Volume 16 - Special Issue on Cognitive Sciences
BCN 2025, 16 - Special Issue on Cognitive Sciences: 273-282 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dehghan F, Alizadeh Zarei M, Khosro Abadi R, Farhangdost H, Soltani Tehrani A A, Joghataei M T. Eye Movements during Sentence Processing in High-Functioning Autistic Children Compared to Neurotypical Peers: An Eye Tracking Study. BCN 2025; 16 (S1) :273-282
URL: http://bcn.iums.ac.ir/article-1-2753-en.html
URL: http://bcn.iums.ac.ir/article-1-2753-en.html
Faezeh Dehghan1 

 , Mehdi Alizadeh Zarei2
, Mehdi Alizadeh Zarei2 
 , Reza Khosro Abadi3
, Reza Khosro Abadi3 
 , Hashem Farhangdost4
, Hashem Farhangdost4 
 , Amir Ali Soltani Tehrani5
, Amir Ali Soltani Tehrani5 
 , Mohamad Taghi Joghataei *1
, Mohamad Taghi Joghataei *1 




 , Mehdi Alizadeh Zarei2
, Mehdi Alizadeh Zarei2 
 , Reza Khosro Abadi3
, Reza Khosro Abadi3 
 , Hashem Farhangdost4
, Hashem Farhangdost4 
 , Amir Ali Soltani Tehrani5
, Amir Ali Soltani Tehrani5 
 , Mohamad Taghi Joghataei *1
, Mohamad Taghi Joghataei *1 


1- Department of Neuroscience, School of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of occupational Therapy, School of Rehabilitation, Iran University of Medical Sciences, Tehran, Iran.
3- The Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
4- Department of Speech Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- School of Electrical and Computer Engineering, University of Tehran, Iran.
2- Department of occupational Therapy, School of Rehabilitation, Iran University of Medical Sciences, Tehran, Iran.
3- The Institute for Cognitive and Brain Sciences, Shahid Beheshti University, Tehran, Iran.
4- Department of Speech Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- School of Electrical and Computer Engineering, University of Tehran, Iran.
Keywords: High-functioning autism, Eye tracking, Reading, Eye movements, Saccadic eye movements, Sensory-motor integration
Full-Text [PDF 622 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Autism spectrum disorders (ASD) are a collection of neurodevelopmental conditions characterized by impairments in social interaction, communication, repetitive behaviors, and limited interests. In 2021, the Centers for Disease Control and Prevention (CDC) reported that approximately 1 in 44 children in the United States is diagnosed with ASD (Baio et al., 2018). ASD is a lifelong condition, and individuals with ASD face significant challenges in all stages of development. Recent research has focused on the academic achievements of individuals with autism, particularly in the early years of education when acquiring reading, writing, and mathematics skills can be highly challenging. These skills are crucial for social participation and achieving independence.
The CDC has stated that approximately 68% of school children with ASD exhibit cognitive abilities beyond the range of intellectual disability, meaning that they have an Intelligence quotient (IQ) above 70 (Seltzer et al., 2004). Despite having normal IQ, these individuals often display limited verbal abilities at the sentence level. However, their academic and social outcomes are far from optimal. Despite having an average IQ, many children with ASD show a discrepancy between their academic performance and achievement, which resembles specific learning disabilities. Notably, difficulties in reading comprehension and understanding written text have been a focal point, even when these students can read individual words proficiently (Huemer & Mann, 2010; Jones et al., 2009).
The “simple reading view” (Gough & Tunmer, 1986) proposes that skilled literacy relies on two closely interconnected processes: Decoding or identifying individual words and engaging in language processes constructing meaningful text. Both processes are necessary for comprehension. Without adequate word-level reading skills, comprehension is likely to fail. However, skilled word-level reading in isolation does not guarantee understanding at the text level. Decoding and comprehension are believed to depend on underlying language skills. Consequently, there may be three potential subgroups that experience difficulties with reading comprehension: Individuals with strong decoding skills but poor oral language skills, individuals with strong oral language skills but deficits in decoding, and individuals who struggle with both oral language and decoding (Catts et al., 2003).
Most previous studies on reading in children with ASD have focused on a specific subgroup characterized by the ability to decode words. Still, difficulty comprehending written text is often referred to as hyperlexia (Newman et al., 2007). These studies have specifically examined inconsistencies in reading comprehension and decoding ability within average samples (Jones et al., 2009). Children with ASD are often reported to have advanced word-reading skills at an early age (Grigorenko et al., 2003). However, these early developments may be hindered by long-term deficits in word reading and reading comprehension (Nation & Snowling, 1999). In a study exploring reading profiles in ASD, Nation et al. (2006) discovered that many children with ASD struggled with both decoding and comprehension. Around 65% of the cohort exhibited poor reading comprehension, often alongside poor word-level reading skills. Notably, the most significant finding of Nation et al.’s (2006) study was the substantial variation observed, with a wide range of performance levels seen across most reading and reading-related measures. However, the extent of this discrepancy varies widely, possibly due to the specific characteristics of the individuals being studied (Saldaña & Frith, 2007; Newman et al., 2007; Ricketts et al., 2013; Nation et al., 2006; Jones et al., 2009).
Eye movements play a crucial role in the reading process, consisting of three primary components: Saccade, fixation, and regression. During fixations, new information is encoded as vision is suppressed during saccades. Although the size of saccades and the duration of fixations may vary, this pattern of eye movements remains consistent across various visual-cognitive tasks driven by the same neural circuitry. As visual limitations heavily influence eye movement behavior, it is unsurprising that general similarities are observed. In reading, linguistic factors such as word frequency, predictability, and syntactic complexity impact moment-to-moment eye movements (Rayner, 1978; Ryaner, 2009). Despite the association between eye movement patterns and reading skills (Irwin, 2004), eye movements are seldom considered the root cause of reading difficulties. Research on eye movements ranges from perceptual issues to theoretical and computational models of eye movement control during reading. One crucial question is whether eye movements in reading are governed by low-level motor strategies or influenced by cognitive processes in real time (Reichle et al., 1998).
While some studies have explored eye movement characteristics in individuals with ASD, they primarily utilized stimuli with limited content, such as solely faces (Arkush et al., 2013; Harms et al., 2010), and few open datasets are available. Eye-tracking research has also examined attention shifting and disengagement in ASD, with recent research (Manyakov et al., 2018) documenting fewer shifts and longer disengagement time in response to pictures considered of high interest (e.g. trains, cars) in adolescents and adults with ASD—a finding consistent with earlier work linking atypical shifting and disengagement to circumscribed interests in children with ASD (Sasson et al, 2011).
The cause of reading comprehension problems in people with ASD is debatable. We have investigated the ability of autistic children to adapt reading strategies based on different reading goals using eye-tracking technology. The effect of reading purposefulness on the number of fixations (Micai et al., 2021; Drysdale et al, 2022), duration of fixations, number of forward fixations, length of the forward saccade was reported (Micai et al., 2021; Micai et al., 2017). Interestingly, more fixations were observed for the purposeful reading of the text compared to the entertainment-only condition in the control group (Micai et al., 2021). Children with high-functioning ASD can make the necessary inferences to understand reading. Eye movement data showed that children with high-functioning ASD spent more time fixating on the text, had more fixations overall, and made more regressions during reading (i.e. moving backward through the text) than neurotypical peers (Sansosti et al., 2013).
Nonetheless, studies indicate that individuals with ASD exhibit differences in decoding written language and lexical acquisition, yet they can read words within sentences. The question remains whether the delayed semantic processing and comprehension of sentences in individuals with ASD are attributable to higher-level language learning phenomena or if lower-level sensorimotor skills when extracting visual information from written language contribute to the performance disparities and subsequent linguistic processing slowness. Consequently, this study aims to investigate the eye movement characteristics and lower sensory motor skills employed while reading sentences in children with autism.
