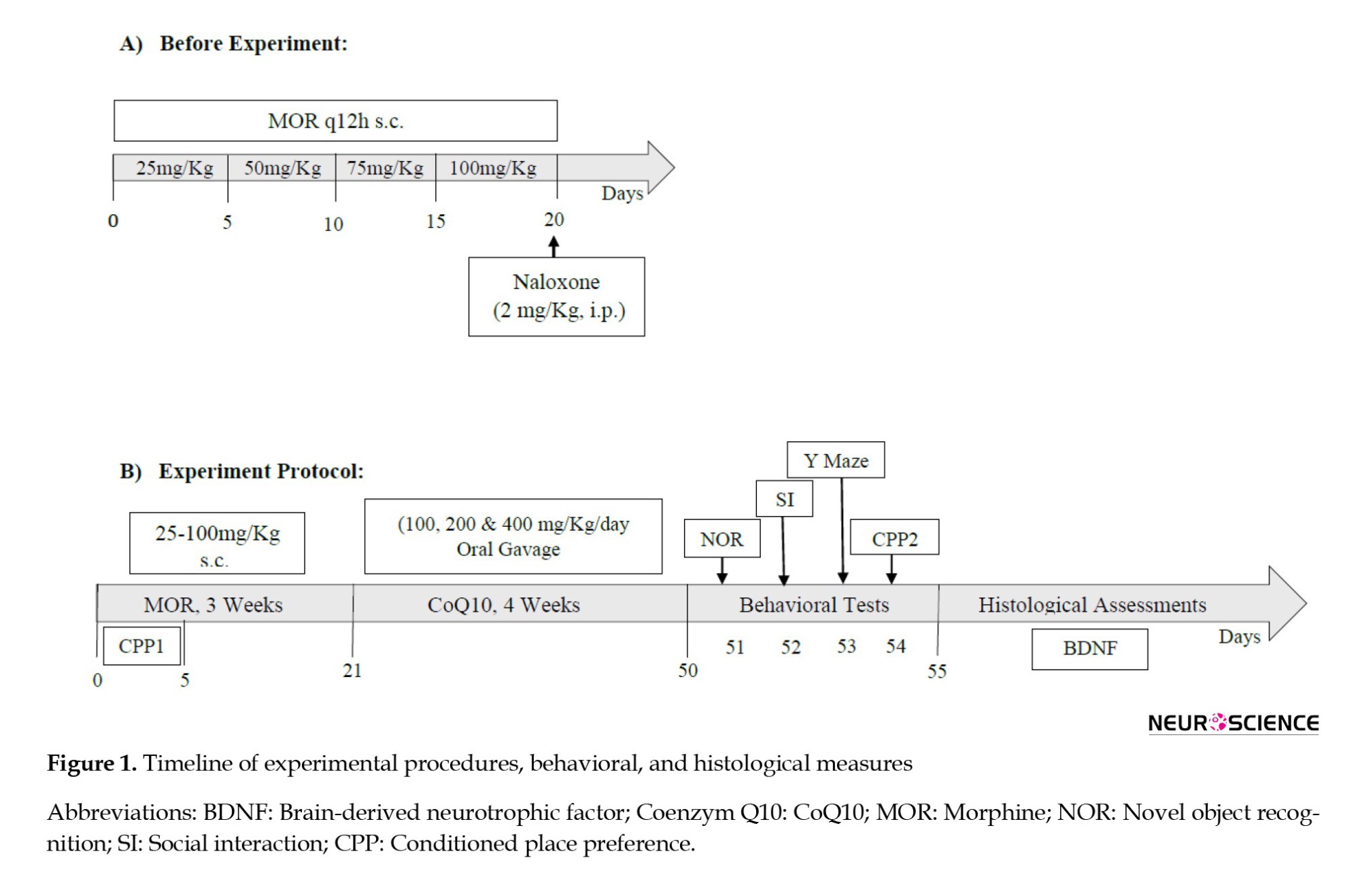
To confirm MOR dependence, on day 21, naloxone (2 mg/kg, IP) was injected intraperitoneally two hours after MOR administration. Withdrawal symptoms were immediately observed (jumping, shaking the head like a dog, grinding teeth, chewing, standing on two legs, scratching the cage, digging) and recorded for 30 minutes (Figure 1A). The frequency of defecation while observing the signs of withdrawal (30 minutes) and the percentage of 24-hour weight loss of the rats were measured. After observing and recording the withdrawal signs, the rats were returned to the cage (Akbari & Mirzaei, 2013). After 21 days of addiction, CoQ10 treatment was administered orally with a gavage syringe number 24 at doses of 100, 200, and 400 mg/kg once a day for one month (Figure 1B). Then, behavioral tests, including NOR test, Y-maze, social interaction, and conditioned place preference (CPP), were performed in all animals of groups, except the animals of the MOR-naloxone group used for the confirmation addiction model. Four animals per group for immunofluorescent staining were used.
NOR test
The investigation of the NOR test was performed in a cube-shaped box measuring 40×50×50 cm3, in which different objects can be placed in a fixed position in two adjacent corners. This test consists of three phases: Habituation, training, and testing. In the habituation phase, the rat is placed in the middle of the box without objects for 10 minutes to investigate the arena thoroughly. This test phase is repeated the next day in the same way. The habituation phase is completed at the end of two consecutive days of the habituation phase.
In the training phase, two identical objects (A & A) are placed in the box, and the animal is allowed to examine the objects for 10 minutes. The animal testing criterion is touching the object with the nose and putting the nose directly at a distance of less than 2 cm from the object. Leaning the animal against the objects or sitting on the objects is not considered part of the time for searching or examining the object. During the training phase, the time each animal spent exploring each object was recorded.
In the testing phase, a new object (A & B) is replaced by one of the previous objects in the box. In this phase, the animal has 10 minutes to examine the objects. It should be noted that the objects are exchanged for the next animal in each test phase to rule out the possibility of preference. After each animal has left the box, the environment and objects in the box are cleaned with 70% alcohol. The time each animal spends with each object is recorded. The DI (the time each animal spends examining the novel object (object B) minus the time spent examining the repeated object (object A), is divided by the total time spent examining both objects (Lissner et al., 2021).
Y-maze
Working memory was assessed using the Y-maze test. This test consisted of three identical arms, each 45 cm long and 10 cm wide and with a wall height of 30 cm. These arms were arranged at an angle of 120° to each other. The test was carried out in low light (5 lx). The performance of the rats during the test was recorded with a video camera attached above the device, and the order of entries into the maze arms (arm entries, AE) was recorded manually. The percentage of spontaneous alternations (SA) was determined as follows (Equation 1):

Only trials with 8 or more arm movements were included in the data analysis (Shcherbakova et al., 2023).
Social interaction (SI)
The SI test was carried out for 10 minutes in a box with dimensions of 43×19×22 cm3) divided into three parts (this space is unfamiliar to the animals, and there is no habituation). Two wired cup-like cages, large enough to accommodate the rat, were placed in two side chambers once an unfamiliar rat was placed. The experiment was conducted during the light phase with an intensity of 650 lx. An observer was carefully observed and recorded the parameters of the test. At the acclimatization stage, empty wire cages were placed in each part, and the rat was placed in the middle part, where it remained for 5 min to achieve habituation. The first stage of the test is the aspect of social tendencies; in this part of the behavioral test, the aim is to check the social tendencies of the rat. The assumption of the test is based on the fact that the natural subject animal has a greater desire to communicate with another animal than to interact and communicate with an empty chamber. At this stage, one of the control rats (stranger one) was placed in one of the chambers. The doors between the parts were removed so that the subject rat placed in the middle part could move between the areas. The following parameters were carefully checked and observed. First, the duration of direct (active) contact with a rat (a stranger) inside the chamber is checked. In the second stage of the test, the level of the animal’s desire to establish new social relationships and the type of its preference is checked. A healthy subject is assumed to show more desire and preference to communicate with a new animal. Therefore, the second animal (stranger 2) was placed in another empty chamber. The parameters stated in the previous stage were observed and checked, and the behavioral differences of the rat in interaction with stranger 1 and compared with stranger 2 were considered and observed. The duration of the two stages was 10 minutes (Kaidanovich-Beilin et al., 2011).
CPP
The CPP was tested in a three-part Plexiglas box (30×40×30 cm3). The CPP procedure comprised a pretest followed by a 3-day conditioning phase and a test divided into three different phases:
Familiarization phase, the conditioning phase, and the test phase. Before making the addiction model (on the first day), each rat was placed in the neutral box for 15 minutes, where it had free access to all three rooms. The time spent in each room was measured, and the preferred room was determined. From the second to the fourth day, the conditioning phase, each rat received an injection of MOR and was placed and confined in the non-preferred room of the CPP device for 45 min while the doors were closed. After 6 hours, each rat received an injection of DW as a vehicle for MOR and was moved to the preferred room of the CPP. On the third day, the protocol was the same as the first day, from morning to afternoon and vice versa.
In the test phase (on the fifth day), each rat was tested for the CPP under MOR-free conditions. The rat was placed in the CPP apparatus for 10 minutes and freely moved to all three rooms. The time spent in each room was recorded. The CPP score was the difference between the time spent in the rooms paired with and without reward (Jamali et al., 2021).
Immunohistochemistry method
To perform an immunohistochemical examination (n=4), rats were anesthetized with xylazine (20 mg/kg) and ketamine (160 mg/kg) and were stabilized by transcranial perfusion. Then, the heads of the rats were separated, and the brains were removed and kept in a 10% buffered formalin solution. About 48 to 72 hours after immersing the brain in a 10% formalin solution, the samples were cut from the hippocampus, and tissue processing and preparation of paraffin blocks were performed. Afterward, 5-µ thick slices were prepared from paraffin blocks by microtome. For immunohistochemical staining, the slides were first deparaffinized, and then other steps were performed in the following order:
Deparaffinizing the slides in Xylenol solution, hydration in descending alcohols at 100, 90, 80, and 70%, respectively, incubation of the slices in standard sodium citrate (SSC 2x) for 2 hours at 65 degrees, incubation in 2N HCL for 30 minutes at 37 °C, soaking in one-tenth normal boric acid (pH=8.5) for 10 minutes, washing in phosphate-buffered saline (PBS), incubation with primary antibody (BDNF) overnight at 4 °C, immersion in three loads in PBS and each time for 10 minutes, incubation with secondary antibody-BDNF for 2 hours, hematoxylin staining for background staining (Counter staining), sticking the slide, observing with a light microscope, examining the slides with ImageE software, and determining the level of BDNF expression in different test groups and preparing images of the tissues by microscope (Sayyah et al., 2022).
