Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 671-682 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abdelnaby R, Ahmed Y H, Zaafar D, Mahmoud M Y, Elsaeed E M, Häger A et al . Structural Changes in Pharyngeal and Tongue Muscles as a Potential Contributor to Dysphagia in Alzheimer Disease Rat Model. BCN 2024; 15 (5) :671-682
URL: http://bcn.iums.ac.ir/article-1-2764-en.html
URL: http://bcn.iums.ac.ir/article-1-2764-en.html
Ramy Abdelnaby *1 

 , Yasmine H. Ahmed2
, Yasmine H. Ahmed2 
 , Dalia Zaafar3
, Dalia Zaafar3 

 , Mohamed Y. Mahmoud4
, Mohamed Y. Mahmoud4 
 , Eman Mohammed Elsaeed5
, Eman Mohammed Elsaeed5 

 , Alexa Häger1
, Alexa Häger1 

 , Heba M. A. Khalil6
, Heba M. A. Khalil6 




 , Yasmine H. Ahmed2
, Yasmine H. Ahmed2 
 , Dalia Zaafar3
, Dalia Zaafar3 

 , Mohamed Y. Mahmoud4
, Mohamed Y. Mahmoud4 
 , Eman Mohammed Elsaeed5
, Eman Mohammed Elsaeed5 

 , Alexa Häger1
, Alexa Häger1 

 , Heba M. A. Khalil6
, Heba M. A. Khalil6 


1- Department of Neurology, RWTH Aachen University, Aachen, Germany.
2- Department of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Modern University for Information and Technology, Cairo, Egypt.
4- Department of Toxicology, Forensic Medicine and Veterinary Regulations, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
5- Department of Human Anatomy and Embryology, Faculty of Medicine, Port Said University, Port Said, Egypt.
6- Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
2- Department of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Modern University for Information and Technology, Cairo, Egypt.
4- Department of Toxicology, Forensic Medicine and Veterinary Regulations, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
5- Department of Human Anatomy and Embryology, Faculty of Medicine, Port Said University, Port Said, Egypt.
6- Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Full-Text [PDF 1964 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Alzheimer disease (AD) is a progressive neurodegenerative disease usually characterized by behavioral and mental alterations, loss of recent memories, cognitive deficits, and the inability to live independently (Waldemar et al., 2007). The most severely affected brain regions are the neocortex and hippocampus, which classically show the main pathological hallmarks of AD, including abnormal accumulation of extracellular amyloid beta-peptide (Aβ) plaques as well as intracellular aggregates of tau-containing neurofibrillary tangles (NFTs) or phosphorylated tau (DeTure & Dickson, 2019; Frisoni et al., 1999). It accounts for 60% of causes of dementia worldwide, with a higher prevalence in females by 1.9 times (Cao et al., 2020). Moreover, the high cost of care and research can affect economies, as the global cost 2018 was estimated to reach $ 1 trillion (Patterson, 2018).
Dysphagia is an impairment in swallowing due to sensory, motor, or behavioral causes or a combination of them (Özsürekci et al., 2020). Neurogenic dysphagia occurs in patients with neurological diseases of different etiologies, such as degenerative, dementia, myopathy, traumatic, and vascular etiologies (Panebianco et al., 2020). It is considered one of the geriatric syndromes associated with impaired quality of life (QoL), malnutrition and weight loss, aspiration pneumonia, aspiration pneumonitis, and mortality (Fernández-Ruiz et al., 2021; Nagamine et al., 2020). No recent publications can accurately point to the prevalence of dysphagia among AD patients (Mira et al., 2022).
Dysphagia typically occurs at later stages of AD; however, subclinical dysphagia can be detected in mild or moderate stages of the disease, which is often underdiagnosed or overlooked (Simoes et al., 2020). AD-associated dysphagia has been explained by the involvement of cortical control of swallowing (Humbert et al., 2010) as well as weakness and atrophy of skeletal muscles causing sarcopenic dysphagia (Özsürekci et al., 2020), which means the involvement of the pharyngeal muscles as a part of the whole-body skeletal muscles.
The cortical deposition of Aβ plaques and NFTs has been proved in AD (Kloskowska et al., 2010), which are the pathological hallmarks of the disease. Although dysphagia is a common comorbidity in AD, no studies have investigated if there are biochemical, histopathological, and immune-histochemical local changes in pharyngeal muscles in AD. Previous studies have found indications for the deposition of neuropathological proteins outside the central nervous system in other degenerative disorders like Parkinson disease. Moreover, deposition of Aβ protein has been detected in specific skeletal muscles. If local deposition of Aβ protein in pharyngeal muscles in AD is proven, we better understand the pathogenesis of dysphagia in AD patients and may improve its management (Kuo et al., 2000). Thus, this study investigated structural changes in rats, including biochemical, histopathological, and immune-histochemical aspects of pharyngeal and tongue muscles in AlCl3/D-gal-induced AD.
2. Materials and Methods
Study animals
Animals were housed according to the regulations approved by the Veterinary Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University (VET- IACUC). Fourteen adult male Wistar rats (150-170 g, 12 weeks) were housed in private plastic cages with softwood chips for bedding and were fed on a balanced commercial diet and water ad libitum. Animals were acclimatized for two weeks before proceeding with the experiment.
Experimental design
Rats were randomly blindly allocated into two groups, each of 7 rats. Group I (control) received distilled water orally and subcutaneously daily for 45 days, while group II (AD) orally received AlCl3 (Sigma-Aldrich Co., USA) (200 mg/kg) and subcutaneously received D-galactose (HEGENG Co., China) (60 mg/kg) daily for 45 days (Chiroma et al., 2018; Ezzat et al., 2022). Aluminum chloride (AlCl3) and D-galactose (D-gal) were used as AD inducers due to their neurotoxicity, including morphological alterations, cognitive impairment, and altered brain neurochemistry in rodents due to its pro-oxidant nature as reported in previous studies (Liaquat et al., 2019; Rebai, & Djebli, 2008). AlCl3 is considered a neurotoxin, and D-gal is used to model subacute aging. Consequently, their combination can create a non-transgenic AD animal model (Xiao et al., 2011).
After the scheduled 45 days, behavioral tests were carried out for 5 days. Then, blood samples were taken for sera separation. Animals were decapitated, and the brains, tongue, and pharynx of each rat were dissected out, washed, and frozen at -20 °C for biochemical analysis or kept in 10% formalin saline for histological and immunopathological tests.
Measurement of rats’ body weight
The rats’ body weight in the two groups was measured at the beginning of the experiment, then twice a week until the end. Body weight change percentage was calculated from the Equation 1:
1. Body Weight at the End of the Experiment (g) - body Weight at the Beginning of the Investigation ×100
The aim of measuring body weight is to monitor any indirect decrease in feed intake.
Validation of AD in rats
Behavioral validation
Open field test
The locomotion and exploratory behavior were evaluated using an open-field test, performed as mentioned by Khalil et al. (2021). The measured parameters were the number of crossing squares and the rearing frequencies.
Y-maze test
The Y-maze test was used to evaluate short-term memory and motor activity. The test was conducted according to Khalil et al. (2020) and Khalil et al. (2021), and the measured parameters were the number of arm entries and the spontaneous alternation percentage (SAP%). The spontaneous alternation percentage depends on the rats’ natural tendency to alternate between three different arms.
Novel object recognition (NOR) test
The NOR test was used to evaluate hippocampal-dependent memory impairment (Lueptow, 2017) and was conducted as mentioned in previous research (Khalil et al., 2021). The calculated parameters were the total exploration time, the discrimination ratio (DR), and the recognition index (RI). DR was calculated using the Equation 2:
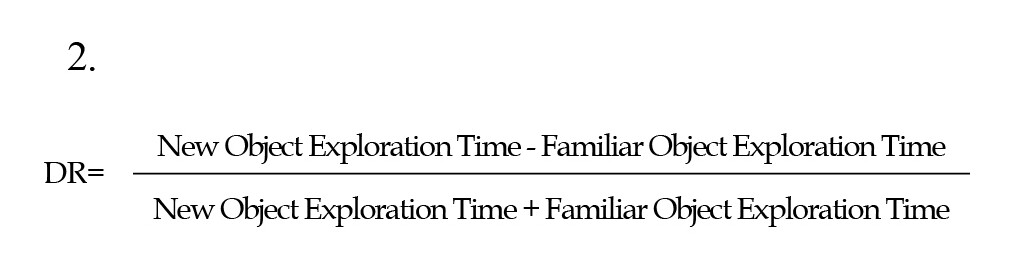
The recognition ratio, the rat’s capacity to determine the same object on different occasions, was calculated using the Equation 3:

(Costa et al., 2008; Lueptow, 2017).
Biochemical validation
Preparation of tissue homogenates
At a concentration of 20% (w/v), hippocampal and pharyngeal tissue homogenates were meticulously prepared in ice-cooled phosphate-buffered saline, employing a homogenizer. Subsequently, these homogenates underwent centrifugation, lasting 15 minutes at 5000×g while being maintained at a temperature of 4 °C. The resultant tissue homogenates were then carefully portioned into aliquots and preserved at -80 °C, pending further analysis for quantifying different biomarkers and mediators as described previously by El-Shoura et al. (2023).
Measurement of tissue malondialdehyde (MDA) concentration
Malondialdehyde (MDA) colorimetric/fluorometric assay kit (K739-100) was purchased from BioVision, USA. It was prepared as described by Halliwell and Chirico (1993).
Measurement of tissue total antioxidant capacity (TAC)
Rat TAC1/Substance P ELISA Kit (LS-F14457) was purchased from LSBio, Inc., USA. It was examined in hippocampal and pharyngeal homogenates as described by Koracevic et al. (2001).
Measurement of tissue brain-derived neurotrophic factor (BDNF)
Rat BDNF ELISA Kit (SEA011Ra) was purchased from Cloud-Clone Corp., USA. It was measured in hippocampal and pharyngeal homogenates, as described by Klein et al. (2011).
Measurement of tissue Aβ
Rat Aβ 1-42 ELISA Kit (LS-F23254) was purchased from LSBio, Inc., USA. Soluble and insoluble/formic acid-soluble Aβ were quantified in hippocampal and pharyngeal homogenates, as mentioned by Liu et al. (2011).
Histological examination
The samples were fixed, dehydrated, cleaned, and embedded in paraffin wax. Rotatory microtome sections of 3–4 µm in thickness were prepared and stained with hematoxylin and eosin (H&E), as Bancroft and Gamble (2008) mentioned.
Immunohistochemical examination
Glial fibrillar acidic protein (GFAP) was used to detect astrocyte protein in the hippocampus. The method was done according to Stoltenburg-Didinger et al. (1996).
Evaluation of immunohistochemical observations (area percentage)
The sections were stained and assessed by the Leica Quin 500 analyzer computer system (Leica Microsystems, Switzerland). The image analyzer was calibrated automatically to convert the pixels into actual micrometer units, then statistically analyzed for each specimen.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 6. Behavioral and biochemical data were expressed as Mean±SEM. An unpaired student t-test was used to compare the means. Histograms were drawn using GraphPad Prism.
3. Results
Body weight change, locomotor activity, and cognitive function
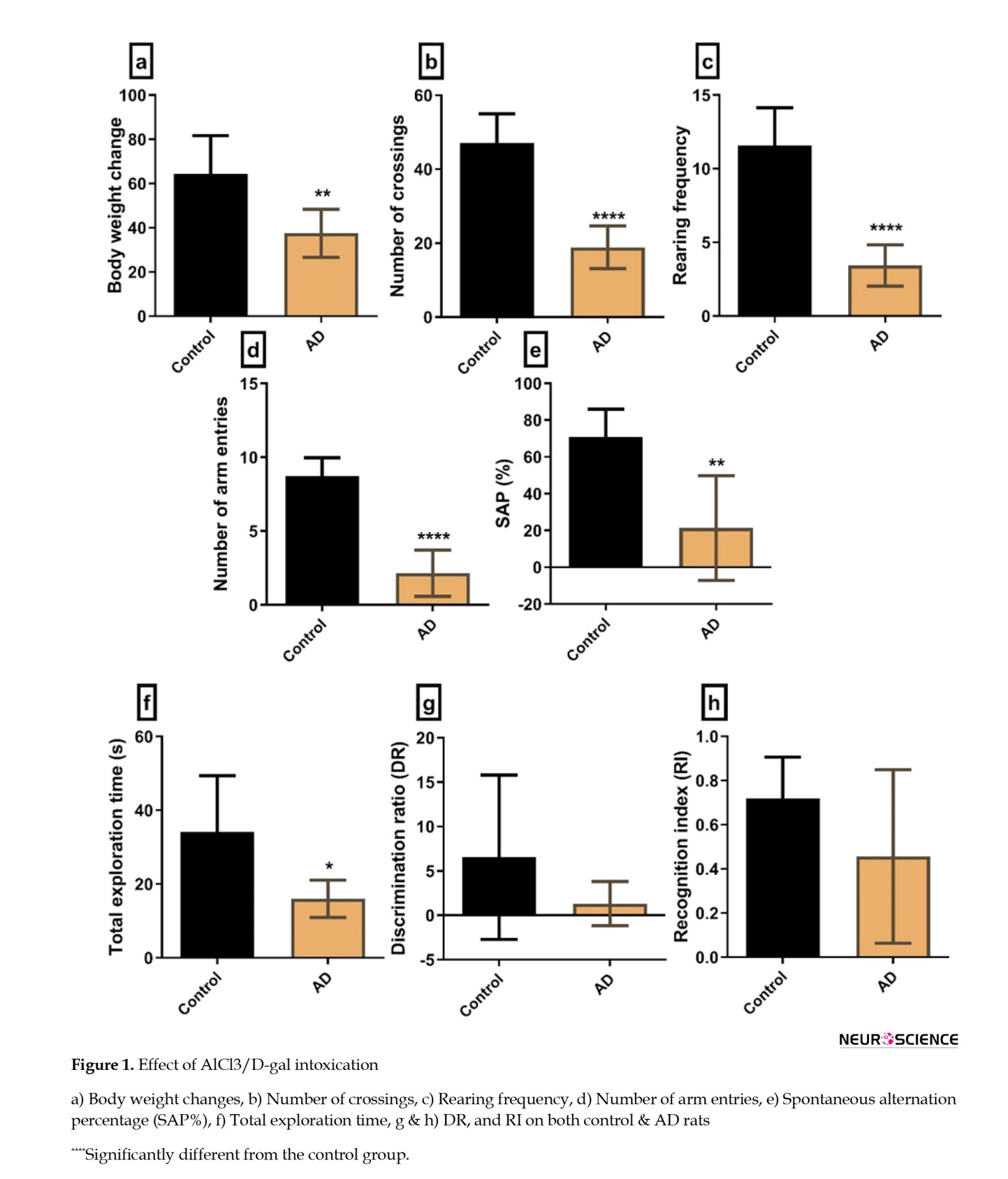
AD rats displayed a significant decrease (P=0.0077) in their body weight compared to control rats. Also, they exhibited a marked reduction in their locomotor activity, as evidenced by decreased crossing squares (P=0.0001) and rearing frequency (P=0.0001) compared to control rats. Regarding cognitive functions, AD rats showed a marked cognitive dysfunction both in the Y maze and the NOR test and displayed a significant decrease in the number of arm entries (P=0.0001) and SAP% (P=0.001). Furthermore, they significantly reduced the total exploration time (P=0.03). However, the decline in the DR and RI was not significant (P=0.17 and P=0.13, respectively) (Figure 1).
Biochemical parameters
Oxidative stress markers
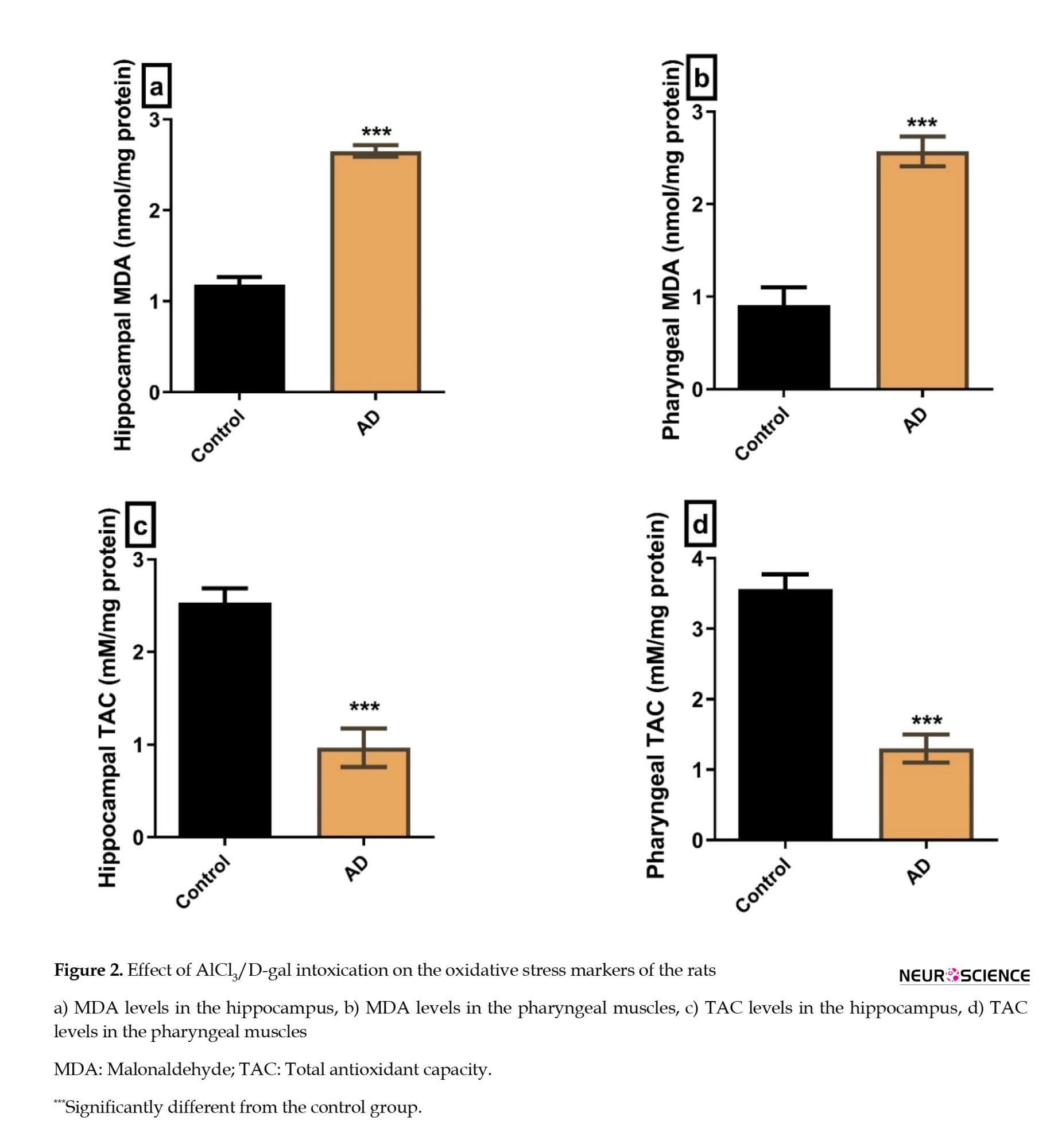
The hippocampal and the pharyngeal MDA were significantly higher in the AD group compared to the controls (P≤0.0001, 0.0003, respectively) (Figures 2A, and 2B). Additionally, the hippocampal and pharyngeal TAC was significantly lower in the AD group compared to the controls (P=0.0005 and P=0.0002, respectively) (Figures 2C, and 2D).
Brain-derived neurotrophic factor
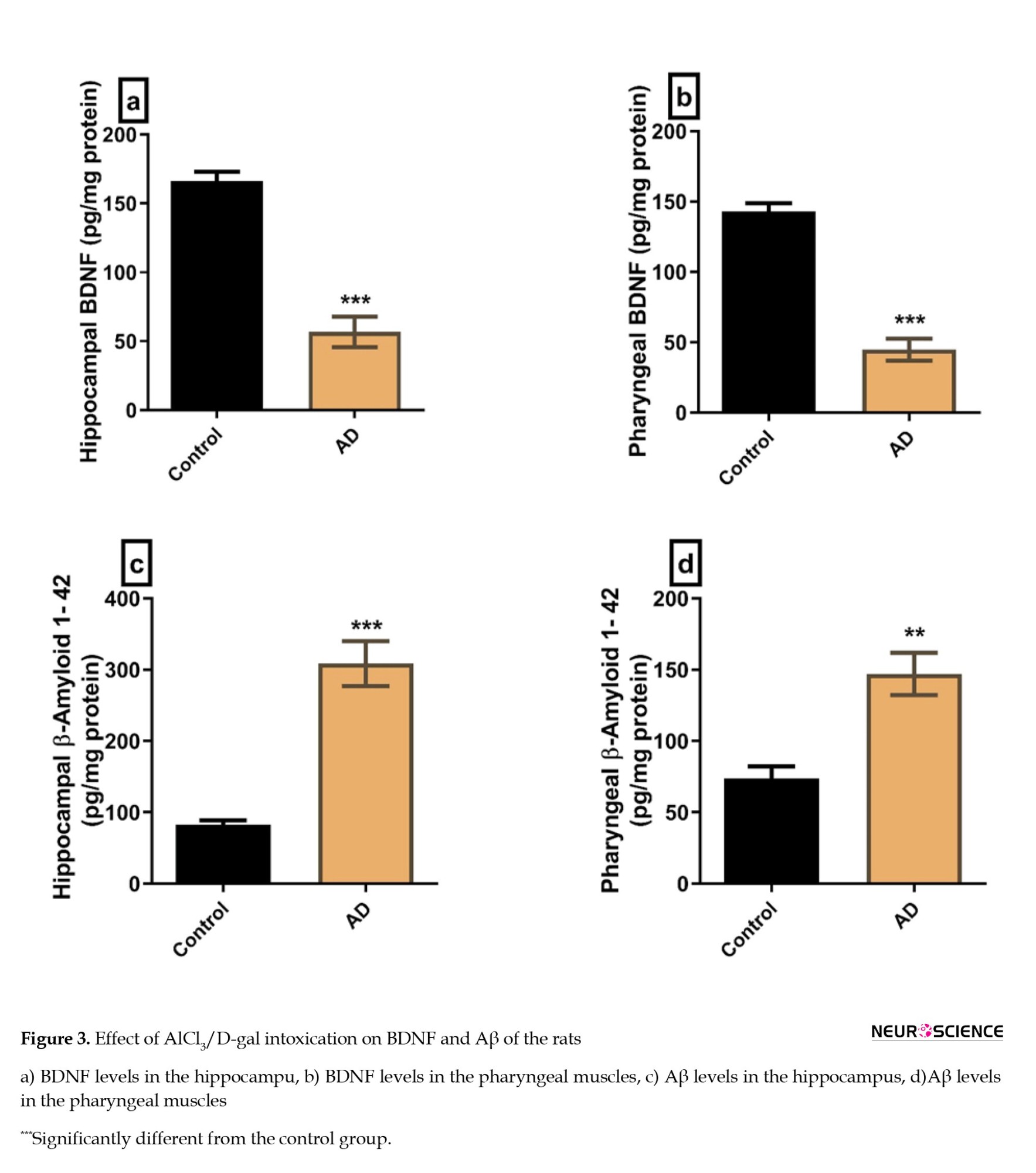
The BDNF was significantly lower in the hippocampus and the pharyngeal muscles of the AD group compared to the control group (P=0.0001 and P<0.0001, respectively) (Figures 3A, and 3B).
Aβs
The Aβ levels in the hippocampus and the pharyngeal muscles were significantly higher in the AD group compared to the control group (P=0.0003 and P=0.0017, respectively) (Figures 3C, and 3D).
Hematoxylin and eosin-stained sections
Hippocampus H&E-stained sections