2. Materials and Methods
Study participants
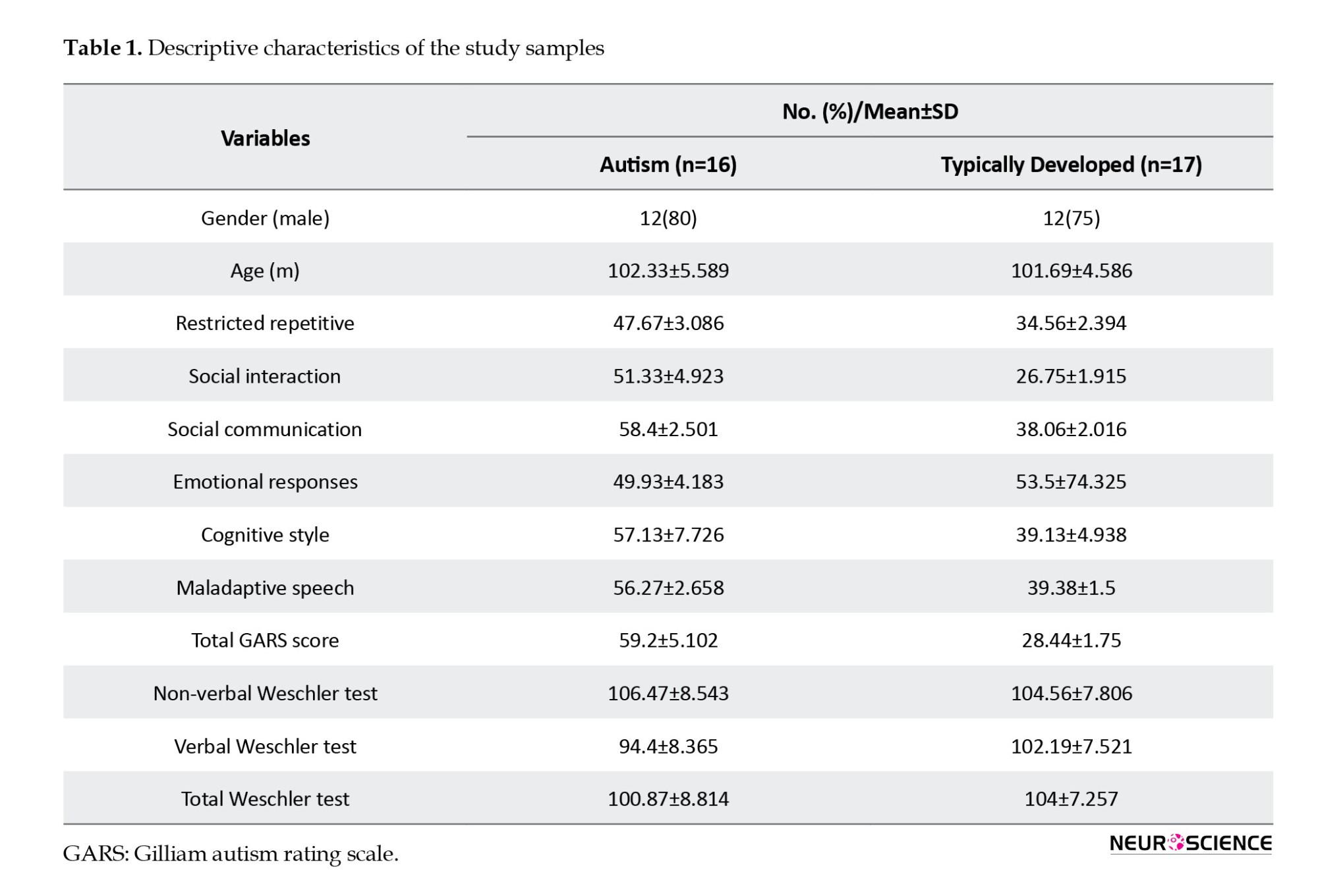
We recruited a group of children diagnosed with high-functioning autism disorder (n=15) with a mean age of 102.3 months. The diagnosis was conducted by a child and adolescent psychiatrist using the diagnostic and statistical manual of mental disorders, fifth edition (DSM-V) diagnostic criteria. In addition to the psychiatrist’s opinion, the Gilliam autism rating scale, third edition (GARS-3), has been used to diagnose autism. On this scale, scoring 50 to 70 is mild autism or level 1. In the articles, high-functioning autism is equivalent to level 1 autism. The control group of typically developing children (n=17) from primary schools, with a mean age of 101.10 months, participated in the study. According to their health records, the control group had no recorded developmental disorders. Written consent was obtained from the participants and their parents for their involvement in the study. Parents were asked to rate their child’s behavior using the GARS. It is important to note that the autistic children did not take any medication during the study.
Study procedure
The research involved a 2-hour session during which the Wechsler IQ test was administered. On a separate day, the children were present to record their eye movements. The children with autism, along with their neurotypical peers, had recently entered public schools after completing six years of education. One of the criteria for entry into the study was that none of the children had repeated any academic classes. This was crucial to minimize any potential learning effects on the task. The autistic children were referred to the researcher from general hospitals in Tehran.
Study measures
The GARS-3
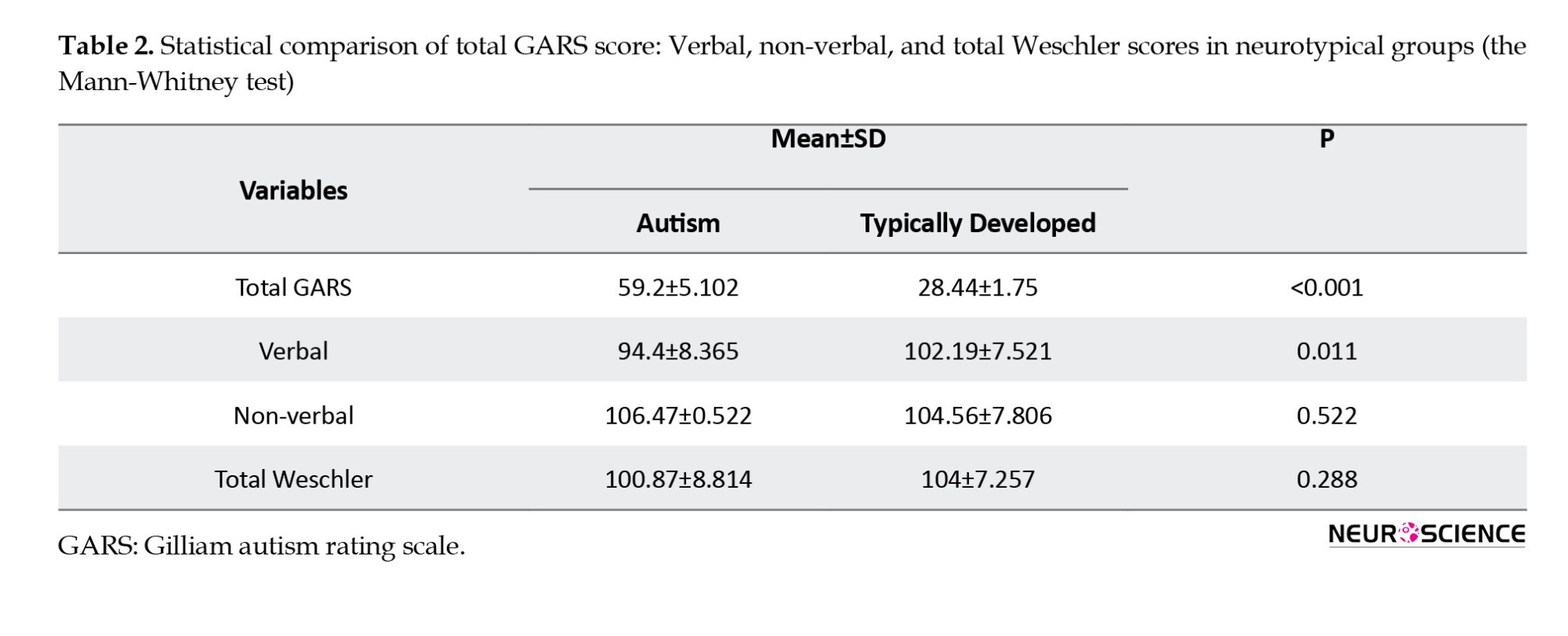
Gilliam developed the GARS-3 scale by extracting questions from the DSM-V diagnostic criteria and the American Autism Association definition (Gilliam, 2014). The aim was to create a tool with high validity and content. The scale consists of 56 questions, rated on a 4-point Likert scale ranging from 0 to 3. The GARS-3 includes 6 subscales: Repetitive/restrictive behaviors, social communication, social interaction, emotional responses, maladaptive speech, and cognitive style. This study utilized the standardized Iranian version of the GARS-3 scale (Minaei & Nazeri, 2018).
The GARS-3 scale was administered to all participants in the research. Individuals with autism who scored between 50 and 70 were diagnosed with ASD-level 1 autism, as shown in Tables 1 and 2. None of the autistic children were prescribed medication.
Wechsler intelligence scale for children
An expert psychologist administered the Wechsler intelligence scale for children (WISC) for both groups. Autistic children who scored above 90 on the Wechsler intelligence test were eligible to participate in the study. The standardized Iranian test version was used (Tables 1 and 2). We have used the standardized version of this test in Iran (Abedi et al., 2015).
Study task
The task consisted of 40 sentences, with 12 sentences containing 8 words, 15 sentences containing 7 words, 9 sentences containing 6 words, and 4 sentences containing 5 words. These sentences were displayed on a monitor screen and were selected from the reading skills book used in the second year of primary school. The chosen words had a high frequency of occurrence as they were taken from the second-grade Persian reading book.
Only nouns and verbs were included among the selected words to ensure control over the semantic load and objectivity. Iranian linguistics and speech therapy experts were consulted to determine the objectivity of the words using a 5-point Likert scale. Words with a score of >2.5 on the scale were agreed upon for use. Subsequently, 130 neurotypical children aged 8 to 10 years were asked to rate these words based on their objectivity. From the initial pool of 280 words, 160 words with objectivity scores >2.5 were selected to form the sentences. These sentences were well-known and had low emotional value.
During the experiment, the participants’ left eye was tracked using a remote monocular eye-tracker (Segal Tracker iSaw, FARMED, Tehran, Iran) with a sampling frequency of 500 Hz. The stimulus presentation was regulated using the PsychoPy framework. The stimuli were displayed on a screen measuring 57 by 43.1 cm, positioned 55 cm in front of the participant, and had a screen resolution of 1920 by 1080 pixels. This setup resulted in a viewing angle of 0.031 degrees for the screen’s pixels.
The task consisted of 40 trials, where the participants were required to read the sentences aloud. After each sentence, the examiner pressed the space button to display the following sentence. If the participants became tired during the trials, the task was temporarily paused and resumed after a break. Both groups of participants in the research performed the task under the same physical conditions.
3. Results
In this research, the data were first analyzed descriptively. Categorical variables were reported as frequency and percentage, quantitative variables with normal distribution were reported with mean and standard deviation, and quantitative variables with skewed distribution were reported with median and interquartile range (IQR). Then, the distribution of quantitative variables was measured with the Shapiro-Wilk test. The independent samples t-test was used to analyze variables with normal distribution, and the Mann-Whitney test for variables that did not follow a normal distribution. We used SPSS software, version 27.0.1
The Pearson correlation test was used to correlate quantitative variables. The analysis was done with SPSS software, and the significance level was considered 0.05 for all tests.
