Volume 16, Issue 1 (January & February 2025)
BCN 2025, 16(1): 115-130 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghantabpour T, Soleimani M, Ahadi R, Karimzadeh F, Moradi A. The Effect of Physical Activity on the Orexin and Brain-derived Neurotrophic Factor Expression on the Kindling Model of Epileptic Rats. BCN 2025; 16 (1) :115-130
URL: http://bcn.iums.ac.ir/article-1-2808-en.html
URL: http://bcn.iums.ac.ir/article-1-2808-en.html
1- Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Physiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Keywords: Epilepsy, Seizure, Exercise, Orexin, Brain-derived neurotrophic factor (BDNF), Pentylenetetrazol
Full-Text [PDF 2420 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Epilepsy, as a neurodegenerative disorder, has affected the lives of 50 million people worldwide. Three main goals of epilepsy treatment are controlling seizure attacks, maintaining life quality, and decreasing the side effects of anti-epileptic drugs (Perucca 2021, Foutz & Wong 2022). Therefore, it is essential to use non-pharmacological treatment approaches for epilepsy (Alqahtani et al., 2020).
Physical activity (PA) has been introduced as a low-cost treatment for improving neurological function available to adults and lacks the intolerable side effects often caused by drug therapy (Matsuda et al., 2009, Perrochon et al., 2019, Bareiss, Johnston et al., 2022, Zhang et al., 2022). Association between epilepsy and physical exercise as a non-pharmacological approach has been noticed, and many researchers have reported it as a non-pharmacological or complementary therapy for epilepsy (Popp et al., 2021; Zhang et al., 2022).
The orexin/hypocretin pathway has been involved in many neurological activities. Prepro-orexin mRNA has been located in the neurons of the lateral hypothalamus as a neuromodulatory system and led to the production of orexin-A (OXA) and -B peptides (Azeez et al., 2021, Abounoori et al., 2022). OXA was usually present in humans and consisted of 33 amino acids bound to two orexin receptors: OXR1 and OXR2. By coupling to the Gq/11-alpha subunit and activating phospholipase C, OXR1 and OXR2 play as members of family G-protein coupled receptors and stimulate the influx of cations, causing neuron depolarization and increasing its excitability (Scammell & Winrow 2011).
It was supposed that orexigenic signaling was involved only in feeding, appetite, and energy homeostasis (Bonnavion & de Lecea 2010). However, recent research indicates that orexin has an essential role in arousal and sleep, and orexin dysregulation causes some sleep disorders, such as narcolepsy and cataplexy, as well as some neurodegenerative disorders (Liblau et al., 2015, Pizza et al., 2022). Orexin is an essential factor in treating cognitive deficits in schizophrenia (Borgland & Labouèbe 2010). OXA and neuronal cell numbers decrease in Alzheimer disease (Um & Lim 2020) and Huntington patients (Petersén et al., 2005).
Evidence shows the signaling of orexin on the occurrence of seizures and epilepsy (Ng, 2017, Sheibani et al., 2023). It has been reported that orexin receptor inactivation in the hippocampus by orexin antagonists reduces pentylenetetrazol (PTZ) induced seizures in male rats (Goudarzi et al., 2015). The serum level of OXA was higher in epileptic individuals compared to healthy and individuals with pseudoseizures (Çikriklar et al., 2020). Intra-cortical injection of the orexins increases penicillin-induced epileptic activity in male rats (Kortunay et al., 2012). The relationship between epilepsy and orexin might be mediated through interaction with gamma-aminobutyric acid (GABA) and glutamate receptors (Razavi et al., 2020; Manavi et al., 2022, Kinboshi et al., 2023).
Brain-derived neurotrophic factor (BDNF) was a member of the neurotrophic family of factors initially recognized as proteins responsible for neuron survival, differentiation, and formation (Kowiański et al., 2018). The BDNF decrease in the brain of Alzheimer patients shows its essential function in the pathological mechanism of this disease (Yulug et al., 2018; Ng et al., 2019). The BDNF has been alleviated and accompanied by an enhancement in the deterioration of dopaminergic neurons in Parkinson disease (Wang et al., 2016, Lin et al., 2017). In contrast, the serum level of BDNF is higher in amyotrophic lateral sclerosis compared to the control group (Riolo et al., 2022).
Several research studies have accentuated the link between epilepsy and BDNF, which has an amelioration effect against hippocampal epilepsy and apoptosis induced by seizure (Yu et al., 2019). The serum level of BDNF is lower in epileptic adults than in healthy people (LaFrance et al., 2010).
Studies have shown some correlation between BDNF and orexin. OXA elevates the protein level of BDNF in the dopaminergic neurons of the substantia nigra in the Parkinson model of mice (Liu et al., 2018). PA increases the levels of BDNF and orexin with antidepressive-like influence and memory-facilitatory function (Ghahfarrokhi et al., 2020). Also, one study reports a relationship between OXA and BDNF in schizophrenic patients after long-term clozapine treatment (Ren et al., 2022).
This study aims to elucidate the effect of PA on orexin signaling and BDNF in PTZ-induced epileptic Wister rats.
2. Materials and Methods
Animals and seizure induction
Thirty male Wistar rats (weighing 250–300 g) were purchased one week before the beginning of the experiment and housed in the Central Animal Facility of Iran University of Medical Sciences, Tehran City, Iran, under a 12-hour light/dark cycle (at 21±2 °C) and free access to food and water.
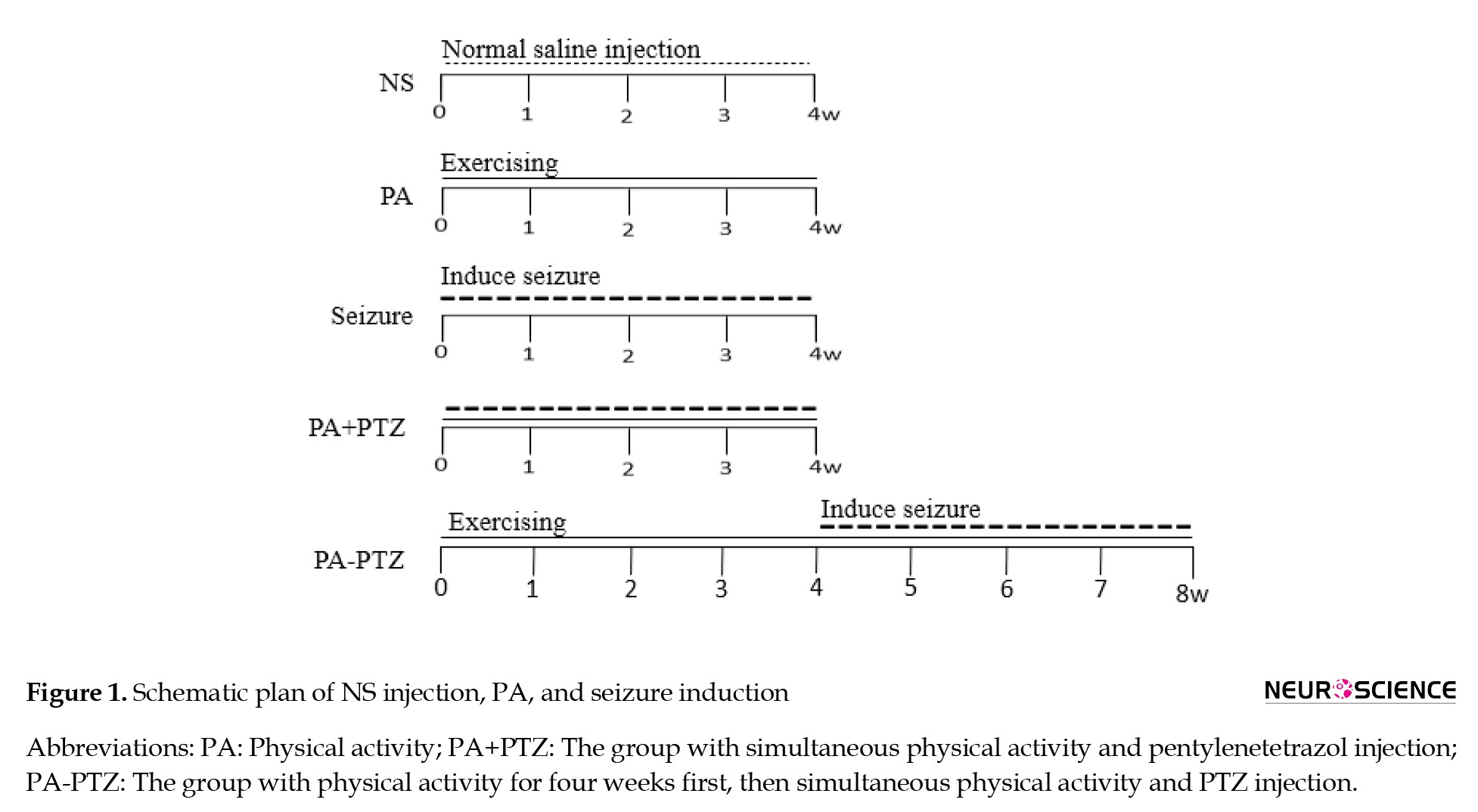
Then, the animals were randomly divided into five groups (Figure 1).
Epilepsy, as a neurodegenerative disorder, has affected the lives of 50 million people worldwide. Three main goals of epilepsy treatment are controlling seizure attacks, maintaining life quality, and decreasing the side effects of anti-epileptic drugs (Perucca 2021, Foutz & Wong 2022). Therefore, it is essential to use non-pharmacological treatment approaches for epilepsy (Alqahtani et al., 2020).
Physical activity (PA) has been introduced as a low-cost treatment for improving neurological function available to adults and lacks the intolerable side effects often caused by drug therapy (Matsuda et al., 2009, Perrochon et al., 2019, Bareiss, Johnston et al., 2022, Zhang et al., 2022). Association between epilepsy and physical exercise as a non-pharmacological approach has been noticed, and many researchers have reported it as a non-pharmacological or complementary therapy for epilepsy (Popp et al., 2021; Zhang et al., 2022).
The orexin/hypocretin pathway has been involved in many neurological activities. Prepro-orexin mRNA has been located in the neurons of the lateral hypothalamus as a neuromodulatory system and led to the production of orexin-A (OXA) and -B peptides (Azeez et al., 2021, Abounoori et al., 2022). OXA was usually present in humans and consisted of 33 amino acids bound to two orexin receptors: OXR1 and OXR2. By coupling to the Gq/11-alpha subunit and activating phospholipase C, OXR1 and OXR2 play as members of family G-protein coupled receptors and stimulate the influx of cations, causing neuron depolarization and increasing its excitability (Scammell & Winrow 2011).
It was supposed that orexigenic signaling was involved only in feeding, appetite, and energy homeostasis (Bonnavion & de Lecea 2010). However, recent research indicates that orexin has an essential role in arousal and sleep, and orexin dysregulation causes some sleep disorders, such as narcolepsy and cataplexy, as well as some neurodegenerative disorders (Liblau et al., 2015, Pizza et al., 2022). Orexin is an essential factor in treating cognitive deficits in schizophrenia (Borgland & Labouèbe 2010). OXA and neuronal cell numbers decrease in Alzheimer disease (Um & Lim 2020) and Huntington patients (Petersén et al., 2005).
Evidence shows the signaling of orexin on the occurrence of seizures and epilepsy (Ng, 2017, Sheibani et al., 2023). It has been reported that orexin receptor inactivation in the hippocampus by orexin antagonists reduces pentylenetetrazol (PTZ) induced seizures in male rats (Goudarzi et al., 2015). The serum level of OXA was higher in epileptic individuals compared to healthy and individuals with pseudoseizures (Çikriklar et al., 2020). Intra-cortical injection of the orexins increases penicillin-induced epileptic activity in male rats (Kortunay et al., 2012). The relationship between epilepsy and orexin might be mediated through interaction with gamma-aminobutyric acid (GABA) and glutamate receptors (Razavi et al., 2020; Manavi et al., 2022, Kinboshi et al., 2023).
Brain-derived neurotrophic factor (BDNF) was a member of the neurotrophic family of factors initially recognized as proteins responsible for neuron survival, differentiation, and formation (Kowiański et al., 2018). The BDNF decrease in the brain of Alzheimer patients shows its essential function in the pathological mechanism of this disease (Yulug et al., 2018; Ng et al., 2019). The BDNF has been alleviated and accompanied by an enhancement in the deterioration of dopaminergic neurons in Parkinson disease (Wang et al., 2016, Lin et al., 2017). In contrast, the serum level of BDNF is higher in amyotrophic lateral sclerosis compared to the control group (Riolo et al., 2022).
Several research studies have accentuated the link between epilepsy and BDNF, which has an amelioration effect against hippocampal epilepsy and apoptosis induced by seizure (Yu et al., 2019). The serum level of BDNF is lower in epileptic adults than in healthy people (LaFrance et al., 2010).
Studies have shown some correlation between BDNF and orexin. OXA elevates the protein level of BDNF in the dopaminergic neurons of the substantia nigra in the Parkinson model of mice (Liu et al., 2018). PA increases the levels of BDNF and orexin with antidepressive-like influence and memory-facilitatory function (Ghahfarrokhi et al., 2020). Also, one study reports a relationship between OXA and BDNF in schizophrenic patients after long-term clozapine treatment (Ren et al., 2022).
This study aims to elucidate the effect of PA on orexin signaling and BDNF in PTZ-induced epileptic Wister rats.
2. Materials and Methods
Animals and seizure induction
Thirty male Wistar rats (weighing 250–300 g) were purchased one week before the beginning of the experiment and housed in the Central Animal Facility of Iran University of Medical Sciences, Tehran City, Iran, under a 12-hour light/dark cycle (at 21±2 °C) and free access to food and water.
Then, the animals were randomly divided into five groups (Figure 1).
Normal saline (NS) group: NS (0.9% saline) was injected intraperitoneally (IP) three days a week for four weeks without any other intervention.
PA group: The animals were forced to run for 30 minutes daily five days/week for four weeks without injections.
Seizure group: Seizures were induced by PTZ injection under the same protocol as the NS group injection.
PA+PTZ group: The animals were forced to run using the same protocol as the PA group. After five hours of running, seizure induction was carried out with the same protocol as the seizure group.
PA-PTZ group: First, rats received PTZ using the same protocol as the seizure group without PA for one month. After four weeks, rats were forced to run and received PTZ with the same protocol as the PA+PTZ group for four weeks.
Seizure induction was performed by IP injection of 35 mg/kg of the PTZ (Sigma Aldrich, Germany) five hours after running in the PA+PTZ and PA-PTZ groups and at the same time and dose in the seizure group.
