Volume 14, Issue 6 (November & December 2023)
BCN 2023, 14(6): 843-856 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Razavi-Toosi S, Asadi Y, Aboutaleb N, Faezi M. Conditioned Medium Derived From the Human Amniotic Membrane Prevents Brain Damage Against Cerebral Ischemia/Reperfusion in Subacute, Acute, and Chronic Phases in a Rat Model of Stroke. BCN 2023; 14 (6) :843-856
URL: http://bcn.iums.ac.ir/article-1-2756-en.html
URL: http://bcn.iums.ac.ir/article-1-2756-en.html
1- Medical Biotechnology Research Center, Faculty of Paramedicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- Physiology Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- Physiology Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1089 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Stroke is a common disease in countries and is the second leading cause of death and leaves the country with a wide range of disabilities (Faezi et al., 2018). Previous studies have shown that in 2016, 5.5 million deaths worldwide were caused by stroke, of which 2.7 million were ischemic strokes and 2.8 million were hemorrhagic strokes (Saini et al., 2021). There are two forms of stroke, ischemic and hemorrhagic; in the ischemic type, the vessels are blocked and the blood flow to an area of the brain is stopped, and it is associated with learning and memory disorders in these people (Adams et al., 1993). During the process of ischemia, the brain undergoes a series of pathological events, including mitochondrial disruption (Azedi et al., 2022), Amplification of reactive oxygen species (ROS) generation, and caspase activation (Azedi et al., 2022), subsequently starting necrosis and apoptosis (Mehrjerdi et al., 2015). Ischemic stroke can affect the strength of the blood-brain barrier (BBB) and increase its permeability through changes in tight junctions and connexin-43 hemichannels. As a result, it increases the penetration of molecules from the blood to the brain and causes edema, increases the volume of brain tissue, and finally brain damage (Krueger et al., 2017). Despite the significant advances in medical science in the treatment of diseases, only a very limited number of drugs are available for the treatment of stroke. For example, currently, tissue plasminogen activator (tPA) is accepted by the Food and Drug Administration (FDA) for ischemic stroke, which is looking for other ways to treat this disorder due to its adverse effects and short effectiveness (Schwamm et al., 2013). Recently, the use of stem cells has shown favorable effects in the treatment of stroke and has attracted the attention of the scientific community (Bang, 2016). Mesenchymal stem cells (MSCs) have unique properties, such as easy access, anti-inflammatory effects, and weak immune response (Shin et al., 2016). The multipotent mesenchymal cells can improve the symptoms of cerebral ischemia due to disadvantages, such as cell number, the type of cell used, the possibility of cell migration, and the creation of ectopic tissue or turning into undesirable cells (Faezi et al., 2018).
During the process of extraction, culture, passage, and transplantation into the recipient tissue, MSCs may be affected by various conditions in terms of temperature stress, nutrition, possible contamination, and hypoxia, which all can increase harmful inflammatory factors in the recipient tissue (Hao et al., 2017). Previous studies have shown that only a limited number of MSCs transplanted to the target tissue survive. Also, Toma et al. presented that only 1% of the MSCs survived 24 hours after transplantation into an organ like a failing heart (Toma et al., 2002); thus, it seems that the useful therapeutic properties observed from pluripotent cells are due to their paracrine effects (Abbasi-Malati et al., 2018).
There is a general view, in which the substances that are secreted by MSCs into their culture medium, are named the scrotum, and their culture medium is called conditioned medium (CM) (Alijani-Ghazyani et al., 2021). The observed therapeutic effects and abilities of MSCs in regenerative medicine can be related to the ability of these cells to secrete growth factors, cytokines, and nutritional factors (Abbasi-Malati et al., 2018).
Many side effects of cell therapy, including the activation of the host immune response or tumorigenesis, are not expected from CM; thus, it seems that CM can be used as a reliable alternative to cell therapy (Joyce et al., 2010). The beneficial therapeutic effects of MSC-CM have been observed in various diseases, such as stroke, spinal cord damage, and neurological disorders (Pawitan, 2014).
As mentioned, paracrine factors secreted from MSCs have been used to improve pathological conditions of cerebral ischemia. Secreted paracrine factors include cytokines, angiogenic and anti-apoptotic factors, such as insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and interleukin 6 (IL-6) (Hung et al., 2007). These agents can be effective in treating neurodegenerative disorders and modulating cell signaling pathways (Pazoki-Toroudi et al., 2016).
Based on the mentioned studies, we investigated the beneficial effects of MSC-CM on neuronal injury in focal cerebral ischemia, and particularly, we investigated whether the MSC-CM will maintain the blood vessel integrity and ameliorate the brain edema and infarct size. Additionally, we investigated how the MSC-CM acts in subacute, acute, and chronic phases and when the best response to medium conditioning occurs in the ischemic brain tissue.
2. Materials and Methods
Preparation and isolation of human amniotic membrane stem cells (hAMSCs)
hAMSCs were derived from our previous research (Tousi et al., 2017). The membrane was carefully separated from the target organ, rinsed several times with phosphate-buffered saline (PBS) to remove blood vessels and blood clots, and turned into small pieces by cutting methods. The tissue pieces were mixed by a homogenizer and placed in a centrifuge (5 minutes/speed 1250 rpm). Next, the supernatant was removed and 30 mL of collagenase was added to the contents of the pellet. The pellet was kept at a temperature of 37°C in an incubator with a wetness of 5% CO2 for 60 minutes. Next, the tissue pieces were centrifuged at 1250 rpm for 5 minutes, the supernatant was discarded, and trypsin (0.25% containing 1 mL of ethylenediaminetetraacetic acid) was poured into the pellets. Then, the samples were incubated for 30 minutes, washed several times, and exposed to tris-ammonium chloride for 10 minutes to eliminate red blood cells. Finally, the pellet containing MSCs in the right volume was placed in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) to obtain the desired cell number.
Preparation of CM derived from hAMSC
MSCs were placed in the correct amount of α-MEM with 10% FBS, 100 μg/mL streptomycin, 2 mM L-glutamine, and 100 U/mL penicillin overnight. In the second quarter, the cells were rinsed with PBS (twice) and placed in the α-MEM medium without serum to provide a CM. To extract the CM, the cells were placed in a normoxic incubator (94% N2, 5% CO2, and 1% O2) for 48 hours. To eliminate the existing cells, the cultured MSC supernatant was extracted and centrifuged at 1200 rpm for 10 minutes. Then, the CM was filtered (0.22 μm). As a result, the ventilated environment was combined, dried, and stored at -80°C for further study. CM was obtained from 2×106 amniotic MSCs (Faezi et al., 2018)
Animals
A total of 105 adult male Wistar rats weighing 250 to 300 g were achieved from the Animal Breeding Center of Gilan University of Medical Sciences. Animals were housed in a temperature-controlled room with a temperature of 22-24°C and 55% humidity, with a light schedule of 12 hours of dark and 12 hours of light, and sufficient food and water. Three categories were considered for the study: Sham (n=15), middle cerebral artery occlusion (MCAO) (n=45), and MCAO+MSC-CM (n=45) (Figure 1). Intraventricular injection of MSC-CM was done 30 minutes after reperfusion.

Animals
Rats were randomly placed into three groups: Damage volume, brain water volume, and BBB strength. Animals in each group were divided into three subgroups: Sham n=5, MCAO n=15, and treatment n=15. The sham group was just under surgical stress and the vehicle and treatment groups were under focal cerebral ischemia for 60 minutes. Intraventricular injection of CM was done for up to 30 minutes after ischemia, and 6, 20, and 30 hours after reperfusion, infarct volume (IV) and behavioral testing were examined in the corresponding group, and using the same way, BBB and brain water content (BWC) were assessed in other groups.
Generation of ischemic rat models and stereotaxic injection of MSC-CM
Rats were anesthetized with an appropriate dose of ketamine hydrochloride (100 mg/kg) along with xylazine (8 mg/kg) intraperitoneally (IP) according to their weight. MCAO was done according to the protocol by Longa et al. (1989).
Briefly, first, the common and external carotid arteries were completely occluded, and then the internal carotid artery was temporarily occluded by a clamp, a small hole was prepared on the common carotid artery by ophthalmic scissors, and a silicone rubber sheath with an oval tip was used to occlude the vein. It was directed to the internal carotid artery up to 20-22 mm above the location of the two branches. This conduction was continued until a slight resistance was felt, which means that the filament entered the anterior cerebral artery and blocked it. After 60 minutes of ischemia and removing the blocking filaments, the blood flow started again in the vein. Rectal temperature (Citizen-513w) was checked and maintained at 37.0°C during surgery. Immediately after MCAO, stereotactic injection of CM was performed. The animal was fixed in the stereotaxic device (Stoelting, USA) by ear and mouth, and then a hole 2 mm in diameter was made on the skull 1 mm posterior and 1.5 mm lateral to the bregma on the right side with a dental drill. For the brain injections, three subgroups were considered (n=5): 1) Sham, 2) MCAO and 3) AMSC-CM. Animals were anesthetized again with a lower dose of ketamine hydrochloride (100 mg/kg) along with xylazine (8 mg/kg). By a Hamilton syringe (2 μL Hamilton, Switzerland) connected to the device, all injections performed in these three subgroups were performed according to the coordinates of the Paxinus atlas (anterior/posterior [AP]=1 mm, dorsal/ventral [DV]=3.5 mm, and lateral [L]=1.5 mm) (Teixeira et al., 2015). The volume of injection into the right cerebral ventricle (ICV) was 0.5 μL with an injection rate of 0.25 μL/min. After the end of each injection, two minutes were allowed to prevent any return to the needle channel. In the end, the needle was removed and the animals were sutured.
Local cerebral blood flow (LCBF) measurement
Before the surgery, the temporal bone in the right hemisphere of the anesthetized animal was considered for the placement of the laser Doppler flowmeter (LDF) probe. Baseline regional blood flow during MCAO and reperfusion was recorded by the probe. Cerebral blood flow was noted constantly every 5 min until 10 min after reperfusion. Local blood flow was recorded at certain times in the ischemic area (15 minutes before MCAO, 45 minutes during MCAO, and 15 minutes after reperfusion) (Bigdeli et al., 2007).
Neurologic deficit score
After 6, 20, and 30 hours of reperfusion (after removing the blocker, animals were returned to their cages), the neurological behavior of the animals was assessed. The neurological injury was scored using a six-point protocol (Longa et al. 1989) as follows: Correct neurological activity=0, forelimb flexion=1, turning the body to the opposite side of the lesion=2, absence of reflex on the right side of the body=3, lack of locomotor activity and hypokinesia=4, and death=5. In case of death, the animals were removed from the study. Reasons, such as asphyxia, pulmonary failure, or subarachnoid hemorrhage.
