Volume 15, Issue 6 (November & December 2024)
BCN 2024, 15(6): 745-758 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadi Machiani S, Rezaei S, Saberi A, Keymoradzadeh A, Bakhshayesh B, Rohampour K. Bihemispheric tDCS Improves Memory and Alters EEG Parameters in Patients With Mild Cognitive Impairment. BCN 2024; 15 (6) :745-758
URL: http://bcn.iums.ac.ir/article-1-2888-en.html
URL: http://bcn.iums.ac.ir/article-1-2888-en.html
Soroush Ahmadi Machiani1 

 , Sajjad Rezaei2
, Sajjad Rezaei2 

 , Alia Saberi3
, Alia Saberi3 

 , Arman Keymoradzadeh1
, Arman Keymoradzadeh1 

 , Babak Bakhshayesh1
, Babak Bakhshayesh1 

 , Kambiz Rohampour *1
, Kambiz Rohampour *1 




 , Sajjad Rezaei2
, Sajjad Rezaei2 

 , Alia Saberi3
, Alia Saberi3 

 , Arman Keymoradzadeh1
, Arman Keymoradzadeh1 

 , Babak Bakhshayesh1
, Babak Bakhshayesh1 

 , Kambiz Rohampour *1
, Kambiz Rohampour *1 


1- Neuroscience Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Psychology, Faculty of Literature and Humanities, University of Guilan, Rasht, Iran.
3- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Psychology, Faculty of Literature and Humanities, University of Guilan, Rasht, Iran.
3- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Keywords: Mild cognitive impairment (MCI), Electroencephalography (EEG), Transcranial direct current stimulation (tDCS), Wechsler memory scale, Coherence
Full-Text [PDF 867 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Mild cognitive impairment (MCI), also known as mild neurocognitive disorder, is characterized by subjective and objective cognitive impairment in older individuals with preserved daily functions (Martin et al., 2019). MCI is a transitional state between normal aging and dementia. The prevalence of MCI in adults older than 60 and the annual rate of progression to dementia are up to 25.2% and 17%, respectively (Jongsiriyanyong & Limpawattana, 2018). Even though MCI is a type of cognitive problem that affects people who are not yet in a state of dementia, it is nevertheless a condition of aging with a likely degenerative etiology that is related to the development of Alzheimer disease (AD) (Okello et al., 2009). The aging brain might trigger compensating cognitive systems. For instance, young individuals who are not cognitively impaired but are in good health and have trouble with executive tasks exhibit overactivation in only one hemisphere of the prefrontal cortex (Reuter-Lorenz et al., 2000). Due to the likelihood that older adults have less attentional and working memory capacities, this brain reaction may be explained by cognitive restructuring in these individuals (Kirova et al., 2015). Working memory and attention problems have been linked to executive functioning decline, which is aggravated by MCI (Kochan et al., 2010).
Studies using positron emission tomography (PET) multimodal imaging data in combination with independent component analysis (ICA) could efficiently distinguish MCI patients from healthy controls. They discovered that MCI patients’ spatial distribution of amyloid beta PET and tau PET significantly differs from that of healthy individuals. These areas are colocalized with the default mode and cognitive control networks (Li et al., 2019). Furthermore, global functional connectivity of the cognitive control network in the resting state has been proposed as a biomarker for cognitive reserve. It is also considered protection for brain function in MCI patients by functional magnetic resonance imaging investigation (Franzmeier et al., 2017). The cognitive control network consists of several interconnected brain regions: The dorsolateral prefrontal cortex (DLPFC), which is involved in working memory (Cieslik et al., 2013), cognitive performance (Cao et al., 2016), attention, and decision-making (Obeso et al., 2021); anterior cingulate cortex, which is involved in monitoring and regulating cognitive processes (Braem et al., 2017); Inferior parietal lobule, involved in spatial attention and working memory (Clower et al., 2001); ventrolateral prefrontal cortex, involved in inhibitory control and decision-making; posterior parietal cortex; and medial prefrontal cortex (Amidfar et al., 2019).
Numerous cognitive processes and the functional connection between various brain regions depend heavily on brain oscillations (Zhang et al., 2018). Theta oscillations, primarily seen in the frontal cortex and regulated by inhibition in other brain regions, are typically associated with memory and executive functions (Huster et al., 2013). An overall boost in the power of the delta and theta frequency ranges is linked to aging (Rossini et al., 2007). Additionally, studies using resting state electroencephalography (EEG) show that lower frequency bands and decreased alpha band power are present in the early stages of AD (Jeong, 2004). Age-related enhancements in theta power have been associated with cerebrospinal fluid (CSF) levels of total and phosphorylated tau (Stomrud et al., 2010).
Additionally, studies show that the functional connection of the brain is disrupted with aging. One of the features of resting state EEG recording in AD is the decrease of fast oscillations throughout the posterior regions and the general enhancement of slow rhythms (Canuet et al., 2015). EEG alterations in pathological conditions can be both a cause and a consequence of the pathology. For instance, in AD, EEG changes often result from neurodegeneration, while in epilepsy, abnormal EEG patterns can trigger seizures, contributing to the condition. Regardless, EEG changes are crucial for diagnosing and monitoring neurological and psychiatric disorders, providing insights into brain function and guiding treatment (Ishii et al., 2017).
Although no approved pharmaceutical treatments exist for MCI (Zhang et al., 2018), most studies on non-invasive treatments show that repetitive transcranial magnetic stimulation of the DLPFC promotes cognitive-enhancing effects in MCI and early AD patients (Huster et al., 2013). Transcranial direct current stimulation (tDCS) is another non-pharmacological, non-invasive form of brain stimulation that has demonstrated neuromodulatory effects. It is utilized in various neurological disorders due to its potential to enhance cognitive functions by modulating neuronal activity. tDCS involves applying a low electrical current to the scalp, which can increase or decrease neuronal excitability in targeted brain regions. This non-invasive method is advantageous because it is relatively safe, well-tolerated, and easy to administer. Moreover, tDCS can be combined with cognitive training to potentially augment its effects, offering a promising therapeutic approach to slow cognitive decline and improve the quality of life in individuals with MCI (Lefaucheur et al., 2017). Anodal stimulation shows an excitatory (depolarizing) effect, while cathodal stimulation inhibits (hyperpolarizes) the affected brain region (Rossini et al., 2007). TDCS can improve cognitive decline by modulating cortical excitability and enhancing network connectivity. By altering neuronal membrane potentials, tDCS increases or decreases cortical neuron excitability, promoting synaptic plasticity essential for learning and memory (Nitsche & Paulus, 2000).
Additionally, tDCS enhances connectivity and coherence within and between brain networks involved in cognitive tasks, improving functional integration (Polanía et al., 2018). Reports suggest that anodal tDCS over the left DLPFC combined with cognitive training improves global cognitive function and memory performance, indicating a synergistic effect of tDCS and cognitive training (Hsu et al., 2015). Similarly, anodal tDCS applied to the temporoparietal cortex enhances performance in verbal episodic memory tasks (Boggio et al., 2012). Thus, the present study aims to investigate the effects of bihemispheric tDCS in the DLPFC on cognitive abilities, brain oscillations, and network connectivity in MCI patients.
2. Materials and Methods
Subject recruitment
Based on the subjective memory evaluation results of the previous research (Das et al., 2019), the sample size needed to determine whether the suggested therapeutic approach is effective at improving memory was calculated by the Equation 1 (Agresti & Finlay, 2009):
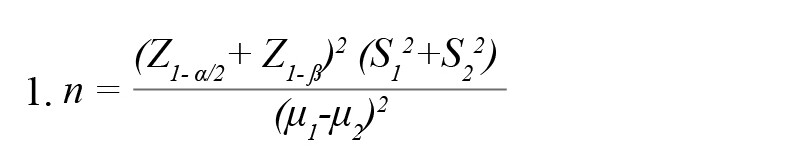
, where μ and S, respectively, indicate the Mean±SD of the memory score in the experimental groups. According to the cited study, μ1=7.83, μ2=10.7, S1=3.28, and S2=2.63. Considering a confidence level of 95% and test power of 80%, 17 participants in each group were estimated.
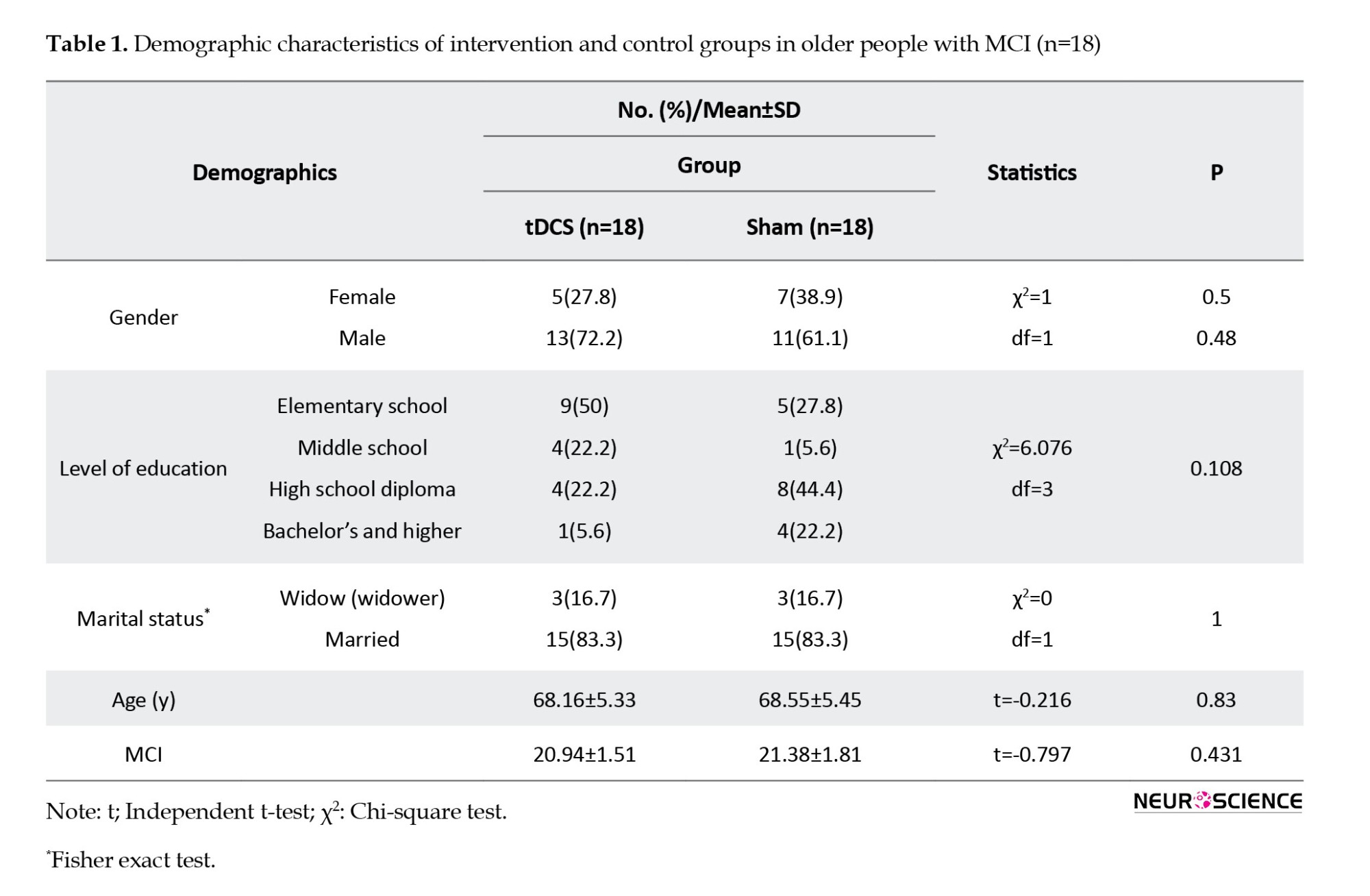
In this study, 36 older people with MCI were evaluated in two groups of 18: Active tDCS and sham tDCS. There was no statistically significant difference between the two groups regarding demographic characteristics, and they were matched in terms of these variables (P>0.05). Table 1 shows the demographic characteristics of the intervention (active) and control (sham) groups.
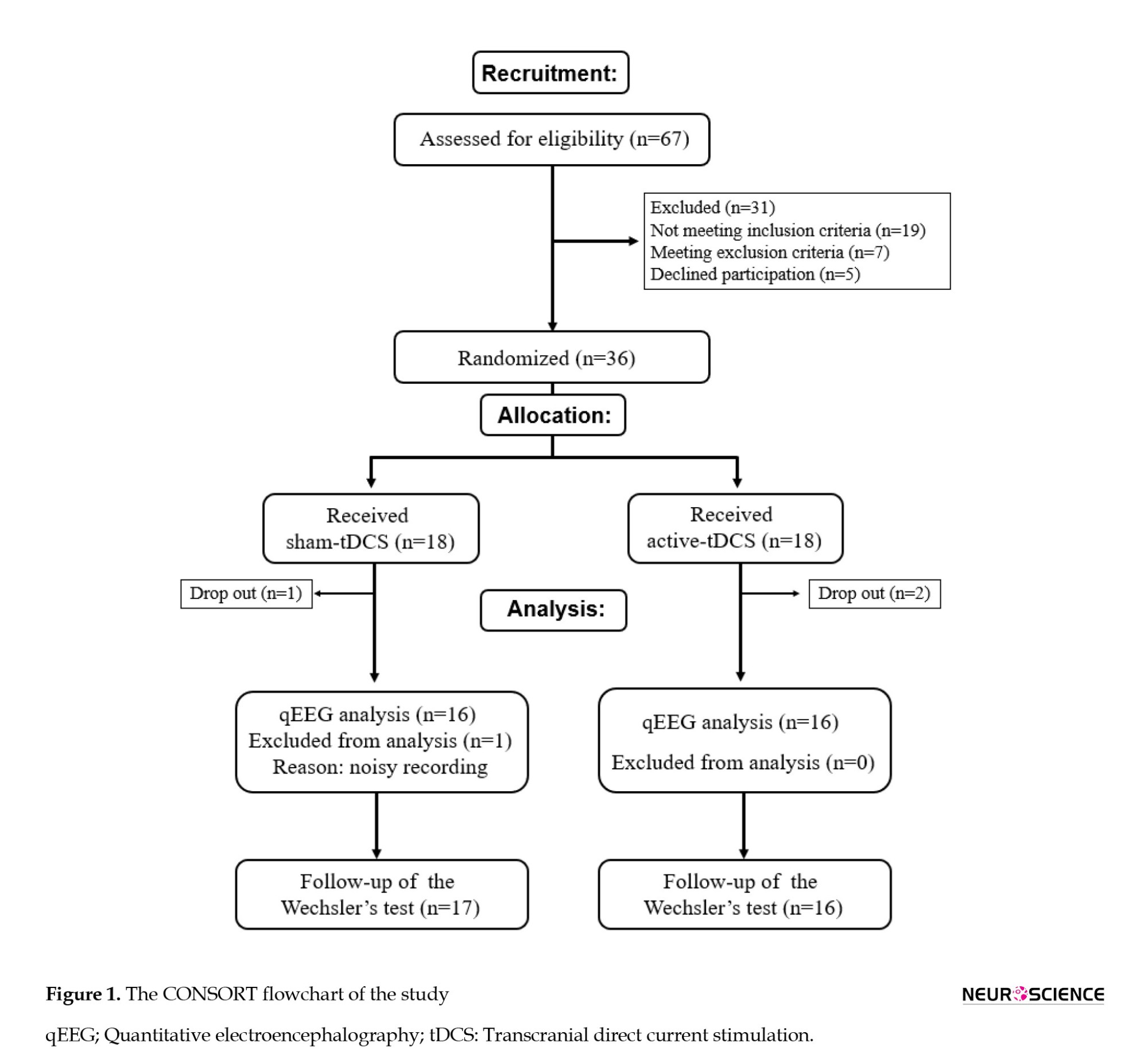
The inclusion criteria were as follows: Diagnosis of MCI by an experienced neurologist based on the 2012 revised criteria of the National Institute on Aging and Alzheimer’s Association (NIA-AA), which included change in cognition recognized by the affected individual or observers, objective impairment in 1 or more cognitive domains, independence in functional activities, and absence of dementia (Morris, 2012); mini-mental state examination (MMSE) result of 19–24 (Park et al., 2019); aged 50–80 years; being right-handed (Das et al., 2019); and ability to read and write Persian. The exclusion criteria were as follows: A history of dementia or intellectual disability; the use of cognitive enhancers (donepezil, rivastigmine, galantamine, and memantine); reversible medical conditions associated with potential cognitive impairment (e.g. a history of vitamin B12 deficiency or hypothyroidism); a diagnosis of current alcohol dependence or DSM-V disorders associated with alcohol use; epilepsy; clinically severe disorders involving the cardiovascular, digestive, respiratory, endocrine, and central nervous systems; history of cerebrovascular surgery; problems associated with direct current stimulating electrode caused by scalp malformation, inflammatory reaction, or other dermatological problem; other contraindications for tDCS medical device (e.g. metal plate inserted into the cephalus, etc.); participatation in other clinical studies within the past 30 days; and expressing difficulty in reading or having a conversation due to vision and hearing issues after wearing assistance (Park et al., 2019). As shown on the CONSORT diagram, 67 patients were assessed for recruitment in the study. Eighteen patients were randomly allocated to the active and sham tDCS group each; considering the dropouts, the results of 16 patients were analyzed. There was a noisy EEG recording in the sham-tDCS group, which was not included in the quantitative EEG (qEEG) analysis (Figure 1).
Intervention
An instrument for tDCS was used for the intervention (OASIS, Mind Alive Company Inc., Alberta, Canada). The electrodes, held in place by a headband, were made of conductive rubber and wrapped with saline-soaked sponges. The anode was placed over the F3 electrode site (overlying the left DLPFC, [L-DLPFC]) and the cathode on the R-DLPFC (F4), as described elsewhere (Yekta et al., 2022). Active tDCS involves an initial ramp up over 30 s and then keeping the current at 2 mA for 29 min. Then, the current was reduced gradually (in 30 s) to zero. For sham tDCS, the current was gradually increased to 2 mA over 30 s, immediately decreased over another 30 s, and maintained without current flow for 29 min. All subjects underwent intervention 3 days a week, up to 10 days.
Memory assessment
Participants’ memory abilities were assessed with the Persian version of the Wechsler memory scale-revised (WMS-R) before (baseline), immediately, and three months after (follow-up) the intervention. David Wechsler first presented this scale in 1947 and revised it in 1987 (Wechsler, 1987). The WMS-R consists of 7 subtests: Mental control, figural memory, logical memory, visual paired associations, visual reproduction, digital span, and visual memory span (Wechsler, 1987). According to the test guide, the memory quotient (MQ) was calculated based on these subtests.
EEG recording