Exercise protocol
The rats of the PA, PA+PTZ, and PA-PTZ groups were adapted to the motorized treadmill (pre-trained on the treadmill for three days (15 min/d) at a speed of 5 m/min. The forced running included 30 minutes on the treadmill five days a week. The rate of the treadmill was started at 5 m/min and gradually increased every 5 min up to 25 m/min at 0 degrees of slope (Barzroodi Pour et al., 2021). Three minutes were considered to warm up and cool down, respectively, in the beginning and at the end.
Assessment of seizure behavior score (SBS)
The SBS was assessed by monitoring the rats from the moment of seizure induction to 45 min later. Convulsive behaviors were scored as follows: 0=normal behavior; 1=immobility; 2=rigid posture; 3=repetitive scratching, circling, or head bobbing; 4=forelimb clonus, rearing, and falling; 5=repeated occurrence of level four behavior, and 6=severe tonic-clonic behavior (Barzroodi Pour et al., 2021).
Tissue preparation
After the last intervention, the rats were anesthetized with an IP injection of ketamine-xylazine (100–2 mg/kg of K-X, Sigma–Aldrich). The corpses perfuse through cardiac circulation using 250 mL of saline and 400 mL of 4% paraformaldehyde solution (PFA, pH 7.4). Animals were decapitated, and their brains were removed. Brain samples were embedded in paraffin blocks, and a microtome took serial sections with 8 μm thickness.
Immunohistofluorescence
Immunohistofluorescence was used to detect the OXA and BDNF expression in the CA1, CA3, and cortex. Three slides of each block with a 30-μm distance between the sections were selected, and after deparaffined, they were rehydrated by a series of xylene and graded alcohol. They were washed with Tris buffer (pH 7.4) three times. The sections were boiled in the citrate buffer (pH 6.0) for 10 min and left to cool. After removing non-specific binding by washing with 1% bovine serum albumin for 10 min, three serial sections were incubated overnight at 4 °C with rabbit polyclonal anti-rat antibody of OXA (Biorbyt Co.), and three sections incubated with rabbit polyclonal anti-rat antibody of BDNF (Biorbyt Co.) at the same condition. Antibodies were diluted 1:250 in Tris buffer at pH 7.4. The sections were washed three times in Tris buffer and incubated with FITC-conjugated goat anti-rabbit IgG antibody (Biorbyt Co.) for two hours at room temperature (Salami et al., 2019). To the specificity of immune staining, negative control was carried out using the same protocol except for primary antibody incubation. Images for assessment were taken with a digital camera (Optika, objective lens ×40) connected to the microscope (Optika Ts100 fluorescence). The FITC-positive neural cells were counted by INFINITY ANALYZE 7 software and calculated in the unit of area (1 mm2).
Statistical analysis
Data were represented as Mean±SEM. The data were analyzed by GraphPad prim software, version 9, multiple t-tests, and one-way analysis of variance (ANOVA) followed by Tukey multiple comparison tests. Normality and lognormality tests were used to prove the normality of the data. A correlation test was used to assay correlations between data from different groups. The group size was six, and the statistical significance was P<0.05.
3. Results
SBS
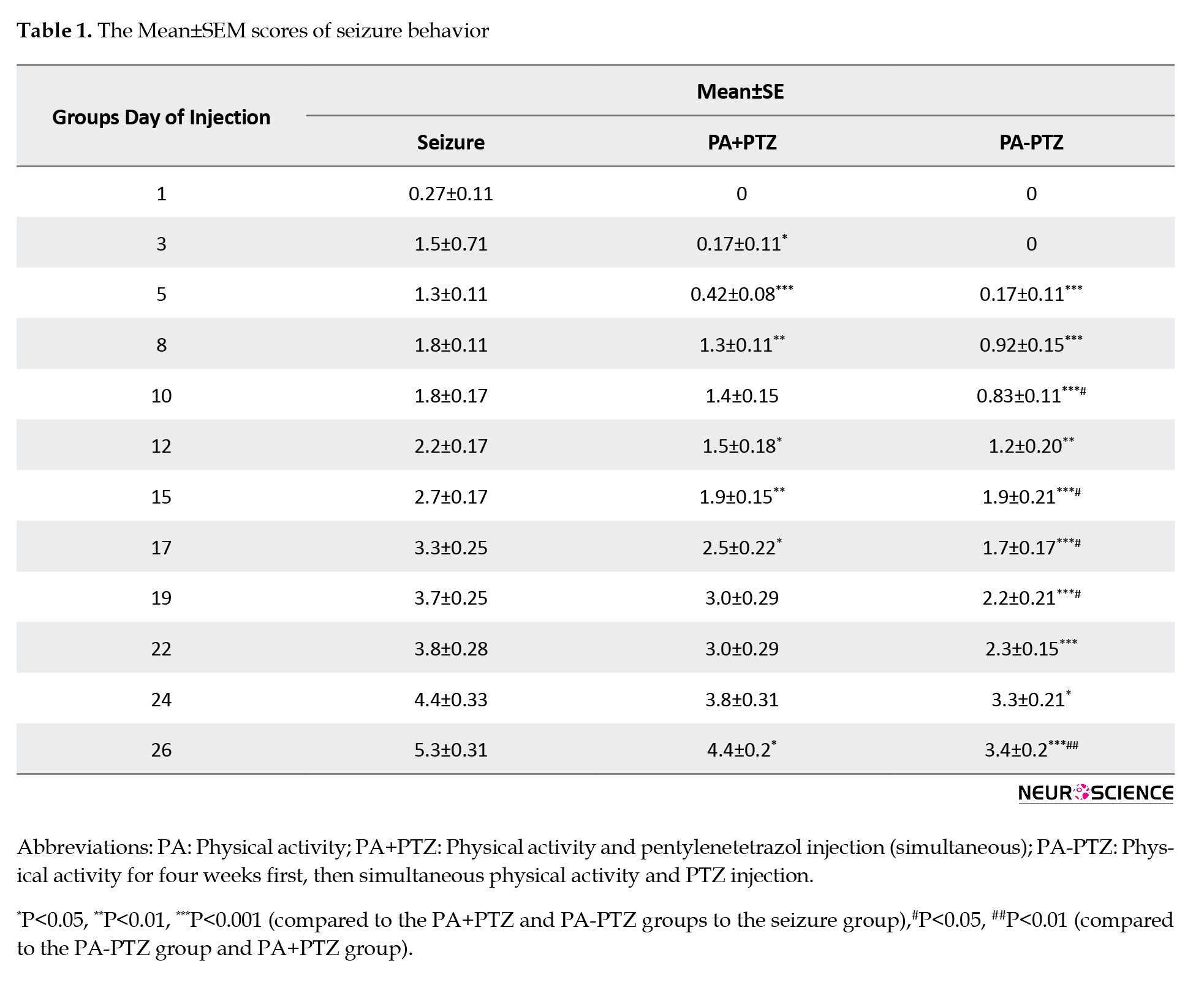
The Mean±SEM scores of the seizure, PA+PTZ, and PA-PTZ groups are mentioned in Table 1.
As shown in Figure 2, SBS was reduced in the PA+PTZ and PA-PTZ groups compared to the seizure group in some days of seizure induction.
PA group: The animals were forced to run for 30 minutes daily five days/week for four weeks without injections.
Seizure group: Seizures were induced by PTZ injection under the same protocol as the NS group injection.
PA+PTZ group: The animals were forced to run using the same protocol as the PA group. After five hours of running, seizure induction was carried out with the same protocol as the seizure group.
PA-PTZ group: First, rats received PTZ using the same protocol as the seizure group without PA for one month. After four weeks, rats were forced to run and received PTZ with the same protocol as the PA+PTZ group for four weeks.
Seizure induction was performed by IP injection of 35 mg/kg of the PTZ (Sigma Aldrich, Germany) five hours after running in the PA+PTZ and PA-PTZ groups and at the same time and dose in the seizure group.
Exercise protocol
The rats of the PA, PA+PTZ, and PA-PTZ groups were adapted to the motorized treadmill (pre-trained on the treadmill for three days (15 min/d) at a speed of 5 m/min. The forced running included 30 minutes on the treadmill five days a week. The rate of the treadmill was started at 5 m/min and gradually increased every 5 min up to 25 m/min at 0 degrees of slope (Barzroodi Pour et al., 2021). Three minutes were considered to warm up and cool down, respectively, in the beginning and at the end.
Assessment of seizure behavior score (SBS)
The SBS was assessed by monitoring the rats from the moment of seizure induction to 45 min later. Convulsive behaviors were scored as follows: 0=normal behavior; 1=immobility; 2=rigid posture; 3=repetitive scratching, circling, or head bobbing; 4=forelimb clonus, rearing, and falling; 5=repeated occurrence of level four behavior, and 6=severe tonic-clonic behavior (Barzroodi Pour et al., 2021).
Tissue preparation
After the last intervention, the rats were anesthetized with an IP injection of ketamine-xylazine (100–2 mg/kg of K-X, Sigma–Aldrich). The corpses perfuse through cardiac circulation using 250 mL of saline and 400 mL of 4% paraformaldehyde solution (PFA, pH 7.4). Animals were decapitated, and their brains were removed. Brain samples were embedded in paraffin blocks, and a microtome took serial sections with 8 μm thickness.
Immunohistofluorescence
Immunohistofluorescence was used to detect the OXA and BDNF expression in the CA1, CA3, and cortex. Three slides of each block with a 30-μm distance between the sections were selected, and after deparaffined, they were rehydrated by a series of xylene and graded alcohol. They were washed with Tris buffer (pH 7.4) three times. The sections were boiled in the citrate buffer (pH 6.0) for 10 min and left to cool. After removing non-specific binding by washing with 1% bovine serum albumin for 10 min, three serial sections were incubated overnight at 4 °C with rabbit polyclonal anti-rat antibody of OXA (Biorbyt Co.), and three sections incubated with rabbit polyclonal anti-rat antibody of BDNF (Biorbyt Co.) at the same condition. Antibodies were diluted 1:250 in Tris buffer at pH 7.4. The sections were washed three times in Tris buffer and incubated with FITC-conjugated goat anti-rabbit IgG antibody (Biorbyt Co.) for two hours at room temperature (Salami et al., 2019). To the specificity of immune staining, negative control was carried out using the same protocol except for primary antibody incubation. Images for assessment were taken with a digital camera (Optika, objective lens ×40) connected to the microscope (Optika Ts100 fluorescence). The FITC-positive neural cells were counted by INFINITY ANALYZE 7 software and calculated in the unit of area (1 mm2).
Statistical analysis
Data were represented as Mean±SEM. The data were analyzed by GraphPad prim software, version 9, multiple t-tests, and one-way analysis of variance (ANOVA) followed by Tukey multiple comparison tests. Normality and lognormality tests were used to prove the normality of the data. A correlation test was used to assay correlations between data from different groups. The group size was six, and the statistical significance was P<0.05.
3. Results
SBS
The Mean±SEM scores of the seizure, PA+PTZ, and PA-PTZ groups are mentioned in Table 1.

As shown in Figure 2, SBS was reduced in the PA+PTZ and PA-PTZ groups compared to the seizure group in some days of seizure induction.
SBS decreased in the PA+PTZ group compared to the seizure group in the 3rd, 5th, 8th, 12th, 15th, 17th, and 26th days.
Also, SBS decreased in the PA-PTZ group compared to the seizure group on all PTZ injection days.
In comparing the PA-PTZ with the PA+PTZ groups, the reduction was indicated in the 10th, 15th, 17th, 19th, and 26th days.
Expression of the OXA in the CA1 area
The mean number of immunofluorescence spots was counted in the CA1 area (Figure 3B).
Also, SBS decreased in the PA-PTZ group compared to the seizure group on all PTZ injection days.
In comparing the PA-PTZ with the PA+PTZ groups, the reduction was indicated in the 10th, 15th, 17th, 19th, and 26th days.
Expression of the OXA in the CA1 area
The mean number of immunofluorescence spots was counted in the CA1 area (Figure 3B).
The expression of the OXA increased in the CA1 areas of the seizure, PA, PA+PTZ, and PA-PTZ groups compared to the NS group (0.11±0.004, 0.084±0.002, 0.081±0.004, and 0.097±0.003 vs 0.058±0.003, respectively; P<0.001).
Also, the OXA expression decreased in the CA1 areas of the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (P<0.001, P<0.001, and P<0.05, respectively).
Additionally, OXA was highly expressed in the CA1 part of the PA-PTZ group compared to the PA (P<0.05) and PA+PTZ (P<0.05). There was no significant difference between the PA+PTZ compared to the PA group.
Expression of the OXA in the CA3 area
Data from the OXA-reacted cells in the CA3 area was analyzed (Figure 3C). The OXA expression increased in the CA3 areas of the seizure, PA, PA+PTZ, and PA-PTZ groups compared to the NS group (0.12±0.002, 0.085±0.002, 0.095±0.002, and 0.11±0.003 vs 0.056±0.003, respectively; P<0.001).
Also, OXA was expressed lower in the CA3 areas of the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (P<0.001, P<0.001, and P<0.01, respectively).
OXA was highly expressed in the CA3 of PA-PTZ compared to the PA (P<0.01) and PA+PTZ (P<0.05). There was no significant difference between the PA+PTZ and PA.
Expression of the OXA in the cortex area
Analyzed data from the OXA-reacted cells in the cortex area (Figure 3D) showed significant differences in different groups.
The cortical expression of the OXA increased in the seizure, PA, PA+PTZ, and PA-PTZ groups compared to the NS group (0.66±0.02, 0.43±0.01, and 0.53±0.02, 0.63±0.02 vs 0.29±0.02, respectively; P<0.001).
The OXA expression decreased in the cortex of the PA and PA+PTZ groups compared to the seizure group (P<0.001 and P<0.01, respectively), but there was no significant difference between the PA-PTZ and seizure groups.
The PA+PTZ and PA-PTZ groups showed higher OXA expression than the PA group (P<0.01 and P<0.001, respectively).
In addition, the PA increased the OXA expression in the PA-PTZ group compared to the PA+PTZ (P<0.05).