Statistical analyses
The behavioral and histological data were analyzed using GraphPad Prism software. One-way analysis of variance (ANOVA), two-way ANOVA, and post hoc Tukey test were used to compare the differences among the experimental groups. Data are presented as Mean±SD, and P<0.05 was considered statistically significant.
3. Results
Effects of CoQ10 on recognition memory
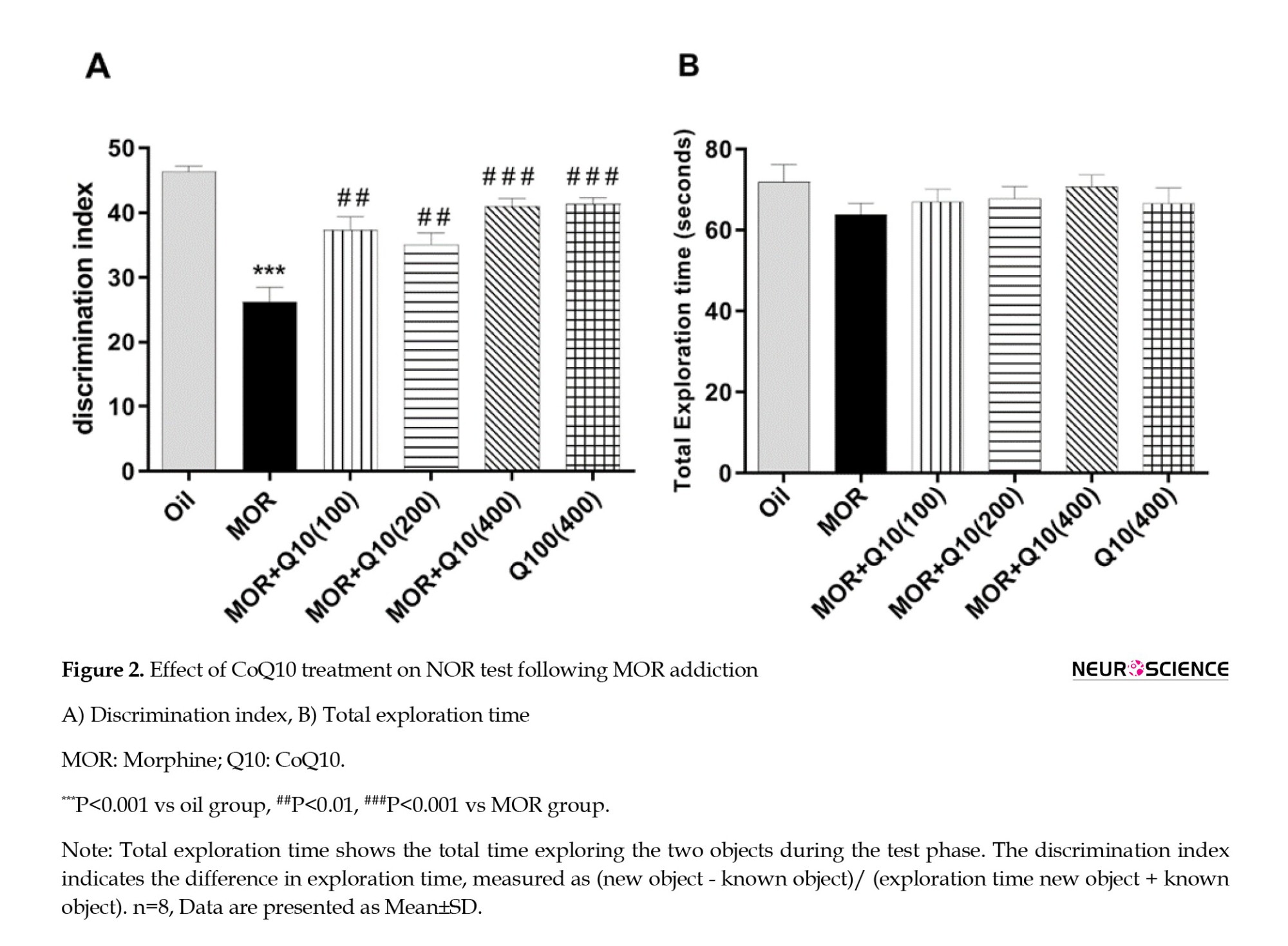
The MOR group showed a significant decrease in DI compared with the oil group (P<0.001, Figure 2A). This finding shows the cognitive dysfunction observed in MOR-addicted rats. DI was significantly increased in the MOR-CoQ10-100, MOR-CoQ10-200, and MOR-CoQ10-400 groups of rats compared with the MOR group (P<0.01, P<0.001, Figure 2A). In addition, Figure 2B indicates no significant difference in total time of exploration in comparison with the MOR group. MOR-addicted rats treated with CoQ10 explored more new objects, and the DI increased, suggesting that CoQ10 has a neuroprotective impact on the recognition memory impairment caused by MOR.
Effects of CoQ10 on working memory in the Y-maze
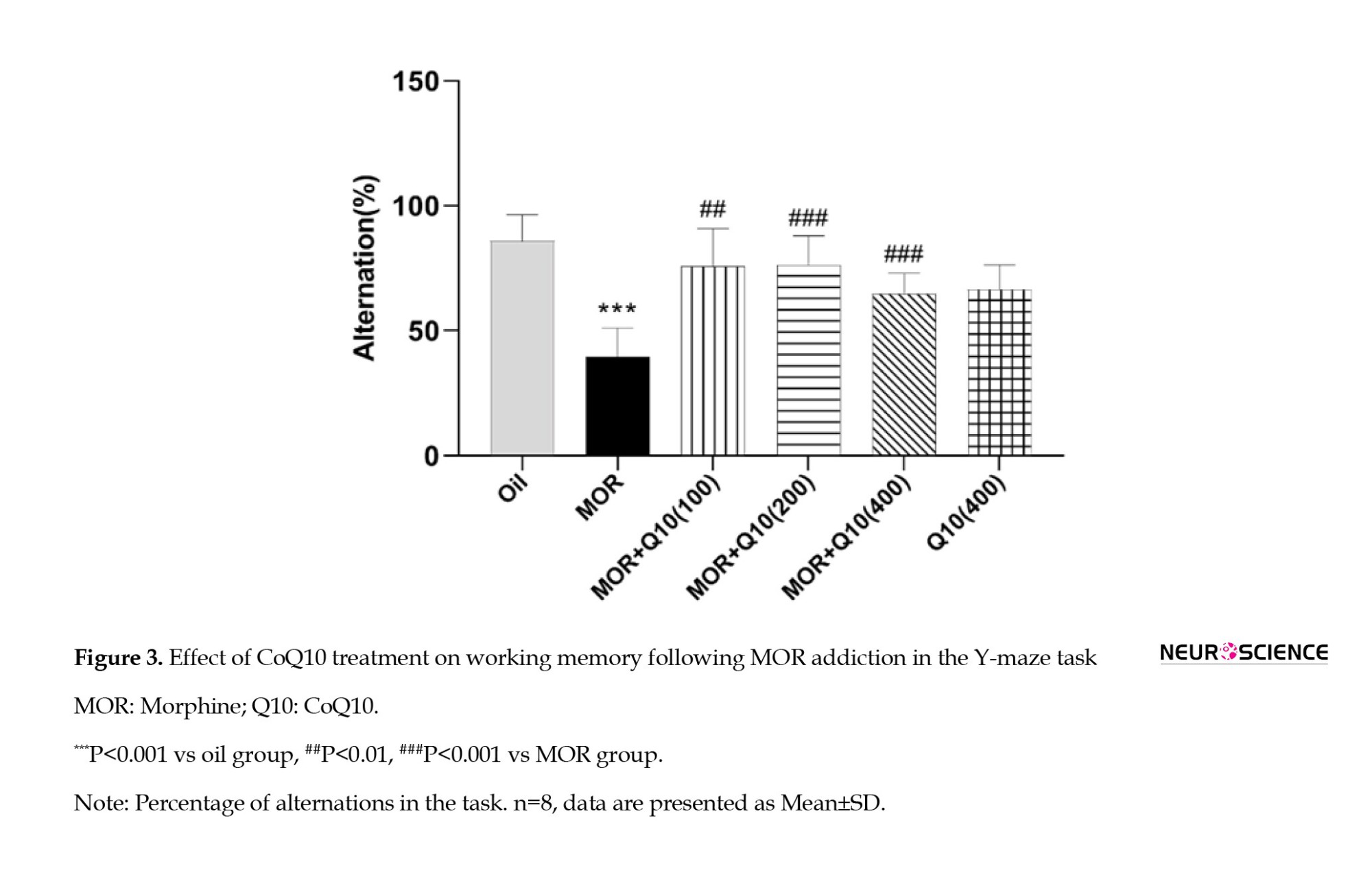
The MOR group showed a significant (P<0.001) decrease in percentage alternations compared to the oil group, indicating a loss of memory in the MOR group. The CoQ10-treated groups showed a statistically significant (P<0.01, P<0.001, Figure 3) increase in percentage alternations compared to the MOR group.
