Volume 16, Issue 5 (September & October 2025)
BCN 2025, 16(5): 845-856 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Noroozi J, Ahmadi M, Shahamati A, Rezayat E, A. Dehaqani M. Development of an Automated Training Device for Macaque Monkeys. BCN 2025; 16 (5) :845-856
URL: http://bcn.iums.ac.ir/article-1-2895-en.html
URL: http://bcn.iums.ac.ir/article-1-2895-en.html
Jalaledin Noroozi1 

 , Mahsa Ahmadi2
, Mahsa Ahmadi2 

 , Atlas Shahamati2
, Atlas Shahamati2 

 , Ehsan Rezayat3
, Ehsan Rezayat3 

 , Mohammad-Reza A. Dehaqani *4
, Mohammad-Reza A. Dehaqani *4 




 , Mahsa Ahmadi2
, Mahsa Ahmadi2 

 , Atlas Shahamati2
, Atlas Shahamati2 

 , Ehsan Rezayat3
, Ehsan Rezayat3 

 , Mohammad-Reza A. Dehaqani *4
, Mohammad-Reza A. Dehaqani *4 


1- Department of Physiology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. & School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran, Iran.
2- School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran, Iran.
3- Department of Psychology, Faculty of Psychology and Education, University of Tehran, Tehran, Iran.
4- School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran, Iran. & School of Electrical and Computer Engineering, College of Engineering, University of Tehran, Tehran, Iran.
2- School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran, Iran.
3- Department of Psychology, Faculty of Psychology and Education, University of Tehran, Tehran, Iran.
4- School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran, Iran. & School of Electrical and Computer Engineering, College of Engineering, University of Tehran, Tehran, Iran.
Full-Text [PDF 2078 kb]
| Abstract (HTML)
Full-Text:
Introduction
Macaques are often chosen for scientific research due to their genetic and physiological similarities to humans, providing valuable insights into biology, medicine, and behavior. Given their high intelligence and complex cognitive abilities, training these animals requires careful consideration of their behavioral, social, and cognitive traits (Hara et al., 2012). Recognizing the challenges and time constraints, we opted to conduct basic training for the macaque within its home cage. This decision aimed to streamline the training process and minimize the duration required for effective skill acquisition.
In typical scenarios, nonhuman primates (NHPs) are often taken temporarily out of their home cages to undergo training in specialized experimental settings. One crucial consideration in this training process is the time devoted to it. To achieve this, experimenters transfer the animal from its home cage to a chair in the laboratory. McMillan et al. (2017) found that the average time required to prepare monkeys for chair restraint usually ranges from 2 to 8 weeks. These differences indicate variations in training approaches, considering factors such as the individual monkey’s temperament (Schapiro, 2017; Coleman, 2012; Coleman et al., 2005) and the use of techniques like pole and collar methods (Bliss et al., 2013; McMillan et al., 2014).
Additionally, some studies have shown that the training period for complex cognitive neuroscience projects can often extend over several months. However, in-chair training in the laboratory is a time-intensive process with inherent limitations. Initially, training inexperienced monkeys can be demanding, as it occurs outside their familiar environment and within a constrained time frame. NHPs, with their diverse temperaments, may exhibit pathological and self-injurious behaviors in response to experimental demands (Novak et al., 1998; Bellanca & Crockett, 2002). Teaching monkeys complex tasks, such as paying attention and temporarily remembering information, is a lengthy process. These tasks require intricate cognitive abilities, such as sustained attention and working memory. Due to these factors, a significant amount of time is necessary to ensure that monkeys learn and effectively display these cognitive skills. To expedite this process, we can build a device that enables all stages of monkey training within their living environment or a simulated game setting.
Several previous experiments have opted for training animals within their home cages rather than using in-chair training in the laboratory (Rumbaugh et al., 1989; Spinelli et al., 2004; Mandell & Sackett, 2009; Truppa et al., 2010; Wagner et al., 2016; Andrews & Rosenblum, 1994). Many of these studies have utilized a computer-based interactive environment interface for housing animals individually or in groups (Rumbaugh et al., 1989; Spinelli et al., 2004; Mandell & Sackett, 2009; Truppa et al., 2010; Wagner et al., 2016; Andrews & Rosenblum, 1994). Additionally, in-cage training systems allow researchers to initiate task training even before animals are accustomed to being transported to the laboratory (Novak et al.,1998). Each system focuses on specific aspects, with its own limitations. None of these systems encompasses all the features, including portability, cost-effective construction, remote control, open-source code, compatibility with different cage types, and real-time observation of monkey behavior during tasks.
We developed a wireless, self-contained touch screen-based training system (Figure 1). It allows remote monitoring and task adjustments while the subject is engaged. The system is cost-effective, lightweight, portable, and sturdy, designed for monitoring subjects without requiring a constant lab presence. It allows for task learning and modification without relocating rhesus NHPs from their natural environment and is adaptable to various cage types. This setup provides rhesus NHPs to engage in tasks at their convenience. Additionally, the system’ Committee. The system was designed exclusively for in-cage macaque training. This portable system can be mounted on NHP cages, enabling in-cage training for all NHPs.
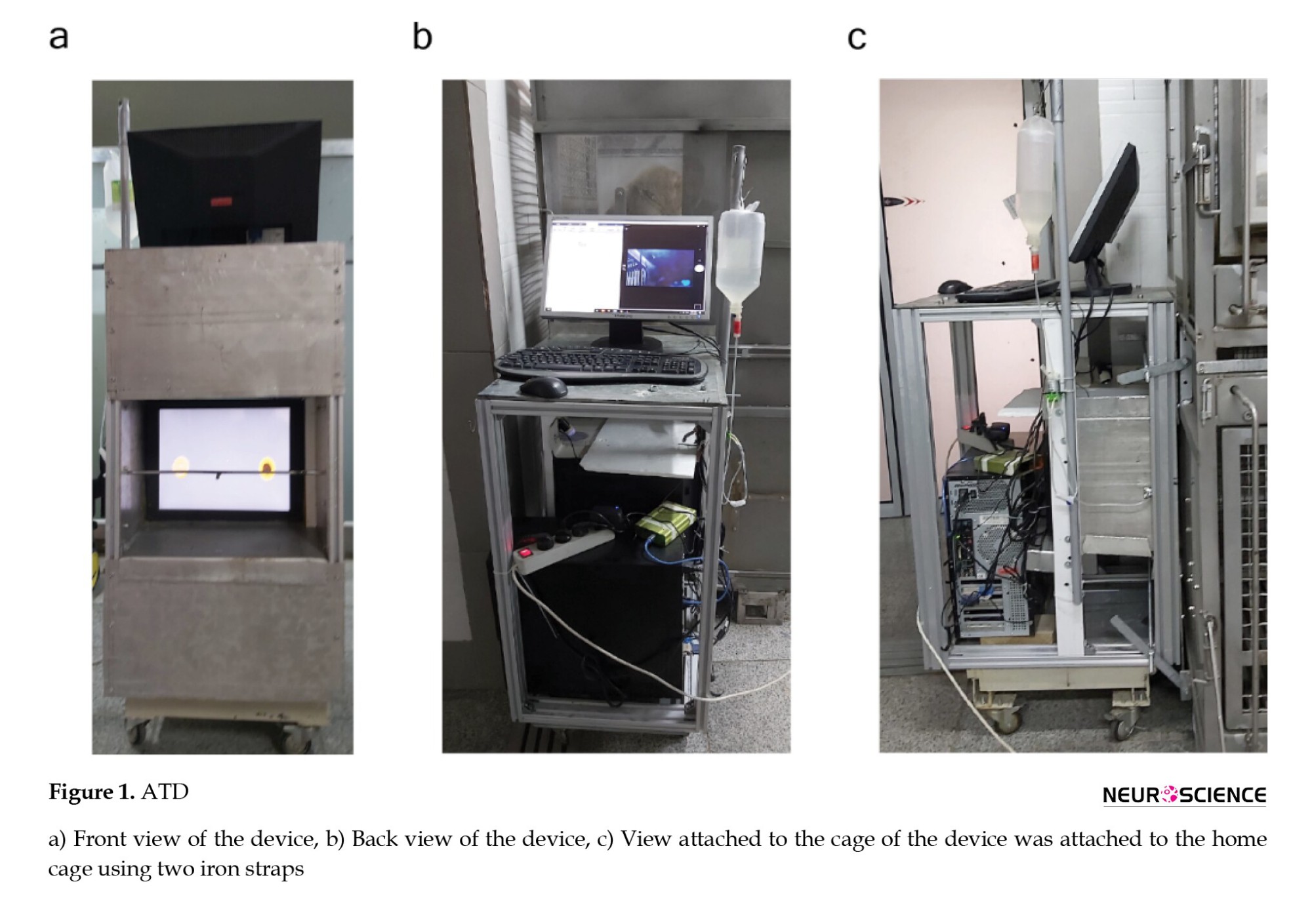
System design
The system consists of three main parts:
Computer setup:
a) Personal computer, and b) touch screen for monkey behavior
Reward system:
c) Peristaltic pump DC 6V/12V, d) DC motor L298N,
e) Arduino UNO, and f) reward source.
Remote control unit:
g) Camera, h) USB Wi-Fi adapter, and i) the fast remote desktop application.
Computer setup
To run software like MATLAB, the personal computer must meet specific minimum specifications, including an INTEL Pentium or Core i3 Processor, 2 GB of DDR3 RAM, and 2 VGA ports for display. The touch screen, serving as a secondary monitor, is linked to the computer via an HDMI cable. The monkey earns a reward by touching and choosing the correct stimulus or condition on the screen.
Reward system
The reward system consists of a DC motor L298N, an Arduino UNO, and a peristaltic pump (Figure 2). Arduino is an open-source hardware and processing platform for programming. The Arduino hardware is programmed using a wiring-based language within a processing-based environment, equipped with all necessary libraries. The Arduino UNO, based on the ATmega328 microcontroller, features 14 digital I/O pins, one USB port, and one power jack supporting 7 to 12 volts.
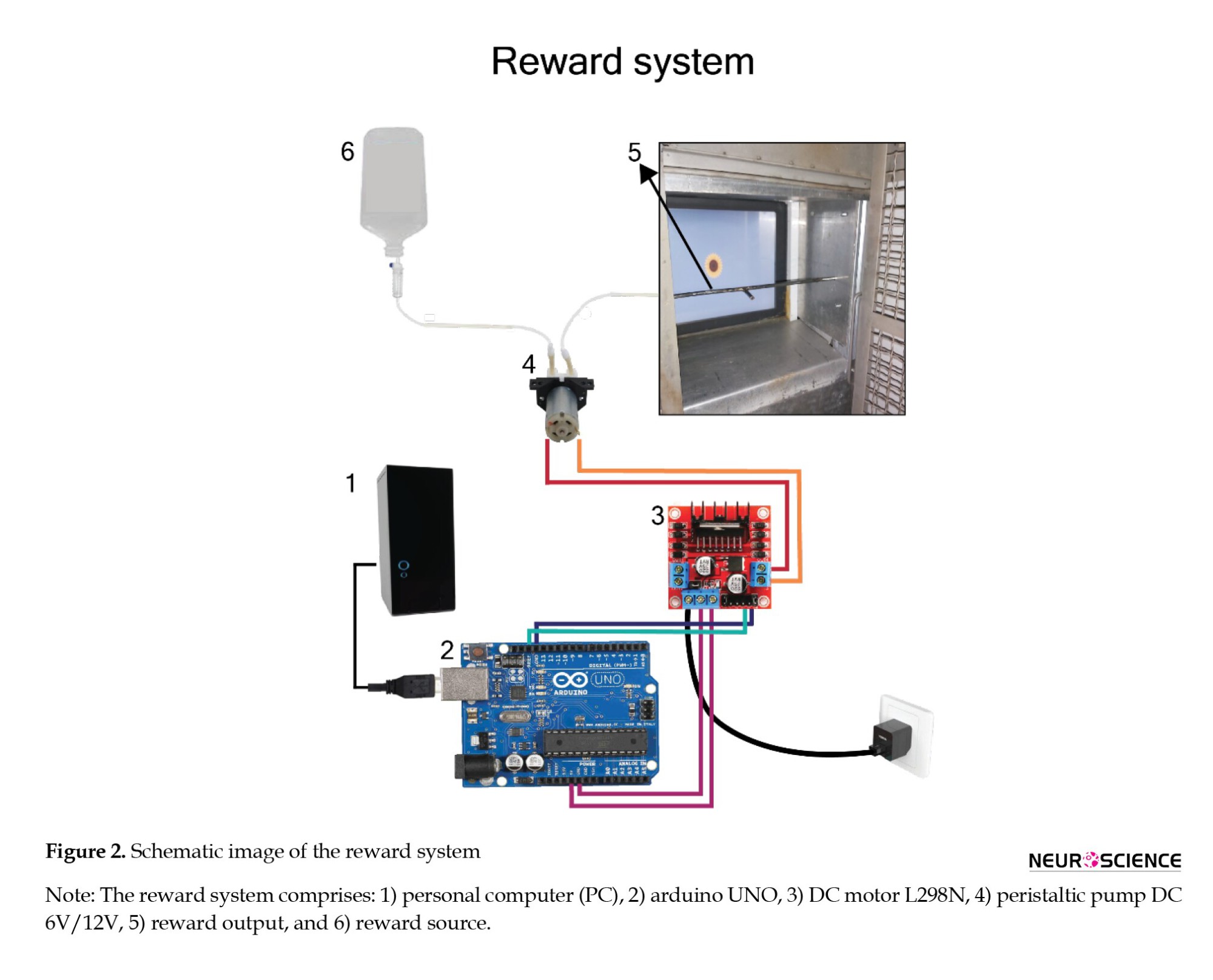
The Arduino is programmed to use the computer’s connected USB port to send the reward trigger signal and control the pump’s activation and deactivation. To prevent interface disconnection and ensure rewards are given only for correct behavior, the trigger must be sent within a maximum time of 3 seconds. The L298N module is a two-channel H-bridge driver for DC and stepper motors, capable of delivering a maximum current of 2 amps per channel. A peristaltic pump, a positive displacement pump type, is employed for pumping various fluids, including fruit juice. After every successful attempt, a signal goes to the microcontroller. Then, the microcontroller turns on the pump for a set time, rewarding the monkey.
