Volume 15, Issue 5 (September & October 2024)
BCN 2024, 15(5): 583-594 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghasempour S, Maghsoudi N, Manaheji H, Ghasemi R, Jaafarisuha A, Zaringhalam J. A New Dipeptide H-MGL Partially Ameliorating Memory Impairment in an STZ-induced Alzheimer Model in Male Rats. BCN 2024; 15 (5) :583-594
URL: http://bcn.iums.ac.ir/article-1-2721-en.html
URL: http://bcn.iums.ac.ir/article-1-2721-en.html
Sarieh Ghasempour1 

 , Nader Maghsoudi2
, Nader Maghsoudi2 

 , Homa Manaheji1
, Homa Manaheji1 

 , Rasoul Ghasemi1
, Rasoul Ghasemi1 

 , Ali Jaafarisuha1
, Ali Jaafarisuha1 

 , Jalal Zaringhalam *1
, Jalal Zaringhalam *1 




 , Nader Maghsoudi2
, Nader Maghsoudi2 

 , Homa Manaheji1
, Homa Manaheji1 

 , Rasoul Ghasemi1
, Rasoul Ghasemi1 

 , Ali Jaafarisuha1
, Ali Jaafarisuha1 

 , Jalal Zaringhalam *1
, Jalal Zaringhalam *1 


1- Department of Physiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Neurobiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Keywords: Alzheimer disease (AD), Dipeptide, hexamethylenediamide bis-(N-monosuccinyl-glutamyl-lysine) (H-MGL), Escape latency, Latency to first
Full-Text [PDF 1218 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Alzheimer disease (AD) is the most common form of dementia, accounting for over half of the cases (Schindowski et al., 2008). AD symptoms are cognitive and emotional disturbances that worsen over time (Kubijanovna, 2023). Specific diets, physical inactivity, midlife hypertension, depression, obesity, smoking, and low educational achievements are some risk factors for AD (Barnes & Yaffe, 2011). There are three main histopathological features associated with AD: Extracellular senile plaques (SPs), especially amyloid beta formation; intracellular neurofibrillary tangles (NFTs); and degeneration of basal forebrain cholinergic neurons. The cause of SPs is the amyloid peptide, whereas the principal cause of NFTs is the accumulation of tau, a microtubule-associated protein (Li et al., 2018). The most affected regions of the brain are the cerebral cortex, entorhinal area, hippocampus, ventral striatum, and basal forebrain (Selkoe, 2001). SPs are depositions of a 40-42 amino acid peptide derived proteolytically from amyloid precursor protein. The amyloid beta formation seems to be the leading cause of AD (Hardy & Higgins, 1992). Microglia and astrocytes are activated around these depositions and produce inflammatory cytokines that contribute to the pathogenesis of AD (Hardy & Higgins, 1992).
Therapeutic approaches regarding cognitive decline and memory dysfunction in AD have targeted several mechanisms. For example, aducanumab, approved by the FDA, clears amyloid plaques from brain tissue, though its effectiveness in improving cognitive deficits has not been proven (Dunn et al., 2021). Another strategy is the application of neurotrophic factors in affected patients. A structurally related family of neurotrophins consists of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 and 4/5, secreted by microglia and neurons. They play an essential role in the growth and survival of neurons and the improvement of cognition, learning, and memory (Sánchez-Sánchez & Arévalo, 2017; Silakarma & Sudewi, 2019). NGF can improve neural cell injuries by enhancing the NGF secretion and suppressing oxidative stress (Lee et al., 2018). Intranasal administration of hNGF-6 (engineered NGF) to AD produces neuroprotective effects (Mitra et al., 2021) and enhances cognition (Eriksdotter-Jönhagen et al., 2012). Moreover, collected evidence showed that BDNF level decreases during dementia and neurodegenerative diseases such as AD (Ng et al., 2019), and BDNF polymorphism increases the vulnerability of the hippocampus-frontal cortex to AD pathology and cognitive decline (Franzmeier et al., 2021).
Neurotrophic factors and their precursors have diverse biological roles depending on their interactions with one of two receptors: Tyrosine receptor kinase (Trk) and or p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor (TNF) receptor superfamily. Neurotrophins interacting with specific Trk receptors (NGF binding to TrkA, BDNF, NT4/5 binding to TrkB, and NT3 binding to TrkC) mediate survival and growth responses. Moreover, their interaction with p75NTR leads to the modulation of brain plasticity and apoptosis in the central nervous system (Bhardwaj & Deshmukh, 2018). Although many advantages have been reported for neurotrophin factors, it has found only a limited application in clinical practice due to its poor stability in biological liquids and several disadvantages of nerve growth factors, including hyperalgesia, severe weight loss, and low blood-brain barrier permeability (Genrikhs et al., 2018; Isaev et al., 2015; Seredenin & Gudasheva, 2015; Mohamed et al., 2011).
Novel dipeptides and proteins are influential in creating new and successful medicines (Lebl & Houghten, 2001). This new technology can somewhat reduce the side effects caused by proteins such as neurotrophins. Dipeptides have advantages such as crossing the blood-brain barrier, less toxicity, and higher bioavailability (Genrikhs et al., 2018; Gudasheva et al., 2018). The active site of peptides and proteins that regulate and interact with the receptor molecule is composed of a specific number of amino acids, which is influenced by the balance between recognition accuracy and the speed at which the peptide receptor complex breaks apart (Gudasheva et al., 2018). Usually, an active site of regulatory peptides represents a peptide chain β turn. Four residues are involved in β turn, which is in the middle. According to geometrical reasons, their side chains immerse most fully into the receptor cavity upon peptide-receptor interaction and, therefore, play a major role in the recognition of whole peptide by receptor. This is a theoretical basis for the dipeptide drug design (Gudasheva et al., 2018).
Hexamethylenediamide bis-(N-monosuccinyl-glutamyl-lysine) (H-MGL), constructed based on the β turn of its fourth loop, is a neurotrophin mimetic with a much smaller size. Because of the expected criteria, H-MGL was selected for development as a potential neuroprotective drug. Given that dipeptides can imitate neurotrophic factors and confer neuroprotective properties, they may hold considerable promise for ameliorating inflammation, neurodegenerative ailments, and memory impairments. However, little research has been done on these fronts. Therefore, in our study, we examined the effect of this dipeptide on spatial memory performance in the Morris water maze (MWM) test in a streptozotocin (STZ)-induced AD model in rats.
2. Materials and Methods
Laboratory animals
The animals were obtained from the Laboratory Animal Center at Shahid Beheshti University of Medical Sciences. Male Wistar rats weighing between 250 and 300 g were accommodated in groups of two or three within Plexiglas enclosures. Throughout the investigation, the animals were meticulously maintained within an animal room wherein a consistent temperature of 25 °C and relative humidity ranging from 60% to 70% were kept. The light within the room was on from 8 AM until 8 PM. The subjects were provided unrestricted access to water and food. All experimental procedures were approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences. In compliance with the guidelines provided by the National Institutes of Health (NIH) on the care and use of laboratory animals, proper protocols were followed for the maintenance and welfare of laboratory animals during the study. The experimental subjects were randomly allocated to one of four groups: A sham group (which is surgery performed), a ST) group; an STZ + H-MGL 1 mg/kg, an STZ+ H-MGL 2 mg/kg, and an STZ + saline as STZ and H-MGL solution. Notably, the findings of the saline group were omitted from the report, as it has been determined within our laboratory that saline did not produce any discernible impact on the rats. Each group consisted of 8 rats.
Chemicals
STZ was purchased from Sigma-Aldrich (St. Louis, MO, USA). H-MGL was designed and synthesized by Hamer Pharmaceutical Co. IR. Other reagents were also obtained from local commercial sources.
Drug administration
STZ intracerebroventricular injection
The rats were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine and injected intraperitoneally. Stereotaxic devices were utilized to settle the animals after anesthesia, and mouth and ear bars were embedded carefully to avoid heat loss (Rostami et al., 2020). To uncover the bregma on the cranium, a 2-mm fine midline cut was made on the skull skin, and the cranium was cleaned with 70% ethanol and tenderly shaved. For getting to the horizontal ventricles, burr gaps were made at 0.8 mm back to bregma, 1.5 mm lateral to the midline, and 3.5 mm underneath the surface of the skull (Javadpour et al., 2021). A single dose of STZ (3 mg/kg) was injected bilaterally intracerebroventricularly right after surgery. STZ was dissolved in sterile 0.9% saline and utilized 2 µL per infusion location.
Administration of H-MGL
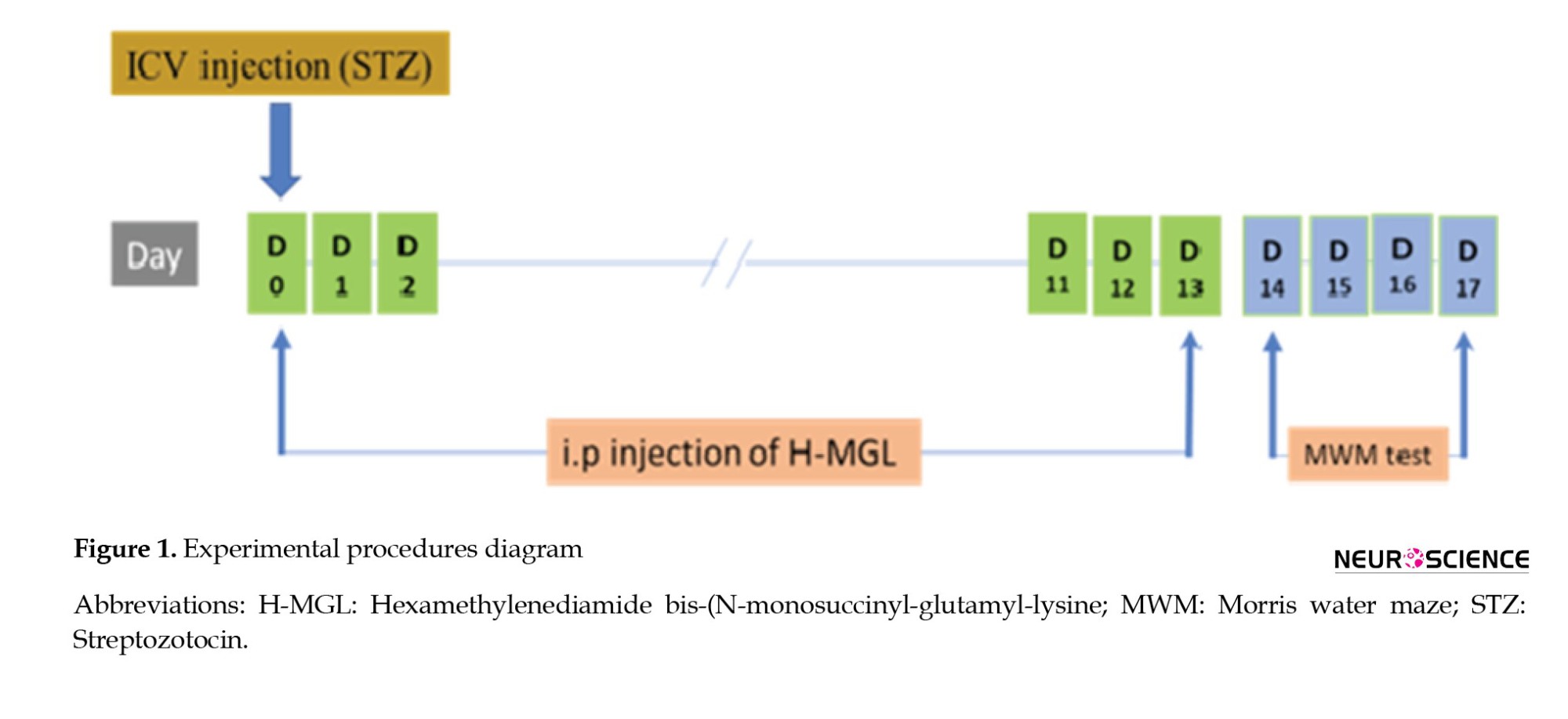
H-MGL (1 or 2 mg/kg) was injected intraperitoneally for 14 successive days (Figure 1), and the vehicle solution got the same volume of sterile 0.9% in the solution group.
Behavioral assessment
Spatial learning and memory performance were evaluated using the MWM test. A dark-colored circular pool with an 80 cm radius and 80 cm height was utilized for the MWM test. Water was filled to a depth of 35 cm warmed to 21±2 °C. The pool was separated into four virtual quadrants, and a stage (diameter: 10 cm) submerged 1.5 cm underneath the surface of the water in one of the quadrants served as a target place. This test was done over four successive days. On each of the three acquisition test days, animals were released within the pool for 90 s and allowed to find the stage. Finding the platform, they were let to remain on it for 20 s and then returned to their home cage. This procedure was done for each trial. Every test day consisted of four trials, each from a different release point. On day four, the probe test trial included picking up the stage and releasing the rats within the pool from the reverse quadrant to the target quadrant, letting them swim for 60 s while their activity was recorded. Rats were assessed for sensorimotor performance after the probe test trial. This procedure was fulfilled by setting the visible stage covered by aluminum foil in a distinctive quadrant and conducting four trials from different release points. On acquisition days, mean escape latency and mean travel distance, and on probe day, duration spent in target and opposite of target zone, frequency platform crossing, and latency to first platform crossing were analyzed. A computerized framework was utilized to prepare and analyze the recorded behaviors of the rats (Noldus EthoVision XT).
Experimental design
On day 0, rats received intracerebroventricular STZ (3 mL/kg). A short time after surgery, H-MGL (1 and 2 mg/kg) was injected intraperitoneally for 14 consecutive days. On day 14, the MWM test was started and conducted through four test days.
Statistical analysis
Data were analyzed by GraphPad Prism software, version 6.07. Behavioral data were analyzed by 2-way or one-way analysis of variance (ANOVA) followed by LSD post-hoc test. Values were reported as Mean±SEM, and P<0.05 was considered significant.
3. Results
The effect of STZ and H-MGL on mean escape latency in MWM
In Figure 2, the mean escape latency was significantly different between the sham, STZ, and STZ+H-MGL1 (F(3, 81)=11.40; P<0.0001). For all three training days, the mean escape latency was significantly greater in the STZ than in the sham (P=0.03, P=0.001, and P=0.01, respectively). In addition, the mean escape latency was significantly greater in the STZ+H-MGL1 than in the sham (P=0.02, P=0.0004, and P=0.001, respectively). H-MGL2 partially reduced escape latency on the first day. However, on the second and third days, there is a significant difference between sham and STZ+H-MGL2 (P=0.006 and P=0.01, respectively) (Figure 2).