Effects of CoQ10 on social interaction
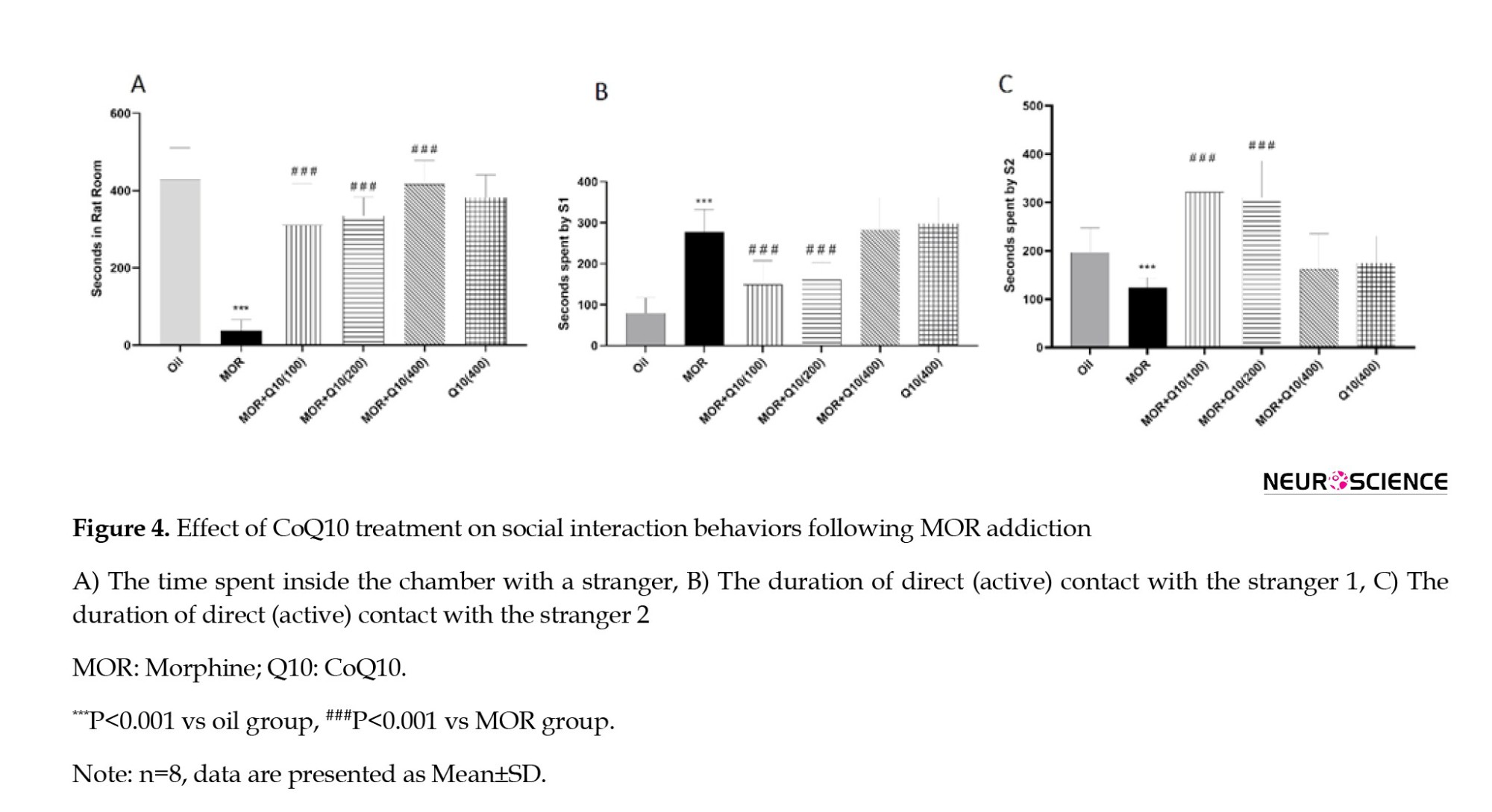
The MOR group showed a significant (P<0.001, Figure 4A) decrease in time spent in social interaction (staying in a rat room). The CoQ10 treated groups showed a statistically significant (P<0.001, Figure 4A) increase in time spent in social interactive behaviors. Figure 4, parts B and C, displays statistically significant (P<0.001) interaction between MOR-CoQ10-100 and MOR-CoQ10-200 groups of rats regarding sociability interaction with trapped stranger 2 (unfamiliar rat) compared with trapped stranger 1 (now-familiar rat).
Effects of CoQ10 on relapse in the CPP
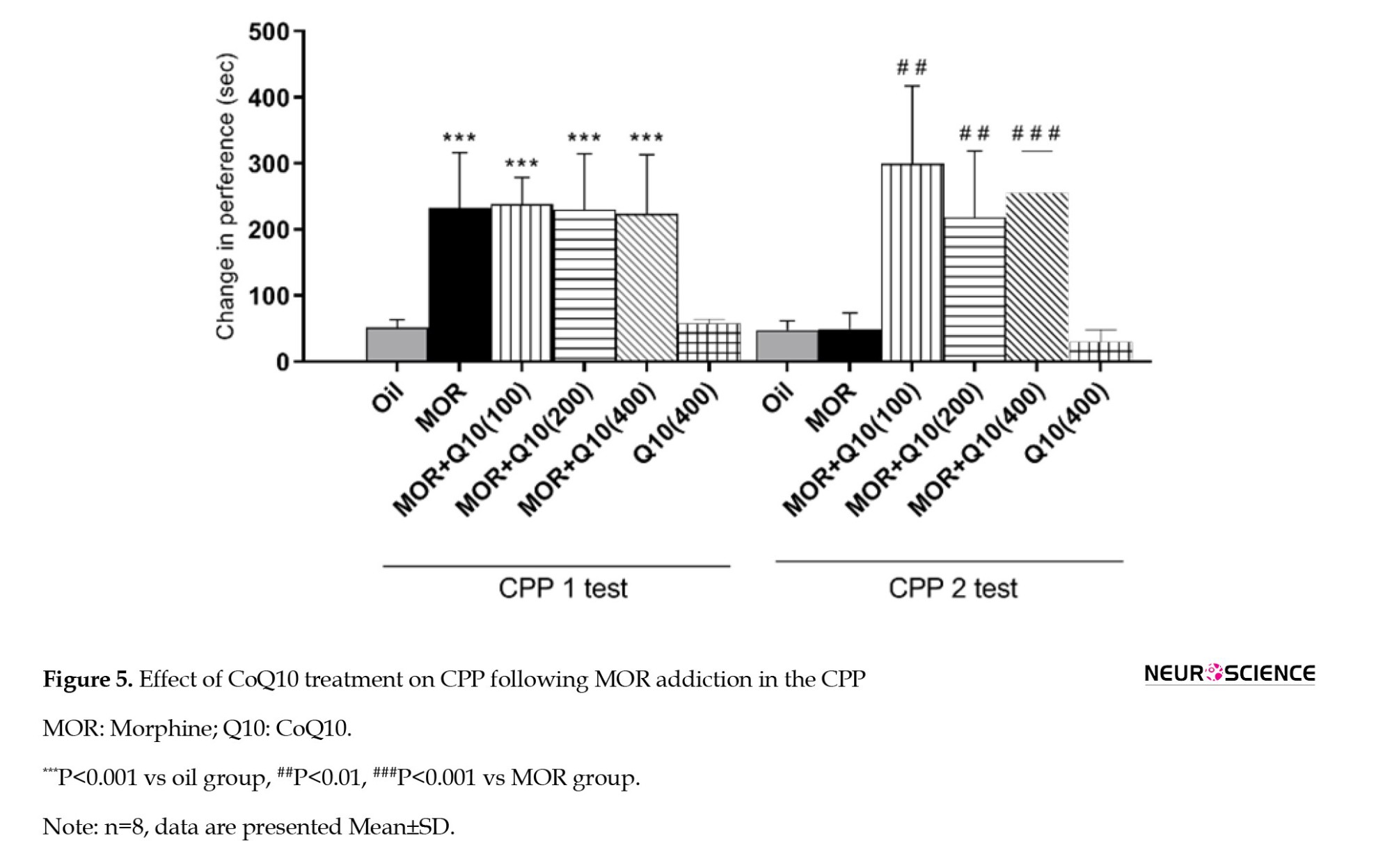
The rats in all groups that received MOR over three days of conditioning showed a significant (P<0.001, Figure 5, CPP1) increase in time spent in the MOR-paired room. This finding shows the place conditioning that occurred in MOR-addicted rats. After CoQ10 treatment in MOR-addicted rats, preference changes significantly increased in CoQ10-treated groups (P<0.01, P<0.001). The finding indicated that the time spent in the MOR-paired chamber reduced significantly (between CPP1 and CPP2), suggesting reduced relapse in CoQ10-treated groups.
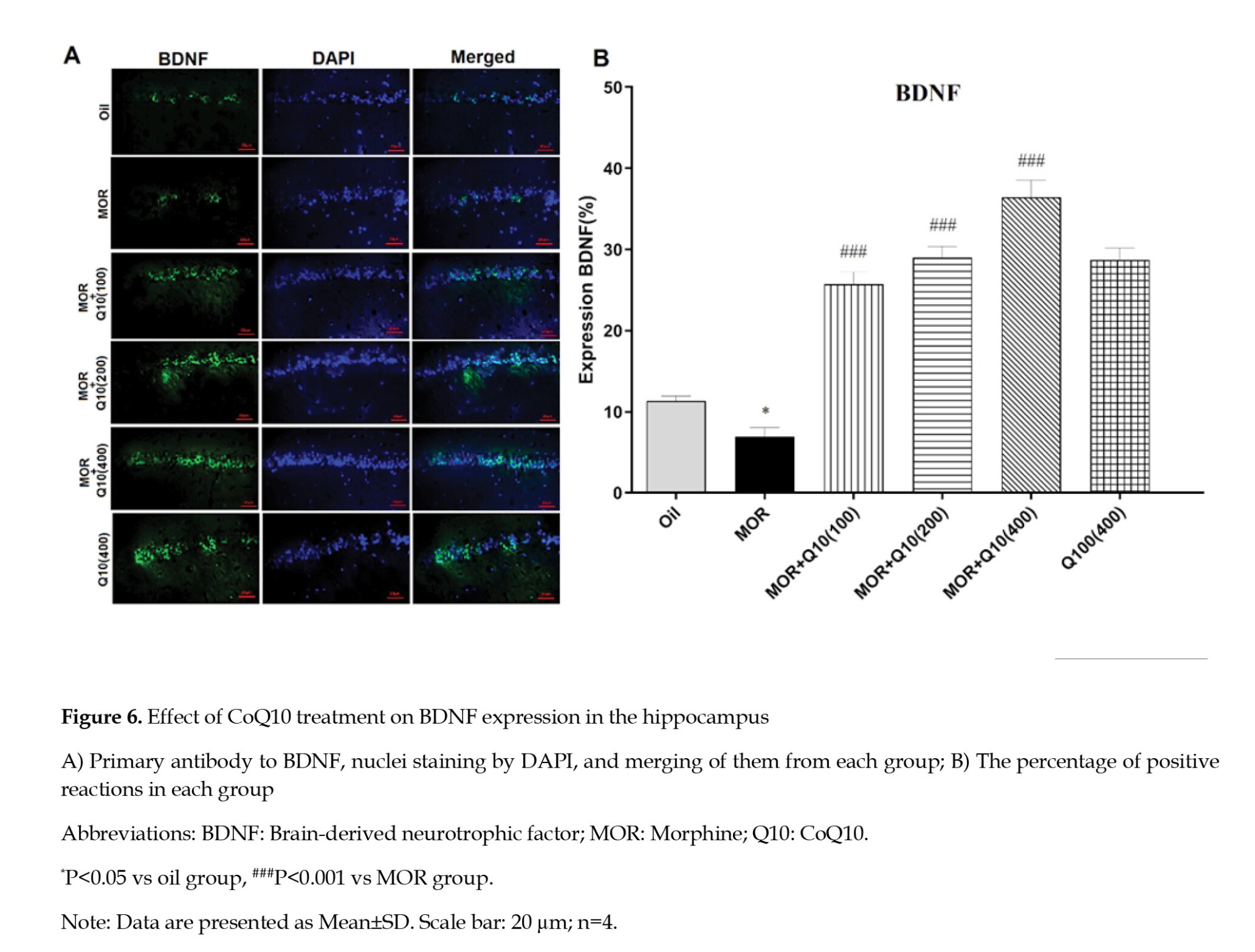
Effects of CoQ10 on BDNF expression in hippocampus
The MOR-addicted group showed a significantly lower BDNF expression level of the hippocampus compared with the oil group (P<0.05, Figure 6). The CoQ10-treated groups showed a statistically significant increase in BDNF expression level compared with the MOR-addicted group (P<0.001, Figure 6).