The H&E-stained hippocampus sections of the control rats revealed the normal histological structure of the molecular layer, pyramidal cell layer, and polymorphic cell layer (Figure 4A). On the other hand, hippocampus sections of AD rats showed histopathological disruption in the form of cellular disorganization of pyramidal cells that had NFTs confirming the induction of AD (Figure 4B).
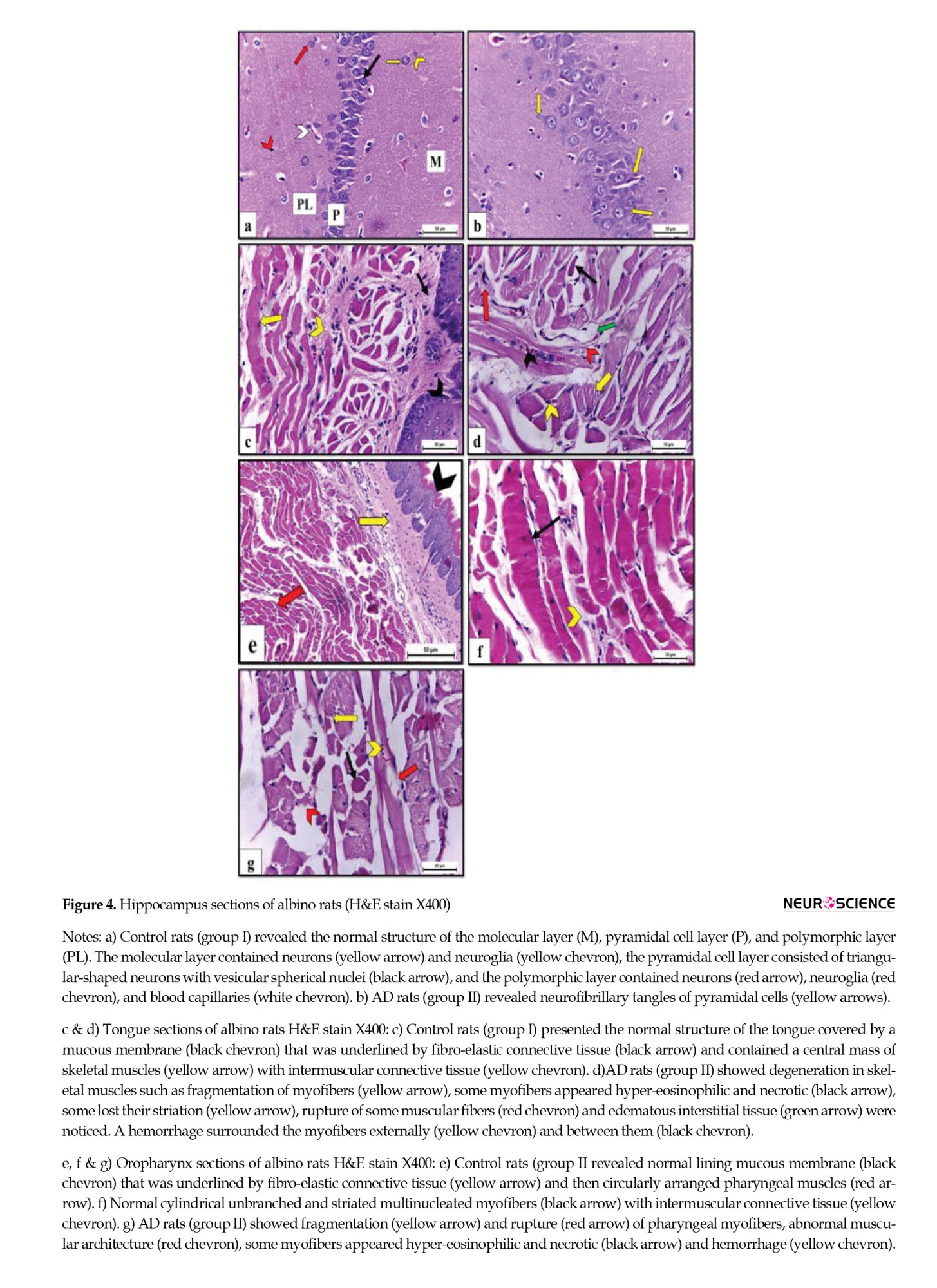
Tongue H&E-stained sections
Tongue sections of control rats showed the normal microscopic structure of a muscular organ covered dorsally by mucous membrane and underlined by propria-submucosa that was formed of fibro-elastic connective tissue and centrally contained skeletal muscle mass with intermuscular fibro-elastic connective tissue in between. Lingual myofibers appeared cylindrical, unbranched, striated, and multinucleated (Figure 4C). On the contrary, tongue sections of AD rats revealed multiple features of degeneration in skeletal muscles, including fragmentation of myofibers. Some myofibers appeared hyper-eosinophilic and necrotic, while others lost their striation. Additionally, rupture of some muscular fibers, edematous interstitial tissue, and inter-/extra- myofibril hemorrhage were observed (Figure 4D).
Pharynx H&E-stained sections
The H&E-stained oropharynx sections of control rats showed normal histological form of the lining mucous membrane that was underlined by fibro-elastic connective tissue in propria-submucosa then circularly arranged pharyngeal muscles (Figure 4E). Pharyngeal muscles were formed of cylindrical, unbranched, striated myofibers that appeared multinucleated and separated from each other by intermuscular connective tissue (Figure 4F). On the other hand, oropharynx sections obtained from AD rats showed abnormal muscular architecture and fragmentation, myofiber rupture, and some myofibers appeared hyper-eosinophilic and necrotic. Hemorrhage was also observed in the myofibers (Figure 4G).
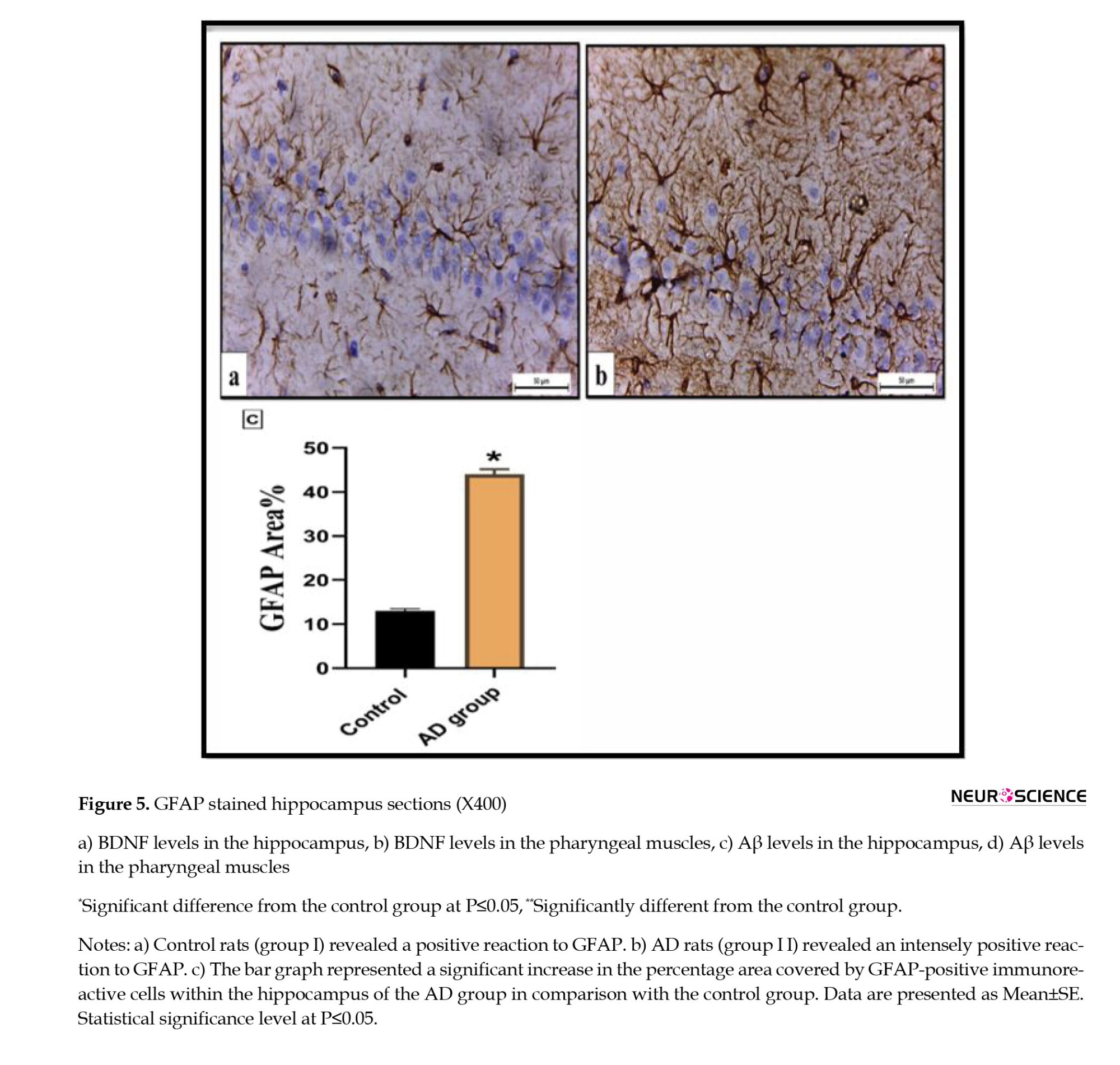
Immunohistochemical-stained sections
Immunohistochemical examination of the hippocampus of the control rats (group I) displayed a positive immunoexpression of the fibrillary astrocyte bodies and processes to GFAP. Meanwhile, the immunoreactivity concentration was significantly increased in the AD rats (group II) (Figures 5A, and 5B). The percentage area covered by GFAP-positive immunoreactive cells within the hippocampus revealed a significant elevation in the AD rats by 44.6 fold compared with the control rats (Figure 5C).
4. Discussion
There is evidence of pathological changes outside the central nervous system in AD, as some studies reported the presence of certain species of phosphorylated tau in peripheral tissues of AD patients, such as the submandibular gland (Dugger et al., 2016) and the deposition of Aβ plaques in the testis and ovary of patients (Miklossy et al., 1999), with evidence of impaired sperm function in vitro (Tavares et al., 2017). In addition, phosphorylated tau has been seen in AD human participants’ peripheral tissues like skin and skeletal muscle (Rodríguez-Leyva et al., 2015). Therefore, this work is meant to assess the histological abnormalities of skeletal muscles in the tongue and oropharynx in AD, in addition to the neurodegenerative impact of the disease.
In this study, AD was confirmed by the impairment of the working spatial and recognition memory, the locomotor disturbance, and the significant increase of hippocampal Aβ levels in AD rats. These results are consistent with Khalil et al. (2020) and Cheignon et al. (2018). Also, AD diagnosis was confirmed by the decreased BDNF expression in the hippocampal homogenates, the significant increase in MDA and decrease in TAC, which is in line with previous studies (Tapia-Arancibia et al., 2008; Tobore, 2019), and the significant increase in hippocampal immunoreactivity to GFAP, which was explained by Kamphuis et al. (2014). Besides, the histopathological changes in the hippocampus established the diagnosis of AD, which agrees with the work of DeTure & Dickson, (2019) and Ryan et al. (2015).
In our study, the development of dysphagia was hypothesized by the significant body weight loss observed in AD rats, as the eating and drinking patterns decreased by 25%-35% in AD versus the control group. Moreover, the histopathology of the tongue and pharynx exhibited myofibers fragmentation and rupture associated with edematous interstitial tissue representing structural affection of the muscles, which may be regarded as a potential contributor to dysphagia. Our results agree with Ogawa et al. (2018), who stated that sarcopenia is closely linked to AD and may be involved in the pathophysiological process of AD, as the involvement of pharyngeal muscles is a part of this generalized skeletal muscle affection. Furthermore, these local changes are associated with significant changes in local oxidative stress markers. Further research is needed to elucidate the possible relation between the pharyngeal local changes in AD and the development of dysphagia.