Remote-control unit
To enable remote control by our researchers, we connected the training system to the internet through a network card. Using desktop remote control applications like AnyDesk, researchers can access the training system from anywhere, enabling observation and control of the monkey through a connected camera. Remote tests or modifications are also possible, encompassing behaviors, reward reception during tasks, the quantity of rewards, and addressing any other issues that may arise.
Cage mounting frame
To effortlessly transport all the mentioned components, we required a frame equipped with wheel bases capable of accommodating and securing all these pieces of equipment. This frame serves as a mobile platform for easy movement and handling. The device comprises three key components: The computer system, reward system, and remote-control units, all strategically arranged on an aluminum frame. The specially designed cage mounting frame boasts dimensions of 50×60×90 cm, providing a sturdy and compact foundation in the shape of a rectangular cube. Constructing this frame involved using four units of each size, totaling 16 components. To enhance convenience in handling, the aluminum frame is thoughtfully equipped with four wheels, facilitating seamless portability. This design feature not only allows for effortless transportation but also ensures the device can be easily maneuvered and used in various locations, adding a practical and flexible dimension to its application.
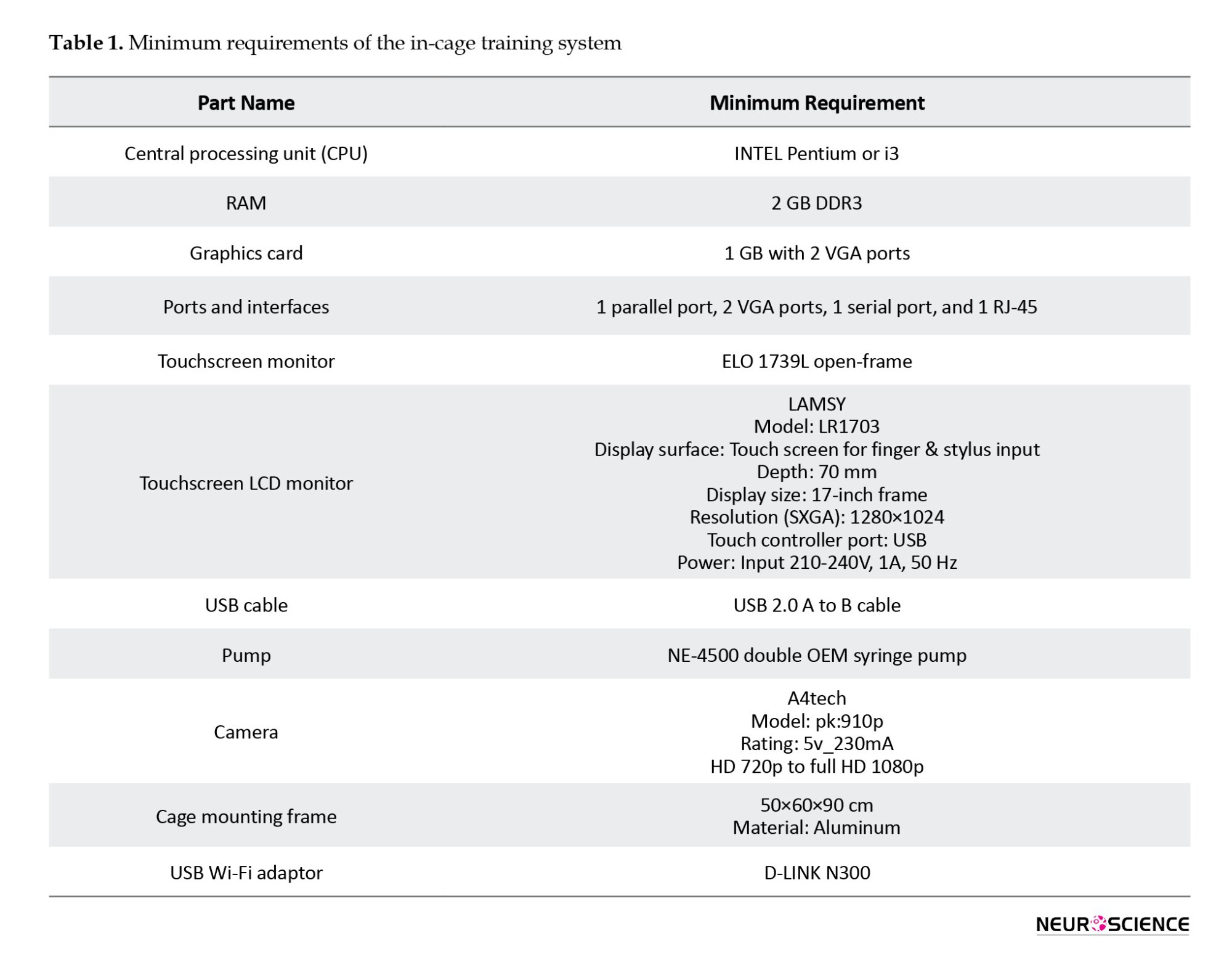
Listed are the various parts of the in-cage training system and the minimum requirement for each part (Table 1).
Results
Behavioral training
To assess the effectiveness and performance of the system, we initiated a training process involving two previously untrained monkeys. We successfully tested the system by training two macaques wirelessly and remotely within their home cages. This training approach avoided any need to remove the monkeys from their cages, eliminated physical contact between the animals and the experimenter, and eliminated the requirement for the monkeys to perform tasks within a designated time frame in a chair. With remote access, we could control the ATD from outside the animal’s living environment. We began training the monkeys by having them touch the monitor, and gradually, we guided them through the step-by-step process of mastering the complex DMS task.
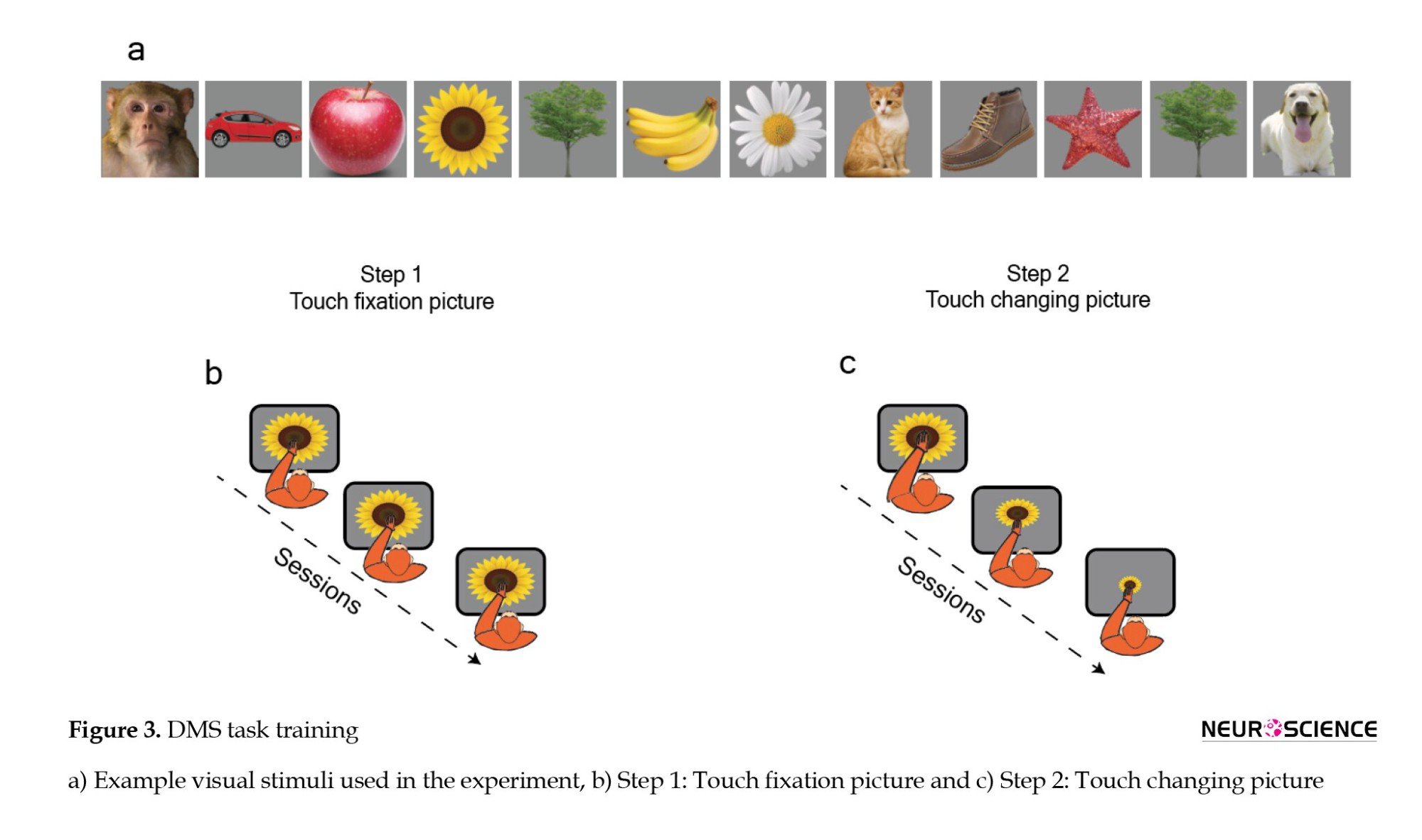
The system was activated at 12 PM and remained operational throughout the day during animal training, being switched off at 5 PM. This setup provides the animals with unrestricted access to the system, allowing them to engage and rest at their convenience. The training sessions took place five days a week, with two designated as vacation days. To teach the DMS task, we employed a structured approach consisting of six steps. In each step, we closely monitored the animal’s performance, ensuring a consistent correctness rate of over 85% for three consecutive days before advancing to the next step in the training process. The stimuli used in this study comprised 44 images divided into two main categories: Faces and objects (Figure 3a). This systematic progression aimed to ensure a thorough and effective learning experience for the animal, establishing a solid foundation before introducing more complex elements of the task.
Step one: Touching the monitor
In the initial step of our training process, our goal was to establish the association between touching the touch screen and receiving a reward. This fundamental connection was quickly acquired through repeated sessions within a single day (every session included 200 trials, lasting approximately 15 to 25 minutes). To achieve this, we presented a sample image covering the entire touch screen, offering the animal a reward of fruit juice upon any touch to the screen. To make this interaction more attractive, we employed various methods known to be appealing to the animal, such as applying honey to the touch screen. This process continued until the animal successfully received juice, as illustrated in Figure 3b. Both monkeys completed step 1, which involved screen touching, in 12 sessions across three days, with a mean performance of 100% in each session. During this stage, the monkeys did not make any wrong trials, resulting in a consistent performance of 100%. This foundational step laid the groundwork for subsequent training stages.
Step two: Resizing the stimuli
In the second step, our objective was to facilitate the animal’s acquisition of the association between image selection and reward reception. To achieve this, we systematically reduced the dimensions of the image continuously. The stimulus varied from one session to another, and if the animal successfully established a proficient association with the stimulus size, the subsequent session featured a smaller size. The gradual adjustment in size was implemented to facilitate effective learning by the monkey. Ultimately, we reduced the stimulus size from 1024×1280 pixels to a final size of 192×192 pixels (Figure 3c). Both Monkeys completed this step in 12 sessions over 3 days, maintaining an average performance of 100%. The overall mean performance across all days for both monkeys individually and their combined average performance was also 100%.
Step three: Random location of stimuli
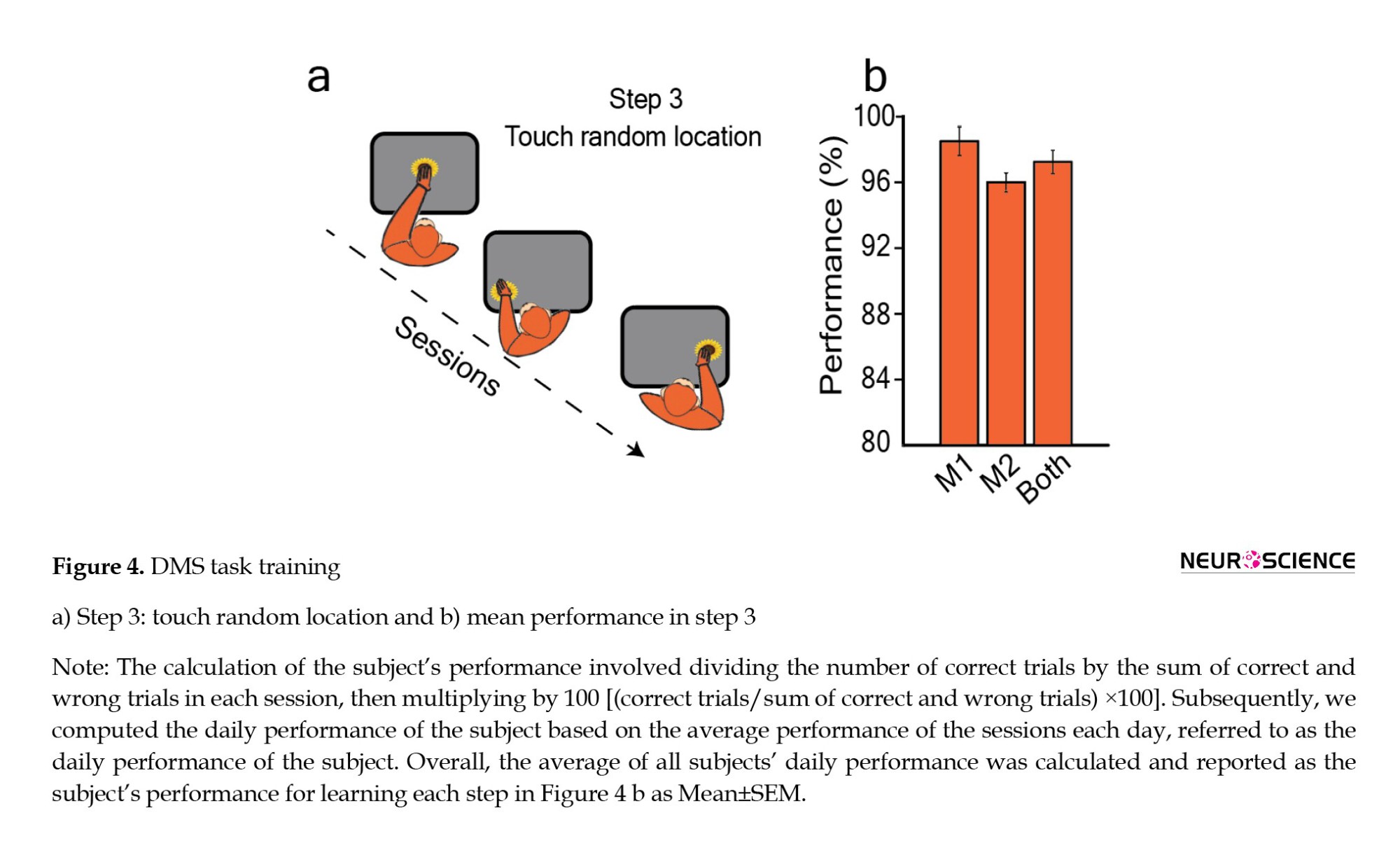
In the third step, our goal was to teach the animal to associate choosing images in various locations on the touchscreen with receiving a reward. Additionally, we wanted to ensure that the animal focused on the sample image rather than just the center of the screen. From this stage onward, the size of the stimuli was set to 192×192 pixels. To fulfill the objective of the third step, we presented stimuli randomly in 9 different locations on the screen. The monkey needed to touch only the location of the displayed stimulus accurately. If the monkey touched anywhere else on the screen, the trial was considered incorrect (Figure 4a). Monkeys completed this step in 12 sessions over 3 days. Monkey M1 had a mean performance of 97±2% on day 1, 98.5±1.4% on day 2, and 100% on day 3. For Monkey M2, the mean performance on day 1 was 95±1.6%, on day 2 was 96±0.9%, and on day 3 was 97±1.5%. Overall, the mean performance for all days reached 98.5±0.87% for Monkey M1, 96±0.5% for Monkey M2, and the combined average performance for both monkeys was 97.25±0.7% (Figure 4b).
After familiarizing the animal with the device in earlier steps, our focus shifted to teaching the DMS task. The DMS task is a cognitive test used in psychology and neuroscience to assess working memory in animals, particularly primates. The task begins with the presentation of a sample stimulus (e.g. an image or object) to the subject. The subject is required to observe and remember this stimulus. After the sample presentation, there is a delay period during which no stimuli are presented. The length of this delay can vary, and it tests the subject’s ability to retain information over a short-term memory interval. Following the delay, the subject is presented with a set of stimuli, which includes the original sample (matching stimulus) and distractors (non-matching stimuli). The subject’s task is to select the stimulus that matches the one presented during the sample phase. A correct response occurs when the subject chooses the stimulus that matches the one from the sample phase. This indicates successful working memory retrieval.
Step four: Visually guided touch
In the fourth training step, our objective was to introduce and teach the DMS task. Initially, a sample image was displayed at the center of the screen, and the animal received a reward for touching the image (sample presentation). Subsequently, a blank screen was presented to the animal for 1000 ms (delay period), after which the same image randomly appeared on either the right or left side of the screen (choice phase). If the animal successfully touched the image, it received a reward (correct response); otherwise, it moved on to the next trial (Figure 5a).
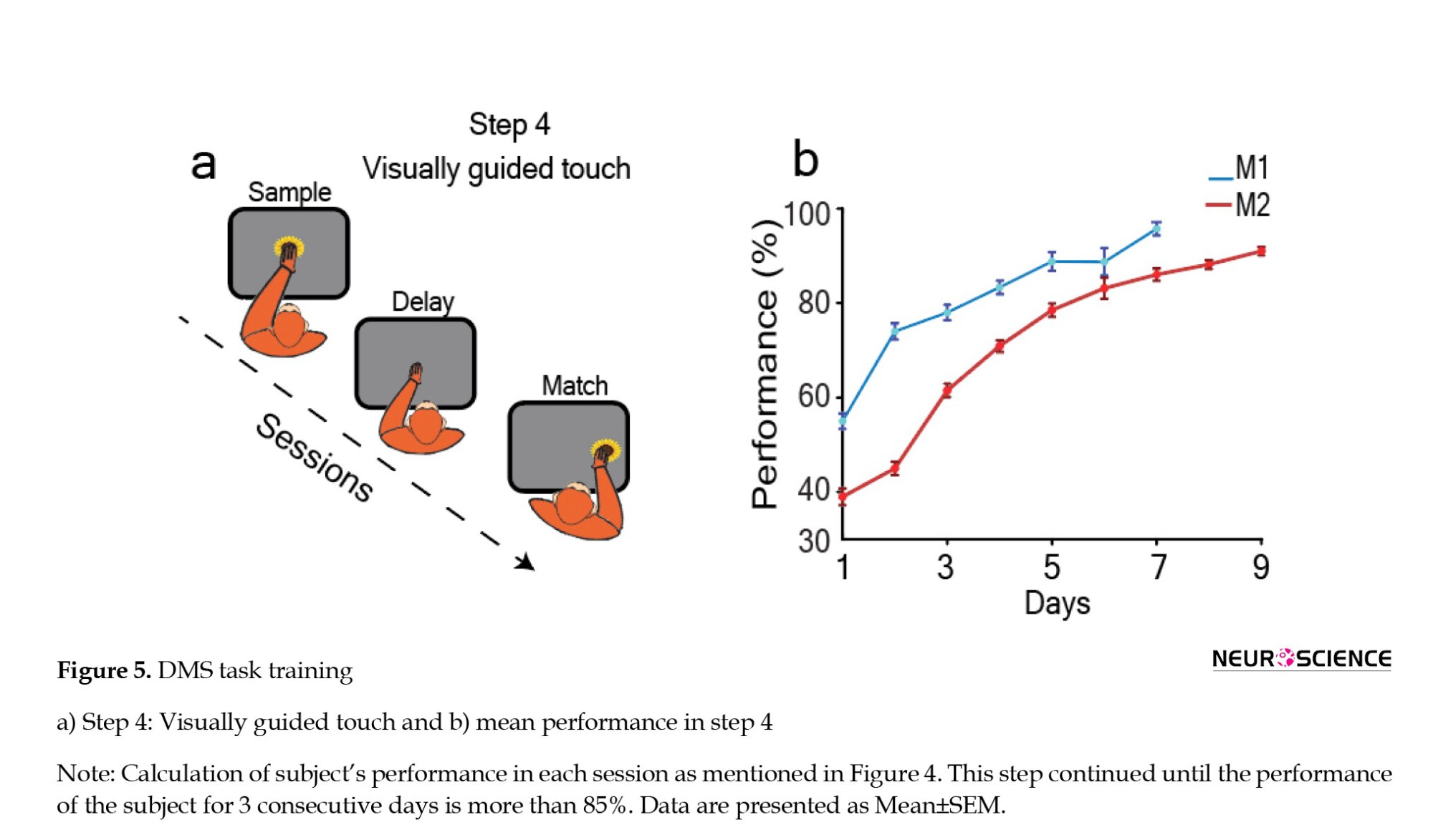
Initially, the reward amount for both the sample presentation and correct response phases was the same. However, over successive sessions, we gradually decreased the reward for the sample presentation phase. Ultimately, only the correct response phase retained a reward. Monkey M1 had a mean performance of 55±1.63% on day 1, 74±1.72% on day 2, 78±1.63% on day 3, 83.3±1.41% on day 4, 88.8±1.98% on day 5, 88.7±2.94% on day 6, and 95.7±1.38% on day 7. Monkey M2 had a mean performance of 39±1.78% on day 1, 45±1.41% on day 2, 61.5±1.44% on day 3, 70.9±1.23% on day 4, 78.5±1.42% on day 5, 83.1±2.22% on day 6, 86.02±1.31% on day 7, 88.17±0.91% on day 8, and 91±0.88% on day 9. Overall, monkey M1 took 28 sessions over 7 days to achieve a performance level exceeding 85%, while for monkey M2, the same training step required 36 sessions spread across 9 days (Figure 5b).
Step five: Teaching the DMS task with transparency
In the fifth step, our goal was to train the association between receiving a reward and selecting a target image that matched the prototype image in the matching-to-sample phase, regardless of the distractor stimulus. Furthermore, we aimed to instill an understanding of the concept of the DMS task.
This step involved presenting a prototype image of the animal for the participant to touch and examine. Afterward, there was a blank screen period of 1000 ms, followed by the appearance of two target pictures on the screen. One of these target images matched the prototype image, while the other served as a distractor image. The animal’s objective was to select the target image that matched the prototype image to receive a reward. This step facilitated the animal’s understanding of both the reward association and the basic concept of the DMS task (Figure 6a). For a trial to be considered correct, the animal needed to choose the target image that matched the initial sample image. To enhance the animal’s learning efficiency, we implemented a strategy by initially making the distracting image transparent. We initiated the image transparency at 0.6, and as the animal’s performance exceeded 80%, we incrementally increased the transparency by 0.1. This approach aimed to optimize the learning process and facilitate a better understanding of the animal.
In the initial phase of this step, when images were introduced with a transparency setting of 0.6, monkey M1 required 32 sessions spread over 8 days to achieve a performance level exceeding 80%. The mean performance of monkey M1 for these 8 days with a transparency setting of 0.6 was as follows: 60.87±1.85% on day 1, 67.49±1.51% on day 2, 62.36±1.86% on day 3, 62.19±3.23% on day 4, 63.25±1.32% on day 5, 74.91±0.82% on day 6, 77.3±2.11% on day 7, and 84.22±2.45% on day 8. Subsequently, we increased the transparency setting by 0.1 each day, and the average daily performance consistently stayed above 80%. On day 9, with a transparency setting of 0.7, monkey M1 achieved a mean performance of 81.16±0.67%, followed by 86.07±3.21% on day 10 for a transparency setting of 0.8, and 90.95±2.14% on day 11 for a transparency setting of 0.9.
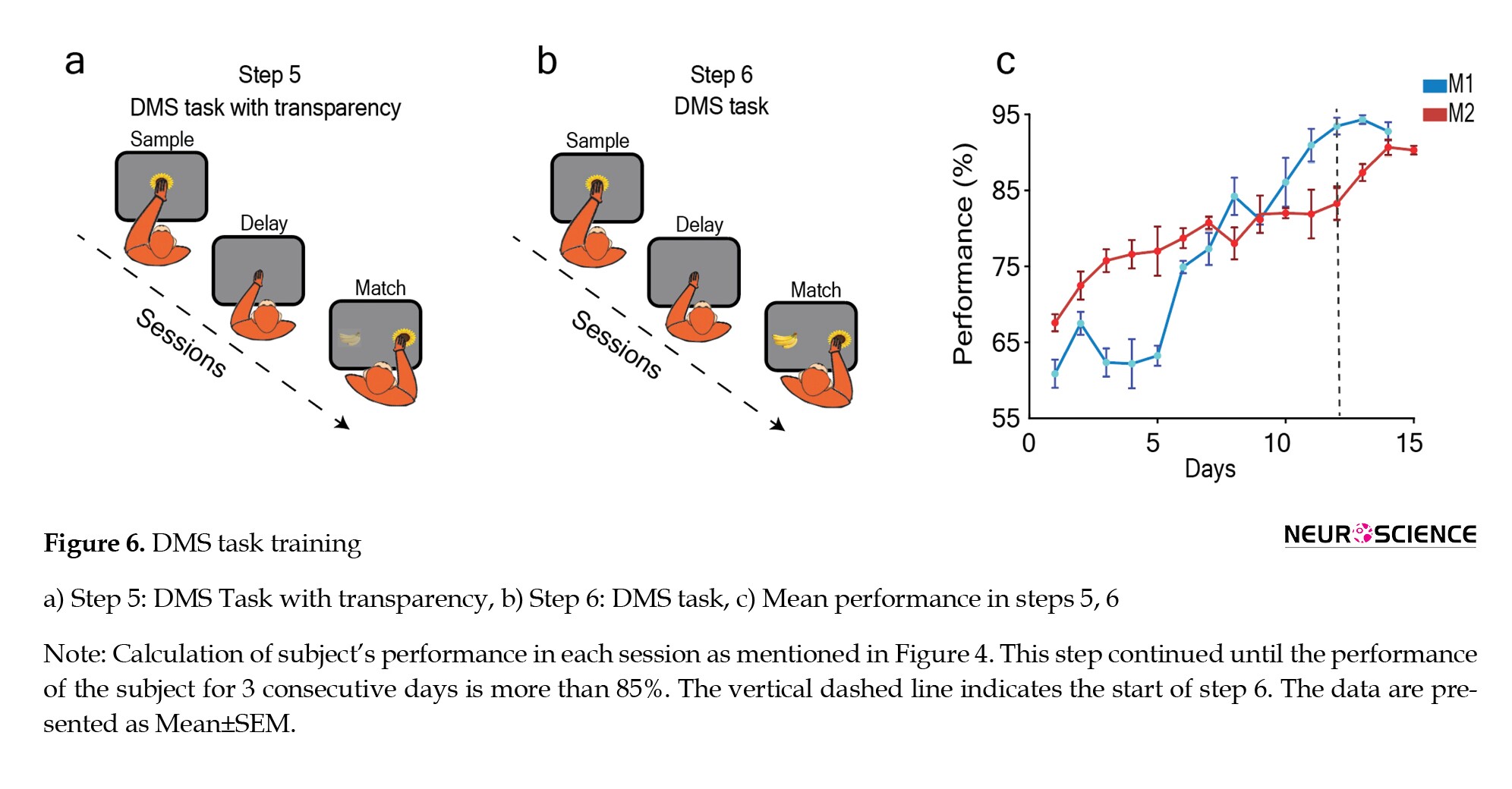
For monkey M2, it took 24 sessions across 7 days to surpass the 80% performance threshold at the initial transparency level of 0.6. The mean performance of monkey M2 for these 7 days was as follows: 67.57±1.13% on day 1, 72.48±1.85% on day 2, 75.75±1.51% on day 3, 76.59±1.86% on day 4, 77±3.23% on day 5, 78.71±1.31% on day 6, and 80.75±0.82% on day 7. Then, in 8 sessions over two days, the performance reached above 80% for a transparency setting of 0.7, with mean performances of 78.02±2.11% on day 8 and 81.86±2.45% on day 9. Like monkey M1, we continued to add 0.1 to transparency daily, and the average daily performance remained above 80%. Monkey M2 had a mean performance of 82±0.67% on day 10 for a transparency setting of 0.8, and 81.89±3.21% on day 11 for a transparency setting of 0.9. In summary, monkey M1 and M2 completed this step in a total of 44 sessions over 11 days (Figure 6c). This information highlights the individual learning trajectories of the monkeys during this specific training step.
Step six: DMS task
In this step, we aimed to master the DMS task. In the preceding step, the animal learned the DMS task with a transparent prototype image. In this current step, the testing procedure mirrored the previous steps, with a notable difference—the distractor image was no longer transparent (Figure 6b). When the transparency of the images reached 1 (indicated by the dashed line on the plot, marking the initiation of the DMS task step), the DMS step commenced. Under this condition, monkey M1 consistently demonstrated a mean performance above 85% for three consecutive days, while monkey M2 maintained this level for four consecutive days. On day 1, monkey M1 exhibited a mean performance of 93.45±1.11, followed by 94.31±0.57 on day 2 and 92.76±1.19% on day 3. Monkey M2’s mean performance on day 1 was 83.28±2.14%, followed by 87.33±1.11% on day 2, 90.67±1.01% on day 3, and 90.29±0.57% on day 4. These consistent performances indicated that the monkeys successfully acquired proficiency in the DMS task (Figure 6c). Overall, monkey M1 learned the DMS task in 30 days, and monkey M2 achieved proficiency in 33 days. This progression signifies the accomplishment of the learning objective in this step.
Discussion
We have developed a touch screen-based training system that’s autonomous, wireless, cost-effective, lightweight, portable, and robust. It is specifically designed for remote subject monitoring, eliminating the need for constant laboratory presence, and can be adapted to various cage setups. This system is user-friendly and customizable by researchers with basic knowledge of hardware and software. Our ATD outperforms previously published touch screen-based systems, offering a broader range of advantages. Touchscreen-based systems are often mentioned for being wireless (Butler & Kennerley, 2019; Calapai et al., 2017), affordable (Butler & Kennerley, 2019), lightweight (Butler & Kennerley, 2019; Calapai et al., 2017), and portable (Butler & Kennerley, 2019; Calapai et al., 2017; Berger et al., 2018; Perdue et al., 2018; Truppa et al., 2010). However, to the best of our knowledge, no published system fulfills all these criteria while also enabling remote subject monitoring and task modification.
Training systems within the animal’s cage have the advantage of enabling researchers to start task training even before the animals are ready to be transported to the laboratory. McMillan et al. (2017) found that the average time needed to prepare monkeys for chair restraint varied, typically taking 2–8 weeks. These differences indicate variations in training approaches, considering factors such as the individual monkey’s temperament (Schapiro, 2017; Coleman, 2012; Coleman et al., 2005) and the use of techniques like pole and collar methods (Bliss et al., 2013; McMillan et al., 2014). Training NHPs before their introduction to research facilities has been noted to enhance their initial performance at the facility (Tulip et al., 2017).
Our study revealed a significant reduction in the number of sessions required for task learning when conducted within the cage (Law & Gold, 2008). In contrast, previous studies have suggested that monkeys generally take months and undergo numerous sessions to master the DMS task. Notably, our findings show that monkey M1 attained proficiency in the DMS task within a mere 30 days (120 sessions), while monkey M2 achieved mastery in 33 days (132 sessions).
These systems lack an eye-tracking feature during training, depending solely on the monkeys’ hand movements (Calapai et al., 2017; Truppa et al., 2010; Curry et al., 2017). Monkeys commonly use their eyes for tasks in the lab (Rezayat et al., 2021; Zhou et al., 2016). It is essential to grasp how monkeys learn through automatic touch screen devices, as it likely impacts their training time in the lab—a factor deserving more exploration. We aim to improve the automatic training system for cage-contained monkeys by adding an eye-tracking system. We expect this addition to enhance monkey training within the cage substantially.
On the other hand, environmental enrichment plays a crucial role in maintaining the welfare of NHPs. However, monkeys may quickly lose interest in unchanging enrichment toys due to habituation (Murphy et al., 2003). Sustaining the engagement of animals requires introducing variations and novelty in their surroundings, along with incorporating primary reinforcers such as food or fluid rewards, diverse stimuli, or novel experiences (Tarou & Bashaw, 2007). Several studies have proposed housing-based training as a valuable tool for the environmental enrichment of captive NHPs (Bennett et al., 2016; Clark, 2017). According to this proposal, cognitive training through an automated protocol that adapts the task difficulty based on the animal’s current skill level could potentially enhance the animal’s motivation for prolonged interaction with the device. Cognitive training through an automated protocol serves as a preventive measure against the onset of depression in animals and enhances the preservation of their mental well-being.
In our study, we demonstrated that two monkeys consistently used the device for several days, extending beyond a month, even when fluid and food intake were not restricted beyond training sessions. This finding suggests that the ATD is a valuable tool for enhancing environmental enrichment (Bennett et al., 2016; Clark, 2017).
Conclusion
In summary, the development of an ATD for macaques represents a significant advancement in enhancing both the well-being of these intelligent animals and the field of scientific research. This innovative device empowers macaques to engage in tasks within their cages, supporting their mental and physical health without external stressors. This breakthrough not only enhances ethical treatment but also unlocks exciting possibilities for scientific exploration. Furthermore, the device’s efficiency facilitates the teaching of various tasks to monkeys without requiring extensive time, financial, or energy investments.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Institute for Research in Fundamental Sciences, Tehran, Iran (Code: 99/60/1/160/1).
Funding
This study was supported by the Institute for Research in Fundamental Sciences, Tehran, Iran.
Authors' contributions
Device development: Jallaledin Noroozi, Ehsan Rezayat, and Atlas Shahamati; Investigation, data analysis, and writing the original draft: Jallaledin Noroozi and Mahsa Ahmadi. Review, editing, and supervision: Mohammad Reza Abolghasemi Dehaqani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Institute for Research in Fundamental Sciences for supporting this study. The authors also acknowledge their colleagues for their constructive feedback during the development of the automated training device.
References
Macaques are often chosen for scientific research due to their genetic and physiological similarities to humans, providing valuable insights into biology, medicine, and behavior. Given their high intelligence and complex cognitive abilities, training these animals requires careful consideration of their behavioral, social, and cognitive traits (Hara et al., 2012). Recognizing the challenges and time constraints, we opted to conduct basic training for the macaque within its home cage. This decision aimed to streamline the training process and minimize the duration required for effective skill acquisition.
In typical scenarios, nonhuman primates (NHPs) are often taken temporarily out of their home cages to undergo training in specialized experimental settings. One crucial consideration in this training process is the time devoted to it. To achieve this, experimenters transfer the animal from its home cage to a chair in the laboratory. McMillan et al. (2017) found that the average time required to prepare monkeys for chair restraint usually ranges from 2 to 8 weeks. These differences indicate variations in training approaches, considering factors such as the individual monkey’s temperament (Schapiro, 2017; Coleman, 2012; Coleman et al., 2005) and the use of techniques like pole and collar methods (Bliss et al., 2013; McMillan et al., 2014).
Additionally, some studies have shown that the training period for complex cognitive neuroscience projects can often extend over several months. However, in-chair training in the laboratory is a time-intensive process with inherent limitations. Initially, training inexperienced monkeys can be demanding, as it occurs outside their familiar environment and within a constrained time frame. NHPs, with their diverse temperaments, may exhibit pathological and self-injurious behaviors in response to experimental demands (Novak et al., 1998; Bellanca & Crockett, 2002). Teaching monkeys complex tasks, such as paying attention and temporarily remembering information, is a lengthy process. These tasks require intricate cognitive abilities, such as sustained attention and working memory. Due to these factors, a significant amount of time is necessary to ensure that monkeys learn and effectively display these cognitive skills. To expedite this process, we can build a device that enables all stages of monkey training within their living environment or a simulated game setting.
Several previous experiments have opted for training animals within their home cages rather than using in-chair training in the laboratory (Rumbaugh et al., 1989; Spinelli et al., 2004; Mandell & Sackett, 2009; Truppa et al., 2010; Wagner et al., 2016; Andrews & Rosenblum, 1994). Many of these studies have utilized a computer-based interactive environment interface for housing animals individually or in groups (Rumbaugh et al., 1989; Spinelli et al., 2004; Mandell & Sackett, 2009; Truppa et al., 2010; Wagner et al., 2016; Andrews & Rosenblum, 1994). Additionally, in-cage training systems allow researchers to initiate task training even before animals are accustomed to being transported to the laboratory (Novak et al.,1998). Each system focuses on specific aspects, with its own limitations. None of these systems encompasses all the features, including portability, cost-effective construction, remote control, open-source code, compatibility with different cage types, and real-time observation of monkey behavior during tasks.
We developed a wireless, self-contained touch screen-based training system (Figure 1). It allows remote monitoring and task adjustments while the subject is engaged. The system is cost-effective, lightweight, portable, and sturdy, designed for monitoring subjects without requiring a constant lab presence. It allows for task learning and modification without relocating rhesus NHPs from their natural environment and is adaptable to various cage types. This setup provides rhesus NHPs to engage in tasks at their convenience. Additionally, the system’ Committee. The system was designed exclusively for in-cage macaque training. This portable system can be mounted on NHP cages, enabling in-cage training for all NHPs.

System design
The system consists of three main parts:
Computer setup:
a) Personal computer, and b) touch screen for monkey behavior
Reward system:
c) Peristaltic pump DC 6V/12V, d) DC motor L298N,
e) Arduino UNO, and f) reward source.
Remote control unit:
g) Camera, h) USB Wi-Fi adapter, and i) the fast remote desktop application.
Computer setup
To run software like MATLAB, the personal computer must meet specific minimum specifications, including an INTEL Pentium or Core i3 Processor, 2 GB of DDR3 RAM, and 2 VGA ports for display. The touch screen, serving as a secondary monitor, is linked to the computer via an HDMI cable. The monkey earns a reward by touching and choosing the correct stimulus or condition on the screen.
Reward system
The reward system consists of a DC motor L298N, an Arduino UNO, and a peristaltic pump (Figure 2). Arduino is an open-source hardware and processing platform for programming. The Arduino hardware is programmed using a wiring-based language within a processing-based environment, equipped with all necessary libraries. The Arduino UNO, based on the ATmega328 microcontroller, features 14 digital I/O pins, one USB port, and one power jack supporting 7 to 12 volts.

The Arduino is programmed to use the computer’s connected USB port to send the reward trigger signal and control the pump’s activation and deactivation. To prevent interface disconnection and ensure rewards are given only for correct behavior, the trigger must be sent within a maximum time of 3 seconds. The L298N module is a two-channel H-bridge driver for DC and stepper motors, capable of delivering a maximum current of 2 amps per channel. A peristaltic pump, a positive displacement pump type, is employed for pumping various fluids, including fruit juice. After every successful attempt, a signal goes to the microcontroller. Then, the microcontroller turns on the pump for a set time, rewarding the monkey.
Remote-control unit
To enable remote control by our researchers, we connected the training system to the internet through a network card. Using desktop remote control applications like AnyDesk, researchers can access the training system from anywhere, enabling observation and control of the monkey through a connected camera. Remote tests or modifications are also possible, encompassing behaviors, reward reception during tasks, the quantity of rewards, and addressing any other issues that may arise.
Cage mounting frame
To effortlessly transport all the mentioned components, we required a frame equipped with wheel bases capable of accommodating and securing all these pieces of equipment. This frame serves as a mobile platform for easy movement and handling. The device comprises three key components: The computer system, reward system, and remote-control units, all strategically arranged on an aluminum frame. The specially designed cage mounting frame boasts dimensions of 50×60×90 cm, providing a sturdy and compact foundation in the shape of a rectangular cube. Constructing this frame involved using four units of each size, totaling 16 components. To enhance convenience in handling, the aluminum frame is thoughtfully equipped with four wheels, facilitating seamless portability. This design feature not only allows for effortless transportation but also ensures the device can be easily maneuvered and used in various locations, adding a practical and flexible dimension to its application.
Listed are the various parts of the in-cage training system and the minimum requirement for each part (Table 1).

Results
Behavioral training
To assess the effectiveness and performance of the system, we initiated a training process involving two previously untrained monkeys. We successfully tested the system by training two macaques wirelessly and remotely within their home cages. This training approach avoided any need to remove the monkeys from their cages, eliminated physical contact between the animals and the experimenter, and eliminated the requirement for the monkeys to perform tasks within a designated time frame in a chair. With remote access, we could control the ATD from outside the animal’s living environment. We began training the monkeys by having them touch the monitor, and gradually, we guided them through the step-by-step process of mastering the complex DMS task.
The system was activated at 12 PM and remained operational throughout the day during animal training, being switched off at 5 PM. This setup provides the animals with unrestricted access to the system, allowing them to engage and rest at their convenience. The training sessions took place five days a week, with two designated as vacation days. To teach the DMS task, we employed a structured approach consisting of six steps. In each step, we closely monitored the animal’s performance, ensuring a consistent correctness rate of over 85% for three consecutive days before advancing to the next step in the training process. The stimuli used in this study comprised 44 images divided into two main categories: Faces and objects (Figure 3a). This systematic progression aimed to ensure a thorough and effective learning experience for the animal, establishing a solid foundation before introducing more complex elements of the task.
Step one: Touching the monitor
In the initial step of our training process, our goal was to establish the association between touching the touch screen and receiving a reward. This fundamental connection was quickly acquired through repeated sessions within a single day (every session included 200 trials, lasting approximately 15 to 25 minutes). To achieve this, we presented a sample image covering the entire touch screen, offering the animal a reward of fruit juice upon any touch to the screen. To make this interaction more attractive, we employed various methods known to be appealing to the animal, such as applying honey to the touch screen. This process continued until the animal successfully received juice, as illustrated in Figure 3b. Both monkeys completed step 1, which involved screen touching, in 12 sessions across three days, with a mean performance of 100% in each session. During this stage, the monkeys did not make any wrong trials, resulting in a consistent performance of 100%. This foundational step laid the groundwork for subsequent training stages.
Step two: Resizing the stimuli
In the second step, our objective was to facilitate the animal’s acquisition of the association between image selection and reward reception. To achieve this, we systematically reduced the dimensions of the image continuously. The stimulus varied from one session to another, and if the animal successfully established a proficient association with the stimulus size, the subsequent session featured a smaller size. The gradual adjustment in size was implemented to facilitate effective learning by the monkey. Ultimately, we reduced the stimulus size from 1024×1280 pixels to a final size of 192×192 pixels (Figure 3c). Both Monkeys completed this step in 12 sessions over 3 days, maintaining an average performance of 100%. The overall mean performance across all days for both monkeys individually and their combined average performance was also 100%.

Step three: Random location of stimuli
In the third step, our goal was to teach the animal to associate choosing images in various locations on the touchscreen with receiving a reward. Additionally, we wanted to ensure that the animal focused on the sample image rather than just the center of the screen. From this stage onward, the size of the stimuli was set to 192×192 pixels. To fulfill the objective of the third step, we presented stimuli randomly in 9 different locations on the screen. The monkey needed to touch only the location of the displayed stimulus accurately. If the monkey touched anywhere else on the screen, the trial was considered incorrect (Figure 4a). Monkeys completed this step in 12 sessions over 3 days. Monkey M1 had a mean performance of 97±2% on day 1, 98.5±1.4% on day 2, and 100% on day 3. For Monkey M2, the mean performance on day 1 was 95±1.6%, on day 2 was 96±0.9%, and on day 3 was 97±1.5%. Overall, the mean performance for all days reached 98.5±0.87% for Monkey M1, 96±0.5% for Monkey M2, and the combined average performance for both monkeys was 97.25±0.7% (Figure 4b).
After familiarizing the animal with the device in earlier steps, our focus shifted to teaching the DMS task. The DMS task is a cognitive test used in psychology and neuroscience to assess working memory in animals, particularly primates. The task begins with the presentation of a sample stimulus (e.g. an image or object) to the subject. The subject is required to observe and remember this stimulus. After the sample presentation, there is a delay period during which no stimuli are presented. The length of this delay can vary, and it tests the subject’s ability to retain information over a short-term memory interval. Following the delay, the subject is presented with a set of stimuli, which includes the original sample (matching stimulus) and distractors (non-matching stimuli). The subject’s task is to select the stimulus that matches the one presented during the sample phase. A correct response occurs when the subject chooses the stimulus that matches the one from the sample phase. This indicates successful working memory retrieval.

Step four: Visually guided touch
In the fourth training step, our objective was to introduce and teach the DMS task. Initially, a sample image was displayed at the center of the screen, and the animal received a reward for touching the image (sample presentation). Subsequently, a blank screen was presented to the animal for 1000 ms (delay period), after which the same image randomly appeared on either the right or left side of the screen (choice phase). If the animal successfully touched the image, it received a reward (correct response); otherwise, it moved on to the next trial (Figure 5a).
Initially, the reward amount for both the sample presentation and correct response phases was the same. However, over successive sessions, we gradually decreased the reward for the sample presentation phase. Ultimately, only the correct response phase retained a reward. Monkey M1 had a mean performance of 55±1.63% on day 1, 74±1.72% on day 2, 78±1.63% on day 3, 83.3±1.41% on day 4, 88.8±1.98% on day 5, 88.7±2.94% on day 6, and 95.7±1.38% on day 7. Monkey M2 had a mean performance of 39±1.78% on day 1, 45±1.41% on day 2, 61.5±1.44% on day 3, 70.9±1.23% on day 4, 78.5±1.42% on day 5, 83.1±2.22% on day 6, 86.02±1.31% on day 7, 88.17±0.91% on day 8, and 91±0.88% on day 9. Overall, monkey M1 took 28 sessions over 7 days to achieve a performance level exceeding 85%, while for monkey M2, the same training step required 36 sessions spread across 9 days (Figure 5b).

Step five: Teaching the DMS task with transparency
In the fifth step, our goal was to train the association between receiving a reward and selecting a target image that matched the prototype image in the matching-to-sample phase, regardless of the distractor stimulus. Furthermore, we aimed to instill an understanding of the concept of the DMS task.
This step involved presenting a prototype image of the animal for the participant to touch and examine. Afterward, there was a blank screen period of 1000 ms, followed by the appearance of two target pictures on the screen. One of these target images matched the prototype image, while the other served as a distractor image. The animal’s objective was to select the target image that matched the prototype image to receive a reward. This step facilitated the animal’s understanding of both the reward association and the basic concept of the DMS task (Figure 6a). For a trial to be considered correct, the animal needed to choose the target image that matched the initial sample image. To enhance the animal’s learning efficiency, we implemented a strategy by initially making the distracting image transparent. We initiated the image transparency at 0.6, and as the animal’s performance exceeded 80%, we incrementally increased the transparency by 0.1. This approach aimed to optimize the learning process and facilitate a better understanding of the animal.
In the initial phase of this step, when images were introduced with a transparency setting of 0.6, monkey M1 required 32 sessions spread over 8 days to achieve a performance level exceeding 80%. The mean performance of monkey M1 for these 8 days with a transparency setting of 0.6 was as follows: 60.87±1.85% on day 1, 67.49±1.51% on day 2, 62.36±1.86% on day 3, 62.19±3.23% on day 4, 63.25±1.32% on day 5, 74.91±0.82% on day 6, 77.3±2.11% on day 7, and 84.22±2.45% on day 8. Subsequently, we increased the transparency setting by 0.1 each day, and the average daily performance consistently stayed above 80%. On day 9, with a transparency setting of 0.7, monkey M1 achieved a mean performance of 81.16±0.67%, followed by 86.07±3.21% on day 10 for a transparency setting of 0.8, and 90.95±2.14% on day 11 for a transparency setting of 0.9.
For monkey M2, it took 24 sessions across 7 days to surpass the 80% performance threshold at the initial transparency level of 0.6. The mean performance of monkey M2 for these 7 days was as follows: 67.57±1.13% on day 1, 72.48±1.85% on day 2, 75.75±1.51% on day 3, 76.59±1.86% on day 4, 77±3.23% on day 5, 78.71±1.31% on day 6, and 80.75±0.82% on day 7. Then, in 8 sessions over two days, the performance reached above 80% for a transparency setting of 0.7, with mean performances of 78.02±2.11% on day 8 and 81.86±2.45% on day 9. Like monkey M1, we continued to add 0.1 to transparency daily, and the average daily performance remained above 80%. Monkey M2 had a mean performance of 82±0.67% on day 10 for a transparency setting of 0.8, and 81.89±3.21% on day 11 for a transparency setting of 0.9. In summary, monkey M1 and M2 completed this step in a total of 44 sessions over 11 days (Figure 6c). This information highlights the individual learning trajectories of the monkeys during this specific training step.
Step six: DMS task
In this step, we aimed to master the DMS task. In the preceding step, the animal learned the DMS task with a transparent prototype image. In this current step, the testing procedure mirrored the previous steps, with a notable difference—the distractor image was no longer transparent (Figure 6b). When the transparency of the images reached 1 (indicated by the dashed line on the plot, marking the initiation of the DMS task step), the DMS step commenced. Under this condition, monkey M1 consistently demonstrated a mean performance above 85% for three consecutive days, while monkey M2 maintained this level for four consecutive days. On day 1, monkey M1 exhibited a mean performance of 93.45±1.11, followed by 94.31±0.57 on day 2 and 92.76±1.19% on day 3. Monkey M2’s mean performance on day 1 was 83.28±2.14%, followed by 87.33±1.11% on day 2, 90.67±1.01% on day 3, and 90.29±0.57% on day 4. These consistent performances indicated that the monkeys successfully acquired proficiency in the DMS task (Figure 6c). Overall, monkey M1 learned the DMS task in 30 days, and monkey M2 achieved proficiency in 33 days. This progression signifies the accomplishment of the learning objective in this step.

Discussion
We have developed a touch screen-based training system that’s autonomous, wireless, cost-effective, lightweight, portable, and robust. It is specifically designed for remote subject monitoring, eliminating the need for constant laboratory presence, and can be adapted to various cage setups. This system is user-friendly and customizable by researchers with basic knowledge of hardware and software. Our ATD outperforms previously published touch screen-based systems, offering a broader range of advantages. Touchscreen-based systems are often mentioned for being wireless (Butler & Kennerley, 2019; Calapai et al., 2017), affordable (Butler & Kennerley, 2019), lightweight (Butler & Kennerley, 2019; Calapai et al., 2017), and portable (Butler & Kennerley, 2019; Calapai et al., 2017; Berger et al., 2018; Perdue et al., 2018; Truppa et al., 2010). However, to the best of our knowledge, no published system fulfills all these criteria while also enabling remote subject monitoring and task modification.
Training systems within the animal’s cage have the advantage of enabling researchers to start task training even before the animals are ready to be transported to the laboratory. McMillan et al. (2017) found that the average time needed to prepare monkeys for chair restraint varied, typically taking 2–8 weeks. These differences indicate variations in training approaches, considering factors such as the individual monkey’s temperament (Schapiro, 2017; Coleman, 2012; Coleman et al., 2005) and the use of techniques like pole and collar methods (Bliss et al., 2013; McMillan et al., 2014). Training NHPs before their introduction to research facilities has been noted to enhance their initial performance at the facility (Tulip et al., 2017).
Our study revealed a significant reduction in the number of sessions required for task learning when conducted within the cage (Law & Gold, 2008). In contrast, previous studies have suggested that monkeys generally take months and undergo numerous sessions to master the DMS task. Notably, our findings show that monkey M1 attained proficiency in the DMS task within a mere 30 days (120 sessions), while monkey M2 achieved mastery in 33 days (132 sessions).
These systems lack an eye-tracking feature during training, depending solely on the monkeys’ hand movements (Calapai et al., 2017; Truppa et al., 2010; Curry et al., 2017). Monkeys commonly use their eyes for tasks in the lab (Rezayat et al., 2021; Zhou et al., 2016). It is essential to grasp how monkeys learn through automatic touch screen devices, as it likely impacts their training time in the lab—a factor deserving more exploration. We aim to improve the automatic training system for cage-contained monkeys by adding an eye-tracking system. We expect this addition to enhance monkey training within the cage substantially.
On the other hand, environmental enrichment plays a crucial role in maintaining the welfare of NHPs. However, monkeys may quickly lose interest in unchanging enrichment toys due to habituation (Murphy et al., 2003). Sustaining the engagement of animals requires introducing variations and novelty in their surroundings, along with incorporating primary reinforcers such as food or fluid rewards, diverse stimuli, or novel experiences (Tarou & Bashaw, 2007). Several studies have proposed housing-based training as a valuable tool for the environmental enrichment of captive NHPs (Bennett et al., 2016; Clark, 2017). According to this proposal, cognitive training through an automated protocol that adapts the task difficulty based on the animal’s current skill level could potentially enhance the animal’s motivation for prolonged interaction with the device. Cognitive training through an automated protocol serves as a preventive measure against the onset of depression in animals and enhances the preservation of their mental well-being.
In our study, we demonstrated that two monkeys consistently used the device for several days, extending beyond a month, even when fluid and food intake were not restricted beyond training sessions. This finding suggests that the ATD is a valuable tool for enhancing environmental enrichment (Bennett et al., 2016; Clark, 2017).
Conclusion
In summary, the development of an ATD for macaques represents a significant advancement in enhancing both the well-being of these intelligent animals and the field of scientific research. This innovative device empowers macaques to engage in tasks within their cages, supporting their mental and physical health without external stressors. This breakthrough not only enhances ethical treatment but also unlocks exciting possibilities for scientific exploration. Furthermore, the device’s efficiency facilitates the teaching of various tasks to monkeys without requiring extensive time, financial, or energy investments.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Institute for Research in Fundamental Sciences, Tehran, Iran (Code: 99/60/1/160/1).
Funding
This study was supported by the Institute for Research in Fundamental Sciences, Tehran, Iran.
Authors' contributions
Device development: Jallaledin Noroozi, Ehsan Rezayat, and Atlas Shahamati; Investigation, data analysis, and writing the original draft: Jallaledin Noroozi and Mahsa Ahmadi. Review, editing, and supervision: Mohammad Reza Abolghasemi Dehaqani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Institute for Research in Fundamental Sciences for supporting this study. The authors also acknowledge their colleagues for their constructive feedback during the development of the automated training device.
References
Andrews, M. W., & Rosenblum, L. A. (1994). Automated recording of individual performance and hand preference during joystick-task acquisition in group-living bonnet macaques (Macaca radiata). Journal of Comparative Psychology (Washington, D.C.: 1983), 108(4), 358–362. [DOI:10.1037/0735-7036.108.4.358] [PMID]
Bellanca, R. U., & Crockett, C. M. (2002). Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. American Journal of Primatology, 58(2), 57–69.[DOI:10.1002/ajp.10052] [PMID]
Bennett, A. J., Perkins, C. M., Tenpas, P. D., Reinebach, A. L., & Pierre, P. J. (2016). Moving evidence into practice: Cost analysis and assessment of macaques' sustained behavioral engagement with videogames and foraging devices. American Journal of Primatology, 78(12), 1250–1264. [DOI:10.1002/ajp.22579] [PMID]
Berger, M., Calapai, A., Stephan, V., Niessing, M., Burchardt, L., & Gail, A., et al. (2018). Standardized automated training of rhesus monkeys for neuroscience research in their housing environment. Journal of Neurophysiology, 119(3), 796–807. [DOI:10.1152/jn.00614.2017] [PMID]
Bliss-Moreau, E., Theil, J. H., & Moadab, G. (2013). Efficient cooperative restraint training with rhesus macaques. Journal of Applied Animal Welfare Science: JAAWS, 16(2), 98–117. [DOI:10.1080/10888705.2013.768897] [PMID]
Butler, J. L., & Kennerley, S. W. (2019). Mymou: A low-cost, wireless touchscreen system for automated training of nonhuman primates. Behavior Research Methods, 51(6), 2559–2572. [DOI:10.3758/s13428-018-1109-5] [PMID]
Calapai, A., Berger, M., Niessing, M., Heisig, K., Brockhausen, R., & Treue, S., et al. (2017). A cage-based training, cognitive testing and enrichment system optimized for rhesus macaques in neuroscience research. Behavior Research Methods, 49(1), 35–45. [DOI:10.3758/s13428-016-0707-3] [PMID]
Clark, F. E. (2017). Cognitive enrichment and welfare: Current approaches and future directions. Animal Behavior and Cognition, 4(1), 52–71. [DOI:10.12966/abc.05.02.2017]
Coleman, K. (2012). Individual differences in temperament and behavioral management practices for nonhuman primates. Applied Animal Behaviour Science, 137(3-4), 106–113. [DOI:10.1016/j.applanim.2011.08.002] [PMID]
Coleman, K., Tully, L. A., & McMillan, J. L. (2005). Temperament correlates with training success in adult rhesus macaques. American Journal of Primatology, 65(1), 63–71. [DOI:10.1002/ajp.20097] [PMID]
Curry, M. D., Zimmermann, A., Parsa, M., Dehaqani, M. R. A., Clark, K. L., & Noudoost, B. (2017). A cage-based training system for non-human primates. AIMS Neuroscience, 4(3), 102-19. [DOI:10.3934/Neuroscience.2017.3.102]
Hara, Y., Rapp, P. R., & Morrison, J. H. (2012). Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Dordrecht, Netherlands), 34(5), 1051–1073. [DOI:10.1007/s11357-011-9278-5] [PMID]
Law, C. T., & Gold, J. I. (2008). Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neuroscience, 11(4), 505–513. [DOI:10.1038/nn2070] [PMID]
Mandell, D. J., & Sackett, G. P. (2009). Comparability of developmental cognitive assessments between standard and computer testing methods. Developmental Psychobiology, 51(1), 1–13. [DOI:10.1002/dev.20329] [PMID]
McMillan, J. L., Bloomsmith, M. A., & Prescott, M. J. (2017). An international survey of approaches to chair restraint of nonhuman primates. Comparative Medicine, 67(5), 442–451. [PMID]
McMillan, J. L., Perlman, J. E., Galvan, A., Wichmann, T., & Bloomsmith, M. A. (2014). Refining the pole-and-collar method of restraint: Emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science: JAALAS, 53(1), 61–68. [PMID]
Murphy, E. S., McSweeney, F. K., Smith, R. G., & McComas, J. J. (2003). Dynamic changes in reinforcer effectiveness: theoretical, methodological, and practical implications for applied research. Journal of Applied Behavior Analysis, 36(4), 421–438. [DOI:10.1901/jaba.2003.36-421] [PMID]
Novak, M. A., Kinsey, J. H., Jorgensen, M. J., & Hazen, T. J. (1998). Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. American Journal of Primatology, 46(3), 213–227. [DOI:10.1002/(SICI)1098-2345(1998)46:3<213::AID-AJP3>3.0.CO;2-L] [PMID]
Perdue, B. M., Beran, M. J., & Washburn, D. A. (2018). A computerized testing system for primates: Cognition, welfare, and the Rumbaughx. Behavioural Processes, 156, 37–50. [DOI:10.1016/j.beproc.2017.12.019] [PMID]
Rezayat, E., Dehaqani, M. A., Clark, K., Bahmani, Z., Moore, T., & Noudoost, B. (2021). Frontotemporal coordination predicts working memory performance and its local neural signatures. Nature Communications, 12(1), 1103. [DOI:10.1038/s41467-021-21151-1] [PMID]
Rumbaugh, D. M., Hopkins, W. D., Washburn, D. A., & Savage-Rumbaugh, E. S. (1989). Lana chimpanzee learns to count by "NUMATH": A summary of a videotaped experimental report. The Psychological Record, 39(4), 459–470. [DOI:10.1007/BF03395074] [PMID]
Spinelli, S., Pennanen, L., Dettling, A. C., Feldon, J., Higgins, G. A., & Pryce, C. R. (2004). Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain research. Cognitive Brain Research, 19(2), 123–137. [DOI:10.1016/j.cogbrainres.2003.11.007] [PMID]
Schapiro, S. J., & Schapiro, S. J. (2017). Handbook of Primate Behavioral Management. Boca Raton: CRC Press; 2017. [DOI:10.1201/9781315120652]
Truppa, V., Garofoli, D., Castorina, G., Piano Mortari, E., Natale, F., & Visalberghi, E. (2010). Identity concept learning in matching-to-sample tasks by tufted capuchin monkeys (Cebus apella). Animal Cognition, 13(6), 835–848. [DOI:10.1007/s10071-010-0332-y] [PMID]
Tulip, J., Zimmermann, J. B., Farningham, D., & Jackson, A. (2017). An automated system for positive reinforcement training of group-housed macaque monkeys at breeding and research facilities. Journal of Neuroscience Methods, 285, 6–18. [DOI:10.1016/j.jneumeth.2017.04.015] [PMID]
Tarou, L. R., & Bashaw, M. J. (2007). Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behavior. Applied Animal Behaviour Science, 102(3-4), 189-204. [DOI:10.1016/j.applanim.2006.05.026]
Wagner, K. E., Hopper, L. M., & Ross, S. R. (2016). Asymmetries in the production of self-directed behavior by chimpanzees and gorillas during a computerized cognitive test. Animal Cognition, 19(2), 343–350. [DOI:10.1007/s10071-015-0937-2] [PMID]
Zhou, X., Zhu, D., Qi, X. L., Li, S., King, S. G., & Salinas, E., et al. (2016). Neural correlates of working memory development in adolescent primates. Nature Communications, 7, 13423. [DOI:10.1038/ncomms13423] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2024/02/28 | Accepted: 2024/04/9 | Published: 2025/09/1
Received: 2024/02/28 | Accepted: 2024/04/9 | Published: 2025/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