4. Discussion
The present research examined the impacts of CoQ10 treatment on cognitive impairments and relapse induced by MOR use and BDNF expression. Based on the findings, CoQ10 significantly improved NOR, working memory, SI, and cue-related reinstatement. Also, it significantly increased the expression of BDNF in the hippocampus of male rats. Our results show that CoQ10 may have a role in the treatment of cognitive deficits and relapse caused by MOR addiction. Our findings are in line with the multiple studies using laboratory animals that have reported the beneficial protective impacts of CoQ10 against various CNS disorders, including Parkinson disease (Ghasemloo et al., 2021; Park et al., 2020), Alzheimer disease (Komaki et al., 2019; Sheykhhasan et al., 2022), aging-induced memory impairments (Hosseini et al., 2022), diabetes-induced memory impairments (Monsef et al., 2019; Omidi et al., 2019), 2,2-dichlorovinyl dimethyl phosphate (DDVP)-induced cognitive impairments and neurodegeneration (Binukumar et al., 2012). Our study is in line with studies showing that MOR impairs NOR memory. Craig and Bajic in a study in 2015, reported that performance on the NOR test of neonatal rats was compromised in the prolonged MOR administration group at a young age (postnatal days 27–31) (Craig & Bajic, 2015). Also, the results of a study by Paris et al. indicated significantly reduced NOR after ICV. The administration of Kappa opioid receptor (KOR) agonists and the pretreatment of KOR antagonists prevented KOR-mediated dysfunction in NOR (Paris et al., 2011). Moreover, in a study, Alipour et al. (2023) reported that paternal MOR use during adolescence can cause a dysfunction in the recognition of new objects and passive avoidance memory in male offspring (Alipour et al., 2023). Also, Ellis et al. (2020) showed paternal MOR addiction led to NOR memory deficit in the female offspring. The findings of the present study, in agreement with a lot of evidence, indicated MOR has a negative impact on working memory (Amirteimoury et al., 2019; Bach et al., 2021; Bonk, 2011; Wang et al., 2013). Conversely, Miladi Gorji et al. (2008) showed that chronic use of MOR did not affect either retention or acquisition of spatial working memory. Also, our data, in agreement with previous research, indicate that MOR administration in adult rats reduces social interaction and leads to less time spent interacting with social peers (Šlamberová et al., 2016). Hughes et al. (2021) demonstrated that MOR reduced social novelty preference and sociability behavior in adolescent rats (postnatal days 28–43).
Our findings reveal the beneficial effects of CoQ10 treatment on cognitive impairments in MOR-addicted male rats. CoQ10 has been studied for its impact on memory and cognitive function in various contexts, and the results are controversial. CoQ10 supplementation is recommended as an adjunct to conventional treatment of migraine, Alzheimer disease, multiple sclerosis, coronary heart disease, and cerebrovascular accidents (Rauchová, 2021). The obtained findings are consistent with the study of Omidi et al. (2019) on rats with streptozotocin-induced diabetes; high-dose supplementation with CoQ10 improved spatial memory and learning, while low-dose supplementation did not have an effect (Omidi et al., 2019). Similarly, in a study on middle-aged rats by Monsef et al. (2019), CoQ10 supplementation improved memory, learning, and cognitive activities in both diabetic and healthy subjects, with higher doses showing better results. In contrast, Maguire et al. (2021) reported that CoQ10 did not affect working memory and attention in patients with schizophrenia and schizoaffective disorder. It should be noted that the FDA has not approved CoQ10 for treating any diseases. It is consumed as a food additive, not a drug (Arenas‐Jal et al., 2020). Moreover, Sumien et al. (2009) found that prolonged consumption of CoQ10 in a low dose has no discernable effect on motor and cognitive activities. In contrast, higher dose intake exacerbates sensory and cognitive dysfunction in old mice.
The present results reveal that our findings support literature indicating that the BDNF levels in the brains of MOR-addicted male rats are altered. Fatahi et al. (2020) reported that BDNF levels increased during delay-based decision-making in the hippocampus of MOR-addicted rats after withdrawal from MOR. Deng et al. (2022) showed that the BDNF-adenoviral vector administered in the VTA by injection decreased MOR-induced CPP with changes in BDNF/ tyrosine kinase B (TrkB)/cAMP-response element-binding protein (CREB) levels in the VTA and NAc. Furthermore, a study conducted by Peregud et al. (2020) revealed that chronic MOR intoxication reduces the binding of HuD, an RNA-binding protein, to the long 3’-UTR of BDNF, and MOR withdrawal stimulates the expression of BDNF in the frontal cortex of rats.
The results of our research suggest that the administration of CoQ10 could increase the expression of BDNF in the hippocampus after chronic use of MOR in male rats. In line with our results, Vaselbehagh et al. (2021) found that CoQ10 treatment significantly increased the expression of BDNF in the CA1 area of the hippocampus in methadone-induced neurotoxicity. Also, Nagib et al. (2019) reported α-tocopherol and or CoQ10 on phenytoin-induced cognitive dysfunction through a change in the BDNF-TrkB-CREB signaling pathway. In addition, Abuelezz et al. (2023) showed a neuroprotective effect of CoQ10 against scopolamine-induced cognitive impairment by activating the phosphoinositide-3 kinase (PI3K)/AKT/glycogen synthase kinase-3β (GSK-3β)/CREB/BDNF/TrKB signaling pathway. Moreover, the investigation carried out by Abuelezz et al. (2023) exhibited the neuroprotective influence of CoQ10 in countering scopolamine-induced cognitive dysfunction through the stimulation of the PI3K/Akt/GSK-3β/CREB/BDNF/TrKB signaling pathway.
CoQ10 transport across the blood-brain barrier involves complex mechanisms primarily mediated by lipoprotein interactions. Studies indicate that CoQ10 is transported via scavenger receptors and the receptor for advanced glycation end products, facilitating its uptake into the brain. However, efflux mechanisms, particularly through low-density lipoprotein receptors, limit net transport (Wainwright et al., 2020). While the primary focus is on CoQ10, its metabolites, particularly ubiquinol, may influence transport dynamics. Studies suggest that the redox state of CoQ10 affects its accumulation in brain tissues, indicating that metabolites could play a role in modulating transport efficiency (Watanabe et al., 2019).
In conclusion, CoQ10 treatment at 100, 200, and 400 mg/kg within 4 weeks could improve cognitive impairments and reduce cue-related reinstatement in MOR-addicted male rats. The histological assays supported the neuroprotective effects of CoQ10 in the hippocampus. CoQ10 may be a potential therapeutic agent for MOR-induced cognitive impairments and relapse.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed under the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1401.029).
Funding
This research was funded by a specific project grant from Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 8951).
Authors' contributions
Conceptualization and data curation: Raheleh Rafaiee, and Somayeh Nazari; Methodology and investigation: Pouria Salehi, Farzaneh Felehkari, Farnam Gohardehi, Mohammad Vahabzadeh-Kebria; Formal analysis: Hamid Jomehpour, Saba Niknamfar, and Mobina Gheibi; Writing: Raheleh Rafaieeand and Hamed Ghazvini; Funding acquisition: Seyedeh Masoumeh Seyedhosseini Tamijani, and Hamid Jomehpour; Supervision; Raheleh Rafaiee; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors gratefully appreciate the Deputy for Research at the Mazandaran University of Medical Sciences, Sari, Iran.
References
Opioids prove to be the most efficient pain relievers for treating various forms of pain (Jaremko et al., 2014). However, the use of opioids is limited by the fears of addiction and tolerance (Heshmatzad et al., 2021). One of the most potent substances that is obtained from opium poppy (Papaver somniferum plant) is morphine (MOR), which can lead to relaxation and euphoria (Raehal & Bohn, 2005). Therefore, MOR and other opioids, such as heroin, have extremely high abusive potential (Ghamati et al., 2014). In recent decades, studies have considered opioid addiction to be a “relapsing chronic disease” that severely affects public health (Leshner, 1998). Of note, the persistence of opioid use disorder is known by the resumption of drug-seeking and drug-abusing behaviors that are activated by stimuli associated with drug use, even long after the withdrawal period (Han et al., 2015). Cue-related reinstatement in MOR-addicted rats refers to the phenomenon where environmental cues associated with drug use trigger a resurgence of drug-seeking behavior after a period of abstinence. Studies show that the insular cortex (IC) and the nucleus accumbens (NAc) are critical in mediating cue-induced reinstatement of MOR-seeking behavior. Activation of glutamatergic projections from the IC to the NAc core is essential for this reinstatement (Zhang et al., 2019).
Opioid addiction severely disrupts cognitive activities such as learning, memory, and mood processes, which can be the reason for returning to drug use. For example, a past study has revealed that chronic MOR significantly impairs working memory in rats (Sala et al., 1994). Also, a previous study has shown that the inhibitory memory of rats is significantly impaired by post-training administration of MOR (2.5, 5, and 7.5 mg/kg) (Tavassoli et al., 2017). Furthermore, it has been shown that long-term MOR addiction strongly attenuates neurogenesis, disrupts memory performance, and changes emotional reactivity and anxiety levels in male rats (Famitafreshi et al., 2015). Previous research has shown that rats exposed to long-term MOR exhibit deficits in spatial memory as assessed by the Morris water maze task (Brolin et al., 2018). In addition, reports indicate that MOR can increase oxidative stress in the rodent brain (Milanesi et al., 2023). As we know, oxidative stress can lead to a wide range of cognitive impairments (Kholghi et al., 2023; Mehrabanifar et al., 2023).