In our study, we show for the first time that degeneration of the tongue and pharyngeal muscles is evident in AD rats in the form of altered muscular structure, rupture and necrosis of the myofibers, and detection of oxidative stress in pharyngeal muscles. AD pharyngeal muscles even exhibited a significant increase in Aβ levels, which could be explained theoretically by the migration/spread of Aβ along cranial nerves, e. g. the vagus nerve. Otherwise, it could be formed in the muscles through metabolic alterations, leading to increased amyloid deposition. Also, the downregulation of BDNF levels in AD pharyngeal muscles could be explained by an injurious effect on the supplying cranial nerves, e. g. the vagus nerve. Further research is still needed to examine these hypotheses.
5. Conclusion
Dysphagia in AD has been discussed as originating mainly from central cerebral affection. So far, there has not been any biochemical, histopathological, or immune-histochemical evidence of AD in the tongue and pharyngeal muscles themselves. In our study, we show for the first time evidence for the presence of AD pathology in these structures, possibly one of the main contributing factors to dysphagia observed in AD. Additional investigations are needed to clarify the underlying processes that could be targeted to decrease the incidence of dysphagia and their direct linkage to clinical impairment. As a potential outlook, targeting AD pathology in these structures might, therefore, prevent dysphagia and potentially enhance the QoL in AD patients from this point. Future studies should explore the other factors contributing to dysphagia and the underlying molecular mechanisms involved in its pathogenesis. However, this is the first study to establish successfully the link between dysphagia and AD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Veterinary Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University (VET- IACUC) (Code: Vet CU28/04/2021/267).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Study design, data interpretation: Ramy Abdelnaby; Data collection and analysis: Heba M. A. Khalil, Yasmine H. Ahmed, Dalia Zaafar, and Mohamed Y. Mahmoud; Writing: Alexa Häger, Ramy Abdelnaby, and Eman Mohammed Elsaeed; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Alzheimer disease (AD) is a progressive neurodegenerative disease usually characterized by behavioral and mental alterations, loss of recent memories, cognitive deficits, and the inability to live independently (Waldemar et al., 2007). The most severely affected brain regions are the neocortex and hippocampus, which classically show the main pathological hallmarks of AD, including abnormal accumulation of extracellular amyloid beta-peptide (Aβ) plaques as well as intracellular aggregates of tau-containing neurofibrillary tangles (NFTs) or phosphorylated tau (DeTure & Dickson, 2019; Frisoni et al., 1999). It accounts for 60% of causes of dementia worldwide, with a higher prevalence in females by 1.9 times (Cao et al., 2020). Moreover, the high cost of care and research can affect economies, as the global cost 2018 was estimated to reach $ 1 trillion (Patterson, 2018).
Dysphagia is an impairment in swallowing due to sensory, motor, or behavioral causes or a combination of them (Özsürekci et al., 2020). Neurogenic dysphagia occurs in patients with neurological diseases of different etiologies, such as degenerative, dementia, myopathy, traumatic, and vascular etiologies (Panebianco et al., 2020). It is considered one of the geriatric syndromes associated with impaired quality of life (QoL), malnutrition and weight loss, aspiration pneumonia, aspiration pneumonitis, and mortality (Fernández-Ruiz et al., 2021; Nagamine et al., 2020). No recent publications can accurately point to the prevalence of dysphagia among AD patients (Mira et al., 2022).