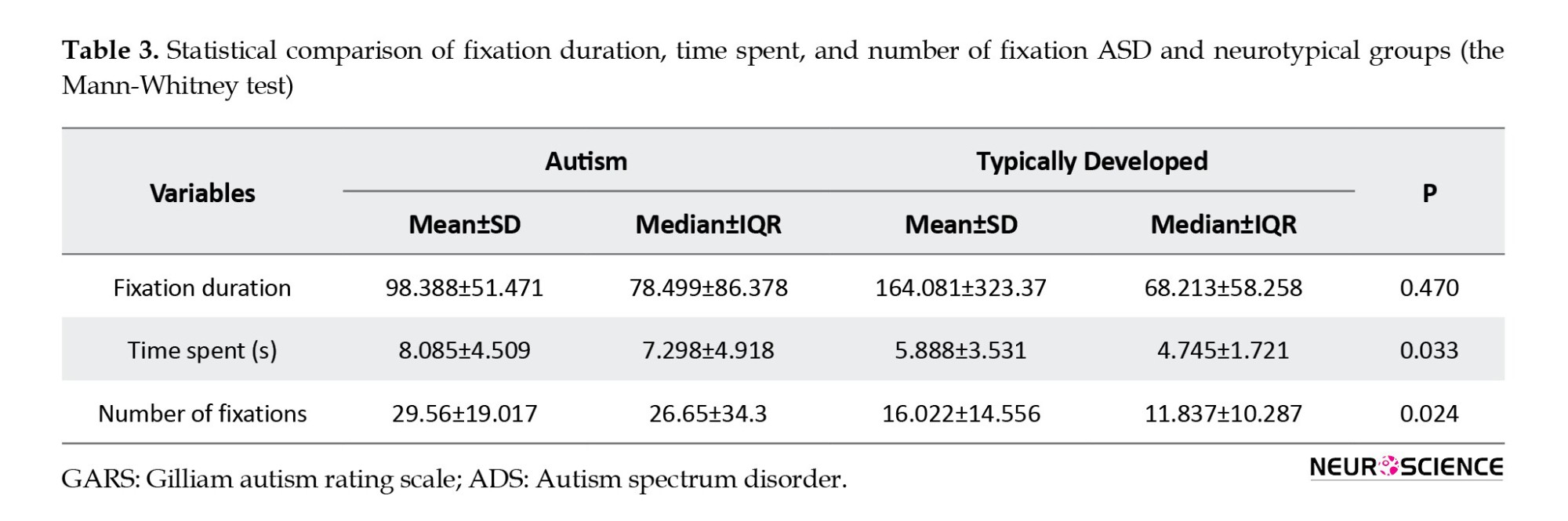
The duration of eye fixation in children diagnosed with ASD (mean=98.38 seconds) was longer than typically developing peers (mean=162.0), although this disparity did not reach statistical significance. The findings indicate that, on average, children with ASD exhibited a significantly higher number of eye fixations while reading sentences when compared to their neurotypical counterparts (Table 3).
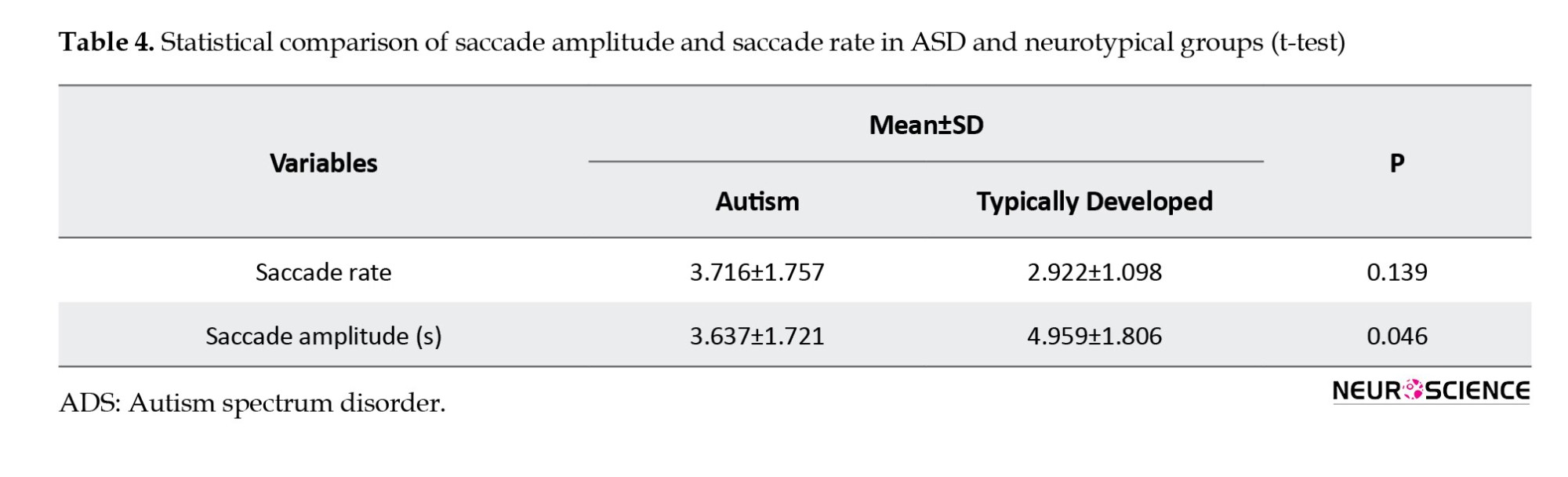
Regarding eye movement, children with ASD demonstrated a higher rate of saccadic movements (mean=3.71) than their neurotypical peers (mean=2.90), but this difference was not statistically significant. However, the amplitude of saccadic movements was significantly greater in autistic children than in their neurotypical peers (Table 4). These results suggest that individuals with autism display a higher frequency of saccades with smaller amplitudes during sentence reading. In comparison, their neurotypical peers exhibit a lower frequency of saccades with larger amplitudes. These findings imply that children with ASD may generate saccades to correct their eye fixation points and compensate for inaccuracies in estimating where their eyes will land (ocular fixation point).
In addition, the mean time required to read a single sentence was compared between the two groups, revealing a statistically significant difference whereby children with ASD spent more time reading than their neurotypical peers (Table 3).
4. Discussion
Children with ASD exhibit increased fixation of the eyes. Specifically, their eyes pause on the text for longer durations, accompanied by numerous saccades with smaller amplitudes than their neurotypical counterparts. The results of the present study indicate that when reading sentences, autistic children display a greater number of eye fixations than their neurotypical peers. However, the duration of each fixation does not differ significantly. The increased occurrence of saccades during sentence reading could be attributed to difficulties in identifying the next fixation point on the subsequent word within the sentence. Saccadic endpoint variability is often attributed to neural noise during sensorimotor processing. Saccadic amplitude refers to the distance the eye covers between two fixation points, where the eyes typically land near the intended target. These findings suggest that autistic individuals experience computational errors in generating saccadic movements during reading, potentially stemming from sensorimotor and perceptual processing impairments.
Research has demonstrated that individuals with autism display atypical visual scanning patterns when observing faces and engaging in social interactions (Klin et al., 2002). They also exhibit abnormalities in sensorimotor control of eye movements when presented with simple visual stimuli, such as illuminated dots on a black screen (Takarae et al., 2004; Takarae et al., 2007) and demonstrated smaller saccadic amplitudes in face perception task (Van der Donck et al., 2021). They also decreased saccade duration and amplitude, independent of human video content (Bast et al., 2021). When making rapid shifts in eye gaze (saccades), individuals with ASD exhibit reduced accuracy (Johnson et al., 2012; Jones et al., 2009; Mercadante et al., 2006) and increased trial-to-trial variability in saccade accuracy (Rosenhallet al., 1988).
Eye movement data is an exceptionally reliable and valuable source for inferring the moment-to-moment processing of words and larger text sections. While it may appear that our eyes move slowly across the screen while reading, it actually consists of a series of saccades, where the eyes jump from one point to another, and fixations, where the eyes remain relatively still (Rayner, 1998).
The difference in parameters of eye movements while reading sentences in the study groups can be explained by two dysfunctions of brain processing: Dysfunction of the cerebellum and dysfunction of the dorsal visual pathway.
The cerebellum plays a crucial role in the precise and adaptable control of eye movements (Ramat et al., 2007). Individuals with cerebellar disorders exhibit various eye movement abnormalities, including saccadic intrusions and oscillations, fixation nystagmus, and slow refixation saccades.
Reading necessitates eye fixation to acquire visual information from words. One’s eyes must systematically move from one word to the next to read a sentence. The saccadic movement must be executed precisely to direct the eye to the correct position on the next word and in the right direction within the sentence. Accurate visual information from the “where” pathway of vision is essential for moving in the correct direction. Multiple studies have reported dysfunctions in this pathway among individuals with autism (Chung & Son, 2020; Hay et al., 2020; Kana et al., 2013; Simmons et al., 2009). The dorsal pathway of the visual system plays a vital role in guiding movements and developing the spatial visual network (Righi & Vettel, 2011). It appears that when children with ASD begin reading a sentence, they struggle to perceive the direction of the line’s continuation and direct their eye movements accordingly. Sequentially reading words in the correct direction to comprehend the sentence becomes a process of trial and error, leading to slower reading speed. Speed and movement accuracy are required to execute the saccadic movement. These parameters are determined within the neural network, where the cerebellum plays a significant role. Even if the location of the next eye fixation is determined correctly if the saccadic movement is either too short or too long, it needs to be corrected.
Saccade dysmetria in individuals with ASD is characterized by increased variability in saccade errors without a corresponding change in mean saccade gain (Barash et al., 1999). A meta-analysis conducted by Johnson et al. (2012) confirmed the presence of saccade dysmetria in ASD, as indicated by greater variability in saccade errors. This dysmetria is typically observed when there is a disruption in the pathway between the cerebellar vermis-fastigial nuclei pathway and the brain stem premotor nuclei, which impairs the ability to recalibrate and reduce saccadic inaccuracies over time (Golla et al., 2008; Barash et al., 1999; Scudder, 2002). Notably, both Johnson et al. (2013), Mosconi et al. (2013), and Johnson et al., 2012) discovered that individuals with ASD had impaired correction of systematic errors in saccade amplitude, induced by classic saccade adaptation paradigms. The pattern of saccade dysmetria in ASD and the impaired ability to correct saccadic errors align with reported morphological abnormalities in the cerebellar vermis (D’Mello et al., 2015; Hashimoto et al., 1993; McKinney et al., 2022; Crucitti et al., 2020; Laidi et al., 2017; Courchesne et al., 1988; Stoodley, 2012), deep cerebellar nuclei (Bauman, 1996; Courchesne et al., 1988), and brainstem in ASD (Hashimoto et al., 1993; Courchesne et al., 1988). Moreover, functional magnetic resonance imaging evidence indicates reduced cerebellar activation during visually guided saccades in individuals with ASD (Takarae et al., 2007).