After recording the participants’ demographic information, which included age, sex, marital status, and other details, all patients were given comfortable seats in a room with little noise and light. The distance between the nasion and inion was first measured to determine the proper size of the EEG cap. For accuracy, the head was measured and marked. The electrodes were placed on the scalp following the EEG 10-20 International System with a 19-channel cap system. The recorded electrodes were as follows: FP1, FP2, F7, F8, F3, F4, and Fz in the frontal region; P3, P4, Pz, T3, T4, T5 and T6 in the parietotemporal area; the C3, C4, and Cz in the central region; and O1 and O2 on the occipital lobe. The electrode impedance was reduced by using electrogel. An impedance of less than 10 kΩ across all electrodes was required for initiating the recordings. While minimizing physical movements, the qEEG recordings were performed at a resting-state, with closed eyes for 7 minutes. The linked ears (LE) were chosen as the default reference electrode, and the forehead was the ground electrode for EEG recording. The brain signals were sent into a 24-channel neurostyle EEG recording system (Neurostyle Pte Ltd., Singapore), where they were captured at a sampling rate of 250 Hz with a bandpass filter of 0.5 to 50 Hz.
Quantitative EEG analysis
The recorded signals were transferred to MATLAB 2021b software (MathWorks Inc., Massachusetts, USA). In the pre-processing stage, the noisy signals were rejected by an automatic artifact rejection technique. Also, the eye blink artifact was removed using an ICA technique. The absolute and relative powers were calculated using the fast Fourier transform (FFT) method; a Hanning window with 50% overlap was applied to the whole EEG signal after artifact rejection. Each frequency band’s power spectral density was calculated for each subject using the Pwelch method. The most commonly studied frequency band ranges were assessed: Delta (0.5–3.5 Hz), theta (4.0–7.5 Hz), alpha (8.0–12.0 Hz), and beta (12.5–25.0 Hz), which is also based on the Neuroguide default settings (Akhoondian et al., 2023).
Additionally, NeuroGuide software, version 3.2.4 (Applied Neuroscience, Inc., USA) was used to create the averaged topographical brain maps, where the intensity of distinct band powers in each brain region is represented by a color gradient. To analyze qEEG data, clean EEG signal epochs were created by a template artifact rejection tool based on 2 standard deviations of the Z-scored EEG signals. Afterwards, the finite impulse response (FIR) filter was applied. Finally, the group analysis feature of the NeuroGuide was used to compare the total signals recorded in each channel of the active tDCS group with the corresponding channel of the sham group at the endpoint of the experiment. Thus, the brain maps represent the mean absolute/relative power difference between the active and sham tDCS groups. The same is true for the coherence brain maps. The significance of the differences was calculated using the NeuroGuide software.
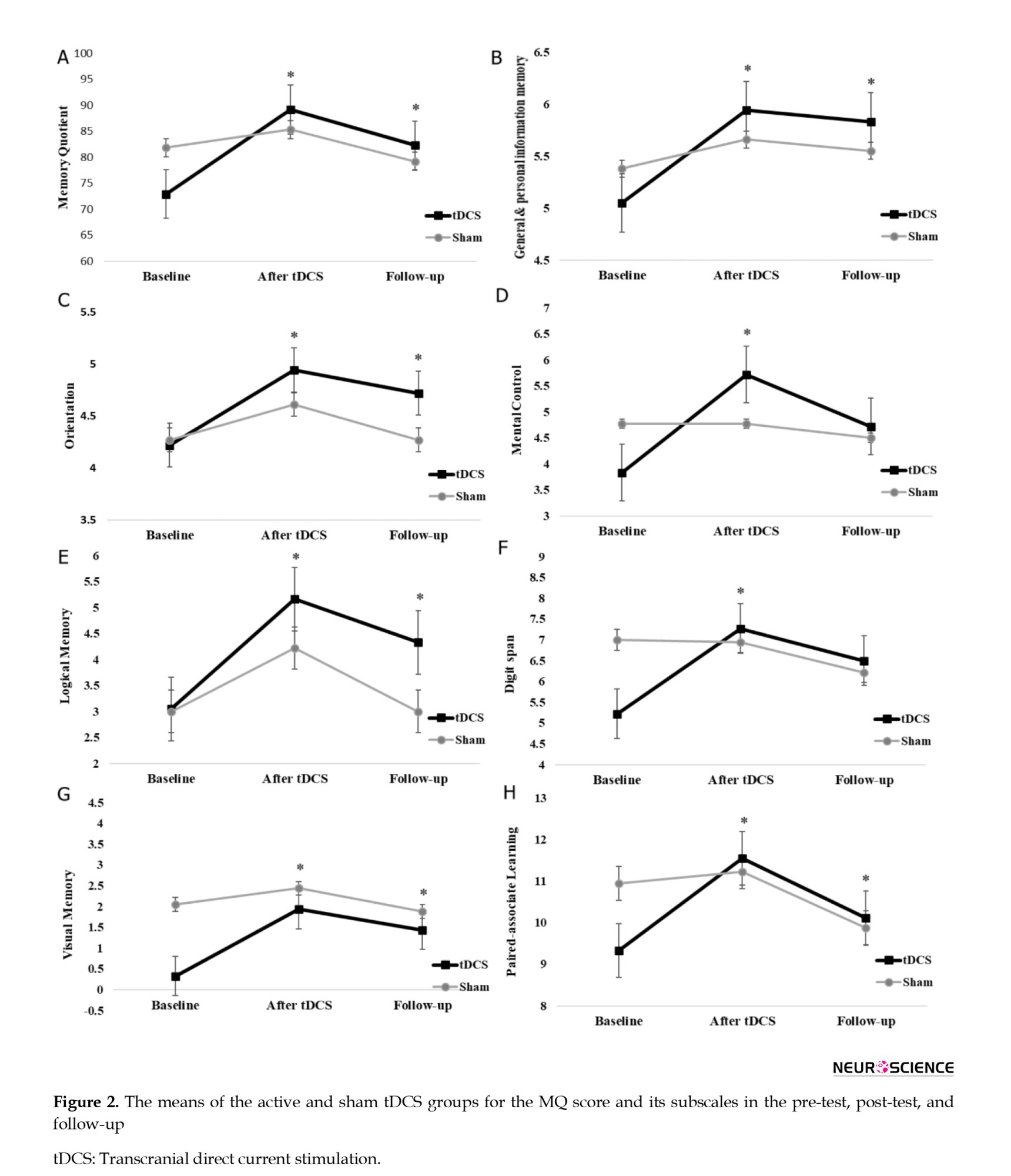
All statistical analyses were performed using the SPSS software version 26 (IBM Corp, USA). Since some values of the WMS-R subscale scores differed between the active and sham tDCS groups at baseline (Figure 2), repeated measures of analysis of covariance (ANCOVA) were used to analyze the data. The pre-test total score and subscales of the WMS-R for the intervention and the control group were considered covariate variables for the measurement of the effect of the intervention in the pre-test, post-test, and a 3-month follow-up. Before covariance analysis, its parametric assumptions such as normality of data distribution, homogeneity of variances, homogeneity of the covariance matrix, homogeneity of regression line slope, Bartlett’s test of sphericity, absence of outlier data and non-collinearity of dependent variables were confirmed. For the analysis of the qEEG data, an unpaired t-test was performed to compare the mean relative power of different frequency bands between the sham and active tDCS groups after the intervention. The qEEG analysis was done in the analysis tab of the NeuroGuide software, and P≤0.05 was considered statistically significant. The coherence in each frequency band range (cross-channel auto-frequency connectivity) was calculated in the NeuroGuide environment. The significant differences between the intervention and control groups were depicted in a brain map.
3. Results
Active tDCS, the memory quotient, and WMS-R Subscales in MCI patients immediately and three months after the application
Table 2 presents the Mean±SD of the memory quotient (MQ) and its 7 subscales according to group membership (receiving active or sham tDCS as a between-subject factor or independent variable) and assessment stages (pre-test, post-test, and follow-up as a within-subject factor).
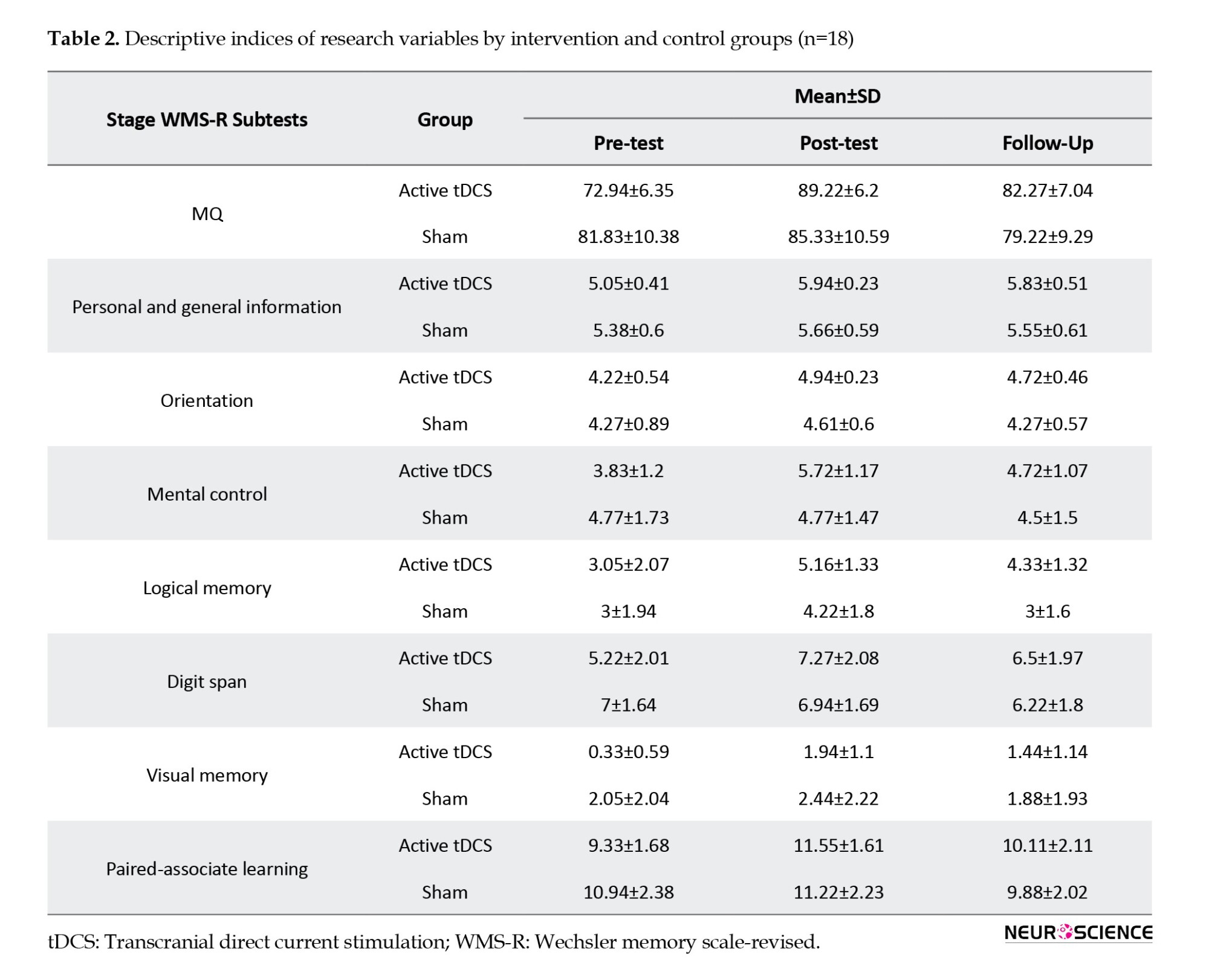
The results of repeated measures of ANCOVA in Table 3 showed that the interaction effect of the time×group and the effect of time on the total score of the MQ was not significant (P>0.05). However, the results indicated that the group’s main effect on the MQ’s total score was significant (P≤0.001).
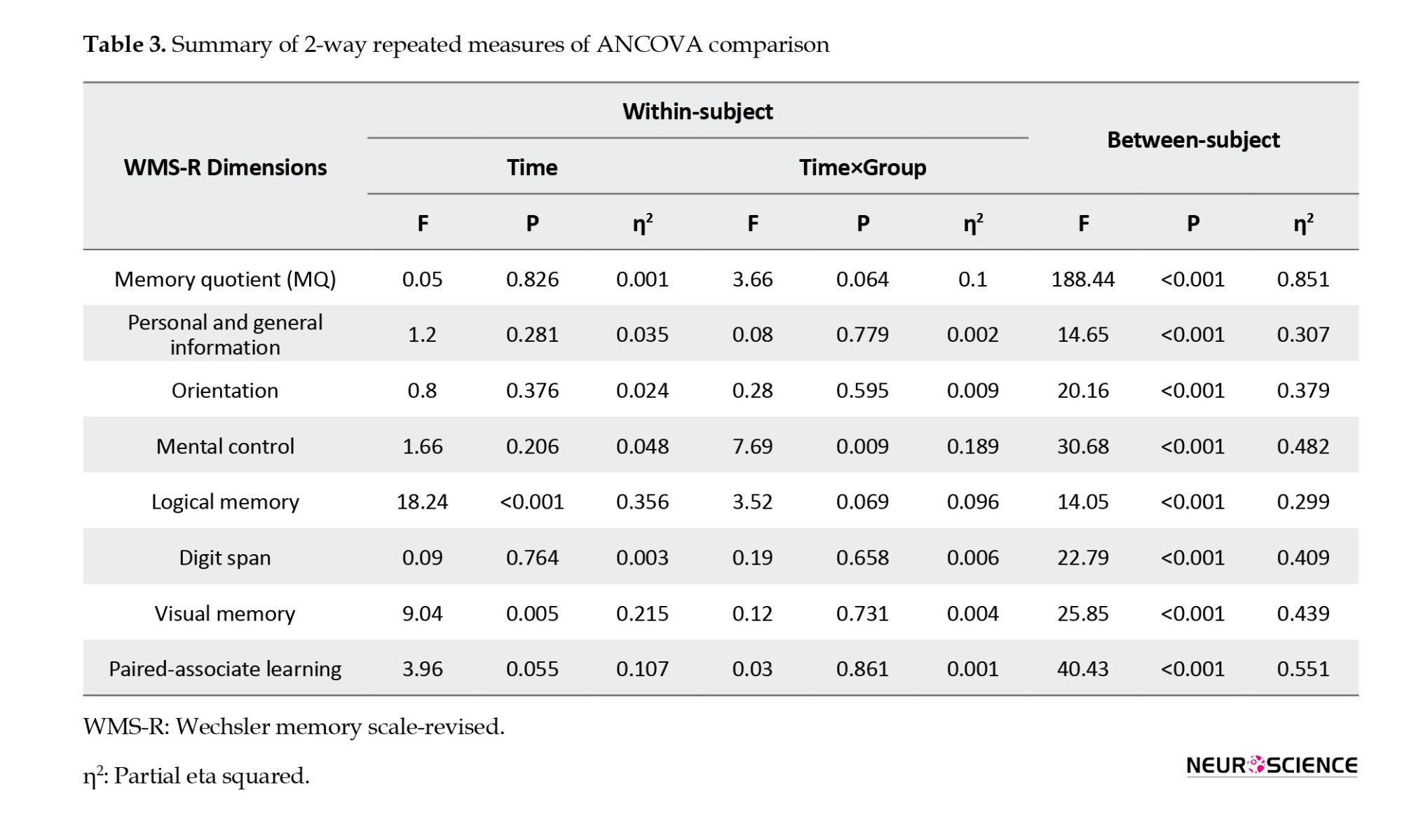
In addition, the results of multiple ANCOVA measures indicated that only the mental control subscale (P=0.009) was significantly affected by the time×group interaction. As a result, the active tDCS group’s degree of mental control was substantially higher than the sham group during the post-test and follow-up phases (Table 3 and Figure 2). Table 3 presents the group and time primary impacts. The findings indicate that only the logical memory and visual memory subscales exhibit a significant time effect (P≤0.05). Moreover, according to the data, all components showed a significant group effect (P≤0.001). Thus, the average of every memory quotient component in the experimental group rose noticeably after the tDCS intervention was implemented compared to the sham group (Table 3 and Figure 2).
Application of active tDCS in MCI patients, the power of the delta and theta band in the left frontal region
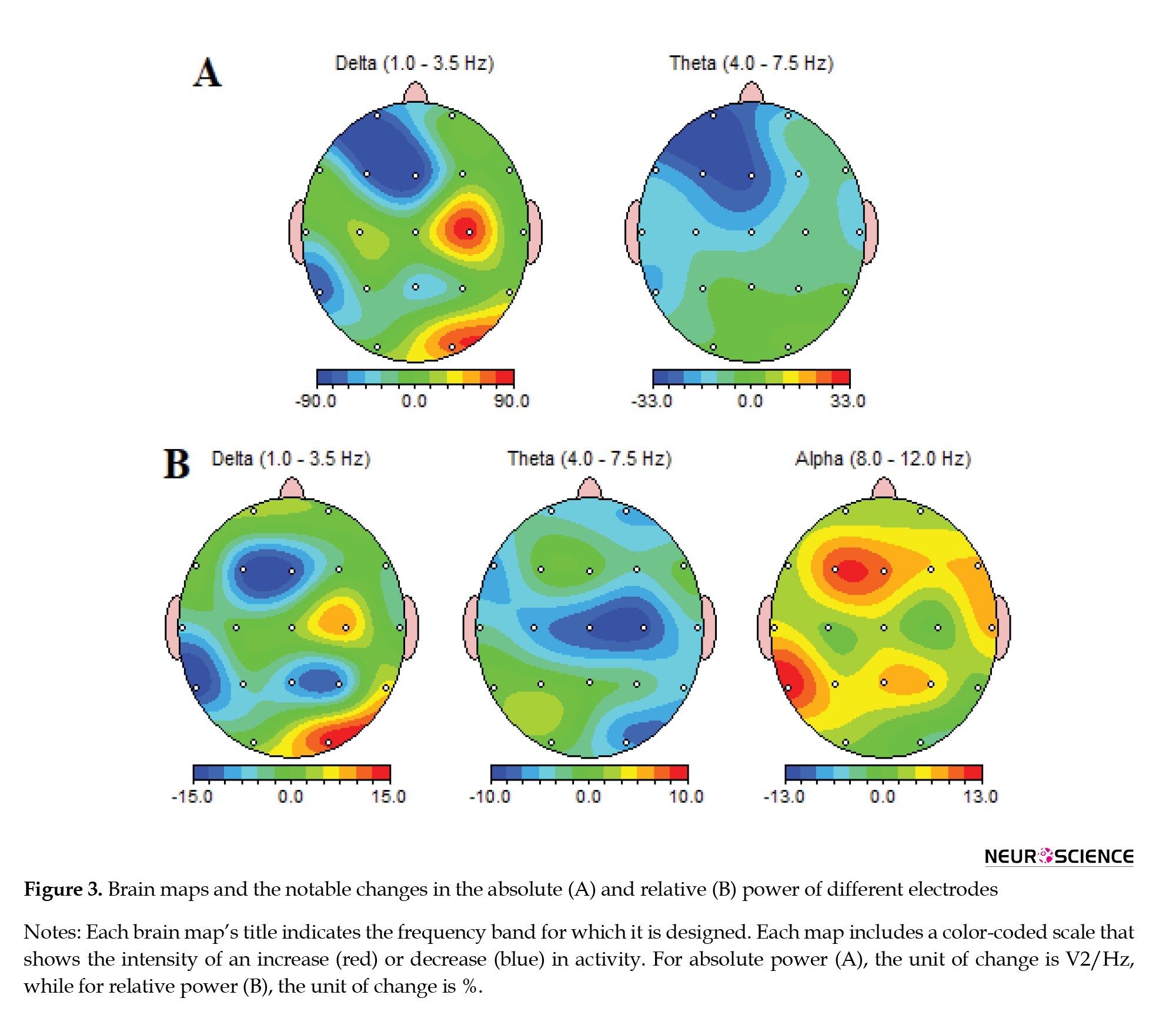
The analysis of EEG recording results after applying 10 sessions of active tDCS compared to the sham tDCS group showed that the absolute power of the delta frequency band decreased in the FP1, F3, Fz, and T5 areas. The decrease in the delta band power of F3 and T5 channels was significant (P<0.05). As depicted in Figure 3A, the absolute power of the theta band range also attenuated in the FP1, F3, and Fz regions (P<0.05). The alterations in the absolute power of other bands were not shown in Figure 3A, as they showed no significant difference.
Active tDCS, the delta and theta relative powers while boosting the alpha band frequency in the frontal and temporal brain regions
Figure 3B shows the alterations in the relative power of the low-frequency band range. After applying active tDCS to MCI patients, the relative delta band power of the Fz (P<0.05) and T5 channels (P<0.01) attenuated significantly. Similarly, the relative theta band power diminished by around 10% in the C4 and Cz regions (P<0.01). On the other hand, the alpha band power was augmented in the F3 and T5 brain regions in patients receiving active tDCS. The alterations in the relative power of the beta and gamma bands were not shown in Figure 3B, as they showed no significant difference.
Application of tDCS, the coherence between frontal and temporal regions, mainly in the delta band frequencies
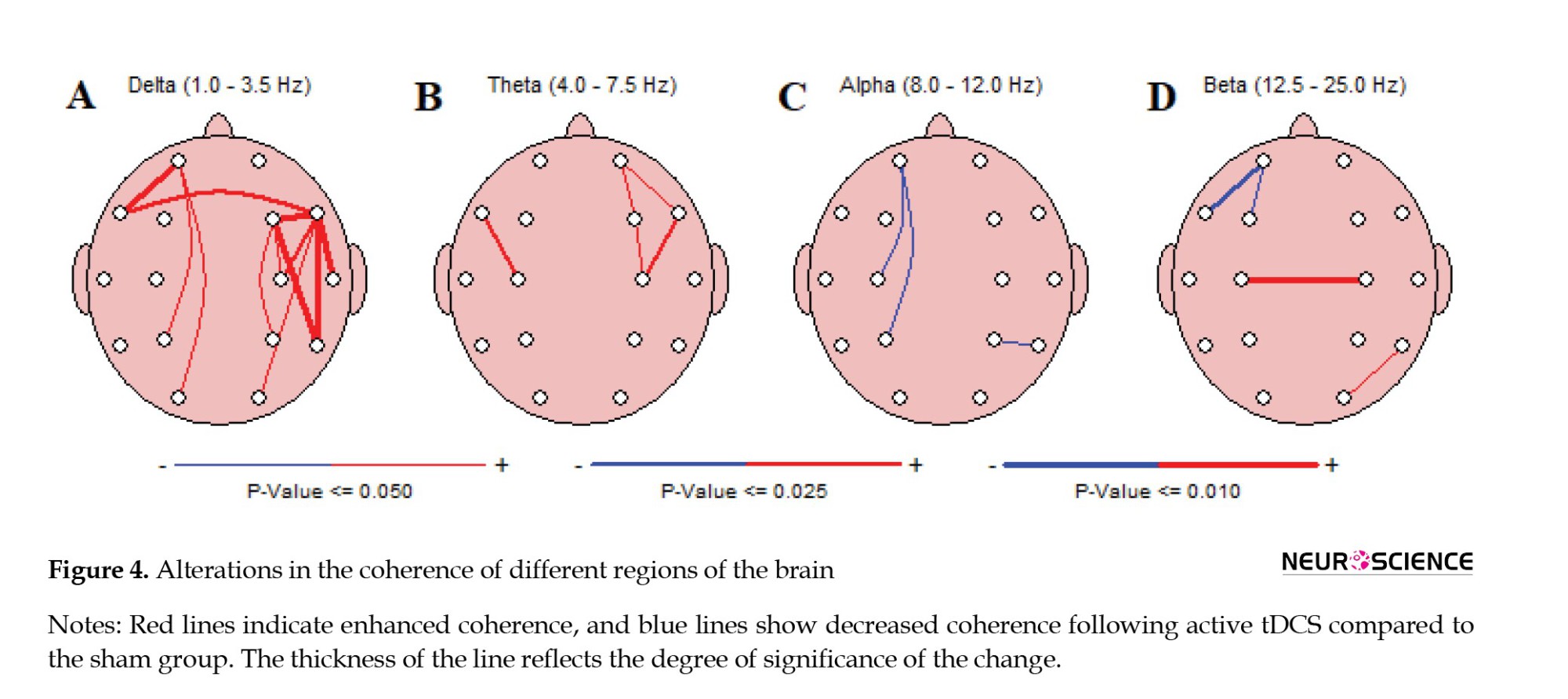
All coherence and functional connectivity results are based on cross-channel auto-frequency analysis. As shown in Figure 4A, the most significant changes (P<0.01) in coherence were detected in the delta band range; the coherence between FP1-F7, F4-F8, F4-T8, F8-T4, and F8-T6 regions increased drastically in the delta band frequencies. On the other hand, in the beta band range, the C3-C4 coherence showed significant attenuation, depicted in Figure 4D. Some other brain regions showed coherence changes with lower significance levels (P<0.05), shown with thinner lines between different channels in Figure 4A-D.
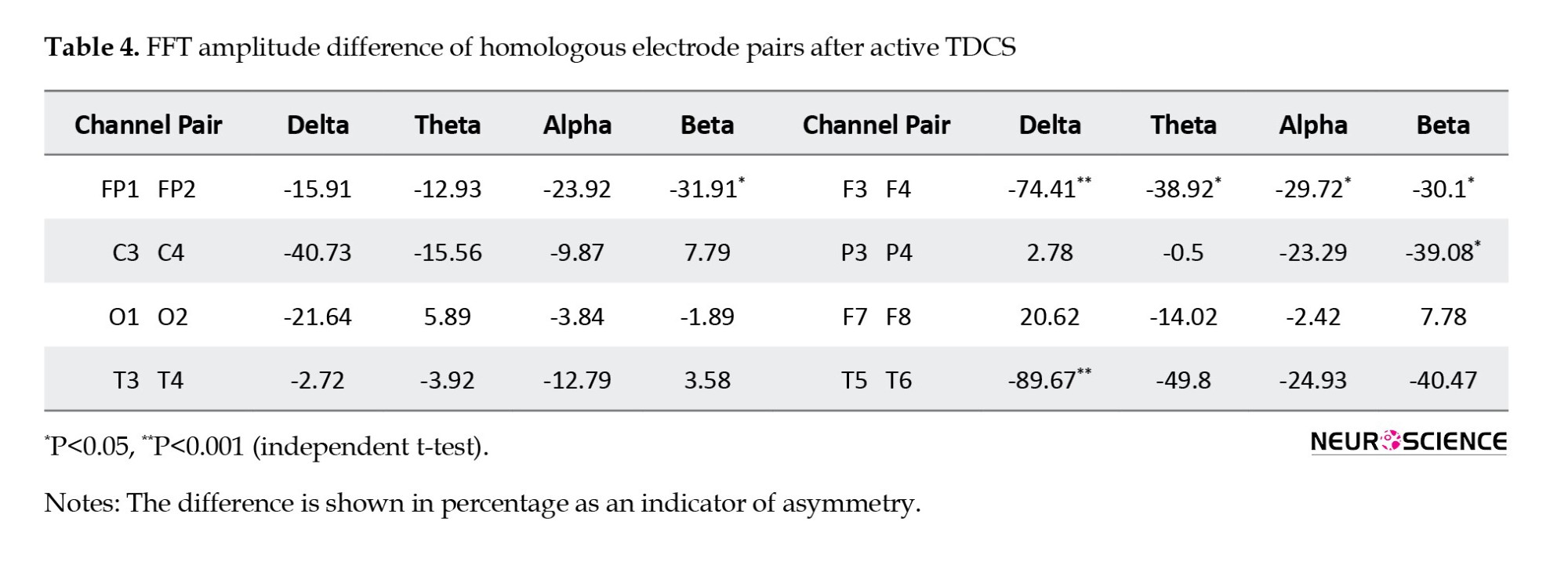
Active tDCS on the F3 region of MCI Patients, frontal and temporal amplitude asymmetry in different band frequencies
The FFT analysis of different frequency bands in distinct brain regions revealed an inter-hemispheric asymmetry after active tDCS intervention. Table 4 indicates a significantly lower amplitude in the left frontal (F3) versus the right frontal (F4) region of MCI patients receiving active tDCS in all frequency ranges (P<0.01 for theta, P<0.05 for other bands). Lower left-side delta band activity is also observed in the T5-T6 region. Also, the P3-P4 and FP1-FP2 channels demonstrated lower beta-band activity in the left hemisphere (P<0.05).
4. Discussion
The results of the present study revealed that 10 sessions of bihemispheric tDCS on the DLPFC regions improved all aspects of Wechsler’s memory scale over three months. Personal and general information memory, digit span, and mental control showed the highest effect sizes. Wu et al. reported improved visuospatial working memory after 10 sessions of anodal tDCS on the right DLPFC (F4) of diabetic polyneuropathy patients (Wu et al., 2016). Gu et al. applied 20-minute anodal tDCS to the left temporal (T3) region for 5 consecutive days in MCI patients. They revealed that picture memory, visual regeneration, logical memory, and memory span increased significantly. Additionally, they noted a favorable relationship between the memory quotient score and the P300 amplitude difference (Gu et al., 2022). The higher effectiveness in the present study can be attributed to the bihemispheric setup implemented on both left and right DLPFCs.
Analysis of qEEG data revealed that after the tDCS treatment period, the absolute power of the delta and theta bands in the left frontal and temporal regions showed a significant decrease compared to the patients receiving sham tDCS. The relative delta power decreased in the frontal and temporal regions, while in the theta range, the relative power decreased in the central areas. Interestingly, in the frequency range of the alpha band, the relative power increased in the frontal and left temporal regions. There have been numerous reports of AD-related alterations in the pattern of the EEG power spectra, including an increase in theta and delta power and a beta reduction, followed by a decline in alpha band power, which results in a general deceleration of the EEG (Dierks et al., 1991; Jafari et al., 2020; Smailovic et al., 2018). Musaeus et al. showed that relative theta power is prominent in AD patients; it is also increased in MCI patients, though to a lesser extent, compared to healthy control individuals. They suggested that the increase in relative theta power could be the first EEG alteration in AD patients (Musaeus et al., 2018). Babiloni et al. demonstrated that MCI patients show a significantly higher delta power in the frontal, parietal, and temporal regions, while their alpha band power is reduced in the temporal and occipital lobes (Babiloni et al., 2018). Wang et al. (2017) reported an enhancement in the gamma band power in addition to the increased delta and decreased alpha power using a wavelet power spectrum analysis (Wang et al., 2017).
Additionally, a general increase in the power of the delta and theta frequency ranges, a notable decrease in the amplitude of alpha activity, and a slowing of background alpha activity are reported as manifestations of physiological aging (Ishii et al., 2017). These reports indicate an augmentation of slow frequency bands in patients with dementia, which can be interpreted as being in line with our results. Bilateral tDCS treatment diminished the spectral power of the delta and theta band ranges and boosted the alpha band power compared to non-treated MCI patients. These reversal patterns observed in the present study could be attributed to boosted excitability in the left DLPFC by anodal tDCS.
On the other hand, Fauzan and Amran (2015) found that MCI patients had higher beta-2 power in the right anterior region compared to normal elderly individuals. Both groups displayed a predominant distribution of theta and alpha in the frontal regions. In contrast, the highest levels of theta were found in the parietal and temporal areas, which is associated with the symptoms of cognitive decline in individuals suffering from MCI (Fauzan and Amran, 2015). According to Luckhaus et al., MCI patients have lower alpha band power, which is significantly linked with worse cognitive function on psychometric tests. The strongest positive predictive accuracy for MCI and AD and the likelihood of cognitive deterioration were found in the alpha power over posterior leads (Luckhaus et al., 2008).