Coenzyme Q10 (2, 3-dimethoxy-5-methyl-6-multiprenyl-1, 4-benzoquinone, [CoQ10]) is a fat-soluble vitamin-like substance and an essential electron carrier in the respiratory chain of the inner mitochondrial membrane for ATP metabolic processes. The nature of CoQ10 in the rapid acquisition and loss of electrons causes the potent antioxidant properties of this compound. CoQ10 prevents lipid peroxidation and protein oxidation by avoiding the generation of peroxyl radicals. Every cell is well equipped with an enzymatic antioxidant defense system to deal with oxidative stress. CoQ10 passes through the blood-brain barrier if taken orally or by intravenous injection. CoQ10 prevents tissue damage and causes exogenous preservation and survival of nerve cells. The medical literature highlights the recurring emphasis on the beneficial impact of CoQ10 on learning and memory. The positive effects of CoQ10 on the learning and memory process are emphasized in the medical literature. A recent study has shown that CoQ10 significantly improves amyloid-beta (Aβ)-induced decline in discrimination index (DI) in the novel object recognition (NOR) test, learning, and spatial memory tested in the Morris water maze, passive avoidance memory and learning, and long-term potentiation deficit in the hippocampus of aged animals (Asadbegi et al., 2023). In another study, CoQ10 showed anti-inflammatory and antioxidant properties.
The change of brain-derived neurotrophic factor (BDNF) in MOR-addicted rats is a significant area of research, revealing complex interactions between substance use and neuroplasticity. MOR dependence leads to a decrease in BDNF levels of cerebrospinal fluid during active addiction. At the same time, withdrawal triggers an increase in BDNF levels (Rezamohammadi et al., 2020). In the hippocampus, BDNF levels were found to enhance during MOR withdrawal, suggesting a compensatory mechanism following addiction (Fatahi et al., 2020). Increased BDNF expression in the ventral tegmental area (VTA) was associated with reduced behavioral sensitization to MOR, indicating a protective role against addiction (Deng et al., 2023). CoQ10 was observed to stimulate the production of BDNF, an essential protein involved in neuroplasticity associated with learning and memory, and SOX2, a transcription factor critical for maintaining self-renewal, in the hippocampus of a rat model of Alzheimer disease (Sheykhhasan et al., 2022). A study has shown that treatment with CoQ10 may lead to neuroprotection against the detrimental effects of Aβ on synaptic plasticity in the hippocampus of rats by improving antioxidant activity (Komaki et al., 2019). Furthermore, it has been shown that CoQ10 can alleviate cognitive impairments induced by Intracerebroventricular (ICV) injection of streptozotocin in experimental rodents (Ishrat et al., 2006). According to these findings, we aim to investigate the effect of CoQ10 on cognitive impairments, cue-related reinstatement, and expression of BDNF in MOR-dependent male rats.
2. Materials and Methods
Study animals
This study used 48 male Wistar rats (200-220 g). Each Plexiglas cage consisted of 4 rats housed under a 12-h light cycle (lights beginning at 7:00 AM), constant humidity, and temperature (23±2 °C). All the rats had free access to food and water, and all the experiments were conducted during the light hours (8:00 AM to 3:00 PM). Also, the rats were bred at the Neuroscience Research Center, Mazandaran University of Medical Sciences, Sari, Iran. Our experimental protocol was designed under the National Institutes of Health Guide for the Care and Use of Animals Lab (Politis et al., 2011).
Drugs
Experimental protocol
This study included 6 experimental groups (n=8) as follows:
1) Oil group: Received sesame oil for one month through oral gavage.
2) MOR+oil group: Received MOR for 3 weeks and normal saline by oral gavage during one month of withdrawal.
3) MOR+CoQ10-100 group: Received MOR for 3 weeks and CoQ10 (100 mg/kg) by oral gavage.
4) MOR+CoQ10-200 group: Received MOR for 3 weeks and CoQ10 (200 mg/kg) for a month by oral gavage.
5) MOR+CoQ10-400 group: Received MOR for 3 weeks and CoQ10 (400 mg/kg) for a month by oral gavage.
6) CoQ10-400 group: Received CoQ10 (400 mg/kg) for a month by oral gavage.
MOR sulfate powder was dissolved in distilled water (DW). Rats were administered increasing doses of MOR (25 to 100 mg/kg, SC) once a day for 21 days: Days 1-5, 5 mg (25 mg/kg, SC); days 6-10, 10 mg (50 mg/kg, SC); days 11-15, 15 mg (75 mg/kg, SC); days 16-21, 20 mg (100 mg/kg/SC). CoQ10 was dissolved in sesame oil and administered through gavage. The volume of oral gavage was 1 mL (Ebrahimi & Esmaeili-Mahani, 2020; Matthews et al., 1998; Paul & Gueven, 2021; Rauscher et al., 2001; Shibani et al., 2019).
To confirm MOR dependence, on day 21, naloxone (2 mg/kg, IP) was injected intraperitoneally two hours after MOR administration. Withdrawal symptoms were immediately observed (jumping, shaking the head like a dog, grinding teeth, chewing, standing on two legs, scratching the cage, digging) and recorded for 30 minutes (Figure 1A). The frequency of defecation while observing the signs of withdrawal (30 minutes) and the percentage of 24-hour weight loss of the rats were measured. After observing and recording the withdrawal signs, the rats were returned to the cage (Akbari & Mirzaei, 2013). After 21 days of addiction, CoQ10 treatment was administered orally with a gavage syringe number 24 at doses of 100, 200, and 400 mg/kg once a day for one month (Figure 1B). Then, behavioral tests, including NOR test, Y-maze, social interaction, and conditioned place preference (CPP), were performed in all animals of groups, except the animals of the MOR-naloxone group used for the confirmation addiction model. Four animals per group for immunofluorescent staining were used.

NOR test
The investigation of the NOR test was performed in a cube-shaped box measuring 40×50×50 cm3, in which different objects can be placed in a fixed position in two adjacent corners. This test consists of three phases: Habituation, training, and testing. In the habituation phase, the rat is placed in the middle of the box without objects for 10 minutes to investigate the arena thoroughly. This test phase is repeated the next day in the same way. The habituation phase is completed at the end of two consecutive days of the habituation phase.
In the training phase, two identical objects (A & A) are placed in the box, and the animal is allowed to examine the objects for 10 minutes. The animal testing criterion is touching the object with the nose and putting the nose directly at a distance of less than 2 cm from the object. Leaning the animal against the objects or sitting on the objects is not considered part of the time for searching or examining the object. During the training phase, the time each animal spent exploring each object was recorded.
In the testing phase, a new object (A & B) is replaced by one of the previous objects in the box. In this phase, the animal has 10 minutes to examine the objects. It should be noted that the objects are exchanged for the next animal in each test phase to rule out the possibility of preference. After each animal has left the box, the environment and objects in the box are cleaned with 70% alcohol. The time each animal spends with each object is recorded. The DI (the time each animal spends examining the novel object (object B) minus the time spent examining the repeated object (object A), is divided by the total time spent examining both objects (Lissner et al., 2021).
Y-maze
Working memory was assessed using the Y-maze test. This test consisted of three identical arms, each 45 cm long and 10 cm wide and with a wall height of 30 cm. These arms were arranged at an angle of 120° to each other. The test was carried out in low light (5 lx). The performance of the rats during the test was recorded with a video camera attached above the device, and the order of entries into the maze arms (arm entries, AE) was recorded manually. The percentage of spontaneous alternations (SA) was determined as follows (Equation 1):

Only trials with 8 or more arm movements were included in the data analysis (Shcherbakova et al., 2023).
Social interaction (SI)
The SI test was carried out for 10 minutes in a box with dimensions of 43×19×22 cm3) divided into three parts (this space is unfamiliar to the animals, and there is no habituation). Two wired cup-like cages, large enough to accommodate the rat, were placed in two side chambers once an unfamiliar rat was placed. The experiment was conducted during the light phase with an intensity of 650 lx. An observer was carefully observed and recorded the parameters of the test. At the acclimatization stage, empty wire cages were placed in each part, and the rat was placed in the middle part, where it remained for 5 min to achieve habituation. The first stage of the test is the aspect of social tendencies; in this part of the behavioral test, the aim is to check the social tendencies of the rat. The assumption of the test is based on the fact that the natural subject animal has a greater desire to communicate with another animal than to interact and communicate with an empty chamber. At this stage, one of the control rats (stranger one) was placed in one of the chambers. The doors between the parts were removed so that the subject rat placed in the middle part could move between the areas. The following parameters were carefully checked and observed. First, the duration of direct (active) contact with a rat (a stranger) inside the chamber is checked. In the second stage of the test, the level of the animal’s desire to establish new social relationships and the type of its preference is checked. A healthy subject is assumed to show more desire and preference to communicate with a new animal. Therefore, the second animal (stranger 2) was placed in another empty chamber. The parameters stated in the previous stage were observed and checked, and the behavioral differences of the rat in interaction with stranger 1 and compared with stranger 2 were considered and observed. The duration of the two stages was 10 minutes (Kaidanovich-Beilin et al., 2011).
CPP
The CPP was tested in a three-part Plexiglas box (30×40×30 cm3). The CPP procedure comprised a pretest followed by a 3-day conditioning phase and a test divided into three different phases:
Familiarization phase, the conditioning phase, and the test phase. Before making the addiction model (on the first day), each rat was placed in the neutral box for 15 minutes, where it had free access to all three rooms. The time spent in each room was measured, and the preferred room was determined. From the second to the fourth day, the conditioning phase, each rat received an injection of MOR and was placed and confined in the non-preferred room of the CPP device for 45 min while the doors were closed. After 6 hours, each rat received an injection of DW as a vehicle for MOR and was moved to the preferred room of the CPP. On the third day, the protocol was the same as the first day, from morning to afternoon and vice versa.
In the test phase (on the fifth day), each rat was tested for the CPP under MOR-free conditions. The rat was placed in the CPP apparatus for 10 minutes and freely moved to all three rooms. The time spent in each room was recorded. The CPP score was the difference between the time spent in the rooms paired with and without reward (Jamali et al., 2021).