Expression of BDNF in the CA1 area
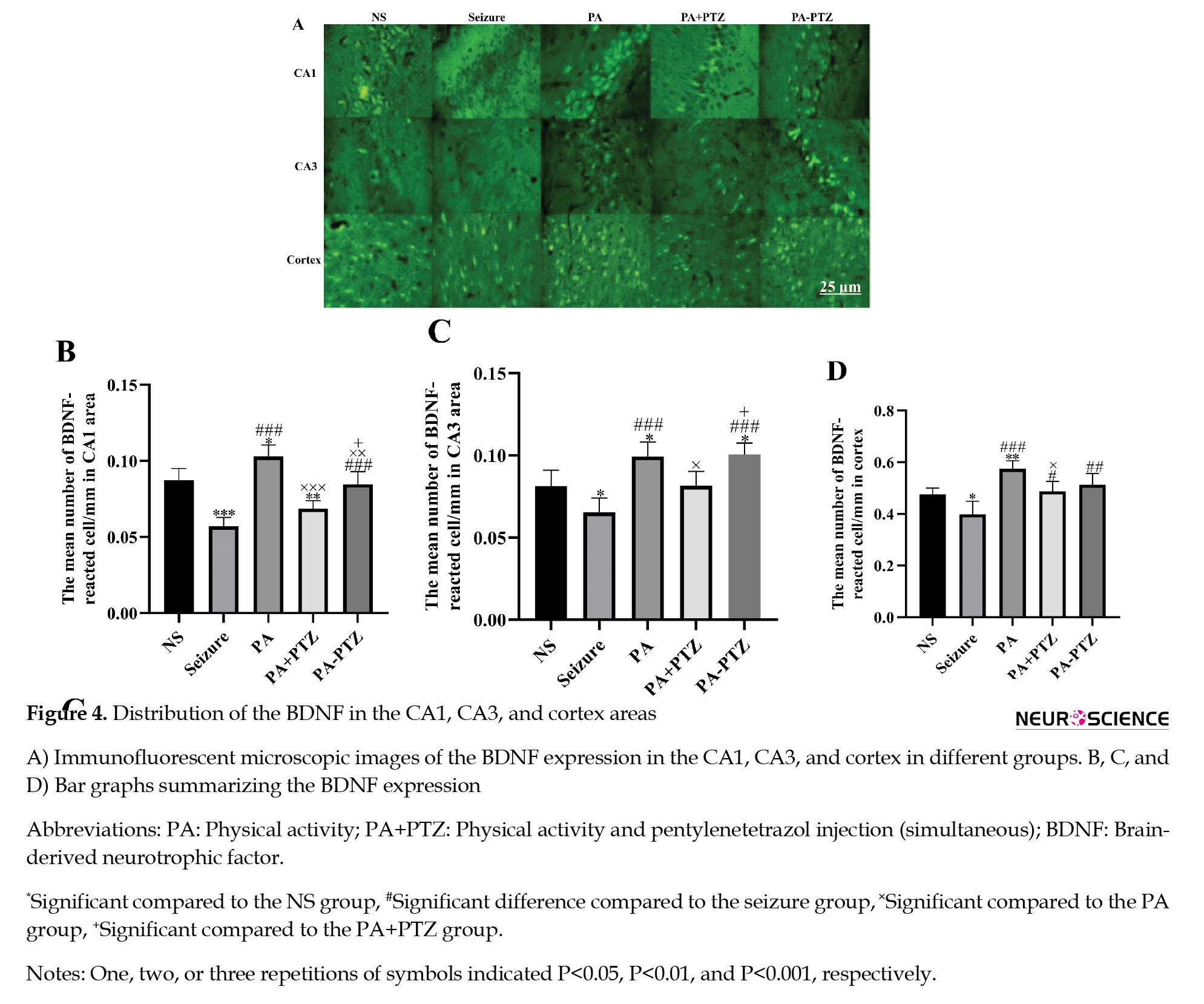
The BDNF expression in the CA1 area was analyzed, and the results are shown in Figure 4B.
Also, the OXA expression decreased in the CA1 areas of the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (P<0.001, P<0.001, and P<0.05, respectively).
Additionally, OXA was highly expressed in the CA1 part of the PA-PTZ group compared to the PA (P<0.05) and PA+PTZ (P<0.05). There was no significant difference between the PA+PTZ compared to the PA group.
Expression of the OXA in the CA3 area
Data from the OXA-reacted cells in the CA3 area was analyzed (Figure 3C). The OXA expression increased in the CA3 areas of the seizure, PA, PA+PTZ, and PA-PTZ groups compared to the NS group (0.12±0.002, 0.085±0.002, 0.095±0.002, and 0.11±0.003 vs 0.056±0.003, respectively; P<0.001).
Also, OXA was expressed lower in the CA3 areas of the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (P<0.001, P<0.001, and P<0.01, respectively).
OXA was highly expressed in the CA3 of PA-PTZ compared to the PA (P<0.01) and PA+PTZ (P<0.05). There was no significant difference between the PA+PTZ and PA.
Expression of the OXA in the cortex area
Analyzed data from the OXA-reacted cells in the cortex area (Figure 3D) showed significant differences in different groups.
The cortical expression of the OXA increased in the seizure, PA, PA+PTZ, and PA-PTZ groups compared to the NS group (0.66±0.02, 0.43±0.01, and 0.53±0.02, 0.63±0.02 vs 0.29±0.02, respectively; P<0.001).
The OXA expression decreased in the cortex of the PA and PA+PTZ groups compared to the seizure group (P<0.001 and P<0.01, respectively), but there was no significant difference between the PA-PTZ and seizure groups.
The PA+PTZ and PA-PTZ groups showed higher OXA expression than the PA group (P<0.01 and P<0.001, respectively).
In addition, the PA increased the OXA expression in the PA-PTZ group compared to the PA+PTZ (P<0.05).
Expression of BDNF in the CA1 area
The BDNF expression in the CA1 area was analyzed, and the results are shown in Figure 4B.
The expression of the BDNF decreased in the CA1 areas of the seizure and PA+PTZ groups (0.057±0.002 and 0.069±0.002, P<0.001 and P<0.01, respectively) and increased in the PA group (0.10±0.003, P<0.05) compared to the NS group (0.087±0.003).
There was no significant difference in the BDNF expression between the PA-PTZ and NS groups. The BDNF expression increased in the CA1 areas of the PA and PA-PTZ groups compared to the seizure group (0.10±0.003 and 0.085±0.003 vs 0.057±0.0029, P<0.001), but the PA+PTZ group had no significant difference compared with the seizure group (0.069±0.002 vs 0.057±0.002, P>0.05).
In the PA+PTZ and PA-PTZ groups, the expression of the BDNF decreased compared to the PA group (0.069±0.002 and 0.085±0.003 vs 0.10±0.003, P<0.001 and P<0.01, respectively).
The BDNF expression significantly increased in the CA1 area of the PA-PTZ compared to the PA+PTZ (P<0.05).
Expression of the BDNF in the CA3 area
The bar graph indicating the results of the BDNF expression in the CA3 area has been illustrated in Figure 4C.
The seizure reduced the BDNF expression in the seizure group compared to the NS group (0.065±0.004 vs 0.081±0.003, P<0.05). The BDNF expression in the PA and PA-PTZ groups increased compared to the NS group (0.099±0.003 and 0.098±0.003 vs 0.081±0.003, respectively; P<0.05), but there was not a significant difference in the PA+PTZ group compared to the NS group (0.089±0.003 vs 0.081±0.003, P>0.05).
The BDNF highly expressed in the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (0.099±0.003, 0.089±0.003, and 0.098±0.003 vs 0.065±0.004, P<0.001, P<0.01, and P<0.001, respectively).
The BDNF expression decreased in the PA+PTZ group compared to the PA group (0.089±0.003 vs 0.099±0.003, P<0.05), but there was not a significant difference between the PA-PTZ and PA (0.098±0.003 vs 0.099±0.003, P>0.05).
The BDNF expression increased in the PA-PTZ compared to the PA+PTZ group (0.098±0.003 vs 0.089±0.003, P<0.05).
Expression of the BDNF in the cortex area
The comparison of different groups is shown in Figure 4D.
The BDNF expression decreased in the seizure group compared to the NS group (0.40±0.02 vs 0.48±0.01, P<0.05) but increased in the PA group compared to the NS group (0.57±0.013 vs 0.48±0.01, P<0.01). There was no significant difference in the PA+PTZ and PA-PTZ groups compared to the NS (0.49±0.01 and 0.51±0.02 vs 0.48±0.01, P>0.05).
The BDNF expression increased in the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (0.57±0.013, 0.49±0.019, and 0.51±0.02 vs 0.4±0.02, P<0.001, P<0.05, and P<0.01, respectively).
The BDNF expression decreased in the PA+PTZ compared to the PA (0.49±0.019 vs 0.57±0.013, P<0.05), but there was no significant difference between the PA-PTZ and PA (0.51±0.02 vs 0.57±0.013, P>0.05).
There was no significant difference between the PA-PTZ and PA+PTZ (0.51±0.02 vs 0.49±0.019, P>0.05).
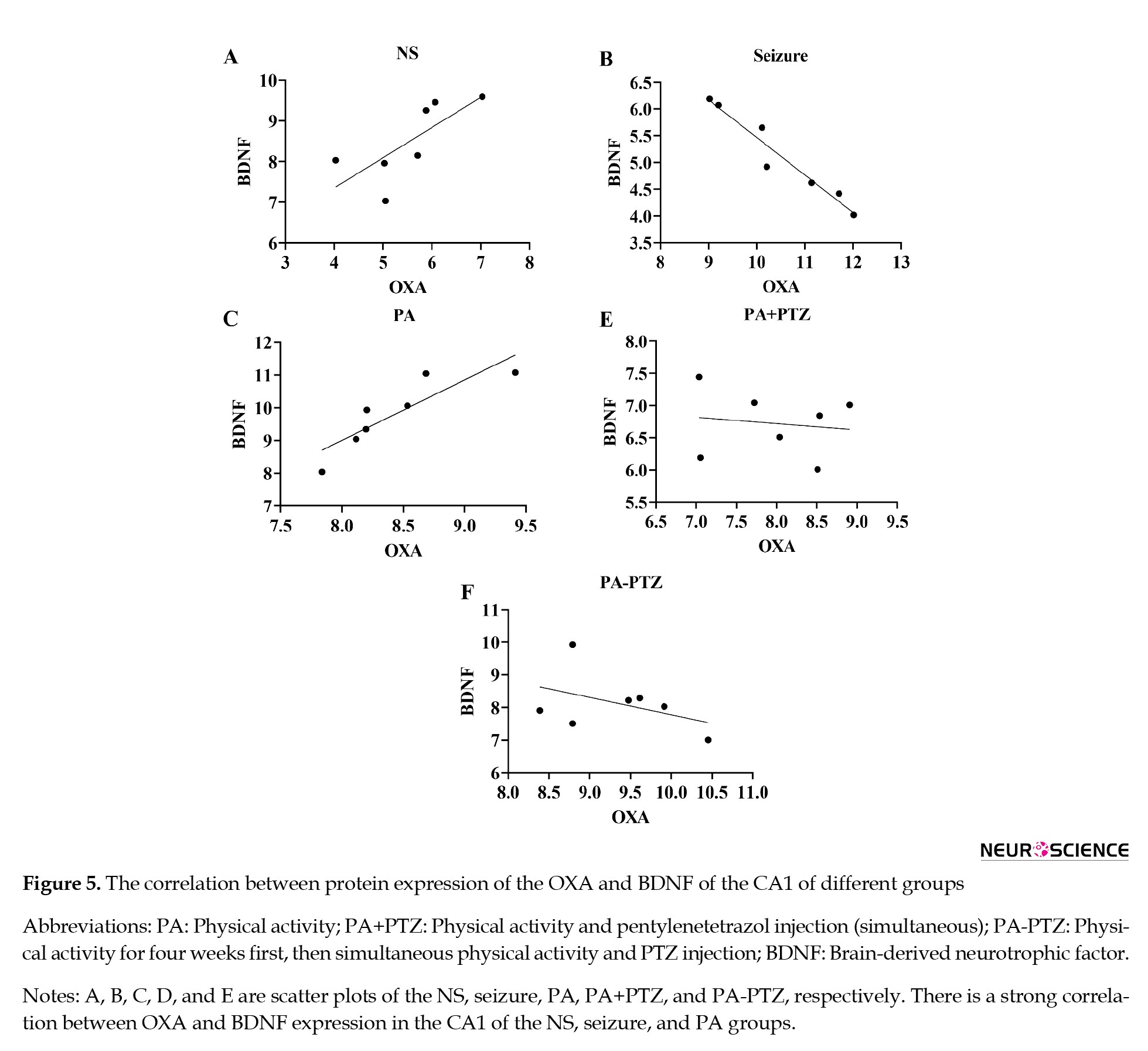
Correlation between the expression of the OXA and BDNF in the CA1, CA3, and cortex areas of the different groups
The relationship between the OXA and BDNF expression in the CA1 regions has been presented in Figure 5.
There was no significant difference in the BDNF expression between the PA-PTZ and NS groups. The BDNF expression increased in the CA1 areas of the PA and PA-PTZ groups compared to the seizure group (0.10±0.003 and 0.085±0.003 vs 0.057±0.0029, P<0.001), but the PA+PTZ group had no significant difference compared with the seizure group (0.069±0.002 vs 0.057±0.002, P>0.05).
In the PA+PTZ and PA-PTZ groups, the expression of the BDNF decreased compared to the PA group (0.069±0.002 and 0.085±0.003 vs 0.10±0.003, P<0.001 and P<0.01, respectively).
The BDNF expression significantly increased in the CA1 area of the PA-PTZ compared to the PA+PTZ (P<0.05).
Expression of the BDNF in the CA3 area
The bar graph indicating the results of the BDNF expression in the CA3 area has been illustrated in Figure 4C.
The seizure reduced the BDNF expression in the seizure group compared to the NS group (0.065±0.004 vs 0.081±0.003, P<0.05). The BDNF expression in the PA and PA-PTZ groups increased compared to the NS group (0.099±0.003 and 0.098±0.003 vs 0.081±0.003, respectively; P<0.05), but there was not a significant difference in the PA+PTZ group compared to the NS group (0.089±0.003 vs 0.081±0.003, P>0.05).
The BDNF highly expressed in the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (0.099±0.003, 0.089±0.003, and 0.098±0.003 vs 0.065±0.004, P<0.001, P<0.01, and P<0.001, respectively).
The BDNF expression decreased in the PA+PTZ group compared to the PA group (0.089±0.003 vs 0.099±0.003, P<0.05), but there was not a significant difference between the PA-PTZ and PA (0.098±0.003 vs 0.099±0.003, P>0.05).
The BDNF expression increased in the PA-PTZ compared to the PA+PTZ group (0.098±0.003 vs 0.089±0.003, P<0.05).
Expression of the BDNF in the cortex area
The comparison of different groups is shown in Figure 4D.