The effect of STZ and H-MGL on mean travel distance in MWM
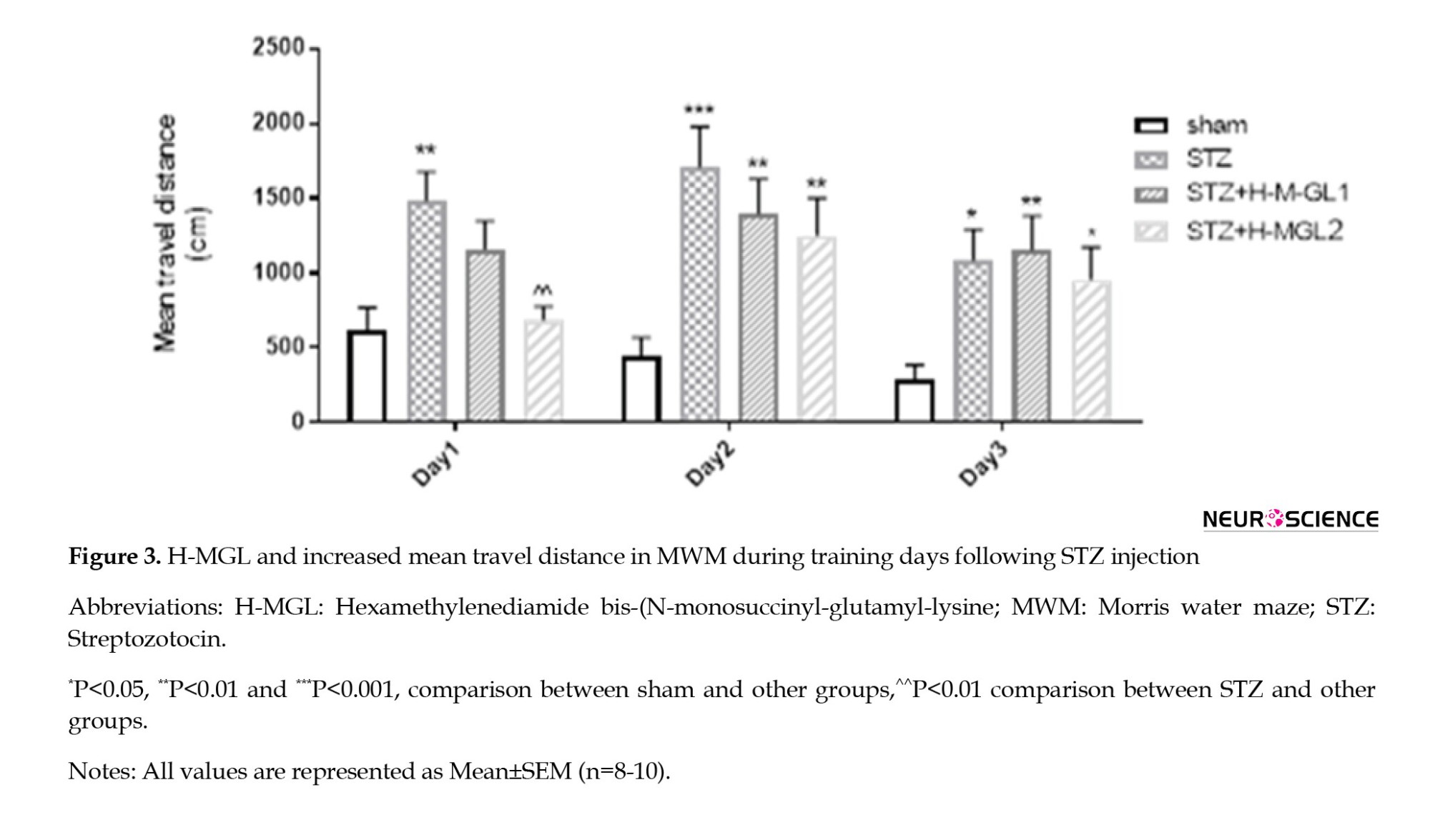
A significant difference in mean travel distance (F(3, 81)=11.72; P<0.0001) was also observed between sham and STZ for each test day (P=0.007, P=0.0001, and P=0.012, respectively) consistent with the results of mean escape latency. However, H-MGL could significantly reduce the increased travel distance in the STZ group (P=0.008) on the first test day. Again, travel distance on the second and third days was significantly higher than sham for STZ+H-MGL1 (P<0.01 and P<0.01, respectively) and STZ+H-MGL2 (P=0.005 and P=0.003, respectively) (Figure 3).
The effect of STZ and H-MGL on memory retention
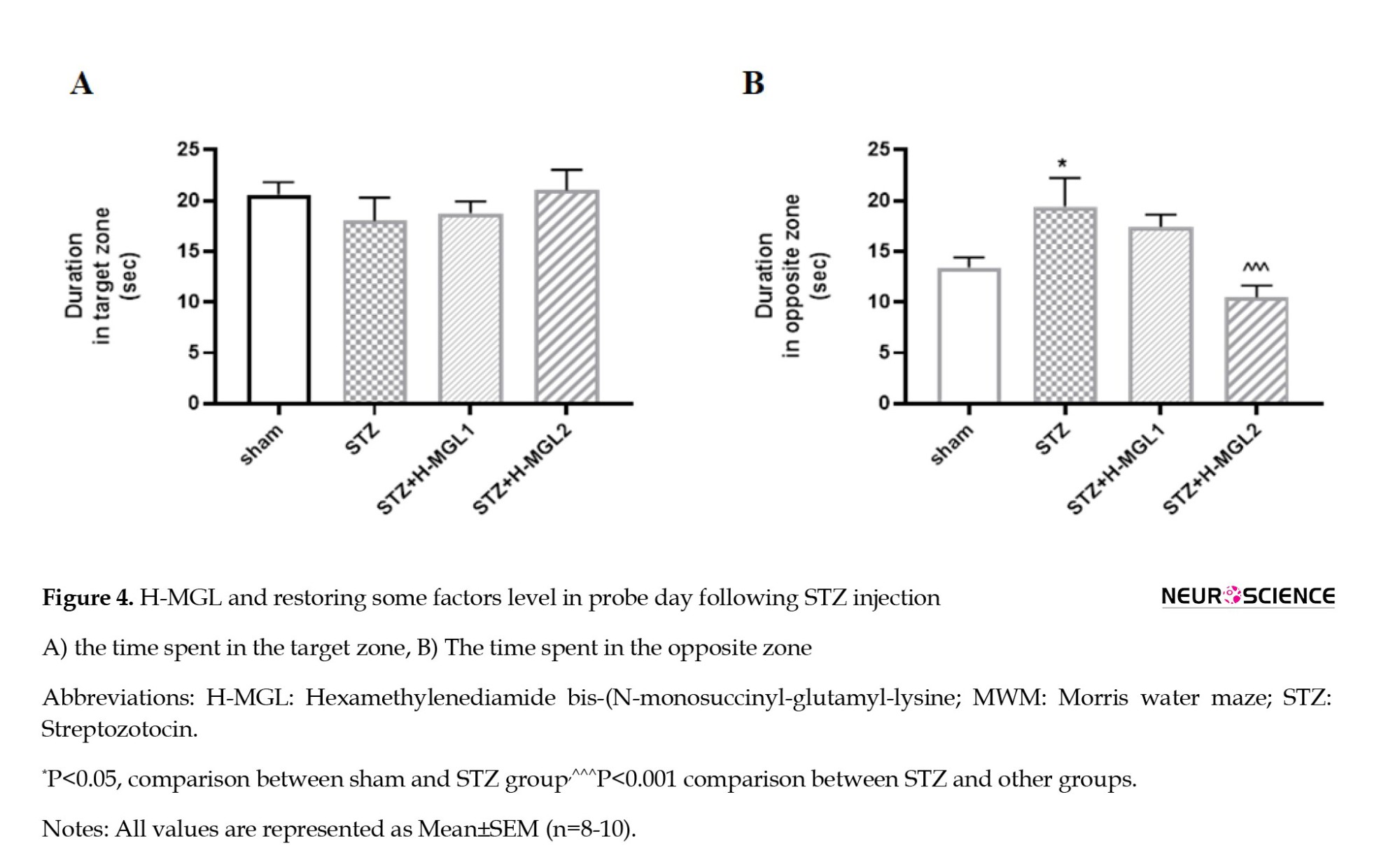
Figure 4 illustrates differences between groups receiving STZ and H-MGL on the probe day. Based on the results, as indicated in Figure 4A, there is no significant difference between the sham and STZ and the H-MGL 1 and 2 in time spent in the target quadrant, though H-MGL2 shows a little nonsignificant elevation (Figure 4A). However, as Figure 4B shows, STZ significantly increases the time spent in opposite quadrant (P<0.05) and H-MGL2 could significantly reduce this increment compared to STZ (P=0.0005) (Figure 4B).
The effect of STZ and H-MGL on frequency crossing and latency to first to platform in probe
As shown in Figure 5A, there is no significant difference between the sham and STZ and the H-MGL 1 and 2 in frequency of platform crossing in the target quadrant, though H-MGL2 shows a little nonsignificant elevation (Figure 5A). However, Figure 5B shows that STZ significantly increased the latency to first crossing platform in the target quadrant (P=0.0002) and H-MGL2 could significantly reduce this increment compared to STZ (P=0.001) (Figure 5B). H-MGL1 has shown no improvement in the latency to first crossing platform and was significantly higher compared to sham (P=0.0003).
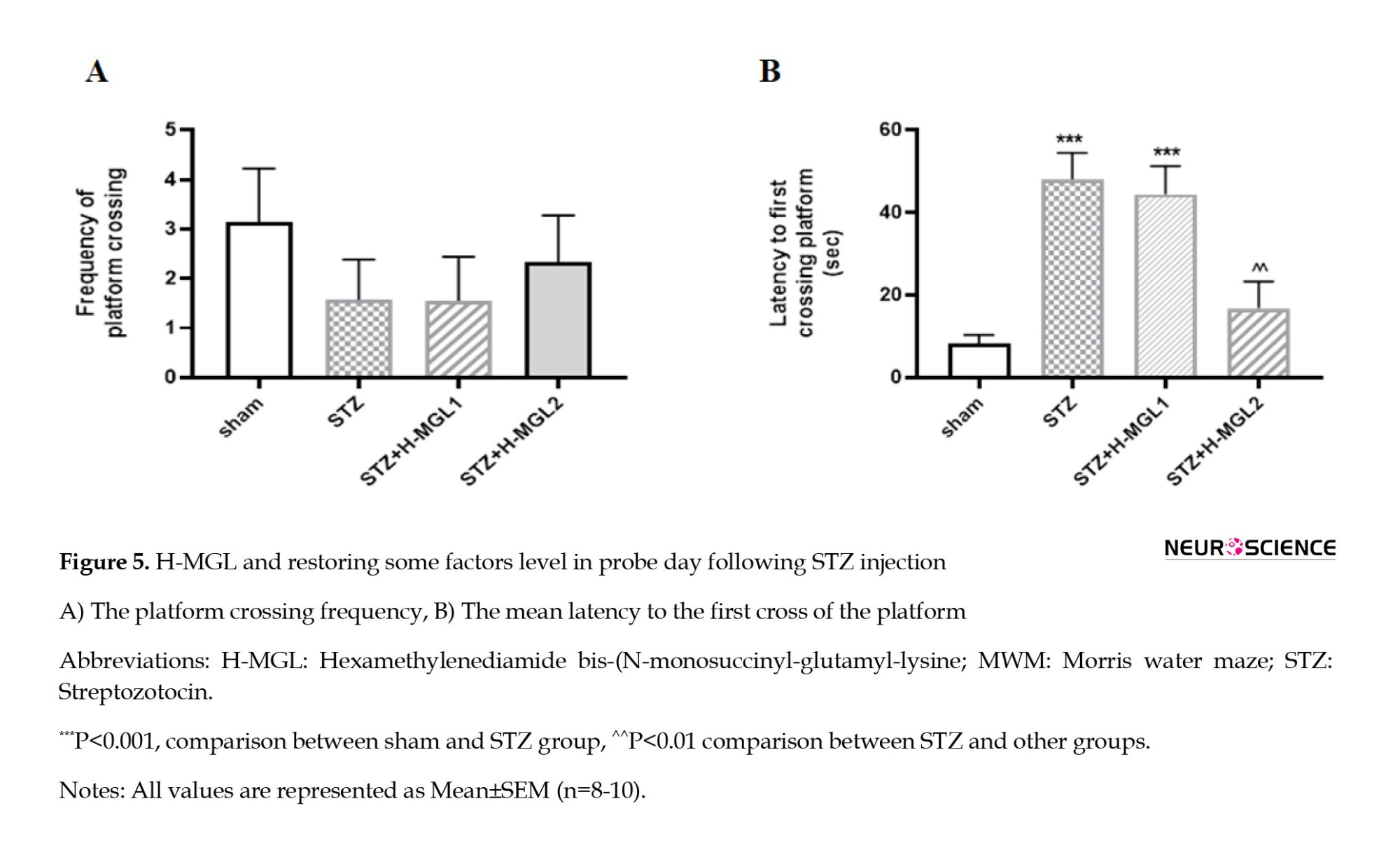
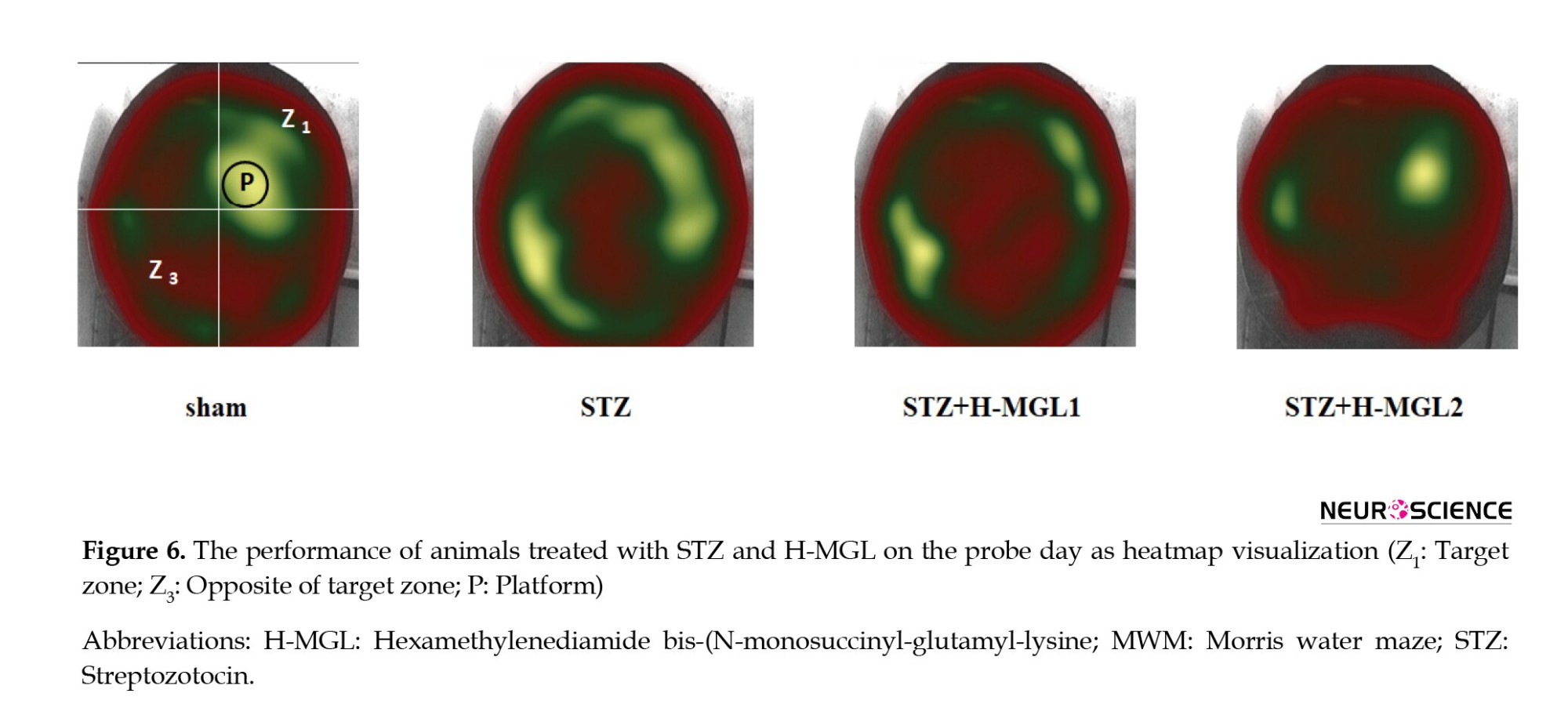
Figure 6 depicts a discernible heat map demonstrating a comparatively elevated recall and superior memory concerning locating the platform in sham and STZ+H-MGL2. The platform’s location in the picture is denoted by the black circle. Each image constitutes a layered depiction emanating from the experimental trial conducted by each respective group. The STZ predominantly navigates the incorrect zone, which is opposite the zone and incorporates the platform’s location. Moreover, this group exhibited a greater tendency towards thigmotaxis. In the context of the experiment, it was observed that the animals that were administered STZ in combination with H-MGL demonstrated a reduction in thigmotactic behavior. Additionally, these rats predominantly displayed swimming behavior in the target area (Z1) and at the periphery of the platform location. The present behavior was perceptibly observed in the STZ+H-MGL2 subject group (Figure 6).
The effect of STZ and H-MGL on somatosensory and visual activity
To ensure that STZ and H-MGL did not harm animals’ ability and motivation to see and move, we examined the latency to first crossing platform (Figure 7A) and swim velocity (Figure 7B) in a visible test.
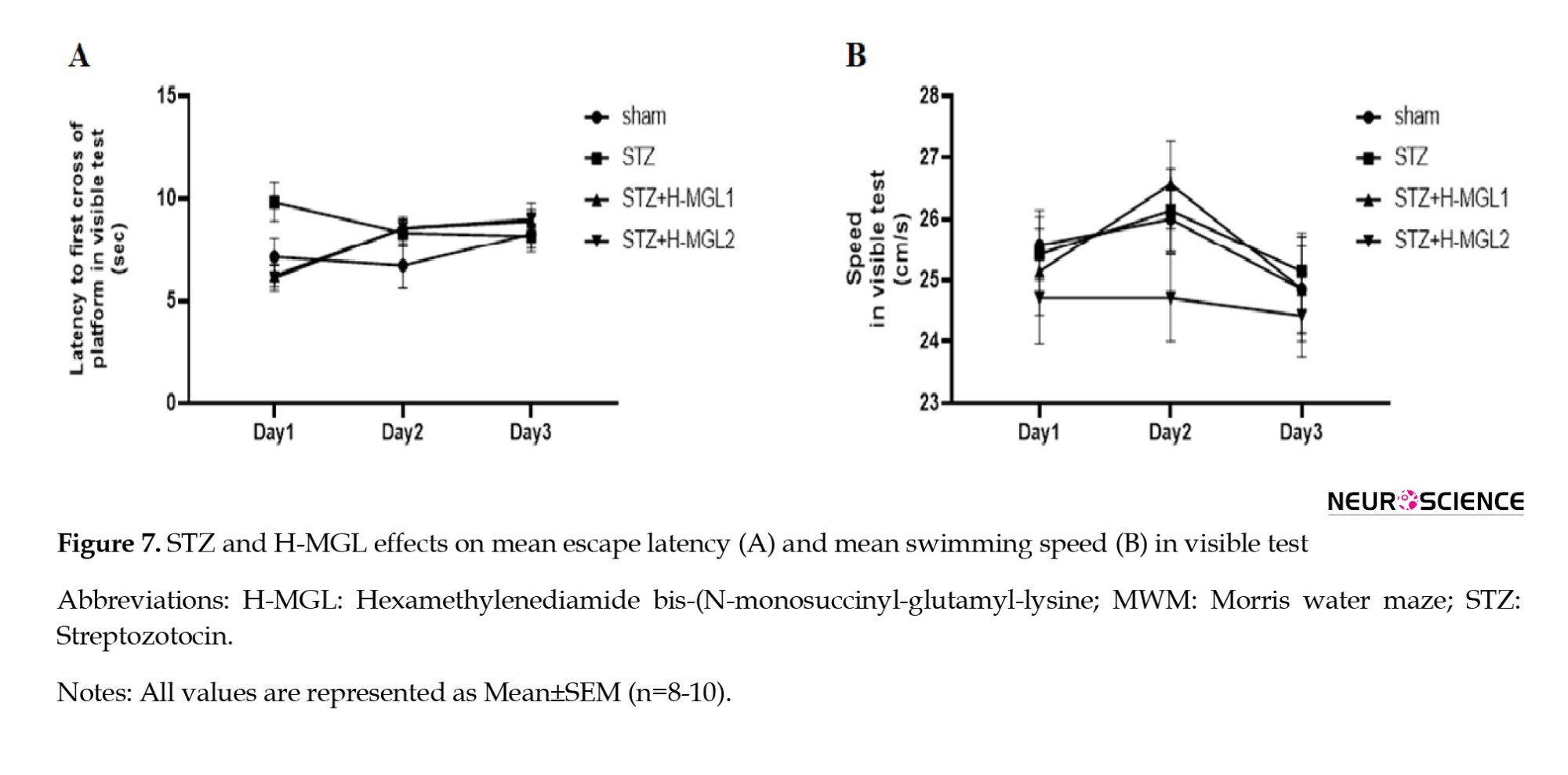
The 2-way ANOVA revealed no significant difference between groups for the latency to first of crossing platform (F(3, 81)=1.082; P=3614) and for swim velocity (F(3, 81)=1.017; P=0.389) and showed that treatment did not influence the coordination of sensory-motor, inspiration, and vision of rats.
4. Discussion
In the present study, we have examined the possible protective role of H-MGL, a dipeptide, in STZ-induced memory impairment as a model of AD. The induction of the model was verified via a significant increase in the escape latency and distance traveled to find the hidden platform during the learning period of the MWM task. Moreover, the administration of STZ decreased the time spent in the target quadrant and the number of passing through the platform location and increased the time spent in the opposite quadrant and the delay in reaching the platform location on probe day. Treatment with H-MGL ameliorated the STZ-induced memory disturbances during acquisition and retrieval trials. However, H-MGL has demonstrated a degree of dose dependency regarding its ameliorative effect on memory performance.
Consistent with previous findings, prior investigations have demonstrated that the central administration of STZ induces cognitive dysfunction (Javadpour et al., 2021). Imipramine alleviates memory impairment and hippocampal apoptosis in STZ-induced sporadic Alzheimer’s rat model: Possible contribution of MAPKs and insulin signaling. Behavioural Brain Research. 2021;408:113260.). The findings of our study indicate that memory impairment in the acquisition phase is mitigated in a dosage-dependent manner through the administration of H-MGL. A lower dose (1 mg/kg) of H-MGL resulted in similar outcomes to those observed in the AD model animals. This finding suggests that the dosage may not be optimal. In contrast to our findings, Zarzhetskii et al. (2015) reported that administering a dipeptide at a dosage of 1 mg/kg exhibited potential efficacy in treating brain injury after resuscitation. In this way, as we used a higher dosage of the dipeptide (2 mg/kg), we observed that the group receiving 2 mg/kg H-MGL demonstrated improved cognitive function by efficiently locating the invisible platform similar to the sham group. These outcomes suggest that the higher dosage of H-MGL dipeptide may be efficacious in alleviating cognitive deficits in AD-like conditions. However, Povarnina et al. (2013) asserted that dipeptide at a 2 mg/kg dosage did not yield any discernible alterations in the acquisition test. It appears that identifying the optimal therapeutic dose while minimizing the adverse effects of the drug may be achieved through strategies to determine the bioavailability of medications. Such measures can potentially mitigate patient discomfort and enhance treatment outcomes.
Neurotrophins have been demonstrated to be implicated in regulating microglial activity, which serves as a primary source of neurotrophin secretion and is susceptible to alterations in AD (Zhou et al., 2016). In this regard, evidence has shown that BDNF has a potential role in hippocampus-dependent memory function. Tyler et al. (2002) have revealed that rats subjected to MWM testing exhibited a greater expression of mRNA encoding BDNF, with a difference of approximately 50%, than their counterparts who underwent the same assessment. In another study involving the chronic constriction injury (CCI) model, the observed decrease in levels of glutamate and BDNF was associated with a significant decline in spatial memory and learning. (Saffarpour et al., 2017). In addition, many researchers reported a direct relationship between the apoptosis process and AD (Zhang et al., 2021; Zhu et al., 2006). Hasegawa et al. (2020) declared neurotrophins have an essential role in Preventing the advance of apoptosis. However, several unfavorable effects have been reported regarding the application of neurotrophic factors. Hence, as dipeptide molecules potentially activate neurotrophic factor receptors, they can be used as a favorable substitute.
Although dipeptides have shown promising therapeutic potential and the fact that dipeptides could mimic neurotrophic factors and their neuroprotective effects in the treatment of neurodegenerative disease with limited side effects, a limited number of investigations have explored the therapeutic properties of the aforementioned substances. In addition, it has some limitations, such as unwanted toxicity and administration because of size and structure, and needs more studies. In accordance, Ostrovskaya et al. (2014) demonstrated the neuroprotective effect of dipeptide in an in-vitro study. In this regard, other studies have indicated that dipeptides exhibit neuroprotective properties in various pathological conditions, including traumatic brain injury and hemorrhagic stroke models (Kraineva et al., 2013; Rai et al., 2014). Povarnina et al. (2011) conducted a study that examined the efficacy of a dipeptide as a treatment for Parkinson disease. The study indicated that treatment with a novel dipeptide known as Gk2 reduced haloperidol catalepsy. Belnik et al. (2007) illustrated the potential of dipeptide Noopept to ameliorate scopolamine-induced spatial memory deficits. Chang et al. (2020) demonstrated that the utilization of IF bioactive dipeptide resulted in a significant elevation of BDNF levels, accompanied by an amelioration of the memory impairment observed in AD. According to Piec et al. (2022), the muramyl dipeptide reduced Aβ accumulation in AD. Identifying the optimal therapeutic dose while minimizing adverse drug effects may be achieved through strategies aimed at determining the bioavailability of medications. Such measures can potentially mitigate patient discomfort and enhance treatment outcomes.
As previously stated, memory deficits in AD have been associated with reduced levels of BDNF at the synaptic cleft. In particular, the peptide Aβ has been shown to induce a reduction in the transcription of the neurotrophic factor BDNF through its detrimental effect on the phosphorylation of CREB (Rosa & Fahnestock, 2015). No evidence shows whether H-MGL dipeptide can perform the same cascade. Neurotrophins such as BDNF and NGF are widely recognized for their therapeutic potential in managing neurodegenerative disorders. However, their clinical application is often overshadowed by several adverse effects, including hyperalgesia, severe weight loss, and impermeability of the blood-brain barrier (BBB) (Genrikhs et al., 2018). Peptide drugs have clear advantages over non-peptide drugs, especially low toxicity and greater permeability (Genrikhs et al., 2018). Regulatory peptides and proteins are vital for designing novel and efficacious pharmaceutical agents (Harithpriya et al., 2023; Ivanov et al., 2022; Mezhlumyan et al., 2022). The binding sites of regulatory peptides and proteins interact with receptors comprising a restricted quantity of amino acids, conferring a critical role in the discernment and precision of receptor recognition. Currently, many investigations are exploring the properties of dipeptides across various fields of study, such as neurology and diabetes.
5. Conclusion
In summary, utilizing H-MGL as an analog of neurotrophins offers several notable advantages, including its favorable bioavailability, potent activity, non-toxic effects, minimal adverse reactions, and capacity to cross the blood-brain barrier. Consequently, it may serve as a promising therapeutic alternative in the future or act as a viable intervention to attenuate the advancement of neurodegenerative disorders. Given the dose-dependent nature of the obtained findings, further investigation involving various dosages is imperative to assess its therapeutic prospects.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethic Committee of Shahid Beheshti University of Medical Sciences (Code: IR. SBMU.MSP.REC.1398.759).
Funding
The study is a part of the PhD dissertation of Sarieh Ghasempour approved by the Department of Physiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (No.: 515). This project was also implemented with the Neurophysiology Research Center at the Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Sarieh Ghasempour and Jalal Zaringhalam: Methodology: Sarieh Ghasempour, Jalal Zaringhalam, Nader Maghsoudi, and Rasoul Ghasemi; Software: Sarieh Ghasempour, Rasoul Ghasemi and Ali Jaafari Suha; Data Curation: Sarieh Ghasempour, Jalal Zaringhalam, and Rasoul Ghasemi; Investigation: Sarieh Ghasempour, Jalal Zaringhalam, and Nader Maghsoudi; Formal analysis: Sarieh Ghasempour, Jalal Zaringhalam, Rasoul Ghasemi and Ali Jaafari Suha; Resources: Jalal Zaringhalam, Nader Maghsoudi, and Homa Manaheji; Writing the Original Draft: Sarieh Ghasempour and Jalal Zaringhalam: Review and EditingL: Sarieh Ghasempour, Jalal Zaringhalam and Rasoul Ghasemi; Validation; All authors; Supervision: Jalal Zaringhalam.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Neurophysiology Research Center at Shahid Beheshti University of Medical Sciences.
References
Alzheimer disease (AD) is the most common form of dementia, accounting for over half of the cases (Schindowski et al., 2008). AD symptoms are cognitive and emotional disturbances that worsen over time (Kubijanovna, 2023). Specific diets, physical inactivity, midlife hypertension, depression, obesity, smoking, and low educational achievements are some risk factors for AD (Barnes & Yaffe, 2011). There are three main histopathological features associated with AD: Extracellular senile plaques (SPs), especially amyloid beta formation; intracellular neurofibrillary tangles (NFTs); and degeneration of basal forebrain cholinergic neurons. The cause of SPs is the amyloid peptide, whereas the principal cause of NFTs is the accumulation of tau, a microtubule-associated protein (Li et al., 2018). The most affected regions of the brain are the cerebral cortex, entorhinal area, hippocampus, ventral striatum, and basal forebrain (Selkoe, 2001). SPs are depositions of a 40-42 amino acid peptide derived proteolytically from amyloid precursor protein. The amyloid beta formation seems to be the leading cause of AD (Hardy & Higgins, 1992). Microglia and astrocytes are activated around these depositions and produce inflammatory cytokines that contribute to the pathogenesis of AD (Hardy & Higgins, 1992).
Therapeutic approaches regarding cognitive decline and memory dysfunction in AD have targeted several mechanisms. For example, aducanumab, approved by the FDA, clears amyloid plaques from brain tissue, though its effectiveness in improving cognitive deficits has not been proven (Dunn et al., 2021). Another strategy is the application of neurotrophic factors in affected patients. A structurally related family of neurotrophins consists of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 and 4/5, secreted by microglia and neurons. They play an essential role in the growth and survival of neurons and the improvement of cognition, learning, and memory (Sánchez-Sánchez & Arévalo, 2017; Silakarma & Sudewi, 2019). NGF can improve neural cell injuries by enhancing the NGF secretion and suppressing oxidative stress (Lee et al., 2018). Intranasal administration of hNGF-6 (engineered NGF) to AD produces neuroprotective effects (Mitra et al., 2021) and enhances cognition (Eriksdotter-Jönhagen et al., 2012). Moreover, collected evidence showed that BDNF level decreases during dementia and neurodegenerative diseases such as AD (Ng et al., 2019), and BDNF polymorphism increases the vulnerability of the hippocampus-frontal cortex to AD pathology and cognitive decline (Franzmeier et al., 2021).
Neurotrophic factors and their precursors have diverse biological roles depending on their interactions with one of two receptors: Tyrosine receptor kinase (Trk) and or p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor (TNF) receptor superfamily. Neurotrophins interacting with specific Trk receptors (NGF binding to TrkA, BDNF, NT4/5 binding to TrkB, and NT3 binding to TrkC) mediate survival and growth responses. Moreover, their interaction with p75NTR leads to the modulation of brain plasticity and apoptosis in the central nervous system (Bhardwaj & Deshmukh, 2018). Although many advantages have been reported for neurotrophin factors, it has found only a limited application in clinical practice due to its poor stability in biological liquids and several disadvantages of nerve growth factors, including hyperalgesia, severe weight loss, and low blood-brain barrier permeability (Genrikhs et al., 2018; Isaev et al., 2015; Seredenin & Gudasheva, 2015; Mohamed et al., 2011).
Novel dipeptides and proteins are influential in creating new and successful medicines (Lebl & Houghten, 2001). This new technology can somewhat reduce the side effects caused by proteins such as neurotrophins. Dipeptides have advantages such as crossing the blood-brain barrier, less toxicity, and higher bioavailability (Genrikhs et al., 2018; Gudasheva et al., 2018). The active site of peptides and proteins that regulate and interact with the receptor molecule is composed of a specific number of amino acids, which is influenced by the balance between recognition accuracy and the speed at which the peptide receptor complex breaks apart (Gudasheva et al., 2018). Usually, an active site of regulatory peptides represents a peptide chain β turn. Four residues are involved in β turn, which is in the middle. According to geometrical reasons, their side chains immerse most fully into the receptor cavity upon peptide-receptor interaction and, therefore, play a major role in the recognition of whole peptide by receptor. This is a theoretical basis for the dipeptide drug design (Gudasheva et al., 2018).
Hexamethylenediamide bis-(N-monosuccinyl-glutamyl-lysine) (H-MGL), constructed based on the β turn of its fourth loop, is a neurotrophin mimetic with a much smaller size. Because of the expected criteria, H-MGL was selected for development as a potential neuroprotective drug. Given that dipeptides can imitate neurotrophic factors and confer neuroprotective properties, they may hold considerable promise for ameliorating inflammation, neurodegenerative ailments, and memory impairments. However, little research has been done on these fronts. Therefore, in our study, we examined the effect of this dipeptide on spatial memory performance in the Morris water maze (MWM) test in a streptozotocin (STZ)-induced AD model in rats.
2. Materials and Methods
Laboratory animals
The animals were obtained from the Laboratory Animal Center at Shahid Beheshti University of Medical Sciences. Male Wistar rats weighing between 250 and 300 g were accommodated in groups of two or three within Plexiglas enclosures. Throughout the investigation, the animals were meticulously maintained within an animal room wherein a consistent temperature of 25 °C and relative humidity ranging from 60% to 70% were kept. The light within the room was on from 8 AM until 8 PM. The subjects were provided unrestricted access to water and food. All experimental procedures were approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences. In compliance with the guidelines provided by the National Institutes of Health (NIH) on the care and use of laboratory animals, proper protocols were followed for the maintenance and welfare of laboratory animals during the study. The experimental subjects were randomly allocated to one of four groups: A sham group (which is surgery performed), a ST) group; an STZ + H-MGL 1 mg/kg, an STZ+ H-MGL 2 mg/kg, and an STZ + saline as STZ and H-MGL solution. Notably, the findings of the saline group were omitted from the report, as it has been determined within our laboratory that saline did not produce any discernible impact on the rats. Each group consisted of 8 rats.
Chemicals
STZ was purchased from Sigma-Aldrich (St. Louis, MO, USA). H-MGL was designed and synthesized by Hamer Pharmaceutical Co. IR. Other reagents were also obtained from local commercial sources.
Drug administration
STZ intracerebroventricular injection
The rats were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine and injected intraperitoneally. Stereotaxic devices were utilized to settle the animals after anesthesia, and mouth and ear bars were embedded carefully to avoid heat loss (Rostami et al., 2020). To uncover the bregma on the cranium, a 2-mm fine midline cut was made on the skull skin, and the cranium was cleaned with 70% ethanol and tenderly shaved. For getting to the horizontal ventricles, burr gaps were made at 0.8 mm back to bregma, 1.5 mm lateral to the midline, and 3.5 mm underneath the surface of the skull (Javadpour et al., 2021). A single dose of STZ (3 mg/kg) was injected bilaterally intracerebroventricularly right after surgery. STZ was dissolved in sterile 0.9% saline and utilized 2 µL per infusion location.
Administration of H-MGL
H-MGL (1 or 2 mg/kg) was injected intraperitoneally for 14 successive days (Figure 1), and the vehicle solution got the same volume of sterile 0.9% in the solution group.

Behavioral assessment
Spatial learning and memory performance were evaluated using the MWM test. A dark-colored circular pool with an 80 cm radius and 80 cm height was utilized for the MWM test. Water was filled to a depth of 35 cm warmed to 21±2 °C. The pool was separated into four virtual quadrants, and a stage (diameter: 10 cm) submerged 1.5 cm underneath the surface of the water in one of the quadrants served as a target place. This test was done over four successive days. On each of the three acquisition test days, animals were released within the pool for 90 s and allowed to find the stage. Finding the platform, they were let to remain on it for 20 s and then returned to their home cage. This procedure was done for each trial. Every test day consisted of four trials, each from a different release point. On day four, the probe test trial included picking up the stage and releasing the rats within the pool from the reverse quadrant to the target quadrant, letting them swim for 60 s while their activity was recorded. Rats were assessed for sensorimotor performance after the probe test trial. This procedure was fulfilled by setting the visible stage covered by aluminum foil in a distinctive quadrant and conducting four trials from different release points. On acquisition days, mean escape latency and mean travel distance, and on probe day, duration spent in target and opposite of target zone, frequency platform crossing, and latency to first platform crossing were analyzed. A computerized framework was utilized to prepare and analyze the recorded behaviors of the rats (Noldus EthoVision XT).
Experimental design
On day 0, rats received intracerebroventricular STZ (3 mL/kg). A short time after surgery, H-MGL (1 and 2 mg/kg) was injected intraperitoneally for 14 consecutive days. On day 14, the MWM test was started and conducted through four test days.
Statistical analysis
Data were analyzed by GraphPad Prism software, version 6.07. Behavioral data were analyzed by 2-way or one-way analysis of variance (ANOVA) followed by LSD post-hoc test. Values were reported as Mean±SEM, and P<0.05 was considered significant.
3. Results
The effect of STZ and H-MGL on mean escape latency in MWM
In Figure 2, the mean escape latency was significantly different between the sham, STZ, and STZ+H-MGL1 (F(3, 81)=11.40; P<0.0001). For all three training days, the mean escape latency was significantly greater in the STZ than in the sham (P=0.03, P=0.001, and P=0.01, respectively). In addition, the mean escape latency was significantly greater in the STZ+H-MGL1 than in the sham (P=0.02, P=0.0004, and P=0.001, respectively). H-MGL2 partially reduced escape latency on the first day. However, on the second and third days, there is a significant difference between sham and STZ+H-MGL2 (P=0.006 and P=0.01, respectively) (Figure 2).

The effect of STZ and H-MGL on mean travel distance in MWM
A significant difference in mean travel distance (F(3, 81)=11.72; P<0.0001) was also observed between sham and STZ for each test day (P=0.007, P=0.0001, and P=0.012, respectively) consistent with the results of mean escape latency. However, H-MGL could significantly reduce the increased travel distance in the STZ group (P=0.008) on the first test day. Again, travel distance on the second and third days was significantly higher than sham for STZ+H-MGL1 (P<0.01 and P<0.01, respectively) and STZ+H-MGL2 (P=0.005 and P=0.003, respectively) (Figure 3).

The effect of STZ and H-MGL on memory retention
Figure 4 illustrates differences between groups receiving STZ and H-MGL on the probe day. Based on the results, as indicated in Figure 4A, there is no significant difference between the sham and STZ and the H-MGL 1 and 2 in time spent in the target quadrant, though H-MGL2 shows a little nonsignificant elevation (Figure 4A). However, as Figure 4B shows, STZ significantly increases the time spent in opposite quadrant (P<0.05) and H-MGL2 could significantly reduce this increment compared to STZ (P=0.0005) (Figure 4B).

The effect of STZ and H-MGL on frequency crossing and latency to first to platform in probe
As shown in Figure 5A, there is no significant difference between the sham and STZ and the H-MGL 1 and 2 in frequency of platform crossing in the target quadrant, though H-MGL2 shows a little nonsignificant elevation (Figure 5A). However, Figure 5B shows that STZ significantly increased the latency to first crossing platform in the target quadrant (P=0.0002) and H-MGL2 could significantly reduce this increment compared to STZ (P=0.001) (Figure 5B). H-MGL1 has shown no improvement in the latency to first crossing platform and was significantly higher compared to sham (P=0.0003).

Figure 6 depicts a discernible heat map demonstrating a comparatively elevated recall and superior memory concerning locating the platform in sham and STZ+H-MGL2. The platform’s location in the picture is denoted by the black circle. Each image constitutes a layered depiction emanating from the experimental trial conducted by each respective group. The STZ predominantly navigates the incorrect zone, which is opposite the zone and incorporates the platform’s location. Moreover, this group exhibited a greater tendency towards thigmotaxis. In the context of the experiment, it was observed that the animals that were administered STZ in combination with H-MGL demonstrated a reduction in thigmotactic behavior. Additionally, these rats predominantly displayed swimming behavior in the target area (Z1) and at the periphery of the platform location. The present behavior was perceptibly observed in the STZ+H-MGL2 subject group (Figure 6).

The effect of STZ and H-MGL on somatosensory and visual activity
To ensure that STZ and H-MGL did not harm animals’ ability and motivation to see and move, we examined the latency to first crossing platform (Figure 7A) and swim velocity (Figure 7B) in a visible test.

The 2-way ANOVA revealed no significant difference between groups for the latency to first of crossing platform (F(3, 81)=1.082; P=3614) and for swim velocity (F(3, 81)=1.017; P=0.389) and showed that treatment did not influence the coordination of sensory-motor, inspiration, and vision of rats.
4. Discussion
In the present study, we have examined the possible protective role of H-MGL, a dipeptide, in STZ-induced memory impairment as a model of AD. The induction of the model was verified via a significant increase in the escape latency and distance traveled to find the hidden platform during the learning period of the MWM task. Moreover, the administration of STZ decreased the time spent in the target quadrant and the number of passing through the platform location and increased the time spent in the opposite quadrant and the delay in reaching the platform location on probe day. Treatment with H-MGL ameliorated the STZ-induced memory disturbances during acquisition and retrieval trials. However, H-MGL has demonstrated a degree of dose dependency regarding its ameliorative effect on memory performance.
Consistent with previous findings, prior investigations have demonstrated that the central administration of STZ induces cognitive dysfunction (Javadpour et al., 2021). Imipramine alleviates memory impairment and hippocampal apoptosis in STZ-induced sporadic Alzheimer’s rat model: Possible contribution of MAPKs and insulin signaling. Behavioural Brain Research. 2021;408:113260.). The findings of our study indicate that memory impairment in the acquisition phase is mitigated in a dosage-dependent manner through the administration of H-MGL. A lower dose (1 mg/kg) of H-MGL resulted in similar outcomes to those observed in the AD model animals. This finding suggests that the dosage may not be optimal. In contrast to our findings, Zarzhetskii et al. (2015) reported that administering a dipeptide at a dosage of 1 mg/kg exhibited potential efficacy in treating brain injury after resuscitation. In this way, as we used a higher dosage of the dipeptide (2 mg/kg), we observed that the group receiving 2 mg/kg H-MGL demonstrated improved cognitive function by efficiently locating the invisible platform similar to the sham group. These outcomes suggest that the higher dosage of H-MGL dipeptide may be efficacious in alleviating cognitive deficits in AD-like conditions. However, Povarnina et al. (2013) asserted that dipeptide at a 2 mg/kg dosage did not yield any discernible alterations in the acquisition test. It appears that identifying the optimal therapeutic dose while minimizing the adverse effects of the drug may be achieved through strategies to determine the bioavailability of medications. Such measures can potentially mitigate patient discomfort and enhance treatment outcomes.
Neurotrophins have been demonstrated to be implicated in regulating microglial activity, which serves as a primary source of neurotrophin secretion and is susceptible to alterations in AD (Zhou et al., 2016). In this regard, evidence has shown that BDNF has a potential role in hippocampus-dependent memory function. Tyler et al. (2002) have revealed that rats subjected to MWM testing exhibited a greater expression of mRNA encoding BDNF, with a difference of approximately 50%, than their counterparts who underwent the same assessment. In another study involving the chronic constriction injury (CCI) model, the observed decrease in levels of glutamate and BDNF was associated with a significant decline in spatial memory and learning. (Saffarpour et al., 2017). In addition, many researchers reported a direct relationship between the apoptosis process and AD (Zhang et al., 2021; Zhu et al., 2006). Hasegawa et al. (2020) declared neurotrophins have an essential role in Preventing the advance of apoptosis. However, several unfavorable effects have been reported regarding the application of neurotrophic factors. Hence, as dipeptide molecules potentially activate neurotrophic factor receptors, they can be used as a favorable substitute.
Although dipeptides have shown promising therapeutic potential and the fact that dipeptides could mimic neurotrophic factors and their neuroprotective effects in the treatment of neurodegenerative disease with limited side effects, a limited number of investigations have explored the therapeutic properties of the aforementioned substances. In addition, it has some limitations, such as unwanted toxicity and administration because of size and structure, and needs more studies. In accordance, Ostrovskaya et al. (2014) demonstrated the neuroprotective effect of dipeptide in an in-vitro study. In this regard, other studies have indicated that dipeptides exhibit neuroprotective properties in various pathological conditions, including traumatic brain injury and hemorrhagic stroke models (Kraineva et al., 2013; Rai et al., 2014). Povarnina et al. (2011) conducted a study that examined the efficacy of a dipeptide as a treatment for Parkinson disease. The study indicated that treatment with a novel dipeptide known as Gk2 reduced haloperidol catalepsy. Belnik et al. (2007) illustrated the potential of dipeptide Noopept to ameliorate scopolamine-induced spatial memory deficits. Chang et al. (2020) demonstrated that the utilization of IF bioactive dipeptide resulted in a significant elevation of BDNF levels, accompanied by an amelioration of the memory impairment observed in AD. According to Piec et al. (2022), the muramyl dipeptide reduced Aβ accumulation in AD. Identifying the optimal therapeutic dose while minimizing adverse drug effects may be achieved through strategies aimed at determining the bioavailability of medications. Such measures can potentially mitigate patient discomfort and enhance treatment outcomes.
As previously stated, memory deficits in AD have been associated with reduced levels of BDNF at the synaptic cleft. In particular, the peptide Aβ has been shown to induce a reduction in the transcription of the neurotrophic factor BDNF through its detrimental effect on the phosphorylation of CREB (Rosa & Fahnestock, 2015). No evidence shows whether H-MGL dipeptide can perform the same cascade. Neurotrophins such as BDNF and NGF are widely recognized for their therapeutic potential in managing neurodegenerative disorders. However, their clinical application is often overshadowed by several adverse effects, including hyperalgesia, severe weight loss, and impermeability of the blood-brain barrier (BBB) (Genrikhs et al., 2018). Peptide drugs have clear advantages over non-peptide drugs, especially low toxicity and greater permeability (Genrikhs et al., 2018). Regulatory peptides and proteins are vital for designing novel and efficacious pharmaceutical agents (Harithpriya et al., 2023; Ivanov et al., 2022; Mezhlumyan et al., 2022). The binding sites of regulatory peptides and proteins interact with receptors comprising a restricted quantity of amino acids, conferring a critical role in the discernment and precision of receptor recognition. Currently, many investigations are exploring the properties of dipeptides across various fields of study, such as neurology and diabetes.
5. Conclusion
In summary, utilizing H-MGL as an analog of neurotrophins offers several notable advantages, including its favorable bioavailability, potent activity, non-toxic effects, minimal adverse reactions, and capacity to cross the blood-brain barrier. Consequently, it may serve as a promising therapeutic alternative in the future or act as a viable intervention to attenuate the advancement of neurodegenerative disorders. Given the dose-dependent nature of the obtained findings, further investigation involving various dosages is imperative to assess its therapeutic prospects.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethic Committee of Shahid Beheshti University of Medical Sciences (Code: IR. SBMU.MSP.REC.1398.759).
Funding
The study is a part of the PhD dissertation of Sarieh Ghasempour approved by the Department of Physiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (No.: 515). This project was also implemented with the Neurophysiology Research Center at the Shahid Beheshti University of Medical Sciences.
Authors' contributions
Conceptualization: Sarieh Ghasempour and Jalal Zaringhalam: Methodology: Sarieh Ghasempour, Jalal Zaringhalam, Nader Maghsoudi, and Rasoul Ghasemi; Software: Sarieh Ghasempour, Rasoul Ghasemi and Ali Jaafari Suha; Data Curation: Sarieh Ghasempour, Jalal Zaringhalam, and Rasoul Ghasemi; Investigation: Sarieh Ghasempour, Jalal Zaringhalam, and Nader Maghsoudi; Formal analysis: Sarieh Ghasempour, Jalal Zaringhalam, Rasoul Ghasemi and Ali Jaafari Suha; Resources: Jalal Zaringhalam, Nader Maghsoudi, and Homa Manaheji; Writing the Original Draft: Sarieh Ghasempour and Jalal Zaringhalam: Review and EditingL: Sarieh Ghasempour, Jalal Zaringhalam and Rasoul Ghasemi; Validation; All authors; Supervision: Jalal Zaringhalam.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Neurophysiology Research Center at Shahid Beheshti University of Medical Sciences.
References
Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer's disease prevalence. The Lancet. Neurology, 10(9), 819–828. [DOI:10.1016/S1474-4422(11)70072-2] [PMID]
Belnik, A., Ostrovskaya, R., & Poletaeva, I. (2007). Dipeptide preparation Noopept prevents scopolamine-induced deficit of spatial memory in BALB/c mice. Bulletin of Experimental Biology and Medicine, 143(4), 431-433. [DOI:10.1007/s10517-007-0148-1] [PMID]
Bhardwaj, R., & Deshmukh, R. (2018). Neurotrophic factors and Parkinson's disease. Clinical-Investigation, 7(4), 53-62. [Link]
Chang, Y. M., Ashok Kumar, K., Ju, D. T., Ho, T. J., Mahalakshmi, B., & Lin, W. T., et al. (2020). Dipeptide IF prevents the effects of hypertension-induced Alzheimer's disease on long-term memory in the cortex of spontaneously hypertensive rats. Environmental Toxicology, 35(5), 570-581. [DOI:10.1002/tox.22892] [PMID]
Dunn, B., Stein, P., & Cavazzoni, P. (2021). Approval of aducanumab for Alzheimer disease-FDA's perspective. JAMA Internal Medicine, 181(10), 1276-1278. [DOI:10.1001/jamainternmed.2021.4607] [PMID]
Eriksdotter-Jönhagen, M., Linderoth, B., Lind, G., Aladellie, L., Almkvist, O., & Andreasen, N., et al. (2012). Encapsulated cell biodelivery of nerve growth factor to the basal forebrain in patients wAlzheimer's disease. Dementia and Geriatric Cognitive Disorders, 33(1), 18-28. [DOI:10.1159/000336051] [PMID]
Franzmeier, N., Ren, J., Damm, A., Monté-Rubio, G., Boada, M., & Ruiz, A., et al. (2021). The BDNF Val66Met SNP modulates the association between beta-amyloid and hippocampal disconnectionAlzheimer's disease. Molecular Psychiatry, 26(2), 614-628. [DOI:10.1038/s41380-019-0404-6] [PMID] [PMCID]
Genrikhs, E. E., Voronkov, D. N., Kapkaeva, M. R., Gudasheva, T. A., Glibka, Y. A., & Isaev, N. K., et al. (2018). The delayed protective effect of GK-2, а dipeptide mimetic of Nerve Growth Factor, in a model of rat traumatic brain injury. Brain Research Bulletin, 140, 148-153. [DOI:10.1016/j.brainresbull.2018.05.002] [PMID]
Gudasheva, T. A., Ostrovskaya, R. U., & Seredenin, S. B. (2018). Novel technologies for dipeptide drugs design and their implantation. Current Pharmaceutical Design, 24(26), 3020-3027. [DOI:10.2174/1381612824666181008105641] [PMID] [PMCID]
Harithpriya, K., Jayasuriya, R., Adhikari, T., Rai, A., & Ramkumar, K. M. (2023). Modulation of transcription factors by small molecules in β-cell development and differentiation. European Journal of Pharmacology, 946, 175606. [DOI: 10.1016/j.ejphar.2023.175606] [PMID]
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science (New York, N.Y.), 256(5054), 184–185. [DOI:10.1126/science.1566067] [PMID]
Hasegawa, Y., Cheng, C., Hayashi, K., Takemoto, Y., & Kim-Mitsuyama, S. (2020). Anti-apoptotic effects of BDNF-TrkB signaling in the treatment of hemorrhagic stroke. Brain Hemorrhages, 1(2), 124-132. [DOI:10.1016/j.hest.2020.04.003]
Ivanov, S. V., Ostrovskaya, R. U., Koliasnikova, K. N., Alchinova, I. B., Demorzhi, M. S., & Gudasheva, T. A., et al. (2022). Low molecular weight NGF mimetic GK-2 normalizes the parameters of glucose and lipid metabolism and exhibits a hepatoprotective effect on a prediabetes model in obese Wistar rats. Clinical and Experimental Pharmacology & Physiology, 49(10), 1116–1125. [DOI:10.1111/1440-1681.13693] [PMID]
Isaev, N. K., Stelmashook, E. V., & Genrikhs, E. E. (2017). Role of nerve growth factor in plasticity of forebrain cholinergic neurons. Biochemistry, 82(3), 291–300. [DOI: 10.1134/S0006297917030075] [PMID]
Javadpour, P., Askari, S., Rashidi, F. S., Dargahi, L., Ahmadiani, A., & Ghasemi, R. (2021). Imipramine alleviates memory impairment and hippocampal apoptosis in STZ-induced sporadic Alzheimer's rat model: Possible contribution of MAPKs and insulin signaling. Behavioural Brain Research, 408, 113260. [DOI: 10.1016/j.bbr.2021.113260] [PMID]
Kraineva, V. A., Gudasheva, T. A., Kotelnikova, S. O., Antipova, T. A., & Seredenin, S. B. (2013). Original nerve growth factor mimetic dipeptide GK-2 limits the manifestations of hemorrhagic stroke in rats. Bulletin of Experimental Biology and Medicine, 154(5), 642-644. [DOI:10.1007/s10517-013-2020-9] [PMID]
Kubijanovna, K. N. (2023). Etiological factors that cause Alzheimer's Disease. IQRO Jurnal, 2(2), 889-892. [Link]
Lebl, M., & Houghten, R. A. (2001). Peptides: The wave of the future. Proceedings of the Second International and the Seventeenth American Peptide Symposium, San Diego, California, U.S.A. June 9–14, 2001, [DOI:10.1007/978-94-010-0464-0]
Lee, H. A., Kim, J. E., Sung, J. E., Yun, W. B., Kim, D. S., & Lee, H. S., et al. (2018). Asparagus cochinchinensis stimulates release of nerve growth factor and abrogates oxidative stress in the Tg2576 model Alzheimer's disease. BMC Complementary and Alternative Medicine, 18(1), 125. [DOI:10.1186/s12906-017-1775-3] [PMID] [PMCID]
Li, T., Shi, H., & Zhao, Y. (2018). Phosphorylation of microtubule-associated protein tau by mitogen-activated protein kinaseAlzheimer's disease. IOP Conference Series: Materials Science and Engineering, 394(2), 1-6. [DOI:10.1088/1757-899X/394/2/022023]
Mezhlumyan, A. G., Tallerova, A. V., Povarnina, P. Y., Tarasiuk, A. V., Sazonova, N. M., & Gudasheva, T. A., et al. (2022). Antidepressant-like effects of BDNF and NGF individual loop dipeptide mimetics depend on the signal transmission patterns associated with Trk. Pharmaceuticals (Basel, Switzerland), 15(3), 284. [DOI: 10.3390/ph15030284] [PMID]
Mitra, S., Gera, R., Linderoth, B., Lind, G., Wahlberg, L., & Almqvist, P., et al. (2021). A review of techniques for biodelivery of Nerve Growth Factor (NGF) to the Brain in Relation to Alzheimer's Disease. Advances in Experimental Medicine and Biology, 1331, 167–191. [DOI:10.1007/978-3-030-74046-7_11] [PMID]
Mohamed, A., Cortez, L., & de Chaves, E. P. (2011). Aggregation state and neurotoxic properties of alzheimer β-amyloid peptide. Current Protein & Peptide Science, 12(3), 235–257. [DOI: 10.2174/138920311795860214] [PMID]
Ng, T. K. S., Ho, C. S. H., Tam, W. W. S., Kua, E. H., & Ho, R. C.-M. (2019). Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients wAlzheimer's disease (AD): A systematic review and meta-analysis. International Journal of Molecular Sciences, 20(2), 257. [DOI:10.3390/ijms20020257] [PMID] [PMCID]
Ostrovskaya, R. U., Vakhitova, Y. V., Kuzmina, U. S.h, Salimgareeva, M. K.h, Zainullina, L. F., & Gudasheva, T. A., et al. (2014). Neuroprotective effect of novel cognitive enhancer noopept on AD-related cellular model involves the attenuation of apoptosis and tau hyperphosphorylation. Journal of Biomedical Science, 21(1), 74. [DOI:10.1186/s12929-014-0074-2] [PMID] [PMCID]
Piec, P. A., Pons, V., Préfontaine, P., & Rivest, S. (2022). Muramyl dipeptide administration delays Alzheimer's Disease physiopathology via NOD2 Receptors. Cells, 11(14), 2241.[DOI:10.3390/cells11142241] [PMID] [PMCID]
Povarnina, P. Y., Gudasheva, T. A., Vorontsova, O. N., Bondarenko, N. A., & Seredenin, S. B. (2011). Antiparkinsonian properties of a nerve growth factor dipeptide mimetic GK-2 in in vivo experiments. Bulletin of Experimental Biology and Medicine, 151(6), 690-693. [DOI:10.1007/s10517-011-1417-6] [PMID]
Povarnina, P. Y., Vorontsova, O. N., Gudasheva, T. A., Ostrovskaya, R. U., & Seredenin, S. B. (2013). Original nerve growth factor mimetic dipeptide GK-2 restores impaired cognitive functions in rat models of Alzheimer's Disease. Acta Naturae, 5(3), 84–91. [DOI:10.32607/20758251-2013-5-3-84-91] [PMID]
Rai, S., Kamat, P. K., Nath, C., & Shukla, R. (2014). Glial activation and post-synaptic neurotoxicity: the key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacology Biochemistry and Behavior, 117, 104-117. [DOI:10.1016/j.pbb.2013.11.035] [PMID]
Rosa, E., & Fahnestock, M. (2015). CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiology of Aging, 36(8), 2406-2413. [DOI:10.1016/j.neurobiolaging.2015.04.014] [PMID]
Rostami, F., Javan, M., Moghimi, A., Haddad-Mashadrizeh, A., & Fereidoni, M. (2020). Prenatal stress promotes icv-STZ-induced sporadic Alzheimer's pathology through central insulin signaling change. Life Sciences, 241, 117154. [DOI:10.1016/j.lfs.2019.117154] [PMID]
Saffarpour, S., Shaabani, M., Naghdi, N., Farahmandfar, M., Janzadeh, A., & Nasirinezhad, F. (2017). In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiology & Behavior, 175, 97-103. [DOI:10.1016/j.physbeh.2017.03.025] [PMID]
Sánchez-Sánchez, J., & Arévalo, J. C. (2017). A review on ubiquitination of neurotrophin receptors: Facts and perspectives. International Journal of Molecular Sciences, 18(3), 630. [DOI:10.3390/ijms18030630] [PMID] [PMCID]
Schindowski, K., Belarbi, K., & Buee, L. (2008). Neurotrophic factors in Alzheimer's disease: Role of axonal transport. Genes, Brain, and Behavior, 7 (Suppl 1(1), 43–56. [DOI:10.1111/j.1601-183X.2007.00378.x] [PMID] [PMCID]
Selkoe, D. J. (2001). Alzheimer's disease: Genes, proteins, and therapy. Physiological Reviews, 81(2), 741–766. [DOI:10.1152/physrev.2001.81.2.741] [PMID]
Seredenin, S. B., & Gudasheva, T. A. (2015). [The development of a pharmacologically active low-molecular mimetic of the nerve growth factor (Russian)]. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova, 115(6), 63–70. [DOI: 10.17116/jnevro20151156163-70] [PMID]
Silakarma, D., & Sudewi, A. A. R. (2019). The role of brain-derived neurotrophic factor (BDNF) in cognitive functions. Bali Medical Journal, 8(2), 518-525. [Link]
Tyler, W. J., Alonso, M., Bramham, C. R., & Pozzo-Miller, L. D. (2002). From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learning & Memory, 9(5), 224-237. [DOI:10.1101/lm.51202] [PMID] [PMCID]
Zarzhetskii, Y. V., Avrushchenko, M. S.h, Moroz, V. V., Gudasheva, T. A., & Seredenin, S. B. (2015). Effectiveness of GK-2, a nerve growth factor mimetic, in preventing post-resuscitation changes in the brain. Bulletin of Experimental Biology and Medicine, 159(4), 453-455. [DOI:10.1007/s10517-015-2989-3] [PMID]
Zhang, L., Qian, Y., Li, J., Zhou, X., Xu, H., & Yan, J., et al. (2021). BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer's disease pathology. Iscience, 24(9), 102942. [DOI:10.1016/j.isci.2021.102942] [PMID] [PMCID]
Type of Study: Original |
Subject:
Behavioral Neuroscience
Received: 2023/05/30 | Accepted: 2023/06/15 | Published: 2024/09/1
Received: 2023/05/30 | Accepted: 2023/06/15 | Published: 2024/09/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