Early synaptic disruption and loss, which result in white matter anomalies and functional connectivity deficiencies, are the main causes of progressive cognitive decline (Rémy et al., 2015). In elderly individuals with biomarker positivity who develop AD, disruption in functional connectivity in the alpha band is documented, in addition to anomalies in alpha source oscillatory activity (Maestú et al., 2015). Rossini et al. demonstrated that a high midline gamma coherence and a significant temporal delta source were related to a faster progression of dementia (Rossini et al., 2006). Compared to healthy control subjects, Toth et al. found that MCI patients had significantly lower levels of local functional connectivity in the left and right frontal regions in the delta band range. Likewise, the left frontotemporal connection and the right and left frontoparietal connectivity decreased in MCI patients (Tóth et al., 2014). The current study’s findings demonstrate a notable improvement in left and right frontal and right frontotemporal connectivity in the delta band range. While other studies reveal that MCI patients have increased alpha-1 connectivity in the right parietal region (Tóth et al., 2014), the tDCS strategy used in the current study increased beta-band coherence in the central brain region.
Martin et al. recently carried out a visual motion direction identification task on MCI patients in an EEG investigation. They only noted a frontal beta asymmetry in the MCI patients rather than any frontal alpha (Martin et al., 2022). According to our findings, frontal asymmetry develops following active tDCS therapy in all frequency bands, with greater oscillation power in the right hemisphere. Additionally, the beta band of the parietal and the delta band of the temporal areas showed the same pattern of asymmetries. Sarica et al. assessed the hippocampus and related structures’ MRI asymmetry index. They demonstrated that compared to healthy controls, AD and progressing MCI patients displayed considerable asymmetry and a greater volume on the right side (Sarica et al., 2018). Similarly, Yang et al. found a rightward asymmetry in global and local network efficiency between the two hemispheres of AD patients using diffusion tensor imaging tractography (Yang et al., 2017).
The primary concern in the present study is the small sample size, which can reduce the generalizability of the findings and increase the risk of statistical anomalies. Additionally, potential biases, including selection bias, can skew the results. Another significant limitation is the short duration of follow-up, which may not capture long-term changes or the full spectrum of brain activity alterations over time. These factors collectively highlight the need to interpret the qEEG findings carefully. Future studies should explore various tDCS protocols, including different intensities, durations, and electrode placements while comparing bihemispheric and unilateral DLPFC stimulation to optimize cognitive benefits in MCI patients. Long-term effects should be assessed through extended follow-up periods and multiple sessions to evaluate sustained cognitive improvements and EEG changes. Increasing sample sizes enhances the generalizability and validity of findings. Detailed EEG analyses and additional functional assessments will deepen understanding of the neurophysiological mechanisms and broader impacts of tDCS, supported by multi-modal neuroimaging techniques.
To translate tDCS findings into clinical practice, standardized protocols and integrating tDCS into cognitive rehabilitation programs are essential. Key challenges include careful patient selection and robust safety monitoring. Long-term efficacy and safety should be assessed through longitudinal studies. Addressing these factors can make tDCS a valuable tool for enhancing cognitive function in MCI patients.
5. Conclusion
This study demonstrates that bihemispheric tDCS of the DLPFC can enhance cognitive functions and modulate brain activity in MCI patients. Anodal stimulation of the left DLPFC and cathodal inhibition of the right one reduces the low-frequency band power and increases the frontotemporal coherence. The cognitive effects of this tDCS protocol lasted for three months and may have implications for preventing and treating dementia.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethic Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1399.596). Upon ethical approval, the study was registered by the Iranian Registry for Clinical Trials (IRCT) (Code: 20190522043673N2).
Funding
This study is derived from the general medical doctorate thesis of Soroush Ahmadi Machiani, approved by the Medical School of Guilan University of Medical Sciences, Rasht, Iran (Code: 242). The research was funded by the Neuroscience Research Center at Guilan University of Medical Sciences, Rasht, Iran (Grant No.: 2724).
Authors' contributions
Conceptualization: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Methodology: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Software: Sajjad Rezaei and Kambiz Rohampour; Data curation: Soroush Ahmadi, Arman Keymoradzadeh and Babak Bakhshayesh; Investigation: Soroush Ahmadi and Arman Keymoradzadeh; Formal analysis: Sajjad Rezaei and Kambiz Rohampour; Resources: Alia Saberi and Kambiz Rohampour; Writing the original draft: Soroush Ahmadi and Arman Keymoradzadeh: Review and editing: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Validation: All authors; Supervision: Kambiz Rohampour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the faculty and staff of the Neuroscience Research Center of Guilan University of Medical Sciences, Rasht, Iran, for their support and contributions throughout the project.
References
Mild cognitive impairment (MCI), also known as mild neurocognitive disorder, is characterized by subjective and objective cognitive impairment in older individuals with preserved daily functions (Martin et al., 2019). MCI is a transitional state between normal aging and dementia. The prevalence of MCI in adults older than 60 and the annual rate of progression to dementia are up to 25.2% and 17%, respectively (Jongsiriyanyong & Limpawattana, 2018). Even though MCI is a type of cognitive problem that affects people who are not yet in a state of dementia, it is nevertheless a condition of aging with a likely degenerative etiology that is related to the development of Alzheimer disease (AD) (Okello et al., 2009). The aging brain might trigger compensating cognitive systems. For instance, young individuals who are not cognitively impaired but are in good health and have trouble with executive tasks exhibit overactivation in only one hemisphere of the prefrontal cortex (Reuter-Lorenz et al., 2000). Due to the likelihood that older adults have less attentional and working memory capacities, this brain reaction may be explained by cognitive restructuring in these individuals (Kirova et al., 2015). Working memory and attention problems have been linked to executive functioning decline, which is aggravated by MCI (Kochan et al., 2010).
Studies using positron emission tomography (PET) multimodal imaging data in combination with independent component analysis (ICA) could efficiently distinguish MCI patients from healthy controls. They discovered that MCI patients’ spatial distribution of amyloid beta PET and tau PET significantly differs from that of healthy individuals. These areas are colocalized with the default mode and cognitive control networks (Li et al., 2019). Furthermore, global functional connectivity of the cognitive control network in the resting state has been proposed as a biomarker for cognitive reserve. It is also considered protection for brain function in MCI patients by functional magnetic resonance imaging investigation (Franzmeier et al., 2017). The cognitive control network consists of several interconnected brain regions: The dorsolateral prefrontal cortex (DLPFC), which is involved in working memory (Cieslik et al., 2013), cognitive performance (Cao et al., 2016), attention, and decision-making (Obeso et al., 2021); anterior cingulate cortex, which is involved in monitoring and regulating cognitive processes (Braem et al., 2017); Inferior parietal lobule, involved in spatial attention and working memory (Clower et al., 2001); ventrolateral prefrontal cortex, involved in inhibitory control and decision-making; posterior parietal cortex; and medial prefrontal cortex (Amidfar et al., 2019).
Numerous cognitive processes and the functional connection between various brain regions depend heavily on brain oscillations (Zhang et al., 2018). Theta oscillations, primarily seen in the frontal cortex and regulated by inhibition in other brain regions, are typically associated with memory and executive functions (Huster et al., 2013). An overall boost in the power of the delta and theta frequency ranges is linked to aging (Rossini et al., 2007). Additionally, studies using resting state electroencephalography (EEG) show that lower frequency bands and decreased alpha band power are present in the early stages of AD (Jeong, 2004). Age-related enhancements in theta power have been associated with cerebrospinal fluid (CSF) levels of total and phosphorylated tau (Stomrud et al., 2010).
Additionally, studies show that the functional connection of the brain is disrupted with aging. One of the features of resting state EEG recording in AD is the decrease of fast oscillations throughout the posterior regions and the general enhancement of slow rhythms (Canuet et al., 2015). EEG alterations in pathological conditions can be both a cause and a consequence of the pathology. For instance, in AD, EEG changes often result from neurodegeneration, while in epilepsy, abnormal EEG patterns can trigger seizures, contributing to the condition. Regardless, EEG changes are crucial for diagnosing and monitoring neurological and psychiatric disorders, providing insights into brain function and guiding treatment (Ishii et al., 2017).
Although no approved pharmaceutical treatments exist for MCI (Zhang et al., 2018), most studies on non-invasive treatments show that repetitive transcranial magnetic stimulation of the DLPFC promotes cognitive-enhancing effects in MCI and early AD patients (Huster et al., 2013). Transcranial direct current stimulation (tDCS) is another non-pharmacological, non-invasive form of brain stimulation that has demonstrated neuromodulatory effects. It is utilized in various neurological disorders due to its potential to enhance cognitive functions by modulating neuronal activity. tDCS involves applying a low electrical current to the scalp, which can increase or decrease neuronal excitability in targeted brain regions. This non-invasive method is advantageous because it is relatively safe, well-tolerated, and easy to administer. Moreover, tDCS can be combined with cognitive training to potentially augment its effects, offering a promising therapeutic approach to slow cognitive decline and improve the quality of life in individuals with MCI (Lefaucheur et al., 2017). Anodal stimulation shows an excitatory (depolarizing) effect, while cathodal stimulation inhibits (hyperpolarizes) the affected brain region (Rossini et al., 2007). TDCS can improve cognitive decline by modulating cortical excitability and enhancing network connectivity. By altering neuronal membrane potentials, tDCS increases or decreases cortical neuron excitability, promoting synaptic plasticity essential for learning and memory (Nitsche & Paulus, 2000).
Additionally, tDCS enhances connectivity and coherence within and between brain networks involved in cognitive tasks, improving functional integration (Polanía et al., 2018). Reports suggest that anodal tDCS over the left DLPFC combined with cognitive training improves global cognitive function and memory performance, indicating a synergistic effect of tDCS and cognitive training (Hsu et al., 2015). Similarly, anodal tDCS applied to the temporoparietal cortex enhances performance in verbal episodic memory tasks (Boggio et al., 2012). Thus, the present study aims to investigate the effects of bihemispheric tDCS in the DLPFC on cognitive abilities, brain oscillations, and network connectivity in MCI patients.
2. Materials and Methods
Subject recruitment
Based on the subjective memory evaluation results of the previous research (Das et al., 2019), the sample size needed to determine whether the suggested therapeutic approach is effective at improving memory was calculated by the Equation 1 (Agresti & Finlay, 2009):
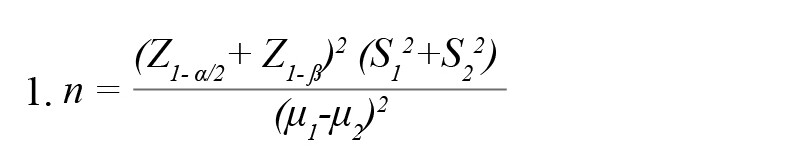
, where μ and S, respectively, indicate the Mean±SD of the memory score in the experimental groups. According to the cited study, μ1=7.83, μ2=10.7, S1=3.28, and S2=2.63. Considering a confidence level of 95% and test power of 80%, 17 participants in each group were estimated.
In this study, 36 older people with MCI were evaluated in two groups of 18: Active tDCS and sham tDCS. There was no statistically significant difference between the two groups regarding demographic characteristics, and they were matched in terms of these variables (P>0.05). Table 1 shows the demographic characteristics of the intervention (active) and control (sham) groups.