The BDNF expression decreased in the seizure group compared to the NS group (0.40±0.02 vs 0.48±0.01, P<0.05) but increased in the PA group compared to the NS group (0.57±0.013 vs 0.48±0.01, P<0.01). There was no significant difference in the PA+PTZ and PA-PTZ groups compared to the NS (0.49±0.01 and 0.51±0.02 vs 0.48±0.01, P>0.05).
The BDNF expression increased in the PA, PA+PTZ, and PA-PTZ groups compared to the seizure group (0.57±0.013, 0.49±0.019, and 0.51±0.02 vs 0.4±0.02, P<0.001, P<0.05, and P<0.01, respectively).
The BDNF expression decreased in the PA+PTZ compared to the PA (0.49±0.019 vs 0.57±0.013, P<0.05), but there was no significant difference between the PA-PTZ and PA (0.51±0.02 vs 0.57±0.013, P>0.05).
There was no significant difference between the PA-PTZ and PA+PTZ (0.51±0.02 vs 0.49±0.019, P>0.05).
Correlation between the expression of the OXA and BDNF in the CA1, CA3, and cortex areas of the different groups
The relationship between the OXA and BDNF expression in the CA1 regions has been presented in Figure 5.
The correlation between the OXA and BDNF expression in the NS group showed that high OXA expression was associated with high BDNF expression (r=0.74, P<0.05). High OXA expression was associated with down BDNF expression in the seizure (r=-0.87, P<0.05) and high BDNF expression in the PA groups (r=0.87, P<0.001). There was no correlation between OXA and BDNF expression in the PA+PTZ and PA-PTZ groups.
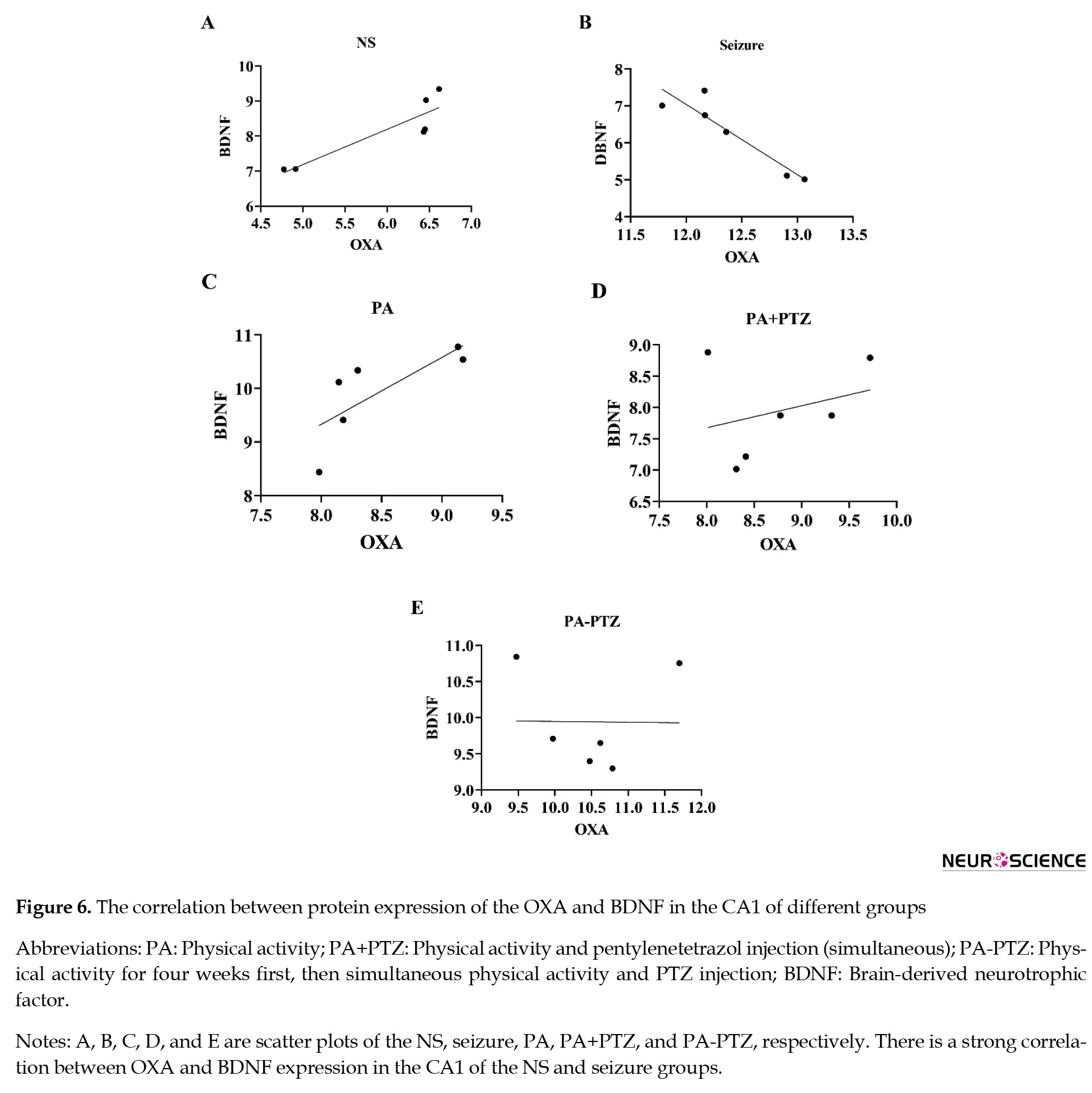
The relationship between OXA and BDNF expression in the CA3 area has been presented in Figure 6.
The relationship between OXA and BDNF expression in the CA3 area has been presented in Figure 6.
The correlation between OXA and BDNF expression in the NS group showed that high OXA expression was associated with high BDNF expression in CA3 of the NS group (r=0.89, P<0.02). High OXA expression was associated with down BDNF expression in the seizure group (r=-0.96, P<0.001). There was no significant correlation between OXA and BDNF expression in the PA, PA+PTZ, and PA-PTZ groups.
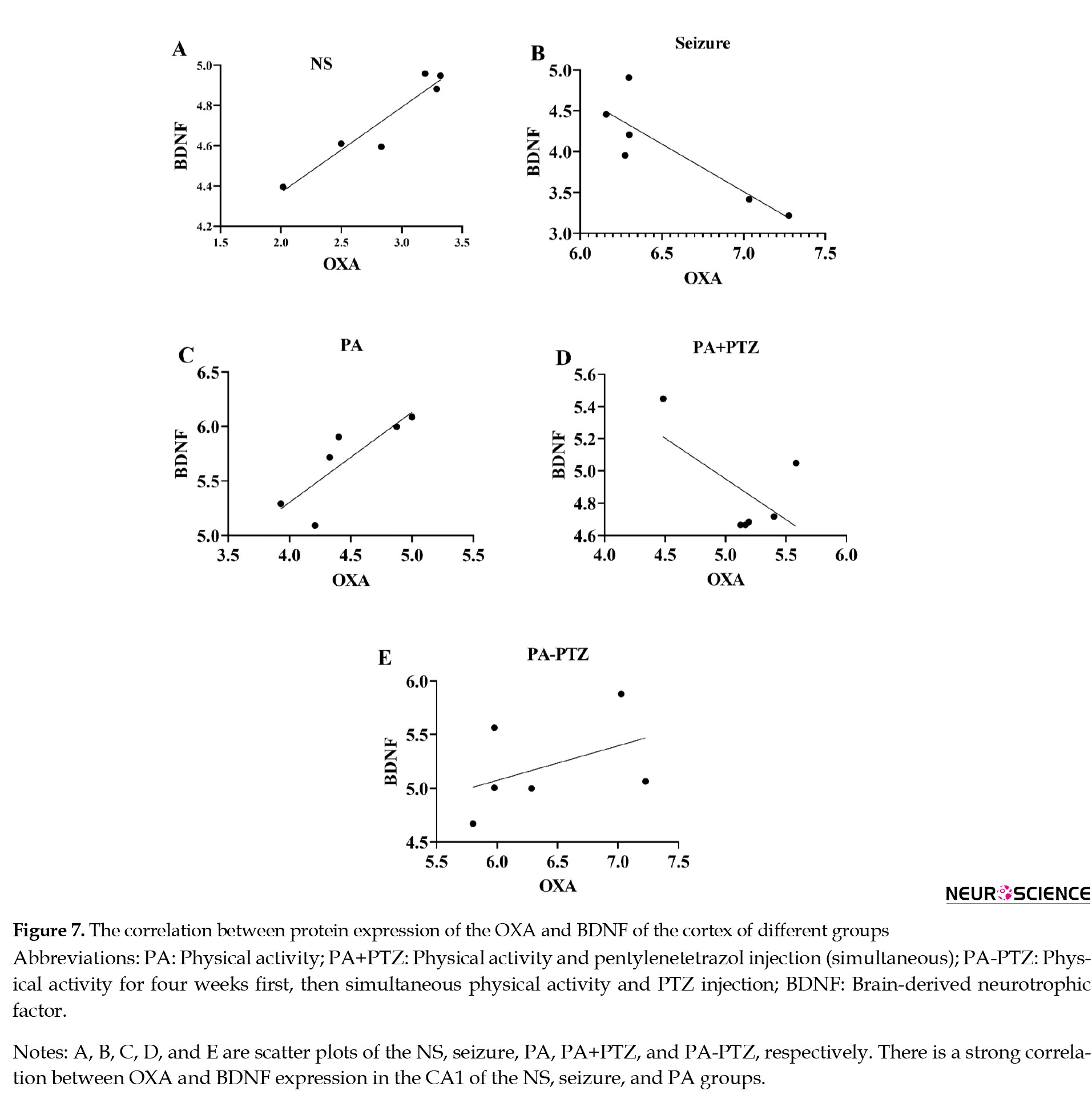
The relationship between the OXA and BDNF expression in the cortex has been presented in Figure 7.
The relationship between the OXA and BDNF expression in the cortex has been presented in Figure 7.
The correlation between the OXA and BDNF expression in the cortex of the NS group showed that high OXA expression was associated with high BDNF expression in the NS group (r=0.95, P<0.003). High OXA expression was associated with down BDNF expression in the seizure group (r=-0.86, P<0.03) and high BDNF expression in the PA group (r=0.83, P<0.04). There was no correlation between the OXA and BDNF expressions in the PA+PTZ and PA-PTZ.
4. Discussion
The present study investigated the effect of aerobic exercise by treadmill as an interventional treatment without medicine therapy on the seizure score, expression of OXA and BDNF, and correlation between OXA and BDNF expression. According to behavioral data, PA could benefit the prevention and treatment of seizures in the epileptic rate. Also, PA could have a different effect on OXA in healthy and epileptic male rats, increasing OXA in healthy rats and decreasing it in PTZ kindling-induced epileptic rat models. Also, as the same effect on OXA in healthy rats, exercise elevates the BDNF in health and compensates for its reduction in epileptic rats. Also, there was a relationship between the OXA and BDNF expression in the CA1, CA3, and cortex areas of some groups.
Our findings indicate that exercise decreases the seizure intensity in epileptic rats. The effects of PA on convulsive behaviors and the quality of life have been studied in individuals with epilepsy (Johnson et al., 2021). Treadmill exercise decreases the severity of PTZ-induced seizures in male rats (Barzroodi Pour et al., 2021). Aerobic training, resistance training, and stretching ameliorate the quality of life, stress levels, and physical fitness of epileptic patients and reduce their seizure frequency (Häfele et al., 2021). PA decreases the seizure frequency in individuals with epilepsy and increases their quality of life (Lee et al., 2022). Swimming reduces the severity of seizures in rats (Souza et al., 2010; Tutkun et al., 2010). Running on the treadmill (10 days) and swimming (6 weeks) diminishes oxidative injury induced by PTZ injection in rats (Souza et al., 2009, Arabaci-Tamer, Kaya et al., 2022).
We illustrated that a 4-week treadmill PA simultaneous with seizure induction attenuated the severity of seizure in the male rats. In addition, PA pre-induction of seizure notably decreased seizure severity.
Our results indicate that exercise as a monotherapy for epilepsy has a potential effect on the severity of seizures. Additionally, it might be a prevention intervention to reduce the severity of the seizure.
There are accumulating studies that investigated the role of orexin in epileptogenesis. Intracortical injections of OXA increases epileptic activity (Kortunay et al., 2012). Results from the Li et al. (2023) study indicate that hypothalamic inhibition of pre-seizure orexin activity reduces seizure severity in epileptic mice. It has been shown that an OX1 receptor antagonist reduced seizure behaviors in the male Albino Swiss mice (Socała et al., 2016). This result suggests that the expression of both orexin types in the hippocampus elevates the excitability of hippocampal neurons during epileptogenesis (Morales et al., 2006). Our results illustrate that seizure increases the OXA in the sedentary male rats’ CA1, CA3, and cortex. The orexin pathway function in the wake-sleep cycle and regulation of the GABA and glutamate system highlight the footprint in the pathogenesis of epilepsy. In this regard, the reduction of orexin activity is related to rapid eye movement (REM) sleep at the outset, and REM sleep is usually protective against epilepsy (Ng, 2017).
Previous studies show the effect of exercise on the orexin pathway. One theory that describes the relationship between the orexinergic system and training is hypothalamic thermoregulation. Enhancing serum level of OXA and rectal temperature after ergometer exercise (Messina et al., 2016) and diminishing the simultaneously PA and core body temperature by blockage orexinergic system (Martin et al., 2019) directed according to this theory.
Another theory about the relationship between exercise and the orexin system is the neurogenesis effect of exercise on the hippocampus. Studies show that the actuation of OXA promotes neuronal proliferation, differentiation, and firing of neurons in the hippocampus and decreases animal immobility (Ito et al., 2008; Zhao et al., 2014; Chen et al., 2017). It has been indicated that the hypothalamic administration of OXA enhanced ambulation and reduced sedentary in the light and dark cycles of Sprague–Dawley rats (Kotz et al., 2002). The injection of OXA in the hypothalamus and substantia nigra has enhanced the ambulation time, and it had a modulatory function on PA (Kotz et al., 2006).
