Volume 16, Issue 3 (May & June 2025)
BCN 2025, 16(3): 569-582 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Banaeeyeh S, Marjan Razavi B, Hosseinzadeh H. Alpha-mangostin Ameliorates Apoptosis, Inflammation and Oxidative Stress in Cuprizone-induced Demyelination in C57BL/6 Mice. BCN 2025; 16 (3) :569-582
URL: http://bcn.iums.ac.ir/article-1-2706-en.html
URL: http://bcn.iums.ac.ir/article-1-2706-en.html
1- Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. & Targeted Drug Delivery Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. & Pharmaceutical Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. & Targeted Drug Delivery Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. & Pharmaceutical Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran.
Keywords: Multiple sclerosis (MS), Cuprizone, Alpha-mangostin (α-MG), Neuroprotection, Corpus callosum (CC)
Full-Text [PDF 1033 kb]
| Abstract (HTML)
Full-Text:
1. Introduction
Multiple sclerosis (MS) is a chronic neurological disorder in which the immune system mistakenly attacks the protective covering of nerve fibers in the central nervous system (CNS), leading to widespread inflammatory lesions (Mauriz et al., 2013). The two principal pathological features of MS are inflammation and demyelination. Repeated inflammatory episodes result in the apoptosis of oligodendrocytes, axonal degeneration, and the activation of astrocytes and microglia (Bjartmar et al., 2003).
The loss of myelin sheaths surrounding axons in the brain and spinal cord leads to impaired signal transmission, contributing to the neurological dysfunction seen in MS (Basoglu et al., 2013; Hurwitz, 2009). This damage is mediated by the infiltration of inflammatory cells—predominantly lymphocytes and macrophages—into the CNS through the blood-brain barrier (BBB). Although the precise etiology of MS remains unclear, it is widely accepted that autoimmune mechanisms play a central role, involving T cells, B cells, macrophages, and dendritic cells (Frohman et al., 2006).
Oxidative stress and mitochondrial dysfunction are also known contributors to the progression and worsening of MS (Smirnova et al., 2011). Remyelination—the regenerative process that restores the damaged myelin sheath—is considered essential in mitigating neurodegeneration. However, this process is often incomplete, insufficient, or fails entirely, especially in progressive stages of the disease (Fiorio et al., 2006).
To investigate the mechanisms of demyelination and remyelination, a variety of experimental models have been developed. These include models based on genetic mutations, immune responses, viral infections, and toxic insults. While none of these fully replicate all stages of MS, they each offer valuable insight into specific pathological features. Among these, toxic demyelination models—particularly the cuprizone (CZ) model—have gained considerable attention and acceptance for their reliability and relevance in MS research.
These models include genetic myelin mutations, immune-mediated demyelination, viral-induced demyelination, and toxic demyelination models. It is important to note that no single model can fully replicate all stages of MS; each mimics only certain aspects of the disease’s complex pathology. Among these, the CZ-induced demyelination model has gained widespread acceptance in recent years due to its specificity and reproducibility (Kipp et al., 2009; Skripuletz et al., 2011).
CZ, chemically known as oxalic acid bis(cyclohexylidene hydrazide) is a well-characterized neurotoxic compound that acts as a selective copper chelator. Its toxicity is particularly directed at oligodendrocytes, leading to cell death and subsequent demyelination in various brain regions, notably the corpus callosum (CC) and superior cerebellar peduncles. As the largest myelinated tract in the brain, the CC is especially vulnerable to CZ-induced damage and is also considered a potential reservoir for neural stem cells. This makes the CC an ideal target for monitoring neuroprotective and remyelination processes (Kipp et al., 2017; Matsushima & Morell, 2001).
Administration of CZ for approximately 5–6 weeks results in acute demyelination of the CC. After withdrawal of CZ and return to a normal diet, partial spontaneous remyelination can occur over the following weeks (Kipp et al., 2009). However, prolonged exposure to CZ (12 weeks or more) significantly impairs remyelination and leads to a state referred to as chronic demyelination (Harsan et al., 2008; Tansey et al., 1996; Torkildsen et al., 2008). The CZ model is thus considered highly effective for studying both the degenerative and regenerative phases of demyelination due to its reliability, simplicity, and consistency (Matsushima & Morell, 2001).
Alpha-mangostin (α-MG) is a polyphenolic compound and the major xanthone extracted from the pericarp of the mangosteen fruit (Garcinia mangostana Linn) (Pedraza-Chaverri et al., 2008). Various components derived from mangosteen—including polyphenols, phenols, and xanthone derivatives—have been shown to exert beneficial effects in numerous disease conditions (Tousian Shandiz et al., 2017).
α-MG, a naturally occurring xanthone derived from plants, has demonstrated a highly selective inhibitory effect on acid sphingomyelinase (ASMase), in addition to a wide range of biological activities (Okudaira et al., 2000; Chairungsrilerd et al., 1996). These include pronounced anti-inflammatory and antioxidant properties (Chen et al., 2008; Devi Sampath & Vijayaraghavan, 2007). In several animal disease models, α-MG has been shown to sustain the activity of key antioxidant enzymes, such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), and the critical intracellular antioxidant glutathione (GSH) (Devi Sampath & Vijayaraghavan, 2007).
Research on Wistar rats has shown that α-MG acts as a neuroprotective agent in models of oxidative brain damage (Márquez-Valadez et al., 2012). Moreover, treatment with α-MG and curcumin in primary cultures of cerebellar granule neurons (CGNs) has been found to confer neuroprotection against iodoacetate-induced oxidative stress, primarily by reducing the production of reactive oxygen species (ROS) (Reyes-Fermín et al., 2012). In another study using rotenone as a cellular model of Parkinson’s disease, α-MG treatment preserved dopaminergic neurons and reduced α-synuclein accumulation, indicating its potential to prevent mitochondrial dysfunction and protein aggregation associated with neurodegeneration (Hao et al., 2017).
In light of these findings, the present study aimed to evaluate the efficacy of α-MG in mitigating clinical symptoms, inflammation, and oxidative stress in a CZ-induced demyelination model of MS using C57BL/6 mice over a 5-week experimental period. To our knowledge, this is the first investigation into the neuroprotective potential of α-MG within this context, including an exploration of the underlying molecular mechanisms.
2. Materials and Methods
Animals
Adult female C57BL/6 mice (8–10 weeks old), weighing 18–22 g, were obtained from the School of Pharmacy at Mashhad University of Medical Sciences, Mashhad, Iran. The animals were housed in groups of six per cage under controlled conditions: A 12-hour light/dark cycle, temperature of 22±2 °C, and relative humidity of 60±5%. All experiments were conducted in accordance with internationally accepted principles for the care and use of laboratory animals (Zimmermann, 1983).
Materials
The following reagents and materials were used in this study:
CZ (CAS No: 370810) was purchased from Sigma-Aldrich (USA), and α-MG (purity >90%) was obtained from Trademax Pharmaceuticals & Chemicals Co. (China).
Bovine serum albumin (BSA; Solarbio, China), dry skim milk (Quetlab, UK), ethylene glycol tetraacetic acid (EGTA; Sigma, USA), ethylenediaminetetraacetic acid (EDTA; Pars Tous Biotechnology, Iran), sodium deoxycholate (Sigma, New Zealand), sodium orthovanadate (Na3VO4; Sigma, India), and other general chemicals were procured from Merck (Germany).
Protease and phosphatase inhibitor cocktail and Pierce ECL Western blotting substrate were sourced from Thermo Fisher Scientific (USA).
The protein assay kit (Bradford reagent) and polyvinylidene difluoride (PVDF) membranes were obtained from Bio-Rad (USA).
Fetal bovine serum was purchased from Gibco (USA).
Malondialdehyde (MDA) and dizinon (DZN) were sourced from Fluka (Switzerland) and Shanghai Tosco Chemical Co. (China), respectively.
Primary antibodies included rabbit polyclonal anti-Bax (#2772, 1:1000), anti-Bcl2 (#2870, 1:1000), anti-cleaved caspase-3 (#9664, 1:1000), and anti-TNF-α (#3707, 1:1000), all obtained from Cell Signaling Technology (USA). Secondary antibodies (HRP-conjugated anti-rabbit and anti-mouse IgG) were also acquired from Cell Signaling.
Imaging was performed using the Alliance 4.7 Gel Doc system (UVtec, UK) and analyzed using UV Tec Software.
Experimental design
The CZ-induced demyelination model was established in adult female C57BL/6 mice (8–10 weeks old) following the protocol described by Kipp et al. (2009). Mice were fed a chow diet containing 0.4% (w/w) CZ for five weeks. α-MG was dissolved in 0.9% normal saline (NS) with two drops of Tween 80 and administered orally by gavage at doses of 20, 40, or 80 mg/kg, starting from day one and continuing daily for five weeks.
The animals were randomly assigned to six experimental groups (n=6 per group):
Control group: Received a normal diet and NS (0.9%) with 2 drops of Tween 80 via gavage for five weeks.
CPZ group: Received 0.4% (w/w) CZ mixed into milled chow for five weeks.
CPZ + α-MG 20 mg/kg group: Received CZ diet + 20 mg/kg α-MG by gavage.
CPZ + α-MG 40 mg/kg group: Received CZ diet + 40 mg/kg α-MG by gavage.
CPZ + α-MG 80 mg/kg group: Received CZ diet + 80 mg/kg α-MG by gavage.
α-MG 80 mg/kg only group: Received a normal diet + 80 mg/kg α-MG by gavage.
Measurement of body weight
Body weight was monitored daily throughout the experiment. However, only the measurements from the first and final days were recorded and reported.
Tissue preparation
At the end of the five-week experimental period, the mice were sacrificed, and the CC was carefully dissected from the brain and immediately stored at −80 °C until further processing.
Determination of MDA level in CC tissue
MDA levels were measured as an indicator of lipid peroxidation, reflecting oxidative damage. The following procedure was employed, adapted from Uchiyama and Mihara (1978):
1. A 10% tissue homogenate was prepared in 1.15% potassium chloride (KCl).
2. 0.5 mL of the homogenate was mixed with: 3 mL of 1% phosphoric acid and 1 mL of 6% thiobarbituric acid (TBA)
3. The mixture was boiled in water for 45 minutes.
4. After boiling, the tubes were cooled to room temperature.
5. 4 mL of n-butanol was added to each tube, and the mixture was vortexed for 1 minute.