Dysphagia typically occurs at later stages of AD; however, subclinical dysphagia can be detected in mild or moderate stages of the disease, which is often underdiagnosed or overlooked (Simoes et al., 2020). AD-associated dysphagia has been explained by the involvement of cortical control of swallowing (Humbert et al., 2010) as well as weakness and atrophy of skeletal muscles causing sarcopenic dysphagia (Özsürekci et al., 2020), which means the involvement of the pharyngeal muscles as a part of the whole-body skeletal muscles.
The cortical deposition of Aβ plaques and NFTs has been proved in AD (Kloskowska et al., 2010), which are the pathological hallmarks of the disease. Although dysphagia is a common comorbidity in AD, no studies have investigated if there are biochemical, histopathological, and immune-histochemical local changes in pharyngeal muscles in AD. Previous studies have found indications for the deposition of neuropathological proteins outside the central nervous system in other degenerative disorders like Parkinson disease. Moreover, deposition of Aβ protein has been detected in specific skeletal muscles. If local deposition of Aβ protein in pharyngeal muscles in AD is proven, we better understand the pathogenesis of dysphagia in AD patients and may improve its management (Kuo et al., 2000). Thus, this study investigated structural changes in rats, including biochemical, histopathological, and immune-histochemical aspects of pharyngeal and tongue muscles in AlCl3/D-gal-induced AD.
2. Materials and Methods
Study animals
Animals were housed according to the regulations approved by the Veterinary Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University (VET- IACUC). Fourteen adult male Wistar rats (150-170 g, 12 weeks) were housed in private plastic cages with softwood chips for bedding and were fed on a balanced commercial diet and water ad libitum. Animals were acclimatized for two weeks before proceeding with the experiment.
Experimental design
Rats were randomly blindly allocated into two groups, each of 7 rats. Group I (control) received distilled water orally and subcutaneously daily for 45 days, while group II (AD) orally received AlCl3 (Sigma-Aldrich Co., USA) (200 mg/kg) and subcutaneously received D-galactose (HEGENG Co., China) (60 mg/kg) daily for 45 days (Chiroma et al., 2018; Ezzat et al., 2022). Aluminum chloride (AlCl3) and D-galactose (D-gal) were used as AD inducers due to their neurotoxicity, including morphological alterations, cognitive impairment, and altered brain neurochemistry in rodents due to its pro-oxidant nature as reported in previous studies (Liaquat et al., 2019; Rebai, & Djebli, 2008). AlCl3 is considered a neurotoxin, and D-gal is used to model subacute aging. Consequently, their combination can create a non-transgenic AD animal model (Xiao et al., 2011).
After the scheduled 45 days, behavioral tests were carried out for 5 days. Then, blood samples were taken for sera separation. Animals were decapitated, and the brains, tongue, and pharynx of each rat were dissected out, washed, and frozen at -20 °C for biochemical analysis or kept in 10% formalin saline for histological and immunopathological tests.
Measurement of rats’ body weight
The rats’ body weight in the two groups was measured at the beginning of the experiment, then twice a week until the end. Body weight change percentage was calculated from the Equation 1:
1. Body Weight at the End of the Experiment (g) - body Weight at the Beginning of the Investigation ×100
The aim of measuring body weight is to monitor any indirect decrease in feed intake.
Validation of AD in rats
Behavioral validation
Open field test
The locomotion and exploratory behavior were evaluated using an open-field test, performed as mentioned by Khalil et al. (2021). The measured parameters were the number of crossing squares and the rearing frequencies.
Y-maze test
The Y-maze test was used to evaluate short-term memory and motor activity. The test was conducted according to Khalil et al. (2020) and Khalil et al. (2021), and the measured parameters were the number of arm entries and the spontaneous alternation percentage (SAP%). The spontaneous alternation percentage depends on the rats’ natural tendency to alternate between three different arms.
Novel object recognition (NOR) test
The NOR test was used to evaluate hippocampal-dependent memory impairment (Lueptow, 2017) and was conducted as mentioned in previous research (Khalil et al., 2021). The calculated parameters were the total exploration time, the discrimination ratio (DR), and the recognition index (RI). DR was calculated using the Equation 2:

The recognition ratio, the rat’s capacity to determine the same object on different occasions, was calculated using the Equation 3:

(Costa et al., 2008; Lueptow, 2017).
Biochemical validation
Preparation of tissue homogenates
At a concentration of 20% (w/v), hippocampal and pharyngeal tissue homogenates were meticulously prepared in ice-cooled phosphate-buffered saline, employing a homogenizer. Subsequently, these homogenates underwent centrifugation, lasting 15 minutes at 5000×g while being maintained at a temperature of 4 °C. The resultant tissue homogenates were then carefully portioned into aliquots and preserved at -80 °C, pending further analysis for quantifying different biomarkers and mediators as described previously by El-Shoura et al. (2023).
Measurement of tissue malondialdehyde (MDA) concentration
Malondialdehyde (MDA) colorimetric/fluorometric assay kit (K739-100) was purchased from BioVision, USA. It was prepared as described by Halliwell and Chirico (1993).
Measurement of tissue total antioxidant capacity (TAC)
Rat TAC1/Substance P ELISA Kit (LS-F14457) was purchased from LSBio, Inc., USA. It was examined in hippocampal and pharyngeal homogenates as described by Koracevic et al. (2001).
Measurement of tissue brain-derived neurotrophic factor (BDNF)
Rat BDNF ELISA Kit (SEA011Ra) was purchased from Cloud-Clone Corp., USA. It was measured in hippocampal and pharyngeal homogenates, as described by Klein et al. (2011).
Measurement of tissue Aβ
Rat Aβ 1-42 ELISA Kit (LS-F23254) was purchased from LSBio, Inc., USA. Soluble and insoluble/formic acid-soluble Aβ were quantified in hippocampal and pharyngeal homogenates, as mentioned by Liu et al. (2011).
Histological examination
The samples were fixed, dehydrated, cleaned, and embedded in paraffin wax. Rotatory microtome sections of 3–4 µm in thickness were prepared and stained with hematoxylin and eosin (H&E), as Bancroft and Gamble (2008) mentioned.
Immunohistochemical examination
Glial fibrillar acidic protein (GFAP) was used to detect astrocyte protein in the hippocampus. The method was done according to Stoltenburg-Didinger et al. (1996).
Evaluation of immunohistochemical observations (area percentage)
The sections were stained and assessed by the Leica Quin 500 analyzer computer system (Leica Microsystems, Switzerland). The image analyzer was calibrated automatically to convert the pixels into actual micrometer units, then statistically analyzed for each specimen.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 6. Behavioral and biochemical data were expressed as Mean±SEM. An unpaired student t-test was used to compare the means. Histograms were drawn using GraphPad Prism.
3. Results
Body weight change, locomotor activity, and cognitive function
AD rats displayed a significant decrease (P=0.0077) in their body weight compared to control rats. Also, they exhibited a marked reduction in their locomotor activity, as evidenced by decreased crossing squares (P=0.0001) and rearing frequency (P=0.0001) compared to control rats. Regarding cognitive functions, AD rats showed a marked cognitive dysfunction both in the Y maze and the NOR test and displayed a significant decrease in the number of arm entries (P=0.0001) and SAP% (P=0.001). Furthermore, they significantly reduced the total exploration time (P=0.03). However, the decline in the DR and RI was not significant (P=0.17 and P=0.13, respectively) (Figure 1).

Biochemical parameters
Oxidative stress markers
The hippocampal and the pharyngeal MDA were significantly higher in the AD group compared to the controls (P≤0.0001, 0.0003, respectively) (Figures 2A, and 2B). Additionally, the hippocampal and pharyngeal TAC was significantly lower in the AD group compared to the controls (P=0.0005 and P=0.0002, respectively) (Figures 2C, and 2D).

Brain-derived neurotrophic factor
The BDNF was significantly lower in the hippocampus and the pharyngeal muscles of the AD group compared to the control group (P=0.0001 and P<0.0001, respectively) (Figures 3A, and 3B).

Aβs
The Aβ levels in the hippocampus and the pharyngeal muscles were significantly higher in the AD group compared to the control group (P=0.0003 and P=0.0017, respectively) (Figures 3C, and 3D).
Hematoxylin and eosin-stained sections
Hippocampus H&E-stained sections
The H&E-stained hippocampus sections of the control rats revealed the normal histological structure of the molecular layer, pyramidal cell layer, and polymorphic cell layer (Figure 4A). On the other hand, hippocampus sections of AD rats showed histopathological disruption in the form of cellular disorganization of pyramidal cells that had NFTs confirming the induction of AD (Figure 4B).

Tongue H&E-stained sections
Tongue sections of control rats showed the normal microscopic structure of a muscular organ covered dorsally by mucous membrane and underlined by propria-submucosa that was formed of fibro-elastic connective tissue and centrally contained skeletal muscle mass with intermuscular fibro-elastic connective tissue in between. Lingual myofibers appeared cylindrical, unbranched, striated, and multinucleated (Figure 4C). On the contrary, tongue sections of AD rats revealed multiple features of degeneration in skeletal muscles, including fragmentation of myofibers. Some myofibers appeared hyper-eosinophilic and necrotic, while others lost their striation. Additionally, rupture of some muscular fibers, edematous interstitial tissue, and inter-/extra- myofibril hemorrhage were observed (Figure 4D).
Pharynx H&E-stained sections
The H&E-stained oropharynx sections of control rats showed normal histological form of the lining mucous membrane that was underlined by fibro-elastic connective tissue in propria-submucosa then circularly arranged pharyngeal muscles (Figure 4E). Pharyngeal muscles were formed of cylindrical, unbranched, striated myofibers that appeared multinucleated and separated from each other by intermuscular connective tissue (Figure 4F). On the other hand, oropharynx sections obtained from AD rats showed abnormal muscular architecture and fragmentation, myofiber rupture, and some myofibers appeared hyper-eosinophilic and necrotic. Hemorrhage was also observed in the myofibers (Figure 4G).
Immunohistochemical-stained sections
Immunohistochemical examination of the hippocampus of the control rats (group I) displayed a positive immunoexpression of the fibrillary astrocyte bodies and processes to GFAP. Meanwhile, the immunoreactivity concentration was significantly increased in the AD rats (group II) (Figures 5A, and 5B). The percentage area covered by GFAP-positive immunoreactive cells within the hippocampus revealed a significant elevation in the AD rats by 44.6 fold compared with the control rats (Figure 5C).