Results illustrate that PA up-regulates the OXA expression in the CA1, CA3, and cortex, but it has a down-expression effect on the expression of OXA in epileptic rats. These findings suggest that PA changed its influence depending on conditions that increased the OXA expression in the healthy nonepileptic rat and decreased it in the epileptic rats. PA acts as a moderator in its effect on OXA expression. Additionally, four weeks of PA before seizure induction and simultaneous PA with seizure induction increased the OXA expression compared to only PA during seizure induction. This finding illustrates that PA increased the OXA expression in the first four weeks, then decreased the OXA expression when it was elevated by seizure. To explain how PA reduced the severity of seizures through OXA, we should review the effect of OXA and its antagonists on GABA and glutamate processes. It has been mentioned that blockage of OXR1 and OXR2 reduces the seizure by decreasing glutamate as excitatory and increasing GABA as inhibitory contents (Goudarzi et al., 2015). Also, a dual orexin antagonist reduces seizure by elevation of GABAergic inhibition (Konduru et al., 2022). Similar to orexin antagonists, it has been reported that PA reduces GABAergic loss in the hippocampus (Lim et al., 2015).
Our findings suggest that PA might improve the imbalance of the inhibitory and excitatory systems through the orexin signaling pathway in epileptic rats.
Previous studies show the involvement of BDNF in epileptogenesis (Egbenya et al., 2023). The serum level of BDNF is lower in individuals with epilepsy (LaFrance et al., 2010). The hippocampus of epileptic animals indicates less BDNF expression than in nonepileptics (Flores-Soto et al., 2021). Similarly, our results illustrate the reduction effect of seizure on BDNF in different brain areas.
Some studies show the up-regulation effect of PA on BDNF expression (Alomari et al., 2013; Fang et al., 2013; Wrann et al., 2013; Venezia et al., 2017). Immobilization reduces BDNF expression in the hippocampus (Nooshinfar et al., 2011), and treadmill exercise improves BDNF deficits (Fang et al., 2013). Forced running exercise restores the down-regulation of the hippocampal BDNF in male Sprague–Dawley rats (Ji et al., 2014). Similarly, our result shows the up-regulation effect of PA on BDNF expression. This suggestion arises from the up-regulation effect of exercise on the 5-HT and noradrenaline (Idorn & thor Straten, 2017), which triggers the cyclic adenosine 3′,5′-monophosphate (cAMP) and its active response element binding protein. Response element binding protein up-regulates the BDNF expression through the tropomyosin receptor kinase B (Rumajogee et al., 2002). Our results demonstrate that PA restores the decrease of the BDNF expression that occurred following seizures. Similarly, resistance exercise restored the BDNF levels reduction in the homogenized hippocampus in the pilocarpine hydrochloride-induced epileptic rat (de Almeida et al., 2017). The reduction in the influence of PA on the BDNF in epilepsy might be explained by the inhibition effect of the BDNF on the GABAergic system in the epileptic brain (Marty et al., 2000). Then, exercise might decrease the severity of seizure by increasing the BDNF, which elevates the density of the inhibitory synapses (Lu et al., 2010). Additionally, our results indicate that running on the treadmill as a pre-induced seizure had a potential effect on restoration of the BDNF reduced by seizure. These findings suggest that the possible reduction effect of PA as pre-induced seizure on the severity of seizure might be due to its possible effect on the BDNF expression.
Studies have shown some correlation between the expression of orexin and BDNF. The OXA and BDNF have anti-depressive effects and improve memory (Chieffi et al., 2017). Individuals with Parkinson express low levels of OXA and BDNF (Drouot et al., 2003; Scalzo et al., 2010). Exercise increases both OXA and BDNF (Messina et al., 2016). Also, a positive correlation between OXA and BDNF is reported in schizophrenia patients (Ren et al., 2022). The OXA increases BDNF expression in Parkinson, and this function of OXA might be mediated through OXR1 in Parkinson disease (Liu et al., 2018). It has been demonstrated that microinjection of OXA in the hippocampus elevates the expression of the BDNF that decreases by capsaicin administration in the capsaicin-induced orofacial pain Wistar rats model (Kooshki et al., 2018).
Our results show a relationship between the OXA and BDNF expression in some groups’ CA1, CA3, and cortex. In the CA1, CA3, and cortex, high-expressed OXA correlates with high-expressed BDNF in the NS group and a negative correlation in the seizure group. In the CA1 and cortex, high-expressed OXA correlates with high-expressed BDNF in the PA group. These findings show that the expression of the OXA and BDNF correlates separately in healthy rats and those that intervened with seizure or PA. The correlation between the OXA and BDNF might indicate the signaling interaction between these two factors. Our results show no correlation between OXA and BDNF expression in epileptic groups with PA. The interruption of cross-talking of OXA and BDNF pathways by exercise in the epileptic animals emphasizes the modulatory effect of PA in the pathological condition.
5. Conclusion
It might be concluded that PA had different roles depending on the pathological and non-pathological conditions. The anticonvulsant effect of PA seemed to depend on disrupting interactions between the orexin and BDNF pathways.
Ethical Considerations
Compliance with ethical guidelines
All experiments were carried out according to the protocol approved by the Animal Ethics of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1401.198).
Funding
This research was supported by Iran University of Medical Sciences, Tehran, Iran (Grant No.: 1401-1-4-20886.
Authors' contributions
Conceptualization: Taha Ghantabpour, Mansoureh Soleimani, and Fariba Karimzadeh; Methodology: All authors; Data collection: Taha Ghantabpour; Data analysis: Taha Ghantabpour and Fariba Karimzadeh; Investigation: Taha Ghantabpour, Reza Ahadi, and Fariba Karimzadeh; Writing the original draft: Taha Ghantabpour; Review and editing: Taha Ghantabpour, Fariba Karimzadeh, and Reza Ahadi; Supervision: Mansoureh Soleimani, Reza Ahadi, and Fariba Karimzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of the Cellular and Molecular Research Center of Iran University of Medical Sciences, Tehran, Iran.
References
Abounoori, M., Maddah, M. M., & Ardeshiri, M. R. (2021). Orexin neuropeptides modulate the hippocampal-dependent memory through basolateral amygdala interconnections. Cerebral circulation-cognition and behavior, 3, 100035.[DOI:10.1016/j.cccb.2021.100035] [PMID]
Alomari, M. A., Khabour, O. F., Alzoubi, K. H., & Alzubi, M. A. (2013). Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behavioural Brain Research, 247, 34–39. [DOI:10.1016/j.bbr.2013.03.007] [PMID]
Alqahtani, F., Imran, I., Pervaiz, H., Ashraf, W., Perveen, N., & Rasool, M. F., et al. (2020). Non-pharmacological interventions for intractable epilepsy. Saudi Pharmaceutical Journal, 28(8), 951–962. [DOI:10.1016/j.jsps.2020.06.016] [PMID]
Azeez, I. A., Igado, O. O., & Olopade, J. O. (2021). An overview of the orexinergic system in different animal species. Metabolic Brain Disease, 36(7), 1419–1444. [DOI:10.1007/s11011-021-00761-0] [PMID]
Bareiss, S. K., Johnston, T., Lu, Q., & Tran, T. D. (2022). The effect of exercise on early sensorimotor performance alterations in the 3xTg-AD model of Alzheimer's disease. Neuroscience Research, 178, 60–68. [DOI:10.1016/j.neures.2022.01.003] [PMID]
Barzroodi Pour, M., Bayat, M., Navazesh, A., Soleimani, M., & Karimzadeh, F. (2021). Exercise improved the anti-epileptic effect of carbamazepine through GABA enhancement in epileptic rats. Neurochemical Research, 46(8), 2112–2130. [DOI:10.1007/s11064-021-03349-3] [PMID]
Bonnavion, P., & de Lecea, L. (2010). Hypocretins in the control of sleep and wakefulness. Current Neurology and Neuroscience Reports, 10(3), 174–179. [DOI:10.1007/s11910-010-0101-y] [PMID]
Borgland, S. L., & Labouèbe, G. (2010). Orexin/hypocretin in psychiatric disorders: Present state of knowledge and future potential. Neuropsychopharmacology, 35(1), 353–354. [DOI:10.1038/npp.2009.119] [PMID]
Chen, X. Y., Chen, L., & Du, Y. F. (2017). Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. Journal of Neuroscience Research, 95(7), 1415–1426. [DOI:10.1002/jnr.23975] [PMID]
Chieffi, S., Carotenuto, M., Monda, V., Valenzano, A., Villano, I., & Precenzano, F., et al. (2017). Orexin system: The key for a healthy life. Frontiers in Physiology, 8, 357. [DOI:10.3389/fphys.2017.00357] [PMID]
Çikriklar, H. I., Kotan, D., Yücel, M., Ceylan, M., Çiftçi, G. G., & Bayraktutan, Ö. F., et al. (2020). The role of orexin-A levels in epileptic seizure. Neuroscience Letters, 734, 135097. [DOI:10.1016/j.neulet.2020.135097] [PMID]
de Almeida, A. A., Gomes da Silva, S., Lopim, G. M., Vannucci Campos, D., & Fernandes, J., et al. (2017). Resistance exercise reduces seizure occurrence, attenuates memory deficits and restores BDNF signaling in rats with chronic epilepsy. Neurochemical Research, 42(4), 1230–1239. [DOI:10.1007/s11064-016-2165-9] [PMID]
Drouot, X., Moutereau, S., Nguyen, J. P., Lefaucheur, J. P., Créange, A., & Remy, P., et al. (2003). Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology, 61(4), 540–543. [DOI:10.1212/01.WNL.0000078194.53210.48] [PMID]
Egbenya, D. L., Hussain, S., Lai, Y. C., Anderson, A. E., & Davanger, S. (2023). Synapse-specific changes in Arc and BDNF in rat hippocampus following chronic temporal lobe epilepsy. Neuroscience Research, 191, 1–12. [DOI:10.1016/j.neures.2022.12.006] [PMID]
Fang, Z. H., Lee, C. H., Seo, M. K., Cho, H., Lee, J. G., & Lee, B. J., et al. (2013). Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neuroscience Research, 76(4), 187–194. [DOI:10.1016/j.neures.2013.04.005] [PMID]
Flores-Soto, M., Romero-Guerrero, C., Vázquez-Hernández, N., Tejeda-Martínez, A., Martín-Amaya-Barajas, F. L., & Orozco-Suárez, S., et al. (2021). Pentylenetetrazol-induced seizures in adult rats are associated with plastic changes to the dendritic spines on hippocampal CA1 pyramidal neurons. Behavioural Brain Research, 406, 113198. [DOI:10.1016/j.bbr.2021.113198] [PMID]
Foutz, T. J., & Wong, M. (2022). Brain stimulation treatments in epilepsy: Basic mechanisms and clinical advances. Biomedical Journal, 45(1), 27–37. [DOI:10.1016/j.bj.2021.08.010] [PMID]
Ghahfarrokhi, M. M., Habibi, A., Alizadeh, A. A., Negaresh, R., Shahi, M. M., & Earnest, C. P. (2020). BDNF and orexin-A response to aerobic exercise are moderated by the meal consumption before exercise in overweight men: Effect of high-carbohydrate, high-protein and high-fat meals. Science & Sports, 35(4), 228-236. [DOI:10.1016/j.scispo.2020.01.011]
Goudarzi, E., Elahdadi Salmani, M., Lashkarbolouki, T., & Goudarzi, I. (2015). Hippocampal orexin receptors inactivation reduces PTZ induced seizures of male rats. Pharmacology, Biochemistry, and Behavior, 130, 77–83. [DOI:10.1016/j.pbb.2015.01.006] [PMID]
Häfele, C. A., Rombaldi, A. J., Feter, N., Häfele, V., Gervini, B. L., & Domingues, M. R., et al. (2021). Effects of an exercise program on health of people with epilepsy: A randomized clinical trial. Epilepsy & Behavior, 117, 107904. [DOI:10.1016/j.yebeh.2021.107904] [PMID]
Idorn, M., & Thor Straten, P. (2017). Exercise and cancer: From "healthy" to "therapeutic"? Cancer Immunology, Immunotherapy, 66(5), 667–671. [DOI:10.1007/s00262-017-1985-z] [PMID]
Ito, N., Yabe, T., Gamo, Y., Nagai, T., Oikawa, T., & Yamada, H., et al. (2008). I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience, 157(4), 720–732. [DOI:10.1016/j.neuroscience.2008.09.042] [PMID]
Ji, J. F., Ji, S. J., Sun, R., Li, K., Zhang, Y., & Zhang, L. Y., et al. (2014). Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochemical and Biophysical Research Communications, 443(2), 646–651. [DOI:10.1016/j.bbrc.2013.12.031] [PMID]
Johnson, E. C., Helen Cross, J., & Reilly, C. (2020). Physical activity in people with epilepsy: A systematic review. Epilepsia, 61(6), 1062–1081. [DOI:10.1111/epi.16517] [PMID]
Kinboshi, M., Shimizu, S., Tokudome, K., Mashimo, T., Serikawa, T., & Ito, H., et al. (2023). Imbalance of glutamatergic and GABAergic neurotransmission in audiogenic seizure-susceptible Leucine-rich glioma-inactivated 1 (Lgi1)-mutant rats. Heliyon, 9(7), e17984. [DOI:10.1016/j.heliyon.2023.e17984] [PMID]
Konduru, S. R., Isaacson, J. R., Lasky, D. J., Zhou, Z., Rao, R. K., & Vattem, S. S., et al. (2022). Dual orexin antagonist normalized sleep homeostatic drive, enhanced GABAergic inhibition, and suppressed seizures after traumatic brain injury. Sleep, 45(12), zsac238. [DOI:10.1093/sleep/zsac238] [PMID]
Kooshki, R., Abbasnejad, M., Esmaeili-Mahani, S., & Raoof, M. (2018). The effect of CA1 administration of orexin-A on hippocampal expression of COX-2 and BDNF in a rat model of orofacial pain. Arquivos de Neuro-Psiquiatria, 76(9), 603–608. [DOI:10.1590/0004-282x20180099] [PMID]