IV analysis
A high dose of chloral hydrate (800 mg/kg) (Merck, Germany) was used for deep anesthesia equivalent to the death of the animals. The heads of the animals were cut and the brains were quickly detached and placed in saline for 15 minutes in a refrigerator at 4°C. Then, the brain was placed in Manrix (Brain Matrix, Iran) and divided into eight sections with 2 mm thick slices. The slices were floated in a 2% solution of 2, 3, 5-triphenyl tetrazolium chloride (Merck, Germany) and placed in an oven at 37°C for 15 minutes. Then, the slices were photographed and the unstained and white areas were defined as infarcts, and the volume of the damaged area was dignified using image analysis software (Image J, version 1.48, US National Institutes of Health, Bethesda, MD, USA). The size of the lesion was measured by calculating the stained parts in each part and multiplying it by the thickness of that slice (2 mm) and then summing the data of all eight parts (Swanson et al., 1990) (Equation 1):
1. Corrected IV=Left hemisphere volume-(Right hemisphere volume-IV)
BWC analysis
After removing the brain and separating the cerebellum and olfactory bulb, wet weight (WW) was measured (Bigdeli et al., 2007). Then, the sections were located in the oven for 6, 20, and 30 hours at 120°C, and the dry weight (DW) was also recorded. Then, the Equation 2 was used to determine the water content in the brain:
2. [(WW-DW)/WW]×100
Analysis of BBB strength
Briefly, 30 minutes after MCAO, 4 mL/kg of 2% Evans blue (EB, Sigma Chemicals) solution in saline was inserted through the tail vein. Then, 6, 20, and 30 hours after recirculation, the animal’s chest was ruptured under deep anesthesia. The animal’s vessels were flushed through the left ventricle with 250 mL of saline to completely clear the EB from the vessels, and this procedure continued until the white fluid was removed from the right atrium. In the next step, the brains were taken out and the brain hemispheres were weighed separately for WW analysis. To measure the presence of EB in the tissue of the right hemispheres, they were homogenized in 2.5 mL of PBS, and 2.5 mL of 60% trichloroacetic acid (TTC) was poured into it to precipitate the protein, and they were vortexed for 5 minutes. Next, the sections were located at 4°C for 30 minutes and centrifuged at 1000 ×g for 30 minutes. Then, the supernatant was extracted and the amount of EB in it was recorded at a wavelength of 610 nm using a spectrophotometer (Perkin-Elmer, U.S.). EB levels were expressed as μg/g of brain tissue against a standard curve (Bigdeli et al., 2007).
Statistical analysis
IV, edema, and BBB health value were shown as Mean±SEM and compared using one-way ANOVA (SPSS software, version 16; post hoc LSD test). Normalized standard deviation (NDS) was expressed as median (range) and analyzed using the Mann–Whitney U test. A P>0.05 was considered significant.
3. Results
Regional cerebral blood flow
Assessment of CBF using laser Doppler flowmetry (LDF) in both the MCAO and MCAO+hAMSC-CM groups showed that cerebral blood flow decreased less than 20% from baseline after MCA occlusion (Figure 2). This was continued throughout the 60 min of MCAO. There was no significant difference between the MCAO and MCAO+hAMSC-CM groups during 60 minutes of MCAO and 30 minutes of reperfusion.

Effects of AMSC-CM on lesion size
As illustrated in Figures 3A, 3B, 3C & 3D, no infarcts occurred in the sham group. However, significant infarct expansion was detected after MCAO. Ischemic damage occurred in the core, subcortex, and penumbra at different times of reperfusion, and the ischemic damage increased with increasing reperfusion time, and the maximum damage was observed 30 minutes after reperfusion.
The therapeutic effect of AMSC-CM was observed in the penumbra area more than in the core area. In the core, subcortex, and penumbra areas, a significant difference was seen between the following groups: MCAO (6, 20, and 30 hours) and sham, sham and treatment (except for the 6-hour group in the penumbra and subcortex areas), and ischemia and treatment.

Effects of AMSC-CM on sensory and practical behavior
Sensorimotor performance was significantly improved after treatment with AMSC-CM. For the neurological deficit score, the MCAO group showed extensive neurological damage 24 hours after recirculation. Among the groups, the group that was reperfused for 30 hours showed more movement injuries. Intraventricular injection of CM changed neurologic scores compared to the MCAO group. Neurological deficit scores were reduced in the treatment group compared to the MCAO group (Figure 4).

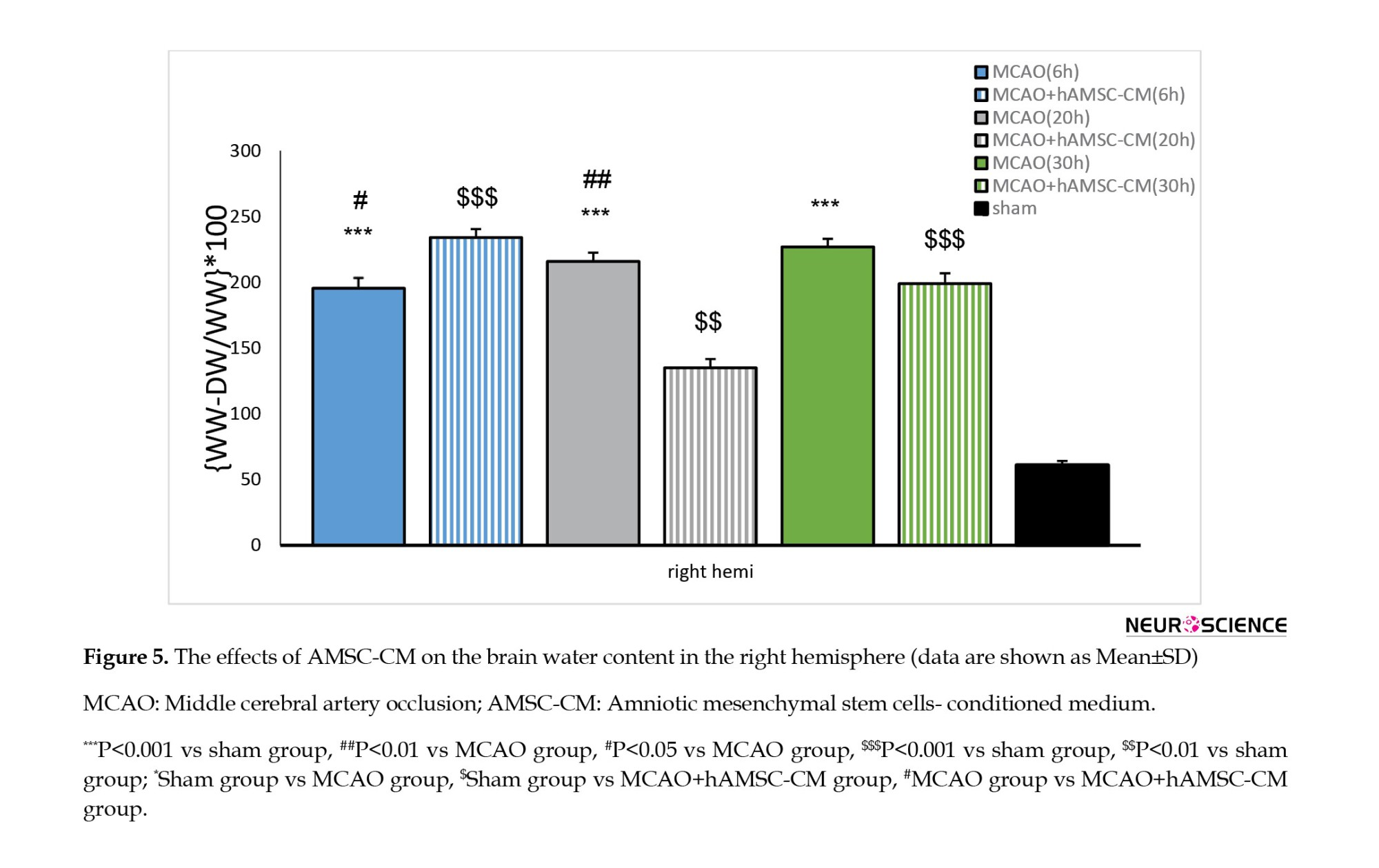
Effects of AMSC-CM on BWC
BWC was increased in the MCAO group at all times of reperfusion and increased by treatment with AMSC-CM (Figure 5). In the right hemisphere, a significant difference was seen between the opposite groups: MCAO (6, 20, and 30 hours) and sham, sham and treatment, and ischemia and treatment (only at 6 and 20 hours).
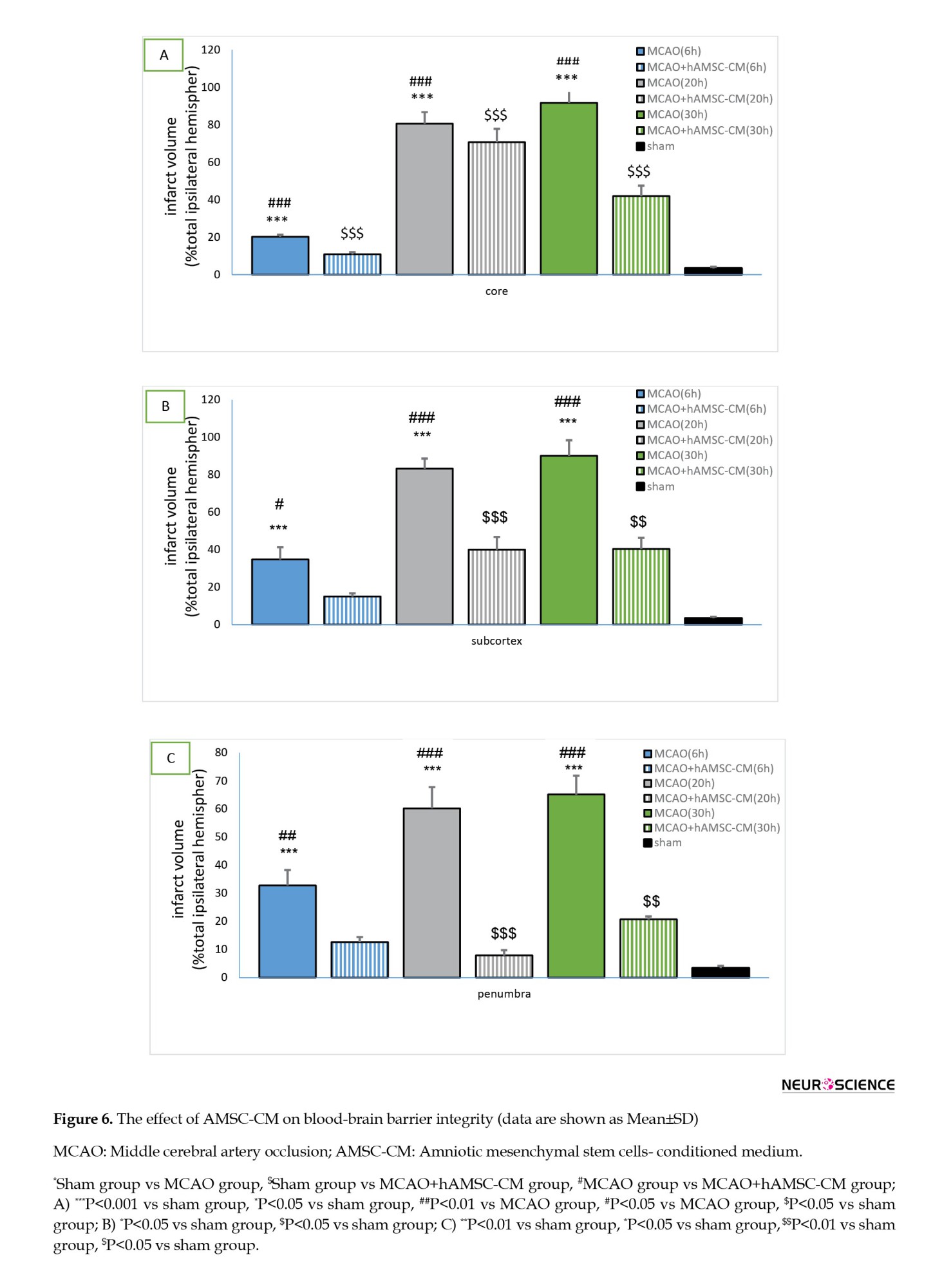
Effects of AMSC-CM on the health of the BBB
The identification factor of BBB disruption was considered based on the amount of EB penetration in the tissue (Figures 6A, 6B and 6C). The amount of EB entry in ischemic areas in the MCAO group (30 h) was strikingly greater than the sham group and treatment (6, 20, and 30 h) in the core, subcortex, and penumbra areas significantly reduced the entry of EB to the ischemic areas.