Immunohistochemistry method
To perform an immunohistochemical examination (n=4), rats were anesthetized with xylazine (20 mg/kg) and ketamine (160 mg/kg) and were stabilized by transcranial perfusion. Then, the heads of the rats were separated, and the brains were removed and kept in a 10% buffered formalin solution. About 48 to 72 hours after immersing the brain in a 10% formalin solution, the samples were cut from the hippocampus, and tissue processing and preparation of paraffin blocks were performed. Afterward, 5-µ thick slices were prepared from paraffin blocks by microtome. For immunohistochemical staining, the slides were first deparaffinized, and then other steps were performed in the following order:
Deparaffinizing the slides in Xylenol solution, hydration in descending alcohols at 100, 90, 80, and 70%, respectively, incubation of the slices in standard sodium citrate (SSC 2x) for 2 hours at 65 degrees, incubation in 2N HCL for 30 minutes at 37 °C, soaking in one-tenth normal boric acid (pH=8.5) for 10 minutes, washing in phosphate-buffered saline (PBS), incubation with primary antibody (BDNF) overnight at 4 °C, immersion in three loads in PBS and each time for 10 minutes, incubation with secondary antibody-BDNF for 2 hours, hematoxylin staining for background staining (Counter staining), sticking the slide, observing with a light microscope, examining the slides with ImageE software, and determining the level of BDNF expression in different test groups and preparing images of the tissues by microscope (Sayyah et al., 2022).
Statistical analyses
The behavioral and histological data were analyzed using GraphPad Prism software. One-way analysis of variance (ANOVA), two-way ANOVA, and post hoc Tukey test were used to compare the differences among the experimental groups. Data are presented as Mean±SD, and P<0.05 was considered statistically significant.
3. Results
Effects of CoQ10 on recognition memory
The MOR group showed a significant decrease in DI compared with the oil group (P<0.001, Figure 2A). This finding shows the cognitive dysfunction observed in MOR-addicted rats. DI was significantly increased in the MOR-CoQ10-100, MOR-CoQ10-200, and MOR-CoQ10-400 groups of rats compared with the MOR group (P<0.01, P<0.001, Figure 2A). In addition, Figure 2B indicates no significant difference in total time of exploration in comparison with the MOR group. MOR-addicted rats treated with CoQ10 explored more new objects, and the DI increased, suggesting that CoQ10 has a neuroprotective impact on the recognition memory impairment caused by MOR.

Effects of CoQ10 on working memory in the Y-maze
The MOR group showed a significant (P<0.001) decrease in percentage alternations compared to the oil group, indicating a loss of memory in the MOR group. The CoQ10-treated groups showed a statistically significant (P<0.01, P<0.001, Figure 3) increase in percentage alternations compared to the MOR group.

Effects of CoQ10 on social interaction
The MOR group showed a significant (P<0.001, Figure 4A) decrease in time spent in social interaction (staying in a rat room). The CoQ10 treated groups showed a statistically significant (P<0.001, Figure 4A) increase in time spent in social interactive behaviors. Figure 4, parts B and C, displays statistically significant (P<0.001) interaction between MOR-CoQ10-100 and MOR-CoQ10-200 groups of rats regarding sociability interaction with trapped stranger 2 (unfamiliar rat) compared with trapped stranger 1 (now-familiar rat).

Effects of CoQ10 on relapse in the CPP
The rats in all groups that received MOR over three days of conditioning showed a significant (P<0.001, Figure 5, CPP1) increase in time spent in the MOR-paired room. This finding shows the place conditioning that occurred in MOR-addicted rats. After CoQ10 treatment in MOR-addicted rats, preference changes significantly increased in CoQ10-treated groups (P<0.01, P<0.001). The finding indicated that the time spent in the MOR-paired chamber reduced significantly (between CPP1 and CPP2), suggesting reduced relapse in CoQ10-treated groups.

Effects of CoQ10 on BDNF expression in hippocampus
The MOR-addicted group showed a significantly lower BDNF expression level of the hippocampus compared with the oil group (P<0.05, Figure 6). The CoQ10-treated groups showed a statistically significant increase in BDNF expression level compared with the MOR-addicted group (P<0.001, Figure 6).

4. Discussion
The present research examined the impacts of CoQ10 treatment on cognitive impairments and relapse induced by MOR use and BDNF expression. Based on the findings, CoQ10 significantly improved NOR, working memory, SI, and cue-related reinstatement. Also, it significantly increased the expression of BDNF in the hippocampus of male rats. Our results show that CoQ10 may have a role in the treatment of cognitive deficits and relapse caused by MOR addiction. Our findings are in line with the multiple studies using laboratory animals that have reported the beneficial protective impacts of CoQ10 against various CNS disorders, including Parkinson disease (Ghasemloo et al., 2021; Park et al., 2020), Alzheimer disease (Komaki et al., 2019; Sheykhhasan et al., 2022), aging-induced memory impairments (Hosseini et al., 2022), diabetes-induced memory impairments (Monsef et al., 2019; Omidi et al., 2019), 2,2-dichlorovinyl dimethyl phosphate (DDVP)-induced cognitive impairments and neurodegeneration (Binukumar et al., 2012). Our study is in line with studies showing that MOR impairs NOR memory. Craig and Bajic in a study in 2015, reported that performance on the NOR test of neonatal rats was compromised in the prolonged MOR administration group at a young age (postnatal days 27–31) (Craig & Bajic, 2015). Also, the results of a study by Paris et al. indicated significantly reduced NOR after ICV. The administration of Kappa opioid receptor (KOR) agonists and the pretreatment of KOR antagonists prevented KOR-mediated dysfunction in NOR (Paris et al., 2011). Moreover, in a study, Alipour et al. (2023) reported that paternal MOR use during adolescence can cause a dysfunction in the recognition of new objects and passive avoidance memory in male offspring (Alipour et al., 2023). Also, Ellis et al. (2020) showed paternal MOR addiction led to NOR memory deficit in the female offspring. The findings of the present study, in agreement with a lot of evidence, indicated MOR has a negative impact on working memory (Amirteimoury et al., 2019; Bach et al., 2021; Bonk, 2011; Wang et al., 2013). Conversely, Miladi Gorji et al. (2008) showed that chronic use of MOR did not affect either retention or acquisition of spatial working memory. Also, our data, in agreement with previous research, indicate that MOR administration in adult rats reduces social interaction and leads to less time spent interacting with social peers (Šlamberová et al., 2016). Hughes et al. (2021) demonstrated that MOR reduced social novelty preference and sociability behavior in adolescent rats (postnatal days 28–43).
Our findings reveal the beneficial effects of CoQ10 treatment on cognitive impairments in MOR-addicted male rats. CoQ10 has been studied for its impact on memory and cognitive function in various contexts, and the results are controversial. CoQ10 supplementation is recommended as an adjunct to conventional treatment of migraine, Alzheimer disease, multiple sclerosis, coronary heart disease, and cerebrovascular accidents (Rauchová, 2021). The obtained findings are consistent with the study of Omidi et al. (2019) on rats with streptozotocin-induced diabetes; high-dose supplementation with CoQ10 improved spatial memory and learning, while low-dose supplementation did not have an effect (Omidi et al., 2019). Similarly, in a study on middle-aged rats by Monsef et al. (2019), CoQ10 supplementation improved memory, learning, and cognitive activities in both diabetic and healthy subjects, with higher doses showing better results. In contrast, Maguire et al. (2021) reported that CoQ10 did not affect working memory and attention in patients with schizophrenia and schizoaffective disorder. It should be noted that the FDA has not approved CoQ10 for treating any diseases. It is consumed as a food additive, not a drug (Arenas‐Jal et al., 2020). Moreover, Sumien et al. (2009) found that prolonged consumption of CoQ10 in a low dose has no discernable effect on motor and cognitive activities. In contrast, higher dose intake exacerbates sensory and cognitive dysfunction in old mice.
The present results reveal that our findings support literature indicating that the BDNF levels in the brains of MOR-addicted male rats are altered. Fatahi et al. (2020) reported that BDNF levels increased during delay-based decision-making in the hippocampus of MOR-addicted rats after withdrawal from MOR. Deng et al. (2022) showed that the BDNF-adenoviral vector administered in the VTA by injection decreased MOR-induced CPP with changes in BDNF/ tyrosine kinase B (TrkB)/cAMP-response element-binding protein (CREB) levels in the VTA and NAc. Furthermore, a study conducted by Peregud et al. (2020) revealed that chronic MOR intoxication reduces the binding of HuD, an RNA-binding protein, to the long 3’-UTR of BDNF, and MOR withdrawal stimulates the expression of BDNF in the frontal cortex of rats.
The results of our research suggest that the administration of CoQ10 could increase the expression of BDNF in the hippocampus after chronic use of MOR in male rats. In line with our results, Vaselbehagh et al. (2021) found that CoQ10 treatment significantly increased the expression of BDNF in the CA1 area of the hippocampus in methadone-induced neurotoxicity. Also, Nagib et al. (2019) reported α-tocopherol and or CoQ10 on phenytoin-induced cognitive dysfunction through a change in the BDNF-TrkB-CREB signaling pathway. In addition, Abuelezz et al. (2023) showed a neuroprotective effect of CoQ10 against scopolamine-induced cognitive impairment by activating the phosphoinositide-3 kinase (PI3K)/AKT/glycogen synthase kinase-3β (GSK-3β)/CREB/BDNF/TrKB signaling pathway. Moreover, the investigation carried out by Abuelezz et al. (2023) exhibited the neuroprotective influence of CoQ10 in countering scopolamine-induced cognitive dysfunction through the stimulation of the PI3K/Akt/GSK-3β/CREB/BDNF/TrKB signaling pathway.
CoQ10 transport across the blood-brain barrier involves complex mechanisms primarily mediated by lipoprotein interactions. Studies indicate that CoQ10 is transported via scavenger receptors and the receptor for advanced glycation end products, facilitating its uptake into the brain. However, efflux mechanisms, particularly through low-density lipoprotein receptors, limit net transport (Wainwright et al., 2020). While the primary focus is on CoQ10, its metabolites, particularly ubiquinol, may influence transport dynamics. Studies suggest that the redox state of CoQ10 affects its accumulation in brain tissues, indicating that metabolites could play a role in modulating transport efficiency (Watanabe et al., 2019).
In conclusion, CoQ10 treatment at 100, 200, and 400 mg/kg within 4 weeks could improve cognitive impairments and reduce cue-related reinstatement in MOR-addicted male rats. The histological assays supported the neuroprotective effects of CoQ10 in the hippocampus. CoQ10 may be a potential therapeutic agent for MOR-induced cognitive impairments and relapse.
Ethical Considerations
Compliance with ethical guidelines
All procedures were performed under the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1401.029).
Funding
This research was funded by a specific project grant from Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 8951).