Kortunay, S., Erken, H. A., Erken, G., Genç, O., Sahiner, M., & Turgut, S., et al. (2012). Orexins increase penicillin-induced epileptic activity. Peptides, 34(2), 419–422. [DOI:10.1016/j.peptides.2012.02.013] [PMID]
Kotz, C. M., Teske, J. A., Levine, J. A., & Wang, C. (2002). Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regulatory Peptides, 104(1-3), 27–32. [DOI:10.1016/S0167-0115(01)00346-9] [PMID]
Kotz, C. M., Wang, C., Teske, J. A., Thorpe, A. J., Novak, C. M., & Kiwaki, K., et al. (2006). Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience, 142(1), 29–36. [DOI:10.1016/j.neuroscience.2006.05.028] [PMID]
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2018). BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38(3), 579–593. [DOI:10.1007/s10571-017-0510-4] [PMID]
LaFrance, W. C., Jr, Leaver, K., Stopa, E. G., Papandonatos, G. D., & Blum, A. S. (2010). Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology, 75(14), 1285–1291. [DOI:10.1212/WNL.0b013e3181f612bb] [PMID]
Lee, Y., Ahn, Y., & Cucullo, L. (2022). Impact of physical activity and medication adherence on the seizure frequency and quality of life of epileptic patients: A population study in West Texas. BioMed Research International, 2022, 4193664. [DOI:10.1155/2022/4193664] [PMID]
Li, H. T., Viskaitis, P., Bracey, E., Peleg-Raibstein, D., & Burdakov, D. (2024). Transient targeting of hypothalamic orexin neurons alleviates seizures in a mouse model of epilepsy. Nature Communications, 15(1), 1249. [DOI:10.1101/2023.05.18.541308]
Liblau, R. S., Vassalli, A., Seifinejad, A., & Tafti, M. (2015). Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. The Lancet. Neurology, 14(3), 318–328. [DOI:10.1016/S1474-4422(14)70218-2] [PMID]
Lim, B. V., Shin, M. S., Lee, J. M., & Seo, J. H. (2015). Treadmill exercise prevents GABAergic neuronal loss with suppression of neuronal activation in the pilocarpine-induced epileptic rats. Journal of Exercise Rehabilitation, 11(2), 80–86. [DOI:10.12965/jer.150193] [PMID]
Lin, J. G., Chen, C. J., Yang, H. B., Chen, Y. H., & Hung, S. Y. (2017). Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of parkinson's disease. International Journal of Molecular Sciences, 18(9), 1846. [DOI:10.3390/ijms18091846] [PMID]
Liu, M. F., Xue, Y., Liu, C., Liu, Y. H., Diao, H. L., & Wang, Y., et al. (2018). Orexin-A exerts neuroprotective effects via OX1R in Parkinson's disease. Frontiers in Neuroscience, 12, 835. [DOI:10.3389/fnins.2018.00835] [PMID]
Lu, H., Cheng, P. L., Lim, B. K., Khoshnevisrad, N., & Poo, M. M. (2010). Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron, 67(5), 821–833. [DOI:10.1016/j.neuron.2010.08.012] [PMID]
Manavi, M. A., Mohammad Jafari, R., Shafaroodi, H., Ejtemaei-Mehr, S., Sharifzadeh, M., & Dehpour, A. R. (2022). Anticonvulsant effects of ivermectin on pentylenetetrazole- and maximal electroshock-induced seizures in mice: The role of GABAergic system and KATP channels. Heliyon, 8(11), e11375. [DOI:10.1016/j.heliyon.2022.e11375] [PMID]
Martin, T., Dauvilliers, Y., Koumar, O. C., Bouet, V., Freret, T., & Besnard, S., et al. (2019). Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Scientific Reports, 9(1), 18432. [DOI:10.1038/s41598-019-54826-3] [PMID]
Marty, S., Wehrlé, R., & Sotelo, C. (2000). Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. The Journal of Neuroscience, 20(21), 8087–8095. [DOI:10.1523/JNEUROSCI.20-21-08087.2000] [PMID]
Matsuda, F., Sakakima, H., Ikutomo, M., & Yoshida, Y. (2009). The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Neuroscience Research 65(Supplement 1), S101. [DOI:10.1016/j.neures.2009.09.440]
Messina, G., Di Bernardo, G., Viggiano, A., De Luca, V., Monda, V., & Messina, A., et al. (2016). Exercise increases the level of plasma orexin A in humans. Journal of Basic and Clinical Physiology and Pharmacology, 27(6), 611–616. [DOI:10.1515/jbcpp-2015-0133] [PMID]
Morales, A., Bonnet, C., Bourgoin, N., Touvier, T., Nadam, J., & Laglaine, A., et al. (2006). Unexpected expression of orexin-B in basal conditions and increased levels in the adult rat hippocampus during pilocarpine-induced epileptogenesis. Brain Research, 1109(1), 164–175. [DOI:10.1016/j.brainres.2006.06.075] [PMID]
Ng M. C. (2017). Orexin and epilepsy: Potential role of REM sleep. Sleep, 40(3), 10.1093/sleep/zsw061. [DOI:10.1093/sleep/zsw061] [PMID]
Ng, T. K. S., Ho, C. S. H., Tam, W. W. S., Kua, E. H., & Ho, R. C. (2019). Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with alzheimer's disease (AD): A systematic review and meta-analysis. International Journal of Molecular Sciences, 20(2), 257. [DOI:10.3390/ijms20020257] [PMID]
Nooshinfar, E., Akbarzadeh-Baghban, A., & Meisami, E. (2011). Effects of increasing durations of immobilization stress on plasma corticosterone level, learning and memory and hippocampal BDNF gene expression in rats. Neuroscience Letters, 500(1), 63–66. [DOI:10.1016/j.neulet.2011.05.243] [PMID]
Perrochon, A., Borel, B., Istrate, D., Compagnat, M., & Daviet, J. C. (2019). Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Annals of Physical and Rehabilitation Medicine, 62(5), 366–378. [DOI:10.1016/j.rehab.2019.04.004] [PMID]
Perucca, E. (2021). The pharmacological treatment of epilepsy: Recent advances and future perspectives. Acta Epileptologica 3(1): 1-11. [DOI:10.1186/s42494-021-00055-z]
Petersén, A., Gil, J., Maat-Schieman, M. L., Björkqvist, M., Tanila, H., & Araújo, I. M., et al. (2005). Orexin loss in huntington's disease. Human Molecular Genetics, 14(1), 39–47. [DOI:10.1093/hmg/ddi004] [PMID]
Pizza, F., Barateau, L., Dauvilliers, Y., & Plazzi, G. (2022). The orexin story, sleep and sleep disturbances. Journal of Sleep Research, 31(4), e13665. [DOI:10.1111/jsr.13665] [PMID]
Popp, J. L., Szaflarski, J. P., Kaur, M., Martin, R. C., Brokamp, G. A., & Terry, D. M., et al. (2021). Relationships between cognitive function, seizure control, and self-reported leisure-time exercise in epilepsy. Epilepsy & Behavior, 118, 107900. [DOI:10.1016/j.yebeh.2021.107900] [PMID]
Razavi, B. M., Farivar, O., Etemad, L., & Hosseinzadeh, H. (2020). Suvorexant, a dual orexin receptor antagonist, protected seizure through interaction with GABAA and glutamate receptors. Iranian Journal of Pharmaceutical Research, 19(2), 383–390. [DOI:10.22037/ijpr.2019.14688.12584] [PMID]
Ren, J., Chen, Y., Fang, X., Wang, D., Wang, Y., & Yu, L., et al. (2022). Correlation of Orexin-A and brain-derived neurotrophic factor levels in metabolic syndrome and cognitive impairment in schizophrenia treated with clozapine. Neuroscience Letters, 782, 136695. [DOI:10.1016/j.neulet.2022.136695] [PMID]
Riolo, G., Ricci, C., De Angelis, N., Marzocchi, C., Guerrera, G., & Borsellino, G., et al. (2022). BDNF and Pro-BDNF in amyotrophic lateral sclerosis: A new perspective for biomarkers of neurodegeneration. Brain Sciences, 12(5), 617. [DOI:10.3390/brainsci12050617] [PMID]
Rumajogee, P., Madeira, A., Vergé, D., Hamon, M., & Miquel, M. C. (2002). Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. Journal of Neurochemistry, 83(6), 1525–1528. [DOI:10.1046/j.1471-4159.2002.01264.x] [PMID]
Salami, M., Bandegi, A. R., Sameni, H. R., Vafaei, A. A., & Pakdel, A. (2019). Hippocampal up-regulation of apolipoprotein d in a rat model of maternal hypo- and hyperthyroidism: Implication of oxidative stress. Neurochemical Research, 44(9), 2190–2201. [DOI:10.1007/s11064-019-02859-5] [PMID]
Scalzo, P., Kümmer, A., Bretas, T. L., Cardoso, F., & Teixeira, A. L. (2010). Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. Journal of Neurology, 257(4), 540–545. [DOI:10.1007/s00415-009-5357-2] [PMID]
Scammell, T. E., & Winrow, C. J. (2011). Orexin receptors: Pharmacology and therapeutic opportunities. Annual Review of Pharmacology and Toxicology, 51, 243–266. [DOI:10.1146/annurev-pharmtox-010510-100528] [PMID]
Sheibani, M., Shayan, M., Khalilzadeh, M., Ghasemi, M., & Dehpour, A. R. (2023). Orexin receptor antagonists in the pathophysiology and treatment of sleep disorders and epilepsy. Neuropeptides, 99, 102335. [DOI:10.1016/j.npep.2023.102335] [PMID]
Socała, K., Szuster-Ciesielska, A., & Wlaź, P. (2016). SB 334867, a selective orexin receptor type 1 antagonist, elevates seizure threshold in mice. Life Sciences, 150, 81–88. [DOI:10.1016/j.lfs.2016.02.075] [PMID]
Souza, M. A., Oliveira, M. S., Furian, A. F., Rambo, L. M., Ribeiro, L. R., & Lima, F. D., et al. (2009). Swimming training prevents pentylenetetrazol-induced inhibition of Na+, K+-ATPase activity, seizures, and oxidative stress. Epilepsia, 50(4), 811–823. [DOI:10.1111/j.1528-1167.2008.01908.x] [PMID]
Tamer, S. A., Kaya, Ö. T. Ç., Yüksel, M., Yıldırım, A., & Yeğen, B. Ç. (2022). A 10-day mild treadmill exercise performed before an epileptic seizure alleviates oxidative injury in the skeletal muscle and brain tissues of the rats. Marmara Medical Journal, 35(1), 1-9. [DOI:10.5472/marumj.1056192]
Tutkun, E., Ayyildiz, M., & Agar, E. (2010). Short-duration swimming exercise decreases penicillin-induced epileptiform ECoG activity in rats. Acta Neurobiologiae Experimentalis, 70(4), 382–389. [DOI:10.55782/ane-2010-1810] [PMID]
Um, Y. H., & Lim, H. K. (2020). Orexin and alzheimer's disease: A new perspective. Psychiatry Investigation, 17(7), 621–626. [DOI:10.30773/pi.2020.0136] [PMID]
Venezia, A. C., Quinlan, E., & Roth, S. M. (2017). A single bout of exercise increases hippocampal Bdnf: Influence of chronic exercise and noradrenaline. Genes, Brain, and Behavior, 16(8), 800–811. [DOI:10.1111/gbb.12394] [PMID]
Wang, Y., Liu, H., Zhang, B. S., Soares, J. C., & Zhang, X. Y. (2016). Low BDNF is associated with cognitive impairments in patients with Parkinson's disease. Parkinsonism & Related Disorders, 29, 66–71. [DOI:10.1016/j.parkreldis.2016.05.023] [PMID]
Wrann, C. D., White, J. P., Salogiannnis, J., Laznik-Bogoslavski, D., Wu, J., & Ma, D., et al. (2013). Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metabolism, 18(5), 649–659. [DOI:10.1016/j.cmet.2013.09.008] [PMID]
Yu, X., Guan, Q., Wang, Y., Shen, H., Zhai, L., & Lu, X., et al. (2019). Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Research, 154, 90–96. [DOI:10.1016/j.eplepsyres.2019.05.007] [PMID]
Yulug, B., Hanoglu, L., Khanmammadov, E., Duz, O. A., Polat, B., & Hanoglu, T., et al. (2018). Beyond the therapeutic effect of rTMS in Alzheimer's disease: A possible neuroprotective role of hippocampal BDNF? A minireview. Mini Reviews in Medicinal Chemistry, 18(17), 1479–1485. [DOI:10.2174/1389557517666170927162537] [PMID]
Zhang, C. Q., Li, H. Y., Wan, Y., Bai, X. Y., Gan, L., & Sun, H. B. (2022). Effect of different physical activity training methods on epilepsy: A protocol for systematic review and meta-analysis. Medicine, 101(11), e29085. [DOI:10.1097/MD.0000000000029085]
Zhang, Y., Huang, Z., Xia, H., Xiong, J., Ma, X., & Liu, C. (2022). The benefits of exercise for outcome improvement following traumatic brain injury: Evidence, pitfalls and future perspectives. Experimental Neurology, 349, 113958. [DOI:10.1016/j.expneurol.2021.113958] [PMID]
Zhao, X., Zhang, R.x, Tang, S., Ren, Y.y, Yang, W.x, & Liu, X.m,et al. (2014). Orexin-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides, 54, 140–147. [DOI:10.1016/j.peptides.2013.11.019] [PMID]
4. Discussion
The present study investigated the effect of aerobic exercise by treadmill as an interventional treatment without medicine therapy on the seizure score, expression of OXA and BDNF, and correlation between OXA and BDNF expression. According to behavioral data, PA could benefit the prevention and treatment of seizures in the epileptic rate. Also, PA could have a different effect on OXA in healthy and epileptic male rats, increasing OXA in healthy rats and decreasing it in PTZ kindling-induced epileptic rat models. Also, as the same effect on OXA in healthy rats, exercise elevates the BDNF in health and compensates for its reduction in epileptic rats. Also, there was a relationship between the OXA and BDNF expression in the CA1, CA3, and cortex areas of some groups.
Our findings indicate that exercise decreases the seizure intensity in epileptic rats. The effects of PA on convulsive behaviors and the quality of life have been studied in individuals with epilepsy (Johnson et al., 2021). Treadmill exercise decreases the severity of PTZ-induced seizures in male rats (Barzroodi Pour et al., 2021). Aerobic training, resistance training, and stretching ameliorate the quality of life, stress levels, and physical fitness of epileptic patients and reduce their seizure frequency (Häfele et al., 2021). PA decreases the seizure frequency in individuals with epilepsy and increases their quality of life (Lee et al., 2022). Swimming reduces the severity of seizures in rats (Souza et al., 2010; Tutkun et al., 2010). Running on the treadmill (10 days) and swimming (6 weeks) diminishes oxidative injury induced by PTZ injection in rats (Souza et al., 2009, Arabaci-Tamer, Kaya et al., 2022).
We illustrated that a 4-week treadmill PA simultaneous with seizure induction attenuated the severity of seizure in the male rats. In addition, PA pre-induction of seizure notably decreased seizure severity.
Our results indicate that exercise as a monotherapy for epilepsy has a potential effect on the severity of seizures. Additionally, it might be a prevention intervention to reduce the severity of the seizure.