The inclusion criteria were as follows: Diagnosis of MCI by an experienced neurologist based on the 2012 revised criteria of the National Institute on Aging and Alzheimer’s Association (NIA-AA), which included change in cognition recognized by the affected individual or observers, objective impairment in 1 or more cognitive domains, independence in functional activities, and absence of dementia (Morris, 2012); mini-mental state examination (MMSE) result of 19–24 (Park et al., 2019); aged 50–80 years; being right-handed (Das et al., 2019); and ability to read and write Persian. The exclusion criteria were as follows: A history of dementia or intellectual disability; the use of cognitive enhancers (donepezil, rivastigmine, galantamine, and memantine); reversible medical conditions associated with potential cognitive impairment (e.g. a history of vitamin B12 deficiency or hypothyroidism); a diagnosis of current alcohol dependence or DSM-V disorders associated with alcohol use; epilepsy; clinically severe disorders involving the cardiovascular, digestive, respiratory, endocrine, and central nervous systems; history of cerebrovascular surgery; problems associated with direct current stimulating electrode caused by scalp malformation, inflammatory reaction, or other dermatological problem; other contraindications for tDCS medical device (e.g. metal plate inserted into the cephalus, etc.); participatation in other clinical studies within the past 30 days; and expressing difficulty in reading or having a conversation due to vision and hearing issues after wearing assistance (Park et al., 2019). As shown on the CONSORT diagram, 67 patients were assessed for recruitment in the study. Eighteen patients were randomly allocated to the active and sham tDCS group each; considering the dropouts, the results of 16 patients were analyzed. There was a noisy EEG recording in the sham-tDCS group, which was not included in the quantitative EEG (qEEG) analysis (Figure 1).