Authors' contributions
Conceptualization and data curation: Raheleh Rafaiee, and Somayeh Nazari; Methodology and investigation: Pouria Salehi, Farzaneh Felehkari, Farnam Gohardehi, Mohammad Vahabzadeh-Kebria; Formal analysis: Hamid Jomehpour, Saba Niknamfar, and Mobina Gheibi; Writing: Raheleh Rafaieeand and Hamed Ghazvini; Funding acquisition: Seyedeh Masoumeh Seyedhosseini Tamijani, and Hamid Jomehpour; Supervision; Raheleh Rafaiee; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors gratefully appreciate the Deputy for Research at the Mazandaran University of Medical Sciences, Sari, Iran.
References
Abuelezz, S. A., & Hendawy, N. (2023). Spotlight on Coenzyme Q10 in scopolamine-induced Alzheimer's disease: Oxidative stress/PI3K/AKT/GSK 3ß/CREB/BDNF/TrKB. The Journal of Pharmacy and Pharmacology, 75(8), 1119–1129. [DOI:10.1093/jpp/rgad048] [PMID]
Akbari, E., & Mirzaei, E. (2013). [The efficacy of oral morphine self-administration to induce dependence, tolerance and hyperalgesia in rat (Persian)]. Feyz Medical Sciences Journal, 16(6), 483-492. [Link]
Alipour, V., Shojaei, A., Rezaei, M., Mirnajafi-Zadeh, J., & Azizi, H. (2023). Intergenerational consequences of adolescent morphine exposure on learning and memory. Neuroscience Letters, 808, 137303. [DOI:10.1016/j.neulet.2023.137303] [PMID]
Amirteimoury, M., Fatemi, I., Hassanshahi, J., & Kaeidi, A. (2019). [Effect of metformin on morphine induced working memory defects in rats (Persian)]. Iranian Journal of Physiology and Pharmacology, 3(2), 117-123. [Link]
Arenas-Jal, M., Suñé-Negre, J. M., & García-Montoya, E. (2020). Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges. Comprehensive Reviews in Food Science and Food Safety, 19(2), 574–594. [DOI:10.1111/1541-4337.12539] [PMID]
Asadbegi, M., Komaki, H., Faraji, N., Taheri, M., Safari, S., & Raoufi, S., et al. (2023). Effectiveness of coenzyme Q10 on learning and memory and synaptic plasticity impairment in an aged Aβ-induced rat model of Alzheimer's disease: A behavioral, biochemical, and electrophysiological study. Psychopharmacology, 240(4), 951–967. [DOI:10.1007/s00213-023-06338-2] [PMID]
Bach, P., Frischknecht, U., Reinhard, I., Bekier, N., Demirakca, T., & Ende, G., et al. (2021). Impaired working memory performance in opioid-dependent patients is related to reduced insula gray matter volume: A voxel-based morphometric study. European Archives of Psychiatry and Clinical Neuroscience, 271(5), 813–822. [DOI:10.1007/s00406-019-01052-7] [PMID]
Binukumar, B. K., Gupta, N., Sunkaria, A., Kandimalla, R., Wani, W. Y., & Sharma, D. R., et al. (2012). Protective efficacy of coenzyme Q10 against DDVP-induced cognitive impairments and neurodegeneration in rats. Neurotoxicity Research, 21(4), 345–357. [DOI:10.1007/s12640-011-9289-0] [PMID]
Bonk, M. J. (2011). Effects of Morphine on Spatial Working Memory Span in Rats [MA thesis]. Carolina: University of North Carolina Wilmington. [Link]
Brolin, E., Zelleroth, S., Jonsson, A., Hallberg, M., Grönbladh, A., & Nyberg, F. (2018). Chronic administration of morphine using mini-osmotic pumps affects spatial memory in the male rat. Pharmacology, Biochemistry, and Behavior, 167, 1–8. [DOI:10.1016/j.pbb.2018.01.007] [PMID]
Craig, M. M., & Bajic, D. (2015). Long-term behavioral effects in a rat model of prolonged postnatal morphine exposure.Behavioral Neuroscience, 129(5), 643–655. [DOI:10.1037/bne0000081] [PMID]
Deng, L., Chu, Z., Li, B., Liu, P., Lei, G., & Yang, L., et al. (2022). BDNF-AAV has protective effects on morphine-induced conditioned place preference through BDNF, TrkB, and CREB concentration changes in the VTA and NAc. Neuroscience Letters, 782, 136701. [DOI:10.1016/j.neulet.2022.136701] [PMID]
Deng, L., Chu, Z., Liu, P., Li, B., Lei, G., & Li, S., et al. (2023). Effects of brain-derived neurotrophic factor and adeno-associated viral vector on morphine-induced condition through target concentration changes in the ventral tegmental area and nucleus accumbens. Behavioural Brain Research, 445, 114385. [DOI:10.1016/j.bbr.2023.114385] [PMID]
Ebrahimi, B., & Esmaeili-Mahani, S. (2020). The effects of hydroalcoholic extract of satureja khuzestanica on naloxone-induced morphine withdrawal symptoms in wistar rats. International Journal of Basic Science in Medicine, 5(1), 16-21. [DOI:10.34172/ijbsm.2020.05]
Ellis, A. S., Toussaint, A. B., Knouse, M. C., Thomas, A. S., Bongiovanni, A. R., & Mayberry, H. L., et al. (2020). Paternal morphine self-administration produces object recognition memory deficits in female, but not male offspring. Psychopharmacology, 237(4), 1209–1221. [DOI:10.1007/s00213-019-05450-6] [PMID]
Famitafreshi, H., Karimian, M., & Marefati, N. (2015). Long-term morphine addiction reduces neurogenesis and memory performance and alters emotional reactivity and anxiety levels in male rats. Open Access Animal Physiology, 7, 129-136. [DOI:10.2147/OAAP.S87674]
Fatahi, Z., Zeinaddini-Meymand, A., Karimi-Haghighi, S., Haghparast, A., Khodagholi, F., & Haghparast, A. (2020). BDNF and p-GSK3β in the hippocampus mediate the impairment of delay-based decision making in morphine-dependent rats. Neuroreport, 31(17), 1208–1214. [DOI:10.1097/WNR.0000000000001535] [PMID]
Ghamati, L., Hajali, V., Sheibani, V., Esmaeilpour, K., Sepehri, G., & Shojaee, M. (2014). Single and repeated ultra-rapid detoxification prevents cognitive impairment in morphine addicted rats: A privilege for single detoxification. Addiction & Health, 6(1-2), 54–64. [PMID]
Ghasemloo, E., Mostafavi, H., Hosseini, M., Forouzandeh, M., Eskandari, M., & Mousavi, S. S. (2021). Neuroprotective effects of coenzyme Q10 in Parkinson's model via a novel Q10/miR-149-5p/MMPs pathway. Metabolic Brain Disease, 36(7), 2089–2100. [DOI:10.1007/s11011-021-00795-4] [PMID]
Han, H., Dong, Z., Jia, Y., Mao, R., Zhou, Q., & Yang, Y., et al. (2015). Opioid addiction and withdrawal differentially drive long-term depression of inhibitory synaptic transmission in the hippocampus. Scientific Reports, 5, 9666. [DOI:10.1038/srep09666] [PMID]
Heshmatzad, K., Nasehi, M., & Vaseghi, S. (2021). Effects of morphine and NeuroAid on the expression levels of GluN2A and GluN3A in the hippocampus and striatum of rats. Iranian Journal of Basic Medical Sciences, 24(4), 469–475. [DOI:10.22038/ijbms.2021.52004.11787] [PMID]
Hosseini, L., Majdi, A., Sadigh-Eteghad, S., Farajdokht, F., Ziaee, M., & Rahigh Aghsan, S., et al. (2022). Coenzyme Q10 ameliorates aging-induced memory deficits via modulation of apoptosis, oxidative stress, and mitophagy in aged rats. Experimental Gerontology, 168, 111950. [DOI:10.1016/j.exger.2022.111950] [PMID]
Hughes, E. M., Calcagno, P., Sanchez, C., Smith, K., Kelly, J. P., & Finn, D. P., et al. (2021). Mu-opioid receptor agonism differentially alters social behaviour and immediate early gene expression in male adolescent rats prenatally exposed to valproic acid versus controls. Brain Research Bulletin, 174, 260–267. [DOI:10.1016/j.brainresbull.2021.06.018] [PMID]
Ishrat, T., Khan, M. B., Hoda, M. N., Yousuf, S., Ahmad, M., & Ansari, M. A., et al. (2006). Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behavioural Brain Research, 171(1), 9–16. [DOI:10.1016/j.bbr.2006.03.009] [PMID]
Jamali, S., Zarrabian, S., & Haghparast, A. (2021). Similar role of mPFC orexin-1 receptors in the acquisition and expression of morphine- and food-induced conditioned place preference in male rats. Neuropharmacology, 198, 108764. [DOI:10.1016/j.neuropharm.2021.108764] [PMID]
Jaremko, K. M., Thompson, N. L., Jr, Reyes, B. A., Jin, J., Ebersole, B., & Jenney, C. B., et al. (2014). Morphine-induced trafficking of a mu-opioid receptor interacting protein in rat locus coeruleus neurons. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 50, 53–65. [DOI:10.1016/j.pnpbp.2013.12.003] [PMID]
Kaidanovich-Beilin, O., Lipina, T., Vukobradovic, I., Roder, J., & Woodgett, J. R. (2011). Assessment of social interaction behaviors. Journal of Visualized Experiments: JoVE, (48), 2473. [PMID]
Kholghi, G., Alipour, V., Rezaie, M., Zarrindast, M. R., & Vaseghi, S. (2023). The interaction effect of sleep deprivation and treadmill exercise in various durations on spatial memory with respect to the oxidative status of rats. Neurochemical research, 48(7), 2077–2092. [DOI:10.1007/s11064-023-03890-3] [PMID]
Komaki, H., Faraji, N., Komaki, A., Shahidi, S., Etaee, F., & Raoufi, S., et al. (2019). Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Research Bulletin, 147, 14-21. [DOI:10.1016/j.brainresbull.2019.01.025] [PMID]
Leshner, A. I. (1998). Drug addiction research: moving toward the 21st century. Drug and Alcohol Dependence, 51(1-2), 5–7.[DOI:10.1016/S0376-8716(98)00061-1] [PMID]