There are accumulating studies that investigated the role of orexin in epileptogenesis. Intracortical injections of OXA increases epileptic activity (Kortunay et al., 2012). Results from the Li et al. (2023) study indicate that hypothalamic inhibition of pre-seizure orexin activity reduces seizure severity in epileptic mice. It has been shown that an OX1 receptor antagonist reduced seizure behaviors in the male Albino Swiss mice (Socała et al., 2016). This result suggests that the expression of both orexin types in the hippocampus elevates the excitability of hippocampal neurons during epileptogenesis (Morales et al., 2006). Our results illustrate that seizure increases the OXA in the sedentary male rats’ CA1, CA3, and cortex. The orexin pathway function in the wake-sleep cycle and regulation of the GABA and glutamate system highlight the footprint in the pathogenesis of epilepsy. In this regard, the reduction of orexin activity is related to rapid eye movement (REM) sleep at the outset, and REM sleep is usually protective against epilepsy (Ng, 2017).
Previous studies show the effect of exercise on the orexin pathway. One theory that describes the relationship between the orexinergic system and training is hypothalamic thermoregulation. Enhancing serum level of OXA and rectal temperature after ergometer exercise (Messina et al., 2016) and diminishing the simultaneously PA and core body temperature by blockage orexinergic system (Martin et al., 2019) directed according to this theory.
Another theory about the relationship between exercise and the orexin system is the neurogenesis effect of exercise on the hippocampus. Studies show that the actuation of OXA promotes neuronal proliferation, differentiation, and firing of neurons in the hippocampus and decreases animal immobility (Ito et al., 2008; Zhao et al., 2014; Chen et al., 2017). It has been indicated that the hypothalamic administration of OXA enhanced ambulation and reduced sedentary in the light and dark cycles of Sprague–Dawley rats (Kotz et al., 2002). The injection of OXA in the hypothalamus and substantia nigra has enhanced the ambulation time, and it had a modulatory function on PA (Kotz et al., 2006).
Results illustrate that PA up-regulates the OXA expression in the CA1, CA3, and cortex, but it has a down-expression effect on the expression of OXA in epileptic rats. These findings suggest that PA changed its influence depending on conditions that increased the OXA expression in the healthy nonepileptic rat and decreased it in the epileptic rats. PA acts as a moderator in its effect on OXA expression. Additionally, four weeks of PA before seizure induction and simultaneous PA with seizure induction increased the OXA expression compared to only PA during seizure induction. This finding illustrates that PA increased the OXA expression in the first four weeks, then decreased the OXA expression when it was elevated by seizure. To explain how PA reduced the severity of seizures through OXA, we should review the effect of OXA and its antagonists on GABA and glutamate processes. It has been mentioned that blockage of OXR1 and OXR2 reduces the seizure by decreasing glutamate as excitatory and increasing GABA as inhibitory contents (Goudarzi et al., 2015). Also, a dual orexin antagonist reduces seizure by elevation of GABAergic inhibition (Konduru et al., 2022). Similar to orexin antagonists, it has been reported that PA reduces GABAergic loss in the hippocampus (Lim et al., 2015).
Our findings suggest that PA might improve the imbalance of the inhibitory and excitatory systems through the orexin signaling pathway in epileptic rats.
Previous studies show the involvement of BDNF in epileptogenesis (Egbenya et al., 2023). The serum level of BDNF is lower in individuals with epilepsy (LaFrance et al., 2010). The hippocampus of epileptic animals indicates less BDNF expression than in nonepileptics (Flores-Soto et al., 2021). Similarly, our results illustrate the reduction effect of seizure on BDNF in different brain areas.
Some studies show the up-regulation effect of PA on BDNF expression (Alomari et al., 2013; Fang et al., 2013; Wrann et al., 2013; Venezia et al., 2017). Immobilization reduces BDNF expression in the hippocampus (Nooshinfar et al., 2011), and treadmill exercise improves BDNF deficits (Fang et al., 2013). Forced running exercise restores the down-regulation of the hippocampal BDNF in male Sprague–Dawley rats (Ji et al., 2014). Similarly, our result shows the up-regulation effect of PA on BDNF expression. This suggestion arises from the up-regulation effect of exercise on the 5-HT and noradrenaline (Idorn & thor Straten, 2017), which triggers the cyclic adenosine 3′,5′-monophosphate (cAMP) and its active response element binding protein. Response element binding protein up-regulates the BDNF expression through the tropomyosin receptor kinase B (Rumajogee et al., 2002). Our results demonstrate that PA restores the decrease of the BDNF expression that occurred following seizures. Similarly, resistance exercise restored the BDNF levels reduction in the homogenized hippocampus in the pilocarpine hydrochloride-induced epileptic rat (de Almeida et al., 2017). The reduction in the influence of PA on the BDNF in epilepsy might be explained by the inhibition effect of the BDNF on the GABAergic system in the epileptic brain (Marty et al., 2000). Then, exercise might decrease the severity of seizure by increasing the BDNF, which elevates the density of the inhibitory synapses (Lu et al., 2010). Additionally, our results indicate that running on the treadmill as a pre-induced seizure had a potential effect on restoration of the BDNF reduced by seizure. These findings suggest that the possible reduction effect of PA as pre-induced seizure on the severity of seizure might be due to its possible effect on the BDNF expression.
Studies have shown some correlation between the expression of orexin and BDNF. The OXA and BDNF have anti-depressive effects and improve memory (Chieffi et al., 2017). Individuals with Parkinson express low levels of OXA and BDNF (Drouot et al., 2003; Scalzo et al., 2010). Exercise increases both OXA and BDNF (Messina et al., 2016). Also, a positive correlation between OXA and BDNF is reported in schizophrenia patients (Ren et al., 2022). The OXA increases BDNF expression in Parkinson, and this function of OXA might be mediated through OXR1 in Parkinson disease (Liu et al., 2018). It has been demonstrated that microinjection of OXA in the hippocampus elevates the expression of the BDNF that decreases by capsaicin administration in the capsaicin-induced orofacial pain Wistar rats model (Kooshki et al., 2018).
Our results show a relationship between the OXA and BDNF expression in some groups’ CA1, CA3, and cortex. In the CA1, CA3, and cortex, high-expressed OXA correlates with high-expressed BDNF in the NS group and a negative correlation in the seizure group. In the CA1 and cortex, high-expressed OXA correlates with high-expressed BDNF in the PA group. These findings show that the expression of the OXA and BDNF correlates separately in healthy rats and those that intervened with seizure or PA. The correlation between the OXA and BDNF might indicate the signaling interaction between these two factors. Our results show no correlation between OXA and BDNF expression in epileptic groups with PA. The interruption of cross-talking of OXA and BDNF pathways by exercise in the epileptic animals emphasizes the modulatory effect of PA in the pathological condition.
5. Conclusion
It might be concluded that PA had different roles depending on the pathological and non-pathological conditions. The anticonvulsant effect of PA seemed to depend on disrupting interactions between the orexin and BDNF pathways.
Ethical Considerations
Compliance with ethical guidelines
All experiments were carried out according to the protocol approved by the Animal Ethics of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1401.198).
Funding
This research was supported by Iran University of Medical Sciences, Tehran, Iran (Grant No.: 1401-1-4-20886.
Authors' contributions
Conceptualization: Taha Ghantabpour, Mansoureh Soleimani, and Fariba Karimzadeh; Methodology: All authors; Data collection: Taha Ghantabpour; Data analysis: Taha Ghantabpour and Fariba Karimzadeh; Investigation: Taha Ghantabpour, Reza Ahadi, and Fariba Karimzadeh; Writing the original draft: Taha Ghantabpour; Review and editing: Taha Ghantabpour, Fariba Karimzadeh, and Reza Ahadi; Supervision: Mansoureh Soleimani, Reza Ahadi, and Fariba Karimzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of the Cellular and Molecular Research Center of Iran University of Medical Sciences, Tehran, Iran.
References
Abounoori, M., Maddah, M. M., & Ardeshiri, M. R. (2021). Orexin neuropeptides modulate the hippocampal-dependent memory through basolateral amygdala interconnections. Cerebral circulation-cognition and behavior, 3, 100035.[DOI:10.1016/j.cccb.2021.100035] [PMID]
Alomari, M. A., Khabour, O. F., Alzoubi, K. H., & Alzubi, M. A. (2013). Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behavioural Brain Research, 247, 34–39. [DOI:10.1016/j.bbr.2013.03.007] [PMID]
Alqahtani, F., Imran, I., Pervaiz, H., Ashraf, W., Perveen, N., & Rasool, M. F., et al. (2020). Non-pharmacological interventions for intractable epilepsy. Saudi Pharmaceutical Journal, 28(8), 951–962. [DOI:10.1016/j.jsps.2020.06.016] [PMID]
Azeez, I. A., Igado, O. O., & Olopade, J. O. (2021). An overview of the orexinergic system in different animal species. Metabolic Brain Disease, 36(7), 1419–1444. [DOI:10.1007/s11011-021-00761-0] [PMID]
Bareiss, S. K., Johnston, T., Lu, Q., & Tran, T. D. (2022). The effect of exercise on early sensorimotor performance alterations in the 3xTg-AD model of Alzheimer's disease. Neuroscience Research, 178, 60–68. [DOI:10.1016/j.neures.2022.01.003] [PMID]
Barzroodi Pour, M., Bayat, M., Navazesh, A., Soleimani, M., & Karimzadeh, F. (2021). Exercise improved the anti-epileptic effect of carbamazepine through GABA enhancement in epileptic rats. Neurochemical Research, 46(8), 2112–2130. [DOI:10.1007/s11064-021-03349-3] [PMID]
Bonnavion, P., & de Lecea, L. (2010). Hypocretins in the control of sleep and wakefulness. Current Neurology and Neuroscience Reports, 10(3), 174–179. [DOI:10.1007/s11910-010-0101-y] [PMID]
Borgland, S. L., & Labouèbe, G. (2010). Orexin/hypocretin in psychiatric disorders: Present state of knowledge and future potential. Neuropsychopharmacology, 35(1), 353–354. [DOI:10.1038/npp.2009.119] [PMID]
Chen, X. Y., Chen, L., & Du, Y. F. (2017). Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. Journal of Neuroscience Research, 95(7), 1415–1426. [DOI:10.1002/jnr.23975] [PMID]
Chieffi, S., Carotenuto, M., Monda, V., Valenzano, A., Villano, I., & Precenzano, F., et al. (2017). Orexin system: The key for a healthy life. Frontiers in Physiology, 8, 357. [DOI:10.3389/fphys.2017.00357] [PMID]
Çikriklar, H. I., Kotan, D., Yücel, M., Ceylan, M., Çiftçi, G. G., & Bayraktutan, Ö. F., et al. (2020). The role of orexin-A levels in epileptic seizure. Neuroscience Letters, 734, 135097. [DOI:10.1016/j.neulet.2020.135097] [PMID]
de Almeida, A. A., Gomes da Silva, S., Lopim, G. M., Vannucci Campos, D., & Fernandes, J., et al. (2017). Resistance exercise reduces seizure occurrence, attenuates memory deficits and restores BDNF signaling in rats with chronic epilepsy. Neurochemical Research, 42(4), 1230–1239. [DOI:10.1007/s11064-016-2165-9] [PMID]
Drouot, X., Moutereau, S., Nguyen, J. P., Lefaucheur, J. P., Créange, A., & Remy, P., et al. (2003). Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology, 61(4), 540–543. [DOI:10.1212/01.WNL.0000078194.53210.48] [PMID]
Egbenya, D. L., Hussain, S., Lai, Y. C., Anderson, A. E., & Davanger, S. (2023). Synapse-specific changes in Arc and BDNF in rat hippocampus following chronic temporal lobe epilepsy. Neuroscience Research, 191, 1–12. [DOI:10.1016/j.neures.2022.12.006] [PMID]
Fang, Z. H., Lee, C. H., Seo, M. K., Cho, H., Lee, J. G., & Lee, B. J., et al. (2013). Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neuroscience Research, 76(4), 187–194. [DOI:10.1016/j.neures.2013.04.005] [PMID]
Flores-Soto, M., Romero-Guerrero, C., Vázquez-Hernández, N., Tejeda-Martínez, A., Martín-Amaya-Barajas, F. L., & Orozco-Suárez, S., et al. (2021). Pentylenetetrazol-induced seizures in adult rats are associated with plastic changes to the dendritic spines on hippocampal CA1 pyramidal neurons. Behavioural Brain Research, 406, 113198. [DOI:10.1016/j.bbr.2021.113198] [PMID]
Foutz, T. J., & Wong, M. (2022). Brain stimulation treatments in epilepsy: Basic mechanisms and clinical advances. Biomedical Journal, 45(1), 27–37. [DOI:10.1016/j.bj.2021.08.010] [PMID]
Ghahfarrokhi, M. M., Habibi, A., Alizadeh, A. A., Negaresh, R., Shahi, M. M., & Earnest, C. P. (2020). BDNF and orexin-A response to aerobic exercise are moderated by the meal consumption before exercise in overweight men: Effect of high-carbohydrate, high-protein and high-fat meals. Science & Sports, 35(4), 228-236. [DOI:10.1016/j.scispo.2020.01.011]
Goudarzi, E., Elahdadi Salmani, M., Lashkarbolouki, T., & Goudarzi, I. (2015). Hippocampal orexin receptors inactivation reduces PTZ induced seizures of male rats. Pharmacology, Biochemistry, and Behavior, 130, 77–83. [DOI:10.1016/j.pbb.2015.01.006] [PMID]