4. Discussion
In this study, we investigated the neuroprotective effects of AMSC-CM against focal ischemia injury and recirculation after 6, 20, and 30 hours, which was done for the first time in the scientific community. The important finding of this study was the neuroprotective effect of AMSC-CM time-dependently against ischemic injuries due to MCAO. The results of our experiments support the fact that AMSC-CM in the initial moments of reperfusion in an animal model may ameliorate the amount of damage to neurons and reduce the process of destruction of the BBB. In addition, the results of this research showed that AMSC-CM can improve nerve function at 6, 20, and 30 h after reperfusion.
Cerebral ischemia leads to a decrease in oxygen and glucose in the brain tissue, and the combination of these factors causes irreparable neurological deficits in the ischemic core in the first minutes after the onset of ischemia (Zhang et al., 2016). In the absence of oxygen, certain areas of the brain turn into a pathological environment where the recirculation of blood leads to the initiation of inflammatory and oxidative pathways and the imbalance of prooxidants and endogenous antioxidants. The excessive production of radicals, toxic free radicals, and ROS, increases the abnormal amount of malondialdehyde (MDA), which is a key index of lipid peroxidation and indicates a degree of membrane damage under oxidative stress conditions (Park et al., 2015). In addition, glutathione (GSH) and superoxide dismutase (SOD) are both harmful free radical absorbers and are seen in brain tissue with ischemia. Ultimately, a pathological environment resulting from ischemia leads to learning and memory impairment as well as loss of structure and function of neurons (Park et al., 2015).
As mentioned, the interruption of blood flow in the vessels leads to a lack of oxygen and then causes the creation of anaerobic infarction areas with a lack of energy in the brain tissues, which finally leads to the necrosis of the brain tissues (Nai et al., 2018).
The loss of neuronal function caused by ischemia begins with the discharge of excitatory neurotransmitters, which leads to membrane depolarization, increased intracellular calcium, and the construction of nitric oxide (NO) and ROS, i.e. radicals. Superoxide anion, hydrogen peroxide, and hydroxyl radical are greatly cytotoxic byproducts (Lee et al., 2010). Oxidative stress plays a central role in the pathogenesis of the acute stage of stroke. The creation of local temporary cerebral ischemia increases the production of free radicals and nitric oxide and decreases the activity of antioxidant enzymes, such as SOD, GSH, and catalase (Zhang et al., 2014). Increasing the levels of free radicals through the direct effect and destruction of cell proteins and lipids, as well as DNA damage, leads to the activation of apoptosis. Also, through an indirect effect, they disrupt the signaling pathway and gene regulation of the cell and finally cell death (Dirnagl et al., 1999).
Many studies have previously attracted the attention of scientists regarding the use of MSCs for the treatment of ischemic stroke. Among different sources of MSCs in the body, such as bone marrow, fat tissue, placenta, and other tissues, AMSCs have unique characteristics that make them have an exceptional place in medicine. Some of these features include an easy and accessible resource after each delivery. There is no legal problem regarding their use (Tousi et al., 2017). Unlike other MSCs that are extracted from other sources in the body, amniotic cells do not express classes 1 and 2, or their expression is very little. Thus, they have high immune tolerance compared to other MSCs (Tsuji et al., 2010). Also, due to their embryonic properties, they have a high proliferation and differentiation power compared to other MSCs (Alviano et al., 2007; Boltze et al., 2014).
It has been well established that MSCs can treat ischemia damage through mechanisms, such as angiogenesis, neurogenesis, and immune response regulation (Eckert et al., 2013).
Despite these advantages, arterial injection of MSCs has been reported to be associated with concerns, such as reduced cerebral blood flow as a result of cellular coagulation and microemboli due to cellular aggregation, which are modulated by dose, rate, and volume of injection (Cui et al., 2015).
At first, the researchers believed that the way MSCs work in tissue healing and renewal is due to their replacement with desired tissue cells, but with more studies and research, the focus shifted to their paracrine actions (Drago et al., 2013). MSCs can release paracrine factors that have immunomodulatory and neuroprotective properties and can help repair and regenerate damaged tissues (Cunningham et al., 2018). The medium prepared from the culture site of MSCs contains substances that have different anti-apoptotic and angiogenic effects, such as exosomes, VEGF, IGF-1, angiogenin, IL-6, IL-8, and hepatocyte growth factor (HGF), which can promote cell proliferation, differentiation, and vascularization. They also affect the generation and survival of cells under oxidative stress (Di Santo et al., 2009; Timmers et al., 2008).
As mentioned, some studies have shown that MSCs can release several paracrine factors. They exert their effects by increasing the expression of materials, such as HGF (Li et al., 2008). In previous studies, it has been shown that HGF exhibits angiogenic and antiapoptotic effects (Guo et al., 2008). In our previous studies, the potency of AMSC-CM in the face of focal cerebral ischemia and the hypothesized mechanisms, by which it reduced brain damage, were also investigated. Also, our results in previous studies showed that treatment with AMSC-CM can exert neuroprotective effects to reduce apoptosis, via decreasing the level of caspase-3 and Bex and increasing the level of Bcl-2 (Faezi et al., 2018).
AMSC-CM exerts its effect through the triggering of BDNF/ERK signaling pathways (Wang et al., 2017). Furthermore, our data demonstrated that the neuroprotective effects of AMSC-CM were thoroughly related to the reduction of injury volume. It has been well established that ERK1/2 and the MAPKs family modulate neuronal viability and cellular apoptosis. The stimulation of this compound leads to the phosphorylation of many cytoplasmic and membrane proteins. Also, the transfer of ERK1/2 from the cytosol to the cell nucleus causes the phosphorylation of various transcription factors, such as CREB protein (Zhao et al., 2017).
In our earlier research, it was shown that intraventricular administration of AMSC-CM at the beginning of recirculation increases the phosphorylation of ERK1/2 and decreases the expression of BDNF protein in the cortical region of infarcted rats, and the results were reversed by AMSC-CM (Faezi et al., 2018).
According to our findings, AMSC-CM can inhibit apoptosis and ultimately cell survival in hypoxic conditions by activating the PI3K-Akt pathway (Hung et al., 2007).
With a slight reduction in cerebral blood flow, brain tissue survives for a relatively long time, while brain damage becomes irreversible when blood flow is completely cut off. In other words, the sooner the blood flow starts again, the more effective it can be in the treatment. In the same study, it was reported that the volume of the penumbra area decreases with the increase of the reperfusion time (Lansberg & Dabus, 2013).
Our experiment showed that by increasing the reperfusion time for 30 hours, the amount of damage and the destruction of the BBB decreases.
During cerebral ischemia, increased expression of aquaporin-4, a type of protein water channel, occurs in the end feet of astrocytes, which causes the lack of strength and increased permeability of the BBB and the formation of cerebral edema (Fu et al., 2007).
Edema in the brain is one of the factors determining the patient’s survival rate in the early hours after a stroke (Dirnagl et al., 1999). Clinical and laboratory studies show that the development of edema following acute localized cerebral ischemia can aggravate the primary ischemic lesion (Shaw et al., 1959). It is said that by creating cerebral edema, the pressure inside the skull increases and by compressing the cerebral vessels, blood flow to the penumbra region is severely reduced and the death of neurons in this region is accelerated (Dirnagl et al., 1999). Rupture of the BBB is the most important cause of cerebral edema (Nishida & Gotoh, 1993). ROS, such as superoxide and hydroxyl radicals play an important role in the development of brain edema and brain lesions (Satoh et al., 2011). The exact mechanisms, by which superoxide anion and hydroxyl radicals participate in edema formation remain unknown. The formation of free radicals, especially super-oxidants and proteases, following cerebral ischemia causes alterations in the BBB and the formation of edema and cell death.
The outcomes of this study indicated that the blockage of the middle cerebral artery caused severe breaking of the BBB and the creation of cerebral edema.
This study showed that the production of free radicals increases cerebral edema in focal cerebral ischemia and the reduction of these free radicals leads to the reduction of cerebral edema. In a study, it was shown that the administration of MSC after cerebral ischemia by inhibiting the expression of aquaporin-4 can increase the strength of the BBB (Baez-Jurado et al., 2019; Tang et al., 2014).
In this paper, we investigated whether the paracrine effects caused by MSCs can increase the strength and integrity of the BBB. Our data demonstrated that MCAO can destroy the BBB, and with increasing reperfusion time, there is an increase in the destruction and permeability of the BBB and subsequent edema creation, which is reversed by AMSC-CM administration.
Also, BM-MSC-CM can recover neuron function after ischemic stroke (Tsai et al., 2014). Consistent with this result, our research showed that post-treatment with AMSC-CM can improve motor performance and neurological deficits 6, 20, and 30 hours after reperfusion.