5. Conclusion
Although the rate of saccades and duration of eye fixations in autistic children do not show statistically significant differences, difficulties in accurately estimating the next fixation point in a sentence hinder proper lexical acquisition. Consequently, the child must readjust the fixation point and repeat the lexical access process, increasing reading time. These findings suggest that to enhance reading skills in individuals with autism, sensory and motor activities should be considered in addition to language parameters. Sensorimotor activities can modify sensory inputs in autism, and exercises that stimulate the motor functions of the cerebellum may aid in improving the acquisition of visual information from words. Semantic and linguistic processing is anticipated to become more efficient by enabling the brain to receive accurate visual information.
In addition to sensorimotor activities, it is recommended that neuro-visual rehabilitation targeting the enhancement of the dorsal visual pathway and improvement of saccades should be considered for preschool-aged children with autism as part of their rehabilitation program.
Limitations
The primary constraint lies in the persistent sensory processing difficulties experienced by children with ASD, prompting the exploration of potential strategies to address this issue. One such strategy involved adjusting the lighting and sound within the room and modifying the texture of the fabric on the chin rest while also considering the rest periods between trials.
Our study aimed to examine eye movements during sentence reading in 8- to 9-year-old children with ASD and their neurotypical counterparts. To extend the applicability of our findings, it is imperative to conduct further investigations encompassing diverse age groups.
Suggestions for future research
In this investigation, we scrutinized the eye movement patterns during sentence reading in autistic children and neurotypical peers. The results demonstrated that although the increased fixation duration and number of saccades in autistic children did not exhibit statistical significance compared to those of typically developing children, the overall time to read a sentence was significantly longer for autistic children. We propose that forthcoming studies on sentence reading should explore eye movement patterns under time-constrained conditions and consider the mean reading time of neurotypical children.
Examining the synchronization between brain waves and eye movements is crucial to elucidate the cognitive mechanisms underlying these disparities. Additionally, it is worth exploring whether these differences persist in autistic individuals as they grow older and their reading skills advance. Consequently, replicating the study with a broader age range would yield intriguing insights.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC 1395.95-03-117-27373).
Funding
The paper was extracted from the PhD dissertation of Faezeh Dehghan, approved by the Department of Neuroscience, School of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran. This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors
Authors' contributions
Conceptualization and supervision: Mohammad Taghi Joghataei, Mehdi Alizadeh Zarei, Faezeh Dehghan, and Reza Khosrowabadi; Methodology: Reza Khosrowabadi; Data collection: Faezeh Dehghan; Data analysis: Faezeh Dehghan, and Mehdi Alizadeh Zarei; Writing: All authors.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all those with whom they have enjoyed working during this and other related projects. They especially thank the Convergent Technologies Research Center at the University of Tehran (NBIC), Tehran, Iran. The authors would especially like to thank the autism families for helping them in this research.
References
Abedi, M., Sadeghi, A., & Rabiei, M. (2015). [Standardization of the Wechsler Intelligence Scale for Children-IV in Chahar Mahal VA Bakhteyri State (Persian)]. Psychological Achievements, 22(2), 99-116. [DOI:10.22055/psy.2016.12310]
Arkush, L., Smith‐Collins, A. P., Fiorentini, C., & Skuse, D. H. (2013). Recognition of face and non‐face stimuli in autistic spectrum disorder. Autism Research, 6(6), 550-560. [DOI:10.1002/aur.1318] [PMID]
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., & Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 67(6), 1–23. [DOI:10.15585/mmwr.ss6706a1] [PMID]
Barash, S., Melikyan, A., Sivakov, A., Zhang, M., Glickstein, M., & Thier, P. (1999). Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. The Journal of Neuroscience: The official Journal of The Society for Neuroscience, 19(24), 10931–10939. [DOI:10.1523/JNEUROSCI.19-24-10931.1999] [PMID]
Bast, N., Mason, L., Freitag, C. M., Smith, T., Portugal, A. M., & Poustka, L., et al. (2021). Saccade dysmetria indicates attenuated visual exploration in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(2), 149–159.[DOI:10.1111/jcpp.13267] [PMID]
Bauman, M. (1996). Observations on the Purkinje cells in the cerebellar vermis in autism. Journal of Neuropathology & Experimental Neurology, 55, 613. [Link]
Catts, H. W., Hogan, T. P., & Fey, M. E. (2003). Subgrouping poor readers on the basis of individual differences in reading-related abilities. Journal of Learning Disabilities, 36(2), 151-164. [DOI:10.1177/002221940303600208] [PMID]
Chung, S., & Son, J. W. (2020). Visual perception in autism spectrum disorder: A review of neuroimaging studies. Journal of Child & Adolescent Psychiatry, 31(3), 105–120. [DOI:10.5765/jkacap.200018] [PMID]
Courchesne, E., Yeung-Courchesne, R., Press, G. A., Hesselink, J. R., & Jernigan, T. L. (1988). Hypoplasia of cerebellar vermal lobules VI and VII in autism. The New England Journal of Medicine, 318(21), 1349–1354. [DOI:10.1056/NEJM198805263182102] [PMID]
Crucitti, J., Hyde, C., Enticott, P. G., & Stokes, M. A. (2020). Are Vermal Lobules VI-VII Smaller in Autism Spectrum Disorder? Cerebellum (London, England), 19(5), 617–628. [DOI:10.1007/s12311-020-01143-5] [PMID]
D’'Mello, A. M., Crocetti, D., Mostofsky, S. H., & Stoodley, C. J. (2015). Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage. Clinical, 7, 631–639. [DOI:10.1016/j.nicl.2015.02.007] [PMID]
Drysdale, B. M., Furlonger, B. E., Anderson, A., & Moore, D. W. (2022). A preliminary study of the eye-gaze patterns and reading comprehension skill of students on the autism spectrum. Advances in Neurodevelopmental Disorders, 6(2), 178-183. [DOI:10.1007/s41252-022-00243-z]
Gilliam J. E. (2014). GARS-3: Gilliam Autism Rating Scale – Third Edition. Austin, TX, USA: Pro-Ed. [Link]
Golla, H., Tziridis, K., Haarmeier, T., Catz, N., Barash, S., & Thier, P. (2008). Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. The European Journal of Neuroscience, 27(1), 132–144. [DOI:10.1111/j.1460-9568.2007.05996.x] [PMID]
Gough, P. B., & Tunmer, W. E. (1986). Decoding, reading, and reading disability. Remedial and Special Education, 7(1), 6-10. [DOI:10.1177/074193258600700104]
Grigorenko, E. L., Klin, A., & Volkmar, F. (2003). Annotation: Hyperlexia: Disability or superability? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(8), 1079–1091. [DOI:10.1111/1469-7610.00193] [PMID]
Harms, M. B., Martin, A., & Wallace, G. L. (2010). Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychology Review, 20(3), 290–322. [DOI:10.1007/s11065-010-9138-6] [PMID]
Hashimoto, T., Tayama, M., Miyazaki, M., Murakawa, K., & Kuroda, Y. (1993). Brainstem and cerebellar vermis involvement in autistic children. Journal of Child Neurology, 8(2), 149–153. [DOI:10.1177/088307389300800207] [PMID]
Hay, I., Dutton, G., Biggar, S., Ibrahim, H., & Assheton, D. (2020). Exploratory study of dorsal visual stream dysfunction in autism; a case series. Research in Autism Spectrum Disorders, 69, 101456. [DOI:10.1016/j.rasd.2019.101456]
Huemer, S. V., & Mann, V. (2010). A comprehensive profile of decoding and comprehension in autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(4), 485–493.[DOI:10.1007/s10803-009-0892-3] [PMID]
Irwin, D. E. (2004). Fixation location and fixation duration as indices of cognitive processing. The interface of language, vision, and action: Eye movements and the visual world (pp. 105-133). London: Psychology Press. [Link]
Johnson, B. P., Rinehart, N. J., Papadopoulos, N., Tonge, B., Millist, L., & White, O., et al. (2012). A closer look at visually guided saccades in autism and Asperger’s disorder. Frontiers in Integrative Neuroscience, 6, 99. [DOI:10.3389/fnint.2012.00099] [PMID]
Johnson, B. P., Rinehart, N. J., White, O., Millist, L., & Fielding, J. (2013). Saccade adaptation in autism and Asperger’s Asperger’s disorder. Neuroscience, 243, 76–87. [DOI:10.1016/j.neuroscience.2013.03.051] [PMID]
Jones, C. R., Happé, F., Golden, H., Marsden, A. J., Tregay, J., & Simonoff, E., et al. (2009). Reading and arithmetic in adolescents with autism spectrum disorders: Peaks and dips in attainment. Neuropsychology, 23(6), 718–728. [DOI:10.1037/a0016360] [PMID]
Kana, R. K., Liu, Y., Williams, D. L., Keller, T. A., Schipul, S. E., Minshew, N. J., & Just, M. A. (2013). The local, global, and neural aspects of visuospatial processing in autism spectrum disorders. Neuropsychologia, 51(14), 2995-3003. [DOI:10.1016/j.neuropsychologia.2013.10.013] [PMID]
Klin, A., Jones, W., Schultz, R., Volkmar, F., & Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809-816. [DOI:10.1001/archpsyc.59.9.809] [PMID]
Laidi, C., Boisgontier, J., Chakravarty, M. M., Hotier, S., d'Albis, M. A., & Mangin, J. F., et al. (2017). Cerebellar anatomical alterations and attention to eyes in autism. Scientific Reports, 7(1), 12008. [DOI:10.1038/s41598-017-11883-w] [PMID]