Intervention
An instrument for tDCS was used for the intervention (OASIS, Mind Alive Company Inc., Alberta, Canada). The electrodes, held in place by a headband, were made of conductive rubber and wrapped with saline-soaked sponges. The anode was placed over the F3 electrode site (overlying the left DLPFC, [L-DLPFC]) and the cathode on the R-DLPFC (F4), as described elsewhere (Yekta et al., 2022). Active tDCS involves an initial ramp up over 30 s and then keeping the current at 2 mA for 29 min. Then, the current was reduced gradually (in 30 s) to zero. For sham tDCS, the current was gradually increased to 2 mA over 30 s, immediately decreased over another 30 s, and maintained without current flow for 29 min. All subjects underwent intervention 3 days a week, up to 10 days.
Memory assessment
Participants’ memory abilities were assessed with the Persian version of the Wechsler memory scale-revised (WMS-R) before (baseline), immediately, and three months after (follow-up) the intervention. David Wechsler first presented this scale in 1947 and revised it in 1987 (Wechsler, 1987). The WMS-R consists of 7 subtests: Mental control, figural memory, logical memory, visual paired associations, visual reproduction, digital span, and visual memory span (Wechsler, 1987). According to the test guide, the memory quotient (MQ) was calculated based on these subtests.
EEG recording
After recording the participants’ demographic information, which included age, sex, marital status, and other details, all patients were given comfortable seats in a room with little noise and light. The distance between the nasion and inion was first measured to determine the proper size of the EEG cap. For accuracy, the head was measured and marked. The electrodes were placed on the scalp following the EEG 10-20 International System with a 19-channel cap system. The recorded electrodes were as follows: FP1, FP2, F7, F8, F3, F4, and Fz in the frontal region; P3, P4, Pz, T3, T4, T5 and T6 in the parietotemporal area; the C3, C4, and Cz in the central region; and O1 and O2 on the occipital lobe. The electrode impedance was reduced by using electrogel. An impedance of less than 10 kΩ across all electrodes was required for initiating the recordings. While minimizing physical movements, the qEEG recordings were performed at a resting-state, with closed eyes for 7 minutes. The linked ears (LE) were chosen as the default reference electrode, and the forehead was the ground electrode for EEG recording. The brain signals were sent into a 24-channel neurostyle EEG recording system (Neurostyle Pte Ltd., Singapore), where they were captured at a sampling rate of 250 Hz with a bandpass filter of 0.5 to 50 Hz.
Quantitative EEG analysis
The recorded signals were transferred to MATLAB 2021b software (MathWorks Inc., Massachusetts, USA). In the pre-processing stage, the noisy signals were rejected by an automatic artifact rejection technique. Also, the eye blink artifact was removed using an ICA technique. The absolute and relative powers were calculated using the fast Fourier transform (FFT) method; a Hanning window with 50% overlap was applied to the whole EEG signal after artifact rejection. Each frequency band’s power spectral density was calculated for each subject using the Pwelch method. The most commonly studied frequency band ranges were assessed: Delta (0.5–3.5 Hz), theta (4.0–7.5 Hz), alpha (8.0–12.0 Hz), and beta (12.5–25.0 Hz), which is also based on the Neuroguide default settings (Akhoondian et al., 2023).
Additionally, NeuroGuide software, version 3.2.4 (Applied Neuroscience, Inc., USA) was used to create the averaged topographical brain maps, where the intensity of distinct band powers in each brain region is represented by a color gradient. To analyze qEEG data, clean EEG signal epochs were created by a template artifact rejection tool based on 2 standard deviations of the Z-scored EEG signals. Afterwards, the finite impulse response (FIR) filter was applied. Finally, the group analysis feature of the NeuroGuide was used to compare the total signals recorded in each channel of the active tDCS group with the corresponding channel of the sham group at the endpoint of the experiment. Thus, the brain maps represent the mean absolute/relative power difference between the active and sham tDCS groups. The same is true for the coherence brain maps. The significance of the differences was calculated using the NeuroGuide software.
All statistical analyses were performed using the SPSS software version 26 (IBM Corp, USA). Since some values of the WMS-R subscale scores differed between the active and sham tDCS groups at baseline (Figure 2), repeated measures of analysis of covariance (ANCOVA) were used to analyze the data. The pre-test total score and subscales of the WMS-R for the intervention and the control group were considered covariate variables for the measurement of the effect of the intervention in the pre-test, post-test, and a 3-month follow-up. Before covariance analysis, its parametric assumptions such as normality of data distribution, homogeneity of variances, homogeneity of the covariance matrix, homogeneity of regression line slope, Bartlett’s test of sphericity, absence of outlier data and non-collinearity of dependent variables were confirmed. For the analysis of the qEEG data, an unpaired t-test was performed to compare the mean relative power of different frequency bands between the sham and active tDCS groups after the intervention. The qEEG analysis was done in the analysis tab of the NeuroGuide software, and P≤0.05 was considered statistically significant. The coherence in each frequency band range (cross-channel auto-frequency connectivity) was calculated in the NeuroGuide environment. The significant differences between the intervention and control groups were depicted in a brain map.