Lissner, L. J., Wartchow, K. M., Toniazzo, A. P., Gonçalves, C. A., & Rodrigues, L. (2021). Object recognition and Morris water maze to detect cognitive impairment from mild hippocampal damage in rats: A reflection based on the literature and experience. Pharmacology, Biochemistry, and Behavior, 210, 173273. [DOI:10.1016/j.pbb.2021.173273] [PMID]
Maguire, Á., Mooney, C., Flynn, G., Ferguson, Y., O'Keane, V., & O'Rourke, D., et al. (2021). No effect of coenzyme Q10 on cognitive function, psychological symptoms, and health-related outcomes in schizophrenia and schizoaffective disorder: Results of a randomized, placebo-controlled trial. Journal of Clinical Psychopharmacology, 41(1), 53–57. [DOI:10.1097/JCP.0000000000001330] [PMID]
Matthews, R. T., Yang, L., Browne, S., Baik, M., & Beal, M. F. (1998). Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8892–8897. [DOI:10.1073/pnas.95.15.8892] [PMID]
Mehrabanifar, S., Hesami-Tackallou, S., Vaseghi, S., & Nasehi, M. (2023). The effect of crocin on cholestasis-induced spatial memory impairment with respect to the expression level of TFAM and PGC-1α and activity of catalase and superoxide dismutase in the hippocampus. Metabolic Brain Disease, 38(4), 1167–1176. [DOI:10.1007/s11011-023-01176-9] [PMID]
Miladi Gorji, H., Rashidy-Pour, A., & Fathollahi, Y. (2008). Effects of morphine dependence on the performance of rats in reference and working versions of the water maze. Physiology & Behavior, 93(3), 622–627. [DOI:10.1016/j.physbeh.2007.11.002] [PMID]
Milanesi, L. H., Rossato, D. R., Rosa, J. L. O., D'avila, L. F., Metz, V. G., & Rampelotto, C. R., et al. (2023). Ferulic acid-loaded nanostructure prevents morphine reinstatement: The involvement of dopamine system, NRF2, and ΔFosB in the striatum brain area of rats. Naunyn-Schmiedeberg's Archives of Pharmacology, 396(7), 1535–1545. [DOI:10.1007/s00210-023-02420-w] [PMID]
Monsef, A., Shahidi, S., & Komaki, A. (2019). Influence of chronic coenzyme q10 supplementation on cognitive function, learning, and memory in healthy and diabetic middle-aged rats. Neuropsychobiology, 77(2), 92–100. [DOI:10.1159/000495520] [PMID]
Nagib, M. M., Tadros, M. G., Rahmo, R. M., Sabri, N. A., Khalifa, A. E., & Masoud, S. I. (2019). Ameliorative Effects of α-Tocopherol and/or Coenzyme Q10 on Phenytoin-Induced Cognitive Impairment in Rats: Role of VEGF and BDNF-TrkB-CREB Pathway. Neurotoxicity Research, 35(2), 451–462. [DOI:10.1007/s12640-018-9971-6] [PMID]
Omidi, G., Karimi, S. A., Rezvani-Kamran, A., Monsef, A., Shahidi, S., & Komaki, A. (2019). Effect of coenzyme Q10 supplementation on diabetes induced memory deficits in rats. Metabolic Brain Disease, 34(3), 833–840. [DOI:10.1007/s11011-019-00402-7] [PMID]
Paris, J. J., Reilley, K. J., & McLaughlin, J. P. (2011). Kappa Opioid Receptor-Mediated Disruption of Novel Object Recognition: Relevance for psychostimulant treatment. Journal of Addiction Research & Therapy, S4, 007. [DOI:10.4172/2155-6105.S4-007] [PMID]
Park, H. W., Park, C. G., Park, M., Lee, S. H., Park, H. R., & Lim, J., et al. (2020). Intrastriatal administration of coenzyme Q10 enhances neuroprotection in a Parkinson's disease rat model. Scientific Reports, 10(1), 9572. [DOI:10.1038/s41598-020-66493-w] [PMID]
Paul, A. K., Gueven, N., & Dietis, N. (2021). Profiling the effects of repetitive morphine administration on motor behavior in rats. Molecules (Basel, Switzerland), 26(14), 4355. [DOI:10.3390/molecules26144355] [PMID]
Peregud, D., Panchenko, L., & Gulyaeva, N. (2022). Chronic morphine intoxication reduces binding of HuD to BDNF long 3'-UTR, while morphine withdrawal stimulates BDNF expression in the frontal cortex of male Wistar rats. The International Journal of Neuroscience, 132(3), 283–295. [DOI:10.1080/00207454.2020.1809395] [PMID]
Politis, M., Oertel, W. H., Wu, K., Quinn, N. P., Pogarell, O., & Brooks, D. J., et al. (2011). Graft-induced dyskinesias in Parkinson's disease: High striatal serotonin/dopamine transporter ratio. Movement Disorders: Official Journal of The Movement Disorder Society, 26(11), 1997–2003. [DOI:10.1002/mds.23743] [PMID]
Raehal, K. M., & Bohn, L. M. (2005). Mu opioid receptor regulation and opiate responsiveness. The AAPS Journal, 7(3), E587–E591. [DOI:10.1208/aapsj070360] [PMID]
Rauchová, H. (2021). Coenzyme Q10 effects in neurological diseases. Physiological Research, 70(Suppl4), S683–S714. [DOI:10.33549/physiolres.934712] [PMID]
Rauscher, F. M., Sanders, R. A., & Watkins, J. B., 3rd (2001). Effects of coenzyme Q10 treatment on antioxidant pathways in normal and streptozotocin-induced diabetic rats. Journal of Biochemical and Molecular Toxicology, 15(1), 41–46. [DOI:10.1002/1099-0461(2001)15:1<41::aid-jbt5>3.0.co;2-z] [PMID]
Rezamohammadi, F., Rahmani, M., Ghanbari, A., Khaleghian, A., & Miladi-Gorji, H. (2020). BDNF receptor antagonism during the induction of morphine dependence exacerbates the severity of physical dependence and ameliorates psychological dependence in rats. Neuroscience Letters, 737, 135332. [DOI:10.1016/j.neulet.2020.135332] [PMID]
Sala, M., Braida, D., Leone, M. P., Calcaterra, P., Frattola, D., & Gori, E. (1994). Chronic morphine affects working memory during treatment and withdrawal in rats: possible residual long-term impairment. Behavioural Pharmacology, 5(6), 570–580. [DOI:10.1097/00008877-199410000-00002] [PMID]
Sayyah, M., Seydyousefi, M., Moghanlou, A. E., Metz, G. A. S., Shamsaei, N., & Faghfoori, M. H., et al. (2022). Activation of BDNF- and VEGF-mediated Neuroprotection by Treadmill Exercise Training in Experimental Stroke. Metabolic Brain Disease, 37(6), 1843–1853. [DOI:10.1007/s11011-022-01003-7] [PMID]
Shcherbakova, K., Schwarz, A., Ivleva, I., Nikitina, V., Krytskaya, D., & Apryatin, S., et al. (2023). Short- and long-term cognitive and metabolic effects of medium-chain triglyceride supplementation in rats. Heliyon, 9(2), e13446. [DOI:10.1016/j.heliyon.2023.e13446] [PMID]
Sheykhhasan, M., Amini, R., Soleimani Asl, S., Saidijam, M., Hashemi, S. M., & Najafi, R. (2022). Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer's disease. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 152, 113224. [DOI:10.1016/j.biopha.2022.113224] [PMID]
Shibani, F., Sahamsizadeh, A., Fatemi, I., Allahtavakoli, M., Hasanshahi, J., & Rahmani, M., et al. (2019). Effect of oleuropein on morphine-induced hippocampus neurotoxicity and memory impairments in rats. Naunyn-Schmiedeberg's Archives of Pharmacology, 392(11), 1383–1391. [DOI:10.1007/s00210-019-01678-3] [PMID]
Šlamberová, R., Mikulecká, A., Macúchová, E., Hrebíčková, I., Ševčíková, M., & Nohejlová, K., et al. (2016). Morphine decreases social interaction of adult male rats, while THC does not affect it. Physiological Research, 65(Suppl 5), S547–S555. [DOI:10.33549/physiolres.933527] [PMID]
Sumien, N., Heinrich, K. R., Shetty, R. A., Sohal, R. S., & Forster, M. J. (2009). Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. The Journal of Nutrition, 139(10), 1926–1932. [DOI:10.3945/jn.109.110437] [PMID]
Tavassoli, M., Alinaghipour, A., & Ardjmand, A. (2017). [Effect of Pentylenetetrazol on Morphine State-Dependent Memory in Rat (Persian)]. Journal of Arak University of Medical Sciences, 20(6), 1-11. [Link]
Vaselbehagh, M., Sadegh, M., Karami, H., Babaie, S., & Sakhaie, M. H. (2021). Coenzyme Q10 Modulates Apoptotic Effects of Chronic Administration of Methadone on NMRI Mouse Hippocampus. Cell Journal (Yakhteh), 23(5), 538-543. [PMID]
Wainwright, L., Hargreaves, I. P., Georgian, A. R., Turner, C., Dalton, R. N., & Abbott, N. J., et al. (2020). CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood-Brain Barrier Transport. Journal of clinical Medicine, 9(10), 3236. [DOI:10.3390/jcm9103236] [PMID]
Wang, J. H., Rizak, J. D., Chen, Y. M., Li, L., Hu, X. T., & Ma, Y. Y. (2013). Interactive effects of morphine and dopaminergic compounds on spatial working memory in rhesus monkeys. Neuroscience Bulletin, 29(1), 37–46. [DOI:10.1007/s12264-013-1305-3] [PMID]
Watanabe, K., Nozaki, S., Goto, M., Kaneko, K. I., Hayashinaka, E., & Irie, S., et al. (2019). PET imaging of 11C-labeled coenzyme Q10: Comparison of biodistribution between [11C]ubiquinol-10 and [11C]ubiquinone-10. Biochemical and Biophysical Research Communications, 512(3), 611–615. [DOI:10.1016/j.bbrc.2019.03.073] [PMID]
Zhang, R., Jia, W., Wang, Y., Zhu, Y., Liu, F., & Li, B., et al. (2019). A glutamatergic insular-striatal projection regulates the reinstatement of cue-associated morphine-seeking behavior in mice. Brain Research Bulletin, 152, 257-264. [DOI:10.1016/j.brainresbull.2019.07.023] [PMID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2024/04/6 | Accepted: 2024/12/10 | Published: 2025/03/1
Received: 2024/04/6 | Accepted: 2024/12/10 | Published: 2025/03/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