Manyakov, N. V., Bangerter, A., Chatterjee, M., Mason, L., Ness, S., & Lewin, D., et al. (2018). Visual exploration in autism spectrum disorder: exploring age differences and dynamic features using recurrence quantification analysis. Autism Research, 11(11), 1554-1566. [DOI:10.1002/aur.2021] [PMID]
McKinney, W. S., Kelly, S. E., Unruh, K. E., Shafer, R. L., Sweeney, J. A., & Styner, M., et al. (2022). Corrigendum: Cerebellar volumes and sensorimotor behavior in autism spectrum disorder. Frontiers in Integrative Neuroscience, 16, 1020980. [DOI:10.3389/fnint.2022.1020980] [PMID]
Mercadante, M. T., Macedo, E. C., Baptista, P. M., Paula, C. S., & Schwartzman, J. S. (2006). Saccadic movements using eye-tracking technology in individuals with autism spectrum disorders: Pilot study. Arquivos de Neuro-Psiquiatria, 64(3A), 559–562. [DOI:10.1590/S0004-282X2006000400003] [PMID]
Micai, M., Joseph, H., Vulchanova, M., & Saldaña, D. (2017). Strategies of readers with autism when responding to inferential questions: An eye‐movement study. Autism Research, 10(5), 888-900. [DOI:10.1002/aur.1731] [PMID]
Micai, M., Vulchanova, M., & Saldaña, D. (2021). Reading goals and executive function in Autism: An eye‐tracking study. Autism Research, 14(5), 1007-1024. [DOI:10.1002/aur.2447] [PMID]
Minaei, A., & Nazeri, S. (2018). [Psychometric properties of the Gilliam Autism Rating Scale-Third Edition (GARS-3) in individuals with autism: A pilot study (Persian)]. Journal of Exceptional Children, 18(2), 113-122. [Link]
Mosconi, M. W., Luna, B., Kay-Stacey, M., Nowinski, C. V., Rubin, L. H., & Scudder, C., et al. (2013). Saccade adaptation abnormalities implicate dysfunction of cerebellar-dependent learning mechanisms in Autism Spectrum Disorders (ASD). Plos One, 8(5), e63709. [PMID]
Nation, K., Clarke, P., Wright, B., & Williams, C. (2006). Patterns of reading ability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 36(7), 911–919.[DOI:10.1007/s10803-006-0130-1] [PMID]
Nation, K., & Snowling, M. J. (1999). Developmental differences in sensitivity to semantic relations among good and poor comprehenders: Evidence from semantic priming. Cognition, 70(1), B1-B13. [DOI:10.1016/S0010-0277(99)00004-9]
Newman, T. M., Macomber, D., Naples, A. J., Babitz, T., Volkmar, F., & Grigorenko, E. L. (2007). Hyperlexia in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(4), 760-774. [DOI:10.1007/s10803-006-0206-y] [PMID]
Ramat, S., Leigh, R. J., Zee, D. S., & Optican, L. M. (2007). What clinical disorders tell us about the neural control of saccadic eye movements. Brain: A Journal of Neurology, 130(Pt 1), 10–35. [DOI:10.1093/brain/awl309] [PMID]
Rayner, K. (1978). Eye movements in reading and information processing. Psychological Bulletin, 85(3), 618-660. [DOI:10.1037/0033-2909.85.3.618] [PMID]
Rayner, K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124(3), 372-422. [DOI:10.1037/0033-2909.124.3.372] [PMID]
Rayner, K. (2009). Eye movements and attention in reading, scene perception, and visual search. Quarterly Journal of Experimental Psychology (2006), 62(8), 1457–1506.[DOI:10.1080/17470210902816461] [PMID]
Reichle, E. D., Pollatsek, A., Fisher, D. L., & Rayner, K. (1998). Toward a model of eye movement control in reading. Psychological Review, 105(1), 125-157. [DOI:10.1037/0033-295X.105.1.125] [PMID]
Ricketts, J., Jones, C. R., Happé, F., & Charman, T. (2013). Reading comprehension in autism spectrum disorders: The role of oral language and social functioning. Journal of Autism and Developmental Disorders, 43(4), 807–816. [DOI:10.1007/s10803-012-1619-4] [PMID]
Righi, G., & Vettel, J. (2011). Dorsal visual pathway. In: J. S. Kreutzer, J. DeLuca &B. Caplan (Eds), Encyclopedia of Clinical Neuropsychology (pp. 887-888). New York: Springer. [DOI:10.1007/978-0-387-79948-3_1358]
Rosenhall, U., Johansson, E., & Gillberg, C. (1988). Oculomotor findings in autistic children. The Journal of Laryngology and Otology, 102(5), 435–439. [DOI:10.1017/S0022215100105286] [PMID]
Saldaña, D., & Frith, U. (2007). Do readers with autism make bridging inferences from world knowledge? Journal of Experimental Child Psychology, 96(4), 310-319. [DOI:10.1016/j.jecp.2006.11.002] [PMID]
Sansosti, F. J., Was, C., Rawson, K. A., & Remaklus, B. L. (2013). Eye movements during processing of text requiring bridging inferences in adolescents with higher functioning autism spectrum disorders: A preliminary investigation. Research in Autism Spectrum Disorders, 7(12), 1535-1542. [DOI:10.1016/j.rasd.2013.09.001]
Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., & Bodfish, J. W. (2011). Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders, 41, 242-247. [DOI:10.1007/s10803-010-1038-3] [PMID]
Scudder, C. A. (2002). Role of the fastigial nucleus in controlling horizontal saccades during adaptation. Annals of the New York Academy of Sciences, 978(1), 63-78. [DOI:10.1111/j.1749-6632.2002.tb07556.x] [PMID]
Seltzer, M. M., Shattuck, P., Abbeduto, L., & Greenberg, J. S. (2004). Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews, 10(4), 234–247. [DOI:10.1002/mrdd.20038] [PMID]
Simmons, D. R., Robertson, A. E., McKay, L. S., Toal, E., McAleer, P., & Pollick, F. E. (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–2739. [DOI:10.1016/j.visres.2009.08.005] [PMID]
Stoodley, C. J. (2012). The cerebellum and cognition: evidence from functional imaging studies. Cerebellum (London, England), 11(2), 352–365. [DOI:10.1007/s12311-011-0260-7] [PMID]
Takarae, Y., Minshew, N., Luna, B., & Sweeney, J. (2004). Oculomotor abnormalities parallel cerebellar histopathology in autism. Journal of Neurology, Neurosurgery, and Psychiatry, 75(9), 1359–1361. [DOI:10.1136/jnnp.2003.022491] [PMID]
Takarae, Y., Minshew, N. J., Luna, B., & Sweeney, J. A. (2007). Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research, 156(2), 117–127. [DOI:10.1016/j.pscychresns.2007.03.008] [PMID]
Van der Donck, S., Vettori, S., Dzhelyova, M., Mahdi, S. S., Claes, P., & Steyaert, J., et al. (2021). Investigating automatic emotion processing in boys with autism via eye tracking and facial mimicry recordings. Autism Research, 14(7), 1404-1420. [DOI:10.1002/aur.2490] [PMID]
Autism spectrum disorders (ASD) are a collection of neurodevelopmental conditions characterized by impairments in social interaction, communication, repetitive behaviors, and limited interests. In 2021, the Centers for Disease Control and Prevention (CDC) reported that approximately 1 in 44 children in the United States is diagnosed with ASD (Baio et al., 2018). ASD is a lifelong condition, and individuals with ASD face significant challenges in all stages of development. Recent research has focused on the academic achievements of individuals with autism, particularly in the early years of education when acquiring reading, writing, and mathematics skills can be highly challenging. These skills are crucial for social participation and achieving independence.
The CDC has stated that approximately 68% of school children with ASD exhibit cognitive abilities beyond the range of intellectual disability, meaning that they have an Intelligence quotient (IQ) above 70 (Seltzer et al., 2004). Despite having normal IQ, these individuals often display limited verbal abilities at the sentence level. However, their academic and social outcomes are far from optimal. Despite having an average IQ, many children with ASD show a discrepancy between their academic performance and achievement, which resembles specific learning disabilities. Notably, difficulties in reading comprehension and understanding written text have been a focal point, even when these students can read individual words proficiently (Huemer & Mann, 2010; Jones et al., 2009).
The “simple reading view” (Gough & Tunmer, 1986) proposes that skilled literacy relies on two closely interconnected processes: Decoding or identifying individual words and engaging in language processes constructing meaningful text. Both processes are necessary for comprehension. Without adequate word-level reading skills, comprehension is likely to fail. However, skilled word-level reading in isolation does not guarantee understanding at the text level. Decoding and comprehension are believed to depend on underlying language skills. Consequently, there may be three potential subgroups that experience difficulties with reading comprehension: Individuals with strong decoding skills but poor oral language skills, individuals with strong oral language skills but deficits in decoding, and individuals who struggle with both oral language and decoding (Catts et al., 2003).
Most previous studies on reading in children with ASD have focused on a specific subgroup characterized by the ability to decode words. Still, difficulty comprehending written text is often referred to as hyperlexia (Newman et al., 2007). These studies have specifically examined inconsistencies in reading comprehension and decoding ability within average samples (Jones et al., 2009). Children with ASD are often reported to have advanced word-reading skills at an early age (Grigorenko et al., 2003). However, these early developments may be hindered by long-term deficits in word reading and reading comprehension (Nation & Snowling, 1999). In a study exploring reading profiles in ASD, Nation et al. (2006) discovered that many children with ASD struggled with both decoding and comprehension. Around 65% of the cohort exhibited poor reading comprehension, often alongside poor word-level reading skills. Notably, the most significant finding of Nation et al.’s (2006) study was the substantial variation observed, with a wide range of performance levels seen across most reading and reading-related measures. However, the extent of this discrepancy varies widely, possibly due to the specific characteristics of the individuals being studied (Saldaña & Frith, 2007; Newman et al., 2007; Ricketts et al., 2013; Nation et al., 2006; Jones et al., 2009).