6. Tubes were centrifuged at 3,500 rpm for 10 minutes.
7. The supernatant was collected and its absorbance was read at 532 nm.
8. A standard curve was created using MDA concentrations ranging from 0–100 nmol/mL.
9. MDA levels were expressed as nmol/g tissue.
Western blot assay
Western blot analysis was performed to assess protein expression related to apoptosis. The frozen CC tissue was lysed in a buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM EDTA, 2 mM EGTA, 10 mM NaF, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate, 0.2% sodium deoxycholate, 1 mM PMSF, and a protease inhibitor cocktail. Samples were homogenized, sonicated on ice (3×10 seconds, with 10-second intervals), and centrifuged at 10,000 g for 10 minutes at 4 °C. Protein concentration was determined using the Bradford assay.
Samples were mixed with 2× SDS sample buffer (1:1 ratio), heated, aliquoted, and stored at −80 °C. For electrophoresis, 100 µg of protein per sample was loaded onto a 12% SDS-PAGE gel, separated, and transferred onto a PVDF membrane.
Membranes were blocked with skim milk for 2 hours, then incubated overnight at 4 °C with primary antibodies: Anti-Bax, anti-Bcl2, anti-cleaved caspase-3, anti-TNF-α, and anti-β-actin (all 1:1,000). After washing, membranes were incubated for 2 hours with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000). Protein bands were visualized using enhanced chemiluminescence, and densitometric analysis was performed using the Alliance 4.7 Gel Doc system and UV Tec Software. Protein expression was normalized to β-actin as a loading control.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software, version 8.0 (GraphPad Inc., San Diego, CA, USA). Data are presented as Mean±SEM. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to assess differences among groups for body weight, Western blot, and lipid peroxidation results. A P<0.05 was considered statistically significant.
3. Results
Effect of α-MG on body weight in CZ-treated mice
At the beginning of the experiment, there were no significant differences in body weight among the experimental groups. However, by the end of the 5-week period, mice in the CZ-treated group exhibited a significant reduction in body weight compared to the control group. Treatment with α-MG at a dose of 80 mg/kg effectively reversed this weight loss, resulting in a significant improvement in body weight compared to the CZ group (Table 1).
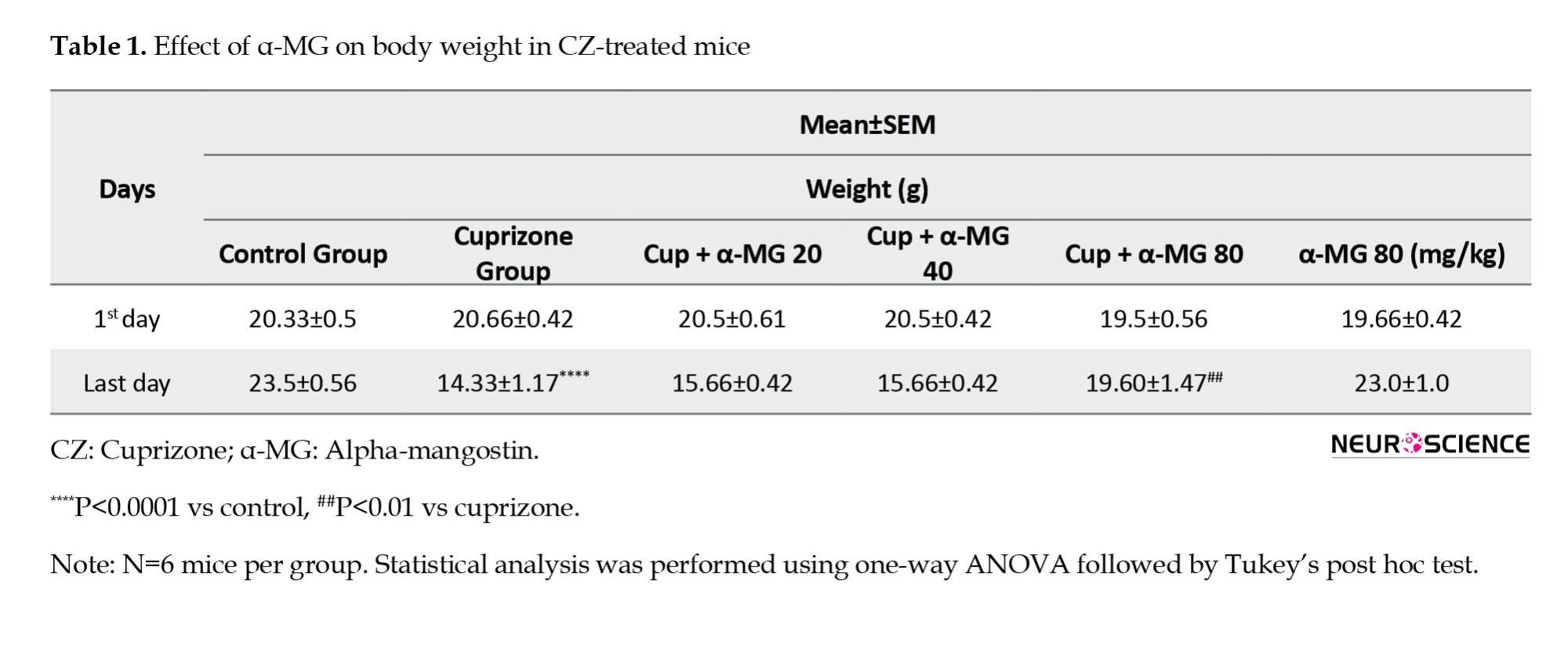
Effect of α-MG on oxidative stress marker in CZ-treated mice
Lipid peroxidation was assessed by measuring MDA levels in CC homogenates. Mice in the CZ group demonstrated a significant increase in MDA levels compared to the control group (P<0.01), indicating elevated oxidative stress. Treatment with α-MG for five weeks at doses of 20, 40, and 80 mg/kg significantly reduced MDA levels (P<0.01, P<0.001, and P<0.001, respectively), suggesting a dose-dependent antioxidative effect of α-MG (Figure 1).
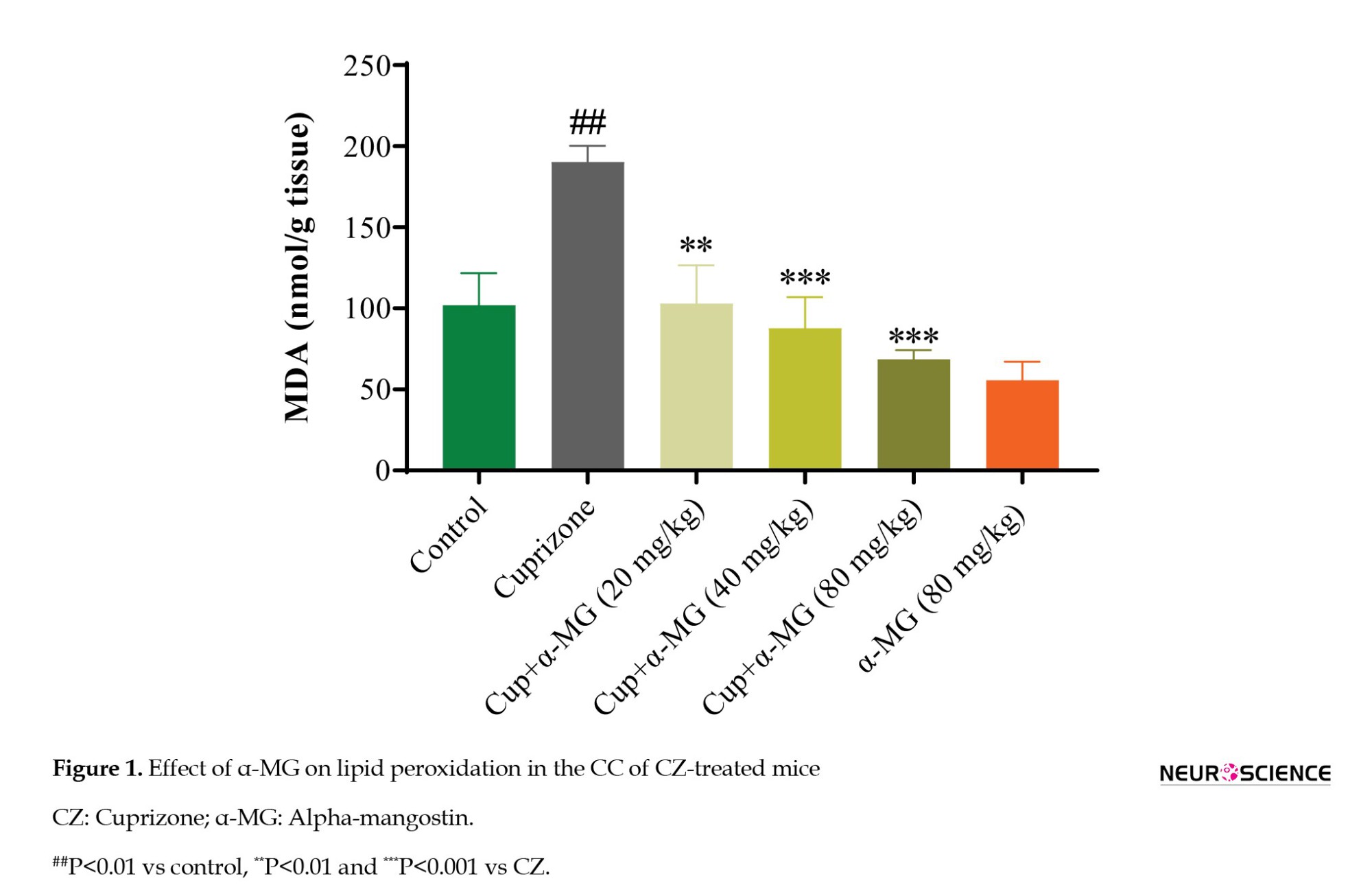
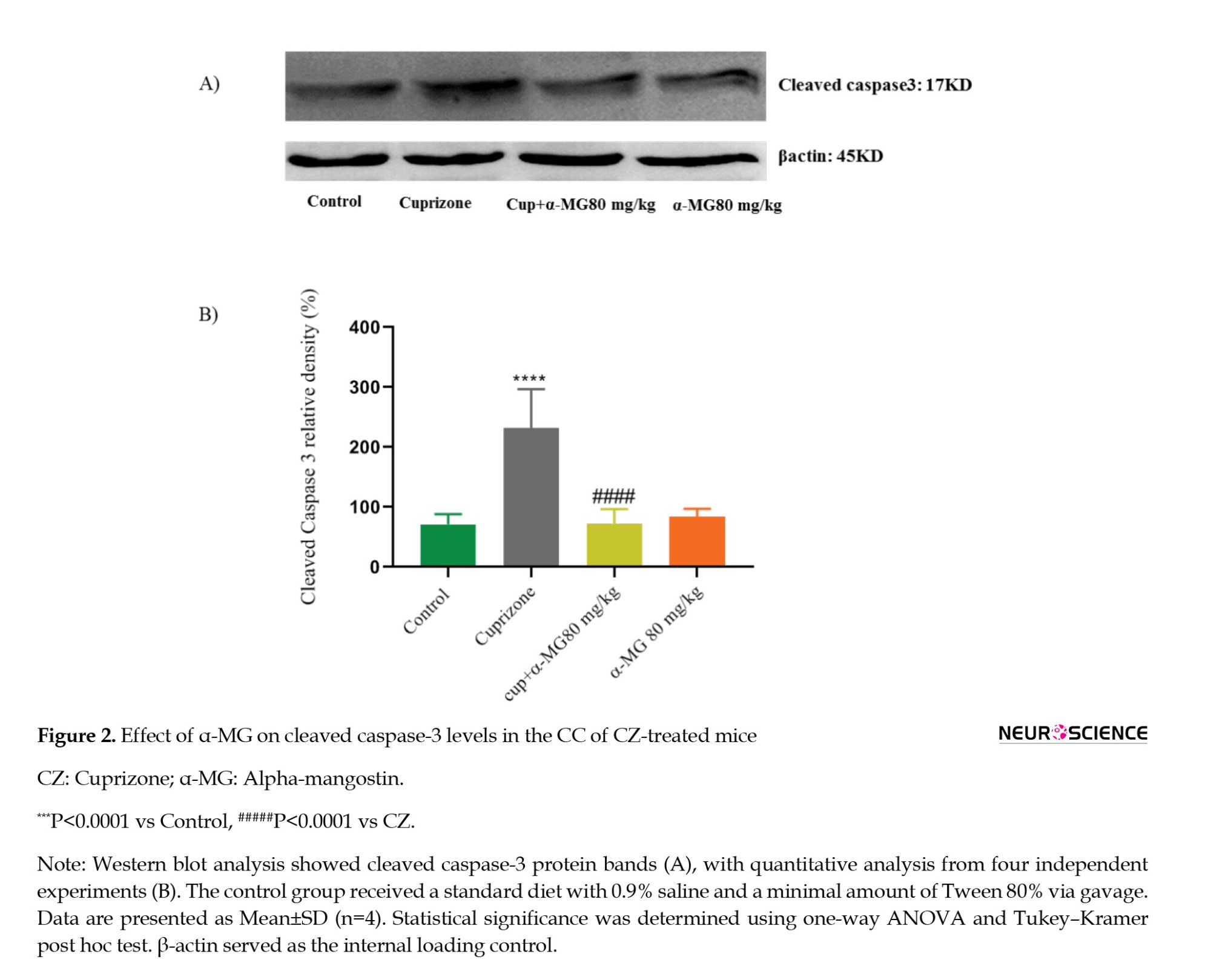
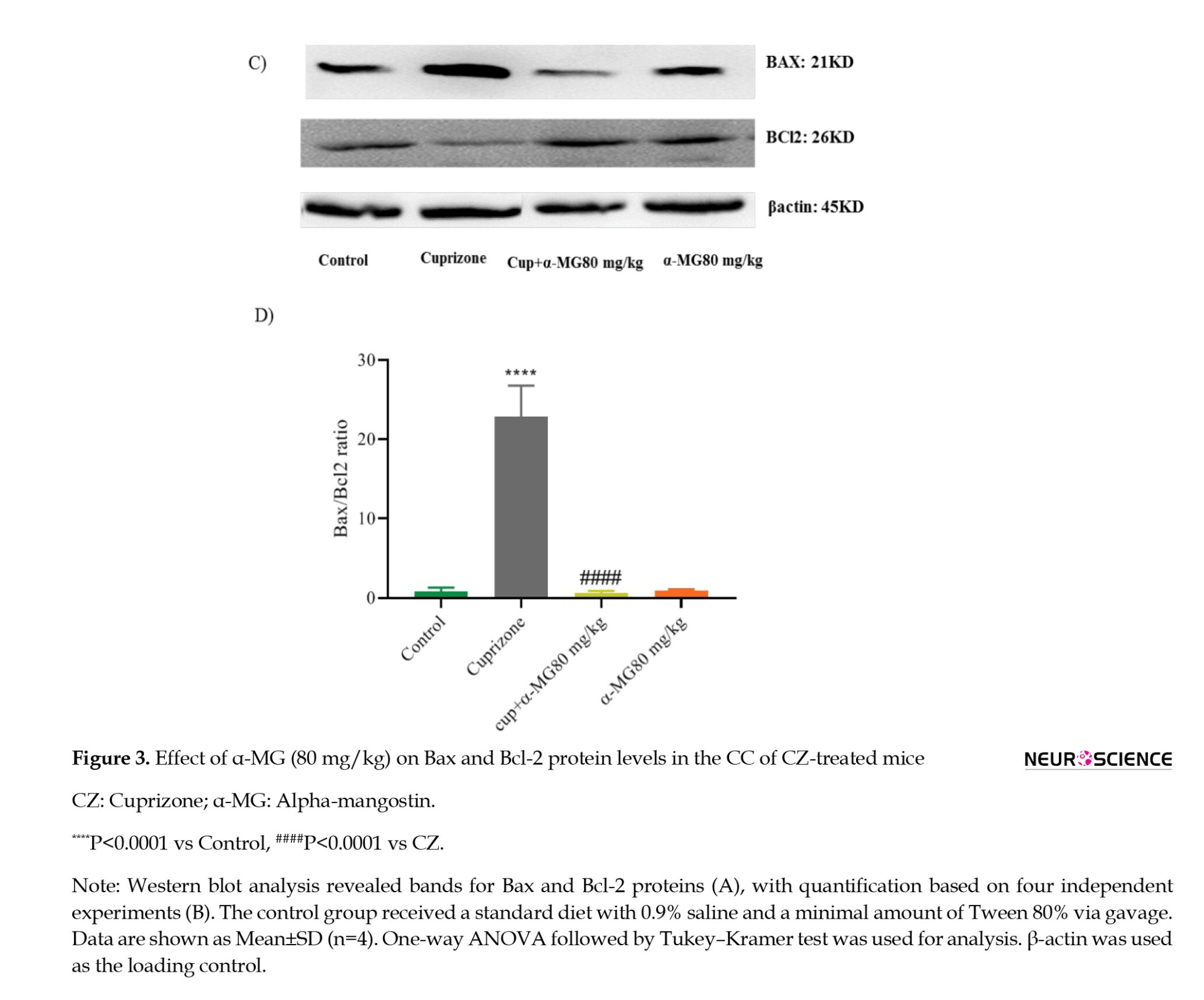
Effect of α-MG on apoptotic markers in CZ-treated mice
Based on its strong antioxidant effect, the 80 mg/kg dose of α-MG was selected for further investigation of apoptotic markers. Western blot analysis was conducted to assess the expression of cleaved caspase-3, Bax, and Bcl-2 in CC tissue. The CZ group showed a significant increase in cleaved caspase-3 expression (P<0.0001) and in the Bax/Bcl-2 ratio (P<0.0001) compared to controls. Treatment with α-MG (80 mg/kg) for five weeks significantly decreased cleaved caspase-3 levels (P<0.0001) (Figures 2A and 2B), as well as the Bax/Bcl-2 ratio (P<0.0001) (Figures 3A and 3B), indicating its anti-apoptotic potential.
Effect of α-MG on the inflammatory marker in CZ-treated mice
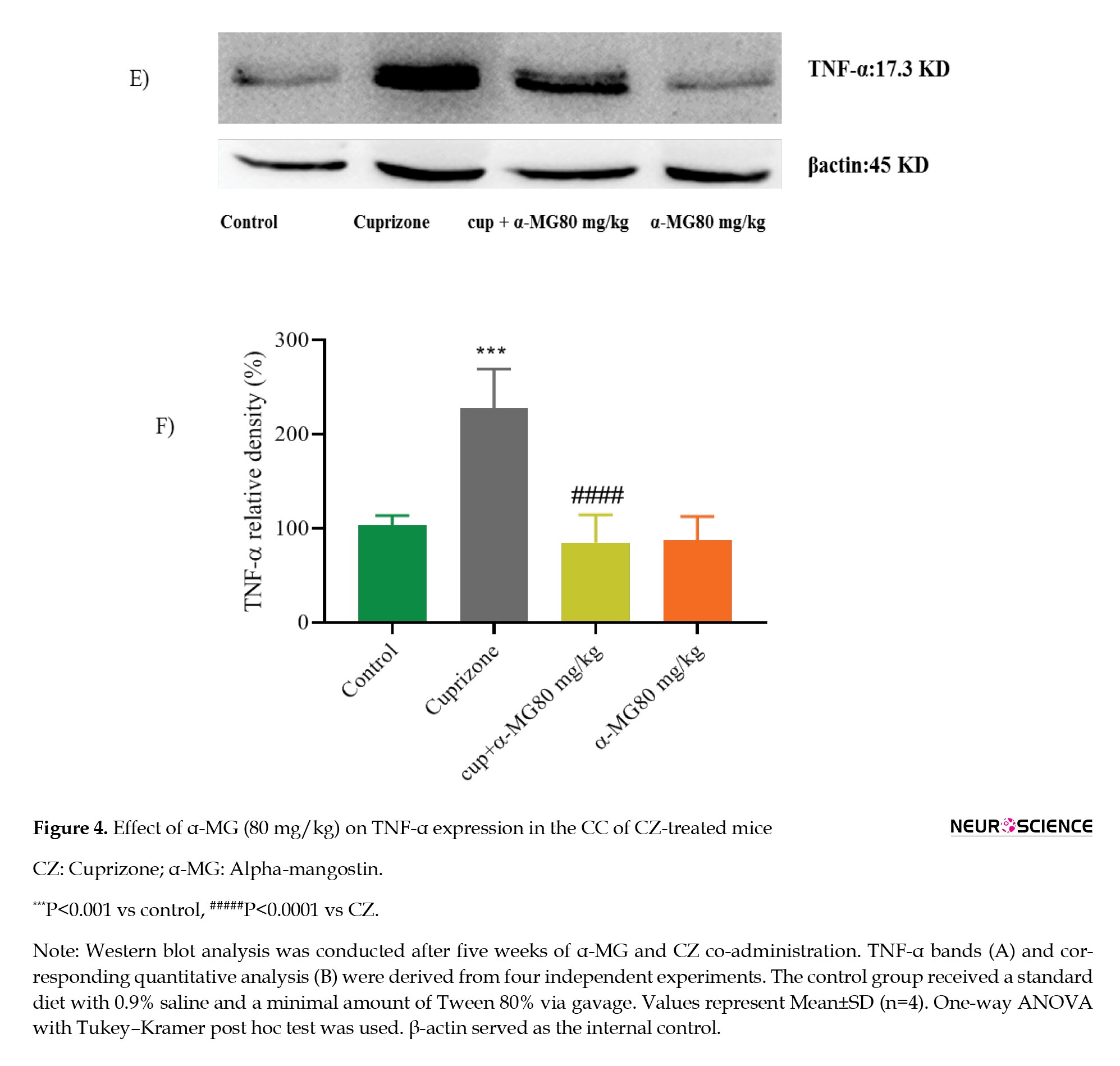
Demyelination induced by CZ was associated with a significant increase in the expression of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) (P<0.001). Treatment with α-MG (80 mg/kg) for five weeks significantly reduced TNF-α levels compared to the CZ group (P<0.0001), indicating a pronounced anti-inflammatory effect (Figures 4A and 4B).
4. Discussion
To our knowledge, this is the first study to investigate the effects of α-MG in a mouse model of CZ-induced MS. Our findings demonstrate that feeding mice a diet containing 0.4% (w/w) CZ led to pronounced neurotoxicity, characterized by reduced body weight and elevated oxidative stress, apoptosis, and inflammation in the CC. Oral administration of α-MG at doses of 20, 40, and 80 mg/kg over five weeks effectively prevented body weight loss and mitigated oxidative stress, apoptotic activity, and inflammatory responses in CZ-exposed mice.
Experimental models are critical for elucidating the mechanisms of MS and evaluating therapeutic interventions. Although no model fully recapitulates the complexity of human MS pathology, the CZ model has emerged as a widely accepted tool. CZ induces demyelination in key white matter regions, including the CC, enabling a clear distinction between demyelination and remyelination phases (Matsushima & Morell, 2001). This separation makes the model suitable for visualizing affected areas and evaluating potential therapeutic compounds that support remyelination and neuronal survival (Abakumova et al., 2015).
Previous studies have consistently shown that dietary CZ causes a dose-dependent and reversible reduction in body weight (Hiremath et al., 1998; Skripuletz et al., 2011; Steelman et al., 2012; Stidworthy et al., 2003). Severe weight loss at CZ concentrations above 0.3% is associated with increased mortality (Hiremath et al., 1998; Stidworthy et al., 2003). As a copper-chelating agent, CZ induces systemic copper deficiency, contributing to weight loss in both rats (Taylor et al., 1988) and mice (Prohaska, 1983). Consistent with these findings, our study observed significant weight loss in CZ-treated animals.
Notably, α-MG administration has previously been shown to reverse weight loss in animal models. For instance, in a rat model of autism, α-MG improved weight outcomes in a dose-dependent manner compared to methylmercury-exposed animals (Sahu et al., 2022; Tiwari et al., 2021). Similarly, our findings indicate that chronic administration of α-MG, particularly at 80 mg/kg, was effective in restoring body weight in CZ-treated mice. This suggests a protective role of α-MG against CZ-induced metabolic and systemic toxicity in the MS model.
According to multiple studies, CZ induces oxidative stress in the CC primarily by disrupting mitochondrial function in oligodendrocytes, a mechanism that closely resembles pattern III lesions observed in MS patients (Kang et al., 2012; Lucchinetti et al., 2000). CZ also impairs the mitochondrial electron transport chain (ETC) and antioxidant systems, leading to diminished production of key antioxidant enzymes such as SOD and GSH (Ghaiad et al., 2017). Furthermore, it increases MDA levels, a marker of lipid peroxidation and oxidative cell injury in the CC, ultimately contributing to cell death (Kashani et al., 2014). In line with previous research, our results show that CZ administration significantly elevated MDA concentrations in brain tissue.
α-MG has been extensively studied for its antioxidant properties. It acts by directly scavenging ROS, such as superoxide and hydrogen peroxide, and indirectly by enhancing endogenous antioxidant defenses (Márquez-Valadez et al., 2012; Martínez et al., 2011). Studies have shown that α-MG effectively reduces lipid peroxidation and boosts the activity of antioxidant enzymes. For instance, a mammary organ culture assay in mice demonstrated that α-MG could scavenge peroxynitrite, highlighting its potential as a botanical dietary antioxidant (Jung et al., 2006). Moreover, α-MG administration has been found to elevate GSH levels and reduce MDA concentrations in various experimental models (Ghasemzadeh Rahbardar et al., 2020; Liu et al., 2018). In vitro studies have confirmed its ability to mitigate oxidative damage in PC12 cells exposed to cadmium and arsenic (Ahmadian et al., 2022). Additionally, α-MG significantly lowered MDA levels and increased GSH in cardiac tissue following doxorubicin-induced cardiotoxicity in rats (Eisvand et al., 2022). Consistent with these findings, our results demonstrate that α-MG treatment at 20, 40, and 80 mg/kg significantly reduced MDA levels during the demyelination phase, confirming its role as a potent antioxidant.
In MS patients, demyelinated regions of the CNS are typically characterized by infiltrates of myelin-specific T cells and B cells from peripheral blood, which generate antibodies targeting myelin components. These findings support the widely accepted view that MS is a chronic autoimmune inflammatory demyelinating disorder of the CNS (Martino & Hartung, 1999). CZ-induced demyelination offers a reliable model for studying neuroinflammation in detail. In this model, inflammatory responses in the CC are marked by the activation and proliferation of astrocytes and microglia. Notably, peripheral immune cell infiltration is minimal, and the inflammatory response is largely mediated by resident microglial cells, which release inflammatory cytokines and pro-oxidative agents (Arnett et al., 2002; Hillis et al., 2016).
Experimental studies have confirmed that dietary CZ elevates levels of inflammatory cytokines—such as IL-1β, IL-6, and TNF-α—in the CC and other brain regions (Berghoff et al., 2017; Elbaz et al., 2018; Ghaiad et al., 2017; Rüther et al., 2017; Sanadgol et al., 2017; Vakilzadeh et al., 2015). In agreement with these findings, our study observed a significant increase in TNF-α levels following CZ treatment. The anti-inflammatory potential of α-MG has been well-documented, with evidence showing its ability to suppress the production of inflammatory mediators such as NO, PGE2, IL-1β, IL-6, and TNF-α (Liu et al., 2012; Mohan et al., 2018; Tiwari et al., 2021; Tousian et al., 2019; Tousian et al., 2020). Our results support these findings, showing that α-MG at 80 mg/kg significantly reduced TNF-α levels during demyelination. This suggests that α-MG has a protective effect against CZ-induced neuroinflammation and may be a viable adjunct therapy alongside conventional treatments (Chen et al., 2008).
Apoptosis is recognized as a primary mechanism of oligodendrocyte damage following acute injury in MS (Barnett & Prineas, 2004). CZ, a copper-chelating agent, induces apoptosis in oligodendrocytes and facilitates myelin degradation through oxidative stress pathways (Denic et al., 2011). Studies using C57BL/6 mice have shown that the earliest pathological event following CZ exposure is the apoptotic death of mature oligodendrocytes in the CC (Acs & Komoly, 2012; Blakemore, 1972). To assess apoptotic activity, the present study evaluated the expression of key regulatory proteins: Bax (pro-apoptotic), Bcl-2 (anti-apoptotic), and cleaved caspase-3 (a marker of apoptosis execution). Our findings are consistent with previous research, showing that CZ administration increases cleaved caspase-3 levels, elevates the Bax/Bcl-2 ratio, and decreases Bcl-2 expression in CC tissue (Sanadgol et al., 2020; Vakilzadeh et al., 2015, Vakilzadeh et al., 2016; Zahednasab et al., 2019).
In contrast, α-MG demonstrated a protective effect against CZ-induced apoptosis. Prior in vitro studies revealed that α-MG (10 µM) mitigates MPP+-induced apoptosis in SH-SY5Y cells by reducing ROS generation, balancing pro- and anti-apoptotic gene expression, and suppressing caspase-3 activation (Janhom & Dharmasaroja, 2015). Similarly, in rat chondrocytes, α-MG at concentrations of 3–12 µM inhibited apoptosis by downregulating Bax and caspase-3 expression (Pan et al., 2017). In a model of doxorubicin-induced cardiotoxicity, α-MG (100 mg/kg) reduced apoptosis through decreased levels of cleaved caspase-3 and a lower Bax/Bcl-2 ratio (Eisvand et al., 2022). These effects are attributed not only to direct modulation of apoptotic markers but also to α-MG’s influence on mitochondrial function—reducing electron leakage and ROS production (Reiter et al., 2001). Furthermore, α-MG has been shown to reduce cadmium-induced apoptosis by downregulating caspase-3 (Ahmadian et al., 2022).
Consistent with these reports, our study demonstrated that administration of α-MG at 80 mg/kg significantly reduced both cleaved caspase-3 levels and the Bax/Bcl-2 ratio in the CC of CZ-treated mice, indicating a marked anti-apoptotic effect.
5. Conclusion
In conclusion, this study provides the first evidence that α-mangostin effectively counteracts CZ-induced demyelination in mice by reducing oxidative stress, inflammation, and apoptosis in the CC. These findings suggest that α-MG may serve as a promising adjuvant therapy to support existing treatments for MS, potentially improving neuroprotection and disease management.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Internal Review Board and Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.SP.1396.189) and conducted in accordance with the Internationally Accepted Principles for Animal Use and Care (Zimmermann, 1983).
Funding
This study was funded by Mashhad University of Medical Sciences through start-up funds allocated to Associate Professor Hossein Hosseinzadeh (Grant No.: 960851).
Authors' contributions
Conceptualization, study design, resources, supervision, review and editing: Hossein Hosseinzadeh and Bibi Marjan Razavi; Material preparation, data collection, analysis and writing the original draft: Sara Banaeeyeh; Funding acquisition: Hossein Hosseinzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the Vice Chancellor of Research at Mashhad University of Medical Sciences, Mashhad, Iran, for financial help.
References
Multiple sclerosis (MS) is a chronic neurological disorder in which the immune system mistakenly attacks the protective covering of nerve fibers in the central nervous system (CNS), leading to widespread inflammatory lesions (Mauriz et al., 2013). The two principal pathological features of MS are inflammation and demyelination. Repeated inflammatory episodes result in the apoptosis of oligodendrocytes, axonal degeneration, and the activation of astrocytes and microglia (Bjartmar et al., 2003).
The loss of myelin sheaths surrounding axons in the brain and spinal cord leads to impaired signal transmission, contributing to the neurological dysfunction seen in MS (Basoglu et al., 2013; Hurwitz, 2009). This damage is mediated by the infiltration of inflammatory cells—predominantly lymphocytes and macrophages—into the CNS through the blood-brain barrier (BBB). Although the precise etiology of MS remains unclear, it is widely accepted that autoimmune mechanisms play a central role, involving T cells, B cells, macrophages, and dendritic cells (Frohman et al., 2006).
Oxidative stress and mitochondrial dysfunction are also known contributors to the progression and worsening of MS (Smirnova et al., 2011). Remyelination—the regenerative process that restores the damaged myelin sheath—is considered essential in mitigating neurodegeneration. However, this process is often incomplete, insufficient, or fails entirely, especially in progressive stages of the disease (Fiorio et al., 2006).
To investigate the mechanisms of demyelination and remyelination, a variety of experimental models have been developed. These include models based on genetic mutations, immune responses, viral infections, and toxic insults. While none of these fully replicate all stages of MS, they each offer valuable insight into specific pathological features. Among these, toxic demyelination models—particularly the cuprizone (CZ) model—have gained considerable attention and acceptance for their reliability and relevance in MS research.
These models include genetic myelin mutations, immune-mediated demyelination, viral-induced demyelination, and toxic demyelination models. It is important to note that no single model can fully replicate all stages of MS; each mimics only certain aspects of the disease’s complex pathology. Among these, the CZ-induced demyelination model has gained widespread acceptance in recent years due to its specificity and reproducibility (Kipp et al., 2009; Skripuletz et al., 2011).
CZ, chemically known as oxalic acid bis(cyclohexylidene hydrazide) is a well-characterized neurotoxic compound that acts as a selective copper chelator. Its toxicity is particularly directed at oligodendrocytes, leading to cell death and subsequent demyelination in various brain regions, notably the corpus callosum (CC) and superior cerebellar peduncles. As the largest myelinated tract in the brain, the CC is especially vulnerable to CZ-induced damage and is also considered a potential reservoir for neural stem cells. This makes the CC an ideal target for monitoring neuroprotective and remyelination processes (Kipp et al., 2017; Matsushima & Morell, 2001).
Administration of CZ for approximately 5–6 weeks results in acute demyelination of the CC. After withdrawal of CZ and return to a normal diet, partial spontaneous remyelination can occur over the following weeks (Kipp et al., 2009). However, prolonged exposure to CZ (12 weeks or more) significantly impairs remyelination and leads to a state referred to as chronic demyelination (Harsan et al., 2008; Tansey et al., 1996; Torkildsen et al., 2008). The CZ model is thus considered highly effective for studying both the degenerative and regenerative phases of demyelination due to its reliability, simplicity, and consistency (Matsushima & Morell, 2001).
Alpha-mangostin (α-MG) is a polyphenolic compound and the major xanthone extracted from the pericarp of the mangosteen fruit (Garcinia mangostana Linn) (Pedraza-Chaverri et al., 2008). Various components derived from mangosteen—including polyphenols, phenols, and xanthone derivatives—have been shown to exert beneficial effects in numerous disease conditions (Tousian Shandiz et al., 2017).
α-MG, a naturally occurring xanthone derived from plants, has demonstrated a highly selective inhibitory effect on acid sphingomyelinase (ASMase), in addition to a wide range of biological activities (Okudaira et al., 2000; Chairungsrilerd et al., 1996). These include pronounced anti-inflammatory and antioxidant properties (Chen et al., 2008; Devi Sampath & Vijayaraghavan, 2007). In several animal disease models, α-MG has been shown to sustain the activity of key antioxidant enzymes, such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), and the critical intracellular antioxidant glutathione (GSH) (Devi Sampath & Vijayaraghavan, 2007).
Research on Wistar rats has shown that α-MG acts as a neuroprotective agent in models of oxidative brain damage (Márquez-Valadez et al., 2012). Moreover, treatment with α-MG and curcumin in primary cultures of cerebellar granule neurons (CGNs) has been found to confer neuroprotection against iodoacetate-induced oxidative stress, primarily by reducing the production of reactive oxygen species (ROS) (Reyes-Fermín et al., 2012). In another study using rotenone as a cellular model of Parkinson’s disease, α-MG treatment preserved dopaminergic neurons and reduced α-synuclein accumulation, indicating its potential to prevent mitochondrial dysfunction and protein aggregation associated with neurodegeneration (Hao et al., 2017).
In light of these findings, the present study aimed to evaluate the efficacy of α-MG in mitigating clinical symptoms, inflammation, and oxidative stress in a CZ-induced demyelination model of MS using C57BL/6 mice over a 5-week experimental period. To our knowledge, this is the first investigation into the neuroprotective potential of α-MG within this context, including an exploration of the underlying molecular mechanisms.
2. Materials and Methods
Animals
Adult female C57BL/6 mice (8–10 weeks old), weighing 18–22 g, were obtained from the School of Pharmacy at Mashhad University of Medical Sciences, Mashhad, Iran. The animals were housed in groups of six per cage under controlled conditions: A 12-hour light/dark cycle, temperature of 22±2 °C, and relative humidity of 60±5%. All experiments were conducted in accordance with internationally accepted principles for the care and use of laboratory animals (Zimmermann, 1983).
Materials
The following reagents and materials were used in this study:
CZ (CAS No: 370810) was purchased from Sigma-Aldrich (USA), and α-MG (purity >90%) was obtained from Trademax Pharmaceuticals & Chemicals Co. (China).
Bovine serum albumin (BSA; Solarbio, China), dry skim milk (Quetlab, UK), ethylene glycol tetraacetic acid (EGTA; Sigma, USA), ethylenediaminetetraacetic acid (EDTA; Pars Tous Biotechnology, Iran), sodium deoxycholate (Sigma, New Zealand), sodium orthovanadate (Na3VO4; Sigma, India), and other general chemicals were procured from Merck (Germany).
Protease and phosphatase inhibitor cocktail and Pierce ECL Western blotting substrate were sourced from Thermo Fisher Scientific (USA).
The protein assay kit (Bradford reagent) and polyvinylidene difluoride (PVDF) membranes were obtained from Bio-Rad (USA).
Fetal bovine serum was purchased from Gibco (USA).
Malondialdehyde (MDA) and dizinon (DZN) were sourced from Fluka (Switzerland) and Shanghai Tosco Chemical Co. (China), respectively.
Primary antibodies included rabbit polyclonal anti-Bax (#2772, 1:1000), anti-Bcl2 (#2870, 1:1000), anti-cleaved caspase-3 (#9664, 1:1000), and anti-TNF-α (#3707, 1:1000), all obtained from Cell Signaling Technology (USA). Secondary antibodies (HRP-conjugated anti-rabbit and anti-mouse IgG) were also acquired from Cell Signaling.
Imaging was performed using the Alliance 4.7 Gel Doc system (UVtec, UK) and analyzed using UV Tec Software.
Experimental design
The CZ-induced demyelination model was established in adult female C57BL/6 mice (8–10 weeks old) following the protocol described by Kipp et al. (2009). Mice were fed a chow diet containing 0.4% (w/w) CZ for five weeks. α-MG was dissolved in 0.9% normal saline (NS) with two drops of Tween 80 and administered orally by gavage at doses of 20, 40, or 80 mg/kg, starting from day one and continuing daily for five weeks.
The animals were randomly assigned to six experimental groups (n=6 per group):
Control group: Received a normal diet and NS (0.9%) with 2 drops of Tween 80 via gavage for five weeks.
CPZ group: Received 0.4% (w/w) CZ mixed into milled chow for five weeks.
CPZ + α-MG 20 mg/kg group: Received CZ diet + 20 mg/kg α-MG by gavage.
CPZ + α-MG 40 mg/kg group: Received CZ diet + 40 mg/kg α-MG by gavage.
CPZ + α-MG 80 mg/kg group: Received CZ diet + 80 mg/kg α-MG by gavage.
α-MG 80 mg/kg only group: Received a normal diet + 80 mg/kg α-MG by gavage.
Measurement of body weight
Body weight was monitored daily throughout the experiment. However, only the measurements from the first and final days were recorded and reported.
Tissue preparation
At the end of the five-week experimental period, the mice were sacrificed, and the CC was carefully dissected from the brain and immediately stored at −80 °C until further processing.
Determination of MDA level in CC tissue
MDA levels were measured as an indicator of lipid peroxidation, reflecting oxidative damage. The following procedure was employed, adapted from Uchiyama and Mihara (1978):
1. A 10% tissue homogenate was prepared in 1.15% potassium chloride (KCl).
2. 0.5 mL of the homogenate was mixed with: 3 mL of 1% phosphoric acid and 1 mL of 6% thiobarbituric acid (TBA)
3. The mixture was boiled in water for 45 minutes.
4. After boiling, the tubes were cooled to room temperature.
5. 4 mL of n-butanol was added to each tube, and the mixture was vortexed for 1 minute.
6. Tubes were centrifuged at 3,500 rpm for 10 minutes.
7. The supernatant was collected and its absorbance was read at 532 nm.
8. A standard curve was created using MDA concentrations ranging from 0–100 nmol/mL.
9. MDA levels were expressed as nmol/g tissue.
Western blot assay
Western blot analysis was performed to assess protein expression related to apoptosis. The frozen CC tissue was lysed in a buffer containing 50 mM Tris-HCl (pH 7.4), 2 mM EDTA, 2 mM EGTA, 10 mM NaF, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate, 0.2% sodium deoxycholate, 1 mM PMSF, and a protease inhibitor cocktail. Samples were homogenized, sonicated on ice (3×10 seconds, with 10-second intervals), and centrifuged at 10,000 g for 10 minutes at 4 °C. Protein concentration was determined using the Bradford assay.
Samples were mixed with 2× SDS sample buffer (1:1 ratio), heated, aliquoted, and stored at −80 °C. For electrophoresis, 100 µg of protein per sample was loaded onto a 12% SDS-PAGE gel, separated, and transferred onto a PVDF membrane.
Membranes were blocked with skim milk for 2 hours, then incubated overnight at 4 °C with primary antibodies: Anti-Bax, anti-Bcl2, anti-cleaved caspase-3, anti-TNF-α, and anti-β-actin (all 1:1,000). After washing, membranes were incubated for 2 hours with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000). Protein bands were visualized using enhanced chemiluminescence, and densitometric analysis was performed using the Alliance 4.7 Gel Doc system and UV Tec Software. Protein expression was normalized to β-actin as a loading control.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software, version 8.0 (GraphPad Inc., San Diego, CA, USA). Data are presented as Mean±SEM. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to assess differences among groups for body weight, Western blot, and lipid peroxidation results. A P<0.05 was considered statistically significant.
3. Results
Effect of α-MG on body weight in CZ-treated mice
At the beginning of the experiment, there were no significant differences in body weight among the experimental groups. However, by the end of the 5-week period, mice in the CZ-treated group exhibited a significant reduction in body weight compared to the control group. Treatment with α-MG at a dose of 80 mg/kg effectively reversed this weight loss, resulting in a significant improvement in body weight compared to the CZ group (Table 1).

Effect of α-MG on oxidative stress marker in CZ-treated mice
Lipid peroxidation was assessed by measuring MDA levels in CC homogenates. Mice in the CZ group demonstrated a significant increase in MDA levels compared to the control group (P<0.01), indicating elevated oxidative stress. Treatment with α-MG for five weeks at doses of 20, 40, and 80 mg/kg significantly reduced MDA levels (P<0.01, P<0.001, and P<0.001, respectively), suggesting a dose-dependent antioxidative effect of α-MG (Figure 1).

Effect of α-MG on apoptotic markers in CZ-treated mice
Based on its strong antioxidant effect, the 80 mg/kg dose of α-MG was selected for further investigation of apoptotic markers. Western blot analysis was conducted to assess the expression of cleaved caspase-3, Bax, and Bcl-2 in CC tissue. The CZ group showed a significant increase in cleaved caspase-3 expression (P<0.0001) and in the Bax/Bcl-2 ratio (P<0.0001) compared to controls. Treatment with α-MG (80 mg/kg) for five weeks significantly decreased cleaved caspase-3 levels (P<0.0001) (Figures 2A and 2B), as well as the Bax/Bcl-2 ratio (P<0.0001) (Figures 3A and 3B), indicating its anti-apoptotic potential.


Effect of α-MG on the inflammatory marker in CZ-treated mice
Demyelination induced by CZ was associated with a significant increase in the expression of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) (P<0.001). Treatment with α-MG (80 mg/kg) for five weeks significantly reduced TNF-α levels compared to the CZ group (P<0.0001), indicating a pronounced anti-inflammatory effect (Figures 4A and 4B).

4. Discussion
To our knowledge, this is the first study to investigate the effects of α-MG in a mouse model of CZ-induced MS. Our findings demonstrate that feeding mice a diet containing 0.4% (w/w) CZ led to pronounced neurotoxicity, characterized by reduced body weight and elevated oxidative stress, apoptosis, and inflammation in the CC. Oral administration of α-MG at doses of 20, 40, and 80 mg/kg over five weeks effectively prevented body weight loss and mitigated oxidative stress, apoptotic activity, and inflammatory responses in CZ-exposed mice.
Experimental models are critical for elucidating the mechanisms of MS and evaluating therapeutic interventions. Although no model fully recapitulates the complexity of human MS pathology, the CZ model has emerged as a widely accepted tool. CZ induces demyelination in key white matter regions, including the CC, enabling a clear distinction between demyelination and remyelination phases (Matsushima & Morell, 2001). This separation makes the model suitable for visualizing affected areas and evaluating potential therapeutic compounds that support remyelination and neuronal survival (Abakumova et al., 2015).
Previous studies have consistently shown that dietary CZ causes a dose-dependent and reversible reduction in body weight (Hiremath et al., 1998; Skripuletz et al., 2011; Steelman et al., 2012; Stidworthy et al., 2003). Severe weight loss at CZ concentrations above 0.3% is associated with increased mortality (Hiremath et al., 1998; Stidworthy et al., 2003). As a copper-chelating agent, CZ induces systemic copper deficiency, contributing to weight loss in both rats (Taylor et al., 1988) and mice (Prohaska, 1983). Consistent with these findings, our study observed significant weight loss in CZ-treated animals.
Notably, α-MG administration has previously been shown to reverse weight loss in animal models. For instance, in a rat model of autism, α-MG improved weight outcomes in a dose-dependent manner compared to methylmercury-exposed animals (Sahu et al., 2022; Tiwari et al., 2021). Similarly, our findings indicate that chronic administration of α-MG, particularly at 80 mg/kg, was effective in restoring body weight in CZ-treated mice. This suggests a protective role of α-MG against CZ-induced metabolic and systemic toxicity in the MS model.
According to multiple studies, CZ induces oxidative stress in the CC primarily by disrupting mitochondrial function in oligodendrocytes, a mechanism that closely resembles pattern III lesions observed in MS patients (Kang et al., 2012; Lucchinetti et al., 2000). CZ also impairs the mitochondrial electron transport chain (ETC) and antioxidant systems, leading to diminished production of key antioxidant enzymes such as SOD and GSH (Ghaiad et al., 2017). Furthermore, it increases MDA levels, a marker of lipid peroxidation and oxidative cell injury in the CC, ultimately contributing to cell death (Kashani et al., 2014). In line with previous research, our results show that CZ administration significantly elevated MDA concentrations in brain tissue.
α-MG has been extensively studied for its antioxidant properties. It acts by directly scavenging ROS, such as superoxide and hydrogen peroxide, and indirectly by enhancing endogenous antioxidant defenses (Márquez-Valadez et al., 2012; Martínez et al., 2011). Studies have shown that α-MG effectively reduces lipid peroxidation and boosts the activity of antioxidant enzymes. For instance, a mammary organ culture assay in mice demonstrated that α-MG could scavenge peroxynitrite, highlighting its potential as a botanical dietary antioxidant (Jung et al., 2006). Moreover, α-MG administration has been found to elevate GSH levels and reduce MDA concentrations in various experimental models (Ghasemzadeh Rahbardar et al., 2020; Liu et al., 2018). In vitro studies have confirmed its ability to mitigate oxidative damage in PC12 cells exposed to cadmium and arsenic (Ahmadian et al., 2022). Additionally, α-MG significantly lowered MDA levels and increased GSH in cardiac tissue following doxorubicin-induced cardiotoxicity in rats (Eisvand et al., 2022). Consistent with these findings, our results demonstrate that α-MG treatment at 20, 40, and 80 mg/kg significantly reduced MDA levels during the demyelination phase, confirming its role as a potent antioxidant.
In MS patients, demyelinated regions of the CNS are typically characterized by infiltrates of myelin-specific T cells and B cells from peripheral blood, which generate antibodies targeting myelin components. These findings support the widely accepted view that MS is a chronic autoimmune inflammatory demyelinating disorder of the CNS (Martino & Hartung, 1999). CZ-induced demyelination offers a reliable model for studying neuroinflammation in detail. In this model, inflammatory responses in the CC are marked by the activation and proliferation of astrocytes and microglia. Notably, peripheral immune cell infiltration is minimal, and the inflammatory response is largely mediated by resident microglial cells, which release inflammatory cytokines and pro-oxidative agents (Arnett et al., 2002; Hillis et al., 2016).
Experimental studies have confirmed that dietary CZ elevates levels of inflammatory cytokines—such as IL-1β, IL-6, and TNF-α—in the CC and other brain regions (Berghoff et al., 2017; Elbaz et al., 2018; Ghaiad et al., 2017; Rüther et al., 2017; Sanadgol et al., 2017; Vakilzadeh et al., 2015). In agreement with these findings, our study observed a significant increase in TNF-α levels following CZ treatment. The anti-inflammatory potential of α-MG has been well-documented, with evidence showing its ability to suppress the production of inflammatory mediators such as NO, PGE2, IL-1β, IL-6, and TNF-α (Liu et al., 2012; Mohan et al., 2018; Tiwari et al., 2021; Tousian et al., 2019; Tousian et al., 2020). Our results support these findings, showing that α-MG at 80 mg/kg significantly reduced TNF-α levels during demyelination. This suggests that α-MG has a protective effect against CZ-induced neuroinflammation and may be a viable adjunct therapy alongside conventional treatments (Chen et al., 2008).
Apoptosis is recognized as a primary mechanism of oligodendrocyte damage following acute injury in MS (Barnett & Prineas, 2004). CZ, a copper-chelating agent, induces apoptosis in oligodendrocytes and facilitates myelin degradation through oxidative stress pathways (Denic et al., 2011). Studies using C57BL/6 mice have shown that the earliest pathological event following CZ exposure is the apoptotic death of mature oligodendrocytes in the CC (Acs & Komoly, 2012; Blakemore, 1972). To assess apoptotic activity, the present study evaluated the expression of key regulatory proteins: Bax (pro-apoptotic), Bcl-2 (anti-apoptotic), and cleaved caspase-3 (a marker of apoptosis execution). Our findings are consistent with previous research, showing that CZ administration increases cleaved caspase-3 levels, elevates the Bax/Bcl-2 ratio, and decreases Bcl-2 expression in CC tissue (Sanadgol et al., 2020; Vakilzadeh et al., 2015, Vakilzadeh et al., 2016; Zahednasab et al., 2019).
In contrast, α-MG demonstrated a protective effect against CZ-induced apoptosis. Prior in vitro studies revealed that α-MG (10 µM) mitigates MPP+-induced apoptosis in SH-SY5Y cells by reducing ROS generation, balancing pro- and anti-apoptotic gene expression, and suppressing caspase-3 activation (Janhom & Dharmasaroja, 2015). Similarly, in rat chondrocytes, α-MG at concentrations of 3–12 µM inhibited apoptosis by downregulating Bax and caspase-3 expression (Pan et al., 2017). In a model of doxorubicin-induced cardiotoxicity, α-MG (100 mg/kg) reduced apoptosis through decreased levels of cleaved caspase-3 and a lower Bax/Bcl-2 ratio (Eisvand et al., 2022). These effects are attributed not only to direct modulation of apoptotic markers but also to α-MG’s influence on mitochondrial function—reducing electron leakage and ROS production (Reiter et al., 2001). Furthermore, α-MG has been shown to reduce cadmium-induced apoptosis by downregulating caspase-3 (Ahmadian et al., 2022).
Consistent with these reports, our study demonstrated that administration of α-MG at 80 mg/kg significantly reduced both cleaved caspase-3 levels and the Bax/Bcl-2 ratio in the CC of CZ-treated mice, indicating a marked anti-apoptotic effect.
5. Conclusion
In conclusion, this study provides the first evidence that α-mangostin effectively counteracts CZ-induced demyelination in mice by reducing oxidative stress, inflammation, and apoptosis in the CC. These findings suggest that α-MG may serve as a promising adjuvant therapy to support existing treatments for MS, potentially improving neuroprotection and disease management.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Internal Review Board and Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.SP.1396.189) and conducted in accordance with the Internationally Accepted Principles for Animal Use and Care (Zimmermann, 1983).
Funding
This study was funded by Mashhad University of Medical Sciences through start-up funds allocated to Associate Professor Hossein Hosseinzadeh (Grant No.: 960851).
Authors' contributions
Conceptualization, study design, resources, supervision, review and editing: Hossein Hosseinzadeh and Bibi Marjan Razavi; Material preparation, data collection, analysis and writing the original draft: Sara Banaeeyeh; Funding acquisition: Hossein Hosseinzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to the Vice Chancellor of Research at Mashhad University of Medical Sciences, Mashhad, Iran, for financial help.
References
Abakumova, T. O., Kuz'kina, A. A., Zharova, M. E., Pozdeeva, D. A., Gubskii, I. L., & Shepeleva, I. I., et al. (2015). Cuprizone Model as a Tool for Preclinical Studies of the Efficacy of Multiple Sclerosis Diagnosis and Therapy. Bulletin of Experimental Biology and Medicine, 159(1), 111–115. [DOI:10.1007/s10517-015-2940-0] [PMID]
Acs, P., & Komoly, S. (2012). Selective ultrastructural vulnerability in the cuprizone-induced experimental demyelination. Ideggyogyaszati Szemle, 65(7-8), 266-270. [PMID]
Ahmadian, R., Heidari, M. R., Razavi, B. M., & Hosseinzadeh, H. (2023). Alpha-mangostin protects PC12 cells against neurotoxicity induced by cadmium and arsenic. Biological Trace Element Research, 201(8), 4008–4021. [DOI:10.1007/s12011-022-03498-8] [PMID]
Arnett, H. A., Hellendall, R. P., Matsushima, G. K., Suzuki, K., Laubach, V. E., & Sherman, P., et al. (2002). The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. The Journal of Immunology, 168(1), 427-433. [DOI:10.4049/jimmunol.168.1.427] [PMID]
Barnett, M. H., & Prineas, J. W. (2004). Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Annals of Neurology, 55(4), 458-468. [DOI:10.1002/ana.20016] [PMID]
Basoglu, H., Boylu, N. T., & Kose, H. (2013). Cuprizone-induced demyelination in Wistar rats; Electrophysiological and histological assessment. European Review for Medical and Pharmacological Sciences, 17(20), 2711-2717. [PMID]
Berghoff, S. A., Düking, T., Spieth, L., Winchenbach, J., Stumpf, S. K., & Gerndt, N., et al. (2017). Blood-brain barrier hyperpermeability precedes demyelination in the cuprizone model. Acta Neuropathologica Communications, 5(1), 94. [DOI:10.1186/s40478-017-0497-6] [PMID]
Bjartmar, C., Wujek, J. R., & Trapp, B. D. (2003). Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. Journal of the Neurological Sciences, 206(2), 165–171. [DOI:10.1016/s0022-510x(02)00069-2] [PMID]
Blakemore, W. F. (1972). Observations on oligodendrocyte degeneration, the resolution of status spongiosus and remyelination in cuprizone intoxication in mice. Journal of Neurocytology, 1(4), 413-426. [DOI:10.1007/BF01102943]
Chairungsrilerd, N., Furukawa, K., Ohta, T., Nozoe, S., & Ohizumi, Y. (1996). Pharmacological properties of alpha-mangostin, a novel histamine H1 receptor antagonist. European Journal of Pharmacology, 314(3), 351–356. [DOI:10.1016/s0014-2999(96)00562-6] [PMID]
Chen, L. G., Yang, L. L., & Wang, C. C. (2008). Anti-inflammatory activity of mangostins from Garcinia mangostana. Food and Chemical Toxicology, 46(2), 688-693. [DOI:10.1016/j.fct.2007.09.096] [PMID]
Denic, A., Johnson, A. J., Bieber, A. J., Warrington, A. E., Rodriguez, M., & Pirko, I. (2011). The relevance of animal models in multiple sclerosis research. Pathophysiology, 18(1), 21-29. [DOI:10.1016/j.pathophys.2010.04.004] [PMID]
Devi Sampath, P., & Vijayaraghavan, K. (2007). Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. Journal of Biochemical and Molecular Toxicology, 21(6), 336–339. [DOI:10.1002/jbt.20199] [PMID]
Eisvand, F., Imenshahidi, M., Ghasemzadeh Rahbardar, M., Tabatabaei Yazdi, S. A., Rameshrad, M., & Razavi, B. M., et al. (2022). Cardioprotective effects of alpha‐mangostin on doxorubicin‐induced cardiotoxicity in rats. Phytotherapy Research, 36(1), 506-524. [DOI:10.1002/ptr.7356] [PMID]
Elbaz, E. M., Senousy, M. A., El-Tanbouly, D. M., & Sayed, R. H. (2018). Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicology and Applied Pharmacology, 352, 153-161. [DOI:10.1016/j.taap.2018.05.035] [PMID]
Fiorio, M., Tinazzi, M., & Aglioti, S. M. (2006). Selective impairment of hand mental rotation in patients with focal hand dystonia. Brain, 129(p1), 47-54. [DOI:10.1093/brain/awh630] [PMID]
Frohman, E. M., Racke, M. K., & Raine, C. S. (2006). Multiple sclerosis-the plaque and its pathogenesis. The New England Journal of Medicine, 354(9), 942-955. [DOI:10.1056/NEJMra052130] [PMID]
Ghaiad, H. R., Nooh, M. M., El-Sawalhi, M. M., & Shaheen, A. A. (2017). Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Molecular Neurobiology, 54(5), 3219-3229. [DOI:10.1007/s12035-016-9891-5] [PMID]
Ghasemzadeh Rahbardar, M., Razavi, B. M., & Hosseinzadeh, H. (2020). Investigating the ameliorative effect of alpha-mangostin on development and existing pain in a rat model of neuropathic pain. Phytotherapy Research, 34(12), 3211–3225. [DOI:10.1002/ptr.6768] [PMID]
Hao, X. M., Li, L. D., Duan, C. L., & Li, Y. J. (2017). Neuroprotective effect of α-mangostin on mitochondrial dysfunction and α-synuclein aggregation in rotenone-induced model of Parkinson's disease in differentiated SH-SY5Y cells. Journal of Asian Natural Products Research, 19(8), 833–845. [DOI:10.1080/10286020.2017.1339349] [PMID]
Harsan, L. A., Steibel, J., Zaremba, A., Agin, A., Sapin, R., & Poulet, P., et al. (2008). Recovery from chronic demyelination by thyroid hormone therapy: Myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. The Journal of Neuroscience, 28(52), 14189–14201. [DOI:10.1523/JNEUROSCI.4453-08.2008] [PMID]
Hillis, J. M., Davies, J., Mundim, M. V., Al-Dalahmah, O., & Szele, F. G. (2016). Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. Journal of Neuroinflammation, 13(1), 190. [DOI:10.1186/s12974-016-0651-2] [PMID]
Hiremath, M. M., Saito, Y., Knapp, G. W., Ting, J. Y., Suzuki, K., & Matsushima, G. K. (1998). Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. Journal of Neuroimmunology, 92(1-2), 38-49. [DOI:10.1016/S0165-5728(98)00168-4]
Hurwitz B. J. (2009). The diagnosis of multiple sclerosis and the clinical subtypes. Annals of Indian Academy of Neurology, 12(4), 226–230. [DOI:10.4103/0972-2327.58276] [PMID]
Janhom, P., & Dharmasaroja, P. (2015). Neuroprotective effects of alpha-mangostin on mpP(+)-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. Journal of Toxicology, 2015, 919058. [DOI:10.1155/2015/919058] [PMID]
Jung, H. A., Su, B. N., Keller, W. J., Mehta, R. G., & Kinghorn, A. D. (2006). Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). Journal of Agricultural and Food Chemistry, 54(6), 2077–2082. [DOI:10.1021/jf052649z] [PMID]
Kang, Z., Liu, L., Spangler, R., Spear, C., Wang, C., Gulen, M. F., et al. (2012). IL-17-induced Act1-mediated signaling is critical for cuprizone-induced demyelination. The Journal of Neuroscience, 32(24), 8284–8292. [DOI:10.1523/JNEUROSCI.0841-12.2012] [PMID]
Kashani, I. R., Rajabi, Z., Akbari, M., Hassanzadeh, G., Mohseni, A., & Eramsadati, M. K., et al. (2014). Protective effects of melatonin against mitochondrial injury in a mouse model of multiple sclerosis. Experimental Brain Research, 232(9), 2835–2846. [DOI:10.1007/s00221-014-3946-5] [PMID]
Kipp, M., Clarner, T., Dang, J., Copray, S., & Beyer, C. (2009). The cuprizone animal model: New insights into an old story. Acta Neuropathologica, 118(6), 723–736. [DOI:10.1007/s00401-009-0591-3] [PMID]
Kipp, M., Nyamoya, S., Hochstrasser, T., & Amor, S. (2017). Multiple sclerosis animal models: A clinical and histopathological perspective. Brain Pathology, 27(2), 123–137. [DOI:10.1111/bpa.12454] [PMID]
Liu, G., Tang, L., She, J., Xu, J., Gu, Y., & Liu, H., et al. (2018). [Alpha-mangostin attenuates focal segmental glomerulosclerosis of mice induced by adriamycin (Chinese)]. Zhong Nan Da Xue Xue Bao, 43(10), 1089–1096. [DOI:10.11817/j.issn.1672-7347.2018.10.008] [PMID]
Liu, S. H., Lee, L. T., Hu, N. Y., Huange, K. K., Shih, Y. C., & Munekazu, I., et al. (2012). Effects of alpha-mangostin on the expression of anti-inflammatory genes in U937 cells. Chinese Medicine, 7(1), 19. [DOI:10.1186/1749-8546-7-19] [PMID]
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. (2000). Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Annals of Neurology. 47(6):707-17. [DOI:10.1002/1531-8249(200006)47:63.0.CO;2-Q]
Márquez-Valadez, B., Maldonado, P. D., Galván-Arzate, S., Méndez-Cuesta, L. A., Pérez-De La Cruz, V., Pedraza-Chaverrí, J., et al. (2012). Alpha-mangostin induces changes in glutathione levels associated with glutathione peroxidase activity in rat brain synaptosomes. Nutritional Neuroscience, 15(5), 13–19. [DOI:10.1179/147683012X13327575416400] [PMID]
Martínez, A., Galano, A., & Vargas, R. (2011). Free radical scavenger properties of α-mangostin: Thermodynamics and kinetics of HAT and RAF mechanisms. The Journal of Physical Chemistry. B, 115(43), 12591–12598. [DOI:10.1021/jp205496u] [PMID]
Martino, G., & Hartung, H. P. (1999). Immunopathogenesis of multiple sclerosis: The role of T cells. Current Opinion in Neurology, 12(3), 309–321. [DOI:10.1097/00019052-199906000-00010] [PMID]
Matsushima, G. K., & Morell, P. (2001). The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathology, 11(1), 107–116. [DOI:10.1111/j.1750-3639.2001.tb00385.x] [PMID]
Mauriz, E., Laliena, A., Vallejo, D., Tuñón, M. J., Rodríguez-López, J. M., & Rodríguez-Pérez, R., et al. (2013). Effects of a low-fat diet with antioxidant supplementation on biochemical markers of multiple sclerosis long-term care residents. Nutricion Hospitalaria, 28(6), 2229-2235. [Link]
Mohan, S., Syam, S., Abdelwahab, S. I., & Thangavel, N., (2018). An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food & Function, 9(7), 3860–3871. [DOI:10.1039/C8FO00439K] [PMID]
Okudaira, C., Ikeda, Y., Kondo, S., Furuya, S., Hirabayashi, Y., & Koyano, T., et al. (2000). Inhibition of acidic sphingomyelinase by xanthone compounds isolated from Garcinia speciosa. Journal of Enzyme Inhibition, 15(2), 129–138.[DOI:10.1080/14756360009030346] [PMID]
Pan, T., Chen, R., Wu, D., Cai, N., Shi, X., & Li, B., et al. (2017). Alpha-Mangostin suppresses interleukin-1β-induced apoptosis in rat chondrocytes by inhibiting the NF-κB signaling pathway and delays the progression of osteoarthritis in a rat model. International Immunopharmacology, 52, 156–162. [DOI:10.1016/j.intimp.2017.08.021] [PMID]
Pedraza-Chaverri, J., Cárdenas-Rodríguez, N., Orozco-Ibarra, M., & Pérez-Rojas, J. M. (2008). Medicinal properties of mangosteen (Garcinia mangostana). Food and Chemical Toxicology, 46(10), 3227–3239. [DOI:10.1016/j.fct.2008.07.024] [PMID]
Prohaska J. R. (1983). Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. The Journal of Nutrition, 113(10), 2048–2058. [DOI:10.1093/jn/113.10.2048] [PMID]
Reiter, R. J., Tan, D. X., Manchester, L. C., & Qi, W. (2001). Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochemistry and Biophysics, 34(2), 237–256. [DOI:10.1385/CBB:34:2:237] [PMID]
Reyes-Fermín, L. M., González-Reyes, S., Tarco-Álvarez, N. G., Hernández-Nava, M., Orozco-Ibarra, M., & Pedraza-Chaverri, J. (2012). Neuroprotective effect of α-mangostin and curcumin against iodoacetate-induced cell death. Nutritional Neuroscience, 15(5), 34–41. [DOI:10.1179/1476830512Y.0000000011] [PMID]
Rüther, B. J., Scheld, M., Dreymueller, D., Clarner, T., Kress, E., Brandenburg, L. O., et al. (2017). Combination of cuprizone and experimental autoimmune encephalomyelitis to study inflammatory brain lesion formation and progression. Glia, 65(12), 1900–1913. [DOI:10.1002/glia.23202] [PMID]
Sahu, R., Mehan, S., Kumar, S., Prajapati, A., Alshammari, A., & Alharbi, M., et al. (2022). Effect of alpha-mangostin in the prevention of behavioural and neurochemical defects in methylmercury-induced neurotoxicity in experimental rats. Toxicology Reports, 9, 977–998. [DOI:10.1016/j.toxrep.2022.04.023] [PMID]
Sanadgol, N., Barati, M., Houshmand, F., Hassani, S., Clarner, T., & Shahlaei, M., et al. (2020). Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacological Reports, 72(3), 641–658. [DOI:10.1007/s43440-019-00019-8] [PMID]
Sanadgol, N., Golab, F., Tashakkor, Z., Taki, N., Moradi Kouchi, S., & Mostafaie, A., et al. (2017). Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharmaceutical Biology, 55(1), 1679–1687. [DOI:10.1080/13880209.2017.1319867] [PMID]
Skripuletz, T., Gudi, V., Hackstette, D., & Stangel, M. (2011). De-and remyelination in the CNS white and grey matter induced by cuprizone: The old, the new, and the unexpected. Histology and Histopathology, 26(12), 2011. [Link]
Smirnova, L. P., Krotenko, N. V., Grishko, E. V., Krotenko, N. M., Alifirova, V. M., & Ivanova, S. A. (2011). The state of the antioxidant system during therapy of patients with multiple sclerosis. Biochemistry (Moscow) Supplement Series B, 5(1), 76-80. [DOI:10.1134/S1990750811010136]
Steelman, A. J., Thompson, J. P., & Li, J. (2012). Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neuroscience Research, 72(1), 32–42. [DOI:10.1016/j.neures.2011.10.002] [PMID]
Stidworthy, M. F., Genoud, S., Suter, U., Mantei, N., & Franklin, R. J. (2003). Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathology, 13(3), 329–339. [DOI:10.1111/j.1750-3639.2003.tb00032.x] [PMID]
Tansey, F. A., Zhang, H., & Cammer, W. (1996). Expression of carbonic anhydrase II mRNA and protein in oligodendrocytes during toxic demyelination in the young adult mouse. Neurochemical Research, 21(4), 411–416. [DOI:10.1007/BF02527704] [PMID]
Taylor, C. G., Bettger, W. J., & Bray, T. M. (1988). Effect of dietary zinc or copper deficiency on the primary free radical defense system in rats. The Journal of Nutrition, 118(5), 613–621. [DOI:10.1093/jn/118.5.613] [PMID]
Tiwari, A., Khera, R., Rahi, S., Mehan, S., Makeen, H. A., & Khormi, Y. H., et al. (2021). Neuroprotective effect of α-mangostin in the ameliorating propionic acid-induced experimental model of autism in wistar rats. Brain Sciences, 11(3), 288. [DOI:10.3390/brainsci11030288] [PMID]
Torkildsen, O., Brunborg, L. A., Myhr, K. M., & Bø, L. (2008). The cuprizone model for demyelination. Acta neurologica Scandinavica. Supplementum, 188, 72–76. [DOI:10.1111/j.1600-0404.2008.01036.x] [PMID]
Tousian, H., Razavi, B. M., & Hosseinzadeh, H. (2020). Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. Daru, 28(1), 45–55. [DOI:10.1007/s40199-019-00305-z] [PMID]
Tousian, H., Razavi, B. M., & Hosseinzadeh, H. (2020). Effects of alpha-mangostin on memory senescence induced by high glucose in human umbilical vein endothelial cells. Iranian Journal of Basic Medical Sciences, 23(10), 1261–1267. [DOI:10.22038/ijbms.2020.40651.9612] [PMID]
Tousian Shandiz, H., Razavi, B. M., & Hosseinzadeh, H. (2017). Review of garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytotherapy Research, 31(8), 1173–1182. [DOI:10.1002/ptr.5862] [PMID]
Uchiyama, M., & Mihara, M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry, 86(1), 271-278. [DOI:10.1016/0003-2697(78)90342-1]
Vakilzadeh, G., Khodagholi, F., Ghadiri, T., Darvishi, M., Ghaemi, A., & Noorbakhsh, F., et al. (2015). Protective effect of a cAMP analogue on behavioral deficits and neuropathological changes in cuprizone model of demyelination. Molecular Neurobiology, 52(1), 130–141. [DOI:10.1007/s12035-014-8857-8] [PMID]
Vakilzadeh, G., Khodagholi, F., Ghadiri, T., Ghaemi, A., Noorbakhsh, F., & Sharifzadeh, M., et al. (2016). The effect of melatonin on behavioral, molecular, and histopathological changes in cuprizone model of demyelination. Molecular Neurobiology, 53(7), 4675–4684. [DOI:10.1007/s12035-015-9404-y] [PMID]
Zahednasab, H., Firouzi, M., Kaboudanian-Ardestani, S., Mojallal-Tabatabaei, Z., Karampour, S., & Keyvani, H. (2019). The protective effect of rifampicin on behavioral deficits, biochemical, and neuropathological changes in a cuprizone model of demyelination. Cytokine, 113, 417–426. [DOI:10.1016/j.cyto.2018.10.016] [PMID]
Zimmermann, M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16,109-110. [DOI: 10.1016/0304-3959(83)90201-4] [PMID]
Type of Study: Original |
Subject:
Cellular and molecular Neuroscience
Received: 2023/05/7 | Accepted: 2024/05/25 | Published: 2025/05/1
Received: 2023/05/7 | Accepted: 2024/05/25 | Published: 2025/05/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