4. Discussion
There is evidence of pathological changes outside the central nervous system in AD, as some studies reported the presence of certain species of phosphorylated tau in peripheral tissues of AD patients, such as the submandibular gland (Dugger et al., 2016) and the deposition of Aβ plaques in the testis and ovary of patients (Miklossy et al., 1999), with evidence of impaired sperm function in vitro (Tavares et al., 2017). In addition, phosphorylated tau has been seen in AD human participants’ peripheral tissues like skin and skeletal muscle (Rodríguez-Leyva et al., 2015). Therefore, this work is meant to assess the histological abnormalities of skeletal muscles in the tongue and oropharynx in AD, in addition to the neurodegenerative impact of the disease.
In this study, AD was confirmed by the impairment of the working spatial and recognition memory, the locomotor disturbance, and the significant increase of hippocampal Aβ levels in AD rats. These results are consistent with Khalil et al. (2020) and Cheignon et al. (2018). Also, AD diagnosis was confirmed by the decreased BDNF expression in the hippocampal homogenates, the significant increase in MDA and decrease in TAC, which is in line with previous studies (Tapia-Arancibia et al., 2008; Tobore, 2019), and the significant increase in hippocampal immunoreactivity to GFAP, which was explained by Kamphuis et al. (2014). Besides, the histopathological changes in the hippocampus established the diagnosis of AD, which agrees with the work of DeTure & Dickson, (2019) and Ryan et al. (2015).
In our study, the development of dysphagia was hypothesized by the significant body weight loss observed in AD rats, as the eating and drinking patterns decreased by 25%-35% in AD versus the control group. Moreover, the histopathology of the tongue and pharynx exhibited myofibers fragmentation and rupture associated with edematous interstitial tissue representing structural affection of the muscles, which may be regarded as a potential contributor to dysphagia. Our results agree with Ogawa et al. (2018), who stated that sarcopenia is closely linked to AD and may be involved in the pathophysiological process of AD, as the involvement of pharyngeal muscles is a part of this generalized skeletal muscle affection. Furthermore, these local changes are associated with significant changes in local oxidative stress markers. Further research is needed to elucidate the possible relation between the pharyngeal local changes in AD and the development of dysphagia.
In our study, we show for the first time that degeneration of the tongue and pharyngeal muscles is evident in AD rats in the form of altered muscular structure, rupture and necrosis of the myofibers, and detection of oxidative stress in pharyngeal muscles. AD pharyngeal muscles even exhibited a significant increase in Aβ levels, which could be explained theoretically by the migration/spread of Aβ along cranial nerves, e. g. the vagus nerve. Otherwise, it could be formed in the muscles through metabolic alterations, leading to increased amyloid deposition. Also, the downregulation of BDNF levels in AD pharyngeal muscles could be explained by an injurious effect on the supplying cranial nerves, e. g. the vagus nerve. Further research is still needed to examine these hypotheses.
5. Conclusion
Dysphagia in AD has been discussed as originating mainly from central cerebral affection. So far, there has not been any biochemical, histopathological, or immune-histochemical evidence of AD in the tongue and pharyngeal muscles themselves. In our study, we show for the first time evidence for the presence of AD pathology in these structures, possibly one of the main contributing factors to dysphagia observed in AD. Additional investigations are needed to clarify the underlying processes that could be targeted to decrease the incidence of dysphagia and their direct linkage to clinical impairment. As a potential outlook, targeting AD pathology in these structures might, therefore, prevent dysphagia and potentially enhance the QoL in AD patients from this point. Future studies should explore the other factors contributing to dysphagia and the underlying molecular mechanisms involved in its pathogenesis. However, this is the first study to establish successfully the link between dysphagia and AD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Veterinary Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University (VET- IACUC) (Code: Vet CU28/04/2021/267).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Study design, data interpretation: Ramy Abdelnaby; Data collection and analysis: Heba M. A. Khalil, Yasmine H. Ahmed, Dalia Zaafar, and Mohamed Y. Mahmoud; Writing: Alexa Häger, Ramy Abdelnaby, and Eman Mohammed Elsaeed; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Bancroft, J. D., & Gamble, M. (2008). Theory and practice of histological techniques. London: Churchill Livingstone. [Link]
Cao, Q., Tan, C. C., Xu, W., Hu, H., Cao, X. P., & Dong, Q., et al. (2020). The prevalence of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 73(3), 1157-1166. [DOI:10.3233/JAD-191092] [PMID]
Cheignon, C., Tomas, M., Bonnefont-Rousselot, D., Faller, P., Hureau, C., & Collin, F. (2018). Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biology, 14, 450-464. [DOI:10.1016/j.redox.2017.10.014] [PMID] [PMCID]
Chiroma, S. M., Mohd Moklas, M. A., Mat Taib, C. N., Baharuldin, M. T. H., & Amon, Z. (2018). D-galactose and aluminium chloride induced rat model with cognitive impairments. Biomedicine & Pharmacotherapy, 103, 1602-1608. [DOI:10.1016/j.biopha.2018.04.152] [PMID]
Costa, M. S., Botton, P. H., Mioranzza, S., Ardais, A. P., Moreira, J. D., & Souza, D. O., et al. (2008). Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho-CREB immunocontent in the hippocampus. Neurochemistry International, 53(3-4), 89-94. [DOI:10.1016/j.neuint.2008.06.006] [PMID]
DeTure, M. A., & Dickson, D. W. (2019). The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration, 14(1), 32. [DOI:10.1186/s13024-019-0333-5] [PMID] [PMCID]
Dugger, B. N., Whiteside, C. M., Maarouf, C. L., Walker, D. G., Beach, T. G., & Sue, L. I., et al. (2016). The presence of select tau species in human peripheral tissues and their relation to Alzheimer’s disease. Journal of Alzheimer’s Disease, 51(2), 345-356. [DOI:10.3233/JAD-150859] [PMID] [PMCID]
El-Shoura, E. A. M., Salem, M. A., Ahmed, Y. H., Ahmed, L. K., & Zaafar, D. (2023). Combined β-sitosterol and trimetazidine mitigate potassium dichromate-induced cardiotoxicity in rats through the interplay between NF-κB/AMPK/mTOR/TLR4 and HO-1/NADPH signaling pathways. Environmental Science and Pollution Research International, 30(25), 67771–67787. [DOI:10.1007/s11356-023-27021-1] [PMID] [PMCID]
Fernández-Ruiz, V. E., Paredes-Ibáñez, R., Armero-Barranco, D., Sánchez-Romera, J. F., & Ferrer, M. (2020). Analysis of quality of life and nutritional status in elderly patients with dysphagia in order to prevent hospital admissions in a COVID-19 pandemic. Life, 11(1), 22. [DOI:10.3390/life11010022] [PMID] [PMCID]
Frisoni, G. B., Laakso, M. P., Beltramello, A., Geroldi, C., Bianchetti, A., & Soininen, H., et al. (1999). Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology, 52(1), 91-100. [DOI:10.1212/WNL.52.1.91] [PMID]
Halliwell, B., & Chirico, S. (1993). Lipid peroxidation: Its mechanism, measurement, and significance. The American Journal of Clinical Nutrition, 57(5 Suppl), 715S–725S. [DOI:10.1093/ajcn/57.5.715S] [PMID]
Humbert, I. A., McLaren, D. G., Kosmatka, K., Fitzgerald, M., Johnson, S., & Porcaro, E., et al. (2010). Early deficits in cortical control of swallowing in Alzheimer’s disease. Journal of Alzheimer’s Disease, 19(4), 1185-1197. [DOI:10.3233/JAD-2010-1316] [PMID] [PMCID]
Kamphuis, W., Middeldorp, J., Kooijman, L., Sluijs, J. A., Kooi, E. J., & Moeton, M., et al. (2014). Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiology of Aging, 35(3), 492-510. [DOI:10.1016/j.neurobiolaging.2013.09.035] [PMID]
Khalil, H. M., Salama, H. H., Al-Mokaddem, A. K., Aljuaydi, S. H., & Edris, A. E. (2020). Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer’s disease in rats. Journal of Functional Foods, 75, 104296. [DOI:10.1016/j.jff.2020.104296]
Khalil, H. M. A., Eliwa, H. A., El-Shiekh, R. A., Al-Mokaddem, A. K., Hassan, M., & Tawfek, A. M., et al. (2021). Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. Journal of Ethnopharmacology, 277, 114141. [DOI:10.1016/j.jep.2021.114141] [PMID]
Klein, A. B., Williamson, R., Santini, M. A., Clemmensen, C., Ettrup, A., & Rios, M., et al. (2011). Blood BDNF concentrations reflect brain-tissue BDNF levels across species. International Journal of Neuropsychopharmacology, 14(3), 347-353. [DOI:10.1017/S1461145710000738] [PMID]
Kloskowska, E., Pham, T. M., Nilsson, T., Zhu, S., Oberg, J., & Codita, A., et al. (2010). Cognitive impairment in the Tg6590 transgenic rat model of Alzheimer’s disease. Journal of Cellular and Molecular Medicine, 14(6b), 1816-1823. [DOI:10.1111/j.1582-4934.2009.00809.x] [PMID] [PMCID]
Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S., & Cosic, V. (2001). Method for the measurement of antioxidant activity in human fluids. Journal of Clinical Pathology, 54(5), 356-361. [DOI:10.1136/jcp.54.5.356] [PMID] [PMCID]
Kuo, Y. M., Kokjohn, T. A., Watson, M. D., Woods, A. S., Cotter, R. J., & Sue, L. I., et al. (2000). Elevated Aβ42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AβPP metabolism. The American Journal of Pathology, 156(3), 797-805. [DOI:10.1016/S0002-9440(10)64947-4] [PMID]
Liaquat, L., Sadir, S., Batool, Z., Tabassum, S., Shahzad, S., & Afzal, A., et al. (2019). Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sciences, 217, 202-211. [DOI:10.1016/j.lfs.2018.12.009] [PMID]
Liu, R. M., van Groen, T., Katre, A., Cao, D., Kadisha, I., & Ballinger, C., et al. (2011). Knockout of plasminogen activator inhibitor 1 gene reduces amyloid beta peptide burden in a mouse model of Alzheimer’s disease. Neurobiology of Aging, 32(6), 1079-1089. [DOI:10.1016/j.neurobiolaging.2009.06.003] [PMID] [PMCID]
Lueptow, L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. Journal of Visualized Experiments: JoVE, (126), 55718. [DOI:10.3791/55718] [PMID]
Ezzat, M. I., Issa, M. Y., Sallam, I. E., Zaafar, D., Khalil, H. M. A., & Mousa, M. R., et al. (2022). Impact of different processing methods on the phenolics and neuroprotective activity of Fragaria ananassa Duch. extracts in a D-galactose and aluminum chloride-induced rat model of aging. Food & Function, 13(14), 7794-7812. [DOI:10.1039/D2FO00645F] [PMID]
Miklossy, J., Taddei, K., Martins, R., Escher, G., Kraftsik, R., & Pillevuit, O., et al. (1999). Alzheimer disease: Curly fibers and tangles in organs other than brain. Journal of Neuropathology and Experimental Neurology, 58(8), 803-814. [DOI:10.1097/00005072-199908000-00003] [PMID]
Mira, A., Gonçalves, R., & Rodrigues, I. T. (2022). Dysphagia in Alzheimer’s disease: A systematic review. Dementia & Neuropsychologia, 16(3), 261–269. [DOI:10.1590/1980-5764-dn-2021-0073] [PMID] [PMCID]
Nagamine, T., Takayama, A., & Matsumoto, Y. (2020). Low-dose and short-term corticosteroid therapy for aspiration-induced lung injury in an elderly patient with Alzheimer’s Disease. International Medical Journal, 27(2), 118-119. [Link]
Ogawa, Y., Kaneko, Y., Sato, T., Shimizu, S., Kanetaka, H., & Hanyu, H. (2018). Sarcopenia and muscle functions at various stages of Alzheimer disease. Frontiers in Neurology, 9, 710. [DOI:10.3389/fneur.2018.00710] [PMID] [PMCID]
Özsürekci, C., Arslan, S. S., Demir, N., Çalışkan, H., Şengül Ayçiçek, G., & Kılınç, H. E., et al. (2020). Timing of dysphagia screening in Alzheimer’s dementia. Journal of Parenteral and Enteral Nutrition, 44(3), 516-524. [DOI:10.1002/jpen.1664] [PMID]
Panebianco, M., Marchese-Ragona, R., Masiero, S., & Restivo, D. A. (2020). Dysphagia in neurological diseases: A literature review. Neurological Sciences, 41(11), 3067–3073. [DOI:10.1007/s10072-020-04495-2] [PMID] [PMCID]
Patterson, C. (2018). World Alzheimer. Report 2018 The state of the art of dementia research: New frontiers. London: Alzheimer’s Disease International. [Link]
Rebai, O., & Djebli, N. E. (2008). Chronic exposure to aluminum chloride in mice: Exploratory behaviors and spatial learning. Advances in Biological Research, 2(1-2), 26-33. [Link]
Rodríguez-Leyva, I., Chi-Ahumada, E., Calderón-Garcidue-as, A. L., Medina-Mier, V., Santoyo Martha, E., & Martel-Gallegos, G. (2015). Presence of phosphorylated tau protein in the skin of Alzheimer’s disease patients. Molecular Biomarkers & Diagnosis, S6, 005-10. [Link]
Ryan, N. S., Rossor, M. N., & Fox, N. C. (2015). Alzheimer's disease in the 100 years since Alzheimer's death. Brain: A Journal of Neurology, 138(Pt 12), 3816–3821. [DOI:10.1093/brain/awv316] [PMID]
Simões, A. L. S., Oliva Filho, A., & Hebling, E. (2020). Signs for early detection of dysphagia in older adults with severe Alzheimer’s disease. The Journal of Nutrition, Health & Aging, 24, 659-664. [DOI:10.1007/s12603-020-1382-8] [PMID]
Stoltenburg-Didinger, G., Pünder, I., Peters, B., Marcinkowski, M., Herbst, H., & Winneke, G., et al. (1996). Glial fibrillary acidic protein and RNA expression in adult rat hippocampus following low-level lead exposure during development. Histochemistry and Cell Biology, 105(6), 431-442. [DOI:10.1007/BF01457656] [PMID]
Tapia-Arancibia, L., Aliaga, E., Silhol, M., & Arancibia, S. (2008). New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Research Reviews, 59(1), 201-220. [DOI:10.1016/j.brainresrev.2008.07.007] [PMID]
Tavares, R. S., Martins, S., Almeida-Santos, T., Sousa, A. P., Ramalho-Santos, J., & da Cruz e Silva, O. A. (2017). Alzheimer’s disease-related amyloid-β 1-42 peptide induces the loss of human sperm function. Cell and Tissue Research, 369(3), 647-651. [DOI:10.1007/s00441-017-2665-1] [PMID]
Tobore, T. O. (2019). On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurological Sciences, 40(8), 1527–1540. [DOI:10.1007/s10072-019-03863-x] [PMID]
Waldemar, G., Dubois, B., Emre, M., Georges, J., McKeith, I. G., & Rossor, M., et al. (2007). Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. European Journal of Neurology, 14(1), e1-e26. [DOI:10.1111/j.1468-1331.2006.01605.x] [PMID]
Type of Study: Original |
Subject:
Clinical Neuroscience
Received: 2023/07/29 | Accepted: 2023/10/29 | Published: 2024/09/1
Received: 2023/07/29 | Accepted: 2023/10/29 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