Häfele, C. A., Rombaldi, A. J., Feter, N., Häfele, V., Gervini, B. L., & Domingues, M. R., et al. (2021). Effects of an exercise program on health of people with epilepsy: A randomized clinical trial. Epilepsy & Behavior, 117, 107904. [DOI:10.1016/j.yebeh.2021.107904] [PMID]
Idorn, M., & Thor Straten, P. (2017). Exercise and cancer: From "healthy" to "therapeutic"? Cancer Immunology, Immunotherapy, 66(5), 667–671. [DOI:10.1007/s00262-017-1985-z] [PMID]
Ito, N., Yabe, T., Gamo, Y., Nagai, T., Oikawa, T., & Yamada, H., et al. (2008). I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience, 157(4), 720–732. [DOI:10.1016/j.neuroscience.2008.09.042] [PMID]
Ji, J. F., Ji, S. J., Sun, R., Li, K., Zhang, Y., & Zhang, L. Y., et al. (2014). Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochemical and Biophysical Research Communications, 443(2), 646–651. [DOI:10.1016/j.bbrc.2013.12.031] [PMID]
Johnson, E. C., Helen Cross, J., & Reilly, C. (2020). Physical activity in people with epilepsy: A systematic review. Epilepsia, 61(6), 1062–1081. [DOI:10.1111/epi.16517] [PMID]
Kinboshi, M., Shimizu, S., Tokudome, K., Mashimo, T., Serikawa, T., & Ito, H., et al. (2023). Imbalance of glutamatergic and GABAergic neurotransmission in audiogenic seizure-susceptible Leucine-rich glioma-inactivated 1 (Lgi1)-mutant rats. Heliyon, 9(7), e17984. [DOI:10.1016/j.heliyon.2023.e17984] [PMID]
Konduru, S. R., Isaacson, J. R., Lasky, D. J., Zhou, Z., Rao, R. K., & Vattem, S. S., et al. (2022). Dual orexin antagonist normalized sleep homeostatic drive, enhanced GABAergic inhibition, and suppressed seizures after traumatic brain injury. Sleep, 45(12), zsac238. [DOI:10.1093/sleep/zsac238] [PMID]
Kooshki, R., Abbasnejad, M., Esmaeili-Mahani, S., & Raoof, M. (2018). The effect of CA1 administration of orexin-A on hippocampal expression of COX-2 and BDNF in a rat model of orofacial pain. Arquivos de Neuro-Psiquiatria, 76(9), 603–608. [DOI:10.1590/0004-282x20180099] [PMID]
Kortunay, S., Erken, H. A., Erken, G., Genç, O., Sahiner, M., & Turgut, S., et al. (2012). Orexins increase penicillin-induced epileptic activity. Peptides, 34(2), 419–422. [DOI:10.1016/j.peptides.2012.02.013] [PMID]
Kotz, C. M., Teske, J. A., Levine, J. A., & Wang, C. (2002). Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regulatory Peptides, 104(1-3), 27–32. [DOI:10.1016/S0167-0115(01)00346-9] [PMID]
Kotz, C. M., Wang, C., Teske, J. A., Thorpe, A. J., Novak, C. M., & Kiwaki, K., et al. (2006). Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience, 142(1), 29–36. [DOI:10.1016/j.neuroscience.2006.05.028] [PMID]
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2018). BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38(3), 579–593. [DOI:10.1007/s10571-017-0510-4] [PMID]
LaFrance, W. C., Jr, Leaver, K., Stopa, E. G., Papandonatos, G. D., & Blum, A. S. (2010). Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology, 75(14), 1285–1291. [DOI:10.1212/WNL.0b013e3181f612bb] [PMID]
Lee, Y., Ahn, Y., & Cucullo, L. (2022). Impact of physical activity and medication adherence on the seizure frequency and quality of life of epileptic patients: A population study in West Texas. BioMed Research International, 2022, 4193664. [DOI:10.1155/2022/4193664] [PMID]
Li, H. T., Viskaitis, P., Bracey, E., Peleg-Raibstein, D., & Burdakov, D. (2024). Transient targeting of hypothalamic orexin neurons alleviates seizures in a mouse model of epilepsy. Nature Communications, 15(1), 1249. [DOI:10.1101/2023.05.18.541308]
Liblau, R. S., Vassalli, A., Seifinejad, A., & Tafti, M. (2015). Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. The Lancet. Neurology, 14(3), 318–328. [DOI:10.1016/S1474-4422(14)70218-2] [PMID]
Lim, B. V., Shin, M. S., Lee, J. M., & Seo, J. H. (2015). Treadmill exercise prevents GABAergic neuronal loss with suppression of neuronal activation in the pilocarpine-induced epileptic rats. Journal of Exercise Rehabilitation, 11(2), 80–86. [DOI:10.12965/jer.150193] [PMID]
Lin, J. G., Chen, C. J., Yang, H. B., Chen, Y. H., & Hung, S. Y. (2017). Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of parkinson's disease. International Journal of Molecular Sciences, 18(9), 1846. [DOI:10.3390/ijms18091846] [PMID]
Liu, M. F., Xue, Y., Liu, C., Liu, Y. H., Diao, H. L., & Wang, Y., et al. (2018). Orexin-A exerts neuroprotective effects via OX1R in Parkinson's disease. Frontiers in Neuroscience, 12, 835. [DOI:10.3389/fnins.2018.00835] [PMID]
Lu, H., Cheng, P. L., Lim, B. K., Khoshnevisrad, N., & Poo, M. M. (2010). Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron, 67(5), 821–833. [DOI:10.1016/j.neuron.2010.08.012] [PMID]
Manavi, M. A., Mohammad Jafari, R., Shafaroodi, H., Ejtemaei-Mehr, S., Sharifzadeh, M., & Dehpour, A. R. (2022). Anticonvulsant effects of ivermectin on pentylenetetrazole- and maximal electroshock-induced seizures in mice: The role of GABAergic system and KATP channels. Heliyon, 8(11), e11375. [DOI:10.1016/j.heliyon.2022.e11375] [PMID]
Martin, T., Dauvilliers, Y., Koumar, O. C., Bouet, V., Freret, T., & Besnard, S., et al. (2019). Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Scientific Reports, 9(1), 18432. [DOI:10.1038/s41598-019-54826-3] [PMID]
Marty, S., Wehrlé, R., & Sotelo, C. (2000). Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. The Journal of Neuroscience, 20(21), 8087–8095. [DOI:10.1523/JNEUROSCI.20-21-08087.2000] [PMID]
Matsuda, F., Sakakima, H., Ikutomo, M., & Yoshida, Y. (2009). The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Neuroscience Research 65(Supplement 1), S101. [DOI:10.1016/j.neures.2009.09.440]
Messina, G., Di Bernardo, G., Viggiano, A., De Luca, V., Monda, V., & Messina, A., et al. (2016). Exercise increases the level of plasma orexin A in humans. Journal of Basic and Clinical Physiology and Pharmacology, 27(6), 611–616. [DOI:10.1515/jbcpp-2015-0133] [PMID]
Morales, A., Bonnet, C., Bourgoin, N., Touvier, T., Nadam, J., & Laglaine, A., et al. (2006). Unexpected expression of orexin-B in basal conditions and increased levels in the adult rat hippocampus during pilocarpine-induced epileptogenesis. Brain Research, 1109(1), 164–175. [DOI:10.1016/j.brainres.2006.06.075] [PMID]
Ng M. C. (2017). Orexin and epilepsy: Potential role of REM sleep. Sleep, 40(3), 10.1093/sleep/zsw061. [DOI:10.1093/sleep/zsw061] [PMID]
Ng, T. K. S., Ho, C. S. H., Tam, W. W. S., Kua, E. H., & Ho, R. C. (2019). Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with alzheimer's disease (AD): A systematic review and meta-analysis. International Journal of Molecular Sciences, 20(2), 257. [DOI:10.3390/ijms20020257] [PMID]
Nooshinfar, E., Akbarzadeh-Baghban, A., & Meisami, E. (2011). Effects of increasing durations of immobilization stress on plasma corticosterone level, learning and memory and hippocampal BDNF gene expression in rats. Neuroscience Letters, 500(1), 63–66. [DOI:10.1016/j.neulet.2011.05.243] [PMID]
Perrochon, A., Borel, B., Istrate, D., Compagnat, M., & Daviet, J. C. (2019). Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Annals of Physical and Rehabilitation Medicine, 62(5), 366–378. [DOI:10.1016/j.rehab.2019.04.004] [PMID]
Perucca, E. (2021). The pharmacological treatment of epilepsy: Recent advances and future perspectives. Acta Epileptologica 3(1): 1-11. [DOI:10.1186/s42494-021-00055-z]
Petersén, A., Gil, J., Maat-Schieman, M. L., Björkqvist, M., Tanila, H., & Araújo, I. M., et al. (2005). Orexin loss in huntington's disease. Human Molecular Genetics, 14(1), 39–47. [DOI:10.1093/hmg/ddi004] [PMID]
Pizza, F., Barateau, L., Dauvilliers, Y., & Plazzi, G. (2022). The orexin story, sleep and sleep disturbances. Journal of Sleep Research, 31(4), e13665. [DOI:10.1111/jsr.13665] [PMID]
Popp, J. L., Szaflarski, J. P., Kaur, M., Martin, R. C., Brokamp, G. A., & Terry, D. M., et al. (2021). Relationships between cognitive function, seizure control, and self-reported leisure-time exercise in epilepsy. Epilepsy & Behavior, 118, 107900. [DOI:10.1016/j.yebeh.2021.107900] [PMID]
Razavi, B. M., Farivar, O., Etemad, L., & Hosseinzadeh, H. (2020). Suvorexant, a dual orexin receptor antagonist, protected seizure through interaction with GABAA and glutamate receptors. Iranian Journal of Pharmaceutical Research, 19(2), 383–390. [DOI:10.22037/ijpr.2019.14688.12584] [PMID]
Ren, J., Chen, Y., Fang, X., Wang, D., Wang, Y., & Yu, L., et al. (2022). Correlation of Orexin-A and brain-derived neurotrophic factor levels in metabolic syndrome and cognitive impairment in schizophrenia treated with clozapine. Neuroscience Letters, 782, 136695. [DOI:10.1016/j.neulet.2022.136695] [PMID]
Riolo, G., Ricci, C., De Angelis, N., Marzocchi, C., Guerrera, G., & Borsellino, G., et al. (2022). BDNF and Pro-BDNF in amyotrophic lateral sclerosis: A new perspective for biomarkers of neurodegeneration. Brain Sciences, 12(5), 617. [DOI:10.3390/brainsci12050617] [PMID]
Rumajogee, P., Madeira, A., Vergé, D., Hamon, M., & Miquel, M. C. (2002). Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. Journal of Neurochemistry, 83(6), 1525–1528. [DOI:10.1046/j.1471-4159.2002.01264.x] [PMID]
Salami, M., Bandegi, A. R., Sameni, H. R., Vafaei, A. A., & Pakdel, A. (2019). Hippocampal up-regulation of apolipoprotein d in a rat model of maternal hypo- and hyperthyroidism: Implication of oxidative stress. Neurochemical Research, 44(9), 2190–2201. [DOI:10.1007/s11064-019-02859-5] [PMID]
Scalzo, P., Kümmer, A., Bretas, T. L., Cardoso, F., & Teixeira, A. L. (2010). Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. Journal of Neurology, 257(4), 540–545. [DOI:10.1007/s00415-009-5357-2] [PMID]
Scammell, T. E., & Winrow, C. J. (2011). Orexin receptors: Pharmacology and therapeutic opportunities. Annual Review of Pharmacology and Toxicology, 51, 243–266. [DOI:10.1146/annurev-pharmtox-010510-100528] [PMID]
Sheibani, M., Shayan, M., Khalilzadeh, M., Ghasemi, M., & Dehpour, A. R. (2023). Orexin receptor antagonists in the pathophysiology and treatment of sleep disorders and epilepsy. Neuropeptides, 99, 102335. [DOI:10.1016/j.npep.2023.102335] [PMID]
Socała, K., Szuster-Ciesielska, A., & Wlaź, P. (2016). SB 334867, a selective orexin receptor type 1 antagonist, elevates seizure threshold in mice. Life Sciences, 150, 81–88. [DOI:10.1016/j.lfs.2016.02.075] [PMID]
Souza, M. A., Oliveira, M. S., Furian, A. F., Rambo, L. M., Ribeiro, L. R., & Lima, F. D., et al. (2009). Swimming training prevents pentylenetetrazol-induced inhibition of Na+, K+-ATPase activity, seizures, and oxidative stress. Epilepsia, 50(4), 811–823. [DOI:10.1111/j.1528-1167.2008.01908.x] [PMID]
Tamer, S. A., Kaya, Ö. T. Ç., Yüksel, M., Yıldırım, A., & Yeğen, B. Ç. (2022). A 10-day mild treadmill exercise performed before an epileptic seizure alleviates oxidative injury in the skeletal muscle and brain tissues of the rats. Marmara Medical Journal, 35(1), 1-9. [DOI:10.5472/marumj.1056192]
Tutkun, E., Ayyildiz, M., & Agar, E. (2010). Short-duration swimming exercise decreases penicillin-induced epileptiform ECoG activity in rats. Acta Neurobiologiae Experimentalis, 70(4), 382–389. [DOI:10.55782/ane-2010-1810] [PMID]
Um, Y. H., & Lim, H. K. (2020). Orexin and alzheimer's disease: A new perspective. Psychiatry Investigation, 17(7), 621–626. [DOI:10.30773/pi.2020.0136] [PMID]
Venezia, A. C., Quinlan, E., & Roth, S. M. (2017). A single bout of exercise increases hippocampal Bdnf: Influence of chronic exercise and noradrenaline. Genes, Brain, and Behavior, 16(8), 800–811. [DOI:10.1111/gbb.12394] [PMID]
Wang, Y., Liu, H., Zhang, B. S., Soares, J. C., & Zhang, X. Y. (2016). Low BDNF is associated with cognitive impairments in patients with Parkinson's disease. Parkinsonism & Related Disorders, 29, 66–71. [DOI:10.1016/j.parkreldis.2016.05.023] [PMID]
Wrann, C. D., White, J. P., Salogiannnis, J., Laznik-Bogoslavski, D., Wu, J., & Ma, D., et al. (2013). Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metabolism, 18(5), 649–659. [DOI:10.1016/j.cmet.2013.09.008] [PMID]
Yu, X., Guan, Q., Wang, Y., Shen, H., Zhai, L., & Lu, X., et al. (2019). Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Research, 154, 90–96. [DOI:10.1016/j.eplepsyres.2019.05.007] [PMID]
Yulug, B., Hanoglu, L., Khanmammadov, E., Duz, O. A., Polat, B., & Hanoglu, T., et al. (2018). Beyond the therapeutic effect of rTMS in Alzheimer's disease: A possible neuroprotective role of hippocampal BDNF? A minireview. Mini Reviews in Medicinal Chemistry, 18(17), 1479–1485. [DOI:10.2174/1389557517666170927162537] [PMID]
Zhang, C. Q., Li, H. Y., Wan, Y., Bai, X. Y., Gan, L., & Sun, H. B. (2022). Effect of different physical activity training methods on epilepsy: A protocol for systematic review and meta-analysis. Medicine, 101(11), e29085. [DOI:10.1097/MD.0000000000029085]
Zhang, Y., Huang, Z., Xia, H., Xiong, J., Ma, X., & Liu, C. (2022). The benefits of exercise for outcome improvement following traumatic brain injury: Evidence, pitfalls and future perspectives. Experimental Neurology, 349, 113958. [DOI:10.1016/j.expneurol.2021.113958] [PMID]
Zhao, X., Zhang, R.x, Tang, S., Ren, Y.y, Yang, W.x, & Liu, X.m,et al. (2014). Orexin-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides, 54, 140–147. [DOI:10.1016/j.peptides.2013.11.019] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2023/10/15 | Accepted: 2023/10/22 | Published: 2025/01/1
Received: 2023/10/15 | Accepted: 2023/10/22 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |