Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 283-298 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghobadi-Azbari P, Moslemi H, Yousefpour M, Hosseini Y. Amygdala Network Dynamics During Drug Cue Processing in Methamphetamine Use Disorder. BCN 2025; 16 (S1) :283-298
URL: http://bcn.iums.ac.ir/article-1-2785-en.html
URL: http://bcn.iums.ac.ir/article-1-2785-en.html
1- Department of Science and Technology Studies, Faculty of Faculty of Neuroscience, AJA University of Command and Staff, Tehran, Iran.
2- Department of Physiology, Faculty of Medicine AJA University of Medical Sciences, Tehran, Iran.
3- Cognitive and Behavioral Research Center, AJA University of Medical Sciences, Tehran, Iran.
2- Department of Physiology, Faculty of Medicine AJA University of Medical Sciences, Tehran, Iran.
3- Cognitive and Behavioral Research Center, AJA University of Medical Sciences, Tehran, Iran.
Keywords: Addiction neuroscience, Craving, Cue reactivity, Dynamic causal modeling (DCM), Generalized psychophysiological interaction (gPPI), Functional magnetic resonance imaging (fMRI), Methamphetamine use disorder (MUD)
Full-Text [PDF 3258 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Methamphetamine use disorder (MUD) continues to be a substantial public health concern, with its profound impact on individuals' physical, psychological, and social well-being (Courtney & Ray, 2014; Hedegaard et al., 2018; Paulus & Stewart, 2020a). Understanding the neural mechanisms that underlie the complex process of addiction is paramount for developing targeted interventions and treatment strategies (Paulus & Stewart, 2020b; Soleimani et al., 2023; Soleimani et al., 2023). Neuroimaging methods, including functional magnetic resonance imaging (fMRI), provide a distinct perspective on the complex operations of the addicted brain, offering insights into the neural networks and pathways that influence drug-related behaviors (Ekhtiari et al., 2022; Jan et al., 2012; Koob & Volkow, 2016; Parvaz et al., 2011; Stewart et al., 2014).
Amygdala, a crucial node in the brain's emotion processing and reward systems, plays a central role in the addiction process (Everitt et al., 1999; Phelps & LeDoux, 2005). Its interactions with various cortical regions, particularly the prefrontal cortex, are instrumental in the emergence of addictive behaviors, including craving and cue reactivity (Goldstein & Volkow, 2011; Zilverstand et al., 2018). Given the amygdala's involvement in processing emotionally salient stimuli and its established relevance to addiction, investigating its connectivity with cortical regions during methamphetamine cue exposure is of paramount importance (Soleimani et al., 2023).
The present study seeks to address this crucial gap in knowledge by utilizing an fMRI approach to explore amygdala-cortical connectivity and effective neural pathways involved in methamphetamine cue reactivity. By employing a combination of task-based fMRI paradigms and advanced connectivity analyses, we aim to shed light on how neural networks interplay in response to methamphetamine cues, providing a more comprehensive understanding of addiction's neurobiological underpinnings.
Our primary objective is to investigate the modulation of amygdala-cortical connectivity during methamphetamine cue exposure compared to neutral cues. Our hypothesis posits that exposure to drug-related cues will increase connectivity between the amygdala and regions associated with reward processing and cognitive control, such as the prefrontal cortex. This investigation will offer valuable insights into the brain's dynamic response to cues that trigger drug craving, unraveling the mechanisms that facilitate the transition from cue exposure to craving and potential relapse.
Furthermore, this study seeks to delve beyond functional connectivity by exploring effective neural pathways using dynamic causal modeling (DCM). This modeling enables the investigation of directed interactions between brain regions, shedding light on the neural network's causal relationships and information flow. By applying DCM to our multimodal fMRI data, we aim to uncover the directional influences and effective pathways that underlie amygdala-cortical interactions during methamphetamine cue reactivity.
In addition to the neural perspectives, this study will explore potential correlations between neural connectivity patterns and psychological variables. We will utilize the Pearson correlation and group factor analysis (GFA) to elucidate potential neuro-behavioral relationships, bridging the gap between brain connectivity and the subjective experiences associated with drug cue reactivity.
In the subsequent sections, we will comprehensively overview the research methodology, findings, and implications. This study aims to contribute to the growing body of knowledge concerning addiction's neurobiological underpinnings by integrating advanced neuroimaging techniques, connectivity analyses, and psychological assessments. Ultimately, our findings may pave the way for more targeted interventions that address the neural circuitry involved in methamphetamine cue reactivity and addiction-related processes.
2. Materials and Methods
Study participants
The present study involved 62 male volunteers diagnosed with MUD. Thirteen subjects were excluded from the study due to non-compliance with the inclusion instructions, as revealed in their screening assessments. The participants were sourced from addiction treatment centers. The exclusion criteria included current or previous comorbid axis I-disorders other than drug dependence, as outlined by the diagnostic and statistical manual of mental disorders, the fourth edition, text revision (DSM-IV-TR) (APA, 2000), psychiatric or neurological disorders, including head trauma, as well as MR-specific exclusion criteria. The inclusion criteria stipulated a diagnosis of methamphetamine dependence for a minimum of 6 months in accordance with DSM-IV-TR criteria. Additionally, participants were required to self-report and test negative for all drugs (except nicotine) in both urine and self-reports for a minimum of one week. Participants’ right-handedness was assessed using the Edinburgh handedness inventory (Oldfield, 1971), and inclusion criteria also specified an age range of 20 to 45 years. The visual acuity of each participant was normal or corrected to normal.
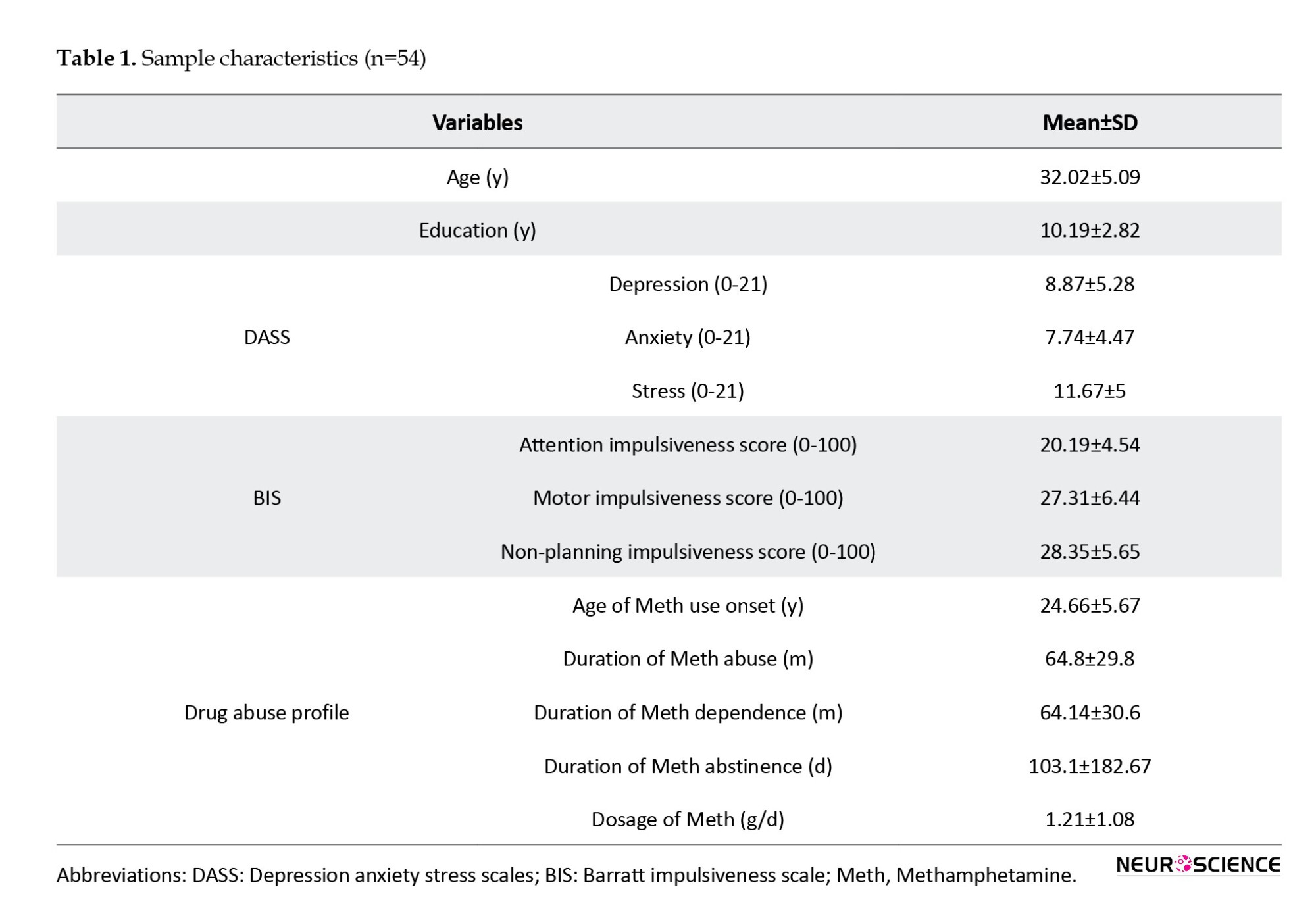
Sixty-two subjects met all the inclusion and exclusion criteria. Nevertheless, 8 participants were excluded from the data analysis due to excessive head motion during scanning. Consequently, the final sample for the analyses comprised 54 male participants diagnosed with MUD, aged 22 to 44 years (Mean±SD 32.02±5.09). Comprehensive behavioral and demographic information about methamphetamine users is provided in Table 1.
Experimental procedures
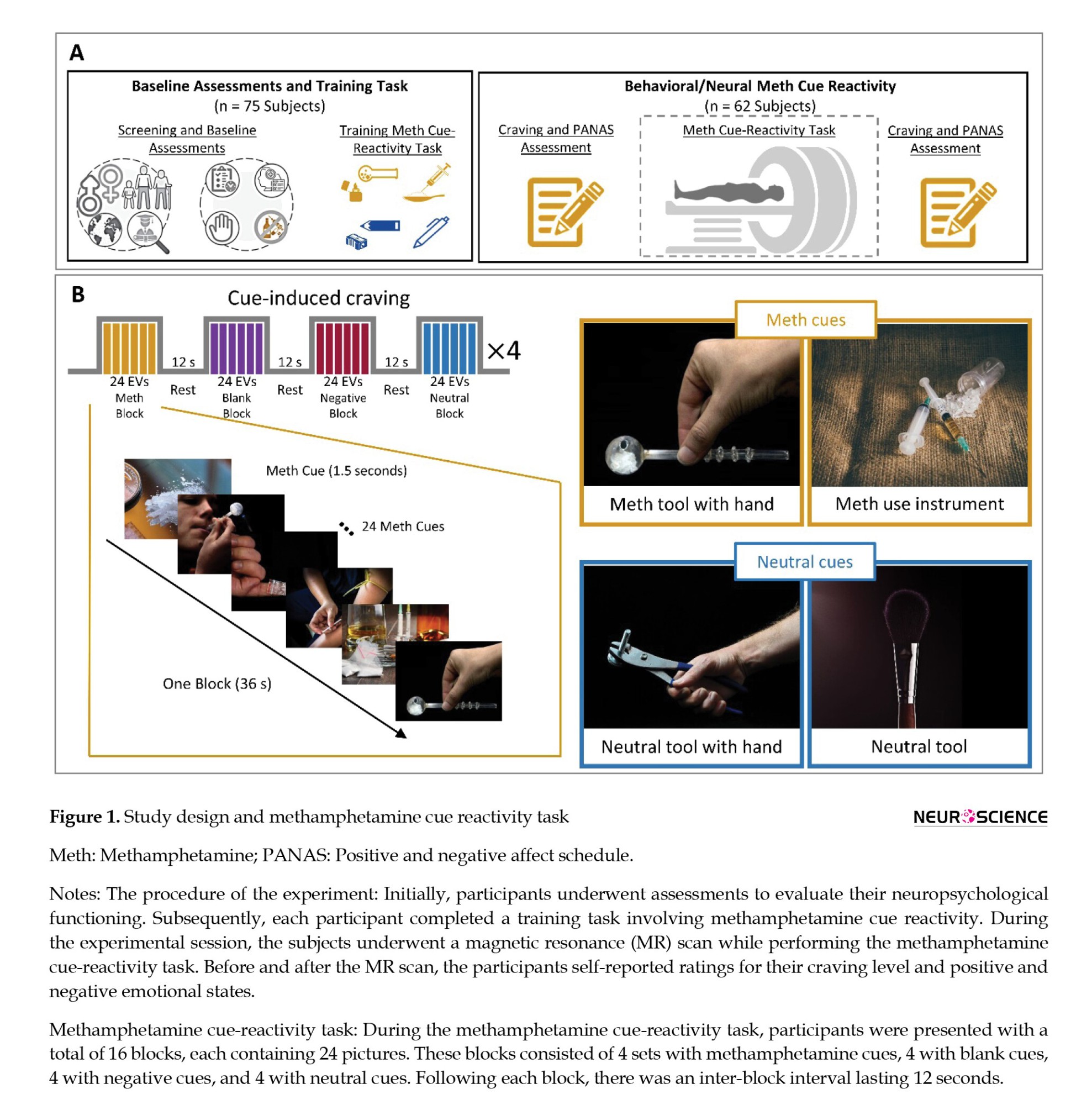
During the screening session, participants diagnosed with MUD underwent a series of baseline assessments. These assessments encompassed demographic information (Ranaei et al., 2022), substance use patterns, mental health evaluations, treatment history, and a risky behaviors profile. This profile included a history of drug injection, engagement in high-risk sexual activities, prior incarcerations, participation in drug sales, and a record of violent altercations. Furthermore, participants completed the Barratt impulsiveness scales-11 (BIS-11) (Barratt, 1994) and the depression anxiety stress scales-21 (DASS-21) (Hosseini et al., 2023; Hosseini & Modarresi Chahardehi, 2021; Osman et al., 2012). They also underwent a training session to familiarize themselves with the drug cue reactivity task before the MRI scan day (Figure 1A).
After successful screening, participants underwent a single fMRI session. Participants arrived for the MRI scans between 8:30 and 10:30 AM on the designated day after abstaining from all drugs (except nicotine) for at least one week. After participants finished self-assessments of their current drug craving using a 0–100 visual analog scale (VAS) and evaluations of positive and negative emotional states using the positive and negative affect schedule (PANAS) (Crawford & Henry, 2004), they proceeded to engage in the methamphetamine cue-reactivity paradigm within the fMRI scanner. After the scans were concluded, the participants were asked to provide another round of ratings for their level of drug craving and assess their positive and negative emotional states (Figure 1A). To reduce the likelihood of post-scanning drug use, participants were recommended to stay at the imaging center for at least one hour for recovery.
Functional methamphetamine cue reactivity paradigm
The methamphetamine cue reactivity paradigm was developed to investigate the brain's functional responses to methamphetamine compared to neutral stimuli (Dakhili et al., 2022; Ekhtiari et al., 2022; Jafakesh et al., 2022) The experiment comprised four runs of the fMRI drug cue reactivity task, with intervals of rest blocks featuring a fixation point. Each run had four blocks lasting 36 seconds each, and each block contained 24 images. The images included blank screens, negative cues, methamphetamine cues, and neutral cues. The stimuli were displayed for 1000 ms, followed by an average inter-stimulus interval of 500 ms. The order of block presentations was pseudo-randomized across the four runs, and after each block, there was a 12-second inter-block interval. Each run lasted approximately 198 s, with 18-s fixation intervals separating the runs. In these fixation periods, a white cross was shown on a black background (Figure 1B). Before the scanning session, participants received training to acquaint themselves with the fMRI environment and the cue reactivity paradigm. The negative and neutral cues were selected from the international affective picture system database (Lang et al., 1997). In contrast, the drug-related cues were sourced from a publicly available and validated cue database (Ekhtiari et al., 2020).
MRI data acquisition
We used a SIEMENS 3.0T scanner (MAGNETOM Trio, SIEMENS, Germany) with a 64-channel head coil to acquire T1 and fMRI sequences. fMRI images were obtained from a T2*-weighted gradient-echo echoplanar imaging (EPI) sequence. Each volume of functional data comprised 40 slices, featuring a repetition time (TR) of 2200 ms, an echo time (TE) of 30 ms, and a flip angle of 90°. The field of view (FOV) was configured to 192×192 mm, and the voxel dimensions were 3×3×3 mm. A total of 367 T2*-weighted functional images were obtained in an interleaved slice acquisition order.
Furthermore, a structural image was obtained using the magnetization-prepared rapid acquisition gradient echo sequence. This sequence parameters were set as follows: TR=1800 ms, TE=3.44 ms, flip angle=7°, FOV=256×256 mm, and voxel size=1×1×1 mm.
Functional MRI data preprocessing
The fMRI data was subjected to preprocessing through the utilization of the CONN toolbox (version 20.b) (Whitfield-Gabrieli & Nieto-Castanon, 2012) in SPM12 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). The standard preprocessing pipeline involved several steps: functional realignment and unwarp, slice-timing correction, outlier identification, direct segmentation, and normalization into the standard Montreal Neurological Institute (MNI) space. Additionally, functional smoothing was applied using an 8 mm full-width at half-maximum Gaussian kernel.
Task-based functional brain activity analysis
The preprocessed fMRI data underwent a general linear model (GLM) analysis. This model was constructed by modeling onset times for the methamphetamine, neutral, negative, and blank conditions using a 36-second block function. These onset times were convolved with a canonical hemodynamic response function to generate the corresponding regressors of interest. Moreover, the six individual motion correction parameters were included as nuisance regressors in the first-level model.
In each participant's case, contrast images illustrating the comparison between the methamphetamine and neutral conditions were subsequently incorporated into second-level one-sample t-test models, all of which were executed using SPM12. The individual contrast maps were initially thresholded at P<0.05 (uncorrected). On the other hand, the whole-brain statistical group maps underwent correction for family-wise error (FWE) to address multiple comparisons. This correction was accomplished using Gaussian random field theory (Nichols & Hayasaka, 2003; Worsley et al., 1996). Furthermore, Brainnetome atlas parcellation was applied to each subject's data. This allowed for the estimation of the average BOLD signal change across the 246 sub-regions present in the atlas. Notably, the specific sub-region of the right medial amygdala, identified through the Brainnetome atlas-based parcellation of the fMRI data, was chosen as the seed region for the ensuing seed-to-whole brain generalized psychophysiological interaction (gPPI) analysis. This analysis aimed to uncover modulations in connectivity influenced by drug cue reactivity.
Task-based functional brain connectivity analysis
The task-based functional brain connectivity analysis was conducted using the CONN toolbox (version 20.b) (Whitfield-Gabrieli & Nieto-Castanon, 2012) integrated within SPM12. A seed-to-whole brain approach was employed in this analytical framework, utilizing gPPI analysis. In this context, the right medial amygdala was chosen as the specified seed region. The primary objective of the PPI analysis was to reveal specific brain regions exhibiting connectivity patterns that demonstrated variability based on the psychological context, with a specific focus on the differential response to methamphetamine stimuli in comparison to neutral stimuli.
At the first level of the gPPI analysis, the psychological regressors of interest encompassed the timing of both methamphetamine and neutral blocks. These timings were convolved with a hemodynamic response function. The physiological regressor was then computed using the average time series derived from the designated seed region, specifically the right medial amygdala, as outlined in the Brainnetome mask. The PPI regressors were constructed as interaction terms involving psychological and physiological regressors. Through this approach, we contrasted the PPI regressors, identifying brain regions where connectivity with the medial amygdala demonstrated significant differences between the methamphetamine and neutral conditions. These PPI regressors were computed for each participant, revealing the voxel-level interaction with the seed region while comparing the methamphetamine and neutral conditions.
Subsequently, a second-level gPPI analysis was conducted to detect significant clusters. This analysis utilized voxel-wise and cluster-extent thresholds, where clusters were deemed active if they exceeded a voxel-level threshold of uncorrected P<0.001 and a cluster-size threshold corrected for false discovery rate (FDR) at P<0.05. The regions demonstrating the most robust PPI connectivity with the amygdala region were subsequently selected as regions of interest for the parametric empirical Bayes (PEB)-DCM analysis. To this end, functional regions of interest (ROIs) with 8 mm spheres were centered on the peak voxels within the amygdala (coordinates: 19, -2, -19), the dlPFC (coordinates: 16, 34, 44), and the PCC (coordinates: -4, -40, 34). These coordinates were derived from our gPPI outcomes concerning cue-reactivity processing.
Task-based dynamic causal modelling
The analysis of task-based effective brain connectivity over the amygdala, dlPFC, and PCC brain regions was conducted utilizing the DCM toolbox integrated within SPM12. The principal eigenvariate time series were derived from predefined unilateral masks of these regions, with adjustments for effects of interest. To explore the effective functional connectivity between these regions, we employed the bilinear DCM approach (Friston et al., 2003), incorporating the stochastic option (Daunizeau et al., 2012; Friston et al., 2011; Li et al., 2011). A full DCM model was constructed, encompassing three components: 1) Fixed connections encompass the extrinsic forward and backward connections between the amygdala and dlPFC, amygdala and PCC, and dlPFC and PCC, as well as intrinsic self-connections within each region, 2) Contextual modulation involves the adjustment of intrinsic self-connections influenced by craving, and 3) exogenous inputs refer to the introduction of methamphetamine and neutral stimuli as driving inputs into the respective nodes. Bayesian model inversion was carried out for each subject, entailing the establishment and inversion of this parent DCM model.
In the group-level DCM analysis, we performed a linear PEB analysis on a fully connected model estimated across all participants. By utilizing a post hoc model selection approach that entailed an extensive exploration of the model space, we ascertained the network structure at the group level. This process involved employing Bayesian model reduction to assess nested PEB models. Using Bayesian parameter averaging, we estimated the probabilities and magnitudes associated with each connectivity parameter. Additionally, we determined the magnitudes and effects related to the modulated connections. We established significant connectivity parameters in the current study using a posterior probability threshold of Pp>0.99.
GFA
We applied the GFA method to uncover latent variables that reveal relationships among distinct variable groups while adhering to a sparsity constraint (Klami et al., 2015). GFA relies on sparse Bayesian estimation to pinpoint latent factors that either expound upon group-specific variations or outline a robust relationship among different groups. In this study, we defined four variable groups: 1) Neural measures, 2) Psychologic measures, 3) Behavioral measures, and 4) Demographic measures. For the neural measures, significant effective connectivity parameters derived from the DCM analysis within three identified regions of interest (amygdala, dlPFC, PCC) were used as constituents of the neural GFA group. Six subscale scores across two self-report questionnaires (DASS subscales encompassing depression, anxiety, stress, and BIS subscales encapsulating attention, motor, and non-planning) were entered as a group for psychological measures.
In contrast, the behavioral group consisted of measurements taken before and after fMRI, encompassing craving intensity and positive and negative scores on the PANAS. Finally, demographic measures were represented by three scores (age, education, and marital status), forming a distinct GFA group. To ensure compatibility with GFA, all variables underwent z-normalization, thereby attaining a mean of zero and a variance of one. This standardized format aligns with the requirements of GFA. We conducted the sparse Bayesian estimation process in ten iterations to mitigate the potential of identifying spurious latent factors. Only the factors that displayed robustness across all ten replicates of GFA were then extracted and deemed suitable for subsequent analysis (Ghobadi-Azbari et al., 2022; Peng et al., 2021; White et al., 2021).
We utilized the Pearson correlation to assess the bivariate relationships between behavioral and neural variables as an additional approach to exploring neurobehavioral associations. The GFA and Pearson correlation were conducted using the statistical software R. The GFA analysis was executed using the ‘gfa’ function within the R software, specifically with the R package GFA (Leppäaho et al., 2017; Team, 2020).
3. Results
Behavioral data
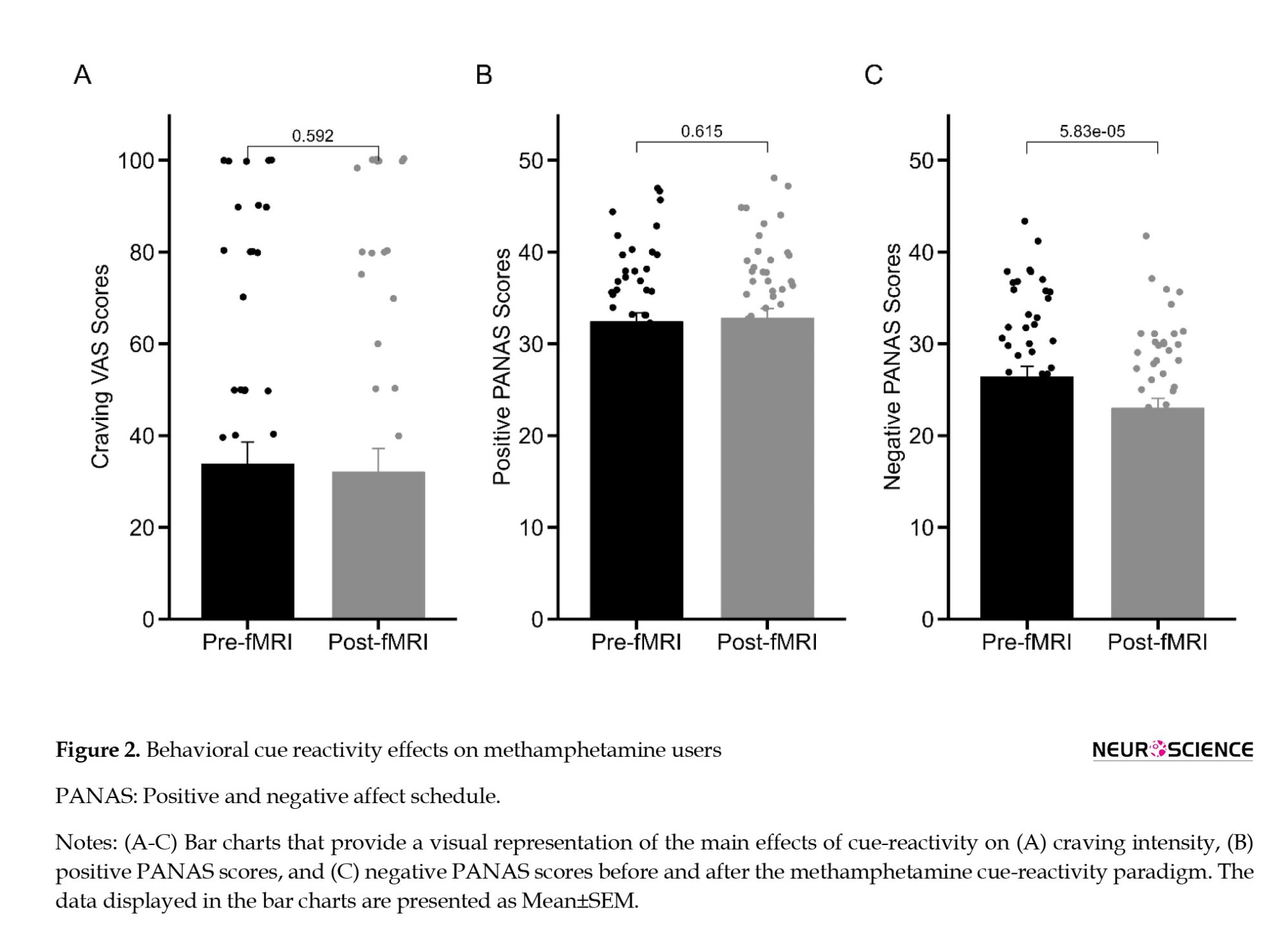
To investigate the behavioral effect of cue reactivity on positive and negative emotional states, as assessed by the PANAS scale and craving intensity, we conducted a comparative analysis by directly comparing data collected before and after the fMRI session. After the completion of the fMRI session, participants did not exhibit a statistically significant reduction in self-reported craving intensity (t53=-0.54, P=0.592; Figure 2A), and there was no notable increase observed in PANAS-PA scores (t53=0.51, P=0.615; Figure 2B) when compared to their pre-scanner state. Nevertheless, a substantial and statistically significant alteration emerged in PANAS-NA scores following the cue-reactivity paradigm (t53=-4.37, P<0.0001) (Figure 2C).
Functional activity analysis of methamphetamine cue reactivity
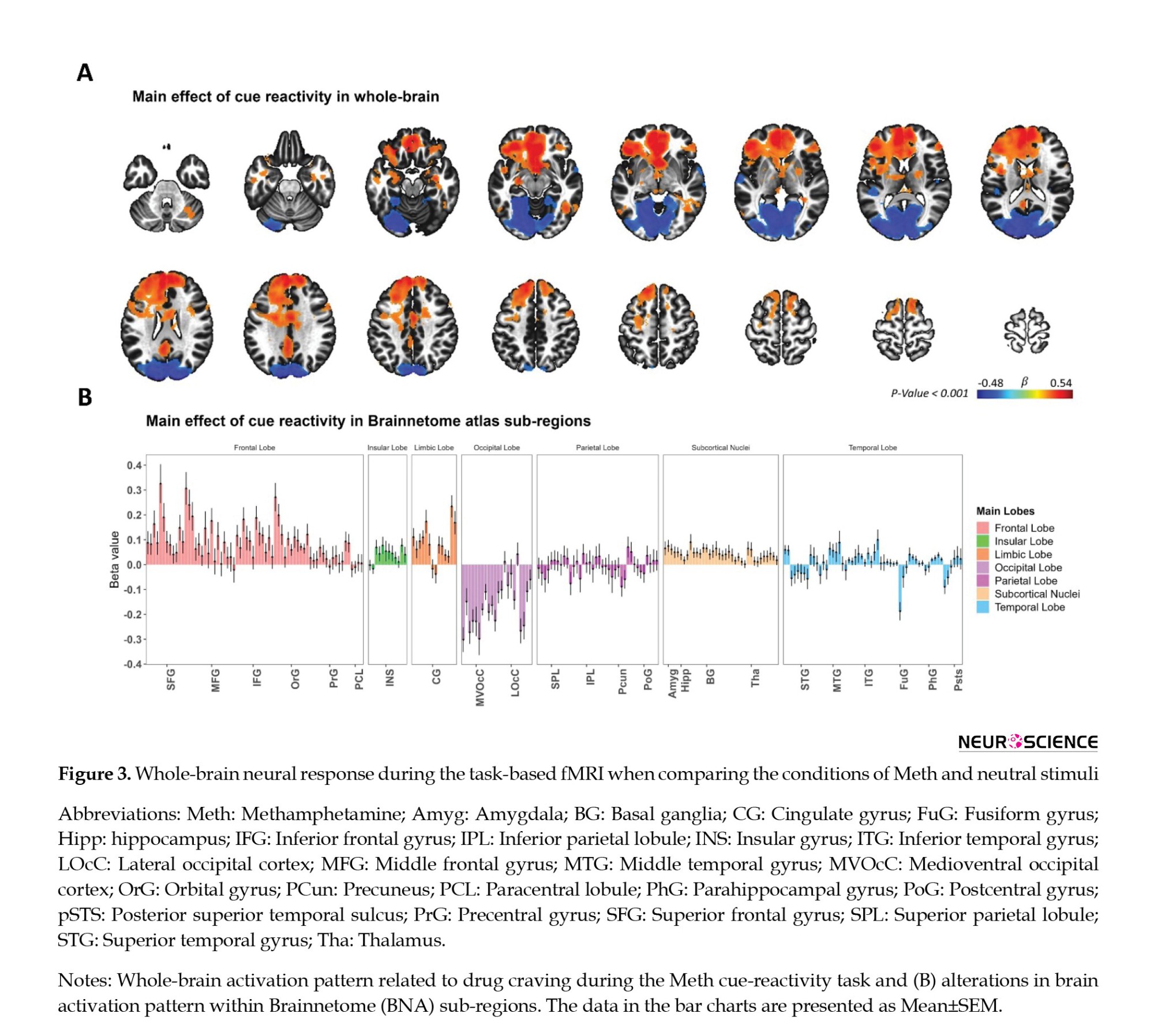
A whole-brain GLM analysis was performed as a quality control measure to validate the activation pattern linked to methamphetamine cue reactivity. This analysis included methamphetamine and neutral cues as fixed regressors, as depicted in Figure 3A. The main effect of cue reactivity (methamphetamine > neutral contrast) yielded significance in multiple clusters, employing a voxel-wise P threshold of P<0.001 and a minimum cluster size of k=40. These clusters included regions in the middle orbital gyrus, superior medial gyrus, posterior cingulate cortex, caudal lingual gyrus, and cerebellum cortex. Furthermore, we presented the brain activation findings across the 246 subregions outlined in the human Brainnetome atlas, as illustrated in Figure 3B.
Functional connectivity analysis of methamphetamine cue reactivity
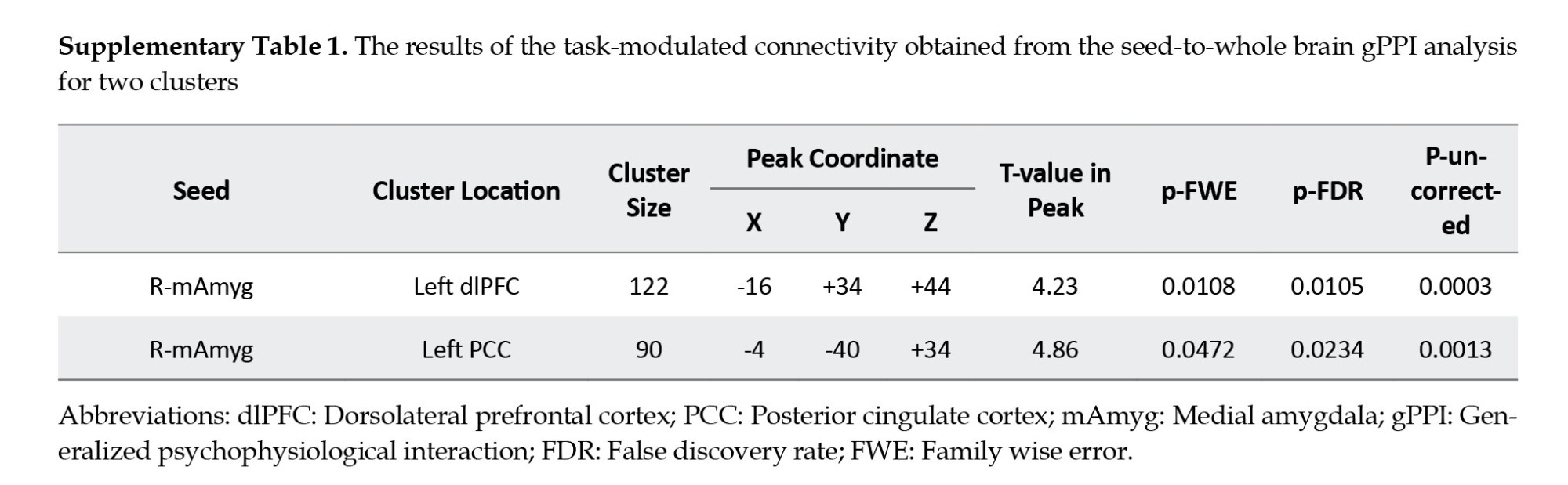
We utilized seed-to-whole brain gPPI analysis to pinpoint target regions exhibiting functional connectivity with the source region, specifically the right medial amygdala (coordinates: X=19, y=-2, z=-19; Figure 4A). In fact, we examined how this connectivity was modulated by drug cue reactivity during the methamphetamine > neutral condition. The gPPI analysis unveiled that task-modulated connectivity was statistically significant in two clusters. This outcome was determined using a voxel-level threshold of uncorrected P<0.001 and a cluster-size threshold of FDR-corrected P<0.05, as depicted in Figure 4B. These clusters are located in the dlPFC (peak at MNI coordinate: -16, 34, 44; 122 voxels; p-FWE=0.01) and the PCC (peak at MNI coordinate: -4, -40, 34; 90 voxels; p-FWE=0.05) (Figure 4C and supplementary Table 1). Our results showed increased PPI strength within the amygdala-dlPFC and amygdala-PCC functional networks during the cue reactivity task in the methamphetamine condition (amygdala-dlPFC: 0.042±0.009; amygdala-PCC: 0.035±0.008; Mean±SE) compared to the neutral condition (amygdala-dlPFC: -0.005±0.011, amygdala-PCC: -0.004±0.009; Mean±SE) (Figures 4D and 4E).
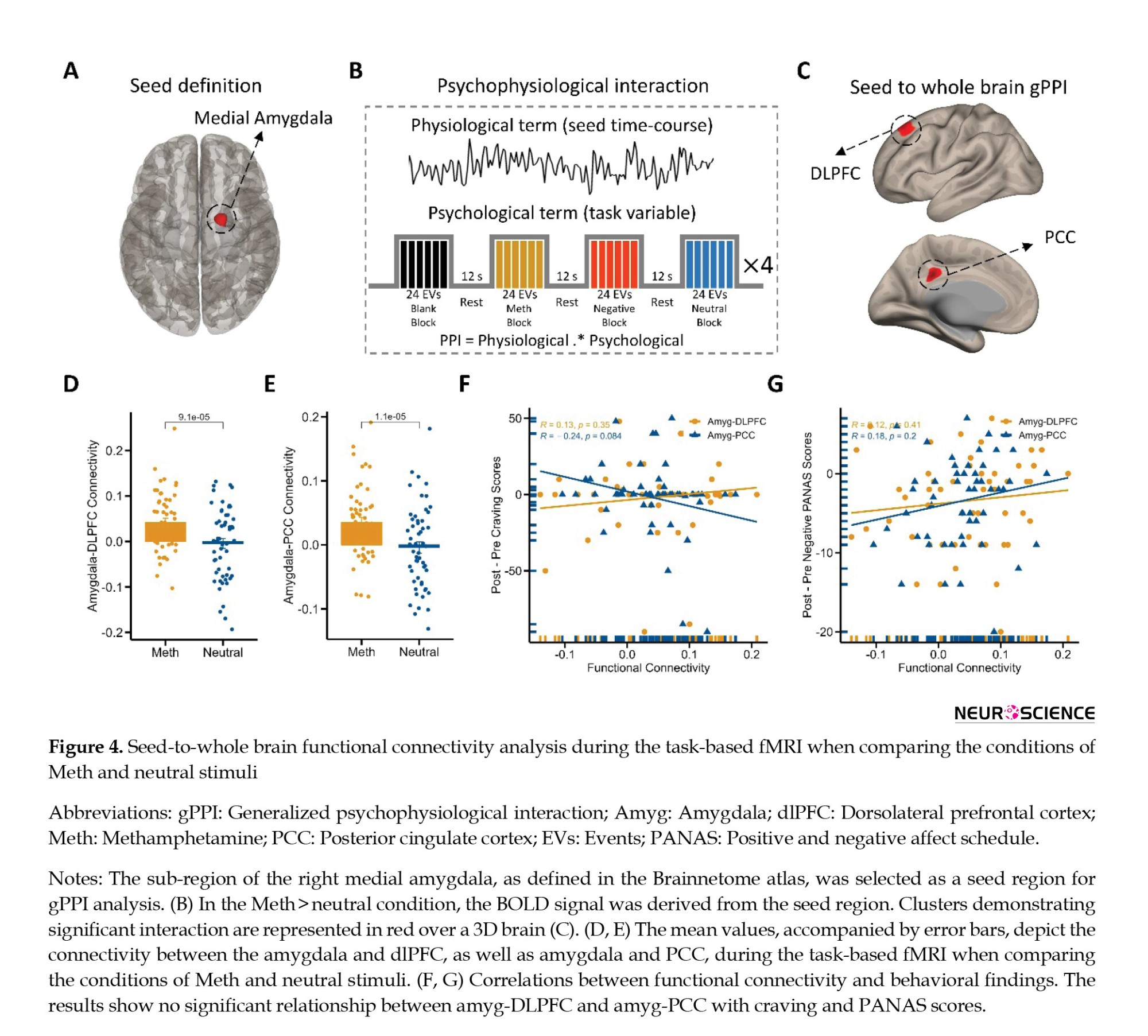
Next, we tested for bivariate correlations between behavioral effects (self-reported craving and positive and negative emotional states) and neural response (amygdala-dlPFC and amygdala-PCC PPI connectivity during cue exposure). Therefore, we tested whether individual estimated functional connectivity parameters are directly associated with behavioral cue-reactivity effects ([post–pre] craving and [post-pre] positive/negative PANAS). Interestingly, behavioral findings did not correlate with functional connectivity on amygdala-dlPFC and amygdala-PCC connections (Figures 4F and 4-G). For example, the individual functional connectivity in the amygdala-dlPFC did not correlate with craving intensity (R=0.13; P=0.35; Figure 4F) and negative PANAS changes (R=0.12; P=0.41; Figure 4G).
Effective connectivity of methamphetamine cue reactivity
Building upon these results, we aimed to examine whether the observed changes in functional connectivity could be validated through effective connectivity analysis. Our functional connectivity analysis determined whether cue reactivity influences forward, backward, or bidirectional projections between the amygdala, dlPFC, and PCC. For this purpose, we applied dynamic causal modeling to the adjusted BOLD time series data originating from the amygdala, dlPFC, and PCC. Using model selection, we established a full model for each participant (Friston et al., 2016). This model encompassed all conceivable modulatory inputs from craving on intrinsic self-connections and visual stimuli acting as driving inputs into the nodes (Figure 5A).
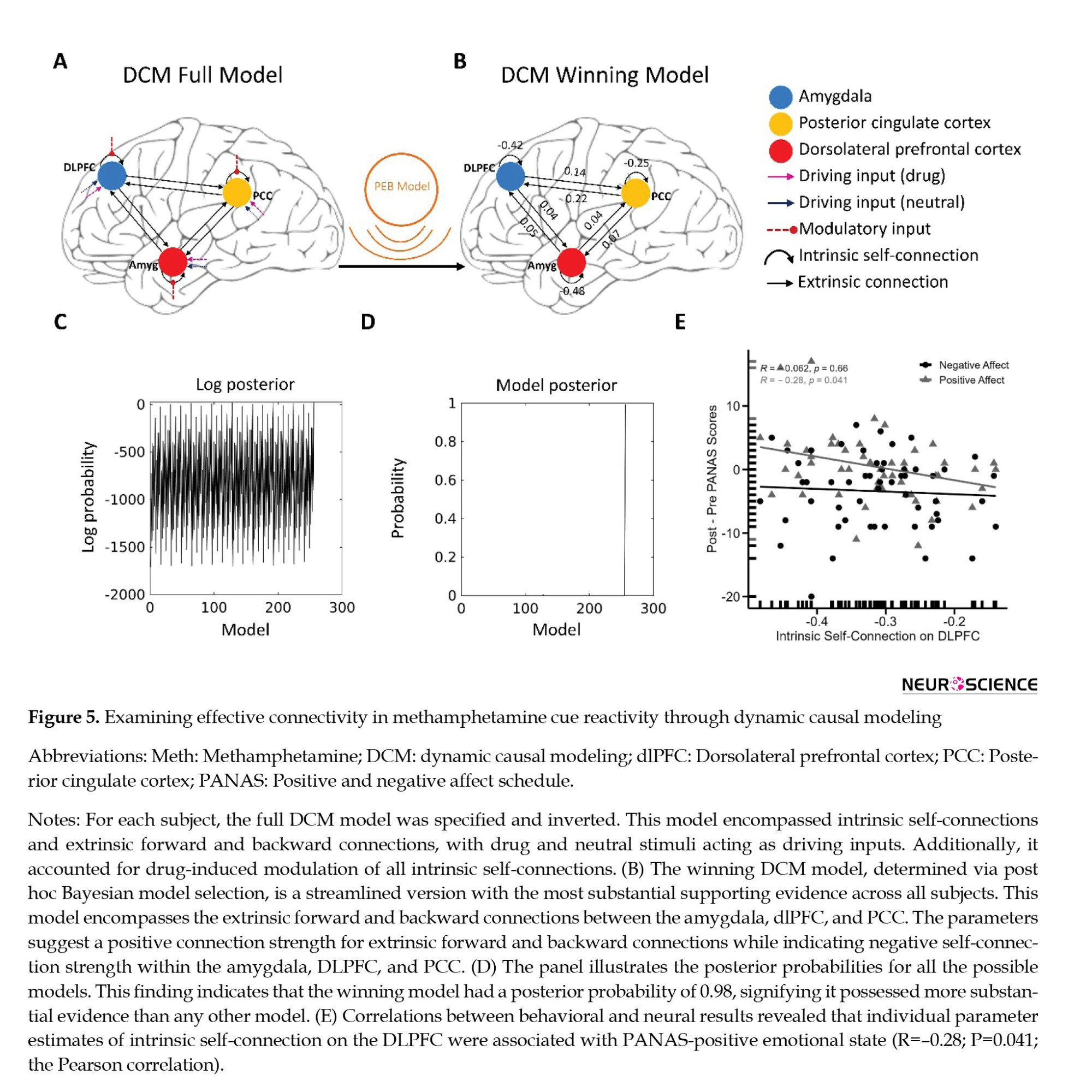
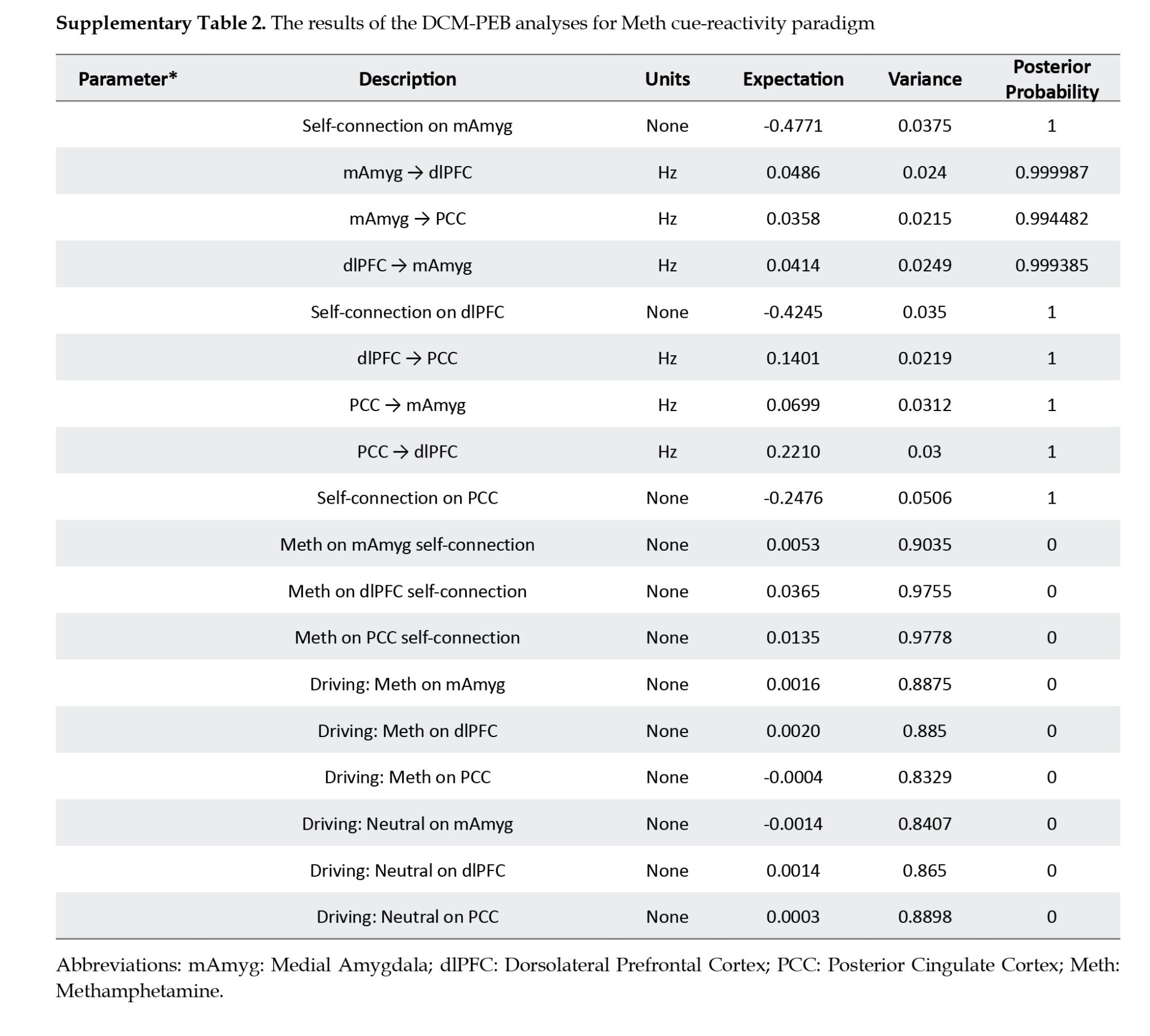
Regarding the Bayesian model selection (Friston et al., 2016) dynamic causal models – and linear models at subsequent (between-subject, we determined the model with the strongest evidence by assessing the data for all potential nested PEB models (Figures 5B, 5C and 5D). The winning model featured an effective neural network characterized by reciprocal positive connections between the amygdala and dlPFC, amygdala, and PCC, as well as dlPFC and PCC. Additionally, it included negative intrinsic connections within the three nodes (Figure 5B and Supplementary Table 2).
We tested whether behavioral effects (craving and positive and negative emotional states) are mediated by estimated significant effective connectivity. Hence, we examined whether the individual effective connectivity parameters from the winning model were directly correlated with behavioral cue reactivity effects, specifically the differences between post- and pre-measurements of craving and positive/negative PANAS scores. The correlational analyses unveiled a noteworthy negative association (R=-0.28; P=0.041, n=54, the Pearson correlation). This finding suggests that the inhibitory intrinsic connection of the dlPFC was predictive of an increase in PANAS positive affect values during cue reactivity (Figure 5E). It is worth noting that behavioral findings did not exhibit any correlation with cue reactivity effects on other intrinsic and extrinsic connections.
Relationships between neural, psychological, behavioral, and demographic variables
The GFA extracted 9 sturdy latent variables (Figure 6), collectively accounting for roughly 38.53% of the variance across variable groups. Notably, no robust cross-unit latent factors were discerned between the neural, psychological, behavioral, and demographic variable groups. In simpler terms, the GFA did not reveal any meaningful association or alignment between the neural group and the behavioral, psychological, and demographic variable groups within the latent variable space.
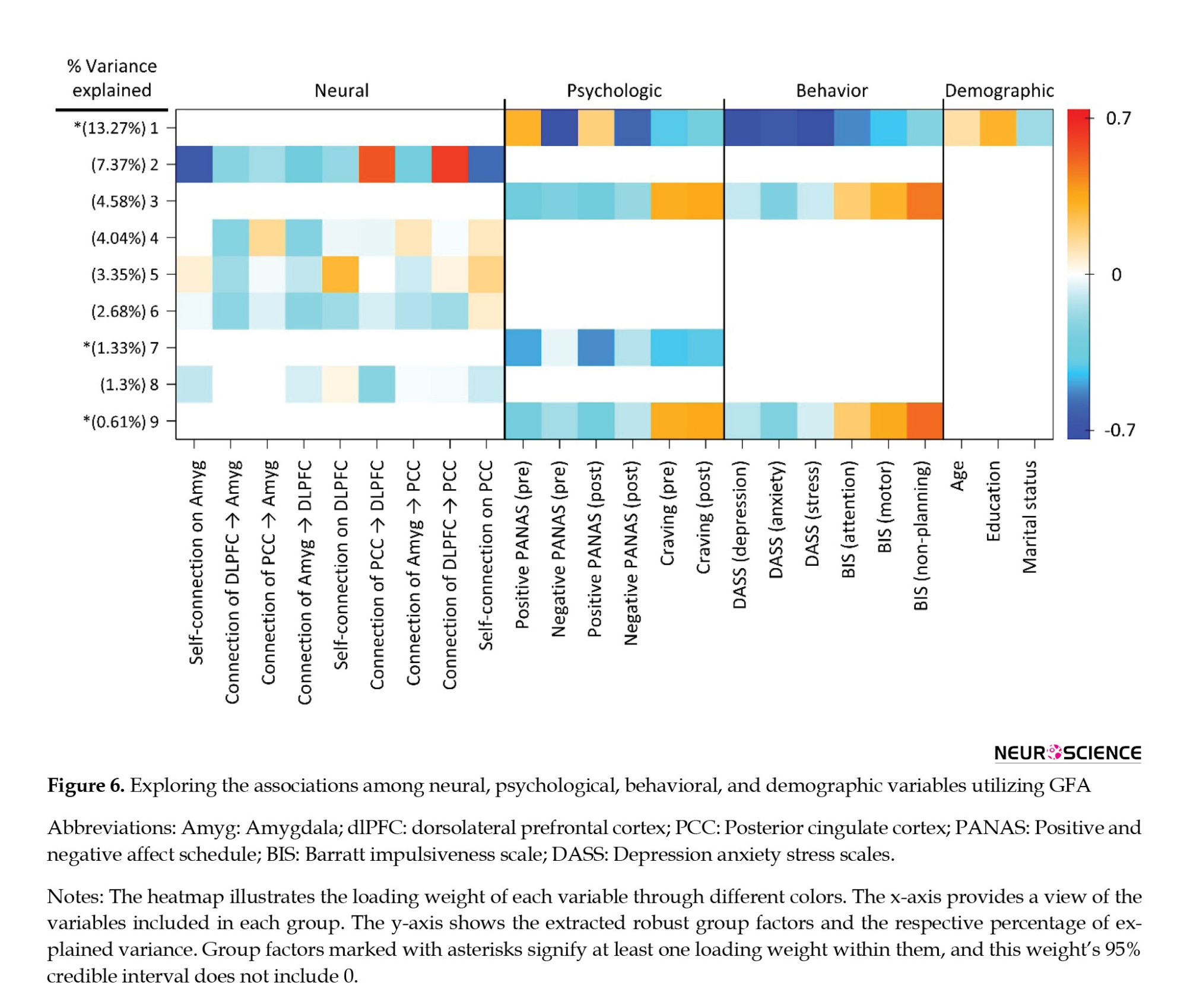
On the contrary, the GFA detected a robust latent factor that exhibited loadings across units of analysis in behavioral, psychological, and demographic domains. Nevertheless, the explained mean-variance differed at 20.05%, 30.88%, and 4.32% within the behavioral, psychological, and demographic variable groups, respectively. Hence, although this latent factor technically encompassed loadings across behavioral, psychological, and demographic levels of analysis, it was primarily influenced by the behavioral and psychological variables, accounting for most of the explained variance.
4. Discussion
The current study delved into the intricate neural mechanisms underlying methamphetamine cue reactivity by examining functional and effective connectivity in the amygdala-cortical pathways. The research aimed to uncover the underlying neural dynamics contributing to drug craving and addiction-related processes in individuals with MUD (Grodin et al., 2019). Our discussion will focus on the implications of the findings, their alignment with existing literature, and their potential contributions to understanding addiction and informing interventions.
Functional connectivity and methamphetamine cue reactivity
The application of seed-to-whole brain gPPI analysis offered valuable insights into the functional interactions between the amygdala and other brain regions when exposed to drug cues. The observed increase in task-modulated connectivity within the amygdala-dlPFC and amygdala-PCC functional networks during methamphetamine cue reactivity supports the notion that these regions play a pivotal role in processing drug-related stimuli. These results are in line with prior research indicating that the dlPFC and PCC play a crucial role in cognitive control processes, emotional regulation, and cue-induced craving in addiction contexts (Goldstein & Volkow, 2002, 2011; Jia et al., 2011; Sinha, 2001). The observed heightened connectivity within the amygdala-dlPFC and amygdala-PCC functional networks during methamphetamine cue reactivity tasks supports the engagement of these regions in the processing of drug-related cues. This engagement potentially contributes to the escalation of craving and the risk of relapse, aligning with the idea that the amygdala holds a central role in encoding emotional significance (Janak & Tye, 2015; Luo et al., 2013; Šimić et al., 2021).
Effective neural pathways and methamphetamine cue reactivity
Dynamic causal modeling offers a more profound comprehension of the effective neural pathways that underlie methamphetamine cue reactivity. The established effective neural network, including reciprocal positive connections between the amygdala-dlPFC, amygdala-PCC, and dlPFC-PCC, aligns with theories highlighting the interplay between emotional processing and cognitive control systems in addiction. The existence of negative intrinsic connections among these nodes further highlights the complexity of the neural network involved in processing drug cues, potentially reflecting an intricate modulatory mechanism to counteract excessive responses to drug cues (Rolls, 2019). These results align with existing literature emphasizing the coordinated activity between the prefrontal cortex, cingulate cortex, and amygdala, mediating cue-induced responses and cognitive control (Etkin et al., 2015; Sotres-Bayon & Quirk, 2010).
Neurobehavioral relationships
The correlation analysis revealed a negative association between intrinsic self-inhibition in the dlPFC and PANAS-positive emotional state. This finding suggests a potential role for dlPFC inhibitory processes in modulating positive affect during methamphetamine cue reactivity. This finding aligns with theories emphasizing the interplay between cognitive control mechanisms and emotional regulation in substance use disorders (Lanteaume et al., 2007; Ochsner & Gross, 2005; Volkow et al., 2016). While this result contributes to our understanding of the intricate relationship between neural connectivity and emotional responses, further research is necessary to elucidate the underlying mechanisms and potential clinical implications.
In contrast to the robust neural connectivity findings, the absence of significant cross-unit latent factors between the functional connectivity patterns and behavioral, psychological, or demographic variables in the GFA analysis is intriguing. This outcome might indicate that the observed alterations in neural connectivity do not have straightforward translations into measurable relationships with these external factors. This discrepancy could be attributed to the multifaceted nature of substance use disorders, which are influenced by a myriad of interacting factors.
Integration with previous research
The present study builds upon a growing body of research that highlights the intricate interplay between brain regions in addiction. The observed connectivity changes in response to methamphetamine cues resonate with previous studies investigating cue reactivity across different substances of abuse. The convergence of findings regarding the involvement of the amygdala, dlPFC, and PCC underscores their roles as core components in the addiction process, supporting the notion of a broader addiction-related neural network.
Clinical implications
Understanding the neural mechanisms underpinning methamphetamine cue reactivity holds significant clinical implications. The identified networks and connections offer potential targets for intervention strategies to mitigate craving and prevent relapse. Interventions targeting the amygdala-cortical circuitry could be designed to enhance cognitive control (Sadeghi et al., 2023) over drug-related cues and emotions, potentially aiding in the management of addiction (Soleimani et al., 2023).
5. Conclusion
In summary, this research enriches our understanding of addiction by investigating the amygdala-cortical connectivity and effective neural pathways associated with methamphetamine cue reactivity. Integrating functional and effective connectivity analyses and neurobehavioral assessments provides a comprehensive picture of the underlying neural dynamics. These findings offer insights into the mechanisms driving addiction-related processes and potential avenues for targeted interventions. As addiction continues to pose a significant public health challenge, these insights may pave the way for innovative strategies aimed at improving treatment outcomes and reducing the burden of addiction on affected individuals and society.
Limitations and future studies
Although the study offers valuable insights, it is essential to acknowledge its limitations. The sample consisted solely of male participants with MUD, potentially limiting the generalizability of the findings. Future studies could include diverse samples and consider the impact of gender and other demographic variables. Additionally, the study's cross-sectional nature restricts our capacity to establish causal relationships between functional connectivity patterns and addiction-related outcomes. Longitudinal studies could shed light on the trajectory of neural changes and their predictive value.
Ethical Considerations
Compliance with ethical guidelines
The Declaration of Helsinki was followed in the design and execution of the study procedure. This study was reviewed and approved by the Ethics Committee of Research at Tehran University of Medical Science, Tehran, Iran (IR.TUMS.REC.93-02-98-23869). Participants in this study provided their written informed consent to participate.
Funding
The study was supported by a research grant from AJA University of Command and Staff, Tehran, Iran.
Authors' contributions
Conceptualization and methodology: Peyman Ghobadi-Azbari, and Yasaman Hosseini; Investigation: All authors; Formal analysis, and visualization: Peyman Ghobadi-Azbari; Writing the original draft: Peyman Ghobadi-Azbari; Review, and editing: Yasaman Hosseini, Hossein Moslemi, and Mitra Yousefpour; Supervision: Yasaman Hosseini and Hossein Moslemi.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Methamphetamine use disorder (MUD) continues to be a substantial public health concern, with its profound impact on individuals' physical, psychological, and social well-being (Courtney & Ray, 2014; Hedegaard et al., 2018; Paulus & Stewart, 2020a). Understanding the neural mechanisms that underlie the complex process of addiction is paramount for developing targeted interventions and treatment strategies (Paulus & Stewart, 2020b; Soleimani et al., 2023; Soleimani et al., 2023). Neuroimaging methods, including functional magnetic resonance imaging (fMRI), provide a distinct perspective on the complex operations of the addicted brain, offering insights into the neural networks and pathways that influence drug-related behaviors (Ekhtiari et al., 2022; Jan et al., 2012; Koob & Volkow, 2016; Parvaz et al., 2011; Stewart et al., 2014).
Amygdala, a crucial node in the brain's emotion processing and reward systems, plays a central role in the addiction process (Everitt et al., 1999; Phelps & LeDoux, 2005). Its interactions with various cortical regions, particularly the prefrontal cortex, are instrumental in the emergence of addictive behaviors, including craving and cue reactivity (Goldstein & Volkow, 2011; Zilverstand et al., 2018). Given the amygdala's involvement in processing emotionally salient stimuli and its established relevance to addiction, investigating its connectivity with cortical regions during methamphetamine cue exposure is of paramount importance (Soleimani et al., 2023).
The present study seeks to address this crucial gap in knowledge by utilizing an fMRI approach to explore amygdala-cortical connectivity and effective neural pathways involved in methamphetamine cue reactivity. By employing a combination of task-based fMRI paradigms and advanced connectivity analyses, we aim to shed light on how neural networks interplay in response to methamphetamine cues, providing a more comprehensive understanding of addiction's neurobiological underpinnings.
Our primary objective is to investigate the modulation of amygdala-cortical connectivity during methamphetamine cue exposure compared to neutral cues. Our hypothesis posits that exposure to drug-related cues will increase connectivity between the amygdala and regions associated with reward processing and cognitive control, such as the prefrontal cortex. This investigation will offer valuable insights into the brain's dynamic response to cues that trigger drug craving, unraveling the mechanisms that facilitate the transition from cue exposure to craving and potential relapse.
Furthermore, this study seeks to delve beyond functional connectivity by exploring effective neural pathways using dynamic causal modeling (DCM). This modeling enables the investigation of directed interactions between brain regions, shedding light on the neural network's causal relationships and information flow. By applying DCM to our multimodal fMRI data, we aim to uncover the directional influences and effective pathways that underlie amygdala-cortical interactions during methamphetamine cue reactivity.
In addition to the neural perspectives, this study will explore potential correlations between neural connectivity patterns and psychological variables. We will utilize the Pearson correlation and group factor analysis (GFA) to elucidate potential neuro-behavioral relationships, bridging the gap between brain connectivity and the subjective experiences associated with drug cue reactivity.
In the subsequent sections, we will comprehensively overview the research methodology, findings, and implications. This study aims to contribute to the growing body of knowledge concerning addiction's neurobiological underpinnings by integrating advanced neuroimaging techniques, connectivity analyses, and psychological assessments. Ultimately, our findings may pave the way for more targeted interventions that address the neural circuitry involved in methamphetamine cue reactivity and addiction-related processes.
2. Materials and Methods
Study participants
The present study involved 62 male volunteers diagnosed with MUD. Thirteen subjects were excluded from the study due to non-compliance with the inclusion instructions, as revealed in their screening assessments. The participants were sourced from addiction treatment centers. The exclusion criteria included current or previous comorbid axis I-disorders other than drug dependence, as outlined by the diagnostic and statistical manual of mental disorders, the fourth edition, text revision (DSM-IV-TR) (APA, 2000), psychiatric or neurological disorders, including head trauma, as well as MR-specific exclusion criteria. The inclusion criteria stipulated a diagnosis of methamphetamine dependence for a minimum of 6 months in accordance with DSM-IV-TR criteria. Additionally, participants were required to self-report and test negative for all drugs (except nicotine) in both urine and self-reports for a minimum of one week. Participants’ right-handedness was assessed using the Edinburgh handedness inventory (Oldfield, 1971), and inclusion criteria also specified an age range of 20 to 45 years. The visual acuity of each participant was normal or corrected to normal.
Sixty-two subjects met all the inclusion and exclusion criteria. Nevertheless, 8 participants were excluded from the data analysis due to excessive head motion during scanning. Consequently, the final sample for the analyses comprised 54 male participants diagnosed with MUD, aged 22 to 44 years (Mean±SD 32.02±5.09). Comprehensive behavioral and demographic information about methamphetamine users is provided in Table 1.

Experimental procedures
During the screening session, participants diagnosed with MUD underwent a series of baseline assessments. These assessments encompassed demographic information (Ranaei et al., 2022), substance use patterns, mental health evaluations, treatment history, and a risky behaviors profile. This profile included a history of drug injection, engagement in high-risk sexual activities, prior incarcerations, participation in drug sales, and a record of violent altercations. Furthermore, participants completed the Barratt impulsiveness scales-11 (BIS-11) (Barratt, 1994) and the depression anxiety stress scales-21 (DASS-21) (Hosseini et al., 2023; Hosseini & Modarresi Chahardehi, 2021; Osman et al., 2012). They also underwent a training session to familiarize themselves with the drug cue reactivity task before the MRI scan day (Figure 1A).
After successful screening, participants underwent a single fMRI session. Participants arrived for the MRI scans between 8:30 and 10:30 AM on the designated day after abstaining from all drugs (except nicotine) for at least one week. After participants finished self-assessments of their current drug craving using a 0–100 visual analog scale (VAS) and evaluations of positive and negative emotional states using the positive and negative affect schedule (PANAS) (Crawford & Henry, 2004), they proceeded to engage in the methamphetamine cue-reactivity paradigm within the fMRI scanner. After the scans were concluded, the participants were asked to provide another round of ratings for their level of drug craving and assess their positive and negative emotional states (Figure 1A). To reduce the likelihood of post-scanning drug use, participants were recommended to stay at the imaging center for at least one hour for recovery.

Functional methamphetamine cue reactivity paradigm
The methamphetamine cue reactivity paradigm was developed to investigate the brain's functional responses to methamphetamine compared to neutral stimuli (Dakhili et al., 2022; Ekhtiari et al., 2022; Jafakesh et al., 2022) The experiment comprised four runs of the fMRI drug cue reactivity task, with intervals of rest blocks featuring a fixation point. Each run had four blocks lasting 36 seconds each, and each block contained 24 images. The images included blank screens, negative cues, methamphetamine cues, and neutral cues. The stimuli were displayed for 1000 ms, followed by an average inter-stimulus interval of 500 ms. The order of block presentations was pseudo-randomized across the four runs, and after each block, there was a 12-second inter-block interval. Each run lasted approximately 198 s, with 18-s fixation intervals separating the runs. In these fixation periods, a white cross was shown on a black background (Figure 1B). Before the scanning session, participants received training to acquaint themselves with the fMRI environment and the cue reactivity paradigm. The negative and neutral cues were selected from the international affective picture system database (Lang et al., 1997). In contrast, the drug-related cues were sourced from a publicly available and validated cue database (Ekhtiari et al., 2020).
MRI data acquisition
We used a SIEMENS 3.0T scanner (MAGNETOM Trio, SIEMENS, Germany) with a 64-channel head coil to acquire T1 and fMRI sequences. fMRI images were obtained from a T2*-weighted gradient-echo echoplanar imaging (EPI) sequence. Each volume of functional data comprised 40 slices, featuring a repetition time (TR) of 2200 ms, an echo time (TE) of 30 ms, and a flip angle of 90°. The field of view (FOV) was configured to 192×192 mm, and the voxel dimensions were 3×3×3 mm. A total of 367 T2*-weighted functional images were obtained in an interleaved slice acquisition order.
Furthermore, a structural image was obtained using the magnetization-prepared rapid acquisition gradient echo sequence. This sequence parameters were set as follows: TR=1800 ms, TE=3.44 ms, flip angle=7°, FOV=256×256 mm, and voxel size=1×1×1 mm.
Functional MRI data preprocessing
The fMRI data was subjected to preprocessing through the utilization of the CONN toolbox (version 20.b) (Whitfield-Gabrieli & Nieto-Castanon, 2012) in SPM12 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom). The standard preprocessing pipeline involved several steps: functional realignment and unwarp, slice-timing correction, outlier identification, direct segmentation, and normalization into the standard Montreal Neurological Institute (MNI) space. Additionally, functional smoothing was applied using an 8 mm full-width at half-maximum Gaussian kernel.
Task-based functional brain activity analysis
The preprocessed fMRI data underwent a general linear model (GLM) analysis. This model was constructed by modeling onset times for the methamphetamine, neutral, negative, and blank conditions using a 36-second block function. These onset times were convolved with a canonical hemodynamic response function to generate the corresponding regressors of interest. Moreover, the six individual motion correction parameters were included as nuisance regressors in the first-level model.
In each participant's case, contrast images illustrating the comparison between the methamphetamine and neutral conditions were subsequently incorporated into second-level one-sample t-test models, all of which were executed using SPM12. The individual contrast maps were initially thresholded at P<0.05 (uncorrected). On the other hand, the whole-brain statistical group maps underwent correction for family-wise error (FWE) to address multiple comparisons. This correction was accomplished using Gaussian random field theory (Nichols & Hayasaka, 2003; Worsley et al., 1996). Furthermore, Brainnetome atlas parcellation was applied to each subject's data. This allowed for the estimation of the average BOLD signal change across the 246 sub-regions present in the atlas. Notably, the specific sub-region of the right medial amygdala, identified through the Brainnetome atlas-based parcellation of the fMRI data, was chosen as the seed region for the ensuing seed-to-whole brain generalized psychophysiological interaction (gPPI) analysis. This analysis aimed to uncover modulations in connectivity influenced by drug cue reactivity.
Task-based functional brain connectivity analysis
The task-based functional brain connectivity analysis was conducted using the CONN toolbox (version 20.b) (Whitfield-Gabrieli & Nieto-Castanon, 2012) integrated within SPM12. A seed-to-whole brain approach was employed in this analytical framework, utilizing gPPI analysis. In this context, the right medial amygdala was chosen as the specified seed region. The primary objective of the PPI analysis was to reveal specific brain regions exhibiting connectivity patterns that demonstrated variability based on the psychological context, with a specific focus on the differential response to methamphetamine stimuli in comparison to neutral stimuli.
At the first level of the gPPI analysis, the psychological regressors of interest encompassed the timing of both methamphetamine and neutral blocks. These timings were convolved with a hemodynamic response function. The physiological regressor was then computed using the average time series derived from the designated seed region, specifically the right medial amygdala, as outlined in the Brainnetome mask. The PPI regressors were constructed as interaction terms involving psychological and physiological regressors. Through this approach, we contrasted the PPI regressors, identifying brain regions where connectivity with the medial amygdala demonstrated significant differences between the methamphetamine and neutral conditions. These PPI regressors were computed for each participant, revealing the voxel-level interaction with the seed region while comparing the methamphetamine and neutral conditions.
Subsequently, a second-level gPPI analysis was conducted to detect significant clusters. This analysis utilized voxel-wise and cluster-extent thresholds, where clusters were deemed active if they exceeded a voxel-level threshold of uncorrected P<0.001 and a cluster-size threshold corrected for false discovery rate (FDR) at P<0.05. The regions demonstrating the most robust PPI connectivity with the amygdala region were subsequently selected as regions of interest for the parametric empirical Bayes (PEB)-DCM analysis. To this end, functional regions of interest (ROIs) with 8 mm spheres were centered on the peak voxels within the amygdala (coordinates: 19, -2, -19), the dlPFC (coordinates: 16, 34, 44), and the PCC (coordinates: -4, -40, 34). These coordinates were derived from our gPPI outcomes concerning cue-reactivity processing.
Task-based dynamic causal modelling
The analysis of task-based effective brain connectivity over the amygdala, dlPFC, and PCC brain regions was conducted utilizing the DCM toolbox integrated within SPM12. The principal eigenvariate time series were derived from predefined unilateral masks of these regions, with adjustments for effects of interest. To explore the effective functional connectivity between these regions, we employed the bilinear DCM approach (Friston et al., 2003), incorporating the stochastic option (Daunizeau et al., 2012; Friston et al., 2011; Li et al., 2011). A full DCM model was constructed, encompassing three components: 1) Fixed connections encompass the extrinsic forward and backward connections between the amygdala and dlPFC, amygdala and PCC, and dlPFC and PCC, as well as intrinsic self-connections within each region, 2) Contextual modulation involves the adjustment of intrinsic self-connections influenced by craving, and 3) exogenous inputs refer to the introduction of methamphetamine and neutral stimuli as driving inputs into the respective nodes. Bayesian model inversion was carried out for each subject, entailing the establishment and inversion of this parent DCM model.
In the group-level DCM analysis, we performed a linear PEB analysis on a fully connected model estimated across all participants. By utilizing a post hoc model selection approach that entailed an extensive exploration of the model space, we ascertained the network structure at the group level. This process involved employing Bayesian model reduction to assess nested PEB models. Using Bayesian parameter averaging, we estimated the probabilities and magnitudes associated with each connectivity parameter. Additionally, we determined the magnitudes and effects related to the modulated connections. We established significant connectivity parameters in the current study using a posterior probability threshold of Pp>0.99.
GFA
We applied the GFA method to uncover latent variables that reveal relationships among distinct variable groups while adhering to a sparsity constraint (Klami et al., 2015). GFA relies on sparse Bayesian estimation to pinpoint latent factors that either expound upon group-specific variations or outline a robust relationship among different groups. In this study, we defined four variable groups: 1) Neural measures, 2) Psychologic measures, 3) Behavioral measures, and 4) Demographic measures. For the neural measures, significant effective connectivity parameters derived from the DCM analysis within three identified regions of interest (amygdala, dlPFC, PCC) were used as constituents of the neural GFA group. Six subscale scores across two self-report questionnaires (DASS subscales encompassing depression, anxiety, stress, and BIS subscales encapsulating attention, motor, and non-planning) were entered as a group for psychological measures.
In contrast, the behavioral group consisted of measurements taken before and after fMRI, encompassing craving intensity and positive and negative scores on the PANAS. Finally, demographic measures were represented by three scores (age, education, and marital status), forming a distinct GFA group. To ensure compatibility with GFA, all variables underwent z-normalization, thereby attaining a mean of zero and a variance of one. This standardized format aligns with the requirements of GFA. We conducted the sparse Bayesian estimation process in ten iterations to mitigate the potential of identifying spurious latent factors. Only the factors that displayed robustness across all ten replicates of GFA were then extracted and deemed suitable for subsequent analysis (Ghobadi-Azbari et al., 2022; Peng et al., 2021; White et al., 2021).
We utilized the Pearson correlation to assess the bivariate relationships between behavioral and neural variables as an additional approach to exploring neurobehavioral associations. The GFA and Pearson correlation were conducted using the statistical software R. The GFA analysis was executed using the ‘gfa’ function within the R software, specifically with the R package GFA (Leppäaho et al., 2017; Team, 2020).
3. Results
Behavioral data
To investigate the behavioral effect of cue reactivity on positive and negative emotional states, as assessed by the PANAS scale and craving intensity, we conducted a comparative analysis by directly comparing data collected before and after the fMRI session. After the completion of the fMRI session, participants did not exhibit a statistically significant reduction in self-reported craving intensity (t53=-0.54, P=0.592; Figure 2A), and there was no notable increase observed in PANAS-PA scores (t53=0.51, P=0.615; Figure 2B) when compared to their pre-scanner state. Nevertheless, a substantial and statistically significant alteration emerged in PANAS-NA scores following the cue-reactivity paradigm (t53=-4.37, P<0.0001) (Figure 2C).

Functional activity analysis of methamphetamine cue reactivity
A whole-brain GLM analysis was performed as a quality control measure to validate the activation pattern linked to methamphetamine cue reactivity. This analysis included methamphetamine and neutral cues as fixed regressors, as depicted in Figure 3A. The main effect of cue reactivity (methamphetamine > neutral contrast) yielded significance in multiple clusters, employing a voxel-wise P threshold of P<0.001 and a minimum cluster size of k=40. These clusters included regions in the middle orbital gyrus, superior medial gyrus, posterior cingulate cortex, caudal lingual gyrus, and cerebellum cortex. Furthermore, we presented the brain activation findings across the 246 subregions outlined in the human Brainnetome atlas, as illustrated in Figure 3B.

Functional connectivity analysis of methamphetamine cue reactivity
We utilized seed-to-whole brain gPPI analysis to pinpoint target regions exhibiting functional connectivity with the source region, specifically the right medial amygdala (coordinates: X=19, y=-2, z=-19; Figure 4A). In fact, we examined how this connectivity was modulated by drug cue reactivity during the methamphetamine > neutral condition. The gPPI analysis unveiled that task-modulated connectivity was statistically significant in two clusters. This outcome was determined using a voxel-level threshold of uncorrected P<0.001 and a cluster-size threshold of FDR-corrected P<0.05, as depicted in Figure 4B. These clusters are located in the dlPFC (peak at MNI coordinate: -16, 34, 44; 122 voxels; p-FWE=0.01) and the PCC (peak at MNI coordinate: -4, -40, 34; 90 voxels; p-FWE=0.05) (Figure 4C and supplementary Table 1). Our results showed increased PPI strength within the amygdala-dlPFC and amygdala-PCC functional networks during the cue reactivity task in the methamphetamine condition (amygdala-dlPFC: 0.042±0.009; amygdala-PCC: 0.035±0.008; Mean±SE) compared to the neutral condition (amygdala-dlPFC: -0.005±0.011, amygdala-PCC: -0.004±0.009; Mean±SE) (Figures 4D and 4E).

Next, we tested for bivariate correlations between behavioral effects (self-reported craving and positive and negative emotional states) and neural response (amygdala-dlPFC and amygdala-PCC PPI connectivity during cue exposure). Therefore, we tested whether individual estimated functional connectivity parameters are directly associated with behavioral cue-reactivity effects ([post–pre] craving and [post-pre] positive/negative PANAS). Interestingly, behavioral findings did not correlate with functional connectivity on amygdala-dlPFC and amygdala-PCC connections (Figures 4F and 4-G). For example, the individual functional connectivity in the amygdala-dlPFC did not correlate with craving intensity (R=0.13; P=0.35; Figure 4F) and negative PANAS changes (R=0.12; P=0.41; Figure 4G).
Effective connectivity of methamphetamine cue reactivity
Building upon these results, we aimed to examine whether the observed changes in functional connectivity could be validated through effective connectivity analysis. Our functional connectivity analysis determined whether cue reactivity influences forward, backward, or bidirectional projections between the amygdala, dlPFC, and PCC. For this purpose, we applied dynamic causal modeling to the adjusted BOLD time series data originating from the amygdala, dlPFC, and PCC. Using model selection, we established a full model for each participant (Friston et al., 2016). This model encompassed all conceivable modulatory inputs from craving on intrinsic self-connections and visual stimuli acting as driving inputs into the nodes (Figure 5A).

Regarding the Bayesian model selection (Friston et al., 2016) dynamic causal models – and linear models at subsequent (between-subject, we determined the model with the strongest evidence by assessing the data for all potential nested PEB models (Figures 5B, 5C and 5D). The winning model featured an effective neural network characterized by reciprocal positive connections between the amygdala and dlPFC, amygdala, and PCC, as well as dlPFC and PCC. Additionally, it included negative intrinsic connections within the three nodes (Figure 5B and Supplementary Table 2).
We tested whether behavioral effects (craving and positive and negative emotional states) are mediated by estimated significant effective connectivity. Hence, we examined whether the individual effective connectivity parameters from the winning model were directly correlated with behavioral cue reactivity effects, specifically the differences between post- and pre-measurements of craving and positive/negative PANAS scores. The correlational analyses unveiled a noteworthy negative association (R=-0.28; P=0.041, n=54, the Pearson correlation). This finding suggests that the inhibitory intrinsic connection of the dlPFC was predictive of an increase in PANAS positive affect values during cue reactivity (Figure 5E). It is worth noting that behavioral findings did not exhibit any correlation with cue reactivity effects on other intrinsic and extrinsic connections.
Relationships between neural, psychological, behavioral, and demographic variables
The GFA extracted 9 sturdy latent variables (Figure 6), collectively accounting for roughly 38.53% of the variance across variable groups. Notably, no robust cross-unit latent factors were discerned between the neural, psychological, behavioral, and demographic variable groups. In simpler terms, the GFA did not reveal any meaningful association or alignment between the neural group and the behavioral, psychological, and demographic variable groups within the latent variable space.

On the contrary, the GFA detected a robust latent factor that exhibited loadings across units of analysis in behavioral, psychological, and demographic domains. Nevertheless, the explained mean-variance differed at 20.05%, 30.88%, and 4.32% within the behavioral, psychological, and demographic variable groups, respectively. Hence, although this latent factor technically encompassed loadings across behavioral, psychological, and demographic levels of analysis, it was primarily influenced by the behavioral and psychological variables, accounting for most of the explained variance.
4. Discussion
The current study delved into the intricate neural mechanisms underlying methamphetamine cue reactivity by examining functional and effective connectivity in the amygdala-cortical pathways. The research aimed to uncover the underlying neural dynamics contributing to drug craving and addiction-related processes in individuals with MUD (Grodin et al., 2019). Our discussion will focus on the implications of the findings, their alignment with existing literature, and their potential contributions to understanding addiction and informing interventions.
Functional connectivity and methamphetamine cue reactivity
The application of seed-to-whole brain gPPI analysis offered valuable insights into the functional interactions between the amygdala and other brain regions when exposed to drug cues. The observed increase in task-modulated connectivity within the amygdala-dlPFC and amygdala-PCC functional networks during methamphetamine cue reactivity supports the notion that these regions play a pivotal role in processing drug-related stimuli. These results are in line with prior research indicating that the dlPFC and PCC play a crucial role in cognitive control processes, emotional regulation, and cue-induced craving in addiction contexts (Goldstein & Volkow, 2002, 2011; Jia et al., 2011; Sinha, 2001). The observed heightened connectivity within the amygdala-dlPFC and amygdala-PCC functional networks during methamphetamine cue reactivity tasks supports the engagement of these regions in the processing of drug-related cues. This engagement potentially contributes to the escalation of craving and the risk of relapse, aligning with the idea that the amygdala holds a central role in encoding emotional significance (Janak & Tye, 2015; Luo et al., 2013; Šimić et al., 2021).
Effective neural pathways and methamphetamine cue reactivity
Dynamic causal modeling offers a more profound comprehension of the effective neural pathways that underlie methamphetamine cue reactivity. The established effective neural network, including reciprocal positive connections between the amygdala-dlPFC, amygdala-PCC, and dlPFC-PCC, aligns with theories highlighting the interplay between emotional processing and cognitive control systems in addiction. The existence of negative intrinsic connections among these nodes further highlights the complexity of the neural network involved in processing drug cues, potentially reflecting an intricate modulatory mechanism to counteract excessive responses to drug cues (Rolls, 2019). These results align with existing literature emphasizing the coordinated activity between the prefrontal cortex, cingulate cortex, and amygdala, mediating cue-induced responses and cognitive control (Etkin et al., 2015; Sotres-Bayon & Quirk, 2010).
Neurobehavioral relationships
The correlation analysis revealed a negative association between intrinsic self-inhibition in the dlPFC and PANAS-positive emotional state. This finding suggests a potential role for dlPFC inhibitory processes in modulating positive affect during methamphetamine cue reactivity. This finding aligns with theories emphasizing the interplay between cognitive control mechanisms and emotional regulation in substance use disorders (Lanteaume et al., 2007; Ochsner & Gross, 2005; Volkow et al., 2016). While this result contributes to our understanding of the intricate relationship between neural connectivity and emotional responses, further research is necessary to elucidate the underlying mechanisms and potential clinical implications.
In contrast to the robust neural connectivity findings, the absence of significant cross-unit latent factors between the functional connectivity patterns and behavioral, psychological, or demographic variables in the GFA analysis is intriguing. This outcome might indicate that the observed alterations in neural connectivity do not have straightforward translations into measurable relationships with these external factors. This discrepancy could be attributed to the multifaceted nature of substance use disorders, which are influenced by a myriad of interacting factors.
Integration with previous research
The present study builds upon a growing body of research that highlights the intricate interplay between brain regions in addiction. The observed connectivity changes in response to methamphetamine cues resonate with previous studies investigating cue reactivity across different substances of abuse. The convergence of findings regarding the involvement of the amygdala, dlPFC, and PCC underscores their roles as core components in the addiction process, supporting the notion of a broader addiction-related neural network.
Clinical implications
Understanding the neural mechanisms underpinning methamphetamine cue reactivity holds significant clinical implications. The identified networks and connections offer potential targets for intervention strategies to mitigate craving and prevent relapse. Interventions targeting the amygdala-cortical circuitry could be designed to enhance cognitive control (Sadeghi et al., 2023) over drug-related cues and emotions, potentially aiding in the management of addiction (Soleimani et al., 2023).
5. Conclusion
In summary, this research enriches our understanding of addiction by investigating the amygdala-cortical connectivity and effective neural pathways associated with methamphetamine cue reactivity. Integrating functional and effective connectivity analyses and neurobehavioral assessments provides a comprehensive picture of the underlying neural dynamics. These findings offer insights into the mechanisms driving addiction-related processes and potential avenues for targeted interventions. As addiction continues to pose a significant public health challenge, these insights may pave the way for innovative strategies aimed at improving treatment outcomes and reducing the burden of addiction on affected individuals and society.
Limitations and future studies
Although the study offers valuable insights, it is essential to acknowledge its limitations. The sample consisted solely of male participants with MUD, potentially limiting the generalizability of the findings. Future studies could include diverse samples and consider the impact of gender and other demographic variables. Additionally, the study's cross-sectional nature restricts our capacity to establish causal relationships between functional connectivity patterns and addiction-related outcomes. Longitudinal studies could shed light on the trajectory of neural changes and their predictive value.
Ethical Considerations
Compliance with ethical guidelines
The Declaration of Helsinki was followed in the design and execution of the study procedure. This study was reviewed and approved by the Ethics Committee of Research at Tehran University of Medical Science, Tehran, Iran (IR.TUMS.REC.93-02-98-23869). Participants in this study provided their written informed consent to participate.
Funding
The study was supported by a research grant from AJA University of Command and Staff, Tehran, Iran.
Authors' contributions
Conceptualization and methodology: Peyman Ghobadi-Azbari, and Yasaman Hosseini; Investigation: All authors; Formal analysis, and visualization: Peyman Ghobadi-Azbari; Writing the original draft: Peyman Ghobadi-Azbari; Review, and editing: Yasaman Hosseini, Hossein Moslemi, and Mitra Yousefpour; Supervision: Yasaman Hosseini and Hossein Moslemi.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.


References
Association, A. P. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR®. American Psychiatric Association.
Barratt, E. S. (1994). Impulsiveness and aggression. In Violence and mental disorder: Developments in risk assessment (pp. 61-79). The University of Chicago Press.