3. Results
Active tDCS, the memory quotient, and WMS-R Subscales in MCI patients immediately and three months after the application
Table 2 presents the Mean±SD of the memory quotient (MQ) and its 7 subscales according to group membership (receiving active or sham tDCS as a between-subject factor or independent variable) and assessment stages (pre-test, post-test, and follow-up as a within-subject factor).

The results of repeated measures of ANCOVA in Table 3 showed that the interaction effect of the time×group and the effect of time on the total score of the MQ was not significant (P>0.05). However, the results indicated that the group’s main effect on the MQ’s total score was significant (P≤0.001).

In addition, the results of multiple ANCOVA measures indicated that only the mental control subscale (P=0.009) was significantly affected by the time×group interaction. As a result, the active tDCS group’s degree of mental control was substantially higher than the sham group during the post-test and follow-up phases (Table 3 and Figure 2). Table 3 presents the group and time primary impacts. The findings indicate that only the logical memory and visual memory subscales exhibit a significant time effect (P≤0.05). Moreover, according to the data, all components showed a significant group effect (P≤0.001). Thus, the average of every memory quotient component in the experimental group rose noticeably after the tDCS intervention was implemented compared to the sham group (Table 3 and Figure 2).
Application of active tDCS in MCI patients, the power of the delta and theta band in the left frontal region
The analysis of EEG recording results after applying 10 sessions of active tDCS compared to the sham tDCS group showed that the absolute power of the delta frequency band decreased in the FP1, F3, Fz, and T5 areas. The decrease in the delta band power of F3 and T5 channels was significant (P<0.05). As depicted in Figure 3A, the absolute power of the theta band range also attenuated in the FP1, F3, and Fz regions (P<0.05). The alterations in the absolute power of other bands were not shown in Figure 3A, as they showed no significant difference.