Eye movements play a crucial role in the reading process, consisting of three primary components: Saccade, fixation, and regression. During fixations, new information is encoded as vision is suppressed during saccades. Although the size of saccades and the duration of fixations may vary, this pattern of eye movements remains consistent across various visual-cognitive tasks driven by the same neural circuitry. As visual limitations heavily influence eye movement behavior, it is unsurprising that general similarities are observed. In reading, linguistic factors such as word frequency, predictability, and syntactic complexity impact moment-to-moment eye movements (Rayner, 1978; Ryaner, 2009). Despite the association between eye movement patterns and reading skills (Irwin, 2004), eye movements are seldom considered the root cause of reading difficulties. Research on eye movements ranges from perceptual issues to theoretical and computational models of eye movement control during reading. One crucial question is whether eye movements in reading are governed by low-level motor strategies or influenced by cognitive processes in real time (Reichle et al., 1998).
While some studies have explored eye movement characteristics in individuals with ASD, they primarily utilized stimuli with limited content, such as solely faces (Arkush et al., 2013; Harms et al., 2010), and few open datasets are available. Eye-tracking research has also examined attention shifting and disengagement in ASD, with recent research (Manyakov et al., 2018) documenting fewer shifts and longer disengagement time in response to pictures considered of high interest (e.g. trains, cars) in adolescents and adults with ASD—a finding consistent with earlier work linking atypical shifting and disengagement to circumscribed interests in children with ASD (Sasson et al, 2011).
The cause of reading comprehension problems in people with ASD is debatable. We have investigated the ability of autistic children to adapt reading strategies based on different reading goals using eye-tracking technology. The effect of reading purposefulness on the number of fixations (Micai et al., 2021; Drysdale et al, 2022), duration of fixations, number of forward fixations, length of the forward saccade was reported (Micai et al., 2021; Micai et al., 2017). Interestingly, more fixations were observed for the purposeful reading of the text compared to the entertainment-only condition in the control group (Micai et al., 2021). Children with high-functioning ASD can make the necessary inferences to understand reading. Eye movement data showed that children with high-functioning ASD spent more time fixating on the text, had more fixations overall, and made more regressions during reading (i.e. moving backward through the text) than neurotypical peers (Sansosti et al., 2013).
Nonetheless, studies indicate that individuals with ASD exhibit differences in decoding written language and lexical acquisition, yet they can read words within sentences. The question remains whether the delayed semantic processing and comprehension of sentences in individuals with ASD are attributable to higher-level language learning phenomena or if lower-level sensorimotor skills when extracting visual information from written language contribute to the performance disparities and subsequent linguistic processing slowness. Consequently, this study aims to investigate the eye movement characteristics and lower sensory motor skills employed while reading sentences in children with autism.
2. Materials and Methods
Study participants
We recruited a group of children diagnosed with high-functioning autism disorder (n=15) with a mean age of 102.3 months. The diagnosis was conducted by a child and adolescent psychiatrist using the diagnostic and statistical manual of mental disorders, fifth edition (DSM-V) diagnostic criteria. In addition to the psychiatrist’s opinion, the Gilliam autism rating scale, third edition (GARS-3), has been used to diagnose autism. On this scale, scoring 50 to 70 is mild autism or level 1. In the articles, high-functioning autism is equivalent to level 1 autism. The control group of typically developing children (n=17) from primary schools, with a mean age of 101.10 months, participated in the study. According to their health records, the control group had no recorded developmental disorders. Written consent was obtained from the participants and their parents for their involvement in the study. Parents were asked to rate their child’s behavior using the GARS. It is important to note that the autistic children did not take any medication during the study.
Study procedure
The research involved a 2-hour session during which the Wechsler IQ test was administered. On a separate day, the children were present to record their eye movements. The children with autism, along with their neurotypical peers, had recently entered public schools after completing six years of education. One of the criteria for entry into the study was that none of the children had repeated any academic classes. This was crucial to minimize any potential learning effects on the task. The autistic children were referred to the researcher from general hospitals in Tehran.
Study measures
The GARS-3
Gilliam developed the GARS-3 scale by extracting questions from the DSM-V diagnostic criteria and the American Autism Association definition (Gilliam, 2014). The aim was to create a tool with high validity and content. The scale consists of 56 questions, rated on a 4-point Likert scale ranging from 0 to 3. The GARS-3 includes 6 subscales: Repetitive/restrictive behaviors, social communication, social interaction, emotional responses, maladaptive speech, and cognitive style. This study utilized the standardized Iranian version of the GARS-3 scale (Minaei & Nazeri, 2018).
The GARS-3 scale was administered to all participants in the research. Individuals with autism who scored between 50 and 70 were diagnosed with ASD-level 1 autism, as shown in Tables 1 and 2. None of the autistic children were prescribed medication.

Wechsler intelligence scale for children
An expert psychologist administered the Wechsler intelligence scale for children (WISC) for both groups. Autistic children who scored above 90 on the Wechsler intelligence test were eligible to participate in the study. The standardized Iranian test version was used (Tables 1 and 2). We have used the standardized version of this test in Iran (Abedi et al., 2015).

Study task
The task consisted of 40 sentences, with 12 sentences containing 8 words, 15 sentences containing 7 words, 9 sentences containing 6 words, and 4 sentences containing 5 words. These sentences were displayed on a monitor screen and were selected from the reading skills book used in the second year of primary school. The chosen words had a high frequency of occurrence as they were taken from the second-grade Persian reading book.
Only nouns and verbs were included among the selected words to ensure control over the semantic load and objectivity. Iranian linguistics and speech therapy experts were consulted to determine the objectivity of the words using a 5-point Likert scale. Words with a score of >2.5 on the scale were agreed upon for use. Subsequently, 130 neurotypical children aged 8 to 10 years were asked to rate these words based on their objectivity. From the initial pool of 280 words, 160 words with objectivity scores >2.5 were selected to form the sentences. These sentences were well-known and had low emotional value.
During the experiment, the participants’ left eye was tracked using a remote monocular eye-tracker (Segal Tracker iSaw, FARMED, Tehran, Iran) with a sampling frequency of 500 Hz. The stimulus presentation was regulated using the PsychoPy framework. The stimuli were displayed on a screen measuring 57 by 43.1 cm, positioned 55 cm in front of the participant, and had a screen resolution of 1920 by 1080 pixels. This setup resulted in a viewing angle of 0.031 degrees for the screen’s pixels.
The task consisted of 40 trials, where the participants were required to read the sentences aloud. After each sentence, the examiner pressed the space button to display the following sentence. If the participants became tired during the trials, the task was temporarily paused and resumed after a break. Both groups of participants in the research performed the task under the same physical conditions.
3. Results
In this research, the data were first analyzed descriptively. Categorical variables were reported as frequency and percentage, quantitative variables with normal distribution were reported with mean and standard deviation, and quantitative variables with skewed distribution were reported with median and interquartile range (IQR). Then, the distribution of quantitative variables was measured with the Shapiro-Wilk test. The independent samples t-test was used to analyze variables with normal distribution, and the Mann-Whitney test for variables that did not follow a normal distribution. We used SPSS software, version 27.0.1
The Pearson correlation test was used to correlate quantitative variables. The analysis was done with SPSS software, and the significance level was considered 0.05 for all tests.
The duration of eye fixation in children diagnosed with ASD (mean=98.38 seconds) was longer than typically developing peers (mean=162.0), although this disparity did not reach statistical significance. The findings indicate that, on average, children with ASD exhibited a significantly higher number of eye fixations while reading sentences when compared to their neurotypical counterparts (Table 3).

Regarding eye movement, children with ASD demonstrated a higher rate of saccadic movements (mean=3.71) than their neurotypical peers (mean=2.90), but this difference was not statistically significant. However, the amplitude of saccadic movements was significantly greater in autistic children than in their neurotypical peers (Table 4). These results suggest that individuals with autism display a higher frequency of saccades with smaller amplitudes during sentence reading. In comparison, their neurotypical peers exhibit a lower frequency of saccades with larger amplitudes. These findings imply that children with ASD may generate saccades to correct their eye fixation points and compensate for inaccuracies in estimating where their eyes will land (ocular fixation point).

In addition, the mean time required to read a single sentence was compared between the two groups, revealing a statistically significant difference whereby children with ASD spent more time reading than their neurotypical peers (Table 3).
4. Discussion
Children with ASD exhibit increased fixation of the eyes. Specifically, their eyes pause on the text for longer durations, accompanied by numerous saccades with smaller amplitudes than their neurotypical counterparts. The results of the present study indicate that when reading sentences, autistic children display a greater number of eye fixations than their neurotypical peers. However, the duration of each fixation does not differ significantly. The increased occurrence of saccades during sentence reading could be attributed to difficulties in identifying the next fixation point on the subsequent word within the sentence. Saccadic endpoint variability is often attributed to neural noise during sensorimotor processing. Saccadic amplitude refers to the distance the eye covers between two fixation points, where the eyes typically land near the intended target. These findings suggest that autistic individuals experience computational errors in generating saccadic movements during reading, potentially stemming from sensorimotor and perceptual processing impairments.
Research has demonstrated that individuals with autism display atypical visual scanning patterns when observing faces and engaging in social interactions (Klin et al., 2002). They also exhibit abnormalities in sensorimotor control of eye movements when presented with simple visual stimuli, such as illuminated dots on a black screen (Takarae et al., 2004; Takarae et al., 2007) and demonstrated smaller saccadic amplitudes in face perception task (Van der Donck et al., 2021). They also decreased saccade duration and amplitude, independent of human video content (Bast et al., 2021). When making rapid shifts in eye gaze (saccades), individuals with ASD exhibit reduced accuracy (Johnson et al., 2012; Jones et al., 2009; Mercadante et al., 2006) and increased trial-to-trial variability in saccade accuracy (Rosenhallet al., 1988).
Eye movement data is an exceptionally reliable and valuable source for inferring the moment-to-moment processing of words and larger text sections. While it may appear that our eyes move slowly across the screen while reading, it actually consists of a series of saccades, where the eyes jump from one point to another, and fixations, where the eyes remain relatively still (Rayner, 1998).