Among the limitations of the present study was that the drug had to be stored and used under certain temperature conditions, and any delay in intraventricular injection or non-observance of temperature balance with the denaturation of functional proteins would cause the inactivation of the drug intervention. In this research, western blot and PCR techniques were not used due to the high division of the protocol and study groups and the need to focus on the tissue process. For this reason, the use of molecular techniques in future studies is suggested to identify proteins and investigate mechanisms at the molecular level.
It is also suggested that in the future, other complementary movement tests, such as the balance test and corner test will be used to more accurately evaluate functional disorders and increase the power of research by examining several inflammatory factors.
5. Conclusion
The use of AMSC-CM reduces lesion volume, cerebrospinal fluid accumulation, and BBB breakdown in three subacute, acute, and chronic phases of focal cerebral ischemia. AMSC-CM may be a hopeful therapeutic case for stroke, but further study is needed to consider the therapeutic nature of AMSC-CM. Finally, the results of this research open several paths that can be studied to investigate the protective effects of the treatment used in this research.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Animal Ethics Committee of Gilan University of Medical Sciences (Code: IR.GUMS.REC.1396.372) and all experiments were performed with the assumption of minimal suffering and harm to rats.
Funding
The present study financially supported by Vice Chancellor of Research and Technology, Guilan University of Medical Sciences (Grant No.: 96071502).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the faculty members of the Department of Physiology, Faculty of Medicine, Gilan University of Medical Sciences for their useful recommendations and cooperation.
Reference
Abbasi-Malati, Z., Roushandeh, A. M., Kuwahara, Y., & Roudkenar, M. H. (2018). Mesenchymal stem cells on horizon: A new arsenal of therapeutic agents. Stem Cell Reviews and Reports, 14(4), 484–499. [DOI:10.1007/s12015-018-9817-x] [PMID]
Aboutaleb, N., Tousi, S. M. T. R., Amirizadeh, N., Nasirinezhad, F., Nikougoftar, M., & Ganjibakhsh, M. (2017). A rapid and cost-effective protocol for isolating mesenchymal stem cells from the human amniotic membrane. Galen Medical Journal, 6(3), e670-e670. [DOI:10.31661/gmj.v6i3.670]
Adams, H. P., Jr, Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., & Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke, 24(1), 35–41 [DOI:10.1161/01.STR.24.1.35] [PMID]
Alijani-Ghazyani, Z., Roushandeh, A. M., Sabzevari, R., Salari, A., Razavi Toosi, M. T., & Jahanian-Najafabadi, A., et al. (2021). Conditioned medium harvested from Hif1α engineered mesenchymal stem cells ameliorates LAD-occlusion -induced injury in rat acute myocardial ischemia model. The International Journal of Biochemistry & Cell Biology, 130, 105897. [DOI:10.1016/j.biocel.2020.105897] [PMID]
Alviano, F., Fossati, V., Marchionni, C., Arpinati, M., Bonsi, L., & Franchina, M., et al. (2007). Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Developmental Biology, 7, 11. [DOI:10.1186/1471-213X-7-11] [PMID] [PMCID]
Azedi, F., Tavakol, S., Ketabforoush, A. H. M. E., Khazaei, G., Bakhtazad, A., & Mousavizadeh, K., et al. (2022). Modulation of autophagy by melatonin via sirtuins in stroke: From mechanisms to therapies. Life Sciences, 307, 120870.[DOI:10.1016/j.lfs.2022.120870] [PMID]
Baez-Jurado, E., Hidalgo-Lanussa, O., Barrera-Bailón, B., Sahebkar, A., & Ashraf, G. M., et al. (2019). Secretome of mesenchymal stem cells and its potential protective effects on brain pathologies. Molecular Neurobiology, 56(10), 6902–6927. [DOI:10.1007/s12035-019-1570-x] [PMID]
Bang O. Y. (2016). Clinical trials of adult stem cell therapy in patients with ischemic stroke. Journal of Clinical Neurology, 12(1), 14–20. [DOI:10.3988/jcn.2016.12.1.14] [PMID]
Bigdeli, M. R., Hajizadeh, S., Froozandeh, M., Rasulian, B., Heidarianpour, A., & Khoshbaten, A. (2007). Prolonged and intermittent normobaric hyperoxia induce different degrees of ischemic tolerance in rat brain tissue. Brain Research, 1152, 228–233. [DOI:10.1016/j.brainres.2007.03.068] [PMID]
Boltze, J., Lukomska, B., Jolkkonen, J., & MEMS–IRBI consortium (2014). Mesenchymal stromal cells in stroke: Improvement of motor recovery or functional compensation?. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1420–1421. [DOI:10.1038/jcbfm.2014.94] [PMID] [PMCID]
Shaw, C. M., Alvord, E. C., Jr, & Berry, R. G. (1959). Swelling of the brain following ischemic infarction with arterial occlusion. Archives of Neurology, 1, 161–177. [DOI:10.1001/archneur.1959.03840020035006] [PMID]
Cui, L. L., Kerkelä, E., Bakreen, A., Nitzsche, F., Andrzejewska, A., & Nowakowski, A., et al. (2015). The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Research & Therapy, 6(1), 11. [DOI:10.1186/scrt544] [PMID] [PMCID]
Cunningham, C. J., Redondo-Castro, E., & Allan, S. M. (2018). The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. Journal of Cerebral Blood Flow and Metabolism, 38(8), 1276–1292. [DOI:10.1177/0271678X18776802] [PMID] [PMCID]
Di Santo, S., Yang, Z., Wyler von Ballmoos, M., Voelzmann, J., Diehm, N., & Baumgartner, I., et al. (2009). Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. Plos One, 4(5), e5643. [DOI:10.1371/journal.pone.0005643] [PMID] [PMCID]
Dirnagl, U., Iadecola, C., & Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends in Neurosciences, 22(9), 391–397. [DOI:10.1016/S0166-2236(99)01401-0] [PMID]
Drago, D., Cossetti, C., Iraci, N., Gaude, E., Musco, G., & Bachi, A., et al. (2013). The stem cell secretome and its role in brain repair. Biochimie, 95(12), 2271–2285. [DOI:10.1016/j.biochi.2013.06.020] [PMID] [PMCID]
Eckert, M. A., Vu, Q., Xie, K., Yu, J., Liao, W., & Cramer, S. C., et al. (2013). Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. Journal of Cerebral Blood Flow and Metabolism, 33(9), 1322–1334. [DOI:10.1038/jcbfm.2013.91] [PMID] [PMCID]
Faezi, M., Nasseri Maleki, S., Aboutaleb, N., & Nikougoftar, M. (2018). The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. Journal of Chemical Neuroanatomy, 94, 21–31. [DOI:10.1016/j.jchemneu.2018.08.004] [PMID]
Fu, X., Li, Q., Feng, Z., & Mu, D. (2007). The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia, 55(9), 935–941. [DOI:10.1002/glia.20515] [PMID]
Guo, Y., He, J., Wu, J., Yang, L., Dai, S., & Tan, X., et al. (2008). Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Archives of Medical Research, 39(2), 179–188. [DOI:10.1016/j.arcmed.2007.11.001] [PMID]
Hao, T., Li, J., Yao, F., Dong, D., Wang, Y., & Yang, B., et al. (2017). Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano, 11(6), 5474–5488. [DOI:10.1021/acsnano.7b00221] [PMID]
Hung, S. C., Pochampally, R. R., Chen, S. C., Hsu, S. C., & Prockop, D. J. (2007). Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells, 25(9), 2363–2370. [DOI:10.1634/stemcells.2006-0686] [PMID]
Joyce, N., Annett, G., Wirthlin, L., Olson, S., Bauer, G., & Nolta, J. A. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative Medicine, 5(6), 933–946. [DOI:10.2217/rme.10.72] [PMID] [PMCID]
Krueger, M., Härtig, W., Frydrychowicz, C., Mueller, W. C., Reichenbach, A., & Bechmann, I., et al. (2017). Stroke-induced blood-brain barrier breakdown along the vascular tree - No preferential affection of arteries in different animal models and in humans. Journal of Cerebral Blood Flow and Metabolism, 37(7), 2539–2554. [DOI:10.1177/0271678X16670922] [PMID] [PMCID]
Lansberg, M. G., & Dabus, G. (2013). Interaction between time to treatment and reperfusion therapy in patients with acute ischemic stroke. Journal of Neurointerventional Surgery, 5(Suppl 1), i48–i51. [DOI:10.1136/neurintsurg-2013-010728] [PMID]
Lee, D. H., Lee, Y. J., & Kwon, K. H. (2010). Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. Journal of Clinical Biochemistry and Nutrition, 47(2), 121–129. [DOI:10.3164/jcbn.10-29] [PMID] [PMCID]
Li, L., Zhang, Y., Li, Y., Yu, B., Xu, Y., & Zhao, S., et al. (2008). Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transplant International, 21(12), 1181–1189. [DOI:10.1111/j.1432-2277.2008.00742.x] [PMID]
Longa, E. Z., Weinstein, P. R., Carlson, S., & Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20(1), 84–91. [DOI:10.1161/01.STR.20.1.84] [PMID]
Mehrjerdi, F. Z., Aboutaleb, N., Pazoki-Toroudi, H., Soleimani, M., Ajami, M., & Khaksari, M., et al. (2015). The protective effect of remote renal preconditioning against hippocampal ischemia reperfusion injury: Role of KATP channels. Journal of Molecular Neuroscience, 57(4), 554–560. [DOI:10.1007/s12031-015-0636-0] [PMID]
Nai, Y., Liu, H., Bi, X., Gao, H., & Ren, C. (2018). Protective effect of astaxanthin on acute cerebral infarction in rats. Human & Experimental Toxicology, 37(9), 929–936. [DOI:10.1177/0960327117745693] [PMID]
Nishida, E., & Gotoh, Y. (1993). The MAP kinase cascade is essential for diverse signal transduction pathways. Trends in Biochemical Sciences, 18(4), 128–131. [DOI:10.1016/0968-0004(93)90019-J] [PMID]
Park, E., Choi, S. K., Kang, S. W., Pak, Y. K., Lee, G. J., & Chung, J. H., et al. (2015). Cerebral ischemia-induced mitochondrial changes in a global ischemic rat model by AFM. Biomedicine & Pharmacotherapy, 71, 15–20. [DOI:10.1016/j.biopha.2015.02.007] [PMID]
Pawitan J. A. (2014). Prospect of stem cell conditioned medium in regenerative medicine. BioMed Research International, 2014, 965849. [DOI:10.1155/2014/965849] [PMID] [PMCID]
Pazoki-Toroudi, H., Amani, H., Ajami, M., Nabavi, S. F., Braidy, N., & Kasi, P. D., et al. (2016). Targeting mTOR signaling by polyphenols: A new therapeutic target for ageing. Ageing Research Reviews, 31, 55–66. [DOI:10.1016/j.arr.2016.07.004] [PMID]
Saini, V., Guada, L., & Yavagal, D. R. (2021). Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology, 97(20 Suppl 2), S6–S16. [DOI:10.1212/WNL.0000000000012781] [PMID]
Satoh, Y., Kobayashi, Y., Takeuchi, A., Pagès, G., Pouysségur, J., & Kazama, T. (2011). Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. The Journal of Neuroscience, 31(3), 1149–1155. [DOI:10.1523/JNEUROSCI.2243-10.2011] [PMID] [PMCID]