Courtney, K. E., & Ray, L. A. (2014). Methamphetamine: An Update on Epidemiology, Pharmacology, Clinical Phenomenology, and Treatment Literature. Drug and Alcohol Dependence, 0, 11-21. [DOI:10.1016/j.drugalcdep.2014.08.003] [PMID]
Crawford, J. R., & Henry, J. D. (2004). The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. The British Journal of Clinical Psychology, 43(Pt 3), 245-265. [DOI:10.1348/0144665031752934] [PMID]
Dakhili, A., Sangchooli, A., Jafakesh, S., Zare-Bidoky, M., Soleimani, G., Batouli, S. A. H., Kazemi, K., Faghiri, A., Oghabian, M. A., & Ekhtiari, H. (2022). Cue-induced craving and negative emotion disrupt response inhibition in methamphetamine use disorder: Behavioral and fMRI results from a mixed Go/No-Go task. Drug and Alcohol Dependence, 233, 109353. [DOI:10.1016/j.drugalcdep.2022.109353] [PMID]
Daunizeau, J., Stephan, K. E., & Friston, K. J. (2012). Stochastic dynamic causal modelling of fMRI data: Should we care about neural noise? NeuroImage, 62(1), 464-481. [DOI:10.1016/j.neuroimage.2012.04.061] [PMID]
Ekhtiari, H., Kuplicki, R., Pruthi, A., & Paulus, M. (2020). Methamphetamine and Opioid Cue Database (MOCD): Development and Validation. Drug and Alcohol Dependence, 209, 107941. [DOI:10.1016/j.drugalcdep.2020.107941] [PMID]
Ekhtiari, H., Zare-Bidoky, M., Sangchooli, A., Janes, A. C., Kaufman, M. J., Oliver, J. A., Prisciandaro, J. J., Wüstenberg, T., Anton, R. F., Bach, P., Baldacchino, A., Beck, A., Bjork, J. M., Brewer, J., Childress, A. R., Claus, E. D., Courtney, K. E., Ebrahimi, M., Filbey, F. M., … Zilverstand, A. (2022). A methodological checklist for fMRI drug cue reactivity studies: Development and expert consensus. Nature Protocols, 17(3), 567-595. [DOI:10.1038/s41596-021-00649-4] [PMID]
Etkin, A., Büchel, C., & Gross, J. J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), Article 11. [DOI:10.1038/nrn4044] [PMID]
Everitt, B. J., Parkinson, J. A., Olmstead, M. C., Arroyo, M., Robledo, P., & Robbins, T. W. (1999). Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences, 877, 412-438. [DOI:10.1111/j.1749-6632.1999.tb09280.x] [PMID]
Friston, K. J., Harrison, L., & Penny, W. (2003). Dynamic causal modelling. NeuroImage, 19(4), 1273-1302. [DOI:10.1016/s1053-8119(03)00202-7] [PMID]
Friston, K. J., Li, B., Daunizeau, J., & Stephan, K. E. (2011). Network discovery with DCM. Neuroimage, 56(3-4), 1202-1221. [DOI:10.1016/j.neuroimage.2010.12.039] [PMID]
Friston, K. J., Litvak, V., Oswal, A., Razi, A., Stephan, K. E., van Wijk, B. C. M., Ziegler, G., & Zeidman, P. (2016). Bayesian model reduction and empirical Bayes for group (DCM) studies. NeuroImage, 128, 413-431. [DOI:10.1016/j.neuroimage.2015.11.015] [PMID]
Ghobadi-Azbari, P., Mahdavifar Khayati, R., Sangchooli, A., & Ekhtiari, H. (2022). Task-Dependent Effective Connectivity of the Reward Network During Food Cue-Reactivity: A Dynamic Causal Modeling Investigation. Frontiers in Behavioral Neuroscience, 16. [DOI:10.3389/fnbeh.2022.899605] [PMID]
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry, 159(10), 1642-1652. [DOI:10.1176/appi.ajp.159.10.1642] [PMID]
Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews. Neuroscience, 12(11), 652-669. [DOI:10.1038/nrn3119] [PMID]
Grodin, E. N., Courtney, K. E., & Ray, L. A. (2019). Drug-Induced Craving for Methamphetamine Is Associated With Neural Methamphetamine Cue Reactivity. Journal of Studies on Alcohol and Drugs, 80(2), 245-251. [DOI:10.15288/jsad.2019.80.245] [PMID]
Hedegaard, H., Bastian, B. A., Trinidad, J. P., Spencer, M., & Warner, M. (2018). Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011-2016. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 67(9), 1-14.
Hosseini, Y., Chahardehi, A. M., Hosseini, F., & Afzali, M. (2023). Frontline Nurses’ Stress, Sleep Quality, and Temperament During the COVID-19 Pandemic: An Intervention Study using Resilience Training and Comics Programs. Life Sciences Student Journal, 1(1), Article 1. [DOI:10.22034/LSSJ.2023.1.57]
Hosseini, Y., & Modarresi Chahardehi, A. (2021, September 5). Stress Among Nurses in COVID-19 Pandemic. 44th ICMM World Congress on Military Medicine, Brussels.
Jafakesh, S., Sangchooli, A., Aarabi, A., Helfroush, M. S., Dakhili, A., Oghabian, M. A., Kazemi, K., & Ekhtiari, H. (2022). Temporally dynamic neural correlates of drug cue reactivity, response inhibition, and methamphetamine-related response inhibition in people with methamphetamine use disorder. Scientific Reports, 12(1), Article 1. [DOI:10.1038/s41598-022-05619-8] [PMID]
Jan, R. K., Kydd, R. R., & Russell, B. R. (2012). Functional and Structural Brain Changes Associated with Methamphetamine Abuse. Brain Sciences, 2(4), 434-482. [DOI:10.3390/brainsci2040434] [PMID]
Janak, P. H., & Tye, K. M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284-292. [DOI:10.1038/nature14188] [PMID]
Jia, Z., Worhunsky, P. D., Carroll, K. M., Rounsaville, B. J., Stevens, M. C., Pearlson, G. D., & Potenza, M. N. (2011). AN INITIAL STUDY OF NEURAL RESPONSES TO MONETARY INCENTIVES AS RELATED TO TREATMENT OUTCOME IN COCAINE DEPENDENCE. Biological Psychiatry, 70(6), 553-560. [DOI:10.1016/j.biopsych.2011.05.008] [PMID]
Klami, A., Virtanen, S., Leppäaho, E., & Kaski, S. (2015). Group Factor Analysis. IEEE Transactions on Neural Networks and Learning Systems, 26(9), 2136-2147. [DOI:10.1109/TNNLS.2014.2376974]
Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760-773. [DOI:10.1016/S2215-0366(16)00104-8]
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 1(39-58), 3.
Lanteaume, L., Khalfa, S., Régis, J., Marquis, P., Chauvel, P., & Bartolomei, F. (2007). Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex (New York, N.Y.: 1991), 17(6), 1307-1313. [DOI:10.1093/cercor/bhl041] [PMID]
Leppäaho, E., Virtanen, S., Ammad-ud-din, M., Khan, S. A., Suvitaival, T., Saarinen, I., & Kaski, S. (2017). GFA: Group Factor Analysis (1.0.3) [Computer software]. [DOI:10.32614/CRAN.package.GFA]
Li, B., Daunizeau, J., Stephan, K. E., Penny, W., Hu, D., & Friston, K. (2011). Generalised filtering and stochastic DCM for fMRI. NeuroImage, 58(2), 442-457. [DOI:10.1016/j.neuroimage.2011.01.085] [PMID]
Luo, Y.-X., Xue, Y.-X., Shen, H.-W., & Lu, L. (2013). Role of amygdala in drug memory. Neurobiology of Learning and Memory, 105, 159-173. [DOI:10.1016/j.nlm.2013.06.017] [PMID]
Nichols, T., & Hayasaka, S. (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12(5), 419-446. [DOI:10.1191/0962280203sm341ra] [PMID]
Ochsner, K. N., & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242-249. [DOI:10.1016/j.tics.2005.03.010] [PMID]
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97-113. [DOI:10.1016/0028-3932(71)90067-4] [PMID]
Osman, A., Wong, J. L., Bagge, C. L., Freedenthal, S., Gutierrez, P. M., & Lozano, G. (2012). The Depression Anxiety Stress Scales-21 (DASS-21): Further examination of dimensions, scale reliability, and correlates. Journal of Clinical Psychology, 68(12), 1322-1338. [DOI:10.1002/jclp.21908] [PMID]
Parvaz, M. A., Alia-Klein, N., Woicik, P. A., Volkow, N. D., & Goldstein, R. Z. (2011). Neuroimaging for drug addiction and related behaviors. Reviews in the Neurosciences, 22(6), 609-624. [DOI:10.1515/RNS.2011.055] [PMID]
Paulus, M. P., & Stewart, J. L. (2020a). Methamphetamine Use Disorder: The Next Addiction Crisis. JAMA Psychiatry, 77(9), 959-966. [DOI:10.1001/jamapsychiatry.2020.0246] [PMID]
Paulus, M. P., & Stewart, J. L. (2020b). Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry, 77(9), 959-966. [DOI:10.1001/jamapsychiatry.2020.0246] [PMID]
Peng, Y., Knotts, J. D., Taylor, C. T., Craske, M. G., Stein, M. B., Bookheimer, S., Young, K. S., Simmons, A. N., Yeh, H.-W., Ruiz, J., & Paulus, M. P. (2021). Failure to Identify Robust Latent Variables of Positive or Negative Valence Processing Across Units of Analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(5), 518-526. [DOI:10.1016/j.bpsc.2020.12.005] [PMID]
Phelps, E. A., & LeDoux, J. E. (2005). Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron, 48(2), 175-187. [DOI:10.1016/j.neuron.2005.09.025] [PMID]
Ranaei, V., Mehrabi, N., Hosseini, Y., & Afzali, M. (2022). INVESTIGATING THE FACTORS RELATED TO GENERAL HEALTH IN NURSES WORKING IN MILITARY HOSPITALS IN IRAN. International Journal of Early Childhood Special Education, 14(05).
Rolls, E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure & Function, 224(9), 3001-3018. [DOI:10.1007/s00429-019-01945-2] [PMID]
Sadeghi, M. A., Nassireslami, E., Yousefi Zoshk, M., Hosseini, Y., Abbasian, K., & Chamanara, M. (2023). Phosphodiesterase inhibitors in psychiatric disorders. Psychopharmacology, 240(6), 1201-1219. [DOI:10.1007/s00213-023-06361-3] [PMID]
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., Olucha-Bordonau, F. E., Vukšić, M., & R. Hof, P. (2021). Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules, 11(6), 823. [DOI:10.3390/biom11060823] [PMID]
Sinha, R. (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158(4), 343-359. [DOI:10.1007/s002130100917] [PMID]
Soleimani, G., Conelea, C. A., Kuplicki, R., Opitz, A., Lim, K. O., Paulus, M. P., & Ekhtiari, H. (2023). Optimizing Individual Targeting of Fronto-Amygdala Network with Transcranial Magnetic Stimulation (TMS): Biophysical, Physiological and Behavioral Variations in People with Methamphetamine Use Disorder (p. 2023.04.02.23288047). medRxiv. [DOI:10.1101/2023.04.02.23288047]
Soleimani, G., Nitsche, M. A., Bergmann, T. O., Towhidkhah, F., Violante, I. R., Lorenz, R., Kuplicki, R., Tsuchiyagaito, A., Mulyana, B., Mayeli, A., Ghobadi-Azbari, P., Mosayebi-Samani, M., Zilverstand, A., Paulus, M. P., Bikson, M., & Ekhtiari, H. (2023). Closing the loop between brain and electrical stimulation: Towards precision neuromodulation treatments. Translational Psychiatry, 13(1), 279. [DOI:10.1038/s41398-023-02565-5] [PMID]
Sotres-Bayon, F., & Quirk, G. J. (2010). Prefrontal control of fear: More than just extinction. Current Opinion in Neurobiology, 20(2), 231-235. [DOI:10.1016/j.conb.2010.02.005] [PMID]
Stewart, J. L., Connolly, C. G., May, A. C., Tapert, S. F., Wittmann, M., & Paulus, M. P. (2014). Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals. Addiction (Abingdon, England), 109(3), 460-471. [DOI:10.1111/add.12403] [PMID]
Team, R. C. (2020). R: A language and environment for statistical programming. R Foundation for Statistical Computing, Vienna, Austria. Www. R-Project. Org.
Volkow, N. D., Koob, G. F., & McLellan, A. T. (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. The New England Journal of Medicine, 374(4), 363-371. [DOI:10.1056/NEJMra1511480] [PMID]
White, E. J., Kuplicki, R., Stewart, J. L., Kirlic, N., Yeh, H.-W., Paulus, M. P., & Aupperle, R. L. (2021). Latent variables for region of interest activation during the monetary incentive delay task. NeuroImage, 230, 117796. [DOI:10.1016/j.neuroimage.2021.117796] [PMID]
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125-141. [DOI:10.1089/brain.2012.0073] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2023/08/30 | Accepted: 2023/09/26 | Published: 2025/03/18
Received: 2023/08/30 | Accepted: 2023/09/26 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