The difference in parameters of eye movements while reading sentences in the study groups can be explained by two dysfunctions of brain processing: Dysfunction of the cerebellum and dysfunction of the dorsal visual pathway.
The cerebellum plays a crucial role in the precise and adaptable control of eye movements (Ramat et al., 2007). Individuals with cerebellar disorders exhibit various eye movement abnormalities, including saccadic intrusions and oscillations, fixation nystagmus, and slow refixation saccades.
Reading necessitates eye fixation to acquire visual information from words. One’s eyes must systematically move from one word to the next to read a sentence. The saccadic movement must be executed precisely to direct the eye to the correct position on the next word and in the right direction within the sentence. Accurate visual information from the “where” pathway of vision is essential for moving in the correct direction. Multiple studies have reported dysfunctions in this pathway among individuals with autism (Chung & Son, 2020; Hay et al., 2020; Kana et al., 2013; Simmons et al., 2009). The dorsal pathway of the visual system plays a vital role in guiding movements and developing the spatial visual network (Righi & Vettel, 2011). It appears that when children with ASD begin reading a sentence, they struggle to perceive the direction of the line’s continuation and direct their eye movements accordingly. Sequentially reading words in the correct direction to comprehend the sentence becomes a process of trial and error, leading to slower reading speed. Speed and movement accuracy are required to execute the saccadic movement. These parameters are determined within the neural network, where the cerebellum plays a significant role. Even if the location of the next eye fixation is determined correctly if the saccadic movement is either too short or too long, it needs to be corrected.
Saccade dysmetria in individuals with ASD is characterized by increased variability in saccade errors without a corresponding change in mean saccade gain (Barash et al., 1999). A meta-analysis conducted by Johnson et al. (2012) confirmed the presence of saccade dysmetria in ASD, as indicated by greater variability in saccade errors. This dysmetria is typically observed when there is a disruption in the pathway between the cerebellar vermis-fastigial nuclei pathway and the brain stem premotor nuclei, which impairs the ability to recalibrate and reduce saccadic inaccuracies over time (Golla et al., 2008; Barash et al., 1999; Scudder, 2002). Notably, both Johnson et al. (2013), Mosconi et al. (2013), and Johnson et al., 2012) discovered that individuals with ASD had impaired correction of systematic errors in saccade amplitude, induced by classic saccade adaptation paradigms. The pattern of saccade dysmetria in ASD and the impaired ability to correct saccadic errors align with reported morphological abnormalities in the cerebellar vermis (D’Mello et al., 2015; Hashimoto et al., 1993; McKinney et al., 2022; Crucitti et al., 2020; Laidi et al., 2017; Courchesne et al., 1988; Stoodley, 2012), deep cerebellar nuclei (Bauman, 1996; Courchesne et al., 1988), and brainstem in ASD (Hashimoto et al., 1993; Courchesne et al., 1988). Moreover, functional magnetic resonance imaging evidence indicates reduced cerebellar activation during visually guided saccades in individuals with ASD (Takarae et al., 2007).
5. Conclusion
Although the rate of saccades and duration of eye fixations in autistic children do not show statistically significant differences, difficulties in accurately estimating the next fixation point in a sentence hinder proper lexical acquisition. Consequently, the child must readjust the fixation point and repeat the lexical access process, increasing reading time. These findings suggest that to enhance reading skills in individuals with autism, sensory and motor activities should be considered in addition to language parameters. Sensorimotor activities can modify sensory inputs in autism, and exercises that stimulate the motor functions of the cerebellum may aid in improving the acquisition of visual information from words. Semantic and linguistic processing is anticipated to become more efficient by enabling the brain to receive accurate visual information.
In addition to sensorimotor activities, it is recommended that neuro-visual rehabilitation targeting the enhancement of the dorsal visual pathway and improvement of saccades should be considered for preschool-aged children with autism as part of their rehabilitation program.
Limitations
The primary constraint lies in the persistent sensory processing difficulties experienced by children with ASD, prompting the exploration of potential strategies to address this issue. One such strategy involved adjusting the lighting and sound within the room and modifying the texture of the fabric on the chin rest while also considering the rest periods between trials.
Our study aimed to examine eye movements during sentence reading in 8- to 9-year-old children with ASD and their neurotypical counterparts. To extend the applicability of our findings, it is imperative to conduct further investigations encompassing diverse age groups.
Suggestions for future research
In this investigation, we scrutinized the eye movement patterns during sentence reading in autistic children and neurotypical peers. The results demonstrated that although the increased fixation duration and number of saccades in autistic children did not exhibit statistical significance compared to those of typically developing children, the overall time to read a sentence was significantly longer for autistic children. We propose that forthcoming studies on sentence reading should explore eye movement patterns under time-constrained conditions and consider the mean reading time of neurotypical children.
Examining the synchronization between brain waves and eye movements is crucial to elucidate the cognitive mechanisms underlying these disparities. Additionally, it is worth exploring whether these differences persist in autistic individuals as they grow older and their reading skills advance. Consequently, replicating the study with a broader age range would yield intriguing insights.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC 1395.95-03-117-27373).
Funding
The paper was extracted from the PhD dissertation of Faezeh Dehghan, approved by the Department of Neuroscience, School of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran. This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors
Authors' contributions
Conceptualization and supervision: Mohammad Taghi Joghataei, Mehdi Alizadeh Zarei, Faezeh Dehghan, and Reza Khosrowabadi; Methodology: Reza Khosrowabadi; Data collection: Faezeh Dehghan; Data analysis: Faezeh Dehghan, and Mehdi Alizadeh Zarei; Writing: All authors.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all those with whom they have enjoyed working during this and other related projects. They especially thank the Convergent Technologies Research Center at the University of Tehran (NBIC), Tehran, Iran. The authors would especially like to thank the autism families for helping them in this research.
References
Abedi, M., Sadeghi, A., & Rabiei, M. (2015). [Standardization of the Wechsler Intelligence Scale for Children-IV in Chahar Mahal VA Bakhteyri State (Persian)]. Psychological Achievements, 22(2), 99-116. [DOI:10.22055/psy.2016.12310]
Arkush, L., Smith‐Collins, A. P., Fiorentini, C., & Skuse, D. H. (2013). Recognition of face and non‐face stimuli in autistic spectrum disorder. Autism Research, 6(6), 550-560. [DOI:10.1002/aur.1318] [PMID]
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., & Warren, Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 67(6), 1–23. [DOI:10.15585/mmwr.ss6706a1] [PMID]
Barash, S., Melikyan, A., Sivakov, A., Zhang, M., Glickstein, M., & Thier, P. (1999). Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. The Journal of Neuroscience: The official Journal of The Society for Neuroscience, 19(24), 10931–10939. [DOI:10.1523/JNEUROSCI.19-24-10931.1999] [PMID]
Bast, N., Mason, L., Freitag, C. M., Smith, T., Portugal, A. M., & Poustka, L., et al. (2021). Saccade dysmetria indicates attenuated visual exploration in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 62(2), 149–159.[DOI:10.1111/jcpp.13267] [PMID]
Bauman, M. (1996). Observations on the Purkinje cells in the cerebellar vermis in autism. Journal of Neuropathology & Experimental Neurology, 55, 613. [Link]
Catts, H. W., Hogan, T. P., & Fey, M. E. (2003). Subgrouping poor readers on the basis of individual differences in reading-related abilities. Journal of Learning Disabilities, 36(2), 151-164. [DOI:10.1177/002221940303600208] [PMID]
Chung, S., & Son, J. W. (2020). Visual perception in autism spectrum disorder: A review of neuroimaging studies. Journal of Child & Adolescent Psychiatry, 31(3), 105–120. [DOI:10.5765/jkacap.200018] [PMID]
Courchesne, E., Yeung-Courchesne, R., Press, G. A., Hesselink, J. R., & Jernigan, T. L. (1988). Hypoplasia of cerebellar vermal lobules VI and VII in autism. The New England Journal of Medicine, 318(21), 1349–1354. [DOI:10.1056/NEJM198805263182102] [PMID]
Crucitti, J., Hyde, C., Enticott, P. G., & Stokes, M. A. (2020). Are Vermal Lobules VI-VII Smaller in Autism Spectrum Disorder? Cerebellum (London, England), 19(5), 617–628. [DOI:10.1007/s12311-020-01143-5] [PMID]
D’'Mello, A. M., Crocetti, D., Mostofsky, S. H., & Stoodley, C. J. (2015). Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage. Clinical, 7, 631–639. [DOI:10.1016/j.nicl.2015.02.007] [PMID]