Schwamm, L. H., Ali, S. F., Reeves, M. J., Smith, E. E., Saver, J. L., & Messe, S., et alC. (2013). Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circulation. Cardiovascular Quality and Outcomes, 6(5), 543–549. [DOI:10.1161/CIRCOUTCOMES.111.000303] [PMID]
Shin, T. H., Phukan, G., Shim, J. S., Nguyen, D. T., Kim, Y., & Oh-Lee, J. D., et al. (2016). Restoration of polyamine metabolic patterns in in vivo and in vitro model of ischemic stroke following human mesenchymal stem cell treatment. Stem Cells International, 2016, 4612531. [DOI:10.1155/2016/4612531] [PMID] [PMCID]
Swanson, R. A., Morton, M. T., Tsao-Wu, G., Savalos, R. A., Davidson, C., & Sharp, F. R. (1990). A semiautomated method for measuring brain infarct volume. Journal of Cerebral Blood Flow and Metabolism, 10(2), 290–293. [DOI:10.1038/jcbfm.1990.47] [PMID]
Tang, G., Liu, Y., Zhang, Z., Lu, Y., Wang, Y., & Huang, J., et al. (2014). Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells, 32(12), 3150–3162. [DOI:10.1002/stem.1808] [PMID]
Teixeira, F. G., Carvalho, M. M., Neves-Carvalho, A., Panchalingam, K. M., Behie, L. A., & Pinto, L., et al. (2015). Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Reviews and Reports, 11(2), 288–297. [DOI:10.1007/s12015-014-9576-2] [PMID]
Timmers, L., Lim, S. K., Arslan, F., Armstrong, J. S., Hoefer, I. E., & Doevendans, P. A., et al. (2007). Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research, 1(2), 129–137. [DOI:10.1016/j.scr.2008.02.002] [PMID]
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation, 105(1), 93–98. [DOI:10.1161/hc0102.101442] [PMID]
Tsai, M. J., Tsai, S. K., Hu, B. R., Liou, D. Y., Huang, S. L., & Huang, M. C., et al. (2014). Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. Journal of Biomedical Science, 21(1), 5. [DOI:10.1186/1423-0127-21-5] [PMID] [PMCID]
Tsuji, H., Miyoshi, S., Ikegami, Y., Hida, N., Asada, H., & Togashi, I., et al. (2010). Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circulation Research, 106(10), 1613–1623. [DOI:10.1161/CIRCRESAHA.109.205260] [PMID]
Wang, J., Zhang, S., Ma, H., Yang, S., Liu, Z., & Wu, X., et al. (2017). Chronic intermittent hypobaric hypoxia pretreatment ameliorates ischemia-induced cognitive dysfunction through activation of ERK1/2-CREB-BDNF pathway in anesthetized mice. Neurochemical Research, 42(2), 501–512. [DOI:10.1007/s11064-016-2097-4] [PMID]
Zhang, R., Liu, C., Liu, X., & Guo, Y. (2016). Protective effect of Spatholobus suberectus on brain tissues in cerebral ischemia. American Journal of Translational Research, 8(9), 3963–3969. [PMID] [PMCID]
Zhang, X. S., Zhang, X., Zhou, M. L., Zhou, X. M., Li, N., & Li, W., et al (2014). Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. Journal of Neurosurgery, 121(1), 42–54. [DOI:10.3171/2014.2.JNS13730] [PMID]
Zhao, Y., Wang, P., Chen, S., Han, C., Yan, Q., & Zheng, L., et al. (2017). Dihydromyricetin protects against cerebral ischemia/reperfusion injury via suppressing microglia-mediated neuroinflammation and activation of ERK1/2-CREB signaling pathway. Journal of Functional Foods, 33, 76-84. [DOI:10.1016/j.jff.2017.03.034]
Stroke is a common disease in countries and is the second leading cause of death and leaves the country with a wide range of disabilities (Faezi et al., 2018). Previous studies have shown that in 2016, 5.5 million deaths worldwide were caused by stroke, of which 2.7 million were ischemic strokes and 2.8 million were hemorrhagic strokes (Saini et al., 2021). There are two forms of stroke, ischemic and hemorrhagic; in the ischemic type, the vessels are blocked and the blood flow to an area of the brain is stopped, and it is associated with learning and memory disorders in these people (Adams et al., 1993). During the process of ischemia, the brain undergoes a series of pathological events, including mitochondrial disruption (Azedi et al., 2022), Amplification of reactive oxygen species (ROS) generation, and caspase activation (Azedi et al., 2022), subsequently starting necrosis and apoptosis (Mehrjerdi et al., 2015). Ischemic stroke can affect the strength of the blood-brain barrier (BBB) and increase its permeability through changes in tight junctions and connexin-43 hemichannels. As a result, it increases the penetration of molecules from the blood to the brain and causes edema, increases the volume of brain tissue, and finally brain damage (Krueger et al., 2017). Despite the significant advances in medical science in the treatment of diseases, only a very limited number of drugs are available for the treatment of stroke. For example, currently, tissue plasminogen activator (tPA) is accepted by the Food and Drug Administration (FDA) for ischemic stroke, which is looking for other ways to treat this disorder due to its adverse effects and short effectiveness (Schwamm et al., 2013). Recently, the use of stem cells has shown favorable effects in the treatment of stroke and has attracted the attention of the scientific community (Bang, 2016). Mesenchymal stem cells (MSCs) have unique properties, such as easy access, anti-inflammatory effects, and weak immune response (Shin et al., 2016). The multipotent mesenchymal cells can improve the symptoms of cerebral ischemia due to disadvantages, such as cell number, the type of cell used, the possibility of cell migration, and the creation of ectopic tissue or turning into undesirable cells (Faezi et al., 2018).
During the process of extraction, culture, passage, and transplantation into the recipient tissue, MSCs may be affected by various conditions in terms of temperature stress, nutrition, possible contamination, and hypoxia, which all can increase harmful inflammatory factors in the recipient tissue (Hao et al., 2017). Previous studies have shown that only a limited number of MSCs transplanted to the target tissue survive. Also, Toma et al. presented that only 1% of the MSCs survived 24 hours after transplantation into an organ like a failing heart (Toma et al., 2002); thus, it seems that the useful therapeutic properties observed from pluripotent cells are due to their paracrine effects (Abbasi-Malati et al., 2018).
There is a general view, in which the substances that are secreted by MSCs into their culture medium, are named the scrotum, and their culture medium is called conditioned medium (CM) (Alijani-Ghazyani et al., 2021). The observed therapeutic effects and abilities of MSCs in regenerative medicine can be related to the ability of these cells to secrete growth factors, cytokines, and nutritional factors (Abbasi-Malati et al., 2018).
Many side effects of cell therapy, including the activation of the host immune response or tumorigenesis, are not expected from CM; thus, it seems that CM can be used as a reliable alternative to cell therapy (Joyce et al., 2010). The beneficial therapeutic effects of MSC-CM have been observed in various diseases, such as stroke, spinal cord damage, and neurological disorders (Pawitan, 2014).
As mentioned, paracrine factors secreted from MSCs have been used to improve pathological conditions of cerebral ischemia. Secreted paracrine factors include cytokines, angiogenic and anti-apoptotic factors, such as insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and interleukin 6 (IL-6) (Hung et al., 2007). These agents can be effective in treating neurodegenerative disorders and modulating cell signaling pathways (Pazoki-Toroudi et al., 2016).
Based on the mentioned studies, we investigated the beneficial effects of MSC-CM on neuronal injury in focal cerebral ischemia, and particularly, we investigated whether the MSC-CM will maintain the blood vessel integrity and ameliorate the brain edema and infarct size. Additionally, we investigated how the MSC-CM acts in subacute, acute, and chronic phases and when the best response to medium conditioning occurs in the ischemic brain tissue.
2. Materials and Methods
Preparation and isolation of human amniotic membrane stem cells (hAMSCs)
hAMSCs were derived from our previous research (Tousi et al., 2017). The membrane was carefully separated from the target organ, rinsed several times with phosphate-buffered saline (PBS) to remove blood vessels and blood clots, and turned into small pieces by cutting methods. The tissue pieces were mixed by a homogenizer and placed in a centrifuge (5 minutes/speed 1250 rpm). Next, the supernatant was removed and 30 mL of collagenase was added to the contents of the pellet. The pellet was kept at a temperature of 37°C in an incubator with a wetness of 5% CO2 for 60 minutes. Next, the tissue pieces were centrifuged at 1250 rpm for 5 minutes, the supernatant was discarded, and trypsin (0.25% containing 1 mL of ethylenediaminetetraacetic acid) was poured into the pellets. Then, the samples were incubated for 30 minutes, washed several times, and exposed to tris-ammonium chloride for 10 minutes to eliminate red blood cells. Finally, the pellet containing MSCs in the right volume was placed in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) to obtain the desired cell number.
Preparation of CM derived from hAMSC
MSCs were placed in the correct amount of α-MEM with 10% FBS, 100 μg/mL streptomycin, 2 mM L-glutamine, and 100 U/mL penicillin overnight. In the second quarter, the cells were rinsed with PBS (twice) and placed in the α-MEM medium without serum to provide a CM. To extract the CM, the cells were placed in a normoxic incubator (94% N2, 5% CO2, and 1% O2) for 48 hours. To eliminate the existing cells, the cultured MSC supernatant was extracted and centrifuged at 1200 rpm for 10 minutes. Then, the CM was filtered (0.22 μm). As a result, the ventilated environment was combined, dried, and stored at -80°C for further study. CM was obtained from 2×106 amniotic MSCs (Faezi et al., 2018)
Animals
A total of 105 adult male Wistar rats weighing 250 to 300 g were achieved from the Animal Breeding Center of Gilan University of Medical Sciences. Animals were housed in a temperature-controlled room with a temperature of 22-24°C and 55% humidity, with a light schedule of 12 hours of dark and 12 hours of light, and sufficient food and water. Three categories were considered for the study: Sham (n=15), middle cerebral artery occlusion (MCAO) (n=45), and MCAO+MSC-CM (n=45) (Figure 1). Intraventricular injection of MSC-CM was done 30 minutes after reperfusion.

Animals
Rats were randomly placed into three groups: Damage volume, brain water volume, and BBB strength. Animals in each group were divided into three subgroups: Sham n=5, MCAO n=15, and treatment n=15. The sham group was just under surgical stress and the vehicle and treatment groups were under focal cerebral ischemia for 60 minutes. Intraventricular injection of CM was done for up to 30 minutes after ischemia, and 6, 20, and 30 hours after reperfusion, infarct volume (IV) and behavioral testing were examined in the corresponding group, and using the same way, BBB and brain water content (BWC) were assessed in other groups.
Generation of ischemic rat models and stereotaxic injection of MSC-CM
Rats were anesthetized with an appropriate dose of ketamine hydrochloride (100 mg/kg) along with xylazine (8 mg/kg) intraperitoneally (IP) according to their weight. MCAO was done according to the protocol by Longa et al. (1989).
Briefly, first, the common and external carotid arteries were completely occluded, and then the internal carotid artery was temporarily occluded by a clamp, a small hole was prepared on the common carotid artery by ophthalmic scissors, and a silicone rubber sheath with an oval tip was used to occlude the vein. It was directed to the internal carotid artery up to 20-22 mm above the location of the two branches. This conduction was continued until a slight resistance was felt, which means that the filament entered the anterior cerebral artery and blocked it. After 60 minutes of ischemia and removing the blocking filaments, the blood flow started again in the vein. Rectal temperature (Citizen-513w) was checked and maintained at 37.0°C during surgery. Immediately after MCAO, stereotactic injection of CM was performed. The animal was fixed in the stereotaxic device (Stoelting, USA) by ear and mouth, and then a hole 2 mm in diameter was made on the skull 1 mm posterior and 1.5 mm lateral to the bregma on the right side with a dental drill. For the brain injections, three subgroups were considered (n=5): 1) Sham, 2) MCAO and 3) AMSC-CM. Animals were anesthetized again with a lower dose of ketamine hydrochloride (100 mg/kg) along with xylazine (8 mg/kg). By a Hamilton syringe (2 μL Hamilton, Switzerland) connected to the device, all injections performed in these three subgroups were performed according to the coordinates of the Paxinus atlas (anterior/posterior [AP]=1 mm, dorsal/ventral [DV]=3.5 mm, and lateral [L]=1.5 mm) (Teixeira et al., 2015). The volume of injection into the right cerebral ventricle (ICV) was 0.5 μL with an injection rate of 0.25 μL/min. After the end of each injection, two minutes were allowed to prevent any return to the needle channel. In the end, the needle was removed and the animals were sutured.
Local cerebral blood flow (LCBF) measurement
Before the surgery, the temporal bone in the right hemisphere of the anesthetized animal was considered for the placement of the laser Doppler flowmeter (LDF) probe. Baseline regional blood flow during MCAO and reperfusion was recorded by the probe. Cerebral blood flow was noted constantly every 5 min until 10 min after reperfusion. Local blood flow was recorded at certain times in the ischemic area (15 minutes before MCAO, 45 minutes during MCAO, and 15 minutes after reperfusion) (Bigdeli et al., 2007).
Neurologic deficit score
After 6, 20, and 30 hours of reperfusion (after removing the blocker, animals were returned to their cages), the neurological behavior of the animals was assessed. The neurological injury was scored using a six-point protocol (Longa et al. 1989) as follows: Correct neurological activity=0, forelimb flexion=1, turning the body to the opposite side of the lesion=2, absence of reflex on the right side of the body=3, lack of locomotor activity and hypokinesia=4, and death=5. In case of death, the animals were removed from the study. Reasons, such as asphyxia, pulmonary failure, or subarachnoid hemorrhage.
IV analysis
A high dose of chloral hydrate (800 mg/kg) (Merck, Germany) was used for deep anesthesia equivalent to the death of the animals. The heads of the animals were cut and the brains were quickly detached and placed in saline for 15 minutes in a refrigerator at 4°C. Then, the brain was placed in Manrix (Brain Matrix, Iran) and divided into eight sections with 2 mm thick slices. The slices were floated in a 2% solution of 2, 3, 5-triphenyl tetrazolium chloride (Merck, Germany) and placed in an oven at 37°C for 15 minutes. Then, the slices were photographed and the unstained and white areas were defined as infarcts, and the volume of the damaged area was dignified using image analysis software (Image J, version 1.48, US National Institutes of Health, Bethesda, MD, USA). The size of the lesion was measured by calculating the stained parts in each part and multiplying it by the thickness of that slice (2 mm) and then summing the data of all eight parts (Swanson et al., 1990) (Equation 1):
1. Corrected IV=Left hemisphere volume-(Right hemisphere volume-IV)
BWC analysis
After removing the brain and separating the cerebellum and olfactory bulb, wet weight (WW) was measured (Bigdeli et al., 2007). Then, the sections were located in the oven for 6, 20, and 30 hours at 120°C, and the dry weight (DW) was also recorded. Then, the Equation 2 was used to determine the water content in the brain:
2. [(WW-DW)/WW]×100
Analysis of BBB strength
Briefly, 30 minutes after MCAO, 4 mL/kg of 2% Evans blue (EB, Sigma Chemicals) solution in saline was inserted through the tail vein. Then, 6, 20, and 30 hours after recirculation, the animal’s chest was ruptured under deep anesthesia. The animal’s vessels were flushed through the left ventricle with 250 mL of saline to completely clear the EB from the vessels, and this procedure continued until the white fluid was removed from the right atrium. In the next step, the brains were taken out and the brain hemispheres were weighed separately for WW analysis. To measure the presence of EB in the tissue of the right hemispheres, they were homogenized in 2.5 mL of PBS, and 2.5 mL of 60% trichloroacetic acid (TTC) was poured into it to precipitate the protein, and they were vortexed for 5 minutes. Next, the sections were located at 4°C for 30 minutes and centrifuged at 1000 ×g for 30 minutes. Then, the supernatant was extracted and the amount of EB in it was recorded at a wavelength of 610 nm using a spectrophotometer (Perkin-Elmer, U.S.). EB levels were expressed as μg/g of brain tissue against a standard curve (Bigdeli et al., 2007).
Statistical analysis
IV, edema, and BBB health value were shown as Mean±SEM and compared using one-way ANOVA (SPSS software, version 16; post hoc LSD test). Normalized standard deviation (NDS) was expressed as median (range) and analyzed using the Mann–Whitney U test. A P>0.05 was considered significant.
3. Results
Regional cerebral blood flow
Assessment of CBF using laser Doppler flowmetry (LDF) in both the MCAO and MCAO+hAMSC-CM groups showed that cerebral blood flow decreased less than 20% from baseline after MCA occlusion (Figure 2). This was continued throughout the 60 min of MCAO. There was no significant difference between the MCAO and MCAO+hAMSC-CM groups during 60 minutes of MCAO and 30 minutes of reperfusion.

Effects of AMSC-CM on lesion size
As illustrated in Figures 3A, 3B, 3C & 3D, no infarcts occurred in the sham group. However, significant infarct expansion was detected after MCAO. Ischemic damage occurred in the core, subcortex, and penumbra at different times of reperfusion, and the ischemic damage increased with increasing reperfusion time, and the maximum damage was observed 30 minutes after reperfusion.
The therapeutic effect of AMSC-CM was observed in the penumbra area more than in the core area. In the core, subcortex, and penumbra areas, a significant difference was seen between the following groups: MCAO (6, 20, and 30 hours) and sham, sham and treatment (except for the 6-hour group in the penumbra and subcortex areas), and ischemia and treatment.

Effects of AMSC-CM on sensory and practical behavior
Sensorimotor performance was significantly improved after treatment with AMSC-CM. For the neurological deficit score, the MCAO group showed extensive neurological damage 24 hours after recirculation. Among the groups, the group that was reperfused for 30 hours showed more movement injuries. Intraventricular injection of CM changed neurologic scores compared to the MCAO group. Neurological deficit scores were reduced in the treatment group compared to the MCAO group (Figure 4).

Effects of AMSC-CM on BWC
BWC was increased in the MCAO group at all times of reperfusion and increased by treatment with AMSC-CM (Figure 5). In the right hemisphere, a significant difference was seen between the opposite groups: MCAO (6, 20, and 30 hours) and sham, sham and treatment, and ischemia and treatment (only at 6 and 20 hours).

Effects of AMSC-CM on the health of the BBB
The identification factor of BBB disruption was considered based on the amount of EB penetration in the tissue (Figures 6A, 6B and 6C). The amount of EB entry in ischemic areas in the MCAO group (30 h) was strikingly greater than the sham group and treatment (6, 20, and 30 h) in the core, subcortex, and penumbra areas significantly reduced the entry of EB to the ischemic areas.

4. Discussion
In this study, we investigated the neuroprotective effects of AMSC-CM against focal ischemia injury and recirculation after 6, 20, and 30 hours, which was done for the first time in the scientific community. The important finding of this study was the neuroprotective effect of AMSC-CM time-dependently against ischemic injuries due to MCAO. The results of our experiments support the fact that AMSC-CM in the initial moments of reperfusion in an animal model may ameliorate the amount of damage to neurons and reduce the process of destruction of the BBB. In addition, the results of this research showed that AMSC-CM can improve nerve function at 6, 20, and 30 h after reperfusion.
Cerebral ischemia leads to a decrease in oxygen and glucose in the brain tissue, and the combination of these factors causes irreparable neurological deficits in the ischemic core in the first minutes after the onset of ischemia (Zhang et al., 2016). In the absence of oxygen, certain areas of the brain turn into a pathological environment where the recirculation of blood leads to the initiation of inflammatory and oxidative pathways and the imbalance of prooxidants and endogenous antioxidants. The excessive production of radicals, toxic free radicals, and ROS, increases the abnormal amount of malondialdehyde (MDA), which is a key index of lipid peroxidation and indicates a degree of membrane damage under oxidative stress conditions (Park et al., 2015). In addition, glutathione (GSH) and superoxide dismutase (SOD) are both harmful free radical absorbers and are seen in brain tissue with ischemia. Ultimately, a pathological environment resulting from ischemia leads to learning and memory impairment as well as loss of structure and function of neurons (Park et al., 2015).
As mentioned, the interruption of blood flow in the vessels leads to a lack of oxygen and then causes the creation of anaerobic infarction areas with a lack of energy in the brain tissues, which finally leads to the necrosis of the brain tissues (Nai et al., 2018).
The loss of neuronal function caused by ischemia begins with the discharge of excitatory neurotransmitters, which leads to membrane depolarization, increased intracellular calcium, and the construction of nitric oxide (NO) and ROS, i.e. radicals. Superoxide anion, hydrogen peroxide, and hydroxyl radical are greatly cytotoxic byproducts (Lee et al., 2010). Oxidative stress plays a central role in the pathogenesis of the acute stage of stroke. The creation of local temporary cerebral ischemia increases the production of free radicals and nitric oxide and decreases the activity of antioxidant enzymes, such as SOD, GSH, and catalase (Zhang et al., 2014). Increasing the levels of free radicals through the direct effect and destruction of cell proteins and lipids, as well as DNA damage, leads to the activation of apoptosis. Also, through an indirect effect, they disrupt the signaling pathway and gene regulation of the cell and finally cell death (Dirnagl et al., 1999).
Many studies have previously attracted the attention of scientists regarding the use of MSCs for the treatment of ischemic stroke. Among different sources of MSCs in the body, such as bone marrow, fat tissue, placenta, and other tissues, AMSCs have unique characteristics that make them have an exceptional place in medicine. Some of these features include an easy and accessible resource after each delivery. There is no legal problem regarding their use (Tousi et al., 2017). Unlike other MSCs that are extracted from other sources in the body, amniotic cells do not express classes 1 and 2, or their expression is very little. Thus, they have high immune tolerance compared to other MSCs (Tsuji et al., 2010). Also, due to their embryonic properties, they have a high proliferation and differentiation power compared to other MSCs (Alviano et al., 2007; Boltze et al., 2014).
It has been well established that MSCs can treat ischemia damage through mechanisms, such as angiogenesis, neurogenesis, and immune response regulation (Eckert et al., 2013).
Despite these advantages, arterial injection of MSCs has been reported to be associated with concerns, such as reduced cerebral blood flow as a result of cellular coagulation and microemboli due to cellular aggregation, which are modulated by dose, rate, and volume of injection (Cui et al., 2015).
At first, the researchers believed that the way MSCs work in tissue healing and renewal is due to their replacement with desired tissue cells, but with more studies and research, the focus shifted to their paracrine actions (Drago et al., 2013). MSCs can release paracrine factors that have immunomodulatory and neuroprotective properties and can help repair and regenerate damaged tissues (Cunningham et al., 2018). The medium prepared from the culture site of MSCs contains substances that have different anti-apoptotic and angiogenic effects, such as exosomes, VEGF, IGF-1, angiogenin, IL-6, IL-8, and hepatocyte growth factor (HGF), which can promote cell proliferation, differentiation, and vascularization. They also affect the generation and survival of cells under oxidative stress (Di Santo et al., 2009; Timmers et al., 2008).
As mentioned, some studies have shown that MSCs can release several paracrine factors. They exert their effects by increasing the expression of materials, such as HGF (Li et al., 2008). In previous studies, it has been shown that HGF exhibits angiogenic and antiapoptotic effects (Guo et al., 2008). In our previous studies, the potency of AMSC-CM in the face of focal cerebral ischemia and the hypothesized mechanisms, by which it reduced brain damage, were also investigated. Also, our results in previous studies showed that treatment with AMSC-CM can exert neuroprotective effects to reduce apoptosis, via decreasing the level of caspase-3 and Bex and increasing the level of Bcl-2 (Faezi et al., 2018).
AMSC-CM exerts its effect through the triggering of BDNF/ERK signaling pathways (Wang et al., 2017). Furthermore, our data demonstrated that the neuroprotective effects of AMSC-CM were thoroughly related to the reduction of injury volume. It has been well established that ERK1/2 and the MAPKs family modulate neuronal viability and cellular apoptosis. The stimulation of this compound leads to the phosphorylation of many cytoplasmic and membrane proteins. Also, the transfer of ERK1/2 from the cytosol to the cell nucleus causes the phosphorylation of various transcription factors, such as CREB protein (Zhao et al., 2017).
In our earlier research, it was shown that intraventricular administration of AMSC-CM at the beginning of recirculation increases the phosphorylation of ERK1/2 and decreases the expression of BDNF protein in the cortical region of infarcted rats, and the results were reversed by AMSC-CM (Faezi et al., 2018).
According to our findings, AMSC-CM can inhibit apoptosis and ultimately cell survival in hypoxic conditions by activating the PI3K-Akt pathway (Hung et al., 2007).
With a slight reduction in cerebral blood flow, brain tissue survives for a relatively long time, while brain damage becomes irreversible when blood flow is completely cut off. In other words, the sooner the blood flow starts again, the more effective it can be in the treatment. In the same study, it was reported that the volume of the penumbra area decreases with the increase of the reperfusion time (Lansberg & Dabus, 2013).
Our experiment showed that by increasing the reperfusion time for 30 hours, the amount of damage and the destruction of the BBB decreases.
During cerebral ischemia, increased expression of aquaporin-4, a type of protein water channel, occurs in the end feet of astrocytes, which causes the lack of strength and increased permeability of the BBB and the formation of cerebral edema (Fu et al., 2007).
Edema in the brain is one of the factors determining the patient’s survival rate in the early hours after a stroke (Dirnagl et al., 1999). Clinical and laboratory studies show that the development of edema following acute localized cerebral ischemia can aggravate the primary ischemic lesion (Shaw et al., 1959). It is said that by creating cerebral edema, the pressure inside the skull increases and by compressing the cerebral vessels, blood flow to the penumbra region is severely reduced and the death of neurons in this region is accelerated (Dirnagl et al., 1999). Rupture of the BBB is the most important cause of cerebral edema (Nishida & Gotoh, 1993). ROS, such as superoxide and hydroxyl radicals play an important role in the development of brain edema and brain lesions (Satoh et al., 2011). The exact mechanisms, by which superoxide anion and hydroxyl radicals participate in edema formation remain unknown. The formation of free radicals, especially super-oxidants and proteases, following cerebral ischemia causes alterations in the BBB and the formation of edema and cell death.
The outcomes of this study indicated that the blockage of the middle cerebral artery caused severe breaking of the BBB and the creation of cerebral edema.
This study showed that the production of free radicals increases cerebral edema in focal cerebral ischemia and the reduction of these free radicals leads to the reduction of cerebral edema. In a study, it was shown that the administration of MSC after cerebral ischemia by inhibiting the expression of aquaporin-4 can increase the strength of the BBB (Baez-Jurado et al., 2019; Tang et al., 2014).
In this paper, we investigated whether the paracrine effects caused by MSCs can increase the strength and integrity of the BBB. Our data demonstrated that MCAO can destroy the BBB, and with increasing reperfusion time, there is an increase in the destruction and permeability of the BBB and subsequent edema creation, which is reversed by AMSC-CM administration.
Also, BM-MSC-CM can recover neuron function after ischemic stroke (Tsai et al., 2014). Consistent with this result, our research showed that post-treatment with AMSC-CM can improve motor performance and neurological deficits 6, 20, and 30 hours after reperfusion.
Among the limitations of the present study was that the drug had to be stored and used under certain temperature conditions, and any delay in intraventricular injection or non-observance of temperature balance with the denaturation of functional proteins would cause the inactivation of the drug intervention. In this research, western blot and PCR techniques were not used due to the high division of the protocol and study groups and the need to focus on the tissue process. For this reason, the use of molecular techniques in future studies is suggested to identify proteins and investigate mechanisms at the molecular level.
It is also suggested that in the future, other complementary movement tests, such as the balance test and corner test will be used to more accurately evaluate functional disorders and increase the power of research by examining several inflammatory factors.
5. Conclusion
The use of AMSC-CM reduces lesion volume, cerebrospinal fluid accumulation, and BBB breakdown in three subacute, acute, and chronic phases of focal cerebral ischemia. AMSC-CM may be a hopeful therapeutic case for stroke, but further study is needed to consider the therapeutic nature of AMSC-CM. Finally, the results of this research open several paths that can be studied to investigate the protective effects of the treatment used in this research.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Animal Ethics Committee of Gilan University of Medical Sciences (Code: IR.GUMS.REC.1396.372) and all experiments were performed with the assumption of minimal suffering and harm to rats.
Funding
The present study financially supported by Vice Chancellor of Research and Technology, Guilan University of Medical Sciences (Grant No.: 96071502).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the faculty members of the Department of Physiology, Faculty of Medicine, Gilan University of Medical Sciences for their useful recommendations and cooperation.
Reference
Abbasi-Malati, Z., Roushandeh, A. M., Kuwahara, Y., & Roudkenar, M. H. (2018). Mesenchymal stem cells on horizon: A new arsenal of therapeutic agents. Stem Cell Reviews and Reports, 14(4), 484–499. [DOI:10.1007/s12015-018-9817-x] [PMID]
Aboutaleb, N., Tousi, S. M. T. R., Amirizadeh, N., Nasirinezhad, F., Nikougoftar, M., & Ganjibakhsh, M. (2017). A rapid and cost-effective protocol for isolating mesenchymal stem cells from the human amniotic membrane. Galen Medical Journal, 6(3), e670-e670. [DOI:10.31661/gmj.v6i3.670]
Adams, H. P., Jr, Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., & Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke, 24(1), 35–41 [DOI:10.1161/01.STR.24.1.35] [PMID]
Alijani-Ghazyani, Z., Roushandeh, A. M., Sabzevari, R., Salari, A., Razavi Toosi, M. T., & Jahanian-Najafabadi, A., et al. (2021). Conditioned medium harvested from Hif1α engineered mesenchymal stem cells ameliorates LAD-occlusion -induced injury in rat acute myocardial ischemia model. The International Journal of Biochemistry & Cell Biology, 130, 105897. [DOI:10.1016/j.biocel.2020.105897] [PMID]
Alviano, F., Fossati, V., Marchionni, C., Arpinati, M., Bonsi, L., & Franchina, M., et al. (2007). Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Developmental Biology, 7, 11. [DOI:10.1186/1471-213X-7-11] [PMID] [PMCID]
Azedi, F., Tavakol, S., Ketabforoush, A. H. M. E., Khazaei, G., Bakhtazad, A., & Mousavizadeh, K., et al. (2022). Modulation of autophagy by melatonin via sirtuins in stroke: From mechanisms to therapies. Life Sciences, 307, 120870.[DOI:10.1016/j.lfs.2022.120870] [PMID]
Baez-Jurado, E., Hidalgo-Lanussa, O., Barrera-Bailón, B., Sahebkar, A., & Ashraf, G. M., et al. (2019). Secretome of mesenchymal stem cells and its potential protective effects on brain pathologies. Molecular Neurobiology, 56(10), 6902–6927. [DOI:10.1007/s12035-019-1570-x] [PMID]
Bang O. Y. (2016). Clinical trials of adult stem cell therapy in patients with ischemic stroke. Journal of Clinical Neurology, 12(1), 14–20. [DOI:10.3988/jcn.2016.12.1.14] [PMID]
Bigdeli, M. R., Hajizadeh, S., Froozandeh, M., Rasulian, B., Heidarianpour, A., & Khoshbaten, A. (2007). Prolonged and intermittent normobaric hyperoxia induce different degrees of ischemic tolerance in rat brain tissue. Brain Research, 1152, 228–233. [DOI:10.1016/j.brainres.2007.03.068] [PMID]
Boltze, J., Lukomska, B., Jolkkonen, J., & MEMS–IRBI consortium (2014). Mesenchymal stromal cells in stroke: Improvement of motor recovery or functional compensation?. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1420–1421. [DOI:10.1038/jcbfm.2014.94] [PMID] [PMCID]
Shaw, C. M., Alvord, E. C., Jr, & Berry, R. G. (1959). Swelling of the brain following ischemic infarction with arterial occlusion. Archives of Neurology, 1, 161–177. [DOI:10.1001/archneur.1959.03840020035006] [PMID]
Cui, L. L., Kerkelä, E., Bakreen, A., Nitzsche, F., Andrzejewska, A., & Nowakowski, A., et al. (2015). The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Research & Therapy, 6(1), 11. [DOI:10.1186/scrt544] [PMID] [PMCID]
Cunningham, C. J., Redondo-Castro, E., & Allan, S. M. (2018). The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. Journal of Cerebral Blood Flow and Metabolism, 38(8), 1276–1292. [DOI:10.1177/0271678X18776802] [PMID] [PMCID]
Di Santo, S., Yang, Z., Wyler von Ballmoos, M., Voelzmann, J., Diehm, N., & Baumgartner, I., et al. (2009). Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. Plos One, 4(5), e5643. [DOI:10.1371/journal.pone.0005643] [PMID] [PMCID]
Dirnagl, U., Iadecola, C., & Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends in Neurosciences, 22(9), 391–397. [DOI:10.1016/S0166-2236(99)01401-0] [PMID]
Drago, D., Cossetti, C., Iraci, N., Gaude, E., Musco, G., & Bachi, A., et al. (2013). The stem cell secretome and its role in brain repair. Biochimie, 95(12), 2271–2285. [DOI:10.1016/j.biochi.2013.06.020] [PMID] [PMCID]
Eckert, M. A., Vu, Q., Xie, K., Yu, J., Liao, W., & Cramer, S. C., et al. (2013). Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. Journal of Cerebral Blood Flow and Metabolism, 33(9), 1322–1334. [DOI:10.1038/jcbfm.2013.91] [PMID] [PMCID]
Faezi, M., Nasseri Maleki, S., Aboutaleb, N., & Nikougoftar, M. (2018). The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. Journal of Chemical Neuroanatomy, 94, 21–31. [DOI:10.1016/j.jchemneu.2018.08.004] [PMID]
Fu, X., Li, Q., Feng, Z., & Mu, D. (2007). The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia, 55(9), 935–941. [DOI:10.1002/glia.20515] [PMID]
Guo, Y., He, J., Wu, J., Yang, L., Dai, S., & Tan, X., et al. (2008). Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Archives of Medical Research, 39(2), 179–188. [DOI:10.1016/j.arcmed.2007.11.001] [PMID]
Hao, T., Li, J., Yao, F., Dong, D., Wang, Y., & Yang, B., et al. (2017). Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano, 11(6), 5474–5488. [DOI:10.1021/acsnano.7b00221] [PMID]
Hung, S. C., Pochampally, R. R., Chen, S. C., Hsu, S. C., & Prockop, D. J. (2007). Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells, 25(9), 2363–2370. [DOI:10.1634/stemcells.2006-0686] [PMID]
Joyce, N., Annett, G., Wirthlin, L., Olson, S., Bauer, G., & Nolta, J. A. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative Medicine, 5(6), 933–946. [DOI:10.2217/rme.10.72] [PMID] [PMCID]
Krueger, M., Härtig, W., Frydrychowicz, C., Mueller, W. C., Reichenbach, A., & Bechmann, I., et al. (2017). Stroke-induced blood-brain barrier breakdown along the vascular tree - No preferential affection of arteries in different animal models and in humans. Journal of Cerebral Blood Flow and Metabolism, 37(7), 2539–2554. [DOI:10.1177/0271678X16670922] [PMID] [PMCID]
Lansberg, M. G., & Dabus, G. (2013). Interaction between time to treatment and reperfusion therapy in patients with acute ischemic stroke. Journal of Neurointerventional Surgery, 5(Suppl 1), i48–i51. [DOI:10.1136/neurintsurg-2013-010728] [PMID]
Lee, D. H., Lee, Y. J., & Kwon, K. H. (2010). Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. Journal of Clinical Biochemistry and Nutrition, 47(2), 121–129. [DOI:10.3164/jcbn.10-29] [PMID] [PMCID]
Li, L., Zhang, Y., Li, Y., Yu, B., Xu, Y., & Zhao, S., et al. (2008). Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transplant International, 21(12), 1181–1189. [DOI:10.1111/j.1432-2277.2008.00742.x] [PMID]
Longa, E. Z., Weinstein, P. R., Carlson, S., & Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20(1), 84–91. [DOI:10.1161/01.STR.20.1.84] [PMID]
Mehrjerdi, F. Z., Aboutaleb, N., Pazoki-Toroudi, H., Soleimani, M., Ajami, M., & Khaksari, M., et al. (2015). The protective effect of remote renal preconditioning against hippocampal ischemia reperfusion injury: Role of KATP channels. Journal of Molecular Neuroscience, 57(4), 554–560. [DOI:10.1007/s12031-015-0636-0] [PMID]
Nai, Y., Liu, H., Bi, X., Gao, H., & Ren, C. (2018). Protective effect of astaxanthin on acute cerebral infarction in rats. Human & Experimental Toxicology, 37(9), 929–936. [DOI:10.1177/0960327117745693] [PMID]
Nishida, E., & Gotoh, Y. (1993). The MAP kinase cascade is essential for diverse signal transduction pathways. Trends in Biochemical Sciences, 18(4), 128–131. [DOI:10.1016/0968-0004(93)90019-J] [PMID]
Park, E., Choi, S. K., Kang, S. W., Pak, Y. K., Lee, G. J., & Chung, J. H., et al. (2015). Cerebral ischemia-induced mitochondrial changes in a global ischemic rat model by AFM. Biomedicine & Pharmacotherapy, 71, 15–20. [DOI:10.1016/j.biopha.2015.02.007] [PMID]
Pawitan J. A. (2014). Prospect of stem cell conditioned medium in regenerative medicine. BioMed Research International, 2014, 965849. [DOI:10.1155/2014/965849] [PMID] [PMCID]
Pazoki-Toroudi, H., Amani, H., Ajami, M., Nabavi, S. F., Braidy, N., & Kasi, P. D., et al. (2016). Targeting mTOR signaling by polyphenols: A new therapeutic target for ageing. Ageing Research Reviews, 31, 55–66. [DOI:10.1016/j.arr.2016.07.004] [PMID]
Saini, V., Guada, L., & Yavagal, D. R. (2021). Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology, 97(20 Suppl 2), S6–S16. [DOI:10.1212/WNL.0000000000012781] [PMID]
Satoh, Y., Kobayashi, Y., Takeuchi, A., Pagès, G., Pouysségur, J., & Kazama, T. (2011). Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. The Journal of Neuroscience, 31(3), 1149–1155. [DOI:10.1523/JNEUROSCI.2243-10.2011] [PMID] [PMCID]
Schwamm, L. H., Ali, S. F., Reeves, M. J., Smith, E. E., Saver, J. L., & Messe, S., et alC. (2013). Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circulation. Cardiovascular Quality and Outcomes, 6(5), 543–549. [DOI:10.1161/CIRCOUTCOMES.111.000303] [PMID]
Shin, T. H., Phukan, G., Shim, J. S., Nguyen, D. T., Kim, Y., & Oh-Lee, J. D., et al. (2016). Restoration of polyamine metabolic patterns in in vivo and in vitro model of ischemic stroke following human mesenchymal stem cell treatment. Stem Cells International, 2016, 4612531. [DOI:10.1155/2016/4612531] [PMID] [PMCID]
Swanson, R. A., Morton, M. T., Tsao-Wu, G., Savalos, R. A., Davidson, C., & Sharp, F. R. (1990). A semiautomated method for measuring brain infarct volume. Journal of Cerebral Blood Flow and Metabolism, 10(2), 290–293. [DOI:10.1038/jcbfm.1990.47] [PMID]
Tang, G., Liu, Y., Zhang, Z., Lu, Y., Wang, Y., & Huang, J., et al. (2014). Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells, 32(12), 3150–3162. [DOI:10.1002/stem.1808] [PMID]
Teixeira, F. G., Carvalho, M. M., Neves-Carvalho, A., Panchalingam, K. M., Behie, L. A., & Pinto, L., et al. (2015). Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Reviews and Reports, 11(2), 288–297. [DOI:10.1007/s12015-014-9576-2] [PMID]
Timmers, L., Lim, S. K., Arslan, F., Armstrong, J. S., Hoefer, I. E., & Doevendans, P. A., et al. (2007). Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research, 1(2), 129–137. [DOI:10.1016/j.scr.2008.02.002] [PMID]
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation, 105(1), 93–98. [DOI:10.1161/hc0102.101442] [PMID]
Tsai, M. J., Tsai, S. K., Hu, B. R., Liou, D. Y., Huang, S. L., & Huang, M. C., et al. (2014). Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. Journal of Biomedical Science, 21(1), 5. [DOI:10.1186/1423-0127-21-5] [PMID] [PMCID]
Tsuji, H., Miyoshi, S., Ikegami, Y., Hida, N., Asada, H., & Togashi, I., et al. (2010). Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circulation Research, 106(10), 1613–1623. [DOI:10.1161/CIRCRESAHA.109.205260] [PMID]
Wang, J., Zhang, S., Ma, H., Yang, S., Liu, Z., & Wu, X., et al. (2017). Chronic intermittent hypobaric hypoxia pretreatment ameliorates ischemia-induced cognitive dysfunction through activation of ERK1/2-CREB-BDNF pathway in anesthetized mice. Neurochemical Research, 42(2), 501–512. [DOI:10.1007/s11064-016-2097-4] [PMID]
Zhang, R., Liu, C., Liu, X., & Guo, Y. (2016). Protective effect of Spatholobus suberectus on brain tissues in cerebral ischemia. American Journal of Translational Research, 8(9), 3963–3969. [PMID] [PMCID]
Zhang, X. S., Zhang, X., Zhou, M. L., Zhou, X. M., Li, N., & Li, W., et al (2014). Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. Journal of Neurosurgery, 121(1), 42–54. [DOI:10.3171/2014.2.JNS13730] [PMID]
Zhao, Y., Wang, P., Chen, S., Han, C., Yan, Q., & Zheng, L., et al. (2017). Dihydromyricetin protects against cerebral ischemia/reperfusion injury via suppressing microglia-mediated neuroinflammation and activation of ERK1/2-CREB signaling pathway. Journal of Functional Foods, 33, 76-84. [DOI:10.1016/j.jff.2017.03.034]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2023/07/22 | Accepted: 2023/08/29 | Published: 2023/11/1
Received: 2023/07/22 | Accepted: 2023/08/29 | Published: 2023/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