Active tDCS, the delta and theta relative powers while boosting the alpha band frequency in the frontal and temporal brain regions
Figure 3B shows the alterations in the relative power of the low-frequency band range. After applying active tDCS to MCI patients, the relative delta band power of the Fz (P<0.05) and T5 channels (P<0.01) attenuated significantly. Similarly, the relative theta band power diminished by around 10% in the C4 and Cz regions (P<0.01). On the other hand, the alpha band power was augmented in the F3 and T5 brain regions in patients receiving active tDCS. The alterations in the relative power of the beta and gamma bands were not shown in Figure 3B, as they showed no significant difference.
Application of tDCS, the coherence between frontal and temporal regions, mainly in the delta band frequencies
All coherence and functional connectivity results are based on cross-channel auto-frequency analysis. As shown in Figure 4A, the most significant changes (P<0.01) in coherence were detected in the delta band range; the coherence between FP1-F7, F4-F8, F4-T8, F8-T4, and F8-T6 regions increased drastically in the delta band frequencies. On the other hand, in the beta band range, the C3-C4 coherence showed significant attenuation, depicted in Figure 4D. Some other brain regions showed coherence changes with lower significance levels (P<0.05), shown with thinner lines between different channels in Figure 4A-D.

Active tDCS on the F3 region of MCI Patients, frontal and temporal amplitude asymmetry in different band frequencies
The FFT analysis of different frequency bands in distinct brain regions revealed an inter-hemispheric asymmetry after active tDCS intervention. Table 4 indicates a significantly lower amplitude in the left frontal (F3) versus the right frontal (F4) region of MCI patients receiving active tDCS in all frequency ranges (P<0.01 for theta, P<0.05 for other bands). Lower left-side delta band activity is also observed in the T5-T6 region. Also, the P3-P4 and FP1-FP2 channels demonstrated lower beta-band activity in the left hemisphere (P<0.05).

4. Discussion
The results of the present study revealed that 10 sessions of bihemispheric tDCS on the DLPFC regions improved all aspects of Wechsler’s memory scale over three months. Personal and general information memory, digit span, and mental control showed the highest effect sizes. Wu et al. reported improved visuospatial working memory after 10 sessions of anodal tDCS on the right DLPFC (F4) of diabetic polyneuropathy patients (Wu et al., 2016). Gu et al. applied 20-minute anodal tDCS to the left temporal (T3) region for 5 consecutive days in MCI patients. They revealed that picture memory, visual regeneration, logical memory, and memory span increased significantly. Additionally, they noted a favorable relationship between the memory quotient score and the P300 amplitude difference (Gu et al., 2022). The higher effectiveness in the present study can be attributed to the bihemispheric setup implemented on both left and right DLPFCs.
Analysis of qEEG data revealed that after the tDCS treatment period, the absolute power of the delta and theta bands in the left frontal and temporal regions showed a significant decrease compared to the patients receiving sham tDCS. The relative delta power decreased in the frontal and temporal regions, while in the theta range, the relative power decreased in the central areas. Interestingly, in the frequency range of the alpha band, the relative power increased in the frontal and left temporal regions. There have been numerous reports of AD-related alterations in the pattern of the EEG power spectra, including an increase in theta and delta power and a beta reduction, followed by a decline in alpha band power, which results in a general deceleration of the EEG (Dierks et al., 1991; Jafari et al., 2020; Smailovic et al., 2018). Musaeus et al. showed that relative theta power is prominent in AD patients; it is also increased in MCI patients, though to a lesser extent, compared to healthy control individuals. They suggested that the increase in relative theta power could be the first EEG alteration in AD patients (Musaeus et al., 2018). Babiloni et al. demonstrated that MCI patients show a significantly higher delta power in the frontal, parietal, and temporal regions, while their alpha band power is reduced in the temporal and occipital lobes (Babiloni et al., 2018). Wang et al. (2017) reported an enhancement in the gamma band power in addition to the increased delta and decreased alpha power using a wavelet power spectrum analysis (Wang et al., 2017).
Additionally, a general increase in the power of the delta and theta frequency ranges, a notable decrease in the amplitude of alpha activity, and a slowing of background alpha activity are reported as manifestations of physiological aging (Ishii et al., 2017). These reports indicate an augmentation of slow frequency bands in patients with dementia, which can be interpreted as being in line with our results. Bilateral tDCS treatment diminished the spectral power of the delta and theta band ranges and boosted the alpha band power compared to non-treated MCI patients. These reversal patterns observed in the present study could be attributed to boosted excitability in the left DLPFC by anodal tDCS.
On the other hand, Fauzan and Amran (2015) found that MCI patients had higher beta-2 power in the right anterior region compared to normal elderly individuals. Both groups displayed a predominant distribution of theta and alpha in the frontal regions. In contrast, the highest levels of theta were found in the parietal and temporal areas, which is associated with the symptoms of cognitive decline in individuals suffering from MCI (Fauzan and Amran, 2015). According to Luckhaus et al., MCI patients have lower alpha band power, which is significantly linked with worse cognitive function on psychometric tests. The strongest positive predictive accuracy for MCI and AD and the likelihood of cognitive deterioration were found in the alpha power over posterior leads (Luckhaus et al., 2008).
Early synaptic disruption and loss, which result in white matter anomalies and functional connectivity deficiencies, are the main causes of progressive cognitive decline (Rémy et al., 2015). In elderly individuals with biomarker positivity who develop AD, disruption in functional connectivity in the alpha band is documented, in addition to anomalies in alpha source oscillatory activity (Maestú et al., 2015). Rossini et al. demonstrated that a high midline gamma coherence and a significant temporal delta source were related to a faster progression of dementia (Rossini et al., 2006). Compared to healthy control subjects, Toth et al. found that MCI patients had significantly lower levels of local functional connectivity in the left and right frontal regions in the delta band range. Likewise, the left frontotemporal connection and the right and left frontoparietal connectivity decreased in MCI patients (Tóth et al., 2014). The current study’s findings demonstrate a notable improvement in left and right frontal and right frontotemporal connectivity in the delta band range. While other studies reveal that MCI patients have increased alpha-1 connectivity in the right parietal region (Tóth et al., 2014), the tDCS strategy used in the current study increased beta-band coherence in the central brain region.
Martin et al. recently carried out a visual motion direction identification task on MCI patients in an EEG investigation. They only noted a frontal beta asymmetry in the MCI patients rather than any frontal alpha (Martin et al., 2022). According to our findings, frontal asymmetry develops following active tDCS therapy in all frequency bands, with greater oscillation power in the right hemisphere. Additionally, the beta band of the parietal and the delta band of the temporal areas showed the same pattern of asymmetries. Sarica et al. assessed the hippocampus and related structures’ MRI asymmetry index. They demonstrated that compared to healthy controls, AD and progressing MCI patients displayed considerable asymmetry and a greater volume on the right side (Sarica et al., 2018). Similarly, Yang et al. found a rightward asymmetry in global and local network efficiency between the two hemispheres of AD patients using diffusion tensor imaging tractography (Yang et al., 2017).
The primary concern in the present study is the small sample size, which can reduce the generalizability of the findings and increase the risk of statistical anomalies. Additionally, potential biases, including selection bias, can skew the results. Another significant limitation is the short duration of follow-up, which may not capture long-term changes or the full spectrum of brain activity alterations over time. These factors collectively highlight the need to interpret the qEEG findings carefully. Future studies should explore various tDCS protocols, including different intensities, durations, and electrode placements while comparing bihemispheric and unilateral DLPFC stimulation to optimize cognitive benefits in MCI patients. Long-term effects should be assessed through extended follow-up periods and multiple sessions to evaluate sustained cognitive improvements and EEG changes. Increasing sample sizes enhances the generalizability and validity of findings. Detailed EEG analyses and additional functional assessments will deepen understanding of the neurophysiological mechanisms and broader impacts of tDCS, supported by multi-modal neuroimaging techniques.
To translate tDCS findings into clinical practice, standardized protocols and integrating tDCS into cognitive rehabilitation programs are essential. Key challenges include careful patient selection and robust safety monitoring. Long-term efficacy and safety should be assessed through longitudinal studies. Addressing these factors can make tDCS a valuable tool for enhancing cognitive function in MCI patients.
5. Conclusion
This study demonstrates that bihemispheric tDCS of the DLPFC can enhance cognitive functions and modulate brain activity in MCI patients. Anodal stimulation of the left DLPFC and cathodal inhibition of the right one reduces the low-frequency band power and increases the frontotemporal coherence. The cognitive effects of this tDCS protocol lasted for three months and may have implications for preventing and treating dementia.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethic Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1399.596). Upon ethical approval, the study was registered by the Iranian Registry for Clinical Trials (IRCT) (Code: 20190522043673N2).
Funding
This study is derived from the general medical doctorate thesis of Soroush Ahmadi Machiani, approved by the Medical School of Guilan University of Medical Sciences, Rasht, Iran (Code: 242). The research was funded by the Neuroscience Research Center at Guilan University of Medical Sciences, Rasht, Iran (Grant No.: 2724).
Authors' contributions
Conceptualization: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Methodology: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Software: Sajjad Rezaei and Kambiz Rohampour; Data curation: Soroush Ahmadi, Arman Keymoradzadeh and Babak Bakhshayesh; Investigation: Soroush Ahmadi and Arman Keymoradzadeh; Formal analysis: Sajjad Rezaei and Kambiz Rohampour; Resources: Alia Saberi and Kambiz Rohampour; Writing the original draft: Soroush Ahmadi and Arman Keymoradzadeh: Review and editing: Sajjad Rezaei, Alia Saberi and Kambiz Rohampour; Validation: All authors; Supervision: Kambiz Rohampour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the faculty and staff of the Neuroscience Research Center of Guilan University of Medical Sciences, Rasht, Iran, for their support and contributions throughout the project.
References
Agresti, A., & Finlay, B. (2009). Statistical methods for the social sciences. London: Pearson Education, Inc. [Link]
Akhoondian, M., Rashtiani, S., Khakpour-Taleghani, B., Rostampour, M., Jafari, A., & Rohampour, K. (2023). Lateral habenula deep brain stimulation alleviates depression-like behaviors and reverses the oscillatory pattern in the nucleus accumbens in an animal model of depression. Brain Research Bulletin, 202, 110745. [DOI:10.1016/j.brainresbull.2023.110745] [PMID]
Amidfar, M., Ko, Y. H., & Kim, Y. K. (2019). Neuromodulation and cognitive control of emotion. Advances in Experimental Medicine and Biology, 1192, 545–564. [DOI:10.1007/978-981-32-9721-0_27] [PMID]
Babiloni, C., Del Percio, C., Lizio, R., Noce, G., Lopez, S., & Soricelli, A., et al. (2018). Abnormalities of resting state cortical EEG rhythms in subjects with mild cognitive impairment due to alzheimer’s and lewy body diseases. Journal of Alzheimer's disease: JAD, 62(1), 247–268. [DOI:10.3233/JAD-170703] [PMID]
Boggio, P. S., Ferrucci, R., Mameli, F., Martins, D., Martins, O., &Vergari, M., et al. (2012). Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimulation, 5(3), 223–230. [DOI:10.1016/j.brs.2011.06.006] [PMID]
Braem, S., King, J. A., Korb, F. M., Krebs, R. M., Notebaert, W., & Egner, T. (2017). The role of anterior cingulate cortex in the affective evaluation of conflict. Journal of Cognitive Neuroscience, 29(1), 137–149. [DOI:10.1162/jocn_a_01023] [PMID]
Canuet, L., Pusil, S., López, M. E., Bajo, R., Pineda-Pardo, J. Á., & Cuesta, P., et al. (2015). Network disruption and cerebrospinal fluid amyloid-Beta and phospho-tau levels in mild cognitive impairment. The Journal of Neuroscience: The Official Journal of The Society for Neuroscience, 35(28), 10325–10330. [DOI:10.1523/JNEUROSCI.0704-15.2015] [PMID]
Cao, W., Cao, X., Hou, C., Li, T., Cheng, Y., & Jiang, L., et al. (2016). Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci, 8, 70. [DOI:10.3389/fnagi.2016.00070]
Cieslik, E. C., Zilles, K., Caspers, S., Roski, C., Kellermann, T. S., & Jakobs, O., et al. (2013). Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex, 23(11), 2677-2689. [DOI:10.1093/cercor/bhs256] [PMID]
Clower, D. M., West, R. A., Lynch, J. C., & Strick, P. L. (2001). The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. The Journal of Neuroscience: The Official Journal of The Society for Neuroscience, 21(16), 6283–6291. [DOI:10.1523/JNEUROSCI.21-16-06283.2001] [PMID]
Das, N., Spence, J. S., Aslan, S., Vanneste, S., Mudar, R., & Rackley, A., et al. (2019). Cognitive training and transcranial direct current stimulation in mild cognitive impairment: A randomized pilot trial. Frontiers in Neuroscience, 13, 307. [DOI:10.3389/fnins.2019.00307] [PMID]
Dierks, T., Perisic, I., Frölich, L., Ihl, R., & Maurer, K. (1991). Topography of the quantitative electroencephalogram in dementia of the Alzheimer type: Relation to severity of dementia. Psychiatry Research, 40(3), 181–194. [DOI:10.1016/0925-4927(91)90009-f] [PMID]
Fauzan, N., & Amran, N. H. (2015). Brain dynamics of Mild Cognitive Impairment (MCI) from EEG Features. Procedia - Social and Behavioral Sciences, 165, 284-290. [DOI:10.1016/j.sbspro.2014.12.633]
Franzmeier, N., Caballero, M. Á. A., Taylor, A. N. W., Simon-Vermot, L., Buerger, K., & Ertl-Wagner, B., et al. (2017). Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging and Behavior, 11(2), 368–382. [DOI:10.1007/s11682-016-9599-1] [PMID]
Gu, J., Li, D., Li, Z., Guo, Y., Qian, F., & Wang, Y., et al. (2022). The effect and mechanism of transcranial direct current stimulation on episodic memory in patients with mild cognitive impairment. Frontiers in Neuroscience, 16, 811403. [DOI:10.3389/fnins.2022.811403] [PMID]
Hsu, W. Y., Zanto, T. P., Anguera, J. A., Lin, Y. Y., & Gazzaley, A. (2015). Delayed enhancement of multitasking performance: Effects of anodal transcranial direct current stimulation on the prefrontal cortex. Cortex; A Journal Devoted to The Study of The Nervous System and Behavior, 69, 175–185. [DOI:10.1016/j.cortex.2015.05.014] [PMID]
Huster, R. J., Enriquez-Geppert, S., Lavallee, C. F., Falkenstein, M., & Herrmann, C. S. (2013). Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International Journal of Psychophysiology, 87(3), 217-233. [DOI:10.1016/j.ijpsycho.2012.08.001] [PMID]
Ishii, R., Canuet, L., Aoki, Y., Hata, M., Iwase, M., & Ikeda, S., et al. (2017). Healthy and pathological brain aging: From the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology, 75(4), 151-161. [DOI:10.1159/000486870] [PMID]
Jafari, Z., Kolb, B. E., & Mohajerani, M. H. (2020). Neural oscillations and brain stimulation in Alzheimer’s disease. Progress in Neurobiology, 194, 101878. [DOI:10.1016/j.pneurobio.2020.101878] [PMID]
Jeong, J. (2004). EEG dynamics in patients with Alzheimer’s disease. Clinical Neurophysiology: Official Journal of The International Federation of Clinical Neurophysiology, 115(7), 1490–1505. [DOI:10.1016/j.clinph.2004.01.001] [PMID]
Jongsiriyanyong, S., & Limpawattana, P. (2018). Mild cognitive impairment in clinical practice: A review article. American Journal of Alzheimer's Disease and Other Dementias, 33(8), 500–507. [DOI:10.1177/1533317518791401] [PMID]
Kirova, A. M., Bays, R. B., & Lagalwar, S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Research International, 2015, 748212. [DOI:10.1155/2015/748212] [PMID]
Kochan, N. A., Breakspear, M., Slavin, M. J., Valenzuela, M., McCraw, S., & Brodaty, H., et al. (2010). Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge.Dementia and Geriatric Cognitive Disorders, 30(6), 553–568.[DOI:10.1159/000322112] [PMID]
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., & Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128(1), 56-92. [DOI:10.1016/j.clinph.2016.10.087] [PMID]
Li, Y., Yao, Z., Yu, Y., Zou, Y., Fu, Y., & Hu, B., et al. (2019). Brain network alterations in individuals with and without mild cognitive impairment: Parallel independent component analysis of AV1451 and AV45 positron emission tomography. BMC Psychiatry, 19(1), 165. [DOI:10.1186/s12888-019-2149-9] [PMID]
Luckhaus, C., Grass-Kapanke, B., Blaeser, I., Ihl, R., Supprian, T., & Winterer, G., et al. (2008). Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): Results of a 1-year follow-up study. International Journal of Geriatric Psychiatry, 23(11), 1148–1155. [DOI:10.1002/gps.2042] [PMID]
Maestú, F., Peña, J. M., Garcés, P., González, S., Bajo, R., & Bagic, A., et al. (2015). A multicenter study of the early detection of synaptic dysfunction in Mild Cognitive Impairment using Magnetoencephalography-derived functional connectivity. NeuroImage. Clinical, 9, 103–109. [DOI:10.1016/j.nicl.2015.07.011] [PMID]
Martin, D. M., Mohan, A., Alonzo, A., Gates, N., Gbadeyan, O., & Meinzer, M., et al. (2019). A pilot double-blind randomized controlled trial of cognitive training combined with transcranial direct current stimulation for amnestic mild cognitive impairment. Journal of Alzheimer's Disease: JAD, 71(2), 503–512. [DOI:10.3233/JAD-190306] [PMID]
Martin, T., Giordani, B., & Kavcic, V. (2022). EEG asymmetry and cognitive testing in MCI identification. International Journal of Psychophysiology, 177, 213-219. [DOI:10.1016/j.ijpsycho.2022.05.012] [PMID]
Morris, J. C. (2012). Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Archives of Neurology, 69(6), 700-8. [DOI:10.1001/archneurol.2011.3152]
Musaeus, C. S., Engedal, K., Høgh, P., Jelic, V., Mørup, M., & Naik, M., et al. (2018). EEG Theta power is an early marker of cognitive decline in dementia due to alzheimer’s disease. Journal of Alzheimer's Disease: JAD, 64(4), 1359–1371. [DOI:10.3233/JAD-180300] [PMID]
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(Pt 3), 633–639. [DOI:10.1111/j.1469-7793.2000.t01-1-00633.x] [PMID]
Obeso, I., Herrero, M. T., Ligneul, R., Rothwell, J. C., & Jahanshahi, M. (2021). A causal role for the right dorsolateral prefrontal cortex in avoidance of risky choices and making advantageous selections. Neuroscience, 458, 166-179. [DOI:10.1016/j.neuroscience.2020.12.035] [PMID]
Okello, A., Koivunen, J., Edison, P., Archer, H. A., Turkheimer, F. E., & Någren, K., et al. (2009). Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology, 73(10), 754-760. [DOI:10.1212/WNL.0b013e3181b23564] [PMID]
Park, J., Oh, Y., Chung, K., Kim, K. J., Kim, C. O., & Park, J. Y. (2019). Effect of home-based transcranial direct current stimulation (tDCS) on cognitive function in patients with mild cognitive impairment: A study protocol for a randomized, double-blind, cross-over study. Trials, 20(1), 278. [DOI:10.1186/s13063-019-3360-1] [PMID]
Polanía, R., Nitsche, M. A., & Ruff, C. C. (2018). Studying and modifying brain function with non-invasive brain stimulation. Nature Neuroscience, 21(2), 174–187. [DOI:10.1038/s41593-017-0054-4] [PMID]
Rémy, F., Vayssière, N., Saint-Aubert, L., Barbeau, E., & Pariente, J. (2015). White matter disruption at the prodromal stage of Alzheimer’s disease: relationships with hippocampal atrophy and episodic memory performance. NeuroImage. Clinical, 7, 482–492. [DOI:10.1016/j.nicl.2015.01.014] [PMID]
Reuter-Lorenz, P. A., Jonides, J., Smith, E. E., Hartley, A., Miller, A., & Marshuetz, C., et al. (2000). Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience, 12(1), 174–187. [DOI:10.1162/089892900561814] [PMID]
Rossini, P. M., Del Percio, C., Pasqualetti, P., Cassetta, E., Binetti, G., & Dal Forno, G., et al. (2006). Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience, 143(3), 793-803. [DOI:10.1016/j.neuroscience.2006.08.049] [PMID]
Rossini, P. M., Rossi, S., Babiloni, C., & Polich, J. (2007). Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Progress in Neurobiology, 83(6), 375–400. [DOI:10.1016/j.pneurobio.2007.07.010] [PMID]
Sarica, A., Vasta, R., Novellino, F., Vaccaro, M. G., Cerasa, A., & Quattrone, A., et al. (2018). MRI Asymmetry Index of Hippocampal Subfields Increases Through the Continuum From the mild cognitive impairment to the alzheimer’s disease. Frontiers in Neuroscience, 12, 576. [DOI:10.3389/fnins.2018.00576] [PMID]
Smailovic, U., Koenig, T., Kåreholt, I., Andersson, T., Kramberger, M. G., & Winblad, B., et al. (2018). Quantitative EEG power and synchronization correlate with Alzheimer’s disease CSF biomarkers. Neurobiology of Aging, 63, 88–95. [DOI:10.1016/j.neurobiolaging.2017.11.005] [PMID]
Stomrud, E., Hansson, O., Minthon, L., Blennow, K., Rosén, I., & Londos, E. (2010). Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiology of Aging, 31(2), 215–223.[DOI:10.1016/j.neurobiolaging.2008.03.025] [PMID]
Tóth, B., File, B., Boha, R., Kardos, Z., Hidasi, Z., & Gaál, Z. A., et al. (2014). EEG network connectivity changes in mild cognitive impairment - Preliminary results. International Journal of Psychophysiology, 92(1), 1-7. [DOI:10.1016/j.ijpsycho.2014.02.001] [PMID]
Wang, J., Fang, Y., Wang, X., Yang, H., Yu, X., & Wang, H. (2017). Enhanced gamma activity and cross-frequency interaction of resting-state electroencephalographic oscillations in patients with Alzheimer’s Disease. Frontiers in Aging Neuroscience, 9, 243. [DOI:10.3389/fnagi.2017.00243] [PMID]
Wechsler, D. (1987). WMS-R: Wechsler Memory Scale-Revised: Manual. San Antonio, TX: Psychological Corporation. [Link]
Wu, Y. J., Tseng, P., Huang, H. W., Hu, J. F., Juan, C. H., Hsu, K. S., & Lin, C. C. (2016). The facilitative effect of transcranial direct current stimulation on visuospatial working memory in patients with diabetic polyneuropathy: A pre-post sham-controlled study. Frontiers in Human Neuroscience, 10, 479. [DOI:10.3389/fnhum.2016.00479]
Yang, C., Zhong, S., Zhou, X., Wei, L., Wang, L., & Nie, S. (2017). The abnormality of topological asymmetry between hemispheric brain white matter networks in alzheimer’s disease and mild cognitive impairment. Frontiers in Aging Neuroscience, 9, 261. [DOI:10.3389/fnagi.2017.00261] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2024/02/16 | Accepted: 2024/07/29 | Published: 2024/11/1
Received: 2024/02/16 | Accepted: 2024/07/29 | Published: 2024/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