Drysdale, B. M., Furlonger, B. E., Anderson, A., & Moore, D. W. (2022). A preliminary study of the eye-gaze patterns and reading comprehension skill of students on the autism spectrum. Advances in Neurodevelopmental Disorders, 6(2), 178-183. [DOI:10.1007/s41252-022-00243-z]
Gilliam J. E. (2014). GARS-3: Gilliam Autism Rating Scale – Third Edition. Austin, TX, USA: Pro-Ed. [Link]
Golla, H., Tziridis, K., Haarmeier, T., Catz, N., Barash, S., & Thier, P. (2008). Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. The European Journal of Neuroscience, 27(1), 132–144. [DOI:10.1111/j.1460-9568.2007.05996.x] [PMID]
Gough, P. B., & Tunmer, W. E. (1986). Decoding, reading, and reading disability. Remedial and Special Education, 7(1), 6-10. [DOI:10.1177/074193258600700104]
Grigorenko, E. L., Klin, A., & Volkmar, F. (2003). Annotation: Hyperlexia: Disability or superability? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(8), 1079–1091. [DOI:10.1111/1469-7610.00193] [PMID]
Harms, M. B., Martin, A., & Wallace, G. L. (2010). Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychology Review, 20(3), 290–322. [DOI:10.1007/s11065-010-9138-6] [PMID]
Hashimoto, T., Tayama, M., Miyazaki, M., Murakawa, K., & Kuroda, Y. (1993). Brainstem and cerebellar vermis involvement in autistic children. Journal of Child Neurology, 8(2), 149–153. [DOI:10.1177/088307389300800207] [PMID]
Hay, I., Dutton, G., Biggar, S., Ibrahim, H., & Assheton, D. (2020). Exploratory study of dorsal visual stream dysfunction in autism; a case series. Research in Autism Spectrum Disorders, 69, 101456. [DOI:10.1016/j.rasd.2019.101456]
Huemer, S. V., & Mann, V. (2010). A comprehensive profile of decoding and comprehension in autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(4), 485–493.[DOI:10.1007/s10803-009-0892-3] [PMID]
Irwin, D. E. (2004). Fixation location and fixation duration as indices of cognitive processing. The interface of language, vision, and action: Eye movements and the visual world (pp. 105-133). London: Psychology Press. [Link]
Johnson, B. P., Rinehart, N. J., Papadopoulos, N., Tonge, B., Millist, L., & White, O., et al. (2012). A closer look at visually guided saccades in autism and Asperger’s disorder. Frontiers in Integrative Neuroscience, 6, 99. [DOI:10.3389/fnint.2012.00099] [PMID]
Johnson, B. P., Rinehart, N. J., White, O., Millist, L., & Fielding, J. (2013). Saccade adaptation in autism and Asperger’s Asperger’s disorder. Neuroscience, 243, 76–87. [DOI:10.1016/j.neuroscience.2013.03.051] [PMID]
Jones, C. R., Happé, F., Golden, H., Marsden, A. J., Tregay, J., & Simonoff, E., et al. (2009). Reading and arithmetic in adolescents with autism spectrum disorders: Peaks and dips in attainment. Neuropsychology, 23(6), 718–728. [DOI:10.1037/a0016360] [PMID]
Kana, R. K., Liu, Y., Williams, D. L., Keller, T. A., Schipul, S. E., Minshew, N. J., & Just, M. A. (2013). The local, global, and neural aspects of visuospatial processing in autism spectrum disorders. Neuropsychologia, 51(14), 2995-3003. [DOI:10.1016/j.neuropsychologia.2013.10.013] [PMID]
Klin, A., Jones, W., Schultz, R., Volkmar, F., & Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809-816. [DOI:10.1001/archpsyc.59.9.809] [PMID]
Laidi, C., Boisgontier, J., Chakravarty, M. M., Hotier, S., d'Albis, M. A., & Mangin, J. F., et al. (2017). Cerebellar anatomical alterations and attention to eyes in autism. Scientific Reports, 7(1), 12008. [DOI:10.1038/s41598-017-11883-w] [PMID]
Manyakov, N. V., Bangerter, A., Chatterjee, M., Mason, L., Ness, S., & Lewin, D., et al. (2018). Visual exploration in autism spectrum disorder: exploring age differences and dynamic features using recurrence quantification analysis. Autism Research, 11(11), 1554-1566. [DOI:10.1002/aur.2021] [PMID]
McKinney, W. S., Kelly, S. E., Unruh, K. E., Shafer, R. L., Sweeney, J. A., & Styner, M., et al. (2022). Corrigendum: Cerebellar volumes and sensorimotor behavior in autism spectrum disorder. Frontiers in Integrative Neuroscience, 16, 1020980. [DOI:10.3389/fnint.2022.1020980] [PMID]
Mercadante, M. T., Macedo, E. C., Baptista, P. M., Paula, C. S., & Schwartzman, J. S. (2006). Saccadic movements using eye-tracking technology in individuals with autism spectrum disorders: Pilot study. Arquivos de Neuro-Psiquiatria, 64(3A), 559–562. [DOI:10.1590/S0004-282X2006000400003] [PMID]
Micai, M., Joseph, H., Vulchanova, M., & Saldaña, D. (2017). Strategies of readers with autism when responding to inferential questions: An eye‐movement study. Autism Research, 10(5), 888-900. [DOI:10.1002/aur.1731] [PMID]
Micai, M., Vulchanova, M., & Saldaña, D. (2021). Reading goals and executive function in Autism: An eye‐tracking study. Autism Research, 14(5), 1007-1024. [DOI:10.1002/aur.2447] [PMID]
Minaei, A., & Nazeri, S. (2018). [Psychometric properties of the Gilliam Autism Rating Scale-Third Edition (GARS-3) in individuals with autism: A pilot study (Persian)]. Journal of Exceptional Children, 18(2), 113-122. [Link]
Mosconi, M. W., Luna, B., Kay-Stacey, M., Nowinski, C. V., Rubin, L. H., & Scudder, C., et al. (2013). Saccade adaptation abnormalities implicate dysfunction of cerebellar-dependent learning mechanisms in Autism Spectrum Disorders (ASD). Plos One, 8(5), e63709. [PMID]
Nation, K., Clarke, P., Wright, B., & Williams, C. (2006). Patterns of reading ability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 36(7), 911–919.[DOI:10.1007/s10803-006-0130-1] [PMID]
Nation, K., & Snowling, M. J. (1999). Developmental differences in sensitivity to semantic relations among good and poor comprehenders: Evidence from semantic priming. Cognition, 70(1), B1-B13. [DOI:10.1016/S0010-0277(99)00004-9]
Newman, T. M., Macomber, D., Naples, A. J., Babitz, T., Volkmar, F., & Grigorenko, E. L. (2007). Hyperlexia in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(4), 760-774. [DOI:10.1007/s10803-006-0206-y] [PMID]
Ramat, S., Leigh, R. J., Zee, D. S., & Optican, L. M. (2007). What clinical disorders tell us about the neural control of saccadic eye movements. Brain: A Journal of Neurology, 130(Pt 1), 10–35. [DOI:10.1093/brain/awl309] [PMID]
Rayner, K. (1978). Eye movements in reading and information processing. Psychological Bulletin, 85(3), 618-660. [DOI:10.1037/0033-2909.85.3.618] [PMID]
Rayner, K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124(3), 372-422. [DOI:10.1037/0033-2909.124.3.372] [PMID]
Rayner, K. (2009). Eye movements and attention in reading, scene perception, and visual search. Quarterly Journal of Experimental Psychology (2006), 62(8), 1457–1506.[DOI:10.1080/17470210902816461] [PMID]
Reichle, E. D., Pollatsek, A., Fisher, D. L., & Rayner, K. (1998). Toward a model of eye movement control in reading. Psychological Review, 105(1), 125-157. [DOI:10.1037/0033-295X.105.1.125] [PMID]
Ricketts, J., Jones, C. R., Happé, F., & Charman, T. (2013). Reading comprehension in autism spectrum disorders: The role of oral language and social functioning. Journal of Autism and Developmental Disorders, 43(4), 807–816. [DOI:10.1007/s10803-012-1619-4] [PMID]
Righi, G., & Vettel, J. (2011). Dorsal visual pathway. In: J. S. Kreutzer, J. DeLuca &B. Caplan (Eds), Encyclopedia of Clinical Neuropsychology (pp. 887-888). New York: Springer. [DOI:10.1007/978-0-387-79948-3_1358]
Rosenhall, U., Johansson, E., & Gillberg, C. (1988). Oculomotor findings in autistic children. The Journal of Laryngology and Otology, 102(5), 435–439. [DOI:10.1017/S0022215100105286] [PMID]
Saldaña, D., & Frith, U. (2007). Do readers with autism make bridging inferences from world knowledge? Journal of Experimental Child Psychology, 96(4), 310-319. [DOI:10.1016/j.jecp.2006.11.002] [PMID]
Sansosti, F. J., Was, C., Rawson, K. A., & Remaklus, B. L. (2013). Eye movements during processing of text requiring bridging inferences in adolescents with higher functioning autism spectrum disorders: A preliminary investigation. Research in Autism Spectrum Disorders, 7(12), 1535-1542. [DOI:10.1016/j.rasd.2013.09.001]
Sasson, N. J., Elison, J. T., Turner-Brown, L. M., Dichter, G. S., & Bodfish, J. W. (2011). Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders, 41, 242-247. [DOI:10.1007/s10803-010-1038-3] [PMID]
Scudder, C. A. (2002). Role of the fastigial nucleus in controlling horizontal saccades during adaptation. Annals of the New York Academy of Sciences, 978(1), 63-78. [DOI:10.1111/j.1749-6632.2002.tb07556.x] [PMID]
Seltzer, M. M., Shattuck, P., Abbeduto, L., & Greenberg, J. S. (2004). Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews, 10(4), 234–247. [DOI:10.1002/mrdd.20038] [PMID]
Simmons, D. R., Robertson, A. E., McKay, L. S., Toal, E., McAleer, P., & Pollick, F. E. (2009). Vision in autism spectrum disorders. Vision Research, 49(22), 2705–2739. [DOI:10.1016/j.visres.2009.08.005] [PMID]
Stoodley, C. J. (2012). The cerebellum and cognition: evidence from functional imaging studies. Cerebellum (London, England), 11(2), 352–365. [DOI:10.1007/s12311-011-0260-7] [PMID]
Takarae, Y., Minshew, N., Luna, B., & Sweeney, J. (2004). Oculomotor abnormalities parallel cerebellar histopathology in autism. Journal of Neurology, Neurosurgery, and Psychiatry, 75(9), 1359–1361. [DOI:10.1136/jnnp.2003.022491] [PMID]
Takarae, Y., Minshew, N. J., Luna, B., & Sweeney, J. A. (2007). Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research, 156(2), 117–127. [DOI:10.1016/j.pscychresns.2007.03.008] [PMID]
Van der Donck, S., Vettori, S., Dzhelyova, M., Mahdi, S. S., Claes, P., & Steyaert, J., et al. (2021). Investigating automatic emotion processing in boys with autism via eye tracking and facial mimicry recordings. Autism Research, 14(7), 1404-1420. [DOI:10.1002/aur.2490] [PMID]
Type of Study: Original |
Subject:
Cognitive Neuroscience
Received: 2023/07/19 | Accepted: 2023/08/28 | Published: 2025/03/18
Received: 2023/07/19 | Accepted: 2023/08/28 | Published: 2025/03/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





